|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
|
16-1531026
|
|
(State of
Incorporation)
|
|
(I.R.S. Employer
Identification No.)
|
|
Title of Each Class:
|
|
Name of Each Exchange on Which Registered:
|
|
Common Stock, Par Value $0.001 Per Share
|
|
New York Stock Exchange
|
|
Large accelerated filer
|
x
|
Accelerated filer
|
¨
|
|
|
Non-accelerated filer
|
¨
|
Smaller reporting company
|
¨
|
|
|
Emerging growth company
|
¨
|
|||
|
Document
|
Part
|
|
|
Proxy Statement for the 2018 Annual Meeting of Stockholders
|
Part III, Item 10
“Directors, Executive Officers and Corporate Governance”
|
|
|
Part III, Item 11
“Executive Compensation”
|
||
|
Part III, Item 12
“Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters”
|
||
|
Part III, Item 13
“Certain Relationships and Related Transactions, and Director Independence”
|
||
|
Part III, Item 14
“Principal Accountant Fees and Services”
|
||
|
PAGE
|
||
|
Item 1.
|
Business
.....................................................................................................................................................................
|
|
|
Item 1A.
|
Risk Factors
...............................................................................................................................................................
|
|
|
Item 1B.
|
Unresolved Staff Comments
......................................................................................................................................
|
|
|
Item 2.
|
Properties
...................................................................................................................................................................
|
|
|
Item 3.
|
Legal Proceedings
.....................................................................................................................................................
|
|
|
Item 4.
|
Mine Safety Disclosures
............................................................................................................................................
|
|
|
Item 5.
|
||
|
Item 6.
|
Selected Financial Data
.............................................................................................................................................
|
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
....................................
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
..................................................................................
|
|
|
Item 8.
|
Financial Statements and Supplementary Data
.........................................................................................................
|
|
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
...................................
|
|
|
Item 9A.
|
Controls and Procedures
............................................................................................................................................
|
|
|
Item 9B.
|
Other Information
......................................................................................................................................................
|
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
........................................................................................
|
|
|
Item 11.
|
Executive Compensation
...........................................................................................................................................
|
|
|
Item 12.
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
..........................................................
|
|
|
Item 14.
|
Principal Accountant Fees and Services
....................................................................................................................
|
|
|
Item 15.
|
Exhibits and Financial Statement Schedules
.............................................................................................................
|
|
|
Item 16.
|
Form 10-K Summary.................................................................................................................................................
|
|
|
Signatures
..................................................................................................................................................................
|
||
|
•
|
Advanced Surgical, Orthopedics & Portable Medical
|
|
•
|
Cardio & Vascular
|
|
•
|
Cardiac & Neuromodulation
|
|
•
|
Electrochem
|
|
Device
|
Principal Illness or Symptom
|
|||
|
Pacemakers
|
Abnormally slow heartbeat (Bradycardia)
|
|||
|
ICDs
|
Rapid and irregular heartbeat (Tachycardia)
|
|||
|
CRT/CRT-Ds
|
Congestive heart failure
|
|||
|
ICMs
|
Unexplained fainting or risk of cardiac arrhythmias
|
|||
|
Neurostimulators
|
Chronic pain, incontinence, movement disorders, epilepsy, obesity or depression
|
|||
|
Cochlear hearing devices
|
Hearing loss
|
|||
|
Product Line
|
Product Development Opportunities
|
|||
|
AS&O
|
Developing a portfolio of products including single use instruments and coated products for the orthopedics market and instruments for the robotics market.
|
|||
|
Cardio & Vascular
|
Developing a portfolio of catheter, introducer, wire-based, sensor and coating products for the cardio and vascular markets.
|
|||
|
Cardiac & Neuromodulation
|
Developing next generation technology programs for our batteries, filtered feedthroughs, high voltage capacitors and lead solutions to reduce the size and cost, while increasing performance for cardiac and neuromodulation devices.
|
|||
|
•
|
future sales, expenses and profitability;
|
|
•
|
future development and expected growth of our business and industry;
|
|
•
|
our ability to execute our business model and our business strategy;
|
|
•
|
our ability to identify trends within our industries and to offer products and services that meet the changing needs of those markets; and
|
|
•
|
projected capital expenditures.
|
|
•
|
a substantial percentage of our costs are fixed in nature, which results in our operations being particularly sensitive to fluctuations in production volumes;
|
|
•
|
changes in the mix of our revenue represented by our various products and customers could result in reductions in our profits if the mix of our revenue represented by lower margin products increases;
|
|
•
|
timing of orders placed by our principal customers who account for a significant portion of our revenues; and
|
|
•
|
increased costs of raw materials or supplies.
|
|
•
|
require us to dedicate a large portion of our cash flow from operations to the servicing and repayment of our outstanding indebtedness, thereby reducing funds available for working capital, capital expenditures, RD&E expenditures and other general corporate requirements;
|
|
•
|
limit our ability to obtain additional financing to fund future working capital, capital expenditures, RD&E expenditures and other general corporate requirements in the future;
|
|
•
|
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate;
|
|
•
|
restrict our ability to make strategic acquisitions or dispositions or to exploit business opportunities;
|
|
•
|
place us at a competitive disadvantage compared to our competitors that have less outstanding indebtedness; and
|
|
•
|
adversely affect the market price of our common stock.
|
|
•
|
managing a larger combined company;
|
|
•
|
consolidating corporate and administrative infrastructures;
|
|
•
|
issues in integrating manufacturing, warehouse and distribution facilities, RD&E and sales forces;
|
|
•
|
difficulties attracting and retaining key personnel;
|
|
•
|
loss of customers and suppliers and inability to attract new customers and suppliers;
|
|
•
|
unanticipated issues in integrating information technology, communications and other systems;
|
|
•
|
incompatibility of purchasing, logistics, marketing, administration and other systems and processes; and
|
|
•
|
unforeseen and unexpected liabilities related to the acquired business.
|
|
•
|
changes in foreign economic conditions and/or regulatory requirements;
|
|
•
|
changes in foreign currency exchange rates;
|
|
•
|
local product preferences and product requirements;
|
|
•
|
outstanding accounts receivables that take longer to collect than is typical in the U.S.;
|
|
•
|
difficulties in enforcing agreements through foreign legal systems;
|
|
•
|
less protection of intellectual property in some countries outside of the U.S.;
|
|
•
|
trade protection measures and import and export licensing requirements;
|
|
•
|
work force instability;
|
|
•
|
political and economic instability; and
|
|
•
|
complex tax and cash management issues.
|
|
Fourth Quarter
|
Third Quarter
|
Second Quarter
|
First Quarter
|
||||||||||||
|
2017
|
|||||||||||||||
|
High
|
$
|
55.20
|
|
$
|
51.65
|
|
$
|
44.25
|
|
$
|
41.80
|
|
|||
|
Low
|
42.75
|
|
40.01
|
|
33.90
|
|
29.00
|
|
|||||||
|
Close
|
45.30
|
|
51.15
|
|
43.25
|
|
40.20
|
|
|||||||
|
2016
|
|||||||||||||||
|
High
|
$
|
31.45
|
|
$
|
33.19
|
|
$
|
39.45
|
|
$
|
52.40
|
|
|||
|
Low
|
18.10
|
|
20.62
|
|
28.55
|
|
30.95
|
|
|||||||
|
Close
|
29.45
|
|
21.69
|
|
32.00
|
|
34.92
|
|
|||||||
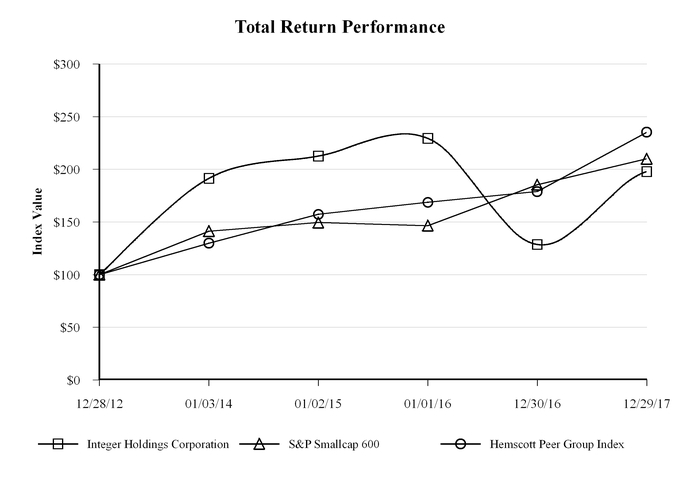
|
Company/Index
|
12/28/12
|
01/03/14
|
01/02/15
|
01/01/16
|
12/30/16
|
12/29/17
|
|||||||||||||
|
Integer Holdings Corporation
|
$
|
100.00
|
|
$
|
191.35
|
|
$
|
212.58
|
|
$
|
229.36
|
|
$
|
128.66
|
|
$
|
197.90
|
|
|
|
S&P Smallcap 600
|
100.00
|
|
141.31
|
|
149.45
|
|
146.50
|
|
185.40
|
|
209.94
|
|
|||||||
|
Hemscott Peer Group Index
|
100.00
|
|
129.89
|
|
157.39
|
|
168.77
|
|
178.91
|
|
235.29
|
|
|||||||
|
2017
(1)(2)(3)
|
2016
(1)(2)
|
2015
(1)(2)
|
2014
(1)(2)
|
2013
(1)
|
|||||||||||||||
|
Summary of Operations for the Fiscal Year:
|
|||||||||||||||||||
|
Sales
|
$
|
1,461,921
|
|
$
|
1,386,778
|
|
$
|
800,414
|
|
$
|
687,787
|
|
$
|
663,945
|
|
||||
|
Net income (loss)
|
66,679
|
|
5,961
|
|
(7,594
|
)
|
55,458
|
|
36,267
|
|
|||||||||
|
Earnings (loss) per share
|
|||||||||||||||||||
|
Basic
|
$
|
2.12
|
|
$
|
0.19
|
|
$
|
(0.29
|
)
|
$
|
2.23
|
|
$
|
1.51
|
|
||||
|
Diluted
|
2.09
|
|
0.19
|
|
(0.29
|
)
|
2.14
|
|
1.43
|
|
|||||||||
|
Financial Position at Year End:
|
|||||||||||||||||||
|
Working capital
|
$
|
322,906
|
|
$
|
332,087
|
|
$
|
360,764
|
|
$
|
242,022
|
|
$
|
190,731
|
|
||||
|
Total assets
|
2,848,345
|
|
2,832,543
|
|
2,982,136
|
|
955,122
|
|
889,629
|
|
|||||||||
|
Long-term obligations
|
1,745,961
|
|
1,922,084
|
|
1,917,671
|
|
233,099
|
|
255,772
|
|
|||||||||
|
(1)
|
From 2013 to 2017, we recorded material charges in Other Operating Expenses (“OOE”), primarily related to our cost savings and consolidation initiatives and our acquisitions. Additional information is set forth in Note 11 “Other Operating Expenses” of the Notes to Consolidated Financial Statements contained in Item 8 of this report.
|
|
(2)
|
On October 27, 2015 and August 12, 2014, we acquired LRM and Centro de Construcción de Cardioestimuladores del Uruguay, respectively. On March 14, 2016, we spun-off a portion of our former QiG segment, which is now an independent, publicly traded company known as Nuvectra. This data includes the results of operations of these acquired companies subsequent to their acquisition and does not include the result of operations of Nuvectra subsequent to the Spin-off. Additional information is set forth in Note 2 “Divestiture and Acquisition” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. Additionally, in connection with our acquisition of LRM we issued approximately $1.8 billion of long-term debt. Additional information is set forth in Note 8 “Debt” of the Notes to Consolidated Financial Statements contained in Item 8 of this report.
|
|
(3)
|
On December 22, 2017, the Tax Reform Act was enacted, which significantly changed existing U.S. tax laws, reducing the federal corporate income tax rate from 35% to 21%, and imposing a deemed repatriation tax on unremitted foreign earnings, as well as other changes. As a result of the Tax Reform Act, our Consolidated Statement of Operations and Comprehensive Income (Loss) reflects a net benefit of $39.4 million in the fourth quarter of fiscal year 2017. Additional information is set forth in Note 12 “Income Taxes” of the Notes to Consolidated Financial Statements contained in Item 8 of this report.
|
|
•
|
Our business
|
|
•
|
Our acquisition and divestiture
|
|
•
|
Use of non-GAAP financial information
|
|
•
|
Strategic overview
|
|
•
|
Financial overview
|
|
•
|
Cost savings and consolidation efforts
|
|
•
|
Inventories
|
|
•
|
Valuation of goodwill, intangible and other long-lived assets
|
|
•
|
Income taxes
|
|
•
|
Fiscal
2017
compared with fiscal
2016
|
|
•
|
Fiscal
2016
compared with fiscal
2015
|
|
•
|
Liquidity and capital resources
|
|
•
|
Off-balance sheet arrangements
|
|
•
|
Contractual obligations
|
|
•
|
Impact of recently issued accounting standards
|
|
•
|
Sales for 2017 increased 5.4% primarily driven by market growth, new business wins, and lower comparables versus 2016 in our Cardio & Vascular, Advanced Surgical, Orthopedics & Portable Medical and Non-Medical product lines. These increases were partially offset by price concessions given to our larger OEM customers in return for long-term volume commitments, which lowered 2017 sales by approximately $16 million in comparison to 2016;
|
|
•
|
Gross profit for 2017 increased $15.3 million primarily due to the increase in sales discussed above, as well as production efficiencies, partially offset by the price concessions given to our larger OEM customers and higher incentive compensation expenses based upon 2017 results;
|
|
•
|
Operating expenses for 2017 were lower by $15.9 million primarily due to the results of Nuvectra not being included after the Spin-off ($4.7 million), lower consolidation and integration charges ($24.4 million), and various efficiencies and synergies gained as a result of our integration and consolidation initiatives partially offset by higher incentive compensation expenses ($8.6 million);
|
|
•
|
Interest expense for 2017 declined $4.8 million primarily due to the amendment of our Term Loan B Facility in 2017, which lowered the interest rate paid on that debt by 100 basis points, as well as the net repayment of $128.6 million of debt during 2017. These reductions were partially offset by the accelerated write-off of deferred fees and original issue discount of $3.6 million due to the accelerated pay down of debt during 2017, as well as the increase in LIBOR during 2017;
|
|
•
|
Other (income) loss, net for 2017 was lower by $14.6 million (higher net loss) due to higher foreign currency exchange rate losses driven by the remeasurement of intercompany loans as a result of the weakening of the U.S. dollar relative to the Euro during 2017, which are primarily non-cash in nature;
|
|
•
|
As a result of the U.S. Tax Cuts and Jobs Act (the “Tax Reform Act”), which was enacted on December 22, 2017, we recognized a $39.4 million net income tax benefit primarily related to the revaluation of our net deferred tax liabilities partially offset by a one-time mandatory tax on the repatriation of undistributed foreign subsidiary earnings and profits;
|
|
•
|
Our weighted average diluted shares increased 915,000 in 2017 primarily due to the issuance of shares under our stock-based compensation programs, as well as the increase in our average stock price during the year. The net result of this increase was a decrease to diluted EPS by $0.06 per share.
|
|
•
|
Sales for 2016 of $1.39 billion increased $586 million or 73% in comparison to 2015. During 2016, incremental sales contributed by LRM were approximately $650 million. Sales for 2016 also include the impact of foreign currency exchange rate fluctuations, which reduced legacy Greatbatch Medical sales by approximately $1 million in comparison to the prior year due to the strengthening dollar versus the Euro. Excluding the impact of these items, as well as the divestiture of $1 million of revenue earned by Nuvectra prior to the Spin-off, organic sales decreased 8% in comparison to the prior year. This decrease was primarily due to 1) the reduction of shipments in a limited number of cardiac rhythm management (“CRM”) customer programs; 2) the 30% decline in Non-Medical sales caused by the slowdown in the energy markets; and 3) contractual price reductions given in exchange for longer-term volume commitments from customers. These decreases were partially offset by growth in sales to our neuromodulation customers during 2016;
|
|
•
|
Gross profit for 2016 increased $143.2 million primarily due to the acquisition of LRM which added $192.7 million to gross profit. 2015 cost of sales includes $23.0 million of inventory step-up amortization recorded as a result of the LRM acquisition, which was fully amortized at the end of 2015;
|
|
•
|
Operating expenses for 2016 increased $48.0 million primarily due to the acquisition of LRM, which added $76.1 million of operating expenses partially offset by lower operating expenses due to the Spin-off of Nuvectra of $20.4 million; and
|
|
•
|
Interest expense for 2016 increased $77.8 million primarily due the $1.8 billion of debt issued in connection with the LRM acquisition. In addition to the debt incurred, we issued 5.0 million shares to the former owners of LRM as part of the consideration paid, which increased weighted average diluted shares outstanding.
|
|
2017
|
2016
|
2015
|
|||||||||||||||||||||||||||||||||
|
Pre-Tax
|
Net
Income
|
Per
Diluted
Share
|
Pre-Tax
|
Net
Income
|
Per
Diluted
Share
|
Pre-Tax
|
Net
Income (Loss)
|
Per
Diluted
Share
|
|||||||||||||||||||||||||||
|
As reported (GAAP)
|
$
|
21,827
|
|
$
|
66,679
|
|
$
|
2.09
|
|
$
|
1,185
|
|
$
|
5,961
|
|
$
|
0.19
|
|
$
|
(15,700
|
)
|
$
|
(7,594
|
)
|
$
|
(0.29
|
)
|
||||||||
|
Adjustments:
|
|||||||||||||||||||||||||||||||||||
|
Amortization of intangibles
(a)
|
44,174
|
|
31,255
|
|
0.98
|
|
37,862
|
|
26,771
|
|
0.86
|
|
17,496
|
|
12,273
|
|
0.45
|
|
|||||||||||||||||
|
IP related litigation (SG&A)
(a)(b)
|
4,375
|
|
2,844
|
|
0.09
|
|
3,040
|
|
1,976
|
|
0.06
|
|
4,417
|
|
2,871
|
|
0.11
|
|
|||||||||||||||||
|
Other operating expenses
(a)
:
|
|||||||||||||||||||||||||||||||||||
|
Consolidation and optimization
(c)
|
13,349
|
|
10,529
|
|
0.33
|
|
26,490
|
|
21,582
|
|
0.69
|
|
26,393
|
|
21,158
|
|
0.77
|
|
|||||||||||||||||
|
Acquisition and integration
(c)
|
10,870
|
|
7,202
|
|
0.22
|
|
28,316
|
|
18,554
|
|
0.59
|
|
33,449
|
|
25,885
|
|
0.95
|
|
|||||||||||||||||
|
Asset dispositions, severance
and other
(c)
|
7,182
|
|
4,808
|
|
0.15
|
|
6,931
|
|
5,760
|
|
0.18
|
|
6,622
|
|
5,099
|
|
0.19
|
|
|||||||||||||||||
|
Strategic reorganization and
alignment
(c)
|
5,891
|
|
3,829
|
|
0.12
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
(Gain) loss on cost and equity
method investments, net
(a)
|
1,565
|
|
1,017
|
|
0.03
|
|
833
|
|
541
|
|
0.02
|
|
(3,350
|
)
|
(2,177
|
)
|
(0.08
|
)
|
|||||||||||||||||
|
Loss on extinguishment of debt
(a)(d)
|
3,525
|
|
2,291
|
|
0.07
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Tax adjustments
(e)
|
—
|
|
(40,281
|
)
|
(1.26
|
)
|
—
|
|
(154
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Nuvectra results prior to
Spin-off
(a)(f)
|
—
|
|
—
|
|
—
|
|
4,037
|
|
2,624
|
|
0.08
|
|
24,103
|
|
15,667
|
|
0.57
|
|
|||||||||||||||||
|
Acquisition related inventory
step-up amortization (COS)
(a)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
22,986
|
|
15,605
|
|
0.57
|
|
|||||||||||||||||
|
Acquisition transaction costs
(a)(g)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
9,463
|
|
6,151
|
|
0.23
|
|
|||||||||||||||||
|
Adjusted (Non-GAAP)
|
$
|
112,758
|
|
$
|
90,173
|
|
$
|
2.81
|
|
$
|
108,694
|
|
$
|
83,615
|
|
$
|
2.68
|
|
$
|
125,879
|
|
$
|
94,938
|
|
$
|
3.48
|
|
||||||||
|
Diluted weighted average shares for adjusted EPS
(h)
|
32,056
|
|
31,222
|
|
27,304
|
|
|||||||||||||||||||||||||||||
|
(a)
|
The difference between pre-tax and net income amounts is the estimated tax impact related to the respective adjustment. Net income amounts are computed using a 35% U.S. tax rate, and the statutory tax rates in Mexico, Germany, France, Netherlands, Uruguay, Ireland and Switzerland, as adjusted for the existence of net operating losses. Expenses that are not deductible for tax purposes (i.e. permanent tax differences) are added back at 100%.
|
|
(b)
|
In 2013, we filed suit against AVX Corporation alleging they were infringing our intellectual property (“IP”). Given the complexity and significant costs incurred pursuing this litigation, we are excluding these litigation expenses from adjusted amounts. Refer to Note 13 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information regarding this litigation.
|
|
(c)
|
Refer to the “Cost Savings and Consolidation Efforts” section of this Item and Note 11 “Other Operating Expenses” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information regarding these initiatives.
|
|
(d)
|
Represents debt extinguishment charges in connection with pre-payments made on our Term B Loan Facility during 2017, which are included in interest expense.
|
|
(e)
|
The tax adjustment for 2017 represents the net tax benefit resulting from the Tax Reform Act, which was signed into law on December 22, 2017. Tax adjustments also include a discrete tax benefit related to certain transaction costs of the LRM acquisition and the spin-off of Nuvectra in 2016 and a tax charge in the fourth quarter of 2016 and 2017 in connection with the enactment of regulations under §987 of the Internal Revenue Code, which resulted in an adjustment to our deferred tax assets.
|
|
(f)
|
Represents the results of Nuvectra prior to its Spin-off on March 14, 2016.
|
|
(g)
|
During 2015, we recorded transaction costs (i.e. debt commitment fees, interest rate swap termination costs, and debt extinguishment charges) in connection with our acquisition of LRM. These expenses are included as a component of interest expense in our Consolidated Statement of Operations and Comprehensive Income (Loss).
|
|
(h)
|
The diluted weighted average shares for adjusted EPS or fiscal years 2017, 2016 and 2015 include 168,000, 249,000 and 941,000, respectively, of potentially dilutive shares not included in the computation of diluted weighted average common shares for GAAP diluted EPS purposes because their effect would have been anti-dilutive in that period.
|
|
2017
|
2016
|
2015
|
|||||||||
|
Net income (loss) as reported (GAAP)
|
$
|
66,679
|
|
$
|
5,961
|
|
$
|
(7,594
|
)
|
||
|
Interest expense
|
106,460
|
|
111,270
|
|
33,513
|
|
|||||
|
Benefit for income taxes
|
(44,852
|
)
|
(4,776
|
)
|
(8,106
|
)
|
|||||
|
Depreciation
|
56,084
|
|
52,662
|
|
27,136
|
|
|||||
|
Amortization excluding OOE
|
44,174
|
|
37,862
|
|
17,496
|
|
|||||
|
EBITDA (Non-GAAP)
|
228,545
|
|
202,979
|
|
62,445
|
|
|||||
|
IP related litigation
|
4,375
|
|
3,040
|
|
4,417
|
|
|||||
|
Stock-based compensation expense excluding OOE
|
12,424
|
|
6,933
|
|
9,287
|
|
|||||
|
Consolidation and optimization expenses
|
13,349
|
|
26,490
|
|
26,393
|
|
|||||
|
Acquisition and integration expenses
|
10,870
|
|
28,316
|
|
33,449
|
|
|||||
|
Asset dispositions, severance and other
|
7,182
|
|
6,931
|
|
6,622
|
|
|||||
|
Strategic reorganization and alignment
|
5,891
|
|
—
|
|
—
|
|
|||||
|
Noncash loss on cost and equity method investments
|
2,965
|
|
1,495
|
|
275
|
|
|||||
|
Nuvectra results prior to Spin-off
|
—
|
|
3,665
|
|
23,517
|
|
|||||
|
Acquisition related inventory step-up amortization
|
—
|
|
—
|
|
22,986
|
|
|||||
|
Adjusted EBITDA (Non-GAAP)
|
$
|
285,601
|
|
$
|
279,849
|
|
$
|
189,391
|
|
||
|
Initiative
|
Expected Expense
|
Expected Capital
|
Expected Annual Cost Savings
(a)
|
Expected Completion Date
|
||||
|
Consolidation and optimization expenses
|
||||||||
|
Manufacturing alignment to support growth
|
$9 - $11
|
$4 - $6
|
$2 - $3
|
2019
|
||||
|
LRM consolidations
|
$18 - $22
|
$5 - $6
|
$12 - $13
|
2018
|
||||
|
Investments in capacity and capabilities
|
$56
|
$23
|
> $20
|
Complete
|
||||
|
Strategic reorganization and alignment
|
$10 - $12
|
-
|
$8 - $12
|
2018
|
||||
|
•
|
potential manufacturing consolidations;
|
|
•
|
continuous improvement;
|
|
•
|
productivity initiatives;
|
|
•
|
direct material and indirect expense savings opportunities; and
|
|
•
|
the establishment of centers of excellence.
|
|
|
Change
|
Change
|
|||||||||||||||||||||||
|
2017 vs. 2016
|
2016 vs. 2015
|
||||||||||||||||||||||||
|
2017
|
2016
|
2015
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Medical Sales:
|
|||||||||||||||||||||||||
|
Cardio & Vascular
|
$
|
536,794
|
|
$
|
490,857
|
|
$
|
131,299
|
|
$
|
45,937
|
|
9
|
%
|
$
|
359,558
|
|
274
|
%
|
||||||
|
Cardiac & Neuromodulation
|
428,349
|
|
439,541
|
|
361,722
|
|
(11,192
|
)
|
(3
|
)%
|
77,819
|
|
22
|
%
|
|||||||||||
|
Advanced Surgical, Orthopedics &
Portable Medical
|
439,810
|
|
414,701
|
|
247,944
|
|
25,109
|
|
6
|
%
|
166,757
|
|
67
|
%
|
|||||||||||
|
Total Medical Sales
|
1,404,953
|
|
1,345,099
|
|
740,965
|
|
59,854
|
|
4
|
%
|
604,134
|
|
82
|
%
|
|||||||||||
|
Non-Medical
|
56,968
|
|
41,679
|
|
59,449
|
|
15,289
|
|
37
|
%
|
(17,770
|
)
|
(30
|
)%
|
|||||||||||
|
Total sales
|
1,461,921
|
|
1,386,778
|
|
800,414
|
|
75,143
|
|
5
|
%
|
586,364
|
|
73
|
%
|
|||||||||||
|
Cost of sales
|
1,068,370
|
|
1,008,479
|
|
565,279
|
|
59,891
|
|
6
|
%
|
443,200
|
|
78
|
%
|
|||||||||||
|
Gross profit
|
393,551
|
|
378,299
|
|
235,135
|
|
15,252
|
|
4
|
%
|
143,164
|
|
61
|
%
|
|||||||||||
|
Gross profit as a % of sales
|
26.9
|
%
|
27.3
|
%
|
29.4
|
%
|
|||||||||||||||||||
|
Selling, general and administrative
expenses (“SG&A”)
|
161,573
|
|
153,291
|
|
102,530
|
|
8,282
|
|
5
|
%
|
50,761
|
|
50
|
%
|
|||||||||||
|
SG&A as a % of sales
|
11.1
|
%
|
11.1
|
%
|
12.8
|
%
|
|||||||||||||||||||
|
Research, development and engineering
costs (“RD&E”)
|
55,247
|
|
55,001
|
|
52,995
|
|
246
|
|
—
|
%
|
2,006
|
|
4
|
%
|
|||||||||||
|
RD&E as a % of sales
|
3.8
|
%
|
4.0
|
%
|
6.6
|
%
|
|||||||||||||||||||
|
Other operating expenses
|
37,292
|
|
61,737
|
|
66,464
|
|
(24,445
|
)
|
(40
|
)%
|
(4,727
|
)
|
(7
|
)%
|
|||||||||||
|
Operating income
|
139,439
|
|
108,270
|
|
13,146
|
|
31,169
|
|
29
|
%
|
95,124
|
|
NM
|
||||||||||||
|
Operating margin
|
9.5
|
%
|
7.8
|
%
|
1.6
|
%
|
|||||||||||||||||||
|
Interest expense
|
106,460
|
|
111,270
|
|
33,513
|
|
(4,810
|
)
|
(4
|
)%
|
77,757
|
|
NM
|
||||||||||||
|
(Gain) loss on cost and equity method
investments, net
|
1,565
|
|
833
|
|
(3,350
|
)
|
732
|
|
88
|
%
|
4,183
|
|
NM
|
||||||||||||
|
Other (income) loss, net
|
9,587
|
|
(5,018
|
)
|
(1,317
|
)
|
14,605
|
|
NM
|
(3,701
|
)
|
NM
|
|||||||||||||
|
Income (loss) before benefit
for income taxes
|
21,827
|
|
1,185
|
|
(15,700
|
)
|
20,642
|
|
16,885
|
|
|||||||||||||||
|
Benefit for income taxes
|
(44,852
|
)
|
(4,776
|
)
|
(8,106
|
)
|
(40,076
|
)
|
NM
|
3,330
|
|
NM
|
|||||||||||||
|
Effective tax rate
|
(205.5
|
)%
|
(403.0
|
)%
|
51.6
|
%
|
|||||||||||||||||||
|
Net income (loss)
|
$
|
66,679
|
|
$
|
5,961
|
|
$
|
(7,594
|
)
|
$
|
60,718
|
|
NM
|
$
|
13,555
|
|
NM
|
||||||||
|
Net margin
|
4.6
|
%
|
0.4
|
%
|
(0.9
|
)%
|
|
|
|||||||||||||||||
|
Diluted earnings (loss) per share
|
$
|
2.09
|
|
$
|
0.19
|
|
$
|
(0.29
|
)
|
$
|
1.90
|
|
NM
|
|
$
|
0.48
|
|
NM
|
|||||||
|
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
Medical Sales:
|
||||||||||||||
|
Cardio & Vascular
|
$
|
536,794
|
|
$
|
490,857
|
|
$
|
45,937
|
|
9.4
|
%
|
|||
|
Cardiac & Neuromodulation
|
428,349
|
|
439,541
|
|
(11,192
|
)
|
(2.5
|
)%
|
||||||
|
Advanced Surgical, Orthopedics & Portable Medical
|
439,810
|
|
414,701
|
|
25,109
|
|
6.1
|
%
|
||||||
|
Total Medical Sales
|
1,404,953
|
|
1,345,099
|
|
59,854
|
|
4.4
|
%
|
||||||
|
Non-Medical
|
56,968
|
|
41,679
|
|
15,289
|
|
36.7
|
%
|
||||||
|
Total sales
|
$
|
1,461,921
|
|
|
$
|
1,386,778
|
|
|
$
|
75,143
|
|
|
5.4
|
%
|
|
% Change
|
||
|
|
2017 vs. 2016
|
|
|
Price
(a)
|
(1.1
|
)%
|
|
Mix
(b)
|
(0.2
|
)%
|
|
Incentive compensation
(c)
|
(0.6
|
)%
|
|
Production efficiencies and volume
(d)
|
1.5
|
%
|
|
Total percentage point change to gross profit as a percentage of sales
|
(0.4
|
)%
|
|
(a)
|
Our Gross Margin for 2017 was negatively impacted by price concessions given to our larger OEM customers in return for long-term volume commitments.
|
|
(b)
|
Our Gross Margin for 2017 was negatively impacted by a higher mix of sales of lower margin products.
|
|
(c)
|
Amount represents the impact to our Gross Margin attributable to our cash and stock incentive programs. Performance-based compensation is accrued based upon actual results achieved.
|
|
(d)
|
Represents various increases and decreases to our Gross Margin. Overall, our Gross Margin for 2017 was positively impacted by production efficiencies and synergies gained as a result of our integration and consolidation initiatives as well as higher volumes in comparison to 2016.
|
|
|
$ Change
|
||
|
2017 vs. 2016
|
|||
|
Nuvectra SG&A
(a)
|
$
|
(1,913
|
)
|
|
Legal expenses
(b)
|
986
|
|
|
|
Intangible asset amortization
(c)
|
6,462
|
|
|
|
Incentive compensation programs
(d)
|
5,569
|
|
|
|
Other
(e)
|
(2,822
|
)
|
|
|
Net increase in SG&A Expenses
|
$
|
8,282
|
|
|
(a)
|
Amount represents the impact to our SG&A related to the overhead costs divested as a result of the Spin-off of Nuvectra in March 2016.
|
|
(b)
|
Amount represents the change in legal costs compared to the prior year period. This variance is primarily due to the timing of legal expenses incurred related to our on-going IP infringement case. Refer to Note 13 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 1 of this report for information related to this IP infringement litigation.
|
|
(c)
|
Amount represents the increase in intangible asset amortization (i.e. customer list), which is amortized based upon the forecasted cash flows at the time of acquisition for the respective asset.
|
|
(d)
|
Amount represents the impact to our SG&A attributable to our cash and stock incentive programs. Performance-based compensation is accrued based upon actual results achieved.
|
|
(e)
|
Represents various increases and decreases to our SG&A. Overall, our SG&A for 2017 was positively impacted by efficiencies and synergies gained as a result of our integration and consolidation initiatives.
|
|
|
$ Change
|
||
|
2017 vs. 2016
|
|||
|
Nuvectra RD&E
(a)
|
$
|
(2,830
|
)
|
|
Incentive compensation programs
(b)
|
2,995
|
|
|
|
Other
(c)
|
81
|
|
|
|
Net increase in RD&E
|
$
|
246
|
|
|
(a)
|
Represents the impact to our RD&E related to the divested costs as a result of the Spin-off of Nuvectra in March 2016.
|
|
(b)
|
Represents the impact to our RD&E attributable to our cash and stock incentive programs. Performance-based compensation is accrued based upon actual results achieved.
|
|
(c)
|
Represents various increases and decreases to our RD&E. Our RD&E for 2017 was positively impacted by efficiencies and synergies gained as a result of our integration and consolidation initiatives, which was offset by our increased investment in projects with a higher growth opportunity.
|
|
2017
|
2016
|
Change
|
|||||||||
|
Consolidation and optimization initiatives
(a)
|
$
|
13,349
|
|
$
|
26,490
|
|
$
|
(13,141
|
)
|
||
|
Acquisition and integration costs
(b)
|
10,870
|
|
28,316
|
|
(17,446
|
)
|
|||||
|
Asset dispositions, severance and other
(c)
|
7,182
|
|
6,931
|
|
251
|
|
|||||
|
Strategic reorganization and alignment
(d)
|
5,891
|
|
—
|
|
$
|
5,891
|
|
||||
|
Total other operating expenses
|
$
|
37,292
|
|
$
|
61,737
|
|
$
|
(24,445
|
)
|
||
|
(a)
|
Refer to the “Cost Savings and Consolidation Efforts” section of this Item and Note 11 “Other Operating Expenses” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information regarding these initiatives.
|
|
(b)
|
During 2017 and 2016, we incurred costs related to the acquisition of LRM, consisting primarily of professional, consulting, severance, retention, relocation, and travel costs. In addition, the 2016 fiscal year included change-in-control payments to former LRM executives.
|
|
(c)
|
During 2017 and 2016, we recorded losses in connection with various asset disposals and/or write-downs. The 2017 amount also includes approximately $5.3 million in expense related to our leadership transitions. Additionally, during 2016 we incurred legal and professional costs in connection with the Spin-off of $4.4 million.
|
|
(d)
|
As a result of the strategic review of our customers, competitors and markets we undertook during the fourth quarter of 2017, we began to take steps to better align our resources in order to invest to grow, protect, preserve and to enhance the profitability of our portfolio of products. This will include focusing our investment in RD&E and manufacturing, improving our business processes and redirecting investments away from projects where the market does not justify the investment. As a result, during the fourth quarter of 2017 we incurred charges related to the initial steps of this initiative, which included lease termination charges and accelerated amortization of certain intangible assets.
|
|
|
U.S.
|
International
|
Combined
|
|||||||||||||||||
|
|
$
|
%
|
$
|
%
|
$
|
%
|
||||||||||||||
|
Income (loss) before provision (benefit) for income taxes
|
$
|
(46,459
|
)
|
$
|
68,286
|
|
$
|
21,827
|
|
|||||||||||
|
Provision (benefit) at statutory rate
|
$
|
(16,261
|
)
|
35.0
|
%
|
$
|
23,900
|
|
35.0
|
%
|
$
|
7,639
|
|
35.0
|
%
|
|||||
|
Federal tax credits
|
(1,850
|
)
|
4.0
|
|
(46
|
)
|
(0.1
|
)
|
(1,896
|
)
|
(8.7
|
)
|
||||||||
|
Foreign rate differential
|
3,063
|
|
(6.6
|
)
|
(14,188
|
)
|
(20.8
|
)
|
(11,125
|
)
|
(50.9
|
)
|
||||||||
|
Uncertain tax positions
|
34
|
|
(0.1
|
)
|
3,483
|
|
5.1
|
|
3,517
|
|
16.1
|
|
||||||||
|
State taxes, net of federal benefit
|
(864
|
)
|
1.9
|
|
—
|
|
—
|
|
(864
|
)
|
(4.0
|
)
|
||||||||
|
Valuation allowance
|
546
|
|
(1.2
|
)
|
484
|
|
0.7
|
|
1,030
|
|
4.7
|
|
||||||||
|
Other
|
(3,732
|
)
|
8.0
|
|
(27
|
)
|
—
|
|
(3,759
|
)
|
(17.2
|
)
|
||||||||
|
Tax expense (benefit) before U.S. Tax Reform items
|
(19,064
|
)
|
41.0
|
|
13,606
|
|
19.9
|
|
(5,458
|
)
|
(25.0
|
)
|
||||||||
|
U.S. Tax Reform items:
|
||||||||||||||||||||
|
Change in tax rates
|
(56,408
|
)
|
121.4
|
|
(45
|
)
|
(0.1
|
)
|
(56,453
|
)
|
(258.6
|
)
|
||||||||
|
Toll charge on unremitted earnings
|
14,719
|
|
(31.7
|
)
|
—
|
|
—
|
|
14,719
|
|
67.4
|
|
||||||||
|
Change in unremitted earnings assertion
|
(545
|
)
|
1.2
|
|
2,885
|
|
4.2
|
|
2,340
|
|
10.7
|
|
||||||||
|
Tax expense related to U.S. Tax Reform items
|
(42,234
|
)
|
90.9
|
|
2,840
|
|
4.1
|
|
(39,394
|
)
|
(180.5
|
)
|
||||||||
|
Provision (benefit) for income taxes
|
$
|
(61,298
|
)
|
131.9
|
%
|
$
|
16,446
|
|
24.1
|
%
|
$
|
(44,852
|
)
|
(205.5
|
)%
|
|||||
|
|
Change
|
|||||||||||||
|
2016
|
2015
|
$
|
%
|
|||||||||||
|
Medical Sales:
|
||||||||||||||
|
Cardio & Vascular
|
$
|
490,857
|
|
$
|
131,299
|
|
$
|
359,558
|
|
273.8
|
%
|
|||
|
Cardiac & Neuromodulation
|
439,541
|
|
361,722
|
|
77,819
|
|
21.5
|
%
|
||||||
|
Advanced Surgical, Orthopedics & Portable Medical
|
414,701
|
|
247,944
|
|
166,757
|
|
67.3
|
%
|
||||||
|
Total Medical Sales
|
1,345,099
|
|
740,965
|
|
604,134
|
|
81.5
|
%
|
||||||
|
Non-Medical
|
41,679
|
|
59,449
|
|
(17,770
|
)
|
(29.9
|
)%
|
||||||
|
Total sales
|
$
|
1,386,778
|
|
|
$
|
800,414
|
|
|
$
|
586,364
|
|
73.3
|
%
|
|
|
% Change
|
||
|
|
2016 vs. 2015
|
|
|
Impact of LRM acquisition
(a)
|
(3.1
|
)%
|
|
Price
(b)
|
(2.1
|
)%
|
|
Production efficiencies, volume and mix
(c)
|
0.1
|
%
|
|
Incentive compensation
(d)
|
0.4
|
%
|
|
Warranty reserves and obsolescence write-offs
(e)
|
(0.3
|
)%
|
|
Inventory step-up amortization
(f)
|
2.9
|
%
|
|
Total percentage point change to gross profit as a percentage of sales
|
(2.1
|
)%
|
|
(b)
|
Our Gross Margin for 2016 was negatively impacted by contractual price reductions given in exchange for longer-term volume commitments.
|
|
(c)
|
Our Gross Margin benefited from production efficiencies gained at our manufacturing facilities as a result of our various lean, supply chain, and integration initiatives, which were offset by a higher sales mix of lower margin products and lower sales volumes.
|
|
(d)
|
Represents the impact to our Gross Margin from the change in cash and stock incentive compensation versus the prior year and is recorded based upon the actual results achieved.
|
|
(e)
|
Cost of sales for fiscal year 2016 includes the impact of various warranty reserves and obsolescence write-offs, including reserves related to various customer returns and field actions that were higher than normal in 2016.
|
|
(f)
|
Represents the impact to Gross Margin in comparison to 2015 related to the $23.0 million of inventory step-up amortization recorded in 2015 as a result of the LRM acquisition. The inventory step-up was fully amortized during fiscal year 2015.
|
|
|
$ Change
|
||
|
2016 vs. 2015
|
|||
|
Impact of LRM acquisition
(a)
|
$
|
56,885
|
|
|
Nuvectra SG&A
(b)
|
(8,628
|
)
|
|
|
Legal fees
(c)
|
(1,553
|
)
|
|
|
Other
(d)
|
4,057
|
|
|
|
Net increase in SG&A
|
$
|
50,761
|
|
|
(a)
|
Represents the incremental SG&A expenses from LRM, which was acquired in October 2015.
|
|
(b)
|
Represents the net decrease in SG&A costs attributable to Nuvectra, which was spun-off in March 2016.
|
|
(c)
|
Represents the change in legal costs in comparison to 2015. Costs associated with our ongoing IP infringement case accounted for approximately $1.4 million of the decrease in SG&A expenses from 2015 to 2016.
|
|
(d)
|
Represents the net impact of various increases and decreases to SG&A costs, including incremental operating costs associated with operating a company that nearly doubled in size at the end of 2015.
|
|
|
$ Change
|
||
|
2016 vs. 2015
|
|||
|
Impact of LRM acquisition
(a)
|
$
|
10,889
|
|
|
Nuvectra RD&E
(b)
|
(12,600
|
)
|
|
|
Other
(c)
|
3,717
|
|
|
|
Net increase in RD&E
|
$
|
2,006
|
|
|
(a)
|
Represents the incremental RD&E expenses from LRM, which was acquired in October 2015.
|
|
(b)
|
Represents the net decrease in RD&E costs attributable to Nuvectra, which was spun-off in March 2016.
|
|
(c)
|
Represents the net impact of various increases and decreases to RD&E costs and includes the impact of normal increases in operating costs, as well as our continued investment in developing our core and new technologies to drive future growth.
|
|
2016
|
2015
|
Change
|
|||||||||
|
Consolidation and optimization initiatives
(a)
|
$
|
26,490
|
|
$
|
26,393
|
|
$
|
97
|
|
||
|
Acquisition and integration costs
(b)
|
28,316
|
|
33,449
|
|
(5,133
|
)
|
|||||
|
Asset dispositions, severance and other
(c)
|
6,931
|
|
6,622
|
|
309
|
|
|||||
|
Total other operating expenses
|
$
|
61,737
|
|
$
|
66,464
|
|
$
|
(4,727
|
)
|
||
|
(a)
|
Refer to the “Cost Savings and Consolidation Efforts” section of this Item and Note 11 “Other Operating Expenses” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information regarding these initiatives.
|
|
(b)
|
During 2016 and 2015, we incurred costs related to the acquisition and integration of LRM consisting primarily of change-in-control payments to former LRM executives, professional and consulting fees, severance, retention, relocation, and travel costs.
|
|
(b)
|
During 2016 and 2015, we recorded losses in connection with various asset disposals and/or write-downs. Additionally, during 2016 and 2015, we incurred legal and professional costs in connection with the Spin-off of $4.4 million and $6.0 million, respectively.
|
|
|
U.S.
|
International
|
Combined
|
|||||||||||||||||
|
|
$
|
%
|
$
|
%
|
$
|
%
|
||||||||||||||
|
Income (loss) before provision (benefit) for income taxes
|
$
|
(52,446
|
)
|
$
|
53,631
|
|
$
|
1,185
|
|
|||||||||||
|
Provision (benefit) at statutory rate
|
$
|
(18,356
|
)
|
35.0
|
%
|
$
|
18,771
|
|
35.0
|
%
|
$
|
415
|
|
35.0
|
%
|
|||||
|
Federal tax credits
|
(1,750
|
)
|
3.3
|
|
(42
|
)
|
(0.1
|
)
|
(1,792
|
)
|
(151.2
|
)
|
||||||||
|
Foreign rate differential
|
3,192
|
|
(6.1
|
)
|
(10,278
|
)
|
(19.2
|
)
|
(7,086
|
)
|
(598.0
|
)
|
||||||||
|
Uncertain tax positions
|
1,464
|
|
(2.8
|
)
|
260
|
|
0.5
|
|
1,724
|
|
145.5
|
|
||||||||
|
State taxes, net of federal benefit
|
(1,068
|
)
|
2.0
|
|
—
|
|
—
|
|
(1,068
|
)
|
(90.1
|
)
|
||||||||
|
Change in foreign tax rates
|
—
|
|
—
|
|
(270
|
)
|
(0.5
|
)
|
(270
|
)
|
(22.8
|
)
|
||||||||
|
Non-deductible transaction costs
|
1,012
|
|
(1.9
|
)
|
—
|
|
—
|
|
1,012
|
|
85.4
|
|
||||||||
|
Valuation allowance
|
811
|
|
(1.5
|
)
|
529
|
|
1.0
|
|
1,340
|
|
113.1
|
|
||||||||
|
Change in Tax law
|
2,630
|
|
(5.0
|
)
|
—
|
|
—
|
|
2,630
|
|
221.9
|
|
||||||||
|
Other
|
(1,703
|
)
|
3.2
|
|
22
|
|
—
|
|
(1,681
|
)
|
(141.8
|
)
|
||||||||
|
Provision (benefit) for income taxes
|
$
|
(13,768
|
)
|
26.3
|
%
|
$
|
8,992
|
|
16.8
|
%
|
$
|
(4,776
|
)
|
(403.0
|
)%
|
|||||
|
(dollars in thousands)
|
December 29, 2017
|
December 30, 2016
|
|||||
|
Cash and cash equivalents
|
$
|
44,096
|
|
$
|
52,116
|
|
|
|
Working capital
|
$
|
322,906
|
|
$
|
332,087
|
|
|
|
Current ratio
|
2.54
|
|
2.79
|
|
|||
|
2017
|
2016
|
||||
|
Cash provided by (used in):
|
|||||
|
Operating activities
|
149,357
|
|
105,532
|
|
|
|
Investing activities
|
(47,936
|
)
|
(63,300
|
)
|
|
|
Financing activities
|
(111,669
|
)
|
(72,146
|
)
|
|
|
Effect of foreign currency exchange rates on cash and cash equivalents
|
2,228
|
|
(448
|
)
|
|
|
Net change in cash and cash equivalents
|
(8,020
|
)
|
(30,362
|
)
|
|
|
|
Payments due by period
|
||||||||||||||||||
|
Total
|
Less than 1 year
|
1-3 years
|
3-5 years
|
More than 5 years
|
|||||||||||||||
|
Total debt obligations
|
$
|
1,642,443
|
|
$
|
30,469
|
|
$
|
149,000
|
|
$
|
1,102,974
|
|
$
|
360,000
|
|
||||
|
Interest on debt
(a)
|
444,006
|
|
92,322
|
|
178,775
|
|
148,271
|
|
24,638
|
|
|||||||||
|
Operating lease obligations
(b)
|
64,548
|
|
12,815
|
|
20,380
|
|
13,310
|
|
18,043
|
|
|||||||||
|
Foreign currency contracts
(b)
|
65,367
|
|
65,367
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Defined benefit plan obligations
(c)
|
3,804
|
|
289
|
|
534
|
|
668
|
|
2,313
|
|
|||||||||
|
Other
(d)
|
81,076
|
|
76,539
|
|
4,526
|
|
11
|
|
—
|
|
|||||||||
|
Total
|
$
|
2,301,244
|
|
$
|
277,801
|
|
$
|
353,215
|
|
$
|
1,265,234
|
|
$
|
404,994
|
|
||||
|
(a)
|
Interest payments in the table above reflect the contractual interest payments on our outstanding debt based upon the balance outstanding and applicable interest rates at December 29, 2017, and exclude the impact of the debt discount amortization and impact of interest rate swap agreements. Refer to Note 8 “Debt” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information regarding long-term debt.
|
|
(b)
|
Refer to Note 13 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our operating lease obligations and foreign currency contracts.
|
|
(c)
|
Refer to Note 9 “Benefit Plans” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our defined benefit plan obligations.
|
|
(d)
|
Amounts include inventory purchase commitments, which are legally binding and specify minimum purchase quantities. These commitments do not include open purchase orders.
|
|
Page
|
|
|
Management’s Report on Internal Control Over Financial Reporting
...................................................................................
|
|
|
Reports of Independent Registered Public Accounting Firm
.................................................................................................
|
|
|
Consolidated Balance Sheets as of December 29, 2017 and December 30, 2016..................................................................
|
|
|
Consolidated Statements of Operations and Comprehensive Income (Loss) for the years ended December 29, 2017, December 30, 2016 and January 1, 2016................................................................................................................................
|
|
|
Consolidated Statements of Cash Flows for the years ended December 29, 2017, December 30, 2016 and January 1, 2016........................................................................................................................................................................................
|
|
|
Consolidated Statements of Stockholders’ Equity for the years ended December 29, 2017, December 30, 2016 and January 1, 2016.......................................................................................................................................................................
|
|
|
Notes to Consolidated Financial Statements
..........................................................................................................................
|
|
|
/s/ Joseph W. Dziedzic
|
|
/s/ Gary J. Haire
|
|
Joseph W. Dziedzic
|
|
Gary J. Haire
|
|
President & Chief Executive Officer
|
|
Executive Vice President & Chief Financial Officer
|
|
(in thousands except share and per share data)
|
December 29,
2017 |
December 30,
2016 |
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
44,096
|
|
$
|
52,116
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $0.8 million and $0.7 million, respectively
|
242,456
|
|
204,626
|
|
|||
|
Inventories
|
227,534
|
|
225,151
|
|
|||
|
Refundable income taxes
|
37
|
|
13,388
|
|
|||
|
Prepaid expenses and other current assets
|
17,786
|
|
22,026
|
|
|||
|
Total current assets
|
531,909
|
|
517,307
|
|
|||
|
Property, plant and equipment, net
|
370,375
|
|
372,042
|
|
|||
|
Goodwill
|
990,238
|
|
967,326
|
|
|||
|
Other intangible assets, net
|
920,393
|
|
940,060
|
|
|||
|
Deferred income taxes
|
4,152
|
|
3,970
|
|
|||
|
Other assets
|
31,278
|
|
31,838
|
|
|||
|
Total assets
|
$
|
2,848,345
|
|
$
|
2,832,543
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Current portion of long-term debt
|
$
|
30,469
|
|
$
|
31,344
|
|
|
|
Accounts payable
|
83,517
|
|
77,896
|
|
|||
|
Income taxes payable
|
13,477
|
|
3,699
|
|
|||
|
Accrued expenses
|
81,540
|
|
72,281
|
|
|||
|
Total current liabilities
|
209,003
|
|
185,220
|
|
|||
|
Long-term debt
|
1,578,696
|
|
1,698,819
|
|
|||
|
Deferred income taxes
|
145,364
|
|
208,579
|
|
|||
|
Other long-term liabilities
|
21,901
|
|
14,686
|
|
|||
|
Total liabilities
|
1,954,964
|
|
2,107,304
|
|
|||
|
Commitments and contingencies (Note 13)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.001 par value, authorized 100,000,000 shares; no shares issued or outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value; 100,000,000 shares authorized; 31,977,953 and 31,059,038 shares issued, respectively; 31,871,427 and 30,925,496 shares outstanding, respectively
|
32
|
|
31
|
|
|||
|
Additional paid-in capital
|
669,756
|
|
637,955
|
|
|||
|
Treasury stock, at cost, 106,526 and 133,542 shares, respectively
|
(4,654
|
)
|
(5,834
|
)
|
|||
|
Retained earnings
|
176,068
|
|
109,087
|
|
|||
|
Accumulated other comprehensive income (loss)
|
52,179
|
|
(16,000
|
)
|
|||
|
Total stockholders’ equity
|
893,381
|
|
725,239
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
2,848,345
|
|
$
|
2,832,543
|
|
|
|
|
Fiscal Year Ended
|
||||||||||
|
(in thousands except per share data)
|
December 29,
2017 |
December 30,
2016 |
January 1,
2016 |
||||||||
|
Sales
|
$
|
1,461,921
|
|
$
|
1,386,778
|
|
$
|
800,414
|
|
||
|
Cost of sales
|
1,068,370
|
|
1,008,479
|
|
565,279
|
|
|||||
|
Gross profit
|
393,551
|
|
378,299
|
|
235,135
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Selling, general and administrative expenses
|
161,573
|
|
153,291
|
|
102,530
|
|
|||||
|
Research, development and engineering costs
|
55,247
|
|
55,001
|
|
52,995
|
|
|||||
|
Other operating expenses
|
37,292
|
|
61,737
|
|
66,464
|
|
|||||
|
Total operating expenses
|
254,112
|
|
270,029
|
|
221,989
|
|
|||||
|
Operating income
|
139,439
|
|
108,270
|
|
13,146
|
|
|||||
|
Interest expense
|
106,460
|
|
111,270
|
|
33,513
|
|
|||||
|
(Gain) loss on cost and equity method investments, net
|
1,565
|
|
833
|
|
(3,350
|
)
|
|||||
|
Other (income) loss, net
|
9,587
|
|
(5,018
|
)
|
(1,317
|
)
|
|||||
|
Income (loss) before benefit for income taxes
|
21,827
|
|
1,185
|
|
(15,700
|
)
|
|||||
|
Benefit for income taxes
|
(44,852
|
)
|
(4,776
|
)
|
(8,106
|
)
|
|||||
|
Net income (loss)
|
$
|
66,679
|
|
$
|
5,961
|
|
$
|
(7,594
|
)
|
||
|
Earnings (loss) per share:
|
|||||||||||
|
Basic
|
$
|
2.12
|
|
$
|
0.19
|
|
$
|
(0.29
|
)
|
||
|
Diluted
|
$
|
2.09
|
|
$
|
0.19
|
|
$
|
(0.29
|
)
|
||
|
Weighted average shares outstanding:
|
|||||||||||
|
Basic
|
31,402
|
|
30,778
|
|
26,363
|
|
|||||
|
Diluted
|
31,888
|
|
30,973
|
|
26,363
|
|
|||||
|
Comprehensive Income (Loss)
|
|||||||||||
|
Net income (loss)
|
$
|
66,679
|
|
$
|
5,961
|
|
$
|
(7,594
|
)
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Foreign currency translation gain (loss)
|
65,860
|
|
(19,269
|
)
|
(7,841
|
)
|
|||||
|
Net change in cash flow hedges, net of tax
|
2,243
|
|
2,478
|
|
108
|
|
|||||
|
Defined benefit plan liability adjustment, net of tax
|
76
|
|
(579
|
)
|
(20
|
)
|
|||||
|
Other comprehensive income (loss), net
|
68,179
|
|
(17,370
|
)
|
(7,753
|
)
|
|||||
|
Comprehensive income (loss)
|
$
|
134,858
|
|
$
|
(11,409
|
)
|
$
|
(15,347
|
)
|
||
|
|
Fiscal Year Ended
|
||||||||||
|
(in thousands)
|
December 29, 2017
|
December 30, 2016
|
January 1, 2016
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income (loss)
|
$
|
66,679
|
|
$
|
5,961
|
|
$
|
(7,594
|
)
|
||
|
Adjustments to reconcile net income (loss) to net cash provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
102,796
|
|
90,524
|
|
44,632
|
|
|||||
|
Debt related charges included in interest expense
|
10,911
|
|
7,278
|
|
11,320
|
|
|||||
|
Inventory step-up amortization
|
—
|
|
—
|
|
22,986
|
|
|||||
|
Stock-based compensation
|
14,680
|
|
8,408
|
|
9,376
|
|
|||||
|
Non-cash loss on cost and equity method investments
|
2,965
|
|
1,495
|
|
275
|
|
|||||
|
Other non-cash losses
|
7,110
|
|
5,216
|
|
1,093
|
|
|||||
|
Deferred income taxes
|
(59,212
|
)
|
(7,350
|
)
|
(10,298
|
)
|
|||||
|
Changes in operating assets and liabilities, net of acquisitions:
|
|||||||||||
|
Accounts receivable
|
(34,597
|
)
|
(2,169
|
)
|
3,684
|
|
|||||
|
Inventories
|
(986
|
)
|
22,170
|
|
(25,752
|
)
|
|||||
|
Prepaid expenses and other assets
|
4,854
|
|
(3,846
|
)
|
(1,861
|
)
|
|||||
|
Accounts payable
|
4,887
|
|
(1,127
|
)
|
3,129
|
|
|||||
|
Accrued expenses
|
14,977
|
|
(13,935
|
)
|
(28,605
|
)
|
|||||
|
Income taxes payable
|
14,293
|
|
(7,093
|
)
|
(9,906
|
)
|
|||||
|
Net cash provided by operating activities
|
149,357
|
|
105,532
|
|
12,479
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Acquisition of property, plant and equipment
|
(47,301
|
)
|
(58,632
|
)
|
(44,616
|
)
|
|||||
|
Proceeds from sale of property, plant and equipment
|
472
|
|
347
|
|
746
|
|
|||||
|
Purchase of cost and equity method investments
|
(1,316
|
)
|
(3,015
|
)
|
(6,300
|
)
|
|||||
|
Acquisitions, net of cash acquired
|
—
|
|
—
|
|
(423,389
|
)
|
|||||
|
Other investing activities
|
209
|
|
(2,000
|
)
|
—
|
|
|||||
|
Net cash used in investing activities
|
(47,936
|
)
|
(63,300
|
)
|
(473,559
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Principal payments of long-term debt
|
(178,558
|
)
|
(46,000
|
)
|
(1,232,175
|
)
|
|||||
|
Proceeds from issuance of long-term debt
|
50,000
|
|
57,000
|
|
1,749,750
|
|
|||||
|
Proceeds from the exercise of stock options
|
19,324
|
|
2,821
|
|
6,583
|
|
|||||
|
Payment of debt issuance costs
|
(2,360
|
)
|
(1,177
|
)
|
(45,933
|
)
|
|||||
|
Distribution of cash and cash equivalents to Nuvectra Corporation
|
—
|
|
(76,256
|
)
|
—
|
|
|||||
|
Purchase of non-controlling interests
|
—
|
|
(6,818
|
)
|
(9,875
|
)
|
|||||
|
Other financing activities
|
(75
|
)
|
(1,716
|
)
|
(440
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(111,669
|
)
|
(72,146
|
)
|
467,910
|
|
|||||
|
Effect of foreign currency exchange rates on cash and cash equivalents
|
2,228
|
|
(448
|
)
|
(1,176
|
)
|
|||||
|
Net (decrease) increase in cash and cash equivalents
|
(8,020
|
)
|
(30,362
|
)
|
5,654
|
|
|||||
|
Cash and cash equivalents, beginning of year
|
52,116
|
|
82,478
|
|
76,824
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
44,096
|
|
$
|
52,116
|
|
$
|
82,478
|
|
||
|
|
Common Stock
|
Additional
Paid-In
Capital
|
Treasury
Stock
|
Retained
Earnings
|
Accumulated
Other Comprehensive
Income (Loss)
|
Total
Stockholders’
Equity
|
|||||||||||||||||||||||
|
(in thousands)
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||
|
January 2, 2015
|
25,099
|
|
$
|
25
|
|
$
|
366,073
|
|
(28
|
)
|
$
|
(1,307
|
)
|
$
|
239,448
|
|
$
|
9,123
|
|
$
|
613,362
|
|
|||||||
|
Comprehensive loss:
|
|||||||||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(7,594
|
)
|
—
|
|
(7,594
|
)
|
|||||||||||||
|
Other comprehensive loss, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(7,753
|
)
|
(7,753
|
)
|
|||||||||||||
|
Share-based compensation plans:
|
—
|
|
|||||||||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
9,364
|
|
—
|
|
—
|
|
—
|
|
—
|
|
9,364
|
|
|||||||||||||
|
Net shares issued (acquired)
|
585
|
|
1
|
|
5,764
|
|
(107
|
)
|
(5,261
|
)
|
—
|
|
—
|
|
504
|
|
|||||||||||||
|
Excess tax benefit on share-based compensation
|
—
|
|
—
|
|
5,639
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5,639
|
|
|||||||||||||
|
Shares contributed to 401(k) Plan
|
—
|
|
—
|
|
452
|
|
72
|
|
3,468
|
|
—
|
|
—
|
|
3,920
|
|
|||||||||||||
|
Shares issued in connection with acquisition
|
4,980
|
|
5
|
|
245,363
|
|
—
|
|
—
|
|
—
|
|
—
|
|
245,368
|
|
|||||||||||||
|
Roll-over options issued in connection with acquisition
|
—
|
|
—
|
|
4,508
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,508
|
|
|||||||||||||
|
Purchase of non-controlling interests in subsidiaries
|
—
|
|
—
|
|
(16,693
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(16,693
|
)
|
|||||||||||||
|
January 1, 2016
|
30,664
|
|
31
|
|
620,470
|
|
(63
|
)
|
(3,100
|
)
|
231,854
|
|
1,370
|
|
850,625
|
|
|||||||||||||
|
Comprehensive loss:
|
|||||||||||||||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5,961
|
|
—
|
|
5,961
|
|
|||||||||||||
|
Other comprehensive loss, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(17,370
|
)
|
(17,370
|
)
|
|||||||||||||
|
Share-based compensation plans:
|
|||||||||||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
8,408
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,408
|
|
|||||||||||||
|
Net shares issued (acquired)
|
395
|
|
—
|
|
1,570
|
|
(71
|
)
|
(2,734
|
)
|
—
|
|
—
|
|
(1,164
|
)
|
|||||||||||||
|
Excess tax benefit on share-based compensation
|
—
|
|
—
|
|
2,266
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,266
|
|
|||||||||||||
|
Spin-off of Nuvectra Corporation
|
—
|
|
—
|
|
5,241
|
|
—
|
|
—
|
|
(128,728
|
)
|
—
|
|
(123,487
|
)
|
|||||||||||||
|
December 30, 2016
|
31,059
|
|
31
|
|
637,955
|
|
(134
|
)
|
(5,834
|
)
|
109,087
|
|
(16,000
|
)
|
725,239
|
|
|||||||||||||
|
Cumulative effect adjustment of the adoption of ASU 2016-09 (Note 1)
|
—
|
|
—
|
|
(812
|
)
|
—
|
|
—
|
|
302
|
|
—
|
|
(510
|
)
|
|||||||||||||
|
Comprehensive income:
|
|
|
|
|
|||||||||||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
66,679
|
|
—
|
|
66,679
|
|
|||||||||||||
|
Other comprehensive income, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
68,179
|
|
68,179
|
|
|||||||||||||
|
Share-based compensation plans:
|
|||||||||||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
14,680
|
|
—
|
|
—
|
|
—
|
|
—
|
|
14,680
|
|
|||||||||||||
|
Net shares issued
|
919
|
|
1
|
|
17,933
|
|
27
|
|
1,180
|
|
—
|
|
—
|
|
19,114
|
|
|||||||||||||
|
December 29, 2017
|
31,978
|
|
$
|
32
|
|
$
|
669,756
|
|
(107
|
)
|
$
|
(4,654
|
)
|
$
|
176,068
|
|
$
|
52,179
|
|
$
|
893,381
|
|
|||||||
|
•
|
recording all tax effects associated with stock-based compensation through the income statement, as opposed to recording certain amounts in other paid-in capital, which eliminates the requirements to calculate a windfall pool;
|
|
•
|
allowing entities to withhold shares to satisfy the employer’s statutory tax withholding requirement up to the highest marginal tax rate applicable to employees rather than the employer’s minimum statutory rate, without requiring liability classification for the award;
|
|
•
|
modifying the requirement to estimate the number of awards that will ultimately vest by providing an accounting policy election to either estimate the number of forfeitures or recognize forfeitures as they occur;
|
|
•
|
changing certain presentation requirements in the statement of cash flows, including removing the requirement to present excess tax benefits as an inflow from financing activities and an outflow from operating activities, and requiring the cash paid to taxing authorities arising from withheld shares to be classified as a financing activity; and
|
|
•
|
the assumed proceeds from applying the treasury stock method when computing EPS is amended to exclude the amount of excess tax benefits that would be recognized in additional paid-in capital.
|
|
Assets divested
|
|||
|
Cash and cash equivalents
|
$
|
76,256
|
|
|
Other current assets
|
977
|
|
|
|
Property, plant and equipment, net
|
4,407
|
|
|
|
Amortizing intangible assets, net
|
1,931
|
|
|
|
Goodwill
|
40,830
|
|
|
|
Deferred income taxes
|
6,446
|
|
|
|
Total assets divested
|
130,847
|
|
|
|
Liabilities transferred
|
|||
|
Current liabilities
|
2,119
|
|
|
|
Net assets divested
|
$
|
128,728
|
|
|
Cash
|
$
|
478,490
|
|
|
Fair value of Integer common stock
|
245,368
|
|
|
|
Replacement stock options attributable to pre-acquisition service
|
4,508
|
|
|
|
Total purchase consideration
|
$
|
728,366
|
|
|
Assets acquired
|
|||
|
Current assets
|
$
|
269,815
|
|
|
Property, plant and equipment
|
216,473
|
|
|
|
Amortizing intangible assets
|
849,000
|
|
|
|
Indefinite-lived intangible assets
|
70,000
|
|
|
|
Goodwill
|
660,670
|
|
|
|
Other non-current assets
|
1,629
|
|
|
|
Total assets acquired
|
2,067,587
|
|
|
|
Liabilities assumed
|
|||
|
Current liabilities
|
103,986
|
|
|
|
Debt assumed
|
1,044,675
|
|
|
|
Other long-term liabilities
|
190,560
|
|
|
|
Total liabilities assumed
|
1,339,221
|
|
|
|
Net assets acquired
|
$
|
728,366
|
|
|
Sales
|
$
|
1,445,689
|
|
|
Net income
|
2,405
|
|
|
|
Earnings per share:
|
|||
|
Basic
|
$
|
0.08
|
|
|
Diluted
|
$
|
0.08
|
|
|
|
2017
|
2016
|
2015
|
||||||||
|
Noncash investing and financing activities:
|
|||||||||||
|
Property, plant and equipment purchases included in accounts payable
|
$
|
3,474
|
|
$
|
3,499
|
|
$
|
7,401
|
|
||
|
Common stock contributed to 401(k) Plan
|
—
|
|
—
|
|
3,920
|
|
|||||
|
Common stock issued in connection with LRM acquisition
|
—
|
|
—
|
|
245,368
|
|
|||||
|
Replacement stock options issued in connection with LRM acquisition
|
—
|
|
—
|
|
4,508
|
|
|||||
|
Purchase of non-controlling interests in subsidiaries included in accrued expenses
|
—
|
|
—
|
|
6,818
|
|
|||||
|
Cash paid (refunded) during the year for:
|
|||||||||||
|
Interest
|
93,839
|
|
106,475
|
|
13,057
|
|
|||||
|
Income taxes
|
(8,185
|
)
|
7,263
|
|
6,312
|
|
|||||
|
Acquisition of noncash assets
|
—
|
|
—
|
|
2,013,604
|
|
|||||
|
Liabilities assumed
|
—
|
|
—
|
|
1,340,339
|
|
|||||
|
December 29,
2017 |
December 30,
2016 |
||||||
|
Raw materials
|
$
|
97,615
|
|
$
|
100,738
|
|
|
|
Work-in-process
|
92,650
|
|
89,224
|
|
|||
|
Finished goods
|
37,269
|
|
35,189
|
|
|||
|
Total
|
$
|
227,534
|
|
$
|
225,151
|
|
|
|
December 29, 2017
|
December 30,
2016 |
||||||
|
Manufacturing machinery and equipment
|
$
|
373,558
|
|
$
|
332,886
|
|
|
|
Buildings and building improvements
|
138,605
|
|
132,277
|
|
|||
|
Information technology hardware and software
|
62,204
|
|
52,467
|
|
|||
|
Leasehold improvements
|
64,675
|
|
59,292
|
|
|||
|
Furniture and fixtures
|
20,555
|
|
18,989
|
|
|||
|
Land and land improvements
|
19,577
|
|
20,046
|
|
|||
|
Construction work in process
|
28,051
|
|
32,252
|
|
|||
|
Other
|
1,146
|
|
1,062
|
|
|||
|
708,371
|
|
649,271
|
|
||||
|
Accumulated depreciation
|
(337,996
|
)
|
(277,229
|
)
|
|||
|
Total
|
$
|
370,375
|
|
$
|
372,042
|
|
|
|
2017
|
2016
|
2015
|
|||||||||
|
Depreciation expense
|
$
|
56,084
|
|
$
|
52,662
|
|
$
|
27,136
|
|
||
|
Medical
|
Non-Medical
|
Total
|
|||||||||
|
December 30, 2016
|
$
|
950,326
|
|
$
|
17,000
|
|
$
|
967,326
|
|
||
|
Foreign currency translation
|
22,912
|
|
—
|
|
22,912
|
|
|||||
|
December 29, 2017
|
$
|
973,238
|
|
$
|
17,000
|
|
$
|
990,238
|
|
||
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Foreign
Currency
Translation
|
Net
Carrying
Amount
|
||||||||||||
|
December 29, 2017
|
|||||||||||||||
|
Definite-lived:
|
|||||||||||||||
|
Purchased technology and patents
|
$
|
256,719
|
|
$
|
(117,695
|
)
|
$
|
2,483
|
|
$
|
141,507
|
|
|||
|
Customer lists
|
759,987
|
|
(87,555
|
)
|
16,103
|
|
688,535
|
|
|||||||
|
Other
|
4,534
|
|
(7,797
|
)
|
3,326
|
|
63
|
|
|||||||
|
Total amortizing intangible assets
|
$
|
1,021,240
|
|
$
|
(213,047
|
)
|
$
|
21,912
|
|
$
|
830,105
|
|
|||
|
Indefinite-lived:
|
|||||||||||||||
|
Trademarks and tradenames
|
$
|
90,288
|
|
||||||||||||
|
December 30, 2016
|
|||||||||||||||
|
Definite-lived:
|
|||||||||||||||
|
Purchased technology and patents
|
$
|
256,719
|
|
$
|
(100,719
|
)
|
$
|
333
|
|
$
|
156,333
|
|
|||
|
Customer lists
|
759,987
|
|
(60,474
|
)
|
(6,269
|
)
|
693,244
|
|
|||||||
|
Other
|
4,534
|
|
(5,142
|
)
|
803
|
|
195
|
|
|||||||
|
Total amortizing intangible assets
|
$
|
1,021,240
|
|
$
|
(166,335
|
)
|
$
|
(5,133
|
)
|
$
|
849,772
|
|
|||
|
Indefinite-lived:
|
|||||||||||||||
|
Trademarks and tradenames
|
$
|
90,288
|
|
||||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Cost of sales
|
$
|
16,586
|
|
$
|
16,769
|
|
$
|
7,403
|
|
||
|
SG&A
|
27,043
|
|
20,581
|
|
9,681
|
|
|||||
|
RD&E
|
545
|
|
512
|
|
412
|
|
|||||
|
Other Operating Expenses
|
2,538
|
|
—
|
|
—
|
|
|||||
|
Total intangible asset amortization expense
|
$
|
46,712
|
|
$
|
37,862
|
|
$
|
17,496
|
|
||
|
2018
|
2019
|
2020
|
2021
|
2022
|
After 2022
|
||||||||||||||||||
|
Amortization Expense
|
$
|
45,316
|
|
$
|
45,433
|
|
$
|
46,051
|
|
$
|
45,176
|
|
$
|
43,142
|
|
$
|
604,987
|
|
|||||
|
December 29, 2017
|
December 30,
2016 |
||||||
|
Salaries and benefits
|
$
|
32,529
|
|
$
|
30,199
|
|
|
|
Profit sharing and bonuses
|
19,244
|
|
3,054
|
|
|||
|
Accrued interest
|
8,523
|
|
6,838
|
|
|||
|
Other
|
21,244
|
|
32,190
|
|
|||
|
Total
|
$
|
81,540
|
|
$
|
72,281
|
|
|
|
|
December 29, 2017
|
December 30,
2016 |
|||||
|
Senior secured term loan A
|
$
|
335,157
|
|
$
|
356,250
|
|
|
|
Senior secured term loan B
|
873,286
|
|
1,014,750
|
|
|||
|
9.125% senior notes due 2023
|
360,000
|
|
360,000
|
|
|||
|
Revolving line of credit
|
74,000
|
|
40,000
|
|
|||
|
Unamortized discount on term loan B and debt issuance costs
|
(33,278
|
)
|
(40,837
|
)
|
|||
|
Total debt
|
1,609,165
|
|
1,730,163
|
|
|||
|
Current portion of long-term debt
|
(30,469
|
)
|
(31,344
|
)
|
|||
|
Total long-term debt
|
$
|
1,578,696
|
|
$
|
1,698,819
|
|
|
|
2018
|
2019
|
2020
|
2021
|
2022
|
After 2022
|
|||||||||||||
|
Future minimum principal payments
|
$
|
30,469
|
|
37,500
|
|
111,500
|
|
229,688
|
|
873,286
|
|
360,000
|
|
|||||
|
January 1, 2016
|
$
|
4,791
|
|
|
Amortization during the period
|
(991
|
)
|
|
|
December 30, 2016
|
3,800
|
|
|
|
Amortization during the period
|
(992
|
)
|
|
|
December 29, 2017
|
$
|
2,808
|
|
|
Debt Issuance Costs
|
Unamortized Discount on TLB Facility
|
Total
|
|||||||||
|
January 1, 2016
|
$
|
35,908
|
|
$
|
10,039
|
|
$
|
45,947
|
|
||
|
Financing costs incurred
|
1,177
|
|
—
|
|
1,177
|
|
|||||
|
Amortization during the period
|
(4,989
|
)
|
(1,298
|
)
|
(6,287
|
)
|
|||||
|
December 30, 2016
|
32,096
|
|
8,741
|
|
40,837
|
|
|||||
|
Financing costs incurred
|
2,360
|
|
—
|
|
2,360
|
|
|||||
|
Write-off of debt issuance costs and unamortized discount
|
(2,421
|
)
|
(1,104
|
)
|
(3,525
|
)
|
|||||
|
Amortization during the period
|
(5,146
|
)
|
(1,248
|
)
|
(6,394
|
)
|
|||||
|
December 29, 2017
|
$
|
26,889
|
|
$
|
6,389
|
|
$
|
33,278
|
|
||
|
Notional Amount
|
Start Date
|
End Date
|
Pay Fixed Rate
|
Receive Current Floating Rate
|
Fair Value
|
Balance Sheet Location
|
|||||||||||
|
$
|
200,000
|
|
Jun-17
|
Jun-20
|
1.1325
|
%
|
1.5521%
|
$
|
4,279
|
|
Other Assets
|
||||||
|
2017
|
2016
|
||||||
|
Change in projected benefit obligation:
|
|||||||
|
Projected benefit obligation at beginning of year
|
$
|
8,728
|
|
$
|
7,992
|
|
|
|
Service cost
|
464
|
|
431
|
|
|||
|
Interest cost
|
162
|
|
174
|
|
|||
|
Plan participants’ contribution
|
75
|
|
75
|
|
|||
|
Actuarial (gain) loss
|
(143
|
)
|
341
|
|
|||
|
Benefits (paid) transferred in, net
|
(160
|
)
|
84
|
|
|||
|
Foreign currency translation
|
1,027
|
|
(369
|
)
|
|||
|
Projected benefit obligation at end of year
|
10,153
|
|
8,728
|
|
|||
|
Change in fair value of plan assets:
|
|||||||
|
Fair value of plan assets at beginning of year
|
1,172
|
|
871
|
|
|||
|
Employer contributions
|
56
|
|
36
|
|
|||
|
Plan participants’ contributions
|
75
|
|
75
|
|
|||
|
Actual loss on plan assets
|
—
|
|
(9
|
)
|
|||
|
Benefits transferred in, net
|
—
|
|
224
|
|
|||
|
Foreign currency translation
|
55
|
|
(25
|
)
|
|||
|
Fair value of plan assets at end of year
|
1,358
|
|
1,172
|
|
|||
|
Projected benefit obligation in excess of plan assets at end of year
|
$
|
8,795
|
|
$
|
7,556
|
|
|
|
Defined benefit liability classified as other current liabilities
|
$
|
120
|
|
$
|
109
|
|
|
|
Defined benefit liability classified as long-term liabilities
|
$
|
8,675
|
|
$
|
7,447
|
|
|
|
Accumulated benefit obligation at end of year
|
$
|
8,322
|
|
$
|
7,115
|
|
|
|
2017
|
2016
|
||||||
|
Net loss occurring during the year
|
$
|
20
|
|
$
|
368
|
|
|
|
Amortization of losses
|
(63
|
)
|
(62
|
)
|
|||
|
Prior service cost
|
1
|
|
1
|
|
|||
|
Amortization of prior service cost
|
(11
|
)
|
(11
|
)
|
|||
|
Pre-tax adjustment (gain) loss
|
(53
|
)
|
296
|
|
|||
|
Taxes
|
(23
|
)
|
283
|
|
|||
|
Net (gain) loss
|
$
|
(76
|
)
|
$
|
579
|
|
|
|
Amortization of net prior service cost
|
$
|
11
|
|
|
Amortization of net loss
|
43
|
|
|
|
2017
|
2016
|
||||||
|
Service cost
|
$
|
464
|
|
$
|
431
|
|
|
|
Interest cost
|
162
|
|
174
|
|
|||
|
Expected return on assets
|
(19
|
)
|
(18
|
)
|
|||
|
Recognized net actuarial loss
|
72
|
|
72
|
|
|||
|
Net pension cost
|
$
|
679
|
|
$
|
659
|
|
|
|
2017
|
2016
|
2015
|
|||
|
Discount rate
|
1.9%
|
2.2%
|
2.3%
|
||
|
Salary growth
|
2.9%
|
2.9%
|
3.0%
|
||
|
Expected rate of return on assets
|
1.5%
|
2.0%
|
2.3%
|
||
|
2017
|
2016
|
2015
|
|||
|
Discount rate
|
2.0%
|
1.9%
|
2.2%
|
||
|
Salary growth
|
2.9%
|
2.9%
|
2.9%
|
||
|
Expected rate of return on assets
|
1.3%
|
1.5%
|
2.0%
|
||
|
Fair Value
|
Quoted
Prices in
Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
||||||||||||
|
December 29, 2017
|
|||||||||||||||
|
Insurance contract
|
$
|
1,358
|
|
$
|
—
|
|
$
|
1,358
|
|
$
|
—
|
|
|||
|
December 30, 2016
|
|||||||||||||||
|
Insurance contract
|
$
|
1,172
|
|
$
|
—
|
|
$
|
1,172
|
|
$
|
—
|
|
|||
|
2018
|
2019
|
2020
|
2021
|
2022
|
2023-2027
|
|||||||||||||
|
Estimated benefit payments
|
$
|
289
|
|
290
|
|
244
|
|
279
|
|
389
|
|
2,313
|
|
|||||
|
2017
|
2016
|
2015
|
|||||||||
|
Stock options
|
$
|
1,716
|
|
$
|
2,499
|
|
$
|
2,708
|
|
||
|
RSAs and RSUs (time-based)
|
5,324
|
|
1,991
|
|
2,027
|
|
|||||
|
PRSUs
|
7,640
|
|
3,918
|
|
4,641
|
|
|||||
|
Total stock-based compensation expense
|
$
|
14,680
|
|
$
|
8,408
|
|
$
|
9,376
|
|
||
|
Cost of sales
|
$
|
1,062
|
|
$
|
332
|
|
$
|
795
|
|
||
|
SG&A
|
10,623
|
|
6,246
|
|
7,510
|
|
|||||
|
RD&E
|
739
|
|
355
|
|
982
|
|
|||||
|
OOE
|
2,256
|
|
1,475
|
|
89
|
|
|||||
|
Total stock-based compensation expense
|
$
|
14,680
|
|
$
|
8,408
|
|
$
|
9,376
|
|
||
|
2017
|
2016
|
2015
|
|||||||||
|
Weighted average fair value of options granted
|
$
|
12.86
|
|
$
|
8.52
|
|
$
|
12.18
|
|
||
|
Assumptions:
|
|||||||||||
|
Expected term (in years)
|
4.5
|
|
4.7
|
|
4.7
|
|
|||||
|
Risk-free interest rate
|
1.77
|
%
|
1.49
|
%
|
1.55
|
%
|
|||||
|
Expected volatility
|
37
|
%
|
27
|
%
|
26
|
%
|
|||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
|||||
|
Number of
Stock
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contractual
Term
(in years)
|
Aggregate
Intrinsic
Value
(in millions)
|
|||||||||
|
Outstanding at December 30, 2016
|
1,739,972
|
|
$
|
28.26
|
|
|||||||
|
Granted
|
125,020
|
|
38.78
|
|
||||||||
|
Exercised
|
(804,064
|
)
|
24.03
|
|
||||||||
|
Forfeited or expired
|
(129,575
|
)
|
45.74
|
|
||||||||
|
Outstanding at December 29, 2017
|
931,353
|
|
$
|
30.89
|
|
6.2
|
$
|
13.9
|
|
|||
|
Vested and expected to vest at December 29, 2017
|
931,353
|
|
$
|
30.89
|
|
6.2
|
$
|
13.9
|
|
|||
|
Exercisable at December 29, 2017
|
798,311
|
|
$
|
30.13
|
|
5.8
|
$
|
12.5
|
|
|||
|
2017
|
2016
|
2015
|
|||||||||
|
Intrinsic value
|
$
|
13,928
|
|
$
|
690
|
|
$
|
8,231
|
|
||
|
Cash received
|
19,324
|
|
2,821
|
|
6,583
|
|
|||||
|
Time-Vested
Restricted Stock Units and Awards
|
Weighted
Average Grant Date
Fair Value
|
|||||
|
Nonvested at December 30, 2016
|
39,394
|
|
$
|
45.51
|
|
|
|
Granted
|
309,107
|
|
34.18
|
|
||
|
Vested
|
(148,299
|
)
|
34.28
|
|
||
|
Forfeited
|
(36,771
|
)
|
38.03
|
|
||
|
Nonvested at December 29, 2017
|
163,431
|
|
$
|
35.96
|
|
|
|
Performance-
Vested
Restricted Stock Units and Awards
|
Weighted
Average Grant Date
Fair Value
|
|||||
|
Nonvested at December 30, 2016
|
356,586
|
|
$
|
31.87
|
|
|
|
Granted
|
419,112
|
|
31.62
|
|
||
|
Forfeited
|
(305,809
|
)
|
30.77
|
|
||
|
Nonvested at December 29, 2017
|
469,889
|
|
$
|
32.37
|
|
|
|
2017
|
2016
|
2015
|
|||||||||
|
Consolidation and optimization initiatives
|
$
|
13,349
|
|
$
|
26,490
|
|
$
|
26,393
|
|
||
|
Acquisition and integration costs
|
10,870
|
|
28,316
|
|
33,449
|
|
|||||
|
Asset dispositions, severance and other
|
7,182
|
|
6,931
|
|
6,622
|
|
|||||
|
Strategic reorganization and alignment
|
5,891
|
|
—
|
|
—
|
|
|||||
|
Total other operating expenses
|
$
|
37,292
|
|
$
|
61,737
|
|
$
|
66,464
|
|
||
|
Severance and Retention
|
Accelerated
Depreciation/
Asset Write-offs
|
Other
|
Total
|
||||||||||||
|
December 30, 2016
|
$
|
795
|
|
$
|
—
|
|
$
|
402
|
|
$
|
1,197
|
|
|||
|
Restructuring charges
|
1,781
|
|
—
|
|
11,568
|
|
13,349
|
|
|||||||
|
Cash payments
|
(1,268
|
)
|
—
|
|
(11,970
|
)
|
(13,238
|
)
|
|||||||
|
December 29, 2017
|
$
|
1,308
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,308
|
|
|||
|
2017
|
2016
|
2015
|
|||||||||
|
U.S.
|
$
|
(46,459
|
)
|
$
|
(52,446
|
)
|
$
|
(42,166
|
)
|
||
|
International
|
68,286
|
|
53,631
|
|
26,466
|
|
|||||
|
Total income (loss) before benefit for income taxes
|
$
|
21,827
|
|
$
|
1,185
|
|
$
|
(15,700
|
)
|
||
|
2017
|
2016
|
2015
|
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
(1,558
|
)
|
$
|
(8,327
|
)
|
$
|
(3,753
|
)
|
||
|
State
|
(29
|
)
|
149
|
|
(367
|
)
|
|||||
|
International
|
15,947
|
|
10,752
|
|
6,312
|
|
|||||
|
14,360
|
|
2,574
|
|
2,192
|
|
||||||
|
Deferred:
|
|||||||||||
|
Federal
|
(58,924
|
)
|
(4,952
|
)
|
(8,144
|
)
|
|||||
|
State
|
(788
|
)
|
(638
|
)
|
(880
|
)
|
|||||
|
International
|
500
|
|
(1,760
|
)
|
(1,274
|
)
|
|||||
|
(59,212
|
)
|
(7,350
|
)
|
(10,298
|
)
|
||||||
|
Total benefit for income taxes
|
$
|
(44,852
|
)
|
$
|
(4,776
|
)
|
$
|
(8,106
|
)
|
||
|
2017
|
2016
|
2015
|
|||||||||||||||
|
Statutory rate
|
$
|
7,639
|
|
35.0
|
%
|
$
|
415
|
|
35.0
|
%
|
$
|
(5,495
|
)
|
35.0
|
%
|
||
|
Federal tax credits
|
(1,896
|
)
|
(8.7
|
)
|
(1,792
|
)
|
(151.2
|
)
|
(1,850
|
)
|
11.8
|
|
|||||
|
Foreign rate differential
|
(11,125
|
)
|
(50.9
|
)
|
(7,086
|
)
|
(598.0
|
)
|
(3,180
|
)
|
20.2
|
|
|||||
|
Uncertain tax positions
|
3,517
|
|
16.1
|
|
1,724
|
|
145.5
|
|
(531
|
)
|
3.4
|
|
|||||
|
State taxes, net of federal benefit
|
(864
|
)
|
(4.0
|
)
|
(1,068
|
)
|
(90.1
|
)
|
(1,490
|
)
|
9.5
|
|
|||||
|
Non-deductible transaction costs
|
—
|
|
—
|
|
1,012
|
|
85.4
|
|
4,867
|
|
(31.0
|
)
|
|||||
|
Valuation allowance
|
1,030
|
|
4.7
|
|
1,340
|
|
113.1
|
|
626
|
|
(4.0
|
)
|
|||||
|
Change in tax rates
|
(56,453
|
)
|
(258.6
|
)
|
(270
|
)
|
(22.8
|
)
|
(91
|
)
|
0.6
|
|
|||||
|
U.S. Tax Reform - Toll charge on unremitted earnings
|
14,719
|
|
67.4
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Change in unremitted earnings assertion
|
2,340
|
|
10.7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Change in tax law (Internal Revenue Code §987)
|
—
|
|
—
|
|
2,630
|
|
221.9
|
|
—
|
|
—
|
|
|||||
|
Other
|
(3,759
|
)
|
(17.2
|
)
|
(1,681
|
)
|
(141.8
|
)
|
(962
|
)
|
6.1
|
|
|||||
|
Effective tax rate
|
$
|
(44,852
|
)
|
(205.5
|
)%
|
$
|
(4,776
|
)
|
(403.0
|
)%
|
$
|
(8,106
|
)
|
51.6
|
%
|
||
|
December 29,
2017 |
December 30,
2016 |
||||||
|
Net operating loss carryforwards
|
$
|
107,005
|
|
$
|
154,706
|
|
|
|
Tax credit carryforwards
|
28,215
|
|
24,646
|
|
|||
|
Inventories
|
4,956
|
|
7,524
|
|
|||
|
Accrued expenses
|
3,815
|
|
5,724
|
|
|||
|
Stock-based compensation
|
5,531
|
|
10,614
|
|
|||
|
Other
|
—
|
|
936
|
|
|||
|
Gross deferred tax assets
|
149,522
|
|
204,150
|
|
|||
|
Less valuation allowance
|
(36,480
|
)
|
(35,391
|
)
|
|||
|
Net deferred tax assets
|
113,042
|
|
168,759
|
|
|||
|
Property, plant and equipment
|
(27,547
|
)
|
(33,069
|
)
|
|||
|
Intangible assets
|
(219,576
|
)
|
(337,722
|
)
|
|||
|
Convertible subordinated notes
|
(806
|
)
|
(2,577
|
)
|
|||
|
Other
|
(6,325
|
)
|
—
|
|
|||
|
Gross deferred tax liabilities
|
(254,254
|
)
|
(373,368
|
)
|
|||
|
Net deferred tax liability
|
$
|
(141,212
|
)
|
$
|
(204,609
|
)
|
|
|
Presented as follows:
|
|||||||
|
Noncurrent deferred tax asset
|
$
|
4,152
|
|
$
|
3,970
|
|
|
|
Noncurrent deferred tax liability
|
(145,364
|
)
|
(208,579
|
)
|
|||
|
Net deferred tax liability
|
$
|
(141,212
|
)
|
$
|
(204,609
|
)
|
|
|
Jurisdiction
|
Tax
Attribute
|
Amount
(in millions)
|
Begin to
Expire
|
|||||
|
U.S. Federal
|
Net operating loss
|
$
|
415.9
|
|
2019
|
|||
|
U.S. State
|
Net operating loss
|
227.3
|
|
2018
|
||||
|
International
|
Net operating loss
|
37.4
|
|
2018
|
||||
|
U.S. Federal
|
Foreign tax credit
|
17.0
|
|
2019
|
||||
|
U.S. Federal and State
|
R&D tax credit
|
7.2
|
|
2018
|
||||
|
U.S. State
|
Investment tax credit
|
6.3
|
|
2018
|
||||
|
2017
|
2016
|
2015
|
|||||||||
|
Balance, beginning of year
|
$
|
10,561
|
|
$
|
9,271
|
|
$
|
2,411
|
|
||
|
Additions based upon tax positions related to the current year
|
3,833
|
|
1,450
|
|
274
|
|
|||||
|
Reductions as a result of a lapse of applicable statute of limitations
|
(510
|
)
|
—
|
|
(470
|
)
|
|||||
|
Revaluation due to change in tax rate (Tax Reform Act)
|
(1,782
|
)
|
—
|
|
—
|
|
|||||
|
Additions (reductions) related to prior period tax returns
|
(14
|
)
|
240
|
|
163
|
|
|||||
|
Reductions (additions) relating to business combinations
|
—
|
|
(400
|
)
|
7,443
|
|
|||||
|
Reductions relating to settlements with tax authorities
|
—
|
|
—
|
|
(550
|
)
|
|||||
|
Balance, end of year
|
$
|
12,088
|
|
$
|
10,561
|
|
$
|
9,271
|
|
||
|
2017
|
2016
|
||||||
|
Beginning balance
|
$
|
3,911
|
|
$
|
3,316
|
|
|
|
Additions to warranty reserve, net of reversals
|
3,449
|
|
3,238
|
|
|||
|
Warranty claims settled
|
(2,615
|
)
|
(2,643
|
)
|
|||
|
Ending balance
|
$
|
4,745
|
|
$
|
3,911
|
|
|
|
2017
|
2016
|
2015
|
|||||||||
|
Operating lease expense
|
$
|
17,513
|
|
$
|
15,357
|
|
$
|
6,516
|
|
||
|
2018
|
2019
|
2020
|
2021
|
2022
|
After 2022
|
|||||||||||||
|
Future minimum lease payments
|
$
|
12,815
|
|
11,468
|
|
8,912
|
|
7,798
|
|
5,512
|
|
18,043
|
|
|||||
|
2017
|
2016
|
2015
|
|||||||||
|
Increase in sales
|
$
|
1,327
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Increase in cost of sales
|
84
|
|
3,516
|
|
1,948
|
|
|||||
|
Ineffective portion of change in fair value
|
—
|
|
—
|
|
—
|
|
|||||
|
Aggregate
Notional
Amount
|
Start
Date
|
End
Date
|
$/Foreign Currency
|
Fair
Value
|
Balance Sheet Location
|
|||||||||||
|
$
|
4,625
|
|
Jan 2018
|
Jun 2018
|
0.0514
|
|
Peso
|
$
|
(127
|
)
|
Accrued Expenses
|
|||||
|
30,398
|
|
Jan 2018
|
Dec 2018
|
0.0507
|
|
Peso
|
(879
|
)
|
Accrued Expenses
|
|||||||
|
30,344
|
|
Jan 2018
|
Dec 2018
|
1.2089
|
|
Euro
|
145
|
|
Accrued Expenses
|
|||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Numerator:
|
|||||||||||
|
Net income (loss)
|
$
|
66,679
|
|
$
|
5,961
|
|
$
|
(7,594
|
)
|
||
|
Denominator for basic EPS:
|
|||||||||||
|
Weighted average shares outstanding
|
31,402
|
|
30,778
|
|
26,363
|
|
|||||
|
Effect of dilutive securities:
|
|||||||||||
|
Stock options, restricted stock and restricted stock units
|
486
|
|
195
|
|
—
|
|
|||||
|
Denominator for diluted EPS
|
31,888
|
|
30,973
|
|
26,363
|
|
|||||
|
Basic EPS
|
$
|
2.12
|
|
$
|
0.19
|
|
$
|
(0.29
|
)
|
||
|
Diluted EPS
|
$
|
2.09
|
|
$
|
0.19
|
|
$
|
(0.29
|
)
|
||
|
2017
|
2016
|
2015
|
||||||
|
Time-vested stock options, restricted stock and restricted stock units
|
676
|
|
657
|
|
1,718
|
|
||
|
Performance-vested stock options and restricted stock units
|
285
|
|
357
|
|
578
|
|
||
|
Defined
Benefit
Plan
Liability
|
Cash
Flow
Hedges
|
Foreign
Currency
Translation
Adjustment
|
Total
Pre-Tax
Amount
|
Tax
|
Net-of-Tax
Amount
|
||||||||||||||||||
|
December 30, 2016
|
$
|
(1,475
|
)
|
$
|
1,420
|
|
$
|
(15,660
|
)
|
$
|
(15,715
|
)
|
$
|
(285
|
)
|
$
|
(16,000
|
)
|
|||||
|
Unrealized gain on cash flow hedges
|
—
|
|
3,707
|
|
—
|
|
3,707
|
|
(353
|
)
|
3,354
|
|
|||||||||||
|
Realized gain on foreign currency hedges
|
—
|
|
(1,243
|
)
|
—
|
|
(1,243
|
)
|
435
|
|
(808
|
)
|
|||||||||||
|
Realized gain on interest rate swap hedges
|
—
|
|
(466
|
)
|
—
|
|
(466
|
)
|
163
|
|
(303
|
)
|
|||||||||||
|
Net defined benefit plan adjustments
|
53
|
|
—
|
|
—
|
|
53
|
|
23
|
|
76
|
|
|||||||||||
|
Foreign currency translation gain
|
—
|
|
—
|
|
65,860
|
|
65,860
|
|
—
|
|
65,860
|
|
|||||||||||
|
December 29, 2017
|
$
|
(1,422
|
)
|
$
|
3,418
|
|
$
|
50,200
|
|
$
|
52,196
|
|
$
|
(17
|
)
|
$
|
52,179
|
|
|||||
|
Defined
Benefit
Plan
Liability
|
Cash
Flow
Hedges
|
Foreign
Currency
Translation
Adjustment
|
Total
Pre-Tax
Amount
|
Tax
|
Net-of-Tax
Amount
|
||||||||||||||||||
|
January 1, 2016
|
$
|
(1,179
|
)
|
$
|
(2,392
|
)
|
$
|
3,609
|
|
$
|
38
|
|
$
|
1,332
|
|
$
|
1,370
|
|
|||||
|
Unrealized gain on cash flow hedges
|
—
|
|
210
|
|
—
|
|
210
|
|
(73
|
)
|
137
|
|
|||||||||||
|
Realized loss on foreign currency hedges
|
—
|
|
3,516
|
|
—
|
|
3,516
|
|
(1,231
|
)
|
2,285
|
|
|||||||||||
|
Realized loss on interest rate swap hedges
|
—
|
|
86
|
|
—
|
|
86
|
|
(30
|
)
|
56
|
|
|||||||||||
|
Net defined benefit plan adjustments
|
(296
|
)
|
—
|
|
—
|
|
(296
|
)
|
(283
|
)
|
(579
|
)
|
|||||||||||
|
Foreign currency translation loss
|
—
|
|
—
|
|
(19,269
|
)
|
(19,269
|
)
|
—
|
|
(19,269
|
)
|
|||||||||||
|
December 30, 2016
|
$
|
(1,475
|
)
|
$
|
1,420
|
|
$
|
(15,660
|
)
|
$
|
(15,715
|
)
|
$
|
(285
|
)
|
$
|
(16,000
|
)
|
|||||
|
|
Fair Value
|
Quoted
Prices in
Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
December 29, 2017
|
|||||||||||||||
|
Assets: Interest rate swap (Note 8)
|
$
|
4,279
|
|
$
|
—
|
|
$
|
4,279
|
|
$
|
—
|
|
|||
|
Liabilities: Foreign currency contracts (Note 13)
|
$
|
861
|
|
$
|
—
|
|
$
|
861
|
|
$
|
—
|
|
|||
|
December 30, 2016
|
|||||||||||||||
|
Assets: Interest rate swaps (Note 8)
|
$
|
3,482
|
|
$
|
—
|
|
$
|
3,482
|
|
$
|
—
|
|
|||
|
Liabilities: Foreign currency contracts (Note 13)
|
$
|
2,063
|
|
$
|
—
|
|
$
|
2,063
|
|
$
|
—
|
|
|||
|
Fair Value
|
Quoted
Prices in
Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
||||||||||||
|
December 29, 2017
|
|||||||||||||||
|
Assets: Cost method investment
|
$
|
180
|
|
$
|
—
|
|
$
|
180
|
|
$
|
—
|
|
|||
|
Assets: Assets Held for Sale
|
490
|
|
—
|
|
490
|
|
—
|
|
|||||||
|
December 30, 2016
|
|||||||||||||||
|
Assets: Cost method investment
|
$
|
430
|
|
$
|
—
|
|
$
|
430
|
|
$
|
—
|
|
|||
|
Assets: Assets Held for Sale
|
794
|
|
—
|
|
794
|
|
—
|
|
|||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Segment sales by product line:
|
|||||||||||
|
Medical
|
|||||||||||
|
Cardio & Vascular
|
$
|
536,794
|
|
$
|
490,857
|
|
$
|
131,299
|
|
||
|
Cardiac & Neuromodulation
|
428,349
|
|
439,541
|
|
361,722
|
|
|||||
|
Advanced Surgical, Orthopedics & Portable Medical
|
439,810
|
|
414,701
|
|
247,944
|
|
|||||
|
Total Medical
|
1,404,953
|
|
1,345,099
|
|
740,965
|
|
|||||
|
Non-Medical
|
56,968
|
|
41,679
|
|
59,449
|
|
|||||
|
Total sales
|
$
|
1,461,921
|
|
$
|
1,386,778
|
|
$
|
800,414
|
|
||
|
|
Sales
|
Accounts Receivable
|
|||||||
|
2017
|
2016
|
2015
|
December 29,
2017 |
December 30,
2016 |
|||||
|
Customer A
|
17%
|
18%
|
17%
|
9%
|
7%
|
||||
|
Customer B
|
17%
|
17%
|
18%
|
18%
|
20%
|
||||
|
Customer C
|
12%
|
12%
|
12%
|
8%
|
4%
|
||||
|
Customer D
|
9%
|
9%
|
5%
|
17%
|
14%
|
||||
|
55%
|
56%
|
52%
|
52%
|
45%
|
|||||
|
2017
|
2016
|
2015
|
|||||||||
|
Segment income from operations:
|
|||||||||||
|
Medical
|
$
|
211,002
|
|
$
|
185,448
|
|
$
|
83,784
|
|
||
|
Non-Medical
|
11,335
|
|
1,513
|
|
7,289
|
|
|||||
|
Total segment income from operations
|
222,337
|
|
186,961
|
|
91,073
|
|
|||||
|
Unallocated operating expenses
|
(82,898
|
)
|
(78,691
|
)
|
(77,927
|
)
|
|||||
|
Operating income
|
139,439
|
|
108,270
|
|
13,146
|
|
|||||
|
Unallocated expenses, net
|
(117,612
|
)
|
(107,085
|
)
|
(28,846
|
)
|
|||||
|
Income (loss) before benefit for income taxes
|
$
|
21,827
|
|
$
|
1,185
|
|
$
|
(15,700
|
)
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Segment depreciation and amortization:
|
|||||||||||
|
Medical
|
$
|
93,927
|
|
$
|
83,184
|
|
$
|
61,618
|
|
||
|
Non-Medical
|
2,675
|
|
2,346
|
|
2,503
|
|
|||||
|
Total depreciation and amortization included in segment
income from operations
|
96,602
|
|
85,530
|
|
64,121
|
|
|||||
|
Unallocated depreciation and amortization
|
6,194
|
|
4,994
|
|
3,497
|
|
|||||
|
Total depreciation and amortization
|
$
|
102,796
|
|
$
|
90,524
|
|
$
|
67,618
|
|
||
|
December 29,
2017 |
December 30,
2016 |
||||||
|
Identifiable assets:
|
|||||||
|
Medical
|
$
|
2,687,227
|
|
$
|
2,638,180
|
|
|
|
Non-Medical
|
54,071
|
|
60,988
|
|
|||
|
Total reportable segments
|
2,741,298
|
|
2,699,168
|
|
|||
|
Unallocated assets
|
107,047
|
|
133,375
|
|
|||
|
Total assets
|
$
|
2,848,345
|
|
$
|
2,832,543
|
|
|
|
|
2017
|
2016
|
2015
|
||||||||
|
Expenditures for tangible long-lived assets, excluding acquisitions:
|
|||||||||||
|
Medical
|
$
|
37,740
|
|
$
|
44,670
|
|
$
|
40,931
|
|
||
|
Non-Medical
|
661
|
|
1,451
|
|
600
|
|
|||||
|
Total reportable segments
|
38,401
|
|
46,121
|
|
41,531
|
|
|||||
|
Unallocated long-lived tangible assets
|
8,783
|
|
8,251
|
|
6,523
|
|
|||||
|
Total expenditures
|
$
|
47,184
|
|
$
|
54,372
|
|
$
|
48,054
|
|
||
|
2017
|
2016
|
2015
|
|||||||||
|
Sales by geographic area:
|
|||||||||||
|
United States
|
$
|
862,290
|
|
$
|
805,742
|
|
$
|
401,380
|
|
||
|
Non-Domestic locations:
|
|||||||||||
|
Puerto Rico
|
133,752
|
|
159,243
|
|
136,898
|
|
|||||
|
Rest of world
|
465,879
|
|
421,793
|
|
262,136
|
|
|||||
|
Total sales
|
$
|
1,461,921
|
|
$
|
1,386,778
|
|
$
|
800,414
|
|
||
|
December 29,
2017 |
December 30,
2016 |
||||||
|
Long-lived tangible assets by geographic area:
|
|||||||
|
United States
|
$
|
252,767
|
|
$
|
258,899
|
|
|
|
Rest of world
|
117,608
|
|
113,143
|
|
|||
|
Total
|
$
|
370,375
|
|
$
|
372,042
|
|
|
|
(in thousands, except per share data)
|
Fourth Quarter
|
Third Quarter
|
Second Quarter
|
First Quarter
|
|||||||||||
|
Fiscal Year 2017
|
|||||||||||||||
|
Sales
|
$
|
390,481
|
|
$
|
363,308
|
|
$
|
362,719
|
|
$
|
345,413
|
|
|||
|
Gross profit
|
104,818
|
|
98,235
|
|
99,272
|
|
91,226
|
|
|||||||
|
Net income (loss)
|
54,338
|
|
13,690
|
|
2,990
|
|
(4,339
|
)
|
|||||||
|
EPS—basic
|
1.71
|
|
0.43
|
|
0.10
|
|
(0.14
|
)
|
|||||||
|
EPS—diluted
|
1.68
|
|
0.43
|
|
0.09
|
|
(0.14
|
)
|
|||||||
|
Fiscal Year 2016
|
|||||||||||||||
|
Sales
|
$
|
359,591
|
|
$
|
346,567
|
|
$
|
348,382
|
|
$
|
332,238
|
|
|||
|
Gross profit
|
92,891
|
|
97,909
|
|
96,031
|
|
91,468
|
|
|||||||
|
Net income (loss)
|
7,933
|
|
11,458
|
|
(770
|
)
|
(12,660
|
)
|
|||||||
|
EPS—basic
|
0.26
|
|
0.37
|
|
(0.03
|
)
|
(0.41
|
)
|
|||||||
|
EPS—diluted
|
0.25
|
|
0.37
|
|
(0.03
|
)
|
(0.41
|
)
|
|||||||
|
a.
|
Evaluation of Disclosure Controls and Procedures
|
|
(1)
|
Financial statements and financial statement schedules filed as part of this Annual Report on Form 10-K. Refer to Part II, Item 8. “Financial Statements and Supplementary Data.”
|
|
(2)
|
The following financial statement schedule is included in this Annual Report on Form 10-K (in thousands):
|
|
|
Col. C—Additions
|
|
|
|
|
|||||||||||||||
|
Column A
Description
|
Col. B Balance at Beginning
of Period
|
Charged to Costs &
Expenses
|
Charged to Other Accounts- Describe
|
|
Col. D Deductions
- Describe
|
|
Col. E Balance at End of
Period
|
|||||||||||||
|
December 29, 2017
|
||||||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
742
|
|
$
|
151
|
|
$
|
31
|
|
(4)
|
$
|
(100
|
)
|
(2)
|
$
|
824
|
|
|||
|
Valuation allowance for deferred tax assets
|
$
|
35,391
|
|
$
|
3,284
|
|
(1)
|
$
|
—
|
|
$
|
(2,195
|
)
|
(2)(5)
|
$
|
36,480
|
|
|||
|
December 30, 2016
|
||||||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
954
|
|
$
|
140
|
|
$
|
245
|
|
(4)
|
$
|
(597
|
)
|
(2)
|
$
|
742
|
|
|||
|
Valuation allowance for deferred tax assets
|
$
|
39,171
|
|
$
|
641
|
|
(1)
|
$
|
(5,135
|
)
|
(3)(4)
|
$
|
714
|
|
(5)
|
$
|
35,391
|
|
||
|
January 1, 2016
|
||||||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
1,411
|
|
$
|
(70
|
)
|
$
|
459
|
|
(3)(4)
|
$
|
(846
|
)
|
(2)
|
$
|
954
|
|
|||
|
Valuation allowance for deferred tax assets
|
$
|
10,709
|
|
$
|
788
|
|
(1)
|
$
|
27,836
|
|
(3)(4)
|
$
|
(162
|
)
|
(5)
|
$
|
39,171
|
|
||
|
(1)
|
Valuation allowance recorded in the provision for income taxes for certain net operating losses and tax credits. The increase in 2017 includes the impact of the adoption of the U.S. Tax Cuts and Jobs Act which increased the value of our state deferred tax assets to which a corresponding valuation allowance was recorded.
|
|
(2)
|
Accounts written off.
|
|
(3)
|
Balance recorded as a part of our 2015 acquisition of LRM. 2016 amount represents measurement-period adjustments related to the acquisition of LRM.
|
|
(4)
|
Includes foreign currency translation effect.
|
|
(5)
|
Includes return to provision adjustments for prior years.
|
|
(3)
|
See exhibits listed under Part (b) below.
|
|
EXHIBIT
NUMBER
|
DESCRIPTION
|
|
|
2.1
|
||
|
2.2
|
||
|
3.1
|
||
|
EXHIBIT
NUMBER
|
DESCRIPTION
|
|
|
3.2
|
||
|
4.1
|
||
|
4.2
|
||
|
10.1#
|
||
|
10.2#
|
||
|
10.3#
|
||
|
10.4#
|
||
|
10.5
|
||
|
10.6
|
||
|
10.7
|
||
|
10.8
|
||
|
10.9#
|
||
|
10.10#
|
||
|
10.11#
|
||
|
10.12#
|
||
|
10.13#
|
||
|
10.14#
|
||
|
10.15#
|
||
|
10.16#
|
||
|
EXHIBIT
NUMBER
|
DESCRIPTION
|
|
|
10.17#
|
||
|
10.18#
|
||
|
10.19#
|
||
|
10.20#
|
||
|
10.21#
|
||
|
10.22#
|
||
|
10.23#
|
||
|
10.24#
|
||
|
10.25
|
||
|
10.26
|
||
|
10.27
|
||
|
10.28
|
||
|
10.29#
|
||
|
10.30#
|
||
|
10.31#*
|
||
|
10.32#*
|
||
|
10.33#*
|
||
|
12.1*
|
||
|
21.1*
|
||
|
23.1*
|
||
|
31.1*
|
||
|
31.2*
|
||
|
32.1**
|
||
|
101.INS*
|
XBRL Instance Document
|
|
|
EXHIBIT
NUMBER
|
DESCRIPTION
|
|
|
101.SCH*
|
XRBL Taxonomy Extension Schema Document
|
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.LAB*
|
XBRL Taxonomy Extension Labels Linkbase Document
|
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
* -
|
Filed herewith.
|
|
** -
|
Furnished herewith.
|
|
# -
|
Indicates exhibits that are management contracts or compensation plans or arrangements required to be filed pursuant to Item 15(b) of Form 10-K.
|
|
INTEGER HOLDINGS CORPORATION
|
|||
|
Dated:
|
February 22, 2018
|
By
|
/s/ Joseph W. Dziedzic
|
|
Joseph W. Dziedzic (Principal Executive Officer)
|
|||
|
President and Chief Executive Officer
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Joseph W. Dziedzic
|
President, Chief Executive Officer and Director
|
February 22, 2018
|
||
|
Joseph W. Dziedzic
|
(Principal Executive Officer)
|
|||
|
/s/ Gary J. Haire
|
Executive Vice President and Chief Financial Officer
|
February 22, 2018
|
||
|
Gary J. Haire
|
(Principal Financial Officer)
|
|||
|
/s/ Tom P. Thomas
|
Vice President, Corporate Controller
|
February 22, 2018
|
||
|
Tom P. Thomas
|
(Principal Accounting Officer)
|
|||
|
/s/ Bill R. Sanford
|
Chairman
|
February 22, 2018
|
||
|
Bill R. Sanford
|
||||
|
/s/ Pamela G. Bailey
|
Director
|
February 22, 2018
|
||
|
Pamela G. Bailey
|
||||
|
Director
|
February 22, 2018
|
|||
|
James F. Hinrichs
|
||||
|
/s/ Jean M. Hobby
|
Director
|
February 22, 2018
|
||
|
Jean M. Hobby
|
||||
|
/s/ M. Craig Maxwell
|
Director
|
February 22, 2018
|
||
|
M. Craig Maxwell
|
||||
|
/s/ Filippo Passerini
|
Director
|
February 22, 2018
|
||
|
Filippo Passerini
|
||||
|
/s/ Peter H. Soderberg
|
Director
|
February 22, 2018
|
||
|
Peter H. Soderberg
|
||||
|
/s/ Donald J. Spence
|
Director
|
February 22, 2018
|
||
|
Donald J. Spence
|
||||
|
/s/ William B. Summers, Jr.
|
Director
|
February 22, 2018
|
||
|
William B. Summers, Jr.
|
||||