|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
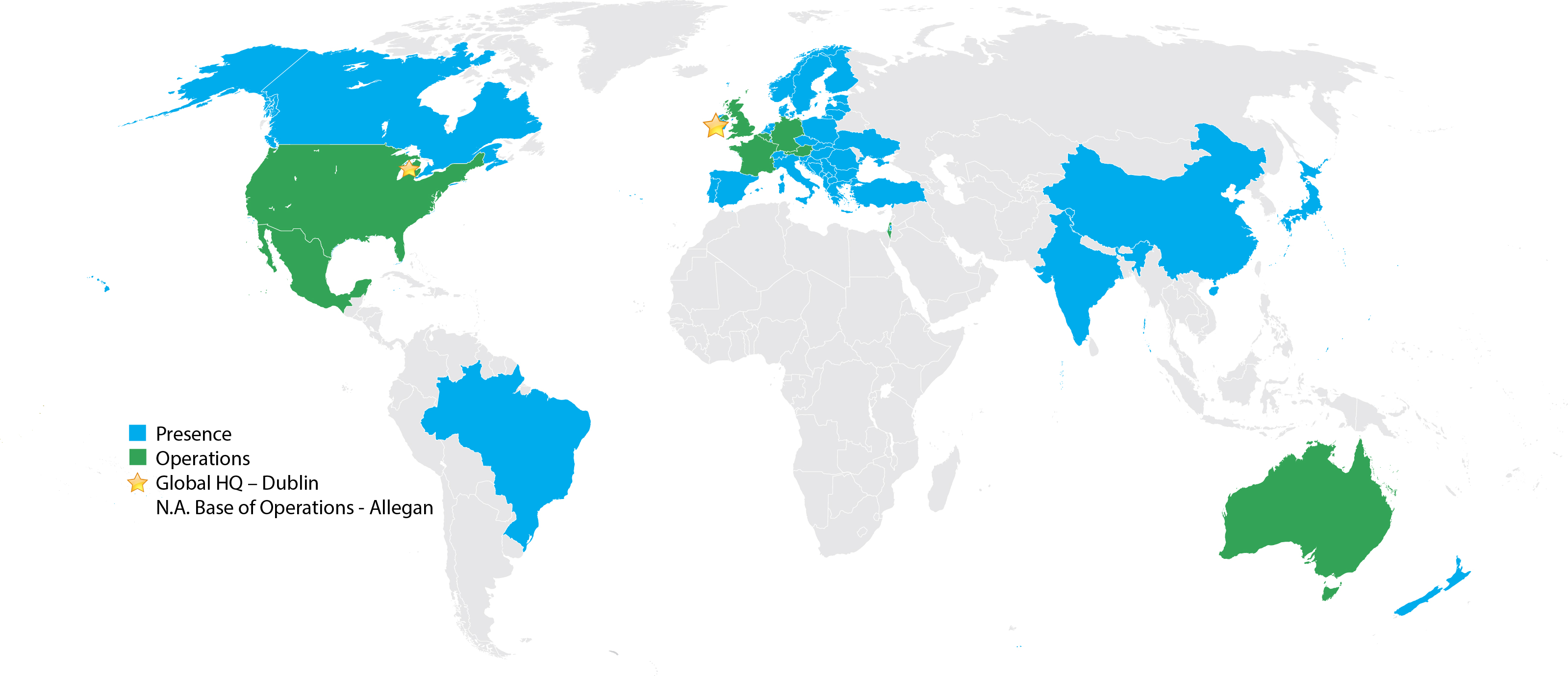
Ireland
|
|
N/A
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
Treasury Building, Lower Grand Canal Street, Dublin 2, Ireland
|
|
-
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Ordinary shares, €0.001 par value
|
|
New York Stock Exchange
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
|
YES
|
[ ]
|
NO
|
[X]
|
|
|
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 of Section 15(d) of the Act.
|
YES
|
[ ]
|
NO
|
[X]
|
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
|
YES
|
[ ]
|
NO
|
[X]
|
|
|
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
|
YES
|
[ ]
|
NO
|
[X]
|
|
|
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
|
[ ]
|
||||
|
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer", "accelerated filer", "smaller reporting company", and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
|||||
|
Large accelerated filer
|
[X]
|
Accelerated filer
|
[ ]
|
Non-accelerated filer
|
[ ]
|
Smaller reporting company
|
[ ]
|
|||
|
Emerging growth company
|
[ ]
|
|||||||||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
|
[ ]
|
|||||||||
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
|
YES
|
[ ]
|
NO
|
[X]
|
|
|
|
|
Page No.
|
|
Explanatory Note
|
||
|
Part I.
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Additional Item.
|
||
|
Part II.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Part III.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Part IV.
|
||
|
Item 15.
|
||
|
ITEM 1.
|
BUSINESS
|
|
•
|
Consumer Healthcare Americas
("
CHCA
")
,
comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
Consumer Healthcare International
("
CHCI
")
,
comprises our legacy Branded Consumer Healthcare segment and now includes our consumer focused businesses in the U.K., Australia, and Israel, which were previously reported in the legacy Consumer Healthcare segment. This segment includes our U.K. liquid licensed products business, which was previously reported in the Prescription Pharmaceuticals segment.
|
|
•
|
Prescription Pharmaceuticals
("
RX
")
,
comprises our U.S. Prescription Pharmaceuticals business.
|
|
•
|
Specialty Sciences
, continued to comprise the Tysabri
®
Royalty Stream.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2015 |
December 27, 2014
(1)
|
June 27,
2015 |
June 28, 2014
(1)
|
|||||||||||||||||
|
$
|
311.1
|
|
$
|
460.9
|
|
$
|
231.1
|
|
$
|
77.3
|
|
$
|
273.8
|
|
$
|
231.4
|
|
|||||
|
•
|
In 2016, we experienced a reduction in pricing expectations within our
CHCA
segment, particularly in the cough/cold, animal health and analgesics categories due to various factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, and competition in specific product categories. We expect this pricing environment to continue to impact our
CHCA
segment for the foreseeable future.
|
|
•
|
On
August 5, 2016
, we completed the sale of our U.S. Vitamin, Minerals, and Supplements ("VMS") business to International Vitamin Corporation for
$61.8 million
inclusive of an estimated working capital adjustment. See
Item 8. Note 2
for additional discussion of the divestiture.
|
|
Product Category
|
Description
|
|
|
Analgesics
|
Pain relievers and fever reducers
|
|
|
Cough/cold/allergy/sinus
|
Cough, cold, allergy, and sinus products
|
|
|
Gastrointestinal
|
Antacids, anti-diarrheal, and anti-heartburn products
|
|
|
Infant nutritionals
|
Infant formula and food products
|
|
|
Smoking cessation
|
Gums, lozenges, and other products designed to help users quit smoking
|
|
|
Animal health
|
Pet health and wellness products
|
|
|
Other
|
Feminine hygiene, diabetes care, dermatological care, diagnostic products, scar management, and other miscellaneous healthcare products
|
|

|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2015 |
December 27,
2014 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||||||
|
$
|
117.4
|
|
$
|
235.2
|
|
$
|
122.9
|
|
$
|
33.2
|
|
$
|
145.5
|
|
$
|
75.5
|
|
|||||
|
•
|
As part of our strategic initiatives, management continues to drive improvements and evaluate the overall cost structures within our
CHCI
segment in the following ways:
|
|
•
|
On December 8, 2016, we announced the cancellation of the unprofitable EuroGenerics NV distribution agreement in Belgium. The cancellation, combined with the exit of certain OTC distribution agreements, is expected to reduce net sales by approximately $200.0 million in 2017.
|
|
•
|
We continue to make progress on our previously announced restructuring plans to right-size the
Omega
business due to the impact of market dynamics on sales volumes. In addition, we made several strategic leadership changes during the year ended December 31, 2016, including appointing new leaders for Belgium, France and Germany as well as a new Executive Vice President of the CHCI segment. Management continues to evaluate the overall cost structure relative to current and expected market dynamics. In 2016, we recognized
$20.9 million
of restructuring expense in the CHCI segment.
|
|
•
|
Management continues to evaluate the most effective business model for each country and has announced strategic evaluations for Russia and Argentina.
|
|
•
|
The
CHCI
segment has been impacted by market dynamics in key countries such as Belgium, France, Germany and Italy due to softness in certain brand categories and by unfavorable foreign currency impacts, primarily in the U.K. related to Brexit. In addition, the segment had been impacted in Belgium by a change in the forecast with a major wholesaler, as management implements improved supply chain efficiencies in this market. The
CHCI
segment has restructured its approach to addressing these markets including: (1) implementing a brand prioritization strategy to address these market dynamics, with an objective to balance the cost of advertising and promotional investments with expected contributions from category sales, (2) restructuring its sales force in each of these markets to more effectively serve customers, and (3) exiting certain unfavorable distribution agreements. The combination of these actions are expected to improve the segment's focus on higher value OTC products, reduce selling costs and improve operating margins in the segment.
|
|
Product Category
|
Description
|
Top 20 Brands
|
||
|
Cough, Cold, and Allergy
|
Products that address respiratory symptoms, including traditional medications and alternative treatments such as aromatherapy solutions.
|
Bittner
®
/Aflubin
®
Bronchodual ® Physiomer ® Phytosun ® /Valda ® Prevalin ® /Beconase ® Solpadeine ® /Antigrippine ® |
||
|
Lifestyle
|
Weight management, pregnancy and fertility kits, pain relief, sleep management, smoking cessation, and eye care.
|
Paravet
®
/Clément-Thékan
®
Niquitin
®
Predictor
®
Silence
®
/Nytol
®
XLS (Medical)
®
|
||
|
Natural Health and VMS
|
Vitamins, minerals, supplements, and various other natural remedies.
|
Biover
®
/Abtei
®
Davitamon ® / Granufink ® /Bional ® |
||
|
Personal Care and Derma-Therapeutics
|
Products for the face and body, including sun care, baby-specific, and feminine hygiene products, and solutions for various skin conditions and allergies such as eczema, psoriasis and rosacea.
|
ACO
®
Bodysol
®
/Galenco
®
Dermalex
®
Lactacyd
®
Wartner
®
|
||
|
Anti-Parasite
|
Products focused on the elimination of parasites in both humans and pets including lice treatment and insect repellent.
|
Jungle Formula
®
Paranix
®
|
||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2015 |
December 27, 2014
(1)
|
June 27,
2015 |
June 28, 2014
(1)
|
|||||||||||||||||
|
$
|
119.0
|
|
$
|
106.8
|
|
$
|
66.8
|
|
$
|
3.7
|
|
$
|
43.8
|
|
$
|
12.2
|
|
|||||
|
•
|
We continue to experience a significant reduction in pricing expectations from historical levels in our
RX
segment due to industry and competitive pressures. This softness in pricing is attributed to various factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future, and we are forecasting a 9% to 11% pricing decline in this segment for the year ended December 31, 2017 compared to the prior year.
|
|
•
|
On January 22, 2016, we acquired a portfolio of generic dosage forms and strengths of Retin-A
®
(tretinoin), a topical prescription acne treatment, from Matawan Pharmaceuticals, LLC, for
$416.4 million
in cash ("Tretinoin Products").
|
|
•
|
On March 1, 2016, we completed the acquisition of two development-stage specialty Rx products to further invest in our specialty Rx portfolio.
|
|
•
|
On August 22, 2016, we purchased the remaining 60.9% ownership rights to a generic Benzaclin
TM
product ("Generic Benzaclin
TM
"), which we developed and marketed in collaboration with Barr Laboratories. As a result of this transaction, we are now entitled to 100% of income from sales of the product.
|
|
•
|
On November 10, 2016, we announced that as part of our portfolio review process we are conducting a comprehensive internal evaluation of the
RX
segment's market position, growth opportunities, and interdependencies with our manufacturing and shared service operations to determine if strategic alternatives should be explored.
|
|
•
|
During the three months ended December 31, 2016, the U.S. market for our Entocort
®
(budesonide) capsules, including both brand and authorized generic capsules, experienced significant and unexpected increased competition, reducing our future revenue stream. This led to an impairment charge of
$342.2 million
related to the Entocort
®
intangible asset acquired in 2015. We expect our 2017 net sales to be negatively affected in an amount of approximately $72.0 million.
|
|
•
|
In December 2016, we transitioned our specialty pharmaceutical commercial activities to our partner, Exeltis, who will lead sales and marketing efforts for this portfolio of products. We do not expect this transition will have an impact on our net sales.
|
|
Generic Name
(1)
|
Comparative Brand-Name Drug
|
|
|
Adapalene cream
|
Differin
®
|
|
|
Bacitracin ophthalmic ointment
|
N/A
|
|
|
Benzoyl peroxide 5% - clindamycin 1% gel
|
BenzaClin
TM
|
|
|
Budesonide
|
Entocort
®
|
|
|
Clindamycin foam
|
Evoclin
®
|
|
|
Clindamycin phosphate and benzoyl peroxide gel
|
Duac
®
|
|
|
Clobetasol foam, lotion and shampoo
|
Olux
®
, Olux-E
®
, Clobex
®
|
|
|
Desonide cream, ointment
|
Desonate
®
, Tridesilon
®
|
|
|
Dihydroergotamine injection
|
D.H.E. 45
|
|
|
Halobetasol ointment and cream
|
Ultravate
®
|
|
|
Hydrocortisone suppositories
|
N/A
|
|
|
Mupirocin ointment
|
Bactroban
®
|
|
|
Nystatin topical powder
|
Mycostatin
®
|
|
|
Permethrin cream
|
Elimite
®
|
|
|
Potassium chloride
|
Klor-Con
®
|
|
|
Tacrolimus
|
Protopic
®
|
|
|
Testosterone 1% gel
|
Androgel
|
|
|
Testosterone cypionate injection
|
Depo
®
, Testosterone
|
|
|
Triamcinolone acetonide nasal spray
|
Nasacort
®
AQ
|
|
|
Triamcinolone cream/ointment
|
Triderm™/Kenalog™
|
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2015 |
December 27,
2014 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||||||
|
$
|
68.0
|
|
$
|
118.6
|
|
$
|
41.2
|
|
$
|
40.4
|
|
$
|
117.8
|
|
$
|
104.1
|
|
|||||
|
•
|
In February 2016, a competitor's pipeline product, Ocrevus
®
, received breakthrough therapy designation from the FDA and was approved in 2017. The product is expected to compete with Tysabri
®
and have a significant negative impact on the Tysabri
®
royalty stream. Although the product has not launched, industry analysts believe that based on released clinical study information, Ocrevus
®
will favorably compete against Tysabri
®
in the relapsing, remitting multiple sclerosis market segment due to its high efficacy and convenient dosage form.
|
|
•
|
On November 15, 2016, Biogen received additional intellectual property protection for Tysabri
®
as they were granted a new patent in the U.S. with coverage to 2027.
|
|
•
|
On
March 27, 2017
, we announced the completed divestment of our Tysabri
®
royalty stream to Royalty Pharma for up to
$2.85 billion
, which consists of
$2.2 billion
in cash and up to
$250.0 million
and
$400.0 million
in milestone payments to us if the royalties on global net sales of Tysabri
®
that are received by Royalty Pharma meet specific thresholds in 2018 and 2020, respectively. As a result of this transaction, we will derecognize the Tysabri
®
financial asset in the first quarter of 2017 and we do not expect the disposition to have a material impact on our results.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2015 |
December 27,
2014 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||||||
|
$
|
184.0
|
|
$
|
186.3
|
|
$
|
88.2
|
|
$
|
89.8
|
|
$
|
187.8
|
|
$
|
152.5
|
|
|||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2015 |
December 27,
2014 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
13
|
%
|
14
|
%
|
13
|
%
|
19
|
%
|
16
|
%
|
19
|
%
|
|||||
|
•
|
Helping consumers access safe, effective and affordable healthcare products;
|
|
•
|
Strong corporate governance;
|
|
•
|
Complying with regulatory and legal requirements;
|
|
•
|
Demonstrating environmental stewardship;
|
|
•
|
Continuously improving packaging sustainability;
|
|
•
|
Protecting human rights of our global employees and challenging our partners to do the same;
|
|
•
|
Diversity of thought, experience and perspective;
|
|
•
|
Providing a safe and healthy work environment for our employees; and
|
|
•
|
Establishing effective community partnerships.
|
|
•
|
Physician Payment Sunshine Act
- This act requires certain pharmaceutical manufacturers to engage in extensive tracking of payments or transfers of value to physicians and teaching hospitals, maintenance of a payment database and public reporting of the payment data.
|
|
•
|
Foreign Corrupt Practices Act of 1977 ("FCPA")
- This act and other similar anti-bribery laws prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties or international organizations with the intent to obtain or retain business or seek a business advantage.
|
|
•
|
Federal Trade Commission ("FTC")
- This agency oversees the advertising and other promotional practices of consumer products marketers. The FTC considers whether a product’s claims are substantiated, truthful and not misleading. The FTC also reviews mergers and acquisitions of companies exceeding specified thresholds and investigates certain business practices relevant to the healthcare industry.
|
|
•
|
International Organization for Standardization ("ISO")
- The ISO Standards specify requirements for a Quality Management System that demonstrates the ability to consistently provide products that meet customer and applicable regulatory standards and includes processes to ensure continuous improvement. Our infant formula manufacturing sites are ISO 9001-2008 Certified for Quality Management Systems. ISO inspections are conducted at least annually.
|
|
•
|
United States Pharmacopeial Convention, Inc. ("USP")
- The USP is a non-governmental, standard-setting organization. By reference, the FFDCA incorporates the USP quality and testing standards and monographs as the standard that must be met for the listed drugs, unless compliance with those standards is specifically disclaimed on the product’s labeling. USP standards exist for most Rx and OTC pharmaceuticals and many nutritional supplements. The FDA typically requires USP compliance as part of cGMP compliance.
|
|
•
|
Health Insurance Portability and Accountability Act ("HIPAA")
- We could be subject to criminal penalties if we knowingly obtain individually identifiable health information from a covered entity in a manner that is not authorized or permitted by HIPAA or for aiding and abetting the violation of HIPAA.
|
|
•
|
Consumer Product Safety Commission ("CPSC")
- The CPSC has published regulations requiring child resistant packaging on certain products including pharmaceuticals and dietary supplements. The manufacturer of any product that is subject to any CPSC rule, ban, standard or regulation must certify that, based on a reasonable testing program, the product complies with CPSC requirements.
|
|
•
|
Other State Agencies
- We are subject to regulation by numerous other state health departments, insurance departments, boards of pharmacy, state controlled substance agencies, state consumer health and safety regulations, and other comparable state agencies, each of which have license requirements and fees that vary by state.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
•
|
As a manufacturer of generic versions of brand-name drugs through our
CHCA
and
RX
segments, we experience competition from brand-name drug companies that may try to prevent, discourage or delay the use of generic versions through various measures, including introduction of new branded products, legislative initiatives, changing dosage forms or dosing regimens, regulatory processes, filing new patents or patent extensions, lawsuits, citizens’ petitions, and negative publicity prior to introduction of a generic product. In addition, brand-name competitors may lower their prices to compete with generic products, increase advertising, or launch, either through an affiliate or licensing arrangements with another company, an authorized generic at or near the time the first generic product is launched, depriving the generic product potential market exclusivity.
|
|
•
|
Our
CHCA
and
RX
segments also experience competition from our generic competitors, some of whom are significantly larger than we are, who may develop their products more rapidly or complete regulatory approval processes sooner, or may market their products earlier than we do. In the U.S., if we are not the first to file our ANDA, the FDA may grant 180-day exclusivity to another company, which would prevent us from selling the product during the exclusivity period. Even if we are the first to file, in certain circumstances, we may not be able to fully exploit our 180-day exclusivity period.
|
|
•
|
Our
CHCA
and
RX
segments may experience increased price competition as other generic companies produce the same product, sometimes for dramatically lower margins in order to gain market share. Other generic companies may introduce new drugs and/or drug delivery techniques that make our current products less desirable. A drug may be subject to competition from alternative therapies during the period of patent protection or regulatory exclusivity, and thereafter we may be subject to further competition from generic products or biosimilars.
|
|
•
|
The pharmaceutical industry is consolidating. This creates larger competitors and places further pressure on prices, development activities, and customer retention. Our animal health category within the
CHCA
segment has seen an increase in direct to consumer advertising by several branded competitors, which may increase in the future, and our nutritionals category has experienced increased competition through alternative channels such as health food stores, direct mail, and direct sales.
|
|
•
|
We develop and distribute branded products primarily through our
CHCI
segment. We experience competition from other brand-name drug companies, many of which are larger and have more resources to devote to advertising and marketing. These direct competitors may be able to adapt more quickly to changes in customer requirements. Our current and future competitors may develop products comparable or superior to those offered by us at more competitive prices. If we are unable to compete successfully, our business will be harmed through loss of customers or increased negative pricing pressure that would adversely affect our ability to generate revenue and adversely affect our operating results.
|
|
•
|
We maintain a diversified product line to function as a primary supplier for our customers. Capital investments are driven by growth, technological advancements, cost improvement and the need for manufacturing flexibility. Our future capital expenditures could vary materially due to the uncertainty of these factors. In addition, if we fail to stay current with the latest manufacturing, information and packaging technology, we may be unable to competitively support the launch of new product introductions.
|
|
•
|
Our product margins may decline over time due to our products' aging life cycles, changes in consumer choice, changes in competition for our existing products, or the introduction of next generation innovative products; therefore, new product introductions are necessary to maintain our current financial condition. If we are unable to continue to create new products, we may lose market share or experience pricing pressure, and our net sales may be negatively impacted.
|
|
•
|
We must prove that the regulated generic drug products in our
CHCA
and
RX
segments are bioequivalent to their branded counterparts, which requires bioequivalence studies, and in the case of topical products, even more extensive clinical endpoint trials to demonstrate their efficacy. The development and commercialization process, particularly with respect to innovative products, is both time consuming and costly, and subject to a high degree of business risk. Products currently under development may require re-design to meet evolving FDA standards, may not perform as expected, may not pass required bioequivalence studies, or may be the subject of intellectual property challenges. Necessary regulatory
|
|
•
|
Our ability to attract and retain scientists proficient in emerging delivery forms and/or contracting with a third party in order to generate new products of this type is critical to our long-term plans. If we fail to attract and retain this talent, our long-term sales growth and profit could be adversely impacted.
|
|
•
|
Even upon the successful development of a product, our customers' failure to launch a product successfully, or delays in manufacturing, could adversely affect our operating results. In addition, the FDA or similar regulatory agency could impose higher standards and additional requirements, such as requiring more supporting data and clinical data than previously required, in order to gain regulatory clearance to launch new formulations into the market, which could negatively impact our future net sales.
|
|
•
|
The future growth and stability of U.S. store brand market share will be impacted, in part, by general economic conditions, which can influence consumers to switch to and from store brand products. Our
CHCA
segment sales could be negatively affected if economic conditions improve and consumers return to purchasing higher-priced brand-name products. Conversely, while store brand products present an alternative to higher-priced branded products, if economic conditions deteriorate, our
CHCA
segment sales could be negatively impacted if consumers forgo obtaining healthcare or reduce their healthcare spending.
|
|
•
|
Our
CHCI
segment's success is dependent on the continued growth in demand for its lifestyle products, which include weight-loss products and various dietary supplements. If demand for these products decreases, our
CHCI
segment's results of operations would be negatively impacted.
|
|
•
|
Our
CHCA
customers may request changes in packaging to meet consumer demands, which could cause us to incur inventory obsolescence charges and redesign costs, which in turn would negatively impact our
CHCA
segment's results of operations.
|
|
•
|
Our infant formula product category within our
CHCA
segment is subject to changing consumer preferences and health and nutrition-related concerns. Our business depends, in part, on consumer preferences and choices, including the number of mothers who choose to use infant formula products rather than breastfeed their babies. To the extent that private, public, and government sources may promote the benefits of breastfeeding over the use of infant formula, there could be a reduced demand for infant formula products. We could also be adversely impacted by an increase in the number of families that are provided with infant formula by the U.S. federal government through the Women, Infants and Children program, as we do not participate in this program.
|
|
•
|
We must obtain approval from the appropriate regulatory agencies in order to manufacture and sell our products in the regions in which we operate. Obtaining this approval can be time consuming and costly. There can be no assurance that, in the event we submit an application for a marketing authorization to any global regulatory agency, we will obtain the approval to market a product and/or that we will obtain it on a timely basis. Laws unique to the U.S. regulatory framework encourage generic competition by providing eligibility for first generic marketing exclusivity if certain conditions are met. If we are granted generic exclusivity, the exclusivity may be shared with other generic companies, including authorized generics; or it is possible that we may forfeit 180-day exclusivity if we do not obtain regulatory approval or begin marketing the product within the statutory requirements. Finally, if we are not the first to file our ANDA, the FDA may grant 180-day exclusivity to another company, thereby effectively delaying the launch of our product.
|
|
•
|
Global regulatory agencies regularly inspect our manufacturing facilities and the facilities of our third-party suppliers. The failure of one of our facilities, or a facility of one of our third-party suppliers, to comply with applicable laws and regulations may lead to a breach of representations made to our customers, or to regulatory or government action against us related to the products made in that facility. Such action could include suspension of or delay in regulatory approvals. If the compliance violations are severe, agencies of the government may initiate product seizure, injunction, recall, suspension of production or distribution of our products, loss of certain licenses or other governmental penalties, or civil or criminal prosecution, thereby impacting the reputation of all of our products.
|
|
•
|
In the U.S., the DSCSA requires development of an electronic pedigree to track and trace each prescription drug at the salable unit level through the distribution system, which will be effective incrementally over a 10-year period beginning on January 1, 2015, for manufacturers, wholesale distributors, and re-packagers, and on July 1, 2015 for dispensers. Similarly, the European Commission passed legislation requiring new product packaging ‘safety features’ to prevent falsification of medicinal products primarily within the prescription medicines sector. The act was adopted February 9, 2016. EU member states (with the exception of Belgium, Italy and Greece), and EEA members Norway, Iceland, Liechtenstein and Switzerland must be in compliance within three years, or by February 9, 2019. Belgium, Italy, and Greece have until February 9, 2025 to comply. Marketing Authorization holders will have three years from the publication date to implement the necessary changes or risk forfeiting their product licenses. Compliance with the new U.S. and EU electronic pedigree requirements may increase our operational expenses and impose significant administrative burdens.
|
|
•
|
Global regulatory agencies highly scrutinize any product application submitted to switch a product from physician prescribed Rx to unsupervised OTC use by the general public. The expansion of Rx-to-OTC switches is critical to our future growth. Reluctance of regulatory agencies to approve Rx-to-OTC switches in new product categories could impact that growth.
|
|
•
|
Several bills have been introduced in U.S. Congress that could, if enacted, affect the manufacture and marketing of Rx and OTC drugs including labeling and packaging. For example, the FDA is proposing to change existing regulations to permit generic drug application holders to revise their labeling without prior FDA review to add new safety information that may differ from the corresponding brand drug. The FDA has delayed publication of the Final Rule until April 2017.
If this proposed regulatory change is adopted without further revision, it may eliminate the preemption of certain failure-to-warn claims, with respect to generic drugs, which could have a material adverse impact on our future operating results. Regulatory bodies outside of the U.S. could enact similar legislation. We cannot predict whether further label restrictions may be required, or whether additional regulations in the U.S. or other countries in which we operate, may be passed.
|
|
•
|
Our infant formula products may be subject to barriers or sanctions imposed by countries or international organizations limiting international trade and dictating the specific content of infant formula products. Governments could enhance regulations on the industry aimed at ensuring the safety and quality of dairy products, including, but not limited to, compulsory batch-by-batch inspection and testing for additional safety and quality issues. Such inspections and testing may increase our operating costs related to infant formula products.
|
|
•
|
On June 10, 2014, the FDA published a final rule ("FR") entitled "Current Good Manufacturing Practices, Quality Control Procedures, Quality Factors, Notification Requirements, and Records and Reports, for Infant Formula." The FR includes, among other things, new or modified requirements related to infant formula manufacturing, quality controls, record-keeping, and clinical trials. Our infant formula manufacturing facilities have been inspected by the FDA after the effective date of the FR and found to be in full compliance with the new GMP regulations with no corrective actions required
|
|
•
|
Some of our pharmaceutical products are marketed through direct interactions with healthcare professionals, which is known as “detailing.” This activity is subject to extensive regulation under a variety of U.S. laws and regulations, including anti-kickback, anti-bribery, and false claims laws; the FFDCA with respect to claims and off-label promotions; and similar laws in non-U.S. jurisdictions. If our marketing activities are found to be improper, we could be subject to civil and governmental actions and penalties. These risks may increase as non-U.S. jurisdictions adopt new anti-bribery laws and regulations.
|
|
•
|
If we are unable to successfully obtain the necessary quota for controlled substances and List I chemicals, we risk having delayed product launches or failing to meet commercial supply obligations. If we are unable to comply with regulatory requirements for controlled substances and List I chemicals, the DEA, or similar regulatory agency, may take regulatory actions, resulting in temporary or permanent interruption of distribution of our products, withdrawal of our products from the market, or other penalties.
|
|
•
|
Changes to the Medical Device Directive are anticipated in 2017, based on a proposal
for new European Medical Device Regulation, which has been under discussion since 2012. These changes are expected to
include increased supervision by the Notified Bodies by Competent Authorities and revisions to documentation requirements. We will monitor the regulation's progress and cannot currently predict how it will impact the future production and sale of products classified as medical devices.
At this time, work is ongoing to translate the final text of the Regulation in all the EU official languages and to correct technical inconsistencies. Final formal adoption is expected both on the Council and the Parliament sides during the first quarter of 2017
.
|
|
•
|
Our operations extend to numerous countries outside the U.S. and are subject to the risks inherent in conducting business globally and under the laws, regulations, and customs of various jurisdictions. These risks include compliance with a variety of national and local laws of countries in which we do business, such as restrictions on the import and export of certain intermediates, drugs, and technologies. We must also comply with a variety of U.S. laws related to doing business outside of the U.S., including Office of Foreign Asset Controls, United Nations and EU sanctions; the Iran Threat Reduction and Syria Human Rights Act of 2012; and rules relating to the use of certain “conflict minerals” under Section 1502 of the Dodd-Frank Wall Street Reform and Consumer Protection Act. Further changes in laws, regulations, and practices affecting the pharmaceutical industry and the healthcare system, including imports, exports, manufacturing, quality, cost, pricing, reimbursement, approval, inspection, and delivery of healthcare, may affect our business and operations.
|
|
•
|
By their nature, these programs require us to provide discounts and rebates and therefore reduce our net product revenues. Further, because the amounts of these discounts are based on our commercial sales practices, it is important that we maintain pricing practices that appropriately take into account these government pricing programs.
|
|
•
|
We are required to report pricing data to CMS, including AMP, on a monthly and quarterly basis and BP and ASP on a quarterly basis. We also are required to report quarterly and annual Non-FAMPs to the VA. If we fail to submit required information, make misrepresentations, or knowingly submit false information to the government as to AMP, ASP, or BP, we may be liable for substantial civil monetary penalties or subject to other enforcement actions, such as under the False Claims Act, and CMS may terminate our Medicaid drug rebate agreement. In that event, U.S. federal payments may not be available under Medicaid or Medicare Part B for our covered outpatient drugs.
|
|
•
|
The Health Reform Law enacted in 2010 requires the use of AMP data to calculate FULs for multiple source drugs and amends the statutory definitions of AMP and "multiple source drug" in a manner that materially affects the calculation of FULs. CMS surveys and publishes retail community pharmacy acquisition cost information to provide state Medicaid agencies with a basis for comparing their own reimbursement and pricing methodologies and rates. CMS’s final Medicaid rebate program also directs states to revise their payment methods to establish payment rates consistent with actual acquisition costs. Based on our initial evaluation, we do not believe that the changes will have a material impact on our business. However, states are continuing to evaluate their payment methods and we cannot predict how the new FUL or state payment methodologies will affect our pharmacy customers or to what extent these customers may seek additional discounts in light of reimbursement changes. We also cannot predict how the sharing of FUL data and retail survey prices may impact competition in the marketplace.
|
|
•
|
Under the 340B program, if we fail to provide required discounts to covered entities, we may be subject to refund claims or civil money penalties under that program.
|
|
•
|
If we inadvertently overcharge the government in connection with our FSS contract or TriCare Agreement, whether due to a misstated FCP or otherwise, we would be required to refund the difference. Failure to make necessary disclosures and/or to identify contract overcharges can result in False Claims Act allegations or potential violations of other laws and regulations. Unexpected refunds to the government, and responses to a government investigation or enforcement action, are expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations, and growth prospects.
|
|
•
|
Our reporting and payment obligations under the Medicaid rebate program and other governmental purchasing and rebate programs are complex and may involve subjective decisions. Our calculations and methodologies are subject to review by the governmental agencies, and it is possible that these reviews could result in challenges to our submissions. If we do not comply with those reporting and payment obligations, we could be subject to civil and/or criminal sanctions, including fines, penalties, and possible exclusion from U.S. federal healthcare programs (including Medicaid and Medicare).
|
|
•
|
We maintain several single-source supplier relationships, either because alternative sources are not available or because the relationship is advantageous due to regulatory, performance, quality, support, or price considerations. Unavailability or delivery delays of single-source components or products could adversely affect our ability to ship the related product in a timely manner. The effect of unavailability or delivery delays would be more severe if associated with our higher-volume or more profitable products. Even where alternative sources of supply are available, qualifying the alternate suppliers and establishing reliable supplies could cost more or result in delays and a loss of net sales. Additionally, global regulatory requirements for obtaining product approvals could substantially lengthen the approval of an alternate material source. As a result, the loss of a single-source supplier could have a material adverse effect on our results of operations.
|
|
•
|
The rapid increase in cost of many raw materials from inflationary forces, such as increased energy costs, and our ability or inability to pass on these increases to our customers could have a negative material impact on our financial results.
|
|
•
|
Our infant formula products require certain key raw ingredients that are derived from raw milk, such as skim milk powder, whey protein powder, and lactose. Our supply of milk-based ingredients may be limited by the ability of individual dairy farmers and cooperatives to provide raw milk in the amount and quality we deem necessary. Raw milk production is influenced by factors beyond our control including seasonal and environmental factors, governmental agricultural and environmental policy, and global demand. We cannot guarantee that there will be sufficient supplies of these key ingredients necessary to produce infant formula.
|
|
•
|
Our products, and the raw materials used to make those products, generally have limited shelf lives. Our inventory levels are based, in part, on expectations regarding future sales. We may experience build-ups in inventory if sales slow. Any significant shortfall in sales may result in higher inventory levels of raw materials and finished products, thereby increasing the risk of inventory spoilage and corresponding inventory write-downs and write-offs. Cargo thefts and/or diversions, and economically or maliciously motivated product tampering on store shelves may occur, causing unexpected shortages, which may have a material impact on our operations.
|
|
•
|
We rely on third parties to source many of our raw materials, as well as to manufacture sterile, injectable products that we distribute. We maintain a strict program of verification and product testing throughout the ingredient sourcing and manufacturing process to identify potential counterfeit ingredients, adulterants, and toxic substances. Nevertheless, discovery of previously unknown problems with the raw materials or product manufacturing processes, or new data suggesting an unacceptable safety risk associated therewith, could result in a voluntary or mandatory withdrawal of the contaminated product from the marketplace, either temporarily or permanently. Any future recall or removal would result in additional costs and lost revenue, harm our reputation, and may give rise to product liability litigation.
|
|
•
|
Changes in regulation could impact the supply of the API and certain other raw materials used in our products. For example, the EU recently promulgated new standards requiring all API imported into the EU be certified as complying with GMP established by the EU. The regulations placed the certification requirement on the regulatory bodies of the exporting countries, which led to an API supply shortage in Europe as certain governments were not willing or able to comply with the regulation in a timely fashion, or at all. A shortage in API or other raw ingredients could cause us to have to cease manufacture of certain products, or to incur costs and delays to qualify other suppliers to substitute for those API manufacturers are unable to export. This could have a material adverse effect on our business, results of operations, financial condition, and cash flow.
|
|
•
|
Breaches or disruptions could impair our ability to develop, meet regulatory approval efforts for, produce, and/or ship products, take and fulfill orders, and/or collect and make payments on a timely basis;
|
|
•
|
Any system issue, whether as a result of an intentional breach or a natural disaster, could damage our reputation and cause us to lose customers, experience lower sales volume, and incur significant liabilities; and
|
|
•
|
We could incur significant expense in addressing a disruption and related data security and privacy concerns.
|
|
•
|
Companies working to develop new therapies or alternative formulations of products for multiple sclerosis that, if successfully developed, would compete with, or could gain greater acceptance than, Tysabri
®
and damage Tysabri
®
’s market share. In February 2016, a competitor's pipeline product, Ocrevus
®
, received breakthrough therapy designation from the FDA, and was approved in 2017. The product is expected to compete with Tysabri
®
and have a significant negative impact on the Tysabri
®
royalty stream;
|
|
•
|
Biogen is the owner of the patents on Tysabri
®
. The loss of protection of these patents, such as a patent invalidation, could adversely affect the royalty stream from Tysabri
®
. In addition, once the Tysabri
®
patents expire, other generic companies may introduce products similar to Tysabri
®
that could adversely affect the royalty stream;
|
|
•
|
Foreign currency movement, which could have a negative impact on Biogen's Tysabri
®
sales, thereby reducing the royalties;
|
|
•
|
Any negative developments relating to Tysabri
®
, such as safety, efficacy, or reimbursement issues, could reduce demand for Tysabri
®
; and
|
|
•
|
Adverse regulatory or legislative developments could limit or prohibit the sale of Tysabri
®
, such as restrictions on the use of Tysabri
®
or safety-related label changes, including enhanced risk management programs, which may significantly reduce expected royalty revenue and require significant expense and management time to address the associated legal and regulatory issues.
|
|
•
|
In April 2016, we announced that our former Chairman and Chief Executive Officer, Joseph C. Papa, resigned from the Company and that John T. Hendrickson, formerly our President, was appointed to serve as our new Chief Executive Officer. Mr. Hendrickson was later appointed to serve as a member of our Board of Directors.
|
|
•
|
In April 2016, we announced that the former Executive Vice President and General Manager of our
CHCI
segment, Marc Coucke, resigned from the Company and that our current Executive Vice President and General Manager, International, Sharon Kochan, would undertake expanded responsibilities that include providing leadership and strategic direction to our
CHCI
segment.
|
|
•
|
On November 8, 2016, we appointed John Wesolowski the General Manager, President
RX
. Mr. Wesolowski served as Acting General Manager,
RX
following the resignation of Doug Boothe on July 20, 2016.
|
|
•
|
On February 27, 2017, we announced that our Executive Vice President, Business Operations and Chief Financial Officer, Judy L. Brown resigned from the Company and Ron Winowiecki, formerly our Senior Vice President, Business Finance, was appointed acting Chief Financial Officer of the Company.
|
|
•
|
On February 27, 2017, we announced the appointment of Svend Andersen to the position of Executive Vice President and President, Consumer Healthcare International.
|
|
•
|
Our products involve risks such as product contamination, spoilage, mislabeling, and tampering that could require us to recall one or more of our products. Serious product quality concerns could also result in governmental actions against us that, among other things, could result in the suspension of production or distribution of our products, product seizures, loss of certain licenses, delays in the issuance of governmental approvals for new products, or other governmental penalties, all of which could be detrimental to our reputation and reduce demand for our products.
|
|
•
|
We cannot guarantee that counterfeiting, imitation or other tampering with our products will not occur or that we will be able to detect and resolve it. Any counterfeiting or contamination of any products could negatively impact our reputation and sales, particularly if counterfeit or imitation products cause death or injury to consumers.
|
|
•
|
Many of the brands we acquired from Omega have European recognition. This recognition is the result of the large investments Omega has made in its products over many years. The quality and safety of the products are critical to our business. If we are unable to effectively manage real or perceived issues,
|
|
•
|
Our
CHCI
segment's financial success is dependent on the success of its brands, and the success of these brands can suffer if marketing plans or product initiatives do not have the desired impact on a brand’s image or its ability to attract consumers and the performance of the segment may be negatively impacted if spending on such plans and initiatives does not generate the returns we anticipate. In addition, given the association of individual products within the commercial network of our
CHCI
segment, an issue with one of our products could negatively affect the reputation of other products, thereby potentially hurting our financial results.
|
|
•
|
Powdered infant formula products are not sterile. All of our infant formula products must be prepared and maintained according to label instruction to retain their flavor and nutritional value and avoid contamination or deterioration. Depending on the product, a risk of contamination or deterioration may exist at each stage of the production cycle, including the purchase and delivery of raw materials, the processing and packaging of food products, and the use and handling by consumers, hospital personnel, and healthcare professionals. In the event that certain of our infant formula products are found or alleged to have suffered contamination or deterioration, whether or not under our control, our reputation and our infant formula product category sales could be materially adversely affected.
|
|
•
|
To the extent that we seek to use social media tools as a means to communicate about our products and/or business, there are uncertainties as to the rules that apply to such communications, or as to the interpretations that authorities will apply to the rules that exist. As a result, despite our efforts to monitor evolving social media communication guidelines and comply with applicable rules, there is risk that our use of social media for such purposes may cause us to be found in violation of them. A violation of such guidelines may damage our reputation as well as cause potential lawsuits and adversely affect our operating activities.
|
|
•
|
Our employees may knowingly or inadvertently make use of social media tools in ways that may not be aligned with our social media strategy, may give rise to liability, or could lead to the loss of trade secrets or other intellectual property, or public exposure of personal information (including sensitive personal information) of our employees, clinical trial patients, customers, and others.
|
|
•
|
Negative posts or comments about us, store brands or generic pharmaceuticals, or our products in social media could seriously damage our reputation and could adversely affect the price of our securities. In addition, negative posts or comments about our products could result in increased pharmacovigilance reporting requirements, which may give rise to liability if we fail to fully comply with such requirements.
|
|
•
|
We have experienced a reduction in pricing expectations during 2016 in comparison to historical patterns in our U.S. businesses, in particular in our
RX
segment, due to industry and competitive pressures in the sector. The reduced pricing is attributable to a variety of factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific product categories, the loss of exclusivity on certain products, the recent increase in the speed and number of approvals from the FDA, and consolidation of certain customers in the
RX
segment. We have seen year-over-year pricing erosion in the second half of 2016 moderate from the levels experienced in the first half of 2016. We expect this pricing environment to continue to impact the Company for the foreseeable future.
|
|
•
|
The
CHCI
segment has been impacted by market dynamics in key countries such as Belgium, France, Germany and Italy due to softness in certain brand categories and by unfavorable foreign currency impacts, primarily in the U.K. related to Brexit. In addition, the segment had been impacted in Belgium by a change in the forecast with a major wholesaler, as management implements improved supply chain efficiencies in this market. The
CHCI
segment has restructured its approach to addressing these markets including: (1) implementing a brand prioritization strategy to address these market dynamics, with an objective to balance the cost of advertising and promotional investments with expected contributions from category sales, (2) restructuring its sales force in each of these markets to more effectively serve customers, and (3) exiting certain unfavorable distribution agreements. The combination of these actions are expected to improve the segment's focus on higher value OTC products, reduce selling costs and improve operating margins in the segment.
|
|
•
|
Difficulty involved with managing the expanded operations of the respective parties, as well as identifying the extent of all weaknesses, risks, and contingent and other liabilities;
|
|
•
|
Uncertainties involved in assessing the value, strengths, and potential profitability of the respective parties, as well as identifying the extent of all weaknesses, risks, and contingent and other liabilities of acquisition targets;
|
|
•
|
Unanticipated changes in the business, industry, market or general economic conditions different from the assumptions underlying our rationale for pursuing the transaction;
|
|
•
|
Difficulties due to a lack of, or limited experience in, any new product or geographic markets we enter;
|
|
•
|
Inability to achieve identified operating and financial synergies, or return on investment, from an acquisition in the amounts or on the time frame anticipated;
|
|
•
|
Substantial demands on our management, operational resources, technology, and financial and internal control systems, which could lead to dissatisfaction and potential loss of key customers, management, or
|
|
•
|
Integration activities that may detract attention from our day-to-day business, and substantial costs associated with the transaction process or other material adverse effects as a result of these integration efforts; and
|
|
•
|
Difficulties, restrictions or increased costs associated with raising future capital in connection with an acquisition may impact our liquidity, credit ratings and financial position, thereby making it more difficult, restrictive or expensive to raise future capital. In addition, the issuance of equity to pay a portion of the purchase price for an acquisition would dilute our existing shareholders.
|
|
•
|
Our success in the European markets in which Omega operates will depend on a number of factors, such as:
|
|
◦
|
Our ability to commercialize new products;
|
|
◦
|
Our ability to adapt to changes in economic and political conditions;
|
|
◦
|
Fluctuations in the value of foreign currencies and interest rates;
|
|
◦
|
Compliance with differing regulatory and legal requirements, including tax laws, trade laws, labor, safety, local content, consumer protection regulation, and import or export licensing requirements; and
|
|
◦
|
Consistency and transparency of foreign tax systems, transfer pricing stability across jurisdictions, and our ability to reinvest earnings and cash as appropriate.
|
|
•
|
While Omega has not historically been subject to U.S. laws and regulations, such as the FCPA, it has been subject to a wide range of European laws and regulations, including the U.K. Bribery Act of 2010. The comparable U.S. laws and regulations to which Omega is now subject may differ from those to which Omega was historically subject. Therefore, it is possible that certain Omega sales or other activities that were permitted while Omega was an independent company may no longer be permitted. While we are continuing to put into place compliance processes and controls intended to ensure compliance with U.S. and global laws that now apply to Omega, if Omega’s operations fail to comply with such laws and regulations, we could be subject to governmental investigations, legal or regulatory proceedings, substantial fines, and/or other legal or equitable penalties.
|
|
•
|
Our ability to effectively transfer liabilities, contracts, facilities and personnel to any purchaser;
|
|
•
|
Fees for legal and transaction-related services;
|
|
•
|
Diversion of management resources; and
|
|
•
|
Loss of key personnel and reduction in revenue.
|
|
•
|
Goodwill impairment charges of
$1.1 billion
related to our Specialty Sciences, BCH-Rest of World ("ROW"), BCH-Belgium, and Animal Health Reporting Units.
|
|
•
|
Indefinite-lived and definite-lived intangible asset impairment charges of
$1.5 billion
related to: trademarks, trade names and brands; developed product technology/formulation and product rights; distribution and license agreements; and supply agreements.
|
|
•
|
Unexpected changes in regulatory requirements;
|
|
•
|
Problems related to markets with different cultural biases or political systems;
|
|
•
|
Possible difficulties in enforcing agreements;
|
|
•
|
Longer payment cycles and shipping lead-times;
|
|
•
|
Difficulties obtaining export or import licenses;
|
|
•
|
Changes to U.S. and foreign trade policies, including the enactment of tariffs on goods imported into the U.S., including but not limited to, goods imported from Mexico; and
|
|
•
|
Imposition of withholding or other taxes.
|
|
•
|
Several emerging market economies are particularly vulnerable to the impact of rising interest rates, inflationary pressures, weaker oil and other commodity prices, and large external deficits. While some of these jurisdictions are showing signs of stabilization or recovery, others, such as Ukraine, Russia and Greece, continue to experience levels of stress and volatility. Risks in one country can limit our opportunities for portfolio growth and negatively affect our operations in another country or countries. As a result, any such unfavorable conditions or developments could have an adverse impact on our operations.
|
|
•
|
While the challenging global economic environment has not had a material impact on our liquidity or capital resources, there can be no assurance that possible future changes in global financial markets and global
|
|
•
|
The challenging economic conditions have also impacted the movements in exchange rates, which have experienced significant recent volatility, including the unfavorable foreign currency impact on the British pound due to Brexit. Uncertainty regarding the future growth rates between countries, the influence of central bank actions, and the changing political environment globally may contribute to continued high levels of exchange rate volatility, which could have an adverse impact on our results.
|
|
•
|
Our customers could be adversely impacted if economic conditions worsen. Our
CHCA
segment does not advertise its products like national brand companies and thus is largely dependent on retailer promotional activities to drive sales volume and increase market share.
If our customers do not have the ability to invest in store brand promotional activities, our sales may suffer. Additionally, while we actively review the credit worthiness of our customers and suppliers, we cannot fully predict to what extent they may be negatively impacted by slowing economic growth.
|
|
•
|
Certain countries and international organizations have refused to do business with companies with Israeli operations. We are also precluded from marketing our products to certain countries due to U.S. and Israeli regulatory restrictions. International economic sanctions and boycotts of our products could negatively impact our sales and ability to export our products.
|
|
•
|
Our facilities in Israel are within a conflict zone. If terrorist acts or military actions were to result in substantial damage to our facilities, our business activities would be disrupted since, with respect to most products, we would need to obtain prior regulatory agency approval for a change in manufacturing site. In addition, our insurance may not adequately compensate us for losses that may occur, and any losses or damages incurred by us could have a material adverse effect on our business.
|
|
•
|
The U.S. Department of State and other governments have at times issued advisories regarding travel to certain countries in which we do business. As a result, regulatory agencies have at various times curtailed or prohibited their inspectors from traveling to inspect facilities. If these inspectors are unable to inspect our facilities, the regulatory agencies could withhold approval for new products intended to be produced at those facilities.
|
|
•
|
Our international operations may be subject to interruption due to travel restrictions, war, terrorist acts, and other armed conflicts. For example, Belgium, France, Turkey, and Eastern Europe may be exposed to further acts of terrorism, which could give rise to travel and increased security restrictions. Also, further threats of armed hostilities in Mexico could limit or disrupt markets and our operations, including disruptions resulting from the cancellation of contracts or the loss of assets. These events could have a material adverse effect on our international business operations.
|
|
•
|
The UK held a referendum on June 23, 2016 on its membership in the EU. A majority of UK voters voted to exit the EU (“Brexit”), and negotiations will commence to determine the future terms of the UK’s relationship with the EU, subject to a negotiation period that could last up to two years after the UK government formally initiates the withdraw process, including the terms of trade between the UK and the EU. Brexit has created significant instability and volatility in the global financial markets, has led to significant weakening of the British pound compared to the U.S. dollar and other currencies, and could adversely affect European or worldwide economic or market conditions. Although it is unknown what those terms will be, they may impair the ability of our operations in the EU to transact business in the future in the UK, and similarly the ability of our UK operations to transact business in the future in the EU. Specifically, it is possible that there will be greater restrictions on imports and exports between the UK and EU countries and increased regulatory complexities. These changes may adversely affect our operations and financial results. In addition, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the UK determines which EU laws to replace or replicate. Further, among other things, Brexit could reduce consumer spending in the UK and the EU, which could result in decreased demand for our products. Any of these effects of Brexit, and others we cannot anticipate, could adversely affect our business, business opportunities, results of operations, financial condition and cash flows.
|
|
•
|
We may be subject to liability if our products violate applicable laws or regulations in the jurisdictions where our products are distributed. The successful assertion of product liability or other product-related claims against us could result in potentially significant monetary damages, and we could incur substantial legal expenses. Even if a product liability or consumer fraud claim is unsuccessful, not merited, or not fully pursued, we may still incur substantial legal expenses defending against such a claim, and our reputation may suffer.
|
|
•
|
We are a defendant in product liability lawsuits arising out of serious adverse events, including deaths, which occurred in patients taking Tysabri
®
. We expect additional product liability lawsuits related to Tysabri
®
usage to be filed. Tysabri
®
's distributor, Biogen, and Perrigo will each be responsible for 50% of losses and expenses arising out of any Tysabri
®
product liability claims. Along with Biogen, we intend to vigorously defend these lawsuits, however, we cannot predict how these cases will be resolved. Adverse results in one or more of these cases could result in substantial monetary judgments not covered by insurance.
|
|
•
|
We may face environmental exposures including, for example, those relating to discharges from and materials handled as part of our operations, the remediation of soil and groundwater contaminated by hazardous substances or wastes, and the health and safety of our employees. While we do not have any material remediation liabilities currently outstanding, we may in the future face liability for the costs of investigation, removal or remediation of certain hazardous substances or petroleum products on, under or in our currently or formerly owned property, or from a third-party disposal facility that we may have used, without regard to whether we knew of, or caused, the presence of the contaminants. The actual or alleged presence of these substances, or the failure to remediate them, could have adverse effects, including, for example, substantial investigative or remedial obligations and limitations on our ability to sell or rent affected property or to borrow funds using affected property as collateral. There can be no assurance that environmental liabilities and costs will not have a material adverse effect on us. See
Item 1. Business - Information Applicable to All Reportable Segments - Environmental
for more information.
|
|
•
|
Our
CHCI
segment regularly makes advertising claims regarding the effectiveness of its products, which we are responsible for defending. An unsuccessful defense of product-related claims could result in potentially significant monetary damages and substantial legal expenses. Even if a claim is unsuccessful, not merited, or not fully pursued, we may still incur substantial legal expenses defending against such a claim, and our reputation could suffer.
|
|
•
|
We are a defendant in a securities class action lawsuit filed in the US District Court for the District of New Jersey in which the complaint alleges violations of Securities Exchange Act sections 10(b) (and Rule 10b‑5) and 14(e) against both Perrigo and our former Chief Executive Officer, Joseph C. Papa, and 20(a) control person liability against Mr. Papa. In general, the allegations concern the actions taken by the Company and the former executive to defend against the hostile takeover bid by Mylan in the period April 21, 2015 through November 13, 2015. The plaintiff also alleges that we provided inadequate disclosure concerning alleged integration problems as a result of the Omega acquisition in the period April 21, 2015 through May 11, 2016. Another securities class action case was also filed in the same court making essentially the same claims on behalf of a class of persons who sold put options in Perrigo shares during the same class period. In December 2016, the court consolidated the two actions. In February 2017, the court selected the lead plaintiffs and the lead counsel to the putative class.
In March 2017, the court entered a scheduling order that sets a deadline for the lead plaintiffs to file an amended complaint. That deadline has not yet passed.
|
|
•
|
We along with certain of our current and former executive officers and board members are also defendants in a securities class action suit where the plaintiff alleges violations of Israeli law in the District Court of Tel Aviv-Jaffa
.
On June 15, 2016, we filed a motion to stay the case pending the outcome of the U.S. securities class actions. The plaintiffs did not oppose the motion. The Israeli court granted the motion on the same day, and the action is stayed.
|
|
•
|
We are a defendant in a securities claims which the complaint commenced an action in the District Court of Tel Aviv-Jaffa asserting securities claims against Perrigo and our auditor Ernst & Young LLP ("EY"). The case is styled
Keinan v. Perrigo Company plc, et al.
The action seeks certification of a class of purchasers of Perrigo shares on the Israeli exchange beginning February 6, 2014. The proposed closing date for the class is not clear. In general, the plaintiff asserts that Perrigo improperly accounted for its stream of royalty income from two drugs: Tysabri
®
and Prialt. The court filings contend that the alleged improper accounting caused the audited financial results for Perrigo to be incorrect for the year ended December 31, 2016, six months ended December 31, 2015, and the years ended June 27, 2015 and June 28, 2014 and the other financial data released by the Company over those years to also be inaccurate. The plaintiff maintains that the defendants are liable under Israeli securities law or, in the alternative, under U.S. securities law principles. The plaintiff indicates an initial, preliminary class damages estimate of
686.0 million
NIS (approximately
$187.0 million
). The response from Perrigo and EY is not yet due. Perrigo is consulting Israeli counsel about its response to these allegations.
|
|
•
|
As a manufacturer of generic pharmaceutical products, the ability of our
CHCA
and
RX
segments to bring new products to market is often limited by third-party patents or proprietary rights and regulatory exclusivity periods awarded on products. Launching new products prior to resolution of intellectual property issues may result in us incurring legal liability if the related litigation is later resolved against us. The cost and time for us to develop prescription and Rx-to-OTC switch products is significantly greater than the rest of the new products that we introduce. Any failure to bring new products to market in a timely manner could cause us to lose market share, and our operating results could suffer.
|
|
•
|
We could have to defend against charges that we violated patents or proprietary rights of third parties. This could require us to incur substantial expense and could divert significant effort of our technical and
|
|
•
|
At times, our
CHCA
or
RX
segments may seek approval to market drug products before the expiration of patents for those products, based upon our belief that such patents are invalid, unenforceable or would not be infringed by our products. In these cases we may face significant patent litigation. Depending upon a complex analysis of a variety of legal and commercial factors, we may, in certain circumstances, elect to market a generic pharmaceutical product while litigation is pending, before any court decision, or while an appeal of a lower court decision is pending, known as an "at risk" launch. The risk involved in an "at risk" launch can be substantial because, if a patent holder ultimately prevails, the remedies available to the patent holder may include, among other things, damages measured by the profits lost by the holder, which are often significantly higher than the profits we make from selling the generic version of the product. By electing to proceed in this manner, we could face substantial damages if we receive an adverse final court decision. In the case where a patent holder is able to prove that our infringement was "willful" or "exceptional," under applicable law, the patent holder may be awarded up to three times the amount of its actual damages or we may be required to pay attorneys’ fees.
|
|
•
|
We have been issued patents covering certain of our products, and we have filed, and expect to continue to file, patent applications seeking to protect newly developed technologies and products in various countries. Any existing or future patents issued to or licensed by us may not provide us with any significant competitive advantages for our products or may even be challenged, invalidated, or circumvented by competitors. In addition, patent rights may not prevent our competitors from developing, using, or commercializing non-infringing products that are similar or functionally equivalent to our products.
|
|
•
|
We also rely on trade secrets, unpatented proprietary know-how, and continuing technological innovation that we seek to protect, in part by confidentiality agreements with licensees, suppliers, employees, and consultants. If these agreements are breached, we may not have adequate remedies for any such breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Furthermore, trade secrets and proprietary technology may otherwise become known or be independently developed by competitors or, if patents are not issued with respect to products arising from research, we may not be able to maintain the value of such intellectual property rights.
|
|
•
|
Insurance costs could increase significantly, or the availability of insurance may decrease, either of which could adversely impact our financial condition;
|
|
•
|
Deductible or retention amounts could increase or our coverage could be reduced in the future and to the extent losses occur, there could be an adverse effect on our financial results depending on the nature of the loss and the level of insurance coverage we maintained;
|
|
•
|
Product liability insurance may not be available to us at an economically reasonable cost (or at all for certain specific products) or our insurance may not adequately cover our liability in connection with product liability claims (see
Item 8. Note 16
for further information related to legal proceedings); and
|
|
•
|
As our business inherently exposes us to claims for injuries allegedly resulting from the use of our products, we may become subject to claims for which we are not adequately insured. Unanticipated payment of a large claim may have a material adverse effect on our business.
|
|
•
|
Changes to the inversion rules in section 7874 of the Code, the IRS Treasury regulations promulgated thereunder, or other IRS guidance; and
|
|
•
|
Legislative proposals aimed at expanding the scope of U.S. corporate tax residence.
|
|
•
|
Income tax rate changes by governments;
|
|
•
|
The jurisdictions in which our profits are determined to be earned and taxed;
|
|
•
|
Changes in the valuation of our deferred tax assets and liabilities;
|
|
•
|
Adjustments to estimated taxes upon finalization of various tax returns;
|
|
•
|
Adjustments to our interpretation of transfer pricing standards, changes in available tax credits, grants and other incentives;
|
|
•
|
Changes in stock-based compensation expense; changes in tax laws or the interpretation of such tax laws (such as proposals for fundamental U.S. international tax reform);
|
|
•
|
Changes in U.S. generally accepted accounting principles;
|
|
•
|
Expiration or the inability to renew tax rulings or tax holiday incentives;
|
|
•
|
Divestitures of current operations; and
|
|
•
|
Repatriation of non-U.S. earnings with respect to which we have not previously provided for U.S. taxes.
|
|
•
|
In the United States, the Internal Revenue Service ("IRS") audit of our fiscal years ended June 27, 2009 and June 26, 2010 had previously concluded with the issuance of a statutory notice of deficiency on August 27, 2014. While we had previously agreed on certain adjustments and made associated payments of $8.0 million (inclusive of interest) in November 2014, the statutory notice of deficiency asserted various additional adjustments, including transfer pricing adjustments. The statutory notice of deficiency's adjustments for fiscal years 2009 and 2010 asserted an incremental tax obligation of approximately $68.9 million, inclusive of interest and penalties. We disagree with the IRS’s positions asserted in the statutory notice of deficiency. To contest the IRS's adjustments, in January 2015 we paid the incremental tax obligation (a prerequisite to contesting the proposed adjustments in U.S. district court), and in June 2015, we filed an administrative request for a refund with the IRS. The IRS subsequently denied our request for a refund. We anticipate filing a complaint in U.S. district court claiming a refund of the paid amounts prior to August 2017. An unfavorable resolution of this matter could have a material impact on our consolidated financial statements in future periods.
|
|
•
|
The IRS issued a statutory notice of deficiency on April 20, 2017 for the IRS audits of our fiscal years ended June 25, 2011 and June 30, 2012. While we agreed to certain adjustments in October 2016 and made minimal associated payments, the statutory notice of deficiency asserted various additional adjustments, including transfer pricing adjustments. The statutory notice of deficiency for fiscal years 2011 and 2012 asserted an incremental tax obligation of approximately
$74.2 million
, inclusive of interest and penalties. We disagree with the IRS's positions asserted in this notice. In anticipation of contesting the IRS's adjustments, in May 2017 we paid the incremental tax obligation (a prerequisite to contesting the proposed adjustments in U.S. district court) and expect to file an administrative request for refund.
|
|
•
|
We received notices of proposed adjustments on December 22, 2016 for the IRS audit of Athena Neurosciences, Inc., a subsidiary of Elan Corporation plc, which Perrigo acquired in December 2013,
|
|
•
|
Our senior credit facilities, the agreements governing our senior notes, and agreements governing our other indebtedness contain a number of restrictions and covenants that limit our ability to make distributions or other payments to our investors and creditors unless certain financial tests or other criteria are satisfied.
|
|
•
|
We also must comply with certain specified financial ratios and tests. These restrictions could affect our ability to operate our business and may limit our ability to take advantage of potential business opportunities, such as acquisitions. If we do not comply with the covenants and restrictions contained in our senior credit facilities, agreements governing our senior notes, and agreements governing our other indebtedness, we could be in default under those agreements, and the debt, together with accrued interest, could then be declared immediately due and payable.
|
|
•
|
While we have announced our intention, consistent with our investment grade philosophy, to pay down a portion of our outstanding indebtedness during 2017 using the proceeds from our sale of the Tysabri
®
royalty stream and residual free cash flow, we cannot provide assurance that our debt pay down strategy will occur on the proposed time line, or at all, or that we will maintain our investment grade rating. Further, if our debt pay down strategy is delayed, we may be required to obtain further amendments or waivers of our financial covenants under our 2014 Revolver and 2014 Term Loan.
|
|
•
|
Any default under our senior credit facilities or agreements governing our senior notes or other indebtedness could lead to an acceleration of debt under other debt instruments that contain cross-acceleration or cross-default provisions. If our indebtedness is accelerated, there can be no assurance that we would be able to repay or refinance our debt or obtain sufficient new financing.
|
|
•
|
Downgrades to our credit ratings may limit our access to capital and materially increase borrowing costs on current or future financing, including via trade payables with vendors. Customers' inclination to purchase goods from us may also be affected by the publicity associated with deterioration of our credit ratings.
|
|
•
|
There are various maturity dates associated with our credit facilities, senior notes, and other debt facilities. There is no assurance that cash, future borrowings or equity financing will be available for the payment or refinancing of our indebtedness. Further, there is no assurance that future refinancing or renegotiation of our senior credit facilities, senior notes or other debt facilities, or additional agreements will not have materially different or more stringent terms. See
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
.
|
|
•
|
Under Irish law, our authorized share capital can be increased by an ordinary resolution of our shareholders, and the directors may issue new ordinary or preferred shares up to a maximum amount equal to the authorized but unissued share capital, without shareholder approval, once authorized to do so by the articles of association or by an ordinary resolution of our shareholders.
|
|
•
|
Subject to specified exceptions, Irish law grants statutory preemption rights to existing shareholders to subscribe for new issuances of shares for cash, but allows shareholders to authorize the waiver of the statutory preemption rights by way of special resolution with respect to any particular allotment of shares.
|
|
•
|
Our articles of association contain, as permitted by Irish company law, a provision authorizing the board to issue new shares for cash without offering preemption rights. The authorization of the directors to issue shares and the authorization of the waiver of the statutory preemption rights must both be renewed by the shareholders at least every five years, and we cannot provide any assurance that these authorizations will always be approved, which could limit our ability to issue equity and thereby adversely affect the holders of our securities.
|
|
•
|
Under Irish law, the duties of directors and officers of a company are generally owed to the company only. As a result, shareholders of Irish companies do not have the right to bring an action against the directors or officers of a company, except in limited circumstances.
|
|
•
|
Depending on the circumstances, shareholders may be subject to different or additional tax consequences under Irish law as a result of the acquisition, ownership and/or disposition of ordinary shares, including, but not limited to, Irish stamp duty, dividend withholding tax, and capital acquisitions tax.
|
|
•
|
There is no treaty between Ireland and the U.S. providing for the reciprocal enforcement of foreign judgments. Before a foreign judgment would be deemed enforceable in Ireland, the judgment must be provided by a court of competent jurisdiction and be for a final and conclusive sum. An Irish court may exercise its right to refuse to recognize and enforce a foreign judgment if the foreign judgment was obtained by fraud, if it violated Irish public policy, if it is in breach of natural justice, or if it is irreconcilable with an earlier judgment.
|
|
•
|
An Irish court may stay proceedings if concurrent proceedings are being brought elsewhere. Judgments of U.S. courts of liabilities predicated upon U.S. federal securities laws may not be enforced by Irish courts if deemed to be contrary to public policy in Ireland.
|
|
•
|
The availability of distributable reserves, as approved by our shareholders and the Irish High Court;
|
|
•
|
Our ability to receive cash dividends and distributions from our subsidiaries;
|
|
•
|
Compliance with applicable laws and debt covenants; and
|
|
•
|
Our financial condition, results of operations, capital requirements, general business conditions, and other factors that our Board of Directors may deem relevant.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
Country
|
Number of Facilities
|
Segment(s) Supported
|
||
|
Ireland
|
1
|
CHCA, CHCI, RX, Specialty Sciences
|
||
|
United States
|
46
|
CHCA, RX, Other
|
||
|
Mexico
|
12
|
CHCA
|
||
|
United Kingdom
|
7
|
CHCI
|
||
|
India
|
7
|
Other
|
||
|
France
|
6
|
CHCI
|
||
|
Belgium
|
5
|
CHCI
|
||
|
Australia
|
4
|
CHCI
|
||
|
Israel
|
3
|
CHCA, CHCI, RX, Other
|
||
|
Austria
|
3
|
CHCI
|
||
|
Germany
|
2
|
CHCI
|
||
|
Switzerland
|
2
|
CHCI
|
||
|
Italy
|
2
|
CHCI
|
||
|
Portugal
|
1
|
CHCI
|
||
|
Russia
|
1
|
CHCI
|
||
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
Title and Business Experience
|
Age
|
|||
|
Svend Andersen
|
Mr. Andersen was named Executive Vice President and President, Consumer Healthcare International in February 2017. Prior to joining Perrigo, Mr. Andersen served as Executive Vice President – Europe for LEO-Pharma. Prior to that, he led Hospira, Inc.’s Europe, Middle East and Africa (“EMEA”) business, was responsible for the Western European division’s pharmaceuticals, generics, OTC and hospital products businesses at Actavis, and also led Alpharma’s EMEA businesses prior to its acquisition by Actavis.
|
55
|
||
|
Thomas M. Farrington
|
Mr. Farrington was named Executive Vice President and Chief Information Officer in November 2015. He formerly served as Senior Vice President and Chief Information Officer from October 2006 to November 2015.
|
59
|
||
|
John T. Hendrickson
|
Mr. Hendrickson was appointed Chief Executive Officer in April 2016 and to the Board of Directors in June 2016. He served as President from October 2015 until April 2016. He was formerly Executive Vice President, Global Operations & Supply Chain of Perrigo Company from March 2007 to October 2015; Executive Vice President and General Manager of Perrigo Consumer Healthcare from 2003 to 2007; Executive Vice President – Operations from 1999 to 2003; Vice President of Manufacturing from 1996 to 1999; Vice President of Customer Service from 1995 to 1996; Director of Engineering from 1993 to 1995; and Process Engineering Manager from 1989 to 1993.
|
54
|
||
|
Ronald Janish
|
Mr. Janish was named Executive Vice President of Global Operations and Supply Chain in October 2015. He served as Senior Vice President of International and Rx Operations from 2012 until 2015 and as Managing Director of Perrigo’s Australian operations from 2010 to 2012. Previously, he held Senior Vice President roles for Perrigo in International Market Development, China Business Development and Global Procurement.
|
51
|
||
|
Todd W. Kingma
|
Mr. Kingma was named Executive Vice President, General Counsel and Secretary in May 2006. He served as Vice President, General Counsel and Secretary from August 2003 to May 2006.
|
57
|
||
|
Sharon Kochan
|
Mr. Kochan was named Executive Vice President and General Manager, Consumer Healthcare International in August 2012. He served as Executive Vice President, General Manager of Prescription Pharmaceuticals from March 2007 to July 2012 and as Senior Vice President of Business Development and Strategy from March 2005 to March 2007. Mr. Kochan was Vice President, Business Development of Agis Industries (1983) Ltd. from July 2001 until the acquisition of Agis by the Company in March 2005.
|
48
|
||
|
James R. Michaud
|
Mr. Michaud was named Executive Vice President, Chief Human Resources Officer in August 2016. Immediately prior to joining Perrigo, Mr. Michaud was President of Human Resources Strategies, a consulting company focused on providing business based human resource strategies to a wide variety of companies in multiple industries. His corporate career spanned senior human resource roles in Alcoa, Arcelor Mittal Steel, and most recently, Cliffs Natural Resources, where he served as Executive Vice President, Chief Human Resources Officer from 2010 to 2014.
|
61
|
||
|
Jeffrey R. Needham
|
Mr. Needham was named Executive Vice President, General Manager of Consumer Healthcare Americas in October 2009. He served as Senior Vice President of Commercial Business Development for Consumer Healthcare from March 2005 through October 2009. Previously, he served as Senior Vice President of International from November 2004 to March 2005. He served as Managing Director of Perrigo’s U.K. operations from May 2002 to November 2004 and as Vice President of Marketing from 1993 to 2002.
|
60
|
||
|
Grainne Quinn
|
Ms. Quinn was named Executive Vice President in July 2016 and has served as Chief Medical Officer since November 2015. Prior to that she served as Vice President and Head of Global Patient Safety from January 2014 until November 2015. Dr. Quinn was Vice President and Head of Global Pharmacovigilance and Risk Management for Elan from April 2009 until December 2013 when the Company acquired Elan.
|
47
|
||
|
Paul Weninger
|
Mr. Weninger was named Executive Vice President of Global Quality Operations in December 2015. He served as Senior Vice President, U.S. Quality Operations from 2013 to 2015; Vice President, Consumer Healthcare and Rx Quality Operations, U.S. and Asia Pacific from 2010 to 2013; Vice President, Global CHC Quality Operations from 2007 to 2010.
|
53
|
||
|
John Wesolowski
|
Mr. Wesolowski was named Executive Vice President, President RX in November 2016. He previously was named as Acting General Manager, RX, in July 2016 and served in that capacity until November 2016. Previously, he served as Senior Vice President of RX Commercial Operations, from 2013 until July 2016. Mr. Wesolowski joined Perrigo in February 2004 as the Vice President, RX Sales and Marketing and was subsequently promoted to the Senior Vice President of RX Sales and Marketing in 2012.
|
49
|
||
|
Ronald L. Winowiecki
|
Mr. Winowiecki was appointed Acting CFO in February 2017. He served as Senior Vice President of Business Finance at Perrigo since January 2014. Before serving as SVP, Mr. Winowiecki was Perrigo’s Vice President for Treasury and Accounting Shared Services from September 2011 to December 2013 and the company’s Corporate Vice President Treasurer from October 2008 to August 2011.
|
50
|
||
|
ITEM 5.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||||||
|
|
December 31, 2016
|
December 31, 2015
|
June 27, 2015
|
||||||||||||||||||||
|
High
|
Low
|
High
|
Low
|
High
|
Low
|
||||||||||||||||||
|
First quarter
|
$
|
152.36
|
|
$
|
122.62
|
|
$
|
198.42
|
|
$
|
158.35
|
|
$
|
160.65
|
|
$
|
135.00
|
|
|||||
|
Second quarter
|
$
|
133.53
|
|
$
|
84.85
|
|
$
|
167.92
|
|
$
|
140.40
|
|
$
|
171.57
|
|
$
|
142.38
|
|
|||||
|
Third quarter
|
$
|
99.14
|
|
$
|
82.50
|
|
N/A
|
|
N/A
|
|
$
|
174.65
|
|
$
|
147.21
|
|
|||||||
|
Fourth quarter
|
$
|
97.17
|
|
$
|
79.72
|
|
N/A
|
|
N/A
|
|
$
|
205.72
|
|
$
|
161.86
|
|
|||||||
|
12/31/2011
|
12/31/2012
|
12/31/2013
|
12/31/2014
|
12/31/2015
|
12/31/2016
|
|
|
Perrigo Company plc
|
$100.00
|
$107.25
|
$158.67
|
$173.32
|
$150.48
|
$87.07
|
|
S&P 500
|
$100.00
|
$116.00
|
$153.58
|
$174.60
|
$177.01
|
$198.18
|
|
S&P Pharmaceuticals
|
$100.00
|
$114.43
|
$154.74
|
$189.12
|
$200.06
|
$196.93
|
|
*
|
$100 invested on December 31, 2011 in stock or index - including reinvestment of dividends. Indexes calculated on month-end basis.
|
|
**
|
Perrigo Company prior to December 18, 2013. Perrigo Company plc beginning December 18, 2013.
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||||||||||
|
December 31, 2016
(1)
|
December 31, 2015
(2)
|
December 27, 2014
(3)
|
June 27, 2015
(4)
|
June 28, 2014
(5)
|
June 29, 2013
(6)
|
June 30, 2012
(7)
|
|||||||||||||||||||||
|
(in millions, except per share amounts)
|
Restated
|
Restated
|
Restated
|
Restated
|
|||||||||||||||||||||||
|
Statements of Operations Data
|
|||||||||||||||||||||||||||
|
Net sales
|
$
|
5,280.6
|
|
$
|
2,632.2
|
|
$
|
1,844.7
|
|
$
|
4,227.1
|
|
$
|
3,914.1
|
|
$
|
3,539.8
|
|
$
|
3,173.2
|
|
||||||
|
Cost of sales
|
3,228.8
|
|
1,553.3
|
|
1,170.9
|
|
2,582.9
|
|
2,462.0
|
|
2,259.8
|
|
2,077.7
|
|
|||||||||||||
|
Gross profit
|
2,051.8
|
|
1,078.9
|
|
673.8
|
|
1,644.2
|
|
1,452.1
|
|
1,280.0
|
|
1,095.6
|
|
|||||||||||||
|
Operating expenses
|
4,051.5
|
|
1,011.3
|
|
384.1
|
|
971.7
|
|
880.7
|
|
600.9
|
|
526.4
|
|
|||||||||||||
|
Operating income (loss)
|
$
|
(1,999.7
|
)
|
$
|
67.6
|
|
$
|
289.7
|
|
$
|
672.5
|
|
$
|
571.4
|
|
$
|
679.1
|
|
$
|
569.2
|
|
||||||
|
Net income (loss)
|
$
|
(4,012.8
|
)
|
$
|
42.5
|
|
$
|
180.6
|
|
$
|
136.1
|
|
$
|
232.8
|
|
$
|
441.9
|
|
$
|
401.6
|
|
||||||
|
Diluted income from continuing operations per share
|
$
|
(28.01
|
)
|
$
|
0.29
|
|
$
|
1.34
|
|
$
|
0.97
|
|
$
|
2.01
|
|
$
|
4.68
|
|
$
|
4.18
|
|
||||||
|
Dividends declared per share
|
$
|
0.58
|
|
$
|
0.25
|
|
$
|
0.21
|
|
$
|
0.46
|
|
$
|
0.39
|
|
$
|
0.35
|
|
$
|
0.31
|
|
||||||
|
(1)
|
Includes the results of operations for assets acquired from Barr Laboratories, Inc. and assets acquired from Matawan Pharmaceuticals, LLC for the five months and eleven months and one week ended
December 31, 2016
, respectively.
|
|
(2)
|
Includes the results of operations of Naturwohl and the GSK, ScarAway
®
, and Entocort
®
asset acquisitions for the two and a half months, three months, three months, and two weeks ended
December 31, 2015
, respectively.
|
|
(3)
|
Includes the results of operations for assets acquired from Lumara Health, Inc. for the two months ended
December 27, 2014
.
|
|
(4)
|
Includes the results of operations for assets acquired from Lumara Health, Inc. and the results of operations of Omega Pharma Invest N.V. and Gelcaps Exportadora de Mexico, S.A. de C.V. for the eight, three, and two months ended
June 27, 2015
, respectively.
|
|
(5)
|
Includes the results of operations for Elan Corporation, plc and results of operations for assets acquired from Fera Pharmaceuticals, LLC (Methazolomide) and Aspen Global Inc. for the six, five and four months ended
June 28, 2014
, respectively.
|
|
(6)
|
Includes the results of operations for assets acquired from Fera Pharmaceuticals, LLC, and results of operations for Velcera, Inc., Rosemont Pharmaceuticals Ltd., Cobrek Pharmaceuticals, Inc., and Sergeant's Pet Care Products, Inc. for the two weeks, and three, five, six and nine months ended
June 29, 2013
, respectively.
|
|
(7)
|
Includes the results of operations for Paddock Laboratories,Inc. and CanAm Care, LLC for the eleven and six months ended June 30, 2012, respectively.
|
|
December 31, 2016
|
December 31, 2015
|
December 27, 2014
|
June 27,
2015 |
June 28, 2014
(1)
|
June 29, 2013
(1)
|
June 30, 2012
(1)
|
|||||||||||||||||||||
|
(in millions)
|
Restated
|
Restated
|
Restated
|
Restated
|
|||||||||||||||||||||||
|
Balance Sheet Data
|
|||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
622.3
|
|
$
|
417.8
|
|
$
|
3,596.1
|
|
$
|
785.6
|
|
$
|
799.5
|
|
$
|
779.9
|
|
$
|
602.5
|
|
||||||
|
Total assets
|
13,870.1
|
|
19,349.6
|
|
16,508.4
|
|
19,591.9
|
|
13,879.1
|
|
5,336.9
|
|
4,013.6
|
|
|||||||||||||
|
Long-term debt, less current portion
|
5,224.5
|
|
4,971.6
|
|
4,439.4
|
|
5,246.9
|
|
3,063.1
|
|
1,927.8
|
|
1,329.2
|
|
|||||||||||||
|
(1)
|
Financial data has been retrospectively adjusted for the change in accounting policy to reclassify deferred financing fees from Other non-current assets to Long-term debt.
|
|
ITEM 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
Consumer Healthcare Americas
("
CHCA
")
,
comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
Consumer Healthcare International
("
CHCI
")
,
comprises our legacy Branded Consumer Healthcare segment and now includes our consumer focused businesses in the U.K., Australia, and Israel, which were previously reported in the legacy Consumer Healthcare segment. This segment includes our U.K. liquid licensed products business, which was previously reported in the Prescription Pharmaceuticals segment.
|
|
•
|
Prescription Pharmaceuticals
("
RX
")
,
comprises our U.S. Prescription Pharmaceuticals business.
|
|
•
|
Specialty Sciences
, continued to comprise the Tysabri
®
Royalty Stream.
|
|
•
|
High quality;
|
|
•
|
Superior customer service;
|
|
•
|
Leading innovation;
|
|
•
|
Best cost; and
|
|
•
|
Empowered people.
|
|
•
|
Leadership in first-to-market product development and product life cycle management;
|
|
•
|
Turn-key regulatory, and promotional capabilities;
|
|
•
|
Management of supply chain complexity and utilizing economies of scale;
|
|
•
|
Quality and cost effectiveness throughout the supply chain creating a sustainable, low-cost network; and
|
|
•
|
Expansive pan-European commercial infrastructure, brand-building capabilities, and diverse product portfolio.
|
|
•
|
Consistent with previously announced actions, we added a number of positions and processes to our Dublin headquarters across a range of corporate functions, including supply chain/global operations, procurement, enterprise risk management, and corporate finance, leveraging the strength of our global platform.
|
|
•
|
We repaid
$500.0 million
outstanding under our 1.300% Senior Notes due 2016 ("1.300% 2016 Notes") on September 29, 2016.
|
|
•
|
We completed the sale of our U.S. Vitamins, Minerals, and Supplements ("VMS") business to International Vitamins Corporation ("IVC") on August 5, 2016.
|
|
•
|
On November 13, 2015, our shareholders rejected an unsolicited tender offer from Mylan N.V. ("Mylan"). During the
six months ended
December 31, 2015
, the total cost to effectively defend against Mylan was
$86.9 million
, which was recorded in
Administration
expense.
|
|
•
|
We expanded our product offerings through targeted acquisitions including:
|
|
•
|
The announced acquisition of
a portfolio of generic dosage forms and strengths of
Retin-A
®
(tretinoin), a topical prescription acne treatment, from Matawan Pharmaceuticals, LLC, which closed in January 2016 and will expand our "prescription only" ("Rx") portfolio.
|
|
•
|
The acquisition of Crohn's disease treatment Entocort
®
(budesonide) capsules and its authorized generic (for sale within the U.S.), from AstraZeneca plc, which expanded our Rx portfolio.
|
|
•
|
The acquisition of Naturwohl Pharma GmbH ("Naturwohl"), a nutritional business known for its leading German dietary supplement brand, Yokebe
®
, and the acquisition of a portfolio of well-established OTC brands, such as Niquitin
®
and Coldrex
®
,
from GlaxoSmithKline Consumer Healthcare (“GSK”). Both of these acquisitions built upon the global platform we established through the Omega Pharma Invest N.V. (
"
Omega") acquisition, leveraging our European market share and expanding our product offerings.
|
|
•
|
The ScarAway
®
brand portfolio acquisition, which served as our entry into the branded OTC business in the U.S.
|
|
•
|
We launched a number of new products across our segments with sales totaling
$231.1 million
for the
six months ended
December 31, 2015
.
|
|
•
|
We repurchased $500.0 million shares as part of our authorized share repurchase plan.
|
|
•
|
We executed initiatives designed to increase operational efficiency and improve our return on invested capital by globalizing our supply chain through global shared service arrangements, streamlining our organizational structure, and disposing of certain assets. During the
six months ended
December 31, 2015
, restructuring charges totaled
$26.9 million
.
|
|
•
|
We realized growth in the following areas:
|
|
•
|
Total net sales of
$4.2 billion
due primarily to current year acquisitions and new products;
|
|
•
|
Gross profit percentage of
38.9%
; and
|
|
•
|
Operating cash flows of
$855.2 million
.
|
|
•
|
We significantly expanded our geographic footprint and product portfolio through the acquisition of Omega, one of Europe's largest healthcare companies, which closed on
March 30, 2015
.
|
|
•
|
The Omega acquisition provided us with a significantly larger product portfolio, broadened our global reach through access to 34 new countries, and enhanced our scale.
|
|
•
|
We expanded our product offerings through targeted acquisitions including:
|
|
•
|
The Lumara Health Inc. ("Lumara") product acquisition, which expanded our women's health offerings within our
RX
segment; and
|
|
•
|
Patheon Inc.'s Mexican operations, Gelcaps Exportadora de Mexico, S.A. de C.V., ("Gelcaps"), which provided us with gelcap manufacturing capabilities and expanded our presence in the Mexican OTC market.
|
|
•
|
We established a differentiated platform for international expansion through the Elan acquisition.
|
|
•
|
The Elan acquisition led to the creation of our new parent company, Perrigo Company plc, incorporated under the laws of Ireland. Our new corporate structure has allowed us to continue to grow in core markets and further expand outside of the U.S. with the parent company serving as a global business hub and providing the scale and resources to drive our strategic initiatives and investments.
|
|
•
|
The acquisition also provided us with our Tysabri
®
royalty stream, enhancing our cash flows. See
Item 1. Business - Specialty Sciences
for more information on Tysabri
®
.
|
|
•
|
We increased our presence in the Australian market through the acquisition of a basket of OTC products from Aspen Global Inc. ("Aspen").
|
|
•
|
We further developed our ophthalmic capabilities with the acquisition of Methazolomide from Fera Pharmaceuticals, LLC ("Fera").
|
|
•
|
We continue to experience a significant reduction in pricing expectations from historical levels in our
RX
segment due to industry and competitive pressures. This softness in pricing is attributed to various factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future, and we are forecasting a 9% to 11% pricing decline in this segment for the year ended December 31, 2017 compared to the prior year.
|
|
•
|
The
CHCI
segment has been impacted by market dynamics in key countries such as Belgium, France, Germany and Italy due to softness in certain brand categories and by unfavorable foreign currency impacts, primarily in the U.K. related to Brexit. In addition, the segment has been impacted in Belgium by a change in the forecast with a major wholesaler, as management implements improved supply chain efficiencies in this market. The
CHCI
segment has restructured its approach to addressing these market dynamics including: (1) implementing a brand prioritization strategy, with an objective to balance the cost of advertising and promotional investments with expected contributions from category sales, (2) restructuring its sales force in each of these markets to more effectively serve customers, and (3) exiting certain unfavorable distribution agreements. The combination of these actions are expected to improve the segment's focus on higher value OTC products, reduce selling costs and improve operating margins in the segment.
|
|
•
|
On December 9, 2016, we announced that we had entered into a definitive agreement to sell our India API business to Strides Shasun Limited. As of December 31, 2016, the net assets of our India API business were classified as "held for sale" as discussed in
Item 8. Note 9
. The sale closed on April 6, 2017 and is not expected to have a material impact on our operations or result in a significant gain or loss when recorded in the second quarter of 2017.
|
|
•
|
During the three months ended December 31, 2016, the U.S. market for our Entocort
®
(budesonide) capsules, including both brand and authorized generic capsules, experienced significant and unexpected increased competition, reducing our future revenue stream. This led to an impairment charge of
$342.2 million
related to the Entocort
®
intangible asset acquired in 2015. We expect our 2017 net sales to be negatively affected in an amount of approximately $72.0 million.
|
|
•
|
On February 27, 2017, we announced we were exploring strategic alternatives for our Israel API operations.
|
|
•
|
On
March 27, 2017
, we announced the completed divestment of our Tysabri
®
royalty stream to Royalty Pharma for up to
$2.85 billion
, which consists of
$2.2 billion
in cash and up to
$250.0 million
and
$400.0 million
in milestone payments to us if the royalties on global net sales of Tysabri
®
that are received by Royalty Pharma meet specific thresholds in 2018 and 2020, respectively. As a result of this transaction, we will derecognize the Tysabri
®
financial asset in the first quarter of 2017 and we do not expect the disposition to have a material impact on our results.
|
|
•
|
On April 6, 2017, we completed the divestment of our India API business to Strides Shasun Limited. As of December 31, 2016, the net assets of our India API business were classified as "held for sale" as discussed in
Item 8. Note 9
. The sale is not expected to have a material impact on our operations or result in a significant gain or (loss) when recorded in the second quarter of 2017.
|
|
Year Ended
|
||||||||||||||||||||||||||||
|
December 31, 2016
|
||||||||||||||||||||||||||||
|
Goodwill
|
Indefinite-Lived Intangible Assets
|
Definite-Lived Intangible Assets
|
Held For Sale
|
IPR&D
|
Fixed Assets
|
Total
|
||||||||||||||||||||||
|
CHCA
(1)
|
$
|
24.5
|
|
$
|
0.4
|
|
$
|
—
|
|
$
|
9.9
|
|
$
|
—
|
|
$
|
3.5
|
|
$
|
38.3
|
|
|||||||
|
CHCI
(2)
|
868.4
|
|
849.1
|
|
321.4
|
|
—
|
|
3.5
|
|
—
|
|
2,042.4
|
|
||||||||||||||
|
RX
(3)
|
—
|
|
—
|
|
342.2
|
|
—
|
|
—
|
|
0.2
|
|
342.4
|
|
||||||||||||||
|
Specialty Sciences
(4)
|
199.6
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
199.6
|
|
||||||||||||||
|
Other
(5)
|
—
|
|
—
|
|
2.0
|
|
6.3
|
|
—
|
|
—
|
|
8.3
|
|
||||||||||||||
|
$
|
1,092.5
|
|
$
|
849.5
|
|
$
|
665.6
|
|
$
|
16.2
|
|
$
|
3.5
|
|
$
|
3.7
|
|
$
|
2,631.0
|
|
||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||||||
|
June 28,
2014 |
June 27,
2015 |
December 27,
2014 |
December 31,
2015 |
December 31,
2015 |
December 31,
2016 |
||||||||||||||||||
|
($ in millions)
|
Restated
|
Restated
|
Restated
|
Restated
|
Restated
|
||||||||||||||||||
|
Net sales
|
$
|
3,914.1
|
|
$
|
4,227.1
|
|
$
|
1,844.7
|
|
$
|
2,632.2
|
|
$
|
5,014.7
|
|
$
|
5,280.6
|
|
|||||
|
Gross profit
|
$
|
1,452.1
|
|
$
|
1,644.2
|
|
$
|
673.8
|
|
$
|
1,078.9
|
|
$
|
2,049.4
|
|
$
|
2,051.8
|
|
|||||
|
Gross profit %
|
37.1
|
%
|
38.9
|
%
|
36.5
|
%
|
41.0
|
%
|
40.9
|
%
|
38.9
|
%
|
|||||||||||
|
Operating expenses
|
$
|
880.7
|
|
$
|
971.7
|
|
$
|
384.1
|
|
$
|
1,011.3
|
|
$
|
1,599.0
|
|
$
|
4,051.5
|
|
|||||
|
Operating expenses %
|
22.5
|
%
|
23.0
|
%
|
20.8
|
%
|
38.4
|
%
|
31.9
|
%
|
76.7
|
%
|
|||||||||||
|
Operating income (loss)
|
$
|
571.4
|
|
$
|
672.5
|
|
$
|
289.7
|
|
$
|
67.6
|
|
$
|
450.4
|
|
$
|
(1,999.7
|
)
|
|||||
|
Operating income (loss) %
|
14.6
|
%
|
15.9
|
%
|
15.7
|
%
|
2.6
|
%
|
9.0
|
%
|
(37.9
|
)%
|
|||||||||||
|
Interest and other, net
|
$
|
267.8
|
|
$
|
412.2
|
|
$
|
79.7
|
|
$
|
58.7
|
|
$
|
391.2
|
|
$
|
2,848.6
|
|
|||||
|
Income tax expense (benefit)
|
$
|
70.8
|
|
$
|
124.2
|
|
$
|
29.4
|
|
$
|
(33.6
|
)
|
$
|
61.1
|
|
$
|
(835.5
|
)
|
|||||
|
Net income (loss)
|
$
|
232.8
|
|
$
|
136.1
|
|
$
|
180.6
|
|
$
|
42.5
|
|
$
|
(1.9
|
)
|
$
|
(4,012.8
|
)
|
|||||

|
*
|
Net sales by geography is derived from the location of the entity that sells to a third party. For geographic information for the
six months ended
December 31, 2015
, and the
years ended
June 27, 2015
and
June 28, 2014
, refer to
Item 8. Note 19
.
|
|
•
|
In 2016, we experienced a reduction in pricing expectations within our
CHCA
segment, particularly in the cough/cold, animal health and analgesics categories due to various factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, and competition in specific product categories. We expect this pricing environment to continue to impact our
CHCA
segment for the foreseeable future.
|
|
•
|
On August 5, 2016, we completed the sale of our U.S. VMS business to IVC for
$61.8 million
inclusive of an estimated working capital adjustment. The below table indicates the sales attributable to the U.S. VMS business for periods presented in this report:
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||||||
|
($ in millions)
|
June 28,
2014 |
June 27,
2015 |
December 27,
2014 |
December 31,
2015 |
December 31,
2015 |
December 31,
2016 |
|||||||||||||||||
|
Net sales
|
$
|
189.5
|
|
$
|
157.9
|
|
$
|
80.8
|
|
$
|
85.2
|
|
$
|
162.3
|
|
$
|
110.2
|
|
|||||

|
|
|||||||
|
Year Ended
|
|||||||
|
December 31,
2015 |
December 31,
2016 |
||||||
|
($ in millions)
|
Restated
|
||||||
|
Net sales
|
$
|
2,554.2
|
|
$
|
2,507.1
|
|
|
|
Gross profit
|
$
|
846.7
|
|
$
|
825.2
|
|
|
|
Gross profit %
|
33.2
|
%
|
32.9
|
%
|
|||
|
Operating income
|
$
|
439.9
|
|
$
|
399.8
|
|
|
|
Operating income %
|
17.2
|
%
|
15.9
|
%
|
|||
|
•
|
Discontinued products of
$61.3 million
related primarily to a label refresh within the infant formula category; and
|
|
•
|
A net
$56.5 million
decrease in existing product sales as a result of:
|
|
•
|
Strong sales in our infant nutrition, and smoking cessation categories; more than offset by
|
|
•
|
A milder cold and flu season in the first and second quarters of 2016, which led to weaker sales in the cough/cold and analgesics categories;
|
|
•
|
Pricing pressure, which impacted sales in the cough/cold, analgesics, and animal health categories in particular;
|
|
•
|
Lower sales in the antacids category; and
|
|
•
|
Timing of promotions in the second and third quarters of 2015 and a milder allergy season in the third quarter of 2016, which had a negative impact on year-over-year sales in the cough/cold category;
|
|
•
|
Lower year-over-year sales of
$52.1 million
attributable to the U.S. VMS business, which was sold in August 2016; and
|
|
•
|
Unfavorable foreign currency movement of
$15.0 million
; offset partially by
|
|
•
|
New product sales of
$117.4 million
related primarily to the launches of fluticasone nasal spray (store brand equivalent to Flonase
®
), certain guaifenesin products (store brand equivalent to Mucinex
®
), several new infant formula and food products, and new animal health products; and
|
|
•
|
Incremental net sales of
$20.3 million
related primarily to the Gelcaps and ScarAway
®
acquisitions.
|
|
•
|
A decrease
of
$21.5 million
in gross profit due to:
|
|
•
|
Pricing pressure as noted above; and
|
|
•
|
Increased intangible asset amortization expense associated primarily with the Gelcaps and ScarAway
®
acquisitions; offset partially by
|
|
•
|
Margin contributions from new products and strong performance in the infant nutrition and smoking cessation categories; and
|
|
•
|
Continued manufacturing and supply chain efficiencies.
|
|
•
|
An increase
of
$18.6 million
in operating expenses due to:
|
|
•
|
A
$24.5 million
goodwill impairment charge related to our Animal Health business, as described in
Item 8. Note 3
;
|
|
•
|
Increased research and development investments of $6.5 million due to timing of clinical trials;
|
|
•
|
A
$6.2 million
impairment charge related to the sale of the U.S. VMS business, as described in
Item 8. Note 9
;
|
|
•
|
A
$3.7 million
impairment charge recorded on the held-for-sale assets associated with our animal health pet treats plant, as described in
Item 8. Note 9
; partially offset by
|
|
•
|
Decreased restructuring expense of $9.9 million; and
|
|
•
|
Decreased selling and administrative expenses due to cost containment.
|
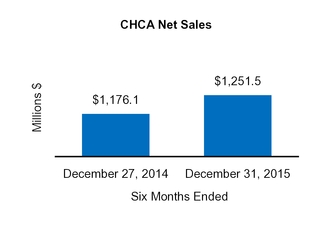
|
|
|||||||
|
Six Months Ended
|
|||||||
|
December 27,
2014 |
December 31,
2015 |
||||||
|
($ in millions)
|
Restated
|
Restated
|
|||||
|
Net sales
|
$
|
1,176.1
|
|
$
|
1,251.5
|
|
|
|
Gross profit
|
$
|
361.2
|
|
$
|
417.9
|
|
|
|
Gross profit %
|
30.7
|
%
|
33.4
|
%
|
|||
|
Operating income
|
$
|
151.1
|
|
$
|
209.2
|
|
|
|
Operating income %
|
12.9
|
%
|
16.7
|
%
|
|||
|
•
|
New product sales of
$122.9 million
related primarily to certain new infant formula products;
|
|
•
|
Incremental net sales due primarily to the Gelcaps and ScarAway
®
acquisitions of $20.2 million; and
|
|
•
|
A
$66.0 million increase in existing sales primarily attributable to increased sales volumes of smoking cessation, cough/cold, and gastrointestinal products; offset partially by
|
|
•
|
A decline of $22.9 million in sales of existing products, primarily in animal health and diabetic care;
|
|
•
|
Discontinued products of $99.6 million related primarily to reformulated infant formula, analgesic, and animal health products; and
|
|
•
|
Unfavorable foreign currency movement of $11.2 million.
|
|
•
|
An increase
of
$56.7 million
in gross profit due to:
|
|
•
|
Improved purchase prices and efficiencies in manufacturing facilities; and
|
|
•
|
Incrementally higher gross profit attributable primarily to the Gelcaps and ScarAway
®
acquisitions; and
|
|
•
|
A decrease
of
$1.4 million
in operating expenses due to:
|
|
•
|
Decreased Research and Development expense ("R&D") spend of $13.6 million due to relative timing of clinical trials; offset partially by
|
|
•
|
An increase in restructuring expense of $10.9 million related to strategic organizational enhancements; and
|
|
•
|
Increased administrative expenses of $1.9 million primarily related to the Gelcaps and ScarAway
®
acquisitions.
|

|
|
|||||||
|
Year Ended
|
|||||||
|
June 28,
2014 |
June 27,
2015 |
||||||
|
($ in millions)
|
Restated
|
Restated
|
|||||
|
Net sales
|
$
|
2,581.2
|
|
$
|
2,478.8
|
|
|
|
Gross profit
|
$
|
814.0
|
|
$
|
790.1
|
|
|
|
Gross profit %
|
31.5
|
%
|
31.9
|
%
|
|||
|
Operating income
|
$
|
402.8
|
|
$
|
381.9
|
|
|
|
Operating income %
|
15.6
|
%
|
15.4
|
%
|
|||
|
•
|
New product sales of
$145.5 million
related primarily to the launches of Fipronil (a generic version of Frontline
®
Plus) and certain new infant formula products;
|
|
•
|
Incremental net sales attributable to the Gelcaps acquisition of $4.5 million; and
|
|
•
|
Increased sales volumes of smoking cessation products totaling $46.9 million due in part to certain national brand products not being available to consumers due to manufacturing and supply issues; more than offset by
|
|
•
|
A decline of $186.6 million in sales of existing products, primarily in contract manufacturing, as well as in sales of VMS, cough/cold, analgesic, gastrointestinal, and animal health products. The decline in contract manufacturing and analgesics was driven by a branded competitor's return to the market. The decline in VMS sales was due primarily to increased competition in the marketplace and pricing pressures;
|
|
•
|
Discontinued products of $104.1 million related primarily to animal health and nutritional products; and
|
|
•
|
Unfavorable foreign currency movement of $7.9 million.
|
|
•
|
A decrease
of
$23.9 million
in gross profit due to:
|
|
•
|
Lower segment sales and incremental amortization expense attributable to the Gelcaps acquisition; offset partially by
|
|
•
|
Improved purchase prices and efficiencies in manufacturing facilities.
|
|
•
|
Offset partially by
a decrease
of
$3.0 million
in operating expenses due to:
|
|
•
|
Decreased animal health advertising expenses; and
|
|
•
|
A
$6.8 million
goodwill impairment charge related to our Mexico operations, as described in
Item 8. Note 3
; offset in part by
|
|
•
|
A $10.0 million option payment related to a collaboration agreement made during the year ended June 27, 2015, as described in
Item 8. Note 17
.
|
|
•
|
The
CHCI
segment has been impacted by market dynamics in key countries such as Belgium, France, Germany and Italy due to softness in certain brand categories and by unfavorable foreign currency impacts, primarily in the U.K. related to Brexit. In addition, the segment had been impacted in Belgium by a change in the forecast with a major wholesaler, as management implements improved supply chain efficiencies in this market. The
CHCI
segment has restructured its approach to addressing these markets including: (1) implementing a brand prioritization strategy to address these market dynamics, with an objective to balance the cost of advertising and promotional investments with expected contributions from category sales, (2) restructuring its sales force in each of these markets to more effectively serve customers, and (3) exiting certain unfavorable distribution agreements. The combination of these actions are expected to improve the segment's focus on higher value OTC products, reduce selling costs and improve operating margins in the segment.
|
|
•
|
As part of our strategic initiatives, management continues to drive improvements and evaluate the overall cost structures within our CHCI segment in the following ways:
|
|
•
|
On December 8, 2016, we announced the cancellation of the unprofitable EuroGenerics NV distribution agreement in Belgium. The cancellation, combined with the exit of certain OTC distribution agreements, is expected to reduce net sales by approximately $200.0 million in 2017.
|
|
•
|
We continue to make progress on our previously announced restructuring plans to right-size the
Omega
business due to the impact of market dynamics on sales volumes. In addition, we made several strategic leadership changes during the year ended December 31, 2016, including appointing new leaders for Belgium, France and Germany as well as a new Executive Vice President of the CHCI segment. Management continues to evaluate the overall cost structure relative to current and expected market dynamics. In 2016, we recognized
$20.9 million
of restructuring expense in the CHCI segment.
|
|
•
|
Management continues to evaluate the most effective business model for each country and has announced strategic evaluations for Russia and Argentina.
|

|
|
|||||||
|
Year Ended
|
|||||||
|
December 31, 2015
(1)
|
December 31,
2016 |
||||||
|
($ in millions)
|
Restated
|
||||||
|
Net sales
|
$
|
1,360.6
|
|
$
|
1,652.2
|
|
|
|
Gross profit
|
$
|
614.7
|
|
$
|
693.4
|
|
|
|
Gross profit %
|
45.2
|
%
|
42.0
|
%
|
|||
|
Operating loss
|
$
|
(124.3
|
)
|
$
|
(2,087.4
|
)
|
|
|
Operating loss %
|
(9.1
|
)%
|
(126.3
|
)%
|
|||
|
•
|
An additional three months of results from operations attributable to Omega;
|
|
•
|
New products totaling
$119.0 million
; and
|
|
•
|
Incremental nets sales due to the Naturwohl and GSK Products acquisitions totaling
$84.2 million
; offset partially by
|
|
•
|
A net
$143.6 million
decrease in sales volumes of existing products due primarily to weaker current year sales in the lifestyle category due in part to a product launch in the prior year, and in the natural health/vitamins category due primarily to timing of promotional activities, and the divestment of a European sports brand, as well as the expiration of a distribution contract in the prior year;
|
|
•
|
Unfavorable foreign currency movement of
$44.1 million
; and
|
|
•
|
Discontinued products of
$8.4 million
.
|
|
•
|
A
$78.7 million
increase in gross profit due to an additional three months of operations attributable to Omega; offset partially by
|
|
•
|
Decreased sales of existing products in the higher-margin lifestyle and natural health/vitamins categories noted above;
|
|
•
|
Weaker performance in Belgium and Germany;
|
|
•
|
Unfavorable foreign currency effect; more than offset by
|
|
•
|
An increase
of
$2.0 billion
in operating expenses due primarily to:
|
|
•
|
Intangible asset and goodwill impairment charges totaling
$2.0 billion
, as described in
Item 8. Note 3
; and
|
|
•
|
Restructuring charges totaling
$20.9 million
related to strategic organizational enhancements;
|
|
•
|
An additional three months of operations from the Omega acquisition; offset partially by
|
|
•
|
Cost control measures.
|

|
Six Months Ended
|
|||||||
|
December 27,
2014 |
December 31,
2015 |
||||||
|
($ in millions)
|
Restated
|
||||||
|
Net sales
|
$
|
177.1
|
|
$
|
833.0
|
|
|
|
Gross profit
|
$
|
55.9
|
|
$
|
386.0
|
|
|
|
Gross profit %
|
31.6
|
%
|
46.3
|
%
|
|||
|
Operating income (loss)
|
$
|
14.1
|
|
$
|
(148.5
|
)
|
|
|
Operating income (loss) %
|
8.0
|
%
|
(17.8
|
)%
|
|||
|
•
|
Incremental net sales attributable to the Omega, Naturwohl and GSK acquisitions totaling
$569.1 million
; and
|
|
•
|
New products totaling
$66.8 million
; offset partially by
|
|
•
|
Unfavorable foreign currency movement of
$14.8 million
; and
|
|
•
|
Discontinued products of
$3.8 million
.
|
|
•
|
A
$330.1 million
increase
in gross profit and a
$492.7 million
increase in operating expenses due to an additional six months of operations attributable to Omega.
|

|
Year Ended
|
|||||||
|
June 28,
2014 |
June 27, 2015
(1)
|
||||||
|
($ in millions)
|
Restated
|
||||||
|
Net sales
|
$
|
331.1
|
|
$
|
704.6
|
|
|
|
Gross profit
|
$
|
97.3
|
|
$
|
284.5
|
|
|
|
Gross profit %
|
29.4
|
%
|
40.4
|
%
|
|||
|
Operating income
|
$
|
17.0
|
|
$
|
38.2
|
|
|
|
Operating income %
|
5.1
|
%
|
5.4
|
%
|
|||
|
•
|
Incremental net sales attributable to the Omega and Aspen Global Inc. ("Aspen") acquisitions totaling $350.2 million; and
|
|
•
|
New products totaling
$43.8 million
; offset partially by
|
|
•
|
Unfavorable foreign currency movement of
$16.9 million
.
|
|
•
|
A
$187.2 million
increase
in gross profit and a
$166.0 million
increase
in operating expenses due to an additional three months of operations attributable to Omega.
|
|
•
|
We continue to experience a significant reduction in pricing expectations from historical levels in our
RX
segment due to industry and competitive pressures. This softness in pricing is attributed to various factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future, and we are forecasting a 9% to 11% pricing decline in this segment for the year ended December 31, 2017 compared to the prior year.
|
|
•
|
On January 22, 2016, we acquired a portfolio of generic dosage forms and strengths of Retin-A
®
(tretinoin), a topical prescription acne treatment, from Matawan Pharmaceuticals, LLC, for
$416.4 million
in cash ("Tretinoin Products").
|
|
•
|
On March 1, 2016, we completed the acquisition of two development-stage specialty Rx products to further invest in our specialty Rx portfolio.
|
|
•
|
On August 22, 2016, we purchased the remaining 60.9% ownership rights to a generic Benzaclin
TM
product ("Generic Benzaclin
TM
"), which we developed and marketed in collaboration with Barr Laboratories. As a result of this transaction, we are now entitled to 100% of income from sales of the product.
|
|
•
|
On November 10, 2016, we announced that as part of our portfolio review process we are conducting a comprehensive internal evaluation of the
RX
segment's market position, growth opportunities, and interdependencies with our manufacturing and shared service operations to determine if strategic alternatives should be explored.
|
|
•
|
During the three months ended December 31, 2016, the U.S. market for our Entocort
®
(budesonide) capsules, including both brand and authorized generic capsules, experienced significant and unexpected increased competition, reducing our future revenue stream. This led to an impairment charge of
$342.2 million
related to the Entocort
®
intangible asset acquired in 2015. We expect our 2017 net sales to be negatively affected in an amount of approximately $72.0 million.
|
|
•
|
In December 2016, we transitioned our specialty pharmaceutical commercial activities to our partner, Exeltis, who will lead sales and marketing efforts for this portfolio of products. We do not expect that this transition will have an impact on our Net sales.
|

|
|
|||||||
|
Year Ended
|
|||||||
|
December 31,
2015 |
December 31,
2016 |
||||||
|
($ in millions)
|
Restated
|
||||||
|
Net sales
|
$
|
1,001.9
|
|
$
|
1,042.8
|
|
|
|
Gross profit
|
$
|
543.3
|
|
$
|
501.1
|
|
|
|
Gross profit %
|
54.2
|
%
|
48.1
|
%
|
|||
|
Operating income (loss)
|
$
|
377.8
|
|
$
|
(0.2
|
)
|
|
|
Operating income %
|
37.7
|
%
|
—
|
%
|
|||
|
•
|
Net sales attributable to the Entocort
®
and Tretinoin Products acquisitions totaling
$150.9 million
; and
|
|
•
|
New product sales of
$68.0 million
due primarily to sales of benzoyl peroxide 5%-clindamycin 1% gel (a generic version of Benzaclin™); offset partially by
|
|
•
|
Decreased sales of existing products of
$174.1 million
due to declined sales volume of certain products, pricing pressure across the portfolio, and the lack of exclusive market position for two key products versus the prior year; and
|
|
•
|
Discontinued products of
$3.9 million
.
|
|
•
|
A decrease
of
$42.2 million
in gross profit due primarily to the pricing pressure noted above, as well as higher amortization expense from the Entocort
®
and Tretinoin Products acquisitions; and
|
|
•
|
An
increase
of
$335.8 million
in operating expenses due primarily to:
|
|
•
|
A
$342.2 million
impairment charge related to the Entocort
®
intangible assets, as described in
Item 8. Note 3
;
|
|
•
|
Increased selling and administration expenses of $9.3 million, and
|
|
•
|
Increased R&D investments of $3.0 million due to timing of clinical trials; offset partially by
|
|
•
|
The absence of an
$18.0 million
R&D payment made in connection with a R&D contractual arrangement in the prior year.
|

|
|
|||||||
|
Six Months Ended
|
|||||||
|
December 27,
2014 |
December 31,
2015 |
||||||
|
($ in millions)
|
Restated
|
||||||
|
Net sales
|
$
|
436.7
|
|
$
|
502.6
|
|
|
|
Gross profit
|
$
|
230.5
|
|
$
|
253.4
|
|
|
|
Gross profit %
|
52.8
|
%
|
50.4
|
%
|
|||
|
Operating income
|
$
|
168.8
|
|
$
|
181.9
|
|
|
|
Operating income %
|
38.6
|
%
|
36.2
|
%
|
|||
|
•
|
New product sales of
$41.2 million
related primarily to the launches of clobetasol propionate 0.05% spray, tacrolimus 0.1% ointment, and testosterone gel 1%; and
|
|
•
|
Net sales attributable to the Lumara Health, Inc. ("Lumara") product acquisition of
$7.0 million
; offset partially by
|
|
•
|
A decrease in volumes of certain existing products.
|
|
•
|
An increase
of
$22.9 million
in gross profit due primarily to:
|
|
•
|
Higher net sales and favorable product mix; and
|
|
•
|
Certain pricing initiatives.
|
|
•
|
Partially offset by a
$9.8 million
increase
in operating expenses due to:
|
|
•
|
Increased selling and administration expense related to the specialty pharmaceuticals sales force; and
|
|
•
|
An increase in restructuring expense of $2.6 million related to our strategic organizational enhancements.
|

|
|
|||||||
|
Year Ended
|
|||||||
|
($ in millions)
|
June 28,
2014 |
June 27,
2015 |
|||||
|
Net sales
|
$
|
864.2
|
|
$
|
936.0
|
|
|
|
Gross profit
|
$
|
463.7
|
|
$
|
520.4
|
|
|
|
Gross profit %
|
53.7
|
%
|
55.6
|
%
|
|||
|
Operating income
|
$
|
341.5
|
|
$
|
364.7
|
|
|
|
Operating income %
|
39.5
|
%
|
39.0
|
%
|
|||
|
•
|
New product sales of
$117.8 million
related primarily to the launches of clobetasol propionate 0.05% spray, tacrolimus 0.1% ointment, and testosterone gel 1%; and
|
|
•
|
Net sales attributable to the Lumara product acquisition of $18.1 million; offset partially by
|
|
•
|
Discontinued products of $28.5 million;
|
|
•
|
Decrease in volumes of certain existing products; and
|
|
•
|
Unfavorable foreign exchange movement of $1.8 million for products manufactured in Israel.
|
|
•
|
An increase
of
$56.7 million
in gross profit due primarily to:
|
|
•
|
Higher net sales and favorable product mix; and
|
|
•
|
Pricing initiatives taken in the first quarter of the year ended June 28, 2014.
|
|
•
|
Partially offset by a
$33.5 million
increase
in operating expenses due to:
|
|
•
|
An R&D payment of $18.0 million made in connection with an R&D contractual arrangement during the year ended June 27, 2015;
|
|
•
|
Increased selling and administration expense related to the specialty pharmaceuticals sales force; and
|
|
•
|
Higher R&D expenses resulting from planned higher spending on new product development.
|
|
•
|
On
March 27, 2017
, we announced the completed divestment of our Tysabri
®
royalty stream to Royalty Pharma for up to
$2.85 billion
, which consists of
$2.2 billion
in cash and up to
$250.0 million
and
$400.0 million
in milestone payments to us if the royalties on global net sales of Tysabri
®
that are received by Royalty Pharma meet specific thresholds in 2018 and 2020, respectively. As a result of this transaction, we will derecognize the Tysabri
®
financial asset in the first quarter of 2017 and we do not expect the disposition to have a material impact on our results.
|
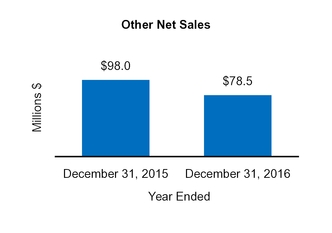
|
|
|||||||
|
Year Ended
|
|||||||
|
($ in millions)
|
December 31,
2015 |
December 31,
2016 |
|||||
|
Net sales
|
$
|
98.0
|
|
$
|
78.5
|
|
|
|
Gross profit
|
$
|
44.7
|
|
$
|
32.3
|
|
|
|
Gross profit %
|
45.5
|
%
|
41.2
|
%
|
|||
|
Operating income (loss)
|
$
|
(7.1
|
)
|
$
|
6.1
|
|
|
|
Operating income (loss) %
|
(7.3
|
)%
|
7.8
|
%
|
|||

|
|
|||||||
|
Six Months Ended
|
|||||||
|
($ in millions)
|
December 27,
2014 |
December 31,
2015 |
|||||
|
Net sales
|
$
|
54.8
|
|
$
|
45.1
|
|
|
|
Gross profit
|
$
|
26.2
|
|
$
|
21.6
|
|
|
|
Gross profit %
|
47.7
|
%
|
47.8
|
%
|
|||
|
Operating income (loss)
|
$
|
14.5
|
|
$
|
(19.5
|
)
|
|
|
Operating income (loss) %
|
26.4
|
%
|
(43.3
|
)%
|
|||

|
Year Ended
|
|||||||
|
($ in millions)
|
June 28,
2014 |
June 27,
2015 |
|||||
|
Net sales
|
$
|
137.6
|
|
$
|
107.7
|
|
|
|
Gross profit
|
$
|
77.1
|
|
$
|
49.2
|
|
|
|
Gross profit %
|
56.0
|
%
|
45.7
|
%
|
|||
|
Operating income
|
$
|
46.1
|
|
$
|
26.8
|
|
|
|
Operating income %
|
33.5
|
%
|
24.9
|
%
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||||
|
June 28,
2014 |
June 27,
2015 |
December 27,
2014 |
December 31,
2015 |
December 31,
2015 |
December 31,
2016 |
|||||||||||||||||
|
Restated
|
Restated
|
|||||||||||||||||||||
|
$
|
173.5
|
|
$
|
121.5
|
|
$
|
49.6
|
|
$
|
149.0
|
|
$
|
220.9
|
|
$
|
116.6
|
|
|||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||||||
|
June 28,
2014 |
June 27,
2015 |
December 27,
2014 |
December 31,
2015 |
December 31,
2015 |
December 31,
2016 |
||||||||||||||||||
|
($ in millions)
|
Restated
|
Restated
|
Restated
|
Restated
|
Restated
|
||||||||||||||||||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
(26.6
|
)
|
$
|
(78.5
|
)
|
$
|
(46.9
|
)
|
$
|
(57.3
|
)
|
$
|
(88.8
|
)
|
$
|
2,608.2
|
|
|||||
|
Interest expense, net
|
$
|
103.5
|
|
$
|
146.0
|
|
$
|
56.7
|
|
$
|
89.9
|
|
$
|
179.1
|
|
$
|
216.6
|
|
|||||
|
Other expense, net
|
$
|
25.1
|
|
$
|
334.2
|
|
$
|
60.3
|
|
$
|
25.2
|
|
$
|
299.1
|
|
$
|
22.7
|
|
|||||
|
Loss on extinguishment of debt
|
$
|
165.8
|
|
$
|
10.5
|
|
$
|
9.6
|
|
$
|
0.9
|
|
$
|
1.8
|
|
$
|
1.1
|
|
|||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||
|
June 28, 2014
|
June 27,
2015 |
December 27,
2014 |
December 31,
2015 |
December 31,
2015 |
December 31,
2016 |
|||||||||||
|
Restated
|
Restated
|
Restated
|
Restated
|
Restated
|
||||||||||||
|
23.3
|
%
|
47.7
|
%
|
14.0
|
%
|
(376.2
|
)%
|
103.3
|
%
|
17.2
|
%
|
|||||

|
Year Ended
|
|||||||||||
|
|
December 31,
2015 |
December 31,
2016 |
Increase/ (Decrease)
|
||||||||
|
Restated
|
|||||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||
|
Net income (loss)
|
$
|
(1.9
|
)
|
$
|
(4,012.8
|
)
|
$
|
(4,010.9
|
)
|
||
|
Non-cash adjustments
|
745.4
|
|
4,769.2
|
|
4,023.8
|
|
|||||
|
Subtotal
|
743.5
|
|
756.4
|
|
12.9
|
|
|||||
|
Increase (decrease) in cash due to:
|
|||||||||||
|
Accounts receivable
|
4.8
|
|
(0.6
|
)
|
(5.4
|
)
|
|||||
|
Inventories
|
(21.5
|
)
|
100.7
|
|
122.2
|
|
|||||
|
Accounts payable
|
(26.7
|
)
|
(75.7
|
)
|
(49.0
|
)
|
|||||
|
Payroll and related taxes
|
(42.0
|
)
|
(41.1
|
)
|
0.9
|
|
|||||
|
Accrued customer programs
|
53.9
|
|
(13.9
|
)
|
(67.8
|
)
|
|||||
|
Accrued liabilities
|
98.9
|
|
(79.5
|
)
|
(178.4
|
)
|
|||||
|
Accrued income taxes
|
(67.9
|
)
|
20.9
|
|
88.8
|
|
|||||
|
Other
|
21.3
|
|
(12.3
|
)
|
(33.6
|
)
|
|||||
|
Subtotal
|
$
|
20.8
|
|
$
|
(101.5
|
)
|
$
|
(122.3
|
)
|
||
|
Net cash from (for) operating activities
|
$
|
764.3
|
|
$
|
654.9
|
|
$
|
(109.4
|
)
|
||
|
•
|
Changes in accrued liabilities due primarily to payment of legal expenses associated with the Mylan defense which were accrued at December 31, 2015, deferred revenue associated with the BCH Belgium Distribution Contracts, and timing of payments;
|
|
•
|
Changes in accrued customer-related programs due to the pricing dynamics in the
RX
segment; and
|
|
•
|
Changes in accounts payable due to changes to the Omega accounts payable structure as discussed below; offset partially by
|
|
•
|
Changes in inventories due to improved inventory management in our
CHCI
and
CHCA
segments and increased sales of cough/cold products at the end of the year ended December 31, 2016; and
|
|
•
|
Changes in accrued income taxes due primarily to the prior year period including a $68.9 million incremental tax payment made in connection with the contested IRS audit described in Note 14.
|
|
Six Months Ended
|
|||||||||||
|
|
December 27,
2014 |
December 31,
2015 |
Increase / (Decrease)
|
||||||||
|
Restated
|
Restated
|
||||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||
|
Net income (loss)
|
$
|
180.6
|
|
$
|
42.5
|
|
$
|
(138.1
|
)
|
||
|
Non-cash adjustments
|
88.6
|
|
279.2
|
|
190.6
|
|
|||||
|
Subtotal
|
269.2
|
|
321.7
|
|
52.5
|
|
|||||
|
Increase (decrease) in cash due to:
|
|||||||||||
|
Accounts receivable
|
(3.4
|
)
|
52.5
|
|
55.9
|
|
|||||
|
Inventories
|
(19.4
|
)
|
(29.6
|
)
|
(10.2
|
)
|
|||||
|
Accounts payable
|
(46.8
|
)
|
(194.1
|
)
|
(147.3
|
)
|
|||||
|
Payroll and related taxes
|
(26.3
|
)
|
(38.2
|
)
|
(11.9
|
)
|
|||||
|
Accrued customer programs
|
51.8
|
|
34.4
|
|
(17.4
|
)
|
|||||
|
Accrued liabilities
|
52.1
|
|
108.1
|
|
56.0
|
|
|||||
|
Accrued income taxes
|
33.1
|
|
(56.8
|
)
|
(89.9
|
)
|
|||||
|
Other
|
(18.3
|
)
|
2.9
|
|
21.2
|
|
|||||
|
Subtotal
|
$
|
22.8
|
|
$
|
(120.8
|
)
|
$
|
(143.6
|
)
|
||
|
Net cash from (for) operating activities
|
$
|
292.0
|
|
$
|
200.9
|
|
$
|
(91.1
|
)
|
||
|
•
|
Changes in accounts payable due primarily to the addition of Omega as well as the impact of Omega's accounts payable structure described above; and
|
|
•
|
Changes in accrued income taxes due primarily to the six months ended December 31, 2015 including a $68.9 million incremental tax payment made in connection with the contested IRS audit described in Note 14; offset partially by
|
|
•
|
Increased net earnings after adjusting for non-cash items such as impairment charges, changes in the fair value of the Tysabri
®
royalty stream, losses on extinguishment of debt, and depreciation and amortization;
|
|
•
|
Changes in accounts receivable due to timing of receipt of payments; and
|
|
•
|
Changes in accrued liabilities due primarily to amounts not yet paid related to our defense against Mylan.
|
|
Year Ended
|
|||||||||||
|
|
June 28,
2014 |
June 27,
2015 |
Increase / (Decrease)
|
||||||||
|
($ in millions)
|
Restated
|
Restated
|
|||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||
|
Net income (loss)
|
$
|
232.8
|
|
$
|
136.1
|
|
$
|
(96.7
|
)
|
||
|
Non-cash adjustments
|
379.7
|
|
554.7
|
|
175.0
|
|
|||||
|
Subtotal
|
612.5
|
|
690.8
|
|
78.3
|
|
|||||
|
Increase (decrease) in cash due to:
|
|||||||||||
|
Accounts receivable
|
(140.5
|
)
|
(51.1
|
)
|
89.4
|
|
|||||
|
Inventories
|
84.7
|
|
(11.4
|
)
|
(96.1
|
)
|
|||||
|
Accounts payable
|
(24.9
|
)
|
120.5
|
|
145.4
|
|
|||||
|
Payroll and related taxes
|
(55.5
|
)
|
(30.2
|
)
|
25.3
|
|
|||||
|
Accrued customer programs
|
113.1
|
|
71.3
|
|
(41.8
|
)
|
|||||
|
Accrued liabilities
|
23.0
|
|
42.8
|
|
19.8
|
|
|||||
|
Accrued income taxes
|
(11.3
|
)
|
21.9
|
|
33.2
|
|
|||||
|
Other
|
31.9
|
|
0.6
|
|
(31.3
|
)
|
|||||
|
Subtotal
|
$
|
20.5
|
|
$
|
164.4
|
|
$
|
143.9
|
|
||
|
Net cash from (for) operating activities
|
$
|
633.0
|
|
$
|
855.2
|
|
$
|
222.2
|
|
||
|
•
|
Changes in accounts payable due primarily to the addition of Omega in the fourth quarter, as Omega structured terms with suppliers based on seasonality of the business as noted above;
|
|
•
|
Changes in accounts receivable due to timing of sales and receipt of payments;
|
|
•
|
Increased net earnings after adjusting for non-cash items such as changes in the fair value of the Tysabri
®
royalty stream, losses on extinguishment of debt, losses on acquisition-related foreign currency derivatives, and depreciation and amortization; offset partially by
|
|
•
|
Changes in inventory due primarily to the addition of Omega.
|
|
Year Ended
|
|||||||||||
|
|
December 31,
2015 |
December 31,
2016 |
Increase/ (Decrease)
|
||||||||
|
($ in millions)
|
Restated
|
||||||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||
|
Proceeds from royalty rights
|
$
|
335.1
|
|
$
|
353.7
|
|
$
|
18.6
|
|
||
|
Acquisitions of businesses, net of cash acquired
|
(2,886.4
|
)
|
(427.4
|
)
|
2,459.0
|
|
|||||
|
Asset acquisitions
|
(4.0
|
)
|
(65.1
|
)
|
(61.1
|
)
|
|||||
|
Settlement of acquisition-related foreign currency derivatives
|
(304.8
|
)
|
—
|
|
304.8
|
|
|||||
|
Proceeds from sale of securities
|
—
|
|
4.5
|
|
4.5
|
|
|||||
|
Additions to property, plant and equipment
|
(166.8
|
)
|
(106.2
|
)
|
60.6
|
|
|||||
|
Proceeds from sale of business
|
—
|
|
69.1
|
|
69.1
|
|
|||||
|
Other investing
|
(2.7
|
)
|
(3.6
|
)
|
(0.9
|
)
|
|||||
|
Net cash from (for) investing activities
|
$
|
(3,029.6
|
)
|
$
|
(175.0
|
)
|
$
|
2,854.6
|
|
||
|
Six Months Ended
|
|||||||||||
|
|
December 27,
2014 |
December 31,
2015 |
Increase / (Decrease)
|
||||||||
|
Restated
|
Restated
|
||||||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||
|
Proceeds from royalty rights
|
$
|
175.8
|
|
$
|
166.3
|
|
$
|
(9.5
|
)
|
||
|
Acquisitions of businesses, net of cash acquired
|
(83.0
|
)
|
(791.6
|
)
|
(708.6
|
)
|
|||||
|
Settlement of acquisition-related foreign currency derivatives
|
(26.4
|
)
|
(1.3
|
)
|
25.1
|
|
|||||
|
Additions to property, plant and equipment
|
(48.0
|
)
|
(77.8
|
)
|
(29.8
|
)
|
|||||
|
Other investing
|
0.8
|
|
(3.7
|
)
|
(4.5
|
)
|
|||||
|
Net cash from (for) investing activities
|
$
|
19.2
|
|
$
|
(708.1
|
)
|
$
|
(727.3
|
)
|
||
|
Year Ended
|
|||||||||||
|
|
June 28,
2014 |
June 27,
2015 |
Increase / (Decrease)
|
||||||||
|
Restated
|
Restated
|
||||||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||
|
Proceeds from royalty rights
|
$
|
60.5
|
|
$
|
344.6
|
|
$
|
284.1
|
|
||
|
Acquisitions of businesses, net of cash acquired
|
(1,605.8
|
)
|
(2,177.8
|
)
|
(572.0
|
)
|
|||||
|
Asset acquisitions
|
—
|
|
(4.0
|
)
|
(4.0
|
)
|
|||||
|
Settlement of acquisition-related foreign currency derivatives
|
—
|
|
(329.9
|
)
|
(329.9
|
)
|
|||||
|
Proceeds from sale of securities
|
81.4
|
|
—
|
|
(81.4
|
)
|
|||||
|
Additions to property, plant and equipment
|
(171.6
|
)
|
(137.0
|
)
|
34.6
|
|
|||||
|
Other investing
|
(8.8
|
)
|
1.8
|
|
10.6
|
|
|||||
|
Net cash from (for) investing activities
|
$
|
(1,644.3
|
)
|
$
|
(2,302.3
|
)
|
$
|
(658.0
|
)
|
||
|
Year Ended
|
|||||||||||
|
|
December 31,
2015 |
December 31,
2016 |
Increase / (Decrease)
|
||||||||
|
($ in millions)
|
Restated
|
||||||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
$
|
666.0
|
|
$
|
(802.5
|
)
|
$
|
(1,468.5
|
)
|
||
|
Issuances of long-term debt
|
—
|
|
1,190.3
|
|
1,190.3
|
|
|||||
|
Payments on long-term debt
|
(917.3
|
)
|
(559.2
|
)
|
358.1
|
|
|||||
|
Premium on early debt retirement
|
—
|
|
(0.6
|
)
|
(0.6
|
)
|
|||||
|
Deferred financing fees
|
(3.6
|
)
|
(2.8
|
)
|
0.8
|
|
|||||
|
Issuance of ordinary shares
|
8.9
|
|
8.3
|
|
(0.6
|
)
|
|||||
|
Equity issuance costs
|
—
|
|
(10.3
|
)
|
(10.3
|
)
|
|||||
|
Repurchase of ordinary shares
|
(500.0
|
)
|
—
|
|
500.0
|
|
|||||
|
Cash dividends
|
(72.2
|
)
|
(83.2
|
)
|
(11.0
|
)
|
|||||
|
Other financing
|
(19.0
|
)
|
(8.7
|
)
|
10.3
|
|
|||||
|
Net cash from (for) financing activities
|
$
|
(837.2
|
)
|
$
|
(268.7
|
)
|
$
|
568.5
|
|
||
|
Six Months Ended
|
|||||||||||
|
|
December 27,
2014 |
December 31,
2015 |
Increase / (Decrease)
|
||||||||
|
($ in millions)
|
Restated
|
||||||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
$
|
(2.1
|
)
|
$
|
718.0
|
|
$
|
720.1
|
|
||
|
Issuances of long-term debt
|
2,504.3
|
|
—
|
|
(2,504.3
|
)
|
|||||
|
Payments on long-term debt
|
(934.5
|
)
|
(28.3
|
)
|
906.2
|
|
|||||
|
Deferred financing fees
|
(24.8
|
)
|
(0.3
|
)
|
24.5
|
|
|||||
|
Issuance of ordinary shares
|
1,039.5
|
|
4.9
|
|
(1,034.6
|
)
|
|||||
|
Equity issuance costs
|
(35.7
|
)
|
—
|
|
35.7
|
|
|||||
|
Repurchase of ordinary shares
|
—
|
|
(500.0
|
)
|
(500.0
|
)
|
|||||
|
Cash dividends
|
(29.0
|
)
|
(36.3
|
)
|
(7.3
|
)
|
|||||
|
Other financing
|
(8.8
|
)
|
(8.4
|
)
|
0.4
|
|
|||||
|
Net cash from (for) financing activities
|
$
|
2,508.9
|
|
$
|
149.6
|
|
$
|
(2,359.3
|
)
|
||
|
Year Ended
|
|||||||||||
|
|
June 28,
2014 |
June 27,
2015 |
Increase / (Decrease)
|
||||||||
|
($ in millions)
|
Restated
|
||||||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
$
|
(3.0
|
)
|
$
|
(54.0
|
)
|
$
|
(51.0
|
)
|
||
|
Issuances of long-term debt
|
3,293.6
|
|
2,504.3
|
|
(789.3
|
)
|
|||||
|
Payments on long-term debt
|
(2,035.0
|
)
|
(1,823.5
|
)
|
211.5
|
|
|||||
|
Premium on early debt retirement
|
(133.5
|
)
|
—
|
|
133.5
|
|
|||||
|
Deferred financing fees
|
(48.8
|
)
|
(28.1
|
)
|
20.7
|
|
|||||
|
Issuance of ordinary shares
|
9.8
|
|
1,043.5
|
|
1,033.7
|
|
|||||
|
Equity issuance costs
|
—
|
|
(35.7
|
)
|
(35.7
|
)
|
|||||
|
Cash dividends
|
(46.1
|
)
|
(64.8
|
)
|
(18.7
|
)
|
|||||
|
Other financing
|
(9.0
|
)
|
(19.3
|
)
|
(10.3
|
)
|
|||||
|
Net cash from (for) financing activities
|
$
|
1,028.0
|
|
$
|
1,522.4
|
|
$
|
494.4
|
|
||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||
|
|
June 28,
2014 |
June 27,
2015 |
December 31,
2015 |
December 27,
2014 |
December 31,
2016 |
|||||||||||||||
|
Dividends paid (in millions)
|
$
|
46.1
|
|
$
|
64.8
|
|
$
|
36.3
|
|
$
|
29.0
|
|
$
|
83.2
|
|
|||||
|
Dividends paid per share
|
$
|
0.39
|
|
$
|
0.46
|
|
$
|
0.25
|
|
$
|
0.21
|
|
$
|
0.58
|
|
|||||
|
Declaration Date
|
Record Date
|
Payable
|
Dividend Declared
|
|||||
|
Year Ended December 31, 2016
|
||||||||
|
November 8, 2016
|
November 25, 2016
|
December 13, 2016
|
$
|
0.145
|
|
|||
|
August 2, 2016
|
August 26, 2016
|
September 13, 2016
|
$
|
0.145
|
|
|||
|
April 26, 2016
|
May 27, 2016
|
June 14, 2016
|
$
|
0.145
|
|
|||
|
February 16, 2016
|
February 26, 2016
|
March 15, 2016
|
$
|
0.145
|
|
|||
|
Six Months Ended December 31, 2015
|
||||||||
|
November 4, 2015
|
November 27, 2015
|
December 15, 2015
|
$
|
0.125
|
|
|||
|
August 12, 2015
|
August 28, 2015
|
September 15, 2015
|
$
|
0.125
|
|
|||
|
Year Ended June 27, 2015
|
||||||||
|
April 28, 2015
|
May 29, 2015
|
June 16, 2015
|
$
|
0.125
|
|
|||
|
January 27, 2015
|
February 27, 2015
|
March 17, 2015
|
$
|
0.125
|
|
|||
|
November 3, 2014
|
November 28, 2014
|
December 16, 2014
|
$
|
0.105
|
|
|||
|
August 13, 2014
|
August 29, 2014
|
September 16, 2014
|
$
|
0.105
|
|
|||
|
•
|
On March 7, 2016, Perrigo Finance issued
$500.0 million
in aggregate principal amount of
3.500%
senior notes due 2021 and
$700.0 million
in aggregate principal amount of
4.375%
senior notes due 2026 (together, the "2016 Notes") and received net proceeds of
$1.2 billion
after fees and market discount, which were used to repay the amounts outstanding under the 2015 Revolver and 2014 Revolver mentioned above.
|
|
•
|
We had
$5.4 billion
and
$4.7 billion
outstanding under our notes and bonds, and
$420.7 million
and
$488.8 million
outstanding under our term loan, as of
December 31, 2016
and
December 31, 2015
, respectively. On September 29, 2016, we repaid the
$500.0 million
outstanding under the 1.300% 2016 Notes.
|
|
•
|
On September 2, 2014, we offered to exchange what were previously private placement senior notes for public bonds registered with the Securities and Exchange Commission. Substantially all of the private placement senior notes have been exchanged.
|
|
•
|
On
December 2, 2014
, Perrigo Finance, our 100% owned finance subsidiary, issued
$500.0 million
in aggregate principal amount of
3.50%
senior notes due
2021
,
$700.0 million
in aggregate principal amount of
3.90%
senior notes due
2024
, and
$400.0 million
in aggregate principal amount of
4.90%
senior notes due
2044
(collectively, the "2014 Bonds").
|
|
•
|
The 2014 Bonds are fully and unconditionally guaranteed on a senior unsecured basis by Perrigo Company plc, and no other subsidiary of Perrigo Company plc guarantees the 2014 Bonds. We may redeem the 2014 Bonds at any time under the terms of the applicable indenture, subject to the payment of a make-whole premium.
|
|
•
|
On
December 5, 2014
, Perrigo Finance entered into a term loan agreement consisting of a
€500.0 million
(
$614.3 million
) tranche maturing December 5, 2019, and Perrigo Company plc entered into a
$300.0 million
term loan tranche maturing December 18, 2015 ("2014 Term Loan").
|
|
•
|
On December 5, 2014, we repaid the remaining
$895.0 million
outstanding under our 2013 Term Loan described below, then terminated it.
|
|
•
|
On June 24, 2015, we repaid the $300.0 million portion of the 2014 Term Loan.
|
|
•
|
On March 30, 2015, we assumed
$20.0 million
in aggregate principal amount of
6.19%
senior notes due
2016
(the "2016 Notes"),
€135.0 million
(
$147.0 million
) aggregate principal amount of
5.1045%
senior notes due
2023
,
€300.0 million
(
$326.7 million
) in aggregate principal amount of
5.125%
retail bonds due
|
|
•
|
The fair value of the 2023 Notes and Retail Bonds
exceeded par value by
€93.6 million
(
$101.9 million
) on the date of the acquisition. As a result, a fair value adjustment was recorded as part of the carrying value of the underlying debt and will be amortized as a reduction of interest expense over the remaining terms of the respective debt instruments. The adjustment does not affect cash interest payments.
|
|
•
|
On May 29, 2015, we repaid the
$20.0 million
in aggregate principal amount of the 2016 Notes.
|
|
|
Payment Due
|
||||||||||||||||||
|
($ in millions)
|
2017
|
2018-2019
|
2020-2021
|
After 2021
|
Total
|
||||||||||||||
|
Short and long-term debt
(1)
|
$
|
781.7
|
|
$
|
1,474.8
|
|
$
|
1,332.2
|
|
$
|
4,339.7
|
|
$
|
7,928.4
|
|
||||
|
Capital lease obligations
|
1.4
|
|
1.0
|
|
0.5
|
|
—
|
|
2.9
|
|
|||||||||
|
Purchase obligations
(2)
|
781.9
|
|
9.7
|
|
—
|
|
—
|
|
791.6
|
|
|||||||||
|
Operating leases
(3)
|
40.2
|
|
54.8
|
|
29.2
|
|
19.8
|
|
144.0
|
|
|||||||||
|
Other contractual liabilities reflected on the consolidated balance sheets:
|
|||||||||||||||||||
|
Deferred compensation and benefits
(4)
|
—
|
|
—
|
|
—
|
|
85.6
|
|
85.6
|
|
|||||||||
|
Other
(5)
|
122.5
|
|
10.6
|
|
7.6
|
|
5.9
|
|
146.6
|
|
|||||||||
|
Total
|
$
|
1,727.7
|
|
$
|
1,550.9
|
|
$
|
1,369.5
|
|
$
|
4,451.0
|
|
$
|
9,099.1
|
|
||||
|
(1)
|
Short-term and long-term debt includes interest payments, which were calculated using the effective interest rate at
December 31, 2016
.
|
|
(2)
|
Consists of commitments for both materials and services.
|
|
(3)
|
Used in normal course of business, principally for warehouse facilities and computer equipment.
|
|
(4)
|
Includes amounts associated with non-qualified plans related to deferred compensation, executive retention and post-employment benefits. Of this amount, we have funded
$52.5 million
, which is recorded in Other non-current assets on the balance sheet. These amounts are assumed payable after five years, although certain circumstances, such as termination, would require earlier payment.
|
|
(5)
|
Primarily includes consulting fees, legal settlements, contingent consideration obligations, restructuring accruals and electrical and gas purchase contracts, which were accrued in Other current liabilities and Other non-current liabilities at
December 31, 2016
for all years.
|
|
Customer-Related Accruals and Allowances
|
|||||||||||||||||||||||
|
Rx Americas
|
All Other Segments *
|
||||||||||||||||||||||
|
(in millions)
|
Chargebacks
|
Medicaid
Rebates |
Returns and Shelf Stock Allowances
|
Admin. Fees and Other Rebates
|
Rebates and Other Allowances
|
Total
|
|||||||||||||||||
|
Balance at June 28, 2014
|
$
|
147.9
|
|
$
|
24.4
|
|
$
|
53.6
|
|
$
|
24.9
|
|
$
|
67.2
|
|
$
|
318.0
|
|
|||||
|
Balances Acquired in Business Acquisitions
|
—
|
|
—
|
|
—
|
|
—
|
|
76.1
|
|
76.1
|
|
|||||||||||
|
Foreign currency translation adjustments
|
|
|
|
|
|
|
|
|
(8.0
|
)
|
(8.0
|
)
|
|||||||||||
|
Provisions / Adjustments
|
1,123.1
|
|
46.8
|
|
35.3
|
|
130.7
|
|
158.6
|
|
1,494.5
|
|
|||||||||||
|
Credits / Payments
|
(1,079.6
|
)
|
(39.6
|
)
|
(26.8
|
)
|
(110.3
|
)
|
(165.1
|
)
|
(1,421.4
|
)
|
|||||||||||
|
Balance at June 27, 2015
|
$
|
191.4
|
|
$
|
31.6
|
|
$
|
62.1
|
|
$
|
45.3
|
|
$
|
128.8
|
|
$
|
459.2
|
|
|||||
|
Foreign currency translation adjustments
|
—
|
|
—
|
|
—
|
|
—
|
|
(3.2
|
)
|
(3.2
|
)
|
|||||||||||
|
Provisions / Adjustments
|
666.3
|
|
11.7
|
|
21.3
|
|
47.8
|
|
144.3
|
|
891.4
|
|
|||||||||||
|
Credits / Payments
|
(632.7
|
)
|
(18.6
|
)
|
(20.6
|
)
|
(53.1
|
)
|
(133.0
|
)
|
(858.0
|
)
|
|||||||||||
|
Balance at December 31, 2015
|
$
|
225.0
|
|
$
|
24.7
|
|
$
|
62.8
|
|
$
|
40.0
|
|
$
|
136.9
|
|
$
|
489.4
|
|
|||||
|
Foreign currency translation adjustments
|
—
|
|
—
|
|
—
|
|
—
|
|
(7.5
|
)
|
(7.5
|
)
|
|||||||||||
|
Provisions / Adjustments
|
1,437.2
|
|
27.4
|
|
48.0
|
|
103.4
|
|
259.6
|
|
1,875.6
|
|
|||||||||||
|
Credits / Payments
|
(1,445.2
|
)
|
(27.5
|
)
|
(33.7
|
)
|
(108.8
|
)
|
(258.0
|
)
|
(1,873.2
|
)
|
|||||||||||
|
Balance at December 31, 2016
|
$
|
217.0
|
|
$
|
24.6
|
|
$
|
77.1
|
|
$
|
34.6
|
|
$
|
131.0
|
|
$
|
484.3
|
|
|||||
|
*
|
CHCA
,
CHCI
, and Specialty Sciences
|
|
•
|
the amount and timing of projected future cash flows, adjusted for the probability of technical and marketing success;
|
|
•
|
the amount and timing of projected costs to develop IPR&D into commercially viable products;
|
|
•
|
the discount rate selected to measure the risks inherent in the future cash flows;
|
|
•
|
the estimate of an appropriate market royalty rate; and
|
|
•
|
an assessment of the asset's life cycle and the competitive trends impacting the asset, including consideration of any technical, legal, regulatory, or economic barriers to entry.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Tysabri
®
Royalty Stream - at fair value
|
|||||||||||
|
Beginning balance
|
$
|
5,310.0
|
|
$
|
5,420.0
|
|
$
|
5,680.0
|
|
||
|
Royalties earned
|
(351.8
|
)
|
(167.3
|
)
|
(338.5
|
)
|
|||||
|
Change in fair value
|
(2,608.2
|
)
|
57.3
|
|
78.5
|
|
|||||
|
Ending balance
|
$
|
2,350.0
|
|
$
|
5,310.0
|
|
$
|
5,420.0
|
|
||
|
Reporting Unit
|
Goodwill Remaining in Reporting Unit
|
Segment
|
||||
|
Animal Health
|
$
|
178.9
|
|
CHCA
|
||
|
BCH-Belgium
|
$
|
63.2
|
|
CHCI
|
||
|
BCH-ROW
|
$
|
816.5
|
|
CHCI
|
||
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
PAGE NO.
|
|
|
1
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
8
|
||
|
9
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
15
|
||
|
16
|
||
|
17
|
||
|
18
|
||
|
19
|
||
|
20
|
||
|
21
|
||
|
22
|
||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
|
December 31, 2016
|
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Net sales
|
$
|
5,280.6
|
|
$
|
2,632.2
|
|
$
|
4,227.1
|
|
$
|
3,914.1
|
|
|||
|
Cost of sales
|
3,228.8
|
|
1,553.3
|
|
2,582.9
|
|
2,462.0
|
|
|||||||
|
Gross profit
|
2,051.8
|
|
1,078.9
|
|
1,644.2
|
|
1,452.1
|
|
|||||||
|
Operating expenses
|
|||||||||||||||
|
Distribution
|
88.3
|
|
47.9
|
|
67.7
|
|
55.3
|
|
|||||||
|
Research and development
|
184.0
|
|
88.2
|
|
187.8
|
|
152.5
|
|
|||||||
|
Selling
|
665.0
|
|
325.9
|
|
319.0
|
|
208.6
|
|
|||||||
|
Administration
|
452.2
|
|
306.8
|
|
385.3
|
|
411.3
|
|
|||||||
|
Impairment charges
|
2,631.0
|
|
215.6
|
|
6.8
|
|
6.0
|
|
|||||||
|
Restructuring
|
31.0
|
|
26.9
|
|
5.1
|
|
47.0
|
|
|||||||
|
Total operating expenses
|
4,051.5
|
|
1,011.3
|
|
971.7
|
|
880.7
|
|
|||||||
|
Operating income (loss)
|
(1,999.7
|
)
|
67.6
|
|
672.5
|
|
571.4
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
2,608.2
|
|
(57.3
|
)
|
(78.5
|
)
|
(26.6
|
)
|
|||||||
|
Interest expense, net
|
216.6
|
|
89.9
|
|
146.0
|
|
103.5
|
|
|||||||
|
Other expense, net
|
22.7
|
|
25.2
|
|
334.2
|
|
25.1
|
|
|||||||
|
Loss on extinguishment of debt
|
1.1
|
|
0.9
|
|
10.5
|
|
165.8
|
|
|||||||
|
Income (loss) before income taxes
|
(4,848.3
|
)
|
8.9
|
|
260.3
|
|
303.6
|
|
|||||||
|
Income tax expense (benefit)
|
(835.5
|
)
|
(33.6
|
)
|
124.2
|
|
70.8
|
|
|||||||
|
Net income (loss)
|
$
|
(4,012.8
|
)
|
$
|
42.5
|
|
$
|
136.1
|
|
$
|
232.8
|
|
|||
|
Income (loss) per share
|
|||||||||||||||
|
Basic
|
$
|
(28.01
|
)
|
$
|
0.29
|
|
$
|
0.97
|
|
$
|
2.02
|
|
|||
|
Diluted
|
$
|
(28.01
|
)
|
$
|
0.29
|
|
$
|
0.97
|
|
$
|
2.01
|
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
143.3
|
|
145.6
|
|
139.3
|
|
115.1
|
|
|||||||
|
Diluted
|
143.3
|
|
146.1
|
|
139.8
|
|
115.6
|
|
|||||||
|
Dividends declared per share
|
$
|
0.58
|
|
$
|
0.25
|
|
$
|
0.46
|
|
$
|
0.39
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||
|
December 31, 2016
|
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
||||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Net income (loss)
|
$
|
(4,012.8
|
)
|
$
|
42.5
|
|
$
|
136.1
|
|
$
|
232.8
|
|
|||
|
Other comprehensive income (loss):
|
|||||||||||||||
|
Foreign currency translation adjustments
|
(63.3
|
)
|
(135.5
|
)
|
(33.5
|
)
|
83.8
|
|
|||||||
|
Change in fair value of derivative financial instruments
(1)
|
(5.3
|
)
|
2.1
|
|
(0.2
|
)
|
(11.6
|
)
|
|||||||
|
Change in fair value of investment securities
(2)
|
8.7
|
|
9.3
|
|
(5.3
|
)
|
2.4
|
|
|||||||
|
Change in post-retirement and pension liability
(3)
|
(6.6
|
)
|
5.3
|
|
2.9
|
|
(12.0
|
)
|
|||||||
|
Other comprehensive income (loss), net of tax
|
(66.5
|
)
|
(118.8
|
)
|
(36.1
|
)
|
62.6
|
|
|||||||
|
Comprehensive income (loss)
|
$
|
(4,079.3
|
)
|
$
|
(76.3
|
)
|
$
|
100.0
|
|
$
|
295.4
|
|
|||
|
(1)
|
Includes tax effect of
$2.1 million
,
$0.4 million
,
$5.7 million
and
$(1.2) million
for the
year ended
December 31, 2016
, the
six months ended
December 31, 2015
, and the
years ended
June 27, 2015
, and
June 28, 2014
, respectively.
|
|
(2)
|
Includes tax effect of
$4.1 million
,
$3.6 million
,
$2.7 million
and
$1.2 million
for the
year ended
December 31, 2016
, the
six months ended
December 31, 2015
, and the
years ended
June 27, 2015
, and
June 28, 2014
, respectively.
|
|
(3)
|
Includes tax effect of
$2.5 million
,
$2.8 million
,
$0.6 million
and
$0.0 million
for the
year ended
December 31, 2016
, the
six months ended
December 31, 2015
, and the
years ended
June 27, 2015
, and
June 28, 2014
, respectively.
|
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Restated
|
Restated
|
||||||||||
|
Assets
|
|||||||||||
|
Cash and cash equivalents
|
$
|
622.3
|
|
$
|
417.8
|
|
$
|
785.6
|
|
||
|
Accounts receivable, net of allowance for doubtful accounts of $6.3 million, $4.5 million, and $2.6 million, respectively
|
1,176.0
|
|
1,189.0
|
|
1,209.4
|
|
|||||
|
Inventories
|
795.0
|
|
898.7
|
|
935.7
|
|
|||||
|
Current deferred income taxes
|
—
|
|
—
|
|
148.2
|
|
|||||
|
Prepaid expenses and other current assets
|
212.0
|
|
286.1
|
|
150.1
|
|
|||||
|
Total current assets
|
2,805.3
|
|
2,791.6
|
|
3,229.0
|
|
|||||
|
Property, plant and equipment, net
|
870.1
|
|
886.2
|
|
932.4
|
|
|||||
|
Tysabri
®
royalty stream - at fair value
|
2,350.0
|
|
5,310.0
|
|
5,420.0
|
|
|||||
|
Goodwill and other indefinite-lived intangible assets
|
4,163.9
|
|
7,069.0
|
|
6,984.3
|
|
|||||
|
Other intangible assets, net
|
3,396.8
|
|
2,973.1
|
|
2,742.8
|
|
|||||
|
Non-current deferred income taxes
|
72.1
|
|
71.4
|
|
50.1
|
|
|||||
|
Other non-current assets
|
211.9
|
|
248.3
|
|
233.3
|
|
|||||
|
Total non-current assets
|
11,064.8
|
|
16,558.0
|
|
16,362.9
|
|
|||||
|
Total assets
|
$
|
13,870.1
|
|
$
|
19,349.6
|
|
$
|
19,591.9
|
|
||
|
Liabilities and Shareholders’ Equity
|
|||||||||||
|
Accounts payable
|
$
|
471.7
|
|
$
|
555.8
|
|
$
|
709.3
|
|
||
|
Payroll and related taxes
|
115.8
|
|
125.3
|
|
133.9
|
|
|||||
|
Accrued customer programs
|
380.3
|
|
396.0
|
|
358.5
|
|
|||||
|
Accrued liabilities
|
263.3
|
|
351.9
|
|
257.5
|
|
|||||
|
Accrued income taxes
|
32.4
|
|
62.7
|
|
56.3
|
|
|||||
|
Current deferred income taxes
|
—
|
|
—
|
|
79.7
|
|
|||||
|
Current indebtedness
|
572.8
|
|
1,060.5
|
|
153.3
|
|
|||||
|
Total current liabilities
|
1,836.3
|
|
2,552.2
|
|
1,748.5
|
|
|||||
|
Long-term debt, less current portion
|
5,224.5
|
|
4,971.6
|
|
5,246.9
|
|
|||||
|
Non-current deferred income taxes
|
389.9
|
|
1,372.7
|
|
1,514.3
|
|
|||||
|
Other non-current liabilities
|
461.8
|
|
346.3
|
|
382.7
|
|
|||||
|
Total non-current liabilities
|
6,076.2
|
|
6,690.6
|
|
7,143.9
|
|
|||||
|
Total liabilities
|
7,912.5
|
|
9,242.8
|
|
8,892.4
|
|
|||||
|
Commitments and contingencies - Note 16
|
|||||||||||
|
Shareholders’ equity
|
|||||||||||
|
Controlling interest:
|
|||||||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
—
|
|
|||||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
8,135.0
|
|
8,142.6
|
|
8,621.9
|
|
|||||
|
Accumulated other comprehensive income (loss)
|
(81.8
|
)
|
(15.3
|
)
|
103.5
|
|
|||||
|
Retained earnings (accumulated deficit)
|
(2,095.1
|
)
|
1,980.1
|
|
1,973.9
|
|
|||||
|
Total controlling interest
|
5,958.1
|
|
10,107.4
|
|
10,699.3
|
|
|||||
|
Noncontrolling interest
|
(0.5
|
)
|
(0.6
|
)
|
0.2
|
|
|||||
|
Total shareholders’ equity
|
5,957.6
|
|
10,106.8
|
|
10,699.5
|
|
|||||
|
Total liabilities and shareholders' equity
|
$
|
13,870.1
|
|
$
|
19,349.6
|
|
$
|
19,591.9
|
|
||
|
Supplemental Disclosures of Balance Sheet Information
|
|||||||||||
|
Preferred shares, issued and outstanding
|
—
|
|
—
|
|
—
|
|
|||||
|
Ordinary shares, issued and outstanding
|
143.4
|
|
143.1
|
|
146.3
|
|
|||||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
|
December 31,
2016 |
December 31,
2015 |
|
June 27,
2015 |
|
June 28,
2014 |
|||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Cash Flows From (For) Operating Activities
|
|
|
|
|
|||||||||||
|
Net income (loss)
|
$
|
(4,012.8
|
)
|
$
|
42.5
|
|
|
$
|
136.1
|
|
|
$
|
232.8
|
|
|
|
Adjustments to derive cash flows
|
|
|
|
|
|||||||||||
|
Depreciation and amortization
|
457.0
|
|
182.4
|
|
|
258.7
|
|
|
206.1
|
|
|||||
|
Loss on acquisition-related foreign currency derivatives
|
—
|
|
—
|
|
326.4
|
|
—
|
|
|||||||
|
Share-based compensation
|
23.0
|
|
22.8
|
|
|
31.6
|
|
|
24.6
|
|
|||||
|
Impairment charges
|
2,631.0
|
|
215.6
|
|
6.8
|
|
6.0
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
2,608.2
|
|
(57.3
|
)
|
(78.5
|
)
|
(26.6
|
)
|
|||||||
|
Loss on extinguishment of debt
|
1.1
|
|
0.9
|
|
|
10.5
|
|
|
165.8
|
|
|||||
|
Restructuring charges
|
31.0
|
|
26.9
|
|
|
5.1
|
|
|
47.0
|
|
|||||
|
Deferred income taxes
|
(990.9
|
)
|
(120.0
|
)
|
|
(16.3
|
)
|
|
(49.7
|
)
|
|||||
|
Amortization of financing fees and debt discount (premium)
|
(24.7
|
)
|
(10.2
|
)
|
0.2
|
|
2.0
|
|
|||||||
|
Other non-cash adjustments
|
33.5
|
|
18.1
|
|
10.2
|
|
4.5
|
|
|||||||
|
Subtotal
|
756.4
|
|
321.7
|
|
|
690.8
|
|
|
612.5
|
|
|||||
|
Increase (decrease) in cash due to:
|
|
|
|
|
|||||||||||
|
Accounts receivable
|
(0.6
|
)
|
52.5
|
|
|
(51.1
|
)
|
|
(140.5
|
)
|
|||||
|
Inventories
|
100.7
|
|
(29.6
|
)
|
|
(11.4
|
)
|
|
84.7
|
|
|||||
|
Accounts payable
|
(75.7
|
)
|
(194.1
|
)
|
|
120.5
|
|
|
(24.9
|
)
|
|||||
|
Payroll and related taxes
|
(41.1
|
)
|
(38.2
|
)
|
|
(30.2
|
)
|
|
(55.5
|
)
|
|||||
|
Accrued customer programs
|
(13.9
|
)
|
34.4
|
|
|
71.3
|
|
|
113.1
|
|
|||||
|
Accrued liabilities
|
(79.5
|
)
|
108.1
|
|
|
42.8
|
|
|
23.0
|
|
|||||
|
Accrued income taxes
|
20.9
|
|
(56.8
|
)
|
|
21.9
|
|
|
(11.3
|
)
|
|||||
|
Other
|
(12.3
|
)
|
2.9
|
|
|
0.6
|
|
|
31.9
|
|
|||||
|
Subtotal
|
(101.5
|
)
|
(120.8
|
)
|
|
164.4
|
|
|
20.5
|
|
|||||
|
Net cash from (for) operating activities
|
654.9
|
|
200.9
|
|
|
855.2
|
|
|
633.0
|
|
|||||
|
Cash Flows From (For) Investing Activities
|
|
|
|
|
|||||||||||
|
Proceeds from royalty rights
|
353.7
|
|
166.3
|
|
344.6
|
|
60.5
|
|
|||||||
|
Acquisitions of businesses, net of cash acquired
|
(427.4
|
)
|
(791.6
|
)
|
|
(2,177.8
|
)
|
|
(1,605.8
|
)
|
|||||
|
Asset acquisitions
|
(65.1
|
)
|
—
|
|
(4.0
|
)
|
—
|
|
|||||||
|
Settlement of acquisition-related foreign currency derivatives
|
—
|
|
(1.3
|
)
|
(329.9
|
)
|
—
|
|
|||||||
|
Proceeds from sale of securities
|
4.5
|
|
—
|
|
|
—
|
|
|
81.4
|
|
|||||
|
Additions to property, plant and equipment
|
(106.2
|
)
|
(77.8
|
)
|
|
(137.0
|
)
|
|
(171.6
|
)
|
|||||
|
Proceeds from sale of business
|
69.1
|
|
—
|
|
|
—
|
|
|
—
|
|
|||||
|
Other investing
|
(3.6
|
)
|
(3.7
|
)
|
1.8
|
|
(8.8
|
)
|
|||||||
|
Net cash from (for) investing activities
|
(175.0
|
)
|
(708.1
|
)
|
|
(2,302.3
|
)
|
|
(1,644.3
|
)
|
|||||
|
Cash Flows From (For) Financing Activities
|
|
|
|
|
|||||||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(802.5
|
)
|
718.0
|
|
|
(54.0
|
)
|
|
(3.0
|
)
|
|||||
|
Issuances of long-term debt
|
1,190.3
|
|
—
|
|
|
2,504.3
|
|
|
3,293.6
|
|
|||||
|
Payments on long-term debt
|
(559.2
|
)
|
(28.3
|
)
|
|
(1,823.5
|
)
|
|
(2,035.0
|
)
|
|||||
|
Premium on early debt retirement
|
(0.6
|
)
|
—
|
|
|
—
|
|
|
(133.5
|
)
|
|||||
|
Deferred financing fees
|
(2.8
|
)
|
(0.3
|
)
|
|
(28.1
|
)
|
|
(48.8
|
)
|
|||||
|
Issuance of ordinary shares
|
8.3
|
|
4.9
|
|
|
1,043.5
|
|
|
9.8
|
|
|||||
|
Equity issuance costs
|
(10.3
|
)
|
—
|
|
|
(35.7
|
)
|
|
—
|
|
|||||
|
Repurchase of ordinary shares
|
—
|
|
(500.0
|
)
|
—
|
|
—
|
|
|||||||
|
Cash dividends
|
(83.2
|
)
|
(36.3
|
)
|
|
(64.8
|
)
|
|
(46.1
|
)
|
|||||
|
Other financing
|
(8.7
|
)
|
(8.4
|
)
|
(19.3
|
)
|
(9.0
|
)
|
|||||||
|
Net cash from (for) financing activities
|
(268.7
|
)
|
149.6
|
|
|
1,522.4
|
|
|
1,028.0
|
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(6.7
|
)
|
(10.2
|
)
|
|
(89.2
|
)
|
|
2.9
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
204.5
|
|
(367.8
|
)
|
|
(13.9
|
)
|
|
19.6
|
|
|||||
|
Cash and cash equivalents, beginning of period
|
417.8
|
|
785.6
|
|
|
799.5
|
|
|
779.9
|
|
|||||
|
Cash and cash equivalents, end of period
|
$
|
622.3
|
|
$
|
417.8
|
|
|
$
|
785.6
|
|
|
$
|
799.5
|
|
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
||||||||||||
|
Supplemental Disclosures of Cash Flow Information
|
|||||||||||||||
|
Cash paid/received during the year for:
|
|||||||||||||||
|
Interest paid
|
$
|
205.1
|
|
$
|
84.2
|
|
$
|
143.2
|
|
$
|
98.4
|
|
|||
|
Interest received
|
$
|
1.2
|
|
$
|
0.7
|
|
$
|
1.1
|
|
$
|
2.4
|
|
|||
|
Income taxes paid
|
$
|
139.5
|
|
$
|
87.8
|
|
$
|
131.0
|
|
$
|
93.2
|
|
|||
|
Income taxes refunded
|
$
|
9.3
|
|
$
|
1.7
|
|
$
|
9.6
|
|
$
|
4.3
|
|
|||
|
|
Ordinary Shares
Issued |
Accumulated
Other Comprehensive Income |
Retained
Earnings (Accumulated Deficit) |
Total
|
||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||
|
Balance at June 29, 2013
|
94.1
|
|
$
|
538.5
|
|
$
|
77.0
|
|
$
|
1,715.9
|
|
$
|
2,331.4
|
|
||||
|
|
|
|||||||||||||||||
|
Net income (restated)
|
—
|
|
—
|
|
—
|
|
232.8
|
|
232.8
|
|
||||||||
|
Other comprehensive income (restated)
|
—
|
|
—
|
|
62.6
|
|
—
|
|
62.6
|
|
||||||||
|
Issuance of common stock under:
|
||||||||||||||||||
|
Elan acquisition
|
39.4
|
|
6,117.2
|
|
—
|
|
—
|
|
6,117.2
|
|
||||||||
|
Stock options
|
0.2
|
|
9.8
|
|
—
|
|
—
|
|
9.8
|
|
||||||||
|
Restricted stock plan
|
0.2
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Compensation for stock options
|
—
|
|
6.5
|
|
—
|
|
—
|
|
6.5
|
|
||||||||
|
Compensation for restricted stock
|
—
|
|
18.1
|
|
—
|
|
—
|
|
18.1
|
|
||||||||
|
Cash dividends, $0.39 per share
|
—
|
|
—
|
|
—
|
|
(46.1
|
)
|
(46.1
|
)
|
||||||||
|
Tax effect from stock transactions
|
—
|
|
8.2
|
|
—
|
|
—
|
|
8.2
|
|
||||||||
|
Shares withheld for payment employee's withholding tax liability
|
(0.1
|
)
|
(7.5
|
)
|
—
|
|
—
|
|
(7.5
|
)
|
||||||||
|
Registration of ordinary shares
|
—
|
|
(5.4
|
)
|
—
|
|
—
|
|
(5.4
|
)
|
||||||||
|
Purchase of noncontrolling interest
|
—
|
|
(7.2
|
)
|
—
|
|
—
|
|
(7.2
|
)
|
||||||||
|
Balance at June 28, 2014 (restated)
|
133.8
|
|
6,678.2
|
|
139.6
|
|
1,902.6
|
|
8,720.4
|
|
||||||||
|
|
|
|
|
|
|
|
||||||||||||
|
Net income (restated)
|
—
|
|
—
|
|
—
|
|
136.1
|
|
136.1
|
|
||||||||
|
Other comprehensive loss (restated)
|
—
|
|
—
|
|
(36.1
|
)
|
—
|
|
(36.1
|
)
|
||||||||
|
Issuance of ordinary shares under:
|
||||||||||||||||||
|
Equity offering
|
6.8
|
|
|
1,035.0
|
|
|
—
|
|
|
—
|
|
|
1,035.0
|
|
||||
|
Omega acquisition
|
5.4
|
|
904.9
|
|
—
|
|
—
|
|
904.9
|
|
||||||||
|
Stock options
|
0.2
|
|
8.5
|
|
—
|
|
—
|
|
8.5
|
|
||||||||
|
Restricted stock plan
|
0.2
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Compensation for stock options
|
—
|
|
6.9
|
|
—
|
|
—
|
|
6.9
|
|
||||||||
|
Compensation for restricted stock
|
—
|
|
24.7
|
|
—
|
|
—
|
|
24.7
|
|
||||||||
|
Cash dividends, $0.46 per share
|
—
|
|
—
|
|
—
|
|
(64.8
|
)
|
(64.8
|
)
|
||||||||
|
Tax effect from stock transactions
|
—
|
|
7.0
|
|
—
|
|
—
|
|
7.0
|
|
||||||||
|
Shares withheld for payment of employee's withholding tax liability
|
(0.1
|
)
|
(7.6
|
)
|
—
|
|
—
|
|
(7.6
|
)
|
||||||||
|
Equity issuance costs
|
—
|
|
|
(35.7
|
)
|
|
—
|
|
|
—
|
|
|
(35.7
|
)
|
||||
|
Balance at June 27, 2015 (restated)
|
146.3
|
|
8,621.9
|
|
103.5
|
|
1,973.9
|
|
10,699.3
|
|
||||||||
|
Net income (restated)
|
—
|
|
—
|
|
—
|
|
42.5
|
|
42.5
|
|
||||||||
|
Other comprehensive loss (restated)
|
—
|
|
—
|
|
(118.8
|
)
|
—
|
|
(118.8
|
)
|
||||||||
|
Issuance of ordinary shares under:
|
||||||||||||||||||
|
Stock options
|
0.1
|
|
4.9
|
|
—
|
|
—
|
|
4.9
|
|
||||||||
|
Restricted stock plan
|
0.1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Compensation for stock options
|
—
|
|
2.5
|
|
—
|
|
—
|
|
2.5
|
|
||||||||
|
Compensation for restricted stock (restated)
|
—
|
|
20.3
|
|
—
|
|
—
|
|
20.3
|
|
||||||||
|
Cash dividends, $0.25 per share
|
—
|
|
—
|
|
—
|
|
(36.3
|
)
|
(36.3
|
)
|
||||||||
|
Tax effect from stock transactions
|
—
|
|
3.3
|
|
—
|
|
—
|
|
3.3
|
|
||||||||
|
Shares withheld for payment of employee's withholding tax liability
|
(0.1
|
)
|
(10.3
|
)
|
—
|
|
—
|
|
(10.3
|
)
|
||||||||
|
Repurchases of ordinary shares
|
(3.3
|
)
|
(500.0
|
)
|
—
|
|
—
|
|
(500.0
|
)
|
||||||||
|
Balance at December 31, 2015 (restated)
|
143.1
|
|
8,142.6
|
|
(15.3
|
)
|
1,980.1
|
|
10,107.4
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(4,012.8
|
)
|
(4,012.8
|
)
|
||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
(66.5
|
)
|
—
|
|
(66.5
|
)
|
||||||||
|
Stock options
|
0.2
|
|
8.3
|
|
—
|
|
—
|
|
8.3
|
|
||||||||
|
|
Ordinary Shares
Issued |
Accumulated
Other Comprehensive Income |
Retained
Earnings (Accumulated Deficit) |
Total
|
||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||
|
Restricted stock plan
|
0.2
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Compensation for stock options
|
—
|
|
5.0
|
|
—
|
|
—
|
|
5.0
|
|
||||||||
|
Compensation for restricted stock
|
—
|
|
18.0
|
|
—
|
|
—
|
|
18.0
|
|
||||||||
|
Cash dividends, $0.58 per share
|
—
|
|
(20.8
|
)
|
—
|
|
(62.4
|
)
|
(83.2
|
)
|
||||||||
|
Tax effect from stock transactions
|
—
|
|
(1.5
|
)
|
—
|
|
—
|
|
(1.5
|
)
|
||||||||
|
Shares withheld for payment of employee's
withholding tax liability |
(0.1
|
)
|
(6.3
|
)
|
—
|
|
—
|
|
(6.3
|
)
|
||||||||
|
Equity issuance costs
|
—
|
|
(10.3
|
)
|
—
|
|
—
|
|
(10.3
|
)
|
||||||||
|
Balance at December 31, 2016
|
143.4
|
|
$
|
8,135.0
|
|
$
|
(81.8
|
)
|
$
|
(2,095.1
|
)
|
$
|
5,958.1
|
|
||||
|
•
|
Consumer Healthcare Americas
("
CHCA
")
,
comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
Consumer Healthcare International
("
CHCI
")
,
comprises our legacy Branded Consumer Healthcare ("BCH") segment and now includes our consumer focused businesses in the U.K., Australia, and Israel, which were previously reported in the legacy Consumer Healthcare segment. This segment includes our U.K. liquid licensed products business, which was previously reported in the Prescription Pharmaceuticals segment.
|
|
•
|
Prescription Pharmaceuticals
("
RX
")
,
comprises our U.S. Prescription Pharmaceuticals business.
|
|
•
|
Specialty Sciences
, continued to comprise the Tysabri
®
Royalty Stream.
|
|
|
Six Months Ended
|
||||||||||||||
|
|
December 31, 2015
|
||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Net sales
|
$
|
2,769.5
|
|
$
|
(166.4
|
)
|
$
|
29.1
|
|
(b)
|
$
|
2,632.2
|
|
||
|
Cost of sales
|
1,661.4
|
|
(145.0
|
)
|
36.9
|
|
(b)
|
1,553.3
|
|
||||||
|
Gross profit (loss)
|
1,108.1
|
|
(21.4
|
)
|
(7.8
|
)
|
1,078.9
|
|
|||||||
|
Operating expenses
|
|||||||||||||||
|
Distribution
|
47.9
|
|
—
|
|
—
|
|
47.9
|
|
|||||||
|
Research and development
|
88.2
|
|
—
|
|
—
|
|
88.2
|
|
|||||||
|
Selling
|
325.9
|
|
—
|
|
—
|
|
325.9
|
|
|||||||
|
Administration
|
309.1
|
|
—
|
|
(2.3
|
)
|
306.8
|
|
|||||||
|
Impairment charges
|
215.6
|
|
—
|
|
—
|
|
215.6
|
|
|||||||
|
Restructuring
|
26.9
|
|
—
|
|
—
|
|
26.9
|
|
|||||||
|
Total operating expenses
|
1,013.6
|
|
—
|
|
(2.3
|
)
|
1,011.3
|
|
|||||||
|
Operating income (loss)
|
94.5
|
|
(21.4
|
)
|
(5.5
|
)
|
67.6
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
—
|
|
(57.3
|
)
|
—
|
|
(57.3
|
)
|
|||||||
|
Interest expense, net
|
89.9
|
|
—
|
|
—
|
|
89.9
|
|
|||||||
|
Other expense (income), net
|
26.9
|
|
0.9
|
|
(2.6
|
)
|
25.2
|
|
|||||||
|
Loss on extinguishment of debt
|
0.9
|
|
—
|
|
—
|
|
0.9
|
|
|||||||
|
Income (loss) before income taxes
|
(23.2
|
)
|
35.0
|
|
(2.9
|
)
|
8.9
|
|
|||||||
|
Income tax expense (benefit)
|
(28.8
|
)
|
4.4
|
|
(9.2
|
)
|
(a)
|
(33.6
|
)
|
||||||
|
Net income
|
$
|
5.6
|
|
$
|
30.6
|
|
$
|
6.3
|
|
$
|
42.5
|
|
|||
|
Income per share
|
|||||||||||||||
|
Basic
|
$
|
0.04
|
|
$
|
0.21
|
|
$
|
0.04
|
|
$
|
0.29
|
|
|||
|
Diluted
|
$
|
0.04
|
|
$
|
0.21
|
|
$
|
0.04
|
|
$
|
0.29
|
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
145.6
|
|
145.6
|
|
|||||||||||
|
Diluted
|
146.1
|
|
146.1
|
|
|||||||||||
|
(a)
|
Adjustments primarily related to certain tax basis intangible assets that existed at the time of the acquisition of Omega on
March 30, 2015
, which reduced the deferred tax liabilities in acquired intangible assets and increased our valuation allowance resulting in a net change to our deferred taxes. The resulting balance sheet reclassification required a reduction of goodwill, offset by a corresponding reduction to net deferred taxes at the date of the Omega acquisition. The adjustment made at the date of the Omega acquisition also had an impact on previously reported goodwill impairment charges. ("BCH Deferred Tax Matters").
|
|
(b)
|
Adjustments primarily related to certain contracts related to a specific Belgium distributor that were consignment in nature due to an option for the distributor to return the product if it was not sold timely. The characterization of the contracts as consignment impacted the timing of revenue recognition in the Consolidated Statement of Operations and, due to the impact on factoring arrangements, required a reclassification between accounts receivable and current liabilities for the amounts factored for these contracts. (“BCH Belgium Distribution Contracts”)
|
|
|
Year Ended
|
||||||||||||||
|
|
June 27, 2015
|
||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Net sales
|
$
|
4,603.9
|
|
$
|
(338.5
|
)
|
$
|
(38.3
|
)
|
(b)
|
$
|
4,227.1
|
|
||
|
Cost of sales
|
2,891.4
|
|
(290.1
|
)
|
(18.4
|
)
|
(b)
|
2,582.9
|
|
||||||
|
Gross profit (loss)
|
1,712.5
|
|
(48.4
|
)
|
(19.9
|
)
|
1,644.2
|
|
|||||||
|
Operating expenses
|
|||||||||||||||
|
Distribution
|
67.7
|
|
—
|
|
—
|
|
67.7
|
|
|||||||
|
Research and development
|
187.8
|
|
—
|
|
—
|
|
187.8
|
|
|||||||
|
Selling
|
319.0
|
|
—
|
|
—
|
|
319.0
|
|
|||||||
|
Administration
|
385.2
|
|
—
|
|
0.1
|
|
385.3
|
|
|||||||
|
Impairment charges
|
—
|
|
—
|
|
6.8
|
|
6.8
|
|
|||||||
|
Restructuring
|
5.1
|
|
—
|
|
—
|
|
5.1
|
|
|||||||
|
Total operating expenses
|
964.8
|
|
—
|
|
6.9
|
|
971.7
|
|
|||||||
|
Operating income (loss)
|
747.7
|
|
(48.4
|
)
|
(26.8
|
)
|
672.5
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
—
|
|
(78.5
|
)
|
—
|
|
(78.5
|
)
|
|||||||
|
Interest expense, net
|
146.0
|
|
—
|
|
—
|
|
146.0
|
|
|||||||
|
Other expense (income), net
|
343.2
|
|
—
|
|
(9.0
|
)
|
334.2
|
|
|||||||
|
Loss on extinguishment of debt
|
10.5
|
|
—
|
|
—
|
|
10.5
|
|
|||||||
|
Income (loss) before income taxes
|
248.0
|
|
30.1
|
|
(17.8
|
)
|
260.3
|
|
|||||||
|
Income tax expense
|
120.0
|
|
3.8
|
|
0.4
|
|
124.2
|
|
|||||||
|
Net income (loss)
|
$
|
128.0
|
|
$
|
26.3
|
|
$
|
(18.2
|
)
|
$
|
136.1
|
|
|||
|
Income per share
|
|||||||||||||||
|
Basic
|
$
|
0.92
|
|
$
|
0.18
|
|
$
|
(0.13
|
)
|
$
|
0.97
|
|
|||
|
Diluted
|
$
|
0.92
|
|
$
|
0.18
|
|
$
|
(0.13
|
)
|
$
|
0.97
|
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
139.3
|
|
139.3
|
|
|||||||||||
|
Diluted
|
139.8
|
|
139.8
|
|
|||||||||||
|
(b)
|
Adjustments primarily related to BCH Belgium Distribution Contracts as described above.
|
|
|
Year Ended
|
||||||||||||||
|
|
June 28, 2014
|
||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Net sales
|
$
|
4,060.8
|
|
$
|
(146.7
|
)
|
$
|
—
|
|
$
|
3,914.1
|
|
|||
|
Cost of sales
|
2,613.1
|
|
(152.8
|
)
|
1.7
|
|
2,462.0
|
|
|||||||
|
Gross profit (loss)
|
1,447.7
|
|
6.1
|
|
(1.7
|
)
|
1,452.1
|
|
|||||||
|
Operating expenses
|
|||||||||||||||
|
Distribution
|
55.3
|
|
—
|
|
—
|
|
55.3
|
|
|||||||
|
Research and development
|
158.5
|
|
—
|
|
(6.0
|
)
|
152.5
|
|
|||||||
|
Selling
|
208.6
|
|
—
|
|
—
|
|
208.6
|
|
|||||||
|
Administration
|
411.3
|
|
—
|
|
—
|
|
411.3
|
|
|||||||
|
Impairment charges
|
—
|
|
—
|
|
6.0
|
|
6.0
|
|
|||||||
|
Restructuring
|
47.0
|
|
—
|
|
—
|
|
47.0
|
|
|||||||
|
Total operating expenses
|
880.7
|
|
—
|
|
—
|
|
880.7
|
|
|||||||
|
Operating income (loss)
|
567.0
|
|
6.1
|
|
(1.7
|
)
|
571.4
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
—
|
|
(26.6
|
)
|
—
|
|
(26.6
|
)
|
|||||||
|
Interest expense, net
|
103.5
|
|
—
|
|
—
|
|
103.5
|
|
|||||||
|
Other expense, net
|
25.1
|
|
—
|
|
—
|
|
25.1
|
|
|||||||
|
Loss on extinguishment of debt
|
165.8
|
|
—
|
|
—
|
|
165.8
|
|
|||||||
|
Income (loss) before income taxes
|
272.6
|
|
32.7
|
|
(1.7
|
)
|
303.6
|
|
|||||||
|
Income tax expense (benefit)
|
67.3
|
|
4.1
|
|
(0.6
|
)
|
70.8
|
|
|||||||
|
Net income (loss)
|
$
|
205.3
|
|
$
|
28.6
|
|
$
|
(1.1
|
)
|
$
|
232.8
|
|
|||
|
Income per share
|
|||||||||||||||
|
Basic
|
$
|
1.78
|
|
$
|
0.25
|
|
$
|
(0.01
|
)
|
$
|
2.02
|
|
|||
|
Diluted
|
$
|
1.77
|
|
$
|
0.25
|
|
$
|
(0.01
|
)
|
$
|
2.01
|
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
115.1
|
|
115.1
|
|
|||||||||||
|
Diluted
|
115.6
|
|
115.6
|
|
|||||||||||
|
December 31, 2015
|
|||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Assets
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
417.8
|
|
$
|
—
|
|
$
|
—
|
|
$
|
417.8
|
|
|||
|
Accounts receivable, net of allowance for doubtful accounts of $4.5 million
|
1,193.1
|
|
—
|
|
(4.1
|
)
|
(b)
|
1,189.0
|
|
||||||
|
Inventories
|
844.4
|
|
—
|
|
54.3
|
|
(b)
|
898.7
|
|
||||||
|
Prepaid expenses and other current assets
|
289.1
|
|
—
|
|
(3.0
|
)
|
286.1
|
|
|||||||
|
Total current assets
|
2,744.4
|
|
—
|
|
47.2
|
|
2,791.6
|
|
|||||||
|
Property, plant and equipment, net
|
886.2
|
|
—
|
|
—
|
|
886.2
|
|
|||||||
|
Tysabri
®
royalty stream - at fair value
|
—
|
|
5,310.0
|
|
—
|
|
5,310.0
|
|
|||||||
|
Goodwill and other indefinite-lived intangible assets
|
7,281.2
|
|
—
|
|
(212.2
|
)
|
(a)(b)
|
7,069.0
|
|
||||||
|
Other intangible assets, net
|
8,190.5
|
|
(5,212.2
|
)
|
(5.2
|
)
|
2,973.1
|
|
|||||||
|
Non-current deferred income taxes
|
54.6
|
|
—
|
|
16.8
|
|
(a)(c)
|
71.4
|
|
||||||
|
Other non-current assets
|
237.0
|
|
—
|
|
11.3
|
|
248.3
|
|
|||||||
|
Total non-current assets
|
16,649.5
|
|
97.8
|
|
(189.3
|
)
|
16,558.0
|
|
|||||||
|
Total assets
|
$
|
19,393.9
|
|
$
|
97.8
|
|
$
|
(142.1
|
)
|
$
|
19,349.6
|
|
|||
|
Liabilities and Shareholders’ Equity
|
|||||||||||||||
|
Accounts payable
|
$
|
554.9
|
|
$
|
—
|
|
$
|
0.9
|
|
(b)
|
$
|
555.8
|
|
||
|
Payroll and related taxes
|
125.3
|
|
—
|
|
—
|
|
125.3
|
|
|||||||
|
Accrued customer programs
|
398.0
|
|
—
|
|
(2.0
|
)
|
(b)
|
396.0
|
|
||||||
|
Accrued liabilities
|
308.4
|
|
—
|
|
43.5
|
|
(b)
|
351.9
|
|
||||||
|
Accrued income taxes
|
85.2
|
|
—
|
|
(22.5
|
)
|
(a)
|
62.7
|
|
||||||
|
Current indebtedness
|
1,018.3
|
|
—
|
|
42.2
|
|
(b)
|
1,060.5
|
|
||||||
|
Total current liabilities
|
2,490.1
|
|
—
|
|
62.1
|
|
2,552.2
|
|
|||||||
|
Long-term debt, less current portion
|
4,971.6
|
|
—
|
|
—
|
|
4,971.6
|
|
|||||||
|
Non-current deferred income taxes
|
1,563.7
|
|
12.2
|
|
(203.2
|
)
|
(a)(b)(c)
|
1,372.7
|
|
||||||
|
Other non-current liabilities
|
332.4
|
|
—
|
|
13.9
|
|
(a)
|
346.3
|
|
||||||
|
Total non-current liabilities
|
6,867.7
|
|
12.2
|
|
(189.3
|
)
|
6,690.6
|
|
|||||||
|
Total liabilities
|
9,357.8
|
|
12.2
|
|
(127.2
|
)
|
9,242.8
|
|
|||||||
|
Commitments and contingencies - Note 16
|
|
|
|
|
|||||||||||
|
Shareholders’ equity
|
|||||||||||||||
|
Controlling interest:
|
|||||||||||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
8,144.6
|
|
—
|
|
(2.0
|
)
|
8,142.6
|
|
|||||||
|
Accumulated other comprehensive (loss)
|
(15.5
|
)
|
—
|
|
0.2
|
|
(b)
|
(15.3
|
)
|
||||||
|
Retained earnings
|
1,907.6
|
|
85.6
|
|
(13.1
|
)
|
1,980.1
|
|
|||||||
|
Total controlling interest
|
10,036.7
|
|
85.6
|
|
(14.9
|
)
|
10,107.4
|
|
|||||||
|
Noncontrolling interest
|
(0.6
|
)
|
—
|
|
—
|
|
(0.6
|
)
|
|||||||
|
Total shareholders’ equity
|
10,036.1
|
|
85.6
|
|
(14.9
|
)
|
10,106.8
|
|
|||||||
|
Total liabilities and shareholders' equity
|
$
|
19,393.9
|
|
$
|
97.8
|
|
$
|
(142.1
|
)
|
$
|
19,349.6
|
|
|||
|
(a)
|
Adjustments primarily related to the BCH Deferred Tax Matters as described above.(Goodwill and other indefinite-lived intangible assets:
$(223.3) million
, Non-current deferred income tax asset:
$272.2 million
, and Non-current deferred income tax liability
$65.4 million
)
|
|
(b)
|
Adjustments primarily related to BCH Belgium Distribution Contracts as described above. (Goodwill and other indefinite-lived intangible assets:
$10.2 million
and Non-current deferred income tax liabili:
$8.7 million
)
|
|
(c)
|
Adjustment related to income tax expense (benefit) for interim period tax accounting required under ASC 740, Accounting for Income Taxes. The balance of the adjustment to deferred taxes relates to jurisdictional netting.
|
|
June 27, 2015
|
|||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Assets
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
785.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
785.6
|
|
|||
|
Accounts receivable, net of allowance for doubtful accounts of $2.6 million
|
1,282.1
|
|
—
|
|
(72.7
|
)
|
(b)(d)
|
1,209.4
|
|
||||||
|
Inventories
|
838.9
|
|
—
|
|
96.8
|
|
(b)
|
935.7
|
|
||||||
|
Current deferred income taxes
|
122.3
|
|
—
|
|
25.9
|
|
(c)
|
148.2
|
|
||||||
|
Prepaid expenses and other current assets
|
154.0
|
|
—
|
|
(3.9
|
)
|
150.1
|
|
|||||||
|
Total current assets
|
3,182.9
|
|
—
|
|
46.1
|
|
3,229.0
|
|
|||||||
|
Property, plant and equipment, net
|
932.4
|
|
—
|
|
—
|
|
932.4
|
|
|||||||
|
Tysabri
®
royalty stream - at fair value
|
—
|
|
5,420.0
|
|
—
|
|
5,420.0
|
|
|||||||
|
Goodwill and other indefinite-lived intangible assets
|
7,235.0
|
|
—
|
|
(250.7
|
)
|
(a)(b)
|
6,984.3
|
|
||||||
|
Other intangible assets, net
|
8,105.6
|
|
(5,357.2
|
)
|
(5.6
|
)
|
2,742.8
|
|
|||||||
|
Non-current deferred income taxes
|
39.6
|
|
—
|
|
10.5
|
|
(a)(c)
|
50.1
|
|
||||||
|
Other non-current assets
|
225.1
|
|
—
|
|
8.2
|
|
233.3
|
|
|||||||
|
Total non-current assets
|
16,537.7
|
|
62.8
|
|
(237.6
|
)
|
16,362.9
|
|
|||||||
|
Total assets
|
$
|
19,720.6
|
|
$
|
62.8
|
|
$
|
(191.5
|
)
|
$
|
19,591.9
|
|
|||
|
Liabilities and Shareholders’ Equity
|
|||||||||||||||
|
Accounts payable
|
$
|
747.5
|
|
$
|
—
|
|
$
|
(38.2
|
)
|
(b)(d)
|
$
|
709.3
|
|
||
|
Payroll and related taxes
|
133.9
|
|
—
|
|
—
|
|
133.9
|
|
|||||||
|
Accrued customer programs
|
368.1
|
|
—
|
|
(9.6
|
)
|
(b)
|
358.5
|
|
||||||
|
Accrued liabilities
|
246.4
|
|
—
|
|
11.1
|
|
(b)
|
257.5
|
|
||||||
|
Accrued income taxes
|
52.6
|
|
—
|
|
3.7
|
|
(a)
|
56.3
|
|
||||||
|
Current deferred income taxes
|
80.6
|
|
—
|
|
(0.9
|
)
|
79.7
|
|
|||||||
|
Current indebtedness
|
64.6
|
|
—
|
|
88.7
|
|
(b)
|
153.3
|
|
||||||
|
Total current liabilities
|
1,693.7
|
|
—
|
|
54.8
|
|
1,748.5
|
|
|||||||
|
Long-term debt, less current portion
|
5,246.9
|
|
—
|
|
—
|
|
5,246.9
|
|
|||||||
|
Non-current deferred income taxes
|
1,745.1
|
|
7.9
|
|
(238.7
|
)
|
(a)(b)(c)
|
1,514.3
|
|
||||||
|
Other non-current liabilities
|
372.1
|
|
—
|
|
10.6
|
|
382.7
|
|
|||||||
|
Total non-current liabilities
|
7,364.1
|
|
7.9
|
|
(228.1
|
)
|
7,143.9
|
|
|||||||
|
Total liabilities
|
9,057.8
|
|
7.9
|
|
(173.3
|
)
|
8,892.4
|
|
|||||||
|
Commitments and contingencies - Note 16
|
|
|
|||||||||||||
|
Shareholders’ equity
|
|||||||||||||||
|
Controlling interest:
|
|||||||||||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
8,621.9
|
|
—
|
|
—
|
|
8,621.9
|
|
|||||||
|
Accumulated other comprehensive income
|
102.4
|
|
—
|
|
1.1
|
|
103.5
|
|
|||||||
|
Retained earnings
|
1,938.3
|
|
54.9
|
|
(19.3
|
)
|
1,973.9
|
|
|||||||
|
Total controlling interest
|
10,662.6
|
|
54.9
|
|
(18.2
|
)
|
10,699.3
|
|
|||||||
|
Noncontrolling interest
|
0.2
|
|
—
|
|
—
|
|
0.2
|
|
|||||||
|
Total shareholders’ equity
|
10,662.8
|
|
54.9
|
|
(18.2
|
)
|
10,699.5
|
|
|||||||
|
Total liabilities and shareholders' equity
|
$
|
19,720.6
|
|
$
|
62.8
|
|
$
|
(191.5
|
)
|
$
|
19,591.9
|
|
|||
|
(a)
|
Adjustments primarily related to the BCH Deferred Tax Matters as described above. (Goodwill and other indefinite-lived intangible assets:
$(262.3) million
, Non-current deferred income tax asset:
$268.9 million
, and Non-current deferred tax liability
$4.0 million
)
|
|
(b)
|
Adjustments primarily related to BCH Belgium Distribution Contracts as described above. (Accounts receivable:
$(39.5) million
, Goodwill and other indefinite-lived intangible assets:
$10.5 million
, Accounts payable:
$(1.0) million
, and Non-current deferred income taxes:
$(8.6) million
)
|
|
(c)
|
Adjustment related to income tax expense (benefit) for interim period tax accounting required under ASC 740, Accounting for Income Taxes. The balance of the adjustment to deferred taxes relates to jurisdictional netting.
|
|
(d)
|
The balance of the adjustments in this category relate to other identified required corrections related to balance sheet reclassifications.
|
|
June 28, 2014
|
|||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Assets
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
799.5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
799.5
|
|
|||
|
Accounts receivable, net of allowance for doubtful accounts of $2.7 million
|
935.1
|
|
—
|
|
(3.6
|
)
|
931.5
|
|
|||||||
|
Inventories
|
631.6
|
|
—
|
|
(1.7
|
)
|
629.9
|
|
|||||||
|
Current deferred income taxes
|
62.8
|
|
—
|
|
—
|
|
62.8
|
|
|||||||
|
Prepaid expenses and other current assets
|
121.9
|
|
—
|
|
—
|
|
121.9
|
|
|||||||
|
Total current assets
|
2,550.9
|
|
—
|
|
(5.3
|
)
|
2,545.6
|
|
|||||||
|
Property, plant and equipment, net
|
779.9
|
|
—
|
|
—
|
|
779.9
|
|
|||||||
|
Tysabri
®
royalty stream - at fair value
|
—
|
|
5,680.0
|
|
—
|
|
5,680.0
|
|
|||||||
|
Goodwill and other indefinite-lived intangible assets
|
3,543.8
|
|
—
|
|
(1.1
|
)
|
3,542.7
|
|
|||||||
|
Other intangible assets, net
|
6,787.0
|
|
(5,647.3
|
)
|
(11.0
|
)
|
|
1,128.7
|
|
||||||
|
Non-current deferred income taxes
|
23.6
|
|
—
|
|
—
|
|
23.6
|
|
|||||||
|
Other non-current assets
|
167.6
|
|
—
|
|
11.0
|
|
178.6
|
|
|||||||
|
Total non-current assets
|
11,301.9
|
|
32.7
|
|
(1.1
|
)
|
11,333.5
|
|
|||||||
|
Total assets
|
$
|
13,852.8
|
|
$
|
32.7
|
|
$
|
(6.4
|
)
|
$
|
13,879.1
|
|
|||
|
Liabilities and Shareholders’ Equity
|
|||||||||||||||
|
Accounts payable
|
$
|
364.3
|
|
$
|
—
|
|
$
|
—
|
|
$
|
364.3
|
|
|||
|
Payroll and related taxes
|
112.3
|
|
—
|
|
—
|
|
112.3
|
|
|||||||
|
Accrued customer programs
|
256.5
|
|
—
|
|
—
|
|
256.5
|
|
|||||||
|
Accrued liabilities
|
179.4
|
|
—
|
|
(5.3
|
)
|
174.1
|
|
|||||||
|
Accrued income taxes
|
17.4
|
|
—
|
|
(0.6
|
)
|
16.8
|
|
|||||||
|
Current deferred income taxes
|
1.1
|
|
—
|
|
—
|
|
1.1
|
|
|||||||
|
Current indebtedness
|
143.7
|
|
—
|
|
—
|
|
143.7
|
|
|||||||
|
Total current liabilities
|
1,074.7
|
|
—
|
|
(5.9
|
)
|
1,068.8
|
|
|||||||
|
Long-term debt, less current portion
|
3,063.1
|
|
—
|
|
—
|
|
3,063.1
|
|
|||||||
|
Non-current deferred income taxes
|
727.9
|
|
4.1
|
|
0.6
|
|
732.6
|
|
|||||||
|
Other non-current liabilities
|
293.4
|
|
—
|
|
—
|
|
293.4
|
|
|||||||
|
Total non-current liabilities
|
4,084.4
|
|
4.1
|
|
0.6
|
|
4,089.1
|
|
|||||||
|
Total liabilities
|
5,159.1
|
|
4.1
|
|
(5.3
|
)
|
5,157.9
|
|
|||||||
|
Commitments and contingencies - Note 16
|
|
|
|||||||||||||
|
Shareholders’ equity
|
|||||||||||||||
|
Controlling interest:
|
|||||||||||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
6,678.2
|
|
—
|
|
—
|
|
6,678.2
|
|
|||||||
|
Accumulated other comprehensive income
|
139.6
|
|
—
|
|
—
|
|
139.6
|
|
|||||||
|
Retained earnings
|
1,875.1
|
|
28.6
|
|
(1.1
|
)
|
1,902.6
|
|
|||||||
|
Total controlling interest
|
8,692.9
|
|
28.6
|
|
(1.1
|
)
|
8,720.4
|
|
|||||||
|
Noncontrolling interest
|
0.8
|
|
—
|
|
—
|
|
0.8
|
|
|||||||
|
Total shareholders’ equity
|
8,693.7
|
|
28.6
|
|
(1.1
|
)
|
8,721.2
|
|
|||||||
|
Total liabilities and shareholders' equity
|
$
|
13,852.8
|
|
$
|
32.7
|
|
$
|
(6.4
|
)
|
$
|
13,879.1
|
|
|||
|
Six Months Ended
|
|||||||||||||||
|
December 31, 2015
|
|||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||||||
|
Net income
|
$
|
5.6
|
|
$
|
30.6
|
|
$
|
6.3
|
|
$
|
42.5
|
|
|||
|
Adjustments to derive cash flows
|
|||||||||||||||
|
Loss on extinguishment of debt
|
0.9
|
|
—
|
|
—
|
|
0.9
|
|
|||||||
|
Restructuring charges
|
26.9
|
|
—
|
|
—
|
|
26.9
|
|
|||||||
|
Depreciation and amortization
|
328.0
|
|
(145.0
|
)
|
(0.6
|
)
|
182.4
|
|
|||||||
|
Impairment charges
|
215.6
|
|
—
|
|
—
|
|
215.6
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
—
|
|
(57.3
|
)
|
—
|
|
(57.3
|
)
|
|||||||
|
Share-based compensation
|
24.8
|
|
—
|
|
(2.0
|
)
|
22.8
|
|
|||||||
|
Deferred income taxes
|
(141.8
|
)
|
4.4
|
|
17.4
|
|
(a)(b)
|
(120.0
|
)
|
||||||
|
Amortization of financing fees and debt premium
|
—
|
|
—
|
|
(10.2
|
)
|
(10.2
|
)
|
|||||||
|
Other non-cash adjustments
|
17.5
|
|
—
|
|
0.6
|
|
18.1
|
|
|||||||
|
Subtotal
|
477.5
|
|
(167.3
|
)
|
11.5
|
|
321.7
|
|
|||||||
|
Increase (decrease) in cash due to:
|
|||||||||||||||
|
Accounts receivable
|
86.1
|
|
2.6
|
|
(36.2
|
)
|
(b)
|
52.5
|
|
||||||
|
Inventories
|
(70.0
|
)
|
—
|
|
40.4
|
|
(b)
|
(29.6
|
)
|
||||||
|
Accounts payable
|
(199.5
|
)
|
—
|
|
5.4
|
|
(b)
|
(194.1
|
)
|
||||||
|
Payroll and related taxes
|
(38.2
|
)
|
—
|
|
—
|
|
(38.2
|
)
|
|||||||
|
Accrued customer programs
|
27.0
|
|
—
|
|
7.4
|
|
(b)
|
34.4
|
|
||||||
|
Accrued liabilities
|
75.6
|
|
—
|
|
32.5
|
|
(b)
|
108.1
|
|
||||||
|
Accrued income taxes
|
(30.5
|
)
|
—
|
|
(26.3
|
)
|
(a)
|
(56.8
|
)
|
||||||
|
Other
|
(4.8
|
)
|
—
|
|
7.7
|
|
2.9
|
|
|||||||
|
Subtotal
|
(154.3
|
)
|
2.6
|
|
30.9
|
|
(120.8
|
)
|
|||||||
|
Net cash from (for) operating activities
|
323.2
|
|
(164.7
|
)
|
42.4
|
|
200.9
|
|
|||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||||||
|
Proceeds from royalty rights
|
—
|
|
164.7
|
|
1.6
|
|
166.3
|
|
|||||||
|
Acquisitions of businesses, net of cash acquired
|
(791.6
|
)
|
—
|
|
—
|
|
(791.6
|
)
|
|||||||
|
Additions to property, plant and equipment
|
(77.8
|
)
|
—
|
|
—
|
|
(77.8
|
)
|
|||||||
|
Other investing
|
(5.0
|
)
|
—
|
|
1.3
|
|
(3.7
|
)
|
|||||||
|
Net cash from (for) investing activities
|
(874.4
|
)
|
164.7
|
|
1.6
|
|
(708.1
|
)
|
|||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||||||
|
Payments on long-term debt
|
(28.3
|
)
|
—
|
|
—
|
|
(28.3
|
)
|
|||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
762.0
|
|
—
|
|
(44.0
|
)
|
(b)
|
718.0
|
|
||||||
|
Deferred financing fees
|
(0.3
|
)
|
—
|
|
—
|
|
(0.3
|
)
|
|||||||
|
Issuance of ordinary shares
|
4.9
|
|
—
|
|
—
|
|
4.9
|
|
|||||||
|
Repurchase of ordinary shares
|
(500.0
|
)
|
—
|
|
—
|
|
(500.0
|
)
|
|||||||
|
Cash dividends
|
(36.3
|
)
|
—
|
|
—
|
|
(36.3
|
)
|
|||||||
|
Other financing
|
(8.4
|
)
|
—
|
|
—
|
|
(8.4
|
)
|
|||||||
|
Net cash from (for) financing activities
|
193.6
|
|
—
|
|
(44.0
|
)
|
149.6
|
|
|||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(10.2
|
)
|
—
|
|
—
|
|
(10.2
|
)
|
|||||||
|
Net increase (decrease) in cash and cash equivalents
|
(367.8
|
)
|
—
|
|
—
|
|
(367.8
|
)
|
|||||||
|
Cash and cash equivalents, beginning of period
|
785.6
|
|
—
|
|
—
|
|
785.6
|
|
|||||||
|
Cash and cash equivalents, end of period
|
$
|
417.8
|
|
$
|
—
|
|
$
|
—
|
|
$
|
417.8
|
|
|||
|
(a)
|
Adjustments primarily related to the BCH Deferred Tax Matters as described above.
|
|
(b)
|
Adjustments primarily related to BCH Belgium Distribution Contracts as described above.
|
|
Year Ended
|
|||||||||||||||
|
June 27, 2015
|
|||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||||||
|
Net income
|
$
|
128.0
|
|
$
|
26.3
|
|
$
|
(18.2
|
)
|
$
|
136.1
|
|
|||
|
Adjustments to derive cash flows
|
|||||||||||||||
|
Loss on extinguishment of debt
|
10.5
|
|
—
|
|
—
|
|
10.5
|
|
|||||||
|
Restructuring charges
|
5.1
|
|
—
|
|
—
|
|
5.1
|
|
|||||||
|
Depreciation and amortization
|
548.8
|
|
(290.1
|
)
|
—
|
|
258.7
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
—
|
|
(78.5
|
)
|
—
|
|
(78.5
|
)
|
|||||||
|
Share-based compensation
|
31.6
|
|
—
|
|
—
|
|
31.6
|
|
|||||||
|
Loss on acquisition-related foreign currency derivatives
|
326.4
|
|
—
|
|
—
|
|
326.4
|
|
|||||||
|
Deferred income taxes
|
(16.4
|
)
|
3.8
|
|
(3.7
|
)
|
(a)
|
(16.3
|
)
|
||||||
|
Amortization of financing fees and debt discount
|
—
|
|
—
|
|
0.2
|
|
0.2
|
|
|||||||
|
Other non-cash adjustments
|
17.0
|
|
—
|
|
(6.8
|
)
|
10.2
|
|
|||||||
|
Subtotal
|
1,051.0
|
|
(338.5
|
)
|
(21.7
|
)
|
690.8
|
|
|||||||
|
Increase (decrease) in cash due to:
|
|||||||||||||||
|
Accounts receivable
|
(81.7
|
)
|
(4.3
|
)
|
34.9
|
|
(b)
|
(51.1
|
)
|
||||||
|
Inventories
|
10.7
|
|
—
|
|
(22.1
|
)
|
(b)
|
(11.4
|
)
|
||||||
|
Accounts payable
|
140.6
|
|
—
|
|
(20.1
|
)
|
(b)
|
120.5
|
|
||||||
|
Payroll and related taxes
|
(30.2
|
)
|
—
|
|
—
|
|
(30.2
|
)
|
|||||||
|
Accrued customer programs
|
69.9
|
|
—
|
|
1.4
|
|
(b)
|
71.3
|
|
||||||
|
Accrued liabilities
|
37.3
|
|
—
|
|
5.5
|
|
(b)
|
42.8
|
|
||||||
|
Accrued income taxes
|
17.5
|
|
—
|
|
4.4
|
|
21.9
|
|
|||||||
|
Other
|
(16.8
|
)
|
—
|
|
17.4
|
|
0.6
|
|
|||||||
|
Subtotal
|
147.3
|
|
(4.3
|
)
|
21.4
|
|
164.4
|
|
|||||||
|
Net cash from (for) operating activities
|
1,198.3
|
|
(342.8
|
)
|
(0.3
|
)
|
855.2
|
|
|||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||||||
|
Proceeds from royalty rights
|
—
|
|
342.8
|
|
1.8
|
|
344.6
|
|
|||||||
|
Acquisitions of businesses, net of cash acquired
|
(2,177.8
|
)
|
—
|
|
—
|
|
(2,177.8
|
)
|
|||||||
|
Asset acquisitions
|
(4.0
|
)
|
—
|
|
—
|
|
(4.0
|
)
|
|||||||
|
Settlement of acquisition-related foreign currency derivatives
|
(329.9
|
)
|
—
|
|
—
|
|
(329.9
|
)
|
|||||||
|
Additions to property, plant and equipment
|
(137.0
|
)
|
—
|
|
—
|
|
(137.0
|
)
|
|||||||
|
Other investing
|
1.8
|
|
—
|
|
—
|
|
1.8
|
|
|||||||
|
Net cash from (for) investing activities
|
(2,646.9
|
)
|
342.8
|
|
1.8
|
|
(2,302.3
|
)
|
|||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||||||
|
Issuances of long-term debt
|
2,504.3
|
|
—
|
|
—
|
|
2,504.3
|
|
|||||||
|
Payments on long-term debt
|
(1,823.5
|
)
|
—
|
|
—
|
|
(1,823.5
|
)
|
|||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(52.5
|
)
|
—
|
|
(1.5
|
)
|
(b)
|
(54.0
|
)
|
||||||
|
Deferred financing fees
|
(28.1
|
)
|
—
|
|
—
|
|
(28.1
|
)
|
|||||||
|
Issuance of ordinary shares
|
1,043.4
|
|
—
|
|
0.1
|
|
1,043.5
|
|
|||||||
|
Equity issuance costs
|
(35.7
|
)
|
—
|
|
—
|
|
(35.7
|
)
|
|||||||
|
Cash dividends
|
(64.8
|
)
|
—
|
|
—
|
|
(64.8
|
)
|
|||||||
|
Purchase of noncontrolling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Other financing
|
(19.2
|
)
|
—
|
|
(0.1
|
)
|
(19.3
|
)
|
|||||||
|
Net cash from (for) financing activities
|
1,523.9
|
|
—
|
|
(1.5
|
)
|
1,522.4
|
|
|||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(89.2
|
)
|
—
|
|
—
|
|
(89.2
|
)
|
|||||||
|
Net increase (decrease) in cash and cash equivalents
|
(13.9
|
)
|
—
|
|
—
|
|
(13.9
|
)
|
|||||||
|
Cash and cash equivalents, beginning of period
|
799.5
|
|
—
|
|
—
|
|
799.5
|
|
|||||||
|
Cash and cash equivalents, end of period
|
$
|
785.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
785.6
|
|
|||
|
Year Ended
|
|||||||||||||||
|
June 28, 2014
|
|||||||||||||||
|
Adjustments
|
|||||||||||||||
|
Previously Reported
|
Tysabri
®
|
Other
|
Restated
|
||||||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||||||
|
Net income
|
$
|
205.3
|
|
$
|
28.6
|
|
$
|
(1.1
|
)
|
$
|
232.8
|
|
|||
|
Adjustments to derive cash flows
|
|||||||||||||||
|
Loss on extinguishment of debt
|
165.8
|
|
—
|
|
—
|
|
165.8
|
|
|||||||
|
Restructuring charges
|
47.0
|
|
—
|
|
—
|
|
47.0
|
|
|||||||
|
Depreciation and amortization
|
358.9
|
|
(152.8
|
)
|
—
|
|
206.1
|
|
|||||||
|
Impairment charges
|
—
|
|
—
|
|
6.0
|
|
6.0
|
|
|||||||
|
Tysabri
®
royalty stream - change in fair value
|
—
|
|
(26.6
|
)
|
—
|
|
(26.6
|
)
|
|||||||
|
Share-based compensation
|
24.6
|
|
—
|
|
—
|
|
24.6
|
|
|||||||
|
Deferred income taxes
|
(53.8
|
)
|
4.1
|
|
—
|
|
(49.7
|
)
|
|||||||
|
Amortization of financing fees and debt discount
|
—
|
|
—
|
|
2.0
|
|
2.0
|
|
|||||||
|
Other non-cash adjustments
|
10.5
|
|
—
|
|
(6.0
|
)
|
4.5
|
|
|||||||
|
Subtotal
|
758.3
|
|
(146.7
|
)
|
0.9
|
|
612.5
|
|
|||||||
|
Increase (decrease) in cash due to:
|
|||||||||||||||
|
Accounts receivable
|
(226.7
|
)
|
86.2
|
|
—
|
|
(140.5
|
)
|
|||||||
|
Inventories
|
83.0
|
|
—
|
|
1.7
|
|
84.7
|
|
|||||||
|
Accounts payable
|
(24.9
|
)
|
—
|
|
—
|
|
(24.9
|
)
|
|||||||
|
Payroll and related taxes
|
(55.5
|
)
|
—
|
|
—
|
|
(55.5
|
)
|
|||||||
|
Accrued customer programs
|
113.1
|
|
—
|
|
—
|
|
113.1
|
|
|||||||
|
Accrued liabilities
|
23.0
|
|
—
|
|
—
|
|
23.0
|
|
|||||||
|
Accrued income taxes
|
(10.7
|
)
|
—
|
|
(0.6
|
)
|
(11.3
|
)
|
|||||||
|
Other
|
33.9
|
|
—
|
|
(2.0
|
)
|
31.9
|
|
|||||||
|
Subtotal
|
(64.8
|
)
|
86.2
|
|
(0.9
|
)
|
20.5
|
|
|||||||
|
Net cash from (for) operating activities
|
693.5
|
|
(60.5
|
)
|
—
|
|
633.0
|
|
|||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||||||
|
Proceeds from royalty rights
|
—
|
|
60.5
|
|
—
|
|
60.5
|
|
|||||||
|
Acquisitions of businesses, net of cash acquired
|
(1,605.8
|
)
|
—
|
|
—
|
|
(1,605.8
|
)
|
|||||||
|
Proceeds from sale of securities
|
81.4
|
|
—
|
|
—
|
|
81.4
|
|
|||||||
|
Additions to property, plant and equipment
|
(171.6
|
)
|
—
|
|
—
|
|
(171.6
|
)
|
|||||||
|
Other investing
|
(8.8
|
)
|
—
|
|
—
|
|
(8.8
|
)
|
|||||||
|
Net cash from (for) investing activities
|
(1,704.8
|
)
|
60.5
|
|
—
|
|
(1,644.3
|
)
|
|||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||||||
|
Issuances of long-term debt
|
3,293.6
|
|
—
|
|
—
|
|
3,293.6
|
|
|||||||
|
Payments on long-term debt
|
(2,035.0
|
)
|
—
|
|
(2,035.0
|
)
|
|||||||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(3.0
|
)
|
—
|
|
—
|
|
(3.0
|
)
|
|||||||
|
Deferred financing fees
|
(48.8
|
)
|
—
|
|
—
|
|
(48.8
|
)
|
|||||||
|
Premium on early debt retirement
|
(133.5
|
)
|
—
|
|
—
|
|
(133.5
|
)
|
|||||||
|
Issuance of ordinary shares
|
9.8
|
|
—
|
|
—
|
|
9.8
|
|
|||||||
|
Cash dividends
|
(46.1
|
)
|
—
|
|
—
|
|
(46.1
|
)
|
|||||||
|
Other financing
|
(9.0
|
)
|
—
|
|
—
|
|
(9.0
|
)
|
|||||||
|
Net cash from (for) financing activities
|
1,028.0
|
|
—
|
|
—
|
|
1,028.0
|
|
|||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
2.9
|
|
—
|
|
—
|
|
2.9
|
|
|||||||
|
Net increase (decrease) in cash and cash equivalents
|
19.6
|
|
—
|
|
—
|
|
19.6
|
|
|||||||
|
Cash and cash equivalents, beginning of period
|
779.9
|
|
—
|
|
—
|
|
779.9
|
|
|||||||
|
Cash and cash equivalents, end of period
|
$
|
799.5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
799.5
|
|
|||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Land
|
$
|
45.0
|
|
$
|
47.5
|
|
$
|
48.7
|
|
||
|
Buildings
|
520.2
|
|
508.2
|
|
528.3
|
|
|||||
|
Machinery and equipment
|
1,094.7
|
|
1,103.3
|
|
1,094.0
|
|
|||||
|
Gross property and equipment
|
1,659.9
|
|
1,659.0
|
|
1,671.0
|
|
|||||
|
Less accumulated depreciation
|
(789.8
|
)
|
(772.8
|
)
|
(738.6
|
)
|
|||||
|
Property and equipment, net
|
$
|
870.1
|
|
$
|
886.2
|
|
$
|
932.4
|
|
||
|
•
|
Relief from royalty method
: This method assumes that if the acquired company did not own the intangible asset or intellectual property, it would be willing to pay a royalty for its use. The benefit of ownership of the intellectual property is valued as the relief from the royalty expense that would otherwise be incurred. We typically use this method for valuing readily transferable intangible assets that have licensing appeal, such as trade names and trademarks and certain technology assets.
|
|
•
|
Multi-period excess earnings method
: This method starts with a forecast of the net cash flows expected to be generated by the asset over its estimated useful life. These cash flows are then adjusted to present value by applying an appropriate discount rate that reflects the risk factors associated with the cash flow streams. We typically use this method for valuing intangible assets such as developed product technology, customer relationships, product formulations and IPR&D.
|
|
•
|
Lost income method
: This method estimates the fair value of an asset by comparing the value of the business, inclusive of the asset, to the hypothetical value of the same business excluding the asset.
|
|
Recently Issued Accounting Standards Adopted
|
||||||
|
Standard
|
Description
|
Date of adoption
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Classification of Certain Cash Receipts and Cash Payments
|
This guidance amends and clarifies the current guidance to reduce diversity in practice of the classification of certain cash receipts and payments in the statement of cash flows.
|
December 31, 2016
|
As of December 31, 2016, we reported $2.6 million of contingent consideration payments in the investing section of the statement of cash flows. This adoption did not affect prior years presented.
|
|||
|
Recently Issued Accounting Standards Not Yet Adopted
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Improvements to Employee Share-Based Payment Accounting
|
This guidance is intended to simplify several aspects of the accounting for share-based payment award transactions. It will require all income tax effects of awards to be recorded through the income statement when they vest or settle as opposed to certain amounts being recorded in additional paid-in capital. An entity will also have to elect whether to account for forfeitures as they occur or by estimating the number of awards expected to be forfeited and adjusting the estimate when it is likely to change (as currently required). The guidance will also increase the amount an employer can withhold to cover income taxes on awards.
|
January 1, 2017
|
We will adopt this standard as of January 1, 2017.
|
|||
|
Clarifying the Definition of a Business
|
This update clarifies the definition of a business and addresses whether transactions should be accounted for as asset acquisitions or business combinations (or divestitures). The guidance includes a screen that an acquired set will not be considered a business if substantially all of the fair value of the assets acquired is concentrated in a single tangible or identifiable intangible asset (or group of similar assets).The guidance also includes a framework to determine whether a substantive process is included in a set of acquired assets and activities. In order to have a substantive process, the acquired set should include an organized workforce, among other factors. Further, the guidance removes language stating that a business need not include all of the inputs and processes that the seller used in operating the business.
|
January 1, 2018
|
We are currently evaluating the implications of adoption on our consolidated financial statements.
|
|||
|
Recently Issued Accounting Standards Not Yet Adopted (continued)
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Revenue from Contracts with Customers
|
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps: identify the contract(s) with a customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) the entity satisfies a performance obligation. This guidance allows for two adoption methods, full retrospective approach or modified retrospective approach.
|
January 1, 2018
|
We are currently evaluating the possible adoption methodologies and the implications of adoption on our consolidated financial statements. We have completed an initial assessment of the adoption and are in the process of completing a detailed review of our various customer contracts. Based on our initial analysis, we do not expect there to be a material impact on our revenue recognition practices. We are currently still evaluating the overall impact of the new revenue standard. We plan to adopt the new revenue standard effective January 1, 2018 using the modified retrospective approach and we continue to evaluate the effect of the standard on the ongoing financial reporting.
|
|||
|
Intra-Entity Asset Transfers of Assets Other Than Inventory
|
Under the new guidance, the tax impact to the seller on the profit from the transfers and the buyer’s deferred tax benefit on the increased tax basis would be recognized when the transfers occur resulting in the recognition of expense sooner, as opposed to historical guidance. The guidance excludes intra-entity transfers of inventory. For intra-entity transfers of inventory, the FASB decided to retain current GAAP, which requires an entity to recognize the income tax consequences when the inventory has been sold to an outside party.
|
January 1, 2018
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Leases
|
This guidance was issued to increase transparency and comparability among organizations by requiring recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. For leases with a term of 12 months or less, lessees are permitted to make an election to not recognize right-of-use assets and lease liabilities. Upon adoption, lessees will apply the new standard as of the beginning of the earliest comparative period presented in the financial statements, however lessees will be able to exclude leases that expire as of the implementation date. Early adoption is permitted.
|
January 1, 2019
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Measurement of Credit Losses on Financial Instruments
|
This guidance changes the impairment model for most financial assets and certain other instruments, replacing the current "incurred loss" approach with an "expected loss" credit impairment model, which will apply to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities, and off-balance sheet credit exposures such as letters of credit. Early adoption is permitted.
|
January 1, 2020
|
We are currently evaluating the new standard for potential impacts on our receivables, debt, and other financial instruments and considering whether to early adopt the standard.
|
|||
|
Tretinoin Products
|
Development-Stage Rx Products
|
All Other
(1)
|
|||||||||
|
Restated
|
|||||||||||
|
Purchase price paid
|
$
|
416.4
|
|
$
|
—
|
|
$
|
17.1
|
|
||
|
Contingent consideration
|
—
|
|
24.9
|
|
26.2
|
|
|||||
|
Total purchase consideration
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
43.3
|
|
||
|
Assets acquired:
|
|||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
—
|
|
$
|
3.8
|
|
||
|
Accounts receivable
|
—
|
|
—
|
|
4.9
|
|
|||||
|
Inventories
|
1.4
|
|
—
|
|
7.1
|
|
|||||
|
Prepaid expenses and other current assets
|
—
|
|
—
|
|
0.1
|
|
|||||
|
Property, plant and equipment
|
—
|
|
—
|
|
1.2
|
|
|||||
|
Goodwill
|
1.7
|
|
—
|
|
—
|
|
|||||
|
Definite-lived intangibles
:
|
|||||||||||
|
Distribution and license agreements, supply agreements
|
—
|
|
—
|
|
1.8
|
|
|||||
|
Developed product technology, formulations, and product rights
|
411.0
|
|
—
|
|
18.0
|
|
|||||
|
Customer relationships and distribution networks
|
—
|
|
—
|
|
8.2
|
|
|||||
|
Non-compete agreements
|
2.3
|
|
—
|
|
—
|
|
|||||
|
Indefinite-lived intangibles
:
|
|||||||||||
|
In-process research and development
|
—
|
|
24.9
|
|
4.9
|
|
|||||
|
Total intangible assets
|
$
|
413.3
|
|
$
|
24.9
|
|
$
|
32.9
|
|
||
|
Total assets
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
50.0
|
|
||
|
Liabilities assumed:
|
|||||||||||
|
Accounts payable
|
$
|
—
|
|
$
|
—
|
|
$
|
2.8
|
|
||
|
Accrued liabilities
|
—
|
|
—
|
|
0.1
|
|
|||||
|
Long-term debt
|
—
|
|
—
|
|
3.3
|
|
|||||
|
Net deferred income tax liabilities
|
—
|
|
—
|
|
0.5
|
|
|||||
|
Total liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
6.7
|
|
||
|
Net assets acquired
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
43.3
|
|
||
|
(1)
|
Consists of
four
product acquisitions in our
CHCA
,
CHCI
and
RX
segments
|
|
Entocort
®
|
Naturwohl
|
ScarAway
®
|
GSK Products
|
All Other
(1)
|
|||||||||||||||
|
Purchase price paid
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
15.3
|
|
||||
|
Contingent consideration
|
—
|
|
—
|
|
—
|
|
—
|
|
13.9
|
|
|||||||||
|
Total purchase consideration
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
29.2
|
|
||||
|
Assets acquired:
|
|||||||||||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
4.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Accounts receivable
|
—
|
|
3.3
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Inventories
|
0.2
|
|
1.5
|
|
1.0
|
|
—
|
|
—
|
|
|||||||||
|
Goodwill
|
—
|
|
61.0
|
|
3.5
|
|
32.6
|
|
—
|
|
|||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||||||
|
Distribution and license agreements, supply agreements
|
—
|
|
21.4
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Developed product technology, formulations, and product rights
|
380.0
|
|
—
|
|
0.5
|
|
—
|
|
—
|
|
|||||||||
|
Customer relationships and distribution networks
|
—
|
|
25.9
|
|
9.8
|
|
61.5
|
|
—
|
|
|||||||||
|
Trademarks, trade names, and brands
|
—
|
|
64.2
|
|
11.4
|
|
129.5
|
|
—
|
|
|||||||||
|
Non-compete agreements
|
—
|
|
0.3
|
|
0.5
|
|
—
|
|
—
|
|
|||||||||
|
Indefinite-lived intangibles
:
|
|||||||||||||||||||
|
In-process research and development
|
—
|
|
—
|
|
—
|
|
—
|
|
29.2
|
|
|||||||||
|
Total intangible assets
|
380.0
|
|
111.8
|
|
22.2
|
|
191.0
|
|
29.2
|
|
|||||||||
|
Total assets
|
380.2
|
|
182.2
|
|
26.7
|
|
223.6
|
|
29.2
|
|
|||||||||
|
Liabilities assumed:
|
|||||||||||||||||||
|
Accounts payable
|
—
|
|
2.8
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Accrued liabilities
|
—
|
|
1.6
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net deferred income tax liabilities
|
—
|
|
27.4
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total liabilities
|
—
|
|
31.8
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net assets acquired
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
29.2
|
|
||||
|
(1)
|
Consists of
eight
product development acquisitions in our
CHCA
,
CHCI
and
RX
segments.
|
|
Perrigo ordinary shares issued
|
5.4
|
|
||
|
Perrigo per share price at transaction close on March 30, 2015
|
$
|
167.64
|
|
|
|
Total value of Perrigo ordinary shares issued
|
$
|
904.9
|
|
|
|
Cash consideration
|
2,078.3
|
|
||
|
Total consideration
|
$
|
2,983.2
|
|
|
|
Year Ended
|
|||
|
Line item
|
June 27, 2015
|
||
|
Administration
|
$
|
29.7
|
|
|
Interest expense, net
|
23.7
|
|
|
|
Other expense, net
|
324.0
|
|
|
|
Loss on extinguishment of debt
|
9.6
|
|
|
|
Total acquisition-related costs
|
$
|
387.0
|
|
|
June 27,
2015 |
Measurement Period Adjustments
|
December 31,
2015 |
|||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||
|
Accounts receivable
|
$
|
227.4
|
|
$
|
(4.5
|
)
|
$
|
222.9
|
|
||
|
Inventories
|
$
|
288.9
|
|
$
|
(11.9
|
)
|
$
|
277.0
|
|
||
|
Property and equipment
|
$
|
121.2
|
|
$
|
9.6
|
|
$
|
130.8
|
|
||
|
Goodwill
|
$
|
1,269.6
|
|
$
|
419.1
|
|
$
|
1,688.7
|
|
||
|
Intangible assets:
|
|||||||||||
|
Developed product technology, formulations, and product rights
|
$
|
36.9
|
|
$
|
(5.5
|
)
|
$
|
31.4
|
|
||
|
Customer relationships and distribution networks
|
$
|
1,342.7
|
|
$
|
(286.4
|
)
|
$
|
1,056.3
|
|
||
|
Definite-lived trademarks, trade names, and brands
|
$
|
282.0
|
|
$
|
5.5
|
|
$
|
287.5
|
|
||
|
Indefinite-lived trademarks, trade names, and brands
|
$
|
2,145.2
|
|
$
|
(141.4
|
)
|
$
|
2,003.8
|
|
||
|
Total intangible assets
|
$
|
3,806.8
|
|
$
|
(427.8
|
)
|
$
|
3,379.0
|
|
||
|
Accrued liabilities
|
$
|
50.0
|
|
$
|
(0.7
|
)
|
$
|
49.3
|
|
||
|
Net deferred income tax liabilities
|
$
|
771.1
|
|
$
|
14.4
|
|
$
|
785.5
|
|
||
|
Other non-current liabilities
|
$
|
88.9
|
|
$
|
(29.0
|
)
|
$
|
59.9
|
|
||
|
Gelcaps
|
Omega
Restated* |
Lumara
|
|||||||||
|
Total purchase consideration
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
83.0
|
|
||
|
Assets acquired:
|
|||||||||||
|
Cash and cash equivalents
|
$
|
4.6
|
|
$
|
14.7
|
|
$
|
—
|
|
||
|
Accounts receivable
|
7.3
|
|
222.9
|
|
2.9
|
|
|||||
|
Inventories
|
7.2
|
|
277.0
|
|
1.5
|
|
|||||
|
Prepaid expenses and other current assets
|
2.1
|
|
51.2
|
|
0.4
|
|
|||||
|
Property and equipment
|
6.0
|
|
130.8
|
|
0.1
|
|
|||||
|
Goodwill
|
6.0
|
|
1,688.7
|
|
—
|
|
|||||
|
Definite-lived intangibles
:
|
|||||||||||
|
Developed product technology, formulations, and product rights
|
—
|
|
31.4
|
|
82.0
|
|
|||||
|
Customer relationships and distribution networks
|
6.6
|
|
1,056.3
|
|
—
|
|
|||||
|
Trademarks, trade names, and brands
|
—
|
|
287.5
|
|
—
|
|
|||||
|
Indefinite-lived intangibles
:
|
|||||||||||
|
Trademarks, trade names, and brands
|
4.4
|
|
2,003.8
|
|
—
|
|
|||||
|
Total intangible assets
|
11.0
|
|
3,379.0
|
|
82.0
|
|
|||||
|
Other non-current assets
|
0.4
|
|
2.4
|
|
—
|
|
|||||
|
Total assets
|
44.6
|
|
5,766.7
|
|
86.9
|
|
|||||
|
Liabilities assumed:
|
|||||||||||
|
Accounts payable
|
3.3
|
|
225.0
|
|
—
|
|
|||||
|
Short-term debt
|
—
|
|
112.6
|
|
—
|
|
|||||
|
Accrued liabilities
|
1.6
|
|
49.3
|
|
3.9
|
|
|||||
|
Payroll and related taxes
|
—
|
|
51.3
|
|
—
|
|
|||||
|
Accrued customer programs
|
—
|
|
28.9
|
|
—
|
|
|||||
|
Long-term debt
|
—
|
|
1,471.0
|
|
—
|
|
|||||
|
Net deferred income tax liabilities
|
1.4
|
|
785.5
|
|
—
|
|
|||||
|
Other non-current liabilities
|
0.4
|
|
59.9
|
|
—
|
|
|||||
|
Total liabilities
|
6.7
|
|
2,783.5
|
|
3.9
|
|
|||||
|
Net assets acquired
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
83.0
|
|
||
|
Elan shares outstanding as of December 18, 2013
|
515.7
|
|
||
|
Exchange ratio per share
|
0.07636
|
|
||
|
Total Perrigo shares issued to Elan shareholders
|
39.4
|
|
||
|
Perrigo per share value at transaction close on December 18, 2013
|
$
|
155.34
|
|
|
|
Total value of Perrigo shares issued to Elan shareholders
|
$
|
6,117.2
|
|
|
|
Cash consideration paid at $6.25 per Elan share
|
3,223.2
|
|
||
|
Cash consideration paid for vested Elan stock options and share awards
|
111.5
|
|
||
|
Total consideration
|
$
|
9,451.9
|
|
|
|
Year Ended
|
||||
|
Line item
|
June 28, 2014
|
|||
|
Administration expense
|
$
|
108.9
|
|
|
|
Interest, net
|
10.0
|
|
||
|
Other expense, net
|
0.2
|
|
||
|
Loss on extinguishment of debt
|
165.8
|
|
||
|
Total acquisition-related costs
|
$
|
284.9
|
|
|
|
Segment
|
Goodwill*
Restated
|
|||
|
CHCA
|
$
|
1,287.4
|
|
|
|
RX
|
845.1
|
|
||
|
Specialty Sciences
|
199.5
|
|
||
|
Total
|
$
|
2,332.0
|
|
|
|
Elan*
Restated
|
All Other
(1)
|
||||||
|
Purchase price paid
|
$
|
9,451.9
|
|
$
|
71.0
|
|
|
|
Contingent consideration
|
—
|
|
0.8
|
|
|||
|
Total purchase consideration
|
$
|
9,451.9
|
|
$
|
71.8
|
|
|
|
Assets acquired:
|
|||||||
|
Cash and cash equivalents
|
$
|
1,807.3
|
|
$
|
—
|
|
|
|
Investment securities
|
100.0
|
|
—
|
|
|||
|
Accounts receivable
|
40.6
|
|
—
|
|
|||
|
Inventories
|
—
|
|
3.0
|
|
|||
|
Prepaid expenses and other assets
|
38.1
|
|
—
|
|
|||
|
Property and equipment
|
9.2
|
|
—
|
|
|||
|
Tysabri
®
royalty stream - at fair value
|
5,800.0
|
|
—
|
|
|||
|
Goodwill
|
2,332.0
|
|
4.6
|
|
|||
|
Definite-lived intangibles
:
|
|||||||
|
Distribution and license agreements, supply agreements
|
—
|
|
17.8
|
|
|||
|
Customer relationships and distribution networks
|
—
|
|
9.8
|
|
|||
|
Trademarks, trade names, and brands
|
—
|
|
34.8
|
|
|||
|
Non-compete agreements
|
—
|
|
1.8
|
|
|||
|
Total intangible assets
|
—
|
|
64.2
|
|
|||
|
Other non-current assets
|
93.4
|
|
—
|
|
|||
|
Total assets
|
10,220.6
|
|
71.8
|
|
|||
|
Liabilities assumed:
|
|||||||
|
Accounts payable
|
2.0
|
|
—
|
|
|||
|
Accrued liabilities
|
115.5
|
|
—
|
|
|||
|
Deferred tax liabilities
|
632.4
|
|
—
|
|
|||
|
Other non-current liabilities
|
18.8
|
|
—
|
|
|||
|
Total liabilities
|
768.7
|
|
—
|
|
|||
|
Net assets acquired
|
$
|
9,451.9
|
|
$
|
71.8
|
|
|
|
(1)
|
Includes opening balance sheet of the Aspen and Fera (Methazolomide) product acquisitions.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||
|
December 31, 2016
|
December 31, 2015
|
June 27,
2015 |
|||||||||
|
(Unaudited)
|
Restated
|
Restated
|
|||||||||
|
Net sales
|
$
|
5,288.6
|
|
$
|
2,748.8
|
|
$
|
5,682.5
|
|
||
|
Net income (loss)
|
$
|
(4,011.0
|
)
|
$
|
81.0
|
|
$
|
250.2
|
|
||
|
CHCA
|
CHCI
|
RX
|
Specialty Sciences
|
Other
|
Total
|
||||||||||||||||||
|
Balance at June 28, 2014 (restated)
|
$
|
1,827.9
|
|
$
|
248.2
|
|
$
|
1,098.7
|
|
$
|
200.7
|
|
$
|
97.6
|
|
$
|
3,473.1
|
|
|||||
|
Business acquisitions
|
4.8
|
|
1,269.6
|
|
—
|
|
—
|
|
—
|
|
1,274.4
|
|
|||||||||||
|
Impairments
|
(6.8
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(6.8
|
)
|
|||||||||||
|
Currency translation adjustment
|
(1.5
|
)
|
12.4
|
|
(8.0
|
)
|
—
|
|
(9.4
|
)
|
(6.5
|
)
|
|||||||||||
|
Purchase accounting adjustments
|
(7.2
|
)
|
—
|
|
(4.7
|
)
|
(1.1
|
)
|
—
|
|
(13.0
|
)
|
|||||||||||
|
Balance at June 27, 2015 (restated)
|
1,817.2
|
|
1,530.2
|
|
1,086.0
|
|
199.6
|
|
88.2
|
|
4,721.2
|
|
|||||||||||
|
Business acquisitions
|
9.7
|
|
87.4
|
|
—
|
|
—
|
|
—
|
|
97.1
|
|
|||||||||||
|
Changes in assets held for sale
|
(13.0
|
)
|
—
|
|
—
|
|
—
|
|
(14.6
|
)
|
(27.6
|
)
|
|||||||||||
|
Currency translation adjustment
|
(0.8
|
)
|
(53.3
|
)
|
(1.9
|
)
|
—
|
|
(2.1
|
)
|
(58.1
|
)
|
|||||||||||
|
Purchase accounting adjustments
|
1.2
|
|
418.9
|
|
—
|
|
—
|
|
—
|
|
420.1
|
|
|||||||||||
|
Balance at December 31, 2015 (restated)
|
1,814.3
|
|
1,983.2
|
|
1,084.1
|
|
199.6
|
|
71.5
|
|
5,152.7
|
|
|||||||||||
|
Business acquisitions
|
—
|
|
—
|
|
1.7
|
|
—
|
|
—
|
|
1.7
|
|
|||||||||||
|
Purchase accounting adjustments
|
17.2
|
|
(16.5
|
)
|
—
|
|
—
|
|
—
|
|
0.7
|
|
|||||||||||
|
Impairments
|
(24.5
|
)
|
(868.4
|
)
|
—
|
|
(199.6
|
)
|
—
|
|
(1,092.5
|
)
|
|||||||||||
|
Changes in assets held for sale
|
4.5
|
|
—
|
|
—
|
|
—
|
|
9.0
|
|
13.5
|
|
|||||||||||
|
Currency translation adjustment
|
(0.9
|
)
|
(27.5
|
)
|
0.8
|
|
—
|
|
0.9
|
|
(26.7
|
)
|
|||||||||||
|
Balance at December 31, 2016
|
$
|
1,810.6
|
|
$
|
1,070.8
|
|
$
|
1,086.6
|
|
$
|
—
|
|
$
|
81.4
|
|
$
|
4,049.4
|
|
|||||
|
Reporting Unit
|
Goodwill Remaining in Reporting Unit
|
Segment
|
||||
|
Animal Health
|
$
|
178.9
|
|
CHCA
|
||
|
BCH-Belgium
|
$
|
63.2
|
|
CHCI
|
||
|
BCH-ROW
|
$
|
816.5
|
|
CHCI
|
||
|
|
December 31, 2016
|
December 31, 2015
|
June 27, 2015
|
||||||||||||||||||||
|
|
Gross
|
Accumulated
Amortization
|
Gross
|
Accumulated
Amortization
|
Gross
|
Accumulated Amortization
|
|||||||||||||||||
|
Restated
|
Restated
|
Restated
|
Restated
|
||||||||||||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||||||||||
|
Distribution and license agreements, supply agreements
|
$
|
305.6
|
|
$
|
120.4
|
|
$
|
242.4
|
|
$
|
77.7
|
|
$
|
218.9
|
|
$
|
58.4
|
|
|||||
|
Developed product technology, formulations, and product rights
|
1,418.1
|
|
526.0
|
|
1,387.6
|
|
426.0
|
|
1,029.6
|
|
383.1
|
|
|||||||||||
|
Customer relationships and distribution networks
|
1,489.9
|
|
307.5
|
|
1,520.7
|
|
193.0
|
|
1,750.0
|
|
146.3
|
|
|||||||||||
|
Trademarks, trade names, and brands
|
1,189.3
|
|
55.3
|
|
539.4
|
|
22.8
|
|
340.8
|
|
11.5
|
|
|||||||||||
|
Non-compete agreements
|
14.3
|
|
11.2
|
|
15.2
|
|
12.7
|
|
14.7
|
|
11.9
|
|
|||||||||||
|
Total definite-lived intangibles
|
$
|
4,417.2
|
|
$
|
1,020.4
|
|
$
|
3,705.3
|
|
$
|
732.2
|
|
$
|
3,354.0
|
|
$
|
611.2
|
|
|||||
|
Indefinite-lived intangibles
:
|
|||||||||||||||||||||||
|
Trademarks, trade names, and brands
|
$
|
50.5
|
|
$
|
—
|
|
$
|
1,868.1
|
|
$
|
—
|
|
$
|
2,257.3
|
|
$
|
—
|
|
|||||
|
In-process research and development
|
64.0
|
|
—
|
|
48.2
|
|
—
|
|
5.8
|
|
—
|
|
|||||||||||
|
Total indefinite-lived intangibles
|
114.5
|
|
—
|
|
1,916.3
|
|
—
|
|
2,263.1
|
|
—
|
|
|||||||||||
|
Total other intangible assets
|
$
|
4,531.7
|
|
$
|
1,020.4
|
|
$
|
5,621.6
|
|
$
|
732.2
|
|
$
|
5,617.1
|
|
$
|
611.2
|
|
|||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||||||
|
December 31, 2016
|
December 31, 2015
|
June 28, 2014
|
|||||||||||||||||
|
($ in millions)
|
Indefinite-Lived Intangible Assets
|
Definite-Lived Intangible Assets
|
IPR&D
|
Indefinite-Lived Intangible Assets
|
IPR&D
|
||||||||||||||
|
CHCA
|
$
|
0.4
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
CHCI
|
849.1
|
|
321.4
|
|
3.5
|
|
185.1
|
|
—
|
|
|||||||||
|
RX
|
—
|
|
342.2
|
|
—
|
|
—
|
|
6.0
|
|
|||||||||
|
Other
|
—
|
|
2.0
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
$
|
849.5
|
|
$
|
665.6
|
|
$
|
3.5
|
|
$
|
185.1
|
|
$
|
6.0
|
|
|||||
|
Amortizable Intangible Asset Category
|
Remaining Weighted-Average Useful Life (Years)
|
|
|
Distribution and license agreements, supply agreements
|
7
|
|
|
Developed product technology, formulations, and product rights
|
12
|
|
|
Customer relationships and distribution networks
|
18
|
|
|
Trademarks, trade names, and brands
|
20
|
|
|
Non-compete agreements
|
3
|
|
|
Year
|
Amount
|
|||
|
2017
|
$
|
339.5
|
|
|
|
2018
|
324.6
|
|
||
|
2019
|
299.7
|
|
||
|
2020
|
266.0
|
|
||
|
2021
|
237.5
|
|
||
|
Thereafter
|
1,929.5
|
|
||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Restated
|
Restated
|
||||||||||
|
Finished goods
|
$
|
431.1
|
|
$
|
537.2
|
|
$
|
563.6
|
|
||
|
Work in process
|
165.7
|
|
151.6
|
|
159.1
|
|
|||||
|
Raw materials
|
198.2
|
|
209.9
|
|
213.0
|
|
|||||
|
Total inventories
|
$
|
795.0
|
|
$
|
898.7
|
|
$
|
935.7
|
|
||
|
Level 1:
|
Quoted prices for identical instruments in active markets.
|
|
Level 2:
|
Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets.
|
|
Level 3:
|
Valuations derived from valuation techniques in which one or more significant inputs are not observable.
|
|
Fair Value
|
||||||||||||||
|
Fair Value Hierarchy
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||||
|
Measured at fair value on a recurring basis:
|
||||||||||||||
|
Assets:
|
||||||||||||||
|
Investment securities
|
Level 1
|
$
|
38.2
|
|
$
|
14.9
|
|
$
|
12.7
|
|
||||
|
Foreign currency forward contracts
|
Level 2
|
3.8
|
|
4.8
|
|
12.4
|
|
|||||||
|
Funds associated with Israeli severance liability
|
Level 2
|
15.9
|
|
17.2
|
|
17.3
|
|
|||||||
|
19.7
|
|
22.0
|
|
29.7
|
|
|||||||||
|
Tysabri
®
royalty stream - at fair value (restated)
|
Level 3
|
2,350.0
|
|
5,310.0
|
|
5,420.0
|
|
|||||||
|
Liabilities:
|
||||||||||||||
|
Interest rate swap agreements
|
Level 2
|
—
|
|
0.3
|
|
—
|
|
|||||||
|
Foreign currency forward contracts
|
Level 2
|
5.0
|
|
3.9
|
|
4.6
|
|
|||||||
|
Total level 2 liabilities
|
5.0
|
|
4.2
|
|
4.6
|
|
||||||||
|
Contingent consideration
|
Level 3
|
$
|
69.9
|
|
$
|
17.9
|
|
$
|
—
|
|
||||
|
Measured at fair value on a non-recurring basis:
|
||||||||||||||
|
Assets:
|
||||||||||||||
|
Goodwill
(1)
|
Level 3
|
$
|
1,148.4
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Indefinite-lived intangible assets
(2)
|
Level 3
|
0.3
|
|
1,031.8
|
|
—
|
|
|||||||
|
Definite-lived intangible assets
(3)
|
Level 3
|
758.0
|
|
—
|
|
—
|
|
|||||||
|
Assets held for sale, net
|
Level 3
|
18.2
|
|
37.5
|
|
—
|
|
|||||||
|
Total level 3 assets
|
$
|
1,924.9
|
|
$
|
1,069.3
|
|
$
|
—
|
|
|||||
|
(1)
|
Goodwill with a carrying amount of
$2.2 billion
was written down to its implied fair value of
$1.1 billion
resulting in a total impairment charge of
$1.1 billion
.
|
|
(2)
|
Indefinite-lived intangible assets with a carrying amount of
$0.7 million
were written down to a fair value of
$0.3 million
resulting in a total impairment charge of
$0.4 million
.
|
|
(3)
|
Definite-lived intangible assets with a carrying amount of
$2.3 billion
were written down to a fair value of
$758.0 million
resulting in a total impairment charge of
$1.5 billion
. Included in this balance are indefinite-lived intangible assets with fair value of
$364.5 million
and
$674.2 million
that were reclassified to definite-lived assets at April 3, 2016 and October 2, 2016, respectively. Total impairment charges recorded on the indefinite-lived intangible assets were
$849.1 million
.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Restated
|
Restated
|
||||||||||
|
Tysabri
®
Royalty Stream - at fair value
|
|||||||||||
|
Beginning balance
|
$
|
5,310.0
|
|
$
|
5,420.0
|
|
$
|
5,680.0
|
|
||
|
Royalties earned
|
(351.8
|
)
|
(167.3
|
)
|
(338.5
|
)
|
|||||
|
Change in fair value
|
(2,608.2
|
)
|
57.3
|
|
78.5
|
|
|||||
|
Ending balance
|
$
|
2,350.0
|
|
$
|
5,310.0
|
|
$
|
5,420.0
|
|
||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Contingent Consideration
|
|||||||||||
|
Beginning balance
|
$
|
17.9
|
|
$
|
—
|
|
$
|
17.4
|
|
||
|
Net realized (gains) losses
|
(2.1
|
)
|
—
|
|
0.9
|
|
|||||
|
Purchases or additions
|
56.7
|
|
17.9
|
|
—
|
|
|||||
|
Foreign currency effect
|
0.1
|
|
—
|
|
—
|
|
|||||
|
Settlements
|
(2.7
|
)
|
—
|
|
(18.3
|
)
|
|||||
|
Ending balance
|
$
|
69.9
|
|
$
|
17.9
|
|
$
|
—
|
|
||
|
Year Ended
|
|||||||||
|
December 31, 2016
|
|||||||||
|
Omega - Lifestyle
|
Omega - XLS
|
Entocort
®
- Branded Products
|
Entocort
®
- AG Products
|
Herron Trade names and Trademarks
|
|||||
|
5-year average growth rate
|
2.5%
|
3.2%
|
(31.7)%
|
(30.4)%
|
4.6%
|
||||
|
Long-term growth rates
|
2.0%
|
NA
|
(10.0)%
|
(4.7)%
|
2.5%
|
||||
|
Discount rate
|
9.3%
|
9.5%
|
13.0%
|
10.5%
|
10.8%
|
||||
|
Royalty rate
|
NA
|
4.0%
|
NA
|
NA
|
11.0%
|
||||
|
Valuation method
|
MPEEM
|
Relief from Royalty
|
MPEEM
|
MPEEM
|
Relief from Royalty
|
||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Net unrealized investment gains (losses):
|
|||||||||||
|
Equity securities, at cost less impairments
|
$
|
16.5
|
|
$
|
6.4
|
|
$
|
17.1
|
|
||
|
Gross unrealized gains
|
21.7
|
|
9.3
|
|
5.7
|
|
|||||
|
Gross unrealized losses
|
—
|
|
(0.8
|
)
|
(10.1
|
)
|
|||||
|
Estimated fair value of equity securities
|
$
|
38.2
|
|
$
|
14.9
|
|
$
|
12.7
|
|
||
|
Asset Derivatives
|
|||||||||||||
|
Fair Value
|
|||||||||||||
|
Balance Sheet Location
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
||||||||||
|
Designated derivatives:
|
|||||||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
3.1
|
|
$
|
3.8
|
|
$
|
3.3
|
|
|||
|
Total designated derivatives
|
$
|
3.1
|
|
$
|
3.8
|
|
$
|
3.3
|
|
||||
|
Non-designated derivatives:
|
|||||||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
0.7
|
|
$
|
1.0
|
|
$
|
9.1
|
|
|||
|
Total non-designated derivatives
|
$
|
0.7
|
|
$
|
1.0
|
|
$
|
9.1
|
|
||||
|
Liability Derivatives
|
|||||||||||||
|
Fair Value
|
|||||||||||||
|
Balance Sheet Location
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
||||||||||
|
Designated derivatives:
|
|||||||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
3.0
|
|
$
|
2.0
|
|
$
|
2.0
|
|
|||
|
Interest rate swap agreements
|
Other non-current liabilities
|
—
|
|
0.3
|
|
—
|
|
||||||
|
Total designated derivatives
|
$
|
3.0
|
|
$
|
2.3
|
|
$
|
2.0
|
|
||||
|
Non-designated derivatives:
|
|||||||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
2.0
|
|
$
|
1.9
|
|
$
|
2.6
|
|
|||
|
Total non-designated derivatives
|
$
|
2.0
|
|
$
|
1.9
|
|
$
|
2.6
|
|
||||
|
Amount of Gain/(Loss) Recorded in OCI
(Effective Portion) |
||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||
|
Designated Cash Flow Hedges
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
||||||||||||
|
Treasury locks
|
$
|
—
|
|
$
|
—
|
|
$
|
(2.7
|
)
|
$
|
—
|
|
||||
|
Interest rate swap agreements
|
(9.0
|
)
|
(0.3
|
)
|
(10.1
|
)
|
7.2
|
|
||||||||
|
Foreign currency forward contracts
|
2.1
|
|
1.7
|
|
(7.7
|
)
|
15.1
|
|
||||||||
|
$
|
(6.9
|
)
|
$
|
1.4
|
|
$
|
(20.5
|
)
|
$
|
22.3
|
|
|||||
|
Amount of Gain/(Loss) Reclassified from AOCI to Income
(Effective Portion) |
||||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||
|
Designated Cash Flow Hedges
|
Income Statement
Location
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||
|
Treasury locks
|
Interest expense, net
|
$
|
(0.1
|
)
|
$
|
—
|
|
$
|
(0.1
|
)
|
$
|
0.2
|
|
|||||
|
Interest rate swap agreements
|
Interest expense, net
|
(2.3
|
)
|
(0.8
|
)
|
(16.4
|
)
|
3.9
|
|
|||||||||
|
Foreign currency forward contracts
|
Net sales (restated)
|
1.3
|
|
(1.8
|
)
|
1.9
|
|
(2.5
|
)
|
|||||||||
|
Cost of sales
|
3.0
|
|
0.8
|
|
(4.2
|
)
|
(6.3
|
)
|
||||||||||
|
Interest expense, net
|
(1.6
|
)
|
(0.4
|
)
|
—
|
|
(0.2
|
)
|
||||||||||
|
Other expense, net (restated)
|
0.4
|
|
1.1
|
|
(4.4
|
)
|
(2.2
|
)
|
||||||||||
|
$
|
0.7
|
|
$
|
(1.1
|
)
|
$
|
(23.2
|
)
|
$
|
(7.1
|
)
|
|||||||
|
Amount of Gain/(Loss) Recognized in Income
(Ineffective Portion) |
||||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||
|
Designated Cash Flow Hedges
|
Income Statement
Location
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||
|
Treasury locks
|
Other expense, net
|
$
|
—
|
|
$
|
—
|
|
$
|
(0.4
|
)
|
$
|
2.3
|
|
|||||
|
Interest rate swap agreements
|
Other expense, net
|
(0.1
|
)
|
—
|
|
(0.7
|
)
|
(5.4
|
)
|
|||||||||
|
Foreign currency forward contracts
|
Net sales (restated)
|
(0.1
|
)
|
(0.1
|
)
|
(0.1
|
)
|
(0.1
|
)
|
|||||||||
|
Cost of sales
|
(0.1
|
)
|
0.2
|
|
0.2
|
|
0.3
|
|
||||||||||
|
Other expense, net (restated)
|
0.6
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Total
|
$
|
0.3
|
|
$
|
0.1
|
|
$
|
(1.0
|
)
|
$
|
(2.9
|
)
|
||||||
|
Amount of Gain/(Loss) Recognized in Income
|
||||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||
|
Designated Fair Value Hedges
|
Income Statement
Location
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||
|
Interest rate swap agreements
|
Other expense, net
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
0.9
|
|
|||||
|
Fixed-rate debt
|
Other expense, net
|
—
|
|
—
|
|
—
|
|
(4.1
|
)
|
|||||||||
|
Net hedge
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(3.2
|
)
|
||||||
|
Amount of Gain/(Loss) Recognized in Income
|
||||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||
|
Non-Designated Derivatives
|
Income Statement
Location
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||||
|
Foreign currency forward contracts
|
Other expense, net
|
$
|
(2.4
|
)
|
$
|
(8.0
|
)
|
$
|
(295.4
|
)
|
$
|
(0.1
|
)
|
|||||
|
Interest expense, net
|
(2.2
|
)
|
(0.7
|
)
|
(3.4
|
)
|
—
|
|
||||||||||
|
Foreign exchange option contracts
|
Other expense, net
|
—
|
|
—
|
|
(26.4
|
)
|
—
|
|
|||||||||
|
Total
|
$
|
(4.6
|
)
|
$
|
(8.7
|
)
|
$
|
(325.2
|
)
|
$
|
(0.1
|
)
|
||||||
|
December 31,
2016 |
December 31,
2015 |
||||||||||||||
|
CHCA
|
Other
|
CHCA
|
Other
|
||||||||||||
|
Assets held for sale
|
|||||||||||||||
|
Current assets
|
$
|
—
|
|
$
|
5.1
|
|
$
|
55.1
|
|
$
|
13.6
|
|
|||
|
Goodwill
|
—
|
|
5.5
|
|
13.0
|
|
14.5
|
|
|||||||
|
Property, plant and equipment
|
13.5
|
|
33.2
|
|
18.8
|
|
37.4
|
|
|||||||
|
Other assets
|
—
|
|
3.8
|
|
—
|
|
3.2
|
|
|||||||
|
Less: impairment reserves
|
(3.7
|
)
|
(35.3
|
)
|
—
|
|
(29.0
|
)
|
|||||||
|
Total assets held for sale
|
$
|
9.8
|
|
$
|
12.3
|
|
$
|
86.9
|
|
$
|
39.7
|
|
|||
|
Liabilities held for sale
|
|||||||||||||||
|
Current liabilities
|
$
|
0.1
|
|
$
|
1.9
|
|
$
|
30.5
|
|
$
|
0.5
|
|
|||
|
Other liabilities
|
—
|
|
1.9
|
|
—
|
|
1.7
|
|
|||||||
|
Total liabilities held for sale
|
$
|
0.1
|
|
$
|
3.8
|
|
$
|
30.5
|
|
$
|
2.2
|
|
|||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||||||
|
Revolving credit agreements
|
|||||||||||||||
|
2015 Revolver
|
$
|
—
|
|
$
|
380.0
|
|
$
|
—
|
|
||||||
|
2014 Revolver
|
—
|
|
300.0
|
|
—
|
|
|||||||||
|
Total revolving credit agreements
|
—
|
|
680.0
|
|
—
|
|
|||||||||
|
Term loans
|
|||||||||||||||
|
*
|
2014 Term loan due December 5, 2019
|
420.7
|
|
488.8
|
|
530.5
|
|
||||||||
|
Notes and bonds
|
|||||||||||||||
|
Coupon
|
Due
|
||||||||||||||
|
1.300%
|
November 8, 2016
|
(2)
|
—
|
|
500.0
|
|
500.0
|
|
|||||||
|
*
|
4.500%
|
May 23, 2017
|
(3)
|
189.3
|
|
195.5
|
|
201.0
|
|
||||||
|
*
|
5.125%
|
December 12, 2017
|
(3)
|
315.6
|
|
325.8
|
|
335.0
|
|
||||||
|
2.300%
|
November 8, 2018
|
(2)
|
600.0
|
|
600.0
|
|
600.0
|
|
|||||||
|
*
|
5.000%
|
May 23, 2019
|
(3)
|
126.2
|
|
130.3
|
|
134.1
|
|
||||||
|
3.500%
|
March 15, 2021
|
(4)
|
500.0
|
|
—
|
|
—
|
|
|||||||
|
3.500%
|
December 15, 2021
|
(1)
|
500.0
|
|
500.0
|
|
500.0
|
|
|||||||
|
*
|
5.105%
|
July 19, 2023
|
(3)
|
142.0
|
|
146.7
|
|
150.8
|
|
||||||
|
4.000%
|
November 15, 2023
|
(2)
|
800.0
|
|
800.0
|
|
800.0
|
|
|||||||
|
3.900%
|
December 15, 2024
|
(1)
|
700.0
|
|
700.0
|
|
700.0
|
|
|||||||
|
4.375%
|
March 15, 2026
|
(4)
|
700.0
|
|
—
|
|
—
|
|
|||||||
|
5.300%
|
November 15, 2043
|
(2)
|
400.0
|
|
400.0
|
|
400.0
|
|
|||||||
|
4.900%
|
December 15, 2044
|
(1)
|
400.0
|
|
400.0
|
|
400.0
|
|
|||||||
|
Total notes and bonds
|
5,373.1
|
|
4,698.3
|
|
4,720.9
|
|
|||||||||
|
Other financing (restated)
|
3.6
|
|
128.2
|
|
101.7
|
|
|||||||||
|
Unamortized premium (discount), net
|
33.0
|
|
73.4
|
|
87.6
|
|
|||||||||
|
Deferred financing fees
|
(33.1
|
)
|
(36.6
|
)
|
(40.5
|
)
|
|||||||||
|
Total borrowings outstanding
|
5,797.3
|
|
6,032.1
|
|
5,400.2
|
|
|||||||||
|
Current indebtedness (restated)
|
(572.8
|
)
|
(1,060.5
|
)
|
(153.3
|
)
|
|||||||||
|
Total long-term debt less current portion
|
$
|
5,224.5
|
|
$
|
4,971.6
|
|
$
|
5,246.9
|
|
||||||
|
(1)
|
Discussed below collectively as the "2014 Notes."
|
|
(2)
|
Discussed below collectively as the "2013 Notes."
|
|
(3)
|
Debt assumed from Omega.
|
|
(4)
|
Discussed below collectively as the "2016 Notes."
|
|
*
|
Debt denominated in euros subject to fluctuations in the euro-to-U.S. dollar exchange rate.
|
|
•
|
$20.0 million
in aggregate principal amount of
6.190%
senior notes due
2016
, which was repaid on May 29, 2015 in full;
|
|
•
|
€135.0 million
(
$147.0 million
) in aggregate principal amount of
5.1045%
senior notes due
2023
(the "2023 Notes");
|
|
•
|
€300.0 million
(
$326.7 million
) in aggregate principal amount of
5.125%
retail bonds due
2017
;
€180.0 million
(
$196.0 million
) in aggregate principal amount of
4.500%
retail bonds due
2017
; and
€120.0 million
(
$130.7 million
) in aggregate principal amount of
5.000%
retail bonds due
2019
(collectively, the "Retail Bonds").
|
|
Make-whole payments
|
$
|
133.5
|
|
|
|
Write-off of financing fees on Bridge Credit Agreements
|
19.0
|
|
||
|
Write-off of deferred financing fees
|
10.5
|
|
||
|
Write-off of unamortized discount
|
2.8
|
|
||
|
Total loss on extinguishment of debt
|
$
|
165.8
|
|
|
|
Payment Due
|
Amount
|
||||
|
2017
|
$
|
559.0
|
|
||
|
2018
|
666.4
|
|
(1)
|
||
|
2019
|
429.3
|
|
|||
|
2020
|
0.6
|
|
|||
|
2021
|
1,000.1
|
|
|||
|
Thereafter
|
3,141.9
|
|
|||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Numerator:
|
|||||||||||||||
|
Net income (loss)
|
$
|
(4,012.8
|
)
|
$
|
42.5
|
|
$
|
136.1
|
|
$
|
232.8
|
|
|||
|
Denominator:
|
|||||||||||||||
|
Weighted average shares outstanding for basic EPS
|
143.3
|
|
145.6
|
|
139.3
|
|
115.1
|
|
|||||||
|
Dilutive effect of share-based awards*
|
—
|
|
0.5
|
|
0.5
|
|
0.5
|
|
|||||||
|
Weighted average shares outstanding for diluted EPS
|
143.3
|
|
146.1
|
|
139.8
|
|
115.6
|
|
|||||||
|
Anti-dilutive share-based awards excluded from computation of diluted EPS*
|
—
|
|
0.1
|
|
0.1
|
|
0.1
|
|
|||||||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
Dividends paid (in millions)
|
$
|
83.2
|
|
$
|
36.3
|
|
$
|
64.8
|
|
$
|
46.1
|
|
|||
|
Dividends paid per share
|
$
|
0.58
|
|
$
|
0.25
|
|
$
|
0.46
|
|
$
|
0.39
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
23.0
|
|
$
|
22.8
|
|
$
|
31.6
|
|
$
|
24.6
|
|
|||
|
|
Number of
Options |
Weighted-Average
Exercise Price Per Share |
Weighted-
Average Remaining Term in Years |
Aggregate
Intrinsic Value |
||||||||
|
Options outstanding at June 27, 2015
|
857
|
|
$
|
97.49
|
|
|||||||
|
Exercised
|
(72
|
)
|
$
|
69.62
|
|
|||||||
|
Forfeited or expired
|
(2
|
)
|
$
|
131.91
|
|
|||||||
|
Options outstanding at December 31, 2015
|
783
|
|
$
|
99.93
|
|
6.3
|
$
|
35.6
|
|
|||
|
Granted
|
344
|
|
$
|
126.67
|
|
|||||||
|
Exercised
|
(122
|
)
|
$
|
67.68
|
|
|||||||
|
Forfeited or expired
|
(256
|
)
|
$
|
126.54
|
|
|||||||
|
Options outstanding December 31, 2016
|
749
|
|
$
|
108.40
|
|
6.6
|
$
|
5.5
|
|
|||
|
Options exercisable
|
473
|
|
$
|
96.89
|
|
5.2
|
$
|
5.5
|
|
|||
|
Options expected to vest
|
266
|
|
$
|
128.17
|
|
9.0
|
$
|
—
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
5.2
|
|
$
|
6.7
|
|
$
|
20.7
|
|
$
|
17.8
|
|
|||
|
|
Year Ended
|
|||||||
|
|
December 31,
2016 |
June 27,
2015 |
June 28,
2014 |
|||||
|
Dividend yield
|
0.5
|
%
|
0.3
|
%
|
0.3
|
%
|
||
|
Volatility, as a percent
|
27.6
|
%
|
27.1
|
%
|
32.7
|
%
|
||
|
Risk-free interest rate
|
1.3
|
%
|
1.7
|
%
|
1.8
|
%
|
||
|
Expected life in years
|
5.5
|
|
5.3
|
|
5.3
|
|
||
|
|
Number of
Non-vested Service- Based Share Units |
Weighted-
Average Grant Date Fair Value Per Share |
Weighted-
Average Remaining Term in Years |
Aggregate
Intrinsic Value |
||||||||
|
Non-vested service-based share units outstanding at June 27, 2015
|
283
|
|
$
|
136.48
|
|
|||||||
|
Granted
|
199
|
|
$
|
165.64
|
|
|||||||
|
Vested
|
(94
|
)
|
$
|
125.03
|
|
|||||||
|
Forfeited
|
(6
|
)
|
$
|
164.56
|
|
|||||||
|
Non-vested service-based share units outstanding at December 31, 2015
|
382
|
|
$
|
154.07
|
|
2.2
|
$
|
55.3
|
|
|||
|
Granted
|
298
|
|
$
|
113.26
|
|
|||||||
|
Vested
|
(92
|
)
|
$
|
137.15
|
|
|||||||
|
Forfeited
|
(120
|
)
|
$
|
151.64
|
|
|||||||
|
Non-vested service-based share units outstanding at December 31, 2016
|
468
|
|
$
|
137.53
|
|
1.7
|
$
|
39.0
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
113.26
|
|
$
|
165.64
|
|
$
|
153.99
|
|
$
|
133.08
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
12.6
|
|
$
|
11.7
|
|
$
|
9.1
|
|
$
|
6.8
|
|
|||
|
|
Number of
Non-vested Performance- Based Share Units |
Weighted-
Average Grant Date Fair Value Per Share |
Weighted-
Average Remaining Term in Years |
Aggregate
Intrinsic Value |
||||||||
|
Non-vested performance-based share units outstanding at June 27, 2015
|
229
|
|
$
|
129.77
|
|
|||||||
|
Granted
|
55
|
|
$
|
184.49
|
|
|||||||
|
Vested
|
(58
|
)
|
$
|
109.20
|
|
|||||||
|
Forfeited
|
(3
|
)
|
$
|
144.73
|
|
|||||||
|
Non-vested performance-based share units outstanding at December 31, 2015
|
223
|
|
$
|
146.31
|
|
1.5
|
$
|
32.3
|
|
|||
|
Granted
|
159
|
|
$
|
126.37
|
|
|||||||
|
Vested
|
(81
|
)
|
$
|
128.74
|
|
|||||||
|
Forfeited
|
(124
|
)
|
$
|
143.64
|
|
|||||||
|
Non-vested performance-based share units outstanding at December 31, 2016
|
177
|
|
$
|
138.29
|
|
1.7
|
$
|
14.8
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
126.37
|
|
$
|
184.49
|
|
$
|
150.14
|
|
$
|
119.85
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
10.4
|
|
$
|
6.4
|
|
$
|
5.1
|
|
$
|
4.6
|
|
|||
|
Fair value of derivative financial instruments, net of tax
|
Foreign currency translation adjustments
|
Fair value of investment securities, net of tax
|
Post-retirement and pension liability adjustments, net of tax
|
Total AOCI
|
|||||||||||||||
|
Balance at June 28, 2014 (restated)
|
$
|
(16.1
|
)
|
$
|
164.4
|
|
$
|
2.4
|
|
$
|
(11.1
|
)
|
$
|
139.6
|
|
||||
|
OCI before reclassifications
|
(15.1
|
)
|
(33.5
|
)
|
(5.3
|
)
|
2.9
|
|
(51.0
|
)
|
|||||||||
|
Amounts reclassified from AOCI
|
14.9
|
|
—
|
|
—
|
|
—
|
|
14.9
|
|
|||||||||
|
Other comprehensive income (loss)
|
(0.2
|
)
|
(33.5
|
)
|
(5.3
|
)
|
2.9
|
|
(36.1
|
)
|
|||||||||
|
Balance at June 27, 2015 (restated)
|
(16.3
|
)
|
130.9
|
|
(2.9
|
)
|
(8.2
|
)
|
103.5
|
|
|||||||||
|
OCI before reclassifications
|
1.1
|
|
(135.5
|
)
|
(1.4
|
)
|
6.7
|
|
(129.1
|
)
|
|||||||||
|
Amounts reclassified from AOCI
|
1.0
|
|
—
|
|
10.7
|
|
(1.4
|
)
|
10.3
|
|
|||||||||
|
Other comprehensive income (loss)
|
2.1
|
|
(135.5
|
)
|
9.3
|
|
5.3
|
|
(118.8
|
)
|
|||||||||
|
Balance at December 31, 2015 (restated)
|
(14.2
|
)
|
(4.6
|
)
|
6.4
|
|
(2.9
|
)
|
(15.3
|
)
|
|||||||||
|
OCI before reclassifications
|
(5.4
|
)
|
(63.3
|
)
|
7.4
|
|
(3.2
|
)
|
(64.5
|
)
|
|||||||||
|
Amounts reclassified from AOCI
|
0.1
|
|
—
|
|
1.3
|
|
(3.4
|
)
|
(2.0
|
)
|
|||||||||
|
Other comprehensive income (loss)
|
(5.3
|
)
|
(63.3
|
)
|
8.7
|
|
(6.6
|
)
|
(66.5
|
)
|
|||||||||
|
Balance at December 31, 2016
|
$
|
(19.5
|
)
|
$
|
(67.9
|
)
|
$
|
15.1
|
|
$
|
(9.5
|
)
|
$
|
(81.8
|
)
|
||||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Pre-tax income (loss):
|
|||||||||||||||
|
Ireland
|
$
|
(3,624.1
|
)
|
$
|
(310.2
|
)
|
$
|
(792.8
|
)
|
$
|
(336.6
|
)
|
|||
|
Other
|
(1,224.2
|
)
|
319.1
|
|
1,053.1
|
|
640.2
|
|
|||||||
|
Total pre-tax income (loss)
|
(4,848.3
|
)
|
8.9
|
|
260.3
|
|
303.6
|
|
|||||||
|
(Benefit) Provision for income taxes:
|
|||||||||||||||
|
Current:
|
|||||||||||||||
|
Ireland
|
0.3
|
|
1.6
|
|
(2.2
|
)
|
2.2
|
|
|||||||
|
United States - federal
|
93.0
|
|
58.9
|
|
77.2
|
|
42.8
|
|
|||||||
|
United States - state
|
0.7
|
|
3.0
|
|
6.8
|
|
9.3
|
|
|||||||
|
Other foreign
|
26.7
|
|
53.0
|
|
67.4
|
|
49.1
|
|
|||||||
|
Subtotal
|
120.7
|
|
116.5
|
|
149.2
|
|
103.4
|
|
|||||||
|
Deferred (credit):
|
|||||||||||||||
|
Ireland
|
(549.4
|
)
|
(23.1
|
)
|
11.1
|
|
(20.1
|
)
|
|||||||
|
United States - federal
|
(7.6
|
)
|
(34.4
|
)
|
(19.9
|
)
|
8.4
|
|
|||||||
|
United States - state
|
(5.1
|
)
|
(3.3
|
)
|
(0.8
|
)
|
(5.8
|
)
|
|||||||
|
Other foreign
|
(394.1
|
)
|
(89.3
|
)
|
(15.4
|
)
|
(15.1
|
)
|
|||||||
|
Subtotal
|
(956.2
|
)
|
(150.1
|
)
|
(25.0
|
)
|
(32.6
|
)
|
|||||||
|
Total (benefit) provision for income taxes
|
$
|
(835.5
|
)
|
$
|
(33.6
|
)
|
$
|
124.2
|
|
$
|
70.8
|
|
|||
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||
|
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
|||||||
|
|
Restated
|
Restated
|
Restated
|
||||||||
|
Provision at statutory rate
|
12.5
|
%
|
12.5
|
%
|
12.5
|
%
|
12.5
|
%
|
|||
|
Ireland tax on non-trading differences
|
(0.4
|
)
|
(207.4
|
)
|
(9.9
|
)
|
2.6
|
|
|||
|
Expenses not deductible for tax purposes/deductions not expensed for book, net
|
(0.7
|
)
|
394.0
|
|
14.7
|
|
10.9
|
|
|||
|
Goodwill impairment not deductible for tax purposes
|
(2.8
|
)
|
—
|
|
—
|
|
—
|
|
|||
|
U.S. Operations:
|
|||||||||||
|
State income taxes, net of federal benefit
|
0.1
|
|
38.4
|
|
(1.0
|
)
|
(0.2
|
)
|
|||
|
Foreign tax credit
|
—
|
|
—
|
|
—
|
|
0.2
|
|
|||
|
Research and development credit
|
—
|
|
(13.2
|
)
|
(0.7
|
)
|
(0.4
|
)
|
|||
|
Other
|
0.4
|
|
112.3
|
|
4.8
|
|
(0.9
|
)
|
|||
|
Other foreign differences (earnings taxed at other than applicable statutory rate)
|
3.3
|
|
(647.2
|
)
|
(16.1
|
)
|
(14.5
|
)
|
|||
|
Intangible Impairment differences
|
4.8
|
|
(397.6
|
)
|
—
|
|
—
|
|
|||
|
Worldwide operations:
|
|||||||||||
|
Valuation allowance changes
|
0.8
|
|
249.3
|
|
25.7
|
|
2.6
|
|
|||
|
Change in unrecognized taxes
|
(0.8
|
)
|
82.7
|
|
17.7
|
|
13.5
|
|
|||
|
Rate change impacts
|
—
|
|
—
|
|
—
|
|
(3.0
|
)
|
|||
|
Effective income tax rate
|
17.2
|
%
|
(376.2
|
)%
|
47.7
|
%
|
23.3
|
%
|
|||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Restated
|
Restated
|
||||||||||
|
Deferred income tax asset (liability):
|
|||||||||||
|
Depreciation and amortization
|
$
|
(765.2
|
)
|
$
|
(1,550.6
|
)
|
$
|
(1,618.1
|
)
|
||
|
Inventory basis differences
|
27.4
|
|
22.8
|
|
32.6
|
|
|||||
|
Accrued liabilities
|
68.5
|
|
50.8
|
|
69.3
|
|
|||||
|
Allowance for doubtful accounts
|
1.7
|
|
1.3
|
|
0.9
|
|
|||||
|
Research and development
|
61.7
|
|
63.7
|
|
62.8
|
|
|||||
|
Loss carryforwards
|
292.4
|
|
244.2
|
|
232.4
|
|
|||||
|
Share-based compensation
|
18.1
|
|
20.6
|
|
14.3
|
|
|||||
|
Foreign tax credit
|
10.6
|
|
10.6
|
|
10.6
|
|
|||||
|
Federal benefit of unrecognized tax positions
|
24.3
|
|
22.8
|
|
26.3
|
|
|||||
|
Interest carryforwards
|
435.3
|
|
334.6
|
|
259.7
|
|
|||||
|
Other, net
|
3.0
|
|
14.7
|
|
30.1
|
|
|||||
|
Subtotal
|
177.8
|
|
(764.5
|
)
|
(879.1
|
)
|
|||||
|
Valuation allowance
|
(495.6
|
)
|
(536.8
|
)
|
(516.6
|
)
|
|||||
|
Net deferred income tax asset (liability):
|
$
|
(317.8
|
)
|
$
|
(1,301.3
|
)
|
$
|
(1,395.7
|
)
|
||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Restated
|
Restated
|
||||||||||
|
Assets
|
$
|
72.1
|
|
$
|
71.4
|
|
$
|
198.3
|
|
||
|
Liabilities
|
(389.9
|
)
|
(1,372.7
|
)
|
(1,594.0
|
)
|
|||||
|
Net deferred income tax (liability) asset
|
$
|
(317.8
|
)
|
$
|
(1,301.3
|
)
|
$
|
(1,395.7
|
)
|
||
|
December 31, 2016
|
|||||||
|
Gross
Carryforwards (1) |
Gross Valuation Allowances
|
||||||
|
U.S. state net operating losses
|
$
|
248.8
|
|
$
|
193.6
|
|
|
|
Worldwide federal net operating losses excluding U.S. states
|
$
|
997.7
|
|
$
|
787.7
|
|
|
|
Worldwide federal capital losses
|
$
|
19.7
|
|
$
|
19.7
|
|
|
|
U.S. federal credits
|
$
|
265.9
|
|
$
|
265.9
|
|
|
|
U.S. state credits
|
$
|
1,462.6
|
|
$
|
1,458.2
|
|
|
|
Unrecognized
Tax Benefits
|
|||
|
Balance at June 28, 2014
|
$
|
160.1
|
|
|
Additions:
|
|||
|
Positions related to the current year
|
38.9
|
|
|
|
Positions related to prior years
|
128.1
|
|
|
|
Reductions:
|
|||
|
Settlements with taxing authorities
|
(1.4
|
)
|
|
|
Lapse of statutes of limitation
|
(1.7
|
)
|
|
|
Balance at June 27, 2015 (restated)
|
324.0
|
|
|
|
Additions:
|
|||
|
Positions related to the current year
|
22.9
|
|
|
|
Reductions:
|
|||
|
Positions related to prior years
|
(43.5
|
)
|
|
|
Settlements with taxing authorities
|
(15.3
|
)
|
|
|
Balance at December 31, 2015 (restated)
|
288.1
|
|
|
|
Additions:
|
|||
|
Positions related to the current year
|
45.5
|
|
|
|
Positions related to prior years
|
8.6
|
|
|
|
Reductions:
|
|||
|
Settlements with taxing authorities
|
(2.4
|
)
|
|
|
Lapse of statutes of limitation
|
(5.3
|
)
|
|
|
Balance at December 31, 2016
|
$
|
334.5
|
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
23.2
|
|
$
|
15.8
|
|
$
|
24.6
|
|
$
|
25.6
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27,
2015 |
June 28,
2014 |
|||||||||||
|
$
|
0.6
|
|
$
|
0.2
|
|
$
|
0.7
|
|
$
|
0.5
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27, 2015 *
|
||||||||
|
$
|
2.3
|
|
$
|
2.9
|
|
$
|
0.6
|
|
||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Pension Benefits
|
Other Benefits
|
||||||||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27, 2015 *
|
December 31,
2016 |
December 31,
2015 |
June 27, 2015 *
|
||||||||||||||||||
|
Restated
|
Restated
|
||||||||||||||||||||||
|
Projected benefit obligation at beginning of period
|
$
|
135.0
|
|
$
|
140.3
|
|
$
|
89.0
|
|
$
|
7.0
|
|
$
|
6.0
|
|
$
|
4.6
|
|
|||||
|
Acquisitions
|
—
|
|
5.6
|
|
70.4
|
|
—
|
|
—
|
|
1.0
|
|
|||||||||||
|
Service costs
|
4.1
|
|
2.2
|
|
0.9
|
|
0.6
|
|
0.3
|
|
0.3
|
|
|||||||||||
|
Interest cost
|
3.6
|
|
1.7
|
|
2.4
|
|
0.2
|
|
0.1
|
|
0.2
|
|
|||||||||||
|
Actuarial (gain) loss
|
22.6
|
|
(10.1
|
)
|
(6.8
|
)
|
(1.9
|
)
|
0.5
|
|
—
|
|
|||||||||||
|
Contributions paid
|
0.3
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Benefits paid
|
(1.7
|
)
|
(0.6
|
)
|
(0.9
|
)
|
(0.1
|
)
|
(0.1
|
)
|
(0.1
|
)
|
|||||||||||
|
Foreign currency translation
|
(5.0
|
)
|
(4.1
|
)
|
(14.7
|
)
|
—
|
|
0.1
|
|
—
|
|
|||||||||||
|
Projected benefit obligation at end of period
|
$
|
158.9
|
|
$
|
135.0
|
|
$
|
140.3
|
|
$
|
5.8
|
|
$
|
7.0
|
|
$
|
6.0
|
|
|||||
|
Fair value of plan assets at beginning of period
|
126.7
|
|
128.1
|
|
99.6
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Acquisitions
|
—
|
|
3.2
|
|
43.5
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Actual return on plan assets
|
9.4
|
|
(1.7
|
)
|
0.2
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Benefits paid
|
(1.7
|
)
|
(0.6
|
)
|
(0.1
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Employer contributions
|
8.2
|
|
1.4
|
|
2.4
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Contributions paid
|
0.3
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Foreign currency translation
|
(4.7
|
)
|
(3.7
|
)
|
(17.5
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Fair value of plan assets at end of period
|
$
|
138.2
|
|
$
|
126.7
|
|
$
|
128.1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Funded (unfunded) status
|
$
|
(20.7
|
)
|
$
|
(8.3
|
)
|
$
|
(12.2
|
)
|
$
|
(5.8
|
)
|
$
|
(7.0
|
)
|
$
|
(6.0
|
)
|
|||||
|
Presented as:
|
|||||||||||||||||||||||
|
Other non-current assets
|
$
|
10.4
|
|
$
|
16.5
|
|
$
|
12.8
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Other non-current liabilities
|
$
|
(31.1
|
)
|
$
|
(24.8
|
)
|
$
|
(25.0
|
)
|
$
|
(5.8
|
)
|
$
|
(7.0
|
)
|
$
|
(6.0
|
)
|
|||||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27, 2015 *
|
||||||||
|
$
|
136.3
|
|
$
|
109.4
|
|
$
|
136.6
|
|
||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27,
2015 |
June 27, 2015 *
|
|||||||||||
|
$
|
(0.7
|
)
|
$
|
(0.4
|
)
|
$
|
0.1
|
|
$
|
(0.1
|
)
|
|||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27, 2015 *
|
||||||||
|
$
|
9.5
|
|
$
|
2.9
|
|
$
|
8.2
|
|
||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Payment Due
|
Pension Benefits
|
Other Benefits
|
||||||
|
2017
|
$
|
1.3
|
|
$
|
0.1
|
|
||
|
2018
|
1.5
|
|
0.2
|
|
||||
|
2019
|
2.3
|
|
0.2
|
|
||||
|
2020
|
2.3
|
|
0.2
|
|
||||
|
2021
|
2.0
|
|
0.3
|
|
||||
|
Thereafter
|
17.1
|
|
1.9
|
|
||||
|
Pension Benefits
|
Other Benefits
|
||||||||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||||||||
|
December 31,
2016 |
December 31, 2015
|
June 27, 2015 *
|
December 31,
2016 |
December 31, 2015
|
June 27, 2015 *
|
||||||||||||||||||
|
Service cost
|
$
|
4.1
|
|
$
|
2.2
|
|
$
|
0.9
|
|
$
|
0.6
|
|
$
|
0.3
|
|
$
|
0.3
|
|
|||||
|
Interest cost
|
3.6
|
|
1.7
|
|
2.4
|
|
0.2
|
|
0.1
|
|
0.2
|
|
|||||||||||
|
Expected return on plan assets
|
(3.9
|
)
|
(1.8
|
)
|
(2.7
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Net actuarial loss
|
0.5
|
|
0.4
|
|
1.0
|
|
—
|
|
—
|
|
0.1
|
|
|||||||||||
|
Net periodic pension cost
|
$
|
4.3
|
|
$
|
2.5
|
|
$
|
1.6
|
|
$
|
0.8
|
|
$
|
0.4
|
|
$
|
0.6
|
|
|||||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Pension Benefits
|
Other Benefits
|
||||||||||||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27, 2015 *
|
December 31,
2016 |
December 31,
2015 |
June 27, 2015 *
|
||||||||||||
|
Discount rate
|
1.76
|
%
|
2.22
|
%
|
2.11
|
%
|
4.00
|
%
|
4.25
|
%
|
4.25
|
%
|
|||||
|
Inflation
|
1.43
|
%
|
2.25
|
%
|
1.93
|
%
|
|||||||||||
|
Expected return on assets
|
2.89
|
%
|
2.93
|
%
|
2.85
|
%
|
|||||||||||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Equities
|
6.2
|
%
|
|
Bonds
|
1.6
|
%
|
|
Absolute return fund
|
4.0
|
%
|
|
Insurance contracts
|
2.8
|
%
|
|
Quoted Prices in Active Markets
|
Other Observable Inputs
|
Unobservable Inputs
|
|||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
||||||||||||
|
Equities
|
$
|
0.1
|
|
$
|
13.6
|
|
$
|
—
|
|
$
|
13.7
|
|
|||
|
Bonds
|
1.6
|
|
22.8
|
|
—
|
|
24.4
|
|
|||||||
|
Property
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Insurance contracts
|
—
|
|
—
|
|
43.4
|
|
43.4
|
|
|||||||
|
Absolute return fund
|
—
|
|
51.5
|
|
—
|
|
51.5
|
|
|||||||
|
Other
|
—
|
|
5.2
|
|
—
|
|
5.2
|
|
|||||||
|
Total
|
$
|
1.7
|
|
$
|
93.1
|
|
$
|
43.4
|
|
$
|
138.2
|
|
|||
|
Quoted Prices in Active Markets
|
Other Observable Inputs
|
Unobservable Inputs
|
|||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
||||||||||||
|
Restated
|
Restated
|
Restated
|
Restated
|
||||||||||||
|
Equities
|
$
|
—
|
|
$
|
14.5
|
|
$
|
—
|
|
$
|
14.5
|
|
|||
|
Bonds
|
—
|
|
38.1
|
|
—
|
|
38.1
|
|
|||||||
|
Property
|
—
|
|
—
|
|
0.3
|
|
0.3
|
|
|||||||
|
Insurance contracts
|
—
|
|
—
|
|
34.9
|
|
34.9
|
|
|||||||
|
Absolute return fund
|
—
|
|
33.7
|
|
—
|
|
33.7
|
|
|||||||
|
Other
|
—
|
|
5.2
|
|
—
|
|
5.2
|
|
|||||||
|
Total
|
$
|
—
|
|
$
|
91.5
|
|
$
|
35.2
|
|
$
|
126.7
|
|
|||
|
Quoted Prices in Active Markets
|
Other Observable Inputs
|
Unobservable Inputs
|
|||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
||||||||||||
|
Restated
|
Restated
|
Restated
|
Restated
|
||||||||||||
|
Equities
|
$
|
—
|
|
$
|
15.4
|
|
$
|
—
|
|
$
|
15.4
|
|
|||
|
Bonds
|
—
|
|
38.0
|
|
—
|
|
38.0
|
|
|||||||
|
Property
|
—
|
|
—
|
|
0.4
|
|
0.4
|
|
|||||||
|
Insurance contracts
|
—
|
|
—
|
|
33.9
|
|
33.9
|
|
|||||||
|
Absolute return fund
|
—
|
|
34.8
|
|
—
|
|
34.8
|
|
|||||||
|
Other
|
—
|
|
5.6
|
|
—
|
|
5.6
|
|
|||||||
|
Total
|
$
|
—
|
|
$
|
93.8
|
|
$
|
34.3
|
|
$
|
128.1
|
|
|||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27, 2015 *
|
|||||||||
|
Restated
|
Restated
|
||||||||||
|
Level 3 assets held at beginning of year
|
$
|
35.2
|
|
$
|
34.3
|
|
$
|
0.8
|
|
||
|
Net Transfers
|
7.6
|
|
—
|
|
—
|
|
|||||
|
Acquisitions
|
—
|
|
—
|
|
33.9
|
|
|||||
|
Unrealized gains
|
0.6
|
|
0.9
|
|
(0.4
|
)
|
|||||
|
Level 3 assets held at end of year
|
$
|
43.4
|
|
$
|
35.2
|
|
$
|
34.3
|
|
||
|
*
|
Includes Omega activity from March 30, 2015 to June 27, 2015.
|
|
Due
|
Amount
|
|||
|
2017
|
$
|
40.2
|
|
|
|
2018
|
30.7
|
|
||
|
2019
|
24.1
|
|
||
|
2020
|
16.7
|
|
||
|
2021
|
12.5
|
|
||
|
Thereafter
|
19.8
|
|
||
|
Balance at June 29, 2013
|
$
|
2.9
|
|
|
Additional charges
|
47.0
|
|
|
|
Payments
|
(28.7
|
)
|
|
|
Non-cash adjustments
|
(4.8
|
)
|
|
|
Balance at June 28, 2014
|
16.4
|
|
|
|
Additional charges
|
5.1
|
|
|
|
Payments
|
(18.5
|
)
|
|
|
Non-cash adjustments
|
(1.4
|
)
|
|
|
Balance at June 27, 2015
|
1.6
|
|
|
|
Additional charges
|
26.9
|
|
|
|
Payments
|
(6.4
|
)
|
|
|
Non-cash adjustments
|
(1.4
|
)
|
|
|
Balance at December 31, 2015
|
20.7
|
|
|
|
Additional charges
|
31.0
|
|
|
|
Payments
|
(35.8
|
)
|
|
|
Non-cash adjustments
|
3.8
|
|
|
|
Balance at December 31, 2016
|
$
|
19.7
|
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
||||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Ireland
|
$
|
89.1
|
|
$
|
11.4
|
|
$
|
7.2
|
|
$
|
—
|
|
|||
|
U.S.
|
3,353.0
|
|
1,686.2
|
|
3,303.2
|
|
3,352.0
|
|
|||||||
|
Europe
|
1,493.0
|
|
758.2
|
|
576.4
|
|
219.7
|
|
|||||||
|
All other countries
(2)
|
345.5
|
|
176.4
|
|
340.3
|
|
342.4
|
|
|||||||
|
$
|
5,280.6
|
|
$
|
2,632.2
|
|
$
|
4,227.1
|
|
$
|
3,914.1
|
|
||||
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Ireland
|
$
|
2.7
|
|
$
|
1.3
|
|
$
|
1.4
|
|
||
|
U.S.
|
556.6
|
|
555.0
|
|
558.6
|
|
|||||
|
Europe
|
144.6
|
|
157.2
|
|
153.8
|
|
|||||
|
Israel
|
114.3
|
|
115.7
|
|
119.8
|
|
|||||
|
All other countries
|
51.9
|
|
57.0
|
|
98.8
|
|
|||||
|
$
|
870.1
|
|
$
|
886.2
|
|
$
|
932.4
|
|
|||
|
CHCA
|
CHCI
(1)
|
RX
|
Specialty Sciences
(2)
|
Other
|
Unallocated
|
Total
|
|||||||||||||||||||||
|
Year Ended December 31, 2016
|
|||||||||||||||||||||||||||
|
Net sales
|
$
|
2,507.1
|
|
$
|
1,652.2
|
|
$
|
1,042.8
|
|
$
|
—
|
|
$
|
78.5
|
|
$
|
—
|
|
$
|
5,280.6
|
|
||||||
|
Operating income (loss)
|
$
|
399.8
|
|
$
|
(2,087.4
|
)
|
$
|
(0.2
|
)
|
$
|
(201.2
|
)
|
$
|
6.1
|
|
$
|
(116.8
|
)
|
$
|
(1,999.7
|
)
|
||||||
|
Operating income (loss) %
|
15.9
|
%
|
(126.3
|
)%
|
—
|
%
|
—
|
%
|
7.8
|
%
|
—
|
%
|
(37.9
|
)%
|
|||||||||||||
|
Total assets
|
$
|
3,351.3
|
|
$
|
4,795.2
|
|
$
|
2,646.4
|
|
$
|
2,775.8
|
|
$
|
301.4
|
|
$
|
—
|
|
$
|
13,870.1
|
|
||||||
|
Capital expenditures
|
$
|
59.1
|
|
$
|
23.7
|
|
$
|
20.4
|
|
$
|
—
|
|
$
|
3.0
|
|
$
|
—
|
|
$
|
106.2
|
|
||||||
|
Property, plant and equip, net
|
$
|
528.3
|
|
$
|
167.2
|
|
$
|
129.7
|
|
$
|
0.4
|
|
$
|
44.5
|
|
$
|
—
|
|
$
|
870.1
|
|
||||||
|
Depreciation/amortization
|
$
|
119.1
|
|
$
|
210.0
|
|
$
|
120.1
|
|
$
|
—
|
|
$
|
7.8
|
|
$
|
—
|
|
$
|
457.0
|
|
||||||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,608.2
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,608.2
|
|
||||||
|
Six Months Ended December 31, 2015 (restated)
|
|||||||||||||||||||||||||||
|
Net sales
|
$
|
1,251.5
|
|
$
|
833.0
|
|
$
|
502.6
|
|
$
|
—
|
|
$
|
45.1
|
|
$
|
—
|
|
$
|
2,632.2
|
|
||||||
|
Operating income (loss)
|
$
|
209.2
|
|
$
|
(148.5
|
)
|
$
|
181.9
|
|
$
|
(6.5
|
)
|
$
|
(19.5
|
)
|
$
|
(149.0
|
)
|
$
|
67.6
|
|
||||||
|
Operating income (loss) %
|
16.7
|
%
|
(17.8
|
)%
|
36.2
|
%
|
—
|
%
|
(43.3
|
)%
|
—
|
%
|
2.6
|
%
|
|||||||||||||
|
Total assets
|
$
|
3,384.8
|
|
$
|
7,083.5
|
|
$
|
2,738.0
|
|
$
|
5,930.2
|
|
$
|
213.1
|
|
$
|
—
|
|
$
|
19,349.6
|
|
||||||
|
Capital expenditures
|
$
|
38.0
|
|
$
|
26.3
|
|
$
|
12.1
|
|
$
|
—
|
|
$
|
1.4
|
|
$
|
—
|
|
$
|
77.8
|
|
||||||
|
Property, plant and equip, net
|
$
|
540.9
|
|
$
|
179.5
|
|
$
|
118.5
|
|
$
|
—
|
|
$
|
47.3
|
|
$
|
—
|
|
$
|
886.2
|
|
||||||
|
Depreciation/amortization
|
$
|
60.9
|
|
$
|
81.9
|
|
$
|
34.3
|
|
$
|
—
|
|
$
|
5.3
|
|
$
|
—
|
|
$
|
182.4
|
|
||||||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(57.3
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
(57.3
|
)
|
||||||
|
Year Ended June 27, 2015 (restated)
|
|||||||||||||||||||||||||||
|
Net sales
|
$
|
2,478.8
|
|
$
|
704.6
|
|
$
|
936.0
|
|
$
|
—
|
|
$
|
107.7
|
|
$
|
—
|
|
$
|
4,227.1
|
|
||||||
|
Operating income (loss)
|
$
|
381.9
|
|
$
|
38.2
|
|
$
|
364.7
|
|
$
|
(17.6
|
)
|
$
|
26.8
|
|
$
|
(121.5
|
)
|
$
|
672.5
|
|
||||||
|
Operating income (loss) %
|
15.4
|
%
|
5.4
|
%
|
39.0
|
%
|
—
|
%
|
24.9
|
%
|
—
|
%
|
15.9
|
%
|
|||||||||||||
|
Total assets
|
$
|
3,763.8
|
|
$
|
7,163.0
|
|
$
|
2,373.4
|
|
$
|
6,040.7
|
|
$
|
251.0
|
|
$
|
—
|
|
$
|
19,591.9
|
|
||||||
|
Capital expenditures
|
$
|
76.8
|
|
$
|
13.1
|
|
$
|
41.0
|
|
$
|
0.5
|
|
$
|
5.6
|
|
$
|
—
|
|
$
|
137.0
|
|
||||||
|
Property, plant and equip, net
|
$
|
556.8
|
|
$
|
176.8
|
|
$
|
113.0
|
|
$
|
—
|
|
$
|
85.8
|
|
$
|
—
|
|
$
|
932.4
|
|
||||||
|
Depreciation/amortization
|
$
|
108.4
|
|
$
|
72.5
|
|
$
|
65.7
|
|
$
|
1.5
|
|
$
|
10.6
|
|
$
|
—
|
|
$
|
258.7
|
|
||||||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(78.5
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
(78.5
|
)
|
||||||
|
Year Ended June 28, 2014 (restated)
|
|||||||||||||||||||||||||||
|
Net sales
|
$
|
2,581.2
|
|
$
|
331.1
|
|
$
|
864.2
|
|
$
|
—
|
|
$
|
137.6
|
|
$
|
—
|
|
$
|
3,914.1
|
|
||||||
|
Operating income (loss)
|
$
|
402.8
|
|
$
|
17.0
|
|
$
|
341.5
|
|
$
|
(62.5
|
)
|
$
|
46.1
|
|
$
|
(173.5
|
)
|
$
|
571.4
|
|
||||||
|
Operating income (loss) %
|
15.6
|
%
|
5.1
|
%
|
39.5
|
%
|
—
|
%
|
33.5
|
%
|
—
|
%
|
14.6
|
%
|
|||||||||||||
|
Total assets
|
$
|
4,510.2
|
|
$
|
767.0
|
|
$
|
2,189.2
|
|
$
|
6,124.7
|
|
$
|
288.0
|
|
$
|
—
|
|
$
|
13,879.1
|
|
||||||
|
Capital expenditures
|
$
|
119.4
|
|
$
|
10.2
|
|
$
|
31.6
|
|
$
|
—
|
|
$
|
10.4
|
|
$
|
—
|
|
$
|
171.6
|
|
||||||
|
Property, plant and equip, net
|
$
|
530.4
|
|
$
|
59.3
|
|
$
|
92.5
|
|
$
|
2.0
|
|
$
|
95.7
|
|
$
|
—
|
|
$
|
779.9
|
|
||||||
|
Depreciation/amortization
|
$
|
93.2
|
|
$
|
33.4
|
|
$
|
66.5
|
|
$
|
1.6
|
|
$
|
11.4
|
|
$
|
—
|
|
$
|
206.1
|
|
||||||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(26.6
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
(26.6
|
)
|
||||||
|
Year Ended
|
Six Months Ended
|
Year Ended
|
|||||||||||||
|
December 31, 2016
|
December 31,
2015 |
June 27,
2015 |
June 28,
2014 |
||||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
CHCA
|
|||||||||||||||
|
Cough/Cold/Allergy/Sinus
(1)
|
$
|
454.6
|
|
$
|
234.6
|
|
$
|
455.6
|
|
$
|
477.6
|
|
|||
|
Analgesics
(1)
|
343.5
|
|
173.1
|
|
375.7
|
|
433.2
|
|
|||||||
|
Gastrointestinal
(1)
|
335.4
|
|
195.8
|
|
384.0
|
|
398.4
|
|
|||||||
|
Infant nutritionals
|
427.0
|
|
200.2
|
|
383.8
|
|
374.8
|
|
|||||||
|
Smoking cessation
|
308.5
|
|
147.5
|
|
284.5
|
|
236.8
|
|
|||||||
|
Vitamins, minerals and dietary supplements
(1)
|
160.4
|
|
105.8
|
|
183.5
|
|
176.9
|
|
|||||||
|
Animal health
|
143.7
|
|
62.3
|
|
157.0
|
|
178.0
|
|
|||||||
|
Other CHCA
(1),(2)
|
334.0
|
|
132.2
|
|
254.7
|
|
305.5
|
|
|||||||
|
Total CHCA
|
2,507.1
|
|
1,251.5
|
|
2,478.8
|
|
2,581.2
|
|
|||||||
|
CHCI
|
|||||||||||||||
|
Branded OTC
|
1,349.2
|
|
665.9
|
|
368.4
|
|
—
|
|
|||||||
|
Other CHCI
(3)
|
303.0
|
|
167.1
|
|
336.2
|
|
331.1
|
|
|||||||
|
Total CHCI
|
1,652.2
|
|
833.0
|
|
704.6
|
|
331.1
|
|
|||||||
|
Generic prescription drugs
|
1,042.8
|
|
502.6
|
|
936.0
|
|
864.2
|
|
|||||||
|
Active pharmaceutical ingredients
|
78.5
|
|
45.1
|
|
107.7
|
|
137.6
|
|
|||||||
|
Total net sales
|
$
|
5,280.6
|
|
$
|
2,632.2
|
|
$
|
4,227.1
|
|
$
|
3,914.1
|
|
|||
|
(2)
|
Consists primarily of feminine hygiene, diabetes care, dermatological care, branded OTC, and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the
CHCA
segment.
|
|
(3)
|
Consists primarily of liquids licensed product, cough/cold/allergy, analgesics and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the CHCI segment.
|
|
Year Ended December 31, 2016
|
First
Quarter (2) |
Second
Quarter (3) |
Third
Quarter (4) |
Fourth
Quarter (5) |
|||||||||||
|
Restated
|
Restated
|
Restated
|
|||||||||||||
|
Net sales
|
$
|
1,347.3
|
|
$
|
1,340.5
|
|
$
|
1,261.6
|
|
$
|
1,331.2
|
|
|||
|
Gross profit
|
$
|
533.1
|
|
$
|
546.5
|
|
$
|
484.5
|
|
$
|
487.7
|
|
|||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
204.4
|
|
$
|
910.8
|
|
$
|
377.4
|
|
$
|
1,115.6
|
|
|||
|
Net loss
|
$
|
(529.2
|
)
|
$
|
(534.3
|
)
|
$
|
(1,590.2
|
)
|
$
|
(1,359.1
|
)
|
|||
|
Loss per share
(1)
:
|
|||||||||||||||
|
Basic
|
$
|
(3.70
|
)
|
$
|
(3.73
|
)
|
$
|
(11.10
|
)
|
$
|
(9.48
|
)
|
|||
|
Diluted
|
$
|
(3.70
|
)
|
$
|
(3.73
|
)
|
$
|
(11.10
|
)
|
$
|
(9.48
|
)
|
|||
|
Weighted average shares outstanding
|
|||||||||||||||
|
Basic
|
143.2
|
|
143.2
|
|
143.3
|
|
143.4
|
|
|||||||
|
Diluted
|
143.2
|
|
143.2
|
|
143.3
|
|
143.4
|
|
|||||||
|
(2)
|
Includes an
intangible asset impairment charges
of
$273.3 million
, and a
goodwill impairment charge
of
$130.5 million
.
|
|
(3)
|
Includes
held-for-sale impairment charges
of
$10.5 million
and
change in fair market value on royalty rights
of
$910.8 million
.
|
|
(4)
|
Includes
intangible asset impairment charges
of
$866.6 million
,
goodwill impairment charges
of
$737.9 million
, and
held-for-sale impairment charges
of
$10.2 million
.
|
|
(5)
|
Includes
intangible asset impairment charges
of
$378.6 million
,
goodwill impairment charges
of
$224.1 million
, and a
reduction in held-for-sale impairment charges
of
$4.5 million
.
|
|
September 26, 2015
(2)
|
December 31, 2015
(3)
|
||||||
|
Six Months Ended December 31, 2015
|
Restated
|
Restated
|
|||||
|
Net sales
|
$
|
1,273.1
|
|
$
|
1,359.1
|
|
|
|
Gross profit
|
$
|
535.2
|
|
$
|
543.7
|
|
|
|
Tysabri
®
royalty stream - change in fair value
|
$
|
(173.8
|
)
|
$
|
116.6
|
|
|
|
Net income (loss)
|
$
|
260.9
|
|
$
|
(218.4
|
)
|
|
|
Income (loss) per share
(1)
:
|
|||||||
|
Basic
|
$
|
1.78
|
|
$
|
(1.51
|
)
|
|
|
Diluted
|
$
|
1.78
|
|
$
|
(1.51
|
)
|
|
|
Weighted-average shares outstanding
|
|||||||
|
Basic
|
146.3
|
|
144.9
|
|
|||
|
Diluted
|
146.9
|
|
144.9
|
|
|||
|
(3)
|
Includes an
intangible asset impairment charge
of
$185.1 million
,
Mylan defense-related fees
of
$71.3 million
,
an
impairment charge on our India API held for sale assets
of
$29.0 million
,
restructuring charges
of
$24.7 million
, and an
investment impairment charge
of
$10.7 million
.
|
|
First
Quarter |
Second
Quarter (2) |
Third
Quarter (3) |
Fourth
Quarter (4) |
||||||||||||
|
Year Ended June 27, 2015
|
Restated
|
Restated
|
Restated
|
Restated
|
|||||||||||
|
Net sales
|
$
|
859.6
|
|
$
|
985.1
|
|
$
|
967.2
|
|
$
|
1,415.2
|
|
|||
|
Gross profit
|
$
|
304.1
|
|
$
|
369.7
|
|
$
|
369.4
|
|
$
|
601.0
|
|
|||
|
Tysabri
®
royalty stream - change in fair value
|
$
|
58.9
|
|
$
|
(105.8
|
)
|
$
|
(100.8
|
)
|
$
|
69.2
|
|
|||
|
Net income (loss)
|
$
|
29.4
|
|
$
|
151.1
|
|
$
|
(22.2
|
)
|
$
|
(22.2
|
)
|
|||
|
Income (loss) per share
(1)
:
|
|||||||||||||||
|
Basic
|
$
|
0.22
|
|
$
|
1.11
|
|
$
|
(0.16
|
)
|
$
|
(0.15
|
)
|
|||
|
Diluted
|
$
|
0.22
|
|
$
|
1.10
|
|
$
|
(0.16
|
)
|
$
|
(0.15
|
)
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
133.9
|
|
136.3
|
|
140.8
|
|
146.3
|
|
|||||||
|
Diluted
|
134.4
|
|
136.8
|
|
140.8
|
|
146.3
|
|
|||||||
|
(2)
|
Includes
losses on derivatives
associated with the Omega acquisition of
$64.7 million
,
Omega transaction expenses
of
$17.8 million
, an
R&D payment made in connection with a collaborative agreement
of
$10.0 million
, a
$9.6 million
loss on extinguishment of debt, partially offset by
income from transfer of rights agreement
of
$12.5 million
.
|
|
(3)
|
Includes
losses on derivatives
associated with the Omega acquisition of
$258.2 million
and
Omega financing fees
of
$18.6 million
.
|
|
(4)
|
Includes
acquisition costs
of
$18.5 million
, an initial
payment made in connection with an R&D agreement
of
$18.0 million
, an
inventory step up related to the Omega acquisition
totaling
$15.6 million
, and
$13.4 million
of Mylan defense-related fees.
|
|
|
Six Months Ended
|
||||||
|
|
December 31,
2015 |
December 27,
2014 |
|||||
|
(Unaudited)
|
|||||||
|
Restated
|
Restated
|
||||||
|
Net sales
|
$
|
2,632.2
|
|
$
|
1,844.7
|
|
|
|
Cost of sales
|
1,553.3
|
|
1,170.9
|
|
|||
|
Gross profit
|
1,078.9
|
|
673.8
|
|
|||
|
Operating expenses
|
|||||||
|
Distribution
|
47.9
|
|
29.2
|
|
|||
|
Research and development
|
88.2
|
|
89.8
|
|
|||
|
Selling
|
325.9
|
|
95.3
|
|
|||
|
Administration
|
306.8
|
|
165.6
|
|
|||
|
Impairment charges
|
215.6
|
|
—
|
|
|||
|
Restructuring
|
26.9
|
|
4.2
|
|
|||
|
Total operating expenses
|
1,011.3
|
|
384.1
|
|
|||
|
Operating income
|
67.6
|
|
289.7
|
|
|||
|
Tysabri
®
royalty stream - change in fair value
|
(57.3
|
)
|
(46.9
|
)
|
|||
|
Interest expense, net
|
89.9
|
|
56.7
|
|
|||
|
Other expense, net
|
25.2
|
|
60.3
|
|
|||
|
Loss on extinguishment of debt
|
0.9
|
|
9.6
|
|
|||
|
Income before income taxes
|
8.9
|
|
210.0
|
|
|||
|
Income tax expense (benefit)
|
(33.6
|
)
|
29.4
|
|
|||
|
Net income
|
$
|
42.5
|
|
$
|
180.6
|
|
|
|
Income per share
|
|||||||
|
Basic
|
$
|
0.29
|
|
$
|
1.34
|
|
|
|
Diluted
|
$
|
0.29
|
|
$
|
1.34
|
|
|
|
Weighted-average shares outstanding
|
|||||||
|
Basic
|
145.6
|
|
135.1
|
|
|||
|
Diluted
|
146.1
|
|
135.6
|
|
|||
|
Dividends declared per share
|
$
|
0.25
|
|
$
|
0.21
|
|
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
•
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
|
|
•
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
•
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
|
•
|
Review the processes and controls in place related to our application of ASC 805 to enhance the effectiveness of the design and operation of those controls to identify assets acquired and liabilities assumed; and
|
|
•
|
Evaluate and enhance management review controls related to business acquisitions.
|
|
•
|
Review the organization structure, resources, processes and controls in place to measure and record income taxes to enhance the effectiveness of the design and operation of those controls;
|
|
•
|
Evaluate the design and operating effectiveness of our controls related to income taxes for business acquisitions and non-routine transactions on an interim and annual basis;
|
|
•
|
Enhance monitoring activities related to income taxes; and
|
|
•
|
Evaluate and enhance the level of precision in the management review controls related to income taxes.
|
|
•
|
Review the design and operation of our controls related to asset group determination in our impairment process on an interim and annual basis; and
|
|
•
|
Evaluate and enhance the management review controls related to impairment.
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
|
|
•
|
Leadership experience
- current and previous executive leadership roles within the private and public sectors.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from service as a director of public, private and non-profit companies.
|
|
•
|
Industry knowledge
- extensive experience and knowledge in management, operations and supply chain as well as the development and marketing of consumer products.
|
|
•
|
Leadership and operating experience
- previous executive leadership roles at Newmont Mining Corporation, Cliffs Natural Resources, Inc., STERIS Corporation, and Office Max.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from current and prior service as a director and committee member on public and non-profit boards.
|
|
•
|
Industry Knowledge
- experience in operations and supply chain and FDA regulated industries.
|
|
•
|
Leadership and operating experience
- previous executive leadership roles at Hillenbrand Industries, Bayer Healthcare AG, Bayer Diagnostics and Pharmacia Corporation.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from current and prior service as a director and committee member on public boards.
|
|
•
|
Industry knowledge
- extensive experience in varying roles within the pharmaceutical industry.
|
|
•
|
Leadership and operating experience
- currently an Executive Vice President at a global medical technology company as well as years of service in previous executive officer roles of varying degrees.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from current and prior service as a director and committee member on public and non-profit company boards.
|
|
•
|
Industry knowledge
- extensive experience in the medical supply and diagnostic equipment industries and in international business.
|
|
•
|
Leadership, operating and marketing experience
- current Chief Executive Officer and various leadership roles at Perrigo.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from current and previous service as a director of private and non-profit companies and organizations.
|
|
•
|
Industry knowledge
- extensive experience and knowledge in operations and supply chain as well as the development and marketing of store brand consumer healthcare products.
|
|
•
|
Leadership and operating experience
- previous executive leadership roles, including IT and cyber security at Biogen, Inc., and Dell, Inc.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from current and prior service as a director and committee member on public boards.
|
|
•
|
Leadership experience
- current and previous executive leadership roles within the private and public sectors.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from service as a director of public, private and non-profit companies.
|
|
•
|
Legal experience
- extensive legal experience in both the public and private sectors.
|
|
•
|
Leadership experience -
former Senior Partner of Pricewaterhouse Coopers.
|
|
•
|
Board and corporate governance experience
- current and prior board and committee experience in the financial, pharmaceutical and other industries.
|
|
•
|
Accounting and financial expertise
- qualified chartered accountant currently designated as an “audit committee financial expert” given his skills and attributes acquired through relevant education and work experience.
|
|
•
|
Leadership experience -
former Chief Financial Officer and investment banking executive.
|
|
•
|
Board and corporate governance experience
- current board and committee experience in the health science industry.
|
|
•
|
Accounting and financial expertise
- designated as an “audit committee financial expert” given his skills and attributes acquired through relevant education and work experience.
|
|
•
|
Leadership experience -
former investment management executive and former co-chair of Children’s Hospital Los Angeles.
|
|
•
|
Board and corporate governance experience
- past and current board and committee experience in the financial and health science industries.
|
|
•
|
Accounting and financial expertise
- extensive accounting and financial skills and attributes acquired through relevant education and work experience.
|
|
•
|
Leadership and operating experience
- current and previous executive leadership roles within the private and public sectors.
|
|
•
|
Board and corporate governance experience
- board and corporate governance experience from service as a director of public and private companies.
|
|
•
|
Accounting and Financial Expertise
- extensive accounting and financial skills and attributes acquired through relevant education and work experience, including involvement in capital markets and investment decision making.
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
•
|
Increased focus on longer-term relative performance comparisons through the addition of a relative TSR metric;
|
|
•
|
Simplified the plan design, aligning it with market practices and our strategic focus - e.g., we removed net operating profit after tax as the primary annual incentive bonus funding mechanism and replaced it with operating income and days of working capital; and
|
|
•
|
Increased the weight of performance-based long-term compensation we provide to executives by eliminating service-based restricted stock units and replaced them with performance shares based on Total Shareholder Return that is measured over a three-year period.
|
|
•
|
The 2016 management incentive bonus plan was funded well below the target level because the metric used to fund that plan, Net Operating Profit after Tax, or NOPAT, was achieved only slightly above the threshold level.
|
|
•
|
Even though the bonus plan was funded at a lower level, our new CEO requested that he not receive any MIB payout for 2016, and the Committee agreed, noting that the decision was not related to personal but overall Company performance. On the same basis, each of our other executive officers received less than the earned amount of his or her annual bonus.
|
|
•
|
Because the Return on Tangible Capital (“ROTC”) in 2016 was below the threshold level, there was zero (0%) vesting credit for the 2016 tranche of the performance-based equity compensation, which will apply to the full three-year vesting credit for the performance-based restricted stock units granted in fiscal 2015, Stub Period 2015, and fiscal 2016.
|
|
•
|
Finally, in 2016, none of our executive officers received a discretionary bonus or special equity award.
|
|
Program Element
|
Change
|
Rationale
|
|
The Management Incentive Bonus Plan (the “MIB Plan”)
|
Remove net operating profit after tax as the primary funding mechanism.
Use achievement of operating income (“OI”) and days of working capital (“DWC”) goals to fund and calculate actual incentive award payouts.
|
Simplifies the formula and is easier to understand.
Aligns with market practices and supports business plans.
|

|
Program Element
|
Change
|
Rationale
|
|
Long-Term Incentive (“LTI”) Plan compensation for the named executive officers
|
Remove service-based restricted stock units (“RSUs”).
Restructure the mix of equity to 70% performance-based restricted stock units (“PRSUs”) and 30% stock options.
Base 50% of the total LTI opportunity on the achievement of Return on Tangible Capital (“ROTC”) and 20% on Total Shareholder Return (“TSR”) relative to the 2017 Executive Compensation Peer Group over a three-year performance cycle.
The LTI design for all other eligible employees remains unchanged.
|
Responds to shareholder feedback.
Increases the focus on relative performance.
Simplifies approach.
Aligns with market practices.
Adds a 3-year performance measurement period.
Increases the performance-based percentage of LTI compensation to 70% (100%, if including options).
|

|
What We Do
|
What We Do Not Do
|
|
Place a significant emphasis on variable, at-risk pay
|
Permit hedging or pledging of Perrigo stock
|
|
Align compensation with shareholder returns through long-term performance
|
Provide significant perquisites
|
|
Include clawback provisions in our incentive agreements
|
Reprice options
|
|
Have rigorous stock ownership guidelines
|
Provide “single trigger” change in control cash severance benefits
|
|
Use an independent compensation consultant
|
|
|
Conduct annual risk assessments
|
|
|
•
|
In April 2016, Joseph C. Papa resigned as Chief Executive Officer and Chairman of the Board.
|
|
•
|
Following Mr. Papa’s resignation, Perrigo’s President, John T. Hendrickson, was appointed Chief Executive Officer.
|
|
•
|
In February 2017, Judy L. Brown resigned as our Executive Vice President, Business Operations and Chief Financial Officer.
|
|
•
|
Ronald L. Winowiecki, Perrigo’s Senior Vice President, Business Finance, was appointed acting Chief Financial Officer following Ms. Brown’s resignation.
|
|
Named Executive Officer
|
Position
|
|
John T. Hendrickson
|
Chief Executive Officer
|
|
Judy L. Brown
|
Executive Vice President, Business Operations and Chief Financial Officer
|
|
Todd W. Kingma
|
Executive Vice President, General Counsel and Secretary
|
|
Jeffrey R. Needham
|
Executive Vice President and President, Consumer Healthcare Americas
|
|
Sharon Kochan
|
Executive Vice President and President, Consumer Healthcare International
|
|
Joseph C. Papa
|
Former Chairman and Chief Executive Officer
|
|
Program Element
|
Committee Decisions
|
|||
|
Annual Base Salary
|
Increased salary by 28.6% to $900,000
|
|||
|
MIB Plan
|
Increased target incentive opportunity from 80% to 115% of base salary
|
|||
|
LTI Plan
|
Granted the following promotional equity awards on June 21, 2016:
|
|||
|
PRSUs
|
Stock Options
|
RSUs
|
|
$1,205,000
|
$723,000
|
$482,000
|
|
•
|
Net sales of $5.3 billion.
|
|
•
|
Operating loss of $2.0 billion, which includes impairment charges of $2.6 billion.
|
|
Program Element
|
Committee Decisions
|
|
Annual Base Salary
|
With the exception of Mr. Hendrickson, the named executive officers’ base salaries increased between
1.5% and 3.6% based on the Committee’s review of the compensation market data and assessment of individual performance, as well as in recognition of increased responsibilities or promotions.
|
|
MIB Plan
|
With the exception of Mr. Hendrickson, the named executive officers received annual incentive awards, based on performance, under the MIB Plan ranging from 0% to 56% of target.
|
|
LTI Plan
|
All of the named executive officers received their target annual LTI Plan award grant for 2016, which consisted of 50% PRSUs that provide no actual value unless threshold ROTC target levels are achieved over 3 years, 30% stock options (vesting over 3 years), and 20% RSUs (vesting over 3 years).
However, based on 2016 performance, there was zero (0%) vesting credit for the 2016 tranche of the performance-based equity compensation, which will apply to the full three-year vesting credit for the PRSUs granted in fiscal 2015, Stub Period 2015, and fiscal 2016.
|
|
•
|
Pay is linked to performance:
A significant portion of total compensation should be variable ("at-risk") and linked to the attainment of specific performance objectives.
|
|
•
|
Pay is shareholder-aligned:
Compensation should be provided through pay elements (base salaries, annual and long-term incentives) designed to drive sustained business performance, build an internal culture of ownership and create long-term value for our shareholders.
|
|
•
|
Pay opportunities are market-competitive:
Provide compensation at levels that will attract, motivate, and retain highly qualified executives who are focused on the long-term best interests of our shareholders.
|
|
Element
|
Form
|
What It Does
|
|
Base Salary
|
Cash
(Fixed)
|
Provides a competitive rate of compensation relative to similar positions in pharmaceutical industry and consumer-goods peers and enables us to attract and retain critical executive talent.
|
|
MIB Plan
|
Cash (Variable)
|
Focuses executives on achieving annual financial and operational goals that drive long-term shareholder value.
|
|
LTI Plan
|
Equity (Variable)
|
Provides incentives for executives to execute on longer-term financial/strategic growth goals that drive value creation and support our retention strategy.
|

|
Abbvie, Inc.
|
Mallinckrodt plc
|
|
Allergan plc
|
Mead Johnson Nutrition Co.
|
|
Allergan, Inc.
|
Mylan, Inc.
|
|
Bristol-Myers Squibb Co.
|
Regeneron Pharmaceuticals
|
|
Celgene Corporation
|
Shire plc (ADR)
|
|
Endo International plc
|
United Therapeutics Corporation
|
|
Hospira, Inc.
|
Valeant Pharmaceuticals International
|
|
Jazz Pharmaceuticals plc
|
Zoetis, Inc.
|
|
Baxter International Inc.
|
Church & Dwight Co., Inc.
|
Conagra Brands, Inc.
|
|
The Clorox Company
|
Endo International plc
|
Henry Schein, Inc.
|
|
Jazz Pharmaceuticals plc
|
Mallinckrodt plc
|
Mylan N.V.
|
|
Patterson Companies, Inc.
|
Prestige Brands Holdings, Inc.
|
Zoetis Inc.
|
|
Shire plc
|
Spectrum Brands Holdings, Inc.
|
TreeHouse Foods. Inc.
|
|
Reckitt Benckiser Group plc
|
||
|
Named Executive Officer
|
2015 Stub Period Annualized Base Salary
|
2016 Base Salary
|
% Increase
|
|
John T. Hendrickson
|
$700,000 (not CEO)
|
$900,000 (CEO)
|
28.6%
|
|
Judy L. Brown
|
$634,500
|
$657,342
|
3.6%
|
|
Todd W. Kingma
|
$511,000
|
$526,330
|
3.0%
|
|
Jeffrey R. Needham
|
$500,000
|
$507,500
|
1.5%
|
|
Sharon Kochan
|
$495,000
|
$502,425
|
1.5%
|
|
Named Executive Officer
|
2016 Target Bonus
(as % of Salary)
|
|
Mr. Hendrickson
|
115%
|
|
Ms. Brown
|
75%
|
|
Mr. Kingma
|
60%
|
|
Mr. Needham
|
60%
|
|
Mr. Kochan
|
60%
|
|
Performance Level
|
Funding Level
|
|
|
Below Threshold:
|
Below 80% of performance target
|
Zero
|
|
Threshold:
|
At 80% of performance target
|
50% funding of target awards
|
|
Between 80% and 100% of performance target
|
50% funding of target awards plus an additional 2.5% of funding for every 1% (or fraction thereof) above the performance target
|
|
|
Target:
|
At 100% of performance target
|
100% funding of target awards
|
|
Between 100% and 120% of performance target
|
100% funding of target awards plus an additional 5% of funding for every 1% (or fraction thereof) above the performance target
|
|
|
Maximum:
|
At or above 120% of performance target
|
200% funding of target awards
|
|
NOPAT Performance Goals ($M)
|
Pool Funding
|
|
|
Maximum
|
$1,884
|
200%
|
|
Target
|
$1,569.6
|
100%
|
|
Threshold
|
$1,256
|
50%
|
|
Actual
|
$1,284.5
|
51.4%
|
|
Performance Metric
|
Weighting
|
Threshold*
|
Target
|
Actual Results
|
|
Corporate OI
|
80%
|
$1,449.35
|
$1,811.69
|
$1,432.40
|
|
Corporate DWC
|
20%
|
113.49
|
94.57 Days
|
96.3
|
|
Named Executive
Officer
|
PRSUs At Target*
|
Stock Options**
|
RSUs*
|
Total Value
|
|
Mr. Hendrickson***
|
9,673
12,494
|
21,943
26,968
|
3,869
4,997
|
$2,500,000
$2,485,000
|
|
Ms. Brown
|
8,125
|
18,432
|
3,250
|
$2,100,000
|
|
Mr. Kingma
|
4,836
|
10,971
|
1,935
|
$1,250,000
|
|
Mr. Needham
|
3,366
|
7,636
|
1,346
|
$870,000
|
|
Mr. Kochan
|
3,289
|
7,461
|
1,315
|
$850,000
|
|
* Except for the promotional grant to Mr. Hendrickson, award amounts for PRSUs and RSUs were determined based on the closing price of Perrigo ordinary shares on the date of grant (February 26, 2016) for all of the named executive officers.
|
||||
|
** Award amounts were calculated based on Black-Scholes values.
|
||||
|
*** Mr. Hendrickson received two grants in fiscal 2016, the annual grant in February 2016 (the first line) and a promotional grant after he became CEO in June 2016 (the second line).
|
||||
|
•
|
January 1, 2016 through December 31, 2016;
|
|
•
|
January 1, 2017 through December 31, 2017; and
|
|
•
|
January 1, 2018 through December 31, 2018
|

|
FY15 Grant
|
Original Measurement Period
|
As Amended
|
|
Year One
|
June 29, 2014 - June 27, 2015
|
June 29, 2014 - June 27, 2015
|
|
Year Two
|
June 28, 2015 - June 25, 2016
|
June 28, 2015 - December 31, 2015
|
|
Year Three
|
June 26, 2016 - July 1, 2017
|
January 1, 2016 - December 31, 2016
|
|
•
|
Chief Executive Officer: 6 times base salary
|
|
•
|
Executive Vice President: 3 times base salary
|
|
•
|
Senior Vice President: 2 times base salary
|
|
Name and Principal
Position
|
Fiscal
Year
|
Salary($)
|
Bonus($)
|
Stock Awards($)
(1)
|
Option Awards($)
(2)
|
Non-Equity Incentive Plan Compensation
($)
(3)
|
All Other Compensation($)
(5)
|
Total
($)
|
|
John T. Hendrickson
Chief Executive Officer |
2016
|
810,521
|
—
|
3,437,040
|
1,473,024
|
—
|
79,312
|
5,799,897
|
|
2015SP
|
283,250
|
—
|
1,450,103
|
—
|
175,822
|
26,908
|
1,936,083
|
|
|
2015
|
490,750
|
—
|
930,677
|
285,023
|
295,912
|
62,730
|
2,065,093
|
|
|
2014
|
470,250
|
—
|
643,937
|
276,234
|
265,328
|
1,299,871
|
2,955,620
|
|
|
Judy L. Brown
Executive Vice President, Chief Financial Officer |
2016
|
651,632
|
—
|
1,469,991
|
630,006
|
—
|
152,800
|
2,904,428
|
|
2015SP
|
312,625
|
375,000
4
|
2,375,083
|
—
|
170,460
|
31,950
|
2,890,118
|
|
|
2015
|
605,950
|
—
|
1,617,124
|
572,404
|
419,968
|
153,904
|
3,369,350
|
|
|
2014
|
563,200
|
—
|
1,206,065
|
517,314
|
340,249
|
3,785,451
|
6,412,279
|
|
|
Todd W. Kingma
Executive Vice President, General Counsel and Secretary |
2016
|
522,498
|
—
|
875,016
|
374,989
|
—
|
92,434
|
1,864,937
|
|
2015SP
|
251,750
|
375,000
4
|
1,475,007
|
—
|
119,601
|
22,837
|
1,869,196
|
|
|
2015
|
490,750
|
—
|
986,231
|
285,023
|
294,812
|
92,914
|
2,149,731
|
|
|
2014
|
470,250
|
—
|
643,937
|
276,234
|
267,855
|
2,400,921
|
4,059,198
|
|
|
Jeffrey R. Needham
Executive Vice President, Consumer Healthcare |
2016
|
505,625
|
—
|
608,932
|
260,998
|
170,000
|
52,869
|
1,598,424
|
|
2015SP
|
248,000
|
—
|
855,146
|
—
|
120,274
|
3,675
|
1,227,095
|
|
|
Sharon Kochan
Executive Vice President |
2016
|
500,587
|
—
|
594,975
|
255,017
|
65,000
|
65,769
|
1,481,347
|
|
2015SP
|
243,150
|
—
|
841,500
|
—
|
110,539
|
3,559
|
1,198,748
|
|
|
2015
|
474,125
|
—
|
575,339
|
240,909
|
279,509
|
47,105
|
1,616,987
|
|
|
2014
|
458,025
|
—
|
559,972
|
240,195
|
243,663
|
1,296,902
|
2,798,756
|
|
|
Joseph C. Papa
Chairman & Chief Executive Officer Resigned April 2016 |
2016
|
464,293
|
—
|
5,914,986
|
2,534,994
|
—
|
196,144
|
9,110,417
|
|
2015SP
|
612,000
|
500,000
4
|
9,599,915
|
—
|
563,995
|
6,544
|
10,782,454
|
|
|
2015
|
1,183,750
|
—
|
5,829,935
|
2,022,335
|
1,410,130
|
167,555
|
10,613,704
|
|
|
2014
|
1,117,500
|
—
|
3,990,351
|
1,711,405
|
1,037,390
|
11,088,894
|
18,945,540
|
|
|
1) Represents the full grant date fair value of stock awards granted in the years shown, calculated in accordance with U.S. GAAP. Stock awards include service-based restricted stock units and performance-based restricted stock units. For the performance-based stock awards, the amounts reported were valued using the closing market price of our ordinary shares on the date of grant assuming payout at target performance of 100%. For 2016 these values were as follows: Mr. Hendrickson, $1,250,042 annual grant, $1,205,046 promotional grant; Ms. Brown, $1,049,994 annual grant; Mr. Kingma, $624,956 annual grant; Mr. Needham, $434,988 annual grant; Mr. Kochan, $425,037 annual grant; and Mr. Papa, $4,225,046 annual grant. The 100% target performance is based on the probable outcome of the relevant performance conditions as of the grant date. See the Grants of Plan-Based Awards for Calendar Year 2016 Table for additional information regarding the full grant date fair value for all stock awards.
|
|
2) Represents the full grant date fair value of stock options granted in the fiscal years shown, calculated in accordance with U.S. GAAP. Stock options were valued using the Black-Scholes model. Additional weighted average valuation assumptions related to option awards are included in the stockholders' equity note of the audited financial statements included in our Annual Reports on Form 10-K for the fiscal years ended December 31, 2016 and June 27, 2015 and the transition report on Form 10-KT for the six month period ended December 31, 2015. No stock options were granted during the 2015 Stub Period.
|
|
3) The compensation amounts set forth in the “Non-Equity Incentive Plan Compensation” column represent the Management Incentive Bonus earned for the relevant fiscal year/stub period as described in the Compensation Discussion and Analysis section entitled Elements of Compensation – Annual Incentive Award Opportunities.
|
|
4) One-time cash bonus awarded for recognizing their key contributions related to Mylan's hostile takeover attempt.
|
|
5) The following table describes the compensation amounts set forth in the “All Other Compensation” column of the Summary Compensation Table:
|
|
Name
|
Perquisites and Other Personal Benefits($)
(1)
|
Registrant Contributions to Defined Contribution Plans ($)
(2)
|
Registrant Contributions to Non-Qualified Plans
|
Executive Long-Term Disability ($)
(3)
|
Total ($)
|
|
John T. Hendrickson
|
24,218
|
16,278
|
34,532
|
4,285
|
79,312
|
|
Judy L. Brown
|
62,452
|
16,278
|
70,677
|
3,392
|
152,800
|
|
Todd W. Kingma
|
15,868
|
16,278
|
55,564
|
4,724
|
92,434
|
|
Jeffrey R. Needham
|
—
|
16,278
|
31,039
|
5,552
|
52,869
|
|
Joseph C. Papa
|
—
|
16,278
|
175,503
|
4,362
|
196,144
|
|
1) Represents an allowance for tax/financial planning services; Employees also receive a reimbursement to cover applicable taxes when they work out of their home state and encounter double taxation in states and localities where they would not be eligible to receive a credit for such taxes when filing their tax returns in their home state. For Ms. Brown, full amount represents financial planning services.
|
|||||
|
2) Represents the Company's contributions to 401(k) and Profit-Sharing Plans.
|
|||||
|
3) Represents long-term disability plan premiums paid by the Company.
|
|||||
|
Estimated Possible Payouts Under Non-Equity Incentive Plan Awards
(3)
|
Estimated Possible Payouts Under Equity Incentive Plans
(4)
|
All Other Stock
|
All Other Option Awards: Number of Securities Underlying
|
Exercise or Base Price of Option
|
Grant Date Fair Value of Stock and Option
|
|||||||||||||||||||||
|
Name
|
Grant
Date
(1)
|
Award
Date
(2)
|
Threshold ($)
|
Target ($)
|
Maximum ($)
|
Threshold (#)
|
Target (#)
|
Maximum (#)
|
Awards (#)
(5)
|
Options(#
)(6)
|
Awards ($/Sh)
|
Awards($)
(7)
|
||||||||||||||
|
John T. Hendrickson
|
2/26/2016
|
419,839
|
|
839,677
|
|
9
|
|
1,679,354
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
9,673
|
|
19,346
|
|
—
|
|
—
|
|
—
|
|
1,250,042
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,869
|
|
—
|
|
—
|
|
499,991
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
21,943
|
|
129.23
|
|
750,012
|
|
|||||||
|
6/21/2016
|
8
|
6/13/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
12,494
|
|
24,988
|
|
1,205,046
|
|
||||||||||
|
6/21/2016
|
8
|
6/13/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,997
|
|
481,961
|
|
||||||||
|
6/21/2016
|
8
|
6/13/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
26,968
|
|
96.45
|
|
723,012
|
|
||||||
|
Judy L. Brown
|
2/26/2016
|
244,362
|
|
488,724
|
|
977,447
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
8,125
|
|
16,250
|
|
—
|
|
—
|
|
—
|
|
1,049,994
|
|
|||||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,250
|
|
—
|
|
—
|
|
419,998
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
18,432
|
|
129.23
|
|
630,006
|
|
|||||
|
Todd W. Kingma
|
2/26/2016
|
156,749
|
|
313,499
|
|
626,997
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
4,836
|
|
9,672
|
|
—
|
|
—
|
|
—
|
|
624,956
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,935
|
|
—
|
|
—
|
|
250,060
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
10,971
|
|
129.23
|
|
374,989
|
|
|||||
|
Jeffrey R. Needham
|
2/26/2016
|
151,687
|
|
303,375
|
|
606,750
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
3,366
|
|
6,732
|
|
—
|
|
—
|
|
—
|
|
434,988
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
1,346
|
|
—
|
|
—
|
|
173,944
|
|
|||||||||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7,636
|
|
129.23
|
|
260,998
|
|
|||||
|
Sharon Kochan
|
2/26/2016
|
150,176
|
|
300,352
|
|
600,704
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
3,289
|
|
6,578
|
|
—
|
|
—
|
|
—
|
|
425,037
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,315
|
|
—
|
|
—
|
|
169,937
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7,461
|
|
129.23
|
|
255,017
|
|
|||||
|
Joseph C. Papa
|
2/26/2016
|
278,576
|
|
557,152
|
|
1,114,304
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
32,694
|
|
65,388
|
|
—
|
|
—
|
|
—
|
|
4,225,046
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
13,077
|
|
—
|
|
—
|
|
1,689,941
|
|
|||||
|
2/26/2016
|
2/15/2016
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
74,166
|
|
129.23
|
|
2,534,994
|
|
|||||
|
1) Actual date of grant.
|
|
2) Date on which the Remuneration Committee approved the award.
|
|
3) These columns show the dollar range of payout targets for fiscal 2016 performance under the Management Incentive Bonus Plan as described in the section titled Elements of Compensation - Annual Incentive Award Opportunities in the Compensation Discussion and Analysis. The target values are based on a percentage of each executive's salary. Beginning in fiscal year 2010, the maximum incentive award opportunity for any individual participant was 200% of the target award. In addition, the Remuneration Committee, or the Board in the case of the CEO, had the discretion to adjust any named executive officer's award up by as much as 50% or down by as much as 100% based on individual performance. The actual payments for fiscal 2016 non-equity incentive awards are shown in the Summary Compensation Table in the column titled "Non-Equity Incentive Plan Compensation."
|
|
4) These columns show the range of performance-based restricted stock units that were granted in fiscal 2016 and that could be earned in Calendar Year 2019 under the LTIP, depending on whether specific financial goals are achieved in each of the three applicable performance periods, as described in the section titled Elements of Compensation - Stock-Based Compensation in the Compensation Discussion and Analysis. Earned awards, if any, can range from 0% to 200% of the target grant. The U.S. GAAP value of the 2016 fiscal performance-based restricted stock units granted on February 26, 2016 was $129.23 per share. These awards, to the extent earned, vest three years from the grant date. Off-cycle grants annotated accordingly.
|
|
5) This column shows the service-based restricted stock units granted during 2016 fiscal year under the LTIP as described in the section titled Elements of Compensation - Stock-Based Compensation in the Compensation Discussion and Analysis. The U.S. GAAP value of the 2016 fiscal year service-based restricted stock units granted on February 26, 2016 was $129.23 per share. Annual awards vest three years from the grant date. Off-cycle grants are annotated accordingly.
|
|
6) This column shows the non-qualified stock options granted during 2016 fiscal year under the LTIP as described in the section titled Elements of Compensation - Stock-Based Compensation in the Compensation Discussion and Analysis. The U.S. GAAP value of the 2016 fiscal year non-qualified stock options granted on February 26, 2016 was $129.23 per share and a Black-Scholes value of $34.18. Annual awards vest ratably over three years beginning on the first anniversary of the grant date. Off-cycle grants are annotated accordingly.
|
|
7) Amounts are computed in accordance with U.S. GAAP and are included in the Summary Compensation Table in the applicable columns titled "Stock Awards" and "Option Awards." For performance-based restricted stock units, the amounts disclosed are computed based on a target performance of 100%, which is the probable outcome of the relevant performance conditions as of the grant date.
|
|
8) Additional grant provided to Mr. Hendrickson as a promotional award in connentation with promotion to CEO during fiscal year 2016.
|
|
9) Prorated target based on 80% of salary through the date of Mr. Hendrickson's promotion to CEO and 115% thereafter.
|
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||
|
Name
|
Option / Stock Award Grant Date
(1)
|
Number of Securities Underlying Unexercised Options (#) Exercisable
(2)
|
Number of Securities Underlying Unexercised Options (#) Unexercisable
(2)
|
Option Exercise Price ($)
|
Option Expiration Date
|
Number of Units of Stock That Have Not Vested (#)
(3)
|
Market Value of Units of Stock That Have Not Vested ($)
(4)
|
Equity Incentive Plan Awards: Number of Unearned Units That Have Not Vested (#)
(5)
|
Equity Incentive Plan Awards: Market or Payout Value of Unearned Units That Have Not Vested ($)
(4)
|
|||||||||||
|
John T. Hendrickson
|
8/22/2013
|
2,394
|
|
—
|
|
119.78
|
|
8/22/2023
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
8/21/2014
|
4,756
|
|
—
|
|
147.75
|
|
8/21/2024
|
|
1,316
|
|
109,531
|
|
2,227
|
|
125,417
|
|
||||
|
6/29/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,987
|
|
248,608
|
|
1,673
|
|
77,963
|
|
||||
|
11/5/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,181
|
|
181,525
|
|
—
|
|
—
|
|
||||
|
2/26/2016
|
—
|
|
21,943
|
|
129.23
|
|
2/26/2026
|
|
3,869
|
|
322,017
|
|
6,481
|
|
361,402
|
|
||||
|
6/21/2016
|
—
|
|
26,968
|
|
96.45
|
|
6/21/2026
|
|
4,997
|
|
415,900
|
|
8,371
|
|
466,800
|
|
||||
|
Judy L. Brown
|
8/23/2012
|
7,474
|
|
—
|
|
108.62
|
|
8/23/2022
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
8/22/2013
|
10,888
|
|
—
|
|
119.78
|
|
8/22/2023
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
8/21/2014
|
9,550
|
|
4,775
|
|
147.75
|
|
8/21/2024
|
|
2,644
|
|
220,060
|
|
4,472
|
|
251,863
|
|
||||
|
6/29/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
5,431
|
|
452,022
|
|
3,041
|
|
141,754
|
|
||||
|
12/28/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,571
|
|
213,984
|
|
—
|
|
—
|
|
||||
|
2/26/2016
|
—
|
|
18,432
|
|
129.23
|
|
2/26/2026
|
|
3,250
|
|
270,498
|
|
5,444
|
|
303,566
|
|
||||
|
Todd W. Kingma
|
8/19/2010
|
2,476
|
|
—
|
|
58.82
|
|
8/18/2020
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
8/23/2011
|
10,064
|
|
—
|
|
90.65
|
|
8/23/2021
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
8/23/2012
|
8,576
|
|
—
|
|
108.62
|
|
8/23/2022
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
8/22/2013
|
7,182
|
|
—
|
|
119.78
|
|
8/22/2023
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
8/21/2014
|
4,756
|
|
2,377
|
|
147.75
|
|
8/21/2024
|
|
1,316
|
|
109,531
|
|
2,227
|
|
125,417
|
|
||||
|
6/29/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,987
|
|
248,608
|
|
1,673
|
|
77,963
|
|
||||
|
12/28/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,571
|
|
213,984
|
|
—
|
|
—
|
|
||||
|
2/26/2016
|
—
|
|
10,971
|
|
129.23
|
|
2/26/2026
|
|
1,935
|
|
161,050
|
|
3,240
|
|
180,682
|
|
||||
|
Jeffrey R. Needham
|
8/23/2012
|
1,962
|
|
—
|
|
108.62
|
|
8/23/2022
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
8/22/2013
|
4,163
|
|
—
|
|
119.78
|
|
8/22/2023
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
8/21/2014
|
4,020
|
|
2,009
|
|
147.75
|
|
8/21/2024
|
|
1,113
|
|
92,635
|
|
1,882
|
|
105,982
|
|
||||
|
5/14/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
515
|
|
42,863
|
|
||||||||
|
6/29/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,322
|
|
193,260
|
|
1,300
|
|
60,606
|
|
||||
|
2/26/2016
|
—
|
|
7,636
|
|
129.23
|
|
2/26/2026
|
|
1,346
|
|
112,028
|
|
2,255
|
|
125,760
|
|
||||
|
Sharon Kochan
|
8/23/2012
|
5,886
|
|
—
|
|
108.62
|
|
8/23/2022
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
8/22/2013
|
6,245
|
|
—
|
|
119.78
|
|
8/22/2023
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
8/21/2014
|
4,020
|
|
2,009
|
|
147.75
|
|
8/21/2024
|
|
1,113
|
|
92,635
|
|
1,882
|
|
105,982
|
|
||||
|
6/29/2015
|
—
|
|
—
|
|
—
|
|
—
|
|
2,285
|
|
190,181
|
|
1,280
|
|
59,641
|
|
||||
|
2/26/2016
|
—
|
|
7,461
|
|
129.23
|
|
2/26/2026
|
|
1,315
|
|
109,447
|
|
2,204
|
|
122,883
|
|
||||
|
1) For better understanding of this table, this column has been added to show the grant date of all stock options and equity awards outstanding at fiscal year end.
|
|
2) All stock option awards vest one-third per year over three years beginning on the anniversary of the grant.
|
|
3) Service-based restricted stock units cliff vest respective to the vesting anniversary provided in the grant agreement.
|
|
4) The market value of these unvested awards was calculated using the closing price of our ordinary shares as of December 31, 2016, which was $83.23.
|
|
5) Performance-based restricted stock units are earned and vest, if at all, three years from the grant date, depending on our performance over a two and a half periods for fiscal 2014 and 2015 grants and three full periods for fiscal 2016, more fully described in the section entitled "Long-Term Incentive Award Opportunities" in the Compensation Discussion and Analysis. As of December 31, 2016, the number of unearned units for the fiscal 2015 award, granted on August 21, 2014, was calculated using vesting credits of 135%, 68% and 0% for fiscal years 2015, 2015 SP and 2016, respectively, based on our actual performance. The number of unearned units for the 2015 SP award, granted on June 29, 2015, was calculated using a vesting credit of 68%, 0% and 100% for fiscal years 2015 SP, 2016 and target for 2017. The number of unearned units for the fiscal 2016 award, granted on February 26, 2016, was calculated using a vesting credit of 0% for fiscal 2016 and a targeted vesting credit of 100% for calendar years 2017 and 2018.
|
|
|
Option Awards
|
|
Stock Awards
|
||
|
Name
|
Number of Shares Acquired on Exercise (#)
|
Value Realized on Exercise ($)
(1)
|
|
Number of Shares Acquired on Vesting (#)
(2)
|
Value Realized on Vesting ($)
(3)
|
|
|
|
|
|
|
|
|
John T. Hendrickson
|
—
|
—
|
|
7,298
|
672,265
|
|
Judy L. Brown
|
33,289
|
1,706,662
|
|
12,193
|
1,122,119
|
|
Todd W. Kingma
|
—
|
—
|
|
7,298
|
672,265
|
|
Jeffrey R. Needham
|
—
|
—
|
|
5,213
|
479,394
|
|
Sharon Kochan
|
—
|
—
|
|
5,890
|
542,240
|
|
|
|
|
|
|
|
|
Joseph C. Papa
|
46,293
|
200,513
|
|
—
|
—
|
|
|
|
|
|
|
|
|
1) The value realized on exercise was calculated using the difference between the exercise price of the option and the closing price of our ordinary shares on the day the awards were exercised.
|
|||||
|
2) Represents service-based restricted stock and units and performance-based restricted stock units issued under the LTIP.
|
|||||
|
3) The value realized on vesting was calculated using the closing price of our ordinary shares on the day the awards vested.
|
|||||
|
Name
|
Executive Contributions in Last FY ($)
(1)
|
Perrigo Contributions in Last FY ($)
(2)
|
Aggregate Earnings (Losses) in Last FY ($)
|
Aggregate Withdrawals/ Distributions ($)
|
Aggregate Balance at Last FYE ($)
(3)
|
|
John T. Hendrickson
|
295,903
|
34,532
|
92,658
|
75,249
|
1,389,000
|
|
Judy L. Brown
|
59,553
|
70,677
|
99,515
|
—
|
1,483,898
|
|
Todd W. Kingma
|
34,370
|
55,564
|
90,924
|
—
|
1,654,460
|
|
Jeffrey R. Needham
|
31,295
|
31,039
|
83,423
|
—
|
1,130,793
|
|
Sharon Kochan
|
105,474
|
30,496
|
64,227
|
—
|
978,322
|
|
Joseph C. Papa
|
102,829
|
175,503
|
436,370
|
5,572,489
|
—
|
|
1) Of the total amounts shown in this column, the following amounts are included in the Summary Compensation Table as 2016 salary: Mr. Hendrickson, $243,156; Ms. Brown, $39,098; Mr. Kingma, $10,450; Mr. Needham, $25,281; Mr. Kochan, $83,366; and Mr. Papa, $46,429; and the following additional amounts are included for 2016 in the Summary Compensation Table in the column entitled Non-Equity Incentive Plan Compensation: Mr. Hendrickson, $52,746; Ms. Brown, $20,455; Mr. Kingma, $23,920; Mr. Needham $6,014; Mr. Kochan, $22,108 and Mr. Papa, $56,400.
|
|
2) These amounts are included in the Summary Compensation Table as All Other Compensation.
|
|
3) In addition to the amounts in footnote 1, this column includes the following amounts included in the Summary Compensation Table in the columns entitled (i) Salary (for stub period 2015): Mr. Hendrickson, $55,075; Ms. Brown, $18,758; Mr. Kingma, $5,035; Mr. Needham, $12,400; Mr. Kochan, 36,473; and Mr. Papa, $61,200; (ii) Non-Equity Incentive Plan Compensation (for stub period 2015): Mr. Hendrickson, $88,774; Ms. Brown, $95,396; Mr. Kingma, $133,962; Mr. Needham $14,056; Mr. Kochan, $55,902; and Mr. Papa, $191,013; (iii) Salary (for fiscal year 2015): Mr. Hendrickson, $89,600; Ms. Brown, $25,980; Mr. Kingma, $9,960; Mr. Needham, $23,826; Mr. Kochan, $59,352 and Mr. Papa, $112,538 (iv) Non-Equity Incentive Plan Compensation (for fiscal year 2015): Mr. Hendrickson, $106,131; Ms. Brown, $40,000; Mr. Kingma, $40,178; Mr. Needham, $11,856; Mr. Kochan, $24,366; and Mr. Papa, $93,365 (v) Salary (for fiscal year 2014): Mr. Hendrickson, $80,000; Ms. Brown, $15,000; Mr. Kingma $10,000; Mr. Needham $22,514; Mr. Kochan, $45,802 and Mr. Papa, $89,575, (vi) Non-Equity Incentive Plan Compensation (for fiscal year 2014): Mr. Hendrickson, $25,026; Ms. Brown, $40,000; Mr. Kingma, $37,540; Mr. Needham, $10,934; Mr Kochan, $24,392 and Mr. Papa, $66,722.
|
|
Name and Benefits
|
Change in Control ($)
|
Death, Disability, Retirement ($)
|
Termination for Cause or Without Good Reason ($)
|
Termination Without Cause or for Good Reason ($)
|
Involuntary Termination for Economic Reasons ($)
|
|||||
|
John T. Hendrickson
|
||||||||||
|
Cash Severance
(1)
|
6,840,000
|
|
1,035,000
|
|
—
|
|
3,870,000
|
|
3,870,000
|
|
|
Equity Awards
|
||||||||||
|
Service-Based Restricted Stock
|
1,381,500
|
|
1,381,500
|
|
—
|
|
314,730
|
|
314,730
|
|
|
Performance-Based Restricted Stock
(3)
|
2,560,050
|
|
2,560,050
|
|
—
|
|
—
|
|
—
|
|
|
Stock Options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
Other Benefits
(4)
|
50,000
|
|
—
|
|
—
|
|
50,000
|
|
50,000
|
|
|
Total Estimated Incremental Value
|
10,831,550
|
|
4,976,550
|
|
—
|
|
4,234,730
|
|
4,234,730
|
|
|
Judy L. Brown
|
||||||||||
|
Cash Severance
(2)
|
2,793,704
|
|
493,007
|
|
—
|
|
1,150,349
|
|
1,150,349
|
|
|
Equity Awards
|
||||||||||
|
Service-Based Restricted Stock
|
1,250,640
|
|
1,250,640
|
|
—
|
|
—
|
|
469,350
|
|
|
Performance-Based Restricted Stock
(3)
|
1,814,850
|
|
1,814,850
|
|
—
|
|
—
|
|
—
|
|
|
Stock Options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
Other Benefits
(4)
|
25,000
|
|
—
|
|
—
|
|
25,000
|
|
25,000
|
|
|
Total Estimated Incremental Value
|
5,884,194
|
|
3,558,497
|
|
—
|
|
1,175,349
|
|
1,644,699
|
|
|
Todd W. Kingma
|
||||||||||
|
Cash Severance
(2)
|
2,000,054
|
|
315,798
|
|
—
|
|
842,128
|
|
842,128
|
|
|
Equity Awards
|
—
|
|
||||||||
|
Service-Based Restricted Stock
|
792,810
|
|
792,810
|
|
—
|
|
—
|
|
349,830
|
|
|
Performance-Based Restricted Stock
(3)
|
1,000,260
|
|
1,000,260
|
|
—
|
|
—
|
|
—
|
|
|
Stock Options
|
77,202
|
|
77,202
|
|
—
|
|
—
|
|
77,202
|
|
|
Other Benefits
(4)
|
25,000
|
|
—
|
|
—
|
|
25,000
|
|
25,000
|
|
|
Total Estimated Incremental Value
|
3,895,326
|
|
2,186,070
|
|
—
|
|
867,128
|
|
1,294,160
|
|
|
Jeffrey R. Needham
|
||||||||||
|
Cash Severance
(2)
|
1,928,500
|
|
304,500
|
|
—
|
|
812,000
|
|
812,000
|
|
|
Equity Awards
|
||||||||||
|
Service-Based Restricted Stock
|
476,640
|
|
476,640
|
|
—
|
|
—
|
|
146,520
|
|
|
Performance-Based Restricted Stock
(3)
|
762,210
|
|
762,210
|
|
—
|
|
—
|
|
—
|
|
|
Stock Options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
Other Benefits
(4)
|
25,000
|
|
—
|
|
—
|
|
25,000
|
|
25,000
|
|
|
Total Estimated Incremental Value
|
3,192,350
|
|
1,543,350
|
|
—
|
|
837,000
|
|
983,520
|
|
|
Sharon Kochan
|
||||||||||
|
Cash Severance
(2)
|
1,909,215
|
|
301,455
|
|
—
|
|
803,880
|
|
803,880
|
|
|
Equity Awards
|
||||||||||
|
Service-Based Restricted Stock
|
424,170
|
|
424,170
|
|
—
|
|
—
|
|
100,170
|
|
|
Performance-Based Restricted Stock
(3)
|
751,950
|
|
751,950
|
|
—
|
|
—
|
|
—
|
|
|
Stock Options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
Other Benefits
(4)
|
25,000
|
|
—
|
|
—
|
|
25,000
|
|
25,000
|
|
|
Total Estimated Incremental Value
|
3,110,335
|
|
1,477,575
|
|
—
|
|
828,880
|
|
929,050
|
|
|
1) Mr. Hendrickson will receive cash severance representing 156 weeks of salary, 156 weeks of target bonus, and a pro rata bonus payment he would have received for the fiscal year if he leaves Perrigo for a change in control. If termination is without cause or for good reason, or involuntary termination for economic reasons Mr. Hendrickson will receive cash severance representing 24 months of salary, 12 months of annual target bonus, and a pro rata bonus payment he would have received for the fiscal year in which his termination occurs. Cash severance represents any earned prorated bonus if his employment is terminated because of death, disability or retirement.
|
|
2) Ms. Brown, Mr. Kingma, Mr. Needham and Mr. Kochan will receive cash severance of 104 weeks annual salary and a bonus prorated for the actual bonus payout they would have received if employment is terminated due to a change in control. They will receive cash severance of 52 weeks annual salary and a bonus prorated for the actual payout they would have received if employment is terminated without cause or involuntary termination for economic reasons. They will receive any earned prorated bonus if their employment is terminated because of death, disability or retirement.
|
|
3) Performance-based restricted stock units were valued based on a full three-year vesting credit of 135%, 68% and 0% for the fiscal 2015, 2015 Stub Period and fiscal 2016 grants, respectively. The full three-year vesting credit was calculated based on the actual average vesting performance for the 2015-2016 fiscal year grants of 68% based on our fiscal ROTC performance. The 2015 Stub Period and 2016 full three-year vesting credit used a target performance of 100% for performance in any future fiscal year.
|
|
4) Other Benefits for Ms. Brown include outplacement/career transition services up to $25,000, and for Messrs. Hendrickson, Kingma, Needham and Kochan include outplacement/career transition services up to $25,000 and 12 months of Company paid COBRA payments.
|
|
•
|
upon Mr. Hendrickson’s death or disability;
|
|
•
|
by Perrigo with or without cause (as defined in the agreement);
|
|
•
|
by mutual agreement; or
|
|
•
|
by Mr. Hendrickson with good reason (as defined in the agreement).
|
|
•
|
a prorated annual bonus for the year of termination (determined based on actual performance);
|
|
•
|
payment of an amount equal to 24 months of his then-current salary and target bonus, payable in a lump sum;
|
|
•
|
a payment of health insurance premiums for 18 months, followed by a cash payment equal to the cost of such premiums for another six months, but only if Mr. Hendrickson is not entitled to health insurance coverage from another employer-provided plan;
|
|
•
|
continued vesting for a period of 24 months of all equity incentive awards granted to him, and in the case of performance-based restricted stock, based on actual Company performance, provided that any portion of such awards that does not vest pursuant to the above is forfeited and no option may be exercised later than the expiration of the option term as specified in the award agreement; and
|
|
•
|
reimbursement of career transition assistance services, up to a value of $50,000.
|
|
•
|
retirement at age 65 or older;
|
|
•
|
retirement at age 60 or older with at least 10 years of service;
|
|
•
|
early retirement of a named executive officer under an early retirement plan;
|
|
•
|
permanent disability as determined by the Remuneration Committee; or
|
|
•
|
death.
|
|
THE REMUNERATION COMMITTEE
|
|
|
Jeffrey B. Kindler, Chair
|
|
|
Geoffrey M. Parker
|
|
|
Theodore R. Samuels
|
|
|
Jeffrey C. Smith
|
|
|
Chairman Annual Cash Retainer:
|
$150,000
|
|
Director Annual Cash Retainer
|
$75,000
|
|
Committee Member Retainer:
|
|
|
Audit
|
$12,500
|
|
Remuneration
|
$12,500
|
|
Nominating & Governance
|
$ 8,000
|
|
Committee Chair Retainer:
|
|
|
Audit
|
$25,000
|
|
Remuneration
|
$25,000
|
|
Nominating & Governance
|
$16,000
|
|
Name
|
Fees Earned or Paid in Cash ($)
|
Stock Awards ($)
(1)
|
Option Awards ($)
(2)
|
All Other Compensation
|
Total ($)
|
|||||
|
Laurie Brlas
|
189,036
|
375,000
|
—
|
—
|
564,036
|
|||||
|
Gary M. Cohen
|
132,000
|
300,000
|
—
|
—
|
432,000
|
|||||
|
Jacqualyn A. Fouse
|
47,868
|
300,000
|
—
|
—
|
347,868
|
|||||
|
Ellen R. Hoffing
|
153,500
|
300,000
|
—
|
—
|
453,500
|
|||||
|
Michael J. Jandernoa
|
116,000
|
300,000
|
—
|
—
|
416,000
|
|||||
|
Gerald K. Kunkle, Jr.
|
128,430
|
300,000
|
—
|
—
|
428,430
|
|||||
|
Herman Morris, Jr.
|
132,390
|
300,000
|
—
|
—
|
432,390
|
|||||
|
Donal O'Connor
|
106,036
|
300,000
|
—
|
—
|
406,036
|
|||||
|
Shlomo Yanai
|
143,500
|
300,000
|
—
|
—
|
443,500
|
|||||
|
Geoffrey M. Parker
|
21,301
|
45,205
|
—
|
—
|
66,506
|
|||||
|
1) Represents the grant date fair value of 3,263 service- based restricted stock units granted to each non-employee director on May 19, 2016 calculated in accordance with U.S. GAAP. The grant date fair value is based on $91.94 per share, the closing price of Perrigo Company plc ordinary shares on the NYSE on the grant date. For Ms. Brlas also includes the grant date fair value of 778 service-based restricted stock units granted for her service as Chairman of the Board on June 21, 2016 calculated in accordance with U.S. GAAP. The grant date fair value is based on $96.45 per share, the closing price of Perrigo Company plc ordinary shares on the NYSE on the grant date. The shares vest on the earlier of one year after the grant date or at the AGM.
|
||||||||||
|
2) As of December 31, 2016, there were no unvested stock options held by non-employee directors.
|
||||||||||
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
Ordinary Shares Beneficially Owned
|
Options Exercisable Within 60 days
|
Total
|
Percent
of Class
|
||||
|
Directors
|
|
|
|
|
|||
|
Bradley A. Alford
|
—
|
|
—
|
|
—
|
|
*
|
|
Laurie Brlas
|
10,457
|
|
7,225
|
|
17,682
|
|
*
|
|
Rolf A. Classon
|
—
|
|
—
|
|
—
|
|
*
|
|
Gary M. Cohen
|
13,351
|
|
10,278
|
|
23,629
|
|
*
|
|
John T Hendrickson
(1)
|
14,774
|
|
23,455
|
|
38,229
|
|
*
|
|
Adriana Karaboutis
|
—
|
|
—
|
|
—
|
|
*
|
|
Jeffrey B. Kindler
|
—
|
|
—
|
|
—
|
|
*
|
|
Donal O'Connor
|
2,810
|
|
—
|
|
2,810
|
|
*
|
|
Geoffrey M. Parker
|
2,650
|
|
—
|
|
2,650
|
|
*
|
|
Theodore R. Samuels
|
2,759
|
|
—
|
|
2,759
|
|
*
|
|
Jeffrey C. Smith
(2)
|
9,641,425
|
|
—
|
|
|
6.7%
|
|
|
Named Executive Officers Other Than Directors
|
|
|
|
|
|||
|
Judy L. Brown
|
10,007
|
|
34,056
|
|
44,063
|
|
*
|
|
Todd W. Kingma
(3)
|
16,560
|
|
43,187
|
|
59,747
|
|
*
|
|
Jeffrey R. Needham
|
10,904
|
|
12,691
|
|
23,595
|
|
*
|
|
Sharon Kochan
|
9,594
|
|
18,638
|
|
28,232
|
|
*
|
|
Joseph C. Papa
(4)
|
118,515
|
|
—
|
|
118,515
|
|
*
|
|
Directors and Executive Officers as a Group (22 Persons)
(5)
|
9,742,572
|
|
145,991
|
|
9,888,563
|
|
6.9%
|
|
* Less than 1%.
|
|
|
|
|
|||
|
(1) Shares owned include 9,879 shares owned by the John T. Hendrickson Trust, of which Mr. Hendrickson is the trustee.
|
|||||||
|
(2) Represents shares held by certain funds and managed accounts for which Starboard Value LP serves as manager or investment manager. Mr. Smith serves as a Managing Member, Chief Executive Officer, and Chief Investment Officer of Starboard Value LP. Mr. Smith has shared voting and shared dispositive power over Starboard’s shares.
|
|||||||
|
(3) Shares owned include 2,000 shares in the Todd Kingma Charitable Remainder Uni-Trust.
|
|||||||
|
(4) Ownership of ordinary shares as of the date of Mr. Papa’s resignation and options as of May 10, 2017.
|
|||||||
|
(5) See footnotes 1 through 3. Includes directors and executive officers as of May 10, 2017. Of these shares, 9,641,425 are beneficially owned indirectly by Jeffrey Smith.
|
|||||||
|
Name and Address
of Beneficial Owner
|
Ordinary Shares
Beneficially Owned
|
Percent
of Class
|
|
The Vanguard Group
(1)
100 Vanguard Blvd.
Malvern, PA 19355
|
14,574,768
|
10.17%
|
|
BlackRock Inc.
(2)
55 East 52nd Street
New York, NY 10055
|
11,617,204
|
8.48%
|
|
Starboard Value LP
(3)
777 Third Avenue, 18
th
Floor
New York, NY 10055
|
9,641,425
|
6.72%
|
|
1)
|
The Vanguard Group, Inc. has sole voting power with respect to 226,541 of the shares, shared voting power with respect to 26,691 of the shares, shared dispositive power with respect to 249,174 of the shares and sole dispositive power with respect to 14,325,514 shares. This information is based on a Schedule 13G/A filed with the SEC on April 10, 2017.
|
|
2)
|
BlackRock Inc. has sole voting power with respect to 10,295,073 of the shares shared voting power with respect to none of the shares and sole dispositive power with respect to all of the shares. This information is based on a Schedule 13G/A filed with the SEC on January 25, 2017.
|
|
3)
|
Based on a Schedule 13D/A filed with the SEC on February 7, 2017, pursuant to which (a) each of Starboard Value LP, Starboard Value GP LLC, Starboard Principal Co LP and Starboard Principal Co GP LLC reported sole voting and dispositive power with respect to 9,641,425 shares; (b) Starboard Value and Opportunity Master Fund Ltd reported sole voting and dispositive power with respect to 3,287,856 shares; (c) each of Starboard Value A LP and Starboard Value A GP LLC reported sole voting and dispositive power with respect 2,366,741 shares; (d) each of Starboard Leaders Kilo LLC and Starboard Leaders Fund LP, Value reported sole voting and dispositive power with respect to 2,001,138 shares; (e) Starboard Value and Opportunity S LLC reported sole voting and dispositive power with respect to 372,738 shares; (f) each of Starboard Leaders Select III LP and Starboard Leaders Select III GP LLC Value reported sole voting and dispositive power with respect 365,603 shares; (g) each of Starboard Value and Opportunity C LP, Starboard Value R LP and Starboard Value R GP LLC reported sole voting and dispositive power with respect to 209,418 shares; and (h) each of Jeffrey C. Smith, Mark R. Mitchell and Peter A. Feld reported shared voting and dispositive power with respect to 9,641,425 shares.
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
2015 Stub Period
|
Calendar Year 2016
|
|||||||
|
Audit Fees
|
$
|
9,808,483
|
|
Audit Fees
|
$
|
14,817,000
|
|
|
|
Audit-Related Fees
(1)
|
1,262,180
|
|
Audit-Related Fees
(1)
|
25,000
|
|
|||
|
Tax Compliance
|
$
|
543,230
|
|
Tax Compliance
|
$
|
1,536,000
|
|
|
|
Tax Consulting & Advisory
|
946,533
|
|
Tax Consulting & Advisory
|
2,664,000
|
|
|||
|
Total Tax Fees
|
$
|
1,489,763
|
|
Total Tax Fees
|
$
|
4,200,000
|
|
|
|
All Other Fees
|
—
|
|
All Other Fees
|
—
|
|
|||
|
Total Fees
|
$
|
12,560,426
|
|
Total Fees
|
$
|
19,042,000
|
|
|
|
(1) Mainly represents attest services provided to the Company in connection with the requirements of the Irish Takeover Panel.
|
||||||||
|
Item 15.
|
Exhibits and Financial Statement Schedules.
|
|
(a)
|
The following documents are filed or incorporated by reference as part of this Form 10-K:
|
|
1.
|
All financial statements. See Index to Consolidated Financial Statements.
|
|
2.
|
Financial Schedules.
|
|
2.1
|
Transaction Agreement, dated as of July 28, 2013, among Perrigo Company, Elan Corporation, plc, Perrigo Company plc, Habsont Limited and Leopard Company (incorporated by reference from Annex A to the joint proxy statement/prospectus included in the Company's Registration Statement on Form S-4/A filed on October 8, 2013) (File No. 333-190859).
|
|
2.2
|
Part A of Appendix I to Rule 2.5 Announcement (Conditions to the Implementation of the Scheme and the Acquisition) (incorporated by reference from Annex B to the joint proxy statement/prospectus included in the Company's Registration Statement on Form S-4/A filed on October 8, 2013) (File No. 333-190859).
|
|
2.3
+
|
Asset Purchase Agreement, dated as of February 5, 2013, by and among Elan Pharma International Limited, Elan Pharmaceuticals, Inc. and Biogen Idec International Holding Ltd (incorporated by reference from Exhibit 4(c) (31) of Elan Corporation, plc’s Annual Report on Form 20-F for the year ended December 31, 2012) (File No. 001-13896).
|
|
2.4
|
Agreement for the Sale and Purchase of 685,348,257 Shares Of Omega Pharma Invest N.V., dated as of November 6, 2014, by and among the Company, Alychlo N.V. and Holdco I BE N.V. (incorporated by reference from Exhibit 10.1 to the Company's Current Report on Form 8-K filed on November 12, 2014) (File No. 001-36353).
|
|
2.5
|
Amendment Agreement dated March 27, 2015 to the Agreement for the Sale and Purchase of 685,348,257 Shares Of Omega Pharma Invest N.V., dated as of November 6, 2014, by and among the Company, Alychlo N.V. and Holdco I BE N.V. (incorporated by reference from Exhibit 2.3 to the Company’s Quarterly Report on Form 10-Q filed on April 29, 2015) (File No. 001-36353).
|
|
2.6
|
Assignment Letter dated March 17, 2015 regarding the Agreement for the Sale and Purchase of 685,348,257 Shares Of Omega Pharma Invest N.V., dated as of November 6, 2014, by and among the Company, Alychlo N.V. and Holdco I BE N.V. (incorporated by reference from Exhibit 2.1 to the Company’s Quarterly Report on Form 10-Q filed on April 29, 2015) (File No. 001-36353).
|
|
2.7
|
Closing Letter dated March 17, 2015 regarding the Agreement for the Sale and Purchase of 685,348,257 Shares Of Omega Pharma Invest N.V., dated as of November 6, 2014, by and among the Company, Alychlo N.V. and Holdco I BE N.V. (incorporated by reference from Exhibit 2.2 to the Company’s Quarterly Report on Form 10-Q filed on April 29, 2015) (File No. 001-36353).
|
|
3.1
|
Certificate of Incorporation of Perrigo Company plc (formerly known as Perrigo Company Limited) (incorporated by reference from Exhibit 4.1 to the Company’s Registration Statement on Form S-8 filed December 19, 2013) (File No. 333-192946).
|
|
3.2
|
Memorandum and Articles of Association of Perrigo Company plc, as amended (incorporated by reference from Exhibit 3.2 to the Company’s Transition Report on Form 10-KT filed on February 25, 2016) (File No. 001-36353).
|
|
4.1
|
Indenture dated as of November 8, 2013, among the Company, the guarantors named therein and Wells Fargo Bank, N.A., as Trustee (incorporated by reference from Exhibit 4.1 to the Company's Current Report on Form 8-K filed on November 12, 2013) (File No. 333-190859).
|
|
4.2
|
First Supplemental Indenture, dated December 18, 2013 to the Indenture dated as of November 8, 2013, among the Company, the guarantors named therein and Wells Fargo Bank, N.A., as Trustee (incorporated by reference from Exhibit 4.1 to the Company's Current Report on Form 8-K filed on December 19, 2013) (File No. 333-190859).
|
|
4.4
|
Base Indenture dated as of December 2, 2014, between Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company and Wells Fargo Bank, National Association, as trustee (incorporated by reference from Exhibit 4.1 to the Company's Current Report on Form 8-K filed on December 2, 2014) (File No. 001-36353).
|
|
4.5
|
First Supplemental Indenture dated as of December 2, 2014, between Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company and Wells Fargo Bank, National Association, as trustee (incorporated by reference from Exhibit 4.2 to the Company's Current Report on Form 8-K filed on December 2, 2014) (File No. 001-36353).
|
|
4.6
|
Supplemental Indenture No. 2, dated as of March 10, 2016, among Perrigo Finance Unlimited Company, the Company and Wells Fargo Bank, National Association, as trustee (incorporated by reference from Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 10, 2016) (File No. 001-36353).
|
|
4.7
|
Form of 3.500% Senior Notes due 2021 (included as Exhibit A-1 to the First Supplemental Indenture dated as of December 2, 2014, between Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company and Wells Fargo Bank, National Association, as trustee) (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on December 2, 2014) (File No. 001-36353).
|
|
4.8
|
Form of 3.900% Senior Notes due 2024 (included as Exhibit A-2 to the First Supplemental Indenture dated as of December 2, 2014, between Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company and Wells Fargo Bank, National Association, as trustee) (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on December 2, 2014) (File No. 001-36353).
|
|
4.9
|
Form of 4.900% Senior Notes due 2044 (included as Exhibit A-3 to the First Supplemental Indenture dated as of December 2, 2014, between Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company and Wells Fargo Bank, National Association, as trustee) (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on December 2, 2014) (File No. 001-36353).
|
|
4.10
|
Form of Global Note representing the 2021 Notes (included in Exhibit 4.6).
|
|
4.11
|
Form of Global Note representing the 2026 Notes (included in Exhibit 4.6).
|
|
4.12
|
Prospectus, dated November 27, 2012, in connection with the public offering of Omega Pharma Invest N.V. of EUR 300,000,000 of 5.125% retail bonds due 2017 (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on April 3, 2015) (File No. 001-36353).
|
|
4.13
|
Prospectus, dated April 23, 2012, in connection with the public offering of Omega Pharma Invest N.V. of EUR 180,000,000 of 4.500% retail bonds due 2017 and EUR 120,000,000 of 5.000% retail bonds due 2019 (incorporated by reference from Exhibit 4.3 to the Company’s Current Report on Form 8-K filed on April 3, 2015) (File No. 001-36353).
|
|
10.1
|
Senior Unsecured Credit Facilities Commitment Letter by and among the Company, J.P. Morgan Securities LLC, JPMorgan Chase Bank, N.A. and Barclays Bank PLC dated as of November 6, 2014 (incorporated by reference from Exhibit 10.3 to the Company's Current Report on Form 8-K filed on November 12, 2014) (File No. 001-36353).
|
|
10.2
|
Revolving Credit Agreement by and among Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company, JP Morgan Chase Bank, N.A., Barclays Bank PLC, and the other lenders party thereto, dated as of December 5, 2014 (incorporated by reference from Exhibit 10.1 to the Company's Current Report on Form 8-K filed on December 9, 2014) (File No. 001-36353).
|
|
10.3
|
Amendment to the Revolving Credit Agreement, dated February 26, 2016, by and among Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company, JPMorgan Chase Bank, N.A., Barclays Bank PLC, and the other lenders party thereto, dated as of December 5, 2014 (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on May 16, 2016) (File No. 001-36353).
|
|
10.4
|
Amendment No. 2, dated September 9, 2016, to the Revolving Credit Agreement by and among Perrigo Finance Unlimited Company, the Company, JPMorgan Chase Bank, N.A. and the other lenders party thereto, dated as of December 5, 2014, as amended by Amendment No. 1, dated as of February 26, 2016 (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 9, 2016) (File No. 001-36353).
|
|
10.5
|
Amendment No. 3, dated December 8, 2016, to the Revolving Credit Agreement by and among Perrigo Finance Unlimited Company, the Company, JPMorgan Chase Bank, N.A. and the other lenders party thereto, dated as of December 5, 2014, as amended by Amendment No. 1, dated as of February 26, 2016, as further amended by Amendment No. 2, dated as of September 9, 2016 (filed herewith).
|
|
10.6
|
Term Loan Credit Agreement by and among Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company, JP Morgan Chase Bank, N.A., Barclays Bank PLC, and the other lenders party thereto, dated as of December 5, 2014 (incorporated by reference from Exhibit 10.2 to the Company's Current Report on Form 8-K filed on December 9, 2014) (File No. 001-36353).
|
|
10.7
|
Amendment to the Term Loan Credit Agreement, dated February 26, 2016, by and among Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, the Company, JPMorgan Chase Bank, N.A., Barclays Bank PLC, and the other lenders party thereto, dated as of December 5, 2014 (incorporated by reference from Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on May 16, 2016) (File No. 001-36353).
|
|
10.8
|
Amendment No. 2, dated September 9, 2016, to the Term Loan Credit Agreement by and among Perrigo Finance Unlimited Company, the Company, JPMorgan Chase Bank, N.A. and the other lenders party thereto, dated as of December 5, 2014, as amended by Amendment No. 1, dated as of February 26, 2016 (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on September 9, 2016) (File No. 001-36353).
|
|
10.9
|
Amendment No. 3, dated December 8, 2016, to the Term Loan Credit Agreement by and among Perrigo Finance Unlimited Company, the Company, JPMorgan Chase Bank, N.A. and the other lenders party thereto, dated as of December 5, 2014, as amended by Amendment No. 1, dated as of February 26, 2016, as further amended by Amendment No. 2, dated as of September 9, 2016 (filed herewith).
|
|
10.10*
|
Annual Incentive Plan, adopted November 4, 2008 (incorporated by reference from Perrigo Company’s Proxy Statement for its 2008 Annual Meeting of Shareholders filed on October 1, 2008) (File No. 000-19725).
|
|
10.11*
|
Amendment No. 1 to Annual Incentive Plan, effective as of June 22, 2015 (incorporated by reference from Exhibit 10.10 to the Company's Annual Report on Form 10-K filed on August 13, 2015) (File No. 001-36353).
|
|
10.12*
|
Amendment No. 2 to the Perrigo Company Annual Incentive Plan, effective June 14, 2016 (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on June 17, 2016) (File No. 001-36353).
|
|
10.13*
|
2003 Long-Term Incentive Plan, effective October 29, 2003, as amended (incorporated by reference from the Appendix to Perrigo Company’s Proxy Statement for its 2003 Annual Meeting of Shareholders filed on September 26, 2003) (File No. 000-19725).
|
|
10.14*
|
Amendment to the 2003 Long-Term Incentive Plan, effective as of October 28, 2005 (incorporated by reference from Exhibit 10(a) to Perrigo Company’s Current Report on Form 8-K filed on November 3, 2005) (File No. 000-19725).
|
|
10.15*
|
2003 Long-Term Incentive Plan, as amended as of February 7, 2007 (incorporated by reference from Exhibit 10(a) to Perrigo Company’s Quarterly Report on Form 10-Q filed on May 8, 2007) (File No. 000-19725).
|
|
10.16*
|
2008 Long-Term Incentive Plan, adopted November 4, 2008 (incorporated by reference from Exhibit 10(b) to Perrigo Company's Quarterly Report on Form 10-Q filed on February 3, 2009) (File No. 000-19725).
|
|
10.17*
|
2013 Long-Term Incentive Plan (incorporated by reference from Annex J to the Company’s Registration Statement on Form S-4/A filed on October 8, 2013) (File No. 333-190859).
|
|
10.18*
|
Amendment No. 1 to the 2013 Long-Term Incentive Plan, dated as of January 29, 2014 (incorporated by reference from Exhibit 10.12 to the Company’s Quarterly Report on Form 10-Q filed on February 6, 2014) (File No. 333-190859).
|
|
10.19*
|
Amendment No. 2 to the 2013 Long-Term Incentive Plan, effective as of July 9, 2015 (incorporated by reference from Exhibit 10.17 to the Company's Annual Report on Form 10-K, filed on August 13, 2015) (File No. 001-36353).
|
|
10.20*
|
Nonqualified Deferred Compensation Plan, as amended as of October 10, 2007 and effective January 1, 2007 (incorporated by reference from Exhibit 10.1 to Perrigo Company’s Current Report on Form 8-K filed on October 11, 2007) (File No. 000-19725).
|
|
10.21*
|
Amendment One to the Nonqualified Deferred Compensation Plan, dated December 3, 2009 (incorporated by reference from Exhibit 10.14 to the Company's Annual Report on Form 10-K filed on August 14, 2014) (File No. 001-36353).
|
|
10.22*
|
Amendment Two to the Nonqualified Deferred Compensation Plan, dated as of October 10, 2012, (incorporated by reference from Exhibit 10.1 to Perrigo Company’s Quarterly Report on Form 10-Q filed on February 1, 2013) (File No. 000-19725).
|
|
10.23*
|
Amendment Three to the Nonqualified Deferred Compensation Plan, dated as of November 13, 2013 (incorporated by reference from Exhibit 10.9 to the Company’s Quarterly Report on Form 10-Q filed on February 6, 2014) (File No. 333-190859).
|
|
10.24*
|
Amendment Four to the Nonqualified Deferred Compensation Plan, dated as of January 31, 2014 (incorporated by reference from Exhibit 10.13 to the Company’s Quarterly Report on Form 10-Q filed on February 6, 2014) (File No. 333-190859).
|
|
10.25*
|
Amendment Five to the Nonqualified Deferred Compensation Plan, dated as of August 17, 2015 (incorporated by reference from Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q filed on November 2, 2015) (File No. 001-36353).
|
|
10.26*
|
Forms of Non-Qualified Stock Option Agreement pursuant to Perrigo Company’s 2008 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.49 to Perrigo Company’s Annual Report on Form 10-K filed on August 18, 2009) (File No. 000-19725).
|
|
10.27*
|
Form of Non-Qualified Stock Option Agreement under Perrigo Company's 2003 Long-Term Incentive Plan (incorporated by reference from Exhibit 10(a) to Perrigo Company’s Quarterly Report on Form 10-Q filed on February 2, 2005) (File No. 000-19725).
|
|
10.28*
|
Forms of Non-Qualified Stock Option Agreement pursuant to Perrigo Company’s 2008 Long-Term Incentive Plan (incorporated by reference from Exhibit 10(c) to Perrigo Company’s Quarterly Report on Form 10-Q filed on February 3, 2009) (File No. 000-19725).
|
|
10.29*
|
Form of Long-Term Incentive Award Agreement under Perrigo Company's 2003 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.1 to Perrigo Company’s Current Report on Form 8-K filed on August 22, 2006) (File No. 000-19725).
|
|
10.30*
|
Form of Long-Term Incentive Award Agreement under Perrigo Company's 2003 Long-Term Incentive Plan (incorporated by reference from Exhibit 10(a) to Perrigo Company’s Quarterly Report on Form 10-Q filed on February 1, 2007) (File No. 000-19725).
|
|
10.31*
|
Form of 2006 Long-Term Incentive Award Agreement, for Approved Section 102 Awards under Perrigo Company’s 2003 Long-Term Incentive Plan (incorporated by reference from Exhibit 10(f) to Perrigo Company’s Quarterly Report on Form 10-Q filed on May 8, 2007) (File No. 000-19725).
|
|
10.32*
|
Form of 2006 Long-Term Incentive Award Agreement under Perrigo Company’s 2003 Long-Term Incentive Plan (incorporated by reference from Exhibit 10(g) to Perrigo Company’s Quarterly Report on Form 10-Q filed on May 8, 2007) (File No. 000-19725).
|
|
10.33*
|
Forms of Restricted Stock Unit Award Agreement pursuant to Perrigo Company’s 2008 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.50 to Perrigo Company’s Annual Report on Form 10-K filed on August 18, 2009) (File No. 000-19725).
|
|
10.34*
|
Forms of Restricted Stock Unit Award Agreement pursuant to Perrigo Company’s 2008 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.52 to Perrigo Company's Annual Report on Form 10-K filed on August 16, 2011) (File No. 000-19725).
|
|
10.35*
|
Forms of Grant Agreement under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.11 to the Company’s Quarterly Report on Form 10-Q filed on February 6, 2014) (File No. 333-190859).
|
|
10.36*
|
Forms of Restricted Stock Unit Award Agreement (Service-Based) under the Company’s 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on November 12, 2014) (File No. 001-36353).
|
|
10.37*
|
Forms of Service-Based and Performance-Based Restricted Stock Unit Award Agreements under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on June 22, 2015) (File No. 001-36353).
|
|
10.38*
|
Forms of Amendments to Performance-Based Restricted Stock Unit Award Agreements under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on June 26, 2015) (File No. 001-36353).
|
|
10.39*
|
Forms of Service-Based and Performance-Based Restricted Stock Unit Award Agreements under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 99.1 to the Company's Current Report on Form 8-K filed on August 12, 2015) (File No. 001-36353).
|
|
10.40*
|
Form of Performance-Based Restricted Stock Unit Award Agreement for Non-U.S. Participants under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on November 2, 2015) (File No. 001-36353).
|
|
10.41*
|
Forms of Amendments to Performance-Based Restricted Stock Unit Award Agreements under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.4 to the Company's Current Report on Form 8-K filed on November 13, 2015) (File No. 001-36353).
|
|
10.42*
|
Forms of Service-Based Restricted Stock Unit Award Agreements under the Company's 2013 Long-Term Incentive Plan (incorporated by reference from Exhibit 10.41 to the Company’s Transition Report on Form 10-KT filed on February 25, 2016) (File No. 001-36353).
|
|
10.43*
|
Employment Agreement, dated as of September 8, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.1 to Perrigo Company’s Current Report on Form 8-K, filed on September 12, 2006) (File No. 000-19725).
|
|
10.44*
|
Amendment No. 1, dated as November 12, 2015, to the Employment Agreement, dated as of September 8, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.1 to the Company's Current Report on Form 8-K filed on November 13, 2015) (File No. 001-36353).
|
|
10.45*
|
Amendment No.2, effective as of October 22, 2015, to the Employment Agreement, dated as of September 8, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.44 to the Company’s Transition Report on Form 10-KT filed on February 25, 2016) (File No. 001-36353).
|
|
10.46*
|
Amendment No. 3, effective as of April 24, 2016, to the Employment Agreement, effective as of October 9, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 25, 2016) (File No. 001-36353).
|
|
10.47*
|
Amendment No. 4, effective as of May 6, 2016, to the Employment Agreement, effective as of October 9, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on May 16, 2016) (File No. 001-36353).
|
|
10.48*
|
Employment Agreement, dated as of August 3, 2016, by and among the Company, Perrigo Management Company and John T. Hendrickson (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on August 5, 2016) (File No. 001-36353).
|
|
10.49
|
Employment agreement, dated October 25, 2016, by and between Perrigo Israel Pharmaceuticals Ltd. and Sharon Kochan (filed herewith).
|
|
10.50*
|
Consultancy Agreement, between Omega Pharma Invest N.V. and Mylecke Management, Art & Invest N.V., represented by Marc Coucke, dated November 5, 2014, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 3, 2015 (File No. 001-36353).
|
|
10.51*
|
Form of Perrigo Company plc Director Indemnity Agreement (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 19, 2013) (File No. 333-190859).
|
|
10.52*
|
Form of Perrigo Company plc Officer Indemnity Agreement (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on December 19, 2013) (File No. 333-190859).
|
|
10.53*
|
Form of Perrigo Company Indemnity Agreement (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on December 19, 2013) (File No. 333-190859).
|
|
10.54*
|
Perrigo Company plc U.S. Severance Policy, as amended and restated effective June 14, 2016 (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 17, 2016) (File No. 001-36353).
|
|
10.55*
|
Perrigo Company plc Change in Control Severance Policy for U.S. Employees, as amended and restated effective June 14, 2016 (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on June 17, 2016) (File No. 001-36353).
|
|
10.56
|
Mutual Agreement dated April 27, 2016 among the Company, Omega Pharma NV, Perrigo Ireland 2 Ltd, Mylecke Management, Art & Invest NV, Alychlo NV and Marc Coucke (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 28, 2016) (File No. 001-36353).
|
|
10.57
|
Amendment dated April 27, 2016 to the Non-Compete Agreement between the Company and Marc Coucke dated March 30, 2015 (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on April 28, 2016) (File No. 001-36353).
|
|
10.58
|
Amendment dated April 27, 2016 to the Lock-up Agreement between the Company and Alychlo NV dated March 30, 2015 (incorporated by reference from Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on April 28, 2016) (File No. 001-36353).
|
|
10.59
|
Amendment dated April 27, 2016 to the Agreement for the Sale and Purchase of 685,348,257 Shares Of Omega Pharma Invest NV, dated as of November 6, 2014, by and among the Company, Alychlo NV and Holdco I BE NV (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 28, 2016) (File No. 001-36353).
|
|
10.60
|
Amendment 1 to Non-Compete Agreement, dated December 30, 2016, by and between Perrigo Ireland 2 Ltd. and Marc Coucke (filed herewith).
|
|
10.61
|
Amendment 2 to Non-Compete Agreement, dated December 30, 2016, by and between Perrigo Ireland 2 Ltd. and Marc Coucke (filed herewith).
|
|
21
|
Subsidiaries of the Registrant.
|
|
23
|
Consent of Ernst & Young LLP.
|
|
24
|
Power of Attorney (see signature page).
|
|
31
|
Rule 13a-14(a) Certifications.
|
|
32
|
Section 1350 Certifications.
|
|
101.INS
|
XBRL Instance Document.
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
+
|
Confidential treatment has been requested for portions of this agreement. A completed copy of the agreement, including the redacted portions, has been filed separately with the SEC.
|
|
*
|
Denotes management contract or compensatory plan or arrangement.
|
|
(b)
|
Exhibits.
|
|
(c)
|
Financial Statement Schedules.
|
|
Year Ended
|
Six Months Ended
|
Year Ended
|
||||||||||
|
Allowance for doubtful accounts
|
December 31,
2016 |
December 31,
2015 |
June 27,
2015 |
|||||||||
|
Balance at beginning of period
|
$
|
4.5
|
|
$
|
2.6
|
|
$
|
2.7
|
|
|||
|
Net bad debt expenses
(1)
|
2.1
|
|
2.5
|
|
0.6
|
|
||||||
|
Additions/(deductions)
(2)
|
(0.3
|
)
|
(0.6
|
)
|
(0.7
|
)
|
||||||
|
Balance at end of period
|
$
|
6.3
|
|
$
|
4.5
|
|
$
|
2.6
|
|
|||
|
(1)
|
Includes effects of changes in foreign exchange rates.
|
|
(2)
|
Uncollectible accounts written off, net of recoveries. Also includes effects of changes in foreign exchange rates.
|
|
PERRIGO COMPANY PLC
|
|
|
By:
|
/s/ John T. Hendrickson
|
|
John T. Hendrickson
|
|
|
Chief Executive Officer
|
|
|
(Principal Executive Officer)
|
|
|
Signature
|
|
Title
|
|
/s/ John T. Hendrickson
|
Chief Executive Officer, Director
|
|
|
John T. Hendrickson
|
(Principal Executive Officer)
|
|
|
/s/ Ronald L. Winowiecki
|
Acting Chief Financial Officer
|
|
|
Ronald L. Winowiecki
|
(Principal Accounting and Financial Officer)
|
|
|
/s/ Laurie Brlas
|
Chairman
|
|
|
Laurie Brlas
|
||
|
/s/ Bradley A. Alford
|
Director
|
|
|
Bradley A. Alford
|
||
|
/s/ Rolf A. Classon
|
Director
|
|
|
Rolf A. Classon
|
||
|
/s/ Adriana Karaboutis
|
Director
|
|
|
Adriana Karaboutis
|
||
|
/s/ Gary M. Cohen
|
Director
|
|
|
Gary M. Cohen
|
||
|
/s/ Jeffrey B. Kindler
|
Director
|
|
|
Jeffrey B. Kindler
|
||
|
/s/ Donal O'Connor
|
Director
|
|
|
Donal O'Connor
|
||
|
/s/ Geoffrey M. Parker
|
Director
|
|
|
Geoffrey M. Parker
|
||
|
/s/ Theodore R. Samuels
|
Director
|
|
|
Theodore R. Samuels
|
||
|
/s/ Jeffrey C. Smith
|
Director
|
|
|
Jeffrey C. Smith
|
||