|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
UNITED STATES
|
Delaware
|
33
-
0336973
|
|
|
(State or other jurisdiction of
incorporation or organization) |
(IRS Employer Identification No.)
|
|
2855 Gazelle Court, Carlsbad, CA
|
92010
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $.001 Par Value
|
The Nasdaq Stock Market, LLC
|
|
Large accelerated filer
|
Accelerated filer
|
|
|
Non-accelerated filer
(Do not check if a smaller reporting company)
|
rr
|
Smaller reporting company
|
|
Emerging growth company
|
| * |
Excludes 22,738,285 shares of common stock held by directors and officers and by stockholders whose beneficial ownership is known by the Registrant to exceed 10 percent of the common stock outstanding at June 30, 2017. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant, or that such person is controlled by or under common control with the Registrant.
|
|
PART I
|
||
|
Page
|
||
|
Item 1.
|
Business
|
4
|
|
Item 1A.
|
Risk Factors
|
37
|
|
Item 1B.
|
Unresolved Staff Comments
|
46
|
|
Item 2.
|
Properties
|
46
|
|
Item 3.
|
Legal Proceedings
|
46
|
|
Item 4.
|
Mine Safety Disclosures
|
46
|
|
PART II
|
||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
46
|
|
Item 6.
|
Selected Financial Data
|
47
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
48
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
69
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
69
|
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
69
|
|
Item 9A.
|
Controls and Procedures
|
69
|
|
Item 9B.
|
Other Information
|
71
|
|
PART III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
71
|
|
Item 11.
|
Executive Compensation
|
71
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
71
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
71
|
|
Item 14.
|
Principal Accounting Fees and Services
|
71
|
|
PART IV
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
72
|
|
Signatures
|
| ● |
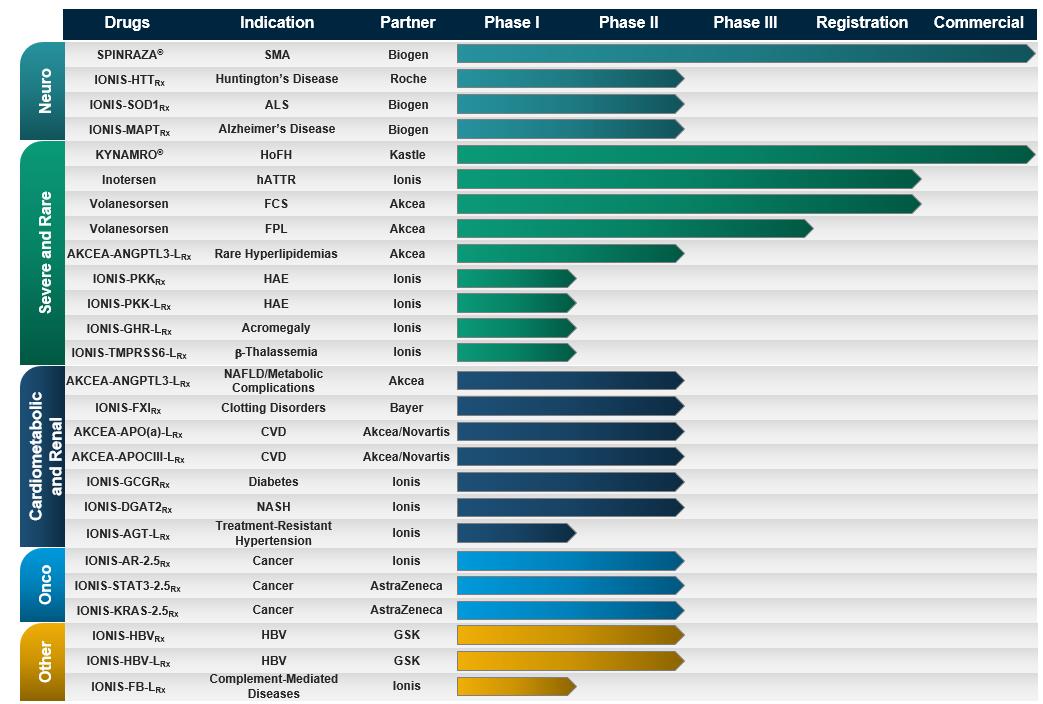
SPINRAZA for SMA
–
one of the most successful orphan drug launches in history
|
| o |
SPINRAZA, commercialized by Biogen, generated 2017 global sales of $884 million
|
| o |
Results from the
ENDEAR study and CHERISH study, in which people with infantile-onset and later-onset SMA, respectively, were treated with SPINRAZA, were published in
The New England Journal of Medicine
|
| o |
Prestigious 2017 Prix Galien USA Award for Best Biotechnology Product awarded to us and Biogen for SPINRAZA
|
| o |
New collaboration with Biogen initiated to discover new antisense drugs with enhanced properties to treat SMA
|
| ● |
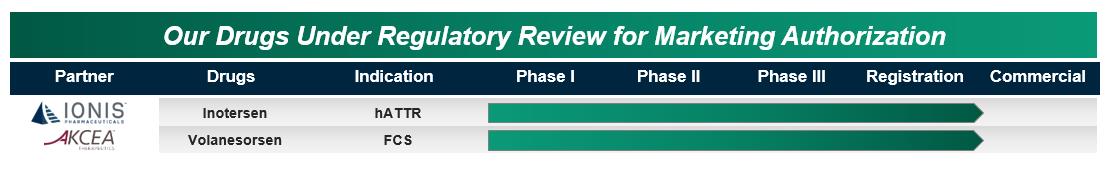
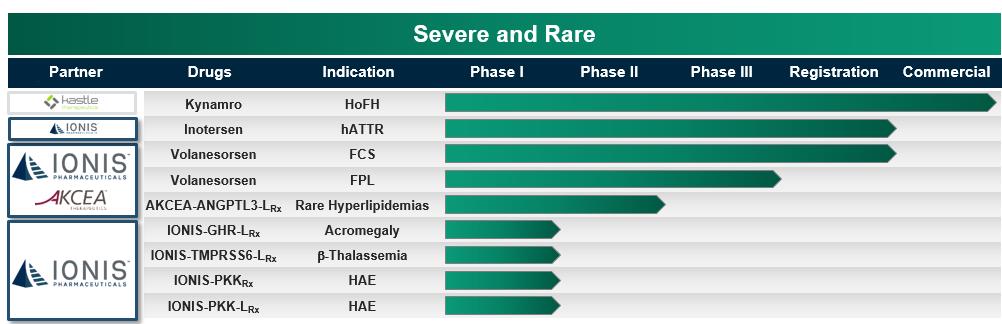
Inotersen for hATTR – potential to transform the lives of people with hATTR
|
| o |
Marketing applications accepted, no FDA Advisory Committee recommended, Priority Review in the U.S. and Accelerated Assessment in the EU
|
| o |
Preparations for g
lobal launch, planned for mid-2018, progressing
|
| o |
Phase 3 NEURO-TTR study met both primary endpoints demonstrating benefit compared to placebo in multiple measures of quality of life and disease severity; 50 percent of inotersen-treated patients experienced improvement from baseline in quality of life
|
| ● |
Volanesorsen for FCS and FPL – potential first treatment for people with FCS
|
| o |
Marketing applications accepted in the U.S., EU and Canada with Promising Innovative Medicine designation in the UK and Priority Review in Canada
|
| o |
Preparations for global launch for FCS, planned for mid-2018, progressing
|
| o |
Phase 3 APPROACH study met primary endpoint of reducing triglyceride levels in people with FCS
|
| ● |
Pipeline Programs (early and mid-stage) –
a
dvancing wholly owned and partnered programs
|
| o |
Positive results from seven Phase 2 studies reported, including:
|
| o |
Robust, dose-dependent reductions of mHTT observed in people with Huntington’s disease treated with IONIS-HTT
Rx
|
| o |
Positive clinical data on five LICA drugs reported, demonstrating consistent, positive performance and sustained target reduction with potential for monthly or less frequent dosing
|
| o |
Positive results from six Phase 1 studies reported
|
| o |
Nine Phase 2 studies and four Phase 1 studies initiated across multiple therapeutic areas to treat people with both broad and rare diseases
|



| ● |
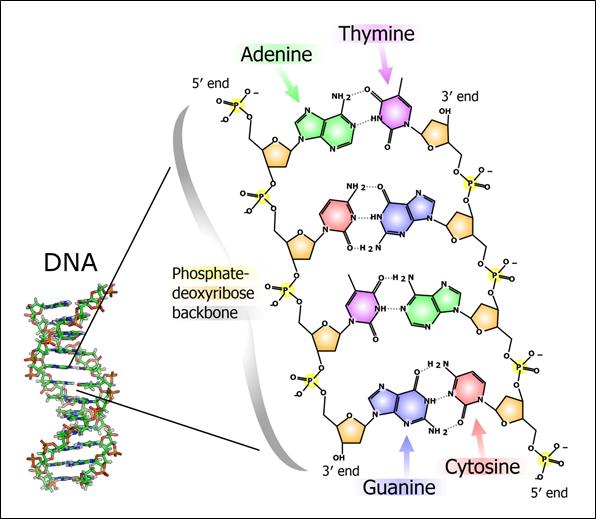
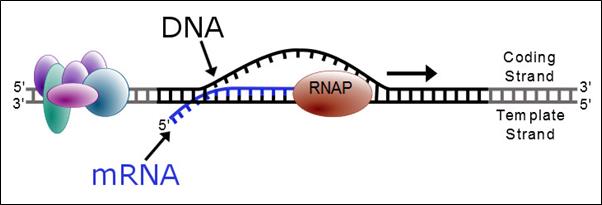
Direct intervention in the disease process at the genetic level by targeting RNA: antisense technology represents a direct route from gene to drug. The explosion in genomic information and RNA biology has led to the discovery of many new disease-causing proteins and RNAs, and has created new opportunities that are only accessible to antisense technology.
|
| ● |
Precise specificity: we design antisense drugs to target a single RNA, which minimizes or eliminates the possibility our drugs will bind to unintended targets which can cause unwanted side effects.
|
| ● |
Good drug properties: antisense drugs distribute well throughout the body without the need for special formulations or vehicles. They also have a relatively long half-life of approximately two to four weeks in most tissues outside of the brain and spinal cord and three to four months in brain and spinal cord, which means patients and/or healthcare providers can dose our drugs weekly, monthly or even less frequently depending on the drug and target tissue.
|
| ● |
Ability to combine with other drugs: because antisense drugs do not interact with the enzymes that metabolize or break down other drugs, physicians can use our drugs in combination with other drugs.
|
| ● |
Broad applications to multiple disease targets, multiple tissues and multiple mechanisms: there are virtually no “undruggable” targets with antisense technology.
|
| ● |
Efficient discovery and early development: because of the efficiency of our antisense technology, our drug discovery and early development costs and success rates compare favorably to small molecule or antibody drug discovery and development.
|
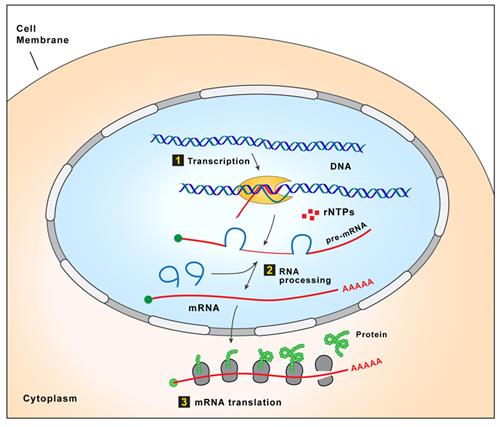
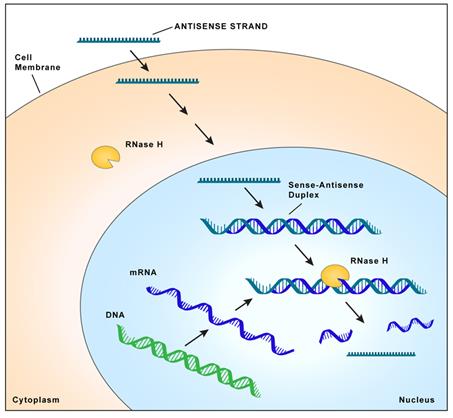
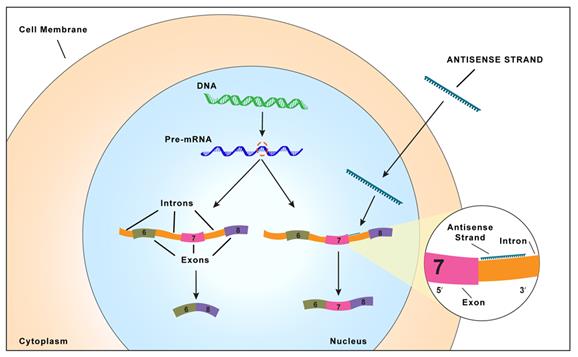
| ● |
We have strategic partnerships through which we can broadly expand our drug discovery efforts to new disease targets in specific therapeutic areas. Our partners provide expertise, tools and resources to complement our drug discovery efforts. For instance, we established a broad strategic alliance with Biogen that pairs Biogen’s extensive resources and expertise in neurodegenerative diseases with our antisense technology. Together we are creating a franchise of novel drugs for neurodegenerative diseases that has the potential to expand both our pipeline and Biogen’s pipeline with promising new drugs. Most recently, we entered into a new collaboration agreement with Biogen to identify
new antisense drugs for the treatment of SMA
.
|
| ● |
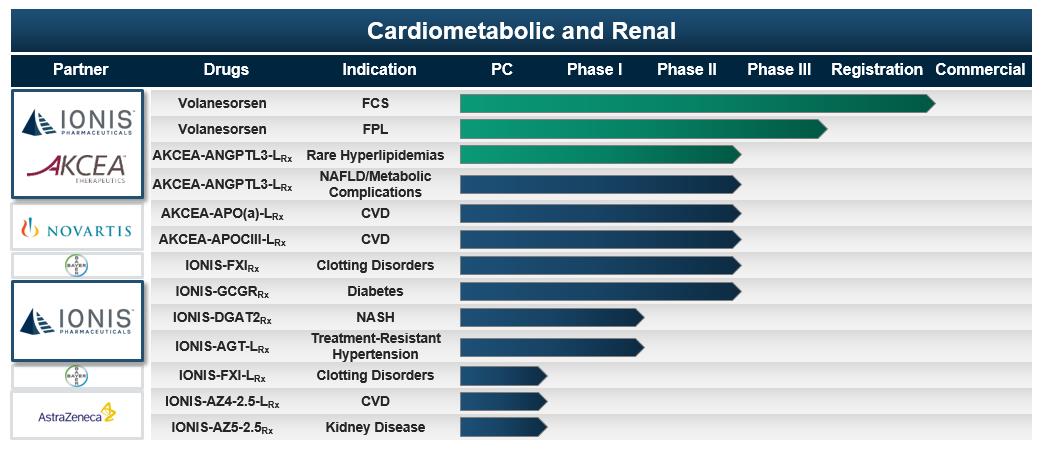
We have partnerships with companies that bring significant expertise and global resources to develop and potentially commercialize drugs for a particular therapeutic area. For example, in January 2017, we and Akcea initiated a collaboration with Novartis to develop and commercialize AKCEA-APO(a)-L
Rx
and AKCEA-APOCIII-L
Rx
. As a leader in the cardiovascular disease space, Novartis brings significant resources and expertise that should support the development and commercialization of these two drugs for significant high-risk patient populations. The collaboration with Novartis should enable us to accelerate the development of these drugs for broader patient populations as Novartis plans to conduct a cardiovascular outcome study for each of these drugs.
|
| ● |
We also form early stage research and development partnerships that allow us to expand the application of our technology to new therapeutic areas. For example, we established a collaboration with Janssen in December 2014, which brings together our RNA-targeted technology platform and Janssen’s expertise in autoimmune disorders and therapeutic formulation to discover and develop antisense drugs to treat autoimmune disorders in the GI tract. Thus far, Janssen has licensed two drugs under our collaboration.
|
| ● |
We also work with a consortium of companies that can exploit our drugs and technologies outside our primary areas of focus. We refer to these companies as satellite companies. Through our satellite company collaborations, we expand the reach and potential of RNA-targeting therapeutics into disease areas that are outside of our core focus. For example, in October 2017, Achaogen submitted an NDA to the FDA for plazomicin. Plazomicin is an aminoglycoside Achaogen discovered based on the technology we licensed to Achaogen and we are eligible to earn milestone payments and royalties under our licensing agreement.
|
|
Type of Patent Claim
|
Description
|
|||
|
1. Chemically Modified Nucleosides and Oligonucleotides
2. Antisense Drug Design Motifs
3. Therapeutic Methods
4. Antisense Sequence
5. Drug Composition
|
|
1. Target and sequence independent
2. Sequence independent
3. Chemistry independent
4. Specific claim to drug candidates
|
|
Jurisdiction
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
|
United States
|
7,101,993
|
|
OLIGONUCLEOTIDES CONTAINING 2’O-MODIFIED PURINES
|
|
2023
|
|
Covers certain MOE nucleosides and oligonucleotides containing these nucleotides.
|
|
|
United States
|
7,399,845
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
|
United States
|
7,741,457
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
|
United States
|
8,022,193
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers oligonucleotides containing cEt nucleoside analogs.
|
|
|
United States
|
7,569,686
|
COMPOUNDS AND METHODS FOR SYNTHESIS OF BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers methods of synthesizing our cEt nucleosides.
|
||||
|
Europe
|
EP1984381
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
||||
|
Europe
|
EP2314594
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt oligonucleotides and methods of use.
|
||||
|
Japan
|
JP5342881
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
Jurisdiction
|
|
Patent/
Application No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
7,015,315
|
|
GAPPED OLIGONUCLEOTIDES
|
|
2023
|
|
2’-O-alkyl-O-alkyl gapmer oligonucleotides.
|
|
Europe
|
|
EP2021472
|
|
COMPOUNDS AND METHODS FOR MODULATING GENE EXPRESSION
|
|
2027
|
|
Short gapmer oligonucleotides, having wings of 2 bicyclic nucleosides, and a gap of 10 deoxynucleotides for the treatment of cardiovascular or metabolic disorders
|
|
United States
|
|
7,750,131
|
|
5’-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
5’-Methy BNA containing gapmer compounds
|
|
Europe
|
|
EP2092065
|
|
ANTISENSE COMPOUNDS
|
|
2027
|
|
Gapmer compounds having wings comprised of 2’-modifed and LNA nucleosides
|
|
Europe
|
EP2410053
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer compounds having wings comprised of 2’-MOE and bicyclic nucleosides
|
||||
|
Japan
|
JP 5665317
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer compounds having wings comprised of 2’-MOE and bicyclic nucleosides
|
||||
|
Europe
|
EP2673361
|
OLIGOMERIC COMPOUNDS COMPRISING BICYCLIC NUCLEOTIDES AND USES THEREOF
|
2032
|
Gapmer having at least one bicyclic nucleoside, 2’-modified nucleoside, and 2’-deoxynucleoside in either the 5’- or 3’-wing.
|
|
Jurisdiction
|
|
Patent/
Application No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
9,127,276
|
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
|
2034
|
|
Covers our primary THA LICA conjugated to any group of nucleosides, including gapmers, double-stranded siRNA compounds, and fully modified oligonucleotides
|
|
United States
|
|
9,181,549
|
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
|
2034
|
|
Covers our primary THA conjugate having our preferred linker and cleavable moiety conjugated to any oligomeric compound or any nucleoside having a 2’-MOE modification or a cEt modification
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
6,210,892
|
|
ALTERATION OF CELLULAR BEHAVIOR BY MODULATION OF MRNA PROCESSING
|
|
2018
|
|
Altering mRNA processing with a fully modified 2’MOE oligonucleotide.
|
|
United States
|
|
8,361,977
|
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
|
2030
|
|
Sequence and chemistry (full 2’-MOE) of SPINRAZA
|
|
Europe
|
|
1910395
|
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
|
2026
|
|
Sequence and chemistry (full 2’-MOE) of SPINRAZA
|
|
United States
|
|
7,838,657
|
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
|
2027
|
|
Oligonucleotides having sequence of SPINRAZA (chemistry independent)
|
|
United States
|
|
8,110,560
|
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
|
2025
|
|
Methods of using antisense oligonucleotides having sequence of SPINRAZA to alter splicing of SMN2 and/or to treat SMA
|
|
United States
|
8,980,853
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2030
|
Methods of administering SPINRAZA
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
9,624,496
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2023
|
Antisense compound specifically hybridizable within the nucleotide region of apoCIII targeted by volanesorsen
|
||||
|
United States
|
|
7,598,227
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Methods of treating hyperlipidemia, lowering cholesterol levels or lowering triglyceride levels with volanesorsen
|
|
United States
|
|
7,750,141
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Antisense sequence and chemistry of volanesorsen
|
|
Europe
|
|
EP1622597
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2024
|
|
Antisense sequence and chemistry of volanesorsen
|
|
Europe
|
EP2441449
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2024
|
Antisense compound specifically hybridizable within the nucleotide region of apoCIII targeted by volanesorsen
|
||||
|
United States
|
9,157,082
|
MODULATION OF APOLIPOPROTEIN CIII (APOCIII) EXPRESSION
|
2032
|
Methods of using APOCIII antisense oligonucleotides for reducing pancreatitis and chylomicronemia and increasing HDL
|
||||
|
Japan
|
JP 6203707
|
MODULATION OF APOLIPOPROTEIN CIII (APOCIII) EXPRESSION
|
2032
|
Methods of using APOCIII antisense oligonucleotides having the sequence of volanesorsen for treating pancreatitis
|
||||
|
United States
|
9,593,333
|
MODULATION OF APOLIPOPROTEIN C-III (APOCIII) EXPRESSION IN LIPOPROTEIN LIPASE DEFICIENT (LPLD) POPULATIONS
|
2034
|
Methods of using APOCIII specific inhibitors for treating lipoprotein lipase deficiency
|
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
8,101,743
|
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
|
2025
|
|
Antisense sequence and chemistry of inotersen
|
|
United States
|
|
8,697,860
|
|
DIAGNOSIS AND TREATMENT OF DISEASE
|
|
2031
|
|
Composition of inotersen
|
|
United States
|
9,061,044
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Sodium salt composition of inotersen
|
||||
|
United States
|
9,399,774
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Methods of treating transthyretin amyloidosis by administering inotersen
|
||||
|
Japan
|
JP5896175
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Composition of inotersen
|
||||
|
Europe
|
EP2563920
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Composition of inotersen
|
|
Drug
|
Company
|
Drug Description
|
Phase
|
Admin/Dosing
|
Efficacy(1)
|
Safety(1)
|
|
AVXS-101
|
AveXis
|
Gene therapy that corrects the SMN1 gene using the
AAV9 Vector
|
Pivotal
|
Infusion
|
As of January 20, 2017, in the Phase 1 OLE, the 12 patients taking the proposed therapeutic dose of AVXS-101 were event free and were a median age of 20.2 months at their last follow up appointment. Additionally, 10 out of the 12 patients achieved the ability to sit unassisted for at least 5 seconds, including one patient whose achievement of this milestone was confirmed after January 20, 2017.
|
Generally well tolerated to date, no new treatment-related SAEs or AEs observed
|
|
RG7916
|
PTC Therapeutics/ Roche/ SMA Foundation
|
A small molecule drug that modulates splicing of the SMN2 gene
|
2
|
Oral
|
None reported
|
Safe and well tolerated at all doses and had no drug-related or safety-related study withdrawals.
|
|
LMI070
|
Novartis
|
A small molecule drug that modulates splicing of the
SMN2 gene
|
1/2
|
Oral
|
None reported
|
Study was placed on clinical hold in May 2016 due to safety findings reported in animal studies. The clinical hold was removed in September 2017 and dosing resumed along with additional monitoring.
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
|
Drug
|
Company
|
Drug Description
|
Phase
|
Admin/Dosing
|
Efficacy(1)
|
Safety(1)
|
|
Metreleptin
|
Novelion Therapeutics
|
A synthetic form of the hormone leptin
|
3
|
Reconstituted subcutaneous injection
|
44.4% mean reduction in triglycerides at four months in patients with abnormal triglyceride levels
|
Anti-metreleptin antibodies, hypoglycemia, hypersensitivity, risk of T-cell lymphoma
|
|
Gemcabene
|
Gemphire Therapeutics
|
Monocalcium salt of a dialkyl ether dicarboxylic acid
|
2
|
Oral, once-daily
|
In a post hoc analysis (n=9) of patients with triglycerides >500 mg/dl, reductions of 59% and 60% from 150mg and 300mg doses, respectively, were observed
|
In a recent study, in the gemcabene-treatment group, the
most frequently occurring adverse events were headache and infection
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
|
Drug
|
Company
|
Drug Description
|
Phase
|
Admin/Dosing
|
Efficacy(1)
|
Safety(1)
|
|
Patisiran
|
Alnylam
|
An RNAi drug formulated with lipid nanoparticles to inhibit TTR mRNA
|
Registration
|
Infusion every 3 weeks with pre-treatment with steroids
|
84.3% mean reduction in TTR at 18 months
|
Most common adverse events more frequently observed in patisiran arm vs. placebo were peripheral edema (29.7% vs.
22.1%) and infusion-related reactions (18.9% vs. 9.1%)
|
|
Tafamidis
|
Pfizer
|
A small molecule drug to stabilize TTR Protein
|
3 to support refiling in the U.S., Approved in the EU
|
Daily oral capsule
|
In 45% of people taking Tafamidis, nerve function either improved or stabilized, compared with 30% of patients taking placebo
|
Urinary tract infection, vaginal infection, upper abdominal pain and diarrhea
|
|
Diflunisal
|
N/A Generic
|
A non-steroid anti-inflammatory agent
|
Approved (but not for ATTR)
|
Daily oral capsule/doses
|
Improved nerve function as shown by lower Neuropathy Impairment Score plus 7 nerve tests, or NIS+7. The NIS+7 score increased by 25.0 points in the placebo group versus 8.7 points in the diflunisal group
|
In two studies repurposing diflunisal for use in TTR amyloidosis, drug-related adverse events that led to discontinuation were: gastrointestinal bleeding, low platelets, deterioration of renal function, congestive heart failure, glaucoma and nausea.
|
|
Tolcapone
|
SOM Biotech
|
Small molecule repurposed generic drug
|
2
|
Daily oral dose
|
Shows binding and stabilization of TTR in humans
|
No drug related adverse events reported
|
|
ALN-TTRsc02
|
Alnylam
|
An RNAi drug conjugated with GalNAC to inhibit TTR mRNA in liver cells
|
1
|
Monthly or quarterly
|
In healthy volunteers, a single dose showed mean max TTR knockdown of 97%
|
Injection site reactions were reported
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations. Diflunisal efficacy and safety came from the published papers of two investigator sponsored studies, Berk JL, Suhr OB, Obici L, et al. Repurposing Diflunisal for Familial Amyloid Polyneuropathy: A Randomized Clinical Trial. JAMA. 2013;310(24):2658-2667 and Sekijima YS, Toja K, Morita H, et al. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid. 2015;22(2):79-83.
|
|
Name
|
|
Age
|
|
Position
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
72
|
|
Chairman, Chief Executive Officer and President
|
|
Brett P. Monia, Ph.D.
|
|
56
|
Chief Operating Officer and Senior Vice President, Drug Discovery and Corporate Development
|
|
|
C. Frank Bennett, Ph.D.
|
|
61
|
|
Senior Vice President, Antisense Research
|
|
Sarah Boyce
|
46
|
Chief Business Officer
|
||
|
Richard S. Geary, Ph.D.
|
|
60
|
|
Senior Vice President, Development
|
|
Elizabeth L. Hougen
|
|
56
|
|
Senior Vice President, Finance and Chief Financial Officer
|
|
Patrick R. O’Neil, Esq.
|
|
44
|
|
Senior Vice President, Legal, General Counsel, Chief Compliance Officer and Corporate Secretary
|
| ● |
receipt and scope of marketing authorizations;
|
| ● |
establishment and demonstration in the medical and patient community of the efficacy and safety of our drugs and their potential advantages over competing products;
|
| ● |
cost and effectiveness of our drugs compared to other available therapies;
|
| ● |
patient convenience of the dosing regimen for our drugs; and
|
| ● |
reimbursement policies of government and third-party payors.
|
| ● |
priced lower than our drugs;
|
| ● |
reimbursed more favorably by government and other third-party payors than our drugs;
|
| ● |
safer than our drugs;
|
| ● |
more effective than our drugs; or
|
| ● |
more convenient to use than our drugs.
|
| ● |
fund our development activities for SPINRAZA;
|
| ● |
seek and obtain regulatory approvals for SPINRAZA; and
|
| ● |
successfully commercialize SPINRAZA.
|
| ● |
the clinical study may produce negative or inconclusive results;
|
| ● |
regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements;
|
| ● |
we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a drug on subjects in the trial;
|
| ● |
we may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies;
|
| ● |
enrollment in our clinical studies may be slower than we anticipate;
|
| ● |
people who enroll in the clinical study may later drop out due to adverse events, a perception they are not benefiting from participating in the study, fatigue with the clinical study process or personal issues;
|
| ● |
the cost of our clinical studies may be greater than we anticipate; and
|
| ● |
the supply or quality of our drugs or other materials necessary to conduct our clinical studies may be insufficient, inadequate or delayed.
|
| ● |
conduct clinical studies;
|
| ● |
seek and obtain marketing authorization; and
|
| ● |
manufacture, market and sell our drugs.
|
| ● |
pursue alternative technologies or develop alternative products that may be competitive with the drug that is part of the collaboration with us;
|
| ● |
pursue higher-priority programs or change the focus of its own development programs; or
|
| ● |
choose to devote fewer resources to our drugs than it does for its own drugs.
|
| ● |
successful commercialization for SPINRAZA;
|
| ● |
marketing approvals for volanesorsen and inotersen;
|
| ● |
the profile and launch timing of our drugs, including volanesorsen and inotersen;
|
| ● |
changes in existing collaborative relationships and our ability to establish and maintain additional collaborative arrangements;
|
| ● |
continued scientific progress in our research, drug discovery and development programs;
|
| ● |
the size of our programs and progress with preclinical and clinical studies;
|
| ● |
the time and costs involved in obtaining marketing authorizations; and
|
| ● |
competing technological and market developments, including the introduction by others of new therapies that address our markets.
|
| ● |
interruption of our research, development and manufacturing efforts;
|
| ● |
injury to our employees and others;
|
| ● |
environmental damage resulting in costly clean up; and
|
| ● |
liabilities under federal, state and local laws and regulations governing health and human safety, as well as the use, storage, handling and disposal of these materials and resultant waste products.
|
|
Property Description
|
Location
|
Square Footage
|
Owned or Leased
|
Initial Lease Term End Date
|
Lease Extension Options
|
|||||
|
Ionis laboratory and office space facility
|
Carlsbad, CA
|
176,000
|
Owned
|
|||||||
|
Ionis manufacturing facility
|
Carlsbad, CA
|
28,700
|
Owned
|
|||||||
|
Ionis manufacturing support facility
|
Carlsbad, CA
|
25,800
|
Leased
|
2021
|
Two, five-year options to extend
|
|||||
|
Akcea office space facility
|
Cambridge, MA
|
6,100
|
Leased
|
2018
|
None
|
|||||
|
Akcea office space facility
|
Cambridge, MA
|
3,100
|
Leased
|
2020
|
None
|
|||||
|
239,700
|
| Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
HIGH
|
LOW
|
||||||
|
2017
|
||||||||
|
First Quarter
|
$
|
56.91
|
$
|
37.29
|
||||
|
Second Quarter
|
$
|
55.73
|
$
|
37.26
|
||||
|
Third Quarter
|
$
|
60.01
|
$
|
43.75
|
||||
|
Fourth Quarter
|
$
|
65.51
|
$
|
50.02
|
||||
|
2016
|
||||||||
|
First Quarter
|
$
|
62.68
|
$
|
30.93
|
||||
|
Second Quarter
|
$
|
46.75
|
$
|
19.59
|
||||
|
Third Quarter
|
$
|
40.82
|
$
|
23.26
|
||||
|
Fourth Quarter
|
$
|
57.00
|
$
|
24.58
|
||||
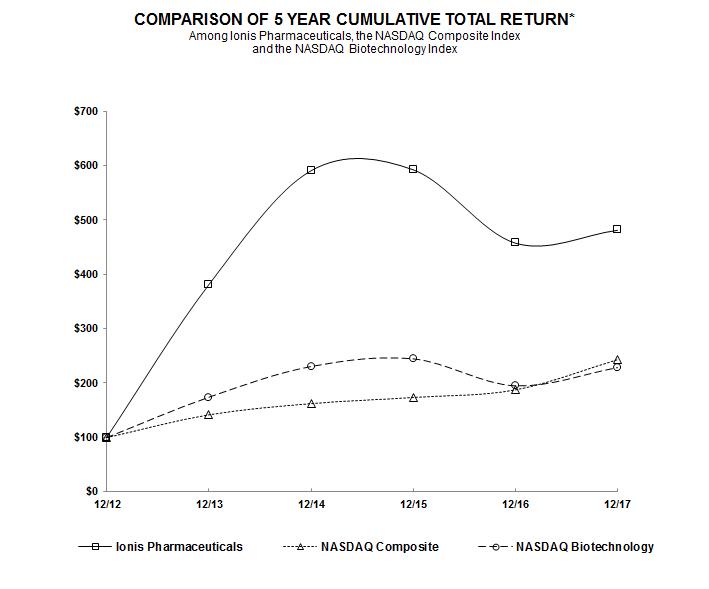
|
|
Dec-12
|
Dec-13
|
Dec-14
|
Dec-15
|
Dec-16
|
Dec-17
|
||||||||||||||||||
|
Ionis Pharmaceuticals, Inc.
|
$
|
100.00
|
$
|
381.61
|
$
|
591.38
|
$
|
593.20
|
$
|
458.14
|
$
|
481.80
|
||||||||||||
|
Nasdaq Composite Index
|
$
|
100.00
|
$
|
141.63
|
$
|
162.09
|
$
|
173.33
|
$
|
187.19
|
$
|
242.29
|
||||||||||||
|
Nasdaq Biotechnology Index
|
$
|
100.00
|
$
|
174.05
|
$
|
230.33
|
$
|
244.29
|
$
|
194.95
|
$
|
228.29
|
||||||||||||
| (1) |
This section is not “soliciting material,” is not deemed “filed” with the SEC, is not subject to the liabilities of Section 18 of the Exchange Act and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
|
|
Years Ended December 31,
|
|||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Consolidated Statement of Operations Data:
|
||||||||||||||||||||
|
Revenue
|
$
|
507,666
|
$
|
346,620
|
$
|
283,703
|
$
|
214,161
|
$
|
147,285
|
||||||||||
|
Research, development and patent expenses
|
$
|
374,644
|
$
|
344,320
|
$
|
322,292
|
$
|
241,751
|
$
|
184,033
|
||||||||||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(5,970
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
$
|
(38,984
|
)
|
$
|
(60,644
|
)
|
|||||
|
Basic net income (loss) per share attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
$
|
(0.33
|
)
|
$
|
(0.55
|
)
|
||||||
|
Diluted net income (loss) per share attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
$
|
(0.33
|
)
|
$
|
(0.55
|
)
|
||||||
|
Shares used in computing basic net income (loss) per share
|
124,016
|
120,933
|
119,719
|
117,691
|
110,502
|
|||||||||||||||
|
Shares used in computing diluted net income (loss) per share
|
126,098
|
120,933
|
119,719
|
117,691
|
110,502
|
|||||||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Consolidated Balance Sheet:
|
||||||||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
1,022,715
|
$
|
665,223
|
$
|
779,183
|
$
|
728,832
|
$
|
656,761
|
||||||||||
|
Working capital
|
$
|
943,243
|
$
|
664,148
|
$
|
688,127
|
$
|
721,265
|
$
|
637,698
|
||||||||||
|
Total assets
|
$
|
1,322,024
|
$
|
912,467
|
$
|
947,900
|
$
|
946,471
|
$
|
843,267
|
||||||||||
|
Long-term debt and other obligations, less current portion
|
$
|
678,564
|
$
|
679,118
|
$
|
598,234
|
$
|
588,896
|
$
|
367,065
|
||||||||||
|
Accumulated deficit
|
$
|
(1,187,398
|
)
|
$
|
(1,181,428
|
)
|
$
|
(1,094,872
|
)
|
$
|
(1,006,594
|
)
|
$
|
(967,610
|
)
|
|||||
|
Stockholders’ equity
|
$
|
418,719
|
$
|
99,565
|
$
|
200,790
|
$
|
257,780
|
$
|
378,390
|
||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Total revenue
|
$
|
507,666
|
$
|
346,620
|
$
|
283,703
|
||||||
|
Total operating expenses
|
$
|
483,132
|
$
|
392,936
|
$
|
359,465
|
||||||
|
Income (loss) from operations
|
$
|
24,534
|
$
|
(46,316
|
)
|
$
|
(75,762
|
)
|
||||
|
Net loss
|
$
|
(17,296
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(5,970
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Cash, cash equivalents and short-term investments
|
$
|
1,022,715
|
$
|
665,223
|
$
|
779,183
|
||||||
| |
Assessing the propriety of revenue recognition and associated deferred revenue;
|
| |
Determining the proper valuation of investments in marketable securities;
|
| |
Determining the appropriate cost estimates for unbilled preclinical studies and clinical development activities;
|
| |
Estimating the impact of the Tax Act and our net deferred income tax asset valuation allowance;
|
| |
Determining the fair value of convertible debt without the conversion feature; and
|
| |
Valuing premiums under our and Akcea’s Novartis collaboration.
|
| ● |
The exclusive license we granted to Bayer to develop and commercialize IONIS-FXI-L
Rx
for the treatment of thrombosis;
|
| ● |
The development services we agreed to perform for IONIS-FXI-L
Rx
and IONIS-FXI
Rx
; and
|
| ● |
The remaining undelivered IONIS-FXI
Rx
API that was part of the original agreement.
|
| ● |
Estimated future product sales;
|
| ● |
Estimated royalties on future product sales;
|
| ● |
Contractual milestone payments;
|
| ● |
Expenses we expect to incur;
|
| ● |
Income taxes; and
|
| ● |
An appropriate discount rate.
|
| ● |
The number of internal hours we will spend performing these services;
|
| ● |
The estimated cost of work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
| ● |
$64.9 million to the IONIS-FXI-L
Rx
exclusive license;
|
| ● |
$11.0 million for development services for IONIS-FXI-L
Rx
and IONIS-FXI
Rx
; and
|
| ● |
$0.4
million for the remaining delivery of
IONIS-FXI
Rx
API.
|
| ● |
We recognized the portion of the consideration attributed to the IONIS-FXI-L
Rx
license in the first quarter of 2017 because we delivered the license and earned the revenue;
|
| ● |
We are recognizing the amount attributed to the development services for IONIS-FXI-L
Rx
and IONIS-FXI
Rx
over the period of time we are performing the services; and
|
| ● |
We are recognizing the amount attributed to the remaining API supply as we deliver it to Bayer.
|
| ● |
Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete.
|
| ● |
Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete.
|
| ● |
Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete.
|
| ● |
Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
|
| ● |
Filing of regulatory applications for marketing authorization such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
|
| ● |
Obtaining marketing authorization in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain authorization from the applicable regulatory agency.
|
| ● |
First commercial sale in a particular market, such as in the United States or Europe.
|
| ● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
|
| ● |
Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
|
| ● |
The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on our performance or the occurrence of a specific outcome resulting from our performance;
|
| ● |
The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
|
| ● |
There is no future performance required to earn the milestone; and
|
| ● |
The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
|
| ● |
$28.4 million for the premium paid by Novartis for its purchase of our common stock in the first quarter of 2017; and
|
| ● |
$5.0 million for the potential premium Novartis would have paid if it had purchased our common stock in the future at a premium.
|
| ● |
$118 million in milestone payments from Biogen, including $90 million in approval milestone payments for SPINRAZA, $15 million in milestone payments for
validating two undisclosed neurological disease targets and $10 million for initiating a Phase 1/2a study of IONIS-MAPT
Rx
;
|
| ● |
$65 million from Bayer for the license of IONIS-FXI-L
Rx
;
|
| ● |
$48 million from Roche primarily for the license of IONIS-HTT
Rx
;
|
| ● |
$10 million from Janssen for the license of IONIS-JBI2-2.5
Rx
and initiation of a Phase 1 study of IONIS-JBI1-2.5
Rx
:
|
| ● |
$115 million from the amortization of upfront fees; and
|
| ● |
$29.6 million primarily from services we performed for our partners.
|
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
305,352
|
$
|
260,233
|
||||
|
Akcea Therapeutics
|
146,332
|
73,363
|
||||||
|
Elimination of intercompany activity
|
(54,527
|
)
|
(12,768
|
)
|
||||
|
Subtotal
|
397,157
|
320,828
|
||||||
|
Non-cash compensation expense related to equity awards
|
85,975
|
72,108
|
||||||
|
Total operating expenses
|
$
|
483,132
|
$
|
392,936
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Research, development and patent expenses, excluding non-cash compensation expense related to equity awards
|
$
|
310,123
|
$
|
289,221
|
||||
|
Non-cash compensation expense related to equity awards
|
64,521
|
55,099
|
||||||
|
Total research, development and patent expenses
|
$
|
374,644
|
$
|
344,320
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
246,390
|
$
|
238,106
|
||||
|
Akcea Therapeutics
|
118,260
|
63,883
|
||||||
|
Elimination of intercompany activity
|
(54,527
|
)
|
(12,768
|
)
|
||||
|
Subtotal
|
310,123
|
289,221
|
||||||
|
Non-cash compensation expense related to equity awards
|
64,521
|
55,099
|
||||||
|
Total research, development and patent expenses
|
$
|
374,644
|
$
|
344,320
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Antisense drug discovery expenses, excluding non-cash compensation expense related to equity awards
|
$
|
56,160
|
$
|
51,028
|
||||
|
Non-cash compensation expense related to equity awards
|
15,203
|
13,589
|
||||||
|
Total antisense drug discovery expenses
|
$
|
71,363
|
$
|
64,617
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
SPINRAZA
|
$
|
10,996
|
$
|
43,868
|
||||
|
Volanesorsen
|
22,524
|
26,285
|
||||||
|
Inotersen
|
24,880
|
22,939
|
||||||
|
Other antisense development projects
|
70,009
|
42,999
|
||||||
|
Development overhead expenses
|
43,784
|
39,398
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
172,193
|
175,489
|
||||||
|
Non-cash compensation expense related to equity awards
|
25,737
|
20,116
|
||||||
|
Total antisense drug development expenses
|
$
|
197,930
|
$
|
195,605
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
122,163
|
$
|
132,418
|
||||
|
Akcea Therapeutics
|
98,425
|
43,071
|
||||||
|
Elimination of intercompany activity
|
(48,395
|
)
|
—
|
|||||
|
Subtotal
|
172,193
|
175,489
|
||||||
|
Non-cash compensation expense related to equity awards
|
25,737
|
20,116
|
||||||
|
Total antisense drug development expenses
|
$
|
197,930
|
$
|
195,605
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Medical affairs expenses, excluding non-cash compensation expense related to equity awards
|
$
|
9,097
|
$
|
3,568
|
||||
|
Non-cash compensation expense related to equity awards
|
2,588
|
1,264
|
||||||
|
Total medical affairs expenses
|
$
|
11,685
|
$
|
4,832
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
1,771
|
$
|
—
|
||||
|
Akcea Therapeutics
|
7,326
|
3,568
|
||||||
|
Subtotal
|
9,097
|
3,568
|
||||||
|
Non-cash compensation expense related to equity awards
|
2,588
|
1,264
|
||||||
|
Total medical affairs expenses
|
$
|
11,685
|
$
|
4,832
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Manufacturing and operations expenses, excluding non-cash compensation expense related to equity awards
|
$
|
43,526
|
$
|
30,148
|
||||
|
Non-cash compensation expense related to equity awards
|
6,904
|
6,113
|
||||||
|
Total manufacturing and operations expenses
|
$
|
50,430
|
$
|
36,261
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
39,098
|
$
|
27,341
|
||||
|
Akcea Therapeutics
|
10,440
|
15,455
|
||||||
|
Elimination of intercompany activity
|
(6,012
|
)
|
(12,648
|
)
|
||||
|
Subtotal
|
43,526
|
30,148
|
||||||
|
Non-cash compensation expense related to equity awards
|
6,904
|
6,113
|
||||||
|
Total manufacturing and operations expenses
|
$
|
50,430
|
$
|
36,261
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Personnel costs
|
$
|
11,432
|
$
|
11,560
|
||||
|
Occupancy
|
8,236
|
7,891
|
||||||
|
Patent expenses
|
2,095
|
3,945
|
||||||
|
Depreciation and amortization
|
249
|
245
|
||||||
|
Insurance
|
1,735
|
1,344
|
||||||
|
Other
|
5,400
|
4,003
|
||||||
|
Total R&D support expenses, excluding non-cash compensation expense related to equity awards
|
29,147
|
28,988
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,089
|
14,017
|
||||||
|
Total R&D support expenses
|
$
|
43,236
|
$
|
43,005
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
27,198
|
$
|
27,319
|
||||
|
Akcea Therapeutics
|
2,069
|
1,789
|
||||||
|
Elimination of intercompany activity
|
(120
|
)
|
(120
|
)
|
||||
|
Subtotal
|
29,147
|
28,988
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,089
|
14,017
|
||||||
|
Total R&D support expenses
|
$
|
43,236
|
$
|
43,005
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Selling, general and administrative expenses, excluding non-cash compensation expense related to equity awards
|
$
|
87,034
|
$
|
31,607
|
||||
|
Non-cash compensation expense related to equity awards
|
21,454
|
17,009
|
||||||
|
Total selling, general and administrative expenses
|
$
|
108,488
|
$
|
48,616
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
58,962
|
$
|
22,127
|
||||
|
Akcea Therapeutics
|
28,072
|
9,480
|
||||||
|
Non-cash compensation expense related to equity awards
|
21,454
|
17,009
|
||||||
|
Total selling general and administrative expenses
|
$
|
108,488
|
$
|
48,616
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Development and patent expenses
|
$
|
118,260
|
$
|
63,883
|
||||
|
General and administrative expenses
|
28,072
|
9,480
|
||||||
|
Total operating expenses, excluding non-cash compensation expense related to equity awards
|
146,332
|
73,363
|
||||||
|
Non-cash compensation expense related to equity awards
|
17,539
|
10,149
|
||||||
|
Total Akcea Therapeutics operating expenses
|
$
|
163,871
|
$
|
83,512
|
||||
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Convertible notes:
|
||||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
$
|
32,536
|
$
|
25,115
|
||||
|
Interest expense payable in cash
|
7,090
|
6,684
|
||||||
|
Non-cash interest expense for long-term financing liability
|
3,352
|
6,693
|
||||||
|
Interest on mortgage for primary R&D and manufacturing facilities
|
1,103
|
—
|
||||||
|
Other
|
671
|
303
|
||||||
|
Total interest expense
|
$
|
44,752
|
$
|
38,795
|
||||
| |
$170 million from Biogen for FDA approval, licensing and advancing the Phase 3 program for SPINRAZA;
|
| |
$53 million from AstraZeneca for advancing and licensing IONIS-KRAS-2.5
Rx
and selecting IONIS-AZ4-2.5-L
Rx
to move into development;
|
| |
$15 million from Janssen for licensing IONIS-JBI1-2.5
Rx
and selecting an additional development candidate;
|
| |
$7.5 million from Biogen for advancing IONIS-SOD1
Rx
, IONIS-BIIB4
Rx
and IONIS-BIIB6
Rx
;
|
| |
$61 million from the amortization of upfront fees; and
|
| |
$19.4 million primarily from the manufacturing services we performed for our partners.
|
| |
During 2016, we were conducting five Phase 3 studies and three open-label extension studies for SPINRAZA, inotersen and volanesorsen. We completed target enrollment in four of these Phase 3 studies at the end of 2015, and as a result, these studies were in their most expensive stage during 2016.
|
| |
Akcea’s operating expenses increased as it continued to build its commercial infrastructure and advance the pre-commercialization activities necessary to successfully launch volanesorsen, if approved for marketing.
|
| |
Our non-cash compensation expense related to equity awards increased due to an increase in the exercise price of the stock options we have granted over the past several years.
|
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
260,233
|
$
|
256,674
|
||||
|
Akcea Therapeutics
|
73,363
|
46,252
|
||||||
|
Elimination of intercompany activity
|
(12,768
|
)
|
(2,775
|
)
|
||||
|
Subtotal
|
320,828
|
300,151
|
||||||
|
Non-cash compensation expense related to equity awards
|
72,108
|
59,314
|
||||||
|
Total operating expenses
|
$
|
392,936
|
$
|
359,465
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Research, development and patent expenses, excluding non-cash compensation expense related to equity awards
|
$
|
289,221
|
$
|
278,654
|
||||
|
Non-cash compensation expense related to equity awards
|
55,099
|
43,638
|
||||||
|
Total research, development and patent expenses
|
$
|
344,320
|
$
|
322,292
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
238,106
|
$
|
240,061
|
||||
|
Akcea Therapeutics
|
63,883
|
41,368
|
||||||
|
Elimination of intercompany activity
|
(12,768
|
)
|
(2,775
|
)
|
||||
|
Subtotal
|
289,221
|
278,654
|
||||||
|
Non-cash compensation expense related to equity awards
|
55,099
|
43,638
|
||||||
|
Total research, development and patent expenses
|
$
|
344,320
|
$
|
322,292
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Antisense drug discovery expenses, excluding non-cash compensation expense related to equity awards
|
$
|
51,028
|
$
|
49,331
|
||||
|
Non-cash compensation expense related to equity awards
|
13,589
|
11,914
|
||||||
|
Total antisense drug discovery expenses
|
$
|
64,617
|
$
|
61,245
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
SPINRAZA
|
$
|
43,868
|
$
|
35,164
|
||||
|
Volanesorsen
|
26,285
|
21,348
|
||||||
|
Inotersen
|
22,939
|
19,560
|
||||||
|
Other antisense development products
|
42,999
|
59,599
|
||||||
|
Development overhead expenses
|
39,398
|
36,117
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
175,489
|
171,788
|
||||||
|
Non-cash compensation expense related to equity awards
|
20,116
|
16,108
|
||||||
|
Total antisense drug development expenses
|
$
|
195,605
|
$
|
187,896
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
132,418
|
$
|
137,092
|
||||
|
Akcea Therapeutics
|
43,071
|
34,696
|
||||||
|
Non-cash compensation expense related to equity awards
|
20,116
|
16,108
|
||||||
|
Total antisense drug development expenses
|
$
|
195,605
|
$
|
187,896
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Medical affairs expenses, excluding non-cash compensation expense related to equity awards
|
$
|
3,568
|
$
|
429
|
||||
|
Non-cash compensation expense related to equity awards
|
1,264
|
100
|
||||||
|
Total medical affairs expenses
|
$
|
4,832
|
$
|
529
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Manufacturing and operations expenses, excluding non-cash compensation expense related to equity awards
|
$
|
30,148
|
$
|
28,588
|
||||
|
Non-cash compensation expense related to equity awards
|
6,113
|
4,563
|
||||||
|
Total manufacturing and operations expenses
|
$
|
36,261
|
$
|
33,151
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
27,341
|
$
|
25,632
|
||||
|
Akcea Therapeutics
|
15,455
|
5,611
|
||||||
|
Elimination of intercompany activity
|
(12,648
|
)
|
(2,655
|
)
|
||||
|
Subtotal
|
30,148
|
28,588
|
||||||
|
Non-cash compensation expense related to equity awards
|
6,113
|
4,563
|
||||||
|
Total manufacturing and operations expenses
|
$
|
36,261
|
$
|
33,151
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Personnel costs
|
$
|
11,560
|
$
|
10,210
|
||||
|
Occupancy
|
7,891
|
7,854
|
||||||
|
Patent expenses
|
3,945
|
2,785
|
||||||
|
Depreciation and amortization
|
245
|
2,911
|
||||||
|
Insurance
|
1,344
|
1,320
|
||||||
|
Other
|
4,003
|
3,438
|
||||||
|
Total R&D support expenses, excluding non-cash compensation expense related to equity awards
|
28,988
|
28,518
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,017
|
10,953
|
||||||
|
Total R&D support expenses
|
$
|
43,005
|
$
|
39,471
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
27,319
|
$
|
28,005
|
||||
|
Akcea Therapeutics
|
1,789
|
633
|
||||||
|
Elimination of intercompany activity
|
(120
|
)
|
(120
|
)
|
||||
|
Subtotal
|
28,988
|
28,518
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,017
|
10,953
|
||||||
|
Total R&D support expenses
|
$
|
43,005
|
$
|
39,471
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
General and administrative expenses, excluding non-cash compensation expense related to equity awards
|
$
|
31,607
|
$
|
21,497
|
||||
|
Non-cash compensation expense related to equity awards
|
17,009
|
15,676
|
||||||
|
Total general and administrative expenses
|
$
|
48,616
|
$
|
37,173
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
22,127
|
$
|
16,613
|
||||
|
Akcea Therapeutics
|
9,480
|
4,884
|
||||||
|
Non-cash compensation expense related to equity awards
|
17,009
|
15,676
|
||||||
|
Total general and administrative expenses
|
$
|
48,616
|
$
|
37,173
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Development and patent expenses
|
$
|
63,883
|
$
|
41,368
|
||||
|
General and administrative expenses
|
9,480
|
4,884
|
||||||
|
Total operating expenses, excluding non-cash compensation expense related to equity awards
|
73,363
|
46,252
|
||||||
|
Non-cash compensation expense related to equity awards
|
10,149
|
6,496
|
||||||
|
Total Akcea Therapeutics operating expenses
|
$
|
83,512
|
$
|
52,748
|
||||
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Convertible notes:
|
||||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
$
|
25,115
|
$
|
23,208
|
||||
|
Interest expense payable in cash
|
6,684
|
6,683
|
||||||
|
Non-cash interest expense for long-term financing liability
|
6,693
|
6,665
|
||||||
|
Other
|
303
|
176
|
||||||
|
Total interest expense
|
$
|
38,795
|
$
|
36,732
|
||||
|
|
Payments Due by Period (in millions)
|
|||||||||||||||||||
|
Contractual Obligations
(selected balances described below)
|
Total
|
Less than
1 year
|
1-3 years
|
3-5 years
|
After
5 years
|
|||||||||||||||
|
Convertible senior notes (principal and interest payable)
|
$
|
712.9
|
$
|
6.9
|
$
|
13.7
|
$
|
692.3
|
$
|
—
|
||||||||||
|
Building mortgage payments
|
$
|
83.2
|
$
|
2.4
|
$
|
4.8
|
$
|
5.1
|
$
|
70.9
|
||||||||||
|
Financing arrangements (principal and interest payable)
|
$
|
13.0
|
$
|
0.3
|
$
|
12.7
|
$
|
—
|
$
|
—
|
||||||||||
|
Other obligations (principal and interest payable)
|
$
|
1.1
|
$
|
0.1
|
$
|
0.1
|
$
|
0.1
|
$
|
0.8
|
||||||||||
|
Operating leases
|
$
|
2.1
|
$
|
0.9
|
$
|
1.1
|
$
|
0.1
|
$
|
—
|
||||||||||
|
Total
|
$
|
812.3
|
$
|
10.6
|
$
|
32.4
|
$
|
697.6
|
$
|
71.7
|
||||||||||
|
1 Percent Convertible
Senior Notes
|
||||
|
Outstanding principal balance
|
$
|
685.5
|
||
|
Original issue date ($500 million of principal)
|
November 2014
|
|||
|
Additional issue date ($185.5 million of principal)
|
December 2016
|
|||
|
Maturity date
|
November 2021
|
|||
|
Interest rate
|
1 percent
|
|||
|
Conversion price per share
|
$
|
66.81
|
||
|
Total shares of common stock subject to conversion
|
10.3
|
|||
|
(i)
|
a floating rate equal to the one-month London Interbank Offered Rate, or LIBOR, in effect plus 1.25 percent per annum;
|
|
(ii)
|
a fixed rate equal to LIBOR plus 1.25 percent for a period of one, two, three, four, six, or twelve months as elected by us; or
|
|
(iii)
|
a fixed rate equal to the LIBOR swap rate during the period of the loan.
|
|
/s/ ERNST & YOUNG LLP
|
|
|
|
|
|
San Diego, California
|
|
|
February 28, 2018
|
|
|
(1)
|
Any information that is included on or linked to our website is not part of this Form 10-K.
|
|
Plan Category
|
Number of Shares
to be Issued Upon Exercise of Outstanding Options |
Weighted Average
Exercise Price of Outstanding Options |
Number of Shares
Remaining Available for Future Issuance |
|
|||||||||
|
Equity compensation plans approved by stockholders(a)
|
9,396,796
|
$
|
44.52
|
8,158,366
|
(b)
|
||||||||
|
Total
|
9,396,796
|
$
|
44.52
|
8,158,366
|
|
||||||||
| (a) |
Consists of four Ionis plans: 1989 Stock Option Plan, Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, 2011 Equity Incentive Plan and Employee Stock Purchase Plan, or ESPP.
|
| (b) |
Of these shares, 668,232 remained available for purchase under the ESPP as of December 31, 2017. The ESPP incorporates an evergreen formula pursuant to which on January 1 of each year, we automatically increase the aggregate number of shares reserved for issuance under the plan by 150,000 shares.
|
|
Exhibit Number
|
Description of Document
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation filed June 19, 1991.
|
|
|
3.2
|
Certificate of Amendment to Restated Certificate of Incorporation
filed June 17, 2014. - Filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2014 Annual Meeting of Stockholders, filed with the SEC on April 25, 2014, and incorporated herein by reference.
|
|
|
3.3
|
Certificate of Amendment to Restated Certificate of Incorporation
filed December 18, 2015. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 18, 2015 and incorporated herein by reference.
|
|
|
3.4
|
Amended and Restated Bylaws
. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 18, 2015 and incorporated herein by reference.
|
|
|
4.1
|
Certificate of Designation of the Series C Junior Participating Preferred Stock
. - Filed as an exhibit to Registrant’s Report on Form 8-K dated filed December 13, 2000 and incorporated herein by reference.
|
|
|
4.2
|
Specimen Common Stock Certificate.
|
|
|
4.3
|
Indenture, dated as of August 13, 2012, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 2¾ percent Convertible Senior Note due 2019
. - Filed as an exhibit to the Registrant’s Report on Form 8-K filed August 13, 2012 and incorporated herein by reference.
|
|
|
4.4
|
Indenture, dated as of November 17, 2014, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 1.00 percent Convertible Senior Note due 2021
. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed November 21, 2014 and incorporated herein by reference.
|
|
|
10.1
|
Form of Indemnity Agreement entered into between the Registrant and its Directors and Officers with related schedule
. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
|
10.2*
|
Registrant’s 1989 Stock Option Plan, as amended
. - Filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2012 Annual Meeting of Stockholders, filed with the SEC on April 16, 2012, and incorporated herein by reference.
|
|
|
10.3*
|
Registrant’s Amended and Restated 2000 Employee Stock Purchase Plan
. - Filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2009 Annual Meeting of Stockholders, filed with the SEC on April 20, 2009, and incorporated herein by reference.
|
|
|
10.4
|
Form of Employee Confidential Information and Inventions Agreement.
|
|
|
10.5
|
Patent Rights Purchase Agreement between the Registrant and Gilead Sciences, Inc., dated December 18, 1998
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1998 and incorporated herein by reference.
|
|
|
10.6
|
Collaboration and License Agreement between the Registrant and Hybridon, Inc., dated May 24, 2001
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s report on Form 10-Q as amended for the quarter ended June 30, 2001 and incorporated herein by reference.
|
|
|
10.7
|
Amendment #1 to the Research, Development and License Agreement dated May 11, 2011 by and between the Registrant and Glaxo Group Limited
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2011 and incorporated herein by reference.
|
|
|
10.8
|
Amended and Restated Collaboration and License Agreement between the Registrant and Antisense Therapeutics Ltd dated February 8, 2008
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2008 and incorporated herein by reference.
|
|
|
10.9
|
Amended and Restated License Agreement between the Registrant and Atlantic Pharmaceuticals Limited dated November 30, 2009
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report as Form 10-K for the year ended December 31, 2009 and incorporated herein by reference.
|
|
10.10
|
Amended and Restated Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen MA Inc. dated October 20, 2017. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.11
|
Stock Purchase Agreement among the Registrant, Akcea Therapeutics, Inc. and Novartis Pharma AG
dated January 5, 2017. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and incorporated herein by reference.
|
|
|
10.12
|
Amendment #1 between the Registrant and Bayer AG dated February 10, 2017
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and incorporated herein by reference.
|
|
|
10.13
|
Registrant’s Amended and Restated 10b5-1 Trading Plan dated September 12, 2013
. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
|
10.14*
|
Registrant’s Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, as amended
. - Filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2014 Annual Meeting of Stockholders, filed with the SEC on April 25, 2014, and incorporated herein by reference.
|
|
10.15*
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the Ionis Pharmaceuticals, Inc. Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan
. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 and incorporated herein by reference.
|
|
|
10.16*
|
Amended and Restated Severance Agreement dated December 3, 2008 between the Registrant and Stanley T. Crooke
.
- Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 5, 2008 and incorporated herein by reference.
|
|
|
Research Collaboration, Option and License Agreement between the Registrant and Biogen MA Inc. dated December 19, 2017. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.18*
|
Ionis Pharmaceuticals, Inc. 2011 Equity Incentive Plan
- Filed as an exhibit to the Registrant’s Notice of 2011 Annual Meeting of Stockholders and Proxy Statement filed with the SEC on April 28, 2011, and incorporated herein by reference.
|
|
|
10.19*
|
Form of Option Agreement under the 2011 Equity Incentive Plan
. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.20*
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the 2011 Equity Incentive Plan
. - Filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed with the SEC on August 8, 2011, and incorporated herein by reference.
|
|
|
10.21
|
Loan Agreement between Ionis Gazelle, LLC and UBS AG dated July 18, 2017
. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.22*
|
Form of Option Agreement under the 1989 Stock Option Plan
. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.23*
|
Form of Option Agreement for Options Granted after March 8, 2005 under the 2002 Non-Employee Director’s Stock Option Plan
. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2004 and incorporated herein by reference.
|
|
|
10.24
|
Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated March 30, 2010
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010 and incorporated herein by reference.
|
|
|
10.25
|
Loan Agreement between Ionis Faraday, LLC and UBS AG
dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.26
|
Research Agreement dated August 10, 2011 between the Registrant and CHDI Foundation, Inc
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011 and incorporated herein by reference.
|
|
|
10.27
|
Guaranty between the Registrant and UBS AG
dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
10.28
|
Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated January 3, 2012
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 and incorporated herein by reference.
|
|
|
10.29
|
DMPK Research, Development, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated June 27, 2012
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 and incorporated herein by reference.
|
|
|
10.30
|
Amendment #2 to Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated October 30, 2012
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
|
10.31
|
Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated December 7, 2012
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
10.32
|
Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated December 10, 2012
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
|
10.33
|
HTT Research, Development, Option and License Agreement among the Registrant, F. Hoffmann-La Roche Ltd and Hoffman-La Roche Inc. dated April 8, 2013
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference.
|
|
|
10.34
|
Letter Agreement between the Registrant and CHDI Foundation, Inc. dated April 8, 2013
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference.
|
|
|
10.35
|
Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated September 5, 2013
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
|
10.36
|
Amendment #1 to Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated August 13, 2013
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
10.37
|
Letter Agreement Amendment between the Registrant and Biogen Idec International Holding Ltd dated January 27, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and incorporated herein by reference.
|
|
|
10.38
|
Amendment No. 3 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated July 10, 2013
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
10.39
|
Amendment #4 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated April 10, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
10.40
|
Amendment #5 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated June 27, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
10.41
|
Exclusive License Agreement between the Registrant and the University of Massachusetts dated January 14, 2010
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
|
10.42
|
Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated October 26, 2011
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
|
|
10.43
|
Amendment to Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated March 14, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
|
|
10.44
|
Amendment #1 to the Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated December 15, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference.
|
|
|
10.45
|
Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 22, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference.
|
|
|
10.46
|
Amendment No.2 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated October 15, 2014
. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference.
|
|
|
10.47
|
Strategic Collaboration Agreement between the Registrant and AstraZeneca AB dated July 31, 2015
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.48
|
Amendment #6 to Research, Development and License Agreement between the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated September 2, 2015
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.49
|
Amendment Number One to the Second Amended and Restated Strategic Collaboration and License Agreement between the Registrant and Alnylam Pharmaceuticals, Inc. dated July 13, 2015
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.50
|
License Agreement between the Registrant and Bayer Pharma AG dated May 1, 2015. Portions of this exhibit have been omitted and separately filed with the SEC
. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 and incorporated herein by reference.
|
|
10.51
|
Line of Credit Agreement between the Registrant and Morgan Stanley Private Bank, National Association dated June 16, 2015
. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 and incorporated herein by reference.
|
|
|
10.52
|
Second Amended and Restated Strategic Collaboration and License Agreement between the Registrant and Alnylam Pharmaceuticals, Inc. dated January 8, 2015
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and incorporated herein by reference.
|
|
|
10.53
|
Amendment #1 to HTT Research, Development, Option and License Agreement between the Registrant, F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. dated January 9, 2015
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and incorporated herein by reference.
|
|
|
10.54
|
Amendment No.1 to Loan Documents between the Registrant and Morgan Stanley Private Bank, National Association dated December 30, 2015
. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed January 5, 2016 and incorporated herein by reference.
|
|
|
10.55
|
Amendment No.2 to Line of Credit Agreement between the Registrant and Morgan Stanley Private Bank, National Association dated February 24, 2016
. Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2015 and incorporated herein by reference.
|
|
|
10.56
|
Amendment No.3 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated January 18, 2016
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and incorporated herein by reference.
|
|
10.57
|
Amendment #7 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated March 4, 2016
. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and incorporated herein by reference.
|
|
|
10.58
|
First Amendment to Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 21, 2016
. Portions of this exhibit have been omitted and separately filed with the SEC.
|
|
|
10.59
|
Letter Agreement between the Registrant and Biogen MA Inc. dated October 28, 2016
. Portions of this exhibit have been omitted and separately filed with the SEC. Portions of this exhibit have been omitted and separately filed with the SEC.
|
|
|
10.60
|
Guaranty between the Registrant and UBS AG dated July 18, 2017.
- Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.61
|
Environmental Indemnity Agreement among the Registrant, Ionis Gazelle, LLC and UBS AG
dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.62
|
Environmental Indemnity Agreement among the Registrant, Ionis Faraday, LLC and UBS AG
dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.63*
|
Amendment to Ionis Pharmaceuticals, Inc. 2011 Equity Incentive Plan
. - Filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2017 Annual Meeting of Stockholders, filed with the SEC on April 10, 2017, and incorporated herein by reference.
|
|
|
14.1
|
Registrant’s Code of Ethics and Business Conduct.
|
|
|
21.1
|
List of Subsidiaries for the Registrant.
|
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
24.1
|
Power of Attorney – Included on the signature page of this Annual Report on Form 10-K.
|
|
|
31.1
|
Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
31.2
|
Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.1
+
|
Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101
|
The following financial statements from the Ionis Pharmaceuticals, Inc. Annual Report on Form 10-K for the year ended December 31, 2016, formatted in Extensive Business Reporting Language (XBRL): (i) consolidated balance sheets, (ii) consolidated statements of operations, (iii) consolidated statements of stockholders’ equity, (iv) consolidated statements of cash flows, and (v) notes to consolidated financial statements (detail tagged).
|
| * |
Indicates management compensatory plans and arrangements as required to be filed as exhibits to this Report pursuant to Item 14(c).
|
| + |
This certification is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 133, as amended, or the Securities Exchange Act of 1934, as amended.
|
|
|
IONIS PHARMACEUTICALS, INC.
|
|
|
|
|
|
|
|
By:
|
/s/ STANLEY T. CROOKE
|
|
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
|
|
Chairman of the Board, President and Chief Executive Officer (Principal executive officer)
|
|
Signatures
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ STANLEY T. CROOKE
|
|
Chairman of the Board, President, and Chief Executive Officer
|
|
February 28, 2018
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
(Principal executive officer)
|
|
|
|
|
|
|
|
|
|
/s/ ELIZABETH L. HOUGEN
|
|
Senior Vice President, Finance and Chief Financial Officer
|
|
February 28, 2018
|
|
Elizabeth L. Hougen
|
|
(Principal financial and accounting officer)
|
|
|
|
/s/ B. LYNNE PARSHALL
|
|
Director and Senior Strategic Advisor
|
|
February 28, 2018
|
|
B. Lynne Parshall, J.D.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ SPENCER R. BERTHELSEN
|
|
Director
|
|
February 28, 2018
|
|
Spencer R. Berthelsen, M.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ BREAUX CASTLEMAN
|
|
Director
|
|
February 28, 2018
|
|
Breaux Castleman
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH KLEIN
|
|
Director
|
|
February 28, 2018
|
|
Joseph Klein, III
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH LOSCALZO
|
|
Director
|
|
February 28, 2018
|
|
Joseph Loscalzo, M.D., Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ FREDERICK T. MUTO
|
|
Director
|
|
February 28, 2018
|
|
Frederick T. Muto, Esq.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH H. WENDER
|
|
Director
|
|
February 28, 2018
|
|
Joseph H. Wender
|
|
|
|
|
|
|
Page
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets at December 31, 2017 and 2016
|
F-3
|
|
Consolidated Statements of Operations for the years ended December 31, 2017, 2016 and 2015
|
F-4
|
|
Consolidated Statements of Comprehensive Loss for the years ended December 31, 2017, 2016 and 2015
|
F-5
|
|
Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2017, 2016 and 2015
|
F-6
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2017, 2016 and 2015
|
F-7
|
|
Notes to Consolidated Financial Statements
|
F-9
|
|
/s/ ERNST & YOUNG LLP
|
|
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
129,630
|
$
|
84,685
|
||||
|
Short-term investments
|
893,085
|
580,538
|
||||||
|
Contracts receivable
|
62,955
|
108,043
|
||||||
|
Inventories
|
9,982
|
7,489
|
||||||
|
Other current assets
|
72,332
|
17,177
|
||||||
|
Total current assets
|
1,167,984
|
797,932
|
||||||
|
Property, plant and equipment, net
|
121,907
|
92,845
|
||||||
|
Patents, net
|
22,004
|
20,365
|
||||||
|
Deposits and other assets
|
10,129
|
1,325
|
||||||
|
Total assets
|
$
|
1,322,024
|
$
|
912,467
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
24,886
|
$
|
21,120
|
||||
|
Accrued compensation
|
25,151
|
24,186
|
||||||
|
Accrued liabilities
|
66,618
|
36,013
|
||||||
|
Current portion of long-term obligations
|
1,621
|
1,185
|
||||||
|
Current portion of deferred contract revenue
|
106,465
|
51,280
|
||||||
|
Total current liabilities
|
224,741
|
133,784
|
||||||
|
Long-term deferred contract revenue
|
72,708
|
91,198
|
||||||
|
1 percent convertible senior notes
|
533,111
|
500,511
|
||||||
|
Long-term obligations, less current portion
|
12,974
|
15,050
|
||||||
|
Long-term financing liability for leased facility
|
—
|
72,359
|
||||||
|
Long-term mortgage debt
|
59,771
|
—
|
||||||
|
Total liabilities
|
903,305
|
812,902
|
||||||
|
Stockholders’ equity:
|
||||||||
|
Common stock, $0.001 par value; 300,000,000 shares authorized, 124,976,373 and 121,636,273 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively
|
125
|
122
|
||||||
|
Additional paid-in capital
|
1,549,904
|
1,311,229
|
||||||
|
Accumulated other comprehensive income (loss)
|
(31,759
|
)
|
(30,358
|
)
|
||||
|
Accumulated deficit
|
(1,187,398
|
)
|
(1,181,428
|
)
|
||||
|
Total Ionis stockholders' equity
|
330,872
|
99,565
|
||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc.
|
87,847
|
—
|
||||||
|
Total stockholders’ equity
|
418,719
|
99,565
|
||||||
|
Total liabilities and stockholders’ equity
|
$
|
1,322,024
|
$
|
912,467
|
||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Revenue:
|
||||||||||||
|
Commercial revenue:
|
||||||||||||
|
SPINRAZA royalties
|
$
|
112,540
|
$
|
883
|
$
|
—
|
||||||
|
Licensing and other royalty revenue
|
9,519
|
19,839
|
2,343
|
|||||||||
|
Total commercial revenue
|
122,059
|
20,722
|
2,343
|
|||||||||
|
Research and development revenue under collaborative agreements
|
385,607
|
325,898
|
281,360
|
|||||||||
|
Total revenue
|
507,666
|
346,620
|
283,703
|
|||||||||
|
Expenses:
|
||||||||||||
|
Research, development and patent
|
374,644
|
344,320
|
322,292
|
|||||||||
|
Selling, general and administrative
|
108,488
|
48,616
|
37,173
|
|||||||||
|
Total operating expenses
|
483,132
|
392,936
|
359,465
|
|||||||||
|
Income (loss) from operations
|
24,534
|
(46,316
|
)
|
(75,762
|
)
|
|||||||
|
Other income (expense):
|
||||||||||||
|
Investment income
|
7,805
|
5,472
|
4,377
|
|||||||||
|
Interest expense
|
(44,752
|
)
|
(38,795
|
)
|
(36,732
|
)
|
||||||
|
Gain on investment in Regulus Therapeutics Inc.
|
374
|
—
|
20,211
|
|||||||||
|
Loss on extinguishment of financing liability for leased facility
|
(7,689
|
)
|
—
|
—
|
||||||||
|
Loss on early retirement of debt
|
—
|
(3,983
|
)
|
—
|
||||||||
|
Other expenses
|
(3,548
|
)
|
—
|
—
|
||||||||
|
Loss before income tax benefit (expense)
|
(23,276
|
)
|
(83,622
|
)
|
(87,906
|
)
|
||||||
|
Income tax benefit (expense)
|
5,980
|
(2,934
|
)
|
(372
|
)
|
|||||||
|
Net loss
|
(17,296
|
)
|
(86,556
|
)
|
(88,278
|
)
|
||||||
|
Net loss attributable to noncontrolling interest in Akcea Therapeutics, Inc.
|
11,326
|
—
|
—
|
|||||||||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(5,970
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Basic net income (loss) per share
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
||||
|
Shares used in computing basic net income (loss) per share
|
124,016
|
120,933
|
119,719
|
|||||||||
|
Diluted net income (loss) per share
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
||||
|
Shares used in computing diluted net income (loss) per share
|
126,098
|
120,933
|
119,719
|
|||||||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Net loss
|
$
|
(17,296
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Unrealized losses on investments, net of tax
|
(960
|
)
|
(17,219
|
)
|
(33,101
|
)
|
||||||
|
Reclassification adjustment for realized (gains) losses included in net loss
|
(374
|
)
|
447
|
(20,211
|
)
|
|||||||
|
Currency translation adjustment
|
(67
|
)
|
(21
|
)
|
—
|
|||||||
|
Comprehensive loss
|
(18,697
|
)
|
(103,349
|
)
|
(141,590
|
)
|
||||||
|
Comprehensive loss attributable to noncontrolling interest in Akcea Therapeutics, Inc.
|
11,421
|
—
|
—
|
|||||||||
|
Comprehensive loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(7,276
|
)
|
$
|
(103,349
|
)
|
$
|
(141,590
|
)
|
|||
|
Common Stock
|
Additional Paid in
|
Accumulated
Other
Comprehensive
|
Accumulated
|
Total Ionis
Stockholders'
|
Noncontrolling
Interest in Akcea
|
Total
Stockholders’
|
||||||||||||||||||||||||||
|
Description
|
Shares
|
Amount
|
Capital
|
Income (Loss)
|
Deficit
|
Equity
|
Therapeutics, Inc.
|
Equity
|
||||||||||||||||||||||||
|
Balance at December 31, 2014
|
118,443
|
$
|
118
|
$
|
1,224,509
|
$
|
39,747
|
$
|
(1,006,594
|
)
|
$
|
257,780
|
$
|
—
|
$
|
257,780
|
||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(88,278
|
)
|
(88,278
|
)
|
—
|
(88,278
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
(53,312
|
)
|
—
|
(53,312
|
)
|
—
|
(53,312
|
)
|
|||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,908
|
2
|
24,888
|
—
|
—
|
24,890
|
—
|
24,890
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
59,314
|
—
|
—
|
59,314
|
—
|
59,314
|
||||||||||||||||||||||||
|
Excess tax benefits from stock-based compensation awards
|
—
|
—
|
396
|
—
|
—
|
396
|
—
|
396
|
||||||||||||||||||||||||
|
Balance at December 31, 2015
|
120,351
|
$
|
120
|
$
|
1,309,107
|
$
|
(13,565
|
)
|
$
|
(1,094,872
|
)
|
$
|
200,790
|
$
|
—
|
$
|
200,790
|
|||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(86,556
|
)
|
(86,556
|
)
|
—
|
(86,556
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
(16,772
|
)
|
—
|
(16,772
|
)
|
—
|
(16,772
|
)
|
|||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
(21
|
)
|
—
|
(21
|
)
|
—
|
(21
|
)
|
|||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,285
|
2
|
13,706
|
—
|
—
|
13,708
|
—
|
13,708
|
||||||||||||||||||||||||
|
2¾ percent convertible senior notes redemption, equity portion
|
—
|
—
|
(128,888
|
)
|
—
|
—
|
(128,888
|
)
|
—
|
(128,888
|
)
|
|||||||||||||||||||||
|
1 percent convertible senior notes, equity portion, net of issuance costs
|
—
|
—
|
43,335
|
—
|
—
|
43,335
|
—
|
43,335
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
72,108
|
—
|
—
|
72,108
|
—
|
72,108
|
||||||||||||||||||||||||
|
Excess tax benefits from stock-based compensation awards
|
—
|
—
|
1,861
|
—
|
—
|
1,861
|
—
|
1,861
|
||||||||||||||||||||||||
|
Balance at December 31, 2016
|
121,636
|
$
|
122
|
$
|
1,311,229
|
$
|
(30,358
|
)
|
$
|
(1,181,428
|
)
|
$
|
99,565
|
$
|
—
|
$
|
99,565
|
|||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(5,970
|
)
|
(5,970
|
)
|
—
|
(5,970
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
(1,334
|
)
|
—
|
(1,334
|
)
|
—
|
(1,334
|
)
|
|||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
(67
|
)
|
—
|
(67
|
)
|
—
|
(67
|
)
|
|||||||||||||||||||||
|
Novartis stock purchase
|
1,631
|
2
|
71,737
|
—
|
—
|
71,739
|
—
|
71,739
|
||||||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,709
|
1
|
22,931
|
—
|
—
|
22,932
|
—
|
22,932
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
85,975
|
—
|
—
|
85,975
|
—
|
85,975
|
||||||||||||||||||||||||
|
Issuance of Akcea Therapeutics, Inc. common stock in conjunction with initial public offering
|
—
|
—
|
157,270
|
—
|
—
|
157,270
|
—
|
157,270
|
||||||||||||||||||||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc. in conjunction with initial public offering
|
—
|
—
|
(90,351
|
)
|
—
|
—
|
(90,351
|
)
|
90,381
|
30
|
||||||||||||||||||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc.
|
—
|
—
|
(8,887
|
)
|
—
|
—
|
(8,887
|
)
|
(2,534
|
)
|
(11,421
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2017
|
124,976
|
$
|
125
|
$
|
1,549,904
|
$
|
(31,759
|
)
|
$
|
(1,187,398
|
)
|
$
|
330,872
|
$
|
87,847
|
$
|
418,719
|
|||||||||||||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Operating activities:
|
||||||||||||
|
Net loss
|
$
|
(17,296
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Adjustments to reconcile net loss to net cash provided by (used in) operating activities:
|
||||||||||||
|
Depreciation
|
6,708
|
7,481
|
6,984
|
|||||||||
|
Amortization of patents
|
1,641
|
1,552
|
1,381
|
|||||||||
|
Amortization of licenses
|
—
|
—
|
1,873
|
|||||||||
|
Amortization of premium on investments, net
|
6,752
|
6,813
|
7,812
|
|||||||||
|
Amortization of debt issuance costs
|
1,616
|
1,225
|
1,133
|
|||||||||
|
Amortization of convertible senior notes discount
|
30,920
|
23,890
|
22,075
|
|||||||||
|
Amortization of long-term financing liability for leased facility
|
3,659
|
6,693
|
6,665
|
|||||||||
|
Stock-based compensation expense
|
85,975
|
72,108
|
59,314
|
|||||||||
|
Gain on investment in Regulus Therapeutics Inc.
|
(374
|
)
|
—
|
(20,211
|
)
|
|||||||
|
Loss on extinguishment of financing liability for leased facility
|
7,689
|
—
|
—
|
|||||||||
|
Loss on early retirement of debt
|
—
|
3,983
|
—
|
|||||||||
|
Non-cash losses related to patents, licensing, property, plant and equipment and strategic investments
|
3,302
|
2,297
|
1,881
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Contracts receivable
|
45,088
|
(96,687
|
)
|
(7,453
|
)
|
|||||||
|
Inventories
|
(2,493
|
)
|
(590
|
)
|
(609
|
)
|
||||||
|
Other current and long-term assets
|
(58,367
|
)
|
1,603
|
(4,394
|
)
|
|||||||
|
Long-term income tax receivable
|
(9,114
|
)
|
—
|
—
|
||||||||
|
Accounts payable
|
1,784
|
(10,677
|
)
|
9,211
|
||||||||
|
Income taxes
|
435
|
1,069
|
—
|
|||||||||
|
Accrued compensation
|
965
|
8,121
|
3,763
|
|||||||||
|
Accrued liabilities and deferred rent
|
28,564
|
4,720
|
(2,140
|
)
|
||||||||
|
Deferred contract revenue
|
36,695
|
(59,150
|
)
|
22,118
|
||||||||
|
Net cash provided by (used in) operating activities
|
174,149
|
(112,105
|
)
|
21,125
|
||||||||
|
Investing activities:
|
||||||||||||
|
Purchases of short-term investments
|
(877,810
|
)
|
(300,912
|
)
|
(493,467
|
)
|
||||||
|
Proceeds from the sale of short-term investments
|
557,369
|
364,572
|
419,584
|
|||||||||
|
Purchases of property, plant and equipment
|
(34,764
|
)
|
(7,107
|
)
|
(7,692
|
)
|
||||||
|
Acquisition of licenses and other assets, net
|
(3,093
|
)
|
(4,421
|
)
|
(4,056
|
)
|
||||||
|
Purchase of strategic investments
|
(2,500
|
)
|
—
|
—
|
||||||||
|
Proceeds from the sale of Regulus Therapeutics, Inc.
|
2,507
|
4,467
|
25,527
|
|||||||||
|
Proceeds from the sale of strategic investments
|
—
|
—
|
52
|
|||||||||
|
Net cash (used in) provided by investing activities
|
(358,291
|
)
|
56,599
|
(60,052
|
)
|
|||||||
|
Financing activities:
|
||||||||||||
|
Proceeds from equity, net
|
22,931
|
13,417
|
24,888
|
|||||||||
|
Proceeds from issuance of common stock in Akcea Therapeutics, Inc. from its initial public offering, net of underwriters' discount
|
110,438
|
—
|
—
|
|||||||||
|
Proceeds from building mortgage debt, net of issuance costs
|
59,750
|
—
|
—
|
|||||||||
|
Proceeds from the issuance of common stock to Novartis
|
71,737
|
—
|
—
|
|||||||||
|
Proceeds from borrowing on line of credit facility
|
—
|
4,000
|
8,500
|
|||||||||
|
Proceeds from the sale of Akcea Therapeutics, Inc. common stock to Novartis in a private placement
|
50,000
|
—
|
—
|
|||||||||
|
Offering costs paid
|
(2,037
|
)
|
(818
|
)
|
—
|
|||||||
|
Payment to settle financing liability for leased facility
|
(80,133
|
)
|
—
|
—
|
||||||||
|
Excess tax benefits from stock-based compensation awards
|
—
|
1,861
|
396
|
|||||||||
|
Principal payments on debt and capital lease obligations
|
(3,599
|
)
|
(7,066
|
)
|
(9,058
|
)
|
||||||
|
Net cash provided by financing activities
|
229,087
|
11,394
|
24,726
|
|||||||||
|
Net increase (decrease) in cash and cash equivalents
|
44,945
|
(44,112
|
)
|
(14,201
|
)
|
|||||||
|
Cash and cash equivalents at beginning of year
|
84,685
|
128,797
|
142,998
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
129,630
|
$
|
84,685
|
$
|
128,797
|
||||||
|
Supplemental disclosures of cash flow information:
|
2017 | 2016 | 2015 | |||||||||
|
Interest paid
|
$
|
8,035
|
$
|
7,313
|
$
|
6,800
|
||||||
|
Supplemental disclosures of non-cash investing and financing activities:
|
||||||||||||
|
Amounts accrued for capital and patent expenditures
|
$
|
1,983
|
$
|
3,439
|
$
|
1,162
|
||||||
|
1 percent convertible senior notes principal issued related to our December 2016 debt exchange
|
$
|
—
|
$
|
185,450
|
$
|
—
|
||||||
|
2¾
percent convertible senior notes principal extinguished related to our December 2016 debt exchange
|
$
|
—
|
$
|
61,099
|
$
|
—
|
||||||
|
Unpaid deferred offering costs
|
$
|
—
|
$
|
291
|
$
|
—
|
||||||
|
Year Ended December 31, 2017
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Loss
Per Share
|
Ionis’
Portion of
Akcea’s Net Loss
|
|||||||||
|
Common shares
|
20,669
|
$
|
(2.82
|
)
|
$
|
(58,332
|
)
|
|||||
|
Preferred shares
|
15,748
|
(1.55
|
)
|
(24,344
|
)
|
|||||||
|
Akcea’s net loss attributable to our ownership
|
$
|
(82,676
|
)
|
|||||||||
|
Ionis’ stand-alone net income
|
92,336
|
|||||||||||
|
Net income available to Ionis common stockholders
|
$
|
9,661
|
||||||||||
|
Weighted average shares outstanding
|
124,016
|
|||||||||||
|
Basic net income per share
|
$
|
0.08
|
||||||||||
|
Year Ended December 31, 2017
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Net income available to Ionis common stockholders
|
$
|
9,661
|
124,016
|
$
|
0.08
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
1,619
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
459
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
4
|
||||||||||
|
Income available to Ionis common stockholders, plus assumed conversions
|
$
|
9,661
|
126,098
|
$
|
0.08
|
|||||||
| |
percent convertible senior notes;
|
| |
2¾ percent convertible senior notes;
|
| |
Dilutive stock options;
|
| |
Unvested restricted stock units; and
|
| |
Employee Stock Purchase Plan, or ESPP.
|
| ● |
The exclusive license we granted to Bayer to develop and commercialize IONIS-FXI-L
Rx
for the treatment of thrombosis;
|
| ● |
The development services we agreed to perform for IONIS-FXI-L
Rx
and IONIS-FXI
Rx
; and
|
| ● |
The remaining undelivered IONIS-FXI
Rx
API that was part of the original agreement.
|
| ● |
Estimated future product sales;
|
| ● |
Estimated royalties on future product sales;
|
| ● |
Contractual milestone payments;
|
| ● |
Expenses we expect to incur;
|
| ● |
Income taxes; and
|
| ● |
An appropriate discount rate.
|
| ● |
The number of internal hours we will spend performing these services;
|
| ● |
The estimated cost of work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
| ● |
$64.9 million to the IONIS-FXI-L
Rx
exclusive license;
|
| ● |
$11.0 million for development services for IONIS-FXI-L
Rx
and IONIS-FXI
Rx
; and
|
| ● |
$0.4
million for the remaining delivery of
IONIS-FXI
Rx
API.
|
| ● |
We recognized the portion of the consideration attributed to the IONIS-FXI-L
Rx
license in the first quarter of 2017 because we delivered the license and earned the revenue;
|
| ● |
We are recognizing the amount attributed to the development services for IONIS-FXI-L
Rx
and IONIS-FXI
Rx
over the period of time we are performing the services; and
|
| ● |
We are recognizing the amount attributed to the remaining API supply as we deliver it to Bayer.
|
| ● |
Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete.
|
| ● |
Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete.
|
| ● |
Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete.
|
| ● |
Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
|
| ● |
Filing of regulatory applications for marketing authorization such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
|
| ● |
Obtaining marketing authorization in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain authorization from the applicable regulatory agency.
|
| ● |
First commercial sale in a particular market, such as in the United States or Europe.
|
| ● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
|
| ● |
Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
|
| ● |
The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on our performance or the occurrence of a specific outcome resulting from our performance;
|
| ● |
The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
|
| ● |
There is no future performance required to earn the milestone; and
|
| ● |
The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
|
|
Years Ending December 31,
|
Amortization
(in millions)
|
|||
|
2018
|
$
|
1.6
|
||
|
2019
|
$
|
1.4
|
||
|
2020
|
$
|
1.3
|
||
|
2021
|
$
|
1.3
|
||
|
2022
|
$
|
1.2
|
||
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Clinical expenses
|
$
|
16,347
|
$
|
23,428
|
||||
|
In-licensing expenses
|
33,790
|
6,430
|
||||||
|
Other miscellaneous expenses
|
16,481
|
6,155
|
||||||
|
Total accrued liabilities
|
$
|
66,618
|
$
|
36,013
|
||||
|
Estimated Useful Lives
|
December 31,
|
|||||||||||
|
(in years)
|
2017
|
2016
|
||||||||||
|
Computer software, laboratory, manufacturing and other equipment
|
3 to 10
|
$
|
66,558
|
$
|
63,287
|
|||||||
|
Building, building improvements and building systems
|
15 to 40
|
92,770
|
48,909
|
|||||||||
|
Land improvements
|
20 |
2,853
|
2,853
|
|||||||||
|
Leasehold improvements
|
5 to 15
|
26,748
|
41,736
|
|||||||||
|
Furniture and fixtures
|
5 to 10
|
6,161
|
5,937
|
|||||||||
|
195,090
|
162,722
|
|||||||||||
|
Less accumulated depreciation
|
(87,676
|
)
|
(80,075
|
)
|
||||||||
|
107,414
|
82,647
|
|||||||||||
|
Land
|
14,493
|
10,198
|
||||||||||
|
Total
|
$
|
121,907
|
$
|
92,845
|
||||||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Beginning balance accumulated other comprehensive (loss) income
|
$
|
(30,358
|
)
|
$
|
(13,565
|
)
|
$
|
39,747
|
||||
|
Unrealized losses on securities, net of tax (1)
|
(960
|
)
|
(17,219
|
)
|
(33,101
|
)
|
||||||
|
Amounts reclassified from accumulated other comprehensive (loss) income (2)
|
(374
|
)
|
447
|
(20,211
|
)
|
|||||||
|
Currency translation adjustment
|
(67
|
)
|
(21
|
)
|
—
|
|||||||
|
Net other comprehensive loss for the period
|
(1,401
|
)
|
(16,793
|
)
|
(53,312
|
)
|
||||||
|
Ending balance accumulated other comprehensive loss
|
$
|
(31,759
|
)
|
$
|
(30,358
|
)
|
$
|
(13,565
|
)
|
|||
| (1) |
There was no tax expense for other comprehensive loss for the years ended December 31, 2017, 2016 or 2015.
|
| (2) |
Amounts for 2015 and 2017 are included in the separate line called “Gain on investment in Regulus Therapeutics Inc.” on our Consolidated Statement of Operations.
|
|
At
December 31, 2017
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
||||||||||
|
Cash equivalents (1)
|
$
|
86,262
|
$
|
86,262
|
$
|
—
|
||||||
|
Corporate debt securities (2)
|
647,461
|
—
|
647,461
|
|||||||||
|
Debt securities issued by U.S. government agencies (3)
|
136,325
|
—
|
136,325
|
|||||||||
|
Debt securities issued by the U.S. Treasury (3)
|
30,818
|
30,818
|
—
|
|||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (4)
|
93,932
|
—
|
93,932
|
|||||||||
|
Total
|
$
|
994,798
|
$
|
117,080
|
$
|
877,718
|
||||||
|
At
December 31, 2016
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
||||||||||
|
Cash equivalents (1)
|
$
|
54,137
|
$
|
54,137
|
$
|
—
|
||||||
|
Corporate debt securities (3)
|
396,221
|
—
|
396,221
|
|||||||||
|
Debt securities issued by U.S. government agencies (3)
|
55,179
|
—
|
55,179
|
|||||||||
|
Debt securities issued by the U.S. Treasury (3)
|
29,286
|
29,286
|
—
|
|||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (5)
|
109,111
|
—
|
109,111
|
|||||||||
|
Investment in Regulus Therapeutics Inc.
|
2,414
|
2,414
|
—
|
|||||||||
|
Total
|
$
|
646,348
|
$
|
85,837
|
$
|
560,511
|
||||||
| (1) |
Included in cash and cash equivalents on our consolidated balance sheet.
|
| (2) |
$11.9 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet.
|
| (3) |
Included in short-term investments on our consolidated balance sheet.
|
| (4) |
$3.5 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet.
|
| (5) |
$9.3 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet.
|
|
Year Ended
December 31, 2017
|
||||
|
Beginning balance of Level 3 instruments
|
$
|
—
|
||
|
Value of the potential premium we will receive from Novartis at inception of the SPA (January 2017)
|
5,035
|
|||
|
Write-off of premium to other expenses
|
(5,035
|
)
|
||
|
Ending balance of Level 3 instruments
|
$
|
—
|
||
|
One year or less
|
71
|
%
|
||
|
After one year but within two years
|
23
|
%
|
||
|
After two years but within three and one half years
|
6
|
%
|
||
|
Total
|
100
|
%
|
|
Gross Unrealized
|
Estimated
|
|||||||||||||||
|
December 31, 2017
|
Cost
(1)
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||
|
Corporate debt securities (2)
|
$
|
500,599
|
$
|
2
|
$
|
(752
|
)
|
$
|
499,849
|
|||||||
|
Debt securities issued by U.S. government agencies
|
83,926
|
—
|
(212
|
)
|
83,714
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
29,428
|
—
|
(17
|
)
|
29,411
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (2)
|
29,240
|
4
|
(122
|
)
|
29,122
|
|||||||||||
|
Total securities with a maturity of one year or less
|
643,193
|
6
|
(1,103
|
)
|
642,096
|
|||||||||||
|
Corporate debt securities
|
148,663
|
8
|
(1,059
|
)
|
147,612
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
52,779
|
—
|
(168
|
)
|
52,611
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
1,409
|
—
|
(2
|
)
|
1,407
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
65,550
|
—
|
(740
|
)
|
64,810
|
|||||||||||
|
Total securities with a maturity of more than one year
|
268,401
|
8
|
(1,969
|
)
|
266,440
|
|||||||||||
|
Total available-for-sale securities
|
$
|
911,594
|
$
|
14
|
$
|
(3,072
|
)
|
$
|
908,536
|
|||||||
|
Gross Unrealized
|
Estimated
|
|||||||||||||||
|
December 31, 2016
|
Cost
(1)
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||
|
Corporate debt securities
|
$
|
195,087
|
$
|
25
|
$
|
(161
|
)
|
$
|
194,951
|
|||||||
|
Debt securities issued by U.S. government agencies
|
26,548
|
—
|
(10
|
)
|
26,538
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
29,298
|
2
|
(14
|
)
|
29,286
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (2)
|
72,775
|
2
|
(134
|
)
|
72,643
|
|||||||||||
|
Total securities with a maturity of one year or less
|
323,708
|
29
|
(319
|
)
|
323,418
|
|||||||||||
|
Corporate debt securities
|
202,408
|
36
|
(1,174
|
)
|
201,270
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
28,807
|
1
|
(167
|
)
|
28,641
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
36,816
|
1
|
(349
|
)
|
36,468
|
|||||||||||
|
Total securities with a maturity of more than one year
|
268,031
|
38
|
(1,690
|
)
|
266,379
|
|||||||||||
|
Total available-for-sale securities
|
$
|
591,739
|
$
|
67
|
$
|
(2,009
|
)
|
$
|
589,797
|
|||||||
|
Equity securities:
|
||||||||||||||||
|
Regulus Therapeutics Inc.
|
$
|
2,133
|
$
|
281
|
$
|
—
|
$
|
2,414
|
||||||||
|
Total equity securities
|
$
|
2,133
|
$
|
281
|
$
|
—
|
$
|
2,414
|
||||||||
|
Total available-for-sale and equity securities
|
$
|
593,872
|
$
|
348
|
$
|
(2,009
|
)
|
$
|
592,211
|
|||||||
| (1) |
Our available-for-sale securities are held at amortized cost.
|
| (2) |
Includes investments classified as cash equivalents on our consolidated balance sheet.
|
|
Less than 12 Months of
Temporary Impairment
|
More than 12 Months of
Temporary Impairment
|
Total Temporary
Impairment
|
||||||||||||||||||||||||||
|
Number of
Investments
|
Estimated
Fair Value
|
Unrealized
Losses
|
Estimated
Fair Value
|
Unrealized
Losses
|
Estimated
Fair Value
|
Unrealized
Losses
|
||||||||||||||||||||||
|
Corporate debt securities
|
476
|
$
|
551,446
|
$
|
(1,236
|
)
|
$
|
74,987
|
$
|
(575
|
)
|
$
|
626,433
|
$
|
(1,811
|
)
|
||||||||||||
|
Debt securities issued by U.S. government agencies
|
45
|
107,788
|
(262
|
)
|
27,538
|
(118
|
)
|
135,326
|
(380
|
)
|
||||||||||||||||||
|
Debt securities issued by the U.S. Treasury
|
7
|
30,818
|
(19
|
)
|
—
|
—
|
30,818
|
(19
|
)
|
|||||||||||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
60
|
62,519
|
(545
|
)
|
24,572
|
(317
|
)
|
87,091
|
(862
|
)
|
||||||||||||||||||
|
Total temporarily impaired securities
|
588
|
$
|
752,571
|
$
|
(2,062
|
)
|
$
|
127,097
|
$
|
(1,010
|
)
|
$
|
879,668
|
$
|
(3,072
|
)
|
||||||||||||
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
1 percent convertible senior notes
|
$
|
533,111
|
$
|
500,511
|
||||
|
Long-term mortgage debt
|
59,771
|
—
|
||||||
|
Long-term financing liability for leased facility
|
—
|
72,359
|
||||||
|
Principal balance of fixed rate note with Morgan Stanley
|
12,500
|
12,500
|
||||||
|
Leases and other obligations
|
2,095
|
3,735
|
||||||
|
Total
|
$
|
607,477
|
$
|
589,105
|
||||
|
Less: current portion
|
(1,621
|
)
|
(1,185
|
)
|
||||
|
Total Long-Term Obligations
|
$
|
605,856
|
$
|
587,920
|
||||
|
1 Percent
Convertible Senior Notes
|
||||
|
Outstanding balance
|
$
|
685.5
|
||
|
Original issue date ($500 million of principal)
|
November 2014
|
|||
|
Additional issue date ($185.5 million of principal)
|
December 2016
|
|||
|
Maturity date
|
November 2021
|
|||
|
Interest rate
|
1 percent
|
|||
|
Conversion price per share
|
$
|
66.81
|
||
|
Total shares of common stock subject to conversion
|
10.3
|
|||
|
1 Percent
Convertible Senior Notes
Issued in November 2014
|
1 Percent
Convertible Senior Notes
Issued in December 2016
|
||
|
Nonconvertible debt borrowing rate
|
7.4 percent
|
6.8 percent
|
|
|
Effective interest rate
|
7.8 percent
|
7.2 percent
|
|
|
Amortization period of debt discount
|
7 years
|
5 years
|
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Fair value of outstanding notes
|
$
|
727,420
|
$
|
700,969
|
||||
|
Principal amount of convertible notes outstanding
|
$
|
685,450
|
$
|
685,450
|
||||
|
Unamortized portion of debt discount
|
$
|
144,112
|
$
|
175,699
|
||||
|
Long-term debt
|
$
|
533,111
|
$
|
500,511
|
||||
|
Carrying value of equity component
|
$
|
219,011
|
$
|
219,011
|
||||
|
(i)
|
a floating rate equal to the one-month London Interbank Offered Rate, or LIBOR, in effect plus 1.25 percent per annum;
|
|
(ii)
|
a fixed rate equal to LIBOR plus 1.25 percent for a period of one, two, three, four, six, or twelve months as elected by us; or
|
|
(iii)
|
a fixed rate equal to the LIBOR swap rate during the period of the loan.
|
|
2018
|
$
|
9,617
|
||
|
2019
|
22,082
|
|||
|
2020
|
9,330
|
|||
|
2021
|
694,774
|
|||
|
2022
|
2,809
|
|||
|
Thereafter
|
71,603
|
|||
|
Subtotal
|
$
|
810,215
|
||
|
Less: current portion
|
(53
|
)
|
||
|
Less: fixed and determinable interest
|
(51,465
|
)
|
||
|
Less: unamortized portion of debt discount
|
(144,791
|
)
|
||
|
Plus: Deferred rent
|
165
|
|||
|
Total
|
$
|
614,071
|
|
Operating
Leases
|
||||
|
2018
|
$
|
864
|
||
|
2019
|
636
|
|||
|
2020
|
477
|
|||
|
2021
|
147
|
|||
|
Total minimum payments
|
$
|
2,124
|
||
| |
arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring entity (or its parent company);
|
| |
arrange for the assignment of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award to the surviving or acquiring corporation (or its parent company);
|
| |
accelerate the vesting and exercisability of a stock award followed by the termination of the stock award;
|
| |
arrange for the lapse of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award;
|
| |
cancel or arrange for the cancellation of a stock award, to the extent not vested or not exercised prior to the effective date of the corporate transaction, in exchange for cash consideration, if any, as the Board, in its sole discretion, may consider appropriate; and
|
| |
arrange for the surrender of a stock award in exchange for a payment equal to the excess of (a) the value of the property the holder of the stock award would have received upon the exercise of the stock award, over (b) any exercise price payable by such holder in connection with such exercise.
|
|
Number of
Shares
|
Weighted
Average Exercise
Price Per Share
|
Average
Remaining
Contractual Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding at December 31, 2016
|
9,178
|
$
|
40.48
|
|||||||||||||
|
Granted
|
3,274
|
$
|
47.76
|
|||||||||||||
|
Exercised
|
(1,345
|
)
|
$
|
15.74
|
||||||||||||
|
Cancelled/forfeited/expired
|
(1,710
|
)
|
$
|
51.71
|
||||||||||||
|
Outstanding at December 31, 2017
|
9,397
|
$
|
44.52
|
4.42
|
$
|
92,288
|
||||||||||
|
Exercisable at December 31, 2017
|
5,182
|
$
|
39.10
|
3.33
|
$
|
80,167
|
||||||||||
|
Number of
Shares
|
Weighted
Average
Grant Date
Fair Value
Per Share
|
|||||||
|
Non-vested at December 31, 2016
|
778
|
$
|
47.68
|
|||||
|
Granted
|
420
|
$
|
48.88
|
|||||
|
Vested
|
(296
|
)
|
$
|
43.79
|
||||
|
Cancelled/forfeited
|
(39
|
)
|
$
|
48.90
|
||||
|
Non-vested at December 31, 2017
|
863
|
$
|
49.55
|
|||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Research, development and patent
|
$
|
64,521
|
$
|
55,099
|
$
|
43,638
|
||||||
|
Selling, general and administrative
|
21,454
|
17,009
|
15,676
|
|||||||||
|
Total
|
$
|
85,975
|
$
|
72,108
|
$
|
59,314
|
||||||
|
December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Risk-free interest rate
|
1.8
|
%
|
1.5
|
%
|
1.5
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
65.9
|
%
|
58.7
|
%
|
53.8
|
%
|
||||||
|
Expected life
|
4.5 years
|
4.5 years
|
4.5 years
|
|||||||||
|
December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Risk-free interest rate
|
2.2
|
%
|
1.3
|
%
|
2.1
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
61.2
|
%
|
53.1
|
%
|
52.2
|
%
|
||||||
|
Expected life
|
6.6 years
|
6.5 years
|
6.9 years
|
|||||||||
|
December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Risk-free interest rate
|
0.8
|
%
|
0.4
|
%
|
0.1
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
59.9
|
%
|
86.4
|
%
|
51.7
|
%
|
||||||
|
Expected life
|
6 months
|
6 months
|
6 months
|
|||||||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
United States
|
$
|
(11,802
|
)
|
$
|
(83,622
|
)
|
$
|
(87,906
|
)
|
|||
|
Foreign
|
(11,474
|
)
|
—
|
—
|
||||||||
|
Loss before income tax (benefit) expense
|
$
|
(23,276
|
)
|
$
|
(83,622
|
)
|
$
|
(87,906
|
)
|
|||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
(7,460
|
)
|
$
|
1,067
|
$
|
379
|
|||||
|
State
|
1,246
|
1,867
|
(7
|
)
|
||||||||
|
Foreign
|
234
|
—
|
—
|
|||||||||
|
Total current income tax (benefit) expense
|
(5,980
|
)
|
2,934
|
372
|
||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
—
|
—
|
—
|
|||||||||
|
State
|
—
|
—
|
—
|
|||||||||
|
Total deferred income tax (benefit) expense
|
—
|
—
|
—
|
|||||||||
|
Total income tax (benefit) expense
|
$
|
(5,980
|
)
|
$
|
2,934
|
$
|
372
|
|||||
|
Years Ended December 31,
|
||||||||||||||||||||||||
|
2017
|
2016
|
2015
|
||||||||||||||||||||||
|
Pre-tax loss
|
$
|
(23,276
|
)
|
$
|
(83,622
|
)
|
$
|
(87,906
|
)
|
|||||||||||||||
|
Statutory rate
|
(8,147
|
)
|
35.0
|
%
|
(29,268
|
)
|
35.0
|
%
|
(30,767
|
)
|
35.0
|
%
|
||||||||||||
|
State income tax net of federal benefit
|
722
|
(3.1
|
)%
|
(276
|
)
|
0.3
|
%
|
1
|
0.0
|
%
|
||||||||||||||
|
Foreign
|
4,299
|
(18.3
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Net change in valuation allowance
|
(76,409
|
)
|
328.3
|
%
|
55,927
|
(66.9
|
)%
|
69,499
|
(79.1
|
)%
|
||||||||||||||
|
Net operating loss expiration
|
3,987
|
(17.0
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Tax credits
|
(32,769
|
)
|
140.8
|
%
|
(26,954
|
)
|
32.2
|
%
|
(41,284
|
)
|
47.0
|
%
|
||||||||||||
|
Deferred tax true-up
|
4,848
|
(20.6
|
)%
|
2,591
|
(3.1
|
)%
|
1,496
|
(1.7
|
)%
|
|||||||||||||||
|
Tax Cuts and Jobs Act
|
107,323
|
(461.1
|
)%
|
—
|
—
|
0.0
|
%
|
|||||||||||||||||
|
Nondeductible items
|
4,123
|
(17.9
|
)%
|
1,149
|
(1.4
|
)%
|
1,055
|
(1.2
|
)%
|
|||||||||||||||
|
Akcea deconsolidation adjustment at IPO
|
469
|
(2.0
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Excess stock-based compensation
|
(14,337
|
)
|
61.0
|
%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
||||||||||||||
|
Other
|
(89
|
)
|
0.6
|
%
|
(235
|
)
|
0.4
|
%
|
372
|
(0.4
|
)%
|
|||||||||||||
|
Effective rate
|
$
|
(5,980
|
)
|
25.7
|
%
|
$
|
2,934
|
(3.5
|
)%
|
$
|
372
|
(0.4
|
)%
|
|||||||||||
|
Years Ended December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Deferred Tax Assets:
|
||||||||
|
Net operating loss carryovers
|
$
|
153,575
|
$
|
194,372
|
||||
|
R&D credits
|
240,290
|
193,845
|
||||||
|
Deferred revenue
|
42,055
|
54,203
|
||||||
|
Stock-based compensation
|
40,090
|
48,209
|
||||||
|
Intangible and capital assets
|
672
|
—
|
||||||
|
Other
|
12,164
|
26,228
|
||||||
|
Total deferred tax assets
|
$
|
488,846
|
$
|
516,857
|
||||
|
Deferred Tax Liabilities:
|
||||||||
|
Convertible debt
|
$
|
(32,391
|
)
|
$
|
(62,669
|
)
|
||
|
Intangible and capital assets
|
—
|
(2,030
|
)
|
|||||
|
Net deferred tax asset
|
$
|
456,455
|
$
|
452,158
|
||||
|
Valuation allowance
|
(456,455
|
)
|
(452,158
|
)
|
||||
|
Total net deferred tax assets and liabilities
|
$
|
—
|
$
|
—
|
||||
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Beginning balance of unrecognized tax benefits
|
$
|
66,999
|
$
|
51,257
|
$
|
27,365
|
||||||
|
Settlement of prior period tax positions
|
—
|
(4,033
|
)
|
—
|
||||||||
|
Increase for prior period tax positions
|
1,520
|
7,928
|
215
|
|||||||||
|
Increase for current period tax positions
|
9,495
|
11,847
|
23,677
|
|||||||||
|
Ending balance of unrecognized tax benefits
|
$
|
78,014
|
$
|
66,999
|
$
|
51,257
|
||||||
| ● |
AstraZeneca may terminate the agreement or any program at any time by providing written notice to us;
|
| ● |
AstraZeneca may terminate the agreement or any program by providing written notice if we undergo a change of control with a third party; and
|
| ● |
Either we or AstraZeneca may terminate the agreement or any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
| ● |
Biogen may terminate the agreement or any program at any time by providing written notice to us;
|
| ● |
Under specific circumstances, if we are acquired by a third party with a product that directly competes with a compound being developed under the agreement, Biogen may terminate the affected program by providing written notice to us;
|
| ● |
If, within a specified period of time, any required clearance of a transaction contemplated by an agreement under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, is not received, then either we or Biogen may terminate the affected program by providing written notice to the other party; and
|
| ● |
Either we or Biogen may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent.
|
| ● |
Bayer may terminate the agreement or any program at any time by providing written notice to us;
|
| ● |
Either we or Bayer may terminate the agreement or any program by providing written notice to the other party upon the other party’s uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
| ● |
GSK may terminate any program, at any time by providing written notice to us; and
|
| ● |
Either we or GSK may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent.
|
| ● |
Janssen may terminate the agreement or any program at any time by providing written notice to us; and
|
| ● |
Either we or Janssen may terminate any program by providing written notice to the other party upon the other party’s uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
| ● |
Novartis may terminate the agreement as a whole or with respect to any drug at any time by providing written notice to us;
|
| ● |
Either we or Novartis may terminate the agreement with respect to any drug by providing written notice to the other party in good faith that we or Novartis has determined that the continued development or commercialization of the drug presents safety concerns that pose an unacceptable risk or threat of harm in humans or would violate any applicable law, ethical principles, or principles of scientific integrity;
|
| ● |
Either we or Novartis may terminate the agreement for a drug by providing written notice to the other party upon the other party’s uncured failure to perform a material obligation related to the drug under the agreement, or the entire agreement if the other party becomes insolvent; and
|
| ● |
We may terminate the agreement if Novartis disputes or assists a third party to dispute the validity of any of our patents.
|
| ● |
Development services for AKCEA-APO(a)-L
Rx
;
|
| ● |
Development services for AKCEA-APOCIII-L
Rx
;
|
| ● |
API for AKCEA-APO(a)-L
Rx
; and
|
| ● |
API for AKCEA-APOCIII-L
Rx
.
|
| ● |
$75 million from the upfront payment;
|
| ● |
$100 million from our common stock Novartis purchased under the SPA, including $28.4 million for the premium paid by Novartis for its purchase of our common stock at a premium in the first quarter of 2017; and
|
| ● |
$5.0 million for the potential premium Novartis would have paid if they purchased our common stock in the future.
|
| ● |
$64.0 million for the development services for
AKCEA-APO(a)-L
Rx
;
|
| ● |
$40.1 million for the development services for
AKCEA-APOCIII-L
Rx
;
|
| ● |
$1.5
million for the delivery of AKCEA-APO(a)-L
Rx
API; and
|
| ● |
$2.8
million for the delivery of AKCEA-APOCIII-L
Rx
API.
|
| ● |
Roche may terminate the agreement at any time by providing written notice to us; and
|
| ● |
Either we or Roche may terminate the agreement by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement or if the other party becomes insolvent.
|
|
2017
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
Revenue:
|
||||||||||||||||
|
Commercial revenue:
|
||||||||||||||||
|
SPINRAZA royalties
|
$
|
112,540
|
$
|
—
|
$
|
—
|
$
|
112,540
|
||||||||
|
Licensing and other royalty revenue
|
9,519
|
—
|
—
|
9,519
|
||||||||||||
|
Total commercial revenue
|
122,059
|
—
|
—
|
122,059
|
||||||||||||
|
R&D revenue under collaborative agreements
|
384,805
|
55,209
|
(54,407
|
)
|
385,607
|
|||||||||||
|
Total segment revenue
|
$
|
506,864
|
$
|
55,209
|
$
|
(54,407
|
)
|
$
|
507,666
|
|||||||
|
Total operating expenses
|
$
|
373,788
|
$
|
163,871
|
$
|
(54,527
|
)
|
$
|
483,132
|
|||||||
|
Income (loss) from operations
|
$
|
133,076
|
$
|
(108,662
|
)
|
$
|
120
|
$
|
24,534
|
|||||||
|
2016
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
Revenue:
|
||||||||||||||||
|
Commercial revenue:
|
||||||||||||||||
|
SPINRAZA royalties
|
$
|
883
|
$
|
—
|
$
|
—
|
$
|
883
|
||||||||
|
Licensing and other royalty revenue
|
19,839
|
—
|
—
|
19,839
|
||||||||||||
|
Total commercial revenue
|
20,722
|
—
|
—
|
20,722
|
||||||||||||
|
R&D revenue under collaborative agreements
|
338,546
|
—
|
(12,648
|
)
|
325,898
|
|||||||||||
|
Total segment revenue
|
$
|
359,268
|
$
|
—
|
$
|
(12,648
|
)
|
$
|
346,620
|
|||||||
|
Total operating expenses
|
$
|
322,192
|
$
|
83,512
|
$
|
(12,768
|
)
|
$
|
392,936
|
|||||||
|
Income (loss) from operations
|
$
|
37,076
|
$
|
(83,512
|
)
|
$
|
120
|
$
|
(46,316
|
)
|
||||||
|
2015
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
Revenue:
|
||||||||||||||||
|
R&D revenue under collaborative agreements
|
$
|
284,015
|
$
|
—
|
$
|
(2,655
|
)
|
$
|
281,360
|
|||||||
|
Licensing and other royalty revenue
|
2,343
|
—
|
—
|
2,343
|
||||||||||||
|
Total segment revenue
|
$
|
286,358
|
$
|
—
|
$
|
(2,655
|
)
|
$
|
283,703
|
|||||||
|
Total operating expenses
|
$
|
309,492
|
$
|
52,748
|
$
|
(2,775
|
)
|
$
|
359,465
|
|||||||
|
Income (loss) from operations
|
$
|
(23,134
|
)
|
$
|
(52,748
|
)
|
$
|
120
|
$
|
(75,762
|
)
|
|||||
|
Total Assets
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
December 31, 2017
|
$
|
1,341,828
|
$
|
268,804
|
$
|
(288,608
|
)
|
$
|
1,322,024
|
|||||||
|
December 31, 2016
|
$
|
1,067,770
|
$
|
10,684
|
$
|
(165,987
|
)
|
$
|
912,467
|
|||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Partner A
|
51
|
%
|
60
|
%
|
37
|
%
|
||||||
|
Partner B
|
14
|
%
|
2
|
%
|
33
|
%
|
||||||
|
Partner C
|
10
|
%
|
2
|
%
|
11
|
%
|
||||||
|
Partner D
|
11
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Partner E
|
3
|
%
|
19
|
%
|
2
|
%
|
||||||
|
Partner F
|
2
|
%
|
4
|
%
|
12
|
%
|
||||||
|
2017 Quarters
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Revenue
|
$
|
110,304
|
$
|
104,152
|
$
|
120,911
|
$
|
172,299
|
||||||||
|
Operating expenses
|
$
|
96,315
|
$
|
105,823
|
$
|
107,002
|
$
|
173,992
|
||||||||
|
Income (loss) from operations
|
$
|
13,989
|
$
|
(1,671
|
)
|
$
|
13,909
|
$
|
(1,693
|
)
|
||||||
|
Net income (loss)
|
$
|
3,468
|
$
|
(11,206
|
)
|
$
|
(4,896
|
)
|
$
|
(4,662
|
)
|
|||||
|
Net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
3,468
|
$
|
(11,206
|
)
|
$
|
(976
|
)
|
$
|
2,744
|
||||||
|
Basic net income (loss) per share (1) (2)
|
$
|
0.03
|
$
|
(0.09
|
)
|
$
|
0.00
|
$
|
0.02
|
|||||||
|
Diluted net income (loss) per share (1) (3)
|
$
|
0.03
|
$
|
(0.09
|
)
|
$
|
0.00
|
$
|
0.02
|
|||||||
|
2016 Quarters
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Revenue
|
$
|
36,874
|
$
|
38,470
|
$
|
110,927
|
$
|
160,349
|
||||||||
|
Operating expenses
|
$
|
91,526
|
$
|
87,397
|
$
|
94,819
|
$
|
119,194
|
||||||||
|
Income (loss) from operations
|
$
|
(54,652
|
)
|
$
|
(48,927
|
)
|
$
|
16,108
|
$
|
41,155
|
||||||
|
Net income (loss)
|
$
|
(62,917
|
)
|
$
|
(56,855
|
)
|
$
|
7,351
|
$
|
25,865
|
||||||
|
Basic net income (loss) per share (1)
|
$
|
(0.52
|
)
|
$
|
(0.47
|
)
|
$
|
0.06
|
$
|
0.21
|
||||||
|
Diluted net income (loss) per share (1) (4) (5)
|
$
|
(0.52
|
)
|
$
|
(0.47
|
)
|
$
|
0.06
|
$
|
0.21
|
||||||
| (1) |
We computed net income (loss) per share independently for each of the quarters presented. Therefore, the sum of the quarterly net income (loss) per share will not necessarily equal the total for the year.
|
| (2) |
As discussed in Note 1,
Organization and Significant Accounting Policies,
we compute basic net income (loss) per share by dividing the total net income (loss) attributable to our common stockholders by our weighted-average number of common shares outstanding during the period. The calculation of total net income (loss) attributable to our common stockholders for the three months ended December 31, 2017 and September 30, 2017 considered our net income for Ionis on a stand-alone basis plus our share of Akcea’s net loss for the periods. To calculate the portion of Akcea’s net loss attributable to our ownership, we multiplied Akcea’s income (loss) per share by the weighted average shares we owned in Akcea during the period.
|
|
Three Months Ended December 31, 2017
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Loss
Per Share
|
Ionis’
Portion of
Akcea’s Net Loss
|
|||||||||
|
Common shares
|
45,448
|
$
|
(0.35
|
)
|
$
|
(15,955
|
)
|
|||||
|
Akcea’s net loss attributable to our ownership
|
$
|
(15,955
|
)
|
|||||||||
|
Ionis’ stand-alone net income
|
18,672
|
|||||||||||
|
Net income available to Ionis common stockholders
|
$
|
2,717
|
||||||||||
|
Weighted average shares outstanding
|
124,818
|
|||||||||||
|
Basic net income per share
|
$
|
0.02
|
||||||||||
|
Three Months Ended December 31, 2017
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Income available to common shareholders
|
$
|
2,717
|
124,818
|
$
|
0.02
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
1,532
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
507
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
5
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
2,717
|
126,862
|
$
|
0.02
|
|||||||
|
Three Months Ended September 30, 2017
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Income (Loss)
Per Share
|
Ionis’
Portion of
Akcea’s Net Loss
|
|||||||||
|
Common shares
|
36,556
|
$
|
(0.27
|
)
|
$
|
(9,870
|
)
|
|||||
|
Preferred shares
|
5,651
|
0.05
|
283
|
|||||||||
|
Akcea’s net loss attributable to our ownership
|
$
|
(9,587
|
)
|
|||||||||
|
Ionis’ stand-alone net income
|
9,168
|
|||||||||||
|
Net loss available to Ionis common stockholders
|
$
|
(419
|
)
|
|||||||||
|
Weighted average shares outstanding
|
124,370
|
|||||||||||
|
Basic net income per share
|
$
|
0.00
|
||||||||||
| (3) |
For the three months ended March 31, 2017 we had net income. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended March 31, 2017 consisted of the following (in thousands):
|
|
Three Months Ended March 31, 2017
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Income available to common shareholders
|
$
|
3,468
|
122,861
|
$
|
0.03
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
1,674
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
377
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
60
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
3,468
|
124,972
|
$
|
0.03
|
|||||||
| (4) |
For the three months ended December 31, 2016, we had net income. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended December 31, 2016 consisted of the following (in thousands):
|
|
Three Months Ended December 31, 2016
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
|
||||||||||||
|
Income available to common shareholders
|
$
|
25,865
|
121,340
|
$
|
0.21
|
|||||||
|
Effect of diluted securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
2,189
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
403
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
21
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
25,865
|
123,953
|
$
|
0.21
|
|||||||
| (5) |
For the three months ended September 30, 2016, we had net income. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended September 30, 2016 consisted of the following (in thousands):
|
|
Three Months Ended September 30, 2016
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
|
||||||||||||
|
Income available to common shareholders
|
$
|
7,351
|
120,989
|
$
|
0.06
|
|||||||
|
Effect of diluted securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
2,129
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
202
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
58
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
7,351
|
123,378
|
$
|
0.06
|
|||||||