|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
|
33
-
0336973
|
|
(State or other jurisdiction of
incorporation or organization) |
|
(IRS Employer Identification No.)
|
|
Large accelerated filer
|
|
Accelerated filer
|
|
|
|
|
|
Non-accelerated filer
(Do not check if a smaller reporting company) |
|
Smaller reporting company
|
| * | Excludes 19,641,255 shares of common stock held by directors and officers and by stockholders whose beneficial ownership is known by the Registrant to exceed 10% of the common stock outstanding at June 30, 2014. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant, or that such person is controlled by or under common control with the Registrant. |
|
PART I
|
||
|
Page
|
||
|
Item 1.
|
Business
|
4 |
|
Item 1A.
|
Risk Factors
|
60 |
|
Item 1B.
|
Unresolved Staff Comments
|
69 |
|
Item 2.
|
Properties
|
70 |
|
Item 3.
|
Legal Proceedings
|
70 |
|
Item 4.
|
Mine Safety Disclosures
|
71 |
|
PART II
|
||
|
Item 5.
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
71 |
|
Item 6.
|
Selected Financial Data
|
73 |
|
Item 7.
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
74 |
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
98 |
|
Item 8.
|
Financial Statements and Supplementary Data
|
98 |
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
98 |
|
Item 9A.
|
Controls and Procedures
|
98 |
|
Item 9B.
|
Other Information
|
101 |
|
PART III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
101 |
|
Item 11.
|
Executive Compensation
|
104 |
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
137 |
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
141 |
|
Item 14.
|
Principal Accounting Fees and Services
|
143 |
|
PART IV
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
144 |
|
Signatures
|
145 | |
| | We and Genzyme reported data from a Phase 3 long-term extension study in patients treated with KYNAMRO (mipomersen sodium) injection. In the study, a retrospective analysis of 104 patients with familial hypercholesterolemia treated with KYNAMRO for a mean of one or two years had a significant reduction in major adverse cardiovascular events, or MACE, compared to two years prior to therapy. |
| o | At the European Society of Cardiology Congress, our KYNAMRO advisor, John Kastelein, M.D., Ph.D., presented an analysis of patients treated with KYNAMRO for one year. These patients experienced a reduction in MACE of 4.85/1000 compared to 25.72/1000 months (in the two years prior to KYNAMRO treatment). |
| o | At the American Heart Association Meeting, a KYNAMRO principal investigator, P. Bart Duell, M.D., presented an analysis of patients treated with KYNAMRO for two years. These patients experienced a seven-fold reduction in MACE of 3.6/1000 compared to 25.72/1000 months (in the two years prior to KYNAMRO treatment). |
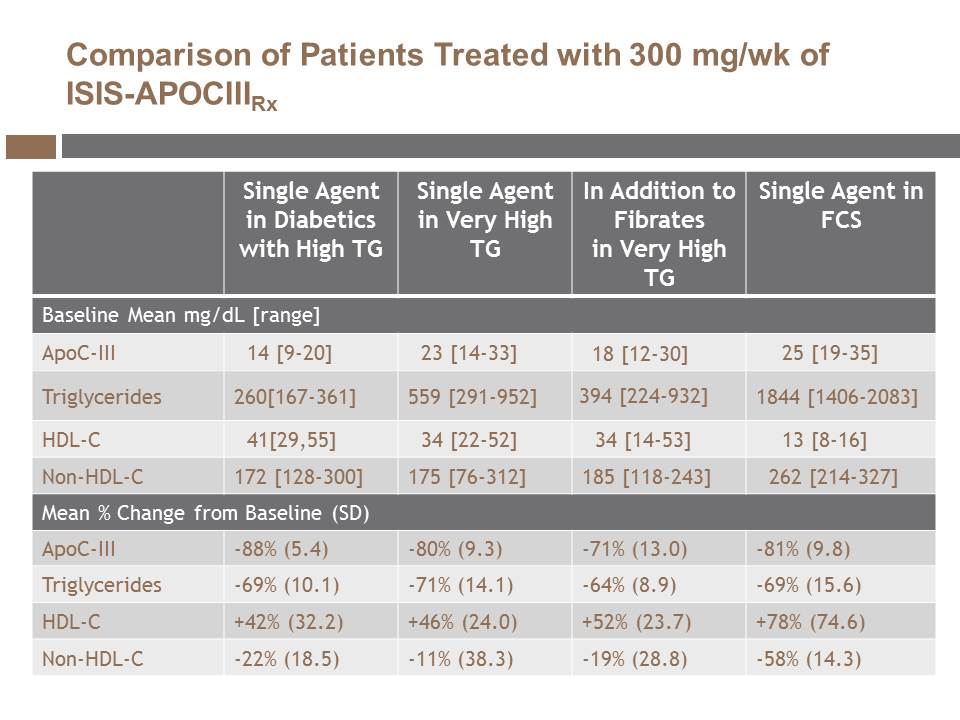
| | We reported Phase 2 data on ISIS-APOCIII Rx in patients with high to extremely high triglyceride levels, in patients with type 2 diabetes and high triglycerides and as a single agent as well as in combination with fibrates. |
| o | At the American College of Cardiology meeting, we presented Phase 2 data on ISIS-APOCIII Rx in combination with fibrates in patients with high triglycerides. In this study, patients achieved statistically significant reductions in triglycerides, apoC-III protein and statistically significant increases in HDL-cholesterol, on top of improvements achieved with each patient's existing therapeutic regimen of triglyceride-lowering drugs. |
| o | At the Arteriosclerosis, Thrombosis and Vascular Biology meeting, we presented Phase 2 data on ISIS-APOCIII Rx in patients with type 2 diabetes and high triglycerides. In this study, patients with diabetes experienced statistically significant decreases in triglyceride levels and statistically significant improvements in glucose control with trends toward enhanced insulin sensitivity. |
| o | At the National Lipid Association meeting, we presented Phase 2 data on ISIS-APOCIII Rx in patients with FCS. FCS is a rare genetic disorder characterized by severely elevated levels of triglycerides. Current treatment options are inadequate and, as a result, patients with FCS have an increased risk of recurrent and potentially fatal pancreatitis and other complications. In this study, patients with extremely high triglycerides experienced substantial reductions of triglycerides that correlated with substantial reductions in triglyceride-rich chylomicrons. We published these data in the New England Journal of Medicine. |
| o | At the 2014 European Society of Cardiology Congress, our collaborator, John Kastelein, M.D., Ph.D. presented an overview of the ISIS-APOCIII Rx Phase 2 program in which treatment with ISIS-APOCIII Rx produced consistent, robust and statistically significant reductions in triglycerides, apoC-III and non-HDL-cholesterol and increases in HDL-cholesterol in all patient populations evaluated. |
|
●
|
At the European Society of Cardiology Congress, Dr. Sotirios Tsimikas, M.D. presented data from the Phase 1 study of ISIS-APO(a)
Rx
in healthy volunteers. In this study, ISIS-APO(a)
Rx
treatment produced dose-dependent and significant reductions in Lp(a) levels in these subjects.
|
| | We reported positive clinical results for ISIS-SMN Rx from two open-label Phase 2 studies in infants and children with SMA, which were consistent with data reported earlier in the year at the American Academy of Neurology meeting. SMA is a severe genetic disease that is the leading genetic cause of infant mortality. |
| o | At the World Muscle Society Congress, we reported the median event-free age of infants with SMA as of September 2, 2014, which compared favorably to that of infants with SMA in the PNCR natural history study. Time- and dose-dependent increases in muscle function scores were observed in both infants and children with SMA in the on-going study. We presented clinical data showing that ISIS-SMN Rx is distributed throughout the spinal cord and neurons with greater amounts of full-length SMN2 mRNA and SMN protein in tissues from ISIS-SMN Rx -treated infants compared to the amounts of full-length SMN2 mRNA and SMN protein in the tissues analyzed from untreated SMA infants. |
| | At the American Diabetes Association Scientific Sessions, we reported Phase 2 data on ISIS-GCGR Rx demonstrating that patients with type 2 diabetes uncontrolled on stable metformin therapy experienced up to a 2.25 percentage point mean reduction in HbA1c levels after 13 weeks of treatment with ISIS-GCGR Rx . |
| | We reported top-line Phase 2 data on ISIS-PTP1B Rx demonstrating that patients with type 2 diabetes who are uncontrolled on metformin with or without sulfonylurea experienced statistically significant mean reductions in body weight and HbA1c (0.7 percentage point) at 36 weeks. |
| | At the American Society of Hematology annual meeting, we reported Phase 2 clinical results for ISIS-FXI Rx in patients undergoing total knee replacement. The results showed that ISIS-FXI Rx -treated patients experienced a seven-fold lower incidence of venous thromboembolism and numerically fewer bleeding events compared to patients treated with enoxaparin. |
| o | These data demonstrate that ISIS-FXI Rx can dissociate the antithrombotic effect from the bleeding risk in patients. This is the first time an antithrombotic drug has demonstrated this profile. We published the Phase 2 clinical data of ISIS-FXI Rx in the New England Journal of Medicine |
| | We reported Phase 2 results showing that ISIS-CRP Rx produced statistically significant mean reductions of CRP protein of 65% with reductions as great as 84% in patients with AF. In addition, two patients who had elevated levels of CRP (>5 mg/L) experienced a reduction of CRP that was associated with a decline to zero in overall AF burden while on treatment. |
| | ATL reported Phase 2 data on ATL1103 in patients with acromegaly. In this study, ATL reported that treatment with ATL1103 produced a statistically significant average reduction in IGF-1, levels at the 400 mg per week dose. |
| | OncoGenex reported top-line Phase 2 data on apatorsen (OGX-427) in patients with metastatic bladder cancer. In this study, the Borealis-1 study, OncoGenex reported that treatment with apatorsen in combination with gemcitabine/cisplatin at the 600 mg dose showed a 14 percent reduction in risk of death and a 17 percent reduction in progressive disease and death. |
|
●
|
AstraZeneca presented data from a Phase 1/2 clinical study of ISIS-STAT3-2.5
Rx
(AZD9150) at the 26
th
European Organization for Research and Treatment of Cancer. In this study, preliminary evidence of antitumor activity was observed in patients with cancer, including advanced/metastatic hepatocellular carcinoma. Additional data presented at the conference demonstrated that ISIS-STAT3-2.5
Rx
reduced STAT3 levels in multiple cell types relevant to cancer growth and survival, clinically and pre-clinically. AstraZeneca also presented preclinical data on ISIS-AR-2.5
Rx
(AZD5312) showing that the drug is active in several tumor models.
|
|
●
|
We reported Phase 1 results showing that ISIS-PKK
Rx
produced significant, dose-dependent reductions of PKK of up to 95 percent in healthy volunteers.
|
| | Regulus reported results from a completed clinical study on RG-101, an anti-miR drug in development to treat patients with hepatitis C virus, or HCV. In this study, a single dose of either 2 mg/kg or 4 mg/kg of RG-101 demonstrated a substantial mean reduction in viral load in patients with varied HCV genotypes and treatment history. |
| | Together with our partners, we continued to advance our pipeline of drugs. |
| o | We initiated ENDEAR and CHERISH, Phase 3 studies evaluating ISIS-SMN Rx in infants and children with SMA, respectively. |
| o | We initiated APPROACH, the Phase 3 study evaluating ISIS-APOCIII Rx in patients with familial chylomicronemia syndrome. |
| o | Achaogen initiated a Phase 3 study of plazomicin in patients with serious multi-drug resistant, gram-negative bacterial infections. |
| o | We initiated a Phase 2 study of ISIS-APO(a) Rx in patients with high levels of lipoprotein(a), an independent risk factor for cardiovascular disease. |
| o | We initiated a Phase 2 study for ISIS-GCCR Rx in patients with type 2 diabetes. |
| o | AstraZeneca initiated a Phase 2 study for ISIS-STAT3-2.5 Rx in patients with hepatocellular carcinoma. |
| o | OncoGenex initiated a Phase 2 study for apatorsen in patients with non-small cell lung cancer. |
| o | We initiated a Phase 1/2 study of ISIS-DMPK-2.5 Rx in healthy volunteers and in patients with myotonic dystrophy type 1. |
| o | Regulus initiated a Phase 1/2 study for RG-101 in healthy volunteers and in patients with HCV. |
| o | We initiated a Phase 1 study of ISIS-ANGPTL3 Rx and ISIS-PKK Rx in healthy volunteers. |
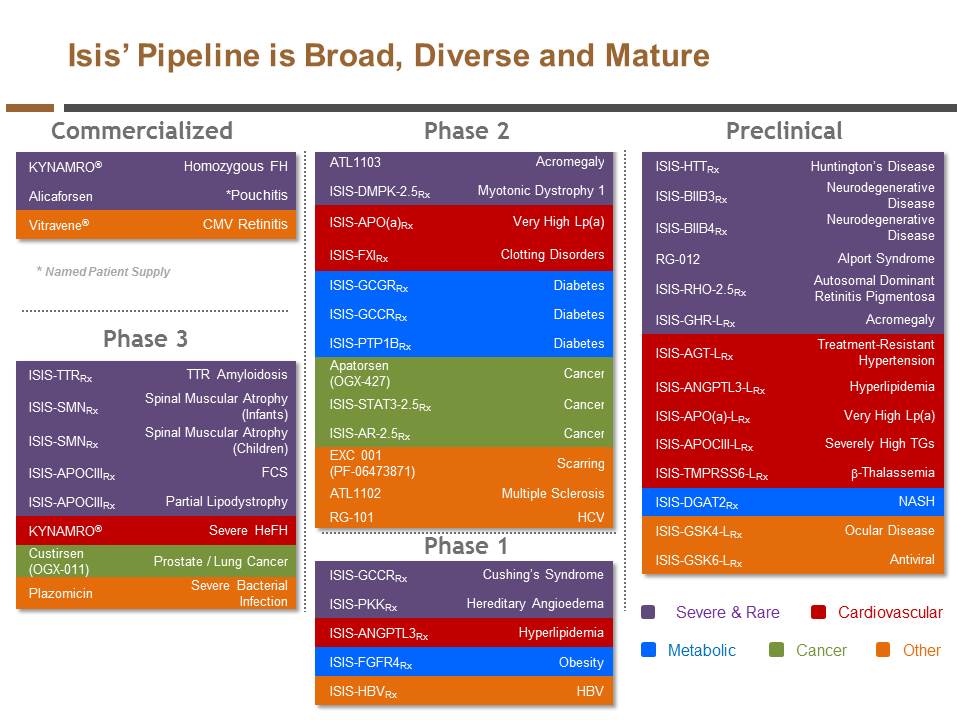
| | We added twelve drugs to our pipeline. |
|
●
|
We received European Orphan Drug Designation for ISIS-APOCIII
Rx
for the treatment of patients with familial chylomicronemia syndrome and for ISIS-TTR
Rx
for the treatment of patients with TTR amyloidosis. We received FDA Orphan Drug Designation for ISIS-DMPK-2.5
Rx
for the treatment of patients with DM1.
|
| | We formed a wholly owned subsidiary, Akcea, to develop and commercialize our lipid drugs, ISIS-APOCIII Rx , ISIS-APO(a) Rx , ISIS-ANGPTL3 Rx and any follow on drugs for these programs. |
| o | We appointed Paula Soteropoulos as president and chief executive officer of Akcea. Ms. Soteropoulos will utilize her expertise in commercializing drugs for severe, rare and cardiovascular diseases in global markets to advance Akcea's novel lipid franchise through development and commercialization. |
| | We formed an alliance with Janssen to discover and develop antisense drugs to treat autoimmune disorders of the GI tract. We received $35 million in upfront payments and are eligible to receive nearly $800 million in development, regulatory and sales milestone payments and license fees for the programs under this alliance. We will also receive tiered royalties up to the low double-digits on sales of drugs successfully commercialized. |
| | We formed an alliance with AstraZeneca to discover and develop novel delivery methods for antisense oligonucleotides. The agreement builds on an existing collaboration between us and AstraZeneca, and supports AstraZeneca's research and development capabilities in the area of antisense oligonucleotide-based therapeutics and RNA biology. |
| | We strengthened our management team with the addition of Sarah Boyce as chief business officer. Ms. Boyce will provide strategic marketing and business expertise from a commercial background to our management team. |
| | Abbott obtained CE Mark and launched the Ibis Biosciences diagnostic platform, now called IRIDICA, in Europe. IRIDICA is available in Europe and other CE-Mark recognized countries. IRIDICA was developed from technology discovered by us and transferred to Ibis Biosciences. |
| | We and Alnylam formed a new agreement that included a cross-license of intellectual property on four disease targets, providing each company with exclusive RNA therapeutic license rights for two programs. |
| | We successfully completed an offering of $500 million aggregate principal amount of 1 percent convertible senior notes due in 2021 in a private placement. We used a significant amount of the net proceeds from the offering to repurchase a large portion of our 2¾ percent convertible senior notes due 2019. |
| | We generated more than $250 million in payments from our partners, including the following: |
| o | $118 million from Biogen Idec, including payments related to advancing ISIS-SMN Rx , initiating a Phase 1 study of ISIS-DMPK-2.5 Rx , validating two undisclosed targets to treat neurological disorders, and selecting two development candidates, ISIS-BIIB3 Rx and ISIS-BIIB4 Rx , to move into our pipeline. |
| o | $36 million from GSK related to the development of ISIS-TTR Rx , ISIS-HBV Rx , ISIS-GSK4-L Rx and ISIS-RHO-2.5 Rx, formerly ISIS-GSK5-2.5 Rx . |
| o | $35 million from Janssen related to our alliance to treat autoimmune disorders of the GI tract. |
| o | $23 million from AstraZeneca related to the development of ISIS-AR-2.5 Rx and ISIS-STAT3-2.5 Rx . |
| o | $10 million from Alnylam related to Alnylam's license of our technology to its partners. |
| o | $4 million from Achaogen for the initiation of a Phase 3 study of Plazomicin. |
| | We received cash through the sale of stock we owned in our satellite company partners of more than $25 million, including more than $20 million from the sale of a portion of our Regulus stock. |
| | We and our partners were recognized by the drug development community for our innovative and collaborative alliances and our commitment to developing drugs to treat patients with serious, unmet medical needs. |
| o | We and Genzyme received the 2014 Partners in Progress Corporate Award from the National Organization for Rare Disorders, or NORD, for the development and approval of KYNAMRO, a drug selected for being a very important orphan therapy to reach the market in the United States. This award honors companies that have brought important and innovative treatments to market for patients with rare disorders. |
| o | Our innovative collaboration with Biogen Idec was voted breakthrough alliance of 2014 by Thomson Reuters Recap. |
| | We added Joseph Loscalzo, M.D., Ph.D. to our Board of Directors. |
| | Our senior vice president of research, Frank Bennett, Ph.D., was awarded the Commitment to a Cure Award by the ALS Association for his research and commitment to develop a treatment for ALS. |
| | Our founder, CEO and chairman of the board of directors, Stanley T. Crooke, M.D., Ph.D., was recognized with several awards. |
|
|
DNA evidence confirming the presence of specific gene mutations associated with a genetic diagnosis of HoFH. However, DNA evidence is generally not necessary for diagnosis and genetic analysis may be inconclusive;
|
|
|
Family history, if known, of premature coronary heart disease and hypercholesterolemia;
|
|
|
Presence of premature heart disease;
|
|
|
Elevated plasma levels of total cholesterol and LDL-C;
|
|
|
Physical examination for signs of cholesterol deposits, including xanthomas on the backs of hands, fingers, face and other areas of the skin. Xanthomas may not be present in every patient; and
|
|
|
Suboptimal response to lipid lowering therapy.
|
|
Drug
|
Indication
|
Partner
|
|
ISIS-HTT
Rx
|
Huntington's Disease
|
Roche
|
|
ISIS-BIIB3
Rx
|
Neurodegenerative Disease
|
Biogen Idec
|
|
ISIS-BIIB4
Rx
|
Neurodegenerative Disease
|
Biogen Idec
|
|
RG-012
|
Alport Syndrome
|
Regulus
|
|
ISIS-RHO-2.5
Rx
|
Autosomal Dominant Retinitis Pigmentosa
|
GSK
|
|
ISIS-GHR-L
Rx
|
Acromegaly
|
Isis owned
|
|
Drug
|
Indication
|
Partner
|
|
ISIS-AGT-L
Rx
|
Treatment-Resistant Hypertension
|
Isis owned
|
|
ISIS-ANGPTL3-L
Rx
|
Hyperlipidemia Disease
|
Akcea
|
|
ISIS-APO(a)-L
Rx
|
Very High Lp(a)
|
Akcea
|
|
ISIS-APOCIII-L
Rx
|
Severely High TGs
|
Akcea
|
|
ISIS-TMPRSS6-L
Rx
|
b-Thalassemia
|
Isis owned
|
|
Drug
|
Indication
|
Partner
|
|
ISIS-DGAT2
Rx
|
NASH
|
Isis owned
|
| ● | Borealis-2 is a study in patients with advanced or metastatic bladder cancer in combination with docetaxel. OncoGenex began enrolling in this study in April 2013. |
| ● | Pacific is an investigator-sponsored study in combination with Zytiga and prednisone in patients with metastatic CRPC who have PSA progression. Enrollment is estimated to be 80 patients and began in December 2012. |
| ● | Spruce is an investigator-sponsored study in combination with carboplatin/pemetrexed therapy in patients with previously untreated Stage IV non-squamous NSCLC. Enrollment is estimated to be 155 patients and began in August 2013. |
| ● | Cedar is an investigator-sponsored study in combination with carboplatin/gemcitabine therapy in patients with previously untreated advanced Non-squamous lung cancer. Enrollment is estimated to be 140 patients and began in August 2014. |
| ● | Rainier is an investigator-sponsored study in combination with ABRAXANE and gemcitabine therapy in patients with previously untreated metastatic pancreatic cancer. Enrollment is estimated to be 130 patients and began in August 2013. |
|
Drug
|
Indication
|
Partner
|
|
ISIS-GSK4-L
Rx
|
Ocular Disease
|
GSK
|
|
ISIS-GSK6-L
Rx
|
Antiviral
|
GSK
|
| | $11.2 million related to the ISIS-AR-2.5 Rx program, which we amortized through March 2014; |
| | $7.6 million related to the option to license three drugs under a separate research program, which we are amortizing through December 2016; and |
| | $0.7 million related to the ISIS-STAT3-2.5 Rx program, which we amortized through February 2015. |
| | AstraZeneca may terminate the agreement or any program at any time by providing written notice to us; |
| | AstraZeneca may terminate the agreement or any program by providing written notice if we undergo a change of control with a third party; and |
| | Either we or AstraZeneca may terminate the agreement or any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent. |
| ● | $3.8 million related to the Phase 2 studies in children and infants with SMA, which we amortized through July 2014; and |
| ● | $7.5 million related to an open-label extension study in children with SMA, which we are amortizing through March 2015. |
| | Biogen Idec may terminate the agreement or any program at any time by providing written notice to us; |
| | Under specific circumstances, if we are acquired by a third party with a product that directly competes with a compound being developed under the agreement, Biogen Idec may terminate the affected program by providing written notice to us; |
| | If, within a specified period of time, any required clearance of a transaction contemplated by an agreement under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, is not received, then either we or Biogen Idec may terminate the affected program by providing written notice to the other party; and |
| | Either we or Biogen Idec may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent. |
| | Genzyme may terminate the license and co-development agreement at any time by providing written notice to Isis; |
| | We may terminate the license and co-development agreement on a country-by-country basis or in its entirety upon Genzyme's uncured failure to use commercially reasonable efforts to develop and commercialize KYNAMRO in the United States, France, Germany, Italy, Spain, the United Kingdom, Japan and Canada; and |
| | Either we or Genzyme may terminate the license and co-development agreement upon the other party's uncured failure to perform a material obligation under the agreement. |
| | GSK may terminate any program, other than the ISIS-TTR Rx program, at any time by providing written notice to us; |
| | GSK may terminate the ISIS-TTR Rx program by providing written notice to us after reviewing specific data from the Phase 3 study for the program; and |
| | Either we or GSK may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent. |
|
●
|
Janssen may terminate the agreement or any program at any time by providing written notice to us; and
|
|
●
|
Either we or Janssen may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
| | Roche may terminate the agreement at any time by providing written notice to us; |
| | Either we or Roche may terminate the agreement by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement or if the other party becomes insolvent; and |
| | Either we or Roche may terminate the brain shuttle program if at least one development candidate is not designated under such program by a mutually agreed deadline. |
|
Type of Patent Claim
|
Breadth |
Description
|
| Broadly Applicable | ||
|
Chemically Modified Nucleosides and Oligonucleotides
Antisense Drug Design Motifs
Therapeutic Methods
Antisense Sequence
Drug Composition
|

|
Target and sequence independent
Sequence independent
Chemistry independent
Specific claim to drug candidates
|
| Specific | ||
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
|
|
|
|
|
|
|
|
|
|
United States
|
|
7,101,993
|
|
OLIGONUCLEOTIDES CONTAINING 2'O-MODIFIED PURINES
|
|
2023
|
|
Covers certain MOE nucleosides and oligonucleotides containing said nucleotides.
|
|
United States
|
|
7,399,845
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
United States
|
|
7,741,457
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
United States
|
|
8,022,193
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
United States
|
7,569,686
|
COMPOUNDS AND METHODS FOR SYNTHESIS OF BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers methods of synthesizing our cEt nucleosides.
|
||||
|
Europe
|
EP1984381
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
Jurisdiction
|
|
Patent/
Application No. |
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
7,015,315
|
|
GAPPED OLIGONUCLEOTIDES
|
|
2023
|
|
Covers 2'-O-alkyl-O-alkyl gapmer oligonucleotides.
|
|
Jurisdiction
|
|
Patent/
Application No. |
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
Europe
|
|
EP2021472
|
|
COMPOUNDS AND METHODS FOR MODULATING GENE EXPRESSION
|
|
2027
|
|
Short gapmer oligonucleotides, 10 to 14 nucleotides in length, with bicyclic nucleosides, which includes cEt locked nucleic acids, in the wings for the treatment of cardiovascular or metabolic disorders
|
|
United States
|
|
7,750,131
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers cEt containing gapmer compounds
|
|
Europe
|
|
EP2092065
|
|
ANTISENSE COMPOUNDS
|
|
2027
|
|
Gapmer compounds having wings comprised of 2'-MOE and bicyclic nucleosides
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
7,407,943
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2021
|
|
Methods of inhibiting expression of apoB, decreasing serum cholesterol, decreasing lipoprotein levels, decreasing serum triglycerides in a human with an antisense compound 12 to 30 nucleotide in length and 100% complementary to human apoB wherein the compound is not a ribozyme.
|
|
Australia
|
|
2002-326481
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2021
|
|
An isolated oligonucleotide compound 12 to 30 nucleobases in length 100% complementary to at least a 12-nucleobase portion of a nucleic acid molecule having nucleotides 151-12820 of SEQ ID 3 (apoB) which is not a ribozyme and use of such compound in therapy
|
|
Japan
|
|
4471650
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2021
|
|
Use of an antisense oligonucleotide 12 to 30 nucleobases in length and 100% complementary to human apoB having one or more modifications and inhibiting expression of apoB by at least 90% in primary hepatocytes when present at a concentration of 300 nM for preparation of a medicament for decreasing serum cholesterol, and decreasing lipoprotein levels in a human
|
|
United States
|
|
7,511,131
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2025
|
|
Antisense sequence and composition of matter of KYNAMRO
|
|
Europe
|
|
EP1569695
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2023
|
|
Antisense sequence and composition of matter of KYNAMRO
|
|
Europe
|
|
EP2336318
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2023
|
|
Antisense sequence and composition of matter of KYNAMRO
|
|
India
|
|
219847
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2023
|
|
Antisense sequence and composition of matter of KYNAMRO
|
|
Australia
|
|
2003294281
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2023
|
|
Antisense sequence and composition of matter of KYNAMRO
|
|
South Africa
|
|
2005/03690
|
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
|
2023
|
|
Antisense sequence and composition of matter of KYNAMRO
|
|
Japan
|
4986109
|
ANTISENSE MODULATION OF APOLIPOPROTEIN B EXPRESSION
|
2023
|
Antisense sequence and composition of matter of KYNAMRO
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
7,598,227
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Methods of treating hyperlipidemia, lowering cholesterol levels and lowering triglyceride levels with an antisense compound comprising an antisense oligonucleotide 15-30 linked nucleosides specifically hybridizable within nucleotides 3253-3558 of SEQ ID 4 (apoCIII)
|
|
United States
|
|
7,750,141
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Antisense sequence and chemistry of ISIS-APOCIII
Rx
|
|
Europe
|
|
EP1622597
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Antisense sequence and chemistry of ISIS-APOCIII
Rx
|
|
Australia
|
|
2004231550
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Compounds 12-50 nucleobases in length specifically hybridizable with SEQ ID 4 (apoCIII), the antisense sequence and chemistry of
ISIS-APOCIII
Rx
and methods of their use in treating hyperlipidemia, lowering cholesterol levels and lowering triglyceride levels
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
6,210,892
|
|
ALTERATION OF CELLULAR BEHAVIOR BY MODULATION OF MRNA PROCESSING
|
|
2018
|
|
Broad claims of altering mRNA processing with a fully-modified 2'MOE oligonucleotide.
|
|
United States
|
|
8,361,977
|
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
|
2030
|
|
Sequence and chemistry (full 2'-MOE) of ISIS-SMN
Rx
|
|
Europe
|
|
1910395
|
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
|
2026
|
|
Sequence and chemistry (full 2'-MOE) of ISIS-SMN
Rx
|
|
United States
|
|
7,838,657
|
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
|
2027
|
|
Oligonucleotides having sequence of ISIS-SMN
Rx
(chemistry independent)
|
|
United States
|
|
8,110,560
|
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
|
2025
|
|
Methods of using antisense oligonucleotides having sequence of SMN
Rx
to alter splicing of SMN2 and/or to treat SMA
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
8,101,743
|
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
|
2025
|
|
Antisense sequence and chemistry of ISIS-TTR
Rx
|
|
United States
|
|
8,697,860
|
|
DIAGNOSIS AND TREATMENT OF DISEASE
|
|
2031
|
|
Composition of ISIS-TTR
Rx
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
5,898,031
|
|
OLIGORIBONUCLEOTIDES FOR CLEAVING RNA
|
|
2016
|
|
Oligonucleotides comprising regions of RNA nucleosides and regions of nucleosides having stabilizing chemical modifications. Such oligonucleotides are suitable for use in single- and double-stranded applications.
|
|
United States
|
|
6,107,094
|
|
OLIGORIBONUCLEOTIDES FOR CLEAVING RNA
|
|
2016
|
|
Compounds and methods that use oligonucleotides having both RNA nucleosides and chemically modified nucleosides, including methods that rely on a dsRNAse to reduce target RNA and compounds having nucleosides with improved affinity and/or stability.
|
|
United States
|
|
7,432,249
|
|
OLIGORIBONUCLEOTIDES FOR CLEAVING RNA
|
|
2016
|
|
Pharmaceutical compositions comprising a diluent or carrier and a single-stranded antisense oligonucleotide having a plurality of RNA nucleosides and at least one sugar modification.
|
|
United States
|
|
7,432,250
|
|
OLIGORIBONUCLEOTIDES FOR CLEAVING RNA
|
|
2016
|
|
Methods for treating a patient by administering an antisense compound having a plurality of RNA nucleosides and at least one sugar modification.
|
|
United States
|
|
7,629,321
|
|
OLIGORIBONUCLEOTIDES FOR CLEAVING RNA
|
|
2016
|
|
Methods for cleaving a target RNA in a cell by contacting the cell with a single-stranded antisense compound having a plurality of RNA nucleosides and at least one sugar modification.
|
|
United States
|
|
7,695,902
|
|
OLIGORIBONUCLEOTIDES FOR CLEAVING RNA
|
|
2016
|
|
Methods of activating a dsRNase by contacting the dsRNase with a double-stranded antisense oligonucleotide where at least one strand has a plurality of RNA nucleosides and at least one sugar modification. The methods may be performed inside a cell.
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
6,900,187
|
|
TRPM-2 ANTISENSE THERAPY USING AN OLIOGNUCLEOTIDE HAVING 2'-O-(2-METHOXY)ETHYL MODIFICATIONS
|
|
2021
|
|
Antisense sequence and composition of custirsen
|
|
Name
|
|
Age
|
|
Position
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
69
|
|
Chairman, Chief Executive Officer and President
|
|
B. Lynne Parshall, J.D.
|
|
60
|
|
Director, Chief Operating Officer and Secretary
|
|
C. Frank Bennett, Ph.D.
|
|
58
|
|
Senior Vice President, Antisense Research
|
|
Sarah Boyce
|
43
|
Chief Business Officer
|
||
|
Richard S. Geary, Ph.D.
|
|
57
|
|
Senior Vice President, Development
|
|
Elizabeth L. Hougen
|
|
53
|
|
Senior Vice President, Finance and Chief Financial Officer
|
|
Brett P. Monia, Ph.D.
|
|
53
|
|
Senior Vice President, Drug Discovery and Corporate Development
|
|
Patrick R. O'Neil, Esq.
|
|
41
|
|
Senior Vice President, Legal and General Counsel
|
| ● | receipt and scope of regulatory approvals; |
| ● | establishment and demonstration in the medical and patient community of the efficacy and safety of our drugs and their potential advantages over competing products; |
| ● | cost and effectiveness of our drugs compared to other available therapies; |
| ● | patient convenience of the dosing regimen for our drugs; and |
| ● | reimbursement policies of government and third-party payors. |
| ● | priced lower than our drugs; |
| ● | safer than our drugs; |
| ● | more effective than our drugs; or |
| ● | more convenient to use than our drugs. |
| ● | KYNAMRO is approved in the United States as an adjunct to lipid-lowering medications and diet to reduce low density lipoprotein-cholesterol, apolipoprotein B, total cholesterol, and non-high density lipoprotein-cholesterol in patients with HoFH; |
| ● | the KYNAMRO label contains a Boxed Warning citing a risk of hepatic toxicity; and |
| ● | KYNAMRO is available only through a Risk Evaluation and Mitigation Strategy called the KYNAMRO REMS. |
| ● | fund some of our development activities for KYNAMRO; |
| ● | seek and obtain regulatory approvals for KYNAMRO; and |
| ● | successfully commercialize KYNAMRO. |
| ● | the clinical study may produce negative or inconclusive results; |
| ● | regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements; |
| ● | we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a drug on subjects in the trial; |
| ● | we may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies; |
| ● | enrollment in our clinical studies may be slower than we anticipate; |
| ● | the cost of our clinical studies may be greater than we anticipate; and |
| ● | the supply or quality of our drugs or other materials necessary to conduct our clinical studies may be insufficient, inadequate or delayed. |
| ● | conduct clinical studies; |
| ● | seek and obtain regulatory approvals; and |
| ● | manufacture, market and sell our drugs. |
| ● | pursue alternative technologies or develop alternative products that may be competitive with the drug that is part of the collaboration with us; |
| ● | pursue higher-priority programs or change the focus of its own development programs; or |
| ● | choose to devote fewer resources to our drugs than it does for its own drugs. |
| ● | additional marketing approvals and successful commercial launch of KYNAMRO; |
| ● | changes in existing collaborative relationships and our ability to establish and maintain additional collaborative arrangements; |
| ● | continued scientific progress in our research, drug discovery and development programs; |
| ● | the size of our programs and progress with preclinical and clinical studies; |
| ● | the time and costs involved in obtaining regulatory approvals; |
| ● | competing technological and market developments, including the introduction by others of new therapies that address our markets; and |
| ● | the profile and launch timing of our drugs, including ISIS-APOCIII Rx , ISIS-SMN Rx and ISIS-TTR Rx . |
| ● | interruption of our research, development and manufacturing efforts; |
| ● | injury to our employees and others; |
| ● | environmental damage resulting in costly clean up; and |
| ● | liabilities under federal, state and local laws and regulations governing health and human safety, as well as the use, storage, handling and disposal of these materials and resultant waste products. |
| Item 1B. | Unresolved Staff Comments |
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
|
|
HIGH
|
LOW
|
||||||
|
2014
|
|
|
||||||
|
First Quarter
|
$
|
62.66
|
$
|
38.04
|
||||
|
Second Quarter
|
$
|
45.04
|
$
|
22.25
|
||||
|
Third Quarter
|
$
|
43.42
|
$
|
27.37
|
||||
|
Fourth Quarter
|
$
|
67.12
|
$
|
35.26
|
||||
|
2013
|
||||||||
|
First Quarter
|
$
|
19.53
|
$
|
10.36
|
||||
|
Second Quarter
|
$
|
28.66
|
$
|
15.92
|
||||
|
Third Quarter
|
$
|
39.83
|
$
|
23.63
|
||||
|
Fourth Quarter
|
$
|
42.69
|
$
|
29.41
|
||||

|
|
Dec-09
|
Dec-10
|
Dec-11
|
Dec-12
|
Dec-13
|
Dec-14
|
||||||||||||||||||
|
Isis Pharmaceuticals, Inc.
|
$
|
100.00
|
$
|
91.09
|
$
|
64.90
|
$
|
93.97
|
$
|
358.60
|
$
|
555.72
|
||||||||||||
|
NASDAQ Composite Index
|
$
|
100.00
|
$
|
117.61
|
$
|
118.70
|
$
|
139.00
|
$
|
196.83
|
$
|
223.74
|
||||||||||||
|
NASDAQ Biotechnology Index
|
$
|
100.00
|
$
|
106.73
|
$
|
122.40
|
$
|
166.72
|
$
|
286.55
|
$
|
379.71
|
||||||||||||
| (1) | This section is not "soliciting material," is not deemed "filed" with the SEC, is not subject to the liabilities of Section 18 of the Exchange Act and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
|
|
Years Ended December 31,
|
|||||||||||||||||||
|
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|||||||||||||||
|
Revenue
|
$
|
214,161
|
$
|
147,285
|
$
|
102,049
|
$
|
99,086
|
$
|
108,473
|
||||||||||
|
Research, development and patent expenses
|
$
|
241,751
|
$
|
184,033
|
$
|
158,458
|
$
|
157,397
|
$
|
145,160
|
||||||||||
|
Net loss attributable to Isis Pharmaceuticals, Inc. common stockholders
|
$
|
(38,984
|
)
|
$
|
(60,644
|
)
|
$
|
(65,478
|
)
|
$
|
(84,801
|
)
|
$
|
(61,251
|
)
|
|||||
|
Basic and diluted net loss per share attributable to Isis Pharmaceuticals, Inc. common stockholders
|
$
|
(0.33
|
)
|
$
|
(0.55
|
)
|
$
|
(0.65
|
)
|
$
|
(0.85
|
)
|
$
|
(0.62
|
)
|
|||||
|
Shares used in computing basic and diluted net loss per share
|
117,691
|
110,502
|
100,576
|
99,656
|
99,143
|
|||||||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Consolidated Balance Sheet:
|
|
|
|
|
|
|||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
728,832
|
$
|
656,761
|
$
|
374,446
|
$
|
343,664
|
$
|
472,353
|
||||||||||
|
Working capital
|
$
|
721,265
|
$
|
637,698
|
$
|
349,116
|
$
|
284,027
|
$
|
377,247
|
||||||||||
|
Investment in Regulus Therapeutics Inc.(1)
|
$
|
81,881
|
$
|
52,096
|
$
|
33,622
|
$
|
4,424
|
$
|
870
|
||||||||||
|
Total assets
|
$
|
955,809
|
$
|
847,156
|
$
|
545,686
|
$
|
484,894
|
$
|
550,477
|
||||||||||
|
Long-term debt and other obligations, less current portion
|
$
|
582,697
|
$
|
370,954
|
$
|
288,598
|
$
|
232,924
|
$
|
199,175
|
||||||||||
|
Accumulated deficit
|
$
|
(1,006,594
|
)
|
$
|
(967,610
|
)
|
$
|
(906,966
|
)
|
$
|
(841,488
|
)
|
$
|
(756,687
|
)
|
|||||
|
Stockholders' equity
|
$
|
257,780
|
$
|
378,390
|
$
|
182,766
|
$
|
171,434
|
$
|
244,542
|
||||||||||
| (1) | In October 2012, Regulus completed an IPO and we changed to accounting for our investment in Regulus at fair value from the equity method because our ownership in Regulus dropped below 20 percent and we no longer had significant influence over Regulus' operating and financial policies. For additional information, see Note 2, Investment in Regulus Therapeutics Inc. in the Notes to the Consolidated Financial Statements. |
| | Assessing the propriety of revenue recognition and associated deferred revenue; |
| | Determining the proper valuation of investments in marketable securities and other equity investments; |
| | Assessing the recoverability of long-lived assets, including property and equipment, intellectual property and licensed technology; |
| | Determining the appropriate cost estimates for unbilled preclinical studies and clinical development activities; |
| | Estimating our net deferred income tax asset valuation allowance; and |
| | Determining the fair value of convertible debt without the conversion feature. |
| | The exclusive license we granted to AstraZeneca to develop and commercialize ISIS-STAT3-2.5 Rx for the treatment of cancer; |
| | The development services we are performing for ISIS-STAT3-2.5 Rx ; |
| | The exclusive license we granted to AstraZeneca to develop and commercialize ISIS-AR-2.5 Rx and the research services we performed for ISIS-AR-2.5 Rx ; and |
| | The option to license up to three drugs under a research program and the research services we are performing for this program. |
| | Estimated future product sales; |
| | Estimated royalties on future product sales; |
| | Contractual milestone payments; |
| | Expenses we expect to incur; |
| | Income taxes; and |
| | An appropriate discount rate. |
| | The number of internal hours we will spend performing these services; |
| | The estimated number and cost of studies we will perform; |
| | The estimated number and cost of studies that we will contract with third parties to perform; and |
| | The estimated cost of drug product we will use in the studies. |
| | In January 2012, we entered into a collaboration agreement with Biogen Idec to develop and commercialize ISIS-SMN Rx for SMA. As part of the collaboration, we received a $29 million upfront payment and we are responsible for global development of ISIS-SMN Rx through completion of Phase 2/3 clinical trials. |
| | In June 2012, we entered into a second and separate collaboration agreement with Biogen Idec to develop and commercialize a novel antisense drug targeting DMPK, or dystrophia myotonica-protein kinase. As part of the collaboration, we received a $12 million upfront payment and we are responsible for global development of the drug through the completion of a Phase 2 clinical trial. |
| | In December 2012, we entered into a third and separate collaboration agreement with Biogen Idec to discover and develop antisense drugs against three targets to treat neurological or neuromuscular disorders. As part of the collaboration, we received a $30 million upfront payment and we are responsible for the discovery of a lead antisense drug for each of three targets. |
| | In September 2013, we entered into a fourth and separate collaboration agreement with Biogen Idec to leverage antisense technology to advance the treatment of neurological diseases. We granted Biogen Idec exclusive rights to the use of our antisense technology to develop therapies for neurological diseases as part of this broad collaboration. We received a $100 million upfront payment and we are responsible for discovery and early development through the completion of a Phase 2 clinical trial for each antisense drug identified during the six year term of this collaboration, while Biogen Idec is responsible for the creation and development of small molecule treatments and biologics. |
| | Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete; |
| | Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete; |
| | Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete; |
| | Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete. |
| | Filing of regulatory applications for marketing approval such as a NDA in the United States or a MAA in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings. |
| | Marketing approval in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain approval from the applicable regulatory agency. |
| | First commercial sale in a particular market, such as in the United States or Europe. |
| | Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product. |
| | Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement; |
| | The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on our performance or the occurrence of a specific outcome resulting from our performance; |
| | The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items; |
| | There is no future performance required to earn the milestone; and |
| | The consideration is reasonable relative to all deliverables and payment terms in the arrangement. |
| | Evidence of decreases in market value; |
| | Changes in the extent or manner in which we use an asset; |
| | Adverse changes in legal factors or in the business climate that would affect the value of an asset; |
| | An adverse action or assessment by a regulator; |
| | An accumulation of costs significantly in excess of amounts originally expected to acquire or construct an asset; |
| | Current period operating or cash flow loss combined with a history of operating or cash flow losses associated with an asset used for the purpose of producing revenue; and |
| | Challenges or potential challenges to our existing patents, the likelihood that the United States Patent and Trademark Office, or foreign equivalent, will issue an application and the scope of our issued patents. |
| | $80 million from Biogen Idec, for advancing ISIS-SMN Rx , including initiating two Phase 3 studies, initiating a Phase 1 study of ISIS-DMPK-2.5 Rx , validating two undisclosed targets to treat neurological disorders under our neurology collaborations, and advancing a third drug into development; |
| | $28.5 million from GSK related to advancing the Phase 2/3 study of ISIS-TTR Rx , and further advancing ISIS-HBV Rx , ISIS-GSK4-L Rx , and ISIS-RHO-2.5 Rx ; |
| | $22.1 million from AstraZeneca related to the initiation of a Phase 1 clinical study of ISIS-AR-2.5 Rx and advancing ISIS-STAT3-2.5 Rx ; and |
| | $4 million from Achaogen when Achaogen initiated a Phase 3 study of plazomicin. |
|
|
Year Ended
December 31, |
|||||||
|
|
2014
|
2013
|
||||||
|
Research, development and patent expenses
|
$
|
215,908
|
$
|
174,360
|
||||
|
Non-cash compensation expense related to equity awards
|
25,843
|
9,673
|
||||||
|
Total research, development and patent expenses
|
$
|
241,751
|
$
|
184,033
|
||||
|
|
Year Ended
December 31, |
|||||||
|
|
2014
|
2013
|
||||||
|
Antisense drug discovery expenses
|
$
|
43,620
|
$
|
42,402
|
||||
|
Non-cash compensation expense related to equity awards
|
7,290
|
2,878
|
||||||
|
Total antisense drug discovery
|
$
|
50,910
|
$
|
45,280
|
||||
|
|
Year Ended
December 31, |
|||||||
|
|
2014
|
2013
|
||||||
|
KYNAMRO
|
$
|
5,359
|
$
|
7,653
|
||||
|
ISIS-TTR
Rx
|
10,927
|
4,174
|
||||||
|
ISIS-SMN
Rx
|
19,064
|
6,938
|
||||||
|
ISIS-APOCIII
Rx
|
9,337
|
5,730
|
||||||
|
Other antisense development products
|
44,913
|
29,129
|
||||||
|
Development overhead costs
|
31,318
|
24,171
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
120,918
|
77,795
|
||||||
|
Non-cash compensation expense related to equity awards
|
9,640
|
3,202
|
||||||
|
Total antisense drug development
|
$
|
130,558
|
$
|
80,997
|
||||
|
|
Year Ended
December 31, |
|||||
|
|
2014
|
2013
|
||||
|
Manufacturing and operations
|
$ |
24,763
|
$ |
20,509
|
||
|
Non-cash compensation expense related to equity awards
|
2,934
|
1,295
|
||||
|
Total manufacturing and operations
|
$ |
27,697
|
$ |
21,804
|
||
|
|
Year Ended
December 31, |
|||||||
|
|
2014
|
2013
|
||||||
|
Personnel costs
|
$
|
9,875
|
$
|
9,571
|
||||
|
Occupancy
|
7,357
|
6,897
|
||||||
|
Patent expenses
|
2,933
|
10,321
|
||||||
|
Depreciation and amortization
|
2,243
|
2,464
|
||||||
|
Insurance
|
1,197
|
1,108
|
||||||
|
Other
|
3,002
|
3,293
|
||||||
|
Total R&D support costs, excluding non-cash compensation expense related to equity awards
|
26,607
|
33,654
|
||||||
|
Non-cash compensation expense related to equity awards
|
5,979
|
2,298
|
||||||
|
Total R&D support costs
|
$
|
32,586
|
$
|
35,952
|
||||
|
|
Year Ended
December 31, |
|||||
|
|
2014
|
2013
|
||||
|
General and administrative expenses
|
$ |
14,600
|
$ |
13,173
|
||
|
Non-cash compensation expense related to equity awards
|
5,540
|
1,745
|
||||
|
Total general and administrative
|
$ |
20,140
|
$ |
14,918
|
||
|
|
Year Ended
December 31, |
|||||||
|
|
2014
|
2013
|
||||||
|
2¾ % convertible notes:
|
|
|
||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
$
|
7,211
|
$
|
6,758
|
||||
|
Interest expense payable in cash
|
5,074
|
5,534
|
||||||
|
1 % convertible notes:
|
||||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
2,365
|
—
|
||||||
|
Interest expense payable in cash
|
597
|
—
|
||||||
|
Non-cash interest expense for long-term financing liability
|
6,622
|
6,568
|
||||||
|
Other
|
340
|
495
|
||||||
|
Total interest expense
|
$
|
22,209
|
$
|
19,355
|
||||
| | $26.5 million from GSK because we advanced ISIS-TTR Rx , ISIS-HBV Rx , formerly ISIS-GSK3 Rx , and ISIS-GSK4-L Rx in development; |
| | $25 million from Genzyme when the FDA approved the KYNAMRO NDA; |
| | $10 million when AstraZeneca added a second development candidate, ISIS-AR-2.5 Rx , to our collaboration; |
| | $17 million from Biogen Idec because we advanced the Phase 2 study of ISIS-SMN Rx in infants and for selecting and advancing ISIS-DMPK-2.5 Rx in development; and |
| | $3.5 million when Xenon licensed XEN701. |
|
|
Year Ended
December 31, |
|||||
|
|
2013
|
2012
|
||||
|
Research, development and patent expenses
|
$ |
174,360
|
$ |
151,212
|
||
|
Non-cash compensation expense related to equity awards
|
9,673
|
7,246
|
||||
|
Total research, development and patent expenses
|
$ |
184,033
|
$ |
158,458
|
||
|
|
Year Ended
December 31, |
|||||
|
|
2013
|
2012
|
||||
|
Antisense drug discovery expenses
|
$ |
42,402
|
$ |
34,035
|
||
|
Non-cash compensation expense related to equity awards
|
2,878
|
2,108
|
||||
|
Total antisense drug discovery
|
$ |
45,280
|
$ |
36,143
|
||
|
|
Year Ended
December 31, |
|||||||
|
|
2013
|
2012
|
||||||
|
KYNAMRO
|
$
|
7,653
|
$
|
9,451
|
||||
|
ISIS-TTR
Rx
|
4,174
|
5,034
|
||||||
|
ISIS-SMN
Rx
|
6,938
|
3,903
|
||||||
|
ISIS-APOCIII
Rx
|
5,730
|
3,104
|
||||||
|
Other antisense development products
|
29,129
|
27,959
|
||||||
|
Development overhead costs
|
24,171
|
21,110
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
77,795
|
70,561
|
||||||
|
Non-cash compensation expense related to equity awards
|
3,202
|
2,482
|
||||||
|
Total antisense drug development
|
$
|
80,997
|
$
|
73,043
|
||||
|
|
Year Ended
December 31, |
|||||||
|
|
2013
|
2012
|
||||||
|
Manufacturing and operations
|
$
|
20,509
|
$
|
19,232
|
||||
|
Non-cash compensation expense related to equity awards
|
1,295
|
999
|
||||||
|
Total manufacturing and operations
|
$
|
21,804
|
$
|
20,231
|
||||
|
|
Year Ended
December 31, |
|||||||
|
|
2013
|
2012
|
||||||
|
Personnel costs
|
$
|
9,571
|
$
|
9,231
|
||||
|
Occupancy
|
6,897
|
6,909
|
||||||
|
Patent expenses
|
10,321
|
3,868
|
||||||
|
Depreciation and amortization
|
2,464
|
3,129
|
||||||
|
Insurance
|
1,108
|
1,143
|
||||||
|
Other
|
3,293
|
3,104
|
||||||
|
Total R&D support costs, excluding non-cash compensation expense related to equity awards
|
33,654
|
27,384
|
||||||
|
Non-cash compensation expense related to equity awards
|
2,298
|
1,657
|
||||||
|
Total R&D support costs
|
$
|
35,952
|
$
|
29,041
|
||||
|
|
Year Ended
December 31, |
|||||||
|
|
2013
|
2012
|
||||||
|
General and administrative expenses
|
$
|
13,173
|
$
|
11,190
|
||||
|
Non-cash compensation expense related to equity awards
|
1,745
|
1,325
|
||||||
|
Total general and administrative
|
$
|
14,918
|
$
|
12,515
|
||||
|
|
Year Ended
December 31, |
|||||||
|
|
2013
|
2012
|
||||||
|
Convertible Notes:
|
|
|
||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
$
|
6,758
|
$
|
9,846
|
||||
|
Interest expense payable in cash
|
5,534
|
4,306
|
||||||
|
Non-cash interest expense for long-term financing liability
|
6,568
|
6,502
|
||||||
|
Other
|
495
|
498
|
||||||
|
Total interest expense
|
$
|
19,355
|
$
|
21,152
|
||||
| | $18.4 million gain we realized in 2012 because of the increase in Regulus' valuation resulting from its IPO; and |
| | $4.8 million loss, $3.6 million of which was non-cash, we recorded in 2012 on the early retirement of our 2⅝ percent convertible subordinated notes. |
|
|
Payments Due by Period (in millions)
|
|||||||||||||||||||
|
Contractual Obligations
(selected balances described below) |
Total
|
Less than
1 year |
1-3 years
|
3-5 years
|
After
5 years |
|||||||||||||||
|
1 percent convertible senior notes (principal and interest payable)
|
$
|
535.0
|
$
|
5.0
|
$
|
10.0
|
$
|
10.0
|
$
|
510.0
|
||||||||||
|
2¾
percent convertible senior notes (principal and interest payable)
|
$
|
69.5
|
$
|
1.5
|
$
|
3.4
|
$
|
64.6
|
$
|
—
|
||||||||||
|
Facility rent payments
|
$
|
131.8
|
$
|
6.2
|
$
|
13.1
|
$
|
13.9
|
$
|
98.6
|
||||||||||
|
Equipment financing arrangements (principal and interest payable)
|
$
|
3.3
|
$
|
2.8
|
$
|
0.5
|
$
|
—
|
$
|
—
|
||||||||||
|
Other obligations (principal and interest payable)
|
$
|
1.3
|
$
|
0.1
|
$
|
0.1
|
$
|
0.1
|
$
|
1.0
|
||||||||||
|
Capital lease
|
$
|
0.2
|
$
|
0.2
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||||||
|
Operating leases
|
$
|
25.1
|
$
|
1.6
|
$
|
3.0
|
$
|
3.0
|
$
|
17.5
|
||||||||||
|
Total
|
$
|
766.2
|
$
|
17.4
|
$
|
30.1
|
$
|
91.6
|
$
|
627.1
|
||||||||||
|
1 Percent Convertible
Senior Notes
|
2¾ Percent Convertible
Senior Notes
|
|||||||
|
Outstanding principal balance
|
$
|
500.0
|
$
|
61.2
|
||||
|
Issue date
|
November 2014
|
August 2012
|
||||||
|
Maturity date
|
November 2021
|
October 2019
|
||||||
|
Interest rate
|
1 percent
|
2¾ percent
|
||||||
|
Conversion price per share
|
$
|
66.81
|
$
|
16.63
|
||||
|
Total shares of common stock subject to conversion
|
7.5
|
3.7
|
||||||
|
|
/s/ ERNST & YOUNG LLP
|
|
|
|
|
San Diego, California
|
|
|
February 27, 2015
|
|
|
Since inception, the Isis mission has been to create a new, more efficient technology for drug discovery and development, antisense technology, and exploit that technology to create a pipeline of first-in-class medicines to treat a wide range of diseases. Today, thanks to the innovation and perseverance of Isis, we believe antisense technology is taking its place as the third platform for drug discovery alongside small molecules and protein therapeutics.
|
Through the efficiency of our technology platform and business strategy we have built a pipeline of 38 drugs in development with under 400 employees, representing a ratio of 1 drug: 12 employees.
|
|
|
Isis is focused on innovation. Isis has implemented a unique business strategy that is intended to support long-term innovation based on the efficiency of antisense technology. Isis has created a unique innovation-focused, science–driven, culture that couples with the technology and business model to ensure long-term productivity and a commitment to the patients we serve.
|
| | create and constantly advance a new, more efficient drug discovery platform, antisense technology; |
| | create a unique business model and culture committed to creating long-term value through innovation; |
| | broaden, deepen and advance our pipeline of antisense drugs; |
| | demand more of every employee - more commitment, more knowledge, more intensity, more innovation and more productivity; |
| | aggressively manage average and below average performance so every employee produces more; and |
| | demand great performance and pay for that performance. |
|
What We Do
|
What We Don't Do
|
|
Demand more of every employee: more commitment, more knowledge, more intensity, more innovation, more productivity
|
Do not guarantee a cash bonus – cash bonuses can, and have been, zero
|
|
Reward productivity and performance
|
Do not provide perquisites for any employees
|
|
Recognize the value of long-term employees and low turnover
|
Do not provide "gross-up" payments, other than for relocation
|
|
Use a balanced mix of fixed and variable cash incentives and long-term equity incentives
|
Do not allow pledging, shorting or hedging against our stock
|
|
Evaluate compensation compared to the 50
th
percentile of our peer group
|
Do not reprice or "cash-out" stock options without stockholder approval
|
|
Design our compensation philosophy and objectives to mitigate unnecessary or imprudent business risk taking
|
|
|
Set explicit and demanding objectives at the beginning of each year from which we measure performance for the year
|
|
|
Place a m aximum limit on Performance MBOs
|
|
|
Set a strict budget for equity awards and salary increases
|
|
|
Set the size of equity awards based on individual and company performance
|
|
|
Require minimum vesting periods for equity awards
|
|
|
Maintain equity holding periods that require our named executive officers and non-employee Board members to hold shares received from their RSUs until they meet certain ownership thresholds or no longer serve the company
|
|
|
Maintain equity holding periods that require our employees to hold ESPP shares for a minimum of six months
|
|
|
Require our executive officers and VPs to trade Isis' stock through Rule 10b5-1 trading plans
|
|
|
Use a "double trigger" for cash payments for change of control
|
|
|
Use an executive "claw-back" policy
|
|
|
Use an independent compensation consultant engaged by the Compensation Committee
|
| | reviewing and approving overall compensation strategy; |
| | reviewing and approving corporate performance goals and objectives relevant to the compensation of our executive officers; |
| | evaluating and recommending to the Board the compensation plans and programs advisable for Isis, as well as modifying or terminating existing plans and programs; |
| | establishing policies with respect to stock compensation arrangements; |
| | reviewing and approving compensation arrangements for our executive officers, including our Chief Executive Officer; |
| | reviewing and approving compensation arrangements for our Directors; |
| | administering our stock-based awards and ESPP; |
| | evaluating risks associated with our compensation policies and practices and assessing whether these risks are reasonably likely to have a material adverse effect on us; |
| | selecting and retaining a qualified, independent compensation consultant; |
| | performing other functions as may be necessary or convenient in the efficient discharge of the foregoing; and |
| | reporting to the Board of Directors from time to time, or whenever it is called upon to do so. |
| | monitor the SEC's adoption of the final rules and definitions; and |
| | adjust Isis' compensation policies as necessary to satisfy the new rules. |
| | selecting the 2014 Executive Peer Group; |
| | evaluating the pay mix for our named executive officers; and |
| | evaluating short-term and long-term incentives for our executive officers. |

| | create and constantly advance a new, more efficient drug discovery platform, antisense technology; |
| | create a unique business model and culture committed to creating long-term value through innovation; |
| | broaden, deepen and advance our pipeline of antisense drugs; |
| | demand more of every employee - more commitment, more knowledge, more intensity, more innovation, more productivity; |
| | aggressively manage average and below average performance so that every employee produces more; and |
| | demand great performance and pay for that performance. |
|
Drug discovery and development across a portfolio of many drugs (currently 38 for Isis) is a long process that spans many years, where decisions we make today can have a positive or negative consequence five years, ten years, and even further into the future. As such, it is essential we set goals that incentivize our employees to execute our long-term strategy, because we believe our long-term strategy should continue to reward our stockholders into the future.
|
Given the uniqueness and complexity of our technology, it is critical to retain the knowledge and experience of outstanding long service employees.
|
| | Long tenure among a dedicated and highly skilled workforce, combined with the highest performance standards, contributes to our leadership in the industry and serves the interests of stockholders. |
| | Our focus on retention is coupled to a strong belief that executive talent most often should be developed and promoted from within Isis. |
| | The long tenure of high-performing executive officers reflects this strategy at all levels of the organization. |
| o | Our named executive officers, or NEOs, who served in 2014 have tenures at Isis ranging from 14 years to 26 years. |
| o | Our other officers who served in 2014 have on average 12 years of tenure at Isis. |
| | Each of the executive officers has been carefully evaluated and selected through a rigorous performance assessment process over a long career. In their current assignments, they remain subject to a challenging annual performance assessment in which they must continue to meet the highest standards or be reassigned or separated from the Company. |
|
Employees in our organization do not share either accountability or responsibility equally for strategic and/or tactical decisions. It is well ingrained in our culture that not everyone should share the same level of risk/reward for the consequences of these decisions. As a result, we have structured the various components of our compensation system to reflect accountability both for the successes and failures (both long-term and short-term) of Isis and our employees. We pay our senior management team for results and their use of judgment in executing the strategies they have established. Therefore, the more senior a person becomes within Isis, the more the person's cash compensation will be "at risk." We compensate the more junior employees for accomplishing their work well and, therefore, a lower portion of their cash compensation is "at risk."
|
The more senior role a person plays, the more that person's cash compensation will be "at risk."
|
| (1) | base salary, |
| (2) | MBO – Performance Based – At Risk Cash Compensation, no portion of which is guaranteed, |
| (3) | stock-based compensation, and |
| (4) | the same benefits, including 401(k) matching, that we provide to all employees. |
| | company-wide performance, including achievement of corporate objectives; |
| | the Compensation Committee's assessment of our CEO's and executive officers' individual performance; |
| | competitive compensation practices; |
| | increased efficiencies and process improvements; |
| | effective collaboration and teamwork; |
| | individual expertise, skills and knowledge; |
| | the need to retain and motivate; |
| | the impact an individual's judgment has on our success or failure; and |
| | the advice of the Compensation Committee's independent compensation consultant. |
| | are similar to Isis in terms of certain factors, including one or more of the following: size (i.e., revenue, market capitalization), industry, and stage of development; |
| | have named executive officer positions that are comparable to ours in terms of breadth, complexity and scope of responsibilities; and |
| | compete with us for executive talent. |
|
Company (ticker)
|
Annual Revenues
(in millions)
|
Market Capitalization
(in millions)
|
Stage of Lead Drug
|
||||||
|
Accorda Therapeutics (ACOR)
|
$
|
336.4
|
$
|
1,589.2
|
Market
|
||||
|
Akorn (AKRX)
|
$
|
317.7
|
$
|
2,094.5
|
Market
|
||||
|
Alkermes (ALKS)
|
$
|
575.6
|
$
|
6,400.5
|
Market
|
||||
|
Alnylam Pharmaceuticals (ALNY)
|
$
|
47.2
|
$
|
4,194.2
|
Phase III
|
||||
|
Arena Pharmaceuticals (ARNA)
|
$
|
81.4
|
$
|
1,462.5
|
Market
|
||||
|
Ariad Pharmaceuticals (ARIA)
|
$
|
45.6
|
$
|
1,501.8
|
Market
|
||||
|
Auxilium Pharmaceuticals (AUXL)
|
$
|
400.7
|
$
|
1,406.3
|
Market
|
||||
|
Exelixis (EXEL)
|
$
|
31.3
|
$
|
718.1
|
Market
|
||||
|
Halozyme Therapeutics (HALO)
|
$
|
54.8
|
$
|
1,505.4
|
Market
|
||||
|
ImmunoGen (IMGN)
|
$
|
35.5
|
$
|
1,255.1
|
Market
|
||||
|
Incyte Corporation (INCY)
|
$
|
354.9
|
$
|
9,015.1
|
Market
|
||||
|
InterMune (ITMN)
|
$
|
70.3
|
$
|
3,307.2
|
Phase III
|
||||
|
Jazz Pharmaceuticals (JAZZ)
|
$
|
872.4
|
$
|
8,137.7
|
Market
|
||||
|
Lexicon Pharmaceuticals (LXRX)
|
$
|
2.2
|
$
|
930.0
|
Phase III
|
||||
|
MannKind (MNKD)
|
$
|
0.0
|
$
|
2,636.7
|
Phase III
|
||||
|
Medivation (MDVN)
|
$
|
272.9
|
$
|
4,980.0
|
Market
|
||||
|
Momenta Pharmaceuticals (MNTA)
|
$
|
35.5
|
$
|
606.1
|
Market
|
||||
|
Nektar Therapeutics (NKTR)
|
$
|
148.9
|
$
|
1,590.7
|
Market
|
||||
|
NPS Pharmaceuticals (NPSP)
|
$
|
155.6
|
$
|
3,104.1
|
Market
|
||||
|
Pacira (PCRX)
|
$
|
85.6
|
$
|
2,326.9
|
Market
|
||||
|
Pharmacyclics (PCYC)
|
$
|
260.2
|
$
|
7,644.9
|
Market
|
||||
|
Seattle Genetics (SGEN)
|
$
|
269.3
|
$
|
5,400.2
|
Market
|
||||
|
The Medicines Company (MDCO)
|
$
|
687.9
|
$
|
1,620.8
|
Market
|
||||
|
Theravance (THRX)
|
$
|
4.8
|
$
|
3,437.7
|
Market
|
||||
|
United Therapeutics (UTHR)
|
$
|
1,117.0
|
$
|
4,874.6
|
Market
|
||||
|
Isis Pharmaceuticals, Inc. (ISIS)
|
$
|
147.3
|
$
|
4,958.2
|
Market
|
||||
|
Isis' Ranking
|
14
|
7
|
NA
|
||||||
|
Isis' Percentile Rank
|
46
|
%
|
75
|
%
|
NA
|
||||
|
2014 Total Cash Compensation
(in thousands)
|
2014 Total Direct Compensation
(in thousands)
|
|||||||||||||||
|
Name
|
NEO
|
50
th
Percentile of Executive Peer Group
|
NEO
|
50
th
Percentile of Executive Peer Group
|
||||||||||||
|
Stanley T. Crooke
|
$
|
1,488,488
|
$
|
1,327,900
|
$
|
6,228,143
|
$
|
4,126,200
|
||||||||
|
Elizabeth L. Hougen
|
$
|
584,600
|
$
|
585,100
|
$
|
1,741,542
|
$
|
1,425,900
|
||||||||
|
B. Lynne Parshall
|
$
|
1,130,924
|
$
|
855,100
|
$
|
3,232,043
|
$
|
2,864,500
|
||||||||
|
Richard Geary
|
$
|
636,066
|
$
|
372,300
|
$
|
1,785,888
|
$
|
575,200
|
||||||||
|
Brett Monia
|
$
|
630,139
|
*
|
$
|
1,787,115
|
*
|
||||||||||
|
Drugs in Clinical Development per Employee
|
Patents per Employee |
|
|
Isis' Ranking
|
1st
|
2
nd
|
|
Executive Peer Group Median
|
1 drug for every 41 employees
|
1.22 patents per employee
|
|
Peer Leader for Drugs in Clinical Development per Employee (ImmunoGen)
|
1 drug for every 19 employees
|
NA
|
|
Peer Leader for Patents per Employee (Alnylam)
|
NA
|
4 patents per employee
|
|
Isis Pharmaceuticals, Inc. (ISIS)
|
1 drug for every 12 employees
|
3 patents per employee
|
| | Salaries were frozen for most NEOs from 2011-2013. The Compensation Committee did not increase salaries for the CEO and most of our NEOs for each of 2011, 2012 and 2013 to allow an increasing percentage of total compensation to be at risk; |
| | A significant portion of cash compensation is at risk . The Compensation Committee structures cash compensation such that a significant proportion of our CEO's, COO's and other NEO's cash compensation is at risk; and |
| | More of total compensation is long-term equity . The Compensation Committee adjusted the total pay mix for our CEO and other NEOs such that more of their compensation is in the form of long-term equity compensation. |
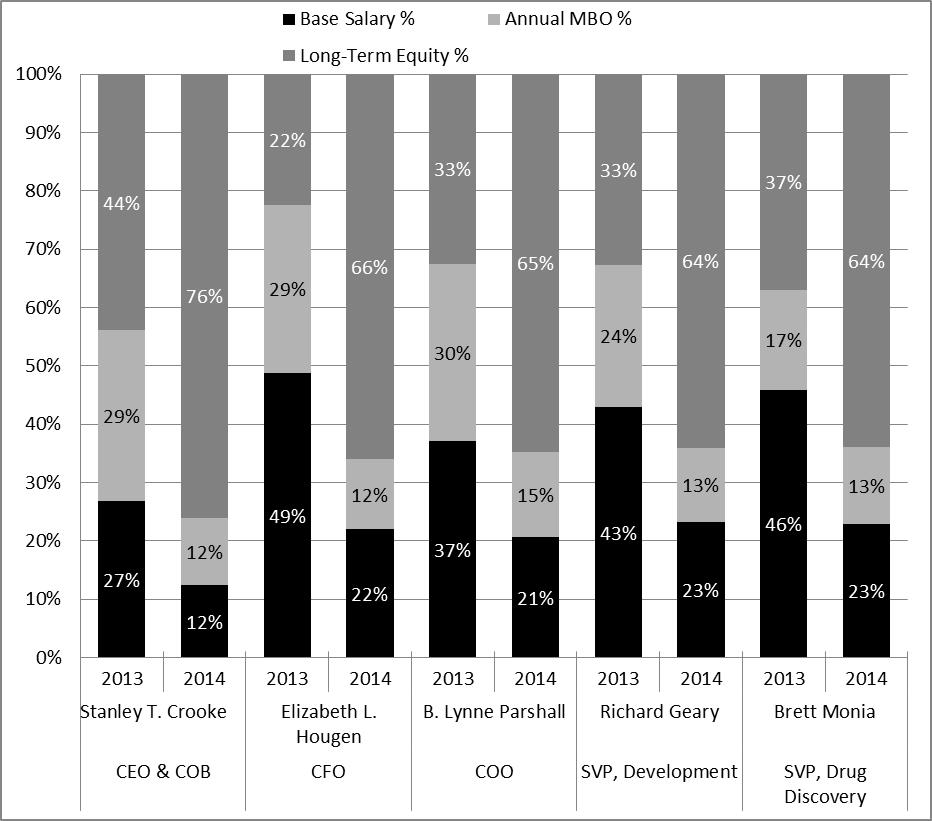
|
Name |
Year |
Base Salary |
Annual Performance MBO |
Long-Term Equity |
Base Salary
% |
Annual Performance
MBO % |
Long-Term Equity
% |
||||||||||||||||||
|
Stanley T. Crooke
|
2013
|
$
|
735,169
|
$
|
803,907
|
$
|
1,203,708
|
27
|
%
|
29
|
%
|
44
|
%
|
||||||||||||
|
CEO & COB
|
2014
|
$
|
768,252
|
$
|
720,236
|
$
|
4,720,669
|
12
|
%
|
12
|
%
|
76
|
%
|
||||||||||||
|
Elizabeth L. Hougen
|
2013
|
$
|
365,496
|
$
|
215,871
|
$
|
167,880
|
49
|
%
|
29
|
%
|
22
|
%
|
||||||||||||
|
CFO
|
2014
|
$
|
377,923
|
$
|
206,677
|
$
|
1,132,961
|
22
|
%
|
12
|
%
|
66
|
%
|
||||||||||||
|
B. Lynne Parshall
|
2013
|
$
|
641,574
|
$
|
526,171
|
$
|
562,945
|
37
|
%
|
30
|
%
|
33
|
%
|
||||||||||||
|
COO
|
2014
|
$
|
664,029
|
$
|
466,895
|
$
|
2,077,096
|
21
|
%
|
15
|
%
|
65
|
%
|
||||||||||||
|
Richard Geary
|
2013
|
$
|
398,444
|
$
|
225,918
|
$
|
562,945
|
34
|
%
|
19
|
%
|
47
|
%
|
||||||||||||
|
SVP, Development
|
2014
|
$
|
411,194
|
$
|
224,872
|
$
|
1,132,961
|
23
|
%
|
13
|
%
|
64
|
%
|
||||||||||||
|
Brett Monia
|
2013
|
$
|
381,288
|
$
|
142,983
|
$
|
307,226
|
46
|
%
|
17
|
%
|
37
|
%
|
||||||||||||
|
SVP, Drug Discovery
|
2014
|
$
|
396,158
|
$
|
233,981
|
$
|
1,132,961
|
23
|
%
|
13
|
%
|
64
|
%
|
||||||||||||
|
We determine base compensation levels for all our employees primarily by market forces. Accordingly, the Compensation Committee believes that it is important when making its compensation decisions to be informed as to the current practices of comparable publicly held companies with which we compete for top talent. To this end, the Compensation Committee reviews market and peer company data, which includes competitive information relating to the mix and levels of compensation for executives in the life sciences industry. We obtain this information for the Executive Peer Group based on recent public filings with the SEC. In addition, we also review data from the Radford Global Life Sciences Survey, which is a summary of compensation data submitted by over 500 life sciences companies. The Committee uses these data to inform and shape its decision-making but does not strictly adhere to quantitative benchmarks. In addition, we assess whether the scope of job responsibilities and internal equity warrant a given base salary.
|
Base salaries for CEO and COO were
frozen
for 2011-2013.
|
|
●
Current Base Salary (x) Merit Increase = Increase to Base Salary
|
Performance MBOs can be, and have in the past been,
zero
.
|
|
| ● Current Base Salary (x) Increase to Base Salary = New Base Salary |
Performance MBOs have a maximum limit.
|
|
2014 Base Salary
|
(x)
|
Merit Increase
|
=
|
Increase to Base Salary
|
|
$768,252
|
(x)
|
4.2%
|
=
|
$32,266
|
|
Current Base Salary
|
(+)
|
Increase to Base Salary
|
=
|
New Base Salary in 2015
|
|
$768,252
|
(+)
|
$32,266
|
=
|
$800,518
|
| | We have a maximum Company Performance Factor of 200% and a maximum Individual Performance Factor of 160%. This range represents the boundary conditions for our Performance Factors and ensures we reward our employees consistent with Isis' success. |
| | We base Target MBO Percentages on position levels within Isis. The Target MBO percentages for 2014 were: Directors 15%; Executive Directors 20%; Vice Presidents 25% or 30%; Senior Vice Presidents 35%, COO 45%; and CEO 60%. |
|
Name
|
Minimum MBO
Percentage of Salary
|
Maximum MBO
Percentage of Salary
|
|
Stanley T. Crooke
|
0%
|
192%
|
|
Elizabeth L. Hougen
|
0%
|
112%
|
|
B. Lynne Parshall
|
0%
|
144%
|
|
Richard Geary
|
0%
|
112%
|
|
Brett Monia
|
0%
|
112%
|
| | Isis' achievement of the approved corporate objectives for the year. At the end of each year, the Compensation Committee meets to evaluate Isis' overall performance. As described below in the chart called "Evaluation of 2014 Corporate Objectives," the Compensation Committee measures Isis' performance based upon the achievement of goals that were set at the beginning of the year and agreed upon by our Board and upper management. |
| | In addition, the Compensation Committee considers our one-, three- and five-year total stockholder returns, and based on these returns may reduce the Individual Performance Factors for our executive officers. |
| | The Compensation Committee then reviews the Company Performance Factor history from the prior ten years to form a comparison for our current year's successes and/or failures. |
| | Finally, the Compensation Committee approves each executive officer's Individual Performance Factor based on the individual's performance. |
|
Evaluation of 2014 Corporate Objectives
|
||
|
Objective & Pre-Approved Measures
|
Evaluation
|
|
|
1
|
Advance Pipeline:
Add four new drugs into pipeline
Initiate Phase 2 Clinical Trials on four drugs
Report positive Phase 2 clinical data on at least three drugs
Initiate Phase 3 Clinical Trials on ISIS-SMN
Rx
(two patient populations)
Initiate Phase 3 Clinical Trials on ISIS-APOCIII
Rx
(two patient populations)
Continue to advance ongoing Phase 3 clinical trial for ISIS-TTR
Rx
Complete discussions with FDA and EMA that are supportive of advancing ISIS-SMN
Rx
and ISIS-APOCIII
Rx
into Phase 3 clinical trials
|
Isis
exceeded
this objective:
Isis added six drugs to its pipeline
Isis and its partners initiated Phase 2 clinical studies for four drugs
Isis reported positive Phase 2 clinical data on seven drugs
Isis initiated ENDEAR and CHERISH, Phase 3 studies for ISIS-SMN
Rx
Isis initiated APPROACH, a Phase 3 study for ISIS-APOCIII
Rx
Isis successfully advanced the ongoing Phase 3 Clinical Trial for ISIS-TTR
Rx
Discussions with FDA and EMA were supportive of advancing ISIS-SMN
Rx
and ISIS-APOCIII
Rx
into Phase 3 clinical trials
|
|
2
|
Stock price increase by a percentage greater than or equal to median of the companies listed in the NASDAQ Biotechnology Index
|
Isis
exceeded
this objective:
Isis' stock price increased over 50% for the year while the median stock price change for companies listed in the NASDAQ Biotechnology Index increased 17%
|
|
3
|
Meet budget and financial projections for the year
|
Isis
exceeded
this objective:
Isis met its budget
Isis significantly exceeded its financial guidance for the year
|
|
Objective & Pre-Approved Measures
|
Evaluation
|
|
|
4
|
Make Biogen Idec relationship successful:
Target sanction of at least two targets
Initiate Phase 1 clinical trial for ISIS-DMPK
Rx
Identify at least two Collaboration Targets
Identify at least two Development Candidates
Achieve $120 million in revenue from relationship
|
Isis
exceeded
this objective:
Isis achieved target sanction for two targets under its Biogen Idec collaborations
Isis initiated a Phase 1/2 study of ISIS-DMPK-2.5
Rx
Isis identified two collaboration targets under its Biogen Idec collaborations
Isis identified two Development Candidates under its Biogen Idec collaborations
Isis recognized over $123 million in revenue during 2014 under its Biogen Idec collaborations
|
|
5
|
Advance at least one LICA development candidate through IND-enabling toxicology studies
|
Isis
met
this objective:
Isis completed IND-enabling toxicology studies for a LICA development candidate
|
|
6
|
Strengthen clinical leadership and organization
Successfully integrate new medical leadership
|
Isis
exceeded
this objective:
Isis hired five new medical doctors into its clinical organization and successfully integrated them
|
|
7
|
Develop and execute long term commercialization/partnering strategy for ISIS-APOCIII
Rx
|
Isis
met
this objective:
Isis established Akcea Therapeutics to develop and commercialize the drugs from its lipid franchise
|
|
8
|
Complete an additional strategic partnership
|
Isis
met
this objective:
Isis formed an alliance with Janssen Biotech, Inc. to discover and develop antisense drugs to treat autoimmune disorders of the GI tract.
|
|
9
|
Achieve $18 million milestone for ISIS-TTR
Rx
|
Isis received an $18 million milestone payment from GSK related to advancing the Phase 2/3 study of ISIS-TTR
Rx
|
|
Unplanned Accomplishments for 2014
|
||
|
10
|
Isis successfully completed an offering of $500 million aggregate principal amount of Convertible Senior Notes due 2021 in a private placement. Isis used a significant amount of the net proceeds from the offering to repurchase a large portion of its 2 ¾% Convertible Senior Notes due 2019.
|
|
|
11
|
Isis appointed Paula Soteropoulos as president and chief executive officer of Akcea Therapeutics.
|
|
|
12
|
Isis strengthened its management team with the addition of Sarah Boyce as chief business officer.
|
|
|
13
|
Isis published the Phase 2 clinical data of ISIS-APOCIII
Rx
in patients with familial chylomicronemia in the New England Journal of Medicine.
|
|
|
14
|
Isis published the Phase 2 clinical data of ISIS-FXI
Rx
in the New England Journal of Medicine.
|
|
|
15
|
Isis formed an alliance with AstraZeneca to discover and develop novel delivery methods for antisense oligonucleotides.
|
|
|
16
|
Isis generated cash from the sale of stock Isis owned in its satellite company partners of more than $25 million, including more than $20 million from the sale of Regulus stock.
|
|
|
17
|
Isis favorably negotiated a tax benefit from the State of California Franchise Tax Board.
|
|
|
Name
|
Base Salary
|
Target MBO %
|
Company Performance Factor
|
Individual Performance Factor
|
Resulting Performance MBO
|
Results Considered When Setting Individual Performance Factor
(1)
|
||||||||||||||||||
|
Stanley T. Crooke
(2)
|
$
|
768,252
|
60
|
%
|
125
|
%
|
125
|
%
|
$
|
720,236
|
1-17
|
|||||||||||||
|
Elizabeth L. Hougen
|
$
|
377,923
|
35
|
%
|
125
|
%
|
125
|
%
|
$
|
206,677
|
2,3,7,8,10 & 15-17
|
|||||||||||||
|
B. Lynne Parshall
(2)
|
$
|
664,029
|
45
|
%
|
125
|
%
|
125
|
%
|
$
|
466,895
|
1-17
|
|||||||||||||
|
Richard S Geary
|
$
|
411,194
|
35
|
%
|
125
|
%
|
125
|
%
|
$
|
224,872
|
1-6, 9, 13 & 14
|
|||||||||||||
|
Brett Monia
|
$
|
396,158
|
35
|
%
|
125
|
%
|
135
|
%
|
$
|
233,981
|
1-5, 8, 9 & 13-15
|
|||||||||||||
| (1) | The numbers correspond to the enumerated objectives in the table entitled "Evaluation of 2014 Corporate Objectives" on pages 119 through 120. The Compensation Committee reviews the individual's contribution towards the corporate objective when setting the Individual Performance Factor. |
| (2) | Since our CEO and COO are ultimately responsible for the Company's performance, their Individual Performance Factors are usually the same as the Company Performance Factor. |
|
We use stock options and RSUs to give all employees, including Isis' executive officers, an economic interest in the long-term appreciation of our common stock. We believe awarding a combination of stock options and RSUs provides a number of benefits. Stock options provide a way to align employee interests with those of upper management and the stockholders because as our stock price increases, so too does the employee's compensation. In 2012, we started granting RSUs as part of the annual merit equity awards. RSUs are a strong retention vehicle for employees as the RSUs vest in annual installments over four years and have value upon vesting, but at the same time, require fewer shares than option awards.
|
Our Stock Awards reward performance and incentivize long-term stock appreciation and increased stockholder returns.
|
|
Executive Officer/Director |
Stock Ownership Guideline
(as a multiple of base salary/annual cash retainer)
|
|
CEO
(1)
|
3 times Base Salary
|
|
COO
|
2 times Base Salary
|
|
All other executive officers
|
1 times Base Salary
|
|
Non-employee Directors
|
4 times Annual Cash Retainer
|
| (1) | Dr. Crooke currently meets these ownership guidelines. |
| | any bonus or other incentive-based or equity-based compensation received by that person from Isis during the 12-month period following the first public issuance or filing with the SEC (whichever first occurs) of the financial document embodying such financial reporting requirement; and |
| | any profits realized from such executive's sale of Isis' securities during that 12-month period. |
|
Name and Principal
Position
|
Year
|
Salary
($)
|
Bonus
(1)
($)
|
Stock Awards
(2)
($)
|
Option Awards
(2)
($)
|
All Other Compensation
(3)
($)
|
Total
($)
|
||||||||||||||||||
|
Stanley T. Crooke
Chairman, President, Chief Executive Officer
|
2014
|
$
|
768,252
|
$
|
720,236
|
$
|
1,489,375
|
$
|
3,231,294
|
$
|
18,986
|
$
|
6,228,143
|
||||||||||||
|
2013
|
$
|
735,169
|
$
|
803,907
|
$
|
325,971
|
$
|
877,737
|
$
|
15,752
|
$
|
2,758,536
|
|||||||||||||
|
2012
|
$
|
735,169
|
$
|
367,585
|
$
|
90,516
|
$
|
344,921
|
$
|
17,815
|
$
|
1,556,006
|
|||||||||||||
|
Elizabeth L. Hougen
Senior Vice President, Finance and Chief Financial Officer
|
2014
|
$
|
377,923
|
$
|
206,677
|
$
|
357,450
|
$
|
775,511
|
$
|
23,981
|
$
|
1,741,542
|
||||||||||||
|
2013
|
$
|
365,496
|
$
|
215,871
|
$ |
(4)
54,419
|
|
$ |
(4)
113,461
|
|
$
|
20,618
|
$
|
769,865
|
|||||||||||
|
2012
|
$
|
337,036
|
$
|
131,444
|
$
|
16,462
|
$
|
62,741
|
$
|
21,148
|
$
|
568,831
|
|||||||||||||
|
B. Lynne Parshall
Director, Chief Operating Officer
|
2014
|
$
|
664,029
|
$
|
466,895
|
$
|
655,325
|
$
|
1,421,771
|
$
|
24,023
|
$
|
3,232,043
|
||||||||||||
|
2013
|
$
|
641,574
|
$
|
526,171
|
$
|
152,482
|
$
|
410,463
|
$
|
20,636
|
$
|
1,751,326
|
|||||||||||||
|
2012
|
$
|
641,574
|
$
|
256,630
|
$
|
52,136
|
$
|
198,675
|
$
|
22,600
|
$
|
1,171,615
|
|||||||||||||
|
Richard S. Geary
Senior Vice President, Development
|
2014
|
$
|
411,194
|
$
|
224,872
|
$
|
357,450
|
$
|
775,511
|
$
|
16,861
|
$
|
1,785,888
|
||||||||||||
|
2013
|
$
|
398,444
|
$
|
225,918
|
$
|
82,117
|
$
|
221,049
|
$
|
14,695
|
$
|
942,223
|
|||||||||||||
|
2012
|
$
|
398,444
|
$
|
143,440
|
$
|
23,583
|
$
|
89,879
|
$
|
17,048
|
$
|
672,394
|
|||||||||||||
|
Brett Monia
(5)
Senior Vice President, Drug Discovery and Corporate Development
|
2014
|
$
|
396,158
|
$
|
233,981
|
$
|
357,450
|
$
|
775,511
|
$
|
24,015
|
$
|
1,787,115
|
||||||||||||
|
2013
|
$
|
381,288
|
$
|
142,983
|
$
|
83,145
|
$
|
224,081
|
$
|
22,834
|
$
|
854,331
|
|||||||||||||
| (1) | We present bonuses in the years they were earned, not in the year paid. Bonuses represent compensation for achievements and are not necessarily paid in the year they are earned; for example, in January 2015 we paid bonuses for 2014 performance. |
| (2) | Amounts represent the aggregate expense recognized for financial statement reporting purposes in accordance with FASB Topic ASC 718 ("ASC 718") for stock and option awards granted to our named executive officers. ASC 718 expense for the option awards is based on the fair value of the awards on the date of grant using an option-pricing model. The fair value of RSUs is based on the market price of our common stock on the date of grant. For more information, please see Note 5, Stockholders' Equity , regarding assumptions underlying valuation of equity awards. |
| (3) | Includes AD&D, Basic Life, Medical, Dental, Vision, and 401(k) matching contributions which are available to all employees. |
| (4) | Ms. Hougen received additional stock options and stock awards due to her promotion to Chief Financial Officer in January 2013. |
| (5) | Mr. Monia was not a named executive officer in 2012 or 2013. We are not disclosing compensation for 2012 as permitted by SEC regulations. |
|
Name
|
Grant Date
|
All Other Stock Awards: Number of Shares of Stock or Units
(#)
|
All Other Option Awards: Number of Securities Underlying Options
(#)
|
Exercise or Base Price of Option Awards
($/Sh)
|
Grant Date Fair Value of Stock and Option Awards
(1)
($)
|
||||||||||||
|
Stanley T. Crooke
|
1/2/14
|
187,500
|
$
|
39.87
|
$
|
3,231,294
|
|||||||||||
|
1/15/14
|
31,250
|
$
|
1,489,375
|
||||||||||||||
|
Elizabeth L. Hougen
|
1/2/14
|
45,000
|
$
|
39.87
|
$
|
775,511
|
|||||||||||
|
1/15/14
|
7,500
|
$
|
357,450
|
||||||||||||||
|
B. Lynne Parshall
|
1/2/14
|
82,500
|
$
|
39.87
|
$
|
1,421,771
|
|||||||||||
|
1/15/14
|
13,750
|
$
|
655,325
|
||||||||||||||
|
Brett Monia
|
1/2/14
|
45,000
|
$
|
39.87
|
$
|
775,511
|
|||||||||||
|
1/15/14
|
7,500
|
$
|
357,450
|
||||||||||||||
|
Richard S. Geary
|
1/2/14
|
45,000
|
$
|
39.87
|
$
|
775,511
|
|||||||||||
|
1/15/14
|
7,500
|
$
|
357,450
|
||||||||||||||
| (1) | Amounts represent the aggregate expense recognized for financial statement reporting purposes in accordance with FASB Topic ASC 718 ("ASC 718") for stock and option awards granted to our named executive Officers. ASC 718 expense for the option awards is based on the fair value of the awards on the date of grant using an option-pricing model. The fair value of RSUs is based on the market price of our common stock on the date of grant. For more information, please see Note 5, Stockholders' Equity , regarding assumptions underlying valuation of equity awards. |
|
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||||
|
Name
|
Grant Date
|
Number of Securities Underlying Unexercised Options (#) Exercisable
(1)
|
Number of Securities Underlying Unexercised Options (#) Unexercisable
|
Option Exercise Price ($)
|
Option Expiration Date
|
Number of Shares or Units of Stock that Have Not Vested
(2
|
Market Value of Shares or Units of Stock that Have Not Vested
(3)
($)
|
||||||||||||||||||
|
Stanley T. Crooke
|
1/4/2010
|
109,163
|
--
|
$
|
11.27
|
1/3/2017
|
--
|
--
|
|||||||||||||||||
|
1/3/2011
|
119,552
|
2,544
|
$
|
10.29
|
1/2/2018
|
--
|
--
|
||||||||||||||||||
|
1/3/2012
|
78,169
|
29,034
|
$
|
7.25
|
1/2/2019
|
--
|
--
|
||||||||||||||||||
|
1/30/2013
|
63,815
|
69,365
|
$
|
14.69
|
1/29/2020
|
--
|
--
|
||||||||||||||||||
|
1/2/2014
|
--
|
187,500
|
$
|
39.87
|
1/1/2021
|
--
|
--
|
||||||||||||||||||
|
1/15/2012
|
--
|
--
|
--
|
--
|
5,954
|
$
|
367,600
|
||||||||||||||||||
|
1/30/2013
|
--
|
--
|
--
|
--
|
16,642
|
$
|
1,027,477
|
||||||||||||||||||
|
1/15/2014
|
--
|
--
|
--
|
--
|
31,250
|
$
|
1,929,375
|
||||||||||||||||||
|
Elizabeth L. Hougen
|
1/2/2009
|
12,657
|
--
|
$
|
14.47
|
1/1/2016
|
--
|
--
|
|||||||||||||||||
|
1/4/2010
|
20,000
|
--
|
$
|
11.27
|
1/3/2017
|
--
|
--
|
||||||||||||||||||
|
1/3/2011
|
21,541
|
459
|
$
|
10.29
|
1/2/2018
|
--
|
--
|
||||||||||||||||||
|
1/3/2012
|
14,218
|
5,282
|
$
|
7.25
|
1/2/2019
|
--
|
--
|
||||||||||||||||||
|
1/2/2013
|
7,522
|
8,178
|
$
|
10.82
|
1/1/2020
|
--
|
--
|
||||||||||||||||||
|
1/2/2013
|
3,593
|
3,907
|
$
|
10.82
|
1/1/2020
|
--
|
--
|
||||||||||||||||||
|
1/2/2014
|
—--
|
45,000
|
$
|
39.87
|
1/1/2021
|
--
|
--
|
||||||||||||||||||
|
1/15/2012
|
--
|
--
|
--
|
--
|
1,082
|
$
|
66,803
|
||||||||||||||||||
|
1/15/2013
|
--
|
--
|
--
|
--
|
937
|
$
|
57,850
|
||||||||||||||||||
|
1/15/2013
|
--
|
--
|
--
|
--
|
1,961
|
$
|
121,072
|
||||||||||||||||||
|
1/15/2014
|
--
|
--
|
--
|
--
|
1,082
|
$
|
66,803
|
||||||||||||||||||
|
B. Lynne Parshall
|
1/3/2011
|
6,074
|
1,519
|
$
|
10.29
|
1/2/2018
|
--
|
--
|
|||||||||||||||||
|
1/3/2012
|
5,146
|
16,724
|
$
|
7.25
|
1/2/2019
|
--
|
--
|
||||||||||||||||||
|
1/30/2013
|
27,332
|
32,438
|
$
|
14.69
|
1/29/2020
|
--
|
--
|
||||||||||||||||||
|
1/2/2014
|
0
|
82,500
|
$
|
39.87
|
1/2/2021
|
--
|
--
|
||||||||||||||||||
|
1/15/2012
|
--
|
--
|
--
|
--
|
3,430
|
$
|
211,768
|
||||||||||||||||||
|
1/30/2013
|
--
|
--
|
--
|
--
|
7,785
|
$
|
480,646
|
||||||||||||||||||
|
1/30/2014
|
--
|
--
|
--
|
--
|
13,750
|
$
|
848,925
|
||||||||||||||||||
|
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||||
|
Name
|
Grant Date
|
Number of Securities Underlying Unexercised Options (#) Exercisable
(1)
|
Number of Securities Underlying Unexercised Options (#) Unexercisable
|
Option Exercise Price ($)
|
Option Expiration Date
|
Number of Shares or Units of Stock that Have Not Vested
(2
|
Market Value of Shares or Units of Stock that Have Not Vested
(3)
($)
|
||||||||||||||||||
|
Brett P. Monia
|
1/3/2011
|
7,129
|
471
|
$
|
10.29
|
1/2/2018
|
--
|
--
|
|||||||||||||||||
|
1/1/2012
|
5,468
|
2,032
|
$
|
7.21
|
12/31/2018
|
--
|
--
|
||||||||||||||||||
|
1/3/2012
|
3,849
|
5,144
|
$
|
7.25
|
1/2/2019
|
--
|
--
|
||||||||||||||||||
|
1/30/2013
|
16,291
|
17,709
|
$
|
14.69
|
1/29/2020
|
--
|
--
|
||||||||||||||||||
|
1/2/2014
|
0
|
45,000
|
$
|
39.87
|
1/1/2021
|
--
|
--
|
||||||||||||||||||
|
1/15/2012
|
--
|
--
|
--
|
--
|
1,054
|
$
|
65,074
|
||||||||||||||||||
|
1/15/2012
|
--
|
--
|
--
|
--
|
416
|
$
|
25,684
|
||||||||||||||||||
|
1/30/2013
|
--
|
--
|
--
|
--
|
4,245
|
$
|
262,086
|
||||||||||||||||||
|
1/15/2014
|
--
|
--
|
--
|
--
|
7,500
|
$
|
463,050
|
||||||||||||||||||
|
Richard S. Geary
|
1/4/2010
|
80
|
--
|
$
|
11.27
|
1/3/2017
|
--
|
--
|
|||||||||||||||||
|
1/3/2011
|
7,729
|
697
|
$
|
10.29
|
1/2/2018
|
--
|
--
|
||||||||||||||||||
|
1/3/2012
|
6,470
|
7,565
|
$
|
7.25
|
1/2/2019
|
--
|
--
|
||||||||||||||||||
|
1/30/2013
|
7,771
|
17,469
|
$
|
14.69
|
1/29/2020
|
--
|
--
|
||||||||||||||||||
|
1/2/2014
|
0
|
45,000
|
$
|
39.87
|
1/1/2021
|
--
|
--
|
||||||||||||||||||
|
1/15/2012
|
--
|
--
|
--
|
--
|
1,551
|
$
|
95,759
|
||||||||||||||||||
|
1/30/2013
|
--
|
--
|
--
|
--
|
4,192
|
$
|
258,814
|
||||||||||||||||||
|
1/15/2014
|
--
|
--
|
--
|
--
|
7,500
|
$
|
463,050
|
||||||||||||||||||
| (1) | The options have a term of seven years and vest at the rate of 25% for the first year and then at the rate of 2.08% per month for 36 months thereafter during the optionee's employment. |
| (2) | The RSUs were granted out of our 2011 Equity Incentive Plan. The RSUs vest at the rate of 25% per year over four years. |
| (3) | Market value of stock awards was determined by multiplying the number of unvested shares by $61.74, which was the closing market price of our common stock on the Nasdaq Global Select Market on December 31, 2014, the last trading day of fiscal 2014. |
|
Option Awards
|
Stock Awards
|
|||||||||||||||
|
Name
|
Number of Shares Acquired on Exercise (#)(1)
|
Value Realized on Exercise ($)
|
Number of Shares Acquired on Vesting (#)
|
Value Realized on Vesting ($)
|
||||||||||||
|
Stanley T. Crooke
|
10,000
|
$
|
309,500
|
8,526
|
$
|
406,349
|
||||||||||
|
3,000
|
$
|
83,250
|
||||||||||||||
|
13,000
|
$
|
325,221
|
||||||||||||||
|
20,000
|
$
|
468,820
|
||||||||||||||
|
15,500
|
$
|
325,252
|
||||||||||||||
|
15,000
|
$
|
240,825
|
||||||||||||||
|
5,000
|
$
|
105,200
|
||||||||||||||
|
3,000
|
$
|
63,240
|
||||||||||||||
|
2,500
|
$
|
52,950
|
||||||||||||||
|
3,000
|
$
|
63,390
|
||||||||||||||
|
2,000
|
$
|
42,220
|
||||||||||||||
|
11,000
|
$
|
226,941
|
||||||||||||||
|
10,000
|
$
|
189,850
|
||||||||||||||
|
7,000
|
$
|
204,246
|
||||||||||||||
|
11,000
|
$
|
281,402
|
||||||||||||||
|
4,000
|
$
|
102,120
|
||||||||||||||
|
25,000
|
$
|
619,575
|
||||||||||||||
|
Elizabeth L. Hougen
|
- -
|
- -
|
1,509
|
$
|
71,919
|
|||||||||||
|
B. Lynne Parshall
(2)
|
2,510
|
$
|
76,382
|
|||||||||||||
|
3,972
|
$
|
149,097
|
||||||||||||||
|
8,561
|
$
|
324,214
|
||||||||||||||
|
443
|
$
|
14,615
|
||||||||||||||
|
15,875
|
$
|
684,435
|
||||||||||||||
|
10,628
|
$
|
318,318
|
4,310
|
$
|
205,415
|
|||||||||||
|
23,635
|
$
|
947,149
|
||||||||||||||
|
31,028
|
$
|
1,195,105
|
||||||||||||||
|
Brett Monia
|
15,000
|
$
|
417,810
|
2,151
|
$
|
102,517
|
||||||||||
|
2,344
|
$
|
62,993
|
||||||||||||||
|
10,000
|
$
|
308,940
|
||||||||||||||
|
Richard S. Geary
|
8,300
|
$
|
293,455
|
2,174
|
$
|
103,613
|
||||||||||
|
7,400
|
$
|
203,966
|
||||||||||||||
|
13,900
|
$
|
594,864
|
||||||||||||||
|
7,600
|
$
|
241,148
|
||||||||||||||
| (1) | Each individual executed each option exercise and resulting sales pursuant to the individual's Rule 10b5-1 trading plan. |
| (2) | Includes options exercised by Ms. Parshall's daughters and son-in-law. |
| - | Dr. Crooke will be eligible to receive a lump sum severance payment equal to 36 months of his then-current base salary in the event his employment is terminated as a result of a change of control of Isis; and |
| - | Ms. Parshall will be eligible to receive a lump sum severance payment equal to: |
|
|
Termination Event
|
|||||||
|
Name
|
Termination Without Cause
|
Termination in a Change of Control
|
||||||
|
Stanley T. Crooke
|
--
|
$
|
2,304,756
|
|||||
|
B. Lynne Parshall
|
$
|
996,044
|
$
|
1,660,073
|
||||
|
Role
|
2014 Cash Compensation
|
|||
|
Board Member (Base)
|
$
|
50,000
|
||
|
Committee Chairs (Additional)
|
||||
|
Audit
|
$
|
24,000
|
||
|
Compensation
|
$
|
15,000
|
||
|
Nominating & Gov.
|
$
|
10,000
|
||
|
Agenda
|
$
|
10,000
|
||
|
Committee Member (Additional)
|
||||
|
Audit
|
$
|
10,000
|
||
|
Compensation
|
$
|
7,500
|
||
|
Nominating & Gov.
|
$
|
5,000
|
||
|
Agenda
|
$
|
5,000
|
||
|
Scientific/Medical
|
$
|
10,000
|
||
|
Name
|
Fees
Earned or Paid in Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
(1)
|
All Other
Compensation
($)
|
Total
($)
|
|||||||||||||||
|
Spencer R. Berthelsen
|
$
|
85,000
|
$
|
94,759
|
$
|
316,388
|
--
|
$
|
496,147
|
|||||||||||
|
Breaux B. Castleman
|
$
|
60,000
|
$
|
94,759
|
$
|
316,388
|
--
|
$
|
471,147
|
|||||||||||
|
Joseph Klein
|
$
|
60,000
|
$
|
94,759
|
$
|
316,388
|
--
|
$
|
471,147
|
|||||||||||
|
Joseph Loscalzo
(2)
|
$
|
65,000
|
$
|
278,846
|
$
|
932,098
|
--
|
$
|
1,275,944
|
|||||||||||
|
Frederick T. Muto
|
$
|
55,000
|
$
|
94,759
|
$
|
316,388
|
--
|
$
|
466,147
|
|||||||||||
|
Joseph H. Wender
|
$
|
86,500
|
$
|
94,759
|
$
|
316,388
|
--
|
$
|
497,647
|
|||||||||||
| (1) | Amounts represent the aggregate expense recognized for financial statement reporting purposes in accordance with FASB Topic ASC 718 ("ASC 718") for stock and option awards granted to the Directors. ASC 718 expense for the option awards is based on the fair value of the awards on the date of grant using an option-pricing model. The fair value of RSUs is based on the market price of our common stock on the date of grant. For more information, please see Note 5, Stockholders' Equity , of the consolidated financial statements in this Form 10-K regarding assumptions underlying valuation of equity awards. |
| (2) | Includes an option to purchase 22,500 shares of our common stock and an RSU for 3,750 shares of our common stock, which were automatically granted to Dr. Loscalzo under the Directors' Plan when he joined our Board. |
|
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||
|
Name
|
Number of Securities Underlying Unexercised Options (#)
Exercisable
(1)
|
Number of Securities Underlying Unexercised Options (#)
Unexercisable
|
Option Exercise Price
($)
|
Option
Expiration
Date
|
Number of Shares or Units of Stock that Have Not Vested
(2) (3)
|
Market Value of Shares or Units of Stock that Have Not Vested
(4)
($)
|
|||||||||||||||
|
Spencer R. Berthelsen
|
10,000
|
0
|
$
|
3.95
|
6/30/15
|
4,697
|
$
|
289,993
|
|||||||||||||
|
12,500
|
0
|
$
|
5.93
|
7/2/16
|
|||||||||||||||||
|
12,500
|
0
|
$
|
9.77
|
7/1/17
|
|||||||||||||||||
|
15,000
|
0
|
$
|
13.88
|
6/30/18
|
|||||||||||||||||
|
15,000
|
0
|
$
|
16.32
|
6/30/19
|
|||||||||||||||||
|
15,000
|
0
|
$
|
9.22
|
6/30/20
|
|||||||||||||||||
|
11,250
|
3,750
|
$
|
9.30
|
6/30/21
|
|||||||||||||||||
|
5,626
|
5,624
|
$
|
12.94
|
7/1/22
|
|||||||||||||||||
|
2,813
|
8,437
|
$
|
28.47
|
6/30/23
|
|||||||||||||||||
|
0
|
16,000
|
$
|
35.53
|
6/30/24
|
|||||||||||||||||
|
Breaux B. Castleman
|
5,625
|
16,875
|
$
|
26.66
|
6/24/23
|
6,885
|
$
|
425,080
|
|||||||||||||
|
2,813
|
8,437
|
$
|
28.47
|
6/30/23
|
|||||||||||||||||
|
0
|
16,000
|
$
|
35.53
|
6/30/24
|
|||||||||||||||||
|
Joseph Klein
|
3,750
|
0
|
$
|
9.22
|
6/30/20
|
4,697
|
$
|
289,993
|
|||||||||||||
|
3,750
|
$
|
9.30
|
6/30/21
|
||||||||||||||||||
|
2,813
|
5,624
|
$
|
12.94
|
7/1/22
|
|||||||||||||||||
|
2,813
|
8,437
|
$
|
28.47
|
6/30/23
|
|||||||||||||||||
|
0
|
16,000
|
$
|
35.53
|
6/30/24
|
|||||||||||||||||
|
Joseph Loscalzo
|
0
|
22,500
|
$
|
49.09
|
2/2/24
|
6,417
|
$
|
396,186
|
|||||||||||||
|
0
|
16,000
|
$
|
35.53
|
6/30/24
|
|||||||||||||||||
|
Frederick T. Muto
|
10,000
|
0
|
$
|
3.95
|
6/30/15
|
4,697
|
$
|
289,993
|
|||||||||||||
|
12,500
|
0
|
$
|
5.93
|
7/2/16
|
|||||||||||||||||
|
12,500
|
0
|
$
|
9.77
|
7/1/17
|
|||||||||||||||||
|
15,000
|
0
|
$
|
13.88
|
6/30/18
|
|||||||||||||||||
|
15,000
|
0
|
$
|
16.32
|
6/30/19
|
|||||||||||||||||
|
15,000
|
0
|
$
|
9.22
|
6/30/20
|
|||||||||||||||||
|
11,250
|
3,750
|
$
|
9.30
|
6/30/21
|
|||||||||||||||||
|
5,626
|
5,624
|
$
|
12.94
|
7/1/22
|
|||||||||||||||||
|
2,813
|
8,437
|
$
|
28.47
|
6/30/23
|
|||||||||||||||||
|
0
|
16,000
|
$
|
35.53
|
6/30/24
|
|||||||||||||||||
|
Joseph H. Wender
|
10,000
|
0
|
$
|
3.95
|
6/30/15
|
4,697
|
$
|
289,993
|
|||||||||||||
|
12,500
|
0
|
$
|
5.93
|
7/2/16
|
|||||||||||||||||
|
12,500
|
0
|
$
|
9.77
|
7/1/17
|
|||||||||||||||||
|
15,000
|
0
|
$
|
13.88
|
6/30/18
|
|||||||||||||||||
|
15,000
|
0
|
$
|
9.22
|
6/30/20
|
|||||||||||||||||
|
11,250
|
3,750
|
$
|
9.30
|
6/30/21
|
|||||||||||||||||
|
5,626
|
5,624
|
$
|
12.94
|
7/1/22
|
|||||||||||||||||
|
2,813
|
8,437
|
$
|
28.47
|
6/30/23
|
|||||||||||||||||
|
0
|
16,000
|
$
|
35.53
|
6/30/24
|
|||||||||||||||||
| (1) | The options were granted out of our Directors' Plan and have a term of ten years and vest at the rate of 25% per year over four years. |
| (2) | The RSUs were granted out of our Directors' Plan and vest at the rate of 25% per year over four years. |
| (3) | All of our non-employee Directors are subject to our Stock Holding and Ownership Guidelines for RSU Shares, which requires each non-employee Director to accumulate and maintain shares of Common Stock issued pursuant to RSUs until he has accumulated shares of Common Stock equal to four times such non-employee Director's base annual cash retainer for service as a Director (but not for service on a Board committee), or until his termination of service. |
| (4) | Market value of stock awards was determined by multiplying the number of unvested shares by $61.74, which was the closing market price of our common stock on the Nasdaq Select Market on December 31, 2014, the last trading day of fiscal 2014. |
|
|
Option Awards
|
Stock Awards
|
||||||||||||||
|
Name
|
Number of
Shares
Acquired
on Exercise
(#)
|
Value Realized
on Exercise
($)
|
Number of
Shares
Acquired
on Vesting
(#)
|
Value Realized
on Vesting
($)
|
||||||||||||
|
Spencer R. Berthelsen
|
10,000
|
$
|
282,900
|
782
|
$
|
27,728
|
||||||||||
|
Breaux B. Castleman
|
--
|
--
|
1,407
|
$
|
49,991
|
|||||||||||
|
Joseph Klein, III
|
10,313
|
$
|
343,039
|
782
|
$
|
27,728
|
||||||||||
|
Frederick T. Muto
|
10,000
|
$
|
228,700
|
782
|
$
|
27,728
|
||||||||||
|
Joseph H. Wender
|
10,000
|
$
|
418,437
|
782
|
$
|
27,728
|
||||||||||
|
Joseph Loscalzo
|
--
|
--
|
--
|
--
|
||||||||||||
| | reviewed and discussed the Compensation Discussion and Analysis included in this Form 10-K with management; and |
| | based on this review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Form 10-K. |
| * | This Section is not "soliciting material," is not deemed filed with the SEC and is not to be incorporated by reference in any filing of Isis under the Securities Act or the Exchange Act. |
| | each Director; |
| | each executive officer named in the Summary Compensation Table under "Executive Compensation--Compensation of Executive Officers"; |
| | all Directors and executive officers as a group; and |
| | every entity that we know beneficially owns more than five percent of our common stock. |
|
|
|
Beneficial Ownership
(1)
|
|
|||||
|
Beneficial Owner
|
|
Number of Shares
|
|
|
Percent of Total
(2)
|
|
||
|
FMR LLC
(3)
|
|
|
17,892,555
|
|
|
|
15.04
|
|
|
245 Summer Street
|
|
|
|
|
|
|
|
|
|
Boston, MA 02210
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BlackRock, Inc.
(4)
|
|
|
9,541,651
|
|
|
8.02
|
|
|
|
55 East 52
nd
Street
|
|
|
|
|
|
|
|
|
|
New York, NY 10022
|
|
|
|
|
|
|
|
|
|
ClearBridge Investments, LLC
(5)
|
|
|
9,034,520
|
|
|
|
7.59
|
|
|
620 8
th
Avenue
|
|
|
|
|
|
|
|
|
|
New York, NY 10018
|
|
|
|
|
|
|
|
|
|
The Vanguard Group
(6)
|
|
|
7,271,871
|
|
|
|
6.11
|
|
|
100 Vanguard Boulevard
|
|
|
|
|
|
|
|
|
|
Malvern, PA 19355
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BB Biotech AG
(7)
|
|
|
5,976,526
|
|
|
|
5.02
|
|
|
Vordergasse 3
|
|
|
|
|
|
|
|
|
|
CH-8200 Schaffhausen, Switzerland
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Spencer R. Berthelsen
(8)
|
|
|
150,154
|
|
|
|
*
|
|
|
Breaux B. Castleman
(9)
|
|
|
9,845
|
|
|
|
*
|
|
|
Stanley T. Crooke
(10)
|
|
|
1,198,094
|
|
|
|
1.0
|
|
|
Joseph Klein, III
(11)
|
|
|
14,321
|
|
|
|
*
|
|
|
Joseph Loscalzo
(12)
|
|
|
6,563
|
|
|
|
*
|
|
|
Frederick T. Muto
(13)
|
|
|
102,284
|
|
|
|
*
|
|
|
B. Lynne Parshall
(14)
|
|
|
73,060
|
|
|
|
*
|
|
|
Joseph H. Wender
(15)
|
|
|
111,906
|
|
|
|
*
|
|
|
Brett Monia
(16)
|
|
|
56,738
|
|
|
|
*
|
|
|
Richard S. Geary
(17)
|
|
|
49,501
|
|
|
|
*
|
|
|
Elizabeth L. Hougen
(18)
|
|
|
100,998
|
|
|
|
*
|
|
|
All Directors and executive officers as a group (fourteen persons)
(19)
|
|
|
2,066,615
|
|
|
|
1.7
|
|
| (1) | We base this table upon information supplied by officers, Directors, principal stockholders and Form 3s, Form 4s, Form 5s, Schedules 13D and 13G filed with the SEC. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, we believe that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. |
| (2) | Applicable percentages are based on 118,974,465 shares of common stock outstanding on February 18, 2015, adjusted as required by rules promulgated by the SEC. |
| (3) | Abigail P. Johnson is a Director, the Vice Chairman, the Chief Executive Officer and the President of FMR LLC. Members of the family of Edward C. Johnson 3d, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders' voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders' voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. |
| (4) | Various persons at BlackRock, Inc. have the right to receive or the power to direct the receipt of dividends from, or the proceeds from the sale of shares of our common stock. |
| (5) | ClearBridge Advisors, LLC, is an investment adviser registered under the Investment Advisers Act . ClearBridge Advisors has sole voting power to direct the vote of 8,778,431 shares and sole power to dispose or direct the disposition of 9,034,520 shares. |
| (6) | The Vanguard Group has sole voting power to direct the vote of 158,221 shares, sole power to dispose or direct the disposition of 7,124,050 shares, and shared dispositive power for 147,821 shares. Vanguard Fiduciary Trust Company , a wholly-owned subsidiary of The Vanguard Group, Inc., is the beneficial owner of 147,821 shares or 0.12% of the Common Stock outstanding of Isis as a result of its serving as investment manager of collective trust accounts. Vanguard Investments Australia, Ltd., a wholly-owned subsidiary of The Vanguard Group, Inc., is the beneficial owner of 10,400 shares of Isis' Common Stock outstanding as a result of its serving as investment manager of Australian investment offerings. |
| (7) | BB Biotech AG shares voting and dispositive powers with its wholly owned subsidiary, Biotech Target N.V. |
| (8) | Includes 70 shares owned by Dr. Berthelsen's daughter for which he disclaims beneficial ownership. Includes 99,689 shares of common stock issuable upon exercise of options held by Dr. Berthelsen that are exercisable on or before April 19, 2015. |
| (9) | Includes 8,438 shares of common stock issuable upon exercise of options held by Mr. Castleman that are exercisable on or before April 19, 2015. |
| (10) | Includes shares of common stock held by Dr. Crooke and 411,868 shares of common stock issuable upon exercise of options held by Dr. Crooke that are exercisable on or before April 19, 2015. Also includes 1,297 shares of common stock and 44,896 shares of common stock issuable upon exercise of options held by Rosanne Crooke, Dr. Crooke's wife, which are exercisable on or before April 19, 2015. Dr. Crooke disclaims beneficial ownership of the shares of common stock owned and issuable upon exercise of options held by his wife. |
| (11) | Includes 100 shares of common stock beneficially owned by Mr. Klein's son and 13,126 shares of common stock issuable upon exercise of options held by Mr. Klein that are exercisable on or before April 19, 2015. |
| (12) | Includes 5,625 shares of common stock issuable upon exercise of options held by Mr. Loscalzo that are exercisable on or before April 19, 2015. |
| (13) | Includes 1,500 shares of common stock beneficially owned through the Cooley LLP Salary Deferral and Profit Sharing Plan and 99,689 shares of common stock issuable upon exercise of options held by Mr. Muto that are exercisable on or before April 19, 2015. |
| (14) | Includes 64,988 shares of common stock issuable upon exercise of options held by Ms. Parshall that are exercisable on or before April 19, 2015. |
| (15) | Includes 74,689 shares of common stock issuable upon exercise of options held by Mr. Wender that are exercisable on or before April 19, 2015. |
| (16) | Includes 52,312 shares of common stock issuable upon exercise of options held by Dr. Monia that are exercisable on or before April 19, 2015. |
| (17) | Includes 41,931 shares of common stock issuable upon exercise of options held by Dr. Geary that are exercisable on or before April 19, 2015. |
| (18) | Includes 100,998 shares of common stock issuable upon exercise of options held by Ms. Hougen that are exercisable on or before April 19, 2015. |
| (19) | Includes an aggregate of 1,194,610 shares issuable upon exercise of options held by all current Directors and executive officers as a group that are exercisable on or before April 19, 2015. |
|
Plan Category
|
Number of Shares
to be Issued Upon Exercise of Outstanding Options |
Weighted Average
Exercise Price of Outstanding Options |
Number of Shares
Remaining Available for Future Issuance |
|||||||||
|
Equity compensation plans approved by stockholders(a)
|
7,122,771
|
$
|
$19.69
|
3,208,151
|
(c)
|
|||||||
|
Equity compensation plans not approved by stockholders(b)
|
256,176
|
$
|
14.75
|
-
|
||||||||
|
Total
|
7,378,947
|
$
|
19.52
|
3,208,151
|
||||||||
| (a) | Consists of four Isis plans: 1989 Stock Option Plan, Amended and Restated 2002 Non-Employee Directors' Stock Option Plan, 2011 Equity Incentive Plan and Employee Stock Purchase Plan, or ESPP. |
| (b) | Consists of the 2000 Broad-Based Equity Incentive Plan, more fully described below. The 2000 Broad-Based Equity Incentive Plan expired on January 5, 2010. |
| (c) | Of these shares, 370,136 remained available for purchase under the ESPP as of December 31, 2014. The ESPP incorporates an evergreen formula pursuant to which on January 1 of each year, we automatically increase the aggregate number of shares reserved for issuance under the plan by 150,000 shares. |
| | a sale, lease or other disposition of all or substantially all of our assets; |
| | a merger or consolidation in which we are not the surviving corporation; or |
| | reverse merger in which we are the surviving corporation but the shares of common stock outstanding immediately preceding the merger are converted by virtue of the merger into other property, whether in the form of securities, cash or otherwise; |
|
Name and Principal
Position
|
Year
|
Salary
($)
|
Bonus
(1)
($)
|
Stock Awards
(2)
($)
|
Option Awards
(2)(4)
($)
|
All Other Compensation
(3)
($)
|
Total
($)
|
||||||||||||||||||
|
Rosanne Crooke
Vice President, Cardiovascular Diseases Drug Discovery Research
|
2014
|
$
|
210,814
|
$
|
82,349
|
$
|
67,487
|
$
|
146,488
|
$
|
5,912
|
$
|
513,050
|
||||||||||||
|
2013
|
$
|
203,685
|
$
|
89,367
|
$
|
23,457
|
$
|
48,906
|
$
|
3,777
|
$
|
369,192
|
|||||||||||||
|
2012
|
$
|
197,369
|
$
|
59,211
|
$
|
9,622
|
$
|
36,692
|
$
|
4,517
|
$
|
307,411
|
|||||||||||||
| (1) | We present bonuses in the years they were earned, not in the year paid. Bonuses represent compensation for achievements and are not necessarily paid in the year they are earned; for example, in January 2015 we paid bonuses for 2014 performance. |
| (2) | Amounts represent the aggregate expense recognized for financial statement reporting purposes in accordance with FASB Topic ASC 718 ("ASC 718") for stock and option awards granted to Dr. Crooke. ASC 718 expense for the option awards is based on the fair value of the awards on the date of grant using an option-pricing model. The fair value of RSUs is based on the market price of our common stock on the date of grant. For more information, please see Note 5, Stockholders' Equity , regarding assumptions underlying valuation of equity awards. |
| (3) | Includes AD&D, Basic Life, Medical, Dental, Vision, and 401(k) matching contributions which are available to all employees. |
| (4) | These amounts represent the estimated fair values of stock option grants we recognized as share-based compensation expense. The estimated fair value amounts were determined using the Black-Scholes option-valuation model and are not indicative of whether Dr. Rosanne Crooke will realize the estimated fair value or any financial benefits from the award. The applicable amounts represent: |
| | 11,404 shares at $7.25 per share received on January 3, 2012; |
| | 10,000 shares at $10.82 per share received on January 2, 2013; and |
| | 8,500 shares at $39.87 per share received on January 2, 2014. |
|
|
ISIS PHARMACEUTICALS, INC.
|
|
|
|
|
|
|
|
By:
|
/s/ STANLEY T. CROOKE
|
|
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
|
|
Chairman of the Board, President and Chief Executive Officer (Principal executive officer)
|
|
Signatures
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ STANLEY T. CROOKE
|
|
Chairman of the Board, President, and Chief Executive Officer
|
|
February 27, 2015
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
(Principal executive officer)
|
|
|
|
|
|
|
|
|
|
/s/ B. LYNNE PARSHALL
|
|
Director, Chief Operating Officer and Secretary
|
|
February 27, 2015
|
|
B. Lynne Parshall, J.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ ELIZABETH L. HOUGEN
|
|
Senior Vice President, Finance and Chief Financial Officer
|
|
February 27, 2015
|
|
Elizabeth L. Hougen
|
|
(Principal financial and accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ SPENCER R. BERTHELSEN
|
|
Director
|
|
February 27, 2015
|
|
Spencer R. Berthelsen, M.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ BREAUX CASTLEMAN
|
|
Director
|
|
February 27, 2015
|
|
Breaux Castleman
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH KLEIN
|
|
Director
|
|
February 27, 2015
|
|
Joseph Klein, III.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH LOSCALZO
|
|
Director
|
|
February 27, 2015
|
|
Joseph Loscalzo, M.D., Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ FREDERICK T. MUTO
|
|
Director
|
|
February 27, 2015
|
|
Frederick T. Muto, Esq.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH H. WENDER
|
|
Director
|
|
February 27, 2015
|
|
Joseph H. Wender
|
|
|
|
|
|
Exhibit
Number |
|
Description of Document
|
|
3.1
|
|
Amended and Restated Certificate of Incorporation filed June 19, 1991. (1)
|
|
|
|
|
|
3.2
|
|
Certificate of Amendment to Restated Certificate of Incorporation filed June 17, 2014. (42)
|
|
|
|
|
|
3.3
|
|
Amended and Restated Bylaws. (13)
|
|
|
|
|
|
4.1
|
|
Certificate of Designation of the Series C Junior Participating Preferred Stock. (12)
|
|
|
|
|
|
4.2
|
|
Specimen Common Stock Certificate. (1)
|
|
|
|
|
|
4.3
|
|
Stock Purchase Agreement between the Registrant and Genzyme Corporation dated January 7, 2008. (5)
|
|
|
|
|
|
4.4
|
|
Indenture, dated as of August 13, 2012, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 2 ¾ percent Convertible Senior Note due 2019. (30)
|
|
4.5
|
Indenture, dated as of November 17, 2014, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 1.00 percent Convertible Senior Note due 2021. (41)
|
|
|
|
|
|
|
10.1
|
|
Form of Indemnification Agreement entered into between the Registrant and its Directors and Officers with related schedule. (37)
|
|
|
|
|
|
10.2*
|
|
Registrant's 1989 Stock Option Plan, as amended. (27)
|
|
|
|
|
|
10.3*
|
|
Registrant's Amended and Restated Employee Stock Purchase Plan. (15)
|
|
|
|
|
|
10.4
|
|
Form of Employee Assignment of Patent Rights. (1)
|
|
|
|
|
|
10.5*
|
|
Registrant's 2000 Broad-Based Equity Incentive Stock Option Plan and related form of option agreement. (7)
|
|
|
|
|
|
10.6
|
|
Drug Development and License Option Agreement dated December 2, 2009 between the Registrant and Eli Lilly and Company. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (24)
|
|
|
|
|
|
10.7
|
|
Patent Rights Purchase Agreement between the Registrant and Gilead Sciences, Inc., dated December 18, 1998. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (6)
|
|
|
|
|
|
10.8
|
|
Collaboration and License Agreement between the Registrant and Hybridon, Inc., dated May 24, 2001. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (14)
|
|
|
|
|
|
10.9
|
|
License and Co-Development Agreement between the Registrant and Genzyme Corporation dated June 24, 2008. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (9)
|
|
|
|
|
|
10.10
|
|
Amendment #1 to the Research, Development and License Agreement dated May 11, 2011 by and between the Registrant and Glaxo Group Limited. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (10)
|
|
|
|
|
|
10.11
|
|
Amended and Restated Collaboration and License Agreement between the Registrant and Antisense Therapeutics Ltd dated February 8, 2008. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (5)
|
|
|
|
|
|
10.12
|
|
Amended and Restated License Agreement between the Registrant and Atlantic Pharmaceuticals Limited dated November 30, 2009. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (24)
|
|
10.13
|
|
Amended and Restated License Agreement dated July 2, 2008 between the Registrant and OncoGenex Technologies Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (17)
|
|
|
|
|
|
10.14
|
|
Lease Agreement between the Registrant and BMR-Gazelle Court LLC dated March 30, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (25)
|
|
|
|
|
|
10.15
|
|
Second Amendment to Lease Agreement dated May 15, 2011 between the Registrant and BMR-Gazelle Court LLC, with First Amendment to Lease Agreement included. (10)
|
|
|
|
|
|
10.16
|
|
Registrant's Amended and Restated Isis Pharmaceuticals, Inc. 10b5-1 Trading Plan dated September 12, 2013. (34)
|
|
|
|
|
|
10.17*
|
|
Registrant's Amended and Restated 2002 Non-Employee Directors' Stock Option Plan, as amended. (42)
|
|
|
|
|
|
10.18*
|
|
Registrant's Form of 2002 Non-Employee Directors' Stock Option Agreement. (21)
|
|
|
|
|
|
10.19*
|
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the Isis Pharmaceuticals, Inc. 2002 Non-Employee Directors' Stock Option Plan. (32)
|
|
|
|
|
|
10.20*
|
|
Amended and Restated Severance Agreement dated December 3, 2008 between the Registrant and Stanley T. Crooke. (16)
|
|
|
|
|
|
10.21*
|
|
Amended and Restated Severance Agreement dated December 3, 2008 between the Registrant and B. Lynne Parshall. (16)
|
|
|
|
|
|
10.22
|
|
Amended and Restated Strategic Collaboration and License Agreement dated April 28, 2009 between the Registrant and Alnylam Pharmaceuticals, Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (20)
|
|
|
|
|
|
10.23*
|
|
Isis Pharmaceuticals, Inc. 2011 Equity Incentive Plan (19)
|
|
|
|
|
|
10.24
|
|
Loan Agreement dated October 15, 2008 between the Registrant and RBS Asset Finance, Inc. (23)
|
|
|
|
|
|
10.25*
|
|
Form of Option Agreement for Options granted under the 2011 Equity Incentive Plan. (29)
|
|
|
|
|
|
10.26*
|
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the 2011 Equity Incentive Plan. (29)
|
|
|
|
|
|
10.27
|
|
Second Amendment to Lease Agreement between the Registrant and BMR-2282 Faraday Avenue LLC dated March 30, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (25)
|
|
|
|
|
|
10.28*
|
|
Form of Option Agreement for Options Granted after March 8, 2005 under the 1989 Stock Option Plan. (11)
|
|
|
|
|
|
10.29*
|
|
Form of Option Agreement for Options Granted after March 8, 2005 under the 2000 Broad-Based Equity Incentive Plan. (11)
|
|
|
|
|
|
10.30*
|
|
Form of Option Agreement for Options Granted after March 8, 2005 under the 2002 Non-Employee Director's Stock Option Plan. (11)
|
|
|
|
|
|
10.31
|
|
Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated March 30, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (25)
|
|
|
|
|
|
10.32
|
|
First Amendment to Loan Agreement between the Registrant and RBS Asset Finance, Inc. dated September 30, 2009. (24)
|
|
|
|
|
|
10.33
|
|
Lease Agreement dated September 6, 2005 between the Registrant and BMR-2282 Faraday Avenue LLC. (18)
|
|
10.34
|
|
Stock Purchase Agreement dated December 17, 2008, among the Registrant, Ibis Biosciences, Inc. and Abbott Molecular Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (23)
|
|
|
|
|
|
10.35
|
|
Research Agreement dated August 10, 2011 between the Registrant and CHDI Foundation, Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (3)
|
|
|
|
|
|
10.36
|
|
Amended and Restated License and Collaboration Agreement among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics LLC dated January 1, 2009. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (4)
|
|
|
|
|
|
10.37
|
|
Amendment No. 1 to Amended and Restated License Agreement between the Registrant and OncoGenex Technologies Inc. dated December 18, 2009. (24)
|
|
|
|
|
|
10.38
|
|
Amendment Number One to the Amended and Restated License and Collaboration Agreement dated June 10, 2010 among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (26)
|
|
|
|
|
|
10.39
|
|
Second Amendment to Loan Agreement dated November 15, 2010 between the Registrant and RBS Asset Finance, Inc. (22)
|
|
|
|
|
|
10.40
|
|
Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated January 3, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (31)
|
|
|
|
|
|
10.41
|
|
Third Amendment to Loan Agreement dated June 24, 2012 between the Registrant and RBS Asset Finance, Inc. (32)
|
|
|
|
|
|
10.42
|
|
DMPK Research, Development, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated June 27, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (32)
|
|
|
|
|
|
10.43
|
|
Letter Agreement Amendment between the Registrant and Alnylam Pharmaceuticals, Inc. dated August 27, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (33)
|
|
|
|
|
|
10.44
|
|
Amendment #2 to Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated October 30, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (37)
|
|
|
|
|
|
10.45
|
|
Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated December 7, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (37)
|
|
|
|
|
|
10.46
|
|
Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated December 10, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (37)
|
|
|
|
|
|
10.47
|
|
HTT Research, Development, Option and License Agreement among the Registrant, F. Hoffmann-La Roche Ltd and Hoffman-La Roche Inc. dated April 8, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (35)
|
|
|
|
|
|
10.48
|
|
Letter Agreement between the Registrant and CHDI Foundation, Inc. dated April 8, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (35)
|
|
|
|
|
|
10.49
|
|
Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated September 5, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (34)
|
|
10.50
|
|
Amendment #1 to Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated August 13, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (34)
|
|
|
|
|
|
10.51
|
|
Amendment Number Three to the Amended and Restated License and Collaboration Agreement among the Registrant, Alnylam Pharmaceuticals, Inc. and Regulus Therapeutics Inc. dated August 2, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (34)
|
|
10.52
|
Letter Agreement Amendment between the Registrant and Biogen Idec International Holding Ltd dated January 27, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. (38)
|
|
|
10.53
|
Amendment No. 3 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated July 10, 2013. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
. (39)
|
|
|
10.54
|
Amendment #4 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated April 10, 2014. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
. (39)
|
|
|
10.55
|
Amendment #5 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated June 27, 2014. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
. (39)
|
|
|
10.56
|
Exclusive License Agreement between the Registrant and the University of Massachusetts dated January 14, 2010. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
. (40)
|
|
|
10.57
|
Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated October 26, 2011. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
. (40)
|
|
|
10.58
|
Amendment to Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated March 14, 2014. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
. (40)
|
|
|
10.59
|
Amendment #1 to the Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated December 15, 2014. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
.
|
|
|
10.60
|
Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 22, 2014. Portions of this exhibit have been omitted and separately filed with the SEC.
|
|
|
10.61
|
Amendment No.2 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated October 15, 2014. Portions of this exhibit have been omitted and separately filed with the SEC
with a request for confidential treatment
.
|
|
|
|
|
|
|
14.1
|
|
Registrant's Code of Ethics and Business Conduct. (36)
|
|
|
|
|
|
21.1
|
|
List of Subsidiaries for the Registrant.
|
|
|
|
|
|
23.1
|
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
|
|
|
24.1
|
|
Power of Attorney. (28)
|
|
|
|
|
|
31.1
|
|
Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
31.2
|
|
Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
32.1
|
|
Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
99.2
|
|
Form of Confidentiality Agreement. (8)
|
|
|
|
|
|
101
|
|
The following financial statements from the Isis Pharmaceuticals, Inc. Annual Report on Form 10-K for the year ended December 31, 2014, formatted in Extensive Business Reporting Language (XBRL): (i) consolidated balance sheets, (ii) consolidated statements of operations, (iii) consolidated statements of stockholders' equity, (iv) consolidated statements of cash flows, and (v) notes to consolidated financial statements (detail tagged).
|

|
(1)
|
|
Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (No. 33-39640) or amendments thereto and incorporated herein by reference.
|
|
|
|
|
|
(2)
|
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2006.
|
|
|
|
|
|
(3)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2011 and incorporated herein by reference.
|
|
|
|
|
|
(4)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2009 and incorporated herein by reference.
|
|
(5)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2008 and incorporated herein by reference.
|
|
|
|
|
|
(6)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 1998 and incorporated herein by reference.
|
|
|
|
|
|
(7)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 1999 and incorporated herein by reference.
|
|
|
|
|
|
(8)
|
|
Filed as an exhibit to the Registrant's Registration Statement on Form S-3 (No. 333-71911) or amendments thereto and incorporated herein by reference.
|
|
|
|
|
|
(9)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2008 and incorporated herein by reference.
|
|
(10)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2011 and incorporated herein by reference.
|
|
|
|
|
|
(11)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2004 and incorporated herein by reference.
|
|
|
|
|
|
(12)
|
|
Filed as an exhibit to Registrant's Report on Form 8-K dated December 8, 2000 and incorporated herein by reference.
|
|
|
|
|
|
(13)
|
|
Filed as an exhibit to the Registrant's Current Report on Form 8-K filed December 14, 2011 and incorporated herein by reference.
|
|
|
|
|
|
(14)
|
|
Filed as an exhibit to the Registrant's report on Form 10-Q as amended for the quarter ended June 30, 2001 and incorporated herein by reference.
|
|
|
|
|
|
(15)
|
|
Filed as an exhibit to Registrant's Notice of Annual Meeting and Proxy Statement for the 2009 Annual Meeting of Stockholders, filed with the SEC on April 20, 2009, and incorporated herein by reference.
|
|
|
|
|
|
(16)
|
|
Filed as an exhibit to the Registrant's Current Report on Form 8-K filed December 5, 2008 and incorporated herein by reference.
|
|
|
|
|
|
(17)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2008 and incorporated herein by reference.
|
|
|
|
|
|
(18)
|
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2005 and incorporated herein by reference.
|
|
|
|
|
|
(19)
|
|
Filed as an exhibit to the Registrant's Notice of 2011 Annual Meeting of Stockholders and Proxy Statement filed with the SEC on April 28, 2011, and incorporated herein by reference.
|
|
|
|
|
|
(20)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2009 and incorporated herein by reference.
|
|
|
|
|
|
(21)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2004 and incorporated herein by reference.
|
|
|
|
|
|
(22)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2010 and incorporated herein by reference.
|
|
|
|
|
|
(23)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2008 and incorporated herein by reference.
|
|
|
|
|
|
(24)
|
|
Filed as an exhibit to the Registrant's Annual Report as Form 10-K for the year ended December 31, 2009 and incorporated herein by reference.
|
|
|
|
|
|
(25)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2010 and incorporated herein by reference.
|
|
|
|
|
|
(26)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 and incorporated herein by reference.
|
|
|
|
|
|
(27)
|
|
Filed as an exhibit to Registrant's Notice of Annual Meeting and Proxy Statement for the 2012 Annual Meeting of Stockholders, filed with the SEC on April 16, 2012, and incorporated herein by reference.
|
|
|
|
|
|
(28)
|
|
Filed as part of the Annual Report on Form 10-K for the year ended December 31, 2013, and incorporated herein by reference.
|
|
|
|
|
|
(29)
|
|
Filed as an exhibit to the Registrant's Registration Statement on Form S-8 filed with the SEC on August 8, 2011, and incorporated herein by reference.
|
|
|
|
|
|
(30)
|
|
Filed as an exhibit to the Registrant's Report on Form 8-K filed August 13, 2012 and incorporated herein by reference.
|
|
(31)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 and incorporated herein by reference.
|
|
|
|
|
|
(32)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 and incorporated herein by reference.
|
|
|
|
|
|
(33)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2012 and incorporated herein by reference.
|
|
|
|
|
|
(34)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
|
|
|
|
(35)
|
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference.
|
|
|
|
|
|
(36)
|
|
Filed as an exhibit to the Registrant's Report on Form 8-K filed on December 9, 2013 and incorporated herein by reference.
|
|
|
|
|
|
(37)
|
|
Filed as an exhibit to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
(38)
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and incorporated herein by reference.
|
|
|
(39)
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
(40)
|
Filed as an exhibit to the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
|
|
(41)
|
Filed as an exhibit to the Registrant's Current Report on Form 8-K filed November 17, 2014 and incorporated herein by reference.
|
|
|
(42)
|
Filed as an exhibit to the Registrant's Notice of Annual Meeting and Proxy Statement, for the 2014 Annual Meeting of Stockholders, filed with the SEC on April 25, 2014, and incorporated herein by reference.
|
|
|
*
|
|
Indicates management compensatory plans and arrangements as required to be filed as exhibits to this Report pursuant to Item 14(c).
|
|
|
Page
|
|
F-2
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-6
|
|
|
F-7
|
|
|
F-9
|
|
|
/s/ ERNST & YOUNG LLP
|
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
142,998
|
$
|
159,973
|
||||
|
Short-term investments
|
585,834
|
496,788
|
||||||
|
Contracts receivable
|
3,903
|
11,102
|
||||||
|
Inventories
|
6,290
|
8,033
|
||||||
|
Investment in Regulus Therapeutics Inc.
|
81,881
|
52,096
|
||||||
|
Other current assets
|
15,691
|
7,518
|
||||||
|
Total current assets
|
836,597
|
735,510
|
||||||
|
Property, plant and equipment, net
|
88,958
|
86,198
|
||||||
|
Licenses, net
|
2,690
|
4,572
|
||||||
|
Patents, net
|
17,186
|
15,517
|
||||||
|
Deposits and other assets
|
10,378
|
5,359
|
||||||
|
Total assets
|
$
|
955,809
|
$
|
847,156
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
17,984
|
$
|
11,009
|
||||
|
Accrued compensation
|
12,302
|
12,168
|
||||||
|
Accrued liabilities
|
30,451
|
22,092
|
||||||
|
Current portion of long-term obligations
|
2,882
|
4,408
|
||||||
|
Current portion of deferred contract revenue
|
51,713
|
48,135
|
||||||
|
Total current liabilities
|
115,332
|
97,812
|
||||||
|
Long-term deferred contract revenue
|
127,797
|
142,790
|
||||||
|
1 percent convertible senior notes
|
327,486
|
—
|
||||||
|
2¾ percent convertible senior notes
|
48,014
|
150,334
|
||||||
|
Long-term obligations, less current portion
|
7,669
|
6,542
|
||||||
|
Long-term financing liability for leased facility
|
71,731
|
71,288
|
||||||
|
Total liabilities
|
698,029
|
468,766
|
||||||
|
Stockholders' equity:
|
||||||||
|
Common stock, $0.001 par value; 300,000,000 shares authorized, 118,442,726 and 116,471,371 shares issued and outstanding at December 31, 2014 and December 31, 2013, respectively
|
118
|
116
|
||||||
|
Additional paid-in capital
|
1,224,509
|
1,324,804
|
||||||
|
Accumulated other comprehensive income
|
39,747
|
21,080
|
||||||
|
Accumulated deficit
|
(1,006,594
|
)
|
(967,610
|
)
|
||||
|
Total stockholders' equity
|
257,780
|
378,390
|
||||||
|
Total liabilities and stockholders' equity
|
$
|
955,809
|
$
|
847,156
|
||||
|
Years Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Revenue:
|
||||||||||||
|
Research and development revenue under collaborative agreements
|
$
|
202,514
|
$
|
144,194
|
$
|
96,415
|
||||||
|
Licensing and royalty revenue
|
11,647
|
3,091
|
5,634
|
|||||||||
|
Total revenue
|
214,161
|
147,285
|
102,049
|
|||||||||
|
Expenses:
|
||||||||||||
|
Research, development and patent expenses
|
241,751
|
184,033
|
158,458
|
|||||||||
|
General and administrative
|
20,140
|
14,918
|
12,515
|
|||||||||
|
Total operating expenses
|
261,891
|
198,951
|
170,973
|
|||||||||
|
Loss from operations
|
(47,730
|
)
|
(51,666
|
)
|
(68,924
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Equity in net loss of Regulus Therapeutics Inc.
|
—
|
—
|
(1,406
|
)
|
||||||||
|
Investment income
|
2,682
|
2,085
|
1,844
|
|||||||||
|
Interest expense
|
(22,209
|
)
|
(19,355
|
)
|
(21,152
|
)
|
||||||
|
Gain on investments, net
|
1,256
|
2,378
|
1,465
|
|||||||||
|
Gain on investment in Regulus Therapeutics Inc.
|
19,902
|
—
|
18,356
|
|||||||||
|
Loss on early retirement of debt
|
(8,292
|
)
|
—
|
(4,770
|
)
|
|||||||
|
Loss before income tax benefit
|
(54,391
|
)
|
(66,558
|
)
|
(74,587
|
)
|
||||||
|
Income tax benefit
|
15,407
|
5,914
|
9,109
|
|||||||||
|
Net loss
|
$
|
(38,984
|
)
|
$
|
(60,644
|
)
|
$
|
(65,478
|
)
|
|||
|
Basic and diluted net loss per share
|
$
|
(0.33
|
)
|
$
|
(0.55
|
)
|
$
|
(0.65
|
)
|
|||
|
Shares used in computing basic and diluted net loss per share
|
117,691
|
110,502
|
100,576
|
|||||||||
|
Years Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Net loss
|
$
|
(38,984
|
)
|
$
|
(60,644
|
)
|
$
|
(65,478
|
)
|
|||
|
Unrealized gains on investments, net of tax
|
40,079
|
10,253
|
13,250
|
|||||||||
|
Reclassification adjustment for realized gains included in net loss
|
(21,412
|
)
|
(1,653
|
)
|
—
|
|||||||
|
Comprehensive loss
|
$
|
(20,317
|
)
|
$
|
(52,044
|
)
|
$
|
(52,228
|
)
|
|||
|
Isis Pharmaceuticals, Inc. Stockholders' Equity
|
||||||||||||||||||||||||
|
Common stock
|
Additional
paid in
|
Accumulated
other
comprehensive
|
Accumulated
|
Total
stockholders'
|
||||||||||||||||||||
|
Description
|
Shares
|
Amount
|
capital
|
income (loss)
|
deficit
|
equity
|
||||||||||||||||||
|
Balance at December 31, 2011
|
100,043
|
$
|
100
|
$
|
1,013,592
|
$
|
(770
|
)
|
$
|
(841,488
|
)
|
$
|
171,434
|
|||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(65,478
|
)
|
(65,478
|
)
|
||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
13,250
|
—
|
13,250
|
||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,438
|
2
|
9,468
|
—
|
—
|
9,470
|
||||||||||||||||||
|
2⅝ percent convertible subordinated notes redemption, equity portion
|
—
|
—
|
(12,041
|
)
|
—
|
—
|
(12,041
|
)
|
||||||||||||||||
|
2¾
percent convertible senior notes, equity portion, net of issuance costs
|
—
|
—
|
57,560
|
—
|
—
|
57,560
|
||||||||||||||||||
|
Share-based compensation expense
|
—
|
—
|
8,571
|
—
|
—
|
8,571
|
||||||||||||||||||
|
Balance at December 31, 2012
|
101,481
|
$
|
102
|
$
|
1,077,150
|
$
|
12,480
|
$
|
(906,966
|
)
|
$
|
182,766
|
||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(60,644
|
)
|
(60,644
|
)
|
||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
8,600
|
—
|
8,600
|
||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
5,372
|
5
|
62,953
|
—
|
—
|
62,958
|
||||||||||||||||||
|
Issuance of public common stock
|
9,618
|
9
|
173,283
|
—
|
—
|
173,292
|
||||||||||||||||||
|
Share-based compensation expense
|
—
|
—
|
11,418
|
—
|
—
|
11,418
|
||||||||||||||||||
|
Balance at December 31, 2013
|
116,471
|
$
|
116
|
$
|
1,324,804
|
$
|
21,080
|
$
|
(967,610
|
)
|
$
|
378,390
|
||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(38,984
|
)
|
(38,984
|
)
|
||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
18,667
|
—
|
18,667
|
||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,972
|
2
|
23,071
|
—
|
—
|
23,073
|
||||||||||||||||||
|
2¾
percent convertible senior notes repurchase, equity portion
|
—
|
—
|
(326,444
|
)
|
—
|
—
|
(326,444
|
)
|
||||||||||||||||
|
1 percent convertible senior notes, equity portion, net of issuance costs
|
—
|
—
|
170,232
|
—
|
—
|
170,232
|
||||||||||||||||||
|
Share-based compensation expense
|
—
|
—
|
31,383
|
—
|
—
|
31,383
|
||||||||||||||||||
|
Excess tax benefits from share-based compensation awards
|
—
|
—
|
1,463
|
—
|
—
|
1,463
|
||||||||||||||||||
|
Balance at December 31, 2014
|
118,443
|
$
|
118
|
$
|
1,224,509
|
$
|
39,747
|
$
|
(1,006,594
|
)
|
$
|
257,780
|
||||||||||||
|
Years Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Operating activities:
|
||||||||||||
|
Net loss
|
$
|
(38,984
|
)
|
$
|
(60,644
|
)
|
$
|
(65,478
|
)
|
|||
|
Adjustments to reconcile net loss to net cash provided by operating activities:
|
||||||||||||
|
Depreciation
|
6,380
|
6,591
|
7,074
|
|||||||||
|
Amortization of patents
|
1,142
|
1,184
|
1,224
|
|||||||||
|
Amortization of licenses
|
1,882
|
2,007
|
2,457
|
|||||||||
|
Amortization of premium on investments, net
|
7,470
|
5,572
|
4,193
|
|||||||||
|
Amortization of debt issuance costs
|
595
|
415
|
619
|
|||||||||
|
Amortization of 2⅝ percent convertible subordinated notes discount
|
—
|
—
|
6,169
|
|||||||||
|
Amortization of 2¾ percent convertible senior notes discount
|
6,723
|
6,344
|
2,268
|
|||||||||
|
Amortization of 1 percent convertible senior notes discount
|
2,256
|
—
|
—
|
|||||||||
|
Amortization of long-term financing liability for leased facility
|
6,622
|
6,567
|
6,503
|
|||||||||
|
Share-based compensation expense
|
31,383
|
11,418
|
8,571
|
|||||||||
|
Equity in net loss of Regulus Therapeutics Inc.
|
—
|
—
|
1,406
|
|||||||||
|
Gain on investment in Regulus Therapeutics Inc.
|
(19,902
|
)
|
—
|
(18,356
|
)
|
|||||||
|
Loss on early retirement of debt
|
8,292
|
—
|
4,770
|
|||||||||
|
Gain on investments, net
|
(1,256
|
)
|
(2,378
|
)
|
(1,465
|
)
|
||||||
|
Non-cash losses related to patents, licensing and property, plant and equipment
|
1,305
|
6,306
|
825
|
|||||||||
|
Tax benefit from other unrealized gains on securities
|
(12,835
|
)
|
(5,914
|
)
|
(9,111
|
)
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Contracts receivable
|
7,199
|
(10,580
|
)
|
6,399
|
||||||||
|
Inventories
|
1,743
|
(1,912
|
)
|
(1,982
|
)
|
|||||||
|
Other current and long-term assets
|
(1,750
|
)
|
(1,091
|
)
|
279
|
|||||||
|
Accounts payable
|
4,824
|
66
|
1,292
|
|||||||||
|
Income taxes
|
(4,034
|
)
|
—
|
—
|
||||||||
|
Accrued compensation
|
134
|
4,290
|
(1,305
|
)
|
||||||||
|
Deferred rent
|
153
|
217
|
255
|
|||||||||
|
Accrued liabilities
|
8,358
|
6,691
|
(3,254
|
)
|
||||||||
|
Deferred contract revenue
|
(11,415
|
)
|
88,344
|
48,523
|
||||||||
|
Net cash provided by operating activities
|
6,285
|
63,493
|
1,876
|
|||||||||
|
Investing activities:
|
||||||||||||
|
Purchases of short-term investments
|
(391,883
|
)
|
(425,554
|
)
|
(217,877
|
)
|
||||||
|
Proceeds from the sale of short-term investments
|
294,727
|
172,762
|
242,659
|
|||||||||
|
Purchases of property, plant and equipment
|
(7,518
|
)
|
(1,552
|
)
|
(1,479
|
)
|
||||||
|
Acquisition of licenses and other assets, net
|
(3,586
|
)
|
(3,810
|
)
|
(3,691
|
)
|
||||||
|
Investment in Regulus Therapeutics Inc.
|
—
|
—
|
(3,000
|
)
|
||||||||
|
Purchases of strategic investments
|
—
|
—
|
(790
|
)
|
||||||||
|
Proceeds from the sale of Regulus Therapeutics, Inc.
|
22,949
|
—
|
—
|
|||||||||
|
Proceeds from the sale of strategic investments
|
2,463
|
2,428
|
2,177
|
|||||||||
|
Net cash (used in) provided by investing activities
|
(82,848
|
)
|
(255,726
|
)
|
17,999
|
|||||||
|
Financing activities:
|
||||||||||||
|
Proceeds from equity awards
|
23,071
|
62,958
|
9,470
|
|||||||||
|
Proceeds from issuance of 2¾ percent convertible senior notes, net of issuance costs
|
—
|
—
|
194,697
|
|||||||||
|
Proceeds from issuance of 1 percent convertible senior notes, net of issuance costs
|
487,035
|
—
|
—
|
|||||||||
|
Principal and premium payment on redemption of the 2⅝ percent convertible subordinated notes
|
—
|
—
|
(163,718
|
)
|
||||||||
|
Repurchase of $140 million principal amount of the 2¾ percent convertible senior notes
|
(441,394
|
)
|
—
|
—
|
||||||||
|
Proceeds from public common stock offering
|
—
|
173,292
|
—
|
|||||||||
|
Proceeds from equipment financing arrangement
|
—
|
2,513
|
9,100
|
|||||||||
|
Excess tax benefits from share-based compensation awards
|
1,463
|
—
|
—
|
|||||||||
|
Principal payments on debt and capital lease obligations
|
(10,587
|
)
|
(11,039
|
)
|
(10,419
|
)
|
||||||
|
Net cash provided by financing activities
|
59,588
|
227,724
|
39,130
|
|||||||||
|
Net (decrease) increase in cash and cash equivalents
|
(16,975
|
)
|
35,491
|
59,005
|
||||||||
|
Cash and cash equivalents at beginning of year
|
159,973
|
124,482
|
65,477
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
142,998
|
$
|
159,973
|
$
|
124,482
|
||||||
|
Supplemental disclosures of cash flow information:
|
||||||||||||
|
Interest paid
|
$
|
6,353
|
$
|
6,000
|
$
|
5,770
|
||||||
|
Supplemental disclosures of non-cash investing and financing activities:
|
||||||||||||
|
Amounts accrued for capital and patent expenditures
|
$
|
2,151
|
$
|
704
|
$
|
647
|
||||||
| | 1 percent convertible senior notes; |
| | 2¾ percent convertible senior notes; |
| | 2⅝ percent convertible subordinated notes; |
| | GSK convertible promissory notes issued by Regulus; |
| | Dilutive stock options; |
| | Unvested restricted stock units; and |
| | Employee Stock Purchase Plan, or ESPP. |
| | The exclusive license we granted to AstraZeneca to develop and commercialize ISIS-STAT3-2.5 Rx for the treatment of cancer; |
| | The development services we are performing for ISIS-STAT3-2.5 Rx ; |
| | The exclusive license we granted to AstraZeneca to develop and commercialize ISIS-AR-2.5 Rx and the research services we performed for ISIS-AR-2.5 Rx ; and |
| | The option to license up to three drugs under a research program and the research services we are performing for this program. |
| | Estimated future product sales; |
| | Estimated royalties on future product sales; |
| | Contractual milestone payments; |
| | Expenses we expect to incur; |
| | Income taxes; and |
| | An appropriate discount rate. |
| | The number of internal hours we will spend performing these services; |
| | The estimated number and cost of studies we will perform; |
| | The estimated number and cost of studies that we will contract with third parties to perform; and |
| | The estimated cost of drug product we will use in the studies. |
| | In January 2012, we entered into a collaboration agreement with Biogen Idec to develop and commercialize ISIS-SMN Rx for spinal muscular atrophy, or SMA. As part of the collaboration, we received a $29 million upfront payment and we are responsible for global development of ISIS-SMN Rx through completion of Phase 2/3 clinical trials. |
| | In June 2012, we entered into a second and separate collaboration agreement with Biogen Idec to develop and commercialize a novel antisense drug targeting DMPK, or dystrophia myotonica-protein kinase. As part of the collaboration, we received a $12 million upfront payment and we are responsible for global development of the drug through the completion of a Phase 2 clinical trial. |
| | In December 2012, we entered into a third and separate collaboration agreement with Biogen Idec to discover and develop antisense drugs against three targets to treat neurological or neuromuscular disorders. As part of the collaboration, we received a $30 million upfront payment and we are responsible for the discovery of a lead antisense drug for each of three targets. |
| | In September 2013, we entered into a fourth and separate collaboration agreement with Biogen Idec to leverage antisense technology to advance the treatment of neurological diseases. We granted Biogen Idec exclusive rights to the use of our antisense technology to develop therapies for neurological diseases as part of this broad collaboration. We received a $100 million upfront payment and we are responsible for discovery and early development through the completion of a Phase 2 clinical trial for each antisense drug identified during the six year term of this collaboration, while Biogen Idec is responsible for the creation and development of small molecule treatments and biologics. |
| | Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete; |
| | Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete; |
| | Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete; |
| | Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete. |
| | Filing of regulatory applications for marketing approval such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings. |
| | Marketing approval in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain approval from the applicable regulatory agency. |
| | First commercial sale in a particular market, such as in the United States or Europe. |
| | Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product. |
| | Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement; |
| | The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on our performance or the occurrence of a specific outcome resulting from our performance; |
| | The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items; |
| | There is no future performance required to earn the milestone; and |
| | The consideration is reasonable relative to all deliverables and payment terms in the arrangement. |
|
Years Ending December 31,
|
Amortization
|
|||
|
(in millions)
|
||||
|
2015
|
$
|
1.2
|
||
|
2016
|
$
|
1.1
|
||
|
2017
|
$
|
1.1
|
||
|
2018
|
$
|
0.9
|
||
|
2019
|
$
|
0.8
|
||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Equipment and computer software
|
$
|
49,772
|
$
|
44,698
|
||||
|
Building and building systems
|
48,521
|
48,132
|
||||||
|
Land improvements
|
2,853
|
2,846
|
||||||
|
Leasehold improvements
|
37,935
|
35,282
|
||||||
|
Furniture and fixtures
|
5,732
|
5,473
|
||||||
|
144,813
|
136,431
|
|||||||
|
Less accumulated depreciation
|
(66,053
|
)
|
(60,431
|
)
|
||||
|
78,760
|
76,000
|
|||||||
|
Land
|
10,198
|
10,198
|
||||||
|
Total
|
$
|
88,958
|
$
|
86,198
|
||||
|
Computer software and hardware
|
3 years
|
|
Other equipment
|
5-7 years
|
|
Furniture and fixtures
|
5-10 years
|
|
Manufacturing equipment
|
10 years
|
|
Building systems and improvements
|
10-25 years
|
|
Land improvements
|
20 years
|
|
Building
|
40 years
|
|
Years Ending December 31,
|
Amortization
|
|||
|
(in millions)
|
||||
|
2015
|
$
|
1.9
|
||
|
2016
|
$
|
0.8
|
||
Accumulated other comprehensive income
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Beginning balance accumulated other comprehensive income (loss)
|
$
|
21,080
|
$
|
12,480
|
$
|
(770
|
)
|
|||||
|
Other comprehensive income before reclassifications, net of tax (1)
|
40,079
|
10,253
|
13,250
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (2)
|
(21,412
|
)
|
(1,653
|
)
|
—
|
|||||||
|
Net current period other comprehensive income
|
18,667
|
8,600
|
13,250
|
|||||||||
|
Ending balance accumulated other comprehensive income
|
$
|
39,747
|
$
|
21,080
|
$
|
12,480
|
||||||
| (1) | Other comprehensive income includes income tax expense of $12.8 million, $5.9 million and $9.1 million for the years ended December 31, 2014 and 2013 and 2012, respectively. |
| (2) | Included in gain on investments, net on our consolidated statement of operations. |
|
At December 31, 2014
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||||
|
Cash equivalents (1)
|
$
|
104,680
|
$
|
104,680
|
$
|
—
|
$
|
—
|
||||||||
|
Corporate debt securities (2)
|
372,002
|
—
|
372,002
|
—
|
||||||||||||
|
Debt securities issued by U.S. government agencies (3)
|
109,855
|
—
|
109,855
|
—
|
||||||||||||
|
Debt securities issued by the U.S. Treasury (4)
|
19,017
|
19,017
|
—
|
—
|
||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states (5)
|
105,033
|
—
|
105,033
|
—
|
||||||||||||
|
Investment in Regulus Therapeutics Inc.
|
81,881
|
—
|
—
|
81,881
|
||||||||||||
|
Total
|
$
|
792,468
|
$
|
123,697
|
$
|
586,890
|
$
|
81,881
|
||||||||
|
At December 31, 2013
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||||
|
Cash equivalents (1)
|
$
|
133,233
|
$
|
133,233
|
$
|
—
|
$
|
—
|
||||||||
|
Corporate debt securities (7)
|
407,897
|
—
|
407,897
|
—
|
||||||||||||
|
Debt securities issued by U.S. government agencies (3)
|
64,432
|
—
|
64,432
|
—
|
||||||||||||
|
Debt securities issued by the U.S. Treasury (3)
|
15,328
|
15,328
|
—
|
—
|
||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states (3)
|
22,255
|
—
|
22,255
|
—
|
||||||||||||
|
Investment in Regulus Therapeutics Inc.
|
52,096
|
52,096
|
—
|
—
|
||||||||||||
|
Equity securities (6)
|
1,276
|
1,276
|
—
|
—
|
||||||||||||
|
Total
|
$
|
696,517
|
$
|
201,933
|
$
|
494,584
|
$
|
—
|
||||||||
| (1) | Included in cash and cash equivalents on our consolidated balance sheet. |
| (2) | $0.8 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet. |
| (3) | Included in short-term investments on our consolidated balance sheet. |
| (4) | $10.0 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet. |
| (5) | $9.3 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet. |
| (6) | Included in other current assets on our consolidated balance sheet. |
|
Year Ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Beginning balance of Level 3 investments
|
$
|
—
|
$
|
34,350
|
||||
|
Transfers into Level 3 investments
|
108,009
|
—
|
||||||
|
Total realized and unrealized gains and (losses):
|
||||||||
|
Included in gain on investments
|
—
|
(1,163
|
)
|
|||||
|
Included in accumulated other comprehensive income (loss)
|
(24,897
|
)
|
32,272
|
|||||
|
Transfers out of Level 3 investments
|
(1,231
|
)
|
(65,419
|
)
|
||||
|
Cost basis of shares sold
|
—
|
(40
|
)
|
|||||
|
Ending balance of Level 3 investments
|
$
|
81,881
|
$
|
—
|
||||
|
One year or less
|
55%
|
|
|
After one year but within two years
|
31%
|
|
|
After two years but within three years
|
14%
|
|
|
Total
|
100%
|
|
Amortized
|
Unrealized
|
Other-Than-
Temporary
Impairment
|
Estimated
|
|||||||||||||||||
|
December 31, 2014
|
Cost
|
Gains
|
Losses
|
Loss
|
Fair Value
|
|||||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||||||
|
Corporate debt securities(1)
|
$
|
219,856
|
$
|
89
|
$
|
(89
|
)
|
$
|
—
|
$
|
219,856
|
|||||||||
|
Debt securities issued by U.S. government agencies
|
47,496
|
7
|
(27
|
)
|
—
|
47,476
|
||||||||||||||
|
Debt securities issued by the U.S. Treasury (1)
|
19,008
|
9
|
—
|
—
|
19,017
|
|||||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states (1)
|
45,196
|
19
|
(53
|
)
|
—
|
45,162
|
||||||||||||||
|
Total securities with a maturity of one year or less
|
331,556
|
124
|
(169
|
)
|
—
|
331,511
|
||||||||||||||
|
Corporate debt securities
|
152,730
|
16
|
(600
|
)
|
—
|
152,146
|
||||||||||||||
|
Debt securities issued by U.S. government agencies
|
62,530
|
—
|
(151
|
)
|
—
|
62,379
|
||||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states
|
60,073
|
32
|
(234
|
)
|
—
|
59,871
|
||||||||||||||
|
Total securities with a maturity of more than one year
|
275,333
|
48
|
(985
|
)
|
—
|
274,396
|
||||||||||||||
|
Total available-for-sale securities
|
$
|
606,889
|
$
|
172
|
$
|
(1,154
|
)
|
$
|
—
|
$
|
605,907
|
|||||||||
|
Cost
|
Unrealized
|
Other-Than-
Temporary
Impairment
|
Estimated
|
|||||||||||||||||
|
December 31, 2014
|
Basis
|
Gains
|
Losses
|
Loss
|
Fair Value
|
|||||||||||||||
|
Equity securities:
|
||||||||||||||||||||
|
Regulus Therapeutics Inc.
|
$
|
12,477
|
$
|
69,404
|
$
|
—
|
$
|
—
|
$
|
81,881
|
||||||||||
|
Total equity securities
|
$
|
12,477
|
$
|
69,404
|
$
|
—
|
$
|
—
|
$
|
81,881
|
||||||||||
|
Total available-for-sale and equity securities
|
$
|
619,366
|
$
|
69,576
|
$
|
(1,154
|
)
|
$
|
—
|
$
|
687,788
|
|||||||||
|
Amortized
|
Unrealized
|
Other-Than-
Temporary
Impairment
|
Estimated
|
|||||||||||||||||
|
December 31, 2013
|
Cost
|
Gains
|
Losses
|
Loss
|
Fair Value
|
|||||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||||||
|
Corporate debt securities(1)
|
$
|
142,096
|
$
|
75
|
$
|
(27
|
)
|
$
|
—
|
$
|
142,144
|
|||||||||
|
Debt securities issued by U.S. government agencies(1)
|
23,242
|
22
|
(16
|
)
|
—
|
23,248
|
||||||||||||||
|
Debt securities issued by the U.S. Treasury
|
6,239
|
6
|
—
|
—
|
6,245
|
|||||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states
|
8,082
|
6
|
(28
|
)
|
—
|
8,060
|
||||||||||||||
|
Total securities with a maturity of one year or less
|
179,659
|
109
|
(71
|
)
|
—
|
179,697
|
||||||||||||||
|
Corporate debt securities
|
265,969
|
177
|
(393
|
)
|
—
|
265,753
|
||||||||||||||
|
Debt securities issued by U.S. government agencies
|
41,308
|
3
|
(127
|
)
|
—
|
41,184
|
||||||||||||||
|
Debt securities issued by the U.S. Treasury
|
9,062
|
21
|
—
|
—
|
9,083
|
|||||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states
|
14,186
|
37
|
(28
|
)
|
—
|
14,195
|
||||||||||||||
|
Total securities with a maturity of more than one year
|
330,525
|
238
|
(548
|
)
|
—
|
330,215
|
||||||||||||||
|
Total available-for-sale securities
|
$
|
510,184
|
$
|
347
|
$
|
(619
|
)
|
$
|
—
|
$
|
509,912
|
|||||||||
|
Cost
|
Unrealized
|
Other-Than-
Temporary
Impairment
|
Estimated
|
|||||||||||||||||
|
December 31, 2013
|
Basis
|
Gains
|
Losses
|
Loss
|
Fair Value
|
|||||||||||||||
|
Equity securities:
|
||||||||||||||||||||
|
Regulus Therapeutics Inc.
|
$
|
15,526
|
$
|
36,570
|
$
|
—
|
$
|
—
|
$
|
52,096
|
||||||||||
|
Securities included in other current assets
|
1,538
|
618
|
—
|
(880
|
)
|
1,276
|
||||||||||||||
|
Securities included deposits and other assets
|
625
|
—
|
—
|
—
|
625
|
|||||||||||||||
|
Total equity securities
|
$
|
17,689
|
$
|
37,188
|
$
|
—
|
$
|
(880
|
)
|
$
|
53,997
|
|||||||||
|
Total available-for-sale and equity securities
|
$
|
527,873
|
$
|
37,535
|
$
|
(619
|
)
|
$
|
(880
|
)
|
$
|
563,909
|
||||||||
| (1) | Includes investments classified as cash equivalents on our consolidated balance sheet. |
|
Less than 12 months of
temporary impairment
|
More than 12 months of
temporary impairment
|
Total temporary
impairment
|
||||||||||||||||||||||||||
|
Number of
Investments
|
Estimated
Fair Value
|
Unrealized
Losses
|
Estimated
Fair Value
|
Unrealized
Losses
|
Estimated
Fair Value
|
Unrealized
Losses
|
||||||||||||||||||||||
|
Corporate debt securities
|
239
|
$
|
242,124
|
$
|
(681
|
)
|
$
|
3,503
|
$
|
(8
|
)
|
$
|
245,627
|
$
|
(689
|
)
|
||||||||||||
|
Debt securities issued by U.S. government agencies
|
16
|
98,342
|
(178
|
)
|
—
|
—
|
98,342
|
(178
|
)
|
|||||||||||||||||||
|
Debt securities issued by states of the United States and political subdivisions of the states
|
46
|
54,292
|
(237
|
)
|
225
|
(50
|
)
|
54,517
|
(287
|
)
|
||||||||||||||||||
|
Total temporarily impaired securities
|
301
|
$
|
394,758
|
$
|
(1,096
|
)
|
$
|
3,728
|
$
|
(58
|
)
|
$
|
398,486
|
$
|
(1,154
|
)
|
||||||||||||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
1 percent convertible senior notes
|
$
|
327,486
|
$
|
—
|
||||
|
2
¾
percent convertible senior notes
|
48,014
|
150,334
|
||||||
|
Long-term financing liability for leased facility
|
71,731
|
71,288
|
||||||
|
Equipment financing arrangement
|
3,226
|
7,461
|
||||||
|
Leases and other obligations
|
7,325
|
3,489
|
||||||
|
Total
|
$
|
457,782
|
$
|
232,572
|
||||
|
Less: current portion
|
(2,882
|
)
|
(4,408
|
)
|
||||
|
Total Long-Term Obligations
|
$
|
454,900
|
$
|
228,164
|
||||
|
1 Percent Convertible
Senior Notes
|
2¾ Percent Convertible
Senior Notes
|
|||||||
|
Outstanding balance
|
$
|
500
|
$
|
61.2
|
||||
|
Issue date
|
November 2014
|
August 2012
|
||||||
|
Maturity date
|
November 2021
|
October 2019
|
||||||
|
Interest rate
|
1 percent
|
2¾ percent
|
||||||
|
Conversion price per share
|
$
|
66.81
|
$
|
16.63
|
||||
|
Total shares of common stock subject to conversion
|
7.5
|
3.7
|
||||||
|
1 Percent Convertible
Senior Notes
|
2¾ Percent Convertible
Senior Notes
|
2⅝ Percent Convertible Subordinated Notes
|
|||||
|
Nonconvertible debt borrowing rate
|
7.4 percent
|
8.0 percent
|
9.3 percent
|
||||
|
Effective interest rate
|
7.8 percent
|
8.8 percent
|
9.8 percent
|
||||
|
Amortization period of debt discount
|
7 years
|
7 years
|
7 years
|
||||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
2¾ Percent Convertible Senior Notes
|
||||||||
|
Fair value of outstanding notes
|
$
|
223,900
|
$
|
505,100
|
||||
|
Principal amount of convertible notes outstanding
|
$
|
61,247
|
$
|
201,250
|
||||
|
Unamortized portion of debt discount
|
$
|
13,233
|
$
|
50,916
|
||||
|
Long-term debt
|
$
|
48,014
|
$
|
150,334
|
||||
|
Carrying value of equity component
|
$
|
18,714
|
$
|
59,528
|
||||
|
1 Percent Convertible Senior Notes
|
||||||||
|
Fair value of outstanding notes
|
$
|
568,000
|
||||||
|
Principal amount of convertible notes outstanding
|
$
|
500,000
|
||||||
|
Unamortized portion of debt discount
|
$
|
172,514
|
||||||
|
Long-term debt
|
$
|
327,486
|
||||||
|
Carrying value of equity component
|
$
|
174,770
|
||||||
|
2015
|
$
|
9,446
|
||
|
2016
|
7,268
|
|||
|
2017
|
6,744
|
|||
|
2018
|
6,744
|
|||
|
2019
|
67,991
|
|||
|
Thereafter
|
511,020
|
|||
|
Subtotal
|
$
|
609,213
|
||
|
Less: current portion
|
(2,882
|
)
|
||
|
Less: fixed and determinable interest
|
(44,222
|
)
|
||
|
Less: unamortized portion of debt discount
|
(185,747
|
)
|
||
|
Plus: Deferred rent
|
1,795
|
|||
|
Total
|
$
|
378,157
|
|
Operating
Leases
|
||||
|
2015
|
$
|
1,527
|
||
|
2016
|
1,538
|
|||
|
2017
|
1,481
|
|||
|
2018
|
1,451
|
|||
|
2019
|
1,474
|
|||
|
Thereafter
|
17,517
|
|||
|
Total minimum payments
|
$
|
24,988
|
||
|
Future Rent
Payments
|
||||
|
2015
|
$
|
6,179
|
||
|
2016
|
6,550
|
|||
|
2017
|
6,550
|
|||
|
2018
|
6,943
|
|||
|
2019
|
6,943
|
|||
|
Thereafter
|
98,565
|
|||
|
Total minimum payments
|
$
|
131,730
|
||
| | a sale, lease or other disposition of all or substantially all of our assets; |
| | a merger or consolidation in which we are not the surviving corporation; or |
| | reverse merger in which we are the surviving corporation but the shares of common stock outstanding immediately preceding the merger are converted by virtue of the merger into other property, whether in the form of securities, cash or otherwise, |
| | arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring entity (or its parent company); |
| | arrange for the assignment of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award to the surviving or acquiring corporation (or its parent company); |
| | accelerate the vesting and exercisability of a stock award followed by the termination of the stock award; |
| | arrange for the lapse of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award; |
| | cancel or arrange for the cancellation of a stock award, to the extent not vested or not exercised prior to the effective date of the corporate transaction, in exchange for cash consideration, if any, as the Board, in its sole discretion, may consider appropriate; and |
| | arrange for the surrender of a stock award in exchange for a payment equal to the excess of (a) the value of the property the holder of the stock award would have received upon the exercise of the stock award, over (b) any exercise price payable by such holder in connection with such exercise. |
|
Number of
Shares
|
Weighted
Average Exercise
Price
Per Share
|
Average
Remaining
Contractual Term
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
(Years)
|
||||||||||||||||
|
Outstanding at December 31, 2013
|
7,279
|
$
|
12.07
|
|||||||||||||
|
Granted
|
2,031
|
$
|
39.88
|
|||||||||||||
|
Exercised
|
(1,816
|
)
|
$
|
12.36
|
||||||||||||
|
Cancelled/forfeited/expired
|
(115
|
)
|
$
|
20.43
|
||||||||||||
|
Outstanding at December 31, 2014
|
7,379
|
$
|
19.52
|
4.41
|
$
|
311,538
|
||||||||||
|
Exercisable at December 31, 2014
|
3,854
|
$
|
11.18
|
3.22
|
$
|
194,858
|
||||||||||
|
Number of
Shares
|
Weighted
Average
Grant Date
Fair Value
Per Share
|
|||||||
|
Non-vested at December 31, 2013
|
425
|
$
|
13.67
|
|||||
|
Granted
|
349
|
$
|
44.94
|
|||||
|
Vested
|
(117
|
)
|
$
|
13.74
|
||||
|
Cancelled/forfeited
|
(19
|
)
|
$
|
22.41
|
||||
|
Non-vested at December 31, 2014
|
638
|
$
|
30.52
|
|||||
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Research, development and patents
|
$
|
25,843
|
$
|
9,673
|
$
|
7,246
|
||||||
|
General and administrative
|
5,540
|
1,745
|
1,325
|
|||||||||
|
Total
|
$
|
31,383
|
$
|
11,418
|
$
|
8,571
|
||||||
|
December 31,
|
|||||
|
2014
|
2013
|
2012
|
|||
|
Risk-free interest rate
|
1.7%
|
1.1%
|
1.1%
|
||
|
Dividend yield
|
0.0%
|
0.0%
|
0.0%
|
||
|
Volatility
|
50.1%
|
51.1%
|
50.7%
|
||
|
Expected life
|
4.7 years
|
5.1 years
|
5.1 years
|
||
|
December 31,
|
|||||
|
2014
|
2013
|
2012
|
|||
|
Risk-free interest rate
|
2.2%
|
2.2%
|
1.3%
|
||
|
Dividend yield
|
0.0%
|
0.0%
|
0.0%
|
||
|
Volatility
|
54.2%
|
52.7%
|
51.3%
|
||
|
Expected life
|
6.9 years
|
7.2 years
|
7.6 years
|
||
|
December 31,
|
|||||
|
2014
|
2013
|
2012
|
|||
|
Risk-free interest rate
|
0.1%
|
0.1%
|
0.1%
|
||
|
Dividend yield
|
0.0%
|
0.0%
|
0.0%
|
||
|
Volatility
|
60.1%
|
62.9%
|
44.5%
|
||
|
Expected life
|
6 months
|
6 months
|
6 months
|
||
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
263
|
$
|
—
|
$
|
—
|
||||||
|
State
|
(4,295
|
)
|
2
|
2
|
||||||||
|
Total current
|
(4,032
|
)
|
2
|
2
|
||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
(8,948
|
)
|
(5,082
|
)
|
(7,827
|
)
|
||||||
|
State
|
(2,427
|
)
|
(834
|
)
|
(1,284
|
)
|
||||||
|
Foreign
|
—
|
—
|
—
|
|||||||||
|
Total deferred
|
(11,375
|
)
|
(5,916
|
)
|
(9,111
|
)
|
||||||
|
Income Tax Benefit
|
$
|
(15,407
|
)
|
$
|
(5,914
|
)
|
$
|
(9,109
|
)
|
|||
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
2014
|
2013
|
2012
|
||||||||||||||||||||||
|
Pre-tax loss
|
$
|
(54,391
|
)
|
$
|
(66,558
|
)
|
$
|
(74,587
|
)
|
|||||||||||||||
|
Statutory rate
|
(19,035
|
)
|
35.0
|
%
|
(23,295
|
)
|
35.0
|
%
|
(26,105
|
)
|
35.0
|
%
|
||||||||||||
|
State income tax net of federal benefit
|
(3,125
|
)
|
5.7
|
%
|
(3,823
|
)
|
5.7
|
%
|
(4,284
|
)
|
5.7
|
%
|
||||||||||||
|
Net change in valuation allowance
|
29,547
|
(54.3
|
)%
|
28,850
|
(43.3
|
)%
|
25,269
|
(33.9
|
)%
|
|||||||||||||||
|
Gain on Investment in Regulus Therapeutics Inc.
|
—
|
—
|
—
|
—
|
(6,353
|
)
|
8.5
|
%
|
||||||||||||||||
|
Loss on debt extinguishment
|
2,406
|
(4.4
|
%)
|
—
|
—
|
—
|
—
|
|||||||||||||||||
|
Tax credits
|
(23,525
|
)
|
43.3
|
%
|
(15,839
|
)
|
23.8
|
%
|
806
|
(1.1
|
)%
|
|||||||||||||
|
California franchise tax refund
|
(2,795
|
)
|
5.1
|
%
|
—
|
—
|
—
|
—
|
||||||||||||||||
|
Deferred tax true-up
|
874
|
(1.6
|
)%
|
8,023
|
(12.1
|
)%
|
839
|
(1.1
|
)%
|
|||||||||||||||
|
Other
|
246
|
(0.5
|
)%
|
170
|
(0.2
|
)%
|
719
|
(0.9
|
)%
|
|||||||||||||||
|
Effective rate
|
$
|
(15,407
|
)
|
28.3
|
%
|
$
|
(5,914
|
)
|
8.9
|
%
|
$
|
(9,109
|
)
|
12.2
|
%
|
|||||||||
|
Year Ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Deferred Tax Assets:
|
||||||||
|
Net operating loss carryovers
|
$
|
231,654
|
$
|
260,462
|
||||
|
R&D credits
|
93,594
|
65,600
|
||||||
|
Capitalized R&D
|
3,088
|
2,736
|
||||||
|
Deferred revenue
|
58,836
|
28,555
|
||||||
|
Accrued restructuring
|
2,374
|
3,304
|
||||||
|
Other
|
3,762
|
7,107
|
||||||
|
Total deferred tax assets
|
$
|
393,308
|
$
|
367,764
|
||||
|
Deferred Tax Liabilities:
|
||||||||
|
Convertible debt
|
$
|
(73,733
|
)
|
$
|
(20,895
|
)
|
||
|
Intangible and capital assets
|
(3,641
|
)
|
(4,614
|
)
|
||||
|
Net deferred tax asset
|
$
|
315,934
|
$
|
342,255
|
||||
|
Valuation allowance
|
(315,934
|
)
|
(342,255
|
)
|
||||
|
Net deferreds
|
$
|
—
|
$
|
—
|
||||
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Beginning balance of unrecognized tax benefits
|
$
|
23,964
|
$
|
10,872
|
$
|
9,834
|
||||||
|
Decrease for prior period tax positions
|
(1,653
|
)
|
—
|
(174
|
)
|
|||||||
|
Increase for prior period tax positions
|
—
|
9,821
|
791
|
|||||||||
|
Increase for current period tax positions
|
5,054
|
3,271
|
421
|
|||||||||
|
Ending balance of unrecognized tax benefits
|
$
|
27,365
|
$
|
23,964
|
$
|
10,872
|
||||||
| | $11.2 million related to the ISIS-AR-2.5 Rx program, which we amortized through March 2014; |
| | $7.6 million related to the option to license three drugs under a separate research program, which we are amortizing through December 2016; and |
| | $0.7 million related to the ISIS-STAT3-2.5 Rx program, which we amortized through February 2015. |
| | AstraZeneca may terminate the agreement or any program at any time by providing written notice to us; |
| | AstraZeneca may terminate the agreement or any program by providing written notice if we undergo a change of control with a third party; and |
| | Either we or AstraZeneca may terminate the agreement or any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent. |
| ● | $3.8 million related to the Phase 2 studies in children and infants with SMA, which we amortized through July 2014; and |
| ● | $7.5 million related to an open-label extension study in children with SMA, which we are amortizing through March 2015. |
| | Biogen Idec may terminate the agreement or any program at any time by providing written notice to us; |
| | Under specific circumstances, if we are acquired by a third party with a product that directly competes with a compound being developed under the agreement, Biogen Idec may terminate the affected program by providing written notice to us; |
| | If, within a specified period of time, any required clearance of a transaction contemplated by an agreement under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, is not received, then either we or Biogen Idec may terminate the affected program by providing written notice to the other party; and |
| | Either we or Biogen Idec may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent. |
| | Genzyme may terminate the license and co-development agreement at any time by providing written notice to Isis; |
| | We may terminate the license and co-development agreement on a country-by-country basis or in its entirety upon Genzyme's uncured failure to use commercially reasonable efforts to develop and commercialize KYNAMRO in the United States, France, Germany, Italy, Spain, the United Kingdom, Japan and Canada; and |
| | Either we or Genzyme may terminate the license and co-development agreement upon the other party's uncured failure to perform a material obligation under the agreement. |
| | GSK may terminate any program, other than the ISIS-TTR Rx program, at any time by providing written notice to us; |
| | GSK may terminate the ISIS-TTR Rx program by providing written notice to us after reviewing specific data from the Phase 3 study for the program; and |
| | Either we or GSK may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent. |
|
●
|
Janssen may terminate the agreement or any program at any time by providing written notice to us; and
|
|
●
|
Either we or Janssen may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
| | Roche may terminate the agreement at any time by providing written notice to us; |
| | Either we or Roche may terminate the agreement by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement or if the other party becomes insolvent; and |
| | Either we or Roche may terminate the brain shuttle program if at least one development candidate is not designated under such program by a mutually agreed deadline. |
|
2014
|
2013
|
2012
|
||||||
|
Partner A
|
58%
|
25%
|
8%
|
|||||
|
Partner B
|
17%
|
24%
|
8%
|
|||||
|
Partner C
|
13%
|
20%
|
9%
|
|||||
|
Partner D
|
0%
|
22%
|
66%
|
|||||
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
|||||||||||||
|
2014 Quarters
|
||||||||||||||||
|
Revenue
|
$
|
28,161
|
$
|
57,076
|
$
|
44,063
|
$
|
84,861
|
||||||||
|
Operating expenses
|
57,828
|
63,726
|
65,556
|
74,781
|
||||||||||||
|
Income (loss) from operations
|
(29,667
|
)
|
(6,650
|
)
|
(21,493
|
)
|
10,080
|
|||||||||
|
Net income (loss)
|
$
|
(31,280
|
)
|
$
|
(12,081
|
)
|
$
|
(26,676
|
)
|
$
|
31,053
|
|||||
|
Basic net income (loss) per share (1)
|
$
|
(0.27
|
)
|
$
|
(0.10
|
)
|
$
|
(0.23
|
)
|
$
|
0.26
|
|||||
|
Diluted net income (loss) per share (1) (2)
|
$
|
(0.27
|
)
|
$
|
(0.10
|
)
|
$
|
(0.23
|
)
|
$
|
0.25
|
|||||
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
|||||||||||||
|
2013 Quarters
|
||||||||||||||||
|
Revenue
|
$
|
43,360
|
$
|
38,092
|
$
|
23,585
|
$
|
42,248
|
||||||||
|
Operating expenses
|
41,735
|
46,020
|
49,090
|
62,106
|
||||||||||||
|
Income (loss) from operations
|
1,625
|
(7,928
|
)
|
(25,505
|
)
|
(19,858
|
)
|
|||||||||
|
Net loss
|
$
|
(1,672
|
)
|
$
|
(10,126
|
)
|
$
|
(24,570
|
)
|
$
|
(24,276
|
)
|
||||
|
Basic and diluted net loss per share (1)
|
$
|
(0.02
|
)
|
$
|
(0.09
|
)
|
$
|
(0.21
|
)
|
$
|
(0.21
|
)
|
||||
|
(1)
|
We computed net income (loss) per share independently for each of the quarters presented. Therefore, the sum of the quarterly net income (loss) per share will not necessarily equal the total for the year.
|
|
(2)
|
For the fourth quarter of 2014, we had net income and as a result we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended December 31, 2014 consisted of:
|
| | 4.2 million shares issuable upon exercise of stock options |
| | 0.4 million shares issuable upon restricted stock award issuance; and |
| | 0.009 million shares issuable related to our ESPP. |
