|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
|
for the fiscal year ended December 31, 2017
|
|
|
or
|
|
|
o
|
Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
|

|
Utah
|
|
0-18592
|
|
87-0447695
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(Commission File No.)
|
|
(IRS Employer Identification No.)
|
|
Large Accelerated Filer
x
|
Accelerated Filer
o
|
Non-Accelerated Filer
o
(Do not check if a smaller reporting company)
|
Smaller Reporting Company
o
|
Emerging Growth Company
o
|
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
•
|
risks relating to managing growth, particularly if accomplished through acquisitions, and the integration of acquired businesses;
|
|
•
|
risks relating to protecting our intellectual property;
|
|
•
|
claims by third parties that we infringe their intellectual property rights which could cause us to incur significant legal or licensing expenses and prevent us from selling our products;
|
|
•
|
greater scrutiny and regulation by governmental authorities, including risks relating to the subpoena we received in October 2016 from the U.S. Department of Justice seeking information on our marketing and promotional practices;
|
|
•
|
risks relating to physicians’ use of our products in unapproved circumstances;
|
|
•
|
FDA regulatory clearance processes and any failure to obtain and maintain required regulatory clearances and approvals;
|
|
•
|
disruption of our critical information systems or material breaches in the security of our systems;
|
|
•
|
failure to comply with export control laws, customs laws, domestic procurement laws, sanctions laws and other laws governing our operations in the U.S. and other countries, which could subject us to civil or criminal penalties, other remedial measures and legal expenses;
|
|
•
|
risks relating to significant adverse changes in, or our failure to comply, with governing regulations;
|
|
•
|
restrictions and limitations in our debt agreements and instruments, which could affect our ability to operate our business and our liquidity;
|
|
•
|
expending significant resources for research, development, testing and regulatory approval or clearance of our products under development and any failure to develop the products, any failure of the products to be effective or any failure to obtain approvals for commercial use;
|
|
•
|
violations of laws targeting fraud and abuse in the healthcare industry:
|
|
•
|
risks relating to healthcare reform legislation negatively affecting our financial results, business, operations or financial condition;
|
|
•
|
changes in the regulatory approval process and requirements in foreign countries, which could force us to incur additional expense or experience delays or uncertainties;
|
|
•
|
loss of key personnel;
|
|
•
|
product liability claims;
|
|
•
|
failure to report adverse medical events to the FDA, which may subject us to sanctions that may materially harm our business;
|
|
•
|
failure to maintain or establish sales capabilities on our own or through third parties, which may result in our inability to commercialize any of our products in countries where we lack direct sales and marketing capabilities;
|
|
•
|
the addressable market for our product groups being smaller than our estimates;
|
|
•
|
demands for price concessions resulting from consolidations in the healthcare industry, group purchasing organizations or public procurement policies;
|
|
•
|
our inability to compete in markets, particularly if there is a significant change in relevant practices or technology;
|
|
•
|
the effect of evolving U.S. and international laws and regulations regarding privacy and data protection;
|
|
•
|
fluctuations in foreign currency exchange rates negatively impacting our financial results;
|
|
•
|
termination or interruption of, or a failure to monitor, our supply relationships or increases in the price of our component parts, finished products, third-party services or raw materials, particularly petroleum-based products;
|
|
•
|
our inability to accurately forecast customer demand for our products or manage our inventory;
|
|
•
|
changes in international and national economic and industry conditions;
|
|
•
|
inability to generate sufficient cash flow to fund our debt obligations, capital expenditures, and ongoing operations;
|
|
•
|
risks relating to our revenues being derived from a few products and medical procedures;
|
|
•
|
volatility of the market price of our common stock;
|
|
•
|
risks relating to work stoppage, transportation interruptions, severe weather and natural disasters;
|
|
•
|
fluctuations in our effective tax rate adversely affecting our business, financial condition or results of operation;
|
|
•
|
limits on reimbursement imposed by governmental and other programs;
|
|
•
|
failure to comply with applicable environmental laws and regulations; and
|
|
•
|
other factors referenced in our press releases and in our reports filed with the Securities and Exchange Commission (the “SEC”).
|
|
Item 1.
|
Business.
|
|
•
|
enhancing growth and profitability through research and development, sales model optimization, cost discipline, and operational focus;
|
|
•
|
optimizing our operational capability through lean processes, cost effective environments and asset utilization;
|
|
•
|
targeting high-growth, high-return opportunities by understanding, innovating, and delivering in our core product groups; and
|
|
•
|
maintaining a highly disciplined, customer-focused enterprise guided by strong core values to globally address unmet or underserved healthcare needs.
|
|
•
|
Peripheral Intervention: $2.3 billion (global)
|
|
•
|
Cardiac Intervention: $1.8 billion (global)
|
|
•
|
Cardiovascular and Critical Care: $3.4 billion (global)
|
|
•
|
Interventional Oncology and Spine: $1.4 billion (global)
|
|
•
|
Endoscopy: $496 million (U.S. domestic)
|
|
•
|
Dallas, Texas
|
|
•
|
Galway, Ireland
|
|
•
|
Jackson Township, New Jersey
|
|
•
|
Malvern, Pennsylvania
|
|
•
|
Paris, France
|
|
•
|
Pearland, Texas
|
|
•
|
San Jose, California
|
|
•
|
Singapore
|
|
•
|
South Jordan, Utah
|
|
•
|
Tijuana, Mexico
|
|
•
|
Venlo, The Netherlands
|
|
•
|
West Jordan, Utah
|
|
•
|
Class I devices are those for which safety and effectiveness can be reasonably assured by adherence to the FDA’s general regulatory controls, which include compliance with the applicable portions of the FDA’s Quality System Regulations (QSRs), facility registration and product listing, reporting of certain adverse medical events and malfunctions, and compliance with the FDA’s restrictions against misbranding and adulteration. While most Class I devices are exempt
|
|
•
|
Class II devices are subject to the FDA’s general controls, including the design control requirements of the QSRs, and any other special controls deemed necessary by the FDA to provide reasonable assurance of the safety and effectiveness of the device. While most Class II devices require premarket review and clearance by the FDA through the 510(k) premarket notification procedure, some Class II devices are exempt from the 510(k) premarket notification process (assuming they are within the limitations of the exemption).
|
|
•
|
Class III devices are those deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or those devices deemed not substantially equivalent to a legally marketed predicate device. Class III devices include those devices for which the FDA has determined that general and special controls alone are insufficient to assure the safety and effectiveness of the device.
|
|
Item 1A.
|
Risk Factors.
|
|
•
|
be developed successfully;
|
|
•
|
be proven safe or effective in clinical trials;
|
|
•
|
offer therapeutic or other improvements over current treatments and products;
|
|
•
|
meet applicable regulatory standards or receive regulatory approvals or clearances;
|
|
•
|
be capable of production in commercial quantities at acceptable costs and in compliance with regulatory requirements;
|
|
•
|
be successfully marketed; or
|
|
•
|
be covered by private or public insurers.
|
|
Item 1B.
|
Unresolved Staff Comments.
|
|
Item 2.
|
Properties.
|
|
Owned
|
Leased
|
Total
|
||||||
|
U.S.
|
552,207
|
|
492,473
|
|
1,044,680
|
|
||
|
International
|
344,181
|
|
554,907
|
|
899,088
|
|
||
|
Total
|
896,388
|
|
1,047,380
|
|
1,943,768
|
|
||
|
Item 3.
|
Legal Proceedings.
|
|
Item 4.
|
Mine Safety Disclosures.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
For the year ended December 31, 2017
|
High
|
Low
|
||||||
|
First Quarter
|
$
|
31.70
|
|
$
|
24.23
|
|
||
|
Second Quarter
|
$
|
38.55
|
|
$
|
28.00
|
|
||
|
Third Quarter
|
$
|
42.60
|
|
$
|
36.25
|
|
||
|
Fourth Quarter
|
$
|
45.90
|
|
$
|
36.21
|
|
||
|
For the year ended December 31, 2016
|
High
|
Low
|
||||||
|
First Quarter
|
$
|
19.49
|
|
$
|
15.47
|
|
||
|
Second Quarter
|
$
|
20.59
|
|
$
|
17.94
|
|
||
|
Third Quarter
|
$
|
25.08
|
|
$
|
19.61
|
|
||
|
Fourth Quarter
|
$
|
26.85
|
|
$
|
20.70
|
|
||
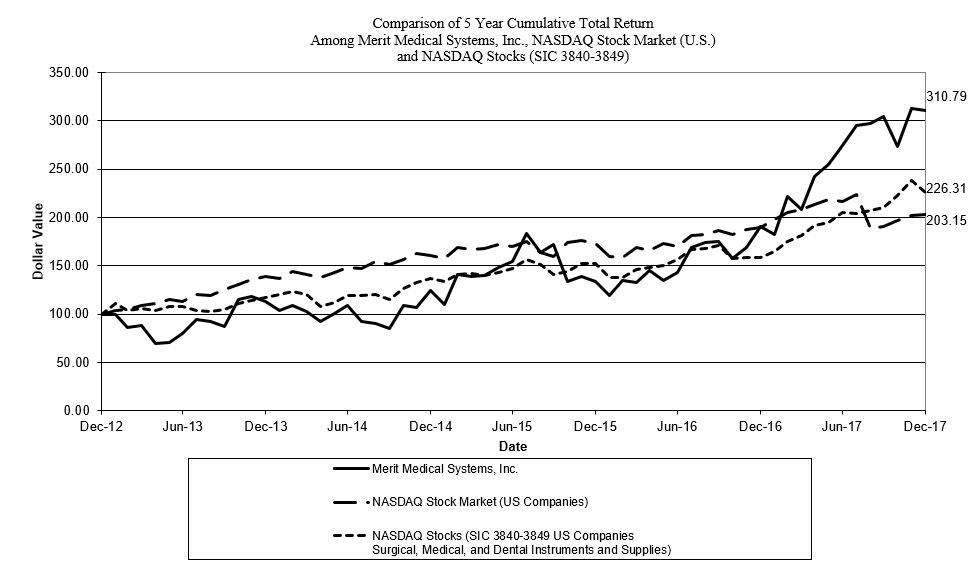
|
|
12/2012
|
12/2013
|
12/2014
|
12/2015
|
12/2016
|
12/2017
|
|||||||||||||||||
|
Merit Medical Systems, Inc.
|
$
|
100
|
|
$
|
113
|
|
$
|
125
|
|
$
|
134
|
|
$
|
191
|
|
$
|
311
|
|
|||||
|
NASDAQ Stock Market (U.S. Companies)
|
100
|
|
139
|
|
161
|
|
173
|
|
190
|
|
203
|
|
|||||||||||
|
NASDAQ Stocks (SIC 3840-3849 U.S. Companies)
|
100
|
|
117
|
|
137
|
|
153
|
|
159
|
|
226
|
|
|||||||||||
|
NOTE:
|
Performance graph data is complete through last fiscal year. Performance graph with peer group uses peer group only performance (excludes only Merit). Peer group indices use beginning of period market capitalization weighting. Index Data: Calculated (or Derived) based from CRSP NASDAQ Stock Market (US Companies), Center for Research in Security Prices (CRSP®), Graduate School of Business, The University of Chicago. Copyright 2018. Used with permission. All rights reserved.
|
|
|
Number of
securities to be
issued upon
exercise of
outstanding
options, warrants
and rights
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
Number of securities
remaining available for
future issuance under
equity compensation plans
(excluding securities
reflected in column (a))
|
||||||
|
Plan category
|
(a)
|
(b)
|
(c)
|
||||||
|
Equity compensation Plans approved by security holders
|
3,623 (1),(3)
|
$
|
20.40
|
|
619 (2),(3)
|
||||
|
Item 6.
|
Selected Financial Data (in thousands, except per share amounts).
|
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
||||||||||||||
|
OPERATING DATA:
|
|
|
|
|
|
|
|||||||||||||
|
Net Sales
|
$
|
727,852
|
|
$
|
603,838
|
|
$
|
542,149
|
|
$
|
509,689
|
|
$
|
449,049
|
|
||||
|
Cost of Sales
|
401,599
|
|
338,813
|
|
306,368
|
|
284,467
|
|
254,682
|
|
|||||||||
|
Gross Profit
|
326,253
|
|
265,025
|
|
235,781
|
|
225,222
|
|
194,367
|
|
|||||||||
|
Operating Expenses:
|
|
|
|
|
|||||||||||||||
|
Selling, general, and administrative
|
229,134
|
|
184,398
|
|
156,348
|
|
147,894
|
|
128,642
|
|
|||||||||
|
Research and development
|
51,403
|
|
45,229
|
|
40,810
|
|
36,632
|
|
33,886
|
|
|||||||||
|
Intangible asset impairment charge
|
809
|
|
—
|
|
—
|
|
1,102
|
|
8,089
|
|
|||||||||
|
Contingent consideration expense (benefit)
|
(298
|
)
|
61
|
|
80
|
|
(572
|
)
|
(4,094
|
)
|
|||||||||
|
Acquired in-process research and development
|
12,136
|
|
461
|
|
1,000
|
|
—
|
|
—
|
|
|||||||||
|
Total operating expenses
|
293,184
|
|
230,149
|
|
198,238
|
|
185,056
|
|
166,523
|
|
|||||||||
|
Income from Operations
|
33,069
|
|
34,876
|
|
37,543
|
|
40,166
|
|
27,844
|
|
|||||||||
|
|
|
||||||||||||||||||
|
Other Income (Expense):
|
|
|
|
|
|||||||||||||||
|
Interest income
|
381
|
|
81
|
|
272
|
|
217
|
|
255
|
|
|||||||||
|
Interest expense
|
(7,736
|
)
|
(8,798
|
)
|
(6,229
|
)
|
(8,829
|
)
|
(8,044
|
)
|
|||||||||
|
Bargain purchase gain
|
11,039
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Other income (expense)
|
(872
|
)
|
(773
|
)
|
(386
|
)
|
18
|
|
(216
|
)
|
|||||||||
|
Other income (expense)—net
|
2,812
|
|
(9,490
|
)
|
(6,343
|
)
|
(8,594
|
)
|
(8,005
|
)
|
|||||||||
|
|
|
||||||||||||||||||
|
Income Before Income Taxes
|
35,881
|
|
25,386
|
|
31,200
|
|
31,572
|
|
19,839
|
|
|||||||||
|
|
|
||||||||||||||||||
|
Income Tax Expense
|
8,358
|
|
5,265
|
|
7,398
|
|
8,598
|
|
3,269
|
|
|||||||||
|
Net Income
|
$
|
27,523
|
|
$
|
20,121
|
|
$
|
23,802
|
|
$
|
22,974
|
|
$
|
16,570
|
|
||||
|
|
|
||||||||||||||||||
|
Earnings Per Common Share:
|
|
|
|
|
|||||||||||||||
|
Diluted
|
$
|
0.55
|
|
$
|
0.45
|
|
$
|
0.53
|
|
$
|
0.53
|
|
$
|
0.39
|
|
||||
|
Average Common Shares:
|
|
|
|
|
|||||||||||||||
|
Diluted
|
50,101
|
|
44,862
|
|
44,511
|
|
43,409
|
|
42,884
|
|
|||||||||
|
BALANCE SHEET DATA:
|
|
|
|
|
|||||||||||||||
|
Working capital
|
$
|
200,501
|
|
$
|
155,092
|
|
$
|
116,093
|
|
$
|
116,910
|
|
$
|
110,321
|
|
||||
|
Total assets
|
1,111,811
|
|
942,803
|
|
778,728
|
|
747,165
|
|
728,283
|
|
|||||||||
|
Long-term debt, less current portion
|
259,013
|
|
314,373
|
|
197,593
|
|
214,490
|
|
238,854
|
|
|||||||||
|
Stockholders’ equity
|
676,334
|
|
498,189
|
|
466,103
|
|
435,259
|
|
405,706
|
|
|||||||||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
|
•
|
Achieve® Automatic Biopsy System
|
|
•
|
Temno® Soft Tissue Biopsy System
|
|
•
|
Tru-Cut® Biopsy Device
|
|
•
|
CorVocet™ Biopsy System
|
|
•
|
Aspira® Pleural Effusion Drainage System
|
|
•
|
Aspira® Peritoneal Drainage System
|
|
•
|
SwiftNINJA® Steerable Microcatheter
|
|
•
|
Elation® GI & Pulmonary Balloons
|
|
•
|
TWISTER® PLUS Rotatable Retrieval Device
|
|
•
|
Prelude IDeal™ Hydrophilic Sheath Introduer
|
|
•
|
Prelude SYNC™ Radial Compression Device
|
|
•
|
Prelude Choice™ Hemostasis Valve Adapter
|
|
•
|
HeRO® Graft
|
|
•
|
Super HeRO®
|
|
•
|
True Form™ Guide Wires
|
|
•
|
Heartspan® Transseptal Sheath
|
|
•
|
Amplatz Guide Wires
|
|
•
|
Critical care products acquired from Argon
|
|
•
|
DualCap® disinfection and protection products acquired from Catheter Connections
|
|
•
|
QuadraSphere® Q2 Microsphere
|
|
•
|
We anticipate continued international expansion through the transition from a distributor-based sales model to a modified direct sales model, which is already in place in a number of markets, including China. We believe this transition will improve revenue growth opportunities by providing us with greater control over the sales channel and improving gross margins, as we move from a wholesale channel to a retail channel. On the other hand, the transition may result in increased costs, primarily as a result of increased compensation expenses for existing and new sales personnel.
|
|
•
|
We also anticipate we will continue to expand product registrations of existing products and introduce new products in new and emerging markets, in an effort to increase the breadth of our product portfolio offered in international markets, thereby supporting revenue growth and margin expansion. Improvement in gross margin remains a key priority for management, through the management of product mix, continued improvement of operational performance and continued new product introductions. However, any reversal in the aforementioned trends could have a negative impact on our future revenue and gross margin.
|
|
•
|
Our revenue growth has been driven by, and we expect our revenue to continue to increase in the future as a result of, the introduction of new products, continued international expansion, and increased physician awareness of our products, among other factors. Any reversal in these trends could have a negative impact on our future revenue. In addition, we have continuously expanded our sales and marketing infrastructure to help us drive and support revenue growth and we intend to continue this expansion.
|
|
•
|
Our revenue may fluctuate, from quarter to quarter, as well as within each quarter, due to a variety of factors, including the seasonality of demand for our products, foreign exchange fluctuations, the timing of new product introductions, competitor product introductions, associated physician evaluations and competitor pricing changes.
|
|
•
|
Our gross margin has been, and we expect it will continue to be, affected by a variety of factors, including product sales mix, geographic sales mix and prices, launch of new products, the impact of distributor relationships and our focus on expanding to a modified direct sales model, production volumes, manufacturing costs and product yields, and the implementation of cost-reduction strategies. As we continue to expand through acquisitions, the acquisitions may be gross margin dilutive. Our gross margins could be negatively affected to the extent that the products acquired have gross margins that differ from ours. For example, the gross margin for the critical care products we acquired from Argon during 2017 is less than our current gross margin. However, improvement in gross margin remains a key priority for management, through the control of product mix, continued improvement of operational performance and continued introductions of new product.
|
|
•
|
The integration of recently completed acquisitions may increase our operating expenses, and it may take time to realize expected revenue from acquisitions. While we expect to integrate our acquired businesses successfully, the expected synergies may not materialize.
|
|
•
|
We continue to experience a variety of financial risks including changes in foreign currency exchange rates, especially when our acquisitions increase the proportion of our revenue from international sales; risks associated with our variable floating rate borrowings, which could negatively affect us in an increasing interest rate environment; and the potentially substantial changes to fiscal, healthcare, trade and tax policies and legislation, which may include comprehensive tax reform and changes to existing trade agreements, including, but not limited to, NAFTA, as well as healthcare reform, including the potential repeal of certain provisions of the Affordable Care Act.
|
|
•
|
On December 22, 2017, the U.S. government enacted the TCJA, which makes changes to the corporate tax rate, business-related deductions and taxation of foreign earnings, among others, that will generally be effective for taxable years beginning after December 31, 2017. We continue to evaluate the TCJA requirements, as well as its applications to our business operations.
|
|
|
2017
|
2016
|
2015
|
||
|
Net sales
|
100%
|
100%
|
100%
|
||
|
Gross profit
|
44.8
|
43.9
|
43.5
|
||
|
Selling, general and administrative expenses
|
31.5
|
30.5
|
28.8
|
||
|
Research and development expenses
|
7.1
|
7.5
|
7.5
|
||
|
Intangible asset impairment charges
|
0.1
|
—
|
—
|
||
|
Contingent consideration expense (benefit)
|
—
|
—
|
—
|
||
|
Acquired in-process research and development expenses
|
1.7
|
0.1
|
0.2
|
||
|
Income from operations
|
4.5
|
5.8
|
6.9
|
||
|
Income before income taxes
|
4.9
|
4.2
|
5.8
|
||
|
Net income
|
3.8
|
3.3
|
4.4
|
||
|
|
% Change
|
2017
|
% Change
|
2016
|
% Change
|
2015
|
|||||||||||
|
Cardiovascular
|
|
|
|
|
|
|
|||||||||||
|
Stand-alone devices
|
44%
|
$
|
275,431
|
|
23%
|
$
|
191,148
|
|
8%
|
$
|
155,414
|
|
|||||
|
Custom kits and procedure trays
|
6%
|
126,114
|
|
2%
|
119,226
|
|
5%
|
116,368
|
|
||||||||
|
Inflation devices
|
8%
|
79,875
|
|
1%
|
73,916
|
|
1%
|
73,373
|
|
||||||||
|
Catheters
|
13%
|
127,747
|
|
17%
|
113,367
|
|
11%
|
96,833
|
|
||||||||
|
Embolization devices
|
8%
|
49,532
|
|
2%
|
46,035
|
|
3%
|
45,025
|
|
||||||||
|
CRM/EP
|
15%
|
41,914
|
|
8%
|
36,459
|
|
3%
|
33,902
|
|
||||||||
|
Total
|
21%
|
700,613
|
|
11%
|
580,151
|
|
6%
|
520,915
|
|
||||||||
|
Endoscopy
|
|
|
|
|
|||||||||||||
|
Endoscopy devices
|
15%
|
27,239
|
|
12%
|
23,687
|
|
18%
|
21,234
|
|
||||||||
|
Total
|
21%
|
$
|
727,852
|
|
11%
|
$
|
603,838
|
|
6%
|
$
|
542,149
|
|
|||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Operating Income
(1)
|
|
|
|
|
|||||||
|
Cardiovascular
|
$
|
24,819
|
|
$
|
30,053
|
|
$
|
34,052
|
|
||
|
Endoscopy
|
8,250
|
|
4,823
|
|
3,491
|
|
|||||
|
Total operating income
|
$
|
33,069
|
|
$
|
34,876
|
|
$
|
37,543
|
|
||
|
|
Payment due by period (in thousands)
|
|||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than 1 Year
|
1-3 Years
|
4-5 Years
|
After 5 Years
|
|||||||||||||||
|
Long-term debt
|
$
|
278,959
|
|
$
|
19,459
|
|
$
|
32,500
|
|
$
|
227,000
|
|
$
|
—
|
|
|||||
|
Interest on long-term debt
(1)
|
38,846
|
|
10,783
|
|
22,043
|
|
6,020
|
|
—
|
|
||||||||||
|
Operating leases
|
104,043
|
|
12,293
|
|
20,544
|
|
13,995
|
|
57,211
|
|
||||||||||
|
Royalty obligations
|
3,728
|
|
284
|
|
774
|
|
605
|
|
2,065
|
|
||||||||||
|
Total contractual cash
|
$
|
425,576
|
|
$
|
42,819
|
|
$
|
75,861
|
|
$
|
247,620
|
|
$
|
59,276
|
|
|||||
|
Covenant Requirement
|
|||
|
Consolidated Total Leverage Ratio (1)
|
|||
|
July 1, 2017 through December 31, 2017
|
3.75 to 1.0
|
||
|
January 1, 2018 through March 31, 2018
|
3.5 to 1.0
|
||
|
April 1, 2018 and thereafter
|
3.25 to 1.0
|
||
|
Consolidated EBITDA (2)
|
1.25 to 1.0
|
||
|
Consolidated Net Income (3)
|
$—
|
||
|
Facility Capital Expenditures (4)
|
$30 million
|
||
|
(1)
|
Maximum Consolidated Total Leverage Ratio (as defined in the Second Amended Credit Agreement) as of any fiscal quarter end.
|
||
|
(2)
|
Minimum ratio of Consolidated EBITDA (as defined in the Second Amended Credit Agreement and adjusted for certain expenditures) to Consolidated Fixed Charges (as defined in the Second Amended Credit Agreement) for any period of four consecutive fiscal quarters.
|
||
|
(3)
|
Minimum level of Consolidated Net Income (as defined in the Second Amended Credit Agreement) for certain periods, and subject to certain adjustments.
|
||
|
(4)
|
Maximum level of the aggregate amount of all Facility Capital Expenditures (as defined in the Second Amended Credit Agreement) in any fiscal year.
|
||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
|
Currency
|
Symbol
|
Forward Notional Amount
|
|
|
Australian Dollar
|
AUD
|
5,600
|
|
|
Brazilian Real
|
BRL
|
8,500
|
|
|
Canadian Dollar
|
CAD
|
2,076
|
|
|
Swiss Franc
|
CHF
|
242
|
|
|
Chinese Renminbi
|
CNY
|
22,990
|
|
|
Danish Krone
|
DKK
|
1,881
|
|
|
Euro
|
EUR
|
23,333
|
|
|
British Pound
|
GBP
|
1,868
|
|
|
Hong Kong Dollar
|
HKD
|
11,000
|
|
|
Japanese Yen
|
JPY
|
178,500
|
|
|
Korean Won
|
KRW
|
1,800,000
|
|
|
Mexican Peso
|
MXN
|
17,540
|
|
|
Swedish Krona
|
SEK
|
4,775
|
|
|
Singapore Dollar
|
SGD
|
5,023
|
|
|
Currency
|
Symbol
|
Forward Notional Amount
|
|
|
Canadian Dollar
|
CAD
|
2,310
|
|
|
Swiss Franc
|
CHF
|
1,375
|
|
|
Chinese Renminbi
|
CNY
|
45,000
|
|
|
Danish Krone
|
DKK
|
14,470
|
|
|
Euro
|
EUR
|
9,165
|
|
|
British Pound
|
GBP
|
3,625
|
|
|
Mexican Peso
|
MXN
|
95,075
|
|
|
Swedish Krona
|
SEK
|
16,330
|
|
|
Item 8.
|
Financial Statements and Supplementary Data.
|
|
/s/ DELOITTE & TOUCHE LLP
|
|
|
|
|
|
Salt Lake City, Utah
|
|
|
March 1, 2018
|
|
|
We have served as the Company's auditor since 1988.
|
|
|
MERIT MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
DECEMBER 31, 2017 AND 2016
(In thousands)
|
|||||||
|
|
2017
|
2016
|
|||||
|
ASSETS
|
|||||||
|
CURRENT ASSETS:
|
|||||||
|
Cash and cash equivalents
|
$
|
32,336
|
|
$
|
19,171
|
|
|
|
Trade receivables — net of allowance for uncollectible accounts — 2017 — $1,769 and 2016 — $1,587
|
105,536
|
|
80,521
|
|
|||
|
Other receivables
|
9,429
|
|
5,643
|
|
|||
|
Inventories
|
155,288
|
|
120,695
|
|
|||
|
Prepaid expenses and other assets
|
9,096
|
|
6,226
|
|
|||
|
Prepaid income taxes
|
3,225
|
|
2,525
|
|
|||
|
Deferred income tax assets
|
—
|
|
8,219
|
|
|||
|
Income tax refund receivables
|
1,211
|
|
423
|
|
|||
|
Total current assets
|
316,121
|
|
243,423
|
|
|||
|
PROPERTY AND EQUIPMENT:
|
|||||||
|
Land and land improvements
|
19,877
|
|
19,379
|
|
|||
|
Buildings
|
147,356
|
|
139,119
|
|
|||
|
Manufacturing equipment
|
197,651
|
|
178,110
|
|
|||
|
Furniture and fixtures
|
49,528
|
|
43,433
|
|
|||
|
Leasehold improvements
|
31,161
|
|
30,413
|
|
|||
|
Construction-in-progress
|
32,896
|
|
28,180
|
|
|||
|
Total property and equipment
|
478,469
|
|
438,634
|
|
|||
|
Less accumulated depreciation
|
(185,649
|
)
|
(162,061
|
)
|
|||
|
Property and equipment — net
|
292,820
|
|
276,573
|
|
|||
|
OTHER ASSETS:
|
|||||||
|
Intangible assets:
|
|||||||
|
Developed technology — net of accumulated amortization — 2017 — $72,420 and 2016 — $52,843
|
167,771
|
|
135,358
|
|
|||
|
Other — net of accumulated amortization — 2017 — $38,127 and 2016 — $30,048
|
59,553
|
|
47,339
|
|
|||
|
Goodwill
|
238,147
|
|
211,927
|
|
|||
|
Deferred income tax assets
|
2,359
|
|
171
|
|
|||
|
Other assets
|
35,040
|
|
28,012
|
|
|||
|
Total other assets
|
502,870
|
|
422,807
|
|
|||
|
TOTAL
|
$
|
1,111,811
|
|
$
|
942,803
|
|
|
|
See notes to consolidated financial statements.
|
(continued)
|
|
|||||
|
MERIT MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2017 AND 2016
(In thousands)
|
|||||||
|
|
2017
|
2016
|
|||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
CURRENT LIABILITIES:
|
|||||||
|
Trade payables
|
$
|
34,931
|
|
$
|
30,619
|
|
|
|
Accrued expenses
|
58,932
|
|
45,519
|
|
|||
|
Current portion of long-term debt
|
19,459
|
|
10,000
|
|
|||
|
Income taxes payable
|
2,298
|
|
2,193
|
|
|||
|
Total current liabilities
|
115,620
|
|
88,331
|
|
|||
|
LONG-TERM DEBT
|
259,013
|
|
314,373
|
|
|||
|
DEFERRED INCOME TAX LIABILITIES
|
23,289
|
|
25,981
|
|
|||
|
LONG-TERM INCOME TAXES PAYABLE
|
4,846
|
|
—
|
|
|||
|
LIABILITIES RELATED TO UNRECOGNIZED TAX BENEFITS
|
2,746
|
|
438
|
|
|||
|
DEFERRED COMPENSATION PAYABLE
|
11,181
|
|
9,211
|
|
|||
|
DEFERRED CREDITS
|
2,403
|
|
2,550
|
|
|||
|
OTHER LONG-TERM OBLIGATIONS
|
16,379
|
|
3,730
|
|
|||
|
Total liabilities
|
435,477
|
|
444,614
|
|
|||
|
COMMITMENTS AND CONTINGENCIES (Notes 2, 7, 8, and 9)
|
|
|
|
|
|||
|
STOCKHOLDERS’ EQUITY:
|
|||||||
|
Preferred stock — 5,000 shares authorized as of December 31, 2017 and 2016; no shares issued
|
—
|
|
—
|
|
|||
|
Common stock, no par value; shares authorized — 2017 and 2016 - 100,000; issued and outstanding as of December 31, 2017 - 50,248 and December 31, 2016 - 44,645
|
353,392
|
|
206,186
|
|
|||
|
Retained earnings
|
321,408
|
|
293,885
|
|
|||
|
Accumulated other comprehensive income (loss)
|
1,534
|
|
(1,882
|
)
|
|||
|
Total stockholders’ equity
|
676,334
|
|
498,189
|
|
|||
|
TOTAL
|
$
|
1,111,811
|
|
$
|
942,803
|
|
|
|
See notes to consolidated financial statements.
|
(concluded)
|
|
|||||
|
MERIT MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
YEARS ENDED DECEMBER 31, 2017, 2016 AND 2015
(In thousands, except per share amounts)
|
|||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
NET SALES
|
$
|
727,852
|
|
$
|
603,838
|
|
$
|
542,149
|
|
||
|
COST OF SALES
|
401,599
|
|
338,813
|
|
306,368
|
|
|||||
|
GROSS PROFIT
|
326,253
|
|
265,025
|
|
235,781
|
|
|||||
|
OPERATING EXPENSES:
|
|
|
|||||||||
|
Selling, general and administrative
|
229,134
|
|
184,398
|
|
156,348
|
|
|||||
|
Research and development
|
51,403
|
|
45,229
|
|
40,810
|
|
|||||
|
Intangible asset impairment charges
|
809
|
|
—
|
|
—
|
|
|||||
|
Contingent consideration expense (benefit)
|
(298
|
)
|
61
|
|
80
|
|
|||||
|
Acquired in-process research and development
|
12,136
|
|
461
|
|
1,000
|
|
|||||
|
Total operating expenses
|
293,184
|
|
230,149
|
|
198,238
|
|
|||||
|
INCOME FROM OPERATIONS
|
33,069
|
|
34,876
|
|
37,543
|
|
|||||
|
OTHER INCOME (EXPENSE):
|
|
|
|||||||||
|
Interest income
|
381
|
|
81
|
|
272
|
|
|||||
|
Interest expense
|
(7,736
|
)
|
(8,798
|
)
|
(6,229
|
)
|
|||||
|
Gain on bargain purchase
|
11,039
|
|
—
|
|
—
|
|
|||||
|
Other income (expense) - net
|
(872
|
)
|
(773
|
)
|
(386
|
)
|
|||||
|
Other income (expense) — net
|
2,812
|
|
(9,490
|
)
|
(6,343
|
)
|
|||||
|
INCOME BEFORE INCOME TAXES
|
35,881
|
|
25,386
|
|
31,200
|
|
|||||
|
INCOME TAX EXPENSE
|
8,358
|
|
5,265
|
|
7,398
|
|
|||||
|
NET INCOME
|
$
|
27,523
|
|
$
|
20,121
|
|
$
|
23,802
|
|
||
|
EARNINGS PER COMMON SHARE:
|
|
|
|||||||||
|
Basic
|
$
|
0.56
|
|
$
|
0.45
|
|
$
|
0.54
|
|
||
|
Diluted
|
$
|
0.55
|
|
$
|
0.45
|
|
$
|
0.53
|
|
||
|
AVERAGE COMMON SHARES:
|
|||||||||||
|
Basic
|
48,805
|
|
44,408
|
|
44,036
|
|
|||||
|
Diluted
|
50,101
|
|
44,862
|
|
44,511
|
|
|||||
|
2017
|
2016
|
2015
|
|||||||||
|
Net income
|
$
|
27,523
|
|
$
|
20,121
|
|
$
|
23,802
|
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Cash flow hedges
|
901
|
|
4,784
|
|
(571
|
)
|
|||||
|
Less income tax benefit (expense)
|
(350
|
)
|
(1,861
|
)
|
222
|
|
|||||
|
Foreign currency translation adjustment
|
3,117
|
|
878
|
|
(3,037
|
)
|
|||||
|
Less income tax benefit (expense)
|
(252
|
)
|
(196
|
)
|
311
|
|
|||||
|
Total other comprehensive income (loss)
|
3,416
|
|
3,605
|
|
(3,075
|
)
|
|||||
|
Total comprehensive income
|
$
|
30,939
|
|
$
|
23,726
|
|
$
|
20,727
|
|
||
|
MERIT MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
YEARS ENDED DECEMBER 31, 2017, 2016 AND 2015
(In thousands)
|
||||||||||||||||||
|
|
|
|
|
|
Accumulated Other
|
|||||||||||||
|
|
|
Common Stock
|
Retained
|
Comprehensive
|
||||||||||||||
|
|
Total
|
Shares
|
Amount
|
Earnings
|
Income (Loss)
|
|||||||||||||
|
BALANCE — January 1, 2015
|
$
|
435,259
|
|
43,614
|
|
$
|
187,709
|
|
$
|
249,962
|
|
$
|
(2,412
|
)
|
||||
|
|
|
|||||||||||||||||
|
Net income
|
23,802
|
|
|
|
|
|
23,802
|
|
|
|
||||||||
|
Other comprehensive loss
|
(3,075
|
)
|
|
|
|
|
|
|
(3,075
|
)
|
||||||||
|
Excess tax benefits from stock-based compensation
|
2,124
|
|
|
|
2,124
|
|
|
|
|
|
||||||||
|
Stock-based compensation expense
|
2,243
|
|
|
|
2,243
|
|
|
|
|
|
||||||||
|
Options exercised
|
10,029
|
|
858
|
|
10,029
|
|
|
|
|
|
||||||||
|
Issuance of common stock under Employee Stock Purchase Plans
|
441
|
|
23
|
|
441
|
|
|
|
|
|
||||||||
|
Shares surrendered in exchange for payment of payroll tax liabilities
|
(918
|
)
|
(43
|
)
|
(918
|
)
|
||||||||||||
|
Shares surrendered in exchange for exercise of stock options
|
(3,802
|
)
|
(185
|
)
|
(3,802
|
)
|
||||||||||||
|
BALANCE — December 31, 2015
|
466,103
|
|
44,267
|
|
197,826
|
|
273,764
|
|
(5,487
|
)
|
||||||||
|
Net income
|
20,121
|
|
|
|
|
|
20,121
|
|
|
|
||||||||
|
Other comprehensive income
|
3,605
|
|
|
|
|
|
|
|
3,605
|
|
||||||||
|
Excess tax benefits from stock-based compensation
|
669
|
|
|
|
669
|
|
|
|
|
|
||||||||
|
Stock-based compensation expense
|
2,506
|
|
|
|
2,506
|
|
|
|
|
|
||||||||
|
Options exercised
|
4,923
|
|
362
|
|
4,923
|
|
||||||||||||
|
Issuance of common stock under Employee Stock Purchase Plans
|
694
|
|
34
|
|
694
|
|
|
|
|
|
||||||||
|
Shares surrendered in exchange for payment of payroll tax liabilities
|
(86
|
)
|
(4
|
)
|
(86
|
)
|
||||||||||||
|
Shares surrendered in exchange for exercise of stock options
|
(346
|
)
|
(14
|
)
|
(346
|
)
|
|
|
|
|
||||||||
|
BALANCE — December 31, 2016
|
498,189
|
|
44,645
|
|
206,186
|
|
293,885
|
|
(1,882
|
)
|
||||||||
|
Net income
|
27,523
|
|
27,523
|
|
||||||||||||||
|
Other comprehensive income
|
3,416
|
|
3,416
|
|
||||||||||||||
|
Stock-based compensation expense
|
4,075
|
|
4,075
|
|
||||||||||||||
|
Options exercised
|
5,689
|
|
404
|
|
5,689
|
|
||||||||||||
|
Issuance of common stock under Employee Stock Purchase Plans
|
836
|
|
24
|
|
836
|
|
||||||||||||
|
Issuance of common stock, net of offering costs
|
136,606
|
|
5,175
|
|
136,606
|
|
||||||||||||
|
BALANCE — December 31, 2017
|
$
|
676,334
|
|
50,248
|
|
$
|
353,392
|
|
$
|
321,408
|
|
$
|
1,534
|
|
||||
|
MERIT MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
YEARS ENDED DECEMBER 31, 2017, 2016 AND 2015
(In thousands)
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|||||||||
|
Net income
|
$
|
27,523
|
|
$
|
20,121
|
|
$
|
23,802
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|||||||||
|
Depreciation and amortization
|
53,582
|
|
43,755
|
|
37,425
|
|
|||||
|
Gain on bargain purchase
|
(11,039
|
)
|
—
|
|
—
|
|
|||||
|
Losses (gains) on sales and/or abandonment of property and equipment
|
427
|
|
530
|
|
(23
|
)
|
|||||
|
Write-off of patents and intangible assets
|
988
|
|
101
|
|
141
|
|
|||||
|
Acquired in-process research and development
|
12,136
|
|
461
|
|
1,000
|
|
|||||
|
Amortization of deferred credits
|
(147
|
)
|
(170
|
)
|
(171
|
)
|
|||||
|
Amortization of long-term debt issuance costs
|
685
|
|
952
|
|
987
|
|
|||||
|
Deferred income taxes
|
(1,304
|
)
|
(962
|
)
|
3,450
|
|
|||||
|
Excess tax benefits from stock-based compensation
|
—
|
|
(669
|
)
|
(2,124
|
)
|
|||||
|
Stock-based compensation expense
|
4,075
|
|
2,506
|
|
2,243
|
|
|||||
|
Changes in operating assets and liabilities, net of effects from acquisitions:
|
|||||||||||
|
Trade receivables
|
(12,844
|
)
|
(6,816
|
)
|
(5,872
|
)
|
|||||
|
Other receivables
|
(3,557
|
)
|
1,161
|
|
335
|
|
|||||
|
Inventories
|
(17,834
|
)
|
(3,656
|
)
|
(13,113
|
)
|
|||||
|
Prepaid expenses and other current assets
|
(1,236
|
)
|
271
|
|
(696
|
)
|
|||||
|
Prepaid income taxes
|
(611
|
)
|
404
|
|
(1,788
|
)
|
|||||
|
Income tax refund receivables
|
(588
|
)
|
406
|
|
(784
|
)
|
|||||
|
Other assets
|
(3,735
|
)
|
(3,763
|
)
|
(362
|
)
|
|||||
|
Trade payables
|
417
|
|
(6,835
|
)
|
14,766
|
|
|||||
|
Accrued expenses
|
6,461
|
|
3,242
|
|
5,873
|
|
|||||
|
Income taxes payable
|
21
|
|
1,451
|
|
2,199
|
|
|||||
|
Long-term income taxes payable
|
4,846
|
|
—
|
|
—
|
|
|||||
|
Liabilities related to unrecognized tax benefits
|
(19
|
)
|
597
|
|
536
|
|
|||||
|
Deferred compensation payable
|
1,970
|
|
712
|
|
(135
|
)
|
|||||
|
Other long-term obligations
|
2,510
|
|
(200
|
)
|
1,769
|
|
|||||
|
Total adjustments
|
35,204
|
|
33,478
|
|
45,656
|
|
|||||
|
Net cash provided by operating activities
|
62,727
|
|
53,599
|
|
69,458
|
|
|||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|||||||||
|
Capital expenditures for:
|
|
|
|||||||||
|
Property and equipment
|
(38,623
|
)
|
(32,837
|
)
|
(50,959
|
)
|
|||||
|
Intangible assets
|
(2,577
|
)
|
(2,217
|
)
|
(1,956
|
)
|
|||||
|
Proceeds from sale-leaseback transactions
|
—
|
|
—
|
|
2,017
|
|
|||||
|
Proceeds from sale of cost method investment
|
—
|
|
1,089
|
|
—
|
|
|||||
|
Proceeds from the sale of property and equipment
|
21
|
|
19
|
|
1,247
|
|
|||||
|
Cash paid in acquisitions, net of cash acquired
|
(105,582
|
)
|
(125,161
|
)
|
(12,368
|
)
|
|||||
|
Net cash used in investing activities
|
(146,761
|
)
|
(159,107
|
)
|
(62,019
|
)
|
|||||
|
See notes to consolidated financial statements.
|
(continued)
|
|
|||||||||
|
MERIT MEDICAL SYSTEMS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2017, 2016 AND 2015
(In thousands)
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|||||||||
|
Proceeds from issuance of common stock
|
$
|
143,810
|
|
$
|
5,271
|
|
$
|
6,668
|
|
||
|
Offering costs
|
(816
|
)
|
—
|
|
—
|
|
|||||
|
Proceeds from issuance of long-term debt
|
197,214
|
|
219,505
|
|
152,375
|
|
|||||
|
Payments on long-term debt
|
(243,214
|
)
|
(102,098
|
)
|
(169,272
|
)
|
|||||
|
Excess tax benefits from stock-based compensation
|
—
|
|
669
|
|
2,124
|
|
|||||
|
Long-term debt issuance costs
|
(416
|
)
|
(1,948
|
)
|
—
|
|
|||||
|
Contingent payments related to acquisitions
|
(61
|
)
|
(218
|
)
|
(1,212
|
)
|
|||||
|
Payment of taxes related to an exchange of common stock
|
—
|
|
(86
|
)
|
(918
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
96,517
|
|
121,095
|
|
(10,235
|
)
|
|||||
|
EFFECT OF EXCHANGE RATES ON CASH
|
682
|
|
(593
|
)
|
(382
|
)
|
|||||
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
13,165
|
|
14,994
|
|
(3,178
|
)
|
|||||
|
CASH AND CASH EQUIVALENTS:
|
|
|
|||||||||
|
Beginning of year
|
19,171
|
|
4,177
|
|
7,355
|
|
|||||
|
End of year
|
$
|
32,336
|
|
$
|
19,171
|
|
$
|
4,177
|
|
||
|
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION
|
|||||||||||
|
Cash paid during the year for:
|
|||||||||||
|
Interest (net of capitalized interest of $513, $460 and $325, respectively)
|
$
|
7,707
|
|
$
|
8,872
|
|
$
|
6,273
|
|
||
|
Income taxes
|
$
|
6,049
|
|
$
|
2,318
|
|
$
|
3,409
|
|
||
|
SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES
|
|
|
|||||||||
|
Property and equipment purchases in accounts payable
|
$
|
1,992
|
|
$
|
2,398
|
|
$
|
3,199
|
|
||
|
Cost method investment converted to intangible asset in acquisition in lieu of additional cash payment
|
$
|
—
|
|
$
|
—
|
|
$
|
1,010
|
|
||
|
Contingent receivable in exchange for sale of cost method investment
|
$
|
—
|
|
$
|
711
|
|
$
|
—
|
|
||
|
Receivable for issuance of common stock associated with option exercises
|
$
|
137
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Acquisition purchases in accrued expenses and other long-term obligations
|
$
|
10,488
|
|
$
|
—
|
|
$
|
1,300
|
|
||
|
Merit common stock surrendered (0, 14 and 185 shares, respectively) in exchange for exercise of stock options
|
$
|
—
|
|
$
|
346
|
|
$
|
3,802
|
|
||
|
See notes to consolidated financial statements.
|
(concluded)
|
|
|||||||||
|
Buildings
|
40 years
|
||
|
Manufacturing equipment
|
4
|
-
|
20 years
|
|
Furniture and fixtures
|
3
|
-
|
20 years
|
|
Land improvements
|
10
|
-
|
20 years
|
|
Leasehold improvements
|
4
|
-
|
25 years
|
|
Assets Acquired
|
||||
|
Trade receivables
|
$
|
1,287
|
|
|
|
Other receivables
|
56
|
|
||
|
Inventories
|
1,808
|
|
||
|
Prepaid expenses and other assets
|
65
|
|
||
|
Property and equipment
|
1,053
|
|
||
|
Intangibles
|
||||
|
Customer lists
|
5,940
|
|
||
|
Goodwill
|
3,740
|
|
||
|
Total assets acquired
|
13,949
|
|
||
|
Liabilities Assumed
|
||||
|
Trade payables
|
(216
|
)
|
||
|
Accrued expenses
|
(542
|
)
|
||
|
Deferred tax liabilities
|
(1,901
|
)
|
||
|
Total liabilities assumed
|
(2,659
|
)
|
||
|
Total net assets acquired
|
$
|
11,290
|
|
|
|
Net Assets Acquired
|
||||
|
Inventories
|
$
|
594
|
|
|
|
Intangibles
|
||||
|
Developed technology
|
14,920
|
|
||
|
Customer list
|
120
|
|
||
|
Goodwill
|
6,366
|
|
||
|
Total net assets acquired
|
$
|
22,000
|
|
|
|
Net Assets Acquired
|
||||
|
Inventories
|
$
|
979
|
|
|
|
Property and equipment
|
58
|
|
||
|
Intangibles
|
||||
|
Developed technology
|
5,400
|
|
||
|
Customer list
|
200
|
|
||
|
Goodwill
|
203
|
|
||
|
Total net assets acquired
|
$
|
6,840
|
|
|
|
Preliminary Allocation
|
Adjustments
(1)
|
Revised Allocation
|
||||||||||
|
Net Assets Acquired
|
||||||||||||
|
Intangibles
|
||||||||||||
|
Developed technology
|
$
|
7,800
|
|
$
|
—
|
|
$
|
7,800
|
|
|||
|
In-process technology
|
850
|
|
70
|
|
920
|
|
||||||
|
Goodwill
|
4,323
|
|
(42
|
)
|
4,281
|
|
||||||
|
Deferred tax liabilities
|
(3,073
|
)
|
(28
|
)
|
(3,101
|
)
|
||||||
|
Total net assets acquired
|
$
|
9,900
|
|
$
|
—
|
|
$
|
9,900
|
|
|||
|
(1)
|
Under U.S. GAAP, measurement period adjustments are recognized on a prospective basis in the period of change, instead of restating prior periods. There was no impact to reported earnings in connection with these measurement period adjustments for the periods presented. Amounts represent adjustments to the preliminary purchase price allocation first presented in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2017 resulting from our ongoing activities, including reassessment of the assets acquired and liabilities assumed, with respect to finalizing our purchase price allocation for this acquisition.
|
|||||||||||
|
Preliminary Allocation
|
Adjustments
(2)
|
Revised Allocation
|
||||||||||
|
Assets Acquired
|
||||||||||||
|
Cash and cash equivalents
|
$
|
1,436
|
|
$
|
—
|
|
$
|
1,436
|
|
|||
|
Trade receivables
|
8,351
|
|
—
|
|
8,351
|
|
||||||
|
Inventories
|
12,217
|
|
(995
|
)
|
11,222
|
|
||||||
|
Prepaid expenses and other assets
|
1,275
|
|
—
|
|
1,275
|
|
||||||
|
Income tax refund receivable
|
—
|
|
165
|
|
165
|
|
||||||
|
Property and equipment
|
2,667
|
|
(348
|
)
|
2,319
|
|
||||||
|
Deferred tax assets
|
184
|
|
18
|
|
202
|
|
||||||
|
Intangibles
|
||||||||||||
|
Developed technology
|
2,600
|
|
(400
|
)
|
2,200
|
|
||||||
|
Customer lists
|
1,300
|
|
200
|
|
1,500
|
|
||||||
|
Trademarks
|
1,500
|
|
(600
|
)
|
900
|
|
||||||
|
Total assets acquired
|
31,530
|
|
(1,960
|
)
|
29,570
|
|
||||||
|
Liabilities Assumed
|
||||||||||||
|
Trade payables
|
(2,306
|
)
|
(108
|
)
|
(2,414
|
)
|
||||||
|
Accrued expenses
|
(5,083
|
)
|
—
|
|
(5,083
|
)
|
||||||
|
Income taxes payable
|
(2
|
)
|
2
|
|
—
|
|
||||||
|
Deferred income tax liabilities
|
(999
|
)
|
65
|
|
(934
|
)
|
||||||
|
Total liabilities assumed
|
(8,390
|
)
|
(41
|
)
|
(8,431
|
)
|
||||||
|
Total net assets acquired
|
23,140
|
|
(2,001
|
)
|
21,139
|
|
||||||
|
Gain on bargain purchase
(1)
|
(12,243
|
)
|
1,204
|
|
(11,039
|
)
|
||||||
|
Total purchase price
|
$
|
10,897
|
|
$
|
(797
|
)
|
$
|
10,100
|
|
|||
|
(1)
|
The total fair value of the net assets acquired from Argon exceeded the purchase price, resulting in a gain on bargain purchase which was recorded within other income (expense) in our consolidated statements of income, and includes a negative adjustment of $1.2 million since the bargain purchase gain was first presented in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2017. We believe the reason for the gain on bargain purchase was a result of the divestiture of a non-strategic, slow-growth critical care business for Argon. It is our understanding that the divestiture allows Argon to focus on its higher growth interventional portfolio.
|
|||||||||||
|
(2)
|
Under U.S. GAAP, measurement period adjustments are recognized on a prospective basis in the period of change, instead of restating prior periods. There was no impact to reported earnings in connection with these measurement period adjustments for the periods presented. Amounts represent adjustments to the preliminary purchase price allocation first presented in our March 31, 2017 Form 10-Q resulting from our ongoing activities, including reassessment of the assets acquired and liabilities assumed, with respect to finalizing our purchase price allocation for this acquisition.
|
|||||||||||
|
Preliminary Allocation
|
Adjustments
(1)
|
Revised Allocation
|
||||||||||
|
Assets Acquired
|
||||||||||||
|
Trade receivables
|
$
|
952
|
|
$
|
6
|
|
$
|
958
|
|
|||
|
Inventories
|
2,244
|
|
(87
|
)
|
2,157
|
|
||||||
|
Prepaid expenses and other assets
|
181
|
|
(96
|
)
|
85
|
|
||||||
|
Property and equipment
|
1,472
|
|
—
|
|
1,472
|
|
||||||
|
Intangibles
|
||||||||||||
|
Developed technology
|
22,900
|
|
(1,800
|
)
|
21,100
|
|
||||||
|
Customer lists
|
100
|
|
600
|
|
700
|
|
||||||
|
Trademarks
|
2,900
|
|
—
|
|
2,900
|
|
||||||
|
Goodwill
|
7,612
|
|
1,377
|
|
8,989
|
|
||||||
|
Total assets acquired
|
38,361
|
|
—
|
|
38,361
|
|
||||||
|
Liabilities Assumed
|
||||||||||||
|
Trade payables
|
(338
|
)
|
—
|
|
(338
|
)
|
||||||
|
Accrued expenses
|
(23
|
)
|
—
|
|
(23
|
)
|
||||||
|
Total liabilities assumed
|
(361
|
)
|
—
|
|
(361
|
)
|
||||||
|
Net assets acquired
|
$
|
38,000
|
|
$
|
—
|
|
$
|
38,000
|
|
|||
|
(1)
|
Under U.S. GAAP, measurement period adjustments are recognized on a prospective basis in the period of change, instead of restating prior periods. There was no impact to reported earnings in connection with these measurement period adjustments for the periods presented. Amounts represent adjustments to the preliminary purchase price first presented in our Quarterly Report on Form 10-Q for the Quarter Ended March 31, 2017, resulting from activities with respect to finalizing our purchase price allocation for this acquisition. The larger adjustments primarily relate to the valuation of the acquired intangible assets.
|
|||||||||||
|
Assets Acquired
|
|||
|
Trade receivables
|
$
|
4,054
|
|
|
Other receivables
|
6
|
|
|
|
Inventories
|
8,585
|
|
|
|
Prepaid expenses
|
630
|
|
|
|
Property and equipment
|
1,630
|
|
|
|
Other long-term assets
|
145
|
|
|
|
Intangibles
|
|||
|
Developed technology
|
67,600
|
|
|
|
Customer lists
|
2,400
|
|
|
|
Trademarks
|
4,400
|
|
|
|
Goodwill
|
24,818
|
|
|
|
Total assets acquired
|
114,268
|
|
|
|
Liabilities Assumed
|
|||
|
Trade payables
|
(1,790
|
)
|
|
|
Accrued expenses
|
(5,298
|
)
|
|
|
Deferred income tax liabilities - current
|
(701
|
)
|
|
|
Deferred income tax liabilities - noncurrent
|
(10,844
|
)
|
|
|
Total liabilities assumed
|
(18,633
|
)
|
|
|
Net assets acquired, net of cash received of $1,327
|
$
|
95,635
|
|
|
Assets Acquired
|
|||
|
Inventories
|
$
|
2,455
|
|
|
Property and equipment
|
290
|
|
|
|
Intangibles
|
|||
|
Developed technology
|
12,100
|
|
|
|
Trademarks
|
700
|
|
|
|
Customers Lists
|
400
|
|
|
|
Goodwill
|
2,555
|
|
|
|
Total assets acquired
|
$
|
18,500
|
|
|
2017
|
2016
|
2015
|
|||||||||||||||||||||
|
As Reported
|
Pro Forma
|
As Reported
|
Pro Forma
|
As Reported
|
Pro Forma
|
||||||||||||||||||
|
Net sales
|
$
|
727,852
|
|
$
|
730,612
|
|
$
|
603,838
|
|
$
|
664,366
|
|
$
|
542,149
|
|
$
|
575,541
|
|
|||||
|
Net income
|
27,523
|
|
17,419
|
|
20,121
|
|
23,068
|
|
23,802
|
|
3,135
|
|
|||||||||||
|
Earnings per common share:
|
|||||||||||||||||||||||
|
Basic
|
$
|
0.56
|
|
$
|
0.36
|
|
$
|
0.45
|
|
$
|
0.52
|
|
$
|
0.54
|
|
$
|
0.07
|
|
|||||
|
Diluted
|
$
|
0.55
|
|
$
|
0.35
|
|
$
|
0.45
|
|
$
|
0.51
|
|
$
|
0.53
|
|
$
|
0.07
|
|
|||||
|
|
2017
|
2016
|
|||||
|
Finished goods
|
$
|
86,555
|
|
$
|
63,852
|
|
|
|
Work-in-process
|
12,799
|
|
11,008
|
|
|||
|
Raw materials
|
55,934
|
|
45,835
|
|
|||
|
Total
|
$
|
155,288
|
|
$
|
120,695
|
|
|
|
|
2017
|
2016
|
|||||
|
Goodwill balance at January 1
|
$
|
211,927
|
|
$
|
184,472
|
|
|
|
Effect of foreign exchange
|
2,641
|
|
82
|
|
|||
|
Additions as the result of acquisitions
|
23,579
|
|
27,373
|
|
|||
|
Goodwill balance at December 31
|
$
|
238,147
|
|
$
|
211,927
|
|
|
|
|
2017
|
||||||||||
|
Gross Carrying
Amount
|
Accumulated
Amortization
|
Net Carrying
Amount
|
|||||||||
|
Patents
|
$
|
16,528
|
|
$
|
(3,737
|
)
|
$
|
12,791
|
|
||
|
Distribution agreements
|
7,262
|
|
(4,686
|
)
|
2,576
|
|
|||||
|
License agreements
|
23,783
|
|
(5,568
|
)
|
18,215
|
|
|||||
|
Trademarks
|
16,224
|
|
(4,686
|
)
|
11,538
|
|
|||||
|
Covenants not to compete
|
1,028
|
|
(968
|
)
|
60
|
|
|||||
|
Customer lists
|
31,935
|
|
(18,482
|
)
|
13,453
|
|
|||||
|
In-process technology
|
920
|
|
—
|
|
920
|
|
|||||
|
Total
|
$
|
97,680
|
|
$
|
(38,127
|
)
|
$
|
59,553
|
|
||
|
|
2016
|
||||||||||
|
Gross Carrying
Amount
|
Accumulated
Amortization
|
Net Carrying
Amount
|
|||||||||
|
Patents
|
$
|
14,130
|
|
$
|
(3,165
|
)
|
$
|
10,965
|
|
||
|
Distribution agreements
|
6,626
|
|
(3,527
|
)
|
3,099
|
|
|||||
|
License agreements
|
20,695
|
|
(3,422
|
)
|
17,273
|
|
|||||
|
Trademarks
|
12,380
|
|
(3,330
|
)
|
9,050
|
|
|||||
|
Covenants not to compete
|
1,028
|
|
(936
|
)
|
92
|
|
|||||
|
Customer lists
|
22,261
|
|
(15,401
|
)
|
6,860
|
|
|||||
|
Royalty agreements
|
267
|
|
(267
|
)
|
—
|
|
|||||
|
Total
|
$
|
77,387
|
|
$
|
(30,048
|
)
|
$
|
47,339
|
|
||
|
Year Ending December 31
|
|
||
|
2018
|
$
|
30,413
|
|
|
2019
|
29,787
|
|
|
|
2020
|
28,373
|
|
|
|
2021
|
21,001
|
|
|
|
2022
|
19,396
|
|
|
|
•
|
The reduction in the U.S. corporate tax rate resulted in a net tax benefit of approximately
$8.4 million
related to the revaluation of our U.S. net deferred tax liability. We are still analyzing certain aspects of the TCJA and refining our calculations, which could potentially affect the measurement of these balances or potentially give rise to new deferred tax amounts.
|
|
•
|
The transition tax resulted in a one-time tax expense of approximately
$10.6 million
. We have not yet completed our calculation of the total post-1986 foreign earnings and profits (“E&P”) for our foreign subsidiaries as E&P will not be finalized until the federal income tax return is filed.
|
|
|
2017
|
2016
|
2015
|
||||||||
|
Domestic
|
$
|
14,531
|
|
$
|
6,174
|
|
$
|
9,470
|
|
||
|
Foreign
|
21,350
|
|
19,212
|
|
21,730
|
|
|||||
|
Total
|
$
|
35,881
|
|
$
|
25,386
|
|
$
|
31,200
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Current expense (benefit):
|
|
|
|
|
|
|
|||||
|
Federal
|
$
|
3,849
|
|
$
|
1,933
|
|
$
|
(17
|
)
|
||
|
State
|
645
|
|
492
|
|
747
|
|
|||||
|
Foreign
|
5,168
|
|
3,802
|
|
3,218
|
|
|||||
|
Total current expense
|
9,662
|
|
6,227
|
|
3,948
|
|
|||||
|
Deferred expense (benefit):
|
|
|
|
|
|
|
|||||
|
Federal
|
(314
|
)
|
(144
|
)
|
3,250
|
|
|||||
|
State
|
(216
|
)
|
(195
|
)
|
294
|
|
|||||
|
Foreign
|
(774
|
)
|
(623
|
)
|
(94
|
)
|
|||||
|
Total deferred (benefit) expense
|
(1,304
|
)
|
(962
|
)
|
3,450
|
|
|||||
|
Total income tax expense
|
$
|
8,358
|
|
$
|
5,265
|
|
$
|
7,398
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Computed federal income tax expense at statutory rate of 35%
|
$
|
12,559
|
|
$
|
8,885
|
|
$
|
10,920
|
|
||
|
State income taxes
|
279
|
|
193
|
|
698
|
|
|||||
|
Tax credits
|
(1,377
|
)
|
(1,164
|
)
|
(1,019
|
)
|
|||||
|
Production activity deduction
|
—
|
|
(53
|
)
|
—
|
|
|||||
|
Foreign tax rate differential
|
(3,329
|
)
|
(3,717
|
)
|
(3,564
|
)
|
|||||
|
Uncertain tax positions
|
(19
|
)
|
597
|
|
536
|
|
|||||
|
Deferred compensation insurance assets
|
(479
|
)
|
(307
|
)
|
182
|
|
|||||
|
Transaction-related expenses
|
90
|
|
274
|
|
—
|
|
|||||
|
U.S. transition tax
|
10,612
|
|
—
|
|
—
|
|
|||||
|
TCJA remeasurement of deferred taxes
|
(8,383
|
)
|
—
|
|
—
|
|
|||||
|
Share-based payments
|
(2,264
|
)
|
—
|
|
—
|
|
|||||
|
Bargain purchase gain
|
(1,570
|
)
|
—
|
|
—
|
|
|||||
|
In-process research and development
|
1,486
|
|
—
|
|
—
|
|
|||||
|
Other — including the effect of graduated rates
|
753
|
|
557
|
|
(355
|
)
|
|||||
|
Total income tax expense
|
$
|
8,358
|
|
$
|
5,265
|
|
$
|
7,398
|
|
||
|
|
2017
|
2016
|
|||||
|
Deferred income tax assets:
|
|
|
|||||
|
Allowance for uncollectible accounts receivable
|
$
|
467
|
|
$
|
645
|
|
|
|
Accrued compensation expense
|
5,154
|
|
6,203
|
|
|||
|
Inventory differences
|
2,505
|
|
1,065
|
|
|||
|
Net operating loss carryforwards
|
15,741
|
|
27,742
|
|
|||
|
Deferred revenue
|
58
|
|
73
|
|
|||
|
Stock-based compensation expense
|
2,281
|
|
2,738
|
|
|||
|
Federal research and development credit carryforward
|
—
|
|
3,524
|
|
|||
|
Foreign tax credits
|
—
|
|
364
|
|
|||
|
Other
|
8,986
|
|
6,984
|
|
|||
|
Total deferred income tax assets
|
35,192
|
|
49,338
|
|
|||
|
Deferred income tax liabilities:
|
|
|
|
|
|||
|
Prepaid expenses
|
(930
|
)
|
(782
|
)
|
|||
|
Property and equipment
|
(20,352
|
)
|
(25,108
|
)
|
|||
|
Intangible assets
|
(28,588
|
)
|
(35,773
|
)
|
|||
|
Other
|
(1,830
|
)
|
(1,480
|
)
|
|||
|
Total deferred income tax liabilities
|
(51,700
|
)
|
(63,143
|
)
|
|||
|
Valuation allowance
|
(4,422
|
)
|
(3,786
|
)
|
|||
|
Net deferred income tax assets (liabilities)
|
$
|
(20,930
|
)
|
$
|
(17,591
|
)
|
|
|
|
|
|
|
||||
|
Reported as:
|
|||||||
|
Deferred income tax assets - Current
|
$
|
—
|
|
$
|
8,219
|
|
|
|
Deferred income tax assets - Long-term
|
2,359
|
|
171
|
|
|||
|
Deferred income tax liabilities - Long-term
|
(23,289
|
)
|
(25,981
|
)
|
|||
|
Net deferred income tax liabilities
|
$
|
(20,930
|
)
|
$
|
(17,591
|
)
|
|
|
Tabular Roll-forward
|
2017
|
2016
|
2015
|
|||||||||
|
Unrecognized tax benefits, opening balance
|
$
|
2,549
|
|
$
|
1,982
|
|
$
|
1,736
|
|
|||
|
Gross increases in tax positions taken in a prior year
|
80
|
|
77
|
|
187
|
|
||||||
|
Gross increases in tax positions taken in the current year
|
403
|
|
856
|
|
763
|
|
||||||
|
Lapse of applicable statute of limitations
|
(283
|
)
|
(366
|
)
|
(704
|
)
|
||||||
|
Unrecognized tax benefits, ending balance
|
$
|
2,749
|
|
$
|
2,549
|
|
$
|
1,982
|
|
|||
|
|
2017
|
2016
|
|||||
|
Payroll and related liabilities
|
$
|
30,225
|
|
$
|
24,429
|
|
|
|
Advances from employees
|
796
|
|
572
|
|
|||
|
Other accrued expenses
|
27,911
|
|
20,518
|
|
|||
|
Total
|
$
|
58,932
|
|
$
|
45,519
|
|
|
|
2017
|
2016
|
||||||
|
2016 Term loan
|
$
|
85,000
|
|
$
|
145,000
|
|
|
|
2016 Revolving credit loans
|
187,000
|
|
180,000
|
|
|||
|
2017 Debt facility
|
6,959
|
|
—
|
|
|||
|
Less unamortized debt issuance costs
|
(487
|
)
|
(627
|
)
|
|||
|
Total long-term debt
|
278,472
|
|
324,373
|
|
|||
|
Less current portion
|
19,459
|
|
10,000
|
|
|||
|
Long-term portion
|
$
|
259,013
|
|
$
|
314,373
|
|
|
|
Covenant Requirement
|
|||
|
Consolidated Total Leverage Ratio (1)
|
|||
|
July 1, 2017 through December 31, 2017
|
3.75 to 1.0
|
||
|
January 1, 2018 through March 31, 2018
|
3.5 to 1.0
|
||
|
April 1, 2018 and thereafter
|
3.25 to 1.0
|
||
|
Consolidated EBITDA (2)
|
1.25 to 1.0
|
||
|
Consolidated Net Income (3)
|
$—
|
||
|
Facility Capital Expenditures (4)
|
$30 million
|
||
|
(1)
|
Maximum Consolidated Total Leverage Ratio (as defined in the Second Amended Credit Agreement) as of any fiscal quarter end.
|
||
|
(2)
|
Minimum ratio of Consolidated EBITDA (as defined in the Second Amended Credit Agreement and adjusted for certain expenditures) to Consolidated Fixed Charges (as defined in the Second Amended Credit Agreement) for any period of four consecutive fiscal quarters.
|
||
|
(3)
|
Minimum level of Consolidated Net Income (as defined in the Second Amended Credit Agreement) for certain periods, and subject to certain adjustments.
|
||
|
(4)
|
Maximum level of the aggregate amount of all Facility Capital Expenditures (as defined in the Second Amended Credit Agreement) in any fiscal year.
|
||
|
Years Ending
|
Future Minimum
|
|||
|
December 31
|
Principal Payments
|
|||
|
2018
|
$
|
19,459
|
|
|
|
2019
|
15,000
|
|
||
|
2020
|
17,500
|
|
||
|
2021
|
227,000
|
|
||
|
Total future minimum principal payments
|
$
|
278,959
|
|
|
|
Currency
|
Symbol
|
Forward Notional Amount
|
|
|
Canadian Dollar
|
CAD
|
2,310
|
|
|
Swiss Franc
|
CHF
|
1,375
|
|
|
Chinese Renminbi
|
CNY
|
45,000
|
|
|
Danish Krone
|
DKK
|
14,470
|
|
|
Euro
|
EUR
|
9,165
|
|
|
British Pound
|
GBP
|
3,625
|
|
|
Mexican Peso
|
MXN
|
95,075
|
|
|
Swedish Krona
|
SEK
|
16,330
|
|
|
Currency
|
Symbol
|
Forward Notional Amount
|
|
|
Australian Dollar
|
AUD
|
5,600
|
|
|
Brazilian Real
|
BRL
|
8,500
|
|
|
Canadian Dollar
|
CAD
|
2,076
|
|
|
Swiss Franc
|
CHF
|
242
|
|
|
Chinese Renminbi
|
CNY
|
22,990
|
|
|
Danish Krone
|
DKK
|
1,881
|
|
|
Euro
|
EUR
|
23,333
|
|
|
British Pound
|
GBP
|
1,868
|
|
|
Hong Kong Dollar
|
HKD
|
11,000
|
|
|
Japanese Yen
|
JPY
|
178,500
|
|
|
Korean Won
|
KRW
|
1,800,000
|
|
|
Mexican Peso
|
MXN
|
17,540
|
|
|
Swedish Krona
|
SEK
|
4,775
|
|
|
Singapore Dollar
|
SGD
|
5,023
|
|
|
Fair Value
|
||||||||||
|
Balance Sheet Location
|
December 31, 2017
|
December 31, 2016
|
||||||||
|
Derivatives designated as hedging instruments
|
||||||||||
|
Assets
|
||||||||||
|
Interest rates swaps
|
Other assets (long-term)
|
$
|
5,749
|
|
$
|
4,991
|
|
|||
|
Foreign currency forward contracts
|
Prepaid expenses and other assets
|
363
|
|
116
|
|
|||||
|
Foreign currency forward contracts
|
Other assets (long-term)
|
35
|
|
18
|
|
|||||
|
Liabilities
|
||||||||||
|
Foreign currency forward contracts
|
Accrued expenses
|
(468
|
)
|
(275
|
)
|
|||||
|
Foreign currency forward contracts
|
Other long-term obligations
|
(82
|
)
|
(18
|
)
|
|||||
|
Derivatives not designated as hedging instruments
|
||||||||||
|
Assets
|
||||||||||
|
Foreign currency forward contracts
|
Prepaid expenses and other assets
|
$
|
223
|
|
$
|
220
|
|
|||
|
Liabilities
|
||||||||||
|
Foreign currency forward contracts
|
Accrued expenses
|
(841
|
)
|
(171
|
)
|
|||||
|
Amount of Gain/(Loss) recognized in OCI
|
Amount of Gain/(Loss) reclassified from AOCI
|
|||||||||||||||
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2017
|
2016
|
2015
|
2017
|
2016
|
2015
|
|||||||||||
|
Derivative instrument
|
Location in statements of income
|
|||||||||||||||
|
Interest rate swaps
|
$853
|
$
|
4,989
|
|
$
|
(571
|
)
|
Interest Expense
|
$95
|
(718
|
)
|
(1,103
|
)
|
|||
|
Foreign currency forward contracts
|
491
|
|
(205
|
)
|
—
|
|
Revenue
|
(277
|
)
|
21
|
|
—
|
|
|||
|
Cost of goods sold
|
625
|
|
(26
|
)
|
—
|
|
||||||||||
|
Year ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Derivative Instrument
|
Location in statements of income
|
||||||||||
|
Foreign currency forward contracts
|
Other expense
|
$
|
(4,746
|
)
|
$
|
69
|
|
$
|
(302
|
)
|
|
|
Years Ending
|
Operating
|
|||
|
December 31
|
Leases
|
|||
|
|
|
|
||
|
2018
|
$
|
12,293
|
|
|
|
2019
|
11,237
|
|
||
|
2020
|
9,307
|
|
||
|
2021
|
7,527
|
|
||
|
2022
|
6,468
|
|
||
|
Thereafter
|
57,211
|
|
||
|
|
|
|
||
|
Total minimum lease payments
|
$
|
104,043
|
|
|
|
Net
Income
|
Shares
|
Per Share
Amount
|
||||||||
|
Year ended December 31, 2017:
|
|
|
|
|
|
|
||||
|
Basic EPS
|
$
|
27,523
|
|
48,805
|
|
$
|
0.56
|
|
||
|
Effect of dilutive stock options and warrants
|
|
|
1,296
|
|
|
|
||||
|
Diluted EPS
|
$
|
27,523
|
|
50,101
|
|
$
|
0.55
|
|
||
|
Year ended December 31, 2016:
|
|
|
|
|
|
|
||||
|
Basic EPS
|
$
|
20,121
|
|
44,408
|
|
$
|
0.45
|
|
||
|
Effect of dilutive stock options and warrants
|
|
|
454
|
|
|
|
||||
|
Diluted EPS
|
$
|
20,121
|
|
44,862
|
|
$
|
0.45
|
|
||
|
Year ended December 31, 2015:
|
|
|
|
|
|
|
||||
|
Basic EPS
|
$
|
23,802
|
|
44,036
|
|
$
|
0.54
|
|
||
|
Effect of dilutive stock options and warrants
|
|
|
475
|
|
|
|
||||
|
Diluted EPS
|
$
|
23,802
|
|
44,511
|
|
$
|
0.53
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Cost of goods sold
|
$
|
632
|
|
$
|
472
|
|
$
|
398
|
|
||
|
Research and development
|
376
|
|
184
|
|
122
|
|
|||||
|
Selling, general, and administrative
|
3,067
|
|
1,850
|
|
1,723
|
|
|||||
|
Stock-based compensation expense before taxes
|
$
|
4,075
|
|
$
|
2,506
|
|
$
|
2,243
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Risk-free interest rate
|
1.77%
|
-
|
1.83%
|
1.15%
|
-
|
1.40%
|
1.53%
|
-
|
1.66%
|
||
|
Expected option life
|
5.0 years
|
5.0 years
|
5.0 years
|
||||||||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
||||||||
|
Expected price volatility
|
33.81%
|
-
|
34.07%
|
34.28%
|
-
|
37.06%
|
33.72%
|
-
|
35.11%
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Total intrinsic value of stock options exercised
|
$
|
9,264
|
|
$
|
3,648
|
|
$
|
7,548
|
|
||
|
Cash received from stock option exercises
|
5,552
|
|
4,577
|
|
6,227
|
|
|||||
|
Excess tax benefit from the exercise of stock options
|
2,264
|
|
669
|
|
2,124
|
|
|||||
|
Number
of Shares
|
Weighted Average
Exercise Price
|
Remaining Contractual
Term (in years)
|
Intrinsic
Value
|
|||||||||
|
Beginning balance
|
2,817
|
|
$
|
15.32
|
|
|
|
|
||||
|
Granted
|
1,297
|
|
29.31
|
|
|
|
|
|||||
|
Exercised
|
(404
|
)
|
14.02
|
|
|
|
|
|||||
|
Forfeited/expired
|
(87
|
)
|
18.79
|
|
|
|
|
|||||
|
Outstanding at December 31
|
3,623
|
|
20.40
|
|
4.57 years
|
$
|
82,615
|
|
||||
|
Exercisable
|
1,110
|
|
14.35
|
|
2.55 years
|
32,019
|
|
|||||
|
Ending vested and expected to vest
|
3,484
|
|
20.23
|
|
4.52 years
|
80,052
|
|
|||||
|
|
Options Outstanding
|
Options Exercisable
|
||||||||||||||
|
Range of Exercise
|
Number Outstanding
|
Weighted Average Remaining Contractual Life (in years)
|
Weighted Average Exercise Price
|
Number Exercisable
|
Weighted Average Exercise Price
|
|||||||||||
|
$9.95
|
-
|
$13.75
|
925
|
2.17 years
|
$
|
12.65
|
|
713
|
$
|
12.84
|
|
|||||
|
$13.77
|
-
|
$17.27
|
991
|
4.38 years
|
$
|
16.18
|
|
294
|
$
|
15.96
|
|
|||||
|
$18.80
|
-
|
$22.00
|
425
|
5.00 years
|
$
|
20.14
|
|
103
|
$
|
20.18
|
|
|||||
|
$28.20
|
-
|
$38.35
|
1,282
|
6.30 years
|
$
|
29.33
|
|
0
|
$
|
—
|
|
|||||
|
$9.95
|
-
|
$38.35
|
3,623
|
|
|
|
1,110
|
|
|
|||||||
|
|
% Change
|
2017
|
% Change
|
2016*
|
% Change
|
2015
|
|||||||||||
|
Cardiovascular
|
|
|
|
|
|
|
|
|
|
||||||||
|
Stand-alone devices
|
44%
|
$
|
275,431
|
|
23%
|
$
|
191,148
|
|
8%
|
$
|
155,414
|
|
|||||
|
Custom kits and procedure trays
|
6%
|
126,114
|
|
2%
|
119,226
|
|
5%
|
116,368
|
|
||||||||
|
Inflation devices
|
8%
|
79,875
|
|
1%
|
73,916
|
|
1%
|
73,373
|
|
||||||||
|
Catheters
|
13%
|
127,747
|
|
17%
|
113,367
|
|
11%
|
96,833
|
|
||||||||
|
Embolization devices
|
8%
|
49,532
|
|
2%
|
46,035
|
|
3%
|
45,025
|
|
||||||||
|
CRM/EP
|
15%
|
41,914
|
|
8%
|
36,459
|
|
3%
|
33,902
|
|
||||||||
|
Total
|
21%
|
700,613
|
|
11%
|
580,151
|
|
6%
|
520,915
|
|
||||||||
|
Endoscopy
|
|
|
|
|
|
|
|
|
|
||||||||
|
Endoscopy devices
|
15%
|
27,239
|
|
12%
|
23,687
|
|
18%
|
21,234
|
|
||||||||
|
Total
|
21%
|
$
|
727,852
|
|
11%
|
$
|
603,838
|
|
6%
|
$
|
542,149
|
|
|||||
|
|
2017
|
2016
|
2015
|
||||||||
|
United States
|
$
|
202,504
|
|
$
|
194,715
|
|
$
|
186,389
|
|
||
|
Ireland
|
45,671
|
|
47,337
|
|
48,896
|
|
|||||
|
Other foreign countries
|
44,645
|
|
34,521
|
|
32,493
|
|
|||||
|
Total
|
$
|
292,820
|
|
$
|
276,573
|
|
$
|
267,778
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net Sales
(1)
|
|
|
|
|
|
|
|||||
|
Cardiovascular
|
$
|
700,613
|
|
$
|
580,151
|
|
$
|
520,915
|
|
||
|
Endoscopy
|
27,239
|
|
23,687
|
|
21,234
|
|
|||||
|
Total net sales
|
727,852
|
|
603,838
|
|
542,149
|
|
|||||
|
Operating expenses
|
|
|
|
|
|
|
|||||
|
Cardiovascular
|
281,095
|
|
218,659
|
|
187,492
|
|
|||||
|
Endoscopy
|
12,089
|
|
11,490
|
|
10,746
|
|
|||||
|
Total operating expenses
|
293,184
|
|
230,149
|
|
198,238
|
|
|||||
|
Operating income (loss)
(1)
|
|
|
|
|
|
|
|||||
|
Cardiovascular
|
24,819
|
|
30,053
|
|
34,052
|
|
|||||
|
Endoscopy
|
8,250
|
|
4,823
|
|
3,491
|
|
|||||
|
Total operating income
|
33,069
|
|
34,876
|
|
37,543
|
|
|||||
|
Total other expense - net
|
2,812
|
|
(9,490
|
)
|
(6,343
|
)
|
|||||
|
Income tax expense
|
8,358
|
|
5,265
|
|
7,398
|
|
|||||
|
Net income
|
$
|
27,523
|
|
$
|
20,121
|
|
$
|
23,802
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Cardiovascular
|
$
|
1,103,806
|
|
$
|
932,927
|
|
$
|
767,952
|
|
||
|
Endoscopy
|
8,005
|
|
9,876
|
|
10,776
|
|
|||||
|
Total
|
$
|
1,111,811
|
|
$
|
942,803
|
|
$
|
778,728
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Cardiovascular
|
$
|
52,700
|
|
$
|
42,806
|
|
$
|
36,474
|
|
||
|
Endoscopy
|
882
|
|
949
|
|
951
|
|
|||||
|
Total
|
$
|
53,582
|
|
$
|
43,755
|
|
$
|
37,425
|
|
||
|
|
2017
|
2016
|
2015
|
||||||||
|
Cardiovascular
|
$
|
38,437
|
|
$
|
32,613
|
|
$
|
50,927
|
|
||
|
Endoscopy
|
186
|
|
224
|
|
32
|
|
|||||
|
Total
|
$
|
38,623
|
|
$
|
32,837
|
|
$
|
50,959
|
|
||
|
|
Quarter Ended
|
||||||||||||||
|
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
2017
|
|
|
|
|
|
|
|
|
|||||||
|
Net sales
|
$
|
171,069
|
|
$
|
186,549
|
|
$
|
179,337
|
|
$
|
190,897
|
|
|||
|
Gross profit
|
75,942
|
|
84,141
|
|
80,514
|
|
85,656
|
|
|||||||
|
Income from operations
|
5,609
|
|
13,362
|
|
879
|
|
13,219
|
|
|||||||
|
Income tax expense
|
690
|
|
1,830
|
|
1,364
|
|
4,474
|
|
|||||||
|
Net income (loss)
|
14,803
|
|
9,483
|
|
(3,569
|
)
|
6,806
|
|
|||||||
|
Basic earnings per common share
|
0.33
|
|
0.19
|
|
(0.07
|
)
|
0.14
|
|
|||||||
|
Diluted earnings per common share
|
0.32
|
|
0.19
|
|
(0.07
|
)
|
0.13
|
|
|||||||
|
2016
|
|
|
|
|
|
|
|
|
|||||||
|
Net sales
|
$
|
138,077
|
|
$
|
151,071
|
|
$
|
156,975
|
|
$
|
157,715
|
|
|||
|
Gross profit
|
60,100
|
|
66,854
|
|
67,815
|
|
70,256
|
|
|||||||
|
Income from operations
|
7,706
|
|
11,581
|
|
2,987
|
|
12,602
|
|
|||||||
|
Income tax expense (benefit)
|
1,555
|
|
2,572
|
|
(978
|
)
|
2,116
|
|
|||||||
|
Net income
|
4,351
|
|
7,290
|
|
973
|
|
7,507
|
|
|||||||
|
Basic earnings per common share
|
0.10
|
|
0.16
|
|
0.02
|
|
0.17
|
|
|||||||
|
Diluted earnings per common share
|
0.10
|
|
0.16
|
|
0.02
|
|
0.17
|
|
|||||||
|
|
Fair Value Measurements Using
|
|||||||||||||||
|
|
Total Fair
|
Quoted prices in
|
Significant other
|
Significant
|
||||||||||||
|
|
Value at
|
active markets
|
observable inputs
|
unobservable inputs
|
||||||||||||
|
Description
|
December 31, 2017
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||||||
|
Interest rate contracts (1)
|
$
|
5,749
|
|
$
|
—
|
|
$
|
5,749
|
|
$
|
—
|
|
||||
|
Foreign currency contract assets, current and long-term (2)
|
$
|
621
|
|
$
|
—
|
|
$
|
621
|
|
$
|
—
|
|
||||
|
Foreign currency contract liabilities, current and long-term (3)
|
$
|
(1,391
|
)
|
$
|
—
|
|
$
|
(1,391
|
)
|
$
|
—
|
|
||||
|
|
Fair Value Measurements Using
|
|||||||||||||||
|
|
Total Fair
|
Quoted prices in
|
Significant other
|
Significant
|
||||||||||||
|
|
Value at
|
active markets
|
observable inputs
|
unobservable inputs
|
||||||||||||
|
Description
|
December 31, 2016
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||||||
|
Interest rate contracts (1)
|
$
|
4,991
|
|
$
|
—
|
|
$
|
4,991
|
|
$
|
—
|
|
||||
|
Foreign currency contract assets, current and long-term (2)
|
$
|
354
|
|
$
|
—
|
|
$
|
354
|
|
$
|
—
|
|
||||
|
Foreign currency contract liabilities, current and long-term (3)
|
$
|
(464
|
)
|
$
|
—
|
|
$
|
(464
|
)
|
$
|
—
|
|
||||
|
2017
|
2016
|
||||||
|
Beginning balance
|
$
|
683
|
|
$
|
1,024
|
|
|
|
Contingent consideration liability recorded as the result of acquisitions (see Note 2)
|
10,400
|
|
—
|
|
|||
|
Fair value adjustments recorded to income during the period
|
(66
|
)
|
(123
|
)
|
|||
|
Contingent payments made
|
(61
|
)
|
(218
|
)
|
|||
|
Ending balance
|
$
|
10,956
|
|
$
|
683
|
|
|
|
Contingent consideration asset or liability
|
Fair value at December 31, 2017
|
Valuation technique
|
Unobservable inputs
|
Range
|
||||||
|
Revenue-based payments
|
$
|
10,956
|
|
Discounted cash flow
|
Discount rate
|
9.9% - 15%
|
||||
|
contingent liability
|
Probability of milestone payment
|
100%
|
||||||||
|
Projected year of payments
|
2018-2037
|
|||||||||
|
Contingent receivable
|
$
|
760
|
|
Discounted cash flow
|
Discount rate
|
10%
|
||||
|
asset
|
Probability of milestone payment
|
75%
|
||||||||
|
Projected year of payments
|
2018-2019
|
|||||||||
|
Contingent consideration asset or liability
|
Fair value at December 31, 2016
|
Valuation technique
|
Unobservable inputs
|
Range
|
||||||
|
Revenue-based payments
|
$
|
683
|
|
Discounted cash flow
|
Discount rate
|
9.9% - 15%
|
||||
|
contingent liability
|
Probability of milestone payment
|
100%
|
||||||||
|
Projected year of payments
|
2017-2028
|
|||||||||
|
Contingent receivable
|
$
|
528
|
|
Discounted cash flow
|
Discount rate
|
10%
|
||||
|
asset
|
Probability of milestone payment
|
57%
|
||||||||
|
Projected year of payments
|
2017-2019
|
|||||||||
|
Item 9A.
|
Controls and Procedures.
|
|
Item 9B.
|
Other Information.
|
|
Item 15.
|
Exhibits and Financial Statement Schedules.
|
|
Description
|
Balance at
Beginning of Year |
Additions Charged to
Costs and Expenses (a) |
Deduction (b)
|
Balance at
End of Year |
||||||||
|
ALLOWANCE FOR UNCOLLECTIBLE ACCOUNTS:
|
|
|
|
|
||||||||
|
2015
|
(893
|
)
|
(607
|
)
|
203
|
|
(1,297
|
)
|
||||
|
2016
|
(1,297
|
)
|
(612
|
)
|
322
|
|
(1,587
|
)
|
||||
|
2017
|
(1,587
|
)
|
(1,012
|
)
|
830
|
|
(1,769
|
)
|
||||
|
Description
|
Balance at
Beginning of Year |
Additions Charged to
Costs and Expenses (c) |
Deduction
|
Balance at
End of Year |
||||||||
|
TAX VALUATION ALLOWANCE:
|
|
|
|
|
|
|
|
|
||||
|
2015
|
(1,603
|
)
|
(378
|
)
|
—
|
|
(1,981
|
)
|
||||
|
2016
|
(1,981
|
)
|
(1,805
|
)
|
—
|
|
(3,786
|
)
|
||||
|
2017
|
(3,786
|
)
|
(636
|
)
|
—
|
|
(4,422
|
)
|
||||
|
|
|
Description
|
|
1.1
|
|
|
|
2.1
|
||
|
2.2
|
||
|
23
|
||
|
3.1
|
||
|
3.2
|
||
|
4.1
|
|
|
|
10.1
|
|
|
|
10.2
|
|
|
|
10.3
|
|
|
|
10.4
|
|
|
|
10.5
|
|
|
|
10.6
|
|
|
|
10.7
|
|
|
|
10.8
|
|
|
|
10.9
|
||
|
10.10
|
||
|
10.11
|
||
|
10.12
|
||
|
10.13
|
||
|
10.14
|
||
|
10.15
|
||
|
10.16
|
||
|
10.17
|
||
|
10.18
|
||
|
10.19
|
||
|
10.20
|
||
|
10.21
|
||
|
10.22
|
||
|
10.23
|
||
|
10.24
|
||
|
10.25
|
|
|
|
10.26
|
|
|
|
10.27
|
|
|
|
10.28
|
|
|
|
10.29
|
|
|
|
10.30
|
||
|
21
|
||
|
23.1
|
||
|
31.1
|
||
|
31.2
|
||
|
32.1
|
||
|
32.2
|
||
|
101
|
The following materials from the Merit Medical Systems, Inc. Annual Report on Form 10-K for the fiscal year ended December 31, 2017, formatted in Extensible Business Reporting Language (XBRL): (i) the Consolidated Statements of Operations, (ii) Consolidated Balance Sheets, (iii) Consolidated Statements of Comprehensive Income (iv) Consolidated Statements of Stockholders' Equity, (v) Consolidated Statements of Cash Flows, and (vi) related notes.
|
|
|
Item 16.
|
Form 10-K Summary.
|
|
|
MERIT MEDICAL SYSTEMS, INC.
|
||
|
|
|
||
|
|
|
||
|
|
By:
|
/s/ FRED P. LAMPROPOULOS
|
|
|
|
|
|
Fred P. Lampropoulos, President and
|
|
|
|
|
Chief Executive Officer
|
|
Signature
|
Capacity in Which Signed
|
|
|
|
|
|
|
/s/: FRED P. LAMPROPOULOS
|
President, Chief Executive Officer and Director
|
|
|
Fred P. Lampropoulos
|
(Principal executive officer)
|
|
|
|
|
|
|
/s/: BERNARD J. BIRKETT
|
Chief Financial Officer, Secretary and Treasurer
|
|
|
Bernard J. Birkett
|
(Principal financial and accounting officer)
|
|
|
|
|
|
|
/s/: A. SCOTT ANDERSON
|
Director
|
|
|
A. Scott Anderson
|
|
|
|
|
|
|
|
/s/: THOMAS J. GUNDERSON
|
Director
|
|
|
Thomas J. Gunderson
|
|
|
|
/s/: NOLAN E. KARRAS
|
Director
|
|
|
Nolan E. Karras
|
|
|
|
/s/: DAVID M. LIU
|
Director
|
|
|
David M. Liu
|
||
|
/s/: FRANKLIN J. MILLER
|
Director
|
|
|
Franklin J. Miller
|
|
|
|
/s/: F. ANN MILLNER
|
Director
|
|
|
F. Ann Millner
|
||
|
/s/: KENT W. STANGER
|
Director
|
|
|
Kent W. Stanger
|
||
|
/s/: MICHAEL E. STILLABOWER
|
Director
|
|
|
Michael E. Stillabower
|
||