|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
13-3757370
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
358 South Main Street,
|
|
|
Burlington, North Carolina
|
27215
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of each class
|
|
Name of exchange on which registered
|
|
Common Stock, $0.10 par value
|
|
New York Stock Exchange
|
|
Large accelerated filer [X]
|
Accelerated filer [ ]
|
|
Non-accelerated filer [ ] (Do not check if a smaller reporting company)
|
Smaller reporting company [ ]
|
|
Emerging growth company [ ]
|
|
|
|
|
Page
|
|
|
|
|
|
Item 1.
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
|
|
|
|
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
|
|
|
|
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
|
|
|
|
|
|
|
Item 15.
|
||
|
Item 16.
|
||
|
Item 1.
|
BUSINESS
|
|
•
|
Quality, timeliness and consistency in reporting test results;
|
|
•
|
Reputation of the laboratory in the medical community or field of specialty;
|
|
•
|
Contractual relationships with MCOs;
|
|
•
|
Service capability and convenience;
|
|
•
|
Number and type of tests performed;
|
|
•
|
Connectivity solutions offered; and
|
|
•
|
Pricing of the laboratory’s services.
|

|
•
|
A physician portal optimized for web and mobile devices;
|
|
•
|
Express electronic ordering for essentially all of LCD's brands and services;
|
|
•
|
Integrated results viewing and enhanced reports;
|
|
•
|
Lab analytics that provide one-click trending of patient, test and population data;
|
|
•
|
CDS tools at the point of testing and resulting;
|
|
•
|
AccuDraw, which provides graphical, step-by-step guidance to help improve accuracy, workflow and turnaround time in the collection and processing of specimens at the point of collection; and
|
|
•
|
Services-oriented architecture with rules-based engines, content aggregation and seamless integration with practice workflow.
|
|
•
|
A patient portal optimized for web and market-leading mobile devices;
|
|
•
|
Integrated results viewing and patient education materials;
|
|
•
|
Online appointment scheduling and bill payment;
|
|
•
|
An online patient cost estimator for select genetic tests; and
|
|
•
|
An option to receive information about clinical trials.
|
|
•
|
Ability to manage large-scale clinical trials both domestically and internationally, including the recruitment of appropriate and sufficient clinical-trial subjects; and
|
|
•
|
Xcellerate Forecasting & Site Selection enables customers to identify the optimal sites and investigators by drawing on the world’s largest proprietary clinical trial knowledge base.
|
|
•
|
Xcellerate Clinical Trial Management provides the foundational operating systems to enable frictionless execution of clinical trials.
|
|
•
|
Xcellerate Monitoring enables customers to improve data quality, clinical trial subject safety and protocol compliance in the execution of clinical trials by proactively identifying and mitigating risks at the study site and clinical trial subject level.
|
|
•
|
Xcellerate Insights enables effective operational oversight by providing interactive, up-to-date views of a broad range of operational metrics and key performance indicators at the study and portfolio levels through a secure collaboration portal.
|
|
•
|
endpoint’s proprietary PULSE
®
platform is made up of pre-validated, configurable study components that enable rapid development and quicker modification to a customer’s existing IRT system. PULSE can help to streamline complex trial randomization methods, improve drug supply management, and simplify site, study, and subject management. The fully digital, mobile-ready system allows access to patient data and outcomes in real time.
|
|
•
|
endpoint’s DRIVE platform provides visibility into supplies management for an entire clinical development portfolio. This enables automated supply functionality to help minimize costs, reduce waste, and manage regulatory compliance across multiple trial sites.
|
|
▪
|
Regional Testing Facility, Raritan, New Jersey - January 2017
|
|
▪
|
Regional Testing Facility, Knoxville, Tennessee - November 2016
|
|
▪
|
Regional Testing Facility, San Antonio, Texas - July 2016
|
|
▪
|
Colorado Coagulation, Denver, Colorado - January 2016
|
|
•
|
Dynacare, Laval, Québec - March 2015
|
|
•
|
Regional Testing Facility, Dublin, Ohio - March 2015
|
|
•
|
Endocrine Sciences, Calabasas, California - January 2015
|
|
•
|
Regional Testing Facility, Dallas, Texas - April 2014
|
|
•
|
Regional Testing Facility, Denver, Colorado - March 2014
|
|
•
|
Integrated Genetics, Santa Fe, New Mexico - October 2013
|
|
•
|
Integrated Genetics, Westborough, Massachusetts - September 2013
|
|
•
|
Dynacare, Montreal, Québec - June 2013
|
|
•
|
Regional Testing Facility, Phoenix, Arizona - April 2013
|
|
•
|
Regional Testing Facility, Birmingham, Alabama - February 2013
|
|
•
|
Integrated Oncology, Brentwood, Tennessee - February 2012
|
|
•
|
ViroMed, Burlington, North Carolina - January 2012
|
|
•
|
Center for Molecular Biology and Pathology (CMBP), Research Triangle Park, North Carolina - February 2011
|
|
•
|
Regional Testing Facility, Tampa, Florida - January 2010
|
|
•
|
Integrated Oncology, Phoenix, Arizona - September 2009
|
|
•
|
Covance Central Laboratory Services Inc., Indianapolis, Indiana - August 2015
|
|
•
|
BML Covance Central Laboratory, Tokyo, Japan - March 2015 (Operated for CDD pursuant to a strategic agreement with BML, Inc.)
|
|
•
|
Covance Pharmaceutical Research and Development (Shanghai) Co. Ltd., Shanghai, China - March 2015
|
|
•
|
Covance (Asia) Pte. Ltd., Singapore - June 2014
|
|
•
|
Covance Central Laboratory Services SARL, Geneva, Switzerland - October 2013
|
|
•
|
The circumstances under which the use and disclosure of PHI are permitted or required without a specific authorization by the patient, including, but not limited to, treatment purposes, activities to obtain payments for the Company’s services, and its healthcare operations activities;
|
|
•
|
A patient’s rights to access, amend and receive an accounting of certain disclosures of PHI;
|
|
•
|
The content of notices of privacy practices for PHI;
|
|
•
|
Administrative, technical and physical safeguards required of entities that use or receive PHI; and
|
|
•
|
The protection of computing systems maintaining electronic PHI.
|
|
•
|
Failure of products to satisfy safety requirements;
|
|
•
|
Unexpected or undesired results of the products;
|
|
•
|
Insufficient clinical trial subject enrollment;
|
|
•
|
Insufficient investigator recruitment;
|
|
•
|
A customer's decision to terminate the development of a product or to end a particular study; and
|
|
•
|
CDD’s failure to perform its duties properly under the contract.
|
|
•
|
Errors or omissions that create harm to clinical trial subjects during a trial or to consumers of a drug after the trial is completed and regulatory approval of the drug has been granted;
|
|
•
|
General risks associated with clinical pharmacology facilities, including negative consequences from the administration of drugs to clinical trial participants or the professional malpractice of clinical pharmacology physicians;
|
|
•
|
Risks that animals in CDD’s breeding facilities may be infected with diseases that may be harmful and even lethal to themselves and humans despite preventive measures contained in CDD's business policies, including those for the quarantine and handling of imported animals; and
|
|
•
|
Errors and omissions during a trial that may undermine the usefulness of a trial or data from the trial or study or may delay the entry of a drug to the market.
|
|
•
|
Changes in the general global economy;
|
|
•
|
Exchange rate fluctuations;
|
|
•
|
The commencement, completion, delay or cancellation of large projects or groups of projects;
|
|
•
|
The progress of ongoing projects;
|
|
•
|
The timing of and charges associated with completed acquisitions or other events; and
|
|
•
|
Changes in the mix of the Company's services.
|
|
•
|
Failure to obtain regulatory clearance, including due to antitrust concerns;
|
|
•
|
Loss of key customers or employees;
|
|
•
|
Difficulty in consolidating redundant facilities and infrastructure and in standardizing information and other systems;
|
|
•
|
Unidentified regulatory problems;
|
|
•
|
Failure to maintain the quality of services that such companies have historically provided;
|
|
•
|
Coordination of geographically separated facilities and workforces; and
|
|
•
|
Diversion of management's attention from the day-to-day business of the Company.
|
|
Location
|
Nature of Occupancy
|
|
Primary Facilities:
|
|
|
Birmingham, Alabama
|
Leased
|
|
Phoenix, Arizona
|
Owned
|
|
Prescott, Arizona
|
Leased
|
|
Calabasas, California
|
Leased
|
|
Los Angeles, California
|
Leased
|
|
Monrovia, California
|
Leased
|
|
San Diego, California
|
Leased
|
|
San Francisco, California
|
Leased
|
|
Tustin, California
|
Leased
|
|
Englewood, Colorado
|
Leased
|
|
Shelton, Connecticut
|
Leased
|
|
Hollywood, Florida
|
Leased
|
|
Tampa, Florida
|
Leased
|
|
Tucker, Georgia
|
Leased
|
|
Chicago, Illinois
|
Leased
|
|
Itasca, Illinois
|
Leased
|
|
Lenexa, Kansas
|
Leased
|
|
Louisville, Kentucky
|
Leased
|
|
Lafayette, Louisiana
|
Owned
|
|
Westborough, Massachusetts
|
Leased
|
|
Battle Creek, Michigan
|
Owned
|
|
Roseville, Minnesota
|
Leased
|
|
St. Paul, Minnesota
|
Owned
|
|
Kansas City, Missouri
|
Owned
|
|
Ewing, New Jersey
|
Leased
|
|
Raritan, New Jersey
|
Owned
|
|
Santa Fe, New Mexico
|
Owned
|
|
New York, New York
|
Leased
|
|
Burlington, North Carolina (5)
|
Owned/Leased
|
|
Charlotte, North Carolina
|
Leased
|
|
Greensboro, North Carolina
|
Leased
|
|
McLeansville, North Carolina
|
Leased
|
|
Raleigh, North Carolina
|
Leased
|
|
Research Triangle Park, North Carolina (3)
|
Leased
|
|
Dublin, Ohio
|
Owned
|
|
Oklahoma City, Oklahoma
|
Leased
|
|
Brentwood, Tennessee
|
Leased
|
|
Knoxville, Tennessee
|
Leased
|
|
Austin, Texas
|
Leased
|
|
Dallas, Texas
|
Leased
|
|
Houston, Texas
|
Leased
|
|
San Antonio, Texas
|
Leased
|
|
Chesapeake, Virginia
|
Leased
|
|
Herndon, Virginia
|
Leased
|
|
Lorton, Virginia
|
Leased
|
|
Seattle, Washington
|
Leased
|
|
Spokane, Washington (2)
|
Leased
|
|
Charleston, West Virginia
|
Leased
|
|
Abingdon, United Kingdom
|
Leased
|
|
Location
|
Nature of Occupancy
|
|
Primary Facilities:
|
|
|
Mechelen, Belgium
|
Leased
|
|
Beijing, China (2)
|
Leased
|
|
Shanghai, China (3)
|
Owned/Leased
|
|
Muenster, Germany
|
Owned
|
|
Bangalore, India
|
Leased
|
|
Singapore
|
Leased
|
|
Harrogate, United Kingdom
|
Owned
|
|
Leeds, United Kingdom
|
Owned
|
|
Maidenhead, United Kingdom
|
Leased
|
|
Slough, United Kingdom
|
Leased
|
|
San Francisco, California
|
Leased
|
|
Indianapolis, Indiana
|
Leased
|
|
Alice, Texas
|
Owned
|
|
Chantilly, Virginia
|
Leased
|
|
Greenfield, Indiana
|
Owned
|
|
Gaithersburg, Maryland
|
Leased
|
|
Cranford, New Jersey
|
Leased
|
|
Princeton, New Jersey
|
Leased
|
|
West Trenton, New Jersey
|
Leased
|
|
Cary, North Carolina
|
Leased
|
|
Denver, Pennsylvania
|
Owned
|
|
Cumberland, Virginia
|
Owned
|
|
Geneva, Switzerland
|
Owned
|
|
Madison, Wisconsin
|
Owned
|
|
Item 3.
|
LEGAL PROCEEDINGS (dollars in millions)
|
|
Item 4.
|
MINE SAFETY DISCLOSURES
|
|
Item 5.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS, AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
|
High
|
Low
|
|||||
|
Year Ended December 31, 2016
|
|
|
|
|
|||
|
First Quarter
|
$
|
123.99
|
|
$
|
97.79
|
|
|
|
Second Quarter
|
$
|
131.99
|
|
$
|
115.98
|
|
|
|
Third Quarter
|
$
|
141.32
|
|
$
|
129.68
|
|
|
|
Fourth Quarter
|
$
|
140.27
|
|
$
|
119.51
|
|
|
|
Year Ended December 31, 2017
|
|
|
|
|
|||
|
First Quarter
|
$
|
145.00
|
|
$
|
128.00
|
|
|
|
Second Quarter
|
$
|
154.82
|
|
$
|
134.19
|
|
|
|
Third Quarter
|
$
|
164.22
|
|
$
|
146.68
|
|
|
|
Fourth Quarter
|
$
|
165.18
|
|
$
|
147.28
|
|
|
|
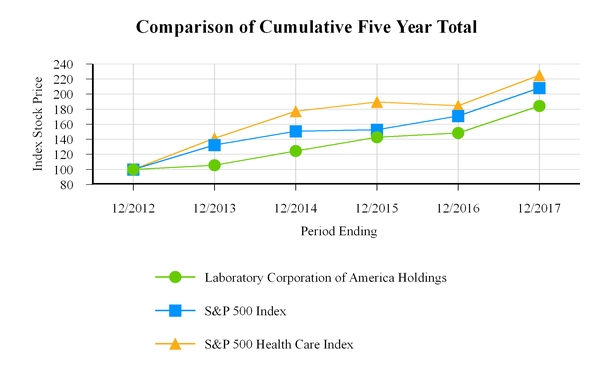
|
12/2012
|
12/2013
|
12/2014
|
12/2015
|
12/2016
|
12/2017
|
|||||||||||||||||
|
Laboratory Corporation of America Holdings
|
$
|
100.00
|
|
$
|
105.48
|
|
$
|
124.57
|
|
$
|
142.74
|
|
$
|
148.21
|
|
$
|
184.15
|
|
|||||
|
S&P 500 Index
|
$
|
100.00
|
|
$
|
132.39
|
|
$
|
150.51
|
|
$
|
152.59
|
|
$
|
170.84
|
|
$
|
208.14
|
|
|||||
|
S&P 500 Health Care Index
|
$
|
100.00
|
|
$
|
141.46
|
|
$
|
177.30
|
|
$
|
189.52
|
|
$
|
184.42
|
|
$
|
225.13
|
|
|||||
|
|
Total Number of Shares Repurchased
|
Average Price Paid Per Share
|
Total Number of Shares Repurchased as Part of Publicly Announced Program
|
Maximum Dollar Value of Shares that May Yet Be Repurchased Under the Program
|
|||||||||
|
October 1 - October 31
|
0.3
|
|
$
|
150.67
|
|
0.3
|
|
$
|
401.4
|
|
|||
|
November 1 - November 30
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
December 1 - December 31
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
0.3
|
|
$
|
150.67
|
|
0.3
|
|
$
|
401.4
|
|
||||
|
Item 6.
|
SELECTED FINANCIAL DATA (in millions, except per share amounts)
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
(a)
2017
|
(b)
2016
|
(c)
2015
|
(d)
2014
|
(e)
2013
|
|||||||||||||||
|
Statement of Operations Data
:
|
|
|
|
|
|
||||||||||||||
|
Net revenues
|
$
|
10,205.9
|
|
$
|
9,437.2
|
|
$
|
8,505.7
|
|
$
|
6,011.6
|
|
$
|
5,808.3
|
|
||||
|
Gross profit
|
3,464.0
|
|
3,180.5
|
|
2,903.3
|
|
2,203.1
|
|
2,223.2
|
|
|||||||||
|
Operating income (i)
|
1,364.2
|
|
1,312.4
|
|
996.8
|
|
904.3
|
|
983.3
|
|
|||||||||
|
Net earnings attributable to Laboratory
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Corporation of America Holdings (j)
|
1,268.2
|
|
732.1
|
|
437.6
|
|
511.2
|
|
573.8
|
|
|||||||||
|
Basic earnings per common share
|
$
|
12.39
|
|
$
|
7.14
|
|
$
|
4.43
|
|
$
|
6.03
|
|
$
|
6.36
|
|
||||
|
Diluted earnings per common share
|
$
|
12.21
|
|
$
|
7.02
|
|
$
|
4.35
|
|
$
|
5.91
|
|
$
|
6.25
|
|
||||
|
Basic weighted average common
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
shares outstanding
|
102.4
|
|
102.5
|
|
98.8
|
|
84.8
|
|
90.2
|
|
|||||||||
|
Diluted weighted average common
|
|
|
|||||||||||||||||
|
shares outstanding
|
103.9
|
|
104.3
|
|
100.6
|
|
86.4
|
|
91.8
|
|
|||||||||
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Cash and cash equivalents, and
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
short-term investments
|
$
|
316.7
|
|
$
|
433.6
|
|
$
|
716.4
|
|
$
|
580.0
|
|
$
|
404.0
|
|
||||
|
Goodwill and intangible assets, net (h)
|
11,870.8
|
|
9,824.9
|
|
9,526.6
|
|
4,575.2
|
|
4,594.8
|
|
|||||||||
|
Total assets (f)
|
16,568.0
|
|
14,247.0
|
|
14,104.7
|
|
7,262.8
|
|
6,939.8
|
|
|||||||||
|
Long-term obligations (f) (g)
|
6,762.1
|
|
5,849.5
|
|
6,364.2
|
|
2,990.8
|
|
2,974.3
|
|
|||||||||
|
Total shareholders' equity
|
6,830.0
|
|
5,505.8
|
|
4,945.1
|
|
2,820.5
|
|
2,491.3
|
|
|||||||||
|
(a)
|
During
2017
, the Company recorded net restructuring charges of
$70.9
. The charges were comprised of
$36.1
in severance and other personnel costs and
$39.9
in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of
$0.5
in unused severance and
$4.6
in unused facility-related costs. The Company also recognized asset impairment losses of $23.5 related to the termination of software development projects within the CDD segment and the forgiveness of certain indebtedness for LCD customers in areas heavily impacted by hurricanes during the third quarter.
|
|
(b)
|
During 2016, the Company recorded net restructuring charges of $58.4. The charges were comprised of $30.9 in severance and other personnel costs and $33.8 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $2.8 in unused severance and $3.5 in unused facility-related costs.
|
|
(c)
|
During 2015, the Company recorded net restructuring charges of $113.9. The charges were comprised of $59.2 in severance and other personnel costs and $55.8 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $1.1 in unused facility-related costs.
|
|
(d)
|
During 2014, the Company recorded net restructuring charges of $17.8. The charges were comprised of $10.5 in severance and other personnel costs and $8.4 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $0.4 in unused severance and $0.7 in unused facility-related costs.
|
|
(e)
|
During 2013, the Company recorded net restructuring charges of $21.8. The charges were comprised of $15.4 in severance and other personnel costs and $9.5 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $0.7 in unused severance and $2.4 in unused facility-related costs.
|
|
(f)
|
During the first quarter of 2016, the Company adopted Accounting Standards Update (ASU) 2015-03, Interest-Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs. In accordance with this guidance, unamortized debt issuance costs of $52.8, $39.0 and $26.1 associated with the Senior Notes and loan obligations have been reclassified from total assets to long-term obligations for fiscal 2015, 2014, 2013, respectively, in the table above.
|
|
(g)
|
Long-term obligations primarily include the Company’s zero-coupon convertible subordinated notes, 5.625% Senior Notes due 2015, 3.125% Senior Notes due 2016, 2.20% Senior Notes due 2017, 2.50% Senior Notes due 2018, 4.625% Senior Notes due 2020, 2.625% Senior Notes due 2020, 3.75% Senior Notes due 2022, 3.20% Senior Notes due 2022, 4.00% Senior Notes due 2023, 3.25% Senior Notes due 2024, 3.60% Senior Notes due 2025, 3.60% Senior Notes due 2027, 4.70% Senior Notes due 2045, 2014 term loan, 2017 term loan, revolving credit facility and other long-term obligations. The accreted balance of the zero-coupon convertible subordinated notes was $8.8, $42.4, $94.5, $93.9, and $110.8 at December 31, 2017, 2016, 2015, 2014, and 2013, respectively. The principal balance of the 5.625% Senior
|
|
(h)
|
During 2016, the Company revised the final purchase price allocation for Covance. As a result, an out of period adjustment of $25.6 was recorded to reduce goodwill and increase a deferred tax asset as of December 31, 2015. The Company concluded that the impact of this adjustment was not material to the current or prior periods.
|
|
(i)
|
The Company changed its financial statement classification for certain gross receipts taxes in 2016, removing these taxes from its provision for income taxes and moving this expense into selling, general and administrative expenses. Certain gross receipts taxes of $6.1, $6.1, and $7.6 were reclassified in 2015, 2014 and 2013, respectively.
|
|
(j)
|
Net earnings attributable to Laboratory Corporation of America Holdings in 2017 includes a provisional net benefit of $519.0 due to the Tax Cuts and Jobs Act (TCJA). For additional information on the TCJA, see Note 13 to the Consolidated Financial Statements.
|
|
Item 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (in millions)
|
|
|
Years Ended December 31,
|
Change
|
|||||||||||||||
|
2017
|
2016
|
2015
|
2017
|
2016
|
|||||||||||||
|
LCD
|
$
|
7,170.5
|
|
$
|
6,593.9
|
|
$
|
6,199.3
|
|
8.7
|
%
|
6.4
|
%
|
||||
|
CDD
|
3,037.2
|
|
2,844.1
|
|
2,306.4
|
|
6.8
|
%
|
23.3
|
%
|
|||||||
|
Intercompany eliminations
|
(1.8
|
)
|
(0.8
|
)
|
—
|
|
125.0
|
%
|
N/A
|
|
|||||||
|
Total
|
$
|
10,205.9
|
|
$
|
9,437.2
|
|
|
$
|
8,505.7
|
|
8.1
|
%
|
11.0
|
%
|
|||
|
Years Ended December 31,
|
Change
|
||||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
Net cost of revenues
|
$
|
6,741.9
|
|
$
|
6,256.7
|
|
$
|
5,602.4
|
|
7.8
|
%
|
11.7
|
%
|
||||
|
Cost of revenues as a % of net revenues
|
66.1
|
%
|
66.3
|
%
|
65.9
|
%
|
|
|
|
|
|||||||
|
|
Years Ended December 31,
|
Change
|
|||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
Selling, general and administrative expenses
|
$
|
1,812.4
|
|
$
|
1,630.2
|
|
$
|
1,628.1
|
|
11.2
|
%
|
0.1
|
%
|
||||
|
SG&A as a % of net revenues
|
17.8
|
%
|
17.3
|
%
|
19.1
|
%
|
|
|
|
|
|||||||
|
|
Years Ended December 31,
|
Change
|
|||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
LCD
|
$
|
116.7
|
|
$
|
93.4
|
|
$
|
82.4
|
|
24.9
|
%
|
13.3
|
%
|
||||
|
CDD
|
99.8
|
|
86.1
|
|
82.1
|
|
15.9
|
%
|
4.9
|
%
|
|||||||
|
Amortization of intangibles and other assets
|
$
|
216.5
|
|
$
|
179.5
|
|
$
|
164.5
|
|
20.6
|
%
|
9.1
|
%
|
||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Restructuring and other special charges
|
$
|
70.9
|
|
$
|
58.4
|
|
$
|
113.9
|
|
||
|
Years Ended December 31,
|
Change
|
||||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
Interest expense
|
$
|
235.1
|
|
$
|
219.1
|
|
$
|
274.9
|
|
7.3
|
%
|
(20.3
|
)%
|
||||
|
|
Years Ended December 31,
|
Change
|
|||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
Equity method income, net
|
$
|
11.3
|
|
$
|
7.9
|
|
$
|
10.0
|
|
43.0
|
%
|
(21.0
|
)%
|
||||
|
|
Years Ended December 31,
|
Change
|
|||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
Other, net
|
$
|
(7.6
|
)
|
$
|
2.6
|
|
$
|
(7.8
|
)
|
392.3
|
%
|
(133.3
|
)%
|
||||
|
Years Ended December 31,
|
|||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Income tax expense
|
$
|
(139.1
|
)
|
$
|
372.3
|
|
$
|
287.3
|
|
||
|
Income tax expense as a % of income before tax
|
(12.3
|
)%
|
33.7
|
%
|
39.6
|
%
|
|||||
|
|
Years Ended December 31,
|
Change
|
|||||||||||||||
|
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||||
|
LCD
|
$
|
1,298.6
|
|
$
|
1,187.6
|
|
$
|
1,053.7
|
|
9.3
|
%
|
12.7
|
%
|
||||
|
LCD operating margin
|
18.1
|
%
|
18.0
|
%
|
17.0
|
%
|
0.1
|
%
|
1.0
|
%
|
|||||||
|
CDD
|
$
|
206.2
|
|
$
|
272.7
|
|
$
|
73.5
|
|
(24.4
|
)%
|
271.0
|
%
|
||||
|
CDD operating margin
|
6.8
|
%
|
9.6
|
%
|
3.2
|
%
|
(2.8
|
)%
|
6.4
|
%
|
|||||||
|
General corporate expenses
|
$
|
(140.6
|
)
|
$
|
(147.9
|
)
|
$
|
(130.4
|
)
|
(4.9
|
)%
|
13.4
|
%
|
||||
|
Total
|
$
|
1,364.2
|
|
$
|
1,312.4
|
|
$
|
996.8
|
|
3.9
|
%
|
31.7
|
%
|
||||
|
|
For the Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net cash provided by operating activities
|
$
|
1,459.4
|
|
$
|
1,175.9
|
|
$
|
982.4
|
|
||
|
Net cash used for investing activities
|
(2,228.7
|
)
|
(795.7
|
)
|
(3,994.9
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
631.9
|
|
(649.8
|
)
|
3,184.6
|
|
|||||
|
Effect of exchange rate on changes in cash and cash equivalents
|
20.5
|
|
(13.2
|
)
|
(35.7
|
)
|
|||||
|
Net change in cash and cash equivalents
|
$
|
(116.9
|
)
|
$
|
(282.8
|
)
|
$
|
136.4
|
|
||
|
Contractual Cash Obligations
|
||||||||||||||||||||
|
Payments Due by Period
|
||||||||||||||||||||
|
|
|
|
2019-
|
2021-
|
2023 and
|
|||||||||||||||
|
|
Total
|
2018
|
2020
|
2022
|
thereafter
|
|||||||||||||||
|
Operating lease obligations
|
$
|
763.4
|
|
$
|
196.1
|
|
$
|
258.7
|
|
$
|
147.8
|
|
$
|
160.8
|
|
|||||
|
Contingent future licensing payments (a)
|
22.4
|
|
3.5
|
|
7.7
|
|
5.6
|
|
9.8
|
|
1.4
|
|
||||||||
|
Minimum royalty payments
|
24.2
|
|
2.5
|
|
8.1
|
|
12.1
|
|
1.5
|
|
||||||||||
|
Purchase obligations
|
29.2
|
|
25.5
|
|
3.7
|
|
—
|
|
—
|
|
||||||||||
|
Capital lease obligations
|
110.4
|
|
15.6
|
|
27.5
|
|
22.3
|
|
45.0
|
|
||||||||||
|
Scheduled interest payments on Senior Notes
|
2,150.7
|
|
218.1
|
|
407.5
|
|
324.3
|
|
1,200.8
|
|
||||||||||
|
Scheduled interest payments on Term Loan
|
117.6
|
|
25.3
|
|
53.8
|
|
38.5
|
|
—
|
|
||||||||||
|
Long-term debt
|
6,741.6
|
|
410.6
|
|
1,258.7
|
|
1,672.3
|
|
3,400.0
|
|
||||||||||
|
Total contractual cash obligations (b) (c)
|
$
|
9,959.5
|
|
$
|
897.2
|
|
$
|
2,025.7
|
|
$
|
2,227.1
|
|
$
|
4,809.5
|
|
|||||
|
(a)
|
Contingent future licensing payments will be made if certain events take place, such as the launch of a specific test, the transfer of certain technology, and the achievement specified revenue milestones.
|
|
(b)
|
The table does not include obligations under the Company’s pension and postretirement benefit plans, which are included in “Note 16 to Consolidated Financial Statements.” Benefits under the Company's postretirement medical plan are made when claims are submitted for payment, the timing of which is not practicable to estimate.
|
|
(c)
|
The table does not include the Company’s reserves for unrecognized tax benefits. The Company had a $27.4 and $28.3 reserve for unrecognized tax benefits, including interest and penalties, at
December 31, 2017
, and
2016
, respectively, which is included in “Note 13 to Consolidated Financial Statements.” Substantially all of these tax reserves are classified in other long-term liabilities in the Company’s Consolidated Balance Sheets at
December 31, 2017
, and
2016
.
|
|
•
|
Revenue recognition and allowance for doubtful accounts;
|
|
•
|
Pension expense;
|
|
•
|
Accruals for self-insurance reserves;
|
|
•
|
Income taxes; and
|
|
•
|
Goodwill and indefinite-lived assets.
|
|
Days Outstanding
|
2017
|
2016
|
|
|
0 – 30
|
50.1%
|
49.1%
|
|
|
31 – 60
|
19.5%
|
17.3%
|
|
|
61 – 90
|
8.9%
|
9.2%
|
|
|
91 – 120
|
6.1%
|
7.5%
|
|
|
121 – 150
|
3.8%
|
4.2%
|
|
|
151 – 180
|
3.7%
|
3.9%
|
|
|
181 – 270
|
6.9%
|
7.4%
|
|
|
271 – 360
|
0.9%
|
1.3%
|
|
|
Over 360
|
0.1%
|
0.1%
|
|
|
•
|
Annual cash flows, on a debt-free basis, arising from future revenues and profitability, changes in working capital, capital spending and income taxes for at least a five-year forecast period.
|
|
•
|
A terminal growth rate for years beyond the forecast period. The terminal growth rate is selected based on consideration of growth rates used in the forecast period, historical performance of the reporting unit and economic conditions.
|
|
•
|
A discount rate that reflects the risks inherent in realizing the forecasted cash flows. A discount rate considers the risk-free rate of return on long-term treasury securities, the risk premium associated with investing in equity securities of comparable companies, the beta obtained from the comparable companies and the cost of debt for investment grade issuers. In addition, the discount rate may consider any company-specific risk in achieving the prospective financial information.
|
|
1.
|
changes in government and third-party payer regulations, reimbursement, or coverage policies or other future reforms in the healthcare system (or in the interpretation of current regulations), new insurance or payment systems, including state, regional or private insurance cooperatives (e.g., health insurance exchanges), affecting governmental and third-party coverage or reimbursement for commercial laboratory testing, including the impact of the Protecting Access to Medicare Act of 2014 (PAMA);
|
|
2.
|
significant monetary damages, fines, penalties, assessments, refunds, repayments, unanticipated compliance expenditures and/or exclusion or disbarment from or ineligibility to participate in government programs, among other adverse consequences, arising from enforcement of anti-fraud and abuse laws and other laws applicable to the Company in jurisdictions in which the Company conducts business;
|
|
3.
|
significant fines, penalties, costs, unanticipated compliance expenditures and/or damage to the Company’s reputation arising from the failure to comply with national, state or local privacy and security laws and regulations, including the Health Insurance Portability and Accountability Act of 1996, the Health Information Technology for Economic and Clinical Health Act, the European Union's General Data Protection Regulation and similar laws and regulations in jurisdictions in which the Company conducts business;
|
|
4.
|
loss or suspension of a license or imposition of a fine or penalties under, or future changes in, or interpretations of applicable national, state or local licensing laws or regulations regarding the operation of clinical laboratories and the delivery of clinical laboratory test results, including, but not limited to, the U.S. Clinical Laboratory Improvement Act of 1967 and the Clinical Laboratory Improvement Amendments of 1988 and similar laws and regulations in jurisdictions in which the Company conducts business;
|
|
5.
|
penalties or loss of license arising from the failure to comply with applicable national, state or local occupational and workplace safety laws and regulations, including the U.S. Occupational Safety and Health Administration requirements and the U.S. Needlestick Safety and Prevention Act and similar laws and regulations in jurisdictions in which the Company conducts business;
|
|
6.
|
fines, unanticipated compliance expenditures, suspension of manufacturing, enforcement actions, injunctions, or criminal prosecution arising from failure to maintain compliance with current good manufacturing practice regulations and similar requirements of various regulatory agencies in jurisdictions in which the Company conducts business;
|
|
7.
|
sanctions or other remedies, including fines, unanticipated compliance expenditures, enforcement actions, injunctions or criminal prosecution arising from failure to comply with the Animal Welfare Act or similar national, state and local laws and regulations in jurisdictions in which the Company conducts business;
|
|
8.
|
changes in testing guidelines or recommendations by government agencies, medical specialty societies and other authoritative bodies affecting the utilization of laboratory tests;
|
|
9.
|
changes in national, state or local government regulations or policies affecting the approval, availability of, and the selling and marketing of diagnostic tests, drug development, or the conduct of drug development and medical device and diagnostic studies and trials, including regulations and policies of the U.S. Food and Drug Administration, the U.S. Department of Agriculture, the Medicine and Healthcare products Regulatory Agency in the U.K., the China Food and Drug Administration, the Pharmaceutical and Medical Devices Agency in Japan, the European Medicines Agency and similar regulations and policies of agencies in jurisdictions in which the Company conducts business;
|
|
10.
|
changes in government regulations or reimbursement pertaining to the biopharmaceutical and medical device and diagnostic industries, changes in reimbursement of biopharmaceutical products or reduced spending on research and development by biopharmaceutical customers;
|
|
11.
|
liabilities that result from the failure to comply with corporate governance requirements;
|
|
12.
|
increased competition, including price competition, potential reduction in rates in response to price transparency and consumerism, competitive bidding and/or changes or reductions to fee schedules and competition from companies that do not comply with existing laws or regulations or otherwise disregard compliance standards in the industry;
|
|
13.
|
changes in payer mix or payment structure, including insurance carrier participation in health insurance exchanges, an increase in capitated reimbursement mechanisms, the impact of a shift to consumer-driven health plans or plans carrying an increased level of member cost-sharing, and adverse changes in payer reimbursement or payer coverage policies (implemented directly or through a third party utilization management organization) related to specific diagnostic tests, categories of testing or testing methodologies;
|
|
14.
|
failure to retain or attract managed care organization (MCO) business as a result of changes in business models, including new risk based or network approaches, out-sourced Laboratory Network Management or Utilization Management companies, or other changes in strategy or business models by MCOs;
|
|
15.
|
failure to obtain and retain new customers, an unfavorable change in the mix of testing services ordered, or a reduction in tests ordered, specimens submitted or services requested by existing customers;
|
|
16.
|
difficulty in maintaining relationships with customers or retaining key employees as a result of uncertainty surrounding the integration of acquisitions and the resulting negative effects on the business of the Company;
|
|
17.
|
consolidation and convergence of MCOs, biopharmaceutical companies, health systems, large physician organizations and other customers, potentially causing material shifts in insourcing, utilization, pricing and reimbursements, including full and partial risk based models;
|
|
18.
|
failure to effectively develop and deploy new systems, system modifications or enhancements required in response to evolving market and business needs;
|
|
19.
|
customers choosing to insource services that are or could be purchased from the Company;
|
|
20.
|
failure to identify, successfully close and effectively integrate and/or manage acquisitions of new businesses;
|
|
21.
|
inability to achieve the expected benefits and synergies of newly acquired businesses, and impact on the Company's cash position, levels of indebtedness and stock price;
|
|
22.
|
termination, loss, delay, reduction in scope or increased costs of contracts, including large contracts and multiple contracts;
|
|
23.
|
liability arising from errors or omissions in the performance of contract research services or other contractual arrangements;
|
|
24.
|
failure to successfully obtain, maintain and enforce intellectual property rights and defend against challenges to the Company’s intellectual property rights;
|
|
25.
|
changes or disruption in services or supplies provided by third parties, including transportation;
|
|
26.
|
damage or disruption to the Company's facilities;
|
|
27.
|
damage to the Company's reputation, loss of business, or other harm from acts of animal rights extremists or potential harm and/or liability arising from animal research activities or the provision of animal research products;
|
|
28.
|
adverse results in litigation matters;
|
|
29.
|
inability to attract and retain experienced and qualified personnel;
|
|
30.
|
failure to develop or acquire licenses for new or improved technologies, such as point-of-care testing, mobile health technologies, and digital pathology, or potential use of new technologies by customers and/or consumers to perform their own tests;
|
|
31.
|
substantial costs arising from the inability to commercialize newly licensed tests or technologies or to obtain appropriate coverage or reimbursement for such tests;
|
|
32.
|
inability to obtain and maintain adequate patent and other proprietary rights for protection of the Company's products and services and successfully enforce the Company's proprietary rights;
|
|
33.
|
scope, validity and enforceability of patents and other proprietary rights held by third parties that may impact the Company's ability to develop, perform, or market the Company's products or services or operate its business;
|
|
34.
|
business interruption or other impact on the business due to adverse weather, fires and/or other natural disasters, acts of war, terrorism or other criminal acts, and/or widespread outbreak of influenza or other pandemic illness;
|
|
35.
|
discontinuation or recalls of existing testing products;
|
|
36.
|
a failure in the Company's information technology systems, including with respect to testing turnaround time and billing processes, or the failure to maintain the security of business information or systems or to protect against cyber security attacks such as denial of service attacks, malware, ransomware and computer viruses, or delays or failures in the development and implementation of the Company’s automation platforms, any of which could result in a negative effect on the Company’s performance of services, a loss of business or increased costs, damages to the Company’s reputation, significant litigation exposure, an inability to meet required financial reporting deadlines, or the failure to meet future regulatory or customer information technology, data security and connectivity requirements;
|
|
37.
|
business interruption, increased costs, and other adverse effects on the Company's operations due to the unionization of employees, union strikes, work stoppages, general labor unrest or failure to comply with labor or employment laws;
|
|
38.
|
failure to maintain the Company's days sales outstanding and/or bad debt expense levels including a negative impact on the Company's reimbursement, cash collections and profitability arising from unfavorable changes in third-party payer policies, payment delays introduced by third party benefit management organizations and increasing levels of patient payment responsibility;
|
|
39.
|
impact on the Company's revenue, cash collections and the availability of credit for general liquidity or other financing needs arising from a significant deterioration in the economy or financial markets or in the Company's credit ratings by Standard & Poor's and/or Moody's;
|
|
40.
|
failure to maintain the expected capital structure for the Company, including failure to maintain the Company's investment grade rating;
|
|
41.
|
changes in reimbursement by foreign governments and foreign currency fluctuations;
|
|
42.
|
inability to obtain certain billing information from physicians, resulting in increased costs and complexity, a temporary disruption in receipts and ongoing reductions in reimbursements and net revenues;
|
|
43.
|
expenses and risks associated with international operations, including, but not limited to, compliance with the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, other global anti-corruption laws and regulations, trade sanction laws and regulations, and economic, political, legal and other operational risks associated with foreign jurisdictions;
|
|
44.
|
failure to achieve expected efficiencies and savings in connection with the Company's business process improvement initiatives;
|
|
45.
|
changes in tax laws and regulations or changes in their interpretation, including the TCJA; and
|
|
46.
|
global economic conditions and government and regulatory changes, including, but not limited to the United Kingdom's announced intention to exit from the European Union.
|
|
Item 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK (in millions)
|
|
1)
|
The Company will pay contingent cash interest on the zero-coupon subordinated notes after September 11, 2006, if the average market price of the notes equals 120% or more of the sum of the issue price, accrued original issue discount and contingent additional principal, if any, for a specified measurement period.
|
|
2)
|
Holders may surrender zero-coupon subordinated notes for conversion during any period in which the rating assigned to the zero-coupon subordinated notes by S&P Ratings Services is BB- or lower.
|
|
Item 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
Item 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
Item 9A.
|
CONTROLS AND PROCEDURES
|
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company;
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the U.S.;
|
|
•
|
provide reasonable assurance that receipts and expenditures of the Company are being made only in accordance with authorization of management and directors of the Company; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements.
|
|
Item 9B.
|
OTHER INFORMATION
|
|
Item 10.
|
DIRECTORS, EXECUTIVE OFFICERS and CORPORATE GOVERNANCE
|
|
Item 11.
|
EXECUTIVE COMPENSATION
|
|
Item 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
Item 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
Item 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
(1)
|
Consolidated Financial Statements and Report of Independent Registered Public Accounting Firm included herein:
|
|
See Index on page F-1
|
|
|
(2)
|
Financial Statement Schedules:
|
|
See Index on page F-1
|
|
|
All other schedules are omitted as they are inapplicable or the required information is furnished in the Consolidated Financial Statements or notes thereto.
|
|
|
(3)
|
Index to and List of Exhibits
|
|
2.1
|
|
|
2.2
|
|
|
3.1
|
|
|
3.2
|
|
|
4.1
|
|
|
4.2
|
|
|
4.3
|
|
|
4.4
|
|
|
4.5
|
|
|
4.6
|
|
|
4.7
|
|
|
4.8
|
|
|
4.9
|
|
|
4.10
|
|
|
4.11
|
|
|
4.12
|
|
|
4.13
|
|
|
4.14
|
|
|
4.15
|
|
|
10.1
|
National Health Laboratories Incorporated Pension Equalization Plan (incorporated herein by reference to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1992).
|
|
10.2
|
|
|
10.3
|
|
|
10.4
|
|
|
10.5
|
National Health Laboratories 1988 Stock Option Plan, as amended (incorporated herein by reference to the Company's Registration Statement on Form S-1, filed with the Commission on July 9, 1990, File No. 33-35782).
|
|
10.6
|
|
|
10.7
|
|
|
10.8
|
|
|
10.9
|
|
|
10.10
|
|
|
10.11
|
|
|
10.12
|
|
|
10.13
|
|
|
10.14
|
|
|
10.15
|
|
|
10.16
|
|
|
10.17
|
|
|
10.18
|
|
|
10.19
|
|
|
10.20
|
|
|
10.21
|
|
|
10.22
|
|
|
10.23
|
|
|
10.24
|
|
|
10.25
|
|
|
10.26
|
|
|
10.27
|
|
|
10.28
|
|
|
10.29
|
|
|
10.30
|
|
|
10.31
|
|
|
10.32
|
|
|
10.33
|
|
|
10.34
|
|
|
10.35
|
|
|
10.36
|
|
|
10.37
|
|
|
10.38
|
|
|
10.39
|
|
|
10.40
|
|
|
10.41
|
|
|
10.42
|
|
|
12.1*
|
|
|
21*
|
|
|
23.1*
|
|
|
24.1*
|
|
|
24.2*
|
|
|
24.3*
|
|
|
24.4*
|
|
|
24.5*
|
|
|
24.6*
|
|
|
24.7*
|
|
|
24.8*
|
|
|
24.9*
|
|
|
31.1*
|
|
|
31.2*
|
|
|
32*
|
|
|
101.INS*
|
XBRL Instance Document
|
|
101.SCH*
|
XBRL Taxonomy Extension Schema
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase
|
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase
|
|
*
|
Filed herewith
|
|
|
By:
|
/s/ DAVID P. KING
|
|
|
|
|
David P. King
|
|
|
|
|
Chairman of the Board, President
|
|
|
|
|
and Chief Executive Officer
|
|
|
Dated:
|
February 27, 2018
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
/s/ DAVID P. KING
|
|
Chairman of the Board, President and Chief
|
|
David P. King
|
|
Executive Officer (Principal Executive Officer)
|
|
|
|
|
|
/s/ GLENN A. EISENBERG
|
|
Executive Vice President, Chief Financial
|
|
Glenn A. Eisenberg
|
|
Officer and Treasurer (Principal Financial Officer)
|
|
/s/ EDWARD T. DODSON
|
|
Chief Accounting Officer (Principal Accounting Officer)
|
|
Edward T. Dodson
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
Kerrii B. Anderson
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
Jean-Luc Bélingard
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
D. Gary Gilliland, M.D., Ph.D.
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
Garheng Kong, M.D., Ph.D.
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
Robert E. Mittelstaedt, Jr.
|
|
|
|
*
|
|
Director
|
|
Peter M. Neupert
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
Richelle Parham
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
Adam H. Schechter
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
R. Sanders Williams, M.D.
|
|
|
|
|
|
|
|
By:
|
/s/ F. SAMUEL EBERTS III
|
|
|
|
F. Samuel Eberts III
|
|
|
|
Attorney-in-fact
|
|
|
|
Page
|
|
|
|
|
|
|
|
Consolidated Financial Statements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Statement Schedule:
|
|
|
|
|
|
December 31,
2017 |
December 31,
2016 |
||||||
|
ASSETS
|
|
|
|||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
316.7
|
|
$
|
433.6
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $260.9 and $235.6 at December 31, 2017 and 2016, respectively
|
1,481.3
|
|
1,328.7
|
|
|||
|
Unbilled services
|
235.6
|
|
190.0
|
|
|||
|
Supplies inventories
|
227.6
|
|
205.2
|
|
|||
|
Prepaid expenses and other
|
421.4
|
|
321.2
|
|
|||
|
Total current assets
|
2,682.6
|
|
2,478.7
|
|
|||
|
Property, plant and equipment, net
|
1,748.9
|
|
1,718.6
|
|
|||
|
Goodwill, net
|
7,530.0
|
|
6,424.4
|
|
|||
|
Intangible assets, net
|
4,340.8
|
|
3,400.5
|
|
|||
|
Joint venture partnerships and equity method investments
|
58.4
|
|
57.6
|
|
|||
|
Deferred income taxes
|
1.9
|
|
2.1
|
|
|||
|
Other assets, net
|
205.4
|
|
165.1
|
|
|||
|
Total assets
|
$
|
16,568.0
|
|
$
|
14,247.0
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable
|
$
|
663.0
|
|
$
|
508.4
|
|
|
|
Accrued expenses and other
|
632.9
|
|
593.7
|
|
|||
|
Unearned revenue
|
332.7
|
|
176.0
|
|
|||
|
Short-term borrowings and current portion of long-term debt
|
417.5
|
|
549.5
|
|
|||
|
Total current liabilities
|
2,046.1
|
|
1,827.6
|
|
|||
|
Long-term debt, less current portion
|
6,344.6
|
|
5,300.0
|
|
|||
|
Deferred income taxes and other tax liabilities
|
948.3
|
|
1,206.4
|
|
|||
|
Other liabilities
|
378.2
|
|
392.0
|
|
|||
|
Total liabilities
|
9,717.2
|
|
8,726.0
|
|
|||
|
Commitments and contingent liabilities
|
|
|
|
|
|||
|
Noncontrolling interest
|
20.8
|
|
15.2
|
|
|||
|
Shareholders’ equity
|
|
|
|
|
|||
|
Common stock, 101.9 and 102.7 shares outstanding at December 31, 2017 and 2016, respectively
|
12.0
|
|
12.1
|
|
|||
|
Additional paid-in capital
|
1,989.8
|
|
2,131.7
|
|
|||
|
Retained earnings
|
6,224.0
|
|
4,955.8
|
|
|||
|
Less common stock held in treasury
|
(1,060.1
|
)
|
(1,012.7
|
)
|
|||
|
Accumulated other comprehensive loss
|
(335.7
|
)
|
(581.1
|
)
|
|||
|
Total shareholders’ equity
|
6,830.0
|
|
5,505.8
|
|
|||
|
Total liabilities and shareholders’ equity
|
$
|
16,568.0
|
|
$
|
14,247.0
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net revenues
|
$
|
10,205.9
|
|
$
|
9,437.2
|
|
$
|
8,505.7
|
|
||
|
Reimbursable out-of-pocket expenses
|
235.5
|
|
204.6
|
|
174.4
|
|
|||||
|
Total revenues
|
10,441.4
|
|
9,641.8
|
|
8,680.1
|
|
|||||
|
Net cost of revenue
|
6,741.9
|
|
6,256.7
|
|
5,602.4
|
|
|||||
|
Reimbursable out-of-pocket expenses
|
235.5
|
|
204.6
|
|
174.4
|
|
|||||
|
Total cost of revenue
|
6,977.4
|
|
6,461.3
|
|
5,776.8
|
|
|||||
|
Gross profit
|
3,464.0
|
|
3,180.5
|
|
2,903.3
|
|
|||||
|
Selling, general and administrative expenses
|
1,812.4
|
|
1,630.2
|
|
1,628.1
|
|
|||||
|
Amortization of intangibles and other assets
|
216.5
|
|
179.5
|
|
164.5
|
|
|||||
|
Restructuring and other special charges
|
70.9
|
|
58.4
|
|
113.9
|
|
|||||
|
Operating income
|
1,364.2
|
|
1,312.4
|
|
996.8
|
|
|||||
|
Other income (expenses):
|
|
|
|
|
|
|
|||||
|
Interest expense
|
(235.1
|
)
|
(219.1
|
)
|
(274.9
|
)
|
|||||
|
Equity method income, net
|
11.3
|
|
7.9
|
|
10.0
|
|
|||||
|
Investment income
|
2.1
|
|
1.7
|
|
1.9
|
|
|||||
|
Other, net
|
(7.6
|
)
|
2.6
|
|
(7.8
|
)
|
|||||
|
Earnings before income taxes
|
1,134.9
|
|
1,105.5
|
|
726.0
|
|
|||||
|
Provision for income taxes
|
(139.1
|
)
|
372.3
|
|
287.3
|
|
|||||
|
Net earnings
|
1,274.0
|
|
733.2
|
|
438.7
|
|
|||||
|
Less: Net earnings attributable to the noncontrolling interest
|
(5.8
|
)
|
(1.1
|
)
|
(1.1
|
)
|
|||||
|
Net earnings attributable to Laboratory Corporation of America Holdings
|
$
|
1,268.2
|
|
$
|
732.1
|
|
$
|
437.6
|
|
||
|
Basic earnings per common share
|
$
|
12.39
|
|
$
|
7.14
|
|
$
|
4.43
|
|
||
|
Diluted earnings per common share
|
$
|
12.21
|
|
$
|
7.02
|
|
$
|
4.35
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net earnings
|
$
|
1,274.0
|
|
$
|
733.2
|
|
$
|
438.7
|
|
||
|
Foreign currency translation adjustments
|
262.3
|
|
(250.0
|
)
|
(370.7
|
)
|
|||||
|
Net benefit plan adjustments
|
20.9
|
|
(40.3
|
)
|
7.7
|
|
|||||
|
Investment adjustments
|
—
|
|
—
|
|
(0.1
|
)
|
|||||
|
Other comprehensive loss before tax
|
283.2
|
|
(290.3
|
)
|
(363.1
|
)
|
|||||
|
Provision for income tax related to items of comprehensive earnings
|
(37.8
|
)
|
(3.8
|
)
|
86.6
|
|
|||||
|
Other comprehensive loss, net of tax
|
245.4
|
|
(294.1
|
)
|
(276.5
|
)
|
|||||
|
Comprehensive earnings
|
1,519.4
|
|
439.1
|
|
162.2
|
|
|||||
|
Less: Net earnings attributable to the noncontrolling interest
|
(5.8
|
)
|
(1.1
|
)
|
(1.1
|
)
|
|||||
|
Net comprehensive earnings attributable to Laboratory Corporation of America Holdings
|
$
|
1,513.6
|
|
$
|
438.0
|
|
$
|
161.1
|
|
||
|
Common
Stock
|
Additional
Paid-in
Capital
|
Retained
Earnings
|
Treasury
Stock
|
Accumulated
Other
Comprehensive
Loss
|
Total
Shareholders’
Equity
|
||||||||||||||||||
|
BALANCE AT DECEMBER 31, 2014
|
$
|
10.4
|
|
$
|
—
|
|
$
|
3,786.1
|
|
$
|
(965.5
|
)
|
$
|
(10.5
|
)
|
$
|
2,820.5
|
|
|||||
|
Net earnings attributable to Laboratory Corporation of America Holdings
|
—
|
|
—
|
|
437.6
|
|
—
|
|
—
|
|
437.6
|
|
|||||||||||
|
Other comprehensive earnings, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
(276.5
|
)
|
(276.5
|
)
|
|||||||||||
|
Issuance of common stock for acquisition consideration
|
1.5
|
|
1,761.0
|
|
—
|
|
—
|
|
—
|
|
1,762.5
|
|
|||||||||||
|
Issuance of common stock under employee stock plans
|
0.1
|
|
98.8
|
|
—
|
|
—
|
|
—
|
|
98.9
|
|
|||||||||||
|
Surrender of restricted stock and performance share awards
|
—
|
|
—
|
|
—
|
|
(12.6
|
)
|
—
|
|
(12.6
|
)
|
|||||||||||
|
Conversion of zero-coupon convertible debt
|
—
|
|
0.4
|
|
—
|
|
—
|
|
—
|
|
0.4
|
|
|||||||||||
|
Stock compensation
|
—
|
|
102.1
|
|
—
|
|
—
|
|
—
|
|
102.1
|
|
|||||||||||
|
Income tax benefit from stock options exercised
|
—
|
|
12.2
|
|
—
|
|
—
|
|
—
|
|
12.2
|
|
|||||||||||
|
BALANCE AT DECEMBER 31, 2015
|
12.0
|
|
$
|
1,974.5
|
|
$
|
4,223.7
|
|
$
|
(978.1
|
)
|
$
|
(287.0
|
)
|
$
|
4,945.1
|
|
||||||
|
Net earnings attributable to Laboratory Corporation of America Holdings
|
—
|
|
—
|
|
732.1
|
|
—
|
|
—
|
|
732.1
|
|
|||||||||||
|
Other comprehensive earnings, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
(294.1
|
)
|
(294.1
|
)
|
|||||||||||
|
Issuance of common stock under employee stock plans
|
0.1
|
|
70.5
|
|
—
|
|
—
|
|
—
|
|
70.6
|
|
|||||||||||
|
Surrender of restricted stock and performance share awards
|
—
|
|
—
|
|
—
|
|
(34.6
|
)
|
—
|
|
(34.6
|
)
|
|||||||||||
|
Conversion of zero-coupon convertible debt
|
—
|
|
21.0
|
|
—
|
|
—
|
|
—
|
|
21.0
|
|
|||||||||||
|
Stock compensation
|
—
|
|
109.6
|
|
—
|
|
—
|
|
—
|
|
109.6
|
|
|||||||||||
|
Purchase of common stock
|
—
|
|
(43.9
|
)
|
—
|
|
—
|
|
—
|
|
(43.9
|
)
|
|||||||||||
|
BALANCE AT DECEMBER 31, 2016
|
12.1
|
|
$
|
2,131.7
|
|
$
|
4,955.8
|
|
$
|
(1,012.7
|
)
|
$
|
(581.1
|
)
|
$
|
5,505.8
|
|
||||||
|
Net earnings attributable to Laboratory Corporation of America Holdings
|
—
|
|
—
|
|
1,268.2
|
|
—
|
|
—
|
|
1,268.2
|
|
|||||||||||
|
Other comprehensive earnings, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
245.4
|
|
245.4
|
|
|||||||||||
|
Issuance of common stock under employee stock plans
|
0.1
|
|
73.5
|
|
—
|
|
—
|
|
—
|
|
73.6
|
|
|||||||||||
|
Surrender of restricted stock and performance share awards
|
—
|
|
—
|
|
—
|
|
(47.4
|
)
|
—
|
|
(47.4
|
)
|
|||||||||||
|
Conversion of zero-coupon convertible debt
|
—
|
|
12.8
|
|
—
|
|
—
|
|
—
|
|
12.8
|
|
|||||||||||
|
Stock compensation
|
—
|
|
109.7
|
|
—
|
|
—
|
|
—
|
|
109.7
|
|
|||||||||||
|
Purchase of common stock
|
(0.2
|
)
|
(337.9
|
)
|
—
|
|
—
|
|
—
|
|
(338.1
|
)
|
|||||||||||
|
BALANCE AT DECEMBER 31, 2017
|
$
|
12.0
|
|
$
|
1,989.8
|
|
$
|
6,224.0
|
|
$
|
(1,060.1
|
)
|
$
|
(335.7
|
)
|
$
|
6,830.0
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
||||||||
|
Net earnings
|
$
|
1,274.0
|
|
$
|
733.2
|
|
$
|
438.7
|
|
||
|
Adjustments to reconcile net earnings to net cash provided by operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation and amortization
|
533.2
|
|
499.2
|
|
457.8
|
|
|||||
|
Stock compensation
|
109.7
|
|
109.6
|
|
102.1
|
|
|||||
|
(Gain) loss on sale of assets
|
1.5
|
|
(9.2
|
)
|
4.6
|
|
|||||
|
Accrued interest on zero-coupon subordinated notes
|
0.3
|
|
1.6
|
|
2.0
|
|
|||||
|
Cumulative earnings less than distributions from equity method investments
|
0.5
|
|
1.2
|
|
0.1
|
|
|||||
|
Asset impairment
|
23.5
|
|
—
|
|
39.7
|
|
|||||
|
Deferred income taxes
|
(525.8
|
)
|
54.7
|
|
(34.1
|
)
|
|||||
|
Change in assets and liabilities (net of effects of acquisitions):
|
|
|
|
|
|
|
|||||
|
Increase in accounts receivable, net
|
(2.1
|
)
|
(85.5
|
)
|
(71.8
|
)
|
|||||
|
Increase in unbilled services
|
(8.3
|
)
|
(33.4
|
)
|
(16.9
|
)
|
|||||
|
Increase in inventories
|
(16.4
|
)
|
(9.6
|
)
|
(0.2
|
)
|
|||||
|
(Increase) decrease in prepaid expenses and other
|
(20.3
|
)
|
(20.5
|
)
|
62.3
|
|
|||||
|
(Decrease) increase in accounts payable
|
85.6
|
|
(8.7
|
)
|
30.7
|
|
|||||
|
Increase in unearned revenue
|
19.0
|
|
29.9
|
|
5.4
|
|
|||||
|
Decrease in accrued expenses and other
|
(15.0
|
)
|
(86.6
|
)
|
(38.0
|
)
|
|||||
|
Net cash provided by operating activities
|
1,459.4
|
|
1,175.9
|
|
982.4
|
|
|||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|||||
|
Capital expenditures
|
(312.9
|
)
|
(278.9
|
)
|
(255.8
|
)
|
|||||
|
Proceeds from sale of assets
|
5.5
|
|
30.8
|
|
0.6
|
|
|||||
|
Proceeds from sale of investments
|
—
|
|
13.5
|
|
8.0
|
|
|||||
|
Acquisition of licensing technology
|
(2.5
|
)
|
—
|
|
—
|
|
|||||
|
Investments in equity affiliates
|
(36.2
|
)
|
(12.5
|
)
|
(11.7
|
)
|
|||||
|
Acquisition of businesses, net of cash acquired
|
(1,882.6
|
)
|
(548.6
|
)
|
(3,736.0
|
)
|
|||||
|
Net cash used for investing activities
|
(2,228.7
|
)
|
(795.7
|
)
|
(3,994.9
|
)
|
|||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|||||
|
Proceeds from Senior Notes offerings
|
1,200.0
|
|
—
|
|
2,900.0
|
|
|||||
|
Proceeds from term loan
|
750.0
|
|
—
|
|
1,000.0
|
|
|||||
|
Payments on term loan
|
(493.0
|
)
|
(150.0
|
)
|
(285.0
|
)
|
|||||
|
Proceeds from revolving credit facilities
|
1,392.2
|
|
139.5
|
|
60.0
|
|
|||||
|
Payments on revolving credit facilities
|
(1,392.2
|
)
|
(139.5
|
)
|
(60.0
|
)
|
|||||
|
Proceeds from bridge loan
|
—
|
|
—
|
|
400.0
|
|
|||||
|
Payments on bridge loan
|
—
|
|
—
|
|
(400.0
|
)
|
|||||
|
Payments on Senior Notes
|
(500.1
|
)
|
(454.7
|
)
|
(500.0
|
)
|
|||||
|
Payments on zero-coupon subordinated notes
|
(33.9
|
)
|
(53.7
|
)
|
(1.3
|
)
|
|||||
|
Payment of debt issuance costs
|
(15.3
|
)
|
—
|
|
(36.7
|
)
|
|||||
|
Payments on long-term lease obligations
|
(7.7
|
)
|
(8.4
|
)
|
(4.3
|
)
|
|||||
|
Noncontrolling interest distributions
|
(1.0
|
)
|
(2.1
|
)
|
—
|
|
|||||
|
Deferred payments on acquisitions
|
(2.6
|
)
|
(7.6
|
)
|
(0.1
|
)
|
|||||
|
Excess tax benefits from stock based compensation
|
—
|
|
—
|
|
13.1
|
|
|||||
|
Net proceeds from issuance of stock to employees
|
73.6
|
|
70.6
|
|
98.9
|
|
|||||
|
Purchase of common stock
|
(338.1
|
)
|
(43.9
|
)
|
—
|
|
|||||
|
Net cash (used for) provided by financing activities
|
631.9
|
|
(649.8
|
)
|
3,184.6
|
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
20.5
|
|
(13.2
|
)
|
(35.7
|
)
|
|||||
|
Net (decrease) increase in cash and cash equivalents
|
(116.9
|
)
|
(282.8
|
)
|
136.4
|
|
|||||
|
Cash and cash equivalents at beginning of year
|
433.6
|
|
716.4
|
|
580.0
|
|
|||||
|
Cash and cash equivalents at end of year
|
$
|
316.7
|
|
$
|
433.6
|
|
$
|
716.4
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
Total revenues
|
2017
|
2016
|
2015
|
||||||||
|
LCD - net revenue
|
$
|
7,170.5
|
|
$
|
6,593.9
|
|
$
|
6,199.3
|
|
||
|
CDD - net revenue
|
3,037.2
|
|
2,844.1
|
|
2,306.4
|
|
|||||
|
CDD - reimbursable out-of-pocket expenses
|
235.5
|
|
204.6
|
|
174.4
|
|
|||||
|
Intercompany eliminations
|
(1.8
|
)
|
(0.8
|
)
|
—
|
|
|||||
|
Total revenues
|
$
|
10,441.4
|
|
$
|
9,641.8
|
|
$
|
8,680.1
|
|
||
|
|
2017
|
2016
|
2015
|
|||||||||||||||||||||||||||||
|
Income
|
Shares
|
Per Share
Amount
|
Income
|
Shares
|
Per Share
Amount
|
Income
|
Shares
|
Per Share
Amount
|
||||||||||||||||||||||||
|
Basic earnings per share
|
$
|
1,268.2
|
|
102.4
|
|
$
|
12.39
|
|
$
|
732.1
|
|
102.5
|
|
$
|
7.14
|
|
$
|
437.6
|
|
98.8
|
|
$
|
4.43
|
|
||||||||
|
Stock options
|
—
|
|
1.4
|
|
|
|
—
|
|
1.5
|
|
|
|
—
|
|
1.2
|
|
|
|
||||||||||||||
|
Restricted stock awards and other
|
—
|
|
—
|
|
|
|
—
|
|
—
|
|
|
|
—
|
|
—
|
|
|
|
||||||||||||||
|
Effect of convertible debt, net of tax
|
—
|
|
0.1
|
|
|
|
—
|
|
0.3
|
|
|
|
—
|
|
0.6
|
|
|
|
||||||||||||||
|
Diluted earnings per share
|
$
|
1,268.2
|
|
103.9
|
|
$
|
12.21
|
|
$
|
732.1
|
|
104.3
|
|
$
|
7.02
|
|
$
|
437.6
|
|
100.6
|
|
$
|
4.35
|
|
||||||||
|
|
Years Ended December 31,
|
||||
|
|
2017
|
2016
|
2015
|
||
|
Stock options
|
0.1
|
—
|
—
|
||
|
|
Years
|
||
|
Buildings and building improvements
|
10
|
-
|
40
|
|
Machinery and equipment
|
3
|
-
|
10
|
|
Furniture and fixtures
|
5
|
-
|
10
|
|
Software
|
3
|
-
|
10
|
|
|
Years
|
||
|
Customer relationships
|
10
|
-
|
36
|
|
Patents, licenses and technology
|
3
|
-
|
15
|
|
Non-compete agreements
|
5
|
-
|
10
|
|
Trade names
|
5
|
-
|
15
|
|
Consideration Transferred
|
||||||||
|
Cash consideration
|
$
|
1,224.5
|
|
|||||
|
Initial
|
Measurement Period Adjustments
|
Preliminary as of December 31, 2017
|
||||||||||
|
Net Assets Acquired
|
||||||||||||
|
Cash and cash equivalents
|
$
|
30.7
|
|
$
|
—
|
|
$
|
30.7
|
|
|||
|
Accounts receivable
|
116.9
|
|
(11.3
|
)
|
105.6
|
|
||||||
|
Unbilled services
|
32.6
|
|
—
|
|
32.6
|
|
||||||
|
Prepaid expenses and other
|
57.9
|
|
—
|
|
57.9
|
|
||||||
|
Property, plant and equipment
|
12.1
|
|
—
|
|
12.1
|
|
||||||
|
Goodwill
|
676.6
|
|
83.9
|
|
760.5
|
|
||||||
|
Customer relationships
|
629.0
|
|
(27.0
|
)
|
602.0
|
|
||||||
|
Trade names and trademarks
|
24.1
|
|
(13.5
|
)
|
10.6
|
|
||||||
|
Technology
|
47.0
|
|
(21.0
|
)
|
26.0
|
|
||||||
|
Favorable leases
|
—
|
|
0.9
|
|
0.9
|
|
||||||
|
Total assets acquired
|
1,626.9
|
|
12.0
|
|
1,638.9
|
|
||||||
|
Accounts payable
|
18.1
|
|
27.0
|
|
45.1
|
|
||||||
|
Accrued expenses and other
|
51.0
|
|
(27.6
|
)
|
23.4
|
|
||||||
|
Unearned revenue
|
124.2
|
|
—
|
|
124.2
|
|
||||||
|
Deferred income taxes
|
208.0
|
|
12.6
|
|
220.6
|
|
||||||
|
Other liabilities
|
1.1
|
|
—
|
|
1.1
|
|
||||||
|
Total liabilities acquired
|
402.4
|
|
12.0
|
|
414.4
|
|
||||||
|
Net assets acquired
|
$
|
1,224.5
|
|
$
|
—
|
|
$
|
1,224.5
|
|
|||
|
Year Ended
|
||||||
|
December 31, 2017
|
December 31, 2016
|
|||||
|
Total net revenues
|
$
|
10,576.3
|
|
$
|
9,937.4
|
|
|
Operating income
|
1,377.0
|
|
1,327.2
|
|
||
|
Net income
|
1,258.3
|
|
714.3
|
|
||
|
Earnings per share:
|
||||||
|
Basic
|
$
|
12.29
|
|
$
|
6.97
|
|
|
Diluted
|
$
|
12.11
|
|
$
|
6.85
|
|
|
Year Ended
December 31, 2015
|
|||
|
Total revenues
|
$
|
9,033.3
|
|
|
Operating income
|
1,117.2
|
|
|
|
Net income
|
547.5
|
|
|
|
Earnings per share:
|
|||
|
Basic
|
$
|
5.05
|
|
|
Diluted
|
$
|
5.03
|
|
|
LCD
|
CDD
|
Total
|
|||||||||||||||||
|
Severance and Other
Employee Costs
|
Lease and Other
Facility Costs
|
Severance and Other
Employee Costs
|
Lease and Other
Facility Costs
|
||||||||||||||||
|
Balance as of December 31, 2016
|
$
|
7.5
|
|
$
|
14.1
|
|
$
|
28.2
|
|
$
|
32.5
|
|
$
|
82.3
|
|
||||
|
Restructuring charges
|
11.4
|
|
6.6
|
|
24.7
|
|
33.3
|
|
76.0
|
|
|||||||||
|
Reduction of prior restructuring accruals
|
(1.1
|
)
|
(0.1
|
)
|
(3.5
|
)
|
(0.4
|
)
|
(5.1
|
)
|
|||||||||
|
Cash payments and other adjustments
|
(16.1
|
)
|
(10.5
|
)
|
(41.1
|
)
|
(30.8
|
)
|
(98.5
|
)
|
|||||||||
|
Balance as of December 31, 2017
|
$
|
1.7
|
|
$
|
10.1
|
|
$
|
8.3
|
|
$
|
34.6
|
|
$
|
54.7
|
|
||||
|
Current
|
|
|
|
|
$
|
22.2
|
|
||||||||||||
|
Non-current
|
|
|
|
|
32.5
|
|
|||||||||||||
|
|
|
|
|
|
$
|
54.7
|
|
||||||||||||
|
Locations
|
Net Investment
|
Interest Owned
|
||||
|
Joint Venture Partnerships
:
|
|
|
||||
|
Alberta, Canada (2)
|
$
|
45.5
|
|
43.37
|
%
|
|
|
Florence, South Carolina
|
10.1
|
|
49.00
|
%
|
||
|
PAML joint ventures
|
$
|
(0.1
|
)
|
various
|
|
|
|
Equity Method Investments
:
|
||||||
|
Various
|
3.7
|
|
various
|
|
||
|
As of December 31:
|
2017
|
2016
|
|||||
|
Current assets
|
$
|
45.7
|
|
$
|
24.3
|
|
|
|
Other assets
|
14.4
|
|
16.9
|
|
|||
|
Total assets
|
$
|
60.1
|
|
$
|
41.2
|
|
|
|
Current liabilities
|
$
|
31.6
|
|
$
|
15.5
|
|
|
|
Other liabilities
|
1.0
|
|
0.3
|
|
|||
|
Total liabilities
|
32.6
|
|
15.8
|
|
|||
|
Partners' equity
|
27.5
|
|
25.4
|
|
|||
|
Total liabilities and partners’ equity
|
$
|
60.1
|
|
$
|
41.2
|
|
|
|
For the period January 1 - December 31:
|
2017
|
2016
|
2015
|
||||||||
|
Net revenues
|
$
|
212.5
|
|
$
|
156.7
|
|
$
|
213.7
|
|
||
|
Gross profit
|
62.6
|
|
45.7
|
|
54.3
|
|
|||||
|
Net earnings
|
25.7
|
|
20.3
|
|
20.1
|
|
|||||
|
December 31, 2017
|
December 31,
2016 |
||||||
|
Gross accounts receivable
|
$
|
1,742.2
|
|
$
|
1,564.3
|
|
|
|
Less allowance for doubtful accounts
|
(260.9
|
)
|
(235.6
|
)
|
|||
|
|
$
|
1,481.3
|
|
$
|
1,328.7
|
|
|
|
December 31, 2017
|
December 31, 2016
|
||||||
|
Land
|
$
|
78.4
|
|
$
|
78.4
|
|
|
|
Buildings and building improvements
|
708.6
|
|
692.8
|
|
|||
|
Machinery and equipment
|
1,181.0
|
|
1,060.1
|
|
|||
|
Software
|
672.2
|
|
626.2
|
|
|||
|
Leasehold improvements
|
333.5
|
|
302.0
|
|
|||
|
Furniture and fixtures
|
90.6
|
|
76.9
|
|
|||
|
Construction in progress
|
185.1
|
|
193.0
|
|
|||
|
Equipment and real estate under capital leases
|
79.2
|
|
81.3
|
|
|||
|
|
3,328.6
|
|
3,110.7
|
|
|||
|
Less accumulated depreciation and amortization of capital lease assets
|
(1,579.7
|
)
|
(1,392.1
|
)
|
|||
|
|
$
|
1,748.9
|
|
$
|
1,718.6
|
|
|
|
LCD
|
CDD
|
Total
|
|||||||||||||||||||||
|
December 31, 2017
|
December 31, 2016
|
December 31, 2017
|
December 31, 2016
|
December 31, 2017
|
December 31, 2016
|
||||||||||||||||||
|
Balance as of January 1
|
$
|
3,644.8
|
|
$
|
3,137.7
|
|
$
|
2,779.6
|
|
$
|
3,064.4
|
|
$
|
6,424.4
|
|
$
|
6,202.1
|
|
|||||
|
Goodwill acquired during the year
|
198.5
|
|
398.3
|
|
811.3
|
|
—
|
|
1,009.8
|
|
398.3
|
|
|||||||||||
|
Foreign currency impact and other adjustments to goodwill
|
1.1
|
|
108.8
|
|
94.7
|
|
(284.8
|
)
|
95.8
|
|
(176.0
|
)
|
|||||||||||
|
Balance at end of year
|
$
|
3,844.4
|
|
$
|
3,644.8
|
|
$
|
3,685.6
|
|
$
|
2,779.6
|
|
$
|
7,530.0
|
|
$
|
6,424.4
|
|
|||||
|
|
December 31, 2017
|
December 31, 2016
|
|||||||||||||||||||||
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
Carrying
Amount
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
Carrying
Amount
|
||||||||||||||||||
|
Customer relationships
|
$
|
4,297.9
|
|
$
|
(1,014.9
|
)
|
$
|
3,283.0
|
|
$
|
3,275.3
|
|
$
|
(855.2
|
)
|
$
|
2,420.1
|
|
|||||
|
Patents, licenses and technology
|
457.9
|
|
(188.6
|
)
|
269.3
|
|
395.3
|
|
(163.3
|
)
|
232.0
|
|
|||||||||||
|
Non-compete agreements
|
79.0
|
|
(49.4
|
)
|
29.6
|
|
53.0
|
|
(42.1
|
)
|
10.9
|
|
|||||||||||
|
Trade names
|
426.3
|
|
(171.4
|
)
|
254.9
|
|
406.3
|
|
(141.6
|
)
|
264.7
|
|
|||||||||||
|
Land use rights
|
10.9
|
|
(2.6
|
)
|
8.3
|
|
10.0
|
|
(1.4
|
)
|
8.6
|
|
|||||||||||
|
Canadian licenses
|
495.7
|
|
—
|
|
495.7
|
|
464.2
|
|
—
|
|
464.2
|
|
|||||||||||
|
|
$
|
5,767.7
|
|
$
|
(1,426.9
|
)
|
$
|
4,340.8
|
|
$
|
4,604.1
|
|
$
|
(1,203.6
|
)
|
$
|
3,400.5
|
|
|||||
|
Amount
|
Weighted Average
Amortization Period
|
||||
|
Customer relationships
|
$
|
955.7
|
|
20.7
|
|
|
Patents, licenses and technology
|
52.9
|
|
6.7
|
||
|
Non-compete agreements
|
27.8
|
|
6.6
|
||
|
Trade names
|
15.7
|
|
5.7
|
||
|
Favorable leases
|
0.9
|
|
3.0
|
||
|
$
|
1,053.0
|
|
19.3
|
||
|
December 31, 2017
|
December 31, 2016
|
||||||
|
Employee compensation and benefits
|
$
|
324.6
|
|
$
|
288.2
|
|
|
|
Self-insurance reserves
|
45.7
|
|
48.2
|
|
|||
|
Accrued taxes payable
|
64.5
|
|
61.2
|
|
|||
|
Royalty and license fees payable
|
6.2
|
|
9.5
|
|
|||
|
Restructuring reserves
|
22.2
|
|
47.7
|
|
|||
|
Acquisition related reserves
|
21.3
|
|
10.3
|
|
|||
|
Interest payable
|
71.7
|
|
58.6
|
|
|||
|
Rebates
|
24.6
|
|
19.5
|
|
|||
|
Other
|
52.1
|
|
50.5
|
|
|||
|
|
$
|
632.9
|
|
$
|
593.7
|
|
|
|
December 31, 2017
|
December 31, 2016
|
||||||
|
Post-retirement benefit obligation
|
$
|
7.4
|
|
$
|
5.8
|
|
|
|
Defined-benefit plan obligation
|
163.4
|
|
195.4
|
|
|||
|
Restructuring reserves
|
32.5
|
|
34.6
|
|
|||
|
Self-insurance reserves
|
28.9
|
|
17.1
|
|
|||
|
Acquisition related reserves
|
3.1
|
|
15.7
|
|
|||
|
Deferred compensation plan obligation
|
64.5
|
|
54.2
|
|
|||
|
Worker's compensation and auto
|
39.0
|
|
33.1
|
|
|||
|
Straight-line rent
|
12.7
|
|
13.3
|
|
|||
|
Escheat liability
|
10.9
|
|
8.3
|
|
|||
|
Other
|
15.8
|
|
14.5
|
|
|||
|
$
|
378.2
|
|
$
|
392.0
|
|
||
|
December 31, 2017
|
December 31, 2016
|
||||||
|
Zero-coupon convertible subordinated notes
|
$
|
8.8
|
|
$
|
42.4
|
|
|
|
2.20% Senior Notes due 2017
|
—
|
|
500.0
|
|
|||
|
2.50% Senior Notes due 2018
|
400.0
|
|
—
|
|
|||
|
Debt issuance costs
|
(1.4
|
)
|
(1.3
|
)
|
|||
|
Capital lease obligation
|
8.3
|
|
8.4
|
|
|||
|
Current portion of note payable
|
1.8
|
|
—
|
|
|||
|
Total short-term borrowings and current portion of long-term debt
|
$
|
417.5
|
|
$
|
549.5
|
|
|
|
December 31, 2017
|
December 31, 2016
|
||||||
|
2.50% Senior Notes due 2018
|
—
|
|
400.0
|
|
|||
|
4.625% Senior Notes due 2020
|
604.1
|
|
614.6
|
|
|||
|
2.625% Senior Notes due 2020
|
500.0
|
|
500.0
|
|
|||
|
3.75% Senior Notes due 2022
|
500.0
|
|
500.0
|
|
|||
|
3.20% Senior Notes due 2022
|
500.0
|
|
500.0
|
|
|||
|
4.00% Senior Notes due 2023
|
300.0
|
|
300.0
|
|
|||
|
3.25% Senior Notes due 2024
|
600.0
|
|
—
|
|
|||
|
3.60% Senior Notes due 2025
|
1,000.0
|
|
1,000.0
|
|
|||
|
3.60% Senior Notes due 2027
|
600.0
|
|
—
|
|
|||
|
4.70% Senior Notes due 2045
|
900.0
|
|
900.0
|
|
|||
|
2014 Term loan
|
72.0
|
|
565.0
|
|
|||
|
2017 Term loan
|
750.0
|
|
—
|
|
|||
|
Debt issuance costs
|
(48.2
|
)
|
(43.0
|
)
|
|||
|
Capital leases
|
57.8
|
|
56.2
|
|
|||
|
Note payable
|
8.9
|
|
7.2
|
|
|||
|
Total long-term debt
|
$
|
6,344.6
|
|
$
|
5,300.0
|
|
|
|
1)
|
The sales price of the Company’s common stock for at least
20
trading days in a period of
30
consecutive trading days ending on the last trading day of the preceding quarter reaches specified thresholds (beginning at
120%
and declining
0.1282%
per quarter until it reaches approximately
110%
for the quarter beginning
July 1, 2021
of the accreted conversion price per share of common stock on the last day of the preceding quarter). The accreted conversion price per share will equal the issue price of a note plus the accrued original issue discount and any accrued contingent additional principal, divided by the number of shares of common stock issuable upon conversion of a note on that day. The conversion trigger price for the fourth quarter of
2017
was
$77.45
.
|
|
2)
|
The credit rating assigned to the notes by S&P Ratings Services is at or below BB-.
|
|
3)
|
The Notes are called for redemption.
|
|
4)
|
Specified corporate transactions have occurred (such as if the Company is party to a consolidation, merger or binding share exchange or a transfer of all or substantially all of its assets).
|
|
Notes and Other
|
Capital Leases
|
Total
|
|||||||||
|
2018
|
$
|
409.2
|
|
$
|
15.6
|
|
$
|
424.8
|
|
||
|
2019
|
35.9
|
|
14.4
|
|
50.3
|
|
|||||
|
2020
|
1,226.9
|
|
13.1
|
|
1,240.0
|
|
|||||
|
2021
|
71.0
|
|
11.7
|
|
82.7
|
|
|||||
|
2022
|
1,601.3
|
|
10.6
|
|
1,611.9
|
|
|||||
|
Thereafter
|
3,351.7
|
|
45.1
|
|
3,396.8
|
|
|||||
|
6,696.0
|
|
110.5
|
|
6,806.5
|
|
||||||
|
Less amounts representing interest
|
—
|
|
(44.4
|
)
|
(44.4
|
)
|
|||||
|
Total long-term debt
|
6,696.0
|
|
66.1
|
|
6,762.1
|
|
|||||
|
Less current portion
|
(409.2
|
)
|
(8.3
|
)
|
(417.5
|
)
|
|||||
|
Long-term debt, due beyond one year
|
$
|
6,286.8
|
|
$
|
57.8
|
|
$
|
6,344.6
|
|
||
|
|
2017
|
2016
|
|||
|
Issued
|
125.1
|
|
125.6
|
|
|
|
In treasury
|
(23.2
|
)
|
(22.9
|
)
|
|
|
Outstanding
|
101.9
|
|
102.7
|
|
|
|
Common Shares Issued
|
|
|
|
|||||
|
|
2017
|
2016
|
2015
|
|||||
|
Common stock issued at January 1
|
125.6
|
|
123.9
|
|
107.1
|
|
||
|
Common stock issued under employee stock plans
|
1.7
|
|
1.6
|
|
1.5
|
|
||
|
Common stock issued upon conversion of zero-coupon subordinated notes
|
0.3
|
|
0.4
|
|
—
|
|
||
|
Common stock issued in conjunction with the Covance acquisition
|
—
|
|
—
|
|
15.3
|
|
||
|
Retirement of common stock
|
(2.5
|
)
|
(0.3
|
)
|
—
|
|
||
|
Common stock issued at December 31
|
125.1
|
|
125.6
|
|
123.9
|
|
||
|
Common Shares Held in Treasury
|
|
|
|
|||||
|
|
2017
|
2016
|
2015
|
|||||
|
Common shares held in treasury at January 1
|
22.9
|
|
22.6
|
|
22.5
|
|
||
|
Surrender of restricted stock and performance share awards
|
0.3
|
|
0.3
|
|
0.1
|
|
||
|
Common shares held in treasury at December 31
|
23.2
|
|
22.9
|
|
22.6
|
|
||
|
Foreign
Currency
Translation
Adjustments
|
Net
Benefit
Plan
Adjustments
|
Unrealized Gains and Losses on Available for Sale Securities
|
Accumulated
Other
Comprehensive
Earnings
|
||||||||||||
|
Balance at December 31, 2014
|
$
|
68.0
|
|
$
|
(78.6
|
)
|
$
|
0.1
|
|
$
|
(10.5
|
)
|
|||
|
Current year adjustments
|
(370.7
|
)
|
19.0
|
|
(0.1
|
)
|
(351.8
|
)
|
|||||||
|
Amounts reclassified from accumulated other comprehensive income
(a) (b)
|
—
|
|
(11.3
|
)
|
—
|
|
(11.3
|
)
|
|||||||
|
Tax effect of adjustments
|
90.1
|
|
(3.5
|
)
|
—
|
|
86.6
|
|
|||||||
|
Balance at December 31, 2015
|
(212.6
|
)
|
(74.4
|
)
|
—
|
|
(287.0
|
)
|
|||||||
|
Current year adjustments
|
(250.0
|
)
|
(3.3
|
)
|
—
|
|
(253.3
|
)
|
|||||||
|
Amounts reclassified from accumulated other comprehensive income
(a)
|
—
|
|
(37.0
|
)
|
—
|
|
(37.0
|
)
|
|||||||
|
Tax effect of adjustments
|
(8.1
|
)
|
4.3
|
|
—
|
|
(3.8
|
)
|
|||||||
|
Balance at December 31, 2016
|
(470.7
|
)
|
(110.4
|
)
|
—
|
|
(581.1
|
)
|
|||||||
|
Current year adjustments
|
262.3
|
|
2.4
|
|
—
|
|
264.7
|
|
|||||||
|
Amounts reclassified from accumulated other comprehensive income
(a)
|
—
|
|
18.5
|
|
—
|
|
18.5
|
|
|||||||
|
Tax effect of adjustments
|
(34.3
|
)
|
(3.5
|
)
|
—
|
|
(37.8
|
)
|
|||||||
|
Balance at December 31, 2017
|
$
|
(242.7
|
)
|
$
|
(93.0
|
)
|
$
|
—
|
|
$
|
(335.7
|
)
|
|||
|
Pre-tax income
|
2017
|
2016
|
2015
|
||||||||
|
Domestic
|
$
|
891.8
|
|
$
|
914.0
|
|
$
|
593.5
|
|
||
|
Foreign
|
243.1
|
|
191.5
|
|
132.5
|
|
|||||
|
Total pre-tax income
|
$
|
1,134.9
|
|
$
|
1,105.5
|
|
$
|
726.0
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Current:
|
|
|
|
||||||||
|
Federal
|
$
|
300.8
|
|
$
|
235.1
|
|
$
|
218.3
|
|
||
|
State
|
32.9
|
|
38.6
|
|
33.7
|
|
|||||
|
Foreign
|
53.0
|
|
43.9
|
|
69.4
|
|
|||||
|
|
$
|
386.7
|
|
$
|
317.6
|
|
$
|
321.4
|
|
||
|
Deferred:
|
|
|
|
|
|
|
|||||
|
Federal
|
$
|
(534.3
|
)
|
$
|
64.4
|
|
$
|
(14.1
|
)
|
||
|
State
|
12.9
|
|
6.0
|
|
(4.2
|
)
|
|||||
|
Foreign
|
(4.4
|
)
|
(15.7
|
)
|
(15.8
|
)
|
|||||
|
|
(525.8
|
)
|
54.7
|
|
(34.1
|
)
|
|||||
|
|
$
|
(139.1
|
)
|
$
|
372.3
|
|
$
|
287.3
|
|
||
|
|
Years Ended December 31,
|
|||||||
|
|
2017
|
2016
|
2015
|
|||||
|
Statutory U.S. rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State and local income taxes, net of U.S. Federal income tax effect
|
2.5
|
|
2.6
|
|
3.2
|
|
||
|
Foreign earnings taxed at lower rates than the statutory U.S. rate
|
(3.5
|
)
|
(3.1
|
)
|
(1.8
|
)
|
||
|
Restructuring and acquisition items
|
0.6
|
|
—
|
|
2.7
|
|
||
|
Share-based compensation
|
(1.7
|
)
|
(1.2
|
)
|
—
|
|
||
|
Re-measurement of deferred taxes
|
(35.0
|
)
|
—
|
|
—
|
|
||
|
Deferred taxes on unremitted foreign earnings
|
(15.8
|
)
|
—
|
|
—
|
|
||
|
Repatriation tax
|
5.0
|
|
—
|
|
—
|
|
||
|
Other
|
0.6
|
|
0.4
|
|
1.1
|
|
||
|
Effective rate
|
(12.3
|
)%
|
33.7
|
%
|
40.2
|
%
|
||
|
December 31, 2017
|
December 31, 2016
|
||||||
|
Deferred tax assets:
|
|
|
|||||
|
Accounts receivable
|
$
|
14.7
|
|
$
|
13.5
|
|
|
|
Employee compensation and benefits
|
113.6
|
|
160.8
|
|
|||
|
Acquisition and restructuring reserves
|
16.8
|
|
38.5
|
|
|||
|
Tax loss carryforwards
|
107.0
|
|
167.7
|
|
|||
|
Other
|
26.0
|
|
44.8
|
|
|||
|
|
278.1
|
|
425.3
|
|
|||
|
Less: valuation allowance
|
(42.8
|
)
|
(31.3
|
)
|
|||
|
Deferred tax assets, net of valuation allowance
|
$
|
235.3
|
|
$
|
394.0
|
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|||
|
Deferred earnings
|
$
|
(5.1
|
)
|
$
|
(193.2
|
)
|
|
|
Intangible assets
|
(913.2
|
)
|
(1,047.4
|
)
|
|||
|
Property, plant and equipment
|
(156.9
|
)
|
(208.8
|
)
|
|||
|
Zero-coupon subordinated notes
|
(10.1
|
)
|
(48.5
|
)
|
|||
|
Currency translation adjustment
|
(0.1
|
)
|
(47.0
|
)
|
|||
|
Other
|
(27.2
|
)
|
(27.9
|
)
|
|||
|
Total gross deferred tax liabilities
|
(1,112.6
|
)
|
(1,572.8
|
)
|
|||
|
Net deferred tax liabilities
|
$
|
(877.3
|
)
|
$
|
(1,178.8
|
)
|
|
|
|
2017
|
2016
|
2015
|
||||||||
|
Balance as of January 1
|
$
|
18.4
|
|
$
|
24.2
|
|
$
|
16.7
|
|
||
|
Increase in reserve for tax positions taken in the current year
|
7.3
|
|
2.3
|
|
4.1
|
|
|||||
|
Increase in reserve as a result of acquisition
|
—
|
|
—
|
|
8.5
|
|
|||||
|
Decrease in reserve as a result of lapses in the statute of limitations
|
(6.2
|
)
|
(8.1
|
)
|
(5.1
|
)
|
|||||
|
Balance as of December 31
|
$
|
19.5
|
|
$
|
18.4
|
|
$
|
24.2
|
|
||
|
Number of
Options
|
Weighted-Average
Exercise Price
per Option
|
Weighted-Average
Remaining
Contractual Term
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Outstanding at December 31, 2016
|
1.6
|
|
$
|
82.43
|
|
|
|
|||||
|
Granted
|
0.1
|
|
130.60
|
|
|
|
||||||
|
Exercised
|
(0.5
|
)
|
83.85
|
|
|
|
||||||
|
Cancelled
|
—
|
|
80.37
|
|
|
|
||||||
|
Outstanding at December 31, 2017
|
1.2
|
|
$
|
86.55
|
|
3.9
|
$
|
85.4
|
|
|||
|
Vested and expected to vest at December 31, 2017
|
1.1
|
|
$
|
81.73
|
|
3.3
|
$
|
82.1
|
|
|||
|
Exercisable at December 31, 2017
|
1.1
|
|
$
|
81.73
|
|
3.3
|
$
|
82.1
|
|
|||
|
|
2017
|
2016
|
2015
|
||||||||
|
Cash received by the Company
|
$
|
43.9
|
|
$
|
52.6
|
|
$
|
82.6
|
|
||
|
Tax benefits realized
|
$
|
13.4
|
|
$
|
13.6
|
|
$
|
16.2
|
|
||
|
Aggregate intrinsic value
|
$
|
34.8
|
|
$
|
35.5
|
|
$
|
42.2
|
|
||
|
Options Outstanding
|
Options Exercisable
|
|||||||||
|
Range of
Exercise Prices
|
Number
Outstanding
|
Weighted Average
|
Number
Exercisable
|
Weighted
Average
Exercise
Price
|
||||||
|
Remaining
Contractual
Life
|
Average
Exercise
Price
|
|||||||||
|
$59.38 - 67.60
|
0.1
|
1.1
|
$60.64
|
0.1
|
$60.64
|
|||||
|
$67.61 - 75.63
|
0.2
|
1.8
|
$72.06
|
0.2
|
$72.06
|
|||||
|
$75.64 - 84.86
|
0.6
|
4.2
|
$84.73
|
0.6
|
$84.73
|
|||||
|
$84.87 - 130.60
|
0.3
|
5.2
|
$105.29
|
0.3
|
$91.13
|
|||||
|
|
1.2
|
3.9
|
$86.55
|
1.2
|
$81.73
|
|||||
|
|
2017
|
2016
|
2015
|
||||
|
Fair value per option
|
$
|
32.75
|
|
N/A
|
N/A
|
||
|
Valuation assumptions
|
|||||||
|
Weighted average expected life (in years)
|
6.0
|
|
N/A
|
N/A
|
|||
|
Risk free interest rate
|
2.1
|
%
|
N/A
|
N/A
|
|||
|
Expected volatility
|
0.2
|
|
N/A
|
N/A
|
|||
|
Expected dividend yield
|
N/A
|
|
N/A
|
N/A
|
|||
|
Number of
Shares
|
Weighted-Average
Grant Date
Fair Value
|
|||||
|
Non-vested at January 1, 2017
|
1.6
|
|
$
|
108.23
|
|
|
|
Granted
|
0.9
|
|
132.48
|
|
||
|
Vested
|
(0.9
|
)
|
100.86
|
|
||
|
Canceled
|
(0.1
|
)
|
118.74
|
|
||
|
Non-vested at December 31, 2017
|
1.5
|
|
$
|
121.36
|
|
|
|
|
2017
|
2016
|
2015
|
||||||||
|
Fair value of the employee’s purchase right
|
$
|
31.54
|
|
$
|
23.32
|
|
$
|
21.95
|
|
||
|
Valuation assumptions
|
|
|
|
|
|
|
|||||
|
Risk free interest rate
|
1.3
|
%
|
0.5
|
%
|
0.3
|
%
|
|||||
|
Expected volatility
|
0.2
|
|
0.2
|
|
0.2
|
|
|||||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
|||||
|
|
Operating
|
||
|
2018
|
$
|
196.1
|
|
|
2019
|
149.0
|
|
|
|
2020
|
109.7
|
|
|
|
2021
|
83.5
|
|
|
|
2022
|
64.2
|
|
|
|
Thereafter
|
160.9
|
|
|
|
Total minimum lease payments
|
763.4
|
|
|
|
Less:
|
|
|
|
|
Amounts included in restructuring and acquisition related accruals
|
(17.6
|
)
|
|
|
Non-cancelable sub-lease income
|
—
|
|
|
|
Total minimum operating lease payments
|
$
|
745.8
|
|
|
|
Year ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Service cost for benefits earned
|
$
|
5.5
|
|
$
|
4.9
|
|
$
|
3.9
|
|
||
|
Interest cost on benefit obligation
|
14.4
|
|
15.5
|
|
15.1
|
|
|||||
|
Expected return on plan assets
|
(16.3
|
)
|
(16.7
|
)
|
(18.3
|
)
|
|||||
|
Net amortization and deferral
|
11.0
|
|
11.2
|
|
11.3
|
|
|||||
|
Defined-benefit plan costs
|
$
|
14.6
|
|
$
|
14.9
|
|
$
|
12.0
|
|
||
|
|
2017
|
2016
|
|||||
|
Balance at January 1
|
$
|
366.5
|
|
$
|
363.1
|
|
|
|
Service cost
|
5.5
|
|
4.9
|
|
|||
|
Interest cost
|
14.4
|
|
15.5
|
|
|||
|
Actuarial loss
|
12.2
|
|
12.1
|
|
|||
|
Benefits and administrative expenses paid
|
(30.6
|
)
|
(29.1
|
)
|
|||
|
Balance at December 31
|
$
|
368.0
|
|
$
|
366.5
|
|
|
|
|
2017
|
2016
|
|||||
|
Fair value of plan assets at beginning of year
|
$
|
247.5
|
|
$
|
250.6
|
|
|
|
Actual return on plan assets
|
29.2
|
|
13.6
|
|
|||
|
Employer contributions
|
17.6
|
|
12.4
|
|
|||
|
Benefits and administrative expenses paid
|
(30.6
|
)
|
(29.1
|
)
|
|||
|
Fair value of plan assets at end of year
|
$
|
263.7
|
|
$
|
247.5
|
|
|
|
2017
|
2016
|
||||||
|
Funded status
|
$
|
104.3
|
|
$
|
119.0
|
|
|
|
Recorded as:
|
|||||||
|
Accrued expenses and other
|
$
|
2.2
|
|
$
|
2.0
|
|
|
|
Other liabilities
|
102.1
|
|
117.0
|
|
|||
|
$
|
104.3
|
|
$
|
119.0
|
|
||
|
|
2017
|
2016
|
2015
|
|||||
|
Discount rate
|
3.7
|
%
|
4.2
|
%
|
4.0
|
%
|
||
|
Expected long term rate of return
|
6.8
|
%
|
6.8
|
%
|
7.0
|
%
|
||
|
Target
Allocation |
Weighted Average
Expected Long-Term
Rate of Return
|
||||
|
Equity securities
|
50.0
|
%
|
5.6
|
%
|
|
|
Fixed income securities
|
45.0
|
%
|
1.1
|
%
|
|
|
Other assets
|
5.0
|
%
|
0.1
|
%
|
|
|
Fair Value Measurements as of
|
|||||||||||||||
|
December 31, 2017
|
|||||||||||||||
|
Fair Value as of December 31, 2017
|
Using Fair Value Hierarchy
|
||||||||||||||
|
Asset Category
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Cash
|
$
|
7.4
|
|
$
|
7.4
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Equity securities:
|
|
|
|
|
|
|
|
|
|||||||
|
U.S. large cap - blend (a)
|
59.9
|
|
—
|
|
59.9
|
|
—
|
|
|||||||
|
U.S. mid cap - blend (b)
|
23.6
|
|
—
|
|
23.6
|
|
—
|
|
|||||||
|
U.S. small cap - blend (c)
|
7.7
|
|
—
|
|
7.7
|
|
—
|
|
|||||||
|
International equity - blend (d)
|
39.1
|
|
—
|
|
39.1
|
|
—
|
|
|||||||
|
Real estate (e)
|
12.8
|
|
—
|
|
12.8
|
|
—
|
|
|||||||
|
Fixed income securities:
|
|
|
|
|
|
|
|
|
|||||||
|
U.S. fixed income (f)
|
107.0
|
|
—
|
|
107.0
|
|
—
|
|
|||||||
|
U.S inflation protection income (g)
|
6.2
|
|
—
|
|
6.2
|
|
—
|
|
|||||||
|
Total fair value of the Company Plan’s assets
|
$
|
263.7
|
|
$
|
7.4
|
|
$
|
256.3
|
|
$
|
—
|
|
|||
|
Fair Value Measurements as of
|
|||||||||||||||
|
December 31, 2016
|
|||||||||||||||
|
Fair Value as of December 31, 2016
|
Using Fair Value Hierarchy
|
||||||||||||||
|
Asset Category
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Cash
|
$
|
6.8
|
|
$
|
6.8
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Equity securities:
|
|
|
|
|
|
|
|
|
|||||||
|
U.S. large cap - blend (a)
|
55.3
|
|
—
|
|
55.3
|
|
—
|
|
|||||||
|
U.S. mid cap - blend (b)
|
21.2
|
|
—
|
|
21.2
|
|
—
|
|
|||||||
|
U.S. small cap - blend (c)
|
7.4
|
|
—
|
|
7.4
|
|
—
|
|
|||||||
|
International equity - blend (d)
|
36.1
|
|
—
|
|
36.1
|
|
—
|
|
|||||||
|
Commodities index (h)
|
12.9
|
|
—
|
|
12.9
|
|
—
|
|
|||||||
|
Fixed income securities:
|
|
|
|
|
|
|
|
|
|||||||
|
U.S. fixed income (f)
|
101.8
|
|
—
|
|
101.8
|
|
—
|
|
|||||||
|
U.S inflation protection income (g)
|
6.0
|
|
—
|
|
6.0
|
|
—
|
|
|||||||
|
Total fair value of the Company Plan’s assets
|
$
|
247.5
|
|
$
|
6.8
|
|
$
|
240.7
|
|
$
|
—
|
|
|||
|
a)
|
This category represents an equity index fund not actively managed that tracks the S&P 500 Index.
|
|
b)
|
This category represents an equity index fund not actively managed that tracks the S&P mid-cap 400 Index.
|
|
c)
|
This category represents an equity index fund not actively managed that tracks the Russell 2000 Index.
|
|
d)
|
This category represents an equity index fund not actively managed that tracks the MSCI ACWI ex USA Index.
|
|
e)
|
This category represents a real estate index fund not actively managed that tracks the MSCI US REIT Index.
|
|
f)
|
This category primarily represents bond index funds not actively managed that track the Barclays Capital U.S. Aggregate Index as well as an actively managed strategy which utilizes the Barclays Capital U.S. Aggregate Bond Index as its primary prospectus benchmark.
|
|
g)
|
This category primarily represents a bond index fund not actively managed that tracks the Barclays Capital U.S. TIPS Index.
|
|
h)
|
This category represents a commodities index fund not actively managed that tracks the Dow Jones - UBS Commodity Index.
|
|
2018
|
$
|
27.2
|
|
|
2019
|
26.6
|
|
|
|
2020
|
26.2
|
|
|
|
2021
|
25.5
|
|
|
|
2022
|
25.4
|
|
|
|
Years 2023 and thereafter
|
118.2
|
|
|
|
Year Ended December 31, 2017
|
Year Ended December 31, 2016
|
||||||
|
Service cost
|
$
|
—
|
|
$
|
—
|
|
|
|
Interest cost
|
0.2
|
|
0.8
|
|
|||
|
Settlement gain
|
(0.3
|
)
|
—
|
|
|||
|
Net periodic pension cost
|
$
|
(0.1
|
)
|
$
|
0.8
|
|
|
|
Assumptions used to determine defined-benefit plan cost
|
|||||||
|
Discount rate
|
4.2
|
%
|
3.8
|
%
|
|||
|
Expected return on assets
|
N/A
|
|
N/A
|
|
|||
|
2017
|
2016
|
||||||
|
Balance at beginning of year
|
$
|
7.5
|
|
$
|
30.9
|
|
|
|
Service cost
|
—
|
|
—
|
|
|||
|
Interest cost
|
0.2
|
|
0.8
|
|
|||
|
Actuarial loss/(gain)
|
0.3
|
|
(0.8
|
)
|
|||
|
Gross benefits paid
|
(3.7
|
)
|
(25.0
|
)
|
|||
|
Termination benefits
|
—
|
|
1.6
|
|
|||
|
Balance at end of year
|
$
|
4.3
|
|
$
|
7.5
|
|
|
|
2017
|
2016
|
||||||
|
Funded status
|
$
|
4.3
|
|
$
|
7.5
|
|
|
|
Recorded as:
|
|||||||
|
Accrued expenses and other
|
$
|
0.9
|
|
$
|
3.7
|
|
|
|
Other liabilities
|
3.4
|
|
3.8
|
|
|||
|
$
|
4.3
|
|
$
|
7.5
|
|
||
|
2018
|
$
|
0.9
|
|
|
2019
|
0.1
|
|
|
|
2020
|
0.1
|
|
|
|
2021
|
0.1
|
|
|
|
2022
|
0.1
|
|
|
|
Year 2023 and thereafter
|
0.9
|
|
|
|
U.K. Plans
|
||||||||
|
Year Ended December 31, 2017
|
Year Ended December 31, 2016
|
|||||||
|
Service cost
|
$
|
5.1
|
|
$
|
4.4
|
|
||
|
Interest cost
|
7.6
|
|
8.4
|
|
||||
|
Expected return on plan assets
|
(11.5
|
)
|
(11.6
|
)
|
||||
|
Net amortization and deferral
|
0.7
|
|
—
|
|
||||
|
Expected participant contributions
|
(1.3
|
)
|
(1.5
|
)
|
||||
|
Defined-benefit plan costs
|
$
|
0.6
|
|
$
|
(0.3
|
)
|
||
|
Assumptions used to determine defined-benefit plan cost:
|
||||||||
|
Discount rate
|
2.7
|
%
|
3.8
|
%
|
||||
|
Expected return on assets
|
4.7
|
%
|
5.6
|
%
|
||||
|
Salary increases
|
3.8
|
%
|
3.6
|
%
|
||||
|
German Plan
|
||||||||
|
Year Ended December 31, 2017
|
Year Ended December 31, 2016
|
|||||||
|
Service cost
|
$
|
1.2
|
|
$
|
0.9
|
|
||
|
Interest cost
|
0.5
|
|
0.6
|
|
||||
|
Net amortization and deferral
|
—
|
|
(0.2
|
)
|
||||
|
Defined-benefit plan costs
|
$
|
1.7
|
|
$
|
1.3
|
|
||
|
Assumptions used to determine defined-benefit plan cost:
|
||||||||
|
Discount rate
|
1.7
|
%
|
2.5
|
%
|
||||
|
Expected return on assets
|
N/A
|
|
N/A
|
|
||||
|
Salary increases
|
2.0
|
%
|
2.0
|
%
|
||||
|
Change in Projected Benefit Obligation:
|
U.K. Plans
|
|||||||
|
2017
|
2016
|
|||||||
|
Balance at beginning of year
|
$
|
278.1
|
|
$
|
246.5
|
|
||
|
Service cost
|
5.1
|
|
4.4
|
|
||||
|
Interest cost
|
7.6
|
|
8.4
|
|
||||
|
Actuarial (gain) loss
|
(7.7
|
)
|
72.6
|
|
||||
|
Benefits paid
|
(6.1
|
)
|
(4.2
|
)
|
||||
|
Foreign currency exchange rate changes
|
26.4
|
|
(49.6
|
)
|
||||
|
Balance at end of year
|
$
|
303.4
|
|
$
|
278.1
|
|
||
|
Change in Projected Benefit Obligation:
|
German Plan
|
|||||||
|
2017
|
2016
|
|||||||
|
Balance at beginning of year
|
$
|
29.0
|
|
$
|
23.6
|
|
||
|
Service cost
|
1.2
|
|
0.9
|
|
||||
|
Interest cost
|
0.5
|
|
0.6
|
|
||||
|
Actuarial (gain) loss
|
1.0
|
|
5.3
|
|
||||
|
Benefits paid
|
(0.2
|
)
|
(0.2
|
)
|
||||
|
Foreign currency exchange rate changes
|
4.2
|
|
(1.2
|
)
|
||||
|
Balance at end of year
|
$
|
35.7
|
|
$
|
29.0
|
|
||
|
Change in Fair Value of Assets:
|
U.K. Plans
|
|||||||
|
2017
|
2016
|
|||||||
|
Balance at beginning of year
|
$
|
233.2
|
|
$
|
226.2
|
|
||
|
Company contributions
|
6.3
|
|
6.8
|
|
||||
|
Participant contributions
|
1.3
|
|
1.5
|
|
||||
|
Actual return on assets
|
23.6
|
|
46.2
|
|
||||
|
Benefits paid
|
(6.1
|
)
|
(4.2
|
)
|
||||
|
Foreign currency exchange rate changes
|
23.6
|
|
(43.3
|
)
|
||||
|
Fair value of plan assets at end of year
|
$
|
281.9
|
|
$
|
233.2
|
|
||
|
U.K. Plans
|
||||||||
|
2017
|
2016
|
|||||||
|
Funded status
|
$
|
21.5
|
|
$
|
44.9
|
|
||
|
Recorded as:
|
||||||||
|
Other liabilities
|
21.5
|
|
44.9
|
|
||||
|
$
|
21.5
|
|
$
|
44.9
|
|
|||
|
German Plan
|
||||||||
|
2017
|
2016
|
|||||||
|
Funded status
|
$
|
35.7
|
|
$
|
29.0
|
|
||
|
Recorded as:
|
||||||||
|
Accrued expenses and other
|
$
|
0.3
|
|
$
|
0.2
|
|
||
|
Other liabilities
|
35.4
|
|
28.8
|
|
||||
|
$
|
35.7
|
|
$
|
29.0
|
|
|||
|
U.K. Plans
|
||||||||
|
2017
|
2016
|
|||||||
|
Net actuarial loss
|
$
|
17.2
|
|
$
|
39.2
|
|
||
|
Less: Tax benefit (deferred tax asset)
|
(2.9
|
)
|
(6.7
|
)
|
||||
|
Accumulated other comprehensive income impact
|
$
|
14.3
|
|
$
|
32.5
|
|
||
|
Assumptions used to determine benefit obligations:
|
||||||||
|
Discount rate
|
2.5
|
%
|
2.7
|
%
|
||||
|
Salary increases
|
3.6
|
%
|
3.8
|
%
|
||||
|
German Plan
|
||||||||
|
2017
|
2016
|
|||||||
|
Net actuarial loss/(gain)
|
$
|
0.7
|
|
$
|
(0.4
|
)
|
||
|
Less: Tax expense (deferred tax liability)
|
(0.2
|
)
|
0.1
|
|
||||
|
Accumulated other comprehensive income impact
|
$
|
0.5
|
|
$
|
(0.3
|
)
|
||
|
Assumptions used to determine benefit obligations:
|
||||||||
|
Discount rate
|
1.7
|
%
|
1.7
|
%
|
||||
|
Salary increases
|
2.0
|
%
|
2.0
|
%
|
||||
|
Equity securities
|
60.0%
|
to
|
70.0%
|
|
|
Debt securities
|
10.0%
|
to
|
15.0%
|
|
|
Annuities
|
10.0%
|
to
|
20.0%
|
|
|
Real estate
|
—%
|
to
|
10.0%
|
|
|
Other
|
—%
|
to
|
5.0%
|
|
|
December 31, 2017
|
||
|
Equity securities
|
66.0%
|
|
|
Debt securities
|
19.0%
|
|
|
Annuities
|
11.0%
|
|
|
Real estate
|
4.0%
|
|
|
Fair Value Measurements as of
|
|||||||||||||||
|
December 31, 2017
|
|||||||||||||||
|
December 31,
2017 |
Using Fair Value Hierarchy
|
||||||||||||||
|
Asset Category
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Cash
|
$
|
0.8
|
|
$
|
0.8
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Mutual funds (a)
|
249.6
|
|
—
|
|
249.6
|
|
—
|
|
|||||||
|
Annuities (b)
|
31.5
|
|
—
|
|
—
|
|
31.5
|
|
|||||||
|
Total fair value of the Company Plan’s assets
|
$
|
281.9
|
|
$
|
0.8
|
|
$
|
249.6
|
|
$
|
31.5
|
|
|||
|
Fair Value Measurements as of
|
|||||||||||||||
|
December 31, 2016
|
|||||||||||||||
|
December 31,
2016 |
Using Fair Value Hierarchy
|
||||||||||||||
|
Asset Category
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Cash
|
$
|
0.9
|
|
$
|
0.9
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Mutual funds (a)
|
202.5
|
|
—
|
|
202.5
|
|
—
|
|
|||||||
|
Annuities (b)
|
29.8
|
|
—
|
|
—
|
|
29.8
|
|
|||||||
|
Total fair value of the Company Plan’s assets
|
$
|
233.2
|
|
$
|
0.9
|
|
$
|
202.5
|
|
$
|
29.8
|
|
|||
|
a)
|
Mutual funds represent pooled investment vehicles offered by investment managers, which are generally comprised of investments in equities, bonds, property and cash. The plans’ trustees hold units in these funds, the value of which is determined by the number of units held multiplied by the unit price calculated by the investment managers. That unit price is derived based on the market value of the securities that comprise the fund, which are determined by quoted prices in active markets. No element of the valuation is based on inputs made by the plans’ trustees.
|
|
b)
|
Annuities represent annuity buy-in insurance policies, whereby the insurer pays the pension payments for the lifetime of the members covered. The annuities are assets of the plan and payments from the insurer are made to the plans’ trustees, who then use those proceeds to pay the pensioners. The cash flows from the annuities are intended to effectively match the payments to the pensioners covered by the policy. As such, these assets are valued actuarially based upon the value of the liabilities with which they are associated. As the valuation of these assets is judgmental, and there are no observable inputs associated with the valuation, these assets are classified as Level 3 in the fair value hierarchy.
|
|
U.K. Plans
|
German Plan
|
|||||||
|
2018
|
$
|
4.4
|
|
$
|
0.3
|
|
||
|
2019
|
5.3
|
|
0.4
|
|
||||
|
2020
|
5.5
|
|
0.6
|
|
||||
|
2021
|
6.0
|
|
0.6
|
|
||||
|
2022
|
7.4
|
|
0.7
|
|
||||
|
Years 2023 and thereafter
|
43.0
|
|
3.7
|
|
||||
|
Year Ended December 31, 2017
|
Year Ended December 31, 2016
|
|||||
|
Interest cost
|
$
|
—
|
|
$
|
—
|
|
|
Actuarial gain
|
(0.1
|
)
|
(0.1
|
)
|
||
|
Prior service credit
|
(1.9
|
)
|
(1.9
|
)
|
||
|
Net periodic post-retirement benefit cost
|
$
|
(2.0
|
)
|
$
|
(2.0
|
)
|
|
Assumptions used to determine net periodic post-retirement benefit cost:
|
||||||
|
Discount rate
|
4.1
|
%
|
4.2
|
%
|
||
|
Healthcare cost trend rate
|
N/A
|
|
7.0
|
%
|
||
|
2017
|
2016
|
|||||
|
Balance at beginning of year
|
$
|
0.8
|
|
$
|
5.2
|
|
|
Interest cost
|
—
|
|
—
|
|
||
|
Participant contributions
|
—
|
|
0.7
|
|
||
|
Actuarial (gain) loss
|
(0.1
|
)
|
0.1
|
|
||
|
Benefits paid
|
—
|
|
(1.3
|
)
|
||
|
Plan amendments
|
—
|
|
(3.9
|
)
|
||
|
Balance at end of year
|
$
|
0.7
|
|
$
|
0.8
|
|
|
2017
|
2016
|
|||||
|
Funded status
|
$
|
0.7
|
|
$
|
0.8
|
|
|
Recorded as:
|
||||||
|
Accrued expenses and other
|
$
|
0.1
|
|
$
|
0.1
|
|
|
Other liabilities
|
0.6
|
|
0.7
|
|
||
|
$
|
0.7
|
|
$
|
0.8
|
|
|
|
Year Ended December 31, 2017
|
Year Ended December 31, 2016
|
|||||
|
Net actuarial gain
|
$
|
(0.7
|
)
|
$
|
(2.6
|
)
|
|
Less: Deferred tax benefit
|
0.3
|
|
0.9
|
|
||
|
Accumulated other comprehensive income impact
|
$
|
(0.4
|
)
|
$
|
(1.7
|
)
|
|
Assumptions used to determine benefit obligation:
|
||||
|
Discount rate
|
3.6
|
%
|
4.1
|
%
|
|
Healthcare cost trend rate
|
N/A
|
|
6.7
|
%
|
|
2018
|
$
|
0.1
|
|
|
2019
|
0.1
|
|
|
|
2020
|
0.1
|
|
|
|
2021
|
0.1
|
|
|
|
2022
|
0.1
|
|
|
|
2023 and thereafter
|
0.2
|
|
|
|
|
Year ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Service cost for benefits earned
|
$
|
—
|
|
$
|
—
|
|
$
|
0.1
|
|
||
|
Interest cost on benefit obligation
|
0.3
|
|
0.3
|
|
1.0
|
|
|||||
|
Net amortization and deferral
|
(6.7
|
)
|
(15.9
|
)
|
(10.4
|
)
|
|||||
|
Post-retirement medical plan costs
|
$
|
(6.4
|
)
|
$
|
(15.6
|
)
|
$
|
(9.3
|
)
|
||
|
|
2017
|
2016
|
|||||
|
Balance at January 1
|
$
|
6.8
|
|
$
|
21.4
|
|
|
|
Service cost for benefits earned
|
—
|
|
—
|
|
|||
|
Interest cost on benefit obligation
|
0.3
|
|
0.3
|
|
|||
|
Actuarial loss
|
2.5
|
|
(0.2
|
)
|
|||
|
Benefits paid
|
(1.0
|
)
|
(1.3
|
)
|
|||
|
Plan amendment
|
—
|
|
(13.4
|
)
|
|||
|
Balance at December 31
|
$
|
8.6
|
|
$
|
6.8
|
|
|
|
Recorded as:
|
|||||||
|
Accrued expenses and other
|
$
|
1.2
|
|
$
|
1.0
|
|
|
|
Other liabilities
|
7.4
|
|
5.8
|
|
|||
|
$
|
8.6
|
|
$
|
6.8
|
|
||
|
2018
|
$
|
1.3
|
|
|
2019
|
1.1
|
|
|
|
2020
|
1.0
|
|
|
|
2021
|
1.0
|
|
|
|
2022
|
0.9
|
|
|
|
Years 2023 and thereafter
|
2.8
|
|
|
|
Fair Value Measurements as of
|
|||||||||||||||
|
December 31, 2017
|
|||||||||||||||
|
Fair Value as of December 31, 2017
|
Using Fair Value Hierarchy
|
||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Noncontrolling interest put
|
$
|
16.7
|
|
$
|
—
|
|
$
|
16.7
|
|
$
|
—
|
|
|||
|
Interest rate swap
|
4.1
|
|
—
|
|
4.1
|
|
—
|
|
|||||||
|
Cash surrender value of life insurance policies
|
64.0
|
|
—
|
|
64.0
|
|
—
|
|
|||||||
|
Deferred compensation liability
|
64.5
|
|
—
|
|
64.5
|
|
—
|
|
|||||||
|
Contingent consideration
|
16.5
|
|
—
|
|
—
|
|
16.5
|
|
|||||||
|
Fair Value Measurements as of
|
|||||||||||||||
|
December 31, 2016
|
|||||||||||||||
|
Fair Value as of December 31, 2016
|
Using Fair Value Hierarchy
|
||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Noncontrolling interest put
|
$
|
15.2
|
|
$
|
—
|
|
$
|
15.2
|
|
$
|
—
|
|
|||
|
Interest rate swap
|
14.6
|
|
—
|
|
14.6
|
|
—
|
|
|||||||
|
Cash surrender value of life insurance policies
|
53.6
|
|
—
|
|
53.6
|
|
—
|
|
|||||||
|
Deferred compensation liability
|
54.2
|
|
—
|
|
54.2
|
|
—
|
|
|||||||
|
Contingent consideration
|
16.8
|
|
—
|
|
—
|
|
16.8
|
|
|||||||
|
1)
|
The Company will pay contingent cash interest on the zero-coupon subordinated notes after September 11, 2006, if the average market price of the notes equals
120%
or more of the sum of the issue price, accrued original issue discount and contingent additional principal, if any, for a specified measurement period.
|
|
2)
|
Holders may surrender zero-coupon subordinated notes for conversion during any period in which the rating assigned to the zero-coupon subordinated notes by S&P’s Ratings Services is BB- or lower.
|
|
Years Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Supplemental schedule of cash flow information:
|
|
|
|
||||||||
|
Cash paid during period for:
|
|
|
|
||||||||
|
Interest
|
$
|
239.1
|
|
$
|
210.7
|
|
$
|
166.1
|
|
||
|
Income taxes, net of refunds
|
348.0
|
|
345.7
|
|
237.6
|
|
|||||
|
Disclosure of non-cash financing and investing activities:
|
|
|
|
|
|
|
|||||
|
Surrender of restricted stock awards and performance shares
|
47.4
|
|
34.6
|
|
12.6
|
|
|||||
|
Conversion of zero-coupon convertible debt
|
35.0
|
|
39.1
|
|
1.1
|
|
|||||
|
Assets acquired under capital leases
|
7.3
|
|
16.0
|
|
22.6
|
|
|||||
|
Accrued property, plant and equipment
|
1.6
|
|
4.4
|
|
4.3
|
|
|||||
|
2017
|
2016
|
2015
|
||||||||||
|
Net Revenues:
|
||||||||||||
|
LCD
|
$
|
7,170.5
|
|
$
|
6,593.9
|
|
$
|
6,199.3
|
|
|||
|
CDD
|
3,037.2
|
|
2,844.1
|
|
2,306.4
|
|
||||||
|
Intercompany eliminations
|
(1.8
|
)
|
(0.8
|
)
|
—
|
|
||||||
|
Total net revenues
|
$
|
10,205.9
|
|
$
|
9,437.2
|
|
$
|
8,505.7
|
|
|||
|
Operating Earnings (Loss):
|
||||||||||||
|
LCD
|
$
|
1,298.6
|
|
$
|
1,187.6
|
|
$
|
1,053.7
|
|
|||
|
CDD
|
206.2
|
|
272.7
|
|
73.5
|
|
||||||
|
General corporate expenses
|
(140.6
|
)
|
(147.9
|
)
|
(130.4
|
)
|
||||||
|
Total operating income
|
1,364.2
|
|
1,312.4
|
|
996.8
|
|
||||||
|
Non-operating expenses, net
|
(229.3
|
)
|
(206.9
|
)
|
(270.8
|
)
|
||||||
|
Earnings before income taxes
|
1,134.9
|
|
1,105.5
|
|
726.0
|
|
||||||
|
Provision for income taxes
|
(139.1
|
)
|
372.3
|
|
287.3
|
|
||||||
|
Net earnings
|
1,274.0
|
|
733.2
|
|
438.7
|
|
||||||
|
Less: Net income attributable to noncontrolling interests
|
(5.8
|
)
|
(1.1
|
)
|
(1.1
|
)
|
||||||
|
Net income attributable to Laboratory Corporation of America Holdings
|
$
|
1,268.2
|
|
$
|
732.1
|
|
$
|
437.6
|
|
|||
|
2017
|
2016
|
2015
|
||||||||||
|
Depreciation and Amortization
|
||||||||||||
|
LCD
|
$
|
304.7
|
|
$
|
270.9
|
|
$
|
245.8
|
|
|||
|
CDD
|
217.4
|
|
219.5
|
|
184.4
|
|
||||||
|
General corporate
|
1.2
|
|
0.1
|
|
4.1
|
|
||||||
|
Total depreciation and amortization
|
$
|
523.3
|
|
$
|
490.5
|
|
$
|
434.3
|
|
|||
|
LCD
|
CDD
|
Intercompany Eliminations
|
Total
|
|||||||||||||
|
Geographic distribution of net revenues
|
||||||||||||||||
|
US
|
$
|
6,808.6
|
|
$
|
1,427.2
|
|
$
|
(1.8
|
)
|
$
|
8,234.0
|
|
||||
|
Canada
|
327.8
|
|
—
|
|
—
|
|
327.8
|
|
||||||||
|
United Kingdom
|
29.6
|
|
272.4
|
|
—
|
|
302.0
|
|
||||||||
|
Switzerland
|
—
|
|
484.4
|
|
—
|
|
484.4
|
|
||||||||
|
Other
|
4.5
|
|
853.2
|
|
—
|
|
857.7
|
|
||||||||
|
Total net revenues
|
$
|
7,170.5
|
|
$
|
3,037.2
|
|
$
|
(1.8
|
)
|
$
|
10,205.9
|
|
||||
|
LCD
|
CDD
|
Total
|
||||||||||
|
Geographic distribution of property, plant and equipment, net
|
||||||||||||
|
U.S.
|
$
|
826.2
|
|
$
|
590.6
|
|
$
|
1,416.8
|
|
|||
|
Canada
|
54.6
|
|
—
|
|
54.6
|
|
||||||
|
U.K.
|
2.1
|
|
120.7
|
|
122.8
|
|
||||||
|
Switzerland
|
—
|
|
81.4
|
|
81.4
|
|
||||||
|
Other
|
3.0
|
|
70.3
|
|
73.3
|
|
||||||
|
Total property, plant and equipment, net
|
$
|
885.9
|
|
$
|
863.0
|
|
$
|
1,748.9
|
|
|||
|
Year Ended December 31, 2017
|
|||||||||||||||||||
|
1st
Quarter
|
2nd
Quarter
|
3rd
Quarter
|
4th
Quarter
(a)
|
Full
Year
|
|||||||||||||||
|
Net revenues
|
$
|
2,408.1
|
|
$
|
2,498.4
|
|
$
|
2,597.9
|
|
$
|
2,701.5
|
|
$
|
10,205.9
|
|
||||
|
Gross profit
|
803.6
|
|
861.8
|
|
882.8
|
|
915.8
|
|
3,464.0
|
|
|||||||||
|
Net earnings attributable to Laboratory Corporation of America Holdings
|
192.2
|
|
188.6
|
|
180.6
|
|
706.8
|
|
1,268.2
|
|
|||||||||
|
Basic earnings per common share
|
1.87
|
|
1.84
|
|
1.77
|
|
6.91
|
|
12.39
|
|
|||||||||
|
Diluted earnings per common share
|
1.84
|
|
1.82
|
|
1.74
|
|
6.81
|
|
12.21
|
|
|||||||||
|
Year Ended December 31, 2016
|
|||||||||||||||||||
|
1st
Quarter
|
2nd
Quarter
|
3rd
Quarter
|
4th
Quarter
|
Full
Year
|
|||||||||||||||
|
Net revenues
|
$
|
2,295.2
|
|
$
|
2,382.0
|
|
$
|
2,372.7
|
|
$
|
2,387.3
|
|
$
|
9,437.2
|
|
||||
|
Gross profit
|
777.3
|
|
826.8
|
|
788.4
|
|
788.0
|
|
3,180.5
|
|
|||||||||
|
Net earnings attributable to Laboratory Corporation of America Holdings
|
164.1
|
|
204.1
|
|
179.5
|
|
184.4
|
|
732.1
|
|
|||||||||
|
Basic earnings per common share
|
1.61
|
|
2.00
|
|
1.74
|
|
1.79
|
|
7.14
|
|
|||||||||
|
Diluted earnings per common share
|
1.58
|
|
1.96
|
|
1.71
|
|
1.75
|
|
7.02
|
|
|||||||||
|
Balance at
beginning
of year
|
Additions
Charged to Costs and Expense
|
(1)
Other (Deductions)Additions |
Balance
at end
of year
|
||||||||||||
|
Year ended December 31, 2017:
|
|
|
|
|
|
||||||||||
|
Applied against asset accounts:
|
|
|
|
|
|
||||||||||
|
Allowance for doubtful accounts
|
$
|
235.6
|
|
$
|
314.7
|
|
$
|
(289.4
|
)
|
$
|
260.9
|
|
|||
|
Valuation allowance-deferred tax assets
|
$
|
31.3
|
|
$
|
11.5
|
|
$
|
—
|
|
$
|
42.8
|
|
|||
|
Year ended December 31, 2016:
|
|
|
|
|
|
|
|
|
|||||||
|
Applied against asset accounts:
|
|
|
|
|
|
|
|
|
|||||||
|
Allowance for doubtful accounts
|
$
|
217.0
|
|
$
|
287.3
|
|
$
|
(268.7
|
)
|
$
|
235.6
|
|
|||
|
Valuation allowance-deferred tax assets
|
$
|
15.1
|
|
$
|
16.2
|
|
$
|
—
|
|
$
|
31.3
|
|
|||
|
Year ended December 31, 2015:
|
|
|
|
|
|
|
|
|
|||||||
|
Applied against asset accounts:
|
|
|
|
|
|
|
|
|
|||||||
|
Allowance for doubtful accounts
|
$
|
211.6
|
|
$
|
265.4
|
|
$
|
(260.0
|
)
|
$
|
217.0
|
|
|||
|
Valuation allowance-deferred tax assets
|
$
|
17.1
|
|
$
|
—
|
|
$
|
(2.0
|
)
|
$
|
15.1
|
|
|||