|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Washington
|
|
91-1663741
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification Number)
|
|
201 Elliott Avenue West
Seattle, Washington
|
|
98119
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Common Stock, $0.01 par value per share
|
The NASDAQ Stock Market LLC
|
|
(Title of each class)
|
(Name of each exchange on which registered)
|
|
Large accelerated filer
|
|
¨
|
|
Accelerated filer
|
|
x
|
|
Non-accelerated filer
|
|
¨
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
|
|
¨
|
|
•
|
our plans for sales, marketing and distribution of OMIDRIA
®
(phenylephrine and ketorolac injection) 1%/0.3%;
|
|
•
|
our expectations regarding our product sales and our estimate regarding how long our existing cash, cash equivalents, short-term investments and revenues will be sufficient to fund our anticipated operating expenses, capital expenditures and interest and principal payments on our outstanding notes under our Loan and Security Agreement, or the Oxford/EWB Loan Agreement, with Oxford Finance LLC, or Oxford, and East West Bank, or EWB;
|
|
•
|
our ability to raise additional capital through the capital markets, including under our at-the-market equity facility with JonesTrading Institutional Services LLC, or JonesTrading, or through one or more corporate partnerships, equity offerings, debt financings, collaborations, licensing arrangements or asset sales;
|
|
•
|
our ability to forecast accurately wholesaler demand as well as our estimates of chargebacks and rebates, distribution fees and estimated product returns;
|
|
•
|
our ability to enter into acceptable arrangements with potential corporate partners, including with respect to OMIDRIA;
|
|
•
|
our expectations regarding the clinical, therapeutic and competitive benefits of OMIDRIA and our product candidates;
|
|
•
|
our anticipation that we will rely on contract manufacturers to manufacture OMIDRIA for commercial sale and to manufacture our product candidates and our expectations regarding product supply and manufacturing of OMIDRIA;
|
|
•
|
our expectations about the commercial competition that OMIDRIA and our product candidates, if commercialized, may face;
|
|
•
|
our expectation that the OMIDRIAssure™ Reimbursement Services Program will increase patient access to OMIDRIA;
|
|
•
|
our expectations regarding cost impacts resulting from hiring sales representatives from Ventiv Commercial Services, LLC;
|
|
•
|
the extent of protection that our patents provide and that our pending patent applications will provide, if patents issue from such applications, for our technologies, programs, products and product candidates;
|
|
•
|
whether the dosing limitations in our OMS824 program may be removed;
|
|
•
|
our ability to design and successfully complete clinical trials and other studies for our products and product candidates and our plans and expectations regarding our clinical trials, including our clinical trials for OMS721 and for OMS824;
|
|
•
|
the expected course and costs of existing claims, legal proceedings and administrative actions, our involvement in potential claims, legal proceedings and administrative actions, and the potential outcomes and effects of both existing and potential claims, legal proceedings and administrative actions, as well as regulatory determinations, on our business, prospects, financial condition and results of operations;
|
|
•
|
our expectations regarding our OMS103 exclusive license agreement including, without limitation, manufacturing and commercialization of OMS103 and the commencement and subsequent continuation of product sales on which we could receive royalty revenue; and
|
|
•
|
our expected financial position, performance, revenues, growth, expenses, magnitude of net losses and the availability of resources.
|
|
|
Page
|
|
ITEM 1.
|
BUSINESS
|
|
•
|
OMIDRIAssure Information Hotline
for physicians and facilities seeking personalized help and information on OMIDRIA coverage and reimbursement for patients;
|
|
•
|
“Equal Access” Patient Assistance Program
providing assistance to financially eligible uninsured or government-insured patients; and
|
|
•
|
“We Pay the Difference” Commercial Reimbursement Program
providing assistance to patients with insufficient commercial insurance.
|
|
Product Candidate/Program
|
Targeted
Procedure/Disease
|
Development Status
|
Next Expected
Milestone
|
Worldwide
Rights
|
|
Clinical Programs
|
|
|
|
|
|
MASP-2 (OMS721) - Lectin Pathway Disorders
|
Atypical Hemolytic Uremic Syndrome (aHUS)
|
Phase 3
|
Initiate Phase 3 Enrollment
|
Omeros (In-licensed)
|
|
MASP-2 (OMS721) - Lectin Pathway Disorders
|
Thrombotic Microangiopathies (TMAs), including Hematopoietic Stem-Cell Transplant-Related TMAs and Thrombotic Thrombocytopenic Purpura (TTP)
|
Phase 2
|
Complete Phase 2 Trial
|
Omeros (In-licensed)
|
|
MASP-2 (OMS721) - Lectin Pathway Disorders
|
IgA Nephropathy and Other Renal Diseases
|
Phase 2
|
Complete Phase 2 Trial
|
Omeros (In-licensed)
|
|
PDE10 (OMS824) - CNS Disorders
|
Huntington’s Disease
|
Phase 2
|
Begin Enrollment in Redesigned Phase 2 Clinical Trials
|
Omeros
|
|
PDE10 (OMS824) - CNS Disorders
|
Schizophrenia
|
Phase 2
(1)
|
Submit Phase 2 Clinical Trial Protocol to FDA
|
Omeros
|
|
PPARγ (OMS405) - Addiction
|
Opioid and Nicotine Addiction
|
Phase 2
|
Analyze Phase 2 Data
|
Omeros
|
|
OMS201 - Urology
|
Ureteroscopy
|
Phase 1/2
|
Determine Commercialization Path
|
Omeros
|
|
Product Candidate/Program
|
Targeted
Procedure/Disease
|
Development Status
|
Next Expected
Milestone
|
Worldwide
Rights
|
|
Preclinical Programs
|
|
|
|
|
|
PDE7 (OMS527)
|
Addictions and Compulsive Disorders; Movement Disorders
|
Preclinical
|
Complete Human Dose-Enabling Toxicology Studies and GMP Manufacturing
|
Omeros (Compounds In-licensed)
|
|
Plasmin (OMS616)
|
Surgical and Traumatic Bleeding
|
Preclinical
|
Complete Human Dose-Enabling Toxicology Studies and GMP Manufacturing
|
Omeros (In-licensed)
|
|
MASP-3 (OMS906) - Alternative Pathway Disorders
|
Paroxysmal nocturnal hemoglobinuria (PNH) and Other Alternative Pathway Disorders
|
Preclinical
|
Complete Manufacturing Scale-up of a Clinical Candidate for IND-Enabling Toxicology Studies
|
Omeros
|
|
GPR17 - CNS Disorders
|
Demyelinating Disorders
|
Preclinical
|
Complete
in vivo
Proof-of-Concept Studies and Compound Optimization
|
Omeros
|
|
GPR101 - Metabolic Disorders
|
Appetite and Eating Disorders
|
Preclinical
|
Complete
in vivo
Proof-of-Concept Studies and Compound Optimization
|
Omeros
|
|
GPR151 - CNS Disorders
|
Neuropathic Pain and Cognition
|
Preclinical
|
Complete
in vivo
Proof-of-Concept Studies and Compound Optimization
|
Omeros
|
|
GPR161 - Cancer
|
Triple-Negative Breast Cancer
|
Preclinical
|
Complete
in vivo
Proof-of-Concept Studies and Compound Optimization
|
Omeros
|
|
GPR174 - Immune Disorders
|
Dysfunction of Regulatory T‑Cell (“T-Reg”) Modulation
|
Preclinical
|
Complete
in vivo
Proof-of-Concept Studies and Compound Optimization
|
Omeros
|
|
GPR183 - Skeletal and Infectious Diseases
|
Osteoporosis and EBV-Related Diseases
|
Preclinical
|
Complete
in vivo
Proof-of-Concept Studies and Compound Optimization
|
Omeros
|
|
GPCR Platform
|
CNS, Metabolic, CV, Oncologic, Musculoskeletal & Other Disorders
|
Preclinical
|
Continue Drug Discovery and Selected Medicinal Chemistry for Class A Orphan and Class B GPCRs
|
Omeros
|
|
Antibody Platform
|
Metabolic, CV, Oncologic, Musculoskeletal & Other Disorders
|
Preclinical
|
Continue Developing Antibodies Targeting Lectin and Alternative Pathway of Complement System and Expanding Antibody Library
|
Omeros (In-licensed)
|
|
(1)
|
Clinical trials evaluating OMS824 in schizophrenia were previously suspended at the request of the FDA and, given that we have not yet submitted a Phase 2 clinical trial protocol to FDA for review, remain suspended. For additional information, see “Clinical Programs-PDE10 Programs-OMS824 for Huntington’s Disease and Schizophrenia.”
|
|
•
|
develop and market products that are less expensive, more effective or safer than our future products;
|
|
•
|
commercialize competing products before we can launch our products;
|
|
•
|
operate larger research and development programs, possess greater manufacturing capabilities or have substantially greater financial resources than we do;
|
|
•
|
initiate or withstand substantial price competition more successfully than we can;
|
|
•
|
have greater success in recruiting skilled technical and scientific workers from the limited pool of available talent;
|
|
•
|
more effectively negotiate third-party licenses and strategic relationships; and
|
|
•
|
take advantage of acquisition or other opportunities more readily than we can.
|
|
•
|
OMIDRIA-Ophthalmology.
OMIDRIA is encompassed by our PharmacoSurgery patent portfolio. The relevant patents and patent applications in this portfolio are directed to combinations of agents, generic and/or proprietary to us or to others, drawn from therapeutic classes such as pain and inflammation inhibitory agents, mydriatic agents and agents that reduce intraocular pressure, delivered locally and intraoperatively to the site of ophthalmological procedures, including cataract and lens replacement surgery. As of March 8, 2016, we owned four issued U.S. patents and four pending U.S. patent applications and 31 issued patents and 55 pending patent applications in foreign markets that are directed to OMIDRIA.
|
|
•
|
OMS103-Arthroscopy.
OMS103 is encompassed by our PharmacoSurgery patent portfolio. The relevant patents and patent applications in this portfolio are directed to combinations of agents, generic and/or proprietary to us or to others, drawn from therapeutic classes such as pain and inflammation inhibitory agents and vasoconstrictive agents, delivered locally and intraoperatively to the site of medical or surgical procedures, including arthroscopy. As of March 8, 2016, we owned three issued U.S. patents, four pending U.S. patent applications, and 12 issued patents and 16 pending patent applications in foreign markets, that are directed to OMS103.
|
|
•
|
OMS201-Urology.
OMS201 is encompassed by our PharmacoSurgery patent portfolio. The relevant patents and patent applications in this portfolio are directed to combinations of agents, generic and/or proprietary to us or to others, drawn from therapeutic classes such as pain and inflammation inhibitory agents and spasm inhibitory agents, delivered locally and intraoperatively to the site of medical or surgical procedures, including uroendoscopy. As of March 8, 2016, we owned one issued U.S. patent, two pending U.S. patent applications, and an additional seven issued patents and two pending patent applications in foreign markets, that are directed to OMS201.
|
|
•
|
MASP-2 Program - OMS721.
We hold worldwide exclusive licenses to rights in connection with MASP-2, the antibodies targeting MASP-2 and the therapeutic applications for those antibodies from the University of Leicester, MRC and Helion. As of March 8, 2016, we exclusively controlled 13 issued patents and 22 pending patent applications in the U.S., and 61 issued patents and 87 pending patent applications in foreign markets, related to our MASP-2 program and associated complement targets.
|
|
•
|
PDE10 Program - OMS824.
As of March 8, 2016, we owned six issued patents and six pending patent application in the U.S., and 12 issued patents and 25 pending patent applications in foreign markets, that are directed to proprietary PDE10 inhibitors.
|
|
•
|
PPARγ Program - OMS405
. As of March 8, 2016, we owned one issued patent and three pending patent applications in the U.S., and 15 issued patents and 25 pending patent applications in foreign markets, directed to our discoveries linking PPARγ and addictive disorders.
|
|
•
|
PDE7 Program - OMS527
. As of March 8, 2016, we owned two issued patents and two pending patent applications in the U.S., and 14 issued patents and 17 pending patent applications in foreign markets directed to our discoveries linking PDE7 to movement disorders, as well as one issued patent and two pending patent applications in the U.S., and three issued patents and 25 pending patent applications in foreign markets directed to the link between PDE7 and addiction and compulsive disorders. Additionally, under a license from Daiichi Sankyo, we exclusively control rights to three issued U.S. patents and 57 issued and five pending patent applications in foreign markets that are directed to proprietary PDE7 inhibitors. For a more detailed description of our agreement with Daiichi Sankyo, see “License and Development Agreements.”
|
|
•
|
Plasmin Program - OMS616
. We hold worldwide exclusive licenses to a series of antifibrinolytic agents from The Regents of the University of California. As of March 8, 2016, we exclusively controlled three issued patents and two pending patent applications in the U.S. and 22 issued and 12 pending patent applications in foreign markets that are directed to these proprietary agents.
|
|
•
|
MASP-3 Program - OMS906
. We own and exclusively control under a license from the University of Leicester all rights to methods of treating various disorders and diseases by inhibiting MASP-3. As of March 8, 2016, we exclusively controlled two pending patent applications in the U.S. and 33 pending patent applications in foreign markets that are directed to these therapeutic methods.
|
|
•
|
GPR17
. As of March 8, 2016, we owned two pending patent applications in the U.S. directed to compounds that functionally interact with GPR17 and their therapeutic applications.
|
|
•
|
GPCR Platform.
As of March 8, 2016, we owned seven issued patents and 13 pending patent applications in the U.S., and 50 issued patents and seven pending patent applications in foreign markets, which are directed to previously unknown links between specific molecular targets in the brain and a series of CNS disorders, to our cellular redistribution assay and to other research tools that are used in our GPCR program, and to orphan GPCRs and other GPCRs for which we have identified functionally interacting compounds using our cellular redistribution assay.
|
|
•
|
Antibody Platform
. As of March 8, 2016, we owned and/or held worldwide exclusive license rights from the UW to four issued patents and four pending patent applications in the U.S., and three issued patents and 14 pending patent applications in foreign markets, directed to our antibody platform and antibodies generated using our platform.
|
|
•
|
PharmacoSurgery Platform.
Our scientific co-founders, Gregory A. Demopulos, M.D. and Pamela Pierce Palmer, M.D., Ph.D., conceived the initial invention underlying our PharmacoSurgery platform and transferred all of their related intellectual property rights to us in 1994. Other than their rights as shareholders, our scientific co-founders have not retained any rights to our PharmacoSurgery platform, except that if we file for liquidation under Chapter 7 of the U.S. Bankruptcy Act or voluntarily liquidate or dissolve, other than in connection with a merger, reorganization, consolidation or sale of assets, our scientific co-founders have the right to repurchase the initial PharmacoSurgery intellectual property at the then-current fair market value. Subsequent developments of the PharmacoSurgery intellectual property were assigned to us by Dr. Demopulos, Dr. Palmer and other of our employees and consultants, without restriction.
|
|
•
|
PDE10 and PDE7 Programs.
We acquired our PDE10 and PDE7 programs and some of our related patents and other intellectual property rights as a result of our acquisition of nura, inc. We hold an exclusive license to certain PDE7 inhibitors claimed in patents and pending patent applications owned by Daiichi Sankyo for use in the treatment of movement, addiction and compulsive disorders as well as other specified indications. For a more detailed description of our agreement with Daiichi Sankyo, see “License and Development Agreements.”
|
|
•
|
MASP Program.
We hold worldwide exclusive licenses to rights related to MASP-2, the antibodies targeting MASP-2 and the therapeutic applications for the antibodies from the University of Leicester, MRC and Helion. We jointly own and hold worldwide exclusive license rights related to therapeutic applications for inhibiting MASP-3 from the University of Leicester. For more detailed descriptions of these licenses, see “License and Development Agreements.”
|
|
•
|
PPARγ Program
. We acquired the patent applications and related intellectual property rights for our PPARγ program in 2009 from Roberto Ciccocioppo, Ph.D., of the Università di Camerino, Italy, pursuant to a patent assignment agreement. For a more detailed description of this agreement, see “License and Development Agreements.”
|
|
•
|
Plasmin Program.
We hold a worldwide exclusive license to patent rights related to certain antifibrinolytics from The Regents of the University of California. For a more detailed description of this agreement, see “License and Development Agreements.”
|
|
•
|
GPCR Platform.
We acquired our GPCR program and some of our related patents and other intellectual property rights as a result of our acquisition of nura, inc. In November of 2010 we acquired intellectual property rights related to an assay technology for our GPCR program from Patobios Limited for approximately $10.8 million.
|
|
•
|
Antibody Platform
. We hold a worldwide exclusive license to patent rights related to our antibody platform from the UW. For a more detailed description of this agreement, see “License and Development Agreements.
|
|
•
|
formulation development and manufacturing process development;
|
|
•
|
preclinical laboratory and animal testing;
|
|
•
|
submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; and in Europe, a CTA is filed according to the country’s local regulations;
|
|
•
|
adequate and well-controlled human clinical trials to establish the efficacy and safety of the product for each indication for which approval is sought;
|
|
•
|
adequate assessment of drug product stability to determine shelf life/expiry dating;
|
|
•
|
in Europe, submission to the EMA or national regulatory authority of a marketing authorization application, or MAA, and in the U.S., submission to the FDA of a New Drug Application, or NDA, in the case of a drug product, or a BLA in the case of a biologic product;
|
|
•
|
satisfactory completion of inspections of clinical sites at which clinical trials with the product were carried out and of the manufacturing facility or facilities at which the product is produced to assess compliance with current Good Clinical Practices, or cGCP, and cGMP; and
|
|
•
|
FDA review and approval of an NDA or BLA, or review and approval of an MAA by the applicable regulatory authorities in the EU.
|
|
•
|
Phase 1 usually involves the initial administration of the investigational product to human subjects, who may or may not have the disease or condition for which the product is being developed, to evaluate the safety, dosage tolerance, pharmacodynamics and, if possible, to gain an early indication of the effectiveness of the product.
|
|
•
|
Phase 2 usually involves trials in a limited patient population with the disease or condition for which the product is being developed to evaluate appropriate dosage, to identify possible adverse side effects and safety risks, and to evaluate preliminarily the effectiveness of the product for specific indications.
|
|
•
|
Phase 3 clinical trials usually further evaluate and confirm effectiveness and test further for safety by administering the product in its final form in an expanded patient population.
|
|
•
|
the federal Anti-Kickback Law, which prohibits offering or paying anything of value to a person or entity to induce the use of a good or service covered by a federal health care program such as Medicare or Medicaid;
|
|
•
|
the federal False Claims Act, which prohibits presenting or causing to be presented a false claim for payment by a federal health care program, and which has been interpreted to include claims caused by improper drug-manufacturer product promotion or the payment of kickbacks;
|
|
•
|
a variety of governmental pricing, price reporting, and rebate requirements, including those under Medicaid and the Veterans Health Care Act; and
|
|
•
|
the so-called Sunshine Act and related provisions of the Affordable Care Act, which require that we report to the federal government information on financial payments that we make to physicians and certain healthcare institutions and also on drug samples that we distribute.
|
|
•
|
a covered benefit under its health plan;
|
|
•
|
safe, effective and medically necessary;
|
|
•
|
appropriate for the specific patient;
|
|
•
|
cost-effective; and
|
|
•
|
neither experimental nor investigational.
|
|
Name
|
Age
|
Position(s)
|
||
|
Executive Officers:
|
|
|
||
|
Gregory A. Demopulos, M.D.
|
57
|
President, Chief Executive Officer and Chairman of the Board of Directors
|
||
|
Michael A. Jacobsen
|
58
|
Vice President, Finance, Chief Accounting Officer and Treasurer
|
||
|
Marcia S. Kelbon, J.D., M.S.
|
56
|
Vice President, Patent, General Counsel and Secretary
|
||
|
Significant Employees:
|
|
|
||
|
Christopher S. Bral, Ph.D.
|
50
|
Vice President, Nonclinical Development
|
||
|
Timothy M. Duffy
|
55
|
Vice President, Business Development
|
||
|
Kenneth M. Ferguson, Ph.D.
|
60
|
Vice President, Development and Chief Development Officer
|
||
|
George A. Gaitanaris, M.D., Ph.D.
|
59
|
Vice President, Science and Chief Scientific Officer
|
||
|
William J. Lambert, Ph.D.
|
57
|
Vice President, Chemistry, Manufacturing and Controls
|
||
|
Catherine A. Melfi, Ph.D.
|
57
|
Vice President, Regulatory Affairs and Quality Systems
|
||
|
Patricia Sandler
|
47
|
Vice President, Sales and Marketing
|
||
|
J. Steven Whitaker, M.D., J.D.
|
60
|
Vice President, Clinical Development and Chief Medical Officer
|
||
|
•
|
a lack of acceptance by physicians, patients, government and private payers and other members of the medical community;
|
|
•
|
our limited experience in marketing, selling and distributing OMIDRIA or any other product;
|
|
•
|
our limited experience managing third-party commercial manufacturing of OMIDRIA or any other product as well as our limited experience managing and maintaining a commercial sales organization;
|
|
•
|
pricing, reimbursement and coverage policies of government and private payers such as Medicare, Medicaid, the Department of Veterans Affairs, or VA, group purchasing organizations, insurance companies, health maintenance organizations and other plan administrators;
|
|
•
|
the availability, relative price and efficacy of the product as compared to alternative treatment options or branded, compounded or generic competing products;
|
|
•
|
an unknown safety risk;
|
|
•
|
the failure to enter into and maintain acceptable partnering arrangements for marketing and distribution of OMIDRIA outside of the U.S.;
|
|
•
|
changed or increased regulatory restrictions in the U.S., EU and other foreign territories; and
|
|
•
|
a lack of adequate financial or other resources.
|
|
•
|
the level and timing of commercial sales of OMIDRIA as well as our product candidates, if and when approved or commercialized;
|
|
•
|
the extent of coverage and reimbursement for OMIDRIA, including following the expiration of pass-through reimbursement effective January 1, 2018, and the amount of OMIDRIA chargebacks, rebates and product returns;
|
|
•
|
the extent of any payments received from collaboration arrangements and development funding as well as the achievement of development and clinical milestones under collaboration and license agreements that we may enter into from time to time and that may vary significantly from quarter to quarter; and
|
|
•
|
the timing, cost and level of investment in our research and development activities as well as expenditures we will or may incur to acquire or develop additional technologies, products and product candidates.
|
|
•
|
continue OMIDRIA sales and marketing;
|
|
•
|
continue research and development in our programs;
|
|
•
|
make principal and interest payments under the Oxford/EWB Loan Agreement;
|
|
•
|
initiate and conduct clinical trials for our programs and product candidates; and
|
|
•
|
commercialize and launch product candidates for which we may receive regulatory approval.
|
|
•
|
discussions with the FDA, the EMA or other foreign authorities regarding the scope or design of our clinical trials;
|
|
•
|
delays or the inability to obtain required approvals from Institutional Review Boards, Ethics Committees or other responsible entities at clinical sites selected for participation in our clinical trials;
|
|
•
|
delays in enrolling patients into clinical trials for any reason including disease severity, trial protocol design, study eligibility criteria, patient population size (
e.g.
, for orphan diseases or for some pediatric indications), proximity and/or availability of clinical trial sites for prospective patients, availability of competing therapies and clinical trials, regional differences in diagnosis and treatment, perceived risks and benefits of the product or product candidate, physician patient referral practices or the ability to monitor patients adequately before and after treatment;
|
|
•
|
lower than anticipated retention rates of patients in clinical trials;
|
|
•
|
the need to repeat or conduct additional clinical trials as a result of inconclusive or negative results, failure to replicate positive early clinical data in subsequent clinical trials, poorly executed testing, a failure of a clinical site to adhere to the clinical protocol, an unacceptable study design or other problems;
|
|
•
|
adverse findings in clinical or nonclinical studies related to the safety of our product candidates in humans;
|
|
•
|
an insufficient supply of product candidate materials or other materials necessary to conduct our clinical trials;
|
|
•
|
the need to qualify new suppliers of product candidate materials for FDA and foreign regulatory approval;
|
|
•
|
an unfavorable inspection or review by the FDA or other regulatory authority of a clinical trial site or records of any clinical investigation;
|
|
•
|
the occurrence of unacceptable drug-related side effects or adverse events experienced by participants in our clinical trials;
|
|
•
|
the suspension by a regulatory agency of a trial put on a clinical hold; and
|
|
•
|
the amendment of clinical trial protocols to reflect changes in regulatory requirements and guidance or other reasons as well as subsequent re-examination of amendments of clinical trial protocols by Institutional Review Boards or Ethics Committees.
|
|
•
|
failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
|
|
•
|
inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities resulting in the imposition of a clinical hold;
|
|
•
|
the failure to remove a clinical hold in a timely manner (which we cannot predict with certainty), if at all;
|
|
•
|
unforeseen safety issues or any determination that a trial presents unacceptable health risks; or
|
|
•
|
lack of adequate funding to continue the clinical trial or development program, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional trials and studies and increased expenses associated with the services of our contract research organizations, or CROs, and other third parties.
|
|
•
|
we may not be able to generate sufficient data to support full patent applications that protect the entire breadth of developments in one or more of our programs, including our GPCR program;
|
|
•
|
it is possible that one or more of our pending patent applications will not become an issued patent or, if issued, that the patent(s) will be sufficient to protect our technology, provide us with a basis for commercially viable products or provide us with any competitive advantages;
|
|
•
|
if our pending applications issue as patents, they may be challenged by third parties as not infringed, invalid or unenforceable under U.S. or foreign laws; or
|
|
•
|
if issued, the patents under which we hold rights may not be valid or enforceable.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Year Ended December 31, 2015
|
High
|
Low
|
||
|
4th Quarter
|
$16.52
|
$10.69
|
||
|
3rd Quarter
|
$30.23
|
$10.48
|
||
|
2nd Quarter
|
$26.64
|
$17.62
|
||
|
1st Quarter
|
$27.64
|
$18.51
|
||
|
Year Ended December 31, 2014
|
High
|
Low
|
||
|
4th Quarter
|
$25.10
|
$11.18
|
||
|
3rd Quarter
|
$18.80
|
$12.12
|
||
|
2nd Quarter
|
$18.01
|
$9.76
|
||
|
1st Quarter
|
$14.69
|
$10.11
|
||
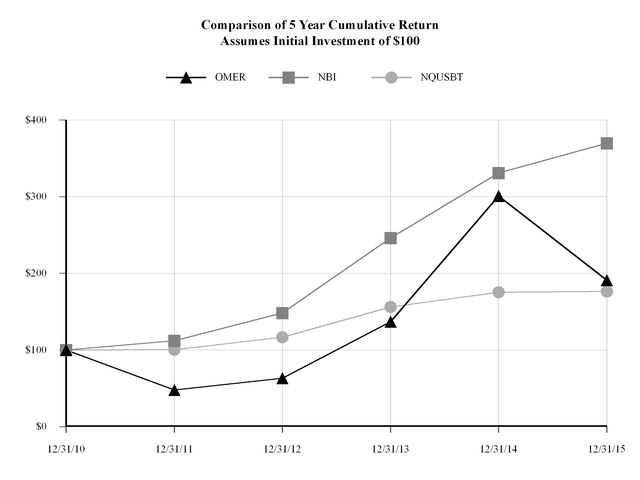
|
ITEM 6.
|
SELECTED CONSOLIDATED FINANCIAL DATA
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||
|
|
(In thousands, except per share and share data)
|
||||||||||||||||||
|
Consolidated Statements of Operations and Comprehensive Loss Data:
|
|||||||||||||||||||
|
Revenue
|
|||||||||||||||||||
|
Product Sales
|
$
|
13,264
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Grant revenue
|
245
|
|
539
|
|
1,600
|
|
6,022
|
|
4,524
|
|
|||||||||
|
Total revenue
|
13,509
|
|
539
|
|
1,600
|
|
6,022
|
|
4,524
|
|
|||||||||
|
Costs and expenses:
|
|
|
|
|
|||||||||||||||
|
Cost of product sales
|
1,041
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Research and development
|
48,379
|
|
47,946
|
|
36,297
|
|
31,922
|
|
23,718
|
|
|||||||||
|
Selling, general and administrative
|
35,327
|
|
22,601
|
|
15,819
|
|
10,985
|
|
8,216
|
|
|||||||||
|
Total costs and expenses
|
84,747
|
|
70,547
|
|
52,116
|
|
42,907
|
|
31,934
|
|
|||||||||
|
Loss from operations
|
(71,238
|
)
|
(70,008
|
)
|
(50,516
|
)
|
(36,885
|
)
|
(27,410
|
)
|
|||||||||
|
Litigation settlement
|
—
|
|
—
|
|
12,500
|
|
—
|
|
—
|
|
|||||||||
|
Interest expense
|
(3,573
|
)
|
(3,470
|
)
|
(2,366
|
)
|
(1,729
|
)
|
(1,884
|
)
|
|||||||||
|
Loss on early extinguishment of debt
|
(1,315
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Other income (expense), net
|
1,030
|
|
(195
|
)
|
586
|
|
170
|
|
748
|
|
|||||||||
|
Net Loss
|
$
|
(75,096
|
)
|
$
|
(73,673
|
)
|
$
|
(39,796
|
)
|
$
|
(38,444
|
)
|
$
|
(28,546
|
)
|
||||
|
Basic and diluted net loss per share
|
$
|
(2.00
|
)
|
$
|
(2.22
|
)
|
$
|
(1.39
|
)
|
$
|
(1.59
|
)
|
$
|
(1.29
|
)
|
||||
|
Denominator for basic and diluted net loss per share
|
37,560,257
|
|
33,234.294
|
|
28,560,360
|
|
24,155,690
|
|
22,212,351
|
|
|||||||||
|
|
As of December 31,
|
||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||
|
|
(In thousands)
|
||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
28,263
|
|
$
|
6,886
|
|
$
|
14,101
|
|
$
|
22,350
|
|
$
|
24,570
|
|
||||
|
Working capital
|
20,893
|
|
(9,274
|
)
|
2,944
|
|
16,341
|
|
6,963
|
|
|||||||||
|
Restricted cash and investments
|
10,679
|
|
679
|
|
679
|
|
679
|
|
193
|
|
|||||||||
|
Total assets
|
48,995
|
|
10,834
|
|
16,535
|
|
26,575
|
|
26,982
|
|
|||||||||
|
Notes payable, net of discount
|
49,842
|
|
32,453
|
|
20,498
|
|
20,103
|
|
19,446
|
|
|||||||||
|
Accumulated deficit
|
(403,142
|
)
|
(328,046
|
)
|
(254,373
|
)
|
(214,577
|
)
|
(176,133
|
)
|
|||||||||
|
Total shareholders’ deficit
|
(26,234
|
)
|
(42,654
|
)
|
(18,384
|
)
|
(6,531
|
)
|
(5,554
|
)
|
|||||||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Product sales, net
|
$
|
13,264
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Grant revenue:
|
|||||||||||
|
Small Business Innovative Research Grants (SBIR)
|
245
|
|
539
|
|
630
|
|
|||||
|
Vulcan Inc.
|
—
|
|
—
|
|
970
|
|
|||||
|
Total revenue
|
$
|
13,509
|
|
$
|
539
|
|
$
|
1,600
|
|
||
|
Chargebacks and Rebates
|
Distribution Fees and Product Return Allowances
|
Total
|
||||||||||
|
(In thousands)
|
||||||||||||
|
Balance as of December 31, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Provision related to current period sales
|
320
|
|
555
|
|
875
|
|
||||||
|
Payments for current period sales
|
(140
|
)
|
(278
|
)
|
(418
|
)
|
||||||
|
Balance as of December 31, 2015
|
$
|
180
|
|
$
|
277
|
|
$
|
457
|
|
|||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Direct external expenses:
|
|||||||||||
|
Clinical research and development:
|
|||||||||||
|
OMS824
|
$
|
1,508
|
|
$
|
10,974
|
|
$
|
7,265
|
|
||
|
OMS721
|
15,852
|
|
8,064
|
|
1,996
|
|
|||||
|
OMIDRIA (OMS302)
|
4,396
|
|
5,294
|
|
4,477
|
|
|||||
|
Other clinical programs
|
37
|
|
144
|
|
439
|
|
|||||
|
Total clinical research and development
|
21,793
|
|
24,476
|
|
14,177
|
|
|||||
|
Preclinical research and development
|
1,383
|
|
2,252
|
|
4,149
|
|
|||||
|
Total direct external expenses
|
23,176
|
|
26,728
|
|
18,326
|
|
|||||
|
Internal, overhead and other expenses
|
20,226
|
|
16,464
|
|
14,383
|
|
|||||
|
Stock-based compensation expense
|
4,977
|
|
4,754
|
|
3,588
|
|
|||||
|
Total research and development expenses
|
$
|
48,379
|
|
$
|
47,946
|
|
$
|
36,297
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Selling, general and administrative expenses, excluding stock-based compensation expense
|
$
|
30,723
|
|
$
|
18,437
|
|
$
|
13,155
|
|
||
|
Stock-based compensation expense
|
4,604
|
|
4,164
|
|
2,664
|
|
|||||
|
Total selling, general and administrative expenses
|
$
|
35,327
|
|
$
|
22,601
|
|
$
|
15,819
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Interest Expense
|
$
|
3,573
|
|
$
|
3,470
|
|
$
|
2,366
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Other Income (Expense), Net
|
$
|
1,030
|
|
$
|
(195
|
)
|
$
|
586
|
|
||
|
|
Years ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Cash provided by (used in):
|
|||||||||||
|
Operating activities
|
$
|
(65,209
|
)
|
$
|
(58,044
|
)
|
$
|
(29,695
|
)
|
||
|
Investing activities
|
(20,606
|
)
|
6,157
|
|
7,909
|
|
|||||
|
Financing activities
|
86,826
|
|
50,857
|
|
21,650
|
|
|||||
|
|
Payments Due Within
|
||||||||||||||||||
|
|
1 Year
|
2-3 Years
|
4-5 Years
|
More than
5 Years
|
Total
|
||||||||||||||
|
|
(In thousands)
|
||||||||||||||||||
|
Operating leases
|
$
|
4,106
|
|
$
|
8,447
|
|
$
|
8,800
|
|
$
|
33,263
|
|
$
|
54,616
|
|
||||
|
Capital leases (principal and interest)
|
86
|
|
165
|
|
62
|
|
—
|
|
313
|
|
|||||||||
|
Notes payable (principal and interest)
|
4,625
|
|
36,030
|
|
22,478
|
|
—
|
|
63,133
|
|
|||||||||
|
Goods & Services
|
$
|
1,396
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
1,396
|
|
|||||
|
Total
|
$
|
10,213
|
|
$
|
44,642
|
|
$
|
31,340
|
|
$
|
33,263
|
|
$
|
119,458
|
|
||||
|
•
|
revenue recognition;
|
|
•
|
research and development expenses, primarily clinical trial expenses; and
|
|
•
|
stock-based compensation.
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDER MATTERS
|
|
|
Number of Securities to be
Issued Upon Exercise of
Outstanding Options,
Warrants and Rights
|
Weighted-Average
Exercise Price of
Outstanding Options,
Warrants and Rights
|
Number of Securities
Remaining Available for
Future Issuance Under
Equity Compensation
Plans
|
||||||
|
Equity compensation plans approved by security holders:
|
|||||||||
|
2008 Equity Incentive Plan (1)
|
6,875,754
|
|
$
|
9.38
|
|
1,724,987
|
|
||
|
Amended and Restated 1998 Stock Option Plan
|
1,434,412
|
|
1.17
|
|
—
|
|
|||
|
nura inc.
|
69
|
|
10.63
|
|
—
|
|
|||
|
Total
|
8,310,235
|
|
$
|
7.97
|
|
1,724,987
|
|
||
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
OMEROS CORPORATION
|
|
/s/ GREGORY A. DEMOPULOS, M.D.
|
|
Gregory A. Demopulos, M.D.
|
|
President, Chief Executive Officer
and Chairman of the Board of Directors |
|
Signature
|
Title
|
Date
|
|
/s/ GREGORY A. DEMOPULOS, M.D.
|
President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer)
|
March 15, 2016
|
|
Gregory A. Demopulos, M.D.
|
||
|
/s/ MICHAEL A. JACOBSEN
|
Vice President, Finance, Chief Accounting Officer and Treasurer (Principal Financial Officer and Principal Accounting Officer)
|
March 15, 2016
|
|
Michael A. Jacobsen
|
||
|
/s/ RAY ASPIRI
|
Director
|
March 15, 2016
|
|
Ray Aspiri
|
||
|
/s/ THOMAS J. CABLE
|
Director
|
March 15, 2016
|
|
Thomas J. Cable
|
||
|
/s/ PETER A. DEMOPULOS, M.D.
|
Director
|
March 15, 2016
|
|
Peter A. Demopulos, M.D.
|
||
|
/s/ ARNOLD C. HANISH
|
Director
|
March 15, 2016
|
|
Arnold C. Hanish
|
||
|
/s/ LEROY E. HOOD, M.D., PH.D.
|
Director
|
March 15, 2016
|
|
Leroy E. Hood, M.D., Ph.D.
|
||
|
/s/ RAJIV SHAH, M.D.
|
Director
|
March 15, 2016
|
|
Rajiv Shah, M.D.
|
||
|
|
Page
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
|||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
1,365
|
|
$
|
354
|
|
|
|
Short-term investments
|
26,898
|
|
6,532
|
|
|||
|
Receivables
|
6,517
|
|
392
|
|
|||
|
Inventory
|
472
|
|
568
|
|
|||
|
Prepaid expense
|
1,894
|
|
1,195
|
|
|||
|
Total current assets
|
37,146
|
|
9,041
|
|
|||
|
Property and equipment, net
|
951
|
|
782
|
|
|||
|
Restricted cash and investments
|
10,679
|
|
679
|
|
|||
|
Other assets
|
219
|
|
332
|
|
|||
|
Total assets
|
$
|
48,995
|
|
$
|
10,834
|
|
|
|
Liabilities and shareholders’ deficit
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
6,428
|
|
$
|
4,915
|
|
|
|
Accrued expenses
|
9,752
|
|
7,070
|
|
|||
|
Current portion of notes payable
|
73
|
|
6,330
|
|
|||
|
Total current liabilities
|
16,253
|
|
18,315
|
|
|||
|
Notes payable, net
|
49,769
|
|
26,123
|
|
|||
|
Deferred rent
|
9,207
|
|
9,050
|
|
|||
|
Commitments and contingencies (Note 9)
|
|
|
|||||
|
Shareholders’ deficit:
|
|||||||
|
Preferred stock, par value $0.01 per share, 20,000,000 authorized and none issued at December 31, 2015 and 2014.
|
—
|
|
—
|
|
|||
|
Common Stock, par value $0.01 per share, 150,000,000 shares authorized at December 31, 2015 and 2014; 38,040,891 and 34,185,464 issued and outstanding at December 31, 2015 and December 31, 2014, respectively.
|
380
|
|
342
|
|
|||
|
Additional paid-in capital
|
376,528
|
|
285,050
|
|
|||
|
Accumulated deficit
|
(403,142
|
)
|
(328,046
|
)
|
|||
|
Total shareholders’ deficit
|
(26,234
|
)
|
(42,654
|
)
|
|||
|
Total liabilities and shareholders’ deficit
|
$
|
48,995
|
|
$
|
10,834
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
2015
|
2014
|
2013
|
|||||||||
|
Revenues:
|
|||||||||||
|
Product sales, net
|
$
|
13,264
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Grant revenue
|
245
|
|
539
|
|
1,600
|
|
|||||
|
Total revenue
|
13,509
|
|
539
|
|
1,600
|
|
|||||
|
Costs and expenses:
|
|||||||||||
|
Cost of product sales
|
1,041
|
|
—
|
|
—
|
|
|||||
|
Research and development
|
48,379
|
|
47,946
|
|
36,297
|
|
|||||
|
Selling, general and administrative
|
35,327
|
|
22,601
|
|
15,819
|
|
|||||
|
Total costs and expenses
|
84,747
|
|
70,547
|
|
52,116
|
|
|||||
|
Loss from operations
|
(71,238
|
)
|
(70,008
|
)
|
(50,516
|
)
|
|||||
|
Litigation settlement
|
—
|
|
—
|
|
12,500
|
|
|||||
|
Interest expense
|
(3,573
|
)
|
(3,470
|
)
|
(2,366
|
)
|
|||||
|
Loss on early extinguishment of debt
|
(1,315
|
)
|
—
|
|
—
|
|
|||||
|
Other income (expense), net
|
1,030
|
|
(195
|
)
|
586
|
|
|||||
|
Net loss
|
$
|
(75,096
|
)
|
$
|
(73,673
|
)
|
$
|
(39,796
|
)
|
||
|
Comprehensive loss
|
$
|
(75,096
|
)
|
$
|
(73,673
|
)
|
$
|
(39,796
|
)
|
||
|
Basic and diluted net loss per share
|
$
|
(2.00
|
)
|
$
|
(2.22
|
)
|
$
|
(1.39
|
)
|
||
|
Weighted-average shares used to compute basic and diluted net loss per share
|
37,560,257
|
|
33,234,294
|
|
28,560,360
|
|
|||||
|
|
Common Stock
|
Additional
Paid-in Capital |
Accumulated
Deficit |
Total
Shareholders’ Deficit |
||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||
|
Balance at December 31, 2012
|
25,897,483
|
|
$
|
259
|
|
$
|
207,787
|
|
$
|
(214,577
|
)
|
$
|
(6,531
|
)
|
||||
|
Issuance of common stock, net of offering costs
|
3,903,004
|
|
39
|
|
16,081
|
|
—
|
|
16,120
|
|
||||||||
|
Issuance of common stock utilizing At-The-Market Agreement, net of commissions
|
373,700
|
|
4
|
|
4,864
|
|
—
|
|
4,868
|
|
||||||||
|
Issuance of common stock upon exercise of stock options
|
185,321
|
|
2
|
|
660
|
|
—
|
|
662
|
|
||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
6,252
|
|
—
|
|
6,252
|
|
||||||||
|
Warrant modification
|
—
|
|
—
|
|
41
|
|
—
|
|
41
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(39,796
|
)
|
(39,796
|
)
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Balance at December 31, 2013
|
30,359,508
|
|
304
|
|
235,685
|
|
(254,373
|
)
|
(18,384
|
)
|
||||||||
|
Issuance of common stock, net of offering costs
|
3,500,000
|
|
35
|
|
37,719
|
|
—
|
|
37,754
|
|
||||||||
|
Issuance of common stock upon exercise of warrants
|
28,653
|
|
—
|
|
68
|
|
—
|
|
68
|
|
||||||||
|
Issuance of common stock upon exercise of stock options
|
297,303
|
|
3
|
|
1,797
|
|
—
|
|
1,800
|
|
||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
8,918
|
|
—
|
|
8,918
|
|
||||||||
|
Warrant modification
|
—
|
|
—
|
|
863
|
|
—
|
|
863
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(73,673
|
)
|
(73,673
|
)
|
||||||||
|
Balance at December 31, 2014
|
34,185,464
|
|
$
|
342
|
|
$
|
285,050
|
|
$
|
(328,046
|
)
|
$
|
(42,654
|
)
|
||||
|
Issuance of common stock and pre-funded warrants, net of offering costs
|
3,444,831
|
|
34
|
|
79,042
|
|
—
|
|
79,076
|
|
||||||||
|
Issuance of common stock upon exercise of warrants
|
133,240
|
|
1
|
|
1,435
|
|
—
|
|
1,436
|
|
||||||||
|
Issuance of common stock upon exercise of stock options
|
277,356
|
|
3
|
|
1,420
|
|
—
|
|
1,423
|
|
||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
9,581
|
|
—
|
|
9,581
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(75,096
|
)
|
(75,096
|
)
|
||||||||
|
Balance at December 31, 2015
|
38,040,891
|
|
$
|
380
|
|
$
|
376,528
|
|
$
|
(403,142
|
)
|
$
|
(26,234
|
)
|
||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
|
2014
|
2013
|
|||||||
|
Operating activities:
|
|||||||||||
|
Net loss
|
$
|
(75,096
|
)
|
$
|
(73,673
|
)
|
$
|
(39,796
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||||||
|
Loss on early extinguishment of debt
|
1,315
|
|
—
|
|
—
|
|
|||||
|
Depreciation and amortization
|
209
|
|
317
|
|
302
|
|
|||||
|
Stock-based compensation expense
|
9,581
|
|
8,918
|
|
6,252
|
|
|||||
|
Non-cash interest expense
|
1,045
|
|
738
|
|
502
|
|
|||||
|
Warrant modification expense
|
—
|
|
863
|
|
41
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Receivables
|
(6,125
|
)
|
(13
|
)
|
1,555
|
|
|||||
|
Inventory
|
96
|
|
(568
|
)
|
—
|
|
|||||
|
Prepaid expenses and noncurrent assets
|
(586
|
)
|
(987
|
)
|
84
|
|
|||||
|
Accounts payable and accrued expenses
|
4,195
|
|
5,459
|
|
(1,169
|
)
|
|||||
|
Deferred revenue
|
—
|
|
—
|
|
(970
|
)
|
|||||
|
Deferred rent
|
157
|
|
902
|
|
3,504
|
|
|||||
|
Net cash used in operating activities
|
(65,209
|
)
|
(58,044
|
)
|
(29,695
|
)
|
|||||
|
Investing activities:
|
|||||||||||
|
Purchases and sales of property and equipment, net
|
(240
|
)
|
(28
|
)
|
(204
|
)
|
|||||
|
Purchases of investments
|
(91,766
|
)
|
(58,849
|
)
|
(47,182
|
)
|
|||||
|
Proceeds from the sale and maturities of investments
|
71,400
|
|
65,034
|
|
55,295
|
|
|||||
|
Net cash provided by (used in) investing activities
|
(20,606
|
)
|
6,157
|
|
7,909
|
|
|||||
|
Financing activities:
|
|||||||||||
|
Proceeds from issuance of common stock and pre-funded warrants, net
|
79,076
|
|
37,754
|
|
20,988
|
|
|||||
|
Proceeds from borrowings under notes payable, net
|
22,329
|
|
12,699
|
|
—
|
|
|||||
|
Increase in restricted cash and investments
|
(10,000
|
)
|
—
|
|
—
|
|
|||||
|
Repayments on notes payable
|
(7,438
|
)
|
(1,464
|
)
|
—
|
|
|||||
|
Proceeds upon exercise of stock options and warrants
|
2,859
|
|
1,868
|
|
662
|
|
|||||
|
Net cash provided by financing activities
|
86,826
|
|
50,857
|
|
21,650
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
1,011
|
|
(1,030
|
)
|
(136)
|
||||||
|
Cash and cash equivalents at beginning of period
|
354
|
|
1,384
|
|
1,520
|
||||||
|
Cash and cash equivalents at end of period
|
$
|
1,365
|
|
$
|
354
|
|
$
|
1,384
|
|
||
|
Supplemental cash flow information
|
|||||||||||
|
Cash paid for interest
|
$
|
4,236
|
|
$
|
2,674
|
|
$
|
1,709
|
|
||
|
Reduction of equipment cost basis due to assets purchased with grant funding
|
$
|
—
|
|
$
|
80
|
|
$
|
—
|
|
||
|
Property acquired under capital lease
|
$
|
137
|
|
$
|
200
|
|
$
|
—
|
|
||
|
Percentage of Total Revenue
|
Percentage of Accounts Receivable
|
|||
|
Distributor A
|
37%
|
45%
|
||
|
Distributor B
|
31%
|
23%
|
||
|
Distributor C
|
28%
|
27%
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Outstanding options to purchase common stock
|
8,310,235
|
|
8,364,469
|
|
6,969,303
|
|
||
|
Warrants and pre-funded warrants to purchase common stock
|
749,250
|
|
551,435
|
|
609,016
|
|
||
|
Total potentially dilutive securities
|
9,059,485
|
|
8,915,904
|
|
7,578,319
|
|
||
|
|
December 31, 2015
|
||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
|
(In thousands)
|
||||||||||||||
|
Assets:
|
|||||||||||||||
|
Money-market funds classified as non-current restricted cash and investments
|
$
|
10,679
|
|
$
|
—
|
|
$
|
—
|
|
$
|
10,679
|
|
|||
|
Money-market funds classified as short-term investments
|
26,898
|
|
—
|
|
—
|
|
26,898
|
|
|||||||
|
Total
|
$
|
37,577
|
|
$
|
—
|
|
$
|
—
|
|
$
|
37,577
|
|
|||
|
|
December 31, 2014
|
||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
|
(In thousands)
|
||||||||||||||
|
Assets:
|
|||||||||||||||
|
Money-market funds classified as non-current restricted cash and investments
|
$
|
679
|
|
$
|
—
|
|
$
|
—
|
|
$
|
679
|
|
|||
|
Money-market funds classified as short-term investments
|
6,532
|
|
—
|
|
—
|
|
6,532
|
|
|||||||
|
Total
|
$
|
7,211
|
|
$
|
—
|
|
$
|
—
|
|
$
|
7,211
|
|
|||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
|
(In thousands)
|
||||||
|
Trade receivables, net
|
$
|
6,208
|
|
$
|
—
|
|
|
|
Grant receivables
|
136
|
|
324
|
|
|||
|
Other receivables
|
173
|
|
68
|
|
|||
|
Total receivables
|
$
|
6,517
|
|
$
|
392
|
|
|
|
December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
(In thousands)
|
|||||||
|
Raw materials
|
$
|
93
|
|
$
|
—
|
|
|
|
Work-in-process
|
158
|
|
—
|
|
|||
|
Finished goods
|
221
|
|
568
|
|
|||
|
Total inventory
|
$
|
472
|
|
$
|
568
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
|
(In thousands)
|
||||||
|
Laboratory equipment
|
$
|
1,735
|
|
$
|
1,636
|
|
|
|
Office equipment and furniture
|
625
|
|
615
|
|
|||
|
Computer equipment
|
482
|
|
403
|
|
|||
|
Capital lease equipment
|
367
|
|
230
|
|
|||
|
Computer software
|
174
|
|
126
|
|
|||
|
Total cost
|
3,383
|
|
3,010
|
|
|||
|
Less accumulated depreciation and amortization
|
(2,432
|
)
|
(2,228
|
)
|
|||
|
Total property and equipment, net
|
$
|
951
|
|
$
|
782
|
|
|
|
December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
|
(In thousands)
|
||||||
|
Contract research and development
|
$
|
2,973
|
|
$
|
1,280
|
|
|
|
Employee compensation
|
2,590
|
|
2,421
|
|
|||
|
Consulting and professional fees
|
2,400
|
|
1,952
|
|
|||
|
Clinical trials
|
1,108
|
|
828
|
|
|||
|
Other accruals
|
681
|
|
589
|
|
|||
|
Total accrued liabilities
|
$
|
9,752
|
|
$
|
7,070
|
|
|
|
Year Ending December 31,
|
Total
|
||
|
|
(In thousands)
|
||
|
2016
|
$
|
73
|
|
|
2017
|
7,627
|
|
|
|
2018
|
19,432
|
|
|
|
2019
|
21,258
|
|
|
|
2020
|
1,891
|
|
|
|
Total future principal payments
|
$
|
50,281
|
|
|
|
Year Ended December 31,
|
||||||||||
|
2015
|
2014
|
2013
|
|||||||||
|
|
(In thousands)
|
||||||||||
|
Small Business Innovative Research Grants (SBIR)
|
$
|
245
|
|
$
|
539
|
|
$
|
630
|
|
||
|
Vulcan Inc.
|
—
|
|
—
|
|
970
|
|
|||||
|
Total revenue
|
$
|
245
|
|
$
|
539
|
|
$
|
1,600
|
|
||
|
Year Ending December 31,
|
Lease
Payments |
Sublease
Receipt |
Net Lease
Payments |
|||||||||
|
|
(In thousands)
|
|||||||||||
|
2016
|
$
|
4,106
|
|
$
|
682
|
|
$
|
3,424
|
|
|||
|
2017
|
4,188
|
|
580
|
|
3,608
|
|
||||||
|
2018
|
4,259
|
|
—
|
|
4,259
|
|
||||||
|
2019
|
4,350
|
|
—
|
|
4,350
|
|
||||||
|
2020
|
4,450
|
|
—
|
|
4,450
|
|
||||||
|
Thereafter
|
33,263
|
|
—
|
|
33,263
|
|
||||||
|
Total
|
$
|
54,616
|
|
$
|
1,262
|
|
$
|
53,354
|
|
|||
|
Options granted and outstanding
|
8,310,235
|
|
|
Options available for future grant
|
1,724,987
|
|
|
Common stock warrants
|
749,250
|
|
|
Total shares reserved
|
10,784,472
|
|
|
Outstanding At
December 31, 2015 |
Expiration Date
|
Exercise Price
|
||
|
749,250
|
February 2, 2022
|
$0.01
|
||
|
•
|
five percent
of the outstanding shares of our common stock on the last day of the preceding year;
|
|
•
|
1,785,714
shares; or
|
|
•
|
such other amount as our board of directors may determine.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Estimated weighted-average fair value
|
$
|
11.31
|
|
$
|
7.39
|
|
$
|
6.87
|
|
||
|
Weighted-average assumptions:
|
|||||||||||
|
Expected volatility
|
71
|
%
|
73
|
%
|
88
|
%
|
|||||
|
Expected term, in years
|
6.0
|
|
5.8
|
|
5.8
|
|
|||||
|
Risk-free interest rate
|
1.68
|
%
|
1.87
|
%
|
1.66
|
%
|
|||||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
|||||
|
(A)
|
Expected Volatility.
Prior to October 14, 2014 the expected volatility rate used to value stock option grants was based on volatilities of a peer group of similar companies whose share prices were publicly available due to our limited trading history. The peer group was developed based on companies in the pharmaceutical and biotechnology industry in a similar stage of development. As of October 2014, we determined it was appropriate to rely 100% on our own historical realized volatility given our own trading history was roughly equivalent to the average expected term of our options and we do not anticipate future volatility will differ significantly from the past. This change in estimate did not have a material impact on our operating income, net income or earnings per share.
|
|
(B)
|
Expected Term.
We elected to utilize the “simplified” method for “plain vanilla” options to determine the expected term of our stock option grants. We will continue to use the simplified method until we have sufficient historical data necessary to provide a reasonable estimate of expected life based on the exercise behavior of our option holders. Under this approach, the weighted-average expected life is presumed to be the average of the vesting term and the contractual term of the option.
|
|
(C)
|
Risk-free Interest Rate.
The risk-free interest rate assumption was based on zero-coupon U.S. Treasury instruments that had terms consistent with the expected term of our stock option grants.
|
|
(D)
|
Expected Dividend Yield.
We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(In thousands)
|
||||||||||
|
Research and development
|
$
|
4,977
|
|
$
|
4,754
|
|
$
|
3,588
|
|
||
|
Selling, general and administrative
|
4,604
|
|
4,164
|
|
2,664
|
|
|||||
|
Total stock-based compensation expense
|
$
|
9,581
|
|
$
|
8,918
|
|
$
|
6,252
|
|
||
|
Options
Outstanding
|
Weighted-
Average
Exercise
Price per
Share
|
Remaining
Contractual Life
(in years)
|
Aggregate
Intrinsic
Value
(In thousands)
|
|||||||||
|
Balance at December 31, 2014
|
8,364,469
|
|
$
|
7.52
|
|
|||||||
|
Granted
|
350,975
|
|
17.90
|
|
||||||||
|
Exercised
|
(277,356
|
)
|
5.13
|
|
||||||||
|
Forfeited and expired
|
(127,853
|
)
|
12.36
|
|
||||||||
|
Balance at December 31, 2015
|
8,310,235
|
|
$
|
7.97
|
|
6.07
|
$
|
65,610
|
|
|||
|
Vested and expected to vest at December 31, 2015
|
8,096,339
|
|
$
|
7.85
|
|
6.00
|
$
|
64,731
|
|
|||
|
Exercisable at December 31, 2015
|
6,217,364
|
|
$
|
6.61
|
|
5.24
|
$
|
56,757
|
|
|||
|
|
December 31, 2015
|
|||||||||||||||
|
|
Options Outstanding
|
Options Exercisable
|
||||||||||||||
|
Range of Exercise Price
|
Number of
Options
|
Weighted-
Average
Remaining
Contractual
Life (Years)
|
Weighted-
Average
Exercise
Price
|
Number of
Options
|
Weighted-
Average
Exercise
Price
|
|||||||||||
|
$0.98-$4.09
|
1,434,412
|
|
1.07
|
$
|
1.17
|
|
1,434,412
|
|
$
|
1.17
|
|
|||||
|
$4.10-$8.00
|
1,975,794
|
|
5.12
|
5.25
|
|
1,958,011
|
|
5.23
|
|
|||||||
|
$8.01-$11.42
|
2,735,818
|
|
7.17
|
9.79
|
|
2,063,009
|
|
9.81
|
|
|||||||
|
$11.43-$25.00
|
2,164,211
|
|
8.86
|
12.66
|
|
761,932
|
|
11.73
|
|
|||||||
|
$0.98-$25.00
|
8,310,235
|
|
6.07
|
$
|
7.97
|
|
6,217,364
|
|
$
|
6.61
|
|
|||||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
|
(In thousands)
|
||||||
|
Deferred tax assets:
|
|
|
|||||
|
Net operating loss carryforwards
|
$
|
110,092
|
|
$
|
88,399
|
|
|
|
Tax credit carryforwards
|
14,758
|
|
9,569
|
|
|||
|
Stock-based compensation
|
8,136
|
|
5,511
|
|
|||
|
Deferred rent
|
3,324
|
|
3,077
|
|
|||
|
Other
|
2,744
|
|
2,084
|
|
|||
|
Total deferred tax assets
|
139,054
|
|
108,640
|
|
|||
|
Less valuation allowance
|
(139,054
|
)
|
(108,640
|
)
|
|||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
|
|
|
Year ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Federal statutory tax rate
|
(34
|
)%
|
(34
|
)%
|
(34
|
)%
|
||
|
State tax rates
|
(2
|
)%
|
—
|
%
|
—
|
%
|
||
|
Other permanent differences
|
1
|
%
|
2
|
%
|
3
|
%
|
||
|
Change in valuation allowance
|
41
|
%
|
35
|
%
|
36
|
%
|
||
|
Tax credits
|
(5
|
)%
|
(4
|
)%
|
(5
|
)%
|
||
|
Other
|
(1
|
)%
|
1
|
%
|
—
|
%
|
||
|
Effective tax rate
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
2015
|
For the Quarter Ended
|
|||||||||||||||
|
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
||||||||||||
|
Revenue
|
$
|
388
|
|
$
|
3,187
|
|
$
|
3,259
|
|
$
|
6,675
|
|
||||
|
Total operating expenses
|
18,318
|
|
19,154
|
|
22,560
|
|
24,715
|
|
||||||||
|
Loss from operations
|
(17,930
|
)
|
(15,967
|
)
|
(19,301
|
)
|
(18,040
|
)
|
||||||||
|
Net loss
|
(18,669
|
)
|
(16,680
|
)
|
(19,921
|
)
|
(19,826
|
)
|
||||||||
|
Basic and diluted net loss per share
|
$
|
(0.51
|
)
|
$
|
(0.44
|
)
|
$
|
(0.53
|
)
|
$
|
(0.52
|
)
|
||||
|
2014
|
For the Quarter Ended
|
|||||||||||||||
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
|||||||||||||
|
Revenue
|
$
|
100
|
|
$
|
45
|
|
$
|
214
|
|
$
|
180
|
|
||||
|
Total operating expenses
|
15,784
|
|
17,262
|
|
17,346
|
|
20,155
|
|
||||||||
|
Loss from operations
|
(15,684
|
)
|
(17,217
|
)
|
(17,132
|
)
|
(19,975
|
)
|
||||||||
|
Net loss
|
(16,642
|
)
|
(17,991
|
)
|
(18,327
|
)
|
(20,713
|
)
|
||||||||
|
Basic and diluted net loss per share
|
$
|
(0.54
|
)
|
$
|
(0.53
|
)
|
$
|
(0.54
|
)
|
$
|
(0.61
|
)
|
||||
|
|
Exhibit Description
|
Incorporated by Reference
|
||||
|
Exhibit
No.
|
Form
|
File No.
|
Exhibit
No.
|
Filing Date
|
Filed
Herewith
|
|
|
3.1
|
Amended and Restated Articles of Incorporation of Omeros Corporation
|
10-K
|
001-34475
|
3.1
|
03/31/2010
|
|
|
3.2
|
Amended and Restated Bylaws of Omeros Corporation
|
10-K
|
001-34475
|
3.2
|
03/31/2010
|
|
|
4.1
|
Form of Omeros Corporation common stock certificate
|
S-1/A
|
333-148572
|
4.1
|
10/02/2009
|
|
|
4.2
|
Form of Omeros Corporation Pre-Funded Warrant to Purchase Common Stock
|
8-K
|
001-34475
|
4.1
|
02/02/2015
|
|
|
10.1*
|
Form of Indemnification Agreement entered into between Omeros Corporation and its directors and officers
|
S-1
|
333-148572
|
10.1
|
01/09/2008
|
|
|
10.2*
|
Second Amended and Restated 1998 Stock Option Plan
|
S-1
|
333-148572
|
10.2
|
01/09/2008
|
|
|
10.3*
|
Form of Stock Option Agreement under the Second Amended and Restated 1998 Stock Option Plan (that does not permit early exercise)
|
S-1
|
333-148572
|
10.3
|
01/09/2008
|
|
|
10.4*
|
nura, inc. 2003 Stock Plan
|
S-1
|
333-148572
|
10.6
|
01/09/2008
|
|
|
10.5*
|
Form of Stock Option Agreement under the nura, inc. 2003 Stock Plan
|
S-1
|
333-148572
|
10.7
|
01/09/2008
|
|
|
10.6*
|
2008 Equity Incentive Plan
|
S-1/A
|
333-148572
|
10.8
|
04/01/2008
|
|
|
10.7*
|
Form of Stock Option Award Agreement under the 2008 Equity Incentive Plan
|
10-Q
|
001-34475
|
10.2
|
11/07/2013
|
|
|
10.8*
|
Second Amended and Restated Employment Agreement between Omeros Corporation and Gregory A. Demopulos, M.D. dated April 7, 2010
|
8-K
|
001-34475
|
10.1
|
04/12/2010
|
|
|
10.9*
|
Offer Letter between Omeros Corporation and Marcia S. Kelbon, Esq. dated August 16, 2001
|
S-1
|
333-148572
|
10.12
|
01/09/2008
|
|
|
10.10*
|
Technology Transfer Agreement between Omeros Corporation and Gregory A. Demopulos, M.D. dated June 16, 1994
|
S-1
|
333-148572
|
10.14
|
01/09/2008
|
|
|
10.11
|
Technology Transfer Agreement between Omeros Corporation and Pamela Pierce, M.D., Ph.D. dated June 16, 1994
|
S-1
|
333-148572
|
10.15
|
01/09/2008
|
|
|
10.12*
|
Second Technology Transfer Agreement between Omeros Corporation and Gregory A. Demopulos, M.D. dated December 11, 2001
|
S-1
|
333-148572
|
10.16
|
01/09/2008
|
|
|
10.13
|
Second Technology Transfer Agreement between Omeros Corporation and Pamela Pierce, M.D., Ph.D. dated March 22, 2002
|
S-1
|
333-148572
|
10.17
|
01/09/2008
|
|
|
10.14*
|
Technology Transfer Agreement between Omeros Corporation and Gregory A. Demopulos, M.D. dated June 16, 1994 (related to tendon splice technology)
|
S-1
|
333-148572
|
10.18
|
01/09/2008
|
|
|
10.15
|
Lease dated January 27, 2012 between Omeros Corporation and BMR-201 Elliott Avenue LLC
|
8-K
|
001-34475
|
10.1
|
02/01/2012
|
|
|
10.16
|
First Amendment to Lease dated November 5, 2012 between Omeros Corporation and BMR-201 Elliott Avenue LLC
|
10-Q
|
001-34475
|
10.2
|
11/09/2012
|
|
|
10.17
|
Second Amendment to Lease dated November 16, 2012 between Omeros Corporation and BMR-201 Elliott Avenue LLC
|
10-K
|
001-34475
|
10.18
|
03/18/2013
|
|
|
10.18
|
Third Amendment to Lease dated October 16, 2013 between Omeros Corporation and BMR-201 Elliott Avenue LLC
|
10-K
|
001-34475
|
10.18
|
03/13/2014
|
|
|
10.19
|
Fourth Amendment to Lease dated September 8, 2015 between Omeros Corporation and BMR-201 Elliott Avenue LLC
|
10-Q
|
001-34475
|
10.3
|
11/09/2015
|
|
|
10.20†
|
Commercial Supply Agreement between Omeros Corporation and Hospira Worldwide, Inc. dated October 9, 2007
|
S-1/A
|
333-148572
|
10.28
|
09/16/2009
|
|
|
10.21†
|
Exclusive License and Sponsored Research Agreement between Omeros Corporation and the University of Leicester dated June 10, 2004
|
S-1/A
|
333-148572
|
10.29
|
09/16/2009
|
|
|
10.22†
|
Research and Development Agreement First Amendment between Omeros Corporation and the University of Leicester dated October 1, 2005
|
S-1
|
333-148572
|
10.30
|
01/09/2008
|
|
|
10.23†
|
Research and Development Agreement Eighth and Ninth Amendments between Omeros Corporation and the University of Leicester dated March 21, 2012 and September 1, 2013
|
10-K
|
001-34475
|
10.24
|
03/16/2015
|
|
|
10.24†
|
Exclusive License and Sponsored Research Agreement between Omeros Corporation and the Medical Research Council dated October 31, 2005
|
S-1/A
|
333-148572
|
10.31
|
09/16/2009
|
|
|
10.25†
|
Amendment dated May 8, 2007 to Exclusive License and Sponsored Research Agreement between Omeros Corporation and the Medical Research Council dated October 31, 2005
|
S-1
|
333-148572
|
10.32
|
01/09/2008
|
|
|
10.26†
|
Funding Agreement between Omeros Corporation and The Stanley Medical Research Institute dated December 18, 2006
|
S-1/A
|
333-148572
|
10.33
|
05/15/2009
|
|
|
10.27†
|
Patent Assignment Agreement between Omeros Corporation and Roberto Ciccocioppo, Ph.D. dated February 23, 2009
|
S-1/A
|
333-148572
|
10.47
|
09/16/2009
|
|
|
10.28†
|
First Amendment to Patent Assignment Agreement between Omeros Corporation and Roberto Ciccocioppo, Ph.D. effective December 31, 2010
|
10-K
|
001-34475
|
10.28
|
03/18/2013
|
|
|
10.29*
|
Omeros Corporation Non-Employee Director Compensation Policy
|
S-1/A
|
333-148572
|
10.50
|
09/16/2009
|
|
|
10.30†
|
License Agreement between Omeros Corporation and Daiichi Sankyo Co., Ltd. (successor-in-interest to Asubio Pharma Co., Ltd.) dated March 3, 2010
|
10-Q
|
001-34475
|
10.1
|
05/12/2010
|
|
|
10.31†
|
Amendment No. 1 to License Agreement with an effective date of January 5, 2011 between Omeros Corporation and Daiichi Sankyo Co., Ltd.
|
10-Q
|
001-34475
|
10.1
|
05/10/2011
|
|
|
10.32†
|
Amendment No. 2 to License Agreement with an effective date of January 25, 2013 between Omeros Corporation and Daiichi Sankyo Co., Ltd.
|
10-Q
|
001-34475
|
10.1
|
05/09/2013
|
|
|
10.33†
|
Exclusive License Agreement between Omeros Corporation and Helion Biotech ApS dated April 20, 2010
|
10-Q
|
001-34475
|
10.2
|
08/10/2010
|
|
|
10.34†
|
Platform Development Funding Agreement between Omeros Corporation and Vulcan Inc. and its affiliate dated October 21, 2010
|
10-K
|
001-34475
|
10.44
|
03/15/2011
|
|
|
10.35†
|
Grant Award Agreement between Omeros Corporation and the Life Sciences Discovery Fund Authority dated October 21, 2010
|
10-K
|
001-34475
|
10.45
|
03/15/2011
|
|
|
10.36†
|
Pharmaceutical Manufacturing and Supply Agreement dated March 5, 2014 by and between Patheon Manufacturing Services, LLC (successor-in-interest to DSM Pharmaceuticals, Inc.) and Omeros Corporation
|
10-Q
|
001-34475
|
10.5
|
05/12/2014
|
|
|
10.37†
|
First Amendment to Pharmaceutical Manufacturing and Supply Agreement between Patheon Manufacturing Services (successor-in-interest to DSM Pharmaceuticals, Inc.) and Omeros Corporation dated July 7, 2015
|
10-Q
|
001-34475
|
10.2
|
11/09/2015
|
|
|
10.38†
|
Master Services Agreement between Omeros Corporation and Ventiv Commercial Services, LLC, made as of May 12, 2014
|
10-Q
|
001-34475
|
10.1
|
08/11/2014
|
|
|
10.39†
|
Project Agreement (Detailing and Sales Operation Services) between Omeros Corporation and Ventiv Commercial Services, LLC, made as of May 12, 2014
|
10-Q
|
001-34475
|
10.2
|
08/11/2014
|
|
|
10.40
|
First Amendment to Project Agreement (Detailing and Sales Operation Services) between Omeros Corporation and Ventiv Commercial Services, LLC, dated June 13, 2014
|
10-Q
|
001-34475
|
10.3
|
08/11/2014
|
|
|
10.41†
|
Second Amendment to Project Agreement (Detailing and Sales Operation Services) between Omeros Corporation and Ventiv Commercial Services, LLC, made as of October 17, 2014
|
10-K
|
001-34475
|
10.45
|
03/16/2015
|
|
|
10.42
|
Project Agreement (Sales Operation Services for Client Sales Teams) between Omeros Corporation and Ventiv Commercial Services, LLC, made as of January 1, 2016
|
X
|
||||
|
10.43†
|
Commercial Supply Agreement among Omeros Corporation, Hospira S.p.A. and Hospira Worldwide, Inc. dated October 3, 2014
|
10-K
|
001-34475
|
10.46
|
03/16/2015
|
|
|
10.44†
|
First Amendment to Commercial Supply Agreement dated August 1, 2015 by and between Omeros Corporation and Hospira Worldwide, Inc.
|
10-Q
|
001-34475
|
10.1
|
11/09/2015
|
|
|
10.45†
|
License Agreement effective as of June 9, 2015 by and between Omeros Corporation, JCB Laboratories, LLC, and Fagron Compounding Services, LLC, d/b/a Fagron Sterile Services
|
10-Q
|
001-34475
|
10.1
|
08/10/2015
|
|
|
10.46
|
At Market Issuance Sales Agreement dated January 6, 2016 between Omeros Corporation and JonesTrading Institutional Services LLC
|
8-K
|
001-34475
|
1.1
|
01/06/2016
|
|
|
10.47
|
Loan and Security Agreement between Omeros and Oxford Finance LLC, as collateral agent and as a lender, and East West Bank, as a lender, dated December 30, 2015
|
8-K
|
001-34475
|
10.1
|
01/06/2016
|
|
|
10.48
|
Form of Secured Promissory Note issued by Omeros to Oxford Finance LLC and to East West Bank, each dated December 30, 2015
|
8-K
|
001-34475
|
10.2
|
01/06/2016
|
|
|
12.1
|
Ratio of Earnings to Fixed Charges
|
X
|
||||
|
23.1
|
Consent of Independent Registered Public Accounting Firm
|
X
|
||||
|
31.1
|
Certification of Principal Executive Officer Pursuant to Rule 13-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
31.2
|
Certification of Principal Financial Officer Pursuant to Rule 13-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
32.1
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
32.2
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
99.1
|
Description of Capital Stock
|
10-K
|
001-34475
|
99.1
|
03/16/2015
|
|
|
101.INS
|
XBRL Instance Document
|
X
|
||||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
X
|
||||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
X
|
||||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
X
|
||||
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document
|
X
|
||||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
X
|
||||
|
*
|
Indicates management contract or compensatory plan or arrangement.
|
|
†
|
Portions of this exhibit are redacted in accordance with a grant of confidential treatment.
|
|
††
|
Portions of this exhibit are redacted in accordance with a request for confidential treatment.
|