|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Nevada
|
75-3254381
|
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(I.R.S. Employer
Identification No.)
|
|
21900 Burbank Blvd, Third Floor, Woodland Hills
|
91367
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
Large accelerated filer
¨
|
Accelerated filer
¨
|
|
Non-accelerated filer
¨
(Do not check if a smaller reporting company)
|
Smaller reporting company
þ
|
|
Item 1.
|
|
1.
|
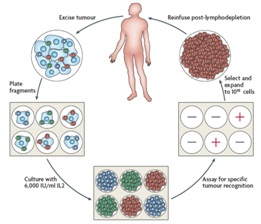
Methods to identify and isolate T-cells and in particular, tumor infiltrating lymphocytes.
|
|
|
|
|
2.
|
Ex Vivo
methods to grow T-cells and in particular, tumor infiltrating lymphocytes.
|
|
|
|
|
3.
|
Methods to use T-cells and in particular, tumor infiltrating lymphocytes, as therapeutic agents for the treatment of metastatic solid tumor cancers, including but not limited to metastatic melanoma.
|
|
·
|
our ability to obtain FDA marketing approval for our product candidates on a timely basis
|
|
·
|
the level of acceptance of our products by physicians, compared to those of competing products or therapies
|
|
·
|
our ability to have our products manufactured on a commercial scale
|
|
·
|
the effectiveness of sales and marketing efforts on behalf of our products
|
|
·
|
our ability to meet demand for our products
|
|
·
|
our ability to secure insurance reimbursement for our products candidates
|
|
·
|
the price of our products relative to competing products or therapies
|
|
·
|
our ability to recruit and retain appropriate management and scientific personnel
|
|
·
|
our ability to develop a commercial scale research and development, manufacturing and marketing infrastructure either on our own or with one or more future strategic partners.
|
|
Item 1A.
|
We currently owe the National Institutes of Health approximately $682,000 under the License Agreement, which obligation may result in the termination of the License Agreement.
|
·
|
our degree of success in developing our adoptive cell therapy products;
|
|
·
|
the rate of progress and cost of our research and development and clinical trial activities;
|
|
·
|
the costs of preparing, filing, prosecuting, maintaining and enforcing patent claims and other intellectual property rights;
|
|
·
|
emergence of competing technologies and other adverse market developments; and
|
|
·
|
the cost of developing and establishing the necessary manufacturing processes and facilities.
|
|
·
|
safety and efficacy results in various human clinical trials reported in scientific and medical literature may not be indicative of results we obtain in our clinical trials;
|
|
·
|
after reviewing test results, we or our collaborators may abandon projects that we might previously have believed to be promising;
|
|
·
|
we, our collaborators or regulators, may suspend or terminate clinical trials if the participating subjects or patients are being exposed to unacceptable health risks; and
|
|
·
|
the effects our potential products have may not be the desired effects or may include undesirable side effects or other characteristics that preclude regulatory approval or limit their commercial use if approved.
|
|
·
|
develop safer or more effective immunotherapeutics and other therapeutic products;
|
|
·
|
reach the market more rapidly, reducing the potential sales of our products; or
|
|
·
|
establish superior proprietary positions.
|
|
·
|
train, manage and motivate our future employees;
|
|
·
|
accurately forecast demand for our products; and
|
|
·
|
acquire and maintain sufficient operational, financial and management information systems.
|
|
·
|
our merger with or into another company;
|
|
·
|
a sale of substantially all of our assets; and
|
|
·
|
amendments to our articles of incorporation.
|
|
·
|
announcements of the results of clinical trials by us or our competitors;
|
|
·
|
developments with respect to patents or proprietary rights;
|
|
·
|
announcements of technological innovations by us or our competitors;
|
|
·
|
announcements of new products or new contracts by us or our competitors;
|
|
·
|
actual or anticipated variations in our operating results due to the level of development expenses and other factors;
|
|
·
|
changes in financial estimates by securities analysts and whether our earnings meet or exceed such estimates;
|
|
·
|
conditions and trends in the pharmaceutical and other industries;
|
|
·
|
new accounting standards;
|
|
·
|
general economic, political and market conditions and other factors; and
|
|
·
|
the occurrence of any of the risks described in this report.
|
|
Item 1B.
|
Unresolved
Staff Comments
|
|
Item 2.
|
|
Item 3.
|
Legal
Proceedings.
|
|
Item 4.
|
Mine
Safety Disclosures.
|
|
Item 5.
|
Market
for Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
YEAR
|
PERIOD
|
HIGH
|
LOW
|
|||||
|
Fiscal Year 2012
|
Fourth Quarter
|
$ |
0.50
|
$ |
0.16
|
|||
|
Third Quarter
|
$ |
0.78
|
$ |
0.27
|
||||
|
Second Quarter
|
$ |
1.22
|
$ |
0.44
|
||||
|
First Quarter
|
$ |
1.35
|
$ |
0.92
|
||||
|
|
||||||||
|
Fiscal Year 2011
|
Fourth Quarter
|
$ |
1.37
|
$ |
0.80
|
|||
|
Third Quarter
|
$ |
1.50
|
$ |
0.82
|
||||
|
Second Quarter
|
$ |
1.59
|
$ |
1.11
|
||||
|
First Quarter
|
$ |
1.26
|
$ |
1.10
|
||||
|
Item 6.
|
Selected
Financial Data
|
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of Operations
|
|
·
|
In 2010, we raised a total of $1,945,000 from the sale of 14,578,309 shares of Common Stock (including warrants). In 2011, we raised a total of $895,000 from the sale of 850,000 shares of Common Stock and five-year Class “C” Warrants to purchase 850,000 shares that exercisable at $1.25 per share. In February 2012 we raised $250,000 from the sale of Common Stock (including warrants).
|
|
·
|
On July 27, 2011, we raised gross proceeds of $5,000,000 from the sale of the Senior Secured Notes and five year warrants (the “Note Warrants”) to purchase 4,000,000 shares of our common stock. The Senior Secured Notes were initially convertible at $1.25 per share, and the Warrants are initially exercisable at $1.25 per share, subject in both cases to anti-dilution adjustments that reduced the exercise price then in effect. The Senior Secured Notes initially were to mature November 30, 2011 but were amended and extended a number of times. The Senior Secured Notes and Note Warrants were extinguished in the Restructuring.
|
|
·
|
In April 2012, we issued two short-term promissory notes in the aggregate amount of $250,000. These promissory notes were exchanged for new 12% Secured Notes issued in May 2012.
|
|
·
|
In May 2012, we issued 12% Secured Notes in the aggregate amount of $1,231,000. These promissory notes were secured by our assets and had a maturity date of December 31, 2012. In addition, we also agreed to issue to the holders of these promissory notes, for no additional consideration, one-half the number of shares of Common Stock for every dollar funded under the 2012 Secured Notes (or 615,625 shares of Common Stock).
|
|
·
|
In September 2012, we received $250,000 loan, which loan was evidenced by promissory note that is due on demand. In addition, we also issued, for no additional consideration, a five year, fully vested warrant to purchase 943,398 shares of common stock at $1.25 per share.
|
|
|
Contractual Obligations
|
|
Payments due by period
|
||||||||||||||||||||
|
Contractual obligations
|
|
Less than 1
|
|
More than
|
||||||||||||||||
| Total |
year
|
1–3 years | 3–5 years |
5 years
|
||||||||||||||||
|
Long-Term Debt Obligations
|
$ |
-
|
$ |
-
|
$ |
-
|
$ |
-
|
$ |
-
|
||||||||||
|
Capital Lease Obligations
|
$ |
-
|
$ |
-
|
$ |
-
|
$ |
-
|
$ |
-
|
||||||||||
|
NIH obligations
|
$ |
115,000
|
$ |
40,000
|
$ |
40,000
|
$ |
35,000
|
$ |
-
|
||||||||||
|
CRADA obligations
|
$ |
3,500,000
|
$ |
1,000,000
|
$ |
2,500,000
|
$ |
-
|
$ |
-
|
||||||||||
| Other Long-Term Liabilities Reflected on the Registrant's Balance Sheet under GAAP | ||||||||||||||||||||
|
Total
|
$ |
3,615,000
|
$ |
1,040,000
|
$ |
2,540,000
|
$ |
35,000
|
$ |
-
|
||||||||||
|
Item 7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
|
Item 8.
|
Financial
Statements and Supplementary Data
|
|
Item 9.
|
Changes
in and Disagreements With Accountants on Accounting and Financial Disclosure
|
|
Item 9A.
|
Controls
and Procedures
|
|
a.
|
The Company did not have sufficient financial reporting personnel to support its financial and operating activities or to maintain sufficient staff to mitigate the risks associated with a lack of segregation of duties. Furthermore, the Company did not have in place formalized finance and accounting policies and procedures; and
|
|
b.
|
The Company did not have effective corporate governance and financial controls to ensure the completeness and accuracy of the accounting for, and the disclosure of, issuance of the Company’s securities such as shares of common stock, options and warrants.
|
|
Item 9B.
|
Other
Information
|
|
Item 10.
|
Directors
, Executive Officers and Corporate Governance
|
|
Name
|
Age
|
Position
|
||
|
Manish Singh
|
45
|
Chief Executive Officer and Chairman of the Board
|
||
|
Merrill A. McPeak
|
77
|
Director
|
||
|
Sanford J. Hillsberg
|
65
|
Director
|
||
|
Jay Venkatesan
|
41
|
Director
|
||
|
Michael Handelman
|
53
|
Chief Financial Officer
|
|
·
|
reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures;
|
|
|
·
|
hiring our independent registered public accounting firm, and coordinating the oversight and review of the adequacy of our internal control over financial reporting with both management and the independent registered public accounting firm; and
|
|
|
·
|
reviewing and, if appropriate, approving all transactions between our company or its subsidiaries and any related party.
|
|
Item 11.
|
Executive
Compensation
|
|
Name and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($) (1)
|
All other
Compen-
sation
($)
|
Total
($)
|
||||||||||||||||||
|
Anthony Cataldo
|
2012
|
$
|
280,500
|
$
|
-
|
$
|
-
|
$
|
280,500
|
||||||||||||||||
|
President and Chief
|
2011
|
275,000
|
–
|
$
|
7,912,037
|
(2)(3)
|
$
|
2,492,750(1)
|
$
|
10,679,787
|
|||||||||||||||
|
Executive Officer(3)
|
-
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Michael Handelman
|
2012
|
180,000
|
–
|
–
|
–
|
180,000
|
|||||||||||||||||||
|
Chief Financial Officer
|
2011
|
110,000
|
–
|
–
|
$
|
2,492,750
|
(1)
|
$
|
2,602,750
|
||||||||||||||||
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
(1)
|
Represents Black-Scholes value of options as determined on the date of grant. Represents options to purchase 2,500,000 shares, which options were granted under the 2011 Equity Compensation Plan, have an exercise price of $1.25 per share, and vest in equal monthly installments over five (5) years.
|
|
(2)
|
On May 27, 2011, as additional compensation for Mr. Cataldo’s services, we issued 3,000,000 shares of our common stock to Mr. Cataldo. The closing price of our common stock on May 27, 2011 was $1.27 per share. The shares were not issued pursuant to any existing stock incentive or option plan.
|
|
|
|
|
(3)
|
Includes the value of 3,501,485 shares (valued at $4,902,037) transferred to Ines Garcia, Mr. Cataldo’s wife, which shares were accounted as additional compensation to Mr. Cataldo.
|
|
Number of Securities Underlying
Unexercised Options
(#)
|
|||||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Option
Exercise Price
($)
|
Option
Expiration Date
|
|||||||||
|
Anthony Cataldo
President and Chief
Executive Officer(1)(2)
|
833,250
|
1,166,750
|
$ |
1.25
|
10/14/2021
|
||||||||
|
Michael Handelman
Chief Financial Officer
and Treasurer(1)
|
833,250
|
1,166,750
|
$ |
1.25
|
10/14/2021
|
||||||||
|
(1)
|
These options vest in equal monthly installments over five (5) years.
|
|
|
(2)
|
Mr. Cataldo has resigned and no longer is this Company’s President and Chief Executive Officer.
|
|
Name(1)
|
Fees
Earned or
Paid in
Cash
($)
|
Stock Awards
($)
|
Option
Awards
($)(1)
|
All Other
Compensation
($)
|
Total
($)
|
|||||||||||||
|
Martin Schroeder
|
$ |
—
|
$ |
—
|
$ |
—
|
$ |
—
|
$ |
—
|
||||||||
|
Dr. L. Stephen Coles
|
$
|
36,000
|
—
|
$
|
—
|
—
|
$
|
36,000
|
||||||||||
|
Dr. William Andrews
|
$
|
36,000
|
—
|
$
|
—
|
—
|
$
|
36,000
|
||||||||||
| Hans Bishop | $ | 111,375 | — | — | — | 111,375 | ||||||||||||
|
Merrill A. McPeak
|
$
|
—
|
—
|
$
|
—
|
—
|
$
|
—
|
||||||||||
|
David Voyticky
|
$
|
—
|
—
|
$
|
—
|
—
|
$
|
—
|
||||||||||
|
Item 12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
Name and address
|
Shares of
Common Stock
Beneficially
Owned (1)
|
Percent of
Common Stock
Beneficially
Owned (1)
|
||||||
|
5% or greater owners:
|
||||||||
|
Ayer Capital Management LP(2)
230 California Street, Suite 600
San Francisco, CA 94111
|
560,401,150 | 37.1 | % | |||||
|
Bristol Investment Fund Ltd. (3)
Bristol Capital Advisors, LLC
10690 Wilshire Boulevard, Suite 1050
Los Angeles, CA 90024
|
415,566,430 | 27.5 | % | |||||
|
Alpha Capital Anstalt
Pradafant 7
9490 Furstentums
Vaduz, Lichtenstein
|
143,218,396 | (4) | 9.5 | % | ||||
|
Directors and executive officers:
|
||||||||
|
Manish Singh
|
120,600,000 | (5) | 8.0 | % | ||||
|
Jay Venkatesan
|
560,401,150 | (6) | 37.1 | % | ||||
|
Michael Handelman
|
833,250 | (7) | * | |||||
|
Merrill A. McPeak
|
35,133,215 | (8) | 2.3 | |||||
|
Sanford J. Hillsberg
|
13,400,000 | (9) | * | |||||
|
All directors and executive officers as a group (5 persons) (10)
|
731,799,865 | 48.5 | % | |||||
|
(1)
|
Applicable percentage ownership is based on 1,509,381,194 shares of Common Stock outstanding at September 23, 2013. The number of shares of Common Stock owned are those “beneficially owned” as determined under the rules of the Securities and Exchange Commission, including any shares of Common Stock as to which a person has sole or shared voting or investment power and any shares of Common Stock which the person has the right to acquire within sixty (60) days through the exercise of any option, warrant or right.
|
|
(2)
|
Based on a Schedule 13G filed with the SEC on June 3, 2013 by Ayer Capital Management, LP, ACM Capital Partners, LLC, Jay Venkatesan, Ayer Capital Partners Master Fund, L.P. and Ayer Capital Partners, LLC. Jay Venkatesan is the Managing Member of ACM Capital Partners, LLC and Ayer Capital Partners Master Fund, L.P.
|
|
(3)
|
Based on a Schedule 13D/A on August 7, 2013, Bristol Investment Fund, Ltd. owns 399,873,215 shares and Bristol Capital, LLC owns 15,696,215 shares. Paul Kessler, as manager of the investment advisor to Bristol Investment Fund, Ltd. ("BIF") and the manager of Bristol Capital, LLC, has power to vote and dispose of the shares owned by these funds. Mr. Kessler disclaims beneficial ownership of the shares owned by BIF.
|
|
(4)
|
Includes 40,000,000 shares of Common Stock that we have agreed to issue, for no additional consideration, to Alpha Capital Anstalt at any time upon the request of Alpha Capital Anstalt. On May 6, 2013, Alpha Capital Anstalt paid us $400,000 for the right to receive these shares. Alpha Capital Anstalt is prohibited from exercising its right to receive these 40,000,000 shares if such issuance would result in Alpha Capital Anstalt owning beneficially more than 9.99% of the outstanding shares of our Common Stock as determined under Section 13(d) of the Securities Exchange Act of 1934.
|
|
(5)
|
Dr. Singh acquired these 120,600,000 shares on July 24, 2013 as consideration for his shares of Common Stock of Lion Biotechnologies, Inc., which company we acquired in the Merger on that date. The merger agreement also provides that during the 12-month period following the Merger, for each $1,000,000 of gross proceeds received by us from any financings, licensing or similar transaction (except from certain listed investors), Dr. Singh will receive 4,050,000 additional shares of Common Stock, up to a maximum of 60,750,000. In addition, under the merger agreement, Dr. Singh is also entitled to 60,750,000 additional shares of Common Stock if, during the 18 months following the closing of the Merger, the closing price per share of our Common Stock equals or exceeds $0.04, as adjusted for any stock split reverse stock split, recapitalization or the like, and $100,000 of our Common Stock is traded for any 10 out of 30 consecutive trading days.
|
|
(6)
|
Represents the 560,401,150 shares owned by Ayer Capital Management LP described in footnote (2) above. Jay Venkatesan is the Managing Member of ACM Capital Partners, LLC and Ayer Capital Partners Master Fund, L.P.
|
|
(7)
|
Consists of options to purchase 833,250 shares of Common Stock that are exercisable currently or within 60 days of September 23, 2013.
|
|
(8)
|
Includes options to purchase 500,000 shares of Common Stock that are exercisable currently or within 60 days of September 23, 2013.
|
|
(9)
|
Mr. Hillsberg acquired these 13,400,000 shares on July 24, 2013 as consideration for his shares of Common Stock of Lion Biotechnologies, Inc., which company we acquired in the Merger on that date. The merger agreement also provides that during the 12-month period following the Merger, for each $1,000,000 of gross proceeds received by us from any financings, licensing or similar transaction (except from certain listed investors), Mr. Hillsberg will receive 450,000 additional shares of Common Stock, up to a maximum of 6,750,000. In addition, under the merger agreement, Mr. Hillsberg is also entitled to 6,750,000 additional shares of Common Stock if, during the 18 months following the closing of the Merger, the closing price per share of our Common Stock equals or exceeds $0.04, as adjusted for any stock split reverse stock split, recapitalization or the like, and $100,000 of our Common Stock is traded for any 10 out of 30 consecutive trading days.
|
|
(10)
|
Includes 730,367,615 shares of Common Stock and options to purchase 1,333,250 shares of Common Stock that are exercisable currently or within 60 days of September 23, 2013.
|
|
Plan Category
|
Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
Number of securities
remaining available
for future issuance
under equity
compensation plans
(excluding securities
reflected in column
(a))
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by stockholders
|
–
|
–
|
–
|
|||||||||
|
Equity compensation plans not approved by stockholders
|
9,375,000
|
$
|
1.085
|
12,125,000
|
||||||||
|
Total
|
9,375,000 | 1.085 | 12,125,000 | |||||||||
|
Item 13.
|
Certain
Relationships and Related Transactions, and Director Independence.
|
|
Item 14.
|
Principal
Accounting Fees and Services
|
|
Year Ended
December 31, 2012
|
Year Ended
December 31, 2011
|
|||||||
|
Audit Fees
|
$
|
152,504
|
$
|
128,175
|
||||
|
Audit-Related Fees
|
–
|
–
|
||||||
|
Tax Fees
|
–
|
–
|
||||||
|
All Other Fees
|
–
|
–
|
||||||
|
$
|
152,504 |
$
|
128,175 | |||||
|
Item 15.
|
Exhibits
, Financial Statements Schedules
|
|
Exhibit
|
Description
|
|
|
2.1
|
Agreement and Plan of Merger between Freight Management Corp. (renamed Genesis Biopharma, Inc.) and Genesis Biopharma, Inc. dated March 15, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 19, 2010).
|
|
|
2.2
|
Asset Purchase Agreement among Freight Management Corp. (renamed Genesis Biopharma, Inc.), Genesis Biopharma, Inc., Hamilton Atlantic and the other signatories thereto dated March 15, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 19, 2010).
|
|
|
3.1
|
Articles of Incorporation filed with the Nevada Secretary of State on September 7, 2007 (incorporated herein by reference to the Registrant’s Registration Statement on Form SB-2 filed with the Commission on January 29, 2008).
|
|
|
3.2
|
Articles of Merger filed with the Nevada Secretary of State on March 15, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 19, 2010).
|
|
|
3.3
|
Certificate of Change to Articles of Incorporation filed with the Nevada Secretary of State on March 15, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 19, 2010).
|
|
|
3.4
|
Bylaws (incorporated herein by reference to the Registrant’s Registration Statement on Form SB-2 filed with the Commission on January 29, 2008).
|
|
|
3.5
|
Amendment to Bylaws (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on May 29, 2013).
|
|
|
4.1
|
Form of Series A Common Stock Purchase Warrant dated September 17, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on September 23, 2010).
|
|
|
4.2
|
Form of Series B Common Stock Purchase Warrant dated September 17, 2010 (incorporated herein by reference to the Registrant’s Form 8-K/A filed with the Commission on July 2, 2010).
|
|
|
4.3
|
Form of Warrant for Consulting Services issued to Emmes Group (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
|
|
4.4
|
Form of Class “C” Warrant (incorporated herein by referenced to the Registrant’s Form 8-K filed with the Commission on April 22, 2011).
|
|
|
4.5
|
Form of Warrant dated July 15, 2011 issued to Bristol Capital, LLC and Theorem Group, LLC (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
|
|
4.6
|
Form of seven (7%) percent senior convertible note effective July 27, 2011 as issued by Genesis Biopharma Inc. to selling stockholders (incorporated herein by referenced to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
4.7
|
Form of seven (7%) percent senior convertible note effective July 27, 2011 as issued by Genesis Biopharma Inc. to selling stockholders (incorporated herein by referenced to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
4.8
|
Form of Warrant as issued to selling stockholders effective July 27, 2011 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
4.9
|
Form of Tranche B seven (7%) percent senior convertible note as issued by Genesis Biopharma Inc. to selling stockholders (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
Exhibit
|
Description
|
|
|
4.10
|
Form of Tranche B Warrant as issued by Genesis Biopharma Inc. to selling stockholders (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
4.11
|
Form of Placement Agent Warrant as issued to Cannacord Genuity, Inc. and Cowen and Company, Inc. effective July 27, 2011 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
4.12
|
Amendment No. 1 to Tranche A Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by referenced to the Registrant’s Form 8-K filed with the Commission on December 5, 2011).
|
|
|
4.13
|
Amendment No. 1 Tranche A Warrants to Purchase Common Stock and Tranche B Warrants to Purchase Common Stock (incorporated herein by referenced to the Registrant’s Form 8-K filed with the Commission on December 5, 2011).
|
|
|
4.14
|
Amendment No. 2 to Tranche A Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by referenced to the Registrant’s Form 8-K/A filed with the Commission on December 22, 2011).
|
|
|
4.15
|
Amendment No. 2 Tranche A Warrants to Purchase Common Stock and Tranche B Warrants to Purchase Common Stock (incorporated herein by referenced to the Registrant’s Form 8-K/A filed with the Commission on December 22, 2011).
|
|
|
4.16
|
Amendment No. 3 to Tranche A Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by referenced to the Registrant’s Form 8-K/A filed with the Commission on January 10, 2011).
|
|
|
4.17
|
Amendment No. 3 Tranche A Warrants to Purchase Common Stock and Tranche B Warrants to Purchase Common Stock (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
|
|
4.18
|
Amendment No. 4 to Tranche A Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
|
|
4.19
|
Amendment No. 4 Tranche A Warrants to Purchase Common Stock and Tranche B Warrants to Purchase Common Stock (incorporated herein by referenced to the Registrant’s Form 8-K/A filed with the Commission on March 6, 2011).
|
|
|
4.20
|
Amendment No. 5 to Tranche A Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by referenced to the Registrant’s Form 8-K/A filed with the Commission on February 6, 2011).
|
|
|
4.21
|
Amendment No. 6 to Tranche A Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by referenced to the Registrant’s Form 8-K/A filed with the Commission on March 6, 2011).
|
|
|
10.1
|
Genesis Biopharma, Inc. 2010 Equity Compensation Plan (incorporated herein by reference to the Registrant’s Annual Report on Form 10-K filed with the Commission on March 31, 2010).
|
|
|
10.2
|
Form of Stock Option Agreement for grants under the Genesis Biopharma Inc 2010 Equity Incentive Plan (incorporated herein by reference to the Registrant’s Annual Report on Form 10-K filed with the Commission on March 31, 2010).
|
|
Exhibit
|
Description
|
|
|
10.3
|
Genesis Biopharma, Inc. 2011 Equity Compensation Plan (incorporated herein by reference to Registrant’s Form 8-K filed with the Commission on October 20, 2011)
|
|
|
10.4
|
Form of ISO Stock Option Agreement for grants under the Genesis Biopharma Inc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.4 of the Registrant’s Form 8-K filed with the Commission on October 20, 2011).
|
|
|
10.5
|
Form of NQSO Stock Option Agreement for grants under the Genesis Biopharma Inc. 2011 Equity Incentive Plan (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on October 20, 2011).
|
|
|
10.6
|
Patent and Know How License between Cancer Research Technology Limited and Genesis Biopharma, Inc. (formerly Freight Management Corp.) dated March 15, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 19, 2010)
|
|
|
10.7
|
Form of Private Placement Subscription Agreement dated September 17, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on September 23, 2010).
|
|
|
10.8
|
Form of Private Placement Subscription Agreement dated October 22, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on October 28, 2010).
|
|
|
10.9
|
Form of Private Placement Subscription Agreement dated December 28, 2010 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on January 3, 2011).
|
|
|
10.10
|
Consulting Agreement, dated February 15, 2011, by and between Emmes Group and Genesis Biopharma, Inc., Amendment No. 1, dated ____, 2011, Amendment No. 2, dated February 12, 2012 (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
|
|
10.11
|
Form of Securities Purchase Agreement, dated April 17, 2011(incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on April 22, 2011).
|
|
|
10.12
|
Consulting Agreement dated July 15, 2011, between Theorem and Genesis Biopharma, Inc. (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
|
|
10.13
|
Consulting Agreement dated July 15, 2011, between Bristol and Genesis Biopharma, Inc. Addendum No. 1, dated ____, 2011 (incorprated herein by reference to the Registrant's Form 10-K filed with the Commission on March 30, 2012).
|
|
|
10.14
|
Form of Securities Purchase Agreement effective July 27, 2011 between Genesis Biopharma, Inc. and selling stockholders (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
10.15
|
Form of Escrow Agreement between Genesis Biopharma Inc. and the selling stockholders effective July 27, 2011 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
10.16
|
Form of Registration Rights Agreement between Genesis Biopharma Inc. and the selling stockholders effective July 27, 2011 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 29, 2011).
|
|
|
10.17
|
Patent License Agreement between the Company and the National Institutes of Health effective October 5, 2011 (incorporated herein by reference to the Registrant’s Form 8-K/A filed with the Commission on December 13, 2011).*
|
|
Exhibit
|
Description
|
|
10.18
|
Cooperative Research and Development Agreement for Intramural-PHS Clinical Research, dated August 5, 2011, between the U.S. Department of Health and Human Services, as represented by the National Cancer Institute and the Company. (incorporated herein by reference to the Registrant’s Form 8-K/A (No.2) filed with the Commission on November 29, 2011).
|
||
|
10.19
|
Employment Agreement dated as of May 1, 2011 between the Company and Anthony J. Cataldo (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on October 20, 2011).
|
||
|
10.20
|
Employment Agreement dated as of May 1, 2011 between the Company and Michael Handelman (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on October 20, 2011).
|
||
|
10.21
|
Lonza Walkersville Inc. Letter of Intent with Genesis Biopharma Inc. effective November 4, 2011 (incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q filed with the Commission on November 21, 2011).
|
||
|
10.22
|
Manufacturing Service Agreement, dated December __, 2011, by and between Lonza Walkersville and Genesis Biopharma, Inc. (incorporated herein by reference to the Registrant’s Form 10-K filed with the Commission on March 30, 2012).
|
||
|
10.23
|
Form of Amendment #3 to Tranche A Senior Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on January 10, 2012).
|
||
|
10.24
|
Form of Amendment No. 5 to Tranche A Senior Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes (incorporated herein by reference to the Registrant’s Form 8-K/A filed with the Commission on February 6, 2012).
|
||
|
10.25
|
Form of Amendment No. 6 to Tranche A Senior Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes, effective as of February 29, 2012 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 6, 2012).
|
||
|
10.26
|
Form of Amendment No. 4 Tranche A Warrants to Purchase Common Stock and Tranche B Warrants to Purchase Common Stock (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on March 6, 2012).
|
||
|
10.27
|
Form of Amendment No. 8 to Tranche A Senior Unsecured Convertible Notes and Tranche B Senior Unsecured Convertible Notes effective March 30, 2012 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on April 5, 2012).
|
||
|
10.28
|
Form of Amendment No. 5 to the Tranche A Warrants to Purchase Common Stock and Tranche B Warrants to Purchase Common Stock effective March 30, 2012 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on April 5, 2012).
|
||
|
10.29
|
Form of two hundred and forty five thousand ($245,000) dollar 12% Promissory Note issued by the Company to Ayer Capital Partners Master Fund, L.P. effective April 5, 2012 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on April 10, 2012).
|
||
|
10.30
|
Form of five thousand ($5,000) dollar 12% Promissory Note issued by the Company to Ayer Capital Partners Kestrel Fund, L.P. effective April 5, 2012 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on April 10, 2012).
|
||
|
10.31
|
Form of Note and Common Stock Subscription Agreement (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on May 11, 2012).
|
||
|
10.32
|
Form of Secured Promissory Note, due June 30, 2012 (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on May 11, 2012).
|
||
|
Exhibit
|
Description
|
|
10.33
|
Form of Maturity Date Extension (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 6, 2012).
|
||
|
10.34
|
Form of Maturity Date Extension (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on October 4, 2012).
|
||
|
10.35
|
Form of Exchange Agreement (incorporated herein by reference to the Registrant’s Form 10-Q filed with the Commission on May 29, 2013).
|
||
|
10.36
|
Form of Stock Purchase Agreement (incorporated herein by reference to the Registrant’s Form 10-Q filed with the Commission on May 29, 2013).
|
||
|
10.37
|
Agreement and Plan of Merger, dated July 24, 2013, between the Company, Lion Biotechnologies, Inc. and Genesis Biopharma Sub, Inc. (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 25, 2013).
|
||
|
10.38
|
Form of Director Stock Award Agreement (incorporated herein by reference to the Registrant’s Form 8-K filed with the Commission on July 25, 2013).
|
||
|
10.39
|
Executive Employment Agreement, dated July 24, 2013, between the Company and Manish Singh (incorporated herein by reference to the Registrant's Form 8-K filed with the Commission on July 25, 2013).
|
||
|
31.1
|
Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer
|
||
|
31.2
|
Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer
|
||
|
32.1
|
Section 1350 Certification of Chief Executive Officer
|
||
|
32.2
|
Section 1350 Certification of Chief Financial Officer
|
||
|
101
|
The following financial information from the Annual Report on Form 10-K of Genesis Biopharma, Inc. for the year ended December 31, 2012, formatted in XBRL (eXtensible Business Reporting Language): (1) Balance Sheets as of December 31, 2012 and 2011; (2) Statements of Income for the years ended December 31, 2012 and 2011; (3) Statements of Comprehensive Income for the years ended December 31, 2012 and 2011; (4) Statements of Shareholders’ Equity for the years ended December 31, 2012 and 2011; (5) Statements of Cash Flows for the years ended December 31, 2012 and 2011; and (6) Notes to Financial Statements
|
||
|
GENESIS BIOPHARMA, INC.
|
|||
|
Date: September 23, 2013
|
By:
|
/s/ Manish Singh
|
|
|
Name:
|
Manish Singh
|
||
|
Title:
|
Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Manish Singh
|
Chief Executive Officer (Principal
|
September 23, 2013
|
||
|
Manish Singh
|
Executive Officer) and Director
|
|||
|
/s/ Michael Handelman
|
Chief Financial Officer
|
September 23, 2013
|
||
|
Michael Handelman
|
(Principal Financial Officer and Principal Accounting Officer)
|
|||
|
/s/ Merrill A. McPeak
|
Director
|
September 23, 2013
|
||
|
Merrill A. McPeak
|
||||
|
/s/ Jay Venkatesan
|
Director
|
September 23, 2013
|
||
|
Jay Venkatesan
|
||||
|
|
Director
|
September __, 2013
|
||
|
Sanford J. Hillsberg
|
|
Page
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Financial Statements
|
|
|
Balance Sheets as of December 31, 2012 and 2011
|
F-2
|
|
Statements of Operations for years ended December 31, 2012 and 2011 and for the period from September 17, 2007 (Date of Inception) through December 31, 2012
|
F-3
|
|
Statements of Stockholders’ Deficiency for the period from September 17, 2007 (Date of Inception) through December 31, 2012
|
F-4
|
|
Statements of Cash Flows for years ended December 31, 2012 and 2011 and for the period from September 17, 2007 (Date of Inception) through December 31, 2012
|
F-5
|
|
Notes to Financial Statements
|
F-6
|
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
-
|
$
|
510,217
|
||||
|
Deposit
|
5,000
|
9,391
|
||||||
|
Prepaid expenses
|
2,275
|
4,473
|
||||||
|
Total Current Assets
|
7,275
|
524,081
|
||||||
|
Property and equipment,
net of accumulated depreciation of $8,915 and $2,704
|
22,138
|
28,349
|
||||||
|
Rent Deposit
|
-
|
16,000
|
||||||
|
Total Assets
|
$
|
29,413
|
$
|
568,430
|
||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIENCY
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable
|
1,098,271
|
190,048
|
||||||
|
Accrued expenses
|
1,740,220
|
67,896
|
||||||
|
7% Senior secured convertible promissory notes
|
5,000,000
|
5,000,000
|
||||||
|
12% Secured promissory note
|
1,231,250
|
-
|
||||||
|
September 2012 secured promissory note
|
250,000
|
-
|
||||||
|
Accrued interest and penalty
|
2,029,148
|
153,611
|
||||||
|
Derivative liabilities
|
-
|
7,937,793
|
||||||
|
Total Current Liabilities
|
11,348,889
|
13,349,348
|
||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders' Deficiency
|
||||||||
|
Common stock, $0.000041666 par value; 1,800,000,000 shares authorized, 81,880,595 and 77,993,591 shares issued and outstanding, respectively
|
3,412
|
3,250
|
||||||
|
Common stock to be issued, 303,125 shares
|
245,153
|
-
|
||||||
|
Additional paid-in capital
|
19,116,154
|
14,592,408
|
||||||
|
Accumulated deficit
|
(30,684,195
|
)
|
(27,376,576
|
)
|
||||
|
Total Stockholders' Deficiency
|
(11,319,476
|
)
|
(12,780,918
|
)
|
||||
|
Total Liabilities and Stockholders' Deficiency
|
$
|
29,413
|
$
|
568,430
|
||||
|
For the Period from
|
||||||||||||
|
September 17, 2007
|
||||||||||||
|
For the Years Ended
|
(Date of Inception)
|
|||||||||||
|
December 31,
|
through
|
|||||||||||
|
2012
|
2011
|
December 31, 2012
|
||||||||||
|
Revenues
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Costs and expenses
|
||||||||||||
|
Operating expenses (including $4,203,254, $14,608,040 and $16,361,663 of non-cash share-based compensation costs)
|
6,476,546
|
19,302,721
|
26,498,038
|
|||||||||
|
Research and development
|
1,656,000
|
1,755,561
|
3,583,045
|
|||||||||
|
Impairment of intangible asset
|
-
|
160,036
|
160,036
|
|||||||||
|
Total costs and expenses
|
8,132,546
|
21,218,318
|
30,241,119
|
|
||||||||
|
Loss from operations
|
(8,132,546
|
)
|
(21,218,318
|
) |
(30,241,119
|
)
|
||||||
|
Other income (expense)
|
||||||||||||
|
Gain from change in fair value of derivative liabilities
|
8,635,147
|
1,596,035
|
10,001,955
|
|||||||||
|
Interest expense
|
(1,922,063
|
)
|
(151,507
|
) |
(2,073,216
|
)
|
||||||
|
Amortization of discount on convertible notes
|
(497,888
|
)
|
(5,000,000
|
) |
(5,497,888
|
)
|
||||||
|
Financing costs
|
(1,390,269
|
)
|
(920,310
|
) |
(2,873,927
|
)
|
||||||
|
Total other income (expense)
|
4,824,927
|
|
(4,475,782
|
) |
(443,076
|
)
|
||||||
|
Net Loss
|
$
|
(3,307,619
|
)
|
$
|
(25,694,100
|
) |
$
|
(30,684,195
|
)
|
|||
|
Net Loss Per Share, Basic and Diluted
|
$
|
(0.04
|
)
|
$
|
(0.34
|
) | ||||||
|
Weighted-Average Common Shares Outstanding, Basic and Diluted
|
79,853,033
|
75,923,905
|
||||||||||
|
Total
|
|||||||||||||||||||||
|
Common
|
Additional
|
Stockholders'
|
|||||||||||||||||||
|
Common Stock
|
Stock to
|
Paid-In
|
Accumulated
|
Equity
|
|||||||||||||||||
|
Shares
|
Amount
|
Be Issued
|
Capital
|
Deficit
|
(Deficiency)
|
||||||||||||||||
|
Initial capitalization, sale of common stock to directors, September 17, 2007
|
12,660,024
|
$
|
528
|
$
|
7,472
|
$
|
-
|
$
|
8,000
|
||||||||||||
|
Private placement, closed December 31, 2007
|
25,440,000
|
1,060
|
51,940
|
-
|
53,000
|
||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
(58,716
|
)
|
(58,716
|
)
|
||||||||||||||
|
Balance - December 31, 2008
|
38,100,024
|
1,588
|
59,412
|
(58,716
|
)
|
2,284
|
|||||||||||||||
|
Net loss
|
-
|
-
|
-
|
(15,772
|
)
|
(15,772
|
)
|
||||||||||||||
|
Balance - December 31, 2009
|
38,100,024
|
1,588
|
59,412
|
(74,488
|
)
|
(13,488
|
)
|
||||||||||||||
|
Common stock sold in private placement at $0.03125 per share, March 2010
|
12,799,968
|
533
|
364,467
|
-
|
365,000
|
||||||||||||||||
|
Common stock issued for intellectual property, March 2010
|
20,960,016
|
873
|
216,535
|
-
|
217,408
|
||||||||||||||||
|
Common stock sold in private placement at $0.75 per share, September 2010
|
933,341
|
39
|
699,961
|
-
|
700,000
|
||||||||||||||||
|
Common stock sold in private placement at $1.00 per share, October 2010
|
250,000
|
10
|
249,990
|
-
|
250,000
|
||||||||||||||||
|
Common stock sold in private placement at $1.00 per share, December 2010
|
595,000
|
25
|
594,975
|
-
|
595,000
|
||||||||||||||||
|
Forgiveness of debt by director
|
-
|
-
|
18,137
|
-
|
18,137
|
||||||||||||||||
|
Fair value of vested stock options
|
-
|
-
|
114,016
|
-
|
114,016
|
||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
(1,607,988
|
)
|
(1,607,988
|
)
|
||||||||||||||
|
Balance - December 31, 2010
|
73,638,349
|
3,068
|
2,317,493
|
(1,682,476
|
)
|
638,085
|
|||||||||||||||
|
Common stock sold in private placement at $1.00 per share, January 2011
|
45,000
|
2
|
44,998
|
-
|
45,000
|
||||||||||||||||
|
Common stock and warrant sold in private placement at $1.00 per share, April to June 2011, net of fair value of warrant derivative
|
850,000
|
35
|
185,669
|
-
|
185,704
|
||||||||||||||||
|
Fair value of common stock issued to consultants for services
|
460,242
|
20
|
498,432
|
-
|
498,452
|
||||||||||||||||
|
Common stock returned for cancelation
|
(3,000,000
|
)
|
(125
|
)
|
125
|
-
|
-
|
||||||||||||||
|
Fair value of common stock issued to officer for services
|
6,000,000
|
250
|
8,009,750
|
-
|
8,010,000
|
||||||||||||||||
|
Fair value of common stock transferred to officer
|
-
|
-
|
702,037
|
-
|
702,037
|
||||||||||||||||
|
Fair value of common stock transferred from CEO to a director
|
-
|
-
|
1,040,000
|
-
|
1,040,000
|
||||||||||||||||
|
Fair value of vested stock options and warrants
|
-
|
-
|
1,793,904
|
-
|
1,793,904
|
||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
(25,694,100
|
)
|
(25,694,100
|
)
|
|||||||||||||
|
Balance - December 31, 2011
|
77,993,591
|
|
3,250
|
-
|
|
14,592,408
|
(27,376,576
|
)
|
(12,780,918
|
) | |||||||||||
|
Common stock sold in private placement at $1.00 per share, net of derivative liability,February 2012
|
250,000
|
|
10
|
-
|
|
67,909
|
|
-
|
|
67,919
|
|||||||||||
|
Common stock issued to consultants for services
|
1,549,504
|
|
65
|
-
|
|
799,935
|
|
-
|
|
800,000
|
|||||||||||
|
Fair value of common stock issued with notes payable recorded as a note discount
|
312,500
|
|
13
|
245,153
|
|
252,722
|
|
-
|
|
497,888
|
|||||||||||
|
Fair value of common stock issued with notes payable recorded as financing cost
|
1,775,000
|
|
74
|
-
|
|
874,926
|
|
- |
|
875,000
|
|||||||||||
|
Fair value of vested stock options and warrants
|
-
|
|
-
|
-
|
|
2,528,254
|
|
-
|
|
2,528,254
|
|||||||||||
|
Net loss
|
-
|
|
-
|
-
|
|
-
|
|
(3,307,619
|
)
|
|
(3,307,619
|
)
|
|||||||||
|
Balance - December 31, 2012
|
81,880,595
|
$
|
3,412
|
245,153
|
$
|
19,116,154
|
$
|
(30,684,195
|
)
|
$
|
(11,319,476
|
)
|
|||||||||
|
For the Period from
|
||||||||||||
|
September 17, 2007
|
||||||||||||
|
For the Years Ended
|
(Date of Inception)
|
|||||||||||
|
December 31,
|
through
|
|||||||||||
|
2012
|
2011
|
December 31, 2012
|
||||||||||
|
Cash Flows From Operating Activities
|
||||||||||||
|
Net loss
|
$
|
(3,307,619
|
)
|
$
|
(25,694,100
|
)
|
$
|
(30,684,195
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
6,211
|
2,704
|
70,287
|
|||||||||
|
Impairment of intangible asset
|
-
|
160,036
|
160,036
|
|||||||||
|
Fair value of vested stock options and warrants
|
2,528,254
|
1,793,904
|
4,436,174
|
|||||||||
|
Fair value of derivative liability recorded upon issuance of warrants
|
-
|
2,563,647
|
2,563,647
|
|||||||||
|
Amortization of discount on convertible notes
|
497,888
|
5,000,000
|
5,497,888
|
|||||||||
|
Private placement costs
|
515,273
|
920,310
|
1,998,931
|
|||||||||
|
Change in fair value of derivative liabilities
|
(8,635,147
|
)
|
(1,596,035
|
)
|
(10,001,955
|
)
|
||||||
|
Common stock issued to officer for services
|
-
|
8,010,000
|
8,010,000
|
|||||||||
|
Common stock issued for services
|
800,000
|
498,452
|
1,298,452
|
|||||||||
|
Common stock issued with note payable reflected as financing cost
|
875,000 |
-
|
875,000
|
|||||||||
|
Fair value of common stock transferred to officer and director
|
-
|
1,742,037
|
1,742,037
|
|||||||||
|
Write off of advances to related party
|
-
|
50,000
|
50,000
|
|||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Prepaid expenses and other assets
|
22,589
|
(21,417
|
)
|
(7,275
|
)
|
|||||||
|
Accounts payable
|
908,223
|
159,756
|
1,098,271
|
|||||||||
|
Accrued expenses
|
1,672,324
|
70,000
|
1,893,831
|
|||||||||
|
Accrued interest and penalty
|
1,875,537
|
151,507
|
1,875,537
|
|||||||||
|
Net Cash Used In Operating Activities
|
(2,241,467
|
)
|
(6,189,199
|
)
|
(9,123,334
|
)
|
||||||
|
Cash Flows From Investing Activities
|
||||||||||||
|
Property and equipment
|
-
|
(31,053
|
)
|
(35,053
|
)
|
|||||||
|
Advances to related party
|
-
|
|
(50,000
|
)
|
(50,000
|
)
|
||||||
|
Net Cash Used In Investing Activities
|
-
|
|
(81,053
|
)
|
(85,053
|
)
|
||||||
|
Cash Flows From Financing Activities
|
||||||||||||
|
Proceeds from the issuance of convertible notes, net
|
-
|
4,615,000
|
4,615,000
|
|||||||||
|
Proceeds from the issuance of promissory notes
|
1,481,250
|
-
|
1,481,250
|
|||||||||
|
Proceeds from the issuance of common stock
|
250,000
|
873,000
|
3,094,000
|
|||||||||
|
Due to director
|
-
|
-
|
18,137
|
|||||||||
|
Net Cash Provided By Financing Activities
|
1,731,250
|
5,488,000
|
9,208,387
|
|||||||||
|
Net Increase (Decrease) In Cash And Cash Equivalents
|
(510,217
|
)
|
(782,252
|
)
|
-
|
|||||||
|
Cash and Cash Equivalents, Beginning Of Year
|
510,217
|
1,292,469
|
-
|
|||||||||
|
Cash and Cash Equivalents, End Of Year
|
$
|
-
|
$
|
510,217
|
$
|
-
|
||||||
|
Supplemental Disclosures of Cash Flow Information:
|
||||||||||||
|
Derivative liability recorded upon issuance of convertible notes and warrants
|
$
|
-
|
$
|
5,535,310
|
$
|
5,535,310
|
||||||
|
Derivative liability recorded as financing cost
|
697,354
|
642,296
|
1,339,650
|
|||||||||
|
Common stock issued for intellectual property
|
-
|
-
|
217,408
|
|||||||||
|
Fair value of common stock issued with notes payable recorded as a note discount
|
497,888
|
-
|
497,888
|
|||||||||
|
Forgiveness of debt by director, treated as contribution of capital
|
-
|
-
|
18,137
|
|||||||||
|
December 31, 2012
|
December 31, 2011
|
|||||||||||||||||||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||||||||||||||
|
Derivative liabilities
|
$ | - | $ | - | $ | - | $ | - | $ | - | $ | 7,937,793 | $ | - | $ | 7,937,793 | ||||||||||||||||
|
Weighted
|
|||||||||||||
|
Weighted
|
Average
|
||||||||||||
|
Shares
|
Average
|
Remaining
|
Aggregate
|
||||||||||
|
Under
|
Exercise
|
Contractual
|
Intrinsic
|
||||||||||
|
Option
|
Price
|
Life
|
Value
|
||||||||||
|
Outstanding at December 31, 2010
|
1,150,000
|
$
|
0.03
|
6.3 years
|
$
|
1,401,563
|
|||||||
|
Granted
|
8,125,000
|
1.23
|
|||||||||||
|
Exercised
|
-
|
-
|
|||||||||||
|
Expired/Forfeited
|
-
|
-
|
|||||||||||
|
Outstanding at December 31, 2011
|
9,275,000
|
1.09
|
8.5 years
|
1,114,063
|
|||||||||
|
Granted
|
300,000
|
1.04
|
|||||||||||
|
Exercised
|
|||||||||||||
|
Expired/Forfeited
|
(200,000
|
) |
0.92
|
||||||||||
|
Outstanding at December 31, 2012
|
9,375,000
|
$
|
1.07
|
7.7 years
|
$
|
217,063
|
|||||||
|
Exercisable at December 31, 2012
|
5,515,830
|
$
|
1.06
|
7.2 years
|
$
|
142,223
|
|||||||
|
Weighted
|
||||||||||||||||
|
Weighted
|
Average
|
|||||||||||||||
|
Shares
|
Average
|
Remaining
|
Aggregate
|
|||||||||||||
|
Under
|
Exercise
|
Contractual
|
Intrinsic
|
|||||||||||||
|
Warrants
|
Price
|
Life
|
Value
|
|||||||||||||
|
Outstanding at December 31, 2010
|
1,050,022
|
$
|
1.00
|
4.7 years
|
$
|
262,506
|
||||||||||
|
Issued
|
8,630,000
|
1.34
|
||||||||||||||
|
Exercised
|
-
|
-
|
||||||||||||||
|
Expired
|
-
|
-
|
||||||||||||||
|
Outstanding at December 31, 2011
|
9,680,022
|
1.22
|
4.5 years
|
-
|
||||||||||||
|
Issued
|
1,193,396
|
1.25
|
||||||||||||||
|
Exercised
|
-
|
|||||||||||||||
|
Expired
|
-
|
|||||||||||||||
|
Outstanding at December 31, 2012
|
10,873,418
|
$
|
1.23
|
3.5 years
|
$
|
-
|
||||||||||
|
December 31, 2012
|
Upon Issuance
|
December 31, 2011
|
||||||||||
|
Average Assumptions:
|
||||||||||||
|
Risk-free interest rate
|
-
|
%
|
0.87
|
%
|
0.46
|
%
|
||||||
|
Expected volatility
|
-
|
%
|
129
|
%
|
86.20
|
%
|
||||||
|
Expected life
|
-
|
5 years
|
4.45 years
|
|||||||||
|
Expected dividend yield
|
-
|
%
|
0.00
|
%
|
0.00
|
%
|
||||||
|
Fair value of conversion feature
|
$
|
-
|
$
|
-
|
$
|
177,258
|
||||||
|
Fair value of warrants
|
|
-
|
|
697,354
|
|
7,760,535
|
||||||
|
Total fair value
|
$
|
-
|
$
|
697,354
|
$
|
7,937,793
|
||||||
On June 19, 2013, the Company entered into a Settlement Agreement and General Release of All Claims (the “Agreement”) with Mr. Anthony Cataldo, the Company’s former chief executive officer (“Cataldo”). Per the Agreement, Mr. Cataldo voluntarily resigned as the Company’s chief executive officer, effective as of June 1, 2013. The Agreement also settles any amounts owed to Mr. Cataldo by the Company, providing that upon the Company achieving its first financing with aggregate proceeds to the Company of greater than $5,000,000 following the date of the Agreement (the “Financing”), the Company shall provide Cataldo with a cash payment equal to $370,000, to be paid out as follows: (a) a payment of $120,000 in cash, less all appropriate federal and state income and employment taxes, payable to Cataldo within ten (10) business days following the closing of the Financing, and (b) a payment of $250,000, less all appropriate federal and state income and employment taxes, payable to Cataldo within ten (10) business days following a closing of the Financing and immediately reinvested by Company on Cataldo’s behalf in the Financing, on the same terms and conditions therein. The Agreement also provides for mutual releases of all claims related in any way to the transactions or occurrences between Cataldo and the Company to date, to the fullest extent permitted by law, including, but not limited to, Cataldo’s employment with Company.
|
December 31, 2012
|
December 31, 2012
|
||||||||||||
|
Pro-forma
|
(Proforma)
|
||||||||||||
|
(As Reported)
|
Adjustments
|
(Unaudited)
|
|||||||||||
|
Cash and cash equivalents
|
$ | - | 3 | 1,240,000 | $ | 1,240,000 | |||||||
|
Other assets
|
29,413 | 29,413 | |||||||||||
|
Total Assets
|
$ | 29,413 | $ | 1,269,413 | |||||||||
|
Accounts Payable
|
$ | 1,098,271 | $ | 1,098,271 | |||||||||
|
Accrued Expenses
|
1,740,220 | 1,740,220 | |||||||||||
|
7% Senior secured promissory notes
|
5,000,000 | 1 | (5,000,000 | ) | - | ||||||||
|
12% Secured promissory note
|
1,231,250 | 1 | (1,231,250 | ) | - | ||||||||
|
September 2012 secured promissory note
|
250,000 | 1 | (250,000 | ) | - | ||||||||
|
Accrued interest and penalty
|
2,029,148 | 1 | (1,891,787 | ) | 137,361 | ||||||||
|
Total Liabilities
|
11,348,889 | 2,975,852 | |||||||||||
|
Common stock and additional
|
19,364,719 | 1 | 8,373,037 | ||||||||||
|
paid in capital
|
2 | 107,734 | |||||||||||
| 3 | 1,240,000 | ||||||||||||
| 4 | 6,700,000 | ||||||||||||
| 5 | 2,002,982 | 37,788,472 | |||||||||||
|
Accumulated deficit
|
(30,684,195 | ) | 2 | (107,734 | ) | ||||||||
| 4 | (6,700,000 | ) | |||||||||||
| 5 | (2,002,982 | ) | |||||||||||
| (39,494,911 | ) | ||||||||||||
|
Total Stockholders' Deficiency
|
(11,319,476 | ) | (1,706,439 | ) | |||||||||
|
|
|||||||||||||
|
Total Liabilities and Stockholders' Deficiency
|
$ | 29,413 | $ | 1,269,413 | |||||||||
|
1 - To record issuance of 837,303,700 shares of common stock with a fair value of $8,373,037 to settle promissory notes and accrued interest and penalty in the aggregate of $8,373,037.
|
||||||||
|
2 - To record issuance of 10,773,418 shares of common stock with a fair value of $107,734 in exchange for the cancellation of 10,773,418 outstanding warrants.
|
||||||||
|
3 - To record issuance of 352,313,447 shares of common stock for cash of $1,349,990, less direct legal costs incurred of $109,990 or net proceeds of $1,240,000.
|
||||||||
|
4 - To record issuance of 134,000,000 shares of common stock with a fair value of $6,700,000 pursuant to the agreement with Lion Biotechnologies, Inc.
|
||||||||
|
5 - To record issuance of 40,059,649 shares of common stock with a fair value of $2,002,982 to 3 members of Board of Directors.
|
||||||||