|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
DELAWARE
|
|
77-0416458
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
|
(I.R.S. Employer Identification Number)
|
|
Title of Each Class:
|
|
Name of Each Exchange on which Registered
|
|
Common Stock, par value $0.001 per share
|
|
The NASDAQ Global Select Market
|
|
Large accelerated filer
|
x
|
Accelerated filer
|
¨
|
|
|
Non-accelerated filer
|
¨
|
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
¨
|
|
Page No.
|
||
|
ITEM 1.
|
BUSINESS
|
|
1.
|
Patient Value.
We believe that the value of a surgical procedure to a patient can be defined as:
Patient Value = Procedure Efficacy/Invasiveness
. We define
procedure efficacy
as a measure of the success of the surgery in resolving the underlying disease and
invasiveness
is how disruptive and painful the treatment is itself. When the patient value of a
da Vinci
procedure is deemed higher than alternate treatment options, patients may seek out surgeons and hospitals that offer that specific
da Vinci
procedure, potentially resulting in a local market share shift for the specific treatment. Adoption occurs procedure by procedure, and is driven by the relative patient value of
da Vinci
procedures compared to alternative treatment options for the same disease state. We believe most patients will place higher value on procedures that are not only more efficacious, but also less invasive than alternative treatments. Our goal is to provide products to surgeons who in turn provide patients with procedure options that are both highly effective and less invasive than other surgical options.
|
|
2.
|
Surgeon Value.
We train surgeons on the use of our
da Vinci
Surgical System and assist them in building their practices by their delivery of superior patient value. We provide an ergonomic platform for surgeons to perform their procedures. We seek to provide surgeons with reliable and easy to use products.
|
|
3.
|
Hospital Value.
We assist hospitals in building value by offering patient value using
da Vinci
thereby increasing surgical revenue and reducing costs through lower complication rates and reduced length of patient stay.
da Vinci surgery
is a cost effective approach to many surgeries as compared to alternative treatment options as demonstrated in many published economic studies.
|
|
1.
|
Convert Candidate Open Procedures to da Vinci
S
urgery.
We believe that our technology has the potential to convert a significant percentage of our targeted open procedures to
da Vinci
Surgery.
|
|
2.
|
Facilitate Difficult MIS Operations.
We believe that several surgical procedures that are seldom performed today using conventional MIS techniques can be performed more routinely using
da Vinci
Surgery. Some procedures have been adopted using MIS techniques but are extremely difficult and are currently performed by a limited number of highly skilled surgeons. We believe our
da Vinci
Surgical System will enable more surgeons at more institutions to perform such procedures.
|
|
3.
|
Offer a Less Invasive Single Port Surgical Option.
We believe that our
da Vinci
Single Site technology has the potential to convert candidate procedures typically performed via multiport laparoscopic technique to single port
da Vinci
Surgery, offering patients less invasive, improved cosmetic outcomes.
|
|
1.
|
a device that has grandfather marketing status because it was legally marketed prior to May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted, or
|
|
2.
|
a device that has previously been cleared through the 510(k) process.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
•
|
failure to obtain the same degree of protection against infringement of our intellectual property rights as we have in the United States;
|
|
•
|
protectionist laws and business practices that favor local competitors, which could slow our growth in international markets;
|
|
•
|
local or national regulations that make it difficult or impractical to market or use our products;
|
|
•
|
inability or regulatory limitations of our ability to move goods across borders;
|
|
•
|
the risks associated with foreign currency exchange rate fluctuations;
|
|
•
|
the expense of establishing facilities and operations in new foreign markets; and
|
|
•
|
building an organization capable of supporting geographically dispersed operations.
|
|
•
|
delays in product shipments;
|
|
•
|
loss of revenue;
|
|
•
|
delay in market acceptance;
|
|
•
|
diversion of our resources;
|
|
•
|
damage to our reputation;
|
|
•
|
product recalls;
|
|
•
|
regulatory actions;
|
|
•
|
increased service or warranty costs; or
|
|
•
|
product liability claims.
|
|
•
|
problems involving production yields;
|
|
•
|
quality control and assurance;
|
|
•
|
component supply shortages;
|
|
•
|
import or export restrictions on components, materials or technology;
|
|
•
|
shortages of qualified personnel; and
|
|
•
|
compliance with state, federal and foreign regulations.
|
|
•
|
the jurisdictions in which profits are determined to be earned and taxed;
|
|
•
|
the resolution of issues arising from tax audits with various tax authorities;
|
|
•
|
changes in valuation of our deferred tax assets and liabilities;
|
|
•
|
increases in expenses not deductible for tax purposes, including write-offs of acquired intangibles and impairment of goodwill in connection with acquisitions;
|
|
•
|
changes in available tax credits and deductions;
|
|
•
|
changes in share-based compensation;
|
|
•
|
changes in tax laws or the interpretation of such tax laws and changes in generally accepted accounting principles; and
|
|
•
|
the repatriation of non-U.S. earnings for which we have not previously provided for U.S. taxes.
|
|
•
|
continued compliance to the QSR, which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the development and manufacturing process;
|
|
•
|
labeling regulations;
|
|
•
|
the FDA’s general prohibition against false or misleading statements in the labeling or promotion of products for unapproved or “off-label” uses;
|
|
•
|
stringent complaint reporting and Medical Device Reporting regulations, which requires that manufacturers keep detailed records of investigations or complaints against their devices and to report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur;
|
|
•
|
adequate use of the Corrective and Preventive Actions process to identify and correct or prevent significant systemic failures of products or processes or in trends which suggest same; and
|
|
•
|
the reporting of Corrections and Removals, which requires that manufacturers report to the FDA recalls and field corrective actions taken to reduce a risk to health or to remedy a violation of the FFDCA that may pose a risk to health.
|
|
•
|
the extent to which our products gain market acceptance;
|
|
•
|
actions relating to regulatory matters;
|
|
•
|
our timing and ability to develop our manufacturing and sales and marketing capabilities;
|
|
•
|
demand for our products;
|
|
•
|
the size and timing of particular sales and any collection delays related to those sales;
|
|
•
|
product quality and supply problems;
|
|
•
|
the progress of surgical training in the use of our products;
|
|
•
|
our ability to develop, introduce and market new or enhanced versions of our products on a timely basis;
|
|
•
|
third-party payor reimbursement policies;
|
|
•
|
our ability to protect our proprietary rights and defend against third party challenges;
|
|
•
|
our ability to license additional intellectual property rights; and
|
|
•
|
the progress and results of clinical trials.
|
|
•
|
announcements about us or our competitors;
|
|
•
|
quarterly variations in operating results;
|
|
•
|
introduction or abandonment of new technologies or products;
|
|
•
|
regulatory approvals and enforcement actions;
|
|
•
|
changes in product pricing policies;
|
|
•
|
changes in earnings estimates by analysts or changes in accounting policies;
|
|
•
|
economic changes and overall market volatility; and
|
|
•
|
political uncertainties.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
|
2013
|
2012
|
|||||||||||||
|
Fiscal
|
High
|
Low
|
High
|
Low
|
|||||||||||
|
First Quarter
|
$
|
583.67
|
|
$
|
459.44
|
|
$
|
546.31
|
|
$
|
440.00
|
|
|||
|
Second Quarter
|
$
|
513.49
|
|
$
|
470.30
|
|
$
|
588.28
|
|
$
|
503.01
|
|
|||
|
Third Quarter
|
$
|
504.01
|
|
$
|
363.89
|
|
$
|
566.61
|
|
$
|
472.48
|
|
|||
|
Fourth Quarter
|
$
|
401.68
|
|
$
|
355.93
|
|
$
|
551.19
|
|
$
|
479.50
|
|
|||
|
Plan Category
|
Number of
securities to be
issued upon
exercise of
outstanding
options,
warrants and
rights (a)
|
Weighted-
average
exercise price
of outstanding
options
|
Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
|
||||||
|
Equity compensation plans approved by security holders
|
4,756,443
|
|
$
|
371.06
|
|
2,099,621
|
|
||
|
Equity compensation plans not approved by security holders
|
802,746
|
|
$
|
437.87
|
|
219,860
|
|
||
|
Total
|
5,559,189
|
|
$
|
380.71
|
|
2,319,481
|
|
||
|
Fiscal Period
|
Total Number of
Shares
Repurchased
|
Average
Price Paid
Per Share
|
Total Number of
Shares Purchased As
Part of a Publicly
Announced Program
|
Approximate Dollar
Amount of Shares That
May Yet be Purchased
Under the Program
|
|||||||||
|
October 1, 2013 to October 31, 2013
|
—
|
|
$
|
—
|
|
—
|
|
$
|
1,000.0
|
|
|||
|
November 1, 2013 to November 30, 2013
|
—
|
|
$
|
—
|
|
—
|
|
$
|
1,000.0
|
|
|||
|
December 1, 2013 to December 31,2013
|
—
|
|
$
|
—
|
|
—
|
|
$
|
1,000.0
|
|
|||
|
Total during quarter ended December 31, 2013
|
—
|
|
$
|
—
|
|
—
|
|
$
|
1,000.0
|
|
|||
|
COMPARISON OF CUMULATIVE TOTAL RETURN AMONG INTUITIVE SURGICAL, NASDAQ
COMPOSITE, S&P HEALTH CARE INDEX, AND S&P 500 INDEX
|
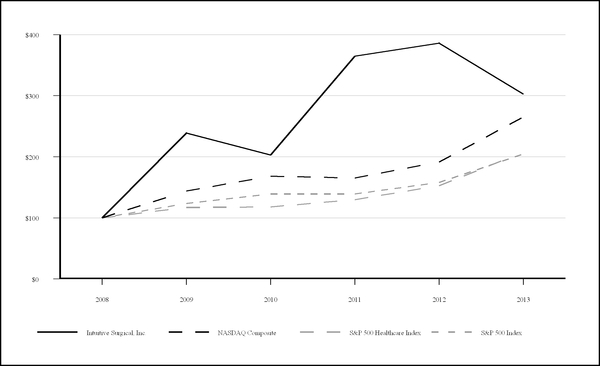
|
December 31,
|
|||||||||||||||||||||||
|
2008
|
2009
|
2010
|
2011
|
2012
|
2013
|
||||||||||||||||||
|
Intuitive Surgical, Inc.
|
$
|
100.00
|
|
$
|
238.94
|
|
$
|
202.97
|
|
$
|
364.60
|
|
$
|
386.15
|
|
$
|
302.45
|
|
|||||
|
NASDAQ Composite
|
$
|
100.00
|
|
$
|
143.89
|
|
$
|
168.22
|
|
$
|
165.19
|
|
$
|
191.47
|
|
$
|
264.84
|
|
|||||
|
S&P 500 Healthcare Index
|
$
|
100.00
|
|
$
|
117.07
|
|
$
|
117.90
|
|
$
|
129.89
|
|
$
|
152.58
|
|
$
|
207.42
|
|
|||||
|
S&P 500 Index
|
$
|
100.00
|
|
$
|
123.45
|
|
$
|
139.23
|
|
$
|
139.23
|
|
$
|
157.90
|
|
$
|
204.63
|
|
|||||
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
|
Years Ended December 31,
|
||||||||||||||||||
|
|
2013
|
2012
|
2011
|
2010
|
2009
|
||||||||||||||
|
|
(In millions, except per share amounts and headcount)
|
||||||||||||||||||
|
Revenue
|
$
|
2,265.1
|
|
$
|
2,178.8
|
|
$
|
1,757.3
|
|
$
|
1,413.0
|
|
$
|
1,052.2
|
|
||||
|
Gross profit
|
$
|
1,594.2
|
|
$
|
1,570.3
|
|
$
|
1,273.8
|
|
$
|
1,030.0
|
|
$
|
751.1
|
|
||||
|
Net income
(1)
|
$
|
671.0
|
|
$
|
656.6
|
|
$
|
495.1
|
|
$
|
381.8
|
|
$
|
232.6
|
|
||||
|
Net income per common share:
|
|||||||||||||||||||
|
Basic
|
$
|
17.12
|
|
$
|
16.50
|
|
$
|
12.63
|
|
$
|
9.74
|
|
$
|
6.07
|
|
||||
|
Diluted
|
$
|
16.73
|
|
$
|
15.98
|
|
$
|
12.32
|
|
$
|
9.47
|
|
$
|
5.93
|
|
||||
|
Shares used in computing basic and diluted net income per share:
|
|||||||||||||||||||
|
Basic
|
$
|
39.2
|
|
$
|
39.8
|
|
$
|
39.2
|
|
$
|
39.2
|
|
$
|
38.3
|
|
||||
|
Diluted
|
$
|
40.1
|
|
$
|
41.1
|
|
$
|
40.2
|
|
$
|
40.3
|
|
$
|
39.2
|
|
||||
|
Cash, cash equivalents and investments
|
$
|
2,753.9
|
|
$
|
2,920.5
|
|
$
|
2,171.8
|
|
$
|
1,608.9
|
|
$
|
1,172.0
|
|
||||
|
Total assets
|
$
|
3,950.3
|
|
$
|
4,059.2
|
|
$
|
3,063.1
|
|
$
|
2,390.4
|
|
$
|
1,809.7
|
|
||||
|
Other long-term liabilities
|
$
|
68.0
|
|
$
|
77.5
|
|
$
|
96.9
|
|
$
|
79.2
|
|
$
|
69.6
|
|
||||
|
Stockholders’ equity
|
$
|
3,501.4
|
|
$
|
3,580.1
|
|
$
|
2,645.6
|
|
$
|
2,037.4
|
|
$
|
1,537.3
|
|
||||
|
Total headcount
|
2,792
|
|
2,362
|
|
1,924
|
|
1,660
|
|
1,263
|
|
|||||||||
|
(1)
|
Net income for the years ended December 31,
2013
,
2012
,
2011
,
2010
, and
2009
included share-based compensation expense of
$110.4 million
,
$105.8 million
,
$93.5 million
,
$78.4 million
and
$70.5 million
, respectively, net of tax, related to employee stock options and employee stock purchases. Net income for the years ended December 31,
2013
,
2012
,
2011
,
2010
, and
2009
included amortization of purchased intellectual property of
$21.3 million
,
$23.1 million
,
$17.8 million
,
$16.7 million
, and
$15.6 million
, respectively. The 2013 tax provision included discrete tax benefits of $26.7 million associated with the reversal of unrecognized tax benefits resulting from expiration of statutes of limitations in multiple jurisdictions. In addition, the 2013 tax provision included a net tax benefit of $8.2 million related to 2012 federal R&D tax credit. The 2012 tax provision includes discrete tax benefits totaling $46.5 million, which is comprised of $38.0 million associated with the third quarter reversal of unrecognized tax benefits in conjunction with the expiration of certain statutes of limitations, and $8.5 million of benefits associated with certain previously unrecognized tax benefits as a result of new IRS guidance issued in the first quarter. The 2012 tax provision excludes a federal R&D tax credit due to its expiration at the end of 2011. There were no significant discrete tax benefits for years ended December 31, 2009 through December 31, 2011.
|
|
ITEM 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
Total revenue increased
4%
to
$2,265.1 million
during the year ended December 31,
2013
, from
$2,178.8 million
during the year ended December 31,
2012
.
|
|
•
|
Approximately
523,000
da Vinci
procedures were performed during the year ended December 31,
2013
, up approximately
16%
from the year ended December 31,
2012
.
|
|
•
|
Instruments and accessories revenue increased
14%
to
$1,032.9 million
during the year ended December 31,
2013
from
$903.3 million
during the year ended December 31,
2012
.
|
|
•
|
Recurring revenue increased
15%
to
$1,430.2 million
during the year ended December 31,
2013
, representing
63%
of total revenue, from
$1,245.9 million
during the year ended December 31,
2012
, representing
57%
of total revenue.
|
|
•
|
We sold
546
da Vinci
Surgical Systems during the year ended December 31,
2013
, compared with
620
for the year ended December 31,
2012
.
|
|
•
|
System revenue decreased
11%
to
$834.9 million
during the year ended December 31,
2013
from
$932.9 million
during the year ended December 31,
2012
.
|
|
•
|
As of December 31, 2013, we had a
da Vinci
Surgical System installed base of
2,966
systems -
2,083
in the U.S.,
476
in Europe,
159
in Japan and
248
in the rest of the world.
|
|
•
|
Operating income decreased
3%
to
$852.5 million
during the year ended December 31,
2013
compared with
$878.1 million
during the year ended December 31,
2012
. Operating income included
$168.9 million
and
$153.3 million
during the years ended December 31,
2013
and
2012
, respectively, of share-based compensation expense related to employee stock programs.
|
|
•
|
We ended fiscal
2013
with
$2,753.9 million
in cash, cash equivalents and investments. Cash, cash equivalents, and investments decreased by
$166.6 million
during
2013
driven by
$1,109.2 million
used to repurchase and retire
2.6 million
shares of common stock and $104.6 million used for capital expenditures, partially offset by cash flow from operations
and
$160.6 million
generated from employee stock programs.
|
|
•
|
We ended fiscal
2013
with
2,792
employees, compared to
2,362
at the end of fiscal
2012
. Headcount additions were made predominantly to our manufacturing, R&D organizations and field sales organizations.
|
|
|
Years Ended December 31,
|
|||||||||||||||||||
|
|
2013
|
% of
total
revenue
|
2012
|
% of
total
revenue
|
2011
|
% of
total
revenue
|
||||||||||||||
|
Revenue:
|
||||||||||||||||||||
|
Product
|
$
|
1,867.8
|
|
82
|
%
|
$
|
1,836.2
|
|
84
|
%
|
$
|
1,478.9
|
|
84
|
%
|
|||||
|
Service
|
397.3
|
|
18
|
%
|
342.6
|
|
16
|
%
|
278.4
|
|
16
|
%
|
||||||||
|
Total revenue
|
2,265.1
|
|
100
|
%
|
2,178.8
|
|
100
|
%
|
1,757.3
|
|
100
|
%
|
||||||||
|
Cost of revenue:
|
||||||||||||||||||||
|
Product
|
543.4
|
|
24
|
%
|
495.3
|
|
23
|
%
|
382.3
|
|
22
|
%
|
||||||||
|
Service
|
127.5
|
|
6
|
%
|
113.2
|
|
5
|
%
|
101.2
|
|
6
|
%
|
||||||||
|
Total cost of revenue
|
670.9
|
|
30
|
%
|
608.5
|
|
28
|
%
|
483.5
|
|
28
|
%
|
||||||||
|
Product gross profit
|
1,324.4
|
|
58
|
%
|
1,340.9
|
|
61
|
%
|
1,096.6
|
|
62
|
%
|
||||||||
|
Service gross profit
|
269.8
|
|
12
|
%
|
229.4
|
|
11
|
%
|
177.2
|
|
10
|
%
|
||||||||
|
Gross profit
|
1,594.2
|
|
70
|
%
|
1,570.3
|
|
72
|
%
|
1,273.8
|
|
72
|
%
|
||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Selling, general and administrative
|
574.0
|
|
25
|
%
|
522.2
|
|
24
|
%
|
438.8
|
|
25
|
%
|
||||||||
|
Research and development
|
167.7
|
|
7
|
%
|
170.0
|
|
8
|
%
|
140.2
|
|
8
|
%
|
||||||||
|
Total operating expenses
|
741.7
|
|
32
|
%
|
692.2
|
|
32
|
%
|
579.0
|
|
33
|
%
|
||||||||
|
Income from operations
|
852.5
|
|
38
|
%
|
878.1
|
|
40
|
%
|
694.8
|
|
39
|
%
|
||||||||
|
Interest and other income, net
|
18.4
|
|
1
|
%
|
15.8
|
|
1
|
%
|
14.9
|
|
1
|
%
|
||||||||
|
Income before taxes
|
870.9
|
|
39
|
%
|
893.9
|
|
41
|
%
|
709.7
|
|
40
|
%
|
||||||||
|
Income tax expense
|
199.9
|
|
9
|
%
|
237.3
|
|
11
|
%
|
214.6
|
|
12
|
%
|
||||||||
|
Net income
|
$
|
671.0
|
|
30
|
%
|
$
|
656.6
|
|
30
|
%
|
$
|
495.1
|
|
28
|
%
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
|
2012
|
|
2011
|
||||||
|
Revenue
|
|||||||||||
|
Instruments and accessories
|
$
|
1,032.9
|
|
$
|
903.3
|
|
$
|
701.1
|
|
||
|
Systems
|
834.9
|
|
932.9
|
|
777.8
|
|
|||||
|
Total product revenue
|
1,867.8
|
|
1,836.2
|
|
1,478.9
|
|
|||||
|
Services
|
397.3
|
|
342.6
|
|
278.4
|
|
|||||
|
Total revenue
|
$
|
2,265.1
|
|
$
|
2,178.8
|
|
$
|
1,757.3
|
|
||
|
Recurring revenue
|
$
|
1,430.2
|
|
$
|
1,245.9
|
|
$
|
979.5
|
|
||
|
% of total revenue
|
63
|
%
|
57
|
%
|
56
|
%
|
|||||
|
Domestic
|
$
|
1,625.9
|
|
$
|
1,726.9
|
|
$
|
1,378.7
|
|
||
|
International
|
639.2
|
|
451.9
|
|
378.6
|
|
|||||
|
Total revenue
|
$
|
2,265.1
|
|
$
|
2,178.8
|
|
$
|
1,757.3
|
|
||
|
% of Revenue—Domestic
|
72
|
%
|
79
|
%
|
78
|
%
|
|||||
|
% of Revenue—International
|
28
|
%
|
21
|
%
|
22
|
%
|
|||||
|
Unit Sales by Region:
|
|||||||||||
|
Domestic Unit Sales
|
342
|
|
476
|
|
400
|
|
|||||
|
International Unit Sales
|
204
|
|
144
|
|
134
|
|
|||||
|
Total Unit Sales
|
546
|
|
620
|
|
534
|
|
|||||
|
Unit Sales by Model:
|
|||||||||||
|
da Vinci Si-e—
Single console Unit Sales (3 arm)
|
30
|
|
26
|
|
16
|
|
|||||
|
da Vinci Si—
Single console Unit Sales (4 arm)
|
365
|
|
449
|
|
384
|
|
|||||
|
da Vinci Si
—Dual console Unit Sales
|
145
|
|
105
|
|
95
|
|
|||||
|
Total
da Vinci Si
Unit Sales
|
540
|
|
580
|
|
495
|
|
|||||
|
da Vinci S
Unit Sales
|
6
|
|
40
|
|
39
|
|
|||||
|
Total Unit Sales
|
546
|
|
620
|
|
534
|
|
|||||
|
Unit Sales involving System Trade-ins:
|
|||||||||||
|
Unit sales involving trade-ins of standard
da Vinci
Surgical Systems
|
28
|
|
51
|
|
65
|
|
|||||
|
Unit sales involving trade-ins of
da Vinci S
Surgical Systems
|
126
|
|
116
|
|
88
|
|
|||||
|
Total unit sales involving trade-ins
|
154
|
|
167
|
|
153
|
|
|||||
|
Unit Sales not involving trade-ins
|
392
|
|
453
|
|
381
|
|
|||||
|
Total Unit Sales
|
546
|
|
620
|
|
534
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
|
(in millions)
|
||||||||||
|
Net cash provided by (used in)
|
|||||||||||
|
Operating activities
|
$
|
880.0
|
|
$
|
814.2
|
|
$
|
677.6
|
|
||
|
Investing activities
|
259.0
|
|
(845.7
|
)
|
(479.0
|
)
|
|||||
|
Financing activities
|
(910.6
|
)
|
119.2
|
|
(12.4
|
)
|
|||||
|
Effect of exchange rates on cash and cash equivalents
|
—
|
|
0.2
|
|
(0.2
|
)
|
|||||
|
Net increase in cash and cash equivalents
|
$
|
228.4
|
|
$
|
87.9
|
|
$
|
186.0
|
|
||
|
1.
|
Our net income included substantial non-cash charges in the form of share-based compensation, amortization of intangible assets, taxes, and depreciation. These non-cash charges totaled
$231.0 million
during the year ended December 31, 2013.
|
|
2.
|
Cash used in working capital during the year ended December 31, 2013 was approximately
$22.0 million
.
|
|
1.
|
Our net income included substantial non-cash charges in the form of share-based compensation, amortization of intangible assets, taxes and depreciation. These non-cash charges totaled $223.1 million during the year ended December 31, 2012.
|
|
2.
|
Cash used in working capital during the year ended December 31, 2012 was approximately $65.5 million.
|
|
1.
|
Our net income included substantial non-cash charges in the form of share-based compensation, amortization of intangible assets, taxes and depreciation. These non-cash charges totaled $202.4 million during the year ended December 31, 2011.
|
|
2.
|
Cash used in working capital during the year ended December 31, 2011 was approximately $19.9 million.
|
|
|
Payments due by period
|
||||||||||||||
|
|
Total
|
Less than
1 year
|
1 to 3 years
|
3 to 5 years
|
|||||||||||
|
Operating leases
|
$
|
11.1
|
|
$
|
4.9
|
|
$
|
5.2
|
|
$
|
1.0
|
|
|||
|
Purchase commitments and obligations
|
268.3
|
|
268.1
|
|
0.2
|
|
—
|
|
|||||||
|
Total contractual obligations
|
$
|
279.4
|
|
$
|
273.0
|
|
$
|
5.4
|
|
$
|
1.0
|
|
|||
|
•
|
the valuation and recognition of investments, which impacts our investment portfolio balance when we assess fair value, and interest and other income, net, when we record impairments;
|
|
•
|
the valuation of revenue and allowance for sales returns and doubtful accounts, which impacts revenue;
|
|
•
|
the estimation of transactions to hedge, which impacts revenue and other expense;
|
|
•
|
the valuation of inventory, which impacts gross margins;
|
|
•
|
the assessment of recoverability of intangibles and the estimated useful lives, which primarily impacts gross margin or operating expenses when we record asset impairments or accelerate their amortization;
|
|
•
|
the valuation and recognition of share-based compensation, which impacts gross margin and operating expenses; and
|
|
•
|
the recognition and measurement of current and deferred income taxes (including the measurement of uncertain tax positions), which impact our provision for taxes.
|
|
•
|
the sufficiency of the trading volume of freely traded options;
|
|
•
|
the ability to reasonably match the terms, such as the date of the grant and the exercise price of the freely traded options to options granted; and
|
|
•
|
the length of the term of the freely traded options used to derive implied volatility.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
Page No.
|
|
|
|
December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
782.1
|
|
$
|
553.7
|
|
|
|
Short-term investments
|
621.4
|
|
770.7
|
|
|||
|
Accounts receivable, net of allowances of $0.5 and $3.0 at December 31, 2013 and 2012, respectively
|
301.4
|
|
370.3
|
|
|||
|
Inventory
|
179.6
|
|
121.5
|
|
|||
|
Prepaids and other current assets
|
38.3
|
|
67.3
|
|
|||
|
Deferred tax assets
|
9.6
|
|
9.3
|
|
|||
|
Total current assets
|
1,932.4
|
|
1,892.8
|
|
|||
|
Property, plant and equipment, net
|
309.9
|
|
241.8
|
|
|||
|
Long-term investments
|
1,350.4
|
|
1,596.1
|
|
|||
|
Long-term deferred tax asset
|
126.1
|
|
87.0
|
|
|||
|
Intangible and other assets, net
|
94.1
|
|
103.4
|
|
|||
|
Goodwill
|
137.4
|
|
138.1
|
|
|||
|
Total assets
|
$
|
3,950.3
|
|
$
|
4,059.2
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
46.2
|
|
$
|
57.6
|
|
|
|
Accrued compensation and employee benefits
|
70.7
|
|
104.0
|
|
|||
|
Deferred revenue
|
200.1
|
|
185.7
|
|
|||
|
Other accrued liabilities
|
63.9
|
|
54.3
|
|
|||
|
Total current liabilities
|
380.9
|
|
401.6
|
|
|||
|
Other long-term liabilities
|
68.0
|
|
77.5
|
|
|||
|
Total liabilities
|
448.9
|
|
479.1
|
|
|||
|
Commitments and contingencies (Note 6)
|
|
|
|
|
|||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, 2.5 shares authorized, $0.001 par value, issuable in series; no shares issued and outstanding as of December 31, 2013 and December 31, 2012, respectively
|
—
|
|
—
|
|
|||
|
Common stock, 100.0 shares authorized, $0.001 par value, 38.2 shares and 40.2 shares issued and outstanding as of December 31, 2013 and December 31, 2012, respectively
|
—
|
|
—
|
|
|||
|
Additional paid-in capital
|
2,519.9
|
|
2,240.1
|
|
|||
|
Retained earnings
|
979.4
|
|
1,333.4
|
|
|||
|
Accumulated other comprehensive income
|
2.1
|
|
6.6
|
|
|||
|
Total stockholders’ equity
|
3,501.4
|
|
3,580.1
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
3,950.3
|
|
$
|
4,059.2
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Revenue:
|
|||||||||||
|
Product
|
$
|
1,867.8
|
|
$
|
1,836.2
|
|
$
|
1,478.9
|
|
||
|
Service
|
397.3
|
|
342.6
|
|
278.4
|
|
|||||
|
Total revenue
|
2,265.1
|
|
2,178.8
|
|
1,757.3
|
|
|||||
|
Cost of revenue:
|
|||||||||||
|
Product
|
543.4
|
|
495.3
|
|
382.3
|
|
|||||
|
Service
|
127.5
|
|
113.2
|
|
101.2
|
|
|||||
|
Total cost of revenue
|
670.9
|
|
608.5
|
|
483.5
|
|
|||||
|
Gross profit
|
1,594.2
|
|
1,570.3
|
|
1,273.8
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Selling, general and administrative
|
574.0
|
|
522.2
|
|
438.8
|
|
|||||
|
Research and development
|
167.7
|
|
170.0
|
|
140.2
|
|
|||||
|
Total operating expenses
|
741.7
|
|
692.2
|
|
579.0
|
|
|||||
|
Income from operations
|
852.5
|
|
878.1
|
|
694.8
|
|
|||||
|
Interest and other income, net
|
18.4
|
|
15.8
|
|
14.9
|
|
|||||
|
Income before taxes
|
870.9
|
|
893.9
|
|
709.7
|
|
|||||
|
Income tax expense
|
199.9
|
|
237.3
|
|
214.6
|
|
|||||
|
Net income
|
$
|
671.0
|
|
$
|
656.6
|
|
$
|
495.1
|
|
||
|
Net income per share:
|
|||||||||||
|
Basic
|
$
|
17.12
|
|
$
|
16.50
|
|
$
|
12.63
|
|
||
|
Diluted
|
$
|
16.73
|
|
$
|
15.98
|
|
$
|
12.32
|
|
||
|
Shares used in computing net income per share:
|
|
|
|
||||||||
|
Basic
|
39.2
|
|
39.8
|
|
39.2
|
|
|||||
|
Diluted
|
40.1
|
|
41.1
|
|
40.2
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Net income
|
$
|
671.0
|
|
$
|
656.6
|
|
$
|
495.1
|
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Change in foreign currency translation gains (losses)
|
—
|
|
0.6
|
|
(0.3
|
)
|
|||||
|
Available-for-sale investments:
|
|||||||||||
|
Change in unrealized gains (losses), net of tax
|
(3.9
|
)
|
5.0
|
|
1.4
|
|
|||||
|
Less: Reclassification adjustment for gains (losses) on investments recognized during the year
|
(0.6
|
)
|
0.1
|
|
(1.6
|
)
|
|||||
|
Net change, net of tax effect
|
(4.5
|
)
|
5.1
|
|
(0.2
|
)
|
|||||
|
Derivative instruments:
|
|||||||||||
|
Change in unrealized gains (losses)
|
(1.8
|
)
|
(1.1
|
)
|
0.5
|
|
|||||
|
Less: Reclassification adjustment for gains (losses) on derivative instruments recognized during the year, net of tax
|
1.8
|
|
1.1
|
|
(0.7
|
)
|
|||||
|
Net change, net of tax effect
|
—
|
|
—
|
|
(0.2
|
)
|
|||||
|
Other comprehensive income (loss)
|
(4.5
|
)
|
5.7
|
|
(0.7
|
)
|
|||||
|
Total comprehensive income
|
$
|
666.5
|
|
$
|
662.3
|
|
$
|
494.4
|
|
||
|
Common Stock
|
Additional
Paid-In
Capital
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Income
|
Total
|
||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||
|
Balances at December 31, 2010
|
38.9
|
|
$
|
—
|
|
$
|
1,316.9
|
|
$
|
718.9
|
|
$
|
1.6
|
|
$
|
2,037.4
|
|
|||||
|
Issuance of common stock upon exercise of options and under stock purchase plan
|
1.4
|
|
260.6
|
|
260.6
|
|
||||||||||||||||
|
Income tax benefit from stock option exercises
|
48.6
|
|
48.6
|
|
||||||||||||||||||
|
Share-based compensation expense related to employee stock plans
|
136.4
|
|
136.4
|
|
||||||||||||||||||
|
Repurchase and retirement of common stock
|
(1.0
|
)
|
(19.7
|
)
|
(312.1
|
)
|
(331.8
|
)
|
||||||||||||||
|
Net income
|
495.1
|
|
495.1
|
|
||||||||||||||||||
|
Other comprehensive income (loss)
|
(0.7
|
)
|
(0.7
|
)
|
||||||||||||||||||
|
Balances at December 31, 2011
|
39.3
|
|
$
|
—
|
|
$
|
1,742.8
|
|
$
|
901.9
|
|
$
|
0.9
|
|
$
|
2,645.6
|
|
|||||
|
Issuance of common stock upon exercise of options and under stock purchase plan
|
1.3
|
|
263.3
|
|
263.3
|
|
||||||||||||||||
|
Income tax benefit from stock option exercises
|
93.9
|
|
93.9
|
|
||||||||||||||||||
|
Share-based compensation expense related to employee stock plans
|
153.3
|
|
153.3
|
|
||||||||||||||||||
|
Repurchase and retirement of common stock
|
(0.4
|
)
|
(13.2
|
)
|
(225.1
|
)
|
(238.3
|
)
|
||||||||||||||
|
Net income
|
656.6
|
|
656.6
|
|
||||||||||||||||||
|
Other comprehensive income (loss)
|
5.7
|
|
5.7
|
|
||||||||||||||||||
|
Balances at December 31, 2012
|
40.2
|
|
$
|
—
|
|
$
|
2,240.1
|
|
$
|
1,333.4
|
|
$
|
6.6
|
|
$
|
3,580.1
|
|
|||||
|
Issuance of common stock upon exercise of options and under stock purchase plan
|
0.6
|
|
160.6
|
|
160.6
|
|
||||||||||||||||
|
Income tax benefit from stock option exercises
|
34.5
|
|
34.5
|
|
||||||||||||||||||
|
Share-based compensation expense related to employee stock plans
|
168.9
|
|
168.9
|
|
||||||||||||||||||
|
Repurchase and retirement of common stock
|
(2.6
|
)
|
(84.2
|
)
|
(1,025.0
|
)
|
(1,109.2
|
)
|
||||||||||||||
|
Net income
|
671.0
|
|
671.0
|
|
||||||||||||||||||
|
Other comprehensive income (loss)
|
(4.5
|
)
|
(4.5
|
)
|
||||||||||||||||||
|
Balances at December 31, 2013
|
38.2
|
|
$
|
—
|
|
$
|
2,519.9
|
|
$
|
979.4
|
|
$
|
2.1
|
|
$
|
3,501.4
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Operating activities:
|
|||||||||||
|
Net income
|
$
|
671.0
|
|
$
|
656.6
|
|
$
|
495.1
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||||||
|
Depreciation
|
46.0
|
|
34.7
|
|
28.7
|
|
|||||
|
Amortization of intangible assets
|
21.3
|
|
23.1
|
|
17.8
|
|
|||||
|
Accretion of discounts and amortization of premiums on investments, net
|
36.8
|
|
33.1
|
|
22.6
|
|
|||||
|
Deferred income taxes
|
(38.5
|
)
|
(20.8
|
)
|
7.1
|
|
|||||
|
Income tax benefits from employee stock option plans
|
34.5
|
|
93.9
|
|
48.6
|
|
|||||
|
Excess tax benefit from share-based compensation
|
(38.0
|
)
|
(94.2
|
)
|
(58.8
|
)
|
|||||
|
Share-based compensation expense
|
168.9
|
|
153.3
|
|
136.4
|
|
|||||
|
Changes in operating assets and liabilities, net of effects of acquisition:
|
|||||||||||
|
Accounts receivable
|
68.9
|
|
(68.9
|
)
|
(51.1
|
)
|
|||||
|
Inventory
|
(70.0
|
)
|
(7.1
|
)
|
(25.3
|
)
|
|||||
|
Prepaids and other assets
|
(5.0
|
)
|
(37.1
|
)
|
(9.4
|
)
|
|||||
|
Accounts payable
|
(8.9
|
)
|
8.4
|
|
10.3
|
|
|||||
|
Accrued compensation and employee benefits
|
(33.3
|
)
|
21.0
|
|
19.6
|
|
|||||
|
Deferred revenue
|
15.2
|
|
30.5
|
|
28.1
|
|
|||||
|
Other accrued liabilities
|
11.1
|
|
(12.3
|
)
|
7.9
|
|
|||||
|
Net cash provided by operating activities
|
880.0
|
|
814.2
|
|
677.6
|
|
|||||
|
Investing activities:
|
|||||||||||
|
Purchase of investments
|
(1,443.7
|
)
|
(1,833.9
|
)
|
(1,532.2
|
)
|
|||||
|
Proceeds from sales of investments
|
984.9
|
|
329.8
|
|
444.3
|
|
|||||
|
Proceeds from maturities of investments
|
822.4
|
|
800.2
|
|
691.8
|
|
|||||
|
Purchase of property, plant and equipment, intellectual property and business
|
(104.6
|
)
|
(141.8
|
)
|
(82.9
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
259.0
|
|
(845.7
|
)
|
(479.0
|
)
|
|||||
|
Financing activities:
|
|||||||||||
|
Proceeds from issuance of common stock, net
|
160.6
|
|
263.3
|
|
260.6
|
|
|||||
|
Excess tax benefit from share-based compensation
|
38.0
|
|
94.2
|
|
58.8
|
|
|||||
|
Repurchase and retirement of common stock
|
(1,109.2
|
)
|
(238.3
|
)
|
(331.8
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(910.6
|
)
|
119.2
|
|
(12.4
|
)
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
—
|
|
0.2
|
|
(0.2
|
)
|
|||||
|
Net increase in cash and cash equivalents
|
228.4
|
|
87.9
|
|
186.0
|
|
|||||
|
Cash and cash equivalents, beginning of year
|
553.7
|
|
465.8
|
|
279.8
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
782.1
|
|
$
|
553.7
|
|
$
|
465.8
|
|
||
|
Supplemental cash flow information:
|
|||||||||||
|
Income taxes paid
|
$
|
194.1
|
|
$
|
226.1
|
|
$
|
152.0
|
|
||
|
|
Useful Lives
|
|
Building
|
Up to 30 years
|
|
Building improvements
|
Up to 15 years
|
|
Leasehold improvements
|
Lesser of useful life or term of lease
|
|
Equipment and furniture
|
5 years
|
|
Computer equipment
|
3 years
|
|
Enterprise-wide software
|
5 years
|
|
Purchased software
|
Lesser of 3 years or life of license
|
|
•
|
System sales.
For systems sold directly to end customers, revenue is recognized when acceptance occurs, which is deemed to have occurred upon the customer acknowledging delivery or installation, depending on the terms of the arrangement. For systems sold through distributors, revenue is recognized when title and risk of loss has transferred, which generally occurs at the time of shipment. Distributors do not have price protection rights and the Company’s system arrangements generally do not provide a rights of return. The
da Vinci
Surgical Systems are delivered with a software component. However, since the software and non-software elements function together to deliver the system’s essential functionality, the Company's arrangements are excluded from being accounted for under software revenue recognition guidance.
|
|
•
|
Instruments and accessories.
Revenue from sales of instruments and accessories is generally recognized at the time of shipment. The Company allows its customers in the normal course of business to return unused products for a limited period of time subsequent to initial purchase and records an allowance against revenue recognized based on historical experience.
|
|
•
|
Service.
Service revenue is recognized ratably over the term of the service period. Revenue related to services performed on a time-and-materials basis is recognized when it is earned and billable.
|
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair
Value
|
Cash and
Cash
Equivalents
|
Short-term
Investments
|
Long-term
Investments
|
|||||||||||||||||||||
|
December 31, 2013
|
|||||||||||||||||||||||||||
|
Cash
|
$
|
247.8
|
|
$
|
—
|
|
$
|
—
|
|
$
|
247.8
|
|
$
|
247.8
|
|
$
|
—
|
|
$
|
—
|
|
||||||
|
Level 1:
|
|||||||||||||||||||||||||||
|
Money market funds
|
516.2
|
|
—
|
|
—
|
|
516.2
|
|
516.2
|
|
—
|
|
—
|
|
|||||||||||||
|
U.S. Treasuries & corporate equity securities
|
65.4
|
|
—
|
|
(0.3
|
)
|
65.1
|
|
—
|
|
25.5
|
|
39.6
|
|
|||||||||||||
|
Subtotal
|
581.6
|
|
—
|
|
(0.3
|
)
|
581.3
|
|
516.2
|
|
25.5
|
|
39.6
|
|
|||||||||||||
|
Level 2:
|
|||||||||||||||||||||||||||
|
Commercial paper
|
100.2
|
|
—
|
|
—
|
|
100.2
|
|
18.1
|
|
82.1
|
|
—
|
|
|||||||||||||
|
Corporate securities
|
844.7
|
|
2.9
|
|
(1.9
|
)
|
845.7
|
|
—
|
|
227.7
|
|
618.0
|
|
|||||||||||||
|
U.S. government agencies
|
352.2
|
|
0.7
|
|
(0.7
|
)
|
352.2
|
|
—
|
|
84.7
|
|
267.5
|
|
|||||||||||||
|
Non-U.S. government securities
|
67.7
|
|
0.2
|
|
(0.1
|
)
|
67.8
|
|
—
|
|
41.2
|
|
26.6
|
|
|||||||||||||
|
Municipal securities
|
550.1
|
|
1.5
|
|
(0.1
|
)
|
551.5
|
|
—
|
|
160.2
|
|
391.3
|
|
|||||||||||||
|
Subtotal
|
1,914.9
|
|
5.3
|
|
(2.8
|
)
|
1,917.4
|
|
18.1
|
|
595.9
|
|
1,303.4
|
|
|||||||||||||
|
Level 3:
|
|||||||||||||||||||||||||||
|
Auction rate securities
|
8.0
|
|
—
|
|
(0.6
|
)
|
7.4
|
|
—
|
|
—
|
|
7.4
|
|
|||||||||||||
|
Subtotal
|
8.0
|
|
—
|
|
(0.6
|
)
|
7.4
|
|
—
|
|
—
|
|
7.4
|
|
|||||||||||||
|
Total assets measured at fair value
|
$
|
2,752.3
|
|
$
|
5.3
|
|
$
|
(3.7
|
)
|
$
|
2,753.9
|
|
$
|
782.1
|
|
$
|
621.4
|
|
$
|
1,350.4
|
|
||||||
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair
Value
|
Cash and
Cash
Equivalents
|
Short-term
Investments
|
Long-term
Investments
|
|||||||||||||||||||||
|
December 31, 2012
|
|||||||||||||||||||||||||||
|
Cash
|
$
|
89.7
|
|
$
|
—
|
|
$
|
—
|
|
$
|
89.7
|
|
$
|
89.7
|
|
$
|
—
|
|
$
|
—
|
|
||||||
|
Level 1:
|
|||||||||||||||||||||||||||
|
Money market funds
|
388.1
|
|
—
|
|
—
|
|
388.1
|
|
388.1
|
|
—
|
|
—
|
|
|||||||||||||
|
U.S. Treasuries & corporate equity securities
|
179.2
|
|
0.2
|
|
—
|
|
179.4
|
|
—
|
|
155.4
|
|
24.0
|
|
|||||||||||||
|
Subtotal
|
567.3
|
|
0.2
|
|
—
|
|
567.5
|
|
388.1
|
|
155.4
|
|
24.0
|
|
|||||||||||||
|
Level 2:
|
|||||||||||||||||||||||||||
|
Commercial paper
|
157.4
|
|
—
|
|
—
|
|
157.4
|
|
75.9
|
|
81.5
|
|
—
|
|
|||||||||||||
|
Corporate securities
|
952.1
|
|
5.8
|
|
(0.4
|
)
|
957.5
|
|
—
|
|
274.6
|
|
682.9
|
|
|||||||||||||
|
U.S. government agencies
|
636.9
|
|
2.6
|
|
—
|
|
639.5
|
|
—
|
|
133.6
|
|
505.9
|
|
|||||||||||||
|
Non-U.S. government securities
|
90.8
|
|
0.5
|
|
—
|
|
91.3
|
|
—
|
|
21.8
|
|
69.5
|
|
|||||||||||||
|
Municipal securities
|
409.3
|
|
1.1
|
|
(0.2
|
)
|
410.2
|
|
—
|
|
103.8
|
|
306.4
|
|
|||||||||||||
|
Subtotal
|
2,246.5
|
|
10.0
|
|
(0.6
|
)
|
2,255.9
|
|
75.9
|
|
615.3
|
|
1,564.7
|
|
|||||||||||||
|
Level 3:
|
|||||||||||||||||||||||||||
|
Auction rate securities
|
8.0
|
|
—
|
|
(0.6
|
)
|
7.4
|
|
—
|
|
—
|
|
7.4
|
|
|||||||||||||
|
Subtotal
|
8.0
|
|
—
|
|
(0.6
|
)
|
7.4
|
|
—
|
|
—
|
|
7.4
|
|
|||||||||||||
|
Total assets measured at fair value
|
$
|
2,911.5
|
|
$
|
10.2
|
|
$
|
(1.2
|
)
|
$
|
2,920.5
|
|
$
|
553.7
|
|
$
|
770.7
|
|
$
|
1,596.1
|
|
||||||
|
Amortized
Cost
|
Fair
Value
|
||||||
|
Mature in less than one year
|
$
|
1,154.6
|
|
$
|
1,155.7
|
|
|
|
Mature in one to five years
|
1,341.9
|
|
1,343.0
|
|
|||
|
Mature in after five years
|
8.0
|
|
7.4
|
|
|||
|
Total
|
$
|
2,504.5
|
|
$
|
2,506.1
|
|
|
|
|
Unrealized losses less
than 12 months
|
Unrealized losses 12
months or greater
|
Total
|
||||||||||||||||||||
|
December 31, 2013
|
Fair
Value
|
Unrealized
Losses
|
Fair
Value
|
Unrealized
Losses
|
Fair
Value
|
Unrealized
Losses
|
|||||||||||||||||
|
Corporate securities
|
$
|
245.3
|
|
$
|
(1.9
|
)
|
$
|
9.5
|
|
$
|
—
|
|
$
|
254.8
|
|
$
|
(1.9
|
)
|
|||||
|
U.S. government and agency securities
|
142.8
|
|
(1.0
|
)
|
—
|
|
—
|
|
142.8
|
|
(1.0
|
)
|
|||||||||||
|
Municipal securities
|
37.6
|
|
(0.1
|
)
|
—
|
|
—
|
|
37.6
|
|
(0.1
|
)
|
|||||||||||
|
Non-U.S. government securities
|
18.7
|
|
(0.1
|
)
|
—
|
|
—
|
|
18.7
|
|
(0.1
|
)
|
|||||||||||
|
Auction rate securities
|
—
|
|
—
|
|
7.4
|
|
(0.6
|
)
|
7.4
|
|
(0.6
|
)
|
|||||||||||
|
$
|
444.4
|
|
$
|
(3.1
|
)
|
$
|
16.9
|
|
$
|
(0.6
|
)
|
$
|
461.3
|
|
$
|
(3.7
|
)
|
||||||
|
December 31, 2012
|
|
|
|
|
|
|
|||||||||||||||||
|
Corporate securities
|
$
|
148.5
|
|
$
|
(0.2
|
)
|
$
|
8.4
|
|
$
|
(0.2
|
)
|
$
|
156.9
|
|
$
|
(0.4
|
)
|
|||||
|
Municipal securities
|
127.0
|
|
(0.2
|
)
|
—
|
|
—
|
|
127.0
|
|
(0.2
|
)
|
|||||||||||
|
Commercial paper
|
27.1
|
|
—
|
|
—
|
|
—
|
|
27.1
|
|
—
|
|
|||||||||||
|
Non-U.S. government securities
|
3.8
|
|
—
|
|
—
|
|
—
|
|
3.8
|
|
—
|
|
|||||||||||
|
U.S. government agencies
|
15.3
|
|
—
|
|
—
|
|
—
|
|
15.3
|
|
—
|
|
|||||||||||
|
Auction rate securities
|
—
|
|
—
|
|
7.4
|
|
(0.6
|
)
|
7.4
|
|
(0.6
|
)
|
|||||||||||
|
$
|
321.7
|
|
$
|
(0.4
|
)
|
$
|
15.8
|
|
$
|
(0.8
|
)
|
$
|
337.5
|
|
$
|
(1.2
|
)
|
||||||
|
|
Fair Value Measurements at
Reporting Date Using
Significant Unobservable
Inputs (Level 3)
|
||
|
|
Auction rate securities
|
||
|
Balance at January 1, 2011
|
$
|
18.6
|
|
|
Sales
|
(2.6
|
)
|
|
|
Total gains or (losses):
|
|||
|
Included in other comprehensive income (loss)
|
0.4
|
|
|
|
Included in earnings
|
—
|
|
|
|
Balance at December 31, 2011
|
$
|
16.4
|
|
|
Sales
|
(12.0
|
)
|
|
|
Total gains or (losses):
|
|||
|
Included in other comprehensive income (loss)
|
3.0
|
|
|
|
Included in earnings
|
—
|
|
|
|
Balance at December 31, 2012
|
$
|
7.4
|
|
|
Sales
|
—
|
|
|
|
Total gains or (losses):
|
|||
|
Included in other comprehensive income (loss)
|
—
|
|
|
|
Included in earnings
|
—
|
|
|
|
Balance at December 31, 2013
|
$
|
7.4
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Recognized gains (losses) in interest and other income, net
|
$
|
(3.4
|
)
|
$
|
(0.3
|
)
|
$
|
(1.2
|
)
|
||
|
Foreign exchange gains (losses) related to re-measurement
|
$
|
3.1
|
|
$
|
0.7
|
|
$
|
0.3
|
|
||
|
Years Ended December 31,
|
||||||||
|
2013
|
2012
|
2011
|
||||||
|
European Euro
|
64.0
|
|
37.6
|
|
35.0
|
|
||
|
Korean Won
|
23,000.0
|
|
4,600.0
|
|
—
|
|
||
|
British Pound
|
7.7
|
|
5.4
|
|
1.8
|
|
||
|
Swiss Franc
|
—
|
|
(1.0
|
)
|
(1.7
|
)
|
||
|
Japanese Yen
|
60.0
|
|
—
|
|
—
|
|
||
|
|
December 31,
|
||||||
|
|
2013
|
|
2012
|
||||
|
Inventory:
|
|||||||
|
Raw materials
|
$
|
67.2
|
|
$
|
41.2
|
|
|
|
Work-in-process
|
12.6
|
|
4.4
|
|
|||
|
Finished goods
|
99.8
|
|
75.9
|
|
|||
|
Total inventory
|
$
|
179.6
|
|
$
|
121.5
|
|
|
|
|
December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
Property, plant and equipment, net:
|
|||||||
|
Land
|
$
|
84.7
|
|
$
|
83.6
|
|
|
|
Building and building/leasehold improvements
|
151.9
|
|
99.1
|
|
|||
|
Machinery and equipment
|
137.2
|
|
105.0
|
|
|||
|
Computer and office equipment
|
27.1
|
|
19.2
|
|
|||
|
Capitalized software
|
66.9
|
|
53.9
|
|
|||
|
Construction-in-process
|
16.4
|
|
15.4
|
|
|||
|
484.2
|
|
376.2
|
|
||||
|
Less: Accumulated depreciation
|
(174.3
|
)
|
(134.4
|
)
|
|||
|
Total property, plant and equipment, net
|
$
|
309.9
|
|
$
|
241.8
|
|
|
|
|
December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
Other accrued liabilities—short term:
|
|||||||
|
Other accrued liabilities
|
$
|
52.8
|
|
$
|
44.6
|
|
|
|
Taxes payable
|
5.1
|
|
5.8
|
|
|||
|
Accrued professional fees
|
6.0
|
|
3.9
|
|
|||
|
Total other accrued liabilities—short-term
|
$
|
63.9
|
|
$
|
54.3
|
|
|
|
|
December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
Other long-term liabilities:
|
|||||||
|
Income taxes—long term
|
$
|
64.5
|
|
$
|
76.7
|
|
|
|
Other long-term liabilities
|
3.5
|
|
0.8
|
|
|||
|
Total other long-term liabilities
|
$
|
68.0
|
|
$
|
77.5
|
|
|
|
Fiscal Year
|
Amount
|
||
|
2014
|
$
|
18.0
|
|
|
2015
|
17.7
|
|
|
|
2016
|
14.7
|
|
|
|
2017
|
8.8
|
|
|
|
2018
|
5.1
|
|
|
|
2019 and thereafter
|
—
|
|
|
|
Total
|
$
|
64.3
|
|
|
Years
|
Amount
|
||
|
2014
|
$
|
4.9
|
|
|
2015
|
3.4
|
|
|
|
2016
|
1.8
|
|
|
|
2017
|
0.7
|
|
|
|
2018
|
0.3
|
|
|
|
2019 and thereafter
|
—
|
|
|
|
$
|
11.1
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Shares repurchased
|
2.6
|
|
0.4
|
|
1.0
|
|
|||||
|
Average price per share
|
$
|
429.09
|
|
$
|
503.05
|
|
$
|
344.72
|
|
||
|
Value of shares repurchased
|
$
|
1,109.2
|
|
$
|
238.3
|
|
$
|
331.8
|
|
||
|
|
Year Ended December 31, 2013
|
||||||||||||||
|
|
Gains (Losses)
on Hedge
Instruments
|
Unrealized Gains
(Losses) on
Available-for-
Sale Securities
|
Foreign
Currency
Translation
Gains (Losses)
|
Total
|
|||||||||||
|
Beginning balance
|
$
|
—
|
|
$
|
6.2
|
|
$
|
0.4
|
|
$
|
6.6
|
|
|||
|
Other comprehensive income before reclassifications
|
(1.8
|
)
|
(3.9
|
)
|
—
|
|
(5.7
|
)
|
|||||||
|
Reclassified from accumulated other comprehensive income
|
1.8
|
|
(0.6
|
)
|
—
|
|
1.2
|
|
|||||||
|
Net current-period other comprehensive income
|
—
|
|
(4.5
|
)
|
—
|
|
(4.5
|
)
|
|||||||
|
Ending balance
|
$
|
—
|
|
$
|
1.7
|
|
$
|
0.4
|
|
$
|
2.1
|
|
|||
|
|
Year Ended December 31, 2012
|
||||||||||||||
|
|
Gains (Losses)
on Hedge
Instruments
|
Unrealized Gains
(Losses) on
Available-for-
Sale Securities
|
Foreign
Currency
Translation
Gains (Losses)
|
Total
|
|||||||||||
|
Beginning balance
|
$
|
—
|
|
$
|
1.1
|
|
$
|
(0.2
|
)
|
$
|
0.9
|
|
|||
|
Other comprehensive income before reclassifications
|
(1.1
|
)
|
5.0
|
|
0.6
|
|
4.5
|
|
|||||||
|
Reclassified from accumulated other comprehensive income
|
1.1
|
|
0.1
|
|
—
|
|
1.2
|
|
|||||||
|
Net current-period other comprehensive income
|
—
|
|
5.1
|
|
0.6
|
|
5.7
|
|
|||||||
|
Ending balance
|
$
|
—
|
|
$
|
6.2
|
|
$
|
0.4
|
|
$
|
6.6
|
|
|||
|
|
|
STOCK OPTIONS OUTSTANDING
|
|||||||
|
|
Shares
Available
for Grant
|
Number
Outstanding
|
Weighted Average
Exercise Price Per
Share
|
||||||
|
Balance at December 31, 2012 (with 2.5 options exercisable at a weighted-average exercise price of $268.31 per share and with 4.8 options vested and expected to vest at a weighted-average exercise price of $340.83 per share)
|
1.6
|
|
4.8
|
|
$
|
340.83
|
|
||
|
Options authorized
|
1.6
|
|
—
|
|
$
|
—
|
|
||
|
Options granted
|
(1.5
|
)
|
1.5
|
|
$
|
471.33
|
|
||
|
Options exercised
|
—
|
|
(0.5
|
)
|
$
|
250.17
|
|
||
|
Options forfeited/expired
|
0.2
|
|
(0.2
|
)
|
$
|
456.64
|
|
||
|
Balance at December 31, 2013
|
1.9
|
|
5.6
|
|
$
|
380.71
|
|
||
|
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||||||
|
Range of
Exercise Prices
|
Number
of Shares
|
Weighted
Average
Remaining
Contractual Life
|
Weighted
Average
Exercise Price
Per Share
|
Aggregate
Intrinsic
Value (1)
|
Number
of Shares
|
Weighted
Average
Remaining
Contractual Life
|
Weighted
Average
Exercise Price
Per Share
|
Aggregate
Intrinsic
Value (1)
|
|||||||||||||||||
|
$18.50-$303.27
|
1.2
|
|
4.23
|
$
|
184.37
|
|
1.2
|
|
$
|
182.68
|
|
||||||||||||||
|
$308.96-$341.19
|
1.4
|
|
6.55
|
$
|
336.63
|
|
1.1
|
|
$
|
335.80
|
|
||||||||||||||
|
$343.83-$504.43
|
1.2
|
|
8.92
|
$
|
400.49
|
|
0.3
|
|
$
|
392.02
|
|
||||||||||||||
|
$505.23-$518.29
|
1.1
|
|
8.26
|
$
|
511.45
|
|
0.5
|
|
$
|
511.37
|
|
||||||||||||||
|
$529.51-$579.24
|
0.7
|
|
8.89
|
$
|
565.89
|
|
0.2
|
|
$
|
565.61
|
|
||||||||||||||
|
TOTAL
|
5.6
|
|
7.17
|
$
|
380.71
|
|
$
|
311.2
|
|
3.3
|
|
6.16
|
$
|
324.60
|
|
$
|
293.3
|
|
|||||||
|
(1)
|
The aggregate intrinsic value represents the total pre-tax intrinsic value, based on the Company’s closing stock price of
$384.08
as of
December 31, 2013
, which would have been received by the option holders had all in-the-money option holders exercised their options as of that date.
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Cost of sales—products
|
$
|
17.6
|
|
$
|
14.1
|
|
$
|
12.3
|
|
||
|
Cost of sales—services
|
12.7
|
|
12.9
|
|
11.0
|
|
|||||
|
Total cost of sales
|
30.3
|
|
27.0
|
|
23.3
|
|
|||||
|
Selling, general and administrative
|
101.4
|
|
93.1
|
|
84.3
|
|
|||||
|
Research and development
|
37.2
|
|
33.2
|
|
28.8
|
|
|||||
|
Share-based compensation expense before income taxes
|
168.9
|
|
153.3
|
|
136.4
|
|
|||||
|
Income tax effect
|
58.5
|
|
47.5
|
|
42.9
|
|
|||||
|
Share-based compensation expense after income taxes
|
$
|
110.4
|
|
$
|
105.8
|
|
$
|
93.5
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
STOCK OPTION PLANS
|
2013
|
2012
|
2011
|
||||||||
|
Average risk free interest rate
|
1.2
|
%
|
0.8
|
%
|
2.2
|
%
|
|||||
|
Average expected term (years)
|
4.5
|
|
4.3
|
|
4.8
|
|
|||||
|
Average volatility
|
30
|
%
|
33
|
%
|
35
|
%
|
|||||
|
Weighted average fair value at grant date
|
$
|
126.50
|
|
$
|
146.26
|
|
$
|
116.03
|
|
||
|
Total share-based compensation expense (in millions)
|
$
|
157.4
|
|
$
|
139.9
|
|
$
|
128.3
|
|
||
|
EMPLOYEE STOCK PURCHASE PLAN
|
|
|
|
||||||||
|
Average risk free interest rate
|
0.2
|
%
|
0.2
|
%
|
0.3
|
%
|
|||||
|
Average expected term (years)
|
1.3
|
|
1.3
|
|
1.3
|
|
|||||
|
Average volatility
|
34
|
%
|
32
|
%
|
33
|
%
|
|||||
|
Weighted average fair value at grant date
|
$
|
153.33
|
|
$
|
138.61
|
|
$
|
99.94
|
|
||
|
Total share-based compensation expense (in millions)
|
$
|
11.5
|
|
$
|
13.4
|
|
$
|
8.1
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
U.S
|
$
|
612.5
|
|
$
|
718.5
|
|
$
|
540.3
|
|
||
|
Foreign
|
258.4
|
|
175.4
|
|
169.4
|
|
|||||
|
Total income before provision for income taxes
|
$
|
870.9
|
|
$
|
893.9
|
|
$
|
709.7
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Current
|
|||||||||||
|
Federal
|
$
|
216.8
|
|
$
|
239.9
|
|
$
|
195.7
|
|
||
|
State
|
17.4
|
|
17.0
|
|
8.1
|
|
|||||
|
Foreign
|
4.3
|
|
3.4
|
|
3.7
|
|
|||||
|
$
|
238.5
|
|
$
|
260.3
|
|
$
|
207.5
|
|
|||
|
Deferred
|
|||||||||||
|
Federal
|
$
|
(36.2
|
)
|
$
|
(20.6
|
)
|
$
|
6.8
|
|
||
|
State
|
(1.9
|
)
|
(1.2
|
)
|
0.4
|
|
|||||
|
Foreign
|
(0.5
|
)
|
(1.2
|
)
|
(0.1
|
)
|
|||||
|
$
|
(38.6
|
)
|
$
|
(23.0
|
)
|
$
|
7.1
|
|
|||
|
Total income tax expense
|
$
|
199.9
|
|
$
|
237.3
|
|
$
|
214.6
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Federal tax at statutory rate
|
$
|
304.8
|
|
$
|
312.9
|
|
$
|
248.3
|
|
||
|
Increase (reduction) in tax resulting from:
|
|||||||||||
|
State taxes, net of federal benefits
|
15.5
|
|
15.8
|
|
8.5
|
|
|||||
|
Foreign rate differential
|
(73.6
|
)
|
(42.8
|
)
|
(47.7
|
)
|
|||||
|
Research and development credit
|
(12.2
|
)
|
—
|
|
(6.6
|
)
|
|||||
|
Share-based compensation not benefited
|
1.9
|
|
5.9
|
|
5.1
|
|
|||||
|
Domestic production activities deduction
|
(9.2
|
)
|
(8.2
|
)
|
—
|
|
|||||
|
Releases due to statute expirations and other
|
(26.7
|
)
|
(46.5
|
)
|
—
|
|
|||||
|
Other
|
(0.6
|
)
|
0.2
|
|
7.0
|
|
|||||
|
$
|
199.9
|
|
$
|
237.3
|
|
$
|
214.6
|
|
|||
|
|
December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
Deferred tax assets:
|
|||||||
|
Share-based compensation expense
|
$
|
123.4
|
|
$
|
85.3
|
|
|
|
Expenses deducted in later years for tax purposes
|
30.3
|
|
25.9
|
|
|||
|
Research and other credits
|
5.8
|
|
5.3
|
|
|||
|
Other
|
1.2
|
|
1.2
|
|
|||
|
Gross deferred tax assets
|
$
|
160.7
|
|
$
|
117.7
|
|
|
|
Valuation allowance
|
(7.2
|
)
|
(6.0
|
)
|
|||
|
Deferred tax assets
|
$
|
153.5
|
|
$
|
111.7
|
|
|
|
Deferred tax liabilities:
|
|||||||
|
Fixed assets
|
$
|
(16.1
|
)
|
$
|
(12.3
|
)
|
|
|
Identified intangible assets related to acquisitions
|
(1.9
|
)
|
(2.2
|
)
|
|||
|
Other
|
(2.1
|
)
|
(3.8
|
)
|
|||
|
Deferred tax liabilities
|
$
|
(20.1
|
)
|
$
|
(18.3
|
)
|
|
|
Net deferred tax assets
|
$
|
133.4
|
|
$
|
93.4
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Beginning balance
|
$
|
88.0
|
|
$
|
98.1
|
|
$
|
78.9
|
|
||
|
Increases related to tax positions taken during the current year
|
22.8
|
|
17.5
|
|
18.1
|
|
|||||
|
Increases related to tax positions taken during a prior year
|
—
|
|
12.9
|
|
1.1
|
|
|||||
|
Decreases related to tax positions taken during a prior year
|
(1.5
|
)
|
(8.3
|
)
|
—
|
|
|||||
|
Decreases related to settlements with tax authorities
|
(6.0
|
)
|
—
|
|
—
|
|
|||||
|
Decreases related to expiration of statute of limitations
|
(29.3
|
)
|
(32.2
|
)
|
—
|
|
|||||
|
Ending balance
|
$
|
74.0
|
|
$
|
88.0
|
|
$
|
98.1
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Net income
|
$
|
671.0
|
|
$
|
656.6
|
|
$
|
495.1
|
|
||
|
Basic:
|
|||||||||||
|
Weighted-average shares outstanding
|
39.2
|
|
39.8
|
|
39.2
|
|
|||||
|
Basic net income per share
|
$
|
17.12
|
|
$
|
16.50
|
|
$
|
12.63
|
|
||
|
Diluted:
|
|||||||||||
|
Weighted-average shares outstanding used in basic calculation
|
39.2
|
|
39.8
|
|
39.2
|
|
|||||
|
Add: Dilutive potential shares
|
0.9
|
|
1.3
|
|
1.0
|
|
|||||
|
Weighted-average shares used in computing diluted net income per share
|
40.1
|
|
41.1
|
|
40.2
|
|
|||||
|
Diluted net income per share
|
$
|
16.73
|
|
$
|
15.98
|
|
$
|
12.32
|
|
||
|
|
2013
|
||||||||||||||
|
|
Q1
|
Q2
|
Q3
|
Q4
|
|||||||||||
|
Revenue
|
$
|
611.4
|
|
$
|
578.5
|
|
$
|
499.0
|
|
$
|
576.2
|
|
|||
|
Gross profit
|
$
|
434.3
|
|
$
|
405.2
|
|
$
|
356.7
|
|
$
|
398.0
|
|
|||
|
Net income
(1)
|
$
|
188.9
|
|
$
|
159.1
|
|
$
|
156.8
|
|
$
|
166.2
|
|
|||
|
Net income per common share
|
|||||||||||||||
|
Basic
|
$
|
4.69
|
|
$
|
3.99
|
|
$
|
4.06
|
|
$
|
4.36
|
|
|||
|
Diluted
|
$
|
4.56
|
|
$
|
3.90
|
|
$
|
3.99
|
|
$
|
4.28
|
|
|||
|
(1) Includes one-time discrete tax benefits as follows:
|
|||||||||||||||
|
Expiration of the statutes of limitations in multiple jurisdictions
|
$
|
—
|
|
$
|
—
|
|
$
|
26.2
|
|
$
|
0.5
|
|
|||
|
Reinstatement of the 2012 federal R&D tax credit
|
$
|
7.5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
0.7
|
|
|||
|
|
2012
|
||||||||||||||
|
|
Q1
|
Q2
|
Q3
|
Q4
|
|||||||||||
|
Revenue
|
$
|
495.2
|
|
$
|
536.5
|
|
$
|
537.8
|
|
$
|
609.3
|
|
|||
|
Gross profit
|
$
|
355.9
|
|
$
|
386.4
|
|
$
|
390.1
|
|
$
|
437.9
|
|
|||
|
Net income
(1)
|
$
|
143.5
|
|
$
|
154.9
|
|
$
|
183.3
|
|
$
|
174.9
|
|
|||
|
Net income per common share
|
|||||||||||||||
|
Basic
|
$
|
3.63
|
|
$
|
3.88
|
|
$
|
4.59
|
|
$
|
4.37
|
|
|||
|
Diluted
|
$
|
3.50
|
|
$
|
3.75
|
|
$
|
4.46
|
|
$
|
4.25
|
|
|||
|
(1) Includes one-time discrete tax benefits as follows:
|
|||||||||||||||
|
Expiration of the statutes of limitations in multiple jurisdictions
|
$
|
—
|
|
$
|
—
|
|
$
|
38.0
|
|
$
|
—
|
|
|||
|
Benefit from new IRS guidance relating to the Section 199 Domestic Production Deduction
|
$
|
8.5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Balance at
Beginning of
Year
|
Additions
|
Deductions
(1)
|
Balance at
End of Year
|
||||||||||||
|
Allowance for doubtful accounts and sales returns
|
|||||||||||||||
|
Year ended December 31, 2013
|
$
|
8.0
|
|
$
|
14.1
|
|
$
|
(16.3
|
)
|
$
|
5.8
|
|
|||
|
Year ended December 31, 2012
|
$
|
5.6
|
|
$
|
15.0
|
|
$
|
(12.6
|
)
|
$
|
8.0
|
|
|||
|
Year ended December 31, 2011
|
$
|
4.8
|
|
$
|
12.3
|
|
$
|
(11.5
|
)
|
$
|
5.6
|
|
|||
|
(1)
|
Primarily represents amounts returned.
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
(i)
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
(ii)
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
(iii)
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULE
|
|
(a)
|
The following documents are filed as part of this Annual Report on Form 10-K
|
|
1)
|
Financial Statements—See Index to Consolidated Financial Statements at Item 8 of this Report on Form 10-K.
|
|
2)
|
The following financial statement schedule of Intuitive Surgical, Inc. is filed as part of this Report and should be read in conjunction with the financial statements of Intuitive Surgical:
|
|
3)
|
Exhibits
|
|
3.1(1)
|
Amended and Restated Certificate of Incorporation of the Company.
|
|
|
3.2(1)
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Company.
|
|
|
3.3(2)
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Company.
|
|
|
3.4(3)
|
Amended and Restated Bylaws of the Company.
|
|
|
4.1(4)
|
Specimen Stock Certificate.
|
|
|
10.1(4)
|
Form of Indemnity Agreement.
|
|
|
10.2(4)
|
2000 Equity Incentive Plan.
|
|
|
10.3(4)
|
2000 Non-Employee Directors’ Stock Option Plan.
|
|
|
10.4(4)
|
2000 Employee Stock Purchase Plan.
|
|
|
10.5(5)
|
2009 Employment Commencement Incentive Plan, as amended and restated.
|
|
|
10.6(6)
|
2010 Incentive Award Plan, as amended and restated.
|
|
|
10.7(7)
|
Severance Plan.
|
|
|
10.8(8)
|
Third Amendment effective as of July 1, 2010, to Employment Agreement between the Company and Lonnie M. Smith, dated February 28, 1997.
|
|
|
10.9(9)
|
Form of Intuitive Surgical, Inc. 2000 Equity Incentive Plan Stock Option Agreement (Incentive and Nonstatutory Stock Options).
|
|
|
21.1(5)
|
Intuitive Surgical, Inc. subsidiaries.
|
|
|
23.1(5)
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
31.1(5)
|
Certification of Principal Executive Officer.
|
|
|
31.2(5)
|
Certification of Principal Financial Officer.
|
|
|
32.1(5)
|
Certification of Chief Executive Officer and Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101(5)
|
The following materials from Intuitive Surgical, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2013, formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Income, (iii) Consolidated Statement of Stockholders’ Equity, (iv) Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements, tagged at Level I through IV.
|
|
|
(1)
|
Incorporated by reference to exhibits filed with the Company’s 2008 Annual Report on Form 10-K filed February 6, 2009 (File No. 000-30713).
|
|
(2)
|
Incorporated by reference to Exhibit A filed with the Company’s Definitive Proxy Statement on Schedule 14A filed March 1, 2012 (File No. 000-30713).
|
|
(3)
|
Incorporated by reference to Exhibit 3.1 filed with the Company’s Current Report on Form 8-K filed April 24, 2012 (File No. 000-30713).
|
|
(4)
|
Incorporated by reference to exhibits filed with the Company’s Registration Statement on Form S-1 filed March 22, 2000 (File No. 333-33016).
|
|
(5)
|
Filed herewith.
|
|
(6)
|
Incorporated by reference to Exhibit 4.1 filed with the Company’s Registration Statement on Form S-8 filed June 17, 2013 (File No. 333-189399).
|
|
(7)
|
Incorporated by reference to Exhibit 10.1 filed with the Company’s Current Report on Form 8-K filed December 2, 2008 (File No. 000-30713).
|
|
(8)
|
Incorporated by reference to Exhibit 10.1 filed with the Company’s Current Report on Form 8-K filed July 26, 2010 (File No. 000-30713).
|
|
(9)
|
Incorporated by reference to Exhibit 10.2 filed with the Company’s Quarterly Report on Form 10-Q filed July 23, 2009 (File No. 000-30713).
|
|
INTUITIVE SURGICAL, INC.
|
||
|
(Registrant)
|
||
|
By:
|
/
S
/ G
ARY
S. G
UTHART
|
|
|
Gary S. Guthart, Ph.D.
President and Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/
S
/ G
ARY
S. G
UTHART
|
President, Chief Executive Officer and Director (Principal Executive Officer)
|
February 3, 2014
|
||
|
Gary S. Guthart, Ph.D.
|
||||
|
/
S
/ M
ARSHALL
L. M
OHR
|
Senior Vice President and Chief Financial Officer (Principal Financial Officer)
|
February 3, 2014
|
||
|
Marshall L. Mohr
|
||||
|
/S/ J
AMIE
E. S
AMATH
|
Vice President, Corporate Controller, and Principal Accounting Officer
|
February 3, 2014
|
||
|
Jamie E. Samath
|
||||
|
/
S
/ L
ONNIE
M. S
MITH
|
Chairman of the Board of Directors
|
February 3, 2014
|
||
|
Lonnie M. Smith
|
||||
|
/
S
/ C
RAIG
H. B
ARRATT
|
Director
|
February 3, 2014
|
||
|
Craig H. Barratt, Ph.D.
|
||||
|
/
S
/ A
MAL
M. J
OHNSON
|
Director
|
February 3, 2014
|
||
|
Amal M. Johnson
|
||||
|
/
S
/ E
RIC
H. H
ALVORSON
|
Director
|
February 3, 2014
|
||
|
Eric H. Halvorson
|
||||
|
/
S
/ A
LAN
J. L
EVY
|
Director
|
February 3, 2014
|
||
|
Alan J. Levy, Ph.D.
|
||||
|
/
S
/ F
LOYD
D. L
OOP
|
Director
|
February 3, 2014
|
||
|
Floyd D. Loop, M.D.
|
||||
|
/
S
/ M
ARK
J. R
UBASH
|
Director
|
February 3, 2014
|
||
|
Mark J. Rubash
|
||||
|
/
S
/ G
EORGE
S
TALK
J
R
.
|
Director
|
February 3, 2014
|
||
|
George Stalk Jr.
|
||||