|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Israel
|
Not applicable
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. employer identification no.)
|
|
|
3 Hatnufa Street, Floor 6, Yokneam Ilit, Israel
|
2069203
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Ordinary Shares, par value NIS 0.01 per share
|
The Nasdaq Stock Market LLC
|
|
Large accelerated filer
o
|
Accelerated filer
x
|
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
o
|
|
Page No
|
||
|
PART I
|
||
|
PART II
|
||
|
PART III
|
||
|
PART IV
|
||
|
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
F-1
|
|
|
•
|
our expectations regarding future growth, including our ability to increase sales in our existing geographic markets and to expand to new markets;
|
|
•
|
our ability to maintain and grow our reputation and the market acceptance of our products;
|
|
•
|
our ability to achieve reimbursement from third-party payors for our products;
|
|
•
|
our expectations as to our clinical research program and clinical results;
|
|
•
|
our ability to improve our products and develop new products;
|
|
•
|
our ability to maintain adequate protection of our intellectual property and to avoid violation of the intellectual property rights of others;
|
|
•
|
our ability to gain and maintain regulatory approvals; and
|
|
•
|
our ability to maintain relationships with existing customers and develop relationships with new customers.
|
|
Spinal Cord Injury
|
|
|
Spinal cord injury is the result of a direct trauma to the nerves themselves or damage to the surrounding bones and soft tissues which ultimately impacts the spinal cord. Spinal cord damage results in a loss of function, such as mobility or feeling. In most people who have spinal cord injury, the spinal cord is intact. Spinal cord injury is not the same as back injury, which may result from pinched nerves or ruptured disks. Even when a person sustains a break in a vertebra or vertebrae, there may not be any spinal cord injury if the spinal cord itself is not affected. There are two types of spinal cord injury – complete and incomplete. In a complete injury, a person loses all ability to feel and voluntarily move below the level of the injury. In an incomplete injury, there is some functioning below the level of the injury.
Upon examination, a patient is assigned a level of injury depending on the location of the spinal cord injury. Cervical level injuries cause paralysis or weakness in both arms and legs and is referred to as quadriplegia. Sometimes this type of injury is accompanied by loss of physical sensation, respiratory issues, bowel, bladder, and sexual dysfunction. Thoracic level injuries can cause paralysis or weakness of the legs (paraplegia) along with loss of physical sensation, bowel, bladder, and sexual dysfunction. In most cases, arms and hands are not affected. Lumbar level injuries result in paralysis or weakness of the legs (paraplegia). Loss of physical sensation, bowel, bladder, and sexual dysfunction can occur. The shoulder, arm, and hand functions are usually unaffected. Sacral level injuries primarily cause loss of bowel and bladder function as well as sexual dysfunction.
|
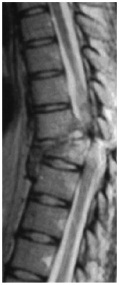
|
|
Image of Separated Spinal Cord of an Adult
|
|
|
The history of exoskeleton development began in the 19
th
century, with the first patent for a mechanical suit appearing in 1890. The use of motors and gears to power these suits is not new, with General Electric developing an early exoskeleton device in the 1960s. Called the Hardiman, it was a hydraulic and electric body suit, but its weight and bulk made practical use prohibitive. Innovation of an advanced exoskeleton that restores a natural walking experience has been a key technological goal of the industry, and the lack of such a system has hindered sector growth. Advances in computer hardware and software and proprietary technological breakthroughs pioneered by us have resulted in the development of an advanced exoskeleton, ReWalk, that restores walking with a natural gait and functional speed.
|
|
|
ReWalk Personal 6.0
|
||
|
•
ReWalk Personal
: intended for everyday use at home, at work or in the community. We began marketing ReWalk Personal in Europe with CE mark clearance at the end of 2012. We received clearance to market ReWalk Personal in the United States in June 2014. ReWalk Personal units are all manufactured according to the same specifications. Each unit is then permanently sized to fit the individual user and the software is configured for the user’s specifications by the rehabilitation center, clinic or distributor.
•
ReWalk Rehabilitation
: designed for the clinical rehabilitation environment, ReWalk Rehabilitation has adjustable sizing enabling multiple patient use. ReWalk Rehabilitation provides a valuable means of exercise and therapy. It also enables individuals to evaluate their capacity for using ReWalk Personal in the future. We began marketing ReWalk Rehabilitation for use in hospitals, rehabilitation centers and stand-alone training centers in the United States, Europe and Asia in 2011. ReWalk Rehabilitation units are all manufactured according to the same specifications and are equipped with adjustable sizing for multi-patient use.
|

|
|
|
•
|
The first study, published in
The Journal of Spinal Cord Medicine
in 2012, included six participants and was designed to assess the safety and tolerance of use of ReWalk by patients with a spinal cord injury. The participants were all able to walk 100 meters with ReWalk. The study found no adverse safety events (which included falls, status of the skin, status of the spine and joints, blood pressure, pulse and electrocardiography) and concluded that use of ReWalk was well-tolerated by participants with no increase in pain and a moderate level of fatigue after use. The participants generally had positive feedback regarding ReWalk. No adverse effects were noted.
|
|
•
|
The second study included 24 participants and was designed to assess the safety and performance of ReWalk in enabling individuals with paraplegia to carry out routine ambulatory functions. Results with respect to a 12-participant subset were published in the
American Journal of Physical Medicine & Rehabilitation
in 2012. The results from this subset demonstrated that all participants were able to independently walk, without assistance from another person, for at least 50 meters and at least five minutes. Some participants reported improvements in pain, bowel function, bladder function and spasticity. All participants had strong positive feedback regarding the emotional and psychosocial benefits of using ReWalk. ReWalk was found to hold significant potential as a safe ambulatory powered orthotic for spinal cord injury patients. Significant performance variability was noted between participants. There were no serious adverse events reported. Five participants reported mild to moderate adverse effects, consisting of skin abrasions, lightheadedness and edema of the lower limbs. These adverse effects were managed by the appropriate use of padding, caffeine intake and adjustment of blood pressure medication, elastic stockings and rest.
|
|
•
|
The third study, published in
The Journal of Spinal Cord Medicine
in 2013, included six participants and found that participants with spinal cord injury, walking independently with ReWalk, demonstrated a stance and gait similar to that of an able-bodied individual. No adverse effects were noted.
|
|
•
|
The fourth study, which is ongoing and includes 30 participants, was designed to assess the mobility skills and levels of training and assistance needed to use and benefit from ReWalk. Results with respect to a seven-participant subset
|
|
•
|
The fifth study, published in International Journal of Physical Therapy and Rehabilitation in November 2014 reported on 16 patients who had undergone gait training using the ReWalk Rehabilitation device. These subjects demonstrated significant increases in joint range of motions for the hip and ankle joints. No adverse results were reported.
|
|
•
|
A sixth study, which was a continuation of the fourth study mentioned above, was presented at a scientific session of the 2015 American Academy of Physical Medicine and Rehabilitation. This study demonstrated improvements in quality of life measurements for pain reduction, fatigue, and improved sleep. Restoration of physiological loading to the legs. Improvements in bowel function, seated balance and reduction in fat mass were also documented.
|
|
•
|
A seventh study published in the Journal of Rehabilitation Research and Development in 2015 assessed heart rate and oxygen demand of powered exoskeleton-assisted walking in person with paraplegia. As part of an ongoing clinical study, eight non-ambulatory persons with paraplegia were trained to ambulate with a powered exoskeleton. Measurements of oxygen uptake and heart rate were recorded for six minutes each during each maneuver while sitting, standing, and walking. The average value of oxygen uptake and heart rate response during walking were significantly higher than for sitting and standing. Persons with paraplegia were able to ambulate efficiently using the powered exoskeleton for over-ground ambulation, providing the potential for functional gain and improved fitness. This report is the first to determine energy expenditure of powered exoskeletal-assisted walking by use of the ReWalk system in persons with SCI. Although the results of this study did not address long-term changes in oxygen demand with habitual use, routine use of the device to increase activity energy expenditure would be expected to have positive cardiopulmonary and metabolic benefits.
|
|
•
|
An eighth study published in Topics in Spinal Cord Injury Rehabilitation in April 2015 assessed in-hospital walking velocity and level of assistance in a powered exoskeleton for persons with SCI. Twelve individuals that had SCI for 1.5 years or more who were wheelchair-users participated, and seven were able to ambulate greater than 0.4 meters per second, which is a velocity that may be conductive to outdoor activity related community ambulation. The maximum velocity recorded was 0.74 meters per second.
|
|
•
|
A ninth publication is a case report on the effects of training with the ReWalk exoskeleton on quality of life in incomplete spinal cord injury. The study was carried out at a hospital for neurological rehabilitation in Germany. One patient, initially unable to walk independently after suffering a traumatic spinal cord injury, was recruited for this study one year after suffering such injury. The progress of the first six months of training was documented and as a primary outcome measure the quality of life was measured using the industry-standard SF-36 questionnaire. At the end of the six-month study period the patient was able to walk independently supervised by one person. Quality of life, mobility, risk of falling, motor skills and control of bladder and bowel functions were improved. A positive effect of robot-assisted gait training on various areas of quality of life was shown.
|
|
•
|
establishment registration and device listing;
|
|
•
|
development of a quality assurance system, including establishing and implementing procedures to design and manufacture devices;
|
|
•
|
labeling regulations that prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and
|
|
•
|
medical device reporting regulations that require manufacturers to report to the FDA if a device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and corrections and removal reporting regulations that require manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the U.S. Food, Drug and Cosmetic Act that may present a risk to health.
|
|
•
|
warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
recalls, withdrawals, or administrative detention or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
•
|
refusal to grant export approvals for our products; or
|
|
•
|
criminal prosecution.
|
|
Years Ended December 31,
|
|||||||||
|
2015
|
2014
|
2013
|
|||||||
|
Revenue:
|
|||||||||
|
United States
|
$
|
2,439
|
|
$
|
2,186
|
|
$
|
941
|
|
|
Europe
|
820
|
|
1,254
|
|
476
|
|
|||
|
Asia - Pacific
|
487
|
|
511
|
|
88
|
|
|||
|
Israel
|
—
|
|
—
|
|
83
|
|
|||
|
Total Revenue
|
$
|
3,746
|
|
$
|
3,951
|
|
$
|
1,588
|
|
|
•
|
lack of sufficient evidence supporting the benefits of ReWalk over competitive products or other available treatment, or lifestyle management, methodologies;
|
|
•
|
results of clinical studies relating to ReWalk or similar products;
|
|
•
|
claims that ReWalk, or any component thereof, infringes on patent or other intellectual property rights of third-parties;
|
|
•
|
perceived risks associated with the use of ReWalk or similar products or technologies;
|
|
•
|
the introduction of new competitive products or greater acceptance of competitive products;
|
|
•
|
adverse regulatory or legal actions relating to ReWalk or similar products or technologies; and
|
|
•
|
problems arising from the outsourcing of our manufacturing capabilities, or our existing manufacturing and supply relationships.
|
|
•
|
a market will not develop for our products;
|
|
•
|
we will not be able to develop scalable products and services, or that, although scalable, our products and services will not be economical to market;
|
|
•
|
we will not be able to establish brand recognition and competitive advantages for our products;
|
|
•
|
we will not receive necessary regulatory clearances or approvals for our products; and
|
|
•
|
our competitors market an equivalent or superior product or hold proprietary rights that preclude us from marketing our products.
|
|
•
|
identify the product features that people with paraplegia or paralysis, their caregivers and healthcare providers are seeking in a medical device that restores upright mobility and successfully incorporate those features into our products;
|
|
•
|
develop and introduce proposed products in sufficient quantities and in a timely manner;
|
|
•
|
adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third-parties;
|
|
•
|
demonstrate the safety, efficacy and health benefits of proposed products; and
|
|
•
|
obtain the necessary regulatory approvals for proposed products.
|
|
•
|
problems assimilating the acquired products or technologies;
|
|
•
|
issues maintaining uniform standards, procedures, controls and policies;
|
|
•
|
unanticipated costs associated with acquisitions;
|
|
•
|
diversion of management’s attention from our existing business;
|
|
•
|
risks associated with entering new markets in which we have limited or no experience; and
|
|
•
|
increased legal and accounting costs relating to the acquisitions or compliance with regulatory matters.
|
|
•
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
customer notifications or repair, replacement or refunds;
|
|
•
|
detention or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
•
|
withdrawing 510(k) marketing clearances or PMA approvals that have already been granted;
|
|
•
|
refusing to provide Certificates for Foreign Government;
|
|
•
|
refusing to grant export approval for our products; or
|
|
•
|
pursuing criminal prosecution.
|
|
•
|
actual or anticipated fluctuations in our growth rate or results of operations or those of our competitors;
|
|
•
|
customer acceptance of our products;
|
|
•
|
announcements by us or our competitors of new products or services, commercial relationships, acquisitions or expansion plans;
|
|
•
|
announcements by us or our competitors of other material developments;
|
|
•
|
our involvement in litigation;
|
|
•
|
changes in government regulation applicable to us and our products;
|
|
•
|
sales, or the anticipation of sales, of our ordinary shares, warrants and debt securities by us, or sales of our ordinary shares by our insiders or other shareholders, including upon expiration of contractual lock-up agreements;
|
|
•
|
developments with respect to intellectual property rights;
|
|
•
|
competition from existing or new technologies and products;
|
|
•
|
changes in key personnel;
|
|
•
|
the trading volume of our ordinary shares;
|
|
•
|
changes in the estimation of the future size and growth rate of our markets; and
|
|
•
|
general economic and market conditions.
|
|
•
|
the composition of our board of directors, which has the authority to direct our business and to appoint and remove our officers;
|
|
•
|
approving or rejecting a merger, consolidation or other business combination;
|
|
•
|
raising future capital; and
|
|
•
|
amending our articles of association, which govern the rights attached to our ordinary shares.
|
|
Square feet(approximate)
|
||
|
Marlborough, Massachusetts
|
3,300
|
|
|
Yokneam, Israel
|
11,080
|
|
|
Berlin, Germany
|
600
|
|
|
Total
|
14,980
|
|
|
High
|
Low
|
||||||
|
2015
|
|||||||
|
Fourth quarter 2015
|
$
|
17.40
|
|
$
|
5.55
|
|
|
|
Third quarter 2015
|
$
|
11.90
|
|
$
|
7.20
|
|
|
|
Second quarter 2015
|
$
|
14.65
|
|
$
|
10.35
|
|
|
|
First quarter 2015
|
$
|
22.74
|
|
$
|
12.03
|
|
|
|
2014
|
|||||||
|
Fourth quarter 2014
|
$
|
34.29
|
|
$
|
18.01
|
|
|
|
Third quarter 2014 (beginning on September 12, 2014)
|
$
|
43.71
|
|
$
|
11.50
|
|
|
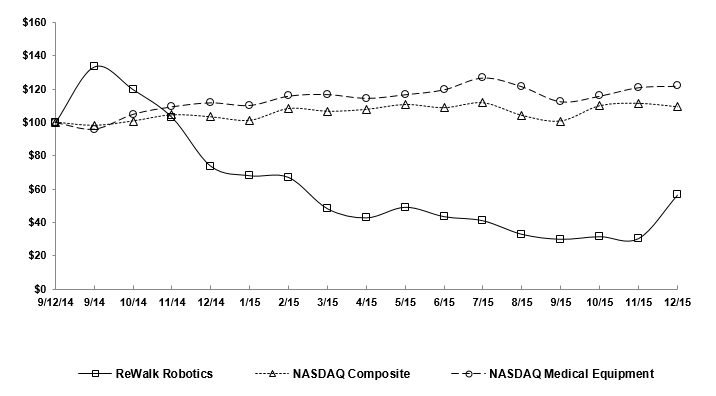
|
Year Ended December 31,
|
|||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Statements of Operations Data:
|
|||||||||||||||
|
Revenues
|
$
|
3,746
|
|
$
|
3,951
|
|
$
|
1,588
|
|
$
|
972
|
|
|||
|
Cost of revenues
|
3,532
|
|
4,106
|
|
2,017
|
|
983
|
|
|||||||
|
Expense related to settlement of BIRD Foundation grants
|
—
|
|
466
|
|
—
|
|
—
|
|
|||||||
|
Gross profit (loss)
|
214
|
|
(621
|
)
|
(429
|
)
|
(11
|
)
|
|||||||
|
Operating expenses:
|
|||||||||||||||
|
Research and development, net
|
5,937
|
|
8,563
|
|
2,463
|
|
1,757
|
|
|||||||
|
Sales and marketing, net
|
13,056
|
|
7,389
|
|
4,091
|
|
2,334
|
|
|||||||
|
General and administrative
|
6,395
|
|
3,352
|
|
1,762
|
|
1,657
|
|
|||||||
|
Total operating expenses
|
25,388
|
|
19,304
|
|
8,316
|
|
5,748
|
|
|||||||
|
Operating loss
|
(25,174
|
)
|
(19,925
|
)
|
(8,745
|
)
|
(5,759
|
)
|
|||||||
|
Financial expenses, net
|
188
|
|
1,698
|
|
3,410
|
|
878
|
|
|||||||
|
Loss before income taxes
|
(25,362
|
)
|
(21,623
|
)
|
(12,155
|
)
|
(6,637
|
)
|
|||||||
|
Income taxes
|
53
|
|
45
|
|
22
|
|
21
|
|
|||||||
|
Net loss
|
$
|
(25,415
|
)
|
$
|
(21,668
|
)
|
$
|
(12,177
|
)
|
$
|
(6,658
|
)
|
|||
|
Net loss per ordinary share, basic and diluted(1)
|
$
|
(2.10
|
)
|
$
|
(6.34
|
)
|
$
|
(74.53
|
)
|
$
|
(41.26
|
)
|
|||
|
Weighted average number of shares used in computing net loss per ordinary share, basic and diluted
|
12,115,038
|
|
3,766,694
|
|
185,688
|
|
185,688
|
|
|||||||
|
As of December 31,
|
|||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Balance Sheet Data:
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
17,869
|
|
$
|
41,829
|
|
$
|
8,860
|
|
$
|
769
|
|
|||
|
Total assets
|
25,574
|
|
47,665
|
|
11,059
|
|
2,094
|
|
|||||||
|
Accumulated deficit
|
(73,989
|
)
|
(48,574
|
)
|
(26,906
|
)
|
(14,729
|
)
|
|||||||
|
Total shareholders’ equity
|
$
|
20,920
|
|
$
|
43,853
|
|
$
|
5,631
|
|
$
|
(2,264
|
)
|
|||
|
(1)
|
Net loss per ordinary share, basic and diluted, is calculated by dividing our net loss excluding dividends accrued on our convertible preferred shares outstanding during the period presented by the weighted average number of shares outstanding during the period presented. See Note 2s to our consolidated financial statements set forth in Item 8. "Financial Statements and Supplementary Data" of this annual report.
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Personal units placed
|
53
|
|
43
|
|
|||
|
Rehabilitation units placed
|
20
|
|
31
|
|
|||
|
Total units placed
|
73
|
|
74
|
|
|||
|
Personal unit revenues
|
$
|
2,766
|
|
$
|
2,191
|
|
|
|
Rehabilitation unit revenues
|
$
|
980
|
|
$
|
1,760
|
|
|
|
Revenues
|
$
|
3,746
|
|
$
|
3,951
|
|
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Gross profit (loss)
|
$
|
214
|
|
$
|
(621
|
)
|
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Research and development expenses, net
|
$
|
5,937
|
|
$
|
8,563
|
|
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Sales and marketing expenses
|
$
|
13,056
|
|
$
|
7,389
|
|
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
General and administrative
|
$
|
6,395
|
|
$
|
3,352
|
|
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Financial expenses, net
|
$
|
188
|
|
$
|
1,698
|
|
|
|
Years Ended December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Income tax
|
$
|
53
|
|
$
|
45
|
|
|
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Personal units placed
|
43
|
|
8
|
|
|||
|
Rehabilitation units placed
|
31
|
|
17
|
|
|||
|
Total units placed
|
74
|
|
25
|
|
|||
|
Personal unit revenues
|
$
|
2,191
|
|
$
|
442
|
|
|
|
Rehabilitation unit revenues
|
$
|
1,760
|
|
$
|
1,146
|
|
|
|
Revenues
|
$
|
3,951
|
|
$
|
1,588
|
|
|
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Gross loss
|
$
|
621
|
|
$
|
429
|
|
|
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Research and development expenses, net
|
$
|
8,563
|
|
$
|
2,463
|
|
|
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Sales and marketing expenses, net
|
$
|
7,389
|
|
$
|
4,091
|
|
|
|
Years Ended December 31,
|
|||||
|
2014
|
2013
|
||||
|
General and administrative
|
3,352
|
|
1,762
|
|
|
|
Years Ended December 31,
|
|||||
|
2014
|
2013
|
||||
|
Financial expenses, net
|
1,698
|
|
3,410
|
|
|
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Income tax
|
$
|
45
|
|
$
|
22
|
|
|
|
Payments due by period (in dollars, in thousands)
|
|||||||||||||
|
Contractual obligations
|
Total
|
Less than 1 year
|
1-3 years
|
3-5 years
|
More than 5 years
|
||||||||
|
Purchase obligations
|
958
|
958
|
|
-
|
|
-
|
|
-
|
|
||||
|
Operating lease obligations
|
3,593
|
322
|
|
972
|
|
1,014
|
|
1,285
|
|
||||
|
Total
|
4,551
|
1,280
|
|
972
|
|
1,014
|
|
1,285
|
|
||||
|
Change in Average Exchange Rate
|
||||||
|
Period
|
NIS against the
U.S. Dollar (%) |
Euro against the
U.S. Dollar (%) |
||||
|
2013
|
(6.4
|
)
|
(3.4
|
)
|
||
|
2014
|
(0.89
|
)
|
(0.01
|
)
|
||
|
2015
|
8.24
|
|
16.44
|
|
||
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements.
|
|
Name
|
Age
|
Position
|
||
|
Larry Jasinski
|
58
|
Chief Executive Officer and Director
|
||
|
Kevin Hershberger
|
51
|
Chief Financial Officer
|
||
|
Ofir Koren
|
46
|
Vice President, Research & Development
|
||
|
Jodi Gricci
|
48
|
Vice President, Global Marketing and Training
|
||
|
John Hamilton
|
62
|
Vice President, Regulatory and Clinical
|
||
|
ReWalk Robotics Ltd.
|
||
|
By:
|
/s/ Larry Jasinski
|
|
|
Larry Jasinski
|
||
|
Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Larry Jasinski
|
Director and Chief Executive Officer
(Principal Executive Officer)
|
February 29, 2016
|
||
|
Larry Jasinski
|
||||
|
/s/ Kevin Hershberger
|
Chief Financial Officer (Principal
Financial Officer and Principal Accounting Officer)
|
February 29, 2016
|
||
|
Kevin Hershberger
|
||||
|
/s/ Jeff Dykan
|
Chairman of the Board
|
February 29, 2016
|
||
|
Jeff Dykan
|
||||
|
/s/ Dr. John William Poduska
|
Director
|
February 29, 2016
|
||
|
Dr. John William Poduska
|
||||
|
/s/ Deborah DiSanzo
|
Director
|
February 29, 2016
|
||
|
Deborah DiSanzo
|
||||
|
/s/ Wayne B. Weisman
|
Director
|
February 29, 2016
|
||
|
Wayne B. Weisman
|
||||
|
/s/ Yasushi Ichiki
|
Director
|
February 29, 2016
|
||
|
Yasushi Ichiki
|
||||
|
/s/ Aryeh Dan
|
Director
|
February 29, 2016
|
||
|
Aryeh Dan
|
||||
|
/s/ Glenn Muir
|
Director
|
February 29, 2016
|
||
|
Glenn Muir
|
||||
|
/s/ Jay Kalish
|
Director
|
February 29, 2016
|
||
|
Jay Kalish
|
||||
|
Number
|
Description
|
|
|
3.1
|
Second Amended and Restated Articles of Association of the Company, as amended by the First Amendment thereto.
|
|
|
4.1
|
Specimen share certificate (incorporated by reference to Exhibit 4.1 to the Company's registration statement on Form F-1/A (File No. 333-197344), filed with the SEC on August 20, 2014).
|
|
|
4.2
|
Amended and Restated Shareholders’ Rights Agreement, dated July 14, 2014, among the Company and the other parties named therein (incorporated by reference to Exhibit 10.9 to the Company's registration statement on Form F-1/A (File No. 333-197344), filed with the SEC on July 16, 2014).
|
|
|
4.3
|
Fourth Amended and Restated Shareholders Agreement, dated July 14, 2014, among the Company and the shareholders party thereto (incorporated by reference to Exhibit 10.10 to the Company's registration statement on Form F-1/A (File No. 333-197344), filed with the SEC on July 16, 2014).
|
|
|
4.4
|
Form of Indenture relating to debt securities (incorporated by reference to Exhibit 4.1 to the Company's registration statement on Form F-3 (File No. 333-207219), filed with the SEC on October 1, 2015).
|
|
|
10.1
|
Letter of Agreement, dated July 11, 2013, between the Company and Sanmina Corporation (incorporated by reference to Exhibit 10.1 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014).*
|
|
|
10.2
|
Strategic Alliance Agreement, dated September 24, 2013, between the Company and Yaskawa Electric Corporation (incorporated by reference to Exhibit 10.2 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014).
|
|
|
10.3
|
Exclusive Distribution Agreement, dated September 24, 2013, between the Company and Yaskawa Electric Corporation (incorporated by reference to Exhibit 10.3 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014).*
|
|
|
10.4
|
Confidentiality and Non-Disclosure Agreement, dated September 24, 2013, between the Company and Yaskawa Electric Corporation (incorporated by reference to Exhibit 10.4 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014).
|
|
|
10.5
|
Side Letter, dated September 30, 2013, between the Company and Yaskawa Electric Corporation (incorporated by reference to Exhibit 10.5 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014).
|
|
|
10.6
|
Series E Preferred Securities Purchase Agreement, dated June 26, 2014, among the Company and the parties named therein (incorporated by reference to Exhibit 10.7 to the Company's registration statement on Form F-1/A (File No. 333-197344), filed with the SEC on July 16, 2014).
|
|
|
10.7
|
Loan Agreement, dated December 30, 2015, between the Company and Kreos Capital V (Expert Fund) Limited (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the SEC on January 4, 2016).
|
|
|
10.8
|
Warrant, dated December 30, 2015, between the Company and Kreos Capital V (Expert Fund) Limited (incorporated by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed with the SEC on January 4, 2016).
|
|
|
10.9
|
Form of indemnification agreement between the Company and each of its directors and executive officers (incorporated by reference to Exhibit 10.11 to the Company's registration statement on Form F-1/A (File No. 333-197344), filed with the SEC on August 20, 2014).
|
|
|
10.10
|
2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.12 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014). **
|
|
|
10.11
|
2012 Israeli Equity Incentive Sub Plan (incorporated by reference to Exhibit 10.13 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014). **
|
|
|
10.12
|
2012 U.S. Equity Incentive Sub Plan (incorporated by reference to Exhibit 10.14 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014). **
|
|
|
10.13
|
2006 Stock Option Plan (incorporated by reference to Exhibit 10.15 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014). **
|
|
|
10.14
|
2014 Equity Incentive Plan (incorporated by reference to Exhibit 10.16 to the Company's registration statement on Form F-1/A (File No. 333-197344), filed with the SEC on August 20, 2014). **
|
|
|
10.15
|
Employment Agreement, dated as of December 17, 2014, between the Company and Kevin Hershberger.**
|
|
|
10.16
|
Executive Employment Agreement, dated as of January 17, 2011, between the Company and Larry Jasinski.**
|
|
|
10.17
|
Employment Agreement, dated of June 18, 2012, between the Company and John Hamilton.**
|
|
|
10.18
|
2014 Incentive Compensation Plan Form of Option Award Agreement.**
|
|
|
10.19
|
2014 Incentive Compensation Plan Form of Restricted Stock Unit Award Agreement for employees and executives.**
|
|
|
10.20
|
2014 Incentive Compensation Plan Form of Restricted Stock Unit Award Agreement for directors.**
|
|
|
10.21
|
ReWalk Robotics Ltd. Compensation Policy for Executive Officers and Non-Executive Directors (incorporated by reference to Exhibit A of Exhibit 99.1 of the Company's Current Report on Form 6-K, furnished to the SEC on November 10, 2014). **
|
|
|
21.1
|
List of subsidiaries of the Company (incorporated by reference to Exhibit 21.1 to the Company's registration statement on Form F-1 (File No. 333-197344), filed with the SEC on July 10, 2014).
|
|
|
23.1
|
Consent of Kost Forer Gabbay & Kasierer.
|
|
|
31.1
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act 2002.
|
|
|
31.2
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act 2002.
|
|
|
32.1
|
Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.***
|
|
|
32.2
|
Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.***
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.PRE
|
XBRL Taxonomy Presentation Linkbase Document
|
|
|
101.CAL
|
XBRL Taxonomy Calculation Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Label Linkbase Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
*
|
Portions of the agreement were omitted and a complete copy of the agreement has been provided separately to the Securities and Exchange Commission pursuant to the Company’s application requesting confidential treatment under Rule 406 of the Securities Act of 1933, as amended.
|
|
**
|
Management contract or compensatory plan, contract or arrangement.
|
|
***
|
Furnished herewith.
|

|
Kost Forer Gabbay & Kasierer
3 Aminadav St.
Tel-Aviv 6706703, Israel
|
Tel: +972-3-6232525
Fax: +972-3-5622555
ey.com
|
|
Tel-Aviv, Israel
|
/s/ KOST FORER GABBAY & KASIERER
|
|
February 29, 2016
|
KOST FORER GABBAY & KASIERER
|
|
|
A Member of Ernst & Young Global
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
ASSETS
|
|
|
|||||
|
CURRENT ASSETS:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
17,869
|
|
$
|
41,829
|
|
|
|
Short-term deposit
|
—
|
|
1,667
|
|
|||
|
Trade receivable, net of allowance for doubtful accounts of $144 and $36, respectively
|
2,146
|
|
1,955
|
|
|||
|
Prepaid expenses and other current assets
|
1,227
|
|
756
|
|
|||
|
Inventories
|
2,534
|
|
777
|
|
|||
|
Total current assets
|
23,776
|
|
46,984
|
|
|||
|
LONG-TERM ASSETS
|
|
|
|
|
|||
|
Other long term assets
|
470
|
|
267
|
|
|||
|
Property and equipment, net
|
1,328
|
|
414
|
|
|||
|
Total long-term assets
|
1,798
|
|
681
|
|
|||
|
Total assets
|
$
|
25,574
|
|
$
|
47,665
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
|
|||||
|
CURRENT LIABILITIES:
|
|
|
|||||
|
Trade payables
|
$
|
2,474
|
|
$
|
1,390
|
|
|
|
Employees and payroll accruals
|
1,221
|
|
872
|
|
|||
|
Deferred revenues and customers advances
|
199
|
|
77
|
|
|||
|
Other current liabilities
|
449
|
|
769
|
|
|||
|
Other liabilities related to settlement of BIRD Foundation grants (see Note 8c)
|
—
|
|
466
|
|
|||
|
Total current liabilities
|
4,343
|
|
3,574
|
|
|||
|
LONG-TERM LIABILITIES
|
|
|
|
|
|||
|
Deferred revenues
|
171
|
|
172
|
|
|||
|
Other long-term liabilities
|
140
|
|
66
|
|
|||
|
Total long-term liabilities
|
311
|
|
238
|
|
|||
|
Total liabilities
|
4,654
|
|
3,812
|
|
|||
|
COMMITMENTS AND CONTINGENT LIABILITIES
|
|
|
|
|
|||
|
Shareholders’ equity:
|
|
|
|
|
|||
|
Share capital
|
|
|
|
|
|||
|
Ordinary share of NIS 0.01 par value-Authorized: 250,000,000 shares at December 31, 2015 and 2014; Issued and outstanding: 12,222,583 and 11,978,554 shares at December 31, 2015 and 2014, respectively
|
33
|
|
32
|
|
|||
|
Additional paid-in capital
|
94,876
|
|
92,395
|
|
|||
|
Accumulated deficit
|
(73,989
|
)
|
(48,574
|
)
|
|||
|
Total shareholders’ equity
|
20,920
|
|
43,853
|
|
|||
|
Total liabilities and shareholders’ equity
|
$
|
25,574
|
|
$
|
47,665
|
|
|
|
|
Year ended
December 31
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Revenues
|
$
|
3,746
|
|
$
|
3,951
|
|
$
|
1,588
|
|
||
|
Cost of revenues
|
3,532
|
|
4,106
|
|
2,017
|
|
|||||
|
Expense related to settlement of BIRD Foundation grants (see Note 8c)
|
—
|
|
466
|
|
—
|
|
|||||
|
Gross profit (loss)
|
214
|
|
(621
|
)
|
(429
|
)
|
|||||
|
Operating expenses:
|
|
|
|
|
|
|
|||||
|
Research and development, net
|
5,937
|
|
8,563
|
|
2,463
|
|
|||||
|
Sales and marketing, net
|
13,056
|
|
7,389
|
|
4,091
|
|
|||||
|
General and administration
|
6,395
|
|
3,352
|
|
1,762
|
|
|||||
|
Total operating expenses
|
25,388
|
|
19,304
|
|
8,316
|
|
|||||
|
Operating loss
|
(25,174
|
)
|
(19,925
|
)
|
(8,745
|
)
|
|||||
|
Financial expenses, net
|
188
|
|
1,698
|
|
3,410
|
|
|||||
|
Loss before income taxes
|
(25,362
|
)
|
(21,623
|
)
|
(12,155
|
)
|
|||||
|
Income taxes
|
53
|
|
45
|
|
22
|
|
|||||
|
Net loss
|
$
|
(25,415
|
)
|
$
|
(21,668
|
)
|
$
|
(12,177
|
)
|
||
|
Net loss per ordinary share, basic and diluted
|
$
|
(2.10
|
)
|
$
|
(6.34
|
)
|
$
|
(74.53
|
)
|
||
|
Weighted average number of shares used in computing net loss per ordinary share, basic and diluted
|
12,115,038
|
|
3,766,694
|
|
185,688
|
|
|||||
|
|
Convertible
Preferred Shares
(1)(3)
|
Ordinary Share
(2)(3)
|
Additional
paid-in
capital
|
Accumulated
deficit
|
Total
shareholders’
equity
(deficiency)
|
||||||||||||||||||
|
|
Number
|
Amount
|
Number
|
Amount
|
|||||||||||||||||||
|
Balance as of January 1, 2013
|
161,718
|
|
*)
|
|
185,688
|
|
*)
|
|
$
|
12,465
|
|
$
|
(14,729
|
)
|
$
|
(2,264
|
)
|
||||||
|
Conversion of convertible loans into Series D convertible preferred share
|
81,677
|
|
*)
|
|
—
|
|
—
|
|
9,896
|
|
—
|
|
9,896
|
|
|||||||||
|
Issuance of Series D convertible preferred share, net of issuance expense in an amount of $204
|
84,008
|
|
*)
|
|
—
|
|
—
|
|
9,961
|
|
—
|
|
9,961
|
|
|||||||||
|
Share-based compensation to employees and non-employees
|
—
|
|
—
|
|
—
|
|
—
|
|
215
|
|
—
|
|
215
|
|
|||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(12,177
|
)
|
(12,177
|
)
|
|||||||||
|
Balance as of December 31, 2013
|
327,403
|
|
*)
|
|
185,688
|
|
*)
|
|
32,537
|
|
(26,906
|
)
|
5,631
|
|
|||||||||
|
Exercise of warrants into Series C Convertible preferred Shares
|
17,705
|
|
*)
|
|
—
|
|
—
|
|
3,825
|
|
—
|
|
3,825
|
|
|||||||||
|
Exercise of warrants into Series D Convertible preferred Shares
|
263
|
|
*)
|
|
—
|
|
—
|
|
57
|
|
—
|
|
57
|
|
|||||||||
|
Issuance of Series D convertible preferred shares
|
4,131
|
|
*)
|
|
—
|
|
—
|
|
1,114
|
|
—
|
|
1,114
|
|
|||||||||
|
Issuance of Series E convertible preferred shares, net of issuance expense in an amount of $212
|
75,695
|
|
*)
|
|
—
|
|
—
|
|
7,895
|
|
—
|
|
7,895
|
|
|||||||||
|
Conversion of convertible preferred shares into ordinary shares
|
(425,197
|
)
|
*)
|
|
7,838,640
|
|
22
|
|
(22
|
)
|
—
|
|
—
|
|
|||||||||
|
Balance as of
Reclassification of liability warrants to equity warrants |
—
|
|
—
|
|
—
|
|
—
|
|
5,555
|
|
—
|
|
5,555
|
|
|||||||||
|
Issuance of ordinary shares in IPO, net
of issuance expenses in an amount of $5,138 |
—
|
|
—
|
|
3,450,000
|
|
9
|
|
36,254
|
|
—
|
|
36,263
|
|
|||||||||
|
Exercise of warrants into ordinary shares
|
—
|
|
—
|
|
157,618
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Share-based compensation to employees and non employees
|
—
|
|
—
|
|
—
|
|
—
|
|
5,179
|
|
—
|
|
5,179
|
|
|||||||||
|
Issuance of ordinary share upon exercise of stock options by employees
|
—
|
|
—
|
|
346,608
|
|
1
|
|
1
|
|
—
|
|
2
|
|
|||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(21,668
|
)
|
(21,668
|
)
|
|||||||||
|
Balance as of December 31, 2014
|
—
|
|
—
|
|
11,978,554
|
|
32
|
|
92,395
|
|
(48,574
|
)
|
43,853
|
|
|||||||||
|
Share-based compensation to employees and non employees
|
—
|
|
—
|
|
—
|
|
—
|
|
2,345
|
|
—
|
|
2,345
|
|
|||||||||
|
Issuance of ordinary share upon exercise of stock options and RSUs by employees and non employees
|
—
|
|
—
|
|
194,345
|
|
1
|
|
136
|
|
—
|
|
137
|
|
|||||||||
|
Cashless exercise of warrants into ordinary shares
|
—
|
|
—
|
|
49,684
|
|
*)
|
|
*)
|
|
—
|
|
—
|
|
|||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(25,415
|
)
|
(25,415
|
)
|
|||||||||
|
Balance as of December 31, 2015
|
—
|
|
—
|
|
12,222,583
|
|
33
|
|
94,876
|
|
(73,989
|
)
|
20,920
|
|
|||||||||
|
*)
|
Represents an amount lower than $1.
|
|
(1)
|
The convertible preferred shares consist of several series, see Note 10b.
|
|
(2)
|
The ordinary shares consist of
two
series, see note 10b.
|
|
(3)
|
All shares amount have been restated to reflect an
18
-for-1 share split, see Note 10a.
|
|
|
Year ended
December 31
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Cash flows from operating activities:
|
|
|
|
||||||||
|
Net loss
|
$
|
(25,415
|
)
|
$
|
(21,668
|
)
|
$
|
(12,177
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation
|
438
|
|
111
|
|
92
|
|
|||||
|
Share-based compensation to employees and non employees
|
2,345
|
|
5,179
|
|
215
|
|
|||||
|
Deferred taxes
|
(61
|
)
|
(60
|
)
|
16
|
|
|||||
|
Financial expenses related to convertible loans
|
—
|
|
—
|
|
2,166
|
|
|||||
|
Revaluation of fair value of warrants to purchase convertible preferred share
|
—
|
|
(776
|
)
|
1,111
|
|
|||||
|
Issuance of Warrants to venture lending
|
—
|
|
835
|
|
—
|
|
|||||
|
Issuance of Warrants to service provider
|
—
|
|
73
|
|
—
|
|
|||||
|
Financial expenses resulted from Issuance of Series D preferred shares to related party
|
—
|
|
1,114
|
|
—
|
|
|||||
|
Changes in assets and liabilities:
|
|
|
|
|
|
|
|||||
|
Trade receivables, net
|
(191
|
)
|
(1,651
|
)
|
(70
|
)
|
|||||
|
Prepaid expenses and other current assets
|
(613
|
)
|
(440
|
)
|
(305
|
)
|
|||||
|
Inventories
|
(2,525
|
)
|
196
|
|
(413
|
)
|
|||||
|
Trade payables
|
1,084
|
|
445
|
|
10
|
|
|||||
|
Employees and payroll accruals
|
349
|
|
308
|
|
269
|
|
|||||
|
Deferred revenues and advances from customers
|
121
|
|
(72
|
)
|
272
|
|
|||||
|
Other liabilities
|
(712
|
)
|
1,105
|
|
2
|
|
|||||
|
Severance pay, net
|
—
|
|
(18
|
)
|
12
|
|
|||||
|
Net cash used in operating activities
|
(25,180
|
)
|
(15,319
|
)
|
(8,800
|
)
|
|||||
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|||||
|
Change in long-term deposits
|
—
|
|
—
|
|
7
|
|
|||||
|
Investment in short-term deposits
|
—
|
|
(10,000
|
)
|
—
|
|
|||||
|
Maturities of short-term deposits
|
1,667
|
|
8,333
|
|
—
|
|
|||||
|
Purchase of property and equipment
|
(584
|
)
|
(169
|
)
|
(187
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
1,083
|
|
(1,836
|
)
|
(180
|
)
|
|||||
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|||||
|
Issuance of convertible loans
|
—
|
|
—
|
|
7,048
|
|
|||||
|
Issuance of ordinary share upon exercise of stock options by employees and non employees
|
137
|
|
2
|
|
—
|
|
|||||
|
Issuance of Series D convertible preferred share, net
|
—
|
|
—
|
|
10,023
|
|
|||||
|
Issuance of Series E convertible preferred shares, including warrants, net
|
—
|
|
12,781
|
|
—
|
|
|||||
|
Exercise of warrants into Series C and D to convertible preferred shares
|
—
|
|
1,078
|
|
—
|
|
|||||
|
REWALK ROBOTICS LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS U.S. dollars in thousands |
|||||||||||
|
Year ended
December 31
|
|||||||||||
|
2015
|
2014
|
2013
|
|||||||||
|
Issuance of ordinary shares in IPO, net of issuance expenses in an amount of $5,138
|
—
|
|
36,263
|
|
—
|
|
|||||
|
Net cash provided by financing activities
|
137
|
|
50,124
|
|
17,071
|
|
|||||
|
Increase (decrease) in cash and cash equivalents
|
(23,960
|
)
|
32,969
|
|
8,091
|
|
|||||
|
Cash and cash equivalents at beginning of period
|
41,829
|
|
8,860
|
|
769
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
17,869
|
|
$
|
41,829
|
|
$
|
8,860
|
|
||
|
Supplemental disclosures of non-cash flow information
|
|
|
|
|
|
|
|||||
|
Conversion of convertible loan into Series D convertible preferred share
|
$
|
—
|
|
$
|
—
|
|
$
|
9,896
|
|
||
|
Warrants to purchase Series D convertible preferred share issued to service provider
|
$
|
—
|
|
$
|
—
|
|
$
|
62
|
|
||
|
Exercise of warrants to purchase preferred shares into Series C and D preferred shares
|
$
|
—
|
|
$
|
2,804
|
|
$
|
—
|
|
||
|
Reclassification of liability warrants to equity warrants
|
$
|
—
|
|
$
|
5,555
|
|
$
|
—
|
|
||
|
Classification of inventory to property and equipment, net
|
$
|
768
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Supplemental disclosures of cash flow information:
|
|||||||||||
|
Cash paid for income taxes
|
$
|
163
|
|
$
|
—
|
|
$
|
123
|
|
||
|
a.
|
ReWalk Robotics Ltd. (“RRL”, and together with its subsidiaries, the “Company”) was incorporated under the laws of the State of Israel on June 20, 2001 and commenced operations on the same date.
|
|
b.
|
RRL has
two
wholly-owned subsidiaries: (i) ReWalk Robotics Inc. (“RRI”) incorporated under the laws of Delaware on February 15, 2012 and (ii) Argo Medical Technologies GmbH (“AMG”) incorporated under the laws of Germany on January 14, 2013.
|
|
c.
|
The Company is designing, developing and commercializing the ReWalk system, an innovative exoskeleton that allow wheelchair-bound persons with mobility impairments or other medical conditions to stand and walk once again. The ReWalk system consists of a light wearable brace support suit which integrates motors at the joints, rechargeable batteries, an array of sensors and a computer-based control system to power knee and hip movement. There are currently
two
types of products: ReWalk Personal and ReWalk Rehabilitation. ReWalk Personal is designed for everyday use by individuals at home and in their communities, and is custom fitted for each user. ReWalk Rehabilitation is designed for the clinical rehabilitation environment where it provides valuable exercise and therapy. It also enables individuals to evaluate their capacity for using ReWalk Personal system in the future.
|
|
d.
|
The Company markets and sells its products directly to institutions and individuals and through third-party distributors. The Company sells its products directly primarily in Germany and the United States, and primarily through distributors in other markets. In its direct markets, the Company has established relationships with rehabilitation centers and the spinal cord injury community, and in its indirect markets, the Company’s distributors maintain these relationships. RRI markets and sells products mainly in the United States and Canada. AMG sell the Company’s products mainly in Germany and Europe.
|
|
e.
|
In September 2014, the Company completed its Initial Public Offering ("IPO") in which the Company issued and sold
|
|
f.
|
The Company depends on
one
contract manufacturer. Reliance on this vendor makes the Company vulnerable to possible capacity constraints and reduced control over component availability, delivery schedules, manufacturing yields and costs. This vendor account for
24%
and
12%
of the Company's total trade payables as of
December 31, 2015
and
2014
, respectively.
|
|
g.
|
The Company has incurred losses in the amount of
$25,415
during the year ended
December 31, 2015
. The Company has an accumulated deficit in the total amount of
$73,989
as of
December 31, 2015
and negative cash flow from operating activity is in the amount of
$25,180
for the year then ended. The Company has sufficient funds to support its operations in
2016
. See note 7 regarding loan received in January 2016.
|
|
a.
|
Use of Estimates
|
|
b.
|
Financial Statements in U.S. Dollars:
|
|
c.
|
Principles of Consolidation:
|
|
d.
|
Cash Equivalents:
|
|
e.
|
Short term deposits:
|
|
f.
|
Inventories:
|
|
g.
|
Related party:
|
|
h.
|
Property and Equipment:
|
|
|
%
|
|
Computer equipment
|
20-33 (mainly 33)
|
|
Office furniture and equipment
|
6 - 10 (mainly 10)
|
|
Machinery and laboratory equipment
|
15
|
|
Field service units
|
50
|
|
Leasehold improvements
|
Over the shorter of the lease
term or estimated useful life |
|
i.
|
Impairment of Long-Lived Assets:
|
|
j.
|
Other long term assets:
|
|
k.
|
Revenue Recognition:
|
|
l.
|
Accounting for Share-Based Compensation:
|
|
|
December 31,
|
||||
|
|
2015
|
2014
|
2013
|
||
|
Expected volatility
|
60%
|
60%-70%
|
70%-75%
|
||
|
Risk-free rate
|
1.60%-1.95%
|
1.74%-1.95%
|
0.95%-2.08%
|
||
|
Dividend yield
|
—%
|
—%
|
—%
|
||
|
Expected term (in years)
|
5.73 - 6.11
|
5.81 - 6.11
|
6.02 - 6.08
|
||
|
Share price
|
$7.30 - $20.97
|
$1.49 - $20.77
|
$3.62 - $5.80
|
||
|
m.
|
Research and Development Costs:
|
|
n.
|
Income Taxes
|
|
o.
|
Warranty:
|
|
p.
|
Concentrations of Credit Risks:
|
|
q.
|
Accrued Severance Pay:
|
|
r.
|
Fair Value Measurements:
|
|
•
|
Level 1.
Observable inputs based on unadjusted quoted prices in active markets for identical assets or liabilities;
|
|
•
|
Level 2.
Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and
|
|
•
|
Level 3.
Unobservable inputs for which there is little or no market data requiring the Company to develop its own assumptions.
|
|
s.
|
Basic and Diluted Net Loss Per Share:
|
|
|
Year ended
December 31
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Net loss
|
$
|
(25,415
|
)
|
$
|
(21,668
|
)
|
$
|
(12,177
|
)
|
||
|
Convertible preferred shares dividend
|
—
|
|
(2,229
|
)
|
(1,663
|
)
|
|||||
|
Net loss attributable to ordinary shares
|
(25,415
|
)
|
(23,897
|
)
|
(13,840
|
)
|
|||||
|
Shares used in computing net loss per ordinary shares, basic and diluted
|
12,115,038
|
|
3,766,694
|
|
185,688
|
|
|||||
|
Net loss per ordinary share, basic and diluted
|
$
|
(2.10
|
)
|
$
|
(6.34
|
)
|
$
|
(74.53
|
)
|
||
|
t.
|
Contingent liabilities
|
|
u.
|
Government grants
|
|
v.
|
New Accounting Pronouncements
|
|
i.
|
Revenue recognition:
|
|
ii.
|
Going Concern:
|
|
iii.
|
Inventory:
|
|
iv.
|
Deferred tax:
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Government institutions
|
$
|
209
|
|
$
|
298
|
|
|
|
Prepaid expenses
|
316
|
|
227
|
|
|||
|
Deposit
|
43
|
|
56
|
|
|||
|
Deferred tax
|
147
|
|
86
|
|
|||
|
Other assets
|
512
|
|
89
|
|
|||
|
|
$
|
1,227
|
|
$
|
756
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Raw materials
|
$
|
450
|
|
$
|
41
|
|
|
|
Finished products
|
2,084
|
|
736
|
|
|||
|
|
$
|
2,534
|
|
$
|
777
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Cost:
|
|
|
|||||
|
Computer equipment
|
$
|
656
|
|
$
|
338
|
|
|
|
Office furniture and equipment
|
286
|
|
144
|
|
|||
|
Machinery and laboratory equipment
|
471
|
|
235
|
|
|||
|
Field service units
|
575
|
|
—
|
|
|||
|
Leasehold improvements
|
113
|
|
32
|
|
|||
|
|
2,101
|
|
749
|
|
|||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Accumulated depreciation
|
773
|
|
335
|
|
|||
|
Property and equipment, net
|
$
|
1,328
|
|
$
|
414
|
|
|
|
|
December 30, 2015
|
|
|
Expected volatility
|
60
|
%
|
|
Risk-free rate
|
2.52
|
%
|
|
Dividend yield
|
—
|
%
|
|
Expected term (in years)
|
10
|
|
|
a.
|
Purchase commitment
|
|
b.
|
Lease commitment: the Company operates from leased facilities in Israel, the Unites States and Germany. These leases expire between 2016 and 2025.
|
|
2016
|
322
|
|
|
|
2017
|
474
|
|
|
|
2018
|
498
|
|
|
|
2019
|
507
|
|
|
|
2020 and Thereafter
|
1,792
|
|
|
|
Total
|
$
|
3,593
|
|
|
c.
|
Royalties:
|
|
e.
|
Legal Claims:
|
|
|
September 17, 2014
(conversion date)
|
December 31, 2013
|
|||
|
Expected volatility
|
70
|
%
|
70
|
%
|
|
|
Risk-free rate
|
0.1
|
%
|
0.1
|
%
|
|
|
Dividend yield
|
—
|
%
|
—
|
%
|
|
|
Expected term (in years)
|
0
|
|
1.25
|
|
|
|
|
September 11,
2014 (IPO date)
|
December 31,
2013 |
September 24,
2013
(issuance date)
|
|||||
|
Expected volatility
|
70
|
%
|
70
|
%
|
70
|
%
|
||
|
Risk-free rate
|
1.7
|
%
|
0.1
|
%
|
0.2
|
%
|
||
|
Dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Expected term (in years)
|
4.80
|
|
1.25
|
|
1.50
|
|
||
|
|
September 11,
2014
(IPO date)
|
June 26, 2014
(issuance date)
|
|||
|
Expected volatility
|
70
|
%
|
70
|
%
|
|
|
Risk-free rate
|
1.4
|
%
|
0.1
|
%
|
|
|
Dividend yield
|
—
|
%
|
—
|
%
|
|
|
Expected term (in years)
|
3.80
|
|
4.00
|
|
|
|
|
Balance at
beginning of
period
|
Issuance of
warrants to
purchase
preferred
share
|
Exercise of
warrants to
purchase
preferred
share
|
Change in fair
value
|
Conversion to Warrants to purchase ordinary
share following IPO
|
Balance at
end of
period
|
|||||||||||||||||
|
December 31, 2014
|
$
|
3,341
|
|
$
|
5,794
|
|
$
|
(2,804
|
)
|
$
|
(776
|
)
|
$
|
(5,555
|
)
|
|
|
||||||
|
December 31, 2013
|
$
|
2,168
|
|
$
|
62
|
|
$
|
—
|
|
$
|
1,111
|
|
$
|
—
|
|
$
|
3,341
|
|
|||||
|
a.
|
All ordinary shares, options, exercise prices and loss per share amounts have been adjusted retroactively for all periods presented in these financial statements, to reflect the
17
-to-one bonus share issuance (equivalent to an
18
-for-1 share split) effected on August 26, 2014.
|
|
b.
|
Composition of convertible preferred share capital and ordinary shares:
|
|
|
Authorized
|
Issued and outstanding
|
|||||||||||||||
|
|
December 31,
|
December 31,
|
|||||||||||||||
|
|
2015
|
2014
|
2013
|
2015
|
2014
|
2013
|
|||||||||||
|
|
Number of shares
|
||||||||||||||||
|
Preferred shares of NIS 0.01 par value:
|
|
|
|
|
|
|
|
|
|||||||||
|
Series A preferred shares
|
—
|
|
—
|
|
11,000
|
|
—
|
|
—
|
|
10,677
|
|
|||||
|
Series B preferred shares
|
—
|
|
—
|
|
100,000
|
|
—
|
|
—
|
|
63,880
|
|
|||||
|
Series C-1 preferred shares
|
—
|
|
—
|
|
200,000
|
|
—
|
|
—
|
|
67,486
|
|
|||||
|
Series C-2 preferred shares
|
—
|
|
—
|
|
40,000
|
|
—
|
|
—
|
|
19,675
|
|
|||||
|
Series D-1 preferred shares
|
—
|
|
—
|
|
100,000
|
|
—
|
|
—
|
|
84,008
|
|
|||||
|
Series D-2 preferred shares
|
—
|
|
—
|
|
69,387
|
|
—
|
|
—
|
|
69,387
|
|
|||||
|
Series D-3 preferred shares
|
—
|
|
—
|
|
10,323
|
|
—
|
|
—
|
|
10,323
|
|
|||||
|
Series D-4 preferred shares
|
—
|
|
—
|
|
1,967
|
|
—
|
|
—
|
|
1,967
|
|
|||||
|
Series E preferred shares
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Total preferred shares
|
—
|
|
—
|
|
532,677
|
|
—
|
|
—
|
|
327,403
|
|
|||||
|
Ordinary shares of NIS 0.01 par value:
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Ordinary shares
|
250,000,000
|
|
250,000,000
|
|
—
|
|
12,222,583
|
|
11,978,554
|
|
—
|
|
|||||
|
Ordinary A shares
|
—
|
|
—
|
|
168,613,056
|
|
—
|
|
—
|
|
180,000
|
|
|||||
|
Ordinary B non-voting shares
|
—
|
|
—
|
|
1,800,000
|
|
—
|
|
—
|
|
5,688
|
|
|||||
|
Total ordinary shares
|
250,000,000
|
|
250,000,000
|
|
170,413,056
|
|
12,222,583
|
|
11,978,554
|
|
185,688
|
|
|||||
|
c.
|
Initial Public Offering:
|
|
d.
|
1. Ordinary shares:
|
|
e.
|
Preferred Share purchase agreements:
|
|
f.
|
Share option plans:
|
|
|
Year Ended December 31, 2015
|
||||||||||
|
|
Number
|
Average
exercise
price
|
Average
remaining
contractual
life (years) (1)
|
Aggregate
intrinsic
value (in
thousands)
|
|||||||
|
Options and RSUs outstanding at the beginning of the year
|
1,350,846
|
|
3.80
|
|
8.27
|
20,373
|
|
||||
|
Options granted
|
709,105
|
|
9.26
|
|
|
|
|
||||
|
RSUs granted
|
43,466
|
|
—
|
|
|
|
|
||||
|
Options exercised
|
(158,101
|
)
|
$
|
0.96
|
|
||||||
|
RSUs vested
|
(19,586
|
)
|
$
|
—
|
|
||||||
|
RSUs forfeited
|
(13,970
|
)
|
$
|
—
|
|
|
|
|
|||
|
Options forfeited
|
(58,391
|
)
|
$
|
3.37
|
|
|
|
|
|||
|
Options and RSUs outstanding at the end of the year
|
1,853,369
|
|
6.12
|
|
8.37
|
17,048
|
|
||||
|
Options and RSUs vested and expected to vest
|
1,807,787
|
|
6.09
|
|
8.33
|
16,699
|
|
||||
|
Options exercisable at the end of the year
|
649,156
|
|
2.65
|
|
7.01
|
7,961
|
|
||||
|
(1)
|
Calculation of weighted average remaining contractual term does not include the RSUs that were granted, which have an indefinite contractual term.
|
|
Range of exercise price
|
Options Outstanding as of December 31, 2015
|
Weighted
average
remaining
contractual
life (years) (1)
|
Options exercisable as of December 31, 2015
|
Weighted
average
remaining
contractual
life (years) (1)
|
||||||
|
—(including options and RSUs)
|
95,599
|
|
3.95
|
7,338
|
|
3.95
|
||||
|
$0.82
|
34,377
|
|
5.04
|
34,377
|
|
5.04
|
||||
|
$1.32
|
400,990
|
|
6.46
|
356,166
|
|
6.42
|
||||
|
$1.48
|
441,412
|
|
8.03
|
207,155
|
|
8.02
|
||||
|
$7.30- $8.99
|
620,027
|
|
9.82
|
—
|
|
0.00
|
||||
|
$19.62-$20.97
|
260,964
|
|
8.98
|
44,120
|
|
8.96
|
||||
|
|
1,853,369
|
|
8.37
|
649,156
|
|
7.01
|
||||
|
(1)
|
Calculation of weighted average remaining contractual term does not include the RSUs that were granted, which have an indefinite contractual term.
|
|
|
g.
|
Options issued to consultants:
|
|
Issuance date
|
Options for
shares of
ordinary
share
|
Exercise
price
per share
|
Options
exercisable
|
Exercisable
through
|
|||||||
|
|
(number)
|
|
(number)
|
|
|||||||
|
March 12, 2007
|
3,454
|
|
$
|
—
|
|
3,454
|
|
March 12, 2017
|
|||
|
|
h.
|
Warrants to purchase ordinary shares
|
|
Issuance date
|
Warrants outstanding
|
Exercise
price per warrant |
Warrants
exercisable |
Contractual term
|
|||||||
|
|
(number)
|
|
(number)
|
|
|||||||
|
July 14, 2014
|
542,506
|
|
$
|
10.08
|
|
542,506
|
|
July 13, 2018
|
|||
|
December 30, 2015
|
119,295
|
|
$
|
9.64
|
|
119,295
|
|
The earlier of: (i) December 30, 2025 (ii) a merger, consolidation, or reorganization of the Company.
|
|||
|
661,801
|
|
661,801
|
|
||||||||
|
|
i.
|
Share-based compensation expense for employees and non-employees:
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Cost of revenues
|
$
|
64
|
|
$
|
33
|
|
$
|
5
|
|
||
|
Research and development, net
|
425
|
|
4,364
|
|
73
|
|
|||||
|
Sales and marketing, net
|
571
|
|
275
|
|
42
|
|
|||||
|
General and administrative
|
1,285
|
|
507
|
|
95
|
|
|||||
|
Total
|
$
|
2,345
|
|
$
|
5,179
|
|
$
|
215
|
|
||
|
Issuance with respect to
|
Warrants
to purchase
|
Issuance
date
|
Number
of
warrants
|
Exercise
price
|
Contractual term
|
||||||||
|
Series C Transaction
|
Preferred C-1
|
8/2/2011
|
7,615
|
|
$
|
105.815
|
|
The earlier of: (i) a merger (ii) the consummation of an initial public offering and (iii) 3 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
Series C Transaction
|
Preferred C-1
|
1/31/2012
|
7,231
|
|
$
|
105.815
|
|
The earlier of: (i) a merger (ii) the consummation of an initial public offering and (iii) 3 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
Series C Transaction
|
Preferred C-1
|
5/10/2012
|
4,252
|
|
$
|
105.815
|
|
The earlier of: (i) a merger (ii) the consummation of an initial public offering and (iii) 3 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
Series C Transaction
|
Preferred C-1
|
8/20/2012
|
5,870
|
|
$
|
105.815
|
|
The earlier of: (i) a merger (ii) the consummation of an initial public offering and (iii) 3 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
Fee agreement with a service provider
|
Preferred D
|
9/24/2013
|
600
|
|
$
|
121
|
|
The earlier of: (i) a merger (ii) the consummation of an initial public offering and (iii) 5 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
Venture loan from Kreos
|
Preferred D
|
6/19/2014
|
5,372
|
|
$
|
206.09
|
|
The earlier of: (i) a merger (ii) 5 years from the consummation of an initial public offering and (iii) 10 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
Series E Transaction
(refer to Note 10d) |
Preferred E
|
6/26/2014
|
37,850
|
|
$
|
206.09
|
|
4 years from the issuance date (all as stipulated in the specific warrant agreement)
|
|||||
|
a.
|
In July 26, 2011, the Company signed a series C convertible preferred share purchase agreement (the “Series C Transaction”) with the Company’s shareholders and new investors (collectively the “Investors”), pursuant to which the Company issued to each of the Investors an aggregate amount of
67,486
convertible preferred C-1 shares, at a price per share of
$105.815
totaling to approximately
$7.1 million
.
|
|
b.
|
On May 30, 2013, the Company entered into a fee agreement with one of its service providers, pursuant to which the Company granted the service provider warrants to purchase shares equal to
5%
of any shares issued upon cash receipt from an external investor identified by the service provider. As part of the Series D Transaction, on September 30, 2013 the service provider was granted warrants to purchase
600
Series D convertible preferred share. The Company accounted for the warrants to purchase Series D convertible preferred shares under ASC 505 and recorded the warrants at fair value as issuance expense, which was determined to be
$62
as of September 24, 2013 (their issuance date), and classified as a liability in the Company’s financial statement.
|
|
c.
|
On June 19, 2014, the Company entered into a loan agreement with Kreos pursuant to which Kreos agreed to grant a line of credit to the Company of
$5.0 million
. The line of credit was available for drawdown until September 30, 2014, with a minimum required drawdown of
$1.0 million
. In October 2014, the Company extended the line of credit until December 31, 2015. Amounts drawn were required to be repaid in
36
monthly installments. The Company did not draw down any funds under this line of credit. Pursuant to the loan agreement, the Company was required to pay a transaction fee of
1.0%
of the total amount of the line of credit upon both the execution and the expiration of the loan agreement. In the year ended
December 31, 2014
the Company paid and accrued, as part of the Company's financial expenses, a transaction fee expenses an amount of
$100
. Pursuant to the loan agreement, the Company granted to Kreos a security interest over all of the Company’s assets, including intellectual property and equity interests in its subsidiaries.
|
|
d.
|
On June 26, 2014, the Company entered into the series E SPA with Gabriel and the other parties named therein (the “Series E SPA”). The transaction closed in July 2014. Pursuant to the Series E SPA, the Company issued warrants to purchase an aggregate of
37,850
preferred E Shares (which, upon the consummation of the IPO, converted on an
18
-for-
1
basis into warrants to purchase the Company’s ordinary shares) to Gabriel and the other investors named in the Series E SPA. The warrants have an exercise price of
$206.09
per share and are exercisable until
four
years from date of grant, subject to certain adjustments.
|
|
e.
|
As of
December 31, 2013
, the fair value of the warrants to purchase convertible preferred shares was
$3,341
. Following the closing of the IPO on September 17, 2014, all outstanding warrants to purchase convertible preferred stock were converted into warrants to purchase ordinary shares which resulted in classification of the remaining warrants liability to additional paid-in capital in the amount of
$5,555
.
|
|
a.
|
Corporate tax rates in Israel:
|
|
b.
|
Profit (loss) before taxes on income is comprised as follows:
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Domestic
|
$
|
(25,485
|
)
|
$
|
(21,743
|
)
|
$
|
(12,219
|
)
|
||
|
Foreign
|
123
|
|
120
|
|
64
|
|
|||||
|
|
$
|
(25,362
|
)
|
$
|
(21,623
|
)
|
$
|
(12,155
|
)
|
||
|
c.
|
Taxes on income are comprised as follows:
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Current
|
$
|
114
|
|
$
|
76
|
|
$
|
6
|
|
||
|
Deferred
|
(61
|
)
|
(60
|
)
|
16
|
|
|||||
|
Prior year taxes
|
—
|
|
29
|
|
—
|
|
|||||
|
|
$
|
53
|
|
$
|
45
|
|
$
|
22
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Domestic
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Foreign
|
53
|
|
45
|
|
22
|
|
|||||
|
|
$
|
53
|
|
$
|
45
|
|
$
|
22
|
|
||
|
d.
|
Deferred income taxes:
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Carry forward tax losses
|
$
|
14,961
|
|
$
|
9,152
|
|
|
|
Research and Development carry forward expenses-temporary differences
|
1,176
|
|
870
|
|
|||
|
Accrual and reserves
|
206
|
|
153
|
|
|||
|
Deferred tax assets before valuation allowance
|
16,343
|
|
10,175
|
|
|||
|
Valuation allowance
|
(16,196
|
)
|
(10,089
|
)
|
|||
|
Net deferred tax assets
|
$
|
147
|
|
$
|
86
|
|
|
|
e.
|
Reconciliation of the theoretical tax expenses:
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Loss before taxes, as reported in the consolidated statements of operations
|
$
|
(25,362
|
)
|
$
|
(21,623
|
)
|
$
|
(12,155
|
)
|
||
|
Statutory tax rate
|
26.5
|
%
|
26.5
|
%
|
25.0
|
%
|
|||||
|
Theoretical tax benefits on the above amount at the Israeli statutory tax rate
|
$
|
(6,721
|
)
|
$
|
(5,730
|
)
|
$
|
(3,039
|
)
|
||
|
Income tax at rate other than the Israeli statutory tax rate
|
16
|
|
12
|
|
7
|
|
|||||
|
Non-deductible expenses including equity based compensation expenses and other
|
651
|
|
1,864
|
|
895
|
|
|||||
|
Operating losses and other temporary differences for which valuation allowance was provided
|
6,107
|
|
3,870
|
|
2,159
|
|
|||||
|
Taxes in respect of prior years
|
—
|
|
29
|
|
—
|
|
|||||
|
Actual tax expense
|
$
|
53
|
|
$
|
45
|
|
$
|
22
|
|
||
|
f.
|
Foreign tax rates:
|
|
g.
|
Tax benefits under the Law for the Encouragement of Capital Investments, 1959 (the “Investment Law”):
|
|
h.
|
Tax assessments:
|
|
i.
|
Net operating carry-forward losses for tax purposes:
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Financial expenses related to convertible loans
|
$
|
—
|
|
$
|
—
|
|
2,166
|
|
|||
|
Issuance of warrants to purchase convertible preferred share
|
—
|
|
835
|
|
—
|
|
|||||
|
Revaluation of fair value of warrants to purchase convertible preferred shares
|
—
|
|
(776
|
)
|
1,111
|
|
|||||
|
Issuance of convertible preferred shares
|
—
|
|
1,114
|
|
—
|
|
|||||
|
Foreign currency transactions and other
|
170
|
|
200
|
|
93
|
|
|||||
|
Financial expenses related to loan agreement with Kreos
|
—
|
|
100
|
|
—
|
|
|||||
|
Issuance cost related to warrants liability
|
—
|
|
128
|
|
—
|
|
|||||
|
Bank commissions
|
36
|
|
36
|
|
40
|
|
|||||
|
(Income) Expenses related to hedging transactions
|
(18
|
)
|
61
|
|
—
|
|
|||||
|
|
$
|
188
|
|
$
|
1,698
|
|
$
|
3,410
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Revenues based on customer’s location:
|
|
|
|
||||||||
|
Israel
|
$
|
—
|
|
$
|
—
|
|
$
|
83
|
|
||
|
United States
|
2,439
|
|
2,186
|
|
941
|
|
|||||
|
Europe
|
820
|
|
1,254
|
|
476
|
|
|||||
|
Asia-Pacific
|
487
|
|
511
|
|
88
|
|
|||||
|
Total revenues
|
$
|
3,746
|
|
$
|
3,951
|
|
$
|
1,588
|
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Long-lived assets by geographic region:
|
|
|
|||||
|
Israel
|
$
|
605
|
|
$
|
279
|
|
|
|
United States
|
483
|
|
88
|
|
|||
|
Germany
|
240
|
|
47
|
|
|||
|
|
$
|
1,328
|
|
$
|
414
|
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2015
|
2014
|
2013
|
||||
|
Customer A
|
14.8
|
%
|
15
|
%
|
*)
|
||