|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
☒
|
Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
|
For the Fiscal Year Ended December 31, 2018
|
||
|
or
|
||
|
☐
|
Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
|
|
For the transition period from
to
|
||
|
Incorporated in New Jersey
|
|
I.R.S. Employer
Identification No. 22-1918501
|
|
Securities Registered pursuant to Section 12(b) of the Act:
|
||
|
Title of Each Class
|
|
Name of Each Exchange on which Registered
|
|
Common Stock ($0.50 par value)
|
|
New York Stock Exchange
|
|
1.125% Notes due 2021
|
New York Stock Exchange
|
|
|
0.500% Notes due 2024
|
New York Stock Exchange
|
|
|
1.875% Notes due 2026
|
New York Stock Exchange
|
|
|
2.500% Notes due 2034
|
New York Stock Exchange
|
|
|
1.375% Notes due 2036
|
New York Stock Exchange
|
|
|
Large accelerated filer
|
☒
|
Accelerated filer
|
☐
|
|
Non-accelerated filer
|
☐
|
Smaller reporting company
|
☐
|
|
Emerging growth company
|
☐
|
||
|
Documents Incorporated by Reference:
|
||
|
Document
|
|
Part of Form 10-K
|
|
Proxy Statement for the Annual Meeting of Shareholders to be held May 28, 2019, to be filed with the
Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this report
|
|
Part III
|
|
|
|
|
Page
|
|
Item 1.
|
|||
|
Item 1A.
|
|||
|
Item 1B.
|
|||
|
Item 2.
|
|||
|
Item 3.
|
|||
|
Item 4.
|
|||
|
Item 5.
|
|||
|
Item 6.
|
|||
|
Item 7.
|
|||
|
Item 7A.
|
|||
|
Item 8.
|
|||
|
(a)
|
|||
|
(b)
|
|||
|
Item 9.
|
|||
|
Item 9A.
|
|||
|
Item 9B.
|
|||
|
Item 10.
|
|||
|
Item 11.
|
|||
|
Item 12.
|
|||
|
Item 13.
|
|||
|
Item 14.
|
|||
|
Item 15.
|
|||
|
Item 16.
|
|||
|
Item 1.
|
Business.
|
|
($ in millions)
|
2018
|
2017
|
2016
|
||||||||
|
Total Sales
|
$
|
42,294
|
|
$
|
40,122
|
|
$
|
39,807
|
|
||
|
Pharmaceutical
|
37,689
|
|
35,390
|
|
35,151
|
|
|||||
|
Keytruda
|
7,171
|
|
3,809
|
|
1,402
|
|
|||||
|
Januvia/Janumet
|
5,914
|
|
5,896
|
|
6,109
|
|
|||||
|
Gardasil/Gardasil
9
|
3,151
|
|
2,308
|
|
2,173
|
|
|||||
|
ProQuad/M-M-R
II
/Varivax
|
1,798
|
|
1,676
|
|
1,640
|
|
|||||
|
Zetia/Vytorin
|
1,355
|
|
2,095
|
|
3,701
|
|
|||||
|
Isentress/Isentress HD
|
1,140
|
|
1,204
|
|
1,387
|
|
|||||
|
Bridion
|
917
|
|
704
|
|
482
|
|
|||||
|
Pneumovax
23
|
907
|
|
821
|
|
641
|
|
|||||
|
NuvaRing
|
902
|
|
761
|
|
777
|
|
|||||
|
Simponi
|
893
|
|
819
|
|
766
|
|
|||||
|
Animal Health
|
4,212
|
|
3,875
|
|
3,478
|
|
|||||
|
Livestock
|
2,630
|
|
2,484
|
|
2,287
|
|
|||||
|
Companion Animals
|
1,582
|
|
1,391
|
|
1,191
|
|
|||||
|
Other Revenues
(1)
|
393
|
|
857
|
|
1,178
|
|
|||||
|
(1)
|
Other revenues are primarily comprised of Healthcare Services segment revenue, third-party manufacturing sales, and miscellaneous corporate revenues, including revenue hedging activities.
|
|
Product
|
Date
|
Approval
|
|
Keytruda
|
December 2018
|
The Japanese Ministry of Health, Labor and Welfare (JMHLW) approved
Keytruda
for three expanded uses in unresectable, advanced or recurrent NSCLC, one in malignant melanoma, as well as a new indication in high microsatellite instability solid tumors.
|
|
December 2018
|
The U.S. Food and Drug Administration (FDA) approved
Keytruda
for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma.
|
|
|
December 2018
|
The European Commission (EC) approved
Keytruda
for the adjuvant treatment of adults with stage III melanoma and lymph node involvement who have undergone complete resection.
|
|
|
November 2018
|
FDA approved
Keytruda
for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
|
|
|
October 2018
|
FDA approved
Keytruda
, in combination with carboplatin and either paclitaxel or nab-paclitaxel, for the first-line treatment of patients with metastatic squamous non-small cell lung cancer (NSCLC).
|
|
|
September 2018
|
EC approved
Keytruda
in combination with pemetrexed and platinum chemotherapy for the first-line treatment of metastatic nonsquamous NSCLC in adults whose tumors have no EGFR or ALK positive mutations.
|
|
|
September 2018
|
EC approved
Keytruda
for the treatment of recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) in adults whose tumors express PD-L1 with a ≥ 50% TPS and progressing on or after platinum-containing chemotherapy.
|
|
|
August 2018
|
FDA approved
Keytruda
in combination with pemetrexed and platinum chemotherapy for the first-line treatment of metastatic nonsquamous NSCLC patients with no EGFR or ALK genomic tumor aberrations.
|
|
|
July 2018
|
The China National Drug Administration (CNDA) approved
Keytruda
for the treatment of adult patients with unresectable or metastatic melanoma following failure of one prior line of therapy.
|
|
|
June 2018
|
FDA approved
Keytruda
for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL), or who have relapsed after two or more prior lines of therapy.
|
|
|
June 2018
|
FDA approved
Keytruda
for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 as determined by an FDA-approved test.
|
|
|
Lynparza
(1)
|
December 2018
|
FDA approved Lynparza for use as maintenance treatment of certain patients with advanced ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based chemotherapy.
|
|
July 2018
|
JMHLW approved Lynparza for use in patients with unresectable or recurrent
BRCA
-mutated, human epidermal growth factor receptor 2 (HER2)-negative breast cancer who have received prior chemotherapy.
|
|
|
May 2018
|
EC approved Lynparza for use as a maintenance therapy in patients with platinum-sensitive relapsed high grade epithelial ovarian, fallopian tube, or primary peritoneal cancer, who are in response (complete or partial) to platinum based chemotherapy regardless of
BRCA
mutation status.
|
|
|
January 2018
|
FDA approved Lynparza for use in patients with
BRCA
-mutated, HER2-negative metastatic breast cancer who have been previously treated with chemotherapy.
|
|
|
January 2018
|
JMHLW approved Lynparza for use as a maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer, regardless of
BRCA
mutation status.
|
|
|
Lenvima
(2)
|
September 2018
|
CNDA approved Lenvima for the treatment of certain patients with hepatocellular carcinoma.
|
|
August 2018
|
FDA approved Lenvima for the treatment of certain patients with hepatocellular carcinoma.
|
|
|
August 2018
|
EC approved Lenvima for the treatment of certain patients with hepatocellular carcinoma.
|
|
|
March 2018
|
JMHLW approved Lenvima for the treatment of certain patients with unresectable hepatocellular carcinoma.
|
|
|
Gardasil
9
|
October 2018
|
FDA approved
Gardasil
9 for an expanded age indication for use in women and men ages 27 to 45 for the prevention of certain cancers and diseases caused by the nine HPV types covered by the vaccine.
|
|
April 2018
|
CNDA approved
Gardasil
9 for use in girls and women ages 16 to 26.
|
|
|
Delstrigo
|
November 2018
|
EC approved
Delstrigo
(doravirine, lamivudine, and tenofovir disoproxil fumarate) for the treatment of adults infected with human immunodeficiency virus (HIV-1) without past or present evidence of resistance to the non-nucleoside reverse transcriptase inhibitor (NNRTI) class, lamivudine, or tenofovir.
|
|
August 2018
|
FDA approved
Delstrigo
for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment experience.
|
|
|
Pifeltro
|
November 2018
|
EC approved
Pifeltro
(doravirine), in combination with other antiretroviral medicinal products, for the treatment of adults infected with HIV-1 without past or present evidence of resistance to the NNRTI class.
|
|
August 2018
|
FDA approved
Pifeltro
for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment experience.
|
|
|
Isentress
|
March 2018
|
EC approved
Isentress
for an extension to the existing indication to cover treatment of neonates.
Isentress
is now indicated in combination with other anti-retroviral medicinal products for the treatment of HIV-1 infection.
|
|
Prevymis
|
January 2018
|
EC approved
Prevymis
(letermovir) for the prophylaxis of cytomegalovirus (CMV) reactivation and disease in adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant.
|
|
Steglatro, Steglujan
and
Segluromet
(3)
|
March 2018
|
EC approved
Steglatro
(ertugliflozin),
Steglujan
(ertugliflozin and sitagliptin) and
Segluromet
(ertugliflozin and metformin hydrochloride) for the treatment of adults aged 18 years and older with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control (as monotherapy in patients for whom the use of metformin is considered inappropriate due to intolerance or contraindications, and in addition to other medicinal products for the treatment of diabetes).
|
|
Vaxelis
|
December 2018
|
FDA approved
Vaxelis
(Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus, Haemophilus b Conjugate [Meningococcal Protein Conjugate] and Hepatitis B [Recombinant] Vaccine) for use in children from 6 weeks through 4 years of age (prior to the 5th birthday)
|
|
(1)
|
In July 2017, Merck and AstraZeneca entered into a global strategic oncology collaboration to co-develop and co-commercialize AstraZeneca’s Lynparza.
|
|
(2)
|
In March 2018,
Merck and Eisai Co., Ltd. announced a strategic collaboration for the worldwide co-development and co-commercialization of Eisai’s Lenvima.
|
|
(3)
|
In 2013, Merck and Pfizer Inc. announced that they entered into a worldwide collaboration, except Japan, for the co-development and co-promotion of ertugliflozin.
|
|
Product
|
Year of Expiration (U.S.)
|
Year of Expiration (EU)
(1)
|
Year of Expiration (Japan)
(3)
|
|
Emend
|
Expired
|
2019
|
2019
|
|
Emend
for Injection
|
2019
|
2020
(2)
|
2020
|
|
Noxafil
|
2019
|
2019
|
N/A
|
|
Vaxelis
(4)
|
2020 (method of making)
|
2021
(5)
(SPCs)
|
Not Marketed
|
|
Januvia
|
2022
(2)
|
2022
(2)
|
2025-2026
|
|
Janumet
|
2022
(2)
|
2023
|
N/A
|
|
Janumet XR
|
2022
(2)
|
N/A
|
N/A
|
|
Isentress
|
2024
|
2022
(2)
|
2022
|
|
Simponi
|
N/A
(6)
|
2025
(7)
|
N/A
(6)
|
|
Lenvima
(8)
|
2025
(2)
(with pending PTE)
|
2021 (patents), 2026
(2)
(SPCs)
|
2026
|
|
Adempas
(9)
|
2026
(2)
|
2028
(2)
|
2027-2028
|
|
Bridion
|
2026
(2)
(with pending PTE)
|
2023
|
2024
|
|
Nexplanon
|
2027 (device)
|
2025 (device)
|
Not Marketed
|
|
Bravecto
|
2027 (with pending PTE)
|
2025 (patents), 2029 (SPCs)
|
2029
|
|
Gardasil
|
2028
|
2021
(2)
|
Expired
|
|
Gardasil
9
|
2028
|
2025 (patents)
,
2030
(2)
(SPCs)
|
N/A
|
|
Keytruda
|
2028
|
2028 (patents), 2030
(2)
(SPCs)
|
2032
|
|
Lynparza
(10)
|
2028
(2)
(with pending PTE)
|
2024 (patents), 2029
(2)
(SPCs)
|
2028-2029 (with pending PTE)
|
|
Zerbaxa
|
2028
(2)
(with pending PTE)
|
2023 (patents), 2028
(2)
(SPCs)
|
N/A
|
|
Sivextro
|
2028
(2)
|
2024 (patents), 2029
(2)
(SPCs)
|
2029 (with pending PTE)
|
|
Belsomra
|
2029
(2)
|
N/A
|
2031
|
|
Prevymis
|
2029
(2)
(with pending PTE)
|
2024 (patents), 2029
(2)
(SPCs)
|
2029 (with pending PTE)
|
|
Steglatro
(11)
|
2031
(2)
(with pending PTE)
|
2029 (patents), 2034
(2)
(SPCs)
|
N/A
|
|
Steglujan
(11)
|
2031
(with pending PTE)
|
2029 (patents), 2034 (SPCs)
|
N/A
|
|
Segluromet
(11)
|
2031 (with pending PTE)
|
2029 (patents), 2034 (SPCs)
|
N/A
|
|
Delstrigo
|
2032 (with pending PTE)
|
2031
(12)
|
N/A
|
|
Pifeltro
|
2032 (with pending PTE)
|
2031
(12)
|
N/A
|
|
N/A:
|
Currently no marketing approval.
|
|
Note:
|
Compound patent unless otherwise noted. Certain of the products listed may be the subject of patent litigation. See Item 8. “Financial Statements and Supplementary Data,” Note 11. “Contingencies and Environmental Liabilities” below.
|
|
(1)
|
The EU date represents the expiration date for the following five countries: France, Germany, Italy, Spain and the United Kingdom (Major EU Markets). If SPC applications have been filed but have not been granted in all Major EU Markets, both the patent expiry date and the SPC expiry date are listed.
|
|
(2)
|
Eligible for 6 months Pediatric Exclusivity.
|
|
(3)
|
The PTE system in Japan allows for a patent to be extended more than once provided the later approval is directed to a different indication from that of the previous approval. This may result in multiple PTE approvals for a given patent, each with its own expiration date.
|
|
(4)
|
Being commercialized in a U.S.-based joint partnership with Sanofi Pasteur.
|
|
(5)
|
SPCs are granted in four Major EU Markets and pending in one, based on a patent that expired in 2016.
|
|
(6)
|
The Company has no marketing rights in the U.S. and Japan.
|
|
(7)
|
Includes Pediatric Exclusivity, which is granted in four Major EU Markets and pending in one.
|
|
(8)
|
Being developed and commercialized in a global strategic oncology collaboration with Eisai.
|
|
(9)
|
Being commercialized in a worldwide collaboration with Bayer AG.
|
|
(10)
|
Being developed and commercialized in a global strategic oncology collaboration with AstraZeneca.
|
|
(11)
|
Being developed and promoted in a worldwide, except Japan, collaboration with Pfizer.
|
|
(12)
|
SPC applications to be filed by May 2019.
|
|
Under Review (in the U.S.)
|
Currently Anticipated
Year of Expiration (in the U.S.)
|
|
V920 (ebola vaccine)
|
2023
|
|
MK-7655A (relebactam + imipenem/cilastatin)
|
2029
|
|
Phase 3 Drug Candidate
|
Currently Anticipated
Year of Expiration (in the U.S.)
|
|
MK-1242 (vericiguat)
(1)
|
2031
|
|
MK-7264 (gefapixant)
|
2027
|
|
V114 (pneumoconjugate vaccine)
|
2031
|
|
(1)
|
Being developed in a worldwide clinical development collaboration with Bayer AG.
|
|
Phase 2
|
Phase 3 (Phase 3 Entry Date)
|
Under Review
|
|
Cancer
|
Cancer
|
New Molecular Entities/Vaccines
|
|
MK-3475
Keytruda
|
MK-3475
Keytruda
|
Bacterial Infection
|
|
Advanced Solid Tumors
|
Breast (October 2015)
|
MK-7655A relebactam+imipenem/cilastatin
|
|
Cutaneous Squamous Cell Carcinoma
|
Cervical (October 2018) (EU)
|
(U.S.)
|
|
Prostate
|
Colorectal (November 2015)
|
Ebola Vaccine
|
|
MK-7902 Lenvima
(1)
|
Esophageal (December 2015)
|
V920
(4)
(U.S.)
|
|
Biliary Tract
|
Gastric (May 2015) (EU)
|
|
|
Non-Small-Cell Lung
|
Hepatocellular (May 2016) (EU)
|
Certain Supplemental Filings
|
|
V937
Cavatak
|
Mesothelioma (May 2018)
|
Cancer
|
|
Melanoma
|
Nasopharyngeal (April 2016)
|
MK-3475
Keytruda
|
|
MK-7690
|
Ovarian (December 2018)
|
•
First-Line Advanced Renal Cell Carcinoma
|
|
Colorectal
(2)
|
Renal (October 2016) (EU)
|
(KEYNOTE-426) (U.S.)
|
|
MK-7339 Lynparza
(1)
|
Small-Cell Lung (May 2017) (EU)
|
•
First-Line Metastatic Squamous Non-Small-
|
|
Advanced Solid Tumors
|
MK-7902 Lenvima
(1,2)
|
Cell Lung Cancer (KEYNOTE-407) (EU)
|
|
Cytomegalovirus Vaccine
|
Endometrial (June 2018)
|
•
First-Line Metastatic Non-Small-Cell Lung
|
|
V160
|
MK-7339 Lynparza
(1)
|
Cancer (KEYNOTE-042) (U.S.) (EU)
|
|
Diabetes Mellitus
|
Pancreatic (December 2014)
|
•
Third-Line Advanced Small-Cell Lung
|
|
MK-8521
(3)
|
Prostate (April 2017)
|
Cancer (KEYNOTE-158) (U.S.)
|
|
HIV-1 Infection
|
Cough
|
•
First-Line Head and Neck Cancer
|
|
MK-8591
|
MK-7264 (gefapixant) (March 2018)
|
(KEYNOTE-048) (U.S.)
|
|
Pediatric Neurofibromatosis Type-1
|
Heart Failure
|
•
Alternative Dosing Regimen
|
|
MK-5618 (selumetinib)
(1)
|
MK-1242 (vericiguat) (September 2016)
(1)
|
(Q6W) (EU)
|
|
Respiratory Syncytial Virus
|
Pneumoconjugate Vaccine
|
MK-7339 Lynparza
(1)
|
|
MK-1654
|
V114 (June 2018)
|
•
Second-Line Metastatic Breast Cancer (EU)
|
|
Schizophrenia
|
•
First-Line Advanced Ovarian Cancer (EU)
|
|
|
MK-8189
|
HABP/VABP
(5)
|
|
|
MK-7625A
Zerbaxa
(U.S.)
|
||
|
Footnotes:
|
||
|
(1)
Being developed in a collaboration.
|
||
|
(2)
Being developed in combination with
|
||
|
Keytruda.
|
||
|
(3)
Development is currently on hold.
|
||
|
(4)
Rolling submission.
|
||
|
(5)
HABP - Hospital-Acquired Bacterial
|
||
|
Pneumonia / VABP - Ventilator-Associated
|
||
|
Bacterial Pneumonia
|
||
|
Item 1A.
|
Risk Factors.
|
|
•
|
findings of ineffectiveness, superior safety or efficacy of competing products, or harmful side effects in clinical or pre-clinical testing;
|
|
•
|
failure to receive the necessary regulatory approvals, including delays in the approval of new products and new indications, or the anticipated labeling, and uncertainties about the time required to obtain regulatory approvals and the benefit/risk standards applied by regulatory agencies in determining whether to grant approvals;
|
|
•
|
failure in certain markets to obtain reimbursement commensurate with the level of innovation and clinical benefit presented by the product;
|
|
•
|
lack of economic feasibility due to manufacturing costs or other factors; and
|
|
•
|
preclusion from commercialization by the proprietary rights of others.
|
|
•
|
results in post-approval Phase 4 trials or other studies;
|
|
•
|
the re-review of products that are already marketed;
|
|
•
|
the recall or loss of marketing approval of products that are already marketed;
|
|
•
|
changing government standards or public expectations regarding safety, efficacy or labeling changes; and
|
|
•
|
greater scrutiny in advertising and promotion.
|
|
•
|
changes in medical reimbursement policies and programs and pricing restrictions in key markets;
|
|
•
|
multiple regulatory requirements that could restrict the Company’s ability to manufacture and sell its products in key markets;
|
|
•
|
trade protection measures and import or export licensing requirements, including the imposition of trade sanctions or similar restrictions by the United States or other governments;
|
|
•
|
foreign exchange fluctuations;
|
|
•
|
diminished protection of intellectual property in some countries; and
|
|
•
|
possible nationalization and expropriation.
|
|
•
|
There may be limited access to, and supply of, normal and diseased tissue samples, cell lines, pathogens, bacteria, viral strains and other biological materials. In addition, government regulations in multiple
|
|
•
|
The development, manufacturing and marketing of biologics and vaccines are subject to regulation by the FDA, the EMA and other regulatory bodies. These regulations are often more complex and extensive than the regulations applicable to other pharmaceutical products. For example, in the United States, a BLA, including both preclinical and clinical trial data and extensive data regarding the manufacturing procedures, is required for human vaccine candidates, and FDA approval is generally required for the release of each manufactured commercial lot.
|
|
•
|
Manufacturing biologics and vaccines, especially in large quantities, is often complex and may require the use of innovative technologies to handle living micro-organisms. Each lot of an approved biologic and vaccine must undergo thorough testing for identity, strength, quality, purity and potency. Manufacturing biologics requires facilities specifically designed for and validated for this purpose, and sophisticated quality assurance and quality control procedures are necessary. Slight deviations anywhere in the manufacturing process, including filling, labeling, packaging, storage and shipping and quality control and testing, may result in lot failures, product recalls or spoilage. When changes are made to the manufacturing process, the Company may be required to provide pre-clinical and clinical data showing the comparable identity, strength, quality, purity or potency of the products before and after such changes.
|
|
•
|
Biologics and vaccines are frequently costly to manufacture because production ingredients are derived from living animal or plant material, and most biologics and vaccines cannot be made synthetically. In particular, keeping up with the demand for vaccines may be difficult due to the complexity of producing vaccines.
|
|
•
|
The use of biologically derived ingredients can lead to variability in the manufacturing process and could lead to allegations of harm, including infections or allergic reactions, which allegations would be reviewed through a standard investigation process that could lead to closure of product facilities due to possible contamination. Any of these events could result in substantial costs.
|
|
Item 1B.
|
Unresolved Staff Comments.
|
|
Item 2.
|
Properties.
|
|
Item 3.
|
Legal Proceedings.
|
|
Item 4.
|
Mine Safety Disclosures.
|
|
Name
|
Age
|
Offices and Business Experience
|
|
Kenneth C. Frazier
|
64
|
Chairman, President and Chief Executive Officer (since December 2011)
|
|
Sanat Chattopadhyay
|
59
|
Executive Vice President and President, Merck Manufacturing Division (since March 2016); Senior Vice President, Operations, Merck Manufacturing Division (November 2009-March 2016)
|
|
Frank Clyburn
|
54
|
Executive Vice President, Chief Commercial Officer (since January 2019); President, Global Oncology Business Unit (October 2013-December 2018); President, Primary Care and Women’s Health Business Line (September 2011-October 2013)
|
|
Robert M. Davis
|
52
|
Executive Vice President, Global Services, and Chief Financial Officer (since April 2016); Executive Vice President and Chief Financial Officer (April 2014-April 2016); Corporate Vice President and President, Medical Products, Baxter International, Inc. (October 2010-March 2014)
|
|
Richard R. DeLuca, Jr.
|
56
|
Executive Vice President and President, Merck Animal Health (since September 2011)
|
|
Julie L. Gerberding
|
62
|
Executive Vice President and Chief Patient Officer, Strategic Communications, Global Public Policy and Population Health (since July 2016); Executive Vice President for Strategic Communications, Global Public Policy and Population Health (January 2015-July 2016); President, Merck Vaccines (January 2010-January 2015)
|
|
Rita A. Karachun
|
55
|
Senior Vice President Finance - Global Controller (since March 2014); Assistant Controller (November 2009-March 2014)
|
|
Steven C. Mizell
|
58
|
Executive Vice President, Chief Human Resources Officer, Human Resources (since October 2018); Executive Vice President, Chief Human Resources Officer (December 2016-October 2018) and Executive Vice President, Human Resources, Monsanto Company (August 2011-December 2016)
|
|
Michael T. Nally
|
43
|
Executive Vice President, Chief Marketing Officer (since January 2019); President, Global Vaccines, Global Human Health (September 2016-January 2019); Managing Director, United Kingdom and Ireland, Global Human Health (January 2014-September 2016); Managing Director, Sweden, Global Human Health (November 2011-January 2014)
|
|
Roger M. Perlmutter, M.D., Ph.D.
|
66
|
Executive Vice President and President, Merck Research Laboratories (since April 2013)
|
|
Jim Scholefield
|
56
|
Executive Vice President, Chief Information and Digital Officer (since October 2018); Chief Information Officer, Nike, Inc (July 2015-October 2018); Chief Technology Officer, The Coca-Cola Company, (November 2010-June 2015)
|
|
Jennifer Zachary
|
41
|
Executive Vice President and General Counsel (since April 2018); Partner, Covington & Burling LLP (January 2013-March 2018)
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
($ in millions)
|
||||||
|
Period
|
Total Number
of Shares
Purchased
(1)
|
Average Price
Paid Per
Share
|
Approximate Dollar Value of Shares
That May Yet Be Purchased
Under the Plans or Programs
(1)
|
|||
|
October 1 — October 31
|
59,154,075
|
$70.56
|
$12,709
(2)
|
|||
|
November 1 — November 30
|
5,279,715
|
$74.64
|
$12,315
|
|||
|
December 1 — December 31
|
4,788,526
|
$76.30
|
$11,949
|
|||
|
Total
|
69,222,316
|
$71.27
|
$11,949
|
|||
|
(1)
|
All shares purchased during the period were made as part of a plan approved by the Board of Directors in November 2017 to purchase up to $10 billion in Merck shares. In October 2018, the Board of Directors authorized additional purchases of up to $10 billion of Merck’s common stock for its treasury. Shares are approximated.
|
|
(2)
|
Amount includes $1.0 billion being held back pending final settlement under the accelerated share repurchase agreements discussed below.
|
|
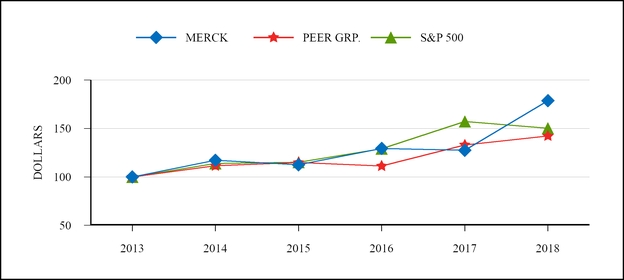
End of
Period Value
|
2018/2013
CAGR**
|
||
|
MERCK
|
$179
|
12%
|
|
|
PEER GRP.**
|
142
|
7%
|
|
|
S&P 500
|
150
|
8%
|
|
|
2013
|
2014
|
2015
|
2016
|
2017
|
2018
|
|
|
MERCK
|
100.00
|
117.10
|
112.40
|
129.40
|
127.40
|
178.70
|
|
PEER GRP.
|
100.00
|
111.40
|
114.80
|
111.20
|
133.00
|
142.20
|
|
S&P 500
|
100.00
|
113.70
|
115.20
|
129.00
|
157.20
|
150.30
|
|
*
|
Compound Annual Growth Rate
|
|
**
|
Peer group average was calculated on a market cap weighted basis.
|
|
Item 6.
|
Selected Financial Data.
|
|
2018
(1)
|
2017
(2)(3)
|
2016
(2)(4)
|
2015
(2)(5)
|
2014
(2)(6)
|
|||||||||||||||
|
Results for Year:
|
|||||||||||||||||||
|
Sales
|
$
|
42,294
|
|
$
|
40,122
|
|
$
|
39,807
|
|
$
|
39,498
|
|
$
|
42,237
|
|
||||
|
Cost of sales
|
13,509
|
|
12,912
|
|
14,030
|
|
15,043
|
|
16,903
|
|
|||||||||
|
Selling, general and administrative
|
10,102
|
|
10,074
|
|
10,017
|
|
10,508
|
|
11,816
|
|
|||||||||
|
Research and development
|
9,752
|
|
10,339
|
|
10,261
|
|
6,796
|
|
7,290
|
|
|||||||||
|
Restructuring costs
|
632
|
|
776
|
|
651
|
|
619
|
|
1,013
|
|
|||||||||
|
Other (income) expense, net
|
(402
|
)
|
(500
|
)
|
189
|
|
1,131
|
|
(12,068
|
)
|
|||||||||
|
Income before taxes
|
8,701
|
|
6,521
|
|
4,659
|
|
5,401
|
|
17,283
|
|
|||||||||
|
Taxes on income
|
2,508
|
|
4,103
|
|
718
|
|
942
|
|
5,349
|
|
|||||||||
|
Net income
|
6,193
|
|
2,418
|
|
3,941
|
|
4,459
|
|
11,934
|
|
|||||||||
|
Less: Net (loss) income attributable to noncontrolling interests
|
(27
|
)
|
24
|
|
21
|
|
17
|
|
14
|
|
|||||||||
|
Net income attributable to Merck & Co., Inc.
|
6,220
|
|
2,394
|
|
3,920
|
|
4,442
|
|
11,920
|
|
|||||||||
|
Basic earnings per common share attributable to Merck & Co., Inc. common shareholders
|
$
|
2.34
|
|
$
|
0.88
|
|
$
|
1.42
|
|
$
|
1.58
|
|
$
|
4.12
|
|
||||
|
Earnings per common share assuming dilution attributable to Merck & Co., Inc. common shareholders
|
$
|
2.32
|
|
$
|
0.87
|
|
$
|
1.41
|
|
$
|
1.56
|
|
$
|
4.07
|
|
||||
|
Cash dividends declared
|
5,313
|
|
5,177
|
|
5,135
|
|
5,115
|
|
5,156
|
|
|||||||||
|
Cash dividends declared per common share
|
$
|
1.99
|
|
$
|
1.89
|
|
$
|
1.85
|
|
$
|
1.81
|
|
$
|
1.77
|
|
||||
|
Capital expenditures
|
2,615
|
|
1,888
|
|
1,614
|
|
1,283
|
|
1,317
|
|
|||||||||
|
Depreciation
|
1,416
|
|
1,455
|
|
1,611
|
|
1,593
|
|
2,471
|
|
|||||||||
|
Average common shares outstanding (millions)
|
2,664
|
|
2,730
|
|
2,766
|
|
2,816
|
|
2,894
|
|
|||||||||
|
Average common shares outstanding assuming dilution (millions)
|
2,679
|
|
2,748
|
|
2,787
|
|
2,841
|
|
2,928
|
|
|||||||||
|
Year-End Position:
|
|||||||||||||||||||
|
Working capital
|
$
|
3,669
|
|
$
|
6,152
|
|
$
|
13,410
|
|
$
|
10,550
|
|
$
|
14,198
|
|
||||
|
Property, plant and equipment, net
|
13,291
|
|
12,439
|
|
12,026
|
|
12,507
|
|
13,136
|
|
|||||||||
|
Total assets
|
82,637
|
|
87,872
|
|
95,377
|
|
101,677
|
|
98,096
|
|
|||||||||
|
Long-term debt
|
19,806
|
|
21,353
|
|
24,274
|
|
23,829
|
|
18,629
|
|
|||||||||
|
Total equity
|
26,882
|
|
34,569
|
|
40,308
|
|
44,767
|
|
48,791
|
|
|||||||||
|
Year-End Statistics:
|
|||||||||||||||||||
|
Number of stockholders of record
|
115,800
|
|
121,700
|
|
129,500
|
|
135,500
|
|
142,000
|
|
|||||||||
|
Number of employees
|
69,000
|
|
69,000
|
|
68,000
|
|
68,000
|
|
70,000
|
|
|||||||||
|
(1)
|
Amounts for 2018 include a charge related to the formation of a collaboration with Eisai Co., Ltd.
|
|
(2)
|
Amounts have been recast as a result of the adoption, on January 1, 2018, of a new accounting standard related to the classification of certain defined benefit plan costs. There was no impact to net income as a result of adopting the new accounting standard.
|
|
(3)
|
Amounts for 2017 include a provisional net tax charge related to the enactment of U.S. tax legislation and a charge related to the formation of a collaboration with AstraZeneca.
|
|
(4)
|
Amounts for 2016 include a charge related to the settlement of worldwide patent litigation related to
Keytruda
.
|
|
(5)
|
Amounts for 2015 include a net charge related to the settlement of
Vioxx
shareholder class action litigation, foreign exchange losses related to Venezuela, gains on the dispositions of businesses and other assets, and the favorable benefit of certain tax items.
|
|
(6)
|
Amounts for 2014 reflect the divestiture of Merck’s Consumer Care business on October 1, 2014, including a gain on the sale, as well as a gain recognized on an option exercise by AstraZeneca, gains on the dispositions of other businesses and assets, and a loss on extinguishment of debt.
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
|
($ in millions)
|
2018
|
Change
|
2017
|
Change
|
2016
|
||||||||||||
|
Cost of sales
|
$
|
13,509
|
|
5
|
%
|
$
|
12,912
|
|
-8
|
%
|
$
|
14,030
|
|
||||
|
Selling, general and administrative
|
10,102
|
|
—
|
%
|
10,074
|
|
1
|
%
|
10,017
|
|
|||||||
|
Research and development
|
9,752
|
|
-6
|
%
|
10,339
|
|
1
|
%
|
10,261
|
|
|||||||
|
Restructuring costs
|
632
|
|
-19
|
%
|
776
|
|
19
|
%
|
651
|
|
|||||||
|
Other (income) expense, net
|
(402
|
)
|
-20
|
%
|
(500
|
)
|
*
|
|
189
|
|
|||||||
|
|
$
|
33,593
|
|
—
|
%
|
$
|
33,601
|
|
-4
|
%
|
$
|
35,148
|
|
||||
|
Segment Profits
|
|||||||||||
|
($ in millions)
|
2018
|
2017
|
2016
|
||||||||
|
Pharmaceutical segment profits
|
$
|
24,292
|
|
$
|
22,495
|
|
$
|
22,141
|
|
||
|
Animal Health segment profits
|
1,659
|
|
1,552
|
|
1,357
|
|
|||||
|
Other non-reportable segment profits
|
103
|
|
275
|
|
146
|
|
|||||
|
Other
|
(17,353
|
)
|
|
(17,801
|
)
|
|
(18,985
|
)
|
|||
|
Income before taxes
|
$
|
8,701
|
|
$
|
6,521
|
|
$
|
4,659
|
|
||
|
($ in millions except per share amounts)
|
2018
|
2017
|
2016
|
||||||||
|
Income before taxes as reported under GAAP
|
$
|
8,701
|
|
$
|
6,521
|
|
$
|
4,659
|
|
||
|
Increase (decrease) for excluded items:
|
|||||||||||
|
Acquisition and divestiture-related costs
|
3,066
|
|
3,760
|
|
7,312
|
|
|||||
|
Restructuring costs
|
658
|
|
927
|
|
1,069
|
|
|||||
|
Other items:
|
|||||||||||
|
Charge related to the formation of an oncology collaboration with Eisai
|
1,400
|
|
—
|
|
—
|
|
|||||
|
Charge related to the termination of a collaboration with Samsung
|
423
|
|
—
|
|
—
|
|
|||||
|
Charge for the acquisition of Viralytics
|
344
|
|
—
|
|
—
|
|
|||||
|
Charge related to the formation of an oncology collaboration with AstraZeneca
|
—
|
|
2,350
|
|
—
|
|
|||||
|
Charge related to the settlement of worldwide
Keytruda
patent litigation
|
—
|
|
—
|
|
625
|
|
|||||
|
Other
|
(57
|
)
|
(16
|
)
|
(67
|
)
|
|||||
|
Non-GAAP income before taxes
|
14,535
|
|
13,542
|
|
13,598
|
|
|||||
|
Taxes on income as reported under GAAP
|
2,508
|
|
4,103
|
|
718
|
|
|||||
|
Estimated tax benefit on excluded items
(1)
|
535
|
|
785
|
|
2,321
|
|
|||||
|
Net tax charge related to the enactment of the TCJA
(2)
|
(160
|
)
|
(2,625
|
)
|
—
|
|
|||||
|
Net tax benefit from the settlement of certain federal income tax issues
|
—
|
|
234
|
|
—
|
|
|||||
|
Tax benefit related to the settlement of a state income tax issue
|
—
|
|
88
|
|
—
|
|
|||||
|
Non-GAAP taxes on income
|
2,883
|
|
|
2,585
|
|
|
3,039
|
|
|||
|
Non-GAAP net income
|
11,652
|
|
10,957
|
|
10,559
|
|
|||||
|
Less: Net (loss) income attributable to noncontrolling interests as reported under GAAP
|
(27
|
)
|
24
|
|
21
|
|
|||||
|
Acquisition and divestiture-related costs attributable to noncontrolling interests
|
(58
|
)
|
—
|
|
—
|
|
|||||
|
Non-GAAP net income attributable to noncontrolling interests
|
31
|
|
|
24
|
|
|
21
|
|
|||
|
Non-GAAP net income attributable to Merck & Co., Inc.
|
$
|
11,621
|
|
|
$
|
10,933
|
|
|
$
|
10,538
|
|
|
EPS assuming dilution as reported under GAAP
|
$
|
2.32
|
|
$
|
0.87
|
|
$
|
1.41
|
|
||
|
EPS difference
(3)
|
2.02
|
|
3.11
|
|
2.37
|
|
|||||
|
Non-GAAP EPS assuming dilution
|
$
|
4.34
|
|
$
|
3.98
|
|
$
|
3.78
|
|
||
|
(1)
|
The estimated tax impact on the excluded items is determined by applying the statutory rate of the originating territory of the non-GAAP adjustments.
|
|
(3)
|
Represents the difference between calculated GAAP EPS and calculated non-GAAP EPS, which may be different than the amount calculated by dividing the impact of the excluded items by the weighted-average shares for the applicable year
.
|
|
Selected Data
|
|||||||||||
|
($ in millions)
|
2018
|
2017
|
2016
|
||||||||
|
Working capital
|
$
|
3,669
|
|
$
|
6,152
|
|
$
|
13,410
|
|
||
|
Total debt to total liabilities and equity
|
30.4
|
%
|
27.8
|
%
|
26.0
|
%
|
|||||
|
Cash provided by operations to total debt
|
0.4:1
|
|
0.3:1
|
|
0.4:1
|
|
|||||
|
Payments Due by Period
|
|||||||||||||||||||
|
($ in millions)
|
Total
|
2019
|
2020—2021
|
2022—2023
|
Thereafter
|
||||||||||||||
|
Purchase obligations
(1)
|
$
|
2,349
|
|
$
|
886
|
|
$
|
1,011
|
|
$
|
407
|
|
$
|
45
|
|
||||
|
Loans payable and current portion of long-term debt
|
5,309
|
|
5,309
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Long-term debt
|
19,882
|
|
—
|
|
4,237
|
|
4,000
|
|
11,645
|
|
|||||||||
|
Interest related to debt obligations
|
7,680
|
|
662
|
|
1,163
|
|
932
|
|
4,923
|
|
|||||||||
|
Unrecognized tax benefits
(2)
|
44
|
|
44
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Transition tax related to the enactment of the TCJA
(3)
|
4,899
|
|
275
|
|
873
|
|
1,217
|
|
2,534
|
|
|||||||||
|
Leases
|
997
|
|
188
|
|
348
|
|
218
|
|
243
|
|
|||||||||
|
|
$
|
41,160
|
|
$
|
7,364
|
|
$
|
7,632
|
|
$
|
6,774
|
|
$
|
19,390
|
|
||||
|
(1)
|
Includes future inventory purchases the Company has committed to in connection with certain divestitures.
|
|
(2)
|
As of
December 31, 2018
, the Company’s Consolidated Balance Sheet reflects liabilities for unrecognized tax benefits, interest and penalties of
$2.3 billion
, including
$44 million
reflected as a current liability. Due to the high degree of uncertainty regarding the timing of future cash outflows of liabilities for unrecognized tax benefits beyond one year, a reasonable estimate of the period of cash settlement for years beyond
2019
cannot be made.
|
|
(3)
|
In connection with the enactment of the TCJA, the Company is required to pay a one-time transition tax, which the Company has elected to pay over a period of eight years as permitted under the TCJA (see Note 16 to the consolidated financial statements).
|
|
($ in millions)
|
2018
|
|||||||||
|
Debt Instrument
|
Par Value of Debt
|
Number of Interest Rate Swaps Held
|
Total Swap Notional Amount
|
|||||||
|
1.85% notes due 2020
|
$
|
1,250
|
|
5
|
|
$
|
1,250
|
|
||
|
3.875% notes due 2021
|
1,150
|
|
5
|
|
1,150
|
|
||||
|
2.40% notes due 2022
|
1,000
|
|
4
|
|
1,000
|
|
||||
|
2.35% notes due 2022
|
1,250
|
|
5
|
|
1,250
|
|
||||
|
($ in millions)
|
2018
|
2017
|
|||||
|
Balance January 1
|
$
|
2,551
|
|
$
|
2,945
|
|
|
|
Current provision
|
10,837
|
|
11,001
|
|
|||
|
Adjustments to prior years
|
(117
|
)
|
(286
|
)
|
|||
|
Payments
|
(10,641
|
)
|
(11,109
|
)
|
|||
|
Balance December 31
|
$
|
2,630
|
|
$
|
2,551
|
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures about Market Risk.
|
|
Item 8.
|
Financial Statements and Supplementary Data.
|
|
(a)
|
Financial Statements
|
|
2018
|
2017
|
2016
|
|||||||||
|
Sales
|
$
|
42,294
|
|
$
|
40,122
|
|
$
|
39,807
|
|
||
|
Costs, Expenses and Other
|
|||||||||||
|
Cost of sales
|
13,509
|
|
12,912
|
|
14,030
|
|
|||||
|
Selling, general and administrative
|
10,102
|
|
10,074
|
|
10,017
|
|
|||||
|
Research and development
|
9,752
|
|
10,339
|
|
10,261
|
|
|||||
|
Restructuring costs
|
632
|
|
776
|
|
651
|
|
|||||
|
Other (income) expense, net
|
(402
|
)
|
(500
|
)
|
189
|
|
|||||
|
|
33,593
|
|
33,601
|
|
35,148
|
|
|||||
|
Income Before Taxes
|
8,701
|
|
6,521
|
|
4,659
|
|
|||||
|
Taxes on Income
|
2,508
|
|
4,103
|
|
718
|
|
|||||
|
Net Income
|
6,193
|
|
2,418
|
|
3,941
|
|
|||||
|
Less: Net (Loss) Income Attributable to Noncontrolling Interests
|
(27
|
)
|
24
|
|
21
|
|
|||||
|
Net Income Attributable to Merck & Co., Inc.
|
$
|
6,220
|
|
$
|
2,394
|
|
$
|
3,920
|
|
||
|
Basic Earnings per Common Share Attributable to Merck & Co., Inc. Common Shareholders
|
$
|
2.34
|
|
$
|
0.88
|
|
$
|
1.42
|
|
||
|
Earnings per Common Share Assuming Dilution Attributable to Merck & Co., Inc. Common Shareholders
|
$
|
2.32
|
|
$
|
0.87
|
|
$
|
1.41
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Net Income Attributable to Merck & Co., Inc.
|
$
|
6,220
|
|
$
|
2,394
|
|
$
|
3,920
|
|
||
|
Other Comprehensive (Loss) Income Net of Taxes:
|
|||||||||||
|
Net unrealized gain (loss) on derivatives, net of reclassifications
|
297
|
|
(446
|
)
|
(66
|
)
|
|||||
|
Net unrealized loss on investments, net of reclassifications
|
(10
|
)
|
(58
|
)
|
(44
|
)
|
|||||
|
Benefit plan net (loss) gain and prior service (cost) credit, net of amortization
|
(425
|
)
|
419
|
|
(799
|
)
|
|||||
|
Cumulative translation adjustment
|
(223
|
)
|
401
|
|
(169
|
)
|
|||||
|
|
(361
|
)
|
316
|
|
(1,078
|
)
|
|||||
|
Comprehensive Income Attributable to Merck & Co., Inc.
|
$
|
5,859
|
|
$
|
2,710
|
|
$
|
2,842
|
|
||
|
2018
|
2017
|
||||||
|
Assets
|
|||||||
|
Current Assets
|
|||||||
|
Cash and cash equivalents
|
$
|
7,965
|
|
$
|
6,092
|
|
|
|
Short-term investments
|
899
|
|
2,406
|
|
|||
|
Accounts receivable (net of allowance for doubtful accounts of $119 in 2018
and $159 in 2017)
|
7,071
|
|
6,873
|
|
|||
|
Inventories (excludes inventories of $1,417 in 2018 and $1,187 in 2017
classified in Other assets - see Note 7)
|
5,440
|
|
5,096
|
|
|||
|
Other current assets
|
4,500
|
|
4,299
|
|
|||
|
Total current assets
|
25,875
|
|
24,766
|
|
|||
|
Investments
|
6,233
|
|
12,125
|
|
|||
|
Property, Plant and Equipment (at cost)
|
|||||||
|
Land
|
333
|
|
365
|
|
|||
|
Buildings
|
11,486
|
|
11,726
|
|
|||
|
Machinery, equipment and office furnishings
|
14,441
|
|
14,649
|
|
|||
|
Construction in progress
|
3,355
|
|
2,301
|
|
|||
|
29,615
|
|
29,041
|
|
||||
|
Less: accumulated depreciation
|
16,324
|
|
16,602
|
|
|||
|
|
13,291
|
|
12,439
|
|
|||
|
Goodwill
|
18,253
|
|
18,284
|
|
|||
|
Other Intangibles, Net
|
11,431
|
|
14,183
|
|
|||
|
Other Assets
|
7,554
|
|
6,075
|
|
|||
|
|
$
|
82,637
|
|
$
|
87,872
|
|
|
|
Liabilities and Equity
|
|||||||
|
Current Liabilities
|
|||||||
|
Loans payable and current portion of long-term debt
|
$
|
5,308
|
|
$
|
3,057
|
|
|
|
Trade accounts payable
|
3,318
|
|
3,102
|
|
|||
|
Accrued and other current liabilities
|
10,151
|
|
10,427
|
|
|||
|
Income taxes payable
|
1,971
|
|
708
|
|
|||
|
Dividends payable
|
1,458
|
|
1,320
|
|
|||
|
Total current liabilities
|
22,206
|
|
18,614
|
|
|||
|
Long-Term Debt
|
19,806
|
|
21,353
|
|
|||
|
Deferred Income Taxes
|
1,702
|
|
2,219
|
|
|||
|
Other Noncurrent Liabilities
|
12,041
|
|
11,117
|
|
|||
|
Merck & Co., Inc. Stockholders’ Equity
|
|||||||
|
Common stock, $0.50 par value
Authorized - 6,500,000,000 shares
Issued - 3,577,103,522 shares in 2018 and 2017
|
1,788
|
|
1,788
|
|
|||
|
Other paid-in capital
|
38,808
|
|
39,902
|
|
|||
|
Retained earnings
|
42,579
|
|
41,350
|
|
|||
|
Accumulated other comprehensive loss
|
(5,545
|
)
|
(4,910
|
)
|
|||
|
77,630
|
|
78,130
|
|
||||
|
Less treasury stock, at cost:
984,543,979 shares in 2018 and 880,491,914 shares in 2017
|
50,929
|
|
43,794
|
|
|||
|
Total Merck & Co., Inc. stockholders’ equity
|
26,701
|
|
34,336
|
|
|||
|
Noncontrolling Interests
|
181
|
|
233
|
|
|||
|
Total equity
|
26,882
|
|
34,569
|
|
|||
|
|
$
|
82,637
|
|
$
|
87,872
|
|
|
|
Common
Stock
|
Other
Paid-In
Capital
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Loss
|
Treasury
Stock
|
Non-
controlling
Interests
|
Total
|
|||||||||||||||||||||
|
Balance January 1, 2016
|
|
$1,788
|
|
$
|
40,222
|
|
$
|
45,348
|
|
$
|
(4,148
|
)
|
$
|
(38,534
|
)
|
$
|
91
|
|
$
|
44,767
|
|
||||||
|
Net income attributable to Merck & Co., Inc.
|
—
|
|
—
|
|
3,920
|
|
—
|
|
—
|
|
—
|
|
3,920
|
|
|||||||||||||
|
Other comprehensive loss, net of taxes
|
—
|
|
—
|
|
—
|
|
(1,078
|
)
|
—
|
|
—
|
|
(1,078
|
)
|
|||||||||||||
|
Cash dividends declared on common stock ($1.85 per share)
|
—
|
|
—
|
|
(5,135
|
)
|
—
|
|
—
|
|
—
|
|
(5,135
|
)
|
|||||||||||||
|
Treasury stock shares purchased
|
—
|
|
—
|
|
—
|
|
—
|
|
(3,434
|
)
|
—
|
|
(3,434
|
)
|
|||||||||||||
|
Acquisition of The StayWell Company LLC
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
124
|
|
124
|
|
|||||||||||||
|
Net income attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
21
|
|
21
|
|
|||||||||||||
|
Distributions attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(16
|
)
|
(16
|
)
|
|||||||||||||
|
Share-based compensation plans and other
|
—
|
|
(283
|
)
|
—
|
|
—
|
|
1,422
|
|
—
|
|
1,139
|
|
|||||||||||||
|
Balance December 31, 2016
|
1,788
|
|
39,939
|
|
44,133
|
|
(5,226
|
)
|
(40,546
|
)
|
220
|
|
40,308
|
|
|||||||||||||
|
Net income attributable to Merck & Co., Inc.
|
—
|
|
—
|
|
2,394
|
|
—
|
|
—
|
|
—
|
|
2,394
|
|
|||||||||||||
|
Other comprehensive income, net of taxes
|
—
|
|
—
|
|
—
|
|
316
|
|
—
|
|
—
|
|
316
|
|
|||||||||||||
|
Cash dividends declared on common stock ($1.89 per share)
|
—
|
|
—
|
|
(5,177
|
)
|
—
|
|
—
|
|
—
|
|
(5,177
|
)
|
|||||||||||||
|
Treasury stock shares purchased
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,014
|
)
|
—
|
|
(4,014
|
)
|
|||||||||||||
|
Acquisition of Vallée S.A.
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7
|
|
7
|
|
|||||||||||||
|
Net income attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
24
|
|
24
|
|
|||||||||||||
|
Distributions attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(18
|
)
|
(18
|
)
|
|||||||||||||
|
Share-based compensation plans and other
|
—
|
|
(37
|
)
|
—
|
|
—
|
|
766
|
|
—
|
|
729
|
|
|||||||||||||
|
Balance December 31, 2017
|
1,788
|
|
39,902
|
|
41,350
|
|
(4,910
|
)
|
(43,794
|
)
|
233
|
|
34,569
|
|
|||||||||||||
|
Net income attributable to Merck & Co., Inc.
|
—
|
|
—
|
|
6,220
|
|
—
|
|
—
|
|
—
|
|
6,220
|
|
|||||||||||||
|
Adoption of new accounting standards (see Note 2)
|
—
|
|
—
|
|
322
|
|
(274
|
)
|
—
|
|
—
|
|
48
|
|
|||||||||||||
|
Other comprehensive loss, net of taxes
|
—
|
|
—
|
|
—
|
|
(361
|
)
|
—
|
|
—
|
|
(361
|
)
|
|||||||||||||
|
Cash dividends declared on common stock ($1.99 per share)
|
—
|
|
—
|
|
(5,313
|
)
|
—
|
|
—
|
|
—
|
|
(5,313
|
)
|
|||||||||||||
|
Treasury stock shares purchased
|
—
|
|
(1,000
|
)
|
—
|
|
—
|
|
(8,091
|
)
|
—
|
|
(9,091
|
)
|
|||||||||||||
|
Net loss attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(27
|
)
|
(27
|
)
|
|||||||||||||
|
Distributions attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(25
|
)
|
(25
|
)
|
|||||||||||||
|
Share-based compensation plans and other
|
—
|
|
(94
|
)
|
—
|
|
—
|
|
956
|
|
—
|
|
862
|
|
|||||||||||||
|
Balance December 31, 2018
|
$
|
1,788
|
|
$
|
38,808
|
|
$
|
42,579
|
|
$
|
(5,545
|
)
|
$
|
(50,929
|
)
|
$
|
181
|
|
$
|
26,882
|
|
||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Cash Flows from Operating Activities
|
|||||||||||
|
Net income
|
$
|
6,193
|
|
$
|
2,418
|
|
$
|
3,941
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
4,519
|
|
4,676
|
|
5,471
|
|
|||||
|
Intangible asset impairment charges
|
296
|
|
646
|
|
3,948
|
|
|||||
|
Charge for future payments related to collaboration license options
|
650
|
|
500
|
|
—
|
|
|||||
|
Provisional charge for one-time transition tax related to the enactment of U.S. tax legislation
|
—
|
|
5,347
|
|
—
|
|
|||||
|
Charge related to the settlement of worldwide
Keytruda
patent litigation
|
—
|
|
—
|
|
625
|
|
|||||
|
Deferred income taxes
|
(509
|
)
|
(2,621
|
)
|
(1,521
|
)
|
|||||
|
Share-based compensation
|
348
|
|
312
|
|
300
|
|
|||||
|
Other
|
978
|
|
190
|
|
213
|
|
|||||
|
Net changes in assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
(418
|
)
|
297
|
|
(619
|
)
|
|||||
|
Inventories
|
(911
|
)
|
(145
|
)
|
206
|
|
|||||
|
Trade accounts payable
|
230
|
|
254
|
|
278
|
|
|||||
|
Accrued and other current liabilities
|
(341
|
)
|
(922
|
)
|
(2,018
|
)
|
|||||
|
Income taxes payable
|
827
|
|
(3,291
|
)
|
124
|
|
|||||
|
Noncurrent liabilities
|
(266
|
)
|
(123
|
)
|
(809
|
)
|
|||||
|
Other
|
(674
|
)
|
(1,087
|
)
|
237
|
|
|||||
|
Net Cash Provided by Operating Activities
|
10,922
|
|
6,451
|
|
10,376
|
|
|||||
|
Cash Flows from Investing Activities
|
|||||||||||
|
Capital expenditures
|
(2,615
|
)
|
(1,888
|
)
|
(1,614
|
)
|
|||||
|
Purchases of securities and other investments
|
(7,994
|
)
|
(10,739
|
)
|
(15,651
|
)
|
|||||
|
Proceeds from sales of securities and other investments
|
15,252
|
|
15,664
|
|
14,353
|
|
|||||
|
Acquisitions, net of cash acquired
|
(431
|
)
|
(396
|
)
|
(780
|
)
|
|||||
|
Other
|
102
|
|
38
|
|
482
|
|
|||||
|
Net Cash Provided by (Used in) Investing Activities
|
4,314
|
|
2,679
|
|
(3,210
|
)
|
|||||
|
Cash Flows from Financing Activities
|
|||||||||||
|
Net change in short-term borrowings
|
5,124
|
|
(26
|
)
|
—
|
|
|||||
|
Payments on debt
|
(4,287
|
)
|
(1,103
|
)
|
(2,386
|
)
|
|||||
|
Proceeds from issuance of debt
|
—
|
|
—
|
|
1,079
|
|
|||||
|
Purchases of treasury stock
|
(9,091
|
)
|
(4,014
|
)
|
(3,434
|
)
|
|||||
|
Dividends paid to stockholders
|
(5,172
|
)
|
(5,167
|
)
|
(5,124
|
)
|
|||||
|
Proceeds from exercise of stock options
|
591
|
|
499
|
|
939
|
|
|||||
|
Other
|
(325
|
)
|
(195
|
)
|
(118
|
)
|
|||||
|
Net Cash Used in Financing Activities
|
(13,160
|
)
|
(10,006
|
)
|
(9,044
|
)
|
|||||
|
Effect of Exchange Rate Changes on Cash, Cash Equivalents and Restricted Cash
|
(205
|
)
|
457
|
|
(131
|
)
|
|||||
|
Net Increase (Decrease) in Cash, Cash Equivalents and Restricted Cash
|
1,871
|
|
(419
|
)
|
(2,009
|
)
|
|||||
|
Cash, Cash Equivalents and Restricted Cash at Beginning of Year (includes $4 million of restricted cash at January 1, 2018 included in Other Assets)
|
6,096
|
|
6,515
|
|
8,524
|
|
|||||
|
Cash, Cash Equivalents and Restricted Cash at End of Year (includes $2 million of restricted cash at December 31, 2018 included in Other Assets)
|
$
|
7,967
|
|
$
|
6,096
|
|
$
|
6,515
|
|
||
|
Year Ended December 31, 2018
|
As Reported
|
Effects of Adopting ASC 606
|
Amounts Without Adoption of ASC 606
|
||||||||
|
Sales
|
$
|
42,294
|
|
$
|
(2
|
)
|
$
|
42,292
|
|
||
|
Cost of sales
|
13,509
|
|
(6
|
)
|
13,503
|
|
|||||
|
Income before taxes
|
8,701
|
|
4
|
|
8,705
|
|
|||||
|
Taxes on income
|
2,508
|
|
1
|
|
2,509
|
|
|||||
|
Net income attributable to Merck & Co., Inc.
|
6,220
|
|
3
|
|
6,223
|
|
|||||
|
December 31, 2018
|
As Reported
|
Effects of Adopting ASC 606
|
Amounts Without Adoption of ASC 606
|
||||||||
|
Assets
|
|||||||||||
|
Accounts receivable
|
$
|
7,071
|
|
$
|
(13
|
)
|
$
|
7,058
|
|
||
|
Inventories
|
5,440
|
|
7
|
|
5,447
|
|
|||||
|
Liabilities
|
|||||||||||
|
Accrued and other current liabilities
|
10,151
|
|
(3
|
)
|
10,148
|
|
|||||
|
Income taxes payable
|
1,971
|
|
(1
|
)
|
1,970
|
|
|||||
|
Equity
|
|||||||||||
|
Retained earnings
|
42,579
|
|
(2
|
)
|
42,577
|
|
|||||
|
($ in millions)
|
ASU 2014-09 (Revenue)
|
ASU 2016-01 (Financial Instruments)
|
ASU 2016-16 (Intra-Entity Transfers of Assets Other than Inventory)
|
ASU 2017-12 (Derivatives and Hedging)
|
ASU 2018-02 (Reclassification of Certain Tax Effects)
|
Total
|
|||||||||||||
|
Assets - Increase (Decrease)
|
|||||||||||||||||||
|
Accounts receivable
|
$
|
5
|
|
$
|
5
|
|
|||||||||||||
|
Liabilities - Increase (Decrease)
|
|||||||||||||||||||
|
Income Taxes Payable
|
(3
|
)
|
(3
|
)
|
|||||||||||||||
|
Debt
|
14
|
|
14
|
|
|||||||||||||||
|
Deferred Income Taxes
|
(54
|
)
|
(54
|
)
|
|||||||||||||||
|
Equity - Increase (Decrease)
|
|||||||||||||||||||
|
Retained earnings
|
5
|
|
8
|
|
54
|
|
(11
|
)
|
266
|
|
322
|
|
|||||||
|
Accumulated other comprehensive loss
|
(8
|
)
|
(266
|
)
|
(274
|
)
|
|||||||||||||
|
Years Ended December 31
|
2018
|
2017
|
|||||
|
Alliance revenue
|
$
|
187
|
|
$
|
20
|
|
|
|
Cost of sales
(1)
|
93
|
|
4
|
|
|||
|
Selling, general and administrative
|
48
|
|
1
|
|
|||
|
Research and development
(2)
|
152
|
|
2,419
|
|
|||
|
December 31
|
2018
|
2017
|
|||||
|
Receivables from AstraZeneca included in
Other current assets
|
$
|
52
|
|
$
|
12
|
|
|
|
Payables to AstraZeneca included in
Accrued and other current liabilities
(3)
|
405
|
|
543
|
|
|||
|
Payables to AstraZeneca included
Other Noncurrent Liabilities
(3)
|
250
|
|
100
|
|
|||
|
Year Ended December 31
|
2018
|
||
|
Alliance revenue
|
$
|
149
|
|
|
Cost of sales
(1)
|
39
|
|
|
|
Selling, general and administrative
|
13
|
|
|
|
Research and development
(2)
|
1,489
|
|
|
|
December 31
|
2018
|
||
|
Receivables from Eisai included in
Other current assets
|
$
|
71
|
|
|
Payables to Eisai included in
Accrued and other current liabilities
(3)
|
375
|
|
|
|
Payables to Eisai included in
Other Noncurrent Liabilities
(3)
|
543
|
|
|
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Net product sales recorded by Merck
|
$
|
190
|
|
$
|
149
|
|
$
|
88
|
|
||
|
Merck’s profit share from sales in Bayer’s marketing territories
|
139
|
|
151
|
|
81
|
|
|||||
|
Total sales
|
329
|
|
300
|
|
169
|
|
|||||
|
Cost of sales
(1)
|
216
|
|
99
|
|
133
|
|
|||||
|
Selling, general and administrative
|
35
|
|
27
|
|
26
|
|
|||||
|
Research and development
|
127
|
|
101
|
|
82
|
|
|||||
|
December 31
|
2018
|
2017
|
|
||||||||
|
Receivables from Bayer included in
Other current assets
|
$
|
32
|
|
$
|
33
|
|
|||||
|
Payables to Bayer included in
Accrued and other current liabilities
(2)
|
—
|
|
350
|
|
|||||||
|
Payables to Bayer included in
Other Noncurrent Liabilities
(2)
|
375
|
|
—
|
|
|||||||
|
Separation
Costs
|
Accelerated
Depreciation
|
Other
|
Total
|
||||||||||||
|
Year Ended December 31, 2018
|
|||||||||||||||
|
Cost of sales
|
$
|
—
|
|
$
|
10
|
|
$
|
11
|
|
$
|
21
|
|
|||
|
Selling, general and administrative
|
—
|
|
2
|
|
1
|
|
3
|
|
|||||||
|
Research and development
|
—
|
|
(13
|
)
|
15
|
|
2
|
|
|||||||
|
Restructuring costs
|
473
|
|
—
|
|
159
|
|
632
|
|
|||||||
|
|
$
|
473
|
|
$
|
(1
|
)
|
$
|
186
|
|
$
|
658
|
|
|||
|
Year Ended December 31, 2017
|
|
|
|
|
|||||||||||
|
Cost of sales
|
$
|
—
|
|
$
|
52
|
|
$
|
86
|
|
$
|
138
|
|
|||
|
Selling, general and administrative
|
—
|
|
2
|
|
—
|
|
2
|
|
|||||||
|
Research and development
|
—
|
|
6
|
|
5
|
|
11
|
|
|||||||
|
Restructuring costs
|
552
|
|
—
|
|
224
|
|
776
|
|
|||||||
|
|
$
|
552
|
|
|
$
|
60
|
|
|
$
|
315
|
|
|
$
|
927
|
|
|
Year Ended December 31, 2016
|
|
|
|
|
|||||||||||
|
Cost of sales
|
$
|
—
|
|
$
|
77
|
|
$
|
104
|
|
$
|
181
|
|
|||
|
Selling, general and administrative
|
—
|
|
8
|
|
87
|
|
95
|
|
|||||||
|
Research and development
|
—
|
|
142
|
|
—
|
|
142
|
|
|||||||
|
Restructuring costs
|
216
|
|
—
|
|
435
|
|
651
|
|
|||||||
|
|
$
|
216
|
|
|
$
|
227
|
|
|
$
|
626
|
|
|
$
|
1,069
|
|
|
Separation
Costs
|
Accelerated
Depreciation
|
Other
|
Total
|
||||||||||||
|
Restructuring reserves January 1, 2017
|
$
|
395
|
|
$
|
—
|
|
$
|
146
|
|
$
|
541
|
|
|||
|
Expenses
|
552
|
|
60
|
|
315
|
|
927
|
|
|||||||
|
(Payments) receipts, net
|
(328
|
)
|
—
|
|
(394
|
)
|
(722
|
)
|
|||||||
|
Non-cash activity
|
—
|
|
(60
|
)
|
61
|
|
1
|
|
|||||||
|
Restructuring reserves December 31, 2017
|
619
|
|
—
|
|
128
|
|
747
|
|
|||||||
|
Expenses
|
473
|
|
(1
|
)
|
186
|
|
658
|
|
|||||||
|
(Payments) receipts, net
|
(649
|
)
|
—
|
|
(238
|
)
|
(887
|
)
|
|||||||
|
Non-cash activity
|
—
|
|
1
|
|
15
|
|
16
|
|
|||||||
|
Restructuring reserves December 31, 2018
(1)
|
$
|
443
|
|
$
|
—
|
|
$
|
91
|
|
$
|
534
|
|
|||
|
(1)
|
The remaining cash outlays are expected to be substantially completed by the end of 2020.
|
|
Amount of Pretax (Gain) Loss Recognized in Other Comprehensive Income
(1)
|
Amount of Pretax (Gain) Loss Recognized in
Other (income) expense, net
for Amounts Excluded from Effectiveness Testing
|
||||||||||||||||||||||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
|||||||||||||||||
|
Net Investment Hedging Relationships
|
|||||||||||||||||||||||
|
Foreign exchange contracts
|
$
|
(18
|
)
|
$
|
—
|
|
$
|
2
|
|
$
|
(11
|
)
|
$
|
—
|
|
$
|
(1
|
)
|
|||||
|
Euro-denominated notes
|
(183
|
)
|
520
|
|
(193
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
2018
|
||||||||||
|
Debt Instrument
|
Par Value of Debt
|
Number of Interest Rate Swaps Held
|
Total Swap Notional Amount
|
|||||||
|
1.85% notes due 2020
|
$
|
1,250
|
|
5
|
|
$
|
1,250
|
|
||
|
3.875% notes due 2021
|
1,150
|
|
5
|
|
1,150
|
|
||||
|
2.40% notes due 2022
|
1,000
|
|
4
|
|
1,000
|
|
||||
|
2.35% notes due 2022
|
1,250
|
|
5
|
|
1,250
|
|
||||
|
Carrying Amount of Hedged Liabilities
|
Cumulative Amount of Fair Value Hedging Adjustment Increase (Decrease) Included in the Carrying Amount
|
||||||||||||||
|
2018
|
2017
|
2018
|
2017
|
||||||||||||
|
Balance Sheet Line Item in which Hedged Item is Included
|
|||||||||||||||
|
Loans payable and current portion of long-term debt
|
$
|
—
|
|
$
|
983
|
|
$
|
—
|
|
$
|
(17
|
)
|
|||
|
Long-Term Debt
(1)
|
4,560
|
|
5,146
|
|
(82
|
)
|
(41
|
)
|
|||||||
|
|
|
2018
|
2017
|
||||||||||||||||||||||
|
|
|
Fair Value of
Derivative
|
U.S. Dollar
Notional
|
Fair Value of
Derivative
|
U.S. Dollar
Notional
|
||||||||||||||||||||
|
|
Balance Sheet Caption
|
Asset
|
Liability
|
Asset
|
Liability
|
||||||||||||||||||||
|
Derivatives Designated as Hedging Instruments
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Interest rate swap contracts
|
Other assets
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2
|
|
$
|
—
|
|
$
|
550
|
|
||||||
|
Interest rate swap contracts
|
Accrued and other current liabilities
|
—
|
|
—
|
|
—
|
|
—
|
|
3
|
|
1,000
|
|
||||||||||||
|
Interest rate swap contracts
|
Other noncurrent liabilities
|
—
|
|
81
|
|
4,650
|
|
—
|
|
52
|
|
4,650
|
|
||||||||||||
|
Foreign exchange contracts
|
Other current assets
|
263
|
|
—
|
|
6,222
|
|
51
|
|
—
|
|
4,216
|
|
||||||||||||
|
Foreign exchange contracts
|
Other assets
|
75
|
|
—
|
|
2,655
|
|
38
|
|
—
|
|
1,936
|
|
||||||||||||
|
Foreign exchange contracts
|
Accrued and other current liabilities
|
—
|
|
7
|
|
774
|
|
—
|
|
71
|
|
2,014
|
|
||||||||||||
|
Foreign exchange contracts
|
Other noncurrent liabilities
|
—
|
|
1
|
|
89
|
|
—
|
|
1
|
|
20
|
|
||||||||||||
|
|
|
$
|
338
|
|
|
$
|
89
|
|
|
$
|
14,390
|
|
|
$
|
91
|
|
|
$
|
127
|
|
|
$
|
14,386
|
|
|
|
Derivatives Not Designated as Hedging Instruments
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Foreign exchange contracts
|
Other current assets
|
$
|
116
|
|
$
|
—
|
|
$
|
5,430
|
|
$
|
39
|
|
$
|
—
|
|
$
|
3,778
|
|
||||||
|
Foreign exchange contracts
|
Accrued and other current liabilities
|
—
|
|
71
|
|
9,922
|
|
—
|
|
90
|
|
7,431
|
|
||||||||||||
|
|
|
$
|
116
|
|
$
|
71
|
|
$
|
15,352
|
|
$
|
39
|
|
$
|
90
|
|
$
|
11,209
|
|
||||||
|
|
|
$
|
454
|
|
$
|
160
|
|
$
|
29,742
|
|
$
|
130
|
|
$
|
217
|
|
$
|
25,595
|
|
||||||
|
2018
|
2017
|
||||||||||||||
|
Asset
|
Liability
|
Asset
|
Liability
|
||||||||||||
|
Gross amounts recognized in the consolidated balance sheet
|
$
|
454
|
|
$
|
160
|
|
$
|
130
|
|
$
|
217
|
|
|||
|
Gross amount subject to offset in master netting arrangements not offset in the consolidated balance sheet
|
(121
|
)
|
(121
|
)
|
(94
|
)
|
(94
|
)
|
|||||||
|
Cash collateral received
|
(107
|
)
|
—
|
|
(3
|
)
|
—
|
|
|||||||
|
Net amounts
|
$
|
226
|
|
$
|
39
|
|
$
|
33
|
|
$
|
123
|
|
|||
|
Sales
|
Other (income) expense, net
(1)
|
Other comprehensive income (loss)
|
|||||||||||||||||||||||||||||||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
||||||||||||||||||||||||
|
Financial Statement Line Items in which Effects of Fair Value or Cash Flow Hedges are Recorded
|
$
|
42,294
|
|
$
|
40,122
|
|
$
|
39,807
|
|
$
|
(402
|
)
|
(500
|
)
|
189
|
|
$
|
(361
|
)
|
$
|
316
|
|
$
|
(1,078
|
)
|
||||||||
|
(Gain) loss on fair value hedging relationships
|
|||||||||||||||||||||||||||||||||
|
Interest rate swap contracts
|
|||||||||||||||||||||||||||||||||
|
Hedged items
|
—
|
|
—
|
|
—
|
|
(27
|
)
|
(48
|
)
|
(29
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Derivatives designated as hedging instruments
|
—
|
|
—
|
|
—
|
|
50
|
|
12
|
|
(35
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Impact of cash flow hedging relationships
|
|||||||||||||||||||||||||||||||||
|
Foreign exchange contracts
|
|||||||||||||||||||||||||||||||||
|
Amount of gain (loss) recognized in
OCI
on derivatives
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
228
|
|
(562
|
)
|
210
|
|
|||||||||||||||
|
(Decrease) increase in
Sales
as a result of
AOCI
reclassifications
|
(160
|
)
|
138
|
|
311
|
|
—
|
|
—
|
|
—
|
|
160
|
|
(138
|
)
|
(311
|
)
|
|||||||||||||||
|
Amount of Derivative Pretax (Gain) Loss Recognized in Income
|
||||||||||||||
|
Years Ended December 31
|
Income Statement Caption
|
2018
|
2017
|
2016
|
||||||||||
|
Derivatives Not Designated as Hedging Instruments
|
||||||||||||||
|
Foreign exchange contracts
(1)
|
Other (income) expense, net
|
$
|
(260
|
)
|
$
|
110
|
|
$
|
132
|
|
||||
|
Foreign exchange contracts
(2)
|
Sales
|
(8
|
)
|
(3
|
)
|
—
|
|
|||||||
|
|
2018
|
2017
|
|||||||||||||||||||||||||||||
|
|
Fair
Value
|
Amortized
Cost
|
Gross Unrealized
|
Fair
Value
|
Amortized
Cost
|
Gross Unrealized
|
|||||||||||||||||||||||||
|
|
Gains
|
Losses
|
Gains
|
Losses
|
|||||||||||||||||||||||||||
|
Corporate notes and bonds
|
$
|
4,920
|
|
$
|
4,985
|
|
$
|
3
|
|
$
|
(68
|
)
|
$
|
9,806
|
|
$
|
9,837
|
|
$
|
9
|
|
$
|
(40
|
)
|
|||||||
|
Asset-backed securities
|
1,275
|
|
1,285
|
|
1
|
|
(11
|
)
|
1,542
|
|
1,548
|
|
1
|
|
(7
|
)
|
|||||||||||||||
|
U.S. government and agency securities
|
892
|
|
895
|
|
2
|
|
(5
|
)
|
2,042
|
|
2,059
|
|
—
|
|
(17
|
)
|
|||||||||||||||
|
Foreign government bonds
|
166
|
|
167
|
|
—
|
|
(1
|
)
|
733
|
|
739
|
|
—
|
|
(6
|
)
|
|||||||||||||||
|
Mortgage-backed securities
|
8
|
|
8
|
|
—
|
|
—
|
|
626
|
|
634
|
|
1
|
|
(9
|
)
|
|||||||||||||||
|
Commercial paper
|
—
|
|
—
|
|
—
|
|
—
|
|
159
|
|
159
|
|
—
|
|
—
|
|
|||||||||||||||
|
Total debt securities
|
7,261
|
|
|
7,340
|
|
|
6
|
|
|
(85
|
)
|
|
14,908
|
|
|
14,976
|
|
|
11
|
|
|
(79
|
)
|
||||||||
|
Publicly traded equity securities
(1)
|
456
|
|
275
|
|
265
|
|
16
|
|
(6
|
)
|
|||||||||||||||||||||
|
Total debt and publicly traded equity securities
|
$
|
7,717
|
|
|
|
|
|
|
|
|
|
|
|
$
|
15,183
|
|
|
$
|
15,241
|
|
|
$
|
27
|
|
|
$
|
(85
|
)
|
|||
|
|
Fair Value Measurements Using
|
Fair Value Measurements Using
|
|||||||||||||||||||||||||||||
|
|
Quoted Prices
In Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total
|
Quoted Prices
In Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total
|
|||||||||||||||||||||||
|
|
2018
|
2017
|
|||||||||||||||||||||||||||||
|
Assets
|
|||||||||||||||||||||||||||||||
|
Investments
|
|||||||||||||||||||||||||||||||
|
Corporate notes and bonds
|
$
|
—
|
|
$
|
4,835
|
|
$
|
—
|
|
$
|
4,835
|
|
$
|
—
|
|
$
|
9,678
|
|
$
|
—
|
|
$
|
9,678
|
|
|||||||
|
Asset-backed securities
(1)
|
—
|
|
1,253
|
|
—
|
|
1,253
|
|
—
|
|
1,476
|
|
—
|
|
1,476
|
|
|||||||||||||||
|
U.S. government and agency securities
|
—
|
|
731
|
|
—
|
|
731
|
|
68
|
|
1,767
|
|
—
|
|
1,835
|
|
|||||||||||||||
|
Foreign government bonds
|
—
|
|
166
|
|
—
|
|
166
|
|
—
|
|
732
|
|
—
|
|
732
|
|
|||||||||||||||
|
Mortgage-backed securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
547
|
|
—
|
|
547
|
|
|||||||||||||||
|
Commercial paper
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
159
|
|
—
|
|
159
|
|
|||||||||||||||
|
Publicly traded equity securities
|
147
|
|
—
|
|
—
|
|
147
|
|
104
|
|
—
|
|
—
|
|
104
|
|
|||||||||||||||
|
|
147
|
|
6,985
|
|
—
|
|
7,132
|
|
172
|
|
14,359
|
|
—
|
|
14,531
|
|
|||||||||||||||
|
Other assets
(2)
|
|||||||||||||||||||||||||||||||
|
U.S. government and agency securities
|
55
|
|
106
|
|
—
|
|
161
|
|
—
|
|
207
|
|
—
|
|
207
|
|
|||||||||||||||
|
Corporate notes and bonds
|
—
|
|
85
|
|
—
|
|
85
|
|
—
|
|
128
|
|
—
|
|
128
|
|
|||||||||||||||
|
Asset-backed securities
(1)
|
—
|
|
22
|
|
—
|
|
22
|
|
—
|
|
66
|
|
—
|
|
66
|
|
|||||||||||||||
|
Mortgage-backed securities
|
—
|
|
8
|
|
—
|
|
8
|
|
—
|
|
79
|
|
—
|
|
79
|
|
|||||||||||||||
|
Foreign government bonds
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1
|
|
—
|
|
1
|
|
|||||||||||||||
|
Publicly traded equity securities
|
309
|
|
—
|
|
—
|
|
309
|
|
171
|
|
—
|
|
—
|
|
171
|
|
|||||||||||||||
|
364
|
|
|
221
|
|
|
—
|
|
|
585
|
|
|
171
|
|
|
481
|
|
|
—
|
|
|
652
|
|
|||||||||
|
Derivative assets
(3)
|
|||||||||||||||||||||||||||||||
|
Forward exchange contracts
|
—
|
|
241
|
|
—
|
|
241
|
|
—
|
|
48
|
|
—
|
|
48
|
|
|||||||||||||||
|
Purchased currency options
|
—
|
|
213
|
|
—
|
|
213
|
|
—
|
|
80
|
|
—
|
|
80
|
|
|||||||||||||||
|
Interest rate swaps
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2
|
|
—
|
|
2
|
|
|||||||||||||||
|
|
—
|
|
|
454
|
|
|
—
|
|
|
454
|
|
|
—
|
|
|
130
|
|
|
—
|
|
|
130
|
|
||||||||
|
Total assets
|
$
|
511
|
|
|
$
|
7,660
|
|
|
$
|
—
|
|
|
$
|
8,171
|
|
|
$
|
343
|
|
|
$
|
14,970
|
|
|
$
|
—
|
|
|
$
|
15,313
|
|
|
Liabilities
|
|||||||||||||||||||||||||||||||
|
Other liabilities
|
|||||||||||||||||||||||||||||||
|
Contingent consideration
|
$
|
—
|
|
$
|
—
|
|
$
|
788
|
|
$
|
788
|
|
$
|
—
|
|
$
|
—
|
|
$
|
935
|
|
$
|
935
|
|
|||||||
|
Derivative liabilities
(3)
|
|||||||||||||||||||||||||||||||
|
Interest rate swaps
|
—
|
|
81
|
|
—
|
|
81
|
|
—
|
|
55
|
|
—
|
|
55
|
|
|||||||||||||||
|
Forward exchange contracts
|
—
|
|
74
|
|
—
|
|
74
|
|
—
|
|
162
|
|
—
|
|
162
|
|
|||||||||||||||
|
Written currency options
|
—
|
|
5
|
|
—
|
|
5
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
—
|
|
|
160
|
|
|
—
|
|
|
160
|
|
|
—
|
|
|
217
|
|
|
—
|
|
|
217
|
|
|||||||||
|
Total liabilities
|
$
|
—
|
|
|
$
|
160
|
|
|
$
|
788
|
|
|
$
|
948
|
|
|
$
|
—
|
|
|
$
|
217
|
|
|
$
|
935
|
|
|
$
|
1,152
|
|
|
(1)
|
Primarily all of the asset-backed securities are highly-rated (Standard & Poor’s rating of AAA and Moody’s Investors Service rating of Aaa), secured primarily by auto loan, credit card and student loan receivables, with weighted-average lives of primarily
5
years or less.
|
|
(2)
|
Investments included in other assets are restricted as to use, primarily for the payment of benefits under employee benefit plans.
|
|
(3)
|
The fair value determination of derivatives includes the impact of the credit risk of counterparties to the derivatives and the Company’s own credit risk, the effects of which were not significant.
|
|
2018
|
2017
|
||||||
|
Fair value January 1
|
$
|
935
|
|
$
|
891
|
|
|
|
Changes in estimated fair value
(1)
|
89
|
|
141
|
|
|||
|
Additions
|
8
|
|
3
|
|
|||
|
Payments
|
(244
|
)
|
(100
|
)
|
|||
|
Fair value December 31
(2)
|
$
|
788
|
|
$
|
935
|
|
|
|
2018
|
2017
|
||||||
|
Finished goods
|
$
|
1,658
|
|
$
|
1,334
|
|
|
|
Raw materials and work in process
|
5,004
|
|
4,703
|
|
|||
|
Supplies
|
194
|
|
201
|
|
|||
|
Total (approximates current cost)
|
6,856
|
|
6,238
|
|
|||
|
Increase to LIFO costs
|
1
|
|
45
|
|
|||
|
|
$
|
6,857
|
|
$
|
6,283
|
|
|
|
Recognized as:
|
|||||||
|
Inventories
|
$
|
5,440
|
|
$
|
5,096
|
|
|
|
Other assets
|
1,417
|
|
1,187
|
|
|||
|
Pharmaceutical
|
Animal Health
|
All Other
|
Total
|
||||||||||||
|
Balance January 1, 2017
|
$
|
16,075
|
|
$
|
1,708
|
|
$
|
379
|
|
$
|
18,162
|
|
|||
|
Acquisitions
|
—
|
|
177
|
|
—
|
|
177
|
|
|||||||
|
Impairments
|
—
|
|
—
|
|
(38
|
)
|
(38
|
)
|
|||||||
|
Other
(1)
|
(9
|
)
|
(8
|
)
|
—
|
|
(17
|
)
|
|||||||
|
Balance December 31, 2017
(2)
|
16,066
|
|
1,877
|
|
341
|
|
18,284
|
|
|||||||
|
Acquisitions
|
—
|
|
17
|
|
24
|
|
41
|
|
|||||||
|
Impairments
|
—
|
|
—
|
|
(144
|
)
|
(144
|
)
|
|||||||
|
Other
(1)
|
96
|
|
(24
|
)
|
—
|
|
72
|
|
|||||||
|
Balance December 31, 2018
(2)
|
$
|
16,162
|
|
$
|
1,870
|
|
$
|
221
|
|
$
|
18,253
|
|
|||
|
|
2018
|
2017
|
|||||||||||||||||||||
|
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
|
|||||||||||||||||
|
Products and product rights
|
$
|
46,615
|
|
$
|
37,585
|
|
$
|
9,030
|
|
$
|
46,693
|
|
$
|
34,950
|
|
$
|
11,743
|
|
|||||
|
IPR&D
|
1,064
|
|
—
|
|
1,064
|
|
1,194
|
|
—
|
|
1,194
|
|
|||||||||||
|
Tradenames
|
209
|
|
107
|
|
102
|
|
209
|
|
97
|
|
112
|
|
|||||||||||
|
Other
|
2,403
|
|
1,168
|
|
1,235
|
|
2,035
|
|
901
|
|
1,134
|
|
|||||||||||
|
|
$
|
50,291
|
|
$
|
38,860
|
|
$
|
11,431
|
|
$
|
50,131
|
|
$
|
35,948
|
|
$
|
14,183
|
|
|||||
|
Products and product rights (8-year useful life)
|
$
|
936
|
|
|
Accounts receivable
|
133
|
|
|
|
Income taxes payable
|
(221
|
)
|
|
|
Deferred income tax liabilities
|
(147
|
)
|
|
|
Other, net
|
47
|
|
|
|
Net assets acquired
|
748
|
|
|
|
Consideration payable to Sanofi, net
|
(392
|
)
|
|
|
Derecognition of Merck’s previously held equity investment in SPMSD
|
(183
|
)
|
|
|
Increase in net assets
|
173
|
|
|
|
Merck’s share of restructuring costs related to the termination
|
(77
|
)
|
|
|
Net gain on termination of SPMSD joint venture
(1)
|
$
|
96
|
|
|
2018
|
2017
|
||||||
|
2.75% notes due 2025
|
$
|
2,490
|
|
$
|
2,488
|
|
|
|
3.70% notes due 2045
|
1,974
|
|
1,973
|
|
|||
|
2.80% notes due 2023
|
1,745
|
|
1,744
|
|
|||
|
4.15% notes due 2043
|
1,237
|
|
1,237
|
|
|||
|
1.85% notes due 2020
|
1,231
|
|
1,232
|
|
|||
|
2.35% notes due 2022
|
1,214
|
|
1,220
|
|
|||
|
1.125% euro-denominated notes due 2021
|
1,134
|
|
1,185
|
|
|||
|
3.875% notes due 2021
|
1,132
|
|
1,140
|
|
|||
|
1.875% euro-denominated notes due 2026
|
1,127
|
|
1,178
|
|
|||
|
2.40% notes due 2022
|
983
|
|
993
|
|
|||
|
6.50% notes due 2033
|
726
|
|
729
|
|
|||
|
Floating-rate notes due 2020
|
699
|
|
699
|
|
|||
|
0.50% euro-denominated notes due 2024
|
565
|
|
591
|
|
|||
|
1.375% euro-denominated notes due 2036
|
561
|
|
587
|
|
|||
|
2.50% euro-denominated notes due 2034
|
560
|
|
585
|
|
|||
|
3.60% notes due 2042
|
490
|
|
489
|
|
|||
|
6.55% notes due 2037
|
414
|
|
415
|
|
|||
|
5.75% notes due 2036
|
338
|
|
338
|
|
|||
|
5.95% debentures due 2028
|
306
|
|
306
|
|
|||
|
5.85% notes due 2039
|
270
|
|
270
|
|
|||
|
6.40% debentures due 2028
|
250
|
|
250
|
|
|||
|
6.30% debentures due 2026
|
135
|
|
135
|
|
|||
|
5.00% notes due 2019
|
—
|
|
1,260
|
|
|||
|
Other
|
225
|
|
309
|
|
|||
|
|
$
|
19,806
|
|
$
|
21,353
|
|
|
|
|
2018
|
2017
|
2016
|
||||||||||||||
|
|
Common
Stock
|
Treasury
Stock
|
Common
Stock
|
Treasury
Stock
|
Common
Stock
|
Treasury
Stock
|
|||||||||||
|
Balance January 1
|
3,577
|
|
880
|
|
3,577
|
|
828
|
|
3,577
|
|
796
|
|
|||||
|
Purchases of treasury stock
|
—
|
|
122
|
|
—
|
|
67
|
|
—
|
|
60
|
|
|||||
|
Issuances
(1)
|
—
|
|
(17
|
)
|
—
|
|
(15
|
)
|
—
|
|
(28
|
)
|
|||||
|
Balance December 31
|
3,577
|
|
985
|
|
3,577
|
|
880
|
|
3,577
|
|
828
|
|
|||||
|
(1)
|
Issuances primarily reflect activity under share-based compensation plans.
|
|
Years Ended December 31
|
2018
|
2017
|
2016
|
|||||
|
Expected dividend yield
|
3.4
|
%
|
3.6
|
%
|
3.8
|
%
|
||
|
Risk-free interest rate
|
2.9
|
%
|
2.0
|
%
|
1.4
|
%
|
||
|
Expected volatility
|
19.1
|
%
|
17.8
|
%
|
19.6
|
%
|
||
|
Expected life (years)
|
6.1
|
|
6.1
|
|
6.2
|
|
||
|
Number
of Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contractual
Term (Years)
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Outstanding January 1, 2018
|
36,274
|
|
$
|
46.77
|
|
|||||||
|
Granted
|
3,520
|
|
58.15
|
|
||||||||
|
Exercised
|
(14,598
|
)
|
40.51
|
|
||||||||
|
Forfeited
|
(1,389
|
)
|
53.80
|
|
|
|
||||||
|
Outstanding December 31, 2018
|
23,807
|
|
$
|
51.89
|
|
5.95
|
$
|
584
|
|
|||
|
Exercisable December 31, 2018
|
16,184
|
|
$
|
48.85
|
|
4.82
|
$
|
446
|
|
|||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Total intrinsic value of stock options exercised
|
$
|
348
|
|
$
|
236
|
|
$
|
444
|
|
||
|
Fair value of stock options vested
|
29
|
|
30
|
|
28
|
|
|||||
|
Cash received from the exercise of stock options
|
591
|
|
499
|
|
939
|
|
|||||
|
|
RSUs
|
PSUs
|
||||||||||||
|
|
Number
of Shares
|
Weighted
Average
Grant Date
Fair Value
|
Number
of Shares
|
Weighted
Average
Grant Date
Fair Value
|
||||||||||
|
Nonvested January 1, 2018
|
13,609
|
|
$
|
59.32
|
|
1,868
|
|
$
|
60.03
|
|
||||
|
Granted
|
7,270
|
|
58.46
|
|
1,081
|
|
57.17
|
|
||||||
|
Vested
|
(3,766
|
)
|
59.66
|
|
(758
|
)
|
57.59
|
|
||||||
|
Forfeited
|
(985
|
)
|
59.30
|
|
(152
|
)
|
60.06
|
|
||||||
|
Nonvested December 31, 2018
|
16,128
|
|
$
|
58.85
|
|
2,039
|
|
$
|
59.42
|
|
||||
|
Pension Benefits
|
|||||||||||||||||||||||||||||||||||
|
U.S.
|
International
|
Other Postretirement Benefits
|
|||||||||||||||||||||||||||||||||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
||||||||||||||||||||||||||
|
Service cost
|
$
|
326
|
|
$
|
312
|
|
$
|
282
|
|
$
|
238
|
|
$
|
252
|
|
$
|
238
|
|
$
|
57
|
|
$
|
57
|
|
$
|
54
|
|
||||||||
|
Interest cost
|
432
|
|
454
|
|
456
|
|
178
|
|
172
|
|
204
|
|
69
|
|
81
|
|
82
|
|
|||||||||||||||||
|
Expected return on plan assets
|
(851
|
)
|
(862
|
)
|
(831
|
)
|
(431
|
)
|
(393
|
)
|
(382
|
)
|
(83
|
)
|
(78
|
)
|
(107
|
)
|
|||||||||||||||||
|
Amortization of unrecognized prior service cost
|
(50
|
)
|
(53
|
)
|
(55
|
)
|
(13
|
)
|
(11
|
)
|
(11
|
)
|
(84
|
)
|
(98
|
)
|
(106
|
)
|
|||||||||||||||||
|
Net loss amortization
|
232
|
|
180
|
|
119
|
|
84
|
|
98
|
|
87
|
|
1
|
|
1
|
|
3
|
|
|||||||||||||||||
|
Termination benefits
|
19
|
|
44
|
|
23
|
|
2
|
|
4
|
|
4
|
|
3
|
|
8
|
|
4
|
|
|||||||||||||||||
|
Curtailments
|
10
|
|
3
|
|
5
|
|
1
|
|
(4
|
)
|
(1
|
)
|
(8
|
)
|
(31
|
)
|
(18
|
)
|
|||||||||||||||||
|
Settlements
|
5
|
|
—
|
|
—
|
|
13
|
|
5
|
|
6
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Net periodic benefit cost (credit)
|
$
|
123
|
|
$
|
78
|
|
$
|
(1
|
)
|
$
|
72
|
|
$
|
123
|
|
$
|
145
|
|
$
|
(45
|
)
|
$
|
(60
|
)
|
$
|
(88
|
)
|
||||||||
|
|
Pension Benefits
|
Other
Postretirement
Benefits
|
|||||||||||||||||||||
|
U.S.
|
International
|
||||||||||||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
2018
|
2017
|
|||||||||||||||||
|
Fair value of plan assets January 1
|
$
|
10,896
|
|
$
|
9,766
|
|
$
|
9,339
|
|
$
|
7,794
|
|
$
|
1,114
|
|
$
|
1,019
|
|
|||||
|
Actual return on plan assets
|
(810
|
)
|
1,723
|
|
(289
|
)
|
677
|
|
(72
|
)
|
161
|
|
|||||||||||
|
Company contributions, net
|
378
|
|
58
|
|
167
|
|
226
|
|
6
|
|
(4
|
)
|
|||||||||||
|
Effects of exchange rate changes
|
—
|
|
—
|
|
(352
|
)
|
843
|
|
—
|
|
—
|
|
|||||||||||
|
Benefits paid
|
(772
|
)
|
(651
|
)
|
(202
|
)
|
(198
|
)
|
(80
|
)
|
(62
|
)
|
|||||||||||
|
Settlements
|
(44
|
)
|
—
|
|
(106
|
)
|
(17
|
)
|
—
|
|
—
|
|
|||||||||||
|
Other
|
—
|
|
—
|
|
23
|
|
14
|
|
—
|
|
—
|
|
|||||||||||
|
Fair value of plan assets December 31
|
$
|
9,648
|
|
$
|
10,896
|
|
$
|
8,580
|
|
$
|
9,339
|
|
$
|
968
|
|
$
|
1,114
|
|
|||||
|
Benefit obligation January 1
|
$
|
11,904
|
|
$
|
10,849
|
|
$
|
9,483
|
|
$
|
8,372
|
|
$
|
1,922
|
|
$
|
1,922
|
|
|||||
|
Service cost
|
326
|
|
312
|
|
238
|
|
252
|
|
57
|
|
57
|
|
|||||||||||
|
Interest cost
|
432
|
|
454
|
|
178
|
|
172
|
|
69
|
|
81
|
|
|||||||||||
|
Actuarial (gains) losses
(1)
|
(1,258
|
)
|
881
|
|
(154
|
)
|
(7
|
)
|
(341
|
)
|
(87
|
)
|
|||||||||||
|
Benefits paid
|
(772
|
)
|
(651
|
)
|
(202
|
)
|
(198
|
)
|
(80
|
)
|
(62
|
)
|
|||||||||||
|
Effects of exchange rate changes
|
—
|
|
—
|
|
(387
|
)
|
916
|
|
(6
|
)
|
3
|
|
|||||||||||
|
Plan amendments
|
—
|
|
—
|
|
10
|
|
(22
|
)
|
(9
|
)
|
—
|
|
|||||||||||
|
Curtailments
|
13
|
|
15
|
|
(2
|
)
|
(3
|
)
|
—
|
|
—
|
|
|||||||||||
|
Termination benefits
|
19
|
|
44
|
|
2
|
|
4
|
|
3
|
|
8
|
|
|||||||||||
|
Settlements
|
(44
|
)
|
—
|
|
(106
|
)
|
(17
|
)
|
—
|
|
—
|
|
|||||||||||
|
Other
|
—
|
|
—
|
|
23
|
|
14
|
|
—
|
|
—
|
|
|||||||||||
|
Benefit obligation December 31
|
$
|
10,620
|
|
$
|
11,904
|
|
$
|
9,083
|
|
$
|
9,483
|
|
$
|
1,615
|
|
$
|
1,922
|
|
|||||
|
Funded status December 31
|
$
|
(972
|
)
|
$
|
(1,008
|
)
|
$
|
(503
|
)
|
$
|
(144
|
)
|
$
|
(647
|
)
|
$
|
(808
|
)
|
|||||
|
Recognized as:
|
|||||||||||||||||||||||
|
Other assets
|
$
|
—
|
|
$
|
—
|
|
$
|
659
|
|
$
|
828
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Accrued and other current liabilities
|
(47
|
)
|
(59
|
)
|
(14
|
)
|
(17
|
)
|
(10
|
)
|
(11
|
)
|
|||||||||||
|
Other noncurrent liabilities
|
(925
|
)
|
(949
|
)
|
(1,148
|
)
|
(955
|
)
|
(637
|
)
|
(797
|
)
|
|||||||||||
|
U.S.
|
International
|
||||||||||||||
|
2018
|
2017
|
2018
|
2017
|
||||||||||||
|
Pension plans with a projected benefit obligation in excess of plan assets
|
|||||||||||||||
|
Projected benefit obligation
|
$
|
10,620
|
|
$
|
11,904
|
|
$
|
6,251
|
|
$
|
3,323
|
|
|||
|
Fair value of plan assets
|
9,648
|
|
10,896
|
|
5,089
|
|
2,352
|
|
|||||||
|
Pension plans with an accumulated benefit obligation in excess of plan assets
|
|||||||||||||||
|
Accumulated benefit obligation
|
$
|
9,702
|
|
$
|
676
|
|
$
|
5,936
|
|
$
|
2,120
|
|
|||
|
Fair value of plan assets
|
8,966
|
|
—
|
|
5,071
|
|
1,346
|
|
|||||||
|
|
Fair Value Measurements Using
|
Fair Value Measurements Using
|
|||||||||||||||||||||||||||||
|
|
Quoted Prices
In Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total
|
Quoted Prices
In Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total
|
|||||||||||||||||||||||
|
2018
|
2017
|
||||||||||||||||||||||||||||||
|
U.S. Pension Plans
|
|||||||||||||||||||||||||||||||
|
Assets
|
|||||||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
40
|
|
$
|
—
|
|
$
|
—
|
|
$
|
40
|
|
$
|
6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
6
|
|
|||||||
|
Investment funds
|
|||||||||||||||||||||||||||||||
|
Developed markets equities
|
169
|
|
—
|
|
—
|
|
169
|
|
390
|
|
—
|
|
—
|
|
390
|
|
|||||||||||||||
|
Emerging markets equities
|
121
|
|
—
|
|
—
|
|
121
|
|
138
|
|
—
|
|
—
|
|
138
|
|
|||||||||||||||
|
Equity securities
|
|||||||||||||||||||||||||||||||
|
Developed markets
|
2,172
|
|
—
|
|
—
|
|
2,172
|
|
2,743
|
|
—
|
|
—
|
|
2,743
|
|
|||||||||||||||
|
Fixed income securities
|
|||||||||||||||||||||||||||||||
|
Government and agency obligations
|
—
|
|
1,509
|
|
—
|
|
1,509
|
|
—
|
|
757
|
|
—
|
|
757
|
|
|||||||||||||||
|
Corporate obligations
|
—
|
|
1,246
|
|
—
|
|
1,246
|
|
—
|
|
900
|
|
—
|
|
900
|
|
|||||||||||||||
|
Mortgage and asset-backed securities
|
—
|
|
262
|
|
—
|
|
262
|
|
—
|
|
240
|
|
—
|
|
240
|
|
|||||||||||||||
|
Other investments
|
—
|
|
—
|
|
13
|
|
13
|
|
—
|
|
—
|
|
15
|
|
15
|
|
|||||||||||||||
|
Net assets in fair value hierarchy
|
$
|
2,502
|
|
$
|
3,017
|
|
|
$
|
13
|
|
|
$
|
5,532
|
|
|
$
|
3,277
|
|
|
$
|
1,897
|
|
|
$
|
15
|
|
|
$
|
5,189
|
|
|
|
Investments measured at NAV
(1)
|
4,116
|
|
5,707
|
|
|||||||||||||||||||||||||||
|
Plan assets at fair value
|
$
|
9,648
|
|
$
|
10,896
|
|
|||||||||||||||||||||||||
|
International Pension Plans
|
|||||||||||||||||||||||||||||||
|
Assets
|
|
|
|||||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
50
|
|
$
|
3
|
|
$
|
—
|
|
$
|
53
|
|
$
|
54
|
|
$
|
19
|
|
$
|
—
|
|
$
|
73
|
|
|||||||
|
Investment funds
|
|
|
|||||||||||||||||||||||||||||
|
Developed markets equities
|
461
|
|
3,071
|
|
—
|
|
3,532
|
|
562
|
|
3,326
|
|
—
|
|
3,888
|
|
|||||||||||||||
|
Emerging markets equities
|
56
|
|
112
|
|
—
|
|
168
|
|
62
|
|
176
|
|
—
|
|
238
|
|
|||||||||||||||
|
Government and agency obligations
|
372
|
|
2,082
|
|
—
|
|
2,454
|
|
249
|
|
2,095
|
|
—
|
|
2,344
|
|
|||||||||||||||
|
Corporate obligations
|
4
|
|
7
|
|
—
|
|
11
|
|
5
|
|
329
|
|
—
|
|
334
|
|
|||||||||||||||
|
Fixed income obligations
|
7
|
|
4
|
|
—
|
|
11
|
|
7
|
|
4
|
|
—
|
|
11
|
|
|||||||||||||||
|
Real estate
(2)
|
—
|
|
1
|
|
1
|
|
2
|
|
—
|
|
1
|
|
2
|
|
3
|
|
|||||||||||||||
|
Equity securities
|
|
|
|||||||||||||||||||||||||||||
|
Developed markets
|
544
|
|
—
|
|
—
|
|
544
|
|
660
|
|
—
|
|
—
|
|
660
|
|
|||||||||||||||
|
Fixed income securities
|
|
|
|||||||||||||||||||||||||||||
|
Government and agency obligations
|
2
|
|
291
|
|
—
|
|
293
|
|
2
|
|
266
|
|
—
|
|
268
|
|
|||||||||||||||
|
Corporate obligations
|
1
|
|
113
|
|
—
|
|
114
|
|
1
|
|
118
|
|
—
|
|
119
|
|
|||||||||||||||
|
Mortgage and asset-backed securities
|
—
|
|
55
|
|
—
|
|
55
|
|
—
|
|
55
|
|
—
|
|
55
|
|
|||||||||||||||
|
Other investments
|
|
|
|||||||||||||||||||||||||||||
|
Insurance contracts
(3)
|
—
|
|
66
|
|
811
|
|
877
|
|
—
|
|
67
|
|
470
|
|
537
|
|
|||||||||||||||
|
Other
|
—
|
|
4
|
|
1
|
|
5
|
|
—
|
|
6
|
|
1
|
|
7
|
|
|||||||||||||||
|
Net assets in fair value hierarchy
|
$
|
1,497
|
|
$
|
5,809
|
|
$
|
813
|
|
$
|
8,119
|
|
$
|
1,602
|
|
$
|
6,462
|
|
$
|
473
|
|
$
|
8,537
|
|
|||||||
|
Investments measured at NAV
(1)
|
461
|
|
802
|
|
|||||||||||||||||||||||||||
|
Plan assets at fair value
|
$
|
8,580
|
|
$
|
9,339
|
|
|||||||||||||||||||||||||
|
(1)
|
Certain investments that were measured at net asset value (NAV) per share or its equivalent as a practical expedient have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the fair value of plan assets at
December 31, 2018
and
2017
.
|
|
(2)
|
The plans’ Level 3 investments in real estate funds are generally valued by market appraisals of the underlying investments in the funds.
|
|
(3)
|
The plans’ Level 3 investments in insurance contracts are generally valued using a crediting rate that approximates market returns and invest in underlying securities whose market values are unobservable and determined using pricing models, discounted cash flow methodologies, or similar techniques.
|
|
|
2018
|
2017
|
|||||||||||||||||||||||||||||
|
|
Insurance
Contracts
|
Real
Estate
|
Other
|
Total
|
Insurance
Contracts
|
Real
Estate
|
Other
|
Total
|
|||||||||||||||||||||||
|
U.S. Pension Plans
|
|||||||||||||||||||||||||||||||
|
Balance January 1
|
$
|
—
|
|
$
|
—
|
|
$
|
15
|
|
$
|
15
|
|
$
|
—
|
|
$
|
—
|
|
$
|
18
|
|
$
|
18
|
|
|||||||
|
Actual return on plan assets:
|
|||||||||||||||||||||||||||||||
|
Relating to assets still held at December 31
|
—
|
|
—
|
|
(3
|
)
|
(3
|
)
|
—
|
|
—
|
|
(2
|
)
|
(2
|
)
|
|||||||||||||||
|
Relating to assets sold during the year
|
—
|
|
—
|
|
4
|
|
4
|
|
—
|
|
—
|
|
4
|
|
4
|
|
|||||||||||||||
|
Purchases and sales, net
|
—
|
|
—
|
|
(3
|
)
|
(3
|
)
|
—
|
|
—
|
|
(5
|
)
|
(5
|
)
|
|||||||||||||||
|
Balance December 31
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
13
|
|
|
$
|
13
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
15
|
|
|
$
|
15
|
|
|
International Pension Plans
|
|||||||||||||||||||||||||||||||
|
Balance January 1
|
$
|
470
|
|
$
|
2
|
|
$
|
1
|
|
$
|
473
|
|
$
|
412
|
|
$
|
4
|
|
$
|
1
|
|
$
|
417
|
|
|||||||
|
Actual return on plan assets:
|
|||||||||||||||||||||||||||||||
|
Relating to assets still held at December 31
|
(32
|
)
|
—
|
|
—
|
|
(32
|
)
|
52
|
|
—
|
|
—
|
|
52
|
|
|||||||||||||||
|
Purchases and sales, net
|
380
|
|
(1
|
)
|
—
|
|
379
|
|
5
|
|
(2
|
)
|
—
|
|
3
|
|
|||||||||||||||
|
Transfers into Level 3
|
(7
|
)
|
—
|
|
—
|
|
(7
|
)
|
1
|
|
—
|
|
—
|
|
1
|
|
|||||||||||||||
|
Balance December 31
|
$
|
811
|
|
|
$
|
1
|
|
|
$
|
1
|
|
|
$
|
813
|
|
|
$
|
470
|
|
|
$
|
2
|
|
|
$
|
1
|
|
|
$
|
473
|
|
|
|
Fair Value Measurements Using
|
Fair Value Measurements Using
|
|||||||||||||||||||||||||||||
|
|
Quoted Prices
In Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total
|
Quoted Prices
In Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total
|
|||||||||||||||||||||||
|
|
2018
|
|
2017
|
|
|||||||||||||||||||||||||||
|
Assets
|
|||||||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
78
|
|
$
|
—
|
|
$
|
—
|
|
$
|
78
|
|
$
|
97
|
|
$
|
—
|
|
$
|
—
|
|
$
|
97
|
|
|||||||
|
Investment funds
|
|||||||||||||||||||||||||||||||
|
Developed markets equities
|
16
|
|
—
|
|
—
|
|
16
|
|
37
|
|
—
|
|
—
|
|
37
|
|
|||||||||||||||
|
Emerging markets equities
|
12
|
|
—
|
|
—
|
|
12
|
|
13
|
|
—
|
|
—
|
|
13
|
|
|||||||||||||||
|
Government and agency obligations
|
1
|
|
—
|
|
—
|
|
1
|
|
1
|
|
—
|
|
—
|
|
1
|
|
|||||||||||||||
|
Equity securities
|
|||||||||||||||||||||||||||||||
|
Developed markets
|
200
|
|
—
|
|
—
|
|
200
|
|
256
|
|
—
|
|
—
|
|
256
|
|
|||||||||||||||
|
Fixed income securities
|
|||||||||||||||||||||||||||||||
|
Government and agency obligations
|
—
|
|
141
|
|
—
|
|
141
|
|
—
|
|
71
|
|
—
|
|
71
|
|
|||||||||||||||
|
Corporate obligations
|
—
|
|
116
|
|
—
|
|
116
|
|
—
|
|
84
|
|
—
|
|
84
|
|
|||||||||||||||
|
Mortgage and asset-backed securities
|
—
|
|
24
|
|
—
|
|
24
|
|
—
|
|
23
|
|
—
|
|
23
|
|
|||||||||||||||
|
Net assets in fair value hierarchy
|
$
|
307
|
|
$
|
281
|
|
$
|
—
|
|
$
|
588
|
|
$
|
404
|
|
$
|
178
|
|
$
|
—
|
|
$
|
582
|
|
|||||||
|
Investments measured at NAV
(1)
|
380
|
|
532
|
|
|||||||||||||||||||||||||||
|
Plan assets at fair value
|
$
|
968
|
|
$
|
1,114
|
|
|||||||||||||||||||||||||
|
(1)
|
Certain investments that were measured at net asset value (NAV) per share or its equivalent as a practical expedient have not been classified in the fair value hierarchy. The fair value amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the fair value of plan assets at
December 31, 2018
and
2017
.
|
|
U.S. Pension Benefits
|
International Pension
Benefits
|
Other
Postretirement
Benefits
|
|||||||||
|
2019
|
$
|
638
|
|
$
|
225
|
|
$
|
91
|
|
||
|
2020
|
661
|
|
213
|
|
95
|
|
|||||
|
2021
|
680
|
|
221
|
|
98
|
|
|||||
|
2022
|
685
|
|
239
|
|
102
|
|
|||||
|
2023
|
709
|
|
249
|
|
105
|
|
|||||
|
2024 — 2028
|
3,805
|
|
1,349
|
|
577
|
|
|||||
|
|
Pension Plans
|
Other Postretirement
Benefit Plans
|
|||||||||||||||||||||||||||||||||
|
U.S.
|
International
|
||||||||||||||||||||||||||||||||||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
||||||||||||||||||||||||||
|
Net (loss) gain arising during the period
|
$
|
(397
|
)
|
$
|
(19
|
)
|
$
|
(743
|
)
|
$
|
(505
|
)
|
$
|
309
|
|
$
|
(380
|
)
|
$
|
186
|
|
$
|
170
|
|
$
|
(45
|
)
|
||||||||
|
Prior service (cost) credit arising during the period
|
(4
|
)
|
(13
|
)
|
(10
|
)
|
(10
|
)
|
22
|
|
(2
|
)
|
2
|
|
(31
|
)
|
(19
|
)
|
|||||||||||||||||
|
|
$
|
(401
|
)
|
$
|
(32
|
)
|
$
|
(753
|
)
|
$
|
(515
|
)
|
$
|
331
|
|
$
|
(382
|
)
|
$
|
188
|
|
$
|
139
|
|
$
|
(64
|
)
|
||||||||
|
Net loss amortization included in benefit cost
|
$
|
232
|
|
$
|
180
|
|
$
|
119
|
|
$
|
84
|
|
$
|
98
|
|
$
|
87
|
|
$
|
1
|
|
$
|
1
|
|
$
|
3
|
|
||||||||
|
Prior service (credit) cost amortization included in benefit cost
|
(50
|
)
|
(53
|
)
|
(55
|
)
|
(13
|
)
|
(11
|
)
|
(11
|
)
|
(84
|
)
|
(98
|
)
|
(106
|
)
|
|||||||||||||||||
|
|
$
|
182
|
|
$
|
127
|
|
$
|
64
|
|
$
|
71
|
|
$
|
87
|
|
$
|
76
|
|
$
|
(83
|
)
|
$
|
(97
|
)
|
$
|
(103
|
)
|
||||||||
|
|
U.S. Pension and Other
Postretirement Benefit Plans
|
International Pension Plans
|
|||||||||||||||
|
December 31
|
2018
|
|
2017
|
|
2016
|
|
2018
|
|
2017
|
|
2016
|
|
|||||
|
Net periodic benefit cost
|
|
|
|
|
|
|
|||||||||||
|
Discount rate
|
3.70
|
%
|
4.30
|
%
|
4.70
|
%
|
2.10
|
%
|
2.20
|
%
|
2.80
|
%
|
|||||
|
Expected rate of return on plan assets
|
8.20
|
%
|
8.70
|
%
|
8.60
|
%
|
5.10
|
%
|
5.10
|
%
|
5.60
|
%
|
|||||
|
Salary growth rate
|
4.30
|
%
|
4.30
|
%
|
4.30
|
%
|
2.90
|
%
|
2.90
|
%
|
2.90
|
%
|
|||||
|
Benefit obligation
|
|
|
|
|
|
|
|||||||||||
|
Discount rate
|
4.40
|
%
|
3.70
|
%
|
4.30
|
%
|
2.20
|
%
|
2.10
|
%
|
2.20
|
%
|
|||||
|
Salary growth rate
|
4.30
|
%
|
4.30
|
%
|
4.30
|
%
|
2.80
|
%
|
2.90
|
%
|
2.90
|
%
|
|||||
|
December 31
|
2018
|
2017
|
|||
|
Health care cost trend rate assumed for next year
|
7.0
|
%
|
7.2
|
%
|
|
|
Rate to which the cost trend rate is assumed to decline
|
4.5
|
%
|
4.5
|
%
|
|
|
Year that the trend rate reaches the ultimate trend rate
|
2032
|
|
2032
|
|
|
|
|
One Percentage Point
|
||||||
|
|
Increase
|
Decrease
|
|||||
|
Effect on total service and interest cost components
|
$
|
11
|
|
$
|
(9
|
)
|
|
|
Effect on benefit obligation
|
88
|
|
(74
|
)
|
|||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Interest income
|
$
|
(343
|
)
|
$
|
(385
|
)
|
$
|
(328
|
)
|
||
|
Interest expense
|
772
|
|
754
|
|
693
|
|
|||||
|
Exchange losses (gains)
|
145
|
|
(11
|
)
|
174
|
|
|||||
|
Income on investments in equity securities, net
(1)
|
(324
|
)
|
(352
|
)
|
(43
|
)
|
|||||
|
Net periodic defined benefit plan (credit) cost other than service cost
|
(512
|
)
|
(512
|
)
|
(531
|
)
|
|||||
|
Other, net
|
(140
|
)
|
6
|
|
224
|
|
|||||
|
|
$
|
(402
|
)
|
$
|
(500
|
)
|
$
|
189
|
|
||
|
|
2018
|
2017
|
2016
|
|||||||||||||||||
|
|
Amount
|
Tax Rate
|
Amount
|
Tax Rate
|
Amount
|
Tax Rate
|
||||||||||||||
|
U.S. statutory rate applied to income before taxes
|
$
|
1,827
|
|
21.0
|
%
|
$
|
2,282
|
|
35.0
|
%
|
$
|
1,631
|
|
35.0
|
%
|
|||||
|
Differential arising from:
|
||||||||||||||||||||
|
Impact of the TCJA
|
289
|
|
3.3
|
|
2,625
|
|
40.3
|
|
—
|
|
—
|
|
||||||||
|
Valuation allowances
|
269
|
|
3.1
|
|
632
|
|
9.7
|
|
(5
|
)
|
(0.1
|
)
|
||||||||
|
Impact of purchase accounting adjustments, including amortization
|
267
|
|
3.1
|
|
713
|
|
10.9
|
|
623
|
|
13.4
|
|
||||||||
|
State taxes
|
201
|
|
2.3
|
|
77
|
|
1.2
|
|
173
|
|
3.7
|
|
||||||||
|
Restructuring
|
56
|
|
0.6
|
|
142
|
|
2.2
|
|
145
|
|
3.1
|
|
||||||||
|
Foreign earnings
|
(245
|
)
|
(2.8
|
)
|
(1,654
|
)
|
(25.4
|
)
|
(1,546
|
)
|
(33.2
|
)
|
||||||||
|
R&D tax credit
|
(96
|
)
|
(1.1
|
)
|
(71
|
)
|
(1.1
|
)
|
(58
|
)
|
(1.3
|
)
|
||||||||
|
Tax settlements
|
(22
|
)
|
(0.3
|
)
|
(356
|
)
|
(5.5
|
)
|
—
|
|
—
|
|
||||||||
|
Other
(1)
|
(38
|
)
|
(0.4
|
)
|
(287
|
)
|
(4.4
|
)
|
(245
|
)
|
(5.2
|
)
|
||||||||
|
|
$
|
2,508
|
|
28.8
|
%
|
$
|
4,103
|
|
62.9
|
%
|
$
|
718
|
|
15.4
|
%
|
|||||
|
(1)
|
Other includes the tax effects of losses on foreign subsidiaries and miscellaneous items.
|
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Domestic
|
$
|
3,717
|
|
$
|
3,483
|
|
$
|
518
|
|
||
|
Foreign
|
4,984
|
|
3,038
|
|
4,141
|
|
|||||
|
|
$
|
8,701
|
|
$
|
6,521
|
|
$
|
4,659
|
|
||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Current provision
|
|||||||||||
|
Federal
|
$
|
536
|
|
$
|
5,585
|
|
$
|
1,166
|
|
||
|
Foreign
|
2,281
|
|
1,229
|
|
916
|
|
|||||
|
State
|
200
|
|
(90
|
)
|
157
|
|
|||||
|
|
3,017
|
|
6,724
|
|
2,239
|
|
|||||
|
Deferred provision
|
|||||||||||
|
Federal
|
(402
|
)
|
(2,958
|
)
|
(1,255
|
)
|
|||||
|
Foreign
|
(64
|
)
|
75
|
|
(225
|
)
|
|||||
|
State
|
(43
|
)
|
262
|
|
(41
|
)
|
|||||
|
|
(509
|
)
|
(2,621
|
)
|
(1,521
|
)
|
|||||
|
|
$
|
2,508
|
|
$
|
4,103
|
|
$
|
718
|
|
||
|
|
2018
|
2017
|
|||||||||||||
|
|
Assets
|
Liabilities
|
Assets
|
Liabilities
|
|||||||||||
|
Product intangibles and licenses
|
$
|
720
|
|
$
|
1,640
|
|
$
|
307
|
|
$
|
2,256
|
|
|||
|
Inventory related
|
32
|
|
377
|
|
29
|
|
499
|
|
|||||||
|
Accelerated depreciation
|
—
|
|
582
|
|
28
|
|
642
|
|
|||||||
|
Pensions and other postretirement benefits
|
565
|
|
151
|
|
498
|
|
192
|
|
|||||||
|
Compensation related
|
291
|
|
—
|
|
314
|
|
—
|
|
|||||||
|
Unrecognized tax benefits
|
174
|
|
—
|
|
156
|
|
—
|
|
|||||||
|
Net operating losses and other tax credit carryforwards
|
715
|
|
—
|
|
654
|
|
—
|
|
|||||||
|
Other
|
621
|
|
66
|
|
909
|
|
52
|
|
|||||||
|
Subtotal
|
3,118
|
|
2,816
|
|
2,895
|
|
3,641
|
|
|||||||
|
Valuation allowance
|
(1,348
|
)
|
|
(900
|
)
|
|
|||||||||
|
Total deferred taxes
|
$
|
1,770
|
|
$
|
2,816
|
|
$
|
1,995
|
|
$
|
3,641
|
|
|||
|
Net deferred income taxes
|
|
$
|
1,046
|
|
|
$
|
1,646
|
|
|||||||
|
Recognized as:
|
|||||||||||||||
|
Other assets
|
$
|
656
|
|
$
|
573
|
|
|||||||||
|
Deferred income taxes
|
|
$
|
1,702
|
|
|
$
|
2,219
|
|
|||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Balance January 1
|
$
|
1,723
|
|
$
|
3,494
|
|
$
|
3,448
|
|
||
|
Additions related to current year positions
|
221
|
|
146
|
|
196
|
|
|||||
|
Additions related to prior year positions
|
142
|
|
520
|
|
75
|
|
|||||
|
Reductions for tax positions of prior years
(1)
|
(73
|
)
|
(1,038
|
)
|
(90
|
)
|
|||||
|
Settlements
(1)
|
(91
|
)
|
(1,388
|
)
|
(92
|
)
|
|||||
|
Lapse of statute of limitations
|
(29
|
)
|
(11
|
)
|
(43
|
)
|
|||||
|
Balance December 31
|
$
|
1,893
|
|
$
|
1,723
|
|
$
|
3,494
|
|
||
|
(1)
|
Amounts reflect the settlements with the IRS as discussed below.
|
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Net income attributable to Merck & Co., Inc.
|
$
|
6,220
|
|
$
|
2,394
|
|
$
|
3,920
|
|
||
|
Average common shares outstanding
|
2,664
|
|
2,730
|
|
2,766
|
|
|||||
|
Common shares issuable
(1)
|
15
|
|
18
|
|
21
|
|
|||||
|
Average common shares outstanding assuming dilution
|
2,679
|
|
2,748
|
|
2,787
|
|
|||||
|
Basic earnings per common share attributable to Merck & Co., Inc. common shareholders
|
$
|
2.34
|
|
$
|
0.88
|
|
$
|
1.42
|
|
||
|
Earnings per common share assuming dilution attributable to Merck & Co., Inc. common shareholders
|
$
|
2.32
|
|
$
|
0.87
|
|
$
|
1.41
|
|
||
|
(1)
|
Issuable primarily under share-based compensation plans.
|
|
Derivatives
|
Investments
|
Employee
Benefit
Plans
|
Cumulative
Translation
Adjustment
|
Accumulated Other
Comprehensive
Income (Loss)
|
|||||||||||||||
|
Balance January 1, 2016, net of taxes
|
$
|
404
|
|
$
|
41
|
|
$
|
(2,407
|
)
|
$
|
(2,186
|
)
|
$
|
(4,148
|
)
|
||||
|
Other comprehensive income (loss) before reclassification adjustments, pretax
|
210
|
|
(38
|
)
|
(1,199
|
)
|
(150
|
)
|
(1,177
|
)
|
|||||||||
|
Tax
|
(72
|
)
|
16
|
|
363
|
|
(19
|
)
|
288
|
|
|||||||||
|
Other comprehensive income (loss) before reclassification adjustments, net of taxes
|
138
|
|
(22
|
)
|
(836
|
)
|
(169
|
)
|
(889
|
)
|
|||||||||
|
Reclassification adjustments, pretax
|
(314
|
)
|
(1)
|
(31
|
)
|
(2)
|
37
|
|
(3)
|
—
|
|
(308
|
)
|
||||||
|
Tax
|
110
|
|
9
|
|
—
|
|
—
|
|
119
|
|
|||||||||
|
Reclassification adjustments, net of taxes
|
(204
|
)
|
(22
|
)
|
37
|
|
—
|
|
(189
|
)
|
|||||||||
|
Other comprehensive income (loss), net of taxes
|
(66
|
)
|
(44
|
)
|
(799
|
)
|
(169
|
)
|
(1,078
|
)
|
|||||||||
|
Balance December 31, 2016, net of taxes
|
338
|
|
(3
|
)
|
(3,206
|
)
|
(2,355
|
)
|
(5,226
|
)
|
|||||||||
|
Other comprehensive income (loss) before reclassification adjustments, pretax
|
(561
|
)
|
212
|
|
438
|
|
235
|
|
324
|
|
|||||||||
|
Tax
|
207
|
|
(35
|
)
|
(106
|
)
|
166
|
|
232
|
|
|||||||||
|
Other comprehensive income (loss) before reclassification adjustments, net of taxes
|
(354
|
)
|
177
|
|
332
|
|
401
|
|
556
|
|
|||||||||
|
Reclassification adjustments, pretax
|
(141
|
)
|
(1)
|
(291
|
)
|
(2)
|
117
|
|
(3)
|
—
|
|
(315
|
)
|
||||||
|
Tax
|
49
|
|
56
|
|
(30
|
)
|
—
|
|
75
|
|
|||||||||
|
Reclassification adjustments, net of taxes
|
(92
|
)
|
(235
|
)
|
87
|
|
—
|
|
(240
|
)
|
|||||||||
|
Other comprehensive income (loss), net of taxes
|
(446
|
)
|
(58
|
)
|
419
|
|
401
|
|
316
|
|
|||||||||
|
Balance December 31, 2017, net of taxes
|
(108
|
)
|
(61
|
)
|
(2,787
|
)
|
(4)
|
(1,954
|
)
|
(4,910
|
)
|
||||||||
|
Other comprehensive income (loss) before reclassification adjustments, pretax
|
228
|
|
(108
|
)
|
(728
|
)
|
(84
|
)
|
(692
|
)
|
|||||||||
|
Tax
|
(55
|
)
|
1
|
|
169
|
|
(139
|
)
|
(24
|
)
|
|||||||||
|
Other comprehensive income (loss) before reclassification adjustments, net of taxes
|
173
|
|
(107
|
)
|
(559
|
)
|
(223
|
)
|
(716
|
)
|
|||||||||
|
Reclassification adjustments, pretax
|
157
|
|
(1)
|
97
|
|
(2)
|
170
|
|
(3)
|
—
|
|
424
|
|
||||||
|
Tax
|
(33
|
)
|
—
|
|
(36
|
)
|
—
|
|
(69
|
)
|
|||||||||
|
Reclassification adjustments, net of taxes
|
124
|
|
97
|
|
134
|
|
—
|
|
355
|
|
|||||||||
|
Other comprehensive income (loss), net of taxes
|
297
|
|
(10
|
)
|
(425
|
)
|
(223
|
)
|
(361
|
)
|
|||||||||
|
Adoption of ASU 2018-02 (see Note 2)
|
(23
|
)
|
1
|
|
(344
|
)
|
100
|
|
(266
|
)
|
|||||||||
|
Adoption of ASU 2016-01 (see Note 2)
|
—
|
|
(8
|
)
|
—
|
|
—
|
|
(8
|
)
|
|||||||||
|
Balance December 31, 2018, net of taxes
|
$
|
166
|
|
|
$
|
(78
|
)
|
|
$
|
(3,556
|
)
|
(4)
|
$
|
(2,077
|
)
|
$
|
(5,545
|
)
|
|
|
(1)
|
Relates to foreign currency cash flow hedges that were reclassified from
AOCI
to
Sales
.
|
|
(2)
|
Represents net realized (gains) losses on the sales of available-for-sale investments that were reclassified from
AOCI
to
Other (income) expense, net
. In 2017 and 2016, these amounts included both investments in debt and equity securities; however, as a result of the adoption of ASU 2016-01 (see Note 2), in 2018, these amounts relate only to investments in available-for-sale debt securities.
|
|
(3)
|
Includes net amortization of prior service cost and actuarial gains and losses included in net periodic benefit cost (see Note 14).
|
|
(4)
|
Includes pension plan net loss of $
4.4 billion
and
$3.5 billion
at December 31,
2018
and
2017
, respectively, and other postretirement benefit plan net (gain) loss of
$(170) million
and
$(16) million
at December 31,
2018
and
2017
, respectively, as well as pension plan prior service credit of
$314 million
and
$326 million
at December 31,
2018
and
2017
, respectively, and other postretirement benefit plan prior service credit of
$375 million
and
$383 million
at December 31,
2018
and
2017
, respectively.
|
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||||||||||||||||||||||||||
|
U.S.
|
Int’l
|
Total
|
U.S.
|
Int’l
|
Total
|
U.S.
|
Int’l
|
Total
|
|||||||||||||||||||||||||||
|
Pharmaceutical:
|
|||||||||||||||||||||||||||||||||||
|
Oncology
|
|||||||||||||||||||||||||||||||||||
|
Keytruda
|
$
|
4,150
|
|
$
|
3,021
|
|
$
|
7,171
|
|
$
|
2,309
|
|
$
|
1,500
|
|
$
|
3,809
|
|
$
|
792
|
|
$
|
610
|
|
$
|
1,402
|
|
||||||||
|
Emend
|
312
|
|
210
|
|
522
|
|
342
|
|
213
|
|
556
|
|
356
|
|
193
|
|
549
|
|
|||||||||||||||||
|
Temodar
|
6
|
|
209
|
|
214
|
|
16
|
|
256
|
|
271
|
|
15
|
|
268
|
|
283
|
|
|||||||||||||||||
|
Alliance revenue - Lynparza
|
127
|
|
61
|
|
187
|
|
—
|
|
20
|
|
20
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Alliance revenue - Lenvima
|
95
|
|
54
|
|
149
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Vaccines
(1)
|
|||||||||||||||||||||||||||||||||||
|
Gardasil/Gardasil
9
|
1,873
|
|
1,279
|
|
3,151
|
|
1,565
|
|
743
|
|
2,308
|
|
1,780
|
|
393
|
|
2,173
|
|
|||||||||||||||||
|
ProQuad/M-M-R II/Varivax
|
1,430
|
|
368
|
|
1,798
|
|
1,374
|
|
303
|
|
1,676
|
|
1,362
|
|
279
|
|
1,640
|
|
|||||||||||||||||
|
Pneumovax 23
|
627
|
|
281
|
|
907
|
|
581
|
|
240
|
|
821
|
|
447
|
|
193
|
|
641
|
|
|||||||||||||||||
|
RotaTeq
|
496
|
|
232
|
|
728
|
|
481
|
|
204
|
|
686
|
|
482
|
|
169
|
|
652
|
|
|||||||||||||||||
|
Zostavax
|
22
|
|
195
|
|
217
|
|
422
|
|
246
|
|
668
|
|
518
|
|
168
|
|
685
|
|
|||||||||||||||||
|
Hospital Acute Care
|
|||||||||||||||||||||||||||||||||||
|
Bridion
|
386
|
|
531
|
|
917
|
|
239
|
|
465
|
|
704
|
|
77
|
|
405
|
|
482
|
|
|||||||||||||||||
|
Noxafil
|
353
|
|
389
|
|
742
|
|
309
|
|
327
|
|
636
|
|
284
|
|
312
|
|
595
|
|
|||||||||||||||||
|
Invanz
|
253
|
|
243
|
|
496
|
|
361
|
|
241
|
|
602
|
|
329
|
|
233
|
|
561
|
|
|||||||||||||||||
|
Cubicin
|
191
|
|
176
|
|
367
|
|
189
|
|
193
|
|
382
|
|
906
|
|
181
|
|
1,087
|
|
|||||||||||||||||
|
Cancidas
|
12
|
|
314
|
|
326
|
|
20
|
|
402
|
|
422
|
|
25
|
|
533
|
|
558
|
|
|||||||||||||||||
|
Primaxin
|
7
|
|
258
|
|
265
|
|
10
|
|
270
|
|
280
|
|
4
|
|
293
|
|
297
|
|
|||||||||||||||||
|
Immunology
|
|||||||||||||||||||||||||||||||||||
|
Simponi
|
—
|
|
893
|
|
893
|
|
—
|
|
819
|
|
819
|
|
—
|
|
766
|
|
766
|
|
|||||||||||||||||
|
Remicade
|
—
|
|
582
|
|
582
|
|
—
|
|
837
|
|
837
|
|
—
|
|
1,268
|
|
1,268
|
|
|||||||||||||||||
|
Neuroscience
|
|||||||||||||||||||||||||||||||||||
|
Belsomra
|
96
|
|
164
|
|
260
|
|
98
|
|
112
|
|
210
|
|
84
|
|
70
|
|
154
|
|
|||||||||||||||||
|
Virology
|
|||||||||||||||||||||||||||||||||||
|
Isentress/Isentress HD
|
513
|
|
627
|
|
1,140
|
|
565
|
|
639
|
|
1,204
|
|
721
|
|
666
|
|
1,387
|
|
|||||||||||||||||
|
Zepatier
|
8
|
|
447
|
|
455
|
|
771
|
|
888
|
|
1,660
|
|
488
|
|
67
|
|
555
|
|
|||||||||||||||||
|
Cardiovascular
|
|||||||||||||||||||||||||||||||||||
|
Zetia
|
45
|
|
813
|
|
857
|
|
352
|
|
992
|
|
1,344
|
|
1,588
|
|
972
|
|
2,560
|
|
|||||||||||||||||
|
Vytorin
|
10
|
|
487
|
|
497
|
|
124
|
|
627
|
|
751
|
|
473
|
|
668
|
|
1,141
|
|
|||||||||||||||||
|
Atozet
|
—
|
|
347
|
|
347
|
|
—
|
|
225
|
|
225
|
|
1
|
|
146
|
|
146
|
|
|||||||||||||||||
|
Adempas
|
—
|
|
329
|
|
329
|
|
—
|
|
300
|
|
300
|
|
—
|
|
169
|
|
169
|
|
|||||||||||||||||
|
Diabetes
|
|||||||||||||||||||||||||||||||||||
|
Januvia
|
1,969
|
|
1,718
|
|
3,686
|
|
2,153
|
|
1,584
|
|
3,737
|
|
2,286
|
|
1,622
|
|
3,908
|
|
|||||||||||||||||
|
Janumet
|
811
|
|
1,417
|
|
2,228
|
|
863
|
|
1,296
|
|
2,158
|
|
984
|
|
1,217
|
|
2,201
|
|
|||||||||||||||||
|
Women’s Health
|
|||||||||||||||||||||||||||||||||||
|
NuvaRing
|
722
|
|
180
|
|
902
|
|
564
|
|
197
|
|
761
|
|
576
|
|
202
|
|
777
|
|
|||||||||||||||||
|
Implanon/Nexplanon
|
495
|
|
208
|
|
703
|
|
496
|
|
191
|
|
686
|
|
420
|
|
186
|
|
606
|
|
|||||||||||||||||
|
Diversified Brands
|
|||||||||||||||||||||||||||||||||||
|
Singulair
|
20
|
|
688
|
|
708
|
|
40
|
|
692
|
|
732
|
|
40
|
|
874
|
|
915
|
|
|||||||||||||||||
|
Cozaar/Hyzaar
|
23
|
|
431
|
|
453
|
|
18
|
|
466
|
|
484
|
|
16
|
|
494
|
|
511
|
|
|||||||||||||||||
|
Nasonex
|
23
|
|
353
|
|
376
|
|
54
|
|
333
|
|
387
|
|
184
|
|
352
|
|
537
|
|
|||||||||||||||||
|
Arcoxia
|
—
|
|
335
|
|
335
|
|
—
|
|
363
|
|
363
|
|
—
|
|
450
|
|
450
|
|
|||||||||||||||||
|
Follistim AQ
|
115
|
|
153
|
|
268
|
|
123
|
|
174
|
|
298
|
|
157
|
|
197
|
|
355
|
|
|||||||||||||||||
|
Dulera
|
186
|
|
28
|
|
214
|
|
261
|
|
26
|
|
287
|
|
412
|
|
24
|
|
436
|
|
|||||||||||||||||
|
Fosamax
|
4
|
|
205
|
|
209
|
|
6
|
|
235
|
|
241
|
|
5
|
|
279
|
|
284
|
|
|||||||||||||||||
|
Other pharmaceutical
(2)
|
1,228
|
|
2,855
|
|
4,090
|
|
1,148
|
|
2,917
|
|
4,065
|
|
1,261
|
|
3,158
|
|
4,420
|
|
|||||||||||||||||
|
Total Pharmaceutical segment sales
|
16,608
|
|
|
21,081
|
|
|
37,689
|
|
|
15,854
|
|
|
19,536
|
|
|
35,390
|
|
|
17,073
|
|
|
18,077
|
|
|
35,151
|
|
|||||||||
|
Animal Health:
|
|||||||||||||||||||||||||||||||||||
|
Livestock
|
528
|
|
2,102
|
|
2,630
|
|
471
|
|
2,013
|
|
2,484
|
|
446
|
|
1,841
|
|
2,287
|
|
|||||||||||||||||
|
Companion Animals
|
710
|
|
872
|
|
1,582
|
|
619
|
|
772
|
|
1,391
|
|
543
|
|
648
|
|
1,191
|
|
|||||||||||||||||
|
Total Animal Health segment sales
|
1,238
|
|
|
2,974
|
|
|
4,212
|
|
|
1,090
|
|
|
2,785
|
|
|
3,875
|
|
|
989
|
|
|
2,489
|
|
|
3,478
|
|
|||||||||
|
Other segment sales
(3)
|
248
|
|
2
|
|
250
|
|
396
|
|
1
|
|
397
|
|
385
|
|
—
|
|
385
|
|
|||||||||||||||||
|
Total segment sales
|
18,094
|
|
|
24,057
|
|
|
42,151
|
|
|
17,340
|
|
|
22,322
|
|
|
39,662
|
|
|
18,447
|
|
|
20,566
|
|
|
39,014
|
|
|||||||||
|
Other
(4)
|
118
|
|
26
|
|
143
|
|
84
|
|
376
|
|
460
|
|
31
|
|
763
|
|
793
|
|
|||||||||||||||||
|
|
$
|
18,212
|
|
$
|
24,083
|
|
|
$
|
42,294
|
|
|
$
|
17,424
|
|
$
|
22,698
|
|
|
$
|
40,122
|
|
|
$
|
18,478
|
|
$
|
21,329
|
|
|
$
|
39,807
|
|
|||
|
(1)
|
On December 31, 2016, Merck and Sanofi terminated their equally-owned joint venture, SPMSD, which marketed vaccines in most major European markets (see Note 9). Accordingly, vaccine sales in 2018 and 2017 include sales in the European markets that were previously part of SPMSD. Amounts for 2016 do not include sales of vaccines sold through SPMSD, the results of which are reflected in equity income from affiliates included in
Other (income) expense, net
. Amounts for 2016 do, however, include supply sales to SPMSD.
|
|
(2)
|
Other pharmaceutical primarily reflects sales of other human health pharmaceutical products, including products within the franchises not listed separately.
|
|
(3)
|
Represents the non-reportable segments of Healthcare Services and Alliances.
|
|
(4)
|
Other is primarily comprised of miscellaneous corporate revenues, including revenue hedging activities, as well as third-party manufacturing sales. Other in 2018, 2017 and 2016 also includes approximately
$95 million
,
$85 million
and
$170 million
, respectively, related to the sale of the marketing rights to certain products.
|
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
United States
|
$
|
18,212
|
|
$
|
17,424
|
|
$
|
18,478
|
|
||
|
Europe, Middle East and Africa
|
12,213
|
|
11,478
|
|
10,953
|
|
|||||
|
Japan
|
3,212
|
|
3,122
|
|
2,846
|
|
|||||
|
Asia Pacific (other than Japan and China)
|
2,909
|
|
2,751
|
|
2,483
|
|
|||||
|
Latin America
|
2,415
|
|
2,339
|
|
2,155
|
|
|||||
|
China
|
2,184
|
|
1,586
|
|
1,435
|
|
|||||
|
Other
|
1,149
|
|
1,422
|
|
1,457
|
|
|||||
|
|
$
|
42,294
|
|
$
|
40,122
|
|
$
|
39,807
|
|
||
|
Years Ended December 31
|
2018
|
2017
|
2016
|
||||||||
|
Segment profits:
|
|||||||||||
|
Pharmaceutical segment
|
$
|
24,292
|
|
$
|
22,495
|
|
$
|
22,141
|
|
||
|
Animal Health segment
|
1,659
|
|
1,552
|
|
1,357
|
|
|||||
|
Other segments
|
103
|
|
275
|
|
146
|
|
|||||
|
Total segment profits
|
26,054
|
|
24,322
|
|
23,644
|
|
|||||
|
Other profits
|
6
|
|
26
|
|
481
|
|
|||||
|
Unallocated:
|
|||||||||||
|
Interest income
|
343
|
|
385
|
|
328
|
|
|||||
|
Interest expense
|
(772
|
)
|
(754
|
)
|
(693
|
)
|
|||||
|
Depreciation and amortization
|
(1,334
|
)
|
(1,378
|
)
|
(1,585
|
)
|
|||||
|
Research and development
|
(8,853
|
)
|
(9,481
|
)
|
(9,218
|
)
|
|||||
|
Amortization of purchase accounting adjustments
|
(2,664
|
)
|
(3,056
|
)
|
(3,692
|
)
|
|||||
|
Restructuring costs
|
(632
|
)
|
(776
|
)
|
(651
|
)
|
|||||
|
Charge related to termination of collaboration agreement with Samsung
|
(423
|
)
|
—
|
|
—
|
|
|||||
|
Loss on extinguishment of debt
|
—
|
|
(191
|
)
|
—
|
|
|||||
|
Gain on sale of certain migraine clinical development programs
|
—
|
|
—
|
|
100
|
|
|||||
|
Charge related to the settlement of worldwide
Keytruda
patent litigation
|
—
|
|
—
|
|
(625
|
)
|
|||||
|
Other unallocated, net
|
(3,024
|
)
|
(2,576
|
)
|
(3,430
|
)
|
|||||
|
|
$
|
8,701
|
|
$
|
6,521
|
|
$
|
4,659
|
|
||
|
Pharmaceutical
|
Animal Health
|
All Other
|
Total
|
||||||||||||
|
Year Ended December 31, 2018
|
|
|
|
||||||||||||
|
Included in segment profits:
|
|||||||||||||||
|
Equity (income) loss from affiliates
|
$
|
4
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4
|
|
|||
|
Depreciation and amortization
|
243
|
|
82
|
|
10
|
|
335
|
|
|||||||
|
Year Ended December 31, 2017
|
|
|
|
||||||||||||
|
Included in segment profits:
|
|||||||||||||||
|
Equity (income) loss from affiliates
|
$
|
7
|
|
$
|
—
|
|
$
|
—
|
|
$
|
7
|
|
|||
|
Depreciation and amortization
|
125
|
|
75
|
|
12
|
|
212
|
|
|||||||
|
Year Ended December 31, 2016
|
|
|
|
||||||||||||
|
Included in segment profits:
|
|||||||||||||||
|
Equity (income) loss from affiliates
|
$
|
(105
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
(105
|
)
|
|||
|
Depreciation and amortization
|
160
|
|
10
|
|
13
|
|
183
|
|
|||||||
|
December 31
|
2018
|
2017
|
2016
|
||||||||
|
United States
|
$
|
8,306
|
|
$
|
8,070
|
|
$
|
8,114
|
|
||
|
Europe, Middle East and Africa
|
3,706
|
|
3,151
|
|
2,732
|
|
|||||
|
Asia Pacific (other than Japan and China)
|
684
|
|
632
|
|
623
|
|
|||||
|
Latin America
|
264
|
|
271
|
|
234
|
|
|||||
|
China
|
167
|
|
150
|
|
152
|
|
|||||
|
Japan
|
159
|
|
158
|
|
164
|
|
|||||
|
Other
|
5
|
|
7
|
|
7
|
|
|||||
|
|
$
|
13,291
|
|
$
|
12,439
|
|
$
|
12,026
|
|
||

|
(b)
|
Supplementary Data
|
|
($ in millions except per share amounts)
|
4th Q
(1)
|
3rd Q
(2)
|
2nd Q
|
1st Q
(3)
|
|||||||||||
|
2018
(4)
|
|
|
|
|
|||||||||||
|
Sales
|
$
|
10,998
|
|
$
|
10,794
|
|
$
|
10,465
|
|
$
|
10,037
|
|
|||
|
Cost of sales
|
3,289
|
|
3,619
|
|
3,417
|
|
3,184
|
|
|||||||
|
Selling, general and administrative
|
2,643
|
|
2,443
|
|
2,508
|
|
2,508
|
|
|||||||
|
Research and development
|
2,214
|
|
2,068
|
|
2,274
|
|
3,196
|
|
|||||||
|
Restructuring costs
|
138
|
|
171
|
|
228
|
|
95
|
|
|||||||
|
Other (income) expense, net
|
110
|
|
(172
|
)
|
(48
|
)
|
(291
|
)
|
|||||||
|
Income before taxes
|
2,604
|
|
2,665
|
|
2,086
|
|
1,345
|
|
|||||||
|
Net income attributable to Merck & Co., Inc.
|
1,827
|
|
1,950
|
|
1,707
|
|
736
|
|
|||||||
|
Basic earnings per common share attributable to Merck & Co., Inc. common shareholders
|
$
|
0.70
|
|
$
|
0.73
|
|
$
|
0.64
|
|
$
|
0.27
|
|
|||
|
Earnings per common share assuming dilution attributable to Merck & Co., Inc. common shareholders
|
$
|
0.69
|
|
$
|
0.73
|
|
$
|
0.63
|
|
$
|
0.27
|
|
|||
|
2017
(4) (5)
|
|
|
|
|
|||||||||||
|
Sales
|
$
|
10,433
|
|
$
|
10,325
|
|
$
|
9,930
|
|
$
|
9,434
|
|
|||
|
Cost of sales
|
3,440
|
|
3,307
|
|
3,116
|
|
3,049
|
|
|||||||
|
Selling, general and administrative
|
2,643
|
|
2,459
|
|
2,500
|
|
2,472
|
|
|||||||
|
Research and development
|
2,314
|
|
4,413
|
|
1,782
|
|
1,830
|
|
|||||||
|
Restructuring costs
|
306
|
|
153
|
|
166
|
|
151
|
|
|||||||
|
Other (income) expense, net
|
(149
|
)
|
(207
|
)
|
(73
|
)
|
(71
|
)
|
|||||||
|
Income before taxes
|
1,879
|
|
200
|
|
2,439
|
|
2,003
|
|
|||||||
|
Net (loss) income attributable to Merck & Co., Inc.
|
(1,046
|
)
|
(56
|
)
|
1,946
|
|
1,551
|
|
|||||||
|
Basic (loss) earnings per common share attributable to Merck & Co., Inc. common shareholders
|
$
|
(0.39
|
)
|
$
|
(0.02
|
)
|
$
|
0.71
|
|
$
|
0.56
|
|
|||
|
(Loss) earnings per common share assuming dilution attributable to Merck & Co., Inc. common shareholders
|
$
|
(0.39
|
)
|
$
|
(0.02
|
)
|
$
|
0.71
|
|
$
|
0.56
|
|
|||
|
(1)
|
Amounts for 2017 include a provisional net tax charge related to the enactment of U.S. tax legislation (see Note 16).
|
|
(2)
|
Amounts for 2017 include a charge related to the formation of a collaboration with AstraZeneca (see Note 4).
|
|
(3)
|
Amounts for 2018 include a charge related to the formation of a collaboration with Eisai (see Note 4).
|
|
Item 9A.
|
Controls and Procedures.
|

|
||

|
||
|
Kenneth C. Frazier
|
|
Robert M. Davis
|
|
Chairman, President
and Chief Executive Officer
|
|
Executive Vice President, Global Services,
and Chief Financial Officer
|
|
Item 9B.
|
Other Information.
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance.
|
|
Item 11.
|
Executive Compensation.
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
|
Plan Category
|
Number of
securities to be
issued upon
exercise of
outstanding
options, warrants
and rights
(a)
|
Weighted-average
exercise price of
outstanding
options, warrants
and rights
(b)
|
Number of
securities remaining
available for future
issuance under equity
compensation plans
(excluding
securities
reflected in column (a))
(c)
|
|||||||
|
Equity compensation plans approved by security holders
(1)
|
23,807,101
(2)
|
|
$
|
51.89
|
|
110,977,283
|
|
|||
|
Equity compensation plans not approved by security holders
|
—
|
|
—
|
|
—
|
|
||||
|
Total
|
23,807,101
|
|
$
|
51.89
|
|
110,977,283
|
|
|||
|
(1)
|
Includes options to purchase shares of Company Common Stock and other rights under the following shareholder-approved plans: the Merck Sharp & Dohme 2004, 2007 and 2010 Incentive Stock Plans, the Merck & Co., Inc. 2006 and 2010 Non-Employee Directors Stock Option Plans, and the Merck & Co., Inc. Schering-Plough 2002 and 2006 Stock Incentive Plans.
|
|
(2)
|
Excludes approximately 16,128,455 shares of restricted stock units and 2,039,065 performance share units (assuming maximum payouts) under the Merck Sharp & Dohme 2004, 2007 and 2010 Incentive Stock Plans. Also excludes 224,599 shares of phantom stock deferred under the MSD Employee Deferral Program and 582,155 shares of phantom stock deferred under the Merck & Co., Inc. Plan for Deferred Payment of Directors’ Compensation.
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
|
Item 14.
|
Principal Accountant Fees and Services.
|
|
Item 15.
|
Exhibits and Financial Statement Schedules.
|
|
Exhibit
Number
|
|
Description
|
||
|
3.1
|
—
|
|||
|
3.2
|
—
|
|||
|
4.1
|
—
|
Indenture, dated as of April 1, 1991, between Merck Sharp & Dohme Corp. (f/k/a Schering Corporation) and U.S. Bank Trust National Association (as successor to Morgan Guaranty Trust Company of New York), as Trustee (the 1991 Indenture) — Incorporated by reference to Exhibit 4 to MSD’s Registration Statement on Form S-3 (No. 33-39349)
|
||
|
4.2
|
—
|
|||
|
4.3
|
—
|
|||
|
4.4
|
—
|
|||
|
4.5
|
—
|
|||
|
4.6
|
—
|
|||
|
4.7
|
—
|
|||
|
Exhibit
Number
|
|
Description
|
||
|
4.8
|
—
|
|||
|
4.9
|
—
|
|||
|
4.10
|
—
|
Long-term debt instruments under which the total amount of securities authorized does not exceed 10% of Merck & Co., Inc.’s total consolidated assets are not filed as exhibits to this report. Merck & Co., Inc. will furnish a copy of these agreements to the Securities and Exchange Commission on request.
|
||
|
*10.1
|
—
|
|||
|
*10.2
|
—
|
|||
|
*10.3
|
—
|
|||
|
*10.4
|
—
|
|||
|
*10.5
|
—
|
|||
|
*10.6
|
—
|
|||
|
*10.7
|
—
|
|||
|
*10.8
|
—
|
|||
|
*10.9
|
—
|
|||
|
*10.10
|
—
|
|||
|
*10.11
|
—
|
|||
|
10.12
|
—
|
|||
|
Exhibit
Number
|
|
Description
|
||
|
*10.13
|
—
|
|||
|
*10.14
|
—
|
|||
|
*10.15
|
—
|
|||
|
*10.16
|
—
|
|||
|
10.17
|
||||
|
*10.18
|
—
|
|||
|
*10.19
|
—
|
|||
|
*10.20
|
—
|
|||
|
*10.21
|
—
|
|||
|
*10.22
|
—
|
|||
|
*10.23
|
—
|
|||
|
10.24
|
—
|
|||
|
10.25
|
—
|
|||
|
10.26
|
—
|
|||
|
10.27
|
—
|
|||
|
10.28
|
—
|
|||
|
21
|
—
|
|||
|
23
|
—
|
|||
|
24.1
|
—
|
|||
|
24.2
|
—
|
|||
|
Exhibit
Number
|
Description
|
|||
|
31.1
|
—
|
|||
|
31.2
|
—
|
|||
|
32.1
|
—
|
|||
|
32.2
|
—
|
|||
|
101
|
—
|
The following materials from Merck & Co., Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2018, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statement of Income, (ii) the Consolidated Statement of Comprehensive Income, (iii) the Consolidated Balance Sheet, (iv) the Consolidated Statement of Equity, (v) the Consolidated Statement of Cash Flows, and (vi) Notes to Consolidated Financial Statements.
|
||
|
*
|
Management contract or compensatory plan or arrangement.
|
|
†
|
Certain portions of the exhibit have been omitted pursuant to a request for confidential treatment. The non-public information has been filed separately with the Securities and Exchange Commission pursuant to rule 24b-2 under the Securities Exchange Act of 1934, as amended.
|
|
MERCK & CO., INC.
|
||
|
By:
|
KENNETH C. FRAZIER
|
|
|
(Chairman, President and Chief Executive Officer)
|
||
|
By:
|
/s/ JENNIFER ZACHARY
|
|
|
Jennifer Zachary
|
||
|
(Attorney-in-Fact)
|
||
|
Signatures
|
Title
|
Date
|
||
|
KENNETH C. FRAZIER
|
Chairman, President and Chief Executive Officer;
Principal Executive Officer; Director
|
February 27, 2019
|
||
|
ROBERT M. DAVIS
|
Executive Vice President, Global Services, and Chief Financial Officer; Principal Financial Officer
|
February 27, 2019
|
||
|
RITA A. KARACHUN
|
Senior Vice President Finance-Global Controller;
Principal Accounting Officer
|
February 27, 2019
|
||
|
LESLIE A. BRUN
|
Director
|
February 27, 2019
|
||
|
THOMAS R. CECH
|
Director
|
February 27, 2019
|
||
|
PAMELA J. CRAIG
|
Director
|
February 27, 2019
|
||
|
THOMAS H. GLOCER
|
Director
|
February 27, 2019
|
||
|
ROCHELLE B. LAZARUS
|
Director
|
February 27, 2019
|
||
|
JOHN H. NOSEWORTHY
|
Director
|
February 27, 2019
|
||
|
PAUL B. ROTHMAN
|
Director
|
February 27, 2019
|
||
|
PATRICIA F. RUSSO
|
Director
|
February 27, 2019
|
||
|
INGE G. THULIN
|
Director
|
February 27, 2019
|
||
|
WENDELL P. WEEKS
|
Director
|
February 27, 2019
|
||
|
PETER C. WENDELL
|
Director
|
February 27, 2019
|
||
|
By:
|
/S/ JENNIFER ZACHARY
|
|
|
Jennifer Zachary
|
||
|
(Attorney-in-Fact)
|
||
|
Exhibit
Number
|
|
Description
|
||
|
3.1
|
—
|
|||
|
3.2
|
—
|
|||
|
4.1
|
—
|
Indenture, dated as of April 1, 1991, between Merck Sharp & Dohme Corp. (f/k/a Schering Corporation) and U.S. Bank Trust National Association (as successor to Morgan Guaranty Trust Company of New York), as Trustee (the 1991 Indenture) — Incorporated by reference to Exhibit 4 to MSD’s Registration Statement on Form S-3 (No. 33-39349)
|
||
|
4.2
|
—
|
|||
|
4.3
|
—
|
|||
|
4.4
|
—
|
|||
|
4.5
|
—
|
|||
|
4.6
|
—
|
|||
|
4.7
|
—
|
|||
|
4.8
|
—
|
|||
|
4.9
|
—
|
|||
|
4.10
|
—
|
Long-term debt instruments under which the total amount of securities authorized does not exceed 10% of Merck & Co., Inc.’s total consolidated assets are not filed as exhibits to this report. Merck & Co., Inc. will furnish a copy of these agreements to the Securities and Exchange Commission on request.
|
||
|
*10.1
|
—
|
|||
|
*10.2
|
—
|
|||
|
*10.3
|
—
|
|||
|
*10.4
|
—
|
|||
|
Exhibit
Number
|
|
Description
|
||
|
*10.5
|
—
|
|||
|
*10.6
|
—
|
|||
|
*10.7
|
—
|
|||
|
*10.8
|
—
|
|||
|
*10.9
|
—
|
|||
|
*10.10
|
—
|
|||
|
*10.11
|
—
|
|||
|
*10.12
|
—
|
|||
|
*10.13
|
—
|
|||
|
*10.14
|
—
|
|||
|
*10.15
|
—
|
|||
|
*10.16
|
—
|
|||
|
*10.17
|
—
|
|||
|
*10.18
|
—
|
|||
|
Exhibit
Number
|
|
Description
|
||
|
*10.19
|
||||
|
*10.20
|
—
|
|||
|
*10.21
|
—
|
|||
|
*10.22
|
—
|
|||
|
*10.23
|
—
|
|||
|
10.24
|
—
|
|||
|
10.25
|
—
|
|||
|
10.26
|
—
|
|||
|
10.27
|
—
|
|||
|
10.28
|
—
|
|||
|
21
|
—
|
|||
|
23
|
—
|
|||
|
24.1
|
—
|
|||
|
24.2
|
—
|
|||
|
31.1
|
—
|
|||
|
31.2
|
—
|
|||
|
32.1
|
—
|
|||
|
32.2
|
—
|
|||
|
101
|
—
|
The following materials from Merck & Co., Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2018, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statement of Income, (ii) the Consolidated Statement of Comprehensive Income, (iii) the Consolidated Balance Sheet, (iv) the Consolidated Statement of Equity, (v) the Consolidated Statement of Cash Flows, and (vi) Notes to Consolidated Financial Statements.
|
||
|
*
|
Management contract or compensatory plan or arrangement.
|
|
†
|
Certain portions of the exhibit have been omitted pursuant to a request for confidential treatment. The non-public information has been filed separately with the Securities and Exchange Commission pursuant to rule 24b-2 under the Securities Exchange Act of 1934, as amended.
|