|
o
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Title of each class
|
Name of each exchange on which registered
|
|
|
Ordinary shares, par value NIS 0.01 per share
|
NASDAQ Global Market
|
|
Large accelerated filer
o
|
Accelerated filer
o
|
Non-accelerated filer
x
|
|
U.S. GAAP
o
|
International Financial Reporting Standards as issued
by the International Accounting Standards Board
x
|
Other
o
|

|
i
|
|
|
i
|
|
|
PART I
|
|
|
1
|
|
|
1
|
|
|
1
|
|
|
28
|
|
|
54
|
|
|
54
|
|
|
66
|
|
|
84
|
|
|
87
|
|
|
88
|
|
|
89
|
|
|
103
|
|
|
104
|
|
|
PART II
|
|
|
105
|
|
|
105
|
|
|
105
|
|
|
106
|
|
|
106
|
|
|
106
|
|
|
106
|
|
|
107
|
|
|
107
|
|
|
107
|
|
|
107
|
|
|
107
|
|
|
PART III
|
|
|
108
|
|
|
108
|
|
|
108
|
|
|
109
|
|
|
F-1
|
|
|
|
·
|
the timing and conduct of our trials of NexoBrid and our pipeline product candidates, including statements regarding the timing, progress and results of current and future preclinical studies and clinical trials, and our research and development programs;
|
|
|
·
|
the clinical utility, potential advantages and timing or likelihood of regulatory filings and approvals of NexoBrid and our pipeline products;
|
|
|
·
|
our expectations regarding future growth, including our ability to develop new products;
|
|
|
·
|
our commercialization, marketing and manufacturing capabilities and strategy and the ability of our marketing team to cover regional burn centers and units;
|
|
|
·
|
our ability to maintain adequate protection of our intellectual property;
|
|
|
·
|
our plans to develop and commercialize our pipeline products;
|
|
|
·
|
our estimates regarding expenses, future revenues, capital requirements and the need for additional financing;
|
|
|
·
|
our estimates regarding the market opportunity for NexoBrid and our pipeline products;
|
|
|
·
|
our expectation regarding the duration of our inventory of intermediate drug substance and products;
|
|
|
·
|
the impact of our research and development expenses as we continue developing product candidates;
|
|
|
·
|
our expectations regarding the time during which we will be an emerging growth company under the JOBS Act; and
|
|
|
·
|
the impact of government laws and regulations.
|
|
|
A.
|
Selected Financial Data
|
|
Year Ended December 31,
|
||||||||||||||||||||
|
2011
|
2012
|
2013
|
2014
|
2015
|
||||||||||||||||
|
(in thousands except share and per share data)
|
||||||||||||||||||||
|
Consolidated statements of operations data:
|
||||||||||||||||||||
|
Revenues
|
$ | — | $ | — | $ | — | $ | 259 | $ | 601 | ||||||||||
|
Cost of revenues (1)
|
— | — | — | 2,785 | 2,519 | |||||||||||||||
|
Gross income (loss)
|
— | — | — | (2,526 | ) | (1,918 | ) | |||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development, gross
|
6,149 | 3,804 | 4,513 | 6,054 | 8,139 | |||||||||||||||
|
Participation by OCS, BARDA and others
|
3,128 | 2,247 | 878 | 705 | 2,118 | |||||||||||||||
|
Research and development, net of participations(1)(2)
|
3,021 | 1,557 | 3,635 | 5,349 | 6,021 | |||||||||||||||
|
Selling and marketing(1)
|
— | — | 2,259 | 8,829 | 9,284 | |||||||||||||||
|
General and administrative(1)
|
1,266 | 1,173 | 1,687 | 4,723 | 4,004 | |||||||||||||||
|
Total operating expenses
|
4,287 | 2,730 | 7,581 | 18,901 | 19,309 | |||||||||||||||
|
Operating loss
|
(4,287 | ) | (2,730 | ) | (7,581 | ) | (21,427 | ) | (21,227 | ) | ||||||||||
|
Financial income (expense), net
|
(532 | ) | 14,715 | (920 | ) | 2,552 | (444 | ) | ||||||||||||
|
Income (loss) from continuing operations
|
(4,819 | ) | 11,985 | (8,501 | ) | (18,875 | ) | (21,671 | ) | |||||||||||
|
Loss from discontinued operation(1)(3)
|
(1,350 | ) | (1,045 | ) | (6,850 | ) | - | (417 | ) | |||||||||||
|
Net income (loss)
|
$ | (6,169 | ) | $ | 10,940 | $ | (15,351 | ) | $ | (18,875 | ) | $ | (22,088 | ) | ||||||
|
Foreign currency translation adjustments
|
— | — | (32 | ) | 14 | 2 | ||||||||||||||
|
Total comprehensive income (loss)
|
$ | (6,169 | ) | $ | 10,940 | $ | (15,383 | ) | $ | (18,861 | ) | $ | (22,086 | ) | ||||||
|
Basic net income (loss) per share(4)
|
$ | (0.39 | ) | $ | 0.70 | $ | (0.98 | ) | $ | (0.95 | ) | (1.02 | ) | |||||||
|
Diluted net income (loss) per share(4)
|
$ | (0.39 | ) | $ | 0.64 | $ | (0.98 | ) | $ | (0.95 | ) | (1.02 | ) | |||||||
|
Weighted average number of ordinary shares used in computing net income (loss) per ordinary share:
|
||||||||||||||||||||
|
Basic:
|
15,683 | 15,683 | 15,671 | 19,940 | 21,718 | |||||||||||||||
|
Diluted:
|
15,683 | 17,199 | 15,671 | 19,940 | 21,718 | |||||||||||||||
|
As of December 31,
|
||||||||||||||||
|
2012
|
2013
|
2014
|
2015
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Consolidated balance sheet data:
|
||||||||||||||||
|
Cash and cash equivalents
and short-term bank deposits
|
$ | 337 | $ | 9,553 | $ | 64,853 | $ | 45,768 | ||||||||
|
Working capital, net(5)
|
(112 | ) | 10,042 | 64,600 | 45,189 | |||||||||||
|
Total assets
|
25,438 | 14,826 | 71,121 | 52,523 | ||||||||||||
|
Total non-current liabilities
|
6,440 | 32,607 | 24,353 | 23,847 | ||||||||||||
|
Total shareholders’ equity (deficit)
|
15,634 | (19,804 | ) | 42,871 | 23,470 | |||||||||||
|
Year Ended December 31,
|
||||||||||||||||||||
|
2011
|
2012
|
2013
|
2014
|
2015
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Cost of revenues
|
$ | — | $ | — | $ | — | $ | 763 | $ | 372 | ||||||||||
|
Research and development
|
182 | 124 | 315 | 657 | 511 | |||||||||||||||
|
Selling and marketing
|
— | — | 24 | 1,430 | 669 | |||||||||||||||
|
General and administrative
|
373 | 210 | 192 | 1,977 | 1,107 | |||||||||||||||
|
Share-based compensation expenses from continuing operations
|
555 | 334 | 531 | 4,827 | 2,659 | |||||||||||||||
|
Discontinued operation
|
109 | 30 | 76 | — | — | |||||||||||||||
|
Total share-based compensation expenses
|
$ | 664 | $ | 364 | $ | 607 | $ | 4,827 | $ | 2,659 | ||||||||||
|
(2)
|
Research and development expenses, net is presented net of participation by the U.S. Biomedical Advanced Research and Development Authority (“BARDA”) and others and net of the change in the fair value of the liability associated with government grants from the Office of the Chief Scientist. Participation by others totaled $2.7 million and $2.2 million for the years ended December 31, 2011 and 2012, a
nd had no effect on subsequent years
. The effect of the participation by the Office of the Chief Scientist totaled $0.4 million, $0.1 million, $0.9 million, $0.7 million and $1.3 million for the years ended December 31, 2011, 2012, 2013, 2014 and 2015, respectively. The effect of the participation by BARDA totaled $0.8 million for the year ended December 31, 2015. See “ITEM 5.A. Operating Results—Research and development” for more information.
|
|
(3)
|
Discontinued operation consists of revenues and expenses related to our exclusive, worldwide license for the development, production and commercialization of the PolyHeal Product, which expired following the termination of our collaboration with Teva. We account for our discontinued operation in accordance with IFRS accounting standard 5, “Non-current Assets Held for Sale and Discontinued Operations.” See “ITEM 5.A. Operating Results—Discontinued operation” for more information.
|
|
(4)
|
Basic and diluted net income (loss) per ordinary share is computed based on the basic and diluted weighted average number of ordinary shares outstanding during each period. For additional information, see Note 21 to our consolidated annual financial statements included elsewhere in this report.
|
|
(5)
|
Working capital, net is defined as total current assets minus total current liabilities.
|
|
|
B.
|
Capitalization and Indebtedness
|
|
|
C.
|
Reasons for the Offer and Use of Proceeds
|
|
|
D.
|
Risk Factors
|
|
|
·
|
the willingness of physicians, burn care teams and hospital administrators to administer our products and their acceptance as part of the medical department routine;
|
|
|
·
|
the consent of hospitals to fund/purchase NexoBrid or obtain third-party coverage or reimbursement for our products;
|
|
|
·
|
the ability to offer NexoBrid and our pipeline products for sale at an attractive value;
|
|
|
·
|
the efficacy and potential advantages of NexoBrid and our pipeline products relative to current standard of care;
|
|
|
·
|
the prevalence and severity of any side effects; and
|
|
|
·
|
the efficacy, potential advantages and timing of introduction to the market of alternative treatments.
|
|
|
·
|
the market acceptance or demand for NexoBrid or any of our pipeline products, if approved;
|
|
|
·
|
the ability to set a price that we believe is fair for NexoBrid or any of our pipeline products, if approved;
|
|
|
·
|
our ability to generate revenues and achieve or maintain profitability;
|
|
|
·
|
the level of taxes that we are required to pay; and
|
|
|
·
|
the availability of capital.
|
|
|
·
|
regulators may not authorize us to conduct a clinical trial within a country or at a prospective trial site or may change the design of a study;
|
|
|
·
|
delays may occur in reaching agreement on acceptable clinical trial terms with regulatory authorities or prospective sites, or obtaining institutional review board approval;
|
|
|
·
|
our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional trials or to abandon strategic projects;
|
|
|
·
|
the number of patients required for our clinical trials may be larger than we anticipate, enrollment in our clinical trials may be slower or more difficult than we expect, or patients may not participate in necessary follow-up visits to obtain required data, any of which would result in significant delays in our clinical testing process;
|
|
|
·
|
our third-party contractors, such as a research institute, may fail to comply with regulatory requirements or meet their contractual obligations to us;
|
|
|
·
|
we may be forced to suspend or terminate our clinical trials if the participants are being exposed, or are thought to be exposed, to unacceptable health risks or if any participant experiences an unexpected serious adverse event;
|
|
|
·
|
regulators or institutional review boards may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements;
|
|
|
·
|
undetected or concealed fraudulent activity by a clinical researcher, if discovered, could preclude the submission of clinical data prepared by that researcher, lead to the suspension or substantive scientific review of one or more of our marketing applications by regulatory agencies, and result in the recall of any approved product distributed pursuant to data determined to be fraudulent;
|
|
|
·
|
the cost of our clinical trials may be greater than we anticipate;
|
|
|
·
|
an audit of preclinical or clinical studies by regulatory authorities may reveal noncompliance with applicable protocols or regulations, which could lead to disqualification of the results and the need to perform additional studies; and
|
|
|
·
|
delays may occur in obtaining our clinical materials.
|
|
|
·
|
accelerate our clinical development activities, particularly with respect to our NexoBrid pediatric clinical trial in severe burns in Europe, our continued clinical development of EscharEx for the debridement of chronic and other hard-to-heal wounds and our clinical trials for our product candidate for the treatment of connective tissue disorders or other indications;
|
|
|
·
|
continue to operate our sales, marketing and distribution infrastructure in Europe and thereafter in the United States to commercialize NexoBrid and any pipeline products for which we obtain marketing approval;
|
|
|
·
|
further scale-up the manufacturing process for NexoBrid;
|
|
|
·
|
seek regulatory and marketing approvals for NexoBrid and any pipeline product that successfully completes clinical trials;
|
|
|
·
|
initiate additional preclinical, clinical or other studies for NexoBrid and our pipeline products and seek to identify and validate new products;
|
|
|
·
|
acquire rights to other product candidates and technologies;
|
|
|
·
|
change or add suppliers;
|
|
|
·
|
maintain, expand and protect our intellectual property portfolio;
|
|
|
·
|
attract and retain skilled personnel; and
|
|
|
·
|
experience any delays or encounter issues with any of the above.
|
|
|
·
|
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market or voluntary or mandatory product recalls;
|
|
|
·
|
fines, warning letters or holds on clinical trials;
|
|
|
·
|
harm to our reputation, reduced demand for our products and loss of market acceptance;
|
|
|
·
|
refusal by the applicable regulatory authority to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals;
|
|
|
·
|
product seizure or detention, or refusal to permit the import or export of products; and
|
|
|
·
|
injunctions or the imposition of civil or criminal penalties.
|
|
|
·
|
delay, scale back or discontinue the development, manufacturing scale-up or commercialization of NexoBrid or our pipeline products;
|
|
|
·
|
seek corporate partners for NexoBrid or one or more of our pipeline products on terms that are less favorable than might otherwise be available; or
|
|
|
·
|
relinquish or license on unfavorable terms, our rights to NexoBrid or our pipeline products that we otherwise would seek to develop or commercialize ourselves.
|
|
|
·
|
the Federal Acquisition Regulations (“FAR”) and agency-specific regulations supplemental to the FAR, which comprehensively regulate the procurement, formation, administration and performance of government contracts;
|
|
|
·
|
business ethics and public integrity obligations, which govern conflicts of interest and the hiring of former government employees, restrict the granting of gratuities and funding of lobbying activities and include other requirements such as the Anti-Kickback Statute and Foreign Corrupt Practices Act;
|
|
|
·
|
export and import control laws and regulations; and
|
|
|
·
|
laws, regulations and executive orders restricting the use and dissemination of information classified for national security purposes and the exportation of certain products and technical data.
|
|
|
·
|
any of our present or future patents or patent claims or other intellectual property rights will not lapse or be invalidated, circumvented, challenged or abandoned;
|
|
|
·
|
our intellectual property rights will provide competitive advantages or prevent competitors from making or selling competing products;
|
|
|
·
|
our ability to assert our intellectual property rights against potential competitors or to settle current or future disputes will not be limited by our agreements with third parties;
|
|
|
·
|
any of our pending or future patent applications will be issued or have the coverage originally sought;
|
|
|
·
|
our intellectual property rights will be enforced in jurisdictions where competition may be intense or where legal protection may be weak; or
|
|
|
·
|
we will not lose the ability to assert our intellectual property rights against, or to license our technology to, others and collect royalties or other payments.
|
|
|
·
|
actual or anticipated variations in our and our competitors’ results of operations and financial condition;
|
|
|
·
|
market acceptance of our products;
|
|
|
·
|
general economic and market conditions and other factors, including factors unrelated to our operating performance;
|
|
|
·
|
the mix of products that we sell and related services that we provide;
|
|
|
·
|
changes in earnings estimates or recommendations by securities analysts, if our ordinary shares continue to be covered by analysts;
|
|
|
·
|
publication of the results of preclinical or clinical trials for NexoBrid or any of our pipeline products;
|
|
|
·
|
failure by us to achieve a publicly announced milestone;
|
|
|
·
|
delays between our expenditures to develop and market new or enhanced products and the generation of sales from those products;
|
|
|
·
|
development of technological innovations or new competitive products by others;
|
|
|
·
|
announcements of technological innovations or new products by us;
|
|
|
·
|
regulatory developments and the decisions of regulatory authorities as to the marketing of our current products or the approval or rejection of new or modified products;
|
|
|
·
|
developments concerning intellectual property rights, including our involvement in litigation;
|
|
|
·
|
changes in our expenditures to develop, acquire or license new products, technologies or businesses;
|
|
|
·
|
changes in our expenditures to promote our products;
|
|
|
·
|
our sale or proposed sale, or the sale by our significant shareholders, of our ordinary shares or other securities in the future;
|
|
|
·
|
changes in key personnel;
|
|
|
·
|
success or failure of our research and development projects or those of our competitors; and
|
|
|
·
|
the trading volume of our ordinary shares.
|
|
|
A.
|
History and Development of the Company
|
|
|
B.
|
Business Overview
|
|
|
·
|
The extent of the surface the burn occupies is usually referred to as percent of total body surface area (“TBSA”). A burn on an adult’s entire palm would generally amount to 1% TBSA, and the average hospitalized patient has a burn covering approximately 10% TBSA. Burns covering more than 15-20% TBSA usually require hospitalization and may result in dehydration, shock and increased risk of mortality.
|
|
|
·
|
The depth of the burn, referred to in terms of “degree” is generally classified into four categories:
|
|
|
o
|
Superficial or first degree burns
. Such burns do not penetrate the basal membrane and usually heal naturally.
|
|
|
o
|
Dermal/partial thickness or second degree burns
. Such burns are characterized by varying amounts of damaged dermis and can be further subdivided into superficial and deep partial-thickness burns. Superficial partial-thickness burns may heal spontaneously after removal of the covering thin eschar. Conversely, deep partial-thickness burns are often difficult for physicians to accurately diagnose before eschar removal and may progress and transform into full-thickness burns if not debrided in a timely manner, depending on the magnitude of latent tissue death of the surrounding skin.
|
|
|
o
|
Full thickness or third degree burns
. Such burns are characterized by death of the entire dermal tissue down to the subcutaneous fat and must be debrided and treated by autografting, which is the process of harvesting skin from healthy donor sites on a patient’s body and transplanting it on the post-debridement, clean wound bed.
|
|
|
o
|
Fourth degree burns
. Such burns, which are rare, extend beyond the subcutaneous fat tissue into the underlying structures, such as muscle or bone, and also require debridement and further substantial treatment.
|
|
|
·
|
Other factors include the age of the victim, the body part where the burn occurred and any co-morbidities of the patient. For example, some patients may require hospitalization regardless of the TBSA or degree of the burn, such as children, the elderly or victims with burns to the extremities, joints or head/neck area or with co-morbidities such as smoke inhalation, diabetes or obesity.
|
|
|
·
|
the prevention of local infection, sepsis (a systemic inflammatory response caused by severe infection) and additional damage to surrounding viable tissue; and
|
|
|
·
|
the initiation of the body’s healing process and scar prevention.
|
|
|
·
|
Surgical debridement
|
|
|
o
|
Surgical debridement predominantly includes tangential excision, a procedure in which a surgeon amputates the entire dead tissue mass, layer after layer, down to healthy, viable tissue. The excision is extended into healthy intact tissue to make sure that no trace of the eschar remains, resulting in up to an estimated 30-50% of healthy tissue being excised during this procedure. Other methods include dermabrasion, in which a mechanically powered, hand-held rotating abrading cylinder is used to slowly scrape off tissue, and hydro surgery, in which a high-pressure flow of water abrades the tissue. These alternative methods have attempted to limit the trauma associated with tangential excision, but entail spray of contaminated eschar or take a significantly longer time to complete than tangential excision.
|
|
|
o
|
The benefits of surgical eschar removal are that it is usually fast and effective. Disadvantages include the significant trauma of the procedure, associated blood loss, risk of surgery in delicate areas of the body such as hands, added costs, and, most importantly, the loss of viable tissue that necessitates additional surgical procedures for harvesting skin from healthy donor sites and autografting.
|
|
|
o
|
Due to the disadvantages of surgery in extensive burns some surgeons limit their debriding surgery to only a part of the affected area in a single session (15-30% TBSA in most centers), thus delaying full debridement by days. After several days, complications related to eschar contamination may begin and some of the benefits of the earlier debridement may not be realized. On the other hand, when excising burns immediately, all suspected necrotic tissue will be excised, inevitably resulting in over-excision, especially in “indeterminate” burns, as after surgical excision, the remaining skin often no longer has any spontaneous healing potential and will heal only by autografting.
|
|
|
·
|
Non-surgical debridement
|
|
|
o
|
Non-surgical debridement includes many different treatment options that do not require direct surgical removal of the skin to remove eschar. With non-surgical debridement, the eschar is naturally, but slowly, removed by contaminant microorganisms, tissue autolysis, or self-decomposition, and the inflammatory process that may lead to serious local and systemic complications. In seeking to facilitate such natural processes, topical medication, anti-microbial agents, enzymes and biological/chemical applications are often applied onto the eschar.
|
|
|
o
|
The benefits of this approach are that it is non-surgical, reduces trauma to the patient and is easier to apply. Disadvantages include numerous dressing changes and mechanical scraping with limited debridement efficacy. This prolongs the eschar removal process, which may lead to death of the tissue surrounding the initial burn wound, causing partial-thickness wounds to transform into full-thickness wounds and forming granulation tissue that may develop into heavy scars.
|
|
|
·
|
Diabetic foot ulcers.
Diabetes can lead to a reduction in blood flow, which can cause patients to lose sensation in their feet and may prevent them from noticing injuries, sometimes leading to the development of DFUs, which are open sores or ulcers on the feet that may take several weeks to heal, if ever. In the United States alone, over 23 million people, or approximately 8% of the population, suffer from diabetes, a chronic, life-threatening disease. Every year, in the United States alone, over 900,000 people develop a DFU and over 600,000 undergo debridement of DFUs.
|
|
|
·
|
Venous leg ulcers.
VLUs develop as a result of vascular insufficiency, or the inability for the vasculature of the leg to return blood back toward the heart properly, and, in the United States alone, affect approximately 1.25 million people per year, out of which over 650,000 undergo debridement of VLUs. These ulcers usually form on the sides of the lower leg, above the ankle and below the calf, and are slow to heal and often recur if preventative steps are not taken. The risk of VLUs can increase as a result of a blood clot forming in the deep veins of the legs, obesity, smoking, lack of physical activity or work that requires many hours of standing.
|
|
|
·
|
Pressure ulcers.
Pressure ulcers form as a result of pressure sores, or bed sores, which are injuries to the skin or the tissue beneath the skin. Constant pressure on an area of skin reduces blood supply to the area and over time can cause the skin to break down and form an open ulcer. These often occur in patients who are hospitalized or confined to a chair or bed, and usually form over bony areas, where there is little cushion between the bone and the skin, such as lower parts of the body. Annually, 2.5 million pressure ulcers are treated in the United States in acute care facilities alone.
|
|
|
·
|
Surgical/traumatic wounds.
Surgical wounds form as a result of various types of surgical procedures such as investigative or corrective, minor or major, open (traditional) or minimal access surgery, elective or emergency, and incisions (simple cuts) or excision (removal of tissue), among others. Traumatic wounds form as a result of cuts, lacerations or puncture wounds, which have caused damage to the skin and underlying tissue. Severe traumatic wounds may require surgical intervention to close the wound and stabilize the patient. Surgical/traumatic hard-to-heal wounds develop for various reasons, such as local surgical complications, suboptimal closure techniques, presence of foreign materials, exposed bones or tendons and infection. In the United States, millions receive post-surgical wound care annually.
|
|
|
·
|
Dupuytren’s disease:
a condition where one or more fingers are permanently flexed, caused by the formation of scar-like tissues below the palmar skin (Palmar Fascia), forming hard “cords” that freeze the fingers in non-functional flexion contraction. This condition affects approximately 6.2 million people in the United States alone.
|
|
|
·
|
Peyronie’s disease:
the development of scar-like tissue, similar to Dupuytren’s cords in the shaft of the penis, causing pain and distortion on erection, preventing intercourse. Peyronie’s disease is typically caused by trauma and affects men over 50 years old. Surgical treatment may be an option in some cases, but can cause complications and may result in a shortening and even greater distortion of the penis. Approximately 3.7% to 7.1% of the male population above the age of 50 suffers from Peyronie’s disease in the United States and approximately 3.2% of such age group suffer from the disease in Europe.
|
|
|
·
|
Frozen shoulder syndrome:
a disorder that causes the smooth tissues of the shoulder capsule to become thick, stiff and inflamed, affecting approximately 2% to 5% of the worldwide population and 10% to 20% of people with diabetes according to industry sources.
|
|
|
·
|
Excessive/unaesthetic scars:
A scar is a mark on the skin which is formed due to infection, injury, surgery, inflammation of tissue, burns, and acne. Scars can be of various sizes, shapes, and colors, depending on the age of the scar, the site of the scar and family history. Scar formation is unpredictable and varies from person to person. Excessive scarring can have unpleasant physical, aesthetic, psychological and social consequences. Estimates indicate that each year around 100 million people in the developed world acquire scars following elective surgery and surgery for trauma. Of these, approximately 15% have excessive or unaesthetic scars.
|
|
Trial 1
|
Trial 2
|
Trial 3
|
Trial 4
|
Trial 5
|
Trial 6
|
|
|
Study Type
|
• Retrospective Phase 2
• Investigator initiated
|
• Dose range Phase 2
|
• Prospective Phase 2
• IND/FDA
|
• Phase 2
• IND/FDA
|
• Phase 3
• EMA
|
• Phase 3b
• EMA
|
|
Design
|
• Data collected from files
of
patients treated with
NexoBrid
|
• Parallel, controlled,
observer-
blind, randomized,
single-center
|
• Parallel, controlled,
observer-
blind, three-arm,
randomized,
multi-center
|
• Parallel, controlled, open
label,
three-arm,
randomized,
single-center
|
• Parallel, controlled, open
label, two-arm, randomized,
multi-center
|
• Parallel, controlled, blinded, two-arm,
multi-center
|
|
Main Objectives
|
• Safety
• Efficacy
|
• Comparison of efficacy and safety
|
• Safety
• Efficacy
|
• Safety
|
• Safety
• Efficacy
|
• Long-term scar assessment
• Quality of life
|
|
Wound Types
|
• Deep partial/full
thickness
thermal
burns
|
• Deep partial/full thickness thermal burns
|
• Deep partial/full thickness
thermal burns
|
• Deep partial/full thickness
thermal burns
|
• Deep partial/full thickness
thermal burns
|
• Scar formation
|
|
Number of Patients
|
• 154
|
• 20
|
• 140
|
• 30
|
• 182
|
• 89
|
|
Study Length
|
• 1985-2000
|
• 2002-2005
|
• 2003-2004
|
• 2006-2007
|
• 2006-2009
|
• 2011
|
|
Location
|
• Israel
|
• Israel
|
• International
|
• United States
|
• International
|
• International
|
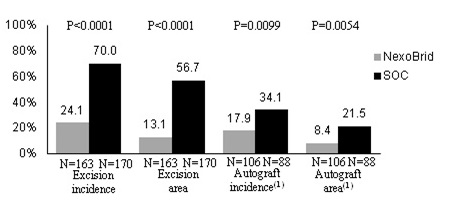
|
|
(1)
|
Only deep partial-thickness wounds are presented, as full-thickness wounds always require autografting due to the lack of viable dermis, regardless of the technique used to remove the eschar.
|
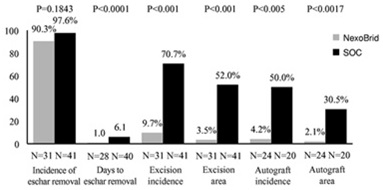

Clinical development overall safety assessment
|
|
·
|
laboratory tests, animal studies and formulation studies all performed in accordance with the applicable E.U. GLP or GMP regulations;
|
|
|
·
|
submission to the relevant national authorities of a clinical trial application (“CTA”), which must be approved before human clinical trials may begin;
|
|
|
·
|
performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication;
|
|
|
·
|
submission to the relevant competent authorities of a marketing authorization application (“MAA”), which includes the data supporting preclinical and clinical safety and efficacy as well as detailed information on the manufacture and composition and control of the product development and proposed labeling as well as other information;
|
|
|
·
|
inspection by the relevant national authorities of the manufacturing facility or facilities and quality systems (including those of third parties) at which the product is produced, to assess compliance with strictly enforced cGMP;
|
|
|
·
|
potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and
|
|
|
·
|
review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product.
|
|
|
·
|
Phase 1 (Most typical kind of study: Human Pharmacology);
|
|
|
·
|
Phase 2 (Most typical kind of study: Therapeutic Exploratory);
|
|
|
·
|
Phase 3 (Most typical kind of study: Therapeutic Confirmatory); and
|
|
|
·
|
Phase 4 (Variety of Studies: Therapeutic Use).
|
|
|
·
|
medicines that have been authorized for marketing in the European Union with the results of PIP studies included in the product information are eligible for an extension of their patent protection by six months. This is the case even when the studies’ results are negative;
|
|
|
·
|
for orphan medicines, such as NexoBrid, the incentive is an additional two years of market exclusivity instead of one;
|
|
|
·
|
scientific advice and protocol assistance at the EMA are free of charge for questions relating to the development of medicines for children; and
|
|
|
·
|
medicines developed specifically for children that are already authorized, but are not protected by a patent or supplementary protection certificate, can apply for a pediatric use marketing authorization (“PUMA”). If a PUMA is granted, the product will benefit from 10 years of market protection as an incentive.
|
|
|
·
|
Mutual recognition procedure.
If an authorization has been granted by one member state, or the Reference Member State, an application may be made for mutual recognition in one or more other member states, or the Concerned Member State(s).
|
|
|
·
|
Decentralized procedure.
The decentralized procedure may be used to obtain a marketing authorization in several European member states when the applicant does not yet have a marketing authorization in any country.
|
|
|
·
|
National procedure.
Applicants following the national procedure will be granted a marketing authorization that is valid only in a single member state. Furthermore, this marketing authorization is not based on recognition of another marketing authorization for the same product awarded by an assessment authority of another member state. If marketing authorization in only one member state is preferred, an application can be filed with the national competent authority of a member state. The national procedure can also serve as the first phase of a mutual recognition procedure.
|
|
|
·
|
completion of laboratory tests, animal studies and formulation studies in compliance with the FDA’s GLP or GMP regulations, as applicable;
|
|
|
·
|
submission to the FDA of an investigational new drug application (“IND”), which must become effective before clinical trials may begin;
|
|
|
·
|
approval by an independent institutional review board (“IRB”) at each clinical site before each trial may be initiated;
|
|
|
·
|
performance of adequate and well-controlled clinical trials in accordance with GCP to establish the safety and efficacy of the product for each indication;
|
|
|
·
|
preparation and submission to the FDA of a BLA or supplemental BLA;
|
|
|
·
|
satisfactory completion of an FDA advisory committee review, if applicable;
|
|
|
·
|
satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMP requirements, and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; and
|
|
|
·
|
payment of user fees and FDA review and approval of the BLA.
|
|
Phase 1:
|
The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
|
|
|
Phase 2:
|
The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
|
|
|
Phase 3:
|
The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
|
|
|
·
|
increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%;
|
|
|
·
|
requires collection of rebates for drugs paid by Medicaid managed care organizations; and
|
|
|
·
|
imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs.
|
|
|
·
|
the federal healthcare Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid;
|
|
|
·
|
the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
|
·
|
the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
|
|
·
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
|
|
·
|
the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
|
|
|
·
|
the federal transparency requirements under the Affordable Care Act require manufacturers of drugs, devices and medical supplies to report to the Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests; and
|
|
|
·
|
analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers.
|
|
|
C.
|
Organizational Structure
|
|
|
D.
|
Property, Plants and Equipment
|
|
|
A.
|
Operating Results
|
|
|
·
|
the scope, rate of progress and expense of our research and development activities;
|
|
|
·
|
preclinical results;
|
|
|
·
|
clinical trial results;
|
|
|
·
|
the terms and timing of regulatory approvals;
|
|
|
·
|
the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; and
|
|
|
·
|
the ability to market, commercialize and achieve market acceptance for NexoBrid or any other product candidate that we may develop in the future.
|
|
Years Ended December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Consolidated statements of operations data:
|
||||||||||||
|
Revenues
|
$ | — | $ | 259 | $ | 601 | ||||||
|
Cost of revenues
|
— | 2,785 | 2,519 | |||||||||
|
Gross loss
|
— | (2,526 | ) | (1,918 | ) | |||||||
|
Operating expenses:
|
||||||||||||
|
Research and development, gross
|
4,513 | 6,054 | 8,139 | |||||||||
|
Participation by OCS and BARDA
|
878 | 705 | 2,118 | |||||||||
|
Research and development, net of participations
|
3,635 | 5,349 | 6,021 | |||||||||
|
Selling and marketing
|
2,259 | 8,829 | 9,284 | |||||||||
|
General and administrative
|
1,687 | 4,723 | 4,004 | |||||||||
|
Total operating expenses
|
7,581 | 18,901 | 19,309 | |||||||||
|
Operating loss
|
7,581 | 21,427 | 21,227 | |||||||||
|
Financial income
|
2,401 | 4,665 | 1,052 | |||||||||
|
Financial expense
|
(3,321 | ) | (2,113 | ) | (1,496 | ) | ||||||
|
Loss from continuing operations
|
8,501 | 18,875 | 21,671 | |||||||||
|
Loss from discontinued operation
|
6,850 | — | 417 | |||||||||
|
Net loss
|
$ | 15,351 | $ | 18,875 | $ | 22,088 | ||||||
|
|
B.
|
Liquidity and Capital Resources
|
|
Issuance of Ordinary Shares and Warrants
|
Net Loans from Shareholders
|
Government Grants and BARDA Funding, net
|
Total
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Year ended December 31, 2015
|
$ | 26 | $ | — | $ | 1,552 | $ | 1,578 | ||||||||
|
Year ended December 31, 2014
|
72,130 | — | 345 | 72,475 | ||||||||||||
|
Year ended December 31, 2013
|
15,950 | 3,015 | 276 | 19,241 | ||||||||||||
|
Year Ended December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Net cash provided by (used in):
|
||||||||||||
|
Continuing operating activities
|
$ | (8,075 | ) | $ | (16,493 | ) | $ | (19,601 | ) | |||
|
Continuing investing activities
|
(2,855 | ) | (37,154 | ) | 36,046 | |||||||
|
Continuing financing activities
|
19,241 | 72,475 | 778 | |||||||||
|
Discontinued operation
|
(1,665 | ) | — | — | ||||||||
|
|
C.
|
Application of Critical Accounting Policies and Estimates
|
|
|
·
|
Fair value of our ordinary shares
. Prior to the completion of our IPO, due to absence of an active market for our ordinary shares, the fair value of our ordinary shares for purposes of determining the exercise price for award grants was determined in good faith by our management and approved by our board of directors. In connection with preparing our financial statements, our management considered the fair value of our ordinary shares based on a number of objective and subjective factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, referred to as the AICPA Practice Aid.
|
|
|
·
|
Volatility
. The expected share price volatility was based on the historical equity volatility of the ordinary shares of comparable companies that are publicly traded.
|
|
|
·
|
Expected term
. The expected term of options granted represents the period of time that options granted are expected to be outstanding. Since adequate historical experience is not available to provide a reasonable estimate, the expected term is determined based on the midpoint between the available exercise dates (the end of the vesting periods) and the last available exercise date (the contracted expiry date).
|
|
|
·
|
Risk-free rate
. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the contractual life of the options.
|
|
|
·
|
Expected dividend yield
. We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
|
|
|
D.
|
Research and Development, Patents and Licenses, etc.
|
|
|
E.
|
Trend Information
|
|
|
F.
|
Off-Balance Sheet Arrangements
|
|
|
G.
|
Contractual Obligations
|
|
Payments Due by Period
|
||||||||||||||||
|
Total
|
2016
|
2017
|
2018 and thereafter
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Operating lease obligations(1)
|
$ | 1,111 | $ | 905 | $ | 116 | $ | 90 | ||||||||
|
(1)
|
Operating lease obligations consist of payments pursuant to lease agreements for office and laboratory facilities, as well as lease agreements for 22 vehicles, which generally run for a period of three years.
|
|
|
A.
|
Directors and Senior Management
|
|
Name
|
Age
|
Position
|
||
|
Executive Officers
|
||||
|
Gal Cohen
|
43
|
President and Chief Executive Officer
|
||
|
Sharon Malka
|
44
|
Chief Financial and Operations Officer
|
||
|
Lior Rosenberg M.D.
|
70
|
Chief Medical Technology Officer
|
||
|
Ety Klinger Ph.D
|
54
|
Chief Research and Development Officer
|
||
|
Carsten Henke
|
50
|
Chief Commercial Officer EU
|
||
|
Yaron Meyer
|
37
|
General Counsel and Corporate Secretary
|
||
|
Nirit Freikorn
|
42
|
Chief Marketing Officer
|
||
|
Directors
|
||||
|
Aharon Yaari
|
64
|
Chairman of the Board of Directors
|
||
|
Ofer Gonen
|
42
|
Director
|
||
|
Marian Gorecki Ph.D (1)(2)(3)
|
75
|
Director
|
||
|
Meron Mann (3)
|
64
|
Director
|
||
|
Sarit Firon (1)(2)(3)(4)
|
49
|
Director
|
||
|
Abraham Havron (1)(2)(3)(4)
|
68
|
Director
|
|
(1)
|
Member of our audit committee
|
|
(2)
|
Member of our compensation committee
|
|
(3)
|
Independent director under the rules of the NASDAQ Stock Market
|
|
(4)
|
External director under the Companies Law.
|
|
|
B.
|
Compensation
|
|
Name and Position
|
Salary & Social Benefits
(1)
|
Bonus
|
Share-Based Payment (2)
|
Other Compensation (3)
|
Total
|
|||||||||||||||
|
(U.S. dollars) (4)
|
||||||||||||||||||||
|
Gal Cohen,
President and Chief Executive Officer
|
353,916 | 221,918 | (5) | 439,964 | 4,910 | 1,020,708 | ||||||||||||||
|
Sharon Malka,
Chief Financial and Operations Officer
|
237,356 | 139,209 | 351,567 | 25,033 | 753,165 | |||||||||||||||
|
Lior Rosenberg, M.D.,
Chief Medical Technology Officer
|
267,738 | 185,362 | 219,309 | 19,084 | 691,493 | |||||||||||||||
|
Carsten Henke,
Chief Commercial Officer EU
&
Managing Director of MediWound Germany GmbH
|
266,380 | 60,879 | 273,716 | 35,076 | 636,051 | |||||||||||||||
|
Ety Klinger,
Chief Research & Development Officer
|
198,445 | 122,835 | 140,776 | 28,452 | 490,509 | |||||||||||||||
|
|
(1)
|
Represents the officer’s gross salary plus payment of mandatory social benefits made by the company on behalf of such officer. Such benefits may include, to the extent applicable to the executive, payments, contributions and/or allocations for savings funds (e.g., Managers’ Life Insurance Policy), education funds (referred to in Hebrew as “keren hishtalmut”), pension, severance, risk insurances (e.g., life or work disability insurance) and payments for social security.
|
|
|
(2)
|
Represents the equity-based compensation expenses recorded in the company’s consolidated financial statements for the year ended December 31, 2015 based on the options’ grant date fair value in accordance with accounting guidance for equity-based compensation.
|
|
|
(3)
|
Represents the other benefits to such officer, which includes either or both of (i) car expenses, including lease costs, gas and maintenance, provided to the officers and (ii) vacation benefits.
|
|
|
(4)
|
Converted (i) from NIS into U.S. dollars at the rate of NIS 3.88 = U.S.$1.00, based on the average representative rate of exchange between the NIS and the U.S. dollar as reported by the Bank of Israel in the year ended December 31, 2015 and (ii) from Euro into U.S. dollars at the rate of Euro 1.1 = U.S$1.00, based on the average representative rate of exchange between the Euro and the U.S. dollar as reported by the Bank of Israel in the year ended December 31, 2015.
|
|
|
(5)
|
Subject to approval by our shareholders at the extraordinary general meeting of our shareholders scheduled for January 28, 2016.
|
|
Name
|
Number of Options
|
Grant Date
|
Exercise Price
|
Vested Options as of January 15, 2016
|
Expiration Date
|
|||||||||
|
Gal Cohen,
(1)
President and Chief Executive Officer
|
208,332 |
11/14/2006
|
$ | 2.63 | 208,332 |
11/13/2016
|
||||||||
| 45,600 |
1/15/2011
|
$ | 9.82 | 45,600 |
1/14/2021
|
|||||||||
| 152,000 |
12/24/2013
|
$ | 12.89 | 72,000 |
12/23/2023
|
|||||||||
| 70,000 | (1) |
1/28/2016
|
$ | 9.58 | 0 |
12/22/2025
|
||||||||
|
Lior Rosenberg,
Chief Medical Technology Officer
|
76,000 |
12/24/2013
|
$ | 12.89 | 38,000 |
12/23/2023
|
||||||||
| 25,000 |
12/23/2015
|
$ | 9.58 | 0 |
12/22/2025
|
|||||||||
|
|
C.
|
Board Practices
|
|
|
·
|
such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding abstentions, to which we refer as a disinterested majority; or
|
|
|
·
|
the total number of shares voted by non-controlling shareholders and by shareholders who do not have a personal interest in the election of the external director against the election of the external director does not exceed 2% of the aggregate voting rights in the company.
|
|
|
(i)
|
his or her service for each such additional term is recommended by one or more shareholders holding at least 1% of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate voting rights in the company, subject to additional restrictions set forth in the Israeli Companies Law with respect to affiliations of external director nominee; or
|
|
|
(ii)
|
his or her service for each such additional term is recommended by the board of directors and is approved at a meeting of shareholders by the same majority required for the initial election of an external director (as described above).
|
|
|
·
|
an employment relationship;
|
|
|
·
|
a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships);
|
|
|
·
|
control; and
|
|
|
·
|
service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director was appointed as a director of the private company in order to serve as an external director following the initial public offering.
|
|
|
·
|
he or she meets the qualifications for being appointed as an external director, except for the requirement (i) that the director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed for trading outside of Israel) and (ii) for accounting and financial expertise or professional qualifications; and
|
|
|
·
|
he or she has not served as a director of the company for a period exceeding nine consecutive years. For this purpose, a break of less than two years in the service shall not be deemed to interrupt the continuation of the service.
|
|
|
·
|
oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors in accordance with Israeli law;
|
|
|
·
|
recommending the engagement or termination of the person filling the office of our internal auditor; and
|
|
|
·
|
recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors.
|
|
|
·
|
determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices;
|
|
|
·
|
determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and whether such transaction is extraordinary or material under the Israeli Companies Law) (see “—Approval of Related Party Transactions Under Israeli Law”);
|
|
|
·
|
establishing the approval process (including, potentially, the approval of the audit committee and conducting a competitive procedure supervised by the audit committee) for certain transactions with a controlling shareholder or in which a controlling shareholder has a personal interest;
|
|
|
·
|
where the board of directors approves the working plan of the internal auditor, examining such working plan before its submission to the board of directors and proposing amendments thereto;
|
|
|
·
|
examining our internal audit controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to fulfill his responsibilities;
|
|
|
·
|
examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment of our auditor; and
|
|
|
·
|
establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
|
|
|
·
|
the knowledge, skills, expertise and accomplishments of the relevant office holder;
|
|
|
·
|
the office holder’s roles and responsibilities and prior compensation agreements with him or her;
|
|
|
·
|
the relationship between the terms offered and the average compensation of the other employees of the company, including those employed through manpower companies;
|
|
|
·
|
the impact of disparities in salary upon work relationships in the company;
|
|
|
·
|
the possibility of reducing variable compensation at the discretion of the board of directors;
|
|
|
·
|
the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and
|
|
|
·
|
as to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
|
|
|
·
|
the link between variable compensation and long-term performance and measurable criteria;
|
|
|
·
|
the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation;
|
|
|
·
|
the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements;
|
|
|
·
|
the minimum holding or vesting period for variable, equity-based compensation; and
|
|
|
·
|
maximum limits for severance compensation.
|
|
|
·
|
recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years);
|
|
|
·
|
recommending to the board of directors periodic updates to the compensation policy and assessing implementation of the compensation policy;
|
|
|
·
|
approving compensation terms of executive officers, directors and employees that require approval of the compensation committee;
|
|
|
·
|
determining whether the compensation terms of a chief executive officer nominee, which were determined pursuant to the compensation policy, will be exempt from approval of the shareholders because such approval would harm the ability to engage with such nominee; and
|
|
|
·
|
determining, subject to the approval of the board and under special circumstances, override a determination of the company’s shareholders regarding certain compensation related issues.
|
|
|
·
|
the responsibilities set forth in the compensation policy;
|
|
|
·
|
reviewing and approving the granting of options and other incentive awards to the extent such authority is delegated by our board of directors; and
|
|
|
·
|
reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors.
|
|
|
·
|
a person (or a relative of a person) who holds 5% or more of the company’s outstanding shares or voting rights;
|
|
|
·
|
a person (or a relative of a person) who has the power to appoint a director or the general manager of the company;
|
|
|
·
|
an office holder (including a director) of the company (or a relative thereof); or
|
|
|
·
|
a member of the company’s independent accounting firm, or anyone on its behalf.
|
|
|
·
|
information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and
|
|
|
·
|
all other important information pertaining to any such action.
|
|
|
·
|
refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or personal affairs;
|
|
|
·
|
refrain from any activity that is competitive with the business of the company;
|
|
|
·
|
refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and
|
|
|
·
|
disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder.
|
|
|
·
|
a transaction other than in the ordinary course of business;
|
|
|
·
|
a transaction that is not on market terms; or
|
|
|
·
|
a transaction that may have a material impact on a company’s profitability, assets or liabilities.
|
|
|
·
|
at least a majority of the shares held by all shareholders who do not have a personal interest in the transaction and who are present and voting at the meeting approves the transaction, excluding abstentions; or
|
|
|
·
|
the shares voted against the transaction by shareholders who have no personal interest in the transaction and who are present and voting at the meeting do not exceed 2% of the voting rights in the company.
|
|
|
·
|
an amendment to the company’s articles of association;
|
|
|
·
|
an increase of the company’s authorized share capital;
|
|
|
·
|
a merger; or
|
|
|
·
|
the approval of related party transactions and acts of office holders that require shareholder approval.
|
|
|
·
|
financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria;
|
|
|
·
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding, and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction; and
|
|
|
·
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent.
|
|
|
·
|
a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
|
|
·
|
a breach of duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder; and
|
|
|
·
|
a financial liability imposed on the office holder in favor of a third party.
|
|
|
·
|
a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
|
|
·
|
a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder;
|
|
|
·
|
an act or omission committed with intent to derive illegal personal benefit; or
|
|
|
·
|
a fine or forfeit levied against the office holder.
|
|
|
D.
|
Employees
|
|
Department
|
As of December 31,
|
|||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Administrative
|
6 | 6 | 6 | |||||||||
|
Research and development
|
10 | 14 | 16 | |||||||||
|
Manufacturing
|
19 | 21 | 20 | |||||||||
|
Sales and marketing
|
8 | 22 | 25 | |||||||||
|
Total
|
43 | 63 | 67 | |||||||||
|
|
E.
|
Share Ownership
|
|
|
A.
|
Major Shareholders
|
|
|
·
|
each person or entity known by us to own beneficially more than 5% of our outstanding shares;
|
|
|
·
|
each of our directors and executive officers individually; and
|
|
|
·
|
all of our executive officers and directors as a group.
|
|
Name of Beneficial Owner
|
Number of Shares
Beneficially Held
|
Percentage of Class
|
||||||
|
Directors and Executive Officers
|
||||||||
|
Aharon Yaari
|
- | - | ||||||
|
Ofer Gonen
|
* | * | ||||||
|
Marian Gorecki
|
* | * | ||||||
|
Meron Mann
|
* | * | ||||||
|
Sarit Firon
|
* | * | ||||||
|
Abraham Havron
|
* | * | ||||||
|
Gal Cohen (1)
|
329,932 | 1.5 | % | |||||
|
Sharon Malka
|
* | * | ||||||
|
Lior Rosenberg (2)
|
1,889,272 | 8.6 | % | |||||
|
Carsten Henke
|
* | * | ||||||
|
Ety Klinger
|
* | * | ||||||
|
Yaron Meyer
|
* | * | ||||||
|
Nirit Freikorn
|
* | * | ||||||
|
All executive officers and directors as a group (14 persons)(3)
|
2,551,210 | 11.3 | % | |||||
|
Principal Shareholders
|
||||||||
|
Clal Biotechnology Industries Ltd.(4)
|
9,789,555 | 44.8 | % | |||||
|
Harel Insurance Investments & Financial Services Ltd. (5)
|
1,366,333 | 6.3 | % | |||||
|
Migdal Insurance & Financial Holdings Ltd. (6)
|
1,685,947 | 7.7 | % | |||||
|
*
|
Less than 1%.
|
|
(1)
|
Shares beneficially owned consist of 329,932 ordinary shares issuable upon exercise of outstanding options.
|
|
(2)
|
Shares beneficially owned consist of: (i) 141,067 ordinary shares held directly by Prof. Rosenberg; (ii) 38,000 ordinary shares issuable upon exercise of outstanding options held directly by Prof. Rosenberg; and (iii) 1,710,205 ordinary shares held by L.R. Research and Development Ltd. in trust for the benefit of Prof. Rosenberg. Prof. Rosenberg is the sole shareholder of L.R. Research and Development Ltd.
|
|
(3)
|
Shares beneficially owned consist of 1,856,729 ordinary shares held directly or indirectly by such executive officers and directors and 694,481 ordinary shares issuable upon exercise of outstanding options.
|
|
(4)
|
Shares beneficially owned consist of: (i) 8,208,973 ordinary shares held by Clal Life Sciences, LP, an Israeli limited partnership, whose managing partner is Clal Application Center Ltd., a wholly-owned subsidiary of CBI; and (ii) 1,580,582 ordinary shares held by CBI, as reported by CBI to the Company. Access Industries Holdings LLC indirectly owns 100% of the outstanding shares of Clal Industries Ltd., which owns the majority of the outstanding shares of, and controls, CBI. The address of Clal Industries Ltd. is the Triangular Tower, 3 Azrieli Center, Tel Aviv 67023, Israel and the address of Access Industries Holdings LLC is c/o Access Industries Group, 730 Fifth Avenue, New York, New York 10019, United States.
|
|
(5)
|
Shares beneficially owned consist of (i) 1,229,016 ordinary shares held by certain subsidiaries of Harel Insurance Investments & Financial Services Ltd. (“Harel”) and (ii) 137,317 ordinary shares beneficially held by Harel for its own account, as reported by Harel to the company. Harel is a widely held public company listed on the Tel Aviv Stock Exchange. The address of Harel is 3 Abba Hillel Road, Ramat Gan 52118, Israel.
|
|
(6)
|
Shares beneficially owned consist of: (i) 1,469,001 ordinary shares held by certain subsidiaries of Migdal Insurance & Financial Holdings Ltd. (“Migdal”) and (ii) 216,946 ordinary shares beneficially held by Migdal for its own account, as reported by Migdal to the company. Migdal is a widely held public company listed on the Tel Aviv Stock Exchange. The address of Migdal is 4 Efal Street, Petah Tikva 49512, Israel.
|
|
|
B.
|
Related Party Transactions
|
|
|
C.
|
Interests of Experts and Counsel
|
|
|
A.
|
Consolidated Statements and Other Financial Information
|
|
|
B.
|
Significant Changes
|
|
|
A.
|
Listing Details
|
|
Low
|
High
|
|||||||
|
Annual:
|
||||||||
|
2015
|
$ | 5.00 | $ | 10.47 | ||||
|
2014 (beginning March 20, 2014)
|
$ | 4.88 | $ | 18.16 | ||||
|
Quarterly:
|
||||||||
|
First Quarter 2016 (through January 15, 2016)
|
$ | 7.37 | $ | 9.29 | ||||
|
Fourth Quarter 2015
|
$ | 7.28 | $ | 10.47 | ||||
|
Third Quarter 2015
|
$ | 6.10 | $ | 8.22 | ||||
|
Second Quarter 2015
|
$ | 5.00 | $ | 7.50 | ||||
|
First Quarter 2015
|
$ | 6.60 | $ | 9.15 | ||||
|
Fourth Quarter 2014
|
$ | 4.88 | $ | 6.81 | ||||
|
Third Quarter 2014
|
$ | 6.01 | $ | 11.64 | ||||
|
Second Quarter 2014
|
$ | 10.10 | $ | 14.13 | ||||
|
First Quarter 2014 (beginning March 20, 2014)
|
$ | 14.41 | $ | 18.16 | ||||
|
Most Recent Six Months:
|
||||||||
|
December 2015
|
$ | 8.39 | $ | 10.31 | ||||
|
November 2015
|
$ | 8.71 | $ | 10.47 | ||||
|
October 2015
|
$ | 7.28 | $ | 9.85 | ||||
|
September 2015
|
$ | 6.10 | $ | 8.22 | ||||
|
August 2015
|
$ | 6.64 | $ | 7.25 | ||||
|
July 2015
|
$ | 6.29 | $ | 7.74 | ||||
|
|
B.
|
Plan of Distribution
|
|
|
C.
|
Markets
|
|
|
D.
|
Selling Shareholders
|
|
|
E.
|
Dilution
|
|
|
F.
|
Expenses of the Issue
|
|
|
A.
|
Share Capital
|
|
|
B.
|
Articles of Association
|
|
|
·
|
amendments to our articles of association;
|
|
|
·
|
appointment or termination of our auditors;
|
|
|
·
|
appointment of external directors;
|
|
|
·
|
approval of certain related party transactions;
|
|
|
·
|
increases or reductions of our authorized share capital;
|
|
|
·
|
a merger; and
|
|
|
·
|
the exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management.
|
|
|
C.
|
Material Contracts
|
|
|
D.
|
Exchange Controls
|
|
|
E.
|
Taxation
|
|
|
·
|
amortization of the cost of purchased a patent, rights to use a patent, and know-how, which are used for the development or advancement of the Industrial Enterprise, over an eight-year period, commencing on the year in which such rights were first exercised;
|
|
|
·
|
under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies controlled by it; and
|
|
|
·
|
expenses related to a public offering are deductible in equal amounts over a three years period commencing on the year of the offering.
|
|
|
·
|
banks, financial institutions or insurance companies;
|
|
|
·
|
real estate investment trusts, regulated investment companies or grantor trusts;
|
|
|
·
|
dealers or traders in securities, commodities or currencies;
|
|
|
·
|
tax-exempt entities or organizations, including an “individual retirement account” or “Roth IRA” as defined in Section 408 or 408A of the Code, respectively;
|
|
|
·
|
certain former citizens or long-term residents of the United States;
|
|
|
·
|
persons that received our shares as compensation for the performance of services;
|
|
|
·
|
persons that holds our shares as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for U.S. federal income tax purposes;
|
|
|
·
|
partnerships (including entities classified as partnerships for U.S. federal income tax purposes) or other pass-through entities, or holders that will hold our shares through such an entity;
|
|
|
·
|
S corporations;
|
|
|
·
|
holders that acquired ordinary shares as a result of holding or owning our preferred shares;
|
|
|
·
|
U.S. Holders (as defined below) whose “functional currency” is not the U.S. dollar; or
|
|
|
·
|
holders that own directly, indirectly or through attribution 10.0% or more of the voting power or value of our shares.
|
|
|
·
|
a citizen or individual resident of the United States;
|
|
|
·
|
a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia;
|
|
|
·
|
an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
|
|
|
·
|
a trust that (1) is subject to the primary supervision of a U.S. Court and one or more U.S. persons that have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
|
|
|
·
|
such gain is effectively connected with your conduct of a trade or business in the United States (or, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment or fixed base that such holder maintains in the United States); or
|
|
|
·
|
you are an individual and have been present in the United States for 183 days or more in the taxable year of such sale or exchange and certain other conditions are met.
|
|
|
·
|
at least 75% of its gross income is “passive income”; or
|
|
|
·
|
at least 50% of the average quarterly value of its total gross assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production of passive income.
|
|
|
F.
|
Dividends and Paying Agents
|
|
|
G.
|
Statement by Experts
|
|
|
H.
|
Documents on Display
|
|
|
I.
|
Subsidiary Information
|
|
Change in Average Exchange Rate
|
||||||||
|
Period
|
Shekel against the
U.S. dollar (%)
|
Euro against the
U.S. dollar (%)
|
||||||
|
2011
|
(7.7 | ) | (3.2 | ) | ||||
|
2012
|
2.3 | 2.0 | ||||||
|
2013
|
7.0 | 4.5 | ||||||
|
2014
|
(12.0 | ) | (11.8 | ) | ||||
|
2015
|
(0.3 | ) | (10.4 | ) | ||||
|
|
·
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
|
·
|
accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
|
·
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
|
2014
|
2015
|
|||||||
|
Audit Fees
|
$ | 140,000 | $ | 140,000 | ||||
|
Audit-Related Fees
|
- | - | ||||||
|
Tax Fees
|
- | - | ||||||
|
Total
|
$ | 140,000 | $ | 140,000 | ||||
|
|
·
|
Quorum.
As permitted under the Israeli Companies Law pursuant to our articles of association, the quorum required for an ordinary meeting of shareholders will consist of at least two shareholders present in person, by proxy or by other voting instrument in accordance with the Israeli Companies Law, who hold at least 25% of the voting power of our shares (and in an adjourned meeting, with some exceptions, at least two shareholders), instead of 33 1/3% of the issued share capital required under the NASDAQ Stock Market rules.
|
|
|
·
|
Nomination of directors.
With the exception of external directors and directors elected by our board of directors due to vacancy, our directors are elected by an annual meeting of our shareholders to hold office until the next annual meeting following one year from his or her election. The nominations for directors, which are presented to our shareholders by our board of directors, are generally made by the board of directors itself, in accordance with the provisions of our articles of association and the Israeli Companies Law. Nominations need not be made by a nominating committee of our board of directors consisting solely of independent directors or otherwise, as required under the NASDAQ Stock Market rules.
|
|
|
·
|
Majority of independent directors.
Under the Companies Law, we are only required to appoint at least two external directors, within the meaning of the Companies Law, to our board of directors. Currently, four of our directors (of which two are external directors, within the meaning of the Companies Law) qualify as independent directors under the rules of the U.S. federal securities laws and the NASDAQ Stock Market rules.
|
|
MediWound Ltd.
|
|||
|
Date: January 25, 2016
|
By:
|
/s/ Sharon Malka
|
|
|
Sharon Malka
|
|||
|
Chief Financial and Operation Officer
|
|||
|
ANNUAL REPORT ON FORM 20-F
INDEX OF EXHIBITS
|
||
|
Exhibit No.
|
Description
|
|
|
1.1
|
Amended and Restated Articles of Association of the Registrant
(1)
|
|
|
1.2
|
First Amendment to the Amended and Restated Articles of Association, effective as of June 12, 2014
(5)
|
|
|
1.3
|
Memorandum of Association of the Registrant
(2)
|
|
|
4.1
|
Form of Registration Rights Agreement by and among the Registrant and certain shareholders of the Registrant
(2)
|
|
|
4.2
|
Form of Information Rights Agreement by and between Clal Biotechnology Industries Ltd. and the Registrant
(2)
|
|
|
4.3
|
Founders Agreement, dated January 2001, by and among Clal Biotechnology Industries Ltd., L.R. R & D Ltd., Professor Lior Rosenberg and the Registrant
(3)
|
|
|
4.4
|
Unprotected Sub-Lease Agreement, dated July 27, 2004, as amended, by and between the Registrant and Clal Life Sciences L.P.
(3)
|
|
|
4.5
|
Patent Purchase Agreement, dated November 24, 2010, by and between the Registrant and L.R. R & D Ltd.
(3)
|
|
|
4.6
|
Form of Indemnification Agreement
(2)
|
|
|
4.7
|
Supply Agreement, dated January 11, 2001, as amended, by and between the Registrant and Challenge Bioproducts Corporation Ltd.†
(3)
|
|
|
4.8
|
License Agreement, dated September 22, 2000, as amended, by and between the Registrant and Mark Klein†
(3)
|
|
|
4.9
|
2003 Israeli Share Option Plan
(3)
|
|
|
4.10
|
2014 Israeli Share Option Plan
(2)
|
|
|
4.11
|
Letter Agreement, dated February 18, 2014, by and between the Registrant and Teva Pharmaceutical Industries Ltd.
(2)
|
|
|
4.12
|
MediWound Ltd.’s Compensation Policy for Executive Officers and Directors
(4)
|
|
|
4.13
|
BARDA Contract, dated September 29, 2015, by and between the Registrant and the U.S. Biomedical Advanced Research and Development Authority†
|
|
|
4.14
|
Modification to the BARDA Contract, dated October 7, 2015, by and between the Registrant and the U.S. Biomedical Advanced Research and Development Authority
|
|
|
8.1
|
List of subsidiaries of the Registrant
(3)
|
|
|
12.1
|
Certificate of Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002
|
|
|
12.2
|
Certificate of Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002
|
|
|
13.1
|
Certificate of Chief Executive Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, furnished herewith
|
|
|
13.2
|
Certificate of Chief Financial Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, furnished herewith
|
|
|
15.1
|
Consent of Kost Forer Gabbay and Kasierer, a member of Ernst & Young Global, an independent registered public accounting firm
|
|
|
†
|
Portions of each agreement were omitted and a complete copy of each agreement has been provided
separately
to the Securities and Exchange Commission pursuant to the company’s application requesting confidential treatment under Rule 406 of the Securities Act of 1993 as amended, or Rule 24b-2 of the Securities Exchange Act of 1934, as amended, as applicaple.
|
|
(1)
|
Previously filed with the SEC on March 14, 2014 pursuant to a registration statement on Form F-1 (File No. 333-193856) and incorporated by reference herein.
|
|
(2)
|
Previously filed with the SEC on March 3, 2014 pursuant to a registration statement on Form F-1 (File No. 333-193856) and incorporated by reference herein.
|
|
(3)
|
Previously filed with the SEC on February 10, 2014 pursuant to a registration statement on Form F-1 (File No. 333-193856) and incorporated by reference herein.
|
|
(4)
|
Previously filed with the SEC on August 5, 2014 as Annex A to Exhibit 99.1 to the Registrant’s Form 6-K and incorporated by reference herein.
|
|
(5)
|
Previously filed with the SEC on February 12, 2015 pursuant to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2014 (File No. 001-36349) and incorporated by reference herein.
|
|
Page
|
|
|
F
-
2
|
|
|
F
-
3
|
|
|
F
-
4
|
|
|
F
-
5
|
|
|
F
-
6 - F-7
|
|
|
F
-
8
-
F
-
42
|

|
Kost Forer Gabbay & Kasierer
3 Aminadav St.
Tel-Aviv 6706703, Israel
|
Tel: +972-3-6232525
Fax: +972-3-5622555
ey.com
|
|
Tel-Aviv, Israel
|
KOST FORER GABBAY & KASIERER
|
|
January 25, 2016
|
A Member of Ernst & Young Global
|
|
December 31,
|
||||||||||||
|
Note
|
2014
|
2015
|
||||||||||
|
CURRENT ASSETS:
|
||||||||||||
|
Cash and cash equivalents
|
5 | 25,422 | 42,502 | |||||||||
|
Short-term bank deposits
|
6 | 39,431 | 3,266 | |||||||||
|
Trade receivables
|
64 | 238 | ||||||||||
|
Inventories
|
7 | 1,421 | 1,715 | |||||||||
|
Other receivables
|
8, 22 | 2,159 | 2,674 | |||||||||
| 68,497 | 50,395 | |||||||||||
|
LONG-TERM ASSETS:
|
||||||||||||
|
Long term deposits
|
168 | 192 | ||||||||||
|
Property, plant and equipment, net
|
9 | 1,088 | 1,040 | |||||||||
|
Intangible assets, net
|
10 | 951 | 896 | |||||||||
|
Other assets
|
19 | 417 | - | |||||||||
| 2,624 | 2,128 | |||||||||||
| 71,121 | 52,523 | |||||||||||
|
CURRENT LIABILITIES:
|
||||||||||||
|
Trade payables
|
1,214 | 1,123 | ||||||||||
|
Other payables
|
11, 22 | 2,683 | 4,083 | |||||||||
| 3,897 | 5,206 | |||||||||||
|
LONG-TERM LIABILITIES:
|
||||||||||||
|
Liabilities in respect of Chief Scientist government grants
|
12,13 | 6,985 | 7,275 | |||||||||
|
Contingent consideration for the purchase of treasury shares
|
13 | 17,361 | 16,475 | |||||||||
|
Severance pay liability, net
|
14 | 7 | 97 | |||||||||
| 24,353 | 23,847 | |||||||||||
|
SHAREHOLDERS' EQUITY:
|
16 | |||||||||||
|
Ordinary shares of NIS 0.01 par value:
|
||||||||||||
|
Authorized: 32,244,508 shares as of December 31, 2014 and 2015; Issued and Outstanding 21,550,300 and 21,850,300 shares respectively.
|
59 | 60 | ||||||||||
|
Share premium
|
109,117 | 111,801 | ||||||||||
|
Foreign currency translation adjustments
|
(18 | ) | (16 | ) | ||||||||
|
Accumulated deficit
|
(66,287 | ) | (88,375 | ) | ||||||||
| 42,871 | 23,470 | |||||||||||
| 71,121 | 52,523 | |||||||||||
|
Year ended
December 31,
|
||||||||||||||||
|
Note
|
2013
|
2014
|
2015
|
|||||||||||||
|
Revenues
|
- | 259 | 601 | |||||||||||||
|
Cost of revenues
|
20a | - | 2,785 | 2,519 | ||||||||||||
|
Gross loss
|
- | (2,526 | ) | (1,918 | ) | |||||||||||
|
Research and development, net of participations
|
20b | 3,635 | 5,349 | 6,021 | ||||||||||||
|
Selling and marketing
|
20c | 2,259 | 8,829 | 9,284 | ||||||||||||
|
General and administrative
|
20d | 1,687 | 4,723 | 4,004 | ||||||||||||
|
Operating loss
|
(7,581 | ) | (21,427 | ) | (21,227 | ) | ||||||||||
|
Financial income
|
20e | 2,401 | 4,665 | 1,052 | ||||||||||||
|
Financial expense
|
20e | (3,321 | ) | (2,113 | ) | (1,496 | ) | |||||||||
|
Loss from continuing operations
|
(8,501 | ) | (18,875 | ) | (21,671 | ) | ||||||||||
|
Loss from discontinued operation
|
19 | (6,850 | ) | - | (417 | ) | ||||||||||
|
Net loss
|
(15,351 | ) | (18,875 | ) | (22,088 | ) | ||||||||||
|
Other comprehensive (loss) income:
|
||||||||||||||||
|
Items to be reclassified to profit or loss in subsequent periods:
|
||||||||||||||||
|
Foreign currency translation adjustments
|
(32 | ) | 14 | 2 | ||||||||||||
|
Total comprehensive loss
|
(15,383 | ) | (18,861 | ) | (22,086 | ) | ||||||||||
|
Basic and diluted net income (loss) per share
|
21 | (0.98 | ) | (0.95 | ) | (1.02 | ) | |||||||||
|
Share capital
|
Share premium
|
Treasury
shares
|
Foreign currency translation reserve
|
Accumulated
deficit
|
Total
equity
|
|||||||||||||||||||
|
Balance as of January 1, 2013
|
9 | 47,686 | - | - | (32,061 | ) | 15,634 | |||||||||||||||||
|
Loss for the period
|
- | - | - | - | (15,351 | ) | (15,351 | ) | ||||||||||||||||
|
Other comprehensive income
|
- | - | - | (32 | ) | (32 | ) | |||||||||||||||||
|
Total comprehensive (loss) income
|
- | - | - | (32 | ) | (15,351 | ) | (15,383 | ) | |||||||||||||||
|
Exercise of options
|
* | ) | 279 | - | - | - | 279 | |||||||||||||||||
|
Purchase of treasury shares
|
- | - | (34,600 | ) | - | - | (34,600 | ) | ||||||||||||||||
|
Share-based compensation
|
- | 607 | - | - | - | 607 | ||||||||||||||||||
|
Issuance of shares, net
|
2 | 13,657 | - | - | - | 13,659 | ||||||||||||||||||
|
Balance as of December 31, 2013
|
11 | 62,229 | (34,600 | ) | (32 | ) | (47,412 | ) | (19,804 | ) | ||||||||||||||
|
Loss for the period
|
- | - | - | - | (18,875 | ) | (18,875 | ) | ||||||||||||||||
|
Other comprehensive income
|
- | - | - | 14 | 14 | |||||||||||||||||||
|
Total comprehensive (loss) income
|
- | - | - | 14 | (18,875 | ) | (18,861 | ) | ||||||||||||||||
|
Exercise of warrants
|
1 | 4,711 | - | - | - | 4,712 | ||||||||||||||||||
|
Exercise of options
|
1 | 305 | - | - | - | 306 | ||||||||||||||||||
|
Cancellation of treasury shares
|
(2 | ) | (34,598 | ) | 34,600 | - | - | - | ||||||||||||||||
|
Effect of share split
|
32 | (32 | ) | - | - | - | - | |||||||||||||||||
|
Share-based compensation
|
- | 4,827 | - | - | - | 4,827 | ||||||||||||||||||
|
Issuance of shares, net
|
16 | 71,675 | - | - | - | 71,691 | ||||||||||||||||||
|
Balance as of December 31, 2014
|
59 | 109,117 | - | (18 | ) | (66,287 | ) | 42,871 | ||||||||||||||||
|
Loss for the period
|
- | - | - | - | (22,088 | ) | (22,088 | ) | ||||||||||||||||
|
Other comprehensive income
|
- | - | - | 2 | - | 2 | ||||||||||||||||||
|
Total comprehensive (loss) income
|
- | - | - | 2 | (22,088 | ) | (22,086 | ) | ||||||||||||||||
|
Exercise of options
|
1 | 25 | - | - | - | 26 | ||||||||||||||||||
|
Share-based compensation
|
- | 2,659 | - | - | - | 2,659 | ||||||||||||||||||
|
Balance as of December 31, 2015
|
60 | 111,801 | - | (16 | ) | (88,375 | ) | (23,470 | ) | |||||||||||||||
U.S. dollars in thousands
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Cash Flows from Operating Activities:
|
||||||||||||
|
Net loss
|
(15,351 | ) | (18,875 | ) | (22,088 | ) | ||||||
|
Adjustments to reconcile net loss to net cash used in continuing operating activities:
|
||||||||||||
|
Adjustments to profit and loss items:
|
||||||||||||
|
Loss from discontinued operation
|
6,850 | - | 417 | |||||||||
|
Depreciation and amortization
|
336 | 492 | 503 | |||||||||
|
Revaluation of warrants to shareholders
|
820 | (4,491 | ) | - | ||||||||
|
Share-based compensation
|
531 | 4,827 | 2,659 | |||||||||
|
Revaluation of liabilities in respect of Chief Scientist government grants
|
(106 | ) | 87 | (474 | ) | |||||||
|
Revaluation of contingent consideration for the purchase of treasury shares
|
(2,400 | ) | 612 | (764 | ) | |||||||
|
Increase in severance pay liability
|
- | - | 90 | |||||||||
|
Accrued interest in respect of financial loans
|
1,669 | - | - | |||||||||
|
Net financing expenses (income)
|
(35 | ) | 226 | (219 | ) | |||||||
| 7,665 | 1,753 | 2,212 | ||||||||||
|
Changes in asset and liability items:
|
||||||||||||
|
Increase in trade receivables
|
- | (67 | ) | (181 | ) | |||||||
|
(Increase) decrease in other receivables
|
(532 | ) | 186 | (556 | ) | |||||||
|
Increase in inventories
|
- | (1,421 | ) | (273 | ) | |||||||
|
Increase (decrease) in trade payables
|
405 | 22 | (76 | ) | ||||||||
|
(Decrease) increase in other payables
|
(262 | ) | 1,909 | 1,361 | ||||||||
| (389 | ) | 629 | 275 | |||||||||
|
Net cash used in continuing operating activities
|
(8,075 | ) | (16,493 | ) | (19,601 | ) | ||||||
|
Net cash used in discontinued operating activities
|
(1,665 | ) | - | - | ||||||||
|
Net cash flows used in operating activities
|
(9,740 | ) | (16,493 | ) | (19,601 | ) | ||||||
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Cash Flows from Investing Activities:
|
||||||||||||
|
Purchase of property and equipment
|
(268 | ) | (366 | ) | (376 | ) | ||||||
|
Purchase of intangible assets
|
(90 | ) | (30 | ) | (30 | ) | ||||||
|
Interest received
|
3 | 173 | 287 | |||||||||
|
Proceeds from (investment in) short term bank deposits, net
|
(2,500 | ) | (36,931 | ) | 36,165 | |||||||
|
Net cash provided by (used in) investing activities
|
(2,855 | ) | (37,154 | ) | 36,046 | |||||||
|
Cash Flows from Financing Activities:
|
||||||||||||
|
Proceeds from exercise of options
|
279 | 306 | 26 | |||||||||
|
Proceeds from issuance of shares and warrants, net
|
15,800 | 71,824 | - | |||||||||
|
Proceeds from shareholders' loans
|
3,930 | - | - | |||||||||
|
Repayment of shareholders' loans
|
(915 | ) | - | - | ||||||||
|
Deferred issuance costs
|
(129 | ) | - | - | ||||||||
|
Proceeds from the Chief Scientist government grants, net of re-payment
|
276 | 345 | 752 | |||||||||
|
Net cash provided by financing activities
|
19,241 | 72,475 | 778 | |||||||||
|
Exchange rate differences on cash and cash equivalent balances
|
70 | (459 | ) | (143 | ) | |||||||
|
Increase in cash and cash equivalents from continuing activities
|
8,311 | 18,828 | 17,223 | |||||||||
|
Decrease in cash and cash equivalents from discontinued activities
|
(1,665 | ) | - | - | ||||||||
|
Balance of cash and cash equivalents at the beginning of the year
|
337 | 7,053 | 25,422 | |||||||||
|
Balance of cash and cash equivalents at the end of the year
|
7,053 | 25,422 | 42,502 | |||||||||
|
Non-cash activities:
|
||||||||||||
|
Treasury shares cancellation against share premium
|
- | 34,600 | - | |||||||||
|
Exercise of cashless warrants into shares
|
- | 4,709 | - | |||||||||
|
Contingent consideration for the purchase of treasury shares
|
19,200 | - | - | |||||||||
|
Exercise of derivative instrument into treasury shares
|
15,400 | - | - | |||||||||
|
Conversion of loans and realization of derivatives into shares and warrants
|
6,239 | - | - | |||||||||
U.S. dollars in thousands (except share and per share data)
|
NOTE 1:
|
GENERAL
|
|
|
a.
|
General description of the company and its operations:
|
|
|
b.
|
The Company has two wholly-owned subsidiaries: MediWound Germany GmbH, acting as EU marketing authorization holder and EU sales and marketing arm and MediWound UK Limited, an inactive company. In addition, the Company owns approximately 7% of PolyHeal Ltd., a private life sciences company ("PolyHeal").
|
|
|
c.
|
On March 25, 2014, the Company closed its initial public offering in the United States and listing on the NASDAQ Global Select Market ("the IPO") of 5,750,000 ordinary shares in the offering, including 750,000 additional shares to cover underwriters over-allotments, which was exercised on March 25, 2014 by the underwriters. As a result, the Company issued and sold a total of 5,750,000 ordinary shares at a price per share of $14.00 with aggregate gross proceeds of $80,500. Under the terms of the offering, the Company incurred aggregate underwriting discounts of approximately $5,635 and expenses of approximately $3,172 in connection with the offering, resulting in net proceeds to the Company of approximately $71,691. Following the IPO the Company's securities are listed for trading on NASDAQ.
|
|
|
d.
|
On September 29, 2015, the Company was awarded a U.S. Biomedical Advanced Research and Development Authority ("BARDA") contract valued up to $112 million for development and procurement of NexoBrid for the U.S. The contract is for the advancement of the development and manufacturing, as well as the procurement of NexoBrid, as a medical countermeasure as part of BARDA preparedness for mass casualty events.
The five-year base contract includes $24 million of funding to support development activities to complete the U.S. Food and Drug Administration (FDA) approval process for NexoBrid for use in thermal burn injuries, as well as $16 million for procurement of NexoBrid, which is contingent upon FDA Emergency Use Authorization (EUA) and/or FDA marketing authorization for NexoBrid. In addition, the contract includes options for further funding of up to $22 million for expanding NexoBrid’s indications and of up to $50 million for additional procurement of NexoBrid.
|
U.S. dollars in thousands (except share and per share data)
|
NOTE 2:
|
SIGNIFICANT ACCOUNTING POLICIES
|
|
|
a.
|
Basis of presentation of financial statements:
|
|
|
b.
|
The Company's operating cycle is one year.
|
|
|
c.
|
Consolidated financial statements include the financial statements of companies that the Company controls (subsidiaries). Control is achieved when the Company is exposed, or has rights, to variable returns from its investment with the investee and has the ability to affect those returns through its power over the investee.
|
|
|
d.
|
Functional currency, reporting currency and foreign currency:
|
|
|
1.
|
Functional currency and reporting currency:
The reporting currency of the financial statements is the U.S. dollar.
The Company determines the functional currency based on the currency in which it primarily generates and expends cash. The Company determined that its functional currency is the U.S. dollar since most of the Company's expenses are in U.S. dollars and the economic environment in which the Company operates in and performs its transactions is mostly affected by the U.S dollar. A certain portion of the Company's costs are denominated in NIS mainly due to payroll and related benefit costs incurred in Israel. To further support the Company's determination, the Company has analyzed the currency in which funds from financing activities are generated or held and the currency in which receipts from operating activities are usually retained. In this respect, funds from financing activities were principally derived from significant funds raised in U.S. dollars including the public offering completed in 2014.
|
U.S. dollars in thousands (except share and per share data)
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
2.
|
Transactions, assets and liabilities in foreign currency:
|
|
|
e.
|
Cash equivalents:
|
|
|
f.
|
Short-term bank deposits:
|
|
|
g.
|
Inventories:
|
|
|
Cost of inventories is determined as follows:
|
|
Raw materials
|
-
|
At cost of purchase using the first-in, first-out method.
|
|
Finished goods
|
-
|
On the basis of average costs including materials, labor and other direct and indirect manufacturing costs based on normal capacity.
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
h.
|
Government support:
|
|
|
i.
|
Property, plant and equipment, net:
|
|
%
|
||||
|
Laboratory equipment
|
15 - 20 | |||
|
Office furniture
|
6 - 15 | |||
|
Computers
|
33 | |||
|
Leasehold improvements
|
See below
|
|||
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
j.
|
Intangible assets, net:
|
|
|
k.
|
Revenue recognition
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
l.
|
Research and development expenses, net of participations:
|
|
|
m.
|
Impairment of non-financial assets:
|
|
|
n.
|
Financial instruments:
|
|
|
1.
|
Financial assets:
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
2.
|
Financial liabilities:
|
|
|
3.
|
Fair value:
|
|
|
4.
|
Offsetting financial instruments:
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
5.
|
Classification of financial instruments by fair value hierarchy:
All assets and liabilities measured at fair value or for which fair value is disclosed are categorized into levels within the fair value hierarchy based on the lowest level input that is significant to the entire fair value measurement:
|
|
Level 1
|
-
|
quoted prices (unadjusted) in active markets for identical assets or liabilities.
|
|
Level 2
|
-
|
inputs other than quoted prices included within level 1 that are observable either directly or indirectly.
|
|
Level 3
|
-
|
inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data).
|
|
|
6.
|
De-recognition of financial instruments:
|
|
|
a)
|
Financial assets:
A financial asset is derecognized when the contractual rights to the cash flows from the financial asset expire or the Company has transferred its contractual rights to receive cash flows from the financial asset or assumes an obligation to pay the cash flows in full without material delay to a third party and has transferred substantially all the risks and rewards of the asset, or has neither transferred nor retained substantially all the risks and rewards of the asset, but has transferred control of the asset.
|
|
|
b)
|
Financial liabilities:
A financial liability is derecognized when it is extinguished, that is when the obligation is discharged or cancelled or expires. A financial liability is extinguished when the debtor (the Company) discharges the liability by paying in cash, other financial assets, goods or services; or is legally released from the liability.
|
|
|
7.
|
Treasury shares:
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
o.
|
Provisions:
|
|
|
p.
|
Severance pay liability, net:
|
|
|
1.
|
Short-term employee benefits:
|
|
|
2.
|
Post-employment benefits:
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
q.
|
Share-based compensation:
|
|
|
r.
|
Discontinued operation:
|
|
|
s.
|
Income (loss) per share:
|
|
NOTE 3:-
|
SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS
|
|
|
•
|
Determining the fair value of share based compensation to employees and directors:
The fair value of share based compensation to employees and directors as well as of warrants to shareholders is determined using acceptable option pricing models.
The assumptions used in the models include the expected volatility, expected life, early exercise factor, expected dividend and risk-free interest rate.
|
|
|
•
|
Chief Scientist government grants:
Government grants received from the OCS are recognized as a liability if future economic benefits are expected from the research and development activity that will result in royalty-bearing sales. There is uncertainty regarding the estimated future cash flows and the estimated discount rate used to measure the amortized cost of the liability.
|
|
|
•
|
Contingent consideration for the purchase of treasury shares:
Contingent consideration for acquisition of treasury shares was first measured at fair value. After initial recognition, the liability is measured at amortized cost using the effective interest method. As the contingent consideration is calculated based on future royalty-bearing sales, there is uncertainty regarding the estimated future cash flows and the estimated discount rate used to measure the fair value of this liability.
|
|
NOTE 4:-
|
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION
|
|
|
IFRS 9-Financial Instruments
:
|
|
|
IF
RS 15-Revenue
recognition
:
|
|
NOTE 5:-
|
CASH AND CASH EQUIVALENTS
|
|
December 31,
|
||||||||
|
2014
|
2015
|
|||||||
|
USD cash for immediate withdrawal
|
20,497 | 34,856 | ||||||
|
Non-USD cash for immediate withdrawl
|
4,925 | 7,646 | ||||||
| 25,422 | 42,502 | |||||||
|
NOTE 6:-
|
SHORT-TERM BANK DEPOSITS
|
|
December 31,
|
||||||||
|
2014
|
2015
|
|||||||
|
USD bank deposits
|
37,001 | - | ||||||
|
EURO bank deposits
|
2,430 | 3,266 | ||||||
| 39,431 | 3,266 | |||||||
|
|
The EURO deposits bear interest of 0.08% and 0.04% for 2015 and 2014, respectively. The USD deposits bear interest ranging from 0.28%-0.76%. The bank deposits are for periods ranging from 106 to 273 days.
|
|
NOTE 7:-
|
INVENTORIES
|
|
Year ended
December 31,
|
||||||||
|
2014
|
2015
|
|||||||
|
Raw materials
|
402 | 387 | ||||||
|
Finished goods
|
1,019 | 1,328 | ||||||
| 1,421 | 1,715 | |||||||
|
NOTE 8:-
|
OTHER RECEIVABLES
|
|
Year ended
December 31,
|
||||||||
|
2014
|
2015
|
|||||||
|
Government authorities
|
218 | 172 | ||||||
|
Related parties
|
136 | 20 | ||||||
|
Former related parties
|
1,597 | 1,477 | ||||||
|
BARDA funds receivable
|
- | 800 | ||||||
|
Prepaid expenses and other
|
208 | 205 | ||||||
| 2,159 | 2,674 | |||||||
|
NOTE 9:-
|
PROPERTY, PLANT AND EQUIPMENT, NET
|
|
|
Balance as of December 31, 2015:
|
|
Office
furniture
|
Electronic
machinery
and lab
equipment
|
Computers
|
Leasehold
improvements
|
Total
|
||||||||||||||||
|
Cost
|
||||||||||||||||||||
|
Balance as of January 1, 2015
|
201 | 1,875 | 185 | 2,015 | 4,276 | |||||||||||||||
|
Disposals
|
- | (136 | ) | (62 | ) | - | (198 | ) | ||||||||||||
|
Additions
|
27 | 216 | 53 | 80 | 376 | |||||||||||||||
|
Foreign currency translation
|
(7 | ) | - | (2 | ) | - | (9 | ) | ||||||||||||
|
Balance as of December 31, 2015
|
221 | 1,955 | 174 | 2,095 | 4,445 | |||||||||||||||
|
Accumulated Depreciation
|
||||||||||||||||||||
|
Balance as of January 1, 2015
|
79 | 1,084 | 93 | 1,932 | 3,188 | |||||||||||||||
|
Disposals
|
- | (136 | ) | (62 | ) | - | (198 | ) | ||||||||||||
|
Additions
|
26 | 257 | 55 | 80 | 418 | |||||||||||||||
|
Foreign currency translation
|
(3 | ) | - | - | - | (3 | ) | |||||||||||||
|
Balance as of December 31, 2015
|
102 | 1,205 | 86 | 2,012 | 3,405 | |||||||||||||||
|
Depreciated cost as of December 31, 2015
|
119 | 750 | 88 | 83 | 1,040 | |||||||||||||||
|
|
Balance as of December 31, 2014:
|
|
Office
furniture
|
Electronic
machinery
and lab
equipment
|
Computers
|
Leasehold
improvements
|
Total
|
||||||||||||||||
|
Cost
|
||||||||||||||||||||
|
Balance as of January 1, 2014
|
169 | 1,723 | 119 | 1,999 | 4,010 | |||||||||||||||
|
Disposals
|
- | (69 | ) | (21 | ) | - | (90 | ) | ||||||||||||
|
Additions
|
41 | 221 | 88 | 16 | 366 | |||||||||||||||
|
Foreign currency translation
|
(9 | ) | - | (1 | ) | - | (10 | ) | ||||||||||||
|
Balance as of December 31, 2014
|
201 | 1,875 | 185 | 2,015 | 4,276 | |||||||||||||||
|
Accumulated Depreciation
|
||||||||||||||||||||
|
Balance as of January 1, 2014
|
54 | 904 | 70 | 1,846 | 2,874 | |||||||||||||||
|
Disposals
|
- | (69 | ) | (21 | ) | - | (90 | ) | ||||||||||||
|
Additions
|
29 | 249 | 45 | 86 | 409 | |||||||||||||||
|
Foreign currency translation
|
(4 | ) | - | (1 | ) | - | (5 | ) | ||||||||||||
|
Balance as of December 31, 2014
|
79 | 1,084 | 93 | 1,932 | 3,188 | |||||||||||||||
|
Depreciated cost as of December 31, 2014
|
122 | 791 | 92 | 83 | 1,088 | |||||||||||||||
|
NOTE 10:-
|
INTANGIBLE ASSETS, NET
|
|
|
Balance as of December 31, 2015
|
|
License and
Knowhow
|
||||
|
Cost
|
||||
|
Balance as of January 1, 2015
|
1,436 | |||
|
Additions
|
30 | |||
|
Balance as of December 31, 2015
|
1,466 | |||
|
Accumulated Amortization
|
||||
|
Balance as of January 1, 2015
|
485 | |||
|
Additions
|
85 | |||
|
Balance as of December 31, 2015
|
570 | |||
|
Amortized cost
|
||||
|
Balance as of December 31, 2015
|
896 | |||
|
|
Balance as of December 31, 2014
|
|
Cost
|
||||
|
Balance as of January 1, 2014
|
1,406 | |||
|
Additions
|
30 | |||
|
Balance as of December 31, 2014
|
1,436 | |||
|
Accumulated Amortization
|
||||
|
Balance as of January 1, 2014
|
402 | |||
|
Additions
|
83 | |||
|
Balance as of December 31, 2014
|
485 | |||
|
Amortized cost
|
||||
|
Balance as of December 31, 2014
|
951 | |||
|
NOTE 11:-
|
OTHER PAYABLES
|
|
Year ended
December 31,
|
||||||||
|
2014
|
2015
|
|||||||
|
Employees and payroll accruals
|
1,038 | 2,453 | ||||||
|
Accrued expenses
|
1,154 | 1,263 | ||||||
|
Current maturities of Chief Scientist government grants
|
49 | 28 | ||||||
|
Related parties
|
442 | 248 | ||||||
|
Deferred revenues
|
- | 91 | ||||||
| 2,683 | 4,083 | |||||||
|
NOTE 12:-
|
CHIEF SCIENTIST GOVERNMENT GRANTS
|
|
Year ended
December 31,
|
||||||||
|
2014
|
2015
|
|||||||
|
Balance as of January 1
|
6,604 | 7,034 | ||||||
|
Grants received
|
348 | 764 | ||||||
|
Royalties payments
|
(5 | ) | (21 | ) | ||||
|
Amounts carried to (profit) or loss
|
87 | (474 | ) | |||||
|
Balance as of Decmber 31
|
7,034 | 7,303 | ||||||
|
Current maturities
|
(49 | ) | (28 | ) | ||||
|
Long term liabilities in respect of Chief Scientist government grants
|
6,985 | 7,275 | ||||||
|
NOTE 13:-
|
FINANCIAL INSTRUMENTS
|
|
|
a.
|
Financial risk factors:
|
|
|
Foreign currency
risk
|
|
|
b.
|
Fair value:
|
|
|
c.
|
Sensitivity tests relating to changes in market factors:
|
|
NOTE 13:-
|
FINANCIAL INSTRUMENTS (Cont.)
|
|
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Sensitivity test to changes in NIS and EURO exchange rates
|
||||||||||||
|
Gain (loss) from change:
|
||||||||||||
|
5% increase in exchange rate
|
$ | 15 | $ | 259 | $ | 361 | ||||||
|
5% decrease in exchange rate
|
$ | (15 | ) | $ | (259 | ) | $ | (361 | ) | |||
|
NOTE 14:-
|
SEVERANCE PAY LIABILTY, NET
|
|
NOTE 14:-
|
SEVERANCE PAY LIABILTY, NET (Cont.)
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Deposits in defined contribution plan
|
42 | 48 | 71 | |||||||||
|
NOTE 15:-
|
CONTINGENT LIABILITIES AND COMMITMENTS
|
|
|
a.
|
In 2000, the Company signed an exclusive license agreement (as amended in 2007) with a third party with regard to its patents and intellectual property. Pursuant to the agreement, the Company received an exclusive license to use the third party's patents and intellectual property, for the purpose of developing, manufacturing, marketing, and commercializing products for treatment of burns and other wounds.
|
|
|
b.
|
Under the Research and Development Law, (the "R&D Law") the Company undertook to pay royalties of 3% - 3.5% on the revenues derived from sales of products or services developed in whole or in part using these OCS grants. The maximum aggregate royalties paid generally cannot exceed 100% of the grants received by the Company, plus annual interest generally equal to the 12-month LIBOR applicable to dollar deposits, as published on the first business day of each calendar year. The maximum royalty amount payable by the Company as of December 31, 2015 is approximately $ 11,509, which represents the total amount of grants actually received by the Company from the OCS including accrued interest, net of royalties actually paid by the Company.
|
|
|
c.
|
On November 24, 2010, the Company signed an agreement with one of its shareholders, to purchase a patent for the production and sale of related products for the treatment of burns. In consideration for the transfer and assignment of all rights and title relating to the patent, the Company paid a one-time payment in the amount of $ 88 and undertook to pay annual fixed payments in the amount of $ 30 as long as the patent is valid in the US and/or in any EU member country. The patent expires in May 2018, and the Company's accumulated outstanding obligation with respect to this agreement as of December 31, 2015 is $ 73.
|
|
|
d.
|
On September 15, 2014, a Statement of Claim was filed against the Company by some shareholders of Polyheal. The plaintiffs allege that the Company is obligated to pay them a total amount of $1,475 in exchange for their respective portion of PolyHeal's shares,
following the commencement of a feasibility study for the next generation of the PolyHeal Product in November 15, 2012, which constituted a milestone under a buyout option agreement between the Company, PolyHeal and its shareholders. For further details, see note 19.
|
|
NOTE 15:-
|
CONTINGENT LIABILITIES AND COMMITMENTS (Cont.)
|
|
|
e.
|
Operating Lease Agreements:
|
|
|
1.
|
The Company's offices and its production facility in Israel are located in a building that the Company leases from its Parent Company, in accordance with a sub lease agreement from July 2004. The sub lease agreement has been amended multiple times, most recently in September 2014. According to the most recently amended sub lease agreement, the Company subleases approximately 1,150 square meters of laboratory, office and clean room space at a monthly rent fee of $54.6. This sub lease agreement expires in December 2016. The Company's subsidiary offices are located in Germany. The monthly rent fee is currently €2.7 (approximately $ 2.9) and the lease agreement expires on April 30, 2019.
|
|
|
2.
|
The Company and its subsidiary have operating lease agreements for 22 vehicles. According to these agreements, the Company leases cars for its employees for a period of three years. As of December 31, 2015, the Company deposited $ 124 in respect of the vehicles operating leases.
|
|
|
3.
|
Minimum future lease fees for both agreements as of December 31, 2015 are as follows:
|
|
2016
|
905 | |||
|
2017
|
116 | |||
|
2018
|
90 | |||
| 1,111 |
|
NOTE 16:-
|
EQUITY
|
|
|
a.
|
Share capital
|
|
|
On March 3, 2014, the Company effected a bonus share distribution under which: (i) two and eight tenths (2.8) bonus shares were issued for each ordinary share outstanding prior to such distribution; and (ii) the conversion rate for each preferred share, option and warrant was adjusted to reflect such bonus share distribution. For accounting purposes, this transaction was recorded as a stock split and accordingly (unless otherwise noted), all ordinary shares, options, warrants and earnings (losses) per share amounts have been adjusted retroactively for all periods presented in these financial statements.
|
|
|
b.
|
Rights attached to shares:
|
|
|
c.
|
In January 2013 and June 2013, the Company and certain of its existing shareholders entered into convertible bridge financing agreements in the amounts of $ 3,000 (of which $ 2,579 were received from Clal Biotechnology Industries Ltd. (the "Parent Company")) and $ 1,585 (of which $ 1,500 were received from the Parent Company),
respectivel
y
. In June 2013, the Company further entered into a share purchase agreement pursuant to which the Company issued 1,530,233 ordinary shares in consideration for $ 15,800 net of issuance expenses. In addition, the Company issued to the investors warrants to purchase 765,127 ordinary shares at an exercise price of $ 10.34 per share. Upon the closing of such share purchase agreement on August 19, 2013, the convertible bridge loans were converted into 603,189 ordinary shares and warrants to purchase 223,131 and 78,477 ordinary shares at exercise prices of $ 6.72 and $ 10.34, respectively.
|
|
|
d.
|
On March 25, 2014 the Company completed its initial public offering in the United States and listing on the NASDAQ Global Select Market of 5,750,000 new ordinary shares at $14 per share. including the underwriters' option to purchase an additional 750,000 shares at the offering price that was exercised prior to closing. The Company's total proceeds from the issuance of the above shares were $71,691 thousands, net of underwriter’s discount and issuance expenses in the amount of $8,809.
|
|
|
e.
|
Transactions between the Company and Teva:
|
|
NOTE 17:-
|
SHARE-BASED COMPENSATION
|
|
|
a.
|
Expense recognized in the financial statements:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Cost of revenues
|
- | 763 | 372 | |||||||||
|
Research and development
|
315 | 657 | 511 | |||||||||
|
Selling and marketing
|
24 | 1,430 | 669 | |||||||||
|
General and administrative
|
192 | 1,977 | 1,107 | |||||||||
|
Expenses attributable to continuing operations
|
531 | 4,827 | 2,659 | |||||||||
|
Expenses attributable to discontinued operation
|
76 | - | - | |||||||||
|
Total share-based compensation
|
607 | 4,827 | 2,659 | |||||||||
|
|
b.
|
Share-based payment plan for employees and directors:
|
|
NOTE 17:-
|
SHARE-BASED COMPENSATION (Cont.)
|
|
|
c.
|
Option grants:
|
|
|
1.
|
On January 6, 2013, the Company granted 62,700 options to purchase ordinary shares under the Plan for an exercise price of $ 13.76 per share to its employees. The total fair value of the options at the date of grant was estimated at $713.
|
|
|
2.
|
On December 24, 2013, the Company granted 904,400 options to purchase ordinary shares under the Plan for an exercise price of $ 12.89 per share to its employees. The total fair value of the options at the date of grant was estimated at $9,570.
|
|
|
3.
|
On July 30, 2014, the Company's Board of Directors approved the grant of 40,000 options to purchase ordinary shares under the Plan for an exercise price of $ 7.26 per share to the external directors of the Company. The board of Directors approval was subject to shareholder approval. On September 22, 2014 the Company's general shareholders meeting approved the grant of the options to the Company's directors. The total fair value of the options at the date of grant was estimated at $155.
|
|
|
4.
|
On January 1, 2015, the Company granted 57,000 options to purchase ordinary shares under the Plan for an exercise price of $ 6.02 per share to its employees. The total fair value of the options at the date of grant was estimated at $269.
|
|
|
5.
|
On May 3, 2015, the Company granted 10,000 options to purchase ordinary shares under the Plan for an exercise price of $ 6.73 per share to its employees. The total fair value of the options at the date of grant was estimated at $39.
|
|
|
6.
|
On December 23, 2015, the Company granted 672,500 options to purchase ordinary shares under the Plan for an exercise price of $ 9.58 per share to its employees. The total fair value of the options at the date of grant was estimated at $4,028.
The board of directors approval of granting 70,000 options to purchase ordinary shares to CEO, is subject to the shareholders approval dated January 28, 2016.
|
|
NOTE 17:-
|
SHARE-BASED COMPENSATION (Cont.)
|
|
|
d.
|
Share options activity:
|
|
2013
|
2014
|
2015
|
||||||||||||||||||||||
|
Number of
options
|
Weighted
Average
Exercise price
|
Number of
options
|
Weighted
Average
Exercise price
|
Number of
options
|
Weighted
Average
Exercise price
|
|||||||||||||||||||
|
Outstanding at beginning of year
|
1,514,946 | 2.14 | 2,376,064 | 6.71 | 1,902,324 | 7.98 | ||||||||||||||||||
|
Granted
|
967,100 | 12.95 | 40,000 | 7.26 | 739,500 | 9.27 | ||||||||||||||||||
|
Exercised
|
(67,268 | ) | 4.15 | (449,714 | ) | 0.68 | (300,000 | ) | 0.09 | |||||||||||||||
|
Forfeited
|
(38,714 | ) | 8.56 | (64,026 | ) | 11.60 | (28,600 | ) | 12.69 | |||||||||||||||
|
Outstanding at end of year
|
2,376,064 | 6.71 | 1,902,324 | 7.98 | 2,313,224 | 9.35 | ||||||||||||||||||
|
Exercisable at end of year
|
1,360,514 | 2.16 | 1,155,584 | 5.03 | 1,535,055 | 9.39 | ||||||||||||||||||
|
Options outstanding as of
December 31, 2015
|
||||||||||||
|
Range of exercise prices ($ )
|
Number of
options
|
Weighted
Average
Remaining
contractual
life
|
Weighted
average exercise
price
|
|||||||||
|
0.09
|
159,773 | 1.95 | 0.09 | |||||||||
|
2.63
|
208,332 | 0.87 | 2.63 | |||||||||
|
7.26 - 9.82
|
1,061,618 | 4.88 | 9.08 | |||||||||
|
12.89 - 13.76
|
883,500 | 7.92 | 12.95 | |||||||||
|
Total
|
2,313,224 | 5.48 | 9.35 | |||||||||
|
|
e.
|
The fair value of the Company's share options granted to employees for the years ended December 31, 2013, 2014 and 2015 was estimated using acceptable option pricing models using the following assumptions:
|
|
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Dividend yield (%)
|
- | - | - | |||||||||
|
Expected volatility of the share prices (%)
|
84 | 75 | 71 | |||||||||
|
Risk-free interest rate (%)
|
1.03 - 2.43 | 0.1-1.80 | 0.25-2.24 | |||||||||
|
Expected life of share options (years)
|
5.5 - 7.0 | - | - | |||||||||
|
Early exercise factor
|
- | 100 | % | 100%-150 | % | |||||||
|
Weighted average share prices (Dollar)
|
$ | 14.41 | $ | 6.91 | $ | 8.98 | ||||||
|
NOTE 17:-
|
SHARE-BASED COMPENSATION (Cont.)
|
|
NOTE 18:-
|
TAXES ON INCOME
|
|
|
a.
|
The Company operates in two main tax jurisdictions: Israel and Germany. As such, the Company is subject to the applicable tax rates in the jurisdictions in which it conducts its business.
|
|
|
b.
|
Corporate tax rates in Israel:
|
|
|
Tax benefits under the Israel Law for the Encouragement of Capital Investments, 1959 (the "Investment Law"):
|
|
NOTE 18:-
|
TAXES ON INCOME (Cont.)
|
|
|
c.
|
Corporate tax rate in Germany:
|
|
|
d.
|
Final tax assessments:
The Company received final tax assessments through 2010.
|
|
|
e.
|
Net operating carryforward losses for tax purposes and other temporary differences:
|
|
|
f.
|
Deferred taxes:
|
|
|
g.
|
Current taxes on income:
|
|
|
h.
|
Theoretical tax:
|
|
NOTE 19:-
|
DISCONTINUED OPERATION
|
|
|
a.
|
In December 2010, the Company, Teva and PolyHeal, entered into a series of agreements to collaborate in the development, manufacturing and commercialization of PolyHeal's wound care product, or the PolyHeal Product(“ 2010 PolyHeal Agreement”).
|
|
NOTE 19:-
|
DISCONTINUED OPERATION (Cont.)
|
|
|
•
|
License agreements:
Under the 2010 PolyHeal Agreement, PolyHeal granted the Company an exclusive global license to manufacture, develop and commercialize all the Polyheal Products in consideration for royalty payments. Concurrently, the Company granted Teva an exclusive global sub license to commercialize the Polyheal Products in consideration for certain royalties and milestone payments. In addition, Teva undertook to finance the Company's future development of the Polyheal Product and all of its manufacturing costs.
|
|
|
•
|
Share purchase agreements:
Under the 2010 PolyHeal Agreement, Teva initially invested $ 6,750 in the Company, and undertook to invest an additional $ 6,750 in the Company subject to the achievement of a development milestone. Concurrent with Teva's investment in the Company, the Company purchased shares of PolyHeal for total consideration of $ 6,750. Additionally, the Company undertook to purchase additional shares of PolyHeal for the same amount, subject to the achievement of the same abovementioned development milestone.
|
|
|
b.
|
On November 15, 2012, the Company informed Teva of the commencement of a feasibility study for the next generation of the PolyHeal Product, which constituted a milestone under the 2010 PolyHeal Agreement. In accordance with the terms of the agreement, Upon achievement of this milestone, Teva was to invest an additional $ 6,750 in exchange of the Company's ordinary shares and the Company was to purchase, following and pending the consummation of this investment, for an identical amount, ordinary shares of PolyHeal from its existing shareholders. As of December 31, 2015, Teva had not made the investment despite the Company’s demand. Consequently, the
Company was not under any obligation to purchase and accordingly has not purchased any of the additional shares of PolyHeal from its shareholders.
|
|
NOTE 19:-
|
DISCONTINUED OPERATION (Cont.)
|
|
|
c.
|
As of December 31, 2012, all of the Company's collaborations with Teva under both the 2007 Teva Agreement and the 2010 PolyHeal agreement were terminated and consequently the Company's exclusive license for the PolyHeal Product expired as a result of the Company's failure to find a substitute strategic successor to Teva within the nine month period following the termination of the Company's agreement with Teva. Following the expiration of the license agreement with PolyHeal, the Company classified the results of PolyHeal operations for all periods presented, and the related cash flows, as a discontinued operation in accordance with IFRS 5. Furthermore, during the year ended December 31, 2013, the Company has fully impaired the license for the PolyHeal Product in the amount of $ 3,657.
|
|
|
d.
|
On September 15, 2014, a Statement of Claim was filed against the Company by certain shareholders of Polyheal. The plaintiffs allege that the Company is obligated to pay them a total amount of $1,475 in exchange for their respective portion of PolyHeal's shares, following the commencement of a milestone under a buyout option agreement between the Company, PolyHeal and its shareholders.
On December 14, 2014, the Company filed its Petition for a Right to Defend, or the Petition, in which it: (i) rejected the arguments raised against it in the Statement of Claim; (ii) emphasized that its obligation under the 2010 Polyheal Agreement to purchase the 7.5% of Polyheal’s ordinary shares is subject to the consumption of the deferred closing, as defined in the buyout agreement, including the receipt of the funds from Teva on a “back to back” basis; and (iii) stated that since no such payment has been made by Teva, the Company is not subject to any obligation to purchase Polyheal shares and/or make any payments to Polyheal’s shareholders.
|
|
|
e.
|
The Company has been acknowledged during 2015 about certain changes in circumstances indicating that the carrying amount of its royalty rights arising from the Company's ownership of shares of Polyheal would not be recoverable. Accordingly, a full impairment of these royalty rights amounting to $417 is included within the loss from discontinued operation.
|
|
NOTE 19:-
|
DISCONTINUED OPERATION (Cont.)
|
|
|
f.
|
As discussed above, the Company decided to classify the results of operations in PolyHeal as discontinued operation.
Below is the data of the operating results attributed to the discontinued operation:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Revenues
|
392 | - | - | |||||||||
|
Cost of revenues *)
|
2,015 | - | - | |||||||||
|
Gross loss
|
(1,623 | ) | - | - | ||||||||
|
Research and development, net of participations
|
607 | - | - | |||||||||
|
Selling and marketing
|
963 | - | - | |||||||||
|
Impairment of intangible assets
|
3,657 | - | 417 | |||||||||
|
Total operating expenses
|
(5,227 | ) | - | (417 | ) | |||||||
|
Operating loss
|
(6,850 | ) | - | (417 | ) | |||||||
|
Loss from discontinued operation
|
(6,850 | ) | - | (417 | ) | |||||||
|
|
*)
|
During the year ended December 31, 2013, the cost of revenues included a write- off of inventory in the amount of $ 490.
|
|
NOTE 20:-
|
SUPPLEMENTARY INFORMATION TO THE STATEMENTS OF COMPREHENSIVE INCOME
|
|
|
a.
|
Cost of revenues:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Salary and benefits (including share-based compensation)
|
- | 2,219 | 1,961 | |||||||||
|
Subcontractors
|
- | 199 | 67 | |||||||||
|
Depreciation and amortization
|
- | 416 | 417 | |||||||||
|
Cost of materials
|
- | 600 | 486 | |||||||||
|
Other manufacturing expenses
|
- | 880 | 934 | |||||||||
|
Increase in inventory of finished products
|
- | (1,529 | ) | (1,346 | ) | |||||||
| - | 2,785 | 2,519 | ||||||||||
|
|
b.
|
Research and development expenses, net of participations:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Salary and benefits (including share-based compensation)
|
2,137 | 2,182 | 2,610 | |||||||||
|
Subcontractors
|
1,372 | 3,362 | 4,464 | |||||||||
|
Depreciation and amortization
|
278 | - | 5 | |||||||||
|
Materials
|
181 | 510 | 841 | |||||||||
|
Other research and development expenses
|
545 | - | 219 | |||||||||
| 4,513 | 6,054 | 8,139 | ||||||||||
|
Participation by the Chief Scientist
|
(878 | ) | (705 | ) | (1,318 | ) | ||||||
|
Participation by BARDA
|
- | - | (800 | ) | ||||||||
| 3,635 | 5,349 | 6,021 | ||||||||||
|
NOTE 20:-
|
SUPPLEMENTARY INFORMATION TO THE STATEMENTS OF COMPREHENSIVE INCOME (Cont.)
|
|
|
c.
|
Selling and marketing expenses:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Salary and benefits (including share based compensation)
|
890 | 4,966 | 5,631 | |||||||||
|
Marketing and advertising
|
1,141 | 3,356 | 2,835 | |||||||||
|
Depreciation and amortization
|
19 | 25 | 22 | |||||||||
|
Shipping and delivery
|
5 | 60 | 180 | |||||||||
|
Registration and marketing fees
|
204 | 422 | 616 | |||||||||
| 2,259 | 8,829 | 9,284 | ||||||||||
|
|
d.
|
General and administrative expenses:
|
|
Salary and benefits (including share-based compensation)
|
951 | 3,521 | 2,670 | |||||||||
|
Professional fees
|
349 | 869 | 1,054 | |||||||||
|
Depreciation and amortization
|
57 | 49 | 59 | |||||||||
|
Other
|
330 | 284 | 221 | |||||||||
| 1,687 | 4,723 | 4,004 |
|
|
e.
|
Financial income and expense:
|
|
Financial income:
|
||||||||||||
|
Interest income
|
1 | 174 | 288 | |||||||||
|
Revaluation of financial derivatives
|
- | 4,491 | - | |||||||||
|
Revaluation of contingent consideration for the purchase of treasury shares
|
2,400 | - | 764 | |||||||||
| 2,401 | 4,665 | 1,052 | ||||||||||
|
Financial expense
|
||||||||||||
|
Interest in respect of Chief Scientist government grants
|
772 | 792 | 844 | |||||||||
|
Revaluation of contingent consideration for the purchase of treasury shares
|
- | 612 | - | |||||||||
|
Revaluation of warrants to shareholders
|
820 | - | - | |||||||||
|
Exchange differences, net
|
44 | 652 | 614 | |||||||||
|
Interest in respect to convertible loans
|
1,669 | - | - | |||||||||
|
Other
|
16 | 57 | 38 | |||||||||
| 3,321 | 2,113 | 1,496 | ||||||||||
|
NOTE 21:-
|
NET LOSS PER SHARE
|
|
|
a.
|
Details of the number of shares and loss used in the computation of loss per share from continuing operations:
|
|
Year ended
December 31,
|
||||||||||||||||||||||||
| 2013 | 2014 | 2015 | ||||||||||||||||||||||
|
Weighted
average
number of shares
|
Loss
|
Weighted
average
number of shares
|
Loss
|
Weighted
average
number of shares
|
Loss
|
|||||||||||||||||||
|
Basic and diluted income (loss)
|
15,671 | (8,501 | ) | 19,940 | (18,875 | ) | 21,718 | (21,671 | ) | |||||||||||||||
|
|
b.
|
Details of the number of shares and loss used in the computation of loss per share from discontinued operation:
|
|
Year ended
December 31,
|
||||||||||||||||||||||||
| 2013 | 2014 | 2015 | ||||||||||||||||||||||
|
Weighted
average
number of shares
|
Loss
|
Weighted
average
number of shares
|
Loss
|
Weighted
average
number of shares
|
Loss
|
|||||||||||||||||||
| Basic and diluted income (loss) | 15,671 | (6,850 | ) | - | - | 21,718 | (417 | ) | ||||||||||||||||
|
|
c.
|
Net loss per share from continuing and discontinued operations:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Basic and Diluted loss per share:
|
||||||||||||
|
Net loss from continuing operations
|
(0.54 | ) | (0.95 | ) | (1.00 | ) | ||||||
|
Loss from discontinued operation
|
(0.44 | ) | - | (0.02 | ) | |||||||
|
Net loss per share
|
(0.98 | ) | (0.95 | ) | (1.02 | ) | ||||||
|
NOTE 22:-
|
BALANCES AND TRANSACTIONS WITH RELATED PARTIES AND KEY OFFICERS
|
|
|
a.
|
Related parties consist of:
|
|
|
•
|
Clal Biotechnologies Industries Ltd.-the Parent Company.
|
|
|
•
|
Teva - a former shareholder which the Company had a collaboration agreement with (see Note 16(e)).
|
|
|
•
|
PolyHeal-in which the Company holds approximately 7% (see Note 19).
|
|
|
•
|
Directors of the Company.
|
|
|
b.
|
Balances with related parties:
|
|
Receivables
|
Payables
|
|||||||
|
Parent Company
(1):
|
||||||||
|
2014
|
- | 151 | ||||||
|
2015
|
- | 207 | ||||||
|
Other related parties:
|
||||||||
|
2014
|
136 | 291 | ||||||
|
2015
|
20 | 41 | ||||||
|
Former related party
(2):
|
||||||||
|
2014
|
1,597 | - | ||||||
|
2015
|
1,477 | - | ||||||
|
|
(1)
|
The Company leases office space and a production facility from the Parent Company in accordance with a sublease agreement for two years with an option for extension (see Note 15 (e)).
|
|
|
(2)
|
Participation by Teva.
|
|
NOTE 22:-
|
BALANCES AND TRANSACTIONS WITH RELATED PARTIES AND KEY OFFICERS (Cont.)
|
|
|
c.
|
Transactions with related parties:
|
|
Professional
Fee (1)
|
Rent expenses
|
Revenues (2)
|
Participations (3)
|
Royalties
|
||||||||||||||||
|
Parent company:
|
||||||||||||||||||||
|
2013
|
- | (612 | ) | - | - | - | ||||||||||||||
|
2014
|
(12 | ) | (576 | ) | - | - | - | |||||||||||||
|
2015
|
(52 | ) | (730 | ) | - | - | - | |||||||||||||
|
Other related parties:
|
||||||||||||||||||||
|
2013
|
- | - | - | 219 | (16 | ) | ||||||||||||||
|
2014
|
(80 | ) | - | - | - | - | ||||||||||||||
|
2015
|
(127 | ) | - | - | - | - | ||||||||||||||
|
Former related party:
|
||||||||||||||||||||
|
2013
|
- | - | 368 | - | - | |||||||||||||||
|
2014
|
- | - | - | - | 51 | |||||||||||||||
|
2015
|
- | - | - | - | 121 | |||||||||||||||
|
|
(1)
|
Proffesional fees do not include short-term employee benefits and share-based compensation to one of the Company's shareholders, who is a key officer, in the amounts of $266, $699 and $619 for the years 2013, 2014 and 2015, respectively.
|
|
|
(2)
|
Attributable to the discontinued operation.
|
|
|
(3)
|
Including certain participation by Teva which is attributable to the discontinued operation.
|
|
|
d.
|
Compensation of key officers of the Company:
|
|
Year ended
December 31,
|
||||||||||||
|
2013
|
2014
|
2015
|
||||||||||
|
Short-term employee benefits
|
1,307 | 2,314 | 2,639 | |||||||||
|
Share-based compensation
|
170 | 2,949 | 1,702 | |||||||||
| 1,477 | 5,263 | 4,341 | ||||||||||
|
Number of key officers
|
6 | 7 | 7 | |||||||||