|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[X]
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Ireland
|
|
Not Applicable
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
Treasury Building, Lower Grand Canal Street, Dublin 2, Ireland
|
|
-
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Large accelerated filer
|
[X]
|
Accelerated filer
|
[ ]
|
Non-accelerated filer
|
[ ]
|
(Do not check if smaller reporting company)
|
||||
|
Smaller reporting company
|
[ ]
|
Emerging growth company
|
[ ]
|
|||||||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
|
[ ]
|
|||||||||
|
PAGE
NUMBER
|
||
|
PART I. FINANCIAL INFORMATION
|
||
|
1
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
5
|
||
|
6
|
||
|
7
|
||
|
8
|
||
|
9
|
||
|
10
|
||
|
11
|
||
|
12
|
||
|
13
|
||
|
14
|
||
|
15
|
||
|
16
|
||
|
PART II. OTHER INFORMATION
|
||
|
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||
|
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||
|
Net sales
|
$
|
1,186.4
|
|
$
|
1,237.9
|
|
$
|
2,403.4
|
|
$
|
2,431.9
|
|
|||
|
Cost of sales
|
715.4
|
|
733.3
|
|
1,439.7
|
|
1,463.0
|
|
|||||||
|
Gross profit
|
471.0
|
|
504.6
|
|
963.7
|
|
968.9
|
|
|||||||
|
Operating expenses
|
|||||||||||||||
|
Distribution
|
23.8
|
|
21.6
|
|
48.5
|
|
42.7
|
|
|||||||
|
Research and development
|
91.9
|
|
42.6
|
|
130.3
|
|
82.3
|
|
|||||||
|
Selling
|
155.2
|
|
155.6
|
|
316.5
|
|
310.6
|
|
|||||||
|
Administration
|
96.8
|
|
98.2
|
|
204.5
|
|
203.6
|
|
|||||||
|
Impairment charges
|
1.7
|
|
27.4
|
|
1.7
|
|
39.6
|
|
|||||||
|
Restructuring
|
3.7
|
|
12.1
|
|
5.2
|
|
50.8
|
|
|||||||
|
Other operating expense (income)
|
3.2
|
|
(1.7
|
)
|
6.1
|
|
(38.0
|
)
|
|||||||
|
Total operating expenses
|
376.3
|
|
355.8
|
|
712.8
|
|
691.6
|
|
|||||||
|
Operating income
|
94.7
|
|
148.8
|
|
250.9
|
|
277.3
|
|
|||||||
|
Change in financial assets
|
(0.6
|
)
|
38.7
|
|
9.0
|
|
21.6
|
|
|||||||
|
Interest expense, net
|
32.1
|
|
45.1
|
|
63.5
|
|
98.4
|
|
|||||||
|
Other expense, net
|
7.9
|
|
6.1
|
|
12.1
|
|
2.5
|
|
|||||||
|
Loss on extinguishment of debt
|
—
|
|
135.2
|
|
0.5
|
|
135.2
|
|
|||||||
|
Income (loss) before income taxes
|
55.3
|
|
(76.3
|
)
|
165.8
|
|
19.6
|
|
|||||||
|
Income tax expense (benefit)
|
19.1
|
|
(6.7
|
)
|
48.8
|
|
17.6
|
|
|||||||
|
Net income (loss)
|
$
|
36.2
|
|
$
|
(69.6
|
)
|
$
|
117.0
|
|
$
|
2.0
|
|
|||
|
Earnings (loss) per share
|
|||||||||||||||
|
Basic
|
$
|
0.26
|
|
$
|
(0.49
|
)
|
$
|
0.84
|
|
$
|
0.01
|
|
|||
|
Diluted
|
$
|
0.26
|
|
$
|
(0.49
|
)
|
$
|
0.84
|
|
$
|
0.01
|
|
|||
|
Weighted-average shares outstanding
|
|||||||||||||||
|
Basic
|
138.1
|
|
143.3
|
|
139.5
|
|
143.3
|
|
|||||||
|
Diluted
|
138.7
|
|
143.3
|
|
140.0
|
|
143.6
|
|
|||||||
|
Dividends declared per share
|
$
|
0.19
|
|
$
|
0.16
|
|
$
|
0.38
|
|
$
|
0.32
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
||||||||||||
|
Net income (loss)
|
$
|
36.2
|
|
$
|
(69.6
|
)
|
$
|
117.0
|
|
$
|
2.0
|
|
|||
|
Other comprehensive income (loss):
|
|||||||||||||||
|
Foreign currency translation adjustments
|
(165.6
|
)
|
154.7
|
|
(92.6
|
)
|
220.1
|
|
|||||||
|
Change in fair value of derivative financial instruments, net of tax
|
(3.5
|
)
|
6.9
|
|
(4.1
|
)
|
8.5
|
|
|||||||
|
Change in fair value of investment securities, net of tax
|
—
|
|
(4.8
|
)
|
—
|
|
(16.3
|
)
|
|||||||
|
Change in post-retirement and pension liability, net of tax
|
(0.2
|
)
|
—
|
|
(0.4
|
)
|
—
|
|
|||||||
|
Other comprehensive income (loss), net of tax
|
(169.3
|
)
|
156.8
|
|
(97.1
|
)
|
212.3
|
|
|||||||
|
Comprehensive income (loss)
|
$
|
(133.1
|
)
|
$
|
87.2
|
|
$
|
19.9
|
|
$
|
214.3
|
|
|||
|
June 30,
2018 |
December 31,
2017 |
||||||
|
Assets
|
|||||||
|
Cash and cash equivalents
|
$
|
526.5
|
|
$
|
678.7
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $6.5 and $6.2, respectively
|
1,129.8
|
|
1,130.8
|
|
|||
|
Inventories
|
883.8
|
|
806.9
|
|
|||
|
Prepaid expenses and other current assets
|
238.4
|
|
203.2
|
|
|||
|
Total current assets
|
2,778.5
|
|
2,819.6
|
|
|||
|
Property, plant and equipment, net
|
811.9
|
|
833.1
|
|
|||
|
Goodwill and other indefinite-lived intangible assets
|
4,227.1
|
|
4,265.7
|
|
|||
|
Other intangible assets, net
|
3,066.0
|
|
3,290.5
|
|
|||
|
Non-current deferred income taxes
|
1.0
|
|
10.4
|
|
|||
|
Other non-current assets
|
341.1
|
|
409.5
|
|
|||
|
Total non-current assets
|
8,447.1
|
|
8,809.2
|
|
|||
|
Total assets
|
$
|
11,225.6
|
|
$
|
11,628.8
|
|
|
|
Liabilities and Shareholders’ Equity
|
|||||||
|
Accounts payable
|
$
|
533.4
|
|
$
|
450.2
|
|
|
|
Payroll and related taxes
|
103.9
|
|
148.8
|
|
|||
|
Accrued customer programs
|
451.9
|
|
419.7
|
|
|||
|
Accrued liabilities
|
192.8
|
|
230.8
|
|
|||
|
Accrued income taxes
|
57.9
|
|
116.1
|
|
|||
|
Current indebtedness
|
197.7
|
|
70.4
|
|
|||
|
Total current liabilities
|
1,537.6
|
|
1,436.0
|
|
|||
|
Long-term debt, less current portion
|
3,085.3
|
|
3,270.8
|
|
|||
|
Non-current deferred income taxes
|
290.8
|
|
321.9
|
|
|||
|
Other non-current liabilities
|
414.6
|
|
429.5
|
|
|||
|
Total non-current liabilities
|
3,790.7
|
|
4,022.2
|
|
|||
|
Total liabilities
|
5,328.3
|
|
5,458.2
|
|
|||
|
Commitments and contingencies - Refer to Note 13
|
|
|
|||||
|
Shareholders’ equity
|
|||||||
|
Controlling interest:
|
|||||||
|
Preferred shares, $0.0001 par value per share, 10 shares authorized
|
—
|
|
—
|
|
|||
|
Ordinary shares, €0.001 par value per share, 10,000 shares authorized
|
7,594.3
|
|
7,892.9
|
|
|||
|
Accumulated other comprehensive income
|
155.0
|
|
253.1
|
|
|||
|
Retained earnings (accumulated deficit)
|
(1,852.2
|
)
|
(1,975.5
|
)
|
|||
|
Total controlling interest
|
5,897.1
|
|
6,170.5
|
|
|||
|
Noncontrolling interest
|
0.2
|
|
0.1
|
|
|||
|
Total shareholders’ equity
|
5,897.3
|
|
6,170.6
|
|
|||
|
Total liabilities and shareholders' equity
|
$
|
11,225.6
|
|
$
|
11,628.8
|
|
|
|
Supplemental Disclosures of Balance Sheet Information
|
|||||||
|
Ordinary shares, issued and outstanding
|
137.6
|
|
140.8
|
|
|||
|
Six Months Ended
|
|||||||
|
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Cash Flows From (For) Operating Activities
|
|||||||
|
Net income
|
$
|
117.0
|
|
$
|
2.0
|
|
|
|
Adjustments to derive cash flows
|
|||||||
|
Depreciation and amortization
|
217.8
|
|
220.8
|
|
|||
|
Share-based compensation
|
22.3
|
|
14.8
|
|
|||
|
Impairment charges
|
1.7
|
|
39.6
|
|
|||
|
Change in financial assets
|
9.0
|
|
21.6
|
|
|||
|
Loss on extinguishment of debt
|
0.5
|
|
135.2
|
|
|||
|
Restructuring charges
|
5.2
|
|
50.8
|
|
|||
|
Deferred income taxes
|
(14.2
|
)
|
(8.1
|
)
|
|||
|
Amortization of debt premium
|
(3.7
|
)
|
(11.8
|
)
|
|||
|
Other non-cash adjustments, net
|
5.1
|
|
(20.6
|
)
|
|||
|
Subtotal
|
360.7
|
|
444.3
|
|
|||
|
Increase (decrease) in cash due to:
|
|||||||
|
Accounts receivable
|
(24.3
|
)
|
51.8
|
|
|||
|
Inventories
|
(99.3
|
)
|
(4.6
|
)
|
|||
|
Accounts payable
|
89.2
|
|
(6.0
|
)
|
|||
|
Payroll and related taxes
|
(48.4
|
)
|
(37.9
|
)
|
|||
|
Accrued customer programs
|
33.9
|
|
(13.8
|
)
|
|||
|
Accrued liabilities
|
(30.4
|
)
|
(49.4
|
)
|
|||
|
Accrued income taxes
|
(20.8
|
)
|
(85.8
|
)
|
|||
|
Other, net
|
(5.9
|
)
|
(13.3
|
)
|
|||
|
Subtotal
|
(106.0
|
)
|
(159.0
|
)
|
|||
|
Net cash from operating activities
|
254.7
|
|
285.3
|
|
|||
|
Cash Flows From (For) Investing Activities
|
|||||||
|
Proceeds from royalty rights
|
10.3
|
|
85.7
|
|
|||
|
Purchase of investment securities
|
(7.5
|
)
|
—
|
|
|||
|
Proceeds from sale of securities
|
—
|
|
—
|
|
|||
|
Additions to property, plant and equipment
|
(33.3
|
)
|
(37.2
|
)
|
|||
|
Net proceeds from sale of business and other assets
|
1.3
|
|
37.2
|
|
|||
|
Proceeds from sale of the Tysabri
®
financial asset
|
—
|
|
2,200.0
|
|
|||
|
Other investing, net
|
—
|
|
(3.7
|
)
|
|||
|
Net cash from (for) investing activities
|
(29.2
|
)
|
2,282.0
|
|
|||
|
Cash Flows From (For) Financing Activities
|
|||||||
|
Issuances of long-term debt
|
431.0
|
|
—
|
|
|||
|
Payments on long-term debt
|
(457.3
|
)
|
(2,229.1
|
)
|
|||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(8.2
|
)
|
—
|
|
|||
|
Deferred financing fees
|
(2.4
|
)
|
(4.0
|
)
|
|||
|
Premium on early debt retirement
|
—
|
|
(116.1
|
)
|
|||
|
Issuance of ordinary shares
|
—
|
|
0.2
|
|
|||
|
Repurchase of ordinary shares
|
(265.0
|
)
|
(58.2
|
)
|
|||
|
Cash dividends
|
(52.8
|
)
|
(46.0
|
)
|
|||
|
Other financing, net
|
(7.5
|
)
|
4.7
|
|
|||
|
Net cash (for) financing activities
|
(362.2
|
)
|
(2,448.5
|
)
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
(15.5
|
)
|
19.7
|
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
(152.2
|
)
|
138.5
|
|
|||
|
Cash and cash equivalents, beginning of period
|
678.7
|
|
622.3
|
|
|||
|
Cash and cash equivalents, end of period
|
$
|
526.5
|
|
$
|
760.8
|
|
|
|
Recently Issued Accounting Standards Not Yet Adopted
|
||||||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial Statements or Other Significant Matters
|
|||
|
ASU 2016-02 Leases
|
This guidance was issued to increase transparency and comparability among organizations by requiring recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. For leases with a term of 12 months or less, lessees are permitted to make an election to not recognize right-of-use assets and lease liabilities. The guidance is required to be adopted using the modified retrospective approach. Early adoption is permitted.
|
January 1, 2019
|
We have completed our initial scoping reviews and assessment phase to identify our leasing processes and go-forward policy that will be impacted by the new standard. We are continuing the design phase of our new lease integration tool and expect our financial statement disclosures will be expanded to present additional details of our leasing arrangements. At this time, we are unable to reasonably estimate the expected increase in assets and liabilities on our Consolidated Balance Sheets or the impacts to our Consolidated Financial Statements upon adoption. We plan to adopt the amended guidance on the effective date, and we expect the right of use asset and corresponding liability will be material and require certain changes to our systems and processes.
|
|||
|
ASU 2018-02 Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income
|
This guidance permits tax effects stranded in accumulated other comprehensive income as a result of tax reform to be reclassified to retained earnings. This reclassification is optional and will require additional disclosure regarding whether reclassification is elected or not.
|
January 1, 2019
|
We are currently evaluating the implications of adoption on our Consolidated Financial Statements.
|
|||
|
ASU 2016-13 Measurement of Credit Losses on Financial Instruments
|
This guidance changes the impairment model for most financial assets and certain other instruments, replacing the current "incurred loss" approach with an "expected loss" credit impairment model, which will apply to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities and off-balance sheet credit exposures such as letters of credit. Early adoption is permitted.
|
January 1, 2020
|
We are currently evaluating the implications of adoption on our Consolidated Financial Statements.
|
|||
|
ASU 2017-04 Intangibles - Goodwill and Other Simplifying the Test for Goodwill
|
The objective of this update is to reduce the cost and complexity of subsequent goodwill accounting by simplifying the impairment test by removing the Step 2 requirement to perform a hypothetical purchase price allocation when the carrying value of a reporting unit exceeds its fair value. If a reporting unit’s carrying value exceeds its fair value, an entity would record an impairment charge based on that difference, limited to the amount of goodwill attributed to that reporting unit. The proposal would not change the guidance on completing Step 1 of the goodwill impairment test. The proposed guidance would be applied prospectively. Early adoption is permitted.
|
January 1, 2020
|
Upon adoption, this guidance eliminates the requirement to calculate the implied fair value of goodwill to measure a goodwill impairment. After adoption, a Step 1 failure will result in an immediate impairment charge based on the carrying value of the reporting unit. We plan to adopt the standard prospectively on the effective date.
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||
|
June 30,
2018 |
June 30,
2018 |
||||||
|
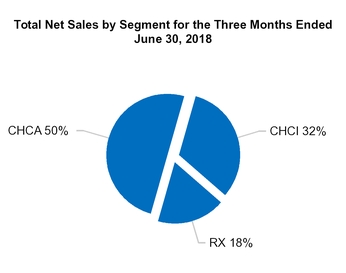
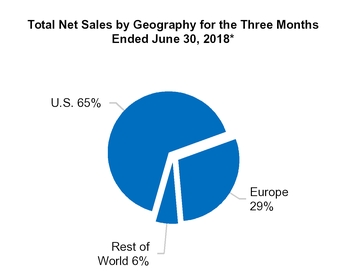
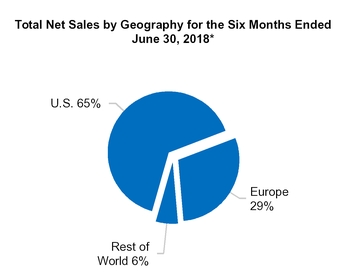
U.S.
|
$
|
772.3
|
|
$
|
1,558.7
|
|
|
|
Europe
(2)
|
344.3
|
|
706.2
|
|
|||
|
All other countries
(3)
|
69.8
|
|
138.5
|
|
|||
|
$
|
1,186.4
|
|
$
|
2,403.4
|
|
||
|
Three Months Ended
|
Six Months Ended
|
||||||
|
June 30,
2018 |
June 30,
2018 |
||||||
|
CHCA
|
|||||||
|
Cough/Cold/Allergy/Sinus
(1)
|
$
|
109.3
|
|
$
|
250.8
|
|
|
|
Infant Nutritionals
|
109.2
|
|
212.6
|
|
|||
|
Gastrointestinal
(1)
|
103.0
|
|
195.2
|
|
|||
|
Analgesics
(1)
|
92.2
|
|
185.9
|
|
|||
|
Smoking Cessation
|
71.2
|
|
137.1
|
|
|||
|
Animal Health
|
31.9
|
|
58.2
|
|
|||
|
Vitamins, Minerals and Dietary Supplements
(1)
|
4.3
|
|
7.3
|
|
|||
|
Other CHCA
(1),(2)
|
75.7
|
|
151.3
|
|
|||
|
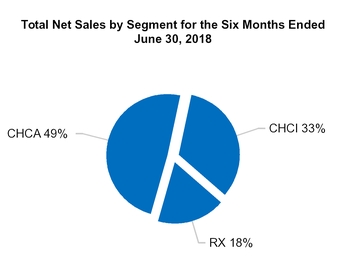
Total CHCA
|
596.8
|
|
1,198.4
|
|
|||
|
CHCI
|
|||||||
|
Lifestyle
|
86.1
|
|
175.8
|
|
|||
|
Cough, Cold, and Allergy
|
84.7
|
|
183.4
|
|
|||
|
Personal Care and Derma-Therapeutics
|
79.7
|
|
155.3
|
|
|||
|
Anti-Parasite
|
30.4
|
|
58.5
|
|
|||
|
Natural Health and Vitamins, Minerals and Dietary Supplements
|
27.9
|
|
61.1
|
|
|||
|
Other CHCI
(3)
|
72.2
|
|
148.3
|
|
|||
|
Total CHCI
|
381.0
|
|
782.4
|
|
|||
|
Total RX
|
208.6
|
|
422.6
|
|
|||
|
Total net sales
|
$
|
1,186.4
|
|
$
|
2,403.4
|
|
|
|
(2)
|
Consists primarily of branded OTC, diabetic care, diagnostic products and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the segment net sales.
|
|
(3)
|
Consists primarily of liquid licensed products, diagnostic products and other miscellaneous or otherwise uncategorized product lines and markets, none of which is greater than 10% of the segment net sales.
|
|
Balance Sheet Location
|
January 1,
2018 |
June 30,
2018 |
|||||||
|
Short-term contract assets
|
Prepaid expenses and other current assets
|
$
|
20.5
|
|
$
|
19.2
|
|
||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||||||||||
|
June 30, 2018
|
June 30, 2018
|
||||||||||||||||||||||
|
(in millions, except per share amounts)
|
As reported
|
Adjustments
|
Before adoption of ASC 606
|
As reported
|
Adjustments
|
Before adoption of ASC 606
|
|||||||||||||||||
|
Net sales
|
$
|
1,186.4
|
|
$
|
6.9
|
|
$
|
1,193.3
|
|
$
|
2,403.4
|
|
$
|
1.3
|
|
$
|
2,404.7
|
|
|||||
|
Cost of sales
|
715.4
|
|
4.2
|
|
719.6
|
|
1,439.7
|
|
1.1
|
|
1,440.8
|
|
|||||||||||
|
Gross profit
|
471.0
|
|
2.7
|
|
473.7
|
|
963.7
|
|
0.2
|
|
963.9
|
|
|||||||||||
|
Operating income
|
94.7
|
|
2.7
|
|
97.4
|
|
250.9
|
|
0.2
|
|
251.1
|
|
|||||||||||
|
Income tax expense (benefit)
|
19.1
|
|
(0.1
|
)
|
19.0
|
|
48.8
|
|
(0.1
|
)
|
48.7
|
|
|||||||||||
|
Net income
|
$
|
36.2
|
|
$
|
2.8
|
|
$
|
39.0
|
|
$
|
117.0
|
|
$
|
0.3
|
|
$
|
117.3
|
|
|||||
|
Earnings per share
|
|||||||||||||||||||||||
|
Basic
|
$
|
0.26
|
|
$
|
0.02
|
|
$
|
0.28
|
|
$
|
0.84
|
|
$
|
—
|
|
$
|
0.84
|
|
|||||
|
Diluted
|
$
|
0.26
|
|
$
|
0.02
|
|
$
|
0.28
|
|
$
|
0.84
|
|
$
|
—
|
|
$
|
0.84
|
|
|||||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||||||||||
|
June 30, 2018
|
June 30, 2018
|
||||||||||||||||||||||
|
(in millions)
|
As reported
|
Adjustments
|
Before adoption of ASC 606
|
As reported
|
Adjustments
|
Before adoption of ASC 606
|
|||||||||||||||||
|
Net income
|
$
|
36.2
|
|
$
|
2.8
|
|
$
|
39.0
|
|
$
|
117.0
|
|
$
|
0.3
|
|
$
|
117.3
|
|
|||||
|
Comprehensive income (loss)
|
$
|
(133.1
|
)
|
$
|
2.8
|
|
$
|
(130.3
|
)
|
$
|
19.9
|
|
$
|
0.3
|
|
$
|
20.2
|
|
|||||
|
June 30, 2018
|
|||||||||||
|
(in millions)
|
As reported
|
Adjustments
|
Before adoption of ASC 606
|
||||||||
|
Assets
|
|||||||||||
|
Inventories
|
$
|
883.8
|
|
$
|
13.7
|
|
$
|
897.5
|
|
||
|
Prepaid expenses and other current assets
|
238.4
|
|
(19.2
|
)
|
219.2
|
|
|||||
|
Total current assets
|
2,778.5
|
|
(5.5
|
)
|
2,773.0
|
|
|||||
|
Total assets
|
$
|
11,225.6
|
|
$
|
(5.5
|
)
|
$
|
11,220.1
|
|
||
|
Liabilities and Shareholders’ Equity
|
|||||||||||
|
Other non-current liabilities
|
$
|
414.6
|
|
$
|
(0.4
|
)
|
$
|
414.2
|
|
||
|
Total non-current liabilities
|
3,790.7
|
|
(0.4
|
)
|
3,790.3
|
|
|||||
|
Total liabilities
|
5,328.3
|
|
(0.4
|
)
|
5,327.9
|
|
|||||
|
Shareholders’ equity
|
|||||||||||
|
Controlling interest:
|
|||||||||||
|
Accumulated deficit
|
(1,852.2
|
)
|
(5.1
|
)
|
(1,857.3
|
)
|
|||||
|
Total controlling interest
|
5,897.1
|
|
(5.1
|
)
|
5,892.0
|
|
|||||
|
Total shareholders’ equity
|
5,897.3
|
|
(5.1
|
)
|
5,892.2
|
|
|||||
|
Total liabilities and shareholders' equity
|
$
|
11,225.6
|
|
$
|
(5.5
|
)
|
$
|
11,220.1
|
|
||
|
Six Months Ended
|
|||||||||||
|
June 30, 2018
|
|||||||||||
|
(in millions)
|
As reported
|
Adjustments
|
Before adoption of ASC 606
|
||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||
|
Net income
|
$
|
117.0
|
|
$
|
0.3
|
|
$
|
117.3
|
|
||
|
(Decreases) in cash due to:
|
|||||||||||
|
Inventories
|
(99.3
|
)
|
1.1
|
|
(98.2
|
)
|
|||||
|
Accrued income taxes
|
(20.8
|
)
|
(0.1
|
)
|
(20.9
|
)
|
|||||
|
Other, net
|
(5.9
|
)
|
(1.3
|
)
|
(7.2
|
)
|
|||||
|
Subtotal
|
(106.0
|
)
|
(0.3
|
)
|
(106.3
|
)
|
|||||
|
Net cash from operating activities
|
$
|
254.7
|
|
$
|
—
|
|
$
|
254.7
|
|
||
|
December 31,
2017 |
Currency translation adjustments
|
June 30,
2018 |
||||||||||
|
CHCA
|
$
|
1,847.4
|
|
$
|
(2.0
|
)
|
$
|
1,845.4
|
|
|||
|
CHCI
|
1,205.7
|
|
(30.6
|
)
|
1,175.1
|
|
||||||
|
RX
|
1,122.3
|
|
(5.3
|
)
|
1,117.0
|
|
||||||
|
Total goodwill
|
$
|
4,175.4
|
|
$
|
(37.9
|
)
|
$
|
4,137.5
|
|
|||
|
|
June 30, 2018
|
December 31, 2017
|
|||||||||||||
|
|
Gross
|
Accumulated Amortization
|
Gross
|
Accumulated Amortization
|
|||||||||||
|
Definite-lived intangibles
:
|
|||||||||||||||
|
Distribution and license agreements and supply agreements
|
$
|
310.0
|
|
$
|
188.3
|
|
$
|
311.2
|
|
$
|
169.8
|
|
|||
|
Developed product technology, formulations, and product rights
|
1,349.8
|
|
647.0
|
|
1,358.4
|
|
598.7
|
|
|||||||
|
Customer relationships and distribution networks
|
1,608.7
|
|
513.2
|
|
1,642.0
|
|
460.6
|
|
|||||||
|
Trademarks, trade names, and brands
|
1,304.3
|
|
159.8
|
|
1,335.4
|
|
129.5
|
|
|||||||
|
Non-compete agreements
|
14.5
|
|
13.0
|
|
14.7
|
|
12.6
|
|
|||||||
|
Total definite-lived intangibles
|
$
|
4,587.3
|
|
$
|
1,521.3
|
|
$
|
4,661.7
|
|
$
|
1,371.2
|
|
|||
|
Indefinite-lived intangibles
:
|
|||||||||||||||
|
Trademarks, trade names, and brands
|
$
|
51.7
|
|
$
|
—
|
|
$
|
52.1
|
|
$
|
—
|
|
|||
|
In-process research and development
|
37.9
|
|
—
|
|
38.2
|
|
—
|
|
|||||||
|
Total indefinite-lived intangibles
|
89.6
|
|
—
|
|
90.3
|
|
—
|
|
|||||||
|
Total other intangible assets
|
$
|
4,676.9
|
|
$
|
1,521.3
|
|
$
|
4,752.0
|
|
$
|
1,371.2
|
|
|||
|
June 30,
2018 |
December 31,
2017 |
||||||
|
Finished goods
|
$
|
485.7
|
|
$
|
454.3
|
|
|
|
Work in process
|
175.6
|
|
152.8
|
|
|||
|
Raw materials
|
222.5
|
|
199.8
|
|
|||
|
Total inventories
|
$
|
883.8
|
|
$
|
806.9
|
|
|
|
June 30, 2018
|
December 31, 2017
|
|||||||||||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||||||||
|
Measured at fair value on a recurring basis:
|
||||||||||||||||||||||||
|
Assets:
|
||||||||||||||||||||||||
|
Investment securities
|
$
|
8.1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
17.0
|
|
$
|
—
|
|
$
|
—
|
|
||||||
|
Foreign currency forward contracts
|
—
|
|
2.7
|
|
—
|
|
—
|
|
6.3
|
|
—
|
|
||||||||||||
|
Funds associated with Israeli severance liability
|
—
|
|
14.2
|
|
—
|
|
—
|
|
16.3
|
|
—
|
|
||||||||||||
|
Royalty Pharma contingent milestone payments
|
—
|
|
—
|
|
125.5
|
|
—
|
|
—
|
|
134.5
|
|
||||||||||||
|
Total assets
|
$
|
8.1
|
|
$
|
16.9
|
|
$
|
125.5
|
|
$
|
17.0
|
|
$
|
22.6
|
|
$
|
134.5
|
|
||||||
|
Liabilities:
|
||||||||||||||||||||||||
|
Foreign currency forward contracts
|
$
|
—
|
|
$
|
9.1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3.8
|
|
$
|
—
|
|
||||||
|
Contingent consideration
|
—
|
|
—
|
|
16.2
|
|
—
|
|
—
|
|
22.0
|
|
||||||||||||
|
Total liabilities
|
$
|
—
|
|
$
|
9.1
|
|
$
|
16.2
|
|
$
|
—
|
|
$
|
3.8
|
|
$
|
22.0
|
|
||||||
|
Measured at fair value on a non-recurring basis:
|
||||||||||||||||||||||||
|
Assets:
|
||||||||||||||||||||||||
|
Definite-lived intangible assets
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
11.5
|
|
||||||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
||||||||||||
|
Royalty Pharma Contingent Milestone Payments
|
|||||||||||||||
|
Beginning balance
|
$
|
124.9
|
|
$
|
184.5
|
|
$
|
134.5
|
|
$
|
—
|
|
|||
|
Additions
|
—
|
|
—
|
|
—
|
|
184.5
|
|
|||||||
|
Foreign currency effect
|
—
|
|
0.5
|
|
—
|
|
0.5
|
|
|||||||
|
Change in fair value
|
0.6
|
|
(39.2
|
)
|
(9.0
|
)
|
(39.2
|
)
|
|||||||
|
Ending balance
|
$
|
125.5
|
|
$
|
145.8
|
|
$
|
125.5
|
|
$
|
145.8
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
||||||||||||
|
Contingent Consideration
|
|||||||||||||||
|
Beginning balance
|
$
|
18.1
|
|
$
|
52.0
|
|
$
|
22.0
|
|
$
|
69.9
|
|
|||
|
Net realized (gains) losses
|
(1.8
|
)
|
(1.3
|
)
|
(1.4
|
)
|
(15.6
|
)
|
|||||||
|
Currency translation adjustments
|
(0.1
|
)
|
1.4
|
|
—
|
|
1.3
|
|
|||||||
|
Settlements
|
—
|
|
(2.4
|
)
|
(4.4
|
)
|
(5.9
|
)
|
|||||||
|
Ending balance
|
$
|
16.2
|
|
$
|
49.7
|
|
$
|
16.2
|
|
$
|
49.7
|
|
|||
|
June 30, 2018
|
December 31, 2017
|
||||||||||||||
|
Level 1
|
Level 2
|
Level 1
|
Level 2
|
||||||||||||
|
Public Bonds
|
|||||||||||||||
|
Carrying Value (excluding discount)
|
$
|
2,600.0
|
|
$
|
2,600.0
|
|
|||||||||
|
Fair value
|
$
|
2,525.9
|
|
$
|
2,650.8
|
|
|||||||||
|
Retail bond and private placement note
|
|||||||||||||||
|
Carrying value (excluding premium)
|
$
|
298.0
|
|
$
|
306.0
|
|
|||||||||
|
Fair value
|
$
|
326.7
|
|
$
|
342.1
|
|
|||||||||
|
Measurement Category
|
Balance Sheet Location
|
June 30,
2018 |
December 31,
2017
(2)
|
|||||||
|
Fair value method
|
Prepaid expenses and other current assets
|
$
|
8.1
|
|
$
|
17.0
|
|
|||
|
Fair value method
(1)
|
Other non-current assets
|
$
|
4.6
|
|
$
|
6.3
|
|
|||
|
Equity method
|
Other non-current assets
|
$
|
13.1
|
|
$
|
4.9
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||||
|
Measurement Category
|
Income Statement Location
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||||
|
Fair value method
|
Other expense, net
|
$
|
6.3
|
|
$
|
—
|
|
$
|
10.6
|
|
$
|
—
|
|
|||||
|
Equity method
|
Other expense, net
|
$
|
(0.9
|
)
|
$
|
(0.2
|
)
|
$
|
(0.7
|
)
|
$
|
(0.3
|
)
|
|||||
|
Asset Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
June 30,
2018 |
December 31,
2017 |
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Prepaid expenses and other current assets
|
$
|
2.1
|
|
$
|
4.1
|
|
||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Prepaid expenses and other current assets
|
$
|
0.6
|
|
$
|
2.2
|
|
||
|
Liability Derivatives
|
|||||||||
|
Balance Sheet Location
|
Fair Value
|
||||||||
|
|
June 30,
2018 |
December 31,
2017 |
|||||||
|
Designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
5.0
|
|
$
|
1.4
|
|
||
|
Non-designated derivatives:
|
|||||||||
|
Foreign currency forward contracts
|
Accrued liabilities
|
$
|
4.1
|
|
$
|
2.4
|
|
||
|
Amount of Gain/(Loss) Recorded in OCI
(Effective Portion) |
||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
Designated Cash Flow Hedges
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
||||||||||||
|
Foreign currency forward contracts
|
$
|
(4.3
|
)
|
$
|
2.7
|
|
$
|
(4.3
|
)
|
$
|
5.2
|
|
||||
|
Amount of Gain/(Loss) Reclassified from AOCI into Earnings
(Effective Portion) |
||||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||||
|
Designated Cash Flow Hedges
|
Income Statement Location
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||||
|
Interest rate swap agreements
|
Interest expense, net
|
$
|
(0.4
|
)
|
$
|
(0.6
|
)
|
$
|
(0.9
|
)
|
$
|
(1.3
|
)
|
|||||
|
Other expense, net
|
—
|
|
(5.9
|
)
|
—
|
|
(5.9
|
)
|
||||||||||
|
Foreign currency forward contracts
|
Net sales
|
—
|
|
0.6
|
|
—
|
|
0.9
|
|
|||||||||
|
Cost of sales
|
1.6
|
|
0.9
|
|
3.9
|
|
1.6
|
|
||||||||||
|
Interest expense, net
|
(1.1
|
)
|
(0.5
|
)
|
(2.0
|
)
|
(1.1
|
)
|
||||||||||
|
Other expense, net
|
(0.1
|
)
|
—
|
|
(0.5
|
)
|
(0.5
|
)
|
||||||||||
|
Total
|
$
|
—
|
|
$
|
(5.5
|
)
|
$
|
0.5
|
|
$
|
(6.3
|
)
|
||||||
|
Amount of Gain/(Loss) Recognized in Earnings
(Ineffective Portion) |
||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||
|
Designated Cash Flow Hedges
|
Income Statement
Location
|
July 1,
2017 |
July 1,
2017 |
|||||||
|
Foreign currency forward contracts
|
Other expense, net
|
$
|
0.1
|
|
$
|
1.0
|
|
|||
|
Amount of Gain/(Loss) Recognized against Earnings
|
||||||||||||||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||||
|
Non-Designated Derivatives
|
Income Statement Location
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||||
|
Foreign currency forward contracts
|
Other expense, net
|
$
|
11.8
|
|
$
|
(5.0
|
)
|
$
|
8.6
|
|
$
|
(13.9
|
)
|
|||||
|
Interest expense, net
|
0.2
|
|
(0.7
|
)
|
(0.7
|
)
|
(1.1
|
)
|
||||||||||
|
Total
|
$
|
12.0
|
|
$
|
(5.7
|
)
|
$
|
7.9
|
|
$
|
(15.0
|
)
|
||||||
|
June 30,
2018 |
December 31,
2017 |
||||||||||
|
Term loans
|
|||||||||||
|
2018 Term loan due March 8, 2020
(1)
|
$
|
383.5
|
|
$
|
—
|
|
|||||
|
2014 Term loan due December 5, 2019
(1)
|
—
|
|
420.0
|
|
|||||||
|
Total term loans
|
383.5
|
|
420.0
|
|
|||||||
|
Notes and Bonds
|
|||||||||||
|
Coupon
|
Due
|
||||||||||
|
5.000%
|
May 23, 2019
(1)
|
140.2
|
|
144.0
|
|
||||||
|
3.500%
|
March 15, 2021
|
280.4
|
|
280.4
|
|
||||||
|
3.500%
|
December 15, 2021
|
309.6
|
|
309.6
|
|
||||||
|
5.105%
|
July 19, 2023
(1)
|
157.8
|
|
162.0
|
|
||||||
|
4.000%
|
November 15, 2023
|
215.6
|
|
215.6
|
|
||||||
|
3.900%
|
December 15, 2024
|
700.0
|
|
700.0
|
|
||||||
|
4.375%
|
March 15, 2026
|
700.0
|
|
700.0
|
|
||||||
|
5.300%
|
November 15, 2043
|
90.5
|
|
90.5
|
|
||||||
|
4.900%
|
December 15, 2044
|
303.9
|
|
303.9
|
|
||||||
|
Total notes and bonds
|
2,898.0
|
|
2,906.0
|
|
|||||||
|
Other financing
|
3.0
|
|
11.7
|
|
|||||||
|
Unamortized premium (discount), net
|
16.6
|
|
21.4
|
|
|||||||
|
Deferred financing fees
|
(18.1
|
)
|
(17.9
|
)
|
|||||||
|
Total borrowings outstanding
|
3,283.0
|
|
3,341.2
|
|
|||||||
|
Current indebtedness
|
(197.7
|
)
|
(70.4
|
)
|
|||||||
|
Total long-term debt less current portion
|
$
|
3,085.3
|
|
$
|
3,270.8
|
|
|||||
|
(1)
|
Debt denominated in euros subject to fluctuations in the euro-to-U.S. dollar exchange rate.
|
|
Premium on debt repayment
|
$
|
116.1
|
|
|
|
Transaction costs
|
3.8
|
|
||
|
Write-off of deferred financing fees
|
10.6
|
|
||
|
Write-off of remaining discount on bond
|
4.7
|
|
||
|
Total loss on extinguishment of debt
|
$
|
135.2
|
|
|
|
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||
|
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||
|
Numerator:
|
|||||||||||||||
|
Net income (loss)
|
$
|
36.2
|
|
$
|
(69.6
|
)
|
$
|
117.0
|
|
$
|
2.0
|
|
|||
|
Denominator:
|
|||||||||||||||
|
Weighted average shares outstanding for basic EPS
|
138.1
|
|
143.3
|
|
139.5
|
|
143.3
|
|
|||||||
|
Dilutive effect of share-based awards*
|
0.6
|
|
—
|
|
0.5
|
|
0.3
|
|
|||||||
|
Weighted average shares outstanding for diluted EPS
|
138.7
|
|
143.3
|
|
140.0
|
|
143.6
|
|
|||||||
|
Anti-dilutive share-based awards excluded from computation of diluted EPS
|
1.7
|
|
—
|
|
0.9
|
|
0.8
|
|
|||||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||
|
74,000
|
|
31,900
|
|
127,000
|
|
46,400
|
|
|||
|
Fair value of derivative financial instruments, net of tax
|
Foreign currency translation adjustments
|
Fair value of investment securities, net of tax
|
Post-retirement and pension liability adjustments, net of tax
|
Total AOCI
|
|||||||||||||||
|
Balance at December 31, 2017
|
$
|
(9.8
|
)
|
$
|
260.6
|
|
$
|
1.0
|
|
$
|
1.3
|
|
$
|
253.1
|
|
||||
|
ASU 2016-01 adoption impact
|
—
|
|
—
|
|
(1.0
|
)
|
—
|
|
(1.0
|
)
|
|||||||||
|
Balance at December 31, 2017 after adoption impact
|
$
|
(9.8
|
)
|
$
|
260.6
|
|
|
$
|
—
|
|
|
$
|
1.3
|
|
|
$
|
252.1
|
|
|
|
OCI before reclassifications
|
(3.6
|
)
|
(92.6
|
)
|
—
|
|
(0.4
|
)
|
(96.6
|
)
|
|||||||||
|
Amounts reclassified from AOCI
|
(0.5
|
)
|
—
|
|
—
|
|
—
|
|
(0.5
|
)
|
|||||||||
|
Other comprehensive income
|
$
|
(4.1
|
)
|
$
|
(92.6
|
)
|
$
|
—
|
|
$
|
(0.4
|
)
|
$
|
(97.1
|
)
|
||||
|
Balance at June 30, 2018
|
$
|
(13.9
|
)
|
$
|
168.0
|
|
$
|
—
|
|
$
|
0.9
|
|
$
|
155.0
|
|
||||
|
Three Months Ended
|
Six Months Ended
|
|||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||
|
34.5
|
%
|
8.7
|
%
|
29.4
|
%
|
89.9
|
%
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
||||||||||||
|
Beginning balance
|
$
|
12.4
|
|
$
|
51.5
|
|
$
|
21.4
|
|
$
|
19.7
|
|
|||
|
Additional charges
|
3.7
|
|
12.1
|
|
5.2
|
|
50.8
|
|
|||||||
|
Payments
|
(3.1
|
)
|
(23.6
|
)
|
(13.8
|
)
|
(30.7
|
)
|
|||||||
|
Non-cash adjustments
|
(0.3
|
)
|
(0.3
|
)
|
(0.1
|
)
|
(0.1
|
)
|
|||||||
|
Ending balance
|
$
|
12.7
|
|
$
|
39.7
|
|
$
|
12.7
|
|
$
|
39.7
|
|
|||
|
•
|
Consumer Healthcare Americas
, comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
Consumer Healthcare International
,
comprises our branded consumer healthcare business primarily in Europe and our consumer focused businesses in the United Kingdom, Australia, and Israel. This segment also includes our U.K. liquid licensed products business.
|
|
•
|
Prescription Pharmaceuticals
,
comprises our U.S. Prescription Pharmaceuticals business.
|
|
Total Assets
|
||||||||
|
June 30,
2018 |
December 31,
2017 |
|||||||
|
CHCA
|
$
|
3,673.0
|
|
$
|
3,786.8
|
|
||
|
CHCI
|
4,799.4
|
|
5,029.0
|
|
||||
|
RX
|
2,753.2
|
|
2,813.0
|
|
||||
|
Total
|
$
|
11,225.6
|
|
$
|
11,628.8
|
|
||
|
Three Months Ended
|
|||||||||||||||||||||||
|
June 30, 2018
|
July 1, 2017
|
||||||||||||||||||||||
|
Net
Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
Net
Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
||||||||||||||||||
|
CHCA
|
$
|
596.8
|
|
$
|
57.4
|
|
$
|
15.3
|
|
$
|
604.9
|
|
$
|
104.2
|
|
$
|
17.0
|
|
|||||
|
CHCI
|
381.0
|
|
5.6
|
|
49.5
|
|
376.5
|
|
3.9
|
|
47.5
|
|
|||||||||||
|
RX
|
208.6
|
|
56.9
|
|
20.5
|
|
240.4
|
|
69.3
|
|
22.3
|
|
|||||||||||
|
Other
|
—
|
|
—
|
|
—
|
|
16.1
|
|
4.1
|
|
0.4
|
|
|||||||||||
|
Unallocated
|
—
|
|
(25.2
|
)
|
—
|
|
—
|
|
(32.7
|
)
|
—
|
|
|||||||||||
|
Total
|
$
|
1,186.4
|
|
$
|
94.7
|
|
$
|
85.3
|
|
$
|
1,237.9
|
|
$
|
148.8
|
|
$
|
87.2
|
|
|||||
|
Six Months Ended
|
|||||||||||||||||||||||
|
June 30, 2018
|
July 1, 2017
|
||||||||||||||||||||||
|
Net
Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
Net
Sales
|
Operating Income (Loss)
|
Intangible Asset Amortization
|
||||||||||||||||||
|
CHCA
|
$
|
1,198.4
|
|
$
|
170.5
|
|
$
|
30.5
|
|
$
|
1,187.6
|
|
$
|
179.2
|
|
$
|
34.2
|
|
|||||
|
CHCI
|
782.4
|
|
20.4
|
|
100.8
|
|
751.5
|
|
4.2
|
|
93.2
|
|
|||||||||||
|
RX
|
422.6
|
|
118.7
|
|
41.1
|
|
457.8
|
|
157.5
|
|
44.6
|
|
|||||||||||
|
Other
|
—
|
|
—
|
|
—
|
|
35.0
|
|
9.7
|
|
0.8
|
|
|||||||||||
|
Unallocated
|
—
|
|
(58.7
|
)
|
—
|
|
—
|
|
(73.3
|
)
|
—
|
|
|||||||||||
|
Total
|
$
|
2,403.4
|
|
$
|
250.9
|
|
$
|
172.4
|
|
$
|
2,431.9
|
|
$
|
277.3
|
|
$
|
172.8
|
|
|||||
|
•
|
Consumer Healthcare Americas
(
"CHCA"
), comprises our U.S., Mexico and Canada consumer healthcare business (OTC, contract, infant formula and animal health categories).
|
|
•
|
Consumer Healthcare International
(
"
CHCI
"
),
comprises our branded consumer healthcare business primarily in Europe and our consumer focused businesses in the United Kingdom, Australia, and Israel. This segment also includes our U.K. liquid licensed products business.
|
|
•
|
Prescription Pharmaceuticals
(
"
RX
"
),
comprises our U.S. Prescription Pharmaceuticals business.
|
|
•
|
On January 1, 2018, we adopted Accounting Standard Updates ("ASU") 2014-09 Revenue from Contracts with Customers and its related amendments (collectively, "ASC 606") using the modified retrospective method. The adoption of ASC 606 represents a change in accounting principle that will more closely align revenue recognition with the transfer of control of products to our customers and will provide financial statement readers with enhanced disclosures (refer to
Item 1. Note 2
).
|
|
•
|
On January 1, 2018, we adopted ASU 2016-01 Financial Instruments - Recognition and Measurement of Financial Assets and Liabilities (refer to
Item 1. Note 7
and
Note 11
).
|
|
•
|
During the
six months ended
June 30, 2018
, we repurchased
$265.0 million
worth of shares as part of our authorized share repurchase plan (refer to
Item 1. Note 10
).
|
|
•
|
On August 9, 2018, we announced a plan to separate our RX business. We intend to begin the preparations for a spin-off of the RX business to shareholders and will also remain open to other value-enhancing options, including a possible sale, merger or other form of separation. We believe the separation, which we currently expect to be completed during the second half of 2019, will enable us to focus on expanding our leading consumer businesses. We anticipate incurring significant costs in connection with the proposed separation, which we cannot quantify until the form of separation is determined.
|
|
•
|
We are performing a growth-driven exercise focused around our core competencies. We are measuring potential business opportunities, including organic and inorganic growth prospects against our strengths and capabilities, as well as identifying gaps and areas for improvement. Following our assessment, we will prioritize potential investment opportunities across the organization and solidify our strategic road-map. Through this exercise, we may further refine our portfolio and the necessary capital resources deployed to deliver consistent shareholder returns.
|
|
|
Three Months Ended
|
||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
1,186.4
|
|
$
|
1,237.9
|
|
|
|
Gross profit
|
$
|
471.0
|
|
$
|
504.6
|
|
|
|
Gross profit %
|
39.7
|
%
|
40.8
|
%
|
|||
|
Operating expenses
|
$
|
376.3
|
|
$
|
355.8
|
|
|
|
Operating expenses %
|
31.7
|
%
|
28.7
|
%
|
|||
|
Operating income
|
$
|
94.7
|
|
$
|
148.8
|
|
|
|
Operating income %
|
8.0
|
%
|
12.0
|
%
|
|||
|
•
|
A net decrease in sales of existing products of
$72.7 million
;
|
|
•
|
The absence of sales of discontinued products of
$16.7 million
;
|
|
•
|
The absence of Active Pharmaceuticals Ingredient ("API") business sales of
$16.1 million
in the former Other segment; and
|
|
•
|
The absence of sales attributable to the exited Russian business and prior year distribution phase out initiatives of
$7.3 million
in the CHCI segment; partially offset by
|
|
•
|
New product sales of
$42.3 million
; and
|
|
•
|
Favorable foreign currency translation of
$19.0 million
.
|
|
•
|
A decrease
in gross profit of
$33.6 million
due primarily to the decrease in net sales and pricing pressure, primarily in the RX segment.
|
|
•
|
An increase
in operating expenses of
$20.5 million
due primarily to:
|
|
•
|
A
$50.0 million
payment to enter into a license agreement with Merck Sharp & Dohme Corp. ("Merck") in our CHCA segment, offset partially by
|
|
•
|
The absence of $23.3 million of impairment charges related to certain definite-lived intangible assets in our RX segment and the Russian business in our CHCI segment in the prior year period; and
|
|
•
|
Decreased
Restructuring
expense of $8.4 million related to the cost reduction initiatives taken in the prior year period.
|
|
|
Six Months Ended
|
||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
2,403.4
|
|
$
|
2,431.9
|
|
|
|
Gross profit
|
$
|
963.7
|
|
$
|
968.9
|
|
|
|
Gross profit %
|
40.1
|
%
|
39.8
|
%
|
|||
|
Operating expenses
|
$
|
712.8
|
|
$
|
691.6
|
|
|
|
Operating expenses %
|
29.7
|
%
|
28.4
|
%
|
|||
|
Operating income
|
$
|
250.9
|
|
$
|
277.3
|
|
|
|
Operating income %
|
10.4
|
%
|
11.4
|
%
|
|||
|
•
|
A net decrease in sales of existing products of
$86.4 million
;
|
|
•
|
The absence of API business sales of
$35.0 million
in the former Other segment;
|
|
•
|
The absence of sales attributable to the exited Russian business and prior year distribution phase out initiatives of
$29.0 million
in the CHCI segment; and
|
|
•
|
The absence of sales of discontinued products of
$25.0 million
, partially offset by
|
|
•
|
New product sales of
$83.5 million
; and
|
|
•
|
Favorable foreign currency translation of
$63.5 million
.
|
|
•
|
A decrease
in gross profit of
$5.2 million
.
|
|
•
|
An increase
in operating expenses of
$21.2 million
due primarily to:
|
|
•
|
A
$50.0 million
payment to enter into a license agreement with Merck in our CHCA segment;
|
|
•
|
The absence of $22.9 million in gains in the prior year period related to certain Abbreviated New Drug Applications ("ANDAs") in our RX segment; and
|
|
•
|
Increased acquisition-related charges and contingent consideration adjustments of $22.5 million; partially offset by
|
|
•
|
Decreased
Restructuring
expense of $45.6 million related to the cost reduction initiatives taken in the prior year period; and
|
|
•
|
The absence of $30.7 million of impairment charges related to certain In-process Research and Development ("IPR&D") and definite-lived intangible assets in our RX segment in the prior year period.
|
|
•
|
On
May 29, 2018
, we entered into a license agreement with Merck that will allow us to develop and commercialize an OTC version of Nasonex-branded products, as well as other products containing the same active ingredient. In connection with this license agreement, we paid an upfront license fee of
$50.0 million
. In addition, if we achieve certain development milestones, we will make future milestone and royalty payments (refer to
Item 1. Note 3
).
|
|
|
Three Months Ended
|
||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
596.8
|
|
$
|
604.9
|
|
|
|
Gross profit
|
$
|
195.3
|
|
$
|
203.8
|
|
|
|
Gross profit %
|
32.7
|
%
|
33.7
|
%
|
|||
|
Operating income
|
$
|
57.4
|
|
$
|
104.2
|
|
|
|
Operating income %
|
9.6
|
%
|
17.2
|
%
|
|||
|
•
|
A net decrease in sales of existing products of
$18.3 million
due primarily to:
|
|
•
|
Lower sales in our animal health and smoking cessation categories; and
|
|
•
|
Ongoing pricing pressure, which we expect to continue for the foreseeable future; partially offset by
|
|
•
|
Higher sales volumes in our cough/cold/allergy/sinus category and infant formula business;
|
|
•
|
The absence of sales of discontinued products of
$4.0 million
; and
|
|
•
|
The effect of unfavorable foreign currency translation of
$1.1 million
; partially offset by
|
|
•
|
New product sales of
$15.3 million
related primarily to esomeprazole magnesium (store brand equivalent to Nexium
®
24HR capsules) and infant formula products.
|
|
•
|
A decrease
of
$8.5 million
in gross profit due primarily to the decrease in net sales and higher cost of sales.
|
|
•
|
An increase
of
$38.3 million
in operating expenses due primarily to:
|
|
•
|
Increased
Research and development
("R&D") expense of $48.4 million related primarily to the
$50.0 million
payment to enter into a license agreement with Merck; partially offset by
|
|
•
|
Decreased
Restructuring
expense of $4.3 million related to the cost reduction initiatives taken in the prior year period;
|
|
•
|
Absence of fixed asset impairments in the prior year period of $4.1 million; and
|
|
•
|
Decreased
Administration
expense of $3.6 million due primarily to decreased legal fees.
|
|
|
|||||||
|
Six Months Ended
|
|||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
1,198.4
|
|
$
|
1,187.6
|
|
|
|
Gross profit
|
$
|
395.7
|
|
$
|
392.1
|
|
|
|
Gross profit %
|
33.0
|
%
|
33.0
|
%
|
|||
|
Operating income
|
$
|
170.5
|
|
$
|
179.2
|
|
|
|
Operating income %
|
14.2
|
%
|
15.1
|
%
|
|||
|
•
|
New product sales of
$26.5 million
related primarily to the launches of esomeprazole magnesium (store brand equivalent to Nexium
®
24HR capsules) and infant formula products; partially offset by
|
|
•
|
A net decrease in sales of existing products of
$9.9 million
due to:
|
|
•
|
Higher sale volumes in our cough/cold/allergy/sinus and infant nutrition categories; more than offset by
|
|
•
|
Lower sales in our animal health and smoking cessation categories; and
|
|
•
|
Ongoing pricing pressure, which we expect to continue for the foreseeable future; and
|
|
•
|
The absence of sales of discontinued products of
$5.9 million
.
|
|
•
|
An increase
of
$3.6 million
in gross profit due primarily to the increase in net sales.
|
|
•
|
An increase
of
$12.3 million
in operating expenses due primarily to:
|
|
•
|
Increased R&D expense of $47.0 million related primarily to the
$50.0 million
payment to enter into the license agreement with Merck; partially offset by
|
|
•
|
Decreased
Restructuring
expense of $27.6 million related to the cost reduction initiatives taken in the prior year period;
|
|
•
|
Decreased
Selling
and
Administration
expense of $4.4 million due primarily to decreased employee-related costs and decreased selling expense in our animal health category; and
|
|
•
|
Absence of fixed asset impairments in the prior year period of $4.1 million.
|
|
|
Three Months Ended
|
||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
381.0
|
|
$
|
376.5
|
|
|
|
Gross profit
|
$
|
181.2
|
|
$
|
174.0
|
|
|
|
Gross profit %
|
47.5
|
%
|
46.2
|
%
|
|||
|
Operating income
|
$
|
5.6
|
|
$
|
3.9
|
|
|
|
Operating income %
|
1.5
|
%
|
1.0
|
%
|
|||
|
•
|
The effect of favorable foreign currency translation of
$20.1 million
; and
|
|
•
|
New product sales of
$19.2 million
; partially offset by
|
|
•
|
A net decrease in sales of existing products of
$19.8 million
due primarily to lower sales in the analgesics, anti-parasite and lifestyle categories; partially offset by higher sales volumes in diagnostic products;
|
|
•
|
The absence of sales of discontinued products of
$7.7 million
; and
|
|
•
|
The absence of
$7.3 million
in sales attributable to the exited Russian business and prior year distribution phase out initiatives.
|
|
•
|
An increas
e
of
$7.2 million
in gross profit due primarily to:
|
|
•
|
Increased net sales and benefits from continued insourcing initiatives; and
|
|
•
|
The effect of favorable foreign currency translation.
|
|
•
|
An increase
of
$5.5 million
in operating expenses due primarily to:
|
|
•
|
Increased
Selling
and
Administration
expense of $11.9 million due primarily to the effect of unfavorable foreign currency translation; offset by
|
|
•
|
Decreased
Restructuring
expense of $6.2 million related to the cost reduction initiatives taken in the prior year.
|
|
•
|
Management continues to implement its previously disclosed strategy for brand prioritization, sales force restructuring, and manufacturing in-sourcing, which is expected to reduce selling costs, improve operating margins and focus on higher value OTC products.
|
|
Six Months Ended
|
|||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
782.4
|
|
$
|
751.5
|
|
|
|
Gross profit
|
$
|
375.7
|
|
$
|
343.5
|
|
|
|
Gross profit %
|
48.0
|
%
|
45.7
|
%
|
|||
|
Operating income
|
$
|
20.4
|
|
$
|
4.2
|
|
|
|
Operating income %
|
2.6
|
%
|
0.6
|
%
|
|||
|
•
|
The effect of favorable foreign currency translation of
$63.3 million
; and
|
|
•
|
New product sales of
$39.5 million
; partially offset by
|
|
•
|
The absence of
$29.0 million
in sales attributable to the exited Russian business and prior year distribution phase out initiatives;
|
|
•
|
A decrease in sales of existing products of
$29.5 million
due primarily to lower sales in the cough/cold/allergy and lifestyle categories, partially offset by improved pricing on certain products; and
|
|
•
|
The absence of sales of discontinued products of
$13.3 million
.
|
|
•
|
An increase
of
$32.2 million
in gross profit due primarily to:
|
|
•
|
Increased net sales and benefits from continued insourcing initiatives; and
|
|
•
|
The effect of favorable foreign currency translation.
|
|
•
|
An increase
of
$16.0 million
in operating expenses due primarily to:
|
|
•
|
Increased
Selling
and
Administration
expense of $23.2 million due primarily to the effect of unfavorable foreign currency translation; and
|
|
•
|
Increased distribution expense of $3.9 million due primarily to a new distribution center and the effect of unfavorable foreign currency translation; offset by
|
|
•
|
Decreased
Restructuring
expense of $8.5 million related to the cost reduction initiatives taken in the prior year; and
|
|
•
|
The absence of a $3.7 million impairment charge related to the Russian business in the prior year period.
|
|
•
|
We continue to experience a significant year-over-year reduction in pricing in our
RX
segment due to competitive pressures. This softness in pricing is attributable to various factors, including increased focus from customers to capture supply chain productivity savings, competition in specific products, and consolidation of certain customers. We expect this softness to continue to impact the segment for the foreseeable future and we are forecasting a 10%-12% pricing decline in this segment for the year ending December 31, 2018.
|
|
•
|
On August 9, 2018, we announced a plan to separate our RX business. We intend to begin the preparations for a spin-off of the RX business to shareholders and will also remain open to other value-enhancing options, including a possible sale, merger or other form of separation. We currently expect the separation of the RX business to be completed during the second half of 2019.
|
|
|
Three Months Ended
|
||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
208.6
|
|
$
|
240.4
|
|
|
|
Gross profit
|
$
|
94.5
|
|
$
|
119.1
|
|
|
|
Gross profit %
|
45.3
|
%
|
49.6
|
%
|
|||
|
Operating income
|
$
|
56.9
|
|
$
|
69.3
|
|
|
|
Operating income %
|
27.3
|
%
|
28.8
|
%
|
|||
|
•
|
Decreased sales of existing products of
$34.6 million
due primarily to pricing pressure; and
|
|
•
|
The absence of sales of discontinued products of
$5.1 million
; offset partially by
|
|
•
|
New product sales of
$7.8 million
due primarily to sales of testosterone 2% topical (generic equivalent to Axiron
®
).
|
|
•
|
A decrease
of
$24.6 million
in gross profit due primarily to lower sales and pricing pressure.
|
|
•
|
A decrease
of
$12.2 million
in operating expenses due primarily to:
|
|
•
|
The absence of $19.6 million of impairment charges related to certain definite-lived intangible assets in the prior year period; offset partially by
|
|
•
|
An increase of $4.5 million in acquisition-related charges and contingent consideration adjustments.
|
|
|
|||||||
|
Six Months Ended
|
|||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
|||||
|
Net sales
|
$
|
422.6
|
|
$
|
457.8
|
|
|
|
Gross profit
|
$
|
192.3
|
|
$
|
215.4
|
|
|
|
Gross profit %
|
45.5
|
%
|
47.0
|
%
|
|||
|
Operating income
|
$
|
118.7
|
|
$
|
157.5
|
|
|
|
Operating income %
|
28.1
|
%
|
34.4
|
%
|
|||
|
•
|
Decreased sales of existing products of
$47.0 million
due primarily to pricing pressure and decreased sales volume of certain products; and
|
|
•
|
The absence of sales of discontinued products of
$5.7 million
; partially offset by
|
|
•
|
New product sales of
$17.5 million
due primarily to sales of testosterone 2% topical (generic equivalent to Axiron
®
).
|
|
•
|
A decrease
of
$23.1 million
in gross profit due primarily to lower net sales and pricing pressure.
|
|
•
|
An increase
of
$15.7 million
in operating expenses due primarily to:
|
|
•
|
The absence of the following:
|
|
•
|
Gain on the sale of certain ANDAs of $22.9 million;
|
|
•
|
Gain related to contingent consideration of $14.8 million; offset partially by
|
|
•
|
An impairment charge related to certain IPR&D assets of $11.1 million; and
|
|
•
|
An impairment charge related to certain definite-lived intangible assets of $19.6 million in the prior year period; and
|
|
•
|
An increase of $2.9 million in R&D expense due primarily to the timing of clinical studies; and
|
|
•
|
Expenses related to acquisition-related charges and contingent consideration adjustments of $8.8 million; partially offset by
|
|
•
|
Decreased
Restructuring
expense of $5.6 million due to the cost reduction initiatives taken in the prior year period.
|
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||
|
$
|
25.2
|
|
$
|
31.9
|
|
$
|
58.7
|
|
$
|
72.5
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
||||||||||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||||||
|
Change in financial assets
|
$
|
(0.6
|
)
|
$
|
38.7
|
|
$
|
9.0
|
|
$
|
21.6
|
|
|||
|
Interest expense, net
|
$
|
32.1
|
|
$
|
45.1
|
|
$
|
63.5
|
|
$
|
98.4
|
|
|||
|
Other expense, net
|
$
|
7.9
|
|
$
|
6.1
|
|
$
|
12.1
|
|
$
|
2.5
|
|
|||
|
Three Months Ended
|
Six Months Ended
|
|||||||||
|
June 30,
2018 |
July 1,
2017 |
June 30,
2018 |
July 1,
2017 |
|||||||
|
34.5
|
%
|
8.7
|
%
|
29.4
|
%
|
89.9
|
%
|
|||
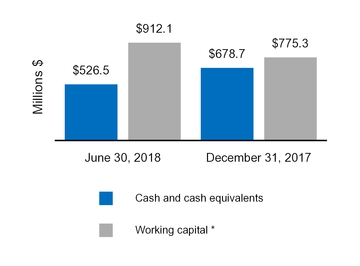
|
Six Months Ended
|
|||||||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
Increase/(Decrease)
|
||||||||
|
Cash Flows From (For) Operating Activities
|
|||||||||||
|
Net income
|
$
|
117.0
|
|
$
|
2.0
|
|
$
|
115.0
|
|
||
|
Non-cash adjustments
|
243.7
|
|
442.3
|
|
(198.6
|
)
|
|||||
|
Subtotal
|
360.7
|
|
444.3
|
|
(83.6
|
)
|
|||||
|
Increase (decrease) in cash due to:
|
|||||||||||
|
Accounts receivable
|
(24.3
|
)
|
51.8
|
|
(76.1
|
)
|
|||||
|
Inventories
|
(99.3
|
)
|
(4.6
|
)
|
(94.7
|
)
|
|||||
|
Accounts payable
|
89.2
|
|
(6.0
|
)
|
95.2
|
|
|||||
|
Payroll and related taxes
|
(48.4
|
)
|
(37.9
|
)
|
(10.5
|
)
|
|||||
|
Accrued customer programs
|
33.9
|
|
(13.8
|
)
|
47.7
|
|
|||||
|
Accrued liabilities
|
(30.4
|
)
|
(49.4
|
)
|
19.0
|
|
|||||
|
Accrued income taxes
|
(20.8
|
)
|
(85.8
|
)
|
65.0
|
|
|||||
|
Other, net
|
(5.9
|
)
|
(13.3
|
)
|
7.4
|
|
|||||
|
Subtotal
|
$
|
(106.0
|
)
|
$
|
(159.0
|
)
|
$
|
53.0
|
|
||
|
Net cash from operating activities
|
$
|
254.7
|
|
$
|
285.3
|
|
$
|
(30.6
|
)
|
||
|
•
|
Decreased net income after non-cash adjustments;
|
|
•
|
Changes in inventory due primarily to increased purchasing and manufacturing volumes related to a strategic build-up of inventories in our CHCA segment and insourcing initiatives in our CHCI segment;
|
|
•
|
Changes in accounts receivable due primarily to the timing of sales and receipt of payments, and the discontinuation of our Belgium accounts receivable factoring program; and
|
|
•
|
Changes in payroll and related taxes due primarily to the timing of annual management and employee bonus payouts; partially offset by
|
|
•
|
Changes in accounts payable due primarily to the timing of payments, mix of payment terms, and an increase in inventory;
|
|
•
|
Changes in accrued income taxes due primarily to Federal tax obligation payments made in the current year period, offset by expected tax refunds (refer to
Item 1. Note 12
);
|
|
•
|
Changes in accrued customer-related programs due primarily to new product launches, which resulted in higher customer related-accruals, pricing dynamics in the RX segment, and the timing of rebate payments; and
|
|
•
|
Changes in accrued liabilities due primarily to legal and professional accruals, and fair market value adjustments related to contingent consideration (refer to
Item 1. Note 6
).
|
|
Six Months Ended
|
|||||||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
Increase/(Decrease)
|
||||||||
|
Cash Flows From (For) Investing Activities
|
|||||||||||
|
Proceeds from royalty rights
|
$
|
10.3
|
|
$
|
85.7
|
|
$
|
(75.4
|
)
|
||
|
Purchase of investment securities
|
(7.5
|
)
|
—
|
|
(7.5
|
)
|
|||||
|
Additions to property, plant and equipment
|
(33.3
|
)
|
(37.2
|
)
|
3.9
|
|
|||||
|
Net proceeds from sale of business and other assets
|
1.3
|
|
37.2
|
|
(35.9
|
)
|
|||||
|
Proceeds from sale of the Tysabri
®
financial asset
|
—
|
|
2,200.0
|
|
(2,200.0
|
)
|
|||||
|
Other investing, net
|
—
|
|
(3.7
|
)
|
3.7
|
|
|||||
|
Net cash from (for) investing activities
|
$
|
(29.2
|
)
|
$
|
2,282.0
|
|
$
|
(2,311.2
|
)
|
||
|
Six Months Ended
|
|||||||||||
|
(in millions)
|
June 30,
2018 |
July 1,
2017 |
Increase/(Decrease)
|
||||||||
|
Cash Flows From (For) Financing Activities
|
|||||||||||
|
Issuances of long-term debt
|
$
|
431.0
|
|
$
|
—
|
|
$
|
431.0
|
|
||
|
Payments on long-term debt
|
(457.3
|
)
|
(2,229.1
|
)
|
1,771.8
|
|
|||||
|
Borrowings (repayments) of revolving credit agreements and other financing, net
|
(8.2
|
)
|
—
|
|
(8.2
|
)
|
|||||
|
Deferred financing fees
|
(2.4
|
)
|
(4.0
|
)
|
1.6
|
|
|||||
|
Premium on early debt retirement
|
—
|
|
(116.1
|
)
|
116.1
|
|
|||||
|
Issuance of ordinary shares
|
—
|
|
0.2
|
|
(0.2
|
)
|
|||||
|
Repurchase of ordinary shares
|
(265.0
|
)
|
(58.2
|
)
|
(206.8
|
)
|
|||||
|
Cash dividends
|
(52.8
|
)
|
(46.0
|
)
|
(6.8
|
)
|
|||||
|
Other financing, net
|
(7.5
|
)
|
4.7
|
|
(12.2
|
)
|
|||||
|
Net cash (for) financing activities
|
$
|
(362.2
|
)
|
$
|
(2,448.5
|
)
|
$
|
2,086.3
|
|
||
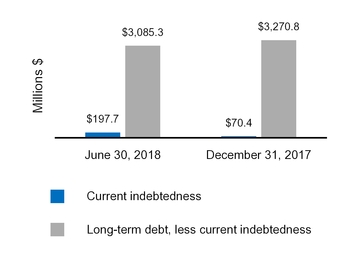
|
•
|
Reviewing our income tax processes and controls and enhancing the overall design and procedures performed in calculating our income tax provision on an interim and annual basis
|
|
•
|
Significantly strengthening our tax capabilities through a combination of key new hires and providing additional resources
|
|
•
|
Re-designing our management review controls and enhancing the precision of review around the key income tax areas
|
|
•
|
Evaluate the sufficiency of our income tax resources and personnel to determine whether additional enhancements are needed
|
|
•
|
Evaluate whether further enhancements are needed to the design of our income tax procedures and controls
|
|
•
|
Demonstrate consistent operating effectiveness of our management review controls over income taxes over a number of quarterly periods
|
|
Total Number of Shares Purchased
|
Average Price Paid per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Plans
|
Value of Shares Available for Purchase
(1)
|
||||||||||
|
April 1 - April 30, 2018
|
1,114,615
|
|
$
|
82.43
|
|
1,114,615
|
|
||||||
|
May 1 - May 31, 2018
|
860,500
|
|
$
|
75.51
|
|
860,500
|
|
||||||
|
June 1 - June 30, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
1.04
|
billion
|
|||
|
Total
|
1,975,115
|
|
$
|
1.04
|
billion
|
||||||||
|
Exhibit
Number
|
Description
|
|
|
3.1
|
||
|
3.2
|
||
|
10.1
|
||
|
10.2
|
||
|
10.3
|
||
|
31.1
|
||
|
31.2
|
||
|
32
|
||
|
101.INS
|
XBRL Instance Document.
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
PERRIGO COMPANY PLC
|
|||
|
(Registrant)
|
|||
|
Date:
|
August 9, 2018
|
/s/
Uwe F.
Roehrhoff
|
|
|
Uwe F. Roehrhoff
|
|||
|
Chief Executive Officer and President
|
|||
|
(Principal Executive Officer)
|
|||
|
Date:
|
August 9, 2018
|
/s/ Ronald L. Winowiecki
|
|
|
Ronald L. Winowiecki
|
|||
|
Chief Financial Officer
|
|||
|
(Principal Accounting and Financial Officer)
|
|||