|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
|
|
Washington, D.C. 20549
|
|
(Mark One)
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Delaware
|
13-5315170
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification Number)
|
|
235 East 42nd Street New York, New York
|
10017
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
(212) 733-2323
(Registrant’s telephone number, including area code)
|
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $.05 par value
|
New York Stock Exchange
|
|
Floating Rate Notes due 2019
|
New York Stock Exchange
|
|
0.000% Notes due 2020
|
New York Stock Exchange
|
|
0.250% Notes due 2022
|
New York Stock Exchange
|
|
1.000% Notes due 2027
|
New York Stock Exchange
|
|
Securities registered pursuant to Section 12(g) of the Act:
None
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
|
Non-accelerated filer
o
|
|
Smaller reporting company
o
|
Emerging growth company
o
|
||
|
DOCUMENTS INCORPORATED BY REFERENCE
|
|
|
Portions of the 2018 Annual Report to Shareholders
|
Parts I, II and IV
|
|
Portions of the Proxy Statement for the 2019 Annual Meeting of Shareholders
|
Part III
|
|
TABLE OF CONTENTS
|
|
|
Page
|
|
Pfizer Inc.
|
2018 Form 10-K
|
i
|
|
DEFINED TERMS
|
|
2018 Financial Report
|
Exhibit 13 to this 2018 Form 10-K
|
|
2018 Form 10-K
|
This Annual Report on Form 10-K for the fiscal year ended December 31, 2018
|
|
2019 Proxy Statement
|
Proxy Statement for the 2019 Annual Meeting of Shareholders
|
|
ACA
|
U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act
|
|
ACIP
|
Advisory Committee on Immunization Practices
|
|
Alliance revenues
|
Revenues from alliance agreements under which we co-promote products discovered or developed by other companies or us
|
|
Anacor
|
Anacor Pharmaceuticals, Inc.
|
|
ANDA
|
Abbreviated New Drug Application
|
|
Astellas
|
Astellas Pharma Inc., Astellas US LLC and Astellas Pharma US, Inc.
|
|
Bain Capital
|
Bain Capital Private Equity and Bain Capital Life Sciences
|
|
BLA
|
Biologics License Application
|
|
BMS
|
Bristol-Myers Squibb Company
|
|
Cerevel
|
Cerevel Therapeutics, LLC
|
|
cGMPs
|
current Good Manufacturing Practices
|
|
DEA
|
U.S. Drug Enforcement Agency
|
|
Developed Markets
|
U.S., Western Europe, Japan, Canada, South Korea, Australia, Scandinavian countries, Finland and New Zealand
|
|
EFPIA
|
European Federation of Pharmaceutical Industries and Associations
|
|
EH
|
Essential Health
|
|
EMA
|
European Medicines Agency
|
|
Emerging Markets
|
Includes, but is not limited to, the following markets: Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa, the Middle East, Central Europe and Turkey
|
|
EU
|
European Union
|
|
Exchange Act
|
Securities Exchange Act of 1934, as amended
|
|
FCPA
|
U.S. Foreign Corrupt Practices Act
|
|
FDA
|
U.S. Food and Drug Administration
|
|
FFDCA
|
U.S. Federal Food, Drug and Cosmetic Act
|
|
GPD
|
Global Product Development organization
|
|
GSK
|
GlaxoSmithKline plc
|
|
HIS
|
Hospira Infusion Systems
|
|
Hospira
|
Hospira, Inc.
|
|
ICU Medical
|
ICU Medical, Inc.
|
|
IH
|
Innovative Health
|
|
IPR&D
|
In-process Research and Development
|
|
LIBOR
|
London Interbank Offered Rate
|
|
LOE
|
Loss of Exclusivity
|
|
MCO
|
Managed Care Organization
|
|
Medivation
|
Medivation, Inc.
|
|
NDA
|
New Drug Application
|
|
NMPA
|
National Medical Product Administration (formerly known as China Food and Drug Administration or CFDA)
|
|
NYSE
|
New York Stock Exchange
|
|
OTC
|
over-the-counter
|
|
PBM
|
Pharmacy Benefit Manager
|
|
PGS
|
Pfizer Global Supply
|
|
PMDA
|
Pharmaceuticals and Medical Device Agency in Japan
|
|
R&D
|
Research and Development
|
|
SEC
|
U.S. Securities and Exchange Commission
|
|
Tax Cuts and Jobs Act
|
Legislation commonly referred to as the U.S. Tax Cuts and Jobs Act of 2017
|
|
U.K.
|
United Kingdom
|
|
U.S.
|
United States
|
|
VAI
|
Voluntary Action Indicated
|
|
WRD
|
Worldwide Research and Development
|
|
Pfizer Inc.
|
2018 Form 10-K
|
ii
|


|
~$53.6 Billion
in Revenues in 2018
|
|
10
Products with Direct Product and/or Alliance Revenues of Greater than $1 Billion in 2018
|

|
2
Distinct Business Segments in 2018
—
Pfizer Innovative Health
(
~$33.4 Billion 2018 Revenues
) /
Pfizer Essential Health
(
~$20.2 Billion 2018 Revenues
)
|

|
6
Primary Therapeutic Areas in Pfizer Innovative Health in 2018
—
Internal Medicine, Vaccines, Oncology, Inflammation & Immunology, Rare Disease and
Consumer Healthcare
|
|
4
Pfizer Essential Health Product Categories in 2018
—
Global Brands
(Legacy Established Products & Peri-LOE Products)
, Sterile Injectable Pharmaceuticals, Biosimilars and Pfizer CentreOne
|

|
>125
Countries Where We Sell Our Products
|

|
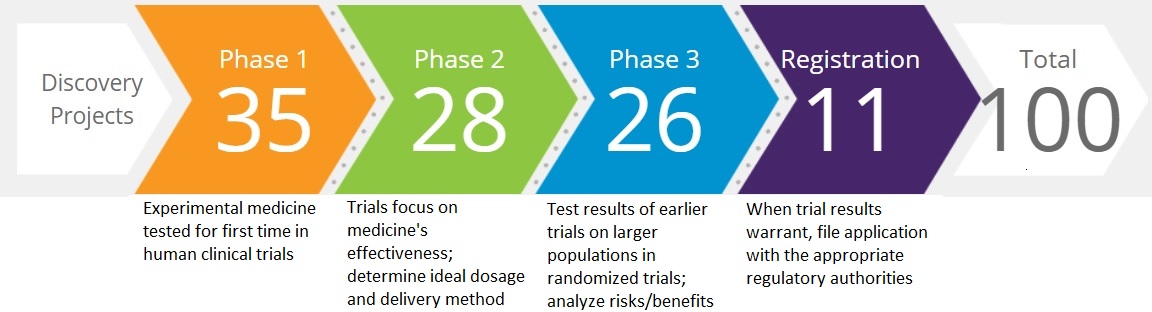
100
Projects in Clinical Research & Development*
|

|
~$8 Billion
2018 R&D Expense
|

|
58
Manufacturing Sites Worldwide Operated by PGS
|

|
~92,400
Employees Globally
|
|
Pfizer Inc.
|
2018 Form 10-K
|
iii
|
|
PART I
|
|
ITEM 1.
|
BUSINESS
|
 ABOUT PFIZER
ABOUT PFIZER
|
•
|
On December 19, 2018, we announced that we entered into a definitive agreement with GSK under which we and GSK have agreed to combine our respective consumer healthcare businesses into a new consumer healthcare joint venture that will operate globally under the GSK Consumer Healthcare name. The joint venture is expected to be a category leader in pain relief, respiratory, vitamin and mineral supplements, digestive health, skin health and therapeutic oral health and will be the largest global OTC consumer healthcare business. In exchange for contributing our Consumer Healthcare business, we will receive a 32% equity stake in the company and GSK will own the remaining 68%. The transaction is expected to close in the second half of 2019, subject to customary closing conditions including GSK shareholder approval and required regulatory approvals.
|
|
•
|
On February 3, 2017, we completed the sale of Pfizer’s global infusion systems net assets, HIS, to ICU Medical for up to approximately
$900 million
,
composed of cash and contingent cash consideration, ICU Medical common stock and seller financing
. HIS, which was acquired as part of the Hospira acquisition in September 2015, includes intravenous pumps, solutions and devices.
|
|
•
|
On December 22, 2016, for
$1,040 million
we acquired the development and commercialization rights to AstraZeneca’s small molecule anti-infectives business, primarily outside the U.S.
, which includes the marketed products Zavicefta™ (ceftazidime-avibactam), Merrem™/Meronem™ (meropenem) and Zinforo™ (ceftaroline fosamil), and the clinical development assets aztreonam-avibactam and ceftaroline fosamil-avibactam.
|
|
•
|
On September 28, 2016, we acquired Medivation for approximately
$14.3 billion
in cash (
$13.9 billion
, net of cash acquired). Medivation is a biopharmaceutical company focused on developing and commercializing small molecules for oncology.
|
|
•
|
On June 24, 2016, we acquired Anacor for approximately
$4.9 billion
in cash (
$4.5 billion
net of cash acquired), plus
$698 million
debt assumed. Anacor is a biopharmaceutical company focused on novel small-molecule therapeutics derived from its boron chemistry platform.
|
|
Pfizer Inc.
|
2018 Form 10-K
|
1
|
|
•
|
Pfizer Biopharmaceuticals Group
- a science-based Innovative Medicines business that includes our Innovative Health business units (except Consumer Healthcare) as well as a new Hospital business unit that commercializes our global portfolio of sterile injectable and anti-infective medicines. We also incorporated our biosimilar portfolio into our Oncology and Inflammation & Immunology therapeutic areas;
|
|
•
|
Upjohn
- an off-patent branded and generic established medicines business headquartered in China that includes 20 of our off-patent solid oral dose legacy brands, including
Lyrica
,
Lipitor
,
Norvasc, Viagra
and
Celebrex
, as well as certain generic medicines; and
|
|
•
|
Consumer Healthcare
- an over-the-counter medicines business, which we announced on December 19, 2018 will be contributed to, and combined with, GSK’s consumer healthcare business to form a new consumer healthcare joint venture.
|

|

|
|
|
IH focused on developing and commercializing novel, value-creating medicines and vaccines that significantly improve patients’ lives, as well as products for consumer healthcare.
Key therapeutic areas included internal medicine, vaccines, oncology, inflammation & immunology, rare disease and consumer healthcare. |
EH included legacy brands that have lost or will soon lose market exclusivity in both developed and emerging markets, branded generics, generic sterile injectable products, biosimilars and select branded products including anti-infectives. EH also included an R&D organization, as well as our contract manufacturing business. Through February 2, 2017, EH also included HIS.
|
|
|
Leading brands included:
- Prevnar 13/Prevenar 13
-
Xeljanz
-
Eliquis
- Lyrica
(U.S., Japan and certain other markets)
-
Enbrel
(outside the U.S. and Canada)
-
Ibrance
-
Xtandi
-
Chantix/Champix
- Several OTC consumer healthcare products
(e.g.,
Centrum
and
Advil
)
|
Leading brands included:
- Lipitor
- Norvasc
- Lyrica
(Europe, Russia, Turkey, Israel and Central Asia countries)
- Celebrex
- Viagra*
-
Inflectra/Remsima
- Sulperazon
-
Several sterile injectable products
|
|
|
Pfizer Inc.
|
2018 Form 10-K
|
2
|
|
Therapeutic Area
|
Description
|
Key Products
|
|
Internal Medicine
|
Included innovative brands from two therapeutic areas, Cardiovascular Metabolic and Pain, as well as regional brands.
|
Lyrica
(outside Europe, Russia, Turkey, Israel and Central Asia countries),
Chantix/Champix
and
Eliquis
(jointly developed and commercialized with BMS)
|
|
Vaccines
|
Included innovative vaccines brands across all ages—infants, adolescents and adults—in pneumococcal disease, meningitis and tick-borne encephalitis, with a pipeline focus on healthcare-acquired infections and maternal health.
|
Prevnar 13/Prevenar 13
(pediatric/adult),
Trumenba
and
FSME-IMMUN
|
|
Oncology
|
Included innovative oncology brands of biologics, small molecules and immunotherapies across a wide range of cancers.
|
Ibrance, Sutent, Xalkori, Inlyta
and
Xtandi
(jointly developed and commercialized with Astellas)
|
|
Inflammation and Immunology
|
Included innovative brands for chronic immune and inflammatory diseases.
|
Enbrel
(outside the U.S. and Canada),
Xeljanz
and
Eucrisa
|
|
Rare Disease
|
Included innovative brands for a number of rare diseases, including hematology, neuroscience, and inherited metabolic disorders.
|
BeneFix
,
Genotropin
and
Refacto AF/Xyntha
|
|
Consumer Healthcare*
|
Included over-the-counter (OTC) brands with a focus on dietary supplements, pain management, gastrointestinal and respiratory and personal care. In 2018, according to Nicholas Hall’s retail sales data (based on moving annual total data through the third quarter of 2018), Pfizer’s Consumer Healthcare business was the fifth-largest branded multi-national, OTC consumer healthcare business in the world and produced two of the ten largest selling consumer healthcare brands (Centrum and Advil) in the world.
|
Dietary Supplements:
Centrum
brands,
Caltrate
and
Emergen-C
Pain Management:
Advil
brands and
ThermaCare
Gastrointestinal:
Nexium 24HR/Nexium Control
and
Preparation H
Respiratory and Personal Care:
Robitussin
,
Advil Cold & Sinus
and
ChapStick
|
|
*
|
On December 19, 2018, we announced that we entered into a definitive agreement with GSK under which we and GSK have agreed to combine our respective consumer healthcare businesses into a new consumer healthcare joint venture, which will operate globally under the GSK Consumer Healthcare name. Assets and liabilities associated with our Consumer Healthcare business were reclassified as held for sale in the consolidated balance sheet as of December 31, 2018. We expect to complete the transaction during the second half of 2019, subject to customary closing conditions, including GSK shareholder approval and required regulatory approvals.
For additional information, see the Notes to Consolidated Financial Statements—
Note 2
C.
Acquisitions, Divestitures, Assets and Liabilities Held for Sale, Licensing Arrangements, Research and Development and Collaborative Arrangements, Equity-Method Investments and Privately Held Investment
:
Assets and Liabilities Held for Sale
.
|
|
Innovative Health $1B+ Products
|
||||
|
2018
|
|
2017
|
|
2016
|
|
Prevnar 13/Prevenar 13
|
|
Prevnar 13/Prevenar 13
|
|
Prevnar 13/Prevenar 13
|
|
Lyrica IH
|
|
Lyrica IH
|
|
Lyrica IH
|
|
Ibrance
|
|
Ibrance
|
|
Enbrel
|
|
Eliquis*
|
|
Eliquis*
|
|
Ibrance
|
|
Enbrel
|
|
Enbrel
|
|
Eliquis*
|
|
Xeljanz
|
|
Xeljanz
|
|
Viagra IH
|
|
Chantix/Champix
|
Sutent
|
Sutent
|
||
|
Sutent
|
||||
|
*
Eliquis
includes alliance revenues and direct sales in 2018, 2017 and 2016.
|
||||
|
Product Category
|
Description
|
Key Products
|
|
Global Brands
—
Legacy Established Products
|
Included products that have lost patent protection (excluding Sterile Injectable Pharmaceuticals and Peri-LOE Products).
|
Lipitor
,
Premarin
family and
Norvasc
|
|
Global Brands
—
Peri-LOE Products
|
Included products that have recently lost or are anticipated to soon lose patent protection.
|
Lyrica
(Europe, Russia, Turkey, Israel and Central Asia),
Viagra*
,
Celebrex
,
Pristiq
,
Zyvox
,
Vfend
,
Revatio
and
Inspra
|
|
Sterile Injectable Pharmaceuticals
|
Included generic injectables and proprietary specialty injectables (excluding Peri-LOE Products).
|
Medrol
,
Sulperazon
,
Fragmin
and
Tygacil
|
|
Biosimilars
|
Included recombinant and monoclonal antibodies, primarily in inflammation, oncology and supportive care.
|
Inflectra
/
Remsima
(biosimilar infliximab) (U.S., Canada, the EU, Australia and certain international markets),
Nivestim/Nivestym
(biosimilar filgrastim) (U.S. and certain European, Asian and Africa/Middle East markets),
Retacrit
(biosimilar epoetin alfa-epbx/epoetin zeta) (U.S. and certain European and Africa/Middle East markets) and
Ixifi Infliximab BS for I.V. Infusion 100mg
(Japan)
|
|
Pfizer CentreOne
|
Included revenues from our contract manufacturing and active pharmaceutical ingredient sales operation, including sterile injectables contract manufacturing, and revenues related to our manufacturing and supply agreements, including with Zoetis Inc.
|
--
|
|
*
|
Viagra lost exclusivity in the U.S. in December 2017. In 2018, revenues for Viagra in the U.S. and Canada, which were reported in IH through 2017, were reported in EH (which reported all other Viagra revenues excluding the U.S. and Canada through 2017). Therefore, in 2018, total Viagra worldwide revenues were reported in EH.
|
|
Essential Health $1B+ Products
|
||||
|
2018
|
2017
|
2016
|
||
|
Lipitor
|
Lipitor
|
Lipitor
|
||
|
Norvasc
|
Premarin
family of products
|
|||
|
Pfizer Inc.
|
2018 Form 10-K
|
3
|
|
•
|
delivering a pipeline of differentiated therapies and vaccines with the greatest medical and commercial potential;
|
|
•
|
advancing our capabilities that can position Pfizer for long-term leadership; and
|
|
•
|
creating new models for biomedical collaboration that will expedite the pace of innovation and productivity.
|
|
•
|
Inflammation and Immunology
;
|
|
•
|
Internal Medicine
;
|
|
•
|
Oncology
;
|
|
•
|
Rare Diseases
;
|
|
•
|
Vaccines
; and
|
|
•
|
Biosimilars.
|
|
•
|
Research Units within our WRD organization were generally responsible for research and early-stage development assets for our IH business (assets that have not yet achieved proof-of-concept).
|
|
•
|
Our R&D organization within the EH business supported the large base of EH products and helped develop potential new sterile injectable drugs and therapeutic solutions, as well as biosimilars.
|
|
•
|
Our Global Product Development organization,
a unified center for late-stage development for our innovative products that was generally responsible for the operational execution of clinical trials for both early-stage assets in the WRD portfolio as well as late-stage assets in the Innovative portfolio. For WRD assets, GPD worked in close collaboration with the Early Clinical Development group, which has expertise in various disciplines such as Biostatistics, Clinical Pharmacology and Digital Medicine.
|
|
•
|
Our science-based and other platform-services organizations, where a significant portion of our R&D spending occurred, provided technical expertise and other services to the various R&D projects, and were organized into science-based functions (which were part of our WRD organization), such as Pharmaceutical Sciences, Medicine Design, Regulatory and Drug Safety, and non-science-based functions, such as Facilities, Business Technology and Finance.
|
|
•
|
WRD is renamed Worldwide Research, Development and Medical (WRDM) as we have created a new Worldwide Medical & Safety organization that incorporates the former Chief Medical Office as well as the Worldwide Safety function;
|
|
•
|
The R&D organization within the EH business has been integrated into the WRDM, GPD and Upjohn organizations, including moving biosimilars into WRDM and GPD and realigning them with the relevant therapeutic areas (e.g., Oncology and Inflammation & Immunology);
|
|
•
|
The Regulatory function has been moved from the WRDM organization into the GPD organization; and
|
|
•
|
Late-stage portfolio spend has been moved from IH to GPD and from EH to GPD and Upjohn.
|
|
Pfizer Inc.
|
2018 Form 10-K
|
4
|
|
Pfizer Inc.
|
2018 Form 10-K
|
5
|
|
Drug
|
U.S. Basic Product Patent Expiration Year
|
Major EU Basic Product Patent Expiration Year
|
Japan Basic Product Patent Expiration Year
|
|||
|
Lyrica
|
2019
(1)
|
2014
(2)
|
2022
(3)
|
|||
|
Chantix/Champix
|
2020
|
2021
|
2022
|
|||
|
Sutent
|
2021
|
2021
|
2024
|
|||
|
Ibrance
|
2023
|
2028
|
2028
|
|||
|
Inlyta
|
2025
|
2025
|
2025
|
|||
|
Xeljanz
|
2025
|
2028
(4)
|
2025
|
|||
|
Prevnar 13/Prevenar 13
|
2026
|
2026
(5)
|
2029
|
|||
|
Eucrisa
|
2026
|
N/A
(6)
|
N/A
(6)
|
|||
|
Eliquis
(7)
|
2026
|
2026
|
2026
|
|||
|
Xtandi
(8)
|
2027
|
*
(8)
|
*
(8)
|
|||
|
Besponsa
|
2027
|
2023
|
2028
(9)
|
|||
|
Xalkori
|
2029
|
2027
|
2028
|
|||
|
Bavencio
(10)
|
2033
|
2032
|
2033
|
|||
|
(1)
|
In November 2018, the FDA granted pediatric exclusivity for Lyrica in the U.S. for an additional six months to June 2019; pediatric exclusivity applies to both the basic product patent for Lyrica and a method of treatment patent, both of which expired in the U.S. in December 2018.
|
|
(2)
|
Lyrica
regulatory exclusivity in the EU expired in July 2014.
|
|
(3)
|
Lyrica
is covered by a Japanese method-of-use patent which expires in 2022. The patent is currently subject to an invalidation action.
|
|
(4)
|
Xeljanz
EU expiry is provided by regulatory exclusivity.
|
|
(5)
|
The EU patent that covers the combination of the 13 serotype conjugates of
Prevenar
13
has been revoked following an opposition proceeding. This first instance decision has been appealed. There are other EU patents and pending applications covering the formulation and various aspects of the manufacturing process of
Prevenar
13
that remain in force.
|
|
(6)
|
Eucrisa
is not approved in the EU or Japan.
|
|
(7)
|
Eliquis
was developed and is being commercialized in collaboration with BMS.
|
|
(8)
|
Xtandi
is being developed and commercialized in collaboration with Astellas, which has exclusive commercialization rights for
Xtandi
outside the U.S.
|
|
(9)
|
Besponsa
Japan expiry is provided by regulatory exclusivity.
|
|
(10)
|
Bavencio
is being developed and commercialized in collaboration with Merck KGaA.
|
|
Pfizer Inc.
|
2018 Form 10-K
|
6
|
|
Pfizer Inc.
|
2018 Form 10-K
|
7
|
|
•
|
environment-related capital expenditures—
$33 million
; and
|
|
•
|
other environment-related expenses—
$162 million
.
|
|
Pfizer Inc.
|
2018 Form 10-K
|
8
|
|
Pfizer Inc.
|
2018 Form 10-K
|
9
|
|
ITEM 1A.
|
RISK FACTORS
|
|
Pfizer Inc.
|
2018 Form 10-K
|
10
|
|
Pfizer Inc.
|
2018 Form 10-K
|
11
|
|
Pfizer Inc.
|
2018 Form 10-K
|
12
|
|
Pfizer Inc.
|
2018 Form 10-K
|
13
|
|
Pfizer Inc.
|
2018 Form 10-K
|
14
|
|
Pfizer Inc.
|
2018 Form 10-K
|
15
|
|
Pfizer Inc.
|
2018 Form 10-K
|
16
|
|
Pfizer Inc.
|
2018 Form 10-K
|
17
|
|
Pfizer Inc.
|
2018 Form 10-K
|
18
|
|
Pfizer Inc.
|
2018 Form 10-K
|
19
|
|
Pfizer Inc.
|
2018 Form 10-K
|
20
|
|
Pfizer Inc.
|
2018 Form 10-K
|
21
|
|
Pfizer Inc.
|
2018 Form 10-K
|
22
|
|
Pfizer Inc.
|
2018 Form 10-K
|
23
|
|
Pfizer Inc.
|
2018 Form 10-K
|
24
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
Pfizer Inc.
|
2018 Form 10-K
|
25
|
|
Name
|
Age
|
Position
|
||
|
Albert Bourla
|
57
|
Chief Executive Officer since January 2019. Chief Operating Officer from January 2018 until December 2018; Group President, Pfizer Innovative Health from June 2016 until December 2017; Group President, Global Innovative Pharma Business (responsible for Vaccines, Oncology and Consumer Healthcare since 2014) from February 2016 until June 2016. President and General Manager of Established Products Business Unit from December 2010 until December 2013. Area President Europe, Africa, Asia and Pacific of Pfizer Animal Health from 2009 until November 2010. Area President Europe, Africa and Middle East of Pfizer Animal Health from 2005 until 2009. Our Director since February 2018. Board member of Pharmaceutical Research and Manufacturers of America (PhRMA). Board member of the Pfizer Foundation, which promotes access to quality healthcare. Member of the Board of Directors of the Partnership for New York City and Catalyst, a global non-profit organization accelerating progress for the advancement of women into leadership.
|
||
|
Frank A. D’Amelio
|
61
|
Chief Financial Officer, Executive Vice President, Business Operations and Global Supply since November 2018. Executive Vice President, Business Operations and Chief Financial Officer from December 2010 until October 2018. Senior Vice President and Chief Financial Officer from September 2007 until December 2010. Prior to joining Pfizer, he was Senior Executive Vice President of Integration and Chief Administrative Officer of Alcatel-Lucent from November 2006 until August 2007. Prior to the Alcatel-Lucent merger, he was Chief Operating Officer of Lucent and before that Chief Financial Officer of Lucent. Director of Zoetis Inc. and of Humana Inc. and Chair of the Humana Audit Committee. Director of the Independent College Fund of New Jersey.
|
||
|
Mikael Dolsten
|
60
|
Chief Scientific Officer, President, Worldwide Research, Development and Medical since January 2019. President of Worldwide Research and Development from December 2010 until December 2018. Senior Vice President; President of Worldwide Research and Development from May 2010 until December 2010. Senior Vice President; President of Pfizer BioTherapeutics Research & Development Group from October 2009 until May 2010. He was Senior Vice President of Wyeth and President, Wyeth Research from June 2008 until October 2009. He was a Private Equity Partner at Orbimed Advisors, LLC from January 2008 until June 2008. Director of Karyopharm Therapeutics Inc. Chairman of the Translational Advisory Board of Apple Tree Partners from 2016 to 2017.
|
||
|
Lidia Fonseca
|
50
|
Chief Digital and Technology Officer, Executive Vice President since January 2019. Chief Information Officer and Senior Vice President of Quest Diagnostics Incorporated from 2014 to 2018. Senior Vice President of Laboratory Corporation of America Holdings from 2008 until March 2013. Director of Tegna, Inc.
|
||
|
Michael Goettler
|
51
|
Group President, Pfizer Upjohn since January 2019. Executive Vice President from July 2018 until December 2018. Global President of Pfizer Inflammation & Immunology from January 2018 until June 2018. Global President of Pfizer Rare Disease from January 2016 until December 2017. Global Commercial Officer, Senior Vice President for Pfizer’s Global Innovative Pharma Business from January 2014 until December 2015. Regional President, Europe for Pfizer Specialty Care and the chair of the European Management Team from June 2012 until December 2013. Regional President Asia - Pacific for Specialty Care from October 2009 until June 2012. Member of the board of directors of PSI (Population Services International).
|
||
|
Angela Hwang
|
53
|
Group President, Pfizer Biopharmaceuticals Group since January 2019. Group President, Pfizer Essential Health from January 2018 until December 2018. Global President, Pfizer Inflammation and Immunology from January 2016 until December 2017. Regional Head, U.S. Vaccines from January 2014 until December 2015. Vice President, Emerging Markets for the Primary Care business from September 2011 until December 2013. Vice President, U.S. Brands business within Essential Health from October 2009 until August 2011.
|
||
|
Rady A. Johnson
|
57
|
Chief Compliance, Quality and Risk Officer, Executive Vice President since January 2019. Executive Vice President, Chief Compliance and Risk Officer from December 2013 until December 2018. Senior Vice President and Associate General Counsel from October 2006 until December 2013.
|
||
|
Douglas M. Lankler
|
53
|
General Counsel, Executive Vice President since December 2013. Corporate Secretary from January 2014 until February 2014. Executive Vice President, Chief Compliance and Risk Officer from February 2011 until December 2013. Executive Vice President, Chief Compliance Officer from December 2010 until February 2011. Senior Vice President and Chief Compliance Officer from January 2010 until December 2010. Senior Vice President, Deputy General Counsel and Chief Compliance Officer from August 2009 until January 2010. Senior Vice President, Associate General Counsel and Chief Compliance Officer from October 2006 until August 2009.
|
||
|
Freda C. Lewis-Hall
|
64
|
Chief Patient Officer, Executive Vice President since January 2019. Executive Vice President, Chief Medical Officer from December 2010 until December 2018. Senior Vice President, Chief Medical Officer from May 2009 until December 2010. Previously, she was Chief Medical Officer and Executive Vice President, Medicines Development at Vertex Pharmaceuticals from June 2008 until May 2009. Dr. Lewis-Hall was Senior Vice President, U.S. Pharmaceuticals, Medical Affairs for Bristol-Myers Squibb Company from 2003 until May 2008. Director of Tenet Healthcare Corporation from December 2014 to May 2017.
|
||
|
A. Rod MacKenzie
|
59
|
Chief Development Officer, Executive Vice President since June 2016. Senior Vice President, Chief Development Officer from March 2016 until June 2016. Group Senior Vice President and Head, Pharma Therapeutics Research and Development from 2010 until March 2016. Senior Vice President, Head of Worldwide Research from 2007 until 2010. Dr. MacKenzie represents Pfizer as a member of the Board of Directors of ViiV Healthcare Limited.
|
||
|
Dawn Rogers
|
54
|
Chief Human Resources Officer, Executive Vice President since January 2019. Executive Vice President, Worldwide Human Resources from June 2018 until December 2018. Senior Vice President, Human Resources for the Chief Operating Officer from November 2017 until May 2018. Senior Vice President of Human Resources for Pfizer Essential Health, Global Product Development, and the Legal and Compliance Divisions from 2016 until November 2017. Senior Vice President of Human Resources for the Global Innovative Pharma Business from 2013 until 2016. Senior Vice President of Human Resources for the Primary Care Business Unit from 2011 until 2013. Senior Vice President of Human Resources for Worldwide Research and Development from 2008 until 2011. Vice President of Human Resources for Pfizer's European Commercial Operations from 2006 to 2008.
|
||
|
Sally Susman
|
57
|
Chief Corporate Affairs Officer, Executive Vice President since January 2019. Executive Vice President, Corporate Affairs (formerly Policy, External Affairs and Communications) from December 2010 until December 2018. Senior Vice President, Policy, External Affairs and Communications from December 2009 until December 2010. Senior Vice President and Chief Communications Officer from February 2008 until December 2009. Prior to joining Pfizer, Ms. Susman held senior level positions at The Est
é
e Lauder Companies, including Executive Vice President from 2004 to January 2008. Director of WPP plc.
|
||
|
John D. Young
|
54
|
Chief Business Officer, Group President since January 2019. Group President, Pfizer Innovative Health from January 2018 until December 2018. Group President, Pfizer Essential Health from June 2016 until December 2017; Group President, Global Established Pharma Business from January 2014 until June 2016. President and General Manager, Pfizer Primary Care from June 2012 until December 2013. Primary Care Business Unit’s Regional President for Europe and Canada from 2009 until June 2012. U.K. Country Manager from 2007 until 2009. Director of Johnson Controls International plc.
|
||
|
Pfizer Inc.
|
2018 Form 10-K
|
26
|
|
PART II
|
|
ITEM 5.
|
MARKET FOR THE COMPANY’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Period
|
Total Number
of Shares
Purchased
(b)
|
Average Price
Paid per
Share
(b)
|
Total Number of
Shares Purchased as
Part of Publicly
Announced Plan
(a)
|
Approximate Dollar Value of Shares
that May Yet Be Purchased
Under the Plan
(a)
|
|||||||||
|
October 1, 2018 through October 28, 2018
|
38,477,427
|
|
$
|
44.25
|
|
38,410,129
|
|
$
|
7,487,879,989
|
|
|||
|
October 29, 2018 through November 30, 2018
|
43,812,603
|
|
$
|
43.50
|
|
43,795,856
|
|
$
|
5,582,880,460
|
|
|||
|
December 1, 2018 through December 31, 2018
|
32,598,112
|
|
$
|
43.77
|
|
32,559,080
|
|
$
|
14,157,881,147
|
|
|||
|
Total
|
114,888,142
|
|
$
|
43.83
|
|
114,765,065
|
|
||||||
|
(a)
|
For additional information, see the Notes to Consolidated Financial Statements
––
Note 12
.
Equity
in our
2018
Financial Report, which is incorporated by reference.
|
|
(b)
|
In addition to the amounts purchased under our share repurchase program, these columns represent (i)
118,667
shares, primarily representing common stock surrendered to the Company to satisfy tax withholding obligations in connection with the vesting of awards under our long-term incentive programs and (ii) the open market purchase by the trustee of
4,410
shares of common stock in connection with the reinvestment of dividends paid on common stock held in trust for employees who were granted performance share awards and who deferred receipt of such awards.
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
Pfizer Inc.
|
2018 Form 10-K
|
27
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
Pfizer Inc.
|
2018 Form 10-K
|
28
|
|
PART III
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
Pfizer Inc.
|
2018 Form 10-K
|
29
|
|
PART IV
|
|
ITEM 15.
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
•
|
Report of Independent Registered Public Accounting Firm on the Consolidated Financial Statements
|
|
•
|
Consolidated Statements of Income
|
|
•
|
Consolidated Statements of Comprehensive Income
|
|
•
|
Consolidated Balance Sheets
|
|
•
|
Consolidated Statements of Equity
|
|
•
|
Consolidated Statements of Cash Flows
|
|
•
|
Notes to Consolidated Financial Statements
|
|
•
|
Selected Quarterly Financial Data (Unaudited)
|
|
Agreement and Plan of Merger, dated as of August 20, 2016, among Pfizer Inc., Montreal, Inc. and Medivation, Inc. is incorporated by reference from our Current Report on Form 8-K filed on August 22, 2016 (File No. 001-03619). (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the Securities and Exchange Commission upon request any omitted schedule or exhibit to the Merger Agreement.)
|
||
|
Stock and Asset Purchase Agreement, dated December 19, 2018, by and among Pfizer Inc., GlaxoSmithKline plc and GlaxoSmithKline Consumer Healthcare Holdings Limited. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the Securities and Exchange Commission upon request any omitted schedule or exhibit to the Stock and Asset Purchase Agreement.)
1
|
||
|
Our Restated Certificate of Incorporation dated April 12, 2004, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended March 28, 2004 (File No. 001-03619).
|
||
|
Amendment dated May 1, 2006 to Restated Certificate of Incorporation dated April 12, 2004, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended July 2, 2006 (File No. 001-03619).
|
||
|
Our By-laws, as amended December 18, 2017, are incorporated by reference from our Current Report on Form 8-K filed on December 21, 2017 (File No. 001-03619).
|
||
|
Indenture, dated as of January 30, 2001, between us and The Chase Manhattan Bank, is incorporated by reference from our Current Report on Form 8-K filed on January 30, 2001 (File No. 001-03619).
|
||
|
First Supplemental Indenture, dated as of March 24, 2009, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended June 28, 2009 (File No. 001-03619).
|
||
|
Second Supplemental Indenture, dated as of June 2, 2009, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K filed on June 3, 2009 (File No. 001-03619).
|
||
|
Third Supplemental Indenture, dated as of June 3, 2013, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K filed on June 3, 2013 (File No. 001-03619).
|
||
|
1
Application has been made to the Securities and Exchange Commission for confidential treatment of certain portions of this exhibit. Omitted material for which confidential treatment has been requested has been separately filed with the Securities and Exchange Commission.
|
||
|
Pfizer Inc.
|
2018 Form 10-K
|
30
|
|
Fourth Supplemental Indenture, dated as of May 15, 2014, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on May 15, 2014 (File No. 001-03619).
|
||
|
Fifth Supplemental Indenture, dated as of October 5, 2015, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on October 6, 2015 (File No. 001-03619).
|
||
|
Sixth Supplemental Indenture, dated as of June 3, 2016, between us and The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on June 3, 2016 (File No. 001-03619).
|
||
|
Seventh Supplemental Indenture, dated as of November 21, 2016, between us and The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on November 21, 2016 (File No. 001-03619).
|
||
|
Eighth Supplemental Indenture, dated as of March 17, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (successor to the Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on March 17, 2017 (File No. 001-03619).
|
||
|
Ninth Supplemental Indenture, dated as of March 6, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent and calculation agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on March 6, 2017 (File No. 001-03619).
|
||
|
Tenth Supplemental Indenture, dated as of December 19, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on December 19, 2017 (File No. 001-03619).
|
||
|
Indenture, dated as of April 10, 1992, between Wyeth (formerly American Home Products Corporation) and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Registration Statement on Form S-3 (File No. 33-57339), filed on January 18, 1995.
|
||
|
Supplemental Indenture, dated as of October 13, 1992, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Registration Statement on Form S-3 (File No. 33-57339), filed on January 18, 1995.
|
||
|
Fifth Supplemental Indenture, dated as of December 16, 2003, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s 2003 Annual Report on Form 10-K (File No. 001-01225).
|
||
|
Sixth Supplemental Indenture, dated as of November 14, 2005, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Current Report on Form 8-K filed on November 15, 2005 (File No. 001-01225).
|
||
|
Seventh Supplemental Indenture, dated as of March 27, 2007, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Current Report on Form 8-K filed on March 28, 2007 (File No. 001-01225).
|
||
|
Eighth Supplemental Indenture, dated as of October 30, 2009, between Wyeth, us and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, formerly The Chase Manhattan Bank), as trustee, to Indenture dated as of April 10, 1992 (as amended on October 13, 1992), is incorporated by reference from our Current Report on Form 8-K filed on November 3, 2009 (File No. 001-03619).
|
||
|
Indenture, dated as of September 7, 2018, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on September 7, 2018 (File No. 001-03619).
|
||
|
First Supplemental Indenture, dated as of September 7, 2018, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on September 7, 2018 (File No. 001-03619).
|
||
|
4.20
|
Except as set forth in Exhibits 4.1-19 above, the instruments defining the rights of holders of long-term debt securities of the Company and its subsidiaries have been omitted.
2
|
|
|
2
We agree to furnish to the Securities and Exchange Commission, upon request, a copy of each instrument with respect to issuances of long-term debt of the Company and its subsidiaries.
|
||
|
Pfizer Inc.
|
2018 Form 10-K
|
31
|
|
2001 Stock and Incentive Plan is incorporated by reference from our Proxy Statement for the 2001 Annual Meeting of Shareholders (File No. 001-03619).
|
||
|
Pfizer Inc. 2004 Stock Plan, as Amended and Restated is incorporated by reference from our 2011 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Pfizer Inc. 2014 Stock Plan is incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Shareholders (File No. 001-03619).
|
||
|
Form of Acknowledgment and Consent and Summary of Key Terms for Stock Option Grants, RSUs and TSRUs is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Form of Executive Grant Letter is incorporated by reference from our 2015 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amendment No. 1 to the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees.
|
||
|
Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended April 3, 2016 (File No. 001-03619).
|
||
|
Amendment No. 1 to the Pfizer Supplemental Savings Plan (Amended and Restated as of January 1, 2016), is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended October 1, 2017 (File No. 001-03619).
|
||
|
Amendment No. 2 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amendment No. 3 to the Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended September 30, 2018 (File No. 001-03619).
|
||
|
Amendment No. 4 to the Pfizer Supplemental Savings Plan.
|
||
|
Amendment No. 5 to the Pfizer Supplemental Savings Plan.
|
||
|
Pfizer Inc. Global Performance Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended October 1, 2017 (File No. 001-03619).
|
||
|
Executive Annual Incentive Plan is incorporated by reference from our 2012 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amended and Restated Deferred Compensation Plan is incorporated by reference from our 2012 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amendment to Amended and Restated Deferred Compensation Plan, dated June 20, 2013, is incorporated by reference from our 2013 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amendment No. 2 to Amended and Restated Deferred Compensation Plan, dated April 27, 2016, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended July 3, 2016 (File No. 001-03619).
|
||
|
Wyeth 2005 (409A) Deferred Compensation Plan (frozen as of January 2012), together with all material Amendments, is incorporated by reference from our 2013 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amended and Restated Wyeth Supplemental Employee Savings Plan (effective as of January 1, 2005 and frozen as of January 2012), together with all material Amendments is incorporated by reference from our 2011 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Amendment to Amended and Restated Wyeth Supplemental Employee Savings Plan, dated June 20, 2013, is incorporated by reference from our 2013 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
The form of Indemnification Agreement with each of our non-employee Directors is incorporated by reference from our 1996 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
The form of Indemnification Agreement with each of the Named Executive Officers identified in our 2018 Proxy Statement is incorporated by reference from our 1997 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Letter to Frank A. D’Amelio regarding replacement pension benefit dated August 22, 2007 is incorporated by reference from our Current Report on Form 8-K filed on August 22, 2007 (File No. 001-03619).
|
||
|
Pfizer Inc. Executive Severance Plan is incorporated by referenced from our Current Report on Form 8-K filed on February 20, 2009 (File No. 001-03619).
|
||
|
Amendment No. 1 to Pfizer Inc. Executive Severance Plan.
|
||
|
Pfizer Inc.
|
2018 Form 10-K
|
32
|
|
Annual Retainer Unit Award Plan (for Non-Employee Directors) (frozen as of March 1, 2006) as amended, is incorporated by reference from our 2008 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Nonfunded Deferred Compensation and Unit Award Plan for Non-Employee Directors, as amended, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended September 28, 2014 (File No. 001-03619).
|
||
|
Form of Special Award Letter Agreement is incorporated by reference from our Current Report on Form 8-K filed on October 28, 2009 (File No. 001-03619).
|
||
|
Offer Letter to G. Mikael Dolsten, dated April 6, 2009, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended April 3, 2011 (File No. 001-03619).
|
||
|
Form of Special Performance-Based Incentive Award Letter is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
Form of Special Performance-Based Incentive Grant Letter is incorporated by reference from our 2017 Annual Report on Form 10-K (File No. 001-03619).
|
||
|
*
10.33
|
Time Sharing Agreement, dated December 17, 2018, by and between Pfizer Inc. and Ian C. Read.
|
|
|
*
13
|
Portions of the 2018 Financial Report, which, except for those sections incorporated by reference, are furnished solely for the information of the SEC and are not to be deemed “filed.”
|
|
|
*
21
|
Subsidiaries of the Company.
|
|
|
*
23
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
*
24
|
Power of Attorney (included as part of signature page).
|
|
|
Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
*
31.2
|
Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
*
32.1
|
Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
*
32.2
|
Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
*101.INS
|
XBRL Instance Document
|
|
|
*101.SCH
|
XBRL Taxonomy Extension Schema
|
|
|
*101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase
|
|
|
*101.LAB
|
XBRL Taxonomy Extension Label Linkbase
|
|
|
*101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase
|
|
|
*101.DEF
|
XBRL Taxonomy Extension Definition Document
|
|
|
ITEM 16.
|
FORM 10-K SUMMARY
|
|
Pfizer Inc.
|
2018 Form 10-K
|
33
|
|
Pfizer Inc.
|
|||
|
Dated: February 28, 2019
|
By:
|
/S/ MARGARET M. MADDEN
|
|
|
Margaret M. Madden
Senior Vice President and Corporate Secretary
Chief Governance Counsel
|
|||
|
Signature
|
|
Title
|
Date
|
|
|
/S/ ALBERT BOURLA
Albert Bourla
|
|
Chief Executive Officer and Director
(Principal Executive Officer)
|
February 27, 2019
|
|
|
/S/ FRANK A. D’AMELIO
Frank A. D’Amelio
|
|
Chief Financial Officer, Executive Vice President, Business Operations and Global Supply
(Principal Financial Officer)
|
February 26, 2019
|
|
|
/S/ LORETTA V. CANGIALOSI
Loretta V. Cangialosi
|
|
Senior Vice President—Controller
(Principal Accounting Officer)
|
February 26, 2019
|
|
|
/S/ IAN C. READ
Ian C. Read
|
Executive Chairman of the Board
|
February 28, 2019
|
||
|
/S/ DENNIS A. AUSIELLO
Dennis A. Ausiello
|
|
Director
|
February 26, 2019
|
|
|
/S/ RONALD E. BLAYLOCK
Ronald E. Blaylock |
|
Director
|
February 27, 2019
|
|
|
/S/ W. DON CORNWELL
W. Don Cornwell |
|
Director
|
|
February 26, 2019
|
|
/S/ JOSEPH J. ECHEVARRIA
Joseph J. Echevarria
|
Director
|
February 26, 2019
|
||
|
/S/ HELEN H. HOBBS
Helen H. Hobbs
|
|
Director
|
February 28, 2019
|
|
|
Signature
|
|
Title
|
Date
|
|
|
/S/ JAMES M. KILTS
James M. Kilts
|
|
Director
|
February 26, 2019
|
|
|
/S/ DAN R. LITTMAN
Dan R. Littman
|
|
Director
|
February 26, 2019
|
|
|
/S/ SHANTANU NARAYEN
Shantanu Narayen
|
Director
|
February 26, 2019
|
||
|
/S/ SUZANNE NORA JOHNSON
Suzanne Nora Johnson
|
Director
|
February 26, 2019
|
||
|
/S/ JAMES C. SMITH
James C. Smith
|
|
Director
|
February 26, 2019
|
|