|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
Annual report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934.
For the fiscal year ended April 29, 2016.
|
|
|
|
|
o
|
Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the transition period from __________ to __________
|

|
Ireland
|
|
98-1183488
|
|
(Jurisdiction of incorporation)
|
|
(I.R.S. Employer Identification No.)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Ordinary shares, par value $0.0001 per share
|
New York Stock Exchange, Inc.
|
|
Item
|
|
Description
|
|
Page
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
|
•
|
Therapy Innovation: Delivering a strong launch cadence of meaningful therapies and procedures.
|
|
•
|
Globalization: Addressing the inequity in health care access globally, primarily in emerging markets.
|
|
•
|
Economic Value: Becoming a leader in value-based health care by offering new services and solutions to improve outcomes and efficiencies, lower costs by reducing hospitalizations, improve remote clinical management, and increase patient engagement.
|
|
•
|
Cardiac Rhythm & Heart Failure
|
|
•
|
Coronary & Structural Heart
|
|
•
|
Aortic & Peripheral Vascular
|
|
•
|
Surgical Solutions
|
|
•
|
Patient Monitoring & Recovery
|
|
•
|
Spine
|
|
•
|
Neuromodulation
|
|
•
|
Surgical Technologies
|
|
•
|
Neurovascular
|
|
•
|
Intensive Insulin Management
|
|
•
|
Non-Intensive Diabetes Therapies
|
|
•
|
Diabetes Service & Solutions
|
|
•
|
product reliability,
|
|
•
|
product performance,
|
|
•
|
product technology,
|
|
•
|
product quality,
|
|
•
|
breadth of product lines,
|
|
•
|
product services,
|
|
•
|
customer support,
|
|
•
|
price, and
|
|
•
|
reimbursement approval from health care insurance providers.
|
|
•
|
take a significant amount of time,
|
|
•
|
require the expenditure of substantial resources,
|
|
•
|
involve stringent clinical and pre-clinical testing, as well as increased post-market surveillance,
|
|
•
|
involve modifications, repairs, or replacements of our products, and
|
|
•
|
result in limitations on the proposed uses of our products.
|
|
•
|
fluctuations in currency exchange rates,
|
|
•
|
healthcare reform legislation,
|
|
•
|
multiple non-U.S. regulatory requirements that are subject to change and that could restrict our ability to manufacture and sell our products,
|
|
•
|
local product preferences and product requirements,
|
|
•
|
longer-term receivables than are typical in the U.S.,
|
|
•
|
trade protection measures and import or export licensing requirements,
|
|
•
|
less intellectual property protection in some countries outside the U.S. than exists in the U.S.,
|
|
•
|
different labor regulations and workforce instability,
|
|
•
|
political instability,
|
|
•
|
the potential payment of U.S. income taxes on earnings of certain controlled foreign subsidiaries subject to U.S. taxation upon repatriation,
|
|
•
|
the expiration and non-renewal of foreign tax rulings and/or grants,
|
|
•
|
potentially negative consequences from changes in or interpretations of tax laws, and
|
|
•
|
economic instability and inflation, recession or interest rate fluctuations.
|
|
•
|
the presence or absence of adequate internal controls and/or significant fraud in the financial systems of acquired companies,
|
|
•
|
our ability or inability to integrate information technology systems of acquired companies in a secure and reliable manner,
|
|
•
|
adverse developments arising out of investigations by governmental entities of the business practices of acquired companies, including potential liability imposed by FCPA,
|
|
•
|
any decrease in customer loyalty and product orders caused by dissatisfaction with the combined companies’ product lines and sales and marketing practices, including price increases,
|
|
•
|
our ability to retain key employees, and
|
|
•
|
the ability of the combined company to achieve synergies among its constituent companies, such as increasing sales of the combined company’s products, achieving cost savings, and effectively combining technologies to develop new products.
|
|
•
|
making it more difficult for us to satisfy our financial obligations;
|
|
•
|
increasing our vulnerability to adverse economic, regulatory and industry conditions, and placing us at a disadvantage compared to our competitors that are less leveraged;
|
|
•
|
limiting our ability to compete and our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate;
|
|
•
|
limiting our ability to borrow additional funds for working capital, capital expenditures, acquisitions and general corporate or other purposes; and
|
|
•
|
exposing us to greater interest rate risk.
|
|
•
|
the diversion of management’s attention to integration matters;
|
|
•
|
difficulties in achieving anticipated cost savings, synergies, business opportunities and growth prospects from combining the businesses;
|
|
•
|
difficulties in the integration of operations and systems;
|
|
•
|
difficulties in the assimilation of employees;
|
|
•
|
difficulties in managing the expanded operations of a significantly larger and more complex company;
|
|
•
|
challenges in keeping existing customers and obtaining new customers; and
|
|
•
|
challenges in attracting and retaining key personnel.
|
|
Location Country or State
|
Square Feet (in thousands)
|
||
|
China
|
1,182
|
|
|
|
South Carolina
|
1,146
|
|
|
|
Connecticut
|
1,098
|
|
|
|
Minnesota
|
1,024
|
|
|
|
Mexico
|
959
|
|
|
|
Puerto Rico
|
831
|
|
|
|
Ireland
|
640
|
|
|
|
Florida
|
550
|
|
|
|
Massachusetts
|
504
|
|
|
|
California
|
502
|
|
|
|
Illinois
|
459
|
|
|
|
Italy
|
454
|
|
|
|
Texas
|
431
|
|
|
|
Switzerland
|
347
|
|
|
|
Arizona
|
294
|
|
|
|
Indiana
|
291
|
|
|
|
Colorado
|
287
|
|
|
|
Nebraska
|
281
|
|
|
|
Georgia
|
236
|
|
|
|
Japan
|
220
|
|
|
|
Dominican Republic
|
217
|
|
|
|
Canada
|
206
|
|
|
|
Fiscal Period
|
Total Number of
Shares Purchased
|
Average Price
Paid per Share
|
Total Number of Shares
Purchased as a Part of
Publicly Announced
Program
|
Maximum Number
of Shares that May
Yet Be Purchased
Under the Program
|
|||||||||
|
1/30/2016-2/26/2016
|
2,700,350
|
|
$
|
74.08
|
|
2,700,350
|
|
77,939,900
|
|
||||
|
2/27/2016-4/1/2016
|
3,710,152
|
|
75.47
|
|
3,710,152
|
|
74,229,748
|
|
|||||
|
4/2/2016-4/29/2016
|
2,351,007
|
|
76.56
|
|
2,351,007
|
|
71,878,741
|
|
|||||
|
Total
|
8,761,509
|
|
$
|
75.34
|
|
8,761,509
|
|
71,878,741
|
|
||||
|
Fiscal
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
||||||||||||
|
2016 High
|
$
|
79.08
|
|
$
|
78.91
|
|
$
|
78.92
|
|
$
|
80.74
|
|
||||
|
2016 Low
|
72.20
|
|
55.54
|
|
72.28
|
|
71.03
|
|
||||||||
|
2015 High
|
65.50
|
|
67.11
|
|
77.39
|
|
79.50
|
|
||||||||
|
2015 Low
|
57.81
|
|
59.83
|
|
65.51
|
|
70.91
|
|
||||||||
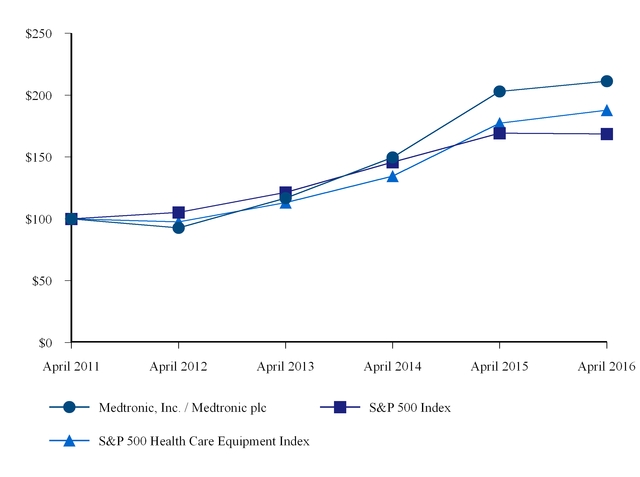
|
Company/Index
|
April 2011
|
April 2012
|
April 2013
|
April 2014
|
April 2015
|
April 2016
|
||||||||||||||||||
|
Medtronic, Inc. / Medtronic plc
|
$
|
100.00
|
|
$
|
92.68
|
|
$
|
116.80
|
|
$
|
149.62
|
|
$
|
203.06
|
|
$
|
211.37
|
|
||||||
|
S&P 500 Index
|
100.00
|
|
105.16
|
|
121.27
|
|
145.85
|
|
169.15
|
|
168.63
|
|
||||||||||||
|
S&P 500 Health Care Equipment Index
|
100.00
|
|
97.46
|
|
113.00
|
|
134.57
|
|
177.23
|
|
187.79
|
|
||||||||||||
|
•
|
in the case of a beneficial owner
of Medtronic shares held in the Depository Trust Company (DTC), the address of the beneficial owner in the records of his or her broker is in the United States and this information is provided by the broker to the Company’s qualifying intermediary; or
|
|
•
|
in the case of a record owner, the record owner has provided to the Company’s transfer agent a valid U.S Certification of Residence (Form 6166) or valid Irish Non-Resident Form V2.
|
|
(in millions, except per share data and additional information)
|
Fiscal Year
|
||||||||||||||||||
|
Operating Results for the Fiscal Year:
|
2016
|
2015
(1)
|
2014
|
2013
|
2012
|
||||||||||||||
|
Net sales
|
$
|
28,833
|
|
$
|
20,261
|
|
$
|
17,005
|
|
$
|
16,590
|
|
$
|
16,184
|
|
||||
|
Cost of products sold
|
9,142
|
|
6,309
|
|
4,333
|
|
4,126
|
|
3,889
|
|
|||||||||
|
Research and development expense
|
2,224
|
|
1,640
|
|
1,477
|
|
1,557
|
|
1,490
|
|
|||||||||
|
Selling, general, and administrative expense
|
9,469
|
|
6,904
|
|
5,847
|
|
5,698
|
|
5,623
|
|
|||||||||
|
Special charges (gains), net
|
70
|
|
(38
|
)
|
40
|
|
—
|
|
—
|
|
|||||||||
|
Restructuring charges, net
|
290
|
|
237
|
|
78
|
|
172
|
|
87
|
|
|||||||||
|
Certain litigation charges, net
|
26
|
|
42
|
|
770
|
|
245
|
|
90
|
|
|||||||||
|
Acquisition-related items
|
283
|
|
550
|
|
117
|
|
(49
|
)
|
12
|
|
|||||||||
|
Amortization of intangible assets
|
1,931
|
|
733
|
|
349
|
|
331
|
|
335
|
|
|||||||||
|
Other expense, net
|
107
|
|
118
|
|
181
|
|
108
|
|
364
|
|
|||||||||
|
Operating profit
|
5,291
|
|
3,766
|
|
3,813
|
|
4,402
|
|
4,294
|
|
|||||||||
|
Operating profit margin percentage
|
18.4
|
%
|
18.6
|
%
|
22.4
|
%
|
26.5
|
%
|
26.5
|
%
|
|||||||||
|
Interest expense, net
|
955
|
|
280
|
|
108
|
|
151
|
|
149
|
|
|||||||||
|
Income from continuing operations before income taxes
|
4,336
|
|
3,486
|
|
3,705
|
|
4,251
|
|
4,145
|
|
|||||||||
|
Provision for income taxes
|
798
|
|
811
|
|
640
|
|
784
|
|
730
|
|
|||||||||
|
Income from continuing operations
|
3,538
|
|
2,675
|
|
3,065
|
|
3,467
|
|
3,415
|
|
|||||||||
|
Income from discontinued operations, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
202
|
|
|||||||||
|
Net income
|
$
|
3,538
|
|
$
|
2,675
|
|
$
|
3,065
|
|
$
|
3,467
|
|
$
|
3,617
|
|
||||
|
Per Ordinary Share:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Basic - Income from continuing operations
|
$
|
2.51
|
|
$
|
2.44
|
|
$
|
3.06
|
|
$
|
3.40
|
|
$
|
3.24
|
|
||||
|
Basic - Net income
|
2.51
|
|
2.44
|
|
3.06
|
|
3.40
|
|
3.43
|
|
|||||||||
|
Diluted - Income from continuing operations
|
2.48
|
|
2.41
|
|
3.02
|
|
3.37
|
|
3.22
|
|
|||||||||
|
Diluted - Net income
|
2.48
|
|
2.41
|
|
3.02
|
|
3.37
|
|
3.41
|
|
|||||||||
|
Cash dividends declared per ordinary share
|
1.52
|
|
1.22
|
|
1.12
|
|
1.04
|
|
0.97
|
|
|||||||||
|
Financial Position at Fiscal Year-end:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Working capital
|
$
|
16,435
|
|
$
|
21,671
|
|
$
|
15,651
|
|
$
|
13,902
|
|
$
|
10,409
|
|
||||
|
Current ratio
|
3.3:1.0
|
3.4:1.0
|
3.8:1.0
|
4.5:1.0
|
2.8:1.0
|
||||||||||||||
|
Total assets
|
$
|
99,782
|
|
$
|
106,685
|
|
$
|
37,943
|
|
$
|
34,900
|
|
$
|
32,818
|
|
||||
|
Long-term debt
|
30,247
|
|
33,752
|
|
10,315
|
|
9,741
|
|
7,359
|
|
|||||||||
|
Shareholders’ equity
|
52,063
|
|
53,230
|
|
19,443
|
|
18,671
|
|
17,113
|
|
|||||||||
|
Additional Information:
(2)
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Full-time employees at year-end
|
88,063
|
|
85,573
|
|
43,305
|
|
42,466
|
|
40,601
|
|
|||||||||
|
Full-time equivalent employees at year-end
|
98,017
|
|
92,500
|
|
49,247
|
|
46,659
|
|
44,944
|
|
|||||||||
|
|
Net Sales
|
|
|||||||||
|
|
Fiscal Year
|
|
|||||||||
|
(dollars in millions; NM - Not Meaningful)
|
2016
|
2015
|
% Change
|
||||||||
|
Cardiac and Vascular Group
|
$
|
10,196
|
|
$
|
9,361
|
|
9
|
%
|
|||
|
Minimally Invasive Therapies Group
(1)
|
9,563
|
|
2,387
|
|
301
|
|
|||||
|
Restorative Therapies Group
|
7,210
|
|
6,751
|
|
7
|
|
|||||
|
Diabetes Group
|
1,864
|
|
1,762
|
|
6
|
|
|||||
|
Total Net Sales
|
$
|
28,833
|
|
$
|
20,261
|
|
42
|
%
|
|||
|
(1)
|
The Minimally Invasive Therapies Group was a new group in the fourth quarter of fiscal year 2015 that contains the majority of Covidien's former operations. Revenue growth is compared to a full year of operations in fiscal year 2016.
|
|
|
Fiscal year ended April 29, 2016
|
||||||||||||||||||||||
|
(in millions)
|
Net Sales
|
Operating Profit
|
Income from Operations Before Income Taxes
|
Net Income
|
Provision for Income Taxes
(1)
|
Effective Tax Rate
|
|||||||||||||||||
|
GAAP
|
$
|
28,833
|
|
$
|
5,291
|
|
$
|
4,336
|
|
$
|
3,538
|
|
$
|
798
|
|
18.4
|
%
|
||||||
|
Non-GAAP Adjustments:
|
|||||||||||||||||||||||
|
Impact of inventory step-up
|
—
|
|
226
|
|
226
|
|
165
|
|
61
|
|
27.0
|
|
|||||||||||
|
Special charges
|
—
|
|
70
|
|
70
|
|
44
|
|
26
|
|
37.1
|
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
299
|
|
299
|
|
221
|
|
78
|
|
26.1
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
26
|
|
26
|
|
17
|
|
9
|
|
34.6
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
283
|
|
283
|
|
212
|
|
71
|
|
25.1
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
1,931
|
|
1,931
|
|
1,467
|
|
464
|
|
24.0
|
|
|||||||||||
|
Loss on previously held forward starting interest rate swaps
|
—
|
|
—
|
|
45
|
|
29
|
|
16
|
|
35.6
|
|
|||||||||||
|
Debt tender premium
|
—
|
|
—
|
|
183
|
|
118
|
|
65
|
|
35.5
|
|
|||||||||||
|
Certain tax adjustments
|
—
|
|
—
|
|
—
|
|
417
|
|
(417
|
)
|
—
|
|
|||||||||||
|
Non-GAAP
|
$
|
28,833
|
|
$
|
8,126
|
|
$
|
7,399
|
|
$
|
6,228
|
|
$
|
1,171
|
|
15.8
|
%
|
||||||
|
(1)
|
The tax effect of each Non-GAAP Adjustment is based on the jurisdictions in which the expense (income) is incurred and the tax laws in effect for each such jurisdiction.
|
|
|
Fiscal year ended April 24, 2015
|
||||||||||||||||||||||
|
(in millions)
|
Net Sales
|
Operating Profit
|
Income from Operations Before Income Taxes
|
Net Income
|
Provision for Income Taxes
(1)
|
Effective Tax Rate
|
|||||||||||||||||
|
GAAP
|
$
|
20,261
|
|
$
|
3,766
|
|
$
|
3,486
|
|
$
|
2,675
|
|
$
|
811
|
|
23.3
|
%
|
||||||
|
Non-GAAP Adjustments:
|
|||||||||||||||||||||||
|
Impact of inventory step-up
|
—
|
|
623
|
|
623
|
|
455
|
|
168
|
|
27.0
|
|
|||||||||||
|
Impact of product technology upgrade commitment
|
—
|
|
74
|
|
74
|
|
61
|
|
13
|
|
17.6
|
|
|||||||||||
|
Special (gains) charges, net
|
—
|
|
(38
|
)
|
(38
|
)
|
(23
|
)
|
(15
|
)
|
39.5
|
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
252
|
|
252
|
|
180
|
|
72
|
|
28.6
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
42
|
|
42
|
|
27
|
|
15
|
|
35.7
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
550
|
|
550
|
|
433
|
|
117
|
|
21.3
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
733
|
|
733
|
|
538
|
|
195
|
|
26.6
|
|
|||||||||||
|
Impact of acquisition on interest expense
|
—
|
|
—
|
|
77
|
|
49
|
|
28
|
|
36.4
|
|
|||||||||||
|
Certain tax adjustments
|
—
|
|
—
|
|
—
|
|
349
|
|
(349
|
)
|
—
|
|
|||||||||||
|
Non-GAAP
|
$
|
20,261
|
|
$
|
6,002
|
|
$
|
5,799
|
|
$
|
4,744
|
|
$
|
1,055
|
|
18.2
|
%
|
||||||
|
(1)
|
The tax effect of each Non-GAAP Adjustment is based on the jurisdictions in which the expense (income) is incurred and the tax laws in effect for each such jurisdiction.
|
|
|
Fiscal year ended April 25, 2014
|
||||||||||||||||||||||
|
(in millions)
|
Net Sales
|
Operating Profit
|
Income from Operations Before Income Taxes
|
Net Income
|
Provision for Income Taxes
(1)
|
Effective Tax Rate
|
|||||||||||||||||
|
GAAP
|
$
|
17,005
|
|
$
|
3,813
|
|
$
|
3,705
|
|
$
|
3,065
|
|
$
|
640
|
|
17.3
|
%
|
||||||
|
Non-GAAP Adjustments:
|
|||||||||||||||||||||||
|
Special charges
|
—
|
|
40
|
|
40
|
|
26
|
|
14
|
|
35.0
|
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
88
|
|
88
|
|
60
|
|
28
|
|
31.8
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
770
|
|
770
|
|
701
|
|
69
|
|
9.0
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
117
|
|
117
|
|
79
|
|
38
|
|
32.5
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
349
|
|
349
|
|
230
|
|
119
|
|
34.1
|
|
|||||||||||
|
Certain tax adjustments
|
—
|
|
—
|
|
—
|
|
(63
|
)
|
63
|
|
—
|
|
|||||||||||
|
Non-GAAP
|
$
|
17,005
|
|
$
|
5,177
|
|
$
|
5,069
|
|
$
|
4,098
|
|
$
|
971
|
|
19.2
|
%
|
||||||
|
(1)
|
The tax effect of each Non-GAAP Adjustment is based on the jurisdictions in which the expense (income) is incurred and the tax laws in effect for each such jurisdiction.
|
|
|
Net Sales
|
|
Net Sales
|
|
|||||||||||||||||
|
|
Fiscal Year
|
|
Fiscal Year
|
|
|||||||||||||||||
|
(dollars in millions; NC - Not Calculable)
|
2016
|
2015
|
% Change
|
2015
|
2014
|
% Change
|
|||||||||||||||
|
Cardiac Rhythm & Heart Failure
|
$
|
5,465
|
|
$
|
5,245
|
|
4
|
%
|
$
|
5,245
|
|
$
|
4,996
|
|
5
|
%
|
|||||
|
Coronary & Structural Heart
|
3,093
|
|
3,038
|
|
2
|
|
3,038
|
|
2,956
|
|
3
|
|
|||||||||
|
Aortic & Peripheral Vascular
(1)
|
1,638
|
|
1,078
|
|
52
|
|
1,078
|
|
895
|
|
20
|
|
|||||||||
|
Total Cardiac and Vascular Group
|
10,196
|
|
9,361
|
|
9
|
|
9,361
|
|
8,847
|
|
6
|
|
|||||||||
|
Surgical Solutions
(1)
|
5,265
|
|
1,293
|
|
307
|
|
1,293
|
|
—
|
|
NC
|
|
|||||||||
|
Patient Monitoring & Recovery
(1)
|
4,298
|
|
1,094
|
|
293
|
|
1,094
|
|
—
|
|
NC
|
|
|||||||||
|
Total Minimally Invasive Therapies Group
(1)
|
9,563
|
|
2,387
|
|
301
|
|
2,387
|
|
—
|
|
NC
|
|
|||||||||
|
Spine
|
2,924
|
|
2,971
|
|
(2
|
)
|
2,971
|
|
3,041
|
|
(2
|
)
|
|||||||||
|
Neuromodulation
|
1,926
|
|
1,977
|
|
(3
|
)
|
1,977
|
|
1,898
|
|
4
|
|
|||||||||
|
Surgical Technologies
|
1,773
|
|
1,671
|
|
6
|
|
1,671
|
|
1,562
|
|
7
|
|
|||||||||
|
Neurovascular
(1)
|
587
|
|
132
|
|
345
|
|
132
|
|
—
|
|
NC
|
|
|||||||||
|
Total Restorative Therapies Group
|
7,210
|
|
6,751
|
|
7
|
|
6,751
|
|
6,501
|
|
4
|
|
|||||||||
|
Diabetes Group
|
1,864
|
|
1,762
|
|
6
|
|
1,762
|
|
1,657
|
|
6
|
|
|||||||||
|
Total
|
$
|
28,833
|
|
$
|
20,261
|
|
42
|
%
|
$
|
20,261
|
|
$
|
17,005
|
|
19
|
%
|
|||||
|
(1)
|
Growth rates are impacted by the acquisition of Covidien in the fourth quarter of fiscal year 2015. Revenue growth is compared to a full year of operations in fiscal year 2016.
|
|
•
|
Increasing competition, fluctuations in currency exchange rates, and continued pricing pressures.
|
|
•
|
Continued acceptance and future growth of the Amplia/Compia/Claria family of MRI Quad CRT-D SureScan systems. The Amplia and Compia MRI Quad CRT-D SureScan systems received U.S. FDA approval in February
|
|
•
|
Continued acceptance and future growth from the Viva/Brava family of CRT-D devices and the Attain Performa portfolio of quadripolar leads. The Viva/Brava family of CRT-D devices utilizes a new algorithm, called AdaptivCRT, which improves patients’ response rates to CRT-D therapy by preserving the patients’ normal heart rhythms and continually adapts to individual patient needs. Paired with Viva/Brava Quad CRT-D, Attain Performa leads provide additional options for physicians to optimize patient therapy. In the second quarter of fiscal year 2015, we received U.S. FDA approval of our Attain Performa quadripolar lead, Viva Quad XT CRT-D, and Viva Quad S CRT-D.
|
|
•
|
Continued acceptance and future growth from the Evera family of ICDs. The Evera family of ICDs has increased battery longevity, advanced shock reduction technology, and a contoured shape with thin, smooth edges that better fits inside the body. Our Evera MRI SureScan ICD received CE Mark approval late in the fourth quarter of fiscal year 2014 and launched in Japan in November 2014. We received U.S. FDA approval of our Evera MRI SureScan ICD in the second quarter of fiscal year 2016.
|
|
•
|
Continued acceptance and future growth from the Advisa DR MRI SureScan pacing system for use in full-body MRI scans. The Advisa DR MRI SureScan is our second-generation MRI pacing system and is the first system to combine advanced pacing technology with proven MRI access. We received U.S. FDA approval of the Advisa SR MRI SureScan single-chamber pacemaker in the first quarter of fiscal year 2016.
|
|
•
|
Continued future growth from the Arctic Front system, including the second generation Arctic Front Advance Cardiac Cryoballoon. The Arctic Front system is a cryoballoon indicated for the treatment of drug refractory paroxysmal atrial fibrillation. The cryoballoon treatment involves a minimally invasive procedure that efficiently creates circumferential lesions around the pulmonary vein, which studies have indicated is the source of erratic electrical signals that cause irregular heartbeat. We received U.S. FDA approval in the first quarter of fiscal year 2016 for the Aortic Front Advance ST Cryoablation Catheter.
|
|
•
|
Continued future growth from Reveal LINQ, our next-generation insertable cardiac monitor launched in international and U.S. markets in the third and fourth quarters of fiscal year 2014, respectively.
|
|
•
|
Acceptance and future growth of our Micra transcatheter pacing system, which received CE Mark approval in April 2015 and U.S. FDA approval in April 2016. Micra is a miniaturized single chamber pacemaker system that is delivered through the femoral vein and is implanted in the right ventricle of the heart. The system does not use a lead and does not have a subcutaneous device pocket underneath the skin as with conventional pacemaker systems.
|
|
•
|
Continued acceptance and future growth from Care Management Service's remote telemonitoring solutions business for the management of chronic diseases such as heart failure, diabetes, and hypertension. Care Management Services has a readmission reduction program focused on minimizing heart failure readmission penalties for U.S. hospitals.
|
|
•
|
Continued acceptance of our CLMS business. CLMS provides a unique service offering, whereby we enter into long-term contracts with hospitals, both within Europe and in certain other regions around the world, to upgrade and more effectively manage their cath lab and hybrid operating rooms. At the end of fiscal year 2016, we had 88 long-term CLMS agreements.
|
|
•
|
Continued acceptance of CoreValve Evolut R, our next-generation recapturable system with differentiated 14-French equivalent delivery system. We have CE Mark approval for the 23 millimeter size of the valve and received CE Mark approval for the 26 and 29 millimeter sizes early in the fourth quarter of fiscal year 2015. We received U.S. FDA approval of the 23, 26, and 29 millimeter sizes in the first quarter of fiscal year 2016.
|
|
•
|
Acceptance of our CoreValve transcatheter heart valve technologies for the replacement of the aortic valve in Japan. We received Japanese regulatory approval in March 2015 and launched in Japan late in the third quarter of fiscal year 2016 following reimbursement approval. We received U.S. FDA approval for valve-in-valve implantation in March 2015.
|
|
•
|
Acceptance of the Resolute Onyx drug-eluting coronary stent, which received CE Mark approval in November 2014. Resolute Onyx builds on the Resolute Integrity drug-eluting coronary stent with thinner struts to improve deliverability and is the first stent to feature our CoreWire technology, allowing greater visibility during the
|
|
•
|
The global stent market continues to experience pricing pressure resulting from government austerity programs and reimbursement cuts in Europe and Japan.
|
|
•
|
Continued worldwide growth of our Euphora Non-Compliant and Semi-Compliant Balloon Dilatation Catheter and our family of coronary guide catheters.
|
|
•
|
Acceptance of the IN.PACT Admiral drug-coated balloon for the treatment of peripheral artery disease in the upper leg. The IN.PACT Admiral drug-coated balloon was launched in the U.S. early in the fourth quarter of fiscal year 2015, and received CE Mark approval in January 2016 for arteriovenous access to help maintain hemodialysis access in patients with end-stage renal disease.
|
|
•
|
Integration of Aptus Endosystems, Inc. (Aptus), acquired in June 2015, into the Aortic & Peripheral division. Aptus is a medical device company focused on developing advanced technology for endovascular aneurysm repair and thoracic endovascular aneurysm repair.
|
|
•
|
Continued and future acceptance of the Endurant family of AAA stent graft products. We received CE Mark and U.S. FDA approval of the Endurant IIs stent graft late in the second quarter of fiscal year 2015. Continued worldwide growth of the Valiant Captivia Thoracic Stent Graft System.
|
|
•
|
Acceptance of our VenaSeal closure system, which was launched in the U.S. in November 2015. The VenaSeal closure system is a minimally invasive procedure that uses a proprietary medical adhesive to close superficial veins of the lower extremities in patients with symptomatic venous reflux.
|
|
•
|
Continued acceptance and future growth of Open-to-Minimally Invasive Surgery (MIS) techniques and tools supported by our efforts to transition open surgery to MIS. The Open to MIS initiative focuses on establishing our presence in and working to optimize open surgery globally, while capturing the market opportunity that exists in transitioning open procedures to MIS, whether through traditional MIS, or advanced technologies like robotics. To achieve this transition, we are focused on product training, surgical skill training and continued therapy innovation to advance MIS.
|
|
•
|
Changes in procedural volumes, competitive pressure, reimbursement challenges, reprocessed products, impacts from changes in the mix of our product offerings, fluctuations in currency exchange rates and pricing pressure, particularly in developed markets.
|
|
•
|
Our ability to create markets and drive product and procedures into emerging markets. We have high quality and cost-effective surgical products designed for customers in emerging markets such as the ReliaMax reusable stapler, which is reposable (part reusable, part disposable), and the ValleyLab LS10 single channel vessel sealing generator, which is compatible with our line of LigaSure instruments and designed for simplified use and affordability.
|
|
•
|
Continued acceptance and future growth within the end stage renal disease market. The population of patients treated for end stage renal disease globally is expected to double over the next decade. We will grow our therapy innovation with scalable and affordable dialysis delivery and investing in vascular creation and maintenance technologies. Our ability to successfully integrate Bellco into Medtronic. Bellco is a pioneer in hemodialysis treatment solutions that we acquired in February 2016.
|
|
•
|
Continued growth due to cross-selling initiatives of Minimally Invasive Therapies Group within other businesses with Medtronic.
|
|
•
|
Continued elevation of the standard of care for respiratory compromise, a progressive condition impacting a patient’s ability to breathe effectively. The Capnostream35 is expected to launch in fiscal year 2017.
|
|
•
|
Creation of less invasive standards of care in diseases and conditions of the gastrointestinal tract and lung to enable earlier diagnosis and intervention.
|
|
•
|
Continued and future acceptance of advanced and general surgical care products from both physicians and patients of open and minimally invasive procedures in Surgical Solutions, including stapling, vessel sealing, and other surgical instruments.
|
|
•
|
Expanding the use of less invasive treatments and furthering our commitment to improving options for women with abnormal uterine bleeding with the fiscal year 2017 acquisition of a highly profitable and fast-growing gynecology business. The addition will expand and strengthen the Minimally Invasive Therapies Group’s offerings and complement the existing global gynecology business.
|
|
•
|
Ability to develop a surgical robotic platform that reduces the variability of surgical procedures and improves the repeatability and reliability of procedures
|
|
•
|
Continued acceptance of other recently launched products including the Endo GIA Reinforced Reload, the LigaSure Maryland jaw laparoscopic sealer and divider, and three additional sizes of the Sonicision Cordless Ultrasonic Dissection Device and the GastriSail Gastric Positioning System.
|
|
•
|
Future acceptance of the Signia Stapling System, expected to launch in fiscal year 2017. The single-hand use increases patient focus and consistent staple lines reduce leaks and tissue trauma.
|
|
•
|
Future acceptance of the HD multi-pass system, expected to launch in fiscal year 2018. The HD multi-pass system reduces infrastructure by requiring less water, has less start-up costs, and offers high quality ultrapure dialysate treatment.
|
|
•
|
Continued acceptance and growth in respiratory care, ventilation and airway management, patient monitoring, and homecare. Key products in this area include the Puritan Bennett 980 ventilator, Microstream Capnography bedside capnography monitor, portable monitor with Nellcor pulse oximetry system with OxiMax technology and the Nellcor Respiratory Compromise monitor with vital signs of SpO2, pulse rate, End-Tidal CO2, and Respiratory Rate.
|
|
•
|
Continued and future acceptance of Early Technologies, including the areas of GI solutions, advanced ablation, and interventional lung solutions. Recently launched products include the PillCam COLON capsule endoscopy, Emprint ablation system with Thermosphere Technology which maintains predictable spherical ablation zones throughout procedures reducing procedure time and cost, and the GenCut core biopsy system and the superDimension Triple Needle Cytology Brush, lung tissue biopsy tools for use with the superDimension navigation system. The superDimension system enables a minimally invasive approach to accessing difficult-to-reach areas of the lung, which can aid in the diagnosis of lung cancer.
|
|
•
|
Ability to generate product innovation and adoption of less invasive surgical techniques to help patients recover faster and at less overall cost to the healthcare system. Opportunities exist to provide advanced solutions that minimize complications and increase efficiency. Our goal is to create localized solutions to improve surgical approaches and increase access to care, address economic and clinical challenges, and advance minimally invasive surgery by minimizing complications, thereby reducing surgical variability and increasing efficiency.
|
|
•
|
Continued and future acceptance of the Valleylab FT10 energy platform, which we launched in fiscal year 2016. The faster sealing of the Valleylab FT10 decreases procedure times and auto-adjusting energy accommodates different tissue types.
|
|
•
|
Changes in procedural volumes, competitive and pricing pressure, reimbursement challenges, impacts from changes in the mix of our product offerings, the timing of product registration approvals, and fluctuations in currency exchange rates.
|
|
•
|
Continued commercial integration in the Restorative Therapies group and market acceptance of our new integrated solutions through the Surgical Synergy program, which integrates our spinal implants and Surgical Technologies' imaging and navigation equipment.
|
|
•
|
Market acceptance and continued adoption of innovative new products, such as our CD Horizon Solera Voyager system, our ELEVATE expandable interbody cage, and our OLIF25 and OLIF51 procedure solutions, both of which have recently been augmented with new implant technology.
|
|
•
|
Continued pricing and competitive pressures on premium balloon kyphoplasty (BKP) within Interventional Spine. Though we remain focused on communicating the clinical and economic benefits for premium BKP, we expect pressure in several markets to continue. We believe opportunities for growth exist in the broader vertebral compression fracture (VCF) and adjacent markets, and continue to pursue the development of other therapies to treat more patients with VCF, including the recent U.S. launches of both the Kyphon V vertebroplasty system and the Osteocool tumor ablation system.
|
|
•
|
Acceptance of Kanghui's broad portfolio of trauma, spine, and large-joint reconstruction products focused on the growing global value segment.
|
|
•
|
Continued acceptance and adoption rates of stimulators and leads approved to treat chronic pain in major markets around the world.
|
|
•
|
Ongoing obligations under the U.S. FDA consent decree entered in April 2015 relating to the SynchroMed drug infusion system and the Neuromodulation quality system. We continue to make progress against our U.S FDA consent decree commitments.
|
|
•
|
Continued and future acceptance of our current indications for Medtronic DBS Therapy for the treatment of movement disorders, epilepsy (approved in Europe), and OCD. The DBS Therapy portfolio includes Activa
|
|
•
|
Continued acceptance of InterStim Therapy for the treatment of the symptoms of overactive bladder, urinary retention, and bowel incontinence.
|
|
•
|
Continued acceptance and growth of our Surgical Technologies therapies, including Advanced Energy products and strategies to focus on its four core markets of orthopedic, spine, breast surgery, and CRDM replacements, Neurosurgery StealthStation S7 and O-Arm Imaging Systems, Midas and ENT power systems, and intraoperative nerve monitoring during surgical procedures utilizing the NIM-Response 3.0 during head and neck surgical procedures. Additionally, continued growth in nerve monitoring utilizing the NIM Eclipse system during spinal surgical procedures.
|
|
•
|
Acceptance of the recently launched NuVent sinus balloon, with built-in surgical EM navigation, used for chronic sinusitis to restore sinus drainage in a minimally invasive way.
|
|
•
|
Continued acceptance and growth of Neurovascular therapies, including the Solitare FR revascularization device for treatment of acute ischemic stroke and the Pipeline Embolization Devices, endovascular treatments for large or giant wide-necked brain aneurysms.
|
|
•
|
Future acceptance of the Medina mesh coil implant launched in European Union in May 2016. Medina Medical was acquired in August 2015 and focuses on the commercialization of treatments for vascular abnormalities of the brain, including cerebral aneurysms.
|
|
•
|
Efficiencies gained from fiscal year 2017 reorganization to provide a stronger focus on the diseases and conditions that we serve to further innovate, integrate platforms and leverage breadth of product portfolio across Restorative Therapies Group. Beginning in the first quarter of fiscal year 2017, the new reporting structure includes Spine, Brain Therapies (consists of Modulation, Neurovascular, and Neurosurgery), Pain Therapies (consists of Stimulation, Pump, and Interventional), and Specialty Therapies (consists of Pelvic Health, Advanced Energy, and ENT).
|
|
•
|
Increasing competition, potential risk of pricing pressures, reduction in reimbursement rates, and fluctuations in currency exchange rates.
|
|
•
|
Changes in medical reimbursement policies and programs. Continued acceptance and improved reimbursement of CGM technologies.
|
|
•
|
Continued acceptance from both physicians and patients of insulin-pump and CGM therapy.
|
|
•
|
Continued acceptance and future growth of the MiniMed 530G System, available in the U.S., which includes the insulin pump and Enlite sensor. This is the first system in the U.S. that assists in protecting against the risk of hypoglycemia by automatically suspending insulin delivery when glucose falls below a specified threshold.
|
|
•
|
Continued acceptance and future growth from our next-generation pump systems, the MiniMed 640G with SmartGuard predictive low-glucose management, which has launched in Europe, Australia, and select Latin
|
|
•
|
Acceptance of MiniMed Connect, which allows users to view their insulin pump and CGM data on a smartphone and provides remote monitoring and text message notifications. The Company received U.S. FDA approval during the first quarter of fiscal 2016.
|
|
•
|
Selection by UnitedHealthcare as the preferred in-network provider of insulin pumps, giving their members access to our advanced diabetes technology and comprehensive support services.
|
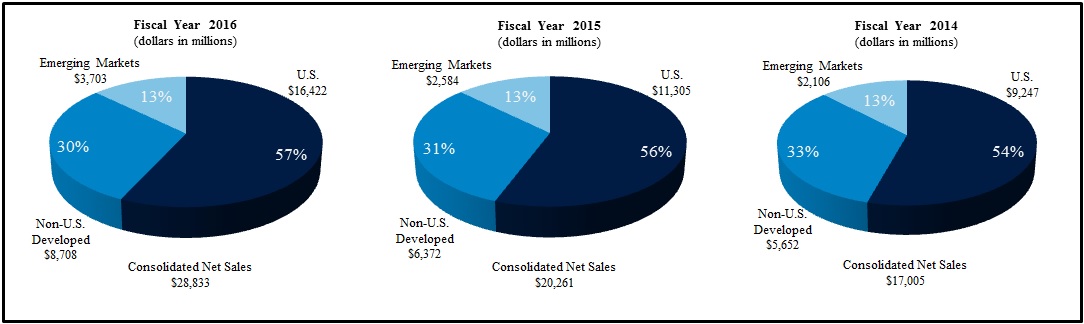
|
Fiscal Year 2016
|
Fiscal Year 2015
|
||||||||||||||||||||||
|
(in millions)
|
U.S.
|
Non-U.S. Developed Markets
|
Emerging Markets
|
U.S.
|
Non-U.S. Developed Markets
|
Emerging Markets
|
|||||||||||||||||
|
Cardiac and Vascular Group
|
$
|
5,347
|
|
$
|
3,283
|
|
$
|
1,566
|
|
$
|
4,435
|
|
$
|
3,412
|
|
$
|
1,514
|
|
|||||
|
Minimally Invasive Therapies Group
|
5,014
|
|
3,299
|
|
1,250
|
|
1,230
|
|
856
|
|
301
|
|
|||||||||||
|
Restorative Therapies Group
|
4,921
|
|
1,542
|
|
747
|
|
4,569
|
|
1,556
|
|
626
|
|
|||||||||||
|
Diabetes Group
|
1,140
|
|
584
|
|
140
|
|
1,071
|
|
548
|
|
143
|
|
|||||||||||
|
Total
|
$
|
16,422
|
|
$
|
8,708
|
|
$
|
3,703
|
|
$
|
11,305
|
|
$
|
6,372
|
|
$
|
2,584
|
|
|||||
|
|
Fiscal Year
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Cost of products sold
|
31.7
|
%
|
31.1
|
%
|
25.5
|
%
|
||
|
Research and development expense
|
7.7
|
|
8.1
|
|
8.7
|
|
||
|
Selling, general, and administrative expense
|
32.8
|
|
34.1
|
|
34.4
|
|
||
|
Fiscal Year
|
|||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Special charges (gains), net
|
$
|
70
|
|
$
|
(38
|
)
|
$
|
40
|
|
||
|
Restructuring charges, net
|
290
|
|
237
|
|
78
|
|
|||||
|
Certain litigation charges, net
|
26
|
|
42
|
|
770
|
|
|||||
|
Acquisition-related items
|
283
|
|
550
|
|
117
|
|
|||||
|
Amortization of intangible assets
|
1,931
|
|
733
|
|
349
|
|
|||||
|
Other expense, net
|
107
|
|
118
|
|
181
|
|
|||||
|
Interest expense, net
|
955
|
|
280
|
|
108
|
|
|||||
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Provision for income taxes
|
$
|
798
|
|
$
|
811
|
|
$
|
640
|
|
||
|
Income from operations before taxes
|
$
|
4,336
|
|
$
|
3,486
|
|
$
|
3,705
|
|
||
|
Effective tax rate
|
18.4
|
%
|
23.3
|
%
|
17.3
|
%
|
|||||
|
Non-GAAP provision for income taxes
|
$
|
1,171
|
|
$
|
1,055
|
|
$
|
971
|
|
||
|
Non-GAAP income from operations before taxes
|
$
|
7,399
|
|
$
|
5,799
|
|
$
|
5,069
|
|
||
|
Non-GAAP Nominal Tax Rate
|
15.8
|
%
|
18.2
|
%
|
19.2
|
%
|
|||||
|
Difference between the effective tax rate and Non-GAAP Nominal Tax Rate
|
(2.6
|
)%
|
(5.1
|
)%
|
1.9
|
%
|
|||||
|
|
Fiscal Year
|
||||||
|
(in millions)
|
2016
|
2015
|
|||||
|
Working capital
|
$
|
16,435
|
|
$
|
21,671
|
|
|
|
Current ratio
(1)
|
3.3:1.0
|
|
3.4:1.0
|
|
|||
|
Cash, cash equivalents, and current investments
|
$
|
12,634
|
|
$
|
19,480
|
|
|
|
Short-term borrowings and long-term debt
|
31,240
|
|
36,186
|
|
|||
|
Net cash position
(2)
|
$
|
(18,606
|
)
|
$
|
(16,706
|
)
|
|
|
Total shareholder's equity
|
$
|
52,063
|
|
$
|
53,230
|
|
|
|
Debt-to-total capital ratio
(3)
|
38
|
%
|
40
|
%
|
|||
|
(1)
|
The ratio of current assets to current liabilities.
|
|
(2)
|
The sum of cash, cash equivalents, and current investments less short-term borrowings and long-term debt and excludes non-current investments that are not considered readily available to fund current operations.
|
|
(3)
|
The ratio of total debt (short-term borrowings and long-term debt) to total capitalization (total debt and total shareholder's equity).
|
|
Rating for Fiscal Year Ended
(1)
|
||||
|
April 29, 2016
|
April 24, 2015
|
|||
|
Standard & Poor's (S&P) Ratings Services
|
||||
|
Long-term debt
|
A
|
A
|
||
|
Short-term debt
|
A-1
|
A-1
|
||
|
Moody's Investors Service (Moody's)
|
||||
|
Long-term debt
|
A3
|
A3
|
||
|
Short-term debt
|
P-2
|
P-2
|
||
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Cash provided by (used in):
|
|
|
|
|
|
|
|||||
|
Operating activities
|
$
|
5,218
|
|
$
|
4,902
|
|
$
|
4,959
|
|
||
|
Investing activities
|
2,245
|
|
(17,058
|
)
|
(3,594
|
)
|
|||||
|
Financing activities
|
(9,543
|
)
|
15,949
|
|
(918
|
)
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
113
|
|
(353
|
)
|
37
|
|
|||||
|
Net change in cash and cash equivalents
|
$
|
(1,967
|
)
|
$
|
3,440
|
|
$
|
484
|
|
||
|
Fiscal Year
|
|||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Net cash provided by operating activities
|
$
|
5,218
|
|
$
|
4,902
|
|
$
|
4,959
|
|
||
|
Net cash provided by (used in) investing activities
|
2,245
|
|
(17,058
|
)
|
(3,594
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(9,543
|
)
|
15,949
|
|
(918
|
)
|
|||||
|
Net cash provided by operating activities
|
5,218
|
|
4,902
|
|
4,959
|
|
|||||
|
Additions to property, plant, and equipment
|
(1,046
|
)
|
(571
|
)
|
(396
|
)
|
|||||
|
Free cash flow
|
$
|
4,172
|
|
$
|
4,331
|
|
$
|
4,563
|
|
||
|
Dividends to shareholders
|
$
|
2,139
|
|
$
|
1,337
|
|
$
|
1,116
|
|
||
|
Repurchase of ordinary shares
|
2,830
|
|
1,920
|
|
2,553
|
|
|||||
|
Issuances of ordinary shares
|
(491
|
)
|
(649
|
)
|
(1,307
|
)
|
|||||
|
Return to shareholders
|
$
|
4,478
|
|
$
|
2,608
|
|
$
|
2,362
|
|
||
|
Return of operating cash flow percentage
|
86
|
%
|
53
|
%
|
48
|
%
|
|||||
|
Return of free cash flow percentage
|
107
|
%
|
60
|
%
|
52
|
%
|
|||||
|
|
Maturity by Fiscal Year
|
|||||||||||||||||||||||||||
|
(in millions)
|
Total
|
2017
|
2018
|
2019
|
2020
|
2021
|
Thereafter
|
|||||||||||||||||||||
|
Contractual obligations related to off-balance sheet arrangements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Operating leases
(1)
|
$
|
544
|
|
$
|
180
|
|
$
|
130
|
|
$
|
90
|
|
$
|
56
|
|
$
|
33
|
|
$
|
55
|
|
|||||||
|
Commitments to fund minority investments/contingent acquisition consideration
(2)
|
520
|
|
89
|
|
72
|
|
155
|
|
48
|
|
41
|
|
115
|
|
||||||||||||||
|
Interest payments
(3)
|
13,925
|
|
1,058
|
|
1,014
|
|
911
|
|
882
|
|
760
|
|
9,300
|
|
||||||||||||||
|
Other
(4)
|
603
|
|
351
|
|
115
|
|
35
|
|
27
|
|
25
|
|
50
|
|
||||||||||||||
|
Contractual obligations related to off-balance sheet arrangements subtotal
|
$
|
15,592
|
|
$
|
1,678
|
|
$
|
1,331
|
|
$
|
1,191
|
|
$
|
1,013
|
|
$
|
859
|
|
$
|
9,520
|
|
|||||||
|
Contractual obligations reflected in the balance sheet:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Long-term debt, including current portion
(5)
|
$
|
30,805
|
|
$
|
887
|
|
$
|
6,188
|
|
$
|
408
|
|
$
|
3,774
|
|
$
|
1,102
|
|
$
|
18,446
|
|
|||||||
|
Capital leases
|
132
|
|
106
|
|
4
|
|
3
|
|
2
|
|
2
|
|
15
|
|
||||||||||||||
|
Contractual obligations reflected in the balance sheet subtotal
|
$
|
30,937
|
|
$
|
887
|
|
$
|
6,188
|
|
$
|
408
|
|
$
|
3,774
|
|
$
|
1,102
|
|
$
|
18,446
|
|
|||||||
|
Total contractual obligations
|
$
|
46,529
|
|
$
|
2,565
|
|
$
|
7,519
|
|
$
|
1,599
|
|
$
|
4,787
|
|
$
|
1,961
|
|
$
|
27,966
|
|
|||||||
|
(1)
|
Certain leases require us to pay real estate taxes, insurance, maintenance, and other operating expenses associated with the leased premises. These future costs are not included in the schedule above.
|
|
(2)
|
Certain commitments related to the funding of cost or equity method investments and/or previous acquisitions are contingent upon the achievement of certain product-related milestones and various other favorable operational conditions, and estimated royalty obligations. While it is not certain if and/or when these payments will be made, the maturity dates included in this table reflect our best estimates. Contingent consideration includes only the maximum potential amount of undiscounted future contingent consideration associated with all completed business combinations or purchases of intellectual property prior to April 24, 2009. See Note 2 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information regarding our debt agreements.
|
|
(3)
|
Interest payments in the table above reflect the contractual interest payments on our outstanding debt, and exclude the impact of the debt discount amortization and impact of interest rate swap agreements. See Note 7 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information regarding our debt agreements.
|
|
(4)
|
We have included inventory purchase commitments which are legally binding and specify minimum purchase quantities. These purchase commitments do not exceed our projected requirements and are in the normal course of business. These commitments do not include open purchase orders. These obligations also include certain research and development arrangements.
|
|
(5)
|
Long-term debt in the table above includes the $3.0 billion Term Loan Credit Agreement, $3.1 billion of CIFSA Senior Notes, $16.9 billion of 2015 Senior Notes, $1.5 billion of 2014 Senior Notes, $1.9 billion of 2013 Senior Notes, $1.1 billion of 2012 Senior Notes, $500 million of 2011 Senior Notes, $1.3 billion of 2010 Senior Notes, and $700 million of 2009 Senior Notes. The table above excludes the debt premium and discount, the fair value impact of outstanding interest rate swap agreements, and the unamortized gains from terminated interest rate swap agreements. See Notes 7 and 8 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information regarding the interest rate swap agreements.
|
|
/s/ PricewaterhouseCoopers LLP
|
|
|
|
Minneapolis, Minnesota
|
|
June 28, 2016
|
|
|
Fiscal Year
|
|||||||||||
|
(in millions, except per share data)
|
2016
|
2015
|
2014
|
|||||||||
|
Net sales
|
$
|
28,833
|
|
$
|
20,261
|
|
$
|
17,005
|
|
|||
|
Costs and expenses:
|
|
|
|
|||||||||
|
Cost of products sold
|
9,142
|
|
6,309
|
|
4,333
|
|
||||||
|
Research and development expense
|
2,224
|
|
1,640
|
|
1,477
|
|
||||||
|
Selling, general, and administrative expense
|
9,469
|
|
6,904
|
|
5,847
|
|
||||||
|
Special charges (gains), net
|
70
|
|
(38
|
)
|
40
|
|
||||||
|
Restructuring charges, net
|
290
|
|
237
|
|
78
|
|
||||||
|
Certain litigation charges, net
|
26
|
|
42
|
|
770
|
|
||||||
|
Acquisition-related items
|
283
|
|
550
|
|
117
|
|
||||||
|
Amortization of intangible assets
|
1,931
|
|
733
|
|
349
|
|
||||||
|
Other expense, net
|
107
|
|
118
|
|
181
|
|
||||||
|
Operating profit
|
5,291
|
|
3,766
|
|
3,813
|
|
||||||
|
Interest income
|
(431
|
)
|
(386
|
)
|
(271
|
)
|
||||||
|
Interest expense
|
1,386
|
|
666
|
|
379
|
|
||||||
|
Interest expense, net
|
955
|
|
280
|
|
108
|
|
||||||
|
Income from operations before income taxes
|
4,336
|
|
3,486
|
|
3,705
|
|
||||||
|
Provision for income taxes
|
798
|
|
811
|
|
640
|
|
||||||
|
Net income
|
$
|
3,538
|
|
$
|
2,675
|
|
$
|
3,065
|
|
|||
|
Basic earnings per share
|
$
|
2.51
|
|
$
|
2.44
|
|
$
|
3.06
|
|
|||
|
Diluted earnings per share
|
$
|
2.48
|
|
$
|
2.41
|
|
$
|
3.02
|
|
|||
|
Basic weighted average shares outstanding
|
1,409.6
|
|
1,095.5
|
|
1,002.1
|
|
||||||
|
Diluted weighted average shares outstanding
|
1,425.9
|
|
1,109.0
|
|
1,013.6
|
|
||||||
|
Cash dividends declared per ordinary share
|
$
|
1.52
|
|
$
|
1.22
|
|
$
|
1.12
|
|
|||
|
|
Fiscal Year
|
|||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
|||||||||
|
Net income
|
$
|
3,538
|
|
$
|
2,675
|
|
$
|
3,065
|
|
|||
|
Other comprehensive loss, net of tax:
|
|
|
|
|
|
|
||||||
|
Unrealized (loss) gain on available-for-sale securities, net of tax (benefit) expense of $(102), $11, and $(58), respectively
|
(121
|
)
|
20
|
|
(103
|
)
|
||||||
|
Translation adjustment
|
(197
|
)
|
(495
|
)
|
13
|
|
||||||
|
Net change in retirement obligations, net of tax (benefit) expense of $(46), $(173), and $72, respectively
|
(66
|
)
|
(366
|
)
|
87
|
|
||||||
|
Unrealized (loss) gain on derivatives, net of tax (benefit) expense of $(172), $146, and $(60), respectively
|
(300
|
)
|
254
|
|
(102
|
)
|
||||||
|
Other comprehensive loss, net of tax
|
(684
|
)
|
(587
|
)
|
(105
|
)
|
||||||
|
Comprehensive income
|
$
|
2,854
|
|
$
|
2,088
|
|
$
|
2,960
|
|
|||
|
(in millions, except per share data)
|
April 29,
2016 |
April 24,
2015 |
||||||
|
ASSETS
|
|
|
||||||
|
Current assets:
|
|
|
||||||
|
Cash and cash equivalents
|
$
|
2,876
|
|
$
|
4,843
|
|
||
|
Investments
|
9,758
|
|
14,637
|
|
||||
|
Accounts receivable, less allowances of $161 and $144, respectively
|
5,562
|
|
5,112
|
|
||||
|
Inventories
|
3,473
|
|
3,463
|
|
||||
|
Tax assets
|
697
|
|
1,335
|
|
||||
|
Prepaid expenses and other current assets
|
1,234
|
|
1,454
|
|
||||
|
Total current assets
|
23,600
|
|
30,844
|
|
||||
|
Property, plant, and equipment, net
|
4,841
|
|
4,699
|
|
||||
|
Goodwill
|
41,500
|
|
40,530
|
|
||||
|
Other intangible assets, net
|
26,899
|
|
28,101
|
|
||||
|
Long-term tax assets
|
1,383
|
|
774
|
|
||||
|
Other assets
|
1,559
|
|
1,737
|
|
||||
|
Total assets
|
$
|
99,782
|
|
$
|
106,685
|
|
||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
|
||||||
|
Current liabilities:
|
|
|
||||||
|
Short-term borrowings
|
$
|
993
|
|
$
|
2,434
|
|
||
|
Accounts payable
|
1,709
|
|
1,610
|
|
||||
|
Accrued compensation
|
1,712
|
|
1,611
|
|
||||
|
Accrued income taxes
|
566
|
|
935
|
|
||||
|
Deferred tax liabilities
|
—
|
|
119
|
|
||||
|
Other accrued expenses
|
2,185
|
|
2,464
|
|
||||
|
Total current liabilities
|
7,165
|
|
9,173
|
|
||||
|
Long-term debt
|
30,247
|
|
33,752
|
|
||||
|
Long-term accrued compensation and retirement benefits
|
1,759
|
|
1,535
|
|
||||
|
Long-term accrued income taxes
|
2,903
|
|
2,476
|
|
||||
|
Long-term deferred tax liabilities
|
3,729
|
|
4,700
|
|
||||
|
Other long-term liabilities
|
1,916
|
|
1,819
|
|
||||
|
Total liabilities
|
47,719
|
|
53,455
|
|
||||
|
Commitments and contingencies (Notes 2, 13, and 15)
|
|
|
||||||
|
Shareholders’ equity:
|
|
|
||||||
|
Ordinary shares— par value $0.0001, 2.6 billion shares authorized, 1,399,018,022 and 1,421,648,005 shares issued and outstanding, respectively
|
—
|
|
—
|
|
||||
|
Retained earnings
|
53,931
|
|
54,414
|
|
||||
|
Accumulated other comprehensive (loss) income
|
(1,868
|
)
|
(1,184
|
)
|
||||
|
Total shareholders’ equity
|
52,063
|
|
53,230
|
|
||||
|
Total liabilities and shareholders’ equity
|
$
|
99,782
|
|
$
|
106,685
|
|
||
|
Ordinary Shares
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Loss
|
Total
Shareholders’
Equity
|
||||||||||||||||
|
(in millions)
|
Number
|
Par Value
|
|||||||||||||||||
|
Balance as of April 26, 2013
|
1,016
|
|
$
|
102
|
|
$
|
19,061
|
|
$
|
(492
|
)
|
$
|
18,671
|
|
|||||
|
Net income
|
—
|
|
—
|
|
3,065
|
|
—
|
|
3,065
|
|
|||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
(105
|
)
|
(105
|
)
|
|||||||||
|
Dividends to shareholders
|
—
|
|
—
|
|
(1,116
|
)
|
—
|
|
(1,116
|
)
|
|||||||||
|
Issuance of shares under stock purchase and award plans
|
31
|
|
3
|
|
1,304
|
|
—
|
|
1,307
|
|
|||||||||
|
Repurchase of ordinary shares
|
(48
|
)
|
(5
|
)
|
(2,548
|
)
|
—
|
|
(2,553
|
)
|
|||||||||
|
Tax benefit from exercise of stock-based awards
|
—
|
|
—
|
|
29
|
|
—
|
|
29
|
|
|||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
145
|
|
—
|
|
145
|
|
|||||||||
|
Balance as of April 25, 2014
|
999
|
|
$
|
100
|
|
$
|
19,940
|
|
$
|
(597
|
)
|
$
|
19,443
|
|
|||||
|
Net income
|
—
|
|
—
|
|
2,675
|
|
—
|
|
2,675
|
|
|||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
(587
|
)
|
(587
|
)
|
|||||||||
|
Ordinary shares issued in connection with the Covidien plc acquisition, net of taxes
|
436
|
|
—
|
|
33,787
|
|
—
|
|
33,787
|
|
|||||||||
|
Result of contribution of Medtronic, Inc. to Medtronic plc
|
—
|
|
(99
|
)
|
99
|
|
—
|
|
—
|
|
|||||||||
|
Dividends to shareholders
|
—
|
|
—
|
|
(1,337
|
)
|
—
|
|
(1,337
|
)
|
|||||||||
|
Issuance of shares under stock purchase and award plans
|
17
|
|
2
|
|
647
|
|
—
|
|
649
|
|
|||||||||
|
Repurchase of ordinary shares
|
(30
|
)
|
(3
|
)
|
(1,917
|
)
|
—
|
|
(1,920
|
)
|
|||||||||
|
Tax benefit from exercise of stock-based awards
|
—
|
|
—
|
|
81
|
|
—
|
|
81
|
|
|||||||||
|
Stock-based compensation
|
|
|
—
|
|
439
|
|
—
|
|
439
|
|
|||||||||
|
Balance as of April 24, 2015
|
1,422
|
|
$
|
—
|
|
$
|
54,414
|
|
$
|
(1,184
|
)
|
$
|
53,230
|
|
|||||
|
Net income
|
—
|
|
—
|
|
3,538
|
|
—
|
|
3,538
|
|
|||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
(684
|
)
|
(684
|
)
|
|||||||||
|
Dividends to shareholders
|
—
|
|
—
|
|
(2,139
|
)
|
—
|
|
(2,139
|
)
|
|||||||||
|
Issuance of shares under stock purchase and award plans
|
15
|
|
—
|
|
491
|
|
—
|
|
491
|
|
|||||||||
|
Repurchase of ordinary shares
|
(38
|
)
|
—
|
|
(2,830
|
)
|
—
|
|
(2,830
|
)
|
|||||||||
|
Tax benefit from exercise of stock-based awards
|
—
|
|
—
|
|
82
|
|
—
|
|
82
|
|
|||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
375
|
|
—
|
|
375
|
|
|||||||||
|
Balance as of April 29, 2016
|
1,399
|
|
$
|
—
|
|
$
|
53,931
|
|
$
|
(1,868
|
)
|
$
|
52,063
|
|
|||||
|
|
Fiscal Year
|
|||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
|||||||||
|
Operating Activities:
|
|
|
|
|||||||||
|
Net income
|
$
|
3,538
|
|
$
|
2,675
|
|
$
|
3,065
|
|
|||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|||||||||
|
Depreciation and amortization
|
2,820
|
|
1,306
|
|
850
|
|
||||||
|
Amortization of debt discount and issuance costs
|
29
|
|
76
|
|
8
|
|
||||||
|
Acquisition-related items
|
218
|
|
634
|
|
110
|
|
||||||
|
Provision for doubtful accounts
|
49
|
|
35
|
|
43
|
|
||||||
|
Deferred income taxes
|
(460
|
)
|
(926
|
)
|
(207
|
)
|
||||||
|
Stock-based compensation
|
375
|
|
439
|
|
145
|
|
||||||
|
Loss on debt extinguishment
|
163
|
|
—
|
|
—
|
|
||||||
|
Other, net
|
(111
|
)
|
(134
|
)
|
(28
|
)
|
||||||
|
Change in operating assets and liabilities, net of acquisitions:
|
|
|
|
|||||||||
|
Accounts receivable, net
|
(435
|
)
|
(413
|
)
|
(70
|
)
|
||||||
|
Inventories
|
(186
|
)
|
(282
|
)
|
(39
|
)
|
||||||
|
Accounts payable and accrued liabilities
|
(65
|
)
|
1,616
|
|
(117
|
)
|
||||||
|
Other operating assets and liabilities
|
(403
|
)
|
643
|
|
444
|
|
||||||
|
Certain litigation charges, net
|
26
|
|
42
|
|
770
|
|
||||||
|
Certain litigation payments
|
(340
|
)
|
(809
|
)
|
(15
|
)
|
||||||
|
Net cash provided by operating activities
|
5,218
|
|
4,902
|
|
4,959
|
|
||||||
|
Investing Activities:
|
|
|
|
|||||||||
|
Acquisitions, net of cash acquired
|
(1,213
|
)
|
(14,884
|
)
|
(385
|
)
|
||||||
|
Additions to property, plant, and equipment
|
(1,046
|
)
|
(571
|
)
|
(396
|
)
|
||||||
|
Purchases of marketable securities
|
(5,406
|
)
|
(7,582
|
)
|
(10,895
|
)
|
||||||
|
Sales and maturities of marketable securities
|
9,924
|
|
5,890
|
|
8,111
|
|
||||||
|
Other investing activities, net
|
(14
|
)
|
89
|
|
(29
|
)
|
||||||
|
Net cash provided by (used in) investing activities
|
2,245
|
|
(17,058
|
)
|
(3,594
|
)
|
||||||
|
Financing Activities:
|
|
|
|
|||||||||
|
Acquisition-related contingent consideration
|
(22
|
)
|
(85
|
)
|
(1
|
)
|
||||||
|
Change in short-term borrowings, net
|
7
|
|
(1
|
)
|
127
|
|
||||||
|
Repayment of short-term borrowings (maturities greater than 90 days)
|
(139
|
)
|
(150
|
)
|
(1,301
|
)
|
||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days)
|
139
|
|
150
|
|
1,176
|
|
||||||
|
Issuance of long-term debt
|
—
|
|
19,942
|
|
1,994
|
|
||||||
|
Payments on long-term debt
|
(5,132
|
)
|
(1,268
|
)
|
(565
|
)
|
||||||
|
Dividends to shareholders
|
(2,139
|
)
|
(1,337
|
)
|
(1,116
|
)
|
||||||
|
Issuance of ordinary shares
|
491
|
|
649
|
|
1,307
|
|
||||||
|
Repurchase of ordinary shares
|
(2,830
|
)
|
(1,920
|
)
|
(2,553
|
)
|
||||||
|
Other financing activities
|
82
|
|
(31
|
)
|
14
|
|
||||||
|
Net cash (used in) provided by financing activities
|
(9,543
|
)
|
15,949
|
|
(918
|
)
|
||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
113
|
|
(353
|
)
|
37
|
|
||||||
|
Net change in cash and cash equivalents
|
(1,967
|
)
|
3,440
|
|
484
|
|
||||||
|
Cash and cash equivalents at beginning of period
|
4,843
|
|
1,403
|
|
919
|
|
||||||
|
Cash and cash equivalents at end of period
|
$
|
2,876
|
|
$
|
4,843
|
|
$
|
1,403
|
|
|||
|
Supplemental Cash Flow Information
|
|
|
|
|||||||||
|
Cash paid for:
|
|
|
|
|||||||||
|
Income taxes
|
$
|
1,379
|
|
$
|
632
|
|
$
|
521
|
|
|||
|
Interest
|
1,266
|
|
578
|
|
394
|
|
||||||
|
(in millions)
|
April 29,
2016 |
April 24,
2015 |
|||||
|
Finished goods
|
$
|
2,242
|
|
$
|
2,268
|
|
|
|
Work in-process
|
499
|
|
509
|
|
|||
|
Raw materials
|
732
|
|
686
|
|
|||
|
Total
|
$
|
3,473
|
|
$
|
3,463
|
|
|
|
(in millions)
|
April 29,
2016 |
April 24,
2015 |
Lives
(in years) |
|||||||
|
Land and land improvements
|
$
|
215
|
|
$
|
217
|
|
Up to 20
|
|
||
|
Buildings and leasehold improvements
|
2,394
|
|
2,314
|
|
Up to 40
|
|
||||
|
Equipment
|
6,328
|
|
5,649
|
|
Generally 3-7, up to 15
|
|
||||
|
Construction in progress
|
777
|
|
683
|
|
—
|
|
||||
|
Subtotal
|
9,714
|
|
8,863
|
|
|
|
||||
|
Less: Accumulated depreciation
|
(4,873
|
)
|
(4,164
|
)
|
|
|
||||
|
Property, plant, and equipment, net
|
$
|
4,841
|
|
$
|
4,699
|
|
|
|
||
|
•
|
Level 1 - Inputs are quoted prices in active markets for identical assets or liabilities.
|
|
•
|
Level 2 - Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly.
|
|
•
|
Level 3 - Inputs are unobservable for the asset or liability.
|
|
(in millions)
|
Warranty Obligation
|
|||
|
Balance as of April 25, 2014
|
$
|
32
|
|
|
|
Fair value of warranty obligation acquired from Covidien
|
23
|
|
||
|
Technology upgrade commitment
|
74
|
|
||
|
Warranty claims provision
|
30
|
|
||
|
Settlements made
|
(24
|
)
|
||
|
Balance as of April 24, 2015
|
$
|
135
|
|
|
|
Warranty claims provision
|
64
|
|
||
|
Settlements made
|
(91
|
)
|
||
|
Balance as of April 29, 2016
|
$
|
108
|
|
|
|
|
Fiscal Year
|
||||||||||
|
(in millions, except per share data)
|
2016
|
2015
|
2014
|
||||||||
|
Numerator:
|
|
|
|
|
|
|
|||||
|
Net income attributable to ordinary shareholders
|
$
|
3,538
|
|
$
|
2,675
|
|
$
|
3,065
|
|
||
|
Denominator:
|
|
|
|
|
|
|
|||||
|
Basic – weighted average shares outstanding
|
1,409.6
|
|
1,095.5
|
|
1,002.1
|
|
|||||
|
Effect of dilutive securities:
|
|
|
|
|
|
|
|||||
|
Employee stock options
|
12.2
|
|
9.1
|
|
7.1
|
|
|||||
|
Employee restricted stock units
|
4.0
|
|
4.3
|
|
4.3
|
|
|||||
|
Other
|
0.1
|
|
0.1
|
|
0.1
|
|
|||||
|
Diluted – weighted average shares outstanding
|
1,425.9
|
|
1,109.0
|
|
1,013.6
|
|
|||||
|
Basic earnings per share
|
$
|
2.51
|
|
$
|
2.44
|
|
$
|
3.06
|
|
||
|
Diluted earnings per share
|
$
|
2.48
|
|
$
|
2.41
|
|
$
|
3.02
|
|
||
|
(in millions, except per share data)
|
||||
|
Cash consideration paid to Covidien shareholders ($35.19 per share)
|
$
|
15,994
|
|
|
|
Cash consideration paid for vested Covidien share awards ($35.19 per share)
|
33
|
|
||
|
Total cash consideration
|
$
|
16,027
|
|
|
|
Covidien shares outstanding as of January 23, 2015
|
455
|
|
||
|
Exchange ratio per share
|
0.956
|
|
||
|
Total Medtronic shares issued to Covidien shareholders
(1)
|
435
|
|
||
|
Medtronic per share value as of January 23, 2015
|
$
|
76.95
|
|
|
|
Fair value of Medtronic shares issued to Covidien shareholders
|
$
|
33,435
|
|
|
|
Fair value of shares issued to Covidien share award holders
(1)
|
70
|
|
||
|
Fair value of share options and awards issued to Covidien share option and award holders
|
456
|
|
||
|
Total fair value of consideration transferred
|
$
|
49,988
|
|
|
|
(estimated in millions)
|
|||
|
Accounts receivable
|
$
|
1,349
|
|
|
Inventories
|
2,219
|
|
|
|
Other current assets
|
3,181
|
|
|
|
Property, plant, and equipment
|
2,293
|
|
|
|
Goodwill
|
29,979
|
|
|
|
Intangible assets
|
26,210
|
|
|
|
Other assets
|
761
|
|
|
|
Total assets acquired
|
65,992
|
|
|
|
|
|||
|
Short-term borrowings
|
1,011
|
|
|
|
Other current liabilities
|
2,434
|
|
|
|
Long-term debt
|
4,623
|
|
|
|
Long-term deferred tax liabilities
|
4,745
|
|
|
|
Other long-term liabilities
|
3,191
|
|
|
|
Total liabilities assumed
|
16,004
|
|
|
|
Net assets acquired
|
$
|
49,988
|
|
|
(in millions)
|
2015
|
2014
|
|||||
|
Pro forma net sales
|
$
|
28,369
|
|
$
|
27,380
|
|
|
|
Pro forma net income
|
$
|
3,944
|
|
$
|
3,280
|
|
|
|
(in millions)
|
Twelve, Inc.
|
RF Surgical Systems, Inc.
|
Medina Medical
|
All Other
|
Total
|
||||||||||||||
|
Other current assets
|
$
|
60
|
|
$
|
40
|
|
$
|
11
|
|
$
|
134
|
|
$
|
245
|
|
||||
|
Property, plant, and equipment
|
—
|
|
2
|
|
—
|
|
39
|
|
41
|
|
|||||||||
|
IPR&D
|
192
|
|
—
|
|
122
|
|
143
|
|
457
|
|
|||||||||
|
Other intangible assets
|
—
|
|
115
|
|
—
|
|
199
|
|
314
|
|
|||||||||
|
Goodwill
|
291
|
|
135
|
|
126
|
|
304
|
|
856
|
|
|||||||||
|
Other assets
|
—
|
|
2
|
|
—
|
|
15
|
|
17
|
|
|||||||||
|
Total assets acquired
|
543
|
|
294
|
|
259
|
|
834
|
|
1,930
|
|
|||||||||
|
|
|||||||||||||||||||
|
Current liabilities
|
37
|
|
27
|
|
6
|
|
91
|
|
161
|
|
|||||||||
|
Long-term deferred tax liabilities, net
|
34
|
|
27
|
|
34
|
|
53
|
|
148
|
|
|||||||||
|
Other liabilities
|
—
|
|
—
|
|
—
|
|
50
|
|
50
|
|
|||||||||
|
Total liabilities assumed
|
71
|
|
54
|
|
40
|
|
194
|
|
359
|
|
|||||||||
|
Net assets acquired
|
$
|
472
|
|
$
|
240
|
|
$
|
219
|
|
$
|
640
|
|
$
|
1,571
|
|
||||
|
(in millions)
|
NGC Medical S.p.A.
|
Sapiens Steering Brain Stimulation
|
All Other
|
Total
|
|||||||||||
|
Other current assets
|
$
|
55
|
|
$
|
3
|
|
$
|
12
|
|
$
|
70
|
|
|||
|
Property, plant, and equipment
|
15
|
|
1
|
|
2
|
|
18
|
|
|||||||
|
IPR&D
|
—
|
|
30
|
|
39
|
|
69
|
|
|||||||
|
Other intangible assets
|
159
|
|
—
|
|
157
|
|
316
|
|
|||||||
|
Goodwill
|
197
|
|
170
|
|
108
|
|
475
|
|
|||||||
|
Other assets
|
3
|
|
3
|
|
49
|
|
55
|
|
|||||||
|
Total assets acquired
|
429
|
|
207
|
|
367
|
|
1,003
|
|
|||||||
|
|
|||||||||||||||
|
Current liabilities
|
34
|
|
4
|
|
6
|
|
44
|
|
|||||||
|
Long-term deferred tax liabilities, net
|
51
|
|
—
|
|
66
|
|
117
|
|
|||||||
|
Other liabilities
|
4
|
|
—
|
|
—
|
|
4
|
|
|||||||
|
Total liabilities assumed
|
89
|
|
4
|
|
72
|
|
165
|
|
|||||||
|
Net assets acquired
|
$
|
340
|
|
$
|
203
|
|
$
|
295
|
|
$
|
838
|
|
|||
|
(in millions)
|
TYRX, Inc.
|
All Other
|
Total
|
||||||||
|
Current assets
|
$
|
6
|
|
$
|
14
|
|
$
|
20
|
|
||
|
Property, plant, and equipment
|
1
|
|
7
|
|
8
|
|
|||||
|
Intangible assets
|
94
|
|
61
|
|
155
|
|
|||||
|
Goodwill
|
132
|
|
123
|
|
255
|
|
|||||
|
Total assets acquired
|
233
|
|
205
|
|
438
|
|
|||||
|
|
|||||||||||
|
Current liabilities
|
4
|
|
12
|
|
16
|
|
|||||
|
Long-term deferred tax liabilities, net
|
7
|
|
—
|
|
7
|
|
|||||
|
Total liabilities assumed
|
11
|
|
12
|
|
23
|
|
|||||
|
Net assets acquired
|
$
|
222
|
|
$
|
193
|
|
$
|
415
|
|
||
|
($ in millions)
|
Fair Value at April 29, 2016
|
Valuation
Technique
|
Unobservable Input
|
Range
|
|||||
|
Discount rate
|
11% - 27%
|
||||||||
|
Revenue-based payments
|
$
|
195
|
|
Discounted cash flow
|
Probability of payment
|
30% - 100%
|
|||
|
|
|
|
|
Projected fiscal year of payment
|
2017 - 2025
|
||||
|
Discount rate
|
0.3% - 5.5%
|
||||||||
|
Product development-based payments
|
$
|
182
|
|
Discounted cash flow
|
Probability of payment
|
75% - 100%
|
|||
|
|
|
|
|
Projected fiscal year of payment
|
2017 - 2025
|
||||
|
|
Fiscal Year
|
||||||
|
(in millions)
|
2016
|
2015
|
|||||
|
Beginning Balance
|
$
|
264
|
|
$
|
68
|
|
|
|
Acquired contingent consideration
|
—
|
|
236
|
|
|||
|
Purchase price contingent consideration
|
149
|
|
40
|
|
|||
|
Contingent consideration payments
|
(22
|
)
|
(85
|
)
|
|||
|
Change in fair value of contingent consideration
|
(14
|
)
|
5
|
|
|||
|
Ending Balance
|
$
|
377
|
|
$
|
264
|
|
|
|
(in millions)
|
Employee
Termination
Costs
|
Asset
Write-downs
|
Other
Costs
|
Total
|
|||||||||||
|
Balance as of April 25, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Restructuring charges
|
213
|
|
28
|
|
7
|
|
248
|
|
|||||||
|
Payments/write-downs
|
(77
|
)
|
(28
|
)
|
—
|
|
(105
|
)
|
|||||||
|
Balance as of April 24, 2015
|
$
|
136
|
|
$
|
—
|
|
$
|
7
|
|
$
|
143
|
|
|||
|
Restructuring charges
|
248
|
|
23
|
|
61
|
|
332
|
|
|||||||
|
Payments/write-downs
|
(153
|
)
|
(23
|
)
|
(31
|
)
|
(207
|
)
|
|||||||
|
Reversal of excess accrual
|
(18
|
)
|
$
|
—
|
|
$
|
—
|
|
(18
|
)
|
|||||
|
Balance as of April 29, 2016
|
$
|
213
|
|
$
|
—
|
|
$
|
37
|
|
$
|
250
|
|
|||
|
|
Covidien Initiative
|
||||||||||
|
(in millions)
|
Employee
Termination
Costs
|
Other
Costs
|
Total
|
||||||||
|
Balance as of January 26, 2015 (Acquisition Date)
|
$
|
76
|
|
$
|
27
|
|
$
|
103
|
|
||
|
Restructuring charges
|
—
|
|
—
|
|
—
|
|
|||||
|
Payments/write-downs
|
(10
|
)
|
(10
|
)
|
(20
|
)
|
|||||
|
Reversal of excess accrual
|
(5
|
)
|
—
|
|
(5
|
)
|
|||||
|
Balance as of April 24, 2015
|
$
|
61
|
|
$
|
17
|
|
$
|
78
|
|
||
|
Restructuring charges
|
—
|
|
—
|
|
—
|
|
|||||
|
Payments/write-downs
|
(49
|
)
|
(12
|
)
|
(61
|
)
|
|||||
|
Reversal of excess accrual
|
(10
|
)
|
—
|
|
(10
|
)
|
|||||
|
Balance as of April 29, 2016
|
$
|
2
|
|
$
|
5
|
|
$
|
7
|
|
||
|
Valuation
|
Balance Sheet Classification
|
||||||||||||||||||||||
|
(in millions)
|
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Investments
|
Other Assets
|
|||||||||||||||||
|
Available-for-sale securities
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Level 1:
|
|||||||||||||||||||||||
|
U.S. government and agency securities
|
$
|
792
|
|
$
|
14
|
|
$
|
(1
|
)
|
$
|
805
|
|
$
|
805
|
|
$
|
—
|
|
|||||
|
Marketable equity securities
|
75
|
|
21
|
|
(11
|
)
|
85
|
|
|
85
|
|
||||||||||||
|
Total Level 1
|
867
|
|
35
|
|
(12
|
)
|
890
|
|
805
|
|
85
|
|
|||||||||||
|
Level 2:
|
|||||||||||||||||||||||
|
Corporate debt securities
|
3,935
|
|
85
|
|
(24
|
)
|
3,996
|
|
3,996
|
|
—
|
|
|||||||||||
|
U.S. government and agency securities
|
902
|
|
2
|
|
—
|
|
904
|
|
904
|
|
—
|
|
|||||||||||
|
Mortgage-backed securities
|
1,016
|
|
17
|
|
(18
|
)
|
1,015
|
|
1,015
|
|
—
|
|
|||||||||||
|
Other asset-backed securities
|
192
|
|
3
|
|
—
|
|
195
|
|
195
|
|
—
|
|
|||||||||||
|
Debt funds
|
3,040
|
|
5
|
|
(281
|
)
|
2,764
|
|
2,764
|
|
—
|
|
|||||||||||
|
Total Level 2
|
9,085
|
|
112
|
|
(323
|
)
|
8,874
|
|
8,874
|
|
—
|
|
|||||||||||
|
Level 3:
|
|||||||||||||||||||||||
|
Corporate debt securities
|
1
|
|
—
|
|
—
|
|
1
|
|
—
|
|
1
|
|
|||||||||||
|
Auction rate securities
|
47
|
|
—
|
|
(3
|
)
|
44
|
|
—
|
|
44
|
|
|||||||||||
|
Total Level 3
|
48
|
|
—
|
|
(3
|
)
|
45
|
|
—
|
|
45
|
|
|||||||||||
|
Total available-for-sale securities
|
10,000
|
|
147
|
|
(338
|
)
|
9,809
|
|
9,679
|
|
130
|
|
|||||||||||
|
Trading securities:
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Level 1:
|
|||||||||||||||||||||||
|
Exchange-traded funds
|
65
|
|
15
|
|
(1
|
)
|
79
|
|
79
|
|
—
|
|
|||||||||||
|
Total Level 1:
|
65
|
|
15
|
|
(1
|
)
|
79
|
|
79
|
|
—
|
|
|||||||||||
|
Total trading securities
|
65
|
|
15
|
|
(1
|
)
|
79
|
|
79
|
|
—
|
|
|||||||||||
|
Cost method, equity method, and other investments:
|
|||||||||||||||||||||||
|
Level 3:
|
|||||||||||||||||||||||
|
Cost method, equity method, and other investments
|
506
|
|
—
|
|
—
|
|
N/A
|
|
—
|
|
506
|
|
|||||||||||
|
Total Level 3:
|
506
|
|
—
|
|
—
|
|
N/A
|
|
—
|
|
506
|
|
|||||||||||
|
Total cost method, equity method, and other investments
|
506
|
|
—
|
|
—
|
|
N/A
|
|
—
|
|
506
|
|
|||||||||||
|
Total investments
|
$
|
10,571
|
|
$
|
162
|
|
$
|
(339
|
)
|
|
$
|
9,888
|
|
$
|
9,758
|
|
$
|
636
|
|
||||
|
Valuation
|
Balance Sheet Classification
|
||||||||||||||||||||||
|
(in millions)
|
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Investments
|
Other Assets
|
|||||||||||||||||
|
Available-for-sale securities:
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Level 1:
|
|||||||||||||||||||||||
|
U.S. government and agency securities
|
$
|
1,525
|
|
$
|
17
|
|
$
|
(1
|
)
|
$
|
1,541
|
|
$
|
1,541
|
|
$
|
—
|
|
|||||
|
Marketable equity securities
|
64
|
|
35
|
|
(19
|
)
|
80
|
|
—
|
|
80
|
|
|||||||||||
|
Total Level 1
|
1,589
|
|
52
|
|
(20
|
)
|
1,621
|
|
1,541
|
|
80
|
|
|||||||||||
|
Level 2:
|
|||||||||||||||||||||||
|
Corporate debt securities
|
6,282
|
|
105
|
|
(10
|
)
|
6,377
|
|
6,377
|
|
—
|
|
|||||||||||
|
U.S. government and agency securities
|
1,597
|
|
4
|
|
(3
|
)
|
1,598
|
|
1,598
|
|
—
|
|
|||||||||||
|
Mortgage-backed securities
|
1,462
|
|
22
|
|
(6
|
)
|
1,478
|
|
1,478
|
|
—
|
|
|||||||||||
|
Non-U.S. government and agency securities
|
85
|
|
—
|
|
—
|
|
85
|
|
85
|
|
—
|
|
|||||||||||
|
Certificates of deposit
|
44
|
|
—
|
|
—
|
|
44
|
|
44
|
|
—
|
|
|||||||||||
|
Other asset-backed securities
|
504
|
|
3
|
|
—
|
|
507
|
|
507
|
|
—
|
|
|||||||||||
|
Debt funds
|
3,061
|
|
19
|
|
(150
|
)
|
2,930
|
|
2,930
|
|
—
|
|
|||||||||||
|
Total Level 2
|
13,035
|
|
153
|
|
(169
|
)
|
13,019
|
|
13,019
|
|
—
|
|
|||||||||||
|
Level 3:
|
|||||||||||||||||||||||
|
Corporate debt securities
|
1
|
|
—
|
|
—
|
|
1
|
|
—
|
|
1
|
|
|||||||||||
|
Auction rate securities
|
109
|
|
—
|
|
(4
|
)
|
105
|
|
—
|
|
105
|
|
|||||||||||
|
Total Level 3
|
110
|
|
—
|
|
(4
|
)
|
106
|
|
—
|
|
106
|
|
|||||||||||
|
Total available-for-sale securities
|
14,734
|
|
205
|
|
(193
|
)
|
14,746
|
|
14,560
|
|
186
|
|
|||||||||||
|
Trading securities:
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Level 1:
|
|||||||||||||||||||||||
|
Exchange-traded funds
|
58
|
|
19
|
|
—
|
|
77
|
|
77
|
|
—
|
|
|||||||||||
|
Total Level 1
|
58
|
|
19
|
|
—
|
|
77
|
|
77
|
|
—
|
|
|||||||||||
|
Total trading securities
|
58
|
|
19
|
|
—
|
|
77
|
|
77
|
|
—
|
|
|||||||||||
|
Cost method, equity method, and other investments:
|
|||||||||||||||||||||||
|
Level 3:
|
|||||||||||||||||||||||
|
Cost method, equity method, and other investments
|
520
|
|
—
|
|
—
|
|
N/A
|
|
—
|
|
520
|
|
|||||||||||
|
Total Level 3
|
520
|
|
—
|
|
—
|
|
N/A
|
|
—
|
|
520
|
|
|||||||||||
|
Total cost method, equity method, and other investments
|
520
|
|
—
|
|
—
|
|
N/A
|
|
—
|
|
520
|
|
|||||||||||
|
Total investments
|
$
|
15,312
|
|
$
|
224
|
|
$
|
(193
|
)
|
$
|
14,823
|
|
$
|
14,637
|
|
$
|
706
|
|
|||||
|
|
April 29, 2016
|
||||||||||||||
|
|
Less than 12 months
|
More than 12 months
|
|||||||||||||
|
(in millions)
|
Fair Value
|
Unrealized
Losses |
Fair Value
|
Unrealized
Losses |
|||||||||||
|
Corporate debt securities
|
$
|
756
|
|
$
|
(18
|
)
|
$
|
136
|
|
$
|
(6
|
)
|
|||
|
Auction rate securities
|
—
|
|
—
|
|
44
|
|
(3
|
)
|
|||||||
|
Mortgage-backed securities
|
196
|
|
(5
|
)
|
92
|
|
(5
|
)
|
|||||||
|
U.S. government and agency securities
|
308
|
|
(4
|
)
|
67
|
|
(5
|
)
|
|||||||
|
Debt funds
|
670
|
|
(26
|
)
|
1,601
|
|
(256
|
)
|
|||||||
|
Marketable equity securities
|
45
|
|
(11
|
)
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
1,975
|
|
$
|
(64
|
)
|
$
|
1,940
|
|
$
|
(275
|
)
|
|||
|
|
April 24, 2015
|
||||||||||||||
|
|
Less than 12 months
|
More than 12 months
|
|||||||||||||
|
(in millions)
|
Fair Value
|
Unrealized
Losses
|
Fair Value
|
Unrealized
Losses
|
|||||||||||
|
Corporate debt securities
|
$
|
944
|
|
$
|
(9
|
)
|
$
|
34
|
|
$
|
(1
|
)
|
|||
|
Auction rate securities
|
—
|
|
—
|
|
105
|
|
(4
|
)
|
|||||||
|
Mortgage-backed securities
|
346
|
|
(3
|
)
|
206
|
|
(3
|
)
|
|||||||
|
U.S. government and agency securities
|
356
|
|
(1
|
)
|
267
|
|
(3
|
)
|
|||||||
|
Debt funds
|
1,291
|
|
(109
|
)
|
559
|
|
(41
|
)
|
|||||||
|
Marketable equity securities
|
4
|
|
(19
|
)
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
2,941
|
|
$
|
(141
|
)
|
$
|
1,171
|
|
$
|
(52
|
)
|
|||
|
|
Valuation Technique
|
Unobservable Input
|
Range (Weighted Average)
|
|
Auction rate securities
|
Discounted cash flow
|
Years to principal recovery
|
2 yrs. - 12 yrs. (3 yrs.)
|
|
Illiquidity premium
|
6%
|
||
|
(in millions)
|
Total Level 3
Investments |
Corporate debt
securities |
Auction rate
securities |
||||||||
|
Balance as of April 24, 2015
|
$
|
106
|
|
$
|
1
|
|
$
|
105
|
|
||
|
Total unrealized gains/(losses) included in other comprehensive income
|
(3
|
)
|
—
|
|
(3
|
)
|
|||||
|
Settlements
|
(58
|
)
|
—
|
|
(58
|
)
|
|||||
|
Balance as of April 29, 2016
|
$
|
45
|
|
$
|
1
|
|
$
|
44
|
|
||
|
(in millions)
|
Total Level 3
Investments |
Corporate debt
securities |
Auction rate
securities |
||||||||
|
Balance as of April 25, 2014
|
$
|
106
|
|
$
|
9
|
|
$
|
97
|
|
||
|
Total realized losses and other-than-temporary impairment losses included in earnings
|
(5
|
)
|
(5
|
)
|
—
|
|
|||||
|
Total unrealized gains/(losses) included in other comprehensive income
|
10
|
|
2
|
|
8
|
|
|||||
|
Settlements
|
(5
|
)
|
(5
|
)
|
—
|
|
|||||
|
Balance as of April 24, 2015
|
$
|
106
|
|
$
|
1
|
|
$
|
105
|
|
||
|
|
Fiscal Year
|
||||||||||||||||||||||
|
|
2016
|
2015
|
2014
|
||||||||||||||||||||
|
(in millions)
|
Debt
(1)
|
Equity
(2)
|
Debt
(1)
|
Equity
(2)(3)
|
Debt
(1)
|
Equity
(2)(3)
|
|||||||||||||||||
|
Proceeds from sales
|
$
|
9,881
|
|
$
|
42
|
|
$
|
5,640
|
|
$
|
250
|
|
$
|
7,991
|
|
$
|
120
|
|
|||||
|
Gross realized gains
|
36
|
|
38
|
|
33
|
|
164
|
|
15
|
|
69
|
|
|||||||||||
|
Gross realized losses
|
(53
|
)
|
—
|
|
(19
|
)
|
—
|
|
(12
|
)
|
—
|
|
|||||||||||
|
Impairment losses recognized
|
—
|
|
(114
|
)
|
—
|
|
(29
|
)
|
(1
|
)
|
(9
|
)
|
|||||||||||
|
(1)
|
Includes available-for-sale debt securities.
|
|
(2)
|
Includes marketable equity securities, cost method, equity method, exchange-traded funds, and other investments.
|
|
(3)
|
As a result of certain acquisitions that occurred during the fiscal year ended
April 29, 2016
, the Company recognized a non-cash realized gain of
$9 million
on its previously-held minority investment included in
other expense, net
on the consolidated statement of income.
|
|
(4)
|
As a result of certain acquisitions that occurred during the fiscal year ended
April 24, 2015
, the Company recognized a non-cash realized gain of $
41 million
on its previously-held minority investments included in
other expense, net
on the consolidated statement of income. Also, a realized gain on an equity method investment totaling $
97 million
is included in
special (gains) charge
s
, net
on the consolidated statement of income.
|
|
(in millions)
|
April 29, 2016
|
||
|
Due in one year or less
|
$
|
899
|
|
|
Due after one year through five years
|
3,181
|
|
|
|
Due after five years through ten years
|
2,792
|
|
|
|
Due after ten years
|
88
|
|
|
|
Total debt securities
|
$
|
6,960
|
|
|
(in millions)
|
Cardiac and
Vascular Group
|
Minimally Invasive Therapies Group
|
Restorative
Therapies Group
|
Diabetes Group
|
Total
|
||||||||||||||
|
Balance as of April 25, 2014
|
$
|
2,881
|
|
$
|
—
|
|
$
|
6,368
|
|
$
|
1,344
|
|
$
|
10,593
|
|
||||
|
Goodwill as a result of Covidien acquisition
|
2,795
|
|
23,399
|
|
2,892
|
|
500
|
|
29,586
|
|
|||||||||
|
Goodwill as a result of other acquisitions
|
245
|
|
—
|
|
218
|
|
9
|
|
472
|
|
|||||||||
|
Other adjustments, net
|
—
|
|
—
|
|
(9
|
)
|
—
|
|
(9
|
)
|
|||||||||
|
Currency adjustment, net
|
(66
|
)
|
—
|
|
(45
|
)
|
(1
|
)
|
(112
|
)
|
|||||||||
|
Balance as of April 24, 2015
|
$
|
5,855
|
|
$
|
23,399
|
|
$
|
9,424
|
|
$
|
1,852
|
|
$
|
40,530
|
|
||||
|
Goodwill as a result of acquisitions
|
393
|
|
264
|
|
199
|
|
—
|
|
856
|
|
|||||||||
|
Measurement period adjustments related to Covidien
|
21
|
|
346
|
|
26
|
|
—
|
|
393
|
|
|||||||||
|
Other adjustments, net
|
—
|
|
(34
|
)
|
3
|
|
—
|
|
(31
|
)
|
|||||||||
|
Currency adjustment, net
|
(26
|
)
|
(191
|
)
|
(32
|
)
|
1
|
|
(248
|
)
|
|||||||||
|
Balance as of April 29, 2016
|
$
|
6,243
|
|
$
|
23,784
|
|
$
|
9,620
|
|
$
|
1,853
|
|
$
|
41,500
|
|
||||
|
Fiscal Year 2016
|
Fiscal Year 2015
|
||||||||||||||
|
(in millions)
|
Gross Carrying Amount
|
Accumulated Amortization
|
Gross Carrying Amount
|
Accumulated Amortization
|
|||||||||||
|
Definite-lived
|
|||||||||||||||
|
Customer-related
|
$
|
18,596
|
|
$
|
(1,331
|
)
|
$
|
18,492
|
|
$
|
(273
|
)
|
|||
|
Purchased technology and patents
|
11,397
|
|
(2,976
|
)
|
11,118
|
|
(2,268
|
)
|
|||||||
|
Trademarks and tradenames
|
854
|
|
(403
|
)
|
640
|
|
(363
|
)
|
|||||||
|
Other
|
72
|
|
(31
|
)
|
79
|
|
(44
|
)
|
|||||||
|
Total
|
$
|
30,919
|
|
$
|
(4,741
|
)
|
$
|
30,329
|
|
$
|
(2,948
|
)
|
|||
|
Indefinite-lived
|
|||||||||||||||
|
IPR&D
|
$
|
721
|
|
$
|
470
|
|
|||||||||
|
Tradenames
|
—
|
|
250
|
|
|||||||||||
|
Total
|
$
|
721
|
|
$
|
720
|
|
|||||||||
|
(in millions)
Fiscal Year
|
Amortization
Expense
|
||
|
2017
|
$
|
1,931
|
|
|
2018
|
1,899
|
|
|
|
2019
|
1,805
|
|
|
|
2020
|
1,757
|
|
|
|
2021
|
1,739
|
|
|
|
(in millions)
|
April 29, 2016
|
April 24, 2015
|
||||||
|
Capital lease obligations
|
$
|
106
|
|
$
|
16
|
|
||
|
Bank borrowings
|
387
|
|
303
|
|
||||
|
Floating rate three-year 2014 senior notes
|
250
|
|
—
|
|
||||
|
0.875 percent three-year 2014 senior notes
|
250
|
|
—
|
|
||||
|
2.625 percent five-year 2011 senior notes
|
—
|
|
500
|
|
||||
|
4.750 percent ten-year 2005 senior notes
|
—
|
|
600
|
|
||||
|
1.350 percent 2012 CIFSA senior notes
|
—
|
|
600
|
|
||||
|
2.800 percent 2010 CIFSA senior notes
|
—
|
|
400
|
|
||||
|
Interest rate swaps
|
—
|
|
10
|
|
||||
|
Debt premium
|
—
|
|
5
|
|
||||
|
Total Short-Term Borrowings
|
$
|
993
|
|
$
|
2,434
|
|
||
|
|
|
April 29, 2016
|
April 24, 2015
|
||||||||||||
|
(in millions, except interest rates)
|
Maturity by
Fiscal Year
|
Payable
|
Effective
Interest
Rate
|
Payable
|
Effective
Interest
Rate
|
||||||||||
|
Floating rate three-year 2014 senior notes
|
2017
|
$
|
—
|
|
—
|
%
|
$
|
250
|
|
0.32
|
%
|
||||
|
0.875 percent three-year 2014 senior notes
|
2017
|
—
|
|
—
|
|
250
|
|
0.91
|
|
||||||
|
6.000 percent ten-year 2008 CIFSA senior notes
|
2018
|
1,150
|
|
1.41
|
|
1,150
|
|
1.41
|
|
||||||
|
1.375 percent five-year 2013 senior notes
|
2018
|
1,000
|
|
1.41
|
|
1,000
|
|
1.41
|
|
||||||
|
1.500 percent three-year 2015 senior notes
|
2018
|
1,000
|
|
1.59
|
|
1,000
|
|
1.59
|
|
||||||
|
5.600 percent ten-year 2009 senior notes
|
2019
|
400
|
|
5.61
|
|
400
|
|
5.61
|
|
||||||
|
4.450 percent ten-year 2010 senior notes
|
2020
|
766
|
|
4.47
|
|
1,250
|
|
4.47
|
|
||||||
|
2.500 percent five-year 2015 senior notes
|
2020
|
2,500
|
|
2.52
|
|
2,500
|
|
2.52
|
|
||||||
|
Floating rate five-year 2015 senior notes
|
2020
|
500
|
|
1.04
|
|
500
|
|
1.04
|
|
||||||
|
4.200 percent ten-year 2010 CIFSA senior notes
|
2021
|
600
|
|
2.22
|
|
600
|
|
2.22
|
|
||||||
|
4.125 percent ten-year 2011 senior notes
|
2021
|
500
|
|
4.19
|
|
500
|
|
4.19
|
|
||||||
|
3.125 percent ten-year 2012 senior notes
|
2022
|
675
|
|
3.16
|
|
675
|
|
3.16
|
|
||||||
|
3.200 percent ten-year 2012 CIFSA senior notes
|
2023
|
650
|
|
2.66
|
|
650
|
|
2.66
|
|
||||||
|
3.150 percent seven-year 2015 senior notes
|
2022
|
2,500
|
|
3.18
|
|
2,500
|
|
3.18
|
|
||||||
|
2.750 percent ten-year 2013 senior notes
|
2023
|
530
|
|
2.78
|
|
1,250
|
|
2.78
|
|
||||||
|
2.950 percent ten-year 2013 CIFSA senior notes
|
2024
|
310
|
|
2.67
|
|
750
|
|
2.67
|
|
||||||
|
3.625 percent ten-year 2014 senior notes
|
2024
|
850
|
|
3.65
|
|
850
|
|
3.65
|
|
||||||
|
3.500 percent ten-year 2015 senior notes
|
2025
|
4,000
|
|
3.61
|
|
4,000
|
|
3.61
|
|
||||||
|
4.375 percent twenty-year 2015 senior notes
|
2035
|
2,382
|
|
4.44
|
|
2,500
|
|
4.44
|
|
||||||
|
6.550 percent thirty-year 2007 CIFSA senior notes
|
2038
|
374
|
|
3.75
|
|
850
|
|
3.75
|
|
||||||
|
6.500 percent thirty-year 2009 senior notes
|
2039
|
300
|
|
6.52
|
|
300
|
|
6.52
|
|
||||||
|
5.550 percent thirty-year 2010 senior notes
|
2040
|
500
|
|
5.56
|
|
500
|
|
5.56
|
|
||||||
|
4.500 percent thirty-year 2012 senior notes
|
2042
|
400
|
|
4.51
|
|
400
|
|
4.51
|
|
||||||
|
4.000 percent thirty-year 2013 senior notes
|
2043
|
325
|
|
4.12
|
|
750
|
|
4.12
|
|
||||||
|
4.625 percent thirty-year 2014 senior notes
|
2044
|
650
|
|
4.67
|
|
650
|
|
4.67
|
|
||||||
|
4.625 percent thirty-year 2015 senior notes
|
2045
|
4,000
|
|
4.64
|
|
4,000
|
|
4.64
|
|
||||||
|
Three-year term loan
|
2018
|
3,000
|
|
1.12
|
|
3,000
|
|
1.12
|
|
||||||
|
Interest rate swaps
|
2021-2022
|
89
|
|
—
|
|
79
|
|
—
|
|
||||||
|
Deferred gains from interest rate swap terminations, net
|
—
|
—
|
|
—
|
|
3
|
|
—
|
|
||||||
|
Capital lease obligations
|
2018-2026
|
26
|
|
4.66
|
|
129
|
|
3.52
|
|
||||||
|
Bank borrowings
|
2018-2021
|
56
|
|
6.46
|
|
17
|
|
—
|
|
||||||
|
Debt premium (discount)
|
2018-2045
|
214
|
|
—
|
|
499
|
|
—
|
|
||||||
|
Total Long-Term Debt
|
|
$
|
30,247
|
|
|
|
$
|
33,752
|
|
|
|
||||
|
(in millions)
Fiscal Year
|
|||
|
2017
|
$
|
993
|
|
|
2018
|
6,176
|
|
|
|
2019
|
411
|
|
|
|
2020
|
3,777
|
|
|
|
2021
|
1,104
|
|
|
|
Thereafter
|
18,476
|
|
|
|
Total debt
|
30,937
|
|
|
|
Less: Current portion of debt
|
993
|
|
|
|
Long-term portion of debt
|
$
|
29,944
|
|
|
(in millions)
|
|
Fiscal Year
|
||||||||||||
|
Derivatives Not Designated as Hedging Instruments
|
Location
|
2016
|
2015
|
2014
|
||||||||||
|
Currency exchange rate contracts
|
Other expense
|
$
|
33
|
|
$
|
210
|
|
$
|
15
|
|
||||
|
April 29, 2016
|
|
|
|
|
|
|||||
|
Gross Gains Recognized in OCI
on Effective Portion of Derivative
|
Effective Portion of Gains (Losses) on Derivative Reclassified from
AOCI into Income
|
|||||||||
|
(in millions)
|
||||||||||
|
Derivatives in Cash Flow Hedging Relationships
|
Amount
|
Location
|
Amount
|
|||||||
|
Currency exchange
rate contracts
|
$
|
(165
|
)
|
Other expense, net
|
$
|
405
|
|
|||
|
|
|
|
Cost of products sold
|
(37
|
)
|
|||||
|
Total
|
$
|
(165
|
)
|
|
$
|
368
|
|
|||
|
April 24, 2015
|
|
|
|
|
|
|||||
|
Gross Losses Recognized in OCI
on Effective Portion of Derivative
|
Effective Portion of Gains (Losses) on Derivative Reclassified from
AOCI into Income
|
|||||||||
|
(in millions)
|
||||||||||
|
Derivatives in Cash Flow Hedging Relationships
|
Amount
|
Location
|
Amount
|
|||||||
|
Currency exchange
rate contracts
|
$
|
707
|
|
Other expense, net
|
$
|
221
|
|
|||
|
|
|
|
Cost of products sold
|
(65
|
)
|
|||||
|
Total
|
$
|
707
|
|
|
$
|
156
|
|
|||
|
April 25, 2014
|
|
|
|
|
|
|||||
|
Gross Gains Recognized in OCI
on Effective Portion of Derivative
|
Effective Portion of Gains (Losses) on Derivative Reclassified from
AOCI into Income
|
|||||||||
|
(in millions)
|
||||||||||
|
Derivatives in Cash Flow Hedging Relationships
|
Amount
|
Location
|
Amount
|
|||||||
|
Currency exchange
rate contracts
|
$
|
(152
|
)
|
Other expense, net
|
$
|
94
|
|
|||
|
|
Cost of products sold
|
(43
|
)
|
|||||||
|
Total
|
$
|
(152
|
)
|
|
$
|
51
|
|
|||
|
April 29, 2016
|
|
|
|
|
|||||||
|
|
Asset Derivatives
|
Liability Derivatives
|
|||||||||
|
(in millions)
|
Balance Sheet Location
|
Fair Value
|
Balance Sheet Location
|
Fair Value
|
|||||||
|
Derivatives designated as hedging instruments
|
|
|
|
|
|
|
|||||
|
Interest rate contracts
|
Prepaid expenses and other current assets
|
$
|
—
|
|
Other accrued expenses
|
$
|
—
|
|
|||
|
Currency exchange rate contracts
|
Prepaid expenses and other current assets
|
123
|
|
Other accrued expenses
|
89
|
|
|||||
|
Interest rate contracts
|
Other assets
|
89
|
|
Other long-term liabilities
|
48
|
|
|||||
|
Currency exchange rate contracts
|
Other assets
|
9
|
|
Other long-term liabilities
|
54
|
|
|||||
|
Total derivatives designated as hedging instruments
|
|
$
|
221
|
|
|
$
|
191
|
|
|||
|
Derivatives not designated as hedging instruments
|
|
|
|
|
|
|
|||||
|
Commodity derivatives
|
Prepaid expenses and other current assets
|
$
|
—
|
|
Other accrued expenses
|
$
|
1
|
|
|||
|
Currency exchange rate contracts
|
Prepaid expenses and other current assets
|
13
|
|
Other accrued expenses
|
23
|
|
|||||
|
Cross currency interest rate contracts
|
Other assets
|
14
|
|
Other long-term liabilities
|
4
|
|
|||||
|
Total derivatives not designated as hedging instruments
|
|
$
|
27
|
|
|
$
|
28
|
|
|||
|
Total derivatives
|
|
$
|
248
|
|
|
$
|
219
|
|
|||
|
April 24, 2015
|
|
|
|
|
|
|
|||||
|
|
Asset Derivatives
|
Liability Derivatives
|
|||||||||
|
(in millions)
|
Balance Sheet Location
|
Fair Value
|
Balance Sheet Location
|
Fair Value
|
|||||||
|
Derivatives designated as hedging instruments
|
|
|
|
|
|
|
|||||
|
Interest rate contracts
|
Prepaid expenses and other current assets
|
$
|
10
|
|
Other accrued expenses
|
$
|
—
|
|
|||
|
Currency exchange rate contracts
|
Prepaid expenses and other current assets
|
382
|
|
Other accrued expenses
|
12
|
|
|||||
|
Interest rate contracts
|
Other assets
|
79
|
|
Other long-term liabilities
|
71
|
|
|||||
|
Currency exchange rate contracts
|
Other assets
|
143
|
|
Other long-term liabilities
|
3
|
|
|||||
|
Total derivatives designated as hedging instruments
|
|
$
|
614
|
|
|
$
|
86
|
|
|||
|
Derivatives not designated as hedging instruments
|
|
|
|
|
|
|
|||||
|
Currency exchange rate contracts
|
Prepaid expenses and other current assets
|
$
|
119
|
|
Other accrued expenses
|
$
|
30
|
|
|||
|
Total derivatives not designated as hedging instruments
|
|
$
|
119
|
|
|
$
|
30
|
|
|||
|
Total derivatives
|
|
$
|
733
|
|
|
$
|
116
|
|
|||
|
April 29, 2016
|
April 24, 2015
|
|||||||||||||||||||||||
|
(in millions)
|
Level 1
|
Level 2
|
Level 3
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||||||||
|
Derivative Assets
|
$
|
145
|
|
$
|
103
|
|
$
|
—
|
|
$
|
644
|
|
$
|
89
|
|
$
|
—
|
|
||||||
|
Derivative Liabilities
|
166
|
|
53
|
|
—
|
|
45
|
|
71
|
|
—
|
|
||||||||||||
|
April 29, 2016
|
Gross Amount Not Offset on the Balance Sheet
|
|||||||||||||||
|
(in millions)
|
Gross Amount of Recognized Assets (Liabilities)
|
Financial Instruments
|
Cash Collateral (Received) or Posted
|
Net Amount
|
||||||||||||
|
Derivative Assets
|
||||||||||||||||
|
Currency exchange rate contracts
|
$
|
145
|
|
$
|
(98
|
)
|
$
|
(1
|
)
|
$
|
46
|
|
||||
|
Interest rate contracts
|
89
|
|
(20
|
)
|
—
|
|
69
|
|
||||||||
|
Cross Currency interest rate contracts
|
14
|
|
—
|
|
—
|
|
14
|
|
||||||||
|
$
|
248
|
|
$
|
(118
|
)
|
$
|
(1
|
)
|
$
|
129
|
|
|||||
|
Derivative Liabilities
|
||||||||||||||||
|
Currency exchange rate contracts
|
$
|
(166
|
)
|
$
|
85
|
|
26
|
|
$
|
(55
|
)
|
|||||
|
Interest rate contracts
|
(48
|
)
|
34
|
|
—
|
|
(14
|
)
|
||||||||
|
Cross currency interest rate contracts
|
(4
|
)
|
—
|
|
—
|
|
(4
|
)
|
||||||||
|
Commodity contracts
|
(1
|
)
|
—
|
|
—
|
|
(1
|
)
|
||||||||
|
$
|
(219
|
)
|
$
|
119
|
|
$
|
26
|
|
$
|
(74
|
)
|
|||||
|
Total
|
$
|
29
|
|
$
|
1
|
|
$
|
25
|
|
$
|
55
|
|
||||
|
April 24, 2015
|
Gross Amount Not Offset on the Balance Sheet
|
|||||||||||||||
|
(in millions)
|
Gross Amount of Recognized Assets (Liabilities)
|
Financial Instruments
|
Cash Collateral (Received) or Posted
|
Net Amount
|
||||||||||||
|
Derivative Assets
|
||||||||||||||||
|
Currency exchange rate contracts
|
$
|
644
|
|
$
|
(61
|
)
|
$
|
(325
|
)
|
$
|
258
|
|
||||
|
Interest rate contracts
|
89
|
|
(10
|
)
|
(13
|
)
|
66
|
|
||||||||
|
$
|
733
|
|
$
|
(71
|
)
|
$
|
(338
|
)
|
$
|
324
|
|
|||||
|
Derivative Liabilities
|
||||||||||||||||
|
Currency exchange rate contracts
|
$
|
(45
|
)
|
$
|
31
|
|
$
|
—
|
|
$
|
(14
|
)
|
||||
|
Interest rate contracts
|
(71
|
)
|
40
|
|
8
|
|
(23
|
)
|
||||||||
|
$
|
(116
|
)
|
$
|
71
|
|
$
|
8
|
|
$
|
(37
|
)
|
|||||
|
Total
|
$
|
617
|
|
$
|
—
|
|
$
|
(330
|
)
|
$
|
287
|
|
||||
|
|
Fiscal Year
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Weighted average fair value of options granted
|
$
|
13.72
|
|
$
|
25.39
|
|
$
|
12.00
|
|
||
|
Assumptions used:
|
|
|
|
|
|
|
|||||
|
Expected life (years)
(1)
|
5.94
|
|
4.24
|
|
6.40
|
|
|||||
|
Risk-free interest rate
(2)
|
1.79
|
%
|
0.99
|
%
|
1.88
|
%
|
|||||
|
Volatility
(3)
|
21.00
|
%
|
21.29
|
%
|
25.20
|
%
|
|||||
|
Dividend yield
(4)
|
1.96
|
%
|
1.66
|
%
|
2.02
|
%
|
|||||
|
(1)
|
Expected life:
The Company analyzes historical employee stock option exercise and termination data to estimate the expected life assumption. The Company calculates the expected life assumption using the midpoint scenario, which combines historical exercise data with hypothetical exercise data, as the Company believes this data currently represents the best estimate of the expected life of a new employee option. The Company also stratifies its employee population into two groups based upon distinctive exercise behavior patterns.
|
|
(2)
|
Risk-free interest rate:
The rate is based on the grant date yield of a zero-coupon U.S. Treasury bond whose maturity period equals the expected term of the option.
|
|
(3)
|
Volatility:
Expected volatility is based on a blend of historical volatility and an implied volatility of the Company’s ordinary shares. Implied volatility is based on market traded options of the Company’s ordinary shares.
|
|
(4)
|
Dividend yield:
The dividend yield rate is calculated by dividing the Company’s annual dividend, based on the most recent quarterly dividend rate, by the closing stock price on the grant date.
|
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Stock options
|
$
|
206
|
|
$
|
140
|
|
$
|
34
|
|
||
|
Restricted stock awards
|
148
|
|
284
|
|
98
|
|
|||||
|
Employees stock purchase plan
|
21
|
|
15
|
|
13
|
|
|||||
|
Total stock-based compensation expense
|
$
|
375
|
|
$
|
439
|
|
$
|
145
|
|
||
|
Cost of products sold
|
$
|
50
|
|
$
|
23
|
|
$
|
14
|
|
||
|
Research and development expense
|
37
|
|
29
|
|
27
|
|
|||||
|
Selling, general, and administrative expense
|
212
|
|
128
|
|
104
|
|
|||||
|
Restructuring charges
|
18
|
|
70
|
|
—
|
|
|||||
|
Acquisition-related items
|
58
|
|
189
|
|
—
|
|
|||||
|
Total stock-based compensation expense
|
375
|
|
439
|
|
145
|
|
|||||
|
Income tax benefits
|
(108
|
)
|
(138
|
)
|
(40
|
)
|
|||||
|
Total stock-based compensation expense, net of tax
|
$
|
267
|
|
$
|
301
|
|
$
|
105
|
|
||
|
|
Options (in thousands)
|
Wtd. Avg.
Exercise
Price
|
Wtd. Avg. Remaining Contractual Term (in years)
|
Aggregate Intrinsic Value (in millions)
|
||||||||
|
Outstanding at April 24, 2015
|
62,021
|
|
$
|
53.27
|
|
|||||||
|
Granted
|
5,785
|
|
77.76
|
|
||||||||
|
Exercised
|
(11,103
|
)
|
41.99
|
|
||||||||
|
Expired/Forfeited
|
(3,733
|
)
|
70.62
|
|
||||||||
|
Outstanding at April 29, 2016
|
52,970
|
|
57.09
|
|
6.47
|
$
|
1,168
|
|
||||
|
Vested and expected to vest at April 29, 2016
|
25,542
|
|
69.91
|
|
8.48
|
236
|
|
|||||
|
Exercisable at April 29, 2016
|
23,383
|
|
40.14
|
|
3.90
|
912
|
|
|||||
|
Fiscal Year
|
|||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Cash proceeds from options exercised
|
$
|
452
|
|
$
|
609
|
|
$
|
1,273
|
|
||
|
Intrinsic value of options exercised
|
374
|
|
329
|
|
249
|
|
|||||
|
Tax benefit related to options exercised
|
131
|
|
106
|
|
78
|
|
|||||
|
|
Awards (in thousands)
|
Wtd. Avg.
Grant
Price
|
||||
|
Nonvested at April 24, 2015
|
10,022
|
|
$
|
53.88
|
|
|
|
Granted
|
2,565
|
|
77.68
|
|
||
|
Vested
|
(3,148
|
)
|
42.96
|
|
||
|
Forfeited
|
(619
|
)
|
59.16
|
|
||
|
Nonvested at April 29, 2016
|
8,820
|
|
$
|
64.33
|
|
|
|
Fiscal Year
|
|||||||||||
|
(in millions, except per share data)
|
2016
|
2015
|
2014
|
||||||||
|
Weighted-average grant-date fair value per restricted stock award
|
$
|
77.68
|
|
$
|
69.30
|
|
$
|
55.62
|
|
||
|
Fair value of restricted stock awards vested
|
276
|
|
174
|
|
142
|
|
|||||
|
Tax benefit related to restricted stock awards vested
|
76
|
|
50
|
|
40
|
|
|||||
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
U.S.
|
$
|
333
|
|
$
|
639
|
|
$
|
1,690
|
|
||
|
International
|
4,003
|
|
2,847
|
|
2,015
|
|
|||||
|
Income from continuing operations before income taxes
|
$
|
4,336
|
|
$
|
3,486
|
|
$
|
3,705
|
|
||
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Current tax expense:
|
|
|
|
|
|
|
|||||
|
U.S.
|
$
|
440
|
|
$
|
1,128
|
|
$
|
532
|
|
||
|
International
|
835
|
|
502
|
|
248
|
|
|||||
|
Total current tax expense
|
1,275
|
|
1,630
|
|
780
|
|
|||||
|
Deferred tax (benefit) expense:
|
|
|
|
|
|
|
|||||
|
U.S.
|
(67
|
)
|
(705
|
)
|
(175
|
)
|
|||||
|
International
|
(410
|
)
|
(114
|
)
|
35
|
|
|||||
|
Net deferred tax benefit
|
(477
|
)
|
(819
|
)
|
(140
|
)
|
|||||
|
Total provision for income taxes
|
$
|
798
|
|
$
|
811
|
|
$
|
640
|
|
||
|
(in millions)
|
April 29, 2016
|
April 24, 2015
|
|||||
|
Deferred tax assets:
|
|
|
|
|
|||
|
Net operating loss, capital loss, and credit carryforwards
|
$
|
7,568
|
|
$
|
5,912
|
|
|
|
Other accrued liabilities
|
619
|
|
585
|
|
|||
|
Accrued compensation
|
358
|
|
330
|
|
|||
|
Pension and post-retirement benefits
|
530
|
|
449
|
|
|||
|
Stock-based compensation
|
316
|
|
418
|
|
|||
|
Other
|
341
|
|
303
|
|
|||
|
Inventory
|
225
|
|
171
|
|
|||
|
Federal and state benefit on uncertain tax positions
|
308
|
|
296
|
|
|||
|
Unrealized loss on available-for-sale securities and derivative financial instruments
|
107
|
|
—
|
|
|||
|
Gross deferred tax assets
|
10,372
|
|
8,464
|
|
|||
|
Valuation allowance
|
(7,032
|
)
|
(5,607
|
)
|
|||
|
Total deferred tax assets
|
3,340
|
|
2,857
|
|
|||
|
Deferred tax liabilities:
|
|
|
|
|
|||
|
Intangible assets
|
(5,173
|
)
|
(5,393
|
)
|
|||
|
Basis impairment
|
(230
|
)
|
(204
|
)
|
|||
|
Realized loss on derivative financial instruments
|
(112
|
)
|
(112
|
)
|
|||
|
Other
|
(179
|
)
|
(96
|
)
|
|||
|
Accumulated depreciation
|
(189
|
)
|
(217
|
)
|
|||
|
Unrealized gain on available-for-sale securities and derivative financial instruments
|
—
|
|
(160
|
)
|
|||
|
Total deferred tax liabilities
|
(5,883
|
)
|
(6,182
|
)
|
|||
|
Prepaid income taxes
|
365
|
|
427
|
|
|||
|
Income tax receivables
|
529
|
|
188
|
|
|||
|
Tax liabilities, net
|
$
|
(1,649
|
)
|
$
|
(2,710
|
)
|
|
|
Reported as (after valuation allowance and jurisdictional netting):
|
|
|
|
|
|||
|
Tax assets
|
$
|
697
|
|
$
|
1,335
|
|
|
|
Long-term tax assets
|
1,383
|
|
774
|
|
|||
|
Deferred tax liabilities
|
—
|
|
(119
|
)
|
|||
|
Long-term deferred tax liabilities
|
(3,729
|
)
|
(4,700
|
)
|
|||
|
Tax liabilities, net
|
$
|
(1,649
|
)
|
$
|
(2,710
|
)
|
|
|
|
Fiscal Year
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
U.S. federal statutory tax rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
Increase (decrease) in tax rate resulting from:
|
|
|
|
|
|
|
||
|
U.S. state taxes, net of federal tax benefit
|
0.9
|
|
0.8
|
|
0.6
|
|
||
|
Research and development credit
|
(1.2
|
)
|
(0.7
|
)
|
(0.5
|
)
|
||
|
Domestic production activities
|
(0.3
|
)
|
(0.4
|
)
|
(0.4
|
)
|
||
|
International
|
(23.4
|
)
|
(24.3
|
)
|
(17.7
|
)
|
||
|
Puerto Rico Excise Tax
|
(1.6
|
)
|
(1.7
|
)
|
(1.6
|
)
|
||
|
Impact of adjustments
(1)
|
11.4
|
|
13.3
|
|
5.6
|
|
||
|
Reversal of excess tax accruals
|
—
|
|
—
|
|
(1.9
|
)
|
||
|
Valuation allowance release
|
(0.9
|
)
|
—
|
|
—
|
|
||
|
Other, net
|
(1.5
|
)
|
1.3
|
|
(1.8
|
)
|
||
|
Effective tax rate
|
18.4
|
%
|
23.3
|
%
|
17.3
|
%
|
||
|
(1)
|
Adjustments include the impact of inventory step-up, impact of product technology upgrade commitment, special charges (gains), net, restructuring charges, net, certain litigation charges, net, acquisition-related items, amortization of intangible assets, and certain tax adjustments.
|
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Gross unrecognized tax benefits at beginning of fiscal year
|
$
|
2,860
|
|
$
|
1,172
|
|
$
|
1,068
|
|
||
|
Gross increases:
|
|
|
|
|
|
|
|||||
|
Prior year tax positions
|
36
|
|
331
|
|
64
|
|
|||||
|
Current year tax positions
|
202
|
|
231
|
|
166
|
|
|||||
|
Acquisitions
|
—
|
|
1,199
|
|
—
|
|
|||||
|
Gross decreases:
|
|
|
|
|
|
|
|||||
|
Prior year tax positions
|
(116
|
)
|
(40
|
)
|
(58
|
)
|
|||||
|
Settlements
|
(275
|
)
|
(33
|
)
|
(66
|
)
|
|||||
|
Statute of limitation lapses
|
(4
|
)
|
—
|
|
(2
|
)
|
|||||
|
Gross unrecognized tax benefits at end of fiscal year
|
$
|
2,703
|
|
$
|
2,860
|
|
$
|
1,172
|
|
||
|
Cash advance paid in connection with proposed settlements
|
(384
|
)
|
(378
|
)
|
—
|
|
|||||
|
Gross unrecognized tax benefits at end of fiscal year, net of cash advance
|
$
|
2,319
|
|
$
|
2,482
|
|
$
|
1,172
|
|
||
|
Jurisdiction
|
Earliest Year Open
|
|
|
United States - federal and state
|
1996
|
|
|
Brazil
|
2011
|
|
|
Canada
|
2005
|
|
|
China
|
2009
|
|
|
Costa Rica
|
2012
|
|
|
Dominican Republic
|
2011
|
|
|
France
|
2011
|
|
|
Germany
|
2009
|
|
|
India
|
2001
|
|
|
Ireland
|
2011
|
|
|
Israel
|
2010
|
|
|
Italy
|
2005
|
|
|
Japan
|
2010
|
|
|
Luxembourg
|
2009
|
|
|
Mexico
|
2005
|
|
|
Puerto Rico
|
2009
|
|
|
Singapore
|
2011
|
|
|
Switzerland
|
2003
|
|
|
United Kingdom
|
2009
|
|
|
|
U.S. Pension Benefits
|
Non-U.S. Pension Benefits
|
|||||||||||||
|
|
Fiscal Year
|
Fiscal Year
|
|||||||||||||
|
(in millions)
|
2016
|
2015
|
2016
|
2015
|
|||||||||||
|
Accumulated benefit obligation at end of year:
|
$
|
2,757
|
|
$
|
2,699
|
|
$
|
1,367
|
|
$
|
1,462
|
|
|||
|
Change in projected benefit obligation:
|
|
|
|
|
|
|
|
|
|||||||
|
Projected benefit obligation at beginning of year
|
$
|
2,956
|
|
$
|
2,203
|
|
$
|
1,647
|
|
$
|
1,031
|
|
|||
|
Service cost
|
120
|
|
104
|
|
81
|
|
60
|
|
|||||||
|
Interest cost
|
122
|
|
105
|
|
31
|
|
33
|
|
|||||||
|
Benefit obligations assumed in Covidien acquisition
|
—
|
|
214
|
|
—
|
|
472
|
|
|||||||
|
Employee contributions
|
—
|
|
—
|
|
16
|
|
16
|
|
|||||||
|
Plan curtailments and settlements
|
(28
|
)
|
—
|
|
(133
|
)
|
(35
|
)
|
|||||||
|
Actuarial (gain) loss
|
(42
|
)
|
391
|
|
(103
|
)
|
354
|
|
|||||||
|
Benefits paid
|
(80
|
)
|
(61
|
)
|
(49
|
)
|
(34
|
)
|
|||||||
|
Currency exchange rate changes and other
|
—
|
|
—
|
|
45
|
|
(250
|
)
|
|||||||
|
Projected benefit obligation at end of year
|
$
|
3,048
|
|
$
|
2,956
|
|
$
|
1,535
|
|
$
|
1,647
|
|
|||
|
Change in plan assets:
|
|
|
|
|
|
|
|
|
|||||||
|
Fair value of plan assets at beginning of year
|
$
|
2,204
|
|
$
|
1,917
|
|
$
|
1,189
|
|
$
|
889
|
|
|||
|
Actual return on plan assets
|
(70
|
)
|
69
|
|
(44
|
)
|
162
|
|
|||||||
|
Plan assets acquired in Covidien acquisition
|
—
|
|
188
|
|
—
|
|
262
|
|
|||||||
|
Employer contributions
|
112
|
|
91
|
|
93
|
|
80
|
|
|||||||
|
Employee contributions
|
—
|
|
—
|
|
16
|
|
16
|
|
|||||||
|
Plan settlements
|
(28
|
)
|
—
|
|
(118
|
)
|
(1
|
)
|
|||||||
|
Benefits paid
|
(80
|
)
|
(61
|
)
|
(49
|
)
|
(34
|
)
|
|||||||
|
Currency exchange rate changes
|
—
|
|
—
|
|
26
|
|
(185
|
)
|
|||||||
|
Fair value of plan assets at end of year
|
$
|
2,138
|
|
$
|
2,204
|
|
$
|
1,113
|
|
$
|
1,189
|
|
|||
|
Funded status at end of year:
|
|
|
|
|
|
|
|
|
|||||||
|
Fair value of plan assets
|
$
|
2,138
|
|
$
|
2,204
|
|
$
|
1,113
|
|
$
|
1,189
|
|
|||
|
Benefit obligations
|
3,048
|
|
2,956
|
|
1,535
|
|
1,647
|
|
|||||||
|
Underfunded status of the plans
|
$
|
(910
|
)
|
$
|
(752
|
)
|
$
|
(422
|
)
|
$
|
(458
|
)
|
|||
|
Recognized liability
|
$
|
(910
|
)
|
$
|
(752
|
)
|
$
|
(422
|
)
|
$
|
(458
|
)
|
|||
|
Amounts recognized on the consolidated
balance sheets consist of:
|
|||||||||||||||
|
Non-current assets
|
$
|
—
|
|
$
|
21
|
|
$
|
20
|
|
$
|
2
|
|
|||
|
Current liabilities
|
(12
|
)
|
(11
|
)
|
(8
|
)
|
(48
|
)
|
|||||||
|
Non-current liabilities
|
(898
|
)
|
(762
|
)
|
(434
|
)
|
(412
|
)
|
|||||||
|
Recognized liability
|
$
|
(910
|
)
|
$
|
(752
|
)
|
$
|
(422
|
)
|
$
|
(458
|
)
|
|||
|
Amounts recognized in accumulated other
comprehensive (loss) income:
|
|||||||||||||||
|
Prior service cost (benefit)
|
$
|
4
|
|
$
|
4
|
|
$
|
(14
|
)
|
$
|
(2
|
)
|
|||
|
Net actuarial loss
|
1,361
|
|
1,253
|
|
359
|
|
372
|
|
|||||||
|
Ending balance
|
$
|
1,365
|
|
$
|
1,257
|
|
$
|
345
|
|
$
|
370
|
|
|||
|
|
Fiscal Year
|
||||||
|
(in millions)
|
2016
|
2015
|
|||||
|
Accumulated benefit obligation
|
$
|
3,922
|
|
$
|
3,678
|
|
|
|
Projected benefit obligation
|
4,333
|
|
4,032
|
|
|||
|
Plan assets at fair value
|
2,981
|
|
2,823
|
|
|||
|
|
Fiscal Year
|
||||||
|
(in millions)
|
2016
|
2015
|
|||||
|
Projected benefit obligation
|
$
|
4,362
|
|
$
|
4,319
|
|
|
|
Plan assets at fair value
|
3,009
|
|
3,086
|
|
|||
|
|
U.S. Pension Benefits
|
Non-U.S. Pension Benefits
|
|||||||||||||||||||||
|
|
Fiscal Year
|
Fiscal Year
|
|||||||||||||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
2016
|
2015
|
2014
|
|||||||||||||||||
|
Service cost
|
$
|
120
|
|
$
|
104
|
|
$
|
107
|
|
$
|
81
|
|
$
|
60
|
|
$
|
54
|
|
|||||
|
Interest cost
|
122
|
|
105
|
|
97
|
|
31
|
|
33
|
|
29
|
|
|||||||||||
|
Expected return on plan assets
|
(180
|
)
|
(160
|
)
|
(141
|
)
|
(48
|
)
|
(41
|
)
|
(35
|
)
|
|||||||||||
|
Amortization of prior service cost
|
—
|
|
—
|
|
1
|
|
—
|
|
—
|
|
1
|
|
|||||||||||
|
Amortization of net actuarial loss
|
98
|
|
65
|
|
85
|
|
20
|
|
12
|
|
11
|
|
|||||||||||
|
Settlement gain
|
$
|
(1
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
(10
|
)
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Net periodic benefit cost
|
$
|
159
|
|
$
|
114
|
|
$
|
149
|
|
$
|
74
|
|
$
|
64
|
|
$
|
60
|
|
|||||
|
(in millions)
|
U.S. Pension
Benefits
|
Non-U.S.
Pension
Benefits
|
|||||
|
Net actuarial loss (gain)
|
205
|
|
(11
|
)
|
|||
|
Amortization of net actuarial loss
|
(98
|
)
|
(12
|
)
|
|||
|
Prior service cost
|
$
|
—
|
|
$
|
(12
|
)
|
|
|
Effect of exchange rates
|
1
|
|
10
|
|
|||
|
Total loss (gain) recognized in accumulated other comprehensive (loss) income
|
$
|
108
|
|
$
|
(25
|
)
|
|
|
Total loss recognized in net periodic benefit cost and accumulated other comprehensive (loss) income
|
$
|
267
|
|
$
|
49
|
|
|
|
|
U.S. Pension Benefits
|
Non-U.S. Pension Benefits
|
|||||||||||||||
|
|
Fiscal Year
|
Fiscal Year
|
|||||||||||||||
|
|
2016
|
2015
|
2014
|
2016
|
2015
|
2014
|
|||||||||||
|
Critical assumptions – projected benefit obligation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Discount rate
|
3.60% - 4.30%
|
|
4.20
|
%
|
4.75
|
%
|
0.25% - 10.20%
|
|
1.88
|
%
|
3.32
|
%
|
|||||
|
Rate of compensation increase
|
3.90
|
%
|
3.90
|
%
|
3.90
|
%
|
2.83
|
%
|
2.92
|
%
|
2.80
|
%
|
|||||
|
Critical assumptions – net periodic benefit cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Discount rate
|
4.20% - 4.80%
|
|
4.75
|
%
|
4.55
|
%
|
0.80% - 9.00%
|
|
3.32
|
%
|
3.52
|
%
|
|||||
|
Expected return on plan assets
|
8.20
|
%
|
8.25
|
%
|
8.25
|
%
|
4.35
|
%
|
4.77
|
%
|
4.76
|
%
|
|||||
|
Rate of compensation increase
|
3.90
|
%
|
3.90
|
%
|
3.90
|
%
|
2.92
|
%
|
2.80
|
%
|
2.78
|
%
|
|||||
|
U.S. Plans
|
|||||
|
|
Target Allocation
|
||||
|
|
April 29, 2016
|
April 24, 2015
|
|||
|
Asset Category
|
|
|
|
|
|
|
Equity securities
|
49
|
%
|
49
|
%
|
|
|
Debt securities
|
23
|
|
23
|
|
|
|
Other
|
28
|
|
28
|
|
|
|
Total
|
100
|
%
|
100
|
%
|
|
|
Non-U.S. Plans
|
|
|
|
|
|
|
|
Target Allocation
|
||||
|
|
April 29, 2016
|
April 24, 2015
|
|||
|
Asset Category
|
|
|
|
|
|
|
Equity securities
|
34
|
%
|
35
|
%
|
|
|
Debt securities
|
27
|
|
29
|
|
|
|
Other
|
39
|
|
36
|
|
|
|
Total
|
100
|
%
|
100
|
%
|
|
|
|
Fair Value
as of
|
|
|||||||||||||
|
|
Fair Value Measurements
Using Inputs Considered as
|
||||||||||||||
|
(in millions)
|
April 29, 2016
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Short-term investments
|
$
|
127
|
|
$
|
127
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S. government securities
|
146
|
|
137
|
|
9
|
|
—
|
|
|||||||
|
Corporate debt securities
|
216
|
|
—
|
|
216
|
|
—
|
|
|||||||
|
Equity mutual funds/commingled trusts
|
956
|
|
—
|
|
763
|
|
193
|
|
|||||||
|
Fixed income mutual funds
|
231
|
|
—
|
|
231
|
|
—
|
|
|||||||
|
Partnership units
|
462
|
|
—
|
|
—
|
|
462
|
|
|||||||
|
|
$
|
2,138
|
|
$
|
264
|
|
$
|
1,219
|
|
$
|
655
|
|
|||
|
|
Fair Value
as of
|
Fair Value Measurements
Using Inputs Considered as
|
|||||||||||||
|
(in millions)
|
April 24, 2015
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Short-term investments
|
$
|
247
|
|
$
|
247
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S. government securities
|
155
|
|
109
|
|
46
|
|
—
|
|
|||||||
|
Corporate debt securities
|
5
|
|
—
|
|
4
|
|
1
|
|
|||||||
|
Equity commingled trusts
|
951
|
|
—
|
|
751
|
|
200
|
|
|||||||
|
Fixed income commingled trusts
|
374
|
|
—
|
|
374
|
|
—
|
|
|||||||
|
Partnership units
|
472
|
|
—
|
|
—
|
|
472
|
|
|||||||
|
|
$
|
2,204
|
|
$
|
356
|
|
$
|
1,175
|
|
$
|
673
|
|
|||
|
(in millions)
|
Total Level 3 Investments
|
Corporate Debt Securities
|
Commingled Trusts
|
Partnership Units
|
|||||||||||
|
Balance as of April 24, 2015
|
$
|
673
|
|
$
|
1
|
|
$
|
200
|
|
$
|
472
|
|
|||
|
Total realized gains included in income
|
10
|
|
—
|
|
—
|
|
10
|
|
|||||||
|
Total unrealized losses included in accumulated other comprehensive (loss) income
|
(151
|
)
|
(1
|
)
|
(7
|
)
|
(143
|
)
|
|||||||
|
Purchases and sales, net
|
123
|
|
—
|
|
—
|
|
123
|
|
|||||||
|
Balance as of April 29, 2016
|
$
|
655
|
|
$
|
—
|
|
$
|
193
|
|
$
|
462
|
|
|||
|
(in millions)
|
Total Level 3 Investments
|
Corporate Debt Securities
|
Commingled Trusts
|
Partnership Units
|
|||||||||||
|
Balance as of April 25, 2014
|
$
|
959
|
|
$
|
1
|
|
$
|
285
|
|
$
|
673
|
|
|||
|
Total realized gains included in income
|
162
|
|
—
|
|
65
|
|
97
|
|
|||||||
|
Total unrealized gains included in accumulated other comprehensive (loss) income
|
(130
|
)
|
—
|
|
(31
|
)
|
(99
|
)
|
|||||||
|
Purchases and sales, net
|
(318
|
)
|
—
|
|
(119
|
)
|
(199
|
)
|
|||||||
|
Balance as of April 24, 2015
|
$
|
673
|
|
$
|
1
|
|
$
|
200
|
|
$
|
472
|
|
|||
|
|
Fair Value
as of
|
Fair Value Measurements
Using Inputs Considered as
|
|||||||||||||
|
(in millions)
|
April 29, 2016
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Registered investment companies
|
$
|
1,037
|
|
$
|
—
|
|
$
|
1,037
|
|
$
|
—
|
|
|||
|
Insurance contracts
|
76
|
|
—
|
|
—
|
|
76
|
|
|||||||
|
|
$
|
1,113
|
|
$
|
—
|
|
$
|
1,037
|
|
$
|
76
|
|
|||
|
|
Fair Value
as of
|
Fair Value Measurements
Using Inputs Considered as
|
|||||||||||||
|
(in millions)
|
April 24, 2015
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Registered investment companies
|
$
|
1,113
|
|
$
|
—
|
|
$
|
1,113
|
|
$
|
—
|
|
|||
|
Insurance contracts
|
60
|
|
—
|
|
—
|
|
60
|
|
|||||||
|
Partnership units
|
16
|
|
—
|
|
—
|
|
16
|
|
|||||||
|
|
$
|
1,189
|
|
$
|
—
|
|
$
|
1,113
|
|
$
|
76
|
|
|||
|
(in millions)
|
Total Level 3 Investments
|
Insurance Contracts
|
Partnership Units
|
||||||||
|
Balance as of April 24, 2015
|
$
|
76
|
|
$
|
60
|
|
$
|
16
|
|
||
|
Total unrealized gains included in accumulated other comprehensive (loss) income
|
—
|
|
—
|
|
—
|
|
|||||
|
Purchases and sales, net
|
(2
|
)
|
14
|
|
(16
|
)
|
|||||
|
Currency exchange rate changes
|
2
|
|
2
|
|
—
|
|
|||||
|
Balance as of April 29, 2016
|
$
|
76
|
|
$
|
76
|
|
$
|
—
|
|
||
|
(in millions)
|
Total Level 3 Investments
|
Insurance Contracts
|
Partnership Units
|
||||||||
|
Balance as of April 25, 2014
|
$
|
21
|
|
$
|
11
|
|
$
|
10
|
|
||
|
Total unrealized gains included in accumulated other comprehensive (loss) income
|
1
|
|
(1
|
)
|
2
|
|
|||||
|
Purchases and sales, net
|
63
|
|
56
|
|
7
|
|
|||||
|
Currency exchange rate changes
|
(9
|
)
|
(6
|
)
|
(3
|
)
|
|||||
|
Balance as of April 24, 2015
|
$
|
76
|
|
$
|
60
|
|
$
|
16
|
|
||
|
(in millions)
|
U.S. Pension Benefits
|
Non-U.S. Pension Benefits
|
|||||
|
Fiscal Year
|
Gross Payments
|
Gross Payments
|
|||||
|
2017
|
$
|
87
|
|
$
|
39
|
|
|
|
2018
|
96
|
|
40
|
|
|||
|
2019
|
105
|
|
39
|
|
|||
|
2020
|
116
|
|
40
|
|
|||
|
2021
|
126
|
|
43
|
|
|||
|
2022 – 2026
|
810
|
|
265
|
|
|||
|
Total
|
$
|
1,340
|
|
$
|
466
|
|
|
|
(in millions)
Fiscal Year
|
Capitalized
Leases
|
Operating
Leases
|
|||||
|
2017
|
$
|
109
|
|
$
|
180
|
|
|
|
2018
|
5
|
|
130
|
|
|||
|
2019
|
4
|
|
90
|
|
|||
|
2020
|
4
|
|
56
|
|
|||
|
2021
|
3
|
|
33
|
|
|||
|
Thereafter
|
16
|
|
55
|
|
|||
|
Total minimum lease payments
|
$
|
141
|
|
$
|
544
|
|
|
|
Less amounts representing interest
|
(9
|
)
|
N/A
|
|
|||
|
Present value of net minimum lease payments
|
$
|
132
|
|
N/A
|
|
||
|
(in millions)
|
Unrealized Gain (Loss) on Available-for-Sale Securities
|
Cumulative Translation Adjustments
(1)
|
Net Change in Retirement Obligations
|
Unrealized Gain (Loss) on Derivatives
|
Total Accumulated Other Comprehensive (Loss) Income
|
||||||||||||||
|
Balance as of April 25, 2014, net of tax
|
$
|
(6
|
)
|
$
|
218
|
|
$
|
(765
|
)
|
$
|
(44
|
)
|
$
|
(597
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications, before tax
|
169
|
|
(495
|
)
|
(617
|
)
|
545
|
|
(398
|
)
|
|||||||||
|
Tax (expense) benefit
|
(60
|
)
|
—
|
|
198
|
|
(199
|
)
|
(61
|
)
|
|||||||||
|
Other comprehensive income (loss) before reclassifications, net of tax
|
109
|
|
(495
|
)
|
(419
|
)
|
346
|
|
(459
|
)
|
|||||||||
|
Reclassifications, before tax
|
(138
|
)
|
—
|
|
78
|
|
(145
|
)
|
(205
|
)
|
|||||||||
|
Tax benefit (expense)
|
49
|
|
—
|
|
(25
|
)
|
53
|
|
77
|
|
|||||||||
|
Reclassifications, net of tax
|
(89
|
)
|
(2)
|
—
|
|
53
|
|
(3)
|
(92
|
)
|
(4)
|
(128
|
)
|
||||||
|
Other comprehensive income (loss), net of tax
|
20
|
|
(495
|
)
|
(366
|
)
|
254
|
|
(587
|
)
|
|||||||||
|
Balance as of April 24, 2015, net of tax
|
14
|
|
(277
|
)
|
(1,131
|
)
|
210
|
|
(1,184
|
)
|
|||||||||
|
Other comprehensive loss before reclassifications, before tax
|
(201
|
)
|
(197
|
)
|
(226
|
)
|
(145
|
)
|
(769
|
)
|
|||||||||
|
Tax benefit
|
94
|
|
—
|
|
85
|
|
51
|
|
230
|
|
|||||||||
|
Other comprehensive loss before reclassifications, net of tax
|
(107
|
)
|
(197
|
)
|
(141
|
)
|
(94
|
)
|
(539
|
)
|
|||||||||
|
Reclassifications, before tax
|
(22
|
)
|
—
|
|
114
|
|
(327
|
)
|
(235
|
)
|
|||||||||
|
Tax benefit (expense)
|
8
|
|
—
|
|
(39
|
)
|
121
|
|
90
|
|
|||||||||
|
Reclassifications, net of tax
|
(14
|
)
|
(2)
|
—
|
|
75
|
|
(3)
|
(206
|
)
|
(4)
|
(145
|
)
|
||||||
|
Other comprehensive loss, net of tax
|
(121
|
)
|
(197
|
)
|
(66
|
)
|
(300
|
)
|
(684
|
)
|
|||||||||
|
Balance as of April 29, 2016, net of tax
|
$
|
(107
|
)
|
$
|
(474
|
)
|
$
|
(1,197
|
)
|
$
|
(90
|
)
|
$
|
(1,868
|
)
|
||||
|
(1)
|
Taxes are not provided on cumulative translation adjustments as substantially all translation adjustments relate to earnings that are intended to be indefinitely reinvested outside the U.S.
|
|
(2)
|
Represents net realized losses on sales of available-for-sale securities that were reclassified from AOCI to
other expense, net
(see Note 5).
|
|
(3)
|
Includes net amortization of prior service costs and actuarial losses included in net periodic benefit cost (see Note 12).
|
|
(4)
|
Relates to cash flow hedges that were reclassified from AOCI to
other expense, net
or
cost of products sold
and forward starting interest rate derivative instruments that were reclassified from AOCI to
interest expense, net
(see Note 8).
|
|
(in millions, except per share data)
|
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Fiscal Year
|
|||||||||||||||
|
Net Sales
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
2016
|
$
|
7,274
|
|
$
|
7,058
|
|
$
|
6,934
|
|
$
|
7,567
|
|
$
|
28,833
|
|
|||||
|
|
2015
|
4,273
|
|
4,366
|
|
4,318
|
|
7,304
|
|
20,261
|
|
||||||||||
|
Gross Profit
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
2016
|
$
|
4,818
|
|
$
|
4,876
|
|
$
|
4,793
|
|
$
|
5,204
|
|
$
|
19,691
|
|
|||||
|
|
2015
|
3,168
|
|
3,224
|
|
3,190
|
|
4,370
|
|
13,952
|
|
||||||||||
|
Net Income (Loss)
|
|||||||||||||||||||||
|
|
2016
|
$
|
820
|
|
$
|
520
|
|
$
|
1,095
|
|
$
|
1,104
|
|
$
|
3,538
|
|
|||||
|
|
2015
|
871
|
|
828
|
|
977
|
|
(1
|
)
|
2,675
|
|
||||||||||
|
Basic Earnings per Share
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
2016
|
$
|
0.58
|
|
$
|
0.37
|
|
$
|
0.78
|
|
$
|
0.79
|
|
2.51
|
|
||||||
|
|
2015
|
0.88
|
|
0.84
|
|
0.99
|
|
—
|
|
2.44
|
|
||||||||||
|
Diluted Earnings per Share
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
2016
|
$
|
0.57
|
|
$
|
0.36
|
|
$
|
0.77
|
|
$
|
0.78
|
|
2.48
|
|
||||||
|
|
2015
|
0.87
|
|
0.83
|
|
0.98
|
|
—
|
|
2.41
|
|
||||||||||
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Cardiac and Vascular Group
|
$
|
10,196
|
|
$
|
9,361
|
|
$
|
8,847
|
|
||
|
Minimally Invasive Therapies Group
|
9,563
|
|
2,387
|
|
—
|
|
|||||
|
Restorative Therapies Group
|
7,210
|
|
6,751
|
|
6,501
|
|
|||||
|
Diabetes Group
|
1,864
|
|
1,762
|
|
1,657
|
|
|||||
|
Total Net Sales
|
$
|
28,833
|
|
$
|
20,261
|
|
$
|
17,005
|
|
||
|
|
Fiscal Year
|
||||||||||
|
(in millions)
|
2016
|
2015
|
2014
|
||||||||
|
Cardiac and Vascular Group
|
$
|
3,182
|
|
$
|
3,140
|
|
$
|
2,982
|
|
||
|
Minimally Invasive Therapies Group
|
1,394
|
|
342
|
|
—
|
|
|||||
|
Restorative Therapies Group
|
1,976
|
|
1,828
|
|
1,821
|
|
|||||
|
Diabetes Group
|
543
|
|
540
|
|
457
|
|
|||||
|
Total Reportable Segments’ Income Before Income Taxes
|
7,095
|
|
5,850
|
|
5,260
|
|
|||||
|
Impact of inventory step-up
|
(226
|
)
|
(623
|
)
|
—
|
|
|||||
|
Impact of product technology upgrade commitment
|
—
|
|
(74
|
)
|
—
|
|
|||||
|
Special (gains) charges, net
|
(70
|
)
|
38
|
|
(40
|
)
|
|||||
|
Restructuring charges, net
(1)
|
(299
|
)
|
(252
|
)
|
(88
|
)
|
|||||
|
Certain litigation charges, net
|
(26
|
)
|
(42
|
)
|
(770
|
)
|
|||||
|
Acquisition-related items
|
(283
|
)
|
(550
|
)
|
(117
|
)
|
|||||
|
Interest expense, net
|
(955
|
)
|
(280
|
)
|
(108
|
)
|
|||||
|
Corporate
|
(900
|
)
|
(581
|
)
|
(432
|
)
|
|||||
|
Total Income From Operations Before Income Taxes
|
$
|
4,336
|
|
$
|
3,486
|
|
$
|
3,705
|
|
||
|
(1)
|
Restructuring charges, net within this table include the impact of amounts recorded within cost of products sold in the consolidated statements of income.
|
|
(in millions)
|
April 29,
2016 |
April 24,
2015 |
|||||
|
Cardiac and Vascular Group
|
$
|
13,563
|
|
$
|
13,642
|
|
|
|
Minimally Invasive Therapies Group
|
52,227
|
|
51,228
|
|
|||
|
Restorative Therapies Group
|
14,564
|
|
15,249
|
|
|||
|
Diabetes Group
|
2,592
|
|
2,597
|
|
|||
|
Total Assets of Reportable Segments
|
82,946
|
|
82,716
|
|
|||
|
Corporate
|
16,836
|
|
23,969
|
|
|||
|
Total Assets
|
$
|
99,782
|
|
$
|
106,685
|
|
|
|
(in millions)
|
Americas
(1)
|
EMEA
(2)
|
Asia Pacific
|
Greater China
|
Consolidated
|
||||||||||||||
|
Fiscal Year 2016
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Net sales to external customers
|
$
|
17,578
|
|
$
|
6,700
|
|
$
|
3,060
|
|
$
|
1,495
|
|
$
|
28,833
|
|
||||
|
Property, plant, and equipment, net
|
$
|
3,728
|
|
$
|
708
|
|
$
|
220
|
|
$
|
185
|
|
$
|
4,841
|
|
||||
|
Fiscal Year 2015
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Net sales to external customers
|
$
|
12,125
|
|
$
|
5,064
|
|
$
|
2,059
|
|
$
|
1,013
|
|
$
|
20,261
|
|
||||
|
Property, plant, and equipment, net
|
$
|
3,626
|
|
$
|
725
|
|
$
|
165
|
|
$
|
183
|
|
$
|
4,699
|
|
||||
|
Fiscal Year 2014
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Net sales to external customers
|
$
|
9,922
|
|
$
|
4,483
|
|
$
|
1,776
|
|
$
|
824
|
|
$
|
17,005
|
|
||||
|
Property, plant, and equipment, net
|
$
|
1,833
|
|
$
|
393
|
|
$
|
74
|
|
$
|
92
|
|
$
|
2,392
|
|
||||
|
(1)
|
The U.S., which is included in the Americas, had net sales to external customers of
$16.4 billion
, $
11.3 billion
, and $
9.2 billion
in fiscal years 2016, 2015, and 2014, respectively. Property, plant, and equipment, net includes
$3.3 billion
,
$3.0 billion
, and
$1.7 billion
in the U.S. in fiscal years
2016
,
2015
, and
2014
respectively.
|
|
(2)
|
EMEA consists of the following regions: Europe, Middle East, and Africa. Sales to Ireland were insignificant during all periods presented. Property, plant, and equipment, net includes
$169 million
,
$151 million
, and
$72 million
in Ireland in fiscal years
2016
,
2015
, and
2014
, respectively.
|
|
•
|
Parent Company Guarantor - Medtronic plc
|
|
•
|
Subsidiary Issuer - Medtronic, Inc.
|
|
•
|
Subsidiary Guarantor - Medtronic Luxco
|
|
•
|
Parent Company Guarantor - Medtronic plc
|
|
•
|
Subsidiary Issuer - CIFSA
|
|
•
|
Subsidiary Guarantors - Medtronic Luxco, Covidien Ltd., and Covidien Group Holdings Ltd.
|
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Net sales
|
$
|
—
|
|
$
|
1,411
|
|
$
|
—
|
|
$
|
28,832
|
|
$
|
(1,410
|
)
|
$
|
28,833
|
|
|||||
|
Costs and expenses:
|
|||||||||||||||||||||||
|
Cost of products sold
|
—
|
|
991
|
|
—
|
|
9,561
|
|
(1,410
|
)
|
9,142
|
|
|||||||||||
|
Research and development expense
|
—
|
|
627
|
|
—
|
|
1,597
|
|
—
|
|
2,224
|
|
|||||||||||
|
Selling, general, and administrative expense
|
10
|
|
991
|
|
—
|
|
8,468
|
|
—
|
|
9,469
|
|
|||||||||||
|
Special (gains) charges, net
|
—
|
|
70
|
|
—
|
|
—
|
|
—
|
|
70
|
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
17
|
|
—
|
|
273
|
|
—
|
|
290
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
—
|
|
—
|
|
26
|
|
—
|
|
26
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
135
|
|
—
|
|
148
|
|
—
|
|
283
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
12
|
|
—
|
|
1,919
|
|
—
|
|
1,931
|
|
|||||||||||
|
Other (income) expense, net
|
112
|
|
(2,329
|
)
|
—
|
|
2,324
|
|
—
|
|
107
|
|
|||||||||||
|
Operating profit (loss)
|
(122
|
)
|
897
|
|
—
|
|
4,516
|
|
—
|
|
5,291
|
|
|||||||||||
|
Interest income
|
—
|
|
(237
|
)
|
(706
|
)
|
(448
|
)
|
960
|
|
(431
|
)
|
|||||||||||
|
Interest expense
|
25
|
|
1,906
|
|
10
|
|
405
|
|
(960
|
)
|
1,386
|
|
|||||||||||
|
Interest expense (income), net
|
25
|
|
1,669
|
|
(696
|
)
|
(43
|
)
|
—
|
|
955
|
|
|||||||||||
|
Equity in net (income) loss of subsidiaries
|
(3,676
|
)
|
4,224
|
|
(2,980
|
)
|
—
|
|
2,432
|
|
—
|
|
|||||||||||
|
Income (loss) from operations before income taxes
|
3,529
|
|
(4,996
|
)
|
3,676
|
|
4,559
|
|
(2,432
|
)
|
4,336
|
|
|||||||||||
|
Provision (benefit) for income taxes
|
(9
|
)
|
(96
|
)
|
—
|
|
903
|
|
—
|
|
798
|
|
|||||||||||
|
Net income (loss)
|
3,538
|
|
(4,900
|
)
|
3,676
|
|
3,656
|
|
(2,432
|
)
|
3,538
|
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(684
|
)
|
(493
|
)
|
(684
|
)
|
(673
|
)
|
1,850
|
|
(684
|
)
|
|||||||||||
|
Total comprehensive income (loss)
|
$
|
2,854
|
|
$
|
(5,393
|
)
|
$
|
2,992
|
|
$
|
2,983
|
|
$
|
(582
|
)
|
$
|
2,854
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Net sales
|
$
|
—
|
|
$
|
1,261
|
|
$
|
—
|
|
$
|
20,261
|
|
$
|
(1,261
|
)
|
$
|
20,261
|
|
|||||
|
Costs and expenses:
|
|||||||||||||||||||||||
|
Cost of products sold
|
—
|
|
895
|
|
—
|
|
6,659
|
|
(1,245
|
)
|
6,309
|
|
|||||||||||
|
Research and development expense
|
—
|
|
552
|
|
—
|
|
1,088
|
|
—
|
|
1,640
|
|
|||||||||||
|
Selling, general, and administrative expense
|
1
|
|
857
|
|
—
|
|
6,046
|
|
—
|
|
6,904
|
|
|||||||||||
|
Special (gains) charges
|
—
|
|
100
|
|
—
|
|
(138
|
)
|
—
|
|
(38
|
)
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
7
|
|
—
|
|
230
|
|
—
|
|
237
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
—
|
|
—
|
|
42
|
|
—
|
|
42
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
312
|
|
—
|
|
238
|
|
—
|
|
550
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
11
|
|
—
|
|
722
|
|
—
|
|
733
|
|
|||||||||||
|
Other (income) expense, net
|
103
|
|
(1,618
|
)
|
—
|
|
1,633
|
|
—
|
|
118
|
|
|||||||||||
|
Operating profit (loss)
|
(104
|
)
|
145
|
|
—
|
|
3,741
|
|
(16
|
)
|
3,766
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Interest income
|
—
|
|
(56
|
)
|
(170
|
)
|
(387
|
)
|
227
|
|
(386
|
)
|
|||||||||||
|
Interest expense
|
—
|
|
762
|
|
—
|
|
131
|
|
(227
|
)
|
666
|
|
|||||||||||
|
Interest expense (income), net
|
—
|
|
706
|
|
(170
|
)
|
(256
|
)
|
—
|
|
280
|
|
|||||||||||
|
Equity in net (income) loss of subsidiaries
|
(2,790
|
)
|
(5,830
|
)
|
(2,620
|
)
|
—
|
|
11,240
|
|
—
|
|
|||||||||||
|
Income (loss) from operations before income taxes
|
2,686
|
|
5,269
|
|
2,790
|
|
3,997
|
|
(11,256
|
)
|
3,486
|
|
|||||||||||
|
Provision (benefit) for income taxes
|
11
|
|
(44
|
)
|
—
|
|
844
|
|
—
|
|
811
|
|
|||||||||||
|
Net income
|
2,675
|
|
5,313
|
|
2,790
|
|
3,153
|
|
(11,256
|
)
|
2,675
|
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(587
|
)
|
(540
|
)
|
(587
|
)
|
(232
|
)
|
1,359
|
|
(587
|
)
|
|||||||||||
|
Total comprehensive income (loss)
|
$
|
2,088
|
|
$
|
4,773
|
|
$
|
2,203
|
|
$
|
2,921
|
|
$
|
(9,897
|
)
|
$
|
2,088
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Net sales
|
$
|
—
|
|
$
|
1,155
|
|
$
|
—
|
|
$
|
17,005
|
|
$
|
(1,155
|
)
|
$
|
17,005
|
|
|||||
|
Costs and expenses:
|
|||||||||||||||||||||||
|
Cost of products sold
|
—
|
|
787
|
|
—
|
|
4,674
|
|
(1,128
|
)
|
4,333
|
|
|||||||||||
|
Research and development expense
|
—
|
|
540
|
|
—
|
|
937
|
|
—
|
|
1,477
|
|
|||||||||||
|
Selling, general, and administrative expense
|
—
|
|
821
|
|
—
|
|
5,026
|
|
—
|
|
5,847
|
|
|||||||||||
|
Special (gains) charges
|
—
|
|
40
|
|
—
|
|
—
|
|
—
|
|
40
|
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
71
|
|
—
|
|
7
|
|
—
|
|
78
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
(24
|
)
|
—
|
|
794
|
|
—
|
|
770
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
—
|
|
—
|
|
117
|
|
—
|
|
117
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
12
|
|
—
|
|
337
|
|
—
|
|
349
|
|
|||||||||||
|
Other (income) expense, net
|
—
|
|
(1,623
|
)
|
—
|
|
1,804
|
|
—
|
|
181
|
|
|||||||||||
|
Operating profit (loss)
|
—
|
|
531
|
|
—
|
|
3,309
|
|
(27
|
)
|
3,813
|
|
|||||||||||
|
Interest income
|
—
|
|
(5
|
)
|
—
|
|
(267
|
)
|
1
|
|
(271
|
)
|
|||||||||||
|
Interest expense
|
—
|
|
317
|
|
—
|
|
63
|
|
(1
|
)
|
379
|
|
|||||||||||
|
Interest expense (income), net
|
—
|
|
312
|
|
—
|
|
(204
|
)
|
—
|
|
108
|
|
|||||||||||
|
Equity in net (income) loss of subsidiaries
|
—
|
|
(3,077
|
)
|
—
|
|
—
|
|
3,077
|
|
—
|
|
|||||||||||
|
Income (loss) from operations before income taxes
|
—
|
|
3,296
|
|
—
|
|
3,513
|
|
(3,104
|
)
|
3,705
|
|
|||||||||||
|
Provision (benefit) for income taxes
|
—
|
|
231
|
|
—
|
|
409
|
|
—
|
|
640
|
|
|||||||||||
|
Net income (loss)
|
—
|
|
3,065
|
|
—
|
|
3,104
|
|
(3,104
|
)
|
3,065
|
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
—
|
|
(105
|
)
|
—
|
|
(286
|
)
|
286
|
|
(105
|
)
|
|||||||||||
|
Total comprehensive income (loss)
|
$
|
—
|
|
$
|
2,960
|
|
$
|
—
|
|
$
|
2,818
|
|
$
|
(2,818
|
)
|
$
|
2,960
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
ASSETS
|
|||||||||||||||||||||||
|
Current assets:
|
|||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
55
|
|
$
|
—
|
|
$
|
2,821
|
|
$
|
—
|
|
$
|
2,876
|
|
|||||
|
Investments
|
—
|
|
—
|
|
—
|
|
9,758
|
|
—
|
|
9,758
|
|
|||||||||||
|
Accounts receivable, net
|
—
|
|
—
|
|
—
|
|
5,562
|
|
—
|
|
5,562
|
|
|||||||||||
|
Inventories
|
—
|
|
162
|
|
—
|
|
3,511
|
|
(200
|
)
|
3,473
|
|
|||||||||||
|
Intercompany receivable
|
389
|
|
161,868
|
|
—
|
|
162,278
|
|
(324,535
|
)
|
—
|
|
|||||||||||
|
Tax assets
|
—
|
|
122
|
|
—
|
|
575
|
|
—
|
|
697
|
|
|||||||||||
|
Prepaid expenses and other current assets
|
24
|
|
149
|
|
—
|
|
1,061
|
|
—
|
|
1,234
|
|
|||||||||||
|
Total current assets
|
413
|
|
162,356
|
|
—
|
|
185,566
|
|
(324,735
|
)
|
23,600
|
|
|||||||||||
|
Property, plant and equipment, net
|
—
|
|
1,139
|
|
—
|
|
3,702
|
|
—
|
|
4,841
|
|
|||||||||||
|
Goodwill
|
—
|
|
—
|
|
—
|
|
41,500
|
|
—
|
|
41,500
|
|
|||||||||||
|
Other intangible assets, net
|
—
|
|
31
|
|
—
|
|
26,868
|
|
—
|
|
26,899
|
|
|||||||||||
|
Long-term tax assets
|
—
|
|
690
|
|
—
|
|
693
|
|
—
|
|
1,383
|
|
|||||||||||
|
Investment in subsidiaries
|
73,108
|
|
63,806
|
|
70,198
|
|
—
|
|
(207,112
|
)
|
—
|
|
|||||||||||
|
Intercompany loans receivable
|
3,000
|
|
8,884
|
|
10,203
|
|
18,140
|
|
(40,227
|
)
|
—
|
|
|||||||||||
|
Other assets
|
—
|
|
644
|
|
—
|
|
915
|
|
—
|
|
1,559
|
|
|||||||||||
|
Total assets
|
$
|
76,521
|
|
$
|
237,550
|
|
$
|
80,401
|
|
$
|
277,384
|
|
$
|
(572,074
|
)
|
$
|
99,782
|
|
|||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||||||||||||||||||
|
Current liabilities:
|
|||||||||||||||||||||||
|
Short-term borrowings
|
$
|
—
|
|
$
|
500
|
|
$
|
—
|
|
$
|
493
|
|
$
|
—
|
|
$
|
993
|
|
|||||
|
Accounts payable
|
—
|
|
288
|
|
—
|
|
1,421
|
|
—
|
|
1,709
|
|
|||||||||||
|
Intercompany payable
|
20,486
|
|
151,687
|
|
—
|
|
152,362
|
|
(324,535
|
)
|
—
|
|
|||||||||||
|
Accrued compensation
|
32
|
|
616
|
|
—
|
|
1,064
|
|
—
|
|
1,712
|
|
|||||||||||
|
Accrued income taxes
|
11
|
|
—
|
|
—
|
|
555
|
|
—
|
|
566
|
|
|||||||||||
|
Deferred tax liabilities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Other accrued expenses
|
1
|
|
243
|
|
—
|
|
1,941
|
|
—
|
|
2,185
|
|
|||||||||||
|
Total current liabilities
|
20,530
|
|
153,334
|
|
—
|
|
157,836
|
|
(324,535
|
)
|
7,165
|
|
|||||||||||
|
Long-term debt
|
—
|
|
26,784
|
|
—
|
|
3,463
|
|
—
|
|
30,247
|
|
|||||||||||
|
Long-term accrued compensation and retirement benefits
|
—
|
|
1,258
|
|
—
|
|
501
|
|
—
|
|
1,759
|
|
|||||||||||
|
Long-term accrued income taxes
|
10
|
|
1,422
|
|
—
|
|
1,471
|
|
—
|
|
2,903
|
|
|||||||||||
|
Long-term intercompany loans payable
|
3,918
|
|
10,128
|
|
14,297
|
|
11,884
|
|
(40,227
|
)
|
—
|
|
|||||||||||
|
Long-term deferred tax liabilities
|
—
|
|
—
|
|
—
|
|
3,729
|
|
—
|
|
3,729
|
|
|||||||||||
|
Other long-term liabilities
|
—
|
|
202
|
|
—
|
|
1,714
|
|
—
|
|
1,916
|
|
|||||||||||
|
Total liabilities
|
24,458
|
|
193,128
|
|
14,297
|
|
180,598
|
|
(364,762
|
)
|
47,719
|
|
|||||||||||
|
Shareholders’ equity
|
52,063
|
|
44,422
|
|
66,104
|
|
96,786
|
|
(207,312
|
)
|
52,063
|
|
|||||||||||
|
Total liabilities and shareholders’ equity
|
$
|
76,521
|
|
$
|
237,550
|
|
$
|
80,401
|
|
$
|
277,384
|
|
$
|
(572,074
|
)
|
$
|
99,782
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
ASSETS
|
|||||||||||||||||||||||
|
Current assets:
|
|||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
263
|
|
$
|
1,071
|
|
$
|
170
|
|
$
|
3,339
|
|
$
|
—
|
|
$
|
4,843
|
|
|||||
|
Investments
|
—
|
|
—
|
|
—
|
|
14,637
|
|
—
|
|
14,637
|
|
|||||||||||
|
Accounts receivable, net
|
—
|
|
—
|
|
—
|
|
5,112
|
|
—
|
|
5,112
|
|
|||||||||||
|
Inventories
|
—
|
|
165
|
|
—
|
|
3,497
|
|
(199
|
)
|
3,463
|
|
|||||||||||
|
Intercompany receivable
|
259
|
|
146,942
|
|
—
|
|
144,638
|
|
(291,839
|
)
|
—
|
|
|||||||||||
|
Tax assets
|
—
|
|
295
|
|
—
|
|
1,040
|
|
—
|
|
1,335
|
|
|||||||||||
|
Prepaid expenses and other current assets
|
4
|
|
128
|
|
—
|
|
1,322
|
|
—
|
|
1,454
|
|
|||||||||||
|
Total current assets
|
526
|
|
148,601
|
|
170
|
|
173,585
|
|
(292,038
|
)
|
30,844
|
|
|||||||||||
|
Property, plant and equipment, net
|
—
|
|
976
|
|
—
|
|
3,723
|
|
—
|
|
4,699
|
|
|||||||||||
|
Goodwill
|
—
|
|
—
|
|
—
|
|
40,530
|
|
—
|
|
40,530
|
|
|||||||||||
|
Other intangible assets, net
|
—
|
|
39
|
|
—
|
|
28,062
|
|
—
|
|
28,101
|
|
|||||||||||
|
Long-term tax assets
|
—
|
|
294
|
|
—
|
|
480
|
|
—
|
|
774
|
|
|||||||||||
|
Investment in subsidiaries
|
70,233
|
|
68,710
|
|
63,063
|
|
—
|
|
(202,006
|
)
|
—
|
|
|||||||||||
|
Intercompany loans receivable
|
3,000
|
|
6,516
|
|
10,000
|
|
10,218
|
|
(29,734
|
)
|
—
|
|
|||||||||||
|
Other assets
|
—
|
|
678
|
|
—
|
|
1,059
|
|
—
|
|
1,737
|
|
|||||||||||
|
Total assets
|
$
|
73,759
|
|
$
|
225,814
|
|
$
|
73,233
|
|
$
|
257,657
|
|
$
|
(523,778
|
)
|
$
|
106,685
|
|
|||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||||||||||||||||||
|
Current liabilities:
|
|||||||||||||||||||||||
|
Short-term borrowings
|
$
|
—
|
|
$
|
1,110
|
|
$
|
—
|
|
$
|
1,324
|
|
$
|
—
|
|
$
|
2,434
|
|
|||||
|
Accounts payable
|
—
|
|
261
|
|
—
|
|
1,349
|
|
—
|
|
1,610
|
|
|||||||||||
|
Intercompany payable
|
20,506
|
|
135,660
|
|
—
|
|
135,673
|
|
(291,839
|
)
|
—
|
|
|||||||||||
|
Accrued compensation
|
1
|
|
490
|
|
—
|
|
1,120
|
|
—
|
|
1,611
|
|
|||||||||||
|
Accrued income taxes
|
19
|
|
—
|
|
—
|
|
916
|
|
—
|
|
935
|
|
|||||||||||
|
Deferred tax liabilities
|
3
|
|
—
|
|
—
|
|
116
|
|
—
|
|
119
|
|
|||||||||||
|
Other accrued expenses
|
—
|
|
628
|
|
—
|
|
1,836
|
|
—
|
|
2,464
|
|
|||||||||||
|
Total current liabilities
|
20,529
|
|
138,149
|
|
—
|
|
142,334
|
|
(291,839
|
)
|
9,173
|
|
|||||||||||
|
Long-term debt
|
—
|
|
29,004
|
|
—
|
|
4,748
|
|
—
|
|
33,752
|
|
|||||||||||
|
Long-term accrued compensation and retirement benefits
|
—
|
|
965
|
|
—
|
|
570
|
|
—
|
|
1,535
|
|
|||||||||||
|
Long-term accrued income taxes
|
—
|
|
1,048
|
|
—
|
|
1,428
|
|
—
|
|
2,476
|
|
|||||||||||
|
Long-term intercompany loans payable
|
—
|
|
10,218
|
|
10,000
|
|
9,516
|
|
(29,734
|
)
|
—
|
|
|||||||||||
|
Long-term deferred tax liabilities
|
—
|
|
—
|
|
—
|
|
4,700
|
|
—
|
|
4,700
|
|
|||||||||||
|
Other long-term liabilities
|
—
|
|
207
|
|
—
|
|
1,612
|
|
—
|
|
1,819
|
|
|||||||||||
|
Total liabilities
|
20,529
|
|
179,591
|
|
10,000
|
|
164,908
|
|
(321,573
|
)
|
53,455
|
|
|||||||||||
|
Shareholders’ equity
|
53,230
|
|
46,223
|
|
63,233
|
|
92,749
|
|
(202,205
|
)
|
53,230
|
|
|||||||||||
|
Total liabilities and shareholders’ equity
|
$
|
73,759
|
|
$
|
225,814
|
|
$
|
73,233
|
|
$
|
257,657
|
|
$
|
(523,778
|
)
|
$
|
106,685
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Operating Activities:
|
|||||||||||||||||||||||
|
Net cash provided by (used in) operating activities
|
$
|
297
|
|
$
|
402
|
|
$
|
696
|
|
$
|
4,635
|
|
$
|
(812
|
)
|
$
|
5,218
|
|
|||||
|
Investing Activities:
|
|||||||||||||||||||||||
|
Acquisitions, net of cash acquired
|
—
|
|
(526
|
)
|
—
|
|
(687
|
)
|
—
|
|
(1,213
|
)
|
|||||||||||
|
Additions to property, plant, and equipment
|
—
|
|
(334
|
)
|
—
|
|
(712
|
)
|
—
|
|
(1,046
|
)
|
|||||||||||
|
Purchases of marketable securities
|
—
|
|
—
|
|
—
|
|
(5,406
|
)
|
—
|
|
(5,406
|
)
|
|||||||||||
|
Sales and maturities of marketable securities
|
—
|
|
—
|
|
—
|
|
9,924
|
|
—
|
|
9,924
|
|
|||||||||||
|
Net (increase) decrease in intercompany loans receivable
|
—
|
|
(2,368
|
)
|
(203
|
)
|
(7,921
|
)
|
10,492
|
|
—
|
|
|||||||||||
|
Capital contributions paid
|
—
|
|
(11
|
)
|
(4,959
|
)
|
(4,900
|
)
|
9,870
|
|
—
|
|
|||||||||||
|
Other investing activities, net
|
—
|
|
—
|
|
—
|
|
(14
|
)
|
—
|
|
(14
|
)
|
|||||||||||
|
Net cash provided by (used in) investing activities
|
—
|
|
(3,239
|
)
|
(5,162
|
)
|
(9,716
|
)
|
20,362
|
|
2,245
|
|
|||||||||||
|
Financing Activities:
|
|||||||||||||||||||||||
|
Acquisition-related contingent consideration
|
—
|
|
—
|
|
—
|
|
(22
|
)
|
—
|
|
(22
|
)
|
|||||||||||
|
Change in short-term borrowings, net
|
—
|
|
—
|
|
—
|
|
7
|
|
—
|
|
7
|
|
|||||||||||
|
Repayment of short-term borrowings (maturities greater than 90 days)
|
—
|
|
—
|
|
(139
|
)
|
—
|
|
—
|
|
(139
|
)
|
|||||||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days)
|
—
|
|
—
|
|
139
|
|
—
|
|
—
|
|
139
|
|
|||||||||||
|
Issuance of long-term debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Payments on long-term debt
|
—
|
|
(2,988
|
)
|
—
|
|
(2,144
|
)
|
—
|
|
(5,132
|
)
|
|||||||||||
|
Dividends to shareholders
|
(2,139
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,139
|
)
|
|||||||||||
|
Issuance of ordinary shares
|
491
|
|
—
|
|
—
|
|
—
|
|
—
|
|
491
|
|
|||||||||||
|
Repurchase of ordinary shares
|
(2,830
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,830
|
)
|
|||||||||||
|
Net intercompany loan borrowings (repayments)
|
3,918
|
|
(91
|
)
|
4,296
|
|
2,369
|
|
(10,492
|
)
|
—
|
|
|||||||||||
|
Intercompany dividend paid
|
—
|
|
—
|
|
—
|
|
(812
|
)
|
812
|
|
—
|
|
|||||||||||
|
Capital contributions received
|
—
|
|
4,900
|
|
—
|
|
4,970
|
|
(9,870
|
)
|
—
|
|
|||||||||||
|
Other financing activities
|
—
|
|
—
|
|
—
|
|
82
|
|
—
|
|
82
|
|
|||||||||||
|
Net cash provided by (used in) financing activities
|
(560
|
)
|
1,821
|
|
4,296
|
|
4,450
|
|
(19,550
|
)
|
(9,543
|
)
|
|||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
—
|
|
—
|
|
—
|
|
113
|
|
—
|
|
113
|
|
|||||||||||
|
Net change in cash and cash equivalents
|
(263
|
)
|
(1,016
|
)
|
(170
|
)
|
(518
|
)
|
—
|
|
(1,967
|
)
|
|||||||||||
|
Cash and cash equivalents at beginning of period
|
263
|
|
1,071
|
|
170
|
|
3,339
|
|
—
|
|
4,843
|
|
|||||||||||
|
Cash and cash equivalents at end of period
|
$
|
—
|
|
$
|
55
|
|
$
|
—
|
|
$
|
2,821
|
|
$
|
—
|
|
$
|
2,876
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Operating Activities:
|
|||||||||||||||||||||||
|
Net cash provided by (used in) operating activities
|
$
|
26
|
|
$
|
1,479
|
|
$
|
170
|
|
$
|
3,640
|
|
$
|
(413
|
)
|
$
|
4,902
|
|
|||||
|
Investing Activities:
|
|||||||||||||||||||||||
|
Acquisitions, net of cash acquired
|
(9,700
|
)
|
(65
|
)
|
—
|
|
(5,119
|
)
|
—
|
|
(14,884
|
)
|
|||||||||||
|
Additions to property, plant, and equipment
|
—
|
|
(187
|
)
|
—
|
|
(384
|
)
|
—
|
|
(571
|
)
|
|||||||||||
|
Purchases of marketable securities
|
—
|
|
—
|
|
—
|
|
(7,582
|
)
|
—
|
|
(7,582
|
)
|
|||||||||||
|
Sales and maturities of marketable securities
|
—
|
|
—
|
|
—
|
|
5,890
|
|
—
|
|
5,890
|
|
|||||||||||
|
Net (increase) decrease in intercompany loans receivable
|
—
|
|
(16,996
|
)
|
—
|
|
53
|
|
16,943
|
|
—
|
|
|||||||||||
|
Other investing activities, net
|
—
|
|
—
|
|
—
|
|
89
|
|
—
|
|
89
|
|
|||||||||||
|
Net cash provided by (used in) investing activities
|
(9,700
|
)
|
(17,248
|
)
|
—
|
|
(7,053
|
)
|
16,943
|
|
(17,058
|
)
|
|||||||||||
|
Financing Activities:
|
|||||||||||||||||||||||
|
Acquisition-related contingent consideration
|
—
|
|
—
|
|
—
|
|
(85
|
)
|
—
|
|
(85
|
)
|
|||||||||||
|
Change in short-term borrowings, net
|
—
|
|
—
|
|
—
|
|
(1
|
)
|
—
|
|
(1
|
)
|
|||||||||||
|
Repayment of short-term borrowings (maturities greater than 90 days)
|
—
|
|
(150
|
)
|
—
|
|
—
|
|
—
|
|
(150
|
)
|
|||||||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days)
|
—
|
|
150
|
|
—
|
|
—
|
|
—
|
|
150
|
|
|||||||||||
|
Issuance of long-term debt
|
—
|
|
19,942
|
|
—
|
|
—
|
|
—
|
|
19,942
|
|
|||||||||||
|
Payments on long-term debt
|
—
|
|
(1,268
|
)
|
—
|
|
—
|
|
—
|
|
(1,268
|
)
|
|||||||||||
|
Dividends to shareholders
|
(435
|
)
|
(902
|
)
|
—
|
|
—
|
|
—
|
|
(1,337
|
)
|
|||||||||||
|
Issuance of ordinary shares
|
172
|
|
477
|
|
—
|
|
—
|
|
—
|
|
649
|
|
|||||||||||
|
Repurchase of ordinary shares
|
(300
|
)
|
(1,620
|
)
|
—
|
|
—
|
|
—
|
|
(1,920
|
)
|
|||||||||||
|
Net intercompany loan borrowings (repayments)
|
10,500
|
|
(53
|
)
|
—
|
|
6,496
|
|
(16,943
|
)
|
—
|
|
|||||||||||
|
Intercompany dividends paid
|
—
|
|
—
|
|
—
|
|
(413
|
)
|
413
|
|
—
|
|
|||||||||||
|
Other financing activities
|
—
|
|
—
|
|
—
|
|
(31
|
)
|
—
|
|
(31
|
)
|
|||||||||||
|
Net cash provided by (used in) financing activities
|
9,937
|
|
16,576
|
|
—
|
|
5,966
|
|
(16,530
|
)
|
15,949
|
|
|||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
—
|
|
—
|
|
—
|
|
(353
|
)
|
—
|
|
(353
|
)
|
|||||||||||
|
Net change in cash and cash equivalents
|
263
|
|
807
|
|
170
|
|
2,200
|
|
—
|
|
3,440
|
|
|||||||||||
|
Cash and cash equivalents at beginning of period
|
—
|
|
264
|
|
—
|
|
1,139
|
|
—
|
|
1,403
|
|
|||||||||||
|
Cash and cash equivalents at end of period
|
$
|
263
|
|
$
|
1,071
|
|
$
|
170
|
|
$
|
3,339
|
|
$
|
—
|
|
$
|
4,843
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (Medtronic, Inc.)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Operating Activities:
|
|||||||||||||||||||||||
|
Net cash provided by (used in) operating activities
|
$
|
—
|
|
$
|
1,384
|
|
$
|
—
|
|
$
|
3,949
|
|
$
|
(374
|
)
|
$
|
4,959
|
|
|||||
|
Investing Activities:
|
|||||||||||||||||||||||
|
Acquisitions, net of cash acquired
|
—
|
|
—
|
|
—
|
|
(385
|
)
|
—
|
|
(385
|
)
|
|||||||||||
|
Additions to property, plant, and equipment
|
—
|
|
(154
|
)
|
—
|
|
(242
|
)
|
—
|
|
(396
|
)
|
|||||||||||
|
Purchases of marketable securities
|
—
|
|
—
|
|
—
|
|
(10,895
|
)
|
—
|
|
(10,895
|
)
|
|||||||||||
|
Sales and maturities of marketable securities
|
—
|
|
—
|
|
—
|
|
8,111
|
|
—
|
|
8,111
|
|
|||||||||||
|
Net (increase) decrease in intercompany loans receivable
|
—
|
|
1
|
|
—
|
|
(12
|
)
|
11
|
|
—
|
|
|||||||||||
|
Other investing activities, net
|
—
|
|
—
|
|
—
|
|
(29
|
)
|
—
|
|
(29
|
)
|
|||||||||||
|
Net cash provided by (used in) investing activities
|
—
|
|
(153
|
)
|
—
|
|
(3,452
|
)
|
11
|
|
(3,594
|
)
|
|||||||||||
|
Financing Activities:
|
|||||||||||||||||||||||
|
Acquisition-related contingent consideration
|
—
|
|
—
|
|
—
|
|
(1
|
)
|
—
|
|
(1
|
)
|
|||||||||||
|
Change in short-term borrowings, net
|
—
|
|
—
|
|
—
|
|
127
|
|
—
|
|
127
|
|
|||||||||||
|
Repayment of short-term borrowings (maturities greater than 90 days)
|
—
|
|
(1,301
|
)
|
—
|
|
—
|
|
—
|
|
(1,301
|
)
|
|||||||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days)
|
—
|
|
1,045
|
|
—
|
|
131
|
|
—
|
|
1,176
|
|
|||||||||||
|
Issuance of long-term debt
|
—
|
|
1,994
|
|
—
|
|
—
|
|
—
|
|
1,994
|
|
|||||||||||
|
Payments on long-term debt
|
—
|
|
(565
|
)
|
—
|
|
—
|
|
—
|
|
(565
|
)
|
|||||||||||
|
Dividends to shareholders
|
—
|
|
(1,116
|
)
|
—
|
|
—
|
|
—
|
|
(1,116
|
)
|
|||||||||||
|
Issuance of ordinary shares
|
—
|
|
1,307
|
|
—
|
|
—
|
|
—
|
|
1,307
|
|
|||||||||||
|
Repurchase of ordinary shares
|
—
|
|
(2,553
|
)
|
—
|
|
—
|
|
—
|
|
(2,553
|
)
|
|||||||||||
|
Net intercompany loan borrowings (repayments)
|
—
|
|
12
|
|
—
|
|
(1
|
)
|
(11
|
)
|
—
|
|
|||||||||||
|
Intercompany dividends paid
|
—
|
|
—
|
|
—
|
|
(374
|
)
|
374
|
|
—
|
|
|||||||||||
|
Other financing activities
|
—
|
|
14
|
|
—
|
|
—
|
|
—
|
|
14
|
|
|||||||||||
|
Net cash provided by (used in) financing activities
|
—
|
|
(1,163
|
)
|
—
|
|
(118
|
)
|
363
|
|
(918
|
)
|
|||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
—
|
|
—
|
|
—
|
|
37
|
|
—
|
|
37
|
|
|||||||||||
|
Net change in cash and cash equivalents
|
—
|
|
68
|
|
—
|
|
416
|
|
—
|
|
484
|
|
|||||||||||
|
Cash and cash equivalents at beginning of period
|
—
|
|
196
|
|
—
|
|
723
|
|
—
|
|
919
|
|
|||||||||||
|
Cash and cash equivalents at end of period
|
$
|
—
|
|
$
|
264
|
|
$
|
—
|
|
$
|
1,139
|
|
$
|
—
|
|
$
|
1,403
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (CIFSA)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Net sales
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
28,833
|
|
$
|
—
|
|
$
|
28,833
|
|
|||||
|
Costs and expenses:
|
|||||||||||||||||||||||
|
Cost of products sold
|
—
|
|
—
|
|
—
|
|
9,142
|
|
—
|
|
9,142
|
|
|||||||||||
|
Research and development expense
|
—
|
|
—
|
|
—
|
|
2,224
|
|
—
|
|
2,224
|
|
|||||||||||
|
Selling, general, and administrative expense
|
10
|
|
1
|
|
3
|
|
9,455
|
|
—
|
|
9,469
|
|
|||||||||||
|
Special (gains) charges, net
|
—
|
|
—
|
|
—
|
|
70
|
|
—
|
|
70
|
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
—
|
|
—
|
|
290
|
|
—
|
|
290
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
—
|
|
—
|
|
26
|
|
—
|
|
26
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
—
|
|
—
|
|
283
|
|
—
|
|
283
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
—
|
|
—
|
|
1,931
|
|
—
|
|
1,931
|
|
|||||||||||
|
Other (income) expense, net
|
112
|
|
1
|
|
(18
|
)
|
12
|
|
—
|
|
107
|
|
|||||||||||
|
Operating profit (loss)
|
(122
|
)
|
(2
|
)
|
15
|
|
5,400
|
|
—
|
|
5,291
|
|
|||||||||||
|
Interest income
|
—
|
|
(434
|
)
|
(710
|
)
|
(451
|
)
|
1,164
|
|
(431
|
)
|
|||||||||||
|
Interest expense
|
25
|
|
138
|
|
10
|
|
2,377
|
|
(1,164
|
)
|
1,386
|
|
|||||||||||
|
Interest (income) expense, net
|
25
|
|
(296
|
)
|
(700
|
)
|
1,926
|
|
—
|
|
955
|
|
|||||||||||
|
Equity in net (income) loss of subsidiaries
|
(3,676
|
)
|
(8,563
|
)
|
(2,961
|
)
|
—
|
|
15,200
|
|
—
|
|
|||||||||||
|
Income (loss) from operations before income taxes
|
3,529
|
|
8,857
|
|
3,676
|
|
3,474
|
|
(15,200
|
)
|
4,336
|
|
|||||||||||
|
Provision (benefit) for income taxes
|
(9
|
)
|
—
|
|
—
|
|
807
|
|
—
|
|
798
|
|
|||||||||||
|
Net income (loss)
|
3,538
|
|
8,857
|
|
3,676
|
|
2,667
|
|
(15,200
|
)
|
3,538
|
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(684
|
)
|
(102
|
)
|
(684
|
)
|
(684
|
)
|
1,470
|
|
(684
|
)
|
|||||||||||
|
Total comprehensive income (loss)
|
$
|
2,854
|
|
$
|
8,755
|
|
$
|
2,992
|
|
$
|
1,983
|
|
$
|
(13,730
|
)
|
$
|
2,854
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (CIFSA)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Net sales
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
20,261
|
|
$
|
—
|
|
$
|
20,261
|
|
|||||
|
Costs and expenses:
|
|||||||||||||||||||||||
|
Cost of products sold
|
—
|
|
—
|
|
—
|
|
6,309
|
|
—
|
|
6,309
|
|
|||||||||||
|
Research and development expense
|
—
|
|
—
|
|
—
|
|
1,640
|
|
—
|
|
1,640
|
|
|||||||||||
|
Selling, general, and administrative expense
|
1
|
|
—
|
|
21
|
|
6,882
|
|
—
|
|
6,904
|
|
|||||||||||
|
Special (gains) charges, net
|
—
|
|
—
|
|
—
|
|
(38
|
)
|
—
|
|
(38
|
)
|
|||||||||||
|
Restructuring charges, net
|
—
|
|
—
|
|
—
|
|
237
|
|
—
|
|
237
|
|
|||||||||||
|
Certain litigation charges, net
|
—
|
|
—
|
|
—
|
|
42
|
|
—
|
|
42
|
|
|||||||||||
|
Acquisition-related items
|
—
|
|
—
|
|
—
|
|
550
|
|
—
|
|
550
|
|
|||||||||||
|
Amortization of intangible assets
|
—
|
|
—
|
|
—
|
|
733
|
|
—
|
|
733
|
|
|||||||||||
|
Other (income) expense, net
|
103
|
|
—
|
|
26
|
|
(11
|
)
|
—
|
|
118
|
|
|||||||||||
|
Operating profit (loss)
|
(104
|
)
|
—
|
|
(47
|
)
|
3,917
|
|
—
|
|
3,766
|
|
|||||||||||
|
Interest income
|
—
|
|
(149
|
)
|
(170
|
)
|
(386
|
)
|
319
|
|
(386
|
)
|
|||||||||||
|
Interest expense
|
—
|
|
29
|
|
—
|
|
956
|
|
(319
|
)
|
666
|
|
|||||||||||
|
Interest (income) expense, net
|
—
|
|
(120
|
)
|
(170
|
)
|
570
|
|
—
|
|
280
|
|
|||||||||||
|
Equity in net (income) loss of subsidiaries
|
(2,790
|
)
|
1,412
|
|
(2,667
|
)
|
—
|
|
4,045
|
|
—
|
|
|||||||||||
|
Income (loss) from operations before income taxes
|
2,686
|
|
(1,292
|
)
|
2,790
|
|
3,347
|
|
(4,045
|
)
|
3,486
|
|
|||||||||||
|
Provision (benefit) for income taxes
|
11
|
|
—
|
|
—
|
|
800
|
|
—
|
|
811
|
|
|||||||||||
|
Net income (loss)
|
2,675
|
|
(1,292
|
)
|
2,790
|
|
2,547
|
|
(4,045
|
)
|
2,675
|
|
|||||||||||
|
Other comprehensive income (loss), net of tax
|
(587
|
)
|
200
|
|
(587
|
)
|
(587
|
)
|
974
|
|
(587
|
)
|
|||||||||||
|
Total comprehensive income (loss)
|
$
|
2,088
|
|
$
|
(1,092
|
)
|
$
|
2,203
|
|
$
|
1,960
|
|
$
|
(3,071
|
)
|
$
|
2,088
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (CIFSA)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
ASSETS
|
|||||||||||||||||||||||
|
Current assets:
|
|||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
208
|
|
$
|
—
|
|
$
|
2,668
|
|
$
|
—
|
|
$
|
2,876
|
|
|||||
|
Investments
|
—
|
|
—
|
|
—
|
|
9,758
|
|
—
|
|
9,758
|
|
|||||||||||
|
Accounts receivable, net
|
—
|
|
—
|
|
—
|
|
5,562
|
|
—
|
|
5,562
|
|
|||||||||||
|
Inventories
|
—
|
|
—
|
|
—
|
|
3,473
|
|
—
|
|
3,473
|
|
|||||||||||
|
Intercompany receivable
|
389
|
|
—
|
|
61
|
|
20,469
|
|
(20,919
|
)
|
—
|
|
|||||||||||
|
Tax assets
|
—
|
|
—
|
|
—
|
|
697
|
|
—
|
|
697
|
|
|||||||||||
|
Prepaid expenses and other current assets
|
24
|
|
—
|
|
—
|
|
1,210
|
|
—
|
|
1,234
|
|
|||||||||||
|
Total current assets
|
413
|
|
208
|
|
61
|
|
43,837
|
|
(20,919
|
)
|
23,600
|
|
|||||||||||
|
Property, plant and equipment, net
|
—
|
|
—
|
|
1
|
|
4,840
|
|
—
|
|
4,841
|
|
|||||||||||
|
Goodwill
|
—
|
|
—
|
|
—
|
|
41,500
|
|
—
|
|
41,500
|
|
|||||||||||
|
Other intangible assets, net
|
—
|
|
—
|
|
—
|
|
26,899
|
|
—
|
|
26,899
|
|
|||||||||||
|
Long-term tax assets
|
—
|
|
—
|
|
—
|
|
1,383
|
|
—
|
|
1,383
|
|
|||||||||||
|
Investment in subsidiaries
|
73,108
|
|
41,582
|
|
68,875
|
|
—
|
|
(183,565
|
)
|
—
|
|
|||||||||||
|
Intercompany loans receivable
|
3,000
|
|
8,253
|
|
11,465
|
|
27,724
|
|
(50,442
|
)
|
—
|
|
|||||||||||
|
Other assets
|
—
|
|
—
|
|
—
|
|
1,559
|
|
—
|
|
1,559
|
|
|||||||||||
|
Total assets
|
$
|
76,521
|
|
$
|
50,043
|
|
$
|
80,402
|
|
$
|
147,742
|
|
$
|
(254,926
|
)
|
$
|
99,782
|
|
|||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||||||||||||||||||
|
Current liabilities:
|
|||||||||||||||||||||||
|
Short-term borrowings
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
993
|
|
$
|
—
|
|
$
|
993
|
|
|||||
|
Accounts payable
|
—
|
|
—
|
|
—
|
|
1,709
|
|
—
|
|
1,709
|
|
|||||||||||
|
Intercompany payable
|
20,486
|
|
—
|
|
—
|
|
433
|
|
(20,919
|
)
|
—
|
|
|||||||||||
|
Accrued compensation
|
32
|
|
—
|
|
—
|
|
1,680
|
|
—
|
|
1,712
|
|
|||||||||||
|
Accrued income taxes
|
11
|
|
—
|
|
—
|
|
555
|
|
—
|
|
566
|
|
|||||||||||
|
Deferred tax liabilities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Other accrued expenses
|
1
|
|
24
|
|
—
|
|
2,160
|
|
—
|
|
2,185
|
|
|||||||||||
|
Total current liabilities
|
20,530
|
|
24
|
|
—
|
|
7,530
|
|
(20,919
|
)
|
7,165
|
|
|||||||||||
|
Long-term debt
|
—
|
|
3,382
|
|
—
|
|
26,865
|
|
—
|
|
30,247
|
|
|||||||||||
|
Long-term accrued compensation and retirement benefits
|
—
|
|
—
|
|
—
|
|
1,759
|
|
—
|
|
1,759
|
|
|||||||||||
|
Long-term accrued income taxes
|
10
|
|
—
|
|
—
|
|
2,893
|
|
—
|
|
2,903
|
|
|||||||||||
|
Long-term intercompany loans payable
|
3,918
|
|
14,689
|
|
14,298
|
|
17,537
|
|
(50,442
|
)
|
—
|
|
|||||||||||
|
Long-term deferred tax liabilities
|
—
|
|
—
|
|
—
|
|
3,729
|
|
—
|
|
3,729
|
|
|||||||||||
|
Other long-term liabilities
|
—
|
|
—
|
|
—
|
|
1,916
|
|
—
|
|
1,916
|
|
|||||||||||
|
Total liabilities
|
24,458
|
|
18,095
|
|
14,298
|
|
62,229
|
|
(71,361
|
)
|
47,719
|
|
|||||||||||
|
Shareholders’ equity
|
52,063
|
|
31,948
|
|
66,104
|
|
85,513
|
|
(183,565
|
)
|
52,063
|
|
|||||||||||
|
Total liabilities and shareholders’ equity
|
$
|
76,521
|
|
$
|
50,043
|
|
$
|
80,402
|
|
$
|
147,742
|
|
$
|
(254,926
|
)
|
$
|
99,782
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (CIFSA)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
ASSETS
|
|||||||||||||||||||||||
|
Current assets:
|
|||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
263
|
|
$
|
728
|
|
$
|
170
|
|
$
|
3,682
|
|
$
|
—
|
|
$
|
4,843
|
|
|||||
|
Investments
|
—
|
|
—
|
|
—
|
|
14,637
|
|
—
|
|
14,637
|
|
|||||||||||
|
Accounts receivable, net
|
—
|
|
—
|
|
—
|
|
5,112
|
|
—
|
|
5,112
|
|
|||||||||||
|
Inventories
|
—
|
|
—
|
|
—
|
|
3,463
|
|
—
|
|
3,463
|
|
|||||||||||
|
Intercompany receivable
|
259
|
|
—
|
|
269
|
|
20,506
|
|
(21,034
|
)
|
—
|
|
|||||||||||
|
Tax assets
|
—
|
|
—
|
|
—
|
|
1,335
|
|
—
|
|
1,335
|
|
|||||||||||
|
Prepaid expenses and other current assets
|
4
|
|
—
|
|
6
|
|
1,444
|
|
—
|
|
1,454
|
|
|||||||||||
|
Total current assets
|
526
|
|
728
|
|
445
|
|
50,179
|
|
(21,034
|
)
|
30,844
|
|
|||||||||||
|
Property, plant and equipment, net
|
—
|
|
—
|
|
1
|
|
4,698
|
|
—
|
|
4,699
|
|
|||||||||||
|
Goodwill
|
—
|
|
—
|
|
—
|
|
40,530
|
|
—
|
|
40,530
|
|
|||||||||||
|
Other intangible assets, net
|
—
|
|
—
|
|
—
|
|
28,101
|
|
—
|
|
28,101
|
|
|||||||||||
|
Long-term tax assets
|
—
|
|
—
|
|
—
|
|
774
|
|
—
|
|
774
|
|
|||||||||||
|
Investment in subsidiaries
|
70,233
|
|
28,663
|
|
61,768
|
|
—
|
|
(160,664
|
)
|
—
|
|
|||||||||||
|
Intercompany loans receivable
|
3,000
|
|
7,401
|
|
11,303
|
|
17,082
|
|
(38,786
|
)
|
—
|
|
|||||||||||
|
Other assets
|
—
|
|
—
|
|
—
|
|
1,737
|
|
—
|
|
1,737
|
|
|||||||||||
|
Total assets
|
$
|
73,759
|
|
$
|
36,792
|
|
$
|
73,517
|
|
$
|
143,101
|
|
$
|
(220,484
|
)
|
$
|
106,685
|
|
|||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||||||||||||||||||
|
Current liabilities:
|
|||||||||||||||||||||||
|
Short-term borrowings
|
$
|
—
|
|
$
|
1,002
|
|
$
|
—
|
|
$
|
1,432
|
|
$
|
—
|
|
$
|
2,434
|
|
|||||
|
Accounts payable
|
—
|
|
—
|
|
2
|
|
1,608
|
|
—
|
|
1,610
|
|
|||||||||||
|
Intercompany payable
|
20,506
|
|
—
|
|
279
|
|
249
|
|
(21,034
|
)
|
—
|
|
|||||||||||
|
Accrued compensation
|
1
|
|
—
|
|
—
|
|
1,610
|
|
—
|
|
1,611
|
|
|||||||||||
|
Accrued income taxes
|
19
|
|
—
|
|
—
|
|
916
|
|
—
|
|
935
|
|
|||||||||||
|
Deferred tax liabilities
|
3
|
|
—
|
|
—
|
|
116
|
|
—
|
|
119
|
|
|||||||||||
|
Other accrued expenses
|
—
|
|
40
|
|
1
|
|
2,423
|
|
—
|
|
2,464
|
|
|||||||||||
|
Total current liabilities
|
20,529
|
|
1,042
|
|
282
|
|
8,354
|
|
(21,034
|
)
|
9,173
|
|
|||||||||||
|
Long-term debt
|
—
|
|
4,581
|
|
—
|
|
29,171
|
|
—
|
|
33,752
|
|
|||||||||||
|
Long-term accrued compensation and retirement benefits
|
—
|
|
—
|
|
—
|
|
1,535
|
|
—
|
|
1,535
|
|
|||||||||||
|
Long-term accrued income taxes
|
—
|
|
—
|
|
—
|
|
2,476
|
|
—
|
|
2,476
|
|
|||||||||||
|
Long-term intercompany loans payable
|
—
|
|
8,385
|
|
10,002
|
|
20,399
|
|
(38,786
|
)
|
—
|
|
|||||||||||
|
Long-term deferred tax liabilities
|
—
|
|
—
|
|
—
|
|
4,700
|
|
—
|
|
4,700
|
|
|||||||||||
|
Other long-term liabilities
|
—
|
|
—
|
|
—
|
|
1,819
|
|
—
|
|
1,819
|
|
|||||||||||
|
Total liabilities
|
20,529
|
|
14,008
|
|
10,284
|
|
68,454
|
|
(59,820
|
)
|
53,455
|
|
|||||||||||
|
Shareholders’ equity
|
53,230
|
|
22,784
|
|
63,233
|
|
74,647
|
|
(160,664
|
)
|
53,230
|
|
|||||||||||
|
Total liabilities and shareholders’ equity
|
$
|
73,759
|
|
$
|
36,792
|
|
$
|
73,517
|
|
$
|
143,101
|
|
$
|
(220,484
|
)
|
$
|
106,685
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (CIFSA)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Operating Activities:
|
|||||||||||||||||||||||
|
Net cash provided by (used in) operating activities
|
$
|
297
|
|
$
|
4,208
|
|
$
|
604
|
|
$
|
4,114
|
|
$
|
(4,005
|
)
|
$
|
5,218
|
|
|||||
|
Investing Activities:
|
|||||||||||||||||||||||
|
Acquisitions, net of cash acquired
|
—
|
|
—
|
|
—
|
|
(1,266
|
)
|
53
|
|
(1,213
|
)
|
|||||||||||
|
Additions to property, plant, and equipment
|
—
|
|
—
|
|
—
|
|
(1,046
|
)
|
—
|
|
(1,046
|
)
|
|||||||||||
|
Purchases of marketable securities
|
—
|
|
—
|
|
—
|
|
(5,406
|
)
|
—
|
|
(5,406
|
)
|
|||||||||||
|
Sales and maturities of marketable securities
|
—
|
|
—
|
|
—
|
|
9,924
|
|
—
|
|
9,924
|
|
|||||||||||
|
Net (increase) decrease in intercompany loans receivable
|
—
|
|
(8,193
|
)
|
(164
|
)
|
(3,302
|
)
|
11,659
|
|
—
|
|
|||||||||||
|
Sales of subsidiaries
|
—
|
|
—
|
|
53
|
|
—
|
|
(53
|
)
|
—
|
|
|||||||||||
|
Capital contributions paid
|
—
|
|
(720
|
)
|
(4,959
|
)
|
—
|
|
5,679
|
|
—
|
|
|||||||||||
|
Other investing activities, net
|
—
|
|
—
|
|
—
|
|
(14
|
)
|
—
|
|
(14
|
)
|
|||||||||||
|
Net cash provided by (used in) investing activities
|
—
|
|
(8,913
|
)
|
(5,070
|
)
|
(1,110
|
)
|
17,338
|
|
2,245
|
|
|||||||||||
|
Financing Activities:
|
|||||||||||||||||||||||
|
Acquisition-related contingent consideration
|
—
|
|
—
|
|
—
|
|
(22
|
)
|
—
|
|
(22
|
)
|
|||||||||||
|
Change in short-term borrowings, net
|
—
|
|
—
|
|
—
|
|
7
|
|
—
|
|
7
|
|
|||||||||||
|
Repayment of short-term borrowings (maturities greater than 90 days)
|
—
|
|
—
|
|
(139
|
)
|
—
|
|
—
|
|
(139
|
)
|
|||||||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days)
|
—
|
|
—
|
|
139
|
|
—
|
|
—
|
|
139
|
|
|||||||||||
|
Issuance of long-term debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Payments on long-term debt
|
—
|
|
(2,121
|
)
|
—
|
|
(3,011
|
)
|
—
|
|
(5,132
|
)
|
|||||||||||
|
Dividends to shareholders
|
(2,139
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,139
|
)
|
|||||||||||
|
Issuance of ordinary shares
|
491
|
|
—
|
|
—
|
|
—
|
|
—
|
|
491
|
|
|||||||||||
|
Repurchase of ordinary shares
|
(2,830
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,830
|
)
|
|||||||||||
|
Net intercompany loan borrowings (repayments)
|
3,918
|
|
6,306
|
|
4,296
|
|
(2,861
|
)
|
(11,659
|
)
|
—
|
|
|||||||||||
|
Intercompany dividend paid
|
—
|
|
—
|
|
—
|
|
(4,005
|
)
|
4,005
|
|
—
|
|
|||||||||||
|
Capital Contributions received
|
—
|
|
—
|
|
—
|
|
5,679
|
|
(5,679
|
)
|
—
|
|
|||||||||||
|
Other financing activities
|
—
|
|
—
|
|
—
|
|
82
|
|
—
|
|
82
|
|
|||||||||||
|
Net cash provided by (used in) financing activities
|
(560
|
)
|
4,185
|
|
4,296
|
|
(4,131
|
)
|
(13,333
|
)
|
(9,543
|
)
|
|||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
—
|
|
—
|
|
—
|
|
113
|
|
—
|
|
113
|
|
|||||||||||
|
Net change in cash and cash equivalents
|
(263
|
)
|
(520
|
)
|
(170
|
)
|
(1,014
|
)
|
—
|
|
(1,967
|
)
|
|||||||||||
|
Cash and cash equivalents at beginning of period
|
263
|
|
728
|
|
170
|
|
3,682
|
|
—
|
|
4,843
|
|
|||||||||||
|
Cash and cash equivalents at end of period
|
$
|
—
|
|
$
|
208
|
|
$
|
—
|
|
$
|
2,668
|
|
$
|
—
|
|
$
|
2,876
|
|
|||||
|
(in millions)
|
Parent Company Guarantor (Medtronic plc)
|
Subsidiary Issuer (CIFSA)
|
Subsidiary Guarantors
|
Subsidiary Non-guarantors
|
Consolidating
Adjustments
|
Total
|
|||||||||||||||||
|
Operating Activities:
|
|||||||||||||||||||||||
|
Net cash provided by (used in) operating activities
|
$
|
26
|
|
$
|
1,238
|
|
$
|
142
|
|
$
|
4,596
|
|
$
|
(1,100
|
)
|
$
|
4,902
|
|
|||||
|
Investing Activities:
|
|||||||||||||||||||||||
|
Acquisitions, net of cash acquired
|
(9,700
|
)
|
440
|
|
—
|
|
(5,624
|
)
|
—
|
|
(14,884
|
)
|
|||||||||||
|
Additions to property, plant, and equipment
|
—
|
|
—
|
|
(1
|
)
|
(570
|
)
|
—
|
|
(571
|
)
|
|||||||||||
|
Purchases of marketable securities
|
—
|
|
—
|
|
—
|
|
(7,582
|
)
|
—
|
|
(7,582
|
)
|
|||||||||||
|
Sales and maturities of marketable securities
|
—
|
|
—
|
|
—
|
|
5,890
|
|
—
|
|
5,890
|
|
|||||||||||
|
Net (increase) decrease in intercompany loans receivable
|
—
|
|
(59
|
)
|
29
|
|
(10,626
|
)
|
10,656
|
|
—
|
|
|||||||||||
|
Capital contributions paid
|
—
|
|
(937
|
)
|
—
|
|
—
|
|
937
|
|
—
|
|
|||||||||||
|
Other investing activities, net
|
—
|
|
—
|
|
—
|
|
89
|
|
—
|
|
89
|
|
|||||||||||
|
Net cash provided by (used in) investing activities
|
(9,700
|
)
|
(556
|
)
|
28
|
|
(18,423
|
)
|
11,593
|
|
(17,058
|
)
|
|||||||||||
|
Financing Activities:
|
|||||||||||||||||||||||
|
Acquisition-related contingent consideration
|
—
|
|
—
|
|
—
|
|
(85
|
)
|
—
|
|
(85
|
)
|
|||||||||||
|
Change in short-term borrowings, net
|
—
|
|
—
|
|
—
|
|
(1
|
)
|
—
|
|
(1
|
)
|
|||||||||||
|
Repayment of short-term borrowings (maturities greater than 90 days)
|
—
|
|
—
|
|
(150
|
)
|
—
|
|
—
|
|
(150
|
)
|
|||||||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days)
|
—
|
|
—
|
|
150
|
|
—
|
|
—
|
|
150
|
|
|||||||||||
|
Issuance of long-term debt
|
—
|
|
—
|
|
—
|
|
19,942
|
|
—
|
|
19,942
|
|
|||||||||||
|
Payments on long-term debt
|
—
|
|
(51
|
)
|
—
|
|
(1,217
|
)
|
—
|
|
(1,268
|
)
|
|||||||||||
|
Dividends to shareholders
|
(435
|
)
|
—
|
|
—
|
|
(902
|
)
|
—
|
|
(1,337
|
)
|
|||||||||||
|
Issuance of ordinary shares
|
172
|
|
—
|
|
—
|
|
477
|
|
—
|
|
649
|
|
|||||||||||
|
Repurchase of ordinary shares
|
(300
|
)
|
—
|
|
—
|
|
(1,620
|
)
|
—
|
|
(1,920
|
)
|
|||||||||||
|
Net intercompany loan borrowings (repayments)
|
10,500
|
|
97
|
|
—
|
|
59
|
|
(10,656
|
)
|
—
|
|
|||||||||||
|
Intercompany dividend paid
|
—
|
|
—
|
|
—
|
|
(1,100
|
)
|
1,100
|
|
—
|
|
|||||||||||
|
Capital contributions received
|
—
|
|
—
|
|
—
|
|
937
|
|
(937
|
)
|
—
|
|
|||||||||||
|
Other financing activities
|
—
|
|
—
|
|
—
|
|
(31
|
)
|
—
|
|
(31
|
)
|
|||||||||||
|
Net cash provided by (used in) financing activities
|
9,937
|
|
46
|
|
—
|
|
16,459
|
|
(10,493
|
)
|
15,949
|
|
|||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
—
|
|
—
|
|
—
|
|
(353
|
)
|
—
|
|
(353
|
)
|
|||||||||||
|
Net change in cash and cash equivalents
|
263
|
|
728
|
|
170
|
|
2,279
|
|
—
|
|
3,440
|
|
|||||||||||
|
Cash and cash equivalents at beginning of period
|
—
|
|
—
|
|
—
|
|
1,403
|
|
—
|
|
1,403
|
|
|||||||||||
|
Cash and cash equivalents at end of period
|
$
|
263
|
|
$
|
728
|
|
$
|
170
|
|
$
|
3,682
|
|
$
|
—
|
|
$
|
4,843
|
|
|||||
|
(a)
|
1. Financial Statement Schedules
|
|
|
Schedule II. Valuation and Qualifying Accounts — years ended April 29, 2016, April 24, 2015, and April 25, 2014.
|
|
|
All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
|
|
|
2. Exhibits
|
|
Exhibit No.
|
Description
|
||
|
2.1
|
Transaction Agreement, dated as of June 15, 2014, among Medtronic, Inc., Covidien plc, Medtronic plc (formerly known as Kalani I Limited), Makani II Limited, Aviation Acquisition Co., Inc., and Aviation Merger Sub, LLC (incorporated by reference to Exhibit 2.1 to Medtronic plc’s Amendment No. 5 to the Registration Statement on Form S-4, filed on November 20, 2014, File No. 333-197406).
|
||
|
2.2
|
Appendix III to the Rule 2.5 Announcement (Conditions Appendix) (incorporated by reference to Exhibit 2.2 to Medtronic, Inc.’s Current Report on Form 8-K, filed on June 16, 2014, File No. 001-07707).
|
||
|
2.3
|
Expenses Reimbursement Agreement, dated as of June 15, 2014, by and between Covidien plc and Medtronic, Inc. (incorporated by reference to Exhibit 2.3 to Medtronic, Inc.’s Current Report on Form 8-K, filed on June 16, 2014, File No. 001-07707).
|
||
|
2.4
|
Separation and Distribution Agreement, dated as of June 29, 2007, by and among Tyco International Ltd., Covidien Ltd. and Tyco Electronics Ltd. (incorporated by reference to Exhibit 2.1 to Covidien plc’s Current Report on Form 8-K, filed on July 5, 2007, File No. 001-33259).
|
||
|
2.5
|
Separation and Distribution Agreement, dated as of June 28, 2013, between Covidien plc and Mallinckrodt plc (incorporated by reference to Exhibit 2.1 to Covidien plc’s Current Report on Form 8-K filed on July 1, 2013, File No. 001-33259).
|
||
|
3.1
|
Certificate of Incorporation of Medtronic plc (incorporated by reference to Exhibit 3.1 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
3.2
|
Amended and Restated Memorandum and Articles of Association of Medtronic plc (incorporated by reference to Exhibit 3.1 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
4.1
|
Form of Indenture between Medtronic, Inc. and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Amendment No. 2 to the Registration Statement on Form S-4, filed on January 10, 2005, File No. 333-121239).
|
||
|
4.2
|
Indenture, dated as of September 15, 2005, between Medtronic, Inc. and Wells Fargo Bank, N. A. (including the Forms of Notes thereof) (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Registration Statement on Form S-4, filed December 6, 2005, File No. 333-130163).
|
||
|
4.3
|
First Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic plc, Medtronic, Inc., Medtronic Global Holdings S.C.A. and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.1 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
4.4
|
Form of Indenture between Medtronic, Inc. and Wells Fargo Bank, National Association regarding 2009 offering (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Registration Statement on Form S-3, filed on March 9, 2009, File No. 333-157777).
|
||
|
4.5
|
First Supplemental Indenture, dated March 12, 2009, between Medtronic, Inc. and Wells Fargo Bank, National Association (including the Forms of Notes thereof) (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Current Report on Form 8-K, filed on March 12, 2009, File No. 001-07707).
|
||
|
4.6
|
Second Supplemental Indenture, dated March 16, 2010, between Medtronic, Inc. and Wells Fargo Bank, National Association (including the Forms of Notes thereof) (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Current Report on Form 8-K, filed on March 16, 2010, File No. 001-07707).
|
||
|
4.7
|
Third Supplemental Indenture, dated March 15, 2011, between Medtronic, Inc. and Wells Fargo Bank, National Association (including the Forms of Notes thereof) (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Current report on Form 8-K, filed on March 16, 2011, File No. 001-07707).
|
||
|
4.8
|
Fourth Supplemental Indenture, dated March 19, 2012, between Medtronic, Inc. and Wells Fargo Bank, National Association (including the Forms of Notes thereof) (incorporated by reference to Exhibit 4.2 to Medtronic, Inc.’s Current Report on Form 8-K, filed on March 20, 2012, File No. 001-07707).
|
||
|
4.9
|
Fifth Supplemental Indenture, dated March 26, 2013, between Medtronic, Inc. and Wells Fargo Bank, National Association (including the Forms of Notes thereof) (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Current Report on Form 8-K, filed on March 26, 2013, File No. 001-07707).
|
||
|
4.10
|
Sixth Supplemental Indenture, dated February 27, 2014, between Medtronic, Inc. and Wells Fargo Bank, National Association (including the Form of Global Note thereof) (incorporated by reference to Exhibit 4.2 to Medtronic, Inc.’s Current Report on Form 8-K, filed on February 27, 2014, File No. 001-07707).
|
||
|
4.11
|
Seventh Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic plc, Medtronic, Inc., Medtronic Global Holdings S.C.A. and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.2 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
4.12
|
Indenture, dated December 10, 2014, between Medtronic, Inc. and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.1 to Medtronic, Inc.’s Current Report on Form 8-K filed with the Commission on December 10, 2014, File No. 001-07707).
|
||
|
4.13
|
First Supplemental Indenture, dated December 10, 2014, between Medtronic, Inc. and Wells Fargo Bank, National Association (including Form of Floating Rate Senior Notes due 2020, Form of 1.500% Senior Notes due 2018, Form of 2.500% Senior Notes due 2020, Form of 3.150% Senior Notes due 2022, Form of 3.500% Senior Notes due 2025, Form of 4.375% Senior Notes due 2035 and Form of 4.625% Senior Notes due 2045) (incorporated by reference to Exhibit 4.2 of Medtronic, Inc.’s Current Report on Form 8-K filed with the Commission on December 10, 2014, File No. 001-07707).
|
||
|
4.14
|
Second Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic plc and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.3 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
4.15
|
Third Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic Global Holdings S.C.A. and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 4.4 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
4.16
|
Indenture, dated as of October 22, 2007, by and among Covidien International Finance S.A., Covidien Ltd. and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1(a) to Covidien plc’s Current Report on Form 8-K filed on October 22, 2007, File No. 001-33259).
|
||
|
4.17
|
First Supplemental Indenture, dated as of October 22, 2007, by and among Covidien International Finance S.A., Covidien Ltd. 1and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1(b) to the Covidien plc’s Current Report on Form 8-K filed on October 22, 2007, File No. 001-33259).
|
||
|
4.18
|
Second Supplemental Indenture, dated as of October 22, 2007, by and among Covidien International Finance S.A., Covidien Ltd. and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1(c) to the Covidien plc’s Current Report on Form 8-K filed on October 22, 2007, File No. 001-33259).
|
||
|
4.19
|
Third Supplemental Indenture, dated as of October 22, 2007, by and among Covidien International Finance S.A., Covidien Ltd. and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1(d) to Covidien plc’s Current Report on Form 8-K filed on October 22, 2007, File No. 001-33259).
|
||
|
4.20
|
Fourth Supplemental Indenture, dated as of October 22, 2007, by and among Covidien International Finance S.A., Covidien Ltd. and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1(e) to Covidien plc’s Current Report on Form 8-K filed on October 22, 2007, File No. 001-33259).
|
||
|
4.21
|
Fifth Supplemental Indenture, dated as of June 4, 2009, by and among Covidien International Finance S.A., Covidien Ltd., Covidien plc and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1 to Covidien plc’s Current Report on Form 8-K12G3 filed on June 5, 2009, File No. 001-33259).
|
||
|
4.22
|
Sixth Supplemental Indenture, dated as of June 28, 2010, among Covidien International Finance S.A., Covidien Ltd., Covidien plc and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1 to Coviden plc’s Current Report on Form 8-K filed on June 28, 2010, File No. 001-33259).
|
||
|
4.23
|
Seventh Supplemental Indenture, dated as of May 30, 2012, among Covidien International Finance S.A., Covidien Ltd., Covidien plc and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1 to Covidien plc’s Current Report on Form 8-K filed on May 30, 2012, File No. 001-33259).
|
||
|
4.24
|
Eighth Supplemental Indenture, dated as of May 16, 2013, among Covidien International Finance S.A., Covidien Ltd., Covidien plc and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.1 to Covidien plc’s Current Report on Form 8-K filed on May 16, 2013, File No. 001-33259).
|
||
|
4.25
|
Ninth Supplemental Indenture, dated as of January 26, 2015, by and among Medtronic plc, Medtronic Global Holdings S.C.A., Covidien public limited company, Covidien International Finance S.A., Covidien Ltd. and Deutsche Bank Trust Company Americas (incorporated by reference to Exhibit 4.5 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
4.26
|
Registration Rights Agreement, dated December 10, 2014, by and among Medtronic, Inc. and Merrill Lynch, Pierce, Fenner & Smith Incorporated, Deutsche Bank Securities Inc. and J.P. Morgan Securities LLC, as representatives of the several initial purchasers (incorporated by reference to Exhibit 4.10 to Medtronic, Inc.’s Current Report on Form 8-K filed with the Commission on December 10, 2014, File No. 001-07707)
|
||
|
4.27
|
Joinder Agreement to the Registration Rights Agreement, dated as of January 26, 2015, by and among Medtronic plc and Medtronic Global Holdings S.C.A. (incorporated by reference to Exhibit 4.6 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
10.1
|
Senior Unsecured Term Loan Credit Agreement, dated as of November 7, 2014, by and among Medtronic, Inc., Medtronic Holdings Limited, Medtronic Global Holdings SCA, the lenders from time to time party thereto and Bank of America, N.A., as administrative agent (incorporated by reference to Exhibit 10.2 to Medtronic Inc.’s Current Report on Form 8-K, filed on November 10, 2014, File No. 001-07707).
|
||
|
10.2
|
Amendment and Restatement Agreement, dated as of November 7, 2014, by and among Medtronic, Inc., Medtronic plc (formerly known as Medtronic Holdings Limited), Medtronic Global Holdings S.C.A., the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent and issuing bank (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Current Report on Form 8-K, filed on November 10, 2014, File No. 001-07707).
|
||
|
10.3
|
Senior Unsecured Bridge Credit Agreement, dated as of November 7, 2014, by and among Medtronic, Inc., Medtronic Holdings Limited, Medtronic Global Holdings SCA, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent (incorporated by reference to Exhibit 10.1 to Medtronic, Inc.’s Current Report on Form 8-K, filed on November 10, 2014, File No. 001-07707).
|
||
|
10.4
|
Senior Unsecured Bridge Credit Agreement, dated as of June 15, 2014, by and among Medtronic, Inc., Kalani I Limited, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent (incorporated by reference to Exhibit 10.1 to Medtronic, Inc.’s Current Report on Form 8-K, filed on June 18, 2014, File No. 001-07707).
|
||
|
10.5
|
Senior Unsecured Cash Bridge Credit Agreement, dated as of June 15, 2014, by and among Makani II Limited, Kalani I Limited, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Current Report on Form 8-K, filed on June 18, 2014, File No. 001-07707).
|
||
|
10.6
|
Amendment dated September 30, 2015, to Senior Unsecured Term Loan Credit Agreement, dated as of November 7, 2014, by and among Medtronic, Inc., Medtronic Holdings Limited, Medtronic Global Holdings, SCA, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent. (incorporated by reference to Exhibit 10.1 to Medtronic plc’s Form 10-Q for the quarter ended October 30, 2015, filed on December 9, 2015, File No. 001-36820).
|
||
|
10.7
|
Amendment dated September 30, 2015, to Amended and Restated Revolving Credit Agreement, dated as of November 7, 2014, by and among Medtronic, Inc., Medtronic Holdings Limited, Medtronic Global Holdings, SCA, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent and issuing bank (incorporated by reference to Exhibit 10.2 to Medtronic plc’s Form 10-Q for the quarter ended October 30, 2015,
filed on December 9, 2015, File No. 001-36820).
|
||
|
10.8
|
Amended and Restated Five-Year Senior Credit Agreement, dated as of May 23, 2014, among Covidien International Finance S.A., Covidien plc, the lenders party thereto and Citibank, N.A., as administrative agent (incorporated by reference to Exhibit 10.1 to Covidien plc’s Current Report on Form 8-K, filed on May 28, 2014, File No. 001-33259).
|
||
|
10.9
|
Tax Sharing Agreement, dated as of June 29, 2007, by and among Tyco International Ltd., Covidien Ltd. and Tyco Electronics Ltd. (incorporated by reference to Exhibit 10.1 to Covidien plc’s Current Report on Form 8-K, filed on July 5, 2007, File No. 001-33259).
|
||
|
10.10
|
Tax Matters Agreement, dated as of June 28, 2013, between Covidien plc and Mallinckrodt plc (incorporated by reference to Exhibit 10.1 to Covidien plc’s Current Report on Form 8-K filed on July 1, 2013, File No. 001-33259).
|
||
|
10.11
|
Employee Matters Agreement, dated as of June 28, 2013, between Covidien plc and Mallinckrodt plc (incorporated by reference to Exhibit 10.2 to Covidien plc’s Current Report on Form 8-K filed on July 1, 2013, File No. 001-33259).
|
||
|
10.12
|
Transition Services Agreement, dated as of June 28, 2013, between Covidien plc and Mallinckrodt plc (incorporated by reference to Exhibit 10.3 to Covidien plc’s Current Report on Form 8-K filed on July 1, 2013, File No. 001-33259).
|
||
|
10.13
|
Form of Deed of Indemnification (incorporated by reference to Exhibit 10.1 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
10.14
|
Form of Indemnification Agreement (incorporated by reference to Exhibit 10.2 to Medtronic plc’s Current Report on Form 8-K12B, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.15
|
Letter Agreement by and between Medtronic, Inc. and Omar Ishrak dated May 11, 2011 (incorporated by reference to Exhibit 10.1 to Medtronic, Inc.’s Current Report on Form 8-K, filed on May 11, 2011, File No. 001-07707).
|
||
|
*10.16
|
Change of Control Severance Plan - Section 16B Officers (as amended and restated as of January 26, 2015) (incorporated by reference to Exhibit 10.14 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.17
|
Amendment to Letter Agreement dated May 11, 2011 by and between Medtronic, Inc. and Omar Ishrak (incorporated by reference to Exhibit 10.1 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 29, 2011, filed September 7, 2011, File No. 001-07707).
|
||
|
*10.18
|
Amendment dated February 12, 2015 to the Letter Agreement by and between Medtronic, Inc. and Omar Ishrak dated May 11, 2011 (incorporated by reference to Exhibit 10.24 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.19
|
Letter Agreement by and between Medtronic, Inc. and Michael J. Coyle dated November 19, 2009 (incorporated by reference to Exhibit 10.55 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 27, 2012, filed on June 26, 2012, File No. 001-07707).
|
||
|
*10.20
|
Letter Agreement by and between Medtronic, Inc. and Carol Surface dated August 22, 2013 (incorporated by reference to Exhibit 10.44 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 25, 2014, filed on June 20, 2014, File No. 001-07707).
|
||
|
*10.21
|
Letter Agreement by and between Medtronic, Inc. and Hooman Hakami dated April 29, 2014 (incorporated by reference to Exhibit 10.5 of Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2014, filed on August 29, 2014, File No. 001-07707)
|
||
|
*10.22
|
Letter Agreement by and between Medtronic, Inc. and Bradley E. Lerman dated May 2, 2014 (incorporated by reference to Exhibit 10.4 of Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2014, filed on August 29, 2014, File No. 001-07707)
|
||
|
*10.23
|
Letter Agreement by and between Medtronic plc and Bryan C. Hanson dated February 12, 2015 (incorporated by reference to Exhibit 10.30 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.24
|
Letter Agreement by and between Medtronic, Inc. and Karen Parkhill dated May 2, 2016 (incorporated by reference to Exhibit 10.1 to Medtronic, plc’s Current Report on Form 8-K, filed on May 4, 2016, File No. 001-36820).
|
||
|
*10.25
|
Form of Offer Letter Amendment (incorporated by reference to Exhibit 10.25 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.26
|
1994 Stock Award Plan (amended and restated as of January 1, 2008) (incorporated by reference to Exhibit 10.1 of Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 28, 2008, filed on March 4, 2008, File No. 001-07707).
|
||
|
*10.27
|
Amendment to the 1994 Stock Award Plan (incorporated by reference to Exhibit 10.7 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.28
|
1998 Outside Director Stock Compensation Plan (as amended and restated effective as of January 1, 2008) (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Current Report on Form 8-K, filed on February 27, 2014, File No. 001-07707)
|
||
|
*10.29
|
Amendment to the 1998 Outside Director Stock Compensation Plan (incorporated by reference to Exhibit 10.2 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.30
|
Form of Initial Option Agreement under the 1998 Outside Director Stock Compensation Plan (incorporated by reference to Exhibit 10.17 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 29, 2005, filed June 29, 2005, File No. 001-07707).
|
||
|
*10.31
|
Form of Annual Option Agreement under the 1998 Outside Director Stock Compensation Plan (incorporated by reference to Exhibit 10.18 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 29, 2005, filed June 29, 2005, File No. 001-07707).
|
||
|
*10.32
|
Form of Replacement Option Agreement under the 1998 Outside Director Stock Compensation Plan (incorporated by reference to Exhibit 10.19 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 29, 2005, filed June 29, 2005, File No. 001-07707).
|
||
|
*10.33
|
Kyphon Inc. 2002 Stock Plan (amended and restated July 26, 2007, as further amended on October 18, 2007) (incorporated by reference to Exhibit 10.6 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 25, 2008, filed on March 4, 2008, File No. 001-07707).
|
||
|
*10.34
|
Addendum: Kyphon Inc. 2002 Stock Plan (dated December 13, 2007) (incorporated by reference to Exhibit 10.7 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 25, 2008, filed on March 4, 2008, File No. 001-07707).
|
||
|
*10.35
|
Amendment to the Kyphon Inc. 2002 Stock Plan (incorporated by reference to Exhibit 10.1 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.36
|
2003 Long-Term Incentive Plan (as amended and restated effective January 1, 2008) (incorporated by reference to Exhibit 10.4 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 28, 2008, filed on March 4, 2008, File No. 001-07707).
|
||
|
*10.37
|
Amendment to the 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.3 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.38
|
Form of Restricted Stock Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 28, 2005, filed on March 7, 2005, File No. 001-07707).
|
||
|
*10.39
|
Form of Non-Qualified Stock Option Agreement under 2003 Long-Term Incentive Plan (four year vesting) (incorporated by reference to Exhibit 10.1 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 28, 2005, filed on March 7, 2005, File No. 001-07707).
|
||
|
*10.40
|
Form of Non-Qualified Stock Option Agreement under 2003 Long-Term Incentive Plan (immediate vesting) (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 28, 2005, filed on March 7, 2005, File No. 001-07707).
|
||
|
*10.41
|
Form of Restricted Stock Units Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.20 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 29, 2005, filed on June 29, 2005, File No. 001-07707).
|
||
|
*10.42
|
Form of Performance Share Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.21 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 29, 2005, filed on June 29, 2005, File No. 001-07707).
|
||
|
*10.43
|
Form of Non-Qualified Stock Option Agreement under 2003 Long-Term Incentive Plan effective June 22, 2006 (incorporated by reference to Exhibit 10.23 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 28, 2006, filed on June 28, 2006, File No. 001-07707).
|
||
|
*10.44
|
Form of Restricted Stock Award Agreement under 2003 Long-Term Incentive Plan effective June 22, 2006 (incorporated by reference to Exhibit 10.24 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 28, 2006, filed on June 28, 2006, File No. 001-07707).
|
||
|
*10.45
|
Form of Restricted Stock Unit Award Agreement under 2003 Long-Term Incentive Plan effective June 22, 2006 (incorporated by reference to Exhibit 10.25 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 28, 2006, filed on June 28, 2006, File No. 001-07707).
|
||
|
*10.46
|
Form of Performance Award Agreement under 2003 Long-Term Incentive Plan effective June 22, 2006 (incorporated by reference to Exhibit 10.26 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 28, 2006, filed on June 28, 2006, File No. 001-07707).
|
||
|
*10.47
|
Form of Restricted Stock Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 26, 2007, filed on December 4, 2007, File No. 001-07707).
|
||
|
*10.48
|
Form of Restricted Stock Unit Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.4 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 26, 2007, filed on December 4, 2007, File No. 001-07707).
|
||
|
*10.49
|
Form of Non-Qualified Stock Option Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.39 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 25, 2008, filed on June 24, 2008, File No. 001-07707).
|
||
|
*10.50
|
Form of Restricted Stock Unit Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.40 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 25, 2008, filed on June 24, 2008, File No. 001-07707).
|
||
|
*10.51
|
Form of Restricted Stock Unit Award Agreement under 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.41 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 25, 2008, filed on June 24, 2008, File No. 001-07707).
|
||
|
*10.52
|
Israeli Amendment to the 2003 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.5 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 25, 2008, filed on March 4, 2008, File No. 001-07707).
|
||
|
*10.53
|
2008 Stock Award and Incentive Plan (as amended and restated effective August 27, 2009) (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 30, 2009, filed on December 9, 2009, File No. 001-07707).
|
||
|
*10.54
|
Amendment to the 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.4 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.55
|
Form of Restricted Stock Unit Award Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2008, filed on September 3, 2008, File No. 001-07707).
|
||
|
*10.56
|
Form of Restricted Stock Award Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2008, filed on September 3, 2008, File No. 001-07707).
|
||
|
*10.57
|
Form of Restricted Stock Award Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.4 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2008, filed on September 3, 2008, File No. 001-07707).
|
||
|
*10.58
|
Form of Restricted Stock Unit Award Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.5 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2008, filed on September 3, 2008, File No. 001-07707).
|
||
|
*10.59
|
Form of Non-Qualified Stock Option Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.6 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended July 25, 2008, filed on September 3, 2008, File No. 001-07707).
|
||
|
*10.60
|
Terms of Non-Employee Director Compensation under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.42 to Medtronic, Inc.’s Annual Report on Form 10-K for the year ended April 27, 2012, filed on June 26, 2012, File No. 001-07707).
|
||
|
*10.61
|
Form of Non-Employee Director Initial Option Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.1 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 24, 2008, filed on December 3, 2008, File No. 001-07707).
|
||
|
*10.62
|
Form of Non-Employee Director Annual Option Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 24, 2008, filed on December 3, 2008, File No. 001-07707).
|
||
|
*10.63
|
Form of Non-Employee Director Deferred Unit Award Agreement under 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 24, 2008, filed on December 3, 2008, File No. 001-07707).
|
||
|
*10.64
|
Form of Non-Employee Restricted Stock Unit Award Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.65 to Medtronic plc’s Annual Report on Form 10-K for the year ended April 24, 2015, filed on June 23, 2015, File No. 001-36820).
|
||
|
*10.65
|
Medtronic Incentive Plan (amended and restated effective January 1, 2008) (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended January 28, 2008, filed on March 4, 2008, File No. 001-07707).
|
||
|
*10.66
|
Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.9 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.67
|
Israeli Amendment to the Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.10 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.68
|
Form of Non-Qualified Stock Option Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.31 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.69
|
Form of Non-Employee Director Deferred Unit Award Agreement under the 2008 Stock Award and Incentive Plan (incorporated by reference to Exhibit 19.3 to Medtronic, Inc.’s Quarterly Report on Form 10-Q for the quarter ended October 24, 2008, filed on December 3, 2008, File No. 001-07707).
|
||
|
*10.70
|
Form of Non-Qualified Stock Option Agreement under 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.2 to Medtronic, Inc.’s Current Report on Form 8-K, filed on August 27, 2013, File No. 001-07707).
|
||
|
*10.71
|
Form of Restricted Stock Unit Award Agreement (U.S. Employees) under 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.3 to Medtronic, Inc.’s Current Report on Form 8-K, filed on August 27, 2013, File No. 001-07707).
|
||
|
*10.72
|
Form of Restricted Stock Unit Award Agreement (Non-U.S. Employees) under 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.4 to Medtronic, Inc.’s Current Report on Form 8-K, filed on August 27, 2013, File No. 001-07707).
|
||
|
*10.73
|
Form of Restricted Stock Unit Award Agreement (Time-Based) under 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.5 to Medtronic, Inc.’s Current Report on Form 8-K, filed on August 27, 2013, File No. 001-07707).
|
||
|
*10.74
|
Form of Restricted Stock Unit Award Agreement (Israeli-Employees) under 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.8 to Medtronic, Inc.’s Current Report on Form 8-K, filed on August 27, 2013, File No. 001-07707).
|
||
|
*10.75
|
Form of Non-Qualified Stock Option Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.48 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.76
|
Form of Restricted Stock Unit Award Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.49 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.77
|
Form of Restricted Stock Unit Award Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.50 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.78
|
Form of Restricted Stock Unit Award Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.51 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.79
|
Form of Stock Option Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.53 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.80
|
Form of Restricted Stock Unit Award Agreement under Amended and Restated 2013 Stock Award and Incentive Plan (incorporated by reference to Exhibit 10.54 to Medtronic plc’s Quarterly Report on Form 10-Q for the quarter ended January 23, 2015, filed on February 27, 2015, File No. 001-36820).
|
||
|
*10.81
|
Medtronic plc 2014 Amended and Restated Employees Stock Purchase Plan (incorporated by reference to Exhibit 10.8 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.82
|
Medtronic plc Incentive Plan (as amended and restated effective January 26, 2015) (incorporated by reference to Exhibit 10.11 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.83
|
Medtronic plc Supplemental Executive Retirement Plan (as restated generally effective January 26, 2015) (incorporated by reference to Exhibit 10.15 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.84
|
Medtronic plc Savings and Investment Plan (as amended and restated generally effective January 26, 2015) (incorporated by reference to Exhibit 4.22 to Medtronic plc’s Registration Statement on Form S-8 filed on January 28, 2015, File No. 333-201737).
|
||
|
*10.85
|
Medtronic plc Puerto Rico Employees’ Savings and Investment Plan (as amended and restated generally effective January 26, 2015) (incorporated by reference to Exhibit 4.23 to Medtronic plc’s Registration Statement on Form S-8 filed on January 28, 2015, File No. 333-201737).
|
||
|
*10.86
|
Medtronic plc Capital Accumulation Plan Deferral Program (as amended and restated generally effective January 26, 2015) (incorporated by reference to Exhibit 10.13 to Medtronic plc’s Current Report on Form 8-K, filed on January 27, 2015, File No. 001-36820).
|
||
|
*10.87
|
Covidien Savings Related Share Plan (incorporated by reference to Exhibit 99.3 to Covidien plc’s Post-Effective Amendment No. 1 to Registration Statement on Form S-8 filed with the Commission on June 5, 2009, File No. 333-144309).
|
||
|
*10.88
|
Covidien Stock and Incentive Plan (incorporated by reference to Exhibit 10.5 to Covidien plc’s Current Report on Form 8-K filed on March 26, 2013, File No. 001-33259).
|
||
|
*10.89
|
Covidien Separation and Distribution Agreement Equity Awards under the Separation and Distribution Agreement, dates as of June 29, 2007, by and among Tyco International Ltd., Covidien Ltd., and Tyco Electronics Ltd. (incorporated by reference to Exhibit 2.1 to Covidien plc’s Current Report on Form 8-K filed on July 5, 2007, File No. 001-33259).
|
||
|
*10.90
|
Covidien Severance Plan for U.S. Officers and Executives, as amended and restated (incorporated by reference to Exhibit 10.1 to Covidien plc’s Current Report on Form 8-K filed on September 23, 2014, File No. 001-33259).
|
||
|
*10.91
|
Covidien Change in Control Severance Plan for Certain U.S. Officers and Executives (incorporated by reference to Exhibit 10.1 to Covidien plc’s Current Report on Form 8-K filed on March 26, 2013, File No. 001-33259).
|
||
|
*10.92
|
Covidien Supplemental Savings and Retirement Plan, as amended and restated (incorporated by reference to Exhibit 10.1 to Covidien plc’s Quarterly Report on Form 10-Q for the quarter ended December 25, 2009, filed on January 26, 2010, File No. 001-33259).
|
||
|
*10.93
|
Form of Non-Competition, Non-Solicitation and Confidentiality Agreement for executive officers and certain key employees (incorporated by reference to Exhibit 10.4 to Covidien plc’s Quarterly Report on Form 10-Q for the quarter ended December 26, 2008, filed on January 29, 2009, File No. 001-33259).
|
||
|
*10.94
|
FY09 Grant U.S. Option Terms and Conditions (incorporated by reference to Exhibit 10.3 to Covidien plc’s Current Report on Form 8-K filed on September 23, 2014, File No. 001-33259).
|
||
|
*10.95
|
FY09 Grant U.S. Restricted Stock Unit Terms and Conditions (incorporated by reference to Exhibit 10.2 to Covidien plc’s Current Report on Form 8-K filed on November 25, 2008, File No. 001-33259).
|
||
|
*10.96
|
Deed Poll of Assumption relating to Covidien Ltd. Employee Equity Plans, dated June 4, 2009 (incorporated by reference to Exhibit 10.3 to Covidien plc’s Current Report on Form 8-K12G3 filed on June 5, 2009, File No. 001-33259).
|
||
|
*10.97
|
Director Grant Restricted Stock Unit Terms and Conditions (incorporated by reference to Exhibit 10.2 to Covidien plc’s Current Report on Form 8-K filed on March 23, 2009, File No. 001-33259).
|
||
|
*10.98
|
Founders’ Grant Standard Option Terms and Conditions (incorporated by reference to Exhibit 10.4 to Covidien plc’s Current Report on Form 8-K filed on September 23, 2014, File No. 001-33259).
|
||
|
*10.99
|
Founders’ Grant Standard Option Terms and Conditions for Directors (incorporated by reference to Exhibit 10.13 to Covidien plc’s Current Report on Form 8-K filed on July 5, 2007, File No. 001-33259).
|
||
|
*10.100
|
Form of Deed of Indemnification by and between Covidien plc and Covidien plc’s Directors and Secretary (incorporated by reference to Exhibit 10.4 to Covidien plc’s Form 10-Q for the quarter ended June 28, 2013, filed on August 5, 2013, File No. 001-33259).
|
||
|
*10.101
|
Form of Terms and Conditions of Option Award (incorporated by reference to Exhibit 10.2 to Covidien plc’s Current Report on Form 8-K filed on September 23, 2014, File No. 001-33259).
|
||
|
*10.102
|
Form of Terms and Conditions of Restricted Unit Award (incorporated by reference to Exhibit 10.3 to Covidien plc’s Quarterly Report on Form 10-Q for the quarter ended December 25, 2009, filed on January 26, 2010, File No. 001-33259).
|
||
|
*10.103
|
Form of Terms and Conditions of Performance Unit Award (incorporated by reference to Exhibit 10.4 to Covidien plc’s Quarterly Report on Form 10-Q for the quarter ended December 25, 2009, filed on January 26, 2010, File No. 001-33259).
|
||
|
*10.104
|
Amended Terms and Conditions of Performance Unit Awards FY12-FY14 (incorporated by reference to Exhibit 10.3 to Covidien plc’s Current Report on Form 8-K filed on March 26, 2013, File No. 001-33259).
|
||
|
*10.105
|
Amended Terms and Conditions of Performance Unit Awards FY13-FY15 (incorporated by reference to Exhibit 10.4 to Covidien plc’s Current Report on Form 8-K filed on March 26, 2013, File No. 001-33259).
|
||
|
*10.106
|
Form of Indemnification Agreement between Covidien Ltd. and Covidien plc’s Directors and Secretary (incorporated by reference to Exhibit 10.5 to Covidien plc’s Form 10-Q for the quarter ended June 28, 2013, filed on August 5, 2013, File No. 001-33259).
|
||
|
12.1
|
Computation of Ratio of Earnings to Fixed Charges.
|
||
|
21
|
List of Subsidiaries of Medtronic plc.
|
||
|
23
|
Consent of Independent Registered Public Accounting Firm.
|
||
|
24
|
Power of Attorney.
|
||
|
31.1
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
31.2
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
32.1
|
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||
|
32.2
|
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||
|
101
|
The following materials from Medtronic plc’s Annual Report on Form 10-K for the year ended April 29, 2016, formatted in Extensible Business Reporting Language (XBRL): (i) consolidated statements of income, (ii) consolidated statements of comprehensive income, (iii) consolidated balance sheets, (iv) consolidated statements of cash flows, (v) consolidated statements of shareholders’ equity, and (vi) the notes to the consolidated financial statements.
|
||
|
*Exhibits that are management contracts or compensatory plans or arrangements.
|
||
|
Additions
|
Deductions
|
|||||||||||||||||
|
Balance at
Beginning of
Fiscal Year
|
Charges to Income
|
Charges to Other Accounts
|
Other Changes (Debit) Credit
|
Balance
at End of
Fiscal Year
|
||||||||||||||
|
Allowance for doubtful accounts:
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Year ended 4/29/16
|
$
|
144
|
|
$
|
49
|
|
$
|
—
|
|
$
|
(28
|
)
|
(b)
|
$
|
161
|
|
||
|
|
|
|
|
|
$
|
(4
|
)
|
(c)
|
|
|
||||||||
|
Year ended 4/24/15
|
$
|
115
|
|
$
|
35
|
|
$
|
34
|
|
(a)
|
$
|
(36
|
)
|
(b)
|
$
|
144
|
|
|
|
|
|
|
|
|
$
|
(4
|
)
|
(c)
|
|
|
||||||||
|
Year ended 4/25/14
|
$
|
98
|
|
$
|
43
|
|
$
|
—
|
|
$
|
(30
|
)
|
(b)
|
$
|
115
|
|
||
|
|
|
|
|
|
$
|
4
|
|
(c)
|
|
|
||||||||
|
Deferred tax valuation allowance:
|
||||||||||||||||||
|
Year ended 4/29/16
|
$
|
5,607
|
|
$
|
1,194
|
|
$
|
4
|
|
(a)
|
$
|
(88
|
)
|
(d)
|
$
|
7,032
|
|
|
|
|
$
|
315
|
|
(c)
|
||||||||||||||
|
Year ended 4/24/15
|
$
|
397
|
|
$
|
40
|
|
$
|
5,660
|
|
(a)
|
$
|
(56
|
)
|
(d)
|
$
|
5,607
|
|
|
|
|
$
|
(434
|
)
|
(c)
|
||||||||||||||
|
Year ended 4/25/14
|
$
|
313
|
|
$
|
104
|
|
$
|
5
|
|
$
|
(29
|
)
|
(d)
|
$
|
397
|
|
||
|
$
|
4
|
|
(c)
|
|||||||||||||||
|
(a) Reflects the impact from acquisitions
|
|
|
(b) Uncollectible accounts written off, less recoveries.
|
|
|
(c) Reflects primarily the effects of currency fluctuations.
|
|
|
(d) Decrease in deferred tax valuation allowance due to carryover attribute utilization and expiration.
|
|
|
|
MEDTRONIC PUBLIC LIMITED COMPANY
|
|
|
|
|
|
|
Dated: June 28, 2016
|
By:
|
/s/
Omar Ishrak
|
|
|
|
Omar Ishrak
|
|
|
|
Chairman and
|
|
|
|
Chief Executive Officer
|
|
|
MEDTRONIC PUBLIC LIMITED COMPANY
|
|
|
|
|
|
|
Dated: June 28, 2016
|
By:
|
/s/
Omar Ishrak
|
|
|
|
Omar Ishrak
|
|
|
|
Chairman and
|
|
|
|
Chief Executive Officer
|
|
|
|
(Principal Executive Officer)
|
|
|
|
|
|
Dated: June 28, 2016
|
By:
|
/s/
Gary L. Ellis
|
|
|
|
Gary L. Ellis
|
|
|
|
Principal Financial and
|
|
|
|
Accounting Officer
|
|
|
|
|
|
|
Directors
|
|
|
|
|
|
|
|
|
Richard H. Anderson*
|
|
Craig Arnold*
|
||
|
Scott C. Donnelly*
|
||
|
|
|
Randall J. Hogan, III*
|
|
|
|
Omar Ishrak*
|
|
|
|
Shirley Ann Jackson, Ph.D*
|
|
|
|
Michael O. Leavitt*
|
|
|
|
James T. Lenehan*
|
|
Elizabeth G. Nabel*
|
||
|
|
|
Denise M. O’Leary*
|
|
|
|
Kendall J. Powell*
|
|
|
|
Robert C. Pozen*
|
|
|
|
Preetha Reddy*
|
|
Dated: June 28, 2016
|
By:
|
/s/ Bradley E. Lerman
|
|
|
|
Bradley E. Lerman
|