|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
|
16-1531026
|
|
(State of
Incorporation)
|
|
(I.R.S. Employer
Identification No.)
|
|
Title of Each Class:
|
|
Name of Each Exchange on Which Registered:
|
|
Common Stock, Par Value $0.001 Per Share
|
|
New York Stock Exchange
|
|
Large accelerated filer
|
x
|
Accelerated filer
|
¨
|
|
|
Non-accelerated filer
|
¨
|
Smaller reporting company
|
¨
|
|
|
Document
|
Part
|
|
|
Proxy Statement for the 2015 Annual Meeting of Stockholders
|
Part III, Item 10
“Directors, Executive Officers and Corporate Governance”
|
|
|
Part III, Item 11
“Executive Compensation”
|
||
|
Part III, Item 12
“Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters”
|
||
|
Part III, Item 13
“Certain Relationships and Related Transactions, and Director Independence”
|
||
|
Part III, Item 14
“Principal Accountant Fees and Services”
|
||
|
ITEM
NUMBER
|
PAGE
NUMBER
|
|
ITEM 1.
|
BUSINESS
|
|
|
Acquisition Date
|
Acquired Company
|
Business at Time of Acquisition
|
|
|
July 1997
|
Wilson Greatbatch Ltd.
|
Founded in 1970, designed and manufactured batteries for implantable medical and commercial applications.
|
|
|
August 1998
|
Hittman Materials and Medical Components, Inc.
|
Founded in 1962, designed and manufactured ceramic and glass feedthroughs and specialized porous coatings for electrodes used in implantable medical devices (“IMDs”).
|
|
|
August 2000
|
Battery Engineering, Inc.
|
Founded in 1983, designed and manufactured high-energy density batteries for industrial, commercial, military and medical applications.
|
|
|
June 2001
|
Sierra-KD Components division of Maxwell Technologies, Inc.
|
Founded in 1986, designed and manufactured ceramic electromagnetic filtering capacitors and integrated them with wire feedthroughs for use in IMDs as well as military, aerospace and commercial applications.
|
|
|
July 2002
|
Globe Tool and Manufacturing Company, Inc.
|
Founded in 1954, designed and manufactured precision enclosures used in IMDs and commercial products used in the aerospace, electronics and automotive sectors.
|
|
|
March 2004
|
NanoGram Devices Corporation
|
Founded in 1996, developed nanoscale materials for battery and medical device applications.
|
|
|
April 2007
|
BIOMEC, Inc.
|
Established in 1998, provided medical device design and component integration to early-stage and established customers.
|
|
|
Acquisition Date
|
Acquired Company
|
Business at Time of Acquisition
|
|
|
June 2007
|
Enpath Medical, Inc.
|
Founded in 1981, designed, developed, and manufactured venous introducers and dilators, implantable leadwires, steerable sheaths and steerable catheters.
|
|
|
October 2007
|
IntelliSensing LLC
|
Founded in 2005, designed and manufactured battery-powered wireless sensing solutions for commercial applications.
|
|
|
November 2007
|
Quan Emerteq LLC
|
Founded in 1998, designed, developed, and manufactured catheters, stimulation leadwires, microcomponents and assemblies.
|
|
|
November 2007
|
Engineered Assemblies Corporation
|
Founded in 1984, designed and integrated custom battery solutions and electronics focused on rechargeable systems for industrial, commercial, military and portable medical applications.
|
|
|
January 2008
|
P Medical Holding SA
|
Founded in 1994, designed, manufactured and supplied delivery systems, instruments and implants for the orthopaedics industry.
|
|
|
February 2008
|
DePuy Orthopaedics’ Chaumont, France manufacturing facility
|
Manufactured hip and shoulder implants for DePuy Orthopaedics.
|
|
|
December 2011
|
Micro Power Electronics, Inc. (“Micro Power”)
|
Founded in 1990, designed custom battery packs, smart chargers and power supplies for industrial, military and portable medical applications.
|
|
|
February 2012
|
NeuroNexus Technologies, Inc.
(“NeuroNexus”)
|
Founded in 2004, medical device design firm specializing in developing neural interface technology, components and systems.
|
|
|
August 2014
|
Centro de Construcción de Cardioestimuladores del Uruguay (“CCC”)
|
Founded in 1969, an active implantable neuromodulation medical device systems developer and manufacturer that produces a range of medical devices including implantable pulse generators, programmer systems, battery chargers, patient wands and leads.
|
|
|
Device
|
Market Size (in billions)
|
Principal Illness or Symptom
|
||
|
Pacemakers
|
$4.0
|
Abnormally slow heartbeat (Bradycardia)
|
||
|
ICDs
|
$3.7
|
Rapid and irregular heartbeat (Tachycardia)
|
||
|
CRT/CRT-Ds
|
$3.0
|
Congestive heart failure
|
||
|
Neurostimulators
|
$2.6
|
Chronic pain, movement disorders, epilepsy, obesity or depression
|
||
|
Cochlear hearing devices
|
$0.8
|
Hearing loss
|
||
|
•
|
Growing patient population
– Implantable pacemakers and ICDs remain primary therapies for a number of critical clinical conditions, most of which are non-elective in nature. As the prevalence of many of these clinical conditions increase with age, underlying population demographics in developed countries will provide an engine for procedure growth.
|
|
•
|
Focus on emerging markets
– OEMs have increased their focus and investment to expand physicians’ awareness of these life changing therapies, which we believe will result in increased utilization to improve quality of life for more patients globally. These growth initiatives will drive increased utilization of existing cardiac technologies and provide an avenue for new device and technology development as device manufacturers look to develop unique products for these markets.
|
|
•
|
Trends in device features
– IMD evolution continues to favor the development of smaller, longer lasting devices with increased functionality and more physiologic shapes. Innovative battery, capacitor, enclosure, and filtering solutions such as those provided by Greatbatch Medical are critical to the realization of these market needs.
|
|
•
|
Growth within neuromodulation
– Neuromodulation applications continue to grow at a faster pace than traditional markets, and are expected to continue to expand as new therapeutic applications are identified. There continues to be growth in clinical data supporting new applications and a growing focus and excitement from clinicians looking for treatment alternatives for challenging patient conditions that have not been traditionally served by implantable stimulation devices. As many cardiac OEM companies are also OEMs in the neuromodulation market, Greatbatch is well positioned to capitalize on these drivers of market growth based on the strength of existing relationships. Additionally, early stage neuromodulation OEMs have begun to receive CE and FDA approvals for their novel device systems and therapies, further fueling incremental growth in the market and providing new potential partners for Greatbatch technology.
|
|
•
|
Innovative and disruptive technologies
– Three innovative and disruptive device technologies (sub-cutaneous ICDs, leadless pacemakers and injectable loop recorders) continued to receive significant attention from OEMs in 2014. These new device technologies will play an important role in increasing utilization of critical therapy and diagnostic tools globally. Our portfolio of technologies and next generation development efforts are vital to the advancement of these new therapy and diagnostic platforms.
|
|
•
|
Aging population in developed markets
– Conditions like osteoarthritis and spine degeneration are underlying drivers of a diverse spectrum of reconstructive therapies, and increase significantly with age. Continued growth in the 65+ population, along with an increased desire to remain active, will provide a driver for procedural growth.
|
|
•
|
Rates of obesity
– Rates of obesity globally have continued to rise, and are expected to do so for the foreseeable future. Excess weight exacerbates wear on joints and will drive the need for replacement and revision procedures.
|
|
•
|
New implant and surgical technology
– The orthopaedic market continues to see a growing focus on minimally invasive procedures across a number of sectors including joint reconstruction and spinal fusion, potentially expanding the use of these therapeutic approaches.
|
|
•
|
Growth in emerging markets
– Growing affluence in emerging markets has provided an opportunity for global growth of a number of orthopaedic procedures. Patient populations outside of developed markets continue to be underpenetrated, and investment from large device manufacturers in these markets will provide for procedural growth of established therapies.
|
|
•
|
Growing global prevalence of vascular disease reflecting both the aging of the population in many developed markets and the continuing growth in the number of people with conditions such as diabetes, hypertension, and obesity.
|
|
•
|
Continued adoption of minimally invasive therapies in emerging markets.
|
|
•
|
Emergence of new minimally invasive therapies expanding patient pools to patients who previously would have remained either untreated or have undergone surgery.
|
|
Product
|
Description
|
Principal Product Attributes
|
|
|
Batteries
|
Lithium iodine (“Li Iodine”)
Lithium silver vanadium oxide (“Li SVO”)
Lithium carbon monoflouride (“Li CFx”)
Lithium ion rechargeable (“Li Ion”)
Lithium SVO/CFx (“Q
HR
” & “Q
MR
”)
|
High reliability and predictability;
Long service life;
Customized configuration;
Light weight;
High energy density, small size
|
|
|
Capacitors
|
Storage for energy generated by a battery before delivery to the heart. Used in ICDs and CRT-Ds.
|
Stores more energy per unit volume (energy density) than other existing technologies; Customized configuration
|
|
|
EMI filters
|
Filters electromagnetic interference to limit undesirable response, malfunctioning or degradation in the performance of electronic equipment
|
High reliability attenuation of EMI RF over wide frequency ranges; Customized design
|
|
|
Feedthroughs
|
Allow electrical signals to be brought from inside hermetically sealed IMD to an electrode
|
Ceramic to metal seal is substantially more durable than traditional seals; Multifunctional
|
|
|
Coated electrodes
|
Deliver electric signal from the feedthrough to a body part undergoing stimulation
|
High quality coated surface; Flexible in utilizing any combination of biocompatible coating surfaces; Customized offering of surfaces and tips
|
|
|
Precision components
|
Machined
Molded and over molded products
|
High level of manufacturing precision;
Broad manufacturing flexibility
|
|
|
Enclosures and related components
|
Titanium
Stainless steel
|
Precision manufacturing, flexibility in configurations and materials
|
|
|
Value-added assemblies
|
Combination of multiple components in a single package/unit
|
Leveraging products and capabilities to provide subassemblies and assemblies;
Provides synergies in component technology and procurement systems
|
|
|
Product
|
Description
|
Principal Product Attributes
|
|
|
Stimulation leads
|
Cardiac, neuromodulation and hearing restoration stimulation leads
|
Custom and unique configurations that increase therapy effectiveness, provide finished device design and manufacturing
|
|
|
Introducers
|
Conduit to deliver CRM leads or placement of dialysis catheters, CVCs, PICCs, and ports
|
Variety of sizes and configurations that facilitate reliable access in vascular access and CRM applications
|
|
|
Steerable sheaths
|
Steerable guide sheath for the delivery of diagnostic and ablation catheters
|
Configurations to enable effective delivery of diagnostic and therapeutic devices in electrophysiology procedures.
|
|
|
Specialty catheter shaft components
|
High performance catheter shafts designed to meet intended clinical performance characteristics
|
Deep catheter design expertise and state-of-the-art manufacturing services
|
|
|
Cases and trays
|
Delivery systems for cleaning and sterilizing orthopaedic instruments and implants
|
High degree of customization;
Short, predictable development and production timelines
|
|
|
Implants
|
Orthopaedic implants for large joint, spine, extremity and trauma procedures
|
Precision manufacturing, leveraging capabilities and product processes including sterile packaging and coatings
|
|
|
Instruments
|
Reusable and single use orthopaedic instruments for large joint, spine, extremity and trauma procedures
|
Designed to improve surgical techniques, reduce surgery time, and increase surgical precision
|
|
|
Primary cells
|
Low-rate
Moderate-rate
High rate (spiral) Wide Range
|
Optimized rate capability, shock and vibration resistant, high and low temperature tolerant, high energy density; Ability to operate in low and high temp applications
|
|
|
Primary and secondary battery packs
|
Highly-customized pack solutions
|
Diverse portfolio of cells in various sizes, temperature ranges and rate capabilities, custom-engineered and designed, value-add charging and battery management systems for secondary packs
|
|
|
Product Line
|
Competitors
|
|
Medical batteries
|
Eagle-Picher
Quallion
|
|
Capacitors
|
AVX (subsidiary of Kyocera) Critical Medical Components
|
|
Feedthroughs
|
Alberox (subsidiary of The Morgan Crucible Co. PLC)
|
|
EMI filtering
|
AVX (subsidiary of Kyocera)
Eurofarad
|
|
Enclosures
|
Heraeus
Hudson
National
|
|
Machined and molded components
|
Numerous
|
|
Value added assembly
|
Numerous
|
|
Catheters
|
Creganna
Teleflex
Vention medical |
|
Product Line
|
Competitors
|
|
Introducers
|
Pressure Products
Theragenics (Galt)
Merit Medical
|
|
Stimulation leads
|
Oscor
|
|
Orthopaedic trays, instruments and implants
|
Accelent
Avalign Technologies
IMDS
Micropulse, Inc.
Juno
Orchid
Sandvik
Symmetry
Paragon
Tecomet
|
|
Primary Power Solutions
|
Tracer Technologies
Engineered Power
Saft
Ultralife
|
|
Secondary Power Solutions
|
Totex
Palladium
ICC/Nexergy
BMZ
Ultralife
Saft
|
|
Manufacturing – U.S.
|
1,810
|
|
|
General and administrative – U.S.
|
134
|
|
|
Sales and marketing – U.S.
|
88
|
|
|
Research, development and engineering – U.S.
|
241
|
|
|
Chaumont, France facility
|
270
|
|
|
Switzerland facility
|
8
|
|
|
Tijuana, Mexico facility
|
969
|
|
|
Montevideo, Uruguay facility
|
170
|
|
|
Total
|
3,690
|
|
|
•
|
future sales, expenses and profitability;
|
|
•
|
future development and expected growth of our business and industry;
|
|
•
|
our ability to execute our business model and our business strategy;
|
|
•
|
our ability to identify trends within our industries and to offer products and services that meet the changing needs of those markets; and
|
|
•
|
projected capital expenditures.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
|
•
|
a substantial percentage of our costs are fixed in nature, which results in our operations being particularly sensitive to fluctuations in production volumes;
|
|
•
|
changes in the mix of our revenue represented by our various products and customers could result in reductions in our profits if the mix of our revenue represented by lower margin products increases;
|
|
•
|
timing of orders placed by our principal customers who account for a significant portion of our revenues; and
|
|
•
|
increased costs of raw materials or supplies.
|
|
•
|
inaccurate assessments of potential liabilities associated with the acquired businesses;
|
|
•
|
the existence of unknown or undisclosed liabilities associated with the acquired businesses;
|
|
•
|
diversion of our management’s attention from our core businesses;
|
|
•
|
potential loss of key employees or customers of the acquired businesses;
|
|
•
|
difficulties in integrating the operations and products of an acquired business or in realizing projected revenue growth, efficiencies and cost savings; and
|
|
•
|
increases in indebtedness and limitation in our ability to access capital if needed.
|
|
•
|
changes in foreign economic conditions and/or regulatory requirements;
|
|
•
|
local product preferences and product requirements;
|
|
•
|
longer-term receivables than are typical in the U.S.;
|
|
•
|
difficulties in enforcing agreements through foreign legal systems;
|
|
•
|
less protection of intellectual property in some countries outside of the U.S.;
|
|
•
|
trade protection measures and import and export licensing requirements;
|
|
•
|
work force instability;
|
|
•
|
political and economic instability; and
|
|
•
|
complex tax and cash management issues.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
|
ITEM 2.
|
PROPERTIES
|
|
|
Location
|
Sq. Ft.
|
Own/Lease
|
Principal Use
|
|||
|
Alden, NY
|
125,000
|
|
Own
|
Medical battery and capacitor manufacturing
|
||
|
Ann Arbor, MI
|
9,970
|
|
Lease
|
Office and lab space for design engineering team
|
||
|
Beaverton, OR
|
62,200
|
|
Lease
|
Commercial battery manufacturing
|
||
|
Biel, Switzerland
|
1,000
|
|
Lease
|
European corporate offices
|
||
|
Blaine, MN
|
32,400
|
|
Own
|
Medical device engineering
|
||
|
Chaumont, France
|
59,200
|
|
Own
|
Manufacturing of orthopaedic implants
|
||
|
Clarence, NY
|
117,800
|
|
Own
|
Corporate offices and RD&E
|
||
|
Clarence, NY
|
20,800
|
|
Own
|
Machining and assembly of components
|
||
|
Clarence, NY
|
18,600
|
|
Lease
|
Machining and assembly of components
|
||
|
Cleveland, OH
|
16,900
|
|
Lease
|
Office and lab space for design engineering team
|
||
|
Fort Wayne, IN
|
81,000
|
|
Own
|
Manufacturing of orthopaedic instruments
|
||
|
Frisco, TX
|
9,200
|
|
Lease
|
Global headquarters – principal executive office
|
||
|
Indianapolis, IN
|
82,600
|
|
Own
|
Manufacturing of orthopaedic cases and trays
|
||
|
Minneapolis, MN
|
72,000
|
|
Own
|
Enclosure manufacturing and engineering
|
||
|
Montevideo, Uruguay
|
21,900
|
|
Lease
|
Active implantable medical device systems assembly
|
||
|
Plymouth, MN
|
122,800
|
|
Lease
|
Introducers, catheters and leads manufacturing
|
||
|
Raynham, MA
|
81,000
|
|
Own
|
Commercial battery manufacturing and RD&E
|
||
|
Tijuana, Mexico
|
190,800
|
|
Lease
|
Feedthrough, catheters and orthopaedic instrument manufacturing and value-added assembly
|
||
|
Tijuana, Mexico
|
144,000
|
|
Lease
|
Portable medical and electronics assembly
|
||
|
Warsaw, IN
|
3,000
|
|
Lease
|
Orthopaedic rapid prototyping design center
|
||
|
•
|
Functions currently performed at our facility in Plymouth, MN to manufacture catheters and introducers will transfer into our existing facility in Tijuana, Mexico by the first half of 2016.
|
|
•
|
Functions currently performed at our facilities in Beaverton, OR and Raynham, MA to manufacture products for the portable medical market will transfer to a new facility in Tijuana, Mexico by the end of 2015.
|
|
•
|
Functions currently performed at our Cleveland, OH facility were transferred to our facilities in Minnesota.
|
|
•
|
Establishing a commercial operations hub at our global headquarters in Frisco, Texas. This initiative will build upon the investment we have made in our global sales and marketing function and is expected to be completed during the first half of 2015.
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
|
High
|
Low
|
Close
|
|||||||||
|
2013
|
|||||||||||
|
First Quarter
|
$
|
30.64
|
|
$
|
22.70
|
|
$
|
29.87
|
|
||
|
Second Quarter
|
34.41
|
|
27.03
|
|
32.79
|
|
|||||
|
Third Quarter
|
38.36
|
|
32.70
|
|
33.69
|
|
|||||
|
Fourth Quarter
|
45.02
|
|
33.24
|
|
43.80
|
|
|||||
|
2014
|
|||||||||||
|
First Quarter
|
$
|
47.78
|
|
$
|
40.02
|
|
$
|
44.85
|
|
||
|
Second Quarter
|
50.65
|
|
43.65
|
|
49.58
|
|
|||||
|
Third Quarter
|
51.64
|
|
42.23
|
|
43.56
|
|
|||||
|
Fourth Quarter
|
50.69
|
|
43.42
|
|
48.66
|
|
|||||
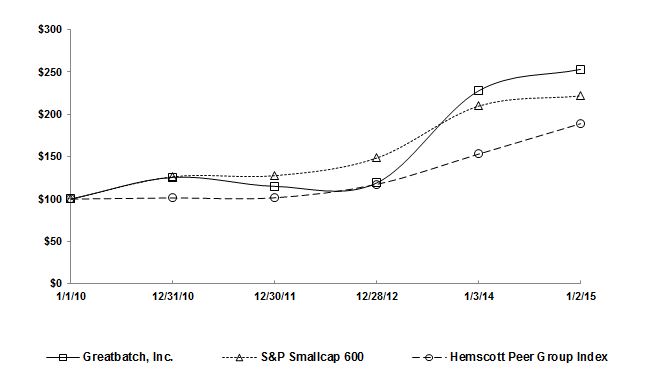
|
Company/Index
|
1/1/10
|
12/31/10
|
12/30/11
|
12/28/12
|
1/3/14
|
1/2/15
|
|
|
Greatbatch, Inc.
|
$ 100.00
|
$ 125.59
|
$ 114.92
|
$ 119.03
|
$ 227.77
|
$ 253.04
|
|
|
S&P Smallcap 600
|
100.00
|
126.31
|
127.59
|
148.42
|
209.74
|
221.81
|
|
|
Hemscott Peer Group Index
|
100.00
|
101.25
|
101.46
|
117.35
|
153.09
|
188.97
|
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
|
|
Years Ended
|
||||||||||||||||||
|
Jan. 2
2015
(1)(2)
|
Jan. 3
2014
(1)
|
Dec. 28,
2012
(1)(2)
|
Dec. 30,
2011
(1)(2)
|
Dec. 31,
2010
(1)(3)
|
|||||||||||||||
|
Statement of Operations Data:
|
|||||||||||||||||||
|
Sales
|
$
|
687,787
|
|
$
|
663,945
|
|
$
|
646,177
|
|
$
|
568,822
|
|
$
|
533,425
|
|
||||
|
Net income (loss)
|
55,458
|
|
36,267
|
|
(4,799
|
)
|
33,122
|
|
33,138
|
|
|||||||||
|
Earnings (loss) per share
|
|||||||||||||||||||
|
Basic
|
$
|
2.23
|
|
$
|
1.51
|
|
$
|
(0.20
|
)
|
$
|
1.42
|
|
$
|
1.44
|
|
||||
|
Diluted
|
2.14
|
|
1.43
|
|
(0.20
|
)
|
1.40
|
|
1.40
|
|
|||||||||
|
Balance Sheet Data:
|
|||||||||||||||||||
|
Working capital
|
$
|
242,022
|
|
$
|
190,731
|
|
$
|
176,376
|
|
$
|
170,907
|
|
$
|
150,922
|
|
||||
|
Total assets
|
956,009
|
|
890,703
|
|
889,875
|
|
881,347
|
|
776,976
|
|
|||||||||
|
Long-term obligations
|
233,986
|
|
256,846
|
|
317,258
|
|
320,015
|
|
289,560
|
|
|||||||||
|
(1)
|
From 2010 to 2014, we recorded material charges in Other Operating Expenses, Net, primarily related to our cost savings and consolidation initiatives. Additional information is set forth in Note 13 “Other Operating Expenses, Net” of the Notes to Consolidated Financial Statements contained in Item 8 of this report.
|
|
(2)
|
On August 12, 2014, February 16, 2012, and December 15, 2011, we acquired Centro de Construcción de Cardioestimuladores del Uruguay, NeuroNexus Technologies, Inc., and Micro Power Electronics, Inc., respectively. This data includes the results of operations of these companies subsequent to their acquisition. Additional information is set forth in Note 2 “Acquisitions” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. During 2014 and 2011, we sold cost method investments, which resulted in pre-tax gains of $3.2 million and $4.5 million, respectively.
|
|
(3)
|
In 2010, we recognized a $9.5 million pre-tax gain in connection with the settlement of an outstanding lawsuit.
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
|
•
|
Our business
|
|
•
|
Our acquisitions
|
|
•
|
Our customers
|
|
•
|
Use of non-GAAP financial information
|
|
•
|
Strategic and financial overview
|
|
•
|
2015
financial guidance
|
|
•
|
Cost savings and consolidation efforts
|
|
•
|
Product development
|
|
•
|
Government regulation
|
|
•
|
Valuation of goodwill and other identifiable intangible assets
|
|
•
|
Stock-based compensation
|
|
•
|
Inventories
|
|
•
|
Tangible long-lived assets
|
|
•
|
Provision for income taxes
|
|
•
|
Fiscal
2014
compared with fiscal
2013
|
|
•
|
Fiscal
2013
compared with fiscal
2012
|
|
•
|
Liquidity and capital resources
|
|
•
|
Off-balance sheet arrangements
|
|
•
|
Litigation
|
|
•
|
Contractual obligations
|
|
•
|
Inflation
|
|
•
|
Impact of recently issued accounting standards
|
|
|
Greatbatch Medical
|
QiG
|
Unallocated
|
Total
|
|||||||||||||||||||||||||||
|
Jan 2,
2015 |
Jan 3,
2014 |
Jan 2,
2015 |
Jan 3,
2014 |
Jan 2,
2015 |
Jan 3,
2014 |
Jan 2,
2015 |
Jan 3,
2014 |
||||||||||||||||||||||||
|
Total sales
|
$
|
678,285
|
|
$
|
660,902
|
|
$
|
9,502
|
|
$
|
3,043
|
|
$
|
—
|
|
$
|
—
|
|
$
|
687,787
|
|
$
|
663,945
|
|
|||||||
|
Operating income (loss) as reported
|
$
|
126,312
|
|
$
|
111,805
|
|
$
|
(23,256
|
)
|
$
|
(30,484
|
)
|
$
|
(27,402
|
)
|
$
|
(19,982
|
)
|
$
|
75,654
|
|
$
|
61,339
|
|
|||||||
|
Adjustments:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Inventory step-up amortization (COS)
|
—
|
|
—
|
|
260
|
|
—
|
|
—
|
|
—
|
|
260
|
|
—
|
|
|||||||||||||||
|
Medical device DVT expenses (RD&E)
(a)
|
—
|
|
—
|
|
—
|
|
5,793
|
|
—
|
|
—
|
|
—
|
|
5,793
|
|
|||||||||||||||
|
Consolidation and optimization costs
|
10,051
|
|
13,388
|
|
882
|
|
86
|
|
255
|
|
1,284
|
|
11,188
|
|
14,758
|
|
|||||||||||||||
|
Acquisition and integration expenses (income)
|
196
|
|
187
|
|
(713
|
)
|
(690
|
)
|
520
|
|
1
|
|
3
|
|
(502
|
)
|
|||||||||||||||
|
Asset dispositions, severance and other
|
2,493
|
|
1,187
|
|
634
|
|
540
|
|
979
|
|
(193
|
)
|
4,106
|
|
1,534
|
|
|||||||||||||||
|
Adjusted operating income (loss)
|
$
|
139,052
|
|
$
|
126,567
|
|
$
|
(22,193
|
)
|
$
|
(24,755
|
)
|
$
|
(25,648
|
)
|
$
|
(18,890
|
)
|
$
|
91,211
|
|
$
|
82,922
|
|
|||||||
|
Adjusted operating margin
|
20.5
|
%
|
19.2
|
%
|
N/A
|
|
N/A
|
|
N/A
|
|
N/A
|
|
13.3
|
%
|
12.5
|
%
|
|||||||||||||||
|
|
Greatbatch Medical
|
QiG
|
Unallocated
|
Total
|
|||||||||||||||||||||||||||
|
Jan 3,
2014 |
Dec 28,
2012 |
Jan 3,
2014 |
Dec 28,
2012 |
Jan 3,
2014 |
Dec 28,
2012 |
Jan 3,
2014 |
Dec 28,
2012 |
||||||||||||||||||||||||
|
Total sales
|
$
|
660,902
|
|
$
|
643,722
|
|
$
|
3,043
|
|
$
|
2,455
|
|
$
|
—
|
|
$
|
—
|
|
$
|
663,945
|
|
$
|
646,177
|
|
|||||||
|
Operating income (loss) as reported
|
$
|
111,805
|
|
$
|
79,093
|
|
$
|
(30,484
|
)
|
$
|
(32,554
|
)
|
$
|
(19,982
|
)
|
$
|
(20,718
|
)
|
$
|
61,339
|
|
$
|
25,821
|
|
|||||||
|
Adjustments:
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
Inventory step-up amortization (COS)
|
—
|
|
532
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
532
|
|
|||||||||||||||
|
Medical device DVT expenses (RD&E)
|
—
|
|
—
|
|
5,793
|
|
5,190
|
|
—
|
|
—
|
|
5,793
|
|
5,190
|
|
|||||||||||||||
|
Consolidation and optimization costs
|
13,388
|
|
34,372
|
|
86
|
|
6
|
|
1,284
|
|
4,670
|
|
14,758
|
|
39,048
|
|
|||||||||||||||
|
Acquisition and integration (income) expenses
|
187
|
|
1,287
|
|
(690
|
)
|
167
|
|
1
|
|
6
|
|
(502
|
)
|
1,460
|
|
|||||||||||||||
|
Asset dispositions, severance and other
|
1,187
|
|
1,073
|
|
540
|
|
57
|
|
(193
|
)
|
708
|
|
1,534
|
|
1,838
|
|
|||||||||||||||
|
Adjusted operating income (loss)
|
$
|
126,567
|
|
$
|
116,357
|
|
$
|
(24,755
|
)
|
$
|
(27,134
|
)
|
$
|
(18,890
|
)
|
$
|
(15,334
|
)
|
$
|
82,922
|
|
$
|
73,889
|
|
|||||||
|
Adjusted operating margin
|
19.2
|
%
|
18.1
|
%
|
N/A
|
|
NA
|
|
N/A
|
|
N/A
|
|
12.5
|
%
|
11.4
|
%
|
|||||||||||||||
|
|
Year Ended
|
||||||||||||||||||||||
|
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||||||||||||||||
|
|
Net
Income
|
Impact
Per
Diluted
Share
|
Net
Income
|
Impact
Per
Diluted
Share
|
Net
Income
(Loss)
|
Impact
Per
Diluted
Share
|
|||||||||||||||||
|
Net income (loss) as reported
|
$
|
55,458
|
|
$
|
2.14
|
|
$
|
36,267
|
|
$
|
1.43
|
|
$
|
(4,799
|
)
|
$
|
(0.20
|
)
|
|||||
|
Adjustments:
|
|||||||||||||||||||||||
|
Inventory step-up amortization (COS)
(a)
|
195
|
|
0.01
|
|
—
|
|
—
|
|
346
|
|
0.01
|
|
|||||||||||
|
Medical device DVT expenses (RD&E)
(a)
|
—
|
|
—
|
|
3,765
|
|
0.15
|
|
3,374
|
|
0.14
|
|
|||||||||||
|
Consolidation and optimization costs
(a)
|
6,567
|
|
0.25
|
|
10,602
|
|
0.42
|
|
28,934
|
|
1.21
|
|
|||||||||||
|
Acquisition and integration expenses (income)
(a)
|
61
|
|
—
|
|
(326
|
)
|
(0.01
|
)
|
949
|
|
0.04
|
|
|||||||||||
|
Asset dispositions, severance and other
(a)
|
3,463
|
|
0.13
|
|
997
|
|
0.04
|
|
1,186
|
|
0.05
|
|
|||||||||||
|
(Gain) loss on cost and equity method investments, net
(a)(b)
|
(2,841
|
)
|
(0.11
|
)
|
451
|
|
0.02
|
|
69
|
|
—
|
|
|||||||||||
|
CSN conversion option discount and deferred fee acceleration amortization
(a)(c)
|
—
|
|
—
|
|
3,007
|
|
0.12
|
|
6,234
|
|
0.26
|
|
|||||||||||
|
R&D Tax Credit
(d)
|
—
|
|
—
|
|
(1,600
|
)
|
(0.06
|
)
|
—
|
|
—
|
|
|||||||||||
|
Swiss tax impact
(e)
|
—
|
|
—
|
|
—
|
|
—
|
|
6,190
|
|
0.26
|
|
|||||||||||
|
Adjusted net income and diluted EPS
(f)
|
$
|
62,903
|
|
$
|
2.42
|
|
$
|
53,163
|
|
$
|
2.10
|
|
$
|
42,483
|
|
$
|
1.77
|
|
|||||
|
Adjusted diluted weighted average shares
(g)
|
25,975
|
|
25,323
|
|
23,947
|
|
|||||||||||||||||
|
(a)
|
Net of tax amounts computed using a 35% U.S. and France statutory tax rates for the 2014, 2013, and 2012 periods and a 0%, 0%, and 22.5% Switzerland tax rate for the 2014, 2013, and 2012 periods, respectively. For 2014, net of tax amounts computed also include a 25% Uruguay statutory tax rate.
|
|
(b)
|
Pre-tax amount is a gain of $4.4 million, loss of $0.7 million, and loss of $0.1 million for 2014, 2013, and 2012, respectively.
|
|
(c)
|
Pre-tax amount is $4.6 million and $9.6 million for 2013 and 2012, respectively.
|
|
(d)
|
The Federal R&D tax credit was enacted for 2014 during the fourth quarter of 2014. The 2013 amount relates to the 2012 portion of the R&D tax credit which was reinstated in the first quarter of 2013 retroactive to the beginning of 2012. As required, the impact of the R&D tax credit relating to 2012 was recognized in the first quarter of 2013.
|
|
(e)
|
Relates to the loss of our Swiss tax holiday due to our decision to transfer manufacturing out of Switzerland, as well as the establishment of a valuation allowance on our Swiss deferred tax assets as it is more likely than not that they will not be fully realized.
|
|
(f)
|
The per share data in this table has been rounded to the nearest $0.01 and therefore may not sum to the total.
|
|
(g)
|
Adjusted diluted weighted average shares for 2012 includes 363,000 shares of dilution related to outstanding stock incentive awards that were not dilutive for GAAP EPS purposes.
|
|
We are estimating the following for 2015:
|
|
|
Sales
|
$715 - $730 million
|
|
GAAP Operating Income as a % of Sales
|
10.7% - 11.0%
|
|
Adjusted Operating Income as a % of Sales
|
13.7% - 14.0%
|
|
Capital Expenditures
|
$35 - $45 million
|
|
GAAP Effective Tax Rate
|
~25%
|
|
Adjusted Effective Tax Rate
|
~26%
|
|
GAAP Diluted EPS
|
$2.02 - $2.12
|
|
Adjusted Diluted EPS
|
$2.61 - $2.71
|
|
Diluted Weighted Average Shares
|
26,500,000
|
|
Initiative
|
Expected Expense
|
Expected Capital
|
Expected Annual Cost Savings
|
Expected Completion Date
|
||||
|
2014 investments in capacity and capabilities
|
$29 - $34
|
$25 - $27
|
> $20
|
2016
|
||||
|
2013 operating unit realignment
|
$6.6
|
—
|
> $7
|
Q4 2014
|
||||
|
Orthopaedic/medical device facilities optimization
|
$45 - $50
|
$43 - $48
|
$10 - $15
|
2016
|
||||
|
Product Line
|
Product Development Opportunities
|
|
|
Cardiac/ Neuromodulation
|
Developing next generation technology programs including Gen 2 Q
HR
battery, next generation filtered feedthroughs, and high voltage capacitors.
|
|
|
Orthopaedic
|
Developing next generation reamers, hip and bone preparation instruments, as well as disposable kits, and power solutions for surgical tools.
|
|
|
Portable Medical
|
Developing power solutions for various surgical, diagnostic and other market categories where device mobility is critical, including sterilized surgical products, wireless power and battery management technologies.
|
|
|
Vascular
|
Developing introducer technologies to expand into new clinical markets, as well as expanding current introducer and catheter platforms to better serve existing clinical markets and customers.
|
|
|
Energy, Military, Environmental
|
Developing power solutions to advance performance and reliability of battery packs in critical environments.
|
|
|
|
Year Ended
|
2014 vs. 2013
|
2013 vs. 2012
|
||||||||||||||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
$
Change
|
%
Change
|
$
Change
|
%
Change
|
|||||||||||||||||||
|
Dollars in thousands, except per share data
|
|||||||||||||||||||||||||
|
Greatbatch Medical Sales
|
|||||||||||||||||||||||||
|
Cardiac/Neuromodulation
|
$
|
321,419
|
|
$
|
325,412
|
|
$
|
306,669
|
|
$
|
(3,993
|
)
|
(1
|
)%
|
$
|
18,743
|
|
6
|
%
|
||||||
|
Orthopaedics
|
147,296
|
|
130,247
|
|
122,061
|
|
17,049
|
|
13
|
%
|
8,186
|
|
7
|
%
|
|||||||||||
|
Portable Medical
|
69,043
|
|
78,743
|
|
81,659
|
|
(9,700
|
)
|
(12
|
)%
|
(2,916
|
)
|
(4
|
)%
|
|||||||||||
|
Vascular
|
58,770
|
|
48,357
|
|
51,980
|
|
10,413
|
|
22
|
%
|
(3,623
|
)
|
(7
|
)%
|
|||||||||||
|
Energy, Military, Environmental
|
81,757
|
|
78,143
|
|
81,353
|
|
3,614
|
|
5
|
%
|
(3,210
|
)
|
(4
|
)%
|
|||||||||||
|
Total Greatbatch Medical
|
678,285
|
|
660,902
|
|
643,722
|
|
17,383
|
|
3
|
%
|
17,180
|
|
3
|
%
|
|||||||||||
|
QiG
|
9,502
|
|
3,043
|
|
2,455
|
|
6,459
|
|
212
|
%
|
588
|
|
24
|
%
|
|||||||||||
|
Total sales
|
687,787
|
|
663,945
|
|
646,177
|
|
23,842
|
|
4
|
%
|
17,768
|
|
3
|
%
|
|||||||||||
|
Cost of sales
|
456,389
|
|
444,632
|
|
444,528
|
|
11,757
|
|
3
|
%
|
104
|
|
—
|
%
|
|||||||||||
|
Gross profit
|
231,398
|
|
219,313
|
|
201,649
|
|
12,085
|
|
6
|
%
|
17,664
|
|
9
|
%
|
|||||||||||
|
Gross profit as a % of sales
|
33.6
|
%
|
33.0
|
%
|
31.2
|
%
|
|||||||||||||||||||
|
Selling, general and administrative expenses (SG&A)
|
90,602
|
|
88,107
|
|
80,992
|
|
2,495
|
|
3
|
%
|
7,115
|
|
9
|
%
|
|||||||||||
|
SG&A as a % of sales
|
13.2
|
%
|
13.3
|
%
|
12.5
|
%
|
|||||||||||||||||||
|
Research, development and engineering costs, net (RD&E)
|
49,845
|
|
54,077
|
|
52,490
|
|
(4,232
|
)
|
(8
|
)%
|
1,587
|
|
3
|
%
|
|||||||||||
|
RD&E as a % of sales
|
7.2
|
%
|
8.1
|
%
|
8.1
|
%
|
|||||||||||||||||||
|
Other operating expenses, net
|
15,297
|
|
15,790
|
|
42,346
|
|
(493
|
)
|
(3
|
)%
|
(26,556
|
)
|
(63
|
)%
|
|||||||||||
|
Operating income
|
75,654
|
|
61,339
|
|
25,821
|
|
14,315
|
|
23
|
%
|
35,518
|
|
138
|
%
|
|||||||||||
|
Operating margin
|
11.0
|
%
|
9.2
|
%
|
4.0
|
%
|
|||||||||||||||||||
|
Interest expense
|
4,252
|
|
11,261
|
|
18,054
|
|
(7,009
|
)
|
(62
|
)%
|
(6,793
|
)
|
(38
|
)%
|
|||||||||||
|
(Gain) loss on cost and equity method investments, net
|
(4,370
|
)
|
694
|
|
106
|
|
(5,064
|
)
|
NA
|
|
588
|
|
NA
|
|
|||||||||||
|
Other (income) expense, net
|
(807
|
)
|
546
|
|
931
|
|
(1,353
|
)
|
NA
|
(385
|
)
|
(41
|
)%
|
||||||||||||
|
Provision for income taxes
|
21,121
|
|
12,571
|
|
11,529
|
|
8,550
|
|
68
|
%
|
1,042
|
|
9
|
%
|
|||||||||||
|
Effective tax rate
|
27.6
|
%
|
25.7
|
%
|
171.3
|
%
|
|||||||||||||||||||
|
Net income (loss)
|
$
|
55,458
|
|
$
|
36,267
|
|
$
|
(4,799
|
)
|
$
|
19,191
|
|
53
|
%
|
$
|
41,066
|
|
NA
|
|||||||
|
Net margin
|
8.1
|
%
|
5.5
|
%
|
(0.7
|
)%
|
|||||||||||||||||||
|
Diluted earnings (loss) per share
|
$
|
2.14
|
|
$
|
1.43
|
|
$
|
(0.20
|
)
|
$
|
0.71
|
|
50
|
%
|
$
|
1.63
|
|
NA
|
|||||||
|
|
Year Ended
|
2014 vs. 2013
|
||||||||||||
|
January 2, 2015
|
January 3,
2014 |
$
Change
|
%
Change
|
|||||||||||
|
Sales:
|
||||||||||||||
|
Greatbatch Medical
|
||||||||||||||
|
Cardiac/Neuromodulation
|
$
|
321,419
|
|
$
|
325,412
|
|
$
|
(3,993
|
)
|
(1
|
)%
|
|||
|
Orthopaedics
|
147,296
|
|
130,247
|
|
17,049
|
|
13
|
%
|
||||||
|
Portable Medical
|
69,043
|
|
78,743
|
|
(9,700
|
)
|
(12
|
)%
|
||||||
|
Vascular
|
58,770
|
|
48,357
|
|
10,413
|
|
22
|
%
|
||||||
|
Energy, Military, Environmental
|
81,757
|
|
78,143
|
|
3,614
|
|
5
|
%
|
||||||
|
Total Greatbatch Medical
|
678,285
|
|
660,902
|
|
17,383
|
|
3
|
%
|
||||||
|
QiG
|
9,502
|
|
3,043
|
|
6,459
|
|
212
|
%
|
||||||
|
Total sales
|
$
|
687,787
|
|
|
$
|
663,945
|
|
|
$
|
23,842
|
|
|
4
|
%
|
|
|
2014-2013
% Point Change |
|
|
Performance-based compensation
(a)
|
0.1
|
%
|
|
Production efficiencies, volume and mix
(b)
|
1.9
|
%
|
|
Impact of acquisition
(c)
|
0.1
|
%
|
|
Price
(d)
|
(1.2
|
)%
|
|
Other
|
(0.3
|
)%
|
|
Total percentage point change to gross profit as a percentage of sales
|
0.6
|
%
|
|
(b)
|
Our gross profit percentage benefited from production efficiencies gained at our manufacturing facilities as a result of our various lean and supply chain initiatives, as well as higher production volumes due to increased sales. Partially offsetting these production efficiencies was an increase in mix of lower margin sales in comparison to the prior year (i.e. higher mix of orthopaedic sales and lower mix of cardiac/neuromodulation sales).
|
|
(c)
|
Amounts represent the impact to our gross profit percentage related to the acquisition of CCC in August 2014.
|
|
(d)
|
Our gross profit percentage was negatively impacted by contractual price concessions to our larger OEM customers, which were given in exchange for long-term contracts and volume commitments.
|
|
|
2014-2013
$ Change
|
||
|
Selling and marketing
(a)
|
$
|
3,408
|
|
|
Performance-based compensation
(b)
|
(991
|
)
|
|
|
Legal fees
(c)
|
2,555
|
|
|
|
G&A personnel costs
(d)
|
(3,096
|
)
|
|
|
Impact of acquisition
(e)
|
911
|
|
|
|
Other
|
(292
|
)
|
|
|
Net increase in SG&A
|
$
|
2,495
|
|
|
(a)
|
Amount represents the incremental costs related to our strategic initiative to increase selling and marketing resources to drive 5% core business growth and sustain a pipeline of revenue generating opportunities.
|
|
(b)
|
Amount represents the change in performance-based compensation versus the prior year and is recorded based upon the actual results achieved.
|
|
(c)
|
Amount represents the increase in legal costs compared to the prior year and includes higher intellectual property related costs, as well as other corporate initiatives.
|
|
(d)
|
Amount represents lower G&A personnel costs in comparison to the prior year and is primarily the result of our various consolidation initiatives including our operating unit realignment that occurred during the second half of 2013.
|
|
(e)
|
Amount represents the incremental SG&A expenses related to the acquisition of CCC in August 2014.
|
|
|
Year Ended
|
|
|||||||||
|
January 2,
2015 |
January 3,
2014 |
Change
|
|||||||||
|
Research, development and engineering costs
|
$
|
58,974
|
|
$
|
62,652
|
|
$
|
(3,678
|
)
|
||
|
Less: cost reimbursements
|
(9,129
|
)
|
(8,575
|
)
|
(554
|
)
|
|||||
|
Total RD&E, net
|
$
|
49,845
|
|
$
|
54,077
|
|
$
|
(4,232
|
)
|
||
|
|
Year Ended
|
|
|||||||||
|
January 2,
2015 |
January 3,
2014 |
Change
|
|||||||||
|
2014 investments in capacity and capabilities
(a)
|
$
|
8,925
|
|
$
|
—
|
|
$
|
8,925
|
|
||
|
2013 operating unit realignment
(a)
|
1,017
|
|
5,625
|
|
(4,608
|
)
|
|||||
|
Orthopaedic facilities optimization
(a)
|
1,317
|
|
8,038
|
|
(6,721
|
)
|
|||||
|
Medical device facility optimization
(a)
|
11
|
|
312
|
|
(301
|
)
|
|||||
|
ERP system upgrade (income) costs
(a)
|
(82
|
)
|
783
|
|
(865
|
)
|
|||||
|
Acquisition and integration (income) costs
(b)
|
3
|
|
(502
|
)
|
505
|
|
|||||
|
Asset dispositions, severance and other
(c)
|
4,106
|
|
1,534
|
|
2,572
|
|
|||||
|
Total other operating expenses, net
|
$
|
15,297
|
|
$
|
15,790
|
|
$
|
(493
|
)
|
||
|
(a)
|
Refer to “Cost Savings and Consolidation Efforts” section of this Item and Note 13 “Other Operating Expenses, Net” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for disclosures related to the timing and level of remaining expenditures for these initiatives.
|
|
(b)
|
During 2014 and 2013, we recognized costs (income) related to the integration of Micro Power Electronics, Inc., NeuroNexus, and CCC. These expenses (income) were primarily for retention bonuses, travel costs in connection with integration efforts, training, severance, and the change in fair value of the contingent consideration recorded in connection with the NeuroNexus acquisition. Refer to Note 18 “Fair Value Measurements” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for disclosures related to the change in fair value of the contingent consideration.
|
|
(c)
|
During 2014 and 2013, we recorded losses in connection with various asset disposals and write-downs. During 2014, we incurred $0.9 million of expense related to the separation of our Senior Vice President, Human Resources. Additionally, during 2014, Greatbatch Medical recorded charges in connection with its business reorganization to align its contract manufacturing operations. Costs incurred primarily related to consulting and IT development. During 2013, Greatbatch Medical recorded a $0.9 million write-off related to its wireless sensing product line and QiG recorded a $0.5 million write-off of NeuroNexus’s in-process research and development “IPR&D”.
|
|
|
U.S.
|
International
|
Combined
|
|||||||||||||||||
|
|
$
|
%
|
$
|
%
|
$
|
%
|
||||||||||||||
|
Income before provision for income taxes
|
$
|
56,801
|
|
$
|
19,778
|
|
$
|
76,579
|
|
|||||||||||
|
Provision at statutory rate
|
$
|
19,881
|
|
35.0
|
%
|
$
|
6,922
|
|
35.0
|
%
|
$
|
26,803
|
|
35.0
|
%
|
|||||
|
Federal tax credits
|
(1,600
|
)
|
(2.8
|
)
|
—
|
|
—
|
|
(1,600
|
)
|
(2.1
|
)
|
||||||||
|
Foreign rate differential
(a)
|
—
|
|
—
|
|
(3,276
|
)
|
(16.6
|
)
|
(3,276
|
)
|
(4.3
|
)
|
||||||||
|
Uncertain tax positions
|
412
|
|
0.7
|
|
—
|
|
—
|
|
412
|
|
0.6
|
|
||||||||
|
State taxes, net of federal benefit
|
507
|
|
0.9
|
|
—
|
|
—
|
|
507
|
|
0.7
|
|
||||||||
|
Change in foreign tax rates
(b)
|
—
|
|
—
|
|
(446
|
)
|
(2.3
|
)
|
(446
|
)
|
(0.6
|
)
|
||||||||
|
Valuation allowance
|
135
|
|
0.2
|
|
(434
|
)
|
(2.2
|
)
|
(299
|
)
|
(0.4
|
)
|
||||||||
|
Other
|
(842
|
)
|
(1.5
|
)
|
(138
|
)
|
(0.7
|
)
|
(980
|
)
|
(1.3
|
)
|
||||||||
|
Provision for income taxes/effective tax rate
|
$
|
18,493
|
|
32.6
|
%
|
$
|
2,628
|
|
13.3
|
%
|
$
|
21,121
|
|
27.6
|
%
|
|||||
|
(a)
|
The tax rate reflects the impact of an increase in foreign source income, which carries a lower overall effective tax rate than U.S. income.
|
|
|
Year Ended
|
2013 vs. 2012
|
||||||||||||
|
January 3,
2014 |
December 28,
2012 |
$
Change
|
%
Change
|
|||||||||||
|
Sales:
|
||||||||||||||
|
Greatbatch Medical
|
||||||||||||||
|
Cardiac/Neuromodulation
|
$
|
325,412
|
|
$
|
306,669
|
|
$
|
18,743
|
|
6
|
%
|
|||
|
Orthopaedics
|
130,247
|
|
122,061
|
|
8,186
|
|
7
|
%
|
||||||
|
Portable Medical
|
78,743
|
|
81,659
|
|
(2,916
|
)
|
(4
|
)%
|
||||||
|
Vascular
|
48,357
|
|
51,980
|
|
(3,623
|
)
|
(7
|
)%
|
||||||
|
Energy, Military, Environmental
|
78,143
|
|
81,353
|
|
(3,210
|
)
|
(4
|
)%
|
||||||
|
Total Greatbatch Medical
|
660,902
|
|
643,722
|
|
17,180
|
|
3
|
%
|
||||||
|
QiG
|
3,043
|
|
2,455
|
|
588
|
|
24
|
%
|
||||||
|
Total sales
|
$
|
663,945
|
|
|
$
|
646,177
|
|
|
$
|
17,768
|
|
3
|
%
|
|
|
|
2013-2012
% Point Change
|
|
|
Impact of Swiss consolidation
(a)
|
0.4
|
%
|
|
Performance-based compensation
(b)
|
(0.5
|
)%
|
|
Cost savings and production efficiencies
(c)
|
2.0
|
%
|
|
Other
|
(0.1
|
)%
|
|
Total percentage point change to gross profit as a percentage of sales
|
1.8
|
%
|
|
(b)
|
Amount represents higher performance-based compensation versus the prior year of approximately $3.4 million and is recorded based upon actual results achieved.
|
|
(c)
|
Our Gross Margin percentage benefited from production efficiencies gained at our manufacturing facilities as a result of our various lean and supply chain initiatives, as well as higher production volumes due to increased sales and inventory levels.
|
|
|
2013-2012
$ Change
|
||
|
Selling and marketing
(a)
|
$
|
3,848
|
|
|
Performance-based compensation
(b)
|
2,680
|
|
|
|
Swiss consolidation
(c)
|
(1,359
|
)
|
|
|
Other
(d)
|
1,946
|
|
|
|
Net increase in SG&A
|
$
|
7,115
|
|
|
(a)
|
Amount represents the incremental costs related to our decision near the end of 2012 to increase selling and marketing resources to drive 5% core business growth and sustain a pipeline of revenue generating opportunities.
|
|
(b)
|
Amount represents the change in performance-based compensation versus the prior year period and is recorded based upon the actual results achieved.
|
|
(c)
|
Amount represents the estimated impact to SG&A costs as a result of the consolidation of our Swiss orthopaedic facilities into other existing Greatbatch facilities, which was completed in the first quarter of 2013.
|
|
(d)
|
Amount represents various cost increases in SG&A expenses that occurred during 2013 including an additional week of operations in comparison to 2012 as the Company utilizes a fifty-two, fifty-three week fiscal year, which ends on the Friday nearest December 31.
|
|
|
Year Ended
|
|
|||||||||
|
January 3,
2014 |
December 28,
2012 |
Change
|
|||||||||
|
Research, development, and engineering costs
|
$
|
62,652
|
|
$
|
62,848
|
|
$
|
(196
|
)
|
||
|
Less cost reimbursements
|
(8,575
|
)
|
(10,358
|
)
|
1,783
|
|
|||||
|
Total RD&E, net
|
$
|
54,077
|
|
$
|
52,490
|
|
$
|
1,587
|
|
||
|
|
Year Ended
|
|
|||||||||
|
January 3,
2014 |
December 28,
2012 |
Change
|
|||||||||
|
2013 operating unit realignment
(a)
|
$
|
5,625
|
|
$
|
—
|
|
$
|
5,625
|
|
||
|
Orthopaedic facilities optimization
(a)
|
8,038
|
|
32,482
|
|
(24,444
|
)
|
|||||
|
Medical device facility optimization
(a)
|
312
|
|
1,525
|
|
(1,213
|
)
|
|||||
|
ERP system upgrade (income) costs
(a)
|
783
|
|
5,041
|
|
(4,258
|
)
|
|||||
|
Acquisition and integration (income) costs
(b)
|
(502
|
)
|
1,460
|
|
(1,962
|
)
|
|||||
|
Asset dispositions, severance and other
(c)
|
1,534
|
|
1,838
|
|
(304
|
)
|
|||||
|
Total other operating expenses, net
|
$
|
15,790
|
|
$
|
42,346
|
|
$
|
(26,556
|
)
|
||
|
(a)
|
Refer to “Cost Savings and Consolidation Efforts” section of this Item and Note 13 “Other Operating Expenses, Net” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for disclosures related to the timing and level of remaining expenditures for these initiatives.
|
|
(b)
|
During 2013 and 2012, we incurred costs (income) related to the integration of Micro Power and NeuroNexus. These expenses were primarily for retention bonuses, travel costs in connection with integration efforts, training, severance and the change in fair value of the contingent consideration recorded in connection with these acquisitions. Refer to Note 18 “Fair Value Measurements” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for disclosures related to the change in fair value of the contingent consideration.
|
|
(c)
|
During 2013 and 2012, we recorded losses in connection with various asset disposals and/or write-downs. Additionally, during 2013, Greatbatch Medical recorded a $0.9 million write-off related to its wireless sensing product line and QiG recorded a $0.5 million write-off of NeuroNexus IPR&D. During 2012, we incurred $1.2 million of costs related to the relocation of our global headquarters to Frisco, Texas.
|
|
|
U.S.
|
International
|
Combined
|
|||||||||||||||||
|
|
$
|
%
|
$
|
%
|
$
|
%
|
||||||||||||||
|
Income before provision for income taxes
|
$
|
42,392
|
|
$
|
6,446
|
|
$
|
48,838
|
|
|||||||||||
|
Provision at statutory rate
|
$
|
14,837
|
|
35.0
|
%
|
$
|
2,256
|
|
35.0
|
%
|
$
|
17,093
|
|
35.0
|
%
|
|||||
|
Federal tax credits
(a)
|
(3,651
|
)
|
(8.6
|
)
|
—
|
|
—
|
|
(3,651
|
)
|
(7.5
|
)
|
||||||||
|
Foreign rate differential
|
—
|
|
—
|
|
(348
|
)
|
(5.4
|
)
|
(348
|
)
|
(0.7
|
)
|
||||||||
|
Uncertain tax positions
|
831
|
|
2.0
|
|
—
|
|
—
|
|
831
|
|
1.7
|
|
||||||||
|
State taxes, net of federal benefit
|
1,147
|
|
2.7
|
|
—
|
|
—
|
|
1,147
|
|
2.3
|
|
||||||||
|
Change in foreign tax rates
(b)
|
—
|
|
—
|
|
(1,807
|
)
|
(28.0
|
)
|
(1,807
|
)
|
(3.7
|
)
|
||||||||
|
Valuation allowance
|
176
|
|
0.4
|
|
10
|
|
0.2
|
|
186
|
|
0.4
|
|
||||||||
|
Other
|
(634
|
)
|
(1.5
|
)
|
(246
|
)
|
(3.8
|
)
|
(880
|
)
|
(1.8
|
)
|
||||||||
|
Provision for income taxes/effective tax rate
|
$
|
12,706
|
|
30.0
|
%
|
$
|
(135
|
)
|
(2.0
|
)%
|
$
|
12,571
|
|
25.7
|
%
|
|||||
|
(a)
|
Amounts relate to the retroactive reinstatement of the U.S. R&D tax credit. On January 2, 2013, the President signed into law the Act, which included a retroactive extension of the R&D tax credit that had expired on December 31, 2011. Under the Act, the R&D credit is extended for two years retroactively from January 1, 2012 through December 31, 2013. As the Act was signed into law on January 2, 2013, the effects of the change in the tax law were recognized as a financial statement event in the financial statement period that includes the date of enactment. As such, we recorded a benefit for the R&D credits earned in 2012 and 2013 through the fiscal 2013 effective tax rate.
|
|
|
At
|
||||||
|
(Dollars in thousands)
|
January 2, 2015
|
January 3, 2014
|
|||||
|
Cash and cash equivalents
|
$
|
76,824
|
|
$
|
35,465
|
|
|
|
Working capital
|
$
|
242,022
|
|
$
|
190,731
|
|
|
|
Current ratio
|
3.23
|
|
3.08
|
|
|||
|
|
Payments due by period
|
||||||||||||||||||
|
CONTRACTUAL OBLIGATIONS
|
Total
|
Less than 1
year
|
1-3 years
|
3-5 years
|
More than 5
years
|
||||||||||||||
|
Debt obligations
(a)
|
$
|
205,998
|
|
$
|
15,898
|
|
$
|
44,745
|
|
$
|
145,355
|
|
$
|
—
|
|
||||
|
Operating lease obligations
(b)
|
36,502
|
|
5,797
|
|
9,860
|
|
6,907
|
|
13,938
|
|
|||||||||
|
Purchase obligations
(b)
|
36,412
|
|
35,117
|
|
1,175
|
|
100
|
|
20
|
|
|||||||||
|
Foreign currency contracts
(b)
|
16,880
|
|
16,880
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Defined benefit plan obligations
(c)
|
1,394
|
|
47
|
|
191
|
|
290
|
|
866
|
|
|||||||||
|
Total contractual obligations
|
$
|
297,186
|
|
$
|
73,739
|
|
$
|
55,971
|
|
$
|
152,652
|
|
$
|
14,824
|
|
||||
|
(a)
|
Includes the annual interest expense on the $187.5 million outstanding on our Term Loan based upon the period end weighted average interest rate of 1.79%, which includes the impact of our interest rate swap agreement. Also includes $5.0 million of deferred federal and state taxes on our convertible subordinated notes that will be due between 2015 and 2018. See Note 9 “Debt” of the Notes to Consolidated Financial Statements contained in Item 8 of this report.
|
|
(b)
|
See Note 15 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our operating leases, purchase obligations and foreign currency contracts.
|
|
(c)
|
See Note 10 “Benefit Plans” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our defined benefit plan obligations.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
|
•
|
Centro de Construcción de Cardioestimuladores del Uruguay
|
|
/s/ Thomas J. Hook
|
|
/s/ Michael Dinkins
|
|
Thomas J. Hook
|
|
Michael Dinkins
|
|
President & Chief Executive Officer
|
|
Executive Vice President & Chief Financial Officer
|
|
|
At
|
||||||
|
(in thousands except share and per share data)
|
January 2,
2015 |
January 3,
2014 |
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
76,824
|
|
$
|
35,465
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $1.4 million in 2014 and $2.0 million in 2013
|
124,953
|
|
113,679
|
|
|||
|
Inventories
|
129,242
|
|
118,358
|
|
|||
|
Refundable income taxes
|
1,716
|
|
2,306
|
|
|||
|
Deferred income taxes
|
6,168
|
|
6,008
|
|
|||
|
Prepaid expenses and other current assets
|
11,780
|
|
6,717
|
|
|||
|
Total current assets
|
350,683
|
|
282,533
|
|
|||
|
Property, plant and equipment, net
|
144,925
|
|
145,773
|
|
|||
|
Amortizing intangible assets, net
|
65,337
|
|
76,122
|
|
|||
|
Indefinite-lived intangible assets
|
20,288
|
|
20,288
|
|
|||
|
Goodwill
|
354,393
|
|
346,656
|
|
|||
|
Deferred income taxes
|
2,626
|
|
2,933
|
|
|||
|
Other assets
|
17,757
|
|
16,398
|
|
|||
|
Total assets
|
$
|
956,009
|
|
$
|
890,703
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Current portion of long-term debt
|
$
|
11,250
|
|
$
|
—
|
|
|
|
Accounts payable
|
46,436
|
|
46,508
|
|
|||
|
Income taxes payable
|
2,003
|
|
—
|
|
|||
|
Deferred income taxes
|
588
|
|
613
|
|
|||
|
Accrued expenses
|
48,384
|
|
44,681
|
|
|||
|
Total current liabilities
|
108,661
|
|
91,802
|
|
|||
|
Long-term debt
|
176,250
|
|
197,500
|
|
|||
|
Deferred income taxes
|
53,195
|
|
52,012
|
|
|||
|
Other long-term liabilities
|
4,541
|
|
7,334
|
|
|||
|
Total liabilities
|
342,647
|
|
348,648
|
|
|||
|
Commitments and contingencies (Note 15)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.001 par value, authorized 100,000,000 shares; no shares issued or outstanding in 2014 or 2013
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value, authorized 100,000,000 shares; 25,099,293 shares issued and 25,070,931 shares outstanding in 2014; 24,459,153 shares issued and 24,422,555 shares outstanding in 2013
|
25
|
|
24
|
|
|||
|
Additional paid-in capital
|
366,073
|
|
344,915
|
|
|||
|
Treasury stock, at cost, 28,362 shares in 2014 and 36,598 shares in 2013
|
(1,307
|
)
|
(1,232
|
)
|
|||
|
Retained earnings
|
239,448
|
|
183,990
|
|
|||
|
Accumulated other comprehensive income
|
9,123
|
|
14,358
|
|
|||
|
Total stockholders’ equity
|
613,362
|
|
542,055
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
956,009
|
|
$
|
890,703
|
|
|
|
|
Year Ended
|
||||||||||
|
(in thousands except per share data)
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||||
|
Sales
|
$
|
687,787
|
|
$
|
663,945
|
|
$
|
646,177
|
|
||
|
Cost of sales
|
456,389
|
|
444,632
|
|
444,528
|
|
|||||
|
Gross profit
|
231,398
|
|
219,313
|
|
201,649
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Selling, general and administrative expenses
|
90,602
|
|
88,107
|
|
80,992
|
|
|||||
|
Research, development and engineering costs, net
|
49,845
|
|
54,077
|
|
52,490
|
|
|||||
|
Other operating expenses, net
|
15,297
|
|
15,790
|
|
42,346
|
|
|||||
|
Total operating expenses
|
155,744
|
|
157,974
|
|
175,828
|
|
|||||
|
Operating income
|
75,654
|
|
61,339
|
|
25,821
|
|
|||||
|
Interest expense
|
4,252
|
|
11,261
|
|
18,054
|
|
|||||
|
(Gain) loss on cost and equity method investments, net
|
(4,370
|
)
|
694
|
|
106
|
|
|||||
|
Other (income) expense, net
|
(807
|
)
|
546
|
|
931
|
|
|||||
|
Income before provision for income taxes
|
76,579
|
|
48,838
|
|
6,730
|
|
|||||
|
Provision for income taxes
|
21,121
|
|
12,571
|
|
11,529
|
|
|||||
|
Net income (loss)
|
$
|
55,458
|
|
$
|
36,267
|
|
$
|
(4,799
|
)
|
||
|
Earnings (loss) per share:
|
|||||||||||
|
Basic
|
$
|
2.23
|
|
$
|
1.51
|
|
$
|
(0.20
|
)
|
||
|
Diluted
|
$
|
2.14
|
|
$
|
1.43
|
|
$
|
(0.20
|
)
|
||
|
Weighted average shares outstanding:
|
|||||||||||
|
Basic
|
24,825
|
|
23,991
|
|
23,584
|
|
|||||
|
Diluted
|
25,975
|
|
25,323
|
|
23,584
|
|
|||||
|
Comprehensive Income (Loss)
|
|||||||||||
|
Net income (loss)
|
$
|
55,458
|
|
$
|
36,267
|
|
$
|
(4,799
|
)
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Foreign currency translation gain (loss)
|
(3,502
|
)
|
1,521
|
|
1,905
|
|
|||||
|
Net change in cash flow hedges, net of tax
|
(1,359
|
)
|
(382
|
)
|
428
|
|
|||||
|
Defined benefit plan liability adjustment, net of tax
|
(374
|
)
|
272
|
|
1,685
|
|
|||||
|
Other comprehensive income (loss)
|
(5,235
|
)
|
1,411
|
|
4,018
|
|
|||||
|
Comprehensive income (loss)
|
$
|
50,223
|
|
$
|
37,678
|
|
$
|
(781
|
)
|
||
|
|
Year Ended
|
||||||||||
|
(in thousands)
|
January 2, 2015
|
January 3, 2014
|
December 28, 2012
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income (loss)
|
$
|
55,458
|
|
$
|
36,267
|
|
$
|
(4,799
|
)
|
||
|
Adjustments to reconcile net income (loss) to net cash provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
37,457
|
|
35,966
|
|
46,368
|
|
|||||
|
Debt related amortization included in interest expense
|
773
|
|
6,366
|
|
12,557
|
|
|||||
|
Stock-based compensation
|
13,186
|
|
14,101
|
|
10,904
|
|
|||||
|
(Gain) loss on cost and equity method investments, net
|
(4,370
|
)
|
694
|
|
106
|
|
|||||
|
Other non-cash (gains) losses, net
|
(3,214
|
)
|
255
|
|
10,788
|
|
|||||
|
Deferred income taxes
|
531
|
|
(29,856
|
)
|
5,733
|
|
|||||
|
Changes in operating assets and liabilities, net of acquisitions:
|
|||||||||||
|
Accounts receivable
|
(11,731
|
)
|
7,379
|
|
(18,834
|
)
|
|||||
|
Inventories
|
(6,726
|
)
|
(11,508
|
)
|
(7,481
|
)
|
|||||
|
Prepaid expenses and other assets
|
(3,281
|
)
|
(353
|
)
|
1,253
|
|
|||||
|
Accounts payable
|
(970
|
)
|
1,307
|
|
5,757
|
|
|||||
|
Accrued expenses
|
1,214
|
|
(1,176
|
)
|
1,459
|
|
|||||
|
Income taxes payable
|
2,949
|
|
(2,687
|
)
|
1,020
|
|
|||||
|
Net cash provided by operating activities
|
81,276
|
|
56,755
|
|
64,831
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Proceeds from sale of orthopaedic product lines
|
2,655
|
|
4,746
|
|
—
|
|
|||||
|
Acquisition of property, plant and equipment
|
(24,823
|
)
|
(18,558
|
)
|
(41,069
|
)
|
|||||
|
Proceeds from sale (purchase) of cost and equity method investments, net
|
2,248
|
|
(3,732
|
)
|
(1,887
|
)
|
|||||
|
Acquisitions, net of cash acquired
|
(16,002
|
)
|
—
|
|
(17,224
|
)
|
|||||
|
Other investing activities, net
|
—
|
|
(740
|
)
|
393
|
|
|||||
|
Net cash used in investing activities
|
(35,922
|
)
|
(18,284
|
)
|
(59,787
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Principal payments of long-term debt
|
(10,000
|
)
|
(458,282
|
)
|
(32,000
|
)
|
|||||
|
Proceeds from issuance of long-term debt
|
—
|
|
425,000
|
|
10,000
|
|
|||||
|
Issuance of common stock
|
8,278
|
|
12,807
|
|
1,263
|
|
|||||
|
Payment of debt issuance costs
|
—
|
|
(2,802
|
)
|
—
|
|
|||||
|
Other financing activities, net
|
(655
|
)
|
(81
|
)
|
(717
|
)
|
|||||
|
Net cash used in financing activities
|
(2,377
|
)
|
(23,358
|
)
|
(21,454
|
)
|
|||||
|
Effect of foreign currency exchange rates on cash and cash equivalents
|
(1,618
|
)
|
68
|
|
186
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
41,359
|
|
15,181
|
|
(16,224
|
)
|
|||||
|
Cash and cash equivalents, beginning of year
|
35,465
|
|
20,284
|
|
36,508
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
76,824
|
|
$
|
35,465
|
|
$
|
20,284
|
|
||
|
|
Common Stock
|
Additional
Paid-In
Capital
|
Treasury
Stock
|
Retained
Earnings
|
Accumulated
Other Comprehensive
Income (Loss)
|
Total
Stockholders’
Equity
|
|||||||||||||||||||||||
|
(in thousands)
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||
|
At December 30, 2011
|
23,466
|
|
$
|
23
|
|
$
|
307,196
|
|
(60
|
)
|
$
|
(1,387
|
)
|
$
|
152,522
|
|
$
|
8,929
|
|
$
|
467,283
|
|
|||||||
|
Stock-based compensation
|
—
|
|
—
|
|
9,019
|
|
—
|
|
—
|
|
—
|
|
—
|
|
9,019
|
|
|||||||||||||
|
Net shares issued under stock incentive plans
|
103
|
|
—
|
|
663
|
|
1
|
|
24
|
|
—
|
|
—
|
|
687
|
|
|||||||||||||
|
Income tax liability from stock options, restricted stock and restricted stock units
|
—
|
|
—
|
|
(141
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(141
|
)
|
|||||||||||||
|
Shares contributed to 401(k) Plan
|
163
|
|
1
|
|
3,881
|
|
39
|
|
911
|
|
—
|
|
4,793
|
|
|||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,799
|
)
|
—
|
|
(4,799
|
)
|
|||||||||||||
|
Total other comprehensive income, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,018
|
|
4,018
|
|
|||||||||||||
|
At December 28, 2012
|
23,732
|
|
24
|
|
320,618
|
|
(20
|
)
|
(452
|
)
|
147,723
|
|
12,947
|
|
480,860
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
9,333
|
|
—
|
|
—
|
|
—
|
|
—
|
|
9,333
|
|
|||||||||||||
|
Net shares issued (acquired) under stock incentive plans
|
636
|
|
—
|
|
12,245
|
|
(17
|
)
|
(780
|
)
|
—
|
|
—
|
|
11,465
|
|
|||||||||||||
|
Income tax benefit from stock options, restricted stock and restricted stock units
|
—
|
|
—
|
|
242
|
|
—
|
|
—
|
|
—
|
|
—
|
|
242
|
|
|||||||||||||
|
Shares contributed to 401(k) Plan
|
91
|
|
—
|
|
2,477
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,477
|
|
|||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
36,267
|
|
—
|
|
36,267
|
|
|||||||||||||
|
Total other comprehensive income, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,411
|
|
1,411
|
|
|||||||||||||
|
At January 3, 2014
|
24,459
|
|
24
|
|
344,915
|
|
(37
|
)
|
(1,232
|
)
|
183,990
|
|
14,358
|
|
542,055
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
8,921
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,921
|
|
|||||||||||||
|
Net shares issued (acquired) under stock incentive plans
|
640
|
|
1
|
|
7,754
|
|
(86
|
)
|
(4,290
|
)
|
—
|
|
—
|
|
3,465
|
|
|||||||||||||
|
Income tax benefit from stock options, restricted stock and restricted stock units
|
—
|
|
—
|
|
4,357
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,357
|
|
|||||||||||||
|
Shares contributed to 401(k) Plan
|
—
|
|
—
|
|
126
|
|
95
|
|
4,215
|
|
—
|
|
—
|
|
4,341
|
|
|||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
55,458
|
|
—
|
|
55,458
|
|
|||||||||||||
|
Total other comprehensive loss, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(5,235
|
)
|
(5,235
|
)
|
|||||||||||||
|
At January 2, 2015
|
25,099
|
|
$
|
25
|
|
$
|
366,073
|
|
(28
|
)
|
$
|
(1,307
|
)
|
$
|
239,448
|
|
$
|
9,123
|
|
$
|
613,362
|
|
|||||||
|
1.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
|
|
2.
|
ACQUISITIONS
|
|
|
Assets acquired
|
|||
|
Current assets
|
$
|
10,670
|
|
|
Property, plant and equipment
|
1,131
|
|
|
|
Amortizing intangible assets
|
6,100
|
|
|
|
Goodwill
|
8,296
|
|
|
|
Total assets acquired
|
26,197
|
|
|
|
Liabilities assumed
|
|||
|
Current liabilities
|
4,842
|
|
|
|
Deferred income taxes
|
1,590
|
|
|
|
Total liabilities assumed
|
6,432
|
|
|
|
Net assets acquired
|
$
|
19,765
|
|
|
Amortizing Intangible Assets
|
Fair
Value
Assigned
|
Weighted
Average
Amortization
Period (Years)
|
Weighted
Average
Discount
Rate
|
|||||
|
Technology
|
$
|
1,400
|
|
10
|
18%
|
|||
|
Customer lists
|
4,600
|
|
10
|
18%
|
||||
|
Trademarks and tradenames
|
100
|
|
2
|
18%
|
||||
|
$
|
6,100
|
|
10
|
18%
|
||||
|
Assets acquired
|
|||
|
Current assets
|
$
|
618
|
|
|
Property, plant and equipment
|
35
|
|
|
|
Amortizing intangible assets
|
2,927
|
|
|
|
Indefinite-lived intangible assets
|
540
|
|
|
|
Goodwill
|
8,924
|
|
|
|
Other assets
|
1,576
|
|
|
|
Total assets acquired
|
14,620
|
|
|
|
Liabilities assumed
|
|||
|
Current liabilities
|
420
|
|
|
|
Deferred income taxes
|
989
|
|
|
|
Total liabilities assumed
|
1,409
|
|
|
|
Net assets acquired
|
$
|
13,211
|
|
|
Fair
Value
Assigned
|
Weighted
Average
Amortization
Period (Years)
|
Estimated
Useful
Life (Years)
|
Weighted
Average
Discount
Rate
|
|||||||
|
Amortizing Intangible Assets
|
||||||||||
|
Technology and patents
|
$
|
1,058
|
|
6
|
10
|
14
|
%
|
|||
|
Customer lists
|
1,869
|
|
7
|
15
|
13
|
%
|
||||
|
$
|
2,927
|
|
7
|
13
|
13
|
%
|
||||
|
Indefinite-lived Intangible Assets
|
||||||||||
|
In-process research and development
|
$
|
540
|
|
N/A
|
12
|
26
|
%
|
|||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Sales
|
$
|
696,357
|
|
$
|
677,657
|
|
$
|
646,617
|
|
||
|
Net income (loss)
|
56,453
|
|
37,612
|
|
(4,973
|
)
|
|||||
|
Earnings (loss) per share:
|
|||||||||||
|
Basic
|
$
|
2.27
|
|
$
|
1.57
|
|
$
|
(0.21
|
)
|
||
|
Diluted
|
$
|
2.17
|
|
$
|
1.49
|
|
$
|
(0.21
|
)
|
||
|
3.
|
SUPPLEMENTAL CASH FLOW INFORMATION
|
|
|
|
Year Ended
|
||||||||||
|
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||||
|
(in thousands)
|
|||||||||||
|
Noncash investing and financing activities:
|
|||||||||||
|
Common stock contributed to 401(k) Plan
|
$
|
4,341
|
|
$
|
2,477
|
|
$
|
4,793
|
|
||
|
Property, plant and equipment purchases included in accounts payable
|
2,926
|
|
2,103
|
|
2,522
|
|
|||||
|
Cash paid during the year for:
|
|||||||||||
|
Interest
|
3,521
|
|
4,989
|
|
6,230
|
|
|||||
|
Income taxes
|
13,565
|
|
44,165
|
|
4,909
|
|
|||||
|
Acquisition of noncash assets
|
22,434
|
|
—
|
|
14,396
|
|
|||||
|
Liabilities assumed
|
6,432
|
|
—
|
|
1,244
|
|
|||||
|
4.
|
INVENTORIES
|
|
|
|
At
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Raw materials
|
$
|
73,354
|
|
$
|
67,939
|
|
|
|
Work-in-process
|
38,930
|
|
36,670
|
|
|||
|
Finished goods
|
16,958
|
|
13,749
|
|
|||
|
Total
|
$
|
129,242
|
|
$
|
118,358
|
|
|
|
5.
|
ASSETS HELD FOR SALE
|
|
|
|
|
At
|
||||||||
|
Asset
|
Business
Segment
|
January 2,
2015 |
January 3,
2014 |
|||||||
|
Building and building improvements
|
Greatbatch Medical
|
$
|
1,635
|
|
$
|
—
|
|
|||
|
6.
|
PROPERTY, PLANT AND EQUIPMENT, NET
|
|
|
|
At
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Manufacturing machinery and equipment
|
$
|
167,173
|
|
$
|
159,542
|
|
|
|
Buildings and building improvements
|
89,258
|
|
87,359
|
|
|||
|
Information technology hardware and software
|
31,725
|
|
28,010
|
|
|||
|
Leasehold improvements
|
31,170
|
|
31,522
|
|
|||
|
Furniture and fixtures
|
14,045
|
|
13,889
|
|
|||
|
Land and land improvements
|
10,816
|
|
13,016
|
|
|||
|
Construction work in process
|
14,129
|
|
7,886
|
|
|||
|
Other
|
629
|
|
633
|
|
|||
|
358,945
|
|
341,857
|
|
||||
|
Accumulated depreciation
|
(214,020
|
)
|
(196,084
|
)
|
|||
|
Total
|
$
|
144,925
|
|
$
|
145,773
|
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Depreciation expense
|
$
|
23,320
|
|
$
|
22,799
|
|
$
|
31,575
|
|
||
|
7.
|
INTANGIBLE ASSETS
|
|
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Foreign
Currency
Translation
|
Net
Carrying
Amount
|
||||||||||||
|
At January 2, 2015
|
|||||||||||||||
|
Purchased technology and patents
|
$
|
95,776
|
|
$
|
(75,894
|
)
|
$
|
1,966
|
|
$
|
21,848
|
|
|||
|
Customer lists
|
72,857
|
|
(31,460
|
)
|
1,374
|
|
42,771
|
|
|||||||
|
Other
|
4,534
|
|
(4,619
|
)
|
803
|
|
718
|
|
|||||||
|
Total amortizing intangible assets
|
$
|
173,167
|
|
$
|
(111,973
|
)
|
$
|
4,143
|
|
$
|
65,337
|
|
|||
|
At January 3, 2014
|
|||||||||||||||
|
Purchased technology and patents
|
$
|
97,376
|
|
$
|
(69,026
|
)
|
$
|
1,980
|
|
$
|
30,330
|
|
|||
|
Customer lists
|
68,257
|
|
(24,671
|
)
|
1,367
|
|
44,953
|
|
|||||||
|
Other
|
4,434
|
|
(4,399
|
)
|
804
|
|
839
|
|
|||||||
|
Total amortizing intangible assets
|
$
|
170,067
|
|
$
|
(98,096
|
)
|
$
|
4,151
|
|
$
|
76,122
|
|
|||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Cost of sales
|
$
|
6,201
|
|
$
|
6,822
|
|
$
|
7,489
|
|
||
|
SG&A
|
7,009
|
|
5,800
|
|
6,227
|
|
|||||
|
RD&E
|
667
|
|
545
|
|
545
|
|
|||||
|
Total intangible asset amortization expense
|
$
|
13,877
|
|
$
|
13,167
|
|
$
|
14,261
|
|
||
|
|
Estimated
Amortization
Expense
|
||
|
2015
|
$
|
12,988
|
|
|
2016
|
10,676
|
|
|
|
2017
|
9,520
|
|
|
|
2018
|
7,232
|
|
|
|
2019
|
5,431
|
|
|
|
Thereafter
|
19,490
|
|
|
|
Total estimated amortization expense
|
$
|
65,337
|
|
|
Trademarks
and
Tradenames
|
|||
|
At January 3, 2014
|
$
|
20,288
|
|
|
At January 2, 2015
|
$
|
20,288
|
|
|
Greatbatch
Medical
|
QiG
|
Total
|
|||||||||
|
At January 3, 2014
|
$
|
304,856
|
|
$
|
41,800
|
|
$
|
346,656
|
|
||
|
Goodwill acquired (Note 2)
|
—
|
|
8,296
|
|
8,296
|
|
|||||
|
Foreign currency translation
|
(559
|
)
|
—
|
|
(559
|
)
|
|||||
|
At January 2, 2015
|
$
|
304,297
|
|
$
|
50,096
|
|
$
|
354,393
|
|
||
|
8.
|
ACCRUED EXPENSES
|
|
|
|
At
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Salaries and benefits
|
$
|
20,770
|
|
$
|
16,311
|
|
|
|
Profit sharing and bonuses
|
18,524
|
|
19,808
|
|
|||
|
Warranty
|
660
|
|
1,819
|
|
|||
|
Other
|
8,430
|
|
6,743
|
|
|||
|
Total
|
$
|
48,384
|
|
$
|
44,681
|
|
|
|
9.
|
DEBT
|
|
|
|
At
|
||||||
|
|
January 2,
2015 |
January 3, 2014
|
|||||
|
Variable rate term loan
|
$
|
187,500
|
|
$
|
197,500
|
|
|
|
Revolving line of credit
|
—
|
|
—
|
|
|||
|
Total debt
|
187,500
|
|
197,500
|
|
|||
|
Less current portion of long-term debt
|
11,250
|
|
—
|
|
|||
|
Total long-term debt
|
$
|
176,250
|
|
$
|
197,500
|
|
|
|
Instrument
|
Type of
Hedge
|
Notional
Amount
|
Start
Date
|
End
Date
|
Pay
Fixed
Rate
|
Current
Receive
Floating
Rate
|
Fair
Value
January 2, 2015
|
Balance
Sheet Location
|
||||||||||||||
|
Interest rate swap
|
Cash flow
|
$
|
100,000
|
|
Feb-13
|
Feb-16
|
0.573
|
%
|
0.155
|
%
|
$
|
(125
|
)
|
Other Long-Term Liabilities
|
||||||||
|
Interest rate swap
|
Cash flow
|
$
|
90,000
|
|
Feb-15
|
Sept-19
|
1.921
|
%
|
N/A
|
$
|
(865
|
)
|
Other Long-Term Liabilities
|
|||||||||
|
2015
|
$
|
11,250
|
|
|
2016
|
16,250
|
|
|
|
2017
|
20,000
|
|
|
|
2018
|
20,000
|
|
|
|
2019
|
120,000
|
|
|
|
Total
|
187,500
|
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Contractual interest
|
$
|
—
|
|
$
|
634
|
|
$
|
4,450
|
|
||
|
Discount amortization
|
—
|
|
5,368
|
|
11,464
|
|
|||||
|
At December 28, 2012
|
$
|
2,056
|
|
|
Financing costs deferred
|
2,802
|
|
|
|
Write-off during the period
|
(156
|
)
|
|
|
Amortization during the period
|
(842
|
)
|
|
|
At January 3, 2014
|
3,860
|
|
|
|
Amortization during the period
|
(773
|
)
|
|
|
At January 2, 2015
|
$
|
3,087
|
|
|
10.
|
BENEFIT PLANS
|
|
|
|
Year Ended
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Change in projected benefit obligation:
|
|||||||
|
Projected benefit obligation at beginning of year
|
$
|
2,422
|
|
$
|
16,215
|
|
|
|
Service cost
|
203
|
|
236
|
|
|||
|
Interest cost
|
75
|
|
138
|
|
|||
|
Prior service cost and plan amendments
|
—
|
|
(45
|
)
|
|||
|
Plan participants’ contribution
|
36
|
|
134
|
|
|||
|
Actuarial (gain) loss
|
630
|
|
(2
|
)
|
|||
|
Benefits transferred in, net
|
155
|
|
434
|
|
|||
|
Settlement/curtailment gain
|
(337
|
)
|
(14,539
|
)
|
|||
|
Foreign currency translation
|
(341
|
)
|
(149
|
)
|
|||
|
Projected benefit obligation at end of year
|
2,843
|
|
2,422
|
|
|||
|
Change in fair value of plan assets:
|
|||||||
|
Fair value of plan assets at beginning of year
|
731
|
|
12,269
|
|
|||
|
Employer contributions (refund)
|
(39
|
)
|
150
|
|
|||
|
Plan participants’ contributions
|
36
|
|
134
|
|
|||
|
Actual loss on plan assets
|
(101
|
)
|
(26
|
)
|
|||
|
Benefits transferred in, net
|
198
|
|
138
|
|
|||
|
Settlements
|
(337
|
)
|
(11,780
|
)
|
|||
|
Foreign currency translation
|
(51
|
)
|
(154
|
)
|
|||
|
Fair value of plan assets at end of year
|
437
|
|
731
|
|
|||
|
Projected benefit obligation in excess of plan assets at end of year
|
$
|
2,406
|
|
$
|
1,691
|
|
|
|
Defined benefit liability classified as other current liabilities
|
$
|
25
|
|
$
|
25
|
|
|
|
Defined benefit liability classified as long-term liabilities
|
$
|
2,381
|
|
$
|
1,666
|
|
|
|
Accumulated benefit obligation at end of year
|
$
|
1,938
|
|
$
|
1,684
|
|
|
|
|
Year Ended
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Net loss occurring during the year
|
$
|
736
|
|
$
|
25
|
|
|
|
Amortization of losses
|
(138
|
)
|
(722
|
)
|
|||
|
Prior service cost
|
(2
|
)
|
150
|
|
|||
|
Amortization of prior service cost
|
(11
|
)
|
33
|
|
|||
|
Foreign currency translation
|
(76
|
)
|
224
|
|
|||
|
Pre-tax adjustment
|
509
|
|
(290
|
)
|
|||
|
Taxes
|
(135
|
)
|
18
|
|
|||
|
Net (gain) loss
|
$
|
374
|
|
$
|
(272
|
)
|
|
|
Amortization of net prior service cost
|
$
|
11
|
|
|
Amortization of net loss
|
45
|
|
|
|
|
Year Ended
|
||||||
|
January 2, 2015
|
January 3, 2014
|
||||||
|
Service cost
|
$
|
203
|
|
$
|
236
|
|
|
|
Interest cost
|
75
|
|
138
|
|
|||
|
Settlements loss
|
105
|
|
—
|
|
|||
|
Expected return on assets
|
(3
|
)
|
—
|
|
|||
|
Recognized net actuarial loss (gain)
|
45
|
|
(1,929
|
)
|
|||
|
Net pension cost (income)
|
$
|
425
|
|
$
|
(1,555
|
)
|
|
|
|
Projected Benefit Obligation
|
Net Pension Cost
|
||||||||||||
|
January 2,
2015 |
January 3,
2014 |
2014
|
2013
|
2012
|
||||||||||
|
Discount rate
|
2.3
|
%
|
3.4
|
%
|
3.4
|
%
|
2.1
|
%
|
2.5
|
%
|
||||
|
Salary growth
|
3.0
|
%
|
3.1
|
%
|
3.1
|
%
|
2.4
|
%
|
2.3
|
%
|
||||
|
Expected rate of return on assets
|
2.3
|
%
|
2.5
|
%
|
2.5
|
%
|
—
|
%
|
3.5
|
%
|
||||
|
|
|
Fair Value Measurements Using
|
|||||||||||||
|
January 2, 2015
|
Quoted
Prices in
Active
Markets for
Identical
Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
||||||||||||
|
Insurance contract
|
$
|
437
|
|
$
|
—
|
|
$
|
437
|
|
$
|
—
|
|
|||
|
Total
|
$
|
437
|
|
$
|
—
|
|
$
|
437
|
|
$
|
—
|
|
|||
|
|
|
Fair Value Measurements Using
|
|||||||||||||
|
January 3,
2014 |
Quoted
Prices in
Active
Markets for
Identical
Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
||||||||||||
|
Insurance contract
|
$
|
731
|
|
$
|
—
|
|
$
|
731
|
|
$
|
—
|
|
|||
|
Total
|
$
|
731
|
|
$
|
—
|
|
$
|
731
|
|
$
|
—
|
|
|||
|
2015
|
$
|
47
|
|
|
2016
|
67
|
|
|
|
2017
|
124
|
|
|
|
2018
|
113
|
|
|
|
2019
|
177
|
|
|
|
2020-2024
|
866
|
|
|
|
11.
|
STOCK-BASED COMPENSATION
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Stock options
|
$
|
2,523
|
|
$
|
3,490
|
|
$
|
2,786
|
|
||
|
Restricted stock and units
|
6,417
|
|
5,843
|
|
6,233
|
|
|||||
|
401(k) stock contribution
|
4,246
|
|
4,768
|
|
1,885
|
|
|||||
|
Total stock-based compensation expense
|
$
|
13,186
|
|
$
|
14,101
|
|
$
|
10,904
|
|
||
|
Cost of sales
|
$
|
3,530
|
|
$
|
3,864
|
|
$
|
2,620
|
|
||
|
Selling, general and administrative expenses
|
7,923
|
|
7,907
|
|
7,684
|
|
|||||
|
Research, development and engineering costs, net
|
1,440
|
|
1,194
|
|
600
|
|
|||||
|
Other operating expenses, net (Note 13)
|
293
|
|
1,136
|
|
—
|
|
|||||
|
Total stock-based compensation expense
|
$
|
13,186
|
|
$
|
14,101
|
|
$
|
10,904
|
|
||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Weighted average grant date fair value
|
$
|
16.43
|
|
$
|
8.38
|
|
$
|
8.20
|
|
||
|
Risk-free interest rate
|
1.73
|
%
|
0.73
|
%
|
0.83
|
%
|
|||||
|
Expected volatility
|
39
|
%
|
39
|
%
|
40
|
%
|
|||||
|
Expected life (in years)
|
5.3
|
|
5.3
|
|
5.3
|
|
|||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
|||||
|
Annual prevesting forfeiture rate
|
9
|
%
|
9
|
%
|
9
|
%
|
|||||
|
Number of
Time-Vested
Stock
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contractual
Life
(In Years)
|
Aggregate
Intrinsic
Value
(In Millions)
|
|||||||||
|
Outstanding at December 30, 2011
|
1,558,771
|
|
$
|
23.42
|
|
|||||||
|
Granted
|
395,978
|
|
22.19
|
|
||||||||
|
Exercised
|
(52,683
|
)
|
20.77
|
|
||||||||
|
Forfeited or expired
|
(126,219
|
)
|
24.21
|
|
||||||||
|
Outstanding at December 28, 2012
|
1,775,847
|
|
23.17
|
|
||||||||
|
Granted
|
372,676
|
|
23.33
|
|
||||||||
|
Exercised
|
(443,428
|
)
|
23.24
|
|
||||||||
|
Forfeited or expired
|
(88,686
|
)
|
28.05
|
|
||||||||
|
Outstanding at January 3, 2014
|
1,616,409
|
|
22.92
|
|
||||||||
|
Granted
|
183,571
|
|
43.84
|
|
||||||||
|
Exercised
|
(295,203
|
)
|
23.42
|
|
||||||||
|
Forfeited or expired
|
(33,279
|
)
|
27.82
|
|
||||||||
|
Outstanding at January 2, 2015
|
1,471,498
|
|
$
|
25.32
|
|
6.1
|
$
|
34.3
|
|
|||
|
Expected to vest at January 2, 2015
|
1,447,519
|
|
$
|
25.10
|
|
6.1
|
$
|
34.1
|
|
|||
|
Exercisable at January 2, 2015
|
1,278,765
|
|
$
|
23.88
|
|
5.8
|
$
|
31.7
|
|
|||
|
Number of
Performance-
Vested Stock
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contractual
Life
(In Years)
|
Aggregate
Intrinsic
Value
(In Millions)
|
|||||||||
|
Outstanding at December 30, 2011
|
478,364
|
|
$
|
24.44
|
|
|||||||
|
Exercised
|
(7,657
|
)
|
22.04
|
|
||||||||
|
Forfeited or expired
|
(185,782
|
)
|
26.35
|
|
||||||||
|
Outstanding at December 28, 2012
|
284,925
|
|
23.26
|
|
||||||||
|
Exercised
|
(107,664
|
)
|
23.23
|
|
||||||||
|
Forfeited or expired
|
—
|
|
—
|
|
||||||||
|
Outstanding at January 3, 2014
|
177,261
|
|
23.27
|
|
||||||||
|
Exercised
|
(58,422
|
)
|
23.35
|
|
||||||||
|
Forfeited or expired
|
—
|
|
—
|
|
||||||||
|
Outstanding at January 2, 2015
|
118,839
|
|
$
|
23.24
|
|
3.0
|
$
|
3.0
|
|
|||
|
Expected to vest at January 2, 2015
|
118,839
|
|
$
|
23.24
|
|
3.0
|
$
|
3.0
|
|
|||
|
Exercisable at January 2, 2015
|
118,839
|
|
$
|
23.24
|
|
3.0
|
$
|
3.0
|
|
|||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Intrinsic value
|
$
|
7,997
|
|
$
|
6,807
|
|
$
|
148
|
|
||
|
Cash received
|
8,278
|
|
12,807
|
|
1,263
|
|
|||||
|
Tax benefit (expense) realized
|
1,704
|
|
727
|
|
(132
|
)
|
|||||
|
Time-Vested
Activity
|
Weighted
Average
Fair Value
|
|||||
|
Nonvested at December 30, 2011
|
69,942
|
|
$
|
22.69
|
|
|
|
Granted
|
92,265
|
|
23.49
|
|
||
|
Vested
|
(74,901
|
)
|
22.83
|
|
||
|
Forfeited
|
(7,037
|
)
|
22.56
|
|
||
|
Nonvested at December 28, 2012
|
80,269
|
|
23.48
|
|
||
|
Granted
|
67,230
|
|
26.76
|
|
||
|
Vested
|
(74,062
|
)
|
23.93
|
|
||
|
Forfeited
|
(5,862
|
)
|
22.26
|
|
||
|
Nonvested at January 3, 2014
|
67,575
|
|
26.37
|
|
||
|
Granted
|
63,817
|
|
44.78
|
|
||
|
Vested
|
(53,568
|
)
|
34.16
|
|
||
|
Forfeited
|
(9,992
|
)
|
35.30
|
|
||
|
Nonvested at January 2, 2015
|
67,832
|
|
$
|
36.22
|
|
|
|
Performance-
Vested
Activity
|
Weighted
Average
Fair Value
|
|||||
|
Nonvested at December 30, 2011
|
529,743
|
|
$
|
16.68
|
|
|
|
Granted
|
332,918
|
|
15.30
|
|
||
|
Vested
|
(15,500
|
)
|
24.64
|
|
||
|
Forfeited
|
(64,715
|
)
|
15.72
|
|
||
|
Nonvested at December 28, 2012
|
782,446
|
|
16.02
|
|
||
|
Granted
|
318,169
|
|
15.86
|
|
||
|
Vested
|
(49,139
|
)
|
14.68
|
|
||
|
Forfeited
|
(271,798
|
)
|
14.94
|
|
||
|
Nonvested at January 3, 2014
|
779,678
|
|
16.41
|
|
||
|
Granted
|
186,825
|
|
31.33
|
|
||
|
Vested
|
(221,470
|
)
|
18.51
|
|
||
|
Forfeited
|
(28,870
|
)
|
18.42
|
|
||
|
Nonvested at January 2, 2015
|
716,163
|
|
$
|
19.57
|
|
|
|
12.
|
RESEARCH, DEVELOPMENT AND ENGINEERING COSTS, NET
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Research, development and engineering costs
|
$
|
58,974
|
|
$
|
62,652
|
|
$
|
62,848
|
|
||
|
Less: cost reimbursements
|
(9,129
|
)
|
(8,575
|
)
|
(10,358
|
)
|
|||||
|
Total research, development and engineering costs, net
|
$
|
49,845
|
|
$
|
54,077
|
|
$
|
52,490
|
|
||
|
13.
|
OTHER OPERATING EXPENSES, NET
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
2014 investments in capacity and capabilities
|
$
|
8,925
|
|
$
|
—
|
|
$
|
—
|
|
||
|
2013 operating unit realignment
|
1,017
|
|
5,625
|
|
—
|
|
|||||
|
Orthopaedic facilities optimization
|
1,317
|
|
8,038
|
|
32,482
|
|
|||||
|
Medical device facility optimization
|
11
|
|
312
|
|
1,525
|
|
|||||
|
ERP system upgrade (income) costs
|
(82
|
)
|
783
|
|
5,041
|
|
|||||
|
Acquisition and integration (income) costs
|
3
|
|
(502
|
)
|
1,460
|
|
|||||
|
Asset dispositions, severance and other
|
4,106
|
|
1,534
|
|
1,838
|
|
|||||
|
Total other operating expenses, net
|
$
|
15,297
|
|
$
|
15,790
|
|
$
|
42,346
|
|
||
|
•
|
Functions currently performed at the Company’s facility in Plymouth, MN to manufacture catheters and introducers will transfer into the Company’s existing facility in Tijuana, Mexico by the first half of 2016.
|
|
•
|
Functions currently performed at the Company’s facilities in Beaverton, OR and Raynham, MA to manufacture products for the portable medical market will transfer to a new facility in Tijuana, Mexico by the end of 2015. Products currently manufactured at the Beaverton facility, which do not serve the portable medical market, are planned to transfer to the Company’s Raynham facility.
|
|
•
|
Establishing a R&D hub in the Minneapolis/St. Paul, MN area for the Company’s Global R&D QiG - Medical Device Systems team, which will serve as the technical center of expertise for active implantable medical device development, implantable leads design, system level design verification testing, and continuation engineering. As part of this initiative, the design engineering responsibilities previously performed at the Company’s Cleveland, OH facility was transferred to the new R&D hub in 2014.
|
|
•
|
Establishing a commercial operations hub at the Company’s global headquarters in Frisco, Texas. This initiative will build upon the investment the Company has made in its global sales and marketing function and is expected to be completed during the first half of 2015.
|
|
•
|
Severance and retention:
$7.0 million
-
$9.0 million
;
|
|
•
|
Accelerated depreciation and asset write-offs:
$2.0 million
-
$3.0 million
; and
|
|
•
|
Other:
$20.0 million
-
$22.0 million
|
|
Severance and Retention
|
Accelerated
Depreciation/
Asset Write-offs
|
Other
|
Total
|
||||||||||||
|
At January 3, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Restructuring charges
|
2,209
|
|
33
|
|
6,683
|
|
8,925
|
|
|||||||
|
Write-offs
|
—
|
|
(33
|
)
|
—
|
|
(33
|
)
|
|||||||
|
Cash payments
|
(1,046
|
)
|
—
|
|
(5,617
|
)
|
(6,663
|
)
|
|||||||
|
At January 2, 2015
|
$
|
1,163
|
|
$
|
—
|
|
$
|
1,066
|
|
$
|
2,229
|
|
|||
|
•
|
Severance and retention:
$5.0 million
; and
|
|
•
|
Other:
$1.6 million
.
|
|
Severance and Retention
|
Other
|
Total
|
|||||||||
|
At January 3, 2014
|
$
|
465
|
|
$
|
746
|
|
$
|
1,211
|
|
||
|
Restructuring charges
|
849
|
|
168
|
|
1,017
|
|
|||||
|
Cash payments
|
(1,314
|
)
|
(914
|
)
|
(2,228
|
)
|
|||||
|
At January 2, 2015
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Severance
and
Retention
|
Accelerated
Depreciation/
Asset Write-offs
|
Other
|
Total
|
||||||||||||
|
At January 3, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
857
|
|
$
|
857
|
|
|||
|
Restructuring charges (income), net
|
—
|
|
(2,255
|
)
|
3,572
|
|
1,317
|
|
|||||||
|
Write-offs
|
—
|
|
(400
|
)
|
—
|
|
(400
|
)
|
|||||||
|
Cash receipts (payments)
|
—
|
|
2,655
|
|
(4,142
|
)
|
(1,487
|
)
|
|||||||
|
At January 2, 2015
|
$
|
—
|
|
$
|
—
|
|
$
|
287
|
|
$
|
287
|
|
|||
|
Production
Inefficiencies,
Moving and
Revalidation
|
Personnel
|
Other
|
Total
|
||||||||||||
|
At January 3, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Restructuring charges
|
—
|
|
1
|
|
10
|
|
11
|
|
|||||||
|
Cash payments
|
—
|
|
(1
|
)
|
(10
|
)
|
(11
|
)
|
|||||||
|
At January 2, 2015
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Training &
Consulting
Costs
|
Accelerated
Depreciation/
Asset Write-offs
|
Total
|
|||||||||
|
At January 3, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Restructuring income
|
(82
|
)
|
—
|
|
(82
|
)
|
|||||
|
Cash receipts
|
82
|
|
—
|
|
82
|
|
|||||
|
At January 2, 2015
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
14.
|
INCOME TAXES
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
U.S.
|
$
|
56,801
|
|
$
|
42,392
|
|
$
|
36,057
|
|
||
|
International
|
19,778
|
|
6,446
|
|
(29,327
|
)
|
|||||
|
Total income before provision for income taxes
|
$
|
76,579
|
|
$
|
48,838
|
|
$
|
6,730
|
|
||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
16,293
|
|
$
|
39,353
|
|
$
|
4,747
|
|
||
|
State
|
1,299
|
|
1,604
|
|
381
|
|
|||||
|
International
|
2,998
|
|
1,470
|
|
668
|
|
|||||
|
20,590
|
|
42,427
|
|
5,796
|
|
||||||
|
Deferred:
|
|||||||||||
|
Federal
|
1,211
|
|
(28,678
|
)
|
6,615
|
|
|||||
|
State
|
(310
|
)
|
427
|
|
175
|
|
|||||
|
International
|
(370
|
)
|
(1,605
|
)
|
(1,057
|
)
|
|||||
|
531
|
|
(29,856
|
)
|
5,733
|
|
||||||
|
Total provision for income taxes
|
$
|
21,121
|
|
$
|
12,571
|
|
$
|
11,529
|
|
||
|
|
Year Ended
|
|||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||
|
Statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
Federal tax credits
|
(2.1
|
)
|
(7.5
|
)
|
—
|
|
||
|
Foreign rate differential
|
(4.3
|
)
|
(0.7
|
)
|
50.7
|
|
||
|
Uncertain tax positions
|
0.6
|
|
1.7
|
|
(10.1
|
)
|
||
|
State taxes, net of federal benefit
|
0.7
|
|
2.3
|
|
4.9
|
|
||
|
Change in tax rate - loss of Swiss tax holiday
|
—
|
|
—
|
|
25.6
|
|
||
|
Change in foreign tax rates
|
(0.6
|
)
|
(3.7
|
)
|
—
|
|
||
|
Valuation allowance
|
(0.4
|
)
|
0.4
|
|
67.6
|
|
||
|
Other
|
(1.3
|
)
|
(1.8
|
)
|
(2.4
|
)
|
||
|
Effective tax rate
|
27.6
|
%
|
25.7
|
%
|
171.3
|
%
|
||
|
|
At
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Tax credits
|
$
|
5,828
|
|
$
|
6,624
|
|
|
|
Net operating loss carryforwards
|
6,721
|
|
9,161
|
|
|||
|
Inventories
|
3,335
|
|
4,202
|
|
|||
|
Accrued expenses
|
4,338
|
|
4,303
|
|
|||
|
Stock-based compensation
|
9,341
|
|
9,194
|
|
|||
|
Other
|
1,659
|
|
573
|
|
|||
|
Gross deferred tax assets
|
31,222
|
|
34,057
|
|
|||
|
Less valuation allowance
|
(10,709
|
)
|
(11,661
|
)
|
|||
|
Net deferred tax assets
|
20,513
|
|
22,396
|
|
|||
|
Property, plant and equipment
|
(2,646
|
)
|
(2,254
|
)
|
|||
|
Intangible assets
|
(57,850
|
)
|
(57,648
|
)
|
|||
|
Convertible subordinated notes
|
(5,006
|
)
|
(6,178
|
)
|
|||
|
Gross deferred tax liabilities
|
(65,502
|
)
|
(66,080
|
)
|
|||
|
Net deferred tax liability
|
$
|
(44,989
|
)
|
$
|
(43,684
|
)
|
|
|
Presented as follows:
|
|||||||
|
Current deferred tax asset
|
$
|
6,168
|
|
$
|
6,008
|
|
|
|
Current deferred tax liability
|
(588
|
)
|
(613
|
)
|
|||
|
Noncurrent deferred tax asset
|
2,626
|
|
2,933
|
|
|||
|
Noncurrent deferred tax liability
|
(53,195
|
)
|
(52,012
|
)
|
|||
|
Net deferred tax liability
|
$
|
(44,989
|
)
|
$
|
(43,684
|
)
|
|
|
Jurisdiction
|
Tax
Attribute
|
Amount
(in millions)
|
|
Begin to
Expire
|
|||
|
International
|
Net Operating Loss
|
48.0
|
|
(1)
|
2015
|
||
|
State
|
Net Operating Loss
|
37.6
|
|
(1)
|
Various
|
||
|
U.S. and State
|
R&D Tax Credit
|
0.7
|
|
(1)
|
Various
|
||
|
State
|
Investment Tax Credit
|
5.3
|
|
|
Various
|
||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Balance, beginning of year
|
$
|
1,858
|
|
$
|
970
|
|
$
|
1,580
|
|
||
|
Additions based upon tax positions related to the current year
|
268
|
|
325
|
|
—
|
|
|||||
|
Additions related to prior period tax positions
|
510
|
|
651
|
|
210
|
|
|||||
|
Reductions relating to settlements with tax authorities
|
(225
|
)
|
(88
|
)
|
(522
|
)
|
|||||
|
Reductions as a result of a lapse of applicable statute of limitations
|
—
|
|
—
|
|
(298
|
)
|
|||||
|
Balance, end of year
|
$
|
2,411
|
|
$
|
1,858
|
|
$
|
970
|
|
||
|
15.
|
COMMITMENTS AND CONTINGENCIES
|
|
|
|
Year Ended
|
||||||
|
January 2,
2015 |
January 3,
2014 |
||||||
|
Beginning balance
|
$
|
1,819
|
|
$
|
2,626
|
|
|
|
Additions to warranty reserve
|
953
|
|
1,624
|
|
|||
|
Warranty claims paid
|
(2,112
|
)
|
(2,431
|
)
|
|||
|
Ending balance
|
$
|
660
|
|
$
|
1,819
|
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Operating lease expense
|
$
|
4,281
|
|
$
|
4,379
|
|
$
|
4,024
|
|
||
|
2015
|
$
|
5,797
|
|
|
2016
|
5,952
|
|
|
|
2017
|
3,908
|
|
|
|
2018
|
3,489
|
|
|
|
2019
|
3,418
|
|
|
|
Thereafter
|
13,938
|
|
|
|
Total estimated operating lease expense
|
$
|
36,502
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Reduction in Cost of Sales
|
$
|
(168
|
)
|
$
|
(1,154
|
)
|
$
|
(79
|
)
|
||
|
Ineffective portion of change in fair value
|
—
|
|
—
|
|
—
|
|
|||||
|
Instrument
|
Type of
Hedge
|
Aggregate
Notional
Amount
|
Start
Date
|
End
Date
|
$/Peso
|
Fair
Value
|
Balance Sheet
Location
|
|||||||||||
|
FX Contract
|
Cash flow
|
$
|
16,880
|
|
Jan-15
|
Dec-15
|
0.0734
|
|
$
|
(1,568
|
)
|
Accrued Expenses
|
||||||
|
16.
|
EARNINGS (LOSS) PER SHARE
|
|
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Numerator for basic EPS:
|
|||||||||||
|
Net income (loss)
|
$
|
55,458
|
|
$
|
36,267
|
|
$
|
(4,799
|
)
|
||
|
Denominator for basic EPS:
|
|||||||||||
|
Weighted average shares outstanding
|
24,825
|
|
23,991
|
|
23,584
|
|
|||||
|
Effect of dilutive securities:
|
|||||||||||
|
Stock options, restricted stock and restricted stock units
|
1,150
|
|
1,332
|
|
—
|
|
|||||
|
Denominator for diluted EPS
|
25,975
|
|
25,323
|
|
23,584
|
|
|||||
|
Basic EPS
|
$
|
2.23
|
|
$
|
1.51
|
|
$
|
(0.20
|
)
|
||
|
Diluted EPS
|
$
|
2.14
|
|
$
|
1.43
|
|
$
|
(0.20
|
)
|
||
|
|
Year Ended
|
|||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||
|
Time-vested stock options, restricted stock and restricted stock units
|
175,549
|
|
18,480
|
|
2,142,000
|
|
||
|
Performance-vested stock options and restricted stock units
|
—
|
|
—
|
|
781,000
|
|
||
|
17.
|
ACCUMULATED OTHER COMPREHENSIVE INCOME
|
|
|
Defined
Benefit
Plan
Liability
|
Cash
Flow
Hedges
|
Foreign
Currency
Translation
Adjustment
|
Total
Pre-Tax
Amount
|
Tax
|
Net-of-Tax
Amount
|
||||||||||||||||||
|
At January 3, 2014
|
$
|
(672
|
)
|
$
|
(468
|
)
|
$
|
14,952
|
|
$
|
13,812
|
|
$
|
546
|
|
$
|
14,358
|
|
|||||
|
Unrealized loss on cash flow hedges
|
—
|
|
(2,372
|
)
|
—
|
|
(2,372
|
)
|
829
|
|
(1,543
|
)
|
|||||||||||
|
Realized gain on foreign currency hedges
|
—
|
|
(168
|
)
|
—
|
|
(168
|
)
|
59
|
|
(109
|
)
|
|||||||||||
|
Realized loss on interest rate swap hedges
|
—
|
|
450
|
|
—
|
|
450
|
|
(157
|
)
|
293
|
|
|||||||||||
|
Net defined benefit plan liability adjustments
|
(509
|
)
|
—
|
|
—
|
|
(509
|
)
|
135
|
|
(374
|
)
|
|||||||||||
|
Foreign currency translation loss
|
—
|
|
—
|
|
(3,502
|
)
|
(3,502
|
)
|
—
|
|
(3,502
|
)
|
|||||||||||
|
At January 2, 2015
|
$
|
(1,181
|
)
|
$
|
(2,558
|
)
|
$
|
11,450
|
|
$
|
7,711
|
|
$
|
1,412
|
|
$
|
9,123
|
|
|||||
|
Defined
Benefit
Plan
Liability
|
Cash
Flow
Hedges
|
Foreign
Currency
Translation
Adjustment
|
Total
Pre-Tax
Amount
|
Tax
|
Net-of-Tax
Amount
|
||||||||||||||||||
|
At December 28, 2012
|
$
|
(962
|
)
|
$
|
120
|
|
$
|
13,431
|
|
$
|
12,589
|
|
$
|
358
|
|
$
|
12,947
|
|
|||||
|
Unrealized gain on cash flow hedges
|
—
|
|
58
|
|
—
|
|
58
|
|
(20
|
)
|
38
|
|
|||||||||||
|
Realized gain on foreign currency hedges
|
—
|
|
(1,154
|
)
|
—
|
|
(1,154
|
)
|
404
|
|
(750
|
)
|
|||||||||||
|
Realized loss on interest rate swap hedges
|
—
|
|
508
|
|
—
|
|
508
|
|
(178
|
)
|
330
|
|
|||||||||||
|
Net defined benefit plan liability adjustments
|
290
|
|
—
|
|
—
|
|
290
|
|
(18
|
)
|
272
|
|
|||||||||||
|
Foreign currency translation gain
|
—
|
|
—
|
|
1,521
|
|
1,521
|
|
—
|
|
1,521
|
|
|||||||||||
|
At January 3, 2014
|
$
|
(672
|
)
|
$
|
(468
|
)
|
$
|
14,952
|
|
$
|
13,812
|
|
$
|
546
|
|
$
|
14,358
|
|
|||||
|
18.
|
FAIR VALUE MEASUREMENTS
|
|
|
At December 28, 2012
|
$
|
1,530
|
|
|
Fair value adjustments
|
(690
|
)
|
|
|
At January 3, 2014
|
840
|
|
|
|
Fair value adjustments
|
(840
|
)
|
|
|
At January 2, 2015
|
$
|
—
|
|
|
|
Fair Value Measurements Using
|
||||||||||||||
|
Description
|
At January 2, 2015
|
Quoted
Prices in
Active Markets
for Identical
Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Liabilities
|
|||||||||||||||
|
Foreign currency contracts (Note 15)
|
$
|
1,568
|
|
$
|
—
|
|
$
|
1,568
|
|
$
|
—
|
|
|||
|
Interest rate swaps (Note 9)
|
990
|
|
—
|
|
990
|
|
—
|
|
|||||||
|
|
Fair Value Measurements Using
|
||||||||||||||
|
Description
|
At January 3,
2014 |
Quoted
Prices in Active Markets for Identical Assets (Level 1) |
Significant
Other Observable Inputs (Level 2) |
Significant
Unobservable Inputs (Level 3) |
|||||||||||
|
Liabilities
|
|||||||||||||||
|
Foreign currency contracts
|
$
|
140
|
|
$
|
—
|
|
$
|
140
|
|
$
|
—
|
|
|||
|
Accrued contingent consideration
|
840
|
|
—
|
|
—
|
|
840
|
|
|||||||
|
Interest rate swap
|
328
|
|
—
|
|
328
|
|
—
|
|
|||||||
|
|
Fair Value Measurements Using
|
||||||||||||||
|
Description
|
At January 2, 2015
|
Quoted
Prices in Active Markets for Identical Assets (Level 1) |
Significant
Other Observable Inputs (Level 2) |
Significant
Unobservable Inputs (Level 3) |
|||||||||||
|
Assets
|
|||||||||||||||
|
Assets Held for Sale (Note 5)
|
$
|
1,635
|
|
$
|
—
|
|
$
|
1,635
|
|
$
|
—
|
|
|||
|
19.
|
BUSINESS SEGMENT, GEOGRAPHIC AND CONCENTRATION RISK INFORMATION
|
|
|
•
|
Cardiac/Neuromodulation:
Products include batteries, capacitors, filtered and unfiltered feed-throughs, engineered components, implantable stimulation leads, and enclosures used in implantable medical devices.
|
|
•
|
Orthopaedics:
Products include implants, instruments and delivery systems for large joint, spine, extremity and trauma procedures.
|
|
•
|
Portable Medical:
Products include life-saving and life-enhancing applications comprising automated external defibrillators, portable oxygen concentrators, ventilators, and powered surgical tools.
|
|
•
|
Vascular:
Products include introducers, steerable sheaths, and catheters that deliver therapies for various markets such as coronary and neurovascular disease, peripheral vascular disease, interventional radiology, vascular access, atrial fibrillation, and interventional cardiology, plus products for medical imaging and pharmaceutical delivery.
|
|
•
|
Energy, Military, Environmental:
Products include primary and rechargeable batteries and battery packs for demanding applications such as down hole drilling tools.
|
|
|
Year Ended
|
||||||||||
|
Sales:
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||||
|
Greatbatch Medical
|
|||||||||||
|
Cardiac/Neuromodulation
|
$
|
321,419
|
|
$
|
325,412
|
|
$
|
306,669
|
|
||
|
Orthopaedics
|
147,296
|
|
130,247
|
|
122,061
|
|
|||||
|
Portable Medical
|
69,043
|
|
78,743
|
|
81,659
|
|
|||||
|
Vascular
|
58,770
|
|
48,357
|
|
51,980
|
|
|||||
|
Energy, Military, Environmental
|
81,757
|
|
78,143
|
|
81,353
|
|
|||||
|
Total Greatbatch Medical
|
678,285
|
|
660,902
|
|
643,722
|
|
|||||
|
QiG
|
9,502
|
|
3,043
|
|
2,455
|
|
|||||
|
Total sales
|
$
|
687,787
|
|
$
|
663,945
|
|
$
|
646,177
|
|
||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Segment income (loss) from operations:
|
|||||||||||
|
Greatbatch Medical
|
$
|
126,312
|
|
$
|
111,805
|
|
$
|
79,093
|
|
||
|
QiG
|
(23,256
|
)
|
(30,484
|
)
|
(32,554
|
)
|
|||||
|
Total segment income from operations
|
103,056
|
|
81,321
|
|
46,539
|
|
|||||
|
Unallocated operating expenses
|
(27,402
|
)
|
(19,982
|
)
|
(20,718
|
)
|
|||||
|
Operating income as reported
|
75,654
|
|
61,339
|
|
25,821
|
|
|||||
|
Unallocated other income (expense), net
|
925
|
|
(12,501
|
)
|
(19,091
|
)
|
|||||
|
Income before provision for income taxes as reported
|
$
|
76,579
|
|
$
|
48,838
|
|
$
|
6,730
|
|
||
|
|
Year Ended
|
||||||||||
|
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||||
|
Depreciation and Amortization:
|
|||||||||||
|
Greatbatch Medical
|
$
|
31,906
|
|
$
|
31,112
|
|
$
|
39,820
|
|
||
|
QiG
|
2,101
|
|
1,539
|
|
630
|
|
|||||
|
Total depreciation and amortization included in segment income from operations
|
34,007
|
|
32,651
|
|
40,450
|
|
|||||
|
Unallocated depreciation and amortization
|
4,223
|
|
9,681
|
|
18,475
|
|
|||||
|
Total depreciation and amortization
|
$
|
38,230
|
|
$
|
42,332
|
|
$
|
58,925
|
|
||
|
|
Year Ended
|
||||||||||
|
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
||||||||
|
Expenditures for tangible long-lived assets, excluding acquisitions:
|
|||||||||||
|
Greatbatch Medical
|
$
|
19,006
|
|
$
|
13,242
|
|
$
|
33,249
|
|
||
|
QiG
|
1,453
|
|
2,134
|
|
3,208
|
|
|||||
|
Total reportable segments
|
20,459
|
|
15,376
|
|
36,457
|
|
|||||
|
Unallocated long-lived tangible assets
|
5,187
|
|
2,798
|
|
4,709
|
|
|||||
|
Total expenditures
|
$
|
25,646
|
|
$
|
18,174
|
|
$
|
41,166
|
|
||
|
|
At
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Identifiable assets:
|
|||||||||||
|
Greatbatch Medical
|
$
|
761,225
|
|
$
|
758,369
|
|
$
|
779,890
|
|
||
|
QiG
|
76,529
|
|
56,245
|
|
57,750
|
|
|||||
|
Total reportable segments
|
837,754
|
|
814,614
|
|
837,640
|
|
|||||
|
Unallocated assets
|
118,255
|
|
76,089
|
|
52,235
|
|
|||||
|
Total assets
|
$
|
956,009
|
|
$
|
890,703
|
|
$
|
889,875
|
|
||
|
|
Year Ended
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Sales by geographic area:
|
|||||||||||
|
United States
|
$
|
312,539
|
|
$
|
325,090
|
|
$
|
330,537
|
|
||
|
Non-Domestic locations:
|
|||||||||||
|
Puerto Rico
|
127,702
|
|
117,961
|
|
105,731
|
|
|||||
|
Belgium
|
65,308
|
|
67,155
|
|
58,043
|
|
|||||
|
Rest of world
|
182,238
|
|
153,739
|
|
151,866
|
|
|||||
|
Total sales
|
$
|
687,787
|
|
$
|
663,945
|
|
$
|
646,177
|
|
||
|
|
At
|
||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
|||||||||
|
Long-lived tangible assets:
|
|||||||||||
|
United States
|
$
|
113,851
|
|
$
|
116,484
|
|
$
|
123,104
|
|
||
|
Rest of world
|
31,074
|
|
29,289
|
|
27,789
|
|
|||||
|
Total
|
$
|
144,925
|
|
$
|
145,773
|
|
$
|
150,893
|
|
||
|
|
Sales
|
Accounts Receivable
|
||||||||||||
|
|
Year Ended
|
At
|
||||||||||||
|
January 2,
2015 |
January 3,
2014 |
December 28,
2012 |
January 2,
2015 |
January 3,
2014 |
||||||||||
|
Customer A
|
18
|
%
|
20
|
%
|
19
|
%
|
4
|
%
|
8
|
%
|
||||
|
Customer B
|
18
|
%
|
16
|
%
|
16
|
%
|
23
|
%
|
19
|
%
|
||||
|
Customer C
|
12
|
%
|
13
|
%
|
11
|
%
|
8
|
%
|
8
|
%
|
||||
|
Customer D
|
6
|
%
|
7
|
%
|
6
|
%
|
12
|
%
|
11
|
%
|
||||
|
54
|
%
|
56
|
%
|
52
|
%
|
47
|
%
|
46
|
%
|
|||||
|
20.
|
QUARTERLY SALES AND EARNINGS DATA—UNAUDITED
|
|
|
4th Qtr.
|
3rd Qtr.
|
2nd Qtr.
|
1st Qtr.
|
||||||||||||
|
|
(in thousands, except per share data)
|
||||||||||||||
|
2014
|
|||||||||||||||
|
Sales
|
$
|
169,726
|
|
$
|
171,699
|
|
$
|
172,081
|
|
$
|
174,281
|
|
|||
|
Gross profit
|
57,214
|
|
58,118
|
|
58,470
|
|
57,596
|
|
|||||||
|
Net income
|
14,176
|
|
14,012
|
|
12,348
|
|
14,922
|
|
|||||||
|
EPS—basic
|
0.57
|
|
0.56
|
|
0.50
|
|
0.61
|
|
|||||||
|
EPS—diluted
|
0.54
|
|
0.54
|
|
0.48
|
|
0.58
|
|
|||||||
|
2013
|
|||||||||||||||
|
Sales
|
$
|
176,619
|
|
$
|
167,730
|
|
$
|
171,331
|
|
$
|
148,265
|
|
|||
|
Gross profit
|
57,385
|
|
55,877
|
|
57,302
|
|
48,749
|
|
|||||||
|
Net income
|
9,781
|
|
11,071
|
|
9,752
|
|
5,663
|
|
|||||||
|
EPS—basic
|
0.40
|
|
0.46
|
|
0.41
|
|
0.24
|
|
|||||||
|
EPS—diluted
|
0.38
|
|
0.44
|
|
0.39
|
|
0.23
|
|
|||||||
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
|
a.
|
Evaluation of Disclosure Controls and Procedures
.
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
|
ITEM 15.
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
|
(a)
|
LIST OF DOCUMENTS FILED AS PART OF THIS REPORT
|
|
1.
|
Financial statements and financial statement schedules filed as part of this Annual Report on Form 10-K. See Part II, Item 8. “Financial Statements and Supplementary Data.”
|
|
2.
|
The following financial statement schedule is included in this Annual Report on Form 10-K (in thousands):
|
|
|
|
Col. C—Additions
|
|
|
|
|
||||||||||||||||
|
Col. A
Description
|
Col. B Balance at Beginning
of Period
|
Charged to Costs &
Expenses
|
Charged to Other Accounts- Describe
|
|
Col. D Deductions
- Describe
|
|
Col. E Balance at End of
Period
|
|||||||||||||||
|
January 2, 2015
|
||||||||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
2,001
|
|
$
|
98
|
|
$
|
14
|
|
(3)(4)
|
$
|
(702
|
)
|
(2)
|
$
|
1,411
|
|
|||||
|
Valuation allowance for deferred income tax assets
|
$
|
11,661
|
|
$
|
(729
|
)
|
(1)
|
$
|
—
|
|
(4)
|
$
|
(223
|
)
|
(1)(5)
|
$
|
10,709
|
|
||||
|
January 3, 2014
|
||||||||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
2,372
|
|
$
|
(93
|
)
|
$
|
(15
|
)
|
(4)
|
$
|
(263
|
)
|
(2)
|
$
|
2,001
|
|
|||||
|
Valuation allowance for deferred income tax assets
|
$
|
12,768
|
|
$
|
(1,263
|
)
|
(1)
|
$
|
32
|
|
(4)
|
$
|
124
|
|
(1)
|
$
|
11,661
|
|
||||
|
December 28, 2012
|
||||||||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
1,930
|
|
$
|
484
|
|
$
|
71
|
|
(3)(4)
|
$
|
(113
|
)
|
(2)
|
$
|
2,372
|
|
|||||
|
Valuation allowance for deferred income tax assets
|
$
|
7,775
|
|
$
|
5,145
|
|
(1)
|
$
|
124
|
|
(4)
|
$
|
(276
|
)
|
(5)
|
$
|
12,768
|
|
||||
|
(1)
|
Valuation allowance recorded in the provision for income taxes for certain net operating losses and tax credits. The net decrease in allowance in 2014 and 2013 primarily relates to the use of net operating loss carryforwards.
|
|
(2)
|
Accounts written off.
|
|
(3)
|
Balance recorded as a part of our 2014 acquisition of Centro de Construcción de Cardioestimuladores del Uruguay and our 2012 acquisition of NeuroNexus Technologies, Inc.
|
|
(4)
|
Includes foreign currency translation effect.
|
|
(5)
|
Primarily relates to return to provision adjustments for prior years.
|
|
3.
|
Exhibits required by Item 601 of Regulation S-K. The exhibits listed on the Exhibit Index of this Annual Report on Form 10-K have been previously filed, are filed herewith or are incorporated herein by reference to other filings.
|
|
Dated:
|
March 3, 2015
|
By
|
/s/ Thomas J. Hook
|
|
Thomas J. Hook (Principal Executive Officer)
|
|||
|
President and Chief Executive Officer
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Thomas J. Hook
|
President, Chief Executive
Officer and Director
(Principal Executive Officer)
|
March 3, 2015
|
||
|
Thomas J. Hook
|
||||
|
/s/ Michael Dinkins
|
Executive Vice President and Chief Financial Officer (Principal Financial Officer)
|
March 3, 2015
|
||
|
Michael Dinkins
|
||||
|
/s/ Thomas J. Mazza
|
Vice President and Corporate Controller (Principal Accounting Officer)
|
March 3, 2015
|
||
|
Thomas J. Mazza
|
||||
|
/s/ Bill R. Sanford
|
Chairman
|
March 3, 2015
|
||
|
Bill R. Sanford
|
||||
|
/s/ Pamela G. Bailey
|
Director
|
March 3, 2015
|
||
|
Pamela G. Bailey
|
||||
|
/s/ Anthony P. Bihl III
|
Director
|
March 3, 2015
|
||
|
Anthony P. Bihl III
|
||||
|
/s/ Joseph W. Dziedzic
|
Director
|
March 3, 2015
|
||
|
Joseph W. Dziedzic
|
||||
|
/s/ Dr. Joseph A. Miller, Jr.
|
Director
|
March 3, 2015
|
||
|
Dr. Joseph A. Miller, Jr.
|
||||
|
/s/ Peter H. Soderberg
|
Director
|
March 3, 2015
|
||
|
Peter H. Soderberg
|
||||
|
/s/ William B. Summers, Jr.
|
Director
|
March 3, 2015
|
||
|
William B. Summers, Jr.
|
||||
|
EXHIBIT
NUMBER
|
DESCRIPTION
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation, as amended (incorporated by reference to Exhibit 3.1 to our Quarterly Report on Form 10-Q for the period ended June 27, 2008).
|
|
|
3.2
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to our Annual Report on Form 10-K for the year ended January 1, 2010).
|
|
|
10.1#
|
1998 Stock Option Plan (including form of “standard” option agreement, form of “special” option agreement and form of “non-standard” option agreement) (incorporated by reference to Exhibit 10.2 to our Registration Statement on Form S-1 filed on May 22, 2000 (File No. 333-37554)).
|
|
|
10.2#
|
Amendment to Greatbatch, Inc. 1998 Stock Option Plan (incorporated by reference to Exhibit 10.2 to our Annual Report on Form 10-K for the period ended January 3, 2014).
|
|
|
10.3#
|
Non-Employee Director Stock Incentive Plan (incorporated by reference to Exhibit A to our Definitive Proxy Statement on Schedule 14-A filed on April 22, 2002).
|
|
|
10.4#
|
Greatbatch, Inc. Executive Short Term Incentive Compensation Plan (incorporated by reference to Exhibit A to our Definitive Proxy Statement on Schedule 14-A filed on April 20, 2012).
|
|
|
10.5
|
License Agreement dated August 8, 1996, between Greatbatch Ltd. and Evans Capacitor Company (incorporated by reference to Exhibit 10.23 to our Registration Statement on Form S-1 filed on May 22, 2000 (File No. 333-37554)).
|
|
|
10.6+
|
Amendment No. 2 dated December 6, 2002, between Greatbatch Technologies, Ltd. and Evans Capacitor Company (incorporated by reference to Exhibit 10.18 to our Annual Report on Form 10-K for the year ended January 3, 2003).
|
|
|
10.7#
|
Form of Change of Control Agreement between Greatbatch, Inc. and its executive officers (Thomas J. Hook, Mauricio Arellano, and Timothy G. McEvoy) (incorporated by reference to Exhibit 10.1 to our Quarterly Report on Form 10-Q for the period ended July 1, 2011).
|
|
|
10.8#
|
Form of Change of Control Agreement between Greatbatch, Inc. and its executive officers (Michael Dinkins, Andrew P. Holman, George M. Cintra, and Thomas K. Hickman) (incorporated by reference to Exhibit 10.8 to our Annual Report on Form 10-K for the year ended December 28, 2012).
|
|
|
10.9
|
Second Amended and Restated Credit Agreement dated September 20, 2013 by and among Greatbatch Ltd., the lenders party thereto and Manufacturers and Traders Trust Company, as administrative agent, Bank of America, N.A., as syndication agent and RBS Citizens, N.A. and Wells Fargo Bank, National Association, as co-documentation agents (incorporated by reference to Exhibit 10.1 to our Current Report on Form 8-K filed on September 23, 2013).
|
|
|
10.10#
|
Employment Agreement dated August 5, 2013 between Greatbatch, Inc. and Thomas J. Hook (incorporated by reference to Exhibit 10.1 to our Current Report on Form 8-K filed on August 9, 2013).
|
|
|
10.11#
|
2005 Stock Incentive Plan (incorporated by reference to Exhibit B to our Definitive Proxy Statement on Schedule 14A filed on April 20, 2007).
|
|
|
10.12#
|
2009 Stock Incentive Plan (incorporated by reference to Exhibit A to our Definitive Proxy Statement on Schedule 14A filed on April 13, 2009).
|
|
|
10.13#
|
2011 Stock Incentive Plan (incorporated by reference to Exhibit A to our Definitive Proxy Statement on Schedule 14A filed on April 14, 2014).
|
|
|
10.14#
|
Amendment to Greatbatch, Inc. 2011 Stock Incentive Plan, Greatbatch, Inc. 2009 Stock Incentive Plan, Greatbatch, Inc. 2005 Stock Incentive Plan (incorporated by reference to Exhibit 10.14 to our Annual Report on Form 10-K for the year ended January 3, 2014).
|
|
|
EXHIBIT
NUMBER
|
DESCRIPTION
|
|
|
10.15#
|
Form of Restricted Stock Award Letter (incorporated by reference to Exhibit 10.15 to our Annual Report on Form 10-K for the year ended January 3, 2014).
|
|
|
10.16#
|
Form of Performance-Based Restricted Stock Units Award Letter (incorporated by reference to Exhibit 10.16 to our Annual Report on Form 10-K for the year ended January 3, 2014).
|
|
|
10.17#
|
Form of Nonqualified Option Award Letter (incorporated by reference to Exhibit 10.17 to our Annual Report on Form 10-K for the year ended January 3, 2014).
|
|
|
10.18#
|
Form of Time-Based Restricted Stock Units Award Letter (incorporated by reference to Exhibit 10.18 to our Annual Report on Form 10-K for the year ended January 3, 2014).
|
|
|
10.19#
|
Separation Agreement and Acknowledgment effective January 3, 2015 between Greatbatch, Inc. and Michelle Graham (incorporated by reference to Exhibit 10.1 to our Quarterly Report on Form 10-Q for the period ended October 3, 2014).
|
|
|
12.1*
|
Ratio of Earnings to Fixed Charges (Unaudited)
|
|
|
21.1*
|
Subsidiaries of Greatbatch, Inc.
|
|
|
23.1*
|
Consent of Independent Registered Public Accounting Firm
|
|
|
31.1*
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act.
|
|
|
31.2*
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act.
|
|
|
32.1**
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS*
|
XBRL Instance Document
|
|
|
101.SCH*
|
XRBL Taxonomy Extension Schema Document
|
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.LAB*
|
XBRL Taxonomy Extension Labels Linkbase Document
|
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
* -
|
Filed herewith.
|
|
** -
|
Furnished herewith.
|
|
# -
|
Indicates exhibits that are management contracts or compensation plans or arrangements required to be filed pursuant to Item 15(b) of Form 10-K.
|