|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the fiscal year ended January 1, 2017
|
Commission file number 1-3215
|
|
New Jersey
|
|
22-1024240
|
|
(State of incorporation)
|
|
(I.R.S. Employer Identification No.)
|
|
One Johnson & Johnson Plaza
New Brunswick, New Jersey
|
|
08933
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Common Stock, Par Value $1.00
|
New York Stock Exchange
New York Stock Exchange
|
|
|
4.75% Notes Due November 2019
|
New York Stock Exchange
|
|
|
0.250% Notes Due January 2022
|
New York Stock Exchange
|
|
|
0.650% Notes Due May 2024
|
New York Stock Exchange
|
|
|
5.50% Notes Due November 2024
|
New York Stock Exchange
|
|
|
1.150% Notes Due November 2028
|
New York Stock Exchange
|
|
|
1.650% Notes Due May 2035
|
New York Stock Exchange
|
|
|
Parts I and III:
|
Portions of registrant’s proxy statement for its 2017 annual meeting of shareholders filed within 120 days after the close of the registrant’s fiscal year (the "Proxy Statement"), are incorporated by reference to this report on Form 10-K (this "Report").
|
|
Item
|
|
Page
|
|
1
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
1A.
|
||
|
1B.
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
|
||
|
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
7A.
|
||
|
8
|
||
|
9
|
||
|
9A.
|
||
|
9B.
|
||
|
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
|
||
|
15
|
||
|
16
|
||
|
|
||
|
|
||
|
•
|
Challenges and uncertainties inherent in innovation and development of new and improved products and technologies on which the Company’s continued growth and success depend, including uncertainty of clinical outcomes, obtaining regulatory approvals, health plan coverage and customer access, and initial and continued commercial success;
|
|
•
|
Challenges to the Company’s ability to obtain and protect adequate patent and other intellectual property rights for new and existing products and technologies in the U.S. and other important markets;
|
|
•
|
The impact of patent expirations, typically followed by the introduction of competing biosimilars and generics and resulting revenue and market share losses;
|
|
•
|
Increasingly aggressive and frequent challenges to the Company’s patents by competitors and others seeking to launch competing generic, biosimilar or other products, potentially resulting in loss of market exclusivity and rapid decline in sales for the relevant product;
|
|
•
|
Competition in research and development of new and improved products, processes and technologies, which can result in product and process obsolescence;
|
|
•
|
Competition to reach agreement with third parties for collaboration, licensing, development and marketing agreements for products and technologies;
|
|
•
|
Competition on the basis of cost-effectiveness, product performance, technological advances and patents attained by competitors; and
|
|
•
|
Allegations that the Company’s products infringe the patents and other intellectual property rights of third parties, which could adversely affect the Company’s ability to sell the products in question and require the payment of money damages and future royalties.
|
|
•
|
Product efficacy or safety concerns, whether or not based on scientific evidence, potentially resulting in product withdrawals, recalls, regulatory action on the part of the U.S. Food and Drug Administration (or international counterparts), declining sales and reputational damage;
|
|
•
|
Impact of significant litigation or government action adverse to the Company, including product liability claims;
|
|
•
|
Increased scrutiny of the health care industry by government agencies and state attorneys general resulting in investigations and prosecutions, which carry the risk of significant civil and criminal penalties, including, but not limited to, debarment from government business;
|
|
•
|
Failure to meet compliance obligations in the McNEIL-PPC, Inc. Consent Decree or the Corporate Integrity Agreements of the Johnson & Johnson Pharmaceutical Affiliates, or any other compliance agreements with governments or government agencies, which could result in significant sanctions;
|
|
•
|
Potential changes to applicable laws and regulations affecting U.S. and international operations, including relating to: approval of new products; licensing and patent rights; sales and promotion of health care products; access to, and reimbursement and pricing for, health care products and services; environmental protection and sourcing of raw materials;
|
|
•
|
Changes in tax laws and regulations, increasing audit scrutiny by tax authorities around the world and exposures to additional tax liabilities potentially in excess of reserves; and
|
|
•
|
Issuance of new or revised accounting standards by the Financial Accounting Standards Board and the Securities and Exchange Commission.
|
|
•
|
Pricing pressures resulting from trends toward health care cost containment, including the continued consolidation among health care providers, trends toward managed care and the shift toward governments increasingly becoming the primary payers of health care expenses;
|
|
•
|
Restricted spending patterns of individual, institutional and governmental purchasers of health care products and services due to economic hardship and budgetary constraints;
|
|
•
|
Challenges to the Company’s ability to realize its strategy for growth including through externally sourced innovations, such as development collaborations, strategic acquisitions, licensing and marketing agreements, and the potential heightened costs of any such external arrangements due to competitive pressures;
|
|
•
|
The potential that the expected strategic benefits and opportunities from any planned or completed acquisition or divestiture by the Company, including the planned acquisition of Actelion Ltd., may not be realized or may take longer to realize than expected;
|
|
•
|
The potential that the expected benefits and opportunities related to the planned restructuring actions in the Medical Device segment may not be realized or may take longer to realize than expected, including due to any required consultation procedures relating to restructuring of workforce; and
|
|
•
|
Market conditions and the possibility that the Company’s share repurchase program may be delayed, suspended or discontinued.
|
|
•
|
Impact of inflation and fluctuations in interest rates and currency exchange rates and the potential effect of such fluctuations on revenues, expenses and resulting margins;
|
|
•
|
Potential changes in export/import and trade laws, regulations and policies of the U.S., U.K. and other countries, including any increased trade restrictions and potential drug reimportation legislation;
|
|
•
|
The impact on international operations from financial instability in international economies, sovereign risk, possible imposition of governmental controls and restrictive economic policies, and unstable international governments and legal systems;
|
|
•
|
Changes to global climate, extreme weather and natural disasters that could affect demand for the Company's products and services, cause disruptions in manufacturing and distribution networks, alter the availability of goods and services within the supply chain, and affect the overall design and integrity of the Company's products and operations; and
|
|
•
|
The impact of armed conflicts and terrorist attacks in the U.S. and other parts of the world including social and economic disruptions and instability of financial and other markets.
|
|
•
|
Difficulties and delays in manufacturing, internally or within the supply chain, that may lead to voluntary or involuntary business interruptions or shutdowns, product shortages, withdrawals or suspensions of products from the market, and potential regulatory action;
|
|
•
|
Interruptions and breaches of the Company's information technology systems, and those of the Company's vendors, could result in reputational, competitive, operational or other business harm as well as financial costs and regulatory action; and
|
|
•
|
Reliance on global supply chains and production and distribution processes that are complex and subject to increasing regulatory requirements that may adversely affect supply, sourcing and pricing of materials used in the Company’s products.
|
|
Item 1.
|
BUSINESS
|
|
1
|
||
|
2
|
||
|
3
|
||
|
Item 1A.
|
RISK FACTORS
|
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
•
|
protective economic policies taken by governments such as trade protection measures and import/export licensing requirements;
|
|
•
|
compliance with local regulations and laws including, in some countries, regulatory requirements restricting the Company’s ability to manufacture or sell its products in the relevant market;
|
|
•
|
diminished protection of intellectual property and contractual rights in certain jurisdictions;
|
|
•
|
potential nationalization or expropriation of the Company’s foreign assets; and
|
|
•
|
disruptions to markets due to war, armed conflict, terrorism, social upheavals or pandemics.
|
|
8
|
||
|
Item 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
Segment
|
Square Feet
(in thousands)
|
||
|
Consumer
|
6,928
|
|
|
|
Pharmaceutical
|
7,463
|
|
|
|
Medical Devices
|
7,087
|
|
|
|
Worldwide Total
|
21,478
|
|
|
|
Geographic Area
|
Number of Facilities
|
Square Feet
(in thousands)
|
||||
|
United States
|
35
|
|
6,015
|
|
||
|
Europe
|
37
|
|
7,770
|
|
||
|
Western Hemisphere, excluding U.S.
|
14
|
|
2,862
|
|
||
|
Africa, Asia and Pacific
|
33
|
|
4,831
|
|
||
|
Worldwide Total
|
119
|
|
21,478
|
|
||
|
9
|
||
|
Item 3.
|
LEGAL PROCEEDINGS
|
|
Item 4.
|
MINE SAFETY DISCLOSURES
|
|
Name
|
Age
|
Position
|
||
|
Dominic J. Caruso
|
59
|
Member, Executive Committee; Executive Vice President; Chief Financial Officer(a)
|
||
|
Joaquin Duato
|
54
|
Member, Executive Committee; Executive Vice President, Worldwide Chairman, Pharmaceuticals(b)
|
||
|
Peter M. Fasolo
|
54
|
Member, Executive Committee; Executive Vice President, Chief Human Resources Officer(c)
|
||
|
Alex Gorsky
|
56
|
Chairman, Board of Directors; Chairman, Executive Committee; Chief Executive Officer
|
||
|
Jorge Mesquita
|
55
|
Member, Executive Committee; Executive Vice President, Worldwide Chairman, Consumer(d)
|
||
|
Sandra E. Peterson
|
58
|
Member, Executive Committee; Executive Vice President, Group Worldwide Chairman(e)
|
||
|
Gary Pruden
|
55
|
Member, Executive Committee; Executive Vice President, Worldwide Chairman, Medical Devices(f)
|
||
|
Paulus Stoffels
|
55
|
Member, Executive Committee; Executive Vice President, Chief Scientific Officer(g)
|
||
|
Michael H. Ullmann
|
58
|
Member, Executive Committee; Executive Vice President, General Counsel(h)
|
||
|
(a)
|
Mr. D. J. Caruso joined the Company in 1999 when the Company acquired Centocor, Inc. At the time of that acquisition, he had been Senior Vice President, Finance of Centocor. Mr. Caruso was named Vice President, Finance of Ortho-McNeil Pharmaceutical, Inc., a subsidiary of the Company, in 2001 and Vice President, Group Finance of the Company’s Medical Devices and Diagnostics Group in 2003. In 2005, Mr. Caruso was named Vice President of the Company’s Group Finance organization. Mr. Caruso became a member of the Executive Committee and Vice President, Finance and Chief Financial Officer in 2007. In April 2016, he was named Executive Vice President, Chief Financial Officer.
|
|
(b)
|
Mr. J. Duato joined the Company in 1989 with Janssen-Farmaceutica S.A. (Spain) and in 1997 became Managing Director of Janssen-Cilag S.p.A. (Italy). In 2000, he led Ortho Biotech Europe before relocating to the United States in 2002 to serve as Vice President, and, in 2005, President of Ortho Biotech Inc. In 2008, he was named Company Group Chairman, Ortho-Clinical Diagnostics, and in 2009 Company Group Chairman, Pharmaceuticals, where he oversaw
|
|
10
|
||
|
(c)
|
Dr. P. M. Fasolo joined the Company in 2004 as Vice President, Worldwide Human Resources for Cordis Corporation, a subsidiary of the Company. He was then named Vice President, Global Talent Management for the Company. He left Johnson & Johnson in 2007 to join Kohlberg Kravis Roberts & Co., as Chief Talent Officer. Dr. Fasolo returned to the Company in 2010 as the Vice President, Global Human Resources, and in 2011, he became a member of the Executive Committee. In April 2016, he was named Executive Vice President, Chief Human Resources Officer.
|
|
(d)
|
Mr. J. Mesquita joined the Company in 2014 as Worldwide Chairman, Consumer. Prior to joining the Company, he served in various marketing and leadership capacities across Latin America, including roles in Oral Care and Beauty, at The Procter & Gamble Company from 1984 to 2013. In April 2016, Mr. Mesquita became a member of the Executive Committee and was named as Executive Vice President, Worldwide Chairman, Consumer.
|
|
(e)
|
Ms. S. E. Peterson joined the Company in 2012 as Group Worldwide Chairman and a member of the Executive Committee. She oversees the Consumer and Consumer Medical Device businesses; the Company's operating infrastructure — Supply Chain, Information Technology, Global Services; Health & Wellness; Global Design; and Health Technology. Prior to joining the Company, Ms. Peterson was Chairman and Chief Executive Officer of Bayer CropScience AG in Germany, previously serving as President and Chief Executive Officer of Bayer Medical Care and President of Bayer HealthCare AG's Diabetes Care Division. Before joining Bayer in 2005, Ms. Peterson held a number of leadership roles at Medco Health Solutions (previously known as Merck-Medco). In April 2016. Ms. Peterson was named Executive Vice President, Group Worldwide Chairman of Johnson & Johnson. Effective June 1, 2017, Ms. Peterson will assume leadership of the Hospital Medical Device business, in addition to her current responsibilities.
|
|
(f)
|
Mr. G. Pruden joined the Company in 1985 with Janssen Pharmaceutica.Inc. and held a number of senior positions in sales, marketing, and strategic account management. In April 2004, he became President of Janssen-Ortho Inc. in Canada. In January 2006, Mr. Pruden was appointed Worldwide President, Ethicon Products, and in 2009 became Company Group Chairman, Ethicon. In 2012, he was named Worldwide Chairman, Global Surgery Group, and in 2015, Worldwide Chairman, Medical Devices. In April 2016. Mr. Pruden became a member of the Executive Committee and was named Executive Vice President, Worldwide Chairman, Medical Devices. Mr. Pruden has announced his intention to retire from the Company effective June 1, 2017.
|
|
(g)
|
Dr. P. Stoffels joined the Company in 2002 with the acquisition of Tibotec Virco NV, where he was Chief Executive Officer of Virco NV and Chairman of Tibotec NV. In 2005, he was appointed Company Group Chairman, Global Virology. In 2006, he assumed the role of Company Group Chairman, Pharmaceuticals, with responsibility for worldwide research and development for the Central Nervous System and Internal Medicine Franchises. Dr. Stoffels was appointed Global Head, Research & Development, Pharmaceuticals in 2009, and in 2011, became Worldwide Chairman, Pharmaceuticals, with responsibility for the Company's therapeutic pipeline through global research and development and strategic business development. In 2012, Dr. Stoffels was also appointed Chief Scientific Officer, with responsibility for enterprise-wide innovation and product safety, and a member of the Executive Committee. In April 2016. Dr. Stoffels was named Executive Vice President, Chief Scientific Officer.
|
|
(h)
|
Mr. M. H. Ullmann joined the Company in 1989 as a corporate attorney in the Law Department. He was appointed Corporate Secretary in 1999 and served in that role until 2006. During that time, he also held various management positions in the Law Department. In 2006, he was named General Counsel, Medical Devices and Diagnostics and was appointed Vice President, General Counsel and a member of the Executive Committee in 2012. In April 2016, Mr. Ullmann was named Executive Vice President, General Counsel.
|
|
11
|
||
|
Item 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Period
|
Total Number
of Shares Purchased
(1)
|
Avg. Price
Paid Per Share
|
Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs
(2)
|
Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Plans or Programs
(3)
|
|||||||
|
October 3, 2016 through October 30, 2016
|
2,485,016
|
|
$
|
116.76
|
|
-
|
-
|
||||
|
October 31, 2016 through November 27, 2016
|
9,324,574
|
|
116.53
|
|
8,775,704
|
-
|
|||||
|
November 28, 2016 through January 1, 2017
|
5,739,190
|
|
113.35
|
|
3,400,003
|
-
|
|||||
|
Total
|
17,548,780
|
|
12,175,707
|
23,543,007
|
|||||||
|
(1)
|
During the fiscal fourth quarter of 2016, the Company repurchased an aggregate of 17,548,780 shares of Johnson & Johnson Common Stock in open-market transactions, of which 12,175,707 shares were purchased pursuant to the repurchase program that was publicly announced on October 13, 2015, and of which 5,373,073 shares were purchased in open-market transactions as part of a systematic plan to meet the needs of the Company’s compensation programs.
|
|
(2)
|
As of January 1, 2017, an aggregate of 65,362,675 shares were purchased for a total of $7.3 billion since the inception of the repurchase program announced on October 13, 2015.
|
|
(3)
|
As of January 1, 2017, the maximum number of shares that may yet be purchased under the plan is 23,543,007 based on the closing price of Johnson & Johnson Common Stock on the New York Stock Exchange on December 30, 2016 of $115.21 per share.
|
|
12
|
||
|
Item 6.
|
SELECTED FINANCIAL DATA
|
|
(Dollars in Millions Except Per Share Amounts)
|
2016
|
2015
|
2014
|
2013
|
2012
|
2011
|
2010
|
2009
|
2008
|
2007
|
2006
|
|||||||||||||||||||
|
Sales to customers — U.S.
|
$37,811
|
35,687
|
34,782
|
|
31,910
|
|
29,830
|
|
28,908
|
|
29,450
|
|
30,889
|
|
32,309
|
|
32,444
|
|
29,775
|
|
||||||||||
|
Sales to customers — International
|
34,079
|
34,387
|
39,549
|
|
39,402
|
|
37,394
|
|
36,122
|
|
32,137
|
|
31,008
|
|
31,438
|
|
28,651
|
|
23,549
|
|
||||||||||
|
Total sales
|
71,890
|
70,074
|
74,331
|
|
71,312
|
|
67,224
|
|
65,030
|
|
61,587
|
|
61,897
|
|
63,747
|
|
61,095
|
|
53,324
|
|
||||||||||
|
Cost of products sold
|
21,685
|
21,536
|
22,746
|
|
22,342
|
|
21,658
|
|
20,360
|
|
18,792
|
|
18,447
|
|
18,511
|
|
17,751
|
|
15,057
|
|
||||||||||
|
Selling, marketing and administrative expenses
|
19,945
|
21,203
|
21,954
|
|
21,830
|
|
20,869
|
|
20,969
|
|
19,424
|
|
19,801
|
|
21,490
|
|
20,451
|
|
17,433
|
|
||||||||||
|
Research and development expense
|
9,095
|
9,046
|
8,494
|
|
8,183
|
|
7,665
|
|
7,548
|
|
6,844
|
|
6,986
|
|
7,577
|
|
7,680
|
|
7,125
|
|
||||||||||
|
In-process research and development
|
29
|
224
|
178
|
|
580
|
|
1,163
|
|
—
|
|
—
|
|
—
|
|
181
|
|
807
|
|
559
|
|
||||||||||
|
Interest income
|
(368)
|
(128)
|
(67
|
)
|
(74
|
)
|
(64
|
)
|
(91
|
)
|
(107
|
)
|
(90
|
)
|
(361
|
)
|
(452
|
)
|
(829
|
)
|
||||||||||
|
Interest expense, net of portion capitalized
|
726
|
552
|
533
|
|
482
|
|
532
|
|
571
|
|
455
|
|
451
|
|
435
|
|
296
|
|
63
|
|
||||||||||
|
Other (income) expense, net
|
484
|
(2,064)
|
(70
|
)
|
2,498
|
|
1,626
|
|
2,743
|
|
(768
|
)
|
(526
|
)
|
(1,015
|
)
|
534
|
|
(671
|
)
|
||||||||||
|
Restructuring
|
491
|
509
|
—
|
|
—
|
|
—
|
|
569
|
|
—
|
|
1,073
|
|
—
|
|
745
|
|
—
|
|
||||||||||
|
|
52,087
|
50,878
|
53,768
|
|
55,841
|
|
53,449
|
|
52,669
|
|
44,640
|
|
46,142
|
|
46,818
|
|
47,812
|
|
38,737
|
|
||||||||||
|
Earnings before provision for taxes on income
|
$19,803
|
19,196
|
20,563
|
|
15,471
|
|
13,775
|
|
12,361
|
|
16,947
|
|
15,755
|
|
16,929
|
|
13,283
|
|
14,587
|
|
||||||||||
|
Provision for taxes on income
|
3,263
|
3,787
|
4,240
|
|
1,640
|
|
3,261
|
|
2,689
|
|
3,613
|
|
3,489
|
|
3,980
|
|
2,707
|
|
3,534
|
|
||||||||||
|
Net earnings
|
16,540
|
15,409
|
16,323
|
|
13,831
|
|
10,514
|
|
9,672
|
|
13,334
|
|
12,266
|
|
12,949
|
|
10,576
|
|
11,053
|
|
||||||||||
|
Add: Net loss attributable to noncontrolling interest
|
—
|
—
|
—
|
|
—
|
|
339
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Net earnings attributable to Johnson & Johnson
|
16,540
|
15,409
|
16,323
|
|
13,831
|
|
10,853
|
|
9,672
|
|
13,334
|
|
12,266
|
|
12,949
|
|
10,576
|
|
11,053
|
|
||||||||||
|
Percent of sales to customers
|
23.0%
|
22.0
|
22.0
|
|
19.4
|
|
16.1
|
|
14.9
|
|
21.7
|
|
19.8
|
|
20.3
|
|
17.3
|
|
20.7
|
|
||||||||||
|
Diluted net earnings per share of common stock
(1)
|
$5.93
|
5.48
|
5.70
|
|
4.81
|
|
3.86
|
|
3.49
|
|
4.78
|
|
4.40
|
|
4.57
|
|
3.63
|
|
3.73
|
|
||||||||||
|
Percent return on average shareholders’ equity
|
23.4%
|
21.9
|
22.7
|
|
19.9
|
|
17.8
|
|
17.0
|
|
24.9
|
|
26.4
|
|
30.2
|
|
25.6
|
|
28.3
|
|
||||||||||
|
Percent increase (decrease) over previous year:
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Sales to customers
|
2.6%
|
(5.7)
|
4.2
|
|
6.1
|
|
3.4
|
|
5.6
|
|
(0.5
|
)
|
(2.9
|
)
|
4.3
|
|
14.6
|
|
5.6
|
|
||||||||||
|
Diluted net earnings per share
|
8.2%
|
(3.9)
|
18.5
|
|
24.6
|
|
10.6
|
|
(27.0
|
)
|
8.6
|
|
(3.7
|
)
|
25.9
|
|
(2.7
|
)
|
11.3
|
|
||||||||||
|
Supplementary balance sheet data:
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Property, plant and equipment, net
|
15,912
|
15,905
|
16,126
|
|
16,710
|
|
16,097
|
|
14,739
|
|
14,553
|
|
14,759
|
|
14,365
|
|
14,185
|
|
13,044
|
|
||||||||||
|
Additions to property, plant and equipment
|
3,226
|
3,463
|
3,714
|
|
3,595
|
|
2,934
|
|
2,893
|
|
2,384
|
|
2,365
|
|
3,066
|
|
2,942
|
|
2,666
|
|
||||||||||
|
Total assets
|
141,208
|
133,411
|
130,358
|
|
131,754
|
|
121,347
|
|
113,644
|
|
102,908
|
|
94,682
|
|
84,912
|
|
80,954
|
|
70,556
|
|
||||||||||
|
Long-term debt
|
22,442
|
12,857
|
15,122
|
|
13,328
|
|
11,489
|
|
12,969
|
|
9,156
|
|
8,223
|
|
8,120
|
|
7,074
|
|
2,014
|
|
||||||||||
|
Operating cash flow
(2)
|
18,767
|
19,569
|
18,710
|
|
17,414
|
|
15,396
|
|
14,298
|
|
16,385
|
|
16,571
|
|
14,972
|
|
15,022
|
|
14,248
|
|
||||||||||
|
Common stock information
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Dividends paid per share
|
$3.15
|
2.95
|
2.76
|
|
2.59
|
|
2.40
|
|
2.25
|
|
2.11
|
|
1.93
|
|
1.795
|
|
1.62
|
|
1.455
|
|
||||||||||
|
Shareholders’ equity per share
|
26.02
|
25.82
|
25.06
|
|
26.25
|
|
23.33
|
|
20.95
|
|
20.66
|
|
18.37
|
|
15.35
|
|
15.25
|
|
13.59
|
|
||||||||||
|
Market price per share (year-end close)
|
$115.21
|
102.72
|
105.06
|
|
92.35
|
|
69.48
|
|
65.58
|
|
61.85
|
|
64.41
|
|
58.56
|
|
67.38
|
|
66.02
|
|
||||||||||
|
Average shares outstanding (millions)
|
||||||||||||||||||||||||||||||
|
— basic
|
2,737.3
|
2,771.8
|
2,815.2
|
|
2,809.2
|
|
2,753.3
|
|
2,736.0
|
|
2,751.4
|
|
2,759.5
|
|
2,802.5
|
|
2,882.9
|
|
2,936.4
|
|
||||||||||
|
— diluted
|
2,788.9
|
2,812.9
|
2,863.9
|
|
2,877.0
|
|
2,812.6
|
|
2,775.3
|
|
2,788.8
|
|
2,789.1
|
|
2,835.6
|
|
2,910.7
|
|
2,961.0
|
|
||||||||||
|
Employees (thousands)
|
126.4
|
127.1
|
126.5
|
|
128.1
|
|
127.6
|
|
117.9
|
|
114.0
|
|
115.5
|
|
118.7
|
|
119.2
|
|
122.2
|
|
||||||||||
|
13
|
||
|
Item 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
|
|
14
|
||
|
Sales increase/(decrease) due to:
|
2016
|
2015
|
2014
|
||||||
|
Volume
|
3.2
|
%
|
1.2
|
%
|
6.3
|
|
|||
|
Price
|
0.7
|
|
0.6
|
|
(0.2
|
)
|
|||
|
Currency
|
(1.3
|
)
|
(7.5
|
)
|
(1.9
|
)
|
|||
|
Total
|
2.6
|
%
|
(5.7
|
)%
|
4.2
|
|
|||
|
15
|
||
|
|
|
|
|
% Change
|
||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
’16 vs. ’15
|
’15 vs. ’14
|
|||||||||||
|
OTC
|
$
|
3,977
|
|
3,895
|
|
4,016
|
|
2.1
|
%
|
(3.0
|
)
|
|||||
|
Beauty**
|
3,897
|
|
3,633
|
|
3,873
|
|
7.3
|
|
(6.2
|
)
|
||||||
|
Baby Care
|
2,001
|
|
2,157
|
|
2,346
|
|
(7.2
|
)
|
(8.1
|
)
|
||||||
|
Oral Care
|
1,568
|
|
1,580
|
|
1,647
|
|
(0.8
|
)
|
(4.1
|
)
|
||||||
|
Women’s Health
|
1,067
|
|
1,200
|
|
1,302
|
|
(11.1
|
)
|
(7.8
|
)
|
||||||
|
Wound Care/Other
|
797
|
|
1,042
|
|
1,312
|
|
(23.5
|
)
|
(20.6
|
)
|
||||||
|
Total Consumer Sales
|
$
|
13,307
|
|
13,507
|
|
14,496
|
|
(1.5
|
)%
|
(6.8
|
)
|
|||||
|
16
|
||
|
% Change
|
||||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
’16 vs. ’15
|
’15 vs. ’14
|
|||||||||||
|
Total Immunology
|
$
|
11,968
|
|
10,402
|
|
10,193
|
|
15.1
|
%
|
2.1
|
|
|||||
|
REMICADE
®
|
6,966
|
|
6,561
|
|
6,868
|
|
6.2
|
|
(4.5
|
)
|
||||||
|
SIMPONI
®
/SIMPONI ARIA
®
|
1,745
|
|
1,328
|
|
1,187
|
|
31.4
|
|
11.9
|
|
||||||
|
STELARA
®
|
3,232
|
|
2,474
|
|
2,072
|
|
30.6
|
|
19.4
|
|
||||||
|
Other Immunology
|
25
|
|
39
|
|
66
|
|
(35.9
|
)
|
(40.9
|
)
|
||||||
|
Total Infectious Diseases
|
3,208
|
|
3,656
|
|
5,599
|
|
(12.3
|
)
|
(34.7
|
)
|
||||||
|
EDURANT
®
/rilpivirine
|
573
|
|
410
|
|
365
|
|
39.8
|
|
12.3
|
|
||||||
|
OLYSIO
®
/SOVRIAD
®
|
106
|
|
621
|
|
2,302
|
|
(82.9
|
)
|
(73.0
|
)
|
||||||
|
PREZISTA
®
/ PREZCOBIX
®
/REZOLSTA
®
|
1,851
|
|
1,810
|
|
1,831
|
|
2.3
|
|
(1.1
|
)
|
||||||
|
Other Infectious Diseases
|
678
|
|
815
|
|
1,101
|
|
(16.8
|
)
|
(26.0
|
)
|
||||||
|
Total Neuroscience
|
6,085
|
|
6,259
|
|
6,487
|
|
(2.8
|
)
|
(3.5
|
)
|
||||||
|
CONCERTA
®
/methylphenidate
|
863
|
|
821
|
|
599
|
|
5.1
|
|
37.1
|
|
||||||
|
INVEGA
®
/paliperidone
|
311
|
|
573
|
|
640
|
|
(45.7
|
)
|
(10.5
|
)
|
||||||
|
INVEGA SUSTENNA
®
/XEPLION
®
/TRINZA
®
|
2,214
|
|
1,830
|
|
1,588
|
|
21.0
|
|
15.2
|
|
||||||
|
RISPERDAL
®
CONSTA
®
|
893
|
|
970
|
|
1,190
|
|
(7.9
|
)
|
(18.5
|
)
|
||||||
|
Other Neuroscience
|
1,804
|
|
2,065
|
|
2,470
|
|
(12.6
|
)
|
(16.4
|
)
|
||||||
|
Total Oncology
|
5,807
|
|
4,695
|
|
4,457
|
|
23.7
|
|
5.3
|
|
||||||
|
DARZALEX
®
|
572
|
|
20
|
|
—
|
|
**
|
|
—
|
|
||||||
|
IMBRUVICA
®
|
1,251
|
|
689
|
|
200
|
|
81.6
|
|
**
|
|
||||||
|
VELCADE
®
|
1,224
|
|
1,333
|
|
1,618
|
|
(8.2
|
)
|
(17.6
|
)
|
||||||
|
ZYTIGA
®
|
2,260
|
|
2,231
|
|
2,237
|
|
1.3
|
|
(0.3
|
)
|
||||||
|
Other Oncology
|
500
|
|
422
|
|
402
|
|
18.5
|
|
5.0
|
|
||||||
|
Cardiovascular / Metabolism / Other
|
6,396
|
|
6,418
|
|
5,577
|
|
(0.3
|
)
|
15.1
|
|
||||||
|
XARELTO
®
|
2,288
|
|
1,868
|
|
1,522
|
|
22.5
|
|
22.7
|
|
||||||
|
INVOKANA
®
/ INVOKAMET
®
|
1,407
|
|
1,308
|
|
586
|
|
7.6
|
|
**
|
|
||||||
|
PROCRIT
®
/EPREX
®
|
1,105
|
|
1,068
|
|
1,238
|
|
3.5
|
|
(13.7
|
)
|
||||||
|
Other
|
1,596
|
|
2,174
|
|
2,231
|
|
(26.6
|
)
|
(2.6
|
)
|
||||||
|
Total Pharmaceutical Sales
|
$
|
33,464
|
|
31,430
|
|
32,313
|
|
6.5
|
%
|
(2.7
|
)
|
|||||
|
17
|
||
|
18
|
||
|
Product Name (Chemical Name)
|
Indication
|
US Approv
|
EU Approv
|
US Filing
|
EU Filing
|
|
DARZALEX
®
(daratumumab)
|
In combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least one prior therapy
|
ü
|
ü
|
||
|
For the treatment of double refractory multiple myeloma
|
ü
|
||||
|
darunavir STR
|
Single tablet regimen for HIV in treatment naive patients and treatment experienced patients
|
ü
|
|||
|
guselkumab
|
Treatment of adults living with moderate to severe plaque psoriasis
|
ü
|
ü
|
||
|
IMBRUVICA
®
(ibrutinib)
|
Additional indication for first-line treatment of chronic lymphocytic leukemia
|
ü
|
ü
|
||
|
Expanded label to include overall survival and combination data in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL)
|
ü
|
||||
|
Expanded label to include treatment for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma in combination with bendamustine and rituximab
|
ü
|
ü
|
|||
|
INVOKAMET
®
(canagliflozin)
|
Initial Therapy FDC with Metformin, Immediate Release
|
ü
|
|||
|
INVOKAMET
®
XR (canagliflozin)
|
A once-daily therapy combining fixed doses of canagliflozin and metformin hydrochloride extended release for the treatment of adults with type 2 diabetes
|
ü
|
|||
|
SIMPONI
®
(golimumab)
|
Treatment of polyarticular juvenile idiopathic arthritis
|
ü
|
|||
|
SIMPONI ARIA
®
(golimumab)
|
Treatment of adults living with active psoriatic arthritis and the treatment of adults living with active ankylosing spondylitis
|
ü
|
|||
|
sirukumab
|
Treatment of Rheumatoid Arthritis
|
ü
|
ü
|
||
|
STELARA
®
(ustekinumab)
|
Treatment of adults with moderately to severely active Crohn's disease
|
ü
|
ü
|
||
|
Treatment of adolescents (12 to 17 years of age) with moderate to severe plaque psoriasis
|
ü
|
||||
|
TREVICTA
®
(paliperidone palmitate a 3 monthly injection)
|
Maintenance treatment of schizophrenia in adult patients
|
ü
|
|||
|
19
|
||
|
|
|
|
|
% Change
|
||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
’16 vs. ’15
|
’15 vs. ’14
|
|||||||||||
|
Orthopaedics
|
$
|
9,334
|
|
9,262
|
|
9,675
|
|
0.8
|
%
|
(4.3
|
)
|
|||||
|
Hips
|
1,361
|
|
1,332
|
|
1,368
|
|
2.2
|
|
(2.6
|
)
|
||||||
|
Knees
|
1,524
|
|
1,496
|
|
1,533
|
|
1.9
|
|
(2.4
|
)
|
||||||
|
Trauma
|
2,569
|
|
2,528
|
|
2,640
|
|
1.6
|
|
(4.2
|
)
|
||||||
|
Spine & Other
|
3,880
|
|
3,906
|
|
4,134
|
|
(0.7
|
)
|
(5.5
|
)
|
||||||
|
Surgery
|
9,296
|
|
9,217
|
|
9,717
|
|
0.9
|
|
(5.1
|
)
|
||||||
|
Advanced
|
3,517
|
|
3,275
|
|
3,237
|
|
7.4
|
|
1.2
|
|
||||||
|
General
|
4,362
|
|
4,482
|
|
4,970
|
|
(2.7
|
)
|
(9.8
|
)
|
||||||
|
Specialty
|
1,417
|
|
1,460
|
|
1,510
|
|
(2.9
|
)
|
(3.3
|
)
|
||||||
|
Vision Care
|
2,785
|
|
2,608
|
|
2,818
|
|
6.8
|
|
(7.5
|
)
|
||||||
|
Cardiovascular
|
1,849
|
|
2,036
|
|
2,208
|
|
(9.2
|
)
|
(7.8
|
)
|
||||||
|
Diabetes Care
|
1,789
|
|
1,928
|
|
2,142
|
|
(7.2
|
)
|
(10.0
|
)
|
||||||
|
Diagnostics*
|
66
|
|
86
|
|
962
|
|
(23.3
|
)
|
(91.1
|
)
|
||||||
|
Total Medical Devices Sales
|
$
|
25,119
|
|
25,137
|
|
27,522
|
|
(0.1
|
)%
|
(8.7
|
)
|
|||||
|
20
|
||
|
% of Sales
|
2016
|
2015
|
2014
|
||||||
|
Cost of products sold
|
30.2
|
%
|
30.7
|
|
30.6
|
|
|||
|
Percent point increase/(decrease) over the prior year
|
(0.5
|
)
|
0.1
|
|
(0.7
|
)
|
|||
|
Selling, marketing and administrative expenses
|
27.7
|
%
|
30.3
|
|
29.5
|
|
|||
|
Percent point increase/(decrease) over the prior year
|
(2.6
|
)
|
0.8
|
|
(1.1
|
)
|
|||
|
21
|
||
|
|
2016
|
2015
|
2014
|
||||||||||||||||
|
(Dollars in Millions)
|
Amount
|
% of Sales*
|
Amount
|
% of Sales*
|
Amount
|
% of Sales*
|
|||||||||||||
|
Consumer
|
$
|
580
|
|
4.4
|
%
|
625
|
|
4.6
|
|
629
|
|
4.3
|
|
||||||
|
Pharmaceutical
|
6,967
|
|
20.8
|
|
6,821
|
|
21.7
|
|
6,213
|
|
19.2
|
|
|||||||
|
Medical Devices
|
1,548
|
|
6.2
|
|
1,600
|
|
6.4
|
|
1,652
|
|
6.0
|
|
|||||||
|
Total research and development expense
|
$
|
9,095
|
|
12.7
|
%
|
9,046
|
|
12.9
|
|
8,494
|
|
11.4
|
|
||||||
|
Percent increase/(decrease) over the prior year
|
0.5
|
%
|
|
|
6.5
|
|
|
|
3.8
|
|
|
|
|||||||
|
*
|
As a percent to segment sales
|
|
22
|
||
|
|
Income Before Tax
|
Segment Sales
|
Percent of Segment Sales
|
||||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
|||||||||||||
|
Consumer
|
$
|
2,441
|
|
1,787
|
|
$
|
13,307
|
|
13,507
|
|
18.3
|
%
|
13.2
|
||||||
|
Pharmaceutical
|
12,827
|
|
11,734
|
|
33,464
|
|
31,430
|
|
38.3
|
|
37.3
|
||||||||
|
Medical Devices
|
5,578
|
|
6,826
|
|
25,119
|
|
25,137
|
|
22.2
|
|
27.2
|
||||||||
|
Total
(1)
|
20,846
|
|
20,347
|
|
71,890
|
|
70,074
|
|
29.0
|
|
29.0
|
||||||||
|
Less: Expenses not allocated to segments
(2)
|
1,043
|
|
1,151
|
|
|
|
|
||||||||||||
|
Earnings before provision for taxes on income
|
$
|
19,803
|
|
19,196
|
|
$
|
71,890
|
|
70,074
|
|
27.5
|
%
|
27.4
|
||||||
|
(1)
|
See Note 18 to the Consolidated Financial Statements for more details.
|
|
(2)
|
Amounts not allocated to segments include interest (income) expense and general corporate (income) expense.
|
|
23
|
||
|
24
|
||
|
25
|
||
|
(Dollars in Millions)
|
Debt Obligations
|
Interest on
Debt Obligations |
Unfunded
Retirement Plans |
Operating Leases
|
Total
|
|||||||||||
|
2017
|
$
|
1,704
|
|
799
|
|
83
|
|
216
|
|
2,802
|
|
|||||
|
2018
|
1,561
|
|
735
|
|
84
|
|
179
|
|
2,559
|
|
||||||
|
2019
|
2,538
|
|
680
|
|
89
|
|
134
|
|
3,441
|
|
||||||
|
2020
|
629
|
|
608
|
|
94
|
|
105
|
|
1,436
|
|
||||||
|
2021
|
1,795
|
|
574
|
|
100
|
|
88
|
|
2,557
|
|
||||||
|
After 2021
|
15,919
|
|
6,956
|
|
610
|
|
100
|
|
23,585
|
|
||||||
|
Total
|
$
|
24,146
|
|
10,352
|
|
1,060
|
|
822
|
|
36,380
|
|
|||||
|
2016
|
2015
|
2014
|
|||||||
|
First quarter
|
$
|
0.75
|
|
0.70
|
|
0.66
|
|
||
|
Second quarter
|
0.80
|
|
0.75
|
|
0.70
|
|
|||
|
Third quarter
|
0.80
|
|
0.75
|
|
0.70
|
|
|||
|
Fourth quarter
|
0.80
|
|
0.75
|
|
0.70
|
|
|||
|
Total
|
$
|
3.15
|
|
2.95
|
|
2.76
|
|
||
|
26
|
||
|
27
|
||
|
(Dollars in Millions)
|
Balance at
Beginning of Period |
Accruals
|
Payments/Credits
|
Balance at
End of Period |
|||||||||
|
2016
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
139
|
|
615
|
|
(618
|
)
|
136
|
|
||||
|
Accrued returns
|
54
|
|
111
|
|
(100
|
)
|
65
|
|
|||||
|
Accrued promotions
|
412
|
|
1,908
|
|
(1,962
|
)
|
358
|
|
|||||
|
Subtotal
|
$
|
605
|
|
2,634
|
|
(2,680
|
)
|
559
|
|
||||
|
Reserve for doubtful accounts
|
18
|
|
12
|
|
(6
|
)
|
24
|
|
|||||
|
Reserve for cash discounts
|
17
|
|
209
|
|
(201
|
)
|
25
|
|
|||||
|
Total
|
$
|
640
|
|
2,855
|
|
(2,887
|
)
|
608
|
|
||||
|
2015
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
122
|
|
581
|
|
(564
|
)
|
139
|
|
||||
|
Accrued returns
|
77
|
|
84
|
|
(107
|
)
|
54
|
|
|||||
|
Accrued promotions
|
241
|
|
1,846
|
|
(1,675
|
)
|
412
|
|
|||||
|
Subtotal
|
$
|
440
|
|
2,511
|
|
(2,346
|
)
|
605
|
|
||||
|
Reserve for doubtful accounts
|
18
|
|
5
|
|
(5
|
)
|
18
|
|
|||||
|
Reserve for cash discounts
|
22
|
|
206
|
|
(211
|
)
|
17
|
|
|||||
|
Total
|
$
|
480
|
|
2,722
|
|
(2,562
|
)
|
640
|
|
||||
|
(1)
|
Includes reserve for customer rebates of $37 million at
January 1, 2017
and $31 million at
January 3, 2016
, recorded as a contra asset.
|
|
(Dollars in Millions)
|
Balance at
Beginning of Period |
Accruals
|
Payments/Credits
(2)
|
Balance at
End of Period |
|||||||||
|
2016
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
3,451
|
|
12,306
|
|
(12,337
|
)
|
3,420
|
|
||||
|
Accrued returns
|
404
|
|
140
|
|
(210
|
)
|
334
|
|
|||||
|
Accrued promotions
|
11
|
|
10
|
|
(21
|
)
|
—
|
|
|||||
|
Subtotal
|
$
|
3,866
|
|
12,456
|
|
(12,568
|
)
|
3,754
|
|
||||
|
Reserve for doubtful accounts
|
46
|
|
2
|
|
(10
|
)
|
38
|
|
|||||
|
Reserve for cash discounts
|
63
|
|
613
|
|
(618
|
)
|
58
|
|
|||||
|
Total
|
$
|
3,975
|
|
13,071
|
|
(13,196
|
)
|
3,850
|
|
||||
|
2015
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
2,717
|
|
10,449
|
|
(9,715
|
)
|
3,451
|
|
||||
|
Accrued returns
|
422
|
|
52
|
|
(70
|
)
|
404
|
|
|||||
|
Accrued promotions
|
34
|
|
127
|
|
(150
|
)
|
11
|
|
|||||
|
Subtotal
|
$
|
3,173
|
|
10,628
|
|
(9,935
|
)
|
3,866
|
|
||||
|
Reserve for doubtful accounts
|
41
|
|
30
|
|
(25
|
)
|
46
|
|
|||||
|
Reserve for cash discounts
|
51
|
|
625
|
|
(613
|
)
|
63
|
|
|||||
|
Total
|
$
|
3,265
|
|
11,283
|
|
(10,573
|
)
|
3,975
|
|
||||
|
(1)
|
Includes reserve for customer rebates of $102 million at
January 1, 2017
and $64 million at
January 3, 2016
, recorded as a contra asset.
|
|
(2)
|
Includes adjustments
|
|
28
|
||
|
(Dollars in Millions)
|
Balance at
Beginning of Period |
Accruals
|
Payments/Credits
|
Balance at
End of Period |
|||||||||
|
2016
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(
1)
|
$
|
1,189
|
|
5,700
|
|
(5,389
|
)
|
1,500
|
|
||||
|
Accrued returns
|
239
|
|
518
|
|
(630
|
)
|
127
|
|
|||||
|
Accrued promotions
|
47
|
|
78
|
|
(93
|
)
|
32
|
|
|||||
|
Subtotal
|
$
|
1,475
|
|
6,296
|
|
(6,112
|
)
|
1,659
|
|
||||
|
Reserve for doubtful accounts
|
204
|
|
21
|
|
(35
|
)
|
190
|
|
|||||
|
Reserve for cash discounts
|
20
|
|
430
|
|
(434
|
)
|
16
|
|
|||||
|
Total
|
$
|
1,699
|
|
6,747
|
|
(6,581
|
)
|
1,865
|
|
||||
|
2015
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
844
|
|
5,216
|
|
(4,871
|
)
|
1,189
|
|
||||
|
Accrued returns
|
188
|
|
556
|
|
(505
|
)
|
239
|
|
|||||
|
Accrued promotions
|
53
|
|
95
|
|
(101
|
)
|
47
|
|
|||||
|
Subtotal
|
$
|
1,085
|
|
5,867
|
|
(5,477
|
)
|
1,475
|
|
||||
|
Reserve for doubtful accounts
|
216
|
|
13
|
|
(25
|
)
|
204
|
|
|||||
|
Reserve for cash discounts
|
16
|
|
877
|
|
(873
|
)
|
20
|
|
|||||
|
Total
|
$
|
1,317
|
|
6,757
|
|
(6,375
|
)
|
1,699
|
|
||||
|
(1)
|
Includes reserve for customer rebates of $430 million at
January 1, 2017
and $411 million at
January 3, 2016
, recorded as a contra asset.
|
|
29
|
||
|
30
|
||
|
31
|
||
|
|
|||||||||||||
|
|
2016
|
2015
|
|||||||||||
|
|
High
|
Low
|
High
|
Low
|
|||||||||
|
First quarter
|
$
|
109.56
|
|
94.28
|
|
$
|
106.50
|
|
97.15
|
|
|||
|
Second quarter
|
121.54
|
|
107.88
|
|
104.48
|
|
97.01
|
|
|||||
|
Third quarter
|
126.07
|
|
117.04
|
|
101.36
|
|
81.79
|
|
|||||
|
Fourth quarter
|
122.50
|
|
109.32
|
|
105.49
|
|
89.90
|
|
|||||
|
Year-end close
|
$115.21
|
$102.72
|
|||||||||||
|
32
|
||
|
Item 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
Index to Audited Consolidated Financial Statements
|
|
|
33
|
||
|
2016
|
2015
|
|||||
|
Assets
|
||||||
|
Current assets
|
|
|
|
|
||
|
Cash and cash equivalents (Notes 1 and 2)
|
$
|
18,972
|
|
13,732
|
|
|
|
Marketable securities (Notes 1 and 2)
|
22,935
|
|
24,644
|
|
||
|
Accounts receivable trade, less allowances for doubtful accounts $252 (2015, $268)
|
11,699
|
|
10,734
|
|
||
|
Inventories (Notes 1 and 3)
|
8,144
|
|
8,053
|
|
||
|
Prepaid expenses and other receivables
|
3,282
|
|
3,047
|
|
||
|
Total current assets
|
65,032
|
|
60,210
|
|
||
|
Property, plant and equipment, net (Notes 1 and 4)
|
15,912
|
|
15,905
|
|
||
|
Intangible assets, net (Notes 1 and 5)
|
26,876
|
|
25,764
|
|
||
|
Goodwill (Notes 1 and 5)
|
22,805
|
|
21,629
|
|
||
|
Deferred taxes on income (Note 8)
|
6,148
|
|
5,490
|
|
||
|
Other assets
|
4,435
|
|
4,413
|
|
||
|
Total assets
|
$
|
141,208
|
|
133,411
|
|
|
|
Liabilities and Shareholders’ Equity
|
|
|
|
|
||
|
Current liabilities
|
|
|
|
|
||
|
Loans and notes payable (Note 7)
|
$
|
4,684
|
|
7,004
|
|
|
|
Accounts payable
|
6,918
|
|
6,668
|
|
||
|
Accrued liabilities
|
5,635
|
|
5,411
|
|
||
|
Accrued rebates, returns and promotions
|
5,403
|
|
5,440
|
|
||
|
Accrued compensation and employee related obligations
|
2,676
|
|
2,474
|
|
||
|
Accrued taxes on income (Note 8)
|
971
|
|
750
|
|
||
|
Total current liabilities
|
26,287
|
|
27,747
|
|
||
|
Long-term debt (Note 7)
|
22,442
|
|
12,857
|
|
||
|
Deferred taxes on income (Note 8)
|
2,910
|
|
2,562
|
|
||
|
Employee related obligations (Notes 9 and 10)
|
9,615
|
|
8,854
|
|
||
|
Other liabilities
|
9,536
|
|
10,241
|
|
||
|
Total liabilities
|
70,790
|
|
62,261
|
|
||
|
Shareholders’ equity
|
|
|
|
|
||
|
Preferred stock — without par value (authorized and unissued 2,000,000 shares)
|
—
|
|
—
|
|
||
|
Common stock — par value $1.00 per share (Note 12) (authorized 4,320,000,000 shares; issued 3,119,843,000 shares)
|
3,120
|
|
3,120
|
|
||
|
Accumulated other comprehensive income (Note 13)
|
(14,901
|
)
|
(13,165
|
)
|
||
|
Retained earnings
|
110,551
|
|
103,879
|
|
||
|
|
98,770
|
|
93,834
|
|
||
|
Less: common stock held in treasury, at cost (Note 12) (413,332,000 shares and 364,681,000 shares)
|
28,352
|
|
22,684
|
|
||
|
Total shareholders’ equity
|
70,418
|
|
71,150
|
|
||
|
Total liabilities and shareholders’ equity
|
$
|
141,208
|
|
133,411
|
|
|
|
34
|
||
|
2016
|
2015
|
2014
|
|||||||
|
Sales to customers
|
$
|
71,890
|
|
70,074
|
|
74,331
|
|
||
|
Cost of products sold
|
21,685
|
|
21,536
|
|
22,746
|
|
|||
|
Gross profit
|
50,205
|
|
48,538
|
|
51,585
|
|
|||
|
Selling, marketing and administrative expenses
|
19,945
|
|
21,203
|
|
21,954
|
|
|||
|
Research and development expense
|
9,095
|
|
9,046
|
|
8,494
|
|
|||
|
In-process research and development
|
29
|
|
224
|
|
178
|
|
|||
|
Interest income
|
(368
|
)
|
(128
|
)
|
(67
|
)
|
|||
|
Interest expense, net of portion capitalized (Note 4)
|
726
|
|
552
|
|
533
|
|
|||
|
Other (income) expense, net
|
484
|
|
(2,064
|
)
|
(70
|
)
|
|||
|
Restructuring (Note 22)
|
491
|
|
509
|
|
—
|
|
|||
|
Earnings before provision for taxes on income
|
19,803
|
|
19,196
|
|
20,563
|
|
|||
|
Provision for taxes on income (Note 8)
|
3,263
|
|
3,787
|
|
4,240
|
|
|||
|
Net earnings
|
$
|
16,540
|
|
15,409
|
|
16,323
|
|
||
|
Net earnings per share (Notes 1 and 15)
|
|||||||||
|
Basic
|
$
|
6.04
|
|
5.56
|
|
5.80
|
|
||
|
Diluted
|
$
|
5.93
|
|
5.48
|
|
5.70
|
|
||
|
Cash dividends per share
|
$
|
3.15
|
|
2.95
|
|
2.76
|
|
||
|
Average shares outstanding (Notes 1 and 15)
|
|||||||||
|
Basic
|
2,737.3
|
|
2,771.8
|
|
2,815.2
|
|
|||
|
Diluted
|
2,788.9
|
|
2,812.9
|
|
2,863.9
|
|
|||
|
35
|
||
|
2016
|
2015
|
2014
|
|||||||
|
Net earnings
|
$
|
16,540
|
|
15,409
|
|
16,323
|
|
||
|
Other comprehensive income (loss), net of tax
|
|||||||||
|
Foreign currency translation
|
(612
|
)
|
(3,632
|
)
|
(4,601
|
)
|
|||
|
Securities:
|
|||||||||
|
Unrealized holding gain (loss) arising during period
|
(52
|
)
|
471
|
|
156
|
|
|||
|
Reclassifications to earnings
|
(141
|
)
|
(124
|
)
|
(5
|
)
|
|||
|
Net change
|
(193
|
)
|
347
|
|
151
|
|
|||
|
Employee benefit plans:
|
|||||||||
|
Prior service credit (cost), net of amortization
|
21
|
|
(60
|
)
|
193
|
|
|||
|
Gain (loss), net of amortization
|
(862
|
)
|
931
|
|
(3,698
|
)
|
|||
|
Effect of exchange rates
|
159
|
|
148
|
|
197
|
|
|||
|
Net change
|
(682
|
)
|
1,019
|
|
(3,308
|
)
|
|||
|
Derivatives & hedges:
|
|||||||||
|
Unrealized gain (loss) arising during period
|
(359
|
)
|
(115
|
)
|
92
|
|
|||
|
Reclassifications to earnings
|
110
|
|
(62
|
)
|
(196
|
)
|
|||
|
Net change
|
(249
|
)
|
(177
|
)
|
(104
|
)
|
|||
|
Other comprehensive income (loss)
|
(1,736
|
)
|
(2,443
|
)
|
(7,862
|
)
|
|||
|
Comprehensive income
|
$
|
14,804
|
|
12,966
|
|
8,461
|
|
||
|
The tax effects in other comprehensive income for the fiscal years ended 2016, 2015 and 2014 respectively: Securities; $104 million, $187 million and $81 million, Employee Benefit Plans; $346 million, $519 million and $1,556 million, Derivatives & Hedges; $134 million, $95 million and $56 million.
|
|
36
|
||
|
Total
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Income
|
Common Stock
Issued Amount
|
Treasury
Stock
Amount
|
|||||||||||
|
Balance, December 29, 2013
|
$
|
74,053
|
|
89,493
|
|
(2,860
|
)
|
3,120
|
|
(15,700
|
)
|
||||
|
Net earnings
|
16,323
|
|
16,323
|
|
|
|
|
|
|
|
|||||
|
Cash dividends paid
|
(7,768
|
)
|
(7,768
|
)
|
|
|
|
|
|
|
|||||
|
Employee compensation and stock option plans
|
2,164
|
|
(769
|
)
|
|
|
|
|
2,933
|
|
|||||
|
Repurchase of common stock
|
(7,124
|
)
|
|
|
|
|
|
|
(7,124
|
)
|
|||||
|
Other
|
(34
|
)
|
(34
|
)
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(7,862
|
)
|
(7,862
|
)
|
|
|
|
|
|||||||
|
Balance, December 28, 2014
|
69,752
|
|
97,245
|
|
(10,722
|
)
|
3,120
|
|
(19,891
|
)
|
|||||
|
Net earnings
|
15,409
|
|
15,409
|
|
|
|
|
|
|
|
|||||
|
Cash dividends paid
|
(8,173
|
)
|
(8,173
|
)
|
|
|
|
|
|
|
|||||
|
Employee compensation and stock option plans
|
1,920
|
|
(577
|
)
|
|
|
|
|
2,497
|
|
|||||
|
Repurchase of common stock
|
(5,290
|
)
|
|
|
|
|
|
|
(5,290
|
)
|
|||||
|
Other
|
(25
|
)
|
(25
|
)
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(2,443
|
)
|
|
|
(2,443
|
)
|
|
|
|
|
|||||
|
Balance, January 3, 2016
|
71,150
|
|
103,879
|
|
(13,165
|
)
|
3,120
|
|
(22,684
|
)
|
|||||
|
Net earnings
|
16,540
|
|
16,540
|
|
|
|
|
|
|
|
|||||
|
Cash dividends paid
|
(8,621
|
)
|
(8,621
|
)
|
|
|
|
|
|
|
|||||
|
Employee compensation and stock option plans
|
2,130
|
|
(1,181
|
)
|
|
|
|
|
3,311
|
|
|||||
|
Repurchase of common stock
|
(8,979
|
)
|
|
|
|
|
|
|
(8,979
|
)
|
|||||
|
Other
|
(66
|
)
|
(66
|
)
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(1,736
|
)
|
|
|
(1,736
|
)
|
|
|
|
|
|||||
|
Balance, January 1, 2017
|
$
|
70,418
|
|
110,551
|
|
(14,901
|
)
|
3,120
|
|
(28,352
|
)
|
||||
|
37
|
||
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
2016
|
2015
|
2014
|
|||||||
|
Cash flows from operating activities
|
|
|
|
|
|||||
|
Net earnings
|
$
|
16,540
|
|
15,409
|
|
16,323
|
|
||
|
Adjustments to reconcile net earnings to cash flows from operating activities:
|
|
|
|
|
|||||
|
Depreciation and amortization of property and intangibles
|
3,754
|
|
3,746
|
|
3,895
|
|
|||
|
Stock based compensation
|
878
|
|
874
|
|
792
|
|
|||
|
Venezuela adjustments
|
—
|
|
122
|
|
87
|
|
|||
|
Asset write-downs
|
283
|
|
624
|
|
410
|
|
|||
|
Net gain on sale of assets/businesses
|
(563
|
)
|
(2,583
|
)
|
(2,383
|
)
|
|||
|
Deferred tax provision
|
(341
|
)
|
(270
|
)
|
441
|
|
|||
|
Accounts receivable allowances
|
(11
|
)
|
18
|
|
(28
|
)
|
|||
|
Changes in assets and liabilities, net of effects from acquisitions and divestitures:
|
|
|
|
|
|||||
|
Increase in accounts receivable
|
(1,065
|
)
|
(433
|
)
|
(247
|
)
|
|||
|
Increase in inventories
|
(249
|
)
|
(449
|
)
|
(1,120
|
)
|
|||
|
Increase in accounts payable and accrued liabilities
|
656
|
|
287
|
|
1,194
|
|
|||
|
Decrease in other current and non-current assets
|
18
|
|
65
|
|
442
|
|
|||
|
(Decrease)/Increase in other current and non-current liabilities
|
(1,133
|
)
|
2,159
|
|
(1,096
|
)
|
|||
|
Net cash flows from operating activities
|
18,767
|
|
19,569
|
|
18,710
|
|
|||
|
Cash flows from investing activities
|
|
|
|
|
|||||
|
Additions to property, plant and equipment
|
(3,226
|
)
|
(3,463
|
)
|
(3,714
|
)
|
|||
|
Proceeds from the disposal of assets/businesses, net
|
1,267
|
|
3,464
|
|
4,631
|
|
|||
|
Acquisitions, net of cash acquired (Note 20)
|
(4,509
|
)
|
(954
|
)
|
(2,129
|
)
|
|||
|
Purchases of investments
|
(33,950
|
)
|
(40,828
|
)
|
(34,913
|
)
|
|||
|
Sales of investments
|
35,780
|
|
34,149
|
|
24,119
|
|
|||
|
Other (primarily intangibles)
|
(123
|
)
|
(103
|
)
|
(299
|
)
|
|||
|
Net cash used by investing activities
|
(4,761
|
)
|
(7,735
|
)
|
(12,305
|
)
|
|||
|
Cash flows from financing activities
|
|
|
|||||||
|
Dividends to shareholders
|
(8,621
|
)
|
(8,173
|
)
|
(7,768
|
)
|
|||
|
Repurchase of common stock
|
(8,979
|
)
|
(5,290
|
)
|
(7,124
|
)
|
|||
|
Proceeds from short-term debt
|
111
|
|
2,416
|
|
1,863
|
|
|||
|
Retirement of short-term debt
|
(2,017
|
)
|
(1,044
|
)
|
(1,267
|
)
|
|||
|
Proceeds from long-term debt
|
12,004
|
|
75
|
|
2,098
|
|
|||
|
Retirement of long-term debt
|
(2,223
|
)
|
(68
|
)
|
(1,844
|
)
|
|||
|
Proceeds from the exercise of stock options/employee withholding tax on stock awards, net
|
1,189
|
|
1,005
|
|
1,543
|
|
|||
|
Other
|
(15
|
)
|
(57
|
)
|
—
|
|
|||
|
Net cash used by financing activities
|
(8,551
|
)
|
(11,136
|
)
|
(12,499
|
)
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
(215
|
)
|
(1,489
|
)
|
(310
|
)
|
|||
|
Increase/(Decrease) in cash and cash equivalents
|
5,240
|
|
(791
|
)
|
(6,404
|
)
|
|||
|
Cash and cash equivalents, beginning of year (Note 1)
|
13,732
|
|
14,523
|
|
20,927
|
|
|||
|
Cash and cash equivalents, end of year (Note 1)
|
$
|
18,972
|
|
13,732
|
|
14,523
|
|
||
|
Supplemental cash flow data
|
|
|
|
|
|
|
|||
|
Cash paid during the year for:
|
|
|
|
|
|
|
|||
|
Interest
|
$
|
730
|
|
617
|
|
603
|
|
||
|
Interest, net of amount capitalized
|
628
|
|
515
|
|
488
|
|
|||
|
Income taxes
|
2,843
|
|
2,865
|
|
3,536
|
|
|||
|
38
|
||
|
Supplemental schedule of non-cash investing and financing activities
|
|
|
|
|
|
|
|||
|
Treasury stock issued for employee compensation and stock option plans, net of cash proceeds/ employee withholding tax on stock awards
|
2,043
|
|
1,486
|
|
1,409
|
|
|||
|
Conversion of debt
|
35
|
|
16
|
|
17
|
|
|||
|
Acquisitions
|
|
|
|
|
|
|
|||
|
Fair value of assets acquired
|
$
|
4,586
|
|
1,174
|
|
2,167
|
|
||
|
Fair value of liabilities assumed and noncontrolling interests
|
(77
|
)
|
(220
|
)
|
(38
|
)
|
|||
|
Net cash paid for acquisitions
|
$
|
4,509
|
|
954
|
|
2,129
|
|
||
|
39
|
||
|
1.
|
Summary of Significant Accounting Policies
|
|
•
|
All excess tax benefits and deficiencies to be recognized as a reduction or an increase to the provision for taxes on income. Previously, the Company recorded these benefits directly to Retained Earnings. The tax benefit for the Company was
$353 million
for the fiscal year 2016. The standard does not permit retroactive presentation of this benefit to prior fiscal years on the Consolidated Statement of Earnings.
|
|
•
|
The tax benefit or deficiency is required to be classified and presented as a cash flow to/from operating activities. It was previously required to be presented as a cash flow to/from financing activities on the Consolidated Statement of Cash Flows. As permitted in the standard, the Company has elected to adopt this reclassification on a prospective basis and therefore prior fiscal years for the Consolidated Statement of Cash Flows have not been recast for this provision.
|
|
•
|
Clarifies that all cash payments made to taxing authorities on employees’ share-based compensation should be classified as a cash outflow from financing activities. This reclassification is required to be recast retrospectively. As a result, for the fiscal year 2016,
$269 million
was classified as a cash outflow from financing activities and
$290 million
and
$239 million
, of cash outflow was reclassified from an operating activity to a financing activity (Proceeds from the exercise of stock options/employee withholding tax on stock awards, net) in the fiscal years 2015 and 2014, respectively.
|
|
•
|
In the diluted net earnings per share calculation, when applying the treasury stock method for shares that could be repurchased, the assumed proceeds no longer include the amount of excess tax benefit. This did not have a material impact on the Company's diluted net earnings per share calculation.
|
|
40
|
||
|
41
|
||
|
42
|
||
|
Building and building equipment
|
20 - 30 years
|
|
Land and leasehold improvements
|
10 - 20 years
|
|
Machinery and equipment
|
2 - 13 years
|
|
43
|
||
|
44
|
||
|
Nature/Type of Collaboration
|
|
Statement of Earnings Presentation
|
|
Third-party sale of product
|
|
Sales to customers
|
|
Royalties/milestones paid to collaborative partner (post-regulatory approval)*
|
|
Cost of products sold
|
|
Royalties received from collaborative partner
|
|
Other income (expense), net
|
|
Upfront payments & milestones paid to collaborative partner (pre-regulatory approval)
|
|
Research and development expense
|
|
Research and development payments to collaborative partner
|
|
Research and development expense
|
|
Research and development payments received from collaborative partner
|
|
Reduction of Research and development expense
|
|
*
|
Milestones are capitalized as intangible assets and amortized to cost of goods sold over the useful life.
|
|
45
|
||
|
46
|
||
|
2.
|
Cash, Cash Equivalents and Current Marketable Securities
|
|
(Dollars in Millions)
|
2016
|
|||||||||||||||||||
|
Carrying Amount
|
Unrecognized Gain
|
Unrecognized Loss
|
Estimated Fair Value
|
Cash & Cash Equivalents
|
Current Marketable Securities
|
|||||||||||||||
|
Cash
|
$
|
1,979
|
|
—
|
|
—
|
|
1,979
|
|
$
|
1,979
|
|
—
|
|
||||||
|
U.S. Gov't Securities
(1)
|
10,832
|
|
—
|
|
(1
|
)
|
10,831
|
|
2,249
|
|
8,583
|
|
||||||||
|
Other Sovereign Securities
(1)
|
1,299
|
|
—
|
|
—
|
|
1,299
|
|
120
|
|
1,179
|
|
||||||||
|
U.S. Reverse repurchase agreements
|
6,103
|
|
—
|
|
—
|
|
6,103
|
|
6,103
|
|
—
|
|
||||||||
|
Other Reverse repurchase agreements
|
240
|
|
—
|
|
—
|
|
240
|
|
240
|
|
—
|
|
||||||||
|
Corporate debt securities
(1)
|
754
|
|
—
|
|
—
|
|
754
|
|
—
|
|
754
|
|
||||||||
|
Money market funds
|
7,187
|
|
—
|
|
—
|
|
7,187
|
|
7,187
|
|
—
|
|
||||||||
|
Time deposits
(1)
|
1,094
|
|
—
|
|
—
|
|
1,094
|
|
1,094
|
|
—
|
|
||||||||
|
Subtotal
|
$
|
29,488
|
|
—
|
|
(1
|
)
|
29,487
|
|
18,972
|
|
10,516
|
|
|||||||
|
Unrealized Gain
|
Unrealized Loss
|
|||||||||||||||||||
|
Gov't Securities
|
$
|
10,277
|
|
5
|
|
(51
|
)
|
10,231
|
|
—
|
|
10,231
|
|
|||||||
|
Other Sovereign Securities
|
90
|
|
—
|
|
—
|
|
90
|
|
90
|
|
||||||||||
|
Corporate debt securities
|
1,777
|
|
1
|
|
(12
|
)
|
1,766
|
|
—
|
|
1,766
|
|
||||||||
|
Equity investments
|
34
|
|
298
|
|
|
332
|
|
332
|
|
|||||||||||
|
Subtotal available for sale
(2)
|
$
|
12,178
|
|
304
|
|
(63
|
)
|
12,419
|
|
—
|
|
12,419
|
|
|||||||
|
Total cash, cash equivalents and current marketable securities
|
|
|
$
|
18,972
|
|
22,935
|
|
|||||||||||||
|
47
|
||
|
(Dollars in Millions)
|
2015
|
|||||||||||||||||||
|
Carrying Amount
|
Unrecognized Gain
|
Unrecognized Loss
|
Estimated Fair Value
|
Cash Equivalents
|
Current Marketable Securities
|
|||||||||||||||
|
Cash
|
$
|
1,832
|
|
—
|
|
—
|
|
1,832
|
|
1,832
|
|
—
|
|
|||||||
|
U.S. Gov't Securities
(1)
|
14,641
|
|
1
|
|
(2
|
)
|
14,640
|
|
650
|
|
13,991
|
|
||||||||
|
Other Sovereign Securities
(1)
|
2,122
|
|
—
|
|
—
|
|
2,122
|
|
933
|
|
1,189
|
|
||||||||
|
U.S. Reverse repurchase agreements
|
1,579
|
|
—
|
|
—
|
|
1,579
|
|
1,579
|
|
—
|
|
||||||||
|
Other Reverse repurchase agreements
|
2,200
|
|
—
|
|
—
|
|
2,200
|
|
2,200
|
|
—
|
|
||||||||
|
Corporate debt securities
(1)
|
2,941
|
|
—
|
|
—
|
|
2,941
|
|
1,793
|
|
1,148
|
|
||||||||
|
Money market funds
|
3,855
|
|
—
|
|
—
|
|
3,855
|
|
3,855
|
|
—
|
|
||||||||
|
Time deposits
(1)
|
890
|
|
—
|
|
—
|
|
890
|
|
890
|
|
—
|
|
||||||||
|
Subtotal
|
$
|
30,060
|
|
1
|
|
(2
|
)
|
30,059
|
|
13,732
|
|
16,328
|
|
|||||||
|
Unrealized Gain
|
Unrealized Loss
|
|||||||||||||||||||
|
Gov't Securities
|
$
|
7,307
|
|
1
|
|
(34
|
)
|
7,274
|
|
—
|
|
7,274
|
|
|||||||
|
Other Sovereign Securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Corporate debt securities
|
1,046
|
|
1
|
|
(5
|
)
|
1,042
|
|
—
|
|
1,042
|
|
||||||||
|
Subtotal available for sale
(2)
|
$
|
8,353
|
|
2
|
|
(39
|
)
|
8,316
|
|
—
|
|
8,316
|
|
|||||||
|
Total cash, cash equivalents and current marketable securities
|
$
|
13,732
|
|
24,644
|
|
|||||||||||||||
|
(Dollars in Millions)
|
Cost Basis
|
Fair Value
|
|||||
|
Due within one year
|
$
|
474
|
|
474
|
|
||
|
Due after one year through five years
|
11,430
|
|
11,381
|
|
|||
|
Due after five years through ten years
|
240
|
|
232
|
|
|||
|
Total debt securities
|
$
|
12,144
|
|
12,087
|
|
||
|
3.
|
Inventories
|
|
(Dollars in Millions)
|
2016
|
2015
|
|||||
|
Raw materials and supplies
|
$
|
952
|
|
936
|
|
||
|
Goods in process
|
2,185
|
|
2,241
|
|
|||
|
Finished goods
|
5,007
|
|
4,876
|
|
|||
|
Total inventories
|
$
|
8,144
|
|
8,053
|
|
||
|
48
|
||
|
4.
|
Property, Plant and Equipment
|
|
(Dollars in Millions)
|
2016
|
2015
|
|||||
|
Land and land improvements
|
$
|
753
|
|
780
|
|
||
|
Buildings and building equipment
|
10,112
|
|
9,829
|
|
|||
|
Machinery and equipment
|
23,554
|
|
22,511
|
|
|||
|
Construction in progress
|
3,354
|
|
3,528
|
|
|||
|
Total property, plant and equipment, gross
|
$
|
37,773
|
|
36,648
|
|
||
|
Less accumulated depreciation
|
21,861
|
|
20,743
|
|
|||
|
Total property, plant and equipment, net
|
$
|
15,912
|
|
15,905
|
|
||
|
5.
|
Intangible Assets and Goodwill
|
|
(Dollars in Millions)
|
2016
|
2015
|
|||||
|
Intangible assets with definite lives:
|
|
|
|
|
|||
|
Patents and trademarks — gross
|
$
|
10,521
|
|
8,299
|
|
||
|
Less accumulated amortization
|
5,076
|
|
4,745
|
|
|||
|
Patents and trademarks — net
|
$
|
5,445
|
|
3,554
|
|
||
|
Customer relationships and other intangibles — gross
|
$
|
17,615
|
|
17,583
|
|
||
|
Less accumulated amortization
|
6,515
|
|
5,816
|
|
|||
|
Customer relationships and other intangibles — net
|
$
|
11,100
|
|
11,767
|
|
||
|
Intangible assets with indefinite lives:
|
|
|
|
|
|||
|
Trademarks
|
$
|
6,888
|
|
7,023
|
|
||
|
Purchased in-process research and development
|
3,443
|
|
3,420
|
|
|||
|
Total intangible assets with indefinite lives
|
$
|
10,331
|
|
10,443
|
|
||
|
Total intangible assets — net
|
$
|
26,876
|
|
25,764
|
|
||
|
(Dollars in Millions)
|
Consumer
|
Pharmaceutical
|
Med Devices
|
Total
|
|||||||||
|
Goodwill at December 28, 2014
|
$
|
7,675
|
|
2,626
|
|
11,531
|
|
21,832
|
|
||||
|
Goodwill, related to acquisitions
|
110
|
|
366
|
|
34
|
|
510
|
|
|||||
|
Goodwill, related to divestitures
|
(119
|
)
|
(17
|
)
|
(57
|
)
|
(193
|
)
|
|||||
|
Currency translation/other
|
(426
|
)
|
(86
|
)
|
(8
|
)
|
(520
|
)
|
|||||
|
Goodwill at January 3, 2016
|
$
|
7,240
|
|
2,889
|
|
11,500
|
|
21,629
|
|
||||
|
Goodwill, related to acquisitions
|
1,362
|
|
—
|
|
210
|
|
1,572
|
|
|||||
|
Goodwill, related to divestitures
|
(63
|
)
|
(12
|
)
|
—
|
|
(75
|
)
|
|||||
|
Currency translation/other
|
(276
|
)
|
(37
|
)
|
(8
|
)
|
(321
|
)
|
|||||
|
Goodwill at January 1, 2017
|
$
|
8,263
|
|
2,840
|
|
11,702
|
|
22,805
|
|
||||
|
49
|
||
|
6.
|
Fair Value Measurements
|
|
50
|
||
|
(Dollars in Millions)
|
Gain/(Loss)
Recognized In Accumulated OCI (1) |
Gain/(Loss) Reclassified From
Accumulated OCI Into Income (1) |
Gain/(Loss) Recognized In
Other Income/Expense (2) |
||||||||||||||||
|
Cash Flow Hedges by Income Statement Caption
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
|||||||||||||
|
Sales to customers
(3)
|
$
|
(65
|
)
|
(83
|
)
|
(47
|
)
|
(126
|
)
|
(1
|
)
|
(5
|
)
|
||||||
|
Cost of products sold
(3)
|
(212
|
)
|
(22
|
)
|
(3
|
)
|
122
|
|
(15
|
)
|
14
|
|
|||||||
|
Research and development expense
(3)
|
(76
|
)
|
(3
|
)
|
(90
|
)
|
6
|
|
—
|
|
1
|
|
|||||||
|
Interest (income)/Interest expense, net
(4)
|
66
|
|
(40
|
)
|
37
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Other (income) expense, net
(3) (5)
|
(72
|
)
|
33
|
|
(7
|
)
|
60
|
|
2
|
|
1
|
|
|||||||
|
Total
|
$
|
(359
|
)
|
(115
|
)
|
(110
|
)
|
62
|
|
(14
|
)
|
11
|
|
||||||
|
(1)
|
Effective portion
|
|
(2)
|
Ineffective portion
|
|
(3)
|
Forward foreign exchange contracts
|
|
(4)
|
Cross currency interest rate swaps
|
|
(5)
|
Includes equity collar contracts
|
|
51
|
||
|
2016
|
2015
|
|||||||||||||||
|
(Dollars in Millions)
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Total
(1)
|
|||||||||||
|
Derivatives designated as hedging instruments:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
(7)
|
$
|
—
|
|
747
|
|
—
|
|
747
|
|
452
|
|
|||||
|
Interest rate contracts
(2)(4)(7)
|
—
|
|
31
|
|
—
|
|
31
|
|
28
|
|
||||||
|
Total
|
—
|
|
778
|
|
—
|
|
778
|
|
480
|
|
||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
(8)
|
—
|
|
723
|
|
—
|
|
723
|
|
358
|
|
||||||
|
Interest rate contracts
(3)(4)(8)
|
—
|
|
382
|
|
—
|
|
382
|
|
241
|
|
||||||
|
Equity collar contracts
(8)
|
—
|
|
57
|
|
—
|
|
57
|
|
—
|
|
||||||
|
Total
|
—
|
|
1,162
|
|
—
|
|
1,162
|
|
599
|
|
||||||
|
Derivatives not designated as hedging instruments:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
(7)
|
—
|
|
34
|
|
—
|
|
34
|
|
33
|
|
||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
(8)
|
—
|
|
57
|
|
—
|
|
57
|
|
41
|
|
||||||
|
Available For Sale Other Investments:
|
||||||||||||||||
|
Equity investments
(5)
|
1,209
|
|
—
|
|
—
|
|
1,209
|
|
1,494
|
|
||||||
|
Debt securities
(6)
|
$
|
—
|
|
12,087
|
|
—
|
|
12,087
|
|
8,316
|
|
|||||
|
(1)
|
2015 assets and liabilities are all classified as Level 2 with the exception of equity investments of
$1,494 million
, which are classified as Level 1.
|
|
(2)
|
Includes
$23 million
and
$20 million
of non-current assets for the fiscal years ending
January 1, 2017
and
January 3, 2016
, respectively.
|
|
(3)
|
Includes
$382 million
and
$239 million
of non-current liabilities for the fiscal years ending
January 1, 2017
and
January 3, 2016
, respectively.
|
|
(4)
|
Includes cross currency interest rate swaps and interest rate swaps.
|
|
(5)
|
Classified as non-current other assets with the exception of
$332 million
of current assets for January 1, 2017. The carrying amount of the equity investments were
$520 million
and
$528 million
as of
January 1, 2017
and
January 3, 2016
, respectively. The unrealized gains were
$757 million
and
$979 million
as of
January 1, 2017
and
January 3, 2016
, respectively. The unrealized losses were
$68 million
and
$13 million
as of
January 1, 2017
and
January 3, 2016
, respectively.
|
|
(6)
|
Classified as current marketable securities.
|
|
(7)
|
Classified as other current assets.
|
|
(8)
|
Classified as accounts payable.
|
|
52
|
||
|
7.
|
Borrowings
|
|
(Dollars in Millions)
|
2016
|
|
Effective Rate %
|
|
2015
|
|
Effective Rate %
|
||||||||
|
2.15% Notes due 2016
|
$
|
—
|
|
—
|
|
900
|
|
2.22
|
|
||||||
|
3 month LIBOR+0.07% FRN due 2016
|
—
|
|
—
|
|
800
|
|
0.48
|
|
|||||||
|
0.70% Notes due 2016
|
—
|
|
—
|
|
398
|
|
0.74
|
|
|||||||
|
5.55% Debentures due 2017
|
1,000
|
|
5.55
|
|
1,000
|
|
5.55
|
|
|||||||
|
1.125% Notes due 2017
|
699
|
|
1.15
|
|
700
|
|
1.15
|
|
|||||||
|
5.15% Debentures due 2018
|
899
|
|
|
5.18
|
|
|
899
|
|
|
5.15
|
|
||||
|
1.65% Notes due 2018
|
600
|
|
1.70
|
|
602
|
|
1.70
|
|
|||||||
|
4.75% Notes due 2019 (1B Euro 1.0449)
(2)
/
(1B Euro 1.0882)
(3)
|
1,041
|
|
(2)
|
5.83
|
|
|
1,085
|
|
(3)
|
5.83
|
|
||||
|
1.875% Notes due 2019
|
499
|
|
1.93
|
|
502
|
|
1.93
|
|
|||||||
|
0.89% Notes due 2019
|
299
|
|
1.20
|
|
—
|
|
—
|
|
|||||||
|
1.125% Notes due 2019
|
699
|
|
1.13
|
|
—
|
|
—
|
|
|||||||
|
3% Zero Coupon Convertible Subordinated Debentures due 2020
|
84
|
|
|
3.00
|
|
|
137
|
|
|
3.00
|
|
||||
|
2.95% Debentures due 2020
|
546
|
|
|
3.15
|
|
|
545
|
|
|
3.15
|
|
||||
|
3.55% Notes due 2021
|
447
|
|
3.67
|
|
448
|
|
3.67
|
|
|||||||
|
2.45% Notes due 2021
|
348
|
|
2.48
|
|
349
|
|
2.48
|
|
|||||||
|
1.65% Notes due 2021
|
997
|
|
1.65
|
|
—
|
|
—
|
|
|||||||
|
0.250% Notes due 2022 (1B Euro 1.0449)
(2)
|
1,041
|
|
(2)
|
0.26
|
|
—
|
|
—
|
|
||||||
|
6.73% Debentures due 2023
|
249
|
|
|
6.73
|
|
|
250
|
|
|
6.73
|
|
||||
|
3.375% Notes due 2023
|
807
|
|
3.17
|
|
811
|
|
3.17
|
|
|||||||
|
2.05% Notes due 2023
|
497
|
|
2.09
|
|
—
|
|
—
|
|
|||||||
|
0.650% Notes due 2024(750MM Euro 1.0449)
(2)
|
779
|
|
(2)
|
0.68
|
|
—
|
|
—
|
|
||||||
|
5.50% Notes due 2024 (500MM GBP 1.2237)
(2)
/(500MM GBP 1.4818)
(3)
|
605
|
|
(2)
|
6.75
|
|
|
737
|
|
(3)
|
6.75
|
|
||||
|
2.45% Notes due 2026
|
1,989
|
|
2.47
|
|
—
|
|
—
|
|
|||||||
|
1.150% Notes due 2028(750MM Euro 1.0449)
(2)
|
775
|
|
(2)
|
1.21
|
|
—
|
|
—
|
|
||||||
|
6.95% Notes due 2029
|
296
|
|
|
7.14
|
|
|
297
|
|
|
7.14
|
|
||||
|
4.95% Debentures due 2033
|
497
|
|
|
4.95
|
|
|
500
|
|
|
4.95
|
|
||||
|
4.375% Notes due 2033
|
857
|
|
4.24
|
|
864
|
|
4.24
|
|
|||||||
|
1.650% Notes due 2035 (1.5B Euro 1.0449)
(2)
|
1,549
|
|
(2)
|
1.68
|
|
—
|
|
—
|
|
||||||
|
3.55% Notes due 2036
|
987
|
|
3.59
|
|
—
|
|
—
|
|
|||||||
|
5.95% Notes due 2037
|
990
|
|
|
5.99
|
|
|
996
|
|
|
5.99
|
|
||||
|
5.85% Debentures due 2038
|
695
|
|
|
5.85
|
|
|
700
|
|
|
5.86
|
|
||||
|
4.50% Debentures due 2040
|
537
|
|
|
4.63
|
|
|
540
|
|
|
4.63
|
|
||||
|
4.85% Notes due 2041
|
296
|
|
4.89
|
|
298
|
|
4.89
|
|
|||||||
|
4.50% Notes due 2043
|
495
|
|
4.52
|
|
499
|
|
4.52
|
|
|||||||
|
3.70% Notes due 2046
|
1,970
|
|
3.74
|
|
—
|
|
—
|
|
|||||||
|
Other
|
77
|
|
|
—
|
|
|
104
|
|
|
—
|
|
||||
|
Subtotal
|
24,146
|
|
(4)
|
3.33
|
%
|
(1)
|
14,961
|
|
(4)
|
4.06
|
|
(1
|
)
|
||
|
Less current portion
|
1,704
|
|
|
|
|
|
2,104
|
|
|
|
|
||||
|
Total long-term debt
|
$
|
22,442
|
|
|
|
|
|
12,857
|
|
|
|
|
|||
|
(1)
|
Weighted average effective rate.
|
|
(2)
|
Translation rate at
January 1, 2017
.
|
|
53
|
||
|
(3)
|
Translation rate at
January 3, 2016
.
|
|
(4)
|
The excess of the fair value over the carrying value of debt was
$1.6 billion
in
2016
and
$1.7 billion
in
2015
.
|
|
(Dollars in Millions)
|
||||||||||
|
2017
|
2018
|
2019
|
2020
|
2021
|
After 2021
|
|||||
|
$1,704
|
1,561
|
2,538
|
629
|
1,795
|
15,919
|
|||||
|
8.
|
Income Taxes
|
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
|||||||
|
Currently payable:
|
||||||||||
|
U.S. taxes
|
$
|
1,896
|
|
2,748
|
|
2,625
|
|
|||
|
International taxes
|
1,708
|
|
1,309
|
|
1,174
|
|
||||
|
Total currently payable
|
3,604
|
|
4,057
|
|
3,799
|
|
||||
|
Deferred:
|
||||||||||
|
U.S. taxes
|
294
|
|
37
|
|
(258
|
)
|
||||
|
International taxes
|
(635
|
)
|
(307
|
)
|
699
|
|
||||
|
Total deferred
|
(341
|
)
|
(270
|
)
|
441
|
|
||||
|
Provision for taxes on income
|
$
|
3,263
|
|
3,787
|
|
4,240
|
|
|||
|
54
|
||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
||||||||
|
U.S.
|
$
|
7,457
|
|
8,179
|
|
8,001
|
|
||||
|
International
|
12,346
|
|
11,017
|
|
12,562
|
|
|||||
|
Earnings before taxes on income:
|
$
|
19,803
|
|
19,196
|
|
20,563
|
|
||||
|
Tax rates:
|
|||||||||||
|
U.S. statutory rate
|
35.0
|
%
|
35.0
|
|
35.0
|
|
|||||
|
International operations excluding Ireland
|
(9.4
|
)
|
(6.7
|
)
|
(7.0
|
)
|
|||||
|
Ireland and Puerto Rico operations
(1)
|
(7.8
|
)
|
(8.7
|
)
|
(6.9
|
)
|
|||||
|
Research and orphan drug tax credits
|
(0.4
|
)
|
(0.2
|
)
|
(0.3
|
)
|
|||||
|
U.S. state and local
|
(0.1
|
)
|
0.4
|
|
1.0
|
|
|||||
|
U.S. manufacturing deduction
|
(0.6
|
)
|
(0.6
|
)
|
(0.6
|
)
|
|||||
|
U.S. tax on international income
|
1.3
|
|
0.2
|
|
1.4
|
|
|||||
|
Additional tax benefits on share based compensation
|
(1.8
|
)
|
—
|
|
—
|
|
|||||
|
U.S. tax benefit on asset/business disposals
|
—
|
|
—
|
|
(1.9
|
)
|
|||||
|
All other
|
0.3
|
|
0.3
|
|
(0.1
|
)
|
|||||
|
Effective tax rate
|
16.5
|
%
|
19.7
|
|
20.6
|
|
|||||
|
55
|
||
|
2016 Deferred Tax
|
2015 Deferred Tax
|
||||||||||||
|
(Dollars in Millions)
|
Asset
|
Liability
|
Asset
|
Liability
|
|||||||||
|
Employee related obligations
|
$
|
2,958
|
|
|
|
2,863
|
|
|
|
||||
|
Stock based compensation
|
749
|
|
|
|
790
|
|
|
|
|||||
|
Depreciation
|
|
|
(219
|
)
|
|
|
(247
|
)
|
|||||
|
Non-deductible intangibles
|
|
|
(6,672
|
)
|
|
|
(6,663
|
)
|
|||||
|
International R&D capitalized for tax
|
1,264
|
|
|
|
1,318
|
|
|
|
|||||
|
Reserves & liabilities
|
1,857
|
|
|
|
1,801
|
|
|
|
|||||
|
Income reported for tax purposes
|
1,309
|
|
|
|
960
|
|
|
|
|||||
|
Net operating loss carryforward international
|
717
|
|
|
|
997
|
|
|
|
|||||
|
Miscellaneous international
|
1,135
|
|
(15
|
)
|
922
|
|
(1)
|
(249
|
)
|
||||
|
Miscellaneous U.S.
|
155
|
|
|
|
436
|
|
|
|
|||||
|
Total deferred income taxes
|
$
|
10,144
|
|
(6,906
|
)
|
10,087
|
|
(7,159
|
)
|
||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
|||||||
|
Beginning of year
|
$
|
3,080
|
|
2,465
|
|
2,729
|
|
|||
|
Increases related to current year tax positions
|
348
|
|
570
|
|
281
|
|
||||
|
Increases related to prior period tax positions
|
11
|
|
182
|
|
295
|
|
||||
|
Decreases related to prior period tax positions
|
(338
|
)
|
(79
|
)
|
(288
|
)
|
||||
|
Settlements
|
(37
|
)
|
(4
|
)
|
(477
|
)
|
||||
|
Lapse of statute of limitations
|
(23
|
)
|
(54
|
)
|
(75
|
)
|
||||
|
End of year
|
$
|
3,041
|
|
3,080
|
|
2,465
|
|
|||
|
56
|
||
|
9.
|
Employee Related Obligations
|
|
(Dollars in Millions)
|
2016
|
2015
|
|||||
|
Pension benefits
|
$
|
4,710
|
|
3,857
|
|
||
|
Postretirement benefits
|
2,733
|
|
2,738
|
|
|||
|
Postemployment benefits
|
2,050
|
|
2,092
|
|
|||
|
Deferred compensation
|
534
|
|
584
|
|
|||
|
Total employee obligations
|
10,027
|
|
9,271
|
|
|||
|
Less current benefits payable
|
412
|
|
417
|
|
|||
|
Employee related obligations — non-current
|
$
|
9,615
|
|
8,854
|
|
||
|
10.
|
Pensions and Other Benefit Plans
|
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
2016
|
2015
|
2014
|
|||||||||||||
|
Service cost
|
$
|
949
|
|
1,037
|
|
882
|
|
224
|
|
257
|
|
211
|
|
||||||
|
Interest cost
|
927
|
|
988
|
|
1,018
|
|
158
|
|
186
|
|
197
|
|
|||||||
|
Expected return on plan assets
|
(1,962
|
)
|
(1,809
|
)
|
(1,607
|
)
|
(6
|
)
|
(7
|
)
|
(7
|
)
|
|||||||
|
Amortization of prior service cost (credit)
|
1
|
|
2
|
|
6
|
|
(34
|
)
|
(33
|
)
|
(34
|
)
|
|||||||
|
Amortization of net transition obligation
|
—
|
|
—
|
|
1
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Recognized actuarial losses
|
496
|
|
745
|
|
460
|
|
135
|
|
201
|
|
136
|
|
|||||||
|
Curtailments and settlements
|
11
|
|
8
|
|
(17
|
)
|
—
|
|
—
|
|
—
|
|
|||||||
|
Net periodic benefit cost
|
$
|
422
|
|
971
|
|
743
|
|
477
|
|
604
|
|
503
|
|
||||||
|
(Dollars in Millions)
|
|
||
|
Amortization of net transition obligation
|
$
|
—
|
|
|
Amortization of net actuarial losses
|
715
|
|
|
|
Amortization of prior service credit
|
28
|
|
|
|
57
|
||
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||
|
Worldwide Benefit Plans
|
2016
|
2015
|
2014
|
2016
|
2015
|
2014
|
|||||||
|
Net Periodic Benefit Cost
|
|||||||||||||
|
Service cost discount rate
|
3.98
|
%
|
3.78
|
4.78
|
4.77
|
4.31
|
5.25
|
||||||
|
Interest cost discount rate
|
4.24
|
%
|
3.78
|
4.78
|
4.10
|
4.31
|
5.25
|
||||||
|
Rate of increase in compensation levels
|
4.02
|
%
|
4.05
|
4.08
|
4.32
|
4.11
|
4.29
|
||||||
|
Expected long-term rate of return on plan assets
|
8.55
|
%
|
8.53
|
8.46
|
|||||||||
|
Benefit Obligation
|
|||||||||||||
|
Discount rate
|
3.78
|
%
|
4.11
|
3.78
|
4.42
|
4.63
|
4.31
|
||||||
|
Rate of increase in compensation levels
|
4.02
|
%
|
4.01
|
4.05
|
4.29
|
4.28
|
4.11
|
||||||
|
Health Care Plans
|
2016
|
2015
|
||||
|
Health care cost trend rate assumed for next year
|
6.32
|
%
|
6.60
|
%
|
||
|
Rate to which the cost trend rate is assumed to decline (ultimate trend)
|
4.50
|
%
|
4.50
|
%
|
||
|
Year the rate reaches the ultimate trend rate
|
2038
|
|
2038
|
|
||
|
One-Percentage-
|
One-Percentage-
|
||||||
|
(Dollars in Millions)
|
Point Increase
|
Point Decrease
|
|||||
|
Health Care Plans
|
|
|
|
|
|||
|
Total interest and service cost
|
$
|
30
|
|
(23
|
)
|
||
|
Post-retirement benefit obligation
|
$
|
401
|
|
(325
|
)
|
||
|
58
|
||
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2016
|
2015
|
|||||||||
|
Change in Benefit Obligation
|
|||||||||||||
|
Projected benefit obligation — beginning of year
|
$
|
25,855
|
|
26,889
|
|
4,669
|
|
5,081
|
|
||||
|
Service cost
|
949
|
|
1,037
|
|
224
|
|
257
|
|
|||||
|
Interest cost
|
927
|
|
988
|
|
158
|
|
186
|
|
|||||
|
Plan participant contributions
|
54
|
|
48
|
|
—
|
|
—
|
|
|||||
|
Amendments
|
(48
|
)
|
60
|
|
—
|
|
—
|
|
|||||
|
Actuarial (gains) losses
|
2,302
|
|
(1,578
|
)
|
(73
|
)
|
(400
|
)
|
|||||
|
Divestitures & acquisitions
|
(24
|
)
|
(5
|
)
|
—
|
|
—
|
|
|||||
|
Curtailments, settlements & restructuring
|
(25
|
)
|
(20
|
)
|
—
|
|
(3
|
)
|
|||||
|
Benefits paid from plan*
|
(1,210
|
)
|
(773
|
)
|
(378
|
)
|
(420
|
)
|
|||||
|
Effect of exchange rates
|
(664
|
)
|
(791
|
)
|
5
|
|
(32
|
)
|
|||||
|
Projected benefit obligation — end of year
|
$
|
28,116
|
|
25,855
|
|
4,605
|
|
4,669
|
|
||||
|
Change in Plan Assets
|
|||||||||||||
|
Plan assets at fair value — beginning of year
|
$
|
22,254
|
|
22,575
|
|
74
|
|
79
|
|
||||
|
Actual return on plan assets
|
2,286
|
|
298
|
|
7
|
|
1
|
|
|||||
|
Company contributions
|
838
|
|
752
|
|
372
|
|
414
|
|
|||||
|
Plan participant contributions
|
54
|
|
48
|
|
—
|
|
—
|
|
|||||
|
Settlements
|
(25
|
)
|
(20
|
)
|
—
|
|
—
|
|
|||||
|
Divestitures & acquisitions
|
(24
|
)
|
(5
|
)
|
—
|
|
—
|
|
|||||
|
Benefits paid from plan assets*
|
(1,210
|
)
|
(773
|
)
|
(378
|
)
|
(420
|
)
|
|||||
|
Effect of exchange rates
|
(540
|
)
|
(621
|
)
|
—
|
|
—
|
|
|||||
|
Plan assets at fair value — end of year
|
$
|
23,633
|
|
22,254
|
|
75
|
|
74
|
|
||||
|
Funded status — end of year
|
$
|
(4,483
|
)
|
(3,601
|
)
|
(4,530
|
)
|
(4,595
|
)
|
||||
|
Amounts Recognized in the Company’s Balance Sheet consist of the following:
|
|||||||||||||
|
Non-current assets
|
$
|
227
|
|
256
|
|
—
|
|
—
|
|
||||
|
Current liabilities
|
(86
|
)
|
(77
|
)
|
(315
|
)
|
(324
|
)
|
|||||
|
Non-current liabilities
|
(4,624
|
)
|
(3,780
|
)
|
(4,215
|
)
|
(4,271
|
)
|
|||||
|
Total recognized in the consolidated balance sheet — end of year
|
$
|
(4,483
|
)
|
(3,601
|
)
|
(4,530
|
)
|
(4,595
|
)
|
||||
|
Amounts Recognized in Accumulated Other Comprehensive Income consist of the following:
|
|||||||||||||
|
Net actuarial loss
|
$
|
7,749
|
|
6,501
|
|
1,804
|
|
2,013
|
|
||||
|
Prior service cost (credit)
|
(12
|
)
|
34
|
|
(150
|
)
|
(185
|
)
|
|||||
|
Unrecognized net transition obligation
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Total before tax effects
|
$
|
7,737
|
|
6,535
|
|
1,654
|
|
1,828
|
|
||||
|
Accumulated Benefit Obligations — end of year
|
$
|
25,319
|
|
23,262
|
|
||||||||
|
*In 2016, the Company offered a voluntary lump-sum payment option below a pre-determined threshold for certain eligible former employees who are vested participants of the U.S. Qualified Defined Benefit Pension Plan. The
distribution of the lump-sums was substantially completed by the end of fiscal 2016. The amount distributed in 2016 was approximately $420 million. These distributions from the plan did not have a material impact on the Company’s financial position.
|
|||||||||||||
|
59
|
||
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2016
|
2015
|
|||||||||
|
Amounts Recognized in Net Periodic Benefit Cost and Other Comprehensive Income
|
|||||||||||||
|
Net periodic benefit cost
|
$
|
422
|
|
971
|
|
477
|
|
604
|
|
||||
|
Net actuarial (gain) loss
|
1,965
|
|
(75
|
)
|
(72
|
)
|
(389
|
)
|
|||||
|
Amortization of net actuarial loss
|
(496
|
)
|
(745
|
)
|
(135
|
)
|
(201
|
)
|
|||||
|
Prior service cost (credit)
|
(48
|
)
|
60
|
|
—
|
|
—
|
|
|||||
|
Amortization of prior service (cost) credit
|
(1
|
)
|
(2
|
)
|
34
|
|
33
|
|
|||||
|
Effect of exchange rates
|
(218
|
)
|
(218
|
)
|
(1
|
)
|
(1
|
)
|
|||||
|
Total recognized in other comprehensive income, before tax
|
$
|
1,202
|
|
(980
|
)
|
(174
|
)
|
(558
|
)
|
||||
|
Total recognized in net periodic benefit cost and other comprehensive income
|
$
|
1,624
|
|
(9
|
)
|
303
|
|
46
|
|
||||
|
U.S. Plans
|
International Plans
|
||||||||||||||||
|
Qualified Plans
|
Non-Qualified Plans
|
Funded Plans
|
Unfunded Plans
|
||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
|||||||||
|
Plan Assets
|
$
|
16,057
|
|
15,113
|
|
—
|
|
—
|
|
7,576
|
|
7,141
|
|
—
|
|
—
|
|
|
Projected Benefit Obligation
|
16,336
|
|
15,280
|
|
1,905
|
|
1,675
|
|
9,502
|
|
8,542
|
|
373
|
|
358
|
|
|
|
Accumulated Benefit Obligation
|
14,759
|
|
13,876
|
|
1,568
|
|
1,411
|
|
8,663
|
|
7,661
|
|
329
|
|
314
|
|
|
|
Over (Under) Funded Status
|
|||||||||||||||||
|
Projected Benefit Obligation
|
$
|
(279
|
)
|
(167
|
)
|
(1,905
|
)
|
(1,675
|
)
|
(1,926
|
)
|
(1,401
|
)
|
(373
|
)
|
(358
|
)
|
|
Accumulated Benefit Obligation
|
1,298
|
|
1,237
|
|
(1,568
|
)
|
(1,411
|
)
|
(1,087
|
)
|
(520
|
)
|
(329
|
)
|
(314
|
)
|
|
|
(Dollars in Millions)
|
2017
|
2018
|
2019
|
2020
|
2021
|
2022-2026
|
|||||||||||||
|
Projected future benefit payments
|
|||||||||||||||||||
|
Retirement plans
|
$
|
897
|
|
908
|
|
958
|
|
1,010
|
|
1,081
|
|
6,416
|
|
||||||
|
Other benefit plans
|
$
|
325
|
|
315
|
|
311
|
|
307
|
|
304
|
|
1,465
|
|
||||||
|
(Dollars in Millions)
|
2017
|
2018
|
2019
|
2020
|
2021
|
2022-2026
|
|||||||||||||
|
Projected future contributions
|
$
|
83
|
|
84
|
|
89
|
|
94
|
|
100
|
|
610
|
|
||||||
|
60
|
||
|
Percent of
Plan Assets
|
Target
Allocation
|
||||||||
|
|
2016
|
2015
|
2017
|
||||||
|
Worldwide Retirement Plans
|
|||||||||
|
Equity securities
|
75
|
%
|
79
|
%
|
73
|
%
|
|||
|
Debt securities
|
25
|
|
21
|
|
27
|
|
|||
|
Total plan assets
|
100
|
%
|
100
|
%
|
100
|
%
|
|||
|
•
|
Short-term investments
— Cash and quoted short-term instruments are valued at the closing price or the amount held on deposit by the custodian bank. Other investments are through investment vehicles valued using the Net Asset Value (NAV) provided by the administrator of the fund. The NAV is based on the value of the underlying assets owned by the fund, minus its liabilities, and then divided by the number of shares outstanding. The NAV is a quoted price in a market that is not active and classified as Level 2.
|
|
•
|
Government and agency securities
— A limited number of these investments are valued at the closing price reported on the major market on which the individual securities are traded. Where quoted prices are available in an active market, the investments are classified within Level 1 of the valuation hierarchy. If quoted market prices are not available for the specific security, then fair values are estimated by using pricing models, quoted prices of securities with similar characteristics or discounted cash flows. When quoted market prices for a security are not available in an active market, they are classified as Level 2.
|
|
•
|
Debt instruments
— A limited number of these investments are valued at the closing price reported on the major market on which the individual securities are traded. Where quoted prices are available in an active market, the investments are classified as Level 1. If quoted market prices are not available for the specific security, then fair values are estimated by using pricing models, quoted prices of securities with similar characteristics or discounted cash flows and are classified as Level 2. Level 3 debt instruments are priced based on unobservable inputs.
|
|
•
|
Equity securities
— Common stocks are valued at the closing price reported on the major market on which the individual securities are traded. Substantially all common stock is classified within Level 1 of the valuation hierarchy.
|
|
•
|
Commingled funds
— These investment vehicles are valued using the NAV provided by the fund administrator. The NAV is based on the value of the underlying assets owned by the fund, minus its liabilities, and then divided by the number of shares outstanding. Assets in the Level 2 category have a quoted market price.
|
|
61
|
||
|
•
|
Insurance contracts
— The instruments are issued by insurance companies. The fair value is based on negotiated value and the underlying investments held in separate account portfolios as well as considering the credit worthiness of the issuer. The underlying investments are government, asset-backed and fixed income securities. In general, insurance contracts are classified as Level 3 as there are no quoted prices nor other observable inputs for pricing.
|
|
•
|
Other assets
— Other assets are represented primarily by limited partnerships and real estate investments, as well as commercial loans and commercial mortgages that are not classified as corporate debt. Other assets that are exchange listed and actively traded are classified as Level 1, while inactively traded assets are classified as Level 2.
|
|
Quoted Prices
in Active
Markets for
Identical Assets
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
(a)
|
Investments Measured at Net Asset Value
(b)
|
|||||||||||||||||||||||||||
|
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total Assets
|
||||||||||||||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
2016
|
2015
|
||||||||||||||||||||
|
Short-term investment funds
|
$
|
145
|
|
184
|
|
652
|
|
312
|
|
—
|
|
—
|
|
—
|
|
—
|
|
797
|
|
496
|
|
|||||||||
|
Government and agency securities
|
—
|
|
—
|
|
2,655
|
|
1,767
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,655
|
|
1,767
|
|
||||||||||
|
Debt instruments
|
—
|
|
—
|
|
1,237
|
|
1,050
|
|
—
|
|
1
|
|
—
|
|
—
|
|
1,237
|
|
1,051
|
|
||||||||||
|
Equity securities
|
11,433
|
|
11,317
|
|
12
|
|
11
|
|
—
|
|
—
|
|
—
|
|
—
|
|
11,445
|
|
11,328
|
|
||||||||||
|
Commingled funds
|
—
|
|
—
|
|
1,316
|
|
1,100
|
|
—
|
|
—
|
|
5,767
|
|
6,122
|
|
7,083
|
|
7,222
|
|
||||||||||
|
Insurance contracts
|
—
|
|
—
|
|
—
|
|
—
|
|
24
|
|
23
|
|
—
|
|
—
|
|
24
|
|
23
|
|
||||||||||
|
Other assets
|
—
|
|
—
|
|
—
|
|
107
|
|
—
|
|
—
|
|
392
|
|
260
|
|
392
|
|
367
|
|
||||||||||
|
Investments at fair value
|
$
|
11,578
|
|
11,501
|
|
5,872
|
|
4,347
|
|
24
|
|
24
|
|
6,159
|
|
6,382
|
|
23,633
|
|
22,254
|
|
|||||||||
|
11.
|
Savings Plan
|
|
62
|
||
|
12.
|
Capital and Treasury Stock
|
|
Treasury Stock
|
|||||||
|
(Amounts in Millions Except Treasury Stock Shares in Thousands)
|
Shares
|
Amount
|
|||||
|
Balance at December 29, 2013
|
299,215
|
|
$
|
15,700
|
|
||
|
Employee compensation and stock option plans
|
(32,302
|
)
|
(2,933
|
)
|
|||
|
Repurchase of common stock
|
69,707
|
|
7,124
|
|
|||
|
Balance at December 28, 2014
|
336,620
|
|
19,891
|
|
|||
|
Employee compensation and stock option plans
|
(24,413
|
)
|
(2,497
|
)
|
|||
|
Repurchase of common stock
|
52,474
|
|
5,290
|
|
|||
|
Balance at January 3, 2016
|
364,681
|
|
22,684
|
|
|||
|
Employee compensation and stock option plans
|
(30,839
|
)
|
(3,311
|
)
|
|||
|
Repurchase of common stock
|
79,490
|
|
8,979
|
|
|||
|
Balance at January 1, 2017
|
413,332
|
|
$
|
28,352
|
|
||
|
13.
|
Accumulated Other Comprehensive Income
|
|
(Dollars in Millions)
|
Foreign
Currency Translation |
Gain/(Loss) On Securities
|
Employee Benefit Plans
|
Gain/
(Loss) On Derivatives & Hedges |
Total
Accumulated Other Comprehensive Income (Loss) |
|||||||||||
|
December 29, 2013
|
$
|
(202
|
)
|
106
|
|
(3,009
|
)
|
245
|
|
(2,860
|
)
|
|||||
|
Net 2014 changes
|
(4,601
|
)
|
151
|
|
(3,308
|
)
|
(104
|
)
|
(7,862
|
)
|
||||||
|
December 28, 2014
|
(4,803
|
)
|
257
|
|
(6,317
|
)
|
141
|
|
(10,722
|
)
|
||||||
|
Net 2015 changes
|
(3,632
|
)
|
347
|
|
1,019
|
|
(177
|
)
|
(2,443
|
)
|
||||||
|
January 3, 2016
|
(8,435
|
)
|
604
|
|
(5,298
|
)
|
(36
|
)
|
(13,165
|
)
|
||||||
|
Net 2016 changes
|
(612
|
)
|
(193
|
)
|
(682
|
)
|
(249
|
)
|
(1,736
|
)
|
||||||
|
January 1, 2017
|
$
|
(9,047
|
)
|
411
|
|
(5,980
|
)
|
(285
|
)
|
(14,901
|
)
|
|||||
|
63
|
||
|
14.
|
International Currency Translation
|
|
15.
|
Earnings Per Share
|
|
(In Millions Except Per Share Amounts)
|
2016
|
2015
|
2014
|
|||||||
|
Basic net earnings per share
|
$
|
6.04
|
|
5.56
|
|
5.80
|
|
|||
|
Average shares outstanding — basic
|
2,737.3
|
|
2,771.8
|
|
2,815.2
|
|
||||
|
Potential shares exercisable under stock option plans
|
142.4
|
|
141.5
|
|
142.6
|
|
||||
|
Less: shares repurchased under treasury stock method
|
(92.1
|
)
|
(102.6
|
)
|
(96.5
|
)
|
||||
|
Convertible debt shares
|
1.3
|
|
2.2
|
|
2.6
|
|
||||
|
Adjusted average shares outstanding — diluted
|
2,788.9
|
|
2,812.9
|
|
2,863.9
|
|
||||
|
Diluted net earnings per share
|
$
|
5.93
|
|
5.48
|
|
5.70
|
|
|||
|
16.
|
Rental Expense and Lease Commitments
|
|
2017
|
2018
|
2019
|
2020
|
2021
|
After 2021
|
Total
|
||||||
|
$216
|
179
|
134
|
105
|
88
|
100
|
822
|
||||||
|
17.
|
Common Stock, Stock Option Plans and Stock Compensation Agreements
|
|
64
|
||
|
2016
|
2015
|
2014
|
||||||
|
Risk-free rate
|
1.51
|
%
|
1.77
|
%
|
1.87
|
%
|
||
|
Expected volatility
|
15.76
|
%
|
15.48
|
%
|
14.60
|
%
|
||
|
Expected life (in years)
|
7.0
|
|
7.0
|
|
6.0
|
|
||
|
Expected dividend yield
|
3.10
|
%
|
2.90
|
%
|
3.10
|
%
|
||
|
(Shares in Thousands)
|
Outstanding Shares
|
Weighted
Average Exercise Price
|
Aggregate
Intrinsic
Value
(Dollars in Millions)
|
||||||||
|
Shares at December 29, 2013
|
119,556
|
|
$
|
64.70
|
|
$
|
3,306
|
|
|||
|
Options granted
|
24,356
|
|
90.44
|
|
|||||||
|
Options exercised
|
(25,319
|
)
|
62.31
|
|
|||||||
|
Options canceled/forfeited
|
(2,881
|
)
|
75.48
|
|
|||||||
|
Shares at December 28, 2014
|
115,712
|
|
70.37
|
|
4,014
|
|
|||||
|
Options granted
|
20,484
|
|
100.06
|
|
|||||||
|
Options exercised
|
(16,683
|
)
|
62.53
|
|
|||||||
|
Options canceled/forfeited
|
(2,996
|
)
|
82.22
|
|
|||||||
|
Shares at January 3, 2016
|
116,517
|
|
76.41
|
|
3,065
|
|
|||||
|
Options granted
|
22,491
|
|
101.87
|
|
|||||||
|
Options exercised
|
(22,547
|
)
|
65.66
|
|
|||||||
|
Options canceled/forfeited
|
(3,006
|
)
|
92.83
|
|
|||||||
|
Shares at January 1, 2017
|
113,455
|
|
$
|
83.16
|
|
$
|
3,636
|
|
|||
|
65
|
||
|
(Shares in Thousands)
|
Outstanding
|
Exercisable
|
||||||||||
|
Exercise Price Range
|
Options
|
Average Life
(1)
|
Average Exercise Price
|
Options
|
Average Exercise Price
|
|||||||
|
$52.13-$58.33
|
7,361
|
|
2.1
|
$58.32
|
7,361
|
|
$58.32
|
|||||
|
$58.34-$62.20
|
11,297
|
|
2.4
|
$61.95
|
11,297
|
|
$61.95
|
|||||
|
$62.62-$65.62
|
14,380
|
|
3.1
|
$64.24
|
14,380
|
|
$64.24
|
|||||
|
$66.07-$72.54
|
18,127
|
|
6.0
|
$72.52
|
17,241
|
|
$72.52
|
|||||
|
$90.44-$101.87
|
62,290
|
|
8.1
|
$97.40
|
135
|
|
$93.73
|
|||||
|
|
113,455
|
|
6.2
|
$83.16
|
50,414
|
|
$65.77
|
|||||
|
(Shares in Thousands)
|
Outstanding Restricted Share Units
|
Outstanding Performance Share Units
|
||||
|
Shares at December 29, 2013
|
30,617
|
|
1,535
|
|
||
|
Granted
|
8,487
|
|
1,113
|
|
||
|
Issued
|
(9,685
|
)
|
(19
|
)
|
||
|
Canceled/forfeited
|
(1,726
|
)
|
(98
|
)
|
||
|
Shares at December 28, 2014
|
27,693
|
|
2,531
|
|
||
|
Granted
|
7,637
|
|
931
|
|
||
|
Issued
|
(10,164
|
)
|
(285
|
)
|
||
|
Canceled/forfeited
|
(1,281
|
)
|
(99
|
)
|
||
|
Shares at January 3, 2016
|
23,885
|
|
3,078
|
|
||
|
Granted
|
7,173
|
|
958
|
|
||
|
Issued
|
(8,913
|
)
|
(1,437
|
)
|
||
|
Canceled/forfeited
|
(1,084
|
)
|
(184
|
)
|
||
|
Shares at January 1, 2017
|
21,061
|
|
2,415
|
|
||
|
66
|
||
|
18.
|
Segments of Business and Geographic Areas
|
|
|
Sales to Customers
|
|||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
|||||||
|
Consumer —
|
||||||||||
|
United States
|
$
|
5,420
|
|
5,222
|
|
5,096
|
|
|||
|
International
|
7,887
|
|
8,285
|
|
9,400
|
|
||||
|
Total
|
13,307
|
|
13,507
|
|
14,496
|
|
||||
|
Pharmaceutical —
|
||||||||||
|
United States
|
20,125
|
|
18,333
|
|
17,432
|
|
||||
|
International
|
13,339
|
|
13,097
|
|
14,881
|
|
||||
|
Total
|
33,464
|
|
31,430
|
|
32,313
|
|
||||
|
Medical Devices —
|
||||||||||
|
United States
|
12,266
|
|
12,132
|
|
12,254
|
|
||||
|
International
|
12,853
|
|
13,005
|
|
15,268
|
|
||||
|
Total
|
25,119
|
|
25,137
|
|
27,522
|
|
||||
|
Worldwide total
|
$
|
71,890
|
|
70,074
|
|
74,331
|
|
|||
|
|
Income Before Tax
|
Identifiable Assets
|
|||||||||||||||
|
(Dollars in Millions)
|
2016
(3)
|
2015
(4)
|
2014
(5)
|
2016
|
2015
|
||||||||||||
|
Consumer
|
$
|
2,441
|
|
1,787
|
|
1,941
|
|
$
|
23,971
|
|
20,772
|
|
|||||
|
Pharmaceutical
|
12,827
|
|
11,734
|
|
11,696
|
|
27,477
|
|
26,144
|
|
|||||||
|
Medical Devices
|
5,578
|
|
6,826
|
|
7,953
|
|
39,773
|
|
40,979
|
|
|||||||
|
Total
|
20,846
|
|
20,347
|
|
21,590
|
|
91,221
|
|
87,895
|
|
|||||||
|
Less: Expense not allocated to segments
(1)
|
1,043
|
|
1,151
|
|
1,027
|
|
|||||||||||
|
General corporate
(2)
|
49,987
|
|
45,516
|
|
|||||||||||||
|
Worldwide total
|
$
|
19,803
|
|
19,196
|
|
20,563
|
|
$
|
141,208
|
|
133,411
|
|
|||||
|
Additions to Property,
Plant & Equipment
|
Depreciation and
Amortization
|
|||||||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
2016
|
2015
|
2014
|
||||||||||||||
|
Consumer
|
$
|
486
|
|
544
|
|
581
|
|
$
|
608
|
|
559
|
|
577
|
|
||||||
|
Pharmaceutical
|
927
|
|
1,063
|
|
977
|
|
886
|
|
929
|
|
1,053
|
|
||||||||
|
Medical Devices
|
1,472
|
|
1,631
|
|
1,807
|
|
1,928
|
|
1,945
|
|
1,974
|
|
||||||||
|
Segments total
|
2,885
|
|
3,238
|
|
3,365
|
|
3,422
|
|
3,433
|
|
3,604
|
|
||||||||
|
General corporate
|
341
|
|
225
|
|
349
|
|
332
|
|
313
|
|
291
|
|
||||||||
|
Worldwide total
|
$
|
3,226
|
|
3,463
|
|
3,714
|
|
$
|
3,754
|
|
3,746
|
|
3,895
|
|
||||||
|
67
|
||
|
|
Sales to Customers
|
Long-Lived Assets
(6)
|
|||||||||||||||
|
(Dollars in Millions)
|
2016
|
2015
|
2014
|
2016
|
2015
|
||||||||||||
|
United States
|
$
|
37,811
|
|
35,687
|
|
34,782
|
|
$
|
36,934
|
|
36,609
|
|
|||||
|
Europe
|
15,770
|
|
15,995
|
|
18,947
|
|
21,996
|
|
20,167
|
|
|||||||
|
Western Hemisphere excluding U.S.
|
5,734
|
|
6,045
|
|
7,160
|
|
2,961
|
|
2,881
|
|
|||||||
|
Asia-Pacific, Africa
|
12,575
|
|
12,347
|
|
13,442
|
|
2,512
|
|
2,493
|
|
|||||||
|
Segments total
|
71,890
|
|
70,074
|
|
74,331
|
|
64,403
|
|
62,150
|
|
|||||||
|
General corporate
|
1,190
|
|
1,148
|
|
|||||||||||||
|
Other non long-lived assets
|
75,615
|
|
70,113
|
|
|||||||||||||
|
Worldwide total
|
$
|
71,890
|
|
70,074
|
|
74,331
|
|
$
|
141,208
|
|
133,411
|
|
|||||
|
(1)
|
Amounts not allocated to segments include interest (income) expense and general corporate (income) expense.
|
|
(2)
|
General corporate includes cash, cash equivalents and marketable securities.
|
|
(3)
|
Includes net litigation expense of
$806 million
and a restructuring related charge of
$685 million
in the Medical Devices segment. The Pharmaceutical segment includes a positive adjustment of
$0.5 billion
to previous reserve estimates, an in-process research and development expense of
$29 million
, and gains from the divestitures of the controlled substance raw material and active pharmaceutical ingredient (API) business and certain anesthetic products in Europe.
|
|
(4)
|
The Medical Devices segment includes a restructuring related charge of
$590 million
, an intangible asset write-down of
$346 million
related to Acclarent, Synthes integration costs of
$196 million
and
$148 million
expense for the cost associated with the DePuy ASR
TM
Hip program. Includes
$224 million
of in-process research and development expense, comprised of
$214 million
and
$10 million
in the Pharmaceutical and Medical Devices segments, respectively. Includes net litigation expense of
$141 million
comprised of
$136 million
in the Pharmaceutical segment and
$5 million
in the Medical Devices segment, which included the gain from the litigation settlement agreement with Guidant for
$600 million
. The Medical Devices Segment includes a gain of
$1.3 billion
from the divestiture of the Cordis business. The Pharmaceutical segment includes a gain of
$981 million
from the U.S. divestiture of NUCYNTA
®
and a positive adjustment of
$0.5 billion
to previous reserve estimates, including Managed Medicaid rebates. The Consumer segment includes a gain of
$229 million
from the divestiture of SPLENDA
®
brand.
|
|
(5)
|
Includes net litigation expense of
$1,253 million
comprised of
$907 million
,
$259 million
and
$87 million
in the Medical Devices, Pharmaceutical and Consumer segments, respectively. Includes
$178 million
of in-process research and development expense, comprised of
$147 million
and
$31 million
in the Pharmaceutical and Medical Devices segments, respectively. The Medical Devices segment includes a net gain of
$1,899 million
from the divestiture of the Ortho-Clinical Diagnostics business, Synthes integration costs of
$754 million
and
$126 million
expense for the cost associated with the DePuy ASR
TM
Hip program. The Pharmaceutical segment includes an additional year of the Branded Prescription Drug Fee of
$220 million
and a positive adjustment of
$0.1 billion
to previous reserve estimates.
|
|
(6)
|
Long-lived assets include property, plant and equipment, net for
2016
, and
2015
of
$15,912
and
$15,905
, respectively, and intangible assets and goodwill, net for
2016
and
2015
of
$49,681
and
$47,393
, respectively.
|
|
68
|
||
|
19.
|
Selected Quarterly Financial Data (unaudited)
|
|
|
2016
|
2015
|
|||||||||||||||||||||||
|
(Dollars in Millions Except Per Share Data)
|
First Quarter
(1)
|
Second Quarter
(2)
|
Third Quarter
(3)
|
Fourth Quarter
(4)
|
First Quarter
(5)
|
Second Quarter
(6)
|
Third Quarter
(7)
|
Fourth Quarter
(8)
|
|||||||||||||||||
|
Segment sales to customers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Consumer
|
$
|
3,195
|
|
3,419
|
|
3,261
|
|
3,432
|
|
3,390
|
|
3,483
|
|
3,314
|
|
3,320
|
|
||||||||
|
Pharmaceutical
|
8,178
|
|
8,654
|
|
8,400
|
|
8,232
|
|
7,726
|
|
7,946
|
|
7,694
|
|
8,064
|
|
|||||||||
|
Medical Devices
|
6,109
|
|
6,409
|
|
6,159
|
|
6,442
|
|
6,258
|
|
6,358
|
|
6,094
|
|
6,427
|
|
|||||||||
|
Total sales
|
17,482
|
|
18,482
|
|
17,820
|
|
18,106
|
|
17,374
|
|
17,787
|
|
17,102
|
|
17,811
|
|
|||||||||
|
Gross profit
|
12,153
|
|
13,146
|
|
12,334
|
|
12,572
|
|
12,092
|
|
12,430
|
|
11,878
|
|
12,138
|
|
|||||||||
|
Earnings before provision for taxes on income
|
5,294
|
|
4,904
|
|
5,281
|
|
4,324
|
|
5,575
|
|
5,741
|
|
4,122
|
|
3,758
|
|
|||||||||
|
Net earnings
|
4,457
|
|
3,997
|
|
4,272
|
|
3,814
|
|
4,320
|
|
4,516
|
|
3,358
|
|
3,215
|
|
|||||||||
|
Basic net earnings per share
|
$
|
1.62
|
|
1.46
|
|
1.56
|
|
1.41
|
|
1.55
|
|
1.63
|
|
1.21
|
|
1.16
|
|
||||||||
|
Diluted net earnings per share
|
$
|
1.59
|
|
1.43
|
|
1.53
|
|
1.38
|
|
1.53
|
|
1.61
|
|
1.20
|
|
1.15
|
|
||||||||
|
(1)
|
The first quarter has been recast to reflect the adoption of ASU 2016-09. See Note 1 to the Consolidated Financial Statements for more details. The first quarter of 2016 includes a restructuring charge of
$120 million
after-tax (
$137 million
before-tax) and net litigation expense of
$56 million
after-tax (
$66 million
before-tax).
|
|
(2)
|
The second quarter of 2016 includes a restructuring charge of
$97 million
after-tax (
$141 million
before-tax) and net litigation expense of
$493 million
after-tax (
$600 million
before-tax).
|
|
(3)
|
The third quarter of 2016 includes a restructuring charge of
$76 million
after-tax (
$109 million
before-tax) and net litigation expense of
$46 million
after-tax (
$55 million
before-tax).
|
|
(4)
|
The fourth quarter of 2016 includes a restructuring charge of
$251 million
after-tax (
$298 million
before-tax) and net litigation expense of
$80 million
after-tax (
$96 million
before-tax).
|
|
(5)
|
The first quarter of 2015 includes a net litigation gain of
$253 million
after-tax (
$402 million
before-tax) and
$122 million
after-tax (
$139 million
before-tax) for costs associated with the DePuy ASR
TM
Hip program.
|
|
(6)
|
The second quarter of 2015 includes net litigation expense of
$23 million
after-tax (
$134 million
before-tax).
|
|
(7)
|
The third quarter of 2015 includes net litigation expense of
$348 million
after-tax (
$409 million
before-tax).
|
|
(8)
|
The fourth quarter of 2015 includes a restructuring charge of
$415 million
after-tax (
$590 million
before-tax),
$156 million
after-tax (
$214 million
before-tax) from impairment of in-process research and development and Synthes integration costs of
$59 million
after-tax (
$83 million
before-tax). Additionally, the fourth quarter of 2015 includes the gain on the Cordis divestiture.
|
|
69
|
||
|
20.
|
Business Combinations and Divestitures
|
|
70
|
||
|
21.
|
Legal Proceedings
|
|
71
|
||
|
72
|
||
|
73
|
||
|
74
|
||
|
75
|
||
|
76
|
||
|
77
|
||
|
78
|
||
|
79
|
||
|
80
|
||
|
81
|
||
|
82
|
||
|
(Dollars in Millions)
|
Severance
|
Asset Write-offs
|
Other**
|
Total
|
|||||
|
2015 restructuring charge
|
$
|
484
|
|
86
|
|
20
|
|
590
|
|
|
2015 activity
|
(86
|
)
|
(3
|
)
|
(89
|
)
|
|||
|
Reserve balance, January 3, 2016
|
484
|
|
—
|
|
17
|
|
501
|
|
|
|
Current year activity:
|
|
|
|
|
|||||
|
Charges
|
—
|
|
249
|
|
436
|
|
685
|
|
|
|
Cash payments
|
(104
|
)
|
—
|
|
(452
|
)
|
(556
|
)
|
|
|
Settled non cash
|
—
|
|
(249
|
)
|
—
|
|
(249
|
)
|
|
|
Reserve balance, January 1, 2017*
|
$
|
380
|
|
—
|
|
1
|
|
381
|
|
|
83
|
||
|
84
|
||
|
/s/ Alex Gorsky
|
/s/ Dominic J. Caruso
|
|
|
Alex Gorsky
|
|
Dominic J. Caruso
|
|
Chairman, Board of Directors
|
|
Executive Vice President, Chief Financial Officer
|
|
Chief Executive Officer
|
|
|
|
85
|
||

|
2011
|
2012
|
2013
|
2014
|
2015
|
2016
|
|
|
Johnson & Johnson
|
$100.00
|
$110.83
|
$149.19
|
$175.05
|
$177.08
|
$204.21
|
|
S&P 500 Index
|
$100.00
|
$115.99
|
$153.55
|
$174.55
|
$176.95
|
$198.10
|
|
S&P Pharmaceutical Index
|
$100.00
|
$114.43
|
$154.73
|
$189.12
|
$200.06
|
$196.93
|
|
S&P Healthcare Equipment Index
|
$100.00
|
$117.27
|
$149.74
|
$189.09
|
$200.39
|
$213.38
|
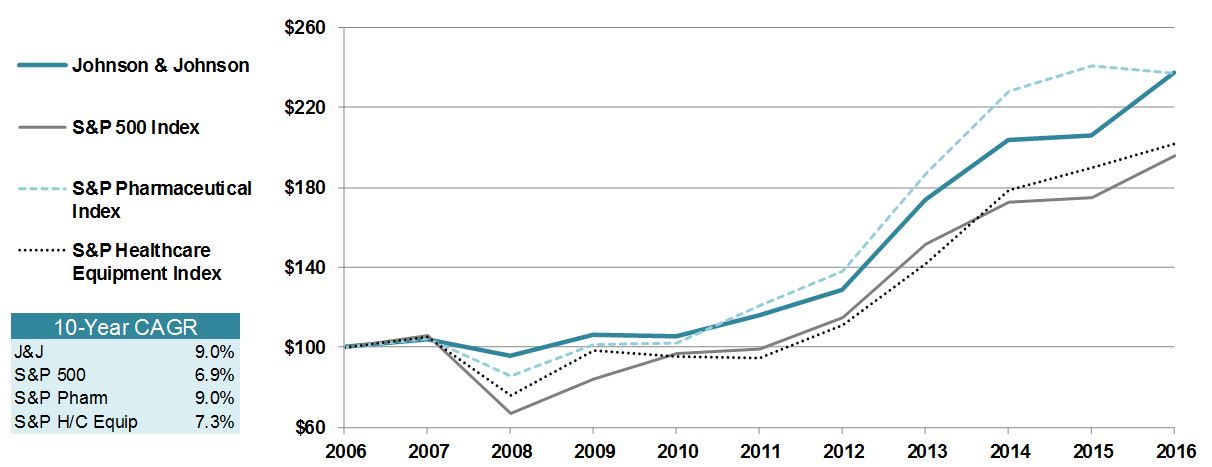
|
2006
|
2007
|
2008
|
2009
|
2010
|
2011
|
2012
|
2013
|
2014
|
2015
|
2016
|
|
|
Johnson & Johnson
|
$100.00
|
$103.61
|
$95.56
|
$106.34
|
$105.72
|
$116.17
|
$128.75
|
$173.32
|
$203.36
|
$205.72
|
$237.24
|
|
S&P 500 Index
|
$100.00
|
$105.57
|
$66.51
|
$84.10
|
$96.76
|
$98.80
|
$114.60
|
$151.71
|
$172.46
|
$174.83
|
$195.72
|
|
S&P Pharmaceutical Index
|
$100.00
|
$104.66
|
$85.61
|
$101.55
|
$102.34
|
$120.51
|
$137.90
|
$186.48
|
$227.91
|
$241.09
|
$237.32
|
|
S&P Healthcare Equipment Index
|
$100.00
|
$105.13
|
$76.07
|
$97.97
|
$95.32
|
$94.55
|
$110.88
|
$141.58
|
$178.79
|
$189.47
|
$201.76
|
|
86
|
||
|
Item 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
Item 9A.
|
CONTROLS AND PROCEDURES
|
|
Item 9B.
|
OTHER INFORMATION
|
|
Item 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
87
|
||
|
Item 11.
|
EXECUTIVE COMPENSATION
|
|
Item 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
Plan Category
|
Number of Securities to
be Issued Upon Exercise of
Outstanding Options and Rights
|
Weighted Average
Exercise Price of
Outstanding Options and Rights
|
Number of Securities
Remaining Available for
Future Issuance Under Equity Compensation Plans
(2)(3)
|
||||||
|
Equity Compensation Plans Approved by Security Holders
(1)
|
137,289,904
|
|
|
$68.72
|
|
439,398,804
|
|
||
|
Equity Compensation Plans Not Approved by Security Holders
|
-
|
|
-
|
|
-
|
|
|||
|
Total
|
137,289,904
|
|
|
$68.72
|
|
439,398,804
|
|
||
|
(1)
|
Included in this category are the following equity compensation plans which have been approved by the Company’s shareholders: 2005 Long-Term Incentive Plan and 2012 Long-Term Incentive Plan.
|
|
(2)
|
This column excludes shares reflected under the column “Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights.”
|
|
(3)
|
The 2005 Long-Term Incentive Plan expired April 26, 2012. All options and restricted shares granted subsequent to that date were under the 2012 Long-Term Incentive Plan.
|
|
Item 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
Item 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
88
|
||
|
Item 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
89
|
||
|
JOHNSON & JOHNSON
|
|
(Registrant)
|
|
By
|
/s/ A. Gorsky
|
|
A. Gorsky, Chairman, Board of Directors,
and Chief Executive Officer |
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ A. Gorsky
|
|
Chairman, Board of Directors
|
|
February 27, 2017
|
|
A. Gorsky
|
Chief Executive Officer
(Principal Executive Officer)
|
|||
|
|
|
|
|
|
|
/s/ D. J. Caruso
|
|
Chief Financial Officer
|
|
February 27, 2017
|
|
D. J. Caruso
|
(Principal Financial Officer)
|
|||
|
|
|
|
|
|
|
/s/ R. A. Kapusta
|
|
Controller and Chief Accounting Officer
|
|
February 27, 2017
|
|
R. A. Kapusta
|
(Principal Accounting Officer)
|
|||
|
|
|
|
|
|
|
/s/ M. C. Beckerle
|
Director
|
February 27, 2017
|
||
|
M. C. Beckerle
|
||||
|
/s/ D. S. Davis
|
|
Director
|
|
February 27, 2017
|
|
D. S. Davis
|
||||
|
|
|
|
|
|
|
/s/ I. E. L. Davis
|
|
Director
|
|
February 27, 2017
|
|
I. E. L. Davis
|
|
|
|
|
|
90
|
||
|
Signature
|
|
Title
|
|
Date
|
|
/s/ M. B. McClellan
|
Director
|
February 27, 2017
|
||
|
M. B. McClellan
|
||||
|
|
|
|
|
|
|
/s/ A. M. Mulcahy
|
|
Director
|
|
February 27, 2017
|
|
A. M. Mulcahy
|
||||
|
|
|
|
|
|
|
/s/ W. D. Perez
|
|
Director
|
|
February 27, 2017
|
|
W. D. Perez
|
||||
|
|
|
|
|
|
|
/s/ C. Prince
|
|
Director
|
|
February 27, 2017
|
|
C. Prince
|
||||
|
|
|
|
|
|
|
/s/ A. E. Washington
|
|
Director
|
|
February 27, 2017
|
|
A. E. Washington
|
||||
|
/s/ R. A. Williams
|
|
Director
|
|
February 27, 2017
|
|
R. A. Williams
|
||||
|
91
|
||
|
Reg. S-K
|
|
|
|
Exhibit Table
|
Description
|
|
|
Item No.
|
of Exhibit
|
|
|
3(i)
|
Restated Certificate of Incorporation effective February 19, 2016 — Incorporated herein by reference to Exhibit 3(i) of the Registrant’s Form 10-K Annual Report for the fiscal year ended January 3, 2016.
|
|
|
3(ii)
|
By-Laws of the Company, as amended effective January 26, 2016 — Incorporated herein by reference to Exhibit 3.1 the Registrant’s Form 8-K Current Report filed January 26, 2016.
|
|
|
4(a)
|
Upon the request of the Securities and Exchange Commission, the Registrant will furnish a copy of all instruments defining the rights of holders of long-term debt of the Registrant.
|
|
|
10(a)
|
2005 Long-Term Incentive Plan — Incorporated herein by reference to Exhibit 4 of the Registrant’s S-8 Registration Statement filed with the Commission on May 10, 2005 (file no. 333-124785).*
|
|
|
10(b)
|
Form of Stock Option Certificate, Restricted Share Unit Certificate and Performance Share Unit Certificate under the 2005 Long-Term Incentive Plan — Incorporated herein by reference to Exhibits 10.1, 10.2 and 10.3 of the Registrant’s Form 8-K Current Report filed January 13, 2012.*
|
|
|
10(c)
|
2012 Long-Term Incentive Plan — Incorporated herein by reference to Appendix A of the Registrant’s Proxy Statement filed with the Commission on March 14, 2012 .*
|
|
|
10(d)
|
Form of Stock Option Certificate, Restricted Share Unit Certificate and Performance Share Unit Certificate under the 2012 Long-Term Incentive Plan — Incorporated herein by reference to Exhibits 10.2, 10.3 and 10.4 of the Registrant’s Form 10-Q Quarterly Report filed May 7, 2012.*
|
|
|
10(e)
|
Executive Incentive Plan (as amended) — Incorporated herein by reference to Exhibit 10(f) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 31, 2000.*
|
|
|
10(f)
|
Domestic Deferred Compensation (Certificate of Extra Compensation) Plan — Incorporated herein by reference to Exhibit 10(g) of the Registrant’s Form 10-K Annual Report for the year ended December 28, 2003.*
|
|
|
10(g)
|
Amendments to the Certificate of Extra Compensation Plan effective as of January 1, 2009 — Incorporated herein by reference to Exhibit 10(j) of the Registrant’s Form 10-K Annual Report for the year ended December 28, 2008.*
|
|
|
10(h)
|
2009 Certificates of Long-Term Performance Plan — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended September 27, 2009.*
|
|
|
10(i)
|
Amended and Restated Deferred Fee Plan for Directors — Incorporated herein by reference to Exhibit 10(k) of the Registrant's Form 10-K Annual Report for the fiscal year ended January 1, 2012.*
|
|
|
10(j)
|
Executive Income Deferral Plan (Amended and Restated) — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended September 30, 2012.*
|
|
|
10(k)
|
Excess Savings Plan — Incorporated herein by reference to Exhibit 10(j) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 29, 1996.*
|
|
|
10(l)
|
Amendments to the Johnson & Johnson Excess Savings Plan effective as of January 1, 2009 — Incorporated herein by reference to Exhibit 10(p) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 28, 2008.*
|
|
|
10(m)
|
Excess Benefit Plan (Supplemental Retirement Plan) — Incorporated herein by reference to Exhibit 10(h) of the Registrant’s Form 10-K Annual Report for the fiscal year ended January 3, 1993.*
|
|
|
10(n)
|
Amendments to the Excess Benefit Plan of Johnson & Johnson and Affiliated Companies effective as of January 1, 2009 — Incorporated herein by reference to Exhibit 10(r) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 28, 2008.*
|
|
|
10(o)
|
Amendment to the Excess Benefit Plan of Johnson & Johnson and Affiliated Companies, effective as of January 1, 2015 — Incorporated herein by reference to Exhibit 10(q) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 28, 2014.*
|
|
|
10(p)
|
Executive Life Plan Agreement — Incorporated herein by reference to Exhibit 10(i) of the Registrant’s Form 10-K Annual Report for the fiscal year ended January 3, 1993.*
|
|
|
10(q)
|
Executive Life Plan Agreement Closure Letter — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended March 29, 2015.*
|
|
|
10(r)
|
Johnson & Johnson Retirement Savings Plan, Johnson & Johnson Savings Plan for Union Represented Employees, and Johnson & Johnson Savings Plan - Incorporated herein by reference to Exhibits 99.1, 99.2 and 99.3 of the Registrant's Form S-8 filed with the Commission on May 6, 2013.*
|
|
|
10(s)
|
Employment Agreement for Dr. Paulus Stoffels - Incorporated herein by reference to Exhibit 10.2 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended September 30, 2012.*
|
|
|
10(t)
|
Summary of Employment Arrangements for Sandra E. Peterson — Incorporated herein by reference to Exhibit 10(t) of the Registrant's Form 10-K Annual Report for the year ended December 30, 2012.*
|
|
|
92
|
||
|
Reg. S-K
|
|
|
|
Exhibit Table
|
Description
|
|
|
Item No.
|
of Exhibit
|
|
|
10(u)
|
Severance Pay Plan of Johnson & Johnson and U.S. Affiliated Companies, Amended and Restated as of October 1, 2014 — Incorporated herein by reference to Exhibit 10.1 of the Registrant's Form 10-Q Quarterly Report for the quarter ended September 28, 2014.*
|
|
|
10(v)
|
First Amendment to the Severance Pay Plan of Johnson & Johnson and U.S. Affiliated Companies (as amended and restated effective October 1, 2014) — Incorporated herein by reference to Exhibit 10.1 of the Registrant's Form 10-Q Quarterly Report for the quarter ended June 28, 2015.*
|
|
|
10(w)
|
Second Amendment to the Severance Pay Plan of Johnson & Johnson and U.S. Affiliated Companies (as amended and restated effective October 1, 2014) — Incorporated herein by reference to Exhibit 10(x) of the Registrant's Form 10-K Annual Report for the fiscal year ended January 3, 2016.*
|
|
|
12
|
Statement of Computation of Ratio of Earnings to Fixed Charges — Filed with this document.
|
|
|
21
|
Subsidiaries - Filed with this document.
|
|
|
23
|
Consent of Independent Registered Public Accounting Firm — Filed with this document.
|
|
|
31(a)
|
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act — Filed with this document.
|
|
|
31(b)
|
Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act — Filed with this document.
|
|
|
32(a)
|
Certification of Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act — Furnished with this document.
|
|
|
32(b)
|
Certification of Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act — Furnished with this document.
|
|
|
101
|
XBRL (Extensible Business Reporting Language) The following materials from this Report for the fiscal year ended January 1, 2017, formatted in Extensive Business Reporting Language (XBRL): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Earnings, (iii) Consolidated Statements of Comprehensive Income, (iv) Consolidated Statements of Equity, (v) Consolidated Statements of Cash Flows, and (vi) Notes to the Consolidated Financial Statements.
|
|
|
*
|
Management contract or compensatory plan.
|
|
93
|
||