|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[X]
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Ireland
|
|
Not Applicable
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
Treasury Building, Lower Grand Canal Street, Dublin 2, Ireland
|
|
-
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Large accelerated filer [X]
|
|
Accelerated filer [ ]
|
|
Non-accelerated filer [ ]
|
|
Smaller reporting company [ ]
|
|
(Do not check if a smaller reporting company)
|
|
|
|
PAGE
NUMBER
|
||
|
PART I. FINANCIAL INFORMATION
|
||
|
1
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
8
|
||
|
9
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
15
|
||
|
16
|
||
|
PART II. OTHER INFORMATION
|
||
|
|
Three Months Ended
|
||||||
|
|
April 2,
2016 |
March 28,
2015 |
|||||
|
Net sales
|
$
|
1,383.2
|
|
$
|
1,049.1
|
|
|
|
Cost of sales
|
860.3
|
|
670.3
|
|
|||
|
Gross profit
|
522.9
|
|
378.8
|
|
|||
|
Operating expenses
|
|||||||
|
Distribution
|
21.8
|
|
14.7
|
|
|||
|
Research and development
|
45.3
|
|
35.4
|
|
|||
|
Selling
|
180.8
|
|
48.8
|
|
|||
|
Administration
|
106.4
|
|
79.6
|
|
|||
|
Impairment charges
|
467.0
|
|
—
|
|
|||
|
Restructuring
|
5.4
|
|
1.1
|
|
|||
|
Total operating expenses
|
826.7
|
|
179.6
|
|
|||
|
Operating income (loss)
|
(303.8
|
)
|
199.2
|
|
|||
|
Interest expense, net
|
51.2
|
|
43.3
|
|
|||
|
Other expense, net
|
3.8
|
|
258.6
|
|
|||
|
Loss on extinguishment of debt
|
0.4
|
|
—
|
|
|||
|
Loss before income taxes
|
(359.2
|
)
|
(102.7
|
)
|
|||
|
Income tax benefit
|
(24.6
|
)
|
(7.8
|
)
|
|||
|
Net loss
|
$
|
(334.6
|
)
|
$
|
(94.9
|
)
|
|
|
Loss per share
|
|||||||
|
Basic loss per share
|
$
|
(2.34
|
)
|
$
|
(0.67
|
)
|
|
|
Diluted loss per share
|
$
|
(2.34
|
)
|
$
|
(0.67
|
)
|
|
|
Weighted-average shares outstanding
|
|||||||
|
Basic
|
143.2
|
|
140.8
|
|
|||
|
Diluted
|
143.2
|
|
140.8
|
|
|||
|
Dividends declared per share
|
$
|
0.145
|
|
$
|
0.125
|
|
|
|
Three Months Ended
|
|||||||
|
April 2,
2016 |
March 28, 2015
|
||||||
|
Net loss
|
$
|
(334.6
|
)
|
$
|
(94.9
|
)
|
|
|
Other comprehensive income (loss):
|
|||||||
|
Foreign currency translation adjustments
|
150.9
|
|
(27.9
|
)
|
|||
|
Change in fair value of derivative financial instruments, net of tax
|
(5.7
|
)
|
0.8
|
|
|||
|
Change in fair value of investment securities, net of tax
|
6.2
|
|
1.2
|
|
|||
|
Change in post-retirement and pension liability adjustments, net of tax
|
0.8
|
|
(0.4
|
)
|
|||
|
Other comprehensive income (loss), net of tax
|
152.2
|
|
(26.3
|
)
|
|||
|
Comprehensive loss
|
$
|
(182.4
|
)
|
$
|
(121.2
|
)
|
|
|
April 2,
2016 |
December 31,
2015 |
||||||
|
Assets
|
|||||||
|
Current assets
|
|||||||
|
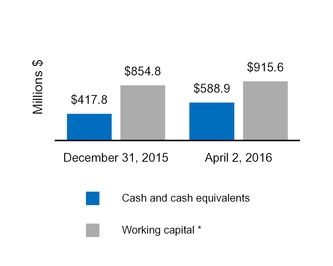
Cash and cash equivalents
|
$
|
588.9
|
|
$
|
417.8
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $3.4 million, and $3.0 million, respectively
|
1,184.2
|
|
1,193.1
|
|
|||
|
Inventories
|
868.8
|
|
844.4
|
|
|||
|
Prepaid expenses and other current assets
|
332.6
|
|
289.1
|
|
|||
|
Total current assets
|
2,974.5
|
|
2,744.4
|
|
|||
|
Property and equipment, net
|
896.3
|
|
886.2
|
|
|||
|
Goodwill and other indefinite-lived intangible assets
|
7,033.0
|
|
7,281.2
|
|
|||
|
Other intangible assets, net
|
8,519.1
|
|
8,190.5
|
|
|||
|
Non-current deferred income taxes
|
78.0
|
|
54.6
|
|
|||
|
Other non-current assets
|
225.3
|
|
237.0
|
|
|||
|
Total non-current assets
|
16,751.7
|
|
16,649.5
|
|
|||
|
Total assets
|
$
|
19,726.2
|
|
$
|
19,393.9
|
|
|
|
Liabilities and Shareholders’ Equity
|
|||||||
|
Accounts payable
|
$
|
559.9
|
|
$
|
554.9
|
|
|
|
Payroll and related taxes
|
92.2
|
|
125.3
|
|
|||
|
Accrued customer programs
|
331.0
|
|
398.0
|
|
|||
|
Accrued liabilities
|
307.5
|
|
308.4
|
|
|||
|
Accrued income taxes
|
179.4
|
|
85.2
|
|
|||
|
Current indebtedness
|
619.2
|
|
1,018.3
|
|
|||
|
Total current liabilities
|
2,089.2
|
|
2,490.1
|
|
|||
|
Long-term debt, less current portion
|
5,902.7
|
|
4,971.6
|
|
|||
|
Non-current deferred income taxes
|
1,487.0
|
|
1,563.7
|
|
|||
|
Other non-current liabilities
|
400.6
|
|
332.4
|
|
|||
|
Total non-current liabilities
|
7,790.3
|
|
6,867.7
|
|
|||
|
Total liabilities
|
9,879.5
|
|
9,357.8
|
|
|||
|
Commitments and contingencies - Note 14
|
|||||||
|
Shareholders’ equity
|
|||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
|||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
8,160.8
|
|
8,144.6
|
|
|||
|
Accumulated other comprehensive income
|
136.7
|
|
(15.5
|
)
|
|||
|
Retained earnings
|
1,549.8
|
|
1,907.6
|
|
|||
|
Total controlling interest
|
9,847.3
|
|
10,036.7
|
|
|||
|
Noncontrolling interest
|
(0.6
|
)
|
(0.6
|
)
|
|||
|
Total shareholders’ equity
|
9,846.7
|
|
10,036.1
|
|
|||
|
Total liabilities and shareholders' equity
|
$
|
19,726.2
|
|
$
|
19,393.9
|
|
|
|
Supplemental Disclosures of Balance Sheet Information
|
|||||||
|
Preferred shares, issued and outstanding
|
—
|
|
—
|
|
|||
|
Ordinary shares, issued and outstanding
|
143.2
|
|
143.1
|
|
|||
|
Three Months Ended
|
|||||||
|
|
April 2,
2016 |
March 28,
2015 |
|||||
|
Cash Flows From (For) Operating Activities
|
|||||||
|
Net loss
|
$
|
(334.6
|
)
|
$
|
(94.9
|
)
|
|
|
Adjustments to derive cash flows
|
|||||||
|
Depreciation and amortization
|
182.5
|
|
127.7
|
|
|||
|
Loss on acquisition-related foreign currency derivatives
|
—
|
|
298.1
|
|
|||
|
Share-based compensation
|
13.8
|
|
7.5
|
|
|||
|
Impairment charges
|
467.0
|
|
—
|
|
|||
|
Loss on extinguishment of debt
|
0.4
|
|
—
|
|
|||
|
Non-cash restructuring charges
|
5.4
|
|
1.1
|
|
|||
|
Deferred income taxes
|
(138.0
|
)
|
(46.3
|
)
|
|||
|
Other non-cash adjustments
|
1.6
|
|
(0.2
|
)
|
|||
|
Subtotal
|
198.1
|
|
293.0
|
|
|||
|
Increase (decrease) in cash due to:
|
|||||||
|
Accounts receivable
|
23.0
|
|
39.4
|
|
|||
|
Inventories
|
(14.8
|
)
|
2.1
|
|
|||
|
Accounts payable
|
0.3
|
|
18.0
|
|
|||
|
Payroll and related taxes
|
(37.4
|
)
|
(1.0
|
)
|
|||
|
Accrued customer programs
|
(69.7
|
)
|
(27.8
|
)
|
|||
|
Accrued liabilities
|
(3.4
|
)
|
(2.5
|
)
|
|||
|
Accrued income taxes
|
99.5
|
|
(51.2
|
)
|
|||
|
Other
|
(25.3
|
)
|
(2.0
|
)
|
|||
|
Subtotal
|
(27.8
|
)
|
(25.0
|
)
|
|||
|
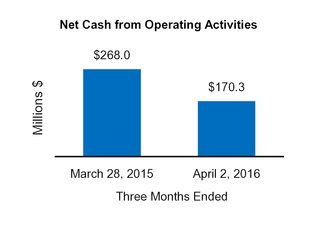
Net cash from (for) operating activities
|
170.3
|
|
268.0
|
|
|||
|
Cash Flows From (For) Investing Activities
|
|||||||
|
Acquisitions of businesses, net of cash acquired
|
(416.4
|
)
|
(4.0
|
)
|
|||
|
Additions to property and equipment
|
(34.7
|
)
|
(31.9
|
)
|
|||
|
Settlement of acquisition-related foreign currency derivatives
|
—
|
|
(298.1
|
)
|
|||
|
Other investing
|
(1.0
|
)
|
—
|
|
|||
|
Net cash from (for) investing activities
|
(452.1
|
)
|
(334.0
|
)
|
|||
|
Cash Flows From (For) Financing Activities
|
|||||||
|
Issuances of long-term debt
|
1,190.3
|
|
—
|
|
|||
|
Payments on long-term debt
|
(14.3
|
)
|
(13.6
|
)
|
|||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(704.3
|
)
|
3.4
|
|
|||
|
Deferred financing fees
|
(1.5
|
)
|
(3.3
|
)
|
|||
|
Issuance of ordinary shares
|
3.1
|
|
1.2
|
|
|||
|
Repurchase of ordinary shares
|
—
|
|
(0.1
|
)
|
|||
|
Cash dividends
|
(20.8
|
)
|
(17.6
|
)
|
|||
|
Other financing
|
(3.5
|
)
|
(1.6
|
)
|
|||
|
Net cash from (for) financing activities
|
449.0
|
|
(31.6
|
)
|
|||
|
Effect of exchange rate changes on cash
|
3.9
|
|
(68.1
|
)
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
171.1
|
|
(165.7
|
)
|
|||
|
Cash and cash equivalents, beginning of period
|
417.8
|
|
3,596.1
|
|
|||
|
Cash and cash equivalents, end of period
|
$
|
588.9
|
|
$
|
3,430.4
|
|
|
|
Supplemental Disclosures of Cash Flow Information
|
|||||||
|
Cash paid/received during the year for:
|
|||||||
|
Interest paid
|
$
|
11.9
|
|
$
|
5.2
|
|
|
|
Interest received
|
$
|
0.4
|
|
$
|
0.2
|
|
|
|
Income taxes paid
|
$
|
34.5
|
|
$
|
92.2
|
|
|
|
Income taxes refunded
|
$
|
0.2
|
|
$
|
1.6
|
|
|
|
Recently Issued Accounting Standards Not Yet Adopted
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Improvements to Employee Share-Based Payment Accounting
|
This guidance is intended to simplify several aspects of the accounting for share-based payment award transactions. It will require all income tax effects of awards to be recorded through the income statement when they vest or settle as opposed to certain amounts being recorded in additional paid-in capital. An entity will also have to elect whether to account for forfeitures as they occur or by estimating the number of awards expected to be forfeited and adjusting the estimate when it is likely to change (as currently required). The guidance will also increase the amount an employer can withhold to cover income taxes on awards. Early adoption is permitted.
|
January 1, 2017
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Revenue from Contracts with Customers
|
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps: identify the contract(s) with a customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) the entity satisfies a performance obligation. This guidance allows for two adoption methods, full retrospective approach or modified retrospective approach. Early adoption is not permitted.
|
January 1, 2018
|
We are currently evaluating the possible adoption methodologies and the implications of adoption on our consolidated financial statements.
|
|||
|
Leases
|
This guidance was issued to increase transparency and comparability among organizations by requiring recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. For leases with a term of 12 months or less, lessees are permitted to make an election to not recognize right-of-use assets and lease liabilities. Upon adoption, lessees will apply the new standard as of the beginning of the earliest comparative period presented in the financial statements, however lessees will be able to exclude leases that expire as of the implementation date. Early adoption is permitted.
|
January 1, 2019
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Tretinoin Products*
|
Development-Stage Rx Products*
|
||||||
|
Purchase price paid
|
$
|
416.4
|
|
$
|
—
|
|
|
|
Contingent consideration
|
—
|
|
29.5
|
|
|||
|
Total purchase consideration
|
$
|
416.4
|
|
$
|
29.5
|
|
|
|
Assets acquired:
|
|||||||
|
Inventories
|
$
|
1.4
|
|
$
|
—
|
|
|
|
Goodwill
|
1.7
|
|
0.5
|
|
|||
|
Definite-lived intangibles
:
|
|||||||
|
Developed product technology, formulations, and product rights
|
411.0
|
|
—
|
|
|||
|
Non-compete agreements
|
2.3
|
|
—
|
|
|||
|
Indefinite-lived intangibles
:
|
|||||||
|
In-process research and development
|
—
|
|
29.0
|
|
|||
|
Total intangible assets
|
413.3
|
|
29.0
|
|
|||
|
Total assets
|
$
|
416.4
|
|
$
|
29.5
|
|
|
|
Perrigo ordinary shares issued
|
5.4
|
|
||
|
Perrigo share price at transaction close on March 30, 2015
|
$
|
167.64
|
|
|
|
Total value of Perrigo ordinary shares issued
|
$
|
904.9
|
|
|
|
Cash consideration
|
2,078.3
|
|
||
|
Total consideration
|
$
|
2,983.2
|
|
|
|
Three months ended
|
||||
|
Line item
|
March 28, 2015
|
|||
|
Administration
|
$
|
2.0
|
|
|
|
Interest expense, net
|
18.7
|
|
||
|
Other expense, net
|
258.2
|
|
||
|
Total acquisition-related costs
|
$
|
278.9
|
|
|
|
Entocort
®
|
Naturwohl
|
ScarAway
®
|
GSK Products
|
Gelcaps
|
Omega
|
All Other
(1)
|
|||||||||||||||||||||
|
Purchase price paid
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
15.3
|
|
||||||
|
Contingent consideration
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
13.9
|
|
|||||||||||||
|
Total purchase consideration
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
29.2
|
|
||||||
|
Assets acquired:
|
|||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
4.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4.6
|
|
$
|
14.7
|
|
$
|
—
|
|
||||||
|
Accounts receivable
|
—
|
|
3.3
|
|
—
|
|
—
|
|
7.3
|
|
260.1
|
|
—
|
|
|||||||||||||
|
Inventories
|
0.2
|
|
1.5
|
|
1.0
|
|
—
|
|
7.2
|
|
202.5
|
|
—
|
|
|||||||||||||
|
Prepaid expenses and other current assets
|
—
|
|
—
|
|
—
|
|
—
|
|
2.1
|
|
39.2
|
|
—
|
|
|||||||||||||
|
Property and equipment
|
—
|
|
—
|
|
—
|
|
—
|
|
6.0
|
|
130.8
|
|
—
|
|
|||||||||||||
|
Goodwill
|
—
|
|
61.0
|
|
3.5
|
|
32.6
|
|
6.0
|
|
1,900.4
|
|
—
|
|
|||||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||||||||||||||
|
Distribution and license agreements, supply agreements
|
—
|
|
21.4
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Developed product technology, formulations, and product rights
|
380.0
|
|
—
|
|
0.5
|
|
—
|
|
—
|
|
27.2
|
|
—
|
|
|||||||||||||
|
Customer relationships and distribution networks
|
—
|
|
25.9
|
|
9.8
|
|
61.5
|
|
6.6
|
|
1,056.3
|
|
—
|
|
|||||||||||||
|
Trademarks, trade names, and brands
|
—
|
|
64.2
|
|
11.4
|
|
129.5
|
|
—
|
|
287.5
|
|
—
|
|
|||||||||||||
|
Non-compete agreements
|
—
|
|
0.3
|
|
0.5
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Indefinite-lived intangibles
:
|
|||||||||||||||||||||||||||
|
Trademarks, trade names, and brands
|
—
|
|
—
|
|
—
|
|
—
|
|
4.4
|
|
2,003.8
|
|
—
|
|
|||||||||||||
|
In-process research and development
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
29.2
|
|
|||||||||||||
|
Total intangible assets
|
380.0
|
|
111.8
|
|
22.2
|
|
191.0
|
|
11.0
|
|
3,374.8
|
|
29.2
|
|
|||||||||||||
|
Other non-current assets
|
—
|
|
—
|
|
—
|
|
—
|
|
0.4
|
|
2.4
|
|
—
|
|
|||||||||||||
|
Total assets
|
380.2
|
|
182.2
|
|
26.7
|
|
223.6
|
|
44.6
|
|
5,924.9
|
|
29.2
|
|
|||||||||||||
|
Liabilities assumed:
|
|||||||||||||||||||||||||||
|
Accounts payable
|
—
|
|
2.8
|
|
—
|
|
—
|
|
3.3
|
|
243.1
|
|
—
|
|
|||||||||||||
|
Short-term debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
24.6
|
|
—
|
|
|||||||||||||
|
Accrued liabilities
|
—
|
|
1.6
|
|
—
|
|
—
|
|
1.6
|
|
43.9
|
|
—
|
|
|||||||||||||
|
Payroll and related taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
51.3
|
|
—
|
|
|||||||||||||
|
Accrued customer programs
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
39.8
|
|
—
|
|
|||||||||||||
|
Long-term debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,471.0
|
|
—
|
|
|||||||||||||
|
Net deferred income tax liabilities
|
—
|
|
27.4
|
|
—
|
|
—
|
|
1.4
|
|
1,014.5
|
|
—
|
|
|||||||||||||
|
Other non-current liabilities
|
—
|
|
—
|
|
—
|
|
—
|
|
0.4
|
|
53.5
|
|
—
|
|
|||||||||||||
|
Total liabilities
|
—
|
|
31.8
|
|
—
|
|
—
|
|
6.7
|
|
2,941.7
|
|
—
|
|
|||||||||||||
|
Net assets acquired
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
29.2
|
|
||||||
|
(1)
|
Consists of
eight
product acquisitions in our CHC, BCH and Rx segments.
|
|
Three Months Ended
|
|||||||
|
(Unaudited)
|
April 2, 2016
|
March 28, 2015
|
|||||
|
Net sales
|
$
|
1,386.5
|
|
$
|
1,377.3
|
|
|
|
Net loss
|
$
|
(333.1
|
)
|
$
|
(75.4
|
)
|
|
|
Reporting Segments:
|
December 31, 2015
|
Business acquisitions
|
Impairments
|
Changes in assets held for sale
|
Currency translation adjustment
|
April 2,
2016 |
||||||||||||||||||
|
CHC
|
$
|
1,890.0
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4.8
|
|
$
|
(1.3
|
)
|
$
|
1,893.5
|
|
||||||
|
BCH
|
1,980.5
|
|
—
|
|
(193.6
|
)
|
—
|
|
100.7
|
|
1,887.6
|
|
||||||||||||
|
Rx
|
1,222.2
|
|
2.2
|
|
—
|
|
—
|
|
(2.7
|
)
|
1,221.7
|
|
||||||||||||
|
Specialty Sciences
|
200.7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
200.7
|
|
||||||||||||
|
Other
|
71.5
|
|
—
|
|
—
|
|
3.7
|
|
2.5
|
|
77.7
|
|
||||||||||||
|
Total goodwill
|
$
|
5,364.9
|
|
$
|
2.2
|
|
$
|
(193.6
|
)
|
$
|
8.5
|
|
$
|
99.2
|
|
$
|
5,281.2
|
|
||||||
|
|
April 2, 2016
|
December 31, 2015
|
|||||||||||||
|
|
Gross
|
Accumulated Amortization
|
Gross
|
Accumulated Amortization
|
|||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||
|
Distribution and license agreements, supply agreements
|
$
|
6,054.8
|
|
$
|
749.8
|
|
$
|
6,053.4
|
|
$
|
667.2
|
|
|||
|
Developed product technology, formulations, and product rights
|
1,795.7
|
|
462.3
|
|
1,383.5
|
|
426.0
|
|
|||||||
|
Customer relationships and distribution networks
|
1,573.2
|
|
229.0
|
|
1,520.7
|
|
193.0
|
|
|||||||
|
Trademarks, trade names, and brands
|
563.6
|
|
31.2
|
|
539.4
|
|
22.8
|
|
|||||||
|
Non-compete agreements
|
17.6
|
|
13.5
|
|
15.2
|
|
12.7
|
|
|||||||
|
Total definite-lived intangibles
|
$
|
10,004.9
|
|
$
|
1,485.8
|
|
$
|
9,512.2
|
|
$
|
1,321.7
|
|
|||
|
Indefinite-lived intangibles
:
|
|||||||||||||||
|
Trademarks, trade names, and brands*
|
$
|
1,682.3
|
|
$
|
—
|
|
$
|
1,868.1
|
|
$
|
—
|
|
|||
|
In-process research and development
|
69.5
|
|
—
|
|
48.2
|
|
—
|
|
|||||||
|
Total indefinite-lived intangibles
|
1,751.8
|
|
—
|
|
1,916.3
|
|
—
|
|
|||||||
|
Total other intangible assets
|
$
|
11,756.7
|
|
$
|
1,485.8
|
|
$
|
11,428.5
|
|
$
|
1,321.7
|
|
|||
|
April 2,
2016 |
December 31,
2015 |
||||||
|
Finished goods
|
$
|
506.1
|
|
$
|
483.4
|
|
|
|
Work in process
|
144.6
|
|
151.4
|
|
|||
|
Raw materials
|
218.1
|
|
209.6
|
|
|||
|
Total inventories
|
$
|
868.8
|
|
$
|
844.4
|
|
|
|
Level 1:
|
Quoted prices for identical instruments in active markets.
|
|
Level 2:
|
Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets.
|
|
Level 3:
|
Valuations derived from valuation techniques in which one or more significant inputs are not observable.
|
|
Fair Value
|
||||||||||
|
Fair Value Hierarchy
|
April 2,
2016 |
December 31,
2015 |
||||||||
|
Measured at fair value on a recurring basis:
|
||||||||||
|
Assets:
|
||||||||||
|
Investment securities
|
Level 1
|
$
|
38.6
|
|
$
|
14.9
|
|
|||
|
Foreign currency forward contracts
|
Level 2
|
$
|
9.4
|
|
$
|
4.8
|
|
|||
|
Funds associated with Israeli post-employment benefits
|
Level 2
|
17.6
|
|
17.2
|
|
|||||
|
Total level 2 assets
|
$
|
27.0
|
|
$
|
22.0
|
|
||||
|
Liabilities:
|
||||||||||
|
Interest rate swap agreements
|
Level 2
|
$
|
—
|
|
$
|
0.3
|
|
|||
|
Foreign currency forward contracts
|
Level 2
|
3.4
|
|
3.9
|
|
|||||
|
Total level 2 liabilities
|
$
|
3.4
|
|
$
|
4.2
|
|
||||
|
Contingent consideration
|
Level 3
|
$
|
48.0
|
|
$
|
17.9
|
|
|||
|
Measured at fair value on a non-recurring basis:
|
||||||||||
|
Assets:
|
||||||||||
|
Goodwill*
|
Level 3
|
$
|
1,761.6
|
|
$
|
—
|
|
|||
|
Indefinite-lived intangible assets
|
Level 3
|
1,082.0
|
|
1,031.8
|
|
|||||
|
Assets held for sale, net
|
Level 3
|
—
|
|
37.5
|
|
|||||
|
Total level 3 assets
|
$
|
2,843.6
|
|
$
|
1,069.3
|
|
||||
|
Three Months Ended
|
|||||||
|
April 2,
2016 |
March 28,
2015 |
||||||
|
Contingent Consideration
|
|||||||
|
Beginning balance:
|
$
|
17.9
|
|
$
|
12.4
|
|
|
|
Net realized losses
|
0.3
|
|
—
|
|
|||
|
Purchases or additions
|
29.5
|
|
—
|
|
|||
|
Foreign currency effect
|
0.3
|
|
—
|
|
|||
|
Ending balance:
|
$
|
48.0
|
|
$
|
12.4
|
|
|
|
April 2,
2016 |
December 31, 2015
|
||||||
|
Equity securities, at cost less impairments
|
$
|
21.9
|
|
$
|
6.4
|
|
|
|
Gross unrealized gains
|
19.2
|
|
9.3
|
|
|||
|
Gross unrealized losses
|
(2.5
|
)
|
(0.8
|
)
|
|||
|
Estimated fair value of equity securities
|
$
|
38.6
|
|
$
|
14.9
|
|
|
|
Asset Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
April 2,
2016 |
December 31, 2015
|
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
4.5
|
|
$
|
3.8
|
|
||
|
Total designated derivatives
|
$
|
4.5
|
|
$
|
3.8
|
|
|||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
4.9
|
|
$
|
1.0
|
|
||
|
Total non-designated derivatives
|
$
|
4.9
|
|
$
|
1.0
|
|
|||
|
Liability Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
April 2,
2016 |
December 31, 2015
|
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
1.7
|
|
$
|
2.0
|
|
||
|
Interest rate swap agreements
|
Other non-current liabilities
|
—
|
|
0.3
|
|
||||
|
Total designated derivatives
|
$
|
1.7
|
|
$
|
2.3
|
|
|||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
1.7
|
|
$
|
1.9
|
|
||
|
Total non-designated derivatives
|
$
|
1.7
|
|
$
|
1.9
|
|
|||
|
Amount of Gain/(Loss) Recorded in OCI
(Effective Portion) |
||||||||
|
Three Months Ended
|
||||||||
|
Designated Cash Flow Hedges
|
April 2,
2016 |
March 28, 2015
|
||||||
|
Interest rate swap agreements
|
$
|
(9.0
|
)
|
$
|
2.0
|
|
||
|
Foreign currency forward contracts
|
1.6
|
|
(3.8
|
)
|
||||
|
$
|
(7.4
|
)
|
$
|
(1.8
|
)
|
|||
|
Amount of Gain/(Loss) Reclassified from AOCI to Income
(Effective Portion) |
||||||||||
|
Three Months Ended
|
||||||||||
|
Designated Cash Flow Hedges
|
Income Statement Location
|
April 2,
2016 |
March 28, 2015
|
|||||||
|
Interest rate swap agreements
|
Interest expense, net
|
$
|
(0.5
|
)
|
$
|
0.8
|
|
|||
|
Foreign currency forward contracts
|
Net sales
|
0.6
|
|
(0.1
|
)
|
|||||
|
Cost of sales
|
0.3
|
|
(2.8
|
)
|
||||||
|
Interest expense, net
|
(0.4
|
)
|
—
|
|
||||||
|
Other expense, net
|
0.1
|
|
(0.4
|
)
|
||||||
|
$
|
0.1
|
|
$
|
(2.5
|
)
|
|||||
|
Amount of Gain/(Loss) Recognized in Income
(Ineffective Portion) |
||||||||||
|
Three Months Ended
|
||||||||||
|
Designated Cash Flow Hedges
|
Income Statement
Location
|
April 2,
2016 |
March 28,
2015 |
|||||||
|
Interest rate swap agreements
|
Other expense, net
|
$
|
(0.1
|
)
|
$
|
—
|
|
|||
|
Foreign currency forward contracts
|
Net sales
|
(0.1
|
)
|
—
|
|
|||||
|
Cost of sales
|
0.1
|
|
(0.1
|
)
|
||||||
|
Total
|
$
|
(0.1
|
)
|
$
|
(0.1
|
)
|
||||
|
Amount of Gain/(Loss) Recognized in Income
|
||||||||||
|
Three Months Ended
|
||||||||||
|
Non-Designated Derivatives
|
Income Statement Location
|
April 2,
2016 |
March 28,
2015 |
|||||||
|
Foreign currency forward contracts
|
Other expense, net
|
$
|
(6.9
|
)
|
$
|
(255.7
|
)
|
|||
|
Interest expense, net
|
0.1
|
|
(2.5
|
)
|
||||||
|
Total
|
$
|
(6.8
|
)
|
$
|
(258.2
|
)
|
||||
|
April 2,
2016 |
December 31,
2015 |
||||||||||||||
|
CHC
|
Other
|
CHC
|
Other
|
||||||||||||
|
Assets held for sale
|
|||||||||||||||
|
Current assets
|
$
|
56.8
|
|
$
|
8.7
|
|
$
|
55.1
|
|
$
|
13.6
|
|
|||
|
Goodwill
|
8.2
|
|
10.9
|
|
13.0
|
|
14.5
|
|
|||||||
|
Property, plant and equipment
|
18.9
|
|
33.7
|
|
18.8
|
|
37.4
|
|
|||||||
|
Other assets
|
0.9
|
|
3.1
|
|
—
|
|
3.2
|
|
|||||||
|
Less: impairment reserves
|
—
|
|
(28.2
|
)
|
—
|
|
(29.0
|
)
|
|||||||
|
Total assets held for sale
|
$
|
84.8
|
|
$
|
28.2
|
|
$
|
86.9
|
|
$
|
39.7
|
|
|||
|
Liabilities held for sale
|
|||||||||||||||
|
Current liabilities
|
$
|
28.5
|
|
$
|
2.8
|
|
$
|
30.5
|
|
$
|
0.5
|
|
|||
|
Other liabilities
|
—
|
|
1.8
|
|
—
|
|
1.7
|
|
|||||||
|
Total liabilities held for sale
|
$
|
28.5
|
|
$
|
4.6
|
|
$
|
30.5
|
|
$
|
2.2
|
|
|||
|
April 2,
2016 |
December 31,
2015 |
||||||||||
|
Revolving credit agreements
|
|||||||||||
|
2015 Revolver
|
$
|
—
|
|
$
|
380.0
|
|
|||||
|
2014 Revolver
|
—
|
|
300.0
|
|
|||||||
|
Total revolving credit agreements
|
—
|
|
680.0
|
|
|||||||
|
Term loans
|
|||||||||||
|
*
|
2014 Term loan due December 5, 2019
|
498.3
|
|
488.8
|
|
||||||
|
Notes and Bonds
|
|||||||||||
|
Coupon
|
Due
|
||||||||||
|
1.300%
|
November 8, 2016
|
(2)
|
500.0
|
|
500.0
|
|
|||||
|
*
|
4.500%
|
May 23, 2017
|
(3)
|
205.0
|
|
195.5
|
|
||||
|
*
|
5.125%
|
December 12, 2017
|
(3)
|
341.7
|
|
325.8
|
|
||||
|
2.300%
|
November 8, 2018
|
(2)
|
600.0
|
|
600.0
|
|
|||||
|
*
|
5.000%
|
May 23, 2019
|
(3)
|
136.7
|
|
130.3
|
|
||||
|
3.500%
|
March 15, 2021
|
(4)
|
500.0
|
|
—
|
|
|||||
|
3.500%
|
December 15, 2021
|
(1)
|
500.0
|
|
500.0
|
|
|||||
|
*
|
5.105%
|
July 19, 2023
|
(3)
|
153.7
|
|
146.7
|
|
||||
|
4.000%
|
November 15, 2023
|
(2)
|
800.0
|
|
800.0
|
|
|||||
|
3.900%
|
December 15, 2024
|
(1)
|
700.0
|
|
700.0
|
|
|||||
|
4.375%
|
March 15, 2026
|
(4)
|
700.0
|
|
—
|
|
|||||
|
5.300%
|
November 15, 2043
|
(2)
|
400.0
|
|
400.0
|
|
|||||
|
4.900%
|
December 15, 2044
|
(1)
|
400.0
|
|
400.0
|
|
|||||
|
Total notes and bonds
|
5,937.1
|
|
4,698.3
|
|
|||||||
|
Other financing
|
65.6
|
|
86.0
|
|
|||||||
|
Unamortized premium (discount), net
|
58.0
|
|
73.4
|
|
|||||||
|
Deferred financing fees
|
(37.1
|
)
|
(36.6
|
)
|
|||||||
|
Total borrowings outstanding
|
6,521.9
|
|
5,989.9
|
|
|||||||
|
Current indebtedness
|
(619.2
|
)
|
(1,018.3
|
)
|
|||||||
|
Total long-term debt less current portion
|
$
|
5,902.7
|
|
$
|
4,971.6
|
|
|||||
|
(1)
|
Discussed below collectively as the "2014 Notes."
|
|
(2)
|
Discussed below collectively as the "2013 Notes."
|
|
(3)
|
Debt assumed from Omega.
|
|
(4)
|
Discussed below collectively as the "2016 Notes."
|
|
*
|
Debt denominated in euros subject to fluctuations in the euro-to-U.S. dollar exchange rate.
|
|
•
|
$20.0 million
in aggregate principal amount of
6.19%
senior notes due
2016
, which was repaid on May 29, 2015 in full;
|
|
•
|
€135.0 million
(
$147.0 million
) in aggregate principal amount of
5.1045%
senior notes due
2023
(the "2023 Notes");
|
|
•
|
€300.0 million
(
$326.7 million
) in aggregate principal amount of
5.125%
retail bonds due
2017
;
€180.0 million
(
$196.0 million
) in aggregate principal amount of
4.500%
retail bonds due
2017
; and
€120.0 million
(
$130.7 million
) in aggregate principal amount of
5.000%
retail bonds due
2019
(collectively, the "Retail Bonds").
|
|
|
Three Months Ended
|
||||||
|
|
April 2,
2016 |
March 28,
2015 |
|||||
|
Numerator:
|
|||||||
|
Net loss
|
$
|
(334.6
|
)
|
$
|
(94.9
|
)
|
|
|
Denominator:
|
|||||||
|
Weighted average shares outstanding for basic EPS
|
143.2
|
|
140.8
|
|
|||
|
Dilutive effect of share-based awards*
|
—
|
|
—
|
|
|||
|
Weighted average shares outstanding for diluted EPS
|
143.2
|
|
140.8
|
|
|||
|
Anti-dilutive share-based awards excluded from computation of diluted EPS
|
0.3
|
|
0.7
|
|
|||
|
Foreign currency translation adjustments
|
Fair value of derivative financial instruments, net of tax
|
Fair value of investment securities, net of tax
|
Post-retirement and pension liability adjustments, net of tax
|
Total AOCI
|
|||||||||||||||
|
Balance at December 31, 2015
|
$
|
(4.4
|
)
|
$
|
(14.2
|
)
|
$
|
6.3
|
|
$
|
(3.2
|
)
|
$
|
(15.5
|
)
|
||||
|
OCI before reclassifications
|
150.9
|
|
(5.8
|
)
|
6.2
|
|
0.8
|
|
152.1
|
|
|||||||||
|
Amounts reclassified from AOCI
|
—
|
|
0.1
|
|
—
|
|
—
|
|
0.1
|
|
|||||||||
|
Other comprehensive income (loss)
|
150.9
|
|
(5.7
|
)
|
6.2
|
|
0.8
|
|
152.2
|
|
|||||||||
|
Balance at April 2, 2016
|
$
|
146.5
|
|
$
|
(19.9
|
)
|
$
|
12.5
|
|
$
|
(2.4
|
)
|
$
|
136.7
|
|
||||
|
Three Months Ended
|
|||||||
|
April 2,
2016 |
March 28,
2015 |
||||||
|
Beginning balance
|
$
|
20.7
|
|
$
|
3.2
|
|
|
|
Additional charges
|
5.4
|
|
1.1
|
|
|||
|
Payments
|
(18.2
|
)
|
(0.7
|
)
|
|||
|
Non-cash adjustments
|
5.1
|
|
—
|
|
|||
|
Ending balance
|
$
|
13.0
|
|
$
|
3.6
|
|
|
|
•
|
CHC
is focused primarily on the global sale of OTC store brand products including cough, cold, allergy, and sinus, analgesic, gastrointestinal, smoking cessation, infant formula and food, VMS, animal health, and diagnostic products.
|
|
•
|
BCH
develops, manufactures, markets and distributes many well-known European OTC brands in the natural health and VMS, cough, cold and allergy, smoking cessation, personal care and derma-therapeutics, lifestyle, and anti-parasite categories.
|
|
•
|
Rx
develops, manufactures and markets a portfolio of generic and specialty pharmaceutical prescription drugs primarily for the U.S. and U.K. markets.
|
|
•
|
Specialty Sciences
is comprised primarily of royalties received from assets focused on the management of multiple sclerosis (Tysabri
®
).
|
|
Three Months Ended
|
April 2,
2016 |
||||||||||||||
|
April 2, 2016
|
|||||||||||||||
|
Net Sales
|
Operating Income (Loss)
|
Amortization of Intangibles
|
Total Assets
|
||||||||||||
|
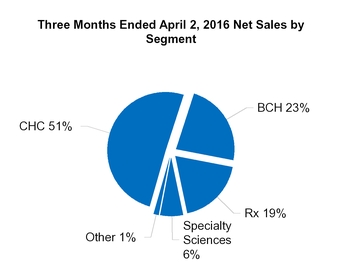
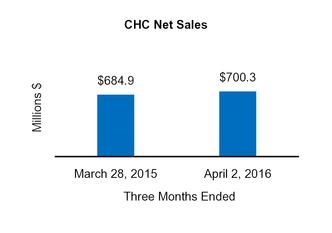
CHC
|
$
|
700.3
|
|
$
|
102.5
|
|
$
|
19.8
|
|
$
|
4,026.5
|
|
|||
|
BCH
|
317.6
|
|
(482.7
|
)
|
35.4
|
|
6,238.2
|
|
|||||||
|
Rx
|
256.7
|
|
87.4
|
|
29.5
|
|
3,384.5
|
|
|||||||
|
Specialty Sciences
|
88.0
|
|
12.9
|
|
72.8
|
|
5,859.6
|
|
|||||||
|
Other
|
20.6
|
|
5.4
|
|
0.5
|
|
217.4
|
|
|||||||
|
Unallocated
|
—
|
|
(29.3
|
)
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
1,383.2
|
|
$
|
(303.8
|
)
|
$
|
158.0
|
|
$
|
19,726.2
|
|
|||
|
Three Months Ended
|
December 31, 2015
|
||||||||||||||
|
March 28, 2015
|
|||||||||||||||
|
Net Sales
|
Operating Income (Loss)
|
Amortization of Intangibles
|
Total Assets
|
||||||||||||
|
CHC
|
$
|
684.9
|
|
$
|
104.3
|
|
$
|
16.2
|
|
$
|
4,007.8
|
|
|||
|
BCH
|
—
|
|
—
|
|
—
|
|
6,324.0
|
|
|||||||
|
Rx
|
251.6
|
|
100.0
|
|
18.3
|
|
3,015.5
|
|
|||||||
|
Specialty Sciences
|
81.9
|
|
5.5
|
|
72.8
|
|
5,833.5
|
|
|||||||
|
Other
|
30.7
|
|
10.5
|
|
0.5
|
|
213.1
|
|
|||||||
|
Unallocated
|
—
|
|
(21.1
|
)
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
1,049.1
|
|
$
|
199.2
|
|
$
|
107.8
|
|
$
|
19,393.9
|
|
|||
|
ITEM 2.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
Consumer Healthcare
(
"CHC"
) is focused primarily on the global sale of OTC store brand products including cough, cold, allergy, and sinus, analgesic, gastrointestinal, smoking cessation, infant formula and food, Vitamins, Minerals and Supplements ("VMS"), animal health, and diagnostic products.
|
|
•
|
Branded Consumer Healthcare
(
"BCH"
) develops, manufactures, markets and distributes many well-known European OTC brands in the natural health and VMS, cough, cold and allergy, smoking cessation, personal care and derma-therapeutics, lifestyle, and anti-parasite categories.
|
|
•
|
Prescription Pharmaceuticals
(
"Rx"
) develops, manufactures and markets a portfolio of generic and specialty pharmaceutical prescription drugs primarily for the U.S. and U.K. markets.
|
|
•
|
Specialty Sciences
is comprised primarily of royalties received from assets focused on the management of multiple sclerosis (Tysabri
®
).
|
|
•
|
Consistent with previously announced actions, we continued leveraging the strength of our global platform by adding a number of positions and processes to our Dublin headquarters across a range of corporate functions, including supply chain/global operations, procurement, enterprise risk management, corporate finance, and information technology;
|
|
•
|
Continued restructuring primarily associated with actions we took to streamline our organization as announced on October 22, 2015;
|
|
•
|
Issued $1.2 billion of senior notes and repaid borrowings under revolving credit facilities;
|
|
•
|
Completed acquisition of a generic Retin-A
®
portfolio, further enhancing our Rx extended topicals strategy; and
|
|
•
|
Completed the acquisition of two development-stage specialty Rx products to further invest in our specialty Rx portfolio.
|
|
•
|
We have experienced a recent reduction in pricing expectations in our U.S. businesses from historical patterns, in particular in our Rx segment due to industry and competitive pressures in the sector. Softness in pricing is attributed to various factors, including increased focus from customers to capture supply chain productivity savings and low raw material commodity pricing, competition in specific product categories, and consolidation of certain customers in the Rx segment. We expect this softness to continue to impact us through the remainder of 2016.
|
|
•
|
Previously anticipated new product sales in 2016 are forecasted to be lower due primarily to changes in our expectations to achieve regulatory approval for certain new products and modified market share penetration assumptions and timing for new products in Europe.
|
|
•
|
In addition to lower forecasted new products sales, our expectations for the BCH segment have been impacted by market dynamics in the lifestyle and natural health/VMS categories. The BCH segment has established a brand prioritization strategy to address these market dynamics with an objective to balance the cost of advertising and promotion investments with expected contributions from category sales.
|
|
|
Three Months Ended
|
% Change
|
||||||||
|
($ in millions)
|
March 28,
2015 |
April 2,
2016 |
||||||||
|
Net sales
|
$
|
1,049.1
|
|
$
|
1,383.2
|
|
32
|
%
|
||
|
Gross profit
|
$
|
378.8
|
|
$
|
522.9
|
|
38
|
%
|
||
|
Gross profit %
|
36.1
|
%
|
37.8
|
%
|
||||||
|
Operating expenses
|
$
|
179.6
|
|
$
|
826.7
|
|
360
|
%
|
||
|
Operating expenses %
|
17.1
|
%
|
59.8
|
%
|
||||||
|
Operating income (loss)
|
$
|
199.2
|
|
$
|
(303.8
|
)
|
(253
|
)%
|
||
|
Operating income (loss) %
|
19.0
|
%
|
(22.0
|
)%
|
||||||
|
Interest and other, net
|
$
|
301.9
|
|
$
|
55.4
|
|
(82
|
)%
|
||
|
Income tax benefit
|
$
|
(7.8
|
)
|
$
|
(24.6
|
)
|
(215
|
)%
|
||
|
Net loss
|
$
|
(94.9
|
)
|
$
|
(334.6
|
)
|
(252
|
)%
|
||
|
•
|
We are pursuing the sale of our U.S. VMS business and expect the sale to take place in the second quarter of 2016. As of
April 2, 2016
, the net assets of our U.S. VMS business were classified as "held for sale" as discussed in
Item 1. Note 9
. Sales attributable to the U.S. VMS business totaled $47.1 million and $37.5 million for the three months ended
April 2, 2016
and
March 28, 2015
, respectively.
|
|
•
|
We have experienced a recent reduction in pricing expectations in our CHC segment primarily attributable to various factors including, increased focus from customers to capture supply chain productivity savings and low raw material commodity pricing, and competition in specific product categories. We expect this softness to continue to impact us through the remainder of 2016.
|
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
March 28, 2015
|
April 2,
2016 |
|||||
|
Net sales
|
$
|
684.9
|
|
$
|
700.3
|
|
|
|
Gross profit
|
$
|
211.9
|
|
$
|
213.9
|
|
|
|
Gross profit %
|
30.9
|
%
|
30.5
|
%
|
|||
|
Operating income
|
$
|
104.3
|
|
$
|
102.5
|
|
|
|
Operating income %
|
15.2
|
%
|
14.6
|
%
|
|||
|
•
|
New product sales of
$30.6 million
related primarily to infant formula and food products;
|
|
•
|
Incremental net sales of
$12.9 million
from acquisitions (primarily the Gelcaps Exportadora de Mexico, S.A. de C.V. ("Gelcaps") and ScarAway
®
acquisitions); and
|
|
•
|
Increased sales volumes of existing products totaling
$17.0 million
due primarily to strong performance in our infant formula and smoking cessation categories, offset partially by weaker sales in our cough/cold and analgesics categories due to a mild cold and flu season; offset partially by
|
|
•
|
Discontinued products of
$32.0 million
due primarily to a label refresh within the infant formula category; and
|
|
•
|
Unfavorable foreign currency movement of
$6.5 million
.
|
|
•
|
An increase
of
$2.0 million
in gross profit due to:
|
|
•
|
Increased new product sales and favorable product mix; and
|
|
•
|
Improved efficiencies in manufacturing facilities; more than offset by
|
|
•
|
An increase of
$3.8 million
in operating expenses due to:
|
|
•
|
An increase in restructuring expense related to strategic organizational enhancements; and
|
|
•
|
Increased selling and administrative expenses related to the Gelcaps and ScarAway
®
acquisitions.
|
|
•
|
We have experienced a recent reduction in our expectations for the BCH segment due to market dynamics in the lifestyle and natural health/VMS categories. The BCH segment has established a brand prioritization strategy to address these market dynamics with an objective to balance the cost of advertising and promotion investments with expected contributions from category sales.
|
|
•
|
Previously anticipated new product sales in 2016 are forecasted to be lower due primarily to changes in our expectations to achieve regulatory approval for certain new products and modified market share penetration assumptions and timing for new products in Europe.
|
|
•
|
During the quarter, we made significant progress on our previously announced restructuring plans to right-size the business due to the impact of market dynamics impacting sales volumes. In addition, we made several strategic leadership changes both during the quarter and subsequent to quarter-end.
|
|
|
Three Months Ended
|
||
|
($ in millions)
|
April 2,
2016 |
||
|
Net sales
|
$
|
317.6
|
|
|
Gross profit
|
$
|
156.6
|
|
|
Gross profit %
|
49.3
|
%
|
|
|
Operating loss
|
$
|
(482.7
|
)
|
|
Operating loss %
|
(152.0
|
)%
|
|
|
•
|
On January 22, 2016, we acquired a portfolio of generic dosage forms and strengths of Retin-A
®
(tretinoin), a topical prescription acne treatment, from Matawan Pharmaceuticals, LLC, for
$416.4 million
in cash ("Tretinoin Products").
|
|
•
|
On March 1, 2016, we completed the acquisition of two development-stage specialty Rx products to further invest in our specialty Rx portfolio.
|
|
•
|
We have experienced a recent reduction in pricing expectations in our Rx segment due to industry and competitive pressures in the sector. Softness in pricing is attributed to various factors including increased focus from customers to capture supply chain productivity savings and low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact us through the remainder of 2016.
|
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
March 28, 2015
|
April 2,
2016 |
|||||
|
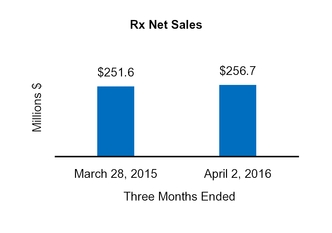
Net sales
|
$
|
251.6
|
|
$
|
256.7
|
|
|
|
Gross profit
|
$
|
141.7
|
|
$
|
127.4
|
|
|
|
Gross profit %
|
56.3
|
%
|
49.6
|
%
|
|||
|
Operating income
|
$
|
100.0
|
|
$
|
87.4
|
|
|
|
Operating income %
|
39.7
|
%
|
34.0
|
%
|
|||
|
•
|
Sales attributable to the Entocort
®
and Tretinoin Products acquisitions totaling
$45.6 million
; and
|
|
•
|
New product sales of
$11.2 million
; offset partially by
|
|
•
|
Decreased sales of existing products of
$50.1 million
due to declined sales volume of certain products, pricing pressure across extended topical products, and the loss of the exclusivity period for a key extended topical product.
|
|
•
|
A decrease
of
$1.7 million
in operating expenses; more than offset by
|
|
•
|
A decrease
of
$14.3 million
in gross profit due primarily to increased amortization expense from the Entocort
®
and Tretinoin Products acquisitions as well as the pricing pressure noted above, offset partially by a gain on the sale of an intangible asset.
|
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
March 28, 2015
|
April 2,
2016 |
|||||
|
Net sales
|
$
|
81.9
|
|
$
|
88.0
|
|
|
|
Gross profit
|
$
|
9.3
|
|
$
|
15.2
|
|
|
|
Gross profit %
|
11.4
|
%
|
17.3
|
%
|
|||
|
Operating income
|
$
|
5.5
|
|
$
|
12.9
|
|
|
|
Operating income %
|
6.7
|
%
|
14.7
|
%
|
|||
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
March 28, 2015
|
April 2,
2016 |
|||||
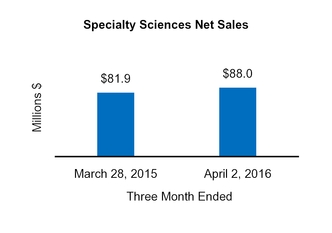
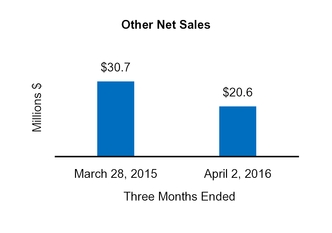
|
Net sales
|
$
|
30.7
|
|
$
|
20.6
|
|
|
|
Gross profit
|
$
|
15.8
|
|
$
|
9.8
|
|
|
|
Gross profit %
|
51.5
|
%
|
47.9
|
%
|
|||
|
Operating income
|
$
|
10.5
|
|
$
|
5.4
|
|
|
|
Operating income %
|
34.1
|
%
|
26.3
|
%
|
|||
|
•
|
A decrease in net earnings after adjusting for
$94.9 million
of non-cash items such as impairment charges and depreciation and amortization;
|
|
•
|
A net increase in cash used for customer-related programs of
$41.9 million
due primarily to the increased pricing pressure we are experiencing in the Rx segment;
|
|
•
|
A net increase in cash used for payroll and related taxes of
$36.4 million
due primarily to severance payments related to the restructuring activities and the addition of Omega operations in the current year period;
|
|
•
|
A net decrease in cash from changes in accounts receivable of
$16.4 million
, inventory of
$16.9 million
, and accounts payable of
$17.7 million
, due primarily to the addition of Omega operations in the current year period; offset partially by
|
|
•
|
A net decrease in cash used for accrued income taxes of
$150.7 million
due primarily to the prior year period including a $68.9 million incremental tax payment made in connection with the contested IRS audit described above under "Income Taxes".
|
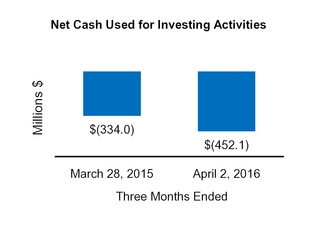
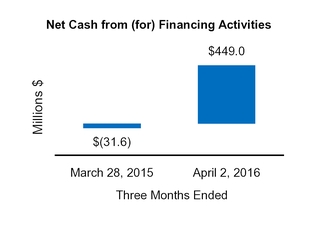
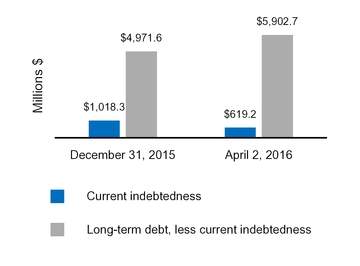
|
|
Payment Due by Period (in millions)
|
||||||||||||||||||
|
2016
(1)
|
2017 - 2018
|
2019 - 2020
|
After 2020
|
Total
|
|||||||||||||||
|
Short and long-term debt
(2)
|
$
|
783.7
|
|
$
|
1,698.5
|
|
$
|
822.5
|
|
$
|
5,512.3
|
|
$
|
8,817.0
|
|
||||
|
(2)
|
Short and long-term debt includes interest payments, which were calculated using the effective interest rate at
April 2, 2016
.
|
|
•
|
Review the processes and controls in place to measure and record income taxes to enhance the efficiency and effectiveness of the design and operation of those controls;
|
|
•
|
Enhance monitoring activities related to income taxes;
|
|
•
|
Evaluate and enhance the level of precision in the management review controls related to income taxes;
|
|
•
|
Test and evaluate the design and operating effectiveness of the control procedures; and
|
|
•
|
Assess the effectiveness of the remediation plan.
|
|
•
|
We have experienced a recent reduction in pricing expectations in our U.S. businesses from historical patterns, in particular in our Rx segment due to industry and competitive pressures in the sector. Softness in pricing is attributed to various factors including increased focus from customers to capture supply chain productivity savings and low raw material commodity pricing, competition in specific product categories, and consolidation of certain customers in the Rx segment. We expect this softness to continue to impact us through calendar 2016.
|
|
•
|
Previously anticipated new product sales in 2016 are forecasted to be lower than previously expected due primarily to changes in our expectations to achieve regulatory approval for certain new products and modified market share penetration assumptions and timing for new products in Europe.
|
|
•
|
We have experienced a recent reduction in our expectations for the BCH segment due to market dynamics in the lifestyle and natural health/VMS categories. The BCH segment has established a brand prioritization strategy to address these market dynamics with an objective to balance the cost of advertising and promotion investments with expected contributions from category sales.
|
|
•
|
Review the processes and controls in place to measure and record income taxes to enhance the efficiency and effectiveness of the design and operation of those controls;
|
|
•
|
Enhance monitoring activities related to income taxes;
|
|
•
|
Evaluate and enhance the level of precision in the management review controls related to income taxes;
|
|
•
|
Test and evaluate the design and operating effectiveness of the control procedures; and
|
|
•
|
Assess the effectiveness of the remediation plan.
|
|
Exhibit
Number
|
Description
|
|
|
3.1
|
Certificate of Incorporation of Perrigo Company plc (formerly known as Perrigo Company Limited) (incorporated by reference from Exhibit 4.1 to our Registration Statement on Form S-8 filed December 19, 2013).
|
|
|
3.2
|
Memorandum and Articles of Association of Perrigo Company plc, as amended (incorporated by reference from Exhibit 3.2 to our Transition Report on Form 10-KT filed on February 25, 2016).
|
|
|
4.1
|
Supplemental Indenture No. 2, dated as of March 10, 2016, among Perrigo Finance Unlimited Company, Perrigo Company plc and Wells Fargo Bank, National Association, as trustee (incorporated by reference from Exhibit 4.1 to our Current Report on Form 8-K filed on March 10, 2016).
|
|
|
4.2
|
Form of Global Note representing the 2021 Notes (included in Exhibit 4.1).
|
|
|
4.3
|
Form of Global Note representing the 2026 Notes (included in Exhibit 4.1).
|
|
|
10.1
|
Amendment to the Revolving Credit Agreement, dated February 26, 2016, by and among Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, Perrigo Company plc, JPMorgan Chase Bank, N.A., Barclays Bank PLC, and the other lenders party thereto, dated as of December 5, 2014 (filed herewith).
|
|
|
10.2
|
Amendment to the Term Loan Credit Agreement, dated February 26, 2016, by and among Perrigo Finance Unlimited Company, formerly known as Perrigo Finance plc, Perrigo Company plc, JPMorgan Chase Bank, N.A., Barclays Bank PLC, and the other lenders party thereto, dated as of December 5, 2014 (filed herewith).
|
|
|
10.3
|
Amendment to the Revolving Credit Agreement, dated February 26, 2016, by and among the Company, Perrigo Finance Unlimited Company, HSBC Bank USA, N.A., Bank of America, N.A. and Morgan Stanley Senior Funding, Inc., and the other lenders party thereto, dated as of December 9, 2015 (filed herewith).
|
|
|
10.4
|
Amendment No. 3, effective as of April 24, 2016, to the Employment Agreement, effective as of October 9, 2006, by and between Perrigo Company and Joseph C. Papa, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 25, 2016 (File No. 001-36353).
|
|
|
10.5
|
Amendment No. 4, effective as of May 6, 2016, to the Employment Agreement, effective as of October 9, 2006, by and between Perrigo Company and Joseph C. Papa (filed herewith).
|
|
|
10.6
|
First Amendment and Consent to Note Purchase Agreement, between Omega Pharma N.V. and the Prudential Insurance Company of America, dated October 7, 2011, in connection with the Note Purchase Agreement, dated May 19, 2011, with respect to the issuance and sale of EUR 135,043,889 aggregate principal amount of Omega’s 5.1045% senior notes due 2023 (filed herewith).
|
|
|
10.7
|
Waiver to Note Purchase Agreement, between Omega Pharma N.V. and the Prudential Insurance Company of America, dated May 16, 2016, in connection with the Note Purchase Agreement, dated May 19, 2011 (as amended by the First Amendment and Consent to the Note Purchase Agreement, dated as of October 7, 2011), with respect to the issuance and sale of EUR 135,043,889 aggregate principal amount of Omega’s 5.1045% senior notes due 2023 (filed herewith).
|
|
|
31.1
|
Rule 13a-14(a) Certification by John T. Hendrickson, Chief Executive Officer (filed herewith).
|
|
|
31.2
|
Rule 13a-14(a) Certification by Judy L. Brown, Executive Vice President, Business Operations and Chief Financial Officer (filed herewith).
|
|
|
32
|
Certification Pursuant to 18 United States Code 1350 and Rule 13a-14(b) of the Securities Exchange Act of 1934 (furnished herewith).
|
|
|
101.INS
|
XBRL Instance Document.
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
PERRIGO COMPANY PLC
|
|||
|
(Registrant)
|
|||
|
Date:
|
May 16, 2016
|
By: /s/ John T. Hendrickson
|
|
|
John T. Hendrickson
|
|||
|
Chief Executive Officer
|
|||
|
(Principal Executive Officer)
|
|||
|
Date:
|
May 16, 2016
|
By: /s/ Judy L. Brown
|
|
|
Judy L. Brown
|
|||
|
Executive Vice President, Business Operations and Chief Financial Officer
|
|||
|
(Principal Accounting and Financial Officer)
|
|||