|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[X]
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2009
or
|
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________to ________
|
|
Texas
(State or other Jurisdiction of Incorporation or Organization)
|
75-2508900
(I.R.S. Employer Identification No.)
|
|
600 S. Royal Lane, Suite 200, Coppell, Texas
(Address of Principal Executive Offices)
|
75019
(Zip Code)
|
|
|
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Sections 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [
]
|
|
|
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes [X] No [ ]
|
|
|
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
|
|
|
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
|
Large accelerated filer [ ]
|
Accelerated filer [X]
|
Non-accelerated filer [ ]
|
Smaller reporting company [ ]
|
|
·
|
management’s plans and objectives for future operations;
|
|
·
|
existing cash flows being adequate to fund future operational needs;
|
|
·
|
future plans related to budgets, future capital requirements, market share growth, and anticipated capital projects and obligations;
|
|
·
|
the realization of net deferred tax assets;
|
|
·
|
the ability to curtail operating expenditures;
|
|
·
|
global statutory tax rates remaining unchanged;
|
|
·
|
the impact of future market changes due to exposure to foreign currency translations;
|
|
·
|
the possibility of certain policies, procedures, and internal processes minimizing exposure to market risk;
|
|
·
|
the impact of new accounting pronouncements on financial condition, results of operations, or cash flows;
|
|
·
|
the outcome of new or existing litigation matters;
|
|
·
|
the outcome of new or existing regulatory inquiries or investigations; and
|
|
·
|
other assumptions described in this report underlying such forward-looking statements.
|
|
·
|
overall growth or lack of growth in the nutritional supplements industry;
|
|
·
|
plans for expected future product development;
|
|
·
|
changes in manufacturing costs;
|
|
·
|
shifts in the mix of packs and products;
|
|
·
|
the future impact of any changes to global associate career and compensation plans or incentives;
|
|
·
|
the ability to attract and retain independent associates and members;
|
|
·
|
new regulatory changes that may affect operations or products;
|
|
·
|
the competitive nature of our business with respect to products and pricing;
|
|
·
|
publicity related to our products or network marketing; and
|
|
·
|
the political, social, and economic climate.
|
|
·
|
In 1994, we developed and began selling our first products containing Manapol
®
, an ingredient formulated to support cell-to-cell communication.
|
|
·
|
In 1996, we enhanced our products based on the study of glycoproteins and our scientists developed our own proprietary compound, Ambrotose
®
complex, which we patented. Our Ambrotose
®
complex is a blend of polysaccharides (composed of monosaccharides) that helps provide support for the immune system.
|
|
·
|
In 2001, we broadened our proprietary ingredients by developing the Ambroglycin
®
blend, a balanced food-mineral matrix which helps deliver nutrients to the body and which is used in our proprietary Catalyst
™
and Glycentials
®
vitamin/mineral supplements.
|
|
·
|
In 2004, we introduced our proprietary blend of antioxidant nutrients, MTech AO Blend
®
, which is used in our proprietary antioxidant Ambrotose AO
®
product.
|
|
·
|
In 2006, we introduced a unique blend of plant-based minerals, natural vitamins, and standardized phytochemicals for use in our proprietary PhytoMatrix
®
product. We also introduced a compound used in reformulated Advanced Ambrotose
®
complex. This compound allows a more potent concentration of the full range of mannose-containing polysaccharides occurring naturally in aloe to be produced in a stable powdered form.
|
|
·
|
In 2007, we introduced into the United States market our skin care line of products that supports skin’s natural texture, beauty, and elasticity. We also launched our PhytoMatrix
®
caplets,
Advanced Ambrotose
®
capsules and
Manna•Bears
™
supplement
into international markets.
|
|
·
|
In 2008, we introduced a proprietary proteolytic enzyme and phytosterol dietary supplement that supports the body’s natural recovery processes associated with physical activity in our BounceBack
™
capsules. We also introduced a proprietary version of whey protein peptide technology which assists targeted fat loss when combined with exercise and a healthy diet in our OsoLean
™
powder.
|
|
·
|
In 2009, we launched some highly notable new products such as:
|
|
§
|
Our Essential Source
™
Omega-3, which features EPA/DHA essential acids.
|
|
§
|
Our PhytoBurst
™
Nutritional Chews, which are formulated with vitamins, minerals and phytonutrients from food-sourced ingredients.
|
|
§
|
Our GI-ProBalance
™
, which is a synbiotic digestive product containing both probiotics and prebiotics, as well as digestive enzymes.
|
|
§
|
Our Advanced Ambrotose
™
products were improved to include beta-Carotene.
|
|
§
|
Several products from our domestic line were launched in many of our current international markets.
|
|
|
Health
,
which offers a variety of nutritional supplements that aid in optimizing overall health and wellness. This category includes a variety of daily nutritional supplements, health solutions for children, and additional nutrients designed to help keep specific body systems at optimal levels.
|
|
|
Weight and Fitness,
which offers products designed to curb appetite and burn fat, build lean muscle tissue, and support recovery from overexertion.
|
|
|
Skin Care
,
which offers several products designed to improve and strengthen the skin’s own natural texture, softness and elasticity including damaged areas, as well as help deliver vital antioxidants to the skin.
|
|
Product Category
|
Representative Products
|
|
Health
|
Ambrotose
®
complex, Ambrotose AO
®
, Advanced Ambrotose
®
, PhytoMatrix
®
, Glyco-Bears
®
, MannaBears
™
, Catalyst
™
, PLUS
™
, Manna-C
™
, CardioBALANCE
®
, ImmunoSTART
®
, BounceBack
™
, MannaCLEANSE
™
, PhytAloe
®
, GI-Pro
®
, and GI-Zyme
®
, Essential Source
™
Omega-3, PhytoBurst
™
Nutritional Chews, and
GI-ProBalance
™
.
|
|
Weight and Fitness
|
OsoLean
™
, Accelerator3
™
, FiberSlim
®
, GlycoSlim
®
, AmbroStart
®
, SPORT
™
, and EM·PACT
®
.
|
|
Skin Care
|
Emprizone
®
, FIRM with Ambrotose
®
, Face Cleansing Cream, Skin Lotion, Skin Serum, Eye Cream, After Shave Milk, Cleansing Oil, and Skin Cream.
|
|
·
|
marketability and proprietary nature of the product;
|
|
·
|
demand for the product;
|
|
·
|
competitors’ products;
|
|
·
|
regulatory considerations;
|
|
·
|
availability of ingredients; and
|
|
·
|
data supporting claims of efficacy and safety.
|
|
Nutrition Industry Sector
|
Projected
2009
|
2008
|
2007
|
|||||||
|
Functional foods
|
36
|
%
|
37
|
%
|
37
|
%
|
||||
|
Nutritional supplements
|
25
|
%
|
25
|
%
|
26
|
%
|
||||
|
Natural and organic foods
|
29
|
%
|
28
|
%
|
28
|
%
|
||||
|
Natural personal care
|
10
|
%
|
10
|
%
|
9
|
%
|
||||
|
Total nutrition industry
|
100
|
%
|
100
|
%
|
100
|
%
|
||||
|
Nutrition Industry Sales Outlet
|
2008
|
2007
|
|||||
|
Grocer, drug, mass merchandise or club
|
56
|
%
|
54
|
%
|
|||
|
Specialty retail
|
30
|
%
|
33
|
%
|
|||
|
Mail order
|
2
|
%
|
2
|
%
|
|||
|
Multi-level marketing/direct selling
|
7
|
%
|
8
|
%
|
|||
|
Practitioner
|
3
|
%
|
2
|
%
|
|||
|
Internet
|
2
|
%
|
1
|
%
|
|||
|
Total sales by sales outlet
|
100
|
%
|
100
|
%
|
|||
|
·
|
the wide acceptance of the Internet and increased access to information by consumers;
|
|
·
|
the rising cost of traditional health care;
|
|
·
|
the growing acceptance and study of the concept of natural-based alternatives;
|
|
·
|
the general aging of the population;
|
|
·
|
the passage of regulatory acts in foreign markets similar to those in the United States, such as the DSHEA; and
|
|
·
|
the innovation of products.
|
|
|
1.
|
High-Quality, Innovative, Proprietary Products.
Our product concept is based on the scientific belief that certain glyconutrients, also known as monosaccharides or sugar molecules, are essential for maintaining a healthy immune system. We believe the addition of effective nutritional supplements to a well-balanced diet, coupled with an effective exercise program, will enhance and help maintain optimal health and wellness. We focus on producing products that are from all-natural sources with no synthetic or chemically derived additives. Our products are formulated with predominately naturally-occurring, plant-derived, carbohydrate-based safe ingredients that are designed to use nutrients working through normal physiology to help achieve and maintain optimal health and wellness, rather than developing common synthetic, carbohydrate-based products.
|
|
|
2.
|
Research and Development Efforts
.
We are steadfast in our commitment to quality-driven research and development. We use systematic processes for the research and development of our unique proprietary product formulas, as well as the identification of quality suppliers and manufacturers. Our research and quality assurance programs are outlined on our corporate websites, https://new.
mannatech.com
,
www.allaboutmannatech.com
, and
www.mannatechscience.org
.
|
|
1)
|
A randomized, double-blind, placebo-controlled study which showed that healthy middle-aged adults who took one teaspoon of Ambrotose
®
complex
twice daily performed significantly better on memory tasks and overall had a more positive mood. The findings of this 12-week study were published in the January 2010 issue of
Developmental Neuropsychology
, a peer-reviewed scientific journal.
|
|
2)
|
A randomized, double-blind, placebo-controlled study showed that healthy young adults who took a single, one-tablespoon serving of Ambrotose
®
complex had significantly improved visual discrimination and working memory. This study, conducted at Howard University, was published in 2009 in the peer-reviewed scientific journal
Perceptual and Motor Skills.
|
|
3)
|
An open-label study showed that OsoLean
™
consumption significantly increased weight loss and waist inches lost, significantly decreased BMI and appetite, and improved quality of life in healthy overweight adults. The findings of this eight week study were published in
The Open Nutraceuticals Journal
in March 2009.
|
|
4)
|
A pre-clinical study showed that rats ingesting Ambrotose
®
complex and Advanced Ambrotose
®
were protected against chemically-induced ulcerative colitis. The findings of this study, conducted by researchers at Eurofins/Product Safety Laboratories, were published in the peer-reviewed journal
Digestive Disease Science
in June 2009.
|
|
5)
|
A randomized, double-blind, placebo-controlled, cross-over study showed on that intake of 2 capsules/day of BounceBack
™
resulted in a significant reduction in pain after strenuous physical activity. This 30 day study, conducted by Medicus Research (Northridge, CA), was published in
Journal of the International Society of Sports Nutrition
in 2009.
|
|
|
3.
|
Quality Assurance Program.
We use qualified manufacturing contractors to produce, test, and package our finished products. These contractors must strictly adhere to our quality assurance program and when necessary be certified by the Therapeutic Goods Administration of Australia (“TGA”). The TGA requires companies that manufacture complementary medicines to comply with its good manufacturing practices regulations. In addition to the TGA regulations, our quality assurance program is designed to comply with the following regulations:
|
|
·
|
the FDA’s current Good Manufacturing Practice (“GMP”) in manufacturing, packaging, labeling, or holding operations for dietary supplements;
|
|
·
|
the FDA’s GMP for human food;
|
|
·
|
the requirements of the Natural Health Products Directorate of Canada; and
|
|
·
|
Korean Food and Drug Administration.
|
|
·
|
Nine products are certified according to the NSF/ANSI 173 Dietary Supplement Standard—the only American National Standard for dietary supplements. This certification ensures that this product contains only the ingredients indicated on the label and is free of impurities, and that Good Manufacturing Practices (GMPs) were used in the manufacturing facility.
|
|
·
|
Eight products are certified Kosher by OUKosher, the trademark with the highest certification standards world-wide.
|
|
·
|
Twenty-three products are certified gluten-free by Covance Laboratories.
|
|
|
4.
|
High-Caliber, Industry-Leading Independent Associates.
Our global team of independent associates are comprised of dedicated, hard working, high-caliber, compliance-oriented individuals, many of whom have been associated with the network marketing industry for decades and have been loyal to us since our beginning in 1993. To capitalize on their wealth of knowledge and experience, we sponsor a panel of independent associates, called the “North American Associate Advisory Council”, and a panel of international independent associates, called the “Global Advisory Council” (collectively called the “Advisory Council”), which help identify and effectively relay the needs of our independent business-building associates to us. The members of the Advisory Council are elected by their peers and serve a three-year term. The Advisory Council meets periodically with our team of senior management to recommend changes, discuss issues, and provide new ideas or concepts, including a full spectrum of innovative ideas for additional quality-driven nutritional supplements aimed at maintaining optimal health and wellness.
|
|
|
5.
|
Support Philosophy for Our Independent Associates and Members.
We are fully committed to providing the highest level of support services to our independent associates and members and believe that we meet expectations and build customer loyalty through the following:
|
|
·
|
providing efficient order processing centers to support operations;
|
|
·
|
offering highly-personalized and responsive customer service;
|
|
·
|
offering a 100% satisfaction guarantee product return policy for the first 180 days following the product’s purchase;
|
|
·
|
providing comprehensive corporate websites (https://new.mannatech.com, www.allaboutmannatech.com, www.mannatechscience.org, www.mannathink.com), that provide instant access to Internet ordering, marketing, technical and educational information, and unique and innovative marketing tools;
|
|
·
|
offering free personalized website development for our independent associates;
|
|
·
|
maintaining an extensive web-based downline management system called Success Tracker™ that provides access to web conferencing and downline organization reporting for our independent associates at minimal costs;
|
|
·
|
offering updated training/orientation and compliance programs for our independent associates;
|
|
·
|
providing strategically based distribution fulfillment centers to ensure products are shipped on time and at minimal cost;
|
|
·
|
inviting their input on innovative product ideas, which are gathered and tabulated on www.mannathink.com (launched at MannaFest 2009);
|
|
·
|
sponsoring comprehensive training about our products and promotional materials, and offering a full spectrum of comprehensive educational materials; and
|
|
·
|
sponsoring several corporate events, which are designed to provide information, education, and motivation for our dedicated business-building associates and to help stimulate business development. These events provide an interactive venue for introducing new products and services and allow interaction between our management teams, outside researchers, and independent associates.
|
|
|
6.
|
Flexible Operating Strategy.
We believe efficiency, focus, and flexibility are paramount to our operations. For over a decade, we have contracted with third parties to produce our proprietary raw materials and to manufacture our proprietary products, which we believe allows us to minimize capital expenditures, capitalize on such parties’ expertise, and build additional resources for strategic alliances in the areas of distribution and logistics, product registration, and export requirements. By contracting with various suppliers and manufacturers and by outsourcing distribution for all of our foreign operations, except Europe, we believe we can quickly adapt operations to current demands in a timely, efficient, and cost-effective manner. We monitor the performance of our third party contractors to ensure they maintain a high quality of service. In addition, we identify alternative sources for our raw materials suppliers and finished goods manufacturers to help prevent any risk of interruption in production should any existing contractors become unable to perform satisfactorily.
|
|
|
7.
|
Experience and Depth of Our Management Team.
We believe our team of executives has extensive experience in all aspects of business operations and is highly-focused on our success. Our Board of Directors is composed of seven directors, including five independent directors. We believe our board members have a wealth of knowledge and experience in most aspects of our business operations and are especially well versed in network marketing, finance, nutritional products, regulatory matters, and corporate governance. Our entire management team is committed to delivering high-quality products and superior service.
|
|
·
|
Attracting New Independent Associates and Retaining Existing Independent Associates.
We continually examine our global associate career and compensation plan and periodically introduce new incentives, such as our annual travel incentives, in order to attract, motivate, and retain independent associates. We believe our global associate career and compensation plan encourages greater associate retention, motivation, and productivity.
|
|
·
|
Carefully Planning and Executing New Market Entries.
In order to expand efficiently around the globe, we must continue to present maximum opportunity to our current associates as well as those who will join us in the future.
|
|
·
|
Developing New Products and Enhancing Existing Products.
We continue to focus on new areas for future product development. We continue our research efforts and strive to ensure that all of our products are made from high-quality, effective ingredients that contain one or more of our proprietary compounds, which we believe contributes to our cutting-edge industry leader goals. We expect that any future products we develop will further complement and enhance our existing products.
|
|
·
|
Strengthening our Financial Results and Adding Value to Our Shareholders and Independent Associates.
We believe we can continue to concentrate on improving financial results by focusing on ways to increase our revenues in both our domestic and foreign operations, and continuing to control operating costs.
|
|
·
|
offering educational meetings and corporate-sponsored events that emphasize business-building and compliance related information;
|
|
·
|
sponsoring various informative and science-based conference calls, web casts, and seminars;
|
|
·
|
providing automated services through the Internet and telephone that offer a full spectrum of information and business-building tools;
|
|
·
|
maintaining an efficient decentralized ordering and distribution system;
|
|
·
|
providing highly personalized and responsive order processing and customer service support accessible by multiple communication channels including telephone, Internet, or e-mail;
|
|
·
|
offering 24-hour, seven days a week access to information and ordering through the Internet;
|
|
·
|
offering Success Tracker
™
, a customized business-building genealogy system, which contains graphs, maps, alerts, reports, and web video conferencing for our independent associates; and
|
|
·
|
providing a wide assortment of business-building and educational materials to help stimulate product sales and simplify enrollment.
|
|
·
|
generating product sales from an independent associate’s global downline to earn certain achievement levels;
|
|
·
|
enrolling new independent associates or members who place a product order;
|
|
·
|
obtaining certain achievement levels and enrolling other independent associates in a downline who place monthly automatic orders;
|
|
·
|
obtaining and developing certain achievement levels within their downline organizations to qualify for additional bonuses;
|
|
·
|
building a team of six qualified independent associates in their global downlines who order products regularly; and
|
|
·
|
various other incentive programs, including periodic travel incentives.
|
|
·
|
Retail Customer Product Return Policy.
Our retail customer product return policy allows a retail customer to return any of our products to the original independent associate who sold the product. Such independent associate will provide the retail customer with a full cash refund for the first 180 days following the product’s purchase. The independent associate may then return or exchange the product based on the independent associate product return policy.
|
|
·
|
Member Product Return Policy.
Our member product return policy allows members to return an order for a full refund within 180 days of the purchase date without termination or restocking fees. After 180 days from the purchase date, the member may not request a refund, and is allowed an exchange only, and may, if abuse of the return policy is found, be terminated as an active member.
|
|
·
|
Independent Associate Product Return Policy.
Our independent associate product return policy allows our independent associates to return an order within one year of the purchase date upon terminating their associate account. If an independent associate returns a product unopened and in good salable condition, the independent associate returning the product may receive a full refund. We may also allow the independent associate to receive a full refund for the first 180 days following the product’s purchase; however, any commissions earned will be deducted from the refund. After 180 days from the purchase date, the independent associate may not request a refund, and is allowed an exchange only. If we discover abuse of the refund policy, we may terminate the associate’s account.
|
|
·
|
minimize the time required to process orders and distribute products;
|
|
·
|
provide customized ordering information;
|
|
·
|
quickly respond to information requests, including providing detailed and accurate information to independent associates about qualification and downline activity;
|
|
·
|
provide detailed reports about paid commissions and incentives;
|
|
·
|
support order processing and customer service departments; and
|
|
·
|
help monitor, analyze, and report operating and financial results.
|
|
·
|
direct selling and network marketing systems;
|
|
·
|
transfer pricing and similar regulations affecting the amount of foreign taxes and customs duties paid;
|
|
·
|
taxation of our independent associates and requirements to collect taxes and maintain appropriate records;
|
|
·
|
how a company manufactures, packages, labels, distributes, imports, sells, and stores products;
|
|
·
|
product ingredients;
|
|
·
|
product claims;
|
|
·
|
product labels;
|
|
·
|
advertising; and
|
|
·
|
the extent to which we may be responsible for claims made by our independent associates.
|
|
·
|
the FDA;
|
|
·
|
the Federal Trade Commission (“FTC”);
|
|
·
|
the Consumer Product Safety Commission;
|
|
·
|
the Department of Agriculture;
|
|
·
|
the Environmental Protection Agency;
|
|
·
|
the United States Postal Service;
|
|
·
|
state attorney general offices; and
|
|
·
|
various agencies of the states and localities in which our products are sold.
|
|
·
|
the identification of dietary or nutritional supplements and their nutrition and ingredient labeling;
|
|
·
|
requirements related to the wording used for claims about nutrients, health claims, and statements of nutritional support;
|
|
·
|
labeling requirements for dietary or nutritional supplements for which “high potency,” “antioxidant,” and “trans-fatty acids” claims are made;
|
|
·
|
notification procedures for statements on dietary and nutritional supplements; and
|
|
·
|
pre-market notification procedures for new dietary ingredients in nutritional supplements.
|
|
|
|
·
|
the National Provincial Laws, Natural Health Product Regulations of Canada, and the Federal Competition Act in Canada;
|
|
·
|
the Therapeutic Goods Administration and the Trade Practices Act in Australia;
|
|
·
|
federal and state regulations in Australia;
|
|
·
|
national regulations including the Local Trading Standards Offices in the United Kingdom;
|
|
·
|
regulations from the Ministry of International Trade and Industry in Japan;
|
|
·
|
regulations from the Commerce Commission and the Fair Trade Act of 1993 in New Zealand;
|
|
·
|
the Fair Trade Commission, which oversees the Door to Door Sales Act and the Health and Functional Food Act enforced by the Korea Food and Drug Administration in the Republic of Korea;
|
|
·
|
the Fair Trade Law, which is enforced by the Taiwan Fair Trade Commission and the Administration of Food Hygiene, Health Food Products Administration Act enforced by the Taiwan Department of Health;
|
|
·
|
the Danish Health Board, the Danish Marketing Practice Act, the Danish Consumer Ombudsman, the Danish Executive Order on Dietary Supplements, the Guidelines for food supplements, and the Danish Act on Foodstuffs in Denmark;
|
|
·
|
the German Unfair Competition Act, German Regulation on food supplements, and German Law on food and feed;
|
|
·
|
regulations governing business practices in South Africa;
|
|
·
|
the Consumer Protection Act, the Sale of Food Act, and various regulations that are governed by the Ministry of Trade and Industry in Singapore;
|
|
·
|
the Austrian Trade Law (1994), the Food Safety and Consumer Protection Law (2006), and the Food Code in Austria;
|
|
·
|
the Food and Consumer Products and the Unfair Trade Practices Act, Door to Door Selling Act and Provisions of the General Dutch Civil Code relating to terms and conditions and misleading advertising in the Netherlands;
|
|
·
|
the Consumer Sales Act, Marketing Practices Act, Distance and Doorstep Sales Act, the Product Liability Act, Product Safety Act, the Companies Act and the Food Act in Sweden;
|
|
·
|
the Law on Marketing and Contract Conditions, the Law on Repentance Right, the Statutory Order on Self Inspection of Food Provisions, the Law on Food products and Food Safety, and various guidelines from the Norwegian Consumers Agency on telephone selling and internet marketing, in Norway; and
|
|
·
|
various European Union (“EU”) regulations and pronouncements address both our selling activities and the sale of food supplements in EU member nations.
|
|
·
|
social security taxes;
|
|
·
|
value added taxes;
|
|
·
|
goods and services taxes;
|
|
·
|
sales taxes;
|
|
·
|
consumption taxes;
|
|
·
|
income taxes;
|
|
·
|
customs duties;
|
|
·
|
employee/independent contractor regulations;
|
|
·
|
employment and severance pay requirements;
|
|
·
|
import/export regulations;
|
|
·
|
federal securities laws; and
|
|
·
|
antitrust laws.
|
|
·
|
claims made about our products;
|
|
·
|
promises or claims of income or other promises or claims by our independent associates; and
|
|
·
|
sales of products in markets where the products have not been approved or licensed.
|
|
·
|
Herbalife Ltd.;
|
|
·
|
Market America, Inc.;
|
|
·
|
Nature’s Sunshine Products, Inc.;
|
|
·
|
Nu Skin Enterprises, Inc.;
|
|
·
|
Reliv, International Inc;
|
|
·
|
Solgar Vitamin and Herb Company, Inc.;
|
|
·
|
Usana Health Sciences, Inc.; and
|
|
·
|
Weider Nutrition.
|
|
·
|
our products can be introduced into the global marketplace at a much lower up-front cost than through conventional methods;
|
|
·
|
our key ingredients and differential components found in our proprietary products can be explained better through network marketing;
|
|
·
|
the network marketing approach can quickly and easily adapt to changing market conditions;
|
|
·
|
consumers appreciate the convenience of ordering from home, through a sales person, by telephone, or on the Internet; and
|
|
·
|
network marketing enables independent associates to earn financial rewards.
|
|
·
|
Amway Corporation;
|
|
·
|
Body Wise International, Inc.;
|
|
·
|
Envion International;
|
|
·
|
Forever Living Products, Inc.;
|
|
·
|
Herbalife International, Inc.;
|
|
·
|
Mary Kay, Inc.;
|
|
·
|
Nature’s Sunshine Products, Inc.;
|
|
·
|
New Vision International;
|
|
·
|
Nu Skin Enterprises, Inc.;
|
|
·
|
Reliv, International Inc.;
|
|
·
|
Shaklee Worldwide; and
|
|
·
|
Usana Health Sciences, Inc.
|
|
·
|
our unique patented, proprietary blend of high-quality products;
|
|
·
|
our 16-year track record in the business of selling nutritional products;
|
|
·
|
our policy of not requiring our independent associates to carry inventory or accounts receivable;
|
|
·
|
our unique and financially rewarding global associate career and compensation plan;
|
|
·
|
our innovative marketing and educational tools; and
|
|
·
|
our easy and convenient delivery system.
|
|
United States
|
Canada
|
Australia
|
United
Kingdom
|
Japan
|
Republic
of Korea
|
Taiwan
|
Switzerland
|
South
Africa
|
Total
|
|
|
2009
|
337
|
1
|
38
|
34
|
33
|
34
|
17
|
7
|
1
|
502
|
|
2008
|
379
|
1
|
39
|
34
|
30
|
33
|
18
|
6
|
—
|
540
|
|
·
|
on-going motivation of our independent associates;
|
|
·
|
general economic conditions;
|
|
·
|
significant changes in the amount of commissions paid;
|
|
·
|
public perception and acceptance of the wellness industry;
|
|
·
|
public perception and acceptance of network marketing;
|
|
·
|
public perception and acceptance of our business and our products, including any negative publicity;
|
|
·
|
the limited number of people interested in pursuing network marketing as a business;
|
|
·
|
our ability to provide proprietary quality-driven products that the market demands; and
|
|
·
|
competition in recruiting and retaining independent associates.
|
|
·
|
Our Ambrotose
®
complex, a glyconutritional dietary supplement ingredient consisting of a blend of monosaccharides, or sugar molecules, used in the majority of our products;
|
|
·
|
The MTech AO Blend
®
, our proprietary, patent-pending antioxidant used in the Ambrotose AO
®
complex; and
|
|
·
|
A compound used in our reformulated Advanced Ambrotose
®
complex that allows for a more potent concentration of the full range of mannose-containing polysaccharides occurring naturally in aloe.
|
|
·
|
the nutritional supplements industry;
|
|
·
|
skeptical consumers;
|
|
·
|
competitors;
|
|
·
|
the safety and quality of our products and/or our ingredients;
|
|
·
|
regulatory investigations of our products or our competitors’ products;
|
|
·
|
the actions of our independent associates;
|
|
·
|
the direct selling/network marketing industry; and
|
|
·
|
scandals within the industries in which we operate.
|
|
·
|
accurately anticipate consumer needs;
|
|
·
|
innovate and develop new products or product enhancements that meet these needs;
|
|
·
|
successfully commercialize new products or product enhancements in a timely manner;
|
|
·
|
price our products competitively;
|
|
·
|
manufacture and deliver our products in sufficient volumes and in a timely manner; and
|
|
·
|
differentiate our product offerings from those of our competitors.
|
|
·
|
misconduct by us or our independent associates;
|
|
·
|
ambiguity in statutes;
|
|
·
|
regulations and related court decisions;
|
|
·
|
the discretion afforded to regulatory authorities and courts interpreting and enforcing laws; and
|
|
·
|
new regulations or interpretations of regulations affecting our business.
|
|
·
|
the formulation, manufacturing, packaging, labeling, distribution, importation, sale, and storage of our products;
|
|
·
|
the health and safety of dietary supplements, cosmetics and foods;
|
|
·
|
trade practice laws and network marketing laws;
|
|
·
|
our product claims and advertising by our independent associates;
|
|
·
|
our network marketing system;
|
|
·
|
pricing restrictions regarding transactions with our foreign subsidiaries or other related parties and similar regulations that affect our level of foreign taxable income;
|
|
·
|
the assessment of customs duties;
|
|
·
|
further taxation of our independent associates, which may obligate us to collect additional taxes and maintain additional records; and
|
|
·
|
export and import restrictions.
|
|
·
|
inflation;
|
|
·
|
the renegotiation or modification of various agreements;
|
|
·
|
increases in custom duties and tariffs;
|
|
·
|
changes and limits in export controls;
|
|
·
|
government regulations and laws;
|
|
·
|
trademark availability and registration issues;
|
|
·
|
changes in exchange rates;
|
|
·
|
changes in taxation;
|
|
·
|
wars and other hostilities; and
|
|
·
|
changes in the perception of network marketing.
|
|
·
|
order processing;
|
|
·
|
supply chain management;
|
|
·
|
customer service;
|
|
·
|
product distribution;
|
|
·
|
commission processing;
|
|
·
|
cash receipts and payments; and
|
|
·
|
financial reporting.
|
|
·
|
broad market fluctuations and general economic conditions;
|
|
·
|
fluctuations in our financial results;
|
|
·
|
future securities offerings;
|
|
·
|
changes in the market’s perception of our products or our business, including false or negative publicity;
|
|
·
|
governmental regulatory actions;
|
|
·
|
the outcome of any lawsuits;
|
|
·
|
financial and business announcements made by us or our competitors;
|
|
·
|
the general condition of the industry; and
|
|
·
|
the sale of large amounts of stock by insiders.
|
25. Concentration Risk
|
2009
|
2008
|
||||||||||
|
Sales by
product
|
% of total
net sales
|
Sales by
product
|
% of total
net sales
|
||||||||
|
Advanced Ambrotose
®
|
$
|
65,360
|
22.6
|
%
|
$
|
85,980
|
25.8
|
%
|
|||
|
Ambrotose
®
|
25,413
|
8.8
|
%
|
33,748
|
10.1
|
%
|
|||||
|
Total
|
$
|
90,773
|
31.4
|
%
|
$
|
119,728
|
35.9
|
%
|
|||
|
Location
|
Size
|
Original
term
|
Expiration
date
|
||||
|
Coppell, Texas (corporate headquarters)
|
110,000
|
sq. feet
|
10
|
years
|
March 2017
|
||
|
Coppell, Texas (distribution center)
|
75,000
|
sq. feet
|
10
|
years
|
March 2017
|
||
|
St. Leonards, Australia (Australian headquarters)
|
850
|
sq. meters
(1)
|
5
|
years
|
August 2013
|
||
|
Didcot, Oxfordshire (combined U.K. headquarters and
distribution center)
|
16,631
|
sq. feet
|
5
|
years
|
July 2011
|
||
|
Minato-ku, Tokyo, Japan (Japanese headquarters)
|
296
|
Tsubos
(2)
|
2
|
years
|
November 2010
|
||
|
Kangnam-gu, Seoul, Korea (Republic of Korea headquarters)
|
625
|
Pyung
(3)
|
2
|
years
|
June 2010
|
||
|
Taipei, Taiwan (Taiwan headquarters)
|
254
|
pings
(4)
|
3
|
years
|
November 2010
|
||
|
Zug, Switzerland (Switzerland headquarters)
|
680
|
sq. meters
(5)
|
5
|
years
|
October 2013
|
||
|
Markham, Ontario (Canada headquarters)
|
3,097
|
sq. feet
|
3
|
years
|
September 2012
|
||
|
Bedfordview, South Africa
|
383
|
sq. meters
(6)
|
5
|
years
|
March 2015
|
||
______________________
|
|
(1)
Approximately 9,149 square feet.
|
|
|
(2)
Approximately 10,538 square feet.
|
|
|
(3)
Approximately 22,190 square feet.
|
|
|
(4)
Approximately 9,021 square feet.
|
|
|
(5)
Approximately 7,324 square feet.
|
|
|
(6)
Approximately 4,119 square feet.
|
|
Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities
|
|
2009:
|
Low
|
High
|
|||||
|
First Quarter
|
$
|
2.50
|
$
|
3.90
|
|||
|
Second Quarter
|
$
|
2.97
|
$
|
4.73
|
|||
|
Third Quarter
|
$
|
3.13
|
$
|
4.80
|
|||
|
Fourth Quarter
|
$
|
2.34
|
$
|
3.89
|
|||
|
2008:
|
|||||||
|
First Quarter
|
$
|
5.09
|
$
|
8.49
|
|||
|
Second Quarter
|
$
|
5.44
|
$
|
7.39
|
|||
|
Third Quarter
|
$
|
3.48
|
$
|
6.96
|
|||
|
Fourth Quarter
|
$
|
1.88
|
$
|
4.41
|
|||
|
Declared date
|
Date of record
|
Date paid
|
Total Amount of
Dividends
|
Dollar amount
paid per common share |
||||||||
|
May 20, 2009
|
June 3, 2009
|
June 29, 2009
|
$
|
0.5
|
million
|
$
|
0.02
|
|||||
|
February 18, 2009
|
March 9, 2009
|
March 26, 2009
|
$
|
0.5
|
million
|
$
|
0.02
|
|||||
|
November 19, 2008
|
December 11, 2008
|
December 29, 2008
|
$
|
0.5
|
million
|
$
|
0.02
|
|||||
|
August 26, 2008
|
September 10, 2008
|
September 29, 2008
|
$
|
0.5
|
million
|
$
|
0.02
|
|||||
|
April 30,2008
|
June 5, 2008
|
June 26, 2008
|
$
|
2.4
|
million
|
$
|
0.09
|
|||||
|
February 22, 2008
|
March 7, 2008
|
March 28, 2008
|
$
|
2.4
|
million
|
$
|
0.09
|
|||||
|
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants, and rights
(a)
|
Weighted-average
exercise price of
outstanding options, warrants, and rights
(b)
|
Number of securities
remaining available for
future issuance under equity
compensation plans
(excluding securities reflected in column (a))
(c)
|
|||
|
Equity compensation plan
|
1,675,060
|
$
|
4.95
|
228,684
|
||
|
Equity compensation plans not approved by Shareholders
|
—
|
—
|
—
|
|||
|
Total
|
1,675,060
|
228,684
|
||||
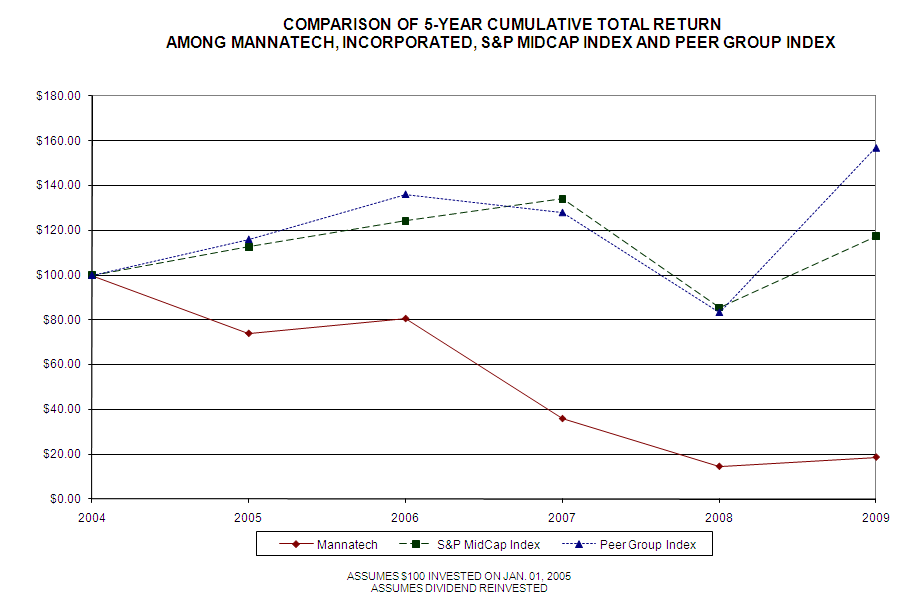
|
Measurement Period
|
Mannatech
|
S&P
Midcap Index
|
Peer
Group Index
|
||||||
|
December 31, 2005
|
$
|
73.92
|
$
|
112.56
|
$
|
116.04
|
|||
|
December 31, 2006
|
$
|
80.55
|
$
|
124.17
|
$
|
136.19
|
|||
|
December 31, 2007
|
$
|
35.85
|
$
|
134.08
|
$
|
128.00
|
|||
|
December 31, 2008
|
$
|
14.49
|
$
|
85.50
|
$
|
83.37
|
|||
|
December 31, 2009
|
$
|
18.62
|
$
|
83.37
|
$
|
156.84
|
|||
|
2009
|
2008
(3)
|
2007
(2)
|
2006
(1)
|
2005
|
||||||||||||||||
|
Consolidated Statements of Operations Data:
|
(in thousands, except per share amounts)
|
|||||||||||||||||||
|
Net sales
|
$ | 289,705 | $ | 332,703 | $ | 412,678 | $ | 410,069 | $ | 389,383 | ||||||||||
|
Gross profit
|
$ | 96,477 | $ | 134,544 | $ | 163,846 | $ | 169,393 | $ | 159,204 | ||||||||||
|
Income (loss) from operations
|
$ | (25,594 | ) | $ | (14,499 | ) | $ | 7,609 | $ | 44,074 | $ | 45,610 | ||||||||
|
Net income (loss)
|
$ | (17,368 | ) | $ | (12,628 | ) | $ | 6,594 | $ | 32,390 | $ | 28,647 | ||||||||
|
Earnings (loss) Per Common Share:
|
||||||||||||||||||||
|
Basic
|
$ | (0.66 | ) | $ | (0.48 | ) | $ | 0.25 | $ | 1.22 | $ | 1.06 | ||||||||
|
Diluted
|
$ | (0.66 | ) | $ | (0.48 | ) | $ | 0.25 | $ | 1.19 | $ | 1.03 | ||||||||
|
Weighted-Average Common Shares Outstanding:
|
||||||||||||||||||||
|
Basic
|
26,467 | 26,461 | 26,443 | 26,598 | 26,990 | |||||||||||||||
|
Diluted
|
26,467 | 26,461 | 26,893 | 27,219 | 27,771 | |||||||||||||||
|
Other Financial Data:
|
||||||||||||||||||||
|
Capital expenditures
|
$ | 4,896 | $ | 5,633 | $ | 13,446 | $ | 27,216 | $ | 13,114 | ||||||||||
|
Dividends declared per common share
|
$ | 0.04 | $ | 0.22 | $ | 0.36 | $ | 0.32 | $ | 0.29 | ||||||||||
|
Consolidated Balance Sheet Data:
|
||||||||||||||||||||
|
Total assets
|
$ | 102,302 | $ | 124,058 | $ | 152,454 | $ | 152,235 | $ | 122,795 | ||||||||||
|
Long-term obligations, excluding current portion
|
$ | 8,339 | $ | 9,813 | $ | 9,431 | $ | 11,402 | $ | 4,964 | ||||||||||
_______________________________
|
2009
|
2008
|
Change
|
||||||||||||||||||||||||||
|
Total
Dollars
|
% of
net sales
|
Total
dollars
|
% of
net sales
|
Dollar
|
Percentage
|
|||||||||||||||||||||||
|
Net sales
|
$ | 289,705 | 100 | % | $ | 332,703 | 100 | % | $ | (42,998 | ) | (12.9 | ) | % | ||||||||||||||
|
Cost of sales
|
46,813 | 16.2 | % | 48,564 | 14.6 | % | (1,751 | ) | (3.6 | ) | % | |||||||||||||||||
|
Commissions and incentives
|
146,415 | 50.5 | % | 149,595 | 45.0 | % | (3,180 | ) | (2.1 | ) | % | |||||||||||||||||
| 193,228 | 66.7 | % | 198,159 | 59.6 | % | (4,931 | ) | (2.5 | ) | % | ||||||||||||||||||
|
Gross profit
|
96,477 | 33.3 | % | 134,544 | 40.4 | % | (38,067 | ) | (28.3 | ) | % | |||||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||||||||||
|
Selling and administrative expenses
|
69,997 | 24.2 | % | 81,077 | 24.4 | % | (11,080 | ) | (13.7 | ) | % | |||||||||||||||||
|
Depreciation and amortization
|
12,333 | 4.3 | % | 12,310 | 3.7 | % | 23 | 0.2 |
|
% | ||||||||||||||||||
|
Other operating costs
|
39,741 | 13.7 | % | 55,656 | 16.7 | % | (15,915 | ) | (28.6 | ) | % | |||||||||||||||||
|
Total operating expenses
|
122,071 | 42.1 | % | 149,043 | 44.8 | % | (26,972 | ) | (18.1 | ) | % | |||||||||||||||||
|
Loss from operations
|
(25,594 | ) | (8.8 | )% | (14,499 | ) | (4.4 | )% | (11,095 | ) | (76.5 | ) | % | |||||||||||||||
|
Interest income
|
473 | 0.2 | % | 1,604 | 0.5 | % | (1,131 | ) | (70.5 | ) | % | |||||||||||||||||
|
Other income (expense), net
|
1,046 | 0.4 | % | (5,303 | ) | (1.6 | )% | 6,349 | 119.7 | % | ||||||||||||||||||
|
Loss before income taxes
|
(24,075 | ) | (8.3 | )% | (18,198 | ) | (5.5 | )% | (5,877 | ) | (32.3 | ) | % | |||||||||||||||
|
Benefit for income taxes
|
6,707 | 2.3 | % | 5,570 | 1.7 | % | 1,137 | 20.4 | % | |||||||||||||||||||
|
Net loss
|
$ | (17,368 | ) | (6.0 | )% | $ | (12,628 | ) | (3.8 | )% | $ | (4,740 | ) | (37.5 | ) | % | ||||||||||||
|
2009
|
2008
|
||||||||||||
|
United States
|
$
|
140.7
|
48.6
|
%
|
$
|
176.9
|
53.1
|
%
|
|||||
|
Japan
|
42.0
|
14.5
|
%
|
44.8
|
13.5
|
%
|
|||||||
|
Republic of Korea
|
26.4
|
9.1
|
%
|
35.7
|
10.7
|
%
|
|||||||
|
Canada
|
23.0
|
7.9
|
%
|
23.6
|
7.1
|
%
|
|||||||
|
Australia
|
22.9
|
7.9
|
%
|
26.1
|
7.8
|
%
|
|||||||
|
South Africa
(1)
|
13.2
|
4.6
|
%
|
5.5
|
1.7
|
%
|
|||||||
|
Taiwan
|
6.6
|
2.3
|
%
|
5.2
|
1.6
|
%
|
|||||||
|
New Zealand
|
4.3
|
1.5
|
%
|
5.2
|
1.6
|
%
|
|||||||
|
United Kingdom
|
3.3
|
1.0
|
%
|
4.7
|
1.4
|
%
|
|||||||
|
Germany
|
3.2
|
1.1
|
%
|
3.8
|
1.1
|
%
|
|||||||
|
Denmark
|
1.6
|
0.6
|
%
|
1.2
|
0.4
|
%
|
|||||||
|
Singapore
(2)
|
1.5
|
0.5
|
%
|
—
|
—
|
%
|
|||||||
|
Austria
(3)
|
0.3
|
0.1
|
%
|
—
|
—
|
%
|
|||||||
|
Norway
(3)
|
0.3
|
0.1
|
%
|
—
|
—
|
%
|
|||||||
|
The Netherlands
(3)
|
0.2
|
0.1
|
%
|
—
|
—
|
%
|
|||||||
|
Sweden
(3)
|
0.2
|
0.1
|
%
|
—
|
—
|
%
|
|||||||
|
Totals
|
$
|
289.7
|
100
|
%
|
$
|
332.7
|
100
|
%
|
|||||
Net sales by country in local currency for the year ended December 31, 2009 and 2008 were as follows (in millions, except percentages) :
|
Change
|
|||||||||||
|
Currency
|
2009
|
2008
|
Local
currency
|
Percentage
|
|||||||
|
Australia and Singapore
(1)
|
AUD
|
31.1
|
31.0
|
0.1
|
0.3
|
%
|
|||||
|
Austria, Germany,
Netherlands
(2)
|
EUR
|
2.6
|
2.6
|
—
|
—
|
||||||
|
Denmark
|
DKK
|
8.6
|
6.3
|
2.3
|
36.5
|
%
|
|||||
|
Japan
|
JPY
|
3,890.7
|
4,584.3
|
(693.6
|
)
|
(15.1
|
)%
|
||||
|
Korea
|
KRW
|
33,366.8
|
38,733.4
|
(5,366.6
|
)
|
(13.9
|
)%
|
||||
|
New Zealand
|
NZD
|
6.9
|
7.3
|
(0.4)
|
(5.5
|
)%
|
|||||
|
Norway
(2)
|
NOK
|
2.0
|
—
|
2.0
|
—
|
||||||
|
South Africa
(3)
|
ZAR
|
109.8
|
47.4
|
62.4
|
131.6
|
%
|
|||||
|
Sweden
(2)
|
SEK
|
1.2
|
—
|
1.2
|
—
|
||||||
|
Taiwan
|
TWD
|
217.4
|
165.4
|
52.0
|
31.4
|
%
|
|||||
|
United Kingdom
|
GBP
|
2.1
|
2.6
|
(0.5
|
)
|
(19.2
|
)%
|
||||
|
·
|
changes in our sales prices;
|
|
·
|
changes in consumer demand;
|
|
·
|
changes in the number of independent associates and members;
|
|
·
|
changes in competitors’ products;
|
|
·
|
changes in economic conditions;
|
|
·
|
changes in regulations;
|
|
·
|
announcements of new scientific studies and breakthroughs;
|
|
·
|
introduction of new products;
|
|
·
|
discontinuation of existing products;
|
|
·
|
adverse publicity;
|
|
·
|
changes in our commissions and incentives programs; and
|
|
·
|
fluctuations in foreign currency exchange rates.
|
|
Change
|
||||||||||||
|
2009
|
2008
|
Dollar
|
Percentage
|
|||||||||
|
Product sales
|
$
|
213.9
|
$
|
260.5
|
$
|
(46.6
|
)
|
(17.9
|
)%
|
|||
|
Pack sales
|
62.1
|
57.7
|
4.4
|
7.6
|
%
|
|||||||
|
Other, including freight
|
13.7
|
14.5
|
(0.8
|
)
|
(5.5
|
)%
|
||||||
|
Total net sales
|
$
|
289.7
|
$
|
332.7
|
$
|
(43.0
|
)
|
(12.9
|
)%
|
|||
|
·
|
Mannatech Optimal Skin Care System products in certain international markets;
|
|
·
|
OsoLean
™
powder and/or OsoLean
™
single use packets in all of our markets;
|
|
·
|
Various promotional packages in United States, Canada, South Africa, Taiwan, and Australia;
|
|
·
|
Health Solutions Starter packs in Australia, Singapore, and New Zealand;
|
|
·
|
GlycoSlim
®
drink mix in certain international markets;
|
|
·
|
Emprizone
®
in Japan;
|
|
·
|
Essential Source
™
Omega 3 in United States, Canada, and South Africa;
|
|
·
|
PhytoBurst
™
Nutritional Chews in United States, Canada and South Africa;
|
|
·
|
GI-ProBalance
™
in South Korea; and
|
|
·
|
Various Optimal Health, Weight and Fitness products in Austria, the Netherlands, Norway and Sweden;
|
|
2009
|
2008
|
|||||||||||||||
|
Number of
independent
associates and
members
|
Pack sales
|
Number of
independent
associates
and members
|
Pack sales
|
Percentage and
dollar change
of pack sales
|
||||||||||||
|
New
|
145,000
|
$
|
39.6
|
133,000
|
$
|
28.0
|
$
|
11.6
|
41.4
|
%
|
||||||
|
Continuing
|
368,000
|
22.5
|
398,000
|
29.7
|
(7.2
|
)
|
(24.2
|
)%
|
||||||||
|
Total
|
513,000
|
$
|
62.1
|
531,000
|
$
|
57.7
|
$
|
4.4
|
7.6
|
%
|
||||||
|
2009
|
2008
|
|||||||||
|
New
|
145,000
|
28
|
%
|
133,000
|
25
|
%
|
||||
|
Continuing
|
368,000
|
72
|
%
|
398,000
|
75
|
%
|
||||
|
Total
|
513,000
|
100
|
%
|
531,000
|
100
|
%
|
||||
|
·
|
registered our most popular products with the appropriate regulatory agencies in all countries of operations;
|
|
·
|
focused on new product development;
|
|
·
|
explored new international markets;
|
|
·
|
launched an aggressive marketing and educational campaign;
|
|
·
|
strengthened compliance initiatives;
|
|
·
|
concentrated on publishing results of research studies and clinical trials related to our products;
|
|
·
|
initiated additional incentives;
|
|
·
|
explored new advertising and educational tools to broaden name recognition;
|
|
·
|
implemented changes to our global associate career and compensation plan;
|
|
·
|
introduced new products in many of our global markets;
|
|
·
|
introduced the $499 Premium/All-Star Pack into United States, Canada, and South Africa in January 2009; and
|
|
·
|
expanded into four new international markets in early fall of 2009.
|
|
Country
|
2009
|
2008
|
|||
|
Australia
|
30.0
|
%
|
30.0
|
%
|
|
|
Canada
|
33.0
|
%
|
33.0
|
%
|
|
|
Denmark
|
25.0
|
%
|
25.0
|
%
|
|
|
Japan
|
42.0
|
%
|
42.0
|
%
|
|
|
Norway
|
28.0
|
%
|
N/A
|
||
|
Republic of Korea
|
24.2
|
%
|
27.5
|
%
|
|
|
Singapore
|
17.0
|
%
|
17.0
|
%
|
|
|
South Africa
|
28.0
|
%
|
28.0
|
%
|
|
|
Sweden
|
26.3
|
%
|
N/A
|
||
|
Switzerland
|
16.2
|
%
|
16.2
|
%
|
|
|
Taiwan
|
25.0
|
%
|
25.0
|
%
|
|
|
United Kingdom
|
28.0
|
%
|
28.0
|
%
|
|
|
United States
|
37.5
|
%
|
37.5
|
%
|
|
|
Country
|
2009
|
2008
|
|||
|
Switzerland
|
$
|
0.3
|
$
|
0.0
|
|
|
Taiwan
(1)
|
0.9
|
0.9
|
|||
|
United States
|
1.1
|
0.0
|
|||
|
Total
|
$
|
2.3
|
$
|
0.9
|
|
|
|
(1)
The 2009 valuation allowance for Taiwan was adjusted to reflect the tax rate change effective for 2010. Without the rate change, the Taiwan valuation allowance would have been $1.1 million.
|
|
2008
|
2007
|
Change
|
||||||||||||||
|
Total
Dollars
|
% of
net sales
|
Total
dollars
|
% of
net sales
|
Dollar
|
Percentage
|
|||||||||||
|
Net sales
|
$
|
332,703
|
100.0
|
%
|
$
|
412,678
|
100.0
|
%
|
$
|
(79,975
|
)
|
(19.4
|
)%
|
|||
|
Cost of sales
|
48,564
|
14.6
|
%
|
59,765
|
14.5
|
%
|
(11,201
|
)
|
(18.7
|
)%
|
||||||
|
Commissions and incentives
|
149,595
|
45.0
|
%
|
189,067
|
45.8
|
%
|
(39,472
|
)
|
(20.9
|
)%
|
||||||
|
198,159
|
59.6
|
%
|
248,832
|
60.3
|
%
|
(50,673
|
)
|
(20.4
|
)%
|
|||||||
|
Gross profit
|
134,544
|
40.4
|
%
|
163,846
|
39.7
|
%
|
(29,302
|
)
|
(17.9
|
)%
|
||||||
|
Operating expenses:
|
||||||||||||||||
|
Selling and administrative expenses
|
81,077
|
24.4
|
%
|
84,298
|
20.4
|
%
|
(3,221
|
)
|
(3.8
|
)%
|
||||||
|
Depreciation and amortization
|
12,310
|
3.7
|
%
|
10,236
|
2.5
|
%
|
2,074
|
20.3
|
%
|
|||||||
|
Other operating costs
|
55,656
|
16.7
|
%
|
61,703
|
15.0
|
%
|
(6,047
|
)
|
(9.8
|
)%
|
||||||
|
Total operating expenses
|
149,043
|
44.8
|
%
|
156,237
|
37.9
|
%
|
(7,194
|
)
|
(4.6
|
)%
|
||||||
|
Income from operations
|
(14,499
|
)
|
(4.4
|
)%
|
7,609
|
1.8
|
%
|
(22,108
|
)
|
(290.6
|
)%
|
|||||
|
Interest income
|
1,604
|
0.5
|
%
|
2,700
|
0.7
|
%
|
(1,096
|
)
|
(40.6
|
)%
|
||||||
|
Other income (expense), net
|
(5,303
|
)
|
(1.6
|
)%
|
180
|
0.0
|
%
|
(5,483
|
)
|
(3046.1
|
)%
|
|||||
|
Income before income taxes
|
(18,198
|
)
|
(5.5
|
)%
|
10,489
|
2.5
|
%
|
(28,687
|
)
|
(273.5
|
)%
|
|||||
|
Provision for income taxes
|
5,570
|
1.7
|
%
|
(3,895
|
)
|
(0.9
|
)%
|
9,465
|
243.0
|
%
|
||||||
|
Net income (loss)
|
$
|
(12,628
|
)
|
(3.8
|
)%
|
$
|
6,594
|
1.6
|
%
|
$
|
(19,222
|
)
|
(291.5
|
)%
|
||
|
2008
|
2007
|
||||||||||||
|
United States
|
$
|
176.9
|
53.1
|
%
|
$
|
244.5
|
59.2
|
%
|
|||||
|
Japan
|
44.8
|
13.5
|
%
|
42.3
|
10.3
|
%
|
|||||||
|
Republic of Korea
|
35.7
|
10.7
|
%
|
44.0
|
10.7
|
%
|
|||||||
|
Australia
|
26.1
|
7.8
|
%
|
29.4
|
7.1
|
%
|
|||||||
|
Canada
|
23.6
|
7.1
|
%
|
27.4
|
6.6
|
%
|
|||||||
|
South Africa
(1)
|
5.5
|
1.7
|
%
|
—
|
—
|
||||||||
|
New Zealand
|
5.2
|
1.6
|
%
|
6.9
|
1.7
|
%
|
|||||||
|
Taiwan
|
5.2
|
1.6
|
%
|
5.4
|
1.3
|
%
|
|||||||
|
United Kingdom
|
4.7
|
1.4
|
%
|
6.7
|
1.6
|
%
|
|||||||
|
Germany
|
3.8
|
1.1
|
%
|
4.6
|
1.1
|
%
|
|||||||
|
Denmark
|
1.2
|
0.4
|
%
|
1.5
|
0.4
|
%
|
|||||||
|
Totals
|
$
|
332.7
|
100
|
%
|
$
|
412.7
|
100
|
%
|
|||||
|
Change
|
|||||||||||
|
Country
|
Currency
|
2008
|
2007
|
Local
currency
|
Percentage
|
||||||
|
Australia and Singapore
(1)
|
AUD
|
31.0
|
35.2
|
(4.2 )
|
(11.9
|
)%
|
|||||
|
Germany
|
EUR
|
2.6
|
3.3
|
(0.7)
|
(21.2
|
)%
|
|||||
|
Denmark
|
DKK
|
6.3
|
8.1
|
(1.8 )
|
(22.2
|
)%
|
|||||
|
Japan
|
JPY
|
4,584.3
|
5,314.5
|
(730.2
|
)
|
(13.7
|
)%
|
||||
|
Korea
|
KRW
|
38,733.4
|
40,563.2
|
(1,829.8
|
)
|
(4.5
|
)%
|
||||
|
New Zealand
|
NZD
|
7.3
|
9.4
|
(2.1)
|
(22.3
|
)%
|
|||||
|
South Africa
(2)
|
ZAR
|
47.4
|
—
|
47.4
|
—
|
||||||
|
Taiwan
|
TWD
|
165.4
|
178.5
|
(13.1
|
)
|
(7.3
|
)%
|
||||
|
United Kingdom
|
GBP
|
2.6
|
3.4
|
(0.8
|
)
|
(23.5
|
)%
|
||||
|
Change
|
||||||||||||
|
2008
|
2007
|
Dollar
|
Percentage
|
|||||||||
|
Product sales
|
$
|
260.5
|
$
|
316.9
|
$
|
(56.4
|
)
|
(17.8
|
)%
|
|||
|
Pack sales
|
57.7
|
79.0
|
$
|
(21.3
|
)
|
(27.0
|
)%
|
|||||
|
Other, including freight*
|
14.5
|
16.8
|
$
|
(2.3
|
)
|
(13.7
|
)%
|
|||||
|
Total net sales
|
$
|
332.7
|
$
|
412.7
|
$
|
(80.0
|
)
|
(19.4
|
)%
|
|||
|
|
*
In April 2007, we began operating our new ERP System, which allowed us to separately quantify deferred revenue associated with sales of packs and products that were shipped but not yet received by customers. As a result, in April 2007, we began recording deferred revenue related to packs with pack sales and deferred revenue associated with products with product sales. For the three months ended March 31, 2007, other sales included $1.9 million related to the change in deferred revenue for packs and products shipped but not yet received by customers, rather than in the applicable pack or product sales category.
|
|
·
|
Mannatech Optimal Skin Care System products in certain international markets;
|
|
·
|
A new sales kit in the United States;
|
|
·
|
PhytoMatrix
®
caplets in Japan, Taiwan, United Kingdom, Denmark, Germany, and South Korea;
|
|
·
|
Bounce Back
™
capsules in United States, Canada, Australia, and New Zealand;
|
|
·
|
OsoLean
™
powder in United States, Canada, Australia, New Zealand, Japan, and Korea;
|
|
·
|
HeartSmart
™
tablets in Taiwan;
|
|
·
|
Various Optimal Health products in Singapore; and
|
|
·
|
Various Optimal Health and Optimal Weight and Fitness products in South Africa.
|
|
2008
|
2007
|
|||||||||||||||
|
Number of
independent
associates and
members
|
Pack sales
|
Number of
independent
associates
and members
|
Pack sales
|
Percentage and
dollar change
of pack sales
|
||||||||||||
|
New
|
133,000
|
$
|
28.0
|
191,000
|
$
|
39.6
|
$
|
(11.6
|
)
|
(29.3
|
)%
|
|||||
|
Continuing
|
398,000
|
29.7
|
384,000
|
39.4
|
(9.7
|
)
|
(24.6
|
)%
|
||||||||
|
Total
|
531,000
|
$
|
57.7
|
575,000
|
$
|
79.0
|
$
|
(21.3
|
)
|
(27.0
|
)%
|
|||||
|
2008
|
2007
|
|||||||||
|
New
|
133,000
|
25
|
%
|
191,000
|
33.2
|
%
|
||||
|
Continuing
|
398,000
|
75
|
%
|
384,000
|
66.8
|
%
|
||||
|
Total
|
531,000
|
100
|
%
|
575,000
|
100
|
%
|
||||
|
·
|
registered our most popular products with the appropriate regulatory agencies in all countries of operations;
|
|
·
|
focused on new product development;
|
|
·
|
launched an aggressive marketing and educational campaign;
|
|
·
|
explored and entered new international markets;
|
|
·
|
strengthened compliance initiatives;
|
|
·
|
initiated additional incentives;
|
|
·
|
explored new advertising and educational tools to broaden name recognition;
|
|
·
|
implemented changes to our global associate career and compensation plan; and
|
|
·
|
introduced new products.
|
|
Country
|
2008
|
2007
|
|||
|
Australia
|
30.0
|
%
|
30.0
|
%
|
|
|
Canada
|
33.0
|
%
|
30.0
|
%
|
|
|
Japan
|
42.0
|
%
|
42.0
|
%
|
|
|
Republic of Korea
|
27.5
|
%
|
27.5
|
%
|
|
|
South Africa
|
28.0
|
%
|
N/A
|
||
|
Switzerland
|
16.2
|
%
|
N/A
|
||
|
Taiwan
|
25.0
|
%
|
25.0
|
%
|
|
|
United Kingdom
|
28.0
|
%
|
30.0
|
%
|
|
|
United States
|
37.5
|
%
|
37.5
|
%
|
|
|
·
|
the timing of the introduction of new products and incentives;
|
|
·
|
our ability to attract and retain associates and members;
|
|
·
|
the timing of our incentives and contests;
|
|
·
|
the general overall economic outlook;
|
|
·
|
government regulations;
|
|
·
|
the outcome of certain lawsuits;
|
|
·
|
the perception and acceptance of network marketing; and
|
|
·
|
the consumer perception of our products and overall operations.
|
|
2009
|
2008
|
2007
|
||||||||||
|
Provided by (used in):
|
||||||||||||
|
Operating activities
|
$
|
(10.3
|
)
|
$
|
(19.9
|
)
|
$
|
17.8
|
||||
|
Investing activities
|
$
|
(1.3
|
)
|
$
|
7.2
|
$
|
(7.8
|
)
|
||||
|
Financing activities
|
$
|
(1.5
|
)
|
$
|
(5.9
|
)
|
$
|
(9.4
|
)
|
|||
|
2010
|
2011
|
2012
|
2013
|
2014
|
Thereafter
|
Total
|
|||||||||||||||
|
Capital lease obligations
|
$
|
913
|
$
|
774
|
$
|
331
|
$
|
2
|
$
|
—
|
$
|
—
|
$
|
2,020
|
|||||||
|
Purchase obligations
(1)
|
11,425
|
4,956
|
2,535
|
1,050
|
1,050
|
1,050
|
22,066
|
||||||||||||||
|
Operating leases
|
2,939
|
1,526
|
1,309
|
1,152
|
786
|
1,741
|
9,453
|
||||||||||||||
|
Post-employment royalty
|
492
|
492
|
492
|
492
|
492
|
—
|
2,460
|
||||||||||||||
|
Employment agreements
|
1,583
|
70
|
—
|
—
|
—
|
—
|
1,653
|
||||||||||||||
|
Texas Attorney General Settlement
|
1,000
|
—
|
—
|
—
|
—
|
—
|
1,000
|
||||||||||||||
|
Total commitments and obligations
|
$
|
18,352
|
$
|
7,818
|
$
|
4,667
|
$
|
2,696
|
$
|
2,328
|
$
|
2,791
|
$
|
38,652
|
|||||||
|
|
(1)
Purchase obligations for the years 2010, 2011, and 2012 include $4.7 million, $2.1 million, and $1.5 million, respectively, of purchase commitments under a contract terminated by the Company for an asserted breach. Pursuant to the terms of the Contract, we are engaged in the arbitration process with the supplier.
|
|
Estimated useful life
|
Net carrying value at
December 31, 2009
|
|||||
|
Office furniture and equipment
|
5 to 7
|
years
|
$
|
2.5 million
|
||
|
Computer hardware and software
|
3 to 5
|
years
|
21.1 million
|
|||
|
Automobiles
|
3 to 5
|
years
|
0.1 million
|
|||
|
Leasehold improvements
|
2 to 10
|
years
(1)
|
3.5 million
|
|||
|
Construction in progress
|
2 to 10
|
years
(2)
|
0.3 million
|
|||
|
Total net carrying value at December 31, 2009
|
$
|
27.5 million
|
||||
|
Sales reserve as of December 31, 2008
|
$
|
719
|
||
|
Current provision related to sales made in 2009
|
3,287
|
|||
|
Current provision related to sales made prior to 2009
|
158
|
|||
|
Actual returns or credits in 2009 related to 2009
|
(2,704
|
)
|
||
|
Actual returns or credits in 2009 related to prior periods
|
(865
|
)
|
||
|
Sales reserve as of December 31, 2009
|
$
|
595
|
|
January
2009
grant
|
February
2009
grant
|
March
2009
grant
|
June
2009
grant
|
September
2009
grant
|
December
2009
grant
|
||||||||||||||
|
Estimated fair value per share of options granted:
|
$
|
1.24
|
$
|
1.64
|
$
|
1.26
|
$
|
1.44
|
$
|
2.01
|
$
|
1.63
|
|||||||
|
Assumptions:
|
|||||||||||||||||||
|
Annualized dividend yield
|
2.85
|
%
|
2.27
|
%
|
2.87
|
%
|
2.67
|
%
|
1.05
|
%
|
1.29
|
%
|
|||||||
|
Risk-free rate of return
|
1.53
|
%
|
1.69
|
%
|
1.72
|
%
|
2.69
|
%
|
2.04
|
%
|
2.24
|
%
|
|||||||
|
Common stock price volatility
|
65.9
|
%
|
66.6
|
%
|
67.6
|
%
|
69.70
|
%
|
70.1
|
%
|
70.8
|
%
|
|||||||
|
Expected average life of stock options (in years)
|
4.5
|
4.5
|
4.5
|
4.5
|
4.5
|
4.5
|
|||||||||||||
|
Country (foreign currency name)
|
Low
|
High
|
Average
|
Spot
|
|||||
|
Australia (Dollar)
|
0.63180
|
0.93490
|
0.79240
|
0.89310
|
|||||
|
Austria, Germany, the Netherlands (Euro)
|
1.25440
|
1.50910
|
1.39463
|
1.43330
|
|||||
|
Canada (Dollar)
|
0.77510
|
0.97420
|
0.88029
|
0.95320
|
|||||
|
Denmark (Krone)
|
0.16860
|
0.20290
|
0.18729
|
0.19270
|
|||||
|
Japan (Yen)
|
0.00992
|
0.01158
|
0.01070
|
0.01084
|
|||||
|
New Zealand (Dollar)
|
0.49490
|
0.75640
|
0.63564
|
0.71770
|
|||||
|
Norway (Krone)
|
0.13900
|
0.18080
|
0.15999
|
0.17220
|
|||||
|
Republic of Korea (Won)
|
0.00064
|
0.00088
|
0.00079
|
0.00086
|
|||||
|
Singapore (Dollar)
|
0.64370
|
0.72460
|
0.68856
|
0.71170
|
|||||
|
South Africa (Rand)
|
0.09531
|
0.13800
|
0.12057
|
0.13510
|
|||||
|
Sweden (Krona)
|
0.10860
|
0.14740
|
0.13167
|
0.13930
|
|||||
|
Switzerland (Franc)
|
0.84170
|
1.00100
|
0.92362
|
0.96360
|
|||||
|
Taiwan (Dollar)
|
0.02846
|
0.03133
|
0.03031
|
0.03102
|
|||||
|
United Kingdom (British Pound)
|
1.37340
|
1.69690
|
1.56593
|
1.59280
|
|
Mar. 31,
2009
|
June 30,
2009
|
Sept. 30,
2009
|
Dec. 31,
2009
(3)
|
Mar. 31,
2008
|
June 30,
2008
(1)
|
Sept. 30,
2008
|
Dec. 31,
2008
(2)
|
||||||||||||||||||
|
(in millions, except per share information)
|
|||||||||||||||||||||||||
|
Net sales
|
$
|
70.7
|
$
|
77.6
|
$
|
71.3
|
$
|
70.1
|
$
|
91.5
|
$
|
86.8
|
$
|
78.0
|
$
|
76.4
|
|||||||||
|
Gross profit
|
$
|
25.2
|
$
|
18.9
|
$
|
24.1
|
$
|
28.2
|
$
|
36.1
|
$
|
32.4
|
$
|
34.5
|
$
|
31.5
|
|||||||||
|
Income (loss) before income taxes
|
$
|
(7.0
|
)
|
$
|
(9.6
|
)
|
$
|
(7.7
|
)
|
$
|
0.2
|
$
|
(2.8
|
)
|
$
|
(16.9
|
)
|
$
|
(0.7
|
)
|
$
|
2.2
|
|||
|
Provision (benefit) for income taxes
|
$
|
(2.2
|
)
|
$
|
(4.1
|
)
|
$
|
1.5
|
$
|
(1.9
|
)
|
$
|
(0.5
|
)
|
$
|
(6.4
|
)
|
$
|
(0.3
|
)
|
$
|
1.6
|
|||
|
Net income (loss)
|
$
|
(4.8
|
)
|
$
|
(5.5
|
)
|
$
|
(9.2
|
)
|
$
|
2.1
|
$
|
(2.3
|
)
|
$
|
(10.5
|
)
|
$
|
(0.4
|
)
|
$
|
0.6
|
|||
|
Earnings (loss) per share:
|
|||||||||||||||||||||||||
|
Basic
|
$
|
(0.18
|
)
|
$
|
(0.21
|
)
|
$
|
(0.35
|
)
|
$
|
0.08
|
$
|
(0.09
|
)
|
$
|
(0.40
|
)
|
$
|
(0.02
|
)
|
$
|
0.02
|
|||
|
Diluted
|
$
|
(0.18
|
)
|
$
|
(0.21
|
)
|
$
|
(0.35
|
)
|
$
|
0.08
|
$
|
(0.09
|
)
|
$
|
(0.40
|
)
|
$
|
(0.02
|
)
|
$
|
0.02
|
|||
|
Mannatech, Incorporated
|
|||
|
Dated: March 11, 2010
|
By:
|
/s/ Stephen D. Fenstermacher
|
|
|
Stephen D. Fenstermacher
Co-Chief Executive Officer and Chief Financial Officer
|
|||
|
Dated: March 11, 2010
|
By:
|
/s/ Robert A. Sinnott
|
|
|
Robert A. Sinnott
Co-Chief Executive Officer and Chief Science Officer
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Stephen D. Fenstermacher
|
Co-Chief Executive Officer and Chief Financial Officer
(principal financial and accounting officer)
|
March 11, 2010
|
||
|
Stephen D. Fenstermacher
|
||||
|
/s/ Robert A. Sinnott
|
Co-Chief Executive Officer and Chief Science Officer
(principal executive officer)
|
March 11, 2010
|
||
|
Robert A. Sinnott
|
||||
|
/s/ J. Stanley Fredrick
|
Chairman of the Board
|
March 11, 2010
|
||
|
J. Stanley Fredrick
|
||||
|
/s/ Patricia A. Wier
|
Director
|
March 11, 2010
|
||
|
Patricia A. Wier
|
||||
|
/s/ Alan D. Kennedy
|
Director
|
March 11, 2010
|
||
|
Alan D. Kennedy
|
||||
|
/s/ Gerald E. Gilbert
|
Director
|
March 11, 2010
|
||
|
Gerald E. Gilbert
|
||||
|
/s/ Marlin Ray Robbins
|
Director
|
March 11, 2010
|
||
|
Marlin Ray Robbins
|
||||
|
/s/ Larry A. Jobe
|
Director
|
March 11, 2010
|
||
|
Larry A. Jobe
|
||||
|
/s/ Robert A. Toth
|
Director
|
March 11, 2010
|
||
|
Robert A. Toth
|
||||
|
Page
|
|
|
December 31,
|
|||||||
|
2009
|
2008
|
||||||
|
ASSETS
|
|||||||
|
Cash and cash equivalents
|
$
|
17,367
|
$
|
30,945
|
|||
|
Restricted cash
|
1,288
|
1,864
|
|||||
|
Accounts receivable, net of allowance of $16.5 and $23 in 2009 and 2008, respectively
|
664
|
291
|
|||||
|
Income tax receivable
|
8,075
|
3,531
|
|||||
|
Inventories, net
|
31,290
|
31,313
|
|||||
|
Prepaid expenses and other current assets
|
3,139
|
3,946
|
|||||
|
Deferred tax assets
|
2,662
|
5,632
|
|||||
|
Total current assets
|
64,485
|
77,522
|
|||||
|
Property and equipment, net
|
27,144
|
36,202
|
|||||
|
Construction in progress
|
317
|
840
|
|||||
|
Long-term restricted cash
|
7,201
|
7,579
|
|||||
|
Other assets
|
2,503
|
1,456
|
|||||
|
Long-term deferred tax assets
|
652
|
459
|
|||||
|
Total assets
|
$
|
102,302
|
$
|
124,058
|
|||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|||||||
|
Current portion of capital leases
|
$
|
847
|
$
|
131
|
|||
|
Accounts payable
|
11,319
|
5,067
|
|||||
|
Accrued expenses
|
14,231
|
24,324
|
|||||
|
Commissions and incentives payable
|
10,624
|
11,453
|
|||||
|
Taxes payable
|
2,577
|
873
|
|||||
|
Current deferred tax liability
|
274
|
192
|
|||||
|
Deferred revenue
|
2,807
|
3,476
|
|||||
|
Total current liabilities
|
42,679
|
45,516
|
|||||
|
Capital leases, excluding current portion
|
1,068
|
155
|
|||||
|
Long-term deferred tax liabilities
|
3,923
|
6,075
|
|||||
|
Other long-term liabilities
|
3,348
|
3,583
|
|||||
|
Total liabilities
|
51,018
|
55,329
|
|||||
|
Commitments and contingencies
|
|||||||
|
Shareholders’ equity:
|
|||||||
|
Preferred stock, $0.01 par value, 1,000,000 shares authorized, no shares issued or outstanding
|
—
|
—
|
|||||
|
Common stock, $0.0001 par value, 99,000,000 shares authorized, 27,687,882 shares issued and 26,480,788
shares outstanding in 2009 and 27,667,882 shares issued and 26,460,788 shares outstanding in 2008
|
3
|
3
|
|||||
|
Additional paid-in capital
|
41,442
|
40,753
|
|||||
|
Retained earnings
|
25,743
|
44,170
|
|||||
|
Accumulated other comprehensive loss
|
(1,113
|
)
|
(1,406
|
)
|
|||
|
Less treasury stock, at cost, 1,207,094 shares in 2009 and 2008
|
(14,791
|
)
|
(14,791
|
)
|
|||
|
Total shareholders’ equity
|
51,284
|
68,729
|
|||||
|
Total liabilities and shareholders’ equity
|
$
|
102,302
|
$
|
124,058
|
|||
|
For the years ended December 31,
|
|||||||||||
|
2009
|
2008
|
2007
|
|||||||||
|
Net sales
|
$
|
289,705
|
$
|
332,703
|
$
|
412,678
|
|||||
|
Cost of sales
|
46,813
|
48,564
|
59,765
|
||||||||
|
Commissions and incentives
|
146,415
|
149,595
|
189,067
|
||||||||
|
193,228
|
198,159
|
248,832
|
|||||||||
|
Gross profit
|
96,477
|
134,544
|
163,846
|
||||||||
|
Operating expenses:
|
|||||||||||
|
Selling and administrative expenses
|
69,997
|
81,077
|
84,298
|
||||||||
|
Depreciation and amortization
|
12,333
|
12,310
|
10,236
|
||||||||
|
Other operating costs
|
39,741
|
55,656
|
61,703
|
||||||||
|
Total operating expenses
|
122,071
|
149,043
|
156,237
|
||||||||
|
Income (loss) from operations
|
(25,594
|
)
|
(14,499
|
)
|
7,609
|
||||||
|
Interest income
|
473
|
1,604
|
2,700
|
||||||||
|
Other income (expense), net
|
1,046
|
(5,303
|
)
|
180
|
|||||||
|
Income (loss) before income taxes
|
(24,075
|
)
|
(18,198
|
)
|
10,489
|
||||||
|
(Provision) benefit for income taxes
|
6,707
|
5,570
|
(3,895
|
)
|
|||||||
|
Net income (loss)
|
$
|
(17,368
|
)
|
$
|
(12,628
|
)
|
$
|
6,594
|
|||
|
Earnings (loss) per common share:
|
|||||||||||
|
Basic
|
$
|
(0.66
|
)
|
$
|
(0.48
|
)
|
$
|
0.25
|
|||
|
Diluted
|
$
|
(0.66
|
)
|
$
|
(0.48
|
)
|
$
|
0.25
|
|||
|
Weighted-average common shares outstanding:
|
|||||||||||
|
Basic
|
26,467
|
26,461
|
26,443
|
||||||||
|
Diluted
|
26,467
|
26,461
|
26,893
|
||||||||
|
Common Stock
Outstanding
|
Additional
paid in
capital
|
Retained
earnings
|
Accumulated
other
comprehensive
income (loss)
|
Treasury stock
|
Total
shareholders’
equity
|
||||||||||||||||||
|
Shares
|
Par
value
|
Shares
|
Amounts
|
||||||||||||||||||||
|
Balance at January 1, 2007
|
26,410
|
$
|
3
|
$
|
38,941
|
$
|
66,393
|
$
|
(1,749
|
)
|
1,207
|
$
|
(14,791
|
)
|
$
|
88,797
|
|||||||
|
Proceeds from stock options exercised
|
51
|
—
|
157
|
—
|
—
|
—
|
—
|
157
|
|||||||||||||||
|
Tax benefit from exercise of stock
options
|
—
|
—
|
100
|
—
|
—
|
—
|
—
|
100
|
|||||||||||||||
|
Charge related to stock-based
compensation
|
—
|
—
|
948
|
—
|
—
|
—
|
—
|
948
|
|||||||||||||||
|
Cumulative impact of a change in
accounting for income tax uncertainties
pursuant to FIN 48
|
—
|
—
|
—
|
(845
|
)
|
—
|
—
|
—
|
(845
|
)
|
|||||||||||||
|
Declared dividends of $0.36 per share
|
—
|
—
|
—
|
(9,522
|
)
|
—
|
—
|
—
|
(9,522
|
)
|
|||||||||||||
|
Components of comprehensive income:
|
|||||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
—
|
613
|
—
|
—
|
613
|
|||||||||||||||
|
Pension obligations, net of tax of $8
|
—
|
—
|
—
|
—
|
12
|
—
|
—
|
12
|
|||||||||||||||
|
Unrealized gain from investments
classified as available-for-sale, net of
tax
|
—
|
—
|
—
|
—
|
1
|
—
|
—
|
1
|
|||||||||||||||
|
Net income
|
—
|
—
|
—
|
6,594
|
—
|
—
|
—
|
6,594
|
|||||||||||||||
|
Total comprehensive income
|
7,220
|
||||||||||||||||||||||
|
Balance at December 31, 2007
|
26,461
|
3
|
40,146
|
62,620
|
(1,123
|
)
|
1,207
|
(14,791
|
)
|
86,855
|
|||||||||||||
|
Tax shortfall from expiration of stock
options
|
—
|
—
|
(120
|
)
|
—
|
—
|
—
|
—
|
(120
|
)
|
|||||||||||||
|
Charge related to stock-based
compensation
|
—
|
—
|
727
|
—
|
—
|
—
|
—
|
727
|
|||||||||||||||
|
Declared dividends of $0.22 per share
|
—
|
—
|
—
|
(5,822
|
)
|
—
|
—
|
—
|
(5,822
|
)
|
|||||||||||||
|
Components of comprehensive loss:
|
|||||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
—
|
(318
|
)
|
—
|
—
|
(318
|
)
|
|||||||||||||
|
Pension obligations, net of tax of $26
|
—
|
—
|
—
|
—
|
35
|
—
|
—
|
35
|
|||||||||||||||
|
Net loss
|
—
|
—
|
—
|
(12,628
|
)
|
—
|
—
|
—
|
(12,628
|
)
|
|||||||||||||
|
Total comprehensive loss
|
(12,911
|
)
|
|||||||||||||||||||||
|
Balance at December 31, 2008
|
26,461
|
3
|
40,753
|
44,170
|
(1,406
|
)
|
1,207
|
(14,791
|
)
|
68,729
|
|||||||||||||
|
Tax shortfall from expiration of stock
options
|
—
|
—
|
(13
|
)
|
—
|
—
|
—
|
—
|
(13
|
)
|
|||||||||||||
|
Proceeds from stock options exercised
|
20
|
—
|
66
|
—
|
—
|
—
|
—
|
66
|
|||||||||||||||
|
Charge related to stock-based
compensation
|
—
|
—
|
636
|
—
|
—
|
—
|
—
|
636
|
|||||||||||||||
|
Declared dividends of $0.04 per share
|
—
|
—
|
—
|
(1,059
|
)
|
—
|
—
|
—
|
(1,059
|
)
|
|||||||||||||
|
Components of comprehensive loss:
|
|||||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
—
|
276
|
—
|
—
|
276
|
|||||||||||||||
|
Pension obligations, net of tax $12
|
17
|
17
|
|||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
(17,368
|
)
|
—
|
—
|
—
|
(17,368
|
)
|
|||||||||||||
|
Total comprehensive loss
|
(17,075
|
)
|
|||||||||||||||||||||
|
Balance at December 31, 2009
|
26,481
|
$
|
3
|
$
|
41,442
|
$
|
25,743
|
$
|
(1,113
|
)
|
1,207
|
$
|
(14,791
|
)
|
$
|
51,284
|
|||||||
|
For the years ended December 31,
|
||||||||||||||
|
2009
|
2008
|
2007
|
||||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||||
|
Net income (loss)
|
$
|
(17,368
|
)
|
$
|
(12,628
|
)
|
$
|
6,594
|
||||||
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities
:
|
||||||||||||||
|
Depreciation and amortization
|
12,333
|
12,310
|
10,236
|
|||||||||||
|
Provision for inventory losses
|
1,544
|
1,321
|
568
|
|||||||||||
|
Provision for doubtful accounts
|
33
|
23
|
877
|
|||||||||||
|
Loss on disposal of assets
|
102
|
468
|
39
|
|||||||||||
|
Accounting charge related to stock-based compensation expense
|
636
|
727
|
948
|
|||||||||||
|
Deferred income taxes
|
761
|
(3,062
|
)
|
(2,440
|
)
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||||
|
Accounts receivable
|
(405
|
)
|
316
|
(495
|
)
|
|||||||||
|
Income tax receivable
|
(4,525
|
)
|
(1,395
|
)
|
28
|
|||||||||
|
Inventories
|
(1,198
|
)
|
(9,512
|
)
|
(337
|
)
|
||||||||
|
Prepaid expenses and other current assets
|
2,821
|
1,927
|
(1,730
|
)
|
||||||||||
|
Other assets
|
(1,019
|
)
|
(9
|
)
|
(76
|
)
|
||||||||
|
Accounts payable
|
6,245
|
1,407
|
276
|
|||||||||||
|
Accrued expenses
|
(10,345
|
)
|
(5,947
|
)
|
3,028
|
|||||||||
|
Taxes payable
|
1,643
|
(4,901
|
)
|
2,618
|
||||||||||
|
Commissions and incentives payable
|
(898
|
)
|
362
|
(4,430
|
)
|
|||||||||
|
Deferred revenue
|
(670
|
)
|
(1,295
|
)
|
2,072
|
|||||||||
|
Net cash provided by (used in) operating activities
|
(10,310
|
)
|
(19,888
|
)
|
17,776
|
|||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||||
|
Acquisition of property and equipment
|
(2,797
|
)
|
(5,614
|
)
|
(13,409
|
)
|
||||||||
|
Proceeds from sale of assets
|
37
|
3
|
—
|
|||||||||||
|
Change in restricted cash
|
1,473
|
(139
|
)
|
(6,854
|
)
|
|||||||||
|
Sale of investments
|
—
|
20,350
|
12,424
|
|||||||||||
|
Purchase of investments
|
—
|
(7,400
|
)
|
—
|
||||||||||
|
Net cash provided by (used in) investing activities
|
(1,287
|
)
|
7,200
|
(7,839
|
)
|
|||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||||
|
Tax benefit from exercise of stock options
|
—
|
—
|
100
|
|||||||||||
|
Proceeds from stock options exercised
|
66
|
—
|
157
|
|||||||||||
|
Payment of cash dividends
|
(1,059
|
)
|
(5,822
|
)
|
(9,522
|
)
|
||||||||
|
Repayment of capital lease obligation
|
(473
|
)
|
(115
|
)
|
(107
|
)
|
||||||||
|
Net cash used in financing activities
|
(1,466
|
)
|
(5,937
|
)
|
(9,372
|
)
|
||||||||
|
Effect of currency exchange rate changes on cash and cash equivalents
|
(515
|
)
|
2,467
|
837
|
||||||||||
|
Net increase (decrease) in cash and cash equivalents
|
(13,578
|
)
|
(16,158
|
)
|
1,402
|
|||||||||
|
Cash and cash equivalents at the beginning of year
|
30,945
|
47,103
|
45,701
|
|||||||||||
|
Cash and cash equivalents at the end of year
|
$
|
17,367
|
$
|
30,945
|
$
|
47,103
|
||||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||||||||
|
Income taxes received (paid), net
|
$
|
2,441
|
$
|
(1,266
|
)
|
$
|
(4,146
|
)
|
||||||
|
Interest paid on capital leases
|
$
|
50
|
$
|
17
|
$
|
21
|
||||||||
|
Summary of non-cash investing and financing activities:
|
||||||||||||||
|
Assets acquired through capital leases
|
$
|
2,099
|
$
|
30
|
$
|
37
|
||||||||
|
Unrealized gains from investments
|
$
|
—
|
$
|
—
|
$
|
1
|
||||||||
|
Estimated useful life
|
||
|
Office furniture and equipment
|
5 to 7
|
years
|
|
Computer hardware and software
|
3 to 5
|
years
|
|
Automobiles
|
3 to 5
|
years
|
|
Leasehold improvements
(1)
|
2 to 10
|
years
|
|
Construction in progress
(2)
|
||
|
(1)
|
We amortize leasehold improvements over the shorter of the useful estimated life of the leased asset or the lease term.
|
|
(2)
|
Construction in process includes leasehold improvements and internally developed software costs. Once placed in service, leasehold improvements will be amortized over the shorter of an asset’s useful life or the remaining lease term. Once the internally-developed software is placed in service, it will be amortized over three to five years.
|
|
2009
|
2008
|
|||||||
|
Sales reserve as of January 1
|
$
|
719
|
$
|
572
|
||||
|
Provision related to sales made in current year
|
3,287
|
4,339
|
||||||
|
Provision related to sales made in prior periods
|
158
|
359
|
||||||
|
Actual returns or credits related to current year
|
(2,704
|
)
|
(3,625
|
)
|
||||
|
Actual returns or credits related to prior periods
|
(865
|
)
|
(926
|
)
|
||||
|
Sales reserve as of December 31
|
$
|
595
|
$
|
719
|
||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||
|
Assets
|
||||||||||||
|
Money Market Funds – Fidelity, US
|
$ $
|
3,266
|
$ $
|
—
|
$ $
|
—
|
$ $
|
3,266
|
||||
|
Interest bearing deposits – various banks, Korea
|
6,309
|
—
|
—
|
6,309
|
||||||||
|
Total assets
|
$
|
9,575
|
$
|
—
|
$
|
—
|
$
|
9,575
|
||||
|
Amounts included in:
|
||||||||||||
|
Cash and cash equivalents
|
$ $
|
3,313
|
$ $
|
—
|
$ $
|
—
|
$ $
|
3,313
|
||||
|
Long-term restricted cash
|
6,262
|
—
|
—
|
6,262
|
||||||||
|
Total
|
$
|
9,575
|
$
|
—
|
$
|
—
|
$
|
9,575
|
||||
|
2009
|
2008
|
|||||||
|
Raw materials
|
$
|
10,819
|
$
|
13,715
|
||||
|
Finished goods
|
21,844
|
18,275
|
||||||
|
Inventory reserves for obsolescence
|
(1,373
|
)
|
(677
|
)
|
||||
|
$
|
31,290
|
$
|
31,313
|
|||||
|
2009
|
2008
|
|||||
|
Office furniture and equipment
|
$
|
10,944
|
$
|
10,951
|
||
|
Computer hardware
|
14,324
|
13,947
|
||||
|
Computer software
|
46,901
|
44,927
|
||||
|
Automobiles
|
115
|
128
|
||||
|
Leasehold improvements
|
11,726
|
11,886
|
||||
|
84,010
|
81,839
|
|||||
|
Less accumulated depreciation and amortization
|
(56,866
|
)
|
(45,637
|
)
|
||
|
Property and equipment, net
|
27,144
|
36,202
|
||||
|
Construction in process
|
317
|
840
|
||||
|
$
|
27,461
|
$
|
37,042
|
|||
|
2010
|
$
|
913
|
|
|
2011
|
774
|
||
|
2012
|
331
|
||
|
2013
|
2
|
||
|
Total future minimum lease payments
|
2,020
|
||
|
Less: Amounts representing interest (effective interest rate 4.2%)
|
(105
|
)
|
|
|
Present value of minimum lease payments
|
1,915
|
||
|
Current portion of capital lease obligations
|
(847
|
)
|
|
|
Long-term portion of capital lease obligations
|
$
|
1,068
|
|
2009
|
2008
|
|||||
|
Accrued inventory purchases
|
$
|
2,562
|
$
|
3,069
|
||
|
Accrued compensation
|
2,579
|
3,841
|
||||
|
Accrued royalties
|
362
|
387
|
||||
|
Accrued sales and other taxes
|
425
|
1,448
|
||||
|
Other accrued operating expenses
|
4,255
|
4,273
|
||||
|
Customer deposits and sales returns
|
607
|
729
|
||||
|
Accrued travel expenses related to corporate events
|
892
|
1,181
|
||||
|
Fixed asset purchases
|
185
|
409
|
||||
|
Accrued legal and accounting fees
|
2,364
|
8,987
|
||||
|
$
|
14,231
|
$
|
24,324
|
|||
|
2009
|
2008
|
2007
|
|||||||||
|
United States
|
$
|
(23,945
|
)
|
$
|
(20,297
|
)
|
$
|
1,747
|
|||
|
Foreign
|
(130
|
)
|
2,099
|
8,742
|
|||||||
|
$
|
(24,075
|
)
|
$
|
(18,198
|
)
|
$
|
10,489
|
||||
|
Current provision (benefit):
|
2009
|
2008
|
2007
|
|||||||||||||
|
Federal
|
$
|
(8,521
|
)
|
$
|
(3,876
|
)
|
$
|
3,022
|
||||||||
|
State
|
(1
|
) |
(95
|
)
|
362
|
|||||||||||
|
Foreign
|
886
|
1,583
|
2,995
|
|||||||||||||
|
(7,636
|
)
|
(2,388
|
)
|
6,379
|
||||||||||||
|
Deferred provision (benefit):
|
||||||||||||||||
|
Federal
|
269
|
(2,411
|
)
|
(2,494
|
)
|
|||||||||||
|
State
|
140
|
(299
|
)
|
(182
|
)
|
|||||||||||
|
Foreign
|
520
|
(472
|
)
|
192
|
||||||||||||
|
929
|
(3,182
|
)
|
(2,484
|
)
|
||||||||||||
|
$
|
(6,707
|
)
|
$
|
(5,570
|
)
|
$
|
3,895
|
|||||||||
A reconciliation of the Company’s effective income tax rate and the United States federal statutory income tax rate is summarized as follows, for the years ended December 31:
|
2009
|
2008
|
2007
|
||||||||
|
Federal statutory income taxes
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||||
|
State income taxes, net of federal benefit
|
1.6
|
0.4
|
1.6
|
|||||||
|
Difference in foreign and United States tax on foreign operations
|
(3.0
|
)
|
(0.6
|
)
|
(0.6
|
)
|
||||
|
Effect of changes in valuation allowance for net operating loss carryforwards
|
(7.2
|
)
|
(1.1
|
)
|
(3.1
|
)
|
||||
|
Effect of change in uncertain tax positions (net)
|
0.9
|
5.5
|
1.1
|
|||||||
|
Other
|
0.6
|
(8.6
|
)
|
3.1
|
||||||
|
27.9
|
%
|
30.6
|
%
|
37.1
|
%
|
|||||
|
2009
|
2008
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Current:
|
||||||||
|
Deferred revenue
|
$
|
1
|
$
|
63
|
||||
|
Inventory capitalization
|
428
|
554
|
||||||
|
Inventory reserves
|
314
|
128
|
||||||
|
Accrued expenses
|
1,953
|
4,314
|
||||||
|
Net operating loss
|
—
|
152
|
||||||
|
Other
|
1,123
|
1,407
|
||||||
|
Total current deferred tax assets
|
3,819
|
6,618
|
||||||
|
Noncurrent:
|
||||||||
|
Depreciation and amortization
|
—
|
—
|
||||||
|
Net operating loss
(1)
|
2,049
|
932
|
||||||
|
Deferred royalty
|
746
|
904
|
||||||
|
Non-cash accounting charges related to stock options and warrants
|
519
|
386
|
||||||
|
Accrued expenses
|
191
|
28
|
||||||
|
Other
|
744
|
156
|
||||||
|
Total noncurrent deferred tax assets
|
4,249
|
2,406
|
||||||
|
Total deferred tax assets
|
8,068
|
9,024
|
||||||
|
Valuation allowance
|
(2,290
|
)
|
(932
|
)
|
||||
|
Total deferred tax assets, net of valuation allowance
|
$
|
5,778
|
$
|
8,092
|
||||
|
Deferred tax liabilities:
|
||||||||
|
Current:
|
||||||||
|
Prepaid expenses
|
$
|
670
|
$
|
789
|
||||
|
Other
|
1,124
|
406
|
||||||
|
Total current deferred tax liabilities
|
1,794
|
1,195
|
||||||
|
Noncurrent:
|
||||||||
|
Internally-developed software
|
2,873
|
7,038
|
||||||
|
Depreciation and amortization
|
1,994
|
35
|
||||||
|
Other
|
—
|
—
|
||||||
|
Total noncurrent deferred tax liabilities
|
4,867
|
7,073
|
||||||
|
Total deferred tax liabilities
|
$
|
6,661
|
$
|
8,268
|
||||
|
|
(1)
The net operating loss for the Company will expire as follows: Taiwan $0.9 million between the years 2016 and 2020; United States (states) $0.6 million between years 2015 and 2030; Switzerland $0.5 million in 2016; and the remainder split between Norway and Sweden does not expire.
|
|
Country
|
2009
|
2008
|
|||
|
Switzerland
|
$
|
0.3
|
$
|
0.0
|
|
|
Taiwan
(1)
|
0.9
|
0.9
|
|||
|
United States
|
1.1
|
0.0
|
|||
|
Total
|
$
|
2.3
|
$
|
0.9
|
|
|
|
(1)
The 2009 valuation allowance for Taiwan was adjusted to reflect the tax rate change effective for 2010. Without the rate change, the Taiwan valuation allowance would have been $1.1 million.
|
|
2009
|
2008
|
|||||||
|
Current deferred tax assets
|
$
|
2,662
|
$
|
5,632
|
||||
|
Noncurrent deferred tax assets
|
652
|
459
|
||||||
|
Current deferred tax liabilities
|
(274
|
)
|
(192
|
)
|
||||
|
Noncurrent deferred tax liabilities
|
(3,923
|
)
|
(6,075
|
)
|
||||
|
Net deferred tax liabilities
|
$
|
(883
|
)
|
$
|
(176
|
)
|
||
|
2009
|
2008
|
||||||
|
Balance as of January 1
|
$
|
596
|
$
|
1,592
|
|||
|
Additions for tax positions related to the current year
|
31
|
17
|
|||||
|
Additions for tax positions of prior years
|
72
|
254
|
|||||
|
Reductions of tax positions of prior years
|
(521
|
)
|
(1,267
|
)
|
|||
|
Balance as of December 31
|
$
|
178
|
$
|
596
|
|||
|
Jurisdiction
|
Open Years
|
|
|
Japan
|
2004-2009
|
|
|
Republic of Korea
|
2005-2009
|
|
|
United States
|
2004-2009
|
|
|
Switzerland
|
2008-2009
|
|
|
Taiwan
|
2005-2009
|
|
2009
|
2008
|
2007
|
|||||||||
|
Sold Products
|
$
|
0.7
|
million
|
$
|
0.8
|
million
|
$
|
1.0
|
million
|
||
|
Contributed Cash Donations
|
$
|
0.3
|
million
|
$
|
0.8
|
million
|
$
|
0.9
|
million
|
||
|
Projected benefit obligation:
|
2009
|
2008
|
||||||
|
Balance, beginning of year
|
$
|
792
|
$
|
553
|
||||
|
Service cost
|
196
|
194
|
||||||
|
Interest cost
|
18
|
16
|
||||||
|
Liability (gains) and losses
|
(23
|
)
|
(56
|
)
|
||||
|
Benefits paid to participants
|
(123
|
)
|
(50
|
)
|
||||
|
Foreign currency
|
(14
|
)
|
135
|
|||||
|
Balance, end of year
|
$
|
846
|
$
|
792
|
||||
|
Plan assets:
|
||||||||
|
Fair value, beginning of year
|
$
|
—
|
$
|
—
|
||||
|
Company contributions
|
123
|
50
|
||||||
|
Benefits paid to participants
|
(123
|
)
|
(50
|
)
|
||||
|
Fair value, end of year
|
$
|
—
|
$
|
—
|
||||
|
Funded status of the Benefit Plan as of December 31
(in thousands)
:
|
2009
|
2008
|
|||||
|
Benefit obligation
|
$
|
(846
|
)
|
$
|
(792
|
)
|
|
|
Fair value of plan assets
|
—
|
—
|
|||||
|
Excess of benefit obligation over fair value of plan assets
|
$
|
(846
|
)
|
$
|
(792
|
)
|
|
|
Amounts recognized in the accompanying Consolidated Balance Sheets consist of,
as of December 31
(in thousands)
:
|
2009
|
2008
|
|||||
|
Accrued benefit liability
|
$
|
(865
|
)
|
$
|
(783
|
)
|
|
|
Transition obligation
|
19
|
(9
|
)
|
||||
|
Net amount recognized in the consolidated balance sheets
|
$
|
(846
|
)
|
$
|
(792
|
)
|
|
|
Non-current liabilities
|
$
|
(846
|
)
|
$
|
(792
|
)
|
|
|
Years Ended December 31,
|
||||||||||||
|
Other changes recognized in other comprehensive income/loss
(in thousands)
|
2009
|
2008
|
2007
|
|||||||||
|
Net periodic cost
|
$
|
219
|
$
|
215
|
$
|
157
|
||||||
|
Other changes in plan assets and benefit obligations
|
—
|
—
|
—
|
|||||||||
|
Current year actuarial gain
|
(23
|
)
|
(56
|
)
|
(17
|
)
|
||||||
|
Current year prior service benefit
|
—
|
—
|
—
|
|||||||||
|
Amortization of actuarial gain
|
—
|
—
|
—
|
|||||||||
|
Amortization of transition obligation
|
(5
|
)
|
(5
|
)
|
(4
|
)
|
||||||
|
Total recognized in other comprehensive income
|
(28
|
)
|
(61
|
)
|
(21
|
)
|
||||||
|
Total
|
$
|
191
|
$
|
154
|
$
|
136
|
||||||
|
As of December 31,
|
||||||||
|
Amounts not yet reflected in net periodic benefit cost and included in accumulated other
comprehensive gain/loss
(in thousands)
:
|
2009
|
2008
|
||||||
|
Net actuarial gain/loss
|
$
|
79
|
$
|
57
|
||||
|
Transition obligation
|
(60
|
)
|
(66
|
)
|
||||
|
Total recognized in accumulated other comprehensive loss
|
$
|
19
|
$
|
(9
|
)
|
|||
|
2010 estimated amounts amortized from accumulated other comprehensive income/loss,
net into net periodic cost
(in thousands)
|
||||
|
Transition obligation
|
$
|
(5
|
)
|
|
|
As
of
December
31,
|
|||||||
|
2009
|
2008
|
||||||
|
Aggregate Benefit Plan information and accumulated benefit obligation in excess
of plan assets
(in thousands)
:
|
|||||||
|
Projected benefit obligation
|
$
|
846
|
$
|
792
|
|||
|
Accumulated benefit obligation
|
491
|
476
|
|||||
|
Fair value of plan assets
|
—
|
—
|
|||||
|
2009
|
2008
|
||||||
|
Discount rate
|
2.25
|
%
|
2.5
|
%
|
|||
|
Rate of increase in compensation levels
|
3.0
|
%
|
3.0
|
%
|
|||
|
2009
|
2008
|
2007
|
||||||||||
|
Service cost
|
$ | 196 | $ | 194 | $ | 142 | ||||||
|
Interest cost
|
18 | 16 | 11 | |||||||||
|
Amortization of transition obligation
|
5 | 5 | 4 | |||||||||
|
Amortization of unrecognized loss
|
— | — | — | |||||||||
|
Total pension expense
|
$ | 219 | $ | 215 | $ | 157 | ||||||
|
2010
|
$
|
5
|
|
2011
|
8
|
|
|
2012
|
10
|
|
|
2013
|
11
|
|
|
2014
|
12
|
|
|
Next five years
|
237
|
|
|
Total expected benefits to be paid
|
$
|
283
|
|
2009
|
|||||||||
|
Number
of
Options
(in thousands)
|
Weighted
average
exercise
price
|
Weighted
average
remaining
contractual life
(in years)
|
Aggregate
intrinsic
value (in
thousands)
|
||||||
|
Outstanding at beginning of year
|
1,570
|
$
|
6.22
|
||||||
|
Granted
|
520
|
$
|
3.15
|
||||||
|
Exercised
|
(20
|
)
|
$
|
2.63
|
|||||
|
Forfeited or expired
|
(531
|
)
|
$
|
6.67
|
|||||
|
Outstanding at end of year
|
1,539
|
$
|
5.07
|
6.2
|
$296
|
||||
|
Options exercisable at year end
|
994
|
$
|
5.96
|
4.6
|
$211
|
||||
|
2009
|
2008
|
2007
|
||||||
|
Dividend yield:
|
1.05
—
2.87
|
%
|
3.2
—
6.1
|
%
|
2.3 — 4.9
|
%
|
||
|
Risk-free interest rate:
|
1.53
—
2.7
|
%
|
1.8
—
3.6
|
%
|
4.2
—
4.7
|
%
|
||
|
Expected market price volatility:
|
65.9
—
70.8
|
%
|
59.8
—
63.8
|
%
|
67.7
—
68.3
|
%
|
||
|
Average expected life of stock options:
|
4.5 years
|
4.5 years
|
4.5 years
|
|||||
|
2009
|
2008
|
2007
|
||||||
|
Selling, general and administrative expenses and income (loss) from operations before income taxes
|
$
|
636
|
$
|
706
|
$
|
1,060
|
||
|
(Provision) Benefit for income taxes
|
134
|
(79
|
)
|
325
|
||||
|
Effect on net income (loss)
|
$
|
502
|
$
|
785
|
$
|
735
|
||
|
Total gross unrecognized
compensation expense
|
Total tax benefit associated
with unrecognized
compensation expense
|
Total net
unrecognized
compensation expense
|
|||||||||
|
2010
|
$
|
0.4
|
$
|
0.1
|
$
|
0.3
|
|||||
|
2011
|
0.2
|
0.1
|
0.1
|
||||||||
|
2012
|
0.1
|
—
|
0.1
|
||||||||
|
$
|
0.7
|
$
|
0.2
|
$
|
0.5
|
||||||
|
Years ending December 31,
|
||
|
2010
|
$
|
2.9
|
|
2011
|
1.5
|
|
|
2012
|
1.3
|
|
|
2013
|
1.2
|
|
|
2014
|
0.8
|
|
|
Thereafter
|
1.8
|
|
|
$
|
9.5
|
|
|
·
|
In May 2008, the Company entered into a supply agreement with Marinova PTY Limited to purchase raw materials used in its products through 2012. On August 13, 2009, the Company terminated the contract for an asserted breach. Pursuant to the terms of the contract, the parties are currently engaged in the arbitration process.
|
|
·
|
In January 2006, the Company entered into a five-year supply agreement with Larex, Inc. to exclusively purchase Arabinogalactan, an important component used in the formulation of its Ambrotose
®
complex. In order to retain exclusive rights to purchase Arabinogalactan, the Company is required to purchase a minimum monthly quantity over the five year agreement. As of December 31, 2009, the Company is required to purchase an aggregate of $0.6 million through 2010.
|
|
·
|
In March 2006, the Company entered into a ten-year supply agreement to purchase plant-derived mineral nutrition products from InB:Biotechnologies, Inc. As of December 31, 2009, the Company is required to purchase an aggregate of $6.7 million through 2016.
|
|
·
|
In June of 2008, the Company entered into a three-year supply agreement with Improve U.S.A. to purchase an aloe vera powder. As of December 31, 2009, under the terms of the agreement, the Company is required to purchase an aggregate of $6.4 million through 2011.
|
|
(i)
|
the revision of the Company’s policies and procedures regarding associate conduct;
|
|
(ii)
|
the engagement of Cyveillance to conduct website monitoring for compliance with the Company’s policies and procedures;
|
|
(iii)
|
the establishment of the Company’s Compliance Committee of the board of directors;
|
|
(iv)
|
the posting of the Company’s Code of Ethics and Business Code of Conduct and committee charters for its Compliance, Audit, Compensation and Stock Option Plan, Nominating and Governance and Science Committees on the Company’s website;
|
|
(v)
|
the requirement that the Company’s general counsel report periodically to the Compliance Committee regarding the Company’s internet website monitoring results;
|
|
(vi)
|
the increase of additional legal and compliance staff to address compliance matters; and
|
|
(vii)
|
the appointment of director Professor Robert Blattberg, a qualified, independent director with no prior affiliations with the Company, who has since resigned from his position as director.
|
The Company also agreed to cover the derivative plaintiffs’ counsels’ fees and expenses up to a sum of $850,000. This settlement payment would be funded by the Company’s insurer. Preliminary approval of the settlement was given on October 2, 2008, and notice of the settlement was subsequently distributed to shareholders. On January 13, 2009, the federal court held a hearing and granted final approval of the settlement and judgment
|
(i)
|
the designation of a corporate level Compliance Officer with oversight over (A) anonymous reporting of compliance violations, (B) responding to compliance-related questions from employees, members and associates, and (C) reviewing and disciplining associates for compliance violations;
|
|
(ii)
|
the disclosure of its policies and procedures and monitoring and compliance program to all employees, associates and members;
|
|
(iii)
|
the monitoring of employees, associates and members for compliance of (A) employee and associate websites used to advertise or promote Company products, (B) meetings held by employees and associates used to advertise or promote Mannatech products, and (C) employee and associate promotional materials not created by the Company; and
|
|
(iv)
|
mandatory investigation of reported compliance violations and termination of employees, members or associates for making claims that Company products can treat, cure, mitigate or prevent any disease unless such claim is de minimus and isolated.
|
|
Month purchased
|
Number of common shares
purchased in the open market
|
Approximate
cost
|
Average price paid
per share
|
|||||
|
May 2005
|
190,850
|
$
|
3.0
|
million
|
$
|
15.71
|
||
|
September 2005
|
182,626
|
2.0
|
million
|
$
|
10.95
|
|||
|
October 2005
|
207,023
|
2.0
|
million
|
$
|
9.66
|
|||
|
May 2006
|
73,955
|
1.0
|
million
|
$
|
13.52
|
|||
|
June 2006
|
253,289
|
3.0
|
million
|
$
|
11.84
|
|||
|
July 2006
|
144,840
|
2.0
|
million
|
$
|
13.81
|
|||
|
August 2006
|
68,861
|
1.0
|
million
|
$
|
14.52
|
|||
|
Total
|
1,121,444
|
$
|
14.0
|
million
|
$
|
12.48
|
||
|
Unrealized
Gain (Loss)
From
Investments
|
Foreign
Currency
Translation
|
Pension
Postretirement
Benefit
Obligation
|
Accumulated
Other
Comprehensive
Income (Loss), Net
|
|||||||||||||
|
Balance as of December 31, 2006
|
$ | (1 | ) | $ | (1,704 | ) | $ | (44 | ) | $ | (1,749 | ) | ||||
|
Current-period change
|
1 | 613 | 12 | 626 | ||||||||||||
|
Balance as of December 31, 2007
|
— | (1,091 | ) | (32 | ) | (1,123 | ) | |||||||||
|
Current-period change
|
— | (318 | ) | 35 | (283 | |||||||||||
|
Balance as of December 31, 2008
|
— | (1,409 | ) | 3 | (1,406 | ) | ||||||||||
|
Current-period change
|
— | 276 | 17 | 293 | ||||||||||||
|
Balance as of December 31, 2009
|
— | $ | (1,133 | ) | $ | 20 | $ | (1,113 | ) | |||||||
|
2009
|
2008
|
2007
|
||||||||||||||||||||||
|
Income/Loss
(Numerator)
|
Shares
(Denominator)
|
Per
Share
Amount)
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per
Share
Amount
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per
Share
Amount
|
||||||||||||||||
|
Basic EPS:
|
||||||||||||||||||||||||
|
Net income (loss)
available to
common
shareholders
|
$
|
(17,368
|
)
|
26,467
|
$
|
(0.66
|
)
|
$
|
(12,628
|
)
|
26,461
|
$
|
(0.48
|
)
|
$
|
6,594
|
26,443
|
$
|
0.25
|
|||||
|
Effect of dilutive
securities –
|
||||||||||||||||||||||||
|
Stock options
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
354
|
—
|
|||||||||||||||
|
Stock warrants
(1)
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
96
|
—
|
|||||||||||||||
|
Diluted EPS:
|
||||||||||||||||||||||||
|
Net income (loss)
available to
common
shareholders plus
assumed
conversions
|
$
|
(17,368
|
)
|
26,467
|
$
|
(0.66
|
)
|
$
|
(12,628
|
)
|
26,461
|
$
|
(0.48
|
)
|
$
|
6,594
|
26,893
|
$
|
0.25
|
|||||
|
|
(1)
In 2001, as part of a separation agreement, the Company granted an officer 213,333 stock warrants for common stock at exercise prices ranging from $1.75 to $4.00 per share. The stock warrants vested immediately and expired on February 28, 2008.
|
|
2009
|
2008
|
2007
|
|||||||||||||||||||||||
|
United States
|
$ | 140.7 | 48.6 | % | $ | 176.9 | 53.1 | % | $ | 244.5 | 59.2 |
%
|
|||||||||||||
|
Japan
|
42.0 | 14.5 | % | 44.8 | 13.5 | % | 42.3 | 10.3 |
%
|
||||||||||||||||
|
Republic of Korea
|
26.4 | 9.1 | % | 35.7 | 10.7 | % | 44.0 | 10.7 |
%
|
||||||||||||||||
|
Canada
|
23.0 | 7.9 | % | 23.6 | 7.1 | % | 27.4 | 6.6 |
%
|
||||||||||||||||
|
Australia
|
22.9 | 7.9 | % | 26.1 | 7.8 | % | 29.4 | 7.1 |
%
|
||||||||||||||||
|
South Africa
(1)
|
13.2 | 4.6 | % | 5.5 | 1.7 | % | — | — |
%
|
||||||||||||||||
|
Taiwan
|
6.6 | 2.3 | % | 5.2 | 1.6 | % | 5.4 | 1.3 |
%
|
||||||||||||||||
|
New Zealand
|
4.3 | 1.5 | % | 5.2 | 1.6 | % | 6.9 | 1.7 |
%
|
||||||||||||||||
|
Germany
|
3.2 | 1.1 | % | 3.8 | 1.1 | % | 4.6 | 1.1 |
%
|
||||||||||||||||
|
United Kingdom
|
3.3 | 1.0 | % | 4.7 | 1.4 | % | 6.7 | 1.6 |
%
|
||||||||||||||||
|
Denmark
|
1.6 | 0.6 | % | 1.2 | 0.4 | % | 1.5 | 0.4 |
%
|
||||||||||||||||
|
Singapore
(2)
|
1.5 | 0.5 | % | — | — | % | — | — |
%
|
||||||||||||||||
|
Austria
(3)
|
0.3 | 0.1 | % | — | — | % | — | — |
%
|
||||||||||||||||
|
The Netherlands
(3)
|
0.2 | 0.1 | % | — | — | % | — | — |
%
|
||||||||||||||||
|
Norway
(3)
|
0.3 | 0.1 | % | — | — | % | — | — |
%
|
||||||||||||||||
|
Sweden
(3)
|
0.2 | 0.1 | % | — | — | % | — | — |
%
|
||||||||||||||||
|
Totals
|
$ | 289.7 | 100 | % | $ | 332.7 | 100 | % | $ | 412.7 | 100 |
%
|
|||||||||||||
|
2009
|
2008
|
2007
|
||||||
|
Consolidated product sales
|
$
|
213.9
|
$
|
260.5
|
$
|
316.9
|
||
|
Consolidated pack sales
|
62.1
|
57.7
|
79.0
|
|||||
|
Consolidated other, including freight*
|
13.7
|
14.5
|
16.8
|
|||||
|
Total
|
$
|
289.7
|
$
|
332.7
|
$
|
412.7
|
||
|
|
*
In April 2007, the Company began operating its new Enterprise Resource Planning (“ERP”) System, which allowed it to separately quantify deferred revenue associated with sales of packs and products that were shipped but not yet received by customers. As a result, in April 2007, the Company began recording deferred revenue related to packs with pack sales and deferred revenue associated with products with product sales. For the three months ended March 31, 2007, other sales included $1.9 million related to the change in deferred revenue for packs and products shipped but not yet received by customers, rather than in the applicable pack or product sales category.
|
|
Country
|
2009
|
2008
|
||||
|
Australia
|
$
|
0.3
|
$
|
0.3
|
||
|
Canada
|
0.1
|
—
|
||||
|
Japan
|
0.3
|
0.2
|
||||
|
Republic of Korea
|
0.6
|
0.8
|
||||
|
Switzerland
|
0.5
|
0.7
|
||||
|
Taiwan
|
0.1
|
0.1
|
||||
|
United Kingdom
|
0.1
|
0.1
|
||||
|
United States
|
25.5
|
34.8
|
||||
|
$
|
27.5
|
$
|
37.0
|
|||
|
Incorporated by Reference
|
|||||
|
Exhibit
Number
|
Exhibit Description
|
Form
|
File No.
|
Exhibit (s)
|
Filing Date
|
|
3.1
|
Amended and Restated Articles of Incorporation of Mannatech, dated
May 19, 1998.
|
S-1
|
333-63133
|
3.1
|
October 28, 1998
|
|
3.2
|
Fourth Amended and Restated Bylaws of Mannatech, dated August 8,
2001 (Corrected).
|
10-K
|
000-24657
|
3.2
|
March 16, 2007
|
|
3.3
|
First Amendment to the Fourth Amended and Restated Bylaws of
Mannatech, effective November 30, 2007.
|
8-K
|
000-24657
|
3.1
|
December 6, 2007
|
|
4.1
|
Specimen Certificate representing Mannatech’s common stock, par
value $0.0001 per share.
|
S-1
|
333-63133
|
4.1
|
October 28, 1998
|
|
10.1
|
Amended and Restated 1997 Stock Option Plan, dated August 7,
2004.
|
10-K
|
000-24657
|
10.1
|
March 15, 2004
|
|
10.2
|
Amended and Restated 1998 Incentive Stock Option Plan, dated
August 7, 2004.
|
10-K
|
000-24657
|
10.1
|
March 15, 2004
|
|
10.3
|
Amended and Restated 2000 Option Plan, dated August 7, 2004.
|
10-K
|
000-24657
|
10.1
|
March 15, 2004
|
|
10.4
|
2008 Stock Incentive Plan.
|
DEF 14A
|
000-24657
|
Appendix B
|
April 29, 2008
|
|
10.5
|
Form of Indemnification Agreement between Mannatech and each
member of the Board of Directors of Mannatech Korea Ltd., dated
March 3, 2004.
|
10-Q
|
000-24657
|
10.2
|
August 9, 2004
|
|
10.6
|
Form of Indemnification Agreement between Mannatech, and its
Board of Directors, dated September 10, 1998.
|
S-1
|
333-63133
|
10.8
|
September 10, 1998
|
|
10.7
|
Commercial Lease Agreement between Mannatech and MEPC
Quorum Properties II Inc., dated November 7, 1996, as amended by
the First Amendment thereto dated May 29, 1997 and the Second
Amendment thereto dated November 13, 1997.
|
S-1
|
333-63133
|
10.13
|
September 10, 1998
|
|
10.8
|
Second Amendment to the Commercial Lease Agreement between
Mannatech and Texas Dugan Limited Partnership, dated September
22, 2005.
|
10-Q
|
000-24657
|
10.1
|
November 9, 2005
|
|
10.9
|
Commercial Lease Agreement between Mannatech and MEPC
Quorum Properties II Inc., dated May 29, 1997 as amended by the
First Amendment thereto dated November 6, 1997.
|
S-1
|
333-63133
|
10.14
|
September 10, 1998
|
|
10.10
|
Third Amendment to the Commercial Lease Agreement between
Mannatech and Texas Dugan Limited Partnership, dated September
22, 2005.
|
10-Q
|
000-24657
|
10.2
|
November 9, 2005
|
|
10.11
|
Trademark License and Supply Agreement between Mannatech and
Carrington Laboratories, Inc., dated January 25, 2007. (Portions
of this exhibit were omitted pursuant to a confidential treatment
request submitted pursuant to Rule 24b-2 of the Exchange Act.)
|
8-K
|
000-24657
|
10.1
|
January 31, 2007
|
|
10.12
|
Supply Agreement between Mannatech (International) Limited and
Marinova Pty. Limited, effective August 9, 2007 and dated May 7,
2007, (Portions of this exhibit were omitted pursuant to a
confidential treatment request submitted pursuant to Rule 24b-2 of
the Exchange Act).
|
10-Q
|
000-24657
|
10.3
|
May 10, 2007
|
|
10.13
|
Amendment to Purchase Agreement between
Mannatech and Marinova PTY, Limited, dated
May 6, 2008 (Portions of this exhibit were omitted
pursuant to a confidential treatment request submitted
pursuant to Rule 24b-2 of the Exchange Act).
|
10-Q
|
000-24657
|
10.4
|
August 11, 2008
|
|
10.14
|
Purchase Agreement between Mannatech and Larex, Inc., dated
January 1, 2006. (Portions of this exhibit were omitted pursuant to a
confidential treatment request submitted pursuant to Rule 24b-2 of
the Exchange Act.)
|
10-K
|
000-24657
|
10.18
|
March 16, 2006
|
|
10.15
|
Purchase Agreement between Mannatech and Wellness Enterprises,
LLC, dated February 1, 2006. (Portions of this exhibit were omitted
pursuant to a confidential treatment request submitted pursuant to
Rule 24b-2 of the Exchange Act.)
|
10-K
|
000-24657
|
10.19
|
March 16, 2006
|
|
Incorporated by Reference
|
|||||
|
Exhibit
Number
|
Exhibit Description
|
Form
|
File No.
|
Exhibit (s)
|
Filing Date
|
|
10.16
|
Supply Agreement between Mannatech and Coradji PTY. Limited,
dated March 29, 2004. (Portions of this exhibit were omitted
pursuant to a confidential treatment request submitted pursuant to
Rule 24b-2 of the Exchange Act.)
|
10-Q/A
|
000-24657
|
10.1
|
March 29, 2005
|
|
10.17
|
Supply License Agreement between Mannatech and
InB:Biotechnologies, Inc., dated March 22, 2006. (Portions of this
exhibit were omitted pursuant to a confidential treatment request
submitted pursuant to Rule 24b-2 of the Exchange Act.)
|
10-Q
|
000-24657
|
10.2
|
May 10, 2006
|
|
10.18
|
Initial Commercial Supply and Manufacturing Agreement between
Mannatech and Fine Chemetics, Inc., dated March 29, 2006.
(Portions of this exhibit were omitted pursuant to a confidential
treatment request submitted pursuant to Rule 24b-2 of the
Exchange Act.)
|
10-Q
|
000-24657
|
10.3
|
May 10, 2006
|
|
10.19
|
Supply Agreement between Mannatech, Incorporated, and Improve
U.S.A., Inc., effective June 1, 2008, and executed May 2, 2008.
(Portions of this exhibit were omitted pursuant to a confidential
treatment request submitted pursuant to Rule 24b-2 of the
Exchange Act.)
|
8-K
|
000-24657
|
10.1
|
May 8, 2008
|
|
10.20
|
Amended and Restated Employment Agreement between Terry L.
Persinger and Mannatech, dated June 16, 2008.
|
8-K
|
000-24657
|
10.1
|
June 20, 2008
|
|
10.21
|
Employment Agreement between Robert A. Sinnott, Ph.D. and
Mannatech, dated October 5, 2007.
|
8-K
|
000-24657
|
10.3
|
October 11, 2007
|
|
10.22
|
Employment Agreement between Mannatech and Mr. Samuel L.
Caster, dated January 23, 2006.
|
10-K
|
000-24657
|
10.32
|
March 16, 2006
|
|
10.23
|
Employment Agreement between Stephen D. Fenstermacher and
Mannatech, dated October 5, 2007.
|
8-K
|
000-24657
|
10.2
|
October 11, 2007
|
|
10.24
|
First Amendment to Employment Agreement between
Stephen D. Fenstermacher and Mannatech, dated
December 18, 2008.
|
10-K
|
000-24657
|
10.24
|
March 12, 2009
|
|
10.25
|
Employment Agreement between Terence L. O’Day and Mannatech,
dated October 5, 2007.
|
8-K
|
000-24657
|
10.1
|
October 11, 2007
|
|
10.26
|
Employment Agreement between B. Keith Clark and Mannatech,
dated October 5, 2007.
|
8-K
|
000-24657
|
10.4
|
October 11, 2007
|
|
10.27
|
Employment Agreement between Wayne L. Badovinus and
Mannatech, dated June 4, 2008.
|
8-K
|
000-24657
|
10.1
|
June 9, 2008
|
|
10.28
|
Employment Agreement between Terri F. Maxwell
and Mannatech, dated August 28, 2008.
|
8-K
|
000-24657
|
10.1
|
September 2, 2008
|
|
10.29
|
Lock-up Agreement between Mannatech and J. Stanley Fredrick,
dated November 6, 2003.
|
10-K
|
000-24657
|
10.36
|
March 15, 2004
|
|
10.30
|
Termination of Lock-up Agreement between Mannatech and
J. Stanley Fredrick, dated March 6, 2009.
|
8-K
|
000-24657
|
10.1
|
March 10, 2009
|
|
10.31
|
Follow-Up Agreement to Letter of Intent Agreement between
Mannatech and Jett, dated September 10, 2001.
|
10-Q
|
000-24657
|
10.4
|
November 14, 2001
|
|
10.32
|
Letter of Understanding between Mannatech and Dr. John Axford,
dated April 19, 2006.
|
8-K
|
000-24657
|
99.1
|
April 21, 2006
|
|
10.33
|
Extension of the Letter of Spokesperson Arrangement between
Mannatech and Dr. John Axford, dated February 18, 2007.
|
8-K
|
000-24657
|
99.1
|
February 21, 2007
|
|
10.34
|
Employment Agreement between Alfredo Bala and Mannatech,
effective October 1, 2007, dated September 18, 2007.
|
8-K
|
000-24657
|
10.1
|
September 24, 2007
|
|
10.35
|
Amendment to Employment Agreement between Alfredo Bala and
Mannatech, dated October 11, 2007.
|
8-K
|
000-24657
|
10.1
|
October 17, 2007
|
|
10.36
|
Clinical Research Agreement dated January 3, 2007 by and between
St. George’s Hospital Medical School (trading as St George’s,
University of London), and Mannatech, Inc.
|
10-K
|
000-24657
|
10.39
|
March 17,2008
|
|
10.37
|
Employment Agreement, effective March 2, 2009, by and between Mannatech and Randy S. Bancino.
|
8-K
|
000-24657
|
10.1
|
March 6, 2009
|
|
10.38
|
First Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and Randy S. Bancino.
|
8-K
|
000-24657
|
10.4
|
December 18, 2009
|
|
Incorporated by Reference
|
|||||
|
Exhibit
Number
|
Exhibit Description
|
Form
|
File No.
|
Exhibit (s)
|
Filing Date
|
|
10.39
|
Consulting Agreement, dated March 17, 2009, between Mannatech, Salinda Enterprises, LLC and Samuel L. Caster.
|
8-K
|
000-24657
|
10.1
|
March 19, 2009
|
|
10.40
|
Separation and Release Agreement, dated July 17, 2009 between Mannatech and Terri F. Maxwell.
|
8-K
|
000-24657
|
10.1
|
July 21, 2009
|
|
10.41
|
Second Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and Stephen D. Fenstermacher.
|
8-K
|
000-24657
|
10.1
|
December 18, 2009
|
|
10.42
|
Second Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and Robert A. Sinnott, Ph.D.
|
8-K
|
000-24657
|
10.2
|
December 18, 2009
|
|
10.43
|
Second Amendment to Employment Agreement, dated as of December 16, 2009, by and between Mannatech and B. Keith Clark.
|
8-K
|
000-24657
|
10.3
|
December 18, 2009
|
|
14.1
|
Code of Ethics.
|
10-K
|
000-24657
|
14.1
|
March 16, 2007
|
|
21*
|
List of Subsidiaries.
|
*
|
*
|
*
|
*
|
|
23.1*
|
Consent of BDO Seidman, LLP.
|
*
|
*
|
*
|
*
|
|
23.2*
|
Report of Independent Registered Public Accounting Firm on
Financial Statement Schedule.
|
*
|
*
|
*
|
*
|
|
24*
|
Power of Attorney, which is included on the signature page of this
annual report on Form 10-K.
|
*
|
*
|
*
|
*
|
|
31.1*
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of
2002, of the Co-Chief Executive Officer of Mannatech.
|
*
|
*
|
*
|
*
|
|
31.2*
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of
2002, of the Co-Chief Financial Officer of Mannatech.
|
*
|
*
|
*
|
*
|
|
32.1*
|
Certification pursuant to Section 906 of the Sarbanes-Oxley Act of
2002, of the Co-Chief Executive Officer of Mannatech.
|
*
|
*
|
*
|
*
|
|
32.2*
|
Certification pursuant to Section 906 of the Sarbanes-Oxley Act of
2002, of the Co-Chief Financial Officer of Mannatech.
|
*
|
*
|
*
|
*
|
|
99.3*
|
Financial Statement schedule regarding Valuation and Qualifying
Accounts.
|
*
|
*
|
*
|
*
|
|
101.INS**
|
XBRL Instance Document
|
**
|
**
|
**
|
**
|
|
101.SCH**
|
XBRL Taxonomy Extension Schema Document
|
**
|
**
|
**
|
**
|
|
101.CAL**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
**
|
**
|
**
|
**
|
|
101.LAB**
|
XBRL Taxonomy Extension Label Linkbase Document
|
**
|
**
|
**
|
**
|
|
101.PRE**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
**
|
**
|
**
|
**
|
|
101.DEF**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
**
|
**
|
**
|
**
|