|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
06-1591613
|
|
(State of organization)
|
|
(I.R.S. Employer Identification Number)
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
Common stock, par value $0.01 per share
|
|
The NASDAQ Stock Market LLC
|
|
Large accelerated filer
☑
|
Accelerated filer ☐
|
Non-accelerated filer
☐
|
Smaller reporting company
☐
|
|
PART I
|
|
|
Item 1
|
|
|
Item 1A
|
|
|
Item 1B
|
|
|
Item 2
|
|
|
Item 3
|
|
|
Item 4
|
|
|
|
|
|
PART II
|
|
|
Item 5
|
|
|
Item 6
|
|
|
Item 7
|
|
|
Item 7A
|
|
|
Item 8
|
|
|
Item 9
|
|
|
Item 9A
|
|
|
Item 9B
|
|
|
|
|
|
PART III
|
|
|
Item 10
|
|
|
Item 11
|
|
|
Item 12
|
|
|
Item 13
|
|
|
Item 14
|
|
|
|
|
|
PART IV
|
|
|
Item 15
|
|
|
Item 16
|
|
|
|
|
|
•
|
our plans to develop and commercialize our product candidates;
|
|
•
|
the outcomes of our ongoing and planned clinical trials and the timing of those outcomes;
|
|
•
|
the timing of and our ability to obtain and maintain regulatory approvals for our product candidates;
|
|
•
|
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
|
|
•
|
our ability to enter into new collaborations or to identify additional products or product candidates with significant commercial potential that are consistent with our commercial objectives;
|
|
•
|
our ability to recover the investment in our manufacturing capabilities;
|
|
•
|
the rate and degree of market acceptance and clinical utility of our products;
|
|
•
|
our commercialization, marketing and manufacturing capabilities and strategy;
|
|
•
|
significant competition in our industry;
|
|
•
|
costs of litigation and the failure to successfully defend lawsuits and other claims against us;
|
|
•
|
economic, political and other risks associated with our international operations;
|
|
•
|
our ability to receive research funding and achieve anticipated milestones under our collaborations;
|
|
•
|
our ability to protect and enforce patents and other intellectual property;
|
|
•
|
costs of compliance and our failure to comply with new and existing governmental regulations including, but not limited to, tax regulations;
|
|
•
|
loss or retirement of key members of management;
|
|
•
|
failure to successfully execute our growth strategy, including any delays in our planned future growth; and
|
|
•
|
our failure to maintain effective internal controls.
|
|
•
|
Therapeutic focus, science driven.
We create therapeutic biological products primarily to treat various types of cancers, including both solid tumors and hematological malignancies. Our proprietary DART and Fc Optimization technology platforms are particularly useful for targeting and harnessing specific elements of the human immune system, allowing us to design molecules that (1) directly target cancer cells and enhance the ability of the immune system to destroy those cells, (2) re-direct effector cells to attack tumors or (3) affect mechanisms that regulate the immune response to cancer, either by stimulating pathways that enhance this response or by blocking pathways that inhibit this response, including checkpoint molecules. This field of scientific discovery, broadly known as immuno-oncology, has been developing rapidly in the last few years, and most therapeutic products to date are largely focused on affecting individual biological pathways. We believe that cancers are sufficiently complex that effective treatments must simultaneously affect more than one pathway. We believe that we are well-positioned, particularly through the adaptability of our DART platform, to be able to create and develop therapeutic molecules designed to simultaneously target more than one pathway.
|
|
•
|
Fully integrated with a deep pipeline.
Our objective is to be a fully-integrated biotechnology company, and we intend to continue to grow and establish all necessary functions from early-stage research through commercialization in at least the United States. At our current stage of development as a company, we have established early-stage discovery, process development, clinical development and clinical-stage manufacturing functions, and we intend to build commercial manufacturing as well as U.S.-based sales and marketing infrastructure as our development pipeline matures.
|
|
•
|
Leveraging collaborations.
Throughout our company's history, we have entered into collaborations with other biopharmaceutical companies and intend to continue to do so. We enter into collaborations when there is a strategic advantage to us to do so and when we believe the financial terms of the collaboration are favorable for meeting our short-term and long-term strategic objectives. We are not dependent upon any one of these collaborations, but in many cases we have rights to receive sales royalties and other significant financial payments if the partnered product candidates achieve certain development and sales milestones. Some of the collaborations also preserve our right to participate in future commercialization, for example by securing co-promotion or profit-sharing rights under certain circumstances.
|
|
•
|
Investments in talent and culture.
One of our most valuable assets is the quality of our employee base. We invest significant effort in selecting and retaining high caliber, talented individuals who reflect our values. As we continue to grow, we continue to seek and develop employees who are strongly committed to delivering life-changing medicines for unmet medical needs through a collaborative work environment.
|

|
•
|
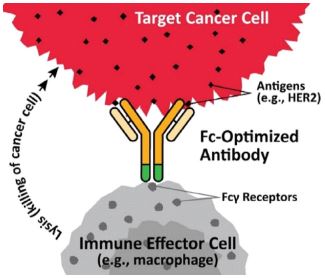
Margetuximab
is a monoclonal antibody that targets HER2-expressing tumors, including certain types of breast and gastroesophageal cancers. HER2 is critical for the growth of many types of tumors. Using our Fc Optimization platform, we have engineered the constant region, or Fc region, of margetuximab to increase its ability to kill tumor cells through an Fc-dependent mechanism, including antibody dependent cell-mediated cytotoxicity, or ADCC.
|
|
•
|
Enoblituzumab
is a monoclonal antibody that targets B7-H3. We engineered enoblituzumab to utilize the same Fc Optimization enhancements that we incorporated in margetuximab to target B7-H3 that is over-expressed on differentiated tumor cells, cancer stem cells and supporting tumor vasculature and underlying tissues. We are currently evaluating enoblituzumab in an ongoing Phase 1 clinical trial as monotherapy in multiple solid tumor types as well as in combination therapy with either an anti-PD-1 antibody or an anti-CTLA-4 antibody.
|
|
•
|
Flotetuzumab (previously known as MGD006)
is a DART molecule that targets both CD123 and CD3. CD123, the Interleukin-3 receptor alpha chain, is expressed on leukemia and leukemic stem cells, but only at very low levels or not at all on normal hematopoietic stem cells. T cells, which express CD3, can destroy tumor cells. In preclinical studies, we have demonstrated the ability of flotetuzumab to recruit, activate, and expand T cell populations to eliminate leukemia cells. We are currently enrolling patients in the United States and Europe in a Phase 1 clinical trial of flotetuzumab in patients with AML or MDS. Under the terms of our collaboration with
|
|
•
|
MGD007
is a DART molecule that targets both the glycoprotein A33 (gpA33) and CD3, and has an Fc domain, which is designed to provide extended pharmacokinetic properties and convenient intermittent dosing. gpA33 is expressed on gastrointestinal tumors, including more than 95% of human colon cancers. We have demonstrated that this molecule is able to mediate T cell killing of gpA33-expressing cancer cells and cancer stem cells in preclinical experiments. We are currently enrolling patients with colorectal cancer in a Phase 1 clinical trial of MGD007. Under the terms of our collaboration with Servier, Servier has an option to obtain exclusive rights to develop and commercialize MGD007 in all countries outside North America, Japan, Korea and India. If the option is exercised, MacroGenics would still retain exclusive rights in those countries.
|
|
•
|
Duvortuxizumab
is a DART molecule that targets both CD19 and CD3 and is being developed for the treatment of B-cell hematological malignancies. CD19, a lymphocyte-specific marker expressed from early B-lymphocyte development through mature memory B cells, is highly represented in B cell malignancies. This makes it attractive for targeted interventions. Duvortuxizumab is designed to redirect T cells, via their CD3 component, to eliminate CD19-expressing cells found in many hematological malignancies. Duvortuxizumab has been engineered to address half-life challenges posed by other programs targeting CD19 and CD3. Like MGD007, this product candidate has an Fc domain, which allows for extended pharmacokinetic properties and convenient dosing at a once-a-week or longer interval. Under our collaboration and license agreement, Janssen is leading the development of this product candidate, subject to our options to co-promote the product in the United States and Canada and to invest in later-stage development in exchange for a United States and Canada profit-share. Janssen has an ongoing Phase 1 study of duvortuxizumab in a variety of B-cell hematological malignancies.
|
|
•
|
MGD009
is the second molecule in our B7-H3 franchise. This DART molecule recognizes B7-H3 and CD3, and has an Fc domain, which is designed to provide extended pharmacokinetic properties. We have demonstrated that this molecule is able to mediate T cell killing of cancer cells in preclinical experiments. We are currently enrolling patients in a Phase 1 clinical trial of MGD009 in patients across a variety of different solid tumors.
|
|
•
|
MGA012
is a monoclonal antibody targeting PD-1. Antibodies targeting PD-1 have shown efficacy against various tumors by releasing the "brakes" on the immune system that are often seen when tumors evade detection by the immune system. We anticipate that MGA012 may be used in combination studies with our other therapeutics. We are currently enrolling patients in a Phase 1 clinical trial of MGA012 in patients across a variety of different solid tumors.
|
|
•
|
MGD013
is a DART molecule that is intended to enable the co-blockade with a single recombinant agent of two immune checkpoint molecules, PD-1 and LAG-3, which may be co-expressed on T cells. We anticipate that MGD013 could be used for the treatment of a wide range of cancers, including both solid tumors and hematological malignancies.
|
|
•
|
MGC018
is a B7-H3 antibody-drug conjugate (ADC) for which we are conducting Investigational New Drug Application (IND)-enabling activities.
MGC018 is based on a MacroGenics proprietary B7-H3 antibody and a duocarmycin-based, linker-drug technology licensed from Synthon Biopharmaceuticals B.V.
|
|
•
|
MGD010
is a DART molecule designed to address limitations of existing B cell-targeted therapies by binding to the CD32B and CD79B proteins found on human B cells. In preclinical studies, this DART molecule modulated the function of human B cells without B cell depletion. In normal conditions, B cells utilize CD32B as one of the key checkpoints or negative regulators to ensure that tolerance to self is maintained and autoimmune disease does not occur. MGD010 is designed to further exploit this mechanism by triggering this inhibitory "immune checkpoint" loop. We believe this molecule preferentially blocks those B cells that are activated to produce the pathogenic antibodies that promote the autoimmune process. We have completed a Phase 1a clinical study in healthy volunteers and observed acceptable safety and pharmacodynamic activity consistent with the expected mechanism of action of MGD010.
|
|
•
|
Teplizumab
is an anti-CD3 monoclonal antibody being developed for the treatment of type 1 diabetes. Teplizumab has been engineered to alter the function of the T cells that mediate the destruction of the insulin-producing beta cells of the islets of the pancreas. Teplizumab potentially represents an advance in the treatment of type 1 diabetes by addressing the underlying disorder, rather than treating the symptoms through insulin replacement therapy. Teplizumab is being evaluated in a Phase 2 clinical trial for potential application to patients
|
|
•
|
MGD014
is a DART molecule that targets the envelope protein of human immunodeficiency virus, or HIV-infected cells (Env) and CD3-expressing T cells. We are developing MGD014 under contract number HHSN272201500032C awarded to us in September 2015 by the National Institute of Allergy and Infectious Diseases, or NIAID, part of the National Institutes of Health. MGD014 is our first DART molecule targeting an infectious agent that is planned for clinical testing. The work under this contract will build on preclinical studies demonstrating that DART molecules targeting the Env and T cells, via their CD3 component, are able to redirect the immune system's T cells to kill HIV-infected cells. DART molecules could be used independently or become a key part of a "shock-and-kill" strategy in conjunction with HIV latency-reversing agents currently under development.
|
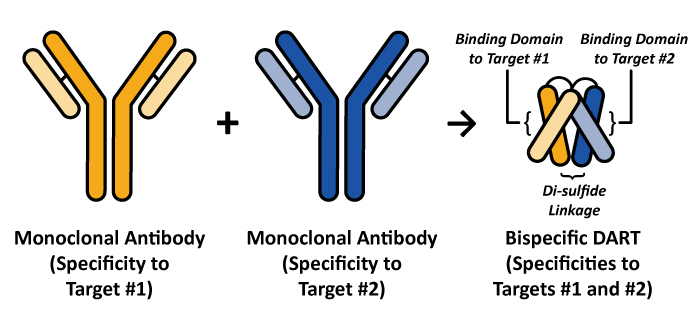
|
•
Redirected T cell activation and killing
. In this version of the DART molecule, we are enabling the cancer-fighting properties of the immune effector cells, such as T lymphocytes to: (1) recognize and bind to structures expressed on a cancer cell (e.g., B7-H3, the first specificity in the example on the right), (2) enable the recruitment of all types of cytotoxic, or cell killing, T cells, irrespective of their ability to recognize cancer cells (e.g., CD3, a common component of the T cell antigen receptor, is the second specificity in the example on the right) and (3) trigger T cell activation, expansion, and cell killing mechanisms to destroy a cancer cell. The outcome is that any of the body's T cells, in theory, could be recruited to destroy a cancer cell and thus, are not limited to the small numbers of specific T cells that might have been generated in response to cancer to kill tumor cells. Furthermore, since any T cell could be recruited for this killing process, only small amounts of a DART molecule are required to trigger this potent immune response. Additionally, the compact structure of the DART protein makes it well suited for maintaining cell-to-cell contact, which we believe contributes to the high level of target cell killing. Similarly, DART molecules targeting CD3 and a viral antigen can be used to recruit T cells to eliminate cells infected by a virus, such as HIV-infected cells.
|

|
|
•
Modulation of receptor signaling
. In another configuration of the DART molecule, we have taken advantage of the two different specificities engineered in a DART structure to bind to particular cells involved in autoimmune processes, such as autoimmune B cells, and to usurp the immune checkpoint signaling pathways programmed within the cells to impede the pathogenic autoimmune responses. Our MGD010 product candidate targets both CD32B, a co-inhibitory molecule, and CD79B, part of the B cell antigen receptor complex, two proteins expressed on the immune system's B cells. Using a single DART molecule, we attempt to promote the interaction of these two receptors, a step required to interrupt the B cell activation and immune response that single antibodies directed against CD32B, CD79B or both cannot accomplish independently.
|

|
|
•
Simultaneous targeting of multiple co-inhibitory receptors or checkpoints, such as those involved in inhibiting T cell responses and B cell responses.
The immune system generally prevents the development of autoimmune phenomena by regulating activated immune cells that have responded to non-self or foreign antigens. This negative feedback loop is triggered by the interactions of co-inhibitory receptors, or checkpoint molecules, expressed on the immune cells with ligands expressed by other cells, such as antigen-presenting cells. This phenomenon is exploited by cancer, whereby tumor cells express checkpoint ligands that block the development of an immune response against the tumor. Antibodies that block the interaction of checkpoint molecules with their ligands have been shown to significantly improve the clinical outcomes of patients with certain advanced cancers. Because of the diversity of immune checkpoint pathways, blockade of a single axis, while clinically significant, as shown in the case of the blockade of the PD-1/PD-L1 axis with pembrolizumab or nivolumab, will not benefit all patients. In fact, combinations of checkpoint inhibitors, such as nivolumab and ipilimumab, a CTLA-4 blocker, have resulted in significantly enhanced benefit compared to ipilimumab or nivolumab alone. We believe that DART molecules targeting two immunoregulatory pathways, such as two checkpoints in a single molecule, could afford the clinical benefit of the combination together with the potential for synergistic activity, as well as significant advantages in manufacturing, simplified clinical development, and enhanced patient convenience.
|

|
|
•
Enhanced effector cell selectivity.
T lymphocytes with lytic effector function belong preferentially to the CD8 lineage, while CD4-positive T cells preferentially provide immune regulatory function, such as the secretion of cytokines or the differentiation into regulatory T cells. Greater selectivity in the recruitment of effector T cells is an example of the range of applications of our TRIDENT technology. By encoding a CD8 recognition arm in addition to the CD3- and tumor antigen-specific arms, our TRIDENT technology allow the preferential engagement of CD8-positive T lymphocytes and redirects them against tumor cells. This strategy allows for retention of lytic effector function, while limiting the CD4 cell engagement and associated effects, such as inflammatory cytokine release.
|

|
|
•
|
MacroGenics-Created Programs
. We have a number of collaborations relating to product candidates that we have created from our internal research efforts. These include Janssen for duvortuxizumab and MGD015; Servier for flotetuzumab and MGD007; and Green Cross Corp., or Green Cross, for margetuximab. In the case of these product candidates, we entered into collaborations because we believed that our collaborator could further enable
|
|
•
|
Joint Research Programs.
We have several programs under which collaborators have sought to utilize some aspect of our protein engineering platforms with new product concepts that are jointly directed, sometimes employing a collaborator's own proprietary technology. These collaborations give us the ability to expand the breadth of our potential products, develop greater scientific expertise and obtain additional funding for research. Pfizer, Inc. and Boehringer Ingelheim GmbH, or Boehringer, are currently advancing projects in their own pipelines based on these types of programs. With these collaborators, we have more limited development or commercial rights related to the product candidates that may emerge from joint research programs, although we will receive royalties from these programs as well as other consideration upon the occurrence of specified development and sales milestones.
|
|
Product Candidate
|
Expiration Date
|
|
margetuximab
|
2029
|
|
enoblituzumab
|
2031
|
|
flotetuzumab
|
2034*
|
|
MGD007
|
2034*
|
|
MGD009
|
2036*
|
|
MGD010
|
2034*
|
|
duvortuxizumab
|
2035*
|
|
MGA012
|
2036*
|
|
•
|
restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials;
|
|
•
|
restrictions on the products, manufacturers, manufacturing facilities or manufacturing process;
|
|
•
|
warning letters;
|
|
•
|
civil and criminal penalties;
|
|
•
|
injunctions;
|
|
•
|
suspension or withdrawal of regulatory approvals;
|
|
•
|
product seizures, detentions or import bans;
|
|
•
|
voluntary or mandatory product recalls and publicity requirements;
|
|
•
|
total or partial suspension of production;
|
|
•
|
imposition of restrictions on operations, including costly new manufacturing requirements; and
|
|
•
|
refusal to approve pending BLAs or supplements to approved BLAs or analogous marketing approvals outside the United States.
|
|
•
|
a product candidate may not be deemed safe or effective;
|
|
•
|
the results may not confirm the positive results from earlier preclinical studies or clinical trials;
|
|
•
|
regulatory agencies may not find the data from preclinical studies and clinical trials sufficient;
|
|
•
|
regulatory agencies might not approve or might require changes to our manufacturing processes or facilities; or
|
|
•
|
regulatory agencies may change their approval policies or adopt new regulations.
|
|
•
|
further discussions with the FDA or other regulatory agencies regarding the scope or design of our clinical trials;
|
|
•
|
the limited number of, and competition for, suitable sites to conduct our clinical trials, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication as our product candidates;
|
|
•
|
any delay or failure to obtain regulatory approval or agreement to commence a clinical trial in any of the countries where enrollment is planned;
|
|
•
|
inability to obtain sufficient funds required for a clinical trial;
|
|
•
|
clinical holds on, or other regulatory objections to, a new or ongoing clinical trial;
|
|
•
|
delay or failure to manufacture sufficient supplies of the product candidate for our clinical trials;
|
|
•
|
delay or failure to reach agreement on acceptable clinical trial agreement terms or clinical trial protocols with prospective sites or clinical research organizations, CROs, the terms of which can be subject to extensive negotiation and may vary significantly among different sites or CROs; and
|
|
•
|
delay or failure to obtain institutional review board, or IRB, approval to conduct a clinical trial at a prospective site.
|
|
•
|
slower than expected rates of patient recruitment and enrollment;
|
|
•
|
failure of patients to complete the clinical trial;
|
|
•
|
unforeseen safety issues, including severe or unexpected drug-related adverse effects experienced by patients, including possible deaths;
|
|
•
|
lack of efficacy during clinical trials;
|
|
•
|
termination of our clinical trials by one or more clinical trial sites;
|
|
•
|
inability or unwillingness of patients or clinical investigators to follow our clinical trial protocols;
|
|
•
|
inability to monitor patients adequately during or after treatment by us and/or our CROs; and
|
|
•
|
the need to repeat or terminate clinical trials as a result of inconclusive or negative results or unforeseen complications in testing.
|
|
•
|
failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
|
|
•
|
unforeseen safety issues or any determination that a clinical trial presents unacceptable health risks;
|
|
•
|
lack of adequate funding to continue the clinical trial due to unforeseen costs or other business decisions; and
|
|
•
|
upon a breach or pursuant to the terms of any agreement with, or for any other reason by, current or future collaborators that have responsibility for the clinical development of any of our product candidates.
|
|
•
|
regulatory authorities may require us to take our approved product off the market;
|
|
•
|
regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field alerts to physicians and pharmacies;
|
|
•
|
we may be required to change the way the product is administered, impose other risk-management measures, conduct additional clinical trials or change the labeling of the product;
|
|
•
|
we may be subject to limitations on how we may promote the product;
|
|
•
|
sales of the product may decrease significantly;
|
|
•
|
we may be subject to litigation or product liability claims; and
|
|
•
|
our reputation may suffer.
|
|
•
|
limitations or warnings contained in the approved labeling for a product candidate;
|
|
•
|
changes in the standard of care for the targeted indications for any of our product candidates;
|
|
•
|
limitations in the approved clinical indications for our product candidates;
|
|
•
|
demonstrated clinical safety and efficacy compared to other products;
|
|
•
|
lack of significant adverse side effects;
|
|
•
|
sales, marketing and distribution support;
|
|
•
|
availability and extent of reimbursement from managed care plans and other third-party payors;
|
|
•
|
timing of market introduction and perceived effectiveness of competitive products;
|
|
•
|
the degree of cost-effectiveness of our product candidates;
|
|
•
|
availability of alternative therapies at similar or lower cost, including generic and over-the-counter products;
|
|
•
|
the extent to which the product candidate is approved for inclusion on formularies of hospitals and managed care organizations;
|
|
•
|
whether the product is designated under physician treatment guidelines as a first-line therapy or as a second- or third-line therapy for particular diseases;
|
|
•
|
adverse publicity about our product candidates or favorable publicity about competitive products;
|
|
•
|
convenience and ease of administration of our products; and
|
|
•
|
potential product liability claims.
|
|
•
|
decreased demand for our future approved products;
|
|
•
|
injury to our reputation;
|
|
•
|
withdrawal of clinical trial participants;
|
|
•
|
termination of clinical trial sites or entire trial programs;
|
|
•
|
increased regulatory scrutiny;
|
|
•
|
significant litigation costs;
|
|
•
|
substantial monetary awards to or costly settlement with patients or other claimants;
|
|
•
|
product recalls or a change in the indications for which they may be used;
|
|
•
|
loss of revenue;
|
|
•
|
diversion of management and scientific resources from our business operations; and
|
|
•
|
the inability to commercialize our product candidates.
|
|
•
|
the number and characteristics of other product candidates and indications that we pursue;
|
|
•
|
the scope, progress, timing, cost and results of research, preclinical development, and clinical trials;
|
|
•
|
the costs, timing and outcome of seeking and obtaining FDA and non-U.S. regulatory approvals;
|
|
•
|
the costs associated with manufacturing our product candidates and establishing sales, marketing, and distribution capabilities;
|
|
•
|
our ability to maintain, expand, and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make in connection with the licensing, filing, defense and enforcement of any patents or other intellectual property rights;
|
|
•
|
our need and ability to hire additional management, scientific, and medical personnel;
|
|
•
|
the effect of competing products that may limit market penetration of our product candidates;
|
|
•
|
our need to implement additional internal systems and infrastructure, including financial and reporting systems; and
|
|
•
|
the economic and other terms, timing of and success of our existing collaborations, and any collaboration, licensing, or other arrangements into which we may enter in the future, including the timing of receipt of any milestone or royalty payments under these agreements.
|
|
•
|
collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations;
|
|
•
|
collaborators may not perform their obligations as expected;
|
|
•
|
collaborators may not pursue development and commercialization of any product candidates that achieve regulatory approval or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborators' strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities;
|
|
•
|
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
|
|
•
|
collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;
|
|
•
|
product candidates discovered in collaboration with us may be viewed by our collaborators as competitive with their own product candidates or products, which may cause collaborators to cease to devote resources to the commercialization of our product candidates;
|
|
•
|
a collaborator with marketing and distribution rights to one or more of our product candidates that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of such product or products;
|
|
•
|
disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time-consuming and expensive;
|
|
•
|
collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
|
|
•
|
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and
|
|
•
|
collaborations may be terminated for the convenience of the collaborator and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable product candidates. For example, each of our collaboration and license agreements may be terminated for convenience upon the completion of a specified notice period.
|
|
•
|
may not perform their obligations as expected;
|
|
•
|
may encounter production difficulties that could constrain the supply of the companion diagnostic;
|
|
•
|
may have difficulties gaining acceptance of the use of the companion diagnostic in the clinical community;
|
|
•
|
may not commit sufficient resources to the marketing and distribution of such product; and
|
|
•
|
may terminate their relationship with us.
|
|
•
|
the possibility of a breach of the manufacturing agreements by the third parties because of factors beyond our control;
|
|
•
|
the possibility of termination or nonrenewal of the agreements by the third parties before we are able to arrange for a qualified replacement third-party manufacturer; and
|
|
•
|
the possibility that we may not be able to secure a manufacturer or manufacturing capacity in a timely manner and on satisfactory terms in order to meet our manufacturing needs.
|
|
•
|
we or our collaborators may initiate litigation or other proceedings against third parties seeking to invalidate the patents held by those third parties or to obtain a judgment that our products or processes do not infringe those third parties' patents;
|
|
•
|
if our competitors file patent applications that claim technology also claimed by us or our licensors, we or our licensors may be required to participate in interference or opposition proceedings to determine the priority of invention, which could jeopardize our patent rights and potentially provide a third party with a dominant patent position;
|
|
•
|
if third parties initiate litigation claiming that our processes or products infringe their patent or other intellectual property rights, we and our collaborators will need to defend against such proceedings; and
|
|
•
|
if a license to necessary technology is terminated, the licensor may initiate litigation claiming that our processes or products infringe or misappropriate their patent or other intellectual property rights and/or that we breached our obligations under the license agreement, and we and our collaborators would need to defend against such proceedings.
|
|
•
|
we or our collaborators may initiate litigation or other proceedings against third parties to enforce our patent rights;
|
|
•
|
third parties may initiate litigation or other proceedings seeking to invalidate patents owned by or licensed to us or to obtain a declaratory judgment that their product or technology does not infringe our patents or patents licensed to us;
|
|
•
|
third parties may initiate opposition, reexamination or inter partes review proceedings challenging the validity or scope of our patent rights, requiring us or our collaborators and/or licensors to participate in such proceedings to defend the validity and scope of our patents;
|
|
•
|
there may be a challenge or dispute regarding inventorship or ownership of patents currently identified as being owned by or licensed to us;
|
|
•
|
the U.S. Patent and Trademark Office may initiate an interference between patents or patent applications owned by or licensed to us and those of our competitors, requiring us or our collaborators and/or licensors to participate in an interference proceeding to determine the priority of invention, which could jeopardize our patent rights; or
|
|
•
|
third parties may seek approval to market biosimilar versions of our future approved products prior to expiration of relevant patents owned by or licensed to us, requiring us to defend our patents, including by filing lawsuits alleging patent infringement.
|
|
•
|
others may be able to develop a platform that is similar to, or better than, ours in a way that is not covered by the claims of our patents;
|
|
•
|
others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of our patents;
|
|
•
|
we might not have been the first to make the inventions covered by patents or pending patent applications;
|
|
•
|
we might not have been the first to file patent applications for these inventions;
|
|
•
|
any patents that we obtain may not provide us with any competitive advantages or may ultimately be found invalid or unenforceable; or
|
|
•
|
we may not develop additional proprietary technologies that are patentable.
|
|
•
|
allow the authorized number of directors to be changed only by resolution of our board of directors;
|
|
•
|
establish a classified board of directors, providing that not all members of the board of directors be elected at one time;
|
|
•
|
authorize our board of directors to issue without stockholder approval blank check preferred stock that, if issued, could operate as a "poison pill" to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors;
|
|
•
|
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent;
|
|
•
|
establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings;
|
|
•
|
limit who may call stockholder meetings; and
|
|
•
|
require the approval of the holders of 75% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our restated certificate of incorporation and restated bylaws.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
|
High
|
Low
|
|||||
|
2016
|
|||||||
|
First Quarter
|
$
|
30.66
|
|
$
|
14.84
|
|
|
|
Second Quarter
|
$
|
28.37
|
|
$
|
16.28
|
|
|
|
Third Quarter
|
$
|
33.30
|
|
$
|
25.25
|
|
|
|
Fourth Quarter
|
$
|
31.85
|
|
$
|
18.22
|
|
|
|
2015
|
|
|
|
|
|||
|
First Quarter
|
$
|
39.90
|
|
$
|
29.50
|
|
|
|
Second Quarter
|
$
|
38.37
|
|
$
|
26.68
|
|
|
|
Third Quarter
|
$
|
39.90
|
|
$
|
20.29
|
|
|
|
Fourth Quarter
|
$
|
36.11
|
|
$
|
19.67
|
|
|

|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
|
(in thousands, except share and per share data)
|
||||||||||||||||||
|
Consolidated Statement of Operations and Comprehensive Income (Loss):
|
|||||||||||||||||||
|
Total revenues
|
$
|
91,880
|
|
$
|
100,854
|
|
$
|
47,797
|
|
$
|
58,035
|
|
$
|
63,826
|
|
||||
|
Cost and expenses:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Research and development
|
122,091
|
|
98,271
|
|
70,186
|
|
46,582
|
|
45,433
|
|
|||||||||
|
General and administrative
|
29,831
|
|
22,765
|
|
15,926
|
|
11,087
|
|
10,188
|
|
|||||||||
|
Total costs and expenses
|
151,922
|
|
121,036
|
|
86,112
|
|
57,669
|
|
55,621
|
|
|||||||||
|
Income (loss) from operations
|
(60,042
|
)
|
(20,182
|
)
|
(38,315
|
)
|
366
|
|
8,205
|
|
|||||||||
|
Other income (expense)
|
1,514
|
|
42
|
|
2
|
|
(627
|
)
|
157
|
|
|||||||||
|
Net income (loss)
|
(58,528
|
)
|
(20,140
|
)
|
(38,313
|
)
|
(261
|
)
|
8,362
|
|
|||||||||
|
Other comprehensive income (loss):
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Unrealized loss on investments
|
(77
|
)
|
(5
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Comprehensive income (loss)
|
$
|
(58,605
|
)
|
$
|
(20,145
|
)
|
$
|
(38,313
|
)
|
$
|
(261
|
)
|
$
|
8,362
|
|
||||
|
Basic and diluted net income (loss) per common share
|
$
|
(1.69
|
)
|
$
|
(0.63
|
)
|
$
|
(1.40
|
)
|
$
|
(0.04
|
)
|
$
|
0.00
|
|
||||
|
Basic and diluted weighted average number of common shares
|
34,685,274
|
|
31,801,645
|
|
27,384,990
|
|
6,847,697
|
|
1,083,276
|
|
|||||||||
|
|
As of December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
|
(in thousands)
|
||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
|||||||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
284,982
|
|
$
|
339,049
|
|
$
|
157,591
|
|
$
|
116,481
|
|
$
|
47,743
|
|
||||
|
Total assets
|
311,263
|
|
359,269
|
|
173,886
|
|
125,782
|
|
53,747
|
|
|||||||||
|
Deferred revenue
|
14,306
|
|
18,497
|
|
30,720
|
|
27,403
|
|
44,080
|
|
|||||||||
|
Total stockholders' equity (deficit)
|
268,751
|
|
313,337
|
|
121,286
|
|
78,914
|
|
(8,237
|
)
|
|||||||||
|
ITEM 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
Janssen
. In December 2014, we entered into a collaboration and license agreement with Janssen for the development and commercialization of duvortuxizumab, a product candidate that incorporates our proprietary DART technology to simultaneously target CD19 and CD3 for the potential treatment of B-cell hematological malignancies. We contemporaneously entered into an agreement with JJDC, an affiliate of Janssen, under which JJDC agreed to purchase 1,923,077 new shares of our common stock for proceeds of $75.0 million. Upon closing, we received a $50.0 million upfront payment from Janssen as well as the $75.0 million investment in our common stock. Janssen is leading the development of this product candidate, subject to our options to co-promote the product in the United States and Canada and to invest in later-stage development in exchange for a United States and Canada profit-share. Janssen initiated a human clinical trial in 2015 for a variety of B-cell hematological malignancies, including diffuse-large B cell lymphoma, follicular lymphoma, mantle-cell lymphoma, chronic lymphocytic leukemia and acute lymphoblastic leukemia. The initiation of this trial triggered a $10.0 million milestone payment to us. Assuming successful development and commercialization, we could receive up to an additional $565.0 million in clinical, regulatory and commercialization milestone payments. If commercialized, we would be eligible to receive low double-digit royalties on any global net sales.
|
|
•
|
Servier.
In September 2012, we entered into an agreement with Servier to develop and commercialize three DART molecules in all countries other than the United States, Canada, Mexico, Japan, South Korea and India. We received a $20.0 million upfront option fee. In addition, we became eligible to receive up to approximately $1.0 billion in additional license fees and clinical, development, regulatory and sales milestone payments for each product Servier successfully develops, obtains regulatory approval for, and commercializes. Additionally, assuming exercise of its options, Servier may share Phase 2 and Phase 3 development costs and would be obligated to pay us low double-digit to mid-teen royalties on product sales in its territories.
|
|
•
|
Boehringer:
In October 2010, we entered into an agreement with Boehringer to discover, develop, and commercialize multiple DART molecules for which we granted an exclusive, worldwide, royalty-bearing license. These DART molecules were evaluated during a five year period that ended in October 2015. We continue to have the potential to earn additional development, regulatory and sales milestone payments that can reach up to approximately $205.0 million for each of the two ongoing programs under development. Boehringer would be required to pay us mid-single digit royalties on product sales.
|
|
•
|
Pfizer
: In October 2010, we entered into an agreement with Pfizer to discover, develop and commercialize multiple DART molecules for which we granted an exclusive, worldwide, royalty-bearing license. We continue to be eligible to receive development and sales milestone payments that can reach up to approximately $200.0 million for the ongoing program under development, PF-06671008, which is being evaluated by Pfizer in a Phase 1 trial. Pfizer would also be required to pay us mid-single digit to low-teen royalties on product sales.
|
|
•
|
Employee-related expenses such as salaries and benefits;
|
|
•
|
Employee-related overhead expenses such as facilities and other allocated items;
|
|
•
|
Stock-based compensation expense to employees and consultants engaged in research and development activities;
|
|
•
|
Depreciation of laboratory equipment, computers and leasehold improvements;
|
|
•
|
Fees paid to consultants, subcontractors, clinical research organizations (CROs) and other third party vendors for work performed under our preclinical and clinical trials including but not limited to investigator grants, laboratory work and analysis, database management, statistical analysis, and other items;
|
|
•
|
Amounts paid to vendors and suppliers for laboratory supplies;
|
|
•
|
Costs related to manufacturing clinical trial materials, including vialing, packaging and testing;
|
|
•
|
License fees and other third party vendor payments related to in-licensed product candidates and technology; and
|
|
•
|
Costs related to compliance with regulatory requirements.
|
|
•
|
Fees paid to CROs for services performed on clinical trials;
|
|
•
|
Fees paid to investigator sites for performance on clinical trials;
|
|
•
|
Fees paid for professional services; and
|
|
•
|
Development expenses incurred by our collaborators which we share.
|
|
•
|
Fair Value of Common Stock
–
Before our entry into the public market on October 10, 2013, our Board of Directors determined the fair value of the common stock. The Board of Directors made determinations of fair value based, in part, upon contemporaneous valuations to determine fair value. The contemporaneous valuations were performed in accordance with applicable methodologies, approaches and assumptions of the technical practice-aid issued by the American Institute of Certified Public Accountants Practice Aid entitled
Valuation of Privately-Held Company Equity Securities Issued as Compensation
.
|
|
•
|
Expected Volatility
– Volatility is a measure of the amount by which a financial variable such as a share price has fluctuated (historical volatility) or is expected to fluctuate (expected volatility) during a period. As we do not yet have sufficient history of our own volatility, we have identified several public entities of similar size, complexity and stage of development and estimate volatility based on the volatility of these companies.
|
|
•
|
Expected Dividend Yield
– We have never declared or paid dividends and have no plans to do so in the foreseeable future.
|
|
•
|
Risk-Free Interest Rate
– This is the U.S. Treasury rate for the week of each option grant during the year, having a term that most closely resembles the expected life of the option.
|
|
•
|
Expected Term
– This is the period of time that the options granted are expected to remain unexercised. Options granted have a maximum term of ten years and we have estimated the expected life of the option term to be 6.25 years. We use a simplified method to calculate the average expected term.
|
|
•
|
Expected Forfeiture Rate
– The forfeiture rate is the estimated percentage of options granted that is expected to be forfeited or canceled on an annual basis before becoming fully vested. We estimate the forfeiture rate based on turnover data with further consideration given to the class of the employees to whom the options were granted.
|
|
Year Ended December 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2016
|
2015
|
|||||||||||||
|
(dollars in millions)
|
||||||||||||||
|
Revenue from collaborative agreements
|
$
|
86.6
|
|
$
|
99.4
|
|
$
|
(12.8
|
)
|
(13
|
)%
|
|||
|
Revenue from government agreements
|
5.3
|
|
1.5
|
|
3.8
|
|
253
|
%
|
||||||
|
Total revenue
|
$
|
91.9
|
|
$
|
100.9
|
|
$
|
(9.0
|
)
|
(9
|
)%
|
|||
|
Year Ended December 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2016
|
2015
|
|||||||||||||
|
(dollars in millions)
|
||||||||||||||
|
Margetuximab
|
$
|
35.4
|
|
$
|
41.2
|
|
$
|
(5.8
|
)
|
(14
|
)%
|
|||
|
Enoblituzumab
|
18.0
|
|
11.9
|
|
6.1
|
|
51
|
%
|
||||||
|
Flotetuzumab (a)
|
3.8
|
|
3.0
|
|
0.8
|
|
27
|
%
|
||||||
|
MGD007
|
3.6
|
|
3.9
|
|
(0.3
|
)
|
(8
|
)%
|
||||||
|
MGD009
|
3.3
|
|
4.0
|
|
(0.7
|
)
|
(18
|
)%
|
||||||
|
MGD010
|
7.8
|
|
7.6
|
|
0.2
|
|
3
|
%
|
||||||
|
MGA012
|
9.3
|
|
3.6
|
|
5.7
|
|
158
|
%
|
||||||
|
MGD013
|
8.7
|
|
5.4
|
|
3.3
|
|
61
|
%
|
||||||
|
Immune checkpoint programs
|
6.3
|
|
—
|
|
6.3
|
|
NA
|
|
||||||
|
Other preclinical and clinical programs, collectively
|
25.9
|
|
17.7
|
|
8.2
|
|
46
|
%
|
||||||
|
Total research and development expense
|
$
|
122.1
|
|
$
|
98.3
|
|
$
|
23.8
|
|
24
|
%
|
|||
|
Year Ended December 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2016
|
2015
|
|||||||||||||
|
(dollars in millions)
|
||||||||||||||
|
General and administrative expense
|
$
|
29.8
|
|
$
|
22.8
|
|
$
|
7.0
|
|
31
|
%
|
|||
|
|
Year Ended December 31,
|
Increase/(Decrease)
|
||||||||||||
|
|
2015
|
2014
|
||||||||||||
|
|
(dollars in millions)
|
|||||||||||||
|
Revenue from collaborative agreements
|
$
|
99.4
|
|
$
|
47.3
|
|
$
|
52.1
|
|
110
|
%
|
|||
|
Revenue from government agreements
|
1.5
|
|
0.5
|
|
1.0
|
|
200
|
%
|
||||||
|
Total revenue
|
$
|
100.9
|
|
$
|
47.8
|
|
$
|
53.1
|
|
111
|
%
|
|||
|
Year Ended December 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2015
|
2014
|
|||||||||||||
|
(dollars in millions)
|
||||||||||||||
|
Margetuximab
|
$
|
41.2
|
|
$
|
19.3
|
|
$
|
21.9
|
|
113
|
%
|
|||
|
Enoblituzumab
|
11.9
|
|
13.6
|
|
(1.7
|
)
|
(13
|
)%
|
||||||
|
Flotetuzumab
|
3.0
|
|
3.5
|
|
(0.5
|
)
|
(14
|
)%
|
||||||
|
MGD007
|
3.9
|
|
4.0
|
|
(0.1
|
)
|
(3
|
)%
|
||||||
|
MGD009
|
4.0
|
|
4.2
|
|
(0.2
|
)
|
(5
|
)%
|
||||||
|
MGD010
|
7.6
|
|
3.9
|
|
3.7
|
|
95
|
%
|
||||||
|
Duvortuxizumab
|
1.7
|
|
5.1
|
|
(3.4
|
)
|
(67
|
)%
|
||||||
|
Preclinical immune checkpoint programs
|
9.0
|
|
1.3
|
|
7.7
|
|
592
|
%
|
||||||
|
Other preclinical and clinical programs, collectively
|
16.0
|
|
15.3
|
|
0.7
|
|
5
|
%
|
||||||
|
Total research and development expense
|
$
|
98.3
|
|
$
|
70.2
|
|
$
|
28.1
|
|
40
|
%
|
|||
|
Year Ended December 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2015
|
2014
|
|||||||||||||
|
(dollars in millions)
|
||||||||||||||
|
General and administrative expense
|
$
|
22.8
|
|
$
|
15.9
|
|
$
|
6.9
|
|
44
|
%
|
|||
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
(dollars in millions)
|
|||||||||||
|
Net cash provided by (used in):
|
|||||||||||
|
Operating activities
|
$
|
(43.7
|
)
|
$
|
(13.7
|
)
|
$
|
(32.8
|
)
|
||
|
Investing activities
|
(70.2
|
)
|
(152.1
|
)
|
(3.6
|
)
|
|||||
|
Financing activities
|
1.9
|
|
204.3
|
|
77.4
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
(112.1
|
)
|
$
|
38.6
|
|
$
|
41.1
|
|
||
|
Total
|
Less than 1 year
|
1 to 3 years
|
3 to 5 years
|
More than 5 years
|
|||||||||||||||
|
(in millions)
|
|||||||||||||||||||
|
Operating Leases
|
$
|
21.7
|
|
$
|
6.3
|
|
$
|
8.0
|
|
$
|
4.7
|
|
$
|
2.7
|
|
||||
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
ITEM 9.
|
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
•
|
pertain to the management of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company;
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements.
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
(1)
|
Consolidated Financial Statements:
|
|
Report of Ernst & Young LLP, Independent Registered Public Accounting Firm on the Audited Consolidated Financial Statements
|
F-2
|
|
Consolidated Balance Sheets
|
F-3
|
|
Consolidated Statements of Operations and Comprehensive Loss
|
F-4
|
|
Consolidated Statements of Stockholders' Equity
|
F-5
|
|
Consolidated Statements of Cash Flows
|
F-6
|
|
Notes to Consolidated Financial Statements
|
F-7
|
|
(2)
|
Financial Statement Schedules:
|
|
(3)
|
Exhibits
|
|
|
MacroGenics, Inc.
|
|
|
|
|
|
|
|
By:
|
/s/ Scott Koenig
|
|
|
|
Scott Koenig, M.D., Ph.D.
|
|
|
|
President and CEO and Director
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ Scott Koenig
|
|
President and CEO and Director
|
|
February 28, 2017
|
|
Scott Koenig, M.D., Ph.D.
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/ James Karrels
|
|
Senior Vice President, Chief Financial
|
|
February 28, 2017
|
|
James Karrels
|
|
Officer and Secretary (Principal Financial Officer)
|
|
|
|
|
|
|
|
|
|
/s/ Lynn Cilinski
|
|
Vice President, Controller and Treasurer
|
|
February 28, 2017
|
|
Lynn Cilinski
|
|
(Principal Accounting Officer)
|
|
|
|
|
|
|
|
|
|
/s/ Paulo Costa
|
|
Director
|
|
February 28, 2017
|
|
Paulo Costa
|
|
|
|
|
|
/s/ Karen Ferrante, M.D.
|
Director
|
February 28, 2017
|
||
|
Karen Ferrante, M.D.
|
||||
|
|
|
|
|
|
|
/s/ Matthew Fust
|
|
Director
|
|
February 28, 2017
|
|
Matthew Fust
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Kenneth Galbraith
|
|
Director
|
|
February 28, 2017
|
|
Kenneth Galbraith
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Edward Hurwitz
|
|
Director
|
|
February 28, 2017
|
|
Edward Hurwitz
|
|
|
|
|
|
/s/ Scott Jackson
|
Director
|
|
February 28, 2017
|
|
|
Scott Jackson
|
||||
|
|
|
|
|
|
|
/s/ David Stump, M.D.
|
|
Director
|
|
February 28, 2017
|
|
David Stump, M.D.
|
|
|
|
|
|
Page
Number
|
|
|
/s/ Ernst & Young LLP
|
|
|
Baltimore, Maryland
|
|
|
February 28, 2017
|
|
|
December 31,
|
|||||||
|
2016
|
2015
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
84,098
|
|
$
|
196,172
|
|
|
|
Marketable securities
|
192,898
|
|
142,877
|
|
|||
|
Accounts receivable
|
2,764
|
|
1,224
|
|
|||
|
Prepaid expenses
|
3,483
|
|
1,806
|
|
|||
|
Other current assets
|
704
|
|
305
|
|
|||
|
Total current assets
|
283,947
|
|
342,384
|
|
|||
|
Property and equipment, net
|
17,961
|
|
14,841
|
|
|||
|
Marketable securities, non-current
|
7,986
|
|
—
|
|
|||
|
Other assets
|
1,369
|
|
2,044
|
|
|||
|
Total assets
|
$
|
311,263
|
|
$
|
359,269
|
|
|
|
Liabilities and stockholders' equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
3,995
|
|
$
|
2,967
|
|
|
|
Accrued expenses
|
16,134
|
|
11,708
|
|
|||
|
Deferred revenue
|
4,261
|
|
5,866
|
|
|||
|
Deferred rent
|
1,319
|
|
914
|
|
|||
|
Lease exit liability
|
1,593
|
|
2,020
|
|
|||
|
Other current liabilities
|
—
|
|
727
|
|
|||
|
Total current liabilities
|
27,302
|
|
24,202
|
|
|||
|
Deferred revenue, net of current portion
|
10,045
|
|
12,631
|
|
|||
|
Deferred rent, net of current portion
|
4,867
|
|
6,406
|
|
|||
|
Lease exit liability, net of current portion
|
298
|
|
2,693
|
|
|||
|
Total liabilities
|
42,512
|
|
45,932
|
|
|||
|
Stockholders' equity:
|
|||||||
|
Common stock, $0.01 par value – 125,000,000 shares authorized, 34,870,607 and 34,345,754 shares outstanding at December 31, 2016 and 2015, respectively
|
349
|
|
343
|
|
|||
|
Additional paid-in capital
|
561,198
|
|
547,185
|
|
|||
|
Accumulated other comprehensive loss
|
(82
|
)
|
(5
|
)
|
|||
|
Accumulated deficit
|
(292,714
|
)
|
(234,186
|
)
|
|||
|
Total stockholders' equity
|
268,751
|
|
313,337
|
|
|||
|
Total liabilities and stockholders' equity
|
$
|
311,263
|
|
$
|
359,269
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Revenues:
|
|||||||||||
|
Revenue from collaborative agreements
|
$
|
86,582
|
|
$
|
99,368
|
|
$
|
47,264
|
|
||
|
Revenue from government agreements
|
5,298
|
|
1,486
|
|
533
|
|
|||||
|
Total revenues
|
91,880
|
|
100,854
|
|
47,797
|
|
|||||
|
Costs and expenses:
|
|
|
|
|
|
|
|||||
|
Research and development
|
122,091
|
|
98,271
|
|
70,186
|
|
|||||
|
General and administrative
|
29,831
|
|
22,765
|
|
15,926
|
|
|||||
|
Total costs and expenses
|
151,922
|
|
121,036
|
|
86,112
|
|
|||||
|
Loss from operations
|
(60,042
|
)
|
(20,182
|
)
|
(38,315
|
)
|
|||||
|
Other income
|
1,514
|
|
42
|
|
2
|
|
|||||
|
Net loss
|
(58,528
|
)
|
(20,140
|
)
|
(38,313
|
)
|
|||||
|
Other comprehensive loss:
|
|
|
|
|
|
|
|||||
|
Unrealized loss on investments
|
(77
|
)
|
(5
|
)
|
—
|
|
|||||
|
Comprehensive loss
|
$
|
(58,605
|
)
|
$
|
(20,145
|
)
|
$
|
(38,313
|
)
|
||
|
Basic and diluted net loss per common share
|
$
|
(1.69
|
)
|
$
|
(0.63
|
)
|
$
|
(1.40
|
)
|
||
|
Basic and diluted weighted average number of common shares
|
34,685,274
|
|
31,801,645
|
|
27,384,990
|
|
|||||
|
Common Stock
|
Treasury Stock
|
Additional
Paid-In
Capital
|
Accumulated
Deficit
|
Accumulated
Other
Comprehensive Income
|
Total
Stockholders'
Equity
|
||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||
|
Balance, December 31, 2013
|
25,177,597
|
|
$
|
252
|
|
14,381
|
|
$
|
(58
|
)
|
$
|
254,453
|
|
$
|
(175,733
|
)
|
$
|
—
|
|
$
|
78,914
|
|
|||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
3,244
|
|
—
|
|
—
|
|
3,244
|
|
|||||||||||||
|
Issuance of common stock, net of offering costs
|
2,250,000
|
|
22
|
|
—
|
|
—
|
|
76,694
|
|
—
|
|
—
|
|
76,716
|
|
|||||||||||||
|
Stock plan related activity
|
568,041
|
|
6
|
|
865
|
|
(19
|
)
|
738
|
|
—
|
|
—
|
|
725
|
|
|||||||||||||
|
Retirement of treasury stock
|
—
|
|
—
|
|
(14,381
|
)
|
58
|
|
(58
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(38,313
|
)
|
—
|
|
(38,313
|
)
|
|||||||||||||
|
Balance, December 31, 2014
|
27,995,638
|
|
280
|
|
865
|
|
(19
|
)
|
335,071
|
|
(214,046
|
)
|
—
|
|
121,286
|
|
|||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
7,847
|
|
—
|
|
—
|
|
7,847
|
|
|||||||||||||
|
Issuance of common stock, net of offering costs
|
5,976,827
|
|
60
|
|
—
|
|
—
|
|
203,407
|
|
—
|
|
—
|
|
203,467
|
|
|||||||||||||
|
Stock plan related activity
|
373,289
|
|
3
|
|
925
|
|
(29
|
)
|
908
|
|
—
|
|
—
|
|
882
|
|
|||||||||||||
|
Retirement of treasury stock
|
—
|
|
—
|
|
(1,790
|
)
|
48
|
|
(48
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Unrealized loss on investments
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(5
|
)
|
(5
|
)
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(20,140
|
)
|
—
|
|
(20,140
|
)
|
|||||||||||||
|
Balance, December 31, 2015
|
34,345,754
|
|
343
|
|
—
|
|
—
|
|
547,185
|
|
(234,186
|
)
|
(5
|
)
|
313,337
|
|
|||||||||||||
|
Share-based compensation
|
|
|
|
|
|
|
|
|
12,165
|
|
|
|
|
|
12,165
|
|
|||||||||||||
|
Stock plan related activity
|
524,853
|
|
6
|
|
1,862
|
|
(39
|
)
|
1,887
|
|
|
|
|
|
1,854
|
|
|||||||||||||
|
Retirement of treasury stock
|
|
|
|
|
(1,862
|
)
|
39
|
|
(39
|
)
|
|
|
|
|
—
|
|
|||||||||||||
|
Unrealized loss on investments
|
|
|
|
|
|
|
|
|
|
|
|
|
(77
|
)
|
(77
|
)
|
|||||||||||||
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
(58,528
|
)
|
|
|
(58,528
|
)
|
|||||||||||||
|
Balance, December 31, 2016
|
34,870,607
|
|
$
|
349
|
|
—
|
|
$
|
—
|
|
$
|
561,198
|
|
$
|
(292,714
|
)
|
$
|
(82
|
)
|
$
|
268,751
|
|
|||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Operating activities
|
|||||||||||
|
Net loss
|
$
|
(58,528
|
)
|
$
|
(20,140
|
)
|
$
|
(38,313
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation and amortization expense
|
7,608
|
|
2,863
|
|
1,822
|
|
|||||
|
Share-based compensation
|
12,165
|
|
7,847
|
|
3,244
|
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|||||
|
Accounts receivable
|
(1,540
|
)
|
1,711
|
|
(931
|
)
|
|||||
|
Prepaid expenses
|
(1,677
|
)
|
2,405
|
|
(3,239
|
)
|
|||||
|
Restricted cash
|
—
|
|
300
|
|
105
|
|
|||||
|
Other assets
|
276
|
|
(285
|
)
|
(1,179
|
)
|
|||||
|
Accounts payable
|
2,232
|
|
(163
|
)
|
(1,500
|
)
|
|||||
|
Accrued expenses
|
4,659
|
|
3,545
|
|
4,346
|
|
|||||
|
Lease exit liability
|
(2,822
|
)
|
(3,293
|
)
|
(1,439
|
)
|
|||||
|
Deferred revenue
|
(4,191
|
)
|
(12,223
|
)
|
3,317
|
|
|||||
|
Deferred rent
|
(1,134
|
)
|
4,650
|
|
(234
|
)
|
|||||
|
Other liabilities
|
(727
|
)
|
(878
|
)
|
1,242
|
|
|||||
|
Net cash used in operating activities
|
(43,679
|
)
|
(13,661
|
)
|
(32,759
|
)
|
|||||
|
Cash flows from investing activities
|
|
|
|
|
|
|
|||||
|
Purchases of marketable securities
|
(347,762
|
)
|
(142,910
|
)
|
—
|
|
|||||
|
Proceeds from sales and maturities of marketable securities
|
288,894
|
|
—
|
|
—
|
|
|||||
|
Purchases of property and equipment
|
(11,381
|
)
|
(9,197
|
)
|
(3,572
|
)
|
|||||
|
Net cash used in investing activities
|
(70,249
|
)
|
(152,107
|
)
|
(3,572
|
)
|
|||||
|
Cash flows from financing activities
|
|
|
|
|
|
|
|||||
|
Proceeds from issuance of common stock, net of offering costs
|
—
|
|
203,467
|
|
76,716
|
|
|||||
|
Proceeds from stock option exercises
|
1,893
|
|
911
|
|
744
|
|
|||||
|
Purchase of treasury stock
|
(39
|
)
|
(29
|
)
|
(19
|
)
|
|||||
|
Net cash provided by financing activities
|
1,854
|
|
204,349
|
|
77,441
|
|
|||||
|
Net change in cash and cash equivalents
|
(112,074
|
)
|
38,581
|
|
41,110
|
|
|||||
|
Cash and cash equivalents at beginning of period
|
196,172
|
|
157,591
|
|
116,481
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
84,098
|
|
$
|
196,172
|
|
$
|
157,591
|
|
||
|
•
|
Level 1 – Fair value is determined by using unadjusted quoted prices that are available in active markets for identical assets and liabilities.
|
|
•
|
Level 2 – Fair value is determined by using inputs other than Level 1 quoted prices that are directly or indirectly observable. Inputs can include quoted prices for similar assets and liabilities in active markets or quoted prices for identical assets and liabilities in inactive markets. Related inputs can also include those used in valuation or other pricing models, such as interest rates and yield curves that can be corroborated by observable market data.
|
|
•
|
Level 3 – Fair value is determined by inputs that are unobservable and not corroborated by market data. Use of these inputs involves significant and subjective judgments to be made by a reporting entity – e.g., determining an appropriate adjustment to a discount factor for illiquidity associated with a given security.
|
|
Fair Value Measurement at December 31, 2016
|
|||||||||||||||
|
Quoted Prices in
Active Markets for
Identical Assets
|
Significant Other
Observable Inputs
|
Significant
Unobservable
Inputs
|
|||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Assets:
|
|||||||||||||||
|
Money market funds
|
$
|
46,781
|
|
$
|
46,781
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S Treasury securities
|
8,826
|
|
—
|
|
8,826
|
|
—
|
|
|||||||
|
Government-sponsored enterprises
|
29,759
|
|
—
|
|
29,759
|
|
—
|
|
|||||||
|
Corporate debt securities
|
166,300
|
|
—
|
|
166,300
|
|
—
|
|
|||||||
|
Total assets measured at fair value
(a)
|
$
|
251,666
|
|
$
|
46,781
|
|
$
|
204,885
|
|
$
|
—
|
|
|||
|
Fair Value Measurement at December 31, 2015
|
|||||||||||||||
|
Quoted Prices in
Active Markets for
Identical Assets
|
Significant Other
Observable Inputs
|
Significant
Unobservable
Inputs
|
|||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Assets:
|
|||||||||||||||
|
Money market funds
|
$
|
62,353
|
|
$
|
62,353
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S Treasury securities
|
9,348
|
|
—
|
|
9,348
|
|
—
|
|
|||||||
|
Government-sponsored enterprises
|
41,202
|
|
—
|
|
41,202
|
|
—
|
|
|||||||
|
Corporate debt securities
|
137,928
|
|
—
|
|
137,928
|
|
—
|
|
|||||||
|
Total assets measured at fair value
(a)
|
$
|
250,831
|
|
$
|
62,353
|
|
$
|
188,478
|
|
$
|
—
|
|
|||
|
Year Ended December 31,
|
||||||||
|
2016
|
2015
|
2014
|
||||||
|
Janssen Biotech, Inc. (Janssen)
|
85
|
%
|
72
|
%
|
*
|
|
||
|
Les Laboratoires Servier and Institut de Reserches Servier (collectively, Servier)
|
*
|
|
*
|
|
36
|
%
|
||
|
Boehringer Ingelheim GmbH (Boehringer)
|
*
|
|
12
|
%
|
29
|
%
|
||
|
Takeda Pharmaceutical Company Limited (Takeda)
|
*
|
|
*
|
|
17
|
%
|
||
|
Gilead Sciences, Inc. (Gilead)
|
*
|
|
*
|
|
11
|
%
|
||
|
December 31,
|
|||||
|
2016
|
2015
|
||||
|
Janssen
|
40
|
%
|
39
|
%
|
|
|
U.S. government
|
19
|
%
|
20
|
%
|
|
|
Servier
|
31
|
%
|
14
|
%
|
|
|
Takeda
|
*
|
|
14
|
%
|
|
|
Eli Lilly
|
*
|
|
13
|
%
|
|
|
Computer equipment
|
3 years
|
|
Software
|
3 years
|
|
Furniture
|
10 years
|
|
Laboratory and office equipment
|
5 years
|
|
Leasehold improvements
|
Shorter of lease term or useful life
|
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
Numerator:
|
|||||||||||
|
Net loss used for calculation of basic and diluted EPS
|
$
|
(58,528
|
)
|
$
|
(20,140
|
)
|
$
|
(38,313
|
)
|
||
|
Denominator:
|
|||||||||||
|
Weighted average shares outstanding, basic
|
34,685,274
|
|
31,801,645
|
|
27,384,990
|
|
|||||
|
Effect of dilutive securities:
|
|||||||||||
|
Stock options and restricted stock units
|
—
|
|
—
|
|
—
|
|
|||||
|
Weighted average shares outstanding, diluted
|
34,685,274
|
|
31,801,645
|
|
27,384,990
|
|
|||||
|
Net loss per share, basic and diluted
|
$
|
(1.69
|
)
|
$
|
(0.63
|
)
|
$
|
(1.40
|
)
|
||
|
Year Ended December 31,
|
||||||||
|
2016
|
2015
|
2014
|
||||||
|
Stock options
|
1,394,608
|
|
1,955,398
|
|
2,094,904
|
|
||
|
December 31, 2016
|
|||||||||||||||
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair
Value
|
||||||||||||
|
U.S. Treasury securities
|
$
|
4,826
|
|
$
|
—
|
|
$
|
(1
|
)
|
$
|
4,825
|
|
|||
|
Government-sponsored enterprises
|
29,764
|
|
5
|
|
(10
|
)
|
29,759
|
|
|||||||
|
Corporate debt securities
|
166,376
|
|
51
|
|
(127
|
)
|
166,300
|
|
|||||||
|
Total
|
$
|
200,966
|
|
$
|
56
|
|
$
|
(138
|
)
|
$
|
200,884
|
|
|||
|
December 31, 2015
|
|||||||||||||||
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair
Value
|
||||||||||||
|
U.S. Treasury securities
|
$
|
9,354
|
|
$
|
1
|
|
$
|
(6
|
)
|
$
|
9,349
|
|
|||
|
Government-sponsored enterprises
|
22,055
|
|
1
|
|
(9
|
)
|
22,047
|
|
|||||||
|
Corporate debt securities
|
111,473
|
|
42
|
|
(34
|
)
|
111,481
|
|
|||||||
|
Total
|
$
|
142,882
|
|
$
|
44
|
|
$
|
(49
|
)
|
$
|
142,877
|
|
|||
|
Amortized Cost
|
Fair Value
|
||||||
|
Mature in one year or less
|
$
|
192,985
|
|
$
|
192,898
|
|
|
|
Mature between one and five years
|
7,981
|
|
7,986
|
|
|||
|
Total
|
200,966
|
|
200,884
|
|
|||
|
December 31,
|
|||||||
|
2016
|
2015
|
||||||
|
Computer equipment
|
$
|
2,520
|
|
$
|
3,069
|
|
|
|
Software
|
2,352
|
|
1,801
|
|
|||
|
Furniture and office equipment
|
897
|
|
919
|
|
|||
|
Lab equipment
|
20,208
|
|
17,306
|
|
|||
|
Leasehold improvements
|
17,807
|
|
11,936
|
|
|||
|
Property and equipment
|
43,784
|
|
35,031
|
|
|||
|
Less accumulated depreciation
|
(25,823
|
)
|
(20,190
|
)
|
|||
|
Property and equipment, net
|
$
|
17,961
|
|
$
|
14,841
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
Research and development
|
$
|
5,778
|
|
$
|
3,623
|
|
$
|
1,562
|
|
||
|
General and administrative
|
6,387
|
|
4,224
|
|
1,682
|
|
|||||
|
Total stock-based compensation expense
|
$
|
12,165
|
|
$
|
7,847
|
|
$
|
3,244
|
|
||
|
Year Ended December 31,
|
|||||
|
2016
|
2015
|
2014
|
|||
|
Expected dividend yield
|
0%
|
0%
|
0%
|
||
|
Expected volatility
|
64% - 69%
|
73% - 75%
|
67%
|
||
|
Risk-free interest rate
|
1.2% - 2.4%
|
1.6% - 2.1%
|
1.8% - 2.3%
|
||
|
Expected term
|
6.25 years
|
6.25 years
|
6.25 years
|
||
|
Shares
|
Weighted-Average
Exercise Price
|
Weighted-Average
Remaining
Contractual Term
(Years)
|
Aggregate Intrinsic Value
(in thousands)
|
|||||||||
|
Outstanding, December 31, 2015
|
4,146,064
|
|
$
|
16.90
|
|
7.4
|
||||||
|
Granted
|
399,949
|
|
23.20
|
|
|
|||||||
|
Options exercised or RSUs vested
|
(526,715
|
)
|
3.59
|
|
|
|||||||
|
Forfeited or expired
|
(181,238
|
)
|
26.53
|
|
|
|||||||
|
Outstanding, December 31, 2016
|
3,838,060
|
|
18.93
|
|
7.0
|
$
|
24,862
|
|
||||
|
December 31, 2016:
|
|
|
|
|
|
|
|
|||||
|
Exercisable
|
2,292,923
|
|
13.71
|
|
6.1
|
22,458
|
|
|||||
|
Vested and expected to vest
|
3,651,523
|
|
18.57
|
|
7.0
|
24,496
|
|
|||||
|
December 31,
|
|||||||
|
2016
|
2015
|
||||||
|
Deferred income tax assets:
|
|||||||
|
Federal U.S. net operating loss carryforward
|
$
|
75,377
|
|
$
|
57,949
|
|
|
|
State net operating loss carryforward
|
6,583
|
|
3,907
|
|
|||
|
Research and development credit, net
|
12,829
|
|
10,278
|
|
|||
|
Orphan drug credit, net
|
19,855
|
|
19,284
|
|
|||
|
Deferred rent
|
2,497
|
|
2,947
|
|
|||
|
Deferred revenue
|
5,098
|
|
6,632
|
|
|||
|
Depreciation
|
2,926
|
|
1,597
|
|
|||
|
Other
|
5,085
|
|
2,532
|
|
|||
|
Gross deferred income tax assets
|
130,250
|
|
105,126
|
|
|||
|
Valuation allowance
|
(128,844
|
)
|
(104,399
|
)
|
|||
|
Net deferred income tax assets
|
1,406
|
|
727
|
|
|||
|
Deferred income tax liabilities:
|
|
|
|
|
|||
|
Prepaid expenditures
|
(1,406
|
)
|
(727
|
)
|
|||
|
Gross deferred income tax liabilities
|
(1,406
|
)
|
(727
|
)
|
|||
|
Net deferred income tax asset/(liability)
|
$
|
—
|
|
$
|
—
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
United States federal tax at statutory rate
|
$
|
(20,489
|
)
|
$
|
(7,049
|
)
|
$
|
(13,410
|
)
|
||
|
State taxes (net of federal benefit)
|
(3,116
|
)
|
(897
|
)
|
(1,608
|
)
|
|||||
|
Deferred income tax adjustments
|
173
|
|
661
|
|
—
|
|
|||||
|
Deferred state blended rate adjustments
|
(32
|
)
|
(493
|
)
|
3,034
|
|
|||||
|
Research credit, net
|
(2,551
|
)
|
(3,296
|
)
|
(2,228
|
)
|
|||||
|
Transaction cost deduction
|
—
|
|
—
|
|
(379
|
)
|
|||||
|
Transaction cost deduction - prior year adjustment
|
—
|
|
—
|
|
(564
|
)
|
|||||
|
Orphan drug credit, net
|
(571
|
)
|
(106
|
)
|
(139
|
)
|
|||||
|
Other permanent items
|
145
|
|
(25
|
)
|
(382
|
)
|
|||||
|
Equity-based compensation
|
1,997
|
|
1,102
|
|
756
|
|
|||||
|
Change in valuation allowance
|
24,444
|
|
10,103
|
|
14,920
|
|
|||||
|
Income tax expense/(benefit)
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
Beginning balance
|
$
|
2,425
|
|
$
|
2,047
|
|
$
|
1,708
|
|
||
|
Increases/(decreases) for current year tax positions
|
308
|
|
357
|
|
242
|
|
|||||
|
Increases/(decreases) for prior year tax positions
|
(268
|
)
|
21
|
|
97
|
|
|||||
|
Ending balance
|
$
|
2,465
|
|
$
|
2,425
|
|
$
|
2,047
|
|
||
|
Accrual balance at December 31, 2014
|
$
|
8,006
|
|
|
Principal payments and other adjustments
|
(3,293
|
)
|
|
|
Accrual balance at December 31, 2015
|
4,713
|
|
|
|
Principal payments and other adjustments (net of sublease receipts)
|
(2,822
|
)
|
|
|
Accrual balance at December 31, 2016
|
$
|
1,891
|
|
|
2017
|
$
|
1,593
|
|
|
2018
|
298
|
|
|
|
Total
|
$
|
1,891
|
|
|
2017
|
$
|
6,314
|
|
|
2018
|
4,210
|
|
|
|
2019
|
3,803
|
|
|
|
2020
|
2,142
|
|
|
|
2021
|
2,574
|
|
|
|
Thereafter
|
2,636
|
|
|
|
$
|
21,679
|
|
|
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
2016
|
|||||||||||||||
|
Revenue
|
$
|
2,846
|
|
$
|
80,673
|
|
$
|
3,255
|
|
$
|
5,106
|
|
|||
|
Net income (loss)
|
(30,363
|
)
|
40,464
|
|
(33,846
|
)
|
(34,783
|
)
|
|||||||
|
Net income (loss) per share, basic
|
$
|
(0.88
|
)
|
$
|
1.17
|
|
$
|
(0.97
|
)
|
$
|
(1.00
|
)
|
|||
|
Net income (loss) per share, diluted
|
$
|
(0.88
|
)
|
$
|
1.12
|
|
$
|
(0.97
|
)
|
$
|
(1.00
|
)
|
|||
|
2015
|
|
|
|
|
|
|
|
|
|||||||
|
Revenue
|
$
|
71,279
|
|
$
|
6,716
|
|
$
|
14,681
|
|
$
|
8,178
|
|
|||
|
Net income (loss)
|
45,129
|
|
(21,376
|
)
|
(15,442
|
)
|
(28,451
|
)
|
|||||||
|
Net income (loss) per share, basic
|
$
|
1.53
|
|
$
|
(0.71
|
)
|
$
|
(0.46
|
)
|
$
|
(0.83
|
)
|
|||
|
Net income (loss) per share, diluted
|
$
|
1.42
|
|
$
|
(0.71
|
)
|
$
|
(0.46
|
)
|
$
|
(0.83
|
)
|
|||
|
Exhibit
No.
|
Description
|
|
|
3.1
|
Restated Certificate of Incorporation of the Company and Certificate of Correction to the Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibits 3.1 and 3.3, respectively, to the Company's Current Report on Form 8-K filed on October 18, 2013)
|
|
|
3.2
|
Amended and Restated By-Laws of the Company (incorporated by reference to Exhibit 3.4 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 1, 2013)
|
|
|
4.1
|
Specimen Stock Certificate (incorporated by reference to Exhibit 4.2 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 9, 2013)
|
|
|
4.2†
|
Investor Agreement by and between Johnson and Johnson Innovation-JJDC, Inc. and the Company, dated December 19, 2014 (incorporated by reference to Exhibit 4.3 to the Company's Annual Report on Form 10-K filed on March 3, 2015)
|
|
|
10.1
|
Form of Indemnification Agreement (incorporated by reference to Exhibit 10.14 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 1, 2013)
|
|
|
10.2†
|
Option for a License Agreement by and between the Company and Les Laboratoires Servier and Institut de Recherches Servier, dated September 19, 2012 (incorporated by reference to Exhibit 10.20 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 4, 2013)
|
|
|
10.3†
|
Collaboration and License Agreement by and between Janssen Biotech, Inc. and the Company, dated December 19, 2014 (incorporated by reference to Exhibit 10.25 to the Company's Annual Report on Form 10-K filed on March 3, 2015)
|
|
|
10.4+
|
Company 2003 Equity Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on September 4, 2013)
|
|
|
10.5+
|
Form of Incentive Stock Option Agreement under 2003 Equity Incentive Plan (incorporated by reference to Exhibit 10.4 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on September 4, 2013)
|
|
|
10.6+
|
Company 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.5 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 1, 2013)
|
|
|
10.7+
|
Form of Incentive Stock Option Agreement under 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.6 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 1, 2013)
|
|
|
10.8+
|
Form of Nonstatutory Stock Option Agreement under 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.7 to the Registration Statement on Form S-1 (File No. 333-190994) filed by the Company on October 1, 2013)
|
|
|
10.9+
|
Form of Restricted Stock Units Grant Notice under 2013 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q filed on May 6, 2015)
|
|
|
10.10+
|
2016 Employee Stock Purchase Plan (incorporated by reference to Exhibit 4.1 to the Registration Statement on Form S-8 (File No. 333-214386) filed by the Company on November 2, 2016)
|
|
|
10.11+
|
Employment Agreement between the Company and Scott Koenig, M.D., Ph.D. (incorporated by reference to Exhibit 10.14 to the Company's Annual Report on Form 10-K filed by the Company on February 29, 2016)
|
|
|
10.12+
|
Employment Agreement between the Company and James Karrels (incorporated by reference to Exhibit 10.15 to the Company's Annual Report on Form 10-K filed by the Company on February 29, 2016)
|
|
|
10.13+
|
Employment Agreement between the Company and Jon Wigginton, M.D. (incorporated by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on May 4, 2016)
|
|
|
10.14+
|
Restricted Stock Units Grant Notice and Agreement between the Company and Jon Wigginton, M.D. (incorporated by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q filed on May 4, 2016)
|
|
|
10.15+
|
Employment Agreement between the Company and Ezio Bonvini, M.D. (incorporated by reference to Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q filed on May 4, 2016)
|
|
|
10.16+
|
Employment Agreement between the Company and Eric Risser
|
|
|
23.1
|
Consent of Ernst & Young, LLP, Independent Registered Public Accounting Firm
|
|
|
31.1
|
Rule 13a-14(a) Certification of Principal Executive Officer
|
|
|
31.2
|
Rule 13a-14(a) Certification of Principal Financial Officer
|
|
|
32.1
|
Section 1350 Certification of Principal Executive Officer
|
|
|
32.2
|
Section 1350 Certification of Principal Financial Officer
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Schema Document
|
|
|
101.CAL
|
XBRL Calculation Linkbase Document
|
|
|
101.DEF
|
XBRL Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Labels Linkbase Document
|
|
|
101.PRE
|
XBRL Presentation Linkbase Document
|
|
|
†
|
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment granted by the SEC.
|
|
+
|
Indicates management contract or compensatory plan.
|