|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
LANTHEUS HOLDINGS, INC.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
35-2318913
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
331 Treble Cove Road, North Billerica, MA
|
01862
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, $0.01 par value per share
|
NASDAQ Global Market
|
|
|
Large accelerated filer
|
☐
|
Accelerated filer
|
þ
|
|||
|
Non-accelerated filer
|
☐
|
Smaller reporting company
|
☐
|
|||
|
Emerging growth company
|
þ
|
|||||
|
Page
|
||
|
•
|
Our ability to continue to grow the appropriate use of DEFINITY in suboptimal echocardiograms in the face of segment competition from other echocardiography contrast agents, including Optison from GE Healthcare Limited (“GE Healthcare”) and Lumason from Bracco Diagnostics Inc. (“Bracco”), and potential generic competition as a result of future patent and regulatory exclusivity expirations;
|
|
•
|
The instability of the global Moly supply, including outages at the NTP Radioisotopes (“NTP”) processing facility in South Africa from late November 2017 until mid-February 2018 and again from early June 2018 through mid-November 2018, resulting in our inability to fill all of the demand for our TechneLite generators on certain manufacturing days during those periods;
|
|
•
|
Risks associated with revenues and unit volumes for Xenon in pulmonary studies as a result of increased competition from Curium;
|
|
•
|
Our dependence upon third parties for the manufacture and supply of a substantial portion of our products, raw materials and components, including DEFINITY at JHS;
|
|
•
|
Our dependence on key customers for our medical imaging products, and our ability to maintain and profitably renew our contracts with those key customers, including Cardinal Health (“Cardinal”), United Pharmacy Partners (“UPPI”), GE Healthcare and Jubilant Drax Image Radiopharmaceuticals (“JDI”) d/b/a Triad Isotopes, Inc. (“Triad”);
|
|
•
|
Our inability to identify and acquire or in-license additional products, businesses or technologies to drive our future growth;
|
|
•
|
Risks associated with the technology transfer programs to secure production of our products at additional contract manufacturer sites, including a modified formulation of DEFINITY at Samsung BioLogics (“SBL”) in South Korea;
|
|
•
|
Risks associated with our lead agent in development, flurpiridaz F 18, which in 2017 we out-licensed to GE Healthcare, including:
|
|
•
|
The ability to successfully complete the Phase 3 development program;
|
|
•
|
The ability to obtain Food and Drug Administration (“FDA”) approval; and
|
|
•
|
The ability to gain post-approval market acceptance and adequate reimbursement;
|
|
•
|
Risks associated with the internal clinical development of DEFINITY for a left ventricular ejection fraction (“LVEF”) indication and LMI 1195 for patient populations that would benefit from molecular imaging of the norepinephrine pathway, including risk stratification of ischemic heart failure patients at risk of sudden cardiac death;
|
|
•
|
Risks associated with the manufacturing and distribution of our products and the regulatory requirements related thereto;
|
|
•
|
Risks associated with our investment in, and construction of, additional specialized manufacturing capabilities at our North Billerica, Massachusetts facility;
|
|
•
|
The dependence of certain of our customers upon third-party healthcare payors and the uncertainty of third-party coverage and reimbursement rates;
|
|
•
|
Uncertainties regarding the impact of on-going U.S. healthcare reform proposals on our business, including related reimbursements for our current and potential future products;
|
|
•
|
Our being subject to extensive government regulation, our potential inability to comply with those regulations and the costs of compliance;
|
|
•
|
Potential liability associated with our marketing and sales practices;
|
|
•
|
The occurrence of any serious or unanticipated side effects with our products;
|
|
•
|
Our exposure to potential product liability claims and environmental, health and safety liability;
|
|
•
|
The extensive costs, time and uncertainty associated with new product development, including further product development relying on external development partners or potentially developed internally;
|
|
•
|
Our inability to introduce new products and adapt to an evolving technology and medical practice landscape;
|
|
•
|
Our inability to protect our intellectual property and the risk of claims that we have infringed on the intellectual property of others;
|
|
•
|
Risks associated with prevailing economic or political conditions and events and financial, business and other factors beyond our control;
|
|
•
|
Risks associated with our international operations;
|
|
•
|
Our inability to adequately operate, maintain and protect our facilities, equipment and technology infrastructure;
|
|
•
|
Our inability to hire or retain skilled employees and key personnel;
|
|
•
|
Our inability to utilize, or limitations in our ability to utilize, net operating loss carryforwards to reduce our future tax liability;
|
|
•
|
Risks related to our outstanding indebtedness and our ability to satisfy those obligations;
|
|
•
|
Costs and other risks associated with the Sarbanes-Oxley Act and the Dodd-Frank Act, including in connection with potentially becoming a large accelerated filer;
|
|
•
|
Risks related to the ownership of our common stock; and
|
|
•
|
Other factors that are described in Part I, Item. 1A. “Risk Factors” of this Annual Report on Form 10-K.
|
|
•
|
U.S. Segment
produces and markets our medical imaging agents and products throughout the U.S. In the U.S., we primarily sell our products to radiopharmacies, integrated delivery networks, hospitals, clinics and group practices.
|
|
•
|
International Segment
operations consist of production and distribution activities in Puerto Rico and some direct distribution activities in Canada. Additionally, within our International Segment, we have established and maintain third-party distribution relationships under which our products are marketed and sold in Europe, Canada, Australia, Asia-Pacific and Latin America.
|
|
•
|
Ultrasound contrast agents are compounds that are used in diagnostic procedures, such as cardiac ultrasounds or echocardiograms, to improve the clarity of the diagnostic image.
|
|
•
|
Medical radiopharmaceuticals are radioactive pharmaceuticals used by clinicians to perform nuclear imaging procedures.
|
|
•
|
In certain circumstances, a radioactive element, or radioisotope, is attached to a chemical compound to form the radiopharmaceutical. This act of attaching the radioisotope to the chemical compound is called radiolabeling, or labeling.
|
|
•
|
In other circumstances, a radioisotope can be used as a radiopharmaceutical without attaching any additional chemical compound.
|
|
•
|
Radioisotopes are most commonly manufactured in a nuclear research reactor, where a target is bombarded with subatomic particles, or in a cyclotron, which is a type of particle accelerator that also creates radioisotopes.
|
|
•
|
Two common forms of nuclear imaging procedures are single-photon emission computed tomography (“SPECT”) which measures gamma rays emitted by a SPECT radiopharmaceutical, and positron emission tomography (“PET”) which measures positrons emitted by a PET radiopharmaceutical.
|
|
•
|
Patents
- We continue to actively pursue additional patents in connection with DEFINITY, both in the U.S. and internationally. In the U.S., we have an Orange Book-listed method of use patent expiring in March 2037. This patent augments an Orange Book-listed composition of matter patent expiring in June 2019, and additional manufacturing patents that are not Orange Book-listed expiring in 2021, 2023 and 2037. Outside of the U.S., our DEFINITY patent protection or regulatory exclusivity currently expires in 2019.
|
|
•
|
LVEF Indication
- We are currently conducting two well-controlled Phase 3 studies designed to demonstrate improved accuracy of LVEF measurements with DEFINITY-enhanced echocardiography versus unenhanced echocardiography. The truth standard in these studies is cardiac magnetic resonance imaging. The studies will be conducted at 20 U.S. sites and will eventually enroll a total of approximately 300 subjects. We believe DEFINITY could improve the accuracy of LVEF calculations, giving clinicians greater confidence in patient management decisions. An LVEF indication could substantially increase the addressable market for contrast-enhanced echocardiography. We believe that DEFINITY, as the market leader, would benefit from the expanded addressable market.
|
|
•
|
Modified Formulation
- We are developing at SBL a modified formulation of DEFINITY. We believe this modified formulation will provide an enhanced product profile by enabling storage as well as shipment at room temperature (DEFINITY’s current formulation requires refrigerated storage), will give clinicians additional choice, and will allow for greater utility of this formulation in broader clinical settings. We were recently granted a composition of matter patent on the modified formulation which runs through December 2035. If the modified formulation is approved by the FDA, then this patent would be eligible to be listed in the Orange Book. We currently believe that, if approved by the FDA, the modified formulation could become commercially available in 2020, although that timing cannot be assured. Given its physical characteristics, the modified formulation may also be better suited for inclusion in kits requiring microbubbles for other indications and applications.
|
|
•
|
New Applications
- As we continue to look for other opportunities to expand our microbubble franchise, we are evaluating new indications and applications beyond echocardiography and contrast imaging generally.
|
|
•
|
In-House Manufacturing
- We are currently building specialized in-house manufacturing capabilities at our North Billerica, Massachusetts facility for DEFINITY and, potentially, other sterile vial products. We believe the investment in these efforts will allow us to better control DEFINITY manufacturing and inventory, reduce our costs in a potentially more price competitive environment, and provide us with supply chain redundancy. We currently expect to be in a position to use this in-house manufacturing capability by early 2021, although that timing cannot be assured.
|
|
•
|
Xenon Xe 133 Gas
(“Xenon”) is a radiopharmaceutical gas that is inhaled and used to assess pulmonary function and also to image cerebral blood flow. Our Xenon is manufactured by a third party as a bi-product of Moly production and is processed and finished by us. We are currently the leading provider of Xenon in the U.S.
|
|
•
|
Neurolite
is an injectable, Technetium-labeled imaging agent used with SPECT technology to identify the area within the brain where blood flow has been blocked or reduced due to stroke. We launched Neurolite in 1995.
|
|
•
|
Cardiolite
, also known by its generic name sestamibi, is an injectable, Technetium-labeled imaging agent used in MPI procedures to assess blood flow to the muscle of the heart using SPECT. Cardiolite was approved by the FDA in 1990 and its market exclusivity expired in July 2008. Included in Cardiolite revenues are branded Cardiolite and generic sestamibi revenues.
|
|
•
|
Thallium TI 201
is an injectable radiopharmaceutical imaging agent used in MPI studies to detect cardiovascular disease. We have marketed Thallium since 1977 and manufacture the agent using cyclotron technology.
|
|
•
|
FDG
is an injectable, fluorine-18-radiolabeled imaging agent used with PET technology to identify and characterize tumors in patients undergoing oncologic diagnostic procedures. We manufacture and distribute FDG from our Puerto Rico radiopharmacy.
|
|
•
|
Gallium (Ga 67)
is an injectable radiopharmaceutical imaging agent used to detect certain infections and cancerous tumors, especially lymphoma. We manufacture Gallium using cyclotron technology.
|
|
•
|
Quadramet
, our only therapeutic product, is an injectable radiopharmaceutical used to treat severe bone pain associated with osteoblastic metastatic bone lesions. We serve as the direct manufacturer and supplier of Quadramet in the U.S.
|
|
•
|
Cobalt (Co 57)
is a non-pharmaceutical radiochemical used in the manufacture of sources for the calibration and maintenance of SPECT imaging cameras.
|
|
Product
|
Approved Markets
|
|
DEFINITY
|
Australia, Canada, European Union, European Economic Area, Israel, India, Mexico, New Zealand, Singapore, South Korea, Taiwan, United States
|
|
TechneLite
|
Australia, Brazil, Canada, China, Colombia, Costa Rica, New Zealand, Panama, South Korea, Taiwan, United States
|
|
Xenon
|
Canada, United States
|
|
Cardiolite
|
Australia, Canada, Costa Rica, Hong Kong, Israel, Japan, New Zealand, Panama, Philippines, South Korea, Taiwan, Thailand, United States
|
|
Neurolite
|
Australia, Austria, Belgium, Canada, Costa Rica, Denmark, Finland, France, Germany, Hong Kong, Italy, Japan, Luxembourg, New Zealand, Philippines, Slovenia, South Korea, Spain, Taiwan, Thailand, United States
|
|
Thallium Tl 201
|
Australia, Canada, Colombia, New Zealand, Pakistan, Panama, South Korea, Taiwan, United States
|
|
Gallium Ga 67
|
Australia, Canada, Colombia, Costa Rica, New Zealand, Pakistan, Panama, South Korea, Taiwan, United States
|
|
FDG
|
United States
|
|
Quadramet
|
United States
|
|
•
|
Cardinal maintains approximately
131
radiopharmacies that are typically located in large, densely populated urban areas in the U.S. We estimate that Cardinal’s radiopharmacies distributed approximately
45%
of the aggregate U.S. SPECT doses sold in the first half of
2018
(the latest information currently available to us). Our written supply agreement with Cardinal relating to TechneLite, Xenon, Neurolite and other products expires on
December 31, 2019
. The agreement specifies pricing levels and requirements to purchase minimum percentages of certain products during certain periods. The agreement may be terminated upon the occurrence of specified events, including a material breach by the other party and certain force majeure events.
|
|
•
|
UPPI is a cooperative purchasing group (roughly analogous to a group purchasing organization) of approximately
68
independently owned or smaller chain radiopharmacies located in the U.S. UPPI’s radiopharmacies are typically broadly dispersed geographically, with some urban presence and a substantial number of radiopharmacies located in suburban and rural areas of the country. We estimate that these independent radiopharmacies, together with approximately
33
unaffiliated, independent radiopharmacies, distributed approximately
24%
of the aggregate U.S. SPECT doses sold in the first half of
2018
. We currently have an agreement with UPPI for the distribution of TechneLite, Xenon and certain other products to radiopharmacies or families of radiopharmacies within the UPPI cooperative purchasing group. The agreement contains specified pricing levels based upon specified purchase amounts for UPPI. We are entitled to terminate the UPPI agreement upon 60 days written notice. The UPPI agreement expires on
December 31, 2019
.
|
|
•
|
GE Healthcare maintains approximately
31
radiopharmacies in the U.S. that purchase our TechneLite generators. We estimate that GE Healthcare distributed approximately
13%
of the aggregate U.S. SPECT doses sold in the first half of
2018
. We currently have an agreement with GE Healthcare for the distribution of TechneLite, Xenon and other products. The agreement provides that GE Healthcare will purchase a minimum percentage of TechneLite generators as well as certain other products from us. Our agreement, which expires on
December 31, 2020
, may be terminated by either party upon the occurrence of specified events including a material breach by either party, bankruptcy by either party, certain irresolvable regulatory changes or economic circumstances, or force majeure events.
|
|
•
|
Triad maintains approximately
56
radiopharmacies in the U.S. that purchase a range of our products. We estimate that Triad distributed approximately
12%
of the aggregate U.S. SPECT doses sold in the first half of
2018
. We currently have an agreement with Triad for the distribution of TechneLite, Xenon, Neurolite and other products. The agreement specifies pricing levels and percentage purchase requirements. The agreement will expire on
December 31, 2020
and may be terminated upon the occurrence of specified events, including a material breach by the other party.
|
|
•
|
DEFINITY
—In February 2012, we entered into a Manufacturing and Supply Agreement with JHS, for the manufacture of DEFINITY. Under the agreement, JHS manufactured DEFINITY for us for an initial term of five years. In September 2016, we extended the agreement through January 2022. The agreement contains automatic renewals for additional one-year periods thereafter. The agreement allows for termination upon the occurrence of certain events such as a material breach or default by either party, or bankruptcy by either party. The agreement also requires us to place orders for a minimum percentage of our requirements for DEFINITY with JHS. Based on our current projections, we believe that we will have sufficient supply of DEFINITY from JHS to meet expected demand.
|
|
•
|
Cardiolite
—In May 2012, we entered into a Manufacturing and Supply Agreement with JHS for the manufacture of Cardiolite products. In the third quarter of 2016, we completed the technology transfer process and received FDA approval to manufacture Cardiolite at JHS. Under the agreement, JHS has agreed to manufacture products for an initial term of five years from the effective date. On November 9, 2017, we extended the term until December 31, 2020, and the agreement can be further extended for three additional one-year periods thereafter so long as the parties, using good faith, reasonable efforts, agree to new pricing for the upcoming additional term. The agreement allows for termination upon the occurrence of specified events, including material breach or bankruptcy by either party. The agreement requires us to place orders for 100% of our requirements for Cardiolite products with JHS during such term. Based on our current projections, we believe that we will have sufficient supply of Cardiolite products from JHS to meet expected demand.
|
|
•
|
Neurolite
—In May 2012, we entered into a Manufacturing and Supply Agreement with JHS for the manufacture of Neurolite, and in January 2015, the FDA granted approval to manufacture Neurolite at JHS. Under the agreement, JHS agreed to manufacture Neurolite for an initial term of five years from the effective date. On November 9, 2017, we extended the term of the agreement until December 31, 2020, and the agreement can be further extended for three additional one-year periods thereafter so long as the parties, using good faith, reasonable efforts, agree to new pricing for the upcoming additional term. The agreement allows for termination upon the occurrence of specified events, including material breach or bankruptcy by either party. The agreement also requires us to place orders for 100% of our requirements for Neurolite during such term. Based on our current projections, we believe that we will have sufficient supply of Neurolite from JHS to meet expected demand.
|
|
•
|
Completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices regulations;
|
|
•
|
Submission to the FDA of an IND which must become effective before human clinical studies may begin, including review and approval by any individual review board (“IRB”), serving any of the institutions participating in the clinical studies;
|
|
•
|
Performance of adequate and well-controlled human clinical studies according to Good Clinical Practices and other requirements, to establish the safety and efficacy of the proposed drug product for its intended use;
|
|
•
|
Submission to the FDA of a new drug application, or NDA, for a new drug;
|
|
•
|
Satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug product is produced to assess compliance with current Good Manufacturing Practices (“cGMPs”) regulations; and
|
|
•
|
FDA review and approval of the NDA.
|
|
•
|
Phase 1.
The agent is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the agent may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients with those diseases.
|
|
•
|
Phase 2.
Involves studies in a limited patient population to identify possible adverse effects and safety risks, to evaluate preliminarily the efficacy of the agent for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule.
|
|
•
|
Phase 3.
Clinical studies are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to collect sufficient safety and efficacy data to support the NDA for FDA approval.
|
|
•
|
increasing the presumed utilization rate for imaging equipment costing $1 million or more in the physician office and free-standing imaging facility setting which reduces the Medicare per procedure medical imaging reimbursement; which rate was further increased by subsequent legislation effective January 1, 2014;
|
|
•
|
increasing drug rebates paid to state Medicaid programs under the Medicaid Drug Rebate Program for brand name prescription drugs and extending those rebates to Medicaid managed care organizations;
|
|
•
|
imposing a non-deductible annual fee on pharmaceutical manufacturers or importers who sell brand name prescription drugs to specified federal government programs;
|
|
•
|
imposing an excise tax on the sale of taxable medical devices, to be paid by the entity that manufactures or imports the device: (which tax applied to applicable sales made from January 1, 2013 through December 31, 2015, but is currently suspended for 2016 through 2019); and
|
|
•
|
amending the federal self-referral laws to require referring physicians ordering certain diagnostic imaging services to inform patients under certain circumstances that the patients may obtain the services from other local and unaffiliated suppliers (which may affect the setting in which a patient obtains services).
|
|
•
|
Limiting payments for imaging services in physician offices and free-standing imaging facility settings based upon rates paid to hospital outpatient departments;
|
|
•
|
Reducing payments for certain imaging procedures when performed together with other imaging procedures in the same family of procedures on the same patient on the same day in the physician office and free-standing imaging facility setting;
|
|
•
|
Making significant revisions to the methodology for determining the practice expense component of the Medicare payment applicable to the physician office and free-standing imaging facility setting which results in a reduction in payment;
|
|
•
|
Revising payment policies and reducing payment amounts for imaging procedures performed in the hospital outpatient setting; and
|
|
•
|
Reducing prospective payment levels for applicable diagnosis-related groups in the hospital inpatient setting.
|
|
•
|
Substantial modifications to our business practices and operations;
|
|
•
|
Significantly reduced demand for our products (if products become ineligible for reimbursement under federal and state healthcare programs);
|
|
•
|
A total or partial shutdown of production in one or more of the facilities where our products are produced while the alleged violation is being remediated;
|
|
•
|
Delays in or the inability to obtain future pre-market clearances or approvals; and
|
|
•
|
Withdrawals or suspensions of our current products from the market.
|
|
•
|
The availability of alternative products from our competitors;
|
|
•
|
The breadth of indications in which alternative products from our competitors can be marketed;
|
|
•
|
The price of our products relative to those of our competitors;
|
|
•
|
The timing of our market entry;
|
|
•
|
Our ability to market and distribute our products effectively;
|
|
•
|
Market acceptance of our products; and
|
|
•
|
Our ability to obtain adequate reimbursement.
|
|
•
|
A reduction of our current financial resources;
|
|
•
|
Incurrence of substantial debt or dilutive issuances of securities to pay for acquisitions;
|
|
•
|
Difficulty or inability to secure financing to fund development activities for those acquired or in-licensed technologies;
|
|
•
|
Higher than expected acquisition and integration costs;
|
|
•
|
Disruption of our business, customer base and diversion of our management’s time and attention to develop acquired products or technologies; and
|
|
•
|
Exposure to unknown liabilities.
|
|
•
|
We might not have been the first to make the inventions covered by each of our pending patent applications and issued patents, and we could lose our patent rights as a result;
|
|
•
|
We might not have been the first to file patent applications for these inventions or our patent applications may not have been timely filed, and we could lose our patent rights as a result;
|
|
•
|
Others may independently develop similar or alternative technologies or duplicate any of our technologies;
|
|
•
|
It is possible that none of our pending patent applications will result in any further issued patents;
|
|
•
|
Our issued patents may not provide a basis for commercially viable drugs, may not provide us with any protection from unauthorized use of our intellectual property by third parties, and may not provide us with any competitive advantages;
|
|
•
|
Our patent applications or patents may be subject to interferences, oppositions, post-grant review, ex-parte re-examinations, inter-partes review or similar administrative proceedings;
|
|
•
|
While we generally apply for patents in those countries where we intend to make, have made, use or sell patented products, we may not be able to accurately predict all of the countries where patent protection will ultimately be desirable and may be precluded from doing so at a later date;
|
|
•
|
We may choose not to seek patent protection in certain countries where the actual cost outweighs the perceived benefit at a certain time;
|
|
•
|
Patents issued in foreign jurisdictions may have different scopes of coverage than our U.S. patents and so our products may not receive the same degree of protection in foreign countries as they would in the U.S.;
|
|
•
|
We may not develop additional proprietary technologies that are patentable; or
|
|
•
|
The patents of others may have an adverse effect on our business.
|
|
•
|
Less stable political and economic environments and changes in a specific country’s or region’s political or economic conditions, including the potential for an unnegotiated exit by the United Kingdom from the EU;
|
|
•
|
Entering into, renewing or enforcing commercial agreements with international governments or provincial authorities or entities directly or indirectly owned or controlled by such governments or authorities, such as our Belgian, Australian and South African isotope suppliers, IRE, ANSTO and NTP, and our Chinese development and commercialization partner, Double-Crane Pharmaceutical Company;
|
|
•
|
International customers which are agencies or institutions owned or controlled by foreign governments;
|
|
•
|
Local business practices which may be in conflict with the U.S. Foreign Corrupt Practices Act and U.K. Bribery Act;
|
|
•
|
Currency fluctuations;
|
|
•
|
Unfavorable labor regulations;
|
|
•
|
Greater difficulties in relying on non-U.S. courts to enforce either local or U.S. laws, particularly with respect to intellectual property;
|
|
•
|
Greater potential for intellectual property piracy;
|
|
•
|
Greater difficulties in managing and staffing non-U.S. operations;
|
|
•
|
The need to ensure compliance with the numerous in-country and international regulatory and legal requirements applicable to our business in each of these jurisdictions and to maintain an effective compliance program to ensure compliance with these requirements, including in connection with the recently enacted GDPR in the EU;
|
|
•
|
Changes in public attitudes about the perceived safety of nuclear facilities;
|
|
•
|
Changes in trade policies, regulatory requirements and other barriers;
|
|
•
|
Civil unrest or other catastrophic events; and
|
|
•
|
Longer payment cycles of non-U.S. customers and difficulty collecting receivables in non-U.S. jurisdictions.
|
|
•
|
Require us to dedicate a substantial portion of cash flow from operations to the payment of interest on and principal of our indebtedness, thereby reducing the funds available for other purposes;
|
|
•
|
Make it more difficult for us to satisfy and comply with our obligations with respect to our outstanding indebtedness, namely the payment of interest and principal;
|
|
•
|
Make it more difficult to refinance the outstanding indebtedness;
|
|
•
|
Subject us to increased sensitivity to interest rate increases;
|
|
•
|
Make us more vulnerable to economic downturns, adverse industry or company conditions or catastrophic external events;
|
|
•
|
Limit our ability to withstand competitive pressures;
|
|
•
|
Reduce our flexibility in planning for or responding to changing business, industry and economic conditions; and
|
|
•
|
Place us at a competitive disadvantage to competitors that have relatively less debt than we have.
|
|
•
|
Maintain net leverage above certain specified levels;
|
|
•
|
Incur additional debt;
|
|
•
|
Pay dividends or make other distributions;
|
|
•
|
Redeem stock;
|
|
•
|
Issue stock of subsidiaries;
|
|
•
|
Make certain investments;
|
|
•
|
Create liens;
|
|
•
|
Enter into transactions with affiliates; and
|
|
•
|
Merge, consolidate or transfer all or substantially all of our assets.
|
|
•
|
Market conditions in the broader stock market;
|
|
•
|
Actual or anticipated fluctuations in our quarterly financial and operating results;
|
|
•
|
Issuance of new or changed securities analysts’ reports or recommendations;
|
|
•
|
Investor perceptions of us and the medical technology and pharmaceutical industries;
|
|
•
|
Sales, or anticipated sales, of large blocks of our stock;
|
|
•
|
Acquisitions or introductions of new products or services by us or our competitors;
|
|
•
|
Additions or departures of key personnel;
|
|
•
|
Regulatory or political developments;
|
|
•
|
Loss of intellectual property protections;
|
|
•
|
Litigation and governmental investigations; and
|
|
•
|
Changing economic conditions.
|
|
Location
|
Purpose
|
Segment
|
Square
Footage
|
Ownership
|
Lease Term
End
|
||||||
|
U.S.
|
|||||||||||
|
North Billerica,
Massachusetts
|
Corporate Headquarters, Manufacturing, Laboratory, Mixed Use and Other Office Space
|
U.S. Segment
|
431,000
|
|
Owned
|
N/A
|
|||||
|
Canada
|
|||||||||||
|
Quebec
|
Mixed Use and Office Space
|
International
Segment |
1,106
|
|
Leased
|
April 2019
|
|||||
|
Quebec
|
Distribution Center and Office Space
|
International
Segment |
1,433
|
|
Leased
|
May 2019
|
|||||
|
Puerto Rico
|
|||||||||||
|
San Juan
|
Manufacturing, Laboratory, Mixed Use and Office Space
|
International
Segment |
9,550
|
|
Leased
|
October 2024
|
|||||
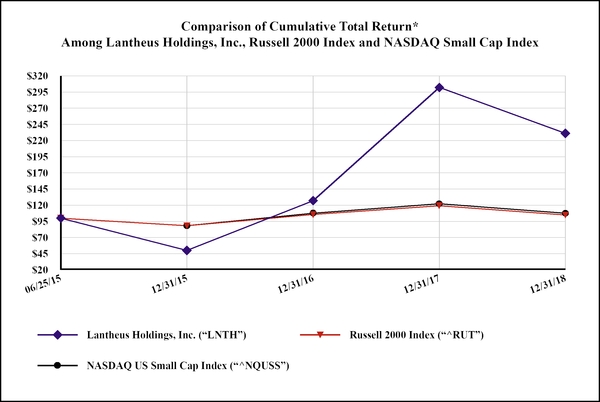
|
Date
|
Lantheus Holdings, Inc. (“LNTH”)
|
Russell 2000 Index (“^RUT”)
|
NASDAQ US Small Cap Index (“^NQUSS”)
|
|||||||||
|
06/25/15
|
$
|
100.00
|
|
$
|
100.00
|
|
$
|
100.00
|
|
|||
|
12/31/15
|
$
|
49.93
|
|
$
|
88.26
|
|
$
|
88.24
|
|
|||
|
12/31/16
|
$
|
127.03
|
|
$
|
105.45
|
|
$
|
107.67
|
|
|||
|
12/31/17
|
$
|
302.07
|
|
$
|
119.31
|
|
$
|
122.28
|
|
|||
|
12/31/18
|
$
|
231.17
|
|
$
|
104.79
|
|
$
|
107.62
|
|
|||
|
Period
|
Total Number of Shares Purchased
|
Average Price Paid per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Programs
|
Approximate Dollar Value of Shares that May Yet Be Purchased Under the Program
|
|||||||
|
October 2018 **
|
737
|
|
$
|
13.27
|
|
*
|
*
|
||||
|
November 2018 **
|
439
|
|
$
|
17.65
|
|
*
|
*
|
||||
|
December 2018 **
|
236
|
|
$
|
16.28
|
|
*
|
*
|
||||
|
Total
|
1,412
|
|
*
|
||||||||
|
Year Ended
December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
Statements of Operations
|
(in thousands, except per share data)
|
||||||||||||||||||
|
Revenues
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
$
|
293,461
|
|
$
|
301,600
|
|
||||
|
Cost of goods sold
|
168,489
|
|
169,243
|
|
164,073
|
|
157,939
|
|
176,081
|
|
|||||||||
|
Sales and marketing
|
43,159
|
|
42,315
|
|
36,542
|
|
34,740
|
|
35,116
|
|
|||||||||
|
General and administrative
|
50,167
|
|
49,842
|
|
38,832
|
|
43,894
|
|
37,313
|
|
|||||||||
|
Research and development
|
17,071
|
|
18,125
|
|
12,203
|
|
14,358
|
|
13,673
|
|
|||||||||
|
Gain on sales of assets
|
—
|
|
—
|
|
6,385
|
|
—
|
|
—
|
|
|||||||||
|
Operating income
|
64,488
|
|
51,853
|
|
56,588
|
|
42,530
|
|
39,417
|
|
|||||||||
|
Interest expense
|
17,405
|
|
18,410
|
|
26,618
|
|
38,715
|
|
42,288
|
|
|||||||||
|
Debt retirement costs
|
—
|
|
—
|
|
1,896
|
|
—
|
|
—
|
|
|||||||||
|
Loss on extinguishment of debt
|
—
|
|
2,442
|
|
—
|
|
15,528
|
|
—
|
|
|||||||||
|
Other income (expense)
|
(2,465
|
)
|
(8,638
|
)
|
(220
|
)
|
65
|
|
(505
|
)
|
|||||||||
|
Income (loss) before income taxes
|
49,548
|
|
39,639
|
|
28,294
|
|
(11,778
|
)
|
(2,366
|
)
|
|||||||||
|
Income tax expense (benefit)
(a)
|
9,030
|
|
(83,746
|
)
|
1,532
|
|
2,968
|
|
1,195
|
|
|||||||||
|
Net income (loss)
|
$
|
40,518
|
|
$
|
123,385
|
|
$
|
26,762
|
|
$
|
(14,746
|
)
|
$
|
(3,561
|
)
|
||||
|
Net income (loss) per common share:
|
|||||||||||||||||||
|
Basic
|
$
|
1.06
|
|
$
|
3.31
|
|
$
|
0.84
|
|
$
|
(0.60
|
)
|
$
|
(0.20
|
)
|
||||
|
Diluted
|
$
|
1.03
|
|
$
|
3.17
|
|
$
|
0.82
|
|
$
|
(0.60
|
)
|
$
|
(0.20
|
)
|
||||
|
Weighted-average common shares:
|
|||||||||||||||||||
|
Basic
|
38,233
|
|
37,276
|
|
32,044
|
|
24,440
|
|
18,081
|
|
|||||||||
|
Diluted
|
39,501
|
|
38,892
|
|
32,656
|
|
24,440
|
|
18,081
|
|
|||||||||
|
(a)
|
The 2017 amount reflects the release of our valuation allowance of
$141.1 million
against its deferred tax assets offset by a provision of
$45.1 million
for remeasuring the Company’s deferred tax assets for the change in tax rates enacted under the Tax Cuts and Jobs Act of 2017.
|
|
Year Ended
December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
Statements of Cash Flows Data
|
(in thousands)
|
||||||||||||||||||
|
Net cash provided by (used in):
|
|||||||||||||||||||
|
Operating activities
|
$
|
61,193
|
|
$
|
54,777
|
|
$
|
49,642
|
|
$
|
21,762
|
|
$
|
11,590
|
|
||||
|
Investing activities
|
$
|
(19,132
|
)
|
$
|
(16,309
|
)
|
$
|
3,281
|
|
$
|
(13,151
|
)
|
$
|
(7,682
|
)
|
||||
|
Financing activities
|
$
|
(4,668
|
)
|
$
|
(13,450
|
)
|
$
|
(30,217
|
)
|
$
|
999
|
|
$
|
(2,297
|
)
|
||||
|
Capital expenditures
|
$
|
20,132
|
|
$
|
17,543
|
|
$
|
7,398
|
|
$
|
13,151
|
|
$
|
8,137
|
|
||||
|
December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
Balance Sheet Data
|
(in thousands)
|
||||||||||||||||||
|
Cash and cash equivalents
|
$
|
113,401
|
|
$
|
76,290
|
|
$
|
51,178
|
|
$
|
28,596
|
|
$
|
19,739
|
|
||||
|
Total assets
|
$
|
439,831
|
|
$
|
383,858
|
|
$
|
255,898
|
|
$
|
242,379
|
|
$
|
243,153
|
|
||||
|
Long-term debt, net
|
$
|
263,709
|
|
$
|
265,393
|
|
$
|
274,460
|
|
$
|
349,858
|
|
$
|
392,863
|
|
||||
|
Total liabilities
|
$
|
368,829
|
|
$
|
360,567
|
|
$
|
362,414
|
|
$
|
427,668
|
|
$
|
482,423
|
|
||||
|
Total stockholders’ equity (deficit)
|
$
|
71,002
|
|
$
|
23,291
|
|
$
|
(106,516
|
)
|
$
|
(185,289
|
)
|
$
|
(239,270
|
)
|
||||
|
•
|
U.S. Segment
produces and markets our medical imaging agents and products throughout the U.S. In the U.S., we primarily sell our products to radiopharmacies, integrated delivery networks, hospitals, clinics and group practices.
|
|
•
|
International Segment
operations consist of production and distribution activities in Puerto Rico and some direct distribution activities in Canada. Additionally, within our International Segment, we have established and maintain third-party distribution relationships under which our products are marketed and sold in Europe, Canada, Australia, Asia-Pacific and Latin America.
|
|
•
|
DEFINITY
is a microbubble contrast agent used in ultrasound exams of the heart, also known as echocardiography exams. DEFINITY contains perflutren-containing lipid microspheres and is indicated in the U.S. for use in patients with suboptimal echocardiograms to assist in imaging the left ventricular chamber and left endocardial border of the heart in ultrasound procedures.
|
|
•
|
TechneLite
is a Technetium generator that provides the essential nuclear material used by radiopharmacies to radiolabel Cardiolite, Neurolite and other Technetium-based radiopharmaceuticals used in nuclear medicine procedures. TechneLite uses Moly as its active ingredient.
|
|
|
Year Ended
December 31,
|
|||||||||||||||||||
|
(in thousands)
|
2018
|
% of
Revenues
|
2017
|
% of
Revenues
|
2016
|
% of
Revenues
|
||||||||||||||
|
DEFINITY
|
$
|
183,073
|
|
53.3
|
%
|
$
|
157,268
|
|
47.5
|
%
|
$
|
131,612
|
|
43.6
|
%
|
|||||
|
TechneLite
|
98,858
|
|
28.8
|
%
|
104,644
|
|
31.6
|
%
|
99,217
|
|
32.9
|
%
|
||||||||
|
Other*
|
61,443
|
|
17.9
|
%
|
69,466
|
|
20.9
|
%
|
71,024
|
|
23.5
|
%
|
||||||||
|
Total revenues
|
$
|
343,374
|
|
100.0
|
%
|
$
|
331,378
|
|
100.0
|
%
|
$
|
301,853
|
|
100.0
|
%
|
|||||
|
•
|
Patents -
We continue to actively pursue additional patents in connection with DEFINITY, both in the U.S. and internationally. In the U.S., we have an Orange Book-listed method of use patent expiring in March 2037. This patent augments an Orange Book-listed composition of matter patent expiring in June 2019, and additional manufacturing patents that are not Orange Book-listed expiring in 2021, 2023 and 2037. Outside of the U.S., our DEFINITY patent protection or regulatory exclusivity currently expires in 2019.
|
|
•
|
LVEF Indication
- We are currently conducting two well-controlled Phase 3 studies designed to demonstrate improved accuracy of LVEF measurements with DEFINITY-enhanced echocardiography versus unenhanced echocardiography. The truth standard in these studies is cardiac magnetic resonance imaging. The studies will be conducted at 20 U.S. sites and will eventually enroll a total of approximately 300 subjects. We believe DEFINITY could improve the accuracy of LVEF calculations, giving clinicians greater confidence in patient management decisions. An LVEF indication could substantially
|
|
•
|
Modified Formulation
- We are developing at SBL a modified formulation of DEFINITY. We believe this modified formulation will provide an enhanced product profile enabling storage as well as shipment at room temperature (DEFINITY’s current formulation requires refrigerated storage), will give clinicians additional choice, and will allow for greater utility of this formulation in broader clinical settings. We were recently granted a composition of matter patent on the modified formulation which runs through December 2035. If the modified formulation is approved by the FDA, then this patent would be eligible to be listed in the Orange Book. We currently believe that, if approved by the FDA, the modified formulation could become commercially available in 2020, although that timing cannot be assured. Given its physical characteristics, the modified formulation may also be better suited for inclusion in kits requiring microbubbles for other indications and applications.
|
|
•
|
New Applications
- As we continue to look for other opportunities to expand our microbubble franchise, we are evaluating new indications and applications beyond echocardiography and contrast imaging generally.
|
|
•
|
In-House Manufacturing
- We are currently building specialized in-house manufacturing capabilities at our North Billerica, Massachusetts facility for DEFINITY and, potentially, other sterile vial products. We believe the investment in these efforts will allow us to better control DEFINITY manufacturing and inventory, reduce our costs in a potentially more price competitive environment, and provide us with supply chain redundancy. We currently expect to be in a position to use this in-house manufacturing capability by early 2021, although that timing cannot be assured.
|
|
•
|
increased revenues for DEFINITY in the suboptimal echocardiogram segment as a result of our continued focused sales efforts which has also resulted in increased gross profit;
|
|
•
|
decreased revenues for TechneLite in the U.S. segment primarily as a result of a temporary supplier disruption;
|
|
•
|
increased revenues for TechneLite in the International segment primarily driven by increased volume as a result of temporary incremental demand which has also resulted in increased gross profit;
|
|
•
|
decreased revenues in other revenue due to the recognition of $5.0 million during the prior year from GE Healthcare in exchange for rights to the continued Phase 3 development and worldwide commercialization of flurpiridaz F 18;
|
|
•
|
decreased depreciation expense as a result of the decommissioning of certain long-lived assets during the prior year period;
|
|
•
|
decreases in other income due to a $5.5 million decrease in tax indemnification income as a result
of the impact of the reduction in the U.S. federal corporate tax rate pursuant to the Tax Cuts and Jobs Act enacted on December 22, 2017; and
|
|
•
|
increased tax expense due to the profit generated during the year ended December 31, 2018 and the fact that we no longer record a valuation allowance against our domestic deferred tax assets offset by the release of our valuation allowance against our Canada deferred tax assets.
|
|
Year Ended
December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$
|
Change
%
|
Change
$
|
Change
% |
||||||||||||||||||
|
Revenues
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
$
|
11,996
|
|
3.6
|
%
|
$
|
29,525
|
|
9.8
|
%
|
||||||
|
Cost of goods sold
|
168,489
|
|
169,243
|
|
164,073
|
|
(754
|
)
|
(0.4
|
)%
|
5,170
|
|
3.2
|
%
|
|||||||||||
|
Gross profit
|
174,885
|
|
162,135
|
|
137,780
|
|
12,750
|
|
7.9
|
%
|
24,355
|
|
17.7
|
%
|
|||||||||||
|
Operating expenses
|
|
|
|
|
|||||||||||||||||||||
|
Sales and marketing
|
43,159
|
|
42,315
|
|
36,542
|
|
844
|
|
2.0
|
%
|
5,773
|
|
15.8
|
%
|
|||||||||||
|
General and administrative
|
50,167
|
|
49,842
|
|
38,832
|
|
325
|
|
0.7
|
%
|
11,010
|
|
28.4
|
%
|
|||||||||||
|
Research and development
|
17,071
|
|
18,125
|
|
12,203
|
|
(1,054
|
)
|
(5.8
|
)%
|
5,922
|
|
48.5
|
%
|
|||||||||||
|
Total operating expenses
|
110,397
|
|
110,282
|
|
87,577
|
|
115
|
|
0.1
|
%
|
22,705
|
|
25.9
|
%
|
|||||||||||
|
Gain on sales of assets
|
—
|
|
—
|
|
6,385
|
|
—
|
|
—
|
%
|
(6,385
|
)
|
(100.0
|
)%
|
|||||||||||
|
Operating income
|
64,488
|
|
51,853
|
|
56,588
|
|
12,635
|
|
24.4
|
%
|
(4,735
|
)
|
(8.4
|
)%
|
|||||||||||
|
Interest expense
|
17,405
|
|
18,410
|
|
26,618
|
|
(1,005
|
)
|
(5.5
|
)%
|
(8,208
|
)
|
(30.8
|
)%
|
|||||||||||
|
Debt retirement costs
|
—
|
|
—
|
|
1,896
|
|
—
|
|
—
|
%
|
(1,896
|
)
|
(100.0
|
)%
|
|||||||||||
|
Loss on extinguishment of debt
|
—
|
|
2,442
|
|
—
|
|
(2,442
|
)
|
(100.0
|
)%
|
2,442
|
|
100.0
|
%
|
|||||||||||
|
Other income
|
(2,465
|
)
|
(8,638
|
)
|
(220
|
)
|
6,173
|
|
(71.5
|
)%
|
(8,418
|
)
|
3,826.4
|
%
|
|||||||||||
|
Income before income taxes
|
49,548
|
|
39,639
|
|
28,294
|
|
9,909
|
|
25.0
|
%
|
11,345
|
|
40.1
|
%
|
|||||||||||
|
Income tax expense (benefit)
|
9,030
|
|
(83,746
|
)
|
1,532
|
|
92,776
|
|
(110.8
|
)%
|
(85,278
|
)
|
(5,566.4
|
)%
|
|||||||||||
|
Net income
|
$
|
40,518
|
|
$
|
123,385
|
|
$
|
26,762
|
|
$
|
(82,867
|
)
|
(67.2
|
)%
|
$
|
96,623
|
|
361.0
|
%
|
||||||
|
Year Ended
December 31, |
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$ |
Change
% |
Change
$ |
Change
% |
||||||||||||||||||
|
U.S.
|
|||||||||||||||||||||||||
|
DEFINITY
|
$
|
178,440
|
|
$
|
153,581
|
|
$
|
128,677
|
|
$
|
24,859
|
|
16.2
|
%
|
$
|
24,904
|
|
19.4
|
%
|
||||||
|
TechneLite
|
74,042
|
|
90,489
|
|
85,412
|
|
(16,447
|
)
|
(18.2
|
)%
|
5,077
|
|
5.9
|
%
|
|||||||||||
|
Other
|
36,098
|
|
45,932
|
|
43,331
|
|
(9,834
|
)
|
(21.4
|
)%
|
2,601
|
|
6.0
|
%
|
|||||||||||
|
Total U.S. Revenues
|
288,580
|
|
290,002
|
|
257,420
|
|
(1,422
|
)
|
(0.5
|
)%
|
32,582
|
|
12.7
|
%
|
|||||||||||
|
International
|
|||||||||||||||||||||||||
|
DEFINITY
|
4,633
|
|
3,687
|
|
2,935
|
|
946
|
|
25.7
|
%
|
752
|
|
25.6
|
%
|
|||||||||||
|
TechneLite
|
24,816
|
|
14,155
|
|
13,805
|
|
10,661
|
|
75.3
|
%
|
350
|
|
2.5
|
%
|
|||||||||||
|
Other
|
25,345
|
|
23,534
|
|
27,693
|
|
1,811
|
|
7.7
|
%
|
(4,159
|
)
|
(15.0
|
)%
|
|||||||||||
|
Total International Revenues
|
54,794
|
|
41,376
|
|
44,433
|
|
13,418
|
|
32.4
|
%
|
(3,057
|
)
|
(6.9
|
)%
|
|||||||||||
|
Total Revenues
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
$
|
11,996
|
|
3.6
|
%
|
$
|
29,525
|
|
9.8
|
%
|
||||||
|
(in thousands)
|
Rebates and
Allowances |
||
|
Balance, January 1, 2016
|
$
|
2,303
|
|
|
Provision related to current period revenues
|
7,255
|
|
|
|
Adjustments relating to prior period revenues
|
(452
|
)
|
|
|
Payments or credits made during the period
|
(6,809
|
)
|
|
|
Balance, December 31, 2016
|
2,297
|
|
|
|
Provision related to current period revenues
|
9,568
|
|
|
|
Adjustments relating to prior period revenues
|
(654
|
)
|
|
|
Payments or credits made during the period
|
(8,351
|
)
|
|
|
Balance, December 31, 2017
|
2,860
|
|
|
|
Provision related to current period revenues
|
13,202
|
|
|
|
Adjustments relating to prior period revenues
|
(361
|
)
|
|
|
Payments or credits made during the period
|
(11,047
|
)
|
|
|
Balance, December 31, 2018
|
$
|
4,654
|
|
|
|
Year Ended
December 31, |
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$ |
Change
% |
Change
$ |
Change
% |
||||||||||||||||||
|
U.S.
|
$
|
161,760
|
|
$
|
154,671
|
|
$
|
128,350
|
|
$
|
7,089
|
|
4.6
|
%
|
$
|
26,321
|
|
20.5
|
%
|
||||||
|
International
|
13,125
|
|
7,464
|
|
9,430
|
|
5,661
|
|
75.8
|
%
|
(1,966
|
)
|
(20.8
|
)%
|
|||||||||||
|
Total Gross profit
|
$
|
174,885
|
|
$
|
162,135
|
|
$
|
137,780
|
|
$
|
12,750
|
|
7.9
|
%
|
$
|
24,355
|
|
17.7
|
%
|
||||||
|
|
Year Ended
December 31, |
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$ |
Change
% |
Change
$ |
Change
% |
||||||||||||||||||
|
U.S.
|
$
|
40,579
|
|
$
|
39,471
|
|
$
|
32,919
|
|
$
|
1,108
|
|
2.8
|
%
|
$
|
6,552
|
|
19.9
|
%
|
||||||
|
International
|
2,580
|
|
2,844
|
|
3,623
|
|
(264
|
)
|
(9.3
|
)%
|
$
|
(779
|
)
|
(21.5
|
)%
|
||||||||||
|
Total Sales and marketing
|
$
|
43,159
|
|
$
|
42,315
|
|
$
|
36,542
|
|
$
|
844
|
|
2.0
|
%
|
$
|
5,773
|
|
15.8
|
%
|
||||||
|
|
Year Ended
December 31, |
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$ |
Change
% |
Change
$ |
Change
% |
||||||||||||||||||
|
U.S.
|
$
|
49,149
|
|
$
|
49,269
|
|
$
|
37,389
|
|
$
|
(120
|
)
|
(0.2
|
)%
|
$
|
11,880
|
|
31.8
|
%
|
||||||
|
International
|
1,018
|
|
573
|
|
1,443
|
|
445
|
|
77.7
|
%
|
(870
|
)
|
(60.3
|
)%
|
|||||||||||
|
Total General and administrative
|
$
|
50,167
|
|
$
|
49,842
|
|
$
|
38,832
|
|
$
|
325
|
|
0.7
|
%
|
$
|
11,010
|
|
28.4
|
%
|
||||||
|
|
Year Ended
December 31, |
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$ |
Change
% |
Change
$ |
Change
% |
||||||||||||||||||
|
U.S.
|
$
|
15,705
|
|
$
|
16,692
|
|
$
|
11,574
|
|
$
|
(987
|
)
|
(5.9
|
)%
|
$
|
5,118
|
|
44.2
|
%
|
||||||
|
International
|
1,366
|
|
1,433
|
|
629
|
|
(67
|
)
|
(4.7
|
)%
|
804
|
|
127.8
|
%
|
|||||||||||
|
Total Research and development
|
$
|
17,071
|
|
$
|
18,125
|
|
$
|
12,203
|
|
$
|
(1,054
|
)
|
(5.8
|
)%
|
$
|
5,922
|
|
48.5
|
%
|
||||||
|
Year Ended
December 31, |
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
Change
$ |
Change
% |
Change
$ |
Change
% |
|||||||||||||||||
|
Income tax expense (benefit)
|
$
|
9,030
|
|
$
|
(83,746
|
)
|
$
|
1,532
|
|
$
|
92,776
|
|
(110.8
|
)%
|
$
|
(85,278
|
)
|
< (1,000)%
|
||||||
|
Year Ended
December 31, |
|||||
|
2018
|
2017
|
2016
|
|||
|
Effective tax rate
|
18.2%
|
(211.3)%
|
5.4%
|
||
|
Year Ended
December 31, |
||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
|||||||||
|
Net cash provided by operating activities
|
$
|
61,193
|
|
$
|
54,777
|
|
$
|
49,642
|
|
|||
|
Net cash (used in) provided by investing activities
|
$
|
(19,132
|
)
|
$
|
(16,309
|
)
|
$
|
3,281
|
|
|||
|
Net cash used in financing activities
|
$
|
(4,668
|
)
|
$
|
(13,450
|
)
|
$
|
(30,217
|
)
|
|||
|
2017 Facility Financial Covenants
|
|
|
Period
|
Consolidated
Leverage Ratio |
|
Q1 2019
|
4.75 to 1.00
|
|
Thereafter
|
4.50 to 1.00
|
|
•
|
The costs of acquiring or in-licensing new products, businesses or technologies, together with the costs of pursuing opportunities that are not eventually consummated;
|
|
•
|
The pricing environment and the level of product sales of our currently marketed products, particularly DEFINITY and any additional products that we may market in the future;
|
|
•
|
Revenue mix shifts and associated volume and selling price changes that could result from contractual status changes with key customers and additional competition;
|
|
•
|
Our investment in the further clinical development and commercialization of existing products and development candidates;
|
|
•
|
The costs of investing in our facilities, equipment and technology infrastructure;
|
|
•
|
The costs and timing of establishing manufacturing and supply arrangements for commercial supplies of our products and raw materials and components;
|
|
•
|
Our ability to have product manufactured and released from JHS and other manufacturing sites in a timely manner in the future;
|
|
•
|
The costs of further commercialization of our existing products, particularly in international markets, including product marketing, sales and distribution and whether we obtain local partners to help share such commercialization costs;
|
|
•
|
The extent to which we choose to establish collaboration, co-promotion, distribution or other similar arrangements for our marketed products;
|
|
•
|
The legal costs relating to maintaining, expanding and enforcing our intellectual property portfolio, pursuing insurance or other claims and defending against product liability, regulatory compliance or other claims; and
|
|
•
|
The cost of interest on any additional borrowings which we may incur under our financing arrangements.
|
|
|
Payments Due by Period
|
||||||||||||||||||
|
(in thousands)
|
Total
|
Less than
1 Year
|
1 - 3 Years
|
3 -5 Years
|
More than
5 Years
|
||||||||||||||
|
Debt obligations (principal)
|
$
|
270,187
|
|
$
|
2,750
|
|
$
|
5,500
|
|
$
|
261,937
|
|
$
|
—
|
|
||||
|
Interest on debt obligations
(a)
|
57,705
|
|
17,101
|
|
33,724
|
|
6,880
|
|
—
|
|
|||||||||
|
Operating lease obligations
(b)
|
1,521
|
|
391
|
|
476
|
|
476
|
|
178
|
|
|||||||||
|
Purchase obligations
(c)
|
1,750
|
|
1,750
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Capital lease obligations
|
144
|
|
123
|
|
21
|
|
—
|
|
—
|
|
|||||||||
|
Other long-term liabilities
(d)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Asset retirement obligations
(e)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total contractual obligations
|
$
|
331,307
|
|
$
|
22,115
|
|
$
|
39,721
|
|
$
|
269,293
|
|
$
|
178
|
|
||||
|
(a)
|
Amount relates to the minimum interest under our 2017 Term Facility.
|
|
(b)
|
Operating leases include minimum payments under leases for our facilities and certain equipment.
|
|
(c)
|
Excludes purchase orders for inventory in the normal course of business.
|
|
(d)
|
Our other long-term liabilities in the consolidated balance sheet include unrecognized tax benefits and related interest and penalties. As of
December 31, 2018
, we had unrecognized tax benefits of
$40.2 million
, which included interest and penalties, classified as noncurrent liabilities. At this time, we are unable to make a reasonably reliable estimate of the timing of payments in individual years in connection with these tax liabilities; therefore, such amounts are not included in the above contractual obligation table.
|
|
(e)
|
We have excluded asset retirement obligations from the table above due to the uncertainty of the timing of the future cash outflows related to the decommissioning of our radioactive operations. As of
December 31, 2018
, the liability, which was approximately
$11.6 million
, was measured at the present value of the obligation expected to be incurred of approximately
$26.9 million
.
|
|
Page
|
|
|
/s/ Deloitte & Touche LLP
|
|
Boston, Massachusetts
|
|
February 20, 2019
|
|
December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Assets
|
|||||||
|
Current assets
|
|||||||
|
Cash and cash equivalents
|
$
|
113,401
|
|
$
|
76,290
|
|
|
|
Accounts receivable, net
|
43,753
|
|
40,259
|
|
|||
|
Inventory
|
33,019
|
|
26,080
|
|
|||
|
Other current assets
|
5,242
|
|
5,221
|
|
|||
|
Total current assets
|
195,415
|
|
147,850
|
|
|||
|
Property, plant and equipment, net
|
107,888
|
|
92,999
|
|
|||
|
Intangibles, net
|
9,133
|
|
11,798
|
|
|||
|
Goodwill
|
15,714
|
|
15,714
|
|
|||
|
Deferred tax assets, net
|
81,449
|
|
87,010
|
|
|||
|
Other long-term assets
|
30,232
|
|
28,487
|
|
|||
|
Total assets
|
$
|
439,831
|
|
$
|
383,858
|
|
|
|
Liabilities and stockholders’ equity
|
|||||||
|
Current liabilities
|
|||||||
|
Current portion of long-term debt
|
$
|
2,750
|
|
$
|
2,750
|
|
|
|
Revolving line of credit
|
—
|
|
—
|
|
|||
|
Accounts payable
|
17,955
|
|
17,464
|
|
|||
|
Accrued expenses and other liabilities
|
32,050
|
|
26,536
|
|
|||
|
Total current liabilities
|
52,755
|
|
46,750
|
|
|||
|
Asset retirement obligations
|
11,572
|
|
10,412
|
|
|||
|
Long-term debt, net
|
263,709
|
|
265,393
|
|
|||
|
Other long-term liabilities
|
40,793
|
|
38,012
|
|
|||
|
Total liabilities
|
368,829
|
|
360,567
|
|
|||
|
Commitments and contingencies (see Note 15)
|
|
|
|||||
|
Stockholders’ equity
|
|||||||
|
Preferred stock ($0.01 par value, 25,000 shares authorized; no shares issued and outstanding)
|
—
|
|
—
|
|
|||
|
Common stock ($0.01 par value, 250,000 shares authorized; 38,466 and 37,765 shares issued and outstanding, respectively)
|
385
|
|
378
|
|
|||
|
Additional paid-in capital
|
239,865
|
|
232,960
|
|
|||
|
Accumulated deficit
|
(168,140
|
)
|
(209,013
|
)
|
|||
|
Accumulated other comprehensive loss
|
(1,108
|
)
|
(1,034
|
)
|
|||
|
Total stockholders’ equity
|
71,002
|
|
23,291
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
439,831
|
|
$
|
383,858
|
|
|
|
|
Year Ended
December 31, |
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Revenues
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
||
|
Cost of goods sold
|
168,489
|
|
169,243
|
|
164,073
|
|
|||||
|
Gross profit
|
174,885
|
|
162,135
|
|
137,780
|
|
|||||
|
Operating expenses
|
|||||||||||
|
Sales and marketing
|
43,159
|
|
42,315
|
|
36,542
|
|
|||||
|
General and administrative
|
50,167
|
|
49,842
|
|
38,832
|
|
|||||
|
Research and development
|
17,071
|
|
18,125
|
|
12,203
|
|
|||||
|
Total operating expenses
|
110,397
|
|
110,282
|
|
87,577
|
|
|||||
|
Gain on sales of assets
|
—
|
|
—
|
|
6,385
|
|
|||||
|
Operating income
|
64,488
|
|
51,853
|
|
56,588
|
|
|||||
|
Interest expense
|
17,405
|
|
18,410
|
|
26,618
|
|
|||||
|
Debt retirement costs
|
—
|
|
—
|
|
1,896
|
|
|||||
|
Loss on extinguishment of debt
|
—
|
|
2,442
|
|
—
|
|
|||||
|
Other income
|
(2,465
|
)
|
(8,638
|
)
|
(220
|
)
|
|||||
|
Income before income taxes
|
49,548
|
|
39,639
|
|
28,294
|
|
|||||
|
Income tax expense (benefit)
|
9,030
|
|
(83,746
|
)
|
1,532
|
|
|||||
|
Net income
|
$
|
40,518
|
|
$
|
123,385
|
|
$
|
26,762
|
|
||
|
Net income per common share:
|
|||||||||||
|
Basic
|
$
|
1.06
|
|
$
|
3.31
|
|
$
|
0.84
|
|
||
|
Diluted
|
$
|
1.03
|
|
$
|
3.17
|
|
$
|
0.82
|
|
||
|
Weighted-average common shares outstanding:
|
|||||||||||
|
Basic
|
38,233
|
|
37,276
|
|
32,044
|
|
|||||
|
Diluted
|
39,501
|
|
38,892
|
|
32,656
|
|
|||||
|
Year Ended
December 31, |
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net income
|
$
|
40,518
|
|
$
|
123,385
|
|
$
|
26,762
|
|
||
|
Other comprehensive (loss) income:
|
|||||||||||
|
Reclassification adjustment for gains on sales of assets included in net income
|
—
|
|
—
|
|
435
|
|
|||||
|
Foreign currency translation
|
(74
|
)
|
(87
|
)
|
603
|
|
|||||
|
Total other comprehensive (loss) income
|
(74
|
)
|
(87
|
)
|
1,038
|
|
|||||
|
Comprehensive income
|
$
|
40,444
|
|
$
|
123,298
|
|
$
|
27,800
|
|
||
|
Common Stock
|
Additional
Paid-In
Capital
|
Accumulated
Deficit
|
Accumulated
Other
Comprehensive
Loss
|
Total
Stockholders’
Equity
(Deficit)
|
|||||||||||||||||||
|
Shares
|
Amount
|
||||||||||||||||||||||
|
Balance, January 1, 2016
|
30,365
|
|
$
|
303
|
|
$
|
175,553
|
|
$
|
(359,160
|
)
|
$
|
(1,985
|
)
|
$
|
(185,289
|
)
|
||||||
|
Issuance of common stock, net of $2,080 issuance costs
|
6,200
|
|
62
|
|
48,758
|
|
—
|
|
—
|
|
48,820
|
|
|||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
26,762
|
|
—
|
|
26,762
|
|
|||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
1,038
|
|
1,038
|
|
|||||||||||
|
Stock option exercises
|
41
|
|
1
|
|
230
|
|
—
|
|
—
|
|
231
|
|
|||||||||||
|
Vesting of restricted stock awards
|
214
|
|
2
|
|
(2
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Shares withheld to cover taxes
|
(64
|
)
|
(1
|
)
|
(601
|
)
|
—
|
|
—
|
|
(602
|
)
|
|||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
2,524
|
|
—
|
|
—
|
|
2,524
|
|
|||||||||||
|
Balance, December 31, 2016
|
36,756
|
|
367
|
|
226,462
|
|
(332,398
|
)
|
(947
|
)
|
(106,516
|
)
|
|||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
123,385
|
|
—
|
|
123,385
|
|
|||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(87
|
)
|
(87
|
)
|
|||||||||||
|
Stock option exercises and employee stock plan purchases
|
478
|
|
5
|
|
3,429
|
|
—
|
|
—
|
|
3,434
|
|
|||||||||||
|
Vesting of restricted stock awards
|
744
|
|
8
|
|
(8
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Shares withheld to cover taxes
|
(214
|
)
|
(2
|
)
|
(2,851
|
)
|
—
|
|
—
|
|
(2,853
|
)
|
|||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
5,928
|
|
—
|
|
—
|
|
5,928
|
|
|||||||||||
|
Balance, December 31, 2017
|
37,765
|
|
378
|
|
232,960
|
|
(209,013
|
)
|
(1,034
|
)
|
23,291
|
|
|||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
40,518
|
|
—
|
|
40,518
|
|
|||||||||||
|
Forfeiture of dividend equivalent right
|
—
|
|
—
|
|
—
|
|
355
|
|
—
|
|
355
|
|
|||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(74
|
)
|
(74
|
)
|
|||||||||||
|
Stock option exercises and employee stock plan purchases
|
223
|
|
2
|
|
1,578
|
|
—
|
|
—
|
|
1,580
|
|
|||||||||||
|
Vesting of restricted stock awards
|
672
|
|
7
|
|
(7
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Shares withheld to cover taxes
|
(194
|
)
|
(2
|
)
|
(3,384
|
)
|
—
|
|
—
|
|
(3,386
|
)
|
|||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
8,718
|
|
—
|
|
—
|
|
8,718
|
|
|||||||||||
|
Balance, December 31, 2018
|
38,466
|
|
$
|
385
|
|
$
|
239,865
|
|
$
|
(168,140
|
)
|
$
|
(1,108
|
)
|
$
|
71,002
|
|
||||||
|
Year Ended
December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Operating activities
|
|||||||||||
|
Net income
|
$
|
40,518
|
|
$
|
123,385
|
|
$
|
26,762
|
|
||
|
Adjustments to reconcile net income to net cash flows from operating activities:
|
|||||||||||
|
Depreciation, amortization and accretion
|
13,929
|
|
19,231
|
|
18,263
|
|
|||||
|
Amortization of debt related costs
|
1,279
|
|
1,361
|
|
1,603
|
|
|||||
|
Provision for bad debt
|
321
|
|
136
|
|
53
|
|
|||||
|
Provision for excess and obsolete inventory
|
2,875
|
|
1,215
|
|
1,342
|
|
|||||
|
Stock-based compensation
|
8,718
|
|
5,928
|
|
2,524
|
|
|||||
|
Gain on sales of assets
|
—
|
|
—
|
|
(6,385
|
)
|
|||||
|
Loss on impairment of land
|
—
|
|
912
|
|
—
|
|
|||||
|
Loss on extinguishment of debt and debt retirement costs
|
—
|
|
2,442
|
|
1,896
|
|
|||||
|
Deferred taxes
|
5,762
|
|
(86,946
|
)
|
(155
|
)
|
|||||
|
Long-term income tax receivable
|
(2,855
|
)
|
(8,413
|
)
|
(200
|
)
|
|||||
|
Long-term income tax payable and other long-term liabilities
|
3,219
|
|
2,793
|
|
565
|
|
|||||
|
Other
|
1,399
|
|
1,049
|
|
1,284
|
|
|||||
|
Increases (decreases) in cash from operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
(3,985
|
)
|
(3,407
|
)
|
(1,059
|
)
|
|||||
|
Inventory
|
(8,690
|
)
|
(9,620
|
)
|
(3,626
|
)
|
|||||
|
Other current assets
|
(661
|
)
|
(388
|
)
|
(155
|
)
|
|||||
|
Accounts payable
|
(2,886
|
)
|
604
|
|
5,700
|
|
|||||
|
Accrued expenses and other liabilities
|
2,250
|
|
4,495
|
|
1,230
|
|
|||||
|
Net cash provided by operating activities
|
61,193
|
|
54,777
|
|
49,642
|
|
|||||
|
Investing activities
|
|||||||||||
|
Capital expenditures
|
(20,132
|
)
|
(17,543
|
)
|
(7,398
|
)
|
|||||
|
Proceeds from sale of assets
|
1,000
|
|
1,234
|
|
10,605
|
|
|||||
|
Other
|
—
|
|
—
|
|
74
|
|
|||||
|
Net cash (used in) provided by investing activities
|
(19,132
|
)
|
(16,309
|
)
|
3,281
|
|
|||||
|
Financing activities
|
|||||||||||
|
Proceeds from issuance of common stock
|
428
|
|
187
|
|
50,900
|
|
|||||
|
Payments for public offering costs
|
—
|
|
(74
|
)
|
(2,006
|
)
|
|||||
|
Proceeds from issuance of long-term debt
|
—
|
|
274,313
|
|
—
|
|
|||||
|
Payments on long-term debt
|
(2,862
|
)
|
(286,694
|
)
|
(78,729
|
)
|
|||||
|
Deferred financing costs
|
—
|
|
(1,576
|
)
|
(11
|
)
|
|||||
|
Proceeds from stock option exercises
|
1,152
|
|
3,247
|
|
231
|
|
|||||
|
Payments for minimum statutory tax withholding related to net share settlement of equity awards
|
(3,386
|
)
|
(2,853
|
)
|
(602
|
)
|
|||||
|
Net cash used in financing activities
|
(4,668
|
)
|
(13,450
|
)
|
(30,217
|
)
|
|||||
|
Effect of foreign exchange rates on cash and cash equivalents
|
(282
|
)
|
94
|
|
(124
|
)
|
|||||
|
Net increase in cash and cash equivalents
|
37,111
|
|
25,112
|
|
22,582
|
|
|||||
|
Cash and cash equivalents, beginning of year
|
76,290
|
|
51,178
|
|
28,596
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
113,401
|
|
$
|
76,290
|
|
$
|
51,178
|
|
||
|
Year Ended
December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Supplemental disclosure of cash flow information
|
|||||||||||
|
Cash paid during the period for:
|
|||||||||||
|
Interest
|
$
|
15,869
|
|
$
|
16,653
|
|
$
|
24,441
|
|
||
|
Income taxes, net of refunds of $35, $17 and $82, respectively
|
$
|
90
|
|
$
|
106
|
|
$
|
265
|
|
||
|
Schedule of non-cash investing and financing activities
|
|||||||||||
|
Additions of property, plant and equipment included in liabilities
|
$
|
7,395
|
|
$
|
2,738
|
|
$
|
4,990
|
|
||
|
Receivable in connection with sale of Australian subsidiary
|
$
|
—
|
|
$
|
—
|
|
$
|
1,479
|
|
||
|
•
|
DEFINITY is a microbubble contrast agent used in ultrasound exams of the heart, also known as echocardiography exams. DEFINITY contains perflutren-containing lipid microspheres and is indicated in the U.S. for use in patients with suboptimal echocardiograms to assist in imaging the left ventricular chamber and left endocardial border of the heart in ultrasound procedures
|
|
•
|
TechneLite is a Technetium generator that provides the essential nuclear material used by radiopharmacies to radiolabel Cardiolite, Neurolite and other Technetium-based radiopharmaceuticals used in nuclear medicine procedures. TechneLite uses Moly as its active ingredient.
|
|
Year Ended
December 31,
|
|||||
|
2018
|
2017
|
2016
|
|||
|
DEFINITY
|
53.3%
|
47.5%
|
43.6%
|
||
|
TechneLite
|
28.8%
|
31.6%
|
32.9%
|
||
|
Class
|
Range of Estimated Useful Lives
|
|
|
Buildings
|
10 - 50 years
|
|
|
Land improvements
|
15 - 40 years
|
|
|
Machinery and equipment
|
3 - 15 years
|
|
|
Furniture and fixtures
|
15 years
|
|
|
Leasehold improvements
|
Lesser of lease term or 15 years
|
|
|
Computer software
|
3 - 5 years
|
|
|
|
Years Ended
December 31,
|
||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
||||||||
|
Foreign currency losses (gains)
|
$
|
557
|
|
$
|
(253
|
)
|
$
|
853
|
|
||
|
Tax indemnification income
|
(2,855
|
)
|
(8,367
|
)
|
(1,055
|
)
|
|||||
|
Other income
|
(167
|
)
|
(18
|
)
|
(18
|
)
|
|||||
|
Total other income
|
$
|
(2,465
|
)
|
$
|
(8,638
|
)
|
$
|
(220
|
)
|
||
|
Standard
|
Description
|
Effective Date
for Company
|
Effect on the
Consolidated Financial
Statements
|
|
Recently Issued Accounting Standards Not Yet Adopted
|
|||
|
ASU 2016-02, Leases (Topic 842)
|
This ASU supersedes existing guidance on accounting for leases in Leases (Topic 840) and generally requires all leases to be recognized on the balance sheet. The provisions of ASU 2016-02 are effective for annual reporting periods beginning after December 15, 2018; early adoption is permitted. In July 2018, an amendment was made that allows companies the option of using the effective date of the new standard as the initial application date (at the beginning of the period in which it is adopted, rather than at the beginning of the earliest comparative period).
|
January 1, 2019
|
The Company has completed its assessment on the impact of the standard, including optional practical expedients and transition methods that the Company will elect upon adoption. The implementation plan included identifying the Company’s lease population, assessing significant leases under the new guidance and identifying changes to processes and controls. The Company concluded that upon adoption of this standard there will not be a significant impact to its Balance Sheet. The Company will utilize the prospective approach of adopting this standard. The Company has identified and implemented appropriate changes to its business processes and controls to support recognition and disclosure under this standard.
|
|
Standard
|
Description
|
Effective Date
for Company
|
Effect on the
Consolidated Financial
Statements
|
|
Accounting Standards Adopted During the Year Ended December 31, 2018
|
|||
|
ASU 2017-09, Compensation—Stock Compensation (Topic 718): Scope of Modification Accounting
|
This ASU clarifies when to account for a change to the terms or conditions of a share-based payment award as a modification. Under the new guidance, modification accounting is required only if the fair value, vesting conditions or classification of the award (as equity or liability) changes as a result of the change in terms or conditions.
The new guidance will be applied prospectively to awards modified on or after the adoption date. The guidance is effective for annual periods, and interim periods within those annual periods, beginning after December 15, 2017 for all entities. |
January 1, 2018
|
The adoption of this standard did not have a material impact on the Company’s consolidated financial statements.
|
|
ASU 2014-09, Revenue from Contracts with Customers (Topic 606) and related amendments
|
This ASU and related amendments affect any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets, unless those contracts are within the scope of other standards. The guidance in this ASU supersedes the revenue recognition requirements in Topic 605, Revenue Recognition and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue upon the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services.
|
January 1, 2018
|
See Note 3, "Revenue from Contracts with Customers" for the required disclosures related to the impact of adopting this standard.
The adoption of this standard did not have a material impact on the Company’s consolidated balance sheets and statements of operations.
|
|
Year Ended December 31, 2018
|
||||||||||||
|
Major Products/Service Lines (in thousands)
|
U.S.
|
International
|
Total
|
|||||||||
|
Product revenue, net
(1)
|
$
|
288,580
|
|
$
|
52,556
|
|
$
|
341,136
|
|
|||
|
License and royalty revenues
|
—
|
|
2,238
|
|
2,238
|
|
||||||
|
Total revenues
|
$
|
288,580
|
|
$
|
54,794
|
|
$
|
343,374
|
|
|||
|
(1)
|
The Company’s principal products include DEFINITY and TechneLite and are categorized within product revenue, net. The Company applies the same revenue recognition policies and judgments for all of its principal products.
|
|
(in thousands)
|
Rebates and
Allowances |
||
|
Balance, January 1, 2018
|
2,860
|
|
|
|
Provision related to current period revenues
|
13,202
|
|
|
|
Adjustments relating to prior period revenues
|
(361
|
)
|
|
|
Payments or credits made during the period
|
(11,047
|
)
|
|
|
Balance, December 31, 2018
|
$
|
4,654
|
|
|
(in thousands)
|
Year Ended December 31, 2018
|
||
|
Amounts included in the contract liability at the beginning of the period
|
$
|
33
|
|
|
Performance obligations satisfied (or partially satisfied) in previous periods
|
$
|
—
|
|
|
•
|
Level 1
— Inputs are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
|
|
•
|
Level 2
— Inputs include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (i.e., interest rates, yield curves, etc.) and inputs that are derived principally from or corroborated by observable market data by correlation or other means (market corroborated inputs).
|
|
•
|
Level 3
— Unobservable inputs that reflect a Company’s estimates about the assumptions that market participants would use in pricing the asset or liability. The Company develops these inputs based on the best information available, including its own data.
|
|
|
December 31, 2018
|
||||||||||||||
|
(in thousands)
|
Total Fair
Value |
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Money market
|
$
|
61,391
|
|
$
|
61,391
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total
|
$
|
61,391
|
|
$
|
61,391
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
|
December 31, 2017
|
||||||||||||||
|
(in thousands)
|
Total Fair
Value
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Money market
|
$
|
8,700
|
|
$
|
8,700
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total
|
$
|
8,700
|
|
$
|
8,700
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Year Ended
December 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
||||||||
|
U.S.
|
$
|
46,945
|
|
$
|
39,559
|
|
$
|
23,736
|
|
||
|
International
|
2,603
|
|
80
|
|
4,558
|
|
|||||
|
Income before income taxes
|
$
|
49,548
|
|
$
|
39,639
|
|
$
|
28,294
|
|
||
|
Year Ended
December 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
||||||||
|
Current
|
|||||||||||
|
Federal
|
$
|
(21
|
)
|
$
|
(58
|
)
|
$
|
(91
|
)
|
||
|
State
|
3,424
|
|
3,242
|
|
1,689
|
|
|||||
|
International
|
(135
|
)
|
16
|
|
(49
|
)
|
|||||
|
3,268
|
|
3,200
|
|
1,549
|
|
||||||
|
Deferred
|
|||||||||||
|
Federal
|
7,821
|
|
(71,742
|
)
|
—
|
|
|||||
|
State
|
1,411
|
|
(15,220
|
)
|
—
|
|
|||||
|
International
|
(3,470
|
)
|
16
|
|
(17
|
)
|
|||||
|
5,762
|
|
(86,946
|
)
|
(17
|
)
|
||||||
|
Income tax expense (benefit)
|
$
|
9,030
|
|
$
|
(83,746
|
)
|
$
|
1,532
|
|
||
|
Year Ended
December 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
||||||||
|
U.S. statutory rate
|
$
|
10,405
|
|
$
|
13,873
|
|
$
|
9,903
|
|
||
|
Permanent items
|
505
|
|
(1,916
|
)
|
(570
|
)
|
|||||
|
Write-off of foreign tax and research credits
|
—
|
|
—
|
|
7,125
|
|
|||||
|
Foreign tax credits
|
—
|
|
—
|
|
(319
|
)
|
|||||
|
Uncertain tax positions
|
3,227
|
|
3,128
|
|
1,529
|
|
|||||
|
Other tax credits
|
(742
|
)
|
(175
|
)
|
90
|
|
|||||
|
State and local taxes
|
2,125
|
|
1,252
|
|
433
|
|
|||||
|
Impact of rate change on deferred taxes
|
—
|
|
45,129
|
|
(383
|
)
|
|||||
|
True-up of prior year tax
|
—
|
|
7
|
|
(2,751
|
)
|
|||||
|
Foreign tax rate differential
|
30
|
|
97
|
|
(242
|
)
|
|||||
|
Valuation allowance
|
(4,073
|
)
|
(141,094
|
)
|
(13,292
|
)
|
|||||
|
Benefit of windfall related to stock compensation
|
(1,760
|
)
|
(2,723
|
)
|
—
|
|
|||||
|
Increase in indemnification deferred tax asset
|
(731
|
)
|
(1,055
|
)
|
—
|
|
|||||
|
Other
|
44
|
|
(269
|
)
|
9
|
|
|||||
|
Income tax expense (benefit)
|
$
|
9,030
|
|
$
|
(83,746
|
)
|
$
|
1,532
|
|
||
|
December 31,
|
|||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Deferred Tax Assets
|
|||||||
|
Federal benefit of state tax liabilities
|
$
|
7,809
|
|
$
|
7,510
|
|
|
|
Reserves, accruals and other
|
11,005
|
|
9,251
|
|
|||
|
Inventory obsolescence
|
428
|
|
239
|
|
|||
|
Capitalized research and development
|
7,491
|
|
9,941
|
|
|||
|
Amortization of intangibles other than goodwill
|
2,809
|
|
3,903
|
|
|||
|
Net operating loss carryforwards
|
55,938
|
|
63,202
|
|
|||
|
Depreciation
|
—
|
|
972
|
|
|||
|
Deferred tax assets
|
85,480
|
|
95,018
|
|
|||
|
Deferred Tax Liabilities
|
|||||||
|
Reserves, accruals and other
|
(1,078
|
)
|
(1,346
|
)
|
|||
|
Customer relationships
|
(986
|
)
|
(1,294
|
)
|
|||
|
Depreciation
|
(727
|
)
|
—
|
|
|||
|
Deferred tax liability
|
(2,791
|
)
|
(2,640
|
)
|
|||
|
Less: valuation allowance
|
(1,240
|
)
|
(5,368
|
)
|
|||
|
$
|
81,449
|
|
$
|
87,010
|
|
||
|
Recorded in the accompanying consolidated balance sheets as:
|
|||||||
|
Noncurrent deferred tax assets
|
$
|
81,449
|
|
$
|
87,010
|
|
|
|
(in thousands)
|
Amount
|
||
|
Balance, January 1, 2017
|
$
|
140,915
|
|
|
Charged to income tax expense (benefit)
|
2,305
|
|
|
|
Adoption of ASU 2016-09
|
2,929
|
|
|
|
Foreign currency
|
313
|
|
|
|
Release valuation allowance
|
(141,094
|
)
|
|
|
Balance, December 31, 2017
|
5,368
|
|
|
|
Charged to income tax expense (benefit)
|
(103
|
)
|
|
|
Foreign currency
|
(56
|
)
|
|
|
Release valuation allowance
|
(3,969
|
)
|
|
|
Balance, December 31, 2018
|
$
|
1,240
|
|
|
(in thousands)
|
Amount
|
||
|
Balance of uncertain tax positions as of January 1, 2017
|
$
|
10,441
|
|
|
Additions related to current year tax positions
|
—
|
|
|
|
Reductions related to prior year tax positions
|
(506
|
)
|
|
|
Settlements
|
—
|
|
|
|
Lapse of statute of limitations
|
(69
|
)
|
|
|
Balance of uncertain tax positions as of December 31, 2017
|
9,866
|
|
|
|
Additions related to current year tax positions
|
—
|
|
|
|
Reductions related to prior year tax positions
|
(4
|
)
|
|
|
Settlements
|
—
|
|
|
|
Lapse of statute of limitations
|
(74
|
)
|
|
|
Balance of uncertain tax positions as of December 31, 2018
|
$
|
9,788
|
|
|
December 31,
|
|||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Raw materials
|
$
|
11,100
|
|
$
|
10,447
|
|
|
|
Work in process
|
4,261
|
|
5,509
|
|
|||
|
Finished goods
|
17,658
|
|
10,124
|
|
|||
|
Total inventory
|
$
|
33,019
|
|
$
|
26,080
|
|
|
|
|
December 31,
|
||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Land
|
$
|
13,450
|
|
$
|
13,450
|
|
|
|
Buildings
|
64,444
|
|
76,059
|
|
|||
|
Machinery, equipment and fixtures
|
69,298
|
|
71,870
|
|
|||
|
Computer software
|
19,266
|
|
20,271
|
|
|||
|
Construction in progress
|
24,169
|
|
7,622
|
|
|||
|
190,627
|
|
189,272
|
|
||||
|
Less: accumulated depreciation and amortization
|
(82,739
|
)
|
(96,273
|
)
|
|||
|
Total property, plant and equipment, net
|
$
|
107,888
|
|
$
|
92,999
|
|
|
|
(in thousands)
|
Amount
|
||
|
Balance, January 1, 2018
|
$
|
10,412
|
|
|
Accretion expense
|
1,160
|
|
|
|
Balance, December 31, 2018
|
$
|
11,572
|
|
|
|
December 31, 2018
|
||||||||||||
|
(in thousands)
|
Amortization
Method |
Cost
|
Accumulated Amortization
|
Net
|
|||||||||
|
Trademarks
|
Straight-Line
|
$
|
13,540
|
|
$
|
(9,856
|
)
|
$
|
3,684
|
|
|||
|
Customer relationships
|
Accelerated
|
98,912
|
|
(93,463
|
)
|
5,449
|
|
||||||
|
Patents
|
Straight-Line
|
6,570
|
|
(6,570
|
)
|
—
|
|
||||||
|
Total
|
$
|
119,022
|
|
$
|
(109,889
|
)
|
$
|
9,133
|
|
||||
|
December 31, 2017
|
|||||||||||||
|
(in thousands)
|
Amortization
Method |
Cost
|
Accumulated Amortization
|
Net
|
|||||||||
|
Trademarks
|
Straight-Line
|
$
|
13,540
|
|
$
|
(9,304
|
)
|
$
|
4,236
|
|
|||
|
Customer relationships
|
Accelerated
|
99,133
|
|
(92,072
|
)
|
7,061
|
|
||||||
|
Patents
|
Straight-Line
|
42,780
|
|
(42,279
|
)
|
501
|
|
||||||
|
Total
|
$
|
155,453
|
|
$
|
(143,655
|
)
|
$
|
11,798
|
|
||||
|
(in thousands)
|
Amount
|
||
|
2019
|
$
|
1,803
|
|
|
2020
|
1,568
|
|
|
|
2021
|
1,309
|
|
|
|
2022
|
1,173
|
|
|
|
2023
|
579
|
|
|
|
2024 and thereafter
|
2,701
|
|
|
|
Total
|
$
|
9,133
|
|
|
|
December 31,
|
||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Compensation and benefits
|
$
|
15,962
|
|
$
|
14,469
|
|
|
|
Freight, distribution and operations
|
7,721
|
|
3,604
|
|
|||
|
Accrued rebates, discounts and chargebacks
|
4,654
|
|
2,860
|
|
|||
|
Accrued professional fees
|
1,673
|
|
2,852
|
|
|||
|
Other
|
2,040
|
|
2,751
|
|
|||
|
Total accrued expenses and other liabilities
|
$
|
32,050
|
|
$
|
26,536
|
|
|
|
(in thousands)
|
Amount
|
||
|
2019
|
2,750
|
|
|
|
2020
|
2,750
|
|
|
|
2021
|
2,750
|
|
|
|
2022
|
261,937
|
|
|
|
Total principal outstanding
|
270,187
|
|
|
|
Unamortized debt discount
|
(1,584
|
)
|
|
|
Unamortized debt issuance costs
|
(2,144
|
)
|
|
|
Total
|
266,459
|
|
|
|
Less: current portion
|
(2,750
|
)
|
|
|
Total long-term debt
|
$
|
263,709
|
|
|
2017 Facility Financial Covenant
|
|
|
Period
|
Consolidated
Leverage Ratio |
|
Q1 2019
|
4.75 to 1.00
|
|
Thereafter
|
4.50 to 1.00
|
|
Year Ended
December 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
||||||||
|
Cost of goods sold
|
$
|
1,140
|
|
$
|
1,692
|
|
$
|
359
|
|
||
|
Sales and marketing
|
1,244
|
|
640
|
|
339
|
|
|||||
|
General and administrative
|
4,990
|
|
2,964
|
|
1,438
|
|
|||||
|
Research and development
|
1,344
|
|
632
|
|
388
|
|
|||||
|
Total stock-based compensation expense
|
$
|
8,718
|
|
$
|
5,928
|
|
$
|
2,524
|
|
||
|
Total
Stock
Options
|
Weighted-
Average
Exercise
Price
|
Weighted-
Average
Remaining
Contractual
Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Balance at January 1, 2018
|
565,425
|
|
$
|
13.65
|
|
|||||||
|
Options granted
|
—
|
|
$
|
—
|
|
|||||||
|
Options exercised
|
(192,550
|
)
|
$
|
5.98
|
|
|||||||
|
Options cancelled and expired
|
(15,800
|
)
|
$
|
20.44
|
|
|||||||
|
Outstanding at December 31, 2018
|
357,075
|
|
$
|
17.50
|
|
4.4
|
535,427
|
|
||||
|
Vested and expected to vest at December 31, 2018
|
357,075
|
|
$
|
17.50
|
|
4.4
|
535,427
|
|
||||
|
Exercisable at December 31, 2018
|
353,049
|
|
$
|
17.56
|
|
4.4
|
522,423
|
|
||||
|
Shares
|
Weighted-
Average Grant
Date Fair Value Per Share
|
|||||
|
Nonvested balance at January 1, 2018
|
1,765,262
|
|
$
|
5.72
|
|
|
|
Granted
|
647,850
|
|
$
|
15.46
|
|
|
|
Vested
|
(672,345
|
)
|
$
|
6.37
|
|
|
|
Forfeited
|
(232,228
|
)
|
$
|
11.87
|
|
|
|
Nonvested balance at December 31, 2018
|
1,508,539
|
|
$
|
9.51
|
|
|
|
Shares
|
Weighted-
Average Grant Date Fair Value Per Share |
|||||
|
Nonvested balance at January 1, 2018
|
291,172
|
|
$
|
16.70
|
|
|
|
Granted
|
—
|
|
$
|
—
|
|
|
|
Vested
|
—
|
|
$
|
—
|
|
|
|
Forfeited
|
(49,292
|
)
|
$
|
16.62
|
|
|
|
Nonvested balance at December 31, 2018
|
241,880
|
|
$
|
16.71
|
|
|
|
Year Ended December 31,
|
|||
|
2018
|
|||
|
Expected volatility
|
84.3
|
%
|
|
|
Risk-free interest rate
|
2.4
|
%
|
|
|
Expected life (in years)
|
2.8
|
|
|
|
Expected dividend yield
|
—
|
|
|
|
Shares
|
Weighted-
Average Grant Date Fair Value Per Share |
|||||
|
Nonvested balance at January 1, 2018
|
—
|
|
$
|
—
|
|
|
|
Granted
|
206,896
|
|
$
|
22.76
|
|
|
|
Vested
|
—
|
|
$
|
—
|
|
|
|
Forfeited
|
(26,983
|
)
|
$
|
22.76
|
|
|
|
Nonvested balance at December 31, 2018
|
179,913
|
|
$
|
22.76
|
|
|
|
|
Year Ended
December 31, |
||||||||||
|
(in thousands, except per share amounts)
|
2018
|
2017
|
2016
|
||||||||
|
Net income
|
$
|
40,518
|
|
$
|
123,385
|
|
$
|
26,762
|
|
||
|
Basic weighted-average common shares outstanding
|
38,233
|
|
37,276
|
|
32,044
|
|
|||||
|
Effect of dilutive stock options
|
61
|
|
288
|
|
612
|
|
|||||
|
Effect of dilutive restricted stock
|
1,207
|
|
1,328
|
|
—
|
|
|||||
|
Diluted weighted-average common shares outstanding
|
39,501
|
|
38,892
|
|
32,656
|
|
|||||
|
Basic income per common share
|
$
|
1.06
|
|
$
|
3.31
|
|
$
|
0.84
|
|
||
|
Diluted income per common share
|
$
|
1.03
|
|
$
|
3.17
|
|
$
|
0.82
|
|
||
|
Antidilutive securities excluded from diluted net income per common share
|
424
|
|
604
|
|
1,563
|
|
|||||
|
(in thousands)
|
Amount
|
||
|
2019
|
$
|
2,264
|
|
|
2020
|
259
|
|
|
|
2021
|
238
|
|
|
|
2022
|
238
|
|
|
|
2023
|
238
|
|
|
|
2024 and thereafter
|
178
|
|
|
|
Total
|
$
|
3,415
|
|
|
Year Ended
December 31, |
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
||||||||
|
Revenues from external customers
|
|||||||||||
|
U.S.
|
$
|
288,580
|
|
$
|
290,002
|
|
$
|
257,420
|
|
||
|
International
|
54,794
|
|
41,376
|
|
44,433
|
|
|||||
|
Total revenues from external customers
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
||
|
Revenues by product
|
|||||||||||
|
DEFINITY
|
$
|
183,073
|
|
$
|
157,268
|
|
$
|
131,612
|
|
||
|
TechneLite
|
98,858
|
|
104,644
|
|
99,217
|
|
|||||
|
Other
|
61,443
|
|
69,466
|
|
71,024
|
|
|||||
|
Total revenues
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
||
|
Geographical revenues
|
|||||||||||
|
U.S.
|
$
|
288,580
|
|
$
|
290,002
|
|
$
|
257,420
|
|
||
|
All other
|
54,794
|
|
41,376
|
|
44,433
|
|
|||||
|
Total revenues
|
$
|
343,374
|
|
$
|
331,378
|
|
$
|
301,853
|
|
||
|
Operating income
|
|||||||||||
|
U.S.
|
$
|
56,327
|
|
$
|
49,239
|
|
$
|
46,909
|
|
||
|
International
|
8,161
|
|
2,614
|
|
9,679
|
|
|||||
|
Operating income
|
64,488
|
|
51,853
|
|
56,588
|
|
|||||
|
Interest expense
|
17,405
|
|
18,410
|
|
26,618
|
|
|||||
|
Debt retirement costs
|
—
|
|
—
|
|
1,896
|
|
|||||
|
Loss on extinguishment of debt
|
—
|
|
2,442
|
|
—
|
|
|||||
|
Other income
|
(2,465
|
)
|
(8,638
|
)
|
(220
|
)
|
|||||
|
Income before income taxes
|
$
|
49,548
|
|
$
|
39,639
|
|
$
|
28,294
|
|
||
|
Depreciation and amortization
|
|||||||||||
|
U.S.
|
$
|
12,278
|
|
$
|
17,672
|
|
$
|
15,995
|
|
||
|
International
|
491
|
|
517
|
|
1,335
|
|
|||||
|
Total depreciation and amortization
|
$
|
12,769
|
|
$
|
18,189
|
|
$
|
17,330
|
|
||
|
|
December 31,
|
||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Long-lived assets
|
|||||||
|
U.S.
|
$
|
106,755
|
|
$
|
91,537
|
|
|
|
International
|
1,133
|
|
1,462
|
|
|||
|
Total long-lived assets
|
$
|
107,888
|
|
$
|
92,999
|
|
|
|
(in thousands)
|
Balance at Beginning of Year
|
Charged to Income
|
Deductions from Reserves
(1)
|
Other Adjustments
|
Balance at End of Year
|
|||||||||||||||
|
Allowance for doubtful accounts
|
||||||||||||||||||||
|
Year ended December 31, 2018
|
$
|
977
|
|
$
|
321
|
|
$
|
(179
|
)
|
$
|
—
|
|
$
|
1,119
|
|
|||||
|
Year ended December 31, 2017
|
$
|
969
|
|
$
|
136
|
|
$
|
(128
|
)
|
$
|
—
|
|
$
|
977
|
|
|||||
|
Year ended December 31, 2016
|
$
|
881
|
|
$
|
53
|
|
$
|
(30
|
)
|
$
|
65
|
|
$
|
969
|
|
|||||
|
Rebates and allowances
|
||||||||||||||||||||
|
Year ended December 31, 2018
|
$
|
2,860
|
|
$
|
13,202
|
|
$
|
(11,047
|
)
|
$
|
(361
|
)
|
$
|
4,654
|
|
|||||
|
Year ended December 31, 2017
|
$
|
2,297
|
|
$
|
9,568
|
|
$
|
(8,351
|
)
|
$
|
(654
|
)
|
$
|
2,860
|
|
|||||
|
Year ended December 31, 2016
|
$
|
2,303
|
|
$
|
7,255
|
|
$
|
(6,809
|
)
|
$
|
(452
|
)
|
$
|
2,297
|
|
|||||
|
(1)
|
Amounts charged to deductions from allowance for doubtful accounts represent the write-off of uncollectible balances and represent payments for rebates and allowances.
|
|
Quarterly Periods During the Year Ended
December 31, 2018 |
|||||||||||||||
|
Q1
|
Q2
|
Q3
|
Q4
|
||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Revenues
|
$
|
82,630
|
|
$
|
85,573
|
|
|
$
|
88,900
|
|
$
|
86,271
|
|
||
|
Gross profit
|
$
|
42,309
|
|
$
|
43,846
|
|
|
$
|
44,885
|
|
$
|
43,845
|
|
||
|
Net income
|
$
|
8,211
|
|
$
|
9,745
|
|
|
$
|
9,269
|
|
$
|
13,293
|
|
||
|
Basic income per weighted-average share
(b)
|
$
|
0.22
|
|
$
|
0.25
|
|
|
$
|
0.24
|
|
$
|
0.35
|
|
||
|
Diluted income per weighted-average share
(b)
|
$
|
0.21
|
|
$
|
0.25
|
|
|
$
|
0.24
|
|
$
|
0.34
|
|
||
|
Quarterly Periods During the Year Ended
December 31, 2017 |
|||||||||||||||
|
Q1
|
Q2
|
Q3
|
Q4
|
||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Revenues
|
$
|
81,359
|
|
$
|
88,837
|
|
$
|
79,941
|
|
$
|
81,241
|
|
|||
|
Gross profit
|
$
|
39,762
|
|
$
|
45,947
|
|
$
|
38,527
|
|
$
|
37,899
|
|
|||
|
Net income
(a)
|
$
|
4,138
|
|
$
|
13,595
|
|
$
|
8,526
|
|
$
|
97,126
|
|
|||
|
Basic income per weighted-average share
(b)
|
$
|
0.11
|
|
$
|
0.37
|
|
$
|
0.23
|
|
$
|
2.58
|
|
|||
|
Diluted income per weighted-average share
(b)
|
$
|
0.11
|
|
$
|
0.35
|
|
$
|
0.22
|
|
$
|
2.47
|
|
|||
|
(a)
|
Net income for the fourth quarter of 2017 reflects the income tax benefit due to the release of the Company’s valuation allowance of
$141.1 million
against its deferred tax assets offset by a provision of
$45.1 million
for remeasuring the Company’s deferred tax assets for the change in tax rates enacted under the Tax Cuts and Jobs Act of 2017.
|
|
(b)
|
Quarterly and annual computations are prepared independently. Accordingly, the sum of each quarter may not necessarily total the fiscal year period amounts noted elsewhere within this Annual Report on Form 10-K.
|
|
▪
|
Exemption from the auditor attestation requirement on the effectiveness of our system of internal control over financial reporting;
|
|
▪
|
Exemption from compliance with any new requirements adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer;
|
|
▪
|
Exemption from the requirement to seek non-binding advisory votes on executive compensation and golden parachute arrangements; and
|
|
▪
|
Reduced disclosure about executive compensation arrangements.
|
|
Page
|
|
|
Incorporated by Reference
|
||||||||||
|
Exhibit
Number
|
Description of Exhibits
|
Form
|
File Number
|
Exhibit
|
Filing Date
|
|||||
|
2.1†
|
10-Q/A
|
001-36569
|
2.1
|
August 25, 2016
|
||||||
|
2.2†
|
10-Q
|
001-36569
|
10.1
|
November 1, 2016
|
||||||
|
3.1
|
8-K
|
001-36569
|
3.1
|
April 27, 2018
|
||||||
|
3.2
|
8-K
|
001-36569
|
3.2
|
April 27, 2018
|
||||||
|
4.1
|
8-K
|
001-36569
|
4.1
|
June 30, 2015
|
||||||
|
10.4†
|
S-4
|
333-169785
|
10.9
|
December 23, 2010
|
||||||
|
10.5†
|
S-4
|
333-169785
|
10.10
|
December 1, 2010
|
||||||
|
10.6†
|
10-Q
|
333-169785
|
10.1
|
May 13, 2011
|
||||||
|
10.9†
|
S-4
|
333-169785
|
10.26
|
December 1, 2010
|
||||||
|
10.10†
|
S-4
|
333-169785
|
10.14
|
December 23, 2010
|
||||||
|
10.11†
|
S-4
|
333-169785
|
10.16
|
December 29, 2010
|
||||||
|
10.12†
|
S-4
|
333-169785
|
10.17
|
December 1, 2010
|
||||||
|
10.13+
|
S-4
|
333-169785
|
10.18
|
October 6, 2010
|
||||||
|
10.14+
|
S-4
|
333-169785
|
10.19
|
October 6, 2010
|
||||||
|
Incorporated by Reference
|
||||||||||
|
Exhibit
Number
|
Description of Exhibits
|
Form
|
File Number
|
Exhibit
|
Filing Date
|
|||||
|
10.15+
|
S-4
|
333-169785
|
10.20
|
October 6, 2010
|
||||||
|
10.16+
|
S-4
|
333-169785
|
10.21
|
October 6, 2010
|
||||||
|
10.17+
|
S-4
|
333-169785
|
10.24
|
October 6, 2010
|
||||||
|
10.18†
|
10-Q
|
333-169785
|
10.1
|
May 15, 2012
|
||||||
|
10.19†
|
10-Q
|
333-169785
|
10.2
|
May 15, 2012
|
||||||
|
10.20†
|
10-Q
|
333-169785
|
10.1
|
August 14, 2012
|
||||||
|
10.22†
|
10-Q
|
001-36569
|
10.53
|
May 2, 2018
|
||||||
|
10.23†
|
10-K
|
333-169785
|
10.54
|
March 29, 2013
|
||||||
|
10.25†
|
10-Q
|
333-169785
|
10.1
|
May 10, 2013
|
||||||
|
10.26+
|
8-K
|
333-169785
|
10.1
|
May 6, 2013
|
||||||
|
10.27+
|
8-K
|
333-169785
|
10.2
|
May 6, 2013
|
||||||
|
10.28+
|
8-K
|
333-169785
|
10.3
|
May 6, 2013
|
||||||
|
10.33+
|
10-K
|
333-169785
|
10.48
|
March 11, 2014
|
||||||
|
10.37+
|
S-1
|
333-196998
|
10.37
|
June 24, 2015
|
||||||
|
10.38+
|
S-1
|
333-196998
|
10.38
|
June 24, 2015
|
||||||
|
10.39+
|
S-1
|
333-196998
|
10.39
|
June 24, 2015
|
||||||
|
10.40+
|
S-1
|
333-196998
|
10.40
|
June 24, 2015
|
||||||
|
10.41+
|
S-1
|
333-196998
|
10.41
|
June 24, 2015
|
||||||
|
10.42+
|
10-Q
|
333-169785
|
10.1
|
May 5, 2015
|
||||||
|
10.47
|
8-K
|
001-36569
|
10.5
|
June 30, 2015
|
||||||
|
10.49+
|
8-K
|
001-36569
|
10.7
|
June 30, 2015
|
||||||
|
10.50+
|
8-K
|
001-36569
|
10.8
|
June 30, 2015
|
||||||
|
10.52+
|
10-Q
|
001-36569
|
10.2
|
November 4, 2015
|
||||||
|
10.53†
|
10-K
|
001-36569
|
10.53
|
March 2, 2016
|
||||||
|
10.57+
|
8-K
|
001-36569
|
10.1
|
April 28, 2016
|
||||||
|
10.58†
|
10-Q
|
001-36569
|
10.2
|
November 1, 2016
|
||||||
|
10.59+
|
8-K
|
001-36569
|
10.1
|
April 28, 2017
|
||||||
|
10.60+
|
8-K
|
001-36569
|
10.2
|
April 28, 2017
|
||||||
|
10.61
|
10-Q
|
001-36569
|
10.1
|
May 2, 2017
|
||||||
|
Incorporated by Reference
|
||||||||||
|
Exhibit
Number
|
Description of Exhibits
|
Form
|
File Number
|
Exhibit
|
Filing Date
|
|||||
|
10.62†
|
10-Q
|
001-36569
|
10.1
|
August 1, 2017
|
||||||
|
10.63†
|
10-Q
|
001-36569
|
10.2
|
August 1, 2017
|
||||||
|
10.64†
|
10-K
|
001-36569
|
10.64
|
February 7, 2018
|
||||||
|
10.65†
|
10-K
|
001-36569
|
10.65
|
February 7, 2018
|
||||||
|
10.66
|
10-Q
|
001-36569
|
10.1
|
October 30, 2018
|
||||||
|
10.67
|
10-Q
|
001-36569
|
10.2
|
October 30, 2018
|
||||||
|
10.68*+
|
||||||||||
|
10.69*+
|
||||||||||
|
10.70*+
|
||||||||||
|
10.71*+
|
||||||||||
|
21.1*
|
||||||||||
|
23.1*
|
||||||||||
|
24.1*
|
||||||||||
|
31.1*
|
||||||||||
|
31.2*
|
||||||||||
|
32.1**
|
||||||||||
|
101.INS*
|
XBRL Instance Document
|
|||||||||
|
101.SCH*
|
XBRL Taxonomy Extension Schema
|
|||||||||
|
101.CAL*
|
XBRL Taxonomy Extension Calculation
|
|||||||||
|
101.DEF*
|
XBRL Taxonomy Extension Definition
|
|||||||||
|
101.LAB*
|
XBRL Taxonomy Extension Labels
|
|||||||||
|
101.PRE*
|
XBRL Taxonomy Extension Presentation
|
|||||||||
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
|
+
|
Indicates management contract or compensatory plan or arrangement.
|
|
†
|
Confidential treatment requested as to certain portions, which portions have been filed separately with the Securities and Exchange Commission
|
|
LANTHEUS HOLDINGS, INC.
|
|
|
By:
|
/S/ MARY ANNE HEINO
|
|
Name:
|
Mary Anne Heino
|
|
Title:
|
President and Chief Executive Officer
|
|
Date:
|
February 20, 2019
|
|
Signature
|
Title
|
Date
|
||
|
/S/ MARY ANNE HEINO
|
Chief Executive Officer, President and Director
(Principal Executive Officer) |
February 20, 2019
|
||
|
Mary Anne Heino
|
||||
|
/S/ ROBERT J. MARSHALL, JR.
|
Chief Financial Officer and Treasurer
(Principal Financial and Accounting Officer) |
February 20, 2019
|
||
|
Robert J. Marshall, Jr.
|
||||
|
/S/ BRIAN MARKISON
|
Chairman of the Board of Directors
|
February 20, 2019
|
||
|
Brian Markison
|
||||
|
/S/ JAMES C. CLEMMER
|
Director
|
February 20, 2019
|
||
|
James C. Clemmer
|
||||
|
/S/ SAMUEL R. LENO
|
Director
|
February 20, 2019
|
||
|
Samuel R. Leno
|
||||
|
/S/ JULIE H. MCHUGH
|
Director
|
February 20, 2019
|
||
|
Julie H. McHugh
|
||||
|
/S/ GARY J. PRUDEN
|
Director
|
February 20, 2019
|
||
|
Gary J. Pruden
|
||||
|
/S/ KENNETH J. PUCEL
|
Director
|
February 20, 2019
|
||
|
Kenneth J. Pucel
|
||||
|
/S/ DR. FREDERICK A. ROBERTSON
|
Director
|
February 20, 2019
|
||
|
Dr. Frederick A. Robertson
|
||||
|
/S/ DR. DERACE L. SCHAFFER
|
Director
|
February 20, 2019
|
||
|
Dr. Derace L. Schaffer
|
||||
|
/S/ DR. JAMES H. THRALL
|
Director
|
February 20, 2019
|
||
|
Dr. James H. Thrall
|
||||