|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
05-0605598
|
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
One Penumbra Place
1351 Harbor Bay Parkway
Alameda, CA
|
94502
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
|
Title of each class
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, Par value $0.001 per share
|
The New York Stock Exchange
|
|
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
|
|
Non-accelerated filer
|
x
|
(Do not check if a smaller reporting Company)
|
Smaller reporting company
|
o
|
|
|
Page
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
•
|
launching our first product, for neurovascular access, in the U. S. in 2007;
|
|
•
|
establishing our direct neuro salesforce in the U. S. and Europe in 2008;
|
|
•
|
launching the first 510(k)-cleared, aspiration-based product for the treatment of ischemic stroke patients in 2008, and launching four subsequent generations of that product;
|
|
•
|
launching our first neurovascular coil for the treatment of brain aneurysms in 2011;
|
|
•
|
launching our first peripheral vascular product in 2013; and
|
|
•
|
establishing our direct peripheral vascular salesforce in the U. S. and Europe in 2014.
|

|
•
|
The Neuron Intracranial Access System
is indicated for the introduction of interventional devices into the peripheral, coronary and neuro vasculature. The system is a two-catheter system comprised of the Neuron Delivery Catheter and the Select Catheter.
|
|
•
|
The Neuron Delivery Catheter
is a variable stiffness, large lumen catheter that combines proximal arch support with a microcatheter-like distal segment that is designed to access the intracranial anatomy. The Neuron can be used individually with a 0.038 inch guidewire, or together with the Neuron Select Catheter, to access the desired location.
|
|
•
|
The Select Catheter
is a single lumen, braid-reinforced, torquable catheter with a radiopaque distal end and a hub on the proximal end. The Select Catheter enables primary access with the Neuron Delivery Catheters, obviating the need for an extra guide catheter.
|
|
•
|
The Neuron MAX System
is an additional configuration to the currently available Neuron Intracranial Access System. The Neuron MAX System is a long sheath catheter with a flexible distal tip for neurovascular use and provides a larger lumen to enable a wide range of device compatibility in neurovascular procedures.
|
|
•
|
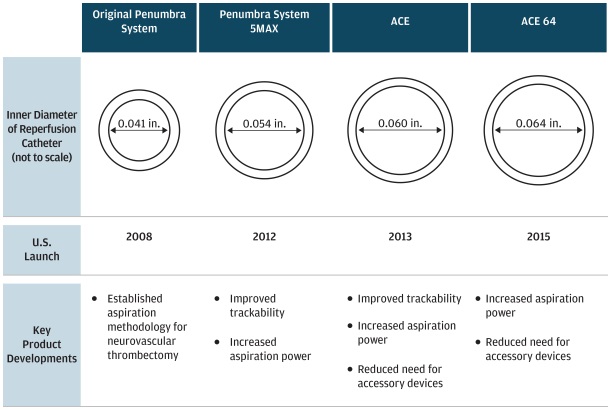
Penumbra Reperfusion Catheters
are the cornerstone of the Penumbra System and are manufactured using a variety of proprietary processes and materials science innovations. We have launched five successive generations of Reperfusion Catheters since 2008.
|
|
•
|
The latest generation of our Reperfusion Catheters, the ACE family of catheters, represents our most powerful and trackable Reperfusion Catheters launched to date. Its design enables specialist physicians to track these large bore aspiration catheters to the distal locations of occluded vessels. Once at the site of the occlusion, ACE provides significantly greater aspiration power than our prior Reperfusion Catheters, which we believe contributes to improved clinical outcomes and reduced procedure times.
|
|
•
|
ACE 64, our latest generation of ACE catheter, is designed to offer enhanced aspiration power relative to prior generations of the product, while maintaining trackability. ACE 64 launched in the U. S. in late May 2015.
|
|
•
|
Penumbra Separators
are a component of the earlier generations of the Penumbra System and enable a physician to remove an aspirated clot that has aggregated in the Reperfusion Catheter during the procedure. The Separators were an important component of our earlier Penumbra System due to the smaller diameter of our original Reperfusion Catheters, which resulted in frequent obstruction of the catheter. With the launch of our larger diameter ACE, Separators are less frequently used by physicians.
|
|
•
|
3D
is a stent retriever component of the Penumbra System that allows a physician to combine direct aspiration with stent retriever technology. 3D is being evaluated in a clinical study pursuant to an Investigational Device Exemption (IDE) to obtain clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act (FD&C Act). With 198 of the anticipated 230 patients enrolled, the Data Safety Monitoring Board for this study recently temporarily suspended further enrollment in order to assess the data. The Board indicated that the suspension was not due to safety issues.
|
|
•
|
Penumbra Aspiration Pumps
are attached to our Reperfusion Catheters and provide the aspirating suction force. Our second generation MAX Aspiration Pump features increased aspiration capabilities and an improved, easier to use design. We have standardized the MAX Aspiration Pump to work with all generations of our Reperfusion Catheters.
|
|
•
|
less retreatments or worsening occlusions;
|
|
•
|
larger aneurysm treatment capabilities;
|
|
•
|
higher packing density; and
|
|
•
|
fewer coils per aneurysm.
|
|
•
|
the Apollo wand that is inserted into the brain through an endoscope, which, in turn, is inserted through a small burr hole into the skull; and
|
|
•
|
a reusable hardware device that delivers vacuum, irrigation and vibrational energy along the disposable wand to the site of the hemorrhage.
|
|
•
|
peripheral vascular embolization;
|
|
•
|
vessel occlusion; and
|
|
•
|
peripheral vascular thrombectomy.
|
|
•
|
active extravasations, or the escape of blood into surrounding tissue;
|
|
•
|
selective embolization in patients with visceral aneurysms;
|
|
•
|
exclusion of branches prior to chemoembolization and radioembolization;
|
|
•
|
embolization in patients with gastrointestinal bleeding;
|
|
•
|
embolization of branches prior to stent graft procedures;
|
|
•
|
procedures after stent grafting in patients with persistent type II endoleaks and sac enlargement;
|
|
•
|
treatment of patients with varicocele and pelvic congestion syndrome;
|
|
•
|
high flow arterial venous malformations;
|
|
•
|
post trans intrahepatic shunt placement;
|
|
•
|
balloon retrograde transvenous obliteration; and
|
|
•
|
exclusion of hepatic branches prior to liver resection.
|
|
•
|
microcatheter deliverability;
|
|
•
|
instant detachment;
|
|
•
|
immediate and precise anchoring;
|
|
•
|
a single device to treat a range of vessel diameters; and
|
|
•
|
dense occlusion in a short segment.
|
|
•
|
Continuous Aspiration Mechanical Thrombectomy Catheters
are the foundation of the system and are ideally suited to reach anatomy below the knee. Much like our MAX and ACE catheters, the CAT catheters are robust, durable, trackable and suited for the peripheral anatomy. The initial launch of the Indigo System included our CAT5 catheter and the device made for more distal access, CAT3, which is able to reach the distal peripheral vessels of the upper and lower extremities. On May 26, 2015, we received FDA clearance for CAT6 and CAT8, two larger sizes of the Indigo System, as well as to market the Indigo System for use in both the peripheral arterial and venous systems.
|
|
•
|
Indigo Separator
enables the peripheral interventionalist to remove a difficult to aspirate clot from the CAT catheter. In the peripheral vessels, clots often form in long segments, and are more resistant to traditional aspiration techniques. The Indigo System with the Separator enables a wide range of clot morphology to be removed from the body. While conclusions should not be drawn from initial results and further results may prove to be worse or inconclusive, we have demonstrated in clinical settings that the Indigo System with the Separator can remove clots that were resistant to hours of revascularization attempts with other technologies and thrombolytic agents.
|
|
•
|
Penumbra Aspiration Pump
is the power source that provides the aspirating suction force to remove waste, such as blood and clots.
|
|
•
|
Neuro:
Interventional neuroradiologists, neurosurgeons and interventional neurologists.
|
|
•
|
Peripheral vascular:
Interventional radiologists and vascular surgeons.
|
|
•
|
significantly greater name recognition;
|
|
•
|
broader or deeper relations with healthcare professionals, customers and third-party payors;
|
|
•
|
more established distribution networks;
|
|
•
|
additional lines of products and the ability to offer rebates or bundle products to offer greater discounts or other incentives to gain a competitive advantage;
|
|
•
|
greater experience in conducting research and development, manufacturing, clinical trials, marketing and obtaining regulatory clearance or approval for products; and
|
|
•
|
greater financial and human resources for product development, sales and marketing and patent litigation.
|
|
•
|
develop innovative, proprietary products that can cost-effectively address significant clinical needs;
|
|
•
|
continue to innovate and develop scientifically advanced technology;
|
|
•
|
obtain and maintain regulatory clearances or approvals;
|
|
•
|
demonstrate efficacy in Penumbra-sponsored and third-party clinical trials and studies;
|
|
•
|
apply technology across product lines and markets;
|
|
•
|
attract and retain skilled research and development and sales personnel; and
|
|
•
|
cost-effectively manufacture and successfully market and sell products.
|
|
•
|
establishment registration and device listing;
|
|
•
|
the QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the manufacturing process;
|
|
•
|
labeling regulations and the FDA prohibitions against the promotion of products for un-cleared, unapproved or “off-label” uses, and other requirements related to promotional activities;
|
|
•
|
medical device reporting regulations, which require that manufactures report to the FDA if their device may have caused or contributed to a death or serious injury or if their device malfunctioned and the device or a similar device marketed by the manufacturer would be likely to cause or contribute to a death or serious injury if the malfunction were to recur;
|
|
•
|
corrections and removal reporting regulations, which require that manufactures report to the FDA field corrections or removals if undertaken to reduce a risk to health posed by a device or to remedy a violation of the FD&C Act that may present a risk to health; and
|
|
•
|
post market surveillance regulations, which apply to certain class II or III devices when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
|
|
•
|
warning or untitled letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
customer notifications, voluntary or mandatory recall or seizure of our products;
|
|
•
|
operating restrictions, partial suspension or total shutdown of production;
|
|
•
|
delay in processing submissions or applications for new products or modifications to existing products;
|
|
•
|
withdrawing approvals that have already been granted; and
|
|
•
|
criminal prosecution.
|
|
•
|
our ability to market and distribute our products effectively;
|
|
•
|
the availability, perceived efficacy and pricing of alternative products from our competitors;
|
|
•
|
the development of new products or alternative treatments by others that render our products and technologies obsolete;
|
|
•
|
the price, quality, effectiveness and reliability of our products;
|
|
•
|
our customer service and reputation;
|
|
•
|
our ability to convince specialist physicians to use our products on their patients; and
|
|
•
|
the timing of market entry of new products or alternative treatments.
|
|
•
|
significantly greater name recognition;
|
|
•
|
broader or deeper relations with healthcare professionals, customers and third-party payors;
|
|
•
|
more established distribution networks;
|
|
•
|
additional lines of products and the ability to offer rebates or bundle products to offer greater discounts or other incentives to gain a competitive advantage;
|
|
•
|
greater experience in conducting research and development, manufacturing, clinical trials, marketing and obtaining regulatory clearance or approval for products; and
|
|
•
|
greater financial and human resources for product development, sales and marketing and patent litigation.
|
|
•
|
develop innovative, proprietary products that can cost-effectively address significant clinical needs;
|
|
•
|
continue to innovate and develop scientifically advanced technology;
|
|
•
|
obtain and maintain regulatory clearances or approvals;
|
|
•
|
demonstrate efficacy in Penumbra-sponsored and third-party clinical trials and studies;
|
|
•
|
apply technology across product lines and markets;
|
|
•
|
attract and retain skilled research and development and sales personnel; and
|
|
•
|
cost-effectively manufacture and successfully market and sell products.
|
|
•
|
capacity constraints;
|
|
•
|
production yields;
|
|
•
|
quality control;
|
|
•
|
equipment availability; and
|
|
•
|
shortages of qualified personnel.
|
|
•
|
continuing to educate hospitals and specialist physicians about the clinical evidence supporting intervention, as well as the use, benefits and cost-effectiveness of our products;
|
|
•
|
improving the speed with which patients are assessed for and receive interventional treatments; and
|
|
•
|
increasing the likelihood that patients are transported to a hospital or stroke center where interventional treatments are available.
|
|
•
|
reliance on distributors;
|
|
•
|
varying coverage and reimbursement policies, processes and procedures;
|
|
•
|
difficulties in staffing and managing international operations from which sales are conducted;
|
|
•
|
difficulties in penetrating markets in which our competitors’ products or alternative procedures that do not use our products are more established;
|
|
•
|
reduced protection for intellectual property rights in some countries;
|
|
•
|
export licensing requirements or restrictions, trade regulations and foreign tax laws;
|
|
•
|
fluctuating foreign currency exchange rates;
|
|
•
|
foreign certification, regulatory requirements and legal requirements;
|
|
•
|
lengthy payment cycles and difficulty in collecting accounts receivable;
|
|
•
|
customs clearance and shipping delays;
|
|
•
|
pricing pressure in international markets;
|
|
•
|
political and economic instability; and
|
|
•
|
preference for locally produced products.
|
|
•
|
others may be able to develop products that are similar to, or better than, ours in a way that is not covered by the claims of our patents;
|
|
•
|
we might not have been the first to make the inventions covered by our patents or pending patent applications;
|
|
•
|
we might not have been the first to file patent applications for these inventions;
|
|
•
|
any patents that we obtain may not provide us with any competitive advantages or may ultimately be found invalid or unenforceable; or
|
|
•
|
we may not develop additional proprietary technologies that are patentable.
|
|
•
|
we or our collaborators may initiate litigation or other proceedings against third parties seeking to invalidate the patents held by those third parties or to obtain a judgment that our products or processes do not infringe those third parties’ patents;
|
|
•
|
we or our collaborators may participate at substantial cost in International Trade Commission proceedings to abate importation of products that would compete unfairly with our products;
|
|
•
|
if our competitors file patent applications that claim technology also claimed by us or our licensors, we or our licensors may be required to participate in interference, derivation or opposition proceedings to determine the priority of invention, which could jeopardize our patent rights and potentially provide a third party with a dominant patent position;
|
|
•
|
if third parties initiate litigation claiming that our processes or products infringe their patent or other intellectual property rights, we and our collaborators will need to defend against such proceedings;
|
|
•
|
if third parties initiate litigation or other proceedings seeking to invalidate patents owned by or licensed to us or to obtain a declaratory judgment that their product or technology does not infringe our patents or patents licensed to us, we will need to defend against such proceedings;
|
|
•
|
we may be subject to ownership disputes relating to intellectual property, including disputes arising from conflicting obligations of consultants or others who are involved in developing our products; and
|
|
•
|
if a license to necessary technology is terminated, the licensor may initiate litigation claiming that our processes or products infringe or misappropriate their patent or other intellectual property rights and/or that we breached our obligations under the license agreement, and we and our collaborators would need to defend against such proceedings.
|
|
•
|
incur substantial monetary liability for infringement or other violations of intellectual property rights, which we may have to pay if a court decides that the product or technology at issue infringes or violates the third party’s rights, and if the court finds that the infringement was willful, we could be ordered to pay treble damages and the third party’s attorneys’ fees;
|
|
•
|
pay substantial damages to our customers or end users to discontinue use or replace infringing technology with non-infringing technology;
|
|
•
|
stop manufacturing, selling, using, exporting or licensing the product or technology incorporating the allegedly infringing technology or stop incorporating the allegedly infringing technology into such product or technology;
|
|
•
|
obtain from the owner of the infringed intellectual property right a license, which may require us to pay substantial upfront fees or royalties to sell or use the relevant technology and which may not be available on commercially reasonable terms, or at all;
|
|
•
|
redesign our products and technology so they do not infringe or violate the third party’s intellectual property rights, which may not be possible or may require substantial monetary expenditures and time;
|
|
•
|
enter into cross-licenses with our competitors, which could weaken our overall intellectual property position;
|
|
•
|
lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property against others;
|
|
•
|
find alternative suppliers for non-infringing products and technologies, which could be costly and create significant delay; or
|
|
•
|
relinquish rights associated with one or more of our patent claims, if our claims are held invalid or otherwise unenforceable.
|
|
•
|
variations in revenue due to the unavailability of specialist physicians who use our products during certain times of the year, such as those periods when there are major conferences on conditions they treat or those periods when high volume users of our products take time off of work;
|
|
•
|
positive or negative media coverage of our products or the procedures or products of our competitors or our industry;
|
|
•
|
publication of clinical trial results or studies by us or our competitors;
|
|
•
|
changes in our sales process due to industry changes, such as changes in the stroke care pathway;
|
|
•
|
delays in receipt of anticipated purchase orders;
|
|
•
|
delays in customers receiving products;
|
|
•
|
performance of our independent distributors;
|
|
•
|
our ability to obtain further regulatory clearances or approvals;
|
|
•
|
the timing of product development and clinical trial activities, including the pace of enrollment;
|
|
•
|
delays in, or failure of, product and component deliveries by our suppliers;
|
|
•
|
changes in reimbursement policies or levels;
|
|
•
|
the number of procedures performed in any given period using our products, which can sometimes vary significantly between periods;
|
|
•
|
customer response to the introduction of new products or alternative treatments, and the degree to we which we are effective in transitioning customers to our products; and
|
|
•
|
fluctuations in foreign currency.
|
|
•
|
price and volume fluctuations in the overall stock market from time to time;
|
|
•
|
volatility in the market prices and trading volumes of medical device company stocks;
|
|
•
|
changes in operating performance and stock market valuations of other medical device companies generally, or those in our industry in particular;
|
|
•
|
sales of shares of our common stock by us or our stockholders;
|
|
•
|
failure of securities analysts to maintain coverage of us, changes in financial estimates by securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors;
|
|
•
|
the financial projections we may provide to the public, any changes in those projections or our failure to meet those projections;
|
|
•
|
announcements by us or our competitors of new products or services;
|
|
•
|
the public’s reaction to our press releases, other public announcements and filings with the SEC;
|
|
•
|
rumors and market speculation involving us or other companies in our industry;
|
|
•
|
actual or anticipated changes in our results of operations or fluctuations in our results of operations;
|
|
•
|
actual or anticipated developments in our business, our competitors’ businesses or the competitive landscape generally;
|
|
•
|
litigation involving us, our industry or both, or investigations by regulators into our operations or those of our competitors;
|
|
•
|
developments or disputes concerning our intellectual property or other proprietary rights;
|
|
•
|
announced or completed acquisitions of businesses or technologies by us or our competitors;
|
|
•
|
new laws or regulations or new interpretations of existing laws or regulations applicable to our business;
|
|
•
|
changes in accounting standards, policies, guidelines, interpretations or principles;
|
|
•
|
any significant change in our management; and
|
|
•
|
general economic conditions and slow or negative growth of our markets.
|
|
•
|
authorizing the issuance of “blank check” preferred stock without any need for action by stockholders;
|
|
•
|
requiring supermajority stockholder voting to effect certain amendments to our restated certificate of incorporation and amended and restated bylaws;
|
|
•
|
eliminating the ability of stockholders to call and bring business before special meetings of stockholders;
|
|
•
|
prohibiting stockholder action by written consent;
|
|
•
|
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings;
|
|
•
|
dividing our board of directors into three classes so that only one third of our directors will be up for election in any given year; and
|
|
•
|
providing that our directors may be removed by our stockholders only for cause.
|
|
•
|
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act;
|
|
•
|
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
|
•
|
reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and
|
|
•
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
|
|
High
|
Low
|
|||||||
|
Year ended December 31, 2015
|
||||||||
|
Third Quarter
|
$
|
43.06
|
|
$
|
38.00
|
|
||
|
Fourth Quarter
|
59.36
|
|
35.31
|
|
||||

|
$100 Investment in stock or index
|
Ticker
|
September 18, 2015
|
September 30, 2015
|
October 30, 2015
|
November 30, 2015
|
December 31, 2015
|
||||||||||||||||
|
Penumbra
|
PEN
|
$
|
100.00
|
|
$
|
97.09
|
|
$
|
89.66
|
|
$
|
121.84
|
|
$
|
130.29
|
|
||||||
|
S&P 500 Health Care Equipment Index
|
XHE
|
100.00
|
|
90.66
|
|
94.65
|
|
99.91
|
|
93.50
|
|
|||||||||||
|
NYSE Composite
|
NYA
|
100.00
|
|
97.69
|
|
104.28
|
|
103.77
|
|
101.11
|
|
|||||||||||
|
•
|
We granted to our directors, officers, employees and consultants options to purchase an aggregate of 1,321,250 shares of common stock under our equity compensation plans, at exercise prices ranging from $22.04 to $30.00 per share.
|
|
•
|
We issued and sold to our directors, officers, employees and consultants an aggregate of 6,500 shares of common stock upon the exercise of options under our equity compensation plans at exercise prices ranging from $1.26 to $3.98 per share, for an aggregate amount of $16,613.
|
|
•
|
We granted to our directors, officers and employees an aggregate of 5,000 shares of restricted stock under our equity compensation plans at a fair market value of $22.04 per share, for an aggregate amount of $110,200.
|
|
Year Ended December 31,
|
||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
|||||||||||||
|
(In thousands, except share and per share amounts)
|
||||||||||||||||
|
Consolidated Statement of Operations Data:
|
||||||||||||||||
|
Revenue
|
$
|
186,095
|
|
$
|
125,510
|
|
$
|
88,848
|
|
$
|
73,141
|
|
||||
|
Cost of revenue
|
62,037
|
|
42,668
|
|
30,972
|
|
24,178
|
|
||||||||
|
Gross profit
|
124,058
|
|
82,842
|
|
57,876
|
|
48,963
|
|
||||||||
|
Operating expenses:
|
||||||||||||||||
|
Research and development
|
18,027
|
|
15,575
|
|
14,084
|
|
12,548
|
|
||||||||
|
Sales, general and administrative
|
101,852
|
|
64,258
|
|
44,918
|
|
32,987
|
|
||||||||
|
Total operating expenses
|
119,879
|
|
79,833
|
|
59,002
|
|
45,535
|
|
||||||||
|
Income (loss) from operations
|
4,179
|
|
3,009
|
|
(1,126
|
)
|
3,428
|
|
||||||||
|
Interest income (expense), net
|
541
|
|
439
|
|
345
|
|
244
|
|
||||||||
|
Other income (expense), net
|
(696
|
)
|
(309
|
)
|
(474
|
)
|
220
|
|
||||||||
|
Income (loss) before provision for (benefit from) income taxes
|
4,024
|
|
3,139
|
|
(1,255
|
)
|
3,892
|
|
||||||||
|
Provision for (benefit from) income taxes
|
1,659
|
|
894
|
|
(5,354
|
)
|
1,934
|
|
||||||||
|
Net income
|
$
|
2,365
|
|
$
|
2,245
|
|
$
|
4,099
|
|
$
|
1,958
|
|
||||
|
Net income (loss) attributable to common stockholders
|
$
|
719
|
|
$
|
(833
|
)
|
$
|
887
|
|
$
|
412
|
|
||||
|
Net income (loss) per share attributable to common
stockholders —Basic |
$
|
0.09
|
|
$
|
(0.18
|
)
|
$
|
0.21
|
|
$
|
0.10
|
|
||||
|
—Diluted
|
$
|
0.08
|
|
$
|
(0.18
|
)
|
$
|
0.14
|
|
$
|
0.07
|
|
||||
|
Weighted average shares used to compute net income (loss) per share attributable to common stockholders
—Basic |
11,993,429
|
|
4,609,375
|
|
4,304,396
|
|
4,153,121
|
|
||||||||
|
—Diluted
|
14,219,650
|
|
4,609,375
|
|
6,500,835
|
|
5,886,126
|
|
||||||||
|
Year Ended December 31,
|
||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
19,547
|
|
$
|
3,290
|
|
$
|
4,131
|
|
$
|
7,435
|
|
||||
|
Marketable investments
|
$
|
129,257
|
|
$
|
48,253
|
|
$
|
9,545
|
|
$
|
11,341
|
|
||||
|
Total assets
|
$
|
263,848
|
|
$
|
121,381
|
|
$
|
71,147
|
|
$
|
63,209
|
|
||||
|
Long-term debt
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4,000
|
|
||||
|
Working capital
|
$
|
216,213
|
|
$
|
94,478
|
|
$
|
46,401
|
|
$
|
41,607
|
|
||||
|
Convertible Preferred stock
|
$
|
—
|
|
$
|
111,467
|
|
$
|
56,222
|
|
$
|
56,222
|
|
||||
|
Total stockholders’ equity (deficit)
|
$
|
232,522
|
|
$
|
(12,370
|
)
|
$
|
(8,062
|
)
|
$
|
(12,751
|
)
|
||||
|
•
|
The rate at which we grow our salesforce and the speed at which newly hired salespeople become fully effective can impact our revenue growth or our costs incurred in anticipation of such growth.
|
|
•
|
Our industry is intensely competitive and, in particular, we compete with a number of large, well-capitalized companies. We must continue to successfully compete in light of our competitors’ existing and future products and their resources to successfully market to the specialist physicians who use our products.
|
|
•
|
We must continue to successfully introduce new products that gain acceptance with specialist physicians and successfully transition from existing products to new products, ensuring adequate supply while avoiding excess inventory of older products and resulting inventory write-downs or write-offs. In addition, as we introduce new products, we generally build our inventory of components and finished goods in advance of sales, which may cause quarterly fluctuations in our financial condition.
|
|
•
|
Publications of clinical results by us, our competitors and other third parties can have a significant influence on whether, and the degree to which, our products are used by specialist physicians and the procedures and treatments those physicians choose to administer for a given condition.
|
|
•
|
The specialist physicians who use our products may not perform procedures during certain times of the year, such as those periods when they are at major medical conferences or are away from their practices for other reasons, the timing of which occurs irregularly during the year and from year to year.
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||||||||||
|
|
||||||||||||||||||||
|
Revenue
|
$
|
186,095
|
|
100.0
|
%
|
$
|
125,510
|
|
100.0
|
%
|
$
|
88,848
|
|
100.0
|
%
|
|||||
|
Cost of revenue
|
62,037
|
|
33.3
|
%
|
42,668
|
|
34.0
|
%
|
30,972
|
|
34.9
|
%
|
||||||||
|
Gross profit
|
124,058
|
|
66.7
|
%
|
82,842
|
|
66.0
|
%
|
57,876
|
|
65.1
|
%
|
||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
18,027
|
|
9.7
|
%
|
15,575
|
|
12.4
|
%
|
14,084
|
|
15.9
|
%
|
||||||||
|
Sales, general and administrative
|
101,852
|
|
54.7
|
%
|
64,258
|
|
51.2
|
%
|
44,918
|
|
50.6
|
%
|
||||||||
|
Total operating expenses
|
119,879
|
|
64.4
|
%
|
79,833
|
|
63.6
|
%
|
59,002
|
|
66.4
|
%
|
||||||||
|
Income (loss) from operations
|
4,179
|
|
2.2
|
%
|
3,009
|
|
2.4
|
%
|
(1,126
|
)
|
(1.3
|
)%
|
||||||||
|
Interest income (expense), net
|
541
|
|
0.3
|
%
|
439
|
|
0.3
|
%
|
345
|
|
0.4
|
%
|
||||||||
|
Other income (expense), net
|
(696
|
)
|
(0.4
|
)%
|
(309
|
)
|
(0.2
|
)%
|
(474
|
)
|
(0.5
|
)%
|
||||||||
|
Income (loss) before provision for (benefit from) income taxes
|
4,024
|
|
2.2
|
%
|
3,139
|
|
2.5
|
%
|
(1,255
|
)
|
(1.4
|
)%
|
||||||||
|
Provision for (benefit from) income taxes
|
1,659
|
|
0.9
|
%
|
894
|
|
0.7
|
%
|
(5,354
|
)
|
(6.0
|
)%
|
||||||||
|
Net income
|
$
|
2,365
|
|
1.3
|
%
|
$
|
2,245
|
|
1.8
|
%
|
$
|
4,099
|
|
4.6
|
%
|
|||||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2015
|
2014
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
Neuro
|
$
|
141,410
|
|
$
|
106,242
|
|
$
|
35,168
|
|
33.1
|
%
|
|||
|
Peripheral Vascular
|
44,685
|
|
19,268
|
|
25,417
|
|
131.9
|
%
|
||||||
|
Total
|
$
|
186,095
|
|
$
|
125,510
|
|
$
|
60,585
|
|
48.3
|
%
|
|||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2015
|
2014
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
Cost of revenue
|
$
|
62,037
|
|
$
|
42,668
|
|
$
|
19,369
|
|
45.4
|
%
|
|||
|
Gross profit
|
$
|
124,058
|
|
$
|
82,842
|
|
$
|
41,216
|
|
49.8
|
%
|
|||
|
Gross margin %
|
66.7
|
%
|
66.0
|
%
|
||||||||||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2015
|
2014
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
R&D
|
$
|
18,027
|
|
$
|
15,575
|
|
$
|
2,452
|
|
15.7
|
%
|
|||
|
R&D as a percentage of revenue
|
9.7
|
%
|
12.4
|
%
|
||||||||||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2015
|
2014
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
SG&A
|
$
|
101,852
|
|
$
|
64,258
|
|
$
|
37,594
|
|
58.5
|
%
|
|||
|
SG&A
as a percentage of revenue
|
54.7
|
%
|
51.2
|
%
|
||||||||||
|
|
Year Ended December 31,
|
Change
|
||||||||||
|
|
2015
|
2014
|
$
|
%
|
||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||
|
Provision for income taxes
|
1,659
|
|
894
|
|
$
|
765
|
|
85.6
|
%
|
|||
|
Effective tax rate
|
41.2
|
%
|
28.5
|
%
|
||||||||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2014
|
2013
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
Neuro
|
$
|
106,242
|
|
$
|
81,343
|
|
$
|
24,899
|
|
30.6
|
%
|
|||
|
Peripheral Vascular
|
19,268
|
|
7,505
|
|
11,763
|
|
156.7
|
%
|
||||||
|
Total
|
$
|
125,510
|
|
$
|
88,848
|
|
$
|
36,662
|
|
41.3
|
%
|
|||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2014
|
2013
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
Cost of revenue
|
$
|
42,668
|
|
$
|
30,972
|
|
$
|
11,696
|
|
37.8
|
%
|
|||
|
Gross profit
|
$
|
82,842
|
|
$
|
57,876
|
|
$
|
24,966
|
|
43.1
|
%
|
|||
|
Gross margin %
|
66.0
|
%
|
65.1
|
%
|
||||||||||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2014
|
2013
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
R&D
|
$
|
15,575
|
|
$
|
14,084
|
|
$
|
1,491
|
|
10.6
|
%
|
|||
|
R&D as a percentage of revenue
|
12.4
|
%
|
15.9
|
%
|
||||||||||
|
|
Year Ended December 31,
|
Change
|
||||||||||||
|
|
2014
|
2013
|
$
|
%
|
||||||||||
|
|
(in thousands, except for percentages)
|
|||||||||||||
|
SG&A
|
$
|
64,258
|
|
$
|
44,918
|
|
$
|
19,340
|
|
43.1
|
%
|
|||
|
SG&A as a percentage of revenue
|
51.2
|
%
|
50.6
|
%
|
||||||||||
|
|
Year Ended December 31,
|
Change
|
|||||||||||
|
|
2014
|
2013
|
$
|
%
|
|||||||||
|
|
(in thousands, except for percentages)
|
||||||||||||
|
Provision for (benefit from) income taxes
|
$
|
894
|
|
$
|
(5,354
|
)
|
$
|
6,248
|
|
nm
|
|||
|
Effective tax rate
|
28.5
|
%
|
nm
|
|
|||||||||
|
|
December 31,
2015 |
December 31,
2014 |
|||||
|
|
(in thousands)
|
||||||
|
Cash and cash equivalents
|
$
|
19,547
|
|
$
|
3,290
|
|
|
|
Marketable investments
|
129,257
|
|
48,253
|
|
|||
|
Accounts receivable, net
|
29,444
|
|
18,912
|
|
|||
|
Accounts payable
|
2,567
|
|
2,348
|
|
|||
|
Accrued liabilities
|
25,581
|
|
18,475
|
|
|||
|
Working capital
(1)
|
216,213
|
|
94,478
|
|
|||
|
(1)
|
Working capital consists of total current assets less total current liabilities.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(in thousands)
|
||||||||||
|
Cash and cash equivalents at beginning of year
|
$
|
3,290
|
|
$
|
4,131
|
|
$
|
7,435
|
|
||
|
Net cash used in operating activities
|
(22,279
|
)
|
(6,389
|
)
|
(3,396
|
)
|
|||||
|
Net cash used in investing activities
|
(85,816
|
)
|
(37,001
|
)
|
(1,251
|
)
|
|||||
|
Net cash provided by financing activities
|
124,424
|
|
42,897
|
|
2,178
|
|
|||||
|
Cash and cash equivalents at end of year
|
19,547
|
|
3,290
|
|
4,131
|
|
|||||
|
|
Payments Due by Period
|
||||||||||||||||||
|
|
Total
|
Less Than
One Year
|
1-3 Years
|
3-5 Years
|
More than
Five Years
|
||||||||||||||
|
|
(in thousands)
|
||||||||||||||||||
|
Rent obligations
(1)
|
$
|
75,535
|
|
$
|
3,803
|
|
$
|
9,351
|
|
$
|
9,826
|
|
$
|
52,555
|
|
||||
|
Equipment lease obligations
(2)
|
953
|
|
252
|
|
387
|
|
314
|
|
—
|
|
|||||||||
|
Purchase commitments
(3)
|
11,820
|
|
10,960
|
|
860
|
|
—
|
|
—
|
|
|||||||||
|
Total
|
$
|
88,308
|
|
$
|
15,015
|
|
$
|
10,598
|
|
$
|
10,140
|
|
$
|
52,555
|
|
||||
|
(1)
|
We lease our corporate headquarters and a manufacturing facility at our campus in Alameda, California, pursuant to lease agreements that expire on various dates from 2029 to 2031. Additionally, we lease offices in Germany, Australia and Brazil. Included in rent obligations
|
|
(2)
|
We lease equipment and automobiles under operating leases. These leases expire at various dates through 2018.
|
|
(3)
|
Purchase commitments consist of contracts with suppliers to purchase raw materials to be used to manufacture products.
|
|
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
19,547
|
|
$
|
3,290
|
|
||
|
Marketable investments
|
129,257
|
|
48,253
|
|
||||
|
Accounts receivable, net of doubtful accounts of $589 and $602 at December 31, 2015 and 2014, respectively
|
29,444
|
|
18,912
|
|
||||
|
Inventories
|
56,761
|
|
33,451
|
|
||||
|
Deferred taxes
|
—
|
|
6,280
|
|
||||
|
Prepaid expenses and other current assets
|
9,352
|
|
5,115
|
|
||||
|
Total current assets
|
244,361
|
|
115,301
|
|
||||
|
Property and equipment, net
|
8,951
|
|
5,181
|
|
||||
|
Deferred taxes
|
10,143
|
|
571
|
|
||||
|
Other non-current assets
|
393
|
|
328
|
|
||||
|
Total assets
|
$
|
263,848
|
|
$
|
121,381
|
|
||
|
Liabilities, Convertible Preferred Stock and Stockholders’ Equity (Deficit)
|
||||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable
|
$
|
2,567
|
|
$
|
2,348
|
|
||
|
Accrued liabilities
|
25,581
|
|
18,475
|
|
||||
|
Total current liabilities
|
28,148
|
|
20,823
|
|
||||
|
Other non-current liabilities
|
3,178
|
|
1,461
|
|
||||
|
Total liabilities
|
31,326
|
|
22,284
|
|
||||
|
Commitments and contingencies (Note 8)
|
|
|
|
|
||||
|
Convertible preferred stock, $0.001 par value per share—none authorized, issued and outstanding at December 31, 2015; 25,000,000 shares authorized, 19,510,410 shares issued and outstanding at December 31, 2014; aggregate liquidation value $149,361 at December 31, 2014
|
—
|
|
111,467
|
|
||||
|
Stockholders’ Equity (Deficit):
|
||||||||
|
Preferred stock, $0.001 par value per share—5,000,000 shares authorized, none issued and outstanding at December 31, 2015; None authorized, issued and outstanding at December 31, 2014
|
—
|
|
—
|
|
||||
|
Common stock, $0.001 par value per share—300,000,000 shares authorized, 29,897,860 issued and outstanding at December 31, 2015; 40,000,000 shares authorized, 4,736,689 issued and outstanding at December 31, 2014
|
30
|
|
5
|
|
||||
|
Additional paid-in capital
|
252,087
|
|
8,446
|
|
||||
|
Notes receivable from stockholders
|
(5
|
)
|
(117
|
)
|
||||
|
Accumulated other comprehensive loss
|
(2,115
|
)
|
(864
|
)
|
||||
|
Accumulated deficit
|
(17,475
|
)
|
(19,840
|
)
|
||||
|
Total stockholders’ equity (deficit)
|
232,522
|
|
(12,370
|
)
|
||||
|
Total liabilities, convertible preferred stock and stockholders’ equity (deficit)
|
$
|
263,848
|
|
$
|
121,381
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2015
|
2014
|
2013
|
|||||||||
|
Revenue
|
$
|
186,095
|
|
$
|
125,510
|
|
$
|
88,848
|
|
||
|
Cost of revenue
|
62,037
|
|
42,668
|
|
30,972
|
|
|||||
|
Gross profit
|
124,058
|
|
82,842
|
|
57,876
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
18,027
|
|
15,575
|
|
14,084
|
|
|||||
|
Sales, general and administrative
|
101,852
|
|
64,258
|
|
44,918
|
|
|||||
|
Total operating expenses
|
119,879
|
|
79,833
|
|
59,002
|
|
|||||
|
Income (loss) from operations
|
4,179
|
|
3,009
|
|
(1,126
|
)
|
|||||
|
Interest income (expense), net
|
541
|
|
439
|
|
345
|
|
|||||
|
Other income (expense), net
|
(696
|
)
|
(309
|
)
|
(474
|
)
|
|||||
|
Income (loss) before provision for (benefit from) income taxes
|
4,024
|
|
3,139
|
|
(1,255
|
)
|
|||||
|
Provision for (benefit from) income taxes
|
1,659
|
|
894
|
|
(5,354
|
)
|
|||||
|
Net income
|
2,365
|
|
2,245
|
|
4,099
|
|
|||||
|
Foreign currency translation adjustments, net of tax
|
(1,308
|
)
|
(1,423
|
)
|
(522
|
)
|
|||||
|
Unrealized gains (losses) on available-for-sale securities, net of tax
|
57
|
|
(237
|
)
|
(84
|
)
|
|||||
|
Comprehensive income
|
$
|
1,114
|
|
$
|
585
|
|
$
|
3,493
|
|
||
|
Net income (loss) attributable to common stockholders (Note 16)
|
$
|
1,084
|
|
$
|
(833
|
)
|
$
|
887
|
|
||
|
Net income (loss) per share attributable to common stockholders
—Basic |
$
|
0.09
|
|
$
|
(0.18
|
)
|
$
|
0.21
|
|
||
|
—Diluted
|
$
|
0.08
|
|
$
|
(0.18
|
)
|
$
|
0.14
|
|
||
|
Weighted average shares used to compute net income (loss) per share attributable to common stockholders
—Basic |
11,993,429
|
|
4,609,375
|
|
4,304,396
|
|
|||||
|
—Diluted
|
14,219,650
|
|
4,609,375
|
|
6,500,835
|
|
|||||
|
Convertible Preferred Stock
|
Common Stock
|
Additional
Paid-in
Capital
|
Notes
Receivable
from
Stockholders
|
Accumulated
Other
Comprehensive
Income (Loss)
|
Accumulated
Deficit
|
Total
Stockholders’
Equity (Deficit)
|
|||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||
|
Balance at December 31, 2012
|
15,594,618
|
|
$
|
56,222
|
|
4,192,745
|
|
$
|
4
|
|
$
|
5,073
|
|
$
|
(138
|
)
|
$
|
1,402
|
|
$
|
(19,092
|
)
|
$
|
(12,751
|
)
|
||||||||||
|
Issuance of common stock
|
—
|
|
—
|
|
198,843
|
|
—
|
|
310
|
|
—
|
|
—
|
|
—
|
|
310
|
|
|||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
886
|
|
—
|
|
—
|
|
—
|
|
886
|
|
|||||||||||||||||
|
Foreign currency translation adjustment, net of tax of $213
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(522
|
)
|
—
|
|
(522
|
)
|
|||||||||||||||||
|
Unrealized loss on investments, net of tax of $10
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(84
|
)
|
—
|
|
(84
|
)
|
|||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,099
|
|
4,099
|
|
|||||||||||||||||
|
Balance at December 31, 2013
|
15,594,618
|
|
56,222
|
|
4,391,588
|
|
4
|
|
6,269
|
|
(138
|
)
|
796
|
|
(14,993
|
)
|
(8,062
|
)
|
|||||||||||||||||
|
Issuance of Series F preferred stock, net of issuance cost of $2,788
|
4,545,455
|
|
57,212
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Repurchase of preferred stock
|
(629,663
|
)
|
(1,967
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,344
|
)
|
(6,344
|
)
|
|||||||||||||||||
|
Repurchase of common stock
|
—
|
|
—
|
|
(115,612
|
)
|
—
|
|
(292
|
)
|
—
|
|
—
|
|
(748
|
)
|
(1,040
|
)
|
|||||||||||||||||
|
Issuance of common stock
|
—
|
|
—
|
|
460,713
|
|
1
|
|
1,036
|
|
—
|
|
—
|
|
—
|
|
1,037
|
|
|||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,433
|
|
—
|
|
—
|
|
—
|
|
1,433
|
|
|||||||||||||||||
|
Forgiven notes receivable from stockholders
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
21
|
|
—
|
|
—
|
|
21
|
|
|||||||||||||||||
|
Foreign currency translation adjustment, net of tax of $245
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,423
|
)
|
—
|
|
(1,423
|
)
|
|||||||||||||||||
|
Unrealized loss on investments, net of tax of $168
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(237
|
)
|
—
|
|
(237
|
)
|
|||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,245
|
|
2,245
|
|
|||||||||||||||||
|
Balance at December 31, 2014
|
19,510,410
|
|
111,467
|
|
4,736,689
|
|
$
|
5
|
|
8,446
|
|
(117
|
)
|
(864
|
)
|
(19,840
|
)
|
(12,370
|
)
|
||||||||||||||||
|
Conversion of convertible preferred stock into common stock upon closing of IPO
|
(19,510,410
|
)
|
(111,467
|
)
|
19,510,410
|
|
19
|
|
111,448
|
|
—
|
|
—
|
|
—
|
|
111,467
|
|
|||||||||||||||||
|
Shares issued upon closing of IPO
|
—
|
|
—
|
|
4,600,000
|
|
5
|
|
124,737
|
|
—
|
|
—
|
|
—
|
|
124,742
|
|
|||||||||||||||||
|
Issuance of common stock
|
—
|
|
—
|
|
1,074,411
|
|
1
|
|
1,125
|
|
—
|
|
—
|
|
—
|
|
1,126
|
|
|||||||||||||||||
|
Shares held for tax withholdings
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,525
|
)
|
—
|
|
—
|
|
—
|
|
(2,525
|
)
|
|||||||||||||||||
|
Repurchase of common stock
|
—
|
|
—
|
|
(23,650
|
)
|
—
|
|
(342
|
)
|
—
|
|
—
|
|
—
|
|
(342
|
)
|
|||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
7,608
|
|
—
|
|
—
|
|
—
|
|
7,608
|
|
|||||||||||||||||
|
Excess tax benefit from stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,590
|
|
—
|
|
—
|
|
—
|
|
1,590
|
|
|||||||||||||||||
|
Forgiven notes receivable from stockholders
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
91
|
|
—
|
|
—
|
|
91
|
|
|||||||||||||||||
|
Note received from a stockholder
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
21
|
|
—
|
|
—
|
|
21
|
|
|||||||||||||||||
|
Foreign currency translation adjustment, net of tax of $117
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,308
|
)
|
—
|
|
(1,308
|
)
|
|||||||||||||||||
|
Unrealized gain on investments, net of tax of $66
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
57
|
|
—
|
|
57
|
|
|||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,365
|
|
2,365
|
|
|||||||||||||||||
|
Balance at December 31, 2015
|
—
|
|
$
|
—
|
|
29,897,860
|
|
$
|
30
|
|
$
|
252,087
|
|
$
|
(5
|
)
|
$
|
(2,115
|
)
|
$
|
(17,475
|
)
|
$
|
232,522
|
|
||||||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|||||||||||
|
Net income
|
$
|
2,365
|
|
$
|
2,245
|
|
$
|
4,099
|
|
||
|
Adjustments to reconcile net income to net cash used in operating activities:
|
|||||||||||
|
Depreciation and amortization
|
1,752
|
|
751
|
|
677
|
|
|||||
|
Amortization of premium on marketable investments
|
83
|
|
—
|
|
—
|
|
|||||
|
Stock-based compensation
|
7,271
|
|
1,433
|
|
886
|
|
|||||
|
Excess tax benefit from stock-based compensation
|
(1,590
|
)
|
—
|
|
—
|
|
|||||
|
Provision for doubtful accounts
|
(13
|
)
|
150
|
|
259
|
|
|||||
|
Inventory write downs
|
1,163
|
|
1,852
|
|
892
|
|
|||||
|
Write off of note receivable
|
91
|
|
21
|
|
—
|
|
|||||
|
Provision for sales returns
|
1,083
|
|
(3
|
)
|
(212
|
)
|
|||||
|
Loss on minority investment
|
—
|
|
150
|
|
—
|
|
|||||
|
Loss on disposal of property and equipment
|
43
|
|
21
|
|
3
|
|
|||||
|
Realized loss on marketable investments
|
541
|
|
—
|
|
160
|
|
|||||
|
Provision for product warranty
|
399
|
|
(8
|
)
|
—
|
|
|||||
|
Release of valuation allowance
|
(243
|
)
|
(321
|
)
|
(4,962
|
)
|
|||||
|
Deferred taxes
|
(2,961
|
)
|
(571
|
)
|
(335
|
)
|
|||||
|
Issuance of common stock to third parties
|
—
|
|
—
|
|
132
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
(11,063
|
)
|
(7,426
|
)
|
(1,550
|
)
|
|||||
|
Inventories
|
(25,126
|
)
|
(9,444
|
)
|
(3,779
|
)
|
|||||
|
Prepaid expenses and other current and non-current assets
|
(4,013
|
)
|
(1,906
|
)
|
(961
|
)
|
|||||
|
Accounts payable
|
132
|
|
1,299
|
|
(451
|
)
|
|||||
|
Accrued expenses and other non-current liabilities
|
7,807
|
|
5,368
|
|
1,746
|
|
|||||
|
Net cash used in operating activities
|
(22,279
|
)
|
(6,389
|
)
|
(3,396
|
)
|
|||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|||||||||||
|
Purchases of marketable investments
|
(135,340
|
)
|
(51,342
|
)
|
(6,858
|
)
|
|||||
|
Proceeds from sales of marketable investments
|
54,998
|
|
18,229
|
|
6,405
|
|
|||||
|
Purchases of property and equipment
|
(5,474
|
)
|
(3,888
|
)
|
(798
|
)
|
|||||
|
Net cash used in investing activities
|
(85,816
|
)
|
(37,001
|
)
|
(1,251
|
)
|
|||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|||||||||||
|
Proceeds from issuance of preferred stock, net of issuance costs
|
—
|
|
57,212
|
|
—
|
|
|||||
|
Proceeds from issuance of common stock issued in initial public offering, net of issuance costs
|
124,742
|
|
—
|
|
—
|
|
|||||
|
Proceeds from exercises of stock options
|
617
|
|
1,036
|
|
178
|
|
|||||
|
Excess tax benefit from stock-based compensation
|
1,590
|
|
—
|
|
—
|
|
|||||
|
Repurchase of preferred stock
|
—
|
|
(8,311
|
)
|
—
|
|
|||||
|
Proceeds from credit facility
|
—
|
|
—
|
|
2,000
|
|
|||||
|
Repayment of credit facility
|
—
|
|
(6,000
|
)
|
—
|
|
|||||
|
Repurchase of common stock and stock options
|
—
|
|
(1,040
|
)
|
—
|
|
|||||
|
Payment of employee taxes related to vested common and restricted stock
|
(2,525
|
)
|
—
|
|
—
|
|
|||||
|
Net cash provided by financing activities
|
124,424
|
|
42,897
|
|
2,178
|
|
|||||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
(72
|
)
|
(348
|
)
|
(835
|
)
|
|||||
|
NET INCREASE IN CASH AND CASH EQUIVALENTS
|
16,257
|
|
(841
|
)
|
(3,304
|
)
|
|||||
|
CASH AND CASH EQUIVALENTS—Beginning of period
|
3,290
|
|
4,131
|
|
7,435
|
|
|||||
|
CASH AND CASH EQUIVALENTS—End of period
|
$
|
19,547
|
|
$
|
3,290
|
|
$
|
4,131
|
|
||
|
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION:
|
|||||||||||
|
Cash paid for interest
|
$
|
1
|
|
$
|
160
|
|
$
|
100
|
|
||
|
Cash paid for income taxes
|
$
|
1,220
|
|
$
|
3,086
|
|
$
|
37
|
|
||
|
NONCASH INVESTING AND FINANCING ACTIVITIES:
|
|||||||||||
|
Conversion of convertible preferred stock into common stock
|
$
|
111,467
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Purchase of property and equipment funded through accounts payable
|
$
|
143
|
|
$
|
44
|
|
$
|
5
|
|
||
|
|
As of December 31, 2015
|
|||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Fair Value
|
||||||||||||
|
Financial Assets
|
||||||||||||||||
|
Cash equivalents:
|
||||||||||||||||
|
Commercial paper
|
$
|
—
|
|
$
|
9,850
|
|
$
|
—
|
|
$
|
9,850
|
|
||||
|
Money market funds
|
252
|
|
—
|
|
—
|
|
252
|
|
||||||||
|
Marketable investments:
|
||||||||||||||||
|
Commercial paper
|
—
|
|
22,332
|
|
—
|
|
22,332
|
|
||||||||
|
U.S. Treasury
|
15,436
|
|
—
|
|
—
|
|
15,436
|
|
||||||||
|
U.S. Agency securities
|
—
|
|
21,464
|
|
—
|
|
21,464
|
|
||||||||
|
U.S. States and Municipalities
|
—
|
|
2,084
|
|
—
|
|
2,084
|
|
||||||||
|
Corporate bonds
|
—
|
|
61,002
|
|
—
|
|
61,002
|
|
||||||||
|
Non-U.S. Government debt securities
|
—
|
|
6,939
|
|
—
|
|
6,939
|
|
||||||||
|
Total
|
$
|
15,688
|
|
$
|
123,671
|
|
$
|
—
|
|
$
|
139,359
|
|
||||
|
|
As of December 31, 2014
|
|||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Fair Value
|
||||||||||||
|
Financial Assets
|
||||||||||||||||
|
Cash equivalents:
|
||||||||||||||||
|
Money market funds
|
$
|
155
|
|
$
|
—
|
|
$
|
—
|
|
$
|
155
|
|
||||
|
Marketable investments:
|
||||||||||||||||
|
Mutual funds
|
8,619
|
|
—
|
|
—
|
|
8,619
|
|
||||||||
|
U.S. Treasury
|
4,009
|
|
—
|
|
—
|
|
4,009
|
|
||||||||
|
U.S. Agency securities
|
—
|
|
6,006
|
|
—
|
|
6,006
|
|
||||||||
|
Corporate bonds
|
—
|
|
29,619
|
|
—
|
|
29,619
|
|
||||||||
|
Total
|
$
|
12,783
|
|
$
|
35,625
|
|
$
|
—
|
|
$
|
48,408
|
|
||||
|
Balance At
Beginning Of Year |
Charged To
Costs And Expenses |
Deductions
|
Balance At
End Of Year |
|||||||||||||
|
For the year ended:
|
||||||||||||||||
|
December 31, 2013
|
$
|
212
|
|
$
|
259
|
|
$
|
—
|
|
$
|
471
|
|
||||
|
December 31, 2014
|
471
|
|
150
|
|
(19
|
)
|
602
|
|
||||||||
|
December 31, 2015
|
602
|
|
(13
|
)
|
—
|
|
589
|
|
||||||||
|
|
December 31,
|
|||||||
|
2015
|
2014
|
|||||||
|
Prepaid expenses
|
$
|
7,442
|
|
$
|
3,130
|
|
||
|
Income tax receivable
|
606
|
|
1,654
|
|
||||
|
Other current assets
|
1,304
|
|
331
|
|
||||
|
Prepaid expenses and other current assets
|
$
|
9,352
|
|
$
|
5,115
|
|
||
|
December 31, 2015
|
||||||||||||||||
|
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
|||||||||||||
|
Commercial paper
|
$
|
22,328
|
|
$
|
5
|
|
$
|
(1
|
)
|
$
|
22,332
|
|
||||
|
U.S. Treasury
|
15,459
|
|
4
|
|
(27
|
)
|
15,436
|
|
||||||||
|
U.S. Agency securities
|
21,497
|
|
1
|
|
(34
|
)
|
21,464
|
|
||||||||
|
U.S. States and Municipalities
|
2,086
|
|
—
|
|
(2
|
)
|
2,084
|
|
||||||||
|
Corporate bonds
|
61,188
|
|
3
|
|
(189
|
)
|
61,002
|
|
||||||||
|
Non-U.S. Government debt securities
|
6,954
|
|
1
|
|
(16
|
)
|
6,939
|
|
||||||||
|
Total
|
$
|
129,512
|
|
$
|
14
|
|
$
|
(269
|
)
|
$
|
129,257
|
|
||||
|
December 31, 2014
|
||||||||||||||||
|
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
|||||||||||||
|
U.S. Agency securities
|
$
|
6,012
|
|
$
|
3
|
|
$
|
(9
|
)
|
$
|
6,006
|
|
||||
|
U.S. Treasury
|
4,011
|
|
—
|
|
(2
|
)
|
4,009
|
|
||||||||
|
Corporate bonds
|
29,834
|
|
4
|
|
(219
|
)
|
29,619
|
|
||||||||
|
Mutual funds
|
8,768
|
|
—
|
|
(149
|
)
|
8,619
|
|
||||||||
|
Total
|
$
|
48,625
|
|
$
|
7
|
|
$
|
(379
|
)
|
$
|
48,253
|
|
||||
|
December 31, 2015
|
||||||||
|
Fair Value
|
Gross Unrealized Losses
|
|||||||
|
Commercial paper
|
$
|
4,746
|
|
$
|
(1
|
)
|
||
|
US Treasury
|
12,453
|
|
(27
|
)
|
||||
|
US Agency securities
|
13,475
|
|
(34
|
)
|
||||
|
US States and Municipalities
|
2,084
|
|
(2
|
)
|
||||
|
Corporate bonds
|
59,163
|
|
(189
|
)
|
||||
|
Non-U.S. government debt securities
|
5,881
|
|
(16
|
)
|
||||
|
Total
|
$
|
97,802
|
|
$
|
(269
|
)
|
||
|
December 31, 2014
|
||||||||
|
Fair Value
|
Gross Unrealized Losses
|
|||||||
|
Mutual Funds
|
$
|
8,619
|
|
$
|
(149
|
)
|
||
|
US Treasury
|
3,008
|
|
(2
|
)
|
||||
|
US Agency Securities
|
5,255
|
|
(9
|
)
|
||||
|
Corporate Bonds
|
26,633
|
|
(219
|
)
|
||||
|
Total
|
$
|
43,515
|
|
$
|
(379
|
)
|
||
|
|
December 31,
|
|||||||
|
2015
|
2014
|
|||||||
|
Marketable Investments
|
Fair Value
|
Fair Value
|
||||||
|
Due in one year
|
$
|
62,983
|
|
$
|
16,442
|
|
||
|
Due in one to five years
|
66,274
|
|
31,811
|
|
||||
|
Total
|
$
|
129,257
|
|
$
|
48,253
|
|
||
|
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Raw materials
|
$
|
9,176
|
|
$
|
5,105
|
|
||
|
Work in process
|
2,746
|
|
543
|
|
||||
|
Finished goods
|
44,839
|
|
27,803
|
|
||||
|
Inventories
|
$
|
56,761
|
|
$
|
33,451
|
|
||
|
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Machinery and equipment
|
$
|
8,559
|
|
$
|
5,089
|
|
||
|
Furniture and fixtures
|
2,091
|
|
519
|
|
||||
|
Leasehold improvements
|
1,564
|
|
379
|
|
||||
|
Software
|
666
|
|
599
|
|
||||
|
Computers
|
565
|
|
153
|
|
||||
|
Construction in progress
|
577
|
|
1,931
|
|
||||
|
Total property and equipment
|
14,022
|
|
8,670
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(5,071
|
)
|
(3,489
|
)
|
||||
|
Property and equipment, net
|
$
|
8,951
|
|
$
|
5,181
|
|
||
|
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Payroll and employee-related expenses
|
$
|
13,653
|
|
$
|
8,221
|
|
||
|
Sales return reserve
|
3,247
|
|
2,164
|
|
||||
|
Preclinical and clinical trial cost
|
1,330
|
|
2,319
|
|
||||
|
Deferred revenue
|
526
|
|
1,591
|
|
||||
|
Product warranty
|
713
|
|
314
|
|
||||
|
Sales tax payable
|
531
|
|
306
|
|
||||
|
Income tax payable
|
308
|
|
332
|
|
||||
|
Other accrued liabilities
|
5,273
|
|
3,228
|
|
||||
|
Total accrued liabilities
|
$
|
25,581
|
|
$
|
18,475
|
|
||
|
December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Balance at the beginning of the year
|
$
|
314
|
|
$
|
323
|
|
$
|
323
|
|
|||
|
Accruals of warranties issued
|
752
|
|
149
|
|
100
|
|
||||||
|
Settlements of warranty claims
|
(353
|
)
|
(158
|
)
|
(100
|
)
|
||||||
|
Balance at the end of the year
|
$
|
713
|
|
$
|
314
|
|
$
|
323
|
|
|||
|
Lease Payments
|
|||
|
Year Ending December 31:
|
|||
|
2016
|
$
|
4,055
|
|
|
2017
|
4,861
|
|
|
|
2018
|
4,877
|
|
|
|
2019
|
5,007
|
|
|
|
2020
|
5,133
|
|
|
|
Thereafter
|
52,555
|
|
|
|
Total future minimum lease payments
|
$
|
76,488
|
|
|
Series
|
Shares
Authorized
|
Shares Issued
and
Outstanding
|
Proceeds,
Net of Issuance
Costs
|
Aggregate
Liquidation
Amount
|
||||||||||
|
Series A Preferred Stock
|
1,000,000
|
|
1,000,000
|
|
$
|
299
|
|
$
|
554
|
|
||||
|
Series B Preferred Stock
|
4,287,486
|
|
4,005,338
|
|
6,536
|
|
11,725
|
|
||||||
|
Series C Preferred Stock
|
4,388,715
|
|
4,168,218
|
|
13,266
|
|
22,238
|
|
||||||
|
Series D Preferred Stock
|
3,944,733
|
|
3,881,459
|
|
19,647
|
|
30,976
|
|
||||||
|
Series E Preferred Stock
|
1,973,684
|
|
1,909,940
|
|
14,507
|
|
21,609
|
|
||||||
|
Series F Preferred Stock
|
5,303,031
|
|
4,545,455
|
|
57,212
|
|
62,259
|
|
||||||
|
Undesignated
|
4,102,351
|
|
—
|
|
—
|
|
—
|
|
||||||
|
Total convertible preferred stock
|
25,000,000
|
|
19,510,410
|
|
$
|
111,467
|
|
$
|
149,361
|
|
||||
|
Number
of Shares
|
Weighted
Average
Exercise
Price
|
Weighted Average Remaining Contractual Life (in Years)
|
Aggregate Intrinsic Value (in thousands)
|
||||||||||
|
Balance, December 31, 2014
|
2,900,676
|
|
$
|
2.66
|
|
||||||||
|
Granted
|
1,809,400
|
|
21.47
|
|
|||||||||
|
Exercised
|
(948,872
|
)
|
0.99
|
|
|||||||||
|
Canceled/Forfeited
|
(5,859
|
)
|
11.15
|
|
|||||||||
|
Balance, December 31, 2015
|
3,755,345
|
|
$
|
12.13
|
|
||||||||
|
Vested and expected to vest—December 31, 2015
|
3,621,919
|
|
$
|
11.79
|
|
6.70
|
$
|
152,187
|
|
||||
|
Exercisable—December 31, 2015
|
1,941,150
|
|
$
|
3.47
|
|
4.20
|
$
|
97,717
|
|
||||
|
Year Ended December 31,
|
||||||
|
2015
|
2014
|
2013
|
||||
|
Expected term (in years)
|
6.08—6.25
|
6.25
|
6.25
|
|||
|
Expected volatility
|
45%
|
45%
|
45%
|
|||
|
Risk-free interest rate
|
1.56%—1.78%
|
1.76%—2.02%
|
0.63%—0.90%
|
|||
|
Expected dividend rate
|
0%
|
0%
|
0%
|
|||
|
•
|
independent third-party valuations;
|
|
•
|
progress of research and development activities;
|
|
•
|
the
Company’s
operating and financial performance, including its levels of available capital resources;
|
|
•
|
rights and preferences of the
Company’s
common stock compared to the rights and preferences of the
Company’s
other outstanding equity securities;
|
|
•
|
equity market conditions affecting comparable public companies, as reflected in comparable companies’ market multiples, IPO valuations and other metrics;
|
|
•
|
the achievement of enterprise milestones, including the
Company’s
progress in clinical trials;
|
|
•
|
the likelihood of achieving a liquidity event for the shares of common stock, such as an IPO given prevailing market and medical device sector conditions;
|
|
•
|
sales of the
Company’s
preferred stock in arms-length transactions;
|
|
•
|
the illiquidity of the
Company’s
securities by virtue of being a private company;
|
|
•
|
business risks; and
|
|
•
|
management and board experience.
|
|
•
|
Market Approach.
The market approach values a business by reference to guideline companies, for which enterprise values are known. This approach has two principal methodologies. The guideline public company methodology derives valuation multiples from the operating data and share prices of similar publicly traded companies. The guideline acquisition methodology focuses on comparisons between the subject company and guideline acquired public or private companies.
|
|
•
|
Option-Pricing Method Backsolve (OPM backsolve).
The OPM backsolve method derives the implied equity value for a company from a recent transaction involving the
Company’s
own securities issued on an arms-length basis.
|
|
•
|
Probability Weighted Expected Return Method (PWERM)
. Using the PWERM method, the value of a company’s common stock is estimated based upon the analysis of future values for the company assuming various possible future liquidity events like an initial public offering, sale or merger. Share value is based upon the probability-weighted present value of expected future net cash flows, considering each of the possible future events, as well as the rights and preferences of each share class.
|
|
•
|
Option-Pricing Method (OPM)
. Under the OPM method, each class of stock is modeled as a call option with a distinct claim on the enterprise value of the company. The option’s exercise prices would be based on a comparison with the enterprise value. The method assumes that a formula, such as the Black-Scholes model, would calculate the fair value when provided with certain values, including share price, expiration date, volatility and the risk free interest rate.
|
|
Number
of Shares
|
Weighted Average
Grant Date
Fair Value
|
||||||
|
Unvested at December 31, 2014
|
367,126
|
|
$
|
7.26
|
|
||
|
Granted
|
765,161
|
|
17.70
|
|
|||
|
Vested
|
(279,716
|
)
|
11.93
|
|
|||
|
Canceled/Forfeited
|
(3,000
|
)
|
7.75
|
|
|||
|
Unvested at December 31, 2015
|
849,571
|
|
15.12
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Cost of sales
|
$
|
316
|
|
$
|
267
|
|
$
|
98
|
|
|||
|
Research and development
|
444
|
|
96
|
|
84
|
|
||||||
|
Sales, general and administrative
|
6,511
|
|
1,070
|
|
704
|
|
||||||
|
$
|
7,271
|
|
$
|
1,433
|
|
$
|
886
|
|
||||
|
Year Ended December 31, 2015
|
Year Ended December 31, 2014
|
|||||||||||||||||||||||
|
Marketable
Investments |
Currency Translation
Adjustments |
Total
|
Marketable
Investments |
Currency Translation
Adjustments |
Total
|
|||||||||||||||||||
|
Balance, beginning of the year
|
$
|
(220
|
)
|
$
|
(644
|
)
|
$
|
(864
|
)
|
$
|
17
|
|
$
|
779
|
|
$
|
796
|
|
||||||
|
Other comprehensive income before reclassifications:
|
||||||||||||||||||||||||
|
Unrealized gains (losses)—marketable investments
|
(417
|
)
|
—
|
|
(417
|
)
|
(388
|
)
|
—
|
|
(388
|
)
|
||||||||||||
|
Foreign currency translation gains (losses)
|
—
|
|
(1,425
|
)
|
(1,425
|
)
|
—
|
|
(1,668
|
)
|
(1,668
|
)
|
||||||||||||
|
Income tax effect—benefit (expense)
|
128
|
|
117
|
|
245
|
|
161
|
|
245
|
|
406
|
|
||||||||||||
|
Net of tax
|
(289
|
)
|
(1,308
|
)
|
(1,597
|
)
|
(227
|
)
|
(1,423
|
)
|
(1,650
|
)
|
||||||||||||
|
Amounts reclassified from accumulated other comprehensive income to earnings:
|
||||||||||||||||||||||||
|
Realized losses—marketable investments
|
540
|
|
—
|
|
540
|
|
(17
|
)
|
—
|
|
(17
|
)
|
||||||||||||
|
Income tax effect—benefit
|
(194
|
)
|
—
|
|
(194
|
)
|
7
|
|
—
|
|
7
|
|
||||||||||||
|
Net of tax
|
346
|
|
—
|
|
346
|
|
(10
|
)
|
—
|
|
(10
|
)
|
||||||||||||
|
Net current-year other comprehensive income (loss)
|
57
|
|
(1,308
|
)
|
(1,251
|
)
|
(237
|
)
|
(1,423
|
)
|
(1,660
|
)
|
||||||||||||
|
Balance, end of the year
|
$
|
(163
|
)
|
$
|
(1,952
|
)
|
$
|
(2,115
|
)
|
$
|
(220
|
)
|
$
|
(644
|
)
|
$
|
(864
|
)
|
||||||
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
United States
|
$
|
2,955
|
|
$
|
2,230
|
|
$
|
(1,142
|
)
|
|||
|
Foreign
|
1,069
|
|
909
|
|
(113
|
)
|
||||||
|
Total income (loss) before provision for (benefit from) income taxes
|
$
|
4,024
|
|
$
|
3,139
|
|
$
|
(1,255
|
)
|
|||
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
3,815
|
|
$
|
1,155
|
|
$
|
(155
|
)
|
|||
|
State
|
603
|
|
274
|
|
87
|
|
||||||
|
Foreign
|
492
|
|
323
|
|
—
|
|
||||||
|
Total current
|
4,910
|
|
1,752
|
|
(68
|
)
|
||||||
|
Deferred:
|
||||||||||||
|
Federal
|
(3,025
|
)
|
(625
|
)
|
(5,087
|
)
|
||||||
|
State
|
(251
|
)
|
(116
|
)
|
(199
|
)
|
||||||
|
Foreign
|
25
|
|
(117
|
)
|
—
|
|
||||||
|
Total deferred
|
(3,251
|
)
|
(858
|
)
|
(5,286
|
)
|
||||||
|
Provision for (benefit from) income taxes
|
$
|
1,659
|
|
$
|
894
|
|
$
|
(5,354
|
)
|
|||
|
Year Ended December 31,
|
|||||||||
|
2015
|
2014
|
2013
|
|||||||
|
Income tax at federal statutory rate
|
34.0
|
%
|
34.0
|
%
|
34.0
|
%
|
|||
|
State income taxes, net of federal benefit
|
1.9
|
|
3.2
|
|
(5.6
|
)
|
|||
|
Foreign taxes differential
|
(9.0
|
)
|
5.2
|
|
5.8
|
|
|||
|
Prepaid tax ASC 810-10
|
2.1
|
|
(8.9
|
)
|
(2.7
|
)
|
|||
|
Foreign exchange gains and losses
|
—
|
|
—
|
|
11.7
|
|
|||
|
IRC 199 deduction
|
(7.4
|
)
|
(7.0
|
)
|
—
|
|
|||
|
Stock-based compensation
|
14.8
|
|
6.0
|
|
(14.1
|
)
|
|||
|
Meals and entertainment
|
5.6
|
|
5.8
|
|
(10.7
|
)
|
|||
|
Imputed interest
|
4.7
|
|
6.6
|
|
(12.1
|
)
|
|||
|
Tax credits
|
(11.6
|
)
|
(12.1
|
)
|
41.8
|
|
|||
|
Other
|
3.6
|
|
2.8
|
|
0.3
|
|
|||
|
Change in valuation allowance
|
2.5
|
|
(7.1
|
)
|
378.2
|
|
|||
|
Effective tax rate
|
41.2
|
%
|
28.5
|
%
|
426.6
|
%
|
|||
|
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Deferred tax assets
|
||||||||
|
Net operating loss carryforwards
|
$
|
1,000
|
|
$
|
1,626
|
|
||
|
Tax credits
|
1,355
|
|
1,779
|
|
||||
|
Accruals and reserves
|
5,048
|
|
3,759
|
|
||||
|
Stock-based compensation
|
1,352
|
|
416
|
|
||||
|
Translation adjustment
|
715
|
|
674
|
|
||||
|
UNICAP adjustments
|
3,840
|
|
2,227
|
|
||||
|
Other
|
870
|
|
—
|
|
||||
|
Gross deferred tax assets
|
14,180
|
|
10,481
|
|
||||
|
Valuation allowance
|
(2,702
|
)
|
(2,945
|
)
|
||||
|
Total deferred tax assets
|
11,478
|
|
7,536
|
|
||||
|
Deferred tax liabilities
|
||||||||
|
Depreciation and amortization
|
(1,335
|
)
|
(685
|
)
|
||||
|
Total deferred tax liabilities
|
(1,335
|
)
|
(685
|
)
|
||||
|
Net deferred tax assets
|
$
|
10,143
|
|
$
|
6,851
|
|
||
|
Deferred income taxes, net - Balance Sheet Classification
|
||||||||
|
Current deferred income tax assets
|
$
|
—
|
|
$
|
6,280
|
|
||
|
Long-term deferred income tax assets
|
10,143
|
|
571
|
|
||||
|
Net deferred tax assets
|
$
|
10,143
|
|
$
|
6,851
|
|
||
|
December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Balance at the beginning of the year
|
$
|
2,945
|
|
$
|
3,860
|
|
$
|
8,742
|
|
|||
|
Release of valuation allowance
|
(243
|
)
|
(321
|
)
|
(4,962
|
)
|
||||||
|
Other reserves and deferrals
|
—
|
|
(594
|
)
|
80
|
|
||||||
|
Balance at the end of the year
|
$
|
2,702
|
|
$
|
2,945
|
|
$
|
3,860
|
|
|||
|
December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Beginning Balance
|
$
|
1,726
|
|
$
|
1,325
|
|
$
|
838
|
|
|||
|
Gross increase for tax positions of current year
|
1,023
|
|
401
|
|
324
|
|
||||||
|
Gross increase for tax positions of prior years
|
1,062
|
|
—
|
|
163
|
|
||||||
|
Settlements
|
—
|
|
—
|
|
—
|
|
||||||
|
Lapse of statute of limitations
|
(192
|
)
|
—
|
|
—
|
|
||||||
|
Ending Balance
|
$
|
3,619
|
|
$
|
1,726
|
|
$
|
1,325
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Net income (loss) per share:
|
||||||||||||
|
Numerator
|
||||||||||||
|
Net income
|
$
|
2,365
|
|
$
|
2,245
|
|
$
|
4,099
|
|
|||
|
Less: Deemed dividend paid to convertible preferred stockholders upon repurchase
|
—
|
|
(6,344
|
)
|
—
|
|
||||||
|
Less: Undistributed income attributable to convertible preferred stockholders
|
(1,281
|
)
|
—
|
|
(3,212
|
)
|
||||||
|
Add: Undistributed loss attributable to convertible preferred stockholders
|
—
|
|
3,266
|
|
—
|
|
||||||
|
Net income (loss) attributable to common stockholders—basic and diluted
|
$
|
1,084
|
|
$
|
(833
|
)
|
$
|
887
|
|
|||
|
Denominator
|
||||||||||||
|
Weighted average shares used to compute net income (loss) attributable to common stockholders—Basic
|
11,993,429
|
|
4,609,375
|
|
4,304,396
|
|
||||||
|
Potential dilutive options, as calculated using treasury stock method
|
1,959,226
|
|
—
|
|
2,021,394
|
|
||||||
|
Potential dilutive common stock warrants, as calculated using treasury stock method
|
—
|
|
—
|
|
86,985
|
|
||||||
|
Potential dilutive restricted stock, as calculated using treasury stock method
|
266,995
|
|
—
|
|
88,060
|
|
||||||
|
Weighted average shares used to compute net income (loss) attributable to common stockholders—Diluted
|
14,219,650
|
|
4,609,375
|
|
6,500,835
|
|
||||||
|
Net income (loss) per share attributable to common stockholders
—Basic
|
$
|
0.09
|
|
$
|
(0.18
|
)
|
$
|
0.21
|
|
|||
|
—Diluted
|
$
|
0.08
|
|
$
|
(0.18
|
)
|
$
|
0.14
|
|
|||
|
|
Year Ended December 31,
|
||||||||
|
|
2015
|
2014
|
2013
|
||||||
|
Options to purchase common stock
|
1,321,250
|
|
2,933,757
|
|
63,750
|
|
|||
|
Restricted stock and restricted stock units
|
96,800
|
|
367,126
|
|
—
|
|
|||
|
Total
|
1,418,050
|
|
3,300,883
|
|
63,750
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
United States
|
$
|
127,311
|
|
$
|
82,965
|
|
$
|
58,305
|
|
|||
|
Japan
|
19,016
|
|
14,699
|
|
12,668
|
|
||||||
|
Other International
|
39,768
|
|
27,846
|
|
17,875
|
|
||||||
|
Total
|
$
|
186,095
|
|
$
|
125,510
|
|
$
|
88,848
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Neuro
|
$
|
141,410
|
|
$
|
106,242
|
|
$
|
81,343
|
|
|||
|
Peripheral Vascular
|
44,685
|
|
19,268
|
|
7,505
|
|
||||||
|
Total
|
$
|
186,095
|
|
$
|
125,510
|
|
$
|
88,848
|
|
|||
|
Quarter Ended
|
||||||||||||||||||||||||||||||||
|
2015
|
2014
|
|||||||||||||||||||||||||||||||
|
December 31
|
September 30
|
June 30
|
March 31
|
December 31
|
September 30
|
June 30
|
March 31
|
|||||||||||||||||||||||||
|
Revenue
|
$
|
54,416
|
|
$
|
50,416
|
|
$
|
42,311
|
|
$
|
38,952
|
|
$
|
35,403
|
|
$
|
32,464
|
|
$
|
31,480
|
|
$
|
26,163
|
|
||||||||
|
Cost of revenue
|
17,958
|
|
16,919
|
|
14,936
|
|
12,224
|
|
11,512
|
|
11,667
|
|
10,780
|
|
8,709
|
|
||||||||||||||||
|
Gross profit
|
$
|
36,458
|
|
$
|
33,497
|
|
$
|
27,375
|
|
$
|
26,728
|
|
$
|
23,891
|
|
$
|
20,797
|
|
$
|
20,700
|
|
$
|
17,454
|
|
||||||||
|
Income (loss) before provision for (benefit from) income taxes
|
$
|
1,876
|
|
$
|
2,084
|
|
$
|
(4,178
|
)
|
$
|
4,242
|
|
$
|
417
|
|
$
|
399
|
|
$
|
526
|
|
$
|
1,797
|
|
||||||||
|
Net income (loss)
|
$
|
1,633
|
|
$
|
901
|
|
$
|
(2,671
|
)
|
$
|
2,502
|
|
$
|
416
|
|
$
|
172
|
|
$
|
391
|
|
$
|
1,266
|
|
||||||||
|
Net income (loss) attributable to common stockholders
|
$
|
1,633
|
|
$
|
276
|
|
$
|
(553
|
)
|
$
|
503
|
|
$
|
81
|
|
$
|
(1,192
|
)
|
$
|
81
|
|
$
|
280
|
|
||||||||
|
Net income (loss) per share attributable to common stockholders—Basic
|
$
|
0.05
|
|
$
|
0.04
|
|
$
|
(0.11
|
)
|
$
|
0.10
|
|
$
|
0.02
|
|
$
|
(0.25
|
)
|
$
|
0.02
|
|
$
|
0.06
|
|
||||||||
|
Net income (loss) per share attributable to common stockholders—Diluted
|
$
|
0.05
|
|
$
|
0.03
|
|
$
|
(0.11
|
)
|
$
|
0.07
|
|
$
|
0.01
|
|
$
|
(0.25
|
)
|
$
|
0.01
|
|
$
|
0.04
|
|
||||||||
|
Weighted average shares used to compute net income (loss) per share attributable to common stockholders—Basic
|
29,890,944
|
|
7,853,730
|
|
5,096,151
|
|
4,903,535
|
|
4,701,999
|
|
4,688,045
|
|
4,608,510
|
|
4,430,824
|
|
||||||||||||||||
|
Weighted average shares used to compute net income (loss) per share attributable to common stockholders—Diluted
|
32,321,410
|
|
10,189,248
|
|
5,096,151
|
|
7,193,452
|
|
7,102,885
|
|
4,688,045
|
|
6,868,889
|
|
6,705,066
|
|
||||||||||||||||
|
1.
|
Financial Statements: The financial statements included in “Index to Consolidated Financial Statements” in Part II, Item 8 are filed as part of this Annual Report on Form 10-K
|
|
2.
|
Exhibits: The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K.
|
|
|
PENUMBRA, INC.
|
|
|
Date: March 8, 2016
|
|
|
|
|
By:
|
/s/ Sri Kosaraju
|
|
|
Sri Kosaraju
|
|
|
|
Chief Financial Officer and Head of Strategy
|
|
|
(Principal Financial and Accounting Officer)
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Adam Elsesser
|
Chairman, Chief Executive Officer and President
|
March 8, 2016
|
||
|
Adam Elsesser
|
(principal executive officer)
|
|||
|
/s/ Sri Kosaraju
|
Chief Financial Officer and Head of Strategy
|
March 8, 2016
|
||
|
Sri Kosaraju
|
(principal financial officer and principal accounting officer)
|
|||
|
/s/ Arani Bose
|
Chief Innovator and Director
|
March 8, 2016
|
||
|
Arani Bose
|
||||
|
/s/ Don Kassing
|
Director
|
March 8, 2016
|
||
|
Don Kassing
|
||||
|
/s/ Walter C. Wang
|
Director
|
March 8, 2016
|
||
|
Walter Charles Wang
|
||||
|
/s/ Harpreet Grewal
|
Director
|
March 8, 2016
|
||
|
Harpreet Grewal
|
||||
|
/s/ Kevin J. Sullivan
|
Director
|
March 8, 2016
|
||
|
Kevin James Sullivan
|
||||
|
Incorporation by Reference
|
||||||||||
|
Exhibit Number
|
Description
|
Form
|
File No.
|
Exhibit(s)
|
Filing Date
|
|||||
|
3.1
|
Restated Certificate of Incorporation of Penumbra, Inc.
|
8-K
|
001-37557
|
3.3
|
September 29, 2015
|
|||||
|
3.2
|
Amended and Restated Bylaws of Penumbra, Inc.
|
8-K
|
001-37557
|
3.4
|
September 29, 2015
|
|||||
|
4.1
|
Specimen Common Stock Certificate
|
S-1/A
|
333-206412
|
4.1
|
September 8, 2015
|
|||||
|
4.2
|
Fourth Amended and Restated Investors’ Rights Agreement, by and among Penumbra, Inc., Adam Elsesser and Arani Bose and the investors listed on Exhibit A thereto, dated May 16, 2014
|
S-1
|
333-206412
|
4.2
|
August 14, 2015
|
|||||
|
10.1
|
Lease for facilities at 1351 Harbor Bay Parkway, Alameda, California, dated November 28, 2007 and amended on May 7, 2008 and June 23, 2011
|
S-1
|
333-206412
|
10.1
|
August 14, 2015
|
|||||
|
10.2
|
Lease for facilities at 1411 Harbor Bay Parkway, Alameda, California, dated September 11, 2014
|
S-1
|
333-206412
|
10.2
|
August 14, 2015
|
|||||
|
10.3
|
Lease for facilities at 1321 Harbor Bay Parkway, Alameda, California, dated September 11, 2014
|
S-1
|
333-206412
|
10.3
|
August 14, 2015
|
|||||
|
10.4*
|
Lease for facilities at 1301, 1311, 1401 and 1431 Harbor Bay Parkway, Alameda, California, dated December 19, 2015
|
|||||||||
|
10.5#
|
Distribution Agreement between Penumbra, Inc. and Medico’s Hirata, dated August 2, 2009, as amended
|
S-1
|
333-206412
|
10.4
|
August 14, 2015
|
|||||
|
10.6†
|
Amended and Restated 2014 Equity Incentive Plan, and forms of Restricted Stock Agreement, Stock Option Agreement and Early Exercise Stock Option Agreement
|
S-1
|
333-206412
|
10.19
|
August 14, 2015
|
|||||
|
10.7†
|
Amended and Restated 2014 Equity Incentive Plan - Restricted Stock Agreement of Penumbra, Inc.
|
10-Q
|
001-37557
|
10.1
|
November 12, 2015
|
|||||
|
10.8†
|
Amended and Restated 2014 Equity Incentive Plan - Stock Option Agreement of Penumbra, Inc.
|
10-Q
|
001-37557
|
10.2
|
November 12, 2015
|
|||||
|
10.9†*
|
Amended and Restated 2014 Equity Incentive Plan - Restricted Stock Unit Agreement of Penumbra, Inc.
|
|||||||||
|
10.10†
|
2014 Equity Incentive Plan, and forms of Restricted Stock Agreement, Stock Option Agreement and Early Exercise Stock Option Agreement
|
S-1
|
333-206412
|
10.5
|
August 14, 2015
|
|||||
|
10.11†
|
2011 Equity Incentive Plan, and forms of Restricted Stock Agreement, Stock Grant Agreement, Stock Option Agreement and Early Exercise Stock Option Agreement
|
S-1
|
333-206412
|
10.6
|
August 14, 2015
|
|||||
|
10.12†
|
2005 Stock Plan, and forms of Notice of Grant and Early Exercise Stock Option Agreement
|
S-1
|
333-206412
|
10.7
|
August 14, 2015
|
|||||
|
10.13†
|
Form of Indemnification Agreement by and between Penumbra, Inc. and each of its directors and executive officers
|
S-1
|
333-206412
|
10.9
|
August 14, 2015
|
|||||
|
10.14†
|
Offer Letter with Adam Elsesser
|
S-1
|
333-206412
|
10.10
|
August 14, 2015
|
|||||
|
10.15†
|
Offer Letter with Arani Bose
|
S-1
|
333-206412
|
10.11
|
August 14, 2015
|
|||||
|
10.16†
|
Offer Letter with Sri Kosaraju
|
S-1
|
333-206412
|
10.12
|
August 14, 2015
|
|||||
|
10.17†
|
Offer Letter with Daniel Davis
|
S-1
|
333-206412
|
10.13
|
August 14, 2015
|
|||||
|
10.18†
|
Offer Letter with James Pray
|
S-1
|
333-206412
|
10.14
|
August 14, 2015
|
|||||
|
10.19†
|
Offer Letter with Lynn Rothman
|
S-1
|
333-206412
|
10.15
|
August 14, 2015
|
|||||
|
10.20†
|
Offer Letter with Robert Evans
|
S-1
|
333-206412
|
10.16
|
August 14, 2015
|
|||||
|
10.21†
|
Form of Employee Nondisclosure and Assignment Agreement
|
S-1
|
333-206412
|
10.17
|
August 14, 2015
|
|||||
|
10.22†
|
Employee Stock Purchase Plan
|
S-1/A
|
333-206412
|
10.18
|
August 31, 2015
|
|||||
|
10.24
|
Engagement Letter between the Penumbra, Inc. and Perella Weinberg Partners LP
|
S-1/A
|
333-206412
|
10.20
|
August 31, 2015
|
|||||
|
21.1
|
Subsidiaries of the Registrant
|
S-1
|
333-206412
|
21.1
|
August 14, 2015
|
|||||
|
23.1*
|
Consent of Deloitte & Touche LLP
|
|||||||||
|
24.1*
|
Power of Attorney (included on signature page)
|
|||||||||
|
31.1*
|
Certification of Principal Executive Officer required under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended.
|
|||||||||
|
31.2*
|
Certification of Principal Financial Officer required under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended.
|
|||||||||
|
32.1*
|
Certification of Principal Executive Officer and Principal Financial Officer required under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. §1350.
|
|||||||||
|
101*
|
The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2015 formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets as of December 31, 2015 and 2014, (ii) Consolidated Statements of Operations and Comprehensive Income for the years ended December 31, 2015, 2014 and 2013, (iii) Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) for the years ended December 31, 2015, 2014 and 2013, (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2014 and 2013, and (v) Notes to Consolidated Financial Statements.
|
|||||||||