|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Ireland
|
|
98-1032470
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Ordinary shares, nominal value $0.0001 per share
|
|
The NASDAQ Stock Market LLC
|
|
Ordinary share purchase rights
|
The NASDAQ Stock Market LLC
|
|
|
Large accelerated filer
x
|
|
Accelerated filer
¨
|
|
Non-accelerated filer
¨
|
|
Smaller reporting company
¨
|
Emerging growth company
¨
|
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|||||
|
Page
|
||
|
PART I
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
PART II
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
PART III
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
PART IV
|
||
|
Item 15.
|
||
|
Item 16.
|
||
|
Item 1.
|
Business
|
|
•
|
Xyrem
®
(sodium oxybate) oral solution
, the only product approved by the U.S. Food and Drug Administration, or FDA, and marketed in the U.S. for the treatment of both cataplexy and excessive daytime sleepiness, or EDS, in patients with narcolepsy;
|
|
•
|
Erwinaze
®
(asparaginase
Erwinia chrysanthemi
)
, a treatment approved in the U.S. and in certain markets in Europe (where it is marketed as Erwinase
®
) for patients with acute lymphoblastic leukemia, or ALL, who have developed hypersensitivity to
E. coli
-derived asparaginase;
|
|
•
|
Defitelio
®
(defibrotide sodium)
, a product approved in the U.S. for the treatment of adult and pediatric patients with hepatic veno-occlusive disease, or VOD, also known as sinusoidal obstruction syndrome, or SOS, with renal or pulmonary dysfunction following hematopoietic stem cell transplantation, or HSCT, and in Europe (where it is marketed as Defitelio
®
(defibrotide)) for the treatment of severe VOD in adults and children undergoing HSCT therapy; and
|
|
•
|
Vyxeos
®
(daunorubicin and cytarabine) liposome for injection
, a product approved in the U.S. for the treatment of adults with newly-diagnosed therapy-related acute myeloid leukemia, or t-AML, or acute myeloid leukemia, or AML, with myelodysplasia-related changes, or AML-MRC.
|
|
•
|
Growing sales of the existing products in our portfolio, including by identifying and investing in growth opportunities such as new treatment indications and new geographic markets;
|
|
•
|
Acquiring or licensing rights to clinically meaningful and differentiated products on the market or product candidates at various stages of development; and
|
|
•
|
Pursuing targeted development of post-discovery differentiated product candidates.
|
|
Project
|
Disease Area
|
Status
|
|
Sleep
|
||
|
Solriamfetol (JZP-110)
|
Excessive sleepiness, or ES, in obstructive sleep apnea, or OSA
|
Submitted a new drug application, or NDA, to the FDA in fourth quarter of 2017; preparing to submit a marketing authorization application, or MAA, to the European Medicines Agency, or EMA, in late 2018
|
|
Solriamfetol (JZP-110)
|
ES in narcolepsy
|
Submitted an NDA to the FDA in fourth quarter of 2017; preparing to submit an MAA to EMA in late 2018
|
|
Solriamfetol (JZP-110)
|
ES in Parkinson’s disease
|
First patient enrolled in Phase 2 trial in first quarter of 2017; targeting completion of enrollment by late 2018
|
|
Xyrem
|
EDS and cataplexy in pediatric narcolepsy patients with cataplexy
|
Expect to submit a supplemental NDA, or sNDA, and pediatric written request report to the FDA in mid-2018
|
|
JZP-507
|
EDS and cataplexy in narcolepsy
|
Expect to be ready to submit an NDA to the FDA as early as mid-2018
|
|
JZP-258
|
EDS and cataplexy in narcolepsy
|
First patient enrolled in Phase 3 trial in first quarter of 2017; expect to complete enrollment in fourth quarter of 2018; subject to results of trial, expect to submit an NDA to the FDA in 2019
|
|
JZP-258
|
Idiopathic hypersomnia, or IH
|
Expect to initiate Phase 3 trial in second half of 2018
|
|
Oxybate once-nightly dosing
|
Narcolepsy
|
Program progressing; evaluation of deuterated oxybate and other formulation options continues as part of once-nightly development
process |
|
Hematology/Oncology
|
||
|
Vyxeos
|
High-risk AML
|
Submitted an MAA to the EMA in fourth quarter of 2017
|
|
Vyxeos
|
Myelodysplastic syndrome, or MDS
|
Preparing for Phase 2 trial with cooperative group with planned initiation in second half of 2018
|
|
Defibrotide
|
Prevention of VOD in high-risk patients following HSCT
|
First patient enrolled in Phase 3 trial in first quarter of 2017
|
|
Defibrotide
|
Prevention of acute Graft versus Host Disease, or aGvHD, following HSCT
|
First patient enrolled in Phase 2 proof of concept trial in first quarter of 2018
|
|
Defibrotide
|
Transplant-associated thrombotic microangiopathy, or TA-TMA
|
Expect to activate sites in pivotal Phase 2 trial in fourth quarter of 2018
|
|
Asparaginase
|
ALL and other hematological malignancies
|
Evaluation of early-stage product candidates
|
|
CombiPlex combinations
|
Oncology/hematological disorders
|
Pre-clinical evaluation of oncology therapeutic combinations
|
|
•
|
Solriamfetol
(JZP-110)
|
|
•
|
Xyrem
|
|
•
|
JZP-507
|
|
•
|
JZP-258
|
|
•
|
Vyxeos
|
|
•
|
Defibrotide
|
|
•
|
Asparaginase Programs
. We are pursuing activities related to the development of improved products, including recombinant crisantaspase, for patients with ALL or other hematological malignancies.
|
|
•
|
CombiPlex
. CombiPlex is a technology platform that enables the design and rapid evaluation of various combinations of therapies to deliver enhanced anti-cancer activity. CombiPlex seeks to identify the most synergistic ratio of drugs in vitro and fix this ratio in a nano-scale delivery complex that maintains the synergistic combination after administration. CombiPlex utilizes two proprietary nano-scale delivery platforms: liposomes to control the release and distribution of water-soluble drugs and drugs that are both water- and fat-soluble (amphipathic), and nanoparticles to control the release and distribution of non-water-soluble (hydrophobic) drugs. We are evaluating the use of CombiPlex in a number of therapeutic combinations in oncology.
|
|
•
|
ImmunoGen
. In August 2017, we entered into a collaboration and option agreement with ImmunoGen, Inc., or ImmunoGen, granting us rights to opt into exclusive, worldwide licenses to develop and commercialize two early-stage, hematology-related antibody-drug conjugate, or ADC, programs, IMGN779 and IMGN632, as well as an additional ADC program to be designated during the term of the agreement. IMGN779 is a CD33-targeted ADC that ImmunoGen is investigating for the treatment of AML, and IMGN632 is a CD123-targeted ADC that ImmunoGen is investigating for the treatment of hematological malignancies, including AML and blastic plasmacytoid dendritic cell neoplasm. Both IMGN779 and IMGN632 are in Phase 1 testing.
|
|
•
|
Pfenex
. In July 2016, we entered into an agreement with Pfenex, Inc., or Pfenex, granting us worldwide rights to develop and commercialize multiple early-stage hematology product candidates and an option for us to negotiate a license for a recombinant pegaspargase product candidate. This agreement was amended in December 2017.
|
|
•
|
XL-protein
. In May 2017, we entered into a license agreement with XL-protein GmbH, or XLp, for the rights to develop, manufacture and commercialize products using XLp’s PASylation
®
technology to extend the plasma half-life of selected asparaginase product candidates.
|
|
•
|
Xyrem
. While Xyrem is the only product approved by the FDA and currently marketed in the U.S. for the treatment of both cataplexy and EDS in patients with narcolepsy, nine companies have filed ANDAs with the FDA seeking approval to market a generic version of Xyrem, the FDA has approved or tentatively approved three ANDAs and we believe that it is likely that the FDA will approve or tentatively approve additional ANDAs. We settled ANDA patent lawsuits against five ANDA filers and, in connection with those settlement agreements, we granted each of the settling filers rights to sell an AG Product and/or its own generic sodium oxybate product as described elsewhere in this Annual Report on Form 10-K. We have ongoing patent lawsuits against the remaining ANDA filers and cannot predict the specific timing or outcome of events with respect to those and other proceedings with such filers or whether there will be additional ANDA filers. Accordingly, the actual timing of any commercial launch of an AG Product or a generic sodium oxybate product is uncertain.
|
|
•
|
Erwinaze
. Erwinaze is a biologic product used in conjunction with chemotherapy and is indicated for patients with ALL who have developed hypersensitivity to
E. coli
-derived asparaginase. While there is currently no direct competition to Erwinaze to treat ALL patients with hypersensitivity to
E. coli
-derived asparaginase, other companies have developed or are developing new treatments for ALL, including new asparaginase treatments that could reduce the rate of hypersensitivity in patients with ALL, and new treatment protocols are being developed for ALL that may not include asparaginase-containing regimens. For example, a number of companies are developing new immunotherapy treatments for relapsed or refractory ALL patients, including one treatment that was recently approved. The development of these new treatments could negatively impact our ability to grow sales of Erwinaze in patient populations where the benefit of an asparaginase-containing regimen is not well established. As a biologic product, Erwinaze also faces potential competition from biosimilar products.
|
|
•
|
Defitelio
.
Defitelio is the only approved treatment in the U.S. for the treatment of adult and pediatric patients with VOD, also known as SOS, with renal or pulmonary dysfunction following HSCT and the only approved treatment in the EU for severe VOD in adults and children undergoing HSCT. Various anti-clotting strategies have been tried by researchers in patients with VOD with mixed results, including Activase (alteplase), a recombinant tissue plasminogen activator marketed by Genentech, Inc., generic heparin sodium injection and Thrombate III (antithrombin III (human)), marketed by Grifols Therapeutics, Inc. While there is currently no direct competition to Defitelio to treat severe VOD, changes in the types of conditioning regimens used as part of HSCT may affect the incidence rate of VOD and demand for Defitelio.
|
|
•
|
Vyxeos
. AML, the cancer indication for which we commercialize Vyxeos, has alternative established therapies. A key consideration in the treatment of AML patients is the patient’s suitability for chemotherapy. The patient population studied in the Vyxeos Phase 3 clinical trial included AML patients deemed able to tolerate chemotherapy. The existing options for the treatment of newly-diagnosed t-AML patients who can tolerate chemotherapy include
|
|
•
|
Solriamfetol
. With respect to solriamfetol, other treatments for ES in patients with narcolepsy include stimulants and wake-promoting agents, such as Provigil and Nuvigil, and generic versions of stimulants and wake-promoting agents. We are also aware of off-label uses of stimulants for ES in patients with OSA. Solriamfetol, if approved by the FDA, will likely face competition from this genericized market. In addition, we are aware of several other products in development for use as potential treatment options for ES in patients with narcolepsy or OSA, including, for example, pitolisant, mazindol, modafinil combinations and Avadel’s once-nightly sodium oxybate formulation.
|
|
•
|
Xyrem.
We have 22 patents in the U.S. relating to Xyrem that expire at various times from December 2019 to March 2033, of which 18 are listed in the FDA’s publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” or the Orange Book. These patents relate to Xyrem’s stable and microbially resistant formulation, its manufacturing process, its method of use, including its restricted distribution system, and its method of administration. Of the patents listed in the Orange Book, five are formulation patents expiring between December 2019 and July 2020; seven are associated with the Xyrem REMS, expiring between December 2022 and June 2024; three are method of use patents covering Xyrem’s use in narcolepsy, which expire in December 2019; and three are method of administration patents relating to a drug-drug interaction, or DDI, between Xyrem and divalproex sodium expiring in March 2033. Four patents are not listed in the Orange Book but also relate to Xyrem: two for methods for making the formulation expiring December 2019, one for a distribution system expiring June 2024 and one for method of administration expiring March 2033. We have received a pediatric written request from the FDA, and we intend to submit the results of a pediatric clinical study in response to this request. If the FDA determines that the submission meets the terms and conditions of the written request, then six months of pediatric exclusivity will be added to the term of each patent listed for Xyrem in the Orange Book. A Xyrem formulation patent has issued in multiple non-U.S. countries and will expire in December 2019. In November 2017, the European Patent Office issued a patent related to the method of administration of Xyrem relating to the DDI between Xyrem and divalproex sodium, expiring in February 2034. In addition to our issued patents, we have patent applications relating to Xyrem pending in the U.S. and other countries.
|
|
•
|
Erwinaze
. Erwinaze has no patent protection. It was granted orphan drug exclusivity by the FDA for the treatment of ALL in the U.S. until November 2018, and we believe that it is protected by exclusivity that prevents approval of a biosimilar in the U.S. through late 2023 under the BPCIA. For more details, see “Business—Government Regulation—Orphan Drug and Other Exclusivities” in this Part I, Item 1.
|
|
•
|
Defitelio
. The unique process of deriving defibrotide from porcine DNA is extensive and uses both chemical and biological processes that rely on complex characterization methods. We have a portfolio of U.S. and non-U.S. patents and patent applications relating to various compositions, methods of use and methods of characterization, some of which have expired and others will expire at various times between now and June 2035. None of these patents are listed in the Orange Book. Defibrotide has been granted orphan drug designation by the EC and the Korean Ministry of Food and Drug Safety to treat and prevent VOD. The Commonwealth of Australia-Department of Health has granted defibrotide orphan drug designation for the treatment of VOD. In addition, the EC has granted orphan drug designation to defibrotide for the prevention of aGvHD.
|
|
•
|
Vyxeos.
We have a portfolio of U.S. and non-U.S. patents and patent applications for Vyxeos and the CombiPlex technology platform relating to various compositions and methods of use. These include three U.S. patents expiring between April 2025 and April 2029 and two U.S. patents covering CombiPlex expiring in January 2027, subject to any patent term extensions. Vyxeos has been granted orphan drug exclusivity by the FDA until August 2024, seven years from its FDA approval, for the treatment of adults with newly-diagnosed t-AML or AML-MRC. In addition, Vyxeos has been granted orphan drug designation by the EC for the treatment of AML.
|
|
•
|
Solriamfetol
. We acquired rights to solriamfetol from Aerial in January 2014, including Aerial’s patent rights relating to solriamfetol, other than in certain jurisdictions in Asia where SK retains rights. We have a portfolio of U.S. and non-U.S. patents and patent applications for solriamfetol relating to various compositions and methods of use. Three U.S. method of use patents covering treatment of sleep-related conditions will expire between June 2026 and August 2027, subject to any patent term extension.
|
|
•
|
JZP-507 and JZP-258.
Certain patents and patent applications relating to Xyrem cover JZP-507 and JZP-258. In addition, JZP-507 and JZP-258 are claimed in formulation patents that will expire in January 2033.
|
|
•
|
preclinical laboratory tests and animal tests;
|
|
•
|
submission to the FDA of an investigational new drug application, or IND, for human clinical testing, which must become effective before human clinical trials commence;
|
|
•
|
adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug or biologic for each indication, as further described below;
|
|
•
|
the submission to the FDA of the NDA or BLA;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facilities at which the product is made, analyzed and stored to assess compliance with cGMP;
|
|
•
|
potential FDA audit of the nonclinical and clinical trial sites that generated the data in support of the application; and
|
|
•
|
FDA review and approval of the application.
|
|
Item 1A.
|
Risk Factors
|
|
•
|
the potential U.S. introduction of a generic version of Xyrem before the entry dates specified in our settlements with certain abbreviated new drug application, or ANDA, filers or on terms that are different from those contemplated by the settlement agreements, as further described below;
|
|
•
|
the potential U.S. introduction of new products that compete with, or otherwise disrupt the market for, Xyrem in the treatment of cataplexy and/or excessive daytime sleepiness, or EDS, in narcolepsy;
|
|
•
|
changes to or uncertainties around regulatory restrictions, including, among other things, changes to our Xyrem risk evaluation and mitigation strategy, or REMS, as further described below;
|
|
•
|
challenges and potential challenges to our intellectual property around Xyrem, including uncertainty in ongoing ANDA litigation or the possibility of new ANDA filers and challenges;
|
|
•
|
any increase in pricing pressure from, changes in policies by, or restrictions on reimbursement imposed by, third party payors;
|
|
•
|
changes in healthcare laws and policy, including changes in requirements for patient assistance programs, rebates, reimbursement and coverage by federal healthcare programs, and changes resulting from increased scrutiny on pharmaceutical pricing and REMS programs by government entities;
|
|
•
|
operational disruptions at the Xyrem central pharmacy or any failure to comply with our REMS obligations to the satisfaction of the U.S. Food and Drug Administration, or FDA;
|
|
•
|
any supply or manufacturing problems, including any problems with our sole source Xyrem active pharmaceutical ingredient, or API, provider;
|
|
•
|
continued acceptance of Xyrem by physicians and patients, including as a result of negative publicity that surfaces from time to time;
|
|
•
|
changes to our label, including new safety warnings or changes to our boxed warning, that further restrict how we market and sell Xyrem; and
|
|
•
|
our U.S.-based API and Xyrem suppliers’ ability to obtain sufficient quotas from the U.S. Drug Enforcement Administration, or DEA, to satisfy our needs for Xyrem.
|
|
•
|
the continued acceptance of Defitelio in the U.S. by hospital pharmacy and therapeutics committees and the continued availability of favorable pricing and adequate coverage and reimbursement by government programs and third party payors;
|
|
•
|
the limited experience of, and need to educate, U.S. physicians in recognizing, diagnosing and treating VOD, particularly in adults;
|
|
•
|
the possibility that physicians recognizing VOD symptoms may not initiate or may delay initiation of treatment while waiting for those symptoms to improve, or may terminate treatment before the end of the recommended dosing schedule;
|
|
•
|
our ability to successfully maintain or grow sales of Defitelio in Europe and other non-U.S. countries;
|
|
•
|
delays or problems in the supply or manufacture of the product;
|
|
•
|
the limited size of the population of VOD patients who are indicated for treatment with Defitelio (particularly if changes in HSCT treatment protocols reduce the incidence of VOD diagnosis);
|
|
•
|
our ability to meet the post-marketing commitments and requirements imposed by the FDA in connection with its approval of our NDA for Defitelio; and
|
|
•
|
our ability to obtain marketing approval in other countries and to develop the product for additional indications.
|
|
•
|
our ability to differentiate Vyxeos from other liposomal chemotherapies and generically available chemotherapy combinations with which physicians and treatment centers are more familiar;
|
|
•
|
delays or problems in the supply or manufacture of the product, including the ability of the third parties upon which we rely to manufacture Vyxeos and its APIs to manufacture sufficient quantities in accordance with applicable specifications;
|
|
•
|
the need to establish pricing and reimbursement support for Vyxeos in the U.S. and in other countries;
|
|
•
|
the acceptance of Vyxeos in the U.S. and other countries by hospital pharmacy and therapeutics committees and the availability of adequate coverage and reimbursement by government programs and third party payors;
|
|
•
|
the approval and use of new and novel compounds in AML that are only approved for use in combination with other agents and that have not been tested in combination with Vyxeos; and
|
|
•
|
the limited size of the population of high-risk AML patients who may potentially be indicated for treatment with Vyxeos, particularly given the ongoing clinical trials by other companies with the same patient population.
|
|
•
|
the clinical indications for which a product is approved and any restrictions placed upon the product in connection with its approval, such as a REMS, patient registry requirements or labeling restrictions;
|
|
•
|
the prevalence of the disease or condition for which the product is approved and its diagnosis;
|
|
•
|
the severity of side effects;
|
|
•
|
acceptance by physicians and patients of each product as a safe and effective treatment;
|
|
•
|
the extent to which the product is approved for inclusion on formularies of hospitals and managed care organizations;
|
|
•
|
the conditions for reimbursement required by, and appropriate pricing and availability of reimbursement from, third party payors;
|
|
•
|
availability of sufficient product inventory to meet demand, particularly with respect to Erwinaze;
|
|
•
|
physicians’ decisions relating to treatment practices based on availability of product inventory, particularly with respect to Erwinaze;
|
|
•
|
perceived advantages over alternative treatments;
|
|
•
|
relative convenience and ease of administration;
|
|
•
|
with respect to Xyrem, physician and patient assessment of the burdens associated with obtaining or maintaining the certifications required under the Xyrem REMS;
|
|
•
|
the cost of treatment in relation to alternative treatments, including generic products; and
|
|
•
|
the availability of financial or other assistance for patients who are uninsured or underinsured.
|
|
•
|
we are unable to obtain and maintain adequate funding to complete the development of, obtain regulatory approval for and commercialize an acquired product candidate;
|
|
•
|
a product candidate proves not to be safe or effective in later clinical trials;
|
|
•
|
a product fails to reach its forecasted commercial potential as a result of pricing pressures or for any other reason;
|
|
•
|
we experience negative publicity regarding actual or potential future price increases for that product or otherwise; or
|
|
•
|
the integration of a product or product candidate gives rise to unforeseen difficulties and expenditures.
|
|
•
|
high acquisition costs;
|
|
•
|
the need to incur substantial debt or engage in dilutive issuances of equity securities to pay for acquisitions;
|
|
•
|
the potential disruption of our historical core business;
|
|
•
|
the strain on, and need to continue to expand, our existing operational, technical, financial and administrative infrastructure;
|
|
•
|
the difficulties in assimilating employees and corporate cultures;
|
|
•
|
the failure to retain key managers and other personnel;
|
|
•
|
the challenges in controlling additional costs and expenses in connection with and as a result of any acquisition;
|
|
•
|
the need to write down assets or recognize impairment charges;
|
|
•
|
the diversion of our management’s attention to integration of operations and corporate and administrative infrastructures; and
|
|
•
|
any unanticipated liabilities for activities of or related to the acquired business or its operations, products or product candidates.
|
|
•
|
delays or failures in obtaining regulatory authorization to commence a trial because of safety concerns of regulators relating to our product candidates or similar product candidates of our competitors or failure to follow regulatory guidelines;
|
|
•
|
delays or failures in obtaining clinical materials and manufacturing sufficient quantities of the product candidate for use in trials;
|
|
•
|
delays or failures in reaching agreement on acceptable terms with prospective study sites;
|
|
•
|
delays or failures in obtaining approval of our clinical trial protocol from an institutional review board, also known as an ethics committee in Europe, to conduct a clinical trial at a prospective study site;
|
|
•
|
delays or failures in recruiting patients to participate in a clinical trial;
|
|
•
|
failure of our clinical trials and clinical investigators to be in compliance with the FDA and other regulatory agencies’ requirements, commonly referred to as good clinical practices;
|
|
•
|
unforeseen safety issues, including negative results from ongoing preclinical studies and clinical trials and adverse events associated with product candidates;
|
|
•
|
inability to monitor patients adequately during or after treatment;
|
|
•
|
difficulty monitoring multiple study sites;
|
|
•
|
difficulty identifying or enrolling eligible patients, in some cases based on the number of clinical trials with enrollment criteria targeting the same patient population;
|
|
•
|
failure of our third party clinical trial managers to satisfactorily perform their contractual duties, comply with regulations or meet expected deadlines; or
|
|
•
|
insufficient funds to complete the trials.
|
|
•
|
the increased complexity and costs inherent in managing international operations;
|
|
•
|
diverse regulatory, financial and legal requirements, and any future changes to such requirements, in one or more countries where we are located or do business;
|
|
•
|
country-specific tax, labor and employment laws and regulations;
|
|
•
|
applicable trade laws, tariffs, export quotas, custom duties or other trade restrictions and any changes to them;
|
|
•
|
challenges inherent in efficiently managing employees in diverse geographies, including the need to adapt systems, policies, benefits and compliance programs to differing labor and other regulations, as well as maintaining positive interactions with unionized employees in one of our international locations;
|
|
•
|
liabilities for activities of, or related to, our international operations, products or product candidates;
|
|
•
|
changes in currency rates; and
|
|
•
|
regulations relating to data security and the unauthorized use of, or access to, commercial and personal information.
|
|
•
|
others may independently develop similar or alternative products without infringing our intellectual property rights, such as products that are not covered by the claims of our patents, or for which we do not have adequate exclusive rights under our license agreements;
|
|
•
|
we or our licensors or partners might not have been the first to invent or file, as appropriate, subject matters covered by our issued patents or pending patent applications or the pending patent applications or issued patents of our licensors or partners;
|
|
•
|
our pending patent applications may not result in issued patents;
|
|
•
|
our issued patents and the issued patents of our licensors or partners may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges by third parties;
|
|
•
|
our issued patents and the issued patents of our licensors or partners may be vulnerable to legal challenges as a result of changes in applicable law;
|
|
•
|
we may not develop additional proprietary products that are patentable; or
|
|
•
|
the patents of others may have an adverse effect on our business.
|
|
•
|
limit our ability to borrow additional funds for working capital, capital expenditures, acquisitions or other general business purposes;
|
|
•
|
limit our ability to use our cash flow or obtain additional financing for working capital, capital expenditures, acquisitions or other general business purposes;
|
|
•
|
require us to use a substantial portion of our cash flow from operations to make debt service payments;
|
|
•
|
limit our flexibility to plan for, or react to, changes in our business and industry;
|
|
•
|
result in dilution to our existing shareholders in the event exchanges of the Exchangeable Senior Notes are settled in our ordinary shares;
|
|
•
|
place us at a competitive disadvantage compared to our less leveraged competitors; and
|
|
•
|
increase our vulnerability to the impact of adverse economic and industry conditions.
|
|
•
|
incur or assume liens or additional debt or provide guarantees in respect of obligations of other persons;
|
|
•
|
issue redeemable preferred stock;
|
|
•
|
pay dividends or distributions or redeem or repurchase capital stock;
|
|
•
|
prepay, redeem or repurchase certain debt;
|
|
•
|
make loans, investments, acquisitions (including acquisitions of exclusive licenses) and capital expenditures;
|
|
•
|
enter into agreements that restrict distributions from our subsidiaries;
|
|
•
|
sell assets and capital stock of our subsidiaries;
|
|
•
|
enter into certain transactions with affiliates; and
|
|
•
|
consolidate or merge with or into, or sell substantially all of our assets to, another person.
|
|
•
|
the revenues from our commercial products, which may be affected by many factors, including the extent of generic or other competition for Xyrem or our other products;
|
|
•
|
the cost of acquiring and/or in-licensing any new products and product candidates;
|
|
•
|
the costs of our commercial operations;
|
|
•
|
the scope, rate of progress, results and costs of our development and clinical activities;
|
|
•
|
the cost and timing of obtaining regulatory approvals and of compliance with laws and regulations;
|
|
•
|
the cost of preparing, filing, prosecuting, defending and enforcing patent claims and other intellectual property rights;
|
|
•
|
the cost of investigations, litigation and/or settlements related to regulatory oversight and third party claims;
|
|
•
|
the costs of integration activities related to any future strategic transactions we may engage in; and
|
|
•
|
the costs arising from changes in laws and regulations, including, for example, healthcare reform legislation.
|
|
•
|
impose advance notice requirements for shareholder proposals and nominations of directors to be considered at shareholder meetings;
|
|
•
|
stagger the terms of our board of directors into three classes;
|
|
•
|
require the approval of a supermajority of the voting power of the shares of our share capital entitled to vote generally at a meeting of shareholders to amend or repeal our articles of association; and
|
|
•
|
permit our board of directors to issue one or more series of preferred shares with rights and preferences, as our shareholders may determine by ordinary resolution.
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
Item 2.
|
Properties
|
|
Item 3.
|
Legal Proceedings
|
|
Item 4.
|
Mine Safety Disclosures.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
High
|
Low
|
||||||
|
Calendar Quarter—2016
|
|||||||
|
First Quarter
|
$
|
139.55
|
|
$
|
108.50
|
|
|
|
Second Quarter
|
$
|
160.00
|
|
$
|
129.00
|
|
|
|
Third Quarter
|
$
|
153.98
|
|
$
|
117.34
|
|
|
|
Fourth Quarter
|
$
|
126.36
|
|
$
|
95.80
|
|
|
|
Calendar Quarter—2017
|
|||||||
|
First Quarter
|
$
|
148.14
|
|
$
|
109.14
|
|
|
|
Second Quarter
|
$
|
163.75
|
|
$
|
139.72
|
|
|
|
Third Quarter
|
$
|
162.59
|
|
$
|
139.28
|
|
|
|
Fourth Quarter
|
$
|
151.45
|
|
$
|
128.58
|
|
|
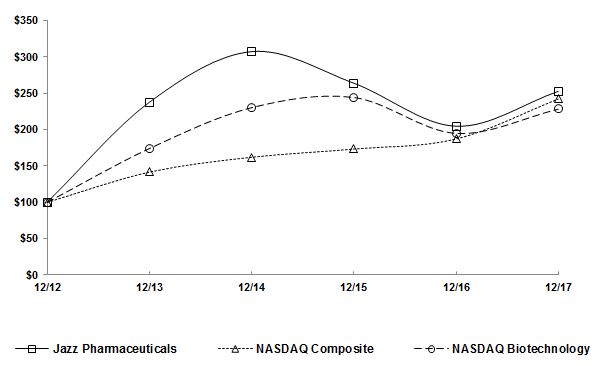
|
(1)
|
This section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, or Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
|
(2)
|
Information used in the graph was obtained from Research Data Group, Inc.
|
|
Total Number of Shares Purchased (1)
|
Average Price Paid per Share (2)
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (3)
|
Maximum Number (or Approximate Dollar Value) of Shares that May Yet Be Purchased Under the Plans or Programs (4)
|
||||||||||
|
October 1 - October 31, 2017
|
90,400
|
|
$
|
143.44
|
|
90,400
|
|
$
|
212,143,010
|
|
|||
|
November 1 - November 30, 2017
|
109,400
|
|
$
|
136.81
|
|
109,400
|
|
$
|
197,178,032
|
|
|||
|
December 1 - December 31, 2017
|
106,100
|
|
$
|
136.10
|
|
106,100
|
|
$
|
182,740,026
|
|
|||
|
Total
|
305,900
|
|
$
|
138.52
|
|
305,900
|
|
||||||
|
(1)
|
This table does not include ordinary shares that we withheld in order to satisfy minimum tax withholding requirements in connection with the vesting of restricted stock units.
|
|
(2)
|
Average price paid per share includes brokerage commissions.
|
|
(3)
|
The ordinary shares reported in the table above were purchased pursuant to our publicly announced share repurchase program. In November 2016, we announced that our board of directors authorized the use of up to $300 million to repurchase our ordinary shares. This authorization has no expiration date.
|
|
(4)
|
The dollar amount shown represents, as of the end of each fiscal month, the approximate dollar value of ordinary shares that may yet be purchased under our publicly announced share repurchase program, exclusive of any brokerage commissions. The timing and amount of repurchases will depend on a variety of factors, including the price of our ordinary shares, alternative investment opportunities, restrictions under our credit agreement, corporate and regulatory requirements and market conditions, and may be modified, suspended or otherwise discontinued at any time without prior notice.
|
|
Item 6.
|
Selected Financial Data
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2017
|
2016(1)
|
2015
|
2014(2)
|
2013
|
||||||||||||||
|
|
(In thousands, except per share amounts)
|
||||||||||||||||||
|
Consolidated Statements of Income Data:
|
|||||||||||||||||||
|
Revenues:
|
|||||||||||||||||||
|
Product sales, net
|
$
|
1,601,399
|
|
$
|
1,477,261
|
|
$
|
1,316,819
|
|
$
|
1,162,716
|
|
$
|
865,398
|
|
||||
|
Royalties and contract revenues
|
17,294
|
|
10,712
|
|
7,984
|
|
10,159
|
|
7,025
|
|
|||||||||
|
Total revenues
|
1,618,693
|
|
1,487,973
|
|
1,324,803
|
|
1,172,875
|
|
872,423
|
|
|||||||||
|
Operating expenses:
|
|||||||||||||||||||
|
Cost of product sales (excluding amortization and impairment of intangible assets)
|
110,188
|
|
105,386
|
|
102,526
|
|
117,418
|
|
102,146
|
|
|||||||||
|
Selling, general and administrative
|
544,156
|
|
502,892
|
|
449,119
|
|
406,114
|
|
304,303
|
|
|||||||||
|
Research and development
|
198,442
|
|
162,297
|
|
135,253
|
|
85,181
|
|
41,632
|
|
|||||||||
|
Acquired in-process research and development
|
85,000
|
|
23,750
|
|
—
|
|
202,626
|
|
4,988
|
|
|||||||||
|
Intangible asset amortization
|
152,065
|
|
101,994
|
|
98,162
|
|
126,584
|
|
79,042
|
|
|||||||||
|
Impairment charges
|
—
|
|
—
|
|
31,523
|
|
39,365
|
|
—
|
|
|||||||||
|
Total operating expenses
|
1,089,851
|
|
896,319
|
|
816,583
|
|
977,288
|
|
532,111
|
|
|||||||||
|
Income from operations
|
528,842
|
|
591,654
|
|
508,220
|
|
195,587
|
|
340,312
|
|
|||||||||
|
Interest expense, net
|
(77,756
|
)
|
(61,942
|
)
|
(56,917
|
)
|
(52,713
|
)
|
(26,916
|
)
|
|||||||||
|
Foreign exchange gain (loss)
|
(9,969
|
)
|
3,372
|
|
1,445
|
|
8,683
|
|
(1,697
|
)
|
|||||||||
|
Loss on extinguishment and modification of debt
|
—
|
|
(638
|
)
|
(16,815
|
)
|
—
|
|
(3,749
|
)
|
|||||||||
|
Income before income tax provision (benefit) and equity in loss of investees
|
441,117
|
|
532,446
|
|
435,933
|
|
151,557
|
|
307,950
|
|
|||||||||
|
Income tax provision (benefit)
|
(47,740
|
)
|
135,236
|
|
106,399
|
|
94,231
|
|
91,638
|
|
|||||||||
|
Equity in loss of investees
|
1,009
|
|
379
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
487,848
|
|
396,831
|
|
329,534
|
|
57,326
|
|
216,312
|
|
|||||||||
|
Net loss attributable to noncontrolling interests
|
—
|
|
—
|
|
(1
|
)
|
(1,061
|
)
|
—
|
|
|||||||||
|
Net income attributable to Jazz Pharmaceuticals plc
|
$
|
487,848
|
|
$
|
396,831
|
|
$
|
329,535
|
|
$
|
58,387
|
|
$
|
216,312
|
|
||||
|
Net income attributable to Jazz Pharmaceuticals plc per ordinary share:
|
|||||||||||||||||||
|
Basic
|
$
|
8.13
|
|
$
|
6.56
|
|
$
|
5.38
|
|
$
|
0.98
|
|
$
|
3.71
|
|
||||
|
Diluted
|
$
|
7.96
|
|
$
|
6.41
|
|
$
|
5.23
|
|
$
|
0.93
|
|
$
|
3.51
|
|
||||
|
Weighted-average ordinary shares used in per share calculations - basic
|
60,018
|
|
60,500
|
|
61,232
|
|
59,746
|
|
58,298
|
|
|||||||||
|
Weighted-average ordinary shares used in per share calculations - diluted
|
61,317
|
|
61,870
|
|
63,036
|
|
62,614
|
|
61,569
|
|
|||||||||
|
|
As of December 31,
|
||||||||||||||||||
|
|
2017
|
2016(1)
|
2015
|
2014(2)
|
2013
|
||||||||||||||
|
|
(In thousands)
|
||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
|||||||||||||||||||
|
Cash, cash equivalents and investments
|
$
|
601,035
|
|
$
|
425,963
|
|
$
|
988,785
|
|
$
|
684,042
|
|
$
|
636,504
|
|
||||
|
Working capital
|
674,330
|
|
490,663
|
|
1,031,025
|
|
799,044
|
|
660,589
|
|
|||||||||
|
Total assets
|
5,123,672
|
|
4,800,227
|
|
3,332,612
|
|
3,308,617
|
|
2,225,900
|
|
|||||||||
|
Long-term debt, current and non-current (1)(2)
|
1,581,038
|
|
2,029,625
|
|
1,188,444
|
|
1,313,161
|
|
539,436
|
|
|||||||||
|
Retained earnings
|
917,956
|
|
528,907
|
|
302,686
|
|
34,704
|
|
18,532
|
|
|||||||||
|
Total Jazz Pharmaceuticals plc shareholders’ equity
|
2,713,097
|
|
1,877,339
|
|
1,598,646
|
|
1,371,144
|
|
1,295,534
|
|
|||||||||
|
(1)
|
On May 27, 2016, we entered into a definitive merger agreement with Celator Pharmaceuticals, Inc., or Celator, pursuant to which we made a cash tender offer of
$30.25
per share for all of the outstanding shares of Celator’s common stock. On July 12, 2016, we completed the acquisition of Celator, which acquisition we refer to in this report as the Celator Acquisition, under the terms of the merger agreement. Celator became an indirect wholly-owned subsidiary of Jazz Pharmaceuticals plc, and each share of Celator common stock then outstanding (other than shares owned by us or Celator) was converted into the right to receive
$30.25
, the same price per share offered in the tender offer. The aggregate cash consideration for the Celator Acquisition was
$1.5 billion
. The results of operations of the acquired Celator business, along with the estimated fair values of the assets acquired and liabilities assumed in the Celator Acquisition, have been included in our consolidated financial statements since the closing of the Celator Acquisition on July 12, 2016. On July 12, 2016, we entered into an amendment to our 2015 credit agreement, which amended agreement we refer to in this report as our amended credit agreement, that provides for a revolving credit facility of
$1.25 billion
, which replaced our prior revolving credit facility of
$750.0 million
, and a
$750.0 million
term loan facility. We used the proceeds of
$1.0 billion
of loans under the revolving credit facility, together with cash on hand, to fund the Celator Acquisition. The maturity date of both our revolving credit facility and term loan facility was extended from June 2020 to July 2021 pursuant to the amended credit facility. In the third quarter of 2017, we completed a private placement of $575.0 million aggregate principal amount of 1.50% exchangeable senior notes due 2024, or the 2024 Notes, resulting in net proceeds to us, after debt issuance costs, of $559.4 million. We used a portion of the net proceeds from the issuance of the 2024 Notes to repay all then outstanding borrowings under the revolving credit facility provided for under the amended credit agreement.
|
|
(2)
|
On January 23, 2014, pursuant to a tender offer, we became the indirect majority shareholder of Gentium S.r.l., or Gentium, acquiring control of Gentium on that date. In February 2014, we completed a subsequent offering period of the tender offer, resulting in total purchases pursuant to the tender offer of approximately
98%
of the fully diluted voting securities of Gentium. As of December 31, 2015, we had acquired the remaining
2%
interest in Gentium for cash consideration of
$17.9 million
, resulting in an aggregate acquisition cost to us of
$994.1 million
, comprising cash payments of
$1,011.2 million
offset by proceeds from the exercise of Gentium share options of
$17.1 million
. The results of operations of the acquired Gentium business, along with the estimated fair values of the assets acquired and liabilities assumed in the transaction, have been included in our consolidated financial statements since the completion of the acquisition of Gentium on January 23, 2014, which is referred to in this report as the Gentium Acquisition. In connection with the Gentium Acquisition, on January 23, 2014, we entered into a second amendment to the credit agreement we entered into in June 2012, or the previous credit agreement. We used the proceeds from incremental term loans of $350.0 million and $300.0 million of loans under the revolving credit facility provided for under the previous credit agreement, together with cash on hand, to finance the Gentium Acquisition. In August 2014, we completed a private placement of $575.0 million aggregate principal amount of 1.875% exchangeable senior notes due 2021, or the 2021 Notes, resulting in net proceeds to us, after debt issuance costs, of $558.9 million. We used a portion of the net proceeds from the issuance of the 2021 Notes to repay all then outstanding borrowings under the revolving credit facility provided for under the previous credit agreement.
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
•
|
Xyrem
®
(sodium oxybate) oral solution
, the only product approved by the U.S. Food and Drug Administration, or FDA, and marketed in the U.S. for the treatment of both cataplexy and excessive daytime sleepiness, or EDS, in patients with narcolepsy;
|
|
•
|
Erwinaze
®
(asparaginase
Erwinia chrysanthemi
)
, a treatment approved in the U.S. and in certain markets in Europe (where it is marketed as Erwinase
®
) for patients with acute lymphoblastic leukemia, or ALL, who have developed hypersensitivity to
E. coli
-derived asparaginase;
|
|
•
|
Defitelio
®
(defibrotide sodium)
, a product approved in the U.S. for the treatment of adult and pediatric patients with hepatic veno-occlusive disease, or VOD, also known as sinusoidal obstruction syndrome, or SOS, with renal or pulmonary dysfunction following hematopoietic stem cell transplantation, or HSCT, and in Europe (where it is marketed as Defitelio
®
(defibrotide)) for the treatment of severe VOD in adults and children undergoing HSCT therapy; and
|
|
•
|
Vyxeos
®
(daunorubicin and cytarabine) liposome for injection
, a product approved in the U.S. for the treatment of adults with newly-diagnosed therapy-related acute myeloid leukemia, or t-AML, or acute myeloid leukemia, or AML, with myelodysplasia-related changes, or AML-MRC.
|
|
•
|
Growing sales of the existing products in our portfolio, including by identifying and investing in growth opportunities such as new treatment indications and new geographic markets;
|
|
•
|
Acquiring or licensing rights to clinically meaningful and differentiated products on the market or product candidates at various stages of development; and
|
|
•
|
Pursuing targeted development of post-discovery differentiated product candidates.
|
|
Project
|
Disease Area
|
Status
|
|
Sleep
|
||
|
Solriamfetol (JZP-110)
|
Excessive sleepiness, or ES, in obstructive sleep apnea, or OSA
|
Submitted a new drug application, or NDA, to the FDA in fourth quarter of 2017; preparing to submit a marketing authorization application, or MAA, to the European Medicines Agency, or EMA, in late 2018
|
|
Solriamfetol (JZP-110)
|
ES in narcolepsy
|
Submitted an NDA to the FDA in fourth quarter of 2017; preparing to submit an MAA to EMA in late 2018
|
|
Solriamfetol (JZP-110)
|
ES in Parkinson’s disease
|
First patient enrolled in Phase 2 trial in first quarter of 2017; targeting completion of enrollment by late 2018
|
|
Project
|
Disease Area
|
Status
|
|
Xyrem
|
EDS and cataplexy in pediatric narcolepsy patients with cataplexy
|
Expect to submit a supplemental NDA, or sNDA, and pediatric written request report to the FDA in mid-2018
|
|
JZP-507
|
EDS and cataplexy in narcolepsy
|
Expect to be ready to submit an NDA to the FDA as early as mid-2018
|
|
JZP-258
|
EDS and cataplexy in narcolepsy
|
First patient enrolled in Phase 3 trial in first quarter of 2017; expect to complete enrollment in fourth quarter of 2018; subject to results of trial, expect to submit an NDA to the FDA in 2019
|
|
JZP-258
|
Idiopathic hypersomnia, or IH
|
Expect to initiate Phase 3 trial in second half of 2018
|
|
Oxybate once-nightly dosing
|
Narcolepsy
|
Program progressing; evaluation of deuterated oxybate and other formulation options continues as part of once-nightly development
process |
|
Hematology/Oncology
|
||
|
Vyxeos
|
High-risk AML
|
Submitted an MAA to the EMA in fourth quarter of 2017
|
|
Vyxeos
|
Myelodysplastic syndrome, or MDS
|
Preparing for Phase 2 trial with cooperative group with planned initiation in second half of 2018
|
|
Defibrotide
|
Prevention of VOD in high-risk patients following HSCT
|
First patient enrolled in Phase 3 trial in first quarter of 2017
|
|
Defibrotide
|
Prevention of acute Graft versus Host Disease, or aGvHD, following HSCT
|
First patient enrolled in Phase 2 proof of concept trial in first quarter of 2018
|
|
Defibrotide
|
Transplant-associated thrombotic microangiopathy, or TA-TMA
|
Expect to activate sites in pivotal Phase 2 trial in fourth quarter of 2018
|
|
Asparaginase
|
ALL and other hematological malignancies
|
Evaluation of early-stage product candidates
|
|
CombiPlex combinations
|
Oncology/hematological disorders
|
Pre-clinical evaluation of oncology therapeutic combinations
|
|
•
|
In December 2017, we submitted an NDA to the FDA for solriamfetol for potential treatment of ES in patients with narcolepsy and OSA.
|
|
•
|
In November 2017, we submitted an MAA for Vyxeos to the EMA for the treatment of t-AML or AML-MRC. We expect to launch Vyxeos, if approved, in the EU on a rolling basis shortly following approval, which could occur as early as mid-2018.
|
|
•
|
In March 2017, we executed license agreements granting Nippon Shinyaku Co., Ltd. exclusive rights to develop and commercialize Defitelio and Vyxeos in Japan.
|
|
•
|
In May 2017, we entered into a license agreement with XL-protein GmbH, or XLp, for the rights to develop, manufacture and commercialize products using XLp’s PASylation® technology to extend the plasma half-life of selected asparaginase product candidates.
|
|
•
|
In August 2017, we entered into a collaboration and option agreement with ImmunoGen granting us rights to opt into exclusive, worldwide licenses to develop and commercialize two early-stage, hematology-related programs, as well as an additional ADC program to be designated during the term of the agreement, as described above.
|
|
•
|
In December 2017, we amended our agreement with Pfenex, Inc., or Pfenex, which agreement grants us worldwide rights to develop and commercialize multiple early-stage hematology product candidates and an option for us to negotiate a license for a recombinant pegaspargase product candidate.
|
|
•
|
the potential U.S. introduction of a generic version of Xyrem before the entry dates specified in our settlements with certain ANDA filers or on terms that are different from those contemplated by the settlement agreements, as further described below;
|
|
•
|
the potential U.S. introduction of new products that compete with, or otherwise disrupt the market for, Xyrem in the treatment of cataplexy and/or EDS in narcolepsy;
|
|
•
|
changes to or uncertainties around regulatory restrictions, including, among other things, changes to our Xyrem risk evaluation and mitigation strategy, or REMS, as further described below;
|
|
•
|
challenges and potential challenges to our intellectual property around Xyrem, including uncertainty in ongoing ANDA litigation or the possibility of new ANDA filers and challenges;
|
|
•
|
any increase in pricing pressure from, changes in policies by, or restrictions on reimbursement imposed by, third party payors;
|
|
•
|
changes in healthcare laws and policy, including changes in requirements for patient assistance programs, rebates, reimbursement and coverage by federal healthcare programs, and changes resulting from increased scrutiny on pharmaceutical pricing and REMS programs by government entities;
|
|
•
|
operational disruptions at the Xyrem central pharmacy or any failure to comply with our REMS obligations to the satisfaction of the FDA;
|
|
•
|
any supply or manufacturing problems, including any problems with our sole source Xyrem active pharmaceutical ingredient, or API, provider;
|
|
•
|
continued acceptance of Xyrem by physicians and patients, including as a result of negative publicity that surfaces from time to time;
|
|
•
|
changes to our label, including new safety warnings or changes to our boxed warning, that further restrict how we market and sell Xyrem; and
|
|
•
|
our U.S.-based API and Xyrem suppliers’ ability to obtain sufficient quotas from the U.S. Drug Enforcement Administration, or DEA, to satisfy our needs for Xyrem.
|
|
•
|
the challenges of protecting and enhancing our intellectual property rights;
|
|
•
|
the challenges of achieving and maintaining commercial success of our products;
|
|
•
|
delays or problems in the supply or manufacture of our products and product candidates, particularly with respect to certain products as to which we maintain limited inventories, our dependence on single source suppliers for most of our products, product candidates and APIs, and the requirement that we and our product suppliers be qualified by the FDA to manufacture product and comply with applicable manufacturing regulations;
|
|
•
|
the need to obtain and maintain appropriate pricing and reimbursement for our products in an increasingly challenging environment due to, among other things, the attention being paid to healthcare cost containment and pharmaceutical pricing in the U.S. and worldwide, including the need to obtain and maintain reimbursement for Xyrem in the U.S. in an environment in which we are subject to increasingly restrictive conditions for reimbursement required by government programs and third party payors;
|
|
•
|
our ability to identify and acquire, in-license or develop additional products or product candidates to grow our business;
|
|
•
|
the challenges of compliance with the requirements of the FDA, the DEA and comparable non-U.S. regulatory agencies, including with respect to product labeling, requirements for distribution, obtaining sufficient DEA quotas where needed, marketing and promotional activities, patient assistance programs, adverse event reporting and product recalls or withdrawals;
|
|
•
|
the difficulty and uncertainty of pharmaceutical product development, including the timing thereof, and the uncertainty of clinical success, such as the risk that results from preclinical studies and/or early clinical trials may not be predictive of results obtained in later and larger clinical trials planned or anticipated to be conducted for our product candidates;
|
|
•
|
the inherent uncertainty associated with the regulatory approval process, especially as we continue to increase investment in our product pipeline development projects and undertake multiple planned regulatory submissions for our product candidates;
|
|
•
|
the risks associated with business combination or product or product candidate acquisition transactions, such as the challenges inherent in the integration of acquired businesses with our historical business, the increase in geographic dispersion among our centers of operation and the risks that we may acquire unanticipated liabilities along with acquired businesses or otherwise fail to realize the anticipated benefits (commercial or otherwise) from such transactions; and
|
|
•
|
possible restrictions on our ability and flexibility to pursue certain future opportunities as a result of our substantial outstanding debt obligations.
|
|
2017
|
Change
|
2016 (1)
|
Change
|
2015
|
|||||||||||||
|
Product sales, net
|
$
|
1,601,399
|
|
8
|
%
|
$
|
1,477,261
|
|
12
|
%
|
$
|
1,316,819
|
|
||||
|
Royalties and contract revenues
|
17,294
|
|
61
|
%
|
10,712
|
|
34
|
%
|
7,984
|
|
|||||||
|
Cost of product sales (excluding amortization and impairment of intangible assets)
|
110,188
|
|
5
|
%
|
105,386
|
|
3
|
%
|
102,526
|
|
|||||||
|
Selling, general and administrative
|
544,156
|
|
8
|
%
|
502,892
|
|
12
|
%
|
449,119
|
|
|||||||
|
Research and development
|
198,442
|
|
22
|
%
|
162,297
|
|
20
|
%
|
135,253
|
|
|||||||
|
Acquired in-process research and development
|
85,000
|
|
258
|
%
|
23,750
|
|
N/A(2)
|
|
—
|
|
|||||||
|
Intangible asset amortization
|
152,065
|
|
49
|
%
|
101,994
|
|
4
|
%
|
98,162
|
|
|||||||
|
Impairment charges
|
—
|
|
N/A(2)
|
|
—
|
|
N/A(2)
|
|
31,523
|
|
|||||||
|
Interest expense, net
|
77,756
|
|
26
|
%
|
61,942
|
|
9
|
%
|
56,917
|
|
|||||||
|
Foreign exchange loss (gain)
|
9,969
|
|
(396
|
)%
|
(3,372
|
)
|
133
|
%
|
(1,445
|
)
|
|||||||
|
Loss on extinguishment and modification of debt
|
—
|
|
N/A(2)
|
|
638
|
|
(96
|
)%
|
16,815
|
|
|||||||
|
Income tax provision (benefit)
|
(47,740
|
)
|
(135
|
)%
|
135,236
|
|
27
|
%
|
106,399
|
|
|||||||
|
Equity in loss of investees
|
1,009
|
|
166
|
%
|
379
|
|
N/A(2)
|
|
—
|
|
|||||||
|
Net loss attributable to noncontrolling interests
|
—
|
|
N/A(2)
|
|
—
|
|
N/A(2)
|
|
(1
|
)
|
|||||||
|
(1)
|
Our financial results include the financial results of the historical Celator business since the closing of the Celator Acquisition on July 12, 2016.
|
|
(2)
|
Comparison to prior period is not meaningful.
|
|
|
2017
|
Change
|
2016
|
Change
|
2015
|
||||||||||||
|
Xyrem
|
$
|
1,186,699
|
|
7
|
%
|
$
|
1,107,616
|
|
16
|
%
|
$
|
955,187
|
|
||||
|
Erwinaze/Erwinase
|
197,340
|
|
(2
|
)%
|
200,678
|
|
(1
|
)%
|
203,261
|
|
|||||||
|
Defitelio/defibrotide
|
133,650
|
|
23
|
%
|
108,952
|
|
54
|
%
|
70,731
|
|
|||||||
|
Vyxeos
|
33,790
|
|
N/A(1)
|
|
—
|
|
N/A(1)
|
|
—
|
|
|||||||
|
Prialt® (ziconotide) intrathecal infusion
|
27,361
|
|
(6
|
)%
|
29,120
|
|
10
|
%
|
26,440
|
|
|||||||
|
Other
|
22,559
|
|
(27
|
)%
|
30,895
|
|
(50
|
)%
|
61,200
|
|
|||||||
|
Product sales, net
|
1,601,399
|
|
8
|
%
|
1,477,261
|
|
12
|
%
|
1,316,819
|
|
|||||||
|
Royalties and contract revenues
|
17,294
|
|
61
|
%
|
10,712
|
|
34
|
%
|
7,984
|
|
|||||||
|
Total revenues
|
$
|
1,618,693
|
|
9
|
%
|
$
|
1,487,973
|
|
12
|
%
|
$
|
1,324,803
|
|
||||
|
(1)
|
Comparison to prior period is not meaningful.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Clinical studies and outside services
|
$
|
93,317
|
|
$
|
100,165
|
|
$
|
63,079
|
|
||
|
Personnel expenses
|
63,941
|
|
47,969
|
|
39,515
|
|
|||||
|
Milestone expenses
|
19,500
|
|
750
|
|
25,000
|
|
|||||
|
Other
|
21,684
|
|
13,413
|
|
7,659
|
|
|||||
|
Total
|
$
|
198,442
|
|
$
|
162,297
|
|
$
|
135,253
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net cash provided by operating activities
|
$
|
693,087
|
|
$
|
592,391
|
|
$
|
531,943
|
|
||
|
Net cash used in investing activities
|
(268,950
|
)
|
(1,751,155
|
)
|
(2,255
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
(409,111
|
)
|
540,987
|
|
(214,323
|
)
|
|||||
|
Effect of exchange rates on cash and cash equivalents
|
5,046
|
|
(5,045
|
)
|
(10,622
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
20,072
|
|
$
|
(622,822
|
)
|
$
|
304,743
|
|
||
|
|
Payments Due by Period
|
||||||||||||||||||
|
Contractual Obligations (1)
|
Total
|
Less than
1 Year
|
1-3 Years
|
3-5 Years
|
More than
5 years
|
||||||||||||||
|
Term loan - principal
|
$
|
676,758
|
|
$
|
40,605
|
|
$
|
135,352
|
|
$
|
500,801
|
|
$
|
—
|
|
||||
|
Term loan - interest (2)
|
73,738
|
|
23,307
|
|
41,275
|
|
9,156
|
|
—
|
|
|||||||||
|
Exchangeable Senior Notes - principal
|
1,150,000
|
|
—
|
|
—
|
|
575,000
|
|
575,000
|
|
|||||||||
|
Exchangeable Senior Notes - interest (3)
|
103,333
|
|
19,239
|
|
38,813
|
|
28,031
|
|
17,250
|
|
|||||||||
|
Revolving credit facility - commitment fee (4)
|
13,417
|
|
3,802
|
|
7,615
|
|
2,000
|
|
—
|
|
|||||||||
|
Commitment to equity method investees
|
28,550
|
|
5,550
|
|
14,000
|
|
9,000
|
|
—
|
|
|||||||||
|
Purchase and other obligations (5)
|
134,702
|
|
46,324
|
|
33,882
|
|
37,358
|
|
17,138
|
|
|||||||||
|
Operating lease obligations (6)
|
46,351
|
|
9,102
|
|
14,277
|
|
10,219
|
|
12,753
|
|
|||||||||
|
Facility lease obligations (7)
|
195,397
|
|
6,297
|
|
23,534
|
|
30,093
|
|
135,473
|
|
|||||||||
|
Total
|
$
|
2,422,246
|
|
$
|
154,226
|
|
$
|
308,748
|
|
$
|
1,201,658
|
|
$
|
757,614
|
|
||||
|
(1)
|
This table does not include potential future milestone payment or royalty obligations to third parties under asset purchase, product development, license and other agreements as the timing and likelihood of such milestone payments are not known, and, in the case of royalty obligations, as the amount of such obligations are not estimable. In 2014, we signed a definitive agreement with Aerial under which we acquired worldwide development, manufacturing and commercial rights to solriamfetol (other than in certain jurisdictions in Asia where SK retains rights). Aerial and SK are currently eligible to receive milestone payments up to an aggregate of $270 million based on development, regulatory and sales milestones and tiered royalties from high single digits to mid-teens based on potential future sales of solriamfetol. In July 2016, we entered into an agreement with Pfenex that granted us worldwide rights to develop and commercialize multiple early-stage hematology product candidates and an option for us to negotiate a license for a recombinant pegaspargase product candidate with Pfenex. This agreement was amended in December 2017. Under the amended agreement, Pfenex received upfront, option and development milestone payments totaling $35.3 million and may be eligible to receive additional payments of up to $189 million based on the achievement of development, regulatory and sales milestones. Potential future milestone payments to other third parties under other agreements could be up to an aggregate of $327 million, of which up to $120 million will become due and payable to Perrigo Company plc (formerly Elan Pharmaceuticals, Inc.) in tiered contingent payments, with the first such payment becoming due if net sales of Prialt of at least $75 million are achieved in a calendar year. The remainder would become due and payable to other third parties upon the achievement of certain developmental, clinical, regulatory and/or commercial milestones, the timing and likelihood of which are not known. We are also obligated under these agreements to pay royalties on net sales of certain products at specified rates, which royalties are dependent on future product sales and are not provided for in the table above as they are not estimable.
|
|
(2)
|
Estimated interest for variable rate debt was calculated based on the interest rates in effect as of
December 31, 2017
. The interest rate for our term loan borrowing was
3.32%
as of
December 31, 2017
.
I
nterest that is fixed, associated with our interest rate swaps, is calculated based on the fixed interest swap rate as of
December 31, 2017
.
|
|
(3)
|
We used the fixed interest rates of 1.875% on the 2021 Notes and 1.50% on the 2024 Notes to estimate interest owed as of
December 31, 2017
until the respective final maturity dates of these notes.
|
|
(4)
|
Our revolving credit facility has a commitment fee payable on the undrawn amount ranging from 0.25% to 0.35% per annum based upon our secured leverage ratio. In the table above, we used a rate of 0.30% and assumed undrawn amounts of
$1.25 billion
as of
December 31, 2017
to estimate commitment fees owed.
|
|
(5)
|
Consists primarily of non-cancelable commitments to ImmunoGen, under our collaboration and option agreement, and to third party manufacturers.
|
|
(6)
|
Consists primarily of the minimum lease payments for our office buildings and automobile lease payments for our sales force. Operating expenses associated with our leased office buildings are not included in table above.
|
|
Rebates Payable
|
Sales Returns Reserve
|
Chargebacks
|
Discounts and Distributor Fees
|
Total
|
|||||||||||||||
|
Balance at December 31, 2014 (1)
|
$
|
44,433
|
|
$
|
14,039
|
|
$
|
4,544
|
|
$
|
5,875
|
|
$
|
68,891
|
|
||||
|
Provision, net
|
124,618
|
|
(4,444
|
)
|
39,124
|
|
46,533
|
|
205,831
|
|
|||||||||
|
Payments/credits
|
(107,013
|
)
|
(3,485
|
)
|
(38,772
|
)
|
(48,684
|
)
|
(197,954
|
)
|
|||||||||
|
Balance at December 31, 2015
|
62,038
|
|
6,110
|
|
4,896
|
|
3,724
|
|
76,768
|
|
|||||||||
|
Provision, net
|
129,608
|
|
(537
|
)
|
40,430
|
|
40,057
|
|
209,558
|
|
|||||||||
|
Payments/credits
|
(123,383
|
)
|
(1,207
|
)
|
(40,577
|
)
|
(39,582
|
)
|
(204,749
|
)
|
|||||||||
|
Balance at December 31, 2016
|
68,263
|
|
4,366
|
|
4,749
|
|
4,199
|
|
81,577
|
|
|||||||||
|
Provision, net
|
144,596
|
|
446
|
|
41,941
|
|
36,642
|
|
223,625
|
|
|||||||||
|
Payments/credits
|
(135,697
|
)
|
(1,161
|
)
|
(43,027
|
)
|
(36,532
|
)
|
(216,417
|
)
|
|||||||||
|
Balance at December 31, 2017
|
$
|
77,162
|
|
$
|
3,651
|
|
$
|
3,663
|
|
$
|
4,309
|
|
$
|
88,785
|
|
||||
|
•
|
estimating the timing of and expected costs to complete the in-process projects;
|
|
•
|
projecting regulatory approvals;
|
|
•
|
estimating future cash flows from product sales resulting from completed products and in-process projects; and
|
|
•
|
developing appropriate discount rates and probability rates by project.
|
|
Year Ended December 31,
|
||||||||
|
2017
|
2016
|
2015
|
||||||
|
Volatility
|
35
|
%
|
39
|
%
|
39
|
%
|
||
|
Expected term (years)
|
4.3
|
|
4.2
|
|
4.2
|
|
||
|
Range of risk-free rates
|
1.6-2.1%
|
|
0.8-1.6%
|
|
1.1-1.5%
|
|
||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
|
Page
|
|
Jazz Pharmaceuticals plc
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Balance Sheets
|
F-2
|
|
Consolidated Statements of Income
|
F-3
|
|
Consolidated Statements of Comprehensive Income
|
F-4
|
|
Consolidated Statements of Shareholders’ Equity
|
F-5
|
|
Consolidated Statements of Cash Flows
|
F-8
|
|
Notes to Consolidated Financial Statements
|
F-10
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|
Item 9A.
|
Controls and Procedures
|
|
1.
|
Our management is responsible for establishing and maintaining adequate internal control over financial reporting.
|
|
2.
|
Our management used the Committee of Sponsoring Organizations of the Treadway Commission Internal Control - Integrated Framework (2013), or the COSO framework, to evaluate the effectiveness of internal control over financial reporting. Management believes that the COSO framework is a suitable framework for its evaluation of financial reporting because it is free from bias, permits reasonably consistent qualitative and quantitative measurements of our internal control over financial reporting, is sufficiently complete so that those relevant factors that would alter a conclusion about the effectiveness of our internal control over financial reporting are not omitted and is relevant to an evaluation of internal control over financial reporting.
|
|
3.
|
Management has assessed the effectiveness of our internal control over financial reporting as of
December 31, 2017
and has concluded that such internal control over financial reporting was effective. There were no material weaknesses in internal control over financial reporting identified by management.
|
|
4.
|
KPMG, our independent registered public accounting firm, has audited the consolidated financial statements of Jazz Pharmaceuticals plc as of and for the year ended
December 31, 2017
, included herein, and has issued an audit report on our internal control over financial reporting, which is included below.
|
|
Item 9B.
|
Other Information
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
•
|
The information relating to our directors and nominees for director is to be included in the section entitled “Proposal 1—Election of Directors;”
|
|
•
|
The information relating to our executive officers is to be included in the section entitled “Executive Officers;”
|
|
•
|
The information relating to our audit committee, audit committee financial expert and procedures by which shareholders may recommend nominees to our board of directors is to be included in the section entitled “Corporate Governance and Board Matters;” and
|
|
•
|
The information regarding compliance with Section 16(a) of the Exchange Act is to be included in the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance.”
|
|
Item 11.
|
Executive Compensation
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
Item 14.
|
Principal Accountant Fees and Services
|
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
|
1.
|
Index to Financial Statements:
|
|
2.
|
Financial Statement Schedules:
|
|
Exhibit
Number
|
Description of Document
|
||
|
2.1
|
|||
|
2.2
|
|||
|
2.3
|
|||
|
2.4
|
|||
|
2.5
|
|||
|
2.6†
|
|||
|
2.7†
|
|||
|
2.8
|
|||
|
2.9
|
|||
|
3.1
|
|||
|
4.1
|
|||
|
4.2
|
|||
|
4.3A
|
|||
|
4.3B
|
|||
|
4.4A
|
|||
|
4.4B
|
|||
|
4.5A
|
|||
|
4.5B
|
|||
|
10.1
|
|||
|
10.2†
|
|||
|
10.3†
|
|||
|
10.4†
|
|||
|
10.5
|
|||
|
10.6†
|
|||
|
10.7†
|
|||
|
10.8†
|
|||
|
10.9A
|
|||
|
10.9B
|
|||
|
10.10A
|
|||
|
10.10B
|
|||
|
10.10C
|
|||
|
10.11
|
|||
|
10.12
|
|||
|
10.13
|
|||
|
10.14+
|
|||
|
10.15+
|
|||
|
10.16+
|
|||
|
10.17A+
|
|||
|
10.17B+
|
|||
|
10.17C+
|
|||
|
10.17D+
|
|||
|
10.18+
|
|||
|
10.19A+
|
|||
|
10.19B+
|
|||
|
10.19C+
|
|||
|
10.19D+
|
|||
|
10.20+
|
|||
|
10.21+
|
|||
|
10.22A+
|
|||
|
10.22B+
|
|||
|
10.22C+
|
|||
|
10.22D+
|
|||
|
10.22E+
|
|||
|
10.22F+
|
|||
|
10.22G+
|
|||
|
10.22H+
|
|||
|
10.23A+
|
|||
|
10.23B+
|
|||
|
10.23C+
|
|||
|
10.23D+
|
|||
|
10.23E+
|
|||
|
10.23F+
|
|||
|
10.23G+
|
|||
|
10.23H+
|
|||
|
10.23I+
|
|||
|
10.23J+
|
|||
|
10.23K+
|
|||
|
10.23L+
|
|||
|
10.23M+
|
|
||
|
10.23N+
|
|||
|
10.23O+
|
|||
|
10.23P+
|
|||
|
10.23Q+
|
|||
|
10.23R+
|
|||
|
10.23S+
|
|||
|
10.24+
|
|||
|
10.24A+
|
|||
|
10.24B+
|
|||
|
10.24C+
|
|||
|
10.24D+
|
|||
|
10.24E+
|
|||
|
10.24F+
|
|||
|
10.24G+
|
|||
|
10.25A+
|
|||
|
10.25B+
|
|||
|
10.26A+
|
|||
|
10.26B+
|
|||
|
10.26C+
|
|||
|
10.27+
|
|||
|
10.28+
|
|||
|
10.29A+
|
|||
|
10.29B+
|
|||
|
21.1
|
|||
|
23.1
|
|||
|
24.1
|
|||
|
31.1
|
|||
|
31.2
|
|||
|
32.1*
|
|||
|
101.INS
|
XBRL Instance Document
|
||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
||
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document
|
||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
||
|
+
|
Indicates management contract or compensatory plan.
|
|
†
|
Confidential treatment has been granted for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
|
|
*
|
The certifications attached as Exhibit 32.1 accompany this Annual Report on Form 10-K pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the Registrant for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
|
|
Item 16.
|
Form 10-K Summary
|
|
Date: February 27, 2018
|
Jazz Pharmaceuticals public limited company
|
|
(Registrant)
|
|
|
/s/ B
RUCE
C. C
OZADD
|
|
|
Bruce C. Cozadd
Chairman and Chief Executive Officer and Director
(Principal Executive Officer)
|
|
|
/s/ M
ATTHEW
P. Y
OUNG
|
|
|
Matthew P. Young
Executive Vice President and Chief Financial Officer (Principal Financial Officer)
|
|
|
/s/ K
AREN
J. W
ILSON
|
|
|
Karen J. Wilson
Senior Vice President, Finance
(Principal Accounting Officer)
|
|
|
Signature
|
Title
|
Date
|
||
|
/s/ B
RUCE
C. C
OZADD
|
Chairman, Chief Executive Officer and Director
(Principal Executive Officer)
|
February 27, 2018
|
||
|
Bruce C. Cozadd
|
||||
|
/s/ M
ATTHEW
P. Y
OUNG
|
Executive Vice President and Chief Financial Officer
(Principal Financial Officer)
|
February 27, 2018
|
||
|
Matthew P. Young
|
||||
|
/s/ K
AREN
J. W
ILSON
|
Senior Vice President, Finance
(Principal Accounting Officer)
|
February 27, 2018
|
||
|
Karen J. Wilson
|
||||
|
/s/ P
AUL
L. B
ERNS
|
Director
|
February 27, 2018
|
||
|
Paul L. Berns
|
||||
|
/s/ P
ATRICK
G. E
NRIGHT
|
Director
|
February 27, 2018
|
||
|
Patrick G. Enright
|
||||
|
/s/ P
ETER
G
RAY
|
Director
|
February 27, 2018
|
||
|
Peter Gray
|
||||
|
/s/ H
EATHER
A
NN
M
CSHARRY
|
Director
|
February 27, 2018
|
||
|
Heather Ann McSharry
|
||||
|
/s/ S
EAMUS
C. M
ULLIGAN
|
Director
|
February 27, 2018
|
||
|
Seamus C. Mulligan
|
||||
|
/s/ K
ENNETH
W. O’
KEEFE
|
Director
|
February 27, 2018
|
||
|
Kenneth W. O’Keefe
|
||||
|
/s/
N
ORBERT
G
.
R
IEDEL,
P
H.
D
.
|
Director
|
February 27, 2018
|
||
|
Norbert G. Riedel, Ph.D.
|
||||
|
/s/ E
LMAR
S
CHNEE
|
Director
|
February 27, 2018
|
||
|
Elmar Schnee
|
||||
|
/s/ C
ATHERINE
A. S
OHN,
P
HARM
.D.
|
Director
|
February 27, 2018
|
||
|
Catherine A. Sohn, Pharm.D.
|
||||
|
/s/ R
ICK
E W
INNINGHAM
|
Director
|
February 27, 2018
|
||
|
Rick E Winningham
|
||||
|
|
December 31,
|
||||||
|
|
2017
|
2016
|
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
386,035
|
|
$
|
365,963
|
|
|
|
Investments
|
215,000
|
|
60,000
|
|
|||
|
Accounts receivable, net of allowances of $4,162 and $5,154 at December 31, 2017 and 2016, respectively
|
224,129
|
|
234,244
|
|
|||
|
Inventories
|
43,245
|
|
34,051
|
|
|||
|
Prepaid expenses
|
23,182
|
|
24,501
|
|
|||
|
Other current assets
|
76,686
|
|
29,310
|
|
|||
|
Total current assets
|
968,277
|
|
748,069
|
|
|||
|
Property and equipment, net
|
170,080
|
|
107,490
|
|
|||
|
Intangible assets, net
|
2,979,127
|
|
3,012,001
|
|
|||
|
Goodwill
|
947,537
|
|
893,810
|
|
|||
|
Deferred tax assets, net
|
34,559
|
|
15,060
|
|
|||
|
Deferred financing costs
|
7,673
|
|
9,737
|
|
|||
|
Other non-current assets
|
16,419
|
|
14,060
|
|
|||
|
Total assets
|
$
|
5,123,672
|
|
$
|
4,800,227
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
24,368
|
|
$
|
22,415
|
|
|
|
Accrued liabilities
|
198,779
|
|
193,268
|
|
|||
|
Current portion of long-term debt
|
40,605
|
|
36,094
|
|
|||
|
Income taxes payable
|
21,577
|
|
4,506
|
|
|||
|
Deferred revenue
|
8,618
|
|
1,123
|
|
|||
|
Total current liabilities
|
293,947
|
|
257,406
|
|
|||
|
Deferred revenue, non-current
|
16,115
|
|
2,601
|
|
|||
|
Long-term debt, less current portion
|
1,540,433
|
|
1,993,531
|
|
|||
|
Deferred tax liabilities, net
|
383,472
|
|
556,733
|
|
|||
|
Other non-current liabilities
|
176,608
|
|
112,617
|
|
|||
|
Commitments and contingencies (Note 13)
|
|
|
|||||
|
Shareholders’ equity:
|
|||||||
|
Ordinary shares, nominal value $0.0001 per share; 300,000 shares authorized; 59,898 and 59,820 shares issued and outstanding at December 31, 2017 and 2016, respectively
|
6
|
|
6
|
|
|||
|
Non-voting euro deferred shares, €0.01 par value per share; 4,000 shares authorized, issued and outstanding at both December 31, 2017 and 2016
|
55
|
|
55
|
|
|||
|
Capital redemption reserve
|
472
|
|
472
|
|
|||
|
Additional paid-in capital
|
1,935,486
|
|
1,665,232
|
|
|||
|
Accumulated other comprehensive loss
|
(140,878
|
)
|
(317,333
|
)
|
|||
|
Retained earnings
|
917,956
|
|
528,907
|
|
|||
|
Total shareholders’ equity
|
2,713,097
|
|
1,877,339
|
|
|||
|
Total liabilities and shareholders’ equity
|
$
|
5,123,672
|
|
$
|
4,800,227
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Revenues:
|
|||||||||||
|
Product sales, net
|
$
|
1,601,399
|
|
$
|
1,477,261
|
|
$
|
1,316,819
|
|
||
|
Royalties and contract revenues
|
17,294
|
|
10,712
|
|
7,984
|
|
|||||
|
Total revenues
|
1,618,693
|
|
1,487,973
|
|
1,324,803
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Cost of product sales (excluding amortization and impairment of intangible assets)
|
110,188
|
|
105,386
|
|
102,526
|
|
|||||
|
Selling, general and administrative
|
544,156
|
|
502,892
|
|
449,119
|
|
|||||
|
Research and development
|
198,442
|
|
162,297
|
|
135,253
|
|
|||||
|
Acquired in-process research and development
|
85,000
|
|
23,750
|
|
—
|
|
|||||
|
Intangible asset amortization
|
152,065
|
|
101,994
|
|
98,162
|
|
|||||
|
Impairment charges
|
—
|
|
—
|
|
31,523
|
|
|||||
|
Total operating expenses
|
1,089,851
|
|
896,319
|
|
816,583
|
|
|||||
|
Income from operations
|
528,842
|
|
591,654
|
|
508,220
|
|
|||||
|
Interest expense, net
|
(77,756
|
)
|
(61,942
|
)
|
(56,917
|
)
|
|||||
|
Foreign exchange gain (loss)
|
(9,969
|
)
|
3,372
|
|
1,445
|
|
|||||
|
Loss on extinguishment and modification of debt
|
—
|
|
(638
|
)
|
(16,815
|
)
|
|||||
|
Income before income tax provision (benefit) and equity in loss of investees
|
441,117
|
|
532,446
|
|
435,933
|
|
|||||
|
Income tax provision (benefit)
|
(47,740
|
)
|
135,236
|
|
106,399
|
|
|||||
|
Equity in loss of investees
|
1,009
|
|
379
|
|
—
|
|
|||||
|
Net income
|
487,848
|
|
396,831
|
|
329,534
|
|
|||||
|
Net loss attributable to noncontrolling interests
|
—
|
|
—
|
|
(1
|
)
|
|||||
|
Net income attributable to Jazz Pharmaceuticals plc
|
$
|
487,848
|
|
$
|
396,831
|
|
$
|
329,535
|
|
||
|
Net income attributable to Jazz Pharmaceuticals plc per ordinary share:
|
|||||||||||
|
Basic
|
$
|
8.13
|
|
$
|
6.56
|
|
$
|
5.38
|
|
||
|
Diluted
|
$
|
7.96
|
|
$
|
6.41
|
|
$
|
5.23
|
|
||
|
Weighted-average ordinary shares used in per share calculations - basic
|
60,018
|
|
60,500
|
|
61,232
|
|
|||||
|
Weighted-average ordinary shares used in per share calculations - diluted
|
61,317
|
|
61,870
|
|
63,036
|
|
|||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net income
|
$
|
487,848
|
|
$
|
396,831
|
|
$
|
329,534
|
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Foreign currency translation adjustments
|
174,973
|
|
(49,861
|
)
|
(145,375
|
)
|
|||||
|
Unrealized gain on hedging activities, net of tax expense of $212, $0 and $0, respectively
|
1,482
|
|
—
|
|
—
|
|
|||||
|
Other comprehensive income (loss)
|
176,455
|
|
(49,861
|
)
|
(145,375
|
)
|
|||||
|
Total comprehensive income
|
664,303
|
|
346,970
|
|
184,159
|
|
|||||
|
Comprehensive loss attributable to noncontrolling interests
|
—
|
|
—
|
|
(1
|
)
|
|||||
|
Comprehensive income attributable to Jazz Pharmaceuticals plc
|
$
|
664,303
|
|
$
|
346,970
|
|
$
|
184,160
|
|
||
|
|
Ordinary Shares
|
Non-voting Euro Deferred
|
Capital Redemp-tion Reserve
|
Additional
Paid-in
Capital
|
Accumu-lated
Other
Compre-hensive
Income (Loss)
|
Retained Earnings
|
Total Jazz Pharma-ceuticals plc
Share-holders’
Equity
|
Non-control-ling interest
|
Total
Equity
|
||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||||||||||
|
Balance at December 31, 2014
|
60,643
|
|
$
|
6
|
|
4,000
|
|
$
|
55
|
|
$
|
471
|
|
$
|
1,458,005
|
|
$
|
(122,097
|
)
|
$
|
34,704
|
|
$
|
1,371,144
|
|
$
|
64
|
|
$
|
1,371,208
|
|
||||||||||
|
Acquisition of noncontrolling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(10
|
)
|
—
|
|
—
|
|
(10
|
)
|
(63
|
)
|
(73
|
)
|
|||||||||||||||||||
|
Issuance of ordinary shares in conjunction with exercise of share options
|
732
|
|
—
|
|
—
|
|
—
|
|
—
|
|
32,982
|
|
—
|
|
—
|
|
32,982
|
|
—
|
|
32,982
|
|
|||||||||||||||||||
|
Issuance of ordinary shares under employee stock purchase plan
|
75
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7,541
|
|
—
|
|
—
|
|
7,541
|
|
—
|
|
7,541
|
|
|||||||||||||||||||
|
Issuance of ordinary shares in conjunction with vesting of restricted stock units
|
265
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||
|
Shares withheld for payment of employee's withholding tax liability
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(26,102
|
)
|
—
|
|
—
|
|
(26,102
|
)
|
—
|
|
(26,102
|
)
|
|||||||||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
91,795
|
|
—
|
|
—
|
|
91,795
|
|
—
|
|
91,795
|
|
|||||||||||||||||||
|
Excess tax benefits from employee share options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,311
|
)
|
—
|
|
—
|
|
(1,311
|
)
|
—
|
|
(1,311
|
)
|
|||||||||||||||||||
|
Shares repurchased
|
(410
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(61,553
|
)
|
(61,553
|
)
|
—
|
|
(61,553
|
)
|
|||||||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(145,375
|
)
|
—
|
|
(145,375
|
)
|
—
|
|
(145,375
|
)
|
|||||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
329,535
|
|
329,535
|
|
(1
|
)
|
329,534
|
|
|||||||||||||||||||
|
Balance at December 31, 2015
|
61,305
|
|
$
|
6
|
|
4,000
|
|
$
|
55
|
|
$
|
471
|
|
$
|
1,562,900
|
|
$
|
(267,472
|
)
|
$
|
302,686
|
|
$
|
1,598,646
|
|
$
|
—
|
|
$
|
1,598,646
|
|
||||||||||
|
|
Ordinary Shares
|
Non-voting Euro Deferred
|
Capital Redemp-tion Reserve
|
Additional
Paid-in
Capital
|
Accumu-lated
Other
Compre-hensive
Income (Loss)
|
Retained Earnings
|
Total
Equity
|
||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||
|
Balance at December 31, 2015
|
61,305
|
|
$
|
6
|
|
4,000
|
|
$
|
55
|
|
$
|
471
|
|
$
|
1,562,900
|
|
$
|
(267,472
|
)
|
$
|
302,686
|
|
$
|
1,598,646
|
|
||||||||
|
Cumulative effect adjustment from adoption of ASU No. 2016-09
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
107,687
|
|
107,687
|
|
|||||||||||||||
|
Issuance of ordinary shares in conjunction with exercise of share options
|
399
|
|
—
|
|
—
|
|
—
|
|
—
|
|
16,880
|
|
—
|
|
—
|
|
16,880
|
|
|||||||||||||||
|
Issuance of ordinary shares under employee stock purchase plan
|
70
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7,294
|
|
—
|
|
—
|
|
7,294
|
|
|||||||||||||||
|
Issuance of ordinary shares in conjunction with vesting of restricted stock units
|
289
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Shares withheld for payment of employee's withholding tax liability
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(21,234
|
)
|
—
|
|
—
|
|
(21,234
|
)
|
|||||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
99,392
|
|
—
|
|
—
|
|
99,392
|
|
|||||||||||||||
|
Shares repurchased
|
(2,243
|
)
|
—
|
|
—
|
|
—
|
|
1
|
|
—
|
|
—
|
|
(278,297
|
)
|
(278,296
|
)
|
|||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(49,861
|
)
|
—
|
|
(49,861
|
)
|
|||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
396,831
|
|
396,831
|
|
|||||||||||||||
|
Balance at December 31, 2016
|
59,820
|
|
$
|
6
|
|
4,000
|
|
$
|
55
|
|
$
|
472
|
|
$
|
1,665,232
|
|
$
|
(317,333
|
)
|
$
|
528,907
|
|
$
|
1,877,339
|
|
||||||||
|
|
Ordinary Shares
|
Non-voting Euro Deferred
|
Capital Redemp-tion Reserve
|
Additional
Paid-in
Capital
|
Accumu-lated
Other
Compre-hensive
Income (Loss)
|
Retained Earnings
|
Total
Equity
|
||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||
|
Balance at December 31, 2016
|
59,820
|
|
$
|
6
|
|
4,000
|
|
$
|
55
|
|
$
|
472
|
|
$
|
1,665,232
|
|
$
|
(317,333
|
)
|
$
|
528,907
|
|
$
|
1,877,339
|
|
||||||||
|
Issuance of Exchangeable Senior Notes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
149,767
|
|
—
|
|
—
|
|
149,767
|
|
|||||||||||||||
|
Issuance of ordinary shares in conjunction with exercise of share options
|
428
|
|
—
|
|
—
|
|
—
|
|
—
|
|
22,683
|
|
—
|
|
—
|
|
22,683
|
|
|||||||||||||||
|
Issuance of ordinary shares under employee stock purchase plan
|
104
|
|
—
|
|
—
|
|
—
|
|
—
|
|
9,141
|
|
—
|
|
—
|
|
9,141
|
|
|||||||||||||||
|
Issuance of ordinary shares in conjunction with vesting of restricted stock units
|
250
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Shares withheld for payment of employee's withholding tax liability
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(18,589
|
)
|
—
|
|
—
|
|
(18,589
|
)
|
|||||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
107,252
|
|
—
|
|
—
|
|
107,252
|
|
|||||||||||||||
|
Shares repurchased
|
(704
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(98,799
|
)
|
(98,799
|
)
|
|||||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
176,455
|
|
—
|
|
176,455
|
|
|||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
487,848
|
|
487,848
|
|
|||||||||||||||
|
Balance at December 31, 2017
|
59,898
|
|
$
|
6
|
|
4,000
|
|
$
|
55
|
|
$
|
472
|
|
$
|
1,935,486
|
|
$
|
(140,878
|
)
|
$
|
917,956
|
|
$
|
2,713,097
|
|
||||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Operating activities
|
|||||||||||
|
Net income
|
$
|
487,848
|
|
$
|
396,831
|
|
$
|
329,534
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||||||
|
Intangible asset amortization
|
152,065
|
|
101,994
|
|
98,162
|
|
|||||
|
Share-based compensation
|
106,900
|
|
98,771
|
|
91,550
|
|
|||||
|
Impairment charges
|
—
|
|
—
|
|
31,523
|
|
|||||
|
Depreciation
|
13,089
|
|
11,786
|
|
9,894
|
|
|||||
|
Acquired in-process research and development
|
85,000
|
|
23,750
|
|
—
|
|
|||||
|
Loss on disposal of property and equipment
|
473
|
|
47
|
|
172
|
|
|||||
|
Deferred income taxes
|
(225,591
|
)
|
(41,163
|
)
|
(68,358
|
)
|
|||||
|
Provision for losses on accounts receivable and inventory
|
2,190
|
|
2,209
|
|
4,062
|
|
|||||
|
Loss on extinguishment and modification of debt
|
—
|
|
638
|
|
16,815
|
|
|||||
|
Amortization of debt discount and deferred financing costs
|
30,026
|
|
22,133
|
|
22,738
|
|
|||||
|
Other non-cash transactions
|
14,321
|
|
(3,741
|
)
|
(5,187
|
)
|
|||||
|
Changes in assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
12,278
|
|
(25,603
|
)
|
(24,841
|
)
|
|||||
|
Inventories
|
(8,667
|
)
|
(17,024
|
)
|
6,271
|
|
|||||
|
Prepaid expenses and other current assets
|
(26,874
|
)
|
(15,700
|
)
|
3,720
|
|
|||||
|
Other long-term assets
|
119
|
|
267
|
|
(4,573
|
)
|
|||||
|
Accounts payable
|
214
|
|
361
|
|
(2,280
|
)
|
|||||
|
Accrued liabilities
|
(6,578
|
)
|
11,989
|
|
2,986
|
|
|||||
|
Income taxes payable
|
16,331
|
|
2,962
|
|
(6,271
|
)
|
|||||
|
Deferred revenue
|
21,009
|
|
(1,315
|
)
|
(536
|
)
|
|||||
|
Other non-current liabilities
|
18,934
|
|
23,199
|
|
26,562
|
|
|||||
|
Net cash provided by operating activities
|
693,087
|
|
592,391
|
|
531,943
|
|
|||||
|
Investing activities
|
|||||||||||
|
Acquisition of investments
|
(385,000
|
)
|
(132,181
|
)
|
—
|
|
|||||
|
Proceeds from maturity of investments
|
230,000
|
|
66,906
|
|
—
|
|
|||||
|
Acquired in-process research and development
|
(85,000
|
)
|
(23,750
|
)
|
—
|
|
|||||
|
Purchases of property and equipment
|
(28,950
|
)
|
(9,687
|
)
|
(35,958
|
)
|
|||||
|
Acquisitions, net of cash acquired
|
—
|
|
(1,502,443
|
)
|
—
|
|
|||||
|
Acquisition of intangible assets
|
—
|
|
(150,000
|
)
|
—
|
|
|||||
|
Net proceeds from sale of business
|
—
|
|
—
|
|
33,703
|
|
|||||
|
Net cash used in investing activities
|
(268,950
|
)
|
(1,751,155
|
)
|
(2,255
|
)
|
|||||
|
Financing activities
|
|||||||||||
|
Net proceeds from issuance of debt
|
559,393
|
|
994,647
|
|
898,642
|
|
|||||
|
Proceeds from employee equity incentive and purchase plans
|
31,824
|
|
24,174
|
|
40,523
|
|
|||||
|
Share repurchases
|
(98,799
|
)
|
(278,296
|
)
|
(61,553
|
)
|
|||||
|
Payment of employee withholding taxes related to share-based awards
|
(18,589
|
)
|
(21,234
|
)
|
(26,102
|
)
|
|||||
|
Repayments of long-term debt
|
(36,094
|
)
|
(28,304
|
)
|
(905,760
|
)
|
|||||
|
Repayments under revolving credit facility
|
(850,000
|
)
|
(150,000
|
)
|
(160,000
|
)
|
|||||
|
Proceeds from tenant improvement allowance on build-to-suit lease
|
3,154
|
|
—
|
|
—
|
|
|||||
|
Acquisition of noncontrolling interests
|
—
|
|
—
|
|
(73
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
(409,111
|
)
|
540,987
|
|
(214,323
|
)
|
|||||
|
Effect of exchange rates on cash and cash equivalents
|
5,046
|
|
(5,045
|
)
|
(10,622
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
20,072
|
|
(622,822
|
)
|
304,743
|
|
|||||
|
Cash and cash equivalents, at beginning of period
|
365,963
|
|
988,785
|
|
684,042
|
|
|||||
|
Cash and cash equivalents, at end of period
|
$
|
386,035
|
|
$
|
365,963
|
|
$
|
988,785
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Supplemental disclosure of cash flow information:
|
|||||||||||
|
Cash paid for interest
|
$
|
44,609
|
|
$
|
39,898
|
|
$
|
40,099
|
|
||
|
Cash paid for income taxes
|
174,124
|
|
160,306
|
|
145,597
|
|
|||||
|
Non-cash investing activities:
|
|||||||||||
|
Amounts capitalized in connection with facility lease obligations
|
40,970
|
|
23,799
|
|
4,351
|
|
|||||
|
•
|
Xyrem
®
(sodium oxybate) oral solution
, the only product approved by the U.S. Food and Drug Administration, or FDA, and marketed in the U.S. for the treatment of both cataplexy and excessive daytime sleepiness, or EDS, in patients with narcolepsy;
|
|
•
|
Erwinaze
®
(asparaginase
Erwinia chrysanthemi
)
, a treatment approved in the U.S. and in certain markets in Europe (where it is marketed as Erwinase
®
) for patients with acute lymphoblastic leukemia, or ALL, who have developed hypersensitivity to
E. coli
-derived asparaginase;
|
|
•
|
Defitelio
®
(defibrotide sodium)
, a product approved in the U.S. for the treatment of adult and pediatric patients with hepatic veno-occlusive disease, or VOD, also known as sinusoidal obstruction syndrome, or SOS, with renal or pulmonary dysfunction following hematopoietic stem cell transplantation, or HSCT, and in Europe (where it is marketed as Defitelio
®
(defibrotide)) for the treatment of severe VOD in adults and children undergoing HSCT therapy; and
|
|
•
|
Vyxeos
®
(daunorubicin and cytarabine) liposome for injection
, a product approved in the U.S. for the treatment of adults with newly-diagnosed therapy-related acute myeloid leukemia, or t-AML, or acute myeloid leukemia, or AML, with myelodysplasia-related changes, or AML-MRC.
|
|
•
|
Growing sales of the existing products in our portfolio, including by identifying and investing in growth opportunities such as new treatment indications and new geographic markets;
|
|
•
|
Acquiring or licensing rights to clinically meaningful and differentiated products on the market or product candidates at various stages of development; and
|
|
•
|
Pursuing targeted development of post-discovery differentiated product candidates.
|
|
Buildings
|
40 years
|
|
Manufacturing equipment and machinery
|
5-10 years
|
|
Computer software and equipment
|
3 years
|
|
Furniture and fixtures
|
5 years
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Numerator:
|
|||||||||||
|
Net income attributable to Jazz Pharmaceuticals plc
|
$
|
487,848
|
|
$
|
396,831
|
|
$
|
329,535
|
|
||
|
Denominator:
|
|||||||||||
|
Weighted-average ordinary shares used in per share calculations - basic
|
60,018
|
|
60,500
|
|
61,232
|
|
|||||
|
Dilutive effect of employee equity incentive and purchase plans
|
1,299
|
|
1,370
|
|
1,804
|
|
|||||
|
Weighted-average ordinary shares used in per share calculations - diluted
|
61,317
|
|
61,870
|
|
63,036
|
|
|||||
|
Net income per ordinary share :
|
|||||||||||
|
Basic
|
$
|
8.13
|
|
$
|
6.56
|
|
$
|
5.38
|
|
||
|
Diluted
|
$
|
7.96
|
|
$
|
6.41
|
|
$
|
5.23
|
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2017
|
2016
|
2015
|
|||||
|
Exchangeable Senior Notes
|
3,805
|
|
2,878
|
|
2,878
|
|
||
|
Options to purchase ordinary shares and RSUs
|
3,319
|
|
3,010
|
|
1,609
|
|
||
|
Ordinary shares under ESPP
|
14
|
|
93
|
|
—
|
|
||
|
Cash and cash equivalents
|
$
|
26,137
|
|
|
Other receivables
|
386
|
|
|
|
Prepaid expenses and deposits
|
151
|
|
|
|
Property and equipment
|
767
|
|
|
|
Intangible assets
|
1,811,250
|
|
|
|
Goodwill
|
252,825
|
|
|
|
Other non-current assets
|
43
|
|
|
|
Accrued liabilities
|
(19,076
|
)
|
|
|
Deferred tax liability, net, non-current
|
(542,901
|
)
|
|
|
Other non-current liabilities
|
(1,002
|
)
|
|
|
Total acquisition consideration - cash paid
|
$
|
1,528,580
|
|
|
•
|
The exclusion of acquisition-related and integration expenses of
$13.6 million
in 2016 and the inclusion of these expenses in 2015.
|
|
•
|
An increase in interest expense of
$13.7 million
in 2016 and
$25.9 million
in 2015 incurred on additional borrowings made to partially fund the Celator Acquisition as if the borrowings had occurred on January 1, 2015.
|
|
|
Year Ended December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Revenues
|
$
|
1,488,118
|
|
$
|
1,326,246
|
|
|
|
Net income attributable to Jazz Pharmaceuticals plc
|
$
|
386,342
|
|
$
|
283,113
|
|
|
|
Net income attributable to Jazz Pharmaceuticals plc per ordinary share - basic
|
$
|
6.39
|
|
$
|
4.62
|
|
|
|
Net income attributable to Jazz Pharmaceuticals plc per ordinary share - diluted
|
$
|
6.24
|
|
$
|
4.49
|
|
|
|
December 31, 2017
|
|||||||||||||||||||||||
|
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Estimated
Fair Value |
Cash and Cash Equivalents
|
Investments
|
||||||||||||||||||
|
Cash
|
$
|
225,235
|
|
$
|
—
|
|
$
|
—
|
|
$
|
225,235
|
|
$
|
225,235
|
|
$
|
—
|
|
|||||
|
Time deposits
|
235,000
|
|
—
|
|
—
|
|
235,000
|
|
20,000
|
|
215,000
|
|
|||||||||||
|
Money market funds
|
140,800
|
|
—
|
|
—
|
|
140,800
|
|
140,800
|
|
—
|
|
|||||||||||
|
Totals
|
$
|
601,035
|
|
$
|
—
|
|
$
|
—
|
|
$
|
601,035
|
|
$
|
386,035
|
|
$
|
215,000
|
|
|||||
|
December 31, 2016
|
|||||||||||||||||||||||
|
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Estimated
Fair Value |
Cash and Cash
Equivalents |
Investments
|
||||||||||||||||||
|
Cash
|
$
|
215,963
|
|
$
|
—
|
|
$
|
—
|
|
$
|
215,963
|
|
$
|
215,963
|
|
$
|
—
|
|
|||||
|
Time deposits
|
210,000
|
|
—
|
|
—
|
|
210,000
|
|
150,000
|
|
60,000
|
|
|||||||||||
|
Totals
|
$
|
425,963
|
|
$
|
—
|
|
$
|
—
|
|
$
|
425,963
|
|
$
|
365,963
|
|
$
|
60,000
|
|
|||||
|
December 31, 2017
|
December 31, 2016
|
||||||||||||||||||
|
Quoted
Prices in Active Markets for Identical Assets (Level 1) |
Significant
Other Observable Inputs (Level 2) |
Total
Estimated Fair Value |
Significant
Other Observable Inputs (Level 2) |
Total
Estimated Fair Value |
|||||||||||||||
|
Assets:
|
|||||||||||||||||||
|
Available-for-sale securities:
|
|||||||||||||||||||
|
Time deposits
|
$
|
—
|
|
$
|
235,000
|
|
$
|
235,000
|
|
$
|
210,000
|
|
$
|
210,000
|
|
||||
|
Money market funds
|
140,800
|
|
—
|
|
140,800
|
|
—
|
|
—
|
|
|||||||||
|
Interest rate contracts
|
—
|
|
2,138
|
|
2,138
|
|
—
|
|
—
|
|
|||||||||
|
Foreign exchange forward contracts
|
—
|
|
15,495
|
|
15,495
|
|
—
|
|
—
|
|
|||||||||
|
Totals
|
$
|
140,800
|
|
$
|
252,633
|
|
$
|
393,433
|
|
$
|
210,000
|
|
$
|
210,000
|
|
||||
|
Liabilities:
|
|||||||||||||||||||
|
Interest rate contracts
|
$
|
—
|
|
$
|
392
|
|
$
|
392
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Foreign exchange forward contracts
|
—
|
|
5,017
|
|
5,017
|
|
—
|
|
—
|
|
|||||||||
|
Totals
|
$
|
—
|
|
$
|
5,409
|
|
$
|
5,409
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Year Ended December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Loss recognized in accumulated other comprehensive loss, net of tax
|
$
|
(213
|
)
|
$
|
—
|
|
|
|
Loss reclassified from accumulated other comprehensive loss to interest expense, net of tax
|
$
|
1,695
|
|
$
|
—
|
|
|
|
December 31, 2017
|
|||||||||||
|
Asset Derivatives
|
Liability Derivatives
|
||||||||||
|
Balance Sheet Location
|
Fair Value
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
Derivatives designated as hedging instruments:
|
|||||||||||
|
Interest rate contracts
|
Other non-current assets
|
$
|
2,138
|
|
Accrued liabilities
|
$
|
392
|
|
|||
|
Derivatives not designated as hedging instruments:
|
|||||||||||
|
Foreign exchange forward contracts
|
Other current assets
|
15,495
|
|
Accrued liabilities
|
5,017
|
|
|||||
|
Total fair value of derivative instruments
|
$
|
17,633
|
|
$
|
5,409
|
|
|||||
|
December 31, 2017
|
|||||||||||||||||||||||
|
Gross Amounts of Recognized Assets/Liabilities
|
Gross Amounts Offset in the Consolidated Balance Sheet
|
Net Amounts of Assets/ Liabilities Presented in the Consolidated Balance Sheet
|
Gross Amounts Not Offset in the Consolidated Balance Sheet
|
||||||||||||||||||||
|
Description
|
Derivative Financial Instruments
|
Cash Collateral Received (Pledged)
|
Net Amount
|
||||||||||||||||||||
|
Derivative assets
|
$
|
1,639
|
|
$
|
—
|
|
$
|
1,639
|
|
$
|
(875
|
)
|
$
|
—
|
|
$
|
764
|
|
|||||
|
Derivative liabilities
|
$
|
(875
|
)
|
$
|
—
|
|
$
|
(875
|
)
|
$
|
875
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Raw materials
|
$
|
3,542
|
|
$
|
1,547
|
|
|
|
Work in process
|
15,692
|
|
18,689
|
|
|||
|
Finished goods
|
24,011
|
|
13,815
|
|
|||
|
Total inventories
|
$
|
43,245
|
|
$
|
34,051
|
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Build-to-suit facility
|
$
|
51,721
|
|
$
|
—
|
|
|
|
Land and buildings
|
46,729
|
|
46,033
|
|
|||
|
Leasehold improvements
|
28,779
|
|
9,328
|
|
|||
|
Manufacturing equipment and machinery
|
23,586
|
|
19,596
|
|
|||
|
Construction-in-progress
|
21,738
|
|
33,427
|
|
|||
|
Computer software
|
19,969
|
|
17,832
|
|
|||
|
Computer equipment
|
12,814
|
|
10,980
|
|
|||
|
Furniture and fixtures
|
5,947
|
|
2,436
|
|
|||
|
Subtotal
|
211,283
|
|
139,632
|
|
|||
|
Less accumulated depreciation and amortization
|
(41,203
|
)
|
(32,142
|
)
|
|||
|
Property and equipment, net
|
$
|
170,080
|
|
$
|
107,490
|
|
|
|
Balance at December 31, 2016
|
$
|
893,810
|
|
|
Foreign exchange
|
53,727
|
|
|
|
Balance at December 31, 2017
|
$
|
947,537
|
|
|
December 31, 2017
|
December 31, 2016
|
||||||||||||||||||||||||
|
Remaining
Weighted- Average Useful Life (In years) |
Gross
Carrying Amount |
Accumulated
Amortization |
Net Book
Value |
Gross
Carrying Amount |
Accumulated
Amortization |
Net Book
Value |
|||||||||||||||||||
|
Acquired developed technologies
|
14.9
|
$
|
3,392,832
|
|
$
|
(562,473
|
)
|
$
|
2,830,359
|
|
$
|
1,477,618
|
|
$
|
(410,523
|
)
|
$
|
1,067,095
|
|
||||||
|
Manufacturing contracts
|
0.1
|
12,824
|
|
(12,634
|
)
|
190
|
|
11,278
|
|
(8,292
|
)
|
2,986
|
|
||||||||||||
|
Trademarks
|
—
|
2,910
|
|
(2,910
|
)
|
—
|
|
2,872
|
|
(2,872
|
)
|
—
|
|
||||||||||||
|
Total finite-lived intangible assets
|
3,408,566
|
|
(578,017
|
)
|
2,830,549
|
|
1,491,768
|
|
(421,687
|
)
|
1,070,081
|
|
|||||||||||||
|
Acquired IPR&D assets
|
148,578
|
|
—
|
|
148,578
|
|
1,941,920
|
|
—
|
|
1,941,920
|
|
|||||||||||||
|
Total intangible assets
|
$
|
3,557,144
|
|
$
|
(578,017
|
)
|
$
|
2,979,127
|
|
$
|
3,433,688
|
|
$
|
(421,687
|
)
|
$
|
3,012,001
|
|
|||||||
|
Year Ending December 31,
|
Estimated Amortization Expense
|
||
|
2018
|
$
|
209,912
|
|
|
2019
|
209,674
|
|
|
|
2020
|
206,706
|
|
|
|
2021
|
205,655
|
|
|
|
2022
|
204,922
|
|
|
|
Thereafter
|
1,793,680
|
|
|
|
Total
|
$
|
2,830,549
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Rebates and other sales deductions
|
$
|
81,368
|
|
$
|
72,344
|
|
|
|
Employee compensation and benefits
|
54,930
|
|
43,363
|
|
|||
|
Royalties
|
8,058
|
|
11,643
|
|
|||
|
Accrued interest
|
7,297
|
|
5,179
|
|
|||
|
Derivative instrument liabilities
|
5,409
|
|
—
|
|
|||
|
Sales returns reserve
|
3,651
|
|
4,366
|
|
|||
|
Professional fees
|
3,213
|
|
4,596
|
|
|||
|
Selling and marketing accruals
|
3,189
|
|
3,924
|
|
|||
|
Inventory-related accruals
|
3,002
|
|
3,350
|
|
|||
|
Accrued construction-in-progress
|
2,827
|
|
1,597
|
|
|||
|
Clinical trial accruals
|
2,181
|
|
10,139
|
|
|||
|
Accrued contract termination fees
|
—
|
|
11,612
|
|
|||
|
Other
|
23,654
|
|
21,155
|
|
|||
|
Total accrued liabilities
|
$
|
198,779
|
|
$
|
193,268
|
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
2021 Notes
|
$
|
575,000
|
|
$
|
575,000
|
|
|
|
Unamortized discount and debt issuance costs on 2021 Notes
|
(81,627
|
)
|
(101,094
|
)
|
|||
|
2021 Notes, net
|
493,373
|
|
473,906
|
|
|||
|
2024 Notes
|
575,000
|
|
—
|
|
|||
|
Unamortized discount and debt issuance costs on 2024 Notes
|
(158,680
|
)
|
—
|
|
|||
|
2024 Notes, net
|
416,320
|
|
—
|
|
|||
|
Term loan
|
671,345
|
|
705,719
|
|
|||
|
Borrowings under revolving credit facility
|
—
|
|
850,000
|
|
|||
|
Total debt
|
1,581,038
|
|
2,029,625
|
|
|||
|
Less current portion
|
40,605
|
|
36,094
|
|
|||
|
Total long-term debt
|
$
|
1,540,433
|
|
$
|
1,993,531
|
|
|
|
Year Ending December 31,
|
Scheduled Long-Term Debt Maturities
|
||
|
2018
|
$
|
40,605
|
|
|
2019
|
58,652
|
|
|
|
2020
|
76,700
|
|
|
|
2021
|
1,075,801
|
|
|
|
Thereafter
|
575,000
|
|
|
|
Total
|
$
|
1,826,758
|
|
|
Year Ending December 31,
|
Lease Payments
|
||
|
2018
|
$
|
6,297
|
|
|
2019
|
9,142
|
|
|
|
2020
|
14,392
|
|
|
|
2021
|
14,824
|
|
|
|
2022
|
15,269
|
|
|
|
Thereafter
|
135,473
|
|
|
|
Total
|
$
|
195,397
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Lease expense
|
$
|
14,982
|
|
$
|
11,600
|
|
$
|
10,479
|
|
||
|
Year ending December 31,
|
Lease
Payments
|
||
|
2018
|
$
|
9,102
|
|
|
2019
|
8,166
|
|
|
|
2020
|
6,111
|
|
|
|
2021
|
5,212
|
|
|
|
2022
|
5,007
|
|
|
|
Thereafter
|
12,753
|
|
|
|
Total
|
$
|
46,351
|
|
|
December 31, 2017
|
||
|
2011 Equity Incentive Plan
|
16,026
|
|
|
Amended and Restated 2007 Non-Employee Directors Stock Award Plan
|
481
|
|
|
2007 Employee Stock Purchase Plan
|
339
|
|
|
Amended and Restated Directors Deferred Compensation Plan
|
178
|
|
|
2007 Equity Incentive Plan
|
17
|
|
|
Total
|
17,041
|
|
|
Net Unrealized
Gain (Loss) From Hedging Activities |
Foreign
Currency Translation Adjustments |
Total
Accumulated Other Comprehensive Loss |
|||||||||
|
Balance at December 31, 2016
|
$
|
—
|
|
$
|
(317,333
|
)
|
$
|
(317,333
|
)
|
||
|
Other comprehensive income (loss) before reclassifications
|
(213
|
)
|
174,973
|
|
174,760
|
|
|||||
|
Amounts reclassified from accumulated other comprehensive loss
|
1,695
|
|
—
|
|
1,695
|
|
|||||
|
Other comprehensive income, net
|
1,482
|
|
174,973
|
|
176,455
|
|
|||||
|
Balance at December 31, 2017
|
$
|
1,482
|
|
$
|
(142,360
|
)
|
$
|
(140,878
|
)
|
||
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Xyrem
|
$
|
1,186,699
|
|
$
|
1,107,616
|
|
$
|
955,187
|
|
||
|
Erwinaze/Erwinase
|
197,340
|
|
200,678
|
|
203,261
|
|
|||||
|
Defitelio/defibrotide
|
133,650
|
|
108,952
|
|
70,731
|
|
|||||
|
Vyxeos
|
33,790
|
|
—
|
|
—
|
|
|||||
|
Prialt
®
(ziconotide) intrathecal infusion
|
27,361
|
|
29,120
|
|
26,440
|
|
|||||
|
Other
|
22,559
|
|
30,895
|
|
61,200
|
|
|||||
|
Product sales, net
|
1,601,399
|
|
1,477,261
|
|
1,316,819
|
|
|||||
|
Royalties and contract revenues
|
17,294
|
|
10,712
|
|
7,984
|
|
|||||
|
Total revenues
|
$
|
1,618,693
|
|
$
|
1,487,973
|
|
$
|
1,324,803
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
United States
|
$
|
1,463,457
|
|
$
|
1,354,921
|
|
$
|
1,192,879
|
|
||
|
Europe
|
122,789
|
|
106,146
|
|
103,614
|
|
|||||
|
All other
|
32,447
|
|
26,906
|
|
28,310
|
|
|||||
|
Total revenues
|
$
|
1,618,693
|
|
$
|
1,487,973
|
|
$
|
1,324,803
|
|
||
|
Year Ended December 31,
|
||||||||
|
2017
|
2016
|
2015
|
||||||
|
Express Scripts
|
73
|
%
|
74
|
%
|
72
|
%
|
||
|
McKesson
|
16
|
%
|
15
|
%
|
7
|
%
|
||
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
United States
|
$
|
95,570
|
|
$
|
35,791
|
|
|
|
Ireland
|
64,343
|
|
62,453
|
|
|||
|
Italy
|
8,220
|
|
7,000
|
|
|||
|
Other
|
1,947
|
|
2,246
|
|
|||
|
Total long-lived assets (1)
|
$
|
170,080
|
|
$
|
107,490
|
|
|
|
(1)
|
Long-lived assets consist of property and equipment.
|
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Grant date fair value
|
$
|
42.72
|
|
$
|
40.45
|
|
$
|
57.19
|
|
||
|
Volatility
|
35
|
%
|
39
|
%
|
39
|
%
|
|||||
|
Expected term (years)
|
4.3
|
|
4.2
|
|
4.2
|
|
|||||
|
Range of risk-free rates
|
1.6-2.1%
|
|
0.8-1.6%
|
|
1.1-1.5%
|
|
|||||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
|||||
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Selling, general and administrative
|
$
|
83,218
|
|
$
|
79,037
|
|
$
|
74,653
|
|
||
|
Research and development
|
17,870
|
|
15,296
|
|
13,356
|
|
|||||
|
Cost of product sales
|
5,812
|
|
4,438
|
|
3,541
|
|
|||||
|
Total share-based compensation expense, pre-tax
|
106,900
|
|
98,771
|
|
91,550
|
|
|||||
|
Income tax benefit from share-based compensation expense (1)
|
(21,792
|
)
|
(30,022
|
)
|
(20,071
|
)
|
|||||
|
Total share-based compensation expense, net of tax
|
$
|
85,108
|
|
$
|
68,749
|
|
$
|
71,479
|
|
||
|
(1)
|
Following adoption of ASU No. 2016-09 “Improvements to Employee Share-Based Payment Accounting” in 2016, the 2017 and 2016 income tax benefit includes excess tax benefits recognized.
|
|
Shares
Subject to
Outstanding
Options
(In thousands)
|
Weighted-
Average
Exercise
Price
|
Weighted-
Average
Remaining
Contractual
Term (Years)
|
Aggregate
Intrinsic
Value
(In thousands)
|
|||||||||
|
Outstanding at January 1, 2017
|
4,513
|
|
$
|
111.52
|
|
|||||||
|
Options granted
|
1,402
|
|
137.46
|
|
||||||||
|
Options exercised
|
(428
|
)
|
53.03
|
|
||||||||
|
Options forfeited
|
(241
|
)
|
140.04
|
|
||||||||
|
Options expired
|
(101
|
)
|
165.49
|
|
||||||||
|
Outstanding at December 31, 2017
|
5,145
|
|
121.06
|
|
6.8
|
$
|
124,032
|
|
||||
|
Vested and expected to vest at December 31, 2017
|
4,896
|
|
120.27
|
|
6.7
|
123,206
|
|
|||||
|
Exercisable at December 31, 2017
|
2,976
|
|
108.45
|
|
5.6
|
116,822
|
|
|||||
|
Number of RSUs (in thousands)
|
Weighted-
Average Grant-Date Fair Value |
Weighted-
Average Remaining Contractual Term (Years) |
Aggregate
Intrinsic Value (In thousands) |
|||||||||
|
Outstanding at January 1, 2017
|
997
|
|
$
|
137.50
|
|
|||||||
|
RSUs granted
|
561
|
|
137.46
|
|
||||||||
|
RSUs released
|
(385
|
)
|
128.19
|
|
||||||||
|
RSUs forfeited
|
(110
|
)
|
141.18
|
|
||||||||
|
Outstanding at December 31, 2017
|
1,063
|
|
140.46
|
|
1.3
|
$
|
143,113
|
|
||||
|
Termination Benefits
|
Facility Closure Costs
|
Total
|
|||||||||
|
Balance at December 31, 2014
|
$
|
1,823
|
|
$
|
118
|
|
$
|
1,941
|
|
||
|
Expense
|
1,469
|
|
172
|
|
1,641
|
|
|||||
|
Payments
|
(2,187
|
)
|
(290
|
)
|
(2,477
|
)
|
|||||
|
Balance at December 31, 2015
|
1,105
|
|
—
|
|
1,105
|
|
|||||
|
Expense
|
1,516
|
|
—
|
|
1,516
|
|
|||||
|
Payments
|
(2,590
|
)
|
—
|
|
(2,590
|
)
|
|||||
|
Balance at December 31, 2016
|
31
|
|
—
|
|
31
|
|
|||||
|
Expense
|
14
|
|
—
|
|
14
|
|
|||||
|
Payments
|
(45
|
)
|
—
|
|
(45
|
)
|
|||||
|
Balance at December 31, 2017
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Ireland
|
$
|
77,476
|
|
$
|
179,570
|
|
$
|
233,785
|
|
||
|
United States
|
271,440
|
|
312,904
|
|
285,420
|
|
|||||
|
Other
|
92,201
|
|
39,972
|
|
(83,272
|
)
|
|||||
|
Total
|
$
|
441,117
|
|
$
|
532,446
|
|
$
|
435,933
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Current
|
|||||||||||
|
Ireland
|
$
|
28,045
|
|
$
|
26,420
|
|
$
|
29,748
|
|
||
|
United States
|
135,608
|
|
140,061
|
|
116,301
|
|
|||||
|
Other
|
14,198
|
|
9,918
|
|
28,708
|
|
|||||
|
Total current tax expense
|
177,851
|
|
176,399
|
|
174,757
|
|
|||||
|
Deferred, exclusive of other components below
|
|||||||||||
|
Ireland
|
(19,709
|
)
|
(7,776
|
)
|
(6,655
|
)
|
|||||
|
United States
|
(27,559
|
)
|
(9,120
|
)
|
332
|
|
|||||
|
Other
|
(19,108
|
)
|
(13,720
|
)
|
(40,532
|
)
|
|||||
|
Total deferred, exclusive of other components
|
(66,376
|
)
|
(30,616
|
)
|
(46,855
|
)
|
|||||
|
Deferred, change in tax rates
|
|||||||||||
|
United States
|
(155,679
|
)
|
109
|
|
294
|
|
|||||
|
Other
|
(3,536
|
)
|
(10,656
|
)
|
(21,797
|
)
|
|||||
|
Total deferred, change in tax rates
|
(159,215
|
)
|
(10,547
|
)
|
(21,503
|
)
|
|||||
|
Total deferred tax benefit
|
(225,591
|
)
|
(41,163
|
)
|
(68,358
|
)
|
|||||
|
Total income tax provision (benefit)
|
$
|
(47,740
|
)
|
$
|
135,236
|
|
$
|
106,399
|
|
||
|
Year Ended December 31,
|
||||||||
|
2017
|
2016
|
2015
|
||||||
|
Statutory income tax rate
|
12.5
|
%
|
12.5
|
%
|
12.5
|
%
|
||
|
Impact of U.S. Tax Act
|
(33.7
|
)%
|
—
|
%
|
—
|
%
|
||
|
Foreign income tax rate differential
|
20.3
|
%
|
16.7
|
%
|
19.1
|
%
|
||
|
Financing costs
|
(5.6
|
)%
|
(2.9
|
)%
|
(0.4
|
)%
|
||
|
Change in unrecognized tax benefits
|
2.8
|
%
|
3.3
|
%
|
3.6
|
%
|
||
|
Change in valuation allowance
|
(2.8
|
)%
|
(0.1
|
)%
|
(0.6
|
)%
|
||
|
Non-deductible compensation
|
2.6
|
%
|
1.8
|
%
|
1.9
|
%
|
||
|
Research and other tax credits
|
(2.6
|
)%
|
(2.8
|
)%
|
(3.8
|
)%
|
||
|
Change in estimates
|
(2.1
|
)%
|
—
|
%
|
(1.0
|
)%
|
||
|
Excess tax benefits from share-based compensation
|
(1.5
|
)%
|
(1.5
|
)%
|
—
|
%
|
||
|
Deduction on subsidiary equity
|
(0.7
|
)%
|
(2.4
|
)%
|
(2.7
|
)%
|
||
|
Change in tax rate
|
(0.4
|
)%
|
(1.8
|
)%
|
(4.5
|
)%
|
||
|
Acquisition-related costs
|
—
|
%
|
2.1
|
%
|
—
|
%
|
||
|
Other
|
0.4
|
%
|
0.5
|
%
|
0.3
|
%
|
||
|
Effective income tax rate
|
(10.8
|
)%
|
25.4
|
%
|
24.4
|
%
|
||
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Deferred tax assets:
|
|||||||
|
Net operating loss carryforwards
|
$
|
125,966
|
|
$
|
202,758
|
|
|
|
Tax credit carryforwards
|
130,782
|
|
114,192
|
|
|||
|
Intangible assets
|
23,536
|
|
15,965
|
|
|||
|
Share-based compensation
|
23,128
|
|
27,522
|
|
|||
|
Accruals
|
45,088
|
|
49,187
|
|
|||
|
Other
|
92,968
|
|
56,739
|
|
|||
|
Total deferred tax assets
|
441,468
|
|
466,363
|
|
|||
|
Valuation allowance
|
(52,144
|
)
|
(53,184
|
)
|
|||
|
Net deferred tax assets
|
389,324
|
|
413,179
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Acquired intangible assets
|
(655,347
|
)
|
(910,460
|
)
|
|||
|
Other
|
(82,890
|
)
|
(44,392
|
)
|
|||
|
Total deferred tax liabilities
|
(738,237
|
)
|
(954,852
|
)
|
|||
|
Net deferred tax liabilities
|
$
|
(348,913
|
)
|
$
|
(541,673
|
)
|
|
|
Year Ended December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Deferred tax assets
|
$
|
34,559
|
|
$
|
15,060
|
|
|
|
Deferred tax liabilities
|
(383,472
|
)
|
(556,733
|
)
|
|||
|
Net deferred tax liabilities
|
$
|
(348,913
|
)
|
$
|
(541,673
|
)
|
|
|
|
December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Balance at the beginning of the year
|
$
|
90,910
|
|
$
|
66,385
|
|
$
|
40,802
|
|
||
|
Increases related to current year tax positions
|
27,875
|
|
26,873
|
|
23,664
|
|
|||||
|
Increases related to prior year tax positions
|
1,620
|
|
1,191
|
|
2,833
|
|
|||||
|
Decreases related to prior year tax positions
|
(1,075
|
)
|
(255
|
)
|
(646
|
)
|
|||||
|
Lapse of the applicable statute of limitations
|
(13,168
|
)
|
(3,284
|
)
|
(268
|
)
|
|||||
|
Balance at the end of the year
|
$
|
106,162
|
|
$
|
90,910
|
|
$
|
66,385
|
|
||
|
|
2017
|
||||||||||||||
|
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
Revenues
|
$
|
376,053
|
|
$
|
394,386
|
|
$
|
411,855
|
|
$
|
436,399
|
|
|||
|
Gross margin (1)
|
348,613
|
|
360,983
|
|
376,768
|
|
404,847
|
|
|||||||
|
Net income
|
86,511
|
|
105,604
|
|
63,526
|
|
232,207
|
|
|||||||
|
Net income per ordinary share, basic
|
1.44
|
|
1.76
|
|
1.06
|
|
3.87
|
|
|||||||
|
Net income per ordinary share, diluted
|
1.41
|
|
1.72
|
|
1.03
|
|
3.79
|
|
|||||||
|
2016
|
|||||||||||||||
|
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
Revenues
|
$
|
336,010
|
|
$
|
381,161
|
|
$
|
374,181
|
|
$
|
396,621
|
|
|||
|
Gross margin (1)
|
310,477
|
|
355,130
|
|
347,310
|
|
358,958
|
|
|||||||
|
Net income
|
75,812
|
|
114,502
|
|
89,828
|
|
116,689
|
|
|||||||
|
Net income per ordinary share, basic
|
1.24
|
|
1.89
|
|
1.49
|
|
1.95
|
|
|||||||
|
Net income per ordinary share, diluted
|
1.21
|
|
1.85
|
|
1.45
|
|
1.91
|
|
|||||||
|
(1)
|
Gross margin is computed by subtracting cost of product sales (excluding amortization and impairment of intangible assets) from product sales, net.
|
|
•
|
Upfront and milestone payments of
$75.0 million
and
$26.5 million
in the third and fourth quarters of 2017, respectively, and
$8.8 million
and
$15.0 million
in the first and third quarters of 2016, respectively;
|
|
•
|
Expenses related to certain legal proceedings and restructuring of
$6.0 million
in the first quarter of 2017 and
$6.1 million
in the first quarter of 2016;
|
|
•
|
Transaction and integration related costs of
$2.2 million
,
$10.8 million
and
$0.7 million
in the second, third and fourth quarters of 2016, respectively;
|
|
•
|
A loss on extinguishment and modification of debt of
$0.6 million
in the third quarter of 2016;
|
|
•
|
A one-time charge of
$11.6 million
in respect of a contract termination in the fourth quarter of 2016; and
|
|
•
|
A net benefit of
$148.8 million
in respect of the impact of the U.S. Tax Act in the fourth quarter of 2017.
|
|
Balance at
beginning
of period
|
Additions
charged to
costs and
expenses
|
Other Additions
|
Deductions
|
Balance at
end of
period
|
|||||||||||||||||
|
For the year ended December 31, 2017
|
|||||||||||||||||||||
|
Allowance for doubtful accounts
|
(1)
|
$
|
287
|
|
$
|
231
|
|
$
|
—
|
|
$
|
(122
|
)
|
$
|
396
|
|
|||||
|
Allowance for sales discounts
|
(1)
|
118
|
|
1,087
|
|
—
|
|
(1,102
|
)
|
103
|
|
||||||||||
|
Allowance for chargebacks
|
(1)
|
4,749
|
|
41,941
|
|
—
|
|
(43,027
|
)
|
3,663
|
|
||||||||||
|
Deferred tax asset valuation allowance
|
(2)(3)(4)
|
53,184
|
|
7,509
|
|
5,581
|
|
(14,130
|
)
|
52,144
|
|
||||||||||
|
For the year ended December 31, 2016
|
|||||||||||||||||||||
|
Allowance for doubtful accounts
|
(1)
|
$
|
489
|
|
$
|
168
|
|
$
|
—
|
|
$
|
(370
|
)
|
$
|
287
|
|
|||||
|
Allowance for sales discounts
|
(1)
|
181
|
|
1,334
|
|
—
|
|
(1,397
|
)
|
118
|
|
||||||||||
|
Allowance for chargebacks
|
(1)
|
3,023
|
|
41,991
|
|
—
|
|
(40,265
|
)
|
4,749
|
|
||||||||||
|
Deferred tax asset valuation allowance
|
(2)(3)(4)
|
33,949
|
|
19,328
|
|
5,544
|
|
(5,637
|
)
|
53,184
|
|
||||||||||
|
For the year ended December 31, 2015
|
|||||||||||||||||||||
|
Allowance for doubtful accounts
|
(1)
|
$
|
530
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(41
|
)
|
$
|
489
|
|
|||||
|
Allowance for sales discounts
|
(1)
|
238
|
|
2,900
|
|
—
|
|
(2,957
|
)
|
181
|
|
||||||||||
|
Allowance for chargebacks
|
(1)
|
2,715
|
|
39,079
|
|
—
|
|
(38,771
|
)
|
3,023
|
|
||||||||||
|
Deferred tax asset valuation allowance
|
(2)(3)(4)
|
29,697
|
|
5,044
|
|
1,888
|
|
(2,680
|
)
|
33,949
|
|
||||||||||
|
(1)
|
Shown as a reduction of accounts receivable. Charges related to sales discounts and chargebacks are reflected as a reduction of revenue.
|
|
(2)
|
Additions to the deferred tax asset valuation allowance relate to movements on certain Irish, U.S. (federal and state) and other foreign deferred tax assets where we continue to maintain a valuation allowance until sufficient positive evidence exists to support reversal.
|
|
(3)
|
Deductions to the deferred tax asset valuation allowance include movements relating to utilization of NOLs and tax credit carryforwards, release in valuation allowance and other movements including adjustments following finalization of tax returns.
|
|
(4)
|
Other additions to the deferred tax asset valuation allowance relate to currency translation adjustments recorded directly in other comprehensive income and a valuation allowance recognized in 2016 on purchase accounting.
|