|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[X]
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Ireland
|
|
Not Applicable
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
Treasury Building, Lower Grand Canal Street, Dublin 2, Ireland
|
|
-
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Large accelerated filer
|
[X]
|
Accelerated filer
|
[ ]
|
Non-accelerated filer
|
[ ]
|
Smaller reporting company
|
[ ]
|
|||
|
Emerging growth company
|
[ ]
|
|||||||||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
|
[ ]
|
|||||||||
|
PAGE
NUMBER
|
||
|
PART I. FINANCIAL INFORMATION
|
||
|
1
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
8
|
||
|
9
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
15
|
||
|
16
|
||
|
17
|
||
|
18
|
||
|
PART II. OTHER INFORMATION
|
||
|
|
Three Months Ended
|
||||||
|
|
April 1,
2017 |
April 2,
2016 |
|||||
|
Net sales
|
$
|
1,194.0
|
|
$
|
1,347.3
|
|
|
|
Cost of sales
|
729.6
|
|
814.2
|
|
|||
|
Gross profit
|
464.4
|
|
533.1
|
|
|||
|
Operating expenses
|
|||||||
|
Distribution
|
21.1
|
|
21.8
|
|
|||
|
Research and development
|
39.8
|
|
45.3
|
|
|||
|
Selling
|
155.0
|
|
180.8
|
|
|||
|
Administration
|
105.4
|
|
107.5
|
|
|||
|
Impairment charges
|
12.2
|
|
403.9
|
|
|||
|
Restructuring
|
38.7
|
|
5.4
|
|
|||
|
Other operating income
|
(36.3
|
)
|
—
|
|
|||
|
Total operating expenses
|
335.9
|
|
764.7
|
|
|||
|
Operating income (loss)
|
128.5
|
|
(231.6
|
)
|
|||
|
Tysabri
®
royalty stream
|
(17.1
|
)
|
204.4
|
|
|||
|
Interest expense, net
|
53.3
|
|
51.2
|
|
|||
|
Other (income) expense, net
|
(3.5
|
)
|
2.5
|
|
|||
|
Loss on extinguishment of debt
|
—
|
|
0.4
|
|
|||
|
Income (loss) before income taxes
|
95.8
|
|
(490.1
|
)
|
|||
|
Income tax expense
|
24.2
|
|
39.1
|
|
|||
|
Net income (loss)
|
$
|
71.6
|
|
$
|
(529.2
|
)
|
|
|
Earnings (loss) per share
|
|||||||
|
Basic
|
$
|
0.50
|
|
$
|
(3.70
|
)
|
|
|
Diluted
|
$
|
0.50
|
|
$
|
(3.70
|
)
|
|
|
Weighted-average shares outstanding
|
|||||||
|
Basic
|
143.4
|
|
143.2
|
|
|||
|
Diluted
|
143.6
|
|
143.2
|
|
|||
|
Dividends declared per share
|
$
|
0.160
|
|
$
|
0.145
|
|
|
|
Three Months Ended
|
|||||||
|
April 1,
2017 |
April 2,
2016 |
||||||
|
Net income (loss)
|
$
|
71.6
|
|
$
|
(529.2
|
)
|
|
|
Other comprehensive income:
|
|||||||
|
Foreign currency translation adjustments
|
65.4
|
|
151.4
|
|
|||
|
Change in fair value of derivative financial instruments, net of tax
|
1.6
|
|
(5.7
|
)
|
|||
|
Change in fair value of investment securities, net of tax
|
(11.4
|
)
|
6.2
|
|
|||
|
Change in post-retirement and pension liability adjustments, net of tax
|
(0.1
|
)
|
0.8
|
|
|||
|
Other comprehensive income, net of tax
|
55.5
|
|
152.7
|
|
|||
|
Comprehensive income (loss)
|
$
|
127.1
|
|
$
|
(376.5
|
)
|
|
|
April 1,
2017 |
December 31,
2016 |
||||||
|
Assets
|
|||||||
|
Cash and cash equivalents
|
$
|
3,077.8
|
|
$
|
622.3
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $6.0 million and $6.3 million, respectively
|
1,050.2
|
|
1,176.0
|
|
|||
|
Inventories
|
800.2
|
|
795.0
|
|
|||
|
Prepaid expenses and other current assets
|
185.2
|
|
212.0
|
|
|||
|
Total current assets
|
5,113.4
|
|
2,805.3
|
|
|||
|
Property, plant and equipment, net
|
875.3
|
|
870.1
|
|
|||
|
Tysabri
®
royalty stream
|
—
|
|
2,350.0
|
|
|||
|
Goodwill and other indefinite-lived intangible assets
|
4,178.0
|
|
4,163.9
|
|
|||
|
Other intangible assets, net
|
3,341.4
|
|
3,396.8
|
|
|||
|
Non-current deferred income taxes
|
76.4
|
|
72.1
|
|
|||
|
Other non-current assets
|
394.9
|
|
211.9
|
|
|||
|
Total non-current assets
|
8,866.0
|
|
11,064.8
|
|
|||
|
Total assets
|
$
|
13,979.4
|
|
$
|
13,870.1
|
|
|
|
Liabilities and Shareholders’ Equity
|
|||||||
|
Accounts payable
|
$
|
476.3
|
|
$
|
471.7
|
|
|
|
Payroll and related taxes
|
146.0
|
|
115.8
|
|
|||
|
Accrued customer programs
|
348.2
|
|
380.3
|
|
|||
|
Accrued liabilities
|
281.6
|
|
263.3
|
|
|||
|
Accrued income taxes
|
57.7
|
|
32.4
|
|
|||
|
Current indebtedness
|
1,175.4
|
|
572.8
|
|
|||
|
Total current liabilities
|
2,485.2
|
|
1,836.3
|
|
|||
|
Long-term debt, less current portion
|
4,618.9
|
|
5,224.5
|
|
|||
|
Non-current deferred income taxes
|
349.4
|
|
389.9
|
|
|||
|
Other non-current liabilities
|
458.6
|
|
461.8
|
|
|||
|
Total non-current liabilities
|
5,426.9
|
|
6,076.2
|
|
|||
|
Total liabilities
|
7,912.1
|
|
7,912.5
|
|
|||
|
Commitments and contingencies - Note 14
|
|||||||
|
Shareholders’ equity
|
|||||||
|
Controlling interest:
|
|||||||
|
Preferred shares, $0.0001 par value, 10 million shares authorized
|
—
|
|
—
|
|
|||
|
Ordinary shares, €0.001 par value, 10 billion shares authorized
|
8,118.1
|
|
8,135.0
|
|
|||
|
Accumulated other comprehensive (loss)
|
(26.3
|
)
|
(81.8
|
)
|
|||
|
Retained earnings (accumulated deficit)
|
(2,024.0
|
)
|
(2,095.1
|
)
|
|||
|
Total controlling interest
|
6,067.8
|
|
5,958.1
|
|
|||
|
Noncontrolling interest
|
(0.5
|
)
|
(0.5
|
)
|
|||
|
Total shareholders’ equity
|
6,067.3
|
|
5,957.6
|
|
|||
|
Total liabilities and shareholders' equity
|
$
|
13,979.4
|
|
$
|
13,870.1
|
|
|
|
Supplemental Disclosures of Balance Sheet Information
|
|||||||
|
Ordinary shares, issued and outstanding
|
143.4
|
|
143.4
|
|
|||
|
Three Months Ended
|
|||||||
|
|
April 1,
2017 |
April 2,
2016 |
|||||
|
Cash Flows From (For) Operating Activities
|
|||||||
|
Net income (loss)
|
$
|
71.6
|
|
$
|
(529.2
|
)
|
|
|
Adjustments to derive cash flows
|
|||||||
|
Depreciation and amortization
|
109.4
|
|
109.7
|
|
|||
|
Share-based compensation
|
6.1
|
|
15.8
|
|
|||
|
Impairment charges
|
12.2
|
|
403.9
|
|
|||
|
Tysabri
®
royalty stream
|
(17.1
|
)
|
204.4
|
|
|||
|
Loss on extinguishment of debt
|
—
|
|
0.4
|
|
|||
|
Restructuring charges
|
38.7
|
|
5.4
|
|
|||
|
Deferred income taxes
|
(46.0
|
)
|
(178.3
|
)
|
|||
|
Amortization of debt discount (premium)
|
(6.4
|
)
|
(6.7
|
)
|
|||
|
Other non-cash adjustments
|
(1.1
|
)
|
1.6
|
|
|||
|
Subtotal
|
167.4
|
|
27.0
|
|
|||
|
Increase (decrease) in cash due to:
|
|||||||
|
Accounts receivable
|
50.1
|
|
17.3
|
|
|||
|
Inventories
|
0.5
|
|
4.4
|
|
|||
|
Accounts payable
|
2.5
|
|
(3.2
|
)
|
|||
|
Payroll and related taxes
|
(10.1
|
)
|
(37.4
|
)
|
|||
|
Accrued customer programs
|
(32.7
|
)
|
(81.7
|
)
|
|||
|
Accrued liabilities
|
2.3
|
|
(12.8
|
)
|
|||
|
Accrued income taxes
|
41.4
|
|
185.7
|
|
|||
|
Other
|
(26.9
|
)
|
(0.8
|
)
|
|||
|
Subtotal
|
27.1
|
|
71.5
|
|
|||
|
Net cash from (for) operating activities
|
194.5
|
|
98.5
|
|
|||
|
Cash Flows From (For) Investing Activities
|
|||||||
|
Proceeds from royalty rights - at fair value
|
85.3
|
|
83.4
|
|
|||
|
Acquisitions of businesses, net of cash acquired
|
—
|
|
(416.4
|
)
|
|||
|
Additions to property and equipment
|
(22.0
|
)
|
(34.7
|
)
|
|||
|
Proceeds from sale of business and other assets
|
25.3
|
|
—
|
|
|||
|
Proceeds from sale of the Tysabri
®
royalty stream
|
2,200.0
|
|
—
|
|
|||
|
Other investing
|
(0.8
|
)
|
(1.0
|
)
|
|||
|
Net cash from (for) investing activities
|
2,287.8
|
|
(368.7
|
)
|
|||
|
Cash Flows From (For) Financing Activities
|
|||||||
|
Issuances of long-term debt
|
—
|
|
1,190.3
|
|
|||
|
Payments on long-term debt
|
(13.6
|
)
|
(14.3
|
)
|
|||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
0.3
|
|
(715.9
|
)
|
|||
|
Deferred financing fees
|
(0.4
|
)
|
(1.5
|
)
|
|||
|
Issuance of ordinary shares
|
—
|
|
3.1
|
|
|||
|
Cash dividends
|
(23.0
|
)
|
(20.8
|
)
|
|||
|
Other financing
|
(0.5
|
)
|
(3.5
|
)
|
|||
|
Net cash from (for) financing activities
|
(37.2
|
)
|
437.4
|
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
10.4
|
|
3.9
|
|
|||
|
Net increase in cash and cash equivalents
|
2,455.5
|
|
171.1
|
|
|||
|
Cash and cash equivalents, beginning of period
|
622.3
|
|
417.8
|
|
|||
|
Cash and cash equivalents, end of period
|
$
|
3,077.8
|
|
$
|
588.9
|
|
|
|
Recently Issued Accounting Standards Adopted
|
||||||
|
Standard
|
Description
|
Date of adoption
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Clarifying the Definition of a Business
|
This update clarifies the definition of a business and addresses whether transactions should be accounted for as asset acquisitions or business combinations (or divestitures). The guidance includes an initial threshold that an acquired set of assets will not be considered a business if substantially all of the fair value of the assets acquired is concentrated in a single tangible or identifiable intangible asset (or group of similar assets). If the acquired set does not pass the initial threshold, then the guidance requires that, to be a business, the set must include an input and a substantive process that together significantly contribute to the ability to create outputs. Different factors are considered to determine whether the set includes a substantive process, such as the inclusion of an organized workforce. Further, the guidance removes language stating that a business need not include all of the inputs and processes that the seller used in operating the business.
|
January 1, 2017
|
We early adopted this new standard and will apply it prospectively when determining whether transactions should be accounted for as asset acquisitions or business combinations (divestitures). During the three months ended April 1, 2017, we applied the new guidance when determining whether certain product divestitures represented sales of assets or businesses. In each case, we determined that the assets sold did not meet the definition of a business under the new rules.
|
|||
|
Improvements to Employee Share-Based Payment Accounting
|
This guidance is intended to simplify several aspects of the accounting for share-based payment award transactions. It will require all income tax effects of awards to be recorded through the income statement when they vest or settle as opposed to certain amounts being recorded in additional paid-in capital. An entity will also have to elect whether to account for forfeitures as they occur or by estimating the number of awards expected to be forfeited and adjusting the estimate when it is likely to change (as currently required). The guidance will also increase the amount an employer can withhold to cover income taxes on awards.
|
January 1, 2017
|
We adopted this standard as of January 1, 2017. We elected to estimate the number of awards expected to be forfeited and adjust the estimate when it is likely to change, consistent with past practice. We did not change the amounts that we withhold to cover income taxes on awards. As the requirement to record all income tax effects of vested or settled awards through the income statement is prospective in nature, there was no cumulative effect of adopting the standard on our balance sheet.
|
|||
|
Recently Issued Accounting Standards Not Yet Adopted
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Revenue from Contracts with Customers
|
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps: identify the contract(s) with a customer; identify the performance obligations in the contract; determine the transaction price; allocate the transaction price to the performance obligations in the contract; and recognize revenue when (or as) the entity satisfies a performance obligation. This guidance allows for two adoption methods, full retrospective approach or modified retrospective approach.
|
January 1, 2018
|
We continue to evaluate the implications of adoption of the new revenue standard on our consolidated financial statements. We have completed an initial assessment of the adoption and are in the process of completing a detailed review of our various customer contracts. Based on our initial analysis, we do not expect there to be a material impact on our revenue recognition practices. We plan to adopt the new revenue standard effective January 1, 2018 using the modified retrospective approach.
|
|||
|
Intra-Entity Asset Transfers of Assets Other Than Inventory
|
Under the new guidance, the tax impact to the seller on the profit from the transfers and the buyer’s deferred tax benefit on the increased tax basis would be recognized when the transfers occur, resulting in the recognition of expense sooner than under historical guidance. The guidance excludes intra-entity transfers of inventory. For intra-entity transfers of inventory, the FASB decided to retain current GAAP, which requires an entity to recognize the income tax consequences when the inventory has been sold to an outside party.
|
January 1, 2018
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Leases
|
This guidance was issued to increase transparency and comparability among organizations by requiring recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. For leases with a term of 12 months or less, lessees are permitted to make an election to not recognize right-of-use assets and lease liabilities. Upon adoption, lessees will apply the new standard as of the beginning of the earliest comparative period presented in the financial statements, however lessees will be able to exclude leases that expire as of the implementation date. Early adoption is permitted.
|
January 1, 2019
|
We are currently evaluating the implications of adoption on our consolidated financial statements.
|
|||
|
Recently Issued Accounting Standards Not Yet Adopted (continued)
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
Measurement of Credit Losses on Financial Instruments
|
This guidance changes the impairment model for most financial assets and certain other instruments, replacing the current "incurred loss" approach with an "expected loss" credit impairment model, which will apply to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities, and off-balance sheet credit exposures such as letters of credit. Early adoption is permitted.
|
January 1, 2020
|
We are currently evaluating the new standard for potential impacts on our receivables, debt, and other financial instruments and considering whether to early adopt the standard.
|
|||
|
Intangibles - Goodwill and Other Simplifying the Test for Goodwill
|
The objective of this update is to reduce the cost and complexity of subsequent goodwill accounting by simplifying the impairment test by removing the Step 2 requirement to perform a hypothetical purchase price allocation when the carrying value of a reporting unit exceeds its fair value. If a reporting unit’s carrying value exceeds its fair value, an entity would record an impairment charge based on that difference, limited to the amount of goodwill attributed to that reporting unit. The proposal would not change the guidance on completing Step 1 of the goodwill impairment test. The proposed guidance would be applied prospectively with an effective date for Perrigo of January 1, 2020, with early adoption permitted as of January 1, 2017.
|
January 1, 2020
|
We are currently evaluating the implications of adoption on our consolidated financial statements and considering whether to early adopt the standard.
|
|||
|
Tretinoin Products
|
Development-Stage Rx Products
|
All Other
(1)
|
|||||||||
|
Purchase price paid
|
$
|
416.4
|
|
$
|
—
|
|
$
|
17.1
|
|
||
|
Contingent consideration
|
—
|
|
24.9
|
|
26.2
|
|
|||||
|
Total purchase consideration
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
43.3
|
|
||
|
Assets acquired:
|
|||||||||||
|
Cash and cash equivalents
|
$
|
—
|
|
$
|
—
|
|
$
|
3.8
|
|
||
|
Accounts receivable
|
—
|
|
—
|
|
4.9
|
|
|||||
|
Inventories
|
1.4
|
|
—
|
|
7.1
|
|
|||||
|
Prepaid expenses and other current assets
|
—
|
|
—
|
|
0.1
|
|
|||||
|
Property and equipment
|
—
|
|
—
|
|
1.2
|
|
|||||
|
Goodwill
|
1.7
|
|
—
|
|
—
|
|
|||||
|
Definite-lived intangibles
:
|
|||||||||||
|
Distribution and license agreements, supply agreements
|
—
|
|
—
|
|
1.8
|
|
|||||
|
Developed product technology, formulations, and product rights
|
411.0
|
|
—
|
|
18.0
|
|
|||||
|
Customer relationships and distribution networks
|
—
|
|
—
|
|
8.2
|
|
|||||
|
Non-compete agreements
|
2.3
|
|
—
|
|
—
|
|
|||||
|
Indefinite-lived intangibles
:
|
|||||||||||
|
In-process research and development
|
—
|
|
24.9
|
|
4.9
|
|
|||||
|
Total intangible assets
|
$
|
413.3
|
|
$
|
24.9
|
|
$
|
32.9
|
|
||
|
Total assets
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
50.0
|
|
||
|
Liabilities assumed:
|
|||||||||||
|
Accounts payable
|
$
|
—
|
|
$
|
—
|
|
$
|
2.8
|
|
||
|
Accrued liabilities
|
—
|
|
—
|
|
0.1
|
|
|||||
|
Long-term debt
|
—
|
|
—
|
|
3.3
|
|
|||||
|
Net deferred income tax liabilities
|
—
|
|
—
|
|
0.5
|
|
|||||
|
Total liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
6.7
|
|
||
|
Net assets acquired
|
$
|
416.4
|
|
$
|
24.9
|
|
$
|
43.3
|
|
||
|
(1)
|
Consists of
four
product acquisitions in our Consumer Healthcare Americas ("
CHCA
"), Consumer Healthcare International ("
CHCI
") and
RX
segments
|
|
Reporting Segments:
|
December 31, 2016
|
Currency translation adjustment
|
April 1,
2017 |
|||||||||
|
CHCA
|
$
|
1,810.6
|
|
$
|
2.3
|
|
$
|
1,812.9
|
|
|||
|
CHCI
|
1,070.8
|
|
14.8
|
|
1,085.6
|
|
||||||
|
RX
|
1,086.6
|
|
4.5
|
|
1,091.1
|
|
||||||
|
Other
|
81.4
|
|
5.1
|
|
86.5
|
|
||||||
|
Total goodwill
|
$
|
4,049.4
|
|
$
|
26.7
|
|
$
|
4,076.1
|
|
|||
|
|
April 1, 2017
|
December 31, 2016
|
|||||||||||||
|
|
Gross
|
Accumulated Amortization
|
Gross
|
Accumulated Amortization
|
|||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||
|
Distribution and license agreements, supply agreements
|
$
|
307.6
|
|
$
|
132.5
|
|
$
|
305.6
|
|
$
|
120.4
|
|
|||
|
Developed product technology, formulations, and product rights
|
1,428.2
|
|
562.5
|
|
1,418.1
|
|
526.0
|
|
|||||||
|
Customer relationships and distribution networks
|
1,505.7
|
|
339.8
|
|
1,489.9
|
|
307.5
|
|
|||||||
|
Trademarks, trade names, and brands
|
1,203.4
|
|
71.5
|
|
1,189.3
|
|
55.3
|
|
|||||||
|
Non-compete agreements
|
14.5
|
|
11.7
|
|
14.3
|
|
11.2
|
|
|||||||
|
Total definite-lived intangibles
|
$
|
4,459.4
|
|
$
|
1,118.0
|
|
$
|
4,417.2
|
|
$
|
1,020.4
|
|
|||
|
Indefinite-lived intangibles
:
|
|||||||||||||||
|
Trademarks, trade names, and brands
|
$
|
50.8
|
|
$
|
—
|
|
$
|
50.5
|
|
$
|
—
|
|
|||
|
In-process research and development
|
51.1
|
|
—
|
|
64.0
|
|
—
|
|
|||||||
|
Total indefinite-lived intangibles
|
101.9
|
|
—
|
|
114.5
|
|
—
|
|
|||||||
|
Total other intangible assets
|
$
|
4,561.3
|
|
$
|
1,118.0
|
|
$
|
4,531.7
|
|
$
|
1,020.4
|
|
|||
|
April 1,
2017 |
December 31,
2016 |
||||||
|
Finished goods
|
$
|
449.3
|
|
$
|
431.1
|
|
|
|
Work in process
|
151.7
|
|
165.7
|
|
|||
|
Raw materials
|
199.2
|
|
198.2
|
|
|||
|
Total inventories
|
$
|
800.2
|
|
$
|
795.0
|
|
|
|
Level 1:
|
Quoted prices for identical instruments in active markets.
|
|
Level 2:
|
Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets.
|
|
Level 3:
|
Valuations derived from valuation techniques in which one or more significant inputs are not observable.
|
|
Fair Value
|
||||||||||
|
Fair Value Hierarchy
|
April 1,
2017 |
December 31,
2016 |
||||||||
|
Measured at fair value on a recurring basis:
|
||||||||||
|
Assets:
|
||||||||||
|
Investment securities
|
Level 1
|
$
|
22.8
|
|
$
|
38.2
|
|
|||
|
Foreign currency forward contracts
|
Level 2
|
$
|
6.5
|
|
$
|
3.8
|
|
|||
|
Funds associated with Israeli severance liability
|
Level 2
|
17.1
|
|
15.9
|
|
|||||
|
Total level 2 assets
|
$
|
23.6
|
|
$
|
19.7
|
|
||||
|
Royalty Pharma contingent milestone payments
|
Level 3
|
$
|
184.5
|
|
$
|
—
|
|
|||
|
Tysabri
®
royalty stream
|
Level 3
|
—
|
|
2,350.0
|
|
|||||
|
Total level 3 assets
|
$
|
184.5
|
|
$
|
2,350.0
|
|
||||
|
Liabilities:
|
||||||||||
|
Foreign currency forward contracts
|
Level 2
|
$
|
2.5
|
|
$
|
5.0
|
|
|||
|
Contingent consideration
|
Level 3
|
$
|
52.0
|
|
$
|
69.9
|
|
|||
|
Measured at fair value on a non-recurring basis:
|
||||||||||
|
Assets:
|
||||||||||
|
Goodwill
(1)
|
Level 3
|
$
|
—
|
|
$
|
1,148.4
|
|
|||
|
Indefinite-lived intangible assets
(2)
|
Level 3
|
13.8
|
|
0.3
|
|
|||||
|
Definite-lived intangible assets
(3)
|
Level 3
|
—
|
|
758.0
|
|
|||||
|
Assets held for sale, net
|
Level 3
|
11.0
|
|
18.2
|
|
|||||
|
Total level 3 assets
|
$
|
24.8
|
|
$
|
1,924.9
|
|
||||
|
(1)
|
As of December 31, 2016, goodwill with a carrying amount of
$2.2 billion
was written down to its implied fair value of
$1.1 billion
.
|
|
(2)
|
As of April 1, 2017, indefinite-lived intangible assets with a carrying amount of
$26.0 million
were written down to a fair value of
$13.8 million
, resulting in a total impairment charge of
$12.2 million
. As of December 31, 2016, indefinite-lived intangible assets with a carrying amount of
$0.7 million
were written down to a fair value of
$0.3 million
.
|
|
(3)
|
As of December 31, 2016, definite-lived intangible assets with a carrying amount of
$2.3 billion
were written down to a fair value of
$758.0 million
resulting in a total impairment charge of
$1.5 billion
. Included in this balance are indefinite-lived intangible assets with fair value of
$364.5 million
and
$674.2 million
that were reclassified to definite-lived assets at April 3, 2016 and October 2, 2016, respectively.
|
|
Three Months Ended
|
|||||||
|
April 1,
2017 |
April 2,
2016 |
||||||
|
Contingent Consideration
|
|||||||
|
Beginning balance
|
$
|
69.9
|
|
$
|
17.9
|
|
|
|
Net realized (gains) losses
|
(14.4
|
)
|
0.3
|
|
|||
|
Purchases or additions
|
—
|
|
29.5
|
|
|||
|
Foreign currency effect
|
(0.1
|
)
|
0.3
|
|
|||
|
Settlements
|
(3.4
|
)
|
—
|
|
|||
|
Ending balance
|
$
|
52.0
|
|
$
|
48.0
|
|
|
|
Year Ended
|
|||||||||
|
December 31, 2016
|
|||||||||
|
Omega - Lifestyle
|
Omega - XLS
|
Entocort
®
- Branded Products
|
Entocort
®
- AG Products
|
Herron Trade names and Trademarks
|
|||||
|
5-year average growth rate
|
2.5%
|
3.2%
|
(31.7)%
|
(30.4)%
|
4.6%
|
||||
|
Long-term growth rates
|
2.0%
|
NA
|
(10.0)%
|
(4.7)%
|
2.5%
|
||||
|
Discount rate
|
9.3%
|
9.5%
|
13.0%
|
10.5%
|
10.8%
|
||||
|
Royalty rate
|
NA
|
4.0%
|
NA
|
NA
|
11.0%
|
||||
|
Valuation method
|
MPEEM
|
Relief from Royalty
|
MPEEM
|
MPEEM
|
Relief from Royalty
|
||||
|
April 1,
2017 |
December 31, 2016
|
||||||
|
Equity securities, at cost less impairments
|
$
|
15.5
|
|
$
|
16.5
|
|
|
|
Gross unrealized gains
|
7.3
|
|
21.7
|
|
|||
|
Estimated fair value of equity securities
|
$
|
22.8
|
|
$
|
38.2
|
|
|
|
Asset Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
April 1,
2017 |
December 31, 2016
|
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
3.0
|
|
$
|
3.1
|
|
||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Other current assets
|
$
|
3.5
|
|
$
|
0.7
|
|
||
|
Liability Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
April 1,
2017 |
December 31, 2016
|
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
1.9
|
|
$
|
3.0
|
|
||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
0.6
|
|
$
|
2.0
|
|
||
|
Amount of Gain/(Loss) Recorded in OCI
(Effective Portion) |
||||||||
|
Three Months Ended
|
||||||||
|
Designated Cash Flow Hedges
|
April 1,
2017 |
April 2,
2016 |
||||||
|
Interest rate swap agreements
|
$
|
—
|
|
$
|
(9.0
|
)
|
||
|
Foreign currency forward contracts
|
2.5
|
|
1.6
|
|
||||
|
Total
|
$
|
2.5
|
|
$
|
(7.4
|
)
|
||
|
Amount of Gain/(Loss) Reclassified from AOCI to Income
(Effective Portion) |
||||||||||
|
Three Months Ended
|
||||||||||
|
Designated Cash Flow Hedges
|
Income Statement Location
|
April 1,
2017 |
April 2,
2016 |
|||||||
|
Interest rate swap agreements
|
Interest expense, net
|
$
|
(0.6
|
)
|
$
|
(0.5
|
)
|
|||
|
Foreign currency forward contracts
|
Net sales
|
0.2
|
|
0.5
|
|
|||||
|
Cost of sales
|
0.7
|
|
0.3
|
|
||||||
|
Interest expense, net
|
(0.6
|
)
|
(0.4
|
)
|
||||||
|
Other (income) expense, net
|
(0.5
|
)
|
0.2
|
|
||||||
|
Total
|
$
|
(0.8
|
)
|
$
|
0.1
|
|
||||
|
Amount of Gain/(Loss) Recognized in Income
(Ineffective Portion) |
||||||||||
|
Three Months Ended
|
||||||||||
|
Designated Cash Flow Hedges
|
Income Statement
Location
|
April 1,
2017 |
April 2,
2016 |
|||||||
|
Interest rate swap agreements
|
Other (income) expense, net
|
$
|
—
|
|
$
|
(0.1
|
)
|
|||
|
Foreign currency forward contracts
|
Net sales
|
—
|
|
(0.1
|
)
|
|||||
|
Cost of sales
|
—
|
|
0.1
|
|
||||||
|
Other (income) expense, net
|
0.9
|
|
—
|
|
||||||
|
Total
|
$
|
0.9
|
|
$
|
(0.1
|
)
|
||||
|
Amount of Gain/(Loss) Recognized in Income
|
||||||||||
|
Three Months Ended
|
||||||||||
|
Non-Designated Derivatives
|
Income Statement Location
|
April 1,
2017 |
April 2,
2016 |
|||||||
|
Foreign currency forward contracts
|
Other (income) expense, net
|
$
|
(8.9
|
)
|
$
|
(6.9
|
)
|
|||
|
Interest expense, net
|
(0.4
|
)
|
0.1
|
|
||||||
|
Total
|
$
|
(9.3
|
)
|
$
|
(6.8
|
)
|
||||
|
April 1,
2017 |
December 31,
2016 |
||||||||||||||
|
CHCA
|
Other
|
CHCA
|
Other
|
||||||||||||
|
Assets held for sale
|
|||||||||||||||
|
Current assets
|
$
|
—
|
|
$
|
4.2
|
|
$
|
—
|
|
$
|
5.1
|
|
|||
|
Goodwill
|
—
|
|
5.5
|
|
—
|
|
5.5
|
|
|||||||
|
Property, plant and equipment
|
2.7
|
|
33.0
|
|
13.5
|
|
33.2
|
|
|||||||
|
Other assets
|
—
|
|
3.2
|
|
—
|
|
3.8
|
|
|||||||
|
Less: impairment reserves
|
—
|
|
(34.5
|
)
|
(3.7
|
)
|
(35.3
|
)
|
|||||||
|
Total assets held for sale
|
$
|
2.7
|
|
$
|
11.4
|
|
$
|
9.8
|
|
$
|
12.3
|
|
|||
|
Liabilities held for sale
|
|||||||||||||||
|
Current liabilities
|
$
|
0.1
|
|
$
|
1.0
|
|
$
|
0.1
|
|
$
|
1.9
|
|
|||
|
Other liabilities
|
—
|
|
2.0
|
|
—
|
|
1.9
|
|
|||||||
|
Total liabilities held for sale
|
$
|
0.1
|
|
$
|
3.0
|
|
$
|
0.1
|
|
$
|
3.8
|
|
|||
|
April 1,
2017 |
December 31,
2016 |
||||||||||
|
Term loans
|
|||||||||||
|
*
|
2014 Term loan due December 5, 2019
|
$
|
412.9
|
|
$
|
420.7
|
|
||||
|
Notes and Bonds
|
|||||||||||
|
Coupon
|
Due
|
||||||||||
|
*
|
4.500%
|
May 23, 2017
|
(3)
|
191.8
|
|
189.3
|
|
||||
|
*
|
5.125%
|
December 12, 2017
|
(3)
|
319.7
|
|
315.6
|
|
||||
|
2.300%
|
November 8, 2018
|
(2)(5)
|
600.0
|
|
600.0
|
|
|||||
|
*
|
5.000%
|
May 23, 2019
|
(3)
|
127.9
|
|
126.2
|
|
||||
|
3.500%
|
March 15, 2021
|
(4)
|
500.0
|
|
500.0
|
|
|||||
|
3.500%
|
December 15, 2021
|
(1)
|
500.0
|
|
500.0
|
|
|||||
|
*
|
5.105%
|
July 19, 2023
|
(3)
|
143.8
|
|
142.0
|
|
||||
|
4.000%
|
November 15, 2023
|
(2)
|
800.0
|
|
800.0
|
|
|||||
|
3.900%
|
December 15, 2024
|
(1)
|
700.0
|
|
700.0
|
|
|||||
|
4.375%
|
March 15, 2026
|
(4)
|
700.0
|
|
700.0
|
|
|||||
|
5.300%
|
November 15, 2043
|
(2)
|
400.0
|
|
400.0
|
|
|||||
|
4.900%
|
December 15, 2044
|
(1)
|
400.0
|
|
400.0
|
|
|||||
|
Total notes and bonds
|
5,383.2
|
|
5,373.1
|
|
|||||||
|
Other financing
|
3.1
|
|
3.6
|
|
|||||||
|
Unamortized premium (discount), net
|
27.1
|
|
33.0
|
|
|||||||
|
Deferred financing fees
|
(32.0
|
)
|
(33.1
|
)
|
|||||||
|
Total borrowings outstanding
|
5,794.3
|
|
5,797.3
|
|
|||||||
|
Current indebtedness
|
(1,175.4
|
)
|
(572.8
|
)
|
|||||||
|
Total long-term debt less current portion
|
$
|
4,618.9
|
|
$
|
5,224.5
|
|
|||||
|
(1)
|
Discussed below collectively as the "2014 Notes."
|
|
(2)
|
Discussed below collectively as the "2013 Notes."
|
|
(3)
|
Debt assumed from Omega.
|
|
(4)
|
Discussed below collectively as the "2016 Notes."
|
|
(5)
|
Inclusive of
$600.0 million
, in aggregate principal amount of 2.300% 2018 Notes, which were repaid May 8, 2017.
|
|
*
|
Debt denominated in Euros subject to fluctuations in the euro-to-U.S. dollar exchange rate.
|
|
•
|
$20.0 million
in aggregate principal amount of
6.190%
senior notes due
2016
, which was repaid on May 29, 2015 in full;
|
|
•
|
€135.0 million
(
$147.0 million
) in aggregate principal amount of
5.1045%
senior notes due
2023
(the "2023 Notes");
|
|
•
|
€300.0 million
(
$326.7 million
) in aggregate principal amount of
5.125%
retail bonds due
2017
;
€180.0 million
(
$196.0 million
) in aggregate principal amount of
4.500%
retail bonds due
2017
; and
|
|
|
Three Months Ended
|
||||||
|
|
April 1,
2017 |
April 2,
2016 |
|||||
|
Numerator:
|
|||||||
|
Net income (loss)
|
$
|
71.6
|
|
$
|
(529.2
|
)
|
|
|
Denominator:
|
|||||||
|
Weighted average shares outstanding for basic EPS
|
143.4
|
|
143.2
|
|
|||
|
Dilutive effect of share-based awards*
|
0.2
|
|
—
|
|
|||
|
Weighted average shares outstanding for diluted EPS
|
143.6
|
|
143.2
|
|
|||
|
Anti-dilutive share-based awards excluded from computation of diluted EPS*
|
1.0
|
|
—
|
|
|||
|
Three Months Ended
|
||||
|
April 1,
2017 |
April 2,
2016 |
|||
|
14,400
|
|
79,000
|
|
|
|
Foreign currency translation adjustments
|
Fair value of derivative financial instruments, net of tax
|
Fair value of investment securities, net of tax
|
Post-retirement and pension liability adjustments, net of tax
|
Total AOCI
|
|||||||||||||||
|
Balance at December 31, 2016
|
$
|
(67.9
|
)
|
$
|
(19.5
|
)
|
$
|
15.1
|
|
$
|
(9.5
|
)
|
$
|
(81.8
|
)
|
||||
|
OCI before reclassifications
|
65.4
|
|
1.5
|
|
(13.0
|
)
|
(0.1
|
)
|
53.8
|
|
|||||||||
|
Amounts reclassified from AOCI
|
—
|
|
0.1
|
|
1.6
|
|
—
|
|
1.7
|
|
|||||||||
|
Other comprehensive income (loss)
|
65.4
|
|
1.6
|
|
(11.4
|
)
|
(0.1
|
)
|
55.5
|
|
|||||||||
|
Balance at April 1, 2017
|
$
|
(2.5
|
)
|
$
|
(17.9
|
)
|
$
|
3.7
|
|
$
|
(9.6
|
)
|
$
|
(26.3
|
)
|
||||
|
Three Months Ended
|
|||||||
|
April 1,
2017 |
April 2,
2016 |
||||||
|
Beginning balance
|
$
|
19.7
|
|
$
|
20.7
|
|
|
|
Additional charges
|
38.7
|
|
5.4
|
|
|||
|
Payments
|
(7.1
|
)
|
(18.2
|
)
|
|||
|
Non-cash adjustments
|
0.2
|
|
5.1
|
|
|||
|
Ending balance
|
$
|
51.5
|
|
$
|
13.0
|
|
|
|
•
|
CHCA
,
comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
CHCI
,
comprises our legacy Branded Consumer Healthcare segment and now includes our consumer focused businesses in the U.K., Australia, and Israel. This segment also includes our U.K. liquid licensed products business.
|
|
•
|
RX
,
comprises our U.S. Prescription Pharmaceuticals business.
|
|
Total Assets
|
April 1,
2017 |
December 31, 2016
|
||||||
|
CHCA
|
$
|
6,295.7
|
|
$
|
3,351.3
|
|
||
|
CHCI
|
4,796.9
|
|
4,795.2
|
|
||||
|
RX
|
2,685.4
|
|
2,646.4
|
|
||||
|
Specialty Sciences
|
—
|
|
2,775.8
|
|
||||
|
Other
|
201.4
|
|
301.4
|
|
||||
|
Total
|
$
|
13,979.4
|
|
$
|
13,870.1
|
|
||
|
Three Months Ended
|
|||||||||||||||||||||||
|
April 1, 2017
|
April 2, 2016
|
||||||||||||||||||||||
|
Net Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
Net Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
||||||||||||||||||
|
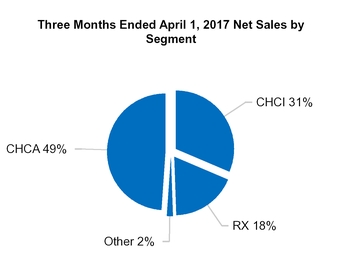
CHCA
|
$
|
582.8
|
|
$
|
75.0
|
|
$
|
17.1
|
|
$
|
639.1
|
|
$
|
100.6
|
|
$
|
18.1
|
|
|||||
|
CHCI
|
374.9
|
|
0.2
|
|
45.7
|
|
439.4
|
|
(396.4
|
)
|
41.2
|
|
|||||||||||
|
RX
|
217.4
|
|
88.2
|
|
22.3
|
|
248.2
|
|
91.4
|
|
25.5
|
|
|||||||||||
|
Specialty Sciences
|
—
|
|
—
|
|
—
|
|
—
|
|
(1.4
|
)
|
—
|
|
|||||||||||
|
Other
|
18.9
|
|
5.7
|
|
0.4
|
|
20.6
|
|
5.5
|
|
0.5
|
|
|||||||||||
|
Unallocated
|
—
|
|
(40.6
|
)
|
—
|
|
—
|
|
(31.3
|
)
|
—
|
|
|||||||||||
|
Total
|
$
|
1,194.0
|
|
$
|
128.5
|
|
$
|
85.5
|
|
$
|
1,347.3
|
|
$
|
(231.6
|
)
|
$
|
85.3
|
|
|||||
|
•
|
Consumer Healthcare Americas
(
"CHCA"
), comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
Consumer Healthcare International
(
"
CHCI
"
),
comprises our legacy Branded Consumer Healthcare segment and now includes our consumer focused businesses in the U.K., Australia, and Israel. This segment also includes our U.K. liquid licensed products business.
|
|
•
|
Prescription Pharmaceuticals
(
"
RX
"
),
comprises our U.S. Prescription Pharmaceuticals business.
|
|
•
|
On February 27, 2017, we announced we were exploring strategic alternatives for our Israel API operations.
|
|
•
|
On
March 27, 2017
, we announced the completed divestment of our Tysabri
®
royalty stream, effective January 1, 2017, to Royalty Pharma for up to
$2.85 billion
, which consists of
$2.2 billion
in cash and up to
$250.0 million
and
$400.0 million
in milestone payments to us if the royalties on global net sales of Tysabri
®
that are received by Royalty Pharma meet specific thresholds in 2018 and 2020, respectively. As a result of this transaction, we derecognized the Tysabri
®
financial asset and recorded a
$17.1 million
gain.
|
|
•
|
On April 6, 2017, we completed the divestment of our India API business to Strides Shasun Limited. As of December 31, 2016, the net assets of our India API business were classified as "held for sale" as discussed in
Item 1. Note 9
. The sale is not expected to have a material impact on our operations or result in a significant gain or (loss) when recorded in the second quarter of 2017.
|
|
•
|
On April 7, 2017, we issued a notice of redemption to redeem all of the
$600.0 million
in aggregate principal amount of the outstanding
2.300%
senior notes due
November 8, 2018
(the “2.300% 2018 Notes”). On May 8, 2017, using proceeds from the sale of the Tysabri
®
royalty stream, we redeemed all of the 2.300% 2018 Notes.
|
|
•
|
On May 23, 2017, we repaid the
€180.0 million
(
$196.0 million
)
4.500%
retail bonds with cash from operations.
|
|
•
|
As discussed above under 2017 Year-to-Date Highlights, on
March 27, 2017
, we announced the completed divestment of our Tysabri
®
royalty stream to Royalty Pharma.
|
|
•
|
We continue to experience a significant reduction in pricing expectations from historical levels in our
RX
segment due to industry and competitive pressures. This softness in pricing is attributed to various factors, including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future, and we are forecasting a 9% - 11% pricing decline in this segment for year ending December 31, 2017.
|
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
April 2,
2016 |
April 1,
2017 |
|||||
|
Net sales
|
$
|
1,347.3
|
|
$
|
1,194.0
|
|
|
|
Gross profit
|
$
|
533.1
|
|
$
|
464.4
|
|
|
|
Gross profit %
|
39.6%
|
38.9
|
%
|
||||
|
Operating expenses
|
$
|
764.7
|
|
$
|
335.9
|
|
|
|
Operating expenses %
|
56.8
|
%
|
28.1
|
%
|
|||
|
Operating income (loss)
|
$
|
(231.6
|
)
|
$
|
128.5
|
|
|
|
Operating income (loss) %
|
(17.2
|
)%
|
10.8
|
%
|
|||
|
Tysabri
®
royalty stream
|
$
|
204.4
|
|
$
|
(17.1
|
)
|
|
|
Interest and other, net
|
$
|
54.1
|
|
$
|
49.8
|
|
|
|
Income tax expense
|
$
|
39.1
|
|
$
|
24.2
|
|
|
|
Net income (loss)
|
$
|
(529.2
|
)
|
$
|
71.6
|
|
|
|
•
|
We have experienced a reduction in pricing expectations within our
CHCA
segment, primarily in the cough/cold, animal health, and analgesics categories due to various factors, including increased focus from customers to capture supply chain productivity savings and competition in specific product categories. We expect this pricing environment to continue to impact our
CHCA
segment for the foreseeable future.
|
|
•
|
We completed the sale of the Animal Health pet treats plant fixed assets on
February 1, 2017
and received
$7.7 million
in proceeds.
|

|
|
Three Months Ended
|
||||||
|
($ in millions)
|
April 2,
2016 |
April 1,
2017 |
|||||
|
Net sales
|
$
|
639.1
|
|
$
|
582.8
|
|
|
|
Gross profit
|
$
|
196.0
|
|
$
|
188.4
|
|
|
|
Gross profit %
|
30.7
|
%
|
32.3
|
%
|
|||
|
Operating income
|
$
|
100.6
|
|
$
|
75.0
|
|
|
|
Operating income %
|
15.7
|
%
|
12.9
|
%
|
|||
|
•
|
New product sales of
$25.3 million
related primarily to the launches of fluticasone nasal spray (store brand equivalent to Flonase
®
) and nicotine replacement gum products; more than offset by
|
|
•
|
Lower year-over-year sales of
$47.1 million
attributable to the U.S. VMS business, which was sold in August 2016;
|
|
•
|
A net decrease in sales of existing products of
$27.7 million
due to:
|
|
•
|
Strong sales in our cough/cold category and contract business; more than offset by
|
|
•
|
Pricing pressures and lower volumes in the antacid and Animal Health categories;
|
|
•
|
Lower volumes in the nicotine replacement and supply constraints in our infant nutrition category;
|
|
•
|
Discontinued products of
$5.0 million
; and
|
|
•
|
Unfavorable foreign currency movement of
$2.0 million
.
|
|
•
|
A decrease
of
$7.6 million
in gross profit due to:
|
|
•
|
Sale of the U.S. VMS business; offset partially by
|
|
•
|
Margin contributions from new products and strong performance in the cough/cold category; and
|
|
•
|
Continued manufacturing and supply chain efficiencies.
|
|
•
|
An increase of
$18.0 million
in operating expenses due to:
|
|
•
|
Increased restructuring expenses of $22.2 million related primarily to cost reduction initiatives; offset by
|
|
•
|
Decreased research and development investments of $2.4 million due to timing of clinical trials; and
|
|
•
|
Decreased selling and administrative expenses of $1.9 million.
|
|
•
|
As part of our strategic initiatives, management continues to drive improvements and evaluate the overall cost structures within our
CHCI
segment in the following ways:
|
|
•
|
On December 8, 2016, we announced the cancellation of the unprofitable EuroGenerics NV distribution agreement in Belgium. The cancellation, combined with the exit of certain OTC distribution agreements, is expected to reduce net sales by approximately $220.0 million in 2017.
|
|
•
|
We continue to make progress on our previously announced restructuring plans to right-size the
Omega
business due to the impact of market dynamics on sales volumes. Management continues to evaluate the overall cost structure relative to current and expected market dynamics. As of April 1, 2017, we recognized
$2.9 million
of restructuring expense in the
CHCI
segment.
|
|
•
|
Management continues to evaluate the most effective business model for each country and has announced strategic evaluations for Russia and Argentina.
|
|
•
|
The combination of these actions are expected to improve the segment's focus on higher value OTC products, reduce selling costs and improve operating margins in the segment.
|
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
April 2,
2016 |
April 1,
2017 |
|||||
|
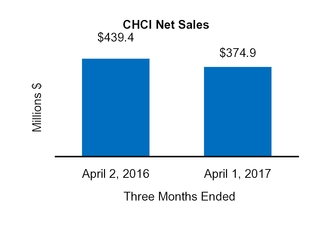
Net sales
|
$
|
439.4
|
|
$
|
374.9
|
|
|
|
Gross profit
|
$
|
199.3
|
|
$
|
169.5
|
|
|
|
Gross profit %
|
45.4%
|
45.2
|
%
|
||||
|
Operating income (loss)
|
$
|
(396.4
|
)
|
$
|
0.2
|
|
|
|
Operating income (loss) %
|
(90.2
|
)%
|
0.1
|
%
|
|||
|
•
|
New product sales of
$19.6 million
; more than offset by
|
|
•
|
The cancellation of unprofitable distribution contracts with a year-over-year impact of
$38.2 million
;
|
|
•
|
Unfavorable foreign currency movement of
$20.4 million
;
|
|
•
|
Decreased sales volumes of existing products totaling
$18.8 million
due primarily to lower sales in Germany and Belgium; and
|
|
•
|
Discontinued products of
$8.4 million
.
|
|
•
|
A decrease
of
$29.8 million
in gross profit due to:
|
|
•
|
Weaker performance in Germany and Belgium; and
|
|
•
|
Unfavorable foreign currency effect; more than offset by
|
|
•
|
A decrease
of
$426.4 million
in operating expenses primarily due to:
|
|
•
|
The absence of an intangible asset and goodwill impairment charges totaling $403.9 million recorded in the prior year period, refer to
Item 1. Note 3
for additional information; and
|
|
•
|
A decrease of advertising and promotional spending of $12.2 million due to previously announced strategic initiatives to better align promotional investments with sales.
|
|
•
|
We continue to experience a significant reduction in pricing expectations from historical levels in our
RX
segment due to industry and competitive pressures. This softness in pricing is attributed to various factors, including increased focus from customers to capture supply chain productivity savings, low raw material commodity pricing, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future and we are forecasting a 9% - 11% pricing decline in this segment for year ending December 31, 2017.
|
|
•
|
On November 10, 2016, we announced that as part of our portfolio review process we are conducting a comprehensive internal evaluation of the
RX
segment's market position, growth opportunities, and interdependencies with our manufacturing and shared service operations to determine if strategic alternatives should be explored.
|
|
•
|
During the three months ended December 31, 2016, the U.S. market for our Entocort
®
(Budesonide) capsules, including both brand and authorized generic capsules, experienced significant and unexpected increased competition, reducing our future revenue stream. We expect our 2017 net sales to be negatively effected by approximately $72.0 million.
|
|
•
|
During the three months ended April 1, 2017, we sold various ANDAs for $21.8 million.
|
|
|
Three Months Ended
|
||||||
|
($ in millions)
|
April 2,
2016 |
April 1,
2017 |
|||||
|
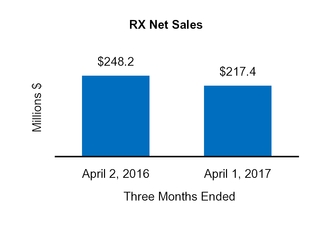
Net sales
|
$
|
248.2
|
|
$
|
217.4
|
|
|
|
Gross profit
|
$
|
127.9
|
|
$
|
96.3
|
|
|
|
Gross profit %
|
51.5
|
%
|
44.3
|
%
|
|||
|
Operating income
|
$
|
91.4
|
|
$
|
88.2
|
|
|
|
Operating income %
|
36.8
|
%
|
40.5
|
%
|
|||
|
•
|
New product sales of
$16.5 million
due primarily to Benzoyl Peroxide 5%-Clindamycin 1% gel (a generic version of Benzaclin™); more than offset by
|
|
•
|
Lower Entocort
®
sales of
$25.2 million
; and
|
|
•
|
Decreased sales of other existing products of
$22.0 million
due primarily to price erosion compared to the prior year.
|
|
•
|
A decrease
of
$31.6 million
in gross profit due primarily to the pricing pressure and lower Entocort
®
sales noted above; offset partially by
|
|
•
|
A decrease
of
$28.4 million
in operating expenses due primarily to sales of certain ANDAs for $21.8 million partially offset by $5.6 million of restructuring expenses related to the specialty pharma sales force.
|

|
|
Three Months Ended
|
||||||
|
($ in millions)
|
April 2,
2016 |
April 1,
2017 |
|||||
|
Net sales
|
$
|
20.6
|
|
$
|
18.9
|
|
|
|
Gross profit
|
$
|
9.9
|
|
$
|
10.2
|
|
|
|
Gross profit %
|
47.9
|
%
|
54.0
|
%
|
|||
|
Operating income
|
$
|
5.5
|
|
$
|
5.7
|
|
|
|
Operating income %
|
26.3
|
%
|
30.1
|
%
|
|||
|
Three Months Ended
|
||||||
|
April 2,
2016 |
April 1,
2017 |
|||||
|
$
|
31.3
|
|
$
|
40.6
|
|
|
|
Three Months Ended
|
|||||||
|
($ in millions)
|
April 2,
2016 |
April 1,
2017 |
|||||
|
Tysabri
®
royalty stream
|
$
|
204.4
|
|
$
|
(17.1
|
)
|
|
|
Interest expense, net
|
$
|
51.2
|
|
$
|
53.3
|
|
|
|
Other (income) expense, net
|
$
|
2.5
|
|
$
|
(3.5
|
)
|
|
|
Loss on extinguishment of debt
|
$
|
0.4
|
|
$
|
—
|
|
|

|
Three Months Ended
|
|||||||||||
|
|
April 2,
2016 |
April 1,
2017 |
Increase/(Decrease)
|
||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||
|
Net income (loss)
|
$
|
(529.2
|
)
|
$
|
71.6
|
|
$
|
600.8
|
|
||
|
Non-cash adjustments
|
556.2
|
|
95.8
|
|
(460.4
|
)
|
|||||
|
Subtotal
|
27.0
|
|
167.4
|
|
140.4
|
|
|||||
|
Increase (decrease) in cash due to:
|
|||||||||||
|
Accounts receivable
|
17.3
|
|
50.1
|
|
32.8
|
|
|||||
|
Inventories
|
4.4
|
|
0.5
|
|
(3.9
|
)
|
|||||
|
Accounts payable
|
(3.2
|
)
|
2.5
|
|
5.7
|
|
|||||
|
Payroll and related taxes
|
(37.4
|
)
|
(10.1
|
)
|
27.3
|
|
|||||
|
Accrued customer programs
|
(81.7
|
)
|
(32.7
|
)
|
49.0
|
|
|||||
|
Accrued liabilities
|
(12.8
|
)
|
2.3
|
|
15.1
|
|
|||||
|
Accrued income taxes
|
185.7
|
|
41.4
|
|
(144.3
|
)
|
|||||
|
Other
|
(0.8
|
)
|
(26.9
|
)
|
(26.1
|
)
|
|||||
|
Subtotal
|
$
|
71.5
|
|
$
|
27.1
|
|
$
|
(44.4
|
)
|
||
|
Net cash from (for) operating activities
|
$
|
98.5
|
|
$
|
194.5
|
|
|
$
|
96.0
|
|
|
|
•
|
Increased net earnings after adjustments for items such as deferred income taxes, impairment charges, restructuring charges, changes in the fair value of the Tysabri
®
royalty stream, and depreciation and amortization;
|
|
•
|
Changes in accrued customer-related programs due to the pricing dynamics in the
RX
segment;
|
|
•
|
Changes in accounts receivable due to timing of receipt of payments; and
|
|
•
|
Changes in payroll and related taxes due primarily to the severance expenses related to restructuring activities; offset primarily by
|
|
•
|
Changes in accrued income taxes due primarily to the effects of the annual effective tax rate computation impacts in the current quarte
r.
|

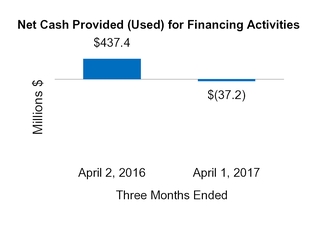
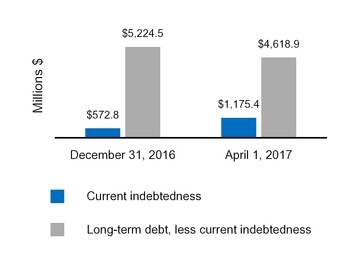
|
•
|
Review the processes and controls in place related to our application of ASC 805 to enhance the effectiveness of the design and operation of those controls to identify assets acquired and liabilities assumed; and
|
|
•
|
Evaluate and enhance management review controls related to business acquisitions.
|
|
•
|
Review the organization structure, resources, processes and controls in place to measure and record income taxes to enhance the effectiveness of the design and operation of those controls;
|
|
•
|
Evaluate the design and operating effectiveness of our controls related to income taxes for business acquisitions and non-routine transactions on an interim and annual basis;
|
|
•
|
Enhance monitoring activities related to income taxes; and
|
|
•
|
Evaluate and enhance the level of precision in the management review controls related to income taxes.
|
|
•
|
Review the design and operation of our controls related to asset group determination in our impairment process on an interim and annual basis; and
|
|
•
|
Evaluate and enhance the management review controls related to impairment
|
|
Exhibit
Number
|
Description
|
|
|
3.1
|
Certificate of Incorporation of Perrigo Company plc (formerly known as Perrigo Company Limited) (incorporated by reference from Exhibit 4.1 to the Company’s Registration Statement on Form S-8 filed on December 19, 2013).
|
|
|
3.2
|
Memorandum and Articles of Association of Perrigo Company plc, as amended (incorporated by reference from Exhibit 3.2 to the Company’s Transition Report on Form 10-KT filed on February 25, 2016).
|
|
|
10.1
|
Perrigo Company plc Change in Control Severance Policy for U.S. Employees, as amended and restated effective February 5, 2017 (filed herewith).
|
|
|
10.2
|
Perrigo Company plc U.S. Severance Policy, as amended and restated effective February 6, 2017 (filed herewith).
|
|
|
10.3
|
Form of Performance-Based Restricted Sock Unit Award Agreement under the Company's 2013 Long-term Incentive Plan (filed herewith).
|
|
|
31.1
|
Rule 13a-14(a) Certification by John T. Hendrickson, Chief Executive Officer (filed herewith).
|
|
|
31.2
|
Rule 13a-14(a) Certification by Ronald L. Winowiecki, Acting Chief Financial Officer (filed herewith).
|
|
|
32
|
Certification Pursuant to 18 United States Code 1350 and Rule 13a-14(b) of the Securities Exchange Act of 1934 (furnished herewith).
|
|
|
101.INS
|
XBRL Instance Document.
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
PERRIGO COMPANY PLC
|
|||
|
(Registrant)
|
|||
|
Date:
|
May 30, 2017
|
By: /s/ John T. Hendrickson
|
|
|
John T. Hendrickson
|
|||
|
Chief Executive Officer
|
|||
|
(Principal Executive Officer)
|
|||
|
Date:
|
May 30, 2017
|
By: /s/ Ronald L. Winowiecki
|
|
|
Ronald L. Winowiecki
|
|||
|
Acting Chief Financial Officer
|
|||
|
(Principal Accounting and Financial Officer)
|
|||