|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[X]
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Ireland
|
|
Not Applicable
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
Treasury Building, Lower Grand Canal Street, Dublin 2, Ireland
|
|
-
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Large accelerated filer [X]
|
|
Accelerated filer [ ]
|
|
Non-accelerated filer [ ]
|
|
Smaller reporting company [ ]
|
|
(Do not check if a smaller reporting company)
|
|
|
|
PAGE
NUMBER
|
||
|
PART I. FINANCIAL INFORMATION
|
||
|
1
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
8
|
||
|
9
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
15
|
||
|
16
|
||
|
17
|
||
|
18
|
||
|
PART II. OTHER INFORMATION
|
||
|
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||
|
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
|||||||||||
|
Net sales
|
$
|
1,481.0
|
|
$
|
1,531.6
|
|
$
|
2,864.2
|
|
$
|
2,580.8
|
|
|||
|
Cost of sales
|
913.8
|
|
903.5
|
|
1,774.1
|
|
1,573.8
|
|
|||||||
|
Gross profit
|
567.2
|
|
628.1
|
|
1,090.1
|
|
1,007.0
|
|
|||||||
|
Operating expenses
|
|||||||||||||||
|
Distribution
|
22.5
|
|
23.7
|
|
44.3
|
|
38.4
|
|
|||||||
|
Research and development
|
47.0
|
|
62.6
|
|
92.2
|
|
98.0
|
|
|||||||
|
Selling
|
171.6
|
|
174.9
|
|
352.4
|
|
223.7
|
|
|||||||
|
Administration
|
101.8
|
|
140.1
|
|
208.2
|
|
219.7
|
|
|||||||
|
Impairment charges (credits)
|
(19.8
|
)
|
—
|
|
447.2
|
|
—
|
|
|||||||
|
Restructuring
|
5.8
|
|
(0.1
|
)
|
11.3
|
|
1.0
|
|
|||||||
|
Total operating expenses
|
328.9
|
|
401.2
|
|
1,155.6
|
|
580.8
|
|
|||||||
|
Operating income (loss)
|
238.3
|
|
226.9
|
|
(65.5
|
)
|
426.2
|
|
|||||||
|
Interest expense, net
|
57.4
|
|
45.9
|
|
108.6
|
|
89.2
|
|
|||||||
|
Other expense, net
|
29.3
|
|
22.7
|
|
33.1
|
|
281.3
|
|
|||||||
|
Loss on extinguishment of debt
|
—
|
|
0.9
|
|
0.4
|
|
0.9
|
|
|||||||
|
Income (loss) before income taxes
|
151.6
|
|
157.4
|
|
(207.6
|
)
|
54.8
|
|
|||||||
|
Income tax expense (benefit)
|
(42.7
|
)
|
101.0
|
|
(67.3
|
)
|
93.2
|
|
|||||||
|
Net income (loss)
|
$
|
194.3
|
|
$
|
56.4
|
|
$
|
(140.3
|
)
|
$
|
(38.4
|
)
|
|||
|
Income (loss) per share
|
|||||||||||||||
|
Basic
|
$
|
1.36
|
|
$
|
0.39
|
|
$
|
(0.98
|
)
|
$
|
(0.27
|
)
|
|||
|
Diluted
|
$
|
1.35
|
|
$
|
0.38
|
|
$
|
(0.98
|
)
|
$
|
(0.27
|
)
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
143.2
|
|
146.3
|
|
143.2
|
|
143.5
|
|
|||||||
|
Diluted
|
143.6
|
|
146.8
|
|
143.2
|
|
143.5
|
|
|||||||
|
Dividends declared per share
|
$
|
0.145
|
|
$
|
0.125
|
|
$
|
0.29
|
|
$
|
0.25
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
||||||||||||
|
Net income (loss)
|
$
|
194.3
|
|
$
|
56.4
|
|
$
|
(140.3
|
)
|
$
|
(38.4
|
)
|
|||
|
Other comprehensive income (loss):
|
|||||||||||||||
|
Foreign currency translation adjustments
|
(106.2
|
)
|
118.6
|
|
44.8
|
|
90.7
|
|
|||||||
|
Change in fair value of derivative financial instruments, net of tax
|
(1.3
|
)
|
4.8
|
|
(7.0
|
)
|
5.5
|
|
|||||||
|
Change in fair value of investment securities, net of tax
|
2.4
|
|
(6.1
|
)
|
8.5
|
|
(4.9
|
)
|
|||||||
|
Change in post-retirement and pension liability adjustments, net of tax
|
(0.3
|
)
|
4.1
|
|
0.5
|
|
3.7
|
|
|||||||
|
Other comprehensive income (loss), net of tax
|
(105.4
|
)
|
121.4
|
|
46.8
|
|
95.0
|
|
|||||||
|
Comprehensive income (loss)
|
$
|
88.9
|
|
$
|
177.8
|
|
$
|
(93.5
|
)
|
$
|
56.6
|
|
|||
|
(Unaudited)
|
|||||||
|
July 2,
2016 |
December 31,
2015 |
||||||
|
Assets
|
|||||||
|
Cash and cash equivalents
|
$
|
641.8
|
|
$
|
417.8
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $4.0 million, and $3.0 million, respectively
|
1,199.1
|
|
1,193.1
|
|
|||
|
Inventories
|
894.6
|
|
844.4
|
|
|||
|
Prepaid expenses and other current assets
|
297.3
|
|
289.1
|
|
|||
|
Total current assets
|
3,032.8
|
|
2,744.4
|
|
|||
|
Property and equipment, net
|
888.6
|
|
886.2
|
|
|||
|
Goodwill and other indefinite-lived intangible assets
|
6,627.1
|
|
7,281.2
|
|
|||
|
Other intangible assets, net
|
8,679.3
|
|
8,190.5
|
|
|||
|
Non-current deferred income taxes
|
100.6
|
|
54.6
|
|
|||
|
Other non-current assets
|
205.2
|
|
237.0
|
|
|||
|
Total non-current assets
|
16,500.8
|
|
16,649.5
|
|
|||
|
Total assets
|
$
|
19,533.6
|
|
$
|
19,393.9
|
|
|
|
Liabilities and Shareholders’ Equity
|
|||||||
|
Liabilities
|
|||||||
|
Accounts payable
|
$
|
514.1
|
|
$
|
554.9
|
|
|
|
Payroll and related taxes
|
98.4
|
|
125.3
|
|
|||
|
Accrued customer programs
|
354.2
|
|
398.0
|
|
|||
|
Accrued liabilities
|
295.7
|
|
308.4
|
|
|||
|
Accrued income taxes
|
72.5
|
|
85.2
|
|
|||
|
Current indebtedness
|
758.1
|
|
1,018.3
|
|
|||
|
Total current liabilities
|
2,093.0
|
|
2,490.1
|
|
|||
|
Long-term debt, less current portion
|
5,652.5
|
|
4,971.6
|
|
|||
|
Non-current deferred income taxes
|
1,473.7
|
|
1,563.7
|
|
|||
|
Other non-current liabilities
|
414.7
|
|
332.4
|
|
|||
|
Total non-current liabilities
|
7,540.9
|
|
6,867.7
|
|
|||
|
Total liabilities
|
9,633.9
|
|
9,357.8
|
|
|||
|
Commitments and contingencies - Note 14
|
|||||||
|
Shareholders’ equity
|
|||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
|||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
8,144.0
|
|
8,144.6
|
|
|||
|
Accumulated other comprehensive income
|
31.3
|
|
(15.5
|
)
|
|||
|
Retained earnings
|
1,725.0
|
|
1,907.6
|
|
|||
|
Total controlling interest
|
9,900.3
|
|
10,036.7
|
|
|||
|
Noncontrolling interest
|
(0.6
|
)
|
(0.6
|
)
|
|||
|
Total shareholders’ equity
|
9,899.7
|
|
10,036.1
|
|
|||
|
Total liabilities and shareholders' equity
|
$
|
19,533.6
|
|
$
|
19,393.9
|
|
|
|
Supplemental Disclosures of Balance Sheet Information
|
|||||||
|
Preferred shares, issued and outstanding
|
—
|
|
—
|
|
|||
|
Ordinary shares, issued and outstanding
|
143.2
|
|
143.1
|
|
|||
|
Six Months Ended
|
|||||||
|
|
July 2,
2016 |
June 27,
2015 |
|||||
|
Cash Flows From (For) Operating Activities
|
|||||||
|
Net income (loss)
|
$
|
(140.3
|
)
|
$
|
(38.4
|
)
|
|
|
Adjustments to derive cash flows
|
|||||||
|
Depreciation and amortization
|
369.3
|
|
295.0
|
|
|||
|
Loss on acquisition-related foreign currency derivatives
|
—
|
|
300.0
|
|
|||
|
Share-based compensation
|
7.6
|
|
15.5
|
|
|||
|
Impairment charges
|
447.2
|
|
—
|
|
|||
|
Loss on extinguishment of debt
|
0.4
|
|
0.9
|
|
|||
|
Non-cash restructuring charges
|
11.3
|
|
1.0
|
|
|||
|
Deferred income taxes
|
(157.1
|
)
|
21.9
|
|
|||
|
Other non-cash adjustments
|
28.2
|
|
12.1
|
|
|||
|
Subtotal
|
566.6
|
|
608.0
|
|
|||
|
Increase (decrease) in cash due to:
|
|||||||
|
Accounts receivable
|
42.3
|
|
(77.2
|
)
|
|||
|
Inventories
|
(50.3
|
)
|
28.4
|
|
|||
|
Accounts payable
|
(41.1
|
)
|
187.5
|
|
|||
|
Payroll and related taxes
|
(39.2
|
)
|
(3.8
|
)
|
|||
|
Accrued customer programs
|
(45.3
|
)
|
18.1
|
|
|||
|
Accrued liabilities
|
(9.8
|
)
|
(14.8
|
)
|
|||
|
Accrued income taxes
|
21.8
|
|
(14.9
|
)
|
|||
|
Other
|
(45.4
|
)
|
(0.6
|
)
|
|||
|
Subtotal
|
(167.0
|
)
|
122.7
|
|
|||
|
Net cash from (for) operating activities
|
399.6
|
|
730.7
|
|
|||
|
Cash Flows From (For) Investing Activities
|
|||||||
|
Acquisitions of businesses, net of cash acquired
|
(419.7
|
)
|
(2,098.8
|
)
|
|||
|
Additions to property and equipment
|
(57.1
|
)
|
(89.0
|
)
|
|||
|
Settlement of acquisition-related foreign currency derivatives
|
—
|
|
(303.5
|
)
|
|||
|
Other investing
|
(1.0
|
)
|
1.0
|
|
|||
|
Net cash from (for) investing activities
|
(477.8
|
)
|
(2,490.3
|
)
|
|||
|
Cash Flows From (For) Financing Activities
|
|||||||
|
Issuances of long-term debt
|
1,190.3
|
|
—
|
|
|||
|
Payments on long-term debt
|
(28.7
|
)
|
(889.0
|
)
|
|||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(803.9
|
)
|
(50.4
|
)
|
|||
|
Deferred financing fees
|
(2.4
|
)
|
(3.3
|
)
|
|||
|
Issuance of ordinary shares
|
3.5
|
|
4.0
|
|
|||
|
Cash dividends
|
(41.6
|
)
|
(35.9
|
)
|
|||
|
Other financing
|
(11.7
|
)
|
(10.6
|
)
|
|||
|
Net cash from (for) financing activities
|
305.5
|
|
(985.2
|
)
|
|||
|
Effect of exchange rate changes on cash
|
(3.3
|
)
|
(65.7
|
)
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
224.0
|
|
(2,810.5
|
)
|
|||
|
Cash and cash equivalents, beginning of period
|
417.8
|
|
3,596.1
|
|
|||
|
Cash and cash equivalents, end of period
|
$
|
641.8
|
|
$
|
785.6
|
|
|
|
Supplemental Disclosures of Cash Flow Information
|
|||||||
|
Cash paid/received during the year for:
|
|||||||
|
Interest paid
|
$
|
89.5
|
|
$
|
86.3
|
|
|
|
Interest received
|
$
|
0.7
|
|
$
|
0.7
|
|
|
|
Income taxes paid
|
$
|
38.0
|
|
$
|
110.2
|
|
|
|
Income taxes refunded
|
$
|
0.3
|
|
$
|
2.1
|
|
|
|
Recently Issued Accounting Standards Not Yet Adopted
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Improvements to Employee Share-Based Payment Accounting
|
This guidance is intended to simplify several aspects of the accounting for share-based payment award transactions. It will require all income tax effects of awards to be recorded through the income statement when they vest or settle as opposed to certain amounts being recorded in additional paid-in capital. An entity will also have to elect whether to account for forfeitures as they occur or by estimating the number of awards expected to be forfeited and adjusting the estimate when it is likely to change (as currently required). The guidance will also increase the amount an employer can withhold to cover income taxes on awards. Early adoption is permitted.
|
January 1, 2017
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Revenue from Contracts with Customers
|
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps: identify the contract(s) with a customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) the entity satisfies a performance obligation. This guidance allows for two adoption methods, full retrospective approach or modified retrospective approach. Early adoption is not permitted.
|
January 1, 2018
|
We are currently evaluating the possible adoption methodologies and the implications of adoption on our consolidated financial statements.
|
|||
|
Leases
|
This guidance was issued to increase transparency and comparability among organizations by requiring recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. For leases with a term of 12 months or less, lessees are permitted to make an election to not recognize right-of-use assets and lease liabilities. Upon adoption, lessees will apply the new standard as of the beginning of the earliest comparative period presented in the financial statements, however lessees will be able to exclude leases that expire as of the implementation date. Early adoption is permitted.
|
January 1, 2019
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Recently Issued Accounting Standards Not Yet Adopted (continued)
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Measurement of Credit Losses on Financial Instruments
|
This guidance changes the impairment model for most financial assets and certain other instruments, replacing the current "incurred loss" approach with an "expected loss" credit impairment model, which will apply to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities,and off-balance sheet credit exposures such as letters of credit. Early adoption is permitted.
|
January 1, 2020
|
We are currently evaluating the new standard for potential impacts on our receivables, debt, and other financial instruments.
|
|||
|
Tretinoin Products
|
Development-Stage Rx Products*
|
All Other
(1)
*
|
|||||||||
|
Purchase price paid
|
$
|
416.4
|
|
$
|
—
|
|
$
|
0.3
|
|
||
|
Contingent consideration
|
—
|
|
24.9
|
|
5.6
|
|
|||||
|
Total purchase consideration
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
5.9
|
|
||
|
Assets acquired:
|
|||||||||||
|
Inventories
|
$
|
1.4
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Goodwill
|
1.7
|
|
—
|
|
—
|
|
|||||
|
Definite-lived intangibles
:
|
|||||||||||
|
Developed product technology, formulations, and product rights
|
411.0
|
|
—
|
|
—
|
|
|||||
|
Non-compete agreements
|
2.3
|
|
—
|
|
—
|
|
|||||
|
Indefinite-lived intangibles
:
|
|||||||||||
|
In-process research and development
|
—
|
|
24.9
|
|
5.9
|
|
|||||
|
Total intangible assets
|
413.3
|
|
24.9
|
|
5.9
|
|
|||||
|
Total assets
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
5.9
|
|
||
|
(1)
|
Consists of
one
product acquisition in the CHC segment
|
|
Perrigo ordinary shares issued
|
5.4
|
|
||
|
Perrigo per share price at transaction close on March 30, 2015
|
$
|
167.64
|
|
|
|
Total value of Perrigo ordinary shares issued
|
$
|
904.9
|
|
|
|
Cash consideration
|
2,078.3
|
|
||
|
Total consideration
|
$
|
2,983.2
|
|
|
|
Three Months Ended
|
Six Months Ended
|
|||||||
|
Line item
|
June 27, 2015
|
|||||||
|
Administration
|
$
|
16.1
|
|
$
|
18.1
|
|
||
|
Interest expense, net
|
—
|
|
18.7
|
|
||||
|
Other expense, net
|
—
|
|
258.2
|
|
||||
|
Total acquisition-related costs
|
$
|
16.1
|
|
$
|
295.0
|
|
||
|
Entocort
®
|
Naturwohl
|
ScarAway
®
|
GSK Products
|
Gelcaps
|
Omega
|
All Other
(1)
|
|||||||||||||||||||||
|
Purchase price paid
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
15.3
|
|
||||||
|
Contingent consideration
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
13.9
|
|
|||||||||||||
|
Total purchase consideration
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
29.2
|
|
||||||
|
Assets acquired:
|
|||||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
4.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4.6
|
|
$
|
14.7
|
|
$
|
—
|
|
||||||
|
Accounts receivable
|
—
|
|
3.3
|
|
—
|
|
—
|
|
7.3
|
|
260.1
|
|
—
|
|
|||||||||||||
|
Inventories
|
0.2
|
|
1.5
|
|
1.0
|
|
—
|
|
7.2
|
|
202.5
|
|
—
|
|
|||||||||||||
|
Prepaid expenses and other current assets
|
—
|
|
—
|
|
—
|
|
—
|
|
2.1
|
|
39.2
|
|
—
|
|
|||||||||||||
|
Property and equipment
|
—
|
|
—
|
|
—
|
|
—
|
|
6.0
|
|
130.8
|
|
—
|
|
|||||||||||||
|
Goodwill
|
—
|
|
61.0
|
|
3.5
|
|
32.6
|
|
6.0
|
|
1,900.4
|
|
—
|
|
|||||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||||||||||||||
|
Distribution and license agreements, supply agreements
|
—
|
|
21.4
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Developed product technology, formulations, and product rights
|
380.0
|
|
—
|
|
0.5
|
|
—
|
|
—
|
|
27.2
|
|
—
|
|
|||||||||||||
|
Customer relationships and distribution networks
|
—
|
|
25.9
|
|
9.8
|
|
61.5
|
|
6.6
|
|
1,056.3
|
|
—
|
|
|||||||||||||
|
Trademarks, trade names, and brands
|
—
|
|
64.2
|
|
11.4
|
|
129.5
|
|
—
|
|
287.5
|
|
—
|
|
|||||||||||||
|
Non-compete agreements
|
—
|
|
0.3
|
|
0.5
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Indefinite-lived intangibles
:
|
|||||||||||||||||||||||||||
|
Trademarks, trade names, and brands
|
—
|
|
—
|
|
—
|
|
—
|
|
4.4
|
|
2,003.8
|
|
—
|
|
|||||||||||||
|
In-process research and development
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
29.2
|
|
|||||||||||||
|
Total intangible assets
|
380.0
|
|
111.8
|
|
22.2
|
|
191.0
|
|
11.0
|
|
3,374.8
|
|
29.2
|
|
|||||||||||||
|
Other non-current assets
|
—
|
|
—
|
|
—
|
|
—
|
|
0.4
|
|
2.4
|
|
—
|
|
|||||||||||||
|
Total assets
|
380.2
|
|
182.2
|
|
26.7
|
|
223.6
|
|
44.6
|
|
5,924.9
|
|
29.2
|
|
|||||||||||||
|
Liabilities assumed:
|
|||||||||||||||||||||||||||
|
Accounts payable
|
—
|
|
2.8
|
|
—
|
|
—
|
|
3.3
|
|
243.1
|
|
—
|
|
|||||||||||||
|
Short-term debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
24.6
|
|
—
|
|
|||||||||||||
|
Accrued liabilities
|
—
|
|
1.6
|
|
—
|
|
—
|
|
1.6
|
|
43.9
|
|
—
|
|
|||||||||||||
|
Payroll and related taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
51.3
|
|
—
|
|
|||||||||||||
|
Accrued customer programs
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
39.8
|
|
—
|
|
|||||||||||||
|
Long-term debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,471.0
|
|
—
|
|
|||||||||||||
|
Net deferred income tax liabilities
|
—
|
|
27.4
|
|
—
|
|
—
|
|
1.4
|
|
1,014.5
|
|
—
|
|
|||||||||||||
|
Other non-current liabilities
|
—
|
|
—
|
|
—
|
|
—
|
|
0.4
|
|
53.5
|
|
—
|
|
|||||||||||||
|
Total liabilities
|
—
|
|
31.8
|
|
—
|
|
—
|
|
6.7
|
|
2,941.7
|
|
—
|
|
|||||||||||||
|
Net assets acquired
|
$
|
380.2
|
|
$
|
150.4
|
|
$
|
26.7
|
|
$
|
223.6
|
|
$
|
37.9
|
|
$
|
2,983.2
|
|
$
|
29.2
|
|
||||||
|
(1)
|
Consists of
eight
product acquisitions in the CHC, BCH, and Rx segments
|
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
(Unaudited)
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
|||||||||||
|
Net sales
|
$
|
1,481.0
|
|
$
|
1,627.9
|
|
$
|
2,867.5
|
|
$
|
3,005.2
|
|
|||
|
Net income (loss)
|
$
|
194.3
|
|
$
|
87.3
|
|
$
|
(138.8
|
)
|
$
|
11.9
|
|
|||
|
Reporting Segments:
|
December 31, 2015
|
Business acquisitions
|
Impairments
|
Changes in assets held for sale
|
Currency translation adjustment
|
July 2,
2016 |
||||||||||||||||||
|
CHC
|
$
|
1,890.0
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4.5
|
|
$
|
(5.7
|
)
|
$
|
1,888.8
|
|
||||||
|
BCH
|
1,980.5
|
|
—
|
|
(163.3
|
)
|
—
|
|
74.6
|
|
1,891.8
|
|
||||||||||||
|
Rx
|
1,222.2
|
|
1.7
|
|
—
|
|
—
|
|
(13.0
|
)
|
1,210.9
|
|
||||||||||||
|
Specialty Sciences
|
200.7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
200.7
|
|
||||||||||||
|
Other
|
71.5
|
|
—
|
|
—
|
|
7.2
|
|
0.9
|
|
79.6
|
|
||||||||||||
|
Total goodwill
|
$
|
5,364.9
|
|
$
|
1.7
|
|
$
|
(163.3
|
)
|
$
|
11.7
|
|
$
|
56.8
|
|
$
|
5,271.8
|
|
||||||
|
|
July 2, 2016
|
December 31, 2015
|
|||||||||||||
|
|
Gross
|
Accumulated Amortization
|
Gross
|
Accumulated Amortization
|
|||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||
|
Distribution and license agreements, supply agreements
|
$
|
6,056.7
|
|
$
|
831.5
|
|
$
|
6,053.4
|
|
$
|
667.2
|
|
|||
|
Developed product technology, formulations, and product rights
|
1,784.6
|
|
492.1
|
|
1,383.5
|
|
426.0
|
|
|||||||
|
Customer relationships and distribution networks
|
1,545.7
|
|
256.6
|
|
1,520.7
|
|
193.0
|
|
|||||||
|
Trademarks, trade names, and brands
|
911.4
|
|
42.6
|
|
539.4
|
|
22.8
|
|
|||||||
|
Non-compete agreements
|
14.6
|
|
10.9
|
|
15.2
|
|
12.7
|
|
|||||||
|
Total definite-lived intangibles
|
$
|
10,313.0
|
|
$
|
1,633.7
|
|
$
|
9,512.2
|
|
$
|
1,321.7
|
|
|||
|
Indefinite-lived intangibles
:
|
|||||||||||||||
|
Trademarks, trade names, and brands
|
$
|
1,288.4
|
|
$
|
—
|
|
$
|
1,868.1
|
|
$
|
—
|
|
|||
|
In-process research and development
|
66.9
|
|
—
|
|
48.2
|
|
—
|
|
|||||||
|
Total indefinite-lived intangibles
|
1,355.3
|
|
—
|
|
1,916.3
|
|
—
|
|
|||||||
|
Total other intangible assets
|
$
|
11,668.3
|
|
$
|
1,633.7
|
|
$
|
11,428.5
|
|
$
|
1,321.7
|
|
|||
|
July 2,
2016 |
December 31,
2015 |
||||||
|
Finished goods
|
$
|
523.4
|
|
$
|
483.4
|
|
|
|
Work in process
|
148.6
|
|
151.4
|
|
|||
|
Raw materials
|
222.6
|
|
209.6
|
|
|||
|
Total inventories
|
$
|
894.6
|
|
$
|
844.4
|
|
|
|
Level 1:
|
Quoted prices for identical instruments in active markets.
|
|
Level 2:
|
Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets.
|
|
Level 3:
|
Valuations derived from valuation techniques in which one or more significant inputs are not observable.
|
|
Fair Value
|
||||||||||
|
Fair Value Hierarchy
|
July 2,
2016 |
December 31,
2015 |
||||||||
|
Measured at fair value on a recurring basis:
|
||||||||||
|
Assets:
|
||||||||||
|
Investment securities
|
Level 1
|
$
|
40.0
|
|
$
|
14.9
|
|
|||
|
Foreign currency forward contracts
|
Level 2
|
$
|
4.3
|
|
$
|
4.8
|
|
|||
|
Funds associated with Israeli post-employment benefits
|
Level 2
|
16.3
|
|
17.2
|
|
|||||
|
Total level 2 assets
|
$
|
20.6
|
|
$
|
22.0
|
|
||||
|
Liabilities:
|
||||||||||
|
Interest rate swap agreements
|
Level 2
|
$
|
—
|
|
$
|
0.3
|
|
|||
|
Foreign currency forward contracts
|
Level 2
|
2.8
|
|
3.9
|
|
|||||
|
Total level 2 liabilities
|
$
|
2.8
|
|
$
|
4.2
|
|
||||
|
Contingent consideration
|
Level 3
|
$
|
44.9
|
|
$
|
17.9
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
||||||||||||
|
Contingent Consideration
|
|||||||||||||||
|
Beginning balance:
|
$
|
48.0
|
|
$
|
12.4
|
|
$
|
17.9
|
|
$
|
12.4
|
|
|||
|
Net realized (gains) losses
|
(0.1
|
)
|
0.9
|
|
—
|
|
0.9
|
|
|||||||
|
Purchases or additions
|
1.0
|
|
—
|
|
30.5
|
|
—
|
|
|||||||
|
Impairments
|
(3.8
|
)
|
(13.3
|
)
|
(3.8
|
)
|
(13.3
|
)
|
|||||||
|
Foreign currency effect
|
(0.2
|
)
|
—
|
|
0.3
|
|
—
|
|
|||||||
|
Ending balance:
|
$
|
44.9
|
|
$
|
—
|
|
$
|
44.9
|
|
$
|
—
|
|
|||
|
Fair Value
|
||||||||||
|
Fair Value Hierarchy
|
July 2,
2016 |
December 31,
2015 |
||||||||
|
Measured at fair value on a non-recurring basis:
|
||||||||||
|
Assets:
|
||||||||||
|
Goodwill
(1)
|
Level 3
|
$
|
1,792.6
|
|
$
|
—
|
|
|||
|
Indefinite-lived intangible assets
(2)
|
Level 3
|
$
|
1,082.0
|
|
$
|
1,031.8
|
|
|||
|
Assets held for sale, net
|
Level 3
|
70.1
|
|
37.5
|
|
|||||
|
Total level 3 assets
|
$
|
2,944.7
|
|
$
|
1,069.3
|
|
||||
|
(1)
|
Goodwill with a carrying amount of
$1,955.9 million
was written down to its implied fair value of
$1,792.6 million
, resulting in total impairment charges of
$163.3 million
for the
six months ended
July 2, 2016
, which are included in
Impairment charges (credits)
on the Condensed Consolidated Statements of Operations. The implied fair value is as of April 2, 2016, the date of the goodwill impairment test.
|
|
(2)
|
Intangible assets estimated fair value at July 2, 2016 is as of April 2, 2016, the date the impairment was taken.
|
|
July 2,
2016 |
December 31, 2015
|
||||||
|
Equity securities, at cost less impairments
|
$
|
20.2
|
|
$
|
6.4
|
|
|
|
Gross unrealized gains
|
19.8
|
|
9.3
|
|
|||
|
Gross unrealized losses
|
—
|
|
(0.8
|
)
|
|||
|
Estimated fair value of equity securities
|
$
|
40.0
|
|
$
|
14.9
|
|
|
|
Asset Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
July 2,
2016 |
December 31, 2015
|
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
2.6
|
|
$
|
3.8
|
|
||
|
Total designated derivatives
|
$
|
2.6
|
|
$
|
3.8
|
|
|||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
1.7
|
|
$
|
1.0
|
|
||
|
Total non-designated derivatives
|
$
|
1.7
|
|
$
|
1.0
|
|
|||
|
Liability Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
July 2,
2016 |
December 31, 2015
|
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
1.0
|
|
$
|
2.0
|
|
||
|
Interest rate swap agreements
|
Other non-current liabilities
|
—
|
|
0.3
|
|
||||
|
Total designated derivatives
|
$
|
1.0
|
|
$
|
2.3
|
|
|||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
1.8
|
|
$
|
1.9
|
|
||
|
Total non-designated derivatives
|
$
|
1.8
|
|
$
|
1.9
|
|
|||
|
Amount of Gain/(Loss) Recorded in OCI
(Effective Portion) |
||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
Designated Cash Flow Hedges
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
||||||||||||
|
Interest rate swap agreements
|
$
|
—
|
|
$
|
(14.0
|
)
|
$
|
(9.0
|
)
|
$
|
(12.0
|
)
|
||||
|
Foreign currency forward contracts
|
(0.3
|
)
|
2.7
|
|
1.3
|
|
(1.1
|
)
|
||||||||
|
Total
|
$
|
(0.3
|
)
|
$
|
(11.3
|
)
|
$
|
(7.7
|
)
|
$
|
(13.1
|
)
|
||||
|
Amount of Gain/(Loss) Reclassified from AOCI to Income
(Effective Portion) |
||||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||||
|
Designated Cash Flow Hedges
|
Income Statement Location
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
|||||||||||||
|
Treasury locks
|
Interest expense, net
|
$
|
—
|
|
$
|
(0.1
|
)
|
$
|
—
|
|
$
|
(0.1
|
)
|
|||||
|
Interest rate swap agreements
|
Interest expense, net
|
(0.6
|
)
|
(19.1
|
)
|
(1.1
|
)
|
(18.2
|
)
|
|||||||||
|
Foreign currency forward contracts
|
Net sales
|
0.2
|
|
2.0
|
|
0.8
|
|
1.9
|
|
|||||||||
|
Cost of sales
|
0.6
|
|
(1.8
|
)
|
0.9
|
|
(4.6
|
)
|
||||||||||
|
Interest expense, net
|
(0.6
|
)
|
—
|
|
(0.9
|
)
|
—
|
|
||||||||||
|
Other expense, net
|
1.4
|
|
(0.2
|
)
|
1.5
|
|
(0.5
|
)
|
||||||||||
|
Total
|
$
|
1.0
|
|
$
|
(19.2
|
)
|
$
|
1.2
|
|
$
|
(21.5
|
)
|
||||||
|
Amount of Gain/(Loss) Recognized in Income
(Ineffective Portion) |
||||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||||
|
Designated Cash Flow Hedges
|
Income Statement
Location
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
|||||||||||||
|
Interest rate swap agreements
|
Other expense, net
|
$
|
—
|
|
$
|
—
|
|
$
|
(0.1
|
)
|
$
|
—
|
|
|||||
|
Foreign currency forward contracts
|
Net sales
|
(0.1
|
)
|
—
|
|
0.1
|
|
(0.3
|
)
|
|||||||||
|
Cost of sales
|
(0.1
|
)
|
0.1
|
|
—
|
|
0.1
|
|
||||||||||
|
Other expense, net
|
0.6
|
|
—
|
|
0.6
|
|
—
|
|
||||||||||
|
Total
|
$
|
0.4
|
|
$
|
0.1
|
|
$
|
0.6
|
|
$
|
(0.2
|
)
|
||||||
|
Amount of Gain/(Loss) Recognized in Income
|
||||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||||
|
Non-Designated Derivatives
|
Income Statement Location
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
|||||||||||||
|
Foreign currency forward contracts
|
Other expense, net
|
$
|
(1.6
|
)
|
$
|
5.2
|
|
$
|
(8.5
|
)
|
$
|
(250.5
|
)
|
|||||
|
Interest expense, net
|
(0.6
|
)
|
(1.0
|
)
|
(0.5
|
)
|
(3.5
|
)
|
||||||||||
|
Total
|
$
|
(2.2
|
)
|
$
|
4.2
|
|
$
|
(9.0
|
)
|
$
|
(254.0
|
)
|
||||||
|
July 2,
2016 |
December 31,
2015 |
||||||||||||||
|
CHC
|
Other
|
CHC
|
Other
|
||||||||||||
|
Assets held for sale
|
|||||||||||||||
|
Current assets
|
$
|
59.4
|
|
$
|
6.8
|
|
$
|
55.1
|
|
$
|
13.6
|
|
|||
|
Goodwill
|
8.5
|
|
7.3
|
|
13.0
|
|
14.5
|
|
|||||||
|
Property, plant and equipment
|
18.9
|
|
34.0
|
|
18.8
|
|
37.4
|
|
|||||||
|
Other assets
|
0.9
|
|
3.2
|
|
—
|
|
3.2
|
|
|||||||
|
Less: impairment reserves
|
(6.2
|
)
|
(32.5
|
)
|
—
|
|
(29.0
|
)
|
|||||||
|
Total assets held for sale
|
$
|
81.5
|
|
$
|
18.8
|
|
$
|
86.9
|
|
$
|
39.7
|
|
|||
|
Liabilities held for sale
|
|||||||||||||||
|
Current liabilities
|
$
|
27.0
|
|
$
|
1.1
|
|
$
|
30.5
|
|
$
|
0.5
|
|
|||
|
Other liabilities
|
—
|
|
2.1
|
|
—
|
|
1.7
|
|
|||||||
|
Total liabilities held for sale
|
$
|
27.0
|
|
$
|
3.2
|
|
$
|
30.5
|
|
$
|
2.2
|
|
|||
|
July 2,
2016 |
December 31,
2015 |
||||||||||
|
Revolving credit agreements
|
|||||||||||
|
2015 Revolver
|
$
|
—
|
|
$
|
380.0
|
|
|||||
|
2014 Revolver
|
—
|
|
300.0
|
|
|||||||
|
Total revolving credit agreements
|
—
|
|
680.0
|
|
|||||||
|
Term loans
|
|||||||||||
|
*
|
2014 Term loan due December 5, 2019
|
473.3
|
|
488.8
|
|
||||||
|
Notes and Bonds
|
|||||||||||
|
Coupon
|
Due
|
||||||||||
|
1.300%
|
November 8, 2016
|
(2)
|
500.0
|
|
500.0
|
|
|||||
|
*
|
4.500%
|
May 23, 2017
|
(3)
|
200.5
|
|
195.5
|
|
||||
|
*
|
5.125%
|
December 12, 2017
|
(3)
|
334.1
|
|
325.8
|
|
||||
|
2.300%
|
November 8, 2018
|
(2)
|
600.0
|
|
600.0
|
|
|||||
|
*
|
5.000%
|
May 23, 2019
|
(3)
|
133.6
|
|
130.3
|
|
||||
|
3.500%
|
March 15, 2021
|
(4)
|
500.0
|
|
—
|
|
|||||
|
3.500%
|
December 15, 2021
|
(1)
|
500.0
|
|
500.0
|
|
|||||
|
*
|
5.105%
|
July 19, 2023
|
(3)
|
150.4
|
|
146.7
|
|
||||
|
4.000%
|
November 15, 2023
|
(2)
|
800.0
|
|
800.0
|
|
|||||
|
3.900%
|
December 15, 2024
|
(1)
|
700.0
|
|
700.0
|
|
|||||
|
4.375%
|
March 15, 2026
|
(4)
|
700.0
|
|
—
|
|
|||||
|
5.300%
|
November 15, 2043
|
(2)
|
400.0
|
|
400.0
|
|
|||||
|
4.900%
|
December 15, 2044
|
(1)
|
400.0
|
|
400.0
|
|
|||||
|
Total notes and bonds
|
5,918.6
|
|
4,698.3
|
|
|||||||
|
Other financing
|
4.6
|
|
86.0
|
|
|||||||
|
Unamortized premium (discount), net
|
49.5
|
|
73.4
|
|
|||||||
|
Deferred financing fees
|
(35.4
|
)
|
(36.6
|
)
|
|||||||
|
Total borrowings outstanding
|
6,410.6
|
|
5,989.9
|
|
|||||||
|
Current indebtedness
|
(758.1
|
)
|
(1,018.3
|
)
|
|||||||
|
Total long-term debt less current portion
|
$
|
5,652.5
|
|
$
|
4,971.6
|
|
|||||
|
(1)
|
Discussed below collectively as the "2014 Notes."
|
|
(2)
|
Discussed below collectively as the "2013 Notes."
|
|
(3)
|
Debt assumed from Omega.
|
|
(4)
|
Discussed below collectively as the "2016 Notes."
|
|
*
|
Debt denominated in euros subject to fluctuations in the euro-to-U.S. dollar exchange rate.
|
|
•
|
$20.0 million
in aggregate principal amount of
6.19%
senior notes due
2016
, which was repaid on May 29, 2015 in full;
|
|
•
|
€135.0 million
(
$147.0 million
) in aggregate principal amount of
5.1045%
senior notes due
2023
(the "2023 Notes");
|
|
•
|
€300.0 million
(
$326.7 million
) in aggregate principal amount of
5.125%
retail bonds due
2017
;
€180.0 million
(
$196.0 million
) in aggregate principal amount of
4.500%
retail bonds due
2017
; and
€120.0 million
(
$130.7 million
) in aggregate principal amount of
5.000%
retail bonds due
2019
(collectively, the "Retail Bonds").
|
|
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||
|
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
|||||||||||
|
Numerator:
|
|||||||||||||||
|
Net income (loss)
|
$
|
194.3
|
|
$
|
56.4
|
|
$
|
(140.3
|
)
|
$
|
(38.4
|
)
|
|||
|
Denominator:
|
|||||||||||||||
|
Weighted average shares outstanding for basic EPS
|
143.2
|
|
146.3
|
|
143.2
|
|
143.5
|
|
|||||||
|
Dilutive effect of share-based awards*
|
0.4
|
|
0.5
|
|
—
|
|
—
|
|
|||||||
|
Weighted average shares outstanding for diluted EPS
|
143.6
|
|
146.8
|
|
143.2
|
|
143.5
|
|
|||||||
|
Anti-dilutive share-based awards excluded from computation of diluted EPS*
|
1.0
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Foreign currency translation adjustments
|
Fair value of derivative financial instruments, net of tax
|
Fair value of investment securities, net of tax
|
Post-retirement and pension liability adjustments, net of tax
|
Total AOCI
|
|||||||||||||||
|
Balance at December 31, 2015
|
$
|
(4.4
|
)
|
$
|
(14.2
|
)
|
$
|
6.3
|
|
$
|
(3.2
|
)
|
$
|
(15.5
|
)
|
||||
|
OCI before reclassifications
|
44.8
|
|
(5.6
|
)
|
7.2
|
|
0.5
|
|
46.9
|
|
|||||||||
|
Amounts reclassified from AOCI
|
—
|
|
(1.4
|
)
|
1.3
|
|
—
|
|
(0.1
|
)
|
|||||||||
|
Other comprehensive income (loss)
|
44.8
|
|
(7.0
|
)
|
8.5
|
|
0.5
|
|
46.8
|
|
|||||||||
|
Balance at July 2, 2016
|
$
|
40.4
|
|
$
|
(21.2
|
)
|
$
|
14.8
|
|
$
|
(2.7
|
)
|
$
|
31.3
|
|
||||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
||||||||||||
|
Beginning balance
|
$
|
13.0
|
|
$
|
3.6
|
|
$
|
20.7
|
|
$
|
3.2
|
|
|||
|
Additional charges
|
5.8
|
|
(0.1
|
)
|
11.3
|
|
1.0
|
|
|||||||
|
Payments
|
(6.6
|
)
|
(1.9
|
)
|
(24.8
|
)
|
(2.6
|
)
|
|||||||
|
Non-cash adjustments
|
—
|
|
—
|
|
5.0
|
|
—
|
|
|||||||
|
Ending balance
|
$
|
12.2
|
|
$
|
1.6
|
|
$
|
12.2
|
|
$
|
1.6
|
|
|||
|
•
|
CHC
is focused primarily on the global sale of OTC store brand products including cough, cold, allergy and sinus, analgesic, gastrointestinal, smoking cessation, infant formula and food, VMS, animal health, and diagnostic products.
|
|
•
|
BCH
develops, manufactures, markets and distributes many well-known European OTC brands in the natural health and vitamins, cough, cold and allergy, smoking cessation, personal care and derma-therapeutics, lifestyle, and anti-parasite categories.
|
|
•
|
Rx
develops, manufactures and markets a portfolio of generic and specialty pharmaceutical prescription drugs primarily for the U.S. and U.K. markets.
|
|
•
|
Specialty Sciences
is comprised primarily of royalties received from assets focused on the management of multiple sclerosis (Tysabri
®
).
|
|
Total Assets
|
July 2,
2016 |
December 31, 2015
|
||||||
|
CHC
|
$
|
4,021.9
|
|
$
|
4,007.8
|
|
||
|
BCH
|
6,211.2
|
|
6,324.0
|
|
||||
|
Rx
|
3,340.1
|
|
3,015.5
|
|
||||
|
Specialty Sciences
|
5,758.4
|
|
5,833.5
|
|
||||
|
Other
|
202.0
|
|
213.1
|
|
||||
|
Total
|
$
|
19,533.6
|
|
$
|
19,393.9
|
|
||
|
Three Months Ended
|
|||||||||||||||||||||||
|
July 2, 2016
|
June 27, 2015
|
||||||||||||||||||||||
|
Net Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
Net Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
||||||||||||||||||
|
CHC
|
$
|
686.3
|
|
$
|
111.2
|
|
$
|
19.0
|
|
$
|
746.4
|
|
$
|
143.3
|
|
$
|
16.6
|
|
|||||
|
BCH
|
393.7
|
|
38.4
|
|
39.5
|
|
401.2
|
|
26.6
|
|
34.2
|
|
|||||||||||
|
Rx
|
293.3
|
|
96.8
|
|
29.9
|
|
278.3
|
|
99.5
|
|
18.5
|
|
|||||||||||
|
Specialty Sciences
|
89.9
|
|
13.3
|
|
72.8
|
|
83.6
|
|
6.4
|
|
72.8
|
|
|||||||||||
|
Other
|
17.8
|
|
(1.3
|
)
|
0.5
|
|
22.1
|
|
1.9
|
|
0.5
|
|
|||||||||||
|
Unallocated
|
—
|
|
(20.1
|
)
|
—
|
|
—
|
|
(50.8
|
)
|
—
|
|
|||||||||||
|
Total
|
$
|
1,481.0
|
|
$
|
238.3
|
|
$
|
161.7
|
|
$
|
1,531.6
|
|
$
|
226.9
|
|
$
|
142.6
|
|
|||||
|
Six Months Ended
|
|||||||||||||||||||||||
|
July 2, 2016
|
June 27, 2015
|
||||||||||||||||||||||
|
Net Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
Net Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
||||||||||||||||||
|
CHC
|
$
|
1,386.6
|
|
$
|
213.6
|
|
$
|
38.8
|
|
$
|
1,431.3
|
|
$
|
247.6
|
|
$
|
32.8
|
|
|||||
|
BCH*
|
711.3
|
|
(444.3
|
)
|
74.9
|
|
401.2
|
|
26.6
|
|
34.2
|
|
|||||||||||
|
Rx
|
550.0
|
|
184.2
|
|
59.4
|
|
529.9
|
|
199.5
|
|
36.9
|
|
|||||||||||
|
Specialty Sciences
|
177.9
|
|
26.3
|
|
145.6
|
|
165.5
|
|
11.9
|
|
145.6
|
|
|||||||||||
|
Other
|
38.4
|
|
4.1
|
|
1.0
|
|
52.9
|
|
12.4
|
|
0.9
|
|
|||||||||||
|
Unallocated
|
—
|
|
(49.4
|
)
|
—
|
|
—
|
|
(71.8
|
)
|
—
|
|
|||||||||||
|
Total*
|
$
|
2,864.2
|
|
$
|
(65.5
|
)
|
$
|
319.7
|
|
$
|
2,580.8
|
|
$
|
426.2
|
|
$
|
250.4
|
|
|||||
|
*
|
The BCH segment was created on March 30, 2015 as a result of the Omega acquisition, thus data for the six months ended June 27, 2015 includes only three months of results from operations attributable to Omega.
|
|
•
|
Consumer Healthcare
(
"CHC"
) is focused primarily on the global sale of OTC store brand products including cough, cold, allergy and sinus, analgesic, gastrointestinal, smoking cessation, infant formula and food, Vitamins, Minerals and Supplements ("VMS"), animal health, and diagnostic products.
|
|
•
|
Branded Consumer Healthcare
(
"BCH"
) develops, manufactures, markets and distributes many well-known European OTC brands in the natural health and vitamins, cough, cold and allergy, smoking cessation, personal care and derma-therapeutics, lifestyle, and anti-parasite categories.
|
|
•
|
Prescription Pharmaceuticals
(
"Rx"
) develops, manufactures and markets a portfolio of generic and specialty pharmaceutical prescription drugs primarily for the U.S. and U.K. markets.
|
|
•
|
Specialty Sciences
is comprised primarily of royalties received from assets focused on the management of multiple sclerosis (Tysabri
®
).
|
|
•
|
We closed the sale of our U.S. VMS business to International Vitamins Corporation ("IVC") on August 5, 2016;
|
|
•
|
Consistent with previously announced actions, we added a number of positions and processes to our Dublin headquarters across a range of corporate functions, including supply chain/global operations, procurement, enterprise risk management, and corporate finance, leveraging the strength of our global platform;
|
|
•
|
We continued restructuring associated primarily with actions we took to streamline our organization as announced on October 22, 2015;
|
|
•
|
We issued $1.2 billion of senior notes and repaid borrowings under revolving credit facilities;
|
|
•
|
We issued a notice of redemption to prepay $500.0 million of senior notes in September 2016;
|
|
•
|
We completed the acquisition of a generic Retin-A
®
portfolio, further enhancing our Rx extended topicals strategy; and
|
|
•
|
We completed the acquisition of two development-stage specialty Rx products to further invest in our specialty Rx portfolio.
|
|
•
|
We have experienced a reduction in pricing expectations during 2016 in comparison to historical patterns in our U.S. businesses, in particular in our Rx segment, due to industry and competitive pressures in the sector. The reduced pricing is attributable to a variety of factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific product categories, and the loss of exclusivity on certain products and consolidation of certain customers in the Rx segment. We expect this pricing environment to continue to impact us at least through the remainder of 2016.
|
|
•
|
Our expectations for the BCH segment continue to be impacted by market dynamics in the lifestyle and natural health/vitamins categories. Factors impacting these categories include softness in key markets due to current macro-economic factors, change in timing of certain advertising and promotional campaigns compared to the prior year, and lower sell-in during the current year due to the re-staging of certain products.The BCH segment has established a brand prioritization strategy to address these market dynamics, with an objective to balance the cost of advertising and promotion investments with expected contributions from category sales.
|
|
•
|
Our expectations for 2016 new product sales are consistent with those communicated in our first quarter Form 10-Q, but continue to remain lower than anticipated as of December 31, 2015. Several new product launches have been delayed due to the regulatory approval process for certain new products in the U.S. and modifications to market share penetration and timing assumptions for new products in our Rx and BCH segments.
|
|
|
Three Months Ended
|
% Change
|
Six Months Ended
|
% Change
|
|||||||||||||||||
|
($ in millions)
|
June 27,
2015 |
July 2,
2016 |
June 27,
2015 |
July 2,
2016 |
|||||||||||||||||
|
Net sales
|
$
|
1,531.6
|
|
$
|
1,481.0
|
|
(3
|
)%
|
$
|
2,580.8
|
|
$
|
2,864.2
|
|
11
|
%
|
|||||
|
Gross profit
|
$
|
628.1
|
|
$
|
567.2
|
|
(10
|
)%
|
$
|
1,007.0
|
|
$
|
1,090.1
|
|
8
|
%
|
|||||
|
Gross profit %
|
41.0
|
%
|
38.3
|
%
|
39.0
|
%
|
38.1
|
%
|
|||||||||||||
|
Operating expenses
|
$
|
401.2
|
|
$
|
328.9
|
|
(18
|
)%
|
$
|
580.8
|
|
$
|
1,155.6
|
|
99
|
%
|
|||||
|
Operating expenses %
|
26.2
|
%
|
22.2
|
%
|
22.5
|
%
|
40.3
|
%
|
|||||||||||||
|
Operating income (loss)
|
$
|
226.9
|
|
$
|
238.3
|
|
5
|
%
|
$
|
426.2
|
|
$
|
(65.5
|
)
|
(115
|
)%
|
|||||
|
Operating income (loss) %
|
14.8
|
%
|
16.1
|
%
|
16.5
|
%
|
(2.3
|
)%
|
|||||||||||||
|
Interest and other, net
|
$
|
69.5
|
|
$
|
86.7
|
|
25
|
%
|
$
|
371.4
|
|
$
|
142.1
|
|
(62
|
)%
|
|||||
|
Income tax expense (benefit)
|
$
|
101.0
|
|
$
|
(42.7
|
)
|
(142
|
)%
|
$
|
93.2
|
|
$
|
(67.3
|
)
|
(172
|
)%
|
|||||
|
Net income (loss)
|
$
|
56.4
|
|
$
|
194.3
|
|
244
|
%
|
$
|
(38.4
|
)
|
$
|
(140.3
|
)
|
(265
|
)%
|
|||||
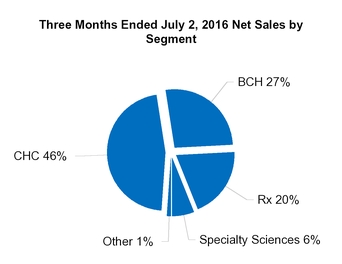
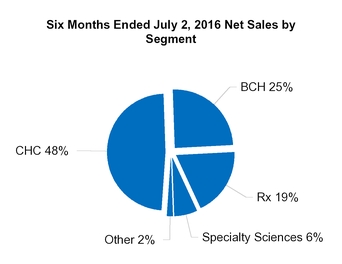
|
•
|
On June 20, 2016, we announced the sale of our U.S. VMS business to IVC, which closed on August 5, 2016.
As of
July 2, 2016
, the net assets of our U.S. VMS business were classified as "held for sale" as discussed in
Item 1. Note 9
. Sales attributable to the U.S. VMS business totaled $42.1 million and $39.6 million for the three months ended
July 2, 2016
and
June 27, 2015
, respectively, and $89.2 million and $77.1 million for the six months ended
July 2, 2016
and
June 27, 2015
, respectively.
|
|
•
|
We have experienced a reduction in pricing expectations in certain categories within our CHC segment in 2016 due to various factors, including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, and competition in specific product categories. We expect this pricing environment to continue to impact our CHC segment at least through the remainder of 2016.
|
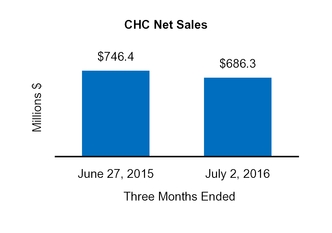
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
June 27, 2015
|
July 2,
2016 |
|||||
|
Net sales
|
$
|
746.4
|
|
$
|
686.3
|
|
|
|
Gross profit
|
$
|
258.3
|
|
$
|
230.3
|
|
|
|
Gross profit %
|
34.6
|
%
|
33.5
|
%
|
|||
|
Operating income
|
$
|
143.3
|
|
$
|
111.2
|
|
|
|
Operating income %
|
19.2
|
%
|
16.2
|
%
|
|||
|
•
|
A net decrease in sales of existing products of
$83.5 million
due to:
|
|
•
|
Strong sales in our infant health and smoking cessation categories, more than offset by
|
|
•
|
Weaker sales in the cough/cold category due to timing of promotions and a weaker allergy season;
|
|
•
|
Pricing pressure primarily in the analgesics category due to increased competition; and
|
|
•
|
Lower sales in the OTC contract manufacturing and animal health categories;
|
|
•
|
Discontinued products of
$12.0 million
due primarily to a label refresh within the infant formula category; and
|
|
•
|
Unfavorable foreign currency movement of
$6.0 million
; offset partially by
|
|
•
|
New product sales of
$32.4 million
related primarily to several new infant formula and food products and the launches of fluticasone nasal spray (generic equivalent to Flonase
®
), guaifenesin extended release (generic equivalent to Mucinex
®
ER); and
|
|
•
|
Incremental net sales of
$11.4 million
from acquisitions (primarily the Gelcaps Exportadora de Mexico, S.A. de C.V. ("Gelcaps") and ScarAway
®
acquisitions).
|
|
•
|
A decrease
of
$28.0 million
in gross profit due to:
|
|
•
|
Decreased sales of existing and discontinued products as described above; and
|
|
•
|
Increased intangible asset amortization expense associated primarily with the Gelcaps and ScarAway
®
acquisitions; offset partially by
|
|
•
|
Margin contributions from new products and strong performance in the infant health and smoking cessation categories; and
|
|
•
|
Continued manufacturing and supply chain efficiencies.
|
|
•
|
An increase of
$4.1 million
in operating expenses due to:
|
|
•
|
A
$6.2 million
impairment charge recorded on the VMS held-for-sale business and
|
|
•
|
Increased research and development investments due to timing of clinical trials; offset partially by
|
|
•
|
Decreased selling and administrative expenses due to cost containment actions taken during the prior year.
|
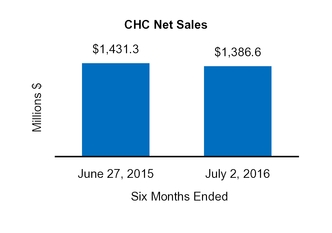
|
|
|||||||
|
Six Months Ended
|
|||||||
|
($ in millions)
|
June 27,
2015 |
July 2,
2016 |
|||||
|
Net sales
|
$
|
1,431.3
|
|
$
|
1,386.6
|
|
|
|
Gross profit
|
$
|
470.2
|
|
$
|
444.1
|
|
|
|
Gross profit %
|
32.9
|
%
|
32.0
|
%
|
|||
|
Operating income
|
$
|
247.6
|
|
$
|
213.6
|
|
|
|
Operating income %
|
17.3
|
%
|
15.4
|
%
|
|||
|
•
|
A net
$66.1 million
decrease in existing product sales due to:
|
|
•
|
Strong sales in our infant health and smoking cessation categories; more than offset by
|
|
•
|
Weaker sales in the cough/cold category due to a mild cold and flu season, weaker allergy season, and timing of promotions;
|
|
•
|
Decreased sales of analgesics due to a mild cold and flu season and pricing pressure due to increased competition; and
|
|
•
|
Lower sales in the OTC contract manufacturing and animal health categories;
|
|
•
|
Discontinued products of
$49.9 million
related primarily to a label refresh within the infant formula category; and
|
|
•
|
Unfavorable foreign currency movement of
$12.6 million
; offset partially by
|
|
•
|
New product sales of
$63.2 million
related primarily to several new infant formula and food products, fluticasone nasal spray (generic equivalent to Flonase
®
), guaifenesin extended release (generic equivalent to Mucinex
®
ER), and several new animal health products; and
|
|
•
|
Incremental net sales of
$24.2 million
due primarily to the Gelcaps and ScarAway
®
acquisitions.
|
|
•
|
A decrease
of
$26.1 million
in gross profit due to:
|
|
•
|
Decreased sales of existing and discontinued products in the first half of 2016 as described above; and
|
|
•
|
Increased intangible asset amortization expense associated primarily with the Gelcaps and ScarAway
®
acquisitions; offset partially by
|
|
•
|
Margin contributions from new products and strong performance in the infant health and smoking cessation categories; and
|
|
•
|
Continued manufacturing and supply chain efficiencies.
|
|
•
|
An increase
of
$7.9 million
in operating expenses due to:
|
|
•
|
A
$6.2 million
impairment charge recorded on the VMS held-for-sale business; and
|
|
•
|
Increased research and development investments due to timing of clinical trials; offset partially by
|
|
•
|
Decreased selling and administrative expenses due to cost containment actions taken during the prior year.
|
|
•
|
Our expectations for the BCH segment continue to be impacted by market dynamics in the lifestyle and natural health/vitamins categories. Factors impacting these categories include softness in key markets due to current macro-economic factors, change in timing of certain advertising and promotional campaigns compared to the prior year, and lower sell-in during the current year due to the re-staging of certain products.The BCH segment has established a brand prioritization strategy to address these market dynamics, with an objective to balance the cost of advertising and promotion investments with expected contributions from category sales.
|
|
•
|
Our expectations for 2016 new product sales are consistent with those communicated in our first quarter Form 10-Q, but continue to remain lower than anticipated as of December 31, 2015. Several new product launches have been delayed due to modifications to market share penetration and timing assumptions for new products in Europe.
|
|
•
|
We continue to make progress on our previously announced restructuring plans to right-size the BCH business due to the impact of market dynamics on sales volumes. In addition, we made several strategic leadership changes during 2016, with additional hiring continuing.
|
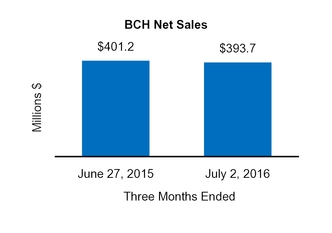
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
June 27,
2015 |
July 2,
2016 |
|||||
|
Net sales
|
$
|
401.2
|
|
$
|
393.7
|
|
|
|
Gross profit
|
$
|
190.1
|
|
$
|
173.1
|
|
|
|
Gross profit %
|
47.4
|
%
|
44.0
|
%
|
|||
|
Operating income
|
$
|
26.6
|
|
$
|
38.4
|
|
|
|
Operating income %
|
6.6
|
%
|
9.8
|
%
|
|||
|
•
|
Decreased sales volumes of existing products totaling
$65.4 million
due primarily to:
|
|
•
|
Weaker current year sales in the lifestyle category due in large part to a product launch in the prior year period and the natural health/vitamins category due primarily to timing of promotional activities and the planned divestment of the Etixx
®
brand in addition to
|
|
•
|
Lower sales in France due to market dynamics; offset largely by
|
|
•
|
New product sales of
$28.3 million
; and
|
|
•
|
Incremental net sales of
$29.1 million
from the Naturwohl Pharma GmbH ("Naturwohl") and GlaxoSmithKline
Consumer Healthcare Product Portfolio ("GSK Products") acquisitions.
|
|
•
|
A decrease
of
$17.0 million
in gross profit due to decreased sales of existing products in the lifestyle and natural health/vitamins categories as noted above, offset by
|
|
•
|
A decrease
of
$28.8 million
in operating expenses due to:
|
|
•
|
The
$30.3 million
reduction to the first quarter estimated goodwill impairment charge recorded in the current quarter; and
|
|
•
|
Decreased advertising and promotional investment due to previously announced strategic initiatives to better align promotional investments with sales; offset partially by
|
|
•
|
Restructuring expense totaling
$4.8 million
in the current quarter related to strategic organizational enhancements.
|

|
Six Months Ended
|
|||||||
|
($ in millions)
|
June 27, 2015*
|
July 2,
2016 |
|||||
|
Net sales
|
$
|
401.2
|
|
$
|
711.3
|
|
|
|
Gross profit
|
$
|
190.1
|
|
$
|
329.7
|
|
|
|
Gross profit %
|
47.4
|
%
|
46.3
|
%
|
|||
|
Operating income (loss)
|
$
|
26.6
|
|
$
|
(444.3
|
)
|
|
|
Operating income (loss) %
|
6.6
|
%
|
(62.5
|
)%
|
|||
|
•
|
An additional three months of results from operations attributable to Omega;
|
|
•
|
New products totaling
$59.5 million
; and
|
|
•
|
Sales from the Naturwohl and GSK Products acquisitions totaling
$66.7 million
; offset partially by
|
|
•
|
Decreased sales volumes of existing products due primarily to:
|
|
•
|
Weaker current year sales in the lifestyle category due in large part to a product launch in the prior year period and the natural health/vitamins category due primarily to timing of promotional activities and the planned divestment of the Etixx
®
brand in addition to
|
|
•
|
Lower sales in France due to market dynamics.
|
|
•
|
A
$139.6 million
increase in gross profit due to an additional three months of operations attributable to Omega offset by
|
|
•
|
An increase
of
$610.5 million
in operating expenses due to:
|
|
•
|
Intangible asset and goodwill impairment charges totaling
$436.7 million
recorded during the six months ended July 2, 2016 described above under "Interim Impairment Testing";
|
|
•
|
An additional three months of operations; and
|
|
•
|
Restructuring charges totaling
$7.1 million
related to strategic organizational enhancements; offset partially by
|
|
•
|
Cost control measures employed to mitigate lower forecasted sales and operating income.
|
|
•
|
We continue to experience a significant reduction in pricing expectations in our Rx segment due to industry and competitive pressures in the sector. This softness in pricing is attributed to various factors, including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact us at least through the remainder of 2016.
|
|
•
|
On January 22, 2016, we acquired a portfolio of generic dosage forms and strengths of Retin-A
®
(tretinoin), a topical prescription acne treatment, from Matawan Pharmaceuticals, LLC, for
$416.4 million
in cash ("Tretinoin Products").
|
|
•
|
On March 1, 2016, we completed the acquisition of two development-stage specialty Rx products to further invest in our specialty Rx portfolio.
|
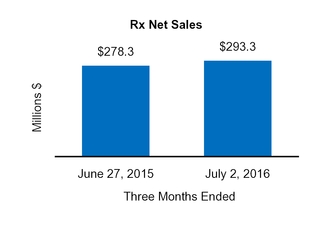
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
June 27, 2015
|
July 2,
2016 |
|||||
|
Net sales
|
$
|
278.3
|
|
$
|
293.3
|
|
|
|
Gross profit
|
$
|
161.4
|
|
$
|
139.3
|
|
|
|
Gross profit %
|
58.0
|
%
|
47.5
|
%
|
|||
|
Operating income
|
$
|
99.5
|
|
$
|
96.8
|
|
|
|
Operating income %
|
35.7
|
%
|
33.0
|
%
|
|||
|
•
|
Sales attributable to the Entocort
®
and Tretinoin Products acquisitions totaling
$43.7 million
; and
|
|
•
|
New product sales of
$25.6 million
due primarily to sales of benzoyl peroxide 5%-clindamycin 1% gel (a generic version of Benzaclin™); offset partially by
|
|
•
|
Decreased sales of existing products of
$50.3 million
due to lower sales volume of certain products, pricing pressure across the portfolio, and the lack of exclusive market position for two key products versus the prior year;
|
|
•
|
Discontinued products of
$2.8 million
; and
|
|
•
|
Unfavorable foreign exchange movement of
$1.1 million
.
|
|
•
|
A decrease
of
$22.1 million
in gross profit due primarily to the pricing pressure noted above as well as higher amortization expense from the Entocort
®
and Tretinoin Products acquisitions; offset largely by
|
|
•
|
A decrease
of
$19.4 million
in operating expenses due primarily to the absence of an
$18.0 million
R&D payment made in connection with a research and development contractual arrangement in the prior year.
|
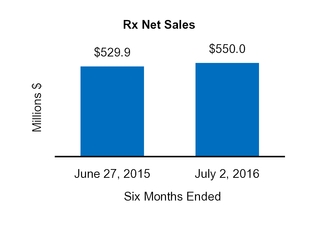
|
|
|||||||
|
Six Months Ended
|
|||||||
|
($ in millions)
|
June 27,
2015 |
July 2,
2016 |
|||||
|
Net sales
|
$
|
529.9
|
|
$
|
550.0
|
|
|
|
Gross profit
|
$
|
303.1
|
|
$
|
266.6
|
|
|
|
Gross profit %
|
57.2
|
%
|
48.5
|
%
|
|||
|
Operating income
|
$
|
199.5
|
|
$
|
184.2
|
|
|
|
Operating income %
|
37.6
|
%
|
33.5
|
%
|
|||
|
•
|
Net sales attributable to the Entocort
®
and Tretinoin Products acquisitions totaling
$89.3 million
; and
|
|
•
|
New product sales of
$36.8 million
due primarily to to sales of benzoyl peroxide 5%-clindamycin 1% gel (a generic version of Benzaclin™); offset partially by
|
|
•
|
Decreased sales of existing products of
$100.4 million
due to declined sales volume of certain products, pricing pressure across the portfolio, and the lack of exclusive market position for two key products versus the prior year;
|
|
•
|
Discontinued products of
$3.5 million
; and
|
|
•
|
Unfavorable foreign exchange movement of
$2.0 million
.
|
|
•
|
A decrease
of
$36.5 million
in gross profit due primarily to the pricing pressure noted above, as well as higher amortization expense from the Entocort
®
and Tretinoin Products acquisitions; offset partially by
|
|
•
|
A decrease
of
$21.2 million
in operating expenses due primarily to the absence of an
$18.0 million
research and development payment made in connection with a research and development contractual arrangement in the prior year.
|
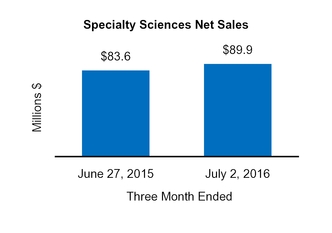
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
June 27, 2015
|
July 2,
2016 |
|||||
|
Net sales
|
$
|
83.6
|
|
$
|
89.9
|
|
|
|
Gross profit
|
$
|
11.1
|
|
$
|
17.0
|
|
|
|
Gross profit %
|
13.3
|
%
|
19.0
|
%
|
|||
|
Operating income
|
$
|
6.4
|
|
$
|
13.3
|
|
|
|
Operating income %
|
7.7
|
%
|
14.8
|
%
|
|||
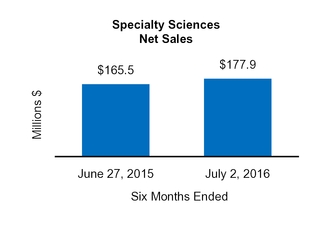
|
|
|||||||
|
Six Months Ended
|
|||||||
|
($ in millions)
|
June 27,
2015 |
July 2,
2016 |
|||||
|
Net sales
|
$
|
165.5
|
|
$
|
177.9
|
|
|
|
Gross profit
|
$
|
20.4
|
|
$
|
32.3
|
|
|
|
Gross profit %
|
12.4
|
%
|
18.2
|
%
|
|||
|
Operating income
|
$
|
11.9
|
|
$
|
26.3
|
|
|
|
Operating income %
|
7.2
|
%
|
14.8
|
%
|
|||
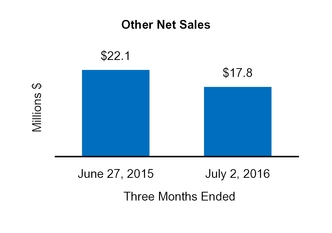
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
June 27, 2015
|
July 2,
2016 |
|||||
|
Net sales
|
$
|
22.1
|
|
$
|
17.8
|
|
|
|
Gross profit
|
$
|
7.2
|
|
$
|
7.5
|
|
|
|
Gross profit %
|
32.8
|
%
|
42.4
|
%
|
|||
|
Operating income (loss)
|
$
|
1.9
|
|
$
|
(1.3
|
)
|
|
|
Operating income (loss) %
|
8.4
|
%
|
(7.1
|
)%
|
|||
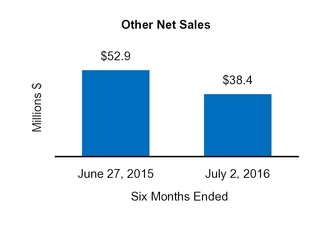
|
|
|||||||
|
Six Months Ended
|
|||||||
|
($ in millions)
|
June 27,
2015 |
July 2,
2016 |
|||||
|
Net sales
|
$
|
52.9
|
|
$
|
38.4
|
|
|
|
Gross profit
|
$
|
23.2
|
|
$
|
17.4
|
|
|
|
Gross profit %
|
43.6
|
%
|
45.3
|
%
|
|||
|
Operating income
|
$
|
12.4
|
|
$
|
4.1
|
|
|
|
Operating income %
|
23.3
|
%
|
10.8
|
%
|
|||
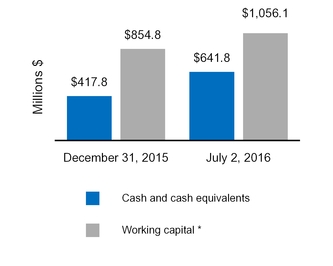
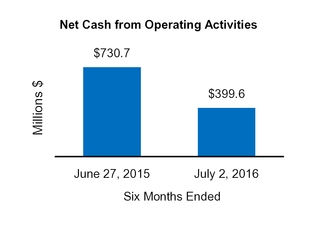
|
•
|
Changes in accounts payable, which contributed to a
$41.1 million
outflow in the first six months of 2016 compared to a
$187.5 million
inflow in the first six months of 2015. The primary reasons for the outflow in 2016 were the addition of Omega operations in the current year period and changes to the Omega accounts payable structure as discussed below.
|
|
•
|
Changes in inventories, which led to a
$50.3 million
outflow in the first six months of 2016 compared to a
$28.4 million
inflow during the first six months of 2015. The current year outflow was due to increased
|
|
•
|
Changes in accrued customer-related programs, which led to a
$45.3 million
outflow in the first six months of 2016 compared to a
$18.1 million
inflow for the first six months of 2015. The primary reason for the outflow in 2016 is the pricing dynamics in the Rx segment.
|
|
•
|
Changes in accrued payroll and related taxes, which contributed to a
$39.2 million
outflow in the first six months of 2016 compared to a
$3.8 million
outflow in the first six months of 2015. The primary reasons for the increased outflow in 2016 were severance payments related to the restructuring activities and the addition of Omega operations in the current year period.
|
|
•
|
Changes in accounts receivable, which contributed to a
$42.3 million
inflow in the first six months of 2016 compared to
$77.2 million
outflow in 2015. The inflow in the first six months of 2016 was due to reduced accounts receivable due to lower sales volumes, offset partially by a reduction of factoring in the current period.
|
|
•
|
Changes in accrued income taxes, which provided a
$21.8 million
inflow in the first six months of 2016 compared to a
$14.9 million
outflow in the first six months of 2015. The six months ended June 27, 2015 included a $68.9 million incremental tax payment made in the first quarter in connection with the contested IRS audit described above under "Income Taxes".
|
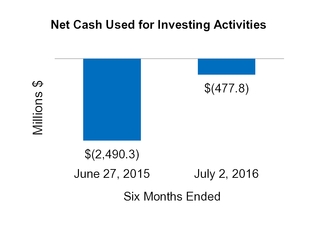
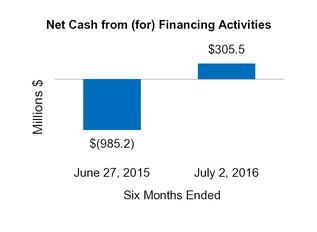
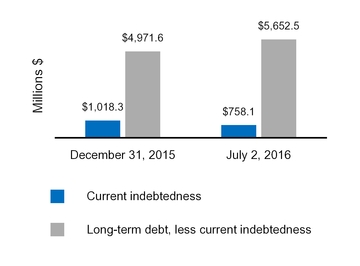
|
|
Payment Due by Period (in millions)
|
||||||||||||||||||
|
2016
(1)
|
2017 - 2018
|
2019 - 2020
|
After 2020
|
Total
|
|||||||||||||||
|
Short and long-term debt
(2)
|
$
|
660.5
|
|
$
|
1,682.1
|
|
$
|
811.6
|
|
$
|
5,508.4
|
|
$
|
8,662.6
|
|
||||
|
(2)
|
Short and long-term debt includes interest payments, which were calculated using the effective interest rate at
July 2, 2016
.
|
|
•
|
Reviewed the processes and controls in place to measure and record income taxes to enhance the efficiency and effectiveness of the design and operation of those controls;
|
|
•
|
Enhanced monitoring activities related to income taxes by adding additional internal controls and checklists; and
|
|
•
|
Evaluated and enhanced the level of precision in the management review controls related to income taxes by adding additional levels of review.
|
|
•
|
In April 2016, we announced that our former Chairman and Chief Executive Officer, Joseph C. Papa, resigned from the Company and that John T. Hendrickson, formerly our President, was appointed to serve as our new Chief Executive Officer. Mr. Hendrickson was later appointed to serve as a member of our Board of Directors.
|
|
•
|
In April 2016, we announced that the former Executive Vice President and General Manager of our BCH segment, Marc Coucke, resigned from the Company and that our current Executive Vice President and General Manager, International, Sharon Kochan, would undertake expanded responsibilities that include providing leadership and strategic direction to our BCH segment.
|
|
•
|
We recently announced the resignation of Doug Boothe, Executive Vice President and General Manager, Rx Pharmaceuticals, and the appointment of John Wesolowski, Acting General Manager, Rx Pharmaceuticals.
|
|
•
|
We have experienced a reduction in pricing expectations during 2016 in comparison to historical patterns in our U.S. businesses, in particular in our Rx segment, due to industry and competitive pressures in the sector. The reduced pricing is attributable to a variety of factors including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific product categories, loss of exclusivity on certain products, and consolidation of certain customers in the Rx segment. We expect this pricing environment to continue to impact us at least through the remainder of 2016.
|
|
•
|
Our expectations for the BCH segment continue to be impacted by market dynamics in the lifestyle and natural health/vitamins categories. Factors impacting these categories include softness in key markets due to current macro-economic factors, change in timing of certain advertising and promotional campaigns compared to the prior year, and lower sell-in during the current year due to the re-staging of certain products.The BCH segment has established a brand prioritization strategy to address these market dynamics, with an objective to balance the cost of advertising and promotion investments with expected contributions from category sales.
|
|
•
|
Our expectations for 2016 new product sales are consistent with those communicated in our first quarter Form 10-Q, but continue to remain lower than anticipated as of December 31, 2015. Several new product launches have been delayed due to the regulatory approval process for certain new products in the U.S. and modifications to market share penetration and timing assumptions for new products in our Rx and BCH segments.
|
|
•
|
Reviewed the processes and controls in place to measure and record income taxes to enhance the efficiency and effectiveness of the design and operation of those controls;
|
|
•
|
Enhanced monitoring activities related to income taxes by adding additional internal controls and checklists; and
|
|
•
|
Evaluated and enhanced the level of precision in the management review controls related to income taxes by adding additional levels of review.
|
|
Exhibit
Number
|
Description
|
|
|
3.1
|
Certificate of Incorporation of Perrigo Company plc (formerly known as Perrigo Company Limited) (incorporated by reference from Exhibit 4.1 to the Company's Registration Statement on Form S-8 filed on December 19, 2013).
|
|
|
3.2
|
Memorandum and Articles of Association of Perrigo Company plc, as amended (incorporated by reference from Exhibit 3.2 to the Company's Transition Report on Form 10-KT filed on February 25, 2016).
|
|
|
10.1
|
Amendment No. 3, effective as of April 24, 2016, to the Employment Agreement, effective as of October 9, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 25, 2016).
|
|
|
10.2
|
Mutual Agreement dated April 27, 2016 among the Company, Omega Pharma NV, Perrigo Ireland 2 Ltd, Mylecke Management, Art & Invest NV, Alychlo NV and Marc Coucke (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 28, 2016).
|
|
|
10.3
|
Amendment dated April 27, 2016 to the Agreement for the Sale and Purchase of 685,348,257 Shares Of Omega Pharma Invest NV, dated as of November 6, 2014, by and among the Company, Alychlo NV and Holdco I BE NV (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 28, 2016).
|
|
|
10.4
|
Amendment dated April 27, 2016 to the Non-Compete Agreement between the Company and Marc Coucke dated March 30, 2015 (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on April 28, 2016).
|
|
|
10.5
|
Amendment dated April 27, 2016 to the Lock-up Agreement between the Company and Alychlo NV dated March 30, 2015 (incorporated by reference from Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on April 28, 2016).
|
|
|
10.6
|
Amendment No. 4, effective as of May 6, 2016, to the Employment Agreement, effective as of October 9, 2006, by and between Perrigo Company and Joseph C. Papa (incorporated by reference from Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on May 16, 2016).
|
|
|
10.7
|
Waiver to Note Purchase Agreement, between Omega Pharma NV and the Prudential Insurance Company of America, dated May 16, 2016, in connection with the Note Purchase Agreement, dated May 19, 2011 (as amended by the First Amendment and Consent to the Note Purchase Agreement, dated as of October 7, 2011), with respect to the issuance and sale of EUR 135,043,889 aggregate principal amount of Omega’s 5.1045% senior notes due 2023 (incorporated by reference from Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q filed on May 16, 2016).
|
|
|
10.8
|
Amendment dated May 23, 2016 to the Waiver to Note Purchase Agreement, between Omega Pharma NV and the Prudential Insurance Company of America, dated May 16, 2016, in connection with the Note Purchase Agreement, dated May 19, 2011 (as amended by the First Amendment and Consent to the Note Purchase Agreement, dated as of October 7, 2011), with respect to the issuance and sale of EUR 135,043,889 aggregate principal amount of Omega’s 5.1045% senior notes due 2023 (filed herewith).
|
|
|
10.9
|
Perrigo Company plc U.S. Severance Policy, as amended and restated effective June 14, 2016 (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 17, 2016).
|
|
|
10.10
|
Perrigo Company plc Change in Control Severance Policy for U.S. Employees, as amended and restated effective June 14, 2016 (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on June 17, 2016).
|
|
|
10.11
|
Amendment No. 2 to the Perrigo Company Annual Incentive Plan, effective June 14, 2016 (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on June 17, 2016).
|
|
|
10.12
|
Second Waiver to Note Purchase Agreement, between Omega Pharma NV and the Prudential Insurance Company of America, dated June 7, 2016, in connection with the Note Purchase Agreement, dated May 19, 2011 (as amended by the First Amendment and Consent to the Note Purchase Agreement, dated as of October 7, 2011 and the Waiver to Note Purchase Agreement, dated as of May 16, 2016, as amended by a letter agreement dated May 23, 2016), with respect to the issuance and sale of EUR 135,043,889 aggregate principal amount of Omega’s 5.1045% senior notes due 2023 (filed herewith).
|
|
|
31.1
|
Rule 13a-14(a) Certification by John T. Hendrickson, Chief Executive Officer (filed herewith).
|
|
|
31.2
|
Rule 13a-14(a) Certification by Judy L. Brown, Executive Vice President, Business Operations and Chief Financial Officer (filed herewith).
|
|
|
32
|
Certification Pursuant to 18 United States Code 1350 and Rule 13a-14(b) of the Securities Exchange Act of 1934 (furnished herewith).
|
|
|
101.INS
|
XBRL Instance Document.
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
PERRIGO COMPANY PLC
|
|||
|
(Registrant)
|
|||
|
Date:
|
August 10, 2016
|
By: /s/ John T. Hendrickson
|
|
|
John T. Hendrickson
|
|||
|
Chief Executive Officer
|
|||
|
(Principal Executive Officer)
|
|||
|
Date:
|
August 10, 2016
|
By: /s/ Judy L. Brown
|
|
|
Judy L. Brown
|
|||
|
Executive Vice President, Business Operations and Chief Financial Officer
|
|||
|
(Principal Accounting and Financial Officer)
|
|||