|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
DELAWARE
|
22-2343568
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
incorporation or organization)
|
Identification No.)
|
|
|
|
|
420 LEXINGTON AVE, SUITE 350
NEW YORK, NEW YORK
|
10170
|
|
(Address of principal executive offices)
|
(zip code)
|
|
|
|
|
|
Title of Each Class
|
|
Name of Each Exchange On Which Registered
|
|
Common Stock, par value $0.001 per share
|
|
NYSE MKT
|
|
Large accelerated filer
o
|
Accelerated filer
o
|
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
x
|
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
||
|
|
||
|
ITEM 1. BUSINESS
|
||
|
ITEM 1A. RISK FACTORS
|
||
|
ITEM 1B. UNRESOLVED STAFF COMMENTS
|
||
|
ITEM 2. PROPERTIES
|
||
|
ITEM 3. LEGAL PROCEEDINGS
|
||
|
ITEM 4. MINE SAFTEY DISCLOSURES
|
||
|
PART II
|
||
|
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
||
|
ITEM 6. SELECTED FINANCIAL DATA
|
||
|
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
||
|
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
||
|
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
||
|
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
||
|
ITEM 9A. CONTROLS AND PROCEDURES
|
||
|
ITEM 9B. OTHER INFORMATION
|
||
|
PART III
|
||
|
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
||
|
ITEM 11. EXECUTIVE COMPENSATION
|
||
|
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
||
|
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
||
|
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
|
||
|
PART IV
|
||
|
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
||

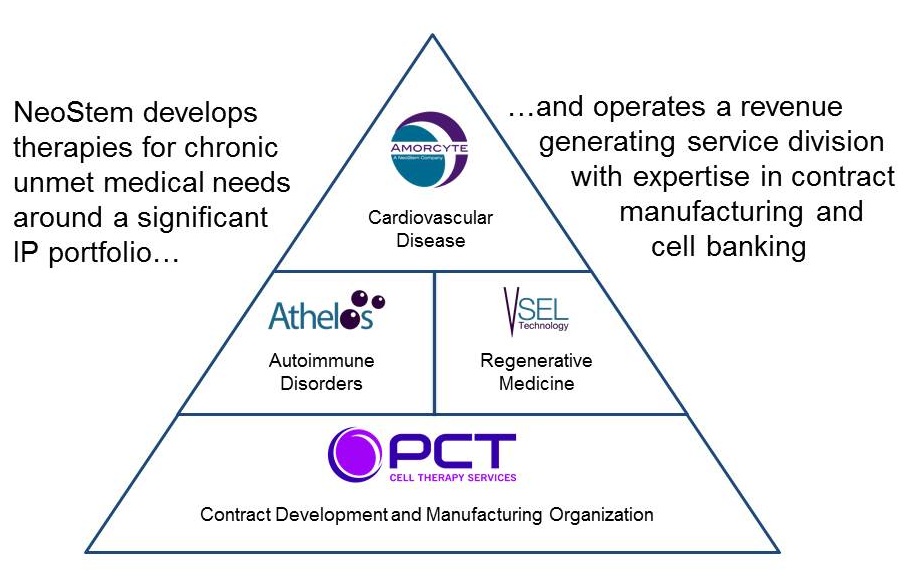
|
Ÿ
|
A patient's own bone marrow is harvested and a sterile pharmaceutical composition enriched for CD34+/CXCR4+ cells is prepared using our patented technology.
|
|
Ÿ
|
The isolated cells are then infused via catheter into the infarct-related artery 6 to 11 days following an AMI, which we believe is the optimal time frame for cellular intervention, after the pro-inflammatory “hot phase” and prior to permanent scar formation, while the heart tissue is actively attracting CD34+/CXCR4+ cells.
|
|
Ÿ
|
The infused cells migrate to the at-risk tissue along a hypoxia-induced Stromal-Derived Factor-1 gradient to a signal emitted from the infarct as described above, inducing angiogenesis and a resultant functional benefit.
|
|
•
|
U.S. Patent No. 7,794,705 covering a chemotactic stem cell product enriched for CD34+ cells that treats injury from AMI;
|
|
•
|
U.S. Patent No. 8,088,370 covering the use of AMR-001 in the repair of injury caused by vascular insufficiency, including all forms of cardiac insufficiency, such as congestive heart failure, chronic myocardial ischemia and, we believe, vascular insufficiency induced ischemic conditions beyond the cardiac setting;
|
|
•
|
U.S. Patent No. 8,343,485 offering expanded breadth in AMR's composition and treatment methods in the vascular insufficiency setting; and
|
|
•
|
U.S. patent application no. 13/285,606 covering a method of treating a progressive myocardial injury caused by an ischemic condition and utilizing a multi-dosing regimen.
|
|
Area of Focus
|
Funding Source
|
Grant Amount
|
Dates of Project
|
Objective
|
|
Wound healing
|
U.S. Department of Defense
|
$700,000
|
6/1/2010- 12/31/2014
|
In collaboration with Roger Williams Medical Center (an affiliate of Boston University), an evaluation of topically applied bone marrow derived adult mesenchymal stem cells for rapid wound healing.
|
|
Osteoporosis
|
U.S. Department of Defense
|
$1,780,500
|
9/30/2011- 12/31/2014
|
In collaboration with the University of Michigan, the development of autologous pluripotent VSEL
TM
stem cells to reverse bone loss associated with osteoporosis.
|
|
Acute Radiation Syndrome
|
National Institutes of Health - National Institute of Allergy and Infectious Disease
|
$600,000
|
3/11/2012- 3/11/2014
|
In collaboration with the University of Louisville, the development of human autologous pluripotent VSEL
TM
stem cells as a countermeasure to acute radiation syndrome.
|
|
Bone Formation
|
National Institutes of Health - National Institute of Dental and Craniofacial Research
|
$1,221,000
|
9/11/2012- 8/31/2014
|
In collaboration with the University of Michigan, an evaluation of human autologous pluripotent VSEL
TM
stem cells in bone formation following tooth extraction for periodontitis.
|
|
Nerve Regeneration
|
U.S. Department of Defense - Telemedicine & Advanced Technology Research Center
|
$50,000
|
2012-2013
|
Evaluation of VSEL
TM
stem cells in regenerating the sciatic nerve.
|
|
•
|
Manufacturing
: Manufacturing cell therapy-based products includes a number of challenges, including the need for substantial capital and resource investment, limited unit sizes and process scalability, short processing turnaround times and stringent and evolving regulatory requirements. PCT's facilities, infrastructure and extensive experience provide a turn-key solution to clients to meet these challenges. In order to bolster our unique expertise and further reduce cost of goods sold for products, PCT continually seeks innovation drivers, including new opportunities for automation in its manufacturing operations.
|
|
•
|
Product and Process Development
: PCT works with clients to develop, optimize, implement and validate various aspects of cell therapy product and process development.
|
|
•
|
Cell and Tissue Processing
: PCT offers a full range of cost-effective cell collection and processing services that meet cGTP standards.
|
|
•
|
Storage, Distribution and Delivery
: PCT offers cryogenic storage facilities for both short- and long-term storage of tissues, primary cells and cell therapy products. In addition, PCT leverages its established logistics and distribution network to ensure a timely, secure and cost-effective point-to-point chain of control and custody.
|
|
•
|
Consulting and Regulatory Support
:
PCT offers its clients a full-range of scientific, technical and regulatory support along the entire spectrum of cell therapy development.
|
|
•
|
Registration and listing requirements for establishments that manufacture HCT/Ps;
|
|
•
|
Requirements for determining donor eligibility, including donor screening and testing;
|
|
•
|
cGTP requirements, which include requirements pertaining to the manufacturer's quality program, personnel, procedures, manufacturing facilities, environmental controls, equipment, supplies and reagents, recovery, processing and process controls, labeling, storage, record-keeping, tracking, complaint files, receipt, pre-distribution shipment, distribution, and donor eligibility determinations, donor screening, and donor testing;
|
|
•
|
Adverse reaction reporting;
|
|
•
|
Labeling of HCT/Ps; and
|
|
•
|
FDA inspection, retention, recall, destruction, and cessation of manufacturing operations.
|
|
•
|
Phase 1
: Studies are initially conducted in a limited population to test the product candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion in healthy humans or, on occasion, in patients, such as cancer patients when the drug or biologic is too toxic to be ethically given to healthy individuals.
|
|
•
|
Phase 2
: Studies are generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the product for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
|
|
•
|
Phase 3
: These are commonly referred to as pivotal studies. When Phase 2 evaluations demonstrate that a dose range of the product is effective and has an acceptable safety profile, Phase 3 clinical trials are undertaken in large patient populations to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically-dispersed clinical trial sites. In most cases FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in rare instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
|
|
•
|
Phase 4
: In some cases, FDA may condition approval of an NDA or BLA for a product candidate on the sponsor's agreement to conduct additional clinical trials after NDA or BLA approval. In other cases, a Sponsor may voluntarily carry out additional trials post approval to gain more information about the drug or biologic. Such post approval trials are typically referred to as Phase 4 studies.
|
|
•
|
Common health care transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures;
|
|
•
|
Unique identifiers for providers, employers, health plans and individuals; and
|
|
•
|
Security and privacy of health information.
|
|
•
|
state and local licensure, registration and regulation of laboratories, the processing and storage of human cells and tissue, and the development and manufacture of pharmaceuticals and biologics;
|
|
•
|
other laws and regulations administered by the United States FDA, including the Federal Food, Drug, and Cosmetic Act and related laws and regulations and the Public Health Service Act and related laws and regulations;
|
|
•
|
laws and regulations administered by the United States Department of Health and Human Services, including the Office for Human Research Protections;
|
|
•
|
state laws and regulations governing human subject research;
|
|
•
|
federal and state coverage and reimbursement laws and regulations, including laws and regulations administered by the Centers for Medicare & Medicaid Services and state Medicaid agencies;
|
|
•
|
the federal Medicare and Medicaid Anti-Kickback Law and similar state laws and regulations;
|
|
•
|
the federal physician self-referral prohibition commonly known as the Stark Law, and state equivalents of the Stark Law;
|
|
•
|
Occupational Safety and Health Administration (“OSHA”) requirements;
|
|
•
|
state and local laws and regulations dealing with the handling and disposal of medical waste; and
|
|
•
|
the Intermediate Sanctions rules of the IRS providing for potential financial sanctions with respect to “Excess Benefit Transactions” with HUMC or other tax-exempt organizations.
|
|
•
|
the scope, progress, results, costs, timing and outcomes of our other cell therapy research and development programs and product candidates;
|
|
•
|
our ability to enter into any collaboration agreements with third parties for our other product candidates and the timing and terms of any such agreements;
|
|
•
|
the timing of and the costs involved in obtaining regulatory approvals for our product candidates, a process which could be particularly lengthy or complex given the FDA's limited experience with marketing approval for cell therapy products; and
|
|
•
|
the costs of maintaining, expanding and protecting our intellectual property portfolio, including potential litigation costs and liabilities.
|
|
•
|
low levels of trading volume for our shares;
|
|
•
|
capital-raising or other transactions that are, or may in the future be, dilutive to existing stockholders or that involve the issuance of debt securities;
|
|
•
|
delays in our clinical trials, negative clinical trial results or adverse regulatory decisions relating to our product candidates;
|
|
•
|
adverse fluctuations in our revenues or operating results or financial results that otherwise fall below the market's expectations;
|
|
•
|
disappointing developments concerning our PCT clients or other potential business partners for our product candidates; and
|
|
•
|
legal challenges, disputes and/or other adverse developments impacting our patents or other proprietary rights that protect our products.
|
|
Ÿ
|
suspensions, delays or changes in the design, initiation, enrollment, implementation or completion of required clinical trials;
|
|
|
Ÿ
|
adverse changes in our financial position or significant and unexpected increases in the cost of our clinical development program;
|
|
|
Ÿ
|
changes or uncertainties in, or additions to, the regulatory approval process that require us to alter our current development strategy;
|
|
|
Ÿ
|
clinical trial results that are negative, inconclusive or even less than desired as to AMR-001's safety and/or efficacy, which could result in the need for additional clinical studies or the termination of the product's development; and
|
|
|
Ÿ
|
delays in the ability to manufacture the product in quantities or in a form that is suitable for any required clinical trials;
|
|
|
Ÿ
|
intellectual property constraints that prevent us from making, using, or commercializing AMR-001; and
|
|
|
Ÿ
|
the supply or quality of our product candidates or other materials necessary to conduct clinical trials of these product candidates may be insufficient or inadequate.
|
|
|
Ÿ
|
obtain approval for indications that are not as broad as the indications we sought;
|
|
|
Ÿ
|
have the product removed from the market after obtaining marketing approval;
|
|
|
Ÿ
|
encounter issues with respect to the manufacturing of commercial supplies;
|
|
|
Ÿ
|
be subject to additional post-marketing testing requirements; and/or
|
|
|
Ÿ
|
be subject to restrictions on how the product is distributed or used.
|
|
|
Ÿ
|
the clinical effectiveness, safety and convenience of AMR-001, particularly in relation to alternative treatments;
|
|
|
Ÿ
|
our ability to distinguish AMR-001 from any ethical and political controversies associated with stem cell products derived from human embryonic or fetal tissue; and
|
|
|
Ÿ
|
the cost of the product, the reimbursement policies of government and third-party payors and our ability to obtain sufficient third-party coverage or reimbursement.
|
|
|
Ÿ
|
the product candidates require significant clinical testing to demonstrate safety and effectiveness before applications for marketing approval can be filed with the FDA and other regulatory authorities;
|
|
|
Ÿ
|
data obtained from preclinical and nonclinical animal testing and clinical trials can be interpreted in different ways, and regulatory authorities may not agree with our respective interpretations or may require us to conduct additional testing;
|
|
|
Ÿ
|
negative or inconclusive results or the occurrence of serious or unexpected adverse events during a clinical trial could cause us to delay or terminate development efforts for a product candidate; and/or
|
|
|
Ÿ
|
FDA and other regulatory authorities may require expansion of the size and scope of the clinical trials.
|
|
|
Ÿ
|
third-party clinical investigators do not perform the clinical trials on the anticipated schedule or consistent with the clinical trial protocol, good clinical practices required by the FDA and other regulatory requirements, or other third parties do not perform data collection and analysis in a timely or accurate manner;
|
|
|
Ÿ
|
inspections of clinical trial sites by the FDA or by institutional review boards of research institutions participating in the clinical trials, reveal regulatory violations that require the sponsor of the trial to undertake corrective action, suspend or terminate one or more sites, or prohibit use of some or all of the data in support of marketing applications; or
|
|
|
Ÿ
|
the FDA or one or more institutional review boards suspends or terminates the trial at an investigational site, or precludes enrollment of additional subjects.
|
|
|
Ÿ
|
warning letters or other actions requiring changes in product manufacturing processes or restrictions on product marketing or distribution;
|
|
|
Ÿ
|
product recalls or seizures or the temporary or permanent withdrawal of a product from the market; and
|
|
|
Ÿ
|
fines, restitution or disgorgement of profits or revenue, the imposition of civil penalties or criminal prosecution.
|
|
|
2012
|
High
|
Low
|
|
First Quarter
|
$0.90
|
$0.37
|
|
Second Quarter
|
$0.61
|
$0.30
|
|
Third Quarter
|
$0.84
|
$0.49
|
|
Fourth Quarter
|
$0.78
|
$0.59
|
|
2011
|
High
|
Low
|
|
First Quarter
|
$2.10
|
$1.14
|
|
Second Quarter
|
$2.08
|
$1.31
|
|
Third Quarter
|
$1.55
|
$0.55
|
|
Fourth Quarter
|
$0.75
|
$0.43
|
|
2010
|
High
|
Low
|
|
First Quarter
|
$2.15
|
$1.26
|
|
Second Quarter
|
$3.50
|
$1.58
|
|
Third Quarter
|
$2.15
|
$1.52
|
|
Fourth Quarter
|
$2.15
|
$1.10
|
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Clinical Services
|
$
|
8,034.8
|
|
$
|
5,503.5
|
|
|
|
Clinical Services Reimbursables
|
3,462.2
|
|
2,599.3
|
|
|||
|
Processing and Storage Services
|
2,644.7
|
|
1,738.3
|
|
|||
|
Other
|
188.2
|
|
208.9
|
|
|||
|
|
$
|
14,329.9
|
|
$
|
10,050.1
|
|
|
|
•
|
Clinical Services, representing process development and clinical manufacturing services provided at PCT to its various clients, were approximately $
8.0 million
for the
year ended
December 31, 2012
compared to $
5.5 million
for the
year ended
December 31, 2011
, representing an increase of approximately $
2.5 million
or
46%
. The increase in clinical services revenue is primarily due to an increased penetration into the cell therapy marketplace along with a general increase in the development of autologous cell therapies in the United States due to enhanced investment and expanded marketing programs in 2011 and 2012. The revenue increase was partially offset by a
$0.3 million
increase in deferred revenue as of December 31, 2012 compared to December 31, 2011, related to ongoing clinical service contracts that had not met revenue recognition completion criteria. In accordance with our revenue recognition policy, revenue is recognized upon contract completion for certain clinical service contracts.
|
|
•
|
Clinical Services Reimbursables, representing reimbursement of expenses for certain consumables incurred on behalf of our clinical service revenue clients, were approximately $
3.5 million
for the
year ended
December 31, 2012
compared to $
2.6 million
for the
year ended
December 31, 2011
, representing an increase of approximately $
0.9 million
or
33%
. Our reimbursable revenue increased as a result of increased manufacturing and process development activity.
|
|
•
|
Processing and Storage Services, representing revenues from our oncology, cord blood, and adult stem cell banking activities, were approximately $
2.6 million
for the
year ended
December 31, 2012
compared to $
1.7 million
for the
year ended
December 31, 2011
, representing an increase of approximately $
0.9 million
or
52%
. The increase is primarily attributable to increased revenue from our oncology stem cell processing service. Additionally, we added hospital clients during 2012 as more hospitals have begun to outsource their oncology stem cell processing.
|
|
•
|
Other Revenue were approximately $
0.2 million
for the
year ended
December 31, 2012
compared to $
0.2 million
for the
year ended
December 31, 2011
. Other revenues primarily represent license fees related to our adult stem cell technology.
|
|
•
|
Research and development expenses were approximately $
10.5 million
for the
year ended
December 31, 2012
compared to $
7.7 million
for the
year ended
December 31, 2011
, representing an increase of approximately $
2.8 million
, or
35%
. Research and development expenses increased by approximately $
6.5 million
in 2012, due to the initiation in January 2012 of our Phase 2 clinical trial for AMR-001 . This increase was partially offset by a $1.2 million in-process research and development charge in 2011, as well as reduced internal research activities following the closing of our research facility in Cambridge, Massachusetts in 2012. Equity-based compensation included in research and development expenses for the
year ended
December 31, 2012
was approximately $
0.4 million
, compared to approximately $
0.9 million
for the
year ended
December 31, 2011
, representing a decrease of $
0.5 million
.
|
|
•
|
Selling, general and administrative expenses were approximately $
22.3 million
for the
year ended
December 31, 2012
compared to $
27.7 million
for the
year ended
December 31, 2011
, representing a decrease of approximately $
5.4 million
, or
19%
. Equity-based compensation included in selling, general and administrative expenses for the
year ended
December 31, 2012
was approximately $
6.1 million
, compared to approximately $
8.9 million
for the
year ended
December 31, 2011
, representing a decrease of $
2.8 million
. General and administrative expenses decreased approximately $
1.7 million
, primarily due to lower overall professional fees, as well as a one-time contribution in 2011 of $0.6 million paid in equity to the Stem for Life Foundation. Selling expenses also decreased $
0.8 million
compared to the prior year period.
|
|
Cash
|
$
|
195.1
|
|
|
Prepaid expenses and other current assets
|
14.9
|
|
|
|
Property, plant and equipment, net
|
1,023.7
|
|
|
|
Other Assets
|
330.5
|
|
|
|
Accounts payable
|
(177.1
|
)
|
|
|
Accrued liabilities
|
(79.2
|
)
|
|
|
Accumulated comprehensive income
|
(169.9
|
)
|
|
|
Loss on exit of segment
|
$
|
1,138.0
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Revenue
|
$
|
52.3
|
|
$
|
274.3
|
|
|
|
Cost of revenues
|
(30.6
|
)
|
(140.6
|
)
|
|||
|
Research and development
|
(103.3
|
)
|
(378.3
|
)
|
|||
|
Selling, general, and administrative
|
(497.3
|
)
|
(3,089.8
|
)
|
|||
|
Other income (expense)
|
(6.8
|
)
|
(9.7
|
)
|
|||
|
Loss on exit of segment
|
(1,138.0
|
)
|
—
|
|
|||
|
Loss from discontinued operations
|
$
|
(1,723.7
|
)
|
$
|
(3,344.1
|
)
|
|
|
Fair value of consideration received
|
$
|
13,397.9
|
|
|
Carrying value of segment non-controlling interest
|
6,015.0
|
|
|
|
Carrying value of segment accumulated comprehensive income
|
4,387.4
|
|
|
|
$
|
23,800.3
|
|
|
|
Less carrying amount of assets and liabilities sold:
|
|||
|
Cash
|
$
|
8,457.5
|
|
|
Restricted Cash
|
2,918.1
|
|
|
|
Accounts Receivable
|
6,130.2
|
|
|
|
Inventories
|
15,077.7
|
|
|
|
Prepaid expenses and other current assets
|
957.8
|
|
|
|
Property, plant and equipment, net
|
38,102.0
|
|
|
|
Other assets
|
5,946.3
|
|
|
|
Accounts payable
|
(9,604.8
|
)
|
|
|
Accrued liabilities
|
(2,008.8
|
)
|
|
|
Bank loans
|
(15,133.5
|
)
|
|
|
Notes payable
|
(6,599.3
|
)
|
|
|
Other liabilities
|
(9,166.8
|
)
|
|
|
Amount due related party
|
(7,859.7
|
)
|
|
|
$
|
27,216.7
|
|
|
|
Loss on exit of segment
|
$
|
(3,416.4
|
)
|
|
Year Ended December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
Revenue
|
$
|
61,703.1
|
|
$
|
63,393.6
|
|
|
|
Cost of revenues
|
(40,245.2
|
)
|
(47,186.8
|
)
|
|||
|
Research and development
|
(1,836.4
|
)
|
(2,904.1
|
)
|
|||
|
Selling, general, and administrative
|
(10,740.0
|
)
|
(11,068.2
|
)
|
|||
|
Other income (expense)
|
(1,045.2
|
)
|
(1,081.4
|
)
|
|||
|
Provision for income taxes
|
(1,794.1
|
)
|
(392.8
|
)
|
|||
|
Asset impairments
|
(31,170.1
|
)
|
(19,432.7
|
)
|
|||
|
Loss on sale of segment
|
(3,416.4
|
)
|
—
|
|
|||
|
Loss from discontinued operations
|
$
|
(28,544.3
|
)
|
$
|
(18,672.4
|
)
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Net cash used in operating activities - continuing operations
|
$
|
(18,759.9
|
)
|
$
|
(21,773.2
|
)
|
|
|
Net cash provided by (used in) investing activities - continuing operations
|
11,748.7
|
|
(251.4
|
)
|
|||
|
Net cash provided by financing activities - continuing operations
|
17,112.0
|
|
17,329.9
|
|
|||
|
•
|
We received gross proceeds of
$6.8 million
, prior to deducting underwriting discounts and offering expenses, for net proceeds of approximately
$6.0 million
in connection with an underwritten public offering of 17,000,000 units (inclusive of exercise of the underwriters over-allotment option) at a purchase price of $0.40 per unit, with each unit consisting of one share of Common Stock and a five year warrant to purchase one share of Common Stock at an exercise price of $0.51 per share.
|
|
•
|
We raised gross proceeds of approximately
$7.1 million
in private placements of an aggregate of approximately
13.4 million
shares of Common Stock and
8.9 million
five year warrants at exercise prices ranging from $0.51 to $0.74.
|
|
•
|
We raised gross proceeds of approximately
$3.3 million
(all of which was raised fourth quarter 2012) through the issuance of
5.3 million
shares of Common Stock under the provisions of our equity line of credit with Aspire.
|
|
•
|
We raised approximately
$6.6 million
from the exercise of approximately
11.1 million
warrants. To induce the exercise of certain of these warrants, we provided consideration to the warrant holders in the form of either cash, stock or additional warrants.
|
|
•
|
we raised an aggregate of approximately
$20.5 million
(or net proceeds of approximately
$19.7 million
) through an underwritten public offering of common stock and warrants, private placements, and warrant exercises.
|
|
•
|
we raised an aggregate of approximately
$3.3 million
under the Purchase Agreement with Aspire Capital, which provides that Aspire Capital is committed to purchase up to $20 million of shares of the Company’s common stock over the term of that Agreement through September 30, 2015, subject to certain terms and conditions, including a floor price.
|
|
•
|
we completed the sale of our 51% interest in Erye for total consideration including approximately
$12.3 million
in cash.
|
|
Total
|
Less than 1 Year
|
1-3 Years
|
3-5 Years
|
More than 5 Years
|
|||||||||||||||
|
Contractual Obligations
|
|||||||||||||||||||
|
Mortgages Payable
|
$
|
3,438.5
|
|
$
|
201.8
|
|
$
|
438.2
|
|
$
|
2,414.8
|
|
$
|
383.7
|
|
||||
|
Capital Lease Obligations
|
254.9
|
|
83.4
|
|
171.5
|
|
—
|
|
—
|
|
|||||||||
|
Operating Lease Obligations
|
3,592.9
|
|
1,143.3
|
|
1,592.5
|
|
857.1
|
|
—
|
|
|||||||||
|
$
|
7,286.3
|
|
$
|
1,428.5
|
|
$
|
2,202.2
|
|
$
|
3,271.9
|
|
$
|
383.7
|
|
|||||
|
•
|
persuasive evidence of an arrangement exists;
|
|
•
|
delivery has occurred or the services have been rendered;
|
|
•
|
the fee is fixed or determinable; and
|
|
•
|
collectability is probable.
|
|
Report of Independent Registered Public Accounting Firm
|
||
|
Financial Statements:
|
||
|
Consolidated Balance Sheet at December 31, 2012 and 2011
|
||
|
Consolidated Statements of Operations Years Ended December 31, 2012 and 2011
|
||
|
Consolidated Statements of Comprehensive Loss Years Ended December 31, 2012 and 2011
|
||
|
Consolidated Statements of Equity Years Ended December 31, 2012 and 2011
|
||
|
Consolidated Statements of Cash Flows Years Ended December 31, 2012 and 2011
|
||
|
Notes to Consolidated Financial Statements
|
||
|
December 31,
2012 |
December 31,
2011 |
||||||
|
ASSETS
|
|
|
|
|
|||
|
Current Assets
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
13,737,452
|
|
$
|
3,935,160
|
|
|
|
Accounts receivable trade, net of allowance for doubtful accounts
of $626,054 and $187,600, respectively
|
1,053,604
|
|
1,010,475
|
|
|||
|
Inventory
|
1,113,025
|
|
647,745
|
|
|||
|
Prepaids and other current assets
|
803,135
|
|
649,739
|
|
|||
|
Assets related to discontinued operations
|
—
|
|
32,367,217
|
|
|||
|
Total current assets
|
16,707,216
|
|
38,610,336
|
|
|||
|
Property, plant and equipment, net
|
11,153,143
|
|
11,616,053
|
|
|||
|
Goodwill
|
11,117,770
|
|
11,117,770
|
|
|||
|
Intangible assets, net
|
14,480,827
|
|
15,086,038
|
|
|||
|
Other assets
|
947,307
|
|
3,326,938
|
|
|||
|
Assets related to discontinued operations
|
—
|
|
75,570,645
|
|
|||
|
$
|
54,406,263
|
|
$
|
155,327,780
|
|
||
|
LIABILITIES AND EQUITY
|
|
|
|
|
|||
|
Current Liabilities
|
|
|
|
|
|||
|
Accounts payable
|
$
|
2,555,240
|
|
$
|
2,287,201
|
|
|
|
Accrued liabilities
|
2,284,813
|
|
1,090,176
|
|
|||
|
Notes payable
|
202,558
|
|
148,062
|
|
|||
|
Mortgages payable
|
3,438,475
|
|
3,635,061
|
|
|||
|
Unearned revenues
|
1,468,341
|
|
1,121,134
|
|
|||
|
Liabilities related to discontinued operations
|
—
|
|
28,165,010
|
|
|||
|
Total current liabilities
|
9,949,427
|
|
36,446,644
|
|
|||
|
Long-term Liabilities
|
|
|
|
|
|||
|
Deferred income taxes
|
3,599,122
|
|
3,774,655
|
|
|||
|
Unearned revenues
|
—
|
|
169,198
|
|
|||
|
Notes payable
|
171,528
|
|
—
|
|
|||
|
Derivative liabilities
|
101,156
|
|
474,463
|
|
|||
|
Acquisition-related contingent consideration
|
7,550,000
|
|
3,130,000
|
|
|||
|
Other long-term liabilities
|
214,871
|
|
—
|
|
|||
|
Liabilities related to discontinued operations
|
—
|
|
26,388,976
|
|
|||
|
Total long-term liabilities
|
11,636,677
|
|
33,937,292
|
|
|||
|
Commitments and Contingencies
|
|
|
|
|
|||
|
Redeemable Securities
|
|
|
|
|
|||
|
Convertible Redeemable Series E Preferred Stock; 10,582,011 shares designated, liquidation value $1.00 per share; issued and outstanding 0 shares and 6,662,748 shares at December 31, 2012 and 2011, respectively
|
—
|
|
4,811,326
|
|
|||
|
EQUITY
|
|
|
|
|
|||
|
Stockholders' Equity
|
|
|
|||||
|
Preferred stock; authorized, 20,000,000 shares
Series B convertible redeemable preferred stock
liquidation value, 1 share of common stock, $.01 par value; 825,000 shares designated; issued and outstanding, 10,000 shares at December 31, 2012 and December 31, 2011
|
100
|
|
100
|
|
|||
|
Common stock, $.001 par value, authorized 500,000,000 shares;
issued and outstanding, 163,753,653 and 109,329,587 shares,
at December 31, 2012 and December 31, 2011, respectively
|
163,754
|
|
109,330
|
|
|||
|
Additional paid-in capital
|
231,071,236
|
|
200,858,638
|
|
|||
|
Treasury stock, at cost
|
(665,600
|
)
|
—
|
|
|||
|
Accumulated deficit
|
(197,392,361
|
)
|
(143,094,854
|
)
|
|||
|
Accumulated other comprehensive income
|
—
|
|
4,152,343
|
|
|||
|
Total NeoStem, Inc. stockholders' equity
|
33,177,129
|
|
62,025,557
|
|
|||
|
Noncontrolling interests
|
(356,970
|
)
|
18,106,961
|
|
|||
|
Total equity
|
32,820,159
|
|
80,132,518
|
|
|||
|
$
|
54,406,263
|
|
$
|
155,327,780
|
|
||
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Revenues
|
$
|
14,329,889
|
|
$
|
10,050,086
|
|
|
|
Cost of revenues
|
11,949,124
|
|
8,646,687
|
|
|||
|
Gross profit
|
2,380,765
|
|
1,403,399
|
|
|||
|
Research and development
|
10,451,070
|
|
7,720,748
|
|
|||
|
Selling, general, and administrative
|
22,315,346
|
|
27,687,162
|
|
|||
|
Operating Expenses
|
32,766,416
|
|
35,407,910
|
|
|||
|
Operating loss
|
(30,385,651
|
)
|
(34,004,511
|
)
|
|||
|
Other income (expense):
|
|||||||
|
Other (expense) income, net
|
(4,314,228
|
)
|
2,085,870
|
|
|||
|
Interest expense
|
(1,576,975
|
)
|
(2,647,692
|
)
|
|||
|
(5,891,203
|
)
|
(561,822
|
)
|
||||
|
Loss from operations before provision for income taxes and noncontrolling interests
|
(36,276,854
|
)
|
(34,566,333
|
)
|
|||
|
Provision (benefit) for income taxes
|
(175,533
|
)
|
—
|
|
|||
|
Net loss from continuing operations
|
(36,101,321
|
)
|
(34,566,333
|
)
|
|||
|
Loss from discontinued operations - net
|
(30,267,990
|
)
|
(22,016,524
|
)
|
|||
|
Net loss
|
(66,369,311
|
)
|
(56,582,857
|
)
|
|||
|
Less - loss from continuing operations attributable to noncontrolling interests
|
(287,181
|
)
|
(299,789
|
)
|
|||
|
Less - loss from discontinued operations attributable to noncontrolling interests
|
(12,312,646
|
)
|
(9,148,599
|
)
|
|||
|
Net loss attributable to NeoStem, Inc.
|
(53,769,484
|
)
|
(47,134,469
|
)
|
|||
|
Warrant inducements
|
(1,012,819
|
)
|
—
|
|
|||
|
Preferred dividends
|
(528,023
|
)
|
(639,765
|
)
|
|||
|
Net loss attributable to NeoStem, Inc. common stockholders
|
$
|
(55,310,326
|
)
|
(47,774,234
|
)
|
||
|
Amounts Attributable to NeoStem, Inc. common stockholders:
|
|||||||
|
Loss from continuing operations
|
$
|
(35,814,140
|
)
|
$
|
(34,266,544
|
)
|
|
|
Loss from discontinued operations - net of taxes
|
(17,955,344
|
)
|
(12,867,925
|
)
|
|||
|
Warrant inducements
|
(1,012,819
|
)
|
—
|
|
|||
|
Preferred dividends
|
(528,023
|
)
|
(639,765
|
)
|
|||
|
Net loss attributable to NeoStem, Inc. common stockholders
|
$
|
(55,310,326
|
)
|
$
|
(47,774,234
|
)
|
|
|
Basic and diluted (loss) per share attributable to NeoStem, Inc. common stockholders:
|
|
|
|
|
|||
|
Continuing operations
|
$
|
(0.26
|
)
|
$
|
(0.39
|
)
|
|
|
Discontinued operations
|
$
|
(0.13
|
)
|
(0.15
|
)
|
||
|
NeoStem, Inc. common stockholders
|
$
|
(0.40
|
)
|
$
|
(0.54
|
)
|
|
|
Weighted average common shares outstanding
|
138,419,965
|
|
88,598,696
|
|
|||
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Net loss
|
$
|
(66,369,311
|
)
|
$
|
(56,582,857
|
)
|
|
|
Other comprehensive income (loss):
|
|
|
|
|
|||
|
Foreign currency translation elimination on exit of segment
|
(169,993
|
)
|
—
|
|
|||
|
Foreign currency translation elimination on sale of segment
|
(4,387,371
|
)
|
—
|
|
|||
|
Foreign currency translation
|
405,021
|
|
2,606,639
|
|
|||
|
Total other comprehensive (loss) income
|
(4,152,343
|
)
|
2,606,639
|
|
|||
|
Comprehensive loss
|
(70,521,654
|
)
|
(53,976,218
|
)
|
|||
|
Noncontrolling interests elimination on sale of segment
|
(6,014,981
|
)
|
—
|
|
|||
|
Comprehensive loss attributable to noncontrolling interests
|
(12,448,950
|
)
|
(8,215,026
|
)
|
|||
|
Comprehensive net loss attributable to NeoStem, Inc. common stockholders
|
$
|
(52,057,723
|
)
|
$
|
(45,761,192
|
)
|
|
|
|
Series B Convertible
Preferred Stock
|
Common Stock
|
Additional
Paid in
Capital
|
Accumulated
Other
Comprehensive
Income
|
Accumulated
Deficit
|
Treasury
Stock
|
Total
NeoStem,
Inc.
Stockholders'
Equity
|
Non-
Controlling
Interest in
Subsidiary
|
Total
Equity
|
||||||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||
|
Balance at December 31, 2010
|
10,000
|
|
$
|
100
|
|
64,221,130
|
|
$
|
63,813
|
|
$
|
141,137,522
|
|
$
|
2,779,066
|
|
$
|
(95,320,620
|
)
|
$
|
—
|
|
$
|
48,659,881
|
|
$
|
37,827,738
|
|
$
|
86,487,619
|
|
||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(47,134,469
|
)
|
—
|
|
(47,134,469
|
)
|
(9,448,388
|
)
|
(56,582,857
|
)
|
|||||||||||||||||||
|
Foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,373,277
|
|
—
|
|
—
|
|
1,373,277
|
|
1,223,710
|
|
2,596,987
|
|
|||||||||||||||||||
|
Exercise of stock options
|
—
|
|
—
|
|
5,000
|
|
5
|
|
7,095
|
|
—
|
|
—
|
|
—
|
|
7,100
|
|
—
|
|
7,100
|
|
|||||||||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
3,824,018
|
|
3,824
|
|
10,262,199
|
|
—
|
|
—
|
|
—
|
|
10,266,023
|
|
—
|
|
10,266,023
|
|
|||||||||||||||||||
|
Proceeds from issuance of common stock
|
—
|
|
—
|
|
19,678,224
|
|
19,678
|
|
21,133,004
|
|
—
|
|
—
|
|
—
|
|
21,152,682
|
|
—
|
|
21,152,682
|
|
|||||||||||||||||||
|
Shares issued for charitable contribution
|
—
|
|
—
|
|
—
|
|
408
|
|
606,955
|
|
—
|
|
—
|
|
—
|
|
607,363
|
|
—
|
|
607,363
|
|
|||||||||||||||||||
|
Repayment of Series E Preferred Principal and Dividends
|
—
|
|
—
|
|
5,157,732
|
|
5,158
|
|
4,254,907
|
|
—
|
|
(639,765
|
)
|
—
|
|
3,620,300
|
|
—
|
|
3,620,300
|
|
|||||||||||||||||||
|
Dividends to related party
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(11,726,099
|
)
|
(11,726,099
|
)
|
|||||||||||||||||||
|
Technology contributed to Athelos by Non-Controlling Interest
|
—
|
|
—
|
|
—
|
|
—
|
|
920,000
|
|
—
|
|
—
|
|
—
|
|
920,000
|
|
230,000
|
|
1,150,000
|
|
|||||||||||||||||||
|
Shares issued in PCT Merger
|
—
|
|
—
|
|
10,600,000
|
|
10,600
|
|
17,189,400
|
|
—
|
|
—
|
|
—
|
|
17,200,000
|
|
—
|
|
17,200,000
|
|
|||||||||||||||||||
|
Shares issued in Amorcyte Merger
|
—
|
|
—
|
|
5,843,483
|
|
5,844
|
|
5,347,556
|
|
—
|
|
—
|
|
—
|
|
5,353,400
|
|
—
|
|
5,353,400
|
|
|||||||||||||||||||
|
Balance at December 31, 2011
|
10,000
|
|
$
|
100
|
|
109,329,587
|
|
$
|
109,330
|
|
$
|
200,858,638
|
|
$
|
4,152,343
|
|
$
|
(143,094,854
|
)
|
$
|
—
|
|
$
|
62,025,557
|
|
$
|
18,106,961
|
|
$
|
80,132,518
|
|
||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(53,769,484
|
)
|
—
|
|
(53,769,484
|
)
|
(12,599,827
|
)
|
(66,369,311
|
)
|
|||||||||||||||||||
|
Foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
235,028
|
|
—
|
|
—
|
|
235,028
|
|
150,877
|
|
385,905
|
|
|||||||||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
3,364,268
|
|
3,364
|
|
6,709,172
|
|
—
|
|
—
|
|
—
|
|
6,712,536
|
|
—
|
|
6,712,536
|
|
|||||||||||||||||||
|
Proceeds from issuance of common stock
|
—
|
|
—
|
|
35,732,289
|
|
35,732
|
|
16,393,095
|
|
—
|
|
—
|
|
—
|
|
16,428,827
|
|
—
|
|
16,428,827
|
|
|||||||||||||||||||
|
Proceeds from warrant exercises
|
—
|
|
—
|
|
11,076,182
|
|
11,077
|
|
6,593,342
|
|
—
|
|
—
|
|
—
|
|
6,604,419
|
|
—
|
|
6,604,419
|
|
|||||||||||||||||||
|
Shares, options and warrants received in Erye sale
|
—
|
|
—
|
|
—
|
|
—
|
|
(452,301
|
)
|
—
|
|
—
|
|
(665,600
|
)
|
(1,117,901
|
)
|
—
|
|
(1,117,901
|
)
|
|||||||||||||||||||
|
Elimination of equity associated with sale of Erye
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,387,371
|
)
|
—
|
|
—
|
|
(4,387,371
|
)
|
(6,014,981
|
)
|
(10,402,352
|
)
|
|||||||||||||||||||
|
Repayment of Series E Preferred Principal and Dividends
|
—
|
|
—
|
|
2,792,375
|
|
2,792
|
|
1,199,425
|
|
—
|
|
(528,023
|
)
|
—
|
|
674,194
|
|
—
|
|
674,194
|
|
|||||||||||||||||||
|
Warrant inducements
|
—
|
|
—
|
|
1,458,952
|
|
1,459
|
|
(230,135
|
)
|
—
|
|
—
|
|
—
|
|
(228,676
|
)
|
—
|
|
(228,676
|
)
|
|||||||||||||||||||
|
Balance at December 31, 2012
|
10,000
|
|
$
|
100
|
|
163,753,653
|
|
$
|
163,754
|
|
$
|
231,071,236
|
|
$
|
—
|
|
$
|
(197,392,361
|
)
|
$
|
(665,600
|
)
|
$
|
33,177,129
|
|
$
|
(356,970
|
)
|
$
|
32,820,159
|
|
||||||||||
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Cash flows from operating activities:
|
|
|
|
|
|||
|
Net loss
|
$
|
(66,369,311
|
)
|
$
|
(56,582,857
|
)
|
|
|
Loss from discontinued operations
|
30,267,990
|
|
22,016,524
|
|
|||
|
Adjustments to reconcile net loss to net cash used in
operating activities:
|
|
|
|
|
|||
|
Common stock, stock options and warrants issued
as payment for compensation, services rendered and interest expense
|
6,712,536
|
|
10,266,023
|
|
|||
|
Depreciation and amortization
|
1,550,571
|
|
1,440,576
|
|
|||
|
Amortization of preferred stock discount and issuance cost
|
1,609,495
|
|
2,440,241
|
|
|||
|
Changes in fair value of derivative liability
|
(373,307
|
)
|
(2,096,904
|
)
|
|||
|
Changes in acquisition-related contingent consideration
|
4,420,000
|
|
—
|
|
|||
|
Write off of acquired in-process research and development
|
—
|
|
1,150,000
|
|
|||
|
Loss on disposal of assets
|
13,653
|
|
—
|
|
|||
|
Contributions paid with common stock
|
—
|
|
607,363
|
|
|||
|
Bad debt expense (recovery)
|
511,755
|
|
(87,773
|
)
|
|||
|
Deferred income taxes
|
(175,533
|
)
|
—
|
|
|||
|
Changes in operating assets and liabilities, net of the effect of acquisitions:
|
|
|
|
|
|||
|
Prepaid expenses and other current assets
|
(178,011
|
)
|
(350,227
|
)
|
|||
|
Accounts receivable
|
(554,884
|
)
|
(443,132
|
)
|
|||
|
Inventory
|
(465,280
|
)
|
(647,745
|
)
|
|||
|
Unearned revenues
|
178,008
|
|
948,452
|
|
|||
|
Other assets
|
2,414,842
|
|
53,211
|
|
|||
|
Accounts payable, accrued expenses and other current liabilities
|
1,677,551
|
|
(486,952
|
)
|
|||
|
Net cash used in operating activities - continuing operations
|
(18,759,925
|
)
|
(21,773,200
|
)
|
|||
|
Net cash provided by operating activities - discontinued operations
|
4,907,407
|
|
845,206
|
|
|||
|
Net cash used in operating activities
|
(13,852,518
|
)
|
(20,927,994
|
)
|
|||
|
Cash flows from investing activities:
|
|
|
|
|
|||
|
Cash received in acquisitions
|
—
|
|
320,863
|
|
|||
|
Cash received in divestiture
|
12,280,000
|
|
—
|
|
|||
|
Change in restricted cash used as collateral for notes payable
|
—
|
|
2,596
|
|
|||
|
Acquisition of property and equipment
|
(531,315
|
)
|
(574,857
|
)
|
|||
|
Net cash provided by (used in) investing activities - continuing operations
|
11,748,685
|
|
(251,398
|
)
|
|||
|
Net cash used in investing activities - discontinued operations
|
(5,660,305
|
)
|
(1,803,182
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
6,088,380
|
|
(2,054,580
|
)
|
|||
|
Cash flows from financing activities:
|
|
|
|
|
|||
|
Net proceeds from exercise of options
|
—
|
|
7,100
|
|
|||
|
Net proceeds from exercise of warrants
|
6,604,418
|
|
—
|
|
|||
|
Net proceeds from issuance of capital stock
|
16,428,827
|
|
21,152,682
|
|
|||
|
Repayment of mortgage loan
|
(196,585
|
)
|
(149,542
|
)
|
|||
|
Proceeds from notes payable
|
666,501
|
|
149,766
|
|
|||
|
Repayment of notes payable
|
(440,477
|
)
|
(180,132
|
)
|
|||
|
Repayment of debt to related party
|
—
|
|
(3,000,000
|
)
|
|||
|
Repayment of preferred stock
|
(5,394,263
|
)
|
(650,000
|
)
|
|||
|
Payment of dividend for preferred stock
|
(327,748
|
)
|
—
|
|
|||
|
Payment for warrant inducement
|
(228,676
|
)
|
—
|
|
|||
|
Net cash provided by financing activities - continuing operations
|
17,111,997
|
|
17,329,874
|
|
|||
|
Net cash (used in) provided by financing activities - discontinued operations
|
(8,370,228
|
)
|
2,739,496
|
|
|||
|
Net cash provided by financing activities
|
8,741,769
|
|
20,069,370
|
|
|||
|
Impact of changes of foreign exchange rates
|
14,389
|
|
46,245
|
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
992,020
|
|
(2,866,959
|
)
|
|||
|
Cash and cash equivalents at beginning of period
|
12,745,432
|
|
15,612,391
|
|
|||
|
Cash and cash equivalents at end of period
|
13,737,452
|
|
12,745,432
|
|
|||
|
Less cash and cash equivalents of discontinued operations at end of period
|
—
|
|
8,810,272
|
|
|||
|
Cash and cash equivalents of continuing operations at end of period
|
$
|
13,737,452
|
|
$
|
3,935,160
|
|
|
|
Supplemental Disclosure of Cash Flow Information:
|
|||||||
|
Cash paid during the period for:
|
|||||||
|
Interest
|
$
|
1,771,800
|
|
$
|
1,522,700
|
|
|
|
Taxes
|
2,100,000
|
|
1,119,500
|
|
|||
|
Supplemental Schedule of non-cash investing activities:
|
|
|
|
|
|||
|
Capitalized interest
|
182,000
|
|
384,300
|
|
|||
|
Common stock, warrants and options received upon sale of Erye
|
1,117,901
|
|
—
|
|
|||
|
Supplemental schedule of non-cash financing activities
|
|||||||
|
Common stock, warrants and contingent consideration issued with the acquisition of Amorcyte
|
—
|
|
8,483,400
|
|
|||
|
Common stock and warrants issued with the acquisition of PCT
|
—
|
|
17,200,000
|
|
|||
|
Common stock issued pursuant to the redemption of Convertible Redeemable Series E 7% Preferred Stock
|
1,026,600
|
|
3,511,200
|
|
|||
|
Common stock issued in payment of dividends for the Convertible Redeemable Series E 7% Preferred Stock
|
175,700
|
|
748,900
|
|
|||
|
Dividend to related party reinvested as loan payable
|
—
|
|
11,726,100
|
|
|||
|
Entity
|
Percentage of Ownership
|
Location
|
||
|
NeoStem, Inc.
|
Parent Company
|
United States of America
|
||
|
NeoStem Therapies, Inc.
|
100%
|
United States of America
|
||
|
Stem Cell Technologies, Inc.
|
100%
|
United States of America
|
||
|
Amorcyte, LLC
|
100%
|
United States of America
|
||
|
CBH Acquisition LLC
|
100%
|
United States of America
|
||
|
China Biopharmaceuticals Holdings, Inc. (CBH)*
|
100% owned by CBH Acquisition LLC
|
United States of America
|
||
|
Progenitor Cell Therapy, LLC (PCT)
|
100%
|
United States of America
|
||
|
NeoStem Family Storage, LLC
|
100% owned by PCT
|
United States of America
|
||
|
Athelos Corporation
|
80.1% owned by PCT
|
United States of America
|
||
|
PCT Allendale, LLC
|
100% owned by PCT
|
United States of America
|
||
|
Building and improvements
|
25-30 years
|
|
Machinery and equipment
|
8-12 years
|
|
Lab equipment
|
5-7 years
|
|
Furniture and fixtures
|
5-12 years
|
|
Software
|
3-5 years
|
|
Leasehold improvements
|
Life of lease
|
|
•
|
persuasive evidence of an arrangement exists;
|
|
•
|
delivery has occurred or the services have been rendered;
|
|
•
|
the fee is fixed or determinable; and
|
|
•
|
collectability is probable.
|
|
i.
|
5,843,483
shares of NeoStem Common Stock (reflecting certain adjustments taken at the closing, and subject to further adjustment following the closing in accordance with the Amorcyte Merger Agreement) (the “Base Stock Consideration”);
|
|
ii.
|
up to
4,092,768
shares of NeoStem Common Stock (the “Contingent Shares”, and together with the Base Stock Consideration, the “Stock Consideration”), which Contingent Shares will be issued only if certain specified business milestones (described below) are accomplished;
|
|
iii.
|
warrants to purchase
1,881,008
shares of NeoStem Common Stock exercisable over a seven (7) year period at an exercise price of
$1.466
per share (the “Warrants”) (such Warrants are redeemable in certain circumstances, and transfer of any shares of NeoStem Common Stock issued upon exercise of the Warrants was restricted until one year after the Closing Date); and
|
|
iv.
|
earn out payments equal to
10%
of the net sales of Amorcyte’s lead product candidate AMR-001 (in the event of and following the date of first commercial sale of AMR-001), provided that in the event NeoStem sublicenses AMR-001, the applicable earn out payment will be equal to
30%
of any sublicensing fees, and provided further that NeoStem will be entitled to recover direct out-of-pocket clinical development costs not previously paid or reimbursed and any costs, expenses, liabilities and settlement amounts arising out of claims of patent infringement or otherwise challenging Amorcyte’s right to use intellectual property, by reducing any earn out payments due by
50%
until such costs have been recouped in full (the “Earn Out Payments”).
|
|
•
|
One-third of the Contingent Shares (
1,364,256
shares) will be issued upon (a) the completion of Phase 2 clinical trial for Amorcyte’s product candidate AMR-001 and (b) issuance of a statistically significant analysis demonstrating satisfaction of the primary clinical end points from the Phase 2 clinical trial, which primary clinical endpoints are described in the Phase 2 clinical trial protocol submitted by Amorcyte to the FDA on July 5, 2011.
|
|
•
|
One-third of the Contingent Shares will be issued following a Type B End of Phase 2/Pre-Phase 3 meeting with the FDA wherein AMR-001 is acknowledged in writing by the FDA to be ready for Phase 3.
|
|
•
|
The remaining one-third of the Contingent Shares will be issued upon the first dosing of the first patient in the pivotal Phase 3 clinical study for AMR-001.
|
|
Cash
|
$
|
92.9
|
|
|
Prepaid Expenses
|
178.2
|
|
|
|
In Process R&D
|
9,400.0
|
|
|
|
Goodwill
|
4,104.5
|
|
|
|
Accounts Payable & Accrued Liabilities
|
1,177.1
|
|
|
|
Deferred Tax Liability
|
3,774.7
|
|
|
|
Amount Due Related Party
|
340.4
|
|
|
|
i.
|
common stock purchase warrants to purchase one million (
1,000,000
) shares of NeoStem Common Stock, exercisable over a seven year period at an exercise price of
$7.00
per share (the “$7.00 Warrants”), and which will vest only if a specified business milestone (described in the PCT Merger Agreement) is accomplished within three (3) years of the Closing Date of the PCT Merger; and
|
|
ii.
|
common stock purchase warrants to purchase one million (
1,000,000
) shares of NeoStem Common Stock exercisable over a seven year term at an exercise price of
$3.00
per share (the “$3.00 Warrants”); and
|
|
iii.
|
common stock purchase warrants to purchase one million (
1,000,000
) shares of NeoStem Common Stock exercisable over a seven year period at an exercise price of
$5.00
per share (the “$5.00 Warrants” and, collectively with the $7.00 Warrants and the $3.00 Warrants, the “Warrants”).
|
|
Cash
|
$
|
227.9
|
|
|
Accounts Receivable
|
451.4
|
|
|
|
Other Current Assets
|
166.2
|
|
|
|
Property, Plant & Equipment
|
11,755.0
|
|
|
|
Intangibles
|
5,700.0
|
|
|
|
Goodwill
|
7,013.5
|
|
|
|
Other Assets
|
581.9
|
|
|
|
Accounts Payable
|
1,370.9
|
|
|
|
Other Liabilities
|
540.5
|
|
|
|
Amount Due Related Party
|
3,000.0
|
|
|
|
Mortgages Payable
|
3,784.6
|
|
|
|
Year Ended December 31,
|
||||||||
|
|
2011
|
2011
|
||||||
|
|
(As Reported)
|
(Pro Forma)
|
||||||
|
Revenues
|
$
|
10,050
|
|
$
|
10,322
|
|
||
|
Cost of revenues
|
8,647
|
|
8,923
|
|
||||
|
Gross profit
|
1,403
|
|
1,400
|
|
||||
|
Research and development
|
7,721
|
|
7,964
|
|
||||
|
Selling, general, and administrative
|
27,687
|
|
29,473
|
|
||||
|
Operating loss
|
(34,005
|
)
|
(36,037
|
)
|
||||
|
Other expense, net
|
(562
|
)
|
(548
|
)
|
||||
|
Net loss from continuing operations
|
(34,566
|
)
|
(36,586
|
)
|
||||
|
Loss from discontinued operations - net
|
(22,017
|
)
|
(22,017
|
)
|
||||
|
Net loss
|
(56,583
|
)
|
(58,602
|
)
|
||||
|
Less – net income attributable to noncontrolling interests
|
(9,448
|
)
|
(9,448
|
)
|
||||
|
Preferred dividends
|
640
|
|
640
|
|
||||
|
Net loss attributable to NeoStem, Inc. common stockholders
|
$
|
(47,774
|
)
|
$
|
(49,794
|
)
|
||
|
Basic and diluted loss per share
|
$
|
(0.54
|
)
|
$
|
(0.53
|
)
|
||
|
Weighted average common shares outstanding
|
88,599
|
|
93,793
|
|
||||
|
December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
Building and improvements
|
$
|
9,897.8
|
|
$
|
9,874.0
|
|
|
|
Machinery and equipment
|
39.5
|
|
17.9
|
|
|||
|
Lab equipment
|
1,800.6
|
|
1,559.1
|
|
|||
|
Furniture and fixtures
|
683.7
|
|
632.4
|
|
|||
|
Software
|
99.5
|
|
98.3
|
|
|||
|
Leasehold improvements
|
654.5
|
|
702.0
|
|
|||
|
Property, plant and equipment, gross
|
13,175.6
|
|
12,883.7
|
|
|||
|
Accumulated depreciation
|
(2,022.5
|
)
|
(1,267.6
|
)
|
|||
|
Property, plant and equipment, net
|
$
|
11,153.1
|
|
$
|
11,616.1
|
|
|
|
|
December 31,
|
||||
|
|
2012
|
2011
|
|||
|
Stock Options
|
21,686,680
|
|
17,143,505
|
|
|
|
Warrants
|
55,287,611
|
|
37,389,825
|
|
|
|
Series E Preferred Stock, Common stock equivalents
|
—
|
|
3,989,669
|
|
|
|
Restricted Shares
|
342,500
|
|
976,668
|
|
|
|
|
December 31, 2012
|
||||||||||
|
|
Fair Value Measurements Using Fair Value Hierarchy
|
||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
||||||||
|
Warrant derivative liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
101.2
|
|
||
|
Contingent consideration
|
—
|
|
—
|
|
7,550.0
|
|
|||||
|
|
December 31, 2011
|
||||||||||
|
|
Fair Value Measurements Using Fair Value Hierarchy
|
||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
||||||||
|
Money market investments
|
$
|
2,497.4
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Embedded derivative liabilities
|
—
|
|
—
|
|
391.7
|
|
|||||
|
Warrant derivative liabilities
|
—
|
|
—
|
|
82.7
|
|
|||||
|
Contingent consideration
|
—
|
|
—
|
|
3,130.0
|
|
|||||
|
Year Ended
|
|||||||||||
|
December 31, 2012
|
|||||||||||
|
|
Embedded Derivatives
|
Warrants
|
Contingent Consideration
|
||||||||
|
Beginning liability balance
|
$
|
391.7
|
|
$
|
82.7
|
|
$
|
3,130.0
|
|
||
|
Change in fair value recorded in earnings
|
(391.7
|
)
|
18.5
|
|
4,420.0
|
|
|||||
|
Ending liability balance
|
$
|
—
|
|
$
|
101.2
|
|
$
|
7,550.0
|
|
||
|
Year Ended
|
|||||||||||
|
December 31, 2011
|
|||||||||||
|
|
Embedded Derivatives
|
Warrants
|
Contingent Consideration
|
||||||||
|
Beginning liability balance
|
$
|
2,281.8
|
|
$
|
289.6
|
|
$
|
—
|
|
||
|
Change in fair value recorded in earnings
|
(1,890.1
|
)
|
(206.9
|
)
|
3,130.0
|
|
|||||
|
Ending liability balance
|
$
|
391.7
|
|
$
|
82.7
|
|
$
|
3,130.0
|
|
||
|
December 31, 2012
|
December 31, 2011
|
||||||||||||||||||||||||
|
Useful Life
|
Gross
|
Accumulated Amortization
|
Net
|
Gross
|
Accumulated Amortization
|
Net
|
|||||||||||||||||||
|
Customer list
|
10 years
|
$
|
1,000.0
|
|
$
|
(195.1
|
)
|
$
|
804.9
|
|
$
|
1,000.0
|
|
$
|
(95.1
|
)
|
$
|
904.9
|
|
||||||
|
Manufacturing technology
|
10 years
|
3,900.0
|
|
(760.9
|
)
|
3,139.1
|
|
3,900.0
|
|
(370.9
|
)
|
3,529.1
|
|
||||||||||||
|
Tradename
|
10 years
|
800.0
|
|
(156.1
|
)
|
643.9
|
|
800.0
|
|
(76.2
|
)
|
723.8
|
|
||||||||||||
|
In process R&D
|
Indefinite
|
9,400.0
|
|
—
|
|
9,400.0
|
|
9,400.0
|
|
—
|
|
9,400.0
|
|
||||||||||||
|
VSEL patent rights
|
19 years
|
669.0
|
|
(176.1
|
)
|
492.9
|
|
669.0
|
|
(140.8
|
)
|
528.2
|
|
||||||||||||
|
Total Intangible Assets
|
$
|
15,769.0
|
|
$
|
(1,288.2
|
)
|
$
|
14,480.8
|
|
$
|
15,769.0
|
|
$
|
(683.0
|
)
|
$
|
15,086.0
|
|
|||||||
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Cost of revenue
|
$
|
390.0
|
|
$
|
370.9
|
|
|
|
Research and development
|
35.2
|
|
35.2
|
|
|||
|
Selling, general and administrative
|
180.0
|
|
171.2
|
|
|||
|
Total
|
$
|
605.2
|
|
$
|
577.3
|
|
|
|
2013
|
$
|
605.2
|
|
|
2014
|
605.2
|
|
|
|
2015
|
605.2
|
|
|
|
2016
|
605.2
|
|
|
|
2017
|
605.2
|
|
|
|
Thereafter
|
11,454.8
|
|
|
|
$
|
14,480.8
|
|
|
|
December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
Salaries, employee benefits and related taxes
|
$
|
1,597.2
|
|
$
|
365.7
|
|
|
|
Professional fees
|
606.6
|
|
240.0
|
|
|||
|
Other
|
81.0
|
|
484.5
|
|
|||
|
$
|
2,284.8
|
|
$
|
1,090.2
|
|
||
|
2003 Equity Plan
|
2009 Equity Plan
|
|||||
|
Shares Authorized for Issuance
|
2,500,000
|
|
33,950,000
|
|
||
|
Outstanding Stock Options
|
(1,390,300
|
)
|
(20,296,380
|
)
|
||
|
Exercised Stock Options
|
(92,500
|
)
|
(5,000
|
)
|
||
|
Restricted stock or equity grants issued under Equity Plans
|
(889,939
|
)
|
(4,606,482
|
)
|
||
|
Total common shares remaining to be issued under the Equity Plans
|
127,261
|
|
9,042,138
|
|
||
|
Stock Options
|
Warrants
|
||||||||
|
Shares
|
Weighted Average Exercise Price
|
Shares
|
Weighted Average Exercise Price
|
||||||
|
Outstanding at December 31, 2010
|
13,032,214
|
|
$1.66
|
21,843,507
|
|
$2.62
|
|||
|
Changes during the Year:
|
|||||||||
|
Granted
|
8,697,600
|
|
$1.50
|
15,993,947
|
|
$2.09
|
|||
|
Exercised
|
(5,000
|
)
|
$1.42
|
—
|
|
—
|
|||
|
Forfeited
|
(1,217,189
|
)
|
$1.96
|
(447,629
|
)
|
$6.18
|
|||
|
Expired
|
(3,364,120
|
)
|
$1.82
|
—
|
|
—
|
|||
|
Outstanding at December 31, 2011
|
17,143,505
|
|
$1.71
|
37,389,825
|
|
$2.35
|
|||
|
Changes during the Year:
|
|||||||||
|
Granted
|
7,787,529
|
|
$0.50
|
31,082,615
|
|
$0.57
|
|||
|
Exercised
|
—
|
|
—
|
(11,076,182
|
)
|
$0.60
|
|||
|
Forfeited
|
(3,020,209
|
)
|
$1.66
|
(790,003
|
)
|
$2.17
|
|||
|
Expired
|
(224,145
|
)
|
$1.88
|
(1,318,644
|
)
|
$4.60
|
|||
|
Outstanding at December 31, 2012
|
21,686,680
|
|
$1.29
|
55,287,611
|
|
$1.57
|
|||
|
Year Ended December 31,
|
||||||||
|
|
2012
|
2011
|
||||||
|
Number of Common Stock Purchase Warrants Issued
|
419,690
|
|
670,000
|
|
||||
|
Value of Common Stock Purchase Warrants Issued
|
$
|
172.2
|
|
$
|
495.1
|
|
||
|
Year Ended December 31,
|
||||||||
|
|
2012
|
2011
|
||||||
|
Number of Restricted Stock Issued
|
2,295,533
|
|
3,467,451
|
|
||||
|
Value of Restricted Stock Issued
|
$
|
1,325.1
|
|
$
|
3,580.6
|
|
||
|
Year Ended December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
Cost of goods sold
|
$
|
195.0
|
|
$
|
96.9
|
|
|
|
Research and development
|
432.9
|
|
867.8
|
|
|||
|
Selling, general and administrative
|
6,084.6
|
|
9,301.3
|
|
|||
|
Total share-based compensation expense
|
$
|
6,712.5
|
|
$
|
10,266.0
|
|
|
|
Stock Options
|
Warrants
|
Restricted Stock
|
|||||||||
|
Unrecognized compensation cost
|
$
|
1,620.1
|
|
$
|
20.5
|
|
$
|
143.0
|
|
||
|
Expected weighted-average period in years of compensation cost to be recognized
|
1.68
|
|
0.44
|
|
0.19
|
|
|||||
|
Stock Options
|
Warrants
|
|||||||||||||||
|
Year Ended December 31,
|
Year Ended December 31,
|
|||||||||||||||
|
2012
|
2011
|
2012
|
2011
|
|||||||||||||
|
Total fair value of shares vested
|
$
|
5,408.0
|
|
$
|
6,194.1
|
|
$
|
171.6
|
|
$
|
269.1
|
|
||||
|
Weighted average estimated fair value of shares granted
|
0.36
|
|
1.06
|
|
0.41
|
|
0.74
|
|
||||||||
|
Stock Options
|
Warrants
|
|||||||
|
Year Ended December 31,
|
Year Ended December 31,
|
|||||||
|
2012
|
2011
|
2012
|
2011
|
|||||
|
Expected term - minimum (in years)
|
2
|
3
|
2
|
3
|
||||
|
Expected term - maximum (in years)
|
10
|
10
|
5
|
5
|
||||
|
Expected volatility - minimum
|
73%
|
79%
|
76%
|
80%
|
||||
|
Expected volatility - maximum
|
84%
|
85%
|
83%
|
86%
|
||||
|
Expected dividend yield
|
—
|
—
|
—
|
—
|
||||
|
Risk-free interest rate - minimum
|
0.28%
|
0.40%
|
0.27%
|
0.78%
|
||||
|
Risk-free interest rate - maximum
|
1.99%
|
3.45%
|
0.88%
|
2.19%
|
||||
|
Years Ended December 31,
|
||||||||
|
|
2012
|
2011
|
||||||
|
U.S. Federal benefit at statutory rate
|
$
|
(12,334.1
|
)
|
$
|
(12,005.5
|
)
|
||
|
State and local benefit net of U.S. federal tax
|
(2,154.1
|
)
|
(2,177.0
|
)
|
||||
|
Permanent non deductible expenses for U.S. taxes
|
(2,781.4
|
)
|
5,907.3
|
|
||||
|
Reduction in deferred tax assets primarily related to deductibility of certain share-based compensation
|
—
|
|
(72.4
|
)
|
||||
|
True-up of prior year net operating loss
|
321.6
|
|
1,367.3
|
|
||||
|
Return to actual
|
(384.8
|
)
|
—
|
|
||||
|
Foreign earnings not permanently reinvested
|
(1,810.3
|
)
|
1,810.3
|
|
||||
|
Effect of change in deferred tax rate
|
525.7
|
|
2,852.1
|
|
||||
|
Valuation allowance for deferred tax assets
|
18,441.9
|
|
2,317.9
|
|
||||
|
Tax provision
|
$
|
(175.5
|
)
|
$
|
—
|
|
||
|
December 31,
|
||||||||
|
|
2012
|
2011
|
||||||
|
Deferred Tax Assets:
|
|
|
|
|
||||
|
Accumulated net operating losses (tax effected)
|
$
|
25,727.7
|
|
$
|
17,816.7
|
|
||
|
Deferred revenue
|
23.1
|
|
212.9
|
|
||||
|
Contingent accounts payable
|
15.2
|
|
13.8
|
|
||||
|
Share-based compensation
|
5,466.7
|
|
3,917.4
|
|
||||
|
Accumulated depreciation
|
348.7
|
|
—
|
|
||||
|
Charitable contributions
|
391.8
|
|
408.2
|
|
||||
|
Bad debt provision
|
239.7
|
|
107.3
|
|
||||
|
Capital loss carryforward
|
6,644.5
|
|
—
|
|
||||
|
Other
|
—
|
|
48.5
|
|
||||
|
Deferred tax assets prior to tax credit carryovers
|
38,857.4
|
|
22,524.8
|
|
||||
|
Deferred Tax Liabilities:
|
||||||||
|
Accumulated depreciation
|
—
|
|
(155.1
|
)
|
||||
|
Intangible and indefinite lived assets
|
(3,311.8
|
)
|
(3,303.0
|
)
|
||||
|
Foreign earnings not permanently reinvested
|
—
|
|
(2,138.5
|
)
|
||||
|
Deferred tax liabilities
|
(3,311.8
|
)
|
(5,596.6
|
)
|
||||
|
|
35,545.6
|
|
16,928.2
|
|
||||
|
Valuation reserve
|
(39,144.7
|
)
|
(20,702.9
|
)
|
||||
|
Net deferred tax liability
|
$
|
(3,599.1
|
)
|
$
|
(3,774.7
|
)
|
||
|
Cash
|
$
|
195.1
|
|
|
Prepaid expenses and other current assets
|
14.9
|
|
|
|
Property, plant and equipment, net
|
1,023.7
|
|
|
|
Other Assets
|
330.5
|
|
|
|
Accounts payable
|
(177.1
|
)
|
|
|
Accrued liabilities
|
(79.2
|
)
|
|
|
Accumulated comprehensive income
|
(169.9
|
)
|
|
|
Loss on exit of segment
|
$
|
1,138.0
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2012
|
2011
|
|||||
|
Revenue
|
$
|
52.3
|
|
$
|
274.3
|
|
|
|
Cost of revenues
|
(30.6
|
)
|
(140.6
|
)
|
|||
|
Research and development
|
(103.3
|
)
|
(378.3
|
)
|
|||
|
Selling, general, and administrative
|
(497.3
|
)
|
(3,089.8
|
)
|
|||
|
Other income (expense)
|
(6.8
|
)
|
(9.7
|
)
|
|||
|
Loss on exit of segment
|
(1,138.0
|
)
|
—
|
|
|||
|
Loss from discontinued operations
|
$
|
(1,723.7
|
)
|
$
|
(3,344.1
|
)
|
|
|
|
December 31, 2011
|
||
|
Assets:
|
|
|
|
|
Cash and cash equivalents
|
$
|
103.3
|
|
|
Prepaid expenses and other current assets
|
284.4
|
|
|
|
Property, plant and equipment, net
|
1,256.8
|
|
|
|
Other Assets
|
149.0
|
|
|
|
|
$
|
1,793.5
|
|
|
Liabilities:
|
|||
|
Accounts payable
|
$
|
177.8
|
|
|
Accrued liabilities
|
31.0
|
|
|
|
|
$
|
208.8
|
|
|
Fair value of consideration received
|
$
|
13,397.9
|
|
|
Carrying value of segment non-controlling interest
|
6,015.0
|
|
|
|
Carrying value of segment accumulated comprehensive income
|
4,387.4
|
|
|
|
$
|
23,800.3
|
|
|
|
Less carrying amount of assets and liabilities sold:
|
|||
|
Cash
|
$
|
8,457.5
|
|
|
Restricted Cash
|
2,918.1
|
|
|
|
Accounts Receivable
|
6,130.2
|
|
|
|
Inventories
|
15,077.7
|
|
|
|
Prepaid expenses and other current assets
|
957.8
|
|
|
|
Property, plant and equipment, net
|
38,102.0
|
|
|
|
Other assets
|
5,946.3
|
|
|
|
Accounts payable
|
(9,604.8
|
)
|
|
|
Accrued liabilities
|
(2,008.8
|
)
|
|
|
Bank loans
|
(15,133.5
|
)
|
|
|
Notes payable
|
(6,599.3
|
)
|
|
|
Other liabilities
|
(9,166.8
|
)
|
|
|
Amount due related party
|
(7,859.7
|
)
|
|
|
$
|
27,216.7
|
|
|
|
Loss on exit of segment
|
$
|
(3,416.4
|
)
|
|
Year Ended December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
Revenue
|
$
|
61,703.1
|
|
$
|
63,393.6
|
|
|
|
Cost of revenues
|
(40,245.2
|
)
|
(47,186.8
|
)
|
|||
|
Research and development
|
(1,836.4
|
)
|
(2,904.1
|
)
|
|||
|
Selling, general, and administrative
|
(10,740.0
|
)
|
(11,068.2
|
)
|
|||
|
Other expense
|
(1,045.2
|
)
|
(1,081.4
|
)
|
|||
|
Provision for income taxes
|
(1,794.1
|
)
|
(392.8
|
)
|
|||
|
Asset impairments
|
(31,170.1
|
)
|
(19,432.7
|
)
|
|||
|
Loss on sale of segment
|
(3,416.4
|
)
|
—
|
|
|||
|
Loss from discontinued operations
|
$
|
(28,544.3
|
)
|
$
|
(18,672.4
|
)
|
|
|
December 31, 2011
|
|||
|
Cash and cash equivalents
|
$
|
8,707.0
|
|
|
Accounts receivable, net
|
5,525.7
|
|
|
|
Inventory
|
16,505.7
|
|
|
|
Deferred income taxes
|
463.7
|
|
|
|
Prepaid expenses and other current assets
|
777.5
|
|
|
|
Property, plant and equipment, net
|
36,490.4
|
|
|
|
Land use rights, net
|
4,872.4
|
|
|
|
Goodwill
|
8,495.7
|
|
|
|
Intangible assets, net
|
21,846.4
|
|
|
|
Other assets
|
2,459.9
|
|
|
|
Total assets
|
$
|
106,144.4
|
|
|
Accounts payable
|
$
|
7,950.3
|
|
|
Accrued liabilities
|
1,705.8
|
|
|
|
Bank loans
|
15,712.0
|
|
|
|
Income tax payable
|
621.6
|
|
|
|
Deferred income taxes
|
6,177.4
|
|
|
|
Unearned revenue
|
1,315.4
|
|
|
|
Amount due related parties
|
20,862.7
|
|
|
|
Total liabilities
|
$
|
54,345.2
|
|
|
Years ended
|
Operating Leases
|
|||
|
2013
|
$
|
1,143.3
|
|
|
|
2014
|
878.8
|
|
||
|
2015
|
713.7
|
|
||
|
2016
|
563.9
|
|
||
|
2017
|
293.2
|
|
||
|
Total minimum lease payments
|
$
|
3,592.9
|
|
|
|
Name
|
Age
|
Director Since
|
|
Robin L. Smith, M.D.(1)
|
48
|
2006
|
|
Richard Berman
|
70
|
2006
|
|
Steven S. Myers
|
66
|
2006
|
|
Eric H.C. Wei
|
56
|
2009
|
|
Drew Bernstein
|
56
|
2009
|
|
Andrew Pecora, M.D., FACP(2)
|
55
|
2011
|
|
Martyn D. Greenacre
|
71
|
2011
|
|
Stephen W. Potter
|
56
|
2013
|
|
Name
|
Age
|
Position
|
|
Robin L. Smith, M.D.(1)
|
48
|
Chief Executive Officer and Chairman of the Board
|
|
Andrew L. Pecora, M.D., F.A.C.P.(1)
|
55
|
Chief Medical Officer of NeoStem, Chief Medical Officer of PCT and Chief Scientific Officer of Amorcyte
|
|
Robert A. Preti, PhD. (1)
|
56
|
President and Chief Scientific Officer of PCT
|
|
Larry A. May (1)
|
63
|
Vice President and Chief Financial Officer
|
|
Catherine M. Vaczy (1)
|
51
|
Vice President and General Counsel
|
|
Joseph Talamo (1)
|
43
|
Vice President, Corporate Controller and Chief Accounting Officer
|
|
Jonathan Sackner-Bernstein, M.D.
|
52
|
Vice President, Clinical Development and Regulatory Affairs
|
|
Martin E. Schmieg
|
51
|
Vice President, Corporate Development
|
|
Jeff Liter
|
56
|
Chief Operating Officer of PCT
|
|
Timothy Fong, PhD.
|
55
|
Vice President, Technology & Product Development of PCT
|
|
•
|
The Board’s review and approval of our business plans and budget (prepared and presented to the Board by the Chief Executive Officer and other management), including the projected opportunities and challenges facing our business;
|
|
•
|
At least quarterly review of our business developments, business plan implementation and financial results;
|
|
•
|
Our Audit Committee’s oversight of our internal controls over financial reporting and its discussions with management and the independent accountants regarding the quality and adequacy of our internal controls and financial reporting; and
|
|
•
|
Our Compensation Committee’s review and recommendations to the Board regarding our executive officer compensation and its relationship to our business plans.
|
|
•
|
serving as an independent and objective party to monitor our financial reporting process, internal control system and disclosure control system;
|
|
•
|
reviewing and appraising the audit efforts of our independent accountants;
|
|
•
|
assuming direct responsibility for the appointment, compensation, retention and oversight of the work of the outside auditors and for the resolution of disputes between the outside auditors and our management regarding financial reporting issues;
|
|
•
|
providing an open avenue of communication among the independent accountants, financial and senior management and the Board; and
|
|
•
|
reviewing and approving all related party transactions.
|
|
•
|
evaluate the performance of the Chief Executive Officer in light of our goals and objectives and determine the Chief Executive Officer's compensation based on this evaluation and such other factors as the Committee shall deem appropriate;
|
|
•
|
approve all salary, bonus, and long-term incentive awards for executive officers;
|
|
•
|
approve the aggregate amounts and methodology for determination of all salary, bonus, and long-term incentive awards for all employees other than executive officers;
|
|
•
|
review and recommend equity-based compensation plans to the full Board of Directors and approve all grants and awards thereunder;
|
|
•
|
review and approve changes to our equity-based compensation plans other than those changes that require stockholder approval under the plans, the requirements of the NYSE MKT or any exchange on which our securities may be listed and/or any applicable law;
|
|
•
|
review and recommend to the full Board changes to our equity-based compensation plans that require stockholder approval under the plans, the requirements of the NYSE MKT or any exchange on which our securities may be listed and/or any applicable law;
|
|
•
|
review and approve changes in our retirement, health, welfare and other benefit programs that result in a material change in costs or the benefit levels provided;
|
|
•
|
administer our equity-based compensation plans; and
|
|
•
|
approve, as required by applicable law, the annual Committee report on executive compensation (if required) for inclusion in our proxy statement.
|
|
•
|
should possess the highest personal and professional standards of integrity and ethical values;
|
|
•
|
must be committed to promoting and enhancing the long term value of our Company for our stockholders;
|
|
•
|
should not have any interests that would materially impair his or her ability to (i) exercise independent judgment or (ii) otherwise discharge the fiduciary duties owed as a director to our Company and our stockholders;
|
|
•
|
must have demonstrated achievement in one of more fields of business, professional, governmental, community, scientific or educational endeavor, and possess mature and objective business judgment and expertise;
|
|
•
|
must have a general appreciation regarding major issues facing public companies of a size and operational scope similar to ours;
|
|
•
|
must have adequate time to devote to the Board of Directors and its committees; and
|
|
•
|
is expected to have sound judgment, derived from management or policy-making experience that demonstrates an ability to function effectively in an oversight role.
|
|
Name and
|
|
|
|
|
Stock
|
|
Option
|
|
All Other
|
|
Total
|
|||||||||||||||
|
Principal Function
|
|
Year
|
|
Salary
|
|
Bonus
|
|
Awards
(1)
|
|
Awards
(1)
|
|
Compensation
|
|
Compensation
|
||||||||||||
|
Robin Smith,
Chief Executive Officer
|
|
2012
|
$
|
412,694
|
|
(2)
|
$
|
363,000
|
|
(3)
|
$
|
183,840
|
|
|
$
|
638,941
|
|
$
|
44,927
|
|
(4)
|
$
|
1,643,403
|
|
||
|
|
2011
|
$
|
375,176
|
|
(5)
|
$
|
330,000
|
|
(6)
|
$
|
—
|
|
$
|
2,912,100
|
|
(7)
|
$
|
30,496
|
|
(8)
|
$
|
3,647,772
|
|
|||
|
Robert Preti,
President and Chief Scientific Officer of PCT
|
|
2012
|
$
|
309,880
|
|
(9)
|
$
|
—
|
|
$
|
—
|
|
$
|
97,578
|
|
$
|
—
|
|
$
|
407,457
|
|
|||||
|
|
2011
|
$
|
300,808
|
|
(10)
|
$
|
—
|
|
$
|
—
|
|
$
|
439,002
|
|
$
|
6,359
|
|
(11)
|
$
|
746,169
|
|
|||||
|
Catherine M. Vaczy,
Vice President and General Counsel
|
|
2012
|
$
|
255,350
|
|
(12)
|
$
|
—
|
|
$
|
6,365
|
|
$
|
123,480
|
|
$
|
17,875
|
|
(13)
|
$
|
403,070
|
|
||||
|
|
2011
|
$
|
221,286
|
|
|
$
|
60,000
|
|
(14)
|
$
|
—
|
|
$
|
293,507
|
|
$
|
16,325
|
|
(15)
|
$
|
591,118
|
|
||||
|
(1)
|
Amounts shown under “Stock Awards” and “Option Awards” represent the aggregate grant date fair value computed in accordance with FASB ASC Topic 718, in accordance with SEC rules. See Note 9 to the Notes to the Consolidated Financial Statements for a discussion of assumptions made in such valuations. All stock awards, option awards and other shares discussed in this table were issued under the Company's Amended & Restated 2009 Equity Compensation Plan (the "Plan"), with a per share price generally equal to the fair market value of a share of common stock on the date of grant.
|
|
(2)
|
Pursuant to an arrangement approved by the Compensation Committee, Dr. Smith elected to receive an aggregate of $
218,090
of her 2012 salary in shares of Common Stock and Options issued under the Plan in lieu of cash.
|
|
(3)
|
On March 6, 2013, Dr. Smith elected to receive a portion of her 2012 bonus in shares of NeoStem, Inc.'s common stock(the “Shares”), $.001 par value. Dr. Smith received 100,000 Shares based on a per share purchase price of $0.53, the fair market value at the time of election.
|
|
(4)
|
Consisted of (i) a car allowance of $
12,000
, (ii) approximately $
1,903
health insurance reimbursement, (iii) approximately $
14,942
paid by us on behalf of Dr. Smith for life and disability insurance; and (iv) $
16,083
for club membership dues.
|
|
(5)
|
Pursuant to an arrangement approved by the Compensation Committee, Dr. Smith elected to receive an aggregate of $172,761 of her 2011 salary, in shares of Common Stock of the Company issued under our 2009 Amended & Restated Equity Compensation Plan at the then-market price.
|
|
(6)
|
In 2011, Dr. Smith elected to accept her entire bonus in shares of Common Stock of the Company.
|
|
(7)
|
Includes $722,900 attributable to the incremental compensation cost recognized for the acceleration of certain of Dr. Smith's stock options on April 4, 2011 in connection with an amendment to her employment agreement.
|
|
(8)
|
Consisted of (i) a car allowance of $12,000, (ii) approximately $15,946 paid by us on behalf of Dr. Smith for life and disability insurance, and (iii) approximately $2,550 for club membership dues.
|
|
(9)
|
Pursuant to an arrangement approved by the Compensation Committee, Dr. Preti elected to receive an aggregate of $
32,761
of his 2012 salary in shares of Common Stock and Options issued under the Plan in lieu of cash.
|
|
(10)
|
As a result of the PCT Merger and Dr. Preti's employment as President of PCT effective upon the PCT Merger, Dr. Preti is considered to be an executive officer of the Company effective January 19, 2011. Salary reflected in this table is pursuant to an employment agreement effective on such date.
|
|
(11)
|
This amount consists of PCT's contribution to Dr. Preti's 401(k).
|
|
(12)
|
Pursuant to an arrangement approved by the Compensation Committee, Ms. Vaczy elected to receive an aggregate of $
37,756
of her 2012 salary in Options issued under the Plan.
|
|
(13)
|
Consisted of (i) a car allowance of $
12,000
, (ii) approximately $
875
health insurance reimbursement; and (iii) $
5,000
for club membership dues.
|
|
(14)
|
Pursuant to a letter agreement dated January 6, 2012, Ms. Vaczy agreed to accept $10,000 of her bonus in shares of common stock issued under the Plan.
|
|
(15)
|
Consisted of (i) a car allowance of $
12,000
; and (ii) $
4,325
for club membership dues.
|
|
Name
|
|
Number of
Securities
Underlying
Unexercised
Options #
Exercisable
|
|
Number of
Securities
Underlying
Unexercised
Options #
Unexercisable
|
|
Equity Incentive
Plan Awards:
Number of
Securities
Underlying
Unexercised
Unearned Options
|
|
Option
Exercise
Price***
|
|
Option
Expiration
Date
|
|||||
|
Robin L. Smith
|
|
15,000
|
|
(1)
|
—
|
|
|
—
|
|
|
$
|
1.90
|
|
|
12/4/2016
|
|
|
|
55,000
|
|
(2)
|
—
|
|
|
—
|
|
|
$
|
1.90
|
|
|
1/17/2017
|
|
|
|
250,000
|
|
(3)
|
—
|
|
|
—
|
|
|
$
|
1.90
|
|
|
9/26/2017
|
|
|
|
120,000
|
|
(4)
|
—
|
|
|
—
|
|
|
$
|
1.63
|
|
|
2/26/2018
|
|
|
|
5,000
|
|
(5)
|
—
|
|
|
—
|
|
|
$
|
1.13
|
|
|
10/30/2018
|
|
|
|
100,000
|
|
(6)
|
—
|
|
|
—
|
|
|
$
|
1.95
|
|
|
5/20/2019
|
|
|
|
500,000
|
|
(7)
|
—
|
|
|
—
|
|
|
$
|
1.71
|
|
|
7/6/2019
|
|
|
|
750,000
|
|
(8)
|
—
|
|
|
—
|
|
|
$
|
2.04
|
|
|
10/28/2019
|
|
|
|
229,678
|
|
(9)
|
—
|
|
|
—
|
|
|
$
|
1.90
|
|
|
10/29/2016
|
|
|
|
200,000
|
|
(10)
|
—
|
|
|
—
|
|
|
$
|
1.66
|
|
|
11/3/2019
|
|
|
|
1,500,000
|
|
(11)
|
—
|
|
—
|
|
|
$
|
1.74
|
|
|
4/3/2021
|
|
|
|
|
790,000
|
|
(12)
|
—
|
|
—
|
|
$
|
0.52
|
|
1/3/2022
|
|||
|
402,627
|
|
(13)
|
—
|
|
—
|
|
$
|
0.36
|
|
4/25/2022
|
|||||
|
700,000
|
|
(14)
|
—
|
|
—
|
|
$
|
0.52
|
|
7/4/2022
|
|||||
|
Catherine M. Vaczy
|
|
1,500
|
|
(15)
|
—
|
|
—
|
|
|
$
|
1.90
|
|
|
4/19/2015
|
|
|
10,000
|
|
(16)
|
—
|
|
—
|
|
$
|
1.90
|
|
6/1/2016
|
|||||
|
15,000
|
|
(17)
|
—
|
|
—
|
|
$
|
1.90
|
|
12/4/2016
|
|||||
|
35,000
|
|
(18)
|
—
|
|
—
|
|
$
|
1.90
|
|
9/26/2017
|
|||||
|
12,000
|
|
(19)
|
—
|
|
—
|
|
$
|
1.70
|
|
12/18/2017
|
|||||
|
36,000
|
|
(20)
|
—
|
|
—
|
|
$
|
1.63
|
|
2/26/2018
|
|||||
|
5,000
|
|
(21)
|
—
|
|
—
|
|
$
|
1.13
|
|
10/30/2018
|
|||||
|
75,000
|
|
(22)
|
—
|
|
—
|
|
$
|
1.95
|
|
5/20/2019
|
|||||
|
200,000
|
|
(23)
|
—
|
|
—
|
|
$
|
1.71
|
|
7/7/2019
|
|||||
|
100,000
|
|
(24)
|
—
|
|
—
|
|
$
|
2.04
|
|
10/28/2019
|
|||||
|
53,955
|
|
(25)
|
—
|
|
—
|
|
$
|
1.90
|
|
10/29/2016
|
|||||
|
100,000
|
|
(26)
|
—
|
|
—
|
|
$
|
1.66
|
|
11/3/2019
|
|||||
|
350,000
|
|
(27)
|
—
|
|
—
|
|
$
|
1.75
|
|
7/6/2020
|
|||||
|
250,000
|
|
(28)
|
—
|
|
—
|
|
$
|
1.74
|
|
4/3/2021
|
|||||
|
66,666
|
|
(29)
|
133,334
|
|
(29)
|
—
|
|
$
|
0.52
|
|
1/3/2022
|
||||
|
|
|
150,000
|
|
(30)
|
—
|
|
—
|
|
|
$
|
0.52
|
|
|
1/5/2022
|
|
|
58,025
|
|
(31)
|
—
|
|
—
|
|
$
|
0.36
|
|
4/25/2022
|
|||||
|
32,234
|
|
(32)
|
32,238
|
|
—
|
|
$
|
0.44
|
|
6/24/2022
|
|||||
|
Robert Preti
|
|
100,000
|
|
(33)
|
300,000
|
|
(33)
|
—
|
|
|
$
|
1.50
|
|
|
1/18/2021
|
|
92,133
|
|
(34)
|
184,267
|
|
(34)
|
—
|
|
$
|
0.52
|
|
1/3/2022
|
||||
|
55,000
|
|
(35)
|
—
|
|
(35)
|
—
|
|
$
|
0.36
|
|
4/25/2022
|
||||
|
**
|
All option awards were made under and are governed by the terms of NeoStem's 2009 Amended & Restated Equity Compensation Plan which was approved by our stockholders at our 2012 annual stockholder meeting on October 5, 2012 . the 2009 Amended & Restated Plan (i) merged the 5,700,000 shares reserved for issuance under the Company's 2009 Non-U.S. Based Equity Compensation Plan (the "Non-U.S. Plan") with and into the 2009 Equity Plan, and (ii) increased by 4,500,000 the aggregate number of shares authorized for issuance under the 2009 Equity Plan.
|
|
(1)
|
Consists of options granted to Dr. Smith by the Compensation Committee on December 5, 2006, which vested as to 10,000 options upon grant and as to 5,000 options on August 9, 2007 upon our Common Stock being listed for trading on the American Stock Exchange (now known as the NYSE Amex).
|
|
(2)
|
This option was granted to Dr. Smith in connection with her entering into an amendment to her employment agreement on January 26, 2007, and vested as to (i) 25,000 options upon the first closings in NeoStem's January 2007 private placement, (ii) 15,000 options on June 30, 2007 and (iii) 15,000 options on December 31, 2007.
|
|
(3)
|
Consists of options granted to Dr. Smith by the Compensation Committee September 27, 2007, which vested as to 150,000 options on the date of grant and as to 100,000 options upon consummation of the Erye Merger on October 30, 2009.
|
|
(4)
|
Consists of options granted to Dr. Smith by the Compensation Committee on February 27, 2008, which vested (i) as to 40,000 options on the date of grant, (ii) as to 30,000 options upon consummation of the Erye Merger on October 30, 2009, (iii) as to 30,000 options on September 2, 2008 upon the achievement of a business milestone, and (iv) as to 20,000 options on October 31, 2008 upon the achievement of a business milestone.
|
|
(5)
|
This option was granted to Dr. Smith by the Compensation Committee on October 31, 2008 and vested on November 2, 2008 upon the achievement of a business milestone.
|
|
(6)
|
This option was granted to Dr. Smith by the Compensation Committee on May 8, 2009 and was vested in its entirety on the date of grant.
|
|
(7)
|
This option was granted to Dr. Smith by the Compensation Committee on July 8, 2009 and vested as to 250,000 options on the date of grant and as to an additional 250,000 options upon consummation of the Erye Merger on October 30, 2009.
|
|
(8)
|
An option was granted to Dr. Smith by the Compensation Committee effective October 29, 2009 upon approval of the Erye Merger and the increase in shares under the 2009 Equity Compensation Plan consisting of an aggregate of 750,000 option shares, and was scheduled to vest as to 250,000 upon the achievement of a specific business milestone, 250,000 on July 8, 2010 and 250,000 on July 8, 2011. On July 7, 2010, the Compensation Committee accelerated the vesting of the 250,000 options originally scheduled to vest upon achievement of a business milestone and the 250,000 options originally scheduled to vest on July 8, 2011. As a result, as of July 8, 2010, this option was fully vested.
|
|
(9)
|
This option was granted to Dr. Smith by the Compensation Committee on October 30, 2009 and was vested in its entirety on the date of grant.
|
|
(10)
|
This option was granted to Dr. Smith by the Compensation Committee on November 4, 2009 and originally scheduled to vest as to one-third of option shares on each one year anniversary of the date of grant. Pursuant to Dr. Smith's April 4, 2011 Employment Agreement amendment, the vesting of this option was accelerated and as of that date the option was fully vested.
|
|
(11)
|
Consists of options granted to Dr. Smith pursuant to the terms of her April 4, 2011 Employment Agreement Amendment which vested as to 500,000 shares on each of the date of grant and December 31, 2011 and and was scheduled to vest as to 500,000 shares on December 31, 2012. The vesting of this option was accelerated pursuant to Dr. Smith's November 13, 2012 Employment Agreement Amendment.
|
|
(12)
|
Consists of options granted to Dr. Smith by the Compensation Committee on January 4, 2012 which vested as to 263,333 options on the date of grant, and was scheduled to vest as to (i) 263,333 options on January 4, 2013, and (ii) 263,334 options on January 4, 2014. The vesting of this option was accelerated pursuant to Dr. Smith's November 13, 2012 Employment Agreement Amendment.
|
|
(13)
|
On April 26, 2012, the Compensation Committee adopted a program (the "2012 Option Program") whereby each participating officer was issued on April 26, 2012 an option (the "Option") to purchase that number of shares of Common Stock equal to that portion of each Participating Officer's gross salary (the "Participating Salary") for the period May 1, 2012 - July 31, 2012 (the "Election Period"). The Option, the issuance of which is in lieu of payment of the Participating Salary vests at the end of the month in which the Participating Salary to which it relates would have been paid and has a term of ten years despite any termination of employment of the Participating Officer. Dr. Smith's Participating Salary for the Election Period was her full salary. Accordingly the options vested as to 134,209 on May 31, 2012, 134,209 on June 30, 2012 and 134,209 on July 31, 2012.
|
|
(14)
|
This option was granted to Dr. Smith by the Compensation Committee on July 5, 2012 and was vested in its entirety on the date of grant.
|
|
(15)
|
This option was granted to Ms. Vaczy pursuant to the terms of her employment agreement dated April 20, 2005 and was originally scheduled to vest as to 500 shares on April 20, 2006; as to an additional 500 shares on April 20, 2007 and as to the remaining 500 shares on April 20, 2008. As a condition of the closing of the June 2006 private placement, Ms. Vaczy entered into a letter agreement with NeoStem pursuant to which she agreed to convert $44,711 in accrued salary into shares of Common Stock at a per share price equal to $4.40 (the price of the shares being sold in the June 2006 private placement) and further agreed to a reduction in her base salary by 25% until the achievement by NeoStem of certain milestones, in partial consideration for which the vesting of this option was accelerated such that it became fully vested as of June 2, 2006, the date of the closing of the June 2006 private placement. This was not considered a material change in the terms of such option and accordingly the fair value was not adjusted.
|
|
(16)
|
This option was granted to Ms. Vaczy pursuant to the letter agreement described in footnote 15, above, and was scheduled to vest as to 33% of the shares upon NeoStem reaching $1,000,000 in cumulative revenues; as to an additional 33% of the shares upon NeoStem reaching $2,000,000 in cumulative revenues; and as to the remaining 34% upon NeoStem reaching $3,000,000 in cumulative revenues. On October 31, 2008, this business milestone was modified pursuant to an action of the Compensation Committee of the Board of Directors and the option vested immediately. This was not considered a material change in the terms of such option and accordingly the fair value was not adjusted.
|
|
(17)
|
This option was granted to Ms. Vaczy by the Compensation Committee on December 5, 2006 and was vested in its entirety in 2007.
|
|
(18)
|
Consists of options granted to Ms. Vaczy by the Compensation Committee on September 27, 2009, which vested (i) as to 15,000 options on the date of grant, (ii) as to 10,000 options on November 13, 2007 upon the achievement of a specific business milestone, and (iii) as to 10,000 options upon consummation of the CBH Merger on October 30, 2009.
|
|
(19)
|
This option was granted to Ms. Vaczy by the Compensation Committee on December 19, 2007 and vested in its entirety on January 1, 2008.
|
|
(20)
|
Consists of options granted to Ms. Vaczy by the Compensation Committee on February 27, 2008, which vested (i) as to 10,000 options on the date of grant, (ii) as to 10,000 options upon consummation of the CBH Merger on October 30, 2009, and (iii) as to 16,000 options on September 2, 2008 upon the achievement of a business milestone.
|
|
(21)
|
This option was granted to Ms. Vaczy by the Compensation Committee on October 31, 2008 and vested on November 2, 2008 upon the achievement of a business milestone.
|
|
(22)
|
This option was granted to Ms. Vaczy by the Compensation Committee on May 21, 2009 and was fully vested on the date of grant.
|
|
(23)
|
This option was granted to Ms. Vaczy by the Compensation Committee on July 8, 2009 and vested as to 100,000 options on July 8, 2009 and 100,000 options vested on October 29, 2009.
|
|
(24)
|
This option was granted to Ms. Vaczy by the Compensation Committee on October 29, 2009 and was fully vested on July 8, 2010.
|
|
(25)
|
This option was granted to Ms. Vaczy by the Compensation Committee on October 30, 2009 and was fully vested on October 30, 2009.
|
|
(26)
|
This option was granted to Ms. Vaczy by the Compensation Committee on November 4, 2009 and vested as to: (i) 33,333 options on November 4, 2010; (ii) 33,333 on November 4, 2011; and (iii) 33,334 on November 4, 2012.
|
|
(27)
|
This option was granted to Ms. Vaczy by the Compensation Committee on July 7, 2010 and vested as to 100,000 options on July 7, 2011, 50,000 options on December 31, 2011 and 200,000 shall vest upon achievement of a certain milestone.
|
|
(28)
|
This option was granted to Ms. Vaczy by the Compensation Committee on April 4, 2011 and vested as to 125,000 options on the date of grant and 125,000 options on April 4, 2012.
|
|
(29)
|
Consists of options granted to Ms. Vaczy by the Compensation Committee on January 4, 2012 which vested as to (i) 66,666 options on the date of grant, (ii) 66,667 options on January 4, 2013, and (iii) 66,667 options are scheduled to vest on January 4, 2014.
|
|
(30)
|
This option was granted to Ms. Vaczy pursuant to a letter agreement dated January 6, 2012 and was fully vested on December 31, 2012.
|
|
(31)
|
On April 26, 2012, the Compensation Committee adopted a program (the "2012 Option Program") whereby each participating officer was issued on April 26, 2012 an option (the "Option") to purchase that number of shares of Common Stock equal to that portion of each Participating Officer's gross salary (the "Participating Salary") for the period May 1, 2012 - July 31, 2012 (the "Election Period"). The Option, the issuance of which is in lieu of payment of the Participating Salary vests at the end of the month in which the Participating Salary to which it relates would have been paid and has a term of ten years despite any termination of employment of the Participating Officer. Ms. Vaczy's Participating Salary for the Election Period was her full salary. Accordingly the options vested as to 19,342 on May 31, 2012, 19,342 on June 30, 2012 and 19,341 on July 31, 2012.
|
|
(32)
|
On July 25, 2012 pursuant to Compensation Committee consent, Ms. Vaczy agreed to accept this option in lieu of her previously agreed upon cash raise which vests as to one twelvth beginning on July 31, 2012 through July 31, 2013.
|
|
(33)
|
Consists of options granted to Dr. Preti pursuant to the terms of his employment agreement dated as of September 23, 2010 and effective on January 19, 2011 upon the closing of the PCT Merger, which are scheduled to vest as to 100,000 shares on each of the first, second, third and fourth one year anniversaries of the effective date of his employment agreement.
|
|
(34)
|
Consists of options granted to Dr. Preti by the Compensation Committee on January 4, 2012, which vested as to: (i) 92,133 on January 4, 2012, (ii) 92,133 on January 4, 2013 and, (iii) 92,334 on January 4, 2014.
|
|
(35)
|
Consists of options granted to Dr. Preti pursuant to the 2012 Option Program which vested as to 27,500 on May 31, 2012 and 27,500 on June 30, 2012.
|
|
|
|
|
Fees Earned
|
|
|
|
|
|||||||||
|
|
|
|
or
|
|
Stock
|
Option
|
|
Total
|
||||||||
|
Name
|
|
Year
|
|
Paid in Cash
|
|
Awards
(1)
|
Awards
(1)
|
|
Compensation
|
|||||||
|
Richard Berman
(2)
|
|
2012
|
$
|
22,500
|
|
$
|
95,900
|
|
$
|
—
|
|
118,400
|
||||
|
Steven S. Myers
(3)
|
|
2012
|
$
|
22,500
|
|
$
|
95,900
|
|
$
|
—
|
|
118,400
|
||||
|
Drew Bernstein
(4)
|
|
2012
|
$
|
22,500
|
|
$
|
—
|
|
$
|
65,361
|
|
87,861
|
||||
|
Edward C. Geehr, M.D.
(5)
|
|
2012
|
$
|
15,000
|
|
$
|
—
|
|
$
|
48,344
|
|
63,344
|
||||
|
Eric C. Wei
(6)
|
|
2012
|
$
|
22,500
|
|
$
|
69,900
|
|
$
|
—
|
|
92,400
|
||||
|
Shi Mingsheng
(7)
|
|
2012
|
$
|
15,000
|
|
$
|
—
|
|
$
|
40,840
|
|
55,840
|
||||
|
Martyn Greenacre
(8)
|
|
2012
|
$
|
22,500
|
|
$
|
69,900
|
|
$
|
—
|
|
92,400
|
||||
|
(1)
|
Amounts shown under “ Stock Awards" and "Option Awards” represent the aggregate grant date fair value computed in accordance with FASB ASC Topic 718, in accordance with SEC rules. See Note 14 for a discussion of assumptions made in such valuations. All stock awards, option awards and other shares discussed in this table were issued under the Company's 2003 Equity Participation Plan, 2009 Equity Compensation Plan or 2009 Non-U.S. Equity Compensation Plan, with a per share price generally equal to the fair market value of a share of common stock on the date of grant.
|
|
(2)
|
At
December 31, 2012
, Mr. Berman had options to purchase
349,387
shares of NeoStem Common Stock outstanding, all of which were vested.
|
|
(3)
|
At
December 31, 2012
, Mr. Myers had options to purchase
349,387
shares of NeoStem Common Stock outstanding, all of which were vested. At
December 31, 2012
, Mr. Myers had a total of
360,306
shares in stock awards outstanding, all of which were vested.
|
|
(4)
|
At
December 31, 2012
, Mr. Bernstein had options to purchase
588,685
shares of NeoStem Common Stock outstanding, all of which were vested.
|
|
(5)
|
At
December 31, 2012
, Dr. Geehr had options to purchase
403,685
shares of NeoStem Common Stock outstanding, all of which were vested. As previously disclosed, Dr. Geehr
did not stand for re-election to our Board at the 2012 annual stockholder meeting.
|
|
(6)
|
At
December 31, 2012
, Mr. Wei had options to purchase
150,000
shares of NeoStem Common Stock outstanding,
150,000
of which were vested.
|
|
(7)
|
Mr. Shi did not participate in the equity portion of the 2009 Board of Directors Compensation Plan. At December 31, 2011, Mr. Shi had options to purchase 400,000 shares of NeoStem Common Stock, 300,000 of which were vested. An additional 100,000 options vested in January 2012 upon achievement of a specified milestone. As previously disclosed and in connection the Erye divestiture, Mr. Shi Mingsheng was not nominated for re-election to our Board of Directors at our 2012 Annual Shareholders meeting, and his options were canceled.
|
|
(8)
|
Mr. Greenacre joined the Board on December 8, 2011. Mr. Greenacre's compensation as a Board member commenced under the 2012 Board of Directors Compensation Plan.
|
|
•
|
each of NeoStem's named executive officers;
|
|
•
|
each of NeoStem's current directors;
|
|
•
|
all of NeoStem's current directors and executive officers as a group; and
|
|
•
|
each person who is known by NeoStem to beneficially own 5% or more of the NeoStem Common Stock.
|
|
Name and Address of Beneficial Holder
|
Number of
Shares
Beneficially
Owned
|
|
Percentage of
Common Stock
Beneficially
Owned
|
|
|
Robin L. Smith, M.D.
Chief Executive Officer and Chairman of the Board
|
7,505,525
|
|
(1)
|
4.3%
|
|
Robert A. Preti, Ph.D.
President and Chief Scientific Officer of PCT
|
2,670,444
|
|
(2)
|
1.6%
|
|
Catherine M. Vaczy
Vice President and General Counsel
|
2,047,528
|
|
(3)
|
1.2%
|
|
Andrew L. Pecora
Chief Medical Officer and Director of NeoStem,
Chief Medical Officer of PCT and Chief Scientific Officer of Amorcyte
|
3,167,598
|
|
(4)
|
1.9%
|
|
Richard Berman
Director
|
496,660
|
|
(5)
|
0.3%
|
|
Steven S. Myers
Director
|
1,789,306
|
|
(6)
|
1.1%
|
|
Drew Bernstein
Director
|
853,685
|
|
(7)
|
0.5%
|
|
Eric H.C. Wei
Director
|
26,785,180
|
|
(8) (9)
|
15.5%
|
|
RimAsia Capital Partners, L.P.
RimAsia Capital Partners GP, L.P.
RimAsia Capital Partners GP, Ltd.
RimAsia Capital Partners Manager, Ltd.
1807 Harbour Centre
25 Harbour Road
Wanchai Hong Kong
|
26,635,180
|
|
(9)
|
15.5%
|
|
Martyn Greenacre
Director
|
755,306
|
|
(10)
|
0.4%
|
|
Stephen Potter
Director
|
153,500
|
|
(11)
|
0.1%
|
|
All Directors and Executive Officers as a group (twelve persons)
|
47,372,996
|
|
(12) (13)
|
25.7%
|
|
(1)
|
Includes (i) options to purchase up to
5,717,305
shares of our common stock which are exercisable within 60 days of
February 20, 2013
and (ii) warrants to purchase up to
38,667
shares of our common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(2)
|
Includes (i) options to purchase up to
499,266
shares of our common stock which are exercisable within 60 days of
February 20, 2013
and (ii) warrants to purchase up to
343,032
shares of our common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(3)
|
Includes (i) options to purchase up to
1,683,166
shares of our common stock which are exercisable within 60 days of
February 20, 2013
and (ii) warrants to purchase up to
9,500
shares of our common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(4)
|
Includes (i) options to purchase up to
716,666
shares of our common stock which are exercisable within 60 days of
February 20, 2013
and (ii) warrants to purchase up to
358,595
shares of our common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(5)
|
Includes options to purchase up to
349,387
shares of our common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(6)
|
Includes options to purchase up to
349,387
shares of common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(7)
|
Includes options to purchase up to
853,685
shares of common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(8)
|
Includes options to purchase up to
150,000
shares of common stock which are exercisable within 60 days of
February 20,
|
|
(9)
|
Includes (i) 22,379,874 shares of common stock held by RimAsia Capital Partners, L.P., a Cayman islands exempted limited partnership ("RimAsia"); (ii) 255,306 shares held by RimAsia Capital Partners Manager, Ltd., a Cayman Islands exempted company ("RimAsia Manager"); and (iii) warrants to purchase up to
4,000,000
which are exercisable within 60 days of
February 20, 2013
which are held by RimAsia. RimAsia Capital Partners GP, L.P. ("RimAsia GP") is the general partner of RimAsia. RimAsia Capital Partners GP, Ltd. ("RimAsia Ltd.") is the general partner of RimAsia GP. RimAsia Manager is the fund manager of RimAsia GP and the manager of RimAsia. Mr. Wei is the managing partner of RimAsia, and indirect partner of RimAsia GP, a director of RimAsia Ltd. and a director of RimAsia Manager.
|
|
(10)
|
Includes warrants to purchase up to
250,000
shares of common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(11)
|
Includes options to purchase up to
93,500
shares of common stock which are exercisable within 60 days of
February 20, 2013
.
|
|
(12)
|
See footnotes 1 - 10. Includes shares and exercisable rights owned by RimAsia Capital Partners as set forth in footnote 8.
|
|
(13)
|
Includes options to purchase up to
1,083,595
shares of common stock which are exercisable within 60 days of
February 20, 2013
held by executive officers not individually listed in this table of the Company and its subsidiaries.
|
|
Plan category
|
|
Number of securities to be issued upon exercise of outstanding options, warrants, and rights (a)
|
|
Weighted-average exercise price of outstanding options, warrants and rights (b)
|
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in columns (a)) (c)
|
||||
|
Equity compensation plans approved by security holders
|
|
21,686,680
|
|
|
$
|
1.29
|
|
|
9,169,399
|
|
|
Equity compensation plans not approved by security holders (1)
|
|
3,583,593
|
|
|
$
|
1.12
|
|
|
—
|
|
|
Total
|
|
25,270,273
|
|
|
$
|
1.26
|
|
|
9,169,399
|
|
|
(1)
|
Consists of individual grants of warrants to seventeen service providers to the Company, no one of which is individually material.
|
|
Fee Category
|
|
Fiscal 2012
Fees
|
|
Fiscal 2011
Fees
|
||||
|
Audit Fees
(1)
|
|
$
|
606,037
|
|
|
$
|
605,521
|
|
|
Audit-Related Fees
(2)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Tax Fees
(3)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
All Other Fees
(4)
|
|
$
|
—
|
|
|
$
|
1,938
|
|
|
Total Fees
|
|
$
|
606,037
|
|
|
$
|
607,459
|
|
|
(1)
|
Audit Fees consist of aggregate fees billed or expected to be billed for professional services rendered for the audit of the Company's annual consolidated financial statements included in the Company's Annual Reports on Form 10-K and review of the interim consolidated financial statements included in Quarterly Reports on Form 10-Q or services that are normally provided by the independent registered public accounting firm in connection with statutory and regulatory filings or engagements for the fiscal years ended December 31, 2012 and December 31, 2011, respectively.
|
|
(2)
|
Audit-Related Fees consist of aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the Company's consolidated financial statements and are not reported under “Audit Fees.”
|
|
(3)
|
Tax Fees consist of aggregate fees billed or expected to be billed for professional services rendered for tax compliance, tax advice and tax planning. These fees related to preparation of the Company's federal and state income tax returns and other tax compliance activities.
|
|
(4)
|
All Other Fees consist of aggregate fees billed for products and services provided by Grant Thornton (as applicable), other than those disclosed above.
|
|
Exhibit
|
Description
|
|
2.1
|
Equity Purchase Agreement, dated as of June 18, 2012, by and among NeoStem, Inc., China Biopharmaceuticals Holdings, Inc., Fullbright Finance Limited, Suzhou Erye Economy & Trading Co., Ltd., and Suzhou Erye Pharmaceutical Co., Ltd. (filed as Exhibit 2.1 to the Company's Current Report on Form 8-K dated June 18, 2012).
|
|
2.2
|
Amendment to Equity Purchase Agreement, dated as of August 14, 2012, by and among NeoStem, Inc., China Biopharmaceuticals Holdings, Inc., Highacheive Holdings Limited, Fullbright Finance Limited, Suzhou Erye Economy & Trading Co., Ltd. and Suzhou Erye Pharmaceutical Co., Ltd. (filed as Exhibit 2.1 to the Company's Current Report on Form 8-K dated August 23, 2012).
|
|
2.3
|
Agreement and Plan of Merger, dated as of July 13, 2011, by and among NeoStem, Inc., Amo Acquisition Company I, Inc., Amo Acquisition Company II, LLC and Amorcyte, Inc. (filed as Exhibit 2.1 to the Company's Current Report on Form 8-K dated July 11, 2011).
|
|
2.4
|
Agreement and Plan of Merger, dated as of September 23, 2010, by and among NeoStem, Inc., NBS Acquisition Company LLC, and Progenitor Cell Therapy, LLC (filed as Exhibit 2.1 to the Company's Current Report on Form 8-K dated September 23, 2010).
|
|
3.1
|
Amended and Restated Certificate of Incorporation, as amended (as certified March 25, 2011) (filed as Exhibit 3.1 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
3.2
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation, filed with the Secretary of State of the State of Delaware on October 14, 2011 (filed as Exhibit 3.2 to the Company's Current Report on Form 8-K dated October 14, 2011).
|
|
3.3
|
Certificate of Elimination of the Series E 7% Senior Convertible Preferred Stock of NeoStem, Inc., filed with the Secretary of State of the State of Delaware on October 25, 2012 (filed as Exhibit 3.1 to the Company's Current Report on Form 8-K dated October 25, 2012).
|
|
3.4
|
Amended and Restated By-Laws dated August 31, 2006 (filed as Exhibit 3.1 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
4.1
|
Form of Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. from January/February 2007 (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated January 26, 2007).
|
|
4.2
|
Form of Non-Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. from January/February 2007 (filed as Exhibit 10.3 to the Company's Current Report on Form 8-K dated January 26, 2007).
|
|
4.3
|
Form of Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. issued to JFS Investments, Inc. (filed as Exhibit 4.15 to the Company's Registration Statement on Form S-3, File No. 333-173853, filed with the SEC on May 2, 2011).
|
|
4.4
|
Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. issued to Solutions in Marketing, Inc. (filed as Exhibit 4.16 to the Company's Registration Statement on Form S-3, File No. 333-173853, filed with the SEC on May 2, 2011).
|
|
4.5
|
Warrant to Purchase Shares of Common Stock of NeoStem, Inc. issued to Wall Street Communications Group, Inc. (filed as Exhibit 4.17 to the Company's Registration Statement on Form S-3, File No. 333-173853, filed with the SEC on May 2, 2011).
|
|
4.6
|
Form of Redeemable Service Provider Warrant (filed as Exhibit 4.19 to the Company's Registration Statement on Form S-3/A, File No. 333.173853, filed with the SEC on September 16, 2011).
|
|
4.7
|
Form of 2011 Redeemable Service Provider Warrant (filed as Exhibit 4.20 to the Company's Registration Statement on Form S-3/A, File No. 333-173853, filed with the SEC on September 16, 2011).
|
|
4.8
|
Form of Redeemable Service Provider Warrant with cashless exercise rights (filed as Exhibit 4.21 to the Company's Registration Statement on Form S-3/A, File No. 333-173853, filed with the SEC on September 16, 2011).
|
|
4.9
|
Form of 2010/2011 Redeemable Service Provider Warrant with cashless exercise rights (filed as Exhibit 4.22 to the Company's Registration Statement on Form S-3/A, File No. 333-173853, filed with the SEC on September 16, 2011).
|
|
4.10
|
Form of Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. from May 2008 (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated May 20, 2008).
|
|
4.11
|
Form of Redeemable Finder's Warrant to Purchase Shares of Common Stock of NeoStem, Inc. from May 2008 (filed as Exhibit 4.6 to the Company's Registration Statement on Form S-3, File No. 333-173853, filed with the SEC on May 2, 2011).
|
|
4.12
|
Form of Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. issued to RimAsia Capital Partners L.P. in September 2008 (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated August 28, 2008).
|
|
4.13
|
Letter Agreement dated December 18, 2008 between NeoStem, Inc. and RimAsia Capital Partners, L.P. (filed as Exhibit 4.1 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2008 as filed with the SEC on March 31, 2009).
|
|
4.14
|
Form of Warrant to Purchase Shares of Common Stock of NeoStem, Inc. from October 2008 (filed as Exhibit 4.2 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2008 as filed with the SEC on March 31, 2009).
|
|
4.15
|
Form of Redeemable Warrant to Purchase Shares of Common Stock of NeoStem, Inc. from November 2008 (filed as Exhibit 4.3 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2008 as filed with the SEC on March 31, 2009).
|
|
4.16
|
Specimen Certificate for Common Stock (filed as Exhibit 4.1 to the Company's Registration Statement on Form S-3, File No. 333-145988, filed with the SEC on September 11, 2007).
|
|
4.17
|
Form of Warrant issued in connection with April and July 2009 private placements (filed as Exhibit 4.2 to the Company's Current Report on Form 8-K dated April 13, 2009).
|
|
4.18
|
Form of Common Stock Purchase Warrant from June 2010 (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated June 25, 2010 and filed with the SEC on June 28, 2010).
|
|
4.19
|
Form of Placement Agent Warrant from June 2010 (filed as Exhibit 4.2 to the Company's Current Report on Form 8-K dated June 25, 2010 and filed with the SEC on June 28, 2010).
|
|
4.20
|
Amended and Restated Warrant, dated March 15, 2010, issued to RimAsia Capital Partners, L.P. (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated March 15, 2010 and filed with the SEC on March 18, 2010).
|
|
4.21
|
Form of Warrant from the November 2010 Common Stock Offering (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated and filed with the SEC on November 16, 2010).
|
|
4.22
|
Form of Warrant from the November 2010 Preferred Stock Offering (filed as Exhibit 4.2 to the Company's Current Report on Form 8-K dated and filed with the SEC on November 16, 2010).
|
|
4.23
|
Warrant Agreement, dated as of January 19, 2011, between NeoStem, Inc. and Continental Stock Transfer & Trust Company, with the forms of $3.00 Warrant, $5.00 Warrant and $7.00 Warrant attached thereto (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated January 18, 2011 and filed with the SEC on January 24, 2011).
|
|
4.24
|
Warrant Agreement, dated as of July 22, 2011, between NeoStem, Inc. and Continental Stock Transfer & Trust Company, with the form of Series NA Warrant attached thereto (filed as Exhibit 4.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2011 as filed with the SEC on November 10, 2011).
|
|
4.25
|
Registration Rights Agreement, dated as of September 28, 2011, by and between NeoStem, Inc. and Aspire Capital Fund, LLC (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated September 28, 2011).
|
|
4.26
|
Warrant Agreement, dated as of October 17, 2011, between NeoStem, Inc. and Continental Stock Transfer & Trust Company, with the form of Global Series AMO Warrant attached thereto (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated October 14, 2011).
|
|
4.27
|
Form of Common Stock Purchase Warrant from the March 2012 Underwritten Offering (filed as Exhibit 4.1 to the Company's Current Report on Form 8-K dated March 29, 2012).
|
|
4.28
|
Form of Common Stock Purchase Warrant for the May-July 2012 private placement (filed as Exhibit 10.5 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 as filed with the SEC on August 14, 2012).
|
|
4.29
|
Form of New Warrant from July 2012 (filed as Exhibit 10.6 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 as filed with the SEC on August 14, 2012).
|
|
4.30
|
Form of Warrant from August 2012 private placement (filed as Exhibit 4.6 to the Company's Registration Statement on Form S-3, File No. 333-183542, filed with the SEC on August 24, 2012).
|
|
4.31
|
Form of 2011/2012 Service Provider Warrant (filed as Exhibit 4.10 to the Company's Registration Statement on Form S-3, File No. 333-183542, filed with the SEC on August 24, 2012).
|
|
4.32
|
Warrant issued to Aspire Capital Fund, LLC in August 2012 (filed as Exhibit 4.9 to the Company's Registration Statement on Form S-3, File No. 333-183542, filed with the SEC on August 24, 2012).
|
|
4.33
|
Form of Warrant for November 2012 Unit private placement (filed as Exhibit 4.4 to the Company's Registration Statement on Form S-3, File No. 333-185346, filed with the SEC on December 7, 2012).
|
|
10.1
|
License Agreement between Stem Cell Technologies, Inc. and the University of Louisville Research Foundation, Inc. (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated November 13, 2007).
(1)
|
|
10.2
|
Amendment No. 1 to Exclusive License Agreement between Stem Cell Technologies, Inc. and the University of Louisville Research Foundation, Inc. (filed as Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2009 as filed with the SEC on May 15, 2009).
|
|
10.3
|
Amendment No. 2 to Exclusive License Agreement between University of Louisville Research Foundation, Inc. and Stem Cell Technologies, Inc. (filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 as filed with the SEC on August 16, 2010).
|
|
10.4
|
Sponsored Research Agreement between NeoStem, Inc. and the University of Louisville Research Foundation, Inc. (filed as Exhibit 10.3 to the Company's Current Report on Form 8-K dated November 13, 2007).
(1)
|
|
10.5
|
Amendment No. 1 to Sponsored Research Agreement between NeoStem, Inc. and the University of Louisville Research Foundation, Inc. (filed as Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2009 as filed with the SEC on May 15, 2009).
|
|
10.6
|
October 2009 English translation of Joint Venture Contract of Suzhou Erye Pharmaceutical Co., Ltd. (filed as Exhibit 10.www to the Company's Annual Report on Form 10-K for the year ended December 31, 2009 as filed with the SEC on March 31, 2010).
|
|
10.7
|
English Translation of Amendment Agreement to Joint Venture Contract of Suzhou Erye Pharmaceutical Co., Ltd. dated May 21, 2010 approved August 16, 2010 (filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 as filed with the SEC on November 12, 2010).
|
|
10.08
|
Consulting Agreement, dated as of May 11, 2010 between NeoStem, Inc. and RimAsia Capital Partners, LP (filed as Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 as filed with the SEC on August 16, 2010).
|
|
10.09
|
Form of Subscription Agreement with respect to private placement consummated on April 5, 2011 (filed as Exhibit 4.13 to the Company's Registration Statement on Form S-3, File No. 333-173853, filed with the SEC on May 2, 2011).
|
|
10.10
|
Common Stock Purchase Agreement, dated as of September 28, 2011, by and between NeoStem, Inc. and Aspire Capital Fund, LLC (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated September 28, 2011).
|
|
10.11
|
Amendment dated as of August 23, 2012 to Common Stock Purchase Agreement dated as of September 28, 2011, by and between NeoStem, Inc. and Aspire Capital Fund, LLC (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated August 23, 2012).
|
|
10.12
|
Form of Subscription Agreement from February 2012 private placement (filed as Exhibit 10.46 to the Company's Annual Report on Form 10-K for the year ended December 31, 2011 as filed with the SEC on March 20, 2012).
|
|
10.13
|
Underwriting Agreement, dated March 29, 2012, by and among NeoStem, Inc. and the underwriters named on Schedule I thereto (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated March 29, 2012).
|
|
10.14
|
Form of Subscription Agreement for the May-July 2012 private placement (filed as Exhibit 10.7 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 as filed with the SEC on August 14, 2012).
|
|
10.15
|
Form of Subscription Agreement from the August 2012 private placement (filed as Exhibit 4.7 to the Company's Registration Statement on Form S-3, File No. 333-183542, filed with the SEC on August 24, 2012).
|
|
10.16
|
Form of Subscription Agreement from the October 2012 private placement (filed as Exhibit 10.10 to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2012 as filed with the SEC on November 13, 2012).
|
|
10.17
|
Form of Subscription Agreement for November 8, 2012 private placement (filed as Exhibit 4.2 to the Company's Registration Statement on Form S-3, File No. 333-185346, filed with the SEC on December 7, 2012).
|
|
10.18
|
Form of Subscription Agreement for November 2012 Unit private placement (filed as Exhibit 4.3 to the Company's Registration Statement on Form S-3, File No. 333-185346, filed with the SEC on December 7, 2012).
|
|
10.19
|
Escrow Agreement, dated as of October 17, 2011, among NeoStem, Inc., Amorcyte, Inc., Paul J. Schmitt, as Amorcyte Representative, and Continental Stock Transfer & Trust Company, as Escrow Agent (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated October 14, 2011).
|
|
10.20
|
Lease dated September 1, 2005 between Vanni Business Park, LLC and Progenitor Cell Therapy, LLC, as amended by First Amendment of Lease effective as of July 1, 2006 (filed as Exhibit 10.48 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
10.21
|
Second Amendment of Lease, executed July 11, 2011 and effective July 1, 2011, by and between Vanni Business Park, LLC and Progenitor Cell Therapy, LLC (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated July 11, 2011).
|
|
10.22
|
Guaranty of Lease, executed July 11, 20911 and effective as of July 1, 2011, by NeoStem, Inc. for the benefit of Vanni Business Park, LLC (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated July 11, 2011).
|
|
10.23
|
Bond Agreement dated as of October 1, 2007 by and among the New Jersey Economic Development Authority, PCT Allendale, LLC and Commerce
Bank/North (filed as Exhibit 10.49 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
10.24
|
Note dated October 31, 2007, made by PCT Allendale, LLC in favor of the New Jersey Economic Development Authority (filed as Exhibit 10.50 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
10.25
|
Mortgage and Security Agreement from PCT Allendale, LLC to New Jersey Economic Development Authority and Commerce Bank/North, dated October 31, 2007 (filed as Exhibit 10.51 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
10.26
|
Mortgage Loan Note dated November 30, 2010, made by PCT Allendale, LLC in favor of TD Bank, N.A. (filed as Exhibit 10.52 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
10.27
|
Mortgage, Security Agreement and Fixture Filing made as of the 30th day of November 2010, between PCT Allendale, LLC and TD Bank, N.A. (filed as Exhibit 10.53 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
10.28
|
Stock Purchase and Assignment Agreement dated March 28, 2011, by and among Progenitor Cell Therapy, LLC, Athelos Corporation and Becton Dickinson and Company (filed as Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2011 as filed with the SEC on May 17, 2011).
|
|
10.29
|
Stockholders' Agreement dated March 28, 2011, by and among Progenitor Cell Therapy, LLC, Athelos Corporation and Becton Dickinson and Company (filed as Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q for the quarter ended March 31, 2011 as filed with the SEC on May 17, 2011).
|
|
10.30
|
NeoStem, Inc. 2003 Equity Participation Plan, as amended (filed as Exhibit 10.2 to the Company's Registration Statement on Form S-1/A, File No. 333-137045, filed with the SEC on November 3, 2006). +
|
|
10.31
|
Form of Stock Option Agreement (filed as Exhibit 10.2 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2003 as filed with the SEC on March 30, 2004). +
|
|
10.32
|
Form of Option Agreement dated July 20, 2005 (filed as Exhibit 10.5 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2005 as filed with the SEC on August 15, 2005). +
|
|
10.33
|
Amended and Restated NeoStem, Inc. 2009 Equity Compensation Plan (as amended and restated as of October 5, 2012) (filed as Appendix B to the Company's Definitive Proxy Statement on Schedule 14A for the 2012 Annual Meeting of Stockholders as filed with the SEC on September 7, 2012). +
|
|
10.34
|
Form of Stock Option Grant Agreement under NeoStem, Inc. 2009 Equity Compensation Plan (filed as Exhibit 10.5 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 as filed with the SEC on August 16, 2010). +
|
|
10.35
|
Description of the NeoStem, Inc. Board of Directors Compensation Plan (incorporated by reference to the first paragraph of Item 5.02 contained within the Company's Current Report on Form 8-K dated January 4, 2012, and the last paragraph appearing under Item 11 of this Annual Report on Form 10-K for the fiscal year ended December 31, 2012). +
|
|
10.36
|
NeoStem, Inc. 2012 Employee Stock Purchase Plan (filed as Appendix A to the Company's Definitive Proxy Statement on Schedule 14A for the 2012 Annual Meeting of Stockholders as filed with the SEC on September 7, 2012). +
|
|
10.37
|
Employment Agreement between Phase III Medical, Inc. and Dr. Robin L. Smith, dated May 26, 2006 (filed as Exhibit 10.4 to the Company's Current Report on Form 8-K dated June 2, 2006). +
|
|
10.38
|
January 26, 2007 Amendment to Employment Agreement of Dr. Robin L. Smith (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated January 26, 2007). +
|
|
10.39
|
September 27, 2007 Amendment to Employment Agreement of Dr. Robin L. Smith (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated September 27, 2007). +
|
|
10.40
|
Letter agreement dated January 9, 2008 with Dr. Robin L. Smith (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated January 9, 2008). +
|
|
10.41
|
Amendment dated July 29, 2009 to Employment Agreement dated May 26, 2006 between NeoStem, Inc. and Dr. Robin L. Smith (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated July 29, 2009). +
|
|
10.42
|
Amendment dated April 4, 2011 to Employment Agreement dated May 26, 2006 between NeoStem, Inc. and Dr. Robin L. Smith (filed as Exhibit 10.66 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011). +
|
|
10.43†
|
Amendment dated November 13, 2012 to Employment Agreement dated May 26, 2006 between NeoStem, Inc. and Dr. Robin L. Smith. +
|
|
10.44
|
Employment Agreement between the Company and Larry A. May dated January 19, 2006 (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated January 19, 2006). +
|
|
10.45
|
Letter Agreement between Phase III Medical, Inc. and Larry A. May effective as of June 2, 2006 (filed as Exhibit 10.7 to the Company's Current Report on Form 8-K dated June 2, 2006). +
|
|
10.46
|
January 26, 2007 Amendment to Employment Agreement of Larry A. May (filed as Exhibit 10.3 to the Company's Current Report on Form 8-K dated January 26, 2007). +
|
|
10.47
|
Letter Agreement, dated April 20, 2005, between Phase III Medical, Inc. and Catherine M. Vaczy (filed as Exhibit 10.3 to the Company's Current Report on Form 8-K dated April 20, 2005). +
|
|
10.48
|
Letter Agreement dated August 12, 2005 with Catherine M. Vaczy (filed as Exhibit 10.7 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2005 as filed with the SEC on August 15, 2005). +
|
|
10.49
|
Letter Agreement dated December 22, 2005 between Phase III Medical, Inc. and Catherine M. Vaczy (filed as Exhibit 10(y) to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2005 as filed with the SEC on April 3, 2006). +
|
|
10.5
|
Letter Agreement dated January 30, 2006 between Phase III Medical, Inc. and Catherine M. Vaczy (filed as Exhibit 10(cc) to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2005 as filed with the SEC on April 3, 2006). +
|
|
10.51
|
Letter Agreement between Phase III Medical, Inc. and Catherine M. Vaczy effective as of June 2, 2006 (filed as Exhibit 10.6 to the Company's Current Report on Form 8-K dated June 2, 2006). +
|
|
10.52
|
January 26, 2007 Employment Agreement with Catherine M. Vaczy (filed as Exhibit 10.4 to the Company's Current Report on Form 8-K dated January 26, 2007). +
|
|
10.53
|
Letter agreement dated January 9, 2008 with Catherine M. Vaczy (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated January 9, 2008). +
|
|
10.54
|
Letter Agreement dated July 8, 2009 between NeoStem, Inc. and Catherine M. Vaczy, Esq. (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated July 6, 2009). +
|
|
10.55
|
Letter Agreement dated July 7, 2010 between NeoStem, Inc. and Catherine M. Vaczy, Esq. (filed as Exhibit 10(a) to the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 as filed with the SEC on November 12, 2010). +
|
|
10.56
|
Letter Agreement dated January 6, 2012 between NeoStem, Inc. and Catherine M. Vaczy, Esq. (filed as Exhibit 10.92 to the Company's Annual Report on Form 10-K for the year ended December 31, 2011 as filed with the SEC on March 20, 2012). +
|
|
10.57†
|
Letter Agreement dated November 13, 2012 between NeoStem, Inc. and Catherine M. Vaczy, Esq. +
|
|
10.58
|
Employment Agreement, dated as of September 23, 2010 and effective on January 19, 2011, by and between Progenitor Cell Therapy, LLC, NeoStem, Inc. and Andrew L. Pecora (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K dated January 18, 2011 and filed with the SEC on January 24, 2011). +
|
|
10.59
|
Amendment dated August 17, 2011 to Employment Agreement dated September 23, 2010 and effective January 19, 2011 between Progenitor Cell Therapy, LLC, NeoStem, Inc. and Andrew L. Pecora (filed as Exhibit 10.95 to the Company's Registration Statement on Form S-4, File No. 333-176673, filed with the SEC on September 2, 2011). +
|
|
10.6
|
Letter Agreement dated April 11, 2012 between NeoStem, Inc. and Andrew Pecora, M.D., F.A.C.P. (filed as Exhibit 10.107 to the Company's Annual Report on Form 10-K/A for the year ended December 31, 2011 as filed with the SEC on April 27, 2012). +
|
|
10.61
|
Employment Agreement, dated as of September 23, 2010 and effective on January 19, 2011, by and between Progenitor Cell Therapy, LLC, NeoStem, Inc. and Robert A. Preti (filed as Exhibit 10.2 to the Company's Current Report on Form 8-K dated January 18, 2011 and filed with the SEC on January 24, 2011). +
|
|
10.62
|
Consulting Agreement, effective March 8, 2011, by and between NeoStem, Inc. and Acute Care Partners (filed as Exhibit 10.87 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011). +
|
|
10.63
|
Form of Indemnification Agreement for directors, officers and certain other employees (filed as Exhibit 10.2 to the Company's Registration Statement on Form S-4/A, File No. 333-160578, filed with the SEC on October 6, 2009).
|
|
10.64
|
Letter Agreement dated June 28, 2011 between NeoStem, Inc. and Joseph Talamo (filed as Exhibit 10.10 to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2011 as filed with the SEC on August 12, 2011). +
|
|
14.1
|
Code of Ethics for Senior Financial Officers (filed as Exhibit 14.1 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2010 as filed with the SEC on April 6, 2011).
|
|
21.1†
|
Subsidiaries of NeoStem, Inc.
|
|
23.1†
|
Consent of Grant Thornton LLP
|
|
31.1†
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
31.2†
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
32.1††
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
32.2††
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
101.INS
|
XBRL Instance Document***
|
|
101.SCH
|
XBRL Taxonomy Extension Schema***
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase***
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase***
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase***
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase***
|
|
+
|
Management contract or compensatory plan, contract or arrangement required to be filed as an exhibit to this Form 10-K pursuant to Item 15(b) of Form 10-K.
|
|
***
|
Users of this interactive data file are advised pursuant to Rule 406T of Regulations S-T that this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections.
|
|
†
|
Filed herewith.
|
|
††
|
Furnished herewith.
|
|
(1)
|
Certain portions of this exhibit were omitted based upon a request for confidential treatment, and the omitted portions were filed separately with the SEC on a confidential basis.
|
|
|
|
|
|
|
|
NEOSTEM, INC.
|
|
|
|
By:
/s/ Robin L. Smith, M.D.
Name: Robin L. Smith, M.D.
Title: Chief Executive Officer
|
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
/s/ Robin L. Smith, M.D.
Robin L. Smith, M.D.
|
|
Director, Chief Executive Officer and
Chairman of the Board (Principal Executive Officer)
|
|
March 8, 2013
|
|
/s/ Larry A. May
Larry A. May
|
|
Chief Financial Officer (Principal Financial Officer)
|
|
March 8, 2013
|
|
/s/ Joseph Talamo
Joseph Talamo
|
|
Vice President, Corporate Controller and Chief
Accounting Officer (Principal Accounting Officer)
|
|
March 8, 2013
|
|
/s/ Richard Berman
Richard Berman
|
|
Director
|
|
March 8, 2013
|
|
/s/ Steven S. Myers
Steven S. Myers
|
|
Director
|
|
March 8, 2013
|
|
/s/ Drew Bernstein
Drew Bernstein
|
|
Director
|
|
March 8, 2013
|
|
/s/ Eric Wei
Eric Wei
|
|
Director
|
|
March 8, 2013
|
|
/s/ Stephen W. Potter
Stephen W. Potter
|
|
Director
|
|
March 8, 2013
|
|
/s/ Andrew L. Pecora, M.D.
Andrew L. Pecora, M.D.
|
|
Director
|
|
March 8, 2013
|
|
/s/ Martyn D. Greenacre
Martyn D. Greenacre
|
|
Director
|
|
March 8, 2013
|