|
FORM 10-K
|

|
Delaware
|
81-3467528
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(IRS Employer Identification No.)
|
|
|
200 Technology Square
Cambridge, Massachusetts
|
02139
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common stock, par value $0.0001 per share
|
The Nasdaq Stock Market LLC
|
|
|
Large accelerated filer
o
|
Accelerated filer
o
|
Non-accelerated filer
x
|
Smaller reporting company
o
|
|||
|
Emerging growth company
x
|
||||||
|
PART I
.
|
Page
|
|
|
Item 1.
|
Business
|
|
|
Item 1A.
|
Risk Factors
|
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
|
Item 2.
|
Properties
|
|
|
Item 3.
|
Legal Proceedings
|
|
|
Item 4.
|
Mine Safety Disclosures
|
|
|
PART II
.
|
||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
Item 6.
|
Selected Consolidated Financial Data
|
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures about Market Risk
|
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|
|
Item 9A.
|
Controls and Procedures
|
|
|
Item 9B.
|
Other Information
|
|
|
PART III
.
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
|
Item 11.
|
Executive Compensation
|
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
|
Item 14.
|
Principal Accountant Fees and Services
|
|
|
PART IV
.
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
|
|
Item 16.
|
Form 10-K Summary
|
|
|
Signatures
|
||
|
•
|
the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs;
|
|
•
|
our ability to identify research priorities and apply a risk-mitigated strategy to efficiently discover and develop development candidates and investigational medicines, including by applying learnings from one program to our other programs and from one modality to our other modalities;
|
|
•
|
our ability and the potential to successfully manufacture our drug substances, delivery vehicles, development candidates, and investigational medicines for preclinical use, for clinical trials and on a larger scale for commercial use, if approved;
|
|
•
|
the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and investigational medicines;
|
|
•
|
our ability to obtain funding for our operations necessary to complete further development and commercialization of our investigational medicines;
|
|
•
|
our ability to obtain and maintain regulatory approval of our investigational medicines;
|
|
•
|
our ability to commercialize our products, if approved;
|
|
•
|
the pricing and reimbursement of our investigational medicines, if approved;
|
|
•
|
the implementation of our business model, and strategic plans for our business, investigational medicines, and technology;
|
|
•
|
the scope of protection we are able to establish and maintain for intellectual property rights covering our investigational medicines and technology;
|
|
•
|
estimates of our future expenses, revenues, capital requirements, and our needs for additional financing;
|
|
•
|
the potential benefits of strategic collaboration agreements, our ability to enter into strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory, and commercialization expertise;
|
|
•
|
future agreements with third parties in connection with the commercialization of our investigational medicines, if approved;
|
|
•
|
the size and growth potential of the markets for our investigational medicines, and our ability to serve those markets;
|
|
•
|
our financial performance;
|
|
•
|
the rate and degree of market acceptance of our investigational medicines;
|
|
•
|
regulatory developments in the United States and foreign countries;
|
|
•
|
our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately;
|
|
•
|
our ability to produce our products or investigational medicines with advantages in turnaround times or manufacturing cost;
|
|
•
|
the success of competing therapies that are or may become available;
|
|
•
|
our ability to attract and retain key scientific or management personnel;
|
|
•
|
the impact of laws and regulations;
|
|
•
|
developments relating to our competitors and our industry; and
|
|
•
|
other risks and uncertainties, including those listed under the caption “Risk Factors.”
|
|
1.
|
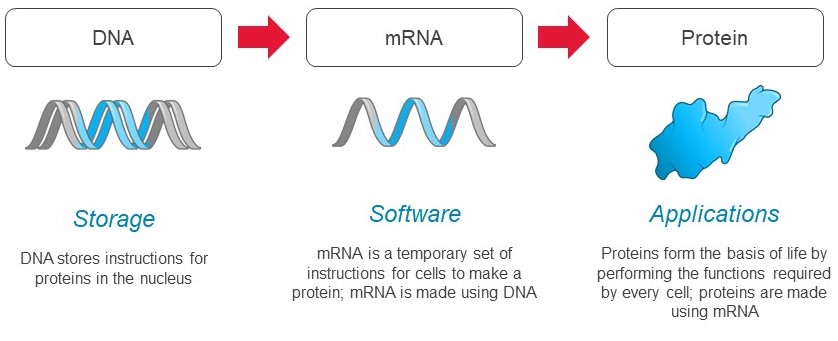
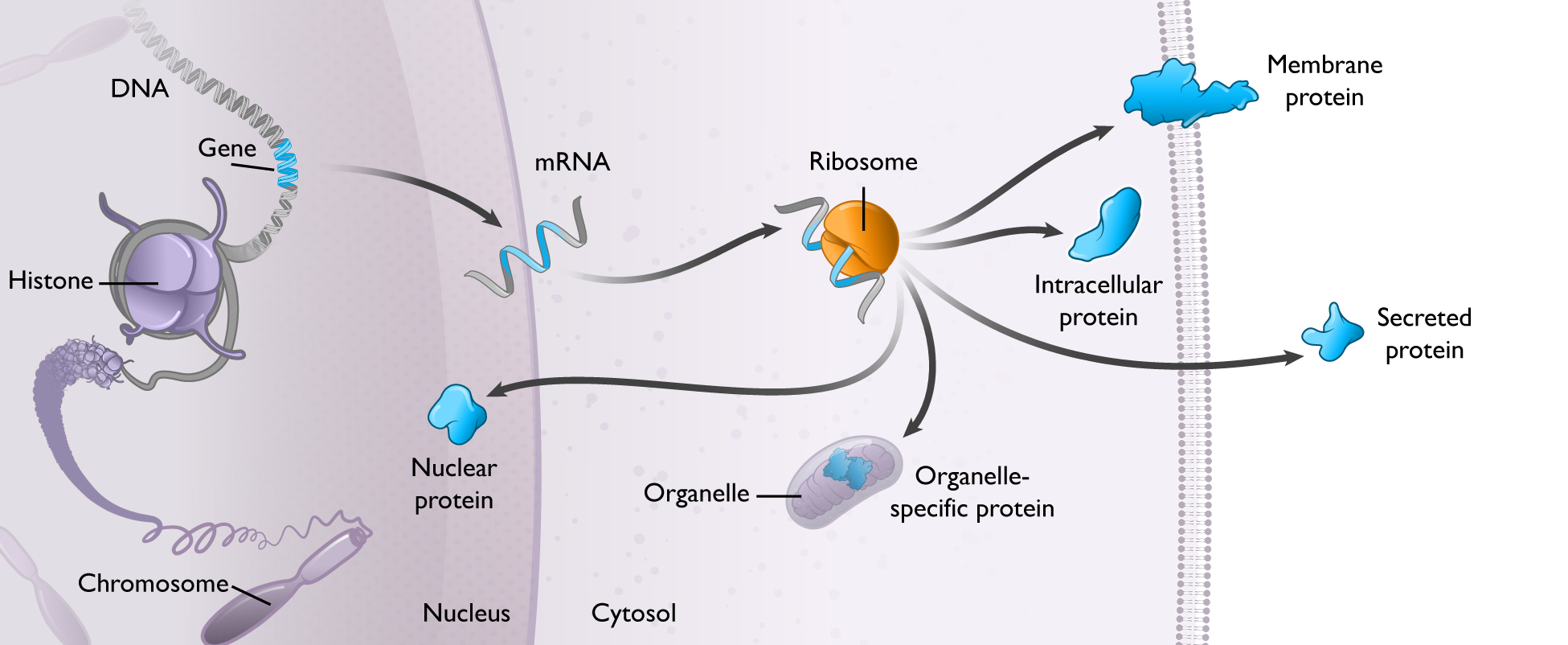
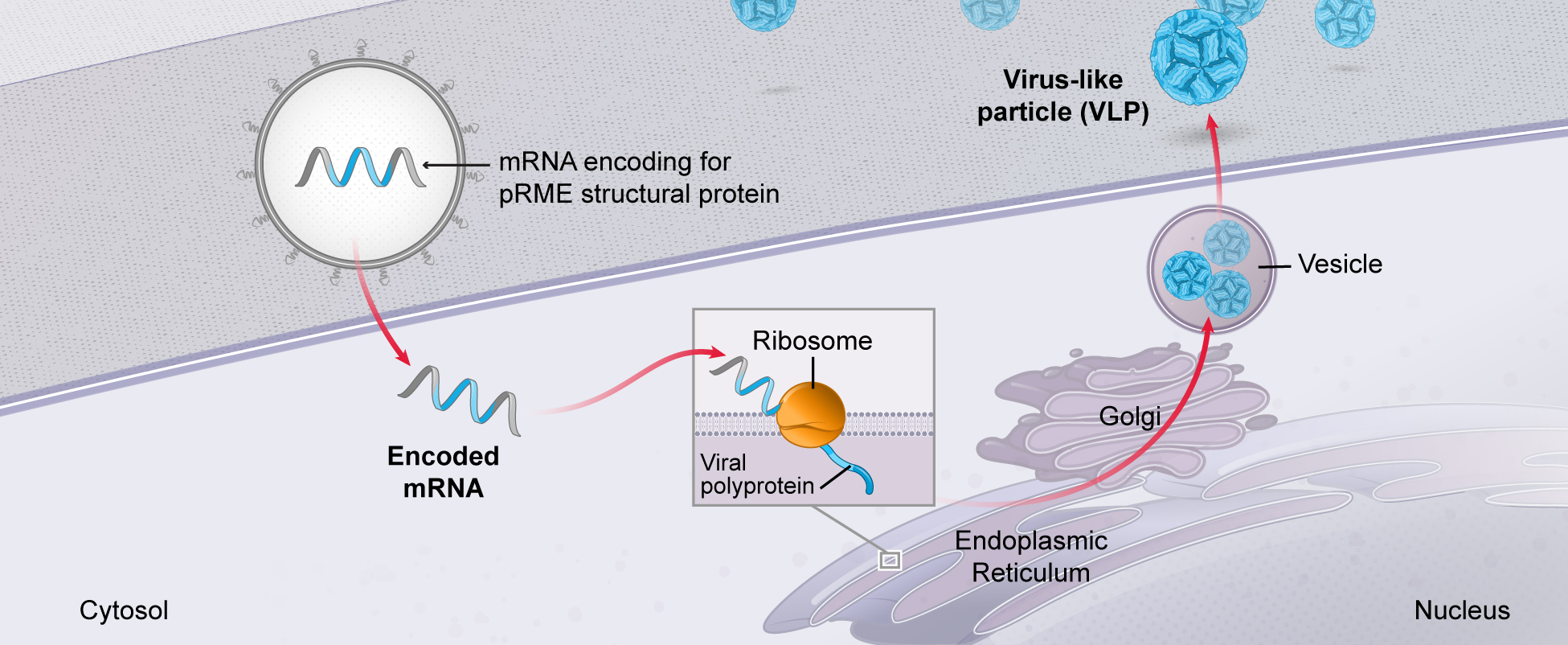
mRNA is used by every cell to produce all proteins:
Cells in the human body use mRNA to make all types of proteins, including secreted, membrane, and intracellular proteins. mRNA is used by cells to vary the quantities of protein produced over time, in different locations, and in various combinations. Given the universal role of mRNA in protein production, we believe that mRNA medicines could have broad applicability across human disease.
|
|
2.
|
Making proteins inside one’s own cells mimics human biology:
Using a person’s own cells to produce protein therapeutics or vaccine antigens could create advantages over existing technologies such as recombinant proteins, which are manufactured using processes that are foreign to the human body. These advantages include the ability to:
|
|
•
|
use multiple mRNAs to produce multiple proteins;
|
|
•
|
reduce or eliminate immunogenicity;
|
|
•
|
create multi-protein complexes;
|
|
•
|
produce therapeutic or vaccine proteins locally;
|
|
•
|
harness native protein folding and glycosylation; and
|
|
•
|
make proteins that are unstable outside the body.
|
|
3.
|
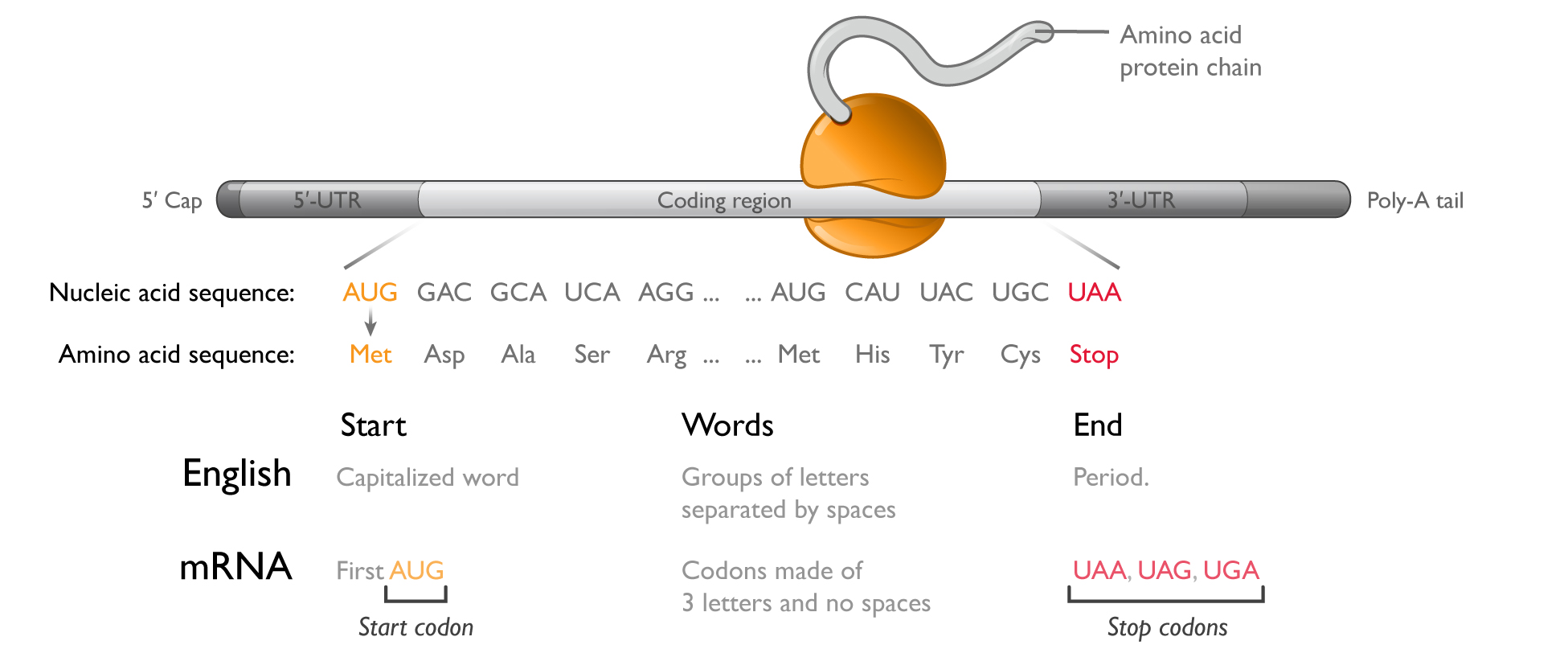
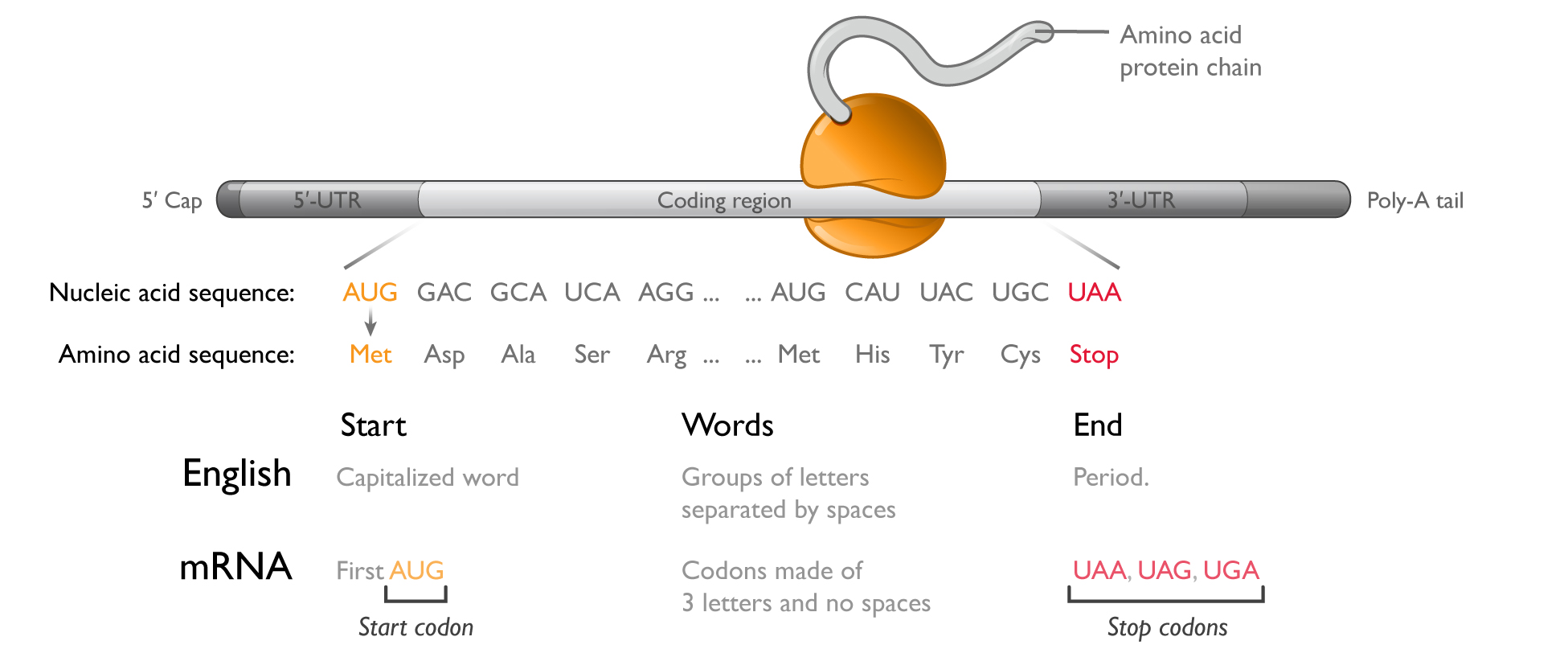
mRNA has a simple and flexible chemical structure:
Each mRNA molecule comprises four chemically similar nucleotides to encode proteins made from up to 20 chemically different amino acids. To make the full diversity of possible proteins, only simple sequence changes are required in mRNA. A vast number of potential mRNA medicines can be developed, therefore, with only minor changes to the underlying chemical structure of the molecule or manufacturing processes, a significant advantage over small molecule or protein therapeutics.
|
|
4.
|
mRNA has the potential for classic pharmacologic features:
The intrinsic properties of mRNA translate into attractive pharmacologic features, including:
|
|
•
|
each mRNA encodes for a specific protein and no other protein;
|
|
•
|
each mRNA molecule can produce many copies of a protein in the cell before being degraded;
|
|
•
|
increasing mRNA levels in a cell generally leads to increasing protein levels; and
|
|
•
|
the effects of mRNA in a cell can be transient and limits risk of irreversible changes to the cell’s DNA.
|
|
1.
|
mRNA has the potential to create an unprecedented abundance and diversity of medicines.
mRNA medicines could be used to provide patients or healthy individuals with any therapeutic protein or vaccine, including those targeting intracellular and membrane proteins. This breadth of applicability has the potential to create an extraordinary number of new mRNA-based medicines that are currently beyond the reach of recombinant protein technology.
|
|
2.
|
Advances in the development of our mRNA medicines can reduce risks across our portfolio.
mRNA medicines share fundamental features that can be used to learn quickly across a portfolio. We believe that once safety and proof of protein production has been established in one program, the technology and biology risks of related programs that use similar mRNA technologies, delivery technologies, and manufacturing processes will decrease significantly.
|
|
3.
|
mRNA technology can accelerate discovery and development.
The software-like features of mRNA enable rapid
in silico
design and the use of automated high-throughput synthesis processes that permit discovery to proceed in parallel rather than sequentially. We believe these mRNA features can also accelerate drug development by allowing the use of shared manufacturing processes and infrastructure.
|
|
4.
|
The ability to leverage shared processes and infrastructure can drive significant capital efficiency over time.
We believe the manufacturing requirements of different mRNA medicines are dramatically more similar than traditional recombinant protein-based drugs across a similarly diverse pipeline. When manufacturing at commercial scale, we believe mRNA medicines will benefit from shared capital expenditures, resulting in lower program-specific capital needs and an advantageous variable cost profile.
|
|
1.
|
We seek to discover and develop a large pipeline in parallel.
Our goal is to address or prevent as many human diseases as our technology, talent, capital, and other resources permit. We do so as rapidly as we can, understanding both the urgency for patients and the need to be disciplined in our approach. We have a diverse pipeline of
20
development candidates,
11
of which are currently in clinical trials, and many of which have the potential to be first-in-class or best-in-class medicines.
|
|
2.
|
We undertake sustained, long-term investment in technology creation.
We aim to improve the performance of mRNA medicines in our current modalities, and to unlock new modalities, through investments within basic and applied science. We are committed to remaining at the forefront of mRNA science, which we believe will take many more years to fully mature.
|
|
3.
|
We focus on the pace and scale of our learning.
We believe that time is a critical resource. We seek to accelerate our progress by solving numerous technical problems in parallel rather than in sequence. Our scientists pursue experiments based on how much we can learn from the results, not just the probability of a positive outcome. We believe negative information is valuable and we can learn from our setbacks. We make significant investments in digital assets and research infrastructure to accelerate the pace and scale of our learning.
|
|
4.
|
We integrate across the most critical parts of our value chain.
mRNA is a complex multicomponent system and we believe it demands integration. We believe that we must be directly engaged in research, drug discovery, drug development, process and analytical development, and manufacturing to accelerate our learning, reduce our risk, and protect our critical know-how. Where appropriate, we seek out strategic collaborators that can augment our capabilities or expand our capacity in specific therapeutic areas, while being careful to resist the fragmentation of our core technology.
|
|
5.
|
We forward invest in core enabling capabilities and infrastructure.
To execute across a broad pipeline, we need to invest at risk before we have all the answers. Our forward investments focus on areas where lead times are long and where early investments can reduce execution risk and accelerate future progress. We proactively decided to invest in a dedicated manufacturing facility in Norwood, MA, to support the anticipated growth of our pipeline.
|
|
1.
|
Technology risk
encompasses the challenges of developing the product features of mRNA medicines, including delivery, controlling interactions with the immune system, optimizing therapeutic index, and manufacturing. We believe the best way to mitigate technology risk is to sustain long-term investments in our platform. In addition, we diversify our technology risk by compartmentalizing our pipeline into groups of programs with shared product features, which we call modalities. Lastly, we stage program development within a modality, leveraging the first program, whether successful or not, to generate insights that accelerate and reduce the risk of subsequent programs within the modality.
|
|
2.
|
Biology risk
entails the risk unique to each program based on its mechanism of action and of clinical development in the target patient population. We believe the best way to manage biology risk is to diversify it by pursuing multiple programs in parallel. In addition, within a modality we seek to initially pursue programs with well-understood biology. Lastly, we may seek strategic collaborators to share risk and upside in disease areas with high inherent biology risk, such as cancer and heart disease.
|
|
3.
|
Execution risk
refers to the challenge of executing against the scale of our mission. We solve for this risk by seeking to hire the right people, the best talent in the industry. We seek to foster a culture of execution with a focus on quick review cycles and high
|
|
4.
|
Financing risk
refers to our ability to access the capital required to fund the current breadth of our endeavor, as well as new opportunities. We manage this risk by attempting to maintain a strong balance sheet with several years of cash runway. As of
December 31, 2018
, we had cash, cash equivalents, and investments of
$1.7 billion
. Cash used in operations and for purchases of property and equipment was
$330.9 million
and
$105.8 million
in
2018
, respectively. Lastly, we may continue to pursue strategic alliances, which provide resources and another source of funding.
|
|
•
|
predictable dose response;
|
|
•
|
reproducible pharmacology, including upon repeat dosing;
|
|
•
|
therapeutic potency, through achieving the intended pharmacologic activity in the target tissue;
|
|
•
|
safety and tolerability; and
|
|
•
|
scalability for development.
|

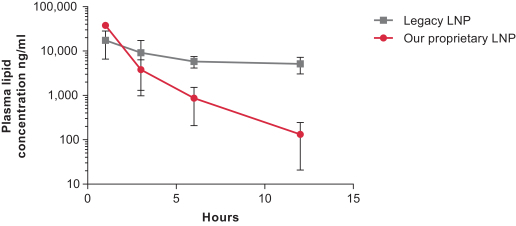
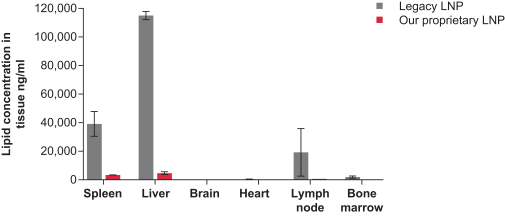
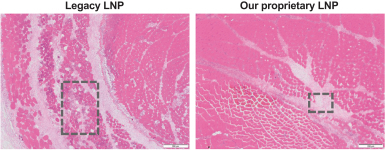
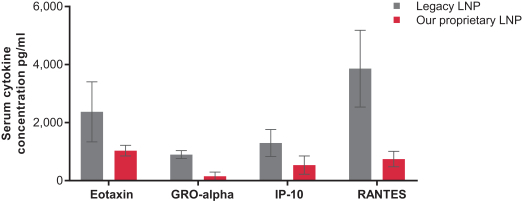
|
|
|
|
Panel (B)
|
Panel (C)
|
|
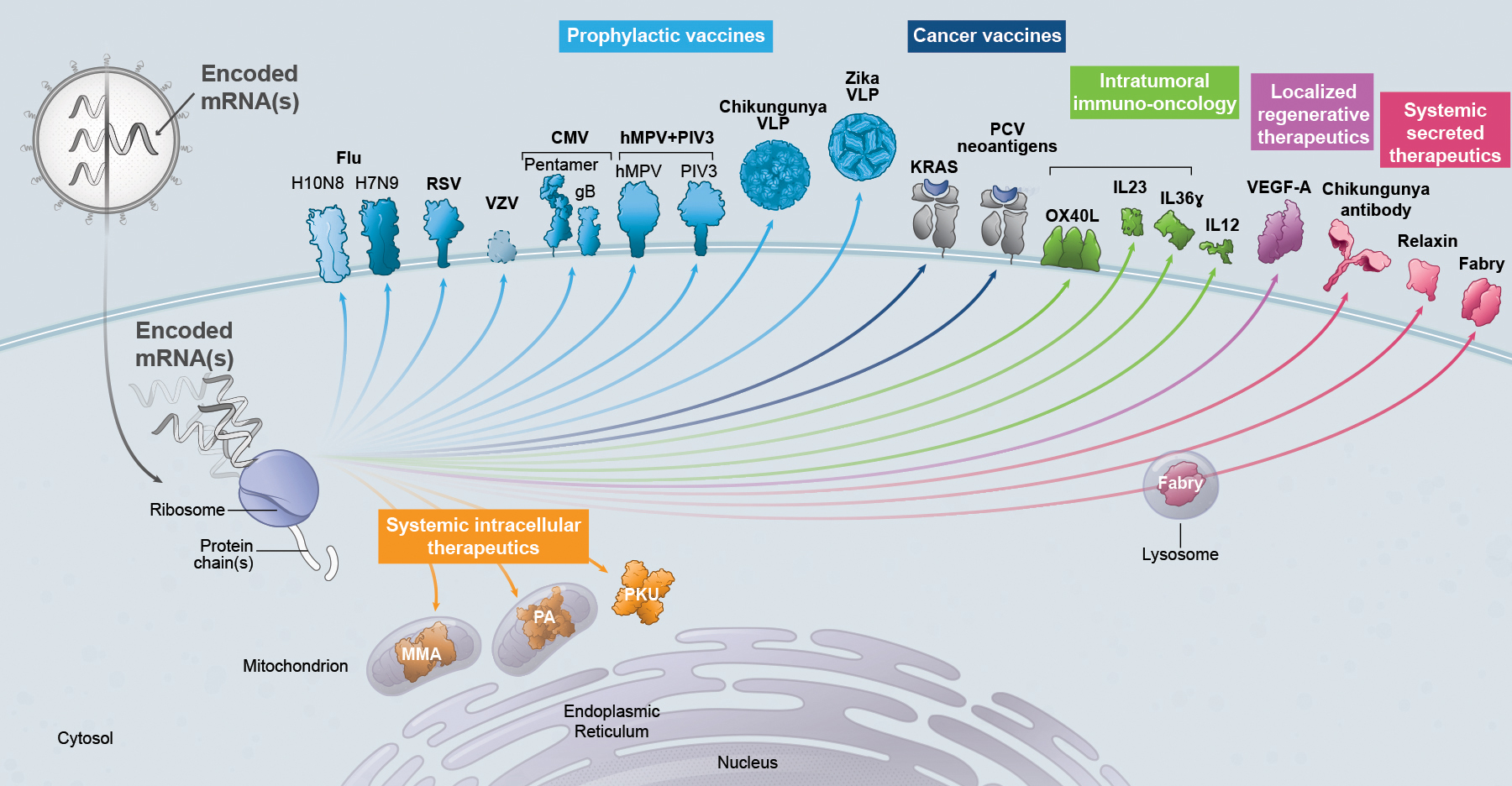
|
•
|
prophylactic vaccines;
|
|
•
|
cancer vaccines;
|
|
•
|
intratumoral immuno-oncology;
|
|
•
|
localized regenerative therapeutics;
|
|
•
|
systemic secreted therapeutics; and
|
|
•
|
systemic intracellular therapeutics.
|
|
•
|
We identify a
first program
(or programs) through which we seek to discover and develop solutions for any modality-specific technological challenges. We then leverage the learnings from this first program to the benefit of all subsequent programs in the modality.
|
|
•
|
We seek to
diversify biology risks
within the modality by advancing multiple programs in parallel, against multiple diseases, following the first program.
|
|
•
|
When we believe a strategic collaborator could significantly de-risk our early efforts in a new modality, we seek a
strategic collaborator to share the risks and benefits
on a specific set of early programs.
|
|
•
|
After experience with the first program (or programs) in a modality, we seek to
rapidly expand our pipeline
within that modality to take full advantage of the opportunity.
|
|
•
|
Prophylactic vaccines;
|
|
•
|
Cancer vaccines;
|
|
•
|
Intratumoral immuno-oncology;
|
|
•
|
Localized regenerative therapeutics;
|
|
•
|
Systemic secreted therapeutics; and
|
|
•
|
Systemic intracellular therapeutics.
|
|
•
|
Ability to mimic many aspects of natural viral infections.
mRNA enters cells and is used to produce viral antigen proteins from within the cell that include natural, post-translational modifications. This mimics the process by which natural viral infections occur, where information from viral genomes is used to produce viral proteins from within a cell. This can potentially enhance the immune response, including improved B and T cell responses.
|
|
•
|
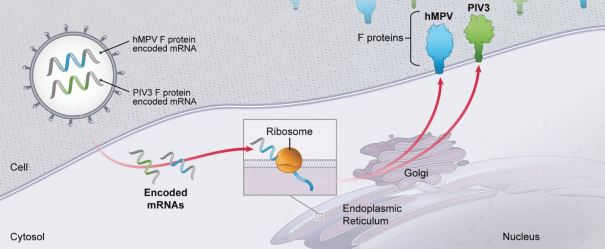
Multiplexing of mRNA for more compelling product profiles
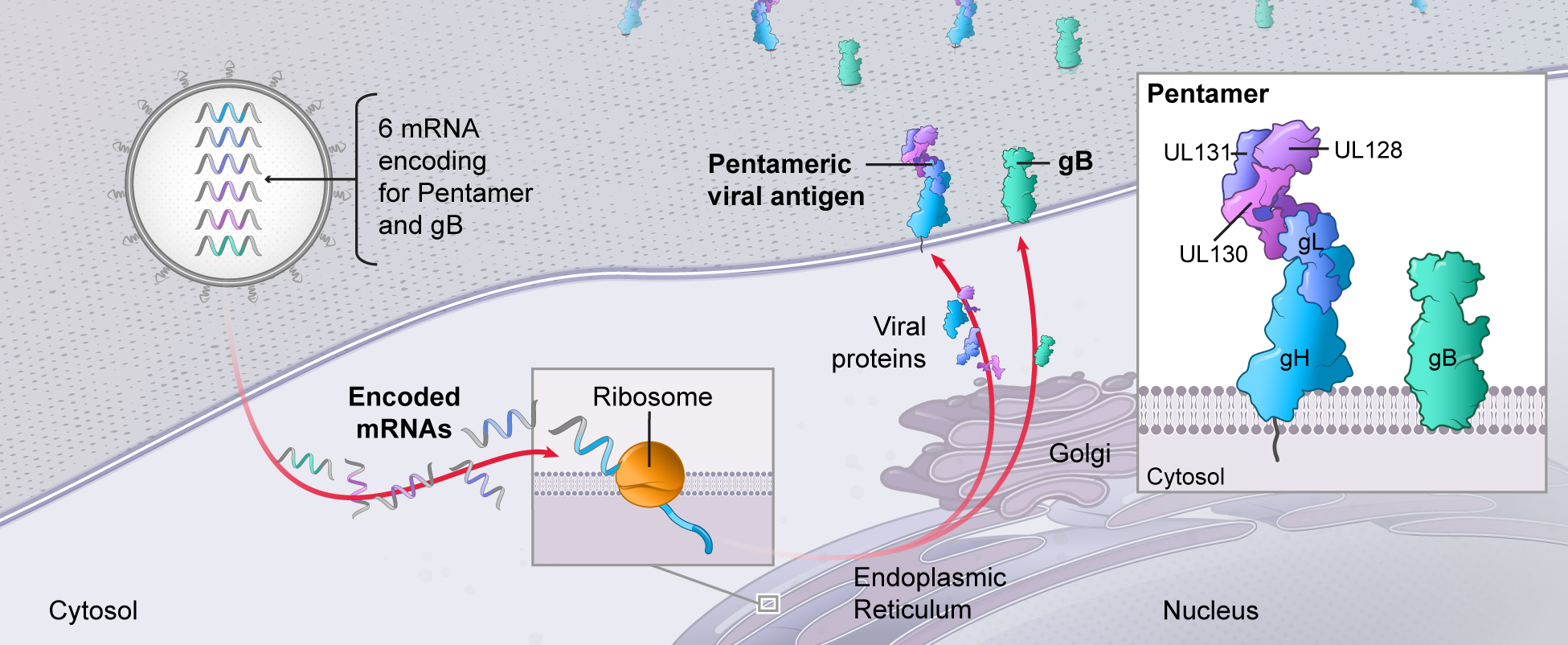
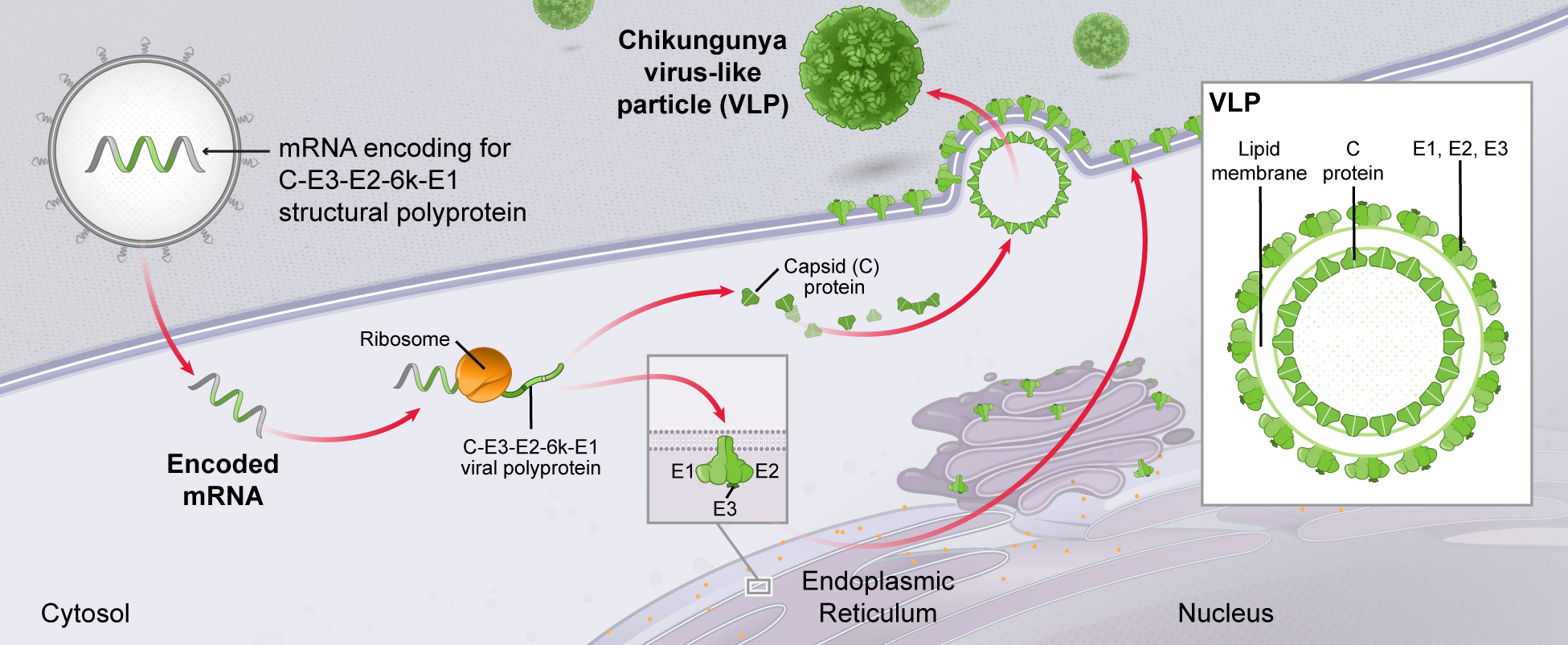
. Multiple mRNAs encoding for multiple viral proteins can be included in a single vaccine, permitting production of complex multimeric antigens that are much more difficult to achieve with traditional technologies. As an example, our CMV vaccine (mRNA-1647) contains six mRNAs, five of which encode five different proteins that combine to form a pentameric protein complex that is a potentially critical antigen for immune protection against CMV.
|
|
•
|
Rapid discovery and advancement of mRNA programs into the clinic
. Many viral antigens are known. However, with traditional vaccines, the target pathogens or antigens have to be produced in dedicated cell-cultures and/or fermentation-based manufacturing production processes in order to initiate testing of potential vaccine constructs. Our ability to design our antigens
in silico
allows us to rapidly produce and test antigens in preclinical models, which can dramatically accelerate our vaccine selection.
|
|
•
|
Capital efficiency and speed from shared manufacturing processes and infrastructure.
Traditional vaccines require product-dedicated production processes, facilities, and operators. Our mRNA vaccines are produced in a manufacturing process that is sufficiently consistent across our pipeline to allow us to use a single facility to produce all of our mRNA vaccines.
|
|
Prophylactic Vaccines Clinical Data Summary
|
||
|
Safety information
|
Immunogenicity information
|
|
|
Approximately 950 subjects dosed in Phase 1 trials at levels up to 300 µg.
|
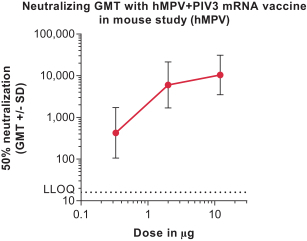
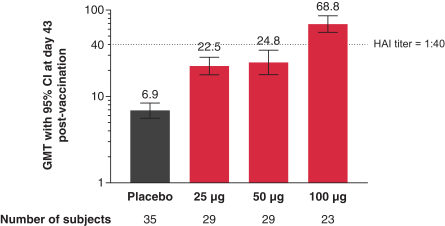
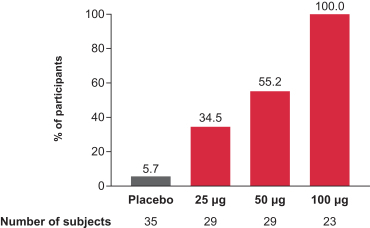
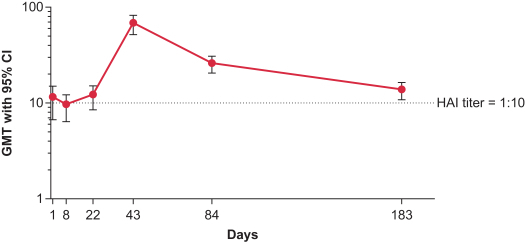
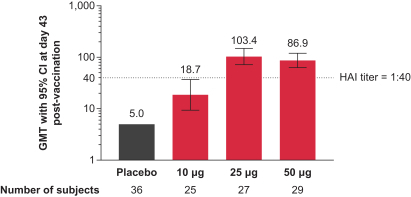
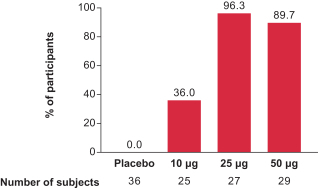
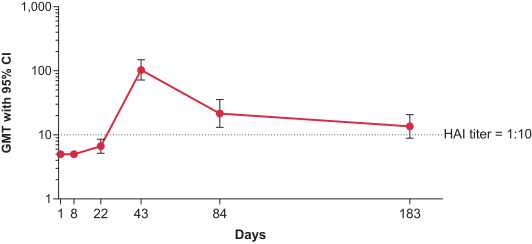
Interim Phase 1 data for our hMPV+PIV3 vaccine (mRNA-1653) showed boosted serum neutralization titers against hMPV and PIV3 at all dose levels tested; Merck has initiated plans for a Phase 2a clinical trial of our RSV vaccine (mRNA-1777); 100% seroresponse was observed for subjects at the 100 µg dose level for our Chikungunya vaccine (mRNA-1388); 96% of subjects at 25 µg achieved hemagglutination inhibition, or HAI, titer
>
1:40 for our H7 influenza vaccine (mRNA-1851); and 100% of subjects at 100 µg achieved HAI titer
>
1:40 for our H10 influenza vaccine (mRNA-1440).
|
|
|
•
|
mRNA vaccines can deliver multiple neoantigens concatenated in a single mRNA molecule
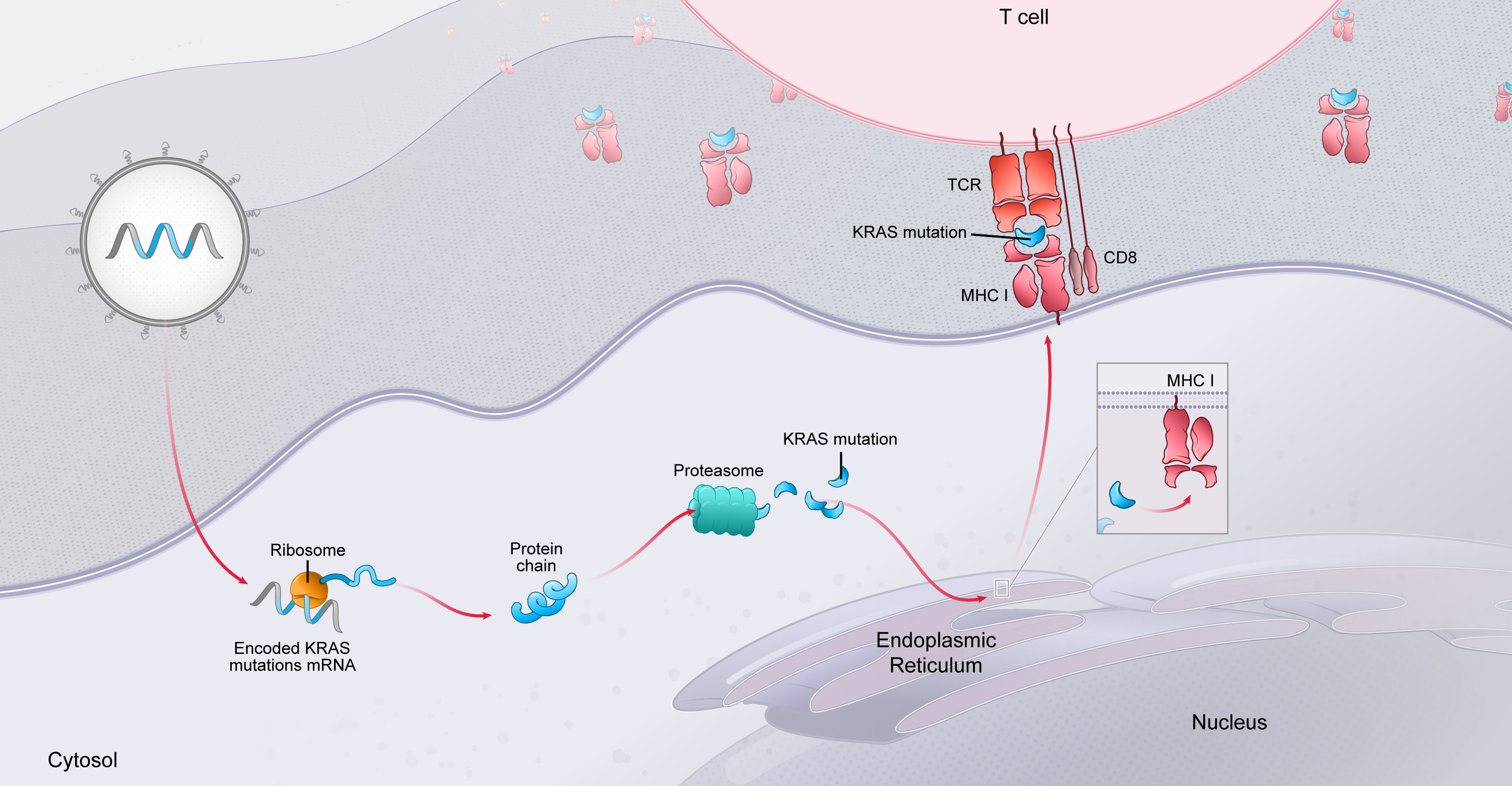
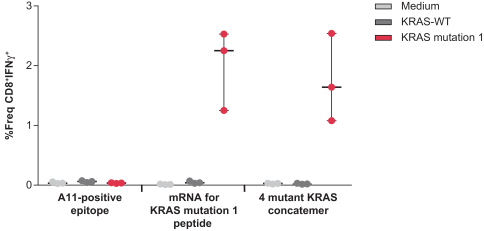
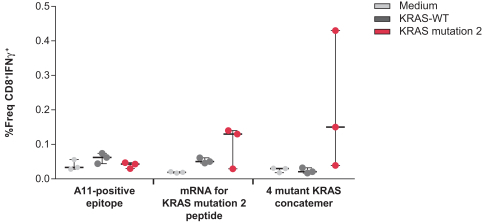
. We currently encode up to 34 neoantigens in one of our personalized cancer vaccines (mRNA-4157), and four KRAS mutations in our KRAS vaccine (mRNA-5671). Given that a T cell response against a single antigen has the potential to eradicate cancer cells, we believe that delivering multiple neoantigens could increase the probability of a successful treatment outcome for a patient.
|
|
•
|
mRNA encoding for neoantigens is translated and processed by patients’ endogenous cellular mechanisms for presentation to the immune system
. Neoantigen peptides are then potentially processed in multiple ways to give rise to different size peptides for presentation by the immune system. We believe this endogenous antigen production and presentation has the potential to drive a more effective immune response.
|
|
•
|
mRNA vaccines can be efficiently personalized.
The shared features of mRNA, combined with our investments in automated manufacturing technology, enable us to manufacture individual cGMP batches of personalized cancer vaccines rapidly, in parallel.
|
|
•
|
mRNA vaccines can be delivered simultaneously with customized immuno-stimulators.
In our KRAS vaccine mRNA-5671, mRNA encoding for KRAS neoantigens can be delivered in conjunction with mRNA encoding for an activated innate immuno-stimulator. The use of such innate immune stimulants has been shown to improve the T cell response against antigens of interest.
|
|
PCV (mRNA-4157) Clinical Data Summary
|
||
|
Safety information
|
Activity information
|
|
|
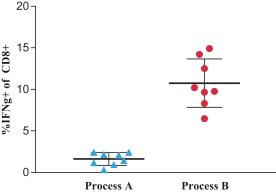
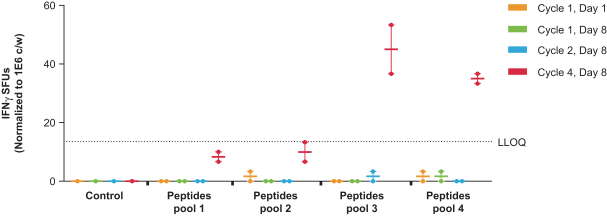
As of February 15, 2019, 33 patients have been dosed with mRNA-4157, including up to the 1 mg dose level. There have been no dose-limiting toxicities or significant related toxicities observed in these patients to date.
|
We have detected antigen-specific T cell responses for one patient at the 0.13 mg dose level in Part A of the Phase 1 clinical trial for PCV (mRNA-4157).
|
|
|
•
|
mRNA focuses and limits exposure of immune stimulatory proteins.
One of the intrinsic properties of mRNA is its transient nature. This allows for short exposure of the proteins encoded by the mRNA in the target tissue thereby enhancing tolerability.
|
|
•
|
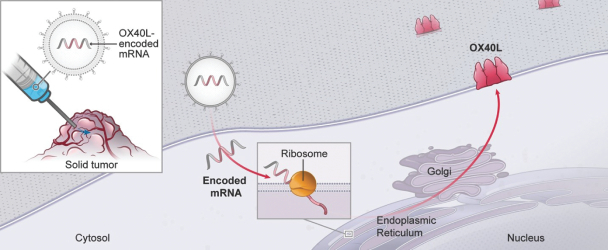
mRNA can produce membrane associated immune stimulatory proteins.
In contrast to recombinant proteins, mRNA administered to a tumor site can lead to the production of either secreted or membrane proteins, depending on the mRNA sequence.
|
|
•
|
Multiplexing of mRNA allows access to multiple immune stimulatory pathways
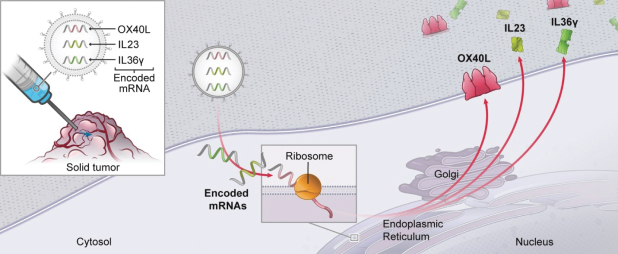
. The ability to combine multiple mRNAs to express multiple proteins allows for activation of several immune pathways simultaneously. For example, OX40L+ IL23+IL36γ (Triplet) (mRNA-2752) encodes for two secreted cytokines (IL23 and IL36γ) and one membrane protein (OX40L).
|
|
•
|
mRNA sequences can be engineered to reduce off-target effects.
Our mRNA can be designed to minimize translation in off-target tissues. For immune-stimulatory proteins this can potentially prevent toxicities.
|
|
•
|
Local administration of mRNA can create a concentration gradient for encoded proteins
. mRNA administered intratumorally allows for the local production of encoded immune-stimulatory proteins, such as cytokines. The mRNA and encoded protein are expected to form a concentration gradient that decreases as a function of the distance from the tumor, thereby potentially lowering undesirable systemic effects and increasing immune-stimulatory effects close to the tumor.
|
|
Intratumoral Immuno-oncology Clinical Data Summary
|
||
|
Safety information
|
Activity information
|
|
|
For OX40L, no safety findings observed that met study pause criteria; rapid onset of multiple grade 2 and one grade 3 transient reversible injection-related reactions were observed, all of which were resolved with standard interventions; three suspected unexpected serious adverse reactions, or SUSARs, were reported.
|
As of February 28, 2019, three patients in this Phase 1 study have been dosed and cleared with the first dose level of 0.25 mg of Triplet (mRNA-2752), and we are enrolling patients for the second 0.5 mg dose level.
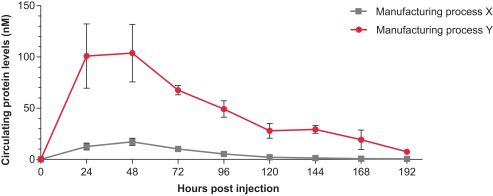
As of February 15, 2019, we have dosed a total of 33 patients with mRNA-2416, and we have not seen any dose limiting toxicities. The Phase 1/2 study is currently in its dose-confirmation phase at doses up to 8mg. As of October 22, 2018, two patients with ovarian cancer have demonstrated clinical observations of regression in certain injected lesions and in an adjacent uninjected lesion. These clinical observations from these two ovarian cancer patients do not meet partial response criteria as per the response evaluation criteria in solid tumors, or RECIST, guidelines version 1.1.
|
|
|
•
|
mRNA can be administered locally to produce the desired protein for an extended, but still limited, duration
. Local exposure to the therapeutic protein encoded by our mRNA is sustained by the ongoing translation of the mRNA into protein, often from hours to days. This pharmacokinetic profile closely mimics the optimal tissue exposure profile for regenerative applications and cannot be achieved by injections of recombinant proteins that rapidly diffuse out of the tissue after injection.
|
|
•
|
Local administration of mRNA allows for focused activity
. mRNA administered to a specific tissue or organ should allow for local production of the encoded protein, which could lead to lower levels of encoded protein in distant or systemic locations. This could help to prevent potential toxicity from production of the encoded protein outside of the targeted tissue.
|
|
•
|
mRNA allows for transient production of the encoded protein
. mRNA therapies should also allow for dose titration and repeat dosing. This provides several advantages over gene therapy. Gene therapy typically results in a permanent change to cellular DNA that may result in uncontrolled or constant production of the desired protein in local tissue or in distant sites, which could cause local or systemic side effects. Further, some gene therapy delivery vehicles are associated with immune responses that limit the ability to repeat dose, preventing dose titration.
|
|
Localized Regenerative Therapeutics Clinical Data Summary
|
||
|
Safety information
|
Activity information
|
|
|
Demonstrated sufficient tolerability in the Phase 1a/b trial at all dose levels (33 patients received AZD8601 for the Phase 1 trial) to warrant advancement to a Phase 2a study.
|
Increase in VEGF-A and bioactivity of VEGF-A protein was observed by increase in blood flow at injection sites up to seven days following a single dose of AZD8601.
|
|
|
•
|
Enzyme replacement therapies, or ERTs, for rare diseases;
|
|
•
|
Antibodies for membrane and extracellular soluble targets; and
|
|
•
|
Circulating modulation factors for common and rare diseases such as growth factors and insulin.
|
|
•
|
mRNA can produce hard-to-make or complex secreted proteins
. Some proteins, due to their folding requirements or complexity, are challenging to make using recombinant technologies, but can potentially be produced by human cells using administered mRNA.
|
|
•
|
Native post-translational modifications are possible through intracellular protein production using mRNA.
mRNA administered to a human cell uses natural secretory pathways inside the cell to make and process the encoded protein. The resulting post-translational modifications, such as glycosylation, are human. With recombinant proteins, these post-translational modifications are native to the non-human cells used for manufacture. These non-human post-translational modifications in recombinant proteins may lead to sub-optimal therapeutic outcomes, side effects, and increased immunogenicity.
|
|
•
|
mRNA can sustain production of proteins, which can increase exposure to proteins with short half-lives.
mRNA can lead to protein production by cells that can last from hours to days depending on design. This feature could increase the levels of short half-life proteins for therapeutic benefit.
|
|
•
|
mRNA allows for desirable pharmacology in rare genetic diseases currently addressed by enzyme replacement therapies.
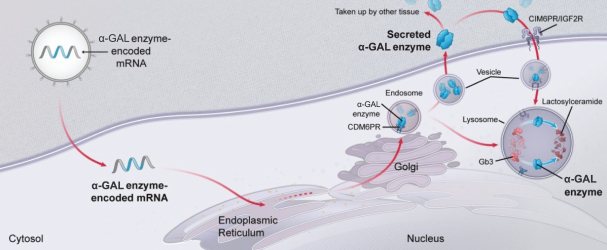
Our mRNA technology potentially permits several differentiated pharmacologic features for treating rare genetic diseases currently addressed by enzyme replacement therapies, including the ability to repeat dose as needed, lower immunogenicity of the replacement protein, the ability to adjust dose levels in real-time based on individual patient needs, and the ability to stop dosing. Gene therapies may also prove to be useful for treating rare genetic diseases; however, mRNA is not limited by pre-existing immunity that may exist for certain gene therapies using viral vectors, and does not localize to the nucleus or require persistent changes to cellular DNA to have the desired effect.
|
|
Systemic Secreted Therapeutics Clinical Data Summary
|
||
|
Safety information
|
Activity information
|
|
|
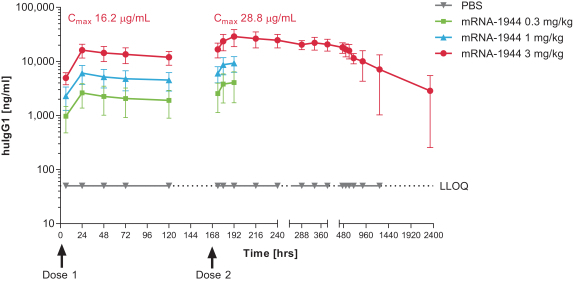
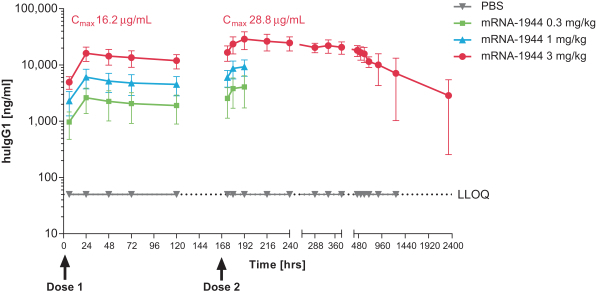
To date, we have not observed any dose-limiting toxicities in the Phase 1 trial of mRNA-1944.
|
As of February 20, 2019, dosing of the first dose level cohort has been completed (0.1 mg/kg, 8 subjects) in the Phase 1 trial of mRNA-1944.
|
|
|
•
|
intracellular pathway proteins;
|
|
•
|
soluble organelle-specific proteins; and
|
|
•
|
organelle-specific membrane proteins.
|
|
•
|
Using mRNA to encode for intracellular and organelle-specific proteins.
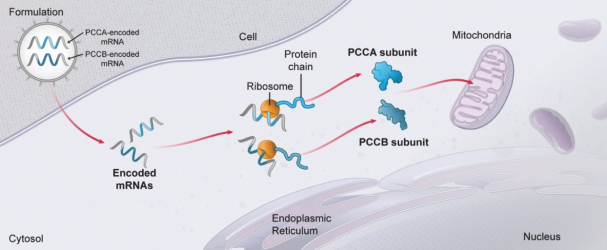
Our modality permits the expression of intracellular proteins, including those that must be directly translated and moved into organelles such as mitochondria. The ability of mRNA to produce protein inside of the cell enables production of these protein types that we believe are beyond the reach of recombinant proteins.
|
|
•
|
mRNA can produce hard-to-make or complex proteins.
For example, some proteins, due to their folding requirements or complexity, are challenging to make using recombinant technologies, but can potentially be produced by human cells using administered mRNA.
|
|
•
|
Native post-translational modifications are possible through intracellular protein production using mRNA.
mRNA administered to a human cell uses natural secretory pathways inside the cell to make and process the encoded protein. The resulting post-translational modifications, such as glycosylation, are human as opposed to recombinant proteins where these post-translational modifications are native to the non-human cells used for manufacture. These non-human post-translational modifications in recombinant proteins may lead to sub-optimal therapeutic outcomes, side effects and increased immunogenicity.
|
|
•
|
mRNA can sustain production of proteins, which can increase exposure to proteins with short half-lives
. mRNA can lead to protein production by cells that can last from hours to days depending on design. This feature could increase the levels of short half-life proteins for therapeutic benefit.
|
|
•
|
mRNA allows for desirable pharmacology in complex metabolic diseases.
Our mRNA technology potentially permits several differentiated pharmacologic features for treating complex metabolic diseases, including the ability to repeat dose as needed, a rapid onset of action, the ability to adjust dose levels real-time based on individual patient needs, and the ability to stop dosing. Gene therapies may also prove to be useful for treating rare genetic diseases; however, mRNA is not limited by pre-existing immunity that may exist for certain gene therapies using viral vectors, and does not localize to the nucleus or require persistent changes to cellular DNA to have the desired effect.
|
|
Systemic Intracellular Therapeutics Data Summary
|
||||
|
Safety information
|
Activity information
|
|||
|
Preclinical
|
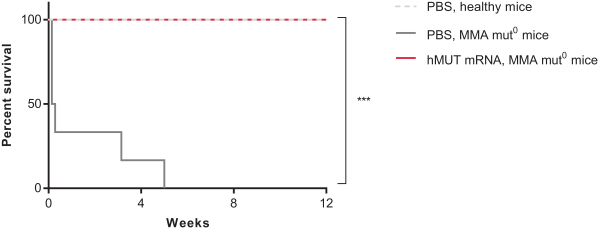
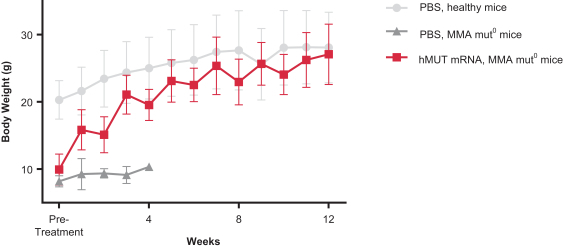
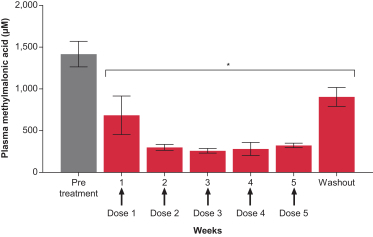
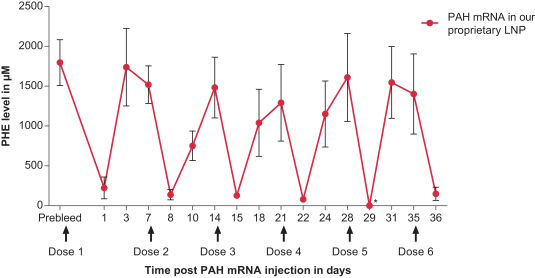
Successfully completed GLP toxicology program for MMA (mRNA-3704) that was included in the open IND to support advancement into the clinic; IND-enabling GLP toxicology programs for PKU (mRNA-3283) and PA (mRNA-3927) are ongoing.
|
Activity measured in animal models for MMA (mRNA-3704), PA (mRNA-3927), and PKU (mRNA-3283); data published for MMA (mRNA-3704).
|
||
|
Clinical
|
—
|
—
|
||

|
•
|
Extracellular soluble ligands (e.g., VEGF, IL12, Relaxin, and erythropoietin);
|
|
•
|
Antibodies (e.g., immunoglobulins, which are composed of two light chain and two heavy chain proteins);
|
|
•
|
Extracellular protein complexes (e.g., Chikungunya virus-like particles);
|
|
•
|
Membrane proteins, in some cases as multimers (e.g., F protein, glycoprotein B, CMV pentamer, and OX40L);
|
|
•
|
Intracellular soluble protein complexes (e.g., methylmalonic-CoA mutase homodimer and propionyl-CoA carboxylase heterododecamer);
|
|
•
|
Intracellular membrane proteins with activating mutations (e.g., STING); and
|
|
•
|
Neoantigens presented to the immune system as short peptides.
|
|
•
|
CMV vaccine (mRNA-1647) in the prophylactic vaccines modality to show the ability to make complex proteins;
|
|
•
|
KRAS vaccine (mRNA-5671) in the cancer vaccines modality to show KRAS neoantigen specific T cells;
|
|
•
|
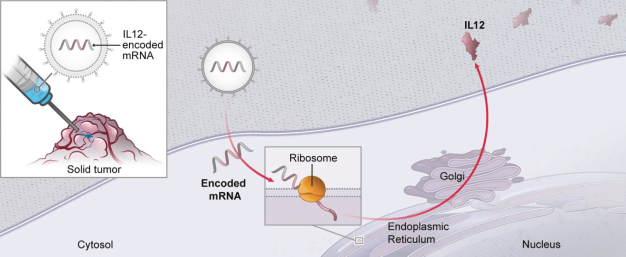
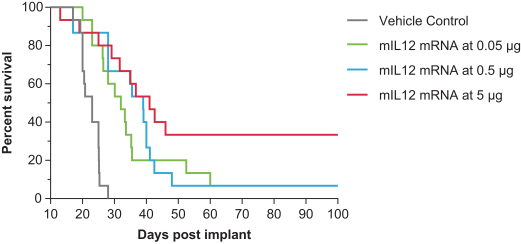
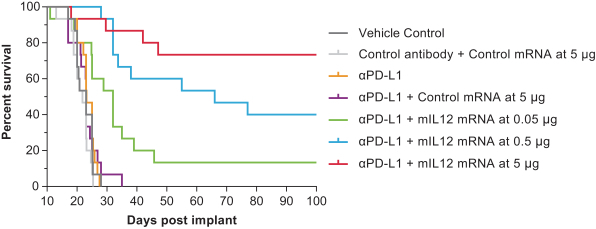
OX40L+IL23+IL36γ (mRNA-2752) and IL12 (MEDI1191) in the intratumoral immuno-oncology modality to show protein levels, although systemic levels may be limited due to the intratumoral nature of the injection;
|
|
•
|
Antibody against Chikungunya virus (mRNA-1944), Relaxin (AZD7970), and Fabry disease (mRNA-3630) in the systemic secreted protein modality to show serum protein levels; and
|
|
•
|
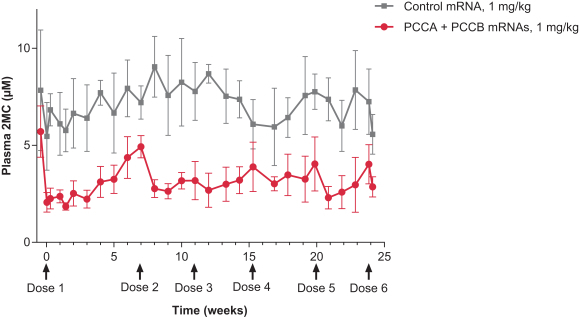
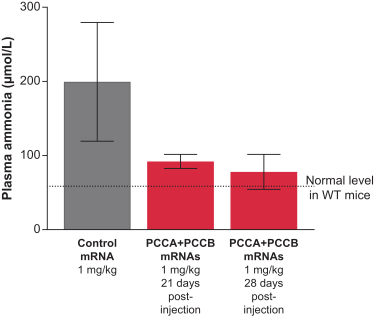
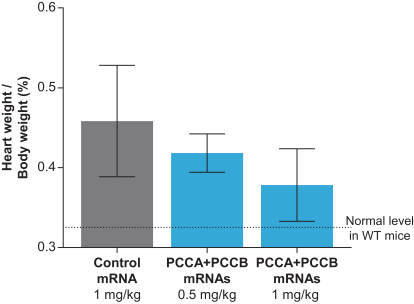
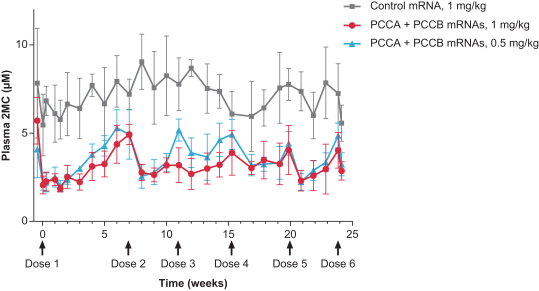
MMA (mRNA-3704), PA (mRNA-3927), and PKU (mRNA-3283) in the systemic intracellular protein modality to show serum changes in metabolites resulting from active protein in these metabolic pathways.
|
|
•
|
Generation of binding antibodies, where the antibodies generated by the vaccine bind to the pathogen antigens being targeted;
|
|
•
|
Generation of neutralizing antibodies, where the antibodies generated by the vaccine are able to prevent the pathogen from infecting cells;
|
|
•
|
Ability of the vaccine to protect vaccinated animals against a pathogen, as measured by reductions in detectable pathogen or by the survival of the challenged animal if the pathogen is lethal; and
|
|
•
|
Generation of an antigen specific T cell response.
|

|
•
|
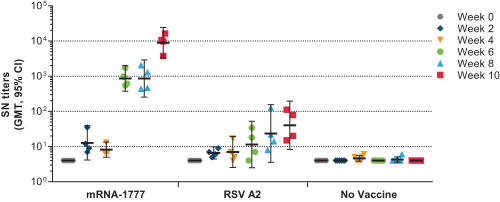
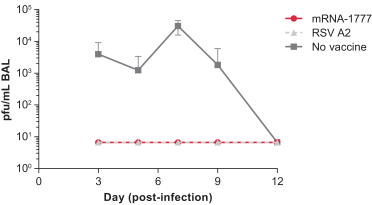
assess the safety and tolerability of mRNA-1777 versus placebo; and
|
|
•
|
determine the immunogenicity of mRNA-1777 by measuring serum neutralizing antibody titers against RSV.
|
|
•
|
direct, by vaccinating adolescents or adults of child-bearing potential (female and male); or
|
|
•
|
indirect, by vaccinating toddlers who could spread CMV to each other, their mothers, and their childcare workers.
|
|
•
|
In CMV seropositive individuals, the majority of neutralizing antibodies target the Pentamer. The CMV Pentamer is made by five CMV glycoproteins that form a membrane-bound complex. The Pentamer is required for CMV entry into epithelial, endothelial, and myeloid cells. The mRNA-expressed Pentamer is displayed on the surface of the cell and stimulates the production of neutralizing antibodies that prevent the virus from entering the cells.
|
|
•
|
gB is a trimeric CMV membrane glycoprotein that abundantly resides on the surface of the viral particles. Fusion between virus and host cells, and hence infection, requires gB. Antibodies to gB can prevent CMV infection. gB has been utilized in some earlier attempts at a CMV vaccine as the sole antigen which had resulted in partial efficacy but not at levels sufficient for approval.
|
|
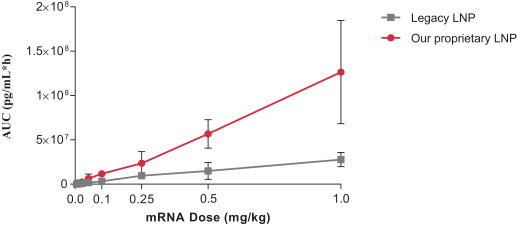
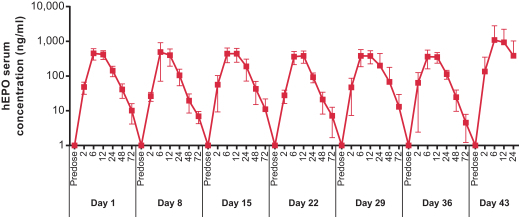
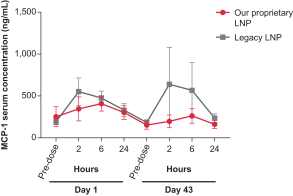
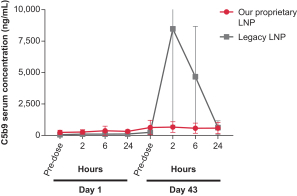
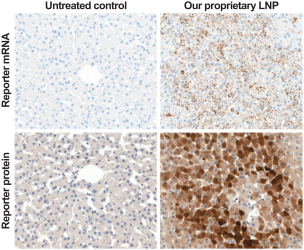
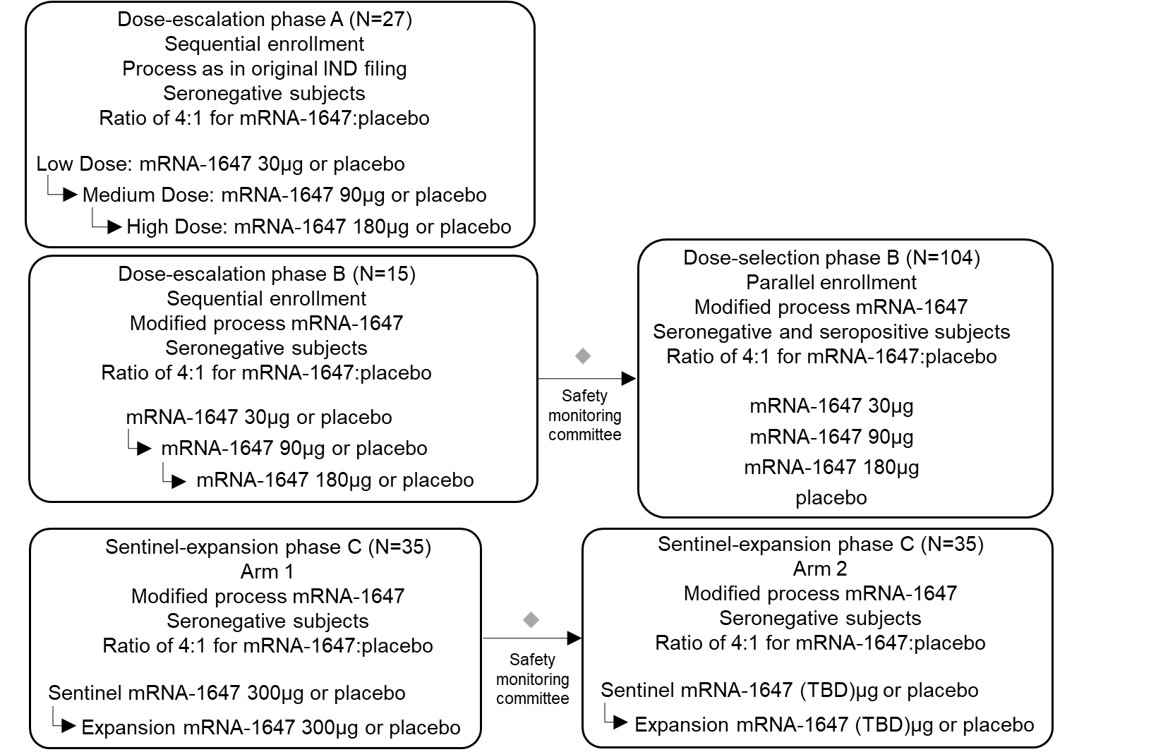
Dose for vaccine including the Pentamer and gB in our proprietary LNP
|
At 41 days
|
|
|
Neutralization titers in
epithelial cell |
||
|
1.2 µg
|
58,336
|
|
|
3.5 µg
|
682,989
|
|
|
10.5 µg
|
457,913
|
|
|
CytoGam comparator (used at maximum concentration of 2 mg/ml observed in human serum)
|
5,905
|
|
|
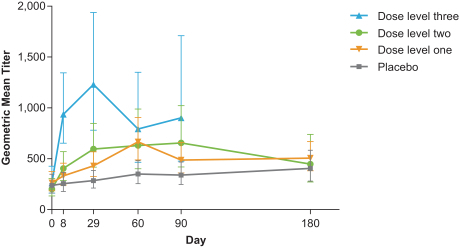
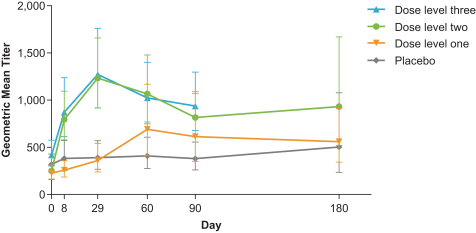
•
|
safety and reactogenicity of different dose levels of mRNA-1647; and
|
|
•
|
neutralizing anti-CMV antibody responses against epithelial cell and fibroblast cell infection following vaccination.
|
|

|
|
|
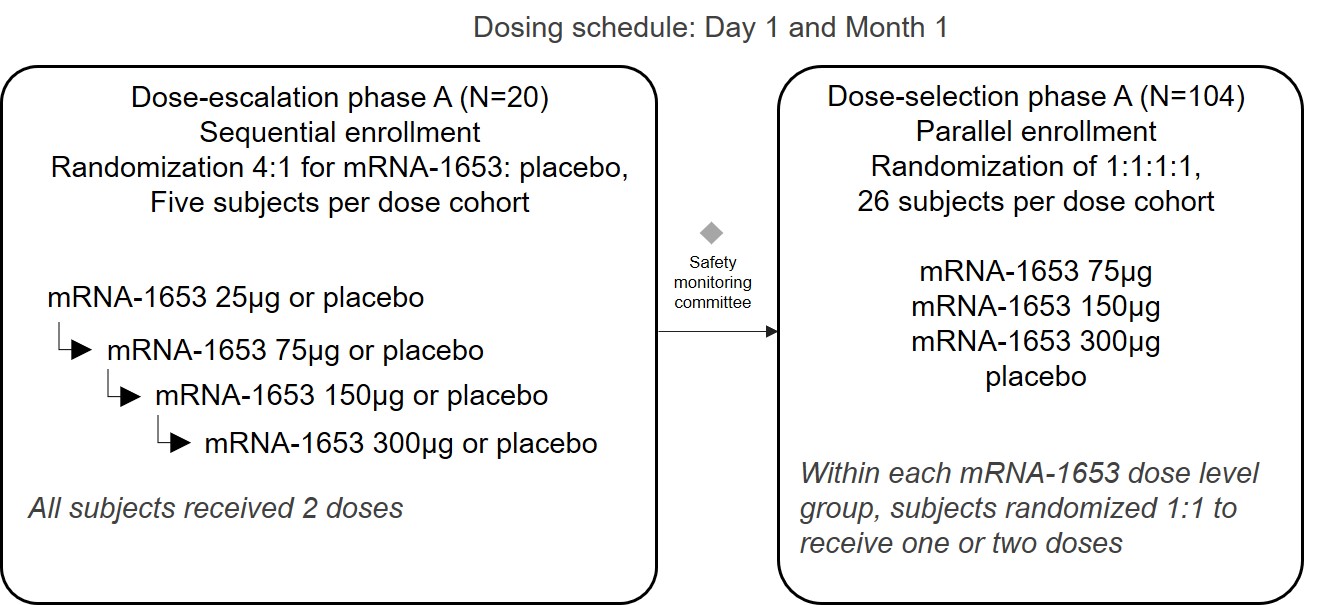
•
|
safety and reactogenicity of mRNA-1653 through 28 days after the last vaccination;
|
|
•
|
humoral immunogenicity of mRNA-1653 through 28 days after the last vaccination;
|
|
•
|
optimal dose and vaccination schedule of mRNA-1653 for further clinical development; and
|
|
•
|
safety of mRNA-1653 through 12 months after the second vaccination.
|
|
•
|
production of vaccines in eggs requires selection of vaccine-virus strains that can be grown in eggs and this strain may not always match the pandemic strain; and
|
|
•
|
growth of the virus in eggs has also been shown to induce structurally relevant mutations that can negatively impact vaccine potency.
|
|
•
|
short time period between strain selection and when the vaccine can be made available; this is enabled by intrinsic features of mRNA and the infrastructure we have built, allowing for shorter research and development and time to manufacture;
|
|
•
|
potential improved vaccine efficacy by avoidance of egg-based manufacture; this prevents the antigenic mismatch due to egg-adapted strains;
|
|
•
|
potential for improved efficacy by way of improved antigen presentation; an mRNA vaccine, upon administration to a cell, produces the antigen in its natural conformation; and
|
|
•
|
combination of multiple antigens into a single vaccine, allowing one to target multiple strains if needed; one of the intrinsic features of mRNA is the ability to utilize multiple mRNA sequences so that the cell produces multiple antigens at the same time.
|
|
25 µg
|
50 µg
|
100 µg
|
Placebo
|
||||
|
Dose 1
|
n=30
|
n=30
|
n=23
|
n=35
|
|||
|
Injection site pain
|
23, 76.7 (0)
|
25, 83.3 (0)
|
19, 82.6 (0)
|
2, 5.7 (0)
|
|||
|
Erythema
|
1, 3.3 (0)
|
0
|
3, 13.0 (0)
|
0
|
|||
|
Injection site swelling
|
2, 6.7 (0)
|
5, 16.7 (0)
|
3, 13.0 (0)
|
0
|
|||
|
Headache
|
5, 16.7 (0)
|
12, 40.0 (0)
|
7, 30.4 (0)
|
5, 14.3 (0)
|
|||
|
Fatigue
|
8, 26.7 (0)
|
13, 43.3 (0)
|
8, 34.8 (0)
|
7, 20.0 (0)
|
|||
|
Myalgia
|
16, 53.3 (0)
|
17, 56.7 (0)
|
12, 52.2 (0)
|
1, 2.9 (0)
|
|||
|
Arthralgia
|
0
|
2, 6.7 (0)
|
2, 8.7 (0)
|
1, 2.9 (0)
|
|||
|
Nausea
|
0
|
1, 3.3 (0)
|
1, 4.3 (0)
|
0
|
|||
|
Fever
|
1, 3.3 (0)
|
1, 3.3 (0)
|
2, 8.7 (0)
|
0
|
|||
|
Dose 2
|
n=28
|
n=29
|
n=23
|
n=27
|
|||
|
Injection site pain
|
22, 78.6 (0)
|
27 93.1 (0)
|
20, 87.0 (0)
|
3, 11.1 (0)
|
|||
|
Erythema
|
0
|
0
|
4, 17.4 (8.7)
|
0
|
|||
|
Injection site swelling
|
2, 7.1 (0)
|
4, 13.8 (0)
|
3, 13.0 (4.3)
|
0
|
|||
|
Headache
|
4, 14.3 (0)
|
14, 48.3 (0)
|
16, 69.6 (0)
|
6, 22.2 (3.7)
|
|||
|
Fatigue
|
8, 28.6 (0)
|
13, 44.8 (0)
|
11, 47.8 (0)
|
4, 14.8 (0)
|
|||
|
Myalgia
|
14, 50.0 (0)
|
17, 58.6 (0)
|
11, 47.8 (0)
|
1, 3.7 (0)
|
|||
|
Arthralgia
|
0
|
2, 6.9 (0)
|
7, 30.4 (0)
|
1, 3.7 (0)
|
|||
|
Nausea
|
1, 3.6 (0)
|
1, 3.4 (0)
|
3, 13.0 (0)
|
0
|
|||
|
Fever
|
1, 3.6 (0)
|
2, 6.9 (0)
|
4, 17.4 (0)
|
1, 3.7 (0)
|
|||
|
*
|
Data represent n, % with solicited AEs (% with severe solicited AEs) in the safety population; 75 µg dose group not shown (2 participants had severe solicited AEs of fatigue and injection site swelling following first vaccination, and no participants received dose 2); 400 µg dose group not shown.
|
|
10 µg
|
25 µg
|
50 µg
|
Placebo
|
||||
|
Dose 1
|
n=30
|
n=30
|
n=30
|
n=36
|
|||
|
Injection site pain
|
22, 73.3 (0)
|
17, 56.7 (0)
|
24, 80.0 (6.7)
|
5, 13.9 (0)
|
|||
|
Erythema
|
0
|
0
|
0
|
0
|
|||
|
Injection site swelling
|
5, 16.7 (0)
|
5, 16.7 (0)
|
9, 30.0 (0)
|
2, 5.6 (0)
|
|||
|
Headache
|
5, 16.7 (0)
|
5, 16.7 (0)
|
7, 23.3 (6.7)
|
6, 16.7 (0)
|
|||
|
Fatigue
|
1, 3.3 (0)
|
4, 13.3 (0)
|
3, 10.0 (0)
|
2, 5.6 (0)
|
|||
|
Myalgia
|
3, 10.0 (0)
|
6, 20.0 (0)
|
8, 26.7 (0)
|
6, 16.7 (0)
|
|||
|
Arthralgia
|
2, 6.7 (0)
|
3, 10.0 (0)
|
3, 10.0 (0)
|
4, 11.1 (0)
|
|||
|
Nausea
|
1, 3.3 (0)
|
1, 3.3 (0)
|
1, 3.3 (0)
|
1, 2.8 (0)
|
|||
|
Fever
|
0
|
1, 3.3 (0)
|
0
|
0
|
|||
|
Dose 2
|
n=30
|
n=30
|
n=30
|
n=36
|
|||
|
Injection site pain
|
14, 46.7 (0)
|
13, 43.3 (0)
|
22,
73.3 (10.0)
|
2, 5.6 (0)
|
|||
|
Erythema
|
0
|
0
|
0
|
0
|
|||
|
Injection site swelling
|
3, 10.0 (0)
|
6, 20.0 (0)
|
6, 20.0 (0)
|
1, 2.8 (0)
|
|||
|
Headache
|
3, 10.0 (0)
|
2, 6.7 (3.3)
|
8, 26.7 (6.7)
|
1, 2.8 (0)
|
|||
|
Fatigue
|
1, 3.3 (0)
|
3, 10.0 (0)
|
4, 13.3 (0)
|
0
|
|||
|
Myalgia
|
3, 10.0 (0)
|
4, 13.3 (0)
|
8, 26.7 (3.3)
|
0
|
|||
|
Arthralgia
|
2, 6.7 (0)
|
1, 3.3 (0)
|
6, 20.0 (3.3)
|
0
|
|||
|
Nausea
|
0
|
0
|
1, 3.3 (0)
|
0
|
|||
|
Fever
|
0
|
0
|
6, 20.0 (6.7)
|
0
|
|||
|
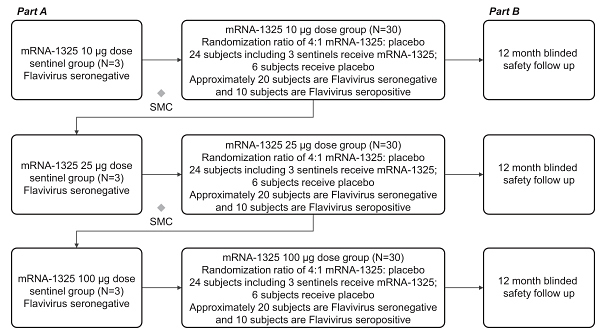
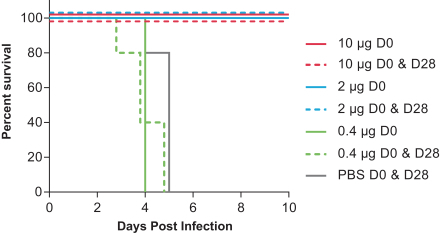
•
|
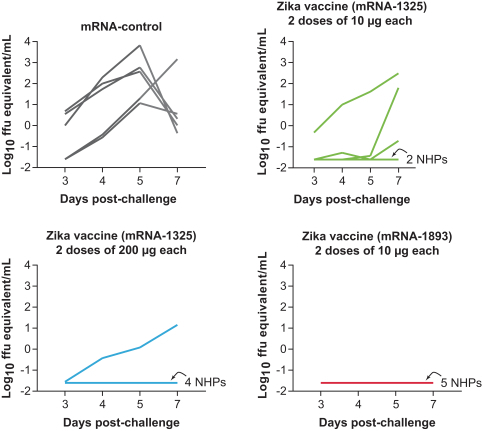
assess the safety of a 2-dose vaccination schedule of mRNA-1325 Zika vaccine, given 28 days apart, across a range of dose levels in flavivirus seronegative and flavivirus seropositive subjects compared with placebo; and
|
|
•
|
assess the immunogenicity of a range of doses of mRNA-1325 Zika vaccine.
|
|
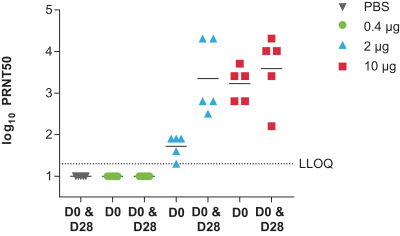
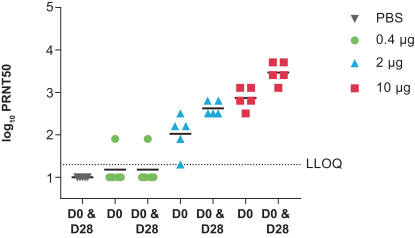
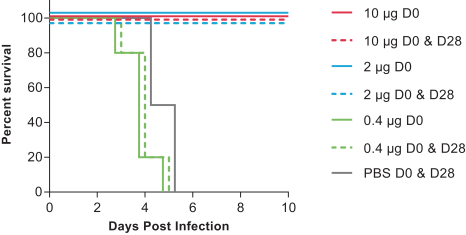
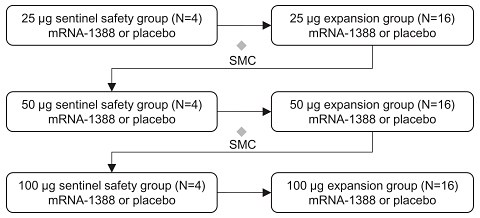
•
|
describe the safety and tolerability of mRNA-1388 relative to placebo;
|
|
•
|
determine the immunogenicity of three dose levels of mRNA-1388 to inform the choice of dose for further development of this vaccine; and
|
|
•
|
assess immunogenicity changes from baseline using serum neutralizing antibody titers to Chikungunya virus and binding antibody titers to Chikungunya-specific proteins.
|
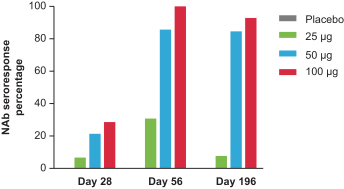
|
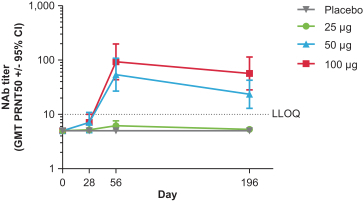
Number of subjects per-protocol subset for clinical data at cut-off date
|
||||||||
|
Cohort
|
Day 0
|
Day 28
|
Day 56
|
Day 196
|
||||
|
25 µg
|
15
|
15
|
13
|
13
|
||||
|
50 µg
|
15
|
14
|
14
|
13
|
||||
|
100 µg
|
14
|
14
|
14
|
14
|
||||
|
Placebo
|
14
|
14
|
13
|
13
|
||||
|
25 µg
|
50 µg
|
100 µg
|
Placebo
|
||||
|
% (No. with severe AE)
|
|||||||
|
Dose 1
|
N=15
|
N=15
|
N=15
|
N=15
|
|||
|
Pain
|
60.0 (0)
|
57.1 (0)
|
73.3 (0)
|
33.3 (0)
|
|||
|
Tenderness
|
66.7 (0)
|
78.6 (0)
|
80.0 (0)
|
40.0 (0)
|
|||
|
Erythema
|
0
|
0
|
0
|
0
|
|||
|
Induration
|
0
|
0
|
0
|
0
|
|||
|
Headache
|
13.3 (0)
|
21.4 (0)
|
20.0 (0)
|
0
|
|||
|
Fatigue
|
0
|
7.1 (0)
|
6.7 (0)
|
6.7 (0)
|
|||
|
Myalgia
|
13.3 (0)
|
14.3 (0)
|
20.0 (0)
|
0
|
|||
|
Arthralgia
|
0
|
0
|
0
|
6.7 (0)
|
|||
|
Nausea
|
0
|
14.3 (0)
|
6.7 (0)
|
0
|
|||
|
Fever
|
0
|
0
|
0
|
0
|
|||
|
Dose 2
|
N=15
|
N=14
|
N=14
|
N=15
|
|||
|
Pain
|
46.7 (0)
|
61.5 (0)
|
85.7 (0)
|
6.7 (0)
|
|||
|
Tenderness
|
53.3 (0)
|
76.9 (0)
|
78.6 (1)
|
6.7 (0)
|
|||
|
Erythema
|
0
|
7.7 (0)
|
7.1 (1)
|
0
|
|||
|
Induration
|
0
|
7.7 (0)
|
0
|
0
|
|||
|
Headache
|
20.0 (0)
|
7.7 (0)
|
28.6 (0)
|
13.3 (0)
|
|||
|
Fatigue
|
6.7 (0)
|
15.4 (0)
|
28.6 (0)
|
13.3 (0)
|
|||
|
Myalgia
|
0
|
7.7 (0)
|
28.6 (0)
|
0
|
|||
|
Arthralgia
|
0
|
0
|
21.4 (0)
|
0
|
|||
|
Nausea
|
0
|
0
|
21.4 (0)
|
0
|
|||
|
Fever
|
0
|
0
|
7.1 (0)
|
13.3 (0)
|
|||
|
25 µg
|
50 µg
|
100 µg
|
Placebo
|
||||
|
No., % of subjects
|
|||||||
|
Dose 1
|
N=15
|
N=15
|
N=15
|
N=15
|
|||
|
Any AE
|
4 (26.7)
|
6 (40.0)
|
3 (20.0)
|
8 (53.3)
|
|||
|
Any related AEs
|
0
|
1 (6.7)
|
1 (6.7)
|
2 (13.3)
|
|||
|
Any grade 3+
|
0
|
2 (13.3)
|
0
|
1 (6.7)
|
|||
|
Any related grade 3+
|
0
|
0
|
0
|
0
|
|||
|
Any MA-AEs
|
1 (6.7)
|
0
|
0
|
0
|
|||
|
SAEs
|
0
|
0
|
0
|
0
|
|||
|
Any related SAE
|
0
|
0
|
0
|
0
|
|||
|
AESIs
|
0
|
0
|
0
|
0
|
|||
|
Dose 2
|
N=15
|
N=14
|
N=14
|
N=15
|
|||
|
Any AE
|
3 (20.0)
|
3 (21.4)
|
6 (42.9)
|
6 (40.0)
|
|||
|
Any related AEs
|
0
|
0
|
4 (28.6)
|
0
|
|||
|
Any grade 3+
|
1 (6.7)
|
2 (14.3)
|
4 (28.6)
|
0
|
|||
|
Any related grade 3+
|
0
|
0
|
3 (21.4)
|
0
|
|||
|
Any MA-AEs
|
0
|
0
|
0
|
1 (6.7)
|
|||
|
SAEs
|
0
|
0
|
1 (7.1)
|
0
|
|||
|
Any related SAE
|
0
|
0
|
1 (7.1)
|
0
|
|||
|
AESIs
|
0
|
0
|
0
|
0
|
|||
|
•
|
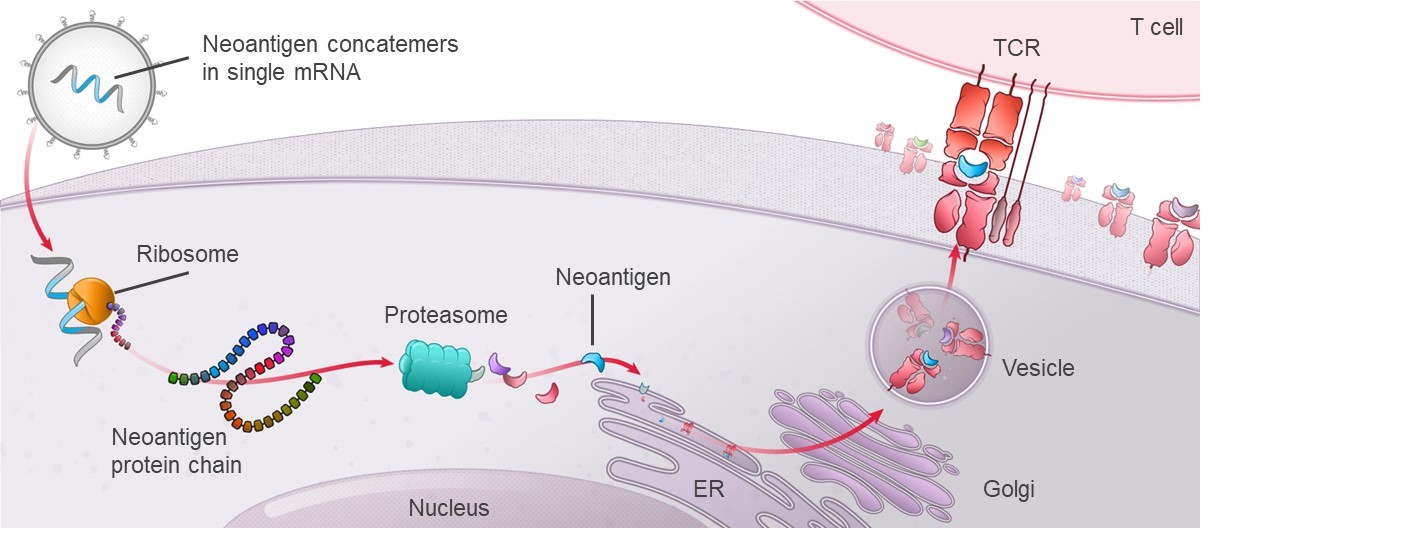
mRNA vaccines can deliver multiple unique and personalized neoantigens in a single mRNA molecule;
|
|
•
|
mRNA vaccines unique to each particular patient can be rapidly designed
in silico
and manufactured with automation in personalized, individual cGMP batches; and
|
|
•
|
mRNA encoding for neoantigens is translated and processed by patients’ endogenous cellular processing and presentation to the immune system.
|
|
1.
|
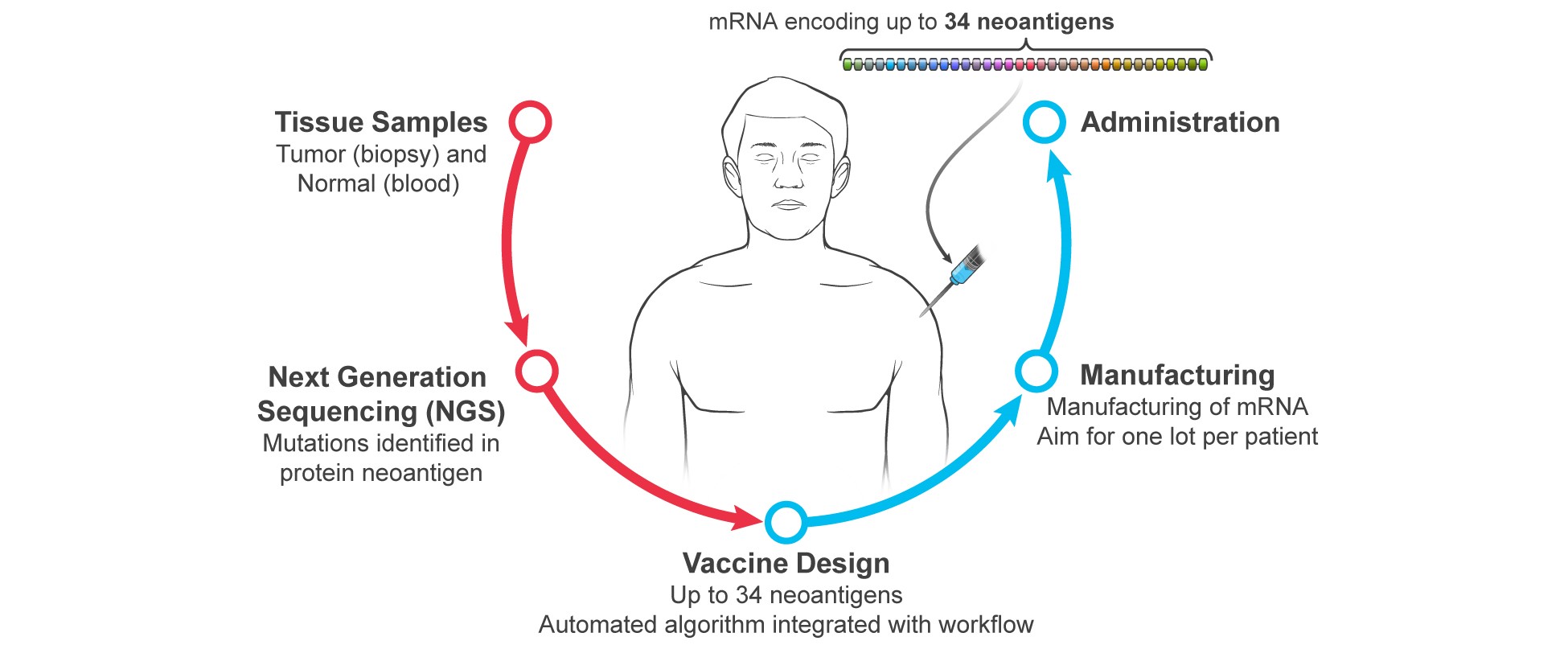
Tumor sample;
|
|
2.
|
Next generation sequencing, or NGS, of tumor DNA and RNA;
|
|
3.
|
Vaccine design using our proprietary bioinformatics algorithm for up to 34 patient-specific neoantigens;
|
|
4.
|
Manufacture of the designed mRNA; and
|
|
5.
|
Administration of the mRNA to the same patient that provided the tumor sample.
|
|
•
|
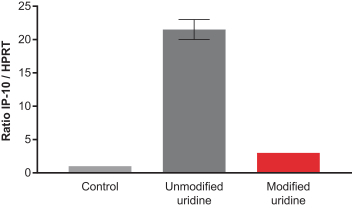
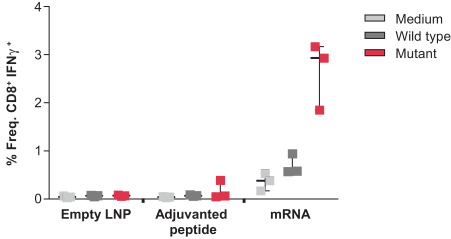
Specific and robust T cell responses to murine neoantigens were observed by vaccinating mice with mRNA vaccines that encode previously published immunogenic epitopes from the MC38 mouse tumor cell line and measuring T cell responses to mutant but not wild type antigens. The responses to mRNA vaccination were also significantly higher than responses to the adjuvanted peptide as per a study we conducted. In this study, mice were vaccinated with either empty LNP, adjuvanted peptides corresponding to previously published data or mRNAs encoding the same neoantigen sequences formulated in LNPs. Mice were vaccinated on day 1, 8, and 15 and T cell responses were measured on day 18 using flow cytometry by re-stimulating splenocytes with either control (medium), wild type or mutant (neoantigens) peptides. In an ideal case, one would see a high T cell response when re-stimulated with mutant neoantigen and would not see an equivalent response for re-stimulation with media and wild type peptide. We believe this would indicate a clear specific response for mutant neoantigens with no response to self. As shown in the figure below, the T cell response by mRNA encoding for neoantigens was much higher than that for peptides. The T cell response for mRNA vaccine re-stimulated with wild type was higher than baseline and close to that with control (medium). The T cell responses for mutant peptide were significantly higher than those against wild type peptide.
|
|
•
|
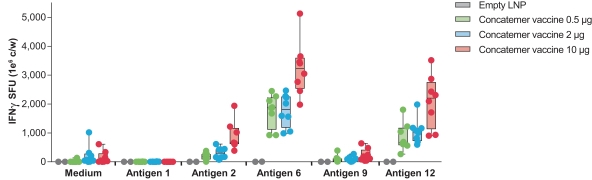
Specific and robust T cell responses to multiple antigens encoded in a single mRNA sequence. The T cell response after vaccinating mice with mRNA vaccine encoding for 16 specific antigens previously reported to be immunogenic in mice as shown in the figure below. mRNA was formulated in a proprietary LNP and delivered intramuscularly to mice on day 1 and day 8. T cell responses were measured on day 15 by re-stimulating splenocytes with either control (medium) or peptides corresponding to each antigen (1, 2, 6, 9, and 12) in the mRNA vaccine and measured by interferon gamma. Measurements are in spot forming units, or SFU, per 1 million cells per well.
|
|
•
|
An mRNA concatemer encoding distinct class I (antigens 6, 9, and 12) and class II antigens (antigen 2) can elicit specific T cell responses to each antigen as shown in the figure below.
|
|
•
|
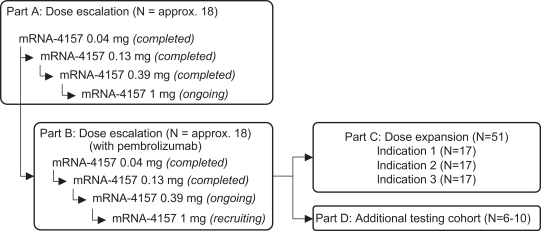
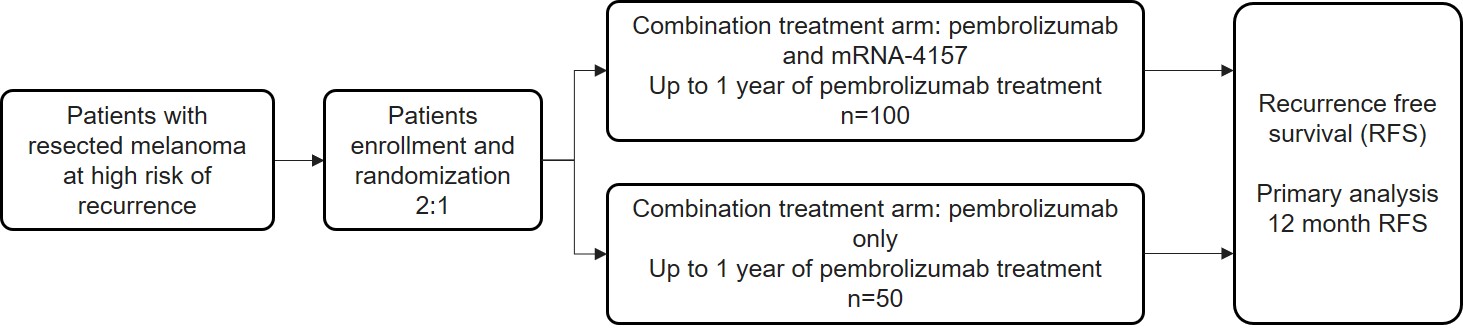
for Part A—To determine the safety and tolerability of mRNA-4157 monotherapy in subjects with resected solid tumors and to assess the immunogenicity of mRNA-4157;
|
|
•
|
for Parts B, C and D—To determine the safety, tolerability, and recommended Phase 2 dose of mRNA-4157 administered in combination with pembrolizumab; and
|
|
•
|
for Part D—To assess the immunogenicity of mRNA-4157 with pembrolizumab from apheresis samples in certain subjects.
|
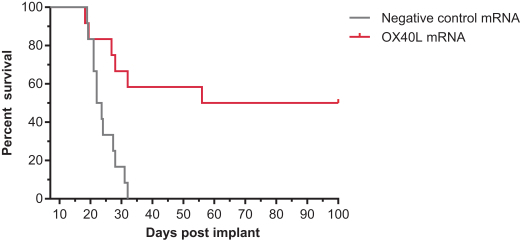
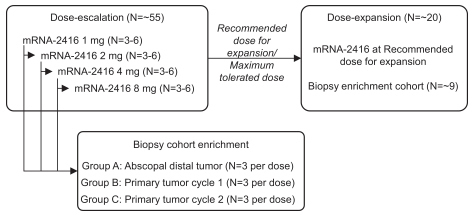
|
A.
|
Baseline biopsy in abscopal distal, untreated tumor, second biopsy within cycle 1 at day 22 to 28 at distal tumor
|
|
B.
|
Baseline biopsy in primary tumor to be treated, second biopsy 24 to 48 hours post-dose cycle 1 day 1 in injected tumor
|
|
C.
|
Baseline biopsy in primary tumor to be treated, second biopsy 24 to 48 hours post-dose cycle 2 day 1 in injected tumor
|
|
|
|
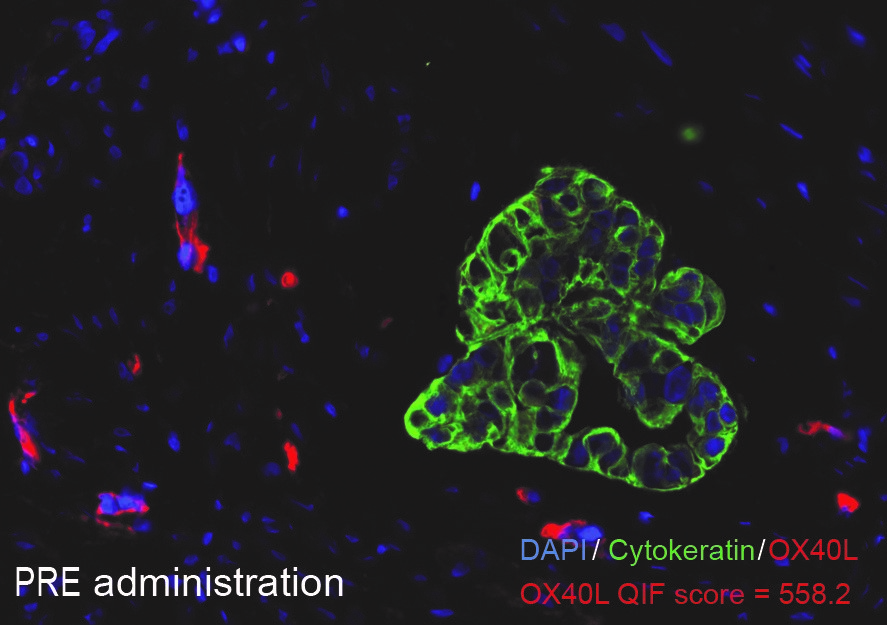
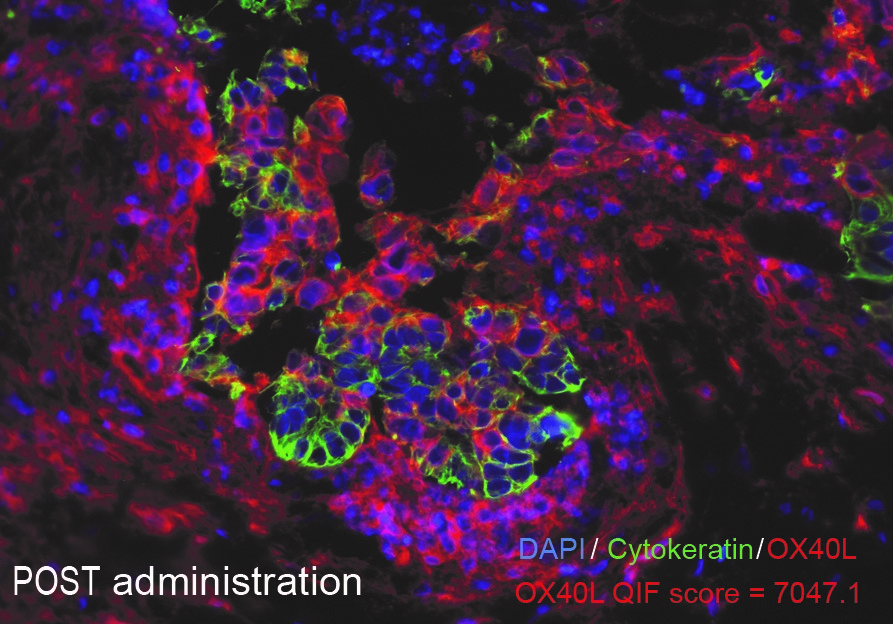
|
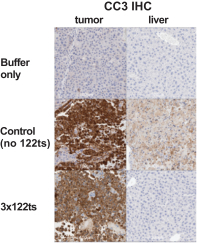
Before treatment with mRNA-2416
|
After treatment with mRNA-2416
|
|
|
•
|
T cell co-stimulation that could strengthen specific anti-cancer adaptive immune responses (mediated by OX40L); and
|
|
•
|
pro-inflammatory cytokines/chemokines to ignite or transform an inflammatory response within the tumor microenvironment (IL23 and IL36γ).
|
|
•
|
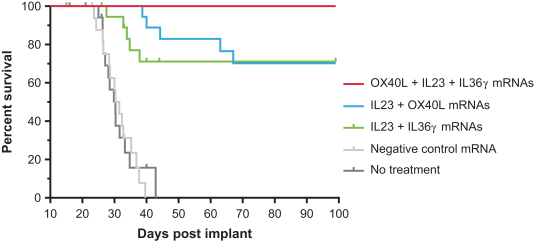
safety and tolerability of Triplet (mRNA-2752) administered alone and in combination with checkpoint inhibitors;
|
|
•
|
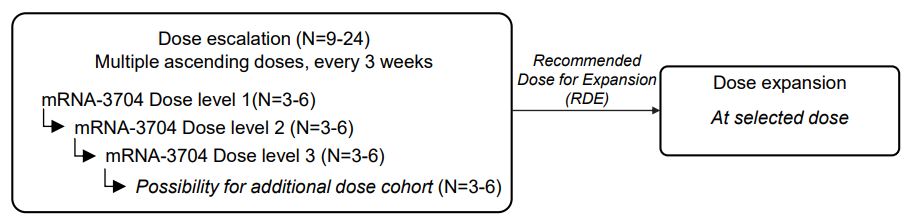
define the maximum tolerated dose, or MTD, and recommended dose for expansion, or RDE, for intratumoral injections of Triplet (mRNA-2752) alone and in combination with checkpoint inhibitors; and
|
|
•
|
assessment of anti-tumor activity, protein expression in tumors, and pharmacokinetics, and exploratory endpoints that include assessment of immunological responses.
|
|
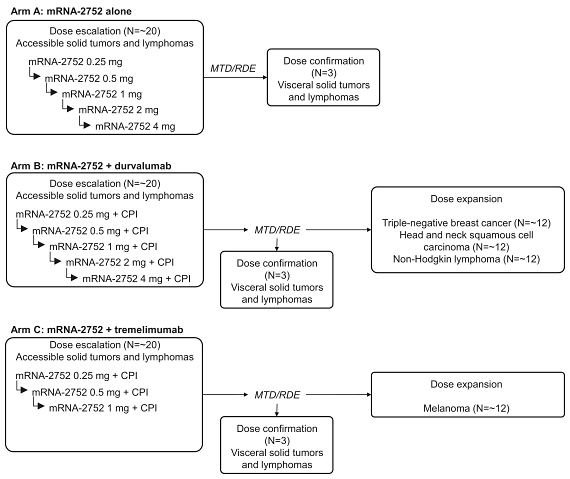
•
|
arm A—Triplet (mRNA-2752) alone;
|
|
•
|
arm B—Triplet (mRNA-2752) in combination with durvalumab, a PD-L1 inhibitor; and
|
|
•
|
arm C—Triplet (mRNA-2752) in combination with tremelimumab, a CTLA-4 inhibitor.
|
|
LVEF, %
|
p-value
|
||
|
Control—Citrate saline
|
47.0 ± 0.8
|
||
|
AZD8601 1 mg dose
|
51.0 ± 0.9
|
<0.01
|
|
|
AZD8601 10 mg dose
|
52.0 ± 1.0
|
<0.01
|
|
|
Part A (n = 27)
|
Part B (n = 15)
|
||||
|
Placebo
only (1) (n = 9) |
VEGF-A mRNA/
placebo (1) (n = 18) |
VEGF-A mRNA/
placebo (2) (n = 15) |
|||
|
Participants with any AE, n (%)
|
5 (55.6)
|
18 (100.0)
|
14 (93.3)
|
||
|
Causally treatment-related, n (%)
|
0
|
18 (100.0)
|
14 (93.3)
|
||
|
Treatment-unrelated, n (%)
|
5 (55.6)
|
0
|
0
|
||
|
Participants with causally treatment-related AEs, n (%)
|
|||||
|
Injection-site reaction [mild]
|
0
|
18 (100.0)
|
14 (93.3)
|
||
|
Participants with treatment-unrelated AEs, n (%)
|
|||||
|
Injection-site reaction [mild]
|
1 (11.1)
|
0
|
0
|
||
|
Injection-site erythema [mild]
|
1 (11.1)
|
2 (11.1)
|
0
|
||
|
Asthenia [mild]
|
0
|
1 (5.6)
|
0
|
||
|
Tinea pedis [mild]
|
0
|
0
|
1 (6.7)
|
||
|
Arthropod bite [mild]
|
0
|
1 (5.6)
|
1 (6.7)
|
||
|
Injury [moderate]
|
0
|
1 (5.6)
|
0
|
||
|
Skin abrasion [mild]
|
0
|
1 (5.6)
|
0
|
||
|
Muscle spasms [mild]
|
0
|
1 (5.6)
|
0
|
||
|
Back pain [mild or moderate]
|
2 (22.2)
|
0
|
0
|
||
|
Myalgia [moderate]
|
0
|
0
|
1 (6.7)
|
||
|
Dizziness [mild]
|
0
|
1 (5.6)
|
0
|
||
|
Headache [mild]
|
1 (11.1)
|
0
|
0
|
||
|
Pruritus [mild]
|
0
|
1 (5.6)
|
0
|
||
|
Tooth extraction [mild]
|
0
|
1 (5.6)
|
0
|
||
|
Nasopharyngitis [moderate]
|
1 (11.1)
|
0
|
0
|
||
|
(1)
|
There are two injection sites and it can be either VEGF-A mRNA/placebo, placebo/VEGF-A mRNA, or placebo/placebo at injection sites 1/2.
|
|
(2)
|
Randomized order of VEGF-A and placebo injections.
|
|
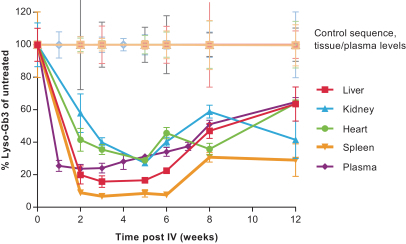
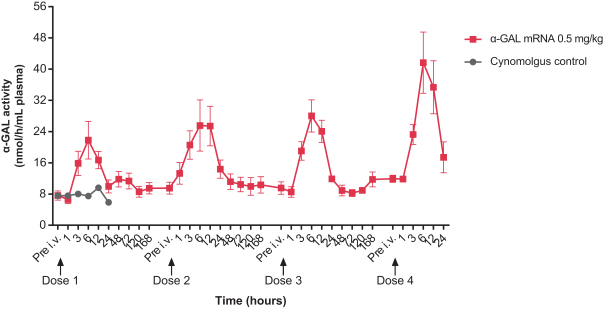
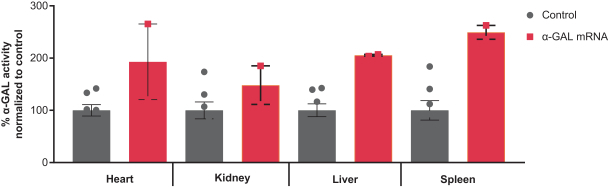
•
|
Enzyme replacement therapies, or ERTs, for rare diseases;
|
|
•
|
Antibodies for membrane and extracellular soluble targets; and
|
|
•
|
Circulating modulation factors for common and rare diseases such as growth factors and insulin.
|
|
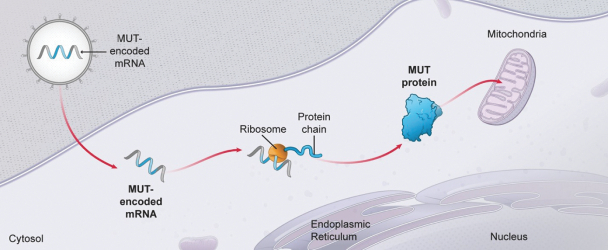
•
|
intracellular pathway proteins;
|
|
•
|
soluble organelle-specific proteins; and
|
|
•
|
organelle-specific membrane proteins.
|
|
•
|
Manufacturing Technology
. Our manufacturing technology development includes state-of-the-art technologies for mRNA and drug product manufacturing and testing to attain robust, consistent supply that matches target product profiles. Manufacturing technology also needs to support scale-up and industrialization of products for ultimate commercial approval.
|
|
•
|
Supply
. The product supply for the Research Engine enables platform research and drug discovery in our therapeutic areas. Within the Early Development Engine, supply is directed towards IND-enabling GLP toxicology programs or current good manufacturing practice, or cGMP, supplies for early clinical studies of our investigational medicines.
|
|
•
|
five cGMP suites for the manufacture of mRNA drug substance and bulk drug product;
|
|
•
|
dedicated cGMP suites for sterile filling;
|
|
•
|
cGMP suites for the manufacture of personalized cancer vaccines, or PCVs;
|
|
•
|
cGMP suites for the manufacture of critical raw materials;
|
|
•
|
space for packaging, labeling, and storage of vialed products;
|
|
•
|
temperature-controlled warehouse for incoming and outgoing products;
|
|
•
|
quality control laboratories;
|
|
•
|
pilot scale manufacturing space for scale-up and manufacture of toxicology supplies;
|
|
•
|
space for the manufacture of research grade mRNA; and
|
|
•
|
clean utilities including purified water and water for injection generation and controlled distribution.
|
|
•
|
utilization of a consistent set of digital building blocks;
|
|
•
|
application of digital technologies in multiple business processes; and
|
|
•
|
rapid iterations for maximum optimization.
|
|
•
|
Quality: Reduction in human errors by enabling automation, repeatability, and seamless integration;
|
|
•
|
Scalability: Growth in our pipeline to
20
programs;
|
|
•
|
Speed: Rapid manufacture of research-grade mRNA from the Research Engine; and
|
|
•
|
Cost efficiencies: Digital infrastructure utilized across our platform, drug discovery, clinical development, and manufacturing to maximize efficiencies.
|
|
•
|
Cloud enablement
is a critical component of our digital infrastructure. We are at the forefront of mRNA technology. We generate complex data sets, and our scientists need computational power and agility to operate without being limited by traditional computing technology. Maintaining digital infrastructure in the cloud provides the benefits of lower costs by simplifying provisioning and administration, flexibility, scalability, ease of maintenance, disaster recovery, and information security.
|
|
•
|
Integration of business processes
enables us to streamline processes and bring data together in a consistent manner, avoiding caches of information and manual intervention. This efficient flow of data between systems enables the automation of our business processes.
|
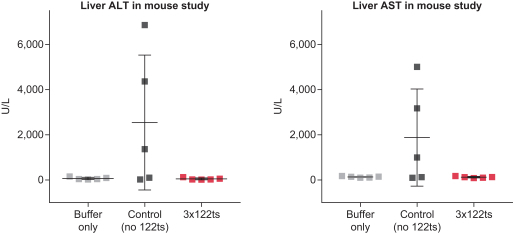
|
•
|
Internet of things
allows for smart interconnected devices that provide real-time synchronization of operations. The data from equipment provides real-time guidance to our scientists and engineers.
|
|
•
|
Automation
allows us to scale our operations reliably and reproducibly. With the help of custom hardware solutions and state-of-the-art robotics, we can continue to increase our operating efficiency, reduce errors, and improve our quality and compliance.
|
|
•
|
Advanced analytics
enable us to draw insights from our data. We are constantly generating large data sets that can provide important insights if mined appropriately and regularly.
|
|
•
|
Artificial intelligence
, or AI, is enabling key breakthroughs in predictive modeling. It will allow us to improve our mRNA design algorithms based on machine learning, and will provide us with critical insights into research, supply chain, manufacturing, and other processes.
|
|
•
|
Neural networks for protein engineering
: One way to optimize the efficacy of the proteins encoded by our mRNA is to engineer the sequence of the protein itself. We use neural networks to analyze and model protein sequences. We train these models by inputting orthologous sequences from thousands of organisms, from which we can generate potential protein sequences optimized for specific attributes.
|
|
•
|
Neural networks for mRNA engineering
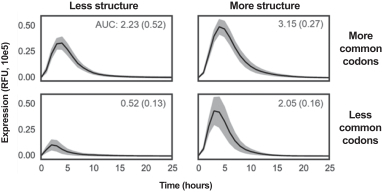
: The redundancy in the genetic code allows for a large number of mRNA sequences that encode the same protein. mRNA sequence may impact translation, thereby impacting the amount of protein produced in circulation. We are developing AI tools to predict mRNA sequences that can enhance protein expression.
|
|
•
|
Bayesian AI for sequencing mRNA
: We analyze the mRNA sequence produced in our Research Engine as part of our quality control requirements. Analysis of sequencing data can be cumbersome and time-consuming. We are developing Bayesian models to accelerate the assessment of sequencing data and more rapidly provide our scientists with high quality mRNA.
|
|
•
|
enterprise Quality Management System, or QMS, to electronically manage deviations, investigation, and correction and preventive actions;
|
|
•
|
Laboratory Information Management System, or LIMS, to manage our analytical development data and automate our manufacturing quality control;
|
|
•
|
computerized maintenance management system to manage equipment maintenance and calibration; and
|
|
•
|
SAP/S4 Hana system for enterprise resource planning, or ERP, manufacturing execution system, and manufacturing control system to manage inventories, track raw material consumption, digitally integrate equipment with manufacturing recipes in batch records, and control automated equipment.
|
|
•
|
Our portfolio application is a digital interface that maintains and tracks the timelines across multiple workstreams for each of our development candidates.
|
|
•
|
The supply application manages the manufacturing schedule of IND-enabling GLP toxicology supplies and cGMP manufacture of clinical supplies to support our programs. This application helps us see how the manufacturing schedule changes over time, identifies supply/demand mismatches, and enables resource planning with real-time alerts should we have any issues.
|
|
•
|
The GLP toxicology application tracks the planned and ongoing IND-enabling GLP toxicology studies and allows us to manage timelines with our external vendors.
|
|
•
|
The regulatory application tracks timelines related to regulatory affairs including, pre-IND meetings, IND/CTA submission dates, and other planned regulatory interactions.
|
|
•
|
Our clinical operations application allows us to track our ongoing trials by accessing clinical operations information in real-time from our CROs. It also has multiple tools and analytics to draw key insights, including, for example, enrollment by trial and enrollment by site to maintain our program timelines.
|
|
•
|
Each patient is provided a unique identifier. We track the entire workflow using a single integrated tracker based on this unique identifier. This is one of many ways we ensure that each patient receives the specific drug product lot manufactured for them.
|
|
•
|
We use neural networks to design the mRNA sequences for the PCV program. Our proprietary vaccine design algorithm selects the top twenty neoantigens to be used and determines their amino acid sequences to trigger the desired immune response.
|
|
•
|
We utilize Monte Carlo simulations of PCV supply/demand to manage our capacity. Since each drug product lot is personalized to a patient, there is a need to manage supply and demand to avoid bottlenecks at any stage of the workflow.
|
|
•
|
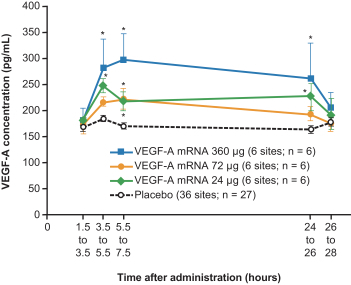
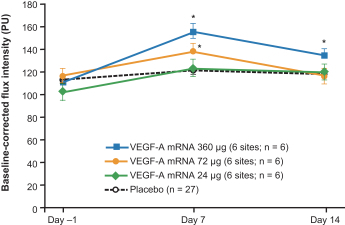
AstraZeneca for the localized regenerative therapeutics modality, such as the VEGF-A (AZD8601) program currently in Phase 2a;
|
|
•
|
AstraZeneca for the intratumoral immuno-oncology modality, such as the IL12 program (MEDI1191);
|
|
•
|
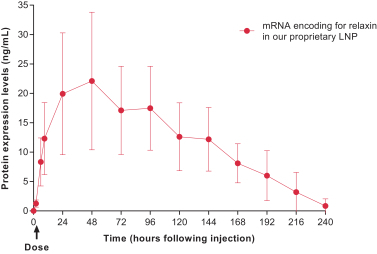
AstraZeneca for the systemic secreted therapeutics modality, such as the Relaxin program (AZD7970);
|
|
•
|
Merck for the prophylactic vaccines modality, such as the RSV vaccine program (mRNA-1777) currently being prepared for a Phase 2;
|
|
•
|
Merck for the cancer vaccines modality, such as the personalized cancer vaccine program (mRNA-4157) currently in Phase 1 using a workflow that enables a rapid turnaround time to bring personalized vaccines to patients, and the KRAS vaccine program (mRNA-5671);
|
|
•
|
DARPA for the systemic secreted therapeutics modality, such as the antibody against Chikungunya virus program (mRNA-1944) currently in Phase 1; and
|
|
•
|
Vertex for the lung delivery modality, such as the CF/CFTR program currently in research.
|
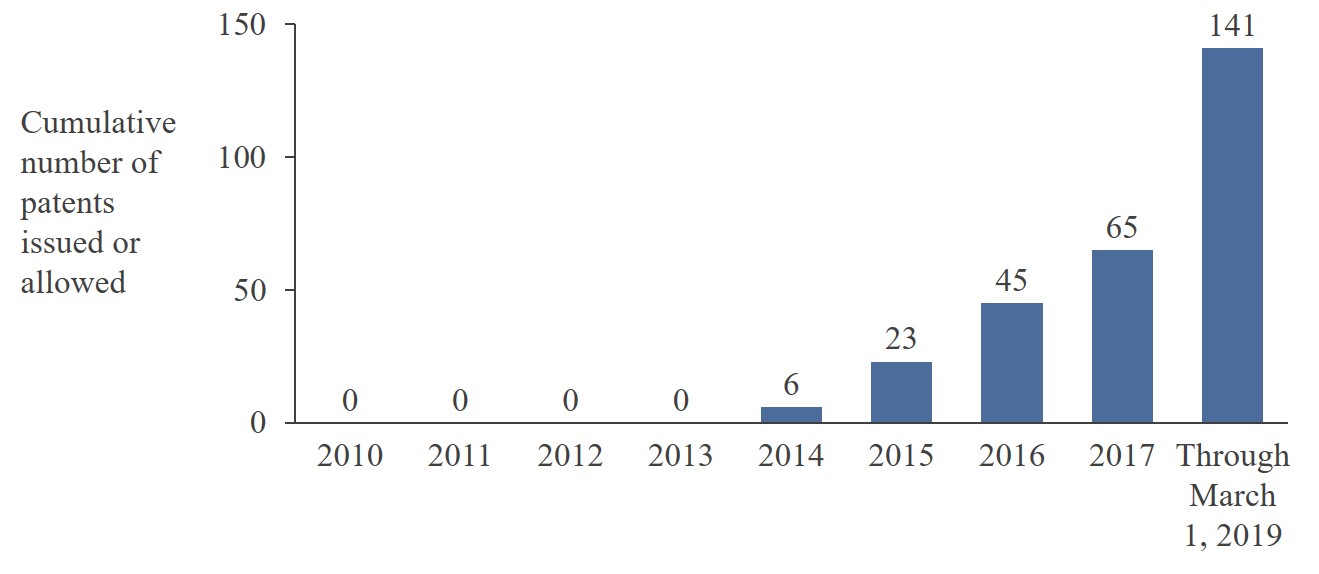
|
•
|
mRNA chemistry imparting improved properties for vaccine and therapeutic uses;
|
|
•
|
methods for mRNA sequence optimization to enhance the levels and fidelity of proteins expressed from our mRNA medicines;
|
|
•
|
methods for identifying epitopes having superior suitability in cancer vaccine contexts;
|
|
•
|
engineering elements tailored to enhance stability and the
in vivo
performance of mRNA medicines;
|
|
•
|
proprietary lipid nanoparticle, or LNP, delivery systems, including novel lipid components designed for optimal expression of both therapeutic and vaccine mRNAs, in particular, prophylactic infectious disease and cancer vaccine mRNAs, intratumoral immuno-oncology therapeutics, local regenerative therapeutics, systemic secreted therapeutics, and systemic intracellular therapeutics; and
|
|
•
|
innovative processes for the manufacture and analysis of mRNA drug substance and formulated drug product.
|
|
•
|
Impact
: Our people will have the opportunity to do work that is unparalleled in terms of its innovation and scope of impact on people’s lives.
|
|
•
|
Growth
: For the intellectually curious, we provide incredible opportunities for growth. We invest in the development of our people as scientists and as leaders.
|
|
•
|
Wellness
: We are committed to the health and wellbeing of our employees and their families by providing family friendly benefits and opportunities to be healthy.
|
|
•
|
Inclusive environment
: We believe in the benefits of bringing together a diverse set of perspectives and backgrounds, and creating an environment where differences are celebrated and leveraged.
|
|
•
|
Compelling rewards
: To attract and retain the best talent, we provide competitive rewards that help to drive groundbreaking work and allow employees to share in the value we will create together.
|
|
•
|
Professional development
: Includes on-site training programs for our employees including for example, leadership, tools to improve interpersonal communication, and project management.
|
|
•
|
Digital learning library
: We have built an online library of videos of a variety of scientific material that our employees can access flexibly. This content includes:
|
|
•
|
Presentations by external speakers to scientific seminars conducted in-house;
|
|
•
|
Scientific courses at external universities; and
|
|
•
|
Peer-to-peer video series in which in-house experts provide an introductory view of complex topics they tackle within their teams.
|
|
•
|
Learning management system
: We have deployed a digital system to track and administer training programs for each of our employees. Training content is developed digitally and offered to our employees.
|
|
•
|
New hire orientation
: This program is designed to onboard all new employees. During this training program, new employees meet with the management team and senior functional leaders to learn about the Company and functional activities.
|
|
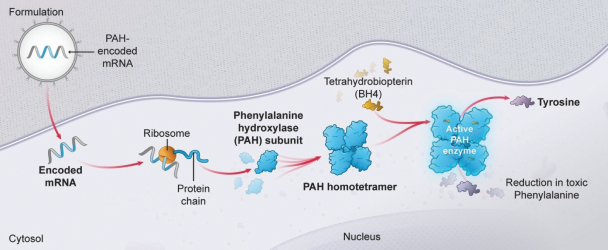
•
|
completion of extensive preclinical studies in accordance with applicable regulations, including studies conducted in accordance with GLP requirements;
|
|
•
|
submission to the FDA of an IND application, which must become effective before human clinical trials may begin;
|
|
•
|
approval by an IRB or independent ethics committee at each clinical trial site before each trial may be initiated;
|
|
•
|
performance of adequate and well-controlled human clinical trials in accordance with applicable IND regulations, good clinical practice, or GCP, requirements and other clinical trial-related regulations to establish the safety and efficacy of the investigational product for each proposed indication;
|
|
•
|
submission to the FDA of a BLA or an NDA;
|
|
•
|
a determination by the FDA within 60 days of its receipt of a BLA or an NDA to accept the filing for review;
|
|
•
|
satisfactory completion of one or more FDA pre-approval inspections of the manufacturing facility or facilities where the biologic or drug will be produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the biologic or drug’s identity, strength, quality and purity;
|
|
•
|
potential FDA audit of the clinical trial sites that generated the data in support of the BLA or NDA;
|
|
•
|
payment of user fees for FDA review of the BLA or NDA; and
|
|
•
|
FDA review and approval of the BLA or NDA, including consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the biologic or drug in the United States.
|
|
•
|
Phase 1 clinical trials generally involve a small number of healthy volunteers or disease-affected patients who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability, and safety of the product candidate.
|
|
•
|
Phase 2 clinical trials generally involve studies in disease-affected patients to evaluate proof of concept and/or determine the dosing regimen(s) for subsequent investigations. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, possible adverse effects and safety risks are identified, and a preliminary evaluation of efficacy is conducted.
|
|
•
|
Phase 3 clinical trials generally involve a large number of patients at multiple sites and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use and to establish the overall benefit/risk relationship of the product, and provide an adequate basis for product labeling.
|
|
•
|
The Anti-Kickback Statute, or AKS, makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer, or pay any remuneration, directly or indirectly, in cash or in kind, that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by up to five years in prison, criminal fines, administrative civil money penalties, and exclusion from participation in federal healthcare programs. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it.
|
|
•
|
The federal False Claims Act imposes civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities (including manufacturers) for, among other things, knowingly presenting, or causing to be presented, false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or avoiding, decreasing, or concealing an obligation to pay money to the federal government. The government may deem manufacturers to have “caused” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Claims which include items or services resulting from a violation of the federal Anti-Kickback Statute are false or fraudulent claims for purposes of the False Claims Act. Our future marketing and activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information, and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our product and any future investigational medicines, are subject to scrutiny under this law.
|
|
•
|
Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for knowingly and willfully executing a scheme, or attempting to execute a scheme, to defraud any healthcare benefit program, including private payors, or falsifying, concealing, or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services.
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, impose, among other things, specified requirements on covered entities and their business associates relating to the privacy and security of individually identifiable health information, including mandatory contractual terms and required implementation of technical safeguards of such information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state Attorneys General new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
|
|
•
|
The Physician Payments Sunshine Act, enacted as part of Act, the ACA, imposed new annual reporting requirements for certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members.
|
|
•
|
Analogous state and foreign fraud and abuse laws and regulations, such as state anti-kickback and false claims laws, which may be broader in scope and apply regardless of payor impose a variety of obligations on. Such laws are enforced by various state agencies and through private actions. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant federal government compliance guidance, require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, and restrict marketing practices or require disclosure of marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances. Such data privacy and security laws may differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts.
|
|
•
|
The Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2025 unless additional Congressional action is taken.
|
|
•
|
The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
|
|
•
|
the initiation, progress, timing, costs, and results of preclinical or nonclinical studies and clinical trials for our development candidates and investigational medicines;
|
|
•
|
results of research and our other platform activities;
|
|
•
|
the clinical development plans we establish for our investigational medicines;
|
|
•
|
the terms of any agreements with our current or future strategic collaborators;
|
|
•
|
the number and characteristics of development candidates and investigational medicines that we develop or may in-license;
|
|
•
|
the outcome, timing, and cost of meeting regulatory requirements established by the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, and other comparable foreign regulatory authorities;
|
|
•
|
the cost of filing, prosecuting, defending, and enforcing our patent claims and other intellectual property, or IP, rights, including patent infringement actions brought by third parties against us regarding our investigational medicines or actions by us challenging the patent or IP rights of others;
|
|
•
|
the effect of competing technological and market developments, including other products that may compete with one or more of our development candidates or investigational medicines;
|
|
•
|
the cost and timing of completion and further expansion of clinical and commercial scale manufacturing activities sufficient to support all of our current and future programs, whether in-house or outsourced; and
|
|
•
|
the cost of establishing sales, marketing, and distribution capabilities for any investigational medicines for which we may receive marketing approval and reimbursement in regions where we choose to commercialize our medicines on our own.
|
|
•
|
discovery efforts at identifying potential mRNA medicines may not be successful;
|
|
•
|
nonclinical or preclinical study results may show potential mRNA medicines to be less effective than desired or to have harmful or problematic side effects;
|
|
•
|
clinical trial results may show potential mRNA medicines to be less effective than expected (e.g., a clinical trial could fail to meet one or more endpoint(s)) or to have unacceptable side effects or toxicities;
|
|
•
|
adverse effects in any one of our clinical programs or adverse effects relating to our mRNA, or lipid nanoparticles, or LNPs, may lead to delays in or termination of one or more of our programs;
|
|
•
|
the insufficient ability of our translational models to reduce risk or predict outcomes in humans, particularly given that each component of our investigational medicines and development candidates may have a dependent or independent effect on safety, tolerability, and efficacy, which may, among other things, be species-dependent;
|
|
•
|
manufacturing failures or insufficient supply of cGMP materials for clinical trials, or higher than expected cost could delay or set back clinical trials, or make mRNA based medicines commercially unattractive;
|
|
•
|
our improvements in the manufacturing processes for this new class of potential medicines may not be sufficient to satisfy the clinical or commercial demand of our investigational medicines or regulatory requirements for clinical trials;
|
|
•
|
changes that we make to optimize our manufacturing, testing or formulating of cGMP materials could impact the safety, tolerability, and efficacy of our investigational medicines and development candidates;
|
|
•
|
pricing or reimbursement issues or other factors that delay clinical trials or make any mRNA medicine uneconomical or noncompetitive with other therapies;
|
|
•
|
failure to timely advance our programs or receive the necessary regulatory approvals or a delay in receiving such approvals, due to, among other reasons, slow or failure to complete enrollment in clinical trials, withdrawal by trial participants from trials, failure to achieve trial endpoints, additional time requirements for data analysis, data integrity issues, biologics license application, or BLA, or the equivalent application, discussions with the FDA or EMA, a regulatory request for additional nonclinical or clinical data, or safety formulation or manufacturing issues may lead to our inability to obtain sufficient funding; and
|
|
•
|
the proprietary rights of others and their competing products and technologies that may prevent our mRNA medicines from being commercialized.
|
|
•
|
continue or expand our research or development of our programs in preclinical development;
|
|
•
|
continue or expand the scope of our mRNA clinical studies for our investigational medicines;
|
|
•
|
initiate additional preclinical, clinical, or other studies for our development candidates and investigational medicines, including under our strategic alliance agreements;
|
|
•
|
continue to invest in our platform to conduct research to identify novel mRNA technology improvements, including identifying novel methods of mRNA delivery, such as LNPs that improve distribution and uptake of mRNA to specific tissues;
|
|
•
|
change or add to internal manufacturing capacity or capability;
|
|
•
|
change or add additional manufacturers or suppliers;
|
|
•
|
add additional infrastructure to our quality control and quality assurance groups to support our operations as we progress our investigational medicines toward commercialization;
|
|
•
|
attract and retain skilled personnel, particularly in Cambridge and Norwood, MA;
|
|
•
|
create additional infrastructure to support our operations as a public company and our product development and planned future commercialization efforts, including new sites in the United States and abroad;
|
|
•
|
seek marketing approvals and reimbursement for our investigational medicines;
|
|
•
|
establish a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
|
|
•
|
seek to identify and validate additional development candidates and investigational medicines;
|
|
•
|
acquire or in-license other development candidates, investigational medicines, and technologies;
|
|
•
|
make milestone or other payments under any in-license agreements;
|
|
•
|
maintain, protect, and expand our IP portfolio; and
|
|
•
|
experience any delays or encounter issues with any of the above.
|
|
•
|
delays or failures in advancement of existing or future development candidates into the clinic or investigational medicines in clinical trials;
|
|
•
|
the feasibility of developing, manufacturing, and commercializing our programs;
|
|
•
|
our ability to manage our growth;
|
|
•
|
the outcomes of research programs, clinical trials, or other product development or approval processes conducted by us and our strategic collaborators;
|
|
•
|
our ability to develop or successfully commercialize mRNA medicines;
|
|
•
|
the ability of our strategic collaborators to develop and successfully commercialize mRNA medicines or other products developed from our IP;
|
|
•
|
our relationships, and any associated exclusivity terms, with strategic collaborators;
|
|
•
|
our contractual or other obligations to provide resources to fund our development candidates and investigational medicines, and to provide resources to our strategic collaborators or to the strategic alliances themselves;
|
|
•
|
our operation in a net loss position for the foreseeable future;
|
|
•
|
risks associated with the international aspects of our business including the conduct of clinical trials in multiple locations and potential commercialization in such locations;
|
|
•
|
our ability to consistently manufacture our development candidates and investigational medicines;
|
|
•
|
our ability to accurately report our financial results in a timely manner;
|
|
•
|
our dependence on, and the need to attract and retain, key management and other personnel;
|
|
•
|
our ability to obtain, protect, and enforce our IP rights;
|
|
•
|
our ability to prevent the theft or misappropriation of our IP, know-how or technologies;
|
|
•
|
potential advantages that our competitors and potential competitors may have in securing funding, obtaining the rights to critical IP or developing competing technologies or products;
|
|
•
|
our ability to obtain additional capital that may be necessary to expand our business;
|
|
•
|
our strategic collaborators’ ability to obtain additional capital that may be necessary to develop and commercialize products under our strategic alliance agreements;
|
|
•
|
business interruptions such as power outages, strikes, acts of terrorism, or natural disasters; and
|
|
•
|
our ability to use our net operating loss carryforwards to offset future taxable income.
|
|
•
|
the FDA, other regulators, institutional review boards, or IRBs, or ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site for any number of reasons, including concerns regarding safety and aspects of the clinical trial design;
|
|
•
|
we may experience delays in reaching, or fail to reach, agreement on favorable terms with prospective trial sites and prospective contract research organizations, or CROs, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
we have in the past and will continue to optimize our manufacturing processes, including through changes to the scale and site of manufacturing, which may lead to potentially significant changes in our clinical trial designs, requiring additional cost and time, and, as a consequence, lead to a delay in plans for progressing one or more investigational medicines;
|
|
•
|
the outcome of our preclinical studies and our early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results;
|
|
•
|
we may be unable to establish clinical endpoints that applicable regulatory authorities would consider clinically meaningful;
|
|
•
|
in an effort to optimize product features, we have in the past and may continue to make changes to our investigational medicines after we commence clinical trials of an investigational medicine which may require us to repeat earlier stages of clinical testing or delay later stage testing of the investigational medicine;
|
|
•
|
clinical trials of any investigational medicines may fail to show safety or efficacy, or produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional nonclinical studies or clinical trials, or we may decide to abandon product development programs;
|
|
•
|
differences in trial design between early stage clinical trials and later-stage clinical trials make it difficult to extrapolate the results of earlier clinical trials to later clinical trials;
|
|
•
|
preclinical and clinical data are often susceptible to varying interpretations and analyses, and many investigational medicines believed to have performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval;
|
|
•
|
our investigational medicines may have undesirable side effects, such as the immunogenicity of the LNPs or their components, the immunogenicity of the protein made by the mRNA, or degradation products, any of which could lead to serious adverse events, or SAEs, or other effects. One or more of such effects or events could cause regulators to impose a clinical hold on the applicable trial, or cause us or our investigators, IRBs, or ethics committees to suspend or terminate the trial of that investigational medicine or any other of our investigational medicines for which a clinical trial may be ongoing;
|
|
•
|
the number of trial participants required for clinical trials of any investigational medicines may be larger than we anticipate, identification of trial participants for such trials may be limited, enrollment in these clinical trials may be slower than we anticipate due to perceived adverse effects, competitive trials, or other reasons, or participants may withdraw from clinical trials or fail to return for post-treatment follow-up at a higher rate than we anticipate;
|
|
•
|
our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, or may deviate from the clinical trial protocol or withdraw from the trial, which may require that we add new clinical trial sites;
|
|
•
|
regulators may elect to impose a clinical hold, or we or our investigators, IRBs, or ethics committees may elect to suspend or terminate clinical research or trials for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable benefit risk ratio;
|
|
•
|
the cost of preclinical or nonclinical testing and studies and clinical trials of any investigational medicines may be greater than we anticipate;
|
|
•
|
the supply or quality of our investigational medicines or other materials necessary to conduct clinical trials may be insufficient or inadequate;
|
|
•
|
safety or efficacy concerns regarding our investigational medicines may result from any safety or efficacy concerns arising from nonclinical or clinical testing of other therapies targeting a similar disease state or other therapies, such as gene therapy, that are perceived as similar to ours; and
|
|
•
|
the FDA or other regulatory authorities may require us to submit additional data such as long-term toxicology studies, or impose other requirements before permitting us to initiate a clinical trial.
|
|
•
|
severity of the disease under investigation;
|
|
•
|
complexity and design of the study protocol;
|
|
•
|
size of the patient population;
|
|
•
|
eligibility criteria for the study in question, including age-based eligibility criteria limiting subject enrollment to adolescent or pediatric populations;
|
|
•
|
proximity and availability of clinical study sites for prospective trial participants;
|
|
•
|
availability of competing therapies and clinical studies, including between our own clinical trials;
|
|
•
|
efforts to facilitate timely enrollment in clinical studies;
|
|
•
|
patient referral practices of physicians;
|
|
•
|
ability to monitor trial participants adequately during and after treatment;
|
|
•
|
ability to recruit clinical trial investigators with the appropriate competencies and experience;
|
|
•
|
clinicians’ and trial participants’ perceptions as to the potential advantages and side effects of the investigational medicine being studied in relation to other available therapies, including any new drugs or treatments that may be approved for the indications we are investigating;
|
|
•
|
the need, in the case of our personalized cancer vaccine, to wait for the manufacture of the personalized drug product;
|
|
•
|
our ability to obtain and maintain participant informed consent; and
|
|
•
|
the risk that trial participants enrolled in clinical trials will not complete a clinical trial.
|
|
•
|
issue a warning letter asserting that we are in violation of the law;
|
|
•
|
seek an injunction or impose civil or criminal penalties or monetary fines;
|
|
•
|
suspend or withdraw regulatory approval or revoke a license;
|
|
•
|
suspend any ongoing clinical studies;
|
|
•
|
refuse to approve a pending BLA or supplements to a BLA submitted by us;
|
|
•
|
seize product; or
|
|
•
|
refuse to allow us to enter into supply contracts, including government contracts.
|
|
•
|
regulatory authorities may suspend or withdraw approvals or revoke licenses of such product;
|
|
•
|
regulatory authorities may require additional warnings on the label;
|
|
•
|
we may be required to change the way a product is administered or conduct additional clinical trials;
|
|
•
|
we could be sued and held liable for harm caused to patients and their children; and
|
|
•
|
our reputation may suffer.
|
|
•
|
termination or non-renewal of supply and service agreements with third parties in a manner or at a time that is costly or damaging to us;
|
|
•
|
disruptions to the operations of these suppliers and service providers caused by conditions unrelated to our business or operations, including the bankruptcy of the supplier or service provider; and
|
|
•
|
inspections of third-party facilities by regulatory authorities that could have a negative outcome and result in delays to or termination of their ability to supply our requirements.
|
|
•
|
critical deviations in the manufacturing process;
|
|
•
|
facility and equipment failures;
|
|
•
|
contamination of the product due to an ineffective quality control strategy;
|
|
•
|
facility contamination as assessed by the facility and utility environmental monitoring program;
|
|
•
|
ineffective process, equipment or analytical change management, resulting in failed lot release criteria;
|
|
•
|
raw material failures due to ineffective supplier qualification or regulatory compliance issues at critical suppliers;
|
|
•
|
ineffective product stability;
|
|
•
|
failed lot release or facility and utility quality control testing;
|
|
•
|
ineffective corrective actions or preventative actions taken to correct or avoid critical deviations due to our developing understanding of the manufacturing process as we scale; and
|
|
•
|
failed or defective components or consumables.
|
|
•
|
logistics associated with the collection of a patient’s tumor, blood or other tissue sample;
|
|
•
|
shipping such samples to a facility for genetic sequencing;
|
|
•
|
next generation sequencing of the tumor mRNA;
|
|
•
|
identification of appropriate tumor-specific mutations;
|
|
•
|
the use of a software program, including proprietary and open source components, which is hosted in the cloud and a part of our investigational medicine, to assist with the design of the patient-specific mRNA, which software must be maintained and secured;
|
|
•
|
effective design of the patient-specific mRNA that encodes for the required neoantigens;
|
|
•
|
batch specific manufacturing failures or issues that arise due to the uniqueness of each patient-specific batch that may not have been foreseen;
|
|
•
|
quality control testing failures;
|
|
•
|
unexpected failures of batches placed on stability;
|
|
•
|
shortages or quality control issues with single-use assemblies, consumables, or critical parts sourced from third-party vendors that must be changed out for each patient-specific batch;
|
|
•
|
significant costs associated with individualized manufacturing that may adversely affect our ability to continue development;
|
|
•
|
successful and timely manufacture and release of the patient-specific batch;
|
|
•
|
shipment issues encountered during transport of the batch to the patient site of care; and
|
|
•
|
the ability to define a consistent safety profile at a given dose when each participant receives a unique vaccine.
|
|
•
|
strategic collaborators may not perform their obligations as expected;
|
|
•
|
the clinical trials conducted as part of such strategic alliance may not be successful;
|
|
•
|
strategic collaborators may not pursue development and commercialization of any investigational medicines that achieve regulatory approval or may elect not to continue or renew development or commercialization of programs based on clinical trial results, changes in the strategic collaborators’ focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities;
|
|
•
|
strategic collaborators may delay clinical trials, provide insufficient funding for clinical trials, stop a clinical trial, abandon an investigational medicine, repeat or conduct new clinical trials, or require a new formulation of an investigational medicine for clinical testing;
|
|
•
|
strategic collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our investigational medicines if the strategic collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;
|
|
•
|
investigational medicines developed in strategic alliances with us may be viewed by our strategic collaborators as competitive with their own investigational medicines or products, which may cause strategic collaborators to cease to devote resources to the development or commercialization of our investigational medicines;
|
|
•
|
a strategic collaborator with marketing and distribution rights to one or more of our investigational medicines that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of any such product;
|
|
•
|
disagreements with strategic collaborators, including disagreements over proprietary rights, contract interpretation, or the preferred course of development of any investigational medicines, may cause delays or termination of the research, development, or commercialization of such investigational medicines, may lead to additional responsibilities for us with respect to such investigational medicines, or may result in litigation or arbitration, any of which would be time-consuming and expensive;
|
|
•
|
strategic collaborators may not properly maintain or defend our IP rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our IP or proprietary information or expose us to potential litigation;
|
|
•
|
disputes may arise with respect to the ownership of IP developed pursuant to our strategic alliances;
|
|
•
|
strategic collaborators may infringe the IP rights of third parties, which may expose us to litigation and potential liability;
|
|
•
|
strategic alliances may be terminated for the convenience of the strategic collaborator and, if terminated, the development of our investigational medicines may be delayed, and we could be required to raise additional capital to pursue further development or commercialization of the applicable investigational medicines;
|
|
•
|
future relationships may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders, or disrupt our management and business;
|
|
•
|
we could face significant competition in seeking appropriate strategic collaborators and the negotiation process is time-consuming and complex; and
|
|
•
|
our international operations through any future collaborations, acquisitions, or joint ventures may expose us to certain operating, legal, and other risks not encountered in the United States.
|
|
•
|
delays to the development timelines for our development candidates or investigational medicines;
|
|
•
|
interruption of supply resulting from modifications to or discontinuation of a supplier’s operations;
|
|
•
|
delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component;
|
|
•
|
a lack of long-term supply arrangements for key components with our suppliers;
|
|
•
|
inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms;
|
|
•
|
difficulty and cost associated with locating and qualifying alternative suppliers for our components in a timely manner;
|
|
•
|
production delays related to the evaluation and testing of components from alternative suppliers, and corresponding regulatory qualifications;
|
|
•
|
delay in delivery due to our suppliers’ prioritizing other customer orders over ours;
|
|
•
|
damage to our reputation caused by defective components produced by our suppliers; and
|
|
•
|
fluctuation in delivery by our suppliers due to changes in demand from us or their other customers.
|
|
•
|
have staffing difficulties;
|
|
•
|
fail to comply with contractual obligations;
|
|
•
|
experience regulatory compliance issues;
|
|
•
|
undergo changes in priorities or become financially distressed;
|
|
•
|
form relationships with other entities, some of which may be our competitors;
|
|
•
|
have human errors; or
|
|
•
|
be subject to cyber attacks.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
whether and the extent to which our technology and processes infringe on IP of the licensor that is not subject to the licensing agreement;
|
|
•
|
our right to sublicense patent and other rights to third parties under collaborative development relationships;
|
|
•
|
our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our investigational medicines, and what activities satisfy those diligence obligations; and
|
|
•
|
the ownership of inventions and know-how resulting from the joint creation or use of IP by our licensors and us and our strategic collaborators.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
the extent to which our technology and processes that are not subject to the licensing agreement infringe on IP of the licensor;
|
|
•
|
the sublicensing of patent and other rights under our collaborative development relationships;
|
|
•
|
our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
|
•
|
the ownership of inventions and know-how resulting from the joint creation or use of IP by our licensors and us and our strategic collaborators; and
|
|
•
|
the priority of invention of patented technology.
|
|
•
|
terminate agreements, in whole or in part, for any reason or no reason;
|
|
•
|
reduce or modify the government’s obligations under such agreements without the consent of the other party;
|
|
•
|
claim rights, including IP rights, in products and data developed under such agreements;
|
|
•
|
audit contract-related costs and fees, including allocated indirect costs;
|
|
•
|
suspend the contractor or grantee from receiving new contracts pending resolution of alleged violations of procurement laws or regulations;
|
|
•
|
impose U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice under such agreements;
|
|
•
|
suspend or debar the contractor or grantee from doing future business with the government;
|
|
•
|
control and potentially prohibit the export of products;
|
|
•
|
pursue criminal or civil remedies under the False Claims Act, False Statements Act, and similar remedy provisions specific to government agreements; and
|
|
•
|
limit the government’s financial liability to amounts appropriated by the U.S. Congress on a fiscal-year basis, thereby leaving some uncertainty about the future availability of funding for a program even after it has been funded for an initial period.
|
|
•
|
specialized accounting systems unique to government contracts and grants;
|
|
•
|
mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent;
|
|
•
|
public disclosures of certain contract and grant information, which may enable competitors to gain insights into our research program; and
|
|
•
|
mandatory socioeconomic compliance requirements, including labor standards, non-discrimination, and affirmative action programs, and environmental compliance requirements.
|
|
•
|
greater financial, technical, and human resources than we have at every stage of the discovery, development, manufacture, and commercialization of products;
|
|
•
|
more extensive experience in preclinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing, and selling drug products;
|
|
•
|
investigational medicines that are based on previously tested or accepted technologies;
|
|
•
|
products that have been approved or are in late stages of development; and
|
|
•
|
collaborative arrangements in our target markets with leading companies and research institutions.
|
|
•
|
the safety and effectiveness of our products relative to alternative therapies, if any;
|
|
•
|
the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration;
|
|
•
|
the timing and scope of regulatory approvals for these products;
|
|
•
|
the availability and cost of manufacturing, marketing, and sales capabilities;
|
|
•
|
the price of any approved mRNA medicine;
|
|
•
|
reimbursement coverage; and
|
|
•
|
patent position.
|
|
•
|
the potential efficacy and potential advantages over alternative treatments;
|
|
•
|
the ability to offer our products, if approved, at competitive prices;
|
|
•
|
the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling;
|
|
•
|
the prevalence and severity of any side effects resulting from checkpoint inhibitors or other drugs or therapies with which our products are co-administered;
|
|
•
|
relative convenience and ease of administration;
|
|
•
|
any restrictions on the use of our products, if approved, together with other medications;
|
|
•
|
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
|
|
•
|
the strength of marketing and distribution support and timing of market introduction of competitive products;
|
|
•
|
publicity concerning our products or competing products and treatments; and
|
|
•
|
sufficient third-party insurance coverage or reimbursement, and patients’ willingness to pay out-of-pocket in the absence of third-party coverage or adequate reimbursement.
|
|
•
|
efforts to develop an international sales, marketing, and distribution organization;
|
|
•
|
changes in a specific country’s or region’s political and cultural climate or economic condition;
|
|
•
|
unexpected changes in foreign laws and regulatory requirements;
|
|
•
|
difficulty of effective enforcement of contractual provisions in local jurisdictions;
|
|
•
|
inadequate IP protection in foreign countries;
|
|
•
|
trade-protection measures, import or export licensing requirements such as Export Administration Regulations promulgated by the U.S. Department of Commerce and fines, penalties, or suspension or revocation of export privileges;
|
|
•
|
the effects of applicable foreign tax structures and potentially adverse tax consequences; and
|
|
•
|
significant adverse changes in foreign currency exchange rates.
|
|
•
|
completing research, preclinical, and clinical development of our development candidates and investigational medicines;
|
|
•
|
seeking and obtaining U.S. and foreign marketing approvals for investigational medicines for which we complete clinical studies;
|
|
•
|
developing a sustainable, stable, consistent, and transferable manufacturing process or processes for our development candidates and investigational medicines;
|
|
•
|
developing a sustainable, scalable, consistent, time sensitive, and transferable manufacturing process for our personalized cancer vaccine investigational medicine;
|
|
•
|
furthering the development of our own manufacturing capabilities and manufacturing relationships with third parties in order to provide adequate (in amount and quality) products and services to support clinical development and the market demand for our investigational medicines, if approved;
|
|
•
|
obtaining market acceptance of our investigational medicines as a treatment option;
|
|
•
|
launching and commercializing investigational medicines for which we obtain marketing approval and reimbursement, either by collaborating with a strategic collaborator or, if launched independently, by establishing a sales force, marketing, and distribution infrastructure;
|
|
•
|
addressing any competing technological and market developments;
|
|
•
|
implementing additional internal systems and infrastructure;
|
|
•
|
negotiating favorable terms in any collaboration, licensing, or other arrangements into which we may enter;
|
|
•
|
maintaining, defending, protecting, and expanding our portfolio of IP rights, including patents, trade secrets and know-how; and
|
|
•
|
attracting, hiring, and retaining qualified personnel.
|
|
•
|
the research methodology used may not be successful in identifying potential investigational medicines;
|
|
•
|
competitors may develop alternatives that render our investigational medicines obsolete;
|
|
•
|
investigational medicines we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
|
|
•
|
an investigational medicine may, on further study, be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
|
|
•
|
an investigational medicine may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
|
|
•
|
an approved product may not be accepted as safe and effective by trial participants, the medical community or third-party payors.
|
|
•
|
decreased demand for any investigational medicine that we may develop;
|
|
•
|
loss of revenue;
|
|
•
|
substantial monetary awards to patients, healthy volunteers, or their children;
|
|
•
|
significant time and costs to defend the related litigation;
|
|
•
|
withdrawal of clinical trial participants;
|
|
•
|
the inability to commercialize any investigational medicine(s) that we may develop; and
|
|
•
|
injury to our reputation and significant negative media attention.
|
|
•
|
The federal Health Care Program Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, in return for the purchase, recommendation, leasing, or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers, and formulary managers on the other. The ACA amends the intent requirement of the federal Anti-Kickback Statute to provide that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it.
|
|
•
|
The federal civil and criminal false claims laws and civil monetary penalty laws prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other government payors that are false or fraudulent. The ACA provides, and recent government cases against pharmaceutical and medical device manufacturers support, the view that federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may implicate the False Claims Act.
|
|
•
|
HIPAA, which created new federal criminal statutes that prohibit a person from knowingly and willfully executing a scheme or making false or fraudulent statements to defraud any healthcare benefit program, regardless of the payor (e.g., public or private).
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and their implementing regulations, imposes certain requirements relating to the privacy, security, and transmission of individually identifiable health information without appropriate authorization by entities subject to the rule, such as health plans, health care clearinghouses, and health care providers.
|
|
•
|
The U.S. Federal Food, Drug and Cosmetic Act, which prohibits, among other things, the adulteration or misbranding of drugs, biologics, and medical devices.
|
|
•
|
Federal transparency laws, including the federal Physician Payment Sunshine Act, which require disclosure of payments and other transfers of value provided to physicians and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations.
|
|
•
|
State law equivalents of each of the above federal laws, state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state laws governing the privacy and security of health information in certain circumstances are also applicable to us and many of them differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts in certain circumstances.
|
|
•
|
increased operating expenses and cash requirements;
|
|
•
|
the assumption of additional indebtedness or contingent liabilities;
|
|
•
|
assimilation of operations, intellectual property, and products of an acquired company, including difficulties associated with integrating new personnel;
|
|
•
|
the diversion of our management’s attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition;
|
|
•
|
retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships;
|
|
•
|
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or investigational medicines and regulatory approvals; and
|
|
•
|
our inability to generate revenue from acquired technology or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
|
|
•
|
results of clinical trials of our investigational medicines or those of our competitors;
|
|
•
|
the success of competitive products or technologies;
|
|
•
|
commencement or termination of strategic alliances;
|
|
•
|
regulatory or legal developments in the United States and other countries;
|
|
•
|
developments or disputes concerning patent applications, issued patents, or other proprietary rights;
|
|
•
|
the recruitment or departure of key personnel;
|
|
•
|
the level of expenses related to any of our investigational medicines or clinical development programs;
|
|
•
|
the results of our efforts to discover, develop, acquire, or in-license additional investigational medicines;
|
|
•
|
actual or anticipated changes in estimates as to financial results, development timelines, or recommendations by securities analysts;
|
|
•
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
•
|
changes in the structure of healthcare payment systems;
|
|
•
|
market conditions in the pharmaceutical and biotechnology sectors;
|
|
•
|
general economic, industry, and market conditions;
|
|
•
|
the numerous programs in our pipeline, the development of which could each generate news or significant adverse events that could impact financial results or recommendations by securities analysts; and
|
|
•
|
public announcements by us or our strategic collaborators regarding the progress of our development candidates or investigational medicines or similar public announcements by our competitors.
|
|
(i)
|
the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more;
|
|
(ii)
|
December 31, 2023, being the last day of the fiscal year following the fifth anniversary of the date of the completion of our initial public offering;
|
|
(iii)
|
the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or
|
|
(iv)
|
the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission or SEC, which means the first day of the year following the first year in which the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last day of our second fiscal quarter. For so long as we remain an EGC, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
|
|
•
|
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002;
|
|
•
|
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
|
•
|
reduced disclosure obligations regarding executive compensation; and
|
|
•
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
|
|
•
|
authorize “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend, and other rights superior to our common stock;
|
|
•
|
create a classified board of directors whose members serve staggered three-year terms;
|
|
•
|
specify that special meetings of our stockholders can be called only by our board of directors, the chairperson of our board of directors, our chief executive officer, or our president;
|
|
•
|
prohibit stockholder action by written consent;
|
|
•
|
establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
|
|
•
|
provide that our directors may be removed only for cause;
|
|
•
|
provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum;
|
|
•
|
specify that no stockholder is permitted to cumulate votes at any election of directors;
|
|
•
|
expressly authorize our board of directors to modify, alter, or repeal our amended and restated by-laws; and
|
|
•
|
require supermajority votes of the holders of our common stock to amend specified provisions of our amended and restated certificate of incorporation and amended and restated by-laws.
|
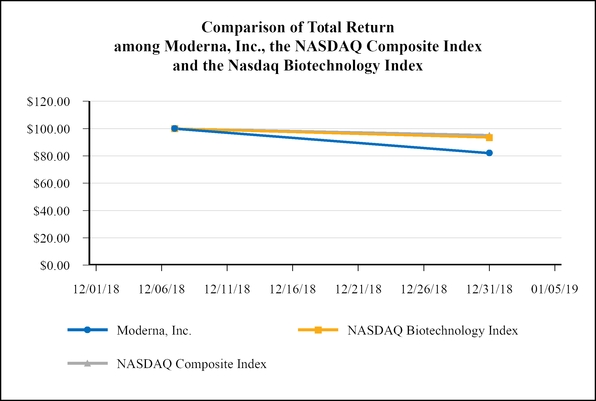
|
1.
|
On January 30, 2018 and February 15, 2018, we issued and sold an aggregate of 55,666,004 shares of our Series G preferred stock to 92 accredited investors at a per share purchase price of $10.06 for aggregate gross consideration of $560 million.
|
|
2.
|
On May 7, 2018, we issued and sold an aggregated of 5,000,000 shares of our Series H preferred stock to one accredited investor at a per share purchase price of $25.00 for aggregate gross consideration of $125 million, which includes a $13 million premium associated with the 2018 amended and restated personalized cancer vaccines agreement with Merck & Co. entered into in conjunction with the Series H issuance.
|
|
3.
|
Between January 1, 2018 and December 6, 2018, under our 2016 Stock Option and Grant Plan and our 2018 Stock Option and Incentive Plan, we issued to certain of our employees, consultants and board members options to purchase an aggregate of 19,223,123 shares of our common stock, with a weighted-average exercise price of $17.01, in exchange for their services to us.
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands, except share and per share data)
|
|||||||||||
|
Statement of Operations Data:
|
|||||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
|
$
|
122,512
|
|
$
|
176,974
|
|
$
|
101,536
|
|
||
|
Grant revenue
|
12,556
|
|
28,851
|
|
6,860
|
|
|||||
|
Total revenue
|
135,068
|
|
205,825
|
|
108,396
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
454,082
|
|
410,459
|
|
274,717
|
|
|||||
|
General and administrative
|
94,252
|
|
64,722
|
|
57,450
|
|
|||||
|
Total operating expenses
|
548,334
|
|
475,181
|
|
332,167
|
|
|||||
|
Loss from operations
|
(413,266
|
)
|
(269,356
|
)
|
(223,771
|
)
|
|||||
|
Interest income
|
27,023
|
|
15,235
|
|
11,312
|
|
|||||
|
Other income (expense), net
|
1,835
|
|
(1,875
|
)
|
(2,709
|
)
|
|||||
|
Loss before provision for (benefit from) income taxes
|
(384,408
|
)
|
(255,996
|
)
|
(215,168
|
)
|
|||||
|
Provision for (benefit from) income taxes
|
326
|
|
(80
|
)
|
1,043
|
|
|||||
|
Net loss
|
(384,734
|
)
|
(255,916
|
)
|
(216,211
|
)
|
|||||
|
Reconciliation of net loss to net loss attributable to common stockholders:
|
|||||||||||
|
Premium paid on repurchases of preferred stock
|
(4,127
|
)
|
—
|
|
—
|
|
|||||
|
Accretion of redeemable convertible preferred units to redemption value
|
—
|
|
—
|
|
(8,663
|
)
|
|||||
|
Cumulative preferred stock dividends
|
(12,996
|
)
|
(13,925
|
)
|
(5,440
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(401,857
|
)
|
$
|
(269,841
|
)
|
$
|
(230,314
|
)
|
||
|
Net loss per share attributable to common stockholders, basic and diluted
(1)
|
$
|
(4.95
|
)
|
$
|
(4.18
|
)
|
$
|
(3.79
|
)
|
||
|
Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted
|
81,114,183
|
|
|
64,497,544
|
|
|
60,747,426
|
|
|||
|
December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Balance Sheet Data:
|
|||||||||||
|
Cash, cash equivalents, and investments
|
$
|
1,694,417
|
|
$
|
901,880
|
|
$
|
1,294,707
|
|
||
|
Working capital
(2)
|
1,340,203
|
|
591,762
|
|
924,350
|
|
|||||
|
Total assets
|
1,962,149
|
|
1,084,489
|
|
1,417,161
|
|
|||||
|
Total deferred revenue
|
274,408
|
|
339,668
|
|
501,989
|
|
|||||
|
Redeemable convertible preferred stock
|
—
|
|
1,176,661
|
|
1,176,661
|
|
|||||
|
Total stockholders’ equity (deficit)
|
1,530,241
|
|
(551,365
|
)
|
(334,810
|
)
|
|||||
|
•
|
continue our platform research and drug discovery and development efforts;
|
|
•
|
conduct clinical studies for our investigational medicines;
|
|
•
|
manufacture clinical study materials and develop large-scale manufacturing capabilities;
|
|
•
|
seek regulatory approval for our investigational medicines;
|
|
•
|
maintain, expand, and protect our intellectual property;
|
|
•
|
hire additional personnel to support our program development effort to obtain regulatory approval and secure additional facilities for operations; and
|
|
•
|
operate as a public company.
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
(1)
|
$
|
122,512
|
|
$
|
176,974
|
|
$
|
101,536
|
|
||
|
Grant revenue
|
12,556
|
28,851
|
6,860
|
||||||||
|
Total revenue
|
$
|
135,068
|
|
$
|
205,825
|
|
$
|
108,396
|
|
||
|
(1)
|
Includes collaboration revenue from an affiliate.
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Collaboration revenue:
|
|||||||||||
|
AstraZeneca
|
$
|
45,993
|
|
$
|
30,021
|
|
$
|
32,427
|
|
||
|
Merck
|
66,082
|
|
62,895
|
|
47,708
|
|
|||||
|
Vertex
|
10,437
|
|
9,138
|
|
3,456
|
|
|||||
|
Alexion
|
—
|
|
74,365
|
|
17,191
|
|
|||||
|
Other
|
—
|
|
555
|
|
754
|
|
|||||
|
Total collaboration revenue
|
$
|
122,512
|
|
$
|
176,974
|
|
$
|
101,536
|
|
||
|
•
|
cost to develop our platform;
|
|
•
|
discovery efforts leading to development candidates;
|
|
•
|
preclinical, nonclinical, and clinical development costs for our programs;
|
|
•
|
cost to develop our manufacturing technology and infrastructure; and
|
|
•
|
digital infrastructure costs.
|
|
•
|
personnel-related expenses, including salaries, benefits, and stock-based compensation expense;
|
|
•
|
expenses incurred under agreements with third parties, such as consultants, investigative sites, contract research organizations, or CROs, that conduct our preclinical and clinical studies, and in-licensing arrangements;
|
|
•
|
costs of acquiring, developing, and manufacturing materials for preclinical and clinical studies, including both internal manufacturing and third-party contract manufacturing organizations, or CMOs;
|
|
•
|
expenses incurred for the procurement of materials, laboratory supplies, and non-capital equipment used in the research and development process; and
|
|
•
|
facilities, depreciation, and amortization, and other direct and allocated expenses incurred as a result of research and development activities.
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Program expenses by modality:
|
|||||||||||
|
Prophylactic vaccines
|
$
|
25,404
|
|
$
|
67,888
|
|
$
|
20,374
|
|
||
|
Cancer vaccines
|
35,891
|
|
31,818
|
|
4,135
|
|
|||||
|
Intratumoral immuno-oncology
|
15,405
|
|
20,340
|
|
8,022
|
|
|||||
|
Localized regenerative therapeutics
|
91
|
|
1,684
|
|
193
|
|
|||||
|
Systemic secreted therapeutics
|
18,207
|
|
7,175
|
|
—
|
|
|||||
|
Systemic intracellular therapeutics
|
45,695
|
|
3,093
|
|
—
|
|
|||||
|
Total program-specific expenses by modality
(1)
|
140,693
|
|
131,998
|
|
32,724
|
|
|||||
|
Other research and development expenses:
|
|||||||||||
|
Discovery programs
|
34,643
|
|
40,190
|
|
52,360
|
|
|||||
|
Platform research
|
91,720
|
|
86,473
|
|
83,414
|
|
|||||
|
Technical development and unallocated manufacturing expenses
|
83,117
|
|
29,606
|
|
36,016
|
|
|||||
|
Shared discovery and development expenses
|
44,250
|
|
47,513
|
|
49,516
|
|
|||||
|
Stock-based compensation
|
37,659
|
|
21,679
|
|
20,687
|
|
|||||
|
Other expenses
(2)
|
22,000
|
|
53,000
|
|
—
|
|
|||||
|
Total research and development expenses
|
$
|
454,082
|
|
$
|
410,459
|
|
$
|
274,717
|
|
||
|
(1)
|
Includes a total of 21, 18 and 12 development candidates at
December 31, 2018
,
2017
and
2016
, respectively. As of February 15, 2019, we have a total of 20 development candidates, as there will be no further development of our first Zika candidate, mRNA-1325. Program-specific expenses include external costs and allocated manufacturing costs of mRNA supply and consumables, and reflect these expenses beginning in the period the program was internally advanced to development.
|
|
(2)
|
Relates to in-licensing agreements entered into in June 2017 with Cellscript, LLC and its affiliate mRNA RiboTherapeutics, Inc. to sublicense certain patent rights.
|
|
•
|
Prophylactic vaccines
included eight development candidates: RSV vaccine (mRNA-1777), CMV vaccine (mRNA-1647), hMPV+PIV3 vaccine (mRNA-1653), VZV vaccine (mRNA-1278), H10N8 vaccine (mRNA-1440), H7N9 vaccine (mRNA-1851), Zika vaccine (mRNA-1893), and Chikungunya vaccine (mRNA-1388). We currently have seven programs for which the Phase 1 trial is either ongoing or has been completed;
|
|
•
|
Cancer vaccines
included two development candidates: Personalized cancer vaccine, or PCV, (mRNA-4157) and KRAS vaccine (mRNA-5671). We are collaborating with Merck on both programs. PCV is in a Phase 1 clinical trial and we and Merck have submitted a protocol to the FDA for a randomized Phase 2 clinical trial of mRNA-4157, and the KRAS vaccine has an open IND;
|
|
•
|
Intratumoral immuno-oncology
included three development candidates: OX40L (mRNA-2416), OX40L+IL23+IL36γ (Triplet) (mRNA-2752), and IL12 (MEDI1191). The OX40L and OX40L+IL23+IL36γ programs are currently in Phase 1 clinical trials and the IND for a Phase 1 clinical trial of IL12 is open;
|
|
•
|
Localized regenerative therapeutics
included one development candidate, VEGF-A (AZD8601). The program is being led by AstraZeneca through clinical development and is in a Phase 2a clinical trial;
|
|
•
|
Systemic secreted therapeutics
included three development candidates: antibody against Chikungunya virus (mRNA-1944), Relaxin (AZD7970), and Fabry disease (mRNA-3630). The antibody against Chikungunya virus development candidate is in collaboration with DARPA and the program is in a Phase 1 clinical trial. The Relaxin program in collaboration with AstraZeneca and the Fabry disease program are both in preclinical development; and
|
|
•
|
Systemic intracellular therapeutics
included three development candidates: MMA (mRNA-3704), PA (mRNA-3927), and PKU (mRNA-3283). The MMA program has an open IND, and the PA and PKU programs are in preclinical development.
|
|
•
|
scope, progress, and expense of developing ongoing and future development candidates and investigational medicines;
|
|
•
|
entry in and completion of related preclinical studies;
|
|
•
|
enrollment in and completion of subsequent clinical trials;
|
|
•
|
safety and efficacy of investigational medicines resulting from these clinical trials;
|
|
•
|
changes in laws or regulations relevant to the investigational medicines in development;
|
|
•
|
receipt of the required regulatory approvals; and
|
|
•
|
commercialization, including establishing manufacturing and marketing capabilities.
|
|
•
|
persuasive evidence of an arrangement;
|
|
•
|
delivery has occurred or services have been rendered;
|
|
•
|
the seller’s price to the buyer is fixed or determinable; and
|
|
•
|
collectability is reasonably assured.
|
|
•
|
CROs to conduct our clinical trials;
|
|
•
|
investigative sites in connection with clinical trials;
|
|
•
|
vendors for laboratory services, supplies, and distribution of materials in connection with clinical trials; and
|
|
•
|
vendors in connection with preclinical development activities.
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
|
$
|
122,512
|
|
$
|
176,974
|
|
$
|
101,536
|
|
||
|
Grant revenue
|
12,556
|
|
28,851
|
|
6,860
|
|
|||||
|
Total revenue
|
135,068
|
|
205,825
|
|
108,396
|
|
|||||
|
Operating Expenses:
|
|||||||||||
|
Research and development
|
454,082
|
|
410,459
|
|
274,717
|
|
|||||
|
General and administrative
|
94,252
|
|
64,722
|
|
57,450
|
|
|||||
|
Total operating expenses
|
548,334
|
|
475,181
|
|
332,167
|
|
|||||
|
Loss from operations
|
(413,266
|
)
|
(269,356
|
)
|
(223,771
|
)
|
|||||
|
Other income, net
|
28,858
|
|
13,360
|
|
8,603
|
|
|||||
|
Loss before provision for (benefit from) income taxes
|
(384,408
|
)
|
(255,996
|
)
|
(215,168
|
)
|
|||||
|
Provision for (benefit from) income taxes
|
326
|
|
(80
|
)
|
1,043
|
|
|||||
|
Net loss
|
$
|
(384,734
|
)
|
$
|
(255,916
|
)
|
$
|
(216,211
|
)
|
||
|
Years Ended December 31,
|
Change 2018 vs. 2017
|
Change 2017 vs. 2016
|
|||||||||||||||||||||||
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Collaboration revenue
|
$
|
122,512
|
|
$
|
176,974
|
|
$
|
101,536
|
|
$
|
(54,462
|
)
|
(31
|
)%
|
$
|
75,438
|
|
74
|
%
|
||||||
|
Grant revenue
|
12,556
|
|
28,851
|
|
6,860
|
|
(16,295
|
)
|
(56
|
)%
|
21,991
|
|
321
|
%
|
|||||||||||
|
Total revenue
|
$
|
135,068
|
|
$
|
205,825
|
|
$
|
108,396
|
|
$
|
(70,757
|
)
|
(34
|
)%
|
$
|
97,429
|
|
90
|
%
|
||||||
|
Years Ended December 31,
|
Change 2018 vs. 2017
|
Change 2017 vs. 2016
|
|||||||||||||||||||||||
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Research and development
|
$
|
454,082
|
|
$
|
410,459
|
|
$
|
274,717
|
|
$
|
43,623
|
|
11
|
%
|
$
|
135,742
|
|
49
|
%
|
||||||
|
General and administrative
|
94,252
|
|
64,722
|
|
57,450
|
|
29,530
|
|
46
|
%
|
7,272
|
|
13
|
%
|
|||||||||||
|
Total operating expenses
|
$
|
548,334
|
|
$
|
475,181
|
|
$
|
332,167
|
|
$
|
73,153
|
|
15
|
%
|
$
|
143,014
|
|
43
|
%
|
||||||
|
Years Ended December 31,
|
Change 2018 vs. 2017
|
Change 2017 vs. 2016
|
|||||||||||||||||||||||
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Interest income
|
$
|
27,023
|
|
$
|
15,235
|
|
$
|
11,312
|
|
$
|
11,788
|
|
77
|
%
|
$
|
3,923
|
|
35
|
%
|
||||||
|
Gain (loss) on investment
|
31
|
|
(1,085
|
)
|
(2,399
|
)
|
1,116
|
|
(103
|
)%
|
1,314
|
|
(55
|
)%
|
|||||||||||
|
Interest expense
|
(3,096
|
)
|
(132
|
)
|
(139
|
)
|
(2,964
|
)
|
2245
|
%
|
7
|
|
(5
|
)%
|
|||||||||||
|
Other income (expense), net
|
4,900
|
|
(658
|
)
|
(171
|
)
|
5,558
|
|
(845
|
)%
|
(487
|
)
|
285
|
%
|
|||||||||||
|
Total other income, net
|
$
|
28,858
|
|
$
|
13,360
|
|
$
|
8,603
|
|
$
|
15,498
|
|
116
|
%
|
$
|
4,757
|
|
55
|
%
|
||||||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net cash provided by (used in):
|
|||||||||||
|
Operating activities
|
$
|
(330,865
|
)
|
$
|
(331,484
|
)
|
$
|
66,734
|
|
||
|
Investing activities
|
(372,472
|
)
|
416,095
|
|
(648,607
|
)
|
|||||
|
Financing activities
|
1,226,842
|
|
168
|
|
472,910
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
523,505
|
|
$
|
84,779
|
|
$
|
(108,963
|
)
|
||
|
•
|
leverage our platform to expand our programs or advance our programs into preclinical and clinical development;
|
|
•
|
further develop our current programs;
|
|
•
|
seek to research and develop additional programs;
|
|
•
|
seek to research and develop additional modalities of mRNA medicines;
|
|
•
|
seek regulatory approvals for any investigational medicines that successfully complete clinical trials;
|
|
•
|
increase manufacturing capacity and production volume;
|
|
•
|
hire additional clinical, manufacturing, quality control, and scientific personnel, expand our operational, financial, and management systems, and increase personnel, including personnel to support our clinical development and manufacturing efforts and our operations as a public company;
|
|
•
|
establish a sales, marketing, medical affairs, and distribution infrastructure to commercialize any investigational medicine for which we may obtain marketing approval and intend to commercialize on our own or jointly;
|
|
•
|
maintain, expand, and protect our intellectual property portfolio; and
|
|
•
|
acquire or in-license other programs and technologies.
|
|
•
|
the rate of progress in the development of our development candidates;
|
|
•
|
the initiation, progress, timing, costs, and results of clinical trials for our investigational medicines and future investigational medicines;
|
|
•
|
the number and characteristics of programs that we develop;
|
|
•
|
the costs of development efforts for our programs that are not subject to reimbursement from our strategic collaborators;
|
|
•
|
the costs of mRNA materials;
|
|
•
|
the costs necessary to obtain regulatory approvals, if any, for our investigational medicines in the United States and other jurisdictions, and the costs of post-marketing studies that could be required by regulatory authorities in jurisdictions where approval is obtained;
|
|
•
|
the continuation of our existing strategic alliances and entry into new collaborations;
|
|
•
|
the cost and timing of completion of additional manufacturing facilities and activities, including potential commercial-scale manufacturing;
|
|
•
|
the costs we incur in maintaining business operations;
|
|
•
|
the costs associated with being a public company;
|
|
•
|
the revenue from any future sales of any approved mRNA medicines for which we are entitled to a profit share, royalties and milestones;
|
|
•
|
the time and unreimbursed costs necessary to commercialize mRNA medicines in territories in which our investigational medicines are approved for sale;
|
|
•
|
the effect of competing technological and market developments; and
|
|
•
|
the costs we incur in the filing, prosecution, maintenance, and defense of our extensive patent portfolio and other intellectual property rights.
|
|
Payments Due by Period
|
|||||||||||||||||||
|
Total
|
Less than 1
year |
1 - 3 years
|
3 - 5 years
|
More than 5
years |
|||||||||||||||
|
Norwood leases
(1)
|
$
|
103,277
|
|
$
|
6,388
|
|
$
|
13,259
|
|
$
|
13,929
|
|
$
|
69,701
|
|
||||
|
Operating leases, excluding Norwood leases
(2)
|
108,492
|
|
13,639
|
|
28,082
|
|
23,513
|
|
43,258
|
|
|||||||||
|
Purchase obligations
(3)
|
2,478
|
|
937
|
|
1,541
|
|
—
|
|
—
|
|
|||||||||
|
License agreement obligations
(4)
|
22,000
|
|
22,000
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total contractual cash obligations
|
$
|
236,247
|
|
$
|
42,964
|
|
$
|
42,882
|
|
$
|
37,442
|
|
$
|
112,959
|
|
||||
|
(1)
|
We lease land and a building located in Norwood, MA. The Norwood leases for the building facilities, land, and adjacent land, will each expire in September 2032 with options to extend each of the terms for two extension periods of ten years each at then market-based rent. The amounts in the table above represent the fixed contractual lease obligations, and do not include the optional extensions. Please refer to Note 7 to our consolidated financial statements.
|
|
(2)
|
We have various lease agreements for office and laboratory space in Cambridge, MA, expiring at various times through December 2027.
|
|
(3)
|
The amounts represent non-cancellable fixed payment obligations under certain clinical service agreements.
|
|
(4)
|
We have license agreements with a non-cancelable fixed payment obligation with Cellscript, LLC and its affiliate mRNA RiboTherapeutics, Inc. We have not included variable and contingent payments including annual license maintenance fees, potential milestone payments, and royalty payments because each of these amounts are not fixed and estimable. Cellscript, LLC and its affiliate mRNA RiboTherapeutics, Inc. are, however, eligible to receive, on a product-by-product basis, milestone payments upon the achievement of development, regulatory and commercial milestones totaling up to $25.5 million for therapeutic and prophylactic products and $0.5 million for diagnostic products. Additionally, we have other in-license agreements with third parties which require us to make future development, regulatory and commercial milestone payments for specified products associated with the agreements. The achievement of these milestones has not occurred and such milestone payments are immaterial.
|
|
Payments Due by Period
|
|||||||||||||||||||
|
Total
|
Less than 1
year |
1 - 3 years
|
3 - 5 years
|
More than 5
years |
|||||||||||||||
|
New Norwood lease, net of sublease income
|
$
|
39,336
|
|
$
|
—
|
|
$
|
5,401
|
|
$
|
6,510
|
|
$
|
27,425
|
|
||||
|
December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
658,364
|
|
$
|
134,859
|
|
|
|
Investments
|
863,063
|
|
621,170
|
|
|||
|
Accounts receivable
|
11,686
|
|
11,881
|
|
|||
|
Accounts receivable from affiliate (Note 14)
|
899
|
|
1,536
|
|
|||
|
Prepaid expenses and other current assets
|
28,399
|
|
12,826
|
|
|||
|
Restricted cash
|
595
|
|
951
|
|
|||
|
Total current assets
|
1,563,006
|
|
783,223
|
|
|||
|
Investments, non-current
|
172,990
|
|
145,851
|
|
|||
|
Property and equipment, net
|
211,977
|
|
139,031
|
|
|||
|
Restricted cash, non-current
|
11,532
|
|
11,798
|
|
|||
|
Other non-current assets
|
2,644
|
|
4,586
|
|
|||
|
Total assets
|
$
|
1,962,149
|
|
$
|
1,084,489
|
|
|
|
Liabilities, Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
31,210
|
|
$
|
20,725
|
|
|
|
Accrued liabilities
|
79,073
|
|
72,715
|
|
|||
|
Deferred revenue
|
109,056
|
|
96,739
|
|
|||
|
Other current liabilities
|
3,464
|
|
1,282
|
|
|||
|
Total current liabilities
|
222,803
|
|
191,461
|
|
|||
|
Deferred revenue, non-current
|
165,352
|
|
242,929
|
|
|||
|
Deferred lease obligation, non-current
|
10,006
|
|
7,586
|
|
|||
|
Lease financing obligation
|
33,489
|
|
15,687
|
|
|||
|
Other non-current liabilities
|
258
|
|
1,530
|
|
|||
|
Total liabilities
|
431,908
|
|
459,193
|
|
|||
|
Commitments and contingencies (Note 7)
|
|
|
|||||
|
Redeemable convertible preferred stock, par value $0.0001; 0 and 448,686,791 shares authorized as of December 31, 2018 and 2017, respectively; 0 and 448,686,791 shares issued and outstanding as of December 31, 2018 and 2017, respectively; aggregate liquidation preference of $0 and $1,209,940 as of December 31, 2018 and 2017, respectively
|
—
|
|
1,176,661
|
|
|||
|
Stockholders’ equity (deficit):
|
|||||||
|
Preferred stock, $0.0001 par value; 162,000,000 and 0 shares authorized at December 31, 2018
and 2017, respectively; 0 shares issued or outstanding at December 31, 2018 and 2017
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.0001; 1,600,000,000 and 696,581,112 shares authorized as of December 31, 2018 and 2017, respectively; 328,798,904 and 65,206,999 shares issued and outstanding as of December 31, 2018 and 2017, respectively
|
33
|
|
6
|
|
|||
|
Additional paid-in capital
|
2,538,155
|
|
71,679
|
|
|||
|
Accumulated other comprehensive loss
|
(1,320
|
)
|
(1,157
|
)
|
|||
|
Accumulated deficit
|
(1,006,627
|
)
|
(621,893
|
)
|
|||
|
Total stockholders’ equity (deficit)
|
1,530,241
|
|
(551,365
|
)
|
|||
|
Total liabilities, redeemable convertible preferred stock and stockholders’ equity
|
$
|
1,962,149
|
|
$
|
1,084,489
|
|
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
|
$
|
76,519
|
|
$
|
146,953
|
|
$
|
69,109
|
|
||
|
Collaboration revenue from affiliate (Note 14)
|
45,993
|
|
30,021
|
|
32,427
|
|
|||||
|
Grant revenue
|
12,556
|
|
28,851
|
|
6,860
|
|
|||||
|
Total revenue
|
135,068
|
|
205,825
|
|
108,396
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
454,082
|
|
410,459
|
|
274,717
|
|
|||||
|
General and administrative
|
94,252
|
|
64,722
|
|
57,450
|
|
|||||
|
Total operating expenses
|
548,334
|
|
475,181
|
|
332,167
|
|
|||||
|
Loss from operations
|
(413,266
|
)
|
(269,356
|
)
|
(223,771
|
)
|
|||||
|
Interest income
|
27,023
|
|
15,235
|
|
11,312
|
|
|||||
|
Other income (expense), net
|
1,835
|
|
(1,875
|
)
|
(2,709
|
)
|
|||||
|
Loss before provision for (benefit from) income taxes
|
(384,408
|
)
|
(255,996
|
)
|
(215,168
|
)
|
|||||
|
Provision for (benefit from) income taxes
|
326
|
|
(80
|
)
|
1,043
|
|
|||||
|
Net loss
|
(384,734
|
)
|
(255,916
|
)
|
(216,211
|
)
|
|||||
|
Reconciliation of net loss to net loss attributable to common stockholders:
|
|||||||||||
|
Premium paid on repurchase of preferred stock
|
(4,127
|
)
|
—
|
|
—
|
|
|||||
|
Accretion of redeemable convertible preferred units to redemption value
|
—
|
|
—
|
|
(8,663
|
)
|
|||||
|
Cumulative preferred stock dividends
|
(12,996
|
)
|
(13,925
|
)
|
(5,440
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(401,857
|
)
|
$
|
(269,841
|
)
|
$
|
(230,314
|
)
|
||
|
Net loss per share attributable to common stockholders, basic and diluted
|
$
|
(4.95
|
)
|
$
|
(4.18
|
)
|
$
|
(3.79
|
)
|
||
|
Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted
|
81,114,183
|
|
64,497,544
|
|
60,747,426
|
|
|||||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net loss
|
$
|
(384,734
|
)
|
$
|
(255,916
|
)
|
$
|
(216,211
|
)
|
||
|
Other comprehensive (loss) income:
|
|||||||||||
|
Unrealized (loss) gain on available-for-sale debt securities
|
(132
|
)
|
(342
|
)
|
223
|
|
|||||
|
Less: Amounts recognized for net realized (gain) included in net loss
|
(31
|
)
|
(412
|
)
|
(60
|
)
|
|||||
|
Total other comprehensive (loss) income
|
(163
|
)
|
(754
|
)
|
163
|
|
|||||
|
Comprehensive loss
|
$
|
(384,897
|
)
|
$
|
(256,670
|
)
|
$
|
(216,048
|
)
|
||
|
Redeemable
Convertible
Preferred Units
|
Redeemable Convertible
Preferred Stock
|
Common Units
|
Common Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
Loss
|
Accumulated
(Deficit) Equity
|
Total
Stockholders’
Equity
(Deficit)
|
|||||||||||||||||||||||||||||||||||||
|
Units
|
Amount
|
Shares
|
Amount
|
Units
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||
|
Balance at December 31, 2015
|
394,685,560
|
|
$
|
695,574
|
|
—
|
|
$
|
—
|
|
59,121,793
|
|
$
|
6
|
|
—
|
|
$
|
—
|
|
$
|
7
|
|
$
|
(566
|
)
|
$
|
(149,207
|
)
|
$
|
(149,760
|
)
|
||||||||||||
|
Vesting of restricted common
stock
|
—
|
|
—
|
|
—
|
|
—
|
|
143,348
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||
|
Exercise of options to
purchase common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
18,348
|
|
—
|
|
—
|
|
—
|
|
6
|
|
—
|
|
—
|
|
6
|
|
||||||||||||||||||||
|
Accretion of redeemable
convertible preferred
units
|
—
|
|
8,663
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(8,663
|
)
|
—
|
|
—
|
|
(8,663
|
)
|
||||||||||||||||||||
|
Distribution to unit holders
|
—
|
|
(1,108
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(363
|
)
|
—
|
|
—
|
|
(363
|
)
|
||||||||||||||||||||
|
Exchange of redeemable
convertible preferred
units and common units
for redeemable
convertible preferred
stock and common
stock, respectively, in
connection with
reorganization
|
(394,685,560
|
)
|
(703,129
|
)
|
394,685,550
|
|
703,129
|
|
(59,283,489
|
)
|
(6
|
)
|
59,244,956
|
|
6
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||
|
Issuance of Series F
redeemable convertible preferred stock, net of issuance costs of $599 |
—
|
|
—
|
|
54,001,241
|
|
473,532
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||
|
Vesting of restricted
common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,291,916
|
|
—
|
|
300
|
|
—
|
|
(300
|
)
|
—
|
|
||||||||||||||||||||
|
Exercise of options to
purchase common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,816
|
|
—
|
|
5
|
|
—
|
|
—
|
|
5
|
|
||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
40,013
|
|
—
|
|
—
|
|
40,013
|
|
||||||||||||||||||||
|
Unrealized gain on
marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
163
|
|
—
|
|
163
|
|
||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(216,211
|
)
|
(216,211
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2016
|
—
|
|
—
|
|
448,686,791
|
|
1,176,661
|
|
—
|
|
—
|
|
63,541,688
|
|
6
|
|
31,305
|
|
(403
|
)
|
(365,718
|
)
|
(334,810
|
)
|
||||||||||||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,644,769
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||
|
Exercise of options to purchase common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
20,542
|
|
—
|
|
212
|
|
—
|
|
—
|
|
212
|
|
||||||||||||||||||||
|
Redeemable convertible preferred stock issuance costs
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(152
|
)
|
—
|
|
—
|
|
(152
|
)
|
||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
40,314
|
|
—
|
|
(259
|
)
|
40,055
|
|
||||||||||||||||||||
|
Unrealized loss on marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(754
|
)
|
—
|
|
(754
|
)
|
||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(255,916
|
)
|
(255,916
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2017
|
—
|
|
—
|
|
448,686,791
|
|
1,176,661
|
|
—
|
|
—
|
|
65,206,999
|
|
6
|
|
71,679
|
|
(1,157
|
)
|
(621,893
|
)
|
(551,365
|
)
|
||||||||||||||||||||
|
Redeemable
Convertible
Preferred Units
|
Redeemable Convertible
Preferred Stock
|
Common Units
|
Common Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
Loss
|
Accumulated
(Deficit) Equity
|
Total
Stockholders’
Equity
(Deficit)
|
||||||||||||||||||||||||||||||||||||
|
Units
|
Amount
|
Shares
|
Amount
|
Units
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
—
|
|
—
|
|
448,686,791
|
|
$
|
1,176,661
|
|
—
|
|
$
|
—
|
|
65,206,999
|
|
$
|
6
|
|
$
|
71,679
|
|
$
|
(1,157
|
)
|
$
|
(621,893
|
)
|
$
|
(551,365
|
)
|
||||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
856,135
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||
|
Issuance of Series G redeemable convertible preferred stock, net of issuance costs of $10,517
|
—
|
|
—
|
|
55,666,004
|
|
549,413
|
|
—
|
|
—
|
|
—
|
|
—
|
|
51
|
|
—
|
|
—
|
|
51
|
|
|||||||||||||||||||
|
Issuance of Series H redeemable convertible preferred stock, net of issuance costs of $474
|
—
|
|
—
|
|
5,000,000
|
|
111,546
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||
|
Repurchase of Series D redeemable convertible preferred stock
|
—
|
|
—
|
|
(269,180
|
)
|
(704
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,009
|
)
|
—
|
|
—
|
|
(2,009
|
)
|
|||||||||||||||||||
|
Repurchase of Series E redeemable convertible preferred stock
|
—
|
|
—
|
|
(544,100
|
)
|
(3,355
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,118
|
)
|
—
|
|
—
|
|
(2,118
|
)
|
|||||||||||||||||||
|
Exercise of options to purchase common stock, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
446,864
|
|
—
|
|
1,427
|
|
—
|
|
—
|
|
1,427
|
|
|||||||||||||||||||
|
Conversion of redeemable convertible
preferred stock into common stock
|
—
|
|
—
|
|
(508,539,515
|
)
|
(1,833,561
|
)
|
—
|
|
—
|
|
236,012,913
|
|
24
|
|
1,833,537
|
|
—
|
|
—
|
|
1,833,561
|
|
|||||||||||||||||||
|
Proceeds of initial public offering, net of
issuance costs of $41,322
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
26,275,993
|
|
3
|
|
563,023
|
|
—
|
|
—
|
|
563,026
|
|
|||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
72,565
|
|
—
|
|
—
|
|
72,565
|
|
|||||||||||||||||||
|
Unrealized loss on marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(163
|
)
|
—
|
|
(163
|
)
|
|||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(384,734
|
)
|
(384,734
|
)
|
|||||||||||||||||||
|
Balance at December 31, 2018
|
—
|
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
328,798,904
|
|
$
|
33
|
|
$
|
2,538,155
|
|
$
|
(1,320
|
)
|
$
|
(1,006,627
|
)
|
$
|
1,530,241
|
|
||||||||||||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Operating activities
|
|||||||||||
|
Net loss
|
$
|
(384,734
|
)
|
$
|
(255,916
|
)
|
$
|
(216,211
|
)
|
||
|
Adjustments to reconcile net loss to net cash (used in) provided by operating activities:
|
|||||||||||
|
Stock-based compensation
|
72,565
|
|
40,055
|
|
39,360
|
|
|||||
|
Depreciation and amortization
|
24,862
|
|
20,537
|
|
15,114
|
|
|||||
|
Amortization of investment premiums and discounts
|
(1,866
|
)
|
1,086
|
|
2,478
|
|
|||||
|
Loss on disposal of property and equipment
|
891
|
|
—
|
|
—
|
|
|||||
|
Changes in assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
195
|
|
1,476
|
|
(8,642
|
)
|
|||||
|
Accounts receivable from affiliate (Note 14)
|
637
|
|
(1,370
|
)
|
60,979
|
|
|||||
|
Prepaid expenses and other assets
|
(5,289
|
)
|
579
|
|
(7,887
|
)
|
|||||
|
Accounts payable
|
15,017
|
|
(12,766
|
)
|
5,993
|
|
|||||
|
Accrued liabilities
|
8,787
|
|
34,369
|
|
5,328
|
|
|||||
|
Deferred revenue
|
(65,260
|
)
|
(162,321
|
)
|
164,129
|
|
|||||
|
Deferred lease obligation
|
2,420
|
|
2,932
|
|
3,828
|
|
|||||
|
Other liabilities
|
910
|
|
(145
|
)
|
1,977
|
|
|||||
|
Deferred income taxes
|
—
|
|
—
|
|
288
|
|
|||||
|
Net cash (used in) provided by operating activities
|
(330,865
|
)
|
(331,484
|
)
|
66,734
|
|
|||||
|
Investing activities
|
|||||||||||
|
Purchases of marketable securities
|
(1,227,709
|
)
|
(727,203
|
)
|
(1,415,461
|
)
|
|||||
|
Proceeds from maturities of marketable securities
|
783,373
|
|
800,438
|
|
675,200
|
|
|||||
|
Proceeds from sales of marketable securities
|
177,008
|
|
402,530
|
|
133,700
|
|
|||||
|
Purchases of property and equipment
|
(105,766
|
)
|
(58,401
|
)
|
(33,144
|
)
|
|||||
|
Decrease (increase) in restricted cash
|
622
|
|
(1,269
|
)
|
(8,902
|
)
|
|||||
|
Net cash (used in) provided by investing activities
|
(372,472
|
)
|
416,095
|
|
(648,607
|
)
|
|||||
|
Financing activities
|
|||||||||||
|
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs
|
661,111
|
|
—
|
|
473,532
|
|
|||||
|
Proceeds from initial public offering of common stock, net of issuance costs
|
563,026
|
|
—
|
|
—
|
|
|||||
|
Repurchases of redeemable convertible preferred stock
|
(8,182
|
)
|
—
|
|
—
|
|
|||||
|
Distributions to preferred and common unit holders
|
—
|
|
(1,483
|
)
|
(633
|
)
|
|||||
|
Proceeds from issuance of common stock through equity plans
|
1,427
|
|
212
|
|
11
|
|
|||||
|
Reimbursement of assets under financing lease obligation
|
11,635
|
|
2,724
|
|
—
|
|
|||||
|
Payments on financing lease obligation
|
(2,175
|
)
|
(1,285
|
)
|
—
|
|
|||||
|
Net cash provided by financing activities
|
1,226,842
|
|
168
|
|
472,910
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
523,505
|
|
84,779
|
|
(108,963
|
)
|
|||||
|
Cash and cash equivalents, beginning of year
|
134,859
|
|
50,080
|
|
159,043
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
658,364
|
|
$
|
134,859
|
|
$
|
50,080
|
|
||
|
Supplemental cash flow information
|
|||||||||||
|
Income taxes paid
|
$
|
294
|
|
$
|
398
|
|
$
|
905
|
|
||
|
Interest in connection with financing lease obligation
|
$
|
2,998
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Non-cash investing and financing activities
|
|||||||||||
|
Issuance costs included in accounts payable and accrued liabilities
|
$
|
2,638
|
|
$
|
152
|
|
$
|
89
|
|
||
|
Purchases of property and equipment included in accounts payable and accrued liabilities
|
$
|
12,892
|
|
$
|
19,959
|
|
$
|
10,014
|
|
||
|
Leasehold improvements included in prepaid and other current assets
|
$
|
10,089
|
|
$
|
1,748
|
|
$
|
—
|
|
||
|
Lease financing obligation (Note 7)
|
$
|
10,089
|
|
$
|
1,748
|
|
$
|
12,500
|
|
||
|
Dividends and accretion of redeemable convertible preferred units
|
$
|
—
|
|
$
|
—
|
|
$
|
8,663
|
|
||
|
Tax distributions to members included in accounts payable and accrued liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
1,464
|
|
||
|
Percentage of Revenue
Years Ended December 31,
|
Percentage of
Accounts Receivable
December 31,
|
|||||||||||||
|
2018
|
2017
|
2016
|
2018
|
2017
|
||||||||||
|
Merck
|
49
|
%
|
31
|
%
|
44
|
%
|
30
|
%
|
13
|
%
|
||||
|
AstraZeneca
|
34
|
%
|
15
|
%
|
30
|
%
|
*
|
|
11
|
%
|
||||
|
Alexion
|
*
|
|
36
|
%
|
16
|
%
|
*
|
|
*
|
|
||||
|
Vertex
|
*
|
|
*
|
|
*
|
|
22
|
%
|
*
|
|
||||
|
BARDA
|
*
|
|
10
|
%
|
*
|
|
13
|
%
|
31
|
%
|
||||
|
DARPA
|
*
|
|
*
|
|
*
|
|
16
|
%
|
18
|
%
|
||||
|
Massachusetts Life Sciences Center
|
*
|
|
*
|
|
*
|
|
12
|
%
|
*
|
|
||||
|
•
|
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
|
|
•
|
Level 2: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; or
|
|
•
|
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
|
|
Estimated Useful Life
|
||
|
Building
|
34 years
|
|
|
Laboratory equipment
|
5 years
|
|
|
Leasehold improvements
|
Lesser of estimated useful life of improvement
or remaining life of related lease
|
|
|
Computer equipment and software
|
3 years
|
|
|
Other assets including automobiles, furniture and fixtures
|
5 years
|
|
|
Unrealized Gain (Loss) on Available-for-Sale Debt
Securities
|
|||
|
Accumulated other comprehensive loss, balance at December 31, 2016
|
$
|
(403
|
)
|
|
Other comprehensive loss
|
(754
|
)
|
|
|
Accumulated other comprehensive loss, balance at December 31, 2017
|
(1,157
|
)
|
|
|
Other comprehensive loss
|
(163
|
)
|
|
|
Accumulated other comprehensive loss, balance at December 31, 2018
|
$
|
(1,320
|
)
|
|
December 31, 2018
|
||||||||||||||||||||||||||||
|
Amortized
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Cash and
Cash
Equivalents
|
Current
Marketable
Securities
|
Non-
Current
Marketable
Securities
|
||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
658,365
|
|
$
|
20
|
|
$
|
(21
|
)
|
$
|
658,364
|
|
$
|
658,364
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Available-for-sale:
|
||||||||||||||||||||||||||||
|
Level 2:
|
||||||||||||||||||||||||||||
|
Certificates of deposit
|
173,102
|
|
42
|
|
(36
|
)
|
173,108
|
|
—
|
|
157,920
|
|
15,188
|
|
||||||||||||||
|
U.S. treasury securities
|
152,205
|
|
18
|
|
(48
|
)
|
152,175
|
|
—
|
|
152,175
|
|
—
|
|
||||||||||||||
|
Debt securities of U.S. government agencies and corporate entities
|
712,065
|
|
40
|
|
(1,335
|
)
|
710,770
|
|
—
|
|
552,968
|
|
157,802
|
|
||||||||||||||
|
$
|
1,695,737
|
|
$
|
120
|
|
$
|
(1,440
|
)
|
$
|
1,694,417
|
|
$
|
658,364
|
|
$
|
863,063
|
|
$
|
172,990
|
|
||||||||
|
December 31, 2017
|
||||||||||||||||||||||||||||
|
Amortized
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Cash and
Cash
Equivalents
|
Current
Marketable
Securities
|
Non-
Current
Marketable
Securities
|
||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
134,859
|
|
$
|
—
|
|
$
|
—
|
|
$
|
134,859
|
|
$
|
134,859
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Available-for-sale:
|
||||||||||||||||||||||||||||
|
Level 2:
|
||||||||||||||||||||||||||||
|
Certificates of deposit
|
245,884
|
|
35
|
|
(218
|
)
|
245,701
|
|
—
|
|
198,398
|
|
47,303
|
|
||||||||||||||
|
U.S. treasury securities
|
118,278
|
|
—
|
|
(354
|
)
|
117,924
|
|
—
|
|
117,924
|
|
—
|
|
||||||||||||||
|
Debt securities of U.S. government agencies and corporate entities
|
404,016
|
|
61
|
|
(681
|
)
|
403,396
|
|
—
|
|
304,848
|
|
98,548
|
|
||||||||||||||
|
$
|
903,037
|
|
$
|
96
|
|
$
|
(1,253
|
)
|
$
|
901,880
|
|
$
|
134,859
|
|
$
|
621,170
|
|
$
|
145,851
|
|
||||||||
|
December 31, 2018
|
||||||||
|
Amortized
Cost
|
Estimated
Fair Value
|
|||||||
|
Due in one year or less
|
$
|
864,003
|
|
$
|
863,063
|
|
||
|
Due after one year through five years
|
173,369
|
|
172,990
|
|
||||
|
Total
|
$
|
1,037,372
|
|
$
|
1,036,053
|
|
||
|
December 31, 2017
|
||||||||
|
Amortized
Cost
|
Estimated
Fair Value
|
|||||||
|
Due in one year or less
|
$
|
622,020
|
|
$
|
621,170
|
|
||
|
Due after one year through five years
|
146,158
|
|
145,851
|
|
||||
|
Total
|
$
|
768,178
|
|
$
|
767,021
|
|
||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Prepaid expenses
|
$
|
10,401
|
|
$
|
7,839
|
|
||
|
Tenant incentives receivables
|
10,089
|
|
1,748
|
|
||||
|
Interest receivable on marketable securities
|
7,909
|
|
3,239
|
|
||||
|
Prepaid expenses and other current assets
|
$
|
28,399
|
|
$
|
12,826
|
|
||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Building
|
$
|
140,442
|
|
$
|
—
|
|
||
|
Laboratory equipment
|
96,907
|
|
77,351
|
|
||||
|
Leasehold improvements
|
13,741
|
|
12,222
|
|
||||
|
Furniture, fixtures and other
|
2,122
|
|
290
|
|
||||
|
Computer equipment and software
|
11,513
|
|
9,420
|
|
||||
|
Internally developed software
|
7,020
|
|
7,020
|
|
||||
|
Construction in progress
|
4,688
|
|
80,759
|
|
||||
|
276,433
|
|
187,062
|
|
|||||
|
Less: Accumulated depreciation
|
(64,456
|
)
|
(48,031
|
)
|
||||
|
Property and equipment, net
|
$
|
211,977
|
|
$
|
139,031
|
|
||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
In-licenses
|
$
|
22,000
|
|
$
|
25,000
|
|
||
|
Property and equipment
|
12,089
|
|
14,624
|
|
||||
|
Compensation-related
|
23,406
|
|
18,221
|
|
||||
|
External goods and services
|
21,578
|
|
14,870
|
|
||||
|
Accrued liabilities
|
$
|
79,073
|
|
$
|
72,715
|
|
||
|
Years ending December 31
(1)
:
|
||||
|
2019
|
$
|
20,027
|
|
|
|
2020
|
20,404
|
|
||
|
2021
|
20,937
|
|
||
|
2022
|
20,208
|
|
||
|
2023
|
17,235
|
|
||
|
Thereafter
|
112,958
|
|
||
|
Total
|
$
|
211,769
|
|
|
|
•
|
Each outstanding redeemable convertible preferred unit of Series A, B, C, D and E of Moderna LLC was exchanged for shares of Series A, B, C, D and E redeemable convertible preferred stock, respectively, of Moderna.
|
|
•
|
Each outstanding common unit of Moderna LLC was exchanged for shares of common stock of Moderna, and if such outstanding unit was subject to vesting at the time of such exchange, then such common stock was issued by Moderna subject to continued vesting to the same extent as such outstanding common unit.
|
|
•
|
Each outstanding incentive unit issued pursuant to Moderna LLC’s 2013 Equity Incentive Plan was exchanged for shares of restricted common stock of Moderna Therapeutics, Inc. under Moderna Therapeutics, Inc.’s 2016 Stock Option and Grant Plan. Additionally, incentive unit holders were granted options to purchase common stock of Moderna
|
|
•
|
Each outstanding option to purchase common units issued pursuant to Moderna LLC’s 2013 Unit Option and Grant Plan was exchanged for an option to purchase common stock of Moderna Therapeutics, Inc. under Moderna Therapeutics, Inc. 2016 Stock Option and Grant Plan, and if such outstanding unit option was subject to vesting at the time of such exchange, then such stock option was issued by Moderna Therapeutics, Inc. subject to continued vesting to the same extent as such outstanding unit option.
|
|
Redeemable
Convertible
Preferred
Shares
Authorized
|
Redeemable
Convertible Preferred Shares Issued and Outstanding |
Carrying
Value
|
Liquidation Preference
As of December 11,
2018
|
|||||||||||
|
Series A redeemable convertible preferred stock
|
42,000,000
|
|
42,000,000
|
|
$
|
182
|
|
$
|
2,859
|
|
||||
|
Series B redeemable convertible preferred stock
|
122,296,280
|
|
122,296,280
|
|
770
|
|
12,493
|
|
||||||
|
Series C redeemable convertible preferred stock
|
85,669,774
|
|
85,669,774
|
|
36,238
|
|
41,750
|
|
||||||
|
Series D redeemable convertible preferred stock
|
63,291,156
|
|
63,021,976
|
|
163,355
|
|
188,840
|
|
||||||
|
Series E redeemable convertible preferred stock
|
81,428,340
|
|
80,884,240
|
|
498,525
|
|
498,814
|
|
||||||
|
Series F redeemable convertible preferred stock
|
54,001,241
|
|
54,001,241
|
|
473,532
|
|
474,131
|
|
||||||
|
Series G redeemable convertible preferred stock
|
55,666,004
|
|
55,666,004
|
|
549,413
|
|
560,000
|
|
||||||
|
Series H redeemable convertible preferred stock
|
5,000,000
|
|
5,000,000
|
|
111,546
|
|
125,000
|
|
||||||
|
Balance at December 11, 2018
|
509,352,795
|
|
508,539,515
|
|
$
|
1,833,561
|
|
$
|
1,903,887
|
|
||||
|
Redeemable
Convertible
Preferred
Shares
Authorized
|
Redeemable
Convertible Preferred Shares Issued and Outstanding |
Liquidation Preference
As of December 31,
2017
|
||||||||||||
|
Carrying
Value
|
||||||||||||||
|
Series A redeemable convertible preferred stock
|
42,000,000
|
|
42,000,000
|
|
$
|
182
|
|
$
|
2,701
|
|
||||
|
Series B redeemable convertible preferred stock
|
122,296,280
|
|
122,296,280
|
|
770
|
|
11,801
|
|
||||||
|
Series C redeemable convertible preferred stock
|
85,669,774
|
|
85,669,774
|
|
36,238
|
|
39,676
|
|
||||||
|
Series D redeemable convertible preferred stock
|
63,291,156
|
|
63,291,156
|
|
164,059
|
|
179,462
|
|
||||||
|
Series E redeemable convertible preferred stock
|
81,428,340
|
|
81,428,340
|
|
501,880
|
|
502,169
|
|
||||||
|
Series F redeemable convertible preferred stock
|
54,001,241
|
|
54,001,241
|
|
473,532
|
|
474,131
|
|
||||||
|
Balance at December 31, 2017
|
448,686,791
|
|
448,686,791
|
|
$
|
1,176,661
|
|
$
|
1,209,940
|
|
||||
|
Series A
Redeemable
Convertible
Preferred
Stock
|
Series B
Redeemable
Convertible
Preferred
Stock
|
Series C
Redeemable
Convertible
Preferred
Stock
|
Series D
Redeemable
Convertible
Preferred
Stock
|
Series E
Redeemable
Convertible
Preferred
Stock
|
Series F
Redeemable
Convertible
Preferred
Stock
|
Series G
Redeemable
Convertible
Preferred
Stock
|
Series H
Redeemable
Convertible
Preferred
Stock
|
Total
Redeemable
Convertible
Preferred
Stock
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||||||||||
|
December 31, 2015
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||||||
|
Exchange of units for stock on 2016 Reorganization (split adjusted)
|
42,000,000
|
|
182
|
|
122,296,280
|
|
770
|
|
85,669,774
|
|
36,238
|
|
63,291,156
|
|
164,059
|
|
81,428,340
|
|
501,880
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
394,685,550
|
|
703,129
|
|
||||||||||||||||||||||||||
|
Issuance of Series F redeemable convertible preferred stock, net of issuance costs of $599
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
54,001,241
|
|
473,532
|
|
—
|
|
—
|
|
—
|
|
—
|
|
54,001,241
|
|
473,532
|
|
||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
42,000,000
|
|
$
|
182
|
|
122,296,280
|
|
$
|
770
|
|
85,669,774
|
|
$
|
36,238
|
|
63,291,156
|
|
$
|
164,059
|
|
81,428,340
|
|
$
|
501,880
|
|
54,001,241
|
|
$
|
473,532
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
448,686,791
|
|
$
|
1,176,661
|
|
|||||||||||||||||
|
Issuance of Series G redeemable convertible preferred stock, net of issuance costs of $10,517
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
55,666,004
|
|
549,413
|
|
—
|
|
—
|
|
55,666,004
|
|
549,413
|
|
||||||||||||||||||||||||||
|
Issuance of Series H redeemable convertible preferred stock, net of issuance costs of $474
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5,000,000
|
|
111,546
|
|
5,000,000
|
|
111,546
|
|
||||||||||||||||||||||||||
|
Repurchase of Series D redeemable convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(269,180
|
)
|
(704
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(269,180
|
)
|
(704
|
)
|
||||||||||||||||||||||||||
|
Repurchase of Series E redeemable convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(544,100
|
)
|
(3,355
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(544,100
|
)
|
(3,355
|
)
|
||||||||||||||||||||||||||
|
Conversion of redeemable convertible preferred stock
|
(42,000,000
|
)
|
(182
|
)
|
(122,296,280
|
)
|
(770
|
)
|
(85,669,774
|
)
|
(36,238
|
)
|
(63,021,976
|
)
|
(163,355
|
)
|
(80,884,240
|
)
|
(498,525
|
)
|
(54,001,241
|
)
|
(473,532
|
)
|
(55,666,004
|
)
|
(549,413
|
)
|
(5,000,000
|
)
|
(111,546
|
)
|
(508,539,515
|
)
|
(1,833,561
|
)
|
||||||||||||||||||||||||||
|
Balance at December 31, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
|||||||||||||||||
|
i.
|
first, to the holders of Series H redeemable convertible preferred stock and Series G redeemable convertible preferred stock, on a pari passu basis, an amount equal to the greater of (a) each respective original issue price plus dividends declared but unpaid or (b) such amount that would be payable had all respective shares been converted to common stock;
|
|
ii.
|
next, to the holders of the Series F redeemable convertible preferred stock an amount equal to the greater of (a) the original issue price plus dividends declared but unpaid or (b) such amount that would be payable had all respective shares been converted to common stock;
|
|
iii.
|
next, to the holders of the Series E redeemable convertible preferred stock an amount equal to the greater of (a) the original issue price plus dividends declared but unpaid or (b) such amount that would be payable had all respective shares been converted to common stock;
|
|
iv.
|
next, to the holders of the Series C redeemable convertible preferred stock an amount equal to the greater of (a) the original issue price plus the Series C redeemable convertible preferred stock dividends accrued but unpaid or (b) such amount that would be payable had all respective shares been converted to common stock;
|
|
v.
|
next,
pari passu
, in relation to the holders of the Series A redeemable convertible preferred stock an amount equal to the greater of (a) the original issue price plus the Series A redeemable convertible preferred stock dividends accrued but unpaid or (b) such amount that would be payable had all respective shares been converted to common stock and in relation to the holders of the Series B redeemable convertible preferred stock an amount equal to the greater of (a) the original issue price plus the Series B redeemable convertible preferred stock dividends accrued but unpaid or (b) such amount that would be payable had all respective shares been converted to common stock;
|
|
vi.
|
next, to the holders of the Series D redeemable convertible preferred stock an amount equal to the Series D redeemable convertible preferred stock dividends accrued but unpaid; and
|
|
vii.
|
finally, to all holders of common stock, pro rata based on the number of shares held by each such holder.
|
|
Number of
Options
|
Weighted-
Average
Exercise
Price per
Share
|
Weighted-
Average
Grant
Date Fair
Value per
Share
|
Weighted-
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic
Value
(1)
(in thousands)
|
||||||||||
|
Outstanding at December 31, 2017
|
33,684,134
|
|
9.31
|
|
4.91
|
|
7.8 years
|
130,587
|
|
|||||
|
Granted
|
19,223,123
|
|
17.01
|
|
9.33
|
|
||||||||
|
Exercised
|
(446,864
|
)
|
4.05
|
|
2.36
|
|
||||||||
|
Canceled/forfeited
|
(1,639,261
|
)
|
12.70
|
|
7.58
|
|
||||||||
|
Outstanding at December 31, 2018
|
50,821,132
|
|
12.16
|
|
6.59
|
|
7.1 years
|
220,434
|
|
|||||
|
Exercisable at December 31, 2018
|
22,118,203
|
|
7.86
|
|
3.53
|
|
5.8 years
|
179,459
|
|
|||||
|
Vested and expected to vest at December 31, 2018
|
28,705,512
|
|
15.48
|
|
8.95
|
|
8.2 years
|
40,983
|
|
|||||
|
Weighted Average
|
||||||||||||
|
Years Ended December 31,
|
||||||||||||
|
2018
|
2017
|
2016
|
||||||||||
|
Risk-free interest rate
|
2.76
|
%
|
2.02
|
%
|
1.65
|
%
|
||||||
|
Expected term
|
6.27 years
|
|
6.21 years
|
|
5.98 years
|
|
||||||
|
Expected volatility
|
63
|
%
|
63
|
%
|
68
|
%
|
||||||
|
Expected dividends
|
—
|
%
|
—
|
%
|
—
|
%
|
||||||
|
Weighted average fair value per share
|
$
|
9.33
|
|
$
|
7.96
|
|
$
|
3.82
|
|
|||
|
Number of
Shares |
Weighted
Average
Grant Date
per Share
|
|||||
|
Outstanding, non-vested at December 31, 2017
|
1,081,205
|
|
$
|
12.15
|
|
|
|
Issued
|
—
|
|
—
|
|
||
|
Vested
|
(856,135
|
)
|
12.15
|
|
||
|
Canceled/forfeited
|
(26,473
|
)
|
12.15
|
|
||
|
Outstanding, non-vested at December 31, 2018
|
198,597
|
|
12.15
|
|
||
|
Units
|
Weighted-Average
Grant Date
per Unit
|
|||||
|
Outstanding, non-vested at December 31, 2017
|
458,715
|
|
$
|
11.93
|
|
|
|
Issued
|
—
|
|
—
|
|
||
|
Vested
(1)
|
(401,371
|
)
|
11.93
|
|
||
|
Canceled/forfeited
|
—
|
|
—
|
|
||
|
Pending settlement
(1)
|
401,371
|
|
11.93
|
|
||
|
Outstanding, non-vested at December 31, 2018
|
458,715
|
|
—
|
|
||
|
Years Ended
December 31, |
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Options
|
$
|
63,288
|
|
$
|
31,724
|
|
$
|
23,607
|
|
||
|
Restricted common stock and units
|
9,277
|
|
8,331
|
|
11,370
|
|
|||||
|
Common
|
—
|
|
—
|
|
4,383
|
|
|||||
|
Total
|
$
|
72,565
|
|
$
|
40,055
|
|
$
|
39,360
|
|
||
|
Research and development
|
$
|
37,659
|
|
$
|
21,679
|
|
$
|
20,687
|
|
||
|
General and administrative
|
34,906
|
|
18,376
|
|
18,673
|
|
|||||
|
Total
|
$
|
72,565
|
|
$
|
40,055
|
|
$
|
39,360
|
|
||
|
Years Ended
December 31, |
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
United States
|
$
|
(380,473
|
)
|
$
|
(247,784
|
)
|
$
|
(211,786
|
)
|
||
|
Foreign
|
(3,935
|
)
|
(8,212
|
)
|
(3,382
|
)
|
|||||
|
Loss before provision for (benefit from) income taxes
|
$
|
(384,408
|
)
|
$
|
(255,996
|
)
|
$
|
(215,168
|
)
|
||
|
Years Ended
December 31, |
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
(26
|
)
|
$
|
(252
|
)
|
$
|
704
|
|
||
|
State
|
352
|
|
172
|
|
51
|
|
|||||
|
Total current
|
326
|
|
(80
|
)
|
755
|
|
|||||
|
Deferred:
|
|
||||||||||
|
Federal
|
—
|
|
—
|
|
288
|
|
|||||
|
Total deferred
|
—
|
|
—
|
|
288
|
|
|||||
|
Total income tax provision for (benefit from) income taxes
|
$
|
326
|
|
$
|
(80
|
)
|
$
|
1,043
|
|
||
|
Years Ended
December 31, |
||||||||
|
2018
|
2017
|
2016
|
||||||
|
Tax effected at statutory rate
|
21.0
|
%
|
34.0
|
%
|
34.0
|
%
|
||
|
State taxes, net of federal benefit
|
6.3
|
%
|
8.5
|
%
|
5.1
|
%
|
||
|
Non-deductible items
|
0.0
|
%
|
(1.3
|
)%
|
(3.0
|
)%
|
||
|
Change in valuation allowance
|
(28.5
|
)%
|
(20.4
|
)%
|
(40.2
|
)%
|
||
|
Federal research and development credits
|
1.5
|
%
|
4.8
|
%
|
4.3
|
%
|
||
|
Foreign tax rate differential
|
(0.2
|
)%
|
(1.1
|
)%
|
(0.5
|
)%
|
||
|
Impact of federal rate change on net deferred taxes
|
0.0
|
%
|
(25.0
|
)%
|
0.0
|
%
|
||
|
Other
|
(0.2
|
)%
|
0.5
|
%
|
(0.2
|
)%
|
||
|
Effective tax rate
|
(0.1
|
)%
|
0.0
|
%
|
(0.5
|
)%
|
||
|
December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Deferred tax assets:
|
|||||||
|
Net operating loss carry-forwards
|
$
|
114,932
|
|
$
|
100,372
|
|
|
|
Stock-based compensation
|
33,138
|
|
15,637
|
|
|||
|
Capitalized licenses, R&D and start-up costs
|
22,143
|
|
18,732
|
|
|||
|
Tax credit carry-forwards
|
53,617
|
|
47,804
|
|
|||
|
Accrued expenses
|
16,443
|
|
16,490
|
|
|||
|
Deferred revenue
|
71,209
|
|
7,103
|
|
|||
|
Lease financing obligation
|
9,149
|
|
—
|
|
|||
|
Other
|
2,870
|
|
1,997
|
|
|||
|
Total gross deferred tax assets
|
323,501
|
|
208,135
|
|
|||
|
Less: valuation allowance
|
(308,273
|
)
|
(198,650
|
)
|
|||
|
Total deferred tax assets, net of valuation allowance
|
15,228
|
|
9,485
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Fixed assets
|
(15,228
|
)
|
(9,485
|
)
|
|||
|
Total deferred tax liabilities
|
(15,228
|
)
|
(9,485
|
)
|
|||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
|
|
Balance as of December 31, 2016
|
$
|
906
|
|
|
Decrease due to prior positions
|
(15
|
)
|
|
|
Increase due to current year tax position
|
49
|
|
|
|
Balance as of December 31, 2017
|
940
|
|
|
|
Decrease due to prior positions
|
(799
|
)
|
|
|
Increase due to current year tax positions
|
—
|
|
|
|
Balance as of December 31, 2018
|
$
|
141
|
|
|
Years Ended
December 31, |
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Numerator:
|
|||||||||||
|
Net loss
|
$
|
(384,734
|
)
|
$
|
(255,916
|
)
|
$
|
(216,211
|
)
|
||
|
Premium paid on repurchase of redeemable convertible preferred stock
|
(4,127
|
)
|
—
|
|
—
|
|
|||||
|
Accretion of redeemable convertible preferred units
|
—
|
|
—
|
|
(8,663
|
)
|
|||||
|
Cumulative dividends on redeemable convertible preferred stock
|
(12,996
|
)
|
(13,925
|
)
|
(5,440
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(401,857
|
)
|
$
|
(269,841
|
)
|
$
|
(230,314
|
)
|
||
|
Denominator:
|
|||||||||||
|
Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted
|
81,114,183
|
|
64,497,544
|
|
60,747,426
|
|
|||||
|
Net loss per share attributable to common stockholders, basic and diluted
|
$
|
(4.95
|
)
|
$
|
(4.18
|
)
|
$
|
(3.79
|
)
|
||
|
December 31,
|
||||||||
|
2018
|
2017
|
2016
|
||||||
|
Redeemable convertible preferred stock
|
—
|
|
205,151,299
|
|
205,151,299
|
|
||
|
Stock options
|
50,821,132
|
|
33,684,134
|
|
25,691,663
|
|
||
|
Restricted common stock
|
198,597
|
|
1,081,205
|
|
2,998,141
|
|
||
|
Restricted common stock units
|
458,715
|
|
458,715
|
|
—
|
|
||
|
51,478,444
|
|
240,375,353
|
|
233,841,103
|
|
|||
|
Name
|
Shares of
Series G Redeemable Convertible Preferred Stock |
Total
Purchase Price |
||||
|
OCHA LLC
(1)
|
50,000
|
$
|
503
|
|
||
|
Viking Global Investors LP and affiliated entities
(2)
|
745,526
|
$
|
7,500
|
|
||
|
(1)
|
OCHA LLC is an entity controlled by an officer.
|
|
(2)
|
Consists of (1)
279,160
shares of Series G redeemable convertible preferred stock held by VGE III Portfolio Ltd.; (2)
148,974
shares of Series G preferred stock held by Viking Global Equities LP; (3)
8,737
shares of Series G redeemable convertible preferred stock held by Viking Global Equities II LP; (4)
129,537
shares of Series G preferred stock held by Viking Global Opportunities Illiquid Investments Sub-Master LP; and (5)
179,118
shares of Series G redeemable convertible preferred stock held by Viking Long Fund Master Ltd.
|
|
Name
|
Shares of
Series F Redeemable Convertible Preferred Stock |
Total
Purchase Price |
||||
|
AstraZeneca and affiliated entities
|
15,945,330
|
$
|
140,000
|
|
||
|
Boston Biotech Ventures LLC
(1)
|
10,000
|
$
|
88
|
|
||
|
Viking Global Investors LP and affiliated entities
(2)
|
5,694,760
|
$
|
50,000
|
|
||
|
(1)
|
Boston Biotech Ventures LLC is an entity controlled by an officer.
|
|
(2)
|
Consists of
5,694,760
shares of Series F preferred stock held by Viking Global Opportunities Illiquid Investments Sub-Master LP.
|
|
2018
|
||||||||||||||||
|
First
Quarter
|
Second Quarter
|
Third
Quarter
|
Fourth
Quarter
|
|||||||||||||
|
Total revenue
|
$
|
29,039
|
|
$
|
28,851
|
|
$
|
41,757
|
|
$
|
35,421
|
|
||||
|
Total operating expenses
|
106,441
|
|
125,866
|
|
127,575
|
|
188,452
|
|
||||||||
|
Loss from operations
|
(77,402
|
)
|
(97,015
|
)
|
(85,818
|
)
|
(153,031
|
)
|
||||||||
|
Net loss per share attributable to common stockholders - basic and diluted
|
$
|
(1.16
|
)
|
$
|
(1.43
|
)
|
$
|
(1.32
|
)
|
$
|
(1.14
|
)
|
||||
|
2017
|
||||||||||||||||
|
First
Quarter
|
Second Quarter
|
Third
Quarter
|
Fourth
Quarter
|
|||||||||||||
|
Total revenue
|
$
|
30,328
|
|
$
|
41,121
|
|
$
|
42,472
|
|
$
|
91,904
|
|
||||
|
Total operating expenses
|
95,791
|
|
135,315
|
|
110,343
|
|
133,732
|
|
||||||||
|
Loss from operations
|
(65,463
|
)
|
(94,194
|
)
|
(67,871
|
)
|
(41,828
|
)
|
||||||||
|
Net loss per share attributable to common stockholders - basic and diluted
|
$
|
(1.03
|
)
|
$
|
(1.47
|
)
|
$
|
(1.05
|
)
|
$
|
(0.64
|
)
|
||||
|
Exhibit No.
|
Exhibit Index
|
|
|
3.1
|
||
|
3.2
|
||
|
4.1
|
||
|
4.2
|
||
|
10.1#
|
||
|
10.2#
|
||
|
10.3#
|
||
|
10.4†
|
||
|
10.5†
|
||
|
10.6†
|
||
|
10.7†
|
||
|
10.8†
|
||
|
10.9
|
||
|
10.10
|
||
|
10.11#
|
||
|
10.12#
|
||
|
10.13#
|
||
|
10.14#
|
||
|
10.15#
|
||
|
10.16#
|
||
|
10.17#
|
||
|
10.18#
|
||
|
10.19#
|
||
|
10.20#
|
||
|
10.21#
|
||
|
10.22#
|
||
|
10.23#
|
||
|
21.1
|
||
|
23.1
|
||
|
31.1
|
||
|
31.2
|
||
|
32.1+
|
||
|
32.2+
|
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Link Document
|
|
|
†
|
Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions.
|
|
#
|
Indicates a management contract or any compensatory plan, contract or arrangement.
|
|
+
|
The certifications furnished in Exhibit 32.1 and 32.2 hereto are deemed to accompany this Annual Report on Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certifications will not be deemed to be incorporated by reference into any filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates it by reference.
|
|
(1)
|
Incorporated by reference to the Registration Statement on Form S-1 (File No. 333-228300) filed with the Securities and Exchange Commission on November 9, 2018.
|
|
(2)
|
Incorporated by reference to the Registration Statement on Form S-1 (File No. 333-228300) filed with the Securities and Exchange Commission on November 28, 2018.
|
|
(3)
|
Incorporated by reference to the Current Report on Form 8-K (File No. 001-38753) filed with the Securities and Exchange Commission on December 14, 2018.
|
|
MODERNA, INC.
|
|||
|
Date:
|
By:
|
/s/ Stéphane Bancel
|
|
|
March 13, 2019
|
|||
|
Stéphane Bancel
|
|||
|
Chief Executive Officer and Director
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Stéphane Bancel
|
Chief Executive Officer and Director
(Principal Executive Officer)
|
March 13, 2019
|
||
|
Stéphane Bancel
|
||||
|
/s/ Lorence Kim, M.D.
|
Chief Financial Officer
(Principal Financial Officer)
|
March 13, 2019
|
||
|
Lorence Kim, M.D.
|
||||
|
/s/ Jennifer Lee
|
Chief Accounting Officer
(Principal Accounting Officer)
|
March 13, 2019
|
||
|
Jennifer Lee
|
||||
|
/s/ Noubar B. Afeyan, Ph.D.
|
Chairman and Director
|
March 13, 2019
|
||
|
Noubar B. Afeyan, Ph.D.
|
||||
|
/s/ Stephen Berenson
|
Director
|
March 13, 2019
|
||
|
Stephen Berenson
|
||||
|
/s/ Peter Barton Hutt, LL.M.
|
Director
|
March 13, 2019
|
||
|
Peter Barton Hutt, LL.M.
|
||||
|
/s/ Robert Langer, Sc.D.
|
Director
|
March 13, 2019
|
||
|
Robert Langer, Sc.D.
|
||||
|
/s/ Elizabeth Nabel, M.D.
|
Director
|
March 13, 2019
|
||
|
Elizabeth Nabel, M.D.
|
||||
|
/s/ Israel Ruiz
|
Director
|
March 13, 2019
|
||
|
Israel Ruiz
|
||||
|
/s/ Paul Sagan
|
Director
|
March 13, 2019
|
||
|
Paul Sagan
|
||||
|
/s/ Moncef Slaoui, Ph.D.
|
Director
|
March 13, 2019
|
||
|
Moncef Slaoui, Ph.D.
|
||||