|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| PetVivo Holdings, Inc. |
| (Exact name of registrant as specified in its charter) |
|
Nevada
|
|
99-0363559
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
12100 Singletree Lane, Suite 186
Eden Prairie, Minnesota
|
55344
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of each class registered:
|
Name of each exchange on which registered:
|
|
|
None
|
None
|
|
Large accelerated filer
|
o |
Accelerated filer
|
o |
|
Non-accelerated filer
|
o |
Smaller reporting company
|
x |
|
(Do not check if a smaller reporting company)
|
|||
|
|
|
Page
|
|||
|
|
|
|
|||
| PART I | |||||
|
|
|
||||
|
Item 1.
|
Business
|
3 | |||
|
Item 1A.
|
Risk Factors
|
15 | |||
|
Item 1B.
|
Unresolved Staff Comments
|
33 | |||
|
Item 2.
|
Properties
|
33 | |||
|
Item 3.
|
Legal Proceedings
|
33 | |||
|
Item 4.
|
Mine Safety Disclosures
|
33 | |||
|
|
|
||||
| PART II | |||||
|
|
|
||||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
34 | |||
|
Item 6.
|
Selected Financial Data
|
37 | |||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operation s
|
38 | |||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
41 | |||
|
Item 8.
|
Financial Statements and Supplementary Data
|
41 | |||
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
42 | |||
|
Item 9A.
|
Controls and Procedures
|
43 | |||
|
Item 9B.
|
Other Information
|
44 | |||
|
|
|
||||
| PART III | |||||
|
|
|
||||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
45 | |||
|
Item 11.
|
Executive Compensation
|
47 | |||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
49 | |||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
50 | |||
|
Item 14.
|
Principal Accounting Fees and Services
|
50 | |||
|
|
|||||
| PART IV | |||||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
51 | |||
|
Gel-Del 2ml Particles
|
Cost
|
6% Royalty
|
Total Cost
|
Sell Price
|
Margin
|
|||||||||||||||
|
PetVivo
|
$ | 68 | $ | 7.80 | $ | 75.80 | $ | 130 | 42 | % | ||||||||||
|
Vet Distributor
|
$ | 130 | $ | 162 | 20 | % | ||||||||||||||
|
Veterinary Clinic
|
$ | 162 | $ | 324 | 50 | % | ||||||||||||||

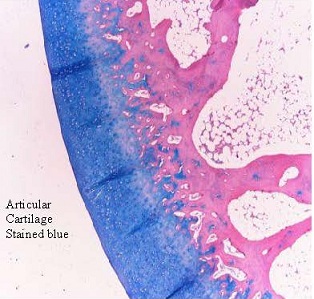

|
Gel-Del Particles
CosmetaLife (GDP) Integration after 12 Weeks
The image at left shows collagen in blue, fibroblasts in red and CosmetaLife (GDP) in gray. Note the typical cellularization and integration
of collagen within the CosmetaLife matrix perimeter. Also notice the fibroblasts (collagen producers) are integrated throughout the
injection site. Microvascularization, indicated by arrowheads, is also present in several locations. There is little to no sign of inflammation.
Trichrome Stain - 20x Objective
CosmetaLife (GDP) Particles
|

|
CosmetaLife (GDP) Particles, shown in figure to the left, were photographed from a light microscope under high magnification. GDP particles were immersed in a saline solution to help disperse them for better viewing. These particles are approximately 100 microns in size (0.1 mm in diameter). |

| Patent No. US 8,153,591 | ||
| Patent App. No. US 13/435,839 | Filed: | 03/30/12 |
| Patent App. No. US 12/344,361 | Filed: | 12/26/08 |
|
•
|
the results of our target animal studies for our current and future product candidates;
|
|
•
|
the amount and timing of our payments required under the License Agreement, including royalties;
|
|
•
|
the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future product candidates;
|
|
•
|
the upfront and other payments, and associated costs, related to the License Agreement;
|
|
•
|
the number and characteristics of the product candidates we pursue;
|
|
•
|
the scope, progress, results and costs of researching and developing any of our current or future product candidates and conducting target animal studies;
|
|
•
|
whether we acquire any other companies, assets, intellectual property or technologies in the future;
|
|
•
|
the cost of commercialization activities, if any of our current or future product candidates are approved for sale, including marketing, sales and distribution costs;
|
|
•
|
the cost of manufacturing our current and future product candidates and any products we successfully commercialize;
|
|
•
|
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements;
|
|
•
|
the expenses needed to attract and retain skilled personnel;
|
|
•
|
the costs associated with being a public company; and
|
|
•
|
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation.
|
|
•
|
our target animal studies or other development activities for our current or future product candidates;
|
|
•
|
our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize any of our current or future product candidates; or
|
|
•
|
our in-licensing and acquisition efforts and expansion of our product portfolio.
|
|
•
|
timely initiation and completion of our target animal studies for our current product candidates, which may be significantly slower than we currently anticipate and will depend substantially upon the satisfactory performance of third-party contractors;
|
|
•
|
our ability to demonstrate to the satisfaction of the CVM, the USDA and the European Medicines Agency, or EMA, or the applicable EU Member State national competent authorities, the safety and efficacy of our product candidates and to obtain regulatory approval in the United States and Europe;
|
|
•
|
our success in educating veterinarians and pet owners about the benefits, administration and use of our product candidates;
|
|
•
|
the prevalence and severity of adverse side effects, including a continued acceptable safety profile of the product following approval;
|
|
•
|
achieving and maintaining compliance with all regulatory requirements applicable to our product candidates;
|
|
•
|
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments;
|
|
•
|
the effectiveness of our marketing, sales and distribution strategy and operations;
|
|
•
|
the ability of our third-party manufacturers to manufacture supplies of any of our current or future product candidates and to develop, validate and maintain commercially viable manufacturing processes that are compliant with current Good Manufacturing Practices, or cGMP;
|
|
•
|
our ability to successfully launch commercial sales of our current product candidates, assuming necessary approvals are obtained, whether alone or in collaboration with others;
|
|
•
|
our ability to enforce our intellectual property rights in and to our product candidates and avoid third-party patent interference, third-party initiated and U.S. PTO-initiated administrative patent proceedings or patent infringement claims; and
|
|
•
|
acceptance of our product candidates as safe and effective by veterinarians, pet owners and the animal health community.
|
|
•
|
the safety of our products as demonstrated in our target animal studies;
|
|
•
|
the indications for which our products are approved;
|
|
•
|
the acceptance by veterinarians and pet owners of the product as a safe and effective treatment;
|
|
•
|
the proper training and administration of our products by veterinarians;
|
|
•
|
the potential and perceived advantages of our product candidates over alternative treatments, including generic medicines and products approved for use by humans that are used off label;
|
|
•
|
the cost of treatment in relation to alternative treatments and willingness to pay for our products, if approved, on the part of veterinarians and pet owners;
|
|
•
|
the willingness of pet owners to pay for our treatments, relative to other discretionary items, especially during economically challenging times;
|
|
•
|
the relative convenience and ease of administration;
|
|
•
|
the prevalence and severity of adverse side effects; and
|
|
•
|
the effectiveness of our sales and marketing efforts and those of our collaborators.
|
|
•
|
the diversion of management’s attention to integration matters;
|
|
•
|
difficulties in achieving anticipated cost savings, synergies, business opportunities and growth prospects from combining the business of Gel-Del Technologies with our company;
|
|
•
|
difficulties in the integration of operations and systems;
|
|
•
|
difficulties in the assimilation of employees; and
|
|
•
|
challenges in attracting and retaining key personnel.
|
|
•
|
reach agreement on acceptable terms with prospective CROs and study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
complete target animal studies due to deviations from study protocol;
|
|
•
|
address any safety concerns that arise during the course of testing;
|
|
•
|
address any conflicts with new or existing laws or regulations;
|
|
•
|
add new study sites; or
|
|
•
|
manufacture sufficient quantities of formulated drug for use in studies.
|
|
•
|
competitors may develop alternatives that render our product candidates obsolete;
|
|
•
|
product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
|
|
•
|
a product candidate may on further study be shown to have harmful side effects in pets or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
|
|
•
|
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
|
|
•
|
a product candidate may not be accepted as safe and effective by veterinarians, pet owners and the pet therapeutic community.
|
|
•
|
manage our target animal studies and other development efforts effectively;
|
|
•
|
identify, recruit, maintain, motivate and integrate additional employees;
|
|
•
|
manage our internal development efforts effectively while carrying out our contractual obligations to third parties; and
|
|
•
|
continue to improve our operational, financial and management controls, reporting systems and procedures.
|
|
•
|
we are unable to demonstrate to the satisfaction of the CVM, the USDA, the EMA or the applicable foreign regulatory body that the product candidate is safe and effective for the requested indication;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may disagree with our interpretation of data from our target animal studies and other development efforts;
|
|
•
|
we may be unable to demonstrate that the product candidate’s benefits outweigh any safety or other perceived risks;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may require additional studies;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may not approve of the formulation, labeling and/or the specifications of our current and future product candidates;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may fail to approve our manufacturing processes or facilities, or the manufacturing processes or facilities of third-party manufacturers with which we contract; and
|
|
•
|
the approval policies or regulations of the CVM, USDA or the applicable foreign regulatory body may significantly change in a manner rendering the data from our studies insufficient for approval.
|
|
•
|
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;
|
|
•
|
fines, warning letters or holds on target animal studies;
|
|
•
|
refusal by the CVM, the USDA or the EMA to approve pending applications or supplements to approved applications filed by us or our strategic collaborators, or suspension or revocation of product license approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of products; and
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
|
•
|
changes to manufacturing methods;
|
|
•
|
recall, replacement, or discontinuance of certain products; and
|
|
•
|
additional record keeping.
|
|
·
|
we are not required to prepare proxy or information statements;
|
|
·
|
we will be subject to only limited portions of the tender offer rules;
|
|
·
|
our officers, directors, and more than ten (10%) percent shareholders are not required to file beneficial ownership reports about their holdings in our company;
|
|
·
|
our officers, directors, and more than ten (10%) percent shareholders are not subject to the short-swing profit recovery provisions of the Exchange Act; and
|
|
·
|
more than five percent (5%) holders of classes of our equity securities will not be required to report information about their ownership positions in the securities.
|
|
Quarter Ended
|
High Bid
|
Low Bid
|
||||||
|
June 30, 2014
|
$ | 0.0650 | $ | 0.0100 | ||||
|
March 31, 2014
|
$ | 0.0299 | $ | 0.0033 | ||||
|
December 31, 2013
|
$ | 0.0050 | $ | 0.0030 | ||||
|
September 30, 2013
|
$ | 0.0299 | $ | 0.0033 | ||||
|
June 30, 2013
|
$ | 0.0299 | $ | 0.0175 | ||||
|
March 31, 2013
|
$ | 0.21 | $ | 0.20 | ||||
|
June 30, 2013
|
$ | 0.16 | $ | 0.09 | ||||
|
Lender
|
Date of Original Loan
|
Principal Amount Loaned
|
||||
|
9185-5643 Quebec Inc.
|
June 16, 2010
|
$ | 21,000.00 | |||
|
Michel St.-Hilaire
|
October 24, 2011
|
$ | 7,500.00 | |||
|
Elden Brochu
|
August 18, 2010
|
$ | 10,500.00 | |||
|
Gina Drouin
|
August 18, 2010
|
$ | 10,500.00 | |||
|
Christian Fontaine
|
October 24, 2011
|
$ | 7,500.00 | |||
|
Ferme Simen Inc.
|
June 14, 2010
|
$ | 21,000.00 | |||
|
·
|
a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading;
|
|
·
|
a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to violation to such duties or other requirements of securities’ laws;
|
|
·
|
a brief, clear, narrative description of a dealer market, including “bid” and “ask” prices for penny stocks and the significance of the spread between the “bid” and “ask” price;
|
|
·
|
a toll-free telephone number for inquiries on disciplinary actions;
|
|
·
|
definitions of significant terms in the disclosure document or in the conduct of trading in penny stocks; and
|
|
·
|
such other information and is in such form (including language, type, size and format), as the SEC shall require by rule or regulation.
|
|
·
|
the bid and offer quotations for the penny stock;
|
|
·
|
the compensation of the broker-dealer and its salesperson in the transaction;
|
|
·
|
the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and
|
|
·
|
monthly account statements showing the market value of each penny stock held in the customer’s account.
|
|
|
|
For Period from August 1, 2013 (inception) to March 31, 2014
|
|
|
|
|
|
|
||
|
Revenues
|
|
$
|
-
|
|
|
|
|
|
|
|
|
Total Operating Expenses
|
|
|
24,648,826
|
|
|
|
|
|
|
|
|
Total Other Income (Expense)
|
|
|
(13,021)
|
|
|
|
|
|
|
|
|
Net Income (loss)
|
|
$
|
(24,661,847
|
)
|
|
|
|
|
|
|
|
Net loss per share - basic and diluted
|
|
$
|
(0.07
|
)
|
|
Reports of Independent Registered Public Accounting Firms
|
F-2 | |||
|
Balance Sheets
|
F-3 | |||
|
Statements of Operations and Comprehendisve Loss
|
F-4 | |||
|
Statements of Stockholders’ Equity [Deficit]
|
F-5 | |||
|
Statements of Cash Flows
|
F-6 | |||
|
Notes to Financial Statements
|
F-7 |
|
PetVivo, Inc.
(Formerly Technologies Scan Corp.)
|
|||||||||||
|
|
|
March 31,
|
March 31,
|
|||||||
|
2014
|
2013
|
|||||||
| Assets: | ||||||||
|
Current Assets
|
||||||||
|
Cash and Cash Equivalents
|
$ | 39,338 | $ | - | ||||
|
Accounts Receivable
|
- | - | ||||||
|
Inventory
|
- | - | ||||||
|
Prepaid Expenses
|
8,000 | - | ||||||
|
Total Current Assets
|
47,338 | - | ||||||
|
Fixed Assets-net
|
- | - | ||||||
|
Other assets, license
|
130,000 | - | ||||||
|
Total Assets
|
$ | 177,338 | $ | - | ||||
|
Liabilities and Stockholders' Deficit:
|
||||||||
|
Current Liabilities:
|
||||||||
|
Derivative Liability
|
186,666 | - | ||||||
|
Accounts Payable and Accrued Expenses
|
83,657 | - | ||||||
|
Convertible Notes Payable
|
58,826 | - | ||||||
|
Note Payable-Debentures
|
150,000 | - | ||||||
|
Total Current Liabilities
|
479,149 | - | ||||||
|
Long Term Debt
|
- | - | ||||||
|
Total Liabilities
|
479,149 | - | ||||||
|
Stockholders' Equity:
|
||||||||
|
Common Stock, Par value $0.001, Authorized 4,000,000,000 shares,
|
||||||||
|
Issued 3,750,946,480 shares
|
3,750,946 | - | ||||||
|
Paid-In Capital
|
20,609,090 | - | ||||||
|
Retained Deficit
|
(24,661,847 | ) | - | |||||
|
Total Stockholders' Equity
|
(301,811 | ) | - | |||||
|
Total Liabilities and Stockholders' Equity
|
$ | 177,338 | $ | - | ||||
|
PetVivo, Inc.
(Formerly Technologies Scan Corp.)
|
|
|
|
From inception 8/1/2013
|
||||
|
To 3/31/2014
|
||||
|
Revenues
|
$ | - | ||
|
Costs of Services
|
- | |||
|
Gross Margin
|
- | |||
|
Expenses:
|
||||
|
Payroll Expenses
|
80,000 | |||
|
Stock for Services
|
24,440,100 | |||
|
Research and Development
|
79,536 | |||
|
General and Administrative
|
49,190 | |||
|
Operating Expenses
|
24,648,826 | |||
|
Operating Income (Loss)
|
(24,648,826 | ) | ||
|
Interest
|
(13,021 | ) | ||
|
Net Loss Before Taxes
|
(24,661,847 | ) | ||
|
Income and Franchise Tax
|
- | |||
|
Net Loss
|
$ | (24,661,847 | ) | |
|
Loss per Share, Basic &
|
||||
|
Diluted
|
$ | (0.07 | ) | |
|
Weighted Average Shares
|
||||
|
Outstanding
|
376,614,610 | |||
|
PetVivo, Inc.
(Formerly Technologies Scan Corp)
|
|||||
|
Statement of Stockholders’ Equity
|
|||||
|
August 1, 2013 to March 31, 2014
|
|
Additional
|
||||||||||||||||||||
|
Common
|
Common
|
Paid in
|
Retained
|
|||||||||||||||||
|
Shares
|
Stock
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
Balance Beginning
|
122,650,000 | $ | 122,650 | (122,650 | ) | - | - | |||||||||||||
|
Shares issued for Debt on 2/25/14
|
24,856,676 | 24,857 | 99,426 | - | 124,283 | |||||||||||||||
|
Shares issued for agreement on 3/14/2014
|
2,310,939,804 | 2,310,940 | (2,310,940 | ) | - | - | ||||||||||||||
|
Shares issued for services on 3/17/2014
|
1,222,000,000 | 1,222,000 | 23,218,000 | - | 24,440,000 | |||||||||||||||
|
Effects of Reverse
|
(274,746 | ) | (274,746 | ) | ||||||||||||||||
|
Shares issued for debt on 3/19/2014
|
70,500,000 | 70,500 | - | 70,500 | ||||||||||||||||
|
Net Loss for the period
|
(24,661,847 | ) | (24,661,847 | ) | ||||||||||||||||
|
|
||||||||||||||||||||
|
Balance March 31, 2014
|
3,750,946,480 | 3,750,946 | 20,609,090 | (24,661,847 | ) | (301,811 | ) | |||||||||||||
|
Petvivo, Inc.
(
Formerly Technologies Scan, Inc.)
|
||||
|
|
||||
|
From Inception August 1, 2013 to March 31, 2014
|
|
2014
|
||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||
|
Net Loss for the Period
|
$ | (24,661,847 | ) | |
|
Shares Issued for Services
|
24,440,100 | |||
|
Adjustments to reconcile net loss to net cash
|
||||
|
provided by operating activities:
|
||||
|
Depreciation and Amortization
|
- | |||
|
Changes in Operating Assets and Liabilities
|
||||
|
Decrease (Increase) in Accounts Receivable
|
- | |||
|
(Increase) Decrease in Prepaids and Deposits
|
(8,000 | ) | ||
|
Increase (Decrease) in Accrued Expenses
|
83,657 | |||
|
Increase in Derivative Liability
|
186,666 | |||
|
|
||||
|
Net Cash Used in Operating Activities
|
40,576 | |||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||
|
Purchase of License
|
(130,000 | ) | ||
|
Net cash provided by Investing Activities
|
(130,000 | ) | ||
|
CASH FLOW FROM FINANCING ACTIVITIES:
|
||||
|
Common Stock issued for Cash
|
- | |||
|
Proceeds from Loans
|
128,762 | |||
|
Reduction of Debt
|
||||
|
Net Cash Provided by Financing Activities
|
128,762 | |||
|
Net (Decrease) Increase in Cash
|
39,338 | |||
|
Cash at Beginning of Period
|
- | |||
|
Cash at End of Period
|
$ | 39,338 | ||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||
|
Cash paid during the year for:
|
||||
|
`Interest
|
$ | - | ||
|
Franchise and Income Taxes
|
$ | - | ||
|
SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING
AND FINANCING ACTIVITIES:
|
||||
|
Accounts Payable Satisfied through Contributed Capital
|
||||
|
and Property and Equipment
|
$ | - | ||
|
Level 1
|
Quoted market prices available in active markets for identical assets or liabilities as of the reporting date.
|
|
|
Level 2
|
Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date.
|
|
|
Level 3
|
Pricing inputs that are generally observable inputs and not corroborated by market data.
|
|
March 31,
2014
|
||||
|
Note Payable to a former shareholder
|
7,500 | |||
|
Notes Payable to an individual interest at 8%, payable on demand, convertible into common shares
|
51,326 | |||
|
Total Owed
|
58,826 | |||
|
1.
|
The Company assumed a 12% convertible debenture in the principal amount of $100,000 to 6287182 Canada Inc., a private corporation organized under the laws of Canada. In accordance with the terms and provisions of the 6287182 Canada Debenture, we may redeem by paying the principal plus accrued interest and 6287182 Canada has the right to convert the principal into shares of our restricted common stock at a per share price equal to 80% of the average closing price for 5 consecutive days prior to notice of conversion. The 6287182 Canada Debenture is due July 31, 2016 and accrues interest at the rate of 12% per annum. The Company is required to pay the accrued interest quarterly commencing on the date of execution and quarterly thereafter.
|
|
2.
|
The Company also assume a second note which is a 12% convertible debenture in the principal amount of $50,000 to Brevets Futek MSM Ltee, a private corporation organized under the laws of Canada. In accordance with the terms and provisions of the convertible debenture, the Company may redeem by paying the principal plus accrued interest. Brevets Futek MSM Ltee has the right to convert the principal into shares of our restricted common stock at a per share price equal to 80% of the average closing price for price for 5 consecutive days prior to notice of conversion. The convertible debenture is due July 17, 2016 and accrues interest at the rate of 12% per annum. The Company is required to pay the accrued interest quarterly commencing on the date of execution and quarterly thereafter
|
|
March 31, 2014
|
||||
|
Deferred Tax Assets – Non-current:
|
||||
|
NOL Carryover
|
$
|
141,066
|
||
|
Payroll Accrual
|
-
|
|||
|
Less valuation allowance
|
|
(141,066)
|
||
|
Deferred tax assets, net of valuation allowance
|
$
|
-
|
||
|
2014
|
||||
|
Book Income
|
$ | (24,661,847 | ) | |
|
Meals and Entertainment
|
681 | |||
|
Stock for Services
|
24,440,100 | |||
|
Payroll
|
80,000 | |||
|
Valuation allowance
|
141,066 | |||
| $ | - | |||
|
1.
|
In April 2014 the company issued 28,595,002 shares of stock for services and debt.
|
|
·
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
|
|
·
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of management and our directors; and
|
|
·
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
|
·
|
Deficiencies in Segregation of Duties.
Lack of proper segregation of functions, duties and responsibilities with respect to our cash and control over the disbursements related thereto due to our very limited staff, including our accounting personnel.
|
|
·
|
Deficiencies in the staffing of our financial accounting department. The number of qualified accounting personnel with experience in public company SEC reporting and GAAP is limited. This weakness does not enable us to maintain adequate controls over our financial accounting and reporting processes regarding the accounting for non-routine and non-systematic transactions. There is a risk that a material misstatement of the financial statements could be caused, or at least not be detected in a timely manner, by this shortage of qualified resources
.
|
|
·
|
Lack of audit committee.
|
|
Name
|
Age
|
Position
|
||
|
John Lai
|
51 |
President/Chief Executive Officer and a Director
|
||
|
John Dolan
|
49 |
Secretary, Treasurer/Chief Financial Officer and a Director
|
CORPORATE GOVERNANCE
|
Summary Compensation Table
|
|||||||||||||||||||||||||||||||||||
|
Name and Principal Position
|
Year
|
Salary ($)
|
Bonus ($)
|
Stock Awards ($)
|
Option Awards ($)
|
Non-Equity Incentive Plan Compensation ($)
|
Nonqualified Deferred Compensation Earnings ($)
|
All Other Compensation ($)
|
Total ($)
|
||||||||||||||||||||||||||
|
John Lai, current President/CEO and Director
|
2014
|
80,000 | 0 | 0 | 0 | 0 | 0 | 0 | 80,000 | ||||||||||||||||||||||||||
|
John F. Dolan, current Secretary, Treasurer/CFO and Director
|
2014
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||
|
Gilbert Pomerleau,
Prior CFO, Treasurer, Secretary and Director
|
2014
2013
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
||||||||||||||||||||||||||
|
Ghislaine St-Hilaire, Prior President and Director
|
2014
2013
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
||||||||||||||||||||||||||
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||||||||||||||||
|
Name
|
Number of Securities Underlying Unexercised Options
# Exercisable
|
# Un-exercisable
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Options
|
Option Exercise Price
|
Option Expiration Date
|
Number of Shares or Units of Stock Not Vested
|
Market Value of Shares or Units Not Vested
|
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights Not Vested
|
Value of Unearned Shares, Units or Other Rights Not Vested
|
|||||||||||||||||||||||||||
|
John Lai, current President/CEO and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
John F. Dolan, current Secretary, Treasurer/CFO and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
Gilbert Pomerleau,
Prior CFO, Treasurer, Secretary and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
Ghislaine St-Hilaire, Prior President and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
|
Fees Earned or Paid in Cash
$
|
Stock Awards
$
|
Option Awards
$
|
Non-Equity Incentive Plan Compensation
$
|
Non-Qualified Deferred Compensation Earnings
$
|
All Other Compensation
$
|
Total
$
|
||||||||||||||
|
John Lai
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
|
John F. Dolan
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
|
Gilbert Pomerleau, Prior director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
|
Ghislaine St-Hilaire, prior Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Name and Address of Beneficial Owner
Officers and Directors
|
Amount and Nature of Beneficial Owner
|
Percent of Class (1)
|
|
|
Common Stock
|
John Lai
12100 Singletree Lane
Suite 186
Eden Prairie, Minnesota 55344
|
1,774,969,312 shares,
President/CEO and Director
|
46.96%
|
|
Common Stock
|
John F. Dolan
12100 Singletree Lane
Suite 186
Eden Prairie, Minnesota 55344
|
360,726,723 shares
Secretary, Treasurer/CFO and Director
|
9.54%
|
|
Common Stock
|
All directors and named executive officers as a group (2 persons)
|
2,135,696,035 shares
|
56.51%
|
|
Name and Address of 5% or Greater Beneficial Owner
|
|||
|
Common Stock
|
Gel-Del Technologies Inc.
1000 Westgate Drive
St. Paul, Minnesota 55114
|
703,055,020 shares,
|
18.60%
|
|
Common Stock
|
Dave Masters
5344 Penn Avenue South
Minneapolis, Minnesota 55419
|
349,761,041 shares
|
9.25%
|
|
Common Stock
|
Randall A. Meyer
4016 Inglewood Avenue South
Edina, Minnesota 55416
|
349,761,041 shares
|
9.25%
|
|
(1)
|
Percentage of beneficial ownership of our common stock is based on 3,779,542,482 shares of common stock outstanding as of the date of this Annual Report.
|
|
(a)
|
Financial Statements.
|
|
(b)
|
Exhibits required by Item 601.
|
|
Exhibit No.
|
Description
|
|
|
3.1
|
Articles of Incorporation, incorporated by reference to Exhibit 3.1 of our Registration Statement on Form S-1 filed on April 18, 2011
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation, incorporated by reference to Exhibit 3.1 of our Registration Statement on Form S-1 filed on April 18, 2011
|
|
|
3.3
|
Certificate of Amendment to Articles of Incorporation incorporated by reference to Exhibit 3.1 of Current Report on Form 8-K filed on March 10, 2014
|
|
|
3.4
|
Certificate of Amendment to Articles of Incorporation incorporated by reference to Exhibit 3.1 of Current Report on Form 8-K filed on April 7, 2014.
|
|
|
3.5
|
Bylaws, incorporated by reference to Exhibit 3.1 of our Registration Statement on Form S-1 filed on April 18, 2011
|
|
|
10.1
|
Letter of Intent between Technologies Scan Corp. and 6285431 Canada Inc. dated September 5, 2012 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on September 11, 2012.
|
|
|
10.2
|
Rescission Agreement between Technologies Scan Corp. and 6285431 Canada Inc. dated April 12, 2013 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on April 18, 2013.
|
|
|
10.3
|
Letter of Intent between Technologies Scan Corp. and Social Geek Media Inc. dated April 6, 2013 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 6, 2013.
|
|
|
10.4
|
Memorandum of Amendment between Technologies Scan Corp. and Social Geek Media Inc. dated May 17, 2013 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 21, 2013.
|
|
|
10.5
|
12% Convertible Debenture of $100,000 between Technologies Scan Corp. and 6287182 Canada Inc. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2013.
|
|
|
10.6
|
12% Convertible Debenture of $100,000 between Technologies Scan Corp. and Brevets Futek MSM Ltee. incorporated by reference to Exhibit 10.2 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2013.
|
|
|
10.7
|
Rescission Agreement dated November 9, 2013 among Social Geek Meda Inc., Patrick Aube and Technologies Scan Corp. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 13, 2013
|
|
|
10.8
|
Letter of Intent dated December 16, 2013 between FedTech Services Inc. and Technologies Scan Corp. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 19, 2013
|
|
|
10.9
|
Term Sheet between Technologies Scan Corp. and PetVivo Inc. dated February 10, 2014 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on February 13, 2014.
|
|
|
10.10
|
Settlement Agreement dated February 2, 2014 between Technologies Scan Corp. and Ghislaine St.-Hilaire incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on February 24, 2014.
|
|
|
10.11
|
Securities Exchange Agreement among Technologies Scan Corp., PetVivo Inc. and shareholders of PetVivo Inc. dated March 21, 2014 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 13, 2014.
|
|
|
10.12
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and 9165-5643 Quebec Inc incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014.
|
|
|
10.13
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Elden Brochu incorporated by reference to Exhibit 10.2 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014.
|
|
|
10.14
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Gina Drouin incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014.
|
|
|
10.15
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Christian Fontaine incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014
|
|
|
10.16
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Ferme Semen Inc. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014
|
|
|
10.17
|
Term Sheet dated June 2, 2014 between Technologies Scan Corp. and Gel-Del Technologies Inc. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on June 2, 2014.
|
|
|
16.1
|
Letter from KBL LLP dated May 24, 2013 incorporated by reference to Exhibit 16.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 28, 2013.
|
|
|
31.1
|
Certification of Principal Executive Officer Required By Rule 13a-14(A) of the Securities Exchange Act of 1934, As Amended, As Adopted Pursuant To Section 302 of the Sarbanes-Oxley Act of 2002*
|
|
|
31.2
|
Certification of Principal Financial Officer Required By Rule 13a-14(A) of the Securities Exchange Act of 1934, As Amended, As Adopted Pursuant To Section 302 of the Sarbanes-Oxley Act of 2002*
|
|
|
32.1
|
Certification of Principal Executive Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
|
32.2
|
Certification of Principal Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
|
101.INS
|
XBRL Instance Document**
|
|
|
101.SCH
|
XBRL Taxonomy Schema**
|
|
|
101.CAL
|
XBRL Taxonomy Calculation Linkbase**
|
|
|
101.DEF
|
XBRL Taxonomy Definition Linkbase**
|
|
|
101.LAB
|
XBRL Taxonomy Label Linkbase**
|
|
|
101.PRE
|
XBRL Taxonomy Presentation Linkbase**
|
|
PetVivo Holdings, Inc.
a Nevada corporation
|
|||
|
July 14, 2014
|
By:
|
/s/ John Lai
|
|
|
|
|
John Lai
|
|
|
|
Its:
|
President, Director
(Principal Executive Officer)
|
|
|
|
|
|
|
|
July 14, 2014
|
By:
|
/s/ John F. Dolan
|
|
|
|
|
John F. Dolan
|
|
|
|
Its:
|
Chief Financial Officer, Secretary, Treasurer, Director
(Principal Financial and Accounting Officer)
|
|
|
July 14, 2014
|
By:
|
/s/ John Lai
|
|
|
|
John Lai
|
|
|
|
Its:
|
President, Director
|
|
|
|
|
(Principal Executive Officer)
|
|
| July 14, 2014 |
By:
|
/s/
John F. Dolan
|
|
|
|
John F. Dolan
|
|
|
|
Its:
|
Chief Financial Officer, Secretary, Treasurer, Director
|
|
|
|
|
(Principal Financial and Accounting Officer)
|
|