|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
or
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
NU SKIN ENTERPRISES, INC.
|
||||
|
(Exact name of registrant as specified in its charter)
|
||||
|
Delaware
|
87-0565309
|
|||
|
(State or other jurisdiction of incorporation or organization)
|
75 WEST CENTER STREET
PROVO UT 84601
|
(IRS Employer Identification No.)
|
||
|
(Address of principal executive offices, including zip code)
|
||||
|
Title of each class
|
Name of exchange on which registered
|
|
Class A common stock, $.001 par value
|
New York Stock Exchange
|
|
Large accelerated filer
þ
|
Accelerated filer
¨
|
|
|
Non-accelerated filer
¨
(Do not check if a smaller reporting company)
|
Smaller Reporting Company
¨
|
|
PART I
|
-1-
|
||
|
ITEM 1.
|
BUSINESS
|
-1-
|
|
|
Overview
|
-2-
|
||
|
Our Difference Demonstrated
|
-2-
|
||
|
Our Product Categories
|
-3-
|
||
|
Sourcing and Production
|
-6-
|
||
|
Research and Development
|
-7-
|
||
|
Intellectual Property
|
-8-
|
||
|
Geographic Sales Regions
|
-8-
|
||
|
Distribution
|
-12-
|
||
|
Our Culture
|
-15-
|
||
|
Competition
|
-16-
|
||
|
Government Regulation
|
-16-
|
||
|
Employees
|
-21-
|
||
|
Available Information
|
-21-
|
||
|
Executive Officers
|
-21-
|
||
|
ITEM 1A.
|
RISK FACTORS
|
-23-
|
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
-41-
|
|
|
ITEM 2.
|
PROPERTIES
|
-41-
|
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
-42-
|
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
-43-
|
|
|
PART II
|
-44-
|
||
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
-44-
|
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
-47-
|
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
-48-
|
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS
|
-69-
|
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
-70-
|
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
-103-
|
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
-103-
|
|
|
ITEM 9B.
|
OTHER INFORMATION
|
-104-
|
|
|
PART III
|
-104-
|
||
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE
|
-104-
|
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
-104-
|
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
-104-
|
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
-104-
|
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
-104-
|
|
|
PART IV
|
-104-
|
||
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
-104-
|
|
|
SIGNATURES
|
-113-
|
||
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
Product Category
|
2009
|
2010
|
2011
|
|||||||||||||||||||||
|
Nu Skin
|
$ | 752.7 | 56.5 | % | $ | 913.8 | 59.4 | % | $ | 964.1 | 55.3 | % | ||||||||||||
|
Pharmanex
|
565.6 | 42.5 | 612.2 | 39.8 | 770.2 | 44.2 | ||||||||||||||||||
|
Other
(2)
|
12.8 | 1.0 | 11.3 | 0.8 | 9.7 | 0.5 | ||||||||||||||||||
| $ | 1,331.1 | 100.0 | % | $ | 1,537.3 | 100.0 | % | $ | 1,744.0 | 100.0 | % | |||||||||||||
|
(1)
|
In 2011, 88% of our sales were transacted in foreign currencies that were then converted to U.S. dollars for financial reporting purposes at weighted-average exchange rates. Foreign currency fluctuations positively impacted reported revenue by approximately 6% in 2011 compared to 2010. Foreign currency fluctuations positively impacted reported revenue by approximately 5% in 2010 compared to 2009.
|
|
(2)
|
We currently offer a limited number of other products and services, including household products and digital content storage.
|
|
Category
|
|
Description
|
|
Selected Products
|
|
Core Systems
|
|
Our core systems provide a solid foundation for individual skin care needs, regardless of skin type. Our core systems target specific skin concerns and are made from ingredients scientifically proven to provide visible results for concerns ranging from aging to acne.
|
ageLOC Transformation Skin Care System
Nu Skin 180º Anti-Aging Skin Therapy System
Nu Skin Tri-Phasic White
Nutricentials
Nu Skin Clear Action Acne Medication System
|
|
|
Targeted Treatments
|
Our customized skin care line allows customer tailored product regimens that help deliver younger looking skin at any age. The products are developed using cutting-edge ingredient technologies that target specific skin care needs.
|
ageLOC Edition Galvanic Spa System II
Galvanic Spa Gels with ageLOC
ageLOC Galvanic Body Spa
ageLOC Galvanic Spa Body Shaping Gel
ageLOC Dermatic Effects Body Contouring Lotion
Tru Face Essence Ultra
Tru Face Line Corrector
Enhancer Skin Conditioning Gel
Celltrex Ultra Recovery Fluid
Celltrex CoQ10 Complete
NAPCA Moisturizer
Polishing Peel Skin Refinisher
|
||
|
Total Care
|
Our total care line addresses body, hair and oral care. The total care line can be used by families and the products are designed to deliver superior benefits from head to toe for the ultimate sense of total body wellness.
|
Body Bar
Liquid Body Lufra
Perennial Intense Body Moisturizer
Dividends Men’s Care
AP-24 Dental Care
Nu Skin Renu Hair Mask
|
||
|
Cosmetic
|
Our
Nu Colour
cosmetic line products are targeted to define and highlight natural beauty.
|
Tinted Moisturizer SPF 15
Finishing Powder
Contouring Lip Gloss
Defining Effects Mascara
|
||
|
Epoch
|
Our
Epoch
line is distinguished by utilizing traditional knowledge of indigenous cultures for skin care. Each
Epoch
product is formulated with botanical ingredients derived from renewable resources found in nature. In addition, we contribute a percentage of our proceeds from
Epoch
sales to charitable causes.
|
Baobab Body Butter
Sole Solution Foot Treatment
Calming Touch Soothing Skin Cream
Glacial Marine Mud
IceDancer Invigorating Leg Gel
Everglide Foaming Shave Gel
Ava puhi moni Shampoo
Epoch Baby Hibiscus Hair & Body Wash
|
|
Category
|
|
Description
|
|
Selected Products
|
|
Nutritional
|
|
Our nutritional supplements supply a broad spectrum of micronutrients needed as a foundation for a lifetime of optimal health.
|
|
ageLOC Vitality
ageLOC R
2
|
|
Anti-aging
|
|
Our anti-aging products are designed to reset genetic expression, which changes with age, to a more youthful level.
|
|
LifePak
family of products
g3
juice
|
|
Solutions
|
|
Our solutions supplements contain standardized levels of botanical and other active ingredients that are formulated to meet the demands of everyday life.
|
|
Tegreen 97
ReishiMax GLp
MarineOmega
Cholestin
CordyMax Cs-4
Cortitrol
Detox Formula
Eye Formula
|
|
Weight Management
|
|
Our weight management products include supplements as well as meal replacement shakes.
|
|
The Right Approach (TRA)
weight management system
MyVictory!
weight management program
|
|
VitaMeal
|
Our VitaMeal is a highly nutritious meal that can be purchased and donated through our Nourish the Children initiative to feed malnourished children or purchased for personal food storage.
|
VitaMeal
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
(U.S. dollars in millions)
|
2009
|
2010
|
2011
|
|||||||||||||||||||||
|
North Asia
|
$ | 606.1 | 45 | % | $ | 686.1 | 45 | % | $ | 751.2 | 43 | % | ||||||||||||
|
Greater China
|
210.4 | 16 | 268.2 | 17 | 341.9 | 20 | ||||||||||||||||||
|
Americas
|
260.9 | 20 | 250.0 | 16 | 252.0 | 14 | ||||||||||||||||||
|
South Asia/Pacific
|
120.1 | 9 | 182.8 | 12 | 236.2 | 14 | ||||||||||||||||||
|
Europe
|
133.6 | 10 | 150.2 | 10 | 162.7 | 9 | ||||||||||||||||||
| $ | 1,331.1 | 100 | % | $ | 1,537.3 | 100 | % | $ | 1,744.0 | 100 | % | |||||||||||||
|
(U.S. dollars in millions)
|
Year Opened
|
2011 Revenue
|
Percentage of
2011 Revenue
|
||
|
Japan
|
1993
|
$
|
472.5
|
27%
|
|
|
South Korea
|
1996
|
$
|
278.7
|
16%
|
|
|
(U.S. dollars in millions)
|
Year Opened
|
2011 Revenue
|
Percentage of
2011 Revenue
|
||
|
China
|
2003
|
$
|
152.5
|
9%
|
|
|
Taiwan
|
1992
|
$
|
108.9
|
6%
|
|
|
Hong Kong
|
1991
|
$
|
80.5
|
5%
|
|
Our Hong Kong and Taiwan markets operate under our global direct selling business model and global compensation plan. However, we currently are unable to operate under our global direct selling business model in China as a result of regulatory restrictions on direct selling activities in this market. Consequently, we have developed a hybrid business model that utilizes retail stores with an employed sales force and contractual sales promoters to sell products through fixed locations, which we supplement with a direct sales opportunity in those locations where we have obtained a direct sales license. We continue to operate this hybrid model because we believe it provides us with more flexibility in the manner in which we can operate throughout China and compensate our contractual sales promoters and employed sales representatives given the restrictions in the direct selling regulations. We rely on our sales force to market and sell products at the various retail locations supported by only minimal advertising and traditional promotional efforts. Our sales force may also refer individuals to join our sales force as sales employees, contractual sales promoters or direct sellers. Our retail model in China is largely based upon our ability to attract customers to our retail stores through our sales force, to educate them about our products through frequent training meetings, and to obtain repeat purchases. We also continue to implement a direct sales opportunity that allows us to engage independent direct sellers who can sell products away from our retail stores. We have received licenses and approvals to engage in direct selling activities in various locations in China, including major cities or districts in ten provinces and three municipalities. We continue to work to obtain the necessary approvals in other locations in China. The direct selling licenses allow us to engage an entry-level, non-employee sales force that can sell products away from fixed retail locations. Our current direct sales model is structured in a manner that we believe complements our existing retail sales model.
|
(U.S. dollars in millions)
|
Year Opened
|
2011 Revenue
|
Percentage of
2011 Revenue
|
||
|
Americas Region
(1)
|
1984
|
$
|
252.0
|
14%
|
|
|
(1)
|
Americas region includes United States, Canada, Colombia, Costa Rica, El Salvador, Guatemala, Honduras, Mexico and Venezuela.
|
|
(U.S. dollars in millions)
|
Year Opened
|
2011 Revenue
|
Percentage of
2011 Revenue
|
|||
|
South Asia/Pacific Region
|
1993
|
$
|
236.2
|
14%
|
||
|
|
(1)
|
South Asia/Pacific region includes Australia, Brunei, French Polynesia, Indonesia, Malaysia, New Caledonia, New Zealand, Philippines, Singapore and Thailand.
|
|
(U.S. dollars in millions)
|
Year Opened
|
2011 Revenue
|
Percentage of
2011 Revenue
|
||
|
Europe Region
(1)
|
1995
|
$
|
162.7
|
9%
|
|
|
|
(1)
|
Europe region includes Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany,
|
Hungary, Ireland, Iceland, Israel, Italy, Luxembourg, the Netherlands, Norway, Poland, Portugal,
|
Romania, Russia, Slovakia, South Africa, Spain, Sweden, Switzerland, Turkey, Ukraine
and the United Kingdom.
|
|
|
•
|
distributors can educate consumers about our products in person, which we believe is more effective for premium-quality, differentiated products than using traditional advertising;
|
|
|
•
|
direct sales allow for actual product demonstrations and testing by potential customers;
|
|
|
•
|
there is greater opportunity for distributor and customer testimonials; and
|
|
|
•
|
as compared to other distribution methods, our distributors can provide customers higher levels of service and encourage repeat purchases.
|
|
As of December 31, 2009
|
As of December 31, 2010
|
As of December 31, 2011
|
||||||||||
|
Active
|
Executive
|
Active
|
Executive
|
Active
|
Executive
|
|||||||
|
North Asia
|
319,000
|
14,144
|
329,000
|
14,687
|
338,000
|
15,293
|
||||||
|
Greater China
|
106,000
|
6,938
|
118,000
|
8,015
|
143,000
|
11,808
|
||||||
|
Americas
|
171,000
|
5,522
|
161,000
|
5,305
|
166,000
|
5,356
|
||||||
|
South Asia/Pacific
|
71,000
|
2,950
|
84,000
|
3,930
|
99,000
|
5,619
|
||||||
|
Europe
|
94,000
|
3,385
|
107,000
|
3,739
|
109,000
|
3,740
|
||||||
|
Total
|
761,000
|
32,939
|
799,000
|
35,676
|
855,000
|
41,816
|
||||||
|
|
•
|
through retail markups on sales of products purchased by distributors at wholesale; and
|
|
|
|
|
|
•
|
through a series of commissions on product sales.
|
|
|
•
|
make retail sales or customer connections to established numbers of retail customers; and
|
|
|
|
|
|
•
|
sell and/or consume at least 80% of personal sales volume.
|
|
|
•
|
impose cancellation/product return, inventory buy-backs and cooling-off rights for consumers and distributors;
|
|
|
•
|
require us or our distributors to register with governmental agencies;
|
|
|
•
|
impose caps on the amount of commission we can pay;
|
|
|
|
|
•
|
impose reporting requirements; and
|
|
|
•
|
impose upon us requirements, such as requiring distributors to maintain levels of retail sales to qualify to receive commissions, to ensure that distributors are being compensated for sales of products and not for recruiting new distributors.
|
|
Name
|
Age
|
Position
|
||
|
Blake Roney
|
53
|
Chairman of the Board
|
||
|
Truman Hunt
|
52
|
President and Chief Executive Officer
|
||
|
Ritch Wood
|
46
|
Chief Financial Officer
|
||
|
Joe Chang
|
59
|
Chief Scientific Officer and Executive Vice President, Product Development
|
||
|
Dan Chard
|
47
|
President, Global Sales and Operations
|
||
|
Scott Schwerdt
|
54
|
President, Americas Region
|
||
|
Matthew Dorny
|
47
|
General Counsel and Secretary
|
|
•
|
any adverse publicity regarding us, our products, our distribution channel, or our competitors;
|
|
•
|
lack of interest in, or the technical failure of, existing or new products;
|
|
•
|
lack of a compelling sponsoring story that generates interest for potential new distributors and effectively draws them into the business;
|
|
•
|
any negative public perception of our products and their ingredients;
|
|
•
|
any negative public perception of our distributors and direct selling businesses in general;
|
|
•
|
our actions to enforce our policies and procedures;
|
|
•
|
any regulatory actions or charges against us or others in our industry;
|
|
•
|
general economic and business conditions; and
|
|
•
|
potential saturation or maturity levels in a given country or market which could negatively impact our ability to attract and retain distributors in such market.
|
|
•
|
continued or increased levels of regulatory and media scrutiny and any regulatory actions taken by regulators, or any adoption of more restrictive regulations, in response to such scrutiny;
|
|
•
|
significant weakening of the Japanese yen;
|
|
•
|
increased regulatory constraints with respect to the claims we can make regarding the efficacy of products and tools, which could limit our ability to effectively market them;
|
|
•
|
risks that the initiatives we have implemented in Japan, which are patterned after successful initiatives implemented in other markets, will not have the same level of success in Japan, may not generate renewed growth or increased productivity among our distributors, and may cost more or require more time to implement than we have anticipated;
|
|
•
|
inappropriate activities by our distributors and any resulting regulatory actions against us or our distributors;
|
|
•
|
improper practices of other direct selling companies or their distributors that increase regulatory and media scrutiny of our industry;
|
|
•
|
any weakness in the economy or consumer confidence; and
|
|
•
|
increased competitive pressures from other direct selling companies and their distributors who actively seek to solicit our distributors to join their businesses.
|
|
•
|
impose order cancellations, product returns, inventory buy-backs and cooling-off rights for consumers and distributors;
|
|
•
|
require us or our distributors to register with government agencies;
|
|
•
|
impose caps on the amount of commissions we can pay; and/or
|
|
•
|
require us to ensure that distributors are not being compensated based upon the recruitment of new distributors.
|
|
•
|
suspicions about the legality and ethics of network marketing;
|
|
•
|
the safety or effectiveness of ingredients in our or our competitors' products;
|
|
•
|
regulatory investigations of us, our competitors and our respective products;
|
|
•
|
the actions of our current or former distributors and employees; and
|
|
•
|
public perceptions of the direct selling industry or the nutritional or personal care industry generally.
|
|
•
|
the possibility that a foreign government might ban or severely restrict our business method of direct selling, or that local civil unrest, political instability or changes in diplomatic or trade relationships might disrupt our operations in an international market;
|
|
•
|
the lack of well-established or reliable legal systems in certain areas where we operate;
|
|
•
|
the presence of high inflation in the economies of international markets in which we operate;
|
|
•
|
the possibility that a government authority might impose legal, tax or other financial burdens on us or our distributors, due, for example, to the structure of our operations in various markets;
|
|
•
|
the possibility that a government authority might challenge the status of our distributors as independent contractors or impose employment or social taxes on our distributors; and
|
|
•
|
the possibility that governments may impose currency remittance restrictions limiting our ability to repatriate cash.
|
|
•
|
difficulties in assimilating acquired operations or products, including the loss of key employees from acquired businesses and disruption to our direct selling channel;
|
|
•
|
diversion of management’s attention from our core business;
|
|
•
|
adverse effects on existing business relationships with suppliers and customers; and
|
|
•
|
risks of entering markets in which we have limited or no prior experience.
|
|
•
|
fluctuations in our quarterly operating results;
|
|
•
|
the sale of shares of Class A common stock by our original or significant stockholders;
|
|
•
|
general trends in the market for our products;
|
|
•
|
acquisitions by us or our competitors;
|
|
•
|
economic and/or currency exchange issues in markets in which we operate;
|
|
•
|
changes in estimates of our operating performance or changes in recommendations by securities analysts; and
|
|
•
|
general business and political conditions.
|
|
•
|
our worldwide headquarters in Provo, Utah;
|
|
•
|
our worldwide distribution center/warehouse in Provo, Utah; and
|
|
•
|
our distribution center in Tokyo, Japan.
|
|
•
|
our nutritional supplement manufacturing facility in Zhejiang Province, China;
|
|
•
|
our personal care manufacturing facility in Shanghai, China;
|
|
•
|
our VitaMeal manufacturing facility in Jixi, Heilongjiang Province, China;
|
|
•
|
our herbal extraction facility in Zhejiang Province, China.
|
We own our corporate headquarters buildings, distribution center and research and development center located in Provo, Utah. We also own personal care and nutritional supplement plants in China, and a few other minor facilities. We currently lease the other properties described above. We believe that our existing and planned facilities are adequate for our current operations in each of our existing markets.
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Quarter Ended
|
High
|
Low
|
||
|
March 31, 2010
|
$ 30.23
|
$ 22.86
|
||
|
June 30, 2010
|
33.99
|
23.12
|
||
|
September 30, 2010
|
29.87
|
23.55
|
||
|
December 31, 2010
|
32.72
|
28.24
|
|
Quarter Ended
|
High
|
Low
|
||
|
March 31, 2011
|
$ 33.08
|
$ 27.50
|
||
|
June 30, 2011
|
39.35
|
28.53
|
||
|
September 30, 2011
|
46.93
|
35.44
|
||
|
December 31, 2011
|
51.67
|
37.67
|
|
|
Purchases of Equity Securities by the Issuer
|
|
(a)
|
(b)
|
(c)
|
(d)
|
|||||
|
Period
|
Total Number
of Shares Purchased
|
Average Price Paid per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs
|
Approximate Dollar Value of Shares that may yet be Purchased Under the Plans or Programs
(in millions)
(1)
|
||||
|
October 1 – 31, 2011
|
231,900
|
$ 41.19
|
231,900
|
$ 93.1
|
||||
|
November 1 – 30, 2011
|
52,919
|
45.05
|
52,683
|
90.7
|
||||
|
December 1 – 31, 2011
|
88,178
|
47.01
|
88,178
|
86.3
|
||||
|
Total
|
372,997
(2)
|
43.11
|
372,761
|
|
(1)
|
In August 1998, our board of directors approved a plan to repurchase $10.0 million of our Class A common stock on the open market or in private transactions. Our board has from time to time increased the amount authorized under the plan and a total amount of approximately $485.0 million is currently authorized. As of December 31, 2011, we had repurchased approximately $398.7 million of shares under the plan. There has been no termination or expiration of the plan since the initial date of approval.
|
|
(2)
|
We have authorized the repurchase of shares acquired by our employees and distributors in certain foreign markets because of regulatory and other issues that make it difficult or costly for these persons to sell such shares in the open market. These shares were awarded or acquired in connection with our initial public offering in 1996. Of the shares listed in this column, in November 236 shares at an average price per share of $49.00 relate to repurchases from such employees and distributors.
|
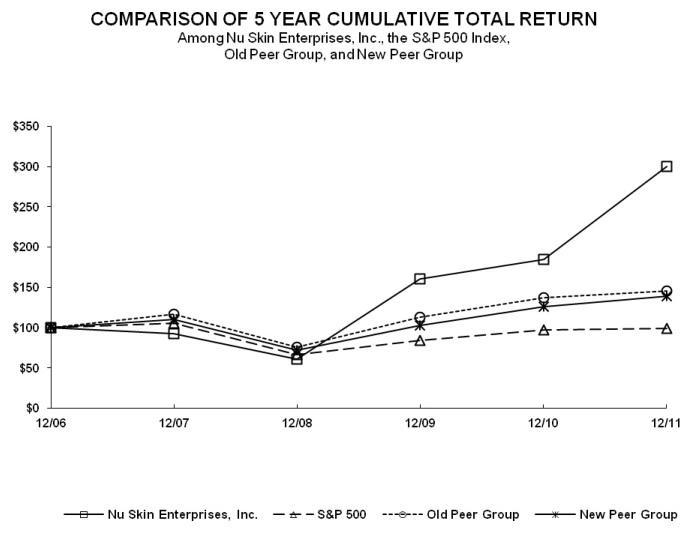
|
Measured Period
|
Company
|
S&P 500 Index
|
New Peer
Group Index
|
Old Peer
Group Index
|
||||
|
December 31, 2006
|
100.00
|
100.00
|
100.00
|
100.00
|
||||
|
December 31, 2007
|
92.44
|
105.49
|
110.51
|
116.59
|
||||
|
December 31, 2008
|
60.50
|
66.46
|
71.55
|
75.47
|
||||
|
December 31, 2009
|
160.70
|
84.05
|
102.74
|
113.01
|
||||
|
December 31, 2010
|
184.22
|
96.71
|
125.81
|
137.10
|
||||
|
December 31, 2011
|
300.26
|
98.75
|
139.22
|
145.23
|
|
Year Ended December 31,
|
||||||||||||||||||||
|
2007
|
2008
|
2009
|
2010 |
2011
|
||||||||||||||||
|
(U.S. dollars in thousands, except per share data and cash dividends)
|
||||||||||||||||||||
|
Income Statement Data
:
|
||||||||||||||||||||
|
Revenue
|
$ | 1,157,667 | $ | 1,247,646 | $ | 1,331,058 | $ | 1,537,259 | $ | 1,743,991 | ||||||||||
|
Cost of sales
|
209,283 | 228,597 | 243,648 | 272,431 | 322,624 | |||||||||||||||
|
Gross profit
|
948,384 | 1,019,049 | 1,087,410 | 1,264,828 | 1,421,367 | |||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Selling expenses
|
499,095 | 533,151 | 559,605 | 646,348 | 751,448 | |||||||||||||||
|
General and administrative expenses
|
358,601 | 360,470 | 369,368 | 401,418 | 436,177 | |||||||||||||||
|
Restructuring charges
|
19,775 | — | 10,724 | — | — | |||||||||||||||
|
Total operating expenses
|
877,471 | 893,621 | 939,697 | 1,047,766 | 1,187,625 | |||||||||||||||
|
Operating income
|
70,913 | 125,428 | 147,713 | 217,062 | 233,742 | |||||||||||||||
|
Other income (expense), net
|
(2,435 | ) | (24,775 | ) | (6,589 | ) | (9,449 | ) | (6,973 | ) | ||||||||||
|
Income before provision for income taxes
|
68,478 | 100,653 | 141,124 | 207,613 | 226,769 | |||||||||||||||
|
Provision for income taxes
|
24,606 | 35,306 | 51,279 | 71,562 | 73,439 | |||||||||||||||
|
Net income
|
$ | 43,872 | $ | 65,347 | $ | 89,845 | $ | 136,051 | $ | 153,330 | ||||||||||
|
Net income per share:
|
||||||||||||||||||||
|
Basic
|
$ | 0.68 | $ | 1.03 | $ | 1.42 | $ | 2.18 | $ | 2.47 | ||||||||||
|
Diluted
|
$ | 0.67 | $ | 1.02 | $ | 1.40 | $ | 2.11 | $ | 2.38 | ||||||||||
|
Weighted-average common shares outstanding (000s):
|
||||||||||||||||||||
|
Basic
|
64,783 | 63,510 | 63,333 | 62,370 | 62,066 | |||||||||||||||
|
Diluted
|
65,584 | 64,132 | 64,296 | 64,547 | 64,546 | |||||||||||||||
|
Balance Sheet Data
(at end of period)
:
|
||||||||||||||||||||
|
Cash and cash equivalents and current investments
|
$ | 92,552 | $ | 114,586 | $ | 158,045 | $ | 230,337 | $ | 290,701 | ||||||||||
|
Working capital
|
95,175 | 124,036 | 152,731 | 206,078 | 288,916 | |||||||||||||||
|
Total assets
|
683,243 | 709,772 | 748,449 | 892,224 | 990,956 | |||||||||||||||
|
Current portion of long-term debt
|
31,441 | 30,196 | 35,400 | 27,865 | 28,608 | |||||||||||||||
|
Long-term debt
|
169,229 | 158,760 | 121,119 | 133,013 | 107,944 | |||||||||||||||
|
Stockholders’ equity
|
275,009 | 316,180 | 375,687 | 471,249 | 574,236 | |||||||||||||||
|
Cash dividends declared
|
0.42 | 0.44 | 0.46 | 0.50 | 0.59 | |||||||||||||||
|
Supplemental Operating Data
(at end of period)
:
|
||||||||||||||||||||
|
Approximate number of active distributors
(1)
|
755,000 | 761,000 | 761,000 | 799,000 | 855,000 | |||||||||||||||
|
Number of executive distributors
(1)
|
30,002 | 30,588 | 32,939 | 35,676 | 41,816 | |||||||||||||||
|
(1)
|
Active distributors include preferred customers and distributors purchasing products directly from us during the three months ended as of the date indicated. An executive distributor is an active distributor who has achieved required personal and group sales volumes.
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
|
•
|
providing compelling initiatives and strong distributor support; and
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
(U.S. dollars in millions)
|
2009
|
2010
|
2011
|
|||||||||||||||||||||
|
North Asia
|
$ | 606.1 | 45 | % | $ | 686.1 | 45 | % | $ | 751.2 | 43 | % | ||||||||||||
|
Greater China
|
210.4 | 16 | 268.2 | 17 | 341.9 | 20 | ||||||||||||||||||
|
Americas
|
260.9 | 20 | 250.0 | 16 | 252.0 | 14 | ||||||||||||||||||
|
South Asia/Pacific
|
120.1 | 9 | 182.8 | 12 | 236.2 | 14 | ||||||||||||||||||
|
Europe
|
133.6 | 10 | 150.2 | 10 | 162.7 | 9 | ||||||||||||||||||
| $ | 1,331.1 | 100 | % | $ | 1,537.3 | 100 | % | $ | 1,744.0 | 100 | % | |||||||||||||
|
Year Ended December 31,
|
||||||||||||
|
2009
|
2010
|
2011
|
||||||||||
|
Revenue
|
100.0 | % | 100.0 | % | 100.0 | % | ||||||
|
Cost of sales
|
18.3 | 17.7 | 18.5 | |||||||||
|
Gross profit
|
81.7 | 82.3 | 81.5 | |||||||||
|
Operating expenses:
|
||||||||||||
|
Selling expenses
|
41.4 | 42.1 | 43.1 | |||||||||
|
General and administrative expenses
|
28.4 | 26.1 | 25.0 | |||||||||
|
Restructuring charges
|
0.8 | — | — | |||||||||
|
Total operating expenses
|
70.6 | 68.2 | 68.1 | |||||||||
|
Operating income
|
11.1 | 14.1 | 13.4 | |||||||||
|
Other income (expense), net
|
(0.5 | ) | (0.6 | ) | (0.4 | ) | ||||||
|
Income before provision for income taxes
|
10.6 | 13.5 | 13.0 | |||||||||
|
Provision for income taxes
|
3.8 | 4.6 | 4.2 | |||||||||
|
Net income
|
6.8 | % | 8.9 | % | 8.8 | % | ||||||
|
|
Revenue
|
|
2010
|
2011
|
Change
|
||||||||||
|
Japan
|
$ | 471.4 | $ | 472.5 | * | |||||||
|
South Korea
|
214.7 | 278.7 | 30% | |||||||||
|
North Asia total
|
$ | 686.1 | $ | 751.2 | 9% | |||||||
|
2010
|
2011
|
Change
|
||||||||||
|
China
|
$ | 91.4 | $ | 152.5 | 67% | |||||||
|
Taiwan
|
107.1 | 108.9 | 2% | |||||||||
|
Hong Kong
|
69.7 | 80.5 | 15% | |||||||||
|
Greater China total
|
$ | 268.2 | $ | 341.9 | 27% | |||||||
|
2010
|
2011
|
Change
|
||||||||||
|
Americas
|
$ | 250.0 | $ | 252.0 | 1% | |||||||
|
2010
|
2011
|
Change
|
||||||||||
|
South Asia/Pacific
|
$ | 182.8 | $ | 236.2 | 29% | |||||||
|
2010
|
2011
|
Change
|
||||||||||
|
Europe
|
$ | 150.2 | $ | 162.7 | 8% | |||||||
|
|
Revenue
|
|
2009
|
2010
|
Change
|
||||||||||
|
Japan
|
$ | 461.9 | $ | 471.4 | 2% | |||||||
|
South Korea
|
144.2 | 214.7 | 49% | |||||||||
|
North Asia total
|
$ | 606.1 | $ | 686.1 | 13% | |||||||
|
2009
|
2010
|
Change
|
||||||||||
|
Taiwan
|
$ | 91.7 | $ | 107.1 | 17% | |||||||
|
China
|
71.1 | 91.4 | 29% | |||||||||
|
Hong Kong
|
47.6 | 69.7 | 46% | |||||||||
|
Greater China total
|
$ | 210.4 | $ | 268.2 | 27% | |||||||
|
2009
|
2010
|
Change
|
||||||||||
|
Americas
|
$ | 260.9 | $ | 250.0 | (4%) | |||||||
|
2009
|
2010
|
Change
|
||||||||||
|
South Asia/Pacific
|
$ | 120.1 | $ | 182.8 | 52% | |||||||
|
2009
|
2010
|
Change
|
||||||||||
|
Europe
|
$ | 133.6 | $ | 150.2 | 12% | |||||||
|
Facility or
Arrangement
(1)
|
Original Principal Amount
|
Balance as of
December 31, 2011
(2)
|
Interest Rate
|
Repayment terms
|
||||
|
2003 $205.0 million multi-currency uncommitted shelf facility:
|
||||||||
|
U.S. dollar
denominated:
|
$40.0 million
|
$28.6 million
|
6.2%
|
Notes due July 2016 with annual principal payments that began in July 2010.
|
||||
|
$20.0 million
|
$17.1 million
|
6.2%
|
Notes due January 2017 with annual principal payments that began in January 2011.
|
|||||
|
Japanese yen
denominated:
|
3.1 billion yen
|
1.3 billion yen ($17.4 million as of December 31, 2011)
|
1.7%
|
Notes due April 2014 with annual principal payments that began in April 2008.
|
||||
|
2.3 billion yen
|
1.9 billion yen ($25.3 million as of December 31, 2011)
|
2.6%
|
Notes due September 2017 with annual principal payments that began in September 2011.
|
|||||
|
2.2 billion yen
|
1.9 billion yen ($24.2 million as of December 31, 2011)
|
3.3%
|
Notes due January 2017 with annual principal payments that began in January 2011.
|
|||||
|
2010 committed loan:
|
||||||||
|
U.S. dollar
denominated:
|
$30.0 million
|
$24.0 million
|
Variable 30 day: 1.29%
|
Amortizes at $1.5 million per quarter.
|
||||
|
2004 $25.0 million revolving credit facility
|
N/A
|
None
|
N/A
|
|||||
|
2009 $100.0 million uncommitted multi-currency shelf facility
|
N/A
|
None
|
N/A
|
|
(1)
|
Each of the credit facilities and arrangements listed in the table are secured by guarantees issued by our material domestic subsidiaries and by pledges of 65% of the outstanding stock of our material foreign subsidiaries. The 2010 committed loan is also secured by deeds of trust with respect to our corporate headquarters and distribution center in Provo, Utah.
|
|
(2)
|
The current portion of our long-term debt (i.e. becoming due in the next 12 months) includes $14.0 million of the balance of our Japanese yen-denominated debt under the 2003 multi-currency uncommitted shelf facility, $8.6 million of the balance on our U.S. dollar denominated debt under the 2003 multi-currency uncommitted shelf facility and $6.0 million of our 2010 committed loan.
|
|
Total
|
2012
|
2013-2014 | 2015-2016 |
Thereafter
|
||||||||||||||||
|
Long-term debt obligations
|
$ | 136,552 | $ | 28,608 | $ | 63,216 | $ | 33,628 | $ | 11,100 | ||||||||||
|
Operating lease obligations
|
63,247 | 16,006 | 26,460 | 19,879 | 902 | |||||||||||||||
|
Purchase obligations
(1)
|
161,868 | 100,905 | 27,917 | 25,023 | 8,023 | |||||||||||||||
|
Other long-term liabilities reflected
on the balance sheet
(2)
|
— | — | — | — | — | |||||||||||||||
|
Total
|
$ | 361,667 | $ | 145,519 | $ | 117,593 | $ | 78,530 | $ | 20,025 | ||||||||||
|
(1)
|
The amounts reported under purchase obligations do not include anticipated expenditures related to ongoing construction projects at our corporate headquarters in Provo, Utah and our Greater China regional headquarters in Shanghai, China. We currently anticipate the Provo and Shanghai facilities will cost approximately $90 million and $55 million, respectively, and anticipate that both facilities will be completed in 2013.
|
|
(2)
|
Other long-term liabilities reflected on the balance sheet of $67.6 million primarily consisting of long-term tax related balances, in which the timing of the commitments is uncertain.
|
|
As of December 31, 2009
|
As of December 31, 2010
|
As of December 31, 2011
|
||||||||||
|
Active
|
Executive
|
Active
|
Executive
|
Active
|
Executive
|
|||||||
|
North Asia
|
319,000
|
14,144
|
329,000
|
14,687
|
338,000
|
15,293
|
||||||
|
Greater China
|
106,000
|
6,938
|
118,000
|
8,015
|
143,000
|
11,808
|
||||||
|
Americas
|
171,000
|
5,522
|
161,000
|
5,305
|
166,000
|
5,356
|
||||||
|
South Asia/Pacific
|
71,000
|
2,950
|
84,000
|
3,930
|
99,000
|
5,619
|
||||||
|
Europe
|
94,000
|
3,385
|
107,000
|
3,739
|
109,000
|
3,740
|
||||||
|
Total
|
761,000
|
32,939
|
799,000
|
35,676
|
855,000
|
41,816
|
||||||
|
2010
|
2011
|
|||||||||||||||||||||||||||||||
|
1
st
Quarter
|
2
nd
Quarter
|
3
rd
Quarter
|
4
th
Quarter
|
1
st
Quarter
|
2
nd
Quarter
|
3
rd
Quarter
|
4
th
Quarter
|
|||||||||||||||||||||||||
|
Revenue
|
$ | 364.1 | $ | 388.4 | $ | 383.6 | $ | 401.2 | $ | 395.8 | $ | 424.4 | $ | 428.4 | $ | 495.3 | ||||||||||||||||
|
Gross profit
|
299.3 | 320.4 | 314.8 | 330.3 | 295.2 | 353.3 | 357.8 | 415.1 | ||||||||||||||||||||||||
|
Operating income
|
46.1 | 59.2 | 52.9 | 58.9 | 24.9 | 66.0 | 67.2 | 75.6 | ||||||||||||||||||||||||
|
Net income
|
31.0 | 32.4 | 35.3 | 37.3 | 15.3 | 41.7 | 46.8 | 49.5 | ||||||||||||||||||||||||
|
Net income per share:
|
||||||||||||||||||||||||||||||||
|
Basic
|
0.50 | 0.51 | 0.57 | 0.60 | 0.25 | 0.67 | 0.75 | 0.80 | ||||||||||||||||||||||||
|
Diluted
|
0.48 | 0.50 | 0.55 | 0.58 | 0.24 | 0.65 | 0.72 | 0.76 | ||||||||||||||||||||||||
|
2010
|
2011 | |||||||||||||||||||||||||||||||
|
1
st
Quarter
|
2
nd
Quarter
|
3
rd
Quarter
|
4
th
Quarter
|
1
st
Quarter
|
2
nd
Quarter
|
3
rd
Quarter
|
4
th
Quarter
|
|||||||||||||||||||||||||
|
Japan
(1)
|
90.6 | 92.0 | 85.7 | 82.6 | 82.3 | 81.5 | 77.7 | 77.3 | ||||||||||||||||||||||||
|
Taiwan
|
31.9 | 31.8 | 31.9 | 30.3 | 29.3 | 28.9 | 29.1 | 30.3 | ||||||||||||||||||||||||
|
Hong Kong
|
7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | ||||||||||||||||||||||||
|
South Korea
|
1,142.0 | 1,163.2 | 1,182.3 | 1,133.5 | 1,119.4 | 1,083.0 | 1,083.4 | 1,146.3 | ||||||||||||||||||||||||
|
Malaysia
|
3.4 | 3.2 | 3.2 | 3.1 | 3.0 | 3.0 | 3.0 | 3.2 | ||||||||||||||||||||||||
|
Thailand
|
32.9 | 32.4 | 31.6 | 30.0 | 30.5 | 30.3 | 30.1 | 31.0 | ||||||||||||||||||||||||
|
China
|
6.8 | 6.8 | 6.8 | 6.7 | 6.6 | 6.5 | 6.4 | 6.4 | ||||||||||||||||||||||||
|
Singapore
|
1.4 | 1.4 | 1.4 | 1.3 | 1.3 | 1.2 | 1.2 | 1.3 | ||||||||||||||||||||||||
|
Canada
|
1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||||||||||||||
|
(1)
|
As of February 1, 2012, the exchange rate of U.S. $1 into the Japanese yen was approximately 76.20
|
|
Year Ended
December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
Revenue as reported
|
$ | 1,537,259 | $ | 1,743,991 | ||||
|
GAAP gross profit as reported
|
$ | 1,264,828 | $ | 1,421,367 | ||||
|
Japan customs expense
|
– |
32,754
|
||||||
|
Gross profit excluding Japan customs expense
|
$ | 1,264,828 | $ | 1,454,121 | ||||
|
Gross profit as a percent of revenue as reported
|
82.3 | % | 81.5 | % | ||||
|
Gross profit as a percent of revenue excluding Japan customs
expense
|
82.3 | % | 83.4 | % | ||||
|
Year Ended
December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
Net income as reported
|
$ | 136,051 | $ | 153,330 | ||||
|
Japan customs expense
|
– |
32,754
|
||||||
|
Tax effect of Japan customs expense
|
– |
(12,275
|
) | |||||
|
Net income excluding Japan customs expense
|
$ | 136,051 | $ | 173,809 | ||||
|
Diluted earnings per share as reported
|
$ | 2.11 | $ | 2.38 | ||||
|
Diluted earnings per share, excluding Japan customs expense
|
$ | 2.11 | $ | 2.69 | ||||
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
1.
|
Financial Statements
. Set forth below is the index to the Financial Statements included in
this Item 8:
|
|
Page
|
||
|
Consolidated Balance Sheets at December 31, 2010 and 2011
|
71
|
|
|
Consolidated Statements of Income for the years ended December 31, 2009, 2010 and 2011
|
72
|
|
|
Consolidated Statements of Stockholders’ Equity and Comprehensive Income for the years ended December 31, 2009, 2010 and 2011
|
73
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2010 and 2011
|
74
|
|
|
Notes to Consolidated Financial Statements
|
75
|
|
|
Report of Independent Registered Public Accounting Firm
|
102
|
|
|
2.
|
Financial Statement Schedules
: Financial statement schedules have been omitted because they are not required or are not applicable, or because the required information is shown in the financial statements or notes thereto.
|
|
December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 230,337 | $ | 272,974 | ||||
|
Current investments
|
─
|
17,727 | ||||||
|
Accounts receivable
|
25,701 | 31,615 | ||||||
|
Inventories, net
|
114,475 | 112,111 | ||||||
|
Prepaid expenses and other
|
52,013 | 95,660 | ||||||
| 422,526 | 530,087 | |||||||
|
Property and equipment, net
|
133,722 | 149,505 | ||||||
|
Goodwill
|
112,446 | 112,446 | ||||||
|
Other intangible assets, net
|
78,270 | 83,333 | ||||||
|
Other assets
|
145,260 | 115,585 | ||||||
|
Total assets
|
$ | 892,224 | $ | 990,956 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$ | 25,480 | $ | 32,181 | ||||
|
Accrued expenses
|
146,108 | 180,382 | ||||||
|
Current portion of long-term debt
|
27,865 | 28,608 | ||||||
|
Related party payable
|
16,995 |
─
|
||||||
|
|
216,448 | 241,171 | ||||||
|
Long-term debt
|
133,013 | 107,944 | ||||||
|
Other liabilities
|
71,514 | 67,605 | ||||||
|
Total liabilities
|
420,975 | 416,720 | ||||||
|
Commitments and contingencies (Notes 10 and 21)
|
||||||||
|
Stockholders’ equity
|
||||||||
|
Class A common stock – 500 million shares authorized,
$.001 par value, 90.6 million shares issued
|
91 | 91 | ||||||
|
Additional paid-in capital
|
256,505 | 292,240 | ||||||
|
Treasury stock, at cost – 28.5 and 28.3 million shares
|
(476,748 | ) | (522,162 | ) | ||||
|
Accumulated other comprehensive loss
|
(58,539 | ) | (62,565 | ) | ||||
|
Retained earnings
|
749,940 | 866,632 | ||||||
| 471,249 | 574,236 | |||||||
|
Total liabilities and stockholders’ equity
|
$ | 892,224 | $ | 990,956 | ||||
|
Year Ended December 31,
|
|||||||||||||
|
2009
|
2010
|
2011
|
|||||||||||
|
Revenue
|
$ | 1,331,058 | $ | 1,537,259 | $ | 1,743,991 | |||||||
|
Cost of sales
|
243,648 | 272,431 | 322,624 | ||||||||||
|
Gross profit
|
1,087,410 | 1,264,828 | 1,421,367 | ||||||||||
|
Operating expenses:
|
|||||||||||||
|
Selling expenses
|
559,605 | 646,348 | 751,448 | ||||||||||
|
General and administrative expenses
|
369,368 | 401,418 | 436,177 | ||||||||||
|
Restructuring charges
|
10,724 | — | — | ||||||||||
|
Total operating expenses
|
939,697 | 1,047,766 | 1,187,625 | ||||||||||
|
Operating income
|
147,713 | 217,062 | 233,742 | ||||||||||
|
Other income (expense), net (Note 25)
|
(6,589 | ) | (9,449 | ) | (6,973 | ) | |||||||
|
Income before provision for income taxes
|
141,124 | 207,613 | 226,769 | ||||||||||
|
Provision for income taxes
|
51,279 | 71,562 | 73,439 | ||||||||||
|
Net income
|
$ | 89,845 | $ | 136,051 | $ | 153,330 | |||||||
|
Net income per share:
|
|||||||||||||
|
Basic
|
$ | 1.42 | $ | 2.18 | $ | 2.47 | |||||||
|
Diluted
|
$ | 1.40 | $ | 2.11 | $ | 2.38 | |||||||
|
Weighted-average common shares outstanding (000s):
|
|||||||||||||
|
Basic
|
63,333 | 62,370 | 62,066 | ||||||||||
|
Diluted
|
64,296 | 64,547 | 64,546 | ||||||||||
|
Class A Common Stock
|
Additional
Paid-in Capital
|
Treasury Stock
|
Accumulated Other Comprehensive Loss
|
Retained Earnings
|
Total
|
|||||||||||||||||||
|
Balance at January 1, 2009
|
$ | 91 | $ | 218,928 | $ | (417,017 | ) | $ | (70,061 | ) | $ | 584,239 | $ | 316,180 | ||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||
|
Net income
|
— | — | — | — | 89,845 | 89,845 | ||||||||||||||||||
|
Foreign currency translation adjustment
|
— | — | — | 1,830 | — | 1,830 | ||||||||||||||||||
|
Net unrealized gains on foreign currency
cash flow hedges
|
— | — | — | 97 | — | 97 | ||||||||||||||||||
|
Total comprehensive income
|
91,772 | |||||||||||||||||||||||
|
Repurchase of Class A common stock (Note 11)
|
— | — | (21,144 | ) | — | — | (21,144 | ) | ||||||||||||||||
|
Exercise of employee stock options (0.6 million
shares)/vesting
of stock awards
|
— | 1,633 | 4,594 | — | — | 6,227 | ||||||||||||||||||
|
Excess tax benefit from equity awards
|
— | 1,669 | — | — | — | 1,669 | ||||||||||||||||||
|
Stock-based compensation
|
— | 9,989 | — | — | — | 9,989 | ||||||||||||||||||
|
Cash dividends
|
— | — | — | — | (29,006 | ) | (29,006 | ) | ||||||||||||||||
|
Balance at December 31, 2009
|
91 | 232,219 | (433,567 | ) | (68,134 | ) | 645,078 | 375,687 | ||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||
|
Net income
|
— | — | — | — | 136,051 | 136,051 | ||||||||||||||||||
|
Foreign currency translation adjustment
|
— | — | — | 9,661 | — | 9,661 | ||||||||||||||||||
|
Net unrealized gains on foreign currency
cash flow hedges
|
— | — | — | 60 | — | 60 | ||||||||||||||||||
|
Less: reclassification adjustment for realized
gains in
current
earnings
|
— | — | — | (126 | ) | — | (126 | ) | ||||||||||||||||
|
Total comprehensive income
|
145,646 | |||||||||||||||||||||||
|
Repurchase of Class A common stock (Note 11)
|
— | — | (58,516 | ) | — | — | (58,516 | ) | ||||||||||||||||
|
Reclassification of treasury shares held
by subsidiary
|
— | 3,122 | (3,122 | ) | — | — | — | |||||||||||||||||
|
Exercise of employee stock options (1.5 million
shares)/vesting
of stock awards
|
— | 2,724 | 18,457 | — | — | 21,181 | ||||||||||||||||||
|
Excess tax benefit from equity awards
|
— | 7,605 | — | — | — | 7,605 | ||||||||||||||||||
|
Stock-based compensation
|
— | 10,835 | — | — | — | 10,835 | ||||||||||||||||||
|
Cash dividends
|
— | — | — | — | (31,189 | ) | (31,189 | ) | ||||||||||||||||
|
Balance at December 31, 2010
|
91 | 256,505 | (476,748 | ) | (58,539 | ) | 749,940 | 471,249 | ||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||
|
Net income
|
— | — | — | — | 153,330 | 153,330 | ||||||||||||||||||
|
Foreign currency translation adjustment
|
— | — | — | (2,985 | ) | — | (2,985 | ) | ||||||||||||||||
|
Net unrealized losses on foreign currency
cash flow hedges
|
— | — | — | (1,954 | ) | — | (1,954 | ) | ||||||||||||||||
|
Less: reclassification adjustment for realized
gains in
current earnings
|
— | — | — | 913 | — | 913 | ||||||||||||||||||
|
Total comprehensive income
|
149,304 | |||||||||||||||||||||||
|
Repurchase of Class A common stock (Note 11)
|
— | — | (67,149 | ) | — | — | (67,149 | ) | ||||||||||||||||
|
Exercise of employee stock options (2.1 million
shares)/vesting
of stock awards
|
— | 7,978 | 21,735 | — | — | 29,713 | ||||||||||||||||||
|
Excess tax benefit from equity awards
|
— | 12,657 | — | — | — | 12,657 | ||||||||||||||||||
|
Stock-based compensation
|
— | 15,100 | — | — | — | 15,100 | ||||||||||||||||||
|
Cash dividends
|
— | — | — | — | (36,638 | ) | (36,638 | ) | ||||||||||||||||
|
Balance at December 31, 2011
|
$ | 91 | $ | 292,240 | $ | (522,162 | ) | $ | (62,565 | ) | $ | 866,632 | $ | 574,236 | ||||||||||
|
Year Ended December 31,
|
||||||||||||
|
2009
|
2010
|
2011
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net income
|
$ | 89,845 | $ | 136,051 | $ | 153,330 | ||||||
|
Adjustments to reconcile net income to net cash provided
by operating activities:
|
||||||||||||
|
Depreciation and amortization
|
28,557 | 29,616 | 32,850 | |||||||||
|
Japan customs expense
|
— | — | 32,754 | |||||||||
|
Foreign currency (gains)/losses
|
(1,966 | ) | 3,681 | 4,162 | ||||||||
|
Stock-based compensation
|
9,989 | 10,835 | 15,450 | |||||||||
|
Deferred taxes
|
12,350 | (13,735 | ) | 108 | ||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
(7,043 | ) | (6,649 | ) | (5,890 | ) | ||||||
|
Inventories, net
|
9,740 | (4,293 | ) | 2,415 | ||||||||
|
Prepaid expenses and other
|
(3,850 | ) | 3,854 | (4,690 | ) | |||||||
|
Other assets
|
(18,690 | ) | (1,631 | ) | (16,809 | ) | ||||||
|
Accounts payable
|
3,602 | (568 | ) | 6,077 | ||||||||
|
Accrued expenses
|
8,598 | 13,777 | 1,624 | |||||||||
|
Other liabilities
|
2,812 | 16,945 | 2,934 | |||||||||
|
Net cash provided by operating activities
|
133,944 | 187,883 | 224,315 | |||||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchase of property and equipment
|
(20,215 | ) | (53,783 | ) | (41,809 | ) | ||||||
|
Proceeds on investment sales
|
— | — | 6,634 | |||||||||
|
Purchases of investments
|
— | — | (24,361 | ) | ||||||||
|
Acquisition of LifeGen (Note 22)
|
— | — | (11,663 | ) | ||||||||
|
Net cash used in investing activities
|
(20,215 | ) | (53,783 | ) | (71,199 | ) | ||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Payment of cash dividends
|
(29,006 | ) | (31,189 | ) | (36,638 | ) | ||||||
|
Repurchase of shares of common stock
|
(21,144 | ) | (58,516 | ) | (67,149 | ) | ||||||
|
Exercise of distributor and employee stock options
|
6,227 | 21,181 | 29,713 | |||||||||
|
Income tax benefit of options exercised
|
1,101 | 6,908 | 12,059 | |||||||||
|
Payments on long-term debt
|
(30,188 | ) | (37,401 | ) | (28,001 | ) | ||||||
|
Related party payment
|
— | — | (16,995 | ) | ||||||||
|
Proceeds from long-term debt
|
— | 30,000 | — | |||||||||
|
Net cash used in financing activities
|
(73,010 | ) | (69,017 | ) | (107,011 | ) | ||||||
|
Effect of exchange rate changes on cash
|
2,740 | 7,209 | (3,468 | ) | ||||||||
|
Net increase in cash and cash equivalents
|
43,459 | 72,292 | 42,637 | |||||||||
|
Cash and cash equivalents, beginning of period
|
114,586 | 158,045 | 230,337 | |||||||||
|
Cash and cash equivalents, end of period
|
$ | 158,045 | $ | 230,337 | $ | 272,974 | ||||||
|
December 31,
|
|||||||||
|
2010
|
2011 | ||||||||
|
Raw materials
|
$ | 31,497 | $ | 24,668 | |||||
|
Finished goods
|
82,978 | 87,443 | |||||||
| $ | 114,475 | $ | 112,111 | ||||||
|
Buildings
|
39 years
|
|||
|
Furniture and fixtures
|
5 - 7 years
|
|||
|
Computers and equipment
|
3 - 5 years
|
|||
|
Leasehold improvements
|
Shorter of estimated useful life or lease term
|
|||
|
Scanners
|
3 years
|
|||
|
Vehicles
|
3 - 5 years
|
|
Gross Balance at January 1, 2009
|
$ | 30,915 | ||
|
Increases related to prior year tax positions
|
2 | |||
|
Increases related to current year tax positions
|
3,618 | |||
|
Settlements
|
(946 | ) | ||
|
Decreases due to lapse of statutes of limitations
|
(4,858 | ) | ||
|
Currency adjustments
|
(456 | ) | ||
|
Gross Balance at December 31, 2009
|
$ | 28,275 | ||
|
Gross Balance at January 1, 2010
|
$ | 28,275 | ||
|
Decreases related to prior year tax positions
|
(1,206 | ) | ||
|
Increases related to current year tax positions
|
2,236 | |||
|
Settlements
|
─
|
|||
|
Decreases due to lapse of statutes of limitations
|
(15,395 | ) | ||
|
Currency adjustments
|
911 | |||
|
Gross Balance at December 31, 2010
|
$ | 14,821 | ||
|
Gross Balance at January 1, 2011
|
$ | 14,821 | ||
|
Decreases related to prior year tax positions
|
(7,138 | ) | ||
|
Increases related to current year tax positions
|
1,415 | |||
|
Settlements
|
(499 | ) | ||
|
Decreases due to lapse of statutes of limitations
|
(1,255 | ) | ||
|
Currency adjustments
|
43 | |||
|
Gross Balance at December 31, 2011
|
$ | 7,387 | ||
|
December 31,
|
|||||||||
|
2010
|
2011 | ||||||||
|
Deferred tax assets
|
$ | 26,094 | $ | 32,867 | |||||
|
Prepaid income taxes
|
─
|
30,223 | |||||||
|
Prepaid inventory
|
7,799 | 12,232 | |||||||
|
Prepaid rent and insurance
|
4,005 | 4,001 | |||||||
|
Prepaid other taxes and duties
|
2,727 | 2,406 | |||||||
|
Deposits
|
5,320 | 4,240 | |||||||
|
Other
|
6,068
|
9,691 |
|
||||||
| $ | 52,013 | $ | 95,660 | ||||||
|
December 31,
|
|||||||||
|
2010
|
2011 |
|
|||||||
|
Land
|
$ | 16,480 | $ | 19,561 | |||||
|
Buildings
|
34,293 | 41,495 | |||||||
|
Construction in progress
|
8,070 | 14,286 | |||||||
|
Furniture and fixtures
|
46,799 | 48,071 | |||||||
|
Computers and equipment
|
87,653 | 92,336 | |||||||
|
Leasehold improvements
|
55,526 | 60,120 | |||||||
|
Scanners
|
18,803 | 15,741 | |||||||
|
Vehicles
|
2,222
|
2,153 |
|
||||||
| 269,846 | 293,763 | ||||||||
|
Less: accumulated depreciation
|
(136,124 | ) | (144,258 | ) | |||||
| $ | 133,722 | $ | 149,505 | ||||||
|
Carrying Amount at
December 31,
|
||||||||
|
Goodwill and indefinite life intangible assets:
|
2010
|
2011
|
||||||
|
Goodwill
|
$ | 112,446 | $ | 112,446 | ||||
|
Trademarks and trade names
|
24,599 | 24,599 | ||||||
| $ | 137,045 | $ | 137,045 | |||||
|
December 31, 2010
|
December 31, 2011
|
||||||||||||||||
|
Finite life intangible assets:
|
Gross Carrying Amount
|
Accumulated Amortization
|
Gross Carrying Amount
|
Accumulated Amortization
|
Weighted-average Amortization Period
|
||||||||||||
|
Scanner technology
|
$ | 46,482 | $ | 18,423 | $ | 46,482 | $ | 21,457 |
18 years
|
||||||||
|
Developed technology
|
22,500 | 13,436 | 22,500 | 14,261 |
20 years
|
||||||||||||
|
Distributor network
|
11,598 | 8,587 | 11,598 | 9,089 |
15 years
|
||||||||||||
|
Trademarks
|
13,323 | 9,524 | 13,401 | 10,214 |
15 years
|
||||||||||||
|
Other
|
32,989 | 23,251 | 46,652 | 26,878 |
8 years
|
||||||||||||
| $ | 126,892 | $ | 73,221 | $ | 140,633 | $ | 81,899 |
15 years
|
|||||||||
|
December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
Deferred taxes
|
$ | 45,027 | $ | 29,661 | ||||
|
Deposits for noncancelable operating leases
|
14,261 | 15,559 | ||||||
|
Deposit for customs assessment (Note 21)
|
65,255 | 50,719 | ||||||
|
Other
|
20,717 | 19,646 | ||||||
| $ | 145,260 | $ | 115,585 | |||||
|
December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
Accrued commissions and other payments to distributors
|
$ | 66,335 | $ | 68,925 | ||||
|
Other taxes payable
|
15,948 | 12,628 | ||||||
|
Accrued payroll and payroll taxes
|
13,063 | 18,039 | ||||||
|
Accrued payable to vendors
|
9,744 | 12,752 | ||||||
|
Deferred revenue
|
2,730 | 22,007 | ||||||
|
Other accrued employee expenses
|
19,704 | 18,588 | ||||||
|
Other
|
18,584 | 27,443 | ||||||
| $ | 146,108 | $ | 180,382 | |||||
|
Facility or
Arrangement
(1)
|
Original Principal Amount
|
Balance as of
December 31, 2010
|
Balance as of
December 31, 2011
(2)
|
Interest Rate
|
Repayment terms
|
|||||
|
2003 $205.0 million multi-currency uncommitted shelf facility:
|
||||||||||
|
U.S. dollar
denominated:
|
$40.0 million
|
$34.3 million
|
$28.6 million
|
6.2%
|
Notes due July 2016 with annual principal payments that began in July 2010.
|
|||||
|
$20.0 million
|
$20.0 million
|
$17.1 million
|
6.2%
|
Notes due January 2017 with annual principal payments that began in January 2011.
|
||||||
|
Japanese yen
denominated:
|
3.1 billion yen
|
1.8 billion yen ($22.0 million as of December 31, 2010)
|
1.3 billion yen ($17.4 million as of December 31, 2011)
|
1.7%
|
Notes due April 2014 with annual principal payments that began in April 2008.
|
|||||
|
2.3 billion yen
|
2.3 billion yen ($27.9 million as of December 31, 2010)
|
1.9 billion yen ($25.3 million as of December 31, 2011)
|
2.6%
|
Notes due September 2017 with annual principal payments that began in September 2011.
|
||||||
|
2.2 billion yen
|
2.2 billion yen ($26.7 million as of December 31, 2010)
|
1.9 billion yen ($24.2 million as of December 31, 2011)
|
3.3%
|
Notes due January 2017 with annual principal payments that began in January 2011.
|
||||||
|
2010 committed loan:
|
||||||||||
|
U.S. dollar
denominated:
|
$30.0 million
|
$30.0 million
|
$24.0 million
|
Variable 30 day: 1.29%
|
Amortizes at $1.5 million per quarter.
|
|||||
|
2004 $25.0 million revolving credit facility
|
N/A
|
None
|
None
|
N/A
|
||||||
|
2009 $100.0 million uncommitted multi-currency shelf facility
|
N/A
|
None
|
None
|
N/A
|
|
(1)
|
Each of the credit facilities and arrangements listed in the table are secured by guarantees issued by the Company's domestic subsidiaries and by pledges of 65% of the outstanding stock of its material foreign subsidiaries. The 2010 committed loan is also secured by deeds of trust with respect to the Company’s corporate headquarters and distribution center in Provo, Utah.
|
|
(2)
|
The current portion of the Company’s long-term debt (i.e. becoming due in the next 12 months) includes $14.0 million of the balance of its Japanese yen-denominated debt under the 2003 multi-currency uncommitted shelf facility, $8.6 million of the balance on its U.S. dollar denominated debt under the 2003 multi-currency uncommitted shelf facility and $6.0 million of its 2010 committed loan.
|
|
Year Ending December 31,
|
||||
|
2012
|
$ | 28,608 | ||
|
2013
|
40,608 | |||
|
2014
|
22,608 | |||
|
2015
|
16,814 | |||
|
2016
|
16,814 | |||
|
Thereafter
|
11,100 | |||
|
Total
|
$ | 136,552 | ||
|
Year Ending December 31,
|
||||
|
2012
|
$ | 16,006 | ||
|
2013
|
14,548 | |||
|
2014
|
11,912 | |||
|
2015
|
10,339 | |||
|
2016
|
9,540 | |||
|
Thereafter
|
902 | |||
|
Total
|
$ | 63,247 | ||
|
Year Ended December 31,
|
|||||||||||||
|
2009
|
2010
|
2011 |
|
||||||||||
|
Basic weighted-average common shares outstanding
|
63,333 | 62,370 | 62,066 | ||||||||||
|
Effect of dilutive securities:
Stock awards and options
|
963 | 2,177 | 2,480 | ||||||||||
|
Diluted weighted-average common shares outstanding
|
64,296 | 64,547 | 64,546 | ||||||||||
|
December 31,
|
||||||||||||
|
Stock Options
:
|
2009
|
2010
|
2011
|
|||||||||
|
Weighted average grant date fair value of grants
|
$ | 2.84 | $ | 8.61 | $ | 9.98 | ||||||
|
Risk-free interest rate
(1)
|
2.3 | % | 1.8 | % | 1.8 | % | ||||||
|
Dividend yield
(2)
|
3.2 | % | 2.6 | % | 2.6 | % | ||||||
|
Expected volatility
(3)
|
40.7 | % | 37.8 | % | 38.4 | % | ||||||
|
Expected life in months
(4)
|
69 months
|
69 months
|
63 months
|
|||||||||
|
(1)
|
The risk-free interest rate is based upon the rate on a zero coupon U.S. Treasury bill, for periods within the contractual life of the option, in effect at the time of the grant.
|
|
(2)
|
The dividend yield is based on the average of historical stock prices and actual dividends paid.
|
|
(3)
|
Expected volatility is based on the historical volatility of our stock price, over a period similar to the expected life of the option.
|
|
(4)
|
The expected term of the option is based on the historical employee exercise behavior, the vesting terms of the respective option, and a contractual life of either seven or ten years.
|
|
Shares
(in thousands)
|
Weighted-average Exercise Price
|
Weighted- average Remaining Contractual Term
(in years)
|
Aggregate Intrinsic Value
(in thousands)
|
|||||||||||||
|
Options activity – service based
|
||||||||||||||||
|
Outstanding at December 31, 2010
|
4,483.9 | $ | 15.82 | |||||||||||||
|
Granted
|
187.5 | 36.10 | ||||||||||||||
|
Exercised
|
(1,149.2 | ) | 16.57 | |||||||||||||
|
Forfeited/cancelled/expired
|
(16.7 | ) | 12.32 | |||||||||||||
|
Outstanding at December 31, 2011
|
3,505.5 | 16.68 | 3.65 | $ | 111,793 | |||||||||||
|
Exercisable at December 31, 2011
|
2,311.4 | 17.02 | 3.10 | 72,930 | ||||||||||||
|
Options activity – performance based
|
||||||||||||||||
|
Outstanding at December 31, 2010
|
3,586.5 | $ | 24.90 | |||||||||||||
|
Granted
|
57.5 | 31.92 | ||||||||||||||
|
Exercised
|
(790.0 | ) | 17.14 | |||||||||||||
|
Forfeited/cancelled/expired
|
(100.0 | ) | 23.72 | |||||||||||||
|
Outstanding at December 31, 2011
|
2,754.0 | 27.32 | 5.20 | $ | 58,532 | |||||||||||
|
Exercisable at December 31, 2011
|
639.4 | 17.25 | 3.01 | 20,027 | ||||||||||||
|
Options activity – all options
|
||||||||||||||||
|
Outstanding at December 31, 2010
|
8,070.4 | $ | 19.86 | |||||||||||||
|
Granted
|
245.0 | 35.12 | ||||||||||||||
|
Exercised
|
(1,939.2 | ) | 16.80 | |||||||||||||
|
Forfeited/cancelled/expired
|
(116.7 | ) | 22.09 | |||||||||||||
|
Outstanding at December 31, 2011
|
6,259.5 | 21.36 | 4.33 | $ | 170,325 | |||||||||||
|
Exercisable at December 31, 2011
|
2,950.8 | 17.07 | 3.09 | 92,957 | ||||||||||||
|
December 31,
|
||||||||||||
|
2009
|
2010
|
2011
|
||||||||||
|
Cash proceeds from stock options exercised
|
$ | 6.2 | $ | 21.2 | $ | 29.7 | ||||||
|
Tax benefit realized for stock options exercised
|
2.9 | 10.3 | 17.4 | |||||||||
|
Intrinsic value of stock options exercised
|
8.2 | 25.4 | 61.6 | |||||||||
|
Number of Shares
(in thousands)
|
Weighted-average Grant Date Fair Value
|
|||||||
|
Nonvested at December 31, 2010
|
530.3 | $ | 22.88 | |||||
|
Granted
|
334.0 | 32.10 | ||||||
|
Vested
|
(185.5 | ) | 21.49 | |||||
|
Forfeited
|
(15.1 | ) | 25.89 | |||||
|
Nonvested at December 31, 2011
|
663.7 | 27.84 | ||||||
|
Fair Value at December 31, 2010
|
||||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Financial assets (liabilities):
|
||||||||||||||||
|
Cash equivalents
|
$ | 41,101 | $ |
─
|
$ |
─
|
$ | 41,101 | ||||||||
|
Forward contracts
|
─
|
45 |
─
|
45 | ||||||||||||
|
Insurance company contracts
|
─
|
─
|
12,967 | 12,967 | ||||||||||||
|
Total
|
$ | 41,101 | $ | 45 | $ | 12,967 | $ | 54.113 | ||||||||
|
Fair Value at December 31, 2011
|
||||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Financial assets (liabilities):
|
||||||||||||||||
|
Cash equivalents
|
$ | 15,733 | $ |
─
|
$ |
─
|
$ | 15,733 | ||||||||
|
Forward contracts
|
─
|
(1,580 | ) |
─
|
(1,580 | ) | ||||||||||
|
Insurance company contracts
|
─
|
─
|
14,925 | 14,925 | ||||||||||||
|
Total
|
$ | 15,733 | $ | (1,580 | ) | $ | 14,925 | $ | 29,078 | |||||||
|
Insurance
Company Contracts
|
||||
|
Beginning balance at December 31, 2009
|
$ | 10,574 | ||
|
Actual return on plan assets:
|
||||
|
Relating to assets still held at the reporting date
|
1,090 | |||
|
Purchases and issuances
|
2,197 | |||
|
Sales and settlements
|
(894 | ) | ||
|
Transfers into Level III
|
─
|
|||
|
Ending balance at December 31, 2010
|
$ | 12,967 | ||
|
Actual return on plan assets:
|
||||
|
Relating to assets still held at the reporting date
|
(365 | ) | ||
|
Purchases and issuances
|
2,883 | |||
|
Sales and settlements
|
(560 | ) | ||
|
Transfers into Level III
|
─
|
|||
|
Ending balance at December 31, 2011
|
$ | 14,925 | ||
|
2009
|
2010 |
2011
|
||||||||||
|
U.S.
|
$ | 71,338 | $ | 141,069 | $ | 142,929 | ||||||
|
Foreign
|
69,786 | 66,544 | 83,840 | |||||||||
|
Total
|
$ | 141,124 | $ | 207,613 | $ | 226,769 | ||||||
|
2009
|
2010
|
2011
|
||||||||||
|
Current
|
||||||||||||
|
Federal
|
$ | 9,409 | $ | 45,761 | $ | 14,723 | ||||||
|
State
|
1,690 | 3,825 | 2,245 | |||||||||
|
Foreign
|
27,784 | 27,450 | 56,973 | |||||||||
| 38,883 | 77,036 | 73,941 | ||||||||||
|
Deferred
|
||||||||||||
|
Federal
|
14,266 | (2,558 | ) | 17,756 | ||||||||
|
State
|
937 | 212 | 582 | |||||||||
|
Foreign
|
(2,807 | ) | (3,128 | ) | (18,840 | ) | ||||||
| 12,396 | (5,474 | ) | (502 | ) | ||||||||
|
Provision for income taxes
|
$ | 51,279 | $ | 71,562 | $ | 73,439 | ||||||
|
Year Ended December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Inventory differences
|
$ | 5,572 | $ | 3,796 | ||||
|
Foreign tax credit and other foreign benefits
|
25,408 | 25,149 | ||||||
|
Stock-based compensation
|
9,632 | 9,674 | ||||||
|
Accrued expenses not deductible until paid
|
28,325 | 37,992 | ||||||
|
Foreign currency exchange
|
17,727 | 16,927 | ||||||
|
Net operating losses
|
12,481 | 11,656 | ||||||
|
Capitalized research and development
|
18,295 | 14,746 | ||||||
|
Asian marketing rights
|
483 |
─
|
||||||
|
Other
|
7,023 | 568 | ||||||
|
Gross deferred tax assets
|
124,946 | 120,508 | ||||||
|
Deferred tax liabilities:
|
||||||||
|
Exchange gains and losses
|
4,763 | 3,300 | ||||||
|
Pharmanex intangibles step-up
|
12,923 | 12,179 | ||||||
|
Amortization of intangibles
|
10,193 | 14,457 | ||||||
|
Foreign outside basis in controlled foreign corporation
|
10,683 | 16,081 | ||||||
|
Prepaid expenses
|
11,239 |
─
|
||||||
|
Other
|
3,921 | 11,431 | ||||||
|
Gross deferred tax liabilities
|
53,722 | 57,448 | ||||||
|
Valuation allowance
|
(11,351 | ) | (11,611 | ) | ||||
|
Deferred taxes, net
|
$ | 59,873 | $ | 51,449 | ||||
|
Year Ended December 31,
|
||||||||
|
2010
|
2011
|
|||||||
|
Net current deferred tax assets
|
$ | 26,094 | $ | 32,867 | ||||
|
Net noncurrent deferred tax assets
|
45,027 | 29,661 | ||||||
|
Total net deferred tax assets
|
71,121 | 62,528 | ||||||
|
Net current deferred tax liabilities
|
8 | 7 | ||||||
|
Net noncurrent deferred tax liabilities
|
11,240 | 11,072 | ||||||
|
Total net deferred tax liabilities
|
11,248 | 11,079 | ||||||
|
Deferred taxes, net
|
$ | 59,873 | $ | 51,449 | ||||
|
Year Ended December 31,
|
|||||||||||||
|
2009
|
2010
|
2011 |
|
||||||||||
|
Income taxes at statutory rate
|
35.00 | % | 35.00 | % | 35.00 | % | |||||||
|
Non-deductible expenses
|
.24 | .10 | .16 | ||||||||||
|
Extraterritorial income tax credit
|
.00 | .00 | (3.39 | ) | |||||||||
|
Other
|
1.10 | (.63 | ) | .62 | |||||||||
| 36.34 | % | 34.47 | % | 32.39 | % | ||||||||
| Year Ended December 31, | ||||||||||||
|
Revenue:
|
2009
|
2010
|
2011
|
|||||||||
|
North Asia
|
$ | 606,113 | $ | 686,073 | $ | 751,165 | ||||||
|
Greater China
|
210,379 | 268,171 | 341,919 | |||||||||
|
Americas
|
260,865 | 250,008 | 251,984 | |||||||||
|
South Asia/Pacific
|
120,123 | 182,796 | 236,212 | |||||||||
|
Europe
|
133,578 | 150,211 | 162,711 | |||||||||
|
Total
|
$ | 1,331,058 | $ | 1,537,259 | $ | 1,743,991 | ||||||
|
Year Ended December 31,
|
||||||||||||
|
Revenue:
|
2009
|
2010
|
2011
|
|||||||||
|
Nu Skin
|
$ | 752,681 | $ | 913,819 | $ | 964,130 | ||||||
|
Pharmanex
|
565,592 | 612,209 | 770,192 | |||||||||
|
Other
|
12,785 | 11,231 | 9,669 | |||||||||
|
Total
|
$ | 1,331,058 | $ | 1,537,259 | $ | 1,743,991 | ||||||
|
Year Ended December 31,
|
||||||||||||
|
Revenue:
|
2009
|
2010
|
2011
|
|||||||||
|
Japan
|
$ | 461,914 | $ | 471,425 | $ | 472,519 | ||||||
|
South Korea
|
144,199 | 214,648 | 278,646 | |||||||||
|
United States
|
218,557 | 212,070 | 211,788 | |||||||||
|
Mainland China
|
71,086 | 91,352 | 152,538 | |||||||||
|
Europe
|
111,862 | 124,497 | 140,497 | |||||||||
|
Taiwan
|
91,727 | 107,133 | 108,857 | |||||||||
|
Hong Kong
|
47,566 | 69,686 | 80,524 | |||||||||
|
December 31,
|
||||||||
|
Long-lived assets:
|
2010
|
2011
|
||||||
|
Japan
|
$ | 12,473 | $ | 14,113 | ||||
|
South Korea
|
9,396 | 11,451 | ||||||
|
United States
|
84,829 | 98,205 | ||||||
|
Europe
|
2,697 | 1,966 | ||||||
|
Mainland China
|
11,646 | 15,135 | ||||||
|
Taiwan
|
2,200 | 1,556 | ||||||
|
Hong Kong
|
1,561 | 1,030 | ||||||
|
2010
|
2011
|
|||||||||||||||||||||||||||||||
|
1
st
Quarter
|
2
nd
Quarter
|
3
rd
Quarter
|
4
th
Quarter
|
1
st
Quarter
|
2
nd
Quarter
|
3
rd
Quarter
|
4
th
Quarter
|
|||||||||||||||||||||||||
|
Revenue
|
$ | 364.1 | $ | 388.4 | $ | 383.6 | $ | 401.2 | $ | 395.8 | $ | 424.4 | $ | 428.4 | $ | 495.3 | ||||||||||||||||
|
Gross profit
|
299.3 | 320.4 | 314.8 | 330.3 | 295.2 | 353.3 | 357.8 | 415.1 | ||||||||||||||||||||||||
|
Operating income
|
46.1 | 59.2 | 52.9 | 58.9 | 24.9 | 66.0 | 67.2 | 75.6 | ||||||||||||||||||||||||
|
Net income
|
31.0 | 32.4 | 35.3 | 37.3 | 15.3 | 41.7 | 46.8 | 49.5 | ||||||||||||||||||||||||
|
Net income per share:
|
||||||||||||||||||||||||||||||||
|
Basic
|
0.50 | 0.51 | 0.57 | 0.60 | 0.25 | 0.67 | 0.75 | 0.80 | ||||||||||||||||||||||||
|
Diluted
|
0.48 | 0.50 | 0.55 | 0.58 | 0.24 | 0.65 | 0.72 | 0.76 | ||||||||||||||||||||||||
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America, and that our receipts and expenditures are being made only in accordance with authorization of management and directors; and
|
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
|
1.
|
Financial Statements
. See Index to Consolidated Financial Statements under Item 8 of Part II.
|
|
|
2.
|
Financial Statement Schedules
. N/A
|
|
|
3.
|
Exhibits
. References to the “Company” shall mean Nu Skin Enterprises, Inc. Unless otherwise noted, the SEC file number for exhibits incorporated by reference is 001-12421.
|
|
*2.1
|
LifeGen Asset Purchase Agreement, dated as of December 13, 2011 between LifeGen Technologies, LLC and Nu Skin International, Inc. (the Company undertakes to furnish a copy of any omitted schedule or similar attachments to the Securities and Exchange Commission upon request.)
|
|
3.1
|
Amended and Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form S-1 (File No. 333-12073) (the “Form S-1”)).
|
|
3.2
|
Certificate of Amendment to the Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.2 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
3.3
|
Certificate of Designation, Preferences and Relative Participating, Optional and Other Special Rights of Preferred Stock and Qualifications, Limitations and Restrictions Thereof (incorporated by reference to Exhibit 3.3 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004).
|
|
3.4
|
Second Amended and Restated Bylaws of Nu Skin Enterprises, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2011)
|
|
4.1
|
Specimen Form of Stock Certificate for Class A Common Stock (incorporated by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-3 (File No. 333-90716)).
|
|
4.2
|
Specimen Form of Stock Certificate for Class B Common Stock (incorporated by reference to Exhibit 4.2 to the Company’s Form S-1).
|
|
10.1
|
Credit Agreement, dated as of May 10, 2001, among the Company, various financial institutions, and Bank of America, N.A., as Administrative Agent (incorporated by reference to Exhibit 10.7 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006).
|
|
10.2
|
First Amendment to Credit Agreement, dated as of December 14, 2001, among the Company, various financial institutions, and Bank of America, N.A. as Administrative Agent (incorporated by reference to Exhibit 10.8 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006).
|
|
10.3
|
Second Amendment to Credit Agreement, dated as of October 22, 2003 between the Company, various financial institutions, and Bank of America, N.A. as Administrative Agent (incorporated by reference to Exhibit 10.11 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003).
|
|
10.4
|
Third Amendment to Credit Agreement, dated as of May 10, 2004, among the Company, various financial institutions, and Bank One, N.A. as Administrative Agent (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004).
|
|
10.5
|
Fourth Amendment to Credit Agreement, dated as of July 28, 2006, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. as Administrative Agent (as successor to Bank One, N.A.) (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K filed on August 23, 2006).
|
|
10.6
|
Fifth Amendment to Credit Agreement, dated as of October 5, 2006, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. as Administrative Agent (as successor to Bank One, N.A.) (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K filed on October 10, 2006).
|
|
10.7
|
Sixth Amendment to Credit Agreement, dated as of August 8, 2007, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. as Administrative Agent (as successor to Bank One, N.A.) (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K filed August 15, 2007).
|
|
10.8
|
Seventh Amendment to Credit Agreement, dated as of November 7, 2007, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. as Administrative Agent (as successor to Bank One, N.A.) (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K filed on November 13, 2007).
|
|
10.9
|
Eighth Amendment to Credit Agreement, dated as of February 29, 2008, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. as Administrative Agent (as successor to Bank One, N.A.) (incorporated by reference to Exhibit 10.87 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
10.10
|
Ninth Amendment to Credit Agreement dated as of August 25, 2009, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. (as successor to Bank One N.A.) as successor administrative agent (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on August 31, 2009).
|
|
10.11
|
Letter Agreement among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. as Administrative Agent (as successor to Bank One, N.A.) (incorporated by reference to Exhibit 99.5 to the Company’s Current Report on Form 8-K filed November 13, 2007).
|
|
10.12
|
Credit Agreement, dated as of December 29, 2010, among the Company and JPMorgan Chase Bank, N.A. (incorporated by reference to Exhibit 10.12 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010.)
|
|
10.13
|
Private Shelf Agreement, dated as of August 26, 2003, between the Company and Prudential Investment Management, Inc. (the “Private Shelf Agreement”) (incorporated by reference to Exhibit 10.20 to the Company’s Annual report on Form 10-K for the year ended December 31, 2008).
|
|
10.14
|
First Amendment to the Private Shelf Agreement, dated as of October 31, 2003 between the Company and Prudential Investment Management, Inc.
(incorporated by reference to Exhibit 10.53 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003).
|
|
10.15
|
Second Amendment to the Private Shelf Agreement, dated as of May 18, 2004, between the Company, Prudential Investment Management, Inc., and the holders of the Series A Senior Notes and Series B Senior Notes issued under the Private Shelf Agreement (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004).
|
|
10.16
|
Third Amendment to the Private Shelf Agreement dated June 13, 2005 between the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005).
|
|
10.17
|
Fourth Amendment to the Private Shelf Agreement dated July 28, 2006 between the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 99.3 to the Company’s Current Report on Form 8-K filed on August 23, 2006).
|
|
10.18
|
Fifth Amendment to the Private Shelf Agreement dated October 5, 2006 between the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 99.3 to the Company’s Current Report on Form 8-K filed on October 10, 2006).
|
|
10.19
|
Sixth Amendment to the Private Shelf Agreement, dated as of November 7, 2007, between the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 99.3 to the Company’s Current Report on Form 8-K filed on November 13, 2007).
|
|
10.20
|
Seventh Amendment to the Private Shelf Agreement, dated as of February 25, 2008, between the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 10.83 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
10.21
|
Multi-Currency Private Shelf Agreement dated as of October 1, 2009, between the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2009).
|
|
10.22
|
Letter Agreement among the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 99.6 to the Company’s Current Report on Form 8-K filed November 13, 2007).
|
|
10.23
|
Letter Agreement dated October 1, 2009, among the Company, Prudential Investment Management, Inc. and certain other lenders (incorporated by reference to Exhibit 10.32 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
10.24
|
Series C Senior Notes Nos. C-1 and C-2 issued February 7, 2005 by the Company to Prudential Investment Management, Inc. and/or its affiliates pursuant to the Private Shelf Agreement (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K filed February 8, 2005).
|
|
10.25
|
Series D Senior Notes Nos. D-1, D-2, D-3 and D-4 issued October 3, 2006 by the Company to Prudential Investment Management, Inc. and/or its affiliates pursuant to the Private Shelf Agreement (incorporated by reference to Exhibit 99.4 to the Company’s Current Report on Form 8-K filed October 10, 2006).
|
|
10.26
|
Series E Senior Notes Nos. E-1, E-2, E-3, E-4 and E-5 issued January 19, 2007 by the Company to Prudential Investment Management, Inc. and/or its affiliates pursuant to the Private Shelf Agreement (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K filed January 25, 2007).
|
|
10.27
|
Series E Senior Note E-6, issued July 20, 2007, by the Company to Prudential Insurance Company of America pursuant to the Private Shelf Agreement (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on 8-K filed January 14, 2008).
|
|
10.28
|
Series EE Senior Note EE-1, issued January 8, 2008, by the Company to Prudential Insurance Company of America pursuant to the Private Shelf Agreement (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on 8-K filed January 14, 2008).
|
|
10.29
|
Series F Senior Notes Nos. F-1 and F-2 issued September 28, 2007 by the Company to Prudential Investment Management, Inc. and/or its affiliates pursuant to the Private Shelf Agreement (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2007).
|
|
10.30
|
Pledge Agreement dated October 12, 2000, by and between the Company and State Street Bank and Trust Company of California, N.A., acting in its capacity as collateral agent (incorporated by reference to Exhibit 10.5 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005).
|
|
10.31
|
Pledge Amendments executed by the Company dated December 31, 2003 (incorporated by reference to Exhibit 10.5 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003).
|
|
10.32
|
Pledge Agreement dated as of January 31, 2005 by and among Nu Skin Asia Investment, Inc., a wholly-owned subsidiary of the Company, and U.S. Bank National Association, as agent for and on behalf of the Benefited Parties under the Amended and Restated Collateral Agency and Intercreditor Agreement (referred to below) (incorporated by reference to Exhibit 99.3 to the Company’s Current Report on Form 8-K/A filed on March 10, 2005).
|
|
10.33
|
Amended and Restated Collateral Agency and Intercreditor Agreement, dated as of August 26, 2003, by and among the Company and various of its subsidiaries, U.S. Bank National Association, as Collateral Agent, and various lending institutions (incorporated by reference to Exhibit 10.40 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008).
|
|
10.34
|
Real Estate Purchase and Sale Agreement, and other ancillary agreements, dated as of December 30, 2010 between Aspen Country, LLC and Nu Skin International, Inc. (incorporated by reference to Exhibit 10.34 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
10.35
|
Real Estate Purchase and Sale Agreement, and other ancillary agreements, dated as of December 30, 2010 between Scrub Oak, LLC and Nu Skin International, Inc. (incorporated by reference to Exhibit 10.35 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
10.36
|
Form of Promissory Notes dated as of December 30, 2010 by Nu Skin International, Inc., with a schedule of material differences (incorporated by reference to Exhibit 10.36 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
10.37
|
Design and Construction Agreements effective March 10, 2011, between Nu Skin International, Inc. and each of Bolin Cywinski Jackson and Okland Construction Company, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2011).
|
|
10.38
|
Tenth Amendment to Credit Agreement dated as of February 11, 2011, among the Company, various financial institutions, and JPMorgan Chase Bank, N.A. (as successor to Bank One N.A.) as successor administrative agent (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2011).
|
|
10.39
|
Form of Termination of Lock-up Agreements dated as of September 1, 2010 between the Company and each of Blake and Nancy Roney, Steven and Kalleen Lund, and Sandra Tillotson (incorporated by reference to Exhibit 10.37 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
#10.40
|
Form of Indemnification Agreement to be entered into between the Company and certain of its officers and directors (incorporated by reference to Exhibit 10.48 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008).
|
|
#10.41
|
Amended and Restated Deferred Compensation Plan, effective as of January 1, 2008 (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2007).
|
|
#10.42
|
Amendment to the Deferred Compensation Plan, effective as of January 1, 2009 (incorporated by reference to Exhibit 10.50 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008).
|
|
#10.43
|
Nu Skin Enterprises, Inc. Nonqualified Deferred Compensation Trust dated December 14, 2005 (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K filed December 19, 2005).
|
|
#10.44
|
Second Amended and Restated Nu Skin Enterprises, Inc. 1996 Stock Incentive Plan (incorporated by reference to Exhibit 10.28 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005).
|
|
#10.45
|
Form of Master Stock Option Agreement (1996 Plan) (incorporated by reference to Exhibit 10.49 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
#10.46
|
Form of Stock Option Agreement for Directors (1996 Plan) (incorporated by reference to Exhibit 10.48 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006).
|
|
#10.47
|
Nu Skin Enterprises, Inc. 2006 Stock Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 1, 2006).
|
|
#10.48
|
Form of Master Stock Option Agreement (2006 Plan) (incorporated by reference to Exhibit 10.10 to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2006).
|
|
#10.49
|
Form of Master Stock Option Agreement (2006 Plan Performance Option (U.S.)) (incorporated by reference to Exhibit 10.54 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
#10.50
|
Form of Master Stock Option Agreement for Directors (2006 Plan) (incorporated by reference to Exhibit 10.59 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008).
|
|
#10.51
|
Form of Director Restricted Stock Unit Agreement (2006 Plan) (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2007).
|
|
#10.52
|
Form of Master Restricted Stock Unit Agreement (2006 Plan) (incorporated by reference to Exhibit 10.11 to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2006).
|
|
#10.53
|
Nu Skin Enterprises, Inc. 2010 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 2, 2010).
|
|
#10.54
|
Form of 2010 Plan U.S. Stock Option Master Agreement and Grant Notice (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 2, 2010).
|
|
#10.55
|
Form of 2010 Plan U.S. Restricted Stock Unit Master Agreement and Grant Notice (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on July 2, 2010).
|
|
#10.56
|
Form of 2010 Plan U.S. Performance Stock Option Master Agreement and Grant Notice (incorporated by reference to Exhibit 10.54 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
#10.57
|
Form of 2010 Plan U.S. Performance Restricted Stock Unit Master Agreement and Grant Notice (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on July 2, 2010).
|
|
#10.58
|
Form of 2010 Plan Director Stock Option Master Agreement and Grant Notice (incorporated by reference to Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2010).
|
|
#10.59
|
Form of 2010 Plan Director Restricted Stock Unit Master Agreement and Grant Notice (incorporated by reference to Exhibit 10.8 to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2010).
|
|
#10.60
|
Nu Skin Enterprises, Inc. 2009 Key Employee Death Benefit Plan (incorporated by reference to Exhibit 10.58 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
#10.61
|
Employment Letter between the Company and Truman Hunt dated January 17, 2003 (incorporated by reference to Exhibit 10.67 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
#10.62
|
Summary of Modifications to Truman Hunt’s Employment Letter (incorporated by reference to Exhibit 10.69 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008).
|
|
#10.63
|
Joseph Y. Chang Employment Agreement dated November 9, 2009, between Mr. Chang and the Company (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2009).
|
|
#10.64
|
Daniel Chard Employment Agreement effective February 13, 2006 between Mr. Chard and the Company (incorporated by reference to Exhibit 10.61 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006).
|
|
#10.65
|
Summary of Modifications to Dan Chard’s Employment Letter (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2009).
|
|
#10.66
|
Event Appearance Bonus Guidelines (Approved for Sandra Tillotson in October 2006) (incorporated by reference to Exhibit 10.68 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006).
|
|
#10.67
|
Ashok Pahwa Settlement and Release Agreement dated April 1, 2010, between Mr. Pahwa and the Company (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2010).
|
|
#10.68
|
Gary Sumihiro Settlement and Release Agreement dated March 1, 2009, between Mr. Sumihiro and the Company (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2009).
|
|
#10.69
|
Gary Sumihiro Consulting Agreement dated March 1, 2009, between Mr. Sumihiro and the Company (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2009).
|
|
#10.70
|
Form of Key Employee Covenants (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2007).
|
|
*21.1
|
Subsidiaries of the Company.
|
|
*23.1
|
Consent of PricewaterhouseCoopers LLP.
|
|
*31.1
|
Certification by M. Truman Hunt, President and Chief Executive Officer, pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
*31.2
|
Certification by Ritch N. Wood, Chief Financial Officer, pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
*32.1
|
Certification by M. Truman Hunt, President and Chief Executive Officer, pursuant to Section 1350, Chapter 63 of Title 18, United States Code, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
*32.2
|
Certification by Ritch N. Wood, Chief Financial Officer, pursuant to Section 1350, Chapter 63 of Title 18, United States Code, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
**101.INS
|
XBRL Instance Document
|
|
**101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
**101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
**101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
**101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
**101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
NU SKIN ENTERPRISES, INC.
|
|||
|
|
By:
|
/s/ M. Truman Hunt | |
| M. Truman Hunt, Chief Executive Officer | |||
|
Signatures
|
Capacity in Which Signed
|
|
|
/s/ Blake M. Roney
|
Chairman of the Board
|
|
|
Blake M. Roney
|
||
|
/s/ M. Truman Hunt
|
President and Chief Executive Officer and Director
|
|
|
M. Truman Hunt
|
(Principal Executive Officer)
|
|
|
/s/ Ritch N. Wood
|
Chief Financial Officer
|
|
|
Ritch N. Wood
|
(Principal Financial Officer and Accounting Officer)
|
|
|
/s/ Sandra N. Tillotson
|
Senior Vice President, Director
|
|
|
Sandra N. Tillotson
|
||
|
/s/ Steven J. Lund
|
Director
|
|
|
Steven J. Lund
|
||
|
/s/ Daniel W. Campbell
|
Director
|
|
|
Daniel W. Campbell
|
||
|
/s/ E.J. “Jake” Garn
|
Director
|
|
|
E. J. “Jake” Garn
|
||
|
/s/ Andrew D. Lipman
|
Director
|
|
|
Andrew D. Lipman
|
||
|
/s/ Patricia A. Negr
ó
n
|
Director
|
|
|
Patricia A. Negr
ó
n
|
||
|
/s/ David D. Ussery
|
Director
|
|
|
David D. Ussery
|
||
|
/s/ Thomas R. Pisano
|
Director
|
|
|
Thomas R. Pisano
|
||
|
/s/ Nevin N. Andersen
|
Director
|
|
|
Nevin N. Andersen
|
||
|
/s/ Neil Offen
|
Director
|
|
|
Neil Offen
|