|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Washington
|
|
91-1663741
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification Number)
|
|
201 Elliott Avenue West
Seattle, Washington
|
|
98119
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
(206) 676-5000
(Registrant’s telephone number, including area code)
|
||
|
Large accelerated filer
|
|
x
|
|
Accelerated filer
|
|
o
|
|
Non-accelerated filer
|
|
o
|
|
Smaller reporting company
|
|
o
|
|
Emerging growth company
|
o
|
|||||
|
Securities Registered pursuant to Section 12(b) of the Securities Exchange Act of 1934:
|
||
|
Common Stock, $0.01 par value per share
|
OMER
|
The Nasdaq Stock Market LLC
|
|
(Title of each class)
|
(Trading symbol)
|
(Name of each exchange on which registered)
|
|
•
|
our expectations relating to demand for OMIDRIA
®
(phenylephrine and ketorolac intraocular solution) 1%/0.3% from wholesalers, ambulatory surgery centers, or ASCs, and hospitals, and our expectations regarding OMIDRIA product sales;
|
|
•
|
our plans for the marketing and distribution of OMIDRIA and our estimates of OMIDRIA chargebacks and rebates, distribution fees and product returns;
|
|
•
|
our estimates regarding how long our existing cash, cash equivalents, short-term investments and revenues will be sufficient to fund our anticipated operating expenses, capital expenditures and debt service obligations;
|
|
•
|
our expectations related to obtaining a permanent separate or similar reimbursement for OMIDRIA from the Centers for Medicare & Medicaid Services, or CMS, particularly for periods after September 30, 2020;
|
|
•
|
our expectations regarding the clinical, therapeutic and competitive benefits and importance of OMIDRIA and our product candidates;
|
|
•
|
our ability to design, initiate and/or successfully complete clinical trials and other studies for our products and product candidates and our plans and expectations regarding our ongoing or planned clinical trials, including for our lead MASP-2 inhibitor, narsoplimab (also referred to as OMS721), and for OMS527 and OMS906;
|
|
•
|
in our narsoplimab clinical programs, our expectations regarding: whether enrollment in any or all ongoing and planned Phase 3 and Phase 2 clinical trials will proceed as expected; whether we can capitalize on the financial and regulatory incentives provided by orphan drug designations granted by the U.S. Food and Drug Administration, or FDA, the European Commission, or EC, or the European Medicines Agency, or EMA; and whether we can capitalize on the regulatory incentives provided by fast-track and/or breakthrough therapy designations granted by the FDA;
|
|
•
|
our expectations regarding clinical plans and anticipated or potential paths to regulatory approval of narsoplimab by the FDA and/or EMA in hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA), Immunoglobulin A (IgA) nephropathy, and/or atypical hemolytic uremic syndrome (aHUS);
|
|
•
|
whether and when a Biologics License Application, or BLA, may be filed with the FDA for narsoplimab in any indication and whether the FDA will grant accelerated or regular (full) approval for narsoplimab in any indication;
|
|
•
|
whether and when a marketing authorization application, or MAA, may be filed with the EMA for narsoplimab in any indication, and whether the EMA will grant approval for narsoplimab in any indication;
|
|
•
|
our expectation that we will rely on contract manufacturers to manufacture OMIDRIA for commercial sale and to manufacture our product candidates for purposes of clinical supply and in anticipation of potential commercialization;
|
|
•
|
our ability to enter into acceptable arrangements with potential corporate partners or contract service providers, including with respect to OMIDRIA or our product candidates, and our ability and plans to effect any such arrangement with respect to OMIDRIA in the European Union, or EU, or in other foreign countries;
|
|
•
|
our ability to raise additional capital through the capital markets or through one or more corporate partnerships, equity offerings, debt financings, collaborations, licensing arrangements or asset sales;
|
|
•
|
our expectations about the commercial competition that OMIDRIA and our product candidates, if commercialized, face or may face;
|
|
•
|
the expected course and costs of existing claims, legal proceedings and administrative actions, our involvement in potential claims, legal proceedings and administrative actions, and the merits, potential outcomes and effects of both existing and potential claims, legal proceedings and administrative actions, as well as regulatory determinations, on our business, prospects, financial condition and results of operations;
|
|
•
|
the extent of protection that our patents provide and that our pending patent applications will provide, if patents are issued from such applications, for our technologies, programs, products and product candidates;
|
|
•
|
the factors on which we base our estimates for accounting purposes and our expectations regarding the effect of changes in accounting guidance or standards on our operating results; and
|
|
•
|
our expected financial position, performance, revenues, growth, costs and expenses, magnitude of net losses and the availability of resources.
|
|
|
Page
|
|
March 31,
2019 |
December 31,
2018 |
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
4,054
|
|
|
$
|
5,861
|
|
|
Short-term investments
|
43,168
|
|
|
54,637
|
|
||
|
Receivables, net
|
24,718
|
|
|
22,818
|
|
||
|
Inventory
|
736
|
|
|
88
|
|
||
|
Prepaid expense and other assets
|
4,195
|
|
|
6,463
|
|
||
|
Total current assets
|
76,871
|
|
|
89,867
|
|
||
|
Property and equipment, net
|
4,479
|
|
|
3,845
|
|
||
|
Right of use assets
|
17,514
|
|
|
—
|
|
||
|
Restricted investments
|
1,154
|
|
|
1,154
|
|
||
|
Advanced payments, non-current
|
1,228
|
|
|
1,070
|
|
||
|
Total assets
|
$
|
101,246
|
|
|
$
|
95,936
|
|
|
Liabilities and shareholders’ deficit
|
|
|
|
|
|
||
|
Current liabilities:
|
|
|
|
|
|
||
|
Accounts payable
|
$
|
7,121
|
|
|
$
|
6,281
|
|
|
Accrued expenses
|
34,823
|
|
|
30,186
|
|
||
|
Current portion of lease liabilities
|
2,561
|
|
|
889
|
|
||
|
Total current liabilities
|
44,505
|
|
|
37,356
|
|
||
|
Lease liabilities, non-current
|
26,578
|
|
|
1,578
|
|
||
|
Unsecured convertible senior notes, net
|
151,182
|
|
|
148,981
|
|
||
|
Deferred rent
|
—
|
|
|
8,177
|
|
||
|
Commitments and contingencies (Note 8)
|
|
|
|
|
|||
|
Shareholders’ deficit:
|
|
|
|
|
|
||
|
Preferred stock, par value $0.01 per share, 20,000,000 shares authorized; none issued and outstanding at March 31, 2019 and December 31, 2018.
|
—
|
|
|
—
|
|
||
|
Common stock, par value $0.01 per share, 150,000,000 shares authorized at March 31, 2019 and December 31, 2018; 49,022,428 and 49,011,684 shares issued and outstanding at March 31, 2019 and December 31, 2018, respectively.
|
490
|
|
|
490
|
|
||
|
Additional paid-in capital
|
552,961
|
|
|
549,479
|
|
||
|
Accumulated deficit
|
(674,470
|
)
|
|
(650,125
|
)
|
||
|
Total shareholders’ deficit
|
(121,019
|
)
|
|
(100,156
|
)
|
||
|
Total liabilities and shareholders’ deficit
|
$
|
101,246
|
|
|
$
|
95,936
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
Revenue:
|
|
|
|
||||
|
Product sales, net
|
$
|
21,779
|
|
|
$
|
1,588
|
|
|
|
|
|
|
||||
|
Costs and expenses:
|
|
|
|
||||
|
Cost of product sales
|
131
|
|
|
203
|
|
||
|
Research and development
|
26,255
|
|
|
18,140
|
|
||
|
Selling, general and administrative
|
14,632
|
|
|
10,934
|
|
||
|
Total costs and expenses
|
41,018
|
|
|
29,277
|
|
||
|
Loss from operations
|
(19,239
|
)
|
|
(27,689
|
)
|
||
|
Interest expense
|
(5,600
|
)
|
|
(2,825
|
)
|
||
|
Other income
|
494
|
|
|
460
|
|
||
|
Net loss
|
$
|
(24,345
|
)
|
|
$
|
(30,054
|
)
|
|
Comprehensive loss
|
$
|
(24,345
|
)
|
|
$
|
(30,054
|
)
|
|
Basic and diluted net loss per share
|
$
|
(0.50
|
)
|
|
$
|
(0.62
|
)
|
|
Weighted-average shares used to compute basic and diluted net loss per share
|
49,014,009
|
|
|
48,284,019
|
|
||
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
|
2018
|
||||
|
Operating activities:
|
|||||||
|
Net loss
|
$
|
(24,345
|
)
|
|
$
|
(30,054
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
||||
|
Stock-based compensation expense
|
3,374
|
|
|
2,966
|
|
||
|
Non-cash interest expense
|
2,201
|
|
|
1,086
|
|
||
|
Depreciation and amortization
|
377
|
|
|
223
|
|
||
|
Changes in operating assets and liabilities:
|
|
|
|
||||
|
Receivables
|
(1,900
|
)
|
|
16,962
|
|
||
|
Inventory
|
(648
|
)
|
|
196
|
|
||
|
Prepaid expenses and other assets
|
2,110
|
|
|
(840
|
)
|
||
|
Accounts payable and accrued expenses
|
5,883
|
|
|
(1,835
|
)
|
||
|
Net cash used in operating activities
|
(12,948
|
)
|
|
(11,296
|
)
|
||
|
Investing activities:
|
|
|
|
||||
|
Purchases of property and equipment
|
(182
|
)
|
|
(183
|
)
|
||
|
Purchases of investments
|
(281
|
)
|
|
(270
|
)
|
||
|
Proceeds from the sale and maturities of investments
|
11,750
|
|
|
9,000
|
|
||
|
Net cash provided by investing activities
|
11,287
|
|
|
8,547
|
|
||
|
Financing activities:
|
|
|
|
||||
|
Proceeds upon exercise of stock options and warrants
|
108
|
|
|
687
|
|
||
|
Payments on finance lease liabilities
|
(254
|
)
|
|
(143
|
)
|
||
|
Net cash provided by (used in) financing activities
|
(146
|
)
|
|
544
|
|
||
|
Net decrease in cash and cash equivalents
|
(1,807
|
)
|
|
(2,205
|
)
|
||
|
Cash and cash equivalents at beginning of period
|
5,861
|
|
|
3,394
|
|
||
|
Cash and cash equivalents at end of period
|
$
|
4,054
|
|
|
$
|
1,189
|
|
|
Supplemental cash flow information
|
|
|
|
||||
|
Cash paid for interest
|
$
|
82
|
|
|
$
|
1,739
|
|
|
Conversion of accrued interest to notes payable
|
$
|
—
|
|
|
$
|
838
|
|
|
|
March 31,
|
||||
|
|
2019
|
2018
|
|||
|
Outstanding options to purchase common stock
|
11,840,521
|
|
9,640,452
|
|
|
|
Outstanding warrants to purchase common stock
|
243,115
|
|
100,602
|
|
|
|
Total potentially dilutive shares excluded from loss per share
|
12,083,636
|
|
9,741,054
|
|
|
|
|
March 31,
2019 |
December 31,
2018 |
|||||
|
|
(In thousands)
|
||||||
|
Trade receivables, net
|
$
|
24,580
|
|
$
|
22,654
|
|
|
|
Sublease and other receivables
|
138
|
|
164
|
|
|||
|
Total accounts receivables, net
|
$
|
24,718
|
|
$
|
22,818
|
|
|
|
|
March 31,
2019 |
December 31,
2018 |
|||||
|
|
(In thousands)
|
||||||
|
Raw materials
|
$
|
43
|
|
|
$
|
83
|
|
|
Work-in-progress
|
392
|
|
|
—
|
|
||
|
Finished goods
|
301
|
|
|
5
|
|
||
|
Total inventory
|
$
|
736
|
|
$
|
88
|
|
|
|
March 31,
2019 |
December 31,
2018 |
||||||
|
|
(In thousands)
|
||||||
|
Finance leases
|
$
|
4,863
|
|
|
$
|
4,034
|
|
|
Laboratory equipment
|
2,717
|
|
|
2,569
|
|
||
|
Computer equipment
|
896
|
|
|
862
|
|
||
|
Office equipment and furniture
|
625
|
|
|
625
|
|
||
|
Total cost
|
9,101
|
|
|
8,090
|
|
||
|
Less accumulated depreciation and amortization
|
(4,622
|
)
|
|
(4,245
|
)
|
||
|
Total property and equipment, net
|
$
|
4,479
|
|
|
$
|
3,845
|
|
|
March 31,
2019 |
December 31,
2018 |
||||||
|
|
(In thousands)
|
||||||
|
Contract research and development
|
$
|
14,460
|
|
|
$
|
12,012
|
|
|
Sales rebates, fees and discounts
|
8,161
|
|
|
8,075
|
|
||
|
Employee compensation
|
2,233
|
|
|
2,714
|
|
||
|
Consulting and professional fees
|
3,344
|
|
|
3,669
|
|
||
|
Interest payable
|
4,995
|
|
|
1,677
|
|
||
|
Clinical trials
|
913
|
|
|
820
|
|
||
|
Other accrued expenses
|
717
|
|
|
1,219
|
|
||
|
Total accrued expenses
|
$
|
34,823
|
|
|
$
|
30,186
|
|
|
|
March 31, 2019
|
||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
|
(In thousands)
|
||||||||||||||
|
Assets:
|
|||||||||||||||
|
Money-market funds classified as non-current restricted cash and investments
|
$
|
1,154
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,154
|
|
|||
|
Money-market funds classified as short-term investments
|
43,168
|
|
—
|
|
—
|
|
43,168
|
|
|||||||
|
Total
|
$
|
44,322
|
|
$
|
—
|
|
$
|
—
|
|
$
|
44,322
|
|
|||
|
|
December 31, 2018
|
||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
|
(In thousands)
|
||||||||||||||
|
Assets:
|
|||||||||||||||
|
Money-market funds classified as non-current restricted cash and investments
|
$
|
1,154
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,154
|
|
|||
|
Money-market funds classified as short-term investments
|
54,637
|
|
—
|
|
—
|
|
54,637
|
|
|||||||
|
Total
|
$
|
55,791
|
|
$
|
—
|
|
$
|
—
|
|
$
|
55,791
|
|
|||
|
|
March 31,
2019 |
December 31,
2018 |
|||||
|
(In thousands)
|
|||||||
|
Principal amount
|
$
|
210,000
|
|
$
|
210,000
|
|
|
|
Unamortized discount
|
(54,130
|
)
|
(56,156
|
)
|
|||
|
Unamortized issuance costs attributable to principal amount
|
(4,688
|
)
|
(4,863
|
)
|
|||
|
Total Convertible Notes, net
|
$
|
151,182
|
|
$
|
148,981
|
|
|
|
Classification on the Balance Sheet
|
March 31, 2019
|
|||||
|
Assets
|
(In thousands)
|
|||||
|
Operating lease assets
|
Right of use assets
|
$
|
17,514
|
|
||
|
Finance lease assets
|
Property and equipment, net
|
3,413
|
|
|||
|
Total lease assets
|
$
|
20,927
|
|
|||
|
Liabilities
|
||||||
|
Current:
|
||||||
|
Operating Leases
|
Current portion of lease liabilities
|
$
|
1,415
|
|
||
|
Finance Lease
|
Current portion of lease liabilities
|
1,146
|
|
|||
|
Non-current:
|
|
|||||
|
Operating
|
Lease liability, non-current
|
24,683
|
|
|||
|
Finance
|
Lease liability, non-current
|
1,895
|
|
|||
|
Total lease liabilities
|
$
|
29,139
|
|
|||
|
Weighted-average remaining lease term
|
||||||
|
Operating leases
|
8.6 years
|
|
||||
|
Finance leases
|
2.9 years
|
|
||||
|
Weighted-average discount rate
|
||||||
|
Operating leases (1)
|
12.85
|
%
|
||||
|
Finance leases
|
12.31
|
%
|
||||
|
Three Months Ended
March 31, 2019 |
|||
|
(In thousands)
|
|||
|
Lease cost
|
|||
|
Operating lease cost
|
$
|
1,031
|
|
|
Finance lease cost:
|
|||
|
Amortization
|
290
|
|
|
|
Interest
|
82
|
|
|
|
Short-term lease cost
|
138
|
|
|
|
Variable lease costs
|
486
|
|
|
|
Sublease income
|
(224
|
)
|
|
|
Total lease cost
|
$
|
1,803
|
|
|
Cash paid for amounts included in the measurement of lease liabilities:
|
|||
|
Operating cash flows from operating leases
|
$
|
1,647
|
|
|
Operating cash flows from finance leases
|
$
|
82
|
|
|
Financing cash flows from finance leases
|
$
|
254
|
|
|
Operating Leases
|
Finance Leases
|
||||||
|
(In thousands)
|
|||||||
|
2019
|
$
|
3,499
|
|
|
$
|
1,060
|
|
|
2020
|
4,770
|
|
|
1,224
|
|
||
|
2021
|
4,880
|
|
|
866
|
|
||
|
2022
|
4,995
|
|
|
290
|
|
||
|
2023
|
5,112
|
|
|
95
|
|
||
|
Thereafter
|
20,728
|
|
|
—
|
|
||
|
Total undiscounted lease payments
|
$
|
43,984
|
|
|
$
|
3,535
|
|
|
Less interest
|
(17,886
|
)
|
|
(494
|
)
|
||
|
Lease liabilities
|
$
|
26,098
|
|
|
$
|
3,041
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
(In thousands)
|
|||||||
|
Beginning and ending common stock
|
$
|
490
|
|
|
$
|
483
|
|
|
|
|
|
|
||||
|
Beginning additional paid-in capital
|
$
|
549,479
|
|
|
$
|
520,071
|
|
|
Exercise of stock options
|
108
|
|
|
687
|
|
||
|
Stock-based compensation expense
|
3,374
|
|
|
2,966
|
|
||
|
Ending additional paid-in capital
|
$
|
552,961
|
|
|
$
|
523,724
|
|
|
|
|
|
|
||||
|
Beginning accumulated deficit
|
$
|
(650,125
|
)
|
|
$
|
(523,368
|
)
|
|
Net loss
|
(24,345
|
)
|
|
(30,054
|
)
|
||
|
Ending accumulated deficit
|
$
|
(674,470
|
)
|
|
$
|
(553,422
|
)
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
|
(In thousands)
|
||||||
|
Research and development
|
$
|
1,494
|
|
$
|
1,200
|
|
|
|
Selling, general and administrative
|
1,880
|
|
|
1,766
|
|
||
|
Total
|
$
|
3,374
|
|
$
|
2,966
|
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
Estimated weighted-average fair value
|
$
|
9.47
|
|
|
$
|
9.79
|
|
|
Weighted-average assumptions
|
|
|
|||||
|
Expected volatility
|
81
|
%
|
|
76
|
%
|
||
|
Expected term, in years
|
6.0
|
|
|
6.1
|
|
||
|
Risk-free interest rate
|
2.48
|
%
|
|
2.54
|
%
|
||
|
Expected dividend yield
|
—
|
%
|
|
—
|
%
|
||
|
Options
Outstanding
|
Weighted-
Average
Exercise
Price per
Share
|
Remaining
Contractual Life
(In years)
|
Aggregate
Intrinsic
Value
(In thousands)
|
|||||||||
|
Balance at December 31, 2018
|
10,313,138
|
|
$
|
11.22
|
|
|||||||
|
Granted
|
1,606,500
|
|
13.49
|
|
||||||||
|
Exercised
|
(10,744
|
)
|
10.01
|
|
||||||||
|
Forfeited
|
(68,373
|
)
|
14.67
|
|
||||||||
|
Balance at March 31, 2019
|
11,840,521
|
|
$
|
11.51
|
|
6.62
|
$
|
71,857
|
|
|||
|
Vested and expected to vest at March 31, 2019
|
11,402,367
|
|
$
|
11.41
|
|
6.53
|
$
|
70,182
|
|
|||
|
Exercisable at March 31, 2019
|
7,815,290
|
|
$
|
10.31
|
|
5.35
|
$
|
56,232
|
|
|||
|
•
|
MASP-2 - narsoplimab (OMS721) - Lectin Pathway Disorders
.
Narsoplimab, also referred to as OMS721, is our lead human monoclonal antibody targeting mannan-binding lectin-associated serine protease-2, or MASP-2. MASP-2 is a novel pro-inflammatory protein target involved in activation of the complement system. The complement system plays a role in the body’s inflammatory response and becomes activated as a result of tissue damage or trauma or microbial pathogen invasion. Inappropriate or uncontrolled activation of the complement system can cause diseases characterized by serious tissue injury. MASP-2 is the effector enzyme of the lectin pathway of the complement system, and the current development focus for narsoplimab is diseases in which the lectin pathway has been shown to contribute to significant tissue injury and pathology. When not treated, these diseases are typically characterized by significant end organ injuries, such as kidney or central nervous system injury. Phase 3 clinical programs are underway for narsoplimab in: hematopoietic stem cell transplant-associated thrombotic microangiopathy, or HSCT-TMA; Immunoglobulin A, or IgA, nephropathy; and atypical hemolytic uremic syndrome, or aHUS. In addition, we have an ongoing Phase 2 clinical trial evaluating narsoplimab in renal diseases, currently focused on patients with IgA nephropathy.
|
|
•
|
HSCT-TMA: In the U.S., the FDA has granted narsoplimab (1) breakthrough therapy designation in patients who have persistent TMA despite modification of immunosuppressive therapy, (2) orphan drug designation for the prevention (inhibition) of complement-mediated TMAs, and (3) orphan drug designation for the treatment of HSCT-TMA. The EC also granted narsoplimab a designation as an orphan medicinal product for treatment in hematopoietic stem cell transplantation.
|
|
•
|
IgA nephropathy: In the U.S., narsoplimab has received from the FDA (1) breakthrough therapy designation for the treatment of IgA nephropathy and (2) orphan drug designation in IgA nephropathy. In Europe, narsoplimab has received from the EC designation as an orphan medicinal product for the treatment of primary IgA nephropathy.
|
|
•
|
aHUS: In the U.S., narsoplimab has received from the FDA (1) fast-track designation for the treatment of patients with aHUS and (2) orphan drug designation for the prevention (inhibition) of complement-mediated thrombotic microangiopathies.
|
|
•
|
PDE7 - OMS527
.
In our phosphodiesterase 7, or PDE7, program, we are developing proprietary compounds to treat addiction and compulsive disorders as well as movement disorders. A Phase 1 single-ascending- and multiple-ascending-dose clinical trial is underway and is designed to assess safety and pharmacokinetics of our lead compound in healthy subjects. We have completed dosing in all six cohorts in the single-ascending-dose portion of the trial, including a cohort to assess whether pharmacokinetics is affected by food. Dosing in three cohorts in the multiple-ascending-dose portion of the trial also recently completed. The compound to date has been well tolerated and pharmacokinetic data support once-daily dosing, with or without food. Completion of the Phase 1 trial is expected in the second or third quarter of 2019. Following Phase 1 completion, if successful, we plan to conduct a Phase 2a study targeting nicotine addiction.
|
|
•
|
MASP-3 - OMS906 - Alternative Pathway Disorders
. As part of our complement target program, we have identified mannan-binding lectin-associated serine protease-3, or MASP-3, which has been shown to be the key activator of the complement system’s alternative pathway, or APC. We believe that we are the first to make this and related discoveries associated with the APC. The complement system is part of the immune system’s innate response, and the APC is considered the amplification loop within the complement system. MASP-3 is responsible for the conversion of pro-
|
|
•
|
Other MASP Inhibitor Preclinical Programs
.
We have generated positive preclinical data from MASP-2 inhibition in
in vivo
models of age-related macular degeneration, myocardial infarction, diabetic neuropathy, stroke, ischemia-reperfusion injury, and other diseases and disorders. We are also developing small-molecule inhibitors of MASP-2 designed for oral administration that we are targeting for initiation of clinical trials in 2020, as well as additional antibodies targeting MASP-2. Development efforts are also directed to small-molecule inhibitors of MASP-3 and bispecific small- and large-molecule inhibitors of MASP-2/-3.
|
|
•
|
GPCR Platform and Programs
. We have developed a proprietary cellular redistribution assay, or CRA, which we use in a high-throughput manner to identify synthetic ligands, including antagonists, agonists and inverse agonists, that bind to and affect the function of orphan GPCRs. We are conducting
in vitro
and
in vivo
preclinical efficacy studies and optimizing compounds for a number of targets including: GPR151, which is linked to schizophrenia and cognition and GPR161, which is associated with triple negative breast cancer and various sarcomas.
One of our priorities in this program is GPR174. In
ex vivo
human studies, our small-molecule inhibitors targeting GPR174 upregulate the production of cytokines (e.g., IL-2, interferon-
γ
), block multiple checkpoint inhibitors (e.g., PDL-1, CTLA-4, LAG‑3) and tumor promoters (e.g., amphiregulin), and suppress regulatory T-cells (Tregs). Based on our data, we believe that GPR174 controls a major pathway in cancer and modulation of the receptor could provide a seminal advance in immuno-oncologic treatments for a wide range of solid and liquid tumors. We continue to focus on GPR174 and several other of our GPCR targets with the objective of moving compounds targeting them into human trials as rapidly as possible.
|
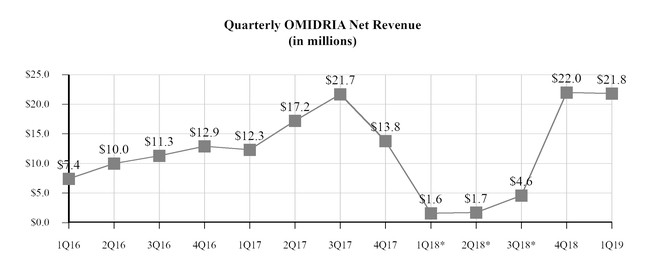
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
|
2018
|
||||
|
|
(In thousands)
|
||||||
|
Product sales, net
|
$
|
21,779
|
|
$
|
1,588
|
|
|
|
Chargebacks and Rebates
|
Distribution Fees and Product Return Allowances
|
Total
|
|||||||||
|
(In thousands)
|
|||||||||||
|
Balance as of December 31, 2018
|
$
|
7,015
|
|
|
$
|
1,485
|
|
|
$
|
8,500
|
|
|
Provisions
|
6,828
|
|
|
1,246
|
|
|
8,074
|
|
|||
|
Payments
|
(6,903
|
)
|
|
(1,119
|
)
|
|
(8,022
|
)
|
|||
|
Balance as of March 31, 2019
|
$
|
6,940
|
|
|
$
|
1,612
|
|
|
$
|
8,552
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
|
(In thousands)
|
||||||
|
Direct external expenses:
|
|||||||
|
Clinical research and development:
|
|||||||
|
MASP-2 Program - OMS721 (narsoplimab)
|
$
|
14,437
|
|
$
|
7,929
|
|
|
|
OMIDRIA - Ophthalmology
|
708
|
|
609
|
|
|||
|
PDE7 - OMS527
|
991
|
|
|
—
|
|
||
|
Other clinical programs
|
375
|
|
210
|
|
|||
|
Total clinical research and development
|
16,511
|
|
8,748
|
|
|||
|
Preclinical research and development
|
726
|
|
1,632
|
|
|||
|
Total direct external expenses
|
17,237
|
|
10,380
|
|
|||
|
Internal, overhead and other expenses
|
7,524
|
|
6,560
|
|
|||
|
Stock-based compensation expense
|
1,494
|
|
|
1,200
|
|
||
|
Total research and development expenses
|
$
|
26,255
|
|
$
|
18,140
|
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
|
(In thousands)
|
||||||
|
Selling, general and administrative expenses, excluding stock-based compensation expense
|
$
|
12,752
|
|
$
|
9,168
|
|
|
|
Stock-based compensation expense
|
1,880
|
|
|
1,766
|
|
||
|
Total selling, general and administrative expenses
|
$
|
14,632
|
|
$
|
10,934
|
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
|
(In thousands)
|
||||||
|
Interest expense
|
$
|
5,600
|
|
$
|
2,825
|
|
|
|
|
Three Months Ended
March 31, |
||||||
|
|
2019
|
2018
|
|||||
|
|
(In thousands)
|
||||||
|
Selected cash flow data
|
|||||||
|
Cash provided by (used in):
|
|||||||
|
Operating activities
|
$
|
(12,948
|
)
|
$
|
(11,296
|
)
|
|
|
Investing activities
|
11,287
|
|
8,547
|
|
|||
|
Financing activities
|
(146
|
)
|
544
|
|
|||
|
Exhibit
Number
|
Description
|
|
31.1
|
|
|
31.2
|
|
|
32.1
|
|
|
32.2
|
|
|
101.INS
|
XBRL Instance Document
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
OMEROS CORPORATION
|
|
|
Dated: May 9, 2019
|
/s/ Gregory A. Demopulos
|
|
Gregory A. Demopulos, M.D.
|
|
|
President, Chief Executive Officer and Chairman of the Board of Directors
|
|
|
Dated: May 9, 2019
|
/s/ Michael A. Jacobsen
|
|
Michael A. Jacobsen
|
|
|
Vice President, Finance, Chief Accounting Officer and Treasurer
|
|