|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the fiscal year ended December 30, 2018
|
Commission file number 1-3215
|
|
New Jersey
|
|
22-1024240
|
|
(State of incorporation)
|
|
(I.R.S. Employer Identification No.)
|
|
One Johnson & Johnson Plaza
New Brunswick, New Jersey
|
|
08933
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Common Stock, Par Value $1.00
|
New York Stock Exchange
|
|
|
4.75% Notes Due November 2019
|
New York Stock Exchange
|
|
|
0.250% Notes Due January 2022
|
New York Stock Exchange
|
|
|
0.650% Notes Due May 2024
|
New York Stock Exchange
|
|
|
5.50% Notes Due November 2024
|
New York Stock Exchange
|
|
|
1.150% Notes Due November 2028
|
New York Stock Exchange
|
|
|
1.650% Notes Due May 2035
|
New York Stock Exchange
|
|
|
Large accelerated filer
þ
|
|
Accelerated filer
o
|
|
|
|
Non-accelerated filer
o
|
|
Smaller reporting company
o
|
|
|
|
Emerging growth company
o
|
||||
|
Parts I and III:
|
Portions of registrant’s proxy statement for its 2019 annual meeting of shareholders filed within 120 days after the close of the registrant’s fiscal year (the "Proxy Statement"), are incorporated by reference to this report on Form 10-K (this "Report").
|
|
Item
|
|
Page
|
|
1
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
1A.
|
||
|
1B.
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
|
||
|
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
7A.
|
||
|
8
|
||
|
9
|
||
|
9A.
|
||
|
9B.
|
||
|
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
|
||
|
15
|
||
|
16
|
||
|
|
||
|
|
||
|
•
|
Challenges and uncertainties inherent in innovation and development of new and improved products and technologies on which the Company’s continued growth and success depend, including uncertainty of clinical outcomes, additional analysis of existing clinical data, obtaining regulatory approvals, health plan coverage and customer access, and initial and continued commercial success;
|
|
•
|
Challenges to the Company’s ability to obtain and protect adequate patent and other intellectual property rights for new and existing products and technologies in the United States and other important markets;
|
|
•
|
The impact of patent expirations, typically followed by the introduction of competing biosimilars and generics and resulting revenue and market share losses;
|
|
•
|
Increasingly aggressive and frequent challenges to the Company’s patents by competitors and others seeking to launch competing generic, biosimilar or other products and increased receptivity of courts, the United States Patent and Trademark Office and other decision makers to such challenges, potentially resulting in loss of market exclusivity and rapid decline in sales for the relevant product sooner than expected;
|
|
•
|
Competition in research and development of new and improved products, processes and technologies, which can result in product and process obsolescence;
|
|
•
|
Competition to reach agreement with third parties for collaboration, licensing, development and marketing agreements for products and technologies;
|
|
•
|
Competition based on cost-effectiveness, product performance, technological advances and patents attained by competitors; and
|
|
•
|
Allegations that the Company’s products infringe the patents and other intellectual property rights of third parties, which could adversely affect the Company’s ability to sell the products in question and require the payment of money damages and future royalties.
|
|
•
|
Product efficacy or safety concerns, whether or not based on scientific evidence, potentially resulting in product withdrawals, recalls, regulatory action on the part of the United States Food and Drug Administration (or international counterparts), declining sales, reputational damage, increased litigation expense and share price impact;
|
|
•
|
Impact, including declining sales and reputational damage, of significant litigation or government action adverse to the Company, including product liability claims and allegations related to pharmaceutical marketing practices and contracting strategies;
|
|
•
|
Impact of an adverse judgment or settlement and the adequacy of reserves related to legal proceedings, including patent litigation, product liability, personal injury claims, securities class actions, government investigations, employment and other legal proceedings;
|
|
•
|
Increased scrutiny of the health care industry by government agencies and state attorneys general resulting in investigations and prosecutions, which carry the risk of significant civil and criminal penalties, including, but not limited to, debarment from government business;
|
|
•
|
Failure to meet compliance obligations in the McNEIL-PPC, Inc. Consent Decree or any other compliance agreements with governments or government agencies, which could result in significant sanctions;
|
|
•
|
Potential changes to applicable laws and regulations affecting United States and international operations, including relating to: approval of new products; licensing and patent rights; sales and promotion of health care products; access to, and reimbursement and pricing for, health care products and services; environmental protection and sourcing of raw materials;
|
|
•
|
Compliance with local regulations and laws that may restrict the Company’s ability to manufacture or sell its products in relevant markets including, requirements to comply with medical device reporting regulations and other requirements such as the European Union's Medical Devices Regulation;
|
|
•
|
Changes in domestic and international tax laws and regulations, including changes related to The Tax Cuts and Jobs Act in the United States, the Federal Act on Tax Reform and AHV Financing in Switzerland, increasing audit scrutiny by tax authorities around the world and exposures to additional tax liabilities potentially in excess of existing reserves; and
|
|
•
|
Issuance of new or revised accounting standards by the Financial Accounting Standards Board and regulations by the Securities and Exchange Commission.
|
|
•
|
Pricing pressures resulting from trends toward health care cost containment, including the continued consolidation among health care providers and other market participants, trends toward managed care, the shift toward governments increasingly becoming the primary payers of health care expenses, significant new entrants to the health care markets seeking to reduce costs and government pressure on companies to voluntarily reduce costs and price increases;
|
|
•
|
Restricted spending patterns of individual, institutional and governmental purchasers of health care products and services due to economic hardship and budgetary constraints;
|
|
•
|
Challenges to the Company’s ability to realize its strategy for growth including through externally sourced innovations, such as development collaborations, strategic acquisitions, licensing and marketing agreements, and the potential heightened costs of any such external arrangements due to competitive pressures;
|
|
•
|
The potential that the expected strategic benefits and opportunities from any planned or completed acquisition or divestiture by the Company may not be realized or may take longer to realize than expected; and
|
|
•
|
The potential that the expected benefits and opportunities related to past and ongoing restructuring actions may not be realized or may take longer to realize than expected.
|
|
•
|
Market conditions and the possibility that the Company’s share repurchase program may be delayed, suspended or discontinued;
|
|
•
|
Impact of inflation and fluctuations in interest rates and currency exchange rates and the potential effect of such fluctuations on revenues, expenses and resulting margins;
|
|
•
|
Potential changes in export/import and trade laws, regulations and policies of the United States and other countries, including any increased trade restrictions or tariffs and potential drug reimportation legislation;
|
|
•
|
The impact on international operations from financial instability in international economies, sovereign risk, possible imposition of governmental controls and restrictive economic policies, and unstable international governments and legal systems;
|
|
•
|
Changes to global climate, extreme weather and natural disasters that could affect demand for the Company's products and services, cause disruptions in manufacturing and distribution networks, alter the availability of goods and services within the supply chain, and affect the overall design and integrity of the Company's products and operations; and
|
|
•
|
The impact of armed conflicts and terrorist attacks in the United States and other parts of the world including social and economic disruptions and instability of financial and other markets.
|
|
•
|
Difficulties and delays in manufacturing, internally through third party providers or otherwise within the supply chain, that may lead to voluntary or involuntary business interruptions or shutdowns, product shortages, withdrawals or suspensions of products from the market, and potential regulatory action;
|
|
•
|
Interruptions and breaches of the Company's information technology systems or those of the Company's vendors which, could result in reputational, competitive, operational or other business harm as well as financial costs and regulatory action;
|
|
•
|
Reliance on global supply chains and production and distribution processes that are complex and subject to increasing regulatory requirements that may adversely affect supply, sourcing and pricing of materials used in the Company’s products; and
|
|
•
|
The potential that the expected benefits and opportunities related to restructuring actions contemplated for the global supply chain may not be realized or may take longer to realize than expected, including due to any required approvals from applicable regulatory authorities. Disruptions associated with the announced global supply chain actions may adversely affect supply and sourcing of materials used in the Company's products.
|
|
Item 1.
|
BUSINESS
|
|
1
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
Item 1A.
|
RISK FACTORS
|
|
5
|
||
|
6
|
||
|
7
|
||
|
•
|
protective economic policies taken by governments such as trade protection measures and import/export licensing requirements;
|
|
•
|
compliance with local regulations and laws including, in some countries, regulatory requirements restricting the Company’s ability to manufacture or sell its products in the relevant market;
|
|
•
|
diminished protection of intellectual property and contractual rights in certain jurisdictions;
|
|
•
|
potential nationalization or expropriation of the Company’s foreign assets; and
|
|
•
|
disruptions to markets due to war, armed conflict, terrorism, social upheavals or pandemics.
|
|
8
|
||
|
9
|
||
|
Item 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
Segment
|
Square Feet
(in thousands)
|
||
|
Consumer
|
6,503
|
|
|
|
Pharmaceutical
|
6,819
|
|
|
|
Medical Devices
|
7,183
|
|
|
|
Worldwide Total
|
20,505
|
|
|
|
Geographic Area
|
Number of Facilities
|
Square Feet
(in thousands)
|
||||
|
United States
|
37
|
|
5,855
|
|
||
|
Europe
|
34
|
|
7,587
|
|
||
|
Western Hemisphere, excluding U.S.
|
12
|
|
2,800
|
|
||
|
Africa, Asia and Pacific
|
28
|
|
4,263
|
|
||
|
Worldwide Total
|
111
|
|
20,505
|
|
||
|
10
|
||
|
Item 3.
|
LEGAL PROCEEDINGS
|
|
Item 4.
|
MINE SAFETY DISCLOSURES
|
|
Name
|
Age
|
Position
|
||
|
Joaquin Duato
|
56
|
Vice Chairman, Executive Committee
(a)
|
||
|
Peter M. Fasolo
|
56
|
Member, Executive Committee; Executive Vice President, Chief Human Resources Officer
(b)
|
||
|
Alex Gorsky
|
58
|
Chairman, Board of Directors; Chairman, Executive Committee; Chief Executive Officer
|
||
|
Ashley McEvoy
|
48
|
Member, Executive Committee; Executive Vice President, Worldwide Chairman, Medical Devices
(c)
|
||
|
Jorge Mesquita
|
57
|
Member, Executive Committee; Executive Vice President, Worldwide Chairman, Consumer
(d)
|
||
|
Thibaut Mongon
|
49
|
Appointee, Member, Executive Committee, Executive Vice President, Worldwide Chairman, Consumer
(e)
|
||
|
Michael E. Sneed
|
59
|
Member, Executive Committee; Executive Vice President, Global Corporate Affairs and Chief Communication Officer
(f)
|
||
|
Paulus Stoffels
|
56
|
Vice Chairman, Executive Committee; Chief Scientific Officer
(g)
|
||
|
Jennifer L. Taubert
|
55
|
Member, Executive Committee; Executive Vice President, Worldwide Chairman, Pharmaceuticals
(h)
|
||
|
Michael H. Ullmann
|
60
|
Member, Executive Committee; Executive Vice President, General Counsel
(i)
|
||
|
Kathryn E. Wengel
|
53
|
Member, Executive Committee; Executive Vice President, Chief Global Supply Chain Officer
(j)
|
||
|
Joseph J. Wolk
|
52
|
Member, Executive Committee; Executive Vice President, Chief Financial Officer
(k)
|
||
|
(a)
|
Mr. J. Duato joined the Company in 1989 with Janssen-Farmaceutica S.A. (Spain), a subsidiary of the Company, and held executive positions of increasing responsibility in the Pharmaceutical sector. In 2009, he was named Company Group Chairman, Pharmaceuticals, and in 2011, he was named Worldwide Chairman, Pharmaceuticals. In 2016, Mr. Duato became a member of the Executive Committee and was named Executive Vice President, Worldwide Chairman,
|
|
11
|
||
|
(b)
|
Dr. P. M. Fasolo joined the Company in 2004 as Vice President, Worldwide Human Resources for Cordis Corporation, a subsidiary of the Company, and was subsequently named Vice President, Global Talent Management for the Company. He left Johnson & Johnson in 2007 to join Kohlberg Kravis Roberts & Co. as Chief Talent Officer. Dr. Fasolo returned to the Company in 2010 as the Vice President, Global Human Resources, and in 2011, he became a member of the Executive Committee. In April 2016, he was named Executive Vice President, Chief Human Resources Officer. Mr. Fasolo has responsibility for global talent, recruiting, diversity, compensation, benefits, employee relations and all aspects of the human resources agenda for the Company.
|
|
(c)
|
Ms. A. McEvoy joined the Company in 1997 as Assistant Brand Manager of McNeil Consumer Health, a subsidiary of the Company, advancing through positions of increasing responsibilities until she was appointed Company Group Chairman, Vision Care in 2012, followed by Company Group Chairman, Consumer Medical Devices in 2014. In July 2018, Ms. McEvoy was promoted to Executive Vice President, Worldwide Chairman, Medical Devices, and became a member of the Executive Committee. She has responsibility for the surgery, orthopaedics, interventional solutions and eye health businesses across Ethicon, DePuy Synthes, Biosense Webster and Johnson & Johnson Vision.
|
|
(d)
|
Mr. J. Mesquita joined the Company in 2014 as Worldwide Chairman, Consumer. Prior to joining the Company, he served in various marketing and leadership capacities across Latin America, including roles in Oral Care and Beauty at The Procter & Gamble Company from 1984 to 2013. In April 2016, Mr. Mesquita became a member of the Executive Committee and was promoted to Executive Vice President, Worldwide Chairman, Consumer. Mr. Mesquita plans to retire from the Company in March 2019.
|
|
(e)
|
Mr. T. Mongon joined the Company in 2000 as Director of Marketing for the Vision Care group in France and subsequently held general management positions as Country Manager France, Belgium and North Africa, Managing Director Latin America, and President Asia-Pacific. Mr. Mongon transitioned to the Pharmaceutical sector in 2012 as the Global Commercial Strategy Leader for the Neuroscience therapeutic area, before joining the consumer sector as Company Group Chairman Asia-Pacific. The Company has announced that Mr. Mongon will be named Executive Vice President and Worldwide Chairman, Consumer, and a member of the Executive Committee, upon the retirement of his predecessor, Mr. Mesquita, effective March 1, 2019. In addition to leading the Consumer business, Mr. Mongon will have responsibility for Johnson & Johnson Southeast Asia.
|
|
(f)
|
Mr. M. E. Sneed joined the Company in 1986 as Product Director for Personal Products, a subsidiary of the Company, and gained increased responsibilities in executive positions across the global enterprise. In 2004, Mr. Sneed was appointed Company Group Chairman, Consumer North America, followed by Company Group Chairman, Vision Care Franchise in 2007. In 2012, he became the Vice President, Global Corporate Affairs and Chief Communications Officer. Mr. Sneed was appointed Executive Vice President, Global Corporate Affairs and Chief Communications Officer in January 2018, and became a member of the Executive Committee in July 2018, leading the corporation's global marketing, communication, design and philanthropy functions.
|
|
(g)
|
Dr. P. Stoffels joined the Company in 2002 with the acquisition of Tibotec Virco NV, where he was Chief Executive Officer of Virco NV and Chairman of Tibotec NV. In 2005, he was appointed Company Group Chairman, Global Virology. In 2006, he assumed the role of Company Group Chairman, Pharmaceuticals. Dr. Stoffels was appointed Global Head, Research & Development, Pharmaceuticals in 2009, and in 2011, became Worldwide Chairman, Pharmaceuticals. In 2012, Dr. Stoffels was appointed Chief Scientific Officer, and became a member of the Executive Committee. In 2016, Dr. Stoffels was named Executive Vice President, Chief Scientific Officer. In 2018, Dr. Stoffels was promoted to Vice Chairman of the Executive Committee, Chief Scientific Officer. He is responsible for the Company’s innovation agenda across the Pharmaceutical, Medical Devices and Consumer sectors, product safety strategy, and the Company’s global public health strategy.
|
|
(h)
|
Ms. J. L. Taubert joined the Company in 2005 as Worldwide Vice President at Johnson & Johnson Pharmaceutical Services, a subsidiary of the Company. She held several executive positions in the Pharmaceutical sector until 2012 when she was appointed Company Group Chairman, North America Pharmaceuticals, and in 2015 became Company Group Chairman, The Americas, Pharmaceuticals. In July 2018, Ms. Taubert was promoted to Executive Vice President, Worldwide Chairman, Pharmaceuticals, and became a member of the Executive Committee.
|
|
(i)
|
Mr. M. H. Ullmann joined the Company in 1989 as a corporate attorney in the Law Department. He was appointed Corporate Secretary in 1999 and served in that role until 2006. During that time, he also held various management positions in the Law Department. In 2006, he was named General Counsel, Medical Devices and Diagnostics and was appointed Vice President, General Counsel and became a member of the Executive Committee in 2012. In April 2016, Mr. Ullmann was named Executive Vice President, General Counsel. Mr. Ullmann has worldwide responsibility for legal, government affairs & policy, global security, aviation and health care compliance & privacy.
|
|
12
|
||
|
(j)
|
Ms. K. E. Wengel joined the Company in 1988 as Project Engineer and Engineering Supervisor at Janssen, a subsidiary of the Company. During her tenure with the Company, she has held a variety of strategic leadership and executive positions across the global enterprise, in roles within operations, quality, engineering, new products, information technology, and other technical and business functions. In 2010, Ms. Wengel became the first Chief Quality Officer of the Company. In 2014, she was promoted to Vice President, Johnson & Johnson Supply Chain. In July 2018, she was promoted to Executive Vice President, Chief Global Supply Chain Officer, and became a member of the Executive Committee.
|
|
(k)
|
Mr. J. J. Wolk joined the Company in 1998 as Finance Manager, Business Development for Ortho-McNeil, a subsidiary of the Company, and through the years held a variety of senior leadership roles in several segments and functions across the Company's subsidiaries, in Pharmaceuticals, Medical Devices and Supply Chain. From 2014 to 2016, he served as Vice President, Finance and Chief Financial Officer of the Janssen Pharmaceutical Companies of Johnson & Johnson. In 2016, Mr. Wolk became the Vice President, Investor Relations. In July 2018, he was appointed Executive Vice President, Chief Financial Officer and became a member of the Executive Committee. Mr. Wolk is responsible for leading the development and execution of the Company's global long-term financial strategy.
|
|
13
|
||
|
Item 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Period
|
Total Number
of Shares Purchased
(1)
|
Avg. Price
Paid Per Share
|
Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs
(2)
|
Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Plans or Programs
(3)
|
|||||||
|
October 1, 2018 through October 28, 2018
|
2,192,500
|
|
$
|
138.74
|
|
-
|
-
|
||||
|
October 29, 2018 through November 25, 2018
|
6,849,298
|
|
143.27
|
|
-
|
-
|
|||||
|
November 26, 2018 through December 30, 2018
|
18,130,189
|
|
139.10
|
|
7,073,136
|
32,131,870
|
|||||
|
Total
|
27,171,987
|
|
|
||||||||
|
(1)
|
During the fiscal fourth quarter of 2018, the Company repurchased an aggregate of 27,171,987 shares of Johnson & Johnson Common Stock in open-market transactions, of which 7,073,136 shares were purchased pursuant to the repurchase program that was publicly announced on December 17, 2018, and of which 20,098,851 shares were purchased in open-market transactions as part of a systematic plan to meet the needs of the Company’s compensation programs.
|
|
(2)
|
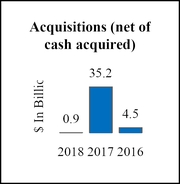
As of December 30, 2018, an aggregate of 7,073,136 shares were purchased for a total of $0.9 billion since the inception of the repurchase program announced on December 17, 2018.
|
|
(3)
|
As of December 30, 2018, the maximum number of shares that may yet be purchased under the plan is 32,131,870 based on the closing price of Johnson & Johnson Common Stock on the New York Stock Exchange on December 28, 2018 of $127.27 per share.
|
|
14
|
||
|
Item 6.
|
SELECTED FINANCIAL DATA
|
|
(Dollars in Millions Except Per Share Amounts)
|
2018
|
2017
|
2016
|
2015
|
2014
|
2013
|
2012
|
2011
|
2010
|
2009
|
2008
|
|||||||||||||||||
|
Sales to customers — U.S.
|
$41,884
|
39,863
|
37,811
|
35,687
|
34,782
|
|
31,910
|
|
29,830
|
|
28,908
|
|
29,450
|
|
30,889
|
|
32,309
|
|
||||||||||
|
Sales to customers — International
|
39,697
|
36,587
|
34,079
|
34,387
|
39,549
|
|
39,402
|
|
37,394
|
|
36,122
|
|
32,137
|
|
31,008
|
|
31,438
|
|
||||||||||
|
Total sales
|
81,581
|
76,450
|
71,890
|
70,074
|
74,331
|
|
71,312
|
|
67,224
|
|
65,030
|
|
61,587
|
|
61,897
|
|
63,747
|
|
||||||||||
|
Cost of products sold
|
27,091
|
25,439
|
21,789
|
21,426
|
22,684
|
|
22,181
|
|
21,515
|
|
20,219
|
|
18,688
|
|
18,380
|
|
18,463
|
|
||||||||||
|
Selling, marketing and administrative expenses
|
22,540
|
21,520
|
20,067
|
21,079
|
21,887
|
|
21,650
|
|
20,697
|
|
20,800
|
|
19,296
|
|
19,712
|
|
21,431
|
|
||||||||||
|
Research and development expense
|
10,775
|
10,594
|
9,143
|
8,999
|
8,471
|
|
8,119
|
|
7,602
|
|
7,486
|
|
6,796
|
|
6,949
|
|
7,554
|
|
||||||||||
|
In-process research and development
|
1,126
|
408
|
29
|
224
|
178
|
|
580
|
|
1,163
|
|
—
|
|
—
|
|
—
|
|
181
|
|
||||||||||
|
Interest income
|
(611)
|
(385)
|
(368)
|
(128)
|
(67
|
)
|
(74
|
)
|
(64
|
)
|
(91
|
)
|
(107
|
)
|
(90
|
)
|
(361
|
)
|
||||||||||
|
Interest expense, net of portion capitalized
|
1,005
|
934
|
726
|
552
|
533
|
|
482
|
|
532
|
|
571
|
|
455
|
|
451
|
|
435
|
|
||||||||||
|
Other (income) expense, net
|
1,405
|
(42)
|
210
|
(1,783)
|
82
|
|
2,903
|
|
2,004
|
|
3,115
|
|
(488
|
)
|
(333
|
)
|
(885
|
)
|
||||||||||
|
Restructuring
|
251
|
309
|
491
|
509
|
—
|
|
—
|
|
—
|
|
569
|
|
—
|
|
1,073
|
|
—
|
|
||||||||||
|
|
63,582
|
58,777
|
52,087
|
50,878
|
53,768
|
|
55,841
|
|
53,449
|
|
52,669
|
|
44,640
|
|
46,142
|
|
46,818
|
|
||||||||||
|
Earnings before provision for taxes on income
|
$17,999
|
17,673
|
19,803
|
19,196
|
20,563
|
|
15,471
|
|
13,775
|
|
12,361
|
|
16,947
|
|
15,755
|
|
16,929
|
|
||||||||||
|
Provision for taxes on income
|
2,702
|
16,373
|
3,263
|
3,787
|
4,240
|
|
1,640
|
|
3,261
|
|
2,689
|
|
3,613
|
|
3,489
|
|
3,980
|
|
||||||||||
|
Net earnings
|
15,297
|
1,300
|
16,540
|
15,409
|
16,323
|
|
13,831
|
|
10,514
|
|
9,672
|
|
13,334
|
|
12,266
|
|
12,949
|
|
||||||||||
|
Add: Net loss attributable to noncontrolling interest
|
—
|
—
|
—
|
—
|
—
|
|
—
|
|
339
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Net earnings attributable to Johnson & Johnson
|
15,297
|
1,300
|
16,540
|
15,409
|
16,323
|
|
13,831
|
|
10,853
|
|
9,672
|
|
13,334
|
|
12,266
|
|
12,949
|
|
||||||||||
|
Percent of sales to customers
|
18.8%
|
1.7
|
23.0
|
22.0
|
22.0
|
|
19.4
|
|
16.1
|
|
14.9
|
|
21.7
|
|
19.8
|
|
20.3
|
|
||||||||||
|
Diluted net earnings per share of common stock
(1)
|
$5.61
|
0.47
|
5.93
|
5.48
|
5.70
|
|
4.81
|
|
3.86
|
|
3.49
|
|
4.78
|
|
4.40
|
|
4.57
|
|
||||||||||
|
Percent return on average shareholders’ equity
|
25.5%
|
2.0
|
23.4
|
21.9
|
22.7
|
|
19.9
|
|
17.8
|
|
17.0
|
|
24.9
|
|
26.4
|
|
30.2
|
|
||||||||||
|
Percent increase (decrease) over previous year:
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
Sales to customers
|
6.7%
|
6.3
|
2.6
|
(5.7)
|
4.2
|
|
6.1
|
|
3.4
|
|
5.6
|
|
(0.5
|
)
|
(2.9
|
)
|
4.3
|
|
||||||||||
|
Diluted net earnings per share
|
N/M
|
(92.1)%
|
8.2
|
(3.9)
|
18.5
|
|
24.6
|
|
10.6
|
|
(27.0
|
)
|
8.6
|
|
(3.7
|
)
|
25.9
|
|
||||||||||
|
Supplementary balance sheet data:
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
Property, plant and equipment, net
|
17,035
|
17,005
|
15,912
|
15,905
|
16,126
|
|
16,710
|
|
16,097
|
|
14,739
|
|
14,553
|
|
14,759
|
|
14,365
|
|
||||||||||
|
Additions to property, plant and equipment
|
3,670
|
3,279
|
3,226
|
3,463
|
3,714
|
|
3,595
|
|
2,934
|
|
2,893
|
|
2,384
|
|
2,365
|
|
3,066
|
|
||||||||||
|
Total assets
|
152,954
|
157,303
|
141,208
|
133,411
|
130,358
|
|
131,754
|
|
121,347
|
|
113,644
|
|
102,908
|
|
94,682
|
|
84,912
|
|
||||||||||
|
Long-term debt
|
27,684
|
30,675
|
22,442
|
12,857
|
15,122
|
|
13,328
|
|
11,489
|
|
12,969
|
|
9,156
|
|
8,223
|
|
8,120
|
|
||||||||||
|
Operating cash flow
|
22,201
|
21,056
|
18,767
|
19,569
|
18,710
|
|
17,414
|
|
15,396
|
|
14,298
|
|
16,385
|
|
16,571
|
|
14,972
|
|
||||||||||
|
Common stock information
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
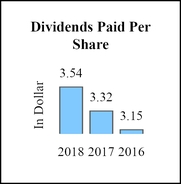
Dividends paid per share
|
$3.54
|
3.32
|
3.15
|
2.95
|
2.76
|
|
2.59
|
|
2.40
|
|
2.25
|
|
2.11
|
|
1.93
|
|
1.795
|
|
||||||||||
|
Shareholders’ equity per share
|
22.44
|
22.43
|
26.02
|
25.82
|
25.06
|
|
26.25
|
|
23.33
|
|
20.95
|
|
20.66
|
|
18.37
|
|
15.35
|
|
||||||||||
|
Market price per share (year-end close)
|
$127.27
|
139.72
|
115.21
|
102.72
|
105.06
|
|
92.35
|
|
69.48
|
|
65.58
|
|
61.85
|
|
64.41
|
|
58.56
|
|
||||||||||
|
Average shares outstanding (millions)
|
||||||||||||||||||||||||||||
|
— basic
|
2,681.5
|
2,692.0
|
2,737.3
|
2,771.8
|
2,815.2
|
|
2,809.2
|
|
2,753.3
|
|
2,736.0
|
|
2,751.4
|
|
2,759.5
|
|
2,802.5
|
|
||||||||||
|
— diluted
|
2,728.7
|
2,745.3
|
2,788.9
|
2,812.9
|
2,863.9
|
|
2,877.0
|
|
2,812.6
|
|
2,775.3
|
|
2,788.8
|
|
2,789.1
|
|
2,835.6
|
|
||||||||||
|
Employees (thousands)
|
135.1
|
134.0
|
126.4
|
127.1
|
126.5
|
|
128.1
|
|
127.6
|
|
117.9
|
|
114.0
|
|
115.5
|
|
118.7
|
|
||||||||||
|
15
|
||
|
Item 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF RESULTS OF OPERATIONS AND FINANCIAL CONDITION
|

|
16
|
||
|
Sales increase/(decrease) due to:
|
2018
|
2017
|
2016
|
||||||
|
Volume
|
8.5
|
%
|
8.0
|
%
|
3.2
|
%
|
|||
|
Price
|
(2.2
|
)
|
(2.0
|
)
|
0.7
|
|
|||
|
Currency
|
0.4
|
|
0.3
|
|
(1.3
|
)
|
|||
|
Total
|
6.7
|
%
|
6.3
|
%
|
2.6
|
%
|
|||
|
17
|
||
|
|
|
|
|
% Change
|
||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
’18 vs. ’17
|
’17 vs. ’16
|
|||||||||||
|
Beauty
|
$
|
4,382
|
|
4,200
|
|
3,897
|
|
4.3
|
%
|
7.8
|
|
|||||
|
OTC
|
4,334
|
|
4,126
|
|
3,977
|
|
5.0
|
|
3.7
|
|
||||||
|
Baby Care
|
1,858
|
|
1,916
|
|
2,001
|
|
(3.0
|
)
|
(4.2
|
)
|
||||||
|
Oral Care
|
1,555
|
|
1,531
|
|
1,568
|
|
1.6
|
|
(2.4
|
)
|
||||||
|
Women’s Health
|
1,049
|
|
1,050
|
|
1,067
|
|
(0.1
|
)
|
(1.6
|
)
|
||||||
|
Wound Care/Other
|
675
|
|
779
|
|
797
|
|
(13.4
|
)
|
(2.3
|
)
|
||||||
|
Total Consumer Sales
|
$
|
13,853
|
|
13,602
|
|
13,307
|
|
1.8
|
%
|
2.2
|
|
|||||
|
18
|
||
|
% Change
|
||||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
’18 vs. ’17
|
’17 vs. ’16
|
|||||||||||
|
Total Immunology
|
$
|
13,120
|
|
12,244
|
|
11,968
|
|
7.2
|
%
|
2.3
|
|
|||||
|
REMICADE
®
|
5,326
|
|
6,315
|
|
6,966
|
|
(15.7
|
)
|
(9.3
|
)
|
||||||
|
SIMPONI
®
/SIMPONI ARIA
®
|
2,084
|
|
1,833
|
|
1,745
|
|
13.7
|
|
5.0
|
|
||||||
|
STELARA
®
|
5,156
|
|
4,011
|
|
3,232
|
|
28.5
|
|
24.1
|
|
||||||
|
TREMFYA
®
|
544
|
|
63
|
|
—
|
|
**
|
|
**
|
|
||||||
|
Other Immunology
|
10
|
|
22
|
|
25
|
|
(54.5
|
)
|
(12.0
|
)
|
||||||
|
Total Infectious Diseases
|
3,304
|
|
3,154
|
|
3,208
|
|
4.8
|
|
(1.7
|
)
|
||||||
|
EDURANT
®
/rilpivirine
|
816
|
|
714
|
|
573
|
|
14.3
|
|
24.6
|
|
||||||
|
PREZISTA
®
/ PREZCOBIX
®
/REZOLSTA
®
/SYMTUZA
®
|
1,955
|
|
1,821
|
|
1,851
|
|
7.4
|
|
(1.6
|
)
|
||||||
|
Other Infectious Diseases
|
533
|
|
619
|
|
784
|
|
(13.9
|
)
|
(21.0
|
)
|
||||||
|
Total Neuroscience
|
6,077
|
|
5,986
|
|
6,085
|
|
1.5
|
|
(1.6
|
)
|
||||||
|
CONCERTA
®
/methylphenidate
|
663
|
|
791
|
|
863
|
|
(16.2
|
)
|
(8.3
|
)
|
||||||
|
INVEGA SUSTENNA
®
/XEPLION
®
/INVEGA TRINZA
®
/TREVICTA
®
|
2,928
|
|
2,569
|
|
2,214
|
|
14.0
|
|
16.0
|
|
||||||
|
RISPERDAL CONSTA
®
|
737
|
|
805
|
|
893
|
|
(8.4
|
)
|
(9.9
|
)
|
||||||
|
Other Neuroscience
|
1,749
|
|
1,821
|
|
2,115
|
|
(4.0
|
)
|
(13.9
|
)
|
||||||
|
Total Oncology
|
9,844
|
|
7,258
|
|
5,807
|
|
35.6
|
|
25.0
|
|
||||||
|
DARZALEX
®
|
2,025
|
|
1,242
|
|
572
|
|
63.0
|
|
**
|
|
||||||
|
IMBRUVICA
®
|
2,615
|
|
1,893
|
|
1,251
|
|
38.1
|
|
51.3
|
|
||||||
|
VELCADE
®
|
1,116
|
|
1,114
|
|
1,224
|
|
0.2
|
|
(9.0
|
)
|
||||||
|
ZYTIGA
®
/abiraterone acetate
|
3,498
|
|
2,505
|
|
2,260
|
|
39.6
|
|
10.8
|
|
||||||
|
Other Oncology
|
590
|
|
504
|
|
500
|
|
17.1
|
|
0.8
|
|
||||||
|
Pulmonary Hypertension
|
2,573
|
|
1,327
|
|
—
|
|
93.9
|
|
***
|
|
||||||
|
OPSUMIT
®
|
1,215
|
|
573
|
|
—
|
|
**
|
|
***
|
|
||||||
|
TRACLEER
®
|
546
|
|
403
|
|
—
|
|
35.5
|
|
***
|
|
||||||
|
UPTRAVI
®
|
663
|
|
263
|
|
—
|
|
**
|
|
***
|
|
||||||
|
Other
|
149
|
|
88
|
|
—
|
|
69.3
|
|
***
|
|
||||||
|
Cardiovascular / Metabolism / Other
|
5,816
|
|
6,287
|
|
6,396
|
|
(7.5
|
)
|
(1.7
|
)
|
||||||
|
XARELTO
®
|
2,477
|
|
2,500
|
|
2,288
|
|
(0.9
|
)
|
9.3
|
|
||||||
|
INVOKANA
®
/ INVOKAMET
®
|
881
|
|
1,111
|
|
1,407
|
|
(20.7
|
)
|
(21.0
|
)
|
||||||
|
PROCRIT
®
/EPREX
®
|
988
|
|
972
|
|
1,105
|
|
1.6
|
|
(12.0
|
)
|
||||||
|
Other
|
1,470
|
|
1,704
|
|
1,596
|
|
(13.7
|
)
|
6.8
|
|
||||||
|
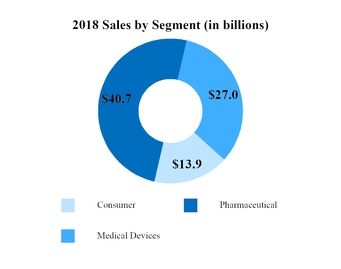
Total Pharmaceutical Sales
|
$
|
40,734
|
|
36,256
|
|
33,464
|
|
12.4
|
%
|
8.3
|
|
|||||
|
19
|
||
|
20
|
||
|
Product Name (Chemical Name)
|
Indication
|
US Approval
|
EU Approval
|
US Filing
|
EU Filing
|
|
DARZALEX
®
(daratumumab)
|
Frontline multiple myeloma transplant ineligible patients in combination with bortezomib, melphalan, and prednisone
|
Ÿ
|
Ÿ
|
||
|
erdafitinib
|
Treatment of locally advanced or metastatic urothelial cancer
|
Ÿ
|
|||
|
ERLEADA™ (apalutamide)
|
Treatment of non-metastatic castration-resistant prostate cancer
|
Ÿ
|
Ÿ
|
||
|
esketamine
|
Antidepressant for treatment-resistant depression in adults
|
Ÿ
|
Ÿ
|
||
|
IMBRUVICA
®
(ibrutinib)
|
Treatment of Waldenstrom's macroglobulinemia used in combination with rituximab
|
Ÿ
|
Ÿ
|
||
|
Treatment for previously untreated Chronic Lymphocytic Leukemia in combination with obinutuzumab
|
Ÿ
|
Ÿ
|
|||
|
INVOKANA
®
(canagliflozin)
|
Reduce the risk of death in type 2 diabetes with established, or risk for, cardiovascular disease. (CANVAS/CANVAS-R )
|
Ÿ
|
Ÿ
|
||
|
JULUCA
®
(rilpivirine and dolutegravir)
|
Single-tablet, once-daily, two-drug regimen for the treatment of HIV-1 infection
|
Ÿ
|
|||
|
OPSUMIT
®
(macitentan)
|
Treatment of adults with inoperable chronic thromboembolic pulmonary hypertension to improve exercise capacity and pulmonary vascular resistance
|
Ÿ
|
Ÿ
|
||
|
STELARA
®
(ustekinumab)
|
Treatment of adults with moderately to severely active ulcerative colitis
|
Ÿ
|
Ÿ
|
||
|
SYMTUZA
®
(darunavir/cobicistat/ emtricitabine/tenofovir alafenamide)
|
A complete darunavir-based single-tablet regimen for the treatment of HIV-1 infection
|
Ÿ
|
|||
|
TREMFYA
®
(guselkumab)
|
Patient controlled injector for the treatment of adults living with moderate to severe plaque psoriasis
|
Ÿ
|
Ÿ
|
||
|
XARELTO
®
(rivaroxaban)
|
Treatment to reduce the risk of major cardiovascular events in people with chronic coronary or peripheral artery disease
|
Ÿ
|
|||
|
For the prevention of venous thromboembolism for medically ill patients
|
Ÿ
|
||||
|
ZYTIGA
®
(abiraterone acetate)
|
Treatment of Hormone Naïve Metastatic Prostate Cancer
|
Ÿ
|
|||
|
21
|
||
|
|
|
|
|
% Change
|
||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
’18 vs. ’17
|
’17 vs. ’16
|
|||||||||||
|
Surgery
|
$
|
9,901
|
|
9,559
|
|
9,296
|
|
3.6
|
%
|
2.8
|
|
|||||
|
Advanced
|
4,002
|
|
3,756
|
|
3,517
|
|
6.5
|
|
6.8
|
|
||||||
|
General
|
4,557
|
|
4,463
|
|
4,362
|
|
2.1
|
|
2.3
|
|
||||||
|
Specialty
|
1,342
|
|
1,340
|
|
1,417
|
|
0.1
|
|
(5.4
|
)
|
||||||
|
Orthopaedics
|
8,885
|
|
9,058
|
|
9,128
|
|
(1.9
|
)
|
(0.8
|
)
|
||||||
|
Hips
|
1,418
|
|
1,394
|
|
1,361
|
|
1.7
|
|
2.4
|
|
||||||
|
Knees
|
1,502
|
|
1,523
|
|
1,524
|
|
(1.4
|
)
|
(0.1
|
)
|
||||||
|
Trauma
|
2,699
|
|
2,616
|
|
2,569
|
|
3.2
|
|
1.8
|
|
||||||
|
Spine & Other
|
3,266
|
|
3,525
|
|
3,674
|
|
(7.3
|
)
|
(4.1
|
)
|
||||||
|
Vision
|
4,553
|
|
4,063
|
|
2,785
|
|
12.1
|
|
45.9
|
|
||||||
|
Contact Lenses/Other
|
3,302
|
|
3,036
|
|
2,785
|
|
8.8
|
|
9.0
|
|
||||||
|
Surgical
|
1,251
|
|
1,027
|
|
—
|
|
21.8
|
|
**
|
|||||||
|
Interventional Solutions
(1)
|
2,646
|
|
2,296
|
|
2,055
|
|
15.2
|
|
11.7
|
|
||||||
|
Diabetes Care
|
1,009
|
|
1,615
|
|
1,789
|
|
(37.5
|
)
|
(9.7
|
)
|
||||||
|
Diagnostics
(2)
|
—
|
|
1
|
|
66
|
|
***
|
***
|
||||||||
|
Total Medical Devices Sales
|
$
|
26,994
|
|
26,592
|
|
25,119
|
|
1.5
|
%
|
5.9
|
|
|||||
|
22
|
||
|
% of Sales
|
2018
|
2017
|
2016
|
||||||
|
Cost of products sold*
|
33.2
|
%
|
33.3
|
|
30.3
|
|
|||
|
Percent point increase/(decrease) over the prior year
|
(0.1
|
)
|
3.0
|
|
(0.3
|
)
|
|||
|
Selling, marketing and administrative expenses*
|
27.6
|
%
|
28.1
|
|
27.9
|
|
|||
|
Percent point increase/(decrease) over the prior year
|
(0.5
|
)
|
0.2
|
|
(2.2
|
)
|
|||
|
23
|
||
|
|
2018
|
2017
|
2016
|
||||||||||||||||
|
(Dollars in Millions)
|
Amount
|
% of Sales**
|
Amount
|
% of Sales**
|
Amount
|
% of Sales**
|
|||||||||||||
|
Consumer
|
$
|
565
|
|
4.1
|
%
|
586
|
|
4.3
|
|
585
|
|
4.4
|
|
||||||
|
Pharmaceutical
|
8,446
|
|
20.7
|
|
8,392
|
|
23.1
|
|
7,001
|
|
20.9
|
|
|||||||
|
Medical Devices
|
1,764
|
|
6.5
|
|
1,616
|
|
6.1
|
|
1,557
|
|
6.2
|
|
|||||||
|
Total research and development expense
|
$
|
10,775
|
|
13.2
|
%
|
10,594
|
|
13.9
|
|
9,143
|
|
12.7
|
|
||||||
|
Percent increase/(decrease) over the prior year
|
1.7
|
%
|
|
|
15.9
|
|
|
|
1.6
|
|
|
|
|||||||
|
*Prior years amounts were reclassified to conform to current year presentation (adoption of ASU 2017-07)
**As a percent to segment sales
|
|
24
|
||
|
|
Income Before Tax
|
Segment Sales
|
Percent of Segment Sales
|
|||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
||||||||||||
|
Consumer
|
$
|
2,320
|
|
2,524
|
|
13,853
|
|
13,602
|
|
16.7
|
%
|
18.6
|
||||||
|
Pharmaceutical
|
12,568
|
|
11,083
|
|
40,734
|
|
36,256
|
|
30.9
|
|
30.6
|
|||||||
|
Medical Devices
|
4,397
|
|
5,392
|
|
26,994
|
|
26,592
|
|
16.3
|
|
20.3
|
|||||||
|
Total
(1)
|
19,285
|
|
18,999
|
|
81,581
|
|
76,450
|
|
23.6
|
|
24.9
|
|||||||
|
Less: Expenses not allocated to segments
(2)
|
1,286
|
|
1,326
|
|
|
|
|
|||||||||||
|
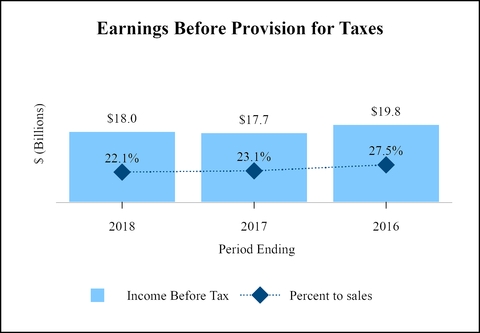
Earnings before provision for taxes on income
|
$
|
17,999
|
|
17,673
|
|
81,581
|
|
76,450
|
|
22.1
|
%
|
23.1
|
||||||
|
(1)
|
See Note 18 to the Consolidated Financial Statements for more details.
|
|
(2)
|
Amounts not allocated to segments include interest (income) expense and general corporate (income) expense.
|
|
25
|
||
|
26
|
||
|
27
|
||
|
(Dollars in Millions)
|
Tax Legislation (TCJA)
|
Debt Obligations
|
Interest on
Debt Obligations |
Unfunded
Retirement Plans |
Operating Leases
|
Total
|
|||||||||||||
|
2019
|
$
|
—
|
|
2,636
|
|
949
|
|
92
|
|
223
|
|
3,900
|
|
||||||
|
2020
|
531
|
|
1,098
|
|
886
|
|
95
|
|
188
|
|
2,798
|
|
|||||||
|
2021
|
812
|
|
1,796
|
|
841
|
|
101
|
|
154
|
|
3,704
|
|
|||||||
|
2022
|
812
|
|
2,134
|
|
796
|
|
108
|
|
116
|
|
3,966
|
|
|||||||
|
2023
|
1,522
|
|
1,553
|
|
764
|
|
115
|
|
76
|
|
4,030
|
|
|||||||
|
After 2023
|
4,565
|
|
21,103
|
|
8,850
|
|
697
|
|
139
|
|
35,354
|
|
|||||||
|
Total
|
$
|
8,242
|
|
30,320
|
|
13,086
|
|
1,208
|
|
896
|
|
53,752
|
|
||||||
|
28
|
||
|
29
|
||
|
(Dollars in Millions)
|
Balance at
Beginning of Period |
Accruals
|
Payments/Credits
|
Balance at
End of Period |
|||||||||
|
2018
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
186
|
|
836
|
|
(751
|
)
|
271
|
|
||||
|
Accrued returns
|
68
|
|
98
|
|
(109
|
)
|
57
|
|
|||||
|
Accrued promotions
|
481
|
|
2,233
|
|
(2,217
|
)
|
497
|
|
|||||
|
Subtotal
|
$
|
735
|
|
3,167
|
|
(3,077
|
)
|
825
|
|
||||
|
Reserve for doubtful accounts
|
31
|
|
10
|
|
(9
|
)
|
32
|
|
|||||
|
Reserve for cash discounts
|
23
|
|
204
|
|
(204
|
)
|
23
|
|
|||||
|
Total
|
$
|
789
|
|
3,381
|
|
(3,290
|
)
|
880
|
|
||||
|
2017
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
136
|
|
638
|
|
(588
|
)
|
186
|
|
||||
|
Accrued returns
|
65
|
|
128
|
|
(125
|
)
|
68
|
|
|||||
|
Accrued promotions
|
358
|
|
2,148
|
|
(2,025
|
)
|
481
|
|
|||||
|
Subtotal
|
$
|
559
|
|
2,914
|
|
(2,738
|
)
|
735
|
|
||||
|
Reserve for doubtful accounts
|
24
|
|
10
|
|
(3
|
)
|
31
|
|
|||||
|
Reserve for cash discounts
|
25
|
|
205
|
|
(207
|
)
|
23
|
|
|||||
|
Total
|
$
|
608
|
|
3,129
|
|
(2,948
|
)
|
789
|
|
||||
|
(1)
|
Includes reserve for customer rebates of $57 million at
December 30, 2018
and $48 million at
December 31, 2017
, recorded as a contra asset.
|
|
(Dollars in Millions)
|
Balance at
Beginning of Period |
Accruals
|
Payments/Credits
(2)
|
Balance at
End of Period |
|||||||||
|
2018
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
4,862
|
|
22,644
|
|
(19,996
|
)
|
7,510
|
|
||||
|
Accrued returns
|
362
|
|
385
|
|
(311
|
)
|
436
|
|
|||||
|
Accrued promotions
|
35
|
|
46
|
|
(68
|
)
|
13
|
|
|||||
|
Subtotal
|
$
|
5,259
|
|
23,075
|
|
(20,375
|
)
|
7,959
|
|
||||
|
Reserve for doubtful accounts
|
77
|
|
37
|
|
(67
|
)
|
47
|
|
|||||
|
Reserve for cash discounts
|
55
|
|
860
|
|
(862
|
)
|
53
|
|
|||||
|
Total
|
$
|
5,391
|
|
23,972
|
|
(21,304
|
)
|
8,059
|
|
||||
|
2017
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
3,420
|
|
16,447
|
|
(15,005
|
)
|
4,862
|
|
||||
|
Accrued returns
|
334
|
|
256
|
|
(228
|
)
|
362
|
|
|||||
|
Accrued promotions
|
—
|
|
69
|
|
(34
|
)
|
35
|
|
|||||
|
Subtotal
|
$
|
3,754
|
|
16,772
|
|
(15,267
|
)
|
5,259
|
|
||||
|
Reserve for doubtful accounts
|
38
|
|
40
|
|
(1
|
)
|
77
|
|
|||||
|
Reserve for cash discounts
|
58
|
|
714
|
|
(717
|
)
|
55
|
|
|||||
|
Total
|
$
|
3,850
|
|
17,526
|
|
(15,985
|
)
|
5,391
|
|
||||
|
(1)
|
Includes reserve for customer rebates of $89 million at
December 30, 2018
and $90 million at
December 31, 2017
, recorded as a contra asset.
|
|
(2)
|
Includes adjustments
|
|
30
|
||
|
(Dollars in Millions)
|
Balance at
Beginning of Period |
Accruals
|
Payments/Credits
|
Balance at
End of Period |
|||||||||
|
2018
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(
1)
|
$
|
1,620
|
|
6,344
|
|
(6,746
|
)
|
1,218
|
|
||||
|
Accrued returns
|
152
|
|
362
|
|
(400
|
)
|
114
|
|
|||||
|
Accrued promotions
|
83
|
|
116
|
|
(157
|
)
|
42
|
|
|||||
|
Subtotal
|
$
|
1,855
|
|
6,822
|
|
(7,303
|
)
|
1,374
|
|
||||
|
Reserve for doubtful accounts
|
183
|
|
29
|
|
(43
|
)
|
169
|
|
|||||
|
Reserve for cash discounts
|
15
|
|
372
|
|
(387
|
)
|
—
|
|
|||||
|
Total
|
$
|
2,053
|
|
7,223
|
|
(7,733
|
)
|
1,543
|
|
||||
|
2017
|
|
|
|
|
|
|
|
|
|||||
|
Accrued rebates
(1)
|
$
|
1,500
|
|
6,407
|
|
(6,287
|
)
|
1,620
|
|
||||
|
Accrued returns
|
127
|
|
729
|
|
(704
|
)
|
152
|
|
|||||
|
Accrued promotions
|
32
|
|
135
|
|
(84
|
)
|
83
|
|
|||||
|
Subtotal
|
$
|
1,659
|
|
7,271
|
|
(7,075
|
)
|
1,855
|
|
||||
|
Reserve for doubtful accounts
|
190
|
|
27
|
|
(34
|
)
|
183
|
|
|||||
|
Reserve for cash discounts
|
16
|
|
389
|
|
(390
|
)
|
15
|
|
|||||
|
Total
|
$
|
1,865
|
|
7,687
|
|
(7,499
|
)
|
2,053
|
|
||||
|
(1)
|
Includes reserve for customer rebates of $632 million at
December 30, 2018
and $501 million at
December 31, 2017
, recorded as a contra asset.
|
|
31
|
||
|
32
|
||
|
Item 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
33
|
||
|
Index to Audited Consolidated Financial Statements
|
|
|
34
|
||
|
2018
|
2017
|
|||||
|
Assets
|
||||||
|
Current assets
|
|
|
|
|
||
|
Cash and cash equivalents (Notes 1 and 2)
|
$
|
18,107
|
|
17,824
|
|
|
|
Marketable securities (Notes 1 and 2)
|
1,580
|
|
472
|
|
||
|
Accounts receivable trade, less allowances for doubtful accounts $248 (2017, $291)
|
14,098
|
|
13,490
|
|
||
|
Inventories (Notes 1 and 3)
|
8,599
|
|
8,765
|
|
||
|
Prepaid expenses and other receivables
|
2,699
|
|
2,537
|
|
||
|
Assets held for sale (Note 20)
|
950
|
|
—
|
|
||
|
Total current assets
|
46,033
|
|
43,088
|
|
||
|
Property, plant and equipment, net (Notes 1 and 4)
|
17,035
|
|
17,005
|
|
||
|
Intangible assets, net (Notes 1 and 5)
|
47,611
|
|
53,228
|
|
||
|
Goodwill (Notes 1 and 5)
|
30,453
|
|
31,906
|
|
||
|
Deferred taxes on income (Note 8)
|
7,640
|
|
7,105
|
|
||
|
Other assets
|
4,182
|
|
4,971
|
|
||
|
Total assets
|
$
|
152,954
|
|
157,303
|
|
|
|
Liabilities and Shareholders’ Equity
|
|
|
|
|
||
|
Current liabilities
|
|
|
|
|
||
|
Loans and notes payable (Note 7)
|
$
|
2,796
|
|
3,906
|
|
|
|
Accounts payable
|
7,537
|
|
7,310
|
|
||
|
Accrued liabilities
|
7,601
|
|
7,304
|
|
||
|
Accrued rebates, returns and promotions
|
9,380
|
|
7,210
|
|
||
|
Accrued compensation and employee related obligations
|
3,098
|
|
2,953
|
|
||
|
Accrued taxes on income (Note 8)
|
818
|
|
1,854
|
|
||
|
Total current liabilities
|
31,230
|
|
30,537
|
|
||
|
Long-term debt (Note 7)
|
27,684
|
|
30,675
|
|
||
|
Deferred taxes on income (Note 8)
|
7,506
|
|
8,368
|
|
||
|
Employee related obligations (Notes 9 and 10)
|
9,951
|
|
10,074
|
|
||
|
Long-term taxes payable (Note 8)
|
8,242
|
|
8,472
|
|
||
|
Other liabilities
|
8,589
|
|
9,017
|
|
||
|
Total liabilities
|
93,202
|
|
97,143
|
|
||
|
Shareholders’ equity
|
|
|
|
|
||
|
Preferred stock — without par value (authorized and unissued 2,000,000 shares)
|
—
|
|
—
|
|
||
|
Common stock — par value $1.00 per share (Note 12) (authorized 4,320,000,000 shares; issued 3,119,843,000 shares)
|
3,120
|
|
3,120
|
|
||
|
Accumulated other comprehensive income (loss) (Note 13)
|
(15,222
|
)
|
(13,199
|
)
|
||
|
Retained earnings
|
106,216
|
|
101,793
|
|
||
|
|
94,114
|
|
91,714
|
|
||
|
Less: common stock held in treasury, at cost (Note 12) (457,519,000 shares and 437,318,000 shares)
|
34,362
|
|
31,554
|
|
||
|
Total shareholders’ equity
|
59,752
|
|
60,160
|
|
||
|
Total liabilities and shareholders’ equity
|
$
|
152,954
|
|
157,303
|
|
|
|
35
|
||
|
2018
|
2017
|
2016
|
|||||||
|
Sales to customers
|
$
|
81,581
|
|
76,450
|
|
71,890
|
|
||
|
Cost of products sold
|
27,091
|
|
25,439
|
|
21,789
|
|
|||
|
Gross profit
|
54,490
|
|
51,011
|
|
50,101
|
|
|||
|
Selling, marketing and administrative expenses
|
22,540
|
|
21,520
|
|
20,067
|
|
|||
|
Research and development expense
|
10,775
|
|
10,594
|
|
9,143
|
|
|||
|
In-process research and development
|
1,126
|
|
408
|
|
29
|
|
|||
|
Interest income
|
(611
|
)
|
(385
|
)
|
(368
|
)
|
|||
|
Interest expense, net of portion capitalized (Note 4)
|
1,005
|
|
934
|
|
726
|
|
|||
|
Other (income) expense, net
|
1,405
|
|
(42
|
)
|
210
|
|
|||
|
Restructuring (Note 22)
|
251
|
|
309
|
|
491
|
|
|||
|
Earnings before provision for taxes on income
|
17,999
|
|
17,673
|
|
19,803
|
|
|||
|
Provision for taxes on income (Note 8)
|
2,702
|
|
16,373
|
|
3,263
|
|
|||
|
Net earnings
|
$
|
15,297
|
|
1,300
|
|
16,540
|
|
||
|
Net earnings per share (Notes 1 and 15)
|
|||||||||
|
Basic
|
$
|
5.70
|
|
0.48
|
|
6.04
|
|
||
|
Diluted
|
$
|
5.61
|
|
0.47
|
|
5.93
|
|
||
|
Average shares outstanding (Notes 1 and 15)
|
|||||||||
|
Basic
|
2,681.5
|
|
2,692.0
|
|
2,737.3
|
|
|||
|
Diluted
|
2,728.7
|
|
2,745.3
|
|
2,788.9
|
|
|||
|
36
|
||
|
2018
|
2017
|
2016
|
|||||||
|
Net earnings
|
$
|
15,297
|
|
1,300
|
|
16,540
|
|
||
|
Other comprehensive income (loss), net of tax
|
|||||||||
|
Foreign currency translation
|
(1,518
|
)
|
1,696
|
|
(612
|
)
|
|||
|
Securities:
(1)
|
|||||||||
|
Unrealized holding gain (loss) arising during period
|
(1
|
)
|
159
|
|
(52
|
)
|
|||
|
Reclassifications to earnings
|
1
|
|
(338
|
)
|
(141
|
)
|
|||
|
Net change
|
—
|
|
(179
|
)
|
(193
|
)
|
|||
|
Employee benefit plans:
|
|||||||||
|
Prior service credit (cost), net of amortization
|
(44
|
)
|
2
|
|
21
|
|
|||
|
Gain (loss), net of amortization
|
(56
|
)
|
29
|
|
(862
|
)
|
|||
|
Effect of exchange rates
|
92
|
|
(201
|
)
|
159
|
|
|||
|
Net change
|
(8
|
)
|
(170
|
)
|
(682
|
)
|
|||
|
Derivatives & hedges:
|
|||||||||
|
Unrealized gain (loss) arising during period
|
(73
|
)
|
(4
|
)
|
(359
|
)
|
|||
|
Reclassifications to earnings
|
(192
|
)
|
359
|
|
110
|
|
|||
|
Net change
|
(265
|
)
|
355
|
|
(249
|
)
|
|||
|
Other comprehensive income (loss)
|
(1,791
|
)
|
1,702
|
|
(1,736
|
)
|
|||
|
Comprehensive income
|
$
|
13,506
|
|
3,002
|
|
14,804
|
|
||
|
(1)
2018 includes the impact from adoption of ASU 2016-01. For further details see Note 1 to the Consolidated Financial Statements
|
|||||||||
|
The tax effects in other comprehensive income for the fiscal years ended 2018, 2017 and 2016 respectively: Foreign Currency Translation $236 million in 2018 due to the enactment of the U.S. Tax Cuts and Jobs Act; Securities: $0 million, $96 million and $104 million, Employee Benefit Plans: $4 million, $83 million and $346 million, Derivatives & Hedges: $70 million, $191 million and $134 million.
|
|
37
|
||
|
Total
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Income
|
Common Stock
Issued Amount
|
Treasury
Stock
Amount
|
||||||||||||
|
Balance, January 3, 2016
|
$
|
71,150
|
|
103,879
|
|
(13,165
|
)
|
3,120
|
|
(22,684
|
)
|
|||||
|
Net earnings
|
16,540
|
|
16,540
|
|
|
|
|
|
|
|
||||||
|
Cash dividends paid ($3.15 per share)
|
(8,621
|
)
|
(8,621
|
)
|
|
|
|
|
|
|
||||||
|
Employee compensation and stock option plans
|
2,130
|
|
(1,181
|
)
|
|
|
|
|
3,311
|
|
||||||
|
Repurchase of common stock
|
(8,979
|
)
|
|
|
|
|
|
|
(8,979
|
)
|
||||||
|
Other
|
(66
|
)
|
(66
|
)
|
||||||||||||
|
Other comprehensive income (loss), net of tax
|
(1,736
|
)
|
(1,736
|
)
|
|
|
|
|
||||||||
|
Balance, January 1, 2017
|
70,418
|
|
110,551
|
|
(14,901
|
)
|
3,120
|
|
(28,352
|
)
|
||||||
|
Net earnings
|
1,300
|
|
1,300
|
|
|
|
|
|
|
|
||||||
|
Cash dividends paid ($3.32 per share)
|
(8,943
|
)
|
(8,943
|
)
|
|
|
|
|
|
|
||||||
|
Employee compensation and stock option plans
|
2,077
|
|
(1,079
|
)
|
|
|
|
|
3,156
|
|
||||||
|
Repurchase of common stock
|
(6,358
|
)
|
|
|
|
|
|
|
(6,358
|
)
|
||||||
|
Other
|
(36
|
)
|
(36
|
)
|
||||||||||||
|
Other comprehensive income (loss), net of tax
|
1,702
|
|
|
|
1,702
|
|
|
|
|
|
||||||
|
Balance, December 31, 2017
|
60,160
|
|
101,793
|
|
(13,199
|
)
|
3,120
|
|
(31,554
|
)
|
||||||
|
Cumulative adjustment
|
(486
|
)
|
(254
|
)
|
(1
|
)
|
(232
|
)
|
||||||||
|
Net earnings
|
15,297
|
|
15,297
|
|
|
|
|
|
|
|
||||||
|
Cash dividends paid ($3.54 per share)
|
(9,494
|
)
|
(9,494
|
)
|
|
|
|
|
|
|
||||||
|
Employee compensation and stock option plans
|
1,949
|
|
(1,111
|
)
|
|
|
|
|
3,060
|
|
||||||
|
Repurchase of common stock
|
(5,868
|
)
|
|
|
|
|
|
|
(5,868
|
)
|
||||||
|
Other
|
(15
|
)
|
(15
|
)
|
||||||||||||
|
Other comprehensive income (loss), net of tax
|
(1,791
|
)
|
|
|
(1,791
|
)
|
|
|
|
|
||||||
|
Balance, December 30, 2018
|
$
|
59,752
|
|
106,216
|
|
(15,222
|
)
|
3,120
|
|
(34,362
|
)
|
|||||
|
38
|
||
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
2018
|
2017
|
2016
|
|||||||
|
Cash flows from operating activities
|
|
|
|
|
|||||
|
Net earnings
|
$
|
15,297
|
|
1,300
|
|
16,540
|
|
||
|
Adjustments to reconcile net earnings to cash flows from operating activities:
|
|
|
|
|
|||||
|
Depreciation and amortization of property and intangibles
|
6,929
|
|
5,642
|
|
3,754
|
|
|||
|
Stock based compensation
|
978
|
|
962
|
|
878
|
|
|||
|
Asset write-downs
|
1,258
|
|
795
|
|
283
|
|
|||
|
Gain on sale of assets/businesses
|
(1,217
|
)
|
(1,307
|
)
|
(563
|
)
|
|||
|
Deferred tax provision
|
(1,016
|
)
|
2,406
|
|
(341
|
)
|
|||
|
Accounts receivable allowances
|
(31
|
)
|
17
|
|
(11
|
)
|
|||
|
Changes in assets and liabilities, net of effects from acquisitions and divestitures:
|
|
|
|
|
|||||
|
Increase in accounts receivable
|
(1,185
|
)
|
(633
|
)
|
(1,065
|
)
|
|||
|
(Increase)/Decrease in inventories
|
(644
|
)
|
581
|
|
(249
|
)
|
|||
|
Increase in accounts payable and accrued liabilities
|
3,951
|
|
2,725
|
|
656
|
|
|||
|
Increase in other current and non-current assets
|
(275
|
)
|
(411
|
)
|
(529
|
)
|
|||
|
(Decrease)/Increase in other current and non-current liabilities
|
(1,844
|
)
|
8,979
|
|
(586
|
)
|
|||
|
Net cash flows from operating activities
|
22,201
|
|
21,056
|
|
18,767
|
|
|||
|
Cash flows from investing activities
|
|
|
|
|
|||||
|
Additions to property, plant and equipment
|
(3,670
|
)
|
(3,279
|
)
|
(3,226
|
)
|
|||
|
Proceeds from the disposal of assets/businesses, net
|
3,203
|
|
1,832
|
|
1,267
|
|
|||
|
Acquisitions, net of cash acquired (Note 20)
|
(899
|
)
|
(35,151
|
)
|
(4,509
|
)
|
|||
|
Purchases of investments
|
(5,626
|
)
|
(6,153
|
)
|
(33,950
|
)
|
|||
|
Sales of investments
|
4,289
|
|
28,117
|
|
35,780
|
|
|||
|
Other (primarily intangibles)
|
(464
|
)
|
(234
|
)
|
(123
|
)
|
|||
|
Net cash used by investing activities
|
(3,167
|
)
|
(14,868
|
)
|
(4,761
|
)
|
|||
|
Cash flows from financing activities
|
|
|
|||||||
|
Dividends to shareholders
|
(9,494
|
)
|
(8,943
|
)
|
(8,621
|
)
|
|||
|
Repurchase of common stock
|
(5,868
|
)
|
(6,358
|
)
|
(8,979
|
)
|
|||
|
Proceeds from short-term debt
|
80
|
|
869
|
|
111
|
|
|||
|
Retirement of short-term debt
|
(2,479
|
)
|
(1,330
|
)
|
(2,017
|
)
|
|||
|
Proceeds from long-term debt,
net of issuance costs
|
5
|
|
8,992
|
|
12,004
|
|
|||
|
Retirement of long-term debt
|
(1,555
|
)
|
(1,777
|
)
|
(2,223
|
)
|
|||
|
Proceeds from the exercise of stock options/employee withholding tax on stock awards, net
|
949
|
|
1,062
|
|
1,189
|
|
|||
|
Other
|
(148
|
)
|
(188
|
)
|
(15
|
)
|
|||
|
Net cash used by financing activities
|
(18,510
|
)
|
(7,673
|
)
|
(8,551
|
)
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
(241
|
)
|
337
|
|
(215
|
)
|
|||
|
Increase/(Decrease) in cash and cash equivalents
|
283
|
|
(1,148
|
)
|
5,240
|
|
|||
|
Cash and cash equivalents, beginning of year (Note 1)
|
17,824
|
|
18,972
|
|
13,732
|
|
|||
|
Cash and cash equivalents, end of year (Note 1)
|
$
|
18,107
|
|
17,824
|
|
18,972
|
|
||
|
Supplemental cash flow data
|
|
|
|
|
|
|
|||
|
Cash paid during the year for:
|
|
|
|
|
|
|
|||
|
Interest
|
$
|
1,049
|
|
960
|
|
730
|
|
||
|
Interest, net of amount capitalized
|
963
|
|
866
|
|
628
|
|
|||
|
Income taxes
|
4,570
|
|
3,312
|
|
2,843
|
|
|||
|
39
|
||
|
Supplemental schedule of non-cash investing and financing activities
|
|
|
|
|
|
|
|||
|
Treasury stock issued for employee compensation and stock option plans, net of cash proceeds/ employee withholding tax on stock awards
|
$
|
2,095
|
|
2,062
|
|
2,043
|
|
||
|
Conversion of debt
|
6
|
|
16
|
|
35
|
|
|||
|
Acquisitions
|
|
|
|
|
|
|
|||
|
Fair value of assets acquired
|
$
|
1,047
|
|
36,937
|
|
4,586
|
|
||
|
Fair value of liabilities assumed and noncontrolling interests
|
(148
|
)
|
(1,786
|
)
|
(77
|
)
|
|||
|
Net cash paid for acquisitions
|
$
|
899
|
|
35,151
|
|
4,509
|
|
||
|
40
|
||
|
1.
|
Summary of Significant Accounting Policies
|
|
Statement of Earnings - For the fiscal year ended December 30, 2018
|
|||||||||
|
(Dollars in millions)
|
As Reported
|
Effect of change
|
Balance without adoption of ASC 606
|
||||||
|
Sales to customers
|
$
|
81,581
|
|
(35
|
)
|
81,546
|
|
||
|
Net earnings
|
15,297
|
|
(28
|
)
|
15,269
|
|
|||
|
Balance Sheet - As of December 30, 2018
|
|||||||||
|
As Reported
|
Effect of change
|
Balance without adoption of ASC 606
|
|||||||
|
Assets
|
152,954
|
|
23
|
|
152,977
|
|
|||
|
Liabilities
|
93,202
|
|
4
|
|
93,206
|
|
|||
|
Equity
|
$
|
59,752
|
|
19
|
|
59,771
|
|
||
|
41
|
||
|
(Dollars In millions)
|
Increase (Decrease) to Net Expense
|
||||||||
|
2018
|
2017
|
2016
|
|||||||
|
Cost of products sold
|
$
|
51
|
|
85
|
|
104
|
|
||
|
Selling, marketing and administrative expenses
|
55
|
|
100
|
|
122
|
|
|||
|
Research and development expense
|
21
|
|
40
|
|
48
|
|
|||
|
Other (income) expense, net
|
(127
|
)
|
(225
|
)
|
(274
|
)
|
|||
|
Earnings before provision for taxes on income
|
$
|
—
|
|
—
|
|
—
|
|
||
|
42
|
||
|
(Dollars in Millions)
|
Cumulative Effect Adjustment Increase (Decrease) to Retained Earnings
|
|||
|
ASU 2014-09 - Revenue from Contracts with Customers
|
$
|
(47
|
)
|
|
|
ASU 2016-01 - Financial Instruments
|
232
|
|
||
|
ASU 2016-16 - Income Taxes: Intra-Entity Transfers
|
(439
|
)
|
||
|
Total
|
$
|
(254
|
)
|
|
|
43
|
||
|
Building and building equipment
|
20 - 30 years
|
|
Land and leasehold improvements
|
10 - 20 years
|
|
Machinery and equipment
|
2 - 13 years
|
|
44
|
||
|
45
|
||
|
Nature/Type of Collaboration
|
|
Statement of Earnings Presentation
|
|
Third-party sale of product & profit share payments received
|
|
Sales to customers
|
|
Royalties/milestones paid to collaborative partner (post-regulatory approval)*
|
|
Cost of products sold
|
|
Royalties received from collaborative partner
|
|
Other income (expense), net
|
|
Upfront payments & milestones paid to collaborative partner (pre-regulatory approval)
|
|
Research and development expense
|
|
Research and development payments to collaborative partner
|
|
Research and development expense
|
|
Research and development payments received from collaborative partner
|
|
Reduction of Research and development expense
|
|
*
|
Milestones are capitalized as intangible assets and amortized to cost of products sold over the useful life.
|
|
46
|
||
|
2.
|
Cash, Cash Equivalents and Current Marketable Securities
|
|
(Dollars in Millions)
|
2018
|
||||||||||||||
|
Carrying Amount
|
Estimated Fair Value
|
Cash & Cash Equivalents
|
Current Marketable Securities
|
||||||||||||
|
Cash
|
$
|
2,619
|
|
2,619
|
|
2,619
|
|
—
|
|
||||||
|
U.S. Reverse repurchase agreements
|
3,009
|
|
3,009
|
|
3,009
|
|
—
|
|
|||||||
|
Other Reverse repurchase agreements
|
443
|
|
443
|
|
443
|
|
—
|
|
|||||||
|
Money market funds
|
3,397
|
|
3,397
|
|
3,397
|
|
—
|
|
|||||||
|
Time deposits
(1)
|
485
|
|
485
|
|
485
|
|
—
|
|
|||||||
|
Subtotal
|
$
|
9,953
|
|
9,953
|
|
9,953
|
|
—
|
|
||||||
|
Gov't Securities
|
$
|
9,474
|
|
9,474
|
|
8,144
|
|
1,330
|
|
||||||
|
Corporate debt securities
|
260
|
|
260
|
|
10
|
|
250
|
|
|||||||
|
Subtotal available for sale
(2)
|
$
|
9,734
|
|
9,734
|
|
8,154
|
|
1,580
|
|
||||||
|
Total cash, cash equivalents and current marketable securities
|
|
|
$
|
18,107
|
|
1,580
|
|
||||||||
|
47
|
||
|
(Dollars in Millions)
|
2017
|
||||||||||||||
|
Carrying Amount
|
Estimated Fair Value
|
Cash & Cash Equivalents
|
Current Marketable Securities
|
||||||||||||
|
Cash
|
$
|
2,929
|
|
2,929
|
|
2,929
|
|
—
|
|
||||||
|
Other Sovereign Securities
(1)
|
279
|
|
279
|
|
219
|
|
60
|
|
|||||||
|
U.S. Reverse repurchase agreements
|
4,025
|
|
4,025
|
|
4,025
|
|
—
|
|
|||||||
|
Corporate debt securities
(1)
|
289
|
|
289
|
|
244
|
|
45
|
|
|||||||
|
Money market funds
|
4,288
|
|
4,288
|
|
4,288
|
|
—
|
|
|||||||
|
Time deposits
(1)
|
1,176
|
|
1,176
|
|
1,175
|
|
1
|
|
|||||||
|
Subtotal
|
$
|
12,986
|
|
12,986
|
|
12,880
|
|
106
|
|
||||||
|
Gov't Securities
|
$
|
4,864
|
|
4,864
|
|
4,833
|
|
31
|
|
||||||
|
Other Sovereign Securities
|
186
|
|
186
|
|
80
|
|
106
|
|
|||||||
|
Corporate debt securities
|
260
|
|
260
|
|
31
|
|
229
|
|
|||||||
|
Subtotal available for sale
(2)
|
$
|
5,310
|
|
5,310
|
|
4,944
|
|
366
|
|
||||||
|
Total cash, cash equivalents and current marketable securities
|
$
|
17,824
|
|
472
|
|
||||||||||
|
(Dollars in Millions)
|
Cost Basis
|
Fair Value
|
|||||
|
Due within one year
|
$
|
9,670
|
|
9,670
|
|
||
|
Due after one year through five years
|
64
|
|
64
|
|
|||
|
Due after five years through ten years
|
—
|
|
—
|
|
|||
|
Total debt securities
|
$
|
9,734
|
|
9,734
|
|
||
|
3.
|
Inventories
|
|
(Dollars in Millions)
|
2018
|
2017
|
|||||
|
Raw materials and supplies
|
$
|
1,114
|
|
1,140
|
|
||
|
Goods in process
|
2,109
|
|
2,317
|
|
|||
|
Finished goods
|
5,376
|
|
5,308
|
|
|||
|
Total inventories
|
$
|
8,599
|
|
(1)
|
8,765
|
|
|
|
48
|
||
|
4.
|
Property, Plant and Equipment
|
|
(Dollars in Millions)
|
2018
|
2017
|
|||||
|
Land and land improvements
|
$
|
807
|
|
829
|
|
||
|
Buildings and building equipment
|
11,176
|
|
11,240
|
|
|||
|
Machinery and equipment
|
25,992
|
|
25,949
|
|
|||
|
Construction in progress
|
3,876
|
|
3,448
|
|
|||
|
Total property, plant and equipment, gross
|
$
|
41,851
|
|
41,466
|
|
||
|
Less accumulated depreciation
|
24,816
|
|
24,461
|
|
|||
|
Total property, plant and equipment, net
|
$
|
17,035
|
|
(1)
|
17,005
|
|
|
|
5.
|
Intangible Assets and Goodwill
|
|
(Dollars in Millions)
|
2018
|
2017
|
|||||
|
Intangible assets with definite lives:
|
|
|
|
|
|||
|
Patents and trademarks — gross
|
$
|
35,194
|
|
36,427
|
|
||
|
Less accumulated amortization
|
9,784
|
|
7,223
|
|
|||
|
Patents and trademarks — net
|
$
|
25,410
|
|
29,204
|
|
||
|
Customer relationships and other intangibles — gross
|
$
|
21,334
|
|
20,204
|
|
||
|
Less accumulated amortization
|
8,323
|
|
7,463
|
|
|||
|
Customer relationships and other intangibles — net
|
$
|
13,011
|
|
12,741
|
|
||
|
Intangible assets with indefinite lives:
|
|
|
|
|
|||
|
Trademarks
|
$
|
6,937
|
|
7,082
|
|
||
|
Purchased in-process research and development
(1)
|
2,253
|
|
4,201
|
|
|||
|
Total intangible assets with indefinite lives
|
$
|
9,190
|
|
11,283
|
|
||
|
Total intangible assets — net
|
$
|
47,611
|
|
53,228
|
|
||
|
49
|
||
|
(Dollars in Millions)
|
Consumer
|
Pharmaceutical
|
Medical Devices
|
Total
|
|||||||||
|
Goodwill at January 1, 2017
|
$
|
8,263
|
|
2,840
|
|
11,702
|
|
22,805
|
|
||||
|
Goodwill, related to acquisitions
(1)
|
102
|
|
6,161
|
|
2,200
|
|
8,463
|
|
|||||
|
Goodwill, related to divestitures
|
(74
|
)
|
(1
|
)
|
(102
|
)
|
(177
|
)
|
|||||
|
Currency translation/other
|
584
|
|
109
|
|
122
|
|
815
|
|
|||||
|
Goodwill at December 31, 2017
|
$
|
8,875
|
|
9,109
|
|
13,922
|
|
31,906
|
|
||||
|
Goodwill, related to acquisitions
|
168
|
|
51
|
|
184
|
|
403
|
|
|||||
|
Goodwill, related to divestitures
|
—
|
|
—
|
|
(1,348
|
)
|
(2)
|
(1,348
|
)
|
||||
|
Currency translation/other
|
(373
|
)
|
(97
|
)
|
(38
|
)
|
(508
|
)
|
|||||
|
Goodwill at December 30, 2018
|
$
|
8,670
|
|
9,063
|
|
12,720
|
|
30,453
|
|
||||
|
6.
|
Fair Value Measurements
|
|
50
|
||
|
51
|
||
|
December 30, 2018
|
December 31, 2017
|
||||||||||||||||||||
|
(Dollars in Millions)
|
Sales
|
Cost of Products Sold
|
R&D Expense
|
Interest (Income) Expense
|
Other (Income) Expense
|
Sales
|
Cost of Products Sold
|
R&D Expense
|
Interest (Income) Expense
|
Other (Income) Expense
|
|||||||||||
|
The effects of fair value, net investment and cash flow hedging:
|
|||||||||||||||||||||
|
Gain (Loss) on fair value hedging relationship:
|
|||||||||||||||||||||
|
Interest rate swaps contracts:
|
|||||||||||||||||||||
|
Hedged items
|
$
|
—
|
|
—
|
|
—
|
|
5
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5
|
|
—
|
|
|
Derivatives designated as hedging instruments
|
—
|
|
—
|
|
—
|
|
(5
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(5
|
)
|
—
|
|
|
|
Gain (Loss) on net investment hedging relationship:
|
|||||||||||||||||||||
|
Cross currency interest rate swaps contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) recognized in income on derivative amount excluded from effectiveness testing
|
—
|
|
—
|
|
—
|
|
56
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Amount of gain or (loss) recognized in AOCI
|
—
|
|
—
|
|
—
|
|
56
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Gain (Loss) on cash flow hedging relationship:
|
|||||||||||||||||||||
|
Forward foreign exchange contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) reclassified from AOCI into income
(1)
|
47
|
|
200
|
|
(220
|
)
|
—
|
|
(24
|
)
|
(31
|
)
|
(159
|
)
|
(165
|
)
|
—
|
|
(87
|
)
|
|
|
Amount of gain or (loss) recognized in AOCI
(1)
|
(32
|
)
|
(17
|
)
|
(193
|
)
|
—
|
|
(4
|
)
|
49
|
|
96
|
|
(199
|
)
|
—
|
|
(60
|
)
|
|
|
Cross currency interest rate swaps contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) reclassified from AOCI into income
|
—
|
|
—
|
|
—
|
|
133
|
|
—
|
|
—
|
|
—
|
|
—
|
|
83
|
|
—
|
|
|
|
Amount of gain or (loss) recognized in AOCI
|
$
|
—
|
|
—
|
|
—
|
|
117
|
|
—
|
|
—
|
|
—
|
|
—
|
|
110
|
|
—
|
|
|
Line item in the Consolidated Balance Sheet in which the hedged item is included
|
Carrying Amount of the Hedged Liability
|
Cumulative Amount of Fair Value Hedging Adjustment Included in the Carrying Amount of the Hedged Liability
|
|||||||||||
|
(Dollars in Millions)
|
December 30, 2018
|
December 31, 2017
|
December 30, 2018
|
December 31, 2017
|
|||||||||
|
Current Portion of Long-term Debt
|
$
|
494
|
|
597
|
|
5
|
|
2
|
|
||||
|
Long-term Debt
|
—
|
|
496
|
|
—
|
|
3
|
|
|||||
|
52
|
||
|
The following table is the effect of derivatives not designated as hedging instrument for the fiscal years ended December 30, 2018 and December 31, 2017:
|
|||||||||||
|
(Dollars in Millions)
|
Location of Gain /(Loss) Recognized in Income on Derivative
|
Gain/(Loss)
Recognized In
Income on Derivative
|
|||||||||
|
Derivatives Not Designated as Hedging Instruments
|
December 30, 2018
|
December 31, 2017
|
|||||||||
|
Foreign Exchange Contracts
|
Other (income) expense
|
(68
|
)
|
(5
|
)
|
||||||
|
The following table is the effect of net investment hedges for the fiscal years ended December 30, 2018 and December 31, 2017:
|
|||||||||||||||
|
Gain/(Loss)
Recognized In
Accumulated OCI
|
Location of Gain or (Loss) Reclassified from Accumulated Other Comprehensive Income Into Income
|
Gain/(Loss) Reclassified From
Accumulated OCI
Into Income
|
|||||||||||||
|
(Dollars in Millions)
|
Fiscal Nine Months Ended
|
||||||||||||||
|
December 30, 2018
|
December 31, 2017
|
December 30, 2018
|
December 31, 2017
|
||||||||||||
|
Debt
|
$
|
218
|
|
(597
|
)
|
Other (income) expense
|
—
|
|
—
|
|
|||||
|
Cross Currency interest rate swaps
|
$
|
150
|
|
—
|
|
Other (income) expense
|
—
|
|
—
|
|
|||||
|
(Dollars in Millions)
|
December 31, 2017
|
December 30, 2018
|
||||||||||||||
|
Carrying Value
|
Changes in Fair Value Reflected in Net Income
(1)
|
Sales/ Purchases/Other
(2)
|
Carrying Value
|
Non Current Other Assets
|
||||||||||||
|
Equity Investments with readily determinable value
|
$
|
751
|
|
(247
|
)
|
7
|
|
511
|
|
511
|
|
|||||
|
Equity Investments without readily determinable value
|
$
|
510
|
|
13
|
|
158
|
|
681
|
|
681
|
|
|||||
|
53
|
||
|
2018
|
2017
|
|||||||||||||||
|
(Dollars in Millions)
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Total
(1)
|
|||||||||||
|
Derivatives designated as hedging instruments:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
|
$
|
—
|
|
501
|
|
—
|
|
501
|
|
418
|
|
|||||
|
Interest rate contracts
(2)(4)
|
—
|
|
161
|
|
—
|
|
161
|
|
7
|
|
||||||
|
Total
|
—
|
|
662
|
|
—
|
|
662
|
|
425
|
|
||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
|
—
|
|
548
|
|
—
|
|
548
|
|
402
|
|
||||||
|
Interest rate contracts
(3)(4)
|
—
|
|
292
|
|
—
|
|
292
|
|
165
|
|
||||||
|
Total
|
—
|
|
840
|
|
—
|
|
840
|
|
567
|
|
||||||
|
Derivatives not designated as hedging instruments:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
|
—
|
|
32
|
|
—
|
|
32
|
|
38
|
|
||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Forward foreign exchange contracts
|
—
|
|
32
|
|
—
|
|
32
|
|
38
|
|
||||||
|
Available For Sale Other Investments:
|
||||||||||||||||
|
Equity investments
(5)
|
511
|
|
—
|
|
—
|
|
511
|
|
751
|
|
||||||
|
Debt securities
(6)
|
$
|
—
|
|
9,734
|
|
—
|
|
9,734
|
|
5,310
|
|
|||||
|
54
|
||
|
Gross to Net Derivative Reconciliation
|
2018
|
2017
|
|||||
|
(Dollars in Millions)
|
|||||||
|
Total Gross Assets
|
$
|
694
|
|
463
|
|
||
|
Credit Support Agreement (CSA)
|
(423
|
)
|
(76
|
)
|
|||
|
Total Net Asset
|
271
|
|
387
|
|
|||
|
Total Gross Liabilities
|
872
|
|
605
|
|
|||
|
Credit Support Agreement (CSA)
|
(605
|
)
|
(238
|
)
|
|||
|
Total Net Liabilities
|
$
|
267
|
|
367
|
|
||
|
(1)
|
2017 assets and liabilities are all classified as Level 2 with the exception of equity investments of
$751 million
, which are classified as Level 1.
|
|
(2)
|
Includes
$6 million
and
$7 million
of non-current assets for the fiscal years ending
December 30, 2018
and
December 31, 2017
, respectively.
|
|
(3)
|
Includes
$3 million
and
$9 million
of non-current liabilities for the fiscal years ending
December 30, 2018
and
December 31, 2017
, respectively.
|
|
(4)
|
Includes cross currency interest rate swaps and interest rate swaps.
|
|
(5)
|
Classified as non-current other assets. The carrying amount of the equity investments were
$511 million
and
$751 million
as of
December 30, 2018
and
December 31, 2017
, respectively.
|
|
(6)
|
Classified as cash equivalents and current marketable securities.
|
|
55
|
||
|
7.
|
Borrowings
|
|
(Dollars in Millions)
|
2018
|
|
Effective Rate %
|
|
2017
|
|
Effective Rate %
|
|||||||
|
5.15% Debentures due 2018
|
$
|
—
|
|
|
|
|
|
900
|
|
|
5.18
|
|||
|
1.65% Notes due 2018
|
—
|
|
|
|
597
|
|
1.70
|
|||||||
|
4.75% Notes due 2019 (1B Euro 1.14)
(2)
/(1B Euro 1.1947)
(3)
|
1,139
|
|
(2)
|
5.83
|
|
|
1,192
|
|
(3)
|
5.83
|
||||
|
1.875% Notes due 2019
|
494
|
|
1.93
|
|
496
|
|
1.93
|
|||||||
|
0.89% Notes due 2019
|
300
|
|
1.32
|
|
300
|
|
1.75
|
|||||||
|
1.125% Notes due 2019
|
699
|
|
1.13
|
|
699
|
|
1.13
|
|||||||
|
3% Zero Coupon Convertible Subordinated Debentures due 2020
|
51
|
|
|
3.00
|
|
|
60
|
|
|
3.00
|
||||
|
2.95% Debentures due 2020
|
548
|
|
|
3.15
|
|
|
547
|
|
|
3.15
|
||||
|
1.950% Notes due 2020
|
499
|
|
1.99
|
|
499
|
|
1.99
|
|||||||
|
3.55% Notes due 2021
|
449
|
|
3.67
|
|
448
|
|
3.67
|
|||||||
|
2.45% Notes due 2021
|
349
|
|
2.48
|
|
349
|
|
2.48
|
|||||||
|
1.65% Notes due 2021
|
998
|
|
1.65
|
|
998
|
|
1.65
|
|||||||
|
0.250% Notes due 2022 (1B Euro 1.14)
(2)
/(1B Euro 1.1947)
(3)
|
1,137
|
|
(2)
|
0.26
|
|
1,191
|
|
(3)
|
0.26
|
|||||
|
2.25% Notes due 2022
|
996
|
|
2.31
|
|
995
|
|
2.31
|
|||||||
|
6.73% Debentures due 2023
|
250
|
|
|
6.73
|
|
|
250
|
|
|
6.73
|
||||
|
3.375% Notes due 2023
|
805
|
|
3.17
|
|
806
|
|
3.17
|
|||||||
|
2.05% Notes due 2023
|
498
|
|
2.09
|
|
498
|
|
2.09
|
|||||||
|
0.650% Notes due 2024 (750MM Euro 1.14)
(2)
/(750MM Euro 1.1947)
(3)
|
851
|
|
(2)
|
0.68
|
|
891
|
|
(3)
|
0.68
|
|||||
|
5.50% Notes due 2024 (500MM GBP 1.2636)
(2)
/(500MM GBP 1.3444)
(3)
|
627
|
|
(2)
|
6.75
|
|
|
666
|
|
(3)
|
6.75
|
||||
|
2.625% Notes due 2025
|
748
|
|
2.63
|
|
747
|
|
2.63
|
|||||||
|
2.45% Notes due 2026
|
1,992
|
|
2.47
|
|
1,990
|
|
2.47
|
|||||||
|
2.95% Notes due 2027
|
996
|
|
2.96
|
|
995
|
|
2.96
|
|||||||
|
1.150% Notes due 2028 (750MM Euro 1.14)
(2)
/(750MM Euro 1.1947)
(3)
|
847
|
|
(2)
|
1.21
|
|
887
|
|
(3)
|
1.21
|
|||||
|
2.900% Notes due 2028
|
1,493
|
|
2.91
|
|
1,492
|
|
2.91
|
|||||||
|
6.95% Notes due 2029
|
297
|
|
|
7.14
|
|
|
296
|
|
|
7.14
|
||||
|
4.95% Debentures due 2033
|
498
|
|
|
4.95
|
|
|
498
|
|
|
4.95
|
||||
|
4.375% Notes due 2033
|
856
|
|
4.24
|
|
856
|
|
4.24
|
|||||||
|
1.650% Notes due 2035 (1.5B Euro 1.14)
(2)
/(1.5B Euro 1.1947)
(3)
|
1,693
|
|
(2)
|
1.68
|
|
1,774
|
|
(3)
|
1.68
|
|||||
|
3.55% Notes due 2036
|
988
|
|
3.59
|
|
987
|
|
3.59
|
|||||||
|
5.95% Notes due 2037
|
991
|
|
|
5.99
|
|
|
991
|
|
|
5.99
|
||||
|
3.625% Notes due 2037
|
1,486
|
|
3.64
|
|
1,486
|
|
3.64
|
|||||||
|
5.85% Debentures due 2038
|
696
|
|
|
5.85
|
|
|
696
|
|
|
5.85
|
||||
|
3.400% Notes due 2038
|
990
|
|
3.42
|
|
990
|
|
3.42
|
|||||||
|
4.50% Debentures due 2040
|
538
|
|
|
4.63
|
|
|
538
|
|
|
4.63
|
||||
|
4.85% Notes due 2041
|
297
|
|
4.89
|
|
296
|
|
4.89
|
|||||||
|
4.50% Notes due 2043
|
495
|
|
4.52
|
|
495
|
|
4.52
|
|||||||
|
3.70% Notes due 2046
|
1,972
|
|
3.74
|
|
1,971
|
|
3.74
|
|||||||
|
3.75% Notes due 2047
|
991
|
|
3.76
|
|
990
|
|
3.76
|
|||||||
|
56
|
||
|
3.500% Notes due 2048
|
742
|
|
3.52
|
|
742
|
|
3.52
|
|||||||
|
Other
|
24
|
|
|
—
|
|
|
75
|
|
|
—
|
||||
|
Subtotal
|
30,320
|
|
(4)
|
3.19
|
%
|
(1)
|
32,174
|
|
(4)
|
3.19
|
(1
|
)
|
||
|
Less current portion
|
2,636
|
|
|
|
|
|
1,499
|
|
|
|
||||
|
Total long-term debt
|
$
|
27,684
|
|
|
|
|
|
30,675
|
|
|
|
|||
|
(1)
|
Weighted average effective rate.
|
|
(2)
|
Translation rate at
December 30, 2018
.
|
|
(3)
|
Translation rate at
December 31, 2017
.
|
|
(4)
|
The excess of the fair value over the carrying value of debt was
$0.3 billion
in
2018
and
$2.0 billion
in
2017
.
|
|
(Dollars in Millions)
|
||||||||||
|
2019
|
2020
|
2021
|
2022
|
2023
|
After 2023
|
|||||
|
$2,636
|
1,098
|
1,796
|
2,134
|
1,553
|
21,103
|
|||||
|
8.
|
Income Taxes
|
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
|||||||
|
Currently payable:
|
||||||||||
|
U.S. taxes
|
$
|
1,081
|
|
11,969
|
|
1,896
|
|
|||
|
U.S. taxes on international operations
|
203
|
|
126
|
|
49
|
|
||||
|
International taxes
|
2,434
|
|
1,872
|
|
1,659
|
|
||||
|
Total currently payable
|
3,718
|
|
13,967
|
|
3,604
|
|
||||
|
Deferred:
|
||||||||||
|
U.S. taxes
|
(148
|
)
|
(1,956
|
)
|
294
|
|
||||
|
U.S. taxes on international operations
|
1,358
|
|
||||||||
|
International taxes
|
(2,226
|
)
|
4,362
|
|
(635
|
)
|
||||
|
Total deferred
|
(1,016
|
)
|
2,406
|
|
(341
|
)
|
||||
|
Provision for taxes on income
|
$
|
2,702
|
|
16,373
|
|
3,263
|
|
|||
|
57
|
||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
|||||||
|
U.S.
|
$
|
5,575
|
|
4,865
|
|
7,457
|
|
|||
|
International
|
12,424
|
|
12,808
|
|
12,346
|
|
||||
|
Earnings before taxes on income:
|
$
|
17,999
|
|
17,673
|
|
19,803
|
|
|||
|
Tax rates:
|
||||||||||
|
U.S. statutory rate
|
21.0
|
%
|
35.0
|
|
35.0
|
|
||||
|
International operations
(1)
|
(3.7
|
)
|
(12.8
|
)
|
(17.2
|
)
|
||||
|
Research and orphan drug tax credits
|
(1.0
|
)
|
(0.4
|
)
|
(0.4
|
)
|
||||
|
U.S. state and local
|
0.8
|
|
0.6
|
|
(0.1
|
)
|
||||
|
U.S. manufacturing deduction
|
—
|
|
(0.8
|
)
|
(0.6
|
)
|
||||
|
U.S. tax on international income
(2)
|
1.4
|
|
0.7
|
|
1.3
|
|
||||
|
Tax benefits on share-based compensation
|
(1.5
|
)
|
(2.1
|
)
|
(1.8
|
)
|
||||
|
U.S. tax benefit on asset/business disposals
|
0.5
|
|
(0.8
|
)
|
—
|
|
||||
|
All other
|
(0.6
|
)
|
(0.1
|
)
|
0.3
|
|
||||
|
TCJA and related impacts
(3)
|
(1.9
|
)
|
(3)
|
73.3
|
|
(4)
|
—
|
|
||
|
Effective Rate
|
15.0
|
%
|
92.6
|
|
16.5
|
|
||||
|
•
|
a
$10.1 billion
charge on previously undistributed foreign earnings as of December 31, 2017
|
|
•
|
a
$4.5 billion
deferred tax liability for foreign local and withholding taxes, offset by a
$1.1 billion
deferred tax asset for U.S. foreign tax credits, for repatriation of substantially all those earnings
|
|
•
|
a
$0.6 billion
tax benefit relating to the remeasurement of U.S. deferred tax assets and liabilities and the impact of the TCJA on unrecognized tax benefits
|
|
•
|
a
$0.1 billion
charge for U.S. state and local taxes on the repatriation of these foreign earnings
|
|
58
|
||
|
•
|
the reduction of the U.S. statutory corporate tax rate including the effects of tax elections which resulted in the acceleration of certain deductions into the 2017 tax return. The impact of these accelerated deductions decreased the annual effective tax rate by approximately
1.7%
|
|
•
|
the impact of the adjustments to the 2017 provisional TCJA charge, including both SAB 118 adjustments and the internal restructuring, decreased the effective tax rate by approximately
1.9%
|
|
•
|
GILTI tax which increased the annual effective tax rate by approximately
1.6%
, which excludes the impact of the SAB 118 adjustment for the adoption of the deferred method for GILTI
|
|
•
|
tax benefits received from stock-based compensation during fiscal 2018 and 2017, reduced the effective tax rate by
1.5%
and
2.0%
, respectively
|
|
•
|
in the fourth quarter of 2018, the Company completed the divestiture of its LifeScan business (Note 20), which increased the Company annual effective tax rate by approximately
0.8%
|
|
•
|
more income in higher tax jurisdictions relative to lower tax jurisdictions as compared to 2017
|
|
59
|
||
|
2018 Deferred Tax
|
2017 Deferred Tax
|
||||||||||||
|
(Dollars in Millions)
|
Asset
|
Liability
|
Asset
|
Liability
|
|||||||||
|
Employee related obligations
|
$
|
2,398
|
|
|
|
2,259
|
|
|
|
||||
|
Stock based compensation
|
639
|
|
|
|
507
|
|
|
|
|||||
|
Depreciation & amortization
|
1,784
|
|
|
|
|
|
(9
|
)
|
|||||
|
Non-deductible intangibles
|
|
|
(5,967
|
)
|
|
|
(6,506
|
)
|
|||||
|
International R&D capitalized for tax
|
1,282
|
|
|
|
1,307
|
|
|
|
|||||
|
Reserves & liabilities
|
1,647
|
|
|
|
1,718
|
|
|
|
|||||
|
Income reported for tax purposes
|
1,104
|
|
|
|
1,316
|
|
|
|
|||||
|
Net operating loss carryforward international
|
786
|
|
|
|
762
|
|
|
|
|||||
|
Undistributed foreign earnings
|
693
|
|
(2,240
|
)
|
1,101
|
|
(4,457
|
)
|
|||||
|
Global intangible low-taxed income
|
(2,971
|
)
|
|||||||||||
|
Miscellaneous international
|
603
|
|
(93
|
)
|
755
|
|
(194
|
)
|
|||||
|
Miscellaneous U.S.
|
469
|
|
|
|
177
|
|
|
|
|||||
|
Total deferred income taxes
|
$
|
11,405
|
|
(11,271
|
)
|
9,902
|
|
(11,166
|
)
|
||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
|||||||
|
Beginning of year
|
$
|
3,151
|
|
3,041
|
|
3,080
|
|
|||
|
Increases related to current year tax positions
|
242
|
|
332
|
|
348
|
|
||||
|
Increases related to prior period tax positions
|
145
|
|
232
|
|
11
|
|
||||
|
Decreases related to prior period tax positions
|
(137
|
)
|
(416
|
)
|
(1)
|
(338
|
)
|
|||
|
Settlements
|
(40
|
)
|
(2
|
)
|
(37
|
)
|
||||
|
Lapse of statute of limitations
|
(35
|
)
|
(36
|
)
|
(23
|
)
|
||||
|
End of year
|
$
|
3,326
|
|
3,151
|
|
3,041
|
|
|||
|
60
|
||
|
9.
|
Employee Related Obligations
|
|
(Dollars in Millions)
|
2018
|
2017
|
|||||
|
Pension benefits
|
$
|
5,327
|
|
5,343
|
|
||
|
Postretirement benefits
|
2,283
|
|
2,331
|
|
|||
|
Postemployment benefits
|
2,330
|
|
2,250
|
|
|||
|
Deferred compensation
|
410
|
|
475
|
|
|||
|
Total employee obligations
|
10,350
|
|
10,399
|
|
|||
|
Less current benefits payable
|
399
|
|
325
|
|
|||
|
Employee related obligations — non-current
|
$
|
9,951
|
|
10,074
|
|
||
|
10.
|
Pensions and Other Benefit Plans
|
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
|||||||||||||
|
Service cost
|
$
|
1,283
|
|
1,080
|
|
949
|
|
269
|
|
247
|
|
224
|
|
||||||
|
Interest cost
|
996
|
|
927
|
|
927
|
|
148
|
|
159
|
|
158
|
|
|||||||
|
Expected return on plan assets
|
(2,212
|
)
|
(2,041
|
)
|
(1,962
|
)
|
(7
|
)
|
(6
|
)
|
(6
|
)
|
|||||||
|
Amortization of prior service cost (credit)
|
3
|
|
2
|
|
1
|
|
(31
|
)
|
(30
|
)
|
(34
|
)
|
|||||||
|
Recognized actuarial losses
|
852
|
|
609
|
|
496
|
|
123
|
|
138
|
|
135
|
|
|||||||
|
Curtailments and settlements
|
1
|
|
17
|
|
11
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Net periodic benefit cost
|
$
|
923
|
|
594
|
|
422
|
|
502
|
|
508
|
|
477
|
|
||||||
|
61
|
||
|
(Dollars in Millions)
|
|
||
|
Amortization of net transition obligation
|
$
|
—
|
|
|
Amortization of net actuarial losses
|
656
|
|
|
|
Amortization of prior service credit
|
27
|
|
|
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||
|
Worldwide Benefit Plans
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
|||||||
|
Net Periodic Benefit Cost
|
|||||||||||||
|
Service cost discount rate
|
3.20
|
%
|
3.59
|
3.98
|
3.85
|
4.63
|
4.77
|
||||||
|
Interest cost discount rate
|
3.60
|
%
|
3.98
|
4.24
|
3.62
|
3.94
|
4.10
|
||||||
|
Rate of increase in compensation levels
|
3.98
|
%
|
4.01
|
4.02
|
4.29
|
4.31
|
4.32
|
||||||
|
Expected long-term rate of return on plan assets
|
8.46
|
%
|
8.43
|
8.55
|
|||||||||
|
Benefit Obligation
|
|||||||||||||
|
Discount rate
|
3.76
|
%
|
3.30
|
3.78
|
4.40
|
3.78
|
4.42
|
||||||
|
Rate of increase in compensation levels
|
3.97
|
%
|
3.99
|
4.02
|
4.29
|
4.30
|
4.29
|
||||||
|
62
|
||
|
Health Care Plans
|
2018
|
2017
|
||||
|
Health care cost trend rate assumed for next year
|
6.12
|
%
|
6.33
|
%
|
||
|
Rate to which the cost trend rate is assumed to decline (ultimate trend)
|
4.55
|
%
|
4.55
|
%
|
||
|
Year the rate reaches the ultimate trend rate
|
2038
|
|
2038
|
|
||
|
One-Percentage-
|
One-Percentage-
|
||||||
|
(Dollars in Millions)
|
Point Increase
|
Point Decrease
|
|||||
|
Health Care Plans
|
|
|
|
|
|||
|
Total interest and service cost
|
$
|
28
|
|
(22
|
)
|
||
|
Post-retirement benefit obligation
|
$
|
340
|
|
(280
|
)
|
||
|
63
|
||
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||
|
Change in Benefit Obligation
|
|||||||||||||
|
Projected benefit obligation — beginning of year
|
$
|
33,221
|
|
28,116
|
|
4,582
|
|
4,605
|
|
||||
|
Service cost
|
1,283
|
|
1,080
|
|
269
|
|
247
|
|
|||||
|
Interest cost
|
996
|
|
927
|
|
148
|
|
159
|
|
|||||
|
Plan participant contributions
|
66
|
|
60
|
|
—
|
|
—
|
|
|||||
|
Amendments
|
26
|
|
(7
|
)
|
—
|
|
(17
|
)
|
|||||
|
Actuarial (gains) losses
|
(2,326
|
)
|
2,996
|
|
(119
|
)
|
(166
|
)
|
|||||
|
Divestitures & acquisitions
|
(29
|
)
|
201
|
|
—
|
|
88
|
|
|||||
|
Curtailments, settlements & restructuring
|
(21
|
)
|
(35
|
)
|
—
|
|
2
|
|
|||||
|
Benefits paid from plan
|
(1,018
|
)
|
(1,050
|
)
|
(383
|
)
|
(351
|
)
|
|||||
|
Effect of exchange rates
|
(528
|
)
|
933
|
|
(17
|
)
|
15
|
|
|||||
|
Projected benefit obligation — end of year
|
$
|
31,670
|
|
33,221
|
|
4,480
|
|
4,582
|
|
||||
|
Change in Plan Assets
|
|||||||||||||
|
Plan assets at fair value — beginning of year
|
$
|
28,404
|
|
23,633
|
|
281
|
|
75
|
|
||||
|
Actual return on plan assets
|
(1,269
|
)
|
4,274
|
|
—
|
|
12
|
|
|||||
|
Company contributions
|
1,140
|
|
664
|
|
282
|
|
545
|
|
|||||
|
Plan participant contributions
|
66
|
|
60
|
|
—
|
|
—
|
|
|||||
|
Settlements
|
(13
|
)
|
(32
|
)
|
—
|
|
—
|
|
|||||
|
Divestitures & acquisitions
|
(17
|
)
|
173
|
|
—
|
|
—
|
|
|||||
|
Benefits paid from plan assets
|
(1,018
|
)
|
(1,050
|
)
|
(383
|
)
|
(351
|
)
|
|||||
|
Effect of exchange rates
|
(475
|
)
|
682
|
|
—
|
|
—
|
|
|||||
|
Plan assets at fair value — end of year
|
$
|
26,818
|
|
28,404
|
|
180
|
|
281
|
|
||||
|
Funded status — end of year
|
$
|
(4,852
|
)
|
(4,817
|
)
|
(4,300
|
)
|
(4,301
|
)
|
||||
|
Amounts Recognized in the Company’s Balance Sheet consist of the following:
|
|||||||||||||
|
Non-current assets
|
$
|
475
|
|
526
|
|
—
|
|
—
|
|
||||
|
Current liabilities
|
(98
|
)
|
(92
|
)
|
(281
|
)
|
(228
|
)
|
|||||
|
Non-current liabilities
|
(5,229
|
)
|
(5,251
|
)
|
(4,019
|
)
|
(4,073
|
)
|
|||||
|
Total recognized in the consolidated balance sheet — end of year
|
$
|
(4,852
|
)
|
(4,817
|
)
|
(4,300
|
)
|
(4,301
|
)
|
||||
|
Amounts Recognized in Accumulated Other Comprehensive Income consist of the following:
|
|||||||||||||
|
Net actuarial loss
|
$
|
8,323
|
|
8,140
|
|
1,263
|
|
1,500
|
|
||||
|
Prior service cost (credit)
|
2
|
|
(25
|
)
|
(106
|
)
|
(137
|
)
|
|||||
|
Unrecognized net transition obligation
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Total before tax effects
|
$
|
8,325
|
|
8,115
|
|
1,157
|
|
1,363
|
|
||||
|
Accumulated Benefit Obligations — end of year
|
$
|
28,533
|
|
29,793
|
|
||||||||
|
64
|
||
|
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||
|
Amounts Recognized in Net Periodic Benefit Cost and Other Comprehensive Income
|
|||||||||||||
|
Net periodic benefit cost
|
$
|
923
|
|
594
|
|
502
|
|
508
|
|
||||
|
Net actuarial (gain) loss
|
1,153
|
|
740
|
|
(111
|
)
|
(169
|
)
|
|||||
|
Amortization of net actuarial loss
|
(852
|
)
|
(609
|
)
|
(123
|
)
|
(138
|
)
|
|||||
|
Prior service cost (credit)
|
26
|
|
(7
|
)
|
—
|
|
(17
|
)
|
|||||
|
Amortization of prior service (cost) credit
|
(3
|
)
|
(2
|
)
|
31
|
|
30
|
|
|||||
|
Effect of exchange rates
|
(114
|
)
|
256
|
|
(3
|
)
|
3
|
|
|||||
|
Total loss/(income) recognized in other comprehensive income, before tax
|
$
|
210
|
|
378
|
|
(206
|
)
|
(291
|
)
|
||||
|
Total recognized in net periodic benefit cost and other comprehensive income
|
$
|
1,133
|
|
972
|
|
296
|
|
217
|
|
||||
|
U.S. Plans
|
International Plans
|
||||||||||||||||
|
Qualified Plans
|
Non-Qualified Plans
|
Funded Plans
|
Unfunded Plans
|
||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
|||||||||
|
Plan Assets
|
$
|
17,725
|
|
18,681
|
|
—
|
|
—
|
|
9,093
|
|
9,723
|
|
—
|
|
—
|
|
|
Projected Benefit Obligation
|
18,609
|
|
19,652
|
|
2,176
|
|
2,257
|
|
10,467
|
|
10,863
|
|
418
|
|
449
|
|
|
|
Accumulated Benefit Obligation
|
16,851
|
|
17,654
|
|
1,793
|
|
1,849
|
|
9,510
|
|
9,893
|
|
379
|
|
397
|
|
|
|
Over (Under) Funded Status
|
|||||||||||||||||
|
Projected Benefit Obligation
|
$
|
(884
|
)
|
(971
|
)
|
(2,176
|
)
|
(2,257
|
)
|
(1,374
|
)
|
(1,140
|
)
|
(418
|
)
|
(449
|
)
|
|
Accumulated Benefit Obligation
|
874
|
|
1,027
|
|
(1,793
|
)
|
(1,849
|
)
|
(417
|
)
|
(170
|
)
|
(379
|
)
|
(397
|
)
|
|
|
(Dollars in Millions)
|
2019
|
2020
|
2021
|
2022
|
2023
|
2024-2028
|
|||||||||||||
|
Projected future benefit payments
|
|||||||||||||||||||
|
Retirement plans
|
$
|
1,062
|
|
1,104
|
|
1,182
|
|
1,257
|
|
1,332
|
|
7,679
|
|
||||||
|
Other benefit plans
|
$
|
375
|
|
397
|
|
411
|
|
428
|
|
413
|
|
2,273
|
|
||||||
|
(Dollars in Millions)
|
2019
|
2020
|
2021
|
2022
|
2023
|
2024-2028
|
|||||||||||||
|
Projected future contributions
|
$
|
92
|
|
95
|
|
101
|
|
108
|
|
115
|
|
697
|
|
||||||
|
65
|
||
|
Percent of
Plan Assets
|
Target
Allocation
|
||||||||
|
|
2018
|
2017
|
2019
|
||||||
|
Worldwide Retirement Plans
|
|||||||||
|
Equity securities
|
71
|
%
|
76
|
%
|
70
|
%
|
|||
|
Debt securities
|
29
|
|
24
|
|
30
|
|
|||
|
Total plan assets
|
100
|
%
|
100
|
%
|
100
|
%
|
|||
|
•
|
Short-term investment funds
— Cash and quoted short-term instruments are valued at the closing price or the amount held on deposit by the custodian bank. Other investments are through investment vehicles valued using the Net Asset Value (NAV) provided by the administrator of the fund. The NAV is based on the value of the underlying assets owned by the fund, minus its liabilities, and then divided by the number of shares outstanding. The NAV is a quoted price in a market that is not active and classified as Level 2.
|
|
•
|
Government and agency securities
— A limited number of these investments are valued at the closing price reported on the major market on which the individual securities are traded. Where quoted prices are available in an active market, the investments are classified within Level 1 of the valuation hierarchy. If quoted market prices are not available for the specific security, then fair values are estimated by using pricing models, quoted prices of securities with similar characteristics or discounted cash flows. When quoted market prices for a security are not available in an active market, they are classified as Level 2.
|
|
•
|
Debt instruments
— A limited number of these investments are valued at the closing price reported on the major market on which the individual securities are traded. Where quoted prices are available in an active market, the investments are classified as Level 1. If quoted market prices are not available for the specific security, then fair values are estimated by using pricing models, quoted prices of securities with similar characteristics or discounted cash flows and are classified as Level 2. Level 3 debt instruments are priced based on unobservable inputs.
|
|
•
|
Equity securities
— Equity securities are valued at the closing price reported on the major market on which the individual securities are traded. Substantially all common stock is classified within Level 1 of the valuation hierarchy.
|
|
•
|
Commingled funds
— These investment vehicles are valued using the NAV provided by the fund administrator. The NAV is based on the value of the underlying assets owned by the fund, minus its liabilities, and then divided by the number of shares outstanding. Assets in the Level 2 category have a quoted market price.
|
|
66
|
||
|
•
|
Insurance contracts
— The instruments are issued by insurance companies. The fair value is based on negotiated value and the underlying investments held in separate account portfolios as well as considering the credit worthiness of the issuer. The underlying investments are government, asset-backed and fixed income securities. In general, insurance contracts are classified as Level 3 as there are no quoted prices nor other observable inputs for pricing.
|
|
•
|
Other assets
— Other assets are represented primarily by limited partnerships and real estate investments, as well as commercial loans and commercial mortgages that are not classified as corporate debt. Other assets that are exchange listed and actively traded are classified as Level 1, while inactively traded assets are classified as Level 2.
|
|
Quoted Prices
in Active
Markets for
Identical Assets
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
(a)
|
Investments Measured at Net Asset Value
|
||||||||||||||||||||||||||||
|
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total Assets
|
|||||||||||||||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
|||||||||||||||||||||
|
Short-term investment funds
|
$
|
122
|
|
429
|
|
529
|
|
427
|
|
—
|
|
—
|
|
—
|
|
—
|
|
651
|
|
856
|
|
||||||||||
|
Government and agency securities
|
—
|
|
—
|
|
3,595
|
|
3,094
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,595
|
|
3,094
|
|
|||||||||||
|
Debt instruments
|
—
|
|
—
|
|
3,105
|
|
2,013
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,105
|
|
2,013
|
|
|||||||||||
|
Equity securities
|
11,298
|
|
13,848
|
|
4
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
11,302
|
|
13,848
|
|
|||||||||||
|
Commingled funds
|
—
|
|
—
|
|
2,304
|
|
1,780
|
|
133
|
|
57
|
|
5,201
|
|
6,158
|
|
7,638
|
|
7,995
|
|
|||||||||||
|
Insurance contracts
|
—
|
|
—
|
|
—
|
|
—
|
|
193
|
|
199
|
|
—
|
|
—
|
|
193
|
|
199
|
|
|||||||||||
|
Other assets
|
—
|
|
—
|
|
33
|
|
121
|
|
—
|
|
—
|
|
301
|
|
278
|
|
334
|
|
399
|
|
|||||||||||
|
Investments at fair value
|
$
|
11,420
|
|
14,277
|
|
9,570
|
|
7,435
|
|
326
|
|
256
|
|
5,502
|
|
6,436
|
|
26,818
|
|
28,404
|
|
||||||||||
|
11.
|
Savings Plan
|
|
67
|
||
|
12.
|
Capital and Treasury Stock
|
|
Treasury Stock
|
|||||||
|
(Amounts in Millions Except Treasury Stock Shares in Thousands)
|
Shares
|
Amount
|
|||||
|
Balance at January 3, 2016
|
364,681
|
|
$
|
22,684
|
|
||
|
Employee compensation and stock option plans
|
(30,839
|
)
|
(3,311
|
)
|
|||
|
Repurchase of common stock
|
79,490
|
|
8,979
|
|
|||
|
Balance at January 1, 2017
|
413,332
|
|
28,352
|
|
|||
|
Employee compensation and stock option plans
|
(25,508
|
)
|
(3,156
|
)
|
|||
|
Repurchase of common stock
|
49,494
|
|
6,358
|
|
|||
|
Balance at December 31, 2017
|
437,318
|
|
31,554
|
|
|||
|
Employee compensation and stock option plans
|
(22,082
|
)
|
(3,060
|
)
|
|||
|
Repurchase of common stock
|
42,283
|
|
5,868
|
|
|||
|
Balance at December 30, 2018
|
457,519
|
|
$
|
34,362
|
|
||
|
13.
|
Accumulated Other Comprehensive Income (Loss)
|
|
(Dollars in Millions)
|
Foreign
Currency Translation |
Gain/(Loss) On Securities
|
Employee Benefit Plans
|
Gain/
(Loss) On Derivatives & Hedges |
Total
Accumulated Other Comprehensive Income (Loss) |
|||||||||||
|
January 3, 2016
|
$
|
(8,435
|
)
|
604
|
|
(5,298
|
)
|
(36
|
)
|
(13,165
|
)
|
|||||
|
Net 2016 changes
|
(612
|
)
|
(193
|
)
|
(682
|
)
|
(249
|
)
|
(1,736
|
)
|
||||||
|
January 1, 2017
|
(9,047
|
)
|
411
|
|
(5,980
|
)
|
(285
|
)
|
(14,901
|
)
|
||||||
|
Net 2017 changes
|
1,696
|
|
(179
|
)
|
(170
|
)
|
355
|
|
1,702
|
|
||||||
|
December 31, 2017
|
(7,351
|
)
|
232
|
|
(6,150
|
)
|
70
|
|
(13,199
|
)
|
||||||
|
Cumulative adjustment to retained earnings
|
(232
|
)
|
(1)
|
(232
|
)
|
|||||||||||
|
Net 2018 changes
|
(1,518
|
)
|
—
|
|
(8
|
)
|
(265
|
)
|
(1,791
|
)
|
||||||
|
December 30, 2018
|
$
|
(8,869
|
)
|
—
|
|
(6,158
|
)
|
(195
|
)
|
(15,222
|
)
|
|||||
|
68
|
||
|
14.
|
International Currency Translation
|
|
15.
|
Earnings Per Share
|
|
(In Millions Except Per Share Amounts)
|
2018
|
2017
|
2016
|
|||||||
|
Basic net earnings per share
|
$
|
5.70
|
|
0.48
|
|
6.04
|
|
|||
|
Average shares outstanding — basic
|
2,681.5
|
|
2,692.0
|
|
2,737.3
|
|
||||
|
Potential shares exercisable under stock option plans
|
139.0
|
|
139.7
|
|
142.4
|
|
||||
|
Less: shares repurchased under treasury stock method
|
(92.5
|
)
|
(87.3
|
)
|
(92.1
|
)
|
||||
|
Convertible debt shares
|
0.7
|
|
0.9
|
|
1.3
|
|
||||
|
Adjusted average shares outstanding — diluted
|
2,728.7
|
|
2,745.3
|
|
2,788.9
|
|
||||
|
Diluted net earnings per share
|
$
|
5.61
|
|
0.47
|
|
5.93
|
|
|||
|
16.
|
Rental Expense and Lease Commitments
|
|
2019
|
2020
|
2021
|
2022
|
2023
|
After 2023
|
Total
|
||||||
|
$223
|
188
|
154
|
116
|
76
|
139
|
896
|
||||||
|
69
|
||
|
17.
|
Common Stock, Stock Option Plans and Stock Compensation Agreements
|
|
2018
|
2017
|
2016
|
||||||
|
Risk-free rate
|
2.77
|
%
|
2.25
|
%
|
1.51
|
%
|
||
|
Expected volatility
|
15.77
|
%
|
15.30
|
%
|
15.76
|
%
|
||
|
Expected life (in years)
|
7.0
|
|
7.0
|
|
7.0
|
|
||
|
Expected dividend yield
|
2.70
|
%
|
2.90
|
%
|
3.10
|
%
|
||
|
70
|
||
|
(Shares in Thousands)
|
Outstanding Shares
|
Weighted
Average Exercise Price
|
Aggregate
Intrinsic
Value
(Dollars in Millions)
|
||||||||
|
Shares at January 3, 2016
|
116,517
|
|
$
|
76.41
|
|
$
|
3,065
|
|
|||
|
Options granted
|
22,491
|
|
101.87
|
|
|||||||
|
Options exercised
|
(22,547
|
)
|
65.66
|
|
|||||||
|
Options canceled/forfeited
|
(3,006
|
)
|
92.83
|
|
|||||||
|
Shares at January 1, 2017
|
113,455
|
|
83.16
|
|
3,636
|
|
|||||
|
Options granted
|
19,287
|
|
115.67
|
|
|||||||
|
Options exercised
|
(18,975
|
)
|
70.87
|
|
|||||||
|
Options canceled/forfeited
|
(2,461
|
)
|
101.40
|
|
|||||||
|
Shares at December 31, 2017
|
111,306
|
|
90.48
|
|
5,480
|
|
|||||
|
Options granted
|
17,115
|
|
129.51
|
|
|||||||
|
Options exercised
|
(16,228
|
)
|
75.44
|
|
|||||||
|
Options canceled/forfeited
|
(2,541
|
)
|
112.90
|
|
|||||||
|
Shares at December 30, 2018
|
109,652
|
|
$
|
98.29
|
|
$
|
3,214
|
|
|||
|
(Shares in Thousands)
|
Outstanding
|
Exercisable
|
||||||||||
|
Exercise Price Range
|
Options
|
Average Life
(1)
|
Average Exercise Price
|
Options
|
Average Exercise Price
|
|||||||
|
$52.13-$65.62
|
13,466
|
|
1.8
|
$62.67
|
13,466
|
|
$62.67
|
|||||
|
$66.07-$72.54
|
12,710
|
|
4.0
|
$72.53
|
12,710
|
|
$72.53
|
|||||
|
$90.44-$100.48
|
29,162
|
|
5.6
|
$95.34
|
28,537
|
|
$95.23
|
|||||
|
$101.87-$115.67
|
37,877
|
|
7.6
|
$108.33
|
111
|
|
$108.04
|
|||||
|
$115.68-$129.51
|
16,437
|
|
9.1
|
$129.51
|
38
|
|
$129.51
|
|||||
|
|
109,652
|
|
6.2
|
$98.29
|
54,862
|
|
$82.03
|
|||||
|
71
|
||
|
(Shares in Thousands)
|
Outstanding Restricted Share Units
|
Outstanding Performance Share Units
|
||||
|
Shares at December 31, 2017
|
20,161
|
|
2,625
|
|
||
|
Granted
|
6,074
|
|
1,142
|
|
||
|
Issued
|
(6,684
|
)
|
(1,151
|
)
|
||
|
Canceled/forfeited/adjusted
|
(1,091
|
)
|
(122
|
)
|
||
|
Shares at December 30, 2018
|
18,460
|
|
2,494
|
|
||
|
72
|
||
|
18.
|
Segments of Business and Geographic Areas
|
|
|
Sales to Customers
|
% Change
|
||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
’18 vs. ’17
|
’17 vs. ’16
|
|||||||||||
|
CONSUMER
|
|
|
|
|||||||||||||
|
Baby Care
|
||||||||||||||||
|
U.S.
|
$
|
422
|
|
449
|
|
488
|
|
(6.0
|
)%
|
(8.0
|
)
|
|||||
|
International
|
1,436
|
|
1,467
|
|
1,513
|
|
(2.1
|
)
|
(3.0
|
)
|
||||||
|
Worldwide
|
1,858
|
|
1,916
|
|
2,001
|
|
(3.0
|
)
|
(4.2
|
)
|
||||||
|
Beauty
|
||||||||||||||||
|
U.S.
|
2,403
|
|
2,335
|
|
2,135
|
|
2.9
|
|
9.4
|
|
||||||
|
International
|
1,979
|
|
1,865
|
|
1,762
|
|
6.1
|
|
5.8
|
|
||||||
|
Worldwide
|
4,382
|
|
4,200
|
|
3,897
|
|
4.3
|
|
7.8
|
|
||||||
|
Oral Care
|
||||||||||||||||
|
U.S.
|
637
|
|
616
|
|
648
|
|
3.4
|
|
(4.9
|
)
|
||||||
|
International
|
918
|
|
915
|
|
920
|
|
0.3
|
|
(0.5
|
)
|
||||||
|
Worldwide
|
1,555
|
|
1,531
|
|
1,568
|
|
1.6
|
|
(2.4
|
)
|
||||||
|
OTC
|
||||||||||||||||
|
U.S.
|
1,850
|
|
1,716
|
|
1,675
|
|
7.8
|
|
2.4
|
|
||||||
|
International
|
2,484
|
|
2,410
|
|
2,302
|
|
3.1
|
|
4.7
|
|
||||||
|
Worldwide
|
4,334
|
|
4,126
|
|
3,977
|
|
5.0
|
|
3.7
|
|
||||||
|
Women's Health
|
||||||||||||||||
|
U.S.
|
13
|
|
12
|
|
19
|
|
8.3
|
|
(36.8
|
)
|
||||||
|
International
|
1,036
|
|
1,038
|
|
1,048
|
|
(0.2
|
)
|
(1.0
|
)
|
||||||
|
Worldwide
|
1,049
|
|
1,050
|
|
1,067
|
|
(0.1
|
)
|
(1.6
|
)
|
||||||
|
Wound Care/Other
|
||||||||||||||||
|
U.S.
|
436
|
|
437
|
|
455
|
|
(0.2
|
)
|
(4.0
|
)
|
||||||
|
International
|
239
|
|
342
|
|
342
|
|
(30.1
|
)
|
0.0
|
|
||||||
|
Worldwide
|
675
|
|
779
|
|
797
|
|
(13.4
|
)
|
(2.3
|
)
|
||||||
|
TOTAL CONSUMER
|
||||||||||||||||
|
U.S.
|
5,761
|
|
5,565
|
|
5,420
|
|
3.5
|
|
2.7
|
|
||||||
|
International
|
8,092
|
|
8,037
|
|
7,887
|
|
0.7
|
|
1.9
|
|
||||||
|
Worldwide
|
13,853
|
|
13,602
|
|
13,307
|
|
1.8
|
|
2.2
|
|
||||||
|
PHARMACEUTICAL
|
||||||||||||||||
|
Immunology
|
||||||||||||||||
|
U.S.
|
9,073
|
|
8,871
|
|
8,846
|
|
2.3
|
|
0.3
|
|
||||||
|
International
|
4,047
|
|
3,373
|
|
3,122
|
|
20.0
|
|
8.0
|
|
||||||
|
Worldwide
|
13,120
|
|
12,244
|
|
11,968
|
|
7.2
|
|
2.3
|
|
||||||
|
REMICADE
®
|
||||||||||||||||
|
U.S.
|
3,664
|
|
4,525
|
|
4,842
|
|
(19.0
|
)
|
(6.5
|
)
|
||||||
|
U.S. Exports
|
436
|
|
563
|
|
782
|
|
(22.6
|
)
|
(28.0
|
)
|
||||||
|
International
|
1,226
|
|
1,227
|
|
1,342
|
|
(0.1
|
)
|
(8.6
|
)
|
||||||
|
Worldwide
|
5,326
|
|
6,315
|
|
6,966
|
|
(15.7
|
)
|
(9.3
|
)
|
||||||
|
73
|
||
|
SIMPONI / SIMPONI ARIA
®
|
||||||||||||||||
|
U.S.
|
1,051
|
|
954
|
|
959
|
|
10.2
|
|
(0.5
|
)
|
||||||
|
International
|
1,033
|
|
879
|
|
786
|
|
17.5
|
|
11.8
|
|
||||||
|
Worldwide
|
2,084
|
|
1,833
|
|
1,745
|
|
13.7
|
|
5.0
|
|
||||||
|
STELARA
®
|
||||||||||||||||
|
U.S.
|
3,469
|
|
2,767
|
|
2,263
|
|
25.4
|
|
22.3
|
|
||||||
|
International
|
1,687
|
|
1,244
|
|
969
|
|
35.6
|
|
28.4
|
|
||||||
|
Worldwide
|
5,156
|
|
4,011
|
|
3,232
|
|
28.5
|
|
24.1
|
|
||||||
|
TREMFAYA
®
|
||||||||||||||||
|
U.S.
|
453
|
|
62
|
|
—
|
|
*
|
*
|
||||||||
|
International
|
91
|
|
1
|
|
—
|
|
*
|
*
|
||||||||
|
Worldwide
|
544
|
|
63
|
|
—
|
|
*
|
*
|
||||||||
|
OTHER IMMUNOLOGY
|
||||||||||||||||
|
U.S.
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|||||||
|
International
|
10
|
|
22
|
|
25
|
|
(54.5
|
)
|
(12.0
|
)
|
||||||
|
Worldwide
|
10
|
|
22
|
|
25
|
|
(54.5
|
)
|
(12.0
|
)
|
||||||
|
Infectious Diseases
|
||||||||||||||||
|
U.S.
|
1,378
|
|
1,358
|
|
1,461
|
|
1.5
|
|
(7.0
|
)
|
||||||
|
International
|
1,926
|
|
1,796
|
|
1,747
|
|
7.2
|
|
2.8
|
|
||||||
|
Worldwide
|
3,304
|
|
3,154
|
|
3,208
|
|
4.8
|
|
(1.7
|
)
|
||||||
|
EDURANT
®
/ rilpivirine
|
||||||||||||||||
|
U.S.
|
58
|
|
58
|
|
52
|
|
0.0
|
|
11.5
|
|
||||||
|
International
|
758
|
|
656
|
|
521
|
|
15.5
|
|
25.9
|
|
||||||
|
Worldwide
|
816
|
|
714
|
|
573
|
|
14.3
|
|
24.6
|
|
||||||
|
PREZISTA
®
/ PREZCOBIX
®
/ REZOLSTA
®
/ SYMTUZA
®
|
||||||||||||||||
|
U.S.
|
1,169
|
|
1,109
|
|
1,143
|
|
5.4
|
|
(3.0
|
)
|
||||||
|
International
|
786
|
|
712
|
|
708
|
|
10.4
|
|
0.6
|
|
||||||
|
Worldwide
|
1,955
|
|
1,821
|
|
1,851
|
|
7.4
|
|
(1.6
|
)
|
||||||
|
OTHER INFECTIOUS DISEASES
|
||||||||||||||||
|
U.S.
|
151
|
|
191
|
|
266
|
|
(20.9
|
)
|
(28.2
|
)
|
||||||
|
International
|
382
|
|
428
|
|
518
|
|
(10.7
|
)
|
(17.4
|
)
|
||||||
|
Worldwide
|
533
|
|
619
|
|
784
|
|
(13.9
|
)
|
(21.0
|
)
|
||||||
|
Neuroscience
|
||||||||||||||||
|
U.S.
|
2,574
|
|
2,630
|
|
2,628
|
|
(2.1
|
)
|
0.1
|
|
||||||
|
International
|
3,503
|
|
3,356
|
|
3,457
|
|
4.4
|
|
(2.9
|
)
|
||||||
|
Worldwide
|
6,077
|
|
5,986
|
|
6,085
|
|
1.5
|
|
(1.6
|
)
|
||||||
|
CONCERTA
®
/ Methylphenidate
|
||||||||||||||||
|
U.S.
|
229
|
|
384
|
|
468
|
|
(40.4
|
)
|
(17.9
|
)
|
||||||
|
International
|
434
|
|
407
|
|
395
|
|
6.6
|
|
3.0
|
|
||||||
|
Worldwide
|
663
|
|
791
|
|
863
|
|
(16.2
|
)
|
(8.3
|
)
|
||||||
|
INVEGA SUSTENNA
®
/ XEPLION
®
/ INVEGA TRINZA
®
/ TREVICTA
®
|
||||||||||||||||
|
U.S.
|
1,791
|
|
1,590
|
|
1,343
|
|
12.6
|
|
18.4
|
|
||||||
|
International
|
1,137
|
|
979
|
|
871
|
|
16.1
|
|
12.4
|
|
||||||
|
Worldwide
|
2,928
|
|
2,569
|
|
2,214
|
|
14.0
|
|
16.0
|
|
||||||
|
74
|
||
|
RISPERDAL CONSTA
®
|
||||||||||||||||
|
U.S.
|
315
|
|
360
|
|
381
|
|
(12.5
|
)
|
(5.5
|
)
|
||||||
|
International
|
422
|
|
445
|
|
512
|
|
(5.2
|
)
|
(13.1
|
)
|
||||||
|
Worldwide
|
737
|
|
805
|
|
893
|
|
(8.4
|
)
|
(9.9
|
)
|
||||||
|
OTHER NEUROSCIENCE
|
||||||||||||||||
|
U.S.
|
239
|
|
296
|
|
436
|
|
(19.3
|
)
|
(32.1
|
)
|
||||||
|
International
|
1,510
|
|
1,525
|
|
1,679
|
|
(1.0
|
)
|
(9.2
|
)
|
||||||
|
Worldwide
|
1,749
|
|
1,821
|
|
2,115
|
|
(4.0
|
)
|
(13.9
|
)
|
||||||
|
Oncology
|
||||||||||||||||
|
U.S.
|
4,331
|
|
3,098
|
|
2,335
|
|
39.8
|
|
32.7
|
|
||||||
|
International
|
5,513
|
|
4,160
|
|
3,472
|
|
32.5
|
|
19.8
|
|
||||||
|
Worldwide
|
9,844
|
|
7,258
|
|
5,807
|
|
35.6
|
|
25.0
|
|
||||||
|
DARZALEX
®
|
||||||||||||||||
|
U.S.
|
1,203
|
|
884
|
|
471
|
|
36.1
|
|
87.7
|
|
||||||
|
International
|
822
|
|
358
|
|
101
|
|
*
|
*
|
||||||||
|
Worldwide
|
2,025
|
|
1,242
|
|
572
|
|
63.0
|
|
*
|
|||||||
|
IMBRUVICA
®
|
||||||||||||||||
|
U.S.
|
1,129
|
|
841
|
|
613
|
|
34.2
|
|
37.2
|
|
||||||
|
International
|
1,486
|
|
1,052
|
|
638
|
|
41.3
|
|
64.9
|
|
||||||
|
Worldwide
|
2,615
|
|
1,893
|
|
1,251
|
|
38.1
|
|
51.3
|
|
||||||
|
VELCADE
®
|
||||||||||||||||
|
U.S.
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|||||||
|
International
|
1,116
|
|
1,114
|
|
1,224
|
|
0.2
|
|
(9.0
|
)
|
||||||
|
Worldwide
|
1,116
|
|
1,114
|
|
1,224
|
|
0.2
|
|
(9.0
|
)
|
||||||
|
ZYTIGA
®
/
abiraterone acetate
|
||||||||||||||||
|
U.S.
|
1,771
|
|
1,228
|
|
1,089
|
|
44.2
|
|
12.8
|
|
||||||
|
International
|
1,727
|
|
1,277
|
|
1,171
|
|
35.2
|
|
9.1
|
|
||||||
|
Worldwide
|
3,498
|
|
2,505
|
|
2,260
|
|
39.6
|
|
10.8
|
|
||||||
|
OTHER ONCOLOGY
|
||||||||||||||||
|
U.S.
|
228
|
|
145
|
|
162
|
|
57.2
|
|
(10.5
|
)
|
||||||
|
International
|
362
|
|
359
|
|
338
|
|
0.8
|
|
6.2
|
|
||||||
|
Worldwide
|
590
|
|
504
|
|
500
|
|
17.1
|
|
0.8
|
|
||||||
|
Pulmonary Hypertension
|
||||||||||||||||
|
U.S.
|
1,651
|
|
773
|
|
—
|
|
*
|
*
|
||||||||
|
International
|
922
|
|
554
|
|
—
|
|
66.4
|
|
*
|
|||||||
|
Worldwide
|
2,573
|
|
1,327
|
|
—
|
|
93.9
|
|
*
|
|||||||
|
OPSUMIT
®
|
||||||||||||||||
|
U.S.
|
700
|
|
320
|
|
—
|
|
*
|
*
|
||||||||
|
International
|
515
|
|
253
|
|
—
|
|
*
|
*
|
||||||||
|
Worldwide
|
1,215
|
|
573
|
|
—
|
|
*
|
*
|
||||||||
|
TRACLEER
®
|
||||||||||||||||
|
U.S.
|
268
|
|
161
|
|
—
|
|
66.5
|
|
*
|
|||||||
|
International
|
278
|
|
242
|
|
—
|
|
14.9
|
|
*
|
|||||||
|
Worldwide
|
546
|
|
403
|
|
—
|
|
35.5
|
|
*
|
|||||||
|
75
|
||
|
UPTRAVI
®
|
||||||||||||||||
|
U.S.
|
598
|
|
238
|
|
—
|
|
*
|
*
|
||||||||
|
International
|
65
|
|
25
|
|
—
|
|
*
|
*
|
||||||||
|
Worldwide
|
663
|
|
263
|
|
—
|
|
*
|
*
|
||||||||
|
OTHER
|
||||||||||||||||
|
U.S.
|
85
|
|
54
|
|
—
|
|
57.4
|
|
*
|
|||||||
|
International
|
64
|
|
34
|
|
—
|
|
88.2
|
|
*
|
|||||||
|
Worldwide
|
149
|
|
88
|
|
—
|
|
69.3
|
|
*
|
|||||||
|
Cardiovascular / Metabolism / Other
|
||||||||||||||||
|
U.S.
|
4,279
|
|
4,744
|
|
4,855
|
|
(9.8
|
)
|
(2.3
|
)
|
||||||
|
International
|
1,537
|
|
1,543
|
|
1,541
|
|
(0.4
|
)
|
0.1
|
|
||||||
|
Worldwide
|
5,816
|
|
6,287
|
|
6,396
|
|
(7.5
|
)
|
(1.7
|
)
|
||||||
|
XARELTO
®
|
||||||||||||||||
|
U.S.
|
2,477
|
|
2,500
|
|
2,288
|
|
(0.9
|
)
|
9.3
|
|
||||||
|
International
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||
|
Worldwide
|
2,477
|
|
2,500
|
|
2,288
|
|
(0.9
|
)
|
9.3
|
|
||||||
|
INVOKANA
®
/ INVOKAMET
®
|
||||||||||||||||
|
U.S.
|
711
|
|
944
|
|
1,273
|
|
(24.7
|
)
|
(25.8
|
)
|
||||||
|
International
|
170
|
|
167
|
|
134
|
|
1.8
|
|
24.6
|
|
||||||
|
Worldwide
|
881
|
|
1,111
|
|
1,407
|
|
(20.7
|
)
|
(21.0
|
)
|
||||||
|
PROCRIT
®
/ EPREX
®
|
||||||||||||||||
|
U.S.
|
674
|
|
675
|
|
767
|
|
(0.1
|
)
|
(12.0
|
)
|
||||||
|
International
|
314
|
|
297
|
|
338
|
|
5.7
|
|
(12.1
|
)
|
||||||
|
Worldwide
|
988
|
|
972
|
|
1,105
|
|
1.6
|
|
(12.0
|
)
|
||||||
|
OTHER
|
||||||||||||||||
|
U.S.
|
417
|
|
625
|
|
527
|
|
(33.3
|
)
|
18.6
|
|
||||||
|
International
|
1,053
|
|
1,079
|
|
1,069
|
|
(2.4
|
)
|
0.9
|
|
||||||
|
Worldwide
|
1,470
|
|
1,704
|
|
1,596
|
|
(13.7
|
)
|
6.8
|
|
||||||
|
TOTAL PHARMACEUTICAL
|
||||||||||||||||
|
U.S.
|
23,286
|
|
21,474
|
|
20,125
|
|
8.4
|
|
6.7
|
|
||||||
|
International
|
17,448
|
|
14,782
|
|
13,339
|
|
18.0
|
|
10.8
|
|
||||||
|
Worldwide
|
40,734
|
|
36,256
|
|
33,464
|
|
12.4
|
|
8.3
|
|
||||||
|
MEDICAL DEVICES
|
||||||||||||||||
|
Diabetes Care
|
||||||||||||||||
|
U.S.
|
371
|
|
612
|
|
739
|
|
(39.4
|
)
|
(17.2
|
)
|
||||||
|
International
|
638
|
|
1,003
|
|
1,050
|
|
(36.4
|
)
|
(4.5
|
)
|
||||||
|
Worldwide
|
1,009
|
|
1,615
|
|
1,789
|
|
(37.5
|
)
|
(9.7
|
)
|
||||||
|
Diagnostics
|
||||||||||||||||
|
U.S.
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||
|
International
|
—
|
|
1
|
|
66
|
|
*
|
*
|
||||||||
|
Worldwide
|
—
|
|
1
|
|
66
|
|
*
|
*
|
||||||||
|
Interventional Solutions
|
||||||||||||||||
|
U.S.
|
1,283
|
|
1,148
|
|
1,031
|
|
11.8
|
|
11.3
|
|
||||||
|
International
|
1,363
|
|
1,148
|
|
1,024
|
|
18.7
|
|
12.1
|
|
||||||
|
Worldwide
|
2,646
|
|
2,296
|
|
2,055
|
|
15.2
|
|
11.7
|
|
||||||
|
76
|
||
|
Orthopaedics
|
||||||||||||||||
|
U.S.
|
5,281
|
|
5,404
|
|
5,438
|
|
(2.3
|
)
|
(0.6
|
)
|
||||||
|
International
|
3,604
|
|
3,654
|
|
3,690
|
|
(1.4
|
)
|
(1.0
|
)
|
||||||
|
Worldwide
|
8,885
|
|
9,058
|
|
9,128
|
|
(1.9
|
)
|
(0.8
|
)
|
||||||
|
HIPS
|
||||||||||||||||
|
U.S.
|
841
|
|
827
|
|
798
|
|
1.7
|
|
3.6
|
|
||||||
|
International
|
577
|
|
567
|
|
563
|
|
1.8
|
|
0.7
|
|
||||||
|
Worldwide
|
1,418
|
|
1,394
|
|
1,361
|
|
1.7
|
|
2.4
|
|
||||||
|
KNEES
|
||||||||||||||||
|
U.S.
|
911
|
|
948
|
|
943
|
|
(3.9
|
)
|
0.5
|
|
||||||
|
International
|
591
|
|
575
|
|
581
|
|
2.8
|
|
(1.0
|
)
|
||||||
|
Worldwide
|
1,502
|
|
1,523
|
|
1,524
|
|
(1.4
|
)
|
(0.1
|
)
|
||||||
|
TRAUMA
|
||||||||||||||||
|
U.S.
|
1,599
|
|
1,576
|
|
1,545
|
|
1.5
|
|
2.0
|
|
||||||
|
International
|
1,100
|
|
1,040
|
|
1,024
|
|
5.8
|
|
1.6
|
|
||||||
|
Worldwide
|
2,699
|
|
2,616
|
|
2,569
|
|
3.2
|
|
1.8
|
|
||||||
|
SPINE & OTHER
|
||||||||||||||||
|
U.S.
|
1,930
|
|
2,053
|
|
2,152
|
|
(6.0
|
)
|
(4.6
|
)
|
||||||
|
International
|
1,336
|
|
1,472
|
|
1,522
|
|
(9.2
|
)
|
(3.3
|
)
|
||||||
|
Worldwide
|
3,266
|
|
3,525
|
|
3,674
|
|
(7.3
|
)
|
(4.1
|
)
|
||||||
|
Surgery
|
||||||||||||||||
|
U.S.
|
4,125
|
|
4,085
|
|
4,026
|
|
1.0
|
|
1.5
|
|
||||||
|
International
|
5,776
|
|
5,474
|
|
5,270
|
|
5.5
|
|
3.9
|
|
||||||
|
Worldwide
|
9,901
|
|
9,559
|
|
9,296
|
|
3.6
|
|
2.8
|
|
||||||
|
ADVANCED
|
||||||||||||||||
|
U.S.
|
1,657
|
|
1,620
|
|
1,524
|
|
2.3
|
|
6.3
|
|
||||||
|
International
|
2,345
|
|
2,136
|
|
1,993
|
|
9.8
|
|
7.2
|
|
||||||
|
Worldwide
|
4,002
|
|
3,756
|
|
3,517
|
|
6.5
|
|
6.8
|
|
||||||
|
GENERAL
|
||||||||||||||||
|
U.S.
|
1,751
|
|
1,728
|
|
1,669
|
|
1.3
|
|
3.5
|
|
||||||
|
International
|
2,806
|
|
2,735
|
|
2,693
|
|
2.6
|
|
1.6
|
|
||||||
|
Worldwide
|
4,557
|
|
4,463
|
|
4,362
|
|
2.1
|
|
2.3
|
|
||||||
|
SPECIALTY
|
||||||||||||||||
|
U.S.
|
717
|
|
737
|
|
833
|
|
(2.7
|
)
|
(11.5
|
)
|
||||||
|
International
|
625
|
|
603
|
|
584
|
|
3.6
|
|
3.3
|
|
||||||
|
Worldwide
|
1,342
|
|
1,340
|
|
1,417
|
|
0.1
|
|
(5.4
|
)
|
||||||
|
Vision
|
||||||||||||||||
|
U.S.
|
1,777
|
|
1,575
|
|
1,032
|
|
12.8
|
|
52.6
|
|
||||||
|
International
|
2,776
|
|
2,488
|
|
1,753
|
|
11.6
|
|
41.9
|
|
||||||
|
Worldwide
|
4,553
|
|
4,063
|
|
2,785
|
|
12.1
|
|
45.9
|
|
||||||
|
CONTACT LENSES / OTHER
|
||||||||||||||||
|
U.S.
|
1,237
|
|
1,122
|
|
1,032
|
|
10.2
|
|
8.7
|
|
||||||
|
International
|
2,065
|
|
1,914
|
|
1,753
|
|
7.9
|
|
9.2
|
|
||||||
|
Worldwide
|
3,302
|
|
3,036
|
|
2,785
|
|
8.8
|
|
9.0
|
|
||||||
|
77
|
||
|
SURGICAL
|
||||||||||||||||
|
U.S.
|
540
|
|
453
|
|
—
|
|
19.2
|
|
*
|
|||||||
|
International
|
711
|
|
574
|
|
—
|
|
23.9
|
|
*
|
|||||||
|
Worldwide
|
1,251
|
|
1,027
|
|
—
|
|
21.8
|
|
*
|
|||||||
|
TOTAL MEDICAL DEVICES
|
|
|
|
|||||||||||||
|
U.S.
|
12,837
|
|
12,824
|
|
12,266
|
|
0.1
|
|
4.5
|
|
||||||
|
International
|
14,157
|
|
13,768
|
|
12,853
|
|
2.8
|
|
7.1
|
|
||||||
|
Worldwide
|
26,994
|
|
26,592
|
|
25,119
|
|
1.5
|
|
5.9
|
|
||||||
|
WORLDWIDE
|
|
|
|
|||||||||||||
|
U.S.
|
41,884
|
|
39,863
|
|
37,811
|
|
5.1
|
|
5.4
|
|
||||||
|
International
|
39,697
|
|
36,587
|
|
34,079
|
|
8.5
|
|
7.4
|
|
||||||
|
Worldwide
|
$
|
81,581
|
|
76,450
|
|
71,890
|
|
6.7
|
%
|
6.3
|
|
|||||
|
|
Income Before Tax
|
Identifiable Assets
|
|||||||||||||||
|
(Dollars in Millions)
|
2018
(3)
|
2017
(4)
|
2016
(5)
|
2018
|
2017
|
||||||||||||
|
Consumer
|
$
|
2,320
|
|
2,524
|
|
2,441
|
|
$
|
25,877
|
|
25,030
|
|
|||||
|
Pharmaceutical
|
12,568
|
|
11,083
|
|
12,827
|
|
56,636
|
|
59,450
|
|
|||||||
|
Medical Devices
|
4,397
|
|
5,392
|
|
5,578
|
|
46,254
|
|
45,413
|
|
|||||||
|
Total
|
19,285
|
|
18,999
|
|
20,846
|
|
128,767
|
|
129,893
|
|
|||||||
|
Less: Expense not allocated to segments
(1)
|
1,286
|
|
1,326
|
|
1,043
|
|
|||||||||||
|
General corporate
(2)
|
24,187
|
|
27,410
|
|
|||||||||||||
|
Worldwide total
|
$
|
17,999
|
|
17,673
|
|
19,803
|
|
$
|
152,954
|
|
157,303
|
|
|||||
|
Additions to Property,
Plant & Equipment
|
Depreciation and
Amortization
|
|||||||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
||||||||||||||
|
Consumer
|
$
|
438
|
|
485
|
|
486
|
|
$
|
688
|
|
674
|
|
608
|
|
||||||
|
Pharmaceutical
|
1,012
|
|
936
|
|
927
|
|
3,802
|
|
2,416
|
|
886
|
|
||||||||
|
Medical Devices
|
1,843
|
|
1,566
|
|
1,472
|
|
2,103
|
|
2,216
|
|
1,928
|
|
||||||||
|
Segments total
|
3,293
|
|
2,987
|
|
2,885
|
|
6,593
|
|
5,306
|
|
3,422
|
|
||||||||
|
General corporate
|
377
|
|
292
|
|
341
|
|
336
|
|
336
|
|
332
|
|
||||||||
|
Worldwide total
|
$
|
3,670
|
|
3,279
|
|
3,226
|
|
$
|
6,929
|
|
5,642
|
|
3,754
|
|
||||||
|
|
Sales to Customers
|
Long-Lived Assets
(6)
|
|||||||||||||||
|
(Dollars in Millions)
|
2018
|
2017
|
2016
|
2018
|
2017
|
||||||||||||
|
United States
|
$
|
41,884
|
|
39,863
|
|
37,811
|
|
$
|
37,117
|
|
38,556
|
|
|||||
|
Europe
|
18,753
|
|
17,126
|
|
15,770
|
|
51,433
|
|
56,677
|
|
|||||||
|
Western Hemisphere excluding U.S.
|
6,113
|
|
6,041
|
|
5,734
|
|
2,752
|
|
2,990
|
|
|||||||
|
Asia-Pacific, Africa
|
14,831
|
|
13,420
|
|
12,575
|
|
2,733
|
|
2,773
|
|
|||||||
|
Segments total
|
81,581
|
|
76,450
|
|
71,890
|
|
94,035
|
|
100,996
|
|
|||||||
|
General corporate
|
1,064
|
|
1,143
|
|
|||||||||||||
|
Other non long-lived assets
|
57,855
|
|
55,164
|
|
|||||||||||||
|
Worldwide total
|
$
|
81,581
|
|
76,450
|
|
71,890
|
|
$
|
152,954
|
|
157,303
|
|
|||||
|
78
|
||
|
(1)
|
Amounts not allocated to segments include interest (income) expense and general corporate (income) expense.
|
|
(2)
|
General corporate includes cash, cash equivalents and marketable securities.
|
|
(3)
|
The Consumer segment includes a gain of
$0.3 billion
from the divestiture of NIZORAL
®
and litigation expense of
$0.3 billion
. The Pharmaceutical segment includes an in-process research and development charge of
$1.1 billion
related to the Alios and XO1 assets and the corresponding XO1 contingent liability reversal of
$0.2 billion
, Actelion acquisition related costs of
$0.2 billion
, unrealized loss on securities of
$0.2 billion
and a gain of
$0.2 billion
from the divestiture of certain non-strategic Pharmaceutical products. The Medical Devices segment includes net litigation expense of
$1.7 billion
, a restructuring related charge of
$0.6 billion
, AMO acquisition related costs of
$0.1 billion
and a gain of
$0.5 billion
from the divestiture of the LifeScan business in the fiscal fourth quarter.
|
|
(4)
|
The Pharmaceutical segment includes
$0.8 billion
for Actelion acquisition related costs, an in-process research and development expense of
$0.4 billion
and litigation expense of
$0.1 billion
. The Medical Devices segment includes litigation expense of
$1.1 billion
, a restructuring related charge of
$0.8 billion
, an asset impairment of
$0.2 billion
primarily related to the insulin pump business and
$0.1 billion
for AMO acquisition related costs. The Medical Devices segment includes a gain of
$0.7 billion
from the divestiture of Codman Neurosurgery. The Consumer segment includes a gain of
$0.5 billion
from the divestiture of COMPEED
®
.
|
|
(5)
|
Includes net litigation expense of
$0.8 billion
and a restructuring related charge of
$0.7 billion
in the Medical Devices segment. The Pharmaceutical segment includes a positive adjustment of
$0.5 billion
to previous reserve estimates and gains from the divestitures of the controlled substance raw material and active pharmaceutical ingredient (API) business and certain anesthetic products in Europe.
|
|
(6)
|
Long-lived assets include property, plant and equipment, net for
2018
, and
2017
of
$17,035
and
$17,005
, respectively, and intangible assets and goodwill, net for
2018
and
2017
of
$78,064
and
$85,134
, respectively.
|
|
79
|
||
|
19.
|
Selected Quarterly Financial Data (unaudited)
|
|
|
2018
|
2017
|
|||||||||||||||||||||||
|
(Dollars in Millions Except Per Share Data)
|
First Quarter
(1)
|
Second Quarter
(2)
|
Third Quarter
(3)
|
Fourth Quarter
(4)
|
First Quarter
(5)
|
Second Quarter
(6)
|
Third Quarter
(7)
|
Fourth Quarter
(8)
|
|||||||||||||||||
|
Segment sales to customers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Consumer
|
$
|
3,398
|
|
3,504
|
|
3,415
|
|
3,536
|
|
3,228
|
|
3,478
|
|
3,356
|
|
3,540
|
|
||||||||
|
Pharmaceutical
|
9,844
|
|
10,354
|
|
10,346
|
|
10,190
|
|
8,245
|
|
8,635
|
|
9,695
|
|
9,681
|
|
|||||||||
|
Medical Devices
|
6,767
|
|
6,972
|
|
6,587
|
|
6,668
|
|
6,293
|
|
6,726
|
|
6,599
|
|
6,974
|
|
|||||||||
|
Total sales
|
20,009
|
|
20,830
|
|
20,348
|
|
20,394
|
|
17,766
|
|
18,839
|
|
19,650
|
|
20,195
|
|
|||||||||
|
Gross profit
|
13,395
|
|
13,903
|
|
13,759
|
|
13,433
|
|
12,357
|
|
12,993
|
|
12,725
|
|
12,936
|
|
|||||||||
|
Earnings before provision for taxes on income
|
5,481
|
|
4,973
|
|
4,423
|
|
3,122
|
|
5,575
|
|
4,748
|
|
4,790
|
|
2,560
|
|
|||||||||
|
Net earnings (loss)
|
4,367
|
|
3,954
|
|
3,934
|
|
3,042
|
|
4,422
|
|
3,827
|
|
3,764
|
|
(10,713
|
)
|
|||||||||
|
Basic net earnings (loss) per share
|
$
|
1.63
|
|
1.47
|
|
1.47
|
|
1.14
|
|
1.63
|
|
1.42
|
|
1.40
|
|
(3.99
|
)
|
||||||||
|
Diluted net earnings (loss) per share
|
$
|
1.60
|
|
1.45
|
|
1.44
|
|
1.12
|
|
1.61
|
|
1.40
|
|
1.37
|
|
(3.99
|
)
|
||||||||
|
(1)
|
The first quarter of 2018 includes an Actelion acquisition related cost of
$92 million
after-tax (
$96 million
before-tax) and a restructuring related charge of
$81 million
after-tax (
$107 million
before-tax).
|
|
(2)
|
The second quarter of 2018 includes a litigation expense of
$609 million
after-tax (
$703 million
before-tax) and a restructuring related charge of
$152 million
after-tax (
$176 million
before-tax).
|
|
(3)
|
The third quarter of 2018 includes an in-process research and development expense of
$859 million
after-tax (
$1,126 million
before-tax) related to the Alios and XO1 assets and the corresponding XO1 contingent liability reversal of
$184 million
after and before tax, a restructuring related charge of
$162 million
after-tax (
$190 million
before-tax) and a
$265 million
benefit after-tax from the impact of tax legislation.
|
|
(4)
|
The fourth quarter of 2018 includes a litigation expense of
$1,113 million
after-tax (
$1,288 million
before-tax), a restructuring related charge of
$190 million
after-tax (
$227 million
before-tax) and a
$137 million
benefit after-tax from the impact of tax legislation.
|
|
(5)
|
The first quarter of 2017 includes a restructuring charge of
$121 million
after-tax (
$161 million
before-tax) and an AMO acquisition related cost of
$251 million
after-tax (
$38 million
before-tax).
|
|
(6)
|
The second quarter of 2017 includes a litigation expense of
$352 million
after-tax (
$493 million
before-tax), Actelion acquisition related costs of
$199 million
after-tax (
$213 million
before-tax) a restructuring charge of
$101 million
after-tax (
$128 million
before-tax) and an asset impairment charge of
$125 million
after-tax (
$182 million
before-tax).
|
|
(7)
|
The third quarter of 2017 includes a litigation expense of
$97 million
after-tax (
$118 million
before-tax), Actelion acquisition related costs of
$255 million
after-tax (
$367 million
before-tax) and a restructuring charge of
$136 million
after-tax (
$187 million
before-tax).
|
|
(8)
|
The fourth quarter of 2017 includes a litigation expense of
$506 million
after-tax (
$645 million
before-tax), Actelion acquisition related costs of
$313 million
after-tax (
$217 million
before-tax), a restructuring charge of
$237 million
after-tax (
$284 million
before-tax), an in-process research and development expense of
$266 million
after-tax (
$408 million
before-tax) and an after-tax benefit of
$116 million
related to the insulin pump business. Additionally, the fourth quarter of 2017 includes a provisional charge of
$13.6 billion
for recently enacted tax legislation.
|
|
80
|
||
|
20.
|
Business Combinations and Divestitures
|
|
81
|
||
|
(Dollars in Millions)
|
||
|
Cash & Cash equivalents
|
469
|
|
|
Inventory
(1)
|
759
|
|
|
Accounts Receivable
|
485
|
|
|
Other current assets
|
93
|
|
|
Property, plant and equipment
|
104
|
|
|
Goodwill
|
6,161
|
|
|
Intangible assets
|
25,010
|
|
|
Deferred Taxes
|
99
|
|
|
Other non-current assets
|
19
|
|
|
Total Assets Acquired
|
33,199
|
|
|
Current liabilities
|
956
|
|
|
Deferred Taxes
|
1,776
|
|
|
Other non-current liabilities
|
413
|
|
|
Total Liabilities Assumed
|
3,145
|
|
|
Net Assets Acquired
|
30,054
|
|
|
(Dollars in Millions)
|
||||
|
Intangible assets with definite lives:
|
||||
|
Patents and trademarks*
|
$
|
24,230
|
|
|
|
Total amortizable intangibles
|
24,230
|
|
||
|
In-process research and development
|
780
|
|
||
|
Total intangible assets
|
$
|
25,010
|
|
|
|
82
|
||
|
Unaudited Pro forma Consolidated Results
|
||||
|
(Dollars in Millions Except Per Share Data)
|
2017
|
2016
|
||
|
Net Sales
|
77,681
|
|
74,339
|
|
|
Net Earnings
|
1,509
|
|
13,916
|
|
|
Diluted Net Earnings per Common Share
|
0.55
|
|
4.99
|
|
|
83
|
||
|
21.
|
Legal Proceedings
|
|
84
|
||
|
85
|
||
|
86
|
||
|
87
|
||
|
88
|
||
|
89
|
||
|
90
|
||
|
91
|
||
|
92
|
||
|
93
|
||
|
94
|
||
|
95
|
||
|
96
|
||
|
97
|
||
|
(Dollars in Millions)
|
Severance
|
Asset Write-offs
|
Other**
|
Total
|
|||||
|
Reserve balance, January 3, 2016
|
$
|
484
|
|
—
|
|
17
|
|
501
|
|
|
2016 activity
|
(104
|
)
|
—
|
|
(16
|
)
|
(120
|
)
|
|
|
Reserve balance, January 1, 2017
|
380
|
|
—
|
|
1
|
|
381
|
|
|
|
2017 activity
|
(151
|
)
|
—
|
|
37
|
|
(114
|
)
|
|
|
Reserve balance, December 31, 2017
|
229
|
|
—
|
|
38
|
|
267
|
|
|
|
Current year activity:
|
|||||||||
|
Charges
|
—
|
|
132
|
|
568
|
|
700
|
|
|
|
Cash payments
|
(35
|
)
|
—
|
|
(558
|
)
|
(593
|
)
|
|
|
Settled non cash
|
—
|
|
(132
|
)
|
—
|
|
(132
|
)
|
|
|
Reserve balance, December 30, 2018*
|
$
|
194
|
|
—
|
|
48
|
|
242
|
|
|
98
|
||
|
99
|
||
|
100
|
||
|
/s/ Alex Gorsky
|
/s/ Joseph J. Wolk
|
|
|
Alex Gorsky
|
|
Joseph J. Wolk
|
|
Chairman, Board of Directors
|
|
Executive Vice President, Chief Financial Officer
|
|
Chief Executive Officer
|
|
|
|
101
|
||
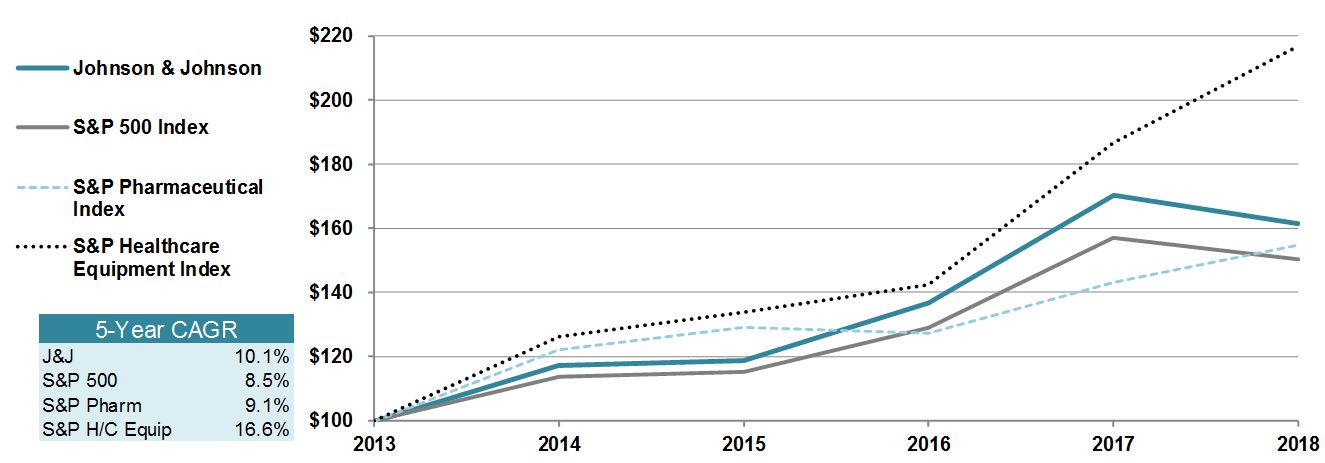
|
2013
|
2014
|
2015
|
2016
|
2017
|
2018
|
|
|
Johnson & Johnson
|
$100.00
|
$117.34
|
$118.69
|
$136.88
|
$170.29
|
$161.54
|
|
S&P 500 Index
|
$100.00
|
$113.68
|
$115.24
|
$129.02
|
$157.17
|
$150.27
|
|
S&P Pharmaceutical Index
|
$100.00
|
$122.22
|
$129.29
|
$127.27
|
$143.27
|
$154.86
|
|
S&P Healthcare Equipment Index
|
$100.00
|
$126.28
|
$133.82
|
$142.50
|
$186.53
|
$216.82
|
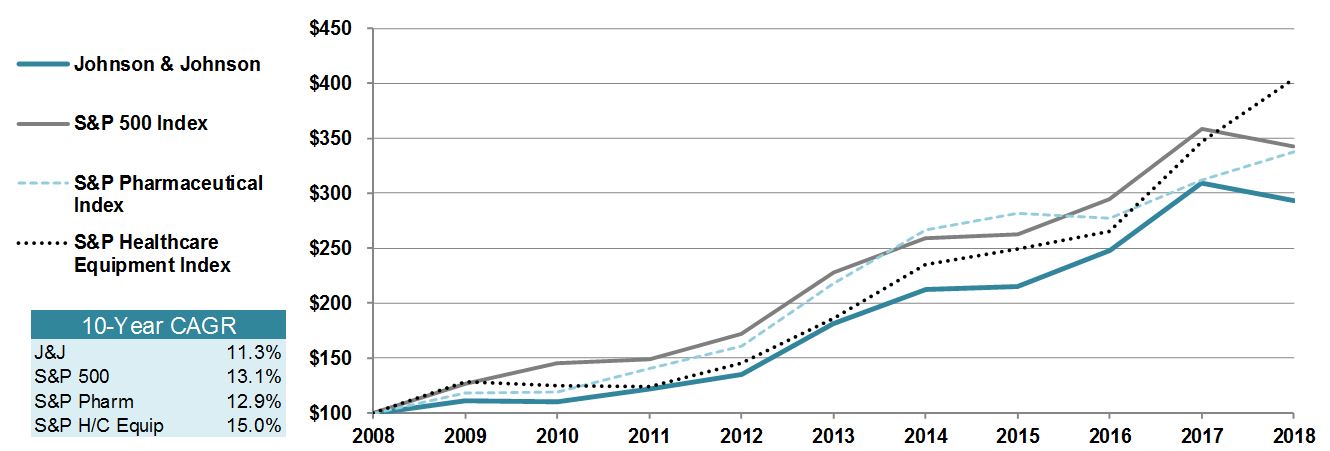
|
2008
|
2009
|
2010
|
2011
|
2012
|
2013
|
2014
|
2015
|
2016
|
2017
|
2018
|
|
|
Johnson & Johnson
|
$100.00
|
$111.28
|
$110.63
|
$121.57
|
$134.73
|
$181.37
|
$212.81
|
$215.28
|
$248.26
|
$308.85
|
$292.99
|
|
S&P 500 Index
|
$100.00
|
$126.45
|
$145.49
|
$148.55
|
$172.31
|
$228.09
|
$259.29
|
$262.86
|
$294.28
|
$358.50
|
$342.75
|
|
S&P Pharmaceutical Index
|
$100.00
|
$118.62
|
$119.54
|
$140.77
|
$161.07
|
$217.82
|
$266.21
|
$281.62
|
$277.21
|
$312.06
|
$337.32
|
|
S&P Healthcare Equipment Index
|
$100.00
|
$128.79
|
$125.30
|
$124.30
|
$145.76
|
$186.12
|
$235.04
|
$249.08
|
$265.23
|
$347.17
|
$403.55
|
|
102
|
||
|
Item 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
Item 9A.
|
CONTROLS AND PROCEDURES
|
|
Item 9B.
|
OTHER INFORMATION
|
|
Item 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
103
|
||
|
Item 11.
|
EXECUTIVE COMPENSATION
|
|
Item 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
Plan Category
|
Number of Securities to
be Issued Upon Exercise of
Outstanding Options and Rights
|
Weighted Average
Exercise Price of
Outstanding Options and Rights
|
Number of Securities
Remaining Available for
Future Issuance Under Equity Compensation Plans
(2)(3)
|
||||||
|
Equity Compensation Plans Approved by Security Holders
(1)
|
130,605,768
|
|
|
$82.52
|
|
351,079,202
|
|
||
|
Equity Compensation Plans Not Approved by Security Holders
|
-
|
|
-
|
|
-
|
|
|||
|
Total
|
130,605,768
|
|
|
$82.52
|
|
351,079,202
|
|
||
|
(1)
|
Included in this category are the following equity compensation plans which have been approved by the Company’s shareholders: 2005 Long-Term Incentive Plan and 2012 Long-Term Incentive Plan.
|
|
(2)
|
This column excludes shares reflected under the column “Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights.”
|
|
(3)
|
The 2005 Long-Term Incentive Plan expired April 26, 2012. All options and restricted shares granted subsequent to that date were under the 2012 Long-Term Incentive Plan.
|
|
Item 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
Item 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
104
|
||
|
Item 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
105
|
||
|
JOHNSON & JOHNSON
|
|
(Registrant)
|
|
By
|
/s/ A. Gorsky
|
|
A. Gorsky, Chairman, Board of Directors,
and Chief Executive Officer |
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ A. Gorsky
|
|
Chairman, Board of Directors
|
|
February 20, 2019
|
|
A. Gorsky
|
Chief Executive Officer
(Principal Executive Officer)
|
|||
|
|
|
|
|
|
|
/s/ J. J. Wolk
|
|
Chief Financial Officer
|
|
February 20, 2019
|
|
J. J. Wolk
|
(Principal Financial Officer)
|
|||
|
|
|
|
|
|
|
/s/ R. A. Kapusta
|
|
Controller and Chief Accounting Officer
|
|
February 20, 2019
|
|
R. A. Kapusta
|
(Principal Accounting Officer)
|
|||
|
|
|
|
|
|
|
/s/ M. C. Beckerle
|
Director
|
February 20, 2019
|
||
|
M. C. Beckerle
|
||||
|
/s/ D. S. Davis
|
|
Director
|
|
February 20, 2019
|
|
D. S. Davis
|
||||
|
|
|
|
|
|
|
/s/ I. E. L. Davis
|
|
Director
|
|
February 20, 2019
|
|
I. E. L. Davis
|
|
|
|
|
|
/s/ J. A. Doudna
|
|
Director
|
|
February 20, 2019
|
|
J. A. Doudna
|
|
|
|
|
|
106
|
||
|
Signature
|
|
Title
|
|
Date
|
|
/s/ M. B. McClellan
|
Director
|
February 20, 2019
|
||
|
M. B. McClellan
|
||||
|
|
|
|
|
|
|
/s/ A. M. Mulcahy
|
|
Director
|
|
February 20, 2019
|
|
A. M. Mulcahy
|
||||
|
|
|
|
|
|
|
/s/ W. D. Perez
|
|
Director
|
|
February 20, 2019
|
|
W. D. Perez
|
||||
|
|
|
|
|
|
|
/s/ C. Prince
|
|
Director
|
|
February 20, 2019
|
|
C. Prince
|
||||
|
|
|
|
|
|
|
/s/ A. E. Washington
|
|
Director
|
|
February 20, 2019
|
|
A. E. Washington
|
||||
|
/s/ R. A. Williams
|
|
Director
|
|
February 20, 2019
|
|
R. A. Williams
|
||||
|
107
|
||
|
Reg. S-K
|
|
|
|
Exhibit Table
|
Description
|
|
|
Item No.
|
of Exhibit
|
|
|
Restated Certificate of Incorporation effective February 19, 2016 — Incorporated herein by reference to Exhibit 3(i) of the Registrant’s Form 10-K Annual Report for the fiscal year ended January 3, 2016.
|
||
|
By-Laws of the Company, as amended effective January 26, 2016 — Incorporated herein by reference to Exhibit 3.1 the Registrant’s Form 8-K Current Report filed January 26, 2016.
|
||
|
4(a)
|
Upon the request of the Securities and Exchange Commission, the Registrant will furnish a copy of all instruments defining the rights of holders of long-term debt of the Registrant.
|
|
|
2005 Long-Term Incentive Plan — Incorporated herein by reference to Exhibit 4 of the Registrant’s S-8 Registration Statement filed with the Commission on May 10, 2005 (file no. 333-124785).*
|
||
|
Form of Stock Option Certificate under the 2005 Long-Term Incentive Plan — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 8-K Current Report filed January 13, 2012.*
|
||
|
2012 Long-Term Incentive Plan — Incorporated herein by reference to Appendix A of the Registrant’s Proxy Statement filed with the Commission on March 15, 2017 .*
|
||
|
Form of Stock Option Certificate, Restricted Share Unit Certificate and Performance Share Unit Certificate under the 2012 Long-Term Incentive Plan — Incorporated herein by reference to Exhibits 10.2, 10.3 and 10.4 of the Registrant’s Form 10-Q Quarterly Report filed May 7, 2012.*
|
||
|
Global NonQualified Stock Option Award Agreement, Global Restricted Share Unit Award Agreement and Global Performance Share Unit Award Agreement under the 2012 Long-Term Incentive Plan — Incorporated herein by reference to Exhibits 10.1, 10.2 and 10.3 of the Registrant’s Form 10-Q Quarterly Report filed May 1, 2018.*
|
||
|
Johnson & Johnson Executive Incentive Plan (as amended) — Incorporated herein by reference to Exhibit 10(f) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 31, 2000.*
|
||
|
Domestic Deferred Compensation (Certificate of Extra Compensation) Plan — Incorporated herein by reference to Exhibit 10(g) of the Registrant’s Form 10-K Annual Report for the year ended December 28, 2003.*
|
||
|
Amendments to the Certificate of Extra Compensation Plan effective as of January 1, 2009 — Incorporated herein by reference to Exhibit 10(j) of the Registrant’s Form 10-K Annual Report for the year ended December 28, 2008.*
|
||
|
2009 Certificates of Long-Term Performance Plan — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended September 27, 2009.*
|
||
|
Amended and Restated Deferred Fee Plan for Directors (Amended as of January 17, 2012) — Incorporated herein by reference to Exhibit 10(k) of the Registrant's Form 10-K Annual Report for the fiscal year ended January 1, 2012.*
|
||
|
The Johnson & Johnson Executive Income Deferral Plan (Amended and Restated Effective January 1, 2010) — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended September 30, 2012.*
|
||
|
Excess Savings Plan (Effective as of January 1, 1996) — Incorporated herein by reference to Exhibit 10(j) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 29, 1996.*
|
||
|
Amendments to the Johnson & Johnson Excess Savings Plan effective as of January 1, 2009 — Incorporated herein by reference to Exhibit 10(p) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 28, 2008.*
|
||
|
10(n)**
|
Excess Benefit Plan (Supplemental Retirement Plan) — Incorporated herein by reference to Exhibit 10(h) of the Registrant’s Form 10-K Annual Report for the fiscal year ended January 3, 1993.*
|
|
|
Amendments to the Excess Benefit Plan of Johnson & Johnson and Affiliated Companies effective as of January 1, 2009 — Incorporated herein by reference to Exhibit 10(r) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 28, 2008.*
|
||
|
Amendment to the Excess Benefit Plan of Johnson & Johnson and Affiliated Companies, effective as of January 1, 2015 — Incorporated herein by reference to Exhibit 10(q) of the Registrant’s Form 10-K Annual Report for the fiscal year ended December 28, 2014.*
|
||
|
10(q)**
|
Executive Life Plan Agreement — Incorporated herein by reference to Exhibit 10(i) of the Registrant’s Form 10-K Annual Report for the fiscal year ended January 3, 1993.*
|
|
|
Executive Life Plan Agreement Closure Letter — Incorporated herein by reference to Exhibit 10.1 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended March 29, 2015.*
|
||
|
Employment Agreement for Dr. Paulus Stoffels - Incorporated herein by reference to Exhibit 10.2 of the Registrant’s Form 10-Q Quarterly Report for the quarter ended September 30, 2012.*
|
||
|
108
|
||
|
Reg. S-K
|
|
|
|
Exhibit Table
|
Description
|
|
|
Item No.
|
of Exhibit
|
|
|
Summary of Employment Arrangements for Sandra E. Peterson — Incorporated herein by reference to Exhibit 10(t) of the Registrant's Form 10-K Annual Report for the year ended December 30, 2012.*
|
||
|
Severance Pay Plan of Johnson & Johnson and U.S. Affiliated Companies, Amended and Restated as of October 1, 2014 — Incorporated herein by reference to Exhibit 10.1 of the Registrant's Form 10-Q Quarterly Report for the quarter ended September 28, 2014.*
|
||
|
First Amendment to the Severance Pay Plan of Johnson & Johnson and U.S. Affiliated Companies (as amended and restated effective October 1, 2014) — Incorporated herein by reference to Exhibit 10.1 of the Registrant's Form 10-Q Quarterly Report for the quarter ended June 28, 2015.*
|
||
|
Second Amendment to the Severance Pay Plan of Johnson & Johnson and U.S. Affiliated Companies (as amended and restated effective October 1, 2014) — Incorporated herein by reference to Exhibit 10(x) of the Registrant's Form 10-K Annual Report for the fiscal year ended January 3, 2016.*
|
||
|
Subsidiaries - Filed with this document.
|
||
|
Consent of Independent Registered Public Accounting Firm — Filed with this document.
|
||
|
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act — Filed with this document.
|
||
|
Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act — Filed with this document.
|
||
|
Certification of Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act — Furnished with this document.
|
||
|
Certification of Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act — Furnished with this document.
|
||
|
101
|
XBRL (Extensible Business Reporting Language) The following materials from this Report for the fiscal year ended December 30, 2018, formatted in Extensive Business Reporting Language (XBRL): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Earnings, (iii) Consolidated Statements of Comprehensive Income, (iv) Consolidated Statements of Equity, (v) Consolidated Statements of Cash Flows, and (vi) Notes to the Consolidated Financial Statements.
|
|
|
*
|
Management contract or compensatory plan.
|
|
**
|
Paper filing.
|
|
109
|
||