|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
þ
|
|
Quarterly Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
for the quarterly period ended September 30, 2018
|
|
|
|
|
|
o
|
|
Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
for the transition period from to
|

|
NEW JERSEY
(State or other jurisdiction of
incorporation or organization)
|
|
22-1024240
(I.R.S. Employer
Identification No.)
|
|
Large accelerated filer
þ
|
|
Accelerated filer
o
|
|
|
|
Non-accelerated filer
o
|
|
Smaller reporting company
o
|
|
|
|
Emerging growth company
o
|
||||
|
|
Page
|
|
|
|
No.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EX-31.1
|
||
|
EX-32.1
|
||
|
EX-101 INSTANCE DOCUMENT
|
||
|
EX-101 SCHEMA DOCUMENT
|
||
|
EX-101 CALCULATION LINKBASE DOCUMENT
|
||
|
EX-101 LABELS LINKBASE DOCUMENT
|
||
|
EX-101 PRESENTATION LINKBASE DOCUMENT
|
||
|
EX-101 DEFINITION LINKBASE DOCUMENT
|
||
|
•
|
Challenges and uncertainties inherent in innovation and development of new and improved products and technologies on which the Company’s continued growth and success depend, including uncertainty of clinical outcomes, additional analysis of existing clinical data, obtaining regulatory approvals, health plan coverage and customer access, and initial and continued commercial success;
|
|
•
|
Challenges to the Company’s ability to obtain and protect adequate patent and other intellectual property rights for new and existing products and technologies in the United States and other important markets;
|
|
•
|
The impact of patent expirations, typically followed by the introduction of competing biosimilars and generics and resulting revenue and market share losses;
|
|
•
|
Increasingly aggressive and frequent challenges to the Company’s patents by competitors and others seeking to launch competing generic, biosimilar or other products and increased receptivity of courts, the United States Patent and Trademark Office and other decision makers to such challenges, potentially resulting in loss of market exclusivity and rapid decline in sales for the relevant product sooner than expected;
|
|
•
|
Competition in research and development of new and improved products, processes and technologies, which can result in product and process obsolescence;
|
|
•
|
Competition to reach agreement with third parties for collaboration, licensing, development and marketing agreements for products and technologies;
|
|
•
|
Competition based on cost-effectiveness, product performance, technological advances and patents attained by competitors; and
|
|
•
|
Allegations that the Company’s products infringe the patents and other intellectual property rights of third parties, which could adversely affect the Company’s ability to sell the products in question and require the payment of money damages and future royalties.
|
|
•
|
Product efficacy or safety concerns, whether or not based on scientific evidence, potentially resulting in product withdrawals, recalls, regulatory action on the part of the United States Food and Drug Administration (or international counterparts), declining sales and reputational damage;
|
|
•
|
Impact, including declining sales and reputational damage, of significant litigation or government action adverse to the Company, including product liability claims and allegations related to pharmaceutical marketing practices and contracting strategies;
|
|
•
|
Increased scrutiny of the health care industry by government agencies and state attorneys general resulting in investigations and prosecutions, which carry the risk of significant civil and criminal penalties, including, but not limited to, debarment from government business;
|
|
•
|
Failure to meet compliance obligations in the McNEIL-PPC, Inc. Consent Decree or the Corporate Integrity Agreements of the Johnson & Johnson Pharmaceutical Affiliates, or any other compliance agreements with governments or government agencies, which could result in significant sanctions;
|
|
•
|
Potential changes to applicable laws and regulations affecting United States and international operations, including relating to: approval of new products; licensing and patent rights; sales and promotion of health care products; access to, and reimbursement and pricing for, health care products and services; environmental protection and sourcing of raw materials;
|
|
•
|
Changes in domestic and international tax laws and regulations, including changes related to The Tax Cuts and Jobs Act in the United States, increasing audit scrutiny by tax authorities around the world and exposures to additional tax liabilities potentially in excess of existing reserves; and
|
|
•
|
Issuance of new or revised accounting standards by the Financial Accounting Standards Board and regulations by the Securities and Exchange Commission.
|
|
•
|
Pricing pressures resulting from trends toward health care cost containment, including the continued consolidation among health care providers and other market participants, trends toward managed care, the shift toward governments increasingly becoming the primary payers of health care expenses and significant new entrants to the health care markets seeking to reduce costs;
|
|
•
|
Restricted spending patterns of individual, institutional and governmental purchasers of health care products and services due to economic hardship and budgetary constraints;
|
|
•
|
Challenges to the Company’s ability to realize its strategy for growth including through externally sourced innovations, such as development collaborations, strategic acquisitions, licensing and marketing agreements, and the potential heightened costs of any such external arrangements due to competitive pressures;
|
|
•
|
The potential that the expected strategic benefits and opportunities from any planned or completed acquisition or divestiture by the Company may not be realized or may take longer to realize than expected; and
|
|
•
|
The potential that the expected benefits and opportunities related to past and future restructuring actions may not be realized or may take longer to realize than expected.
|
|
•
|
Impact of inflation and fluctuations in interest rates and currency exchange rates and the potential effect of such fluctuations on revenues, expenses and resulting margins;
|
|
•
|
Potential changes in export/import and trade laws, regulations and policies of the United States and other countries, including any increased trade restrictions or tariffs and potential drug reimportation legislation;
|
|
•
|
The impact on international operations from financial instability in international economies, sovereign risk, possible imposition of governmental controls and restrictive economic policies, and unstable international governments and legal systems;
|
|
•
|
Changes to global climate, extreme weather and natural disasters that could affect demand for the Company's products and services, cause disruptions in manufacturing and distribution networks, alter the availability of goods and services within the supply chain, and affect the overall design and integrity of the Company's products and operations; and
|
|
•
|
The impact of armed conflicts and terrorist attacks in the United States and other parts of the world including social and economic disruptions and instability of financial and other markets.
|
|
•
|
Difficulties and delays in manufacturing, internally through third party providers or otherwise within the supply chain, that may lead to voluntary or involuntary business interruptions or shutdowns, product shortages, withdrawals or suspensions of products from the market, and potential regulatory action;
|
|
•
|
Interruptions and breaches of the Company's information technology systems or those of the Company's vendors which, could result in reputational, competitive, operational or other business harm as well as financial costs and regulatory action;
|
|
•
|
Reliance on global supply chains and production and distribution processes that are complex and subject to increasing regulatory requirements that may adversely affect supply, sourcing and pricing of materials used in the Company’s products; and
|
|
•
|
The potential that the expected benefits and opportunities related to restructuring actions contemplated for the global supply chain may not be realized or may take longer to realize than expected, including due to any required approvals from
|
|
September 30, 2018
|
December 31, 2017
|
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
16,056
|
|
17,824
|
|
||
|
Marketable securities
|
3,308
|
|
472
|
|
|||
|
Accounts receivable, trade, less allowances for doubtful accounts $281 (2017, $291)
|
14,048
|
|
13,490
|
|
|||
|
Inventories (Note 2)
|
8,678
|
|
8,765
|
|
|||
|
Prepaid expenses and other
|
2,896
|
|
2,537
|
|
|||
|
Assets held for sale (Note 10)
|
2,208
|
|
—
|
|
|||
|
Total current assets
|
47,194
|
|
43,088
|
|
|||
|
Property, plant and equipment at cost
|
41,520
|
|
41,466
|
|
|||
|
Less: accumulated depreciation
|
(24,891
|
)
|
(24,461
|
)
|
|||
|
Property, plant and equipment, net
|
16,629
|
|
17,005
|
|
|||
|
Intangible assets, net (Note 3)
|
48,637
|
|
53,228
|
|
|||
|
Goodwill (Note 3)
|
30,702
|
|
31,906
|
|
|||
|
Deferred taxes on income
|
8,076
|
|
7,105
|
|
|||
|
Other assets
|
4,465
|
|
4,971
|
|
|||
|
Total assets
|
$
|
155,703
|
|
157,303
|
|
||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|
|
|||||
|
Loans and notes payable
|
$
|
1,773
|
|
3,906
|
|
||
|
Accounts payable
|
7,000
|
|
7,310
|
|
|||
|
Accrued liabilities
|
6,044
|
|
7,304
|
|
|||
|
Accrued rebates, returns and promotions
|
8,684
|
|
7,210
|
|
|||
|
Accrued compensation and employee related obligations
|
2,840
|
|
2,953
|
|
|||
|
Accrued taxes on income
|
1,096
|
|
1,854
|
|
|||
|
Total current liabilities
|
27,437
|
|
30,537
|
|
|||
|
Long-term debt (Note 4)
|
29,480
|
|
30,675
|
|
|||
|
Deferred taxes on income
|
7,711
|
|
8,368
|
|
|||
|
Employee related obligations
|
9,374
|
|
10,074
|
|
|||
|
Long-term taxes payable
|
8,537
|
|
8,472
|
|
|||
|
Other liabilities
|
8,538
|
|
9,017
|
|
|||
|
Total liabilities
|
91,077
|
|
97,143
|
|
|||
|
Shareholders’ equity:
|
|
|
|||||
|
Common stock — par value $1.00 per share (authorized 4,320,000,000 shares; issued 3,119,843,000 shares)
|
$
|
3,120
|
|
3,120
|
|
||
|
Accumulated other comprehensive income (loss) (Note 7)
|
(14,647
|
)
|
(13,199
|
)
|
|||
|
Retained earnings
|
107,617
|
|
101,793
|
|
|||
|
Less: common stock held in treasury, at cost (436,688,000 and 437,318,000 shares)
|
31,464
|
|
31,554
|
|
|||
|
Total shareholders’ equity
|
64,626
|
|
60,160
|
|
|||
|
Total liabilities and shareholders' equity
|
$
|
155,703
|
|
157,303
|
|
||
|
|
Fiscal Third Quarters Ended
|
|||||||||||||
|
September 30,
2018 |
Percent
to Sales
|
October 1,
2017 |
Percent
to Sales
|
|||||||||||
|
Sales to customers (Note 9)
|
$
|
20,348
|
|
100.0
|
%
|
$
|
19,650
|
|
100.0
|
%
|
||||
|
Cost of products sold
|
6,589
|
|
32.4
|
|
6,925
|
|
35.2
|
|
||||||
|
Gross profit
|
13,759
|
|
67.6
|
|
12,725
|
|
64.8
|
|
||||||
|
Selling, marketing and administrative expenses
|
5,543
|
|
27.3
|
|
5,423
|
|
27.6
|
|
||||||
|
Research and development expense
|
2,508
|
|
12.3
|
|
2,585
|
|
13.2
|
|
||||||
|
In-process research and development
|
1,126
|
|
5.6
|
|
—
|
|
—
|
|
||||||
|
Interest income
|
(175
|
)
|
(0.9
|
)
|
(74
|
)
|
(0.4
|
)
|
||||||
|
Interest expense, net of portion capitalized
|
243
|
|
1.2
|
|
229
|
|
1.2
|
|
||||||
|
Other (income) expense, net
|
3
|
|
0.0
|
|
(297
|
)
|
(1.5
|
)
|
||||||
|
Restructuring (Note 12)
|
88
|
|
0.4
|
|
69
|
|
0.3
|
|
||||||
|
Earnings before provision for taxes on income
|
4,423
|
|
21.7
|
|
4,790
|
|
24.4
|
|
||||||
|
Provision for taxes on income (Note 5)
|
489
|
|
2.4
|
|
1,026
|
|
5.2
|
|
||||||
|
NET EARNINGS
|
$
|
3,934
|
|
19.3
|
%
|
$
|
3,764
|
|
19.2
|
%
|
||||
|
NET EARNINGS PER SHARE (Note 8)
|
|
|
|
|
||||||||||
|
Basic
|
$
|
1.47
|
|
|
$
|
1.40
|
|
|
||||||
|
Diluted
|
$
|
1.44
|
|
|
$
|
1.37
|
|
|
||||||
|
CASH DIVIDENDS PER SHARE
|
$
|
0.90
|
|
|
$
|
0.84
|
|
|
||||||
|
AVG. SHARES OUTSTANDING
|
|
|
|
|
||||||||||
|
Basic
|
2,683.2
|
|
|
2,684.6
|
|
|
||||||||
|
Diluted
|
2,727.6
|
|
|
2,737.7
|
|
|
||||||||
|
Fiscal Nine Months Ended
|
||||||||||||||
|
September 30,
2018 |
Percent
to Sales
|
October 1,
2017 |
Percent
to Sales
|
|||||||||||
|
Sales to customers (Note 9)
|
$
|
61,187
|
|
100.0
|
%
|
$
|
56,255
|
|
100.0
|
%
|
||||
|
Cost of products sold
|
20,130
|
|
32.9
|
|
18,180
|
|
32.3
|
|
||||||
|
Gross profit
|
41,057
|
|
67.1
|
|
38,075
|
|
67.7
|
|
||||||
|
Selling, marketing and administrative expenses
|
16,549
|
|
27.1
|
|
15,475
|
|
27.5
|
|
||||||
|
Research and development expense
|
7,551
|
|
12.3
|
|
6,951
|
|
12.4
|
|
||||||
|
In-process research and development
|
1,126
|
|
1.8
|
|
—
|
|
0.0
|
|
||||||
|
Interest income
|
(415
|
)
|
(0.6
|
)
|
(300
|
)
|
(0.5
|
)
|
||||||
|
Interest expense, net of portion capitalized
|
755
|
|
1.2
|
|
660
|
|
1.1
|
|
||||||
|
Other (income) expense, net
|
427
|
|
0.7
|
|
11
|
|
0.0
|
|
||||||
|
Restructuring expense (Note 12)
|
187
|
|
0.3
|
|
165
|
|
0.3
|
|
||||||
|
Earnings before provision for taxes on income
|
14,877
|
|
24.3
|
|
15,113
|
|
26.9
|
|
||||||
|
Provision for taxes on income (Note 5)
|
2,622
|
|
4.3
|
|
3,100
|
|
5.5
|
|
||||||
|
NET EARNINGS
|
$
|
12,255
|
|
20.0
|
%
|
$
|
12,013
|
|
21.4
|
%
|
||||
|
NET EARNINGS PER SHARE (Note 8)
|
|
|
|
|
||||||||||
|
Basic
|
$
|
4.57
|
|
|
$
|
4.46
|
|
|
||||||
|
Diluted
|
$
|
4.49
|
|
|
$
|
4.37
|
|
|
||||||
|
CASH DIVIDENDS PER SHARE
|
$
|
2.64
|
|
|
$
|
2.48
|
|
|
||||||
|
AVG. SHARES OUTSTANDING
|
|
|
|
|
||||||||||
|
Basic
|
2,682.6
|
|
|
2,694.4
|
|
|
||||||||
|
Diluted
|
2,729.6
|
|
|
2,746.4
|
|
|
||||||||
|
Prior year amounts have been reclassified to conform to current year presentation
|
||||||||||||||
|
See Notes to Consolidated Financial Statements
|
||||||||||||||
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
||||||||||||
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
||||||||||
|
Net earnings
|
$
|
3,934
|
|
3,764
|
|
$
|
12,255
|
|
12,013
|
|
|||
|
Other comprehensive income (loss), net of tax
|
|||||||||||||
|
Foreign currency translation
|
(151
|
)
|
359
|
|
(1,718
|
)
|
1,597
|
|
|||||
|
Securities:
(1)
|
|||||||||||||
|
Unrealized holding gain (loss) arising during period
|
—
|
|
14
|
|
—
|
|
150
|
|
|||||
|
Reclassifications to earnings
|
(1
|
)
|
(99
|
)
|
(1
|
)
|
(292
|
)
|
|||||
|
Net change
|
(1
|
)
|
(85
|
)
|
(1
|
)
|
(142
|
)
|
|||||
|
Employee benefit plans:
|
|||||||||||||
|
Prior service cost amortization during period
|
(6
|
)
|
(4
|
)
|
(17
|
)
|
(13
|
)
|
|||||
|
Gain (loss) amortization during period
|
192
|
|
124
|
|
574
|
|
370
|
|
|||||
|
Net change
|
186
|
|
120
|
|
557
|
|
357
|
|
|||||
|
Derivatives & hedges:
|
|||||||||||||
|
Unrealized gain (loss) arising during period
|
262
|
|
62
|
|
37
|
|
(8
|
)
|
|||||
|
Reclassifications to earnings
|
(166
|
)
|
31
|
|
(91
|
)
|
350
|
|
|||||
|
Net change
|
96
|
|
93
|
|
(54
|
)
|
342
|
|
|||||
|
Other comprehensive income (loss)
|
130
|
|
487
|
|
(1,216
|
)
|
2,154
|
|
|||||
|
Comprehensive income
|
$
|
4,064
|
|
4,251
|
|
$
|
11,039
|
|
14,167
|
|
|||
|
(1)
2018 includes the impact from the adoption of ASU 2016-01. For further details see Note 1 to the Consolidated Financial Statements
|
|||||||||||||
|
The tax effects in other comprehensive income for the fiscal third quarters were as follows for 2018 and 2017, respectively: Foreign Currency Translation: $104 million in 2018 due to the enactment of the U.S. Tax Cuts and Jobs Act; Securities: $0 million and $45 million; Employee Benefit Plans: $52 million and $61 million; Derivatives & Hedges: $26 million and $50 million.
|
|
The tax effects in other comprehensive income for the fiscal nine months were as follows for 2018 and 2017, respectively: Foreign Currency Translation: $79 million in 2018 due to the enactment of the U.S. Tax Cuts and Jobs Act; Securities: $0 million and $76 million; Employee Benefit Plans: $155 million and $181 million; Derivatives & Hedges:$14 million and $184 million.
|
|
JOHNSON & JOHNSON AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited; Dollars in Millions)
|
|||||||
|
|
Fiscal Nine Months Ended
|
||||||
|
September 30,
2018 |
October 1,
2017 |
||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
|
|
|||||
|
Net earnings
|
$
|
12,255
|
|
12,013
|
|
||
|
Adjustments to reconcile net earnings to cash flows from operating activities:
|
|
|
|||||
|
Depreciation and amortization of property and intangibles
|
5,194
|
|
3,773
|
|
|||
|
Stock based compensation
|
822
|
|
758
|
|
|||
|
Asset write-downs
|
1,226
|
|
309
|
|
|||
|
Net gain on sale of assets/businesses
|
(443
|
)
|
(527
|
)
|
|||
|
Deferred tax provision
|
53
|
|
(407
|
)
|
|||
|
Accounts receivable allowances
|
(3
|
)
|
59
|
|
|||
|
Changes in assets and liabilities, net of effects from acquisitions and divestitures:
|
|
|
|||||
|
Increase in accounts receivable
|
(1,040
|
)
|
(300
|
)
|
|||
|
Increase in inventories
|
(777
|
)
|
(193
|
)
|
|||
|
Increase in accounts payable and accrued liabilities
|
731
|
|
339
|
|
|||
|
Increase in other current and non-current assets
|
(904
|
)
|
(555
|
)
|
|||
|
Decrease in other current and non-current liabilities
|
(1,157
|
)
|
(318
|
)
|
|||
|
NET CASH FLOWS FROM OPERATING ACTIVITIES
|
15,957
|
|
14,951
|
|
|||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
|
|
|||||
|
Additions to property, plant and equipment
|
(2,352
|
)
|
(2,039
|
)
|
|||
|
Proceeds from the disposal of assets/businesses, net
|
895
|
|
726
|
|
|||
|
Acquisitions, net of cash acquired
|
(897
|
)
|
(34,646
|
)
|
|||
|
Purchases of investments
|
(4,155
|
)
|
(5,798
|
)
|
|||
|
Sales of investments
|
1,162
|
|
27,511
|
|
|||
|
Other
|
(48
|
)
|
(117
|
)
|
|||
|
NET CASH USED BY INVESTING ACTIVITIES
|
(5,395
|
)
|
(14,363
|
)
|
|||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
|
|
|||||
|
Dividends to shareholders
|
(7,083
|
)
|
(6,687
|
)
|
|||
|
Repurchase of common stock
|
(2,060
|
)
|
(5,543
|
)
|
|||
|
Proceeds from short-term debt
|
40
|
|
4,760
|
|
|||
|
Retirement of short-term debt
|
(2,365
|
)
|
(936
|
)
|
|||
|
Proceeds from long-term debt, net of issuance costs
|
6
|
|
4,465
|
|
|||
|
Retirement of long-term debt
|
(910
|
)
|
(1,024
|
)
|
|||
|
Proceeds from the exercise of stock options/employee withholding tax on stock awards, net
|
480
|
|
854
|
|
|||
|
Other
|
(229
|
)
|
(25
|
)
|
|||
|
NET CASH USED BY FINANCING ACTIVITIES
|
(12,121
|
)
|
(4,136
|
)
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
(209
|
)
|
297
|
|
|||
|
Decrease in cash and cash equivalents
|
(1,768
|
)
|
(3,251
|
)
|
|||
|
Cash and Cash equivalents, beginning of period
|
17,824
|
|
18,972
|
|
|||
|
CASH AND CASH EQUIVALENTS, END OF PERIOD
|
$
|
16,056
|
|
15,721
|
|
||
|
Acquisitions
|
|
|
|||||
|
Fair value of assets acquired
|
$
|
1,046
|
|
36,494
|
|
||
|
Fair value of liabilities assumed and noncontrolling interests
|
(149
|
)
|
(1,848
|
)
|
|||
|
Net cash paid for acquisitions
|
$
|
897
|
|
34,646
|
|
||
|
Statement of Earnings - For the fiscal nine months ended September 30, 2018
|
|||||||||
|
(Dollars in millions)
|
As Reported
|
Effect of change
|
Balance without adoption of ASC 606
|
||||||
|
Sales to customers
|
$
|
61,187
|
|
(18
|
)
|
61,169
|
|
||
|
Net earnings
|
12,255
|
|
(14
|
)
|
12,241
|
|
|||
|
Statement of Earnings - For the fiscal third quarter ended September 30, 2018
|
|||||||||
|
(Dollars in millions)
|
As Reported
|
Effect of change
|
Balance without adoption of ASC 606
|
||||||
|
Sales to customers
|
$
|
20,348
|
|
22
|
|
20,370
|
|
||
|
Net earnings
|
3,934
|
|
19
|
|
3,953
|
|
|||
|
|
|||||||||
|
As Reported
|
Effect of change
|
Balance without adoption of ASC 606
|
|||||||
|
Assets
|
155,703
|
|
24
|
|
155,727
|
|
|||
|
Liabilities
|
91,077
|
|
(7
|
)
|
91,070
|
|
|||
|
Equity
|
$
|
64,626
|
|
31
|
|
64,657
|
|
||
|
(Dollars In millions)
|
Increase (Decrease) to Net Expense
|
||||||
|
Fiscal Third Quarter Ended
|
Fiscal Nine Months Ended
|
||||||
|
Cost of products sold
|
$
|
23
|
|
69
|
|
||
|
Selling, marketing and administrative expenses
|
27
|
|
80
|
|
|||
|
Research and development expense
|
11
|
|
32
|
|
|||
|
Other (income) expense, net
|
(61
|
)
|
(181
|
)
|
|||
|
Earnings before provision for taxes on income
|
$
|
—
|
|
—
|
|
||
|
(Dollars in Millions)
|
Cumulative Effect Adjustment Increase (Decrease) to Retained Earnings
|
|||
|
ASU 2014-09 - Revenue from Contracts with Customers
|
$
|
(47
|
)
|
|
|
ASU 2016-01 - Financial Instruments
|
232
|
|
||
|
ASU 2016-16 - Income Taxes: Intra-Entity Transfers
|
1,311
|
|
||
|
Total
|
$
|
1,496
|
|
|
|
(Dollars in Millions)
|
September 30, 2018
|
December 31, 2017
|
|||||
|
Raw materials and supplies
|
$
|
1,202
|
|
1,140
|
|
||
|
Goods in process
|
1,986
|
|
2,317
|
|
|||
|
Finished goods
|
5,490
|
|
5,308
|
|
|||
|
Total inventories
(1)
|
$
|
8,678
|
|
8,765
|
|
||
|
(Dollars in Millions)
|
September 30, 2018
|
December 31, 2017
|
|||||
|
Intangible assets with definite lives:
|
|
|
|||||
|
Patents and trademarks — gross
|
$
|
35,478
|
|
36,427
|
|
||
|
Less accumulated amortization
|
9,077
|
|
7,223
|
|
|||
|
Patents and trademarks — net
(1)
|
26,401
|
|
29,204
|
|
|||
|
Customer relationships and other intangibles — gross
|
21,176
|
|
20,204
|
|
|||
|
Less accumulated amortization
|
8,168
|
|
7,463
|
|
|||
|
Customer relationships and other intangibles — net
|
13,008
|
|
12,741
|
|
|||
|
Intangible assets with indefinite lives:
|
|
|
|||||
|
Trademarks
|
7,002
|
|
7,082
|
|
|||
|
Purchased in-process research and development
(2)
|
2,226
|
|
4,201
|
|
|||
|
Total intangible assets with indefinite lives
|
9,228
|
|
11,283
|
|
|||
|
Total intangible assets — net
|
$
|
48,637
|
|
53,228
|
|
||
|
(Dollars in Millions)
|
Consumer
|
Pharm
|
Med Devices
|
Total
|
|||||||||
|
Goodwill, net at December 31, 2017
|
$
|
8,875
|
|
9,109
|
|
13,922
|
|
31,906
|
|
||||
|
Goodwill, related to acquisitions
|
169
|
|
51
|
|
208
|
|
428
|
|
|||||
|
Goodwill, related to divestitures
|
—
|
|
—
|
|
(1,246
|
)
|
(1)
|
(1,246
|
)
|
||||
|
Currency translation/Other
|
(304
|
)
|
(48
|
)
|
(34
|
)
|
(386
|
)
|
|||||
|
Goodwill, net at September 30, 2018
|
$
|
8,740
|
|
9,112
|
|
12,850
|
|
30,702
|
|
||||
|
September 30, 2018
|
October 1, 2017
|
||||||||||||||||||||
|
(Dollars in Millions)
|
Sales
|
Cost of Products Sold
|
R&D Expense
|
Interest (Income) Expense
|
Other (Income) Expense
|
Sales
|
Cost of Products Sold
|
R&D Expense
|
Interest (Income) Expense
|
Other (Income) Expense
|
|||||||||||
|
The effects of fair value, net investment and cash flow hedging:
|
|||||||||||||||||||||
|
Gain (Loss) on fair value hedging relationship:
|
|||||||||||||||||||||
|
Interest rate swaps contracts:
|
|||||||||||||||||||||
|
Hedged items
|
$
|
—
|
|
—
|
|
—
|
|
(7
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(4
|
)
|
—
|
|
|
Derivatives designated as hedging instruments
|
—
|
|
—
|
|
—
|
|
7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4
|
|
—
|
|
|
|
Gain (Loss) on net investment hedging relationship:
|
|||||||||||||||||||||
|
Cross currency interest rate swaps contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) recognized in income on derivative amount excluded from effectiveness testing
|
—
|
|
—
|
|
—
|
|
25
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Amount of gain or (loss) recognized in AOCI
|
—
|
|
—
|
|
—
|
|
25
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Gain (Loss) on cash flow hedging relationship:
|
|||||||||||||||||||||
|
Forward foreign exchange contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) reclassified from AOCI into income
(1)
|
4
|
|
97
|
|
10
|
|
—
|
|
(3
|
)
|
5
|
|
(63
|
)
|
(30
|
)
|
—
|
|
(49
|
)
|
|
|
Amount of gain or (loss) recognized in AOCI
(1)
|
15
|
|
192
|
|
(4
|
)
|
—
|
|
(1
|
)
|
18
|
|
(16
|
)
|
(39
|
)
|
—
|
|
(15
|
)
|
|
|
Cross currency interest rate swaps contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) reclassified from AOCI into income
|
—
|
|
—
|
|
—
|
|
34
|
|
—
|
|
—
|
|
|
—
|
|
106
|
|
—
|
|
||
|
Amount of gain or (loss) recognized in AOCI
|
$
|
—
|
|
—
|
|
—
|
|
35
|
|
—
|
|
—
|
|
—
|
|
—
|
|
114
|
|
—
|
|
|
September 30, 2018
|
October 1, 2017
|
||||||||||||||||||||
|
(Dollars in Millions)
|
Sales
|
Cost of Products Sold
|
R&D Expense
|
Interest (Income) Expense
|
Other (Income) Expense
|
Sales
|
Cost of Products Sold
|
R&D Expense
|
Interest (Income) Expense
|
Other (Income) Expense
|
|||||||||||
|
The effects of fair value, net investment and cash flow hedging:
|
|||||||||||||||||||||
|
Gain (Loss) on fair value hedging relationship:
|
|||||||||||||||||||||
|
Interest rate swaps contracts:
|
|||||||||||||||||||||
|
Hedged items
|
$
|
—
|
|
—
|
|
—
|
|
3
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6
|
)
|
—
|
|
|
Derivatives designated as hedging instruments
|
—
|
|
—
|
|
—
|
|
(3
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
6
|
|
—
|
|
|
|
Gain (Loss) on net investment hedging relationship:
|
|||||||||||||||||||||
|
Cross currency interest rate swaps contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) recognized in income on derivative amount excluded from effectiveness testing
|
—
|
|
—
|
|
—
|
|
27
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Amount of gain or (loss) recognized in AOCI
|
—
|
|
—
|
|
—
|
|
27
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Gain (Loss) on cash flow hedging relationship:
|
|||||||||||||||||||||
|
Forward foreign exchange contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) reclassified from AOCI into income
(1)
|
50
|
|
175
|
|
(242
|
)
|
—
|
|
(24
|
)
|
(34
|
)
|
(162
|
)
|
(131
|
)
|
—
|
|
(86
|
)
|
|
|
Amount of gain or (loss) recognized in AOCI
(1)
|
(3
|
)
|
138
|
|
(220
|
)
|
—
|
|
(16
|
)
|
40
|
|
105
|
|
(167
|
)
|
—
|
|
(59
|
)
|
|
|
Cross currency interest rate swaps contracts:
|
|||||||||||||||||||||
|
Amount of gain or (loss) reclassified from AOCI into income
|
—
|
|
—
|
|
—
|
|
106
|
|
—
|
|
—
|
|
—
|
|
—
|
|
63
|
|
—
|
|
|
|
Amount of gain or (loss) recognized in AOCI
|
$
|
—
|
|
—
|
|
—
|
|
111
|
|
—
|
|
—
|
|
—
|
|
—
|
|
73
|
|
—
|
|
|
Line item in the Consolidated Balance Sheet in which the hedged item is included
|
Carrying Amount of the Hedged Liability
|
Cumulative Amount of Fair Value Hedging Adjustment Included in the Carrying Amount of the Hedged Liability
|
|||||||||||
|
(Dollars in Millions)
|
September 30, 2018
|
December 31, 2017
|
September 30, 2018
|
December 31, 2017
|
|||||||||
|
Current Portion of Long-term Debt
|
$
|
601
|
|
597
|
|
1
|
|
2
|
|
||||
|
Long-term Debt
|
495
|
|
496
|
|
(4
|
)
|
3
|
|
|||||
|
The following table is the effect of derivatives not designated as hedging instrument for the fiscal third quarters and fiscal nine months in 2018 and 2017:
|
||||||||||||||
|
Gain/(Loss)
Recognized In
Income on Derivative
|
Gain/(Loss)
Recognized In
Income on Derivative
|
|||||||||||||
|
(Dollars in Millions)
|
Location of Gain /(Loss) Recognized in Income on Derivative
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
|||||||||||
|
Derivatives Not Designated as Hedging Instruments
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
||||||||||
|
Foreign Exchange Contracts
|
Other (income) expense
|
$
|
49
|
|
(12
|
)
|
(23
|
)
|
22
|
|
||||
|
The following table is the effect of net investment hedges for the fiscal third quarters in 2018 and 2017:
|
|||||||||||||||
|
Gain/(Loss)
Recognized In
Accumulated
OCI
|
Location of Gain or (Loss) Reclassified from Accumulated Other Comprehensive Income Into Income
|
Gain/(Loss) Reclassified From
Accumulated OCI
Into Income
|
|||||||||||||
|
(Dollars in Millions)
|
Fiscal Third Quarters Ended
|
||||||||||||||
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
||||||||||||
|
Debt
|
$
|
(50
|
)
|
(151
|
)
|
Other (income) expense
|
—
|
|
—
|
|
|||||
|
Cross Currency interest rate swaps
|
$
|
(75
|
)
|
—
|
|
Other (income) expense
|
—
|
|
—
|
|
|||||
|
The following table is the effect of net investment hedges for the fiscal nine months in 2018 and 2017:
|
|||||||||||||||
|
Gain/(Loss)
Recognized In
Accumulated OCI
|
Location of Gain or (Loss) Reclassified from Accumulated Other Comprehensive Income Into Income
|
Gain/(Loss) Reclassified From
Accumulated OCI
Into Income
|
|||||||||||||
|
(Dollars in Millions)
|
Fiscal Nine Months Ended
|
||||||||||||||
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
||||||||||||
|
Debt
|
$
|
106
|
|
(529
|
)
|
Other (income) expense
|
—
|
|
—
|
|
|||||
|
Cross Currency interest rate swaps
|
$
|
(37
|
)
|
—
|
|
Other (income) expense
|
—
|
|
—
|
|
|||||
|
(Dollars in Millions)
|
December 31, 2017
|
September 30, 2018
|
||||||||||||||
|
Carrying Value
|
Changes in Fair Value Reflected in Net Income
(1)
|
Sales/ Purchases/Other
(2)
|
Carrying Value
|
Non Current Other Assets
|
||||||||||||
|
Equity Investments with readily determinable value
|
$
|
751
|
|
(35
|
)
|
(19
|
)
|
697
|
|
697
|
|
|||||
|
Equity Investments without readily determinable value
|
$
|
510
|
|
7
|
|
122
|
|
639
|
|
639
|
|
|||||
|
|
September 30, 2018
|
|
December 31, 2017
|
|||||||||||||
|
(Dollars in Millions)
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Total
(1)
|
|||||||||||
|
Derivatives designated as hedging instruments:
|
|
|
|
|
|
|||||||||||
|
Assets:
|
|
|
|
|
|
|||||||||||
|
Forward foreign exchange contracts
|
$
|
—
|
|
551
|
|
—
|
|
551
|
|
418
|
|
|||||
|
Interest rate contracts
(2)(4)
|
—
|
|
19
|
|
—
|
|
19
|
|
7
|
|
||||||
|
Total
|
—
|
|
570
|
|
—
|
|
570
|
|
425
|
|
||||||
|
Liabilities:
|
|
|
|
|
|
|||||||||||
|
Forward foreign exchange contracts
|
—
|
|
401
|
|
—
|
|
401
|
|
402
|
|
||||||
|
Interest rate contracts
(3)(4)
|
—
|
|
269
|
|
—
|
|
269
|
|
165
|
|
||||||
|
Total
|
—
|
|
670
|
|
—
|
|
670
|
|
567
|
|
||||||
|
Derivatives not designated as hedging instruments:
|
|
|
|
|
|
|||||||||||
|
Assets:
|
|
|
|
|
|
|||||||||||
|
Forward foreign exchange contracts
|
—
|
|
28
|
|
—
|
|
28
|
|
38
|
|
||||||
|
Liabilities:
|
|
|
|
|
|
|||||||||||
|
Forward foreign exchange contracts
|
—
|
|
45
|
|
—
|
|
45
|
|
38
|
|
||||||
|
Other Investments:
|
||||||||||||||||
|
Equity investments
(5)
|
697
|
|
—
|
|
—
|
|
697
|
|
751
|
|
||||||
|
Debt securities
(6)
|
$
|
—
|
|
11,155
|
|
—
|
|
11,155
|
|
5,310
|
|
|||||
|
Gross to Net Derivative Reconciliation
|
September 30, 2018
|
December 31, 2017
|
|||||
|
(Dollars in Millions)
|
|||||||
|
Total Gross Assets
|
$
|
598
|
|
463
|
|
||
|
Credit Support Agreement (CSA)
|
(290
|
)
|
(76
|
)
|
|||
|
Total Net Asset
|
308
|
|
387
|
|
|||
|
Total Gross Liabilities
|
715
|
|
605
|
|
|||
|
Credit Support Agreement (CSA)
|
(443
|
)
|
(238
|
)
|
|||
|
Total Net Liabilities
|
$
|
272
|
|
367
|
|
||
|
(1)
|
2017 assets and liabilities are all classified as Level 2 with the exception of equity investments of
$751 million
, which are classified as Level 1.
|
|
(2)
|
Includes
$4 million
and
$7 million
of non-current other assets for
September 30, 2018
and
December 31, 2017
, respectively.
|
|
(3)
|
Includes
$6 million
and
$9 million
of non-current other liabilities for
September 30, 2018
and
December 31, 2017
, respectively.
|
|
(4)
|
Includes cross currency interest rate swaps and interest rate swaps.
|
|
(5)
|
Classified as non-current other assets. The carrying amount of the equity investments were
$697 million
and
$751 million
as of
September 30, 2018
and
December 31, 2017
, respectively.
|
|
(6)
|
Classified as cash equivalents and current marketable securities.
|
|
September 30, 2018
|
||||||||||||||||||
|
(Dollars in Millions)
|
Carrying Amount
|
Unrecognized Gain
|
Unrecognized Loss
|
Estimated Fair Value
|
Cash & Cash Equivalents
|
Current Marketable Securities
|
||||||||||||
|
Cash
|
$
|
2,575
|
|
—
|
|
—
|
|
2,575
|
|
2,575
|
|
|||||||
|
Other sovereign securities
(1)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
||||||
|
U.S. reverse repurchase agreements
|
2,260
|
|
—
|
|
—
|
|
2,260
|
|
2,260
|
|
|
|||||||
|
Other reverse repurchase agreements
|
479
|
|
—
|
|
—
|
|
479
|
|
479
|
|
||||||||
|
Corporate debt securities
(1)
|
200
|
|
—
|
|
—
|
|
200
|
|
200
|
|
—
|
|
||||||
|
Money market funds
|
1,763
|
|
—
|
|
—
|
|
1,763
|
|
1,763
|
|
||||||||
|
Time deposits
(1)
|
932
|
|
—
|
|
—
|
|
932
|
|
932
|
|
||||||||
|
Subtotal
|
8,209
|
|
—
|
|
—
|
|
8,209
|
|
8,209
|
|
—
|
|
||||||
|
Unrealized Gain
|
Unrealized Loss
|
|||||||||||||||||
|
Government securities
|
10,885
|
|
—
|
|
(1
|
)
|
10,884
|
|
7,835
|
|
3,049
|
|
||||||
|
Other sovereign securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||
|
Corporate debt securities
|
271
|
|
—
|
|
—
|
|
271
|
|
12
|
|
259
|
|
||||||
|
Subtotal available for sale debt
(2)
|
$
|
11,156
|
|
—
|
|
(1
|
)
|
11,155
|
|
7,847
|
|
3,308
|
|
|||||
|
Total cash, cash equivalents and current marketable securities
|
|
|
|
|
|
|
|
|
16,056
|
|
3,308
|
|
||||||
|
(Dollars in Millions)
|
Cost Basis
|
Fair Value
|
|||||
|
Due within one year
|
$
|
11,079
|
|
11,078
|
|
||
|
Due after one year through five years
|
77
|
|
77
|
|
|||
|
Due after five years through ten years
|
—
|
|
—
|
|
|||
|
Total debt securities
|
$
|
11,156
|
|
11,155
|
|
||
|
(Dollars in Millions)
|
Carrying Amount
|
Estimated Fair Value
|
|||||
|
Financial Liabilities
|
|
|
|||||
|
Current Debt
|
$
|
1,773
|
|
1,773
|
|
||
|
Non-Current Debt
|
|
|
|||||
|
4.75% Notes due 2019 (1B Euro 1.1681)
|
1,166
|
|
1,231
|
|
|||
|
1.875% Notes due 2019
|
495
|
|
490
|
|
|||
|
3% Zero Coupon Convertible Subordinated Debentures due in 2020
|
52
|
|
100
|
|
|||
|
1.950% Notes due 2020
|
499
|
|
490
|
|
|||
|
2.95% Debentures due 2020
|
548
|
|
551
|
|
|||
|
3.55% Notes due 2021
|
448
|
|
454
|
|
|||
|
2.45% Notes due 2021
|
349
|
|
345
|
|
|||
|
1.65% Notes due 2021
|
998
|
|
967
|
|
|||
|
0.250% Notes due 2022 (1B Euro 1.1681)
|
1,165
|
|
1,172
|
|
|||
|
2.25% Notes due 2022
|
996
|
|
973
|
|
|||
|
6.73% Debentures due 2023
|
250
|
|
291
|
|
|||
|
3.375% Notes due 2023
|
805
|
|
829
|
|
|||
|
2.05% Notes due 2023
|
498
|
|
477
|
|
|||
|
0.650% Notes due 2024 (750MM Euro 1.1681)
|
872
|
|
881
|
|
|||
|
5.50% Notes due 2024 (500 MM GBP 1.3123)
|
651
|
|
793
|
|
|||
|
2.625% Notes due 2025
|
748
|
|
719
|
|
|||
|
2.45% Notes due 2026
|
1,991
|
|
1,884
|
|
|||
|
2.95% Notes due 2027
|
996
|
|
956
|
|
|||
|
2.90% Notes due 2028
|
1,493
|
|
1,420
|
|
|||
|
1.150% Notes due 2028 (750MM Euro 1.1681)
|
868
|
|
874
|
|
|||
|
6.95% Notes due 2029
|
296
|
|
385
|
|
|||
|
4.95% Debentures due 2033
|
498
|
|
559
|
|
|||
|
4.375% Notes due 2033
|
856
|
|
912
|
|
|||
|
1.650% Notes due 2035 (1.5B Euro 1.1681)
|
1,735
|
|
1,774
|
|
|||
|
3.55% Notes due 2036
|
988
|
|
945
|
|
|||
|
5.95% Notes due 2037
|
991
|
|
1,252
|
|
|||
|
3.625% Notes due 2037
|
1,486
|
|
1,433
|
|
|||
|
3.40% Notes due 2038
|
990
|
|
926
|
|
|||
|
5.85% Debentures due 2038
|
696
|
|
874
|
|
|||
|
4.50% Debentures due 2040
|
538
|
|
573
|
|
|||
|
4.85% Notes due 2041
|
297
|
|
330
|
|
|||
|
4.50% Notes due 2043
|
495
|
|
532
|
|
|||
|
3.70% Notes due 2046
|
1,971
|
|
1,884
|
|
|||
|
3.75% Notes due 2047
|
991
|
|
951
|
|
|||
|
3.50% Notes due 2048
|
742
|
|
689
|
|
|||
|
Other
|
22
|
|
22
|
|
|||
|
Total Non-Current Debt
|
$
|
29,480
|
|
29,938
|
|
||
|
•
|
$0.1 billion
increase to the transition tax on previously undistributed foreign earnings as of December 31, 2017 due to U.S. Treasury Department’s issuance of Notice 2018-13 on January 19, 2018, Notice 2018-26 on April 2, 2018, Notice 2018-78 on October 1, 2018 and updates to prior estimates
|
|
•
|
$0.3 billion
decrease to the deferred tax liability for foreign withholding and local taxes, partially offset by a decrease of
$0.2 billion
in deferred tax assets for U.S. foreign tax credits due to updated estimates from the amounts recorded in 2017.
|
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
||||||||||||||||||||||||
|
|
Retirement Plans
|
Other Benefit Plans
|
Retirement Plans
|
Other Benefit Plans
|
|||||||||||||||||||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
|||||||||||||||||
|
Service cost
|
$
|
307
|
|
266
|
|
67
|
|
62
|
|
925
|
|
772
|
|
202
|
|
185
|
|
||||||||
|
Interest cost
|
247
|
|
232
|
|
37
|
|
40
|
|
748
|
|
693
|
|
112
|
|
119
|
|
|||||||||
|
Expected return on plan assets
|
(550
|
)
|
(514
|
)
|
(1
|
)
|
(2
|
)
|
(1,664
|
)
|
(1,528
|
)
|
(5
|
)
|
(5
|
)
|
|||||||||
|
Amortization of prior service cost/(credit)
|
1
|
|
1
|
|
(7
|
)
|
(8
|
)
|
2
|
|
2
|
|
(23
|
)
|
(23
|
)
|
|||||||||
|
Recognized actuarial losses
|
213
|
|
154
|
|
31
|
|
34
|
|
641
|
|
456
|
|
92
|
|
103
|
|
|||||||||
|
Curtailments and settlements
|
—
|
|
2
|
|
—
|
|
—
|
|
(2
|
)
|
1
|
|
—
|
|
—
|
|
|||||||||
|
Net periodic benefit cost
|
$
|
218
|
|
141
|
|
127
|
|
126
|
|
650
|
|
396
|
|
378
|
|
379
|
|
||||||||
|
|
Foreign
|
Gain/(Loss)
|
Employee
|
Gain/(Loss)
|
Total Accumulated
|
|||||||||||
|
Currency
|
On
|
Benefit
|
On Derivatives
|
Other Comprehensive
|
||||||||||||
|
(Dollars in Millions)
|
Translation
|
Securities
|
Plans
|
& Hedges
|
Income (Loss)
|
|||||||||||
|
December 31, 2017
|
$
|
(7,351
|
)
|
232
|
|
(6,150
|
)
|
70
|
|
(13,199
|
)
|
|||||
|
Net change
|
(1,718
|
)
|
(1
|
)
|
557
|
|
(54
|
)
|
(1,216
|
)
|
||||||
|
Cumulative adjustment to retained earnings
|
(232
|
)
|
(1)
|
(232
|
)
|
|||||||||||
|
September 30, 2018
|
$
|
(9,069
|
)
|
(1
|
)
|
(5,593
|
)
|
16
|
|
(14,647
|
)
|
|||||
|
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
|||||||||||
|
(Shares in Millions)
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
|||||||||
|
Basic net earnings per share
|
$
|
1.47
|
|
1.40
|
|
4.57
|
|
4.46
|
|
||||
|
Average shares outstanding — basic
|
2,683.2
|
|
2,684.6
|
|
2,682.6
|
|
2,694.4
|
|
|||||
|
Potential shares exercisable under stock option plans
|
140.3
|
|
139.3
|
|
140.9
|
|
141.2
|
|
|||||
|
Less: shares which could be repurchased under treasury stock method
|
(96.7
|
)
|
(87.2
|
)
|
(94.7
|
)
|
(90.2
|
)
|
|||||
|
Convertible debt shares
|
0.8
|
|
1.0
|
|
0.8
|
|
1.0
|
|
|||||
|
Average shares outstanding — diluted
|
2,727.6
|
|
2,737.7
|
|
2,729.6
|
|
2,746.4
|
|
|||||
|
Diluted net earnings per share
|
$
|
1.44
|
|
1.37
|
|
4.49
|
|
4.37
|
|
||||
|
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
||||||||||||||||||
|
(Dollars in Millions)
|
September 30,
2018 |
October 1,
2017 |
Percent
Change
|
September 30,
2018 |
October 1,
2017 |
Percent Change
|
||||||||||||||
|
CONSUMER
|
|
|
|
|||||||||||||||||
|
Baby Care
|
||||||||||||||||||||
|
U.S.
|
$
|
120
|
|
100
|
|
20.0
|
%
|
$
|
306
|
|
326
|
|
(6.1
|
)%
|
||||||
|
International
|
352
|
|
377
|
|
(6.6
|
)
|
1,079
|
|
1,100
|
|
(1.9
|
)
|
||||||||
|
Worldwide
|
472
|
|
477
|
|
(1.0
|
)
|
1,385
|
|
1,426
|
|
(2.9
|
)
|
||||||||
|
Beauty
|
||||||||||||||||||||
|
U.S.
|
543
|
|
523
|
|
3.8
|
|
1,791
|
|
1,739
|
|
3.0
|
|
||||||||
|
International
|
535
|
|
510
|
|
4.9
|
|
1,480
|
|
1,351
|
|
9.5
|
|
||||||||
|
Worldwide
|
1,078
|
|
1,033
|
|
4.4
|
|
3,271
|
|
3,090
|
|
5.9
|
|
||||||||
|
Oral Care
|
||||||||||||||||||||
|
U.S.
|
158
|
|
154
|
|
2.6
|
|
472
|
|
460
|
|
2.6
|
|
||||||||
|
International
|
226
|
|
228
|
|
(0.9
|
)
|
684
|
|
678
|
|
0.9
|
|
||||||||
|
Worldwide
|
384
|
|
382
|
|
0.5
|
|
1,156
|
|
1,138
|
|
1.6
|
|
||||||||
|
OTC
|
||||||||||||||||||||
|
U.S.
|
440
|
|
401
|
|
9.7
|
|
1,359
|
|
1,310
|
|
3.7
|
|
||||||||
|
International
|
608
|
|
601
|
|
1.2
|
|
1,827
|
|
1,711
|
|
6.8
|
|
||||||||
|
Worldwide
|
1,048
|
|
1,002
|
|
4.6
|
|
3,186
|
|
3,021
|
|
5.5
|
|
||||||||
|
Women's Health
|
||||||||||||||||||||
|
U.S.
|
3
|
|
3
|
|
0.0
|
|
10
|
|
9
|
|
11.1
|
|
||||||||
|
International
|
266
|
|
267
|
|
(0.4
|
)
|
782
|
|
779
|
|
0.4
|
|
||||||||
|
Worldwide
|
269
|
|
270
|
|
(0.4
|
)
|
792
|
|
788
|
|
0.5
|
|
||||||||
|
Wound Care/Other
|
||||||||||||||||||||
|
U.S.
|
106
|
|
104
|
|
1.9
|
|
344
|
|
342
|
|
0.6
|
|
||||||||
|
International
|
58
|
|
88
|
|
(34.1
|
)
|
183
|
|
257
|
|
(28.8
|
)
|
||||||||
|
Worldwide
|
164
|
|
192
|
|
(14.6
|
)
|
527
|
|
599
|
|
(12.0
|
)
|
||||||||
|
TOTAL CONSUMER
|
||||||||||||||||||||
|
U.S.
|
1,370
|
|
1,285
|
|
6.6
|
|
4,282
|
|
4,186
|
|
2.3
|
|
||||||||
|
International
|
2,045
|
|
2,071
|
|
(1.3
|
)
|
6,035
|
|
5,876
|
|
2.7
|
|
||||||||
|
Worldwide
|
3,415
|
|
3,356
|
|
1.8
|
|
10,317
|
|
10,062
|
|
2.5
|
|
||||||||
|
PHARMACEUTICAL
|
||||||||||||||||||||
|
Immunology
|
||||||||||||||||||||
|
U.S.
|
2,400
|
|
2,420
|
|
(0.8
|
)
|
6,717
|
|
6,644
|
|
1.1
|
|
||||||||
|
International
|
998
|
|
849
|
|
17.6
|
|
3,061
|
|
2,514
|
|
21.8
|
|
||||||||
|
Worldwide
|
3,398
|
|
3,269
|
|
3.9
|
|
9,778
|
|
9,158
|
|
6.8
|
|
||||||||
|
REMICADE
®
|
||||||||||||||||||||
|
U.S.
|
987
|
|
1,206
|
|
(18.2
|
)
|
2,821
|
|
3,452
|
|
(18.3
|
)
|
||||||||
|
U.S. Exports
|
100
|
|
156
|
|
(35.9
|
)
|
346
|
|
448
|
|
(22.8
|
)
|
||||||||
|
International
|
292
|
|
285
|
|
2.5
|
|
921
|
|
949
|
|
(3.0
|
)
|
||||||||
|
Worldwide
|
1,379
|
|
1,647
|
|
(16.3
|
)
|
4,088
|
|
4,849
|
|
(15.7
|
)
|
||||||||
|
SIMPONI / SIMPONI ARIA
®
|
||||||||||||||||||||
|
U.S.
|
281
|
|
242
|
|
16.1
|
|
779
|
|
701
|
|
11.1
|
|
||||||||
|
International
|
255
|
|
234
|
|
9.0
|
|
823
|
|
642
|
|
28.2
|
|
||||||||
|
Worldwide
|
536
|
|
476
|
|
12.6
|
|
1,602
|
|
1,343
|
|
19.3
|
|
||||||||
|
STELARA
®
|
||||||||||||||||||||
|
U.S.
|
889
|
|
800
|
|
11.1
|
|
2,460
|
|
2,027
|
|
21.4
|
|
||||||||
|
International
|
421
|
|
324
|
|
29.9
|
|
1,252
|
|
903
|
|
38.6
|
|
||||||||
|
Worldwide
|
1,310
|
|
1,124
|
|
16.5
|
|
3,712
|
|
2,930
|
|
26.7
|
|
||||||||
|
OTHER IMMUNOLOGY
|
||||||||||||||||||||
|
U.S.
|
143
|
|
16
|
|
*
|
311
|
|
16
|
|
*
|
||||||||||
|
International
|
30
|
|
6
|
|
*
|
65
|
|
20
|
|
*
|
||||||||||
|
Worldwide
|
173
|
|
22
|
|
*
|
376
|
|
36
|
|
*
|
||||||||||
|
Infectious Diseases
|
||||||||||||||||||||
|
U.S.
|
345
|
|
353
|
|
(2.3
|
)
|
1,006
|
|
1,020
|
|
(1.4
|
)
|
||||||||
|
International
|
478
|
|
460
|
|
3.9
|
|
1,496
|
|
1,334
|
|
12.1
|
|
||||||||
|
Worldwide
|
823
|
|
813
|
|
1.2
|
|
2,502
|
|
2,354
|
|
6.3
|
|
||||||||
|
EDURANT
®
/ rilpivirine
|
||||||||||||||||||||
|
U.S.
|
13
|
|
15
|
|
(13.3
|
)
|
42
|
|
44
|
|
(4.5
|
)
|
||||||||
|
International
|
189
|
|
179
|
|
5.6
|
|
581
|
|
478
|
|
21.5
|
|
||||||||
|
Worldwide
|
202
|
|
194
|
|
4.1
|
|
623
|
|
522
|
|
19.3
|
|
||||||||
|
PREZISTA
®
/ PREZCOBIX
®
/ REZOLSTA
®
/ SYMTUZA
®
|
||||||||||||||||||||
|
U.S.
|
297
|
|
287
|
|
3.5
|
|
847
|
|
824
|
|
2.8
|
|
||||||||
|
International
|
193
|
|
180
|
|
7.2
|
|
613
|
|
527
|
|
16.3
|
|
||||||||
|
Worldwide
|
490
|
|
467
|
|
4.9
|
|
1,460
|
|
1,351
|
|
8.1
|
|
||||||||
|
OTHER INFECTIOUS DISEASES
|
||||||||||||||||||||
|
U.S.
|
35
|
|
51
|
|
(31.4
|
)
|
117
|
|
152
|
|
(23.0
|
)
|
||||||||
|
International
|
96
|
|
101
|
|
(5.0
|
)
|
302
|
|
329
|
|
(8.2
|
)
|
||||||||
|
Worldwide
|
131
|
|
152
|
|
(13.8
|
)
|
419
|
|
481
|
|
(12.9
|
)
|
||||||||
|
Neuroscience
|
||||||||||||||||||||
|
U.S.
|
651
|
|
647
|
|
0.6
|
|
1,914
|
|
1,931
|
|
(0.9
|
)
|
||||||||
|
International
|
839
|
|
851
|
|
(1.4
|
)
|
2,663
|
|
2,531
|
|
5.2
|
|
||||||||
|
Worldwide
|
1,490
|
|
1,498
|
|
(0.5
|
)
|
4,577
|
|
4,462
|
|
2.6
|
|
||||||||
|
CONCERTA
®
/ Methylphenidate
|
||||||||||||||||||||
|
U.S.
|
57
|
|
100
|
|
(43.0
|
)
|
191
|
|
284
|
|
(32.7
|
)
|
||||||||
|
International
|
100
|
|
98
|
|
2.0
|
|
322
|
|
304
|
|
5.9
|
|
||||||||
|
Worldwide
|
157
|
|
198
|
|
(20.7
|
)
|
513
|
|
588
|
|
(12.8
|
)
|
||||||||
|
INVEGA SUSTENNA
®
/ XEPLION
®
/ TRINZA
®
/ TREVICTA
®
|
||||||||||||||||||||
|
U.S.
|
468
|
|
395
|
|
18.5
|
|
1,306
|
|
1,154
|
|
13.2
|
|
||||||||
|
International
|
281
|
|
248
|
|
13.3
|
|
859
|
|
722
|
|
19.0
|
|
||||||||
|
Worldwide
|
749
|
|
643
|
|
16.5
|
|
2,165
|
|
1,876
|
|
15.4
|
|
||||||||
|
RISPERDAL CONSTA
®
|
||||||||||||||||||||
|
U.S.
|
76
|
|
87
|
|
(12.6
|
)
|
238
|
|
273
|
|
(12.8
|
)
|
||||||||
|
International
|
99
|
|
107
|
|
(7.5
|
)
|
321
|
|
335
|
|
(4.2
|
)
|
||||||||
|
Worldwide
|
175
|
|
194
|
|
(9.8
|
)
|
559
|
|
608
|
|
(8.1
|
)
|
||||||||
|
OTHER NEUROSCIENCE
|
||||||||||||||||||||
|
U.S.
|
50
|
|
65
|
|
(23.1
|
)
|
179
|
|
220
|
|
(18.6
|
)
|
||||||||
|
International
|
359
|
|
398
|
|
(9.8
|
)
|
1,161
|
|
1,170
|
|
(0.8
|
)
|
||||||||
|
Worldwide
|
409
|
|
463
|
|
(11.7
|
)
|
1,340
|
|
1,390
|
|
(3.6
|
)
|
||||||||
|
Oncology
|
||||||||||||||||||||
|
U.S.
|
1,250
|
|
846
|
|
47.8
|
|
3,268
|
|
2,207
|
|
48.1
|
|
||||||||
|
International
|
1,338
|
|
1,052
|
|
27.2
|
|
4,087
|
|
3,012
|
|
35.7
|
|
||||||||
|
Worldwide
|
2,588
|
|
1,898
|
|
36.4
|
|
7,355
|
|
5,219
|
|
40.9
|
|
||||||||
|
DARZALEX
®
|
||||||||||||||||||||
|
U.S.
|
318
|
|
230
|
|
38.3
|
|
880
|
|
643
|
|
36.9
|
|
||||||||
|
International
|
180
|
|
87
|
|
*
|
561
|
|
228
|
|
*
|
||||||||||
|
Worldwide
|
498
|
|
317
|
|
57.1
|
|
1,441
|
|
871
|
|
65.4
|
|
||||||||
|
IMBRUVICA
®
|
||||||||||||||||||||
|
U.S.
|
334
|
|
230
|
|
45.2
|
|
811
|
|
622
|
|
30.4
|
|
||||||||
|
International
|
371
|
|
282
|
|
31.6
|
|
1,101
|
|
749
|
|
47.0
|
|
||||||||
|
Worldwide
|
705
|
|
512
|
|
37.7
|
|
1,912
|
|
1,371
|
|
39.5
|
|
||||||||
|
VELCADE
®
|
||||||||||||||||||||
|
U.S.
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
International
|
271
|
|
273
|
|
(0.7
|
)
|
864
|
|
843
|
|
2.5
|
|
||||||||
|
Worldwide
|
271
|
|
273
|
|
(0.7
|
)
|
864
|
|
843
|
|
2.5
|
|
||||||||
|
ZYTIGA
®
|
||||||||||||||||||||
|
U.S.
|
527
|
|
352
|
|
49.7
|
|
1,420
|
|
826
|
|
71.9
|
|
||||||||
|
International
|
431
|
|
317
|
|
36.0
|
|
1,292
|
|
924
|
|
39.8
|
|
||||||||
|
Worldwide
|
958
|
|
669
|
|
43.2
|
|
2,712
|
|
1,750
|
|
55.0
|
|
||||||||
|
OTHER ONCOLOGY
|
||||||||||||||||||||
|
U.S.
|
71
|
|
34
|
|
*
|
157
|
|
116
|
|
35.3
|
|
|||||||||
|
International
|
85
|
|
93
|
|
(8.6
|
)
|
269
|
|
268
|
|
0.4
|
|
||||||||
|
Worldwide
|
156
|
|
127
|
|
22.8
|
|
426
|
|
384
|
|
10.9
|
|
||||||||
|
Pulmonary Hypertension
|
||||||||||||||||||||
|
U.S.
|
425
|
|
371
|
|
14.6
|
1,215
|
|
408
|
|
*
|
||||||||||
|
International
|
231
|
|
261
|
|
(11.5)
|
691
|
|
309
|
|
*
|
||||||||||
|
Worldwide
|
656
|
|
632
|
|
3.8
|
1,906
|
|
717
|
|
*
|
||||||||||
|
OPSUMIT
®
|
||||||||||||||||||||
|
U.S.
|
182
|
|
150
|
|
21.3
|
511
|
|
174
|
|
*
|
||||||||||
|
International
|
128
|
|
109
|
|
17.4
|
381
|
|
130
|
|
*
|
||||||||||
|
Worldwide
|
310
|
|
259
|
|
19.7
|
892
|
|
304
|
|
*
|
||||||||||
|
TRACLEER
®
|
||||||||||||||||||||
|
U.S.
|
69
|
|
83
|
|
(16.9)
|
208
|
|
85
|
|
*
|
||||||||||
|
International
|
70
|
|
127
|
|
(44.9)
|
214
|
|
151
|
|
*
|
||||||||||
|
Worldwide
|
139
|
|
210
|
|
(33.8)
|
422
|
|
236
|
|
*
|
||||||||||
|
UPTRAVI
®
|
||||||||||||||||||||
|
U.S.
|
154
|
|
113
|
|
36.3
|
433
|
|
121
|
|
*
|
||||||||||
|
International
|
17
|
|
11
|
|
54.5
|
49
|
|
12
|
|
*
|
||||||||||
|
Worldwide
|
171
|
|
124
|
|
37.9
|
482
|
|
133
|
|
*
|
||||||||||
|
OTHER
|
||||||||||||||||||||
|
U.S.
|
20
|
|
25
|
|
(20.0)
|
63
|
|
28
|
|
*
|
||||||||||
|
International
|
16
|
|
14
|
|
14.3
|
47
|
|
16
|
|
*
|
||||||||||
|
Worldwide
|
36
|
|
39
|
|
(7.7)
|
110
|
|
44
|
|
*
|
||||||||||
|
Cardiovascular / Metabolism / Other
|
||||||||||||||||||||
|
U.S.
|
1,026
|
|
1,179
|
|
(13.0
|
)
|
3,230
|
|
3,488
|
|
(7.4
|
)
|
||||||||
|
International
|
365
|
|
406
|
|
(10.1
|
)
|
1,196
|
|
1,177
|
|
1.6
|
|
||||||||
|
Worldwide
|
1,391
|
|
1,585
|
|
(12.2
|
)
|
4,426
|
|
4,665
|
|
(5.1
|
)
|
||||||||
|
XARELTO
®
|
||||||||||||||||||||
|
U.S.
|
612
|
|
635
|
|
(3.6
|
)
|
1,869
|
|
1,790
|
|
4.4
|
|
||||||||
|
International
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Worldwide
|
612
|
|
635
|
|
(3.6
|
)
|
1,869
|
|
1,790
|
|
4.4
|
|
||||||||
|
INVOKANA
®
/ INVOKAMET
®
|
||||||||||||||||||||
|
U.S.
|
150
|
|
220
|
|
(31.8
|
)
|
523
|
|
723
|
|
(27.7
|
)
|
||||||||
|
International
|
40
|
|
45
|
|
(11.1
|
)
|
130
|
|
121
|
|
7.4
|
|
||||||||
|
Worldwide
|
190
|
|
265
|
|
(28.3
|
)
|
653
|
|
844
|
|
(22.6
|
)
|
||||||||
|
PROCRIT
®
/ EPREX
®
|
||||||||||||||||||||
|
U.S.
|
178
|
|
168
|
|
6.0
|
|
523
|
|
511
|
|
2.3
|
|
||||||||
|
International
|
77
|
|
70
|
|
10.0
|
|
244
|
|
229
|
|
6.6
|
|
||||||||
|
Worldwide
|
255
|
|
238
|
|
7.1
|
|
767
|
|
740
|
|
3.6
|
|
||||||||
|
OTHER
|
||||||||||||||||||||
|
U.S.
|
86
|
|
156
|
|
(44.9
|
)
|
315
|
|
464
|
|
(32.1
|
)
|
||||||||
|
International
|
248
|
|
291
|
|
(14.8
|
)
|
822
|
|
827
|
|
(0.6
|
)
|
||||||||
|
Worldwide
|
334
|
|
447
|
|
(25.3
|
)
|
1,137
|
|
1,291
|
|
(11.9
|
)
|
||||||||
|
TOTAL PHARMACEUTICAL
|
|
|
||||||||||||||||||
|
U.S.
|
6,097
|
|
5,816
|
|
4.8
|
|
17,350
|
|
15,698
|
|
10.5
|
|
||||||||
|
International
|
4,249
|
|
3,879
|
|
9.5
|
|
13,194
|
|
10,877
|
|
21.3
|
|
||||||||
|
Worldwide
|
10,346
|
|
9,695
|
|
6.7
|
|
30,544
|
|
26,575
|
|
14.9
|
|
||||||||
|
MEDICAL DEVICES
|
||||||||||||||||||||
|
Diabetes Care
|
||||||||||||||||||||
|
U.S.
|
125
|
|
168
|
|
(25.6
|
)
|
371
|
|
482
|
|
(23.0
|
)
|
||||||||
|
International
|
190
|
|
237
|
|
(19.8
|
)
|
638
|
|
743
|
|
(14.1
|
)
|
||||||||
|
Worldwide
|
315
|
|
405
|
|
(22.2
|
)
|
1,009
|
|
1,225
|
|
(17.6
|
)
|
||||||||
|
Diagnostics
|
||||||||||||||||||||
|
U.S.
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
International
|
—
|
|
—
|
|
—
|
|
—
|
|
1
|
|
*
|
|||||||||
|
Worldwide
|
—
|
|
—
|
|
—
|
|
—
|
|
1
|
|
*
|
|||||||||
|
Interventional Solutions
|
||||||||||||||||||||
|
U.S.
|
320
|
|
279
|
|
14.7
|
|
947
|
|
843
|
|
12.3
|
|
||||||||
|
International
|
333
|
|
274
|
|
21.5
|
|
1,013
|
|
832
|
|
21.8
|
|
||||||||
|
Worldwide
|
653
|
|
553
|
|
18.1
|
|
1,960
|
|
1,675
|
|
17.0
|
|
||||||||
|
Orthopaedics
|
||||||||||||||||||||
|
U.S.
|
1,284
|
|
1,308
|
|
(1.8
|
)
|
3,923
|
|
4,034
|
|
(2.8
|
)
|
||||||||
|
International
|
827
|
|
896
|
|
(7.7
|
)
|
2,700
|
|
2,738
|
|
(1.4
|
)
|
||||||||
|
Worldwide
|
2,111
|
|
2,204
|
|
(4.2
|
)
|
6,623
|
|
6,772
|
|
(2.2
|
)
|
||||||||
|
HIPS
|
||||||||||||||||||||
|
U.S.
|
201
|
|
195
|
|
3.1
|
|
621
|
|
612
|
|
1.5
|
|
||||||||
|
International
|
129
|
|
133
|
|
(3.0
|
)
|
432
|
|
418
|
|
3.3
|
|
||||||||
|
Worldwide
|
330
|
|
328
|
|
0.6
|
|
1,053
|
|
1,030
|
|
2.2
|
|
||||||||
|
KNEES
|
||||||||||||||||||||
|
U.S.
|
215
|
|
220
|
|
(2.3
|
)
|
672
|
|
702
|
|
(4.3
|
)
|
||||||||
|
International
|
126
|
|
123
|
|
2.4
|
|
438
|
|
424
|
|
3.3
|
|
||||||||
|
Worldwide
|
341
|
|
343
|
|
(0.6
|
)
|
1,110
|
|
1,126
|
|
(1.4
|
)
|
||||||||
|
TRAUMA
|
||||||||||||||||||||
|
U.S.
|
395
|
|
398
|
|
(0.8
|
)
|
1,196
|
|
1,179
|
|
1.4
|
|
||||||||
|
International
|
259
|
|
264
|
|
(1.9
|
)
|
829
|
|
768
|
|
7.9
|
|
||||||||
|
Worldwide
|
654
|
|
662
|
|
(1.2
|
)
|
2,025
|
|
1,947
|
|
4.0
|
|
||||||||
|
SPINE & OTHER
|
||||||||||||||||||||
|
U.S.
|
473
|
|
495
|
|
(4.4
|
)
|
1,434
|
|
1,541
|
|
(6.9
|
)
|
||||||||
|
International
|
313
|
|
376
|
|
(16.8
|
)
|
1,001
|
|
1,128
|
|
(11.3
|
)
|
||||||||
|
Worldwide
|
786
|
|
871
|
|
(9.8
|
)
|
2,435
|
|
2,669
|
|
(8.8
|
)
|
||||||||
|
Surgery
|
||||||||||||||||||||
|
U.S.
|
1,016
|
|
1,002
|
|
1.4
|
|
3,031
|
|
3,009
|
|
0.7
|
|
||||||||
|
International
|
1,360
|
|
1,344
|
|
1.2
|
|
4,283
|
|
3,992
|
|
7.3
|
|
||||||||
|
Worldwide
|
2,376
|
|
2,346
|
|
1.3
|
|
7,314
|
|
7,001
|
|
4.5
|
|
||||||||
|
ADVANCED
|
||||||||||||||||||||
|
U.S.
|
421
|
|
398
|
|
5.8
|
|
1,216
|
|
1,190
|
|
2.2
|
|
||||||||
|
International
|
555
|
|
525
|
|
5.7
|
|
1,731
|
|
1,543
|
|
12.2
|
|
||||||||
|
Worldwide
|
976
|
|
923
|
|
5.7
|
|
2,947
|
|
2,733
|
|
7.8
|
|
||||||||
|
GENERAL
|
||||||||||||||||||||
|
U.S.
|
423
|
|
430
|
|
(1.6
|
)
|
1,282
|
|
1,276
|
|
0.5
|
|
||||||||
|
International
|
657
|
|
675
|
|
(2.7
|
)
|
2,094
|
|
2,017
|
|
3.8
|
|
||||||||
|
Worldwide
|
1,080
|
|
1,105
|
|
(2.3
|
)
|
3,376
|
|
3,293
|
|
2.5
|
|
||||||||
|
SPECIALTY
|
||||||||||||||||||||
|
U.S.
|
172
|
|
174
|
|
(1.1
|
)
|
533
|
|
543
|
|
(1.8
|
)
|
||||||||
|
International
|
148
|
|
144
|
|
2.8
|
|
458
|
|
432
|
|
6.0
|
|
||||||||
|
Worldwide
|
320
|
|
318
|
|
0.6
|
|
991
|
|
975
|
|
1.6
|
|
||||||||
|
Vision
|
||||||||||||||||||||
|
U.S.
|
452
|
|
432
|
|
4.6
|
|
1,351
|
|
1,142
|
|
18.3
|
|
||||||||
|
International
|
680
|
|
659
|
|
3.2
|
|
2,069
|
|
1,802
|
|
14.8
|
|
||||||||
|
Worldwide
|
1,132
|
|
1,091
|
|
3.8
|
|
3,420
|
|
2,944
|
|
16.2
|
|
||||||||
|
CONTACT LENSES / OTHER
|
||||||||||||||||||||
|
U.S.
|
319
|
|
302
|
|
5.6
|
|
948
|
|
832
|
|
13.9
|
|
||||||||
|
International
|
516
|
|
498
|
|
3.6
|
|
1,538
|
|
1,404
|
|
9.5
|
|
||||||||
|
Worldwide
|
835
|
|
800
|
|
4.4
|
|
2,486
|
|
2,236
|
|
11.2
|
|
||||||||
|
SURGICAL
|
||||||||||||||||||||
|
U.S.
|
133
|
|
130
|
|
2.3
|
|
403
|
|
310
|
|
30.0
|
|
||||||||
|
International
|
164
|
|
161
|
|
1.9
|
|
531
|
|
398
|
|
33.4
|
|
||||||||
|
Worldwide
|
297
|
|
291
|
|
2.1
|
|
934
|
|
708
|
|
31.9
|
|
||||||||
|
TOTAL MEDICAL DEVICES
|
|
|
|
|
||||||||||||||||
|
U.S.
|
3,197
|
|
3,189
|
|
0.3
|
|
9,623
|
|
9,510
|
|
1.2
|
|
||||||||
|
International
|
3,390
|
|
3,410
|
|
(0.6
|
)
|
10,703
|
|
10,108
|
|
5.9
|
|
||||||||
|
Worldwide
|
6,587
|
|
6,599
|
|
(0.2
|
)
|
20,326
|
|
19,618
|
|
3.6
|
|
||||||||
|
WORLDWIDE
|
|
|
|
|
|
|
||||||||||||||
|
U.S.
|
10,664
|
|
10,290
|
|
3.6
|
|
31,255
|
|
29,394
|
|
6.3
|
|
||||||||
|
International
|
9,684
|
|
9,360
|
|
3.5
|
|
29,932
|
|
26,861
|
|
11.4
|
|
||||||||
|
Worldwide
|
$
|
20,348
|
|
19,650
|
|
3.6
|
%
|
$
|
61,187
|
|
56,255
|
|
8.8
|
%
|
||||||
|
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
||||||||||||||||||
|
(Dollars in Millions)
|
September 30,
2018 |
October 1,
2017 |
Percent
Change
|
September 30,
2018 |
October 1,
2017 |
Percent Change
|
||||||||||||||
|
Consumer
(1)
|
$
|
510
|
|
878
|
|
(41.9
|
)%
|
$
|
1,887
|
|
2,132
|
|
(11.5
|
)%
|
||||||
|
Pharmaceutical
(2)
|
2,876
|
|
2,857
|
|
0.7
|
|
10,193
|
|
9,934
|
|
2.6
|
|
||||||||
|
Medical Devices
(3)
|
1,267
|
|
1,383
|
|
(8.4
|
)
|
3,642
|
|
3,938
|
|
(7.5
|
)
|
||||||||
|
Segment earnings before provision for taxes
|
4,653
|
|
5,118
|
|
(9.1
|
)
|
15,722
|
|
16,004
|
|
(1.8
|
)
|
||||||||
|
Less: Expense not allocated to segments
(4)
|
230
|
|
328
|
|
|
845
|
|
891
|
|
|
|
|||||||||
|
Worldwide income before tax
|
$
|
4,423
|
|
4,790
|
|
(7.7
|
)%
|
$
|
14,877
|
|
15,113
|
|
(1.6
|
)%
|
||||||
|
|
Fiscal Third Quarters Ended
|
Fiscal Nine Months Ended
|
||||||||||||||||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Percent
Change
|
September 30, 2018
|
October 1, 2017
|
Percent Change
|
||||||||||||||
|
United States
|
$
|
10,664
|
|
10,290
|
|
3.6
|
%
|
$
|
31,255
|
|
29,394
|
|
6.3
|
%
|
||||||
|
Europe
|
4,416
|
|
4,308
|
|
2.5
|
|
14,023
|
|
12,398
|
|
13.1
|
|
||||||||
|
Western Hemisphere, excluding U.S.
|
1,550
|
|
1,569
|
|
(1.2
|
)
|
4,657
|
|
4,522
|
|
3.0
|
|
||||||||
|
Asia-Pacific, Africa
|
3,718
|
|
3,483
|
|
6.7
|
|
11,252
|
|
9,941
|
|
13.2
|
|
||||||||
|
Total
|
$
|
20,348
|
|
19,650
|
|
3.6
|
%
|
$
|
61,187
|
|
56,255
|
|
8.8
|
%
|
||||||
|
(Dollars in Millions)
|
||
|
Cash & Cash equivalents
|
469
|
|
|
Inventory
(1)
|
759
|
|
|
Accounts Receivable
|
485
|
|
|
Other current assets
|
93
|
|
|
Property, plant and equipment
|
104
|
|
|
Goodwill
|
6,161
|
|
|
Intangible assets
|
25,010
|
|
|
Deferred Taxes
|
99
|
|
|
Other non-current assets
|
19
|
|
|
Total Assets Acquired
|
33,199
|
|
|
Current liabilities
|
956
|
|
|
Deferred Taxes
|
1,776
|
|
|
Other non-current liabilities
|
413
|
|
|
Total Liabilities Assumed
|
3,145
|
|
|
Net Assets Acquired
|
30,054
|
|
|
(Dollars in Millions)
|
||||
|
Intangible assets with definite lives:
|
||||
|
Patents and trademarks*
|
$
|
24,230
|
|
|
|
Total amortizable intangibles
|
24,230
|
|
||
|
In-process research and development
|
780
|
|
||
|
Total intangible assets
|
$
|
25,010
|
|
|
|
Unaudited Pro forma Consolidated Results
|
||||
|
(Dollars in Millions Except Per Share Data)
|
Fiscal Nine Months Ended
|
|||
|
October 1, 2017
|
||||
|
Net Sales
|
$
|
57,486
|
|
|
|
Net Earnings
|
11,909
|
|
||
|
Diluted Net Earnings per Common Share
|
$
|
4.34
|
|
|
|
(Dollars in Millions)
|
Severance
|
Asset Write-offs
|
Other**
|
Total
|
|||||
|
Reserve balance, December 31, 2017
|
$
|
229
|
|
—
|
|
38
|
|
267
|
|
|
Current year activity:
|
|
|
|
|
|||||
|
Charges
|
—
|
|
100
|
|
373
|
|
473
|
|
|
|
Cash payments
|
(29
|
)
|
—
|
|
(388
|
)
|
(417
|
)
|
|
|
Settled non cash
|
—
|
|
(100
|
)
|
—
|
|
(100
|
)
|
|
|
Reserve balance, September 30, 2018*
|
$
|
200
|
|
—
|
|
23
|
|
223
|
|
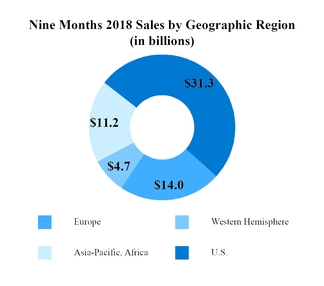

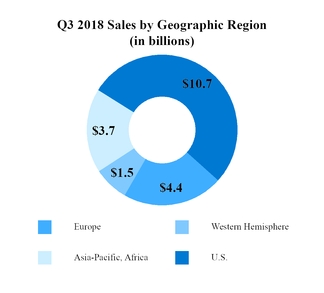
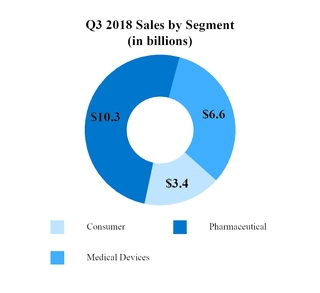
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Total
Change |
Operations
Change |
Currency
Change |
||||||||||||
|
Beauty
|
$
|
3,271
|
|
$
|
3,090
|
|
5.9
|
%
|
5.1
|
%
|
0.8
|
%
|
|||||
|
OTC
|
3,186
|
|
3,021
|
|
5.5
|
|
3.8
|
|
1.7
|
|
|||||||
|
Baby Care
|
1,385
|
|
1,426
|
|
(2.9
|
)
|
(1.8
|
)
|
(1.1
|
)
|
|||||||
|
Oral Care
|
1,156
|
|
1,138
|
|
1.6
|
|
0.7
|
|
0.9
|
|
|||||||
|
Women’s Health
|
792
|
|
788
|
|
0.5
|
|
2.1
|
|
(1.6
|
)
|
|||||||
|
Wound Care/Other
|
527
|
|
599
|
|
(12.0
|
)
|
(12.5
|
)
|
0.5
|
|
|||||||
|
Total Consumer Sales
|
$
|
10,317
|
|
$
|
10,062
|
|
2.5
|
%
|
1.9
|
%
|
0.6
|
%
|
|||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Total
Change |
Operations
Change |
Currency
Change |
||||||||||||
|
Beauty
|
$
|
1,078
|
|
$
|
1,033
|
|
4.4
|
%
|
6.5
|
%
|
(2.1
|
)%
|
|||||
|
OTC
|
1,048
|
|
1,002
|
|
4.6
|
|
6.8
|
|
(2.2
|
)
|
|||||||
|
Baby Care
|
472
|
|
477
|
|
(1.0
|
)
|
4.3
|
|
(5.3
|
)
|
|||||||
|
Oral Care
|
384
|
|
382
|
|
0.5
|
|
3.2
|
|
(2.7
|
)
|
|||||||
|
Women’s Health
|
269
|
|
270
|
|
(0.4
|
)
|
7.9
|
|
(8.3
|
)
|
|||||||
|
Wound Care/Other
|
164
|
|
192
|
|
(14.6
|
)
|
(13.3
|
)
|
(1.3
|
)
|
|||||||
|
Total Consumer Sales
|
$
|
3,415
|
|
$
|
3,356
|
|
1.8
|
%
|
4.9
|
%
|
(3.1
|
)%
|
|||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Total
Change
|
Operations
Change
|
Currency
Change
|
||||||||||||
|
Total Immunology
|
$
|
9,778
|
|
$
|
9,158
|
|
6.8
|
%
|
5.9
|
%
|
0.9
|
%
|
|||||
|
REMICADE
®
|
4,088
|
|
4,849
|
|
(15.7
|
)
|
(15.9
|
)
|
0.2
|
|
|||||||
|
SIMPONI
®
/ SIMPONI ARIA
®
|
1,602
|
|
1,343
|
|
19.3
|
|
17.9
|
|
1.4
|
|
|||||||
|
STELARA
®
|
3,712
|
|
2,930
|
|
26.7
|
|
24.9
|
|
1.8
|
|
|||||||
|
Other Immunology
|
376
|
|
36
|
|
**
|
**
|
**
|
||||||||||
|
Total Infectious Diseases
|
2,502
|
|
2,354
|
|
6.3
|
|
4.0
|
|
2.3
|
|
|||||||
|
EDURANT
®
/rilpivirine
|
623
|
|
522
|
|
19.3
|
|
12.6
|
|
6.7
|
|
|||||||
|
PREZISTA
®
/ PREZCOBIX
®
/ REZOLSTA
®/
SYMTUZA
®
|
1,460
|
|
1,351
|
|
8.1
|
|
7.0
|
|
1.1
|
|
|||||||
|
Other Infectious Diseases
|
419
|
|
481
|
|
(12.9
|
)
|
(13.7
|
)
|
0.8
|
|
|||||||
|
Total Neuroscience
|
4,577
|
|
4,462
|
|
2.6
|
|
0.9
|
|
1.7
|
|
|||||||
|
CONCERTA
®
/methylphenidate
|
513
|
|
588
|
|
(12.8
|
)
|
(13.6
|
)
|
0.8
|
|
|||||||
|
INVEGA SUSTENNA
®
/ XEPLION
®
/ TRINZA
®/
TREVICTA
®
|
2,165
|
|
1,876
|
|
15.4
|
|
13.5
|
|
1.9
|
|
|||||||
|
RISPERDAL CONSTA
®
|
559
|
|
608
|
|
(8.1
|
)
|
(9.8
|
)
|
1.7
|
|
|||||||
|
Other Neuroscience
|
1,340
|
|
1,390
|
|
(3.6
|
)
|
(5.4
|
)
|
1.8
|
|
|||||||
|
Total Oncology
|
7,355
|
|
5,219
|
|
40.9
|
|
38.1
|
|
2.8
|
|
|||||||
|
DARZALEX
®
|
1,441
|
|
871
|
|
65.4
|
|
63.6
|
1.8
|
|
||||||||
|
IMBRUVICA
®
|
1,912
|
|
1,371
|
|
39.5
|
|
36.9
|
|
2.6
|
|
|||||||
|
VELCADE
®
|
864
|
|
843
|
|
2.5
|
|
(1.7
|
)
|
4.2
|
|
|||||||
|
ZYTIGA
®
|
2,712
|
|
1,750
|
|
55.0
|
|
52.0
|
|
3.0
|
|
|||||||
|
Other Oncology
|
426
|
|
384
|
|
10.9
|
|
8.9
|
|
2.0
|
|
|||||||
|
Pulmonary Hypertension***
|
1,906
|
|
717
|
|
**
|
**
|
**
|
||||||||||
|
OPSUMIT
®
|
892
|
|
304
|
|
**
|
**
|
**
|
||||||||||
|
TRACLEER
®
|
422
|
|
236
|
|
**
|
**
|
**
|
||||||||||
|
UPTRAVI
®
|
482
|
|
133
|
|
**
|
**
|
**
|
||||||||||
|
Other
|
110
|
|
44
|
|
**
|
**
|
**
|
||||||||||
|
Cardiovascular / Metabolism / Other
|
4,426
|
|
4,665
|
|
(5.1
|
)
|
(5.8
|
)
|
0.7
|
|
|||||||
|
XARELTO
®
|
1,869
|
|
1,790
|
|
4.4
|
|
4.4
|
|
—
|
|
|||||||
|
INVOKANA
®
/ INVOKAMET
®
|
653
|
|
844
|
|
(22.6
|
)
|
(23.0
|
)
|
0.4
|
|
|||||||
|
PROCRIT
®
/EPREX
®
|
767
|
|
740
|
|
3.6
|
|
2.4
|
|
1.2
|
|
|||||||
|
Other
|
1,137
|
|
1,291
|
|
(11.9
|
)
|
(13.5
|
)
|
1.6
|
|
|||||||
|
Total Pharmaceutical Sales
|
$
|
30,544
|
|
$
|
26,575
|
|
14.9
|
%
|
13.4
|
%
|
1.5
|
%
|
|||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Total
Change
|
Operations
Change
|
Currency
Change
|
||||||||||||
|
Total Immunology
|
$
|
3,398
|
|
$
|
3,269
|
|
3.9
|
%
|
5.0
|
%
|
(1.1
|
)%
|
|||||
|
REMICADE
®
|
1,379
|
|
1,647
|
|
(16.3
|
)
|
(15.3
|
)
|
(1.0
|
)
|
|||||||
|
SIMPONI
®
/ SIMPONI ARIA
®
|
536
|
|
476
|
|
12.6
|
|
14.8
|
|
(2.2
|
)
|
|||||||
|
STELARA
®
|
1,310
|
|
1,124
|
|
16.5
|
|
17.4
|
|
(0.9
|
)
|
|||||||
|
Other Immunology
|
173
|
|
22
|
|
**
|
**
|
**
|
||||||||||
|
Total Infectious Diseases
|
823
|
|
813
|
|
1.2
|
|
3.2
|
|
(2.0
|
)
|
|||||||
|
EDURANT
®
/rilpivirine
|
202
|
|
194
|
|
4.1
|
|
5.2
|
|
(1.1
|
)
|
|||||||
|
PREZISTA
®
/ PREZCOBIX
®
/ REZOLSTA
®/
SYMTUZA
®
|
490
|
|
467
|
|
4.9
|
|
6.9
|
|
(2.0
|
)
|
|||||||
|
Other Infectious Diseases
|
131
|
|
152
|
|
(13.8
|
)
|
(10.7
|
)
|
(3.1
|
)
|
|||||||
|
Total Neuroscience
|
1,490
|
|
1,498
|
|
(0.5
|
)
|
1.5
|
|
(2.0
|
)
|
|||||||
|
CONCERTA
®
/ methylphenidate
|
157
|
|
198
|
|
(20.7
|
)
|
(18.1
|
)
|
(2.6
|
)
|
|||||||
|
INVEGA SUSTENNA
®
/ XEPLION
®
/ TRINZA
®/
TREVICTA
®
|
749
|
|
643
|
|
16.5
|
|
17.8
|
|
(1.3
|
)
|
|||||||
|
RISPERDAL CONSTA
®
|
175
|
|
194
|
|
(9.8
|
)
|
(7.9
|
)
|
(1.9
|
)
|
|||||||
|
Other Neuroscience
|
409
|
|
463
|
|
(11.7
|
)
|
(9.0
|
)
|
(2.7
|
)
|
|||||||
|
Total Oncology
|
2,588
|
|
1,898
|
|
36.4
|
|
38.6
|
|
(2.2
|
)
|
|||||||
|
DARZALEX
®
|
498
|
|
317
|
|
57.1
|
|
60.0
|
|
(2.9
|
)
|
|||||||
|
IMBRUVICA
®
|
705
|
|
512
|
|
37.7
|
|
40.4
|
|
(2.7
|
)
|
|||||||
|
VELCADE
®
|
271
|
|
273
|
|
(0.7
|
)
|
1.8
|
|
(2.5
|
)
|
|||||||
|
ZYTIGA
®
|
958
|
|
669
|
|
43.2
|
|
44.5
|
|
(1.3
|
)
|
|||||||
|
Other Oncology
|
156
|
|
127
|
|
22.8
|
|
25.6
|
|
(2.8
|
)
|
|||||||
|
Pulmonary Hypertension
|
656
|
|
632
|
|
3.8
|
|
4.9
|
|
(1.1
|
)
|
|||||||
|
OPSUMIT
®
|
310
|
|
259
|
|
19.7
|
|
21.2
|
|
(1.5
|
)
|
|||||||
|
TRACLEER
®
|
139
|
|
210
|
|
(33.8
|
)
|
(33.0
|
)
|
(0.8
|
)
|
|||||||
|
UPTRAVI
®
|
171
|
|
124
|
|
37.9
|
|
38.1
|
|
(0.2
|
)
|
|||||||
|
Other
|
36
|
|
39
|
|
(7.7
|
)
|
(5.2
|
)
|
(2.5
|
)
|
|||||||
|
Cardiovascular / Metabolism / Other
|
1,391
|
|
1,585
|
|
(12.2
|
)
|
(11.3
|
)
|
(0.9
|
)
|
|||||||
|
XARELTO
®
|
612
|
|
635
|
|
(3.6
|
)
|
(3.6
|
)
|
—
|
|
|||||||
|
INVOKANA
®
/ INVOKAMET
®
|
190
|
|
265
|
|
(28.3
|
)
|
(27.6
|
)
|
(0.7
|
)
|
|||||||
|
PROCRIT
®
/ EPREX
®
|
255
|
|
238
|
|
7.1
|
|
7.9
|
|
(0.8
|
)
|
|||||||
|
Other
|
334
|
|
447
|
|
(25.3
|
)
|
(22.9
|
)
|
(2.4
|
)
|
|||||||
|
Total Pharmaceutical Sales
|
$
|
10,346
|
|
$
|
9,695
|
|
6.7
|
%
|
8.2
|
%
|
(1.5
|
)%
|
|||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Total
Change
|
Operations
Change
|
Currency
Change
|
||||||||||||
|
Surgery
|
$
|
7,314
|
|
$
|
7,001
|
|
4.5
|
%
|
3.2
|
%
|
1.3
|
%
|
|||||
|
Advanced
|
2,947
|
|
2,733
|
|
7.8
|
|
6.3
|
|
1.5
|
|
|||||||
|
General
|
3,376
|
|
3,293
|
|
2.5
|
|
1.1
|
|
1.4
|
|
|||||||
|
Specialty
|
991
|
|
975
|
|
1.6
|
|
1.1
|
|
0.5
|
|
|||||||
|
Orthopaedics
|
6,623
|
|
6,772
|
|
(2.2
|
)
|
(3.5
|
)
|
1.3
|
|
|||||||
|
Hips
|
1,053
|
|
1,030
|
|
2.2
|
|
0.9
|
|
1.3
|
|
|||||||
|
Knees
|
1,110
|
|
1,126
|
|
(1.4
|
)
|
(2.6
|
)
|
1.2
|
|
|||||||
|
Trauma
|
2,025
|
|
1,947
|
|
4.0
|
|
2.6
|
|
1.4
|
|
|||||||
|
Spine & Other
|
2,435
|
|
2,669
|
|
(8.8
|
)
|
(10.1
|
)
|
1.3
|
|
|||||||
|
Vision
|
3,420
|
|
2,944
|
|
16.2
|
|
14.9
|
|
1.3
|
|
|||||||
|
Contact Lenses/Other
|
2,486
|
|
2,236
|
|
11.2
|
|
9.9
|
|
1.3
|
|
|||||||
|
Surgical
|
934
|
|
708
|
|
31.9
|
|
30.5
|
|
1.4
|
|
|||||||
|
Interventional Solutions
(1)
|
1,960
|
|
1,675
|
|
17.0
|
|
14.9
|
|
2.1
|
|
|||||||
|
Diabetes Care
|
1,009
|
|
1,225
|
|
(17.6
|
)
|
(19.0
|
)
|
1.4
|
|
|||||||
|
Diagnostics
(2)
|
—
|
|
1
|
|
**
|
**
|
**
|
||||||||||
|
Total Medical Devices Sales
|
$
|
20,326
|
|
$
|
19,618
|
|
3.6
|
%
|
2.2
|
%
|
1.4
|
%
|
|||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
Total
Change
|
Operations
Change
|
Currency
Change
|
||||||||||||
|
Surgery
|
$
|
2,376
|
|
$
|
2,346
|
|
1.3
|
%
|
3.8
|
%
|
(2.5
|
)%
|
|||||
|
Advanced
|
976
|
|
923
|
|
5.7
|
|
8.1
|
|
(2.4
|
)
|
|||||||
|
General
|
1,080
|
|
1,105
|
|
(2.3
|
)
|
0.3
|
(2.6
|
)
|
||||||||
|
Specialty
|
320
|
|
318
|
|
0.6
|
|
2.9
|
|
(2.3
|
)
|
|||||||
|
Orthopaedics
|
2,111
|
|
2,204
|
|
(4.2
|
)
|
(2.9
|
)
|
(1.3
|
)
|
|||||||
|
Hips
|
330
|
|
328
|
|
0.6
|
|
2.2
|
|
(1.6
|
)
|
|||||||
|
Knees
|
341
|
|
343
|
|
(0.6)
|
1.0
|
|
(1.6
|
)
|
||||||||
|
Trauma
|
654
|
|
662
|
|
(1.2
|
)
|
0.0
|
|
(1.2
|
)
|
|||||||
|
Spine & Other
|
786
|
|
871
|
|
(9.8
|
)
|
(8.6
|
)
|
(1.2
|
)
|
|||||||
|
Vision
|
1,132
|
|
1,091
|
|
3.8
|
|
5.6
|
|
(1.8
|
)
|
|||||||
|
Contact Lenses/Other
|
835
|
|
800
|
|
4.4
|
|
6.2
|
|
(1.8
|
)
|
|||||||
|
Surgical
|
297
|
|
291
|
|
2.1
|
|
4.1
|
|
(2.0
|
)
|
|||||||
|
Interventional Solutions
(1)
|
653
|
|
553
|
|
18.1
|
|
19.4
|
|
(1.3
|
)
|
|||||||
|
Diabetes Care
|
315
|
|
405
|
|
(22.2
|
)
|
(20.0
|
)
|
(2.2
|
)
|
|||||||
|
Total Medical Devices Sales
|
$
|
6,587
|
|
$
|
6,599
|
|
(0.2
|
)%
|
1.7
|
%
|
(1.9
|
)%
|
|||||
|
|
Income Before Tax
|
Segment Sales
|
Percent of Segment Sales
|
|||||||||||||||||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
||||||||||||||||
|
Consumer
|
$
|
1,887
|
|
$
|
2,132
|
|
$
|
10,317
|
|
$
|
10,062
|
|
18.3
|
%
|
21.2
|
%
|
||||||
|
Pharmaceutical
|
10,193
|
|
9,934
|
|
30,544
|
|
26,575
|
|
33.4
|
|
37.4
|
|
||||||||||
|
Medical Devices
|
3,642
|
|
3,938
|
|
20,326
|
|
19,618
|
|
17.9
|
|
20.1
|
|
||||||||||
|
Segment total
|
15,722
|
|
16,004
|
|
61,187
|
|
56,255
|
|
25.7
|
|
28.4
|
|
||||||||||
|
Less: Expenses not allocated to segments
(1)
|
845
|
|
891
|
|
|
|
|
|
||||||||||||||
|
Worldwide total
|
$
|
14,877
|
|
$
|
15,113
|
|
$
|
61,187
|
|
$
|
56,255
|
|
24.3
|
%
|
26.9
|
%
|
||||||
|
|
Income Before Tax
|
Segment Sales
|
Percent of Segment Sales
|
|||||||||||||||||||
|
(Dollars in Millions)
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
September 30, 2018
|
October 1, 2017
|
||||||||||||||||
|
Consumer
|
$
|
510
|
|
$
|
878
|
|
$
|
3,415
|
|
$
|
3,356
|
|
14.9
|
%
|
26.2
|
%
|
||||||
|
Pharmaceutical
|
2,876
|
|
2,857
|
|
10,346
|
|
9,695
|
|
27.8
|
|
29.5
|
|
||||||||||
|
Medical Devices
|
1,267
|
|
1,383
|
|
6,587
|
|
6,599
|
|
19.2
|
|
21.0
|
|
||||||||||
|
Segment earnings before provision for taxes
|
4,653
|
|
5,118
|
|
20,348
|
|
19,650
|
|
22.9
|
|
26.0
|
|
||||||||||
|
Less: Expenses not allocated to segments
(1)
|
230
|
|
328
|
|
|
|
|
|
||||||||||||||
|
Worldwide income before tax
|
$
|
4,423
|
|
$
|
4,790
|
|
$
|
20,348
|
|
$
|
19,650
|
|
21.7
|
%
|
24.4
|
%
|
||||||
|
Period
|
Total Number
of Shares Purchased
(1)
|
Avg. Price
Per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs
|
Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs
|
|||||||
|
July 2, 2018 through July 29, 2018
|
200,000
|
|
128.19
|
|
—
|
|
—
|
||||
|
July 30, 2018 through August 26, 2018
|
265,958
|
|
135.10
|
|
—
|
|
—
|
||||
|
August 27, 2018 through September 30, 2018
|
2,958,890
|
|
138.58
|
|
—
|
|
—
|
||||
|
Total
|
3,424,848
|
|
—
|
|
|
||||||
|
|
JOHNSON & JOHNSON
(Registrant)
|
|
Date: October 31, 2018
|
By /s/ J. J. WOLK
|
|
J. J. WOLK
|
|
|
|
Executive Vice President, Chief Financial Officer (Principal Financial Officer)
|
|
|
|
|
Date: October 31, 2018
|
By /s/ R. A. KAPUSTA
|
|
|
R. A. KAPUSTA
|
|
|
Controller (Principal Accounting Officer)
|