|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Masimo Corporation
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
33-0368882
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification Number)
|
|
|
52 Discovery, Irvine, California
|
92618
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
|
Title of each class:
|
Name of each exchange on which registered:
|
|
|
Common Stock, par value $0.001
|
The NASDAQ Global Select Market
|
|
|
Large accelerated filer
|
ý
|
Accelerated filer
|
¨
|
Non accelerated filer
|
o
|
Smaller reporting company
|
o
|
||||||||
|
|
(Do not check if a smaller reporting company)
|
||||||||||||||
|
Page
|
||
|
Item 1
|
||
|
Item 1A
|
||
|
Item 1B
|
||
|
Item 2
|
||
|
Item 3
|
||
|
Item 4
|
||
|
Item 5
|
||
|
Item 6
|
||
|
Item 7
|
||
|
Item 7A
|
||
|
Item 8
|
||
|
Item 9
|
||
|
Item 9A
|
||
|
Item 9B
|
||
|
Item 10
|
||
|
Item 11
|
||
|
Item 12
|
||
|
Item 13
|
||
|
Item 14
|
||
|
Item 15
|
||
|
ITEM 1.
|
BUSINESS
|
|
•
|
Fewer false alarms and better true alarm detection
. Over 100 independent studies demonstrate the advantages of Masimo SET
®
during challenging conditions in adult, pediatric and neonatal patients.
|
|
•
|
Fewer arterial blood gas measurements, faster oxygen weaning time, and lower length of stay in the ICU.
Due to the ability of Masimo SET
®
to monitor patients during challenging conditions, studies have shown that Masimo SET
®
helps clinicians reduce the need for arterial blood gas, weaning times from the ventilator, and length of stay.
|
|
•
|
Lower sensor utilization.
Masimo SET
®
sensors provide enhanced durability for greater sensor longevity, and the underlying performance of Masimo SET
®
in challenging conditions makes it easier to obtain measurements on digits with low perfusion, which reduces the use of multiple sensors on the same patient.
|
|
•
|
Increased detection of critical congenital heart disease through newborn screening
. Four studies totaling 118,000 patients have shown that adding Masimo SET
®
to the standard physical exam helps clinicians to increase the detection of critical congenital heart disease, a potentially fatal disease, before the newborn leaves the hospital. The published evidence for Masimo SET
®
led the American Academy of Pediatrics and the U.S. Department of Health and Human Services to recommend mandatory screening for all newborns using “motion-tolerant pulse oximeters that report functional oxygen saturation and have been validated in low perfusion conditions”. In 2012, we received FDA 510(k) clearance for Masimo SET
®
pulse oximeters and neonatal sensors with labeling for screening newborns for CCHD, marking the first time the FDA cleared specific labeling for the use of pulse oximeters, in conjunction with a physical exam, to screen newborns for CCHD.
|
|
•
|
Reduced retinopathy of prematurity in very low birth weight neonates.
In a two-phased study of two centers that previously used competing pulse oximetry, both centers simultaneously changed their neonatal oxygen targeting policy, and one of the centers switched to Masimo SET
®
pulse oximetry. In the first phase of the study, there was no decrease in retinopathy of prematurity at the center using competing pulse oximetry but there was a 58% reduction in significant retinopathy of prematurity and a 40% reduction in the need for laser eye treatment at the center using Masimo SET
®
. In the second phase of the study, the center still using competing pulse oximetry switched to Masimo SET
®
and it experienced results similar to the center already using Masimo SET
®
|
|
•
|
Allows standalone device information to be remotely viewed with Patient SafetyNet
™
, transmitted through notification systems or sent to electronic health record systems to facilitate better patient care and meaningful use.
|
|
•
|
Designed to leverage existing network infrastructures and reduce costs while enhancing clinical workflows and decision support to improve patient safety, wherever the clinician is located.
|
|
•
|
Flexible and cost-effective platform, avoiding installation of costly, separate systems.
|
|
•
|
Brings all the data together to facilitate assessment and decision support.
|
|
•
|
Continue to Expand our Market Share in Pulse Oximetry.
We grew our product revenue to
$556.8 million
in
2014
from
$406.5 million
in 2011, representing a three year compound annual growth rate of 11.1%. This growth can be attributed to strong, independent clinical evidence that demonstrates the benefits of our technology, the increased access to pulse oximetry customers through our agreements with group purchasing organizations (GPOs), our expanding list of OEM partners and the continued expansion of our worldwide direct sales force. We supplement our direct sales to hospitals and other low acuity healthcare facilities through various U.S. and international distributors. Combined sales through our direct and distributor sales channels increased to
$472.7 million
, or
84.9%
of product revenue in
2014
, from
$342.9 million
, or
84.4%
of product revenue in 2011.
|
|
•
|
Expand the Pulse Oximetry Market to Other Patient Care Settings.
We believe the ability to continuously and accurately monitor patients outside of critical care settings, including the general, medical and surgical floors of the hospital, are currently unmet medical needs and have the potential to significantly improve patient care and increase the size of the pulse oximetry market. We believe the ability of Masimo SET
®
to accurately monitor and address the limitations of conventional pulse oximetry has enabled, and will continue to enable, us to expand into non-critical care settings and therefore, significantly expand the market for our products. To further support our expansion into the general care areas, we market Patient SafetyNet
™
, which enables continuous monitoring of up to
80
patients’ oxygen saturation, pulse rate and with rainbow
®
SET
®
, noninvasive hemoglobin and respiration rate.
|
|
•
|
Expand the Use of rainbow
®
Technology in Hospital Settings.
We believe the noninvasive measurement of rainbow
®
Pulse CO-Oximetry (SpHb
®
, SpCO
®
, SpMet
®
, PVI
®
,
SpfO
2
™
, SPOC
™
,
and ORI
™
), rainbow Acoustic Monitoring
™
(RRa
®
), and the Halo Index
™
, as well as future measurements, will provide an excellent opportunity to leverage existing customer relationships into new opportunities to improve patient care and, at the same time, expand our product revenue opportunities through a greater ability to convert non-Masimo hospitals to Masimo hospitals due to our expanded rainbow
®
measurement capabilities.
|
|
•
|
Expand the Use of rainbow
®
Technology in the Non-Hospital Setting.
We believe the noninvasive measurement of hemoglobin creates a significant opportunity in markets such as the physician office and emergency departments, and the noninvasive measurement of carboxyhemoglobin creates a significant opportunity in the fire/alternate care market.
|
|
•
|
Utilize our Customer Base and OEM Relationships to Market our Masimo rainbow
®
SET
®
Products Incorporating Licensed rainbow
®
Technology.
We are currently selling our rainbow
®
SET
®
products through our direct sales force and distributors. We include our MX circuit boards in our pulse oximeters and sell them to our OEM partners, equipped with circuitry to support rainbow
®
Pulse CO-Oximetry measurements that can be activated at time of sale or through a subsequent software upgrade. We believe that, over time, the clinical need of these measurements along with our installed customer base will help drive the adoption of our rainbow
®
Pulse CO-Oximetry products.
|
|
•
|
Continue to Innovate and Maintain Our Technology Leadership Position.
We invented and pioneered what we believe is the first pulse oximeter to accurately measure arterial blood oxygen saturation level and pulse rate in the presence of motion artifact and low perfusion. In addition, we launched our rainbow
®
SET
®
platform that enabled what we believe is the first noninvasive monitoring of carboxyhemoglobin, methemoglobin and hemoglobin, as well as PVI
®
, all of which were previously only available with invasive and/or complicated testing. With our introduction of RRa
®
with rainbow Acoustic Monitoring
™
technology, we believe we have launched the first platform to enable noninvasive and continuous respiration monitoring through an easy-to-use single patient adhesive acoustic sensor. We plan to continue to innovate and develop new technologies and products, internally and through our collaboration with Cercacor, from whom we currently license certain rainbow
®
technologies.
|
|
Patient Monitoring Solutions:
|
||||
|
Circuit Boards and Modules
(e.g., MX-1
®
, MX-3
®
, MX-5, MS-2011, MS-2040, uSpO2
®
, SedLine
®
, ISA
™
, and IRMA
™
)
|
• Signal processing apparatus for all Masimo technology platforms
• Mainstream and sidestream capnography and gas monitors
|
• Incorporated and sold to OEM partners who incorporate our circuit boards into their patient monitoring systems
|
||
|
Monitors and Devices
(e.g., Radical-7
®
, Pronto, Pronto-7
®
, Rad-57
®
, Root
®
, Radius-7
™
, and EMMA
™
)
|
• Bedside, handheld and wireless monitoring devices that incorporate Masimo SET
®
with and without licensed Masimo rainbow
®
SET
®
technology
• Compact and self-contained capnometer which monitors CO
2
concentration
|
• Sold directly to end-users and through distributors and in some cases to our OEM partners who sell to end-users
|
||
|
Patient Monitoring and Connectivity Platforms
(e.g., Root
®
, Radius-7
™
)
|
• Multi-specialty measurement monitor with connected and wireless capabilities
• Ability to connect third-party devices such as IV pumps, ventilators, beds, and other patient monitors to the electronic health record
|
• Sold directly to end-users and through distributors
|
||
|
Sensors
(e.g., SET
®
, rainbow
®
Pulse CO-Oximetry, rainbow Acoustic
™
Sensors
™
, and SedLine
®
)
|
• Extensive line of both single-patient, reusable and rainbow ReSposable
®
sensors
• Patient cables, as well as adapter cables that enable the use of our sensors on certain competitive monitors
|
• Sold directly to end-users and through distributors and to OEM partners who sell to end-users
|
||
|
Line Filters, and Mainstream Adapters
(e.g., capnography and gas disposables)
|
• Line of disposables to measure
mainstream and sidestream capnography and gas parameters
|
• Sold directly to end-users and through distributors and to OEM partners who sell to end-users
|
||
|
Remote Alarm and Monitoring Solutions
(e.g., Patient SafetyNet ™ ) |
• Network-linked, wired or wireless,
multiple patient floor monitoring solutions
• Standalone wireless alarm notification solutions
|
• Sold directly to end-users
|
||
|
Proprietary Measurements
(e.g., SpHb ® , SpCO ® , SpMet ® , PVI ® , RRa ® , ORI ™ , 3D Alarms, Adaptive Threshold Alarm and Halo Index ™ ) |
• Rainbow
®
measurements and other
proprietary features sold to installed monitors |
• Sold directly to end-users and through OEM partners who sell to end-users
|
||
|
Connectivity
(e.g., Root ® , Patient SafetyNet ™ ) |
• Software and hardware enabling
third-party devices to connect through Patient SafetyNet™ to clinicians and for documentation to the electronic health record |
• Sold directly to end-users
|
||
|
Consumer Monitoring Solutions:
|
||||
|
Devices
(e.g., iSpO
2
®
, MightySat
™
)
|
• Pulse oximeter cable and sensor for use with an iPhone, iPad, iPod touch, and select Android smart phones
|
• Sold directly to consumers through on-line websites
|
||
|
•
|
a standalone device for bedside monitoring;
|
|
•
|
a detachable, battery-operated handheld unit for easy portable monitoring; and
|
|
•
|
a monitor interface via SatShare
®
, a proprietary technology allowing our products to work with certain competitor products, to upgrade existing conventional multiparameter patient monitors to Masimo SET
®
while displaying rainbow
®
measurements on the Radical-7
®
itself.
|
|
•
|
Patient safety may be compromised by using imitation Masimo sensors and cables because they are not produced with comparable components, do not provide proper shielding from ambient interferences, create electrostatic noise caused by motion, do not have our quality and performance controls, and are not tested or warranted to work within a Masimo system;
|
|
•
|
We design our sensors and cables to last well beyond their warranty and customer feedback indicates our sensors and cables last significantly longer than competing products, but cable and sensor reliability may still be compromised when used beyond the life they were reliably designed for, affecting patient care and causing clinicians and biomedical engineers to spend time troubleshooting intermittent cable and sensor issues; and
|
|
•
|
We believe that third-party reprocessed pulse oximetry sensors introduce challenges in the clinical environment due to potential quality issues. Internal Masimo testing indicates that 91% of a leading third-party reprocessor’s sensors that were
|
|
End User Markets
|
||||
|
Measurements
|
Professional Caregiver and
Alternate Care Market
|
Patient and Pharmacist
|
||
|
Vital Signs
(1)
|
Masimo
(owns)
|
Cercacor
(non-exclusive license)
|
||
|
Non-Vital Signs
(2)
|
Masimo
(exclusive license)
|
Cercacor
(owns or exclusive license)
|
||
|
(1)
|
Vital Signs measurements include, but are not limited to, SpO
2
, peripheral venous oxygen saturation, mixed venous oxygen saturation, fetal oximetry, sudden infant death syndrome, ECG, blood pressure (noninvasive blood pressure, invasive blood pressure and continuous noninvasive blood pressure), temperature, respiration rate, CO
2
, pulse rate, cardiac output, EEG, perfusion index, depth of anesthesia, cerebral oximetry, tissue oximetry and/or EMG, and associated features derived from these measurements, such as 3-D alarms, PVI
®
and other features.
|
|
(2)
|
Non-Vital Signs measurements include the body fluid constituents other than vital signs measurements and include, but are not limited to, carbon monoxide, methemoglobin, blood glucose, hemoglobin and bilirubin.
|
|
•
|
if the surviving or acquiring entity ceases to use “Masimo” as a company name and trademark, all rights to the “Masimo” trademark will be assigned to Cercacor;
|
|
•
|
the option to license technology developed by Cercacor for use in blood glucose monitoring will be deemed automatically exercised and a $2.5 million license fee for this technology will become immediately payable to Cercacor; and
|
|
•
|
the minimum aggregate annual royalties payable to Cercacor for carbon monoxide, methemoglobin, fractional arterial oxygen saturation, hemoglobin and/or glucose will increase to $15.0 million per year until the exclusivity period of the agreement ends, plus up to $2.0 million for each additional measurement with no maximum ceiling for non-vital sign measurements.
|
|
•
|
the sale of all or substantially all of either company’s assets to a non-affiliated third-party;
|
|
•
|
the acquisition by a non-affiliated third-party of 50% or more of the voting power of either company;
|
|
•
|
Joe Kiani, our Chief Executive Officer and the Chief Executive Officer of Cercacor, resigns or is terminated from his position with either company; and
|
|
•
|
the merger or consolidation of either company with a non-affiliated third-party.
|
|
•
|
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
|
|
•
|
QSR and current good manufacturing practices, which requires manufacturers, including third-party manufacturers, to follow stringent design control, testing, change control, documentation and other quality assurance procedures during all aspects of the development and manufacturing process, including requirements for packaging, labeling and record keeping, complaint handling, corrective and preventive actions and internal auditing;
|
|
•
|
labeling control and advertising regulations, including FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses or indications;
|
|
•
|
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices;
|
|
•
|
approval of product modifications that affect the safety or effectiveness of one of our future approved devices;
|
|
•
|
medical device reporting (MDR), regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
|
|
•
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
•
|
post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
|
|
•
|
the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of its conditions of approval, governing laws and/or regulations;
|
|
•
|
regulations pertaining to voluntary recalls; and
|
|
•
|
notices of corrections or removals.
|
|
•
|
an FDA Form 483, which is issued by the FDA at the conclusion of an inspection when an investigator has observed any conditions that may constitute violations of the FDCA and related Acts;
|
|
•
|
a public warning letter outlining potential violations of the FDCA;
|
|
•
|
fines and civil penalties against us and/or OEM partners;
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
•
|
delays in clearing or approving, or refusal to clear or approve, our products;
|
|
•
|
withdrawal or suspension of clearances and/or approvals of our products or those of our third-party suppliers by the FDA or other regulatory bodies;
|
|
•
|
product recall;
|
|
•
|
product detention or seizure;
|
|
•
|
interruption of production;
|
|
•
|
refusal to provide export certificates, which may be necessary to permit the export of devices from the U.S. to other countries;
|
|
•
|
operating restrictions;
|
|
•
|
injunctions of future violations (including those agreed to in a consent decree); and
|
|
•
|
criminal prosecution.
|
|
•
|
accurate monitoring during both patient motion and low perfusion;
|
|
•
|
ability to introduce other clinically beneficial measurements related to oxygenation and respiration, such as noninvasive and continuous hemoglobin and acoustic respiration rate;
|
|
•
|
competitive pricing, including bundling practices;
|
|
•
|
brand recognition and perception of innovation abilities;
|
|
•
|
sales and marketing capability;
|
|
•
|
access to hospitals which are members of GPOs;
|
|
•
|
recent proliferation of integrated delivery networks;
|
|
•
|
access to OEM partners; and
|
|
•
|
patent protection.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
•
|
perceived clinical benefits from our products;
|
|
•
|
perceived cost effectiveness of our products;
|
|
•
|
perceived safety and effectiveness of our products;
|
|
•
|
reimbursement available through Centers for Medicare and Medicaid Services (CMS) programs for using some of our products; and
|
|
•
|
introduction and acceptance of competing products or technologies.
|
|
•
|
an exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET
®
owned by us, including all improvements on this technology, for the monitoring of non-vital signs parameters and to develop and sell devices incorporating Masimo SET
®
for monitoring non-vital signs parameters in any product market in which a product is intended to be used by a patient or pharmacist rather than by a professional medical caregiver, which we refer to as the Cercacor Market; and
|
|
•
|
a non-exclusive, perpetual and worldwide license, with sublicense rights, to use all Masimo SET
®
for measurement of vital signs in the Cercacor Market.
|
|
•
|
controls on reimbursement for health care services and price controls on medical products and services;
|
|
•
|
limitations on coverage and reimbursement for new medical technologies and procedures; and
|
|
•
|
the introduction of managed care and prospective payment systems in which health care providers contract to provide comprehensive health care for a fixed reimbursement amount per person or per procedure.
|
|
•
|
increase the cost of our products;
|
|
•
|
be expensive and time consuming to defend;
|
|
•
|
result in us being required to pay significant damages to third parties;
|
|
•
|
force us to cease making or selling products that incorporate the challenged intellectual property;
|
|
•
|
require us to redesign, reengineer or rebrand our products, product candidates and technologies;
|
|
•
|
require us to enter into royalty or licensing agreements in order to obtain the right to use a third-party’s intellectual property on terms that may not be favorable or acceptable to us;
|
|
•
|
require us to indemnify third parties pursuant to contracts in which we have agreed to provide indemnification for intellectual property infringement claims;
|
|
•
|
divert the attention of our management and other key employees;
|
|
•
|
result in our customers or potential customers deferring or limiting their purchase or use of the affected products impacted by the claims until the claims are resolved; and
|
|
•
|
otherwise have a material adverse effect on our business, financial condition and results of operations.
|
|
•
|
warning letters or untitled letters issued by the FDA;
|
|
•
|
fines, civil penalties, in rem forfeiture proceedings, injunctions and criminal prosecution;
|
|
•
|
import alerts;
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
•
|
delays in clearing or approving, or refusal to clear or approve, our products;
|
|
•
|
withdrawal or suspension of clearance or approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies;
|
|
•
|
product recall or seizure;
|
|
•
|
orders for physician notification or device repair, replacement or refund;
|
|
•
|
interruption of production or inability to export to certain foreign countries; and
|
|
•
|
operating restrictions.
|
|
•
|
the Federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any bribe, kickback or other remuneration intended to induce the purchase, order or recommendation of an item or service reimbursable under a federal health care program (such as the Medicare or Medicaid programs);
|
|
•
|
federal false claims laws which prohibit, among other things, knowingly and willfully presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payers that are false or fraudulent;
|
|
•
|
the provisions of the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which established federal crimes for knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services; and
|
|
•
|
state laws analogous to each of the above federal laws, such as state anti-kickback and false claims laws that may apply to items or services reimbursed by non-governmental third-party payers, including commercial insurers, and state laws governing the privacy of certain patient identifiable health information (PHI).
|
|
•
|
delays or interruptions in manufacturing and shipping of our products;
|
|
•
|
varying demand for and market acceptance of our technologies and products;
|
|
•
|
delayed acceptance of our new products, negatively impacting the carrying value of our inventory;
|
|
•
|
design, technology or other market changes that could negatively impact the carrying value of our inventory;
|
|
•
|
the effect of competing technological and market developments resulting in lower selling prices or significant promotional costs;
|
|
•
|
changes in the timing of product orders and the volume of sales to our OEM partners;
|
|
•
|
actions taken by GPOs;
|
|
•
|
delays in hospital conversions to our products and declines in hospital patient census;
|
|
•
|
our legal expenses, particularly those related to litigation matters;
|
|
•
|
changes in our product or customer mix;
|
|
•
|
movements in foreign currency exchange rates;
|
|
•
|
market seasonality of our sales due to quarterly fluctuations in hospital and other alternative care admissions;
|
|
•
|
our ability to renew existing long-term sensor contract commitments;
|
|
•
|
changes in the total dollar amount of annual contract renewal activities;
|
|
•
|
changes in the mix and, therefore, the related costs of products that we supply at no upfront costs to our customers as part of their long-term sensor commitments;
|
|
•
|
changes in hospital and other alternative care admission levels;
|
|
•
|
our inability to efficiently scale operations and establish processes to accommodate business growth;
|
|
•
|
unanticipated delays or problems in the introduction of new products, including delays in obtaining clearance or approval from the FDA;
|
|
•
|
high levels of returns and repairs; and
|
|
•
|
change in reimbursement rates for SpHb
®
, SpCO
®
and SpMet
®
parameters.
|
|
•
|
difficulties in integrating any acquired companies, personnel, products and other assets into our existing business;
|
|
•
|
delays in realizing the benefits of the acquired company, products or other assets;
|
|
•
|
diversion of our management’s time and attention from other business concerns;
|
|
•
|
limited or no direct prior experience in new markets or countries we may enter;
|
|
•
|
higher costs of integration than we anticipated;
|
|
•
|
difficulties in retaining key employees of the acquired business who are necessary to manage these acquisitions; and
|
|
•
|
changes in the overall financial model as certain acquired companies may have a different revenue, gross profit margin or operating expense profile.
|
|
•
|
the imposition of additional U.S. and foreign governmental controls or regulations;
|
|
•
|
the imposition of costly and lengthy new export licensing requirements;
|
|
•
|
a shortage of high-quality sales people and distributors;
|
|
•
|
loss of any key personnel that possess proprietary knowledge, or who are otherwise important to our success in certain international markets;
|
|
•
|
changes in duties and tariffs, license obligations and other non-tariff barriers to trade;
|
|
•
|
the imposition of new trade restrictions;
|
|
•
|
the imposition of restrictions on the activities of foreign agents, representatives and distributors;
|
|
•
|
scrutiny of foreign tax authorities which could result in significant fines, penalties and additional taxes being imposed on us;
|
|
•
|
pricing pressure that we may experience internationally;
|
|
•
|
changes in foreign currency exchange rates;
|
|
•
|
laws and business practices favoring local companies;
|
|
•
|
political instability and actual or anticipated military or political conflicts;
|
|
•
|
financial and civil unrest worldwide;
|
|
•
|
longer payment cycles; and
|
|
•
|
difficulties in enforcing or defending intellectual property rights.
|
|
•
|
incur specified types of additional indebtedness (including guarantees or other contingent obligations);
|
|
•
|
pay dividends on, repurchase, or make distributions in respect to our common stock or make other restricted payments, subject to specified exceptions;
|
|
•
|
make specified investments (including loans and advances);
|
|
•
|
sell or transfer certain assets;
|
|
•
|
create certain liens;
|
|
•
|
consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; and
|
|
•
|
enter into certain transactions with any of our affiliates.
|
|
•
|
actual or anticipated fluctuations in our operating results or future prospects;
|
|
•
|
our announcements or our competitors’ announcements of new products;
|
|
•
|
the public’s reaction to our press releases, our other public announcements and our filings with the SEC;
|
|
•
|
strategic actions by us or our competitors, such as acquisitions or restructurings;
|
|
•
|
new laws or regulations or new interpretations of existing laws or regulations applicable to our business;
|
|
•
|
changes in accounting standards, policies, guidance, interpretations or principles;
|
|
•
|
changes in our growth rates or our competitors’ growth rates;
|
|
•
|
developments regarding our patents or proprietary rights or those of our competitors;
|
|
•
|
ongoing legal proceedings;
|
|
•
|
our inability to raise additional capital as needed;
|
|
•
|
concerns or allegations as to the safety or efficacy of our products;
|
|
•
|
changes in financial markets or general economic conditions, including the effects of recession or slow economic growth in the U.S. and abroad;
|
|
•
|
sales of stock by us or members of our management team, our board of directors or certain institutional stockholders; and
|
|
•
|
changes in stock market analyst recommendations or earnings estimates regarding our stock, other comparable companies or our industry generally.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
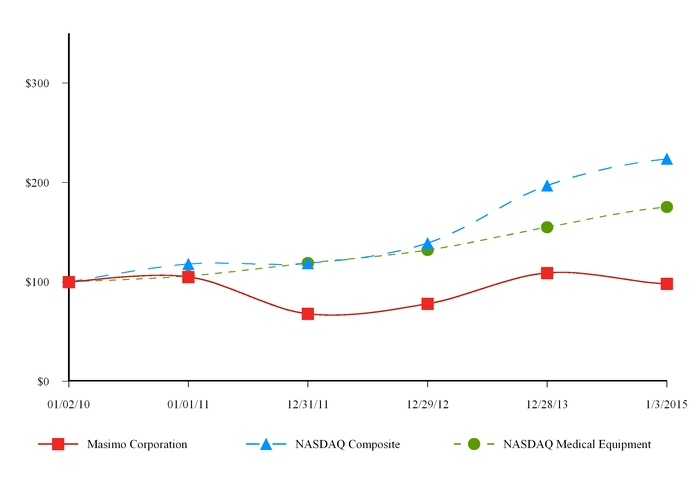
ITEM 5.
|
MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Fiscal 2014
|
Fiscal 2013
|
|||||||||||||||
|
High
|
Low
|
High
|
Low
|
|||||||||||||
|
Fiscal:
|
||||||||||||||||
|
First Quarter
|
$
|
31.88
|
|
$
|
25.37
|
|
$
|
21.33
|
|
$
|
19.51
|
|
||||
|
Second Quarter
|
$
|
27.90
|
|
$
|
22.03
|
|
$
|
22.50
|
|
$
|
19.04
|
|
||||
|
Third Quarter
|
$
|
24.64
|
|
$
|
20.69
|
|
$
|
27.04
|
|
$
|
21.55
|
|
||||
|
Fourth Quarter
|
$
|
27.00
|
|
$
|
21.07
|
|
$
|
29.61
|
|
$
|
25.62
|
|
||||
|
Period
|
Total Number of Shares Purchased
|
Average Cost Paid Per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs
|
Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs
(1)
|
|||||||||
|
September 28, 2014 to October 25, 2014
|
27,659
|
|
$
|
21.01
|
|
—
|
|
3,545,151
|
|
||||
|
October 26, 2014 to November 29, 2014
|
—
|
|
—
|
|
—
|
|
3,545,151
|
|
|||||
|
November 30, 2014 to January 3, 2015
|
—
|
|
—
|
|
—
|
|
3,545,151
|
|
|||||
|
Total
|
27,659
|
|
$
|
21.01
|
|
—
|
|
3,545,151
|
|
||||
|
(1)
|
In October 2014, our Board increased the number of shares of the Company’s common stock authorized for repurchase by 3.0 million shares, bringing the total number of shares of the Company’s common stock authorized under such repurchase program to 9.0 million.
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
Year ended
December 31, 2011 |
Year ended
January 1, 2011 |
|||||||||||||||
|
(dollars in thousands)
|
|||||||||||||||||||
|
Statement of Comprehensive Income Data
(1)
:
|
|||||||||||||||||||
|
Revenue:
|
|||||||||||||||||||
|
Product
|
$
|
556,764
|
|
$
|
517,429
|
|
$
|
464,928
|
|
$
|
406,487
|
|
$
|
356,422
|
|
||||
|
Royalty
|
29,879
|
|
29,816
|
|
28,305
|
|
32,501
|
|
48,985
|
|
|||||||||
|
Total revenue
|
586,643
|
|
547,245
|
|
493,233
|
|
438,988
|
|
405,407
|
|
|||||||||
|
Cost of goods sold
|
195,864
|
|
188,418
|
|
166,982
|
|
144,854
|
|
119,825
|
|
|||||||||
|
Gross profit
|
390,779
|
|
358,827
|
|
326,251
|
|
294,134
|
|
285,582
|
|
|||||||||
|
Operating expenses:
|
|||||||||||||||||||
|
Selling, general and administrative
|
241,016
|
|
215,469
|
|
193,948
|
|
169,205
|
|
174,089
|
|
|||||||||
|
Research and development
|
56,581
|
|
55,631
|
|
47,077
|
|
38,412
|
|
36,000
|
|
|||||||||
|
Litigation award and defense costs
|
(10,331
|
)
|
8,010
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Antitrust litigation expense
(2)
|
—
|
|
—
|
|
—
|
|
—
|
|
(30,728
|
)
|
|||||||||
|
Total operating expenses
|
287,266
|
|
279,110
|
|
241,025
|
|
207,617
|
|
179,361
|
|
|||||||||
|
Operating income
|
103,513
|
|
79,717
|
|
85,226
|
|
86,517
|
|
106,221
|
|
|||||||||
|
Non-operating (income) expense
|
1,472
|
|
3,991
|
|
1,405
|
|
(14
|
)
|
(1,348
|
)
|
|||||||||
|
Income before provision for income taxes
|
102,041
|
|
75,726
|
|
83,821
|
|
86,531
|
|
107,569
|
|
|||||||||
|
Provision for income taxes
|
27,678
|
|
20,005
|
|
21,883
|
|
22,478
|
|
34,164
|
|
|||||||||
|
Net income including noncontrolling interests
|
74,363
|
|
55,721
|
|
61,938
|
|
64,053
|
|
73,405
|
|
|||||||||
|
Net income (loss) attributable to noncontrolling interests
|
1,845
|
|
(2,660
|
)
|
(334
|
)
|
353
|
|
(125
|
)
|
|||||||||
|
Net income attributable to Masimo Corporation stockholders
|
72,518
|
|
58,381
|
|
62,272
|
|
63,700
|
|
73,530
|
|
|||||||||
|
Other comprehensive income (loss), net of tax:
|
|||||||||||||||||||
|
Foreign currency translation adjustments
|
(6,088
|
)
|
453
|
|
2,268
|
|
349
|
|
862
|
|
|||||||||
|
Comprehensive income attributable to Masimo Corporation stockholders
|
$
|
66,430
|
|
$
|
58,834
|
|
$
|
64,540
|
|
$
|
64,049
|
|
$
|
74,392
|
|
||||
|
Net income per common share attributable to Masimo Corporation stockholders
(3)
:
|
|||||||||||||||||||
|
Basic
|
$
|
1.33
|
|
$
|
1.03
|
|
$
|
1.08
|
|
$
|
1.07
|
|
$
|
1.25
|
|
||||
|
Diluted
|
$
|
1.30
|
|
$
|
1.02
|
|
$
|
1.07
|
|
$
|
1.05
|
|
$
|
1.21
|
|
||||
|
Weighted-average number of common shares:
|
|||||||||||||||||||
|
Basic
|
54,708
|
|
56,690
|
|
57,445
|
|
59,659
|
|
58,769
|
|
|||||||||
|
Diluted
|
55,571
|
|
57,480
|
|
58,374
|
|
60,845
|
|
60,609
|
|
|||||||||
|
(1)
|
Pursuant to authoritative accounting guidance, our variable interest entity, Cercacor, is consolidated within our financial statements. Accordingly, all intercompany royalties, option and licensing fees, and other charges between us and Cercacor have been eliminated in the consolidation. For additional discussion of accounting for Cercacor, see Note 3 to our accompanying consolidated financial statements.
|
|
(2)
|
During the year ended January 1, 2011, we completed negotiations to resolve the merits of our antitrust litigation with Covidien. As a result, we recovered a total of $30.8 million in litigation expenses from Covidien.
|
|
(3)
|
See Note 2 to our accompanying consolidated financial statements for a description of the method used to compute basic and diluted net income per common share.
|
|
January 3,
2015 |
December 28,
2013 |
December 29,
2012 |
December 31,
2011 |
January 1,
2011 |
|||||||||||||||
|
(in thousands, except dividends declared per common share)
|
|||||||||||||||||||
|
Balance Sheet Data:
|
|||||||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
134,453
|
|
$
|
95,466
|
|
$
|
71,554
|
|
$
|
129,882
|
|
$
|
88,305
|
|
||||
|
Working capital
|
191,247
|
|
168,008
|
|
129,808
|
|
186,982
|
|
147,408
|
|
|||||||||
|
Total assets
|
565,006
|
|
438,662
|
|
374,661
|
|
366,104
|
|
310,235
|
|
|||||||||
|
Total debt
|
125,224
|
|
336
|
|
115
|
|
122
|
|
172
|
|
|||||||||
|
Total equity
|
307,741
|
|
326,401
|
|
275,668
|
|
279,666
|
|
230,039
|
|
|||||||||
|
Dividends declared per common share
(4)
|
$
|
—
|
|
$
|
—
|
|
$
|
1.00
|
|
$
|
—
|
|
$
|
2.75
|
|
||||
|
(4)
|
During the years ended December 29, 2012 and January 1, 2011, our Board evaluated a variety of options to return value to stockholders, including acquisition opportunities, stock buy-back programs and dividends. After considering all available options during those periods, our Board concluded that the best and most direct way to reward stockholders for their continued investment and confidence in Masimo was through the declaration of three special cash dividends. In February 2010, our Board declared a special dividend of $2.00 per share, or $117.5 million, which was paid in March 2010. In November 2010, our Board declared a second special dividend of $0.75 per share, or $44.5 million, which was paid in December 2010. In October 2012, our Board declared a third special dividend of $1.00 per share, or $57.3 million, which was paid in December 2012.
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
||||||||||||||||||
|
Amount
|
% of
Revenue
|
Amount
|
% of
Revenue
|
Amount
|
% of
Revenue
|
|||||||||||||||
|
(dollars in thousands)
|
||||||||||||||||||||
|
Revenue:
|
||||||||||||||||||||
|
Product
|
$
|
556,764
|
|
94.9
|
%
|
$
|
517,429
|
|
94.6
|
%
|
$
|
464,928
|
|
94.3
|
%
|
|||||
|
Royalty
|
29,879
|
|
5.1
|
|
29,816
|
|
5.4
|
|
28,305
|
|
5.7
|
|
||||||||
|
Total revenue
|
586,643
|
|
100.0
|
|
547,245
|
|
100.0
|
|
493,233
|
|
100.0
|
|
||||||||
|
Cost of goods sold
|
195,864
|
|
33.4
|
|
188,418
|
|
34.4
|
|
166,982
|
|
33.9
|
|
||||||||
|
Gross profit
|
390,779
|
|
66.6
|
|
358,827
|
|
65.6
|
|
326,251
|
|
66.1
|
|
||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Selling, general and administrative
|
241,016
|
|
41.1
|
|
215,469
|
|
39.4
|
|
193,948
|
|
39.3
|
|
||||||||
|
Research and development
|
56,581
|
|
9.6
|
|
55,631
|
|
10.2
|
|
47,077
|
|
9.5
|
|
||||||||
|
Litigation award and defense costs
|
(10,331
|
)
|
(1.8
|
)
|
8,010
|
|
1.5
|
|
—
|
|
—
|
|
||||||||
|
Total operating expenses
|
287,266
|
|
49.0
|
|
279,110
|
|
51.0
|
|
241,025
|
|
48.9
|
|
||||||||
|
Operating income
|
103,513
|
|
17.6
|
|
79,717
|
|
14.6
|
|
85,226
|
|
17.3
|
|
||||||||
|
Non-operating expense
|
1,472
|
|
0.3
|
|
3,991
|
|
0.7
|
|
1,405
|
|
0.3
|
|
||||||||
|
Income before provision for income taxes
|
102,041
|
|
17.4
|
|
75,726
|
|
13.8
|
|
83,821
|
|
17.0
|
|
||||||||
|
Provision for income taxes
|
27,678
|
|
4.7
|
|
20,005
|
|
3.7
|
|
21,883
|
|
4.4
|
|
||||||||
|
Net income including noncontrolling interests
|
74,363
|
|
12.7
|
|
55,721
|
|
10.2
|
|
61,938
|
|
12.6
|
|
||||||||
|
Net income (loss) attributable to noncontrolling interests
|
1,845
|
|
0.3
|
|
(2,660
|
)
|
(0.5
|
)
|
(334
|
)
|
(0.1
|
)
|
||||||||
|
Net income attributable to Masimo Corporation stockholders
|
$
|
72,518
|
|
12.4
|
%
|
$
|
58,381
|
|
10.7
|
%
|
$
|
62,272
|
|
12.6
|
%
|
|||||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Increase/
(Decrease)
|
Percentage
Change
|
|||||||||||||||||
|
North and South America
|
$
|
398,066
|
|
71.5
|
%
|
$
|
378,894
|
|
73.2
|
%
|
$
|
19,172
|
|
5.1
|
%
|
|||||
|
Europe, Middle East and Africa
|
100,747
|
|
18.1
|
|
83,338
|
|
16.1
|
|
17,409
|
|
20.9
|
|
||||||||
|
Asia and Australia
|
57,951
|
|
10.4
|
|
55,197
|
|
10.7
|
|
2,754
|
|
5.0
|
|
||||||||
|
Total Product Revenue
|
$
|
556,764
|
|
100.0
|
%
|
$
|
517,429
|
|
100.0
|
%
|
$
|
39,335
|
|
7.6
|
%
|
|||||
|
Royalty
|
29,879
|
|
29,816
|
|
63.0
|
|
||||||||||||||
|
Total Revenue
|
$
|
586,643
|
|
$
|
547,245
|
|
$
|
39,398
|
|
|||||||||||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Increase/
(Decrease) |
Percentage
Change |
|||||||||||||||||
|
Direct/Distribution
|
$
|
472,711
|
|
84.9
|
%
|
$
|
438,819
|
|
84.8
|
%
|
$
|
33,892
|
|
7.7
|
%
|
|||||
|
OEM
|
84,053
|
|
15.1
|
|
78,610
|
|
15.2
|
|
5,443
|
|
6.9
|
|
||||||||
|
Total Product Revenue
|
$
|
556,764
|
|
100.0
|
%
|
$
|
517,429
|
|
100.0
|
%
|
$
|
39,335
|
|
7.6
|
%
|
|||||
|
Gross Profit
|
||||||||||||||||||||
|
Year ended
January 3, 2015 |
Percentage of
Net Revenues
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues
|
Increase/
(Decrease) |
Percentage
Change |
|||||||||||||||
|
Product Gross Profit
|
$
|
360,900
|
|
64.8
|
%
|
$
|
329,011
|
|
63.6
|
%
|
$
|
31,889
|
|
9.7
|
%
|
|||||
|
Royalty Gross Profit
|
29,879
|
|
100.0
|
|
29,816
|
|
100.0
|
|
63
|
|
0.2
|
|
||||||||
|
Total Gross Profit
|
$
|
390,779
|
|
66.6
|
%
|
$
|
358,827
|
|
65.6
|
%
|
$
|
31,952
|
|
8.9
|
%
|
|||||
|
Selling, General and Administrative
|
|||||
|
Year ended
January 3, 2015 |
Percentage of
Net Revenues |
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$241,016
|
41.1%
|
$215,469
|
39.4%
|
$25,547
|
11.9%
|
|
Research and Development
|
|||||
|
Year ended
January 3, 2015 |
Percentage of
Net Revenues |
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$56,581
|
9.6%
|
$55,631
|
10.2%
|
$950
|
1.7%
|
|
Litigation Award and Defense Costs
|
|||||
|
Year ended
January 3, 2015 |
Percentage of
Net Revenues |
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$(10,331)
|
(1.8)%
|
$8,010
|
1.5%
|
$(18,341)
|
(229.0)%
|
|
Non-operating expense
|
|||||
|
Year ended
January 3, 2015 |
Percentage of
Net Revenues |
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$1,472
|
0.3%
|
$3,991
|
0.7%
|
$(2,519)
|
(63.1)%
|
|
Provision for Income Taxes
|
|||||
|
Year ended
January 3, 2015 |
Percentage of
Net Revenues |
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$27,678
|
4.7%
|
$20,005
|
3.7%
|
$7,673
|
38.4%
|
|
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
Increase/
(Decrease) |
Percentage
Change |
|||||||||||||||||
|
North and South America
|
$
|
378,894
|
|
73.2
|
%
|
$
|
341,672
|
|
73.5
|
%
|
$
|
37,222
|
|
10.9
|
%
|
|||||
|
Europe, Middle East and Africa
|
83,338
|
|
16.1
|
|
68,010
|
|
14.6
|
|
15,328
|
|
22.5
|
|
||||||||
|
Asia and Australia
|
55,197
|
|
10.7
|
|
55,246
|
|
11.9
|
|
(49
|
)
|
(0.1
|
)
|
||||||||
|
Total Product Revenue
|
$
|
517,429
|
|
100.0
|
%
|
$
|
464,928
|
|
100.0
|
%
|
$
|
52,501
|
|
11.3
|
%
|
|||||
|
Royalty
|
29,816
|
|
28,305
|
|
1,511
|
|
||||||||||||||
|
Total Revenue
|
$
|
547,245
|
|
$
|
493,233
|
|
$
|
54,012
|
|
|||||||||||
|
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
Increase/
(Decrease) |
Percentage
Change |
|||||||||||||||||
|
Direct/Distribution
|
$
|
438,819
|
|
84.8
|
%
|
$
|
396,218
|
|
85.2
|
%
|
$
|
42,601
|
|
10.8
|
%
|
|||||
|
OEM
|
78,610
|
|
15.2
|
|
68,710
|
|
14.8
|
|
9,900
|
|
14.4
|
|
||||||||
|
Total Product Revenue
|
$
|
517,429
|
|
100.0
|
%
|
$
|
464,928
|
|
100.0
|
%
|
$
|
52,501
|
|
11.3
|
%
|
|||||
|
Gross Profit
|
||||||||||||||||||||
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues
|
Year ended
December 29, 2012 |
Percentage of
Net Revenues
|
Increase/
(Decrease) |
Percentage
Change |
|||||||||||||||
|
Product Gross Profit
|
$
|
329,011
|
|
63.6
|
%
|
$
|
297,946
|
|
64.1
|
%
|
$
|
31,065
|
|
10.4
|
%
|
|||||
|
Royalty Gross Profit
|
29,816
|
|
100.0
|
|
28,305
|
|
100.0
|
|
1,511
|
|
5.3
|
|
||||||||
|
Total Gross Profit
|
$
|
358,827
|
|
65.6
|
%
|
$
|
326,251
|
|
66.1
|
%
|
$
|
32,576
|
|
10.0
|
%
|
|||||
|
Selling, General and Administrative
|
|||||
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Year ended
December 29, 2012 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$215,469
|
39.4%
|
$193,948
|
39.3%
|
$21,521
|
11.1%
|
|
Research and Development
|
|||||
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Year ended
December 29, 2012 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$55,631
|
10.2%
|
$47,077
|
9.5%
|
$8,554
|
18.2%
|
|
Litigation Award and Defense Costs
|
|||||
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Year ended
December 29, 2012 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$8,010
|
1.5%
|
$—
|
—%
|
$8,010
|
100%
|
|
Non-operating expense
|
|||||
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Year ended
December 29, 2012 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$3,991
|
0.7%
|
$1,405
|
0.3%
|
$2,586
|
184.1%
|
|
Provision for Income Taxes
|
|||||
|
Year ended
December 28, 2013 |
Percentage of
Net Revenues |
Year ended
December 29, 2012 |
Percentage of
Net Revenues |
Increase/
(Decrease) |
Percentage
Change |
|
$20,005
|
3.7%
|
$21,883
|
4.4%
|
$(1,878)
|
(8.6)%
|
|
The following table summarizes our cash flows (in thousands):
|
||||||||
|
Year Ended
|
||||||||
|
January 3, 2015
|
December 28, 2013
|
|||||||
|
Net cash provided by (used in):
|
||||||||
|
Operating activities
|
$
|
95,459
|
|
$
|
54,587
|
|
||
|
Investing activities
|
(78,414
|
)
|
(13,286
|
)
|
||||
|
Financing activities
|
26,246
|
|
(17,941
|
)
|
||||
|
Effect of foreign currency exchange rates on cash
|
(4,304
|
)
|
552
|
|
||||
|
Increase in cash and cash equivalents
|
$
|
38,987
|
|
$
|
23,912
|
|
||
|
Payments Due By Period
|
|||||||||||||||||||
|
Less than
1 year
|
Between
1-3 years
|
Between
3-5 years
|
More than
5 years
|
Total
|
|||||||||||||||
|
Operating Leases
(1)
|
$
|
4,990
|
|
$
|
6,584
|
|
$
|
3,800
|
|
$
|
1,033
|
|
$
|
16,407
|
|
||||
|
Capital Leases (including interest)
(2)
|
87
|
|
155
|
|
—
|
|
—
|
|
242
|
|
|||||||||
|
Line of credit
|
—
|
|
—
|
|
125,000
|
|
—
|
|
125,000
|
|
|||||||||
|
Purchase Commitments
(3)
|
67,085
|
|
—
|
|
—
|
|
—
|
|
67,085
|
|
|||||||||
|
Total Contractual Obligations
|
$
|
72,162
|
|
$
|
6,739
|
|
$
|
128,800
|
|
$
|
1,033
|
|
$
|
208,734
|
|
||||
|
(1)
|
Facility, equipment and automobile leases.
|
|
(2)
|
Leased office equipment.
|
|
(3)
|
Certain inventory items under non-cancellable purchase orders.
|
|
Payments Due By Period
|
|||||||||||||
|
Less than
1 year
|
Between
1-3 years
|
Between
3-5 years
|
More than
5 years
|
||||||||||
|
Minimum royalty commitment to Cercacor
|
$
|
5,000
|
|
$
|
10,000
|
|
$
|
10,000
|
|
(1)
|
|||
|
(1)
|
Subsequent to 2019, the royalty arrangement requires a $5.0 million minimum annual royalty payment unless the agreement is amended, restated or terminated.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
Exhibit
Number
|
Description of Document
|
|
|
3.1(1)
|
Amended and Restated Certificate of Incorporation (Exhibit 3.2)
|
|
|
3.2(2)
|
Certificate of Designation of Series A Junior Participating Preferred Stock (Exhibit 3.1)
|
|
|
3.3(11)
|
Amended and Restated Bylaws (Exhibit 3.2)
|
|
|
4.1(1)
|
Form of Common Stock Certificate (Exhibit 4.1)
|
|
|
4.2(1)
|
Fifth Amended and Restated Registration Rights Agreement made and entered into as of September 14, 1999 between the Registrant and certain of its stockholders (Exhibit 4.2)
|
|
|
4.3(2)
|
Rights Agreement, dated November 9, 2007, between the Registrant and Computershare Trust Company, N.A., as Rights Agent (Exhibit 4.1)
|
|
|
4.4(4)#
|
Masimo Retirement Savings Plan (Exhibit 4.7)
|
|
|
10.1(1)#
|
Form of Indemnity Agreement between the Registrant and its officers and directors (Exhibit 10.1)
|
|
|
10.2(5)#
|
Amended and Restated Employment Agreement, dated February 7, 2012, between Joe Kiani and the Registrant) (Exhibit 10.2)
|
|
|
10.3(1)#
|
Offer Letter, dated February 15, 1996, between Yongsam Lee and the Registrant (Exhibit 10.7)
|
|
|
10.4(6)#
|
Offer Letter, dated May 21, 2004, between Rick Fishel and the Registrant (Exhibit 10.13)
|
|
|
10.5(1)#
|
Offer Letter, dated June 9, 2006, between Mark P. de Raad and the Registrant (Exhibit 10.9)
|
|
|
10.6(1)#
|
Offer Letter, dated March 30, 2007, between Anand Sampath and the Registrant (Exhibit 10.8)
|
|
|
10.7(6)#
|
Offer Letter, dated July 23, 2008, between Jon Coleman and the Registrant (Exhibit 10.9)
|
|
|
10.8(9)#
|
Offer Letter, dated December 27, 2007 between Paul Jansen and the Registrant (Exhibit 10.8)
|
|
|
10.9(9)#
|
Offer Letter, dated March 31, 2010 between Tom McClenahan and the Registrant (Exhibit 10.8)
|
|
|
10.10(10)#
|
Executive Annual Cash Bonus Award Plan, effective January 1, 2007 (Exhibit 10.2)
|
|
|
10.11*#
|
Executive Restated Annual Cash Bonus Award Plan, effective March 13, 2014
|
|
|
10.12*#
|
Executive Multi-Year Cash Bonus Award Plan, effective March 13, 2014
|
|
|
10.13(8)#
|
CEO and Executive Officer Equity Award Compensation Policy, effective January 4, 2008 (Exhibit 10.53)
|
|
|
10.14(9)#
|
Amended and Restated 2007 Severance Protection Plan and Summary Plan Description, effective December 31, 2008 (Exhibit 10.13)
|
|
|
10.15(10)#
|
2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between the Registrant and Mark P. de Raad (Exhibit 10.2)
|
|
|
Exhibit
Number
|
Description of Document
|
||
|
10.16(10)#
|
2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between the Registrant and Yongsam Lee (Exhibit 10.3)
|
||
|
10.17(6)#
|
2007 Severance Protection Plan Participation Agreement, dated January 11, 2008, by and between the Registrant and Rick Fishel (Exhibit 10.57)
|
||
|
10.18(9)#
|
Amended and Restated 2007 Severance Protection Plan Agreement, dated November 12, 2013, by and between the Registrant and Jon Coleman (Exhibit 10.17)
|
||
|
10.19(9)#
|
Amended and Restated 2007 Severance Protection Plan Agreement, dated December 9, 2013, by and between the Registrant and Anand Sampath (Exhibit 10.18)
|
||
|
10.20(9)#
|
Amended and Restated 2007 Severance Protection Plan Agreement, dated November 12, 2013, by and between the Registrant and Paul Jansen (Exhibit 10.19)
|
||
|
10.21#*
|
Amended and Restated 2007 Severance Protection Plan Agreement, dated November 3, 2014, by and between the Registrant and Tom McClenahan
|
||
|
10.22(1)#
|
Third Amended and Restated 1996 Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan of the Registrant, as amended, and forms of agreements related thereto (Exhibit 10.31)
|
||
|
10.23(1)#
|
2004 Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan of the Registrant, as amended, and forms of agreements related thereto (Exhibit 10.32)
|
||
|
10.24(1)#
|
2007 Stock Incentive Plan of the Registrant, and forms of agreements related thereto (Exhibit 10.33)
|
||
|
10.25(1)+
|
Purchase Agreement, dated July 26, 2001, between Jabil Circuit, Inc. and the Registrant (Exhibit 10.15)
|
||
|
10.26(1)+
|
Shelter Labor Services Agreement, dated December 27, 2000, between Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant (Exhibit 10.11)
|
||
|
10.27(11)+
|
Lease Agreement effective as of September 1, 2007, by and among Industrias Asociadas Maquiladoras, S.A. de C.V., Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant, as guarantor (Exhibit 10.1)
|
||
|
10.28++
|
First Amendment, Lease Agreement effective as of December 17, 2013, by and among Industrias Asociadas Maquiladoras, S.A. de C.V., Industrial Vallera de Mexicali, S.A. de C.V. and the Registrant, as guarantor
|
||
|
10.29(12)+
|
Lease Agreement, relating to the premises at 40 Parker, effective as of November 1, 2009, between the Registrant and Northwestern Mutual Life Insurance Company (Exhibit 10.1)
|
||
|
10.30*++
|
Amendment No. 3
to the November 1, 2009
Lease Agreement, relating to the premises at 40 Parker, between the Registrant and Northwestern Mutual Life Insurance Company
|
||
|
10.31(12)+
|
Amendment No. 1 to Lease Agreement, relating to the premises at 50 Parker, dated April 30, 2009, between the Registrant and Northwestern Mutual Life Insurance Company (Exhibit 10.3)
|
||
|
10.32*++
|
Amendment to August 1, 2009 Lease Agreement, related to the premises at 50 Parker, between the Registrant and Northwestern Mutual Life Insurance Company
|
||
|
10.33(12)+
|
Lease Agreement, relating to the premises at 60 Parker, effective as of August 1, 2009, between the Registrant and Northwestern Mutual Life Insurance Company (Exhibit 10.2)
|
||
|
10.34*++
|
Second Amendment to June 22, 2012 Lease Agreement, relating to the premises at 9600 Jeronimo, between the Registrant and Irvine Company, LLC
|
||
|
10.35(1)
|
Settlement Agreement and Release of Claims, dated January 17, 2006, between Cercacor Laboratories, Inc., Nellcor Puritan Bennett, Inc., Mallinckrodt, Inc., Tyco Healthcare Group LP, Tyco International Ltd., Tyco International (US) Inc. and the Registrant (Exhibit 10.30)
|
||
|
10.36(13)
|
Second Amendment to the January 17, 2006 Settlement Agreement and Release of Claims, as amended pursuant to the January 24, 2006 Amendment to Settlement Agreement and Release of Claims, dated January 28, 2011, by and among Masimo Corporation, Masimo Laboratories, Inc., Nellcor Puritan Bennett LLC, Mallinckrodt Inc., Tyco Healthcare Group LP and Covidien Inc. (Exhibit 10.1)
|
||
|
10.37(1)+
|
Amended and Restated Cross-Licensing Agreement, effective January 1, 2007, between Cercacor Laboratories, Inc. and the Registrant (Exhibit 10.34)
|
||
|
10.38(1)
|
Services Agreement, effective January 1, 2007, between Cercacor Laboratories, Inc. and the Registrant (Exhibit 10.35)
|
|
|
10.39(14)
|
Agreement of Purchase and Sale and Escrow Instructions, dated as of November 1, 2013, by and between the Company and Nikken, Inc. (Exhibit 10.1)
|
|
|
10.40(14)
|
First Amendment to Purchase and Sale Agreement, made and entered into effective as of January 8, 2014, by and between the Company and Nikken, Inc. (Exhibit 10.2)
|
|
|
10.41(14)
|
Second Amendment to Purchase and Sale Agreement, made and entered into effective as of January 10, 2014, by and between the Company and Nikken, Inc. (Exhibit 10.3)
|
|
|
10.42(14)
|
Third Amendment to Purchase and Sale Agreement, made and entered into effective as of March 10, 2014, by and between the Company and Nikken, Inc. (Exhibit 10.4)
|
|
|
10.43(14)
|
Fourth Amendment to Purchase and Sale Agreement, made and entered into effective as of March 12, 2014, by and between the Company and Nikken, Inc. (Exhibit 10.5)
|
|
|
10.44(14)++
|
Credit Agreement dated as of April 23, 2014, among Masimo Corporation, the lenders party thereto and JPMorgan Chase Bank, National Association, as administrative agent (Exhibit 10.1)
|
|
|
10.45*++
|
Amendment No. 1 to Credit Agreement, dated as of September 29, 2014, among Masimo Corporation, and the Lenders party hereto and JPMorgan Chase Bank, N.A., as Administrative Agent
|
|
|
12.1*
|
Statement Regarding the Computation of Ratio of Earnings to Fixed Charges
|
|
|
21.1*
|
List of Registrant’s subsidiaries
|
|
|
23.1*
|
Consent of Independent Registered Public Accounting Firm
|
|
|
31.1*
|
Certification of Joe Kiani, Chief Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
31.2*
|
Certification of Mark P. de Raad, Chief Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.1*
|
Certification of Joe Kiani, Chief Executive Officer, and Mark P. de Raad, Chief Financial Officer, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
(1)
|
Incorporated by reference to the exhibits to the Registrant’s Registration Statement on Form S-1 (No. 333-142171), originally filed on April 17, 2007. The number given in parenthesis indicates the corresponding exhibit number in such Form S-1, as amended.
|
|
(2)
|
Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, filed on November 9, 2007. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K.
|
|
(3)
|
Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, filed on October 26, 2011. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K.
|
|
(4)
|
Incorporated by reference to the exhibit to the Registrant’s Registration Statement on Form S-8, filed on February 11, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form S-8.
|
|
(5)
|
Incorporated by reference to the exhibit to the Registrant’s Annual Report on Form 10-K, filed on February 17, 2012. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-K.
|
|
(6)
|
Incorporated by reference to the exhibit to the Registrant’s Annual Report on Form 10-K, filed on March 4, 2009. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-K.
|
|
(7)
|
Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, filed on May 4, 2011. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-Q.
|
|
(8)
|
Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q filed on August 1, 2013. The number given in parentheses indicates the corresponding exhibit number in such Form 10-Q.
|
|
(9)
|
Incorporated by reference to the exhibit to the Registrant’s Annual Report on Form 10-K filed on February 15, 2013. The number given in parentheses indicates the corresponding exhibit number in such Form 10-K.
|
|
(10)
|
Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, filed on January 17, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K.
|
|
(11)
|
Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, filed on June 5, 2008. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K.
|
|
(12)
|
Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, filed on November 4, 2009. The number given in parenthesis indicates the corresponding exhibit number in such Form 10-Q.
|
|
(13)
|
Incorporated by reference to the exhibit to the Registrant’s Current Report on Form 8-K, filed on January 31, 2011. The number given in parenthesis indicates the corresponding exhibit number in such Form 8-K.
|
|
(14)
|
Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, filed on May 1, 2014. The number given in parenthesis indicates the corresponding exhibit number fin such Form 10-Q.
|
|
(15)
|
Incorporated by reference to the exhibit to the Registrant’s Quarterly Report on Form 10-Q, filed on August 7, 2014. The number given in parenthesis indicates the corresponding exhibit number fin such Form 10-Q.
|
|
*
|
Filed herewith.
|
|
#
|
Indicates management contract or compensatory plan.
|
|
+
|
The SEC has granted confidential treatment with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
++
|
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
Date:
|
February 17, 2015
|
By:
|
/s/ J
OE
K
IANI
|
|
|
Joe Kiani
Chairman of the Board & Chief Executive Officer
|
||||
|
SIGNATURE
|
TITLE(S)
|
DATE
|
||
|
/s/ J
OE
K
IANI
|
Chairman of the Board & Chief Executive Officer (Principal Executive Officer)
|
February 17, 2015
|
||
|
Joe Kiani
|
||||
|
/s/ M
ARK
P.
DE
. R
AAD
|
Executive Vice President & Chief Financial Officer (Principal Financial and Accounting Officer)
|
February 17, 2015
|
||
|
Mark P. de Raad
|
||||
|
/s/ S
TEVEN
B
ARKER
, M.D. P
H
.D.
|
Director
|
February 17, 2015
|
||
|
Steven Barker, M.D., Ph.D.
|
||||
|
/s/ R
OBERT
C
OLEMAN
, P
H
.D.
|
Director
|
February 17, 2015
|
||
|
Robert Coleman, Ph.D.
|
||||
|
/s/ S
ANFORD
F
ITCH
|
Director
|
February 17, 2015
|
||
|
Sanford Fitch
|
||||
|
/s/ J
ACK
L
ASERSOHN
|
Director
|
February 17, 2015
|
||
|
Jack Lasersohn
|
||||
|
/s/ C
RAIG
R
EYNOLDS
|
Director
|
February 17, 2015
|
||
|
Craig Reynolds
|
||||
|
|
|
|
Consolidated Financial Statements
|
|
|
Schedule
|
|
|
January 3,
2015 |
December 28,
2013 |
||||||
|
ASSETS
|
|||||||
|
Current assets
|
|||||||
|
Cash and cash equivalents
|
$
|
134,453
|
|
$
|
95,466
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $1,890 and $1,833 at January 3, 2015 and December 28, 2013, respectively
|
71,017
|
|
76,759
|
|
|||
|
Inventories
|
69,718
|
|
56,813
|
|
|||
|
Prepaid income taxes
|
417
|
|
3,740
|
|
|||
|
Other current assets
|
21,471
|
|
19,384
|
|
|||
|
Deferred income taxes, current
|
18,065
|
|
19,636
|
|
|||
|
Total current assets
|
315,141
|
|
271,798
|
|
|||
|
Deferred cost of goods sold
|
67,485
|
|
61,714
|
|
|||
|
Property and equipment, net
|
101,952
|
|
24,866
|
|
|||
|
Intangible assets, net
|
27,771
|
|
28,104
|
|
|||
|
Goodwill
|
20,979
|
|
22,793
|
|
|||
|
Deferred income taxes, noncurrent
|
24,193
|
|
22,565
|
|
|||
|
Other assets
|
7,485
|
|
6,822
|
|
|||
|
Total assets
|
$
|
565,006
|
|
$
|
438,662
|
|
|
|
LIABILITIES AND EQUITY
|
|||||||
|
Current liabilities
|
|||||||
|
Accounts payable
|
$
|
38,045
|
|
$
|
28,004
|
|
|
|
Accrued compensation
|
33,600
|
|
29,486
|
|
|||
|
Accrued liabilities
|
24,541
|
|
23,028
|
|
|||
|
Income taxes payable
|
6,562
|
|
2,406
|
|
|||
|
Deferred revenue
|
21,067
|
|
20,755
|
|
|||
|
Current portion of capital lease obligations
|
79
|
|
111
|
|
|||
|
Total current liabilities
|
123,894
|
|
103,790
|
|
|||
|
Deferred revenue
|
453
|
|
566
|
|
|||
|
Long term debt
|
125,145
|
|
225
|
|
|||
|
Other liabilities
|
7,773
|
|
7,680
|
|
|||
|
Total liabilities
|
257,265
|
|
112,261
|
|
|||
|
Commitments and contingencies (Note 15)
|
|
|
|||||
|
Equity
|
|||||||
|
Masimo Corporation stockholders’ equity:
|
|||||||
|
Preferred stock, $0.001 par value; 5,000 shares authorized at January 3, 2015 and December 28, 2013; 0 shares issued and outstanding at January 3, 2015 and December 28, 2013
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value, 100,000 shares authorized at January 3, 2015 and December 28, 2013; 52,594 and 56,623 shares outstanding at January 3, 2015 and December 28, 2013, respectively
|
52
|
|
57
|
|
|||
|
Treasury stock, 8,611 and 4,156 shares at January 3, 2015 and December 28, 2013, respectively
|
(185,906
|
)
|
(83,454
|
)
|
|||
|
Additional paid-in capital
|
288,686
|
|
273,129
|
|
|||
|
Accumulated other comprehensive (loss) income
|
(2,093
|
)
|
3,995
|
|
|||
|
Retained earnings
|
205,260
|
|
132,742
|
|
|||
|
Total Masimo Corporation stockholders’ equity
|
305,999
|
|
326,469
|
|
|||
|
Noncontrolling interest
|
1,742
|
|
(68
|
)
|
|||
|
Total equity
|
307,741
|
|
326,401
|
|
|||
|
Total liabilities and equity
|
$
|
565,006
|
|
$
|
438,662
|
|
|
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
|||||||||
|
Revenue:
|
|||||||||||
|
Product
|
$
|
556,764
|
|
$
|
517,429
|
|
$
|
464,928
|
|
||
|
Royalty
|
29,879
|
|
29,816
|
|
28,305
|
|
|||||
|
Total revenue
|
586,643
|
|
547,245
|
|
493,233
|
|
|||||
|
Cost of goods sold
|
195,864
|
|
188,418
|
|
166,982
|
|
|||||
|
Gross profit
|
390,779
|
|
358,827
|
|
326,251
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Selling, general and administrative
|
241,016
|
|
215,469
|
|
193,948
|
|
|||||
|
Research and development
|
56,581
|
|
55,631
|
|
47,077
|
|
|||||
|
Litigation award and defense costs
|
(10,331
|
)
|
8,010
|
|
—
|
|
|||||
|
Total operating expenses
|
287,266
|
|
279,110
|
|
241,025
|
|
|||||
|
Operating income
|
103,513
|
|
79,717
|
|
85,226
|
|
|||||
|
Non-operating expense
|
1,472
|
|
3,991
|
|
1,405
|
|
|||||
|
Income before provision for income taxes
|
102,041
|
|
75,726
|
|
83,821
|
|
|||||
|
Provision for income taxes
|
27,678
|
|
20,005
|
|
21,883
|
|
|||||
|
Net income including noncontrolling interest
|
74,363
|
|
55,721
|
|
61,938
|
|
|||||
|
Net income (loss) attributable to noncontrolling interest
|
1,845
|
|
(2,660
|
)
|
(334
|
)
|
|||||
|
Net income attributable to Masimo Corporation stockholders
|
72,518
|
|
58,381
|
|
62,272
|
|
|||||
|
Other comprehensive (loss) income, net of tax:
|
|||||||||||
|
Foreign currency translation adjustments
|
(6,088
|
)
|
453
|
|
2,268
|
|
|||||
|
Comprehensive income attributable to Masimo Corporation stockholders
|
$
|
66,430
|
|
$
|
58,834
|
|
$
|
64,540
|
|
||
|
Net income per share attributable to Masimo Corporation stockholders:
|
|||||||||||
|
Basic
|
$
|
1.33
|
|
$
|
1.03
|
|
$
|
1.08
|
|
||
|
Diluted
|
$
|
1.30
|
|
$
|
1.02
|
|
$
|
1.07
|
|
||
|
Weighted-average shares used in per share calculations:
|
|||||||||||
|
Basic
|
54,708
|
|
56,690
|
|
57,445
|
|
|||||
|
Diluted
|
55,571
|
|
57,480
|
|
58,374
|
|
|||||
|
Cash dividend declared per share
|
$
|
—
|
|
$
|
—
|
|
$
|
1.00
|
|
||
|
Masimo Corporation Stockholders
|
|||||||||||||||||||||||||||||||||
|
Common Stock
|
Treasury Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
Income
|
Retained
Earnings
|
Noncontrolling
Interest
|
Total
Equity
|
|||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||
|
Balance at December 31, 2011
|
58,247
|
|
$
|
58
|
|
2,001
|
|
$
|
(37,396
|
)
|
$
|
243,528
|
|
$
|
1,274
|
|
$
|
69,364
|
|
$
|
2,838
|
|
$
|
279,666
|
|
||||||||
|
Stock options exercised
|
216
|
|
—
|
|
—
|
|
—
|
|
1,642
|
|
—
|
|
—
|
|
—
|
|
1,642
|
|
|||||||||||||||
|
Income tax deficit from exercise of stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
(410
|
)
|
—
|
|
—
|
|
—
|
|
(410
|
)
|
|||||||||||||||
|
Compensation related to stock option grants to employees
|
—
|
|
—
|
|
—
|
|
—
|
|
14,022
|
|
—
|
|
—
|
|
75
|
|
14,097
|
|
|||||||||||||||
|
Repurchases of common stock
|
(1,155
|
)
|
(1
|
)
|
1,155
|
|
(26,268
|
)
|
1
|
|
—
|
|
—
|
|
—
|
|
(26,268
|
)
|
|||||||||||||||
|
Dividend declared
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(57,275
|
)
|
—
|
|
(57,275
|
)
|
|||||||||||||||
|
Issuance of shares in noncontrolling interest entity, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
10
|
|
10
|
|
|||||||||||||||
|
Net income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
62,272
|
|
(334
|
)
|
61,938
|
|
|||||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,100
|
|
—
|
|
—
|
|
2,100
|
|
|||||||||||||||
|
Income tax benefit on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
168
|
|
—
|
|
—
|
|
168
|
|
|||||||||||||||
|
Balance at December 29, 2012
|
57,308
|
|
57
|
|
3,156
|
|
(63,664
|
)
|
258,783
|
|
3,542
|
|
74,361
|
|
2,589
|
|
275,668
|
|
|||||||||||||||
|
Stock options exercised
|
315
|
|
1
|
|
—
|
|
—
|
|
3,289
|
|
—
|
|
—
|
|
—
|
|
3,290
|
|
|||||||||||||||
|
Income tax deficit from exercise of stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
(615
|
)
|
—
|
|
—
|
|
—
|
|
(615
|
)
|
|||||||||||||||
|
Compensation related to stock option grants to employees
|
—
|
|
—
|
|
—
|
|
—
|
|
11,672
|
|
—
|
|
—
|
|
2
|
|
11,674
|
|
|||||||||||||||
|
Repurchases of common stock
|
(1,000
|
)
|
(1
|
)
|
1,000
|
|
(19,790
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(19,791
|
)
|
|||||||||||||||
|
Issuance of shares in noncontrolling interest entity, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1
|
|
1
|
|
|||||||||||||||
|
Net income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
58,381
|
|
(2,660
|
)
|
55,721
|
|
|||||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
453
|
|
—
|
|
—
|
|
453
|
|
|||||||||||||||
|
Balance at December 28, 2013
|
56,623
|
|
57
|
|
4,156
|
|
(83,454
|
)
|
273,129
|
|
3,995
|
|
132,742
|
|
(68
|
)
|
326,401
|
|
|||||||||||||||
|
Stock options exercised
|
426
|
|
—
|
|
—
|
|
—
|
|
4,683
|
|
—
|
|
—
|
|
—
|
|
4,683
|
|
|||||||||||||||
|
Income tax deficit from exercise of stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
(132
|
)
|
—
|
|
—
|
|
—
|
|
(132
|
)
|
|||||||||||||||
|
Compensation related to stock option grants to employees
|
—
|
|
—
|
|
—
|
|
—
|
|
11,002
|
|
—
|
|
—
|
|
3
|
|
11,005
|
|
|||||||||||||||
|
Repurchases of common stock
|
(4,455
|
)
|
(5
|
)
|
4,455
|
|
(102,452
|
)
|
4
|
|
—
|
|
—
|
|
—
|
|
(102,453
|
)
|
|||||||||||||||
|
Purchase of treasury shares by noncontrolling interest entity, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(38
|
)
|
(38
|
)
|
|||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
72,518
|
|
1,845
|
|
74,363
|
|
|||||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,088
|
)
|
—
|
|
—
|
|
(6,088
|
)
|
|||||||||||||||
|
Income tax benefit on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Balance at January 3, 2015
|
52,594
|
|
$
|
52
|
|
8,611
|
|
$
|
(185,906
|
)
|
$
|
288,686
|
|
$
|
(2,093
|
)
|
$
|
205,260
|
|
$
|
1,742
|
|
$
|
307,741
|
|
||||||||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
|||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income including noncontrolling interest
|
$
|
74,363
|
|
$
|
55,721
|
|
$
|
61,938
|
|
||
|
Adjustments to reconcile net income including noncontrolling interest to net cash provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
12,818
|
|
11,421
|
|
9,369
|
|
|||||
|
Share-based compensation
|
11,005
|
|
11,674
|
|
14,097
|
|
|||||
|
Loss on disposal of property and equipment
|
368
|
|
249
|
|
—
|
|
|||||
|
Provision for doubtful accounts
|
583
|
|
728
|
|
231
|
|
|||||
|
Benefit from deferred income taxes
|
(320
|
)
|
(8,613
|
)
|
(6,806
|
)
|
|||||
|
Income tax benefit from exercise of stock options granted prior to January 1, 2006
|
264
|
|
693
|
|
338
|
|
|||||
|
Excess tax deficit from share-based compensation arrangements
|
396
|
|
1,308
|
|
748
|
|
|||||
|
Realized foreign exchange gain on forward contracts
|
—
|
|
—
|
|
(586
|
)
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Decrease (increase) in accounts receivable
|
4,862
|
|
(9,576
|
)
|
(10,130
|
)
|
|||||
|
(Increase) decrease in inventories
|
(13,434
|
)
|
(9,453
|
)
|
539
|
|
|||||
|
Increase in deferred cost of goods sold
|
(5,888
|
)
|
(9,594
|
)
|
(409
|
)
|
|||||
|
Decrease (increase) in prepaid income taxes
|
3,316
|
|
(1,660
|
)
|
1,255
|
|
|||||
|
Increase in other assets
|
(2,619
|
)
|
(756
|
)
|
(2,035
|
)
|
|||||
|
(Decrease) increase in accounts payable
|
(1,375
|
)
|
1,238
|
|
(2,037
|
)
|
|||||
|
Increase in accrued compensation
|
4,948
|
|
4,557
|
|
4,827
|
|
|||||
|
Increase in accrued liabilities
|
1,837
|
|
6,406
|
|
2,939
|
|
|||||
|
Increase (decrease) in income taxes payable
|
3,909
|
|
(381
|
)
|
198
|
|
|||||
|
Increase in deferred revenue
|
199
|
|
1,467
|
|
2,850
|
|
|||||
|
Increase (decrease) in other liabilities
|
227
|
|
(842
|
)
|
(2,203
|
)
|
|||||
|
Net cash provided by operating activities
|
95,459
|
|
54,587
|
|
75,123
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Purchases of property and equipment
|
(75,061
|
)
|
(9,360
|
)
|
(10,517
|
)
|
|||||
|
Increase in intangible assets
|
(3,353
|
)
|
(3,926
|
)
|
(3,664
|
)
|
|||||
|
Cash paid for acquisitions, net of cash acquired
|
—
|
|
—
|
|
(37,399
|
)
|
|||||
|
Net cash used in investing activities
|
(78,414
|
)
|
(13,286
|
)
|
(51,580
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Borrowings under revolving line of credit
|
125,000
|
|
—
|
|
—
|
|
|||||
|
Debt issuance costs
|
(436
|
)
|
—
|
|
—
|
|
|||||
|
Repayments on capital lease obligations
|
(111
|
)
|
(132
|
)
|
(26
|
)
|
|||||
|
Proceeds from issuance of common stock
|
4,680
|
|
3,289
|
|
1,642
|
|
|||||
|
Excess tax deficit from share-based compensation arrangements
|
(396
|
)
|
(1,308
|
)
|
(748
|
)
|
|||||
|
Dividends paid
|
—
|
|
—
|
|
(57,275
|
)
|
|||||
|
Repurchases of common stock
|
(102,453
|
)
|
(19,790
|
)
|
(26,268
|
)
|
|||||
|
Repurchases of equity by noncontrolling interest, net of equity issued
|
(38
|
)
|
—
|
|
—
|
|
|||||
|
Net proceeds from settlement of forward contracts
|
—
|
|
—
|
|
586
|
|
|||||
|
Net cash provided by (used in) financing activities
|
26,246
|
|
(17,941
|
)
|
(82,089
|
)
|
|||||
|
Effect of foreign currency exchange rates on cash
|
(4,304
|
)
|
552
|
|
218
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
38,987
|
|
23,912
|
|
(58,328
|
)
|
|||||
|
Cash and cash equivalents at beginning of period
|
95,466
|
|
71,554
|
|
129,882
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
134,453
|
|
$
|
95,466
|
|
$
|
71,554
|
|
||
|
•
|
Level 1-Quoted prices in active markets for
identical
assets or liabilities.
|
|
•
|
Level 2-Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for
similar
assets or liabilities, quoted prices in markets that are not active or other inputs that can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
•
|
Level 3-Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
|
|
January 3, 2015
|
Adjusted Basis
Cost |
Gross Unrealized
Gains |
Gross Unrealized
(Losses) |
Estimated
Fair Value |
Cash and Cash
Equivalents |
||||||||||||||
|
Cash
|
$
|
92,888
|
|
$
|
—
|
|
$
|
—
|
|
$
|
92,888
|
|
$
|
92,888
|
|
||||
|
Level 1:
|
|||||||||||||||||||
|
Bank Time Deposits
|
40,500
|
|
—
|
|
—
|
|
40,500
|
|
40,500
|
|
|||||||||
|
U.S. Treasuries
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Money Market Funds
|
1,065
|
|
—
|
|
—
|
|
1,065
|
|
1,065
|
|
|||||||||
|
Subtotal
|
41,565
|
|
—
|
|
—
|
|
41,565
|
|
41,565
|
|
|||||||||
|
Level 2:
|
|||||||||||||||||||
|
None
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Level 3:
|
|||||||||||||||||||
|
None
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total assets measured at fair value
|
$
|
134,453
|
|
$
|
—
|
|
$
|
—
|
|
$
|
134,453
|
|
$
|
134,453
|
|
||||
|
December 28, 2013
|
Adjusted Basis
Cost |
Gross Unrealized
Gains |
Gross Unrealized
(Losses) |
Estimated
Fair Value |
Cash and Cash
Equivalents |
||||||||||||||
|
Cash
|
$
|
67,676
|
|
$
|
—
|
|
$
|
—
|
|
$
|
67,676
|
|
$
|
67,676
|
|
||||
|
Level 1:
|
|||||||||||||||||||
|
Bank Time Deposits
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
U.S. Treasuries
|
25,997
|
|
—
|
|
—
|
|
25,997
|
|
25,997
|
|
|||||||||
|
Money Market Funds
|
1,793
|
|
—
|
|
—
|
|
1,793
|
|
1,793
|
|
|||||||||
|
Subtotal
|
27,790
|
|
—
|
|
—
|
|
27,790
|
|
27,790
|
|
|||||||||
|
Level 2:
|
|||||||||||||||||||
|
None
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Level 3:
|
|||||||||||||||||||
|
None
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total assets measured at fair value
|
$
|
95,466
|
|
$
|
—
|
|
$
|
—
|
|
$
|
95,466
|
|
$
|
95,466
|
|
||||
|
Useful Lives
|
|
|
Building
|
39 years
|
|
Building improvements
|
7 years
|
|
Leasehold improvements
|
Lesser of useful life or term of lease
|
|
Machinery and equipment
|
5 years
|
|
Vehicles
|
5 years
|
|
Tooling
|
3 years
|
|
Computer equipment
|
2 to 6 years
|
|
Furniture and office equipment
|
2 to 6 years
|
|
Demonstration units
|
3 years
|
|
Years Ended
|
|||||||||||
|
January 3,
2015 |
December 28,
2013 |
December 29,
2012 |
|||||||||
|
Warranty accrual, beginning of period
|
$
|
1,161
|
|
$
|
838
|
|
$
|
623
|
|
||
|
Accrued warranties assumed with the acquisition of PHASEIN AB
|
—
|
|
—
|
|
170
|
|
|||||
|
Accrual for warranties issued
|
1,144
|
|
1,560
|
|
639
|
|
|||||
|
Changes in pre-existing warranties (including changes in estimates)
|
138
|
|
52
|
|
128
|
|
|||||
|
Settlements made
|
(1,027
|
)
|
(1,289
|
)
|
(722
|
)
|
|||||
|
Warranty accrual, end of period
|
$
|
1,416
|
|
$
|
1,161
|
|
$
|
838
|
|
||
|
Years ended
|
|||||||||||
|
January 3,
2015 |
December 28,
2013 |
December 29,
2012 |
|||||||||
|
Net income attributable to stockholders of Masimo Corporation:
|
|||||||||||
|
Net income including noncontrolling interest
|
$
|
74,363
|
|
$
|
55,721
|
|
$
|
61,938
|
|
||
|
Net income (loss) attributable to the noncontrolling interest
|
1,845
|
|
(2,660
|
)
|
(334
|
)
|
|||||
|
Net income attributable to Masimo Corporation stockholders
|
$
|
72,518
|
|
$
|
58,381
|
|
$
|
62,272
|
|
||
|
Basic net income per share attributable to Masimo Corporation stockholders:
|
|||||||||||
|
Net income attributable to Masimo Corporation stockholders
|
$
|
72,518
|
|
$
|
58,381
|
|
$
|
62,272
|
|
||
|
Weighted-average shares outstanding - basic
|
54,708
|
|
56,690
|
|
57,445
|
|
|||||
|
Basic net income per share attributable to Masimo Corporation stockholders
|
$
|
1.33
|
|
$
|
1.03
|
|
$
|
1.08
|
|
||
|
Diluted net income per share attributable to Masimo Corporation stockholders:
|
|||||||||||
|
Weighted-average shares outstanding
|
54,708
|
|
56,690
|
|
57,445
|
|
|||||
|
Diluted share equivalent: stock options
|
863
|
|
790
|
|
929
|
|
|||||
|
Weighted-average shares outstanding - diluted
|
55,571
|
|
57,480
|
|
58,374
|
|
|||||
|
Diluted net income per share attributable to Masimo Corporation stockholders
|
$
|
1.30
|
|
$
|
1.02
|
|
$
|
1.07
|
|
||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
||||||||||
|
Cash paid during the year for:
|
||||||||||||
|
Interest (net of amounts capitalized)
|
$
|
469
|
|
$
|
28
|
|
$
|
44
|
|
|||
|
Income taxes
|
$
|
19,863
|
|
$
|
29,979
|
|
$
|
28,691
|
|
|||
|
Noncash investing and financing activities:
|
||||||||||||
|
Assets acquired under capital leases
|
$
|
—
|
|
$
|
352
|
|
$
|
21
|
|
|||
|
Unpaid purchases of property, plant and equipment
|
$
|
12,155
|
|
$
|
507
|
|
$
|
776
|
|
|||
|
January 3, 2015
|
December 28, 2013
|
||||||||||||||||||||||||||||||
|
Balance Sheets:
|
Masimo Corp
|
Cercacor
|
Cercacor Elim
|
Total
|
Masimo Corp
|
Cercacor
|
Cercacor Elim
|
Total
|
|||||||||||||||||||||||
|
ASSETS
|
|||||||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
133,509
|
|
$
|
944
|
|
$
|
—
|
|
$
|
134,453
|
|
$
|
95,296
|
|
$
|
170
|
|
$
|
—
|
|
$
|
95,466
|
|
|||||||
|
Accounts receivable, net
|
71,017
|
|
|
|
|
|
71,017
|
|
76,759
|
|
—
|
|
—
|
|
76,759
|
|
|||||||||||||||
|
Inventories
|
69,718
|
|
—
|
|
—
|
|
69,718
|
|
56,813
|
|
—
|
|
—
|
|
56,813
|
|
|||||||||||||||
|
Prepaid income taxes
|
324
|
|
93
|
|
—
|
|
417
|
|
3,732
|
|
8
|
|
—
|
|
3,740
|
|
|||||||||||||||
|
Deferred income taxes, current
|
18,065
|
|
—
|
|
—
|
|
18,065
|
|
19,636
|
|
—
|
|
—
|
|
19,636
|
|
|||||||||||||||
|
Other current assets
|
21,446
|
|
203
|
|
(178
|
)
|
21,471
|
|
19,207
|
|
177
|
|
—
|
|
19,384
|
|
|||||||||||||||
|
Deferred cost of goods sold
|
67,485
|
|
—
|
|
—
|
|
67,485
|
|
61,714
|
|
—
|
|
—
|
|
61,714
|
|
|||||||||||||||
|
Property and equipment, net
|
100,730
|
|
1,222
|
|
—
|
|
101,952
|
|
22,931
|
|
1,935
|
|
—
|
|
24,866
|
|
|||||||||||||||
|
Intangible assets, net
|
29,564
|
|
4,738
|
|
(6,531
|
)
|
27,771
|
|
30,452
|
|
4,683
|
|
(7,031
|
)
|
28,104
|
|
|||||||||||||||
|
Goodwill
|
20,979
|
|
—
|
|
—
|
|
20,979
|
|
22,793
|
|
—
|
|
—
|
|
22,793
|
|
|||||||||||||||
|
Deferred income taxes, noncurrent
|
24,193
|
|
|
|
—
|
|
24,193
|
|
22,565
|
|
—
|
|
—
|
|
22,565
|
|
|||||||||||||||
|
Other assets
|
7,450
|
|
2,021
|
|
(1,986
|
)
|
7,485
|
|
6,787
|
|
2,021
|
|
(1,986
|
)
|
6,822
|
|
|||||||||||||||
|
Total assets
|
$
|
564,480
|
|
$
|
9,221
|
|
$
|
(8,695
|
)
|
$
|
565,006
|
|
$
|
438,685
|
|
$
|
8,994
|
|
$
|
(9,017
|
)
|
$
|
438,662
|
|
|||||||
|
LIABILITIES
|
|||||||||||||||||||||||||||||||
|
Accounts payable
|
$
|
38,003
|
|
$
|
42
|
|
$
|
—
|
|
$
|
38,045
|
|
$
|
27,418
|
|
$
|
586
|
|
$
|
—
|
|
$
|
28,004
|
|
|||||||
|
Accrued compensation
|
32,985
|
|
615
|
|
—
|
|
33,600
|
|
28,317
|
|
1,169
|
|
—
|
|
29,486
|
|
|||||||||||||||
|
Accrued liabilities
|
24,492
|
|
227
|
|
(178
|
)
|
24,541
|
|
22,888
|
|
140
|
|
—
|
|
23,028
|
|
|||||||||||||||
|
Income taxes payable
|
6,350
|
|
212
|
|
—
|
|
6,562
|
|
2,205
|
|
201
|
|
—
|
|
2,406
|
|
|||||||||||||||
|
Deferred revenue
|
21,067
|
|
500
|
|
(500
|
)
|
21,067
|
|
20,755
|
|
500
|
|
(500
|
)
|
20,755
|
|
|||||||||||||||
|
Current portion of capital lease obligations
|
79
|
|
—
|
|
—
|
|
79
|
|
111
|
|
—
|
|
—
|
|
111
|
|
|||||||||||||||
|
Deferred revenue
|
453
|
|
6,031
|
|
(6,031
|
)
|
453
|
|
566
|
|
6,531
|
|
(6,531
|
)
|
566
|
|
|||||||||||||||
|
Long term debt
|
125,145
|
|
—
|
|
—
|
|
125,145
|
|
225
|
|
—
|
|
—
|
|
225
|
|
|||||||||||||||
|
Other liabilities
|
9,634
|
|
125
|
|
(1,986
|
)
|
7,773
|
|
9,459
|
|
207
|
|
(1,986
|
)
|
7,680
|
|
|||||||||||||||
|
EQUITY (DEFICIT)
|
|||||||||||||||||||||||||||||||
|
Common stock
|
52
|
|
11
|
|
(11
|
)
|
52
|
|
57
|
|
11
|
|
(11
|
)
|
57
|
|
|||||||||||||||
|
Treasury stock
|
(185,906
|
)
|
(100
|
)
|
100
|
|
(185,906
|
)
|
(83,454
|
)
|
|
|
—
|
|
(83,454
|
)
|
|||||||||||||||
|
Additional paid-in capital
|
288,686
|
|
491
|
|
(491
|
)
|
288,686
|
|
273,129
|
|
427
|
|
(427
|
)
|
273,129
|
|
|||||||||||||||
|
Accumulated other comprehensive income (loss)
|
(2,093
|
)
|
—
|
|
—
|
|
(2,093
|
)
|
3,995
|
|
—
|
|
—
|
|
3,995
|
|
|||||||||||||||
|
Retained earnings (deficit)
|
205,533
|
|
1,067
|
|
(1,340
|
)
|
205,260
|
|
133,014
|
|
(778
|
)
|
506
|
|
132,742
|
|
|||||||||||||||
|
Total Masimo Corporation stockholders’ equity (deficit)
|
306,272
|
|
1,469
|
|
(1,742
|
)
|
305,999
|
|
326,741
|
|
(340
|
)
|
68
|
|
326,469
|
|
|||||||||||||||
|
Noncontrolling interest
|
—
|
|
—
|
|
1,742
|
|
1,742
|
|
—
|
|
—
|
|
(68
|
)
|
(68
|
)
|
|||||||||||||||
|
Total equity
|
306,272
|
|
1,469
|
|
—
|
|
307,741
|
|
326,741
|
|
(340
|
)
|
—
|
|
326,401
|
|
|||||||||||||||
|
Total liabilities and equity (deficit)
|
$
|
564,480
|
|
$
|
9,221
|
|
$
|
(8,695
|
)
|
$
|
565,006
|
|
$
|
438,685
|
|
$
|
8,994
|
|
$
|
(9,017
|
)
|
$
|
438,662
|
|
|||||||
|
Year ended January 3, 2015
|
Year ended December 28, 2013
|
Year ended December 29, 2012
|
|||||||||||||||||||||||||||||||||||||||||||||
|
Statements of Comprehensive Income:
|
Masimo
Corp |
Cercacor
|
Cercacor
Elim |
Total
|
Masimo
Corp |
Cercacor
|
Cercacor
Elim |
Total
|
Masimo
Corp
|
Cercacor
|
Cercacor
Elim
|
Total
|
|||||||||||||||||||||||||||||||||||
|
Total revenue
|
$
|
586,643
|
|
$
|
5,970
|
|
$
|
(5,970
|
)
|
$
|
586,643
|
|
$
|
547,245
|
|
$
|
5,732
|
|
$
|
(5,732
|
)
|
$
|
547,245
|
|
$
|
493,233
|
|
$
|
5,375
|
|
$
|
(5,375
|
)
|
$
|
493,233
|
|
|||||||||||
|
Cost of goods sold
|
201,334
|
|
—
|
|
(5,470
|
)
|
195,864
|
|
193,775
|
|
—
|
|
(5,357
|
)
|
188,418
|
|
171,982
|
|
—
|
|
(5,000
|
)
|
166,982
|
|
|||||||||||||||||||||||
|
Gross profit (loss)
|
385,309
|
|
5,970
|
|
(500
|
)
|
390,779
|
|
353,470
|
|
5,732
|
|
(375
|
)
|
358,827
|
|
321,251
|
|
5,375
|
|
(375
|
)
|
326,251
|
|
|||||||||||||||||||||||
|
Operating expenses:
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Selling, general and administrative
|
238,674
|
|
2,842
|
|
(500
|
)
|
241,016
|
|
213,374
|
|
2,470
|
|
(375
|
)
|
215,469
|
|
191,870
|
|
2,453
|
|
(375
|
)
|
193,948
|
|
|||||||||||||||||||||||
|
Research and development
|
53,449
|
|
3,132
|
|
—
|
|
56,581
|
|
51,762
|
|
3,869
|
|
—
|
|
55,631
|
|
43,412
|
|
3,665
|
|
—
|
|
47,077
|
|
|||||||||||||||||||||||
|
Litigation award and defense costs
|
(8,010
|
)
|
(2,321
|
)
|
—
|
|
(10,331
|
)
|
8,010
|
|
—
|
|
—
|
|
8,010
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Total operating expenses
|
284,113
|
|
3,653
|
|
(500
|
)
|
287,266
|
|
273,146
|
|
6,339
|
|
(375
|
)
|
279,110
|
|
235,282
|
|
6,118
|
|
(375
|
)
|
241,025
|
|
|||||||||||||||||||||||
|
Operating income
|
101,196
|
|
2,317
|
|
—
|
|
103,513
|
|
80,324
|
|
(607
|
)
|
—
|
|
79,717
|
|
85,969
|
|
(743
|
)
|
—
|
|
85,226
|
|
|||||||||||||||||||||||
|
Non-operating expense (income)
|
1,505
|
|
(33
|
)
|
—
|
|
1,472
|
|
3,991
|
|
—
|
|
—
|
|
3,991
|
|
1,404
|
|
1
|
|
—
|
|
1,405
|
|
|||||||||||||||||||||||
|
Income (loss) before provision for income taxes
|
99,691
|
|
2,350
|
|
—
|
|
102,041
|
|
76,333
|
|
(607
|
)
|
—
|
|
75,726
|
|
84,565
|
|
(744
|
)
|
—
|
|
83,821
|
|
|||||||||||||||||||||||
|
Provision for (benefit from) income taxes
|
27,173
|
|
505
|
|
—
|
|
27,678
|
|
17,952
|
|
2,053
|
|
—
|
|
20,005
|
|
22,293
|
|
(410
|
)
|
—
|
|
21,883
|
|
|||||||||||||||||||||||
|
Net income (loss) including noncontrolling interests
|
72,518
|
|
1,845
|
|
—
|
|
74,363
|
|
58,381
|
|
(2,660
|
)
|
—
|
|
55,721
|
|
62,272
|
|
(334
|
)
|
—
|
|
61,938
|
|
|||||||||||||||||||||||
|
Net income (loss) attributable to noncontrolling interests
|
—
|
|
1,845
|
|
1,845
|
|
—
|
|
—
|
|
(2,660
|
)
|
(2,660
|
)
|
—
|
|
—
|
|
(334
|
)
|
(334
|
)
|
|||||||||||||||||||||||||
|
Net income (loss) attributable to Masimo Corporation stockholders
|
72,518
|
|
1,845
|
|
(1,845
|
)
|
72,518
|
|
58,381
|
|
(2,660
|
)
|
2,660
|
|
58,381
|
|
62,272
|
|
(334
|
)
|
334
|
|
62,272
|
|
|||||||||||||||||||||||
|
Other comprehensive income (loss), net of tax:
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
(6,088
|
)
|
—
|
|
—
|
|
(6,088
|
)
|
453
|
|
—
|
|
—
|
|
453
|
|
2,268
|
|
—
|
|
—
|
|
2,268
|
|
|||||||||||||||||||||||
|
Comprehensive income attributable to Masimo Corporation stockholders
|
$
|
66,430
|
|
$
|
1,845
|
|
$
|
(1,845
|
)
|
$
|
66,430
|
|
$
|
58,834
|
|
$
|
(2,660
|
)
|
$
|
2,660
|
|
$
|
58,834
|
|
$
|
64,540
|
|
$
|
(334
|
)
|
$
|
334
|
|
$
|
64,540
|
|
|||||||||||
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Raw materials
|
$
|
33,056
|
|
$
|
26,758
|
|
|
|
Work-in-process
|
6,020
|
|
6,310
|
|
|||
|
Finished goods
|
30,642
|
|
23,745
|
|
|||
|
Total
|
$
|
69,718
|
|
$
|
56,813
|
|
|
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Royalties receivable
|
$
|
7,200
|
|
$
|
7,300
|
|
|
|
Prepaid expenses
|
9,816
|
|
9,243
|
|
|||
|
Employee loans and advances
|
385
|
|
290
|
|
|||
|
Other current assets
|
4,070
|
|
2,551
|
|
|||
|
Total other current assets
|
$
|
21,471
|
|
$
|
19,384
|
|
|
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Machinery and equipment
|
$
|
38,588
|
|
$
|
33,315
|
|
|
|
Building and improvements
|
30,678
|
|
—
|
|
|||
|
Land
|
22,894
|
|
—
|
|
|||
|
Computer equipment
|
13,035
|
|
11,039
|
|
|||
|
Tooling
|
12,317
|
|
11,636
|
|
|||
|
Leasehold improvements
|
9,912
|
|
8,974
|
|
|||
|
Furniture and office equipment
|
4,864
|
|
4,921
|
|
|||
|
Demonstration units
|
972
|
|
956
|
|
|||
|
Vehicles
|
45
|
|
45
|
|
|||
|
Construction-in-progress
|
25,731
|
|
3,395
|
|
|||
|
Total property and equipment
|
159,036
|
|
74,281
|
|
|||
|
Accumulated depreciation and amortization
|
(57,084
|
)
|
(49,415
|
)
|
|||
|
Total
|
$
|
101,952
|
|
$
|
24,866
|
|
|
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Cost
|
|||||||
|
Patents
|
$
|
20,459
|
|
$
|
18,750
|
|
|
|
Customer relationships
|
7,669
|
|
7,669
|
|
|||
|
Acquired technology
|
5,580
|
|
5,580
|
|
|||
|
Trademarks
|
3,562
|
|
3,338
|
|
|||
|
Capitalized software development costs
|
2,066
|
|
1,612
|
|
|||
|
Other
|
1,450
|
|
969
|
|
|||
|
Total cost
|
40,786
|
|
37,918
|
|
|||
|
Accumulated amortization
|
|||||||
|
Patents
|
(6,649
|
)
|
(5,679
|
)
|
|||
|
Customer relationships
|
(1,853
|
)
|
(1,086
|
)
|
|||
|
Acquired technology
|
(1,392
|
)
|
(834
|
)
|
|||
|
Trademarks
|
(866
|
)
|
(653
|
)
|
|||
|
Capitalized software development costs
|
(1,440
|
)
|
(1,270
|
)
|
|||
|
Other
|
(815
|
)
|
(292
|
)
|
|||
|
Total accumulated amortization
|
(13,015
|
)
|
(9,814
|
)
|
|||
|
Net carrying amount
|
$
|
27,771
|
|
$
|
28,104
|
|
|
|
Fiscal year
|
Amount
|
||
|
2015
|
$
|
3,359
|
|
|
2016
|
3,215
|
|
|
|
2017
|
3,042
|
|
|
|
2018
|
2,690
|
|
|
|
2019
|
2,455
|
|
|
|
Thereafter
|
13,010
|
|
|
|
Total
|
$
|
27,771
|
|
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Goodwill, beginning of period
|
$
|
22,793
|
|
$
|
22,824
|
|
|
|
Foreign currency translation adjustment
|
(1,814
|
)
|
(31
|
)
|
|||
|
Goodwill, end of period
|
$
|
20,979
|
|
$
|
22,793
|
|
|
|
January 3,
2015 |
December 28,
2013 |
|||||||
|
Revolving line of credit
|
$
|
125,000
|
|
$
|
—
|
|
||
|
Long term portion of capital lease obligations acquisition
|
145
|
|
225
|
|
||||
|
Total long term debt
|
$
|
125,145
|
|
$
|
225
|
|
||
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Unrecognized tax benefit
|
$
|
6,812
|
|
$
|
5,769
|
|
|
|
Unfavorable lease liability related to the Spire acquisition
|
726
|
|
1,358
|
|
|||
|
Deferred rent, long-term
|
230
|
|
533
|
|
|||
|
Other
|
5
|
|
20
|
|
|||
|
Total other liabilities, long-term
|
$
|
7,773
|
|
$
|
7,680
|
|
|
|
Years Ended
|
|||||||||||
|
January 3, 2015
|
December 28, 2013
|
December 29, 2012
|
|||||||||
|
Shares repurchased
|
4,455
|
|
1,000
|
|
1,155
|
|
|||||
|
Average cost per share
|
$
|
23.00
|
|
$
|
19.79
|
|
$
|
22.74
|
|
||
|
Value of shares repurchased
|
$
|
102,453
|
|
$
|
19,791
|
|
$
|
26,268
|
|
||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
||||||||||||||||||
|
Shares
|
Average
Exercise Price |
Shares
|
Average
Exercise
Price
|
Shares
|
Average
Exercise Price |
|||||||||||||||
|
Options outstanding, beginning of period
|
8,911
|
|
$
|
22.76
|
|
8,368
|
|
$
|
22.78
|
|
8,277
|
|
$
|
22.68
|
|
|||||
|
Granted
|
1,887
|
|
$
|
24.83
|
|
1,653
|
|
$
|
21.17
|
|
754
|
|
$
|
22.17
|
|
|||||
|
Canceled
|
(416
|
)
|
$
|
24.46
|
|
(795
|
)
|
$
|
24.53
|
|
(447
|
)
|
$
|
27.30
|
|
|||||
|
Expired
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
|||||
|
Exercised
|
(426
|
)
|
$
|
10.95
|
|
(315
|
)
|
$
|
10.49
|
|
(216
|
)
|
$
|
7.62
|
|
|||||
|
Options outstanding, end of period
|
9,956
|
|
$
|
23.59
|
|
8,911
|
|
$
|
22.76
|
|
8,368
|
|
$
|
22.78
|
|
|||||
|
Options exercisable, end of period
|
5,859
|
|
$
|
23.63
|
|
5,188
|
|
$
|
22.69
|
|
4,632
|
|
$
|
21.29
|
|
|||||
|
Options available for grant, end of period
|
5,759
|
|
5,795
|
|
4,934
|
|
||||||||||||||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
|||
|
Risk-free interest rate
|
1.4% to 1.9%
|
0.7% to 1.8%
|
0.7% to 1.3%
|
||
|
Expected term
|
5.1 years to 5.5 years
|
5.1 years to 5.5 years
|
5.5 years
|
||
|
Estimated volatility
|
31.7% to 36.5%
|
31.2% to 39.6%
|
36.6% to 42.6%
|
||
|
Expected dividends
|
0%
|
0%
|
0%
|
||
|
Weighted-average fair value of options granted
|
$7.85 per share
|
$7.53 per share
|
$7.98 per share
|
||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
|||||||||
|
Cost of goods sold
|
$
|
436
|
|
$
|
354
|
|
$
|
480
|
|
||
|
Selling, general and administrative
|
8,812
|
|
9,407
|
|
10,775
|
|
|||||
|
Research and development
|
1,757
|
|
1,913
|
|
2,842
|
|
|||||
|
Total
|
$
|
11,005
|
|
$
|
11,674
|
|
$
|
14,097
|
|
||
|
January 3, 2015
|
December 28, 2013
|
||||||||||||||
|
Options Outstanding
|
Options
Exercisable
|
Options Outstanding
|
Options
Exercisable
|
||||||||||||
|
Range of Exercise Prices
|
Number of
Options
|
Average
Remaining
Contractual
Life
|
Number of
Options
|
Number of
Options
|
Average
Remaining
Contractual
Life
|
Number of
Options
|
|||||||||
|
$2.75 to $4.00
|
67
|
|
0.31
|
67
|
|
279
|
|
1.01
|
279
|
|
|||||
|
$4.01 to $12.00
|
639
|
|
1.44
|
639
|
|
696
|
|
2.46
|
696
|
|
|||||
|
$12.01 to $16.00
|
539
|
|
2.37
|
539
|
|
549
|
|
3.39
|
549
|
|
|||||
|
$16.01 to $23.98
|
4,256
|
|
7.58
|
1,580
|
|
3,635
|
|
8.06
|
986
|
|
|||||
|
$23.99 to $28.99
|
2,426
|
|
6.54
|
1,320
|
|
1,708
|
|
6.20
|
1,077
|
|
|||||
|
$29.00 to $31.99
|
1,744
|
|
4.46
|
1,435
|
|
1,734
|
|
5.37
|
1,311
|
|
|||||
|
$32.00 to $38.30
|
97
|
|
3.87
|
92
|
|
117
|
|
5.28
|
98
|
|
|||||
|
$38.31 to $41.51
|
188
|
|
3.40
|
187
|
|
193
|
|
4.40
|
192
|
|
|||||
|
Total
|
9,956
|
|
5.94
|
5,859
|
|
8,911
|
|
6.12
|
5,188
|
|
|||||
|
Fiscal year
|
Operating
Leases |
Capital
Leases |
Total
|
||||||||
|
2015
|
$
|
4,990
|
|
$
|
87
|
|
$
|
5,077
|
|
||
|
2016
|
4,088
|
|
80
|
|
4,168
|
|
|||||
|
2017
|
2,496
|
|
75
|
|
2,571
|
|
|||||
|
2018
|
1,930
|
|
—
|
|
1,930
|
|
|||||
|
2019
|
1,870
|
|
—
|
|
1,870
|
|
|||||
|
Thereafter
|
1,033
|
|
—
|
|
1,033
|
|
|||||
|
Total
|
$
|
16,407
|
|
$
|
242
|
|
$
|
16,649
|
|
||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
||||||||||||||||||
|
Geographic Area by Destination
|
||||||||||||||||||||
|
North and South America
|
$
|
398,066
|
|
71.5
|
%
|
$
|
378,894
|
|
73.2
|
%
|
$
|
341,672
|
|
73.5
|
%
|
|||||
|
Europe, Middle East and Africa
|
100,747
|
|
18.1
|
|
83,338
|
|
16.1
|
|
68,010
|
|
14.6
|
|
||||||||
|
Asia and Australia
|
57,951
|
|
10.4
|
|
55,197
|
|
10.7
|
|
55,246
|
|
11.9
|
|
||||||||
|
Total Product Revenue
|
$
|
556,764
|
|
100.0
|
%
|
$
|
517,429
|
|
100.0
|
%
|
$
|
464,928
|
|
100.0
|
%
|
|||||
|
United States
|
$
|
380,232
|
|
$
|
361,630
|
|
$
|
327,574
|
|
|||||||||||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
|||||||||
|
United States
|
$
|
69,282
|
|
$
|
50,782
|
|
$
|
59,216
|
|
||
|
Foreign
|
32,759
|
|
24,944
|
|
24,605
|
|
|||||
|
Total
|
$
|
102,041
|
|
$
|
75,726
|
|
$
|
83,821
|
|
||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
22,553
|
|
$
|
24,488
|
|
$
|
26,332
|
|
||
|
State
|
2,736
|
|
2,426
|
|
2,411
|
|
|||||
|
Foreign
|
2,709
|
|
1,704
|
|
(54
|
)
|
|||||
|
27,998
|
|
28,618
|
|
28,689
|
|
||||||
|
Deferred:
|
|||||||||||
|
Federal
|
342
|
|
(7,281
|
)
|
(5,546
|
)
|
|||||
|
State
|
(811
|
)
|
(970
|
)
|
(1,458
|
)
|
|||||
|
Foreign
|
149
|
|
(362
|
)
|
198
|
|
|||||
|
(320
|
)
|
(8,613
|
)
|
(6,806
|
)
|
||||||
|
Total
|
$
|
27,678
|
|
$
|
20,005
|
|
$
|
21,883
|
|
||
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
Year ended
December 29, 2012 |
||||||
|
Statutory regular federal income tax rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State provision, net of federal benefit
|
1.2
|
|
1.3
|
|
0.7
|
|
||
|
Nondeductible items
|
1.3
|
|
0.9
|
|
1.0
|
|
||
|
Foreign tax rate differential
|
(8.2
|
)
|
(9.8
|
)
|
(10.1
|
)
|
||
|
Tax credits
|
(1.5
|
)
|
(3.5
|
)
|
(0.5
|
)
|
||
|
Change in federal valuation allowance
|
(0.1
|
)
|
3.0
|
|
—
|
|
||
|
Other
|
(0.6
|
)
|
(0.5
|
)
|
—
|
|
||
|
Total
|
27.1
|
%
|
26.4
|
%
|
26.1
|
%
|
||
|
January 3,
2015 |
December 28,
2013 |
||||||
|
Deferred tax assets:
|
|||||||
|
Tax credits
|
$
|
3,260
|
|
$
|
3,203
|
|
|
|
Deferred revenue
|
4,943
|
|
4,234
|
|
|||
|
Acquired intangibles
|
437
|
|
507
|
|
|||
|
Net operating losses
|
8
|
|
277
|
|
|||
|
Accrued liabilities
|
14,723
|
|
17,036
|
|
|||
|
Share-based compensation
|
21,594
|
|
19,385
|
|
|||
|
Property and equipment
|
584
|
|
670
|
|
|||
|
Other
|
2,162
|
|
2,149
|
|
|||
|
Total
|
47,711
|
|
47,461
|
|
|||
|
Valuation allowance
|
(3,365
|
)
|
(3,563
|
)
|
|||
|
Total deferred tax assets
|
44,346
|
|
43,898
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
State taxes and other
|
(2,088
|
)
|
(1,697
|
)
|
|||
|
Total deferred tax liabilities
|
(2,088
|
)
|
(1,697
|
)
|
|||
|
Net deferred tax assets
|
$
|
42,258
|
|
$
|
42,201
|
|
|
|
Current net deferred tax asset
|
18,065
|
|
19,636
|
|
|||
|
Long-term net deferred tax asset
|
24,193
|
|
22,565
|
|
|||
|
Net deferred tax assets
|
$
|
42,258
|
|
$
|
42,201
|
|
|
|
Year ended
January 3, 2015 |
Year ended
December 28, 2013 |
||||||
|
Unrecognized tax benefits (gross), beginning of period
|
$
|
6,630
|
|
$
|
6,685
|
|
|
|
Increase from tax positions in prior period
|
830
|
|
265
|
|
|||
|
Increase from tax positions in current period
|
958
|
|
695
|
|
|||
|
Settlements
|
—
|
|
(443
|
)
|
|||
|
Lapse of statute of limitations
|
(394
|
)
|
(572
|
)
|
|||
|
Unrecognized tax benefits (gross), end of period
|
$
|
8,024
|
|
$
|
6,630
|
|
|
|
Quarters Ended
|
|||||||||||||||
|
Fiscal 2014
|
March 29,
2014 |
June 28,
2014 |
September 27,
2014 |
January 3,
2015 |
|||||||||||
|
Total revenue
|
$
|
139,814
|
|
$
|
140,923
|
|
$
|
144,118
|
|
$
|
161,788
|
|
|||
|
Gross profit
|
92,301
|
|
93,095
|
|
96,224
|
|
109,159
|
|
|||||||
|
Operating income
|
30,193
|
|
18,405
|
|
22,268
|
|
32,647
|
|
|||||||
|
Net income attributable to Masimo Corporation stockholders
|
22,632
|
|
13,802
|
|
14,863
|
|
21,221
|
|
|||||||
|
Net income per share attributable to Masimo Corporation stockholders:
|
|||||||||||||||
|
Basic
|
$
|
0.40
|
|
$
|
0.25
|
|
$
|
0.28
|
|
$
|
0.40
|
|
|||
|
Diluted
|
$
|
0.39
|
|
$
|
0.24
|
|
$
|
0.27
|
|
$
|
0.40
|
|
|||
|
Quarters Ended
|
|||||||||||||||
|
Fiscal 2013
|
March 30,
2013 |
June 29,
2013 |
September 28,
2013 |
December 28,
2013 |
|||||||||||
|
Total revenue
|
$
|
135,942
|
|
$
|
137,422
|
|
$
|
131,447
|
|
$
|
142,435
|
|
|||
|
Gross profit
|
89,581
|
|
91,232
|
|
87,479
|
|
90,536
|
|
|||||||
|
Operating income
|
23,141
|
|
23,180
|
|
20,743
|
|
12,653
|
|
|||||||
|
Net income attributable to Masimo Corporation stockholders
|
16,428
|
|
17,038
|
|
15,602
|
|
9,313
|
|
|||||||
|
Net income per share attributable to Masimo Corporation stockholders:
|
|||||||||||||||
|
Basic
|
$
|
0.29
|
|
$
|
0.30
|
|
$
|
0.28
|
|
$
|
0.16
|
|
|||
|
Diluted
|
$
|
0.28
|
|
$
|
0.30
|
|
$
|
0.27
|
|
$
|
0.16
|
|
|||
|
MASIMO CORPORATION
VALUATION AND QUALIFYING ACCOUNTS
Years ended January 3, 2015, December 28, 2013 and December 29, 2012
|
|||||||||||||
|
Description
|
Balance at beginning of period
|
Additions charged to expense and other accounts
|
Amounts charged against reserve
|
Balance at end of period
|
|||||||||
|
Year ended January 3, 2015
|
|||||||||||||
|
Allowance for doubtful accounts........................................
|
$
|
1,833
|
|
$
|
583
|
|
$
|
(526
|
)
|
$
|
1,890
|
|
|
|
Sales returns, allowance and reserves.................................
|
$
|
429
|
|
$
|
1,832
|
|
$
|
(1,789
|
)
|
$
|
472
|
|
|
|
Valuation allowance on deferred tax asset..........................
|
$
|
(3,563
|
)
|
$
|
—
|
|
$
|
198
|
|
$
|
(3,365
|
)
|
|
|
Year ended December 28, 2013
|
|||||||||||||
|
Allowance for doubtful accounts........................................
|
$
|
1,956
|
|
$
|
728
|
|
$
|
(851
|
)
|
$
|
1,833
|
|
|
|
Sales returns, allowance and reserves.................................
|
$
|
516
|
|
$
|
1,881
|
|
$
|
(1,968
|
)
|
$
|
429
|
|
|
|
Valuation allowance on deferred tax asset..........................
|
$
|
(2,441
|
)
|
$
|
(3,163
|
)
|
$
|
2,041
|
|
$
|
(3,563
|
)
|
|
|
Year ended December 29, 2012
|
|||||||||||||
|
Allowance for doubtful accounts........................................
|
$
|
1,798
|
|
$
|
231
|
|
$
|
(73
|
)
|
$
|
1,956
|
|
|
|
Sales returns, allowance and reserves.................................
|
$
|
421
|
|
$
|
1,967
|
|
$
|
(1,872
|
)
|
$
|
516
|
|
|
|
Valuation allowance on deferred tax asset..........................
|
$
|
(400
|
)
|
$
|
(2,041
|
)
|
$
|
—
|
|
$
|
(2,441
|
)
|
|