|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Mark One)
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2013
|
|
|
OR
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from to
|
|
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
94-3166458
(IRS Employer
Identification No.)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $0.001 par value
|
The NASDAQ Stock Market LLC
|
|
|
Large accelerated filer
o
|
Accelerated filer
ý
|
Non-accelerated filer
o
(Do not check if a
smaller reporting company)
|
Smaller reporting company
o
|
|||
|
Page No.
|
||
|
OTHER
|
||
|
•
|
our expectations regarding our future product bookings, which consist of all firm orders, as evidenced by a contract and purchase order for equipment and software and, generally, by a purchase order for consumables. Equipment and software bookings are installable within 12 months and consumables are generally recorded as revenue within one month.;
|
|
•
|
the extent and timing of future revenues, including the amounts of our current backlog, which represents firm orders that have not completed installation and therefore have not been recognized as revenue;
|
|
•
|
the size or growth of our market or market share;
|
|
•
|
the opportunity presented by new products, emerging markets and international market;
|
|
•
|
our ability to align our cost structure and headcount with our current business expectations;
|
|
•
|
the operating margins or earnings per share goals we may set;
|
|
•
|
our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others;
|
|
•
|
our ability to generate cash from operations and our estimates regarding the sufficiency of our cash resources; and
|
|
•
|
our ability to acquire companies, businesses, products or technologies on commercially reasonable terms and integrate such acquisitions effectively.
|
|
•
|
Further penetrating existing markets through technological leadership by:
|
|
•
|
Increasing penetration of new markets, such as non-acute care and international markets by:
|
|
•
|
Expanding our product offering through acquisitions and partnerships.
|
|
•
|
Providing a full service, positive experience for our hospital customers in the solution sales process, the timing and implementation of our product installations and the responsiveness of our support services;
|
|
•
|
Delivering solutions that are designed to provide our customers with the best experience in the healthcare industry, as measured by customer input and third party surveys;
|
|
•
|
Innovating products to address patient safety and cost-containment pressures facing healthcare facilities while improving clinician workflow and overall operating efficiency;
|
|
•
|
Incorporating a broad range of clinical input into our product solution development to accommodate needs ranging from those of institutional pharmacies and stand-alone community hospitals to multi-hospital entities and integrated delivery networks ("IDNs"); and
|
|
•
|
Developing new solutions to enhance our customers' existing systems and protect our customers' investments by preserving, leveraging and upgrading their existing information systems, as well as striving to provide integration of our products with the other healthcare information systems used by our customers.
|
|
•
|
Providing flexibility in our systems that can be tailored to specific customer needs through modular upgrades, thereby protecting our customers' investments.
|
|
•
|
In 2012, The Joint Commission updated its medication management standards which include MM.03.01.01 requiring that medication storage is designed to assist in maintaining medication integrity, promote the availability of medications when needed, minimize the risk of medication diversion, and reduce potential dispensing errors. Law and regulation and manufacturers’ guidelines further define the hospital’s approach to medication storage. The Joint Commission audits all healthcare facilities seeking accreditation for proper medication handling control and reviews all exceptions to control procedures.
|
|
•
|
In 2010, the FDA updated its guidance that requires linear bar codes on most prescription drugs. Drug manufacturers, re-packagers, re-labelers and private label distributors are subject to the rule. The FDA had estimated that the bar code rule, once implemented, would result in a 50% reduction in medication errors, 500,000 fewer adverse drug events over the subsequent 20 years, $93 billion in cost savings and other economic benefits.
|
|
•
|
In 2008, and updated in 2009, the ISMP published guidelines for the Interdisciplinary Safe Use of Automated Dispensing Cabinets.
|
|
•
|
In June 2006, the IOM issued a report which augmented a series of reports issued between 1999 and 2005 highlighting the prevalence of medication errors and indicated that an estimated 1.5 million medication errors occur annually in the United States.
|
|
•
|
The Joint Commission first established the National Patient Safety Goals ("NPSG") in 2002. In 2010, NPSG 03.04.01, National Patient Safety Goals on Labeling Medications, specified the need for labeling all medications, medication containers (i.e. syringes, medicine cups, basins, etc.) and other solutions on and off the sterile field in perioperative and other procedural settings.
|
|
•
|
In 2011, CVS Caremark Corporation published a study in "Health Affairs" that found that patients who take medications as directed by physicians may save the healthcare system as much as $7,800 per patient annually. The study also found that these patients experienced fewer emergency room visits and inpatient hospital stays.
|
|
•
|
In September 2011, the second annual Medication Adherence e-survey, indicated a slight uptick in the previous 12 months in the number of programs designed to improve non-adherence as well as an increasing reliance on community or retail pharmacists to help individuals understand and adhere to their medication regimens.
|
|
Product
|
Use in Hospital
|
Description
|
||
|
OmniRx
|
Any nursing area in a hospital department that administers medications
|
Secure dispensing system that automates the management and dispensing of medications at the point of use.
|
||
|
SinglePointe
|
Any nursing area in a hospital department that administers medications
|
Software product for use in conjunction with the OmniRx product that controls medications on a patient-specific basis, allowing automated control of up to 100% of the medications used in a hospital.
|
||
|
AnywhereRN
|
Any nursing area in a hospital department that administers medications
|
Software that allows nurses to remotely operate automated dispensing cabinets from virtually any workstation in the hospital.
|
||
|
Pandora Analytics
|
Hospital central pharmacy and general hospital management
|
Advanced reporting and data analytics tools.
|
||
|
Savvy Mobile System
|
Any nursing area in a hospital department that administers medications
|
Mobile wireless computer and dispensing system that provides a mobile platform for hospital information systems and a convenient and secure method for nurses to move medication and supplies.
|
||
|
OmniLinkRx
|
Hospital central pharmacy
|
Prescription routing system that allows nurses and doctors to scan handwritten prescription orders for electronic delivery to pharmacists for approval and filling.
|
||
|
WorkflowRx
|
Hospital central pharmacy
|
Automated pharmacy storage, retrieval and packaging systems.
|
||
|
Controlled Substance Management
|
Hospital central pharmacy
|
Controlled substance inventory management system.
|
||
|
Anesthesia Workstation
|
Operating room
|
Secure dispensing system for the management of anesthesia supplies and medications.
|
||
|
Product
|
Use in Hospital
|
Description
|
||
|
Omnicell Supply Solution
|
Any nursing area in a hospital department that uses patient care supplies
|
Secure dispensing system that automates the management and dispensing of medical and surgical supplies at the point of use.
|
||
|
Omnicell Open Supply Solution
|
Areas that require the management of high volume/low dollar inventory as well as areas where space restrictions limit the ability to install closed cabinets and other areas such as offsite clinics.
|
Ability to expand inventory management capabilities by providing efficient workflow and flexibility to enable either remote inventory management from closed supply cabinets or completely open shelf inventory management from a touchscreen PC and Scanner.
|
||
|
Supply/Rx Combination Solution
|
Any nursing area in a hospital department that uses patient care supplies and administers medications
|
Secure dispensing system that manages both supplies and medications from the same cabinets, using the same user interface screens, in medical and surgical units and specialty areas.
|
||
|
Omnicell Tissue Center
|
Perioperative areas of the hospital
|
Manages the chain of custody for bone and tissue specimens from the donor to the patient in the operating room.
|
||
|
OptiFlex SS
|
Perioperative areas of the hospital
|
Specialty modules for the perioperative areas.
|
||
|
OptiFlex CL
|
Procedure areas in the hospital including the cardiac catheterization lab
|
Specialty modules for the cardiac catheterization lab and other procedure areas.
|
||
|
OptiFlex MS
|
Any nursing area in a hospital department that administers supplies
|
System for the management of medical and surgical supplies that provides the flexibility of utilizing bar code control in an open shelf environment.
|
||
|
•
|
Heat Seal Punch Cards
come in a variety of formats that will fit various packaging requirements. Our heat seal cards require a heat sealer such as the MTS Autobond. Punch cards come in a variety of configurations, from 14- to 90-day dose. Heat seal cards provide the strongest seal available, helping pharmacists assure consistency of the medication under nearly any environmental condition.
|
|
•
|
Cold Seal Cards,
also known as pressure sensitive cards, are both efficient and reliable and do not require heat sealing equipment to be sealed. They are ideal for emergency orders, for heat sensitive medications or when the use of a heat sealer is not practical. Cold seal punch cards come in a variety of configurations, from 14- to 90-day dose.
|
|
•
|
MultiMed Cards
allow the packaging of multiple drugs into a single blister cavity. These products are primarily used in community-based pharmacies to assist in organizing complex medication regimes into a simple to use solution that enhances medication adherence. Multi-Med cards are sold in a variety of formats to fit the needs of pharmacists and patients.
|
|
•
|
The
SureSeal
is a programmable, manual sealer utilizing heat only. It is designed as a cost-effective, entry level sealer for low volume sealing of medication punch cards.
|
|
•
|
The
Autobond
is a programmable, semi-automated heat and pressure sealer operating off of electricity and compressed air. Autobond provides temperature and time controls for a consistent quality sealing.
|
|
•
|
The
AutoGen
is a programmable, semi-automated heat and pressure sealer operating off of electricity only.
|
|
•
|
The
Gemini
is a compact all electric heat and pressure sealer.
|
|
•
|
The
MTS-350
is a tabletop machine capable of filling a wide range of medications and features an ergonomic design and easy-to-use controls. The 350 provides a semi-automated mechanism for filling blister cards and a sealer utilizing compressed air and heat.
|
|
•
|
The
MTS-400
is
ergonomically designed for high pre-pack volume for the medium to large pharmacy. The 400 provides a portable workstation with built-in compressor and storage so as not to take up valuable counter space. Fully configured, the MTS-400 allows a single operator to perform the functions of filling, inspection, sealing and labeling simultaneously.
|
|
•
|
The
MTS-500
is designed to automate pre-packaging in the pharmacy and is capable of producing up to 960 pre-packaged punch cards per hour. It includes an integrated label applicator and conveyor to optimize output.
|
|
•
|
AccuFlex
uses robotic technology to accurately and efficiently fill a variety of single-dose medication dispensing systems.
|
|
•
|
OnDemand 400 for RxMap
is an automation system designed specifically for multi-med adherence packaging. It fills multiple medication prescriptions into a single punch card.
|
|
•
|
OnDemand Express II
optimizes robotic technology for very high-speed and accurate fulfillment of single-dose punch cards and reclaimable packaging.
|
|
Name
|
Age
|
Position
|
||
|
Randall A. Lipps
|
56
|
President, Chief Executive Officer, and Chairman of the Board of Directors
|
||
|
J. Christopher Drew
|
48
|
Executive Vice President, Field Operations
|
||
|
Robin G. Seim
|
54
|
Executive Vice President Finance, Administration and Manufacturing, Chief Financial Officer
|
||
|
Dan S. Johnston
|
50
|
Executive Vice President and General Counsel
|
||
|
Nhat H. Ngo
|
41
|
Executive Vice President, Strategy and Business Development
|
||
|
Marga Ortigas-Wedekind
|
52
|
Executive Vice President, Global Marketing and Product Development
|
||
|
Jorge R. Taborga
|
54
|
Executive Vice President, Engineering
|
||
|
•
|
certain competitors may offer or have the ability to offer a broader range of solutions in the marketplace that we are unable to match;
|
|
•
|
certain competitors may develop alternative solutions to the customer problems our products are designed to solve that may provide a better customer outcome or a lower cost of operation;
|
|
•
|
certain competitors may develop new features or capabilities for their products not previously offered that could compete directly with our products;
|
|
•
|
competitive pressures could result in increased price competition for our products and services, fewer customer orders and reduced gross margins, any of which could harm our business;
|
|
•
|
current and potential competitors may make strategic acquisitions or establish cooperative relationships among themselves or with third parties, including larger, more established healthcare supply companies, thereby increasing their ability to develop and offer products and services to address the needs of our prospective customers;
|
|
•
|
our competitors may develop, license or incorporate new or emerging technologies or devote greater resources to the development, promotion and sale of their products and services than we do;
|
|
•
|
certain competitors have greater brand name recognition and a more extensive installed base of medication and supply dispensing systems or other products and services than we do, and such advantages could be used to increase their market share;
|
|
•
|
certain competitors may have existing business relationships with our current and potential customers, which may cause these customers to purchase medication and supply dispensing systems or automation solutions from these competitors;
|
|
•
|
other established or emerging companies may enter the medication management and supply chain solutions market; and
|
|
•
|
our competitors may secure products and services from suppliers on more favorable terms or secure exclusive arrangements with suppliers or buyers that may impede the sales of our products and services.
|
|
•
|
difficulties in combining previously separate businesses into a single unit and the complexity of managing a more dispersed organization as sites are acquired;
|
|
•
|
the substantial costs that may be incurred and the substantial diversion of management's attention from day-to-day business when evaluating and negotiating such transactions and then integrating an acquired business;
|
|
•
|
discovery, after completion of the acquisition, of liabilities assumed from the acquired business or of assets acquired that are broader in scope and magnitude or are more difficult to manage than originally assumed;
|
|
•
|
failure to achieve anticipated benefits such as cost savings and revenue enhancements;
|
|
•
|
difficulties related to assimilating the products or key personnel of an acquired business; and
|
|
•
|
failure to understand and compete effectively in markets in which we have limited previous experience.
|
|
•
|
our reliance on distributors for the sale and post-sale support of our automated dispensing systems outside the United States and Canada;
|
|
•
|
the difficulty of managing an organization operating in various countries;
|
|
•
|
political sentiment against international outsourcing of production;
|
|
•
|
reduced protection for intellectual property rights, particularly in jurisdictions that have less developed intellectual property regimes;
|
|
•
|
changes in foreign regulatory requirements;
|
|
•
|
the requirement to comply with a variety of international laws and regulations, including privacy, labor, import, export, environmental standards, tax, anti-bribery and employment laws and changes in tariff rates;
|
|
•
|
fluctuations in currency exchange rates and difficulties in repatriating funds from certain countries;
|
|
•
|
additional investment, coordination and lead-time necessary to successfully interface our automation solutions with the existing information systems of our customers or potential customers outside of the United States; and
|
|
•
|
political unrest, terrorism and the potential for other hostilities in areas in which we have facilities.
|
|
•
|
incur or assume liens or additional debt or provide guarantees in respect of obligations or other persons;
|
|
•
|
issue redeemable preferred stock;
|
|
•
|
pay dividends or distributions or redeem or repurchase capital stock;
|
|
•
|
prepay, redeem or repurchase certain debt;
|
|
•
|
make loans, investments, acquisitions (including acquisitions of exclusive licenses) and capital expenditures;
|
|
•
|
enter into agreements that restrict distributions from our subsidiaries;
|
|
•
|
sell assets and capital stock of our subsidiaries;
|
|
•
|
enter into certain transactions with affiliates; and
|
|
•
|
consolidate or merge with or into, or sell substantially all of our assets to, another person.
|
|
•
|
our ability to successfully install our products on a timely basis and meet other contractual obligations necessary to recognize revenue;
|
|
•
|
the size, product mix and timing of orders for our medication and supply dispensing systems, and our medication packaging systems, and their installation and integration;
|
|
•
|
the overall demand for healthcare medication management and supply chain solutions;
|
|
•
|
changes in pricing policies by us or our competitors;
|
|
•
|
the number, timing and significance of product enhancements and new product announcements by us or our competitors;
|
|
•
|
the timing and significance of any acquisition or business development transactions that we may consider or negotiate and the revenues, costs and earnings that may be associated with these transactions;
|
|
•
|
the relative proportions of revenues we derive from products and services;
|
|
•
|
fluctuations in the percentage of sales attributable to our international business;
|
|
•
|
our customers' budget cycles;
|
|
•
|
changes in our operating expenses and our ability to stabilize expenses;
|
|
•
|
expenses incurred to remediate product quality or safety issues
|
|
•
|
our ability to generate cash from our accounts receivable on a timely basis;
|
|
•
|
the performance of our products;
|
|
•
|
changes in our business strategy;
|
|
•
|
macroeconomic and political conditions, including fluctuations in interest rates, tax increases and availability of credit markets; and
|
|
•
|
volatility in our stock price and its effect on equity-based compensation expense.
|
|
•
|
changes in our operating results;
|
|
•
|
developments in our relationships with corporate customers;
|
|
•
|
changes in the ratings of our common stock by securities analysts;
|
|
•
|
announcements by us or our competitors of technological innovations or new products;
|
|
•
|
announcements by us or our competitors of acquisitions of businesses, products or technologies; or
|
|
•
|
general economic and market conditions.
|
|
Site
|
Major Activity
|
Segment
|
Approximate Square Footage
|
|
St. Petersburg, Florida
|
Administration, marketing, research and development and manufacturing
|
Non-Acute Care
|
132,500
|
|
Mountain View, California
|
Administration, marketing, and research and development
|
Acute Care
|
100,000
|
|
Milpitas, California
|
Manufacturing
|
Acute Care
|
46,000
|
|
Waukegan, Illinois
|
Technical support and training
|
Acute Care
|
38,000
|
|
Nashville, Tennessee
|
Research and development and marketing
|
Acute Care
|
25,000
|
|
Leeds, United Kingdom
|
Sales, marketing and distribution center
|
Acute Care and Non-Acute Care
|
16,500
|
|
Fiscal Year Ended December 31, 2013
|
High
|
Low
|
|||||
|
Fourth Quarter
|
$
|
25.89
|
|
$
|
20.88
|
|
|
|
Third Quarter
|
$
|
25.22
|
|
$
|
19.29
|
|
|
|
Second Quarter
|
$
|
20.88
|
|
$
|
17.01
|
|
|
|
First Quarter
|
$
|
20.00
|
|
$
|
14.68
|
|
|
|
Fiscal Year Ended December 31, 2012
|
High
|
Low
|
|||||
|
Fourth Quarter
|
$
|
16.13
|
|
$
|
12.61
|
|
|
|
Third Quarter
|
$
|
15.03
|
|
$
|
12.33
|
|
|
|
Second Quarter
|
$
|
15.51
|
|
$
|
12.74
|
|
|
|
First Quarter
|
$
|
17.94
|
|
$
|
14.10
|
|
|
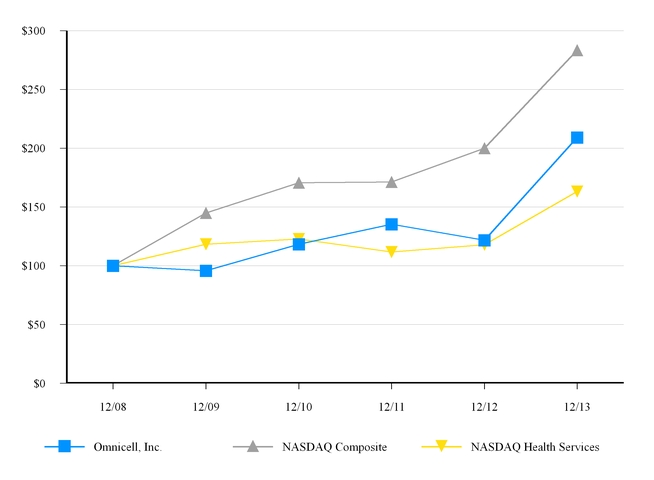
|
12/08
|
12/09
|
12/10
|
12/11
|
12/12
|
12/13
|
|||||||||
|
Omnicell, Inc.
|
100.00
|
|
95.74
|
|
118.35
|
|
135.30
|
121.79
|
209.09
|
|||||
|
NASDAQ Composite
|
100.00
|
|
144.88
|
|
170.58
|
|
171.30
|
199.99
|
283.39
|
|||||
|
NASDAQ Health Services
|
100.00
|
|
118.31
|
|
122.82
|
|
111.62
|
117.99
|
163.12
|
|||||
|
(1)
|
This section is not deemed "soliciting material" or to be "filed" with the SEC and is not to be incorporated by reference into any filing of Omnicell, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
|
Total Number of Shares Purchased
(1)
|
Average Price Paid per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Programs
(1)
|
Maximum Dollar Value of Shares That May Yet Be Purchased Under the Plans or Programs
(2)
|
||||||||||
|
October 1, 2013 to October 31, 2013
|
—
|
|
—
|
|
—
|
|
$
|
50,000,000
|
|
||||
|
November 1, 2013 to November 30, 2013
|
251,618
|
|
$
|
23.40
|
|
251,618
|
|
$
|
44,111,304
|
|
|||
|
December 1, 2013 to December 31, 2013
|
633,027
|
|
$
|
23.77
|
|
633,027
|
|
$
|
29,064,093
|
|
|||
|
Years Ended December 31,
|
|||||||||||||||||||
|
2013
|
2012
|
2011
|
2010
|
2009
|
|||||||||||||||
|
(in thousands, except per share amounts)
|
|||||||||||||||||||
|
Total revenues
|
$
|
380,585
|
|
$
|
314,027
|
|
$
|
245,535
|
|
$
|
222,407
|
|
$
|
213,457
|
|
||||
|
Gross profit
|
$
|
203,399
|
|
$
|
170,588
|
|
$
|
135,784
|
|
$
|
117,917
|
|
$
|
105,221
|
|
||||
|
Income from operations(1)
|
$
|
35,299
|
|
$
|
27,126
|
|
$
|
16,222
|
|
$
|
9,526
|
|
$
|
669
|
|
||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
$
|
4,892
|
|
$
|
444
|
|
||||
|
Net income per share:
|
|||||||||||||||||||
|
Basic
|
$
|
0.69
|
|
$
|
0.49
|
|
$
|
0.31
|
|
$
|
0.15
|
|
$
|
0.01
|
|
||||
|
Diluted
|
$
|
0.67
|
|
$
|
0.47
|
|
$
|
0.30
|
|
$
|
0.15
|
|
$
|
0.01
|
|
||||
|
Shares used in per shares calculations:
|
|||||||||||||||||||
|
Basic
|
34,736
|
|
33,307
|
|
33,123
|
|
32,651
|
|
31,691
|
|
|||||||||
|
Diluted
|
35,777
|
|
34,213
|
|
34,103
|
|
33,513
|
|
32,063
|
|
|||||||||
|
Cash dividends declared per share
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
At December 31,
|
|||||||||||||||||||
|
2013
|
2012
|
2011
|
2010
|
2009
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Total assets
|
$
|
492,501
|
|
$
|
441,819
|
|
$
|
363,849
|
|
$
|
343,224
|
|
$
|
322,260
|
|
||||
|
Long-term obligations, net of current portion
|
$
|
51,100
|
|
$
|
51,192
|
|
$
|
20,305
|
|
$
|
19,846
|
|
$
|
21,405
|
|
||||
|
Total stockholders' equity
|
$
|
348,997
|
|
$
|
307,550
|
|
$
|
282,914
|
|
$
|
265,214
|
|
$
|
242,304
|
|
||||
|
Years Ended December 31,
|
|||||||||||||||||||
|
2013
|
2012
|
2011
|
2010
|
2009
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Share-based compensation expense
|
$
|
11,151
|
|
$
|
9,214
|
|
$
|
9,499
|
|
$
|
9,015
|
|
$
|
9,725
|
|
||||
|
Quarters Ended
|
|||||||||||||||
|
December 31, 2013
|
September 30, 2013
|
June 30, 2013
|
March 31, 2013
|
||||||||||||
|
(in thousands, except per share data)
(unaudited)
|
|||||||||||||||
|
2013
|
|||||||||||||||
|
Total revenues
|
105,750
|
|
94,039
|
|
93,686
|
|
87,110
|
|
|||||||
|
Gross profit
|
56,624
|
|
52,040
|
|
49,368
|
|
45,367
|
|
|||||||
|
Income from operations
|
11,055
|
|
10,717
|
|
9,359
|
|
4,169
|
|
|||||||
|
Net income
|
$
|
6,823
|
|
$
|
7,755
|
|
$
|
6,016
|
|
$
|
3,385
|
|
|||
|
Net income per share:
|
|||||||||||||||
|
Basic(1)
|
$
|
0.19
|
|
$
|
0.22
|
|
$
|
0.17
|
|
$
|
0.10
|
|
|||
|
Diluted(1)
|
$
|
0.19
|
|
$
|
0.21
|
|
$
|
0.17
|
|
$
|
0.10
|
|
|||
|
Quarters Ended
|
|||||||||||||||
|
December 31, 2012
|
September 30, 2012
|
June 30, 2012
|
March 31, 2012
|
||||||||||||
|
(in thousands, except per share data)
(unaudited)
|
|||||||||||||||
|
2012
|
|||||||||||||||
|
Total revenues
|
90,169
|
|
84,331
|
|
75,384
|
|
64,143
|
|
|||||||
|
Gross profit
|
49,376
|
|
46,087
|
|
39,376
|
|
35,749
|
|
|||||||
|
Income from operations
|
9,834
|
|
11,226
|
|
2,431
|
|
3,635
|
|
|||||||
|
Net income
|
$
|
5,532
|
|
$
|
6,920
|
|
$
|
1,375
|
|
$
|
2,351
|
|
|||
|
Net income per share:
|
|
|
|
|
|
|
|
|
|||||||
|
Basic(1)
|
$
|
0.17
|
|
$
|
0.21
|
|
$
|
0.04
|
|
$
|
0.07
|
|
|||
|
Diluted(1)
|
$
|
0.16
|
|
$
|
0.20
|
|
$
|
0.04
|
|
$
|
0.07
|
|
|||
|
(1)
|
Quarterly net income per share figures may not total to annual net income per share, due to rounding and fluctuations in the number of options included or omitted from diluted calculations based on the stock price or option exercise prices and/or net losses recorded in quarterly periods.
|
|
•
|
Development of differentiated products.
We invest in the development of products that we believe bring patient safety and workflow efficiency to our customers’ operations that they cannot get from other competing solutions. These differentiators may be as small as how a transaction operates or information provided on a report or as large as the entire automation of a workflow that would otherwise be completed manually. We intend to continue our focus on differentiating our products, and we carefully assess our investments regularly as we strive to assure those investments provide the solutions most valuable to our customers.
|
|
•
|
Deliver our solutions to new markets
.
Areas of healthcare where work is done manually may benefit from our existing solutions. These areas include hospitals that continue to utilize manual operations, healthcare segments of the US market outside hospitals, and markets outside the US. We weigh the cost of entering these new markets against the expected benefits and focus on the markets that we believe are most likely to adopt our products.
|
|
•
|
Expansion of our solutions through acquisitions and partnerships.
Our acquisitions have generally been focused on automation of manual workflows or data analytics, which is the enhancement of data for our customers’ decision-
|
|
•
|
Our expectation that the overall market demand for healthcare services will increase as the population grows, life expectancies continue to increase, the quality and availability of healthcare services increases;
|
|
•
|
Our expectation that the environment of increased patient safety awareness, increased regulatory control and increased need for workflow efficiency through the adoption of technology in the healthcare industry will make our solutions a priority in the capital budgets of healthcare facilities; and
|
|
•
|
Our belief that healthcare customers will continue to value a consultative customer experience from their suppliers.
|
|
•
|
Products
—Software-enabled equipment that manages and regulates the storage and dispensing of pharmaceuticals, consumable blister cards and packaging equipment and other medical supplies.
|
|
•
|
Software
—Additional software applications that enable incremental functionality of our equipment.
|
|
•
|
Installation
—Installation of equipment as integrated systems at customers' sites.
|
|
•
|
Post-installation technical support
—Phone support, on-site service, parts and access to unspecified software upgrades and enhancements, if and when available.
|
|
•
|
Professional services
—Other customer services, such as training and consulting.
|
|
•
|
Persuasive evidence of an arrangement exists.
We use signed customer contracts and signed customer purchase orders as evidence of an arrangement for leases and sales. For service engagements, we use a signed services agreement and a statement of work to evidence an arrangement.
|
|
•
|
Delivery has occurred.
Equipment and embedded software product delivery is deemed to occur upon successful installation and receipt of a signed and dated customer confirmation of installation letter, providing evidence that we have delivered what a customer ordered. In instances of a customer self-installation, product delivery is deemed to have occurred upon receipt of a signed and dated customer confirmation letter. If a sale does not require installation, we recognize revenue on delivery of products to the customer, including transfer of title and risk of loss, assuming all other revenue criteria are met. For existing distributors, where installation of equipment training has been previously provided and the distributor is certified to install our equipment at end user customer facility, we recognize revenue from sales of products to the distributor upon shipment assuming all other revenue criteria are met since we do not allow for rights of return or refund. For new distributors, where we have not provided installation of equipment training, revenue on the sales of products to the distributor is deferred until the distributor has completed the Distributor Training Program and has been certified to install our equipment at the
|
|
•
|
Fee is fixed or determinable.
We assess whether a fee is fixed or determinable at the outset of the arrangement based on the payment terms associated with the transaction. We have established a history of collecting under the original contract without providing concessions on payments, products or services.
|
|
•
|
Collection is probable.
We assess the probability of collecting from each customer at the outset of the arrangement based on a number of factors, including the customer's payment history and its current creditworthiness. If, in our judgment, collection of a fee is not probable, we defer the revenue until the uncertainty is removed, which generally means revenue is recognized upon our receipt of cash payment assuming all other revenue criteria are met. Our historical experience has been that collection from our customers is generally probable.
|
|
•
|
Macroeconomic conditions such as general economic conditions, limitations on accessing capital, fluctuations in foreign exchange rates or other developments in equity and credit markets;
|
|
•
|
Industry and market considerations such as changes in the environment in which we operate, an increased competitive environment, a decline in market-dependent multiples or metrics (consider in both absolute terms and relative to peers), a change in the market for our products or services, or a regulatory or political development;
|
|
•
|
Cost factors such as increases in raw materials, labor, or other costs that have a negative effect on earnings and cash flows;
|
|
•
|
Overall financial performance such as negative or declining cash flows or a decline in actual or planned revenue or earnings compared with actual and projected results of relevant prior periods;
|
|
•
|
Other relevant entity-specific events such as changes in management, key personnel, strategy, or customers; contemplation of bankruptcy or litigation; and
|
|
•
|
Events affecting a reporting unit such as a change in the composition or carrying amount of its net assets, a more-likely-than-not expectation of selling or disposing all, or a portion, of a reporting unit, the testing for recoverability of a significant asset group within a reporting unit or recognition of a goodwill impairment loss in the financial statements of a subsidiary that is a component of a reporting unit.
|
|
•
|
identifying a triggering event that arises from a change in circumstances;
|
|
•
|
forecasting future operating results; and
|
|
•
|
estimating the proceeds from the disposition of long-lived or intangible assets.
|
|
Years Ended December 31,
|
||||||||||||||||||||
|
2013
|
% of Revenue
|
2012
|
% of Revenue
|
2011
|
% of Revenue
|
|||||||||||||||
|
(in thousands, except percentages)
|
||||||||||||||||||||
|
Revenues:
|
||||||||||||||||||||
|
Product revenues
|
$
|
307,189
|
|
80.7
|
%
|
$
|
247,654
|
|
78.9
|
%
|
$
|
185,864
|
|
75.7
|
%
|
|||||
|
Service and other revenues
|
73,396
|
|
19.3
|
%
|
66,373
|
|
21.1
|
%
|
59,671
|
|
24.3
|
%
|
||||||||
|
Total revenues
|
380,585
|
|
100.0
|
%
|
314,027
|
|
100.0
|
%
|
245,535
|
|
100.0
|
%
|
||||||||
|
Cost of revenues:
|
|
|
|
|
|
|||||||||||||||
|
Cost of product revenues
|
144,997
|
|
38.1
|
%
|
112,369
|
|
35.8
|
%
|
79,567
|
|
32.4
|
%
|
||||||||
|
Cost of service and other revenues
|
32,189
|
|
8.5
|
%
|
31,070
|
|
9.9
|
%
|
30,184
|
|
12.3
|
%
|
||||||||
|
Total cost of revenues
|
177,186
|
|
46.6
|
%
|
143,439
|
|
45.7
|
%
|
109,751
|
|
44.7
|
%
|
||||||||
|
Gross profit
|
203,399
|
|
53.4
|
%
|
170,588
|
|
54.3
|
%
|
135,784
|
|
55.3
|
%
|
||||||||
|
Operating expenses:
|
|
|
|
|
|
|||||||||||||||
|
Research and development
|
29,105
|
|
7.6
|
%
|
23,726
|
|
7.6
|
%
|
22,042
|
|
9.0
|
%
|
||||||||
|
Selling, general and administrative
|
138,995
|
|
36.5
|
%
|
119,736
|
|
38.1
|
%
|
97,520
|
|
39.7
|
%
|
||||||||
|
Income from operations
|
35,299
|
|
9.3
|
%
|
27,126
|
|
8.6
|
%
|
16,222
|
|
6.6
|
%
|
||||||||
|
Interest and other income (expense), net
|
(270
|
)
|
(0.1
|
)%
|
(51
|
)
|
—
|
%
|
(133
|
)
|
(0.1
|
)%
|
||||||||
|
Income before provision for income taxes
|
35,029
|
|
9.2
|
%
|
27,075
|
|
8.6
|
%
|
16,089
|
|
6.6
|
%
|
||||||||
|
Provision for income taxes
|
11,050
|
|
2.9
|
%
|
10,897
|
|
3.5
|
%
|
5,700
|
|
2.3
|
%
|
||||||||
|
Net income
|
$
|
23,979
|
|
6.3
|
%
|
$
|
16,178
|
|
5.2
|
%
|
$
|
10,389
|
|
4.2
|
%
|
|||||
|
Years Ended December 31,
|
2013 to 2012
|
2012 to 2011
|
|||||||||||||||||||||||
|
Change in
|
Change in
|
||||||||||||||||||||||||
|
2013
|
2012
|
2011
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Product revenues
|
$
|
307,189
|
|
$
|
247,654
|
|
$
|
185,864
|
|
$
|
59.5
|
|
24.0
|
%
|
$
|
61.8
|
|
33.2
|
%
|
||||||
|
Cost of product revenues
|
144,997
|
|
112,369
|
|
79,567
|
|
32.6
|
|
29.0
|
%
|
32.8
|
|
41.2
|
%
|
|||||||||||
|
Gross profit
|
$
|
162,192
|
|
$
|
135,285
|
|
$
|
106,297
|
|
$
|
26.9
|
|
19.9
|
%
|
$
|
29.0
|
|
27.3
|
%
|
||||||
|
Years Ended December 31,
|
2013 to 2012
|
2012 to 2011
|
|||||||||||||||||||||||
|
Change in
|
Change in
|
||||||||||||||||||||||||
|
2013
|
2012
|
2011
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Service and other revenues
|
$
|
73,396
|
|
$
|
66,373
|
|
$
|
59,671
|
|
$
|
7.0
|
|
10.6
|
%
|
$
|
6.7
|
|
11.2
|
%
|
||||||
|
Cost of service and other revenues
|
32,189
|
|
31,070
|
|
30,184
|
|
1.1
|
|
3.6
|
%
|
0.9
|
|
2.9
|
%
|
|||||||||||
|
Gross profit
|
$
|
41,207
|
|
$
|
35,303
|
|
$
|
29,487
|
|
$
|
5.9
|
|
16.7
|
%
|
$
|
5.8
|
|
19.7
|
%
|
||||||
|
|
Years Ended December 31,
|
2013 to 2012
|
2012 to 2011
|
||||||||||||||||||||||
|
|
Change in
|
Change in
|
|||||||||||||||||||||||
|
|
2013
|
2012
|
2011
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Research and development
|
$
|
29,105
|
|
$
|
23,726
|
|
$
|
22,042
|
|
$
|
5.4
|
|
22.7
|
%
|
$
|
1.7
|
|
7.6
|
%
|
||||||
|
Selling, general and administrative
|
138,995
|
|
119,736
|
|
97,520
|
|
19.3
|
|
16.1
|
%
|
22.2
|
|
22.8
|
%
|
|||||||||||
|
Total operating expenses
|
$
|
168,100
|
|
$
|
143,462
|
|
$
|
119,562
|
|
$
|
24.6
|
|
17.2
|
%
|
$
|
23.9
|
|
20.0
|
%
|
||||||
|
|
Years Ended December 31,
|
2013 to 2012
|
2012 to 2011
|
||||||||||||||||||||||
|
|
Change in
|
Change in
|
|||||||||||||||||||||||
|
|
2013
|
2012
|
2011
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Interest income
|
$
|
30
|
|
$
|
77
|
|
$
|
266
|
|
$
|
(47.0
|
)
|
(61.0
|
)%
|
$
|
(189.0
|
)
|
(71.1
|
)%
|
||||||
|
Interest expense
|
(122
|
)
|
(29
|
)
|
(62
|
)
|
93.0
|
|
320.7
|
%
|
33.0
|
|
(53.2
|
)%
|
|||||||||||
|
Other income (expense)
|
(178
|
)
|
(99
|
)
|
(337
|
)
|
79.0
|
|
79.8
|
%
|
(238.0
|
)
|
(70.6
|
)%
|
|||||||||||
|
Interest and other income (expense), net
|
$
|
(270
|
)
|
$
|
(51
|
)
|
$
|
(133
|
)
|
$
|
219.0
|
|
429.4
|
%
|
$
|
82.0
|
|
(61.7
|
)%
|
||||||
|
Years Ended December 31,
|
2013 to 2012
|
2012 to 2011
|
|||||||||||||||||||||||
|
|
Change in
|
Change in
|
|||||||||||||||||||||||
|
|
2013
|
2012
|
2011
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Provision for income taxes
|
$
|
11,050
|
|
$
|
10,897
|
|
$
|
5,700
|
|
$
|
153
|
|
1.4
|
%
|
$
|
5,197
|
|
91.2
|
%
|
||||||
|
Years Ended December 31,
|
|||||||||||
|
2013
|
2012
|
2011
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Net cash provided by operating activities
|
$
|
55,263
|
|
$
|
39,484
|
|
$
|
31,243
|
|
||
|
Net cash used in investing activities
|
(20,452
|
)
|
(168,711
|
)
|
(13,066
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
7,374
|
|
(232
|
)
|
(1,840
|
)
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
33
|
|
10
|
|
(210
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
42,218
|
|
$
|
(129,449
|
)
|
$
|
16,127
|
|
||
|
Total
|
Less than
one year
|
One to
three years
|
Three to
five years
|
More than
five years
|
|||||||||||||||
|
Operating leases(1)(2)
|
$
|
41,779
|
|
$
|
5,787
|
|
$
|
10,625
|
|
$
|
8,765
|
|
$
|
16,602
|
|
||||
|
Commitments to contract manufacturers and suppliers(3)
|
7,382
|
|
7,382
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total(4)
|
$
|
49,161
|
|
$
|
13,169
|
|
$
|
10,625
|
|
$
|
8,765
|
|
$
|
16,602
|
|
||||
|
(1)
|
Commitments under operating leases relate primarily to leasehold property and office equipment. Rent expense was
$8.1 million
,
$5.7 million
and
$3.3 million
for the years ended December 31, 2013, 2012 and 2011
, respectively.
|
|
(2)
|
In
October 2011
, we entered into a lease agreement for approximately
100,000
square feet of office space. Pursuant to the lease agreement, the landlord has constructed a single, three-story building of rentable space in Mountain View, California which we now lease and which serves as our headquarters. The term of the lease agreement, which commenced in November 2012, is for a period of
10 years
, with a base lease commitment of approximately
$40.0 million
. We have two options to extend the term of the lease agreement at market rates. Each extension is for an additional 60 month term.
|
|
(3)
|
We purchase components from a variety of suppliers and use contract manufacturers to provide manufacturing services for our products. During the normal course of business, we issue purchase orders with estimates of our requirements several months ahead of the delivery dates.
|
|
(4)
|
At
December 31, 2013
, we have recorded
$5.0 million
for uncertain tax positions under long term liabilities, in accordance with U.S. GAAP, summarized under the section entitled "Critical Accounting Policies and Estimates" of this Annual Report on Form 10-K. As these liabilities do not reflect actual tax assessments, the timing and amount of payments we might be required to make will depend upon a number of factors. Accordingly, as the timing and amount of payment cannot be estimated, the
$5.0 million
of uncertain tax position liabilities has not been included in the contractual obligations table above.
|
|
Index to Financial Statements:
|
Page
|
|
The foregoing additional financial statement schedule should be considered in conjunction with our consolidated financial statements. All other schedules have been omitted because the required information is either not applicable or not sufficiently material to require submission of the schedule.
|
|
|
December 31,
2013 |
December 31,
2012 |
||||||
|
|
|||||||
|
ASSETS
|
|
|
|
|
|||
|
Current assets:
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
104,531
|
|
$
|
62,313
|
|
|
|
Accounts receivable, net of allowances of $490 and $722 at December 31, 2013 and December 31, 2012, respectively
|
58,597
|
|
55,116
|
|
|||
|
Inventories
|
31,457
|
|
26,903
|
|
|||
|
Prepaid expenses
|
18,883
|
|
15,392
|
|
|||
|
Deferred tax assets
|
12,635
|
|
11,860
|
|
|||
|
Other current assets
|
7,675
|
|
9,172
|
|
|||
|
Total current assets
|
233,778
|
|
180,756
|
|
|||
|
Property and equipment, net
|
35,254
|
|
34,107
|
|
|||
|
Non-current net investment in sales-type leases
|
11,485
|
|
13,228
|
|
|||
|
Goodwill
|
111,343
|
|
111,407
|
|
|||
|
Other intangible assets
|
81,602
|
|
85,550
|
|
|||
|
Non-current deferred tax assets
|
1,102
|
|
993
|
|
|||
|
Other assets
|
17,937
|
|
15,778
|
|
|||
|
Total assets
|
$
|
492,501
|
|
$
|
441,819
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable
|
$
|
16,471
|
|
$
|
18,255
|
|
|
|
Accrued compensation
|
19,604
|
|
11,613
|
|
|||
|
Accrued liabilities
|
13,746
|
|
11,988
|
|
|||
|
Deferred service revenue
|
22,626
|
|
20,449
|
|
|||
|
Deferred gross profit
|
19,957
|
|
20,772
|
|
|||
|
Total current liabilities
|
92,404
|
|
83,077
|
|
|||
|
Non-current deferred service revenue
|
17,763
|
|
19,892
|
|
|||
|
Non-current deferred tax liabilities
|
28,162
|
|
26,491
|
|
|||
|
Other long-term liabilities
|
5,175
|
|
4,809
|
|
|||
|
Total liabilities
|
143,504
|
|
134,269
|
|
|||
|
Commitments and contingencies
|
|||||||
|
Stockholders’ equity:
|
|
|
|
|
|||
|
Preferred stock, $0.001 par value; 5,000,000 shares authorized; none issued
|
—
|
|
—
|
|
|||
|
Common Stock, $0.001 par value; 100,000,000 shares authorized; 41,840,298 and 35,003,677 shares issued and outstanding, respectively, at December 31, 2013 and 39,493,469 and 33,541,493 shares issued and outstanding, respectively, at December 31 2012
|
41
|
|
39
|
|
|||
|
Treasury stock, at cost, outstanding: 6,836,621 and 5,951,976 shares at December 31, 2013 and 2012, respectively
|
(110,962
|
)
|
(90,000
|
)
|
|||
|
Additional paid-in capital
|
421,232
|
|
382,844
|
|
|||
|
Retained earnings
|
38,515
|
|
14,536
|
|
|||
|
Accumulated other comprehensive income
|
171
|
|
131
|
|
|||
|
Total stockholders’ equity
|
348,997
|
|
307,550
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
492,501
|
|
$
|
441,819
|
|
|
|
|
Years Ended December 31,
|
|||||||||||
|
|
2013
|
2012
|
2011
|
|||||||||
|
Revenues:
|
|
|
|
|
|
|
||||||
|
Product revenues
|
$
|
307,189
|
|
$
|
247,654
|
|
$
|
185,864
|
|
|||
|
Services and other revenues
|
73,396
|
|
66,373
|
|
59,671
|
|
||||||
|
Total revenues
|
380,585
|
|
314,027
|
|
245,535
|
|
||||||
|
Cost of revenues:
|
|
|
|
|
|
|
||||||
|
Cost of product revenues
|
144,997
|
|
112,369
|
|
79,567
|
|
||||||
|
Cost of services and other revenues
|
32,189
|
|
31,070
|
|
30,184
|
|
||||||
|
Total cost of revenues
|
177,186
|
|
143,439
|
|
109,751
|
|
||||||
|
Gross profit
|
203,399
|
|
170,588
|
|
135,784
|
|
||||||
|
Operating expenses:
|
|
|
|
|
|
|
||||||
|
Research and development
|
29,105
|
|
23,726
|
|
22,042
|
|
||||||
|
Selling, general and administrative
|
138,995
|
|
119,736
|
|
97,520
|
|
||||||
|
Total operating expenses
|
168,100
|
|
143,462
|
|
119,562
|
|
||||||
|
Income from operations
|
35,299
|
|
27,126
|
|
16,222
|
|
||||||
|
Interest and other income (expense), net
|
(270
|
)
|
(51
|
)
|
(133
|
)
|
||||||
|
Income before provision for income taxes
|
35,029
|
|
27,075
|
|
16,089
|
|
||||||
|
Provision for income taxes
|
11,050
|
|
10,897
|
|
5,700
|
|
||||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
|||
|
Net income per share-basic
|
$
|
0.69
|
|
$
|
0.49
|
|
$
|
0.31
|
|
|||
|
Net income per share-diluted
|
$
|
0.67
|
|
$
|
0.47
|
|
$
|
0.30
|
|
|||
|
Weighted average shares outstanding:
|
|
|
|
|
|
|
||||||
|
Basic
|
34,736
|
|
33,307
|
|
33,123
|
|
||||||
|
Diluted
|
35,777
|
|
34,213
|
|
34,103
|
|
||||||
|
|
Years Ended December 31,
|
|||||||||||
|
|
2013
|
2012
|
2011
|
|||||||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
|||
|
Other comprehensive income:
|
|
|||||||||||
|
Unrealized holding (losses) gains arising during the period
|
—
|
|
(1
|
)
|
1
|
|
||||||
|
Changes in fair value of foreign currency forward hedges
|
(65
|
)
|
65
|
|
—
|
|
||||||
|
Foreign currency translation adjustment
|
105
|
|
66
|
|
—
|
|
||||||
|
Other comprehensive income
|
40
|
|
130
|
|
1
|
|
||||||
|
Comprehensive income
|
$
|
24,019
|
|
$
|
16,308
|
|
$
|
10,390
|
|
|||
|
Common
|
Treasury
|
||||||||||||||||||||||||||||
|
Shares
|
Stock
Amount
|
Shares
|
Stock
Amount
|
Additional
Paid In
Capital
|
Retained Earnings (Accumulated
Deficit)
|
Accumulated
Other
Comprehensive
Income
|
Total
Stockholders'
Equity
|
||||||||||||||||||||||
|
Balance at December 31, 2010
|
37,148,706
|
|
$
|
37
|
|
(4,121,123
|
)
|
$
|
(65,064
|
)
|
$
|
342,272
|
|
$
|
(12,031
|
)
|
$
|
—
|
|
$
|
265,214
|
|
|||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
10,389
|
|
—
|
|
10,389
|
|
|||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1
|
|
1
|
|
|||||||||||||
|
Share repurchases
|
—
|
|
—
|
|
(889,511
|
)
|
(12,573
|
)
|
—
|
|
—
|
|
—
|
|
(12,573
|
)
|
|||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
9,499
|
|
—
|
|
—
|
|
9,499
|
|
|||||||||||||
|
Common stock issued under stock option and stock award plans, net of employees' taxes paid related to restricted stock units
|
641,074
|
|
1
|
|
(43,174
|
)
|
—
|
|
2,736
|
|
—
|
|
—
|
|
2,737
|
|
|||||||||||||
|
Issuance of stock under employee stock purchase plan
|
445,965
|
|
—
|
|
—
|
|
—
|
|
4,050
|
|
—
|
|
—
|
|
4,050
|
|
|||||||||||||
|
Income tax benefits realized from employee stock plans
|
—
|
|
—
|
|
—
|
|
—
|
|
3,597
|
|
—
|
|
—
|
|
3,597
|
|
|||||||||||||
|
Balance at December 31, 2011
|
38,235,745
|
|
$
|
38
|
|
(5,053,808
|
)
|
$
|
(77,637
|
)
|
$
|
362,154
|
|
$
|
(1,642
|
)
|
$
|
1
|
|
$
|
282,914
|
|
|||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
16,178
|
|
—
|
|
16,178
|
|
|||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
130
|
|
130
|
|
|||||||||||||
|
Share repurchases
|
—
|
|
—
|
|
(898,168
|
)
|
(12,363
|
)
|
—
|
|
—
|
|
—
|
|
(12,363
|
)
|
|||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
9,214
|
|
—
|
|
—
|
|
9,214
|
|
|||||||||||||
|
Common stock issued under stock option and stock award plans, net of employees' taxes paid related to restricted stock units
|
879,875
|
|
1
|
|
—
|
|
—
|
|
4,547
|
|
—
|
|
—
|
|
4,548
|
|
|||||||||||||
|
Issuance of stock under employee stock purchase plan
|
377,849
|
|
—
|
|
—
|
|
—
|
|
4,402
|
|
—
|
|
—
|
|
4,402
|
|
|||||||||||||
|
Income tax benefits realized from employee stock plans
|
—
|
|
—
|
|
—
|
|
—
|
|
2,527
|
|
—
|
|
—
|
|
2,527
|
|
|||||||||||||
|
Balance at December 31, 2012
|
39,493,469
|
|
$
|
39
|
|
(5,951,976
|
)
|
$
|
(90,000
|
)
|
$
|
382,844
|
|
$
|
14,536
|
|
$
|
131
|
|
$
|
307,550
|
|
|||||||
|
Net income
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
|
|
|
23,979
|
|
|
—
|
|
|
23,979
|
|
||||||
|
Other comprehensive income
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
40
|
|
|
40
|
|
||||||
|
Share repurchases
|
—
|
|
|
—
|
|
|
(884,645
|
)
|
|
(20,962
|
)
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(20,962
|
)
|
||||||
|
Share-based compensation
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
11,151
|
|
|
—
|
|
|
—
|
|
|
11,151
|
|
||||||
|
Common stock issued under stock option and stock award plans, net of employees' taxes paid related to restricted stock units
|
1,897,931
|
|
|
2
|
|
|
—
|
|
|
—
|
|
|
18,882
|
|
|
—
|
|
|
—
|
|
|
18,884
|
|
||||||
|
Issuance of stock under employee stock purchase plan
|
450,713
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
5,779
|
|
|
—
|
|
|
—
|
|
|
5,779
|
|
||||||
|
Income tax benefits realized from employee stock plans
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
2,576
|
|
|
—
|
|
|
—
|
|
|
2,576
|
|
||||||
|
Balance at December 31, 2013
|
41,842,113
|
|
|
$
|
41
|
|
|
(6,836,621
|
)
|
|
$
|
(110,962
|
)
|
|
$
|
421,232
|
|
|
$
|
38,515
|
|
|
$
|
171
|
|
|
$
|
348,997
|
|
|
Years Ended December 31,
|
||||||||||
|
2013
|
2012
|
2011
|
||||||||
|
Cash flows from operating activities:
|
|
|
||||||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
|
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|
|
|
||||
|
Depreciation and amortization
|
18,365
|
|
13,325
|
|
7,983
|
|
||||
|
Loss on disposal of fixed assets
|
345
|
|
66
|
|
—
|
|
||||
|
Asset impairment charge
|
1,759
|
|
—
|
|
—
|
|
||||
|
Provision for (recovery of) receivable allowance
|
110
|
|
582
|
|
(155
|
)
|
||||
|
Gain on sale of note receivable
|
—
|
|
—
|
|
(473
|
)
|
||||
|
Share-based compensation expense
|
11,151
|
|
9,214
|
|
9,499
|
|
||||
|
Income tax benefits from employee stock plans
|
2,576
|
|
2,527
|
|
3,597
|
|
||||
|
Excess tax benefits from employee stock plans
|
(3,673
|
)
|
(3,182
|
)
|
(3,946
|
)
|
||||
|
Provision for excess and obsolete inventories
|
856
|
|
394
|
|
1,112
|
|
||||
|
Foreign currency remeasurement loss
|
—
|
|
—
|
|
210
|
|
||||
|
Deferred income taxes
|
787
|
|
2,718
|
|
589
|
|
||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
||||
|
Accounts receivable, net
|
(3,609
|
)
|
(9,311
|
)
|
5,863
|
|
||||
|
Inventories
|
(5,410
|
)
|
2,536
|
|
(9,434
|
)
|
||||
|
Prepaid expenses
|
(3,491
|
)
|
(4,897
|
)
|
1,464
|
|
||||
|
Other current assets
|
1,566
|
|
(1,114
|
)
|
(594
|
)
|
||||
|
Net investment in sales-type leases
|
1,723
|
|
(4,154
|
)
|
1,036
|
|
||||
|
Other assets
|
630
|
|
(3,831
|
)
|
339
|
|
||||
|
Accounts payable
|
(1,784
|
)
|
1,751
|
|
(2,242
|
)
|
||||
|
Accrued compensation
|
7,991
|
|
4,285
|
|
(403
|
)
|
||||
|
Accrued liabilities
|
1,758
|
|
674
|
|
(342
|
)
|
||||
|
Deferred service revenue
|
82
|
|
2,914
|
|
3,596
|
|
||||
|
Deferred gross profit
|
(815
|
)
|
6,562
|
|
2,491
|
|
||||
|
Other long-term liabilities
|
367
|
|
2,247
|
|
664
|
|
||||
|
Net cash provided by operating activities
|
55,263
|
|
39,484
|
|
31,243
|
|
||||
|
Cash flows from investing activities:
|
|
|
|
|
||||||
|
Purchases of short-term investments
|
—
|
|
—
|
|
(8,097
|
)
|
||||
|
Maturities of short-term investments
|
—
|
|
8,122
|
|
8,143
|
|
||||
|
Acquisition of intangible assets and intellectual property
|
(356
|
)
|
(373
|
)
|
(235
|
)
|
||||
|
Software development for external use
|
(7,761
|
)
|
(5,028
|
)
|
(4,192
|
)
|
||||
|
Purchases of property and equipment
|
(12,335
|
)
|
(15,120
|
)
|
(8,685
|
)
|
||||
|
Business acquisition, net of cash acquired
|
—
|
|
(156,312
|
)
|
—
|
|
||||
|
Net cash used in investing activities
|
(20,452
|
)
|
(168,711
|
)
|
(13,066
|
)
|
||||
|
Cash flows from financing activities:
|
|
|
|
|
|
|
||||
|
Proceeds from issuance of common stock under employee stock purchase and stock option plans, net of employees' taxes paid related to restricted stock units
|
24,663
|
|
8,949
|
|
6,787
|
|
||||
|
Stock repurchases
|
(20,962
|
)
|
(12,363
|
)
|
(12,573
|
)
|
||||
|
Excess tax benefits from employee stock plans
|
3,673
|
|
3,182
|
|
3,946
|
|
||||
|
Net cash provided from (used in) financing activities
|
7,374
|
|
(232
|
)
|
(1,840
|
)
|
||||
|
Effect of exchange rate changes on cash and cash equivalents
|
33
|
|
10
|
|
(210
|
)
|
||||
|
Net (decrease) increase in cash and cash equivalents
|
42,218
|
|
(129,449
|
)
|
16,127
|
|
||||
|
Cash and cash equivalents at beginning of period
|
62,313
|
|
191,762
|
|
175,635
|
|
||||
|
Cash and cash equivalents at end of period
|
$
|
104,531
|
|
$
|
62,313
|
|
$
|
191,762
|
|
|
|
Supplemental disclosure of cash flow information:
|
|
|
|
|
|
|
||||
|
Cash paid for interest
|
$
|
122
|
|
$
|
28
|
|
$
|
62
|
|
|
|
Cash paid for taxes
|
$
|
7,062
|
|
$
|
6,676
|
|
$
|
253
|
|
|
|
Supplemental disclosure of non-cash investing and operating activities
|
||||||||||
|
Purchases of property and equipment
|
$
|
196
|
|
$
|
—
|
|
$
|
—
|
|
|
|
Satisfaction of acquired legal contingency with indemnification asset (Note 2)
|
$
|
—
|
|
$
|
—
|
|
$
|
(1,200
|
)
|
|
|
•
|
Products
—Software-enabled equipment that manages and regulates the storage and dispensing of pharmaceuticals, consumable blister cards and packaging equipment and other medical supplies.
|
|
•
|
Software
—Additional software applications that enable incremental functionality of our equipment.
|
|
•
|
Installation
—Installation of equipment as integrated systems at customers' sites.
|
|
•
|
Post-installation technical support
—Phone support, on-site service, parts and access to unspecified software upgrades and enhancements, if and when available.
|
|
•
|
Professional services
—Other customer services such as training and consulting.
|
|
•
|
Persuasive evidence of an arrangement exists.
We use signed customer contracts and signed customer purchase orders as evidence of an arrangement for leases and sales. For service engagements, we use a signed services agreement and a statement of work to evidence an arrangement.
|
|
•
|
Delivery has occurred.
Equipment and embedded software product delivery is deemed to occur upon successful installation and receipt of a signed and dated customer confirmation of installation letter, providing evidence that we have delivered what a customer ordered. In instances of a customer self-installation, product delivery is deemed to have occurred upon receipt of a signed and dated customer confirmation letter. If a sale does not require installation, we recognize revenue on delivery of products to the customer, including transfer of title and risk of loss, assuming all other revenue criteria are met. For existing distributors, where installation of equipment training has been previously provided and the distributor is certified to install our equipment at end user customer facility, we recognize revenue from sales of products to the distributor upon shipment assuming all other revenue criteria are met since we do not allow for rights of return or refund. For new distributors, where we have not provided installation of equipment training, revenue on the sales of products to the distributor is deferred until the distributor has completed the Distributor Training Program and has been certified to install our equipment at the end user facility. For the sale of consumable blister cards, we recognize revenue when title and risk of loss of the products shipped have transferred to the customer, which usually occurs upon shipment from our facilities. Assuming all other revenue criteria are met, we recognize revenue for support services ratably over the related support services contract period. We recognize revenue on training and professional services as they are performed.
|
|
•
|
Fee is fixed or determinable.
We assess whether a fee is fixed or determinable at the outset of the arrangement based on the payment terms associated with the transaction. We have established a history of collecting under the original contract without providing concessions on payments, products or services.
|
|
•
|
Collection is probable.
We assess the probability of collecting from each customer at the outset of the arrangement based on a number of factors, including the customer's payment history and its current creditworthiness. If, in our judgment, collection of a fee is not probable, we defer the revenue until the uncertainty is removed, which generally means revenue is recognized upon our receipt of cash payment assuming all other revenue criteria are met. Our historical experience has been that collection from our customers is generally probable.
|
|
Computer equipment and related software
|
3 - 5 years
|
|
Leasehold and building improvements
|
Shorter of the lease term or the estimated useful life
|
|
Furniture and fixtures
|
5 years
|
|
Equipment
|
3 - 5 years
|
|
•
|
Macroeconomic conditions such as general economic conditions, limitations on accessing capital, fluctuations in foreign exchange rates or other developments in equity and credit markets;
|
|
•
|
Industry and market considerations such as changes in the environment in which we operate, an increased competitive environment, a decline in market-dependent multiples or metrics (consider in both absolute terms and relative to peers), a change in the market for our products or services, or a regulatory or political development;
|
|
•
|
Cost factors such as increases in raw materials, labor, or other costs that have a negative effect on earnings and cash flows;
|
|
•
|
Overall financial performance such as negative or declining cash flows or a decline in actual or planned revenue or earnings compared with actual and projected results of relevant prior periods;
|
|
•
|
Other relevant entity-specific events such as changes in management, key personnel, strategy, or customers; contemplation of bankruptcy or litigation; and
|
|
•
|
Events affecting a reporting unit such as a change in the composition or carrying amount of its net assets, a more-likely-than-not expectation of selling or disposing all, or a portion, of a reporting unit, the testing for recoverability of a significant asset group within a reporting unit or recognition of a goodwill impairment loss in the financial statements of a subsidiary that is a component of a reporting unit.
|
|
•
|
identifying a triggering event that arises from a change in circumstances;
|
|
•
|
forecasting future operating results; and
|
|
•
|
estimating the proceeds from the disposition of long-lived or intangible assets.
|
|
|
Fair value acquired
|
|
|||
|
Cash including restricted cash
|
$
|
2,000
|
|
||
|
Accounts receivable
|
7,403
|
|
|||
|
Inventory
|
11,726
|
|
|||
|
Deferred tax assets and other current assets
|
2,894
|
|
|||
|
Total current assets
|
24,023
|
|
|||
|
Property and equipment
|
9,807
|
|
|||
|
Intangible assets
|
83,900
|
|
|||
|
Goodwill
|
82,800
|
|
|||
|
Other non-current assets
|
308
|
|
|||
|
Total assets
|
200,838
|
|
|||
|
Current liabilities
|
(7,917
|
)
|
|||
|
Non-current deferred tax liabilities
|
(33,386
|
)
|
|||
|
Other non-current liabilities
|
(1,223
|
)
|
|||
|
Net assets acquired
|
$
|
158,312
|
|
||
|
Cash consideration, fair value
|
$
|
158,312
|
|
||
|
Fair value acquired
|
|
Useful Life (years)
|
First year amortization expense
|
|
|||||||
|
Trade name
|
$
|
6,800
|
|
12
|
$
|
567
|
|
||||
|
Customer relationships
|
50,500
|
|
28 to 30
|
1,707
|
|
||||||
|
Acquired technology
|
26,600
|
|
20
|
1,330
|
|
||||||
|
Intangibles acquired
|
$
|
83,900
|
|
$
|
3,604
|
|
|||||
|
Weighted average life of intangibles
|
25.14
|
||||||||||
|
|
Years Ended December 31
|
|||||||||||
|
|
2013
|
2012
|
2011
|
|||||||||
|
Basic:
|
|
|
|
|
|
|
||||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
|||
|
Weighted average shares outstanding — basic
|
34,736
|
|
33,307
|
|
33,123
|
|
||||||
|
Net income per share — basic
|
$
|
0.69
|
|
$
|
0.49
|
|
$
|
0.31
|
|
|||
|
Diluted:
|
|
|
|
|
|
|
||||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
|||
|
Weighted average shares outstanding — basic
|
34,736
|
|
33,307
|
|
33,123
|
|
||||||
|
Add: Dilutive effect of employee stock plans
|
1,041
|
|
906
|
|
980
|
|
||||||
|
Weighted average shares outstanding — diluted
|
35,777
|
|
34,213
|
|
34,103
|
|
||||||
|
Net income per share — diluted
|
$
|
0.67
|
|
$
|
0.47
|
|
$
|
0.30
|
|
|||
|
|
December 31, 2013
|
|
||||||||||||||||||||
|
|
Amortized
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Cash / Cash
Equivalents
|
Security
Classification
|
||||||||||||||||
|
Cash
|
$
|
38,823
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
38,823
|
|
|
$
|
38,823
|
|
|
|
N/A
|
|
Money market funds
|
65,708
|
|
|
—
|
|
|
—
|
|
|
65,708
|
|
|
65,708
|
|
|
|
Available for sale
|
|||||
|
Total cash and cash equivalents
|
$
|
104,531
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
104,531
|
|
|
$
|
104,531
|
|
|
|
|
|
|
December 31, 2012
|
|
||||||||||||||||||||
|
|
Amortized
Cost
|
Unrealized
Gains
|
Unrealized
Losses
|
Fair Value
|
Cash / Cash
Equivalents
|
Security
Classification
|
||||||||||||||||
|
Cash
|
$
|
23,422
|
|
$
|
—
|
|
$
|
—
|
|
$
|
23,422
|
|
$
|
23,422
|
|
N/A
|
||||||
|
Money market funds
|
38,892
|
|
—
|
|
1
|
|
38,891
|
|
38,891
|
|
Available for sale
|
|||||||||||
|
Total cash and cash equivalents
|
$
|
62,314
|
|
$
|
—
|
|
$
|
1
|
|
$
|
62,313
|
|
$
|
62,313
|
|
|
||||||
|
|
Quoted Prices in Active
Markets for Identical
Instruments
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Total Fair
Value
|
|||||||||||
|
At December 31, 2013
|
$
|
—
|
|
|
|
|
|
|
|
||||||
|
Money market funds
|
$
|
65,708
|
|
$
|
—
|
|
$
|
—
|
|
$
|
65,708
|
|
|||
|
Total
|
$
|
65,708
|
|
$
|
—
|
|
$
|
—
|
|
$
|
65,708
|
|
|||
|
At December 31, 2012
|
|
|
|
|
|
|
|
|
|||||||
|
Money market funds
|
$
|
38,891
|
|
$
|
—
|
|
$
|
—
|
|
$
|
38,891
|
|
|||
|
Total
|
$
|
38,891
|
|
$
|
—
|
|
$
|
—
|
|
$
|
38,891
|
|
|||
|
December 31,
2013 |
December 31,
2012 |
||||||
|
Raw materials
|
$
|
10,765
|
|
$
|
9,994
|
|
|
|
Work in process
|
534
|
|
385
|
|
|||
|
Finished goods
|
20,158
|
|
16,524
|
|
|||
|
Total
|
$
|
31,457
|
|
$
|
26,903
|
|
|
|
|
December 31,
2013 |
December 31,
2012 |
|||||
|
Equipment
|
$
|
40,180
|
|
$
|
32,528
|
|
|
|
Furniture and fixtures
|
5,260
|
|
5,126
|
|
|||
|
Leasehold improvements
|
7,394
|
|
6,992
|
|
|||
|
Purchased software
|
20,199
|
|
19,870
|
|
|||
|
Construction in process
|
2,649
|
|
2,693
|
|
|||
|
|
75,682
|
|
67,209
|
|
|||
|
Accumulated depreciation and amortization
|
(40,428
|
)
|
(33,102
|
)
|
|||
|
Property and equipment, net
|
$
|
35,254
|
|
$
|
34,107
|
|
|
|
December 31,
2013 |
December 31,
2012 |
||||||
|
Net minimum lease payments to be received
|
$
|
18,172
|
|
$
|
19,665
|
|
|
|
Less unearned interest income portion
|
1,455
|
|
1,205
|
|
|||
|
Net investment in sales-type leases
|
16,717
|
|
18,460
|
|
|||
|
Less current portion(1)
|
5,232
|
|
5,232
|
|
|||
|
Non-current net investment in sales-type leases(2)
|
$
|
11,485
|
|
$
|
13,228
|
|
|
|
2014
|
$5,851
|
||
|
2015
|
4,857
|
|
|
|
2016
|
3,618
|
|
|
|
2017
|
2,710
|
|
|
|
2018
|
1,136
|
|
|
|
Total
|
$18,172
|
||
|
|
Allowance for Credit Losses
|
Recorded Investment
in Sales-type Leases Gross
|
Recorded Investment
in Sales-type Leases Net
|
||||||||
|
Credit loss disclosure for December 31, 2013:
|
|
|
|
|
|
|
|||||
|
Accounts individually evaluated for impairment
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Accounts collectively evaluated for impairment
|
167
|
|
16,884
|
|
16,717
|
|
|||||
|
Ending balances: December 31, 2013
|
$
|
167
|
|
$
|
16,884
|
|
$
|
16,717
|
|
||
|
Credit loss disclosure for December 31, 2012:
|
|
|
|
|
|
|
|||||
|
Accounts individually evaluated for impairment
|
$
|
489
|
|
$
|
489
|
|
$
|
—
|
|
||
|
Accounts collectively evaluated for impairment
|
118
|
|
18,578
|
|
18,460
|
|
|||||
|
Ending balances: December 31, 2012
|
$
|
607
|
|
$
|
19,067
|
|
$
|
18,460
|
|
||
|
Year Ended
December 31, 2013
|
||
|
Allowance for credit losses, December 31, 2012
|
607
|
|
|
Current period provision (reversal)
|
49
|
|
|
Direct write-downs charged against the allowance
|
(413
|
)
|
|
Recoveries of amounts previously charged off
|
(76
|
)
|
|
Allowance for credit losses at December 31, 2013
|
167
|
|
|
Goodwill at December 31, 2012
|
Adjustments to Goodwill
|
Goodwill at December 31, 2013
|
|||||||||
|
Reporting units:
|
|||||||||||
|
Acute Care
|
$
|
28,543
|
|
$
|
—
|
|
$
|
28,543
|
|
||
|
Non-Acute Care
|
82,864
|
|
(64
|
)
|
82,800
|
|
|||||
|
Total
|
$
|
111,407
|
|
$
|
(64
|
)
|
$
|
111,343
|
|
||
|
|
December 31, 2013
|
December 31, 2012
|
|
||||||||||||||||
|
|
Gross
|
|
Net
|
Gross
|
|
Net
|
|
||||||||||||
|
Carrying
Amount
|
Accumulated
Amortization
|
Carrying
Amount
|
Carrying
Amount
|
Accumulated
Amortization
|
Carrying
Amount
|
Amortization
Life
|
|||||||||||||
|
Finite-lived intangibles:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Customer relationships
|
54,730
|
|
5,236
|
|
49,494
|
|
54,730
|
|
3,081
|
|
51,649
|
|
5-30 years
|
||||||
|
Acquired technology
|
27,580
|
|
2,598
|
|
24,982
|
|
27,580
|
|
1,128
|
|
26,452
|
|
3-20 years
|
||||||
|
Patents
|
1,493
|
|
254
|
|
1,239
|
|
1,217
|
|
259
|
|
958
|
|
20 years
|
||||||
|
Trade name
|
6,890
|
|
1,003
|
|
5,887
|
|
6,890
|
|
414
|
|
6,476
|
|
3-12 years
|
||||||
|
Non-compete agreements
|
60
|
|
60
|
|
—
|
|
60
|
|
45
|
|
15
|
|
3 years
|
||||||
|
Total finite-lived intangibles
|
90,753
|
|
9,151
|
|
81,602
|
|
90,477
|
|
4,927
|
|
85,550
|
|
|
||||||
|
2014
|
4,229
|
|
|
|
2015
|
4,204
|
|
|
|
2016
|
3,854
|
|
|
|
2017
|
3,818
|
|
|
|
2018
|
3,711
|
|
|
|
Thereafter
|
61,786
|
|
|
|
Total
|
$
|
81,602
|
|
|
December 31,
|
|||||||
|
2013
|
2012
|
||||||
|
Capitalized software development costs, net of accumulated amortization of $10,547 and $7,329 in 2013 and 2012, respectively
|
$
|
13,660
|
|
$
|
11,037
|
|
|
|
Technology license
|
2,350
|
|
2,800
|
|
|||
|
Long-term deposits
|
682
|
|
694
|
|
|||
|
Other assets
|
1,245
|
|
1,247
|
|
|||
|
Total
|
$
|
17,937
|
|
$
|
15,778
|
|
|
|
|
December 31,
2013 |
December 31,
2012 |
|||||
|
Rebates and lease buyouts
|
$
|
1,699
|
|
$
|
3,179
|
|
|
|
Advance payments from customers
|
4,971
|
|
2,829
|
|
|||
|
Accrued Group Purchasing Organization (GPO) fees
|
2,324
|
|
2,278
|
|
|||
|
Technology license purchase obligation, current portion
|
1,500
|
|
1,750
|
|
|||
|
Taxes payable
|
1,664
|
|
555
|
|
|||
|
Other
|
1,588
|
|
1,397
|
|
|||
|
Total
|
$
|
13,746
|
|
$
|
11,988
|
|
|
|
|
December 31,
2013 |
December 31,
2012 |
|||||
|
Sales of medication and supply dispensing systems and packaging equipment, which have been delivered and invoiced but not yet installed
|
$
|
29,040
|
|
$
|
30,138
|
|
|
|
Cost of revenues, excluding installation costs
|
(9,083
|
)
|
(9,366
|
)
|
|||
|
Deferred gross profit
|
$
|
19,957
|
|
$
|
20,772
|
|
|
|
2014
|
$
|
5,787
|
|
|
2015
|
5,476
|
|
|
|
2016
|
5,150
|
|
|
|
2017
|
4,468
|
|
|
|
2018
|
4,298
|
|
|
|
Thereafter
|
16,601
|
|
|
|
Total
|
$
|
41,780
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Domestic
|
$
|
34,678
|
|
$
|
25,794
|
|
$
|
16,177
|
|
||
|
Foreign
|
351
|
|
1,281
|
|
(88
|
)
|
|||||
|
Total income before provision for income taxes
|
$
|
35,029
|
|
$
|
27,075
|
|
$
|
16,089
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Current:
|
|
|
|
||||||||
|
Federal
|
$
|
8,218
|
|
$
|
7,181
|
|
$
|
4,285
|
|
||
|
State
|
1,621
|
|
1,006
|
|
896
|
|
|||||
|
Foreign
|
447
|
|
154
|
|
(70
|
)
|
|||||
|
Total current
|
10,286
|
|
8,341
|
|
5,111
|
|
|||||
|
Deferred:
|
|
|
|
||||||||
|
Federal
|
1,287
|
|
2,169
|
|
1,116
|
|
|||||
|
State
|
(263
|
)
|
651
|
|
(527
|
)
|
|||||
|
Foreign
|
(260
|
)
|
(264
|
)
|
—
|
|
|||||
|
Total deferred
|
764
|
|
2,556
|
|
589
|
|
|||||
|
Total provision for income taxes
|
$
|
11,050
|
|
$
|
10,897
|
|
$
|
5,700
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
U.S. federal tax provision at statutory rate
|
$
|
12,260
|
|
$
|
9,476
|
|
$
|
5,631
|
|
||
|
State taxes
|
883
|
|
1,077
|
|
240
|
|
|||||
|
Non-deductible expenses
|
297
|
|
530
|
|
481
|
|
|||||
|
Acquisition costs
|
—
|
|
431
|
|
—
|
|
|||||
|
Share-based compensation expense
|
407
|
|
403
|
|
443
|
|
|||||
|
Research tax credits
|
(1,430
|
)
|
—
|
|
(755
|
)
|
|||||
|
Domestic production deduction
|
(816
|
)
|
(601
|
)
|
(271
|
)
|
|||||
|
Other
|
(551
|
)
|
(419
|
)
|
(69
|
)
|
|||||
|
Total
|
$
|
11,050
|
|
$
|
10,897
|
|
$
|
5,700
|
|
||
|
|
December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
Deferred tax assets (liabilities):
|
|
|
|||||
|
Tax credit carry forwards
|
$
|
3,160
|
|
$
|
2,990
|
|
|
|
Inventory related items
|
2,947
|
|
2,900
|
|
|||
|
Deferred revenue
|
11,074
|
|
11,497
|
|
|||
|
Stock compensation
|
7,447
|
|
9,331
|
|
|||
|
Loss carry forwards
|
64
|
|
53
|
|
|||
|
Other, net
|
5
|
|
69
|
|
|||
|
Subtotal
|
24,697
|
|
26,840
|
|
|||
|
Less: valuation allowance
|
(39
|
)
|
(39
|
)
|
|||
|
Total net deferred tax assets
|
24,658
|
|
26,801
|
|
|||
|
Reserves and accruals
|
(353
|
)
|
(573
|
)
|
|||
|
Depreciation and amortization
|
(38,681
|
)
|
(39,840
|
)
|
|||
|
Total deferred tax liabilities
|
(39,034
|
)
|
(40,413
|
)
|
|||
|
|
|||||||
|
Net deferred tax liabilities
|
$
|
(14,376
|
)
|
$
|
(13,612
|
)
|
|
|
Balance as of December 31, 2010
|
$
|
5,431
|
|
|
Decreases related to tax positions taken during the prior period
|
(88
|
)
|
|
|
Increases related to tax positions taken during the current period
|
453
|
|
|
|
Balance as of December 31, 2011
|
$
|
5,796
|
|
|
Increases related to tax positions taken during a prior period
|
43
|
|
|
|
Increases related to tax positions related to MTS
|
1,066
|
||
|
Increases related to tax positions taken during the current period
|
422
|
|
|
|
Decreases related to settlements
|
(33
|
)
|
|
|
Decreases related to expiration of statute of limitations
|
(379
|
)
|
|
|
Balance as of December 31, 2012
|
$
|
6,915
|
|
|
Increases related to tax positions taken during a prior period
|
406
|
||
|
Decreases related to tax positions taken during the prior period
|
(79
|
)
|
|
|
Increases related to tax positions taken during the current period
|
764
|
|
|
|
Decreases related to expiration of statute of limitations
|
(32
|
)
|
|
|
Balance as of December 31, 2013
|
$
|
7,974
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Cost of product and service revenues
|
$
|
1,241
|
|
$
|
1,011
|
|
$
|
1,398
|
|
||
|
Research and development
|
1,359
|
|
889
|
|
1,269
|
|
|||||
|
Selling, general and administrative
|
8,551
|
|
7,314
|
|
6,832
|
|
|||||
|
Total share-based compensation expense
|
$
|
11,151
|
|
$
|
9,214
|
|
$
|
9,499
|
|
||
|
|
Years Ended December 31,
|
|||||||
|
Stock Option Plans
|
2013
|
2012
|
2011
|
|||||
|
Risk-free interest rate(1)
|
1.2
|
%
|
0.9
|
%
|
1.6
|
%
|
||
|
Dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Volatility(2)
|
43.1
|
%
|
45.8
|
%
|
48.5
|
%
|
||
|
Expected life(3)
|
5.3 yrs
|
|
5.2 yrs
|
|
5.2 yrs
|
|
||
|
|
Years Ended December 31,
|
|||||||
|
Employee Stock Purchase Plan
|
2013
|
2012
|
2011
|
|||||
|
Risk-free interest rate(1)
|
0.2
|
%
|
0.2
|
%
|
0.5
|
%
|
||
|
Dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Volatility(2)
|
35.1
|
%
|
38.5
|
%
|
40.2
|
%
|
||
|
Expected life(3)
|
0.5 - 2 yrs
|
|
0.5 - 2 yrs
|
|
0.5 - 2 yrs
|
|
||
|
(1)
|
The risk-free interest rate for both stock options and the ESPP is based on the zero-coupon U.S. Treasury rate curve in effect at the time of the option grant or at the beginning of the ESPP offering period.
|
|
(2)
|
Expected volatility for both stock options and the ESPP reflects a combination of historical and market-based implied volatility consistent with ASC 718 and SEC Staff Accounting Bulletin 107. We determined that the combination of historical and market-based implied volatility provides a more accurate reflection of our market conditions and is more representative of future stock price trends than employing solely historical volatility.
|
|
(3)
|
Represents the period of time that options granted are expected to be outstanding, which is derived from historical data on employee exercise and post-vesting employment termination behavior.
|
|
Options:
|
Number of Shares
|
Weighted Average
Exercise Price
|
||||
|
|
(in thousands)
|
|
||||
|
Outstanding at December 31, 2010
|
4,740
|
|
$
|
12.86
|
|
|
|
Granted
|
494
|
|
$
|
14.57
|
|
|
|
Exercised
|
(413
|
)
|
$
|
8.30
|
|
|
|
Expired
|
(86
|
)
|
$
|
13.59
|
|
|
|
Forfeited
|
(42
|
)
|
$
|
20.76
|
|
|
|
Outstanding at December 31, 2011
|
4,693
|
|
$
|
13.36
|
|
|
|
Granted
|
645
|
|
$
|
14.85
|
|
|
|
Exercised
|
(669
|
)
|
$
|
8.65
|
|
|
|
Expired
|
(84
|
)
|
$
|
14.02
|
|
|
|
Forfeited
|
(115
|
)
|
$
|
21.44
|
|
|
|
Outstanding at December 31, 2012
|
4,470
|
|
$
|
14.06
|
|
|
|
Granted
|
502
|
|
$
|
20.25
|
|
|
|
Exercised
|
(1,686
|
)
|
$
|
12.53
|
|
|
|
Expired
|
(56
|
)
|
$
|
15.66
|
|
|
|
Forfeited
|
(87
|
)
|
$
|
14.68
|
|
|
|
Outstanding at December 31, 2013
|
3,143
|
|
$
|
15.82
|
|
|
|
Vested and expected to vest at December 31, 2013
|
3,114
|
|
$
|
15.79
|
|
|
|
Exercisable at December 31, 2013
|
2,078
|
|
$
|
15.11
|
|
|
|
Range of Exercise Prices
|
Number
Outstanding |
Weighted
Average Exercise Price of Outstanding Options |
Number
Exercisable
|
Weighted
Average Exercise
Price of
Exercisable
Options
|
|||||||||
|
|
(in thousands)
|
|
(in thousands)
|
|
|||||||||
|
$6.40 - $10.41
|
332
|
|
$
|
8.71
|
|
332
|
|
$
|
8.71
|
|
|||
|
$10.58 - $10.75
|
384
|
|
$
|
10.65
|
|
384
|
|
$
|
10.65
|
|
|||
|
$11.27 - $13.53
|
347
|
|
$
|
12.84
|
|
291
|
|
$
|
12.77
|
|
|||
|
$13.67 - $14.10
|
358
|
|
$
|
13.86
|
|
143
|
|
$
|
13.94
|
|
|||
|
$14.16 - $15.04
|
364
|
|
$
|
14.58
|
|
167
|
|
$
|
14.67
|
|
|||
|
$15.06 - $17.29
|
382
|
|
$
|
16.79
|
|
106
|
|
$
|
16.51
|
|
|||
|
$17.49 - $20.95
|
434
|
|
$
|
19.84
|
|
369
|
|
$
|
20.12
|
|
|||
|
$21.07 - $23.19
|
330
|
|
$
|
22.52
|
|
104
|
|
$
|
21.50
|
|
|||
|
$23.41 - $26.99
|
136
|
|
$
|
24.61
|
|
106
|
|
$
|
24.76
|
|
|||
|
$29.16 - $29.16
|
76
|
|
$
|
29.16
|
|
76
|
|
$
|
29.16
|
|
|||
|
$6.40 - $29.16
|
3,143
|
|
$
|
15.82
|
|
2,078
|
|
$
|
15.11
|
|
|||
|
Shares of
Restricted Stock
|
Weighted-Average Grant
Date Fair Value Per Share
|
Restricted Stock Units
|
Weighted-Average Grant
Date Fair Value
|
||||||||||
|
|
(in thousands)
|
|
(in thousands)
|
|
|||||||||
|
Nonvested at December 31, 2010
|
77
|
|
$
|
12.91
|
|
308
|
|
$
|
12.98
|
|
|||
|
Granted
|
68
|
|
$
|
14.71
|
|
145
|
|
$
|
14.39
|
|
|||
|
Vested
|
(77
|
)
|
$
|
12.91
|
|
(152
|
)
|
$
|
14.26
|
|
|||
|
Forfeited
|
—
|
|
—
|
|
(14
|
)
|
$
|
12.82
|
|
||||
|
Nonvested at December 31, 2011
|
68
|
|
$
|
14.71
|
|
287
|
|
$
|
13.03
|
|
|||
|
Granted
|
67
|
|
$
|
14.19
|
|
274
|
|
$
|
14.58
|
|
|||
|
Vested
|
(78
|
)
|
$
|
14.64
|
|
(153
|
)
|
$
|
12.90
|
|
|||
|
Forfeited
|
—
|
|
—
|
|
(19
|
)
|
$
|
14.55
|
|
||||
|
Nonvested at December 31, 2012
|
58
|
|
$
|
14.19
|
|
389
|
|
$
|
14.09
|
|
|||
|
Granted
|
$
|
55
|
|
$
|
18.20
|
|
190
|
|
19.87
|
|
|||
|
Vested
|
$
|
(61
|
)
|
$
|
14.23
|
|
(195
|
)
|
14.31
|
|
|||
|
Forfeited
|
—
|
|
—
|
|
(22
|
)
|
14.08
|
||||||
|
Nonvested at December 31, 2013
|
$
|
52
|
|
$
|
18.43
|
|
362
|
|
17.15
|
|
|||
|
Percentile Placement of Our Total Shareholder Return
|
% of PSUs Eligible for Time-
Based Vesting |
|
|
Below the 35th percentile
|
—%
|
|
|
At least the 35th percentile, but below the 50th percentile
|
50%
|
|
|
At least the 50th percentile, but below the 65th percentile
|
100%
|
|
|
At least the 65th percentile, but below the 75th percentile (1)
|
110% to 119%
|
|
|
At or above the 75th percentile
|
120%
|
|
|
Percentile Placement of Our Total Shareholder Return
|
% of PSUs Eligible for Time-
Based Vesting |
|
Below the 35th percentile
|
—%
|
|
At least the 35th percentile, but below the 50th percentile
|
50%
|
|
At least the 50th percentile
|
100%
|
|
Performance-based Stock Units
|
Number of Units
|
Weighted-
Average
Grant Date
Fair Value Per
Unit
|
||||
|
|
(in thousands)
|
|
||||
|
Non-vested, December 31, 2011
|
100
|
|
$
|
11.15
|
|
|
|
Granted
|
135
|
|
$
|
10.94
|
|
|
|
Vested
|
(60
|
)
|
$
|
11.15
|
|
|
|
Forfeited
|
—
|
|
$
|
—
|
|
|
|
Non-vested, December 31, 2012
|
175
|
|
$
|
11.00
|
|
|
|
Granted
|
142
|
|
$
|
14.68
|
|
|
|
Vested
|
(57
|
)
|
$
|
11.15
|
|
|
|
Forfeited
|
(35
|
)
|
$
|
10.93
|
|
|
|
Non-vested, December 31, 2013
|
225
|
|
$
|
13.32
|
|
|
|
December 31,
|
December 31,
|
December 31,
|
|||||||||||||||||||||||||||||
|
2013
|
2012
|
2011
|
|||||||||||||||||||||||||||||
|
Acute Care
|
Non-Acute Care
|
Total
|
Acute Care
|
Non-Acute Care (1)
|
Total
|
Acute Care
|
Total
|
||||||||||||||||||||||||
|
Net revenues from external customers
|
$
|
284,666
|
|
$
|
95,919
|
|
$
|
380,585
|
|
$
|
260,160
|
|
$
|
53,867
|
|
$
|
314,027
|
|
$
|
245,535
|
|
$
|
245,535
|
|
|||||||
|
Cost of revenues
|
122,816
|
|
54,370
|
|
177,186
|
|
111,599
|
|
31,840
|
|
143,439
|
|
109,751
|
|
109,751
|
|
|||||||||||||||
|
Gross profit
|
$
|
161,850
|
|
$
|
41,549
|
|
$
|
203,399
|
|
$
|
148,561
|
|
$
|
22,027
|
|
$
|
170,588
|
|
$
|
135,784
|
|
$
|
135,784
|
|
|||||||
|
Gross margin %
|
56.9
|
%
|
43.3
|
%
|
53.4
|
%
|
57.1
|
%
|
40.9
|
%
|
54.3
|
%
|
55.3
|
%
|
55.3
|
%
|
|||||||||||||||
|
|
|
||||||||||||||||||||||||||||||
|
Operating expenses
|
131,811
|
|
36,289
|
|
168,100
|
|
127,467
|
|
15,995
|
|
143,462
|
|
119,562
|
|
119,562
|
|
|||||||||||||||
|
Income from operations
|
$
|
30,039
|
|
$
|
5,260
|
|
$
|
35,299
|
|
$
|
21,094
|
|
$
|
6,032
|
|
$
|
27,126
|
|
$
|
16,222
|
|
$
|
16,222
|
|
|||||||
|
Operating margin %
|
10.6
|
%
|
5.5
|
%
|
9.3
|
%
|
8.1
|
%
|
11.2
|
%
|
8.6
|
%
|
6.6
|
%
|
6.6
|
%
|
|||||||||||||||
|
|
|
|
|||||||||||||||||||||||||||||
|
Interest and other income (expense), net
|
(270
|
)
|
(51
|
)
|
(133
|
)
|
|||||||||||||||||||||||||
|
Income before provision for income taxes
|
35,029
|
|
27,075
|
|
16,089
|
|
|||||||||||||||||||||||||
|
Provision for income taxes
|
11,050
|
|
10,897
|
|
5,700
|
|
|||||||||||||||||||||||||
|
Net income
|
$
|
23,979
|
|
$
|
16,178
|
|
$
|
10,389
|
|
||||||||||||||||||||||
|
(1) Non-Acute Care segment includes MTS results from May 21, 2012, the closing date of acquisition.
|
|||||||||||||||||||||||||||||||
|
December 31,
|
December 31,
|
December 31,
|
|||||||||||||||||||||||||||||
|
2013
|
2012
|
2011
|
|||||||||||||||||||||||||||||
|
Acute Care
|
Non-Acute Care (1)
|
Total
|
Acute Care
|
Non-Acute Care (1)
|
Total
|
Acute Care
|
Total
|
||||||||||||||||||||||||
|
Segment Assets
|
$
|
263,154
|
|
$
|
229,347
|
|
$
|
492,501
|
|
$
|
235,186
|
|
$
|
206,633
|
|
$
|
441,819
|
|
$
|
363,849
|
|
$
|
363,849
|
|
|||||||
|
Depreciation/Amortization
|
$
|
11,236
|
|
$
|
7,129
|
|
$
|
18,365
|
|
$
|
9,017
|
|
$
|
4,308
|
|
13,325
|
|
7,983
|
|
7,983
|
|
||||||||||
|
Capital Expenditures
|
$
|
4,392
|
|
$
|
7,943
|
|
$
|
12,335
|
|
$
|
13,234
|
|
$
|
1,953
|
|
$
|
15,187
|
|
$
|
8,685
|
|
$
|
8,685
|
|
|||||||
|
(1) Non-Acute Care segment includes MTS results from May 21, 2012, the date of acquisition.
|
|||||||||||||||||||||||||||||||
|
Allowances deducted from assets:
|
Balance at
beginning of year |
Additions
charged to costs and expenses (2) |
Charged
(credited) to other accounts |
Describe
charged to other accounts |
Deductions
|
Describe
deductions |
Balance at
end of year |
|||||||||||||||||
|
For the year ended December 31, 2011
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Accounts receivable(1)
|
$
|
497
|
|
$
|
63
|
|
$
|
(96
|
)
|
(3)
|
$
|
(21
|
)
|
(4)
|
$
|
443
|
|
|||||||
|
Investment in sales-type leases(1)
|
411
|
|
—
|
|
(22
|
)
|
(5)
|
(105
|
)
|
(4)
|
284
|
|
||||||||||||
|
Total allowances deducted from assets
|
$
|
908
|
|
$
|
63
|
|
$
|
(118
|
)
|
|
$
|
(126
|
)
|
|
$
|
727
|
|
|||||||
|
For the year ended December 31, 2012
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Accounts receivable(1)
|
$
|
443
|
|
$
|
316
|
|
$
|
(57
|
)
|
(3)
|
$
|
20
|
|
(4)
|
$
|
722
|
|
|||||||
|
Investment in sales-type leases(1)
|
284
|
|
425
|
|
—
|
|
(3)
|
(102
|
)
|
(4)
|
607
|
|
||||||||||||
|
Total allowances deducted from assets
|
$
|
727
|
|
$
|
741
|
|
$
|
(57
|
)
|
$
|
(82
|
)
|
$
|
1,329
|
|
|||||||||
|
For the year ended December 31, 2013
|
||||||||||||||||||||||||
|
Accounts receivable(1)
|
$
|
722
|
|
195
|
|
(67
|
)
|
(3)
|
(360
|
)
|
(4)
|
490
|
|
|||||||||||
|
Investment in sales-type leases (1)
|
607
|
|
49
|
|
—
|
|
(489
|
)
|
(4)
|
167
|
||||||||||||||
|
Total allowances deducted from assets
|
$
|
1,329
|
|
$
|
244
|
|
$
|
(67
|
)
|
$
|
(849
|
)
|
$
|
657
|
|
|||||||||
|
(1)
|
Allowance for doubtful accounts.
|
|
(2)
|
Represents amounts charged to bad debt expense.
|
|
(3)
|
Represents amounts credited to bad debt expense.
|
|
(4)
|
Represents amounts written-off, net of recoveries.
|
|
(5)
|
Represents amounts credited to bad debt expense and lease receivable adjustment.
|
|
March 17, 2014
|
OMNICELL, INC.
|
||
|
By:
|
/s/ ROBIN G. SEIM
|
||
|
Robin G. Seim,
Chief Financial Officer and Executive Vice President Finance, Administration and Manufacturing |
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ RANDALL A. LIPPS
|
Chief Executive Officer, President and Chairman of the Board (Principal Executive Officer)
|
March 17, 2014
|
||
|
Randall A. Lipps
|
||||
|
/s/ ROBIN G. SEIM
|
Chief Financial Officer and Executive Vice President Finance, Administration and Manufacturing (Principal Accounting and Financial Officer)
|
March 17, 2014
|
||
|
Robin G. Seim
|
||||
|
/s/ JOANNE B. BAUER
|
March 17, 2014
|
|||
|
Joanne B. Bauer
|
Director
|
|||
|
/s/ JAMES T. JUDSON
|
March 17, 2014
|
|||
|
James T. Judson
|
Director
|
|||
|
/s/ RANDY D. LINDHOLM
|
March 17, 2014
|
|||
|
Randy D. Lindholm
|
Director
|
|||
|
/s/ VANCE B. MOORE
|
March 17, 2014
|
|||
|
Vance B. Moore
|
Director
|
|||
|
/s/ MARK W. PARRISH
|
March 17, 2014
|
|||
|
Mark W. Parrish
|
Director
|
|||
|
/s/ GARY S. PETERSMEYER
|
March 17, 2014
|
|||
|
Gary S. Petersmeyer
|
Director
|
|||
|
/s/ DONALD C. WEGMILLER
|
March 17, 2014
|
|||
|
Donald C. Wegmiller
|
Director
|
|||
|
/s/ SARA J. WHITE
|
March 17, 2014
|
|||
|
Sara J. White
|
Director
|
|||
|
Incorporation By Reference
|
|||||||||
|
Exhibit
Number
|
|
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
|
Filing Date
|
||
|
2.1
|
|
Agreement and Plan of Merger, dated as of April 26, 2012, by and among Omnicell, Inc., Mercury Acquisition Corp, MedPak Holdings, Inc., and Excellere Capital Management, LLC
|
8-K
|
000-33043
|
2.1
|
|
5/2/2012
|
||
|
2.2
|
|
Agreement for the sale and purchase of the entire issued share capital of Surgichem Limited, by and among Omnicell, Inc., BUPA Care Homes (CFG) Plc, and MTS Medication Technologies, Inc.
|
8-K
|
000-33043
|
2.1
|
|
12/9/2013
|
||
|
3.1
|
|
Amended and Restated Certificate of Incorporation of Omnicell, Inc.
|
S-1
|
333-57024
|
3.1
|
|
3/14/2001
|
||
|
3.2
|
|
Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Omnicell, Inc.
|
10-Q
|
000-33043
|
3.2
|
|
8/9/2010
|
||
|
3.3
|
|
Certificate of Designation of Series A Junior Participating Preferred Stock
|
10-K
|
000-33043
|
3.2
|
|
3/28/2003
|
||
|
3.4
|
|
Bylaws of Omnicell, Inc., as amended
|
10-Q
|
000-33043
|
3.3
|
|
8/9/2007
|
||
|
4.1
|
|
Reference is made to Exhibits 3.1, 3.2 , 3.3 and 3.4
|
|||||||
|
4.2
|
|
Form of Common Stock Certificate
|
S-1
|
333-57024
|
4.1
|
|
3/14/2001
|
||
|
10.1*
|
|
2012 Executive Officer Annual Base Salaries
|
8-K
|
000-33043
|
10.1
|
|
2/13/2012
|
||
|
10.2*
|
|
2013 Executive Officer Annual Base Salaries
|
8-K
|
000-33043
|
10.1
|
|
2/7/2013
|
||
|
10.3*
|
2014 Executive Officer Annual Base Salaries
|
8-K
|
000-33043
|
10.1
|
|
2/7/2014
|
|||
|
10.4
|
|
Lease, effective July 1, 1999, between AMLI Commercial Properties Limited Partnership and Omnicell, Inc.
|
S-1
|
333-57024
|
10.2
|
|
3/14/2001
|
||
|
Incorporation By Reference
|
|||||||||
|
Exhibit
Number
|
|
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
|
Filing Date
|
||
|
10.5
|
|
First Amendment to Lease, dated September 30, 1999, between AMLI Commercial Properties Limited Partnership and Omnicell, Inc.
|
10-K
|
000-33043
|
10.6
|
|
3/8/2012
|
||
|
10.6
|
|
Lease, dated April 14, 2010, between Point Place II, LLC and Omnicell, Inc.
|
10-K
|
000-33043
|
10.10
|
|
3/11/2011
|
||
|
10.7
|
|
Lease Agreement, dated October 20, 2011, between Middlefield Station Associates, LLC and Omnicell, Inc.
|
10-K
|
000-33043
|
10.9
|
|
3/8/2012
|
||
|
10.8
|
|
Form of Director and Officer Indemnity Agreement
|
S-1
|
333-57024
|
10.12
|
|
3/14/2001
|
||
|
10.9*
|
|
1997 Employee Stock Purchase Plan, as amended
|
10-Q
|
000-33043
|
10.2
|
|
8/5/2009
|
||
|
10.10*
|
|
2003 Equity Incentive Plan, as amended
|
10-K
|
000-33043
|
10.14
|
|
3/23/2007
|
||
|
10.11*
|
|
2009 Equity Incentive Plan, as amended
|
10-Q
|
000-33043
|
10.2
|
|
8/9/2013
|
||
|
10.12*
|
|
Form of Option Grant Notice and Form of Option Agreement for 2009 Equity Incentive Plan, as amended
|
10-K
|
000-33043
|
10.16
|
|
3/11/2011
|
||
|
10.13*
|
|
Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement for 2009 Equity Incentive Plan, as amended
|
10-K
|
000-33043
|
10.17
|
|
3/11/2011
|
||
|
10.14*
|
|
Form of Restricted Stock Bonus Grant Notice and Form of Restricted Stock Bonus Agreement for 2009 Equity Incentive Plan, as amended
|
10-K
|
000-33043
|
10.18
|
|
3/11/2011
|
||
|
10.15*
|
|
Form of Change of Control Agreement
|
10-K
|
000-33043
|
10.26
|
|
3/16/2006
|
||
|
Incorporation By Reference
|
|||||||||
|
Exhibit
Number
|
|
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
|
Filing Date
|
||
|
10.16*
|
|
Addendum to Form of Change of Control Agreement dated December 30, 2010
|
10-K
|
000-33043
|
10.24
|
|
3/11/2011
|
||
|
10.17*
|
|
2010 Omnicell Quarterly Executive Bonus Plan
|
8-K
|
000-33043
|
10.1
|
|
3/17/2010
|
||
|
10.18*
|
|
Employment Agreement, dated October 31, 2003, between Omnicell and Dan S. Johnston
|
10-K
|
000-33043
|
10.26
|
|
3/8/2004
|
||
|
10.19*
|
|
Addendum to Offer Letter, dated December 30, 2010, between Omnicell and Dan S. Johnston
|
10-K
|
000-33043
|
10.14
|
|
3/11/2011
|
||
|
10.20*
|
|
Employment Agreement, dated November 28, 2005, between Omnicell and Robin G. Seim
|
8-K
|
000-33043
|
10.1
|
|
1/24/2006
|
||
|
10.21*
|
|
Addendum to Offer Letter, dated December 30, 2010, between Omnicell and Robin G. Seim
|
10-K
|
000-33043
|
10.21
|
|
3/11/2011
|
||
|
10.22*
|
|
Addendum to Change in Control Severance Letter between Omnicell and Robin G. Seim dated December 30, 2010
|
10-K
|
000-33043
|
10.22
|
|
3/11/2011
|
||
|
10.23*
|
|
Employment Agreement, dated October 17, 2008, between Omnicell and Nhat H. Ngo
|
10-K
|
000-33043
|
10.29
|
|
2/24/2009
|
||
|
10.24*
|
|
Addendum to Change in Control Severance Letter between Omnicell and Nhat H. Ngo dated December 30, 2010
|
10-K
|
000-33043
|
10.28
|
|
3/11/2011
|
||
|
10.25*
|
|
Employment Agreement, dated December 5, 2008, between Omnicell and Marga Ortigas-Wedekind
|
10-K
|
000-33043
|
10.31
|
|
2/24/2009
|
||
|
Incorporation By Reference
|
|||||||||
|
Exhibit
Number
|
|
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
|
Filing Date
|
||
|
10.26*
|
|
Addendum to Change in Control Severance Letter between Omnicell and Marga Ortigas-Wedekind dated December 30, 2010
|
10-K
|
000-33043
|
10.30
|
|
3/11/2011
|
||
|
10.27
|
|
Lease between Omnicell, Inc. and Sycamore Drive Holdings, LLC, dated March 16, 2012
|
8-K
|
000-33043
|
10.1
|
|
3/20/2012
|
||
|
10.28*
|
|
Omnicell, Inc. Amended and Restated Severance Benefit Plan
|
10-Q
|
000-33043
|
10.1
|
|
8/9/2012
|
||
|
10.29*
|
|
Form of Restricted Stock Unit Award Agreement for the 2009 Equity Incentive Plan, as amended
|
10-Q
|
000-33043
|
10.4
|
|
8/9/2012
|
||
|
10.30*
|
|
Form of Performance Cash Award Grant Notice and Form of Performance Cash Award Agreement for the 2009 Equity Incentive Plan, as amended
|
10-Q
|
000-33043
|
10.5
|
|
8/9/2012
|
||
|
10.31
|
|
Lease, between Medical Technologies Systems, Inc. and Gateway Business Centre, Ltd., dated March 31, 2004
|
10-Q
|
000-33043
|
10.6
|
|
8/9/2012
|
||
|
10.32
|
|
First Lease Amendment, between Medical Technologies Systems, Inc. and Gateway Business Centre, Ltd., dated July 26, 2004
|
10-Q
|
000-33043
|
10.7
|
|
8/9/2012
|
||
|
Incorporation By Reference
|
|||||||||
|
Exhibit
Number
|
|
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
|
Filing Date
|
||
|
10.33
|
|
Lease, between MTS Medication Technologies, Ltd. and SAL Pension Fund, Ltd., dated June 9, 2011
|
10-Q
|
000-33043
|
10.8
|
|
8/9/2012
|
||
|
10.34
|
|
Third Amendment to Lease, between PR Amhurst Lake LLC and Omnicell, Inc., dated July 1, 2013
|
10-Q
|
000-33043
|
10.1
|
|
8/9/2013
|
||
|
10.35
|
|
Credit Agreement between Omnicell, Inc., and lenders, dated September 25, 2013
|
8-K
|
000-33043
|
10.1
|
|
9/26/2013
|
||
|
21.1
+
|
|
Subsidiaries of the Registrant
|
|||||||
|
23.1
+
|
|
Consent of Independent Registered Public Accounting Firm
|
|||||||
|
24.1
+
|
|
Power of Attorney (included on the signature pages hereto)
|
|||||||
|
31.1
+
|
|
Certification of Chief Executive Officer, as required by Rule 13a-14(a) or Rule 15d-14(a)
|
|||||||
|
31.2
+
|
|
Certification of Chief Financial Officer, as required by Rule 13a-14(a) or Rule 15d-14(a)
|
|||||||
|
32.1
+
|
|
Certification of Chief Executive Officer, as required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350)
(1)
|
|||||||
|
32.2
+
|
|
Certification of Chief Financial Officer, as required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350)
(1)
|
|||||||
|
101.INS
+
|
|
XBRL Instance Document
(2)
|
|||||||
|
Incorporation By Reference
|
|||||||||
|
Exhibit
Number
|
|
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
|
Filing Date
|
||
|
101.SCH
+
|
|
XBRL Taxonomy Extension Schema Document
(2)
|
|||||||
|
101.CAL
+
|
|
XBRL Taxonomy Extension Calculation Linkbase Document
(2)
|
|||||||
|
101.DEF
+
|
|
XBRL Taxonomy Extension Definition Linkbase Document
(2)
|
|||||||
|
101.LAB
+
|
|
XBRL Taxonomy Extension Labels Linkbase Document
(2)
|
|||||||
|
101.PRE
+
|
|
XBRL Taxonomy Extension Presentation Linkbase Document
(2)
|
|||||||
|
*
|
Indicates a management contract or compensation plan or arrangement.
|
|
+
|
Filed herewith
|
|
(1)
|
This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of the Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.
|
|
(2)
|
Pursuant to applicable securities laws and regulations, the Registrant is deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and is not subject to liability under any anti-fraud provisions of the federal securities laws as long as the Registrant has made a good faith attempt to comply with the submission requirements and promptly amends the interactive data files after becoming aware that the interactive data files fail to comply with the submission requirements. These interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under these sections.
|