|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Mark One)
|
|
|
T
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2018
|
|
|
OR
|
|
|
£
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from to
|
|
|
Cayman Islands
(State or Other Jurisdiction of
Incorporation or Organization)
|
98‑1209416
(I.R.S. Employer
Identification No.)
|
|
|
|
|
c/o Mourant Governance Services (Cayman) Limited
94 Solaris Avenue, Camana Bay
Grand Cayman
Cayman Islands
(Address of Principal Executive Offices)
|
KY1‑1108
(Zip Code)
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
American Depositary Shares, each representing 13 Ordinary Shares, par
value $0.0001 per share
|
|
The NASDAQ Global Select Market
|
|
Ordinary Shares, par value $0.0001 per share*
|
|
The Stock Exchange of Hong Kong Limited
|
|
Large accelerated filer
T
|
Accelerated filer
£
|
Non‑accelerated filer
£
|
Smaller reporting company
£
Emerging growth company
£
|
|
|
|
Page
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
•
|
the initiation, timing, progress and results of our preclinical studies and clinical trials and our research and development programs;
|
|
•
|
our ability to advance our drug candidates into, and successfully complete, clinical trials;
|
|
•
|
our reliance on the success of our clinical‑stage drug candidates;
|
|
•
|
our plans, expected milestones and the timing or likelihood of regulatory filings and approvals;
|
|
•
|
the commercialization of our drugs and drug candidates, if approved;
|
|
•
|
our ability to further develop sales and marketing capabilities and launch new drugs, if approved;
|
|
•
|
the pricing and reimbursement of our drugs and drug candidates, if approved;
|
|
•
|
the implementation of our business model, strategic plans for our business, drugs, drug candidates and technology;
|
|
•
|
the scope of protection we (or our licensors) are able to establish and maintain for intellectual property rights covering our drugs, drug candidates and technology;
|
|
•
|
our ability to operate our business without infringing, misappropriating or otherwise violating the intellectual property rights and proprietary technology of third parties;
|
|
•
|
costs associated with enforcing or defending against intellectual property infringement, misappropriation or violation, product liability and other claims;
|
|
•
|
regulatory developments in the United States, China, the United Kingdom, the European Union and other jurisdictions;
|
|
•
|
the accuracy of our estimates regarding expenses, revenues, capital requirements and our need for additional financing;
|
|
•
|
the potential benefits of strategic collaboration and licensing agreements and our ability to enter into strategic arrangements;
|
|
•
|
our ability to maintain and establish collaborations or licensing agreements;
|
|
•
|
our reliance on third parties to conduct drug development, manufacturing and other services;
|
|
•
|
our ability to manufacture and supply, or have manufactured and supplied, drug candidates for clinical development and drugs for commercial sale;
|
|
•
|
the rate and degree of market access and acceptance and reimbursement of our drugs and drug candidates, if approved;
|
|
•
|
developments relating to our competitors and our industry, including competing therapies;
|
|
•
|
the size of the potential markets for our drugs and drug candidates and our ability to serve those markets;
|
|
•
|
our ability to effectively manage our growth;
|
|
•
|
our ability to attract and retain qualified employees and key personnel;
|
|
•
|
statements regarding future revenue, hiring plans, expenses, capital expenditures, capital requirements and share performance;
|
|
•
|
the future trading price of our American Depositary Shares, or ADSs, and ordinary shares, and impact of securities analysts’ reports on these prices; and
|
|
•
|
other risks and uncertainties, including those listed under “Part I-Item 1A-Risk Factors.”
|
|
•
|
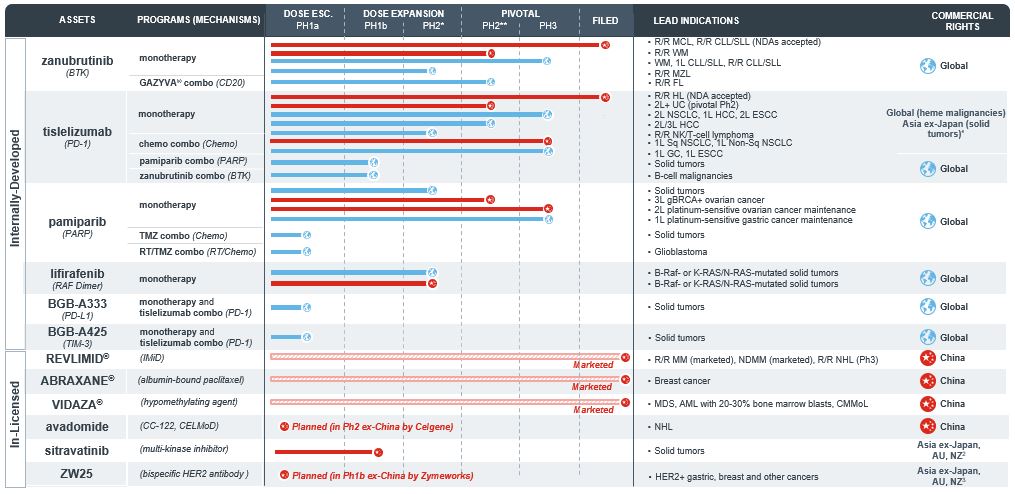
Zanubrutinib (BGB-3111)
- a potentially best-in-class investigational small molecule inhibitor of Bruton’s tyrosine kinase, or BTK, designed to maximize BTK occupancy and minimize off-target effects, that is currently being evaluated in a broad pivotal clinical program in China and in other markets, including the United States, Europe and Australia, which we refer to as globally, for which we submitted for approval in China in 2018 initially for the treatment of patients with relapsed or refractory (R/R) mantle cell lymphoma, or MCL, and chronic lymphocytic leukemia or small lymphocytic lymphoma, or CLL/SLL. We subsequently received priority review in China for both R/R MCL and R/R CLL/SLL. We also plan to submit in 2019 or early 2020 a new drug application, or NDA, to the U.S. Food and Drug Administration, or the FDA, and an NDA in China for Waldenström’s Macroglobulinemia, or WM. In the United States, the FDA has granted zanubrutinib Fast Track status in WM and Breakthrough Therapy designation for the treatment of adult patients with MCL who have received at least one prior therapy. We plan to launch zanubrutinib in China and the United States if we receive approval from the relevant regulatory authorities;
|
|
•
|
Tislelizumab (BGB-A317)
- an investigational humanized IgG4 monoclonal antibody against the immune checkpoint receptor programmed cell death protein 1, or PD-1, specifically designed to minimize binding to FcγR on macrophages, that is currently being evaluated in a broad pivotal clinical program for both solid tumor and hematological indications, both globally and in China, for which we submitted for approval in China in 2018 initially for the treatment of R/R classical Hodgkin’s lymphoma, or cHL. We subsequently received priority review in China, and we plan to launch tislelizumab in China if we receive approval. We also plan to file an NDA in China for the treatment of urothelial bladder cancer, or UBC; and
|
|
•
|
Pamiparib (BGB-290)
- an investigational small molecule inhibitor of poly ADP-ribose polymerase 1, or PARP1, and PARP2 enzymes that is being evaluated in two pivotal clinical trials in China, a global Phase 3 trial, and earlier-stage trials in solid tumor cancers.
|
|
•
|
Globally Develop and Commercialize Zanubrutinib, a Potentially Best-in-Class BTK Inhibitor.
Zanubrutinib is an investigational small molecule inhibitor of BTK that is currently being evaluated both as a monotherapy and in combination with other therapies to treat various lymphomas. Our clinical experience to date suggests a potentially best-in-class profile. To pursue this opportunity, we are conducting a broad pivotal clinical program globally and in China. We have submitted for approval in China for two indications based on single-arm Phase 2 clinical trials in patients with R/R CLL/SLL and R/R MCL. Both applications have been accepted and are being reviewed under priority review status. In addition, we are conducting three global Phase 3 trials: head-to-head against ibrutinib, an approved BTK inhibitor, for patients with WM; against bendamustine plus rituximab for patients with treatment naïve, or TN, CLL/SLL; and head-to-head against ibrutinib for patients with R/R CLL/SLL. Further, we are conducting a global pivotal Phase 2 trial in combination with obinutuzumab in follicular lymphoma, or FL, a pivotal Phase 2 trial in China in WM, and we have recently begun a global study in R/R marginal zone lymphoma, or MZL. Subject to the successful completion and satisfactory results of these trials, we expect to submit for approval of zanubrutinib in the United States in 2019 or early 2020, where it has been granted Fast Track status for patients with WM and Breakthrough Therapy designation for patients with R/R MCL. We also plan to file an NDA in China for patients with WM.
|
|
•
|
Develop and Commercialize Our Investigational Checkpoint Inhibitor, Tislelizumab, in a Rapidly and Favorably Evolving China Market and Other Markets.
We believe that there is a large and growing opportunity for novel cancer therapeutics in China and that the market opportunity for PD-1/PD-L1 antibody therapies may be especially attractive, as this class of agents has demonstrated anti-tumor activity in all four of the most common tumors in China: lung cancer, gastric cancer (GC), liver cancer and esophageal cancer (EC). We believe that we are uniquely positioned to capture this opportunity with our strong presence and experience in China and our integrated global clinical development capabilities in China and other Asia-Pacific countries, the United States, Europe and Australia. We have submitted an NDA in China to market tislelizumab for the treatment of patients with R/R cHL, and the application has been accepted and is being reviewed under priority review status. We are currently running 11 registration or potentially registration-enabling trials in six tumor types and expect to commence additional global pivotal trials in 2019 and 2020. We also plan to submit an NDA in China for patients with UBC. We have additional earlier stage exploratory studies ongoing, and we plan to initiate other studies.
|
|
•
|
Establish a Leadership Position by Further Expanding Our Capabilities.
Although we believe that we have significant integrated capabilities in research and clinical development, manufacturing and commercialization, we plan
|
|
•
|
Take Advantage of Significant Regulatory Reforms in China to Accelerate Global Drug Development.
Historically, the regulatory environment in China has been considered highly challenging, with clinical development significantly delayed and regulatory approvals taking much longer than in the United States and Europe. To address these challenges, the NMPA has issued a series of reform policies and opinions, which, among many things, are expected to expand access to clinical patients and expedite development and approval by removing delays and creating an environment with international quality standards for drug development, manufacturing and commercialization in China. We expect that these regulatory reforms will allow clinical trials in China to play a major role in global drug development programs. We also believe that the ability to effectively operate in China and integrate trials conducted in China with those in the rest of the world will be of increasing strategic importance. We are already taking advantage of these opportunities by conducting and leading dual-purpose global / China registration trials.
|
|
•
|
Expand Our Product Portfolio and Pipeline Through Collaborations with Other Biopharmaceutical Companies to Complement Our Internal Research.
We expect to further expand our portfolio of drugs and drug candidates, in oncology as well as potentially in other therapeutic areas, through internal research and external collaborations, such as our collaborations with Celgene, Mirati and Zymeworks. We intend to pursue collaborations with other biopharmaceutical companies both in China and globally by leveraging our strong clinical development capabilities globally and our commercial capabilities in China. We have pursued and plan to continue to pursue business development opportunities in which development in China is expected to contribute to, and potentially accelerate, the global development program. We believe that there will be increasing interest by international biopharmaceutical companies in seeking collaborations in Asia, particularly in oncology, because clinical recruitment is a major bottleneck in new drug development.
|
|
Indication
|
MZL
|
FL
|
FL
|
DLBCL
|
|
Source
|
ASH 2017
1
|
ASH 2017
1
|
CSCO 2018
2
|
ASH 2017
1
|
|
n
|
9
|
17
|
26
|
26
|
|
Follow-up
|
7.0 mo
|
7.8 mo
|
9.5 mo
|
4.2 mo
|
|
Prior Lines
|
2 (1-8)
|
2 (1-8)
|
3 (1-9)
|
2 (1-10)
|
|
ORR
|
78%
|
41%
|
42%
|
31%
|
|
CR
|
—%
|
18%
|
8%
|
15%
|
|
VGPR
|
—
|
—
|
—
|
—
|
|
PR/PR-L
|
78%
|
24%
|
35%
|
15%
|
|
MR
|
—
|
—
|
—
|
—
|
|
Tumor Type
|
Gastric Cancer
|
Esophageal Cancer
|
Head & Neck SCC
|
Ovarian Cancer
|
Hepato-cellular Carcinoma
|
Urothelial Cancer
|
NSCLC
|
MSI-H / dMMR
|
|
Source
|
ESMO-IO 2018
1
|
ESMO-IO 2018
1
|
ESMO 2017
2
|
ESMO 2017
3
|
ESMO-IO 2018
1
|
ESMO-IO 2018
4
|
ESMO-IO 2018
1
|
CSCO 2018
5
|
|
Median Treatment Duration
|
—
|
—
|
104 days (30-339)
|
71 days (29-540)
|
—
|
4.1 mo (0.7-26.3)
|
—
|
2.2 mo (0.69-11.1)
|
|
Median Follow-up Time
|
4.9 mo (0.9-25.4)
|
5.2 mo (0.2-22.7)
|
—
|
—
|
10.8 mo (0.7-31.6)
|
—
|
11.2 mo (0.5-25.9)
|
4.4 mo (0.1-10.7)
|
|
Median Duration of Response
|
8.5 mo
|
NR
|
—
|
—
|
15.7 mo
|
18.7 mo (6.2-18.7)
|
NR
|
—
|
|
Evaluable Patients
|
N=54
|
N=54
|
N=17
|
N=50
|
N=49
|
N=17
|
N=46
|
N=14
|
|
CR (Confirmed)
|
—
|
1
|
—
|
—
|
—
|
1
|
—
|
—
|
|
PR
|
7
|
5
|
3
|
2
|
6
|
4
|
6
|
4
|
|
SD
|
9
|
14
|
6
|
20
|
19
|
3
|
23
|
4
|
|
Patients Remaining on Treatment *
|
3
|
3
|
3
|
6
|
5
|
2
|
7
|
9
|
|
System Organ Class
Preferred Term
|
Phase 1a
|
Phase 1b
|
Total
|
|
N=116
n (%)
|
N=335
n (%)
|
N=451
n (%)
|
|
|
Patients with at least one TEAE
|
114 (25.3)
|
322 (71.4)
|
436 (96.7)
|
|
Fatigue
|
47 (10.4)
|
78 (17.3)
|
125 (27.7)
|
|
Nausea
|
41 (9.1)
|
68 (15.1)
|
109 (24.2)
|
|
Decreased appetite
|
19 (4.2)
|
71 (15.7)
|
90 (20.0)
|
|
Diarrhea
|
32 (7.1)
|
49 (10.9)
|
81 (18.0)
|
|
Constipation
|
26 (5.8)
|
50 (11.1)
|
76 (16.9)
|
|
Abdominal pain
|
26 (5.8)
|
38 (8.4)
|
64 (14.2)
|
|
Vomiting
|
20 (4.4)
|
43 (9.5)
|
63 (14.0)
|
|
Back pain
|
22 (4.9)
|
40 (8.9)
|
62 (13.7)
|
|
Cough
|
15 (3.3)
|
45 (10.0)
|
60 (13.3)
|
|
Rash
|
23 (5.1)
|
37 (8.2)
|
60 (13.3)
|
|
Dyspnea
|
12 (2.7)
|
33 (7.3)
|
45 (10.0)
|
|
•
|
completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices, or GLP, regulations;
|
|
•
|
submission to the FDA of an investigational new drug, or IND, application, which must become effective before human clinical trials may begin;
|
|
•
|
performance of adequate and well-controlled human clinical trials according to GCP, to establish the safety and efficacy of the proposed drug or safety, purity and potency of the proposed biologic, for the intended use;
|
|
•
|
preparation and submission to the FDA of an NDA for a drug or a BLA for a biologic;
|
|
•
|
a determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application for review;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMP;
|
|
•
|
FDA audits of some clinical trial sites to ensure compliance with GCPs; and
|
|
•
|
FDA review and approval of the NDA or licensing of the BLA.
|
|
•
|
Phase 1.
The product is initially introduced into a small number of healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients.
|
|
•
|
Phase 2.
Involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule.
|
|
•
|
Phase 3.
Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population. These clinical trials are intended to evaluate the overall risk/benefit relationship of the product and provide an adequate basis for product labeling.
|
|
•
|
the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs;
|
|
•
|
federal civil and criminal false claims and civil monetary penalty laws, such as the federal False Claims Act, which impose criminal and civil penalties and authorize civil whistleblower or qui tam actions, against individuals or entities for, among other things: knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent; making a false statement or record material to a false or fraudulent claim or obligation to pay or transmit money or property to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay money to the federal government. In addition, the government
|
|
•
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans and healthcare clearinghouses as well as their respective business associates who perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information;
|
|
•
|
the federal transparency requirements under the ACA, including the provision commonly referred to as the Physician Payments Sunshine Act, which require manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the U.S. Department of Health and Human Services information related to payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and
|
|
•
|
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers.
|
|
•
|
successful enrollment in, and completion of, clinical trials, as well as completion of preclinical studies;
|
|
•
|
favorable safety and efficacy data from our clinical trials and other studies;
|
|
•
|
receipt of regulatory approvals;
|
|
•
|
establishing commercial manufacturing capabilities, either by building facilities ourselves or making arrangements with third-party manufacturers;
|
|
•
|
the performance by contract research organizations, or CROs, or other third parties we may retain of their duties to us in a manner that complies with our protocols and applicable laws and that protects the integrity of the resulting data;
|
|
•
|
obtaining and maintaining patent, trade secret and other intellectual property protection and regulatory exclusivity;
|
|
•
|
ensuring we do not infringe, misappropriate or otherwise violate the patent, trade secret or other intellectual property rights of third parties;
|
|
•
|
successfully launching our drug candidates, if and when approved;
|
|
•
|
obtaining favorable reimbursement from third-party payors for drugs, if and when approved;
|
|
•
|
competition with other products;
|
|
•
|
continued acceptable safety profile following regulatory approval; and
|
|
•
|
manufacturing or obtaining sufficient supplies of our drugs, drug candidates and any competitor drug products that may be necessary for use in clinical trials for evaluation of our drug candidates and commercialization of our drugs.
|
|
•
|
be delayed in obtaining regulatory approval for our drug candidates;
|
|
•
|
not obtain regulatory approval at all;
|
|
•
|
obtain approval for indications that are not as broad as intended;
|
|
•
|
have the drug removed from the market after obtaining regulatory approval;
|
|
•
|
be subject to additional post-marketing testing requirements;
|
|
•
|
be subject to warning labels or restrictions on how the drug is distributed or used; or
|
|
•
|
be unable to obtain reimbursement for use of the drug.
|
|
•
|
failure to begin or complete clinical trials due to disagreements with regulatory authorities;
|
|
•
|
failure to demonstrate that a drug candidate is safe and effective or that a biologic candidate is safe, pure, and potent for its proposed indication;
|
|
•
|
failure of clinical trial results to meet the level of statistical significance required for approval;
|
|
•
|
reporting or data integrity issues related to our clinical trials;
|
|
•
|
disagreement with our interpretation of data from preclinical studies or clinical trials;
|
|
•
|
changes in approval policies or regulations that render our preclinical and clinical data insufficient for approval or require us to amend our clinical trial protocols;
|
|
•
|
regulatory requests for additional analyses, reports, data, nonclinical studies and clinical trials, or questions regarding interpretations of data and results and the emergence of new information regarding our drug candidates or other products;
|
|
•
|
failure to satisfy regulatory conditions regarding endpoints, patient population, available therapies and other requirements for our clinical trials in order to support marketing approval on an accelerated basis or at all;
|
|
•
|
our failure to conduct a clinical trial in accordance with regulatory requirements or our clinical trial protocols; and
|
|
•
|
clinical sites, investigators or other participants in our clinical trials deviating from a trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial.
|
|
•
|
regulatory authorities could delay or halt pending clinical trials;
|
|
•
|
we may suspend, delay or alter development of the drug candidate or marketing of the drug;
|
|
•
|
regulatory authorities may withdraw approvals or revoke licenses of the drug, or we may determine to do so even if not required;
|
|
•
|
regulatory authorities may require additional warnings on the label;
|
|
•
|
we may be required to develop a Risk Evaluation Mitigation Strategy, or REMS, for the drug, as is the case with REVLIMID®, or, if a REMS is already in place, to incorporate additional requirements under the REMS, or to develop a similar strategy as required by a comparable regulatory authority;
|
|
•
|
we may be required to conduct post-market studies; and
|
|
•
|
we could be sued and held liable for harm caused to subjects or patients.
|
|
•
|
restrictions on the marketing or manufacturing of our drugs, withdrawal of the product from the market, or voluntary or mandatory product recalls;
|
|
•
|
fines, untitled or warning letters, or holds on clinical trials;
|
|
•
|
refusal by the FDA, NMPA, EMA or comparable regulatory authorities to approve pending applications or supplements to approved applications filed by us or suspension or revocation of license approvals or withdrawal of approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of our drugs and drug candidates; and
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
|
•
|
the clinical indications for which our drugs and drug candidates are approved;
|
|
•
|
physicians, hospitals, cancer treatment centers and patients considering our drugs and drug candidates as a safe and effective treatment;
|
|
•
|
the potential and perceived advantages of our drugs and drug candidates over alternative treatments;
|
|
•
|
the prevalence and severity of any side effects;
|
|
•
|
product labeling or product insert requirements of regulatory authorities;
|
|
•
|
limitations or warnings contained in the labeling approved by regulatory authorities;
|
|
•
|
the timing of market introduction of our drugs and drug candidates as well as competitive drugs;
|
|
•
|
the cost of treatment in relation to alternative treatments;
|
|
•
|
the availability of adequate coverage, reimbursement and pricing by third-party payors and government authorities;
|
|
•
|
the willingness of patients to pay out-of-pocket in the absence of coverage and reimbursement by third-party payors and government authorities; and
|
|
•
|
the effectiveness of our sales and marketing efforts.
|
|
•
|
efforts to enter into collaboration or licensing arrangements with third parties in connection with our international sales, marketing and distribution efforts may increase our expenses or divert our management’s attention from the acquisition or development of drug candidates;
|
|
•
|
difficulty of effective enforcement of contractual provisions in local jurisdictions;
|
|
•
|
potential third-party patent rights or potentially reduced protection for intellectual property rights;
|
|
•
|
unexpected changes in tariffs, trade barriers and regulatory requirements, including the loss of normal trade status between China and the United States;
|
|
•
|
economic weakness, including inflation;
|
|
•
|
compliance with tax, employment, immigration and labor laws for employees traveling abroad;
|
|
•
|
the effects of applicable non-U.S. tax structures and potentially adverse tax consequences;
|
|
•
|
currency fluctuations, which could result in increased operating expenses and reduced revenue;
|
|
•
|
workforce uncertainty and labor unrest;
|
|
•
|
failure of our employees and contracted third parties to comply with Office of Foreign Asset Control rules and regulations and the Foreign Corrupt Practices Act and other anti-bribery and corruption laws; and
|
|
•
|
business interruptions resulting from geo-political actions, including trade disputes, war and terrorism, or natural disasters, including earthquakes, volcanoes, typhoons, floods, hurricanes and fires.
|
|
•
|
the progress, timing, scope and costs of our clinical trials, including the ability to timely enroll patients in our planned and potential future clinical trials;
|
|
•
|
the outcome, timing and cost of regulatory approvals of our drug candidates;
|
|
•
|
the number and characteristics of drug candidates that we may in-license and develop;
|
|
•
|
the amount and timing of the milestone and royalty payments we receive from our collaborators;
|
|
•
|
the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
|
|
•
|
selling and marketing costs associated with our drugs in China and any future drug candidates that may be approved, including the cost and timing of expanding our marketing and sales capabilities;
|
|
•
|
the terms and timing of any potential future collaborations, licensing or other arrangements that we may establish;
|
|
•
|
cash requirements of any future acquisitions, licensing and/or the development of other drug candidates;
|
|
•
|
the cost and timing of development and completion of commercial-scale internal or outsourced manufacturing activities; and
|
|
•
|
our headcount growth and associated costs.
|
|
•
|
we may be unable to identify manufacturers on acceptable terms or at all because the number of potential manufacturers is limited and the FDA, NMPA, EMA or other comparable regulatory authorities must evaluate and/or approve any manufacturers as part of their regulatory oversight of our drug candidates. This evaluation would require new testing and cGMP-compliance inspections by FDA, NMPA, EMA or other comparable regulatory authorities;
|
|
•
|
our manufacturers may have little or no experience with manufacturing our drug candidates, and therefore may require a significant amount of support from us in order to implement and maintain the infrastructure and processes required to manufacture our drug candidates;
|
|
•
|
our third-party manufacturers might be unable to timely manufacture our drugs and drug candidates or produce the quantity and quality required to meet our clinical and commercial needs, if any;
|
|
•
|
manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies in the United States to ensure strict compliance with cGMPs and other government regulations and by other comparable regulatory authorities for corresponding non-U.S. requirements. We do not have control over third-party manufacturers’ compliance with these regulations and requirements;
|
|
•
|
we may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our drug candidates and drugs;
|
|
•
|
raw materials and components used in the manufacturing process, particularly those for which we have no other source or supplier, may not be available or may not be suitable or acceptable for use due to material or component defects; and
|
|
•
|
our contract manufacturers and critical drug component suppliers may be subject to disruptions in their business, including inclement weather, as well as natural or man-made disasters.
|
|
•
|
collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration;
|
|
•
|
collaborators may not pursue development and commercialization of our drug candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in their strategic focus due to the acquisition of competitive drugs, availability of funding, or other external factors, such as a business combination that diverts resources or creates competing priorities;
|
|
•
|
collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a drug candidate, repeat or conduct new clinical trials, or require a new formulation of a drug candidate for clinical testing;
|
|
•
|
collaborators could independently develop, or develop with third parties, drugs that compete directly or indirectly with our drugs or drug candidates;
|
|
•
|
a collaborator with marketing and distribution rights to one or more drugs may not commit sufficient resources to their marketing and distribution;
|
|
•
|
collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
|
|
•
|
disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our drug candidates, or that result in costly litigation or arbitration that diverts management attention and resources;
|
|
•
|
collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable drug candidates; and
|
|
•
|
collaborators may own or co-own intellectual property covering our drugs that results from our collaborating with them, and in such cases, we would not have the exclusive right to commercialize such intellectual property.
|
|
•
|
identifying, recruiting, integrating, maintaining, and motivating additional employees;
|
|
•
|
managing our internal development efforts effectively, including the clinical and regulatory authority review process for our drug candidates, while complying with our contractual obligations to contractors and other third parties; and
|
|
•
|
improving our operational, financial and management controls, reporting systems and procedures.
|
|
•
|
increased operating expenses and cash requirements;
|
|
•
|
the assumption of additional indebtedness or contingent or unforeseen liabilities;
|
|
•
|
the issuance of our equity securities;
|
|
•
|
assimilation of operations, intellectual property and products of an acquired company, including difficulties associated with integrating new personnel;
|
|
•
|
the diversion of our management’s attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition;
|
|
•
|
retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships;
|
|
•
|
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or drug candidates and regulatory approvals; and
|
|
•
|
our inability to generate revenue from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
|
|
•
|
achieving adequate or clinical-grade materials that meet FDA, NMPA, EMA or other comparable regulatory agency standards or specifications with consistent and acceptable production yield and costs;
|
|
•
|
shortages of qualified personnel, raw materials or key contractors; and
|
|
•
|
ongoing compliance with cGMP regulations and other requirements of the FDA, NMPA, EMA or other comparable regulatory agencies.
|
|
•
|
we have failed to timely provide the depositary with our notice of meeting and related voting materials;
|
|
•
|
we have instructed the depositary that we do not wish a discretionary proxy to be given;
|
|
•
|
we have informed the depositary that there is substantial opposition as to a matter to be voted on at the meeting; or
|
|
•
|
a matter to be voted on at the meeting would have a material adverse impact on shareholders.
|

|
|
2/3/16
|
3/31/16
|
6/30/16
|
9/30/16
|
12/31/16
|
3/31/17
|
6/30/17
|
9/30/17
|
12/31/17
|
3/31/18
|
6/30/18
|
9/30/18
|
12/31/18
|
||||||||||||||||||||||||||||||||||||||
|
BeiGene, Ltd.
|
$
|
100.00
|
|
$
|
103.50
|
|
$
|
105.23
|
|
$
|
108.79
|
|
$
|
107.20
|
|
$
|
129.27
|
|
$
|
158.90
|
|
$
|
365.32
|
|
$
|
345.06
|
|
$
|
593.22
|
|
$
|
542.83
|
|
$
|
608.12
|
|
$
|
495.27
|
|
||||||||||||
|
NASDAQ Composite
|
100.00
|
|
105.84
|
|
105.58
|
|
115.84
|
|
117.20
|
|
129.17
|
|
134.58
|
|
142.37
|
|
151.45
|
|
155.11
|
|
164.94
|
|
176.31
|
|
145.93
|
|
|||||||||||||||||||||||||
|
NASDAQ Biotechnology
|
100.00
|
|
99.09
|
|
96.20
|
|
106.06
|
|
98.61
|
|
108.14
|
|
114.15
|
|
124.31
|
|
117.43
|
|
115.99
|
|
119.12
|
|
132.67
|
|
106.51
|
|
|||||||||||||||||||||||||
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2018
|
2017
|
2016
|
2015
|
2014
|
||||||||||||||
|
|
(in thousands, except share and per share data)
|
||||||||||||||||||
|
Statements of Operations:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Revenue:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Product revenue, net
|
$
|
130,885
|
|
$
|
24,428
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Collaboration revenue
|
67,335
|
|
213,959
|
|
1,070
|
|
8,816
|
|
13,035
|
|
|||||||||
|
Total revenues
|
198,220
|
|
238,387
|
|
1,070
|
|
8,816
|
|
13,035
|
|
|||||||||
|
Expenses
|
|||||||||||||||||||
|
Cost of sales - product
|
(28,705
|
)
|
(4,974
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Research and development (1)
|
(679,005
|
)
|
(269,018
|
)
|
(98,033
|
)
|
(58,250
|
)
|
(21,862
|
)
|
|||||||||
|
Selling, general and administrative
|
(195,385
|
)
|
(62,602
|
)
|
(20,097
|
)
|
(7,311
|
)
|
(6,930
|
)
|
|||||||||
|
Amortization of intangible assets
|
(894
|
)
|
(250
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total expenses
|
(903,989
|
)
|
(336,844
|
)
|
(118,130
|
)
|
(65,561
|
)
|
(28,792
|
)
|
|||||||||
|
Loss from operations
|
(705,769
|
)
|
(98,457
|
)
|
(117,060
|
)
|
(56,745
|
)
|
(15,757
|
)
|
|||||||||
|
Interest income (expense), net
|
13,947
|
|
(4,108
|
)
|
383
|
|
559
|
|
(3,512
|
)
|
|||||||||
|
Changes in fair value of financial instruments
|
—
|
|
—
|
|
(1,514
|
)
|
(1,826
|
)
|
(2,760
|
)
|
|||||||||
|
Gain on debt extinguishment
|
—
|
|
—
|
|
—
|
|
—
|
|
2,883
|
|
|||||||||
|
Other income, net
|
1,993
|
|
11,501
|
|
(972
|
)
|
910
|
|
600
|
|
|||||||||
|
Loss before income tax expense
|
(689,829
|
)
|
(91,064
|
)
|
(119,163
|
)
|
(57,102
|
)
|
(18,546
|
)
|
|||||||||
|
Income tax benefit (expense)
|
15,796
|
|
(2,235
|
)
|
(54
|
)
|
—
|
|
—
|
|
|||||||||
|
Net loss
|
(674,033
|
)
|
(93,299
|
)
|
(119,217
|
)
|
(57,102
|
)
|
(18,546
|
)
|
|||||||||
|
Less: net loss attributable to noncontrolling interest
|
(264
|
)
|
(194
|
)
|
—
|
|
—
|
|
(268
|
)
|
|||||||||
|
Net loss attributable to BeiGene, Ltd.
|
$
|
(673,769
|
)
|
$
|
(93,105
|
)
|
$
|
(119,217
|
)
|
$
|
(57,102
|
)
|
$
|
(18,278
|
)
|
||||
|
Loss per share attributable to BeiGene, Ltd, basic and diluted (2)
|
$
|
(0.93
|
)
|
$
|
(0.17
|
)
|
$
|
(0.30
|
)
|
$
|
(0.52
|
)
|
$
|
(0.18
|
)
|
||||
|
Weighted-average shares outstanding, basic and diluted
|
720,753,819
|
|
543,185,460
|
|
403,619,446
|
|
110,597,263
|
|
99,857,623
|
|
|||||||||
|
(1)
|
Included in research and development expense in 2018 is $89 million of upfront payments related to collaboration agreements with Zymeworks (see Note 3) and Mirati, and the termination of our collaboration agreement with Merck KGaA.
|
|
(2)
|
See Note 18 to our audited consolidated financial statements appearing elsewhere in this Annual Report for a description of the method used to calculate basic and diluted loss per share of ordinary shares.
|
|
|
As of December 31,
|
||||||||||||||||||
|
|
2018
|
2017
|
2016
|
2015
|
2014
|
||||||||||||||
|
|
(in thousands)
|
||||||||||||||||||
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Cash, cash equivalents, and restricted cash
|
$
|
740,713
|
|
$
|
239,602
|
|
$
|
87,514
|
|
$
|
17,869
|
|
$
|
13,898
|
|
||||
|
Short-term investments
|
1,068,509
|
|
597,914
|
|
280,660
|
|
82,617
|
|
30,497
|
|
|||||||||
|
Working capital
|
1,697,390
|
|
763,509
|
|
339,341
|
|
71,097
|
|
33,817
|
|
|||||||||
|
Total assets
|
2,249,684
|
|
1,046,479
|
|
405,813
|
|
116,764
|
|
53,621
|
|
|||||||||
|
Total liabilities
|
496,037
|
|
362,248
|
|
52,906
|
|
42,445
|
|
27,853
|
|
|||||||||
|
Preferred shares
|
—
|
|
—
|
|
—
|
|
176,084
|
|
78,809
|
|
|||||||||
|
Noncontrolling interest
|
14,445
|
|
14,422
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total equity (deficit)
|
1,753,647
|
|
684,231
|
|
352,907
|
|
(101,765
|
)
|
(53,041
|
)
|
|||||||||
|
•
|
expenses incurred under agreements with contract research organizations, or CROs, contract manufacturing organizations, and consultants that conduct and support clinical trials and preclinical studies;
|
|
•
|
costs of comparator drugs in certain of our clinical trials;
|
|
•
|
manufacturing costs related to pre-commercial activities;
|
|
•
|
costs associated with preclinical activities and development activities;
|
|
•
|
costs associated with regulatory operations;
|
|
•
|
employee‑related expenses, including salaries, benefits, travel and share‑based compensation expense for research and development personnel;
|
|
•
|
in-process research and development costs expensed as part of collaboration agreements entered into; and
|
|
•
|
other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other supplies used in research and development activities.
|
|
•
|
zanubrutinib, an investigational small molecule inhibitor of BTK;
|
|
•
|
tislelizumab, an investigational humanized monoclonal antibody against PD‑1;
|
|
•
|
pamiparib, an investigational small molecule inhibitor of PARP1 and PARP2;
|
|
•
|
lifirafenib, a novel small molecule inhibitor of both the monomer and dimer forms of BRAF;
|
|
•
|
BGB-A333, an investigational humanized monoclonal antibody against PD-L1; and
|
|
•
|
BGB-A425, an investigational humanized monoclonal antibody against TIM-3.
|
|
•
|
sitravatinib, an investigational, spectrum-selective kinase inhibitor in clinical development by Mirati Therapeutics, Inc., and
|
|
•
|
ZW25 and ZW49, two bispecific antibody-based product candidates targeting HER2, under development by Zymeworks Inc.
|
|
•
|
successful enrollment in and completion of clinical trials;
|
|
•
|
establishing an appropriate safety profile;
|
|
•
|
establishing commercial manufacturing capabilities or making arrangements with third‑party manufacturers;
|
|
•
|
receipt of marketing approvals from applicable regulatory authorities;
|
|
•
|
successfully launching and commercializing our drug candidates, if and when approved, whether as monotherapies or in combination with our internally discovered drug candidates or third‑party products;
|
|
•
|
obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our drug candidates;
|
|
•
|
continued acceptable safety profiles of the products following approval;
|
|
•
|
competition from competing products; and
|
|
•
|
retention of key personnel.
|
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2018
|
2017
|
$
|
%
|
||||||||||
|
|
(dollars in thousands)
|
|||||||||||||
|
Product revenue, net
|
$
|
130,885
|
|
$
|
24,428
|
|
$
|
106,457
|
|
436
|
%
|
|||
|
Collaboration revenue
|
67,335
|
|
213,959
|
|
(146,624
|
)
|
(69
|
)%
|
||||||
|
Total revenues
|
198,220
|
|
238,387
|
|
(40,167
|
)
|
(17
|
)%
|
||||||
|
Expenses
|
|
|
|
|
|
|
|
|
||||||
|
Cost of sales - product
|
(28,705
|
)
|
(4,974
|
)
|
(23,731
|
)
|
477
|
%
|
||||||
|
Research and development
|
(679,005
|
)
|
(269,018
|
)
|
(409,987
|
)
|
152
|
%
|
||||||
|
Selling, general and administrative
|
(195,385
|
)
|
(62,602
|
)
|
(132,783
|
)
|
212
|
%
|
||||||
|
Amortization of intangible assets
|
(894
|
)
|
(250
|
)
|
(644
|
)
|
258
|
%
|
||||||
|
Total expenses
|
(903,989
|
)
|
(336,844
|
)
|
(567,145
|
)
|
168
|
%
|
||||||
|
Loss from operations
|
(705,769
|
)
|
(98,457
|
)
|
(607,312
|
)
|
617
|
%
|
||||||
|
Interest (expense) income, net
|
13,947
|
|
(4,108
|
)
|
18,055
|
|
NM
|
|
||||||
|
Other income, net
|
1,993
|
|
11,501
|
|
(9,508
|
)
|
(83
|
)%
|
||||||
|
Loss before income tax expense
|
(689,829
|
)
|
(91,064
|
)
|
(598,765
|
)
|
658
|
%
|
||||||
|
Income tax expense
|
15,796
|
|
(2,235
|
)
|
18,031
|
|
NM
|
|
||||||
|
Net loss
|
(674,033
|
)
|
(93,299
|
)
|
(580,734
|
)
|
622
|
%
|
||||||
|
Less: Net loss attributable to noncontrolling interest
|
(264
|
)
|
(194
|
)
|
(70
|
)
|
36
|
%
|
||||||
|
Net loss attributable to BeiGene, Ltd.
|
$
|
(673,769
|
)
|
$
|
(93,105
|
)
|
$
|
(580,664
|
)
|
624
|
%
|
|||
|
|
Year Ended
|
|
|
|||||||||||
|
|
December 31,
|
Changes
|
||||||||||||
|
|
2018
|
2017
|
$
|
%
|
||||||||||
|
Product revenue
|
$
|
130,885
|
|
$
|
24,428
|
|
$
|
106,457
|
|
436
|
%
|
|||
|
Collaboration revenue:
|
|
|
|
|
|
|||||||||
|
License revenue
|
—
|
|
211,391
|
|
(211,391
|
)
|
(100
|
)%
|
||||||
|
Reimbursement of research and development costs
|
56,776
|
|
—
|
|
56,776
|
|
NM
|
|
||||||
|
Research and development service revenue
|
10,559
|
|
2,568
|
|
7,991
|
|
311
|
%
|
||||||
|
Total collaboration revenue
|
67,335
|
|
213,959
|
|
(146,624
|
)
|
(69
|
)%
|
||||||
|
Total
|
$
|
198,220
|
|
$
|
238,387
|
|
$
|
(40,167
|
)
|
(17
|
)%
|
|||
|
|
Year Ended
|
|
|
|
||||||||||
|
|
December 31,
|
|
Changes
|
|||||||||||
|
|
2018
|
|
2017
|
|
$
|
%
|
||||||||
|
|
(dollars in thousands)
|
|||||||||||||
|
External cost of clinical-stage programs
|
$
|
291,176
|
|
|
$
|
131,485
|
|
|
$
|
159,691
|
|
121
|
%
|
|
|
In-process research and development expense
|
89,000
|
|
—
|
|
89,000
|
|
—
|
%
|
||||||
|
External cost of non-clinical-stage programs
|
55,600
|
|
|
9,244
|
|
|
46,356
|
|
501
|
%
|
||||
|
Internal research and development expenses
|
243,229
|
|
|
128,289
|
|
|
114,940
|
|
90
|
%
|
||||
|
Total research and development expenses
|
$
|
679,005
|
|
|
$
|
269,018
|
|
|
$
|
409,987
|
|
152
|
%
|
|
|
•
|
Increases of approximately $54.2 million, $81.0 million, $20.0 million and $5.0 million, respectively, for zanubrutinib, tislelizumab, pamiparib and sitravatinib, partially offset by a decrease of approximately $0.5 million for lifirafenib. The expense increases were primarily due to the expansion of clinical trials for these candidates, including the initiation or continuation of pivotal trials;
|
|
•
|
Increase of $89.0 million related to in-process research and development expense including $10 million of our in-license of sitravatinib with Mirati for the Asia (excluding Japan), Australia and New Zealand territories, $60 million of upfront and milestone payments to Zymeworks, Inc., in order to obtain exclusive license to develop and commercialize ZW25 in the Asia (excluding Japan), Australia and New Zealand territories, and $19 million for the termination of the PARP collaboration agreement with Merck KGaA Darmstadt Germany; and
|
|
•
|
Approximately $46.4 million increase in external spending for our non-clinical-stage programs, primarily related to manufacturing costs and costs associated with advancing our preclinical candidates toward clinical trials.
|
|
•
|
$59.1 million increase of employee salary and benefits, which was primarily attributable to hiring more research and development personnel to support our expanding research and clinical activities;
|
|
•
|
$23.8 million increase of share-based compensation expense, primarily attributable to our increased headcount and higher share price;
|
|
•
|
$1.7 million increase of materials and reagent expenses, mainly in connection with the in-house manufacture of drug candidates used for clinical purposes, that were previously outsourced and recorded as external cost;
|
|
•
|
$15.1 million increase of consulting fees, which was mainly attributable to increased scientific, regulatory and development consulting activities, in connection with the advancement of our pipeline; and
|
|
•
|
$15.2 million increase of facilities, office expense, rental fee and other expenses to support the growth of our organization.
|
|
•
|
$46.5 million increase of employee salary and benefits, which was primarily attributable to the hiring of more personnel to support our growing organization, including the acquired workforce in the acquisition of Celgene’s China operations;
|
|
•
|
$20.5 million increase of share-based compensation expense, primarily attributable to our increased headcount and higher share price;
|
|
•
|
$13.3 million increase of professional fees for legal, consulting, recruiting and audit services, mainly in connection with our patent prosecution activities, consulting services, business development activities, compliance, recruiting services and the preparation of periodic reports and filings with the SEC and HKEx;
|
|
•
|
$9.2 million increase of IT expense, which was primarily attributable to increased headcount and upgrades to our IT infrastructure for human resources, financial systems and compliance management, and
|
|
•
|
$43.3 million increase of selling, facility, travel expenses, rental fees and other administrative expenses, primarily attributable to the global expansion of our business, including the post-combination operating costs of our commercial operations in China.
|
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2017
|
2016
|
$
|
%
|
||||||||||
|
|
(dollars in thousands)
|
|||||||||||||
|
Product revenue, net
|
$
|
24,428
|
|
$
|
—
|
|
$
|
24,428
|
|
—
|
|
|||
|
Collaboration revenue
|
213,959
|
|
1,070
|
|
212,889
|
|
19,896
|
%
|
||||||
|
Total revenues
|
238,387
|
|
1,070
|
|
237,317
|
|
22,179
|
%
|
||||||
|
Expenses
|
|
|
|
|
|
|
|
|
||||||
|
Cost of sales - product
|
(4,974
|
)
|
—
|
|
(4,974
|
)
|
—
|
|
||||||
|
Research and development
|
(269,018
|
)
|
(98,033
|
)
|
(170,985
|
)
|
174
|
%
|
||||||
|
Selling, general and administrative
|
(62,602
|
)
|
(20,097
|
)
|
(42,505
|
)
|
211
|
%
|
||||||
|
Amortization of intangible assets
|
(250
|
)
|
—
|
|
(250
|
)
|
—
|
|
||||||
|
Total expenses
|
(336,844
|
)
|
(118,130
|
)
|
(218,714
|
)
|
185
|
%
|
||||||
|
Loss from operations
|
(98,457
|
)
|
(117,060
|
)
|
18,603
|
|
-16
|
%
|
||||||
|
Interest (expense) income, net
|
(4,108
|
)
|
383
|
|
(4,491
|
)
|
-1,173
|
%
|
||||||
|
Changes in fair value of financial instruments
|
—
|
|
(1,514
|
)
|
1,514
|
|
-100
|
%
|
||||||
|
Other income, net
|
11,501
|
|
(972
|
)
|
12,473
|
|
NM
|
|
||||||
|
Loss before income tax expense
|
(91,064
|
)
|
(119,163
|
)
|
28,099
|
|
-24
|
%
|
||||||
|
Income tax expense
|
(2,235
|
)
|
(54
|
)
|
(2,181
|
)
|
4,039
|
%
|
||||||
|
Net loss
|
(93,299
|
)
|
(119,217
|
)
|
25,918
|
|
-22
|
%
|
||||||
|
Less: Net loss attributable to noncontrolling interest
|
(194
|
)
|
—
|
|
(194
|
)
|
—
|
|
||||||
|
Net loss attributable to BeiGene, Ltd.
|
$
|
(93,105
|
)
|
$
|
(119,217
|
)
|
$
|
26,112
|
|
-22
|
%
|
|||
|
|
Year Ended
|
|
|
|||||||||||
|
|
December 31,
|
Changes
|
||||||||||||
|
|
2017
|
2016
|
$
|
%
|
||||||||||
|
Product revenue
|
$
|
24,428
|
|
$
|
—
|
|
$
|
24,428
|
|
—
|
|
|||
|
Collaboration revenue:
|
|
|
|
|
|
|||||||||
|
License revenue
|
211,391
|
|
—
|
|
211,391
|
|
—
|
|
||||||
|
Research and development service revenue
|
2,568
|
|
1,070
|
|
1,498
|
|
140
|
%
|
||||||
|
Total collaboration revenue
|
213,959
|
|
1,070
|
|
212,889
|
|
19,896
|
%
|
||||||
|
Total
|
$
|
238,387
|
|
$
|
1,070
|
|
$
|
237,317
|
|
22,179
|
%
|
|||
|
|
Year Ended
|
|
|
|
||||||||||
|
|
December 31,
|
|
Changes
|
|||||||||||
|
|
2017
|
|
2016
|
|
$
|
%
|
||||||||
|
|
(dollars in thousands)
|
|||||||||||||
|
External cost of clinical-stage programs
|
$
|
131,485
|
|
|
$
|
54,373
|
|
|
$
|
77,112
|
|
142
|
%
|
|
|
External cost of preclinical-stage programs
|
9,244
|
|
|
6,068
|
|
|
3,176
|
|
52
|
%
|
||||
|
Internal research and development expenses
|
128,289
|
|
|
37,592
|
|
|
90,697
|
|
241
|
%
|
||||
|
Total research and development expenses
|
$
|
269,018
|
|
|
$
|
98,033
|
|
|
$
|
170,985
|
|
174
|
%
|
|
|
•
|
Increases of approximately $40.1 million, $27.1 million and $12.9 million, respectively, for zanubrutinib, tislelizumab and pamiparib, partially offset by a decrease of approximately $3.0 million for lifirafenib. The expense increases were primarily due to the expansion of clinical trials for these candidates, including the initiation or continuation of pivotal trials; and
|
|
•
|
Approximately $3.2 million increase in external spending for our preclinical-stage programs, primarily related to costs associated with advancing our preclinical candidates toward clinical trials.
|
|
•
|
$33.8 million increase of employee salary and benefits, which was primarily attributable to hiring more research and development personnel to support our expanding research and clinical activities;
|
|
•
|
$22.5 million increase of share-based compensation expense, primarily attributable to our increased headcount, as well as the increased valuation of non-employee equity compensation grants due to a higher share price;
|
|
•
|
$15.3 million increase of materials and reagent expenses, mainly in connection with the in-house manufacture of drug candidates used for clinical purposes, that were previously outsourced and recorded as external cost;
|
|
•
|
$9.8 million increase of consulting fees, which was mainly attributable to increased scientific, regulatory and development consulting activities, in connection with the advancement of our pipeline; and
|
|
•
|
$9.3 million increase of facilities, office expense, rental fee and other expenses to support the growth of our organization.
|
|
•
|
$12.6 million increase of employee salary and benefits, which was primarily attributable to the hiring of more personnel to support our growing organization, including the acquired workforce in the acquisition of Celgene’s China operations;
|
|
•
|
$9.7 million increase of share-based compensation expense, primarily attributable to our increased headcount;
|
|
•
|
$8.7 million increase of professional fees for legal, consulting, recruiting and audit services, mainly in connection with our patent prosecution activities, consulting services, business development activities, including the Celgene transactions, recruiting services and the preparation of periodic reports; and
|
|
•
|
$11.5 million increase of selling, facility, travel expenses, rental fees and other administrative expenses, primarily attributable to the global expansion of our business, including the post-combination operating costs of our commercial operations in China.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
|
(in thousands)
|
||||||||||
|
Cash, cash equivalents and restricted cash at beginning of period
|
$
|
239,602
|
|
$
|
87,514
|
|
$
|
17,869
|
|
||
|
Net cash (used in) provided by operating activities
|
(547,717
|
)
|
12,752
|
|
(89,513
|
)
|
|||||
|
Net cash used in investing activities
|
(637,613
|
)
|
(356,319
|
)
|
(221,848
|
)
|
|||||
|
Net cash provided by financing activities
|
1,690,537
|
|
490,356
|
|
380,902
|
|
|||||
|
Net effect of foreign exchange rate changes
|
(4,096
|
)
|
5,299
|
|
104
|
|
|||||
|
Net increase in cash, cash equivalents and restricted cash
|
501,111
|
|
152,088
|
|
69,645
|
|
|||||
|
Cash, cash equivalents and restricted cash at end of period
|
$
|
740,713
|
|
$
|
239,602
|
|
$
|
87,514
|
|
||
|
•
|
the costs, timing and outcome of regulatory reviews and approvals;
|
|
•
|
the ability of our drug candidates to progress through clinical development successfully;
|
|
•
|
the initiation, progress, timing, costs and results of nonclinical studies and clinical trials for our other programs and potential drug candidates;
|
|
•
|
the number and characteristics of the drug candidates we pursue;
|
|
•
|
the costs of establishing commercial manufacturing capabilities or securing necessary supplies from third-party manufacturers;
|
|
•
|
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property‑related claims;
|
|
•
|
the costs of establishing and expanding our commercial operations and the success of those operations;
|
|
•
|
the extent to which we acquire or in‑license other products and technologies; and
|
|
•
|
our ability to maintain and establish collaboration arrangements on favorable terms, if at all.
|
|
|
Payments Due by Period
|
||||||||||||||||||
|
|
|
Less Than
|
|
|
More Than
|
||||||||||||||
|
|
Total
|
1 Year
|
1–3 Years
|
3–5 Years
|
5 Years
|
||||||||||||||
|
|
(in thousands)
|
||||||||||||||||||
|
Contractual obligations
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Operating lease commitments
|
$
|
33,809
|
|
$
|
10,752
|
|
$
|
17,777
|
|
$
|
5,175
|
|
$
|
105
|
|
||||
|
Debt obligations
|
198,399
|
|
8,727
|
|
140
|
|
152,960
|
|
36,572
|
|
|||||||||
|
Purchase commitments
|
9,747
|
|
9,747
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Capital commitments
|
45,910
|
|
45,910
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total
|
$
|
287,865
|
|
$
|
75,136
|
|
$
|
17,917
|
|
$
|
158,135
|
|
$
|
36,677
|
|
||||
|
|
Year Ended December 31,
|
||||
|
|
2018
|
2017
|
2016
|
||
|
Risk-free interest rate
|
2.5% ~ 3.1%
|
2.2% ~ 2.6%
|
1.5% ~ 2.6%
|
||
|
Expected exercise multiple
|
2.2 ~ 2.8
|
2.2 ~ 2.8
|
2.2 ~ 2.8
|
||
|
Expected volatility
|
60% ~ 64%
|
99% ~ 100%
|
98% ~ 102%
|
||
|
Expected dividend yield
|
0%
|
0%
|
0%
|
||
|
Contractual life (years)
|
10
|
10
|
10
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
|
(in thousands)
|
||||||||||
|
Research and development
|
$
|
54,384
|
|
$
|
30,610
|
|
$
|
8,076
|
|
||
|
Selling, general and administration
|
32,743
|
|
12,253
|
|
2,549
|
|
|||||
|
Total
|
$
|
87,127
|
|
$
|
42,863
|
|
$
|
10,625
|
|
||
|
|
|
|
|
Page
|
|
|
|
As of December 31,
|
|||||
|
|
Note
|
2018
|
2017
|
||||
|
|
|
$
|
$
|
||||
|
Assets
|
|
|
|
|
|||
|
Current assets:
|
|
|
|
|
|||
|
Cash and cash equivalents
|
712,937
|
|
239,602
|
|
|||
|
Short-term restricted cash
|
5
|
14,544
|
|
—
|
|
||
|
Short-term investments
|
6
|
1,068,509
|
|
597,914
|
|
||
|
Accounts receivable
|
41,056
|
|
29,428
|
|
|||
|
Unbilled receivable
|
8,612
|
|
—
|
|
|||
|
Inventories
|
7
|
16,242
|
|
10,930
|
|
||
|
Prepaid expenses and other current assets
|
13
|
81,942
|
|
35,623
|
|
||
|
Total current assets
|
1,943,842
|
|
913,497
|
|
|||
|
Long-term restricted cash
|
5
|
13,232
|
|
—
|
|
||
|
Property and equipment, net
|
9
|
157,061
|
|
62,568
|
|
||
|
Land use right, net
|
10
|
45,058
|
|
12,465
|
|
||
|
Intangible assets, net
|
11
|
7,172
|
|
7,250
|
|
||
|
Goodwill
|
109
|
|
109
|
|
|||
|
Deferred tax assets
|
12
|
29,542
|
|
7,675
|
|
||
|
Other non-current assets
|
13
|
53,668
|
|
42,915
|
|
||
|
Total non-current assets
|
305,842
|
|
132,982
|
|
|||
|
Total assets
|
2,249,684
|
|
1,046,479
|
|
|||
|
Liabilities and shareholders' equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
113,283
|
|
69,779
|
|
|||
|
Accrued expenses and other payables
|
13
|
100,414
|
|
49,598
|
|
||
|
Deferred revenue, current portion
|
18,140
|
|
12,233
|
|
|||
|
Tax payable
|
12
|
5,888
|
|
9,156
|
|
||
|
Current portion of long-term bank loan
|
15
|
8,727
|
|
9,222
|
|
||
|
Total current liabilities
|
246,452
|
|
149,988
|
|
|||
|
Non-current liabilities:
|
|||||||
|
Long-term bank loan
|
15
|
40,785
|
|
9,222
|
|
||
|
Shareholder loan
|
16
|
148,888
|
|
146,271
|
|
||
|
Deferred revenue, non-current portion
|
9,842
|
|
24,808
|
|
|||
|
Deferred tax liabilities
|
12
|
11,139
|
|
—
|
|
||
|
Other long-term liabilities
|
13
|
38,931
|
|
31,959
|
|
||
|
Total non-current liabilities
|
249,585
|
|
212,260
|
|
|||
|
Total liabilities
|
496,037
|
|
362,248
|
|
|||
|
Commitments and contingencies
|
24
|
|
|
||||
|
Equity:
|
|||||||
|
Ordinary shares, $0.0001 par value per share; 9,500,000,000 shares authorized; 776,263,184 and 592,072,330 shares issued and outstanding as of December 31, 2018 and 2017, respectively
|
77
|
|
59
|
|
|||
|
Additional paid-in capital
|
2,744,814
|
|
1,000,747
|
|
|||
|
Accumulated other comprehensive income (loss)
|
20
|
1,526
|
|
(480
|
)
|
||
|
Accumulated deficit
|
(1,007,215
|
)
|
(330,517
|
)
|
|||
|
Total BeiGene, Ltd. shareholders’ equity
|
1,739,202
|
|
669,809
|
|
|||
|
Noncontrolling interest
|
14,445
|
|
14,422
|
|
|||
|
Total equity
|
1,753,647
|
|
684,231
|
|
|||
|
Total liabilities and equity
|
2,249,684
|
|
1,046,479
|
|
|||
|
|
|
Year Ended December 31,
|
||||||||
|
|
Note
|
2018
|
2017
|
2016
|
||||||
|
|
|
$
|
$
|
$
|
||||||
|
Revenue
|
|
|
|
|
|
|
|
|||
|
Product revenue, net
|
17
|
130,885
|
|
24,428
|
|
—
|
|
|||
|
Collaboration revenue
|
3
|
67,335
|
|
213,959
|
|
1,070
|
|
|||
|
Total revenues
|
198,220
|
|
238,387
|
|
1,070
|
|
||||
|
Expenses
|
||||||||||
|
Cost of sales - product
|
(28,705
|
)
|
(4,974
|
)
|
—
|
|
||||
|
Research and development
|
(679,005
|
)
|
(269,018
|
)
|
(98,033
|
)
|
||||
|
Selling, general and administrative
|
(195,385
|
)
|
(62,602
|
)
|
(20,097
|
)
|
||||
|
Amortization of intangible assets
|
(894
|
)
|
(250
|
)
|
—
|
|
||||
|
Total expenses
|
(903,989
|
)
|
(336,844
|
)
|
(118,130
|
)
|
||||
|
Loss from operations
|
(705,769
|
)
|
(98,457
|
)
|
(117,060
|
)
|
||||
|
Interest income (expense), net
|
13,947
|
|
(4,108
|
)
|
383
|
|
||||
|
Changes in fair value of financial instruments
|
14
|
—
|
|
—
|
|
(1,514
|
)
|
|||
|
Other income (expense), net
|
1,993
|
|
11,501
|
|
(972
|
)
|
||||
|
Loss before income tax expense
|
(689,829
|
)
|
(91,064
|
)
|
(119,163
|
)
|
||||
|
Income tax benefit (expense)
|
12
|
15,796
|
|
(2,235
|
)
|
(54
|
)
|
|||
|
Net loss
|
(674,033
|
)
|
(93,299
|
)
|
(119,217
|
)
|
||||
|
Less: net loss attributable to noncontrolling interests
|
(264
|
)
|
(194
|
)
|
—
|
|
||||
|
Net loss attributable to BeiGene, Ltd.
|
(673,769
|
)
|
(93,105
|
)
|
(119,217
|
)
|
||||
|
Net loss per share attributable to BeiGene, Ltd., basic and diluted
|
18
|
(0.93
|
)
|
(0.17
|
)
|
(0.30
|
)
|
|||
|
Weighted-average shares outstanding, basic and diluted
|
18
|
720,753,819
|
|
543,185,460
|
|
403,619,446
|
|
|||
|
Net loss per American Depositary Share (“ADS”), basic and diluted
|
(12.15
|
)
|
(2.23
|
)
|
(3.84
|
)
|
||||
|
Weighted-average ADSs outstanding, basic and diluted
|
55,442,601
|
|
41,783,497
|
|
31,047,650
|
|
||||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Net loss
|
(674,033
|
)
|
(93,299
|
)
|
(119,217
|
)
|
||
|
Other comprehensive loss, net of tax of nil:
|
||||||||
|
Foreign currency translation adjustments
|
(478
|
)
|
851
|
|
(245
|
)
|
||
|
Unrealized holding gain (loss), net
|
2,133
|
|
(296
|
)
|
1,108
|
|
||
|
Comprehensive loss
|
(672,378
|
)
|
(92,744
|
)
|
(118,354
|
)
|
||
|
Less: comprehensive loss attributable to noncontrolling interests
|
(352
|
)
|
(105
|
)
|
—
|
|
||
|
Comprehensive loss attributable to BeiGene, Ltd.
|
(672,026
|
)
|
(92,639
|
)
|
(118,354
|
)
|
||
|
|
|
Year Ended December 31,
|
||||||||
|
|
Note
|
2018
|
2017
|
2016
|
||||||
|
|
|
$
|
$
|
$
|
||||||
|
Cash flows from operating activities:
|
|
|
|
|
||||||
|
Net loss
|
(674,033
|
)
|
(93,299
|
)
|
(119,217
|
)
|
||||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||
|
Depreciation and amortization expense
|
10,388
|
|
4,758
|
|
1,909
|
|
||||
|
Share-based compensation expenses
|
19
|
87,127
|
|
42,863
|
|
10,625
|
|
|||
|
Acquired in-process research and development
|
70,000
|
|
—
|
|
—
|
|
||||
|
Changes in fair value of financial instruments
|
—
|
|
—
|
|
1,514
|
|
||||
|
Loss on disposal of property and equipment
|
126
|
|
85
|
|
—
|
|
||||
|
Non-cash interest expense
|
7,820
|
|
7,035
|
|
121
|
|
||||
|
Deferred income tax benefits
|
(21,949
|
)
|
(5,845
|
)
|
(768
|
)
|
||||
|
Disposal (gain) loss on available-for-sale securities
|
(1,948
|
)
|
(44
|
)
|
1,415
|
|
||||
|
Non-cash amortization of bond discount
|
(8,034
|
)
|
—
|
|
—
|
|
||||
|
Changes in operating assets and liabilities:
|
||||||||||
|
Accounts receivable
|
(11,628
|
)
|
(29,428
|
)
|
—
|
|
||||
|
Unbilled receivable
|
7,695
|
|
—
|
|
—
|
|
||||
|
Inventories
|
(5,312
|
)
|
(10,930
|
)
|
—
|
|
||||
|
Prepaid expenses and other current assets
|
(46,302
|
)
|
(28,880
|
)
|
(2,070
|
)
|
||||
|
Other non-current assets
|
(40,228
|
)
|
(29,701
|
)
|
112
|
|
||||
|
Accounts payable
|
23,470
|
|
55,298
|
|
2,707
|
|
||||
|
Accrued expenses and other payables
|
50,543
|
|
24,978
|
|
13,946
|
|
||||
|
Tax payable
|
(3,355
|
)
|
7,426
|
|
804
|
|
||||
|
Deferred revenue
|
(9,059
|
)
|
37,041
|
|
(1,070
|
)
|
||||
|
Other long-term liabilities
|
16,962
|
|
31,395
|
|
459
|
|
||||
|
Net cash (used in) provided by operating activities
|
(547,717
|
)
|
12,752
|
|
(89,513
|
)
|
||||
|
Cash flows from investing activities:
|
||||||||||
|
Purchases of property and equipment
|
(70,283
|
)
|
(46,374
|
)
|
(23,502
|
)
|
||||
|
Purchase of intangible assets
|
(553
|
)
|
—
|
|
—
|
|
||||
|
Payment for asset acquisition, net of cash acquired
|
4
|
(38,298
|
)
|
—
|
|
—
|
|
|||
|
Payment for the acquisition of land use right
|
—
|
|
(12,354
|
)
|
—
|
|
||||
|
Cash acquired in business combination, net of cash paid
|
4
|
—
|
|
19,916
|
|
—
|
|
|||
|
Purchases of investments
|
(2,635,686
|
)
|
(741,296
|
)
|
(382,093
|
)
|
||||
|
Proceeds from sale or maturity of available-for-sale securities
|
2,177,207
|
|
423,789
|
|
183,743
|
|
||||
|
Purchase of in-process research and development
|
(70,000
|
)
|
—
|
|
—
|
|
||||
|
Proceeds from disposal of property and equipment
|
—
|
|
—
|
|
4
|
|
||||
|
Net cash used in investing activities
|
(637,613
|
)
|
(356,319
|
)
|
(221,848
|
)
|
||||
|
Cash flows from financing activities:
|
||||||||||
|
Proceeds from public offering, net of underwriter discount
|
21
|
758,001
|
|
189,191
|
|
368,877
|
|
|||
|
Payment of public offering cost
|
21
|
(414
|
)
|
(674
|
)
|
(2,218
|
)
|
|||
|
Proceeds from public offering and HK IPO, net of underwriter discount
|
21
|
875,368
|
|
—
|
|
—
|
|
|||
|
Payment of public offering and HK IPO costs
|
21
|
(5,659
|
)
|
—
|
|
—
|
|
|||
|
Proceeds from sale of ordinary shares, net of cost
|
21
|
—
|
|
149,928
|
|
—
|
|
|||
|
Proceeds from long-term loan
|
15
|
42,315
|
|
—
|
|
12,048
|
|
|||
|
Repayment of long-term loan
|
(8,736
|
)
|
—
|
|
—
|
|
||||
|
Proceeds from short-term loan
|
—
|
|
2,470
|
|
—
|
|
||||
|
Repayment of short-term loan
|
—
|
|
(2,470
|
)
|
—
|
|
||||
|
Capital contribution from noncontrolling interest
|
—
|
|
14,527
|
|
—
|
|
||||
|
Proceeds from shareholder loan
|
16
|
—
|
|
132,757
|
|
—
|
|
|||
|
Proceeds from exercise of warrants and rental deferral option
|
21
|
—
|
|
—
|
|
2,115
|
|
|||
|
Proceeds from option exercises
|
29,662
|
|
4,627
|
|
80
|
|
||||
|
Net cash provided by financing activities
|
1,690,537
|
|
490,356
|
|
380,902
|
|
||||
|
Effect of foreign exchange rate changes, net
|
(4,096
|
)
|
5,299
|
|
104
|
|
||||
|
Net increase in cash, cash equivalents, and restricted cash
|
501,111
|
|
152,088
|
|
69,645
|
|
||||
|
Cash, cash equivalents, and restricted cash, beginning of year
|
239,602
|
|
87,514
|
|
17,869
|
|
||||
|
Cash, cash equivalents, and restricted cash, end of year
|
740,713
|
|
239,602
|
|
87,514
|
|
||||
|
|
|
Year Ended December 31,
|
||||||||
|
|
Note
|
2018
|
2017
|
2016
|
||||||
|
|
|
$
|
$
|
$
|
||||||
|
Supplemental cash flow disclosures:
|
||||||||||
|
Cash and cash equivalents
|
712,937
|
|
239,602
|
|
87,514
|
|
||||
|
Short-term restricted cash
|
14,544
|
|
—
|
|
—
|
|
||||
|
Long-term restricted cash
|
13,232
|
|
—
|
|
—
|
|
||||
|
Income taxes paid
|
12,361
|
|
29,286
|
|
25
|
|
||||
|
Interest paid
|
2,209
|
|
1,260
|
|
826
|
|
||||
|
Non-cash activities:
|
||||||||||
|
Discount provided on sale of ordinary shares for business combination
|
4
|
—
|
|
23,606
|
|
—
|
|
|||
|
Conversion of senior promissory note
|
—
|
|
—
|
|
14,693
|
|
||||
|
Conversion of deferred rental
|
—
|
|
—
|
|
980
|
|
||||
|
Conversion of convertible preferred shares
|
—
|
|
—
|
|
176,084
|
|
||||
|
Exercise of warrants and option
|
—
|
|
—
|
|
3,687
|
|
||||
|
Follow-on public offering costs accrued in accounts payable
|
—
|
|
—
|
|
269
|
|
||||
|
Acquisitions of equipment included in accounts payable
|
22,105
|
|
2,215
|
|
2,153
|
|
||||
|
Purchase of in-process research and development included in accounts payable
|
19,000
|
|
—
|
|
—
|
|
||||
|
Changes in operating assets and liabilities adjusted through accumulated deficit
|
2,291
|
|
—
|
|
—
|
|
||||
|
|
Attributable to BeiGene, Ltd.
|
|
|
||||||||||||||||||||
|
|
Ordinary Shares
|
Additional
Paid-In
Capital
|
Accumulated
OCI
|
Accumulated
Deficit
|
Total
|
Non-
Controlling
Interests
|
|
||||||||||||||||
|
|
Shares
|
Amount
|
Total
|
||||||||||||||||||||
|
Balance at December 31, 2015
|
116,174,094
|
|
12
|
|
18,227
|
|
(1,809
|
)
|
(118,195
|
)
|
(101,765
|
)
|
—
|
|
(101,765
|
)
|
|||||||
|
Issuance of ordinary shares in connection with initial public offering
|
98,670,000
|
|
10
|
|
166,127
|
|
—
|
|
—
|
|
166,137
|
|
—
|
|
166,137
|
|
|||||||
|
Issuance of ordinary shares in connection with follow-on public offering
|
86,206,250
|
|
9
|
|
198,617
|
|
—
|
|
—
|
|
198,626
|
|
—
|
|
198,626
|
|
|||||||
|
Conversion of senior promissory note (Note 21)
|
7,942,314
|
|
1
|
|
14,692
|
|
—
|
|
—
|
|
14,693
|
|
—
|
|
14,693
|
|
|||||||
|
Exercise of warrants in connection with convertible promissory note (Note 21)
|
621,637
|
|
—
|
|
1,513
|
|
—
|
|
—
|
|
1,513
|
|
—
|
|
1,513
|
|
|||||||
|
Exercise of option to purchase shares by rental deferred (note 21)
|
1,451,586
|
|
—
|
|
3,519
|
|
—
|
|
—
|
|
3,519
|
|
—
|
|
3,519
|
|
|||||||
|
Exercise of warrants by Baker Bros. (Note 21)
|
2,592,593
|
|
—
|
|
1,750
|
|
—
|
|
—
|
|
1,750
|
|
—
|
|
1,750
|
|
|||||||
|
Issuance of shares reserved for share options exercise
|
271,284
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Conversion of preferred shares to ordinary shares (Note 21)
|
199,990,641
|
|
20
|
|
176,064
|
|
—
|
|
—
|
|
176,084
|
|
—
|
|
176,084
|
|
|||||||
|
Share-based compensation
|
1,913,210
|
|
—
|
|
10,704
|
|
—
|
|
—
|
|
10,704
|
|
—
|
|
10,704
|
|
|||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(119,217
|
)
|
(119,217
|
)
|
—
|
|
(119,217
|
)
|
|||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
863
|
|
—
|
|
863
|
|
—
|
|
863
|
|
|||||||
|
Balance at December 31, 2016
|
515,833,609
|
|
52
|
|
591,213
|
|
(946
|
)
|
(237,412
|
)
|
352,907
|
|
—
|
|
352,907
|
|
|||||||
|
Issuance of ordinary shares in secondary follow-on offering, net of transaction costs
|
36,851,750
|
|
4
|
|
188,513
|
|
—
|
|
—
|
|
188,517
|
|
—
|
|
188,517
|
|
|||||||
|
Proceeds from sale of ordinary shares, net of cost
|
32,746,416
|
|
3
|
|
149,925
|
|
—
|
|
—
|
|
149,928
|
|
—
|
|
149,928
|
|
|||||||
|
Discount on the sale of ordinary shares
|
—
|
|
—
|
|
23,606
|
|
—
|
|
—
|
|
23,606
|
|
—
|
|
23,606
|
|
|||||||
|
Contributions from shareholders (Note 8)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
14,527
|
|
14,527
|
|
|||||||
|
Share-based compensation
|
—
|
|
—
|
|
42,863
|
|
—
|
|
—
|
|
42,863
|
|
—
|
|
42,863
|
|
|||||||
|
Issuance of shares reserved for share option exercises
|
787,571
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Exercise of options
|
5,852,984
|
|
—
|
|
4,627
|
|
—
|
|
—
|
|
4,627
|
|
—
|
|
4,627
|
|
|||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
466
|
|
—
|
|
466
|
|
89
|
|
555
|
|
|||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(93,105
|
)
|
(93,105
|
)
|
(194
|
)
|
(93,299
|
)
|
|||||||
|
Balance at December 31, 2017
|
592,072,330
|
|
59
|
|
1,000,747
|
|
(480
|
)
|
(330,517
|
)
|
669,809
|
|
14,422
|
|
684,231
|
|
|||||||
|
Adjustment to opening balance of equity
|
—
|
|
—
|
|
—
|
|
263
|
|
(2,929
|
)
|
(2,666
|
)
|
375
|
|
(2,291
|
)
|
|||||||
|
Balance at January 1, 2018
|
592,072,330
|
|
59
|
|
1,000,747
|
|
(217
|
)
|
(333,446
|
)
|
667,143
|
|
14,797
|
|
681,940
|
|
|||||||
|
Issuance of ordinary shares in connection with follow-on public offering
|
102,970,400
|
|
10
|
|
757,577
|
|
—
|
|
—
|
|
757,587
|
|
—
|
|
757,587
|
|
|||||||
|
Issuance of ordinary shares in connection with global offering and HK IPO
|
65,600,000
|
|
7
|
|
869,702
|
|
—
|
|
—
|
|
869,709
|
|
—
|
|
869,709
|
|
|||||||
|
Issuance of shares reserved for share option exercises
|
1,299,186
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Share-based compensation
|
—
|
|
—
|
|
87,127
|
|
—
|
|
—
|
|
87,127
|
|
—
|
|
87,127
|
|
|||||||
|
Exercise of options and release of RSUs
|
14,321,268
|
|
1
|
|
29,661
|
|
—
|
|
—
|
|
29,662
|
|
—
|
|
29,662
|
|
|||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
1,743
|
|
—
|
|
1,743
|
|
(88
|
)
|
1,655
|
|
|||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(673,769
|
)
|
(673,769
|
)
|
(264
|
)
|
(674,033
|
)
|
|||||||
|
Balance at December 31, 2018
|
776,263,184
|
|
77
|
|
2,744,814
|
|
1,526
|
|
(1,007,215
|
)
|
1,739,202
|
|
14,445
|
|
1,753,647
|
|
|||||||
|
Name of Company
|
Place of Incorporation
|
Date of
Incorporation
|
Percentage of
Ownership by
the Company
|
Principal Activities
|
|||||
|
BeiGene 101
|
Cayman Islands
|
August 30, 2012
|
100
|
%
|
Medical and pharmaceutical research
|
||||
|
BeiGene AUS Pty Ltd. ("BeiGene Australia")
|
Australia
|
July 15, 2013
|
100
|
%
|
Clinical trial activities
|
||||
|
BeiGene (Beijing) Co., Ltd. ("BeiGene Beijing")
|
The People’s Republic of China (“PRC” or “China”)
|
January 24, 2011
|
100
|
%
|
Medical and pharmaceutical research
|
||||
|
BeiGene Biologics Co., Ltd. ("BeiGene Biologics")
|
PRC
|
January 25, 2017
|
95
|
%
|
Biologics manufacturing
|
||||
|
BeiGene Guangzhou Biologics Manufacturing Co., Ltd. ("BeiGene Guangzhou Factory")*
|
PRC
|
March 3, 2017
|
95
|
%
|
Biologics manufacturing
|
||||
|
BeiGene (Guangzhou) Co., Ltd. (“BeiGene Guangzhou”)
|
PRC
|
July 11, 2017
|
100
|
%
|
Medical and pharmaceutical research
|
||||
|
BeiGene (Hong Kong) Co., Limited. ("BeiGene HK")
|
Hong Kong
|
November 22, 2010
|
100
|
%
|
Investment holding
|
||||
|
Beijing Innerway Bio-tech Co., Ltd. ("Innerway")
|
PRC
|
August 9, 2004
|
100
|
%
|
Medical and pharmaceutical research and manufacturing
|
||||
|
BeiGene Ireland Limited ("BeiGene Ireland")
|
Republic of Ireland
|
August 11, 2017
|
100
|
%
|
Clinical trial activities
|
||||
|
BeiGene Pharmaceuticals (Guangzhou) Co., Ltd. ("BeiGene Pharmaceutical (Guangzhou)")
|
PRC
|
April 14, 1999
|
100
|
%
|
Medical and pharmaceutical research and manufacturing
|
||||
|
BeiGene Pharmaceutical (Shanghai) Co., Ltd. ("BeiGene Pharmaceutical (Shanghai)")
|
PRC
|
December 15, 2009
|
100
|
%
|
Medical and pharmaceutical consulting,
marketing and promotional services
|
||||
|
BeiGene (Shanghai) Co., Ltd. (“BeiGene Shanghai”)*
|
PRC
|
September 11, 2015
|
95
|
%
|
Medical and pharmaceutical research
|
||||
|
BeiGene (Suzhou) Co., Ltd. (“BeiGene Suzhou”)
|
PRC
|
April 9, 2015
|
100
|
%
|
Medical and pharmaceutical research and manufacturing
|
||||
|
BeiGene Switzerland GmbH (“BeiGene Switzerland”)
|
Switzerland
|
September 1, 2017
|
100
|
%
|
Clinical trial activities and commercial
|
||||
|
BeiGene UK, Ltd. ("BeiGene UK")
|
United Kingdom
|
December 14, 2018
|
100
|
%
|
Research, development, manufacture and distribution or licensing of pharmaceutical and related products
|
||||
|
BeiGene USA, Inc. ("BeiGene USA")
|
United States
|
July 8, 2015
|
100
|
%
|
Clinical trial activities
|
||||
|
|
Useful Life
|
|
Building
|
20 years
|
|
Office Equipment
|
5 years
|
|
Electronic Equipment
|
3 years
|
|
Manufacturing equipment
|
3 to 10 years
|
|
Laboratory Equipment
|
3 to 5 years
|
|
Computer Software
|
3 to 5 years
|
|
Leasehold Improvements
|
Lesser of useful life or lease term
|
|
As of December 31, 2018
|
Quoted Price
in Active
Market for
Identical
Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
||||||
|
|
$
|
$
|
$
|
||||||
|
Short-term investment (Note 6):
|
|
|
|
|
|||||
|
U.S. treasury securities
|
1,068,509
|
|
—
|
|
—
|
|
|||
|
Cash equivalents
|
|||||||||
|
Money market funds
|
159,810
|
|
—
|
|
—
|
|
|||
|
Total
|
1,228,319
|
|
—
|
|
—
|
|
|||
|
As of December 31, 2017
|
Quoted Price
in Active Market for Identical Assets (Level 1) |
Significant
Other Observable Inputs (Level 2) |
Significant
Unobservable Inputs (Level 3) |
||||||
|
|
$
|
$
|
$
|
||||||
|
Short-term investment (Note 6):
|
|
|
|
||||||
|
U.S. treasury securities
|
561,327
|
|
—
|
|
—
|
|
|||
|
U.S. agency securities
|
17,663
|
|
—
|
|
—
|
|
|||
|
Time deposits
|
18,924
|
|
—
|
|
—
|
|
|||
|
Cash equivalents
|
|||||||||
|
Money market funds
|
44,730
|
|
—
|
|
—
|
|
|||
|
Total
|
642,644
|
|
—
|
|
—
|
|
|||
|
|
Balance at
|
Adjustments
|
Adjustments
|
Balance at
|
||||||||
|
|
December 31,
|
Due to
|
Due to
|
January 1,
|
||||||||
|
|
2017
|
Revenue ASUs
|
ASU 2016-16
|
2018
|
||||||||
|
|
$
|
$
|
$
|
$
|
||||||||
|
Assets:
|
|
|
|
|
|
|
|
|
||||
|
Unbilled receivable
|
—
|
|
16,307
|
|
—
|
|
16,307
|
|
||||
|
Other non-current assets
|
42,915
|
|
—
|
|
(28,588
|
)
|
14,327
|
|
||||
|
Liabilities:
|
||||||||||||
|
Other long-term liabilities
|
31,959
|
|
—
|
|
(9,990
|
)
|
21,969
|
|
||||
|
Equity:
|
||||||||||||
|
Accumulated other comprehensive loss
|
(480
|
)
|
—
|
|
263
|
|
(217
|
)
|
||||
|
Accumulated deficit
|
(330,517
|
)
|
16,307
|
|
(19,236
|
)
|
(333,446
|
)
|
||||
|
Noncontrolling interest
|
14,422
|
|
—
|
|
375
|
|
14,797
|
|
||||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
License revenue
|
—
|
|
211,391
|
|
—
|
|
||
|
Reimbursement of research and development costs
|
56,776
|
|
—
|
|
—
|
|
||
|
Research and development service revenue
|
10,559
|
|
2,568
|
|
1,070
|
|
||
|
Total
|
67,335
|
|
213,959
|
|
1,070
|
|
||
|
|
Purchase Price
|
||
|
Cash paid to acquire Celgene Shanghai
|
$
|
4,532
|
|
|
Discount on Share Subscription Agreement
|
23,606
|
|
|
|
Total purchase price
|
$
|
28,138
|
|
|
|
Amount
|
||
|
Cash and cash equivalents
|
$
|
24,448
|
|
|
Other current assets
|
518
|
|
|
|
Property and equipment, net
|
204
|
|
|
|
Intangible assets
|
7,500
|
|
|
|
Deferred tax asset
|
1,069
|
|
|
|
Total identifiable assets
|
33,739
|
|
|
|
|
|||
|
Current liabilities
|
(5,710
|
)
|
|
|
Total liabilities assumed
|
(5,710
|
)
|
|
|
|
|||
|
Goodwill
|
109
|
|
|
|
Total fair value of consideration transferred
|
$
|
28,138
|
|
|
Investing activities
|
|
||
|
Cash acquired
|
$
|
24,448
|
|
|
Cash paid to acquire Celgene Shanghai
|
(4,532
|
)
|
|
|
Cash acquired in business combination, net of cash paid
|
$
|
19,916
|
|
|
|
|||
|
Non-cash activities
|
|||
|
Discount provided on sale of ordinary shares for business combination
|
$
|
(23,606
|
)
|
|
|
Amount
|
||
|
Land use right
|
$
|
33,783
|
|
|
Building
|
15,874
|
|
|
|
Deferred tax liability
|
(11,221
|
)
|
|
|
Other
|
429
|
|
|
|
Total cost
|
38,865
|
|
|
|
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
(Net Carrying
Amount)
|
|||||||
|
|
$
|
$
|
$
|
$
|
|||||||
|
U.S. treasury securities
|
1,066,770
|
|
1,802
|
|
63
|
|
1,068,509
|
|
|||
|
Total
|
1,066,770
|
|
1,802
|
|
63
|
|
1,068,509
|
|
|||
|
|
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Fair Value
(Net Carrying Amount) |
|||||||
|
|
$
|
$
|
$
|
$
|
|||||||
|
U.S. treasury securities
|
561,733
|
|
—
|
|
406
|
|
561,327
|
|
|||
|
U.S. agency securities
|
17,651
|
|
12
|
|
—
|
|
17,663
|
|
|||
|
Time deposits
|
18,924
|
|
—
|
|
—
|
|
18,924
|
|
|||
|
Total
|
598,308
|
|
12
|
|
406
|
|
597,914
|
|
|||
|
|
As of December 31,
|
||||
|
|
2018
|
2017
|
|||
|
|
$
|
$
|
|||
|
Laboratory equipment
|
22,636
|
|
15,596
|
|
|
|
Leasehold improvements
|
18,048
|
|
15,298
|
|
|
|
Building
|
15,857
|
|
—
|
|
|
|
Manufacturing equipment
|
16,048
|
|
15,737
|
|
|
|
Office equipment
|
2,216
|
|
1,597
|
|
|
|
Electronic equipment
|
1,229
|
|
1,244
|
|
|
|
Computer software
|
1,262
|
|
598
|
|
|
|
Property and equipment, at cost
|
77,296
|
|
50,070
|
|
|
|
Less: Accumulated depreciation
|
(19,722
|
)
|
(13,627
|
)
|
|
|
Construction in progress
|
99,487
|
|
26,125
|
|
|
|
Property and equipment, net
|
157,061
|
|
62,568
|
|
|
|
|
As of December 31,
|
||||
|
|
2018
|
2017
|
|||
|
|
$
|
$
|
|||
|
Land use rights, cost
|
45,701
|
|
12,633
|
|
|
|
Accumulated amortization
|
(643
|
)
|
(168
|
)
|
|
|
Land use rights, net
|
45,058
|
|
12,465
|
|
|
|
|
December 31, 2018
|
December 31, 2017
|
|||||||||||||||
|
|
Gross
carrying
amount
|
Accumulated
amortization
|
Intangible
assets, net
|
Gross
carrying amount |
Accumulated
amortization |
Intangible
assets, net |
|||||||||||
|
Finite-lived intangible assets:
|
|
|
|
|
|
|
|
|
|
||||||||
|
Product distribution rights
|
7,500
|
|
(1,000
|
)
|
6,500
|
|
7,500
|
|
(250
|
)
|
7,250
|
|
|||||
|
Trading license
|
816
|
|
(144
|
)
|
672
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Total finite-lived intangible assets
|
8,316
|
|
(1,144
|
)
|
7,172
|
|
7,500
|
|
(250
|
)
|
7,250
|
|
|||||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
PRC
|
(130,552
|
)
|
(59,590
|
)
|
(7,352
|
)
|
||
|
U.S.
|
15,036
|
|
6,928
|
|
678
|
|
||
|
Other
|
(574,313
|
)
|
(38,402
|
)
|
(112,489
|
)
|
||
|
Total
|
(689,829
|
)
|
(91,064
|
)
|
(119,163
|
)
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
|
|||||
|
Current Tax Expense (Benefit):
|
|
|
|
|||||
|
PRC
|
6,890
|
|
2,477
|
|
—
|
|
||
|
U.S.
|
(377
|
)
|
5,695
|
|
822
|
|
||
|
Total
|
6,513
|
|
8,172
|
|
822
|
|
||
|
Deferred Tax Expense (Benefit):
|
||||||||
|
PRC
|
(2,682
|
)
|
115
|
|
—
|
|
||
|
U.S.
|
(19,627
|
)
|
(6,052
|
)
|
(768
|
)
|
||
|
Total
|
(22,309
|
)
|
(5,937
|
)
|
(768
|
)
|
||
|
Income Tax Expense (Benefit)
|
(15,796
|
)
|
2,235
|
|
54
|
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Loss before tax
|
(689,829
|
)
|
(91,064
|
)
|
(119,163
|
)
|
||
|
China statutory tax rate
|
25
|
%
|
25
|
%
|
25
|
%
|
||
|
Expected taxation at China statutory tax rate
|
(172,457
|
)
|
(22,766
|
)
|
(29,791
|
)
|
||
|
Foreign tax rate differential
|
134,673
|
|
23,275
|
|
27,830
|
|
||
|
Non-deductible expenses
|
4,471
|
|
1,608
|
|
593
|
|
||
|
Impact of U.S. statutory tax rate change
|
1,538
|
|
2,642
|
|
—
|
|
||
|
Deductible intellectual property from intercompany transfer
|
—
|
|
(29,438
|
)
|
—
|
|
||
|
Change in valuation allowance
|
34,009
|
|
30,356
|
|
1,627
|
|
||
|
Research and orphan drug tax credits
|
(12,659
|
)
|
(5,431
|
)
|
(205
|
)
|
||
|
Share-based compensation expense
|
(5,371
|
)
|
1,989
|
|
—
|
|
||
|
Taxation for the year
|
(15,796
|
)
|
2,235
|
|
54
|
|
||
|
Effective tax rate
|
2.3
|
%
|
-2.5
|
%
|
-0.1
|
%
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Deferred Tax Assets:
|
|
|
|
|
|
|
||
|
Accruals and reserves
|
19,193
|
|
7,756
|
|
1,102
|
|
||
|
Net operating losses carryforward
|
61,266
|
|
29,801
|
|
6,987
|
|
||
|
Stock compensation
|
8,642
|
|
4,639
|
|
—
|
|
||
|
Research and orphan drug tax credits
|
13,608
|
|
2,449
|
|
—
|
|
||
|
Depreciation and amortization
|
158,639
|
|
—
|
|
—
|
|
||
|
Gross deferred tax assets
|
261,348
|
|
44,645
|
|
8,089
|
|
||
|
Less valuation allowance
|
(242,945
|
)
|
(36,600
|
)
|
(7,307
|
)
|
||
|
Total deferred tax assets
|
18,403
|
|
8,045
|
|
782
|
|
||
|
Deferred tax liabilities:
|
||||||||
|
Depreciation and amortization
|
—
|
|
(370
|
)
|
(14
|
)
|
||
|
Total deferred tax liabilities
|
—
|
|
(370
|
)
|
(14
|
)
|
||
|
Net deferred tax asset
|
18,403
|
|
7,675
|
|
768
|
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Beginning balance, as of January 1
|
918
|
|
110
|
|
—
|
|
||
|
Additions based on tax positions related to prior tax years
|
11
|
|
234
|
|
—
|
|
||
|
Reductions based on tax positions related to prior tax years
|
(44
|
)
|
(91
|
)
|
—
|
|
||
|
Additions based on tax positions related to the current tax year
|
1,410
|
|
665
|
|
110
|
|
||
|
Ending balance, as of December 31
|
2,295
|
|
918
|
|
110
|
|
||
|
|
As of December 31,
|
||||
|
|
2018
|
2017
|
|||
|
|
$
|
$
|
|||
|
Prepaid research and development costs
|
58,673
|
|
21,156
|
|
|
|
Prepaid taxes
|
14,588
|
|
9,894
|
|
|
|
Interest receivable
|
3,096
|
|
1,557
|
|
|
|
Other
|
5,585
|
|
3,016
|
|
|
|
Total
|
81,942
|
|
35,623
|
|
|
|
|
As of December 31,
|
||||
|
|
2018
|
2017
|
|||
|
|
$
|
$
|
|||
|
Prepayment of property and equipment
|
11,981
|
|
12,867
|
|
|
|
Payment of facility capacity expansion activities (1)
|
25,193
|
|
—
|
|
|
|
Tax on intra-entity contribution of subsidiary
|
—
|
|
28,588
|
|
|
|
Prepaid VAT
|
14,671
|
|
—
|
|
|
|
Rental deposits and other
|
1,823
|
|
1,460
|
|
|
|
Total
|
53,668
|
|
42,915
|
|
|
|
|
As of December 31,
|
||||
|
|
2018
|
2017
|
|||
|
|
$
|
$
|
|||
|
Compensation related
|
35,887
|
|
17,051
|
|
|
|
External research and development activities related
|
34,588
|
|
18,721
|
|
|
|
Commercial activities
|
10,433
|
|
2,350
|
|
|
|
Individual income tax and other taxes
|
8,030
|
|
5,088
|
|
|
|
Sales rebates and returns related
|
4,749
|
|
3,997
|
|
|
|
Other
|
6,727
|
|
2,391
|
|
|
|
Total accrued expenses and other payables
|
100,414
|
|
49,598
|
|
|
|
|
As of December 31,
|
||||
|
|
2018
|
2017
|
|||
|
|
$
|
$
|
|||
|
Deferred government grant income
|
37,851
|
|
31,804
|
|
|
|
Other
|
1,080
|
|
155
|
|
|
|
Total other long-term liabilities
|
38,931
|
|
31,959
|
|
|
|
|
Year Ended
December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Product revenue - gross
|
138,046
|
|
28,428
|
|
—
|
|
||
|
Less: Rebates and sales returns
|
(7,161
|
)
|
(4,000
|
)
|
—
|
|
||
|
Product revenue - net
|
130,885
|
|
24,428
|
|
—
|
|
||
|
|
Sales Rebates and Returns
|
|
|
|
$
|
|
|
Balance as of December 31, 2016
|
—
|
|
|
Accrual
|
4,000
|
|
|
Payment
|
(3
|
)
|
|
Balance as of December 31, 2017
|
3,997
|
|
|
Accrual
|
7,161
|
|
|
Payment
|
(6,409
|
)
|
|
Balance as of December 31, 2018
|
4,749
|
|
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Numerator:
|
|
|
|
|
|
|
||
|
Net loss attributable to BeiGene, Ltd.
|
(673,769
|
)
|
(93,105
|
)
|
(119,217
|
)
|
||
|
Denominator:
|
||||||||
|
Weighted average shares outstanding for computing basic and diluted loss per share
|
720,753,819
|
|
543,185,460
|
|
403,619,446
|
|
||
|
Net loss per share attributable to BeiGene, Ltd., basic and diluted
|
(0.93
|
)
|
(0.17
|
)
|
(0.30
|
)
|
||
|
|
Number of
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Grant
Date Fair
Value
|
Weighted
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic Value
|
||||||||
|
|
|
$
|
$
|
Years
|
$
|
||||||||
|
Outstanding at December 31, 2015
|
44,109,990
|
|
0.35
|
|
|||||||||
|
Granted
|
38,921,219
|
|
2.32
|
|
1.60
|
|
|||||||
|
Exercised
|
(610,116
|
)
|
0.10
|
|
1,353
|
|
|||||||
|
Forfeited
|
(5,341,350
|
)
|
0.92
|
|
|||||||||
|
Outstanding at December 31, 2016
|
77,079,743
|
|
1.31
|
|
|||||||||
|
Granted
|
62,085,462
|
|
3.73
|
|
2.65
|
|
|||||||
|
Exercised
|
(5,887,193
|
)
|
0.82
|
|
24,723
|
|
|||||||
|
Forfeited
|
(6,275,115
|
)
|
2.52
|
|
|||||||||
|
Outstanding at December 31, 2017
|
127,002,897
|
|
2.45
|
|
|||||||||
|
Granted
|
9,387,885
|
|
12.32
|
|
7.08
|
|
|||||||
|
Exercised
|
(13,841,036
|
)
|
2.23
|
|
132,687
|
|
|||||||
|
Forfeited
|
(6,467,099
|
)
|
3.59
|
|
|||||||||
|
Outstanding at December 31, 2018
|
116,082,647
|
|
3.21
|
|
7.63
|
894,871
|
|
||||||
|
Exercisable as of December 31, 2018
|
53,829,397
|
|
1.84
|
|
6.95
|
481,796
|
|
||||||
|
Vested and expected to vest at December 31, 2018
|
109,857,323
|
|
3.15
|
|
7.59
|
853,563
|
|
||||||
|
|
Year Ended December 31,
|
||||
|
|
2018
|
2017
|
2016
|
||
|
Fair value of ordinary share
|
4.30 ~ 8.85
|
2.39 ~ 8.71
|
1.85 ~ 2.84
|
||
|
Risk-free interest rate
|
2.5% ~ 3.1%
|
2.2% ~ 2.6%
|
1.5% ~ 2.6%
|
||
|
Expected exercise multiple
|
2.2 ~ 2.8
|
2.2 ~ 2.8
|
2.2 ~ 2.8
|
||
|
Expected volatility
|
60% ~ 64%
|
99% ~ 100%
|
98% ~ 102%
|
||
|
Expected dividend yield
|
0%
|
0%
|
0%
|
||
|
Contractual life
|
10 years
|
10 years
|
10 years
|
||
|
|
Numbers
of Shares
|
Weighted-Average
Grant Date Fair Value
|
|||
|
|
|
$
|
|||
|
Outstanding at December 31, 2015
|
44,445
|
|
0.05
|
|
|
|
Granted
|
1,075,000
|
|
2.16
|
|
|
|
Vested
|
(44,445
|
)
|
0.05
|
|
|
|
Forfeited
|
—
|
|
—
|
|
|
|
Outstanding at December 31, 2016
|
1,075,000
|
|
2.16
|
|
|
|
Granted
|
300,000
|
|
2.95
|
|
|
|
Vested
|
(268,750
|
)
|
2.04
|
|
|
|
Forfeited
|
(300,000
|
)
|
2.95
|
|
|
|
Outstanding at December 31, 2017
|
806,250
|
|
2.16
|
|
|
|
Granted
|
—
|
|
—
|
|
|
|
Vested
|
(387,500
|
)
|
2.12
|
|
|
|
Forfeited
|
(118,750
|
)
|
2.04
|
|
|
|
Outstanding at December 31, 2018
|
300,000
|
|
2.25
|
|
|
|
Expected to vest at December 31, 2018
|
270,000
|
|
2.25
|
|
|
|
|
Numbers
of Shares
|
Weighted-Average
Grant Date Fair Value
|
|||
|
|
|
$
|
|||
|
Outstanding at December 31, 2016
|
—
|
|
—
|
|
|
|
Granted
|
1,469,442
|
|
7.55
|
|
|
|
Vested
|
—
|
|
—
|
|
|
|
Forfeited
|
—
|
|
—
|
|
|
|
Outstanding at December 31, 2017
|
1,469,442
|
|
—
|
|
|
|
Granted
|
14,079,598
|
|
12.07
|
|
|
|
Vested
|
(689,130
|
)
|
8.33
|
|
|
|
Forfeited
|
(757,458
|
)
|
10.89
|
|
|
|
Outstanding at December 31, 2018
|
14,102,452
|
|
11.85
|
|
|
|
Expected to vest at December 31, 2018
|
12,692,207
|
|
11.85
|
|
|
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Research and development
|
54,384
|
|
30,610
|
|
8,076
|
|
||
|
Selling, general and administrative
|
32,743
|
|
12,253
|
|
2,549
|
|
||
|
Total
|
87,127
|
|
42,863
|
|
10,625
|
|
||
|
|
Foreign Currency
Translation
Adjustments
|
Unrealized
Gains/Losses on
Available-for-Sale
Securities
|
Total
|
|||||
|
|
$
|
$
|
$
|
|||||
|
Balance as of December 31, 2016
|
(847
|
)
|
(99
|
)
|
(946
|
)
|
||
|
Other comprehensive income (loss) before reclassifications
|
762
|
|
(252
|
)
|
510
|
|
||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
(44
|
)
|
(44
|
)
|
||
|
Net-current period other comprehensive income (loss)
|
762
|
|
(296
|
)
|
466
|
|
||
|
Balance as of December 31, 2017
|
(85
|
)
|
(395
|
)
|
(480
|
)
|
||
|
Adjustment for the opening balance of accumulated other comprehensive loss
|
263
|
|
—
|
|
263
|
|
||
|
Balance as of January 1, 2018
|
178
|
|
(395
|
)
|
(217
|
)
|
||
|
Other comprehensive income before reclassifications
|
(390
|
)
|
4,081
|
|
3,691
|
|
||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
(1,948
|
)
|
(1,948
|
)
|
||
|
Net-current period other comprehensive income
|
(390
|
)
|
2,133
|
|
1,743
|
|
||
|
Balance as of December 31, 2018
|
(212
|
)
|
1,738
|
|
1,526
|
|
||
|
|
$
|
|
|
Year ending December 31:
|
|
|
|
2019
|
10,752
|
|
|
2020
|
9,972
|
|
|
2021
|
7,805
|
|
|
2022
|
3,923
|
|
|
2023 and thereafter
|
1,357
|
|
|
Total
|
33,809
|
|
|
|
Quarter Ended
|
||||||||||
|
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
|||||||
|
2018
|
$
|
$
|
$
|
$
|
|||||||
|
Revenue
|
32,544
|
|
52,804
|
|
54,202
|
|
58,670
|
|
|||
|
Loss from operations
|
(110,809
|
)
|
(163,050
|
)
|
(151,102
|
)
|
(280,808
|
)
|
|||
|
Net loss
|
(105,116
|
)
|
(157,715
|
)
|
(144,492
|
)
|
(266,710
|
)
|
|||
|
Net loss attributable to ordinary shareholders
|
(104,596
|
)
|
(156,887
|
)
|
(144,031
|
)
|
(268,255
|
)
|
|||
|
Basic and diluted net loss per share(1)
|
(0.16
|
)
|
(0.22
|
)
|
(0.19
|
)
|
(0.35
|
)
|
|||
|
|
Quarter Ended
|
||||||||||
|
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
|||||||
|
2017
|
$
|
$
|
$
|
$
|
|||||||
|
Revenue
|
—
|
|
—
|
|
220,213
|
|
18,174
|
|
|||
|
(Loss) /income from operations
|
(51,542
|
)
|
(58,022
|
)
|
114,905
|
|
(103,798
|
)
|
|||
|
Net (loss) /income
|
(50,623
|
)
|
(60,680
|
)
|
117,284
|
|
(99,280
|
)
|
|||
|
Net (loss) /income attributable to ordinary shareholders
|
(50,623
|
)
|
(60,545
|
)
|
117,386
|
|
(99,323
|
)
|
|||
|
Basic net (loss) /income per share(1)
|
(0.10
|
)
|
(0.12
|
)
|
0.21
|
|
(0.17
|
)
|
|||
|
Diluted net (loss) /income per share(1)
|
(0.10
|
)
|
(0.12)
|
0.20
|
|
(0.17)
|
|||||
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
2016
|
|||||
|
|
$
|
$
|
$
|
|||||
|
PRC
|
132,385
|
|
24,428
|
|
—
|
|
||
|
U.S.
|
42,793
|
|
138,423
|
|
—
|
|
||
|
Other
|
23,042
|
|
75,536
|
|
1,070
|
|
||
|
Total
|
198,220
|
|
238,387
|
|
1,070
|
|
||
|
Exhibit No.
|
Exhibit Description
|
Filed/ Furnished
Herewith
|
Incorporated by Reference
Herein from Form or Schedule
|
Filing Date
|
SEC File/
Reg. Number
|
|
|
3.1
|
8-K
(Exhibit 3.1)
|
12/12/2018
|
001-37686
|
|||
|
4.1
|
.1
|
8-K
(Exhibit 4.1)
|
2/11/2016
|
001-37686
|
||
|
.2
|
8-K
(Exhibit 4.1)
|
4/11/2016
|
001-37686
|
|||
|
.3
|
10-Q
(Exhibit 4.7)
|
8/10/2016
|
001-37686
|
|||
|
.4
|
10-Q
(Exhibit 4.9)
|
5/10/2017
|
001-37686
|
|||
|
4.2
|
Form of American Depositary Receipt (included in Exhibit 4.1.1)
|
|||||
|
4.3
|
S-1
(Exhibit 4.3)
|
12/9/2015
|
333-207459
|
|||
|
4.4
|
.1
|
S-1
(Exhibit 4.4)
|
10/16/2015
|
333-207459
|
||
|
.2
|
S-1
(Exhibit 10.21)
|
1/27/2016
|
333-207459
|
|||
|
4.5
|
8-K
(Exhibit 4.1)
|
11/17/2016
|
001-37686
|
|||
|
Lease Agreements
|
||||||
|
10.1
|
S-1
(Exhibit 10.4) |
10/16/2015
|
333-207459
|
|||
|
10.2
|
10-Q
(Exhibit 10.5) |
5/12/2016
|
001-37686
|
|||
|
Exhibit No.
|
Exhibit Description
|
Filed/ Furnished
Herewith
|
Incorporated by Reference
Herein from Form or Schedule
|
Filing Date
|
SEC File/
Reg. Number
|
|
|
Collaboration, License and Commercial Agreements
|
||||||
|
10.3#
|
S-1
(Exhibit 10.13) |
10/16/2015
|
333-207459
|
|||
|
10.4#
|
10-Q
(Exhibit 10.4) |
8/10/2016
|
001-37686
|
|||
|
10.5#
|
10-Q
(Exhibit 10.1) |
5/10/2017
|
001-37686
|
|||
|
10.6#
|
10-Q
(Exhibit 10.2) |
5/10/2017
|
001-37686
|
|||
|
10.7#
|
10-Q
(Exhibit 10.3) |
5/10/2017
|
001-37686
|
|||
|
10.8#
|
10-Q
(Exhibit 10.2) |
11/13/2017
|
001-37686
|
|||
|
10.9#
|
10-Q
(Exhibit 10.3) |
11/13/2017
|
001-37686
|
|||
|
10.10
|
8-K
(Exhibit 10.1) |
7/6/2017
|
001-37686
|
|||
|
Exhibit No.
|
Exhibit Description
|
Filed/ Furnished
Herewith
|
Incorporated by Reference
Herein from Form or Schedule
|
Filing Date
|
SEC File/
Reg. Number
|
|
|
Equity and Other Compensation Plans
|
||||||
|
10.11†
|
S-1
(Exhibit 10.1) |
10/16/2015
|
333-207459
|
|||
|
10.12
|
.1†
|
8-K
(Exhibit 10.1) |
12/12/2018
|
001-37686
|
||
|
.2†
|
10-Q
(Exhibit 10.7) |
8/9/2018
|
001-37686
|
|||
|
.3†
|
10-Q (Exhibit 10.2)
|
11/8/2018
|
001-37686
|
|||
|
10.13
|
.1†
|
8-K
(Exhibit 10.1) |
8/13/2018
|
001-37686
|
||
|
.2†
|
8-K
(Exhibit 10.3) |
6/8/2018
|
001-37686
|
|||
|
.3†
|
10-Q
(Exhibit 10.5) |
8/9/2018
|
001-37686
|
|||
|
10.14†
|
8-K
(Exhibit 10.2) |
12/12/2018
|
001-37686
|
|||
|
10.15†
|
S-1
(Exhibit 10.19) |
1/19/2016
|
333-207459
|
|||
|
10.16†
|
8-K
(Exhibit 10.5) |
6/8/2018
|
001-37686
|
|||
|
Agreements with Executive Officers and Directors
|
||||||
|
10.17†
|
S-1
(Exhibit 10.3) |
1/19/2016
|
333-207459
|
|||
|
10.18†
|
8-K
(Exhibit 10.1) |
4/26/2017
|
001-37686
|
|||
|
10.19†
|
10-Q
(Exhibit 10.1) |
8/9/2018
|
001-37686
|
|||
|
10.20†
|
S-1
(Exhibit 10.9) |
10/16/2015
|
333-207459
|
|||
|
10.21†
|
10-Q
(Exhibit 10.1) |
11/10/2016
|
001-37686
|
|||
|
10.22†
|
10-Q
(Exhibit 10.2) |
11/10/2016
|
001-37686
|
|||
|
10.23†
|
10-Q
(Exhibit 10.8) |
8/9/2018
|
001-37686
|
|||
|
Exhibit No.
|
Exhibit Description
|
Filed/ Furnished
Herewith
|
Incorporated by Reference
Herein from Form or Schedule
|
Filing Date
|
SEC File/
Reg. Number
|
|
|
21.1
|
X
|
|||||
|
23.1
|
X
|
|||||
|
31.1
|
X
|
|||||
|
31.2
|
X
|
|||||
|
32.1*
|
X
|
|||||
|
101
|
The following financial statements from the Registrant’s Annual Report on Form 10‑K for the year ended December 31, 2018, formatted in XBRL: (i) Consolidated Balance Sheets (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Loss, (iv) Consolidated Statements of Cash Flows, (v) Consolidated Statements of Shareholders’ Equity (Deficit), and (vi) Notes to the Consolidated Financial Statements
|
X
|
||||
|
#
|
Confidential treatment has been granted by the U.S. Securities and Exchange Commission as to certain portions of this exhibit omitted and filed separately.
|
|
BEIGENE, LTD.
|
||
|
Date: February 28, 2019
|
By:
|
/s/ JOHN V. OYLER
|
|
|
|
|
|
|
|
John V. Oyler
|
|
|
|
Chief Executive Officer and Chairman
|
|
(Principal Executive Officer)
|
||
|
Signature
|
|
Title
|
|
Date
|
|
/s/ JOHN V. OYLER
|
|
Chief Executive Officer and Chairman
|
|
February 28, 2019
|
|
John V. Oyler
|
(Principal Executive Officer)
|
|||
|
|
|
|
|
|
|
/s/ HOWARD LIANG
|
|
Chief Financial Officer and Chief Strategy Officer
|
|
February 28, 2019
|
|
Howard Liang
|
(Principal Financial and Accounting Officer)
|
|||
|
|
|
|
|
|
|
/s/ TIMOTHY CHEN
|
|
Director
|
|
February 28, 2019
|
|
Timothy Chen
|
||||
|
|
|
|
|
|
|
/s/ DONALD W. GLAZER
|
|
Director
|
|
February 28, 2019
|
|
Donald W. Glazer
|
||||
|
|
|
|
|
|
|
/s/ MICHAEL GOLLER
|
|
Director
|
|
February 28, 2019
|
|
Michael Goller
|
||||
|
|
|
|
|
|
|
/s/ RANJEEV KRISHANA
|
|
Director
|
|
February 28, 2019
|
|
Ranjeev Krishana
|
||||
|
|
|
|
|
|
|
/s/ THOMAS MALLEY
|
|
Director
|
|
February 28, 2019
|
|
Thomas Malley
|
||||
|
|
|
|
|
|
|
/s/ XIAODONG WANG
|
|
Director
|
|
February 28, 2019
|
|
Xiaodong Wang
|
||||
|
/s/ JING-SHYH (SAM) SU
|
|
Director
|
|
February 28, 2019
|
|
Jing-Shyh (Sam) Su
|
||||
|
/s/ QINGQING YI
|
|
Director
|
|
February 28, 2019
|
|
Qingqing Yi
|
||||