|
FORM 10-Q
|

|
Delaware
|
81-3467528
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(IRS Employer Identification No.)
|
|
|
200 Technology Square
Cambridge, Massachusetts
|
02139
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
|
Large accelerated filer
o
|
Accelerated filer
o
|
Non-accelerated filer
x
|
Smaller reporting company
o
|
|||
|
Emerging growth company
x
|
||||||
|
Title of each class
|
Trading symbol(s)
|
Name of each exchange on which registered
|
|
Common stock, par value $0.0001 per share
|
MRNA
|
The NASDAQ Stock Market LLC
|
|
•
|
the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs;
|
|
•
|
our ability to identify research priorities and apply a risk-mitigated strategy to efficiently discover and develop development candidates and investigational medicines, including by applying learnings from one program to our other programs and from one modality to our other modalities;
|
|
•
|
our ability and the potential to successfully manufacture our drug substances, delivery vehicles, development candidates, and investigational medicines for preclinical use, for clinical trials and on a larger scale for commercial use, if approved;
|
|
•
|
the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and investigational medicines;
|
|
•
|
our ability to obtain funding for our operations necessary to complete further development and commercialization of our investigational medicines;
|
|
•
|
our ability to obtain and maintain regulatory approval of our investigational medicines;
|
|
•
|
our ability to commercialize our products, if approved;
|
|
•
|
the pricing and reimbursement of our investigational medicines, if approved;
|
|
•
|
the implementation of our business model, and strategic plans for our business, investigational medicines, and technology;
|
|
•
|
the scope of protection we are able to establish and maintain for intellectual property rights covering our investigational medicines and technology;
|
|
•
|
estimates of our future expenses, revenues, capital requirements, and our needs for additional financing;
|
|
•
|
the potential benefits of strategic collaboration agreements, our ability to enter into strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory, and commercialization expertise;
|
|
•
|
future agreements with third parties in connection with the commercialization of our investigational medicines, if approved;
|
|
•
|
the size and growth potential of the markets for our investigational medicines, and our ability to serve those markets;
|
|
•
|
our financial performance;
|
|
•
|
the rate and degree of market acceptance of our investigational medicines;
|
|
•
|
regulatory developments in the United States and foreign countries;
|
|
•
|
our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately;
|
|
•
|
our ability to produce our products or investigational medicines with advantages in turnaround times or manufacturing cost;
|
|
•
|
the success of competing therapies that are or may become available;
|
|
•
|
our ability to attract and retain key scientific or management personnel;
|
|
•
|
the impact of laws and regulations;
|
|
•
|
developments relating to our competitors and our industry; and
|
|
•
|
other risks and uncertainties, including those discussed in Part II, Item 1A - Risk Factors in this Form 10-Q.
|
|
PART I
.
|
Page
|
|
|
Item 1.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
PART II
.
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 2
|
||
|
Item 6.
|
||
|
March 31,
|
December 31,
|
||||||
|
2019
|
2018
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
502,934
|
|
$
|
658,364
|
|
|
|
Investments
|
605,990
|
|
863,063
|
|
|||
|
Accounts receivable
|
5,646
|
|
11,686
|
|
|||
|
Accounts receivable from related party
|
965
|
|
899
|
|
|||
|
Prepaid expenses and other current assets
|
25,292
|
|
28,399
|
|
|||
|
Restricted cash
|
—
|
|
595
|
|
|||
|
Total current assets
|
1,140,827
|
|
1,563,006
|
|
|||
|
Investments, non-current
|
437,659
|
|
172,990
|
|
|||
|
Property and equipment, net
|
213,460
|
|
211,977
|
|
|||
|
Restricted cash, non-current
|
11,823
|
|
11,532
|
|
|||
|
Other non-current assets
|
2,438
|
|
2,644
|
|
|||
|
Total assets
|
$
|
1,806,207
|
|
$
|
1,962,149
|
|
|
|
Liabilities and Stockholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
38,509
|
|
$
|
31,210
|
|
|
|
Accrued liabilities
|
42,183
|
|
79,073
|
|
|||
|
Deferred revenue
|
73,958
|
|
109,056
|
|
|||
|
Other current liabilities
|
4,509
|
|
3,464
|
|
|||
|
Total current liabilities
|
159,159
|
|
222,803
|
|
|||
|
Deferred revenue, non-current
|
156,862
|
|
165,352
|
|
|||
|
Deferred lease obligation, non-current
|
10,556
|
|
10,006
|
|
|||
|
Lease financing obligation
|
33,384
|
|
33,489
|
|
|||
|
Other non-current liabilities
|
213
|
|
258
|
|
|||
|
Total liabilities
|
360,174
|
|
431,908
|
|
|||
|
Commitments and contingencies (Note 7)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, par value $0.0001; 162,000,000 shares authorized as of March 31, 2019
and December 31, 2018; no shares issued or outstanding at March 31, 2019 and
December 31, 2018
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.0001; 1,600,000,000 shares authorized as of March 31, 2019 and December 31, 2018; 328,853,340 and 328,798,904 shares issued and outstanding as of March 31, 2019 and December 31, 2018, respectively
|
33
|
|
33
|
|
|||
|
Additional paid-in capital
|
2,556,709
|
|
2,538,155
|
|
|||
|
Accumulated other comprehensive income (loss)
|
591
|
|
(1,320
|
)
|
|||
|
Accumulated deficit
|
(1,111,300
|
)
|
(1,006,627
|
)
|
|||
|
Total stockholders’ equity
|
1,446,033
|
|
1,530,241
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
1,806,207
|
|
$
|
1,962,149
|
|
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Revenue:
|
|||||||
|
Collaboration revenue
|
$
|
13,301
|
|
$
|
20,110
|
|
|
|
Collaboration revenue from related party
|
814
|
|
7,350
|
|
|||
|
Grant revenue
|
1,910
|
|
1,579
|
|
|||
|
Total revenue
|
16,025
|
|
29,039
|
|
|||
|
Operating expenses:
|
|||||||
|
Research and development
|
130,575
|
|
90,124
|
|
|||
|
General and administrative
|
27,283
|
|
16,317
|
|
|||
|
Total operating expenses
|
157,858
|
|
106,441
|
|
|||
|
Loss from operations
|
(141,833
|
)
|
(77,402
|
)
|
|||
|
Interest income
|
10,972
|
|
5,209
|
|
|||
|
Other expense, net
|
(1,820
|
)
|
(183
|
)
|
|||
|
Loss before benefit from income taxes
|
(132,681
|
)
|
(72,376
|
)
|
|||
|
Benefit from income taxes
|
(24
|
)
|
—
|
|
|||
|
Net loss
|
$
|
(132,657
|
)
|
$
|
(72,376
|
)
|
|
|
Net loss attributable to common stockholders (Note 11)
|
$
|
(132,657
|
)
|
$
|
(75,857
|
)
|
|
|
Net loss per share attributable to common stockholders, basic and diluted
|
$
|
(0.40
|
)
|
$
|
(1.16
|
)
|
|
|
Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted
|
328,809,986
|
|
65,430,835
|
|
|||
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net loss
|
$
|
(132,657
|
)
|
$
|
(72,376
|
)
|
|
|
Other comprehensive income (loss):
|
|||||||
|
Unrealized gain (loss) on available-for-sale debt securities, net of tax, $540 and $0
|
1,908
|
|
(1,999
|
)
|
|||
|
Less: Amounts recognized for net realized loss included in net loss
|
3
|
|
6
|
|
|||
|
Total other comprehensive income (loss)
|
1,911
|
|
(1,993
|
)
|
|||
|
Comprehensive loss
|
$
|
(130,746
|
)
|
$
|
(74,369
|
)
|
|
|
Redeemable Convertible
Preferred Stock
|
Common Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
(Loss) Income
|
Accumulated
Deficit
|
Total
Stockholders’
Equity
|
|||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||
|
Balance at December 31, 2018
|
—
|
|
$
|
—
|
|
328,798,904
|
|
$
|
33
|
|
$
|
2,538,155
|
|
$
|
(1,320
|
)
|
$
|
(1,006,627
|
)
|
$
|
1,530,241
|
|
||||||||||
|
Transition adjustment from adoption of ASU Topic 606 (Note 2)
|
—
|
|
—
|
|
—
|
|
—
|
|
27,984
|
|
27,984
|
|
||||||||||||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
48,911
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Exercise of options to purchase common stock, net
|
—
|
|
—
|
|
5,525
|
|
—
|
|
57
|
|
—
|
|
—
|
|
57
|
|
||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
18,497
|
|
—
|
|
—
|
|
18,497
|
|
||||||||||||||||
|
Unrealized gain on marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,911
|
|
—
|
|
1,911
|
|
||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(132,657
|
)
|
(132,657
|
)
|
||||||||||||||||
|
Balance at March 31, 2019
|
—
|
|
$
|
—
|
|
328,853,340
|
|
$
|
33
|
|
$
|
2,556,709
|
|
$
|
591
|
|
$
|
(1,111,300
|
)
|
$
|
1,446,033
|
|
||||||||||
|
Redeemable Convertible
Preferred Stock
|
Common Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
Loss
|
Accumulated
Deficit
|
Total
Stockholders’
Deficit
|
|||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
448,686,791
|
|
$
|
1,176,661
|
|
65,206,999
|
|
$
|
6
|
|
$
|
71,679
|
|
$
|
(1,157
|
)
|
$
|
(621,893
|
)
|
$
|
(551,365
|
)
|
||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
335,053
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Issuance of Series G redeemable convertible preferred stock, net of issuance costs of $10,517
|
55,666,004
|
|
549,413
|
|
—
|
|
—
|
|
152
|
|
—
|
|
—
|
|
152
|
|
||||||||||||||||
|
Exercise of options to purchase common stock, net
|
—
|
|
—
|
|
1,789
|
|
—
|
|
22
|
|
—
|
|
—
|
|
22
|
|
||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
11,851
|
|
—
|
|
—
|
|
11,851
|
|
||||||||||||||||
|
Unrealized loss on marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,993
|
)
|
—
|
|
(1,993
|
)
|
||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(72,376
|
)
|
(72,376
|
)
|
||||||||||||||||
|
Balance at March 31, 2018
|
504,352.795
|
|
$
|
1,726,074
|
|
65,543,841
|
|
$
|
6
|
|
$
|
83,704
|
|
$
|
(3,150
|
)
|
$
|
(694,269
|
)
|
$
|
(613,709
|
)
|
||||||||||
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Operating activities
|
|||||||
|
Net loss
|
$
|
(132,657
|
)
|
$
|
(72,376
|
)
|
|
|
Adjustments to reconcile net loss to net cash (used in) operating activities:
|
|||||||
|
Stock-based compensation
|
18,497
|
|
11,851
|
|
|||
|
Depreciation and amortization
|
7,328
|
|
5,153
|
|
|||
|
Amortization of investment premiums
|
(1,005
|
)
|
(83
|
)
|
|||
|
Loss on disposal of property and equipment
|
19
|
|
—
|
|
|||
|
Changes in assets and liabilities:
|
|||||||
|
Accounts receivable
|
6,040
|
|
3,876
|
|
|||
|
Accounts receivable from related party
|
(66
|
)
|
(28,529
|
)
|
|||
|
Prepaid expenses and other assets
|
3,313
|
|
(2,241
|
)
|
|||
|
Accounts payable
|
1,731
|
|
1,467
|
|
|||
|
Accrued liabilities
|
(32,557
|
)
|
(37,031
|
)
|
|||
|
Deferred revenue
|
(15,604
|
)
|
6,193
|
|
|||
|
Deferred lease obligation
|
550
|
|
152
|
|
|||
|
Other liabilities
|
484
|
|
183
|
|
|||
|
Net cash used in operating activities
|
(143,927
|
)
|
(111,385
|
)
|
|||
|
Investing activities
|
|||||||
|
Purchases of marketable securities
|
(429,517
|
)
|
(660,239
|
)
|
|||
|
Proceeds from maturities of marketable securities
|
403,940
|
|
189,814
|
|
|||
|
Proceeds from sales of marketable securities
|
21,413
|
|
18,630
|
|
|||
|
Purchases of property and equipment
|
(7,595
|
)
|
(31,909
|
)
|
|||
|
Net cash used in investing activities
|
(11,759
|
)
|
(483,704
|
)
|
|||
|
Financing activities
|
|||||||
|
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs
|
—
|
|
549,531
|
|
|||
|
Proceeds from issuance of common stock through equity plans
|
57
|
|
22
|
|
|||
|
Reimbursement of assets under lease financing obligation
|
—
|
|
1,727
|
|
|||
|
Payments on financing lease obligation
|
(105
|
)
|
(1,285
|
)
|
|||
|
Net cash (used in) provided by financing activities
|
(48
|
)
|
549,995
|
|
|||
|
Net decrease in cash, cash equivalents and restricted cash
|
(155,734
|
)
|
(45,094
|
)
|
|||
|
Cash, cash equivalents and restricted cash, beginning of year
|
670,491
|
|
147,608
|
|
|||
|
Cash, cash equivalents and restricted cash, end of period
|
$
|
514,757
|
|
$
|
102,514
|
|
|
|
Non-cash investing and financing activities
|
|||||||
|
Issuance costs included in accounts payable and accrued liabilities
|
$
|
796
|
|
$
|
—
|
|
|
|
Purchases of property and equipment included in accounts payable and accrued liabilities
|
$
|
14,127
|
|
$
|
28,527
|
|
|
|
Leasehold improvements included in prepaid and other current assets
|
$
|
10,089
|
|
$
|
9,889
|
|
|
|
Lease financing obligation
|
$
|
10,089
|
|
$
|
9,889
|
|
|
|
Unrealized Gain on Available-for-Sale Debt Securities
|
|||
|
Accumulated other comprehensive loss, balance at December 31, 2018
|
$
|
(1,320
|
)
|
|
Other comprehensive income
|
1,911
|
|
|
|
Accumulated other comprehensive loss, balance at March 31, 2019
|
$
|
591
|
|
|
Condensed Consolidated Balance Sheet
|
Balance at December 31, 2018
|
Adjustments
|
Balance at
January 1, 2019
|
|||||||||
|
Deferred revenue, current
|
$
|
109,056
|
|
$
|
(27,281
|
)
|
$
|
81,775
|
|
|||
|
Deferred revenue, non-current
|
165,352
|
|
(3,441
|
)
|
161,911
|
|
||||||
|
Accounts receivable
|
11,686
|
|
(2,738
|
)
|
8,948
|
|
||||||
|
Accumulated deficit
|
(1,006,627
|
)
|
27,984
|
|
(978,643
|
)
|
||||||
|
March 31, 2019
|
||||||||||||
|
Condensed Consolidated Balance Sheet
|
As reported under ASC 606
|
Adjustments
|
Balance without adoption of ASC 606
|
|||||||||
|
Deferred revenue, current
|
$
|
73,958
|
|
$
|
36,452
|
|
$
|
110,410
|
|
|||
|
Deferred revenue, non-current
|
156,862
|
|
(29,840
|
)
|
127,022
|
|
||||||
|
Accumulated deficit
|
(1,111,300
|
)
|
(6,077
|
)
|
(1,117,377
|
)
|
||||||
|
Three Months Ended March 31, 2019
|
||||||||||||
|
Condensed Consolidated Statement of Operations
|
As reported under ASC 606
|
Adjustments
|
Amount without adoption of ASC 606
|
|||||||||
|
Revenue:
|
||||||||||||
|
Collaboration revenue
|
$
|
13,301
|
|
$
|
(1,786
|
)
|
$
|
11,515
|
|
|||
|
Collaboration revenue from related party
|
814
|
|
24,377
|
|
25,191
|
|
||||||
|
Total revenue
|
16,025
|
|
21,907
|
|
37,932
|
|
||||||
|
Loss from operations
|
(141,833
|
)
|
21,907
|
|
(119,926
|
)
|
||||||
|
Loss before benefit from income taxes
|
(132,681
|
)
|
21,907
|
|
(110,774
|
)
|
||||||
|
Net (loss) income
|
(132,657
|
)
|
21,907
|
|
(110,750
|
)
|
||||||
|
Net loss per share - basic and diluted
|
(0.40
|
)
|
0.06
|
|
(0.34
|
)
|
||||||
|
As of March 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Cash and cash equivalents
|
$
|
502,934
|
|
$
|
90,479
|
|
||
|
Restricted cash
|
—
|
|
237
|
|
||||
|
Restricted cash, non-current
|
11,823
|
|
11,798
|
|
||||
|
Total cash, cash equivalents and restricted cash shown in the condensed consolidated
statements of cash flows
|
$
|
514,757
|
|
$
|
102,514
|
|
||
|
Three Months Ended
|
||||||||||||
|
Collaboration Revenue by Strategic Collaborator:
|
March 31, 2019
as reported
(under ASU 606)
|
March 31, 2019
without adoption of 606
(under ASC 605)
|
March 31, 2018
as reported
(under ASC 605)
|
|||||||||
|
Merck
|
$
|
10,687
|
|
$
|
11,515
|
|
$
|
15,967
|
|
|||
|
AstraZeneca
|
814
|
|
25,191
|
|
7,350
|
|
||||||
|
Vertex
|
2,614
|
|
—
|
|
4,143
|
|
||||||
|
Total collaborative revenue
|
$
|
14,115
|
|
$
|
36,706
|
|
$
|
27,460
|
|
|||
|
January 1, 2019
|
Additions
|
Deductions
|
March 31, 2019
|
|||||||||||||
|
Contract Assets:
|
||||||||||||||||
|
Accounts receivable
|
$
|
4,612
|
|
$
|
2,735
|
|
$
|
(3,747
|
)
|
$
|
3,600
|
|
||||
|
Contract Liabilities:
|
||||||||||||||||
|
Deferred revenue
|
$
|
240,924
|
|
$
|
1,137
|
|
$
|
(13,582
|
)
|
$
|
228,479
|
|
||||
|
Revenue recognized in the period from:
|
Three Months Ended March 31, 2019
|
|||
|
Amounts included in contract liabilities at the beginning of the period
(1)
|
$
|
13,582
|
|
|
|
March 31, 2019
|
||||||||||||||||||||||||||||
|
Amortized
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Cash and
Cash
Equivalents
|
Current
Marketable
Securities
|
Non-
Current
Marketable
Securities
|
||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
502,935
|
|
$
|
—
|
|
$
|
(1
|
)
|
$
|
502,934
|
|
$
|
502,934
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Available-for-sale:
|
||||||||||||||||||||||||||||
|
Level 2:
|
||||||||||||||||||||||||||||
|
Certificates of deposit
|
84,114
|
|
72
|
|
(1
|
)
|
84,185
|
|
—
|
|
80,483
|
|
3,702
|
|
||||||||||||||
|
U.S. treasury securities
|
119,347
|
|
155
|
|
(4
|
)
|
119,498
|
|
—
|
|
79,475
|
|
40,023
|
|
||||||||||||||
|
Debt securities of U.S. government agencies and corporate entities
|
839,055
|
|
1,129
|
|
(218
|
)
|
839,966
|
|
—
|
|
446,032
|
|
393,934
|
|
||||||||||||||
|
$
|
1,545,451
|
|
$
|
1,356
|
|
$
|
(224
|
)
|
$
|
1,546,583
|
|
$
|
502,934
|
|
$
|
605,990
|
|
$
|
437,659
|
|
||||||||
|
December 31, 2018
|
||||||||||||||||||||||||||||
|
Amortized
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Cash and
Cash
Equivalents
|
Current
Marketable
Securities
|
Non-
Current
Marketable
Securities
|
||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
658,365
|
|
$
|
20
|
|
$
|
(21
|
)
|
$
|
658,364
|
|
$
|
658,364
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Available-for-sale:
|
||||||||||||||||||||||||||||
|
Level 2:
|
||||||||||||||||||||||||||||
|
Certificates of deposit
|
173,102
|
|
42
|
|
(36
|
)
|
173,108
|
|
—
|
|
157,920
|
|
15,188
|
|
||||||||||||||
|
U.S. treasury securities
|
152,205
|
|
18
|
|
(48
|
)
|
152,175
|
|
—
|
|
152,175
|
|
—
|
|
||||||||||||||
|
Debt securities of U.S. government agencies and corporate entities
|
712,065
|
|
40
|
|
(1,335
|
)
|
710,770
|
|
—
|
|
552,968
|
|
157,802
|
|
||||||||||||||
|
$
|
1,695,737
|
|
$
|
120
|
|
$
|
(1,440
|
)
|
$
|
1,694,417
|
|
$
|
658,364
|
|
$
|
863,063
|
|
$
|
172,990
|
|
||||||||
|
March 31, 2019
|
||||||||
|
Amortized
Cost
|
Estimated
Fair Value
|
|||||||
|
Due in one year or less
|
$
|
605,687
|
|
$
|
605,990
|
|
||
|
Due after one year through five years
|
436,829
|
|
437,659
|
|
||||
|
Total
|
$
|
1,042,516
|
|
$
|
1,043,649
|
|
||
|
December 31, 2018
|
||||||||
|
Amortized
Cost
|
Estimated
Fair Value
|
|||||||
|
Due in one year or less
|
$
|
864,003
|
|
$
|
863,063
|
|
||
|
Due after one year through five years
|
173,369
|
|
172,990
|
|
||||
|
Total
|
$
|
1,037,372
|
|
$
|
1,036,053
|
|
||
|
March 31,
|
December 31,
|
|||||||
|
2019
|
2018
|
|||||||
|
Prepaid expenses
|
$
|
7,895
|
|
$
|
10,401
|
|
||
|
Tenant incentives receivables
|
11,336
|
|
10,089
|
|
||||
|
Interest receivable on marketable securities
|
6,061
|
|
7,909
|
|
||||
|
Prepaid expenses and other current assets
|
$
|
25,292
|
|
$
|
28,399
|
|
||
|
March 31,
|
December 31,
|
|||||||
|
2019
|
2018
|
|||||||
|
Building
|
$
|
140,442
|
|
$
|
140,442
|
|
||
|
Laboratory equipment
|
98,891
|
|
96,907
|
|
||||
|
Leasehold improvements
|
15,861
|
|
13,741
|
|
||||
|
Furniture, fixtures and other
|
2,122
|
|
2,122
|
|
||||
|
Computer equipment and software
|
9,456
|
|
11,513
|
|
||||
|
Internally developed software
|
7,020
|
|
7,020
|
|
||||
|
Construction in progress
|
11,403
|
|
4,688
|
|
||||
|
285,195
|
|
276,433
|
|
|||||
|
Less: Accumulated depreciation
|
(71,735
|
)
|
(64,456
|
)
|
||||
|
Property and equipment, net
|
$
|
213,460
|
|
$
|
211,977
|
|
||
|
March 31,
|
December 31,
|
|||||||
|
2019
|
2018
|
|||||||
|
In-licenses
|
$
|
—
|
|
$
|
22,000
|
|
||
|
Property and equipment
|
7,756
|
|
12,089
|
|
||||
|
Compensation-related
|
11,577
|
|
23,406
|
|
||||
|
External goods and services
|
22,850
|
|
21,578
|
|
||||
|
Accrued liabilities
|
$
|
42,183
|
|
$
|
79,073
|
|
||
|
Fiscal Year
|
Minimum Lease Payments
|
|||
|
2019
|
(remainder of the year)
|
$
|
15,190
|
|
|
2020
|
22,874
|
|
||
|
2021
|
24,105
|
|
||
|
2022
|
23,455
|
|
||
|
2023
|
20,498
|
|
||
|
Thereafter
|
140,384
|
|
||
|
Total
|
$
|
246,506
|
|
|
|
Number of
Options
|
Weighted-
Average
Exercise
Price per
Share
|
Weighted-
Average
Grant
Date Fair
Value per
Share
|
Weighted-
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic
Value
(1)
(in thousands)
|
||||||||||
|
Outstanding at December 31, 2018
|
50,821,132
|
|
12.16
|
|
6.59
|
|
7.1 years
|
220,434
|
|
|||||
|
Granted
|
5,443,286
|
|
20.59
|
|
12.16
|
|
||||||||
|
Exercised
|
(5,525
|
)
|
9.62
|
|
5.62
|
|
||||||||
|
Canceled/forfeited
|
(2,987,612
|
)
|
14.29
|
|
8.48
|
|
||||||||
|
Outstanding at March 31, 2019
|
53,271,281
|
|
12.92
|
|
6.99
|
|
7.5 years
|
412,480
|
|
|||||
|
Exercisable at March 31, 2019
|
24,769,502
|
|
8.54
|
|
4.05
|
|
5.7 years
|
292,602
|
|
|||||
|
Vested and expected to vest at March 31, 2019
|
28,501,493
|
|
16.73
|
|
9.55
|
|
9.0 years
|
119,876
|
|
|||||
|
Weighted Average
|
||||||||
|
Three Months Ended March 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Risk-free interest rate
|
2.48
|
%
|
2.71
|
%
|
||||
|
Expected term
|
6.12 years
|
|
6.06 years
|
|
||||
|
Expected volatility
|
62
|
%
|
63
|
%
|
||||
|
Expected dividends
|
—
|
%
|
—
|
%
|
||||
|
Weighted average fair value per share
|
$
|
12.16
|
|
$
|
8.48
|
|
||
|
Number of
Shares |
Weighted
Average
Grant Date
per Share
|
|||||
|
Outstanding, non-vested at December 31, 2018
|
198,597
|
|
$
|
12.15
|
|
|
|
Issued
|
—
|
|
—
|
|
||
|
Vested
|
(48,911
|
)
|
12.15
|
|
||
|
Canceled, forfeited and adjustments, net
|
711
|
|
12.15
|
|
||
|
Outstanding, non-vested at March 31, 2019
|
150,397
|
|
12.15
|
|
||
|
Units
|
Weighted-Average
Grant Date
per Unit
|
|||||
|
Outstanding, non-vested at December 31, 2018
|
458,715
|
|
$
|
11.93
|
|
|
|
Issued
|
558,208
|
|
20.77
|
|
||
|
Vested
(1)
|
14,334
|
|
11.93
|
|
||
|
Canceled/forfeited
|
(5,440
|
)
|
20.93
|
|
||
|
Pending settlement
(1)
|
(14,334
|
)
|
11.93
|
|
||
|
Outstanding, non-vested at March 31, 2019
|
1,011,483
|
|
16.76
|
|
||
|
Three Months Ended
March 31, |
|||||||
|
2019
|
2018
|
||||||
|
Options
|
$
|
17,487
|
|
$
|
10,048
|
|
|
|
Restricted common stock and units
|
1,010
|
|
1,803
|
|
|||
|
Total
|
$
|
18,497
|
|
$
|
11,851
|
|
|
|
Research and development
|
$
|
10,783
|
|
$
|
7,356
|
|
|
|
General and administrative
|
7,714
|
|
4,495
|
|
|||
|
Total
|
$
|
18,497
|
|
$
|
11,851
|
|
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Numerator:
|
|||||||
|
Net loss
|
$
|
(132,657
|
)
|
$
|
(72,376
|
)
|
|
|
Cumulative dividends on redeemable convertible preferred stock
|
—
|
|
(3,481
|
)
|
|||
|
Net loss attributable to common stockholders
|
$
|
(132,657
|
)
|
$
|
(75,857
|
)
|
|
|
Denominator:
|
|||||||
|
Weighted average common shares used in net loss per share attributable to common stockholders, basic and diluted
|
328,809,986
|
|
65,430,835
|
|
|||
|
Net loss per share attributable to common stockholders, basic and diluted
|
$
|
(0.40
|
)
|
$
|
(1.16
|
)
|
|
|
March 31,
|
|||||
|
2019
|
2018
|
||||
|
Redeemable convertible preferred stock
|
—
|
|
260,817,303
|
|
|
|
Stock options
|
53,271,281
|
|
43,135,998
|
|
|
|
Restricted common stock
|
150,397
|
|
729,877
|
|
|
|
Restricted common stock units
|
1,011,483
|
|
458,715
|
|
|
|
54,433,161
|
|
305,141,893
|
|
||
|
•
|
continue our platform research and drug discovery and development efforts;
|
|
•
|
conduct clinical studies for our investigational medicines;
|
|
•
|
manufacture clinical study materials and develop large-scale manufacturing capabilities;
|
|
•
|
seek regulatory approval for our investigational medicines;
|
|
•
|
maintain, expand, and protect our intellectual property;
|
|
•
|
hire additional personnel to support our program development effort to obtain regulatory approval and secure additional facilities for operations; and
|
|
•
|
operate as a public company.
|

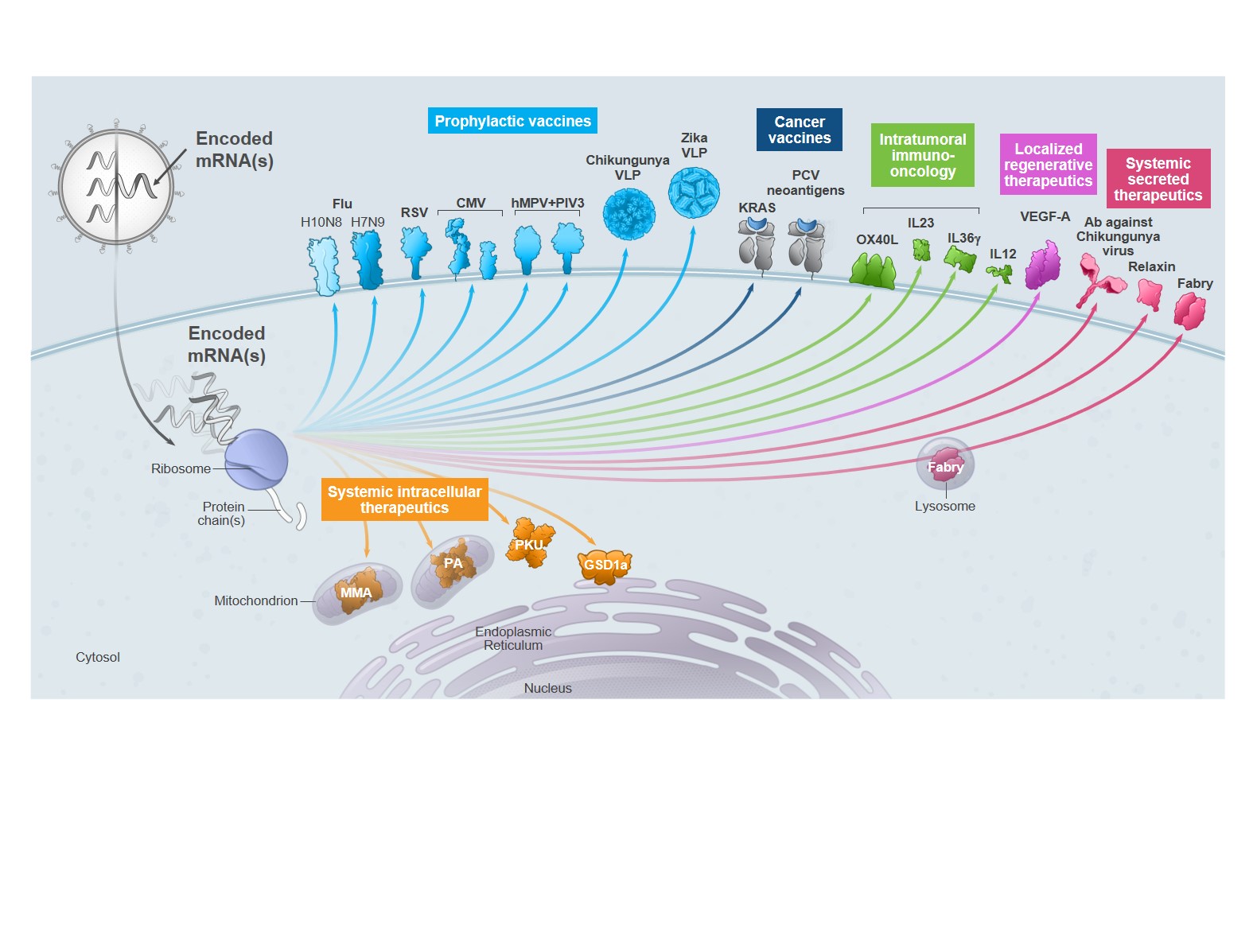
|
•
|
Prophylactic vaccines
included eight development candidates: RSV vaccine (mRNA-1777 and mRNA-1172), CMV vaccine (mRNA-1647), hMPV+PIV3 vaccine (mRNA-1653), H10N8 vaccine (mRNA-1440), H7N9 vaccine (mRNA-1851), Zika vaccine (mRNA-1893), and Chikungunya vaccine (mRNA-1388). We currently have six programs for which the Phase 1 trial is either ongoing or has been completed;
|
|
•
|
Cancer vaccines
included two development candidates: Personalized cancer vaccine, or PCV, (mRNA-4157) and KRAS vaccine (mRNA-5671). We are collaborating with Merck on both programs. PCV is in a Phase 1 clinical trial and we and Merck have submitted a protocol to the U.S. Food and Drug Administration (FDA) for a randomized Phase 2 clinical trial of mRNA-4157, and the KRAS vaccine has an open investigational new drug application (IND) ;
|
|
•
|
Intratumoral immuno-oncology
included three development candidates: OX40L (mRNA-2416), OX40L+IL23+IL36γ (Triplet) (mRNA-2752), and IL12 (MEDI1191). The OX40L and OX40L+IL23+IL36γ programs are currently in Phase 1 clinical trials and the IND for a Phase 1 clinical trial of IL12 is open;
|
|
•
|
Localized regenerative therapeutics
included one development candidate, VEGF-A (AZD8601). The program is being led by AstraZeneca through clinical development and is in a Phase 2a clinical trial;
|
|
•
|
Systemic secreted therapeutics
included three development candidates: antibody against Chikungunya virus (mRNA-1944), Relaxin (AZD7970), and Fabry disease (mRNA-3630). The antibody against Chikungunya virus
|
|
•
|
Systemic intracellular therapeutics
included four development candidates: MMA (mRNA-3704), PA (mRNA-3927), PKU (mRNA-3283), and GSD1a (mRNA-3745). The MMA program has an open IND, and the PA, PKU, and GSD1a programs are in preclinical development.
|
|
•
|
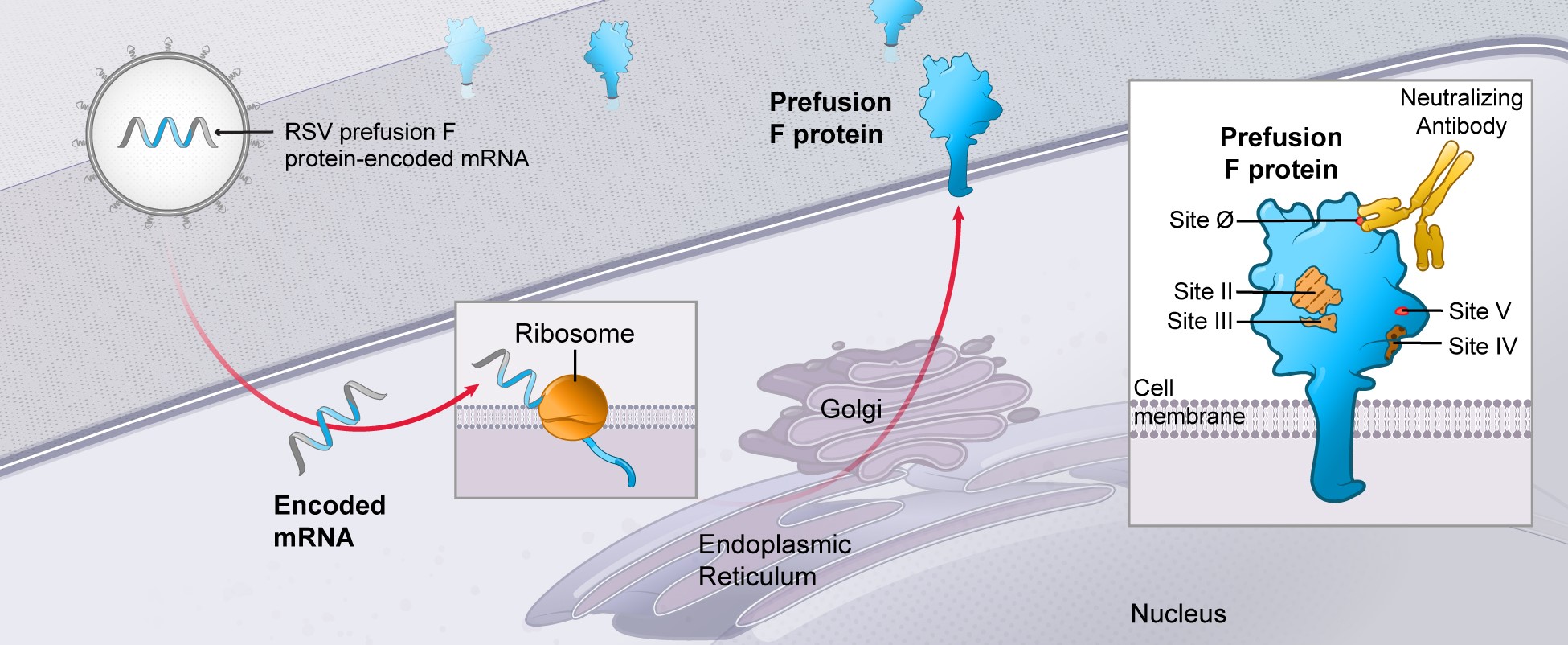
RSV vaccine (mRNA-1777 and mRNA-1172):
Merck has filed an IND with the FDA and plans to run a Phase 1 study for a follow-on development candidate (mRNA-1172), a vaccine for RSV which has shown enhanced potency in preclinical studies and uses a Merck proprietary formulation. As a result, further development of mRNA-1777 has been paused and next steps will be determined based on data from the new mRNA-1172 Phase 1 study.
|
|
•
|
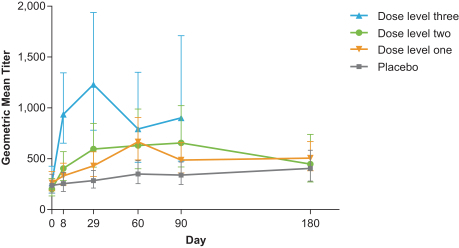
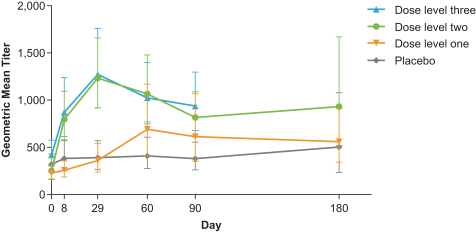
CMV vaccine (mRNA-1647):
The first three dose levels in our ongoing study of mRNA-1647 are fully enrolled, and the study is currently enrolling subjects into the fourth (300 µg) dose cohort. The Phase 1 study is randomized, observer-blind and placebo-controlled with the goal of evaluating the safety and immunogenicity of mRNA-1647, a vaccine against the pentamer and gB complexes of CMV.
|
|
•
|
VZV vaccine (mRNA-1278):
Merck has discontinued preclinical development of mRNA-1278, an investigational vaccine for VZV (the virus that causes shingles). Merck has returned rights to us, and we will not continue development at this time
|
|
•
|
Antibody against Chikungunya virus (mRNA-1944):
We completed enrollment of the second dose level cohort (0.3 mg/kg, 8 subjects) in the Phase 1 trial of mRNA-1944 in healthy adults.
|
|
•
|
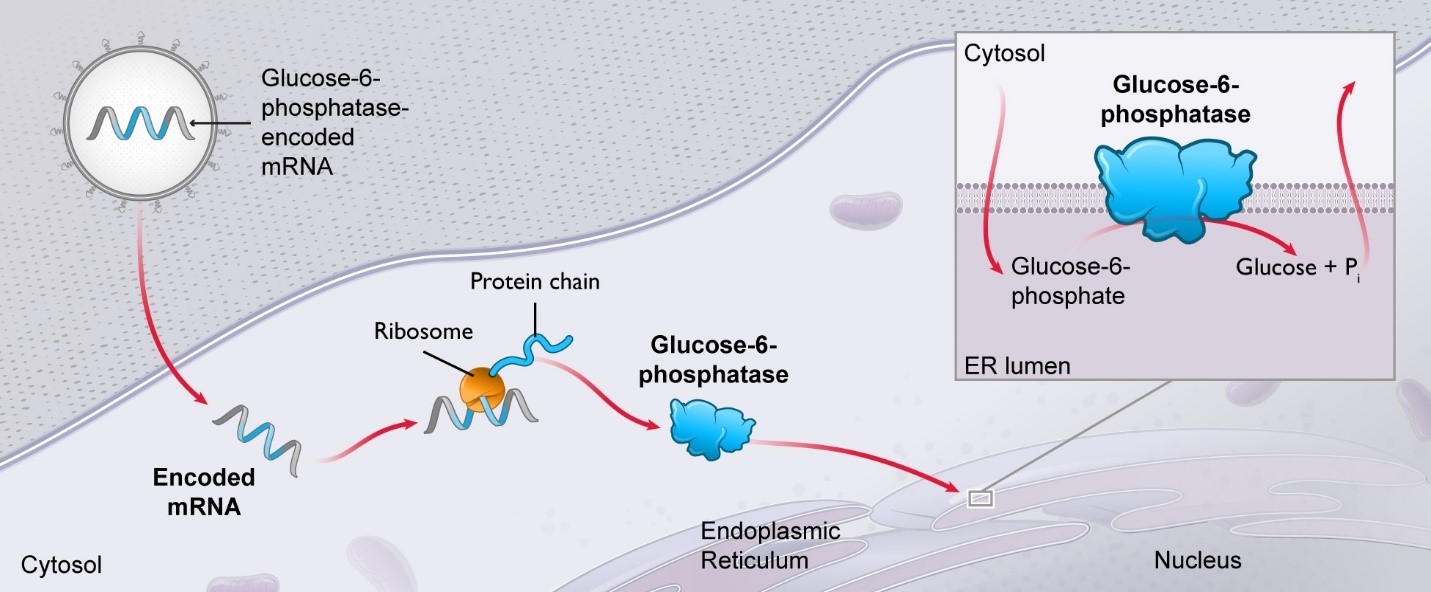
Propionic acidemia (PA) (mRNA-3927):
The European Commission has adopted the recommendation from the Committee for Orphan Medicinal Products for orphan drug designation for mRNA-3927, a development candidate for propionic acidemia (PA).
|
|
•
|
Glycogen storage disease type 1a (GSD1a) (mRNA-3745):
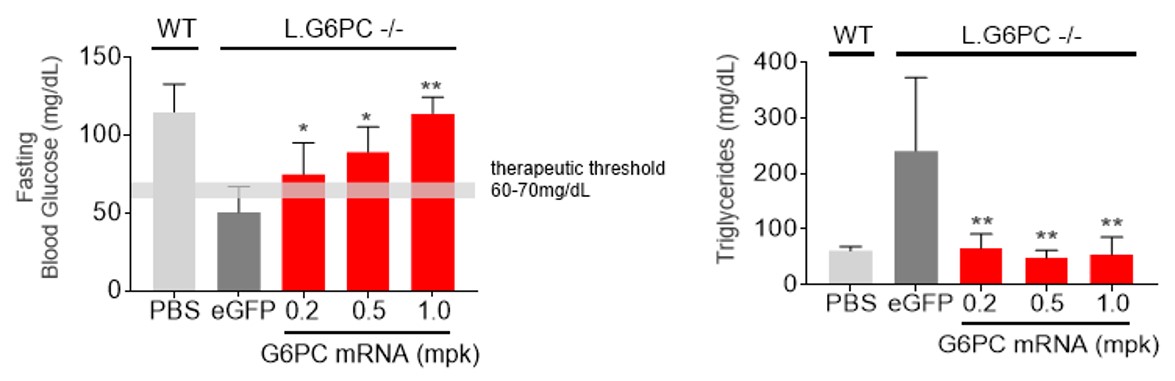
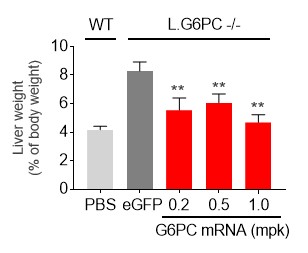
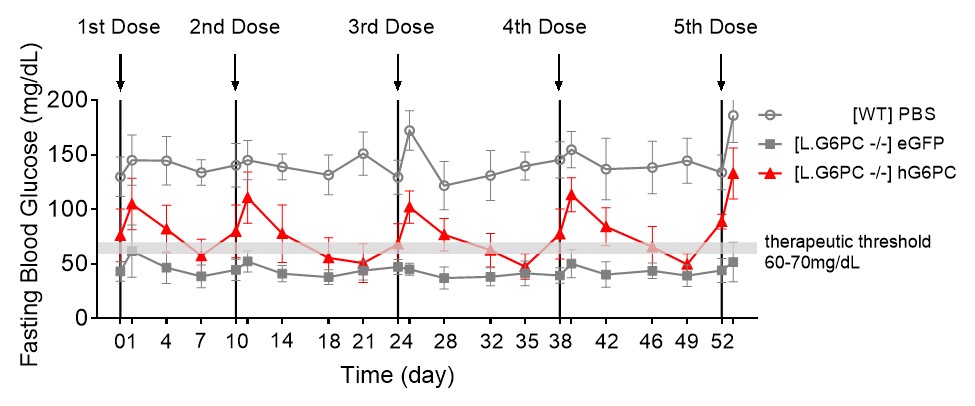
We designated mRNA-3745 as a new development candidate for the rare inherited metabolic disease GSD1a. Please refer to the section of this Form 10-Q entitled “Overview-New Development Candidate in our Systemic Intracellular Therapeutics Modality” for the program description for mRNA-3745.
|
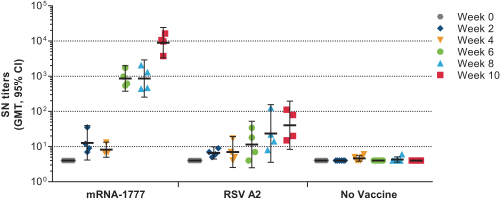
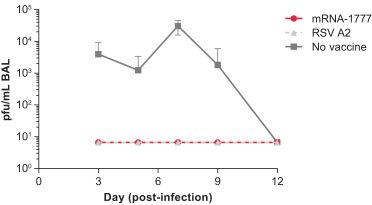
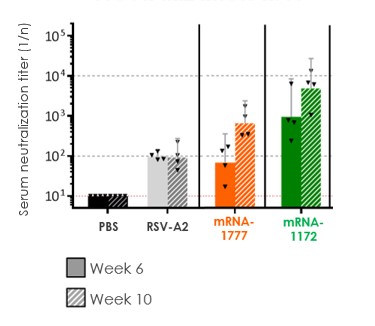
|
•
|
assess the safety and tolerability of mRNA-1777 versus placebo; and
|
|
•
|
determine the immunogenicity of mRNA-1777 by measuring serum neutralizing antibody titers against RSV.
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Collaboration revenue:
|
|||||||
|
Merck
|
$
|
10,687
|
|
$
|
15,967
|
|
|
|
Vertex
|
2,614
|
|
4,143
|
|
|||
|
AstraZeneca
|
814
|
|
7,350
|
|
|||
|
Total collaboration revenue
|
$
|
14,115
|
|
$
|
27,460
|
|
|
|
•
|
cost to develop our platform;
|
|
•
|
discovery efforts leading to development candidates;
|
|
•
|
preclinical, nonclinical, and clinical development costs for our programs;
|
|
•
|
cost to develop our manufacturing technology and infrastructure; and
|
|
•
|
digital infrastructure costs.
|
|
•
|
personnel-related expenses, including salaries, benefits, and stock-based compensation expense;
|
|
•
|
expenses incurred under agreements with third parties, such as consultants, investigative sites, contract research organizations, or CROs, that conduct our preclinical and clinical studies, and in-licensing arrangements;
|
|
•
|
costs of acquiring, developing, and manufacturing materials for preclinical and clinical studies, including both internal manufacturing and third-party contract manufacturing organizations, or CMOs;
|
|
•
|
expenses incurred for the procurement of materials, laboratory supplies, and non-capital equipment used in the research and development process; and
|
|
•
|
facilities, depreciation, and amortization, and other direct and allocated expenses incurred as a result of research and development activities.
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Program expenses by modality:
|
|||||||
|
Prophylactic vaccines
|
$
|
20,262
|
|
$
|
5,591
|
|
|
|
Cancer vaccines
|
10,086
|
|
7,848
|
|
|||
|
Intratumoral immuno-oncology
|
4,418
|
|
5,489
|
|
|||
|
Localized regenerative therapeutics
|
8
|
|
75
|
|
|||
|
Systemic secreted therapeutics
|
4,633
|
|
5,349
|
|
|||
|
Systemic intracellular therapeutics
|
6,743
|
|
8,150
|
|
|||
|
Total program-specific expenses by modality
(1)
|
46,150
|
|
32,502
|
|
|||
|
Other research and development expenses:
|
|||||||
|
Discovery programs
|
12,915
|
|
9,146
|
|
|||
|
Platform research
|
24,497
|
|
21,135
|
|
|||
|
Technical development and unallocated manufacturing expenses
|
21,347
|
|
13,129
|
|
|||
|
Shared discovery and development expenses
|
14,883
|
|
6,856
|
|
|||
|
Stock-based compensation
|
10,783
|
|
7,356
|
|
|||
|
Total research and development expenses
|
$
|
130,575
|
|
$
|
90,124
|
|
|
|
(1)
|
Includes a total of 20 and 18 development candidates at
March 31, 2019
and
2018
, respectively. Program-specific expenses include external costs and allocated manufacturing costs of mRNA supply and consumables, and reflect these expenses beginning in the period the program was internally advanced to development or these expenses ending in the period the program was ceased.
|
|
•
|
scope, progress, and expense of developing ongoing and future development candidates and investigational medicines;
|
|
•
|
entry in and completion of related preclinical studies;
|
|
•
|
enrollment in and completion of subsequent clinical trials;
|
|
•
|
safety and efficacy of investigational medicines resulting from these clinical trials;
|
|
•
|
changes in laws or regulations relevant to the investigational medicines in development;
|
|
•
|
receipt of the required regulatory approvals; and
|
|
•
|
commercialization, including establishing manufacturing and marketing capabilities.
|
|
Three Months Ended March 31,
|
Change 2019 vs. 2018
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
Revenue:
|
||||||||||||||
|
Collaboration revenue
|
$
|
14,115
|
|
$
|
27,460
|
|
$
|
(13,345
|
)
|
(49
|
)%
|
|||
|
Grant revenue
|
1,910
|
|
1,579
|
|
331
|
|
21
|
%
|
||||||
|
Total revenue
|
16,025
|
|
29,039
|
|
(13,014
|
)
|
(45
|
)%
|
||||||
|
Operating Expenses:
|
|
|||||||||||||
|
Research and development
|
130,575
|
|
90,124
|
|
40,451
|
|
45
|
%
|
||||||
|
General and administrative
|
27,283
|
|
16,317
|
|
10,966
|
|
67
|
%
|
||||||
|
Total operating expenses
|
157,858
|
|
106,441
|
|
51,417
|
|
48
|
%
|
||||||
|
Loss from operations
|
(141,833
|
)
|
(77,402
|
)
|
(64,431
|
)
|
83
|
%
|
||||||
|
Other income, net
|
9,152
|
|
5,026
|
|
4,126
|
|
82
|
%
|
||||||
|
Loss before benefit from income taxes
|
(132,681
|
)
|
(72,376
|
)
|
(60,305
|
)
|
83
|
%
|
||||||
|
Benefit from income taxes
|
(24
|
)
|
—
|
|
(24
|
)
|
|
|||||||
|
Net loss
|
$
|
(132,657
|
)
|
$
|
(72,376
|
)
|
$
|
(60,281
|
)
|
83
|
%
|
|||
|
Three Months Ended March 31,
|
Change 2019 vs. 2018
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
Interest income
|
$
|
10,972
|
|
$
|
5,209
|
|
$
|
5,763
|
|
111
|
%
|
|||
|
(Loss) gain on investment
|
(3
|
)
|
58
|
|
(61
|
)
|
(105
|
)%
|
||||||
|
Interest expense
|
(1,533
|
)
|
(27
|
)
|
(1,506
|
)
|
5,578
|
%
|
||||||
|
Other expense, net
|
(284
|
)
|
(214
|
)
|
(70
|
)
|
33
|
%
|
||||||
|
Total other income, net
|
$
|
9,152
|
|
$
|
5,026
|
|
$
|
4,126
|
|
82
|
%
|
|||
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net cash (used in) provided by:
|
|||||||
|
Operating activities
|
$
|
(143,927
|
)
|
$
|
(111,385
|
)
|
|
|
Investing activities
|
(11,759
|
)
|
(483,704
|
)
|
|||
|
Financing activities
|
(48
|
)
|
549,995
|
|
|||
|
Net decrease in cash and cash equivalents
|
$
|
(155,734
|
)
|
$
|
(45,094
|
)
|
|
|
Exhibit No.
|
Exhibit Index
|
|
|
10.1#
|
||
|
31.1
|
||
|
31.2
|
||
|
32.1+
|
||
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Link Document
|
|
|
+
|
The certification furnished in Exhibit 32.1 hereto is deemed to accompany this Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. Such certification will not be deemed to be incorporated by reference into any filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates it by reference.
|
|
#
|
Indicates a management contract or any compensatory plan, contract or arrangement.
|
|
MODERNA, INC.
|
|||
|
Date:
|
By:
|
/s/ Stéphane Bancel
|
|
|
May 9, 2019
|
|||
|
Stéphane Bancel
|
|||
|
Chief Executive Officer and Director
|
|||
|
(
Principal Executive Officer)
|
|||
|
Date:
|
By:
|
/s/ Lorence Kim, M.D.
|
|
|
May 9, 2019
|
|||
|
Lorence Kim, M.D.
|
|||
|
Chief Financial Officer
|
|||
|
(
Principal Financial Officer)
|
|||