SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
|
FORM 20-F
|
| ☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| ☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| ☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

(Exact name of Registrant as specified in its charter)
(Jurisdiction of incorporation or organization)
Yavne, 8122745 Israel
(Address of principal executive offices)
General Counsel and Corporate Secretary
Telephone: +972 (77) 971-4100
E-mail: yaronm@mediwound.com
MediWound Ltd.
42 Hayarkon Street
Yavne, 8122745 Israel
(Name, telephone, e-mail and/or facsimile number and address of company contact person)
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Ordinary shares, par value NIS 0.01 per share
|
NASDAQ Global Market
|
|
Large accelerated filer
☐
|
Accelerated filer
☒
|
Non-accelerated filer
☐
|
|
U.S. GAAP
☐
|
International Financial Reporting Standards as issued
by the International Accounting Standards Board ☒ |
Other
☐
|

|
FORM 20-F
ANNUAL REPORT FOR THE FISCAL YEAR ENDED DECEMBER 31, 2016 |
|
i
|
|
|
i
|
|
|
PART I
|
|
|
1
|
|
|
1
|
|
|
1
|
|
|
30
|
|
|
59
|
|
|
59
|
|
|
71
|
|
|
89
|
|
|
93
|
|
|
94
|
|
|
95
|
|
|
110
|
|
|
111
|
|
|
PART II
|
|
|
112
|
|
|
112
|
|
|
112
|
|
|
113
|
|
|
113
|
|
|
113
|
|
|
113
|
|
|
114
|
|
|
114
|
|
|
114
|
|
|
114
|
|
|
114
|
|
|
PART III
|
|
|
115
|
|
|
115
|
|
|
115
|
|
|
116
|
|
|
F-1
|
|
| · |
the timing and conduct of our trials of NexoBrid, EscharEx and our pipeline product candidates, including statements regarding the timing, progress and results of current and future preclinical studies and clinical trials, and our research and development programs;
|
| · |
the clinical utility, potential advantages and timing or likelihood of regulatory filings and approvals of NexoBrid, EscharEx and our pipeline products;
|
| · |
our expectations regarding future growth, including our ability to develop new products;
|
| · |
our commercialization, marketing and manufacturing capabilities and strategy and the ability of our marketing team to cover regional burn centers and units;
|
| · |
our ability to maintain adequate protection of our intellectual property;
|
| · |
our plans to develop and commercialize NexoBrid, EscharEx and our pipeline products;
|
| · |
our estimates regarding expenses, future revenues, capital requirements and the need for additional financing;
|
| · |
our estimates regarding the market opportunity for NexoBrid, EscharEx and our pipeline products;
|
| · |
our expectation regarding the duration of our inventory of intermediate drug substance and products;
|
| · |
the impact of our research and development expenses as we continue developing product candidates;
|
| · |
our expectations regarding the time during which we will be an emerging growth company under the JOBS Act; and
|
| · |
the impact of government laws and regulations.
|
| A. |
Selected Financial Data
|
|
Year Ended December 31,
|
||||||||||||||||||||
|
2012
|
2013
|
2014
|
2015
|
2016
|
||||||||||||||||
|
(in thousands, except per share data)
|
||||||||||||||||||||
|
Consolidated statements of operations data:
|
||||||||||||||||||||
|
Revenues
|
$
|
—
|
$
|
—
|
$
|
259
|
$
|
601
|
$
|
1,558
|
||||||||||
|
Cost of revenues (1)
|
—
|
—
|
2,785
|
2,519
|
2,158
|
|||||||||||||||
|
Gross loss
|
—
|
—
|
(2,526
|
)
|
(1,918
|
)
|
(600
|
)
|
||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development, gross
|
3,804
|
4,513
|
6,054
|
8,139
|
14,779
|
|||||||||||||||
|
Participation by BARDA and the Israeli Innovation Authority
|
(2,247
|
) |
(878
|
) |
(705
|
) |
(2,118
|
) |
(7,711
|
) | ||||||||||
|
Research and development, net of participations(1)(2)
|
1,557
|
3,635
|
5,349
|
6,021
|
7,068
|
|||||||||||||||
|
Selling and marketing(1)
|
—
|
2,259
|
8,829
|
9,284
|
8,403
|
|||||||||||||||
|
General and administrative(1)
|
1,173
|
1,687
|
4,723
|
4,004
|
4,084
|
|||||||||||||||
|
Operating loss
|
(2,730
|
)
|
(7,581
|
)
|
(21,427
|
)
|
(21,227
|
)
|
(20,155
|
)
|
||||||||||
|
Financial income (expense), net
|
14,715
|
(920
|
)
|
2,552
|
(444
|
)
|
1,270
|
|||||||||||||
|
Income (loss) from continuing operations
|
11,985
|
(8,501
|
)
|
(18,875
|
)
|
(21,671
|
)
|
(18,885
|
)
|
|||||||||||
|
Loss from discontinued operation(1)(3)
|
(1,045
|
)
|
(6,850
|
)
|
-
|
(417
|
)
|
-
|
||||||||||||
|
Net income (loss)
|
$
|
10,940
|
$
|
(15,351
|
)
|
$
|
(18,875
|
)
|
$
|
(22,088
|
)
|
$
|
(18,885
|
)
|
||||||
|
Foreign currency translation adjustments
|
—
|
(32
|
)
|
14
|
2
|
7
|
||||||||||||||
|
Total comprehensive income (loss)
|
$
|
10,940
|
$
|
(15,383
|
)
|
$
|
(18,861
|
)
|
$
|
(22,086
|
)
|
$
|
(18,878
|
)
|
||||||
|
Basic net income (loss) per share(4)
|
$
|
0.70
|
$
|
(0.98
|
)
|
$
|
(0.95
|
)
|
$
|
(1.02
|
)
|
$
|
(0.86
|
)
|
||||||
|
Diluted net income (loss) per share(4)
|
$
|
0.64
|
$
|
(0.98
|
)
|
$
|
(0.95
|
)
|
$
|
(1.02
|
)
|
$
|
(0.86
|
)
|
||||||
|
Weighted average number of ordinary shares used in computing net income (loss) per ordinary share (in thousands):
|
||||||||||||||||||||
|
Basic:
|
15,683
|
15,671
|
19,940
|
21,718
|
21,862
|
|||||||||||||||
|
Diluted:
|
17,199
|
15,671
|
19,940
|
21,718
|
21,862
|
|||||||||||||||
|
As of December 31,
|
||||||||||||||||
|
2013
|
2014
|
2015
|
2016
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Consolidated balance sheet data:
|
||||||||||||||||
|
Cash and cash equivalents and short-term bank deposits
|
$
|
9,553
|
$
|
64,853
|
$
|
45,768
|
$
|
30,029
|
||||||||
|
Working capital, net(5)
|
10,042
|
64,600
|
45,189
|
28,232
|
||||||||||||
|
Total assets
|
14,826
|
71,121
|
52,523
|
35,764
|
||||||||||||
|
Total non-current liabilities
|
32,607
|
24,353
|
23,847
|
22,614
|
||||||||||||
|
Total shareholders’ equity (deficit)
|
(19,804
|
)
|
42,871
|
23,470
|
7,770
|
|||||||||||
|
(1)
|
Includes share-based compensation expense as follows:
|
|
Year Ended December 31,
|
||||||||||||||||||||
|
2012
|
2013
|
2014
|
2015
|
2016
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Cost of revenues
|
$
|
—
|
$
|
—
|
$
|
763
|
$
|
372
|
$
|
504
|
||||||||||
|
Research and development
|
124
|
315
|
657
|
511
|
752
|
|||||||||||||||
|
Selling and marketing
|
—
|
24
|
1,430
|
669
|
765
|
|||||||||||||||
|
General and administrative
|
210
|
192
|
1,977
|
1,107
|
1,150
|
|||||||||||||||
|
Share-based compensation expenses from continuing operations
|
334
|
531
|
4,827
|
2,659
|
3,171
|
|||||||||||||||
|
Discontinued operation (3)
|
30
|
76
|
—
|
—
|
—
|
|||||||||||||||
|
Total share-based compensation expenses
|
$
|
364
|
$
|
607
|
$
|
4,827
|
$
|
2,659
|
$
|
3,171
|
||||||||||
| (2) |
Research and development expenses, net is presented net of participation by the U.S. Biomedical Advanced Research and Development Authority (“BARDA”) and others and net of the change in the fair value of the liability associated with government grants from the Israeli Innovation Authority (IIA) (formerly the Office of Chief Scientist). Participation by others totaled $2.2 million for the year ended December 31, 2012, and had no effect on subsequent years. The effect of the participation by IIA totaled $0.1 million, $0.9 million, $0.7 million, $1.3 million and $2.1 million for the years ended December 31, 2012, 2013, 2014, 2015, and 2016, respectively. The effect of the participation by BARDA totaled $0.8 million and $5.6 million for the years ended December 31, 2015 and 2016, respectively. See “ITEM 5.B.
Liquidity and Capital Resources
” for more information.
|
| (3) |
Discontinued operation consists of revenues and expenses related to our exclusive, worldwide license for the development, production and commercialization of the PolyHeal Product, which expired following the termination of our collaboration with Teva. We account for our discontinued operation in accordance with IFRS accounting standard 5, “Non-current Assets Held for Sale and Discontinued Operations.” See “ITEM 5.A. Operating Results—Discontinued operation” for more information.
|
| (4) |
Basic and diluted net income (loss) per ordinary share is computed based on the basic and diluted weighted average number of ordinary shares outstanding during each period. For additional information, see Note 21 to our consolidated annual financial statements included elsewhere in this report.
|
|
(5)
|
Working capital, net is defined as total current assets minus total current liabilities.
|
| B. |
Capitalization and Indebtedness
|
| C. |
Reasons for the Offer and Use of Proceeds
|
| D. |
Risk Factors
|
| · |
regulators may not authorize us to conduct a clinical trial within a country or at a prospective trial site or may change the design of a study;
|
| · |
delays may occur in reaching agreement on acceptable clinical trial terms with regulatory authorities or prospective sites, or obtaining institutional review board approval;
|
| · |
our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional trials or to abandon strategic projects;
|
| · |
the number of patients required for our clinical trials may be larger than we anticipate, enrollment in our clinical trials may be slower or more difficult than we expect, or patients may not participate in necessary follow-up visits to obtain required data, any of which would result in significant delays in our clinical testing process;
|
| · |
our third-party contractors, such as a research institute, may fail to comply with regulatory requirements or meet their contractual obligations to us;
|
| · |
we may be forced to suspend or terminate our clinical trials if the participants are being exposed, or are thought to be exposed, to unacceptable health risks or if any participant experiences an unexpected serious adverse event;
|
| · |
regulators or institutional review boards may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements;
|
| · |
undetected or concealed fraudulent activity by a clinical researcher, if discovered, could preclude the submission of clinical data prepared by that researcher, lead to the suspension or substantive scientific review of one or more of our marketing applications by regulatory agencies, and result in the recall of any approved product distributed pursuant to data determined to be fraudulent;
|
| · |
the cost of our clinical trials may be greater than we anticipate;
|
| · |
an audit of preclinical or clinical studies by regulatory authorities may reveal noncompliance with applicable protocols or regulations, which could lead to disqualification of the results and the need to perform additional studies; and
|
| · |
delays may occur in obtaining our clinical materials.
|
| · |
the market acceptance or demand for NexoBrid, EscharEx or any of our pipeline products, if approved;
|
| · |
the ability to set a price that we believe is fair for NexoBrid, EscharEx or any of our pipeline products, if approved;
|
| · |
our ability to generate revenues and achieve or maintain profitability;
|
| · |
the level of taxes that we are required to pay; and
|
| · |
the availability of capital.
|
| · |
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs;
|
| · |
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13.0% of the average manufacturer price for branded and generic drugs, respectively;
|
| · |
addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
|
| · |
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D;
|
| · |
extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations;
|
| · |
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level beginning in 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability;
|
| · |
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
| · |
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and
|
| · |
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research.
|
| · |
the willingness of physicians, burn care teams and hospital administrators to administer our products and their acceptance as part of the medical department routine;
|
| · |
the consent of hospitals to fund/purchase NexoBrid or obtain third-party coverage or reimbursement for our products;
|
| · |
the ability to offer NexoBrid, EscharEx and our pipeline products for sale at an attractive value;
|
| · |
the efficacy and potential advantages of NexoBrid, EscharEx and our pipeline products relative to current standard of care;
|
| · |
the prevalence and severity of any side effects; and
|
| · |
the efficacy, potential advantages and timing of introduction to the market of alternative treatments.
|
| · |
delay, scale back or discontinue the development, manufacturing scale-up or commercialization of NexoBrid, EscharEx or our pipeline products;
|
| · |
seek corporate partners for NexoBrid, EscharEx or one or more of our pipeline products on terms that are less favorable than might otherwise be available; or
|
| · |
relinquish or license on unfavorable terms, our rights to NexoBrid, EscharEx or our pipeline products that we otherwise would seek to develop or commercialize ourselves.
|
| · |
accelerate our clinical development activities, particularly with respect to our NexoBrid pediatric clinical trial in severe burns in Europe, our continued clinical development of EscharEx for the debridement of chronic and other hard-to-heal wounds and our clinical trials for our product candidate for the treatment of connective tissue disorders or other indications;
|
| · |
continue to operate our sales, marketing and distribution infrastructure in Europe and thereafter in the United States to commercialize NexoBrid and any pipeline products for which we obtain marketing approval;
|
| · |
further scale-up the manufacturing process for NexoBrid;
|
| · |
seek regulatory and marketing approvals for NexoBrid and any pipeline product that successfully completes clinical trials;
|
| · |
initiate additional preclinical, clinical or other studies for NexoBrid, EscharEx and our pipeline products and seek to identify and validate new products;
|
| · |
acquire rights to other product candidates and technologies;
|
| · |
change or add suppliers;
|
| · |
maintain, expand and protect our intellectual property portfolio;
|
| · |
attract and retain skilled personnel; and
|
| · |
experience any delays or encounter issues with any of the above.
|
| · |
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market or voluntary or mandatory product recalls;
|
| · |
fines, warning letters or holds on clinical trials;
|
| · |
harm to our reputation, reduced demand for our products and loss of market acceptance;
|
| · |
refusal by the applicable regulatory authority to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals;
|
| · |
product seizure or detention, or refusal to permit the import or export of products; and
|
| · |
injunctions or the imposition of civil or criminal penalties.
|
| · |
the Federal Acquisition Regulations (“FAR”) and agency-specific regulations supplemental to the FAR, which comprehensively regulate the procurement, formation, administration and performance of government contracts;
|
| · |
business ethics and public integrity obligations, which govern conflicts of interest and the hiring of former government employees, restrict the granting of gratuities and funding of lobbying activities and include other requirements such as the Anti-Kickback Statute and Foreign Corrupt Practices Act;
|
| · |
export and import control laws and regulations; and
|
| · |
laws, regulations and executive orders restricting the use and dissemination of information classified for national security purposes and the exportation of certain products and technical data.
|
| · |
any of our present or future patents or patent claims or other intellectual property rights will not lapse or be invalidated, circumvented, challenged or abandoned;
|
| · |
our intellectual property rights will provide competitive advantages or prevent competitors from making or selling competing products;
|
| · |
our ability to assert our intellectual property rights against potential competitors or to settle current or future disputes will not be limited by our agreements with third parties;
|
| · |
any of our pending or future patent applications will be issued or have the coverage originally sought;
|
| · |
our intellectual property rights will be enforced in jurisdictions where competition may be intense or where legal protection may be weak; or
|
| · |
we will not lose the ability to assert our intellectual property rights against, or to license our technology to, others and collect royalties or other payments.
|
| · |
actual or anticipated variations in our and our competitors’ results of operations and financial condition;
|
| · |
market acceptance of our products;
|
| · |
general economic and market conditions and other factors, including factors unrelated to our operating performance;
|
| · |
the mix of products that we sell and related services that we provide;
|
| · |
changes in earnings estimates or recommendations by securities analysts, if our ordinary shares continue to be covered by analysts;
|
| · |
publication of the results of preclinical or clinical trials for NexoBrid, EscharEx or any of our pipeline products;
|
| · |
failure by us to achieve a publicly announced milestone;
|
| · |
delays between our expenditures to develop and market new or enhanced products and the generation of sales from those products;
|
| · |
development of technological innovations or new competitive products by others;
|
| · |
announcements of technological innovations or new products by us;
|
| · |
regulatory developments and the decisions of regulatory authorities as to the marketing of our current products or the approval or rejection of new or modified products;
|
| · |
developments concerning intellectual property rights, including our involvement in litigation;
|
| · |
changes in our expenditures to develop, acquire or license new products, technologies or businesses;
|
| · |
changes in our expenditures to promote our products;
|
| · |
our sale or proposed sale, or the sale by our significant shareholders, of our ordinary shares or other securities in the future;
|
| · |
changes in key personnel;
|
| · |
success or failure of our research and development projects or those of our competitors; and
|
| · |
the trading volume of our ordinary shares.
|
| A. |
History and Development of the Company
|
| B. |
Business Overview
|
| · |
The extent of the surface the burn occupies is usually referred to as percent of total body surface area (“TBSA”). A burn on an adult’s entire palm would generally amount to 1% TBSA, and the average hospitalized patient has a burn covering approximately 10% TBSA. Burns covering more than 15-20% TBSA usually require hospitalization and may result in dehydration, shock and increased risk of mortality.
|
| · |
The depth of the burn, referred to in terms of “degree” is generally classified into four categories:
|
| ○ |
Superficial or first degree burns.
Such burns do not penetrate the basal membrane and usually heal naturally.
|
| ○ |
Dermal/partial thickness or second degree burns.
Such burns are characterized by varying amounts of damaged dermis and can be further subdivided into superficial and deep partial-thickness burns. Superficial partial-thickness burns may heal spontaneously after removal of the covering thin eschar. Conversely, deep partial-thickness burns are often difficult for physicians to accurately diagnose before eschar removal and may progress and transform into full-thickness burns if not debrided in a timely manner, depending on the magnitude of latent tissue death of the surrounding skin.
|
| ○ |
Full thickness or third degree burns.
Such burns are characterized by death of the entire dermal tissue down to the subcutaneous fat and must be debrided and treated by autografting, which is the process of harvesting skin from healthy donor sites on a patient’s body and transplanting it on the post-debridement, clean wound bed.
|
| ○ |
Fourth degree burns.
Such burns, which are rare, extend beyond the subcutaneous fat tissue into the underlying structures, such as muscle or bone, and also require debridement and further substantial treatment.
|
| · |
Other factors include the age of the victim, the body part where the burn occurred and any co-morbidities of the patient. For example, some patients may require hospitalization regardless of the TBSA or degree of the burn, such as children, the elderly or victims with burns to the extremities, joints or head/neck area or with co-morbidities such as smoke inhalation, diabetes or obesity.
|
| · |
the prevention of local infection, sepsis (a systemic inflammatory response caused by severe infection) and additional damage to surrounding viable tissue; and
|
| · |
the initiation of the body’s healing process and scar prevention.
|
| · |
Surgical debridement
|
| ○ |
Surgical debridement predominantly includes tangential excision, a procedure in which a surgeon amputates the entire dead tissue mass, layer after layer, down to healthy, viable tissue. The excision is extended into healthy intact tissue to make sure that no trace of the eschar remains, resulting in up to an estimated 30-50% of healthy tissue being excised during this procedure. Other methods include dermabrasion, in which a mechanically powered, hand-held rotating abrading cylinder is used to slowly scrape off tissue, and hydro surgery, in which a high-pressure flow of water abrades the tissue. These alternative methods have attempted to limit the trauma associated with tangential excision, but entail spray of contaminated eschar or take a significantly longer time to complete than tangential excision.
|
| ○ |
The benefits of surgical eschar removal are that it is usually fast and effective. Disadvantages include the significant trauma of the procedure, associated blood loss, risk of surgery in delicate areas of the body such as hands, added costs, and, most importantly, the loss of viable tissue that necessitates additional surgical procedures for harvesting skin from healthy donor sites and autografting.
|
| ○ |
Due to the disadvantages of surgery in extensive burns some surgeons limit their debriding surgery to only a part of the affected area in a single session (15-30% TBSA in most centers), thus delaying full debridement by days. After several days, complications related to eschar contamination may begin and some of the benefits of the earlier debridement may not be realized. On the other hand, when excising burns immediately, all suspected necrotic tissue will be excised, inevitably resulting in over-excision, especially in “indeterminate” burns, as after surgical excision, the remaining skin often no longer has any spontaneous healing potential and will heal only by autografting.
|
| · |
Non-surgical debridement
|
| ○ |
Non-surgical debridement includes many different treatment options that do not require direct surgical removal of the skin to remove eschar. With non-surgical debridement, the eschar is naturally, but slowly, removed by contaminant microorganisms, tissue autolysis, or self-decomposition, and the inflammatory process that may lead to serious local and systemic complications. In seeking to facilitate such natural processes, topical medication, anti-microbial agents, enzymes and biological/chemical applications are often applied onto the eschar.
|
| ○ |
The benefits of this approach are that it is non-surgical, reduces trauma to the patient and is easier to apply. Disadvantages include numerous dressing changes and mechanical scraping with limited debridement efficacy. This prolongs the eschar removal process, which may lead to death of the tissue surrounding the initial burn wound, causing partial-thickness wounds to transform into full-thickness wounds and forming granulation tissue that may develop into heavy scars.
|
| · |
Diabetic foot ulcers
. Diabetes can lead to a reduction in blood flow, which can cause patients to lose sensation in their feet and may prevent them from noticing injuries, sometimes leading to the development of DFUs, which are open sores or ulcers on the feet that may take several weeks to heal, if ever. In the United States alone, over 23 million people, or approximately 8% of the population, suffer from diabetes, a chronic, life-threatening disease. Based on our comprehensive market research study on EscharEx that involved more than 200 healthcare professionals in the U.S. and Europe, every year, in the United States alone, over 900,000 people develop a DFU and over 600,000 undergo debridement of DFUs.
|
| · |
Venous leg ulcers
. VLUs develop as a result of vascular insufficiency, or the inability for the vasculature of the leg to return blood back toward the heart properly. Based on our comprehensive market research study on EscharEx that involved more than 200 healthcare professionals in the U.S. and Europe, in the United States alone, affect approximately 1.25 million people per year, out of which over 650,000 undergo debridement of VLUs. These ulcers usually form on the sides of the lower leg, above the ankle and below the calf, and are slow to heal and often recur if preventative steps are not taken. The risk of VLUs can increase as a result of a blood clot forming in the deep veins of the legs, obesity, smoking, lack of physical activity or work that requires many hours of standing.
|
| · |
Pressure ulcers.
Pressure ulcers form as a result of pressure sores, or bed sores, which are injuries to the skin or the tissue beneath the skin. Constant pressure on an area of skin reduces blood supply to the area and over time can cause the skin to break down and form an open ulcer. These often occur in patients who are hospitalized or confined to a chair or bed, and usually form over bony areas, where there is little cushion between the bone and the skin, such as lower parts of the body. Annually, 2.5 million pressure ulcers are treated in the United States in acute care facilities alone.
|
| · |
Surgical/traumatic wounds.
Surgical wounds form as a result of various types of surgical procedures such as investigative or corrective, minor or major, open (traditional) or minimal access surgery, elective or emergency, and incisions (simple cuts) or excision (removal of tissue), among others. Traumatic wounds form as a result of cuts, lacerations or puncture wounds, which have caused damage to the skin and underlying tissue. Severe traumatic wounds may require surgical intervention to close the wound and stabilize the patient. Surgical/traumatic hard-to-heal wounds develop for various reasons, such as local surgical complications, suboptimal closure techniques, presence of foreign materials, exposed bones or tendons and infection. In the United States, millions receive post-surgical wound care annually.
|
| · |
Dupuytren’s disease:
a condition where one or more fingers are permanently flexed, caused by the formation of scar-like tissues below the palmar skin (Palmar Fascia), forming hard “cords” that freeze the fingers in non-functional flexion contraction. This condition affects approximately 6.2 million people in the United States alone.
|
| · |
Peyronie’s disease:
the development of scar-like tissue, similar to Dupuytren’s cords in the shaft of the penis, causing pain and distortion on erection, preventing intercourse. Peyronie’s disease is typically caused by trauma and affects men over 50 years old. Surgical treatment may be an option in some cases, but can cause complications and may result in a shortening and even greater distortion of the penis. Approximately 3.7% to 7.1% of the male population above the age of 50 suffers from Peyronie’s disease in the United States and approximately 3.2% of such age group suffer from the disease in Europe.
|
| · |
Frozen shoulder syndrome:
a disorder that causes the smooth tissues of the shoulder capsule to become thick, stiff and inflamed, affecting approximately 2% to 5% of the worldwide population and 10% to 20% of people with diabetes according to industry sources.
|
| · |
Excessive/unaesthetic scars:
A scar is a mark on the skin which is formed due to infection, injury, surgery, inflammation of tissue, burns, and acne. Scars can be of various sizes, shapes, and colors, depending on the age of the scar, the site of the scar and family history. Scar formation is unpredictable and varies from person to person. Excessive scarring can have unpleasant physical, aesthetic, psychological and social consequences. Estimates indicate that each year around 100 million people in the developed world acquire scars following elective surgery and surgery for trauma. Of these, approximately 15% have excessive or unaesthetic scars.
|
|
Trial 1
|
Trial 2
|
Trial 3
|
Trial 4
|
Trial 5
|
Trial 6
|
Trial 7
|
|
|
Study Type
|
• Retrospective Phase 2
•
Investigator initiated
|
•
Dose range Phase 2
|
• Prospective Phase 2
•
IND/FDA
|
•
Phase 2
•
IND/FDA
|
•
Phase 3
•
EMA
|
•
Phase 3b
•
EMA
|
•
Phase 2
•
EMA
|
|
Design
|
•
Data collected from files of patients treated with NexoBrid
|
•
Parallel, controlled, observer- blind, randomized, single-center
|
•
Parallel, controlled, observer- blind, three-arm, randomized, multi- center
|
•
Parallel, controlled, open label, three-arm, randomized, single- center
|
•
Parallel, controlled, open label, two-arm, randomized, multi-center
|
•
Parallel, controlled, blinded, two-arm, multi-center
|
• Open label,
single-arm, multi-center |
|
Main Objectives
|
•
Safety
•
Efficacy
|
• Comparison of efficacy and safety
|
•
Safety
•
Efficacy
|
•
Safety
|
•
Safety
•
Efficacy
|
•
Long-term scar assessment
•
Quality of life
|
•
Safety and pharmacokinetics
•
Efficacy
|
|
Wound Types
|
•
Deep partial/full thickness thermal burns
|
•
Deep partial/full thickness thermal burns
|
•
Deep partial/full thickness thermal burns
|
•
Deep partial/full thickness thermal burns
|
•
Deep partial/full thickness thermal burns
|
•
Scar formation
|
• Deep partial/full thickness thermal burns
|
|
Number of Patients
|
•
154
|
•
20
|
•
140
|
•
30
|
•
182
|
•
89
|
•
36
|
|
Study Length
|
•
1985-2000
|
•
2002-2005
|
•
2003-2004
|
•
2006-2007
|
•
2006-2009
|
•
2011
|
•
2009-2015
|
|
Location
|
•
Israel
|
•
Israel
|
•
International
|
•
United States
|
•
International
|
•
International
|
•
International
|
| (1) |
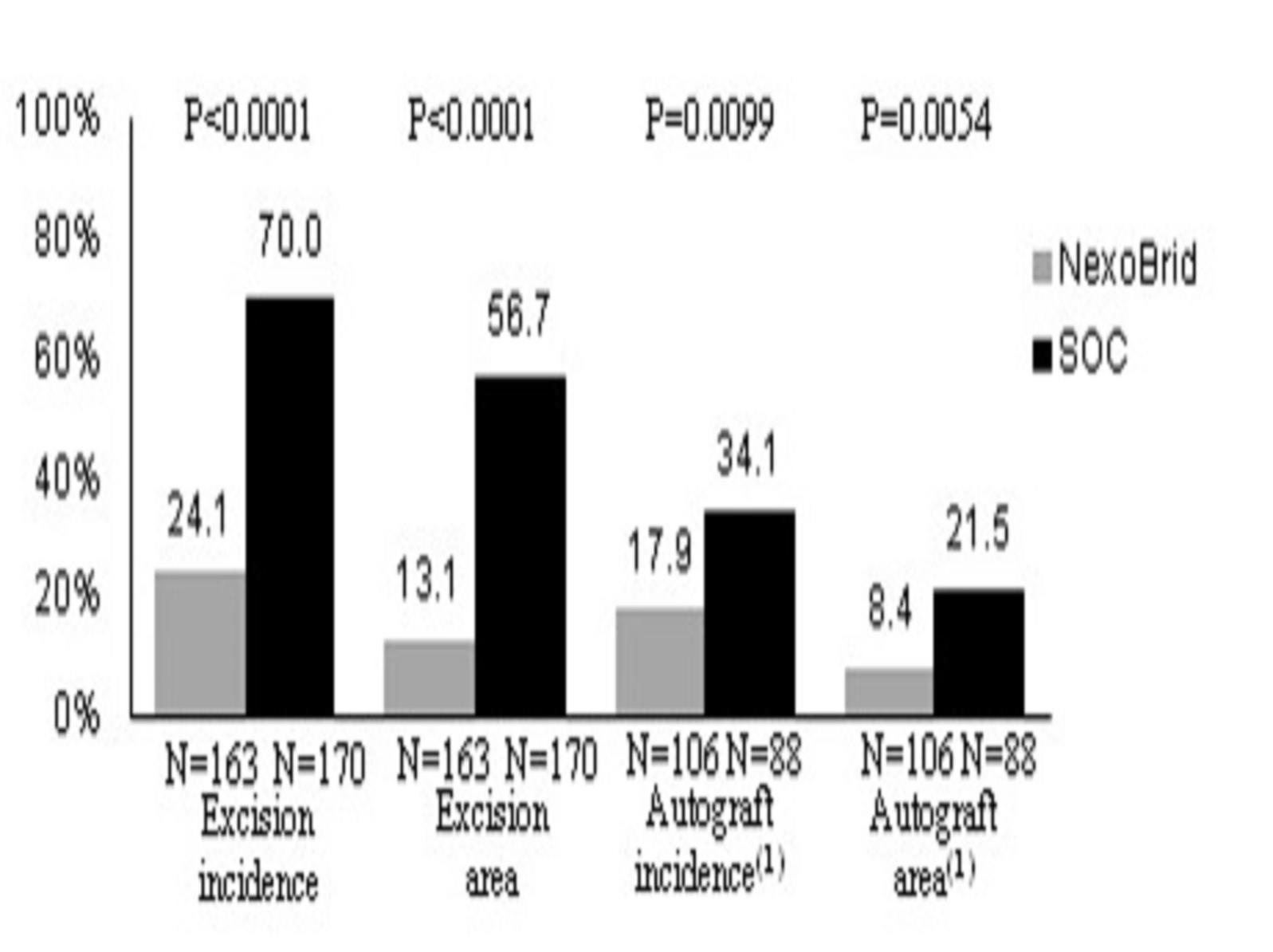
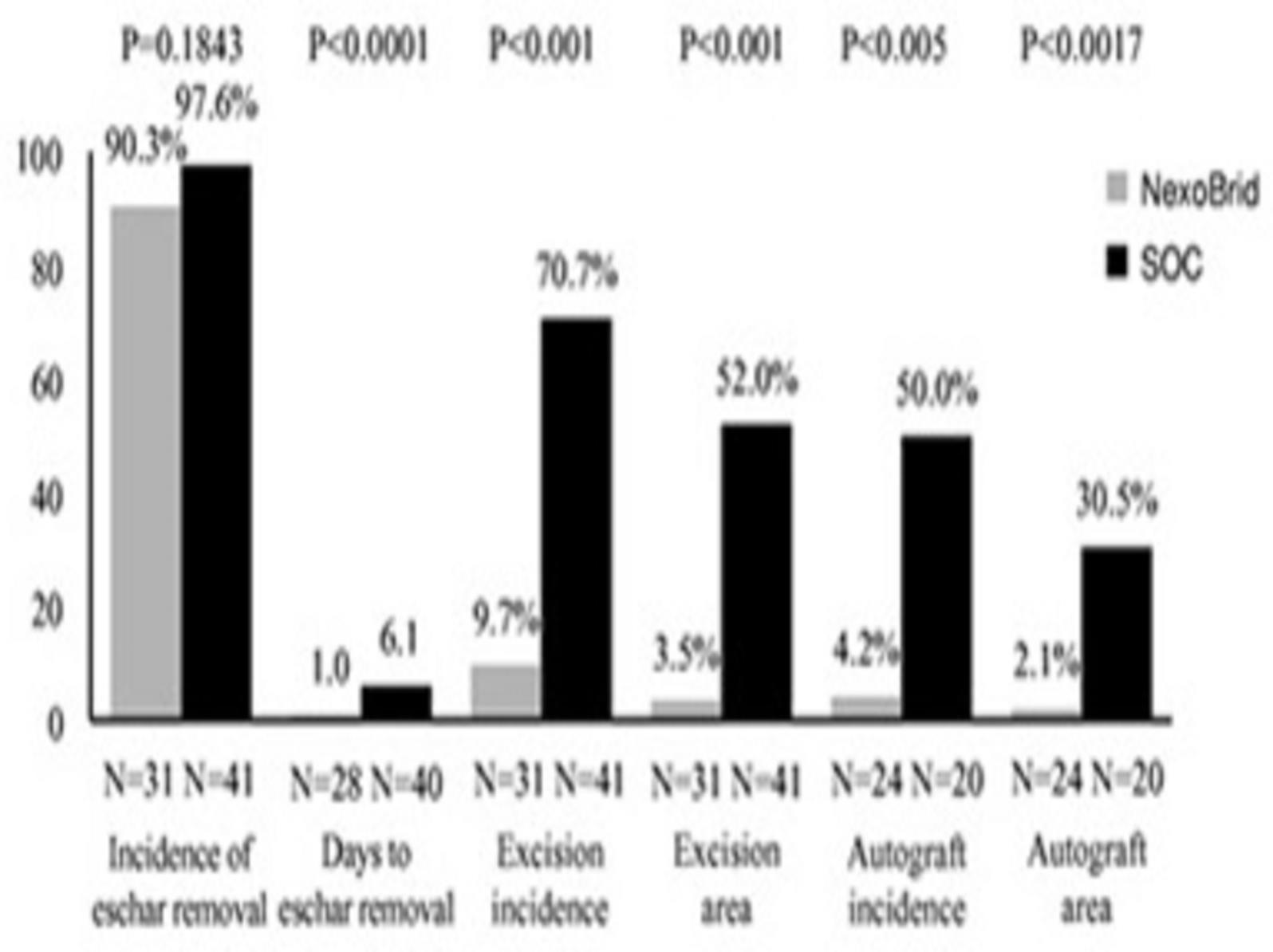
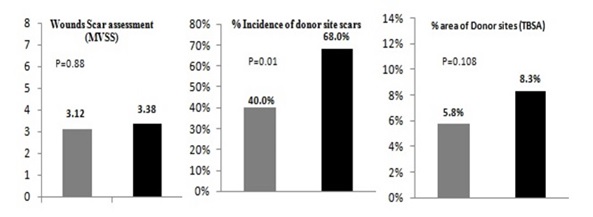
Only deep partial-thickness wounds are presented, as full-thickness wounds always require autografting due to the lack of viable dermis, regardless of the technique used to remove the eschar.
|
| · |
laboratory tests, animal studies and formulation studies all performed in accordance with the applicable E.U. GLP or GMP regulations;
|
| · |
submission to the relevant national authorities of a clinical trial application (“CTA”), which must be approved before human clinical trials may begin;
|
| · |
performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication;
|
| · |
submission to the relevant competent authorities of a marketing authorization application (“MAA”), which includes the data supporting preclinical and clinical safety and efficacy as well as detailed information on the manufacture and composition and control of the product development and proposed labeling as well as other information;
|
| · |
inspection by the relevant national authorities of the manufacturing facility or facilities and quality systems (including those of third parties) at which the product is produced, to assess compliance with strictly enforced cGMP;
|
| · |
potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and
|
| · |
review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product.
|
| · |
Phase 1 (Most typical kind of study: Human Pharmacology);
|
| · |
Phase 2 (Most typical kind of study: Therapeutic Exploratory);
|
| · |
Phase 3 (Most typical kind of study: Therapeutic Confirmatory); and
|
| · |
Phase 4 (Variety of Studies: Therapeutic Use).
|
| · |
medicines that have been authorized for marketing in the European Union with the results of PIP studies included in the product information are eligible for an extension of their patent protection by six months. This is the case even when the studies’ results are negative;
|
| · |
for orphan medicines, such as NexoBrid, the incentive is an additional two years of market exclusivity instead of one;
|
| · |
scientific advice and protocol assistance at the EMA are free of charge for questions relating to the development of medicines for children; and
|
| · |
medicines developed specifically for children that are already authorized, but are not protected by a patent or supplementary protection certificate, can apply for a pediatric use marketing authorization (“PUMA”). If a PUMA is granted, the product will benefit from 10 years of market protection as an incentive.
|
| · |
Mutual recognition procedure.
If an authorization has been granted by one member state, or the Reference Member State, an application may be made for mutual recognition in one or more other member states, or the Concerned Member State(s).
|
| · |
Decentralized procedure.
The decentralized procedure may be used to obtain a marketing authorization in several European member states when the applicant does not yet have a marketing authorization in any country.
|
| · |
National procedure.
Applicants following the national procedure will be granted a marketing authorization that is valid only in a single member state. Furthermore, this marketing authorization is not based on recognition of another marketing authorization for the same product awarded by an assessment authority of another member state. If marketing authorization in only one member state is preferred, an application can be filed with the national competent authority of a member state. The national procedure can also serve as the first phase of a mutual recognition procedure.
|
| · |
completion of laboratory tests, animal studies and formulation studies in compliance with the FDA’s GLP or GMP regulations, as applicable;
|
| · |
submission to the FDA of an investigational new drug application (“IND”), which must become effective before clinical trials may begin;
|
| · |
approval by an independent institutional review board (“IRB”) at each clinical site before each trial may be initiated;
|
| · |
performance of adequate and well-controlled clinical trials in accordance with GCP to establish the safety and efficacy of the product for each indication;
|
| · |
preparation and submission to the FDA of a BLA or supplemental BLA;
|
| · |
satisfactory completion of an FDA advisory committee review, if applicable;
|
| · |
satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMP requirements, and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; and
|
| · |
payment of user fees and FDA review and approval of the BLA.
|
| Phase 1: |
The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
|
| Phase 2: |
The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
|
| Phase 3: |
The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
|
| · |
increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%;
|
| · |
requires collection of rebates for drugs paid by Medicaid managed care organizations; and
|
| · |
imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs.
|
| · |
the federal healthcare Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid;
|
| · |
the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
| · |
the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
| · |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
| · |
the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
|
| · |
the federal physician payment transparency requirements under the Affordable Care Act require certain manufacturers of drugs, devices and medical supplies to report to Centers for Medicare & Medicaid Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests; and
|
| · |
analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers.
|
| C. |
Organizational Structure
|
| D. |
Property, Plants and Equipment
|
| A. |
Operating Results
|
| · |
the scope, rate of progress and expense of our research and development activities;
|
| · |
preclinical results;
|
| · |
clinical trial results;
|
| · |
the terms and timing of regulatory approvals;
|
| · |
the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; and
|
| · |
the ability to market, commercialize and achieve market acceptance for NexoBrid or any other product candidate that we may develop in the future.
|
|
Years Ended December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Consolidated statements of operations data:
|
||||||||||||
|
Revenues
|
$
|
259
|
$
|
601
|
$
|
1,558
|
||||||
|
Cost of revenues
|
2,785
|
2,519
|
2,158
|
|||||||||
|
Gross loss
|
(2,526
|
) |
(1,918
|
) |
(600
|
) | ||||||
|
Operating expenses:
|
||||||||||||
|
Research and development, gross
|
6,054
|
8,139
|
14,779
|
|||||||||
|
Participation by IIA and BARDA
|
(705
|
) |
(2,118
|
) |
(7,711
|
) | ||||||
|
Research and development, net of participations
|
5,349
|
6,021
|
7,068
|
|||||||||
|
Selling and marketing
|
8,829
|
9,284
|
8,403
|
|||||||||
|
General and administrative
|
4,723
|
4,004
|
4,084
|
|||||||||
|
Operating loss
|
(21,427
|
) |
(21,227
|
) |
(20,155
|
) | ||||||
|
Financial income
|
4,665
|
1,052
|
2,166
|
|||||||||
|
Financial expense
|
(2,113
|
) |
(1,496
|
) |
(896
|
) | ||||||
|
Loss from continuing operations
|
(18,875
|
) |
(21,671
|
) |
(18,885
|
) | ||||||
|
Loss from discontinued operation
|
—
|
(417
|
) |
—
|
||||||||
|
Net loss
|
$
|
(18,875
|
) |
$
|
(22,088
|
) |
$
|
(18,885
|
) | |||
| B. |
Liquidity and Capital Resources
|
|
Issuance
of Ordinary Shares and Warrants |
Net Loans
from Shareholders |
Government
Grants and BARDA Funding, net |
Total
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Year ended December 31, 2016
|
$
|
7
|
$
|
—
|
$
|
6,466
|
$
|
6,473
|
||||||||
|
Year ended December 31, 2015
|
26
|
—
|
$
|
1,552
|
1,578
|
|||||||||||
|
Year ended December 31, 2014
|
72,130
|
—
|
345
|
72,475
|
||||||||||||
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Net cash provided by (used in):
|
||||||||||||
|
Continuing operating activities
|
$
|
(16,493
|
)
|
$
|
(19,601
|
)
|
$
|
(16,445
|
)
|
|||
|
Continuing investing activities
|
(37,154
|
)
|
36,046
|
1,816
|
||||||||
|
Continuing financing activities
|
72,475
|
778
|
907
|
|||||||||
| · |
Fair value of our ordinary shares
. After March 20, 2014, the date our ordinary shares began trading on NASDAQ, the grant date fair value for equity-based awards is based on the closing price of our ordinary shares on NASDAQ on the date of grant and fair value for all other purposes related to share-based awards is the closing price of our ordinary shares on NASDAQ on the relevant date.
|
| · |
Volatility
. The expected share price volatility was based on the historical equity volatility of the ordinary shares of comparable companies that are publicly traded.
|
| · |
Early exercise factor
. Since adequate historical experience is not available to provide a reasonable estimate, the early exercise factor is determined based on peer group imperial studies.
|
| · |
Risk-free rate
. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the contractual life of the options.
|
| · |
Expected dividend yield
. We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
|
| C. |
Research and Development, Patents and Licenses, etc.
|
| D. |
Trend Information
|
| E. |
Off-Balance Sheet Arrangements
|
| F. |
Contractual Obligations
|
|
Payments Due by Period
|
||||||||||||||||
|
Total
|
2017
|
2018
|
2019 and
thereafter
|
|||||||||||||
|
(in thousands
)
|
||||||||||||||||
|
Operating lease obligations(1)
|
$
|
1,107
|
$
|
862
|
$
|
159
|
$
|
86
|
||||||||
| (1) |
Operating lease obligations consist of payments pursuant to lease agreements for office and laboratory facilities, as well as lease agreements for 19 vehicles, which generally run for a period of three years.
|
| A. |
Directors and Senior Management
|
|
Name
|
Age
|
Position
|
||
|
Executive Officers
|
||||
|
Gal Cohen
|
44
|
President and Chief Executive Officer
|
||
|
Sharon Malka
|
45
|
Chief Financial and Operations Officer
|
||
|
Lior Rosenberg M.D.
|
71
|
Chief Medical Technology Officer
|
||
|
Ety Klinger Ph.D.
|
55
|
Chief Research and Development Officer
|
||
|
Carsten Henke
|
51
|
Chief Commercial Officer EU
|
||
|
Yaron Meyer
|
38
|
General Counsel and Corporate Secretary
|
||
|
Directors
|
||||
|
Aharon Yaari
|
65
|
Chairman of the Board of Directors
|
||
|
Ofer Gonen
|
43
|
Director
|
||
|
Marian Gorecki Ph.D. (1)(2)(3)
|
76
|
Director
|
||
|
Meron Mann (3)
|
65
|
Director
|
||
|
Sarit Firon (1)(2)(3)(4)
|
50
|
Director
|
||
|
Abraham Havron (1)(2)(3)(4)
|
69
|
Director
|
| (1) |
Member of our audit committee.
|
| (2) |
Member of our compensation committee.
|
| (3) |
Independent director under the rules of the NASDAQ Stock Market.
|
| (4) |
External director under the Companies Law.
|
| B. |
Compensation
|
|
Name and Position
|
Salary & Social
Benefits
(1)
|
Bonus
|
Share-Based
Payment (2)
|
Other
Compensation (3)
|
Total
|
|||||||||||||||
|
(U.S. dollars) (4)
|
||||||||||||||||||||
|
Gal Cohen,
President and Chief Executive Officer
|
358,760
|
88,542
|
414,969
|
25,357
|
889,875
|
|||||||||||||||
|
Sharon Malka,
Chief Financial and Operations Officer
|
240,324
|
62,500
|
342,889
|
23,279
|
668,992
|
|||||||||||||||
|
Lior Rosenberg, M.D.,
Chief Medical Technology Officer
|
269,559
|
41,667
|
195,264
|
30,533
|
537,023
|
|||||||||||||||
|
Carsten Henke,
Chief Commercial Officer EU & Managing Director of MediWound Germany GmbH
|
264,776
|
70,784
|
225,040
|
34,286
|
594,886
|
|||||||||||||||
|
Ety Klinger,
Chief Research & Development Officer
|
204,401
|
19,531
|
195,112
|
18,149
|
437,193
|
|||||||||||||||
| (1) |
Represents the officer’s gross salary plus payment of mandatory social benefits made by the company on behalf of such officer. Such benefits may include, to the extent applicable to the executive, payments, contributions and/or allocations for savings funds (e.g., Managers’ Life Insurance Policy), education funds (referred to in Hebrew as “keren hishtalmut”), pension, severance, risk insurances (e.g., life or work disability insurance) and payments for social security.
|
| (2) |
Represents the equity-based compensation expenses recorded in the company’s consolidated financial statements for the year ended December 31, 2016 based on the options’ grant date fair value in accordance with accounting guidance for equity-based compensation.
|
| (3) |
Represents the other benefits to such officer, which includes either or both of (i) car expenses, including lease costs, gas and maintenance, provided to the officers and (ii) vacation benefits.
|
| (4) |
Converted (i) from NIS into U.S. dollars at the rate of 3.84 = U.S.$1.00, based on the average representative rate of exchange between the NIS and the U.S. dollar as reported by the Bank of Israel in the year ended December 31, 2016 and (ii) from Euro into U.S. dollars at the rate of Euro 1.11 = U.S$1.00, based on the average representative rate of exchange between the Euro and the U.S. dollar as reported by the Bank of Israel in the year ended December 31, 2016.
|
|
Name
|
Number of
Options
|
Grant Date
|
Exercise
Price |
Vested Options
as of
February
15, 2017
|
Expiration Date
|
|||||||||
|
Gal Cohen,
|
||||||||||||||
|
President and Chief Executive Officer
|
208,332
|
11/14/2006
|
$
|
2.63
|
208,332
|
11/13/2018
|
||||||||
|
45,600
|
1/15/2011
|
$
|
9.82
|
45,600
|
1/14/2021
|
|||||||||
|
152,000
|
12/24/2013
|
$
|
12.89
|
114,000
|
12/23/2023
|
|||||||||
|
70,000
|
1/28/2016
|
$
|
9.58
|
17,500
|
12/22/2025
|
|||||||||
|
Lior Rosenberg,
|
||||||||||||||
|
Chief Medical Technology Officer
|
76,000
|
12/24/2013
|
$
|
12.89
|
57,000
|
12/23/2023
|
||||||||
|
25,000
|
12/23/2015
|
$
|
9.58
|
6,250
|
12/22/2025
|
|||||||||
| C. |
Board Practices
|
| · |
such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding abstentions, to which we refer as a disinterested majority; or
|
| · |
the total number of shares voted by non-controlling shareholders and by shareholders who do not have a personal interest in the election of the external director against the election of the external director does not exceed 2% of the aggregate voting rights in the company.
|
| (i) |
his or her service for each such additional term is recommended by one or more shareholders holding at least 1% of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate voting rights in the company, subject to additional restrictions set forth in the Israeli Companies Law with respect to affiliations of external director nominee; or
|
| (ii) |
his or her service for each such additional term is recommended by the board of directors and is approved at a meeting of shareholders by the same majority required for the initial election of an external director (as described above).
|
| · |
an employment relationship;
|
| · |
a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships);
|
| · |
control; and
|
| · |
service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director was appointed as a director of the private company in order to serve as an external director following the initial public offering.
|
| · |
he or she meets the qualifications for being appointed as an external director, except for the requirement (i) that the director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed for trading outside of Israel) and (ii) for accounting and financial expertise or professional qualifications; and
|
| · |
he or she has not served as a director of the company for a period exceeding nine consecutive years. For this purpose, a break of less than two years in the service shall not be deemed to interrupt the continuation of the service.
|
| · |
oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors in accordance with Israeli law;
|
| · |
recommending the engagement or termination of the person filling the office of our internal auditor; and
|
| · |
recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors.
|
| · |
determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices;
|
| · |
determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and whether such transaction is extraordinary or material under the Israeli Companies Law) (see “—Approval of Related Party Transactions Under Israeli Law”);
|
| · |
establishing the approval process (including, potentially, the approval of the audit committee and conducting a competitive procedure supervised by the audit committee) for certain transactions with a controlling shareholder or in which a controlling shareholder has a personal interest;
|
| · |
where the board of directors approves the working plan of the internal auditor, examining such working plan before its submission to the board of directors and proposing amendments thereto;
|
| · |
examining our internal audit controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to fulfill his responsibilities;
|
| · |
examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment of our auditor; and
|
| · |
establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
|
| · |
the knowledge, skills, expertise and accomplishments of the relevant office holder;
|
| · |
the office holder’s roles and responsibilities and prior compensation agreements with him or her;
|
| · |
the relationship between the terms offered and the average compensation of the other employees of the company, including those employed through manpower companies;
|
| · |
the impact of disparities in salary upon work relationships in the company;
|
| · |
the possibility of reducing variable compensation at the discretion of the board of directors;
|
| · |
the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and
|
| · |
as to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
|
| · |
the link between variable compensation and long-term performance, which variable compensation shall, other than office holder who report to the CEO, be primarily based on measurable criteria;
|
| · |
the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation;
|
| · |
the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements;
|
| · |
the minimum holding or vesting period for variable, equity-based compensation; and
|
| · |
maximum limits for severance compensation.
|
| · |
recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years, other than following a company’s initial public offering, in which case such approval must occur within 5 years of the initial public offering);
|
| · |
recommending to the board of directors periodic updates to the compensation policy and assessing implementation of the compensation policy;
|
| · |
approving compensation terms of executive officers, directors and employees that require approval of the compensation committee;
|
| · |
determining whether the compensation terms of a chief executive officer nominee, which were determined pursuant to the compensation policy, will be exempt from approval of the shareholders because such approval would harm the ability to engage with such nominee; and
|
| · |
determining, subject to the approval of the board and under special circumstances, whether to override a determination of the company’s shareholders regarding certain compensation related issues.
|
| · |
the responsibilities set forth in the compensation policy;
|
| · |
reviewing and approving the granting of options and other incentive awards to the extent such authority is delegated by our board of directors; and
|
| · |
reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors.
|
| · |
a person (or a relative of a person) who holds 5% or more of the company’s outstanding shares or voting rights;
|
| · |
a person (or a relative of a person) who has the power to appoint a director or the general manager of the company;
|
| · |
an office holder (including a director) of the company (or a relative thereof); or
|
| · |
a member of the company’s independent accounting firm, or anyone on its behalf.
|
| · |
information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and
|
| · |
all other important information pertaining to any such action.
|
| · |
refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or personal affairs;
|
| · |
refrain from any activity that is competitive with the business of the company;
|
| · |
refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and
|
| · |
disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder.
|
| · |
a transaction other than in the ordinary course of business;
|
| · |
a transaction that is not on market terms; or
|
| · |
a transaction that may have a material impact on a company’s profitability, assets or liabilities.
|
| · |
at least a majority of the shares held by all shareholders who do not have a personal interest in the transaction and who are present and voting at the meeting approves the transaction, excluding abstentions; or
|
| · |
the shares voted against the transaction by shareholders who have no personal interest in the transaction and who are present and voting at the meeting do not exceed 2% of the voting rights in the company.
|
| · |
an amendment to the company’s articles of association;
|
| · |
an increase of the company’s authorized share capital;
|
| · |
a merger; or
|
| · |
the approval of related party transactions and acts of office holders that require shareholder approval.
|
| · |
financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria;
|
| · |
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding, and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction; and
|
| · |
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent.
|
| · |
a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
| · |
a breach of duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder; and
|
| · |
a financial liability imposed on the office holder in favor of a third party.
|
| · |
a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
| · |
a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder;
|
| · |
an act or omission committed with intent to derive illegal personal benefit; or
|
| · |
a fine or forfeit levied against the office holder.
|
| D. |
Employees
|
|
Department
|
As of December 31,
|
|||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Administrative
|
6
|
6
|
7
|
|||||||||
|
Research and development
|
14
|
16
|
22
|
|||||||||
|
Manufacturing
|
21
|
20
|
23
|
|||||||||
|
Sales and marketing
|
22
|
25
|
20
|
|||||||||
|
Total
|
63
|
67
|
72
|
|||||||||
| E. |
Share Ownership
|
| A. |
Major
Shareholders
|
| · |
each person or entity known by us to own beneficially more than 5% of our outstanding shares;
|
| · |
each of our directors and executive officers individually; and
|
| · |
all of our executive officers and directors as a group.
|
|
Name of Beneficial Owner
|
Number of
Shares Beneficially Held |
Percentage
of Class |
||||||
|
Directors and Executive Officers
|
||||||||
|
Aharon Yaari
|
*
|
*
|
||||||
|
Ofer Gonen
|
*
|
*
|
||||||
|
Marian Gorecki
|
*
|
*
|
||||||
|
Meron Mann
|
*
|
*
|
||||||
|
Sarit Firon
|
*
|
*
|
||||||
|
Abraham Havron
|
*
|
*
|
||||||
|
Gal Cohen (1)
|
385,432
|
1.7
|
%
|
|||||
|
Sharon Malka
|
*
|
*
|
||||||
|
Lior Rosenberg (2)
|
1,913,822
|
8.7
|
%
|
|||||
|
Carsten Henke
|
*
|
*
|
||||||
|
Ety Klinger
|
*
|
*
|
||||||
|
Yaron Meyer
|
*
|
*
|
||||||
|
All executive officers and directors as a group (12 persons)(3)
|
2,752,409
|
12.6
|
%
|
|||||
|
Principal Shareholders
|
||||||||
|
Clal Biotechnology Industries Ltd.(4)
|
9,389,555
|
42.8
|
%
|
|||||
|
Wellington Management Group LLP (5)
|
1,944,094
|
8.9
|
%
|
|||||
|
Migdal Insurance & Financial Holdings Ltd. (6)
|
1,685,947
|
7.7
|
%
|
|||||
| * |
Less than 1%.
|
| (1) |
Shares beneficially owned consist of 385,432 ordinary shares issuable upon exercise of outstanding options.
|
| (2) |
Shares beneficially owned consist of: (i) 140,367 ordinary shares held directly by Prof. Rosenberg; (ii) 63,250 ordinary shares issuable upon exercise of outstanding options held directly by Prof. Rosenberg; and (iii) 1,710,205 ordinary shares held by L.R. Research and Development Ltd. in trust for the benefit of Prof. Rosenberg. Prof. Rosenberg is the sole shareholder of L.R. Research and Development Ltd.
|
| (3) |
Shares beneficially owned consist of 1,856,029 ordinary shares held directly or indirectly by such executive officers and directors and 896,380 ordinary shares issuable upon exercise of outstanding options.
|
| (4) |
Shares beneficially owned consist of: (i) 8,208,973 ordinary shares held by Clal Life Sciences, LP, an Israeli limited partnership, whose managing partner is Clal Application Center Ltd., a wholly-owned subsidiary of CBI; and (ii) 1,180,582 ordinary shares held by CBI, as reported by CBI on a Schedule 13G/A filed on February 7, 2017. As reported on a Schedule 13G/A filed on February 14, 2017 by Access Industries Holdings LLC Access Industries Holdings LLC indirectly owns 100% of the outstanding shares of Clal Industries Ltd., which owns the majority of the outstanding shares of, and controls, CBI. The address of Clal Industries Ltd. is the Triangular Tower, 3 Azrieli Center, Tel Aviv 67023, Israel and the address of Access Industries Holdings LLC is c/o Access Industries Group, 730 Fifth Avenue, New York, New York 10019, United States.
|
| (5) |
Shares beneficially owned consist of 1,944,094 owned of record by clients of one or more investment advisers directly or indirectly owned by Wellington Management Group LLP. As reported on a Schedule 13G/A filed on February 9, 2017, of the 1,944,094 shares beneficially owned, Wellington Management Group LLP has shared voting power with respect to 1,769,527 ordinary shares and shared dispositive power with respect to all 1,944,094 ordinary shares. The address of Wellington Management Group is c/o Wellington Management Company LLP, 280 Congress Street, Boston, MA 02210.
|
| (6) |
Shares beneficially owned consist of: (i) 469,001 ordinary Shares held for members of the public through, among others, provident funds, mutual funds, pension funds and insurance policies, which are managed by subsidiaries of
Migdal Insurance & Financial Holdings Ltd (“Migdal”)
, according to the following segmentation: 959,864 ordinary Shares are held by Profit participating life assurance accounts 509,137 ordinary Shares are held by Provident funds and companies that manage provident funds, and 0 ordinary Shares are held by companies for the management of funds for joint investments in trusteeship, each of which subsidiaries operates under independent management and makes independent voting and investment decisions, and (ii) 216,946 are beneficially held for their own account (Nostro account), as reported by Migdal on a Schedule 13G filed on January 26, 2017. Migdal is a widely held public company listed on the Tel Aviv Stock Exchange. The address of Migdal is 4 Efal Street, Petah Tikva 49512, Israel.
|
| B. |
Related Party Transactions
|
| C. |
Interests of Experts and Counsel
|
| A. |
Consolidated Statements and Other Financial Information
|
| B. |
Significant Changes
|
| A. |
Listing Details
|
|
Low
|
High
|
|||||||
|
Annual:
|
||||||||
|
2016
|
$
|
4.25
|
$
|
9.28
|
||||
|
2015
|
$
|
5.00
|
$
|
10.47
|
||||
|
2014 (beginning March 20, 2014)
|
$
|
4.88
|
$
|
18.16
|
||||
|
Quarterly:
|
||||||||
|
First Quarter 2017 (through February 15, 2017)
|
$
|
4.55
|
$
|
6.30
|
||||
|
Fourth Quarter 2016
|
$
|
4.25
|
$
|
7.91
|
||||
|
Third Quarter 2016
|
$
|
6.32
|
$
|
8.58
|
||||
|
Second Quarter 2016
|
$
|
7.40
|
$
|
8.90
|
||||
|
First Quarter 2016
|
$
|
7.37
|
$
|
9.29
|
||||
|
Fourth Quarter 2015
|
$
|
7.28
|
$
|
10.47
|
||||
|
Third Quarter 2015
|
$
|
6.10
|
$
|
8.22
|
||||
|
Second Quarter 2015
|
$
|
5.00
|
$
|
7.50
|
||||
|
First Quarter 2015
|
$
|
6.60
|
$
|
9.15
|
||||
|
Most Recent Six Months:
|
||||||||
|
December 2016
|
$
|
4.25
|
$
|
5.50
|
||||
|
November 2016
|
$
|
5.00
|
$
|
6.75
|
||||
|
October 2016
|
$
|
6.25
|
$
|
7.91
|
||||
|
September 2016
|
$
|
7.15
|
$
|
8.58
|
||||
|
August 2016
|
$
|
6.32
|
$
|
8.40
|
||||
|
July 2016
|
$
|
7.39
|
$
|
8.00
|
||||
| B. |
Plan of Distribution
|
| C. |
Markets
|
| D. |
Selling Shareholders
|
| E. |
Dilution
|
| F. |
Expenses of the Issue
|
| A. |
Share Capital
|
| B. |
Articles of Association
|
| · |
amendments to our articles of association;
|
| · |
appointment or termination of our auditors;
|
| · |
appointment of external directors;
|
| · |
approval of certain related party transactions;
|
| · |
increases or reductions of our authorized share capital;
|
| · |
a merger; and
|
| · |
the exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management.
|
| C. |
Material Contracts
|
| D. |
Exchange Controls
|
| E. |
Taxation
|
| · |
amortization of the cost of purchased a patent, rights to use a patent, and know-how, which are used for the development or advancement of the Industrial Enterprise, over an eight-year period, commencing on the year in which such rights were first exercised;
|
| · |
under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies controlled by it; and
|
| · |
expenses related to a public offering are deductible in equal amounts over a three years period commencing on the year of the offering.
|
| · |
banks, financial institutions or insurance companies;
|
| · |
real estate investment trusts, regulated investment companies or grantor trusts;
|
| · |
dealers or traders in securities, commodities or currencies;
|
| · |
tax-exempt entities or organizations, including an “individual retirement account” or “Roth IRA” as defined in Section 408 or 408A of the Code, respectively;
|
| · |
certain former citizens or long-term residents of the United States;
|
| · |
persons that received our shares as compensation for the performance of services;
|
| · |
persons that holds our shares as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for U.S. federal income tax purposes;
|
| · |
partnerships (including entities classified as partnerships for U.S. federal income tax purposes) or other pass-through entities, or holders that will hold our shares through such an entity;
|
| · |
S corporations;
|
| · |
holders that acquired ordinary shares as a result of holding or owning our preferred shares;
|
| · |
U.S. Holders (as defined below) whose “functional currency” is not the U.S. dollar;
|
| · |
persons that are residents of ordinarily resident in or have a permanent establishment in a jurisdiction outside the United States; or
|
| · |
holders that own directly, indirectly or through attribution 10.0% or more of the voting power or value of our shares.
|
| · |
a citizen or individual resident of the United States;
|
| · |
a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia;
|
| · |
an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
|
| · |
a trust that (1) is subject to the primary supervision of a U.S. Court and one or more U.S. persons that have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
|
| · |
such gain is effectively connected with your conduct of a trade or business in the United States (or, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment or fixed base that such holder maintains in the United States); or
|
| · |
you are an individual and have been present in the United States for 183 days or more in the taxable year of such sale or exchange and certain other conditions are met.
|
| · |
at least 75% of its gross income is “passive income”; or
|
| · |
at least 50% of the average quarterly value of its total gross assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production of passive income.
|
| F. | Dividends and Paying Agents |
| G. |
Statement by Experts
|
| H. |
Documents on Display
|
| I. |
Subsidiary Information
|
|
Change in
|
Exchange Rate
|
|||||||
|
Period
|
Shekel against
the U.S. dollar (%) |
Euro against
the U.S. dollar (%) |
||||||
|
2012
|
2.3
|
2.0
|
||||||
|
2013
|
7.0
|
4.5
|
||||||
|
2014
|
(12.0
|
)
|
(11.8
|
)
|
||||
|
2015
|
(0.3
|
)
|
(10.4
|
)
|
||||
|
2016
|
1.5
|
(3.4
|
)
|
|||||
| · |
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
| · |
accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
| · |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
|
2015
|
2016
|
|||||||
|
Audit Fees
|
$
|
140,000
|
$
|
140,000
|
||||
|
Audit-Related Fees
|
-
|
-
|
||||||
|
Tax Fees
|
-
|
-
|
||||||
|
Total
|
$
|
140,000
|
$
|
140,000
|
||||
| · |
Quorum
. As permitted under the Israeli Companies Law pursuant to our articles of association, the quorum required for an ordinary meeting of shareholders will consist of at least two shareholders present in person, by proxy or by other voting instrument in accordance with the Israeli Companies Law, who hold at least 25% of the voting power of our shares (and in an adjourned meeting, with some exceptions, at least two shareholders), instead of 33 1/3% of the issued share capital required under the NASDAQ Stock Market rules.
|
| · |
Nomination of directors
. With the exception of external directors and directors elected by our board of directors due to vacancy, our directors are elected by an annual meeting of our shareholders to hold office until the next annual meeting following one year from his or her election. The nominations for directors, which are presented to our shareholders by our board of directors, are generally made by the board of directors itself, in accordance with the provisions of our articles of association and the Israeli Companies Law. Nominations need not be made by a nominating committee of our board of directors consisting solely of independent directors or otherwise, as required under the NASDAQ Stock Market rules.
|
| · |
Majority of independent directors
. Under the Companies Law, we are only required to appoint at least two external directors, within the meaning of the Companies Law, to our board of directors. Currently, four of our directors (of which two are external directors, within the meaning of the Companies Law) qualify as independent directors under the rules of the U.S. federal securities laws and the NASDAQ Stock Market rules. If at any time we no longer have a controlling shareholder, we will no longer be required to have external directors; provided that we comply with the majority Board independence requirements of the Nasdaq Stock Market.
|
|
MediWound Ltd.
|
|||
|
Date: February 21, 2017
|
By:
|
/s/ Sharon Malka
|
|
|
Sharon Malka
|
|||
|
Chief Financial and Operation Officer
|
|||
|
Exhibit
No. |
Description
|
||
|
1.1
|
Amended and Restated Articles of Association of the Registrant(1)
|
||
|
1.2
|
First Amendment to the Amended and Restated Articles of Association, effective as of June 12, 2014(5)
|
||
|
1.3
|
Memorandum of Association of the Registrant(2)
|
||
|
4.1
|
Form of Registration Rights Agreement by and among the Registrant and certain shareholders of the Registrant(2)
|
||
|
4.2
|
Form of Information Rights Agreement by and between Clal Biotechnology Industries Ltd. and the Registrant(2)
|
||
|
4.3
|
Founders Agreement, dated January 2001, by and among Clal Biotechnology Industries Ltd., L.R. R & D Ltd., Professor Lior Rosenberg and the Registrant(3)
|
||
|
4.4
|
Unprotected Sub-Lease Agreement, dated July 27, 2004, as amended, by and between the Registrant and Clal Life Sciences L.P.(3)
|
||
|
4.5
|
Patent Purchase Agreement, dated November 24, 2010, by and between the Registrant and L.R. R & D Ltd.(3)
|
||
|
4.6
|
Form of Indemnification Agreement(2)
|
||
|
4.7
|
Supply Agreement, dated January 11, 2001, as amended, by and between the Registrant and Challenge Bioproducts Corporation Ltd.†(3)
|
||
|
4.8
|
License Agreement, dated September 22, 2000, as amended, by and between the Registrant and Mark Klein†(3)
|
||
|
4.9
|
2003 Israeli Share Option Plan(3)
|
||
|
4.10
|
2014 Israeli Share Option Plan(2)
|
||
|
4.11
|
Letter Agreement, dated February 18, 2014, by and between the Registrant and Teva Pharmaceutical Industries Ltd.(2)
|
||
|
4.12
|
MediWound Ltd.’s Compensation Policy for Executive Officers and Directors(4)
|
||
|
4.13
|
BARDA Contract, dated September 29, 2015, by and between the Registrant and the U.S. Biomedical Advanced Research and Development Authority†(6)
|
||
|
4.14
|
Modification to the BARDA Contract, dated October 7, 2015, by and between the Registrant and the U.S. Biomedical Advanced Research and Development Authority(6)
|
||
|
4.15
|
Modification to the BARDA Contract, dated January 29, 2017, by and between the Registrant and the U.S. Biomedical Advanced Research and Development Authority
|
||
|
4.16
|
License Agreement, dated November11, 2016 by and between the registrant and L.R. Research and Development Ltd.
|
||
|
8.1
|
List of subsidiaries of the Registrant(3)
|
||
|
12.1
|
Certificate of Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002
|
||
|
12.2
|
Certificate of Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002
|
||
|
13.1
|
Certificate of Chief Executive Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, furnished herewith
|
||
|
13.2
|
Certificate of Chief Financial Officer pursuant to 18 U.S.C. §1350, as adopted pursuant to §906 of the Sarbanes-Oxley Act of 2002, furnished herewith
|
||
|
15.1
|
Consent of Kost Forer Gabbay and Kasierer, a member of Ernst & Young Global, an independent registered public accounting firm
|
||
| † |
Portions of each agreement were omitted and a complete copy of each agreement has been provided separately to the Securities and Exchange Commission pursuant to the company’s application requesting confidential treatment under Rule 406 of the Securities Act of 1993 as amended, or Rule 24b-2 of the Securities Exchange Act of 1934, as amended, as applicable.
|
| (1) |
Previously filed with the SEC on March 14, 2014 pursuant to a registration statement on Form F-1 (File No. 333-193856) and incorporated by reference herein.
|
| (2) |
Previously filed with the SEC on March 3, 2014 pursuant to a registration statement on Form F-1 (File No. 333-193856) and incorporated by reference herein.
|
| (3) |
Previously filed with the SEC on February 10, 2014 pursuant to a registration statement on Form F-1 (File No. 333-193856) and incorporated by reference herein.
|
| (4) |
Previously filed with the SEC on August 5, 2014 as Annex A to Exhibit 99.1 to the Registrant’s Form 6-K and incorporated by reference herein.
|
| (5) |
Previously filed with the SEC on February 12, 2015 pursuant to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2014 (File No. 001-36349) and incorporated by reference herein.
|
| (6) |
Previously filed with the SEC on January 25, 2016 pursuant to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2015 (File No. 001-36349) and incorporated by reference herein.
|
|
Page
|
|
|
F
-
2
|
|
|
F
-
3
|
|
|
F
-
4
|
|
|
F
-
5
|
|
|
F
-
6 - F-7
|
|
|
F
-
8
-
F
-
40
|

|
Kost Forer Gabbay & Kasierer
3 Aminadav St.
Tel-Aviv 6706703, Israel
|
Tel: +972-3-6232525
Fax: +972-3-5622555
ey.com
|
| /s/ Kost Forer Gabbay & Kasierer | |
|
Tel-Aviv, Israel
|
KOST FORER GABBAY & KASIERER
|
|
February 21, 2017
|
A Member of Ernst & Young Global
|
|
December 31,
|
|||||||||||
|
Note
|
2015
|
2016
|
|||||||||
|
CURRENT ASSETS:
|
|||||||||||
|
Cash and cash equivalents
|
5
|
42,502
|
28,866
|
||||||||
|
Short-term bank deposits
|
6
|
3,266
|
1,163
|
||||||||
|
Trade receivables
|
238
|
332
|
|||||||||
|
Inventories
|
7
|
1,715
|
844
|
||||||||
|
Other receivables
|
8, 22
|
2,674
|
2,407
|
||||||||
|
50,395
|
33,612
|
||||||||||
|
LONG-TERM ASSETS:
|
|||||||||||
|
Long term deposits
|
192
|
103
|
|||||||||
|
Property, plant and equipment, net
|
9
|
1,040
|
1,276
|
||||||||
|
Intangible assets, net
|
10
|
896
|
773
|
||||||||
|
2,128
|
2,152
|
||||||||||
|
52,523
|
35,764
|
||||||||||
|
CURRENT LIABILITIES:
|
|||||||||||
|
Trade payables
|
1,123
|
1,456
|
|||||||||
|
Other payables
|
11, 22
|
4,083
|
3,924
|
||||||||
|
5,206
|
5,380
|
||||||||||
|
LONG‑TERM LIABILITIES:
|
|||||||||||
|
Deferred revenues
|
-
|
1,023
|
|||||||||
|
Liabilities in respect of IIA grants
|
12,13
|
7,275
|
6,839
|
||||||||
|
Contingent consideration for the purchase of shares
|
13,16
|
16,475
|
14,533
|
||||||||
|
Severance pay liability, net
|
14
|
97
|
219
|
||||||||
|
23,847
|
22,614
|
||||||||||
|
SHAREHOLDERS' EQUITY:
|
16
|
||||||||||
|
Ordinary shares of NIS 0.01 par value:
|
|||||||||||
|
Authorized: 32,244,508 shares as of December 31, 2015 and 2016; Issued and Outstanding 21,850,300 and 21,930,449 shares respectively.
|
60
|
60
|
|||||||||
|
Share premium
|
111,801
|
114,979
|
|||||||||
|
Foreign currency translation adjustments
|
(16
|
)
|
(9
|
)
|
|||||||
|
Accumulated deficit
|
(88,375
|
)
|
(107,260
|
)
|
|||||||
|
23,470
|
7,770
|
||||||||||
|
52,523
|
35,764
|
||||||||||
|
Year ended
December 31,
|
|||||||||||||||
|
Note
|
2014
|
2015
|
2016
|
||||||||||||
|
Revenues
|
259
|
601
|
1,558
|
||||||||||||
|
Cost of revenues
|
20a
|
2,785
|
2,519
|
2,158
|
|||||||||||
|
Gross loss
|
(2,526
|
)
|
(1,918
|
)
|
(600
|
)
|
|||||||||
|
Research and development, net of participations
|
20b
|
5,349
|
6,021
|
7,068
|
|||||||||||
|
Selling and marketing
|
20c
|
8,829
|
9,284
|
8,403
|
|||||||||||
|
General and administrative
|
20d
|
4,723
|
4,004
|
4,084
|
|||||||||||
|
Operating loss
|
(21,427
|
)
|
(21,227
|
)
|
(20,155
|
)
|
|||||||||
|
Financial income
|
20e
|
4,665
|
1,052
|
2,166
|
|||||||||||
|
Financial expense
|
20e
|
(2,113
|
)
|
(1,496
|
)
|
(896
|
)
|
||||||||
|
Loss from continuing operations
|
(18,875
|
)
|
(21,671
|
)
|
(18,885
|
)
|
|||||||||
|
Loss from discontinued operation
|
19
|
-
|
(417
|
)
|
-
|
||||||||||
|
Net loss
|
(18,875
|
)
|
(22,088
|
)
|
(18,885
|
)
|
|||||||||
|
Other comprehensive income:
|
|||||||||||||||
|
Items to be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||
|
Foreign currency translation adjustments
|
14
|
2
|
7
|
||||||||||||
|
Total comprehensive loss
|
(18,861
|
)
|
(22,086
|
)
|
(18,878
|
)
|
|||||||||
|
Basic and diluted net loss per share
|
21
|
(0.95
|
)
|
(1.02
|
)
|
(0.86
|
)
|
||||||||
|
Share capital
|
Share premium
|
Treasury
shares
|
Foreign
currency
translation
Adjustments
|
Accumulated
deficit
|
Total
Equity
(Deficiency)
|
|||||||||||||||||||
|
Balance as of January 1, 2014
|
11
|
62,229
|
(34,600
|
)
|
(32
|
)
|
(47,412
|
)
|
(19,804
|
)
|
||||||||||||||
|
Loss for the period
|
-
|
-
|
-
|
-
|
(18,875
|
)
|
(18,875
|
)
|
||||||||||||||||
|
Other comprehensive income
|
-
|
-
|
-
|
14
|
-
|
14
|
||||||||||||||||||
|
Total comprehensive (loss) income
|
-
|
-
|
-
|
14
|
(18,875
|
)
|
(18,861
|
)
|
||||||||||||||||
|
Exercise of warrants
|
1
|
4,711
|
-
|
-
|
-
|
4,712
|
||||||||||||||||||
|
Exercise of options
|
1
|
305
|
-
|
-
|
-
|
306
|
||||||||||||||||||
|
Purchase of treasury shares
|
(2
|
)
|
(34,598
|
)
|
34,600
|
-
|
-
|
-
|
||||||||||||||||
|
Effect of share split
|
32
|
(32
|
)
|
-
|
-
|
-
|
-
|
|||||||||||||||||
|
Share-based compensation
|
-
|
4,827
|
-
|
-
|
-
|
4,827
|
||||||||||||||||||
|
Issuance of shares, net
|
16
|
71,675
|
-
|
-
|
-
|
71,691
|
||||||||||||||||||
|
Balance as of December 31, 2014
|
59
|
109,117
|
-
|
(18
|
)
|
(66,287
|
)
|
42,871
|
||||||||||||||||
|
Loss for the period
|
-
|
-
|
-
|
-
|
(22,088
|
)
|
(22,088
|
)
|
||||||||||||||||
|
Other comprehensive income
|
-
|
-
|
-
|
2
|
-
|
2
|
||||||||||||||||||
|
Total comprehensive (loss) income
|
-
|
-
|
-
|
2
|
(22,088
|
)
|
(22,086
|
)
|
||||||||||||||||
|
Exercise of options
|
1
|
25
|
-
|
-
|
-
|
26
|
||||||||||||||||||
|
Share-based compensation
|
-
|
2,659
|
-
|
-
|
-
|
2,659
|
||||||||||||||||||
|
Balance as of December 31, 2015
|
60
|
111,801
|
-
|
(16
|
)
|
(88,375
|
)
|
23,470
|
||||||||||||||||
|
Loss for the period
|
-
|
-
|
-
|
-
|
(18,885
|
)
|
(18,885
|
)
|
||||||||||||||||
|
Other comprehensive income
|
-
|
-
|
-
|
7
|
-
|
7
|
||||||||||||||||||
|
Total comprehensive (loss) income
|
-
|
-
|
-
|
7
|
(18,885
|
)
|
(18,878
|
)
|
||||||||||||||||
|
Exercise of options
|
*
|
7
|
-
|
-
|
-
|
7
|
||||||||||||||||||
|
Share-based compensation
|
-
|
3,171
|
-
|
-
|
-
|
3,171
|
||||||||||||||||||
|
Balance as of December 31, 2016
|
60
|
114,979
|
-
|
(9
|
)
|
(107,260
|
)
|
7,770
|
||||||||||||||||
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Cash Flows from Operating Activities:
|
||||||||||||
|
Net loss
|
(18,875
|
)
|
(22,088
|
)
|
(18,885
|
)
|
||||||
|
Adjustments to reconcile net loss to net cash used in continuing operating activities:
|
||||||||||||
|
Adjustments to profit and loss items:
|
||||||||||||
|
Loss from discontinued operation
|
-
|
417
|
-
|
|||||||||
|
Depreciation and amortization
|
492
|
503
|
589
|
|||||||||
|
Revaluation of warrants to shareholders
|
(4,491
|
)
|
-
|
-
|
||||||||
|
Share-based compensation
|
4,827
|
2,659
|
3,171
|
|||||||||
|
Revaluation of liabilities in respect of IIA grants
|
87
|
(474
|
)
|
(1,298
|
)
|
|||||||
|
Revaluation of contingent consideration for the purchase of treasury shares
|
612
|
(764
|
)
|
(1,621
|
)
|
|||||||
|
Increase in severance pay liability, net
|
-
|
90
|
125
|
|||||||||
|
Net financing expenses (income)
|
226
|
(219
|
)
|
(508
|
)
|
|||||||
|
1,753
|
2,212
|
458
|
||||||||||
|
Changes in asset and liability items:
|
||||||||||||
|
Increase in trade receivables
|
(67
|
)
|
(181
|
)
|
(107
|
)
|
||||||
|
Decrease (increase) in inventories
|
(1,421
|
)
|
(273
|
)
|
873
|
|||||||
|
Decrease (increase) in other receivables and long term deposits
|
186
|
(556
|
)
|
33
|
||||||||
|
Increase (decrease) in trade payables
|
22
|
(76
|
)
|
331
|
||||||||
|
Increase in other payables and deferred revenues
|
1,909
|
1,361
|
852
|
|||||||||
|
629
|
275
|
1,982
|
||||||||||
|
Net cash flow used in operating activities
|
(16,493
|
)
|
(19,601
|
)
|
(16,445
|
)
|
||||||
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Cash Flows from Investing Activities:
|
||||||||||||
|
Purchase of property and equipment
|
(366
|
)
|
(376
|
)
|
(671
|
)
|
||||||
|
Purchase of intangible assets
|
(30
|
)
|
(30
|
)
|
(30
|
)
|
||||||
|
Interest received
|
173
|
287
|
407
|
|||||||||
|
Proceeds from (investment in) short term bank deposits, net
|
(36,931
|
)
|
36,165
|
2,110
|
||||||||
|
Net cash provided by (used in) investing activities
|
(37,154
|
)
|
36,046
|
1,816
|
||||||||
|
Cash Flows from Financing Activities:
|
||||||||||||
|
Proceeds from exercise of options
|
306
|
26
|
7
|
|||||||||
|
Proceeds from issuance of shares and warrants, net
|
71,824
|
-
|
-
|
|||||||||
|
Proceeds from the IIA grants, net of re-payment
|
345
|
752
|
900
|
|||||||||
|
Net cash provided by financing activities
|
72,475
|
778
|
907
|
|||||||||
|
Exchange rate differences on cash and cash equivalent balances
|
(459
|
)
|
(143
|
)
|
86
|
|||||||
|
Increase (decrease) in cash and cash equivalents
|
18,369
|
17,080
|
(13,636
|
)
|
||||||||
|
Balance of cash and cash equivalents at the beginning of the year
|
7,053
|
25,422
|
42,502
|
|||||||||
|
Balance of cash and cash equivalents at the end of the year
|
25,422
|
42,502
|
28,866
|
|||||||||
|
Non-cash activities:
|
||||||||||||
|
Treasury shares cancellation against share premium
|
34,600
|
-
|
-
|
|||||||||
|
Exercise of cashless warrants into shares
|
4,709
|
-
|
-
|
|||||||||
| NOTE 1: |
GENERAL
|
| a. |
General description of the company and its operations:
|
| b. |
The Company has two wholly‑owned subsidiaries: MediWound Germany GmbH, acting as EU marketing authorization holder and EU sales and marketing arm and MediWound UK Limited, an inactive company. In addition, the Company owns approximately 6.5% of PolyHeal Ltd., a private life sciences company ("PolyHeal").
|
| c. |
On March 25, 2014, the Company closed its initial public offering in the United States and listing on the NASDAQ Global Market ("the IPO") of 5,750,000 ordinary shares at a price per share of $14.00 with aggregate gross proceeds of $80,500. Under the terms of the offering, the Company incurred aggregate underwriting discounts of approximately $5,635 and expenses of approximately $3,172 in connection with the offering, resulting in net proceeds to the Company of approximately $71,691. Following the IPO the Company's securities are listed for trading on NASDAQ.
|
| d. |
On September 29, 2015, the Company was awarded a U.S. Biomedical Advanced Research and Development Authority ("BARDA") contract for development and procurement of NexoBrid for the U.S. The contract is for the advancement of the development and manufacturing, as well as the procurement of NexoBrid, as a medical countermeasure as part of BARDA preparedness for mass casualty events.
|
| a. |
Basis of presentation of financial statements:
|
| b. |
The Company's operating cycle is one year.
|
| c. |
Consolidated financial statements include the financial statements of companies that the Company controls (subsidiaries). Control is achieved when the Company is exposed, or has rights, to variable returns from its investment with the investee and has the ability to affect those returns through its power over the investee.
|
| d. |
Functional currency, reporting currency and foreign currency:
|
| 1. |
Functional currency and reporting currency:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| 2. |
Transactions, assets and liabilities in foreign currency:
|
| e. |
Cash equivalents:
|
| f. |
Short-term bank deposits:
|
| g. |
Inventories:
|
|
Raw materials
|
-
|
At cost of purchase using the first-in, first-out method.
|
|
Finished goods
|
-
|
On the basis of average costs including materials, labor and other direct and indirect manufacturing costs based on normal capacity.
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| h. |
Participation by governments support:
|
| i. |
Leases:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| j. |
Property, plant and equipment, net:
|
|
%
|
||
|
Office furniture
|
6 - 15
|
|
|
Electronic machinery and laboratory equipment
|
15 - 20
|
|
|
Computers
|
33
|
|
|
Leasehold improvements
|
See below
|
| k. |
Intangible assets, net:
|
|
Revenue recognition
|
| m. |
Research and development expenses:
|
| n. |
Impairment of non-financial assets:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| o. |
Financial instruments:
|
| 1. |
Financial assets:
|
| 2. |
Financial liabilities:
|
| 3. |
Fair value:
|
| 4. |
Offsetting financial instruments:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
All assets and liabilities measured at fair value or for which fair value is disclosed are categorized into levels within the fair value hierarchy based on the lowest level input that is significant to the entire fair value measurement:
|
|
Level 1
|
-
|
quoted prices (unadjusted) in active markets for identical assets or liabilities.
|
|
Level 2
|
-
|
inputs other than quoted prices included within level 1 that are observable either directly or indirectly.
|
|
Level 3
|
-
|
inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data).
|
| 6. |
De-recognition of financial instruments:
|
| a) |
Financial assets:
|
| b) |
Financial liabilities:
|
| 7. |
Treasury shares:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| p. |
Provisions:
|
| q. |
Short-term employee benefits and severance pay liability, net:
|
| 1. |
Short-term employee benefits:
|
| 2. |
Post-employment benefits:
|
| r. |
Share-based compensation:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| s. |
Discontinued operation:
|
| t. |
Loss per share:
|
| NOTE 3:- |
SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS
|
| • |
Determining the fair value of share based compensation to employees and directors:
|
| NOTE 3:- |
SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS (Cont.)
|
| • |
Liabilities in respect to Israeli Innovation Authority grants:
|
| • |
Contingent consideration for the purchase of treasury shares:
|
| • |
Legal claims:
|
In estimating the likelihood of outcome of legal claims filed against the Company and its investees, the companies rely on the opinion of their legal counsel. These estimates are based on the legal counsel's best professional judgment, taking into account the stage of proceedings and legal precedents in respect of the different issues. Since the outcome of the claims will be determined in courts, the results could differ from these estimates.
| NOTE 4:- |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION
|
| a. |
IFRS 15, "Revenue from Contracts with Customers":
|
| NOTE 4:- |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION (Cont.)
|
| 1. |
Revenue from the sale of goods:
|
| 2. |
Revenue from distribution agreements with multiple-element:
|
| NOTE 4:- |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION (Cont.)
|
| 3. |
Disclosure and presentation:
|
| b. |
IFRS 16, "Leases":
|
| NOTE 4:- |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION (Cont.)
|
| NOTE 5:- |
CASH AND CASH EQUIVALENTS
|
|
December 31,
|
||||||||
|
2015
|
2016
|
|||||||
|
USD cash for immediate withdrawal
|
34,856
|
25,863
|
||||||
|
Non-USD cash for immediate withdrawal
|
7,646
|
3,003
|
||||||
|
42,502
|
28,866
|
|||||||
| NOTE 6:- |
SHORT-TERM BANK DEPOSITS
|
|
December 31,
|
||||||||
|
2015
|
2016
|
|||||||
|
USD bank deposits
|
-
|
1,163
|
||||||
|
EURO bank deposits
|
3,266
|
-
|
||||||
|
3,266
|
1,163
|
|||||||
|
Year ended
December 31,
|
||||||||
|
2015
|
2016
|
|||||||
|
Raw materials
|
387
|
296
|
||||||
|
Finished goods
|
1,328
|
548
|
||||||
|
1,715
|
844
|
|||||||
| NOTE 8:- |
OTHER RECEIVABLES
|
|
Year ended
December 31,
|
||||||||
|
2015
|
2016
|
|||||||
|
Government authorities
|
172
|
61
|
||||||
|
Related parties
|
20
|
-
|
||||||
|
Former shareholder, net (see Note 16)
|
1,477
|
1,169
|
||||||
|
BARDA funds
|
800
|
953
|
||||||
|
Prepaid expenses and other
|
205
|
224
|
||||||
|
2,674
|
2,407
|
|||||||
| NOTE 9:- |
PROPERTY, PLANT AND EQUIPMENT, NET
|
|
Office
furniture
|
Electronic
machinery
and lab
equipment
|
Computers
|
Leasehold
improvements
|
Total
|
||||||||||||||||
|
Cost
|
||||||||||||||||||||
|
Balance as of January 1, 2016
|
221
|
1,955
|
174
|
2,095
|
4,445
|
|||||||||||||||
|
Disposals
|
-
|
-
|
(27
|
)
|
-
|
(27
|
)
|
|||||||||||||
|
Additions
|
12
|
596
|
38
|
25
|
671
|
|||||||||||||||
|
Foreign currency translation
|
(6
|
)
|
-
|
-
|
-
|
(6
|
)
|
|||||||||||||
|
Balance as of December 31, 2016
|
227
|
2,551
|
185
|
2,120
|
5,083
|
|||||||||||||||
|
Accumulated Depreciation
|
||||||||||||||||||||
|
Balance as of January 1, 2016
|
102
|
1,205
|
86
|
2,012
|
3,405
|
|||||||||||||||
|
Disposals
|
-
|
-
|
(27
|
)
|
-
|
(27
|
)
|
|||||||||||||
|
Additions
|
27
|
303
|
59
|
47
|
436
|
|||||||||||||||
|
Foreign currency translation
|
(7
|
)
|
-
|
-
|
-
|
(7
|
)
|
|||||||||||||
|
Balance as of December 31, 2016
|
126
|
1,508
|
118
|
2,057
|
3,809
|
|||||||||||||||
|
Depreciated cost as of December 31, 2016
|
105
|
1,043
|
67
|
61
|
1,276
|
|||||||||||||||
|
Office
furniture
|
Electronic
machinery
and lab
equipment
|
Computers
|
Leasehold
improvements
|
Total
|
||||||||||||||||
|
Cost
|
||||||||||||||||||||
|
Balance as of January 1, 2015
|
201
|
1,875
|
185
|
2,015
|
4,276
|
|||||||||||||||
|
Disposals
|
-
|
(136
|
)
|
(62
|
)
|
-
|
(198
|
)
|
||||||||||||
|
Additions
|
27
|
216
|
53
|
80
|
376
|
|||||||||||||||
|
Foreign currency translation
|
(7
|
)
|
-
|
(2
|
)
|
-
|
(9
|
)
|
||||||||||||
|
Balance as of December 31, 2015
|
221
|
1,955
|
174
|
2,095
|
4,445
|
|||||||||||||||
|
Accumulated Depreciation
|
||||||||||||||||||||
|
Balance as of January 1, 2015
|
79
|
1,084
|
93
|
1,932
|
3,188
|
|||||||||||||||
|
Disposals
|
-
|
(136
|
)
|
(62
|
)
|
-
|
(198
|
)
|
||||||||||||
|
Additions
|
26
|
257
|
55
|
80
|
418
|
|||||||||||||||
|
Foreign currency translation
|
(3
|
)
|
-
|
-
|
-
|
(3
|
)
|
|||||||||||||
|
Balance as of December 31, 2015
|
102
|
1,205
|
86
|
2,012
|
3,405
|
|||||||||||||||
|
Depreciated cost as of December 31, 2015
|
119
|
750
|
88
|
83
|
1,040
|
|||||||||||||||
| NOTE 10:- |
INTANGIBLE ASSETS, NET
|
|
License and
Knowhow
|
||||
|
Cost
|
||||
|
Balance as of January 1, 2016
|
1,466
|
|||
|
Additions
|
30
|
|||
|
Balance as of December 31, 2016
|
1,496
|
|||
|
Accumulated Amortization
|
||||
|
Balance as of January 1, 2016
|
570
|
|||
|
Additions
|
153
|
|||
|
Balance as of December 31, 2016
|
723
|
|||
|
Amortized cost
|
||||
|
Balance as of December 31, 2016
|
773
|
|||
|
License and
Knowhow
|
||||
|
Cost
|
||||
|
Balance as of January 1, 2015
|
1,436
|
|||
|
Additions
|
30
|
|||
|
Balance as of December 31, 2015
|
1,466
|
|||
|
Accumulated Amortization
|
||||
|
Balance as of January 1, 2015
|
485
|
|||
|
Additions
|
85
|
|||
|
Balance as of December 31, 2015
|
570
|
|||
|
Amortized cost
|
||||
|
Balance as of December 31, 2015
|
896
|
|||
| NOTE 11:- |
OTHER PAYABLES
|
|
Year ended
December 31,
|
||||||||
|
2015
|
2016
|
|||||||
|
Employees and payroll accruals
|
2,453
|
1,566
|
||||||
|
Accrued expenses
|
1,263
|
1,892
|
||||||
|
Current maturities of IIA grants
|
28
|
49
|
||||||
|
Related parties
|
248
|
295
|
||||||
|
Deferred revenues
|
91
|
122
|
||||||
|
4,083
|
3,924
|
|||||||
| NOTE 12:- |
ISRAELI INNOVATION AUTHORITY GRANTS
|
|
Year ended
December 31,
|
||||||||
|
2015
|
2016
|
|||||||
|
Balance as of January 1
|
7,034
|
7,303
|
||||||
|
Grants received
|
764
|
929
|
||||||
|
Royalties
|
(21
|
)
|
(46
|
)
|
||||
|
Amounts carried to Profit
|
(474
|
)
|
(1,298
|
)
|
||||
|
Balance as of Decmber 31
|
7,303
|
6,888
|
||||||
|
Current maturities
|
(28
|
)
|
(49
|
)
|
||||
|
Long term liabilities in respect of IIA grants
|
7,275
|
6,839
|
||||||
| NOTE 13:- |
FINANCIAL INSTRUMENTS
|
| a. |
Financial risk factors:
|
| b. |
Fair value:
|
| c. |
Sensitivity tests relating to changes in market factors:
|
| NOTE 13:- |
FINANCIAL INSTRUMENTS (Cont.)
|
|
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Sensitivity test to changes in NIS and EURO exchange rates
|
||||||||||||
|
Gain (loss) from change:
|
||||||||||||
|
5% increase in exchange rate
|
$
|
259
|
$
|
361
|
$
|
11
|
||||||
|
5% decrease in exchange rate
|
$
|
(259
|
)
|
$
|
(361
|
)
|
$
|
(11
|
)
|
|||
| NOTE 14:- |
SEVERANCE PAY LIABILTY, NET
|
| NOTE 15:- |
CONTINGENT LIABILITIES AND COMMITMENTS
|
| a. |
In 2000, the Company signed an exclusive license agreement (as amended in 2007) with a third party with regard to its patents and intellectual property. Pursuant to the agreement, the Company received an exclusive license to use the third party's patents and intellectual property, for the purpose of developing, manufacturing, marketing, and commercializing products for treatment of burns and other wounds.
|
| b. |
Under the Research and Development Law, (the "R&D Law") the Company undertook to pay royalties of 3% - 3.5% on the revenues derived from sales of products or services developed in whole or in part using these Israeli Innovation Authority grants. The maximum aggregate royalties paid generally cannot exceed 100% of the grants received by the Company, plus annual interest generally equal to the 12-month LIBOR applicable to dollar deposits, as published on the first business day of each calendar year. The maximum royalty amount payable by the Company as of December 31, 2016 is approximately $ 12,718, which represents the total amount of grants actually received by the Company from the Israeli Innovation Authority including accrued interest, net of royalties actually paid by the Company,( see also Note 12).
|
| c. |
On November 24, 2010, the Company signed an agreement with one of its shareholders, to purchase a patent for the production and sale of related products for the treatment of burns. In consideration for the transfer and assignment of all rights and title relating to the patent, the Company paid a one-time payment in the amount of $ 88 and undertook to pay annual fixed payments in the amount of $ 30 as long as the patent is valid in the US and/or in any EU member country. The patent expires in May 2018, and the Company's accumulated outstanding obligation with respect to this agreement as of December 31, 2016 is $ 43.
|
| d. |
On September 15, 2014, a Statement of Claim was filed against the Company by some shareholders of Polyheal. The plaintiffs allege that the Company is obligated to pay them a total amount of $1,475 in exchange for their respective portion of PolyHeal's shares, following the commencement of a feasibility study for the next generation of the PolyHeal Product in November 15, 2012, which constituted a milestone under a buyout option agreement between the Company, PolyHeal and its shareholders. For further details, see note 19.
|
| NOTE 15:- |
CONTINGENT LIABILITIES AND COMMITMENTS (Cont.)
|
| e. |
Operating Lease Agreements:
|
| 1. |
The Company's offices and its production facility in Israel are located in a building that the Company leases from its Parent Company, in accordance with a sub-lease agreement from July 2004. The sub-lease agreement has been amended multiple times, most recently in September 2016. According to the most recently amended sub lease agreement, the Company subleases approximately 1,205 square meters of laboratory, office and clean room space at a monthly rent fee of $56. This sub-lease agreement expires in December 2017. The Company's subsidiary offices are located in Germany. The monthly rent fee is currently €2,800 (approximately $ 3.1) and the lease agreement expires on April 30, 2019.
|
| 2. |
The Company and its subsidiary have operating lease agreements for 19 vehicles. According to these agreements, the Company leases cars for its employees for a period of three years. As of December 31, 2016, the Company deposited $ 69 in respect of the vehicles operating leases.
|
| 3. |
Minimum future lease fees for both agreements as of December 31, 2016 are as follows:
|
|
2017
|
862
|
|||
|
2018
|
159
|
|||
|
2019
|
86
|
|||
|
1,107
|
| NOTE 16:- |
EQUITY
|
| a. |
Share capital
|
| b. |
Rights attached to shares:
|
| c. |
On March 25, 2014 the Company completed its initial public offering in the United States and listing on the NASDAQ Global Select Market of 5,750,000 new ordinary shares at $14 per share including the underwriters' option to purchase an additional 750,000 shares at the offering price that was exercised prior to closing. The Company's total proceeds from the issuance of the above shares were $71,691 thousands, net of underwriter’s discount and issuance expenses in the amount of $ 8,809.
|
|
Upon the closing of the IPO, the Company issued 336,591 ordinary shares pursuant to the exercise of 1,066,735 warrants held by certain of the Company shareholders, including (1) the exercise of 433 warrants into 433 ordinary shares at an exercise price of $6.72 per share and the receipt of proceeds by us related to such exercise and (2) the cashless exercise of 1,066,302 warrants into 336,158 ordinary shares at a weighted average exercise price of $9.58 per share.
|
| NOTE 16:- |
EQUITY (Cont.)
|
| NOTE 17:- |
SHARE‑BASED COMPENSATION
|
| a. |
Expense recognized in the financial statements:
|
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Cost of revenues
|
763
|
372
|
504
|
|||||||||
|
Research and development
|
657
|
511
|
752
|
|||||||||
|
Selling and marketing
|
1,430
|
669
|
765
|
|||||||||
|
General and administrative
|
1,977
|
1,107
|
1,150
|
|||||||||
|
Total share-based compensation
|
4,827
|
2,659
|
3,171
|
|||||||||
| b. |
Share-based payment plan for employees and directors:
|
| NOTE 17:- |
SHARE‑BASED COMPENSATION (Cont.)
|
| c. |
Share options activity:
|
|
2014
|
2015
|
2016
|
||||||||||||||||||||||
|
Number of
options
|
Weighted
Average
Exercise price
|
Number of
options
|
Weighted
Average
Exercise price
|
Number of
options
|
Weighted
Average
Exercise price
|
|||||||||||||||||||
|
Outstanding at beginning of year
|
2,376,064
|
6.71
|
1,902,324
|
7.98
|
2,313,224
|
9.35
|
||||||||||||||||||
|
Granted
|
40,000
|
7.26
|
739,500
|
9.27
|
47,500
|
8.56
|
||||||||||||||||||
|
Exercised
|
(449,714
|
)
|
0.68
|
(300,000
|
)
|
0.09
|
(80,149
|
)
|
0.09
|
|||||||||||||||
|
Forfeited
|
(64,026
|
)
|
11.60
|
(28,600
|
)
|
12.69
|
(99,500
|
)
|
10.80
|
|||||||||||||||
|
Outstanding at end of year
|
1,902,324
|
7.98
|
2,313,224
|
9.35
|
2,181,075
|
9.62
|
||||||||||||||||||
|
Exercisable at end of year
|
1,155,584
|
5.03
|
1,535,055
|
9.39
|
1,401,866
|
9.35
|
||||||||||||||||||
|
Options outstanding as of
December 31, 2016
|
|||||||||||||
|
Range of exercise prices ($ )
|
Number of
options
|
Weighted
Average
Remaining
contractual
life
|
Weighted
average exercise
price
|
||||||||||
|
0.09
|
79,624
|
0.95
|
0.09
|
||||||||||
|
2.63
|
208,332
|
1.86
|
2.63
|
||||||||||
|
7.26 ‑ 9.82
|
1,047,619
|
7.05
|
9.05
|
||||||||||
|
12.89 ‑ 13.76
|
845,500
|
6.92
|
12.95
|
||||||||||
|
Total
|
2,181,075
|
6.28
|
9.62
|
||||||||||
|
1.
|
On December 23, 2015, the Company's Board of Directors approved the grant of 70,000 options to purchase ordinary shares under the Plan for an exercise price of $ 9.58 per share to the CEO of the Company, which was approved by the shareholders' general meeting dated January 28th, 2016 .
|
|
2.
|
On June 9, 2016, the shareholder's general meeting of the Company approved to extend the exercise period of certain options previously granted to CEO. The Fair Value of the extension of the Options, as of the modification date, was estimated at approximately $39.
|
| d. |
The fair value of the Company's share options granted to employees for the years ended December 31, 2014, 2015 and 2016 was estimated using acceptable option pricing models using the following assumptions:
|
|
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Dividend yield (%)
|
0
|
0
|
0
|
|||||||||
|
Expected volatility of the share prices (%)
|
75
|
71
|
72
|
|||||||||
|
Risk‑free interest rate (%)
|
0.1-1.80
|
0.25-2.24
|
0.28-2.0
|
|||||||||
|
Early exercise factor (%)
|
100
|
100-150
|
|
100-150
|
||||||||
|
Weighted average share prices (Dollar)
|
6.91
|
8.98
|
8.56
|
|||||||||
|
Forfeiture rate (%)
|
0
|
0
|
0
|
|||||||||
| NOTE 18:- |
TAXES ON INCOME
|
| a. |
The Company operates in two main tax jurisdictions: Israel and Germany. As such, the Company is subject to the applicable tax rates in the jurisdictions in which it conducts its business.
|
| b. |
Corporate tax rates in Israel:
|
| · |
The Israeli corporate income tax rate was 25% in 2016, 26.5% in 2015 and 2014.
|
| NOTE 18:- |
TAXES ON INCOME (Cont.)
|
| · |
Tax benefits under the Israel Law for the Encouragement of Capital Investments, 1959 (the "Investment Law"):
|
| c. |
Corporate tax rate in Germany:
|
| d. |
Final tax assessments:
|
| e. |
Net operating carryforward losses for tax purposes and other temporary differences:
|
| NOTE 18:- |
TAXES ON INCOME (Cont.)
|
| f. |
Deferred taxes:
|
| g. |
Current taxes on income:
|
| h. |
Theoretical tax:
|
| NOTE 19:- |
DISCONTINUED OPERATION
|
| a. |
In December 2010, the Company, Teva and PolyHeal, entered into a series of agreements to collaborate in the development, manufacturing and commercialization of PolyHeal's wound care product, or the PolyHeal Product(“ 2010 PolyHeal Agreement”).
|
| b. |
The Company has accounted this transaction as an acquisition of a group of assets since the assets acquired did not constitute a business as defined in IFRS 3. The Company allocated the consideration paid for the group of assets acquired based on their fair value to two identifiable assets: the license for the Polyheal Products in the amount of $ 6,333 and royalty rights arising from the Company's ownership of shares of PolyHeal in the amount of $ 417.
|
| c. | The Company has been acknowledged during 2015 about certain changes in circumstances indicating that the carrying amount of its royalty rights arising from the Company's ownership of shares of Polyheal would not be recoverable. Accordingly, a full impairment of these royalty rights amounting to $417 is included within the loss from discontinued operation for the year ended December 31, 2015. |
| NOTE 20:- |
SUPPLEMENTARY INFORMATION TO THE STATEMENTS OF PROFIT AND LOST AND OTHER COMPREHENSIVE LOSS
|
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Salary and benefits (including share-based compensation)
|
2,219
|
1,961
|
2,112
|
|||||||||
|
Subcontractors
|
199
|
67
|
66
|
|||||||||
|
Depreciation and amortization
|
416
|
417
|
475
|
|||||||||
|
Cost of materials
|
600
|
486
|
410
|
|||||||||
|
Other manufacturing expenses
|
880
|
657
|
892
|
|||||||||
|
Decrease (increase) in inventory of finished products
|
(1,019
|
)
|
(309
|
)
|
780
|
|||||||
|
Allotment of manufacturing costs to R&D
|
(510
|
)
|
(760
|
)
|
(2,577
|
)
|
||||||
|
2,785
|
2,519
|
2,158
|
||||||||||
| b. |
Research and development expenses, net of participations:
|
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Salary and benefits (including share-based compensation)
|
2,182
|
2,610
|
3,171
|
|||||||||
|
Subcontractors
|
3,294
|
4,464
|
8,517
|
|||||||||
|
Depreciation and amortization
|
-
|
5
|
28
|
|||||||||
|
Cost of materials
|
-
|
81
|
351
|
|||||||||
|
Allotment of manufacturing costs
|
510
|
760
|
2,577
|
|||||||||
|
Other research and development expenses
|
68
|
219
|
135
|
|||||||||
|
6,054
|
8,139
|
14,779
|
||||||||||
|
Participations:
|
||||||||||||
|
Revaluation of liabilities in respect of IIA grants
|
(705
|
)
|
(1,318
|
)
|
(2,145
|
)
|
||||||
|
BARDA funds
|
-
|
(800
|
)
|
(5,566
|
)
|
|||||||
|
5,349
|
6,021
|
7,068
|
||||||||||
| NOTE 20:- |
SUPPLEMENTARY INFORMATION TO THE STATEMENTS OF COMPREHENSIVE LOSS (Cont.)
|
| c. |
Selling and marketing expenses:
|
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Salary and benefits (including share based compensation)
|
4,966
|
5,631
|
5,438
|
|||||||||
|
Marketing and sales
|
3,356
|
2,835
|
2,444
|
|||||||||
|
Depreciation and amortization
|
25
|
22
|
18
|
|||||||||
|
Shipping and delivery
|
60
|
180
|
111
|
|||||||||
|
Registration and marketing license fees
|
422
|
616
|
392
|
|||||||||
|
8,829
|
9,284
|
8,403
|
||||||||||
| d. |
General and administrative expenses:
|
|
Salary and benefits (including share‑based compensation)
|
3,521
|
2,670
|
2,361
|
|||||||||
|
Professional fees
|
869
|
1,054
|
1,241
|
|||||||||
|
Depreciation and amortization
|
49
|
59
|
66
|
|||||||||
|
Other
|
284
|
221
|
416
|
|||||||||
|
4,723
|
4,004
|
4,084
|
||||||||||
| e. |
Financial income and expense:
|
|
Financial income:
|
||||||||||||
|
Interest income
|
174
|
288
|
414
|
|||||||||
|
Revaluation of financial derivatives
|
4,491
|
-
|
-
|
|||||||||
|
Revaluation of contingent consideration for the purchase of shares
|
-
|
764
|
1,621
|
|||||||||
|
Exchange differences, net
|
-
|
-
|
131
|
|||||||||
|
4,665
|
1,052
|
2,166
|
||||||||||
|
Financial expense:
|
||||||||||||
|
Interest in respect of IIA grants
|
792
|
844
|
847
|
|||||||||
|
Revaluation of contingent consideration for the purchase of shares
|
612
|
-
|
-
|
|||||||||
|
Exchange differences, net
|
652
|
614
|
-
|
|||||||||
|
Other
|
57
|
38
|
49
|
|||||||||
|
2,113
|
1,496
|
896
|
||||||||||
| NOTE 21:- |
NET LOSS PER SHARE
|
| a. |
Details of the number of shares and loss used in the computation of loss per share from continuing operations and from discontinued operation:
|
|
Year ended
December 31,
|
||||||||||||||||||
|
2014
|
2015
|
2016
|
||||||||||||||||
|
Weighted
average
number of shares
|
Loss
|
Weighted
average
number of shares
|
Loss
|
Weighted
average
number of shares
|
Loss
|
|||||||||||||
|
Basic and diluted loss from continuing operation
|
19,939,528
|
(18,875
|
) |
21,718,401
|
(21,671
|
) |
21,862,169
|
(18,885
|
) | |||||||||
|
Basic and diluted loss from discontinued operation
|
-
|
-
|
21,718,401
|
(417
|
) |
-
|
-
|
|||||||||||
| b. |
Net loss per share from continuing and discontinued operations:
|
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Basic and Diluted loss per share:
|
||||||||||||
|
Net loss from continuing operations
|
(0.95
|
)
|
(1.00
|
)
|
(0.86
|
)
|
||||||
|
Loss from discontinued operation
|
-
|
(0.02
|
)
|
-
|
||||||||
|
Net loss per share
|
(0.95
|
)
|
(1.02
|
)
|
(0.86
|
)
|
||||||
| NOTE 22:- |
BALANCES AND TRANSACTIONS WITH RELATED PARTIES AND OFFICERS
|
| a. |
Related parties consist of:
|
| • |
Clal Biotechnologies Industries Ltd.-the Parent Company.
|
| • |
PolyHeal-in which the Company holds approximately 6.5% (see Note 19).
|
| • |
Directors of the Company.
|
| b. |
Balances of related parties:
|
|
Receivables
|
Payables
|
|||||||
|
Parent Company
(1):
|
||||||||
|
As of December 31, 2015
|
-
|
207
|
||||||
|
As of December 31, 2016
|
-
|
218
|
||||||
|
Other related parties:
|
||||||||
|
As of December 31, 2015
|
20
|
41
|
||||||
|
As of December 31, 2016
|
-
|
77
|
||||||
| (1) |
The Company leases office space and a production facility from the Parent Company in accordance with a sublease agreement (see Note 15 (e)).
|
| c. |
Transactions with related parties:
|
|
Professional
Fee (1)
|
Rent expenses
|
|||||||
|
Parent company:
|
||||||||
|
2014
|
12
|
|
576
|
|
||||
|
2015
|
52
|
|
730
|
|
||||
|
2016
|
27
|
|
804
|
|
||||
|
Other related parties:
|
||||||||
|
2014
|
80
|
|
-
|
|||||
|
2015
|
127
|
|
-
|
|||||
|
2016
|
159
|
|
-
|
|||||
| (1) |
Professional fees do not include short-term employee benefits and share-based compensation to one of the Company's shareholders, who is a key officer, in the amounts of $699, $691 and $537 for the years 2014, 2015 and 2016, respectively, as well as payment for the purchasing of a patent in amount of $30, $30 and $30 for the years 2014, 2015 and 2016, respectively ( see note 15c).
|
| NOTE 22:- |
BALANCES AND TRANSACTIONS WITH RELATED PARTIES AND OFFICERS (Cont.)
|
| d. |
Compensation of officers of the Company:
|
|
Year ended
December 31,
|
||||||||||||
|
2014
|
2015
|
2016
|
||||||||||
|
Short-term employee benefits
|
2,314
|
2,639
|
2,108
|
|||||||||
|
Share-based compensation
|
2,949
|
1,702
|
1,445
|
|||||||||
|
5,263
|
4,341
|
3,553
|
||||||||||
|
Number of officers
|
7
|
7
|
7
|
|||||||||