|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
91-1789357
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
|
(IRS Employer
Identification Number)
|
|
12325 Emmet Street
Omaha, NE 68164
|
|
68164
|
|
(Address of Principal Executive Offices)
|
|
(Zip Code)
|
|
Title of Each Class
|
|
Name of Each Exchange On Which Registered
|
|
None
|
|
N/A
|
|
PART I
|
|||
|
Item 1.
|
Business
|
||
|
Item 1A.
|
Risk Factors
|
||
|
Item 1B.
|
Unresolved Staff Comments
|
||
|
Item 2.
|
Properties
|
||
|
Item 3.
|
Legal Proceedings
|
||
|
Item 4.
|
Mine Safety Disclosures
|
||
|
PART II
|
|||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
||
|
Item 6.
|
Selected Consolidated Financial Data
|
||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
||
|
Item 8.
|
Financial Statements and Supplementary Data
|
||
|
Report of Independent Registered Public Accounting Firm
|
|||
|
Consolidated Balance Sheets as of December 31, 2011 and 2010
|
|||
|
Consolidated Statements of Operations for the Years Ended December 31, 2011, 2010 and 2009
|
|||
|
Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2011, 2010 and 2009
|
|||
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2011, 2010 and 2009
|
|||
|
Notes to the Consolidated Financial Statements for the Years Ended December 31, 2011, 2010 and 2009
|
|||
|
Item 9
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures
|
||
|
Item 9A.
|
Controls and Procedures
|
||
|
Item 9B.
|
Other Information
|
||
|
PART III
|
|||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
||
|
Item 11.
|
Executive Compensation
|
||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
||
|
Item 14.
|
Principal Accounting Fees and Services
|
||
|
PART IV
|
|||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
||
|
SIGNATURES
|
|||
|
Item 1.
|
Our Business
|
|
•
|
Clinical Laboratories. Our clinical laboratories specialize in genetic testing for cardiology, neurology, mitochondrial disorders, and oncology. Located in New Haven, Connecticut and Omaha, Nebraska the molecular clinical reference laboratories are certified under the Clinical Laboratory Improvement Amendment (CLIA) as high complexity labs and our Omaha facility is also accredited by the College of American Pathologists (CAP).
|
|
•
|
Pharmacogenomics Services. Our Contract Research Organization located in Omaha, Nebraska provides pharmacogenomics research services supporting Phase II and Phase III clinical trials conducted by our pharmaceutical customers. This lab specializes in pharmacogenomic, biomarker and mutation discovery research serving the pharmaceutical and biomedical industries worldwide for disease research, drug and diagnostic development and clinical trial support.
|
|
•
|
Diagnostic Tools. Our proprietary product is the WAVE
®
System which has broad applicability to genetic variation detection in both molecular genetic research and molecular diagnostics. There is a worldwide installed base of over 1,500 WAVE Systems as of
December 31, 2011
. We also distribute bioinstruments produced by other manufacturers (“OEM Equipment”) through our sales and distribution network. Service contracts to maintain installed systems are sold and supported by our technical support personnel. The installed WAVE base and some OEM Equipment platforms generate a demand for consumables that are required for the continued operation of the bioinstruments. We develop, manufacture and sell these consumable products. In addition, we manufacture and sell consumable products that can be used on multiple, independent platforms. These products include SURVEYOR
®
Nuclease and a range of chromatography columns.
|
|
•
|
Developing new technologies and products internally
|
|
•
|
Acquire all or parts of other companies
|
|
•
|
Entering into joint-development efforts with other companies
|
|
•
|
Reselling other companies' products
|
|
•
|
Help diagnose a patient's disease
|
|
•
|
Guide treatment options
|
|
•
|
Determine whether family members are at risk
|
|
|
December 31,
|
|||||
|
|
2011
|
2010
|
||||
|
Manufacturing and Laboratory
|
68
|
|
62
|
|
||
|
Sales, Marketing and Administration
|
92
|
|
88
|
|
||
|
Research and Development
|
9
|
|
12
|
|
||
|
169
|
|
162
|
|
|||
|
|
December 31,
|
|||||
|
|
2011
|
2010
|
||||
|
United States
|
148
|
|
136
|
|
||
|
Europe (other than the United Kingdom)
|
10
|
|
15
|
|
||
|
United Kingdom
|
11
|
|
10
|
|
||
|
Canada
|
—
|
|
1
|
|
||
|
169
|
|
162
|
|
|||
|
Item 1A.
|
Risk Factors
|
|
•
|
Difficulties in integrating the operations, technologies, products and personnel of the acquired entities;
|
|
•
|
The risk of diverting management’s attention from normal daily operations of the business;
|
|
•
|
Potential difficulties in completing projects associated with in-process research and development;
|
|
•
|
Risks of entering markets in which we have no or limited direct prior experience and where competitors in such markets have stronger market positions;
|
|
•
|
Initial dependence on unfamiliar supply chains or relatively small supply partners;
|
|
•
|
Unexpected expenses resulting from the acquisition;
|
|
•
|
Potential unknown liabilities associated with acquired businesses;
|
|
•
|
Insufficient revenues to offset increased expenses associated with the acquisition; and
|
|
•
|
The potential loss of key employees of the acquired entities.
|
|
•
|
payment cycles in foreign markets are typically longer than in the U.S., and capital spending budgets for research agencies can vary over time with foreign governments;
|
|
•
|
changes in foreign currency exchange rates can make our products more costly in local currencies since our foreign sales are typically paid for in British Pounds or the Euro;
|
|
•
|
the potential for changes in U.S. and foreign laws or regulations that result in additional import or export restrictions, higher tariffs or other taxes, more burdensome licensing requirements or similar impediments to our ability to sell products and services profitably in these markets; and
|
|
•
|
the fluctuation of foreign currency to the US Dollar and the Euro to the British Pound can cause our net sales and expenses to increase or decrease, which adds risk to our financial statements.
|
|
•
|
a broker or dealer approve a person’s account for transactions in penny stocks; and
|
|
•
|
the broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
|
|
•
|
obtain financial information and investment experience objectives of the person; and
|
|
•
|
make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
|
|
•
|
sets forth the basis on which the broker or dealer made the suitability determination; and
|
|
•
|
that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
Item 2.
|
Properties
|
|
Location
|
Function
|
Square
Footage
|
2012
Scheduled
Rent
|
Lease Term
Expires
|
|||||||
|
Omaha, Nebraska
|
WAVE and Consumable Manufacturing
|
25,000
|
|
$
|
139
|
|
July 2016
|
||||
|
San Jose, California
|
Consumable Manufacturing
|
9,110
|
|
$
|
57
|
|
February 2016
|
||||
|
Glasgow, Scotland
|
Multi Functional
(1)
|
5,059
|
|
$
|
36
|
|
March 2017
|
||||
|
Omaha, Nebraska
|
Multi Functional
(1)
|
18,265
|
|
$
|
204
|
|
July 2022
|
||||
|
New Haven, Connecticut
|
Laboratory
|
22,459
|
|
$
|
472
|
|
March 2018
|
||||
|
(1)
|
Multi Functional facilities include functions related to manufacturing, services, sales and marketing, research and development and/or administration.
|
|
Item 3.
|
Legal Proceedings.
|
|
Item 4.
|
Mine Safety Disclosures
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
High
|
Low
|
|||||||
|
Year Ended December 31, 2011
|
||||||||
|
First Quarter
|
$
|
0.90
|
|
$
|
0.61
|
|
||
|
Second Quarter
|
$
|
1.75
|
|
$
|
0.82
|
|
||
|
Third Quarter
|
$
|
1.77
|
|
$
|
1.00
|
|
||
|
Fourth Quarter
|
$
|
1.44
|
|
$
|
1.07
|
|
||
|
Year Ended December 31, 2010
|
||||||||
|
First Quarter
|
$
|
0.88
|
|
$
|
0.61
|
|
||
|
Second Quarter
|
$
|
0.86
|
|
$
|
0.49
|
|
||
|
Third Quarter
|
$
|
0.59
|
|
$
|
0.33
|
|
||
|
Fourth Quarter
|
$
|
0.71
|
|
$
|
0.32
|
|
||
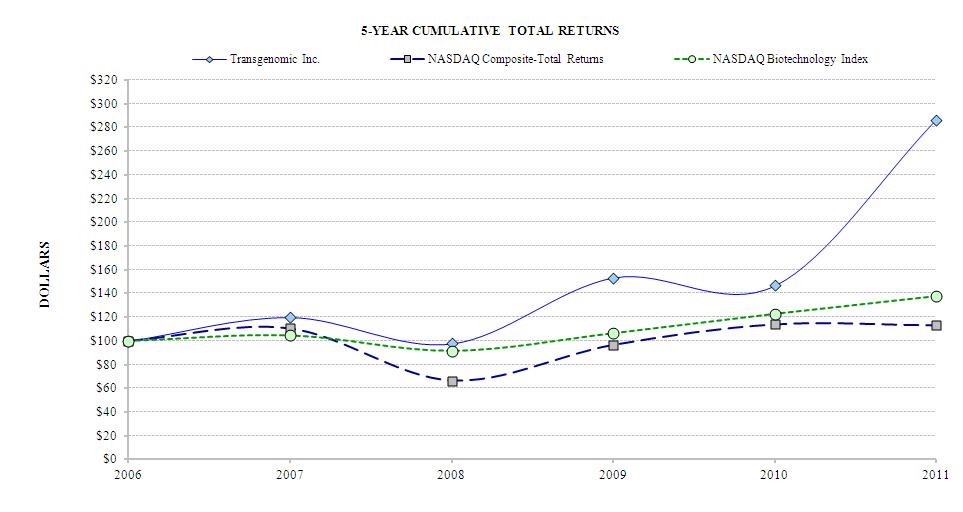
|
Item 6.
|
Selected Consolidated Financial Data.
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2011
|
2010
|
2009
|
2008
|
2007
|
|||||||||||||||
|
Statement of Operations Data:
|
||||||||||||||||||||
|
Net sales
|
$
|
31,971
|
|
$
|
20,048
|
|
$
|
22,023
|
|
$
|
23,993
|
|
$
|
23,176
|
|
|||||
|
Cost of good sold
|
13,534
|
|
10,284
|
|
10,418
|
|
10,345
|
|
10,483
|
|
||||||||||
|
Gross profit
|
18,437
|
|
9,764
|
|
11,605
|
|
13,648
|
|
12,693
|
|
||||||||||
|
Selling, general and administrative
|
19,150
|
|
10,933
|
|
10,319
|
|
10,795
|
|
11,466
|
|
||||||||||
|
Research and development
|
2,218
|
|
2,305
|
|
3,182
|
|
2,465
|
|
3,033
|
|
||||||||||
|
Restructuring charges
(1)
|
41
|
|
138
|
|
—
|
|
118
|
|
1,516
|
|
||||||||||
|
Impairment charges
(2)
|
—
|
|
—
|
|
—
|
|
638
|
|
—
|
|
||||||||||
|
Operating expenses
|
21,409
|
|
13,376
|
|
13,501
|
|
14,016
|
|
16,015
|
|
||||||||||
|
Other income (expense)
(3)
|
(6,765
|
)
|
628
|
|
18
|
|
86
|
|
1,391
|
|
||||||||||
|
Loss before income taxes
|
(9,737
|
)
|
(2,984
|
)
|
(1,878
|
)
|
(282
|
)
|
(1,931
|
)
|
||||||||||
|
Income tax expense
|
45
|
|
150
|
|
42
|
|
213
|
|
243
|
|
||||||||||
|
Loss from continuing operations
|
(9,782
|
)
|
(3,134
|
)
|
(1,920
|
)
|
(495
|
)
|
(2,174
|
)
|
||||||||||
|
Gain from discontinued operations, net of tax
(4)
|
—
|
|
—
|
|
—
|
|
—
|
|
1,374
|
|
||||||||||
|
Net loss
|
$
|
(9,782
|
)
|
$
|
(3,134
|
)
|
$
|
(1,920
|
)
|
$
|
(495
|
)
|
$
|
(800
|
)
|
|||||
|
Preferred stock dividends and accretion
(5)
|
(1,010
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Net loss available to common stockholders
|
$
|
(10,792
|
)
|
$
|
(3,134
|
)
|
$
|
(1,920
|
)
|
$
|
(495
|
)
|
$
|
(800
|
)
|
|||||
|
Basic and diluted loss per share:
|
||||||||||||||||||||
|
From continuing operations
|
$
|
(0.22
|
)
|
$
|
(0.06
|
)
|
$
|
(0.04
|
)
|
$
|
(0.01
|
)
|
$
|
(0.05
|
)
|
|||||
|
From discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
0.03
|
|
||||||||||
|
$
|
(0.22
|
)
|
$
|
(0.06
|
)
|
$
|
(0.04
|
)
|
$
|
(0.01
|
)
|
$
|
(0.02
|
)
|
||||||
|
Basic and diluted weighted average shares outstanding
|
49,362
|
|
49,244
|
|
49,190
|
|
49,190
|
|
49,190
|
|
||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
2011
|
2010
|
2009
|
2008
|
2007
|
||||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Working capital
|
$
|
870
|
|
$
|
6,781
|
|
$
|
10,351
|
|
$
|
11,350
|
|
$
|
11,316
|
|
|||||
|
Total assets
|
33,562
|
|
32,027
|
|
16,004
|
|
17,556
|
|
19,090
|
|
||||||||||
|
Total liabilities and mezzanine equity
|
22,514
|
|
23,527
|
|
4,342
|
|
4,351
|
|
4,988
|
|
||||||||||
|
Total stockholders' equity
(6)
|
11,048
|
|
8,500
|
|
11,662
|
|
13,205
|
|
14,102
|
|
||||||||||
|
(1)
|
Restructuring plans were implemented in 2010, 2008 and 2007 to reduce and align our expenses with current business prospects. The plans included employee terminations, office closures, termination of collaborations and write-offs of abandoned intellectual property. As a result, restructuring charges were recorded and are included in operating expenses.
|
|
(2)
|
Impairment charges in 2008 relate to the impairment of goodwill.
|
|
(3)
|
Other income (expense) for all years presented primarily includes interest expense, interest income and in 2011, expense associated with the "Series A Preferred Stock" and warrants to purchase shares of Series A Preferred Stock (the "Series A Warrants") of $6.1 million, which is due to the change in fair value of the preferred stock conversion feature. The expense associated with the change in value of the preferred stock conversion feature is a non-cash item. Other income in 2011 and 2010 includes $0.2 million and $0.6 million net of consulting fees, respectively, awarded in a federal grant under the Qualifying Therapeutic Discovery Project Program related to 2009 projects. Other income in 2007 includes $0.9 million from the sale of an investment security and $0.2 million in insurance proceeds related to equipment destroyed in a fire at our Cramlington, England facility.
|
|
(4)
|
Discontinued Operations include a reclassification of $1.3 million for an adjustment to other comprehensive income related to the closure of the Nucleic Acids segment. In the fourth quarter of 2005, we implemented a plan to exit the Nucleic Acids operating segment which was primarily engaged in the manufacture of phosphoramadites and the raw materials to produce phosphoramadites which are used to produce synthetic DNA. The Nucleic Acids operating segment consisted primarily of a manufacturing facility in Glasgow, Scotland.
|
|
(5)
|
For 2011, includes accrued dividends on Series A Preferred Stock of $0.6 million and Series A Preferred Stock accretion of $0.4 million.
|
|
(6)
|
Reference Footnote Q. "Subsequent Events" to our accompanying consolidated financial statements for a pro forma analysis of our total stockholders' equity as of December 31, 2011 as the result of a private placement offering performed in February 2012 by the Company.
|
|
•
|
Clinical Laboratories. Our clinical laboratories specialize in genetic testing for cardiology, neurology, mitochondrial disorders, and oncology. Located in New Haven, Connecticut and Omaha, Nebraska the molecular clinical reference laboratories are certified under the Clinical Laboratory Improvement Amendment (CLIA) as high complexity labs and our Omaha facility is also accredited by the College of American Pathologists (CAP).
|
|
•
|
Pharmacogenomics Services. Our Contract Research Organization located in Omaha, Nebraska provides pharmacogenomics research services supporting Phase II and Phase III clinical trials conducted by our pharmaceutical customers. This lab specializes in pharmacogenomic, biomarker and mutation discovery research serving the pharmaceutical and biomedical industries world-wide for disease research, drug and diagnostic development and clinical trial support.
|
|
•
|
Diagnostic Tools. Our proprietary product is the WAVE
®
System which has broad applicability to genetic variation detection in both molecular genetic research and molecular diagnostics. There is a worldwide installed base of over 1,500 WAVE Systems as of December 31, 2011. We also distribute bioinstruments produced by other manufacturers
|
|
2011 vs. 2010
|
Dollars in Thousands
|
|||||||||||||
|
|
Years Ended
|
Change
|
||||||||||||
|
|
2011
|
2010
|
$
|
%
|
||||||||||
|
Net sales
|
$
|
31,971
|
|
$
|
20,048
|
|
$
|
11,923
|
|
59
|
%
|
|||
|
Gross profit
|
18,437
|
|
9,764
|
|
8,673
|
|
89
|
%
|
||||||
|
Preferred stock and warrant expense
|
6,066
|
|
—
|
|
(6,066
|
)
|
nm
|
|
||||||
|
Net loss
|
(9,782
|
)
|
(3,134
|
)
|
(6,648
|
)
|
212
|
%
|
||||||
|
2011 vs. 2010
|
Dollars in Thousands
|
|||||||||||||
|
|
Years Ended
|
Change
|
||||||||||||
|
|
2011
|
2010
|
$
|
%
|
||||||||||
|
Clinical Laboratories
|
$
|
16,038
|
|
$
|
3,606
|
|
$
|
12,432
|
|
345
|
%
|
|||
|
Pharmacogenomic Services
|
2,280
|
|
1,373
|
|
907
|
|
66
|
%
|
||||||
|
Diagnostic Tools
|
13,653
|
|
15,069
|
|
(1,416
|
)
|
(9
|
)%
|
||||||
|
Total net sales
|
$
|
31,971
|
|
$
|
20,048
|
|
$
|
11,923
|
|
59
|
%
|
|||
|
2010 vs. 2009
|
Dollars in Thousands
|
|||||||||||||
|
|
Years Ended
|
Change
|
||||||||||||
|
|
2010
|
2009
|
$
|
%
|
||||||||||
|
Clinical Laboratories
|
$
|
3,606
|
|
$
|
3,541
|
|
$
|
65
|
|
2
|
%
|
|||
|
Pharmacogenomic Services
|
1,373
|
|
1,025
|
|
348
|
|
34
|
%
|
||||||
|
Diagnostic Tools
|
15,069
|
|
17,457
|
|
(2,388
|
)
|
(14
|
)%
|
||||||
|
Total net sales
|
$
|
20,048
|
|
$
|
22,023
|
|
$
|
(1,975
|
)
|
(9
|
)%
|
|||
|
2011 vs. 2010
|
Dollars in Thousands
|
||||||||||||
|
|
Years Ended
|
Margin %
|
|||||||||||
|
|
2011
|
2010
|
2011
|
2010
|
|||||||||
|
Clinical Laboratories
|
$
|
9,478
|
|
$
|
1,481
|
|
59
|
%
|
41
|
%
|
|||
|
Pharmacogenomic Services
|
1,050
|
|
(43
|
)
|
46
|
%
|
(3
|
)%
|
|||||
|
Diagnostic Tools
|
7,909
|
|
8,326
|
|
58
|
%
|
55
|
%
|
|||||
|
Gross profit
|
$
|
18,437
|
|
$
|
9,764
|
|
58
|
%
|
49
|
%
|
|||
|
2010 vs. 2009
|
Dollars in Thousands
|
||||||||||||
|
|
Years Ended
|
Margin %
|
|||||||||||
|
|
2010
|
2009
|
2010
|
2009
|
|||||||||
|
Clinical Laboratories
|
$
|
1,481
|
|
$
|
1,523
|
|
41
|
%
|
43
|
%
|
|||
|
Pharmacogenomic Services
|
(43
|
)
|
205
|
|
(3
|
)%
|
20
|
%
|
|||||
|
Diagnostic Tools
|
8,326
|
|
9,877
|
|
55
|
%
|
57
|
%
|
|||||
|
Gross profit
|
$
|
9,764
|
|
$
|
11,605
|
|
49
|
%
|
53
|
%
|
|||
|
Dollars in Thousands
|
||||||||||||
|
|
Years Ended
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Selling, general and administrative
|
$
|
19,150
|
|
$
|
10,933
|
|
$
|
10,319
|
|
|||
|
Research and development
|
2,218
|
|
2,305
|
|
3,182
|
|
||||||
|
Restructuring charges
|
41
|
|
138
|
|
—
|
|
||||||
|
Total
|
$
|
21,409
|
|
$
|
13,376
|
|
$
|
13,501
|
|
|||
|
Dollars in Thousands
|
||||||||||||
|
|
Years Ended
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Interest income (expense)
|
$
|
(958
|
)
|
$
|
(4
|
)
|
$
|
15
|
|
|||
|
Expense on preferred stock and warrants
|
(6,066
|
)
|
—
|
|
—
|
|
||||||
|
Other, net
|
259
|
|
632
|
|
3
|
|
||||||
|
Total other income (expense), net
|
$
|
(6,765
|
)
|
$
|
628
|
|
$
|
18
|
|
|||
|
|
December 31,
|
|
||||||||||
|
|
2011
|
2010
|
Change
|
|||||||||
|
Current assets
(including cash and cash equivalents of $4,946 and $3,454 respectively)
|
$
|
17,198
|
|
$
|
15,034
|
|
$
|
2,164
|
|
|||
|
Current liabilities
|
16,328
|
|
8,253
|
|
(8,075
|
)
|
||||||
|
Working capital
|
$
|
870
|
|
$
|
6,781
|
|
$
|
(5,911
|
)
|
|||
|
(amounts in thousands)
|
|||||||||||
|
2011
|
2010
|
2009
|
|||||||||
|
Net cash provided by (used for):
|
|||||||||||
|
Operating activities
|
$
|
220
|
|
$
|
(1,718
|
)
|
$
|
1,267
|
|
||
|
Investing activities
|
(508
|
)
|
(6,226
|
)
|
(377
|
)
|
|||||
|
Financing activities
|
1,726
|
|
5,761
|
|
—
|
|
|||||
|
Effect of exchange rates on cash
|
54
|
|
(5
|
)
|
(19
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
1,492
|
|
$
|
(2,188
|
)
|
$
|
871
|
|
||
|
|
(Amounts in thousands)
|
||||||||||||||||||||||||||
|
|
2012
|
2013
|
2014
|
2015
|
|
2016
|
|
After 2016
|
|
Total
|
|||||||||||||||||
|
Short term debt
(1)
|
$
|
3,082
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,082
|
|
||||||
|
Long term debt
(1)
|
3,703
|
|
4,937
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,640
|
|
|||||||||||||
|
Interest
(1)
|
900
|
|
307
|
|
1,207
|
|
|||||||||||||||||||||
|
Capital lease obligations
(2)
|
378
|
|
312
|
|
97
|
|
—
|
|
—
|
|
—
|
|
787
|
|
|||||||||||||
|
Operating lease obligations
(3)
|
1,103
|
|
1,023
|
|
978
|
|
914
|
|
866
|
|
2,094
|
|
6,978
|
|
|||||||||||||
|
Purchase obligations
(4)
|
1,271
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,271
|
|
|||||||||||||
|
|
$
|
10,437
|
|
|
$
|
6,579
|
|
$
|
1,075
|
|
|
$
|
914
|
|
|
$
|
866
|
|
|
$
|
2,094
|
|
|
$
|
21,965
|
|
|
|
•
|
Persuasive evidence of an arrangement exists
|
|
•
|
Delivery has occurred or services have been rendered
|
|
•
|
The seller’s price to the buyer is fixed or determinable, and
|
|
•
|
Collectability is reasonably assured.
|
|
Item 7A.
|
Quantitative and Qualitative Disclosure about Market Risk.
|
|
2011
|
2010
|
||||||
|
ASSETS
|
|||||||
|
CURRENT ASSETS:
|
|||||||
|
Cash and cash equivalents
|
$
|
4,946
|
|
$
|
3,454
|
|
|
|
Accounts receivable (net of allowances for bad debts of $1,088 and $334, respectively)
|
7,573
|
|
7,601
|
|
|||
|
Inventories (net of allowances for obsolescence of $511 and $518, respectively)
|
3,859
|
|
3,344
|
|
|||
|
Other current assets
|
820
|
|
635
|
|
|||
|
Total current assets
|
17,198
|
|
15,034
|
|
|||
|
PROPERTY AND EQUIPMENT:
|
|||||||
|
Equipment
|
10,143
|
|
9,820
|
|
|||
|
Furniture, fixtures & leasehold improvements
|
3,682
|
|
3,479
|
|
|||
|
13,825
|
|
13,299
|
|
||||
|
Less: accumulated depreciation
|
(11,969
|
)
|
(11,697
|
)
|
|||
|
1,856
|
|
1,602
|
|
||||
|
OTHER ASSETS:
|
|||||||
|
Goodwill
|
6,440
|
|
6,275
|
|
|||
|
Intangibles (net of accumulated amortization of $1,437 and $519, respectively)
|
7,966
|
|
8,962
|
|
|||
|
Other assets
|
102
|
|
154
|
|
|||
|
$
|
33,562
|
|
$
|
32,027
|
|
||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|||||||
|
CURRENT LIABILITIES:
|
|||||||
|
Accounts payable
|
$
|
2,609
|
|
$
|
1,360
|
|
|
|
Accrued compensation
|
1,133
|
|
875
|
|
|||
|
Short term debt
|
3,082
|
|
989
|
|
|||
|
Current maturities of long term debt
|
3,703
|
|
—
|
|
|||
|
Accrued expenses
|
3,839
|
|
3,231
|
|
|||
|
Other Liabilities
|
1,042
|
|
1,628
|
|
|||
|
Current portion of lease obligations
|
320
|
|
170
|
|
|||
|
Accrued preferred stock dividend
|
600
|
|
—
|
|
|||
|
Total current liabilities
|
16,328
|
|
8,253
|
|
|||
|
LONG TERM LIABILITIES:
|
|||||||
|
Long term debt less current maturities
|
4,937
|
|
8,640
|
|
|||
|
Preferred stock conversion feature
|
—
|
|
1,983
|
|
|||
|
Preferred stock warrant liability
|
—
|
|
2,351
|
|
|||
|
Other long-term liabilities
|
1,249
|
|
843
|
|
|||
|
Total liabilities
|
22,514
|
|
22,070
|
|
|||
|
Redeemable Series A convertible preferred stock, $.01 par value, 3,879,307 shares authorized, 0 and 2,586,205 shares issued and outstanding, respectively
|
—
|
|
1,457
|
|
|||
|
STOCKHOLDERS’ EQUITY:
|
|||||||
|
Series A preferred stock, $.01 par value, 15,000,000 shares authorized, 2,586,205 and 0 shares issued and outstanding, respectively
|
26
|
|
—
|
|
|||
|
Common stock, $.01 par value, 100,000,000 shares authorized, 49,625,725 and 49,289,672 shares issued and outstanding, respectively
|
501
|
|
498
|
|
|||
|
Additional paid-in capital
|
152,987
|
|
139,730
|
|
|||
|
Accumulated other comprehensive income
|
336
|
|
1,589
|
|
|||
|
Accumulated deficit
|
(142,802
|
)
|
(133,317
|
)
|
|||
|
Total stockholders’ equity
|
11,048
|
|
8,500
|
|
|||
|
$
|
33,562
|
|
$
|
32,027
|
|
||
|
|
|||||||||||
|
|
2011
|
2010
|
2009
|
||||||||
|
NET SALES
|
$
|
31,971
|
|
$
|
20,048
|
|
$
|
22,023
|
|
||
|
COST OF GOODS SOLD
|
13,534
|
|
10,284
|
|
10,418
|
|
|||||
|
Gross profit
|
18,437
|
|
9,764
|
|
11,605
|
|
|||||
|
OPERATING EXPENSES:
|
|||||||||||
|
Selling, general and administrative
|
19,150
|
|
10,933
|
|
10,319
|
|
|||||
|
Research and development
|
2,218
|
|
2,305
|
|
3,182
|
|
|||||
|
Restructuring charges
|
41
|
|
138
|
|
—
|
|
|||||
|
21,409
|
|
13,376
|
|
13,501
|
|
||||||
|
LOSS FROM OPERATIONS
|
(2,972
|
)
|
(3,612
|
)
|
(1,896
|
)
|
|||||
|
OTHER INCOME (EXPENSE):
|
|||||||||||
|
Interest income (expense), net
|
(958
|
)
|
(4
|
)
|
15
|
|
|||||
|
Expense on preferred stock
|
(6,066
|
)
|
—
|
|
—
|
|
|||||
|
Other, net
|
259
|
|
632
|
|
3
|
|
|||||
|
(6,765
|
)
|
628
|
|
18
|
|
||||||
|
LOSS BEFORE INCOME TAXES
|
(9,737
|
)
|
(2,984
|
)
|
(1,878
|
)
|
|||||
|
INCOME TAX EXPENSE
|
45
|
|
150
|
|
42
|
|
|||||
|
NET LOSS
|
$
|
(9,782
|
)
|
$
|
(3,134
|
)
|
$
|
(1,920
|
)
|
||
|
PREFERRED STOCK DIVIDENDS AND ACCRETION
|
(1,010
|
)
|
—
|
|
—
|
|
|||||
|
NET LOSS AVAILABLE TO COMMON STOCKHOLDERS
|
$
|
(10,792
|
)
|
$
|
(3,134
|
)
|
$
|
(1,920
|
)
|
||
|
BASIC AND DILUTED LOSS PER COMMON SHARE
|
$
|
(0.22
|
)
|
$
|
(0.06
|
)
|
$
|
(0.04
|
)
|
||
|
BASIC AND DILUTED WEIGHTED AVERAGE SHARES OF COMMON STOCK OUTSTANDING
|
49,361,632
|
|
49,243,839
|
|
49,189,672
|
|
|||||
|
|
Preferred Stock
|
Common Stock
|
|
|
|
|
|||||||||||||||||||||||
|
|
Outstanding
Shares
|
Par
Value
|
Outstanding
Shares
|
Par
Value
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Accumulated
Other
Comprehensive
Income (Loss)
|
Total
|
|||||||||||||||||||||
|
Balance, January 1, 2009
|
—
|
|
—
|
|
49,189,672
|
|
$
|
497
|
|
$
|
139,501
|
|
$
|
(128,263
|
)
|
$
|
1,470
|
|
$
|
13,205
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,920
|
)
|
(1,920
|
)
|
(1,920
|
)
|
|||||||||||||
|
Other comprehensive income (loss):
|
|||||||||||||||||||||||||||||
|
Foreign currency translation adjustment, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
175
|
|
175
|
|
|||||||||||||
|
Comprehensive loss
|
$
|
(1,745
|
)
|
||||||||||||||||||||||||||
|
Non-cash stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
202
|
|
—
|
|
—
|
|
202
|
|
|||||||||||||
|
Balance, December 31, 2009
|
—
|
|
—
|
|
49,189,672
|
|
$
|
497
|
|
$
|
139,703
|
|
$
|
(130,183
|
)
|
$
|
1,645
|
|
$
|
11,662
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(3,134
|
)
|
$
|
(3,134
|
)
|
$
|
(3,134
|
)
|
||||||||
|
Other comprehensive income (loss):
|
|
|
|||||||||||||||||||||||||||
|
Foreign currency translation adjustment, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(56
|
)
|
(56
|
)
|
|||||||||||||
|
Comprehensive loss
|
$
|
(3,190
|
)
|
||||||||||||||||||||||||||
|
Non-cash stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
(14
|
)
|
—
|
|
—
|
|
(14
|
)
|
|||||||||||||
|
Issuance of shares of stock
|
—
|
|
—
|
|
100,000
|
|
1
|
|
41
|
|
—
|
|
—
|
|
42
|
|
|||||||||||||
|
Balance, December 31, 2010
|
—
|
|
—
|
|
49,289,672
|
|
$
|
498
|
|
$
|
139,730
|
|
$
|
(133,317
|
)
|
$
|
1,589
|
|
$
|
8,500
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(9,782
|
)
|
(9,782
|
)
|
(9,782
|
)
|
|||||||||||||
|
Other comprehensive income (loss):
|
|||||||||||||||||||||||||||||
|
Foreign currency translation adjustment, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
54
|
|
54
|
|
|||||||||||||
|
Comprehensive loss
|
$
|
(9,728
|
)
|
||||||||||||||||||||||||||
|
Non-cash stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,010
|
|
—
|
|
—
|
|
1,010
|
|
|||||||||||||
|
Issuance of shares of common stock
|
—
|
|
—
|
|
90,150
|
|
1
|
|
23
|
|
—
|
|
—
|
|
24
|
|
|||||||||||||
|
Preferred stock accretion
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(410
|
)
|
—
|
|
(410
|
)
|
|||||||||||||
|
Amendment of preferred stock agreement
|
2,586,205
|
|
26
|
|
245,903
|
|
2
|
|
12,224
|
|
—
|
|
—
|
|
12,252
|
|
|||||||||||||
|
Reclassification of other comprehensive income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,307
|
|
(1,307
|
)
|
—
|
|
|||||||||||||
|
Dividends on preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(600
|
)
|
—
|
|
(600
|
)
|
|||||||||||||
|
Balance, December 31, 2011
|
2,586,205
|
|
$
|
26
|
|
49,625,725
|
|
$
|
501
|
|
$
|
152,987
|
|
$
|
(142,802
|
)
|
$
|
336
|
|
$
|
11,048
|
|
|||||||
|
|
2011
|
2010
|
2009
|
||||||||
|
CASH FLOWS PROVIDED BY (USED IN) OPERATING ACTIVITIES:
|
|||||||||||
|
Net loss
|
$
|
(9,782
|
)
|
$
|
(3,134
|
)
|
$
|
(1,920
|
)
|
||
|
Adjustments to reconcile net loss to net cash flows provided by (used in) operating activities:
|
|||||||||||
|
Depreciation and amortization
|
2,101
|
|
708
|
|
852
|
|
|||||
|
Non-cash, stock based compensation
|
1,010
|
|
(14
|
)
|
202
|
|
|||||
|
Provision for losses on doubtful accounts
|
1,738
|
|
28
|
|
(8
|
)
|
|||||
|
Provision for losses on inventory obsolescence
|
48
|
|
100
|
|
482
|
|
|||||
|
Preferred stock revaluation
|
6,066
|
|
—
|
|
—
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
(2,212
|
)
|
44
|
|
1,121
|
|
|||||
|
Inventories
|
(620
|
)
|
(3
|
)
|
808
|
|
|||||
|
Prepaid expenses and other current assets
|
243
|
|
95
|
|
(60
|
)
|
|||||
|
Accounts payable
|
1,028
|
|
364
|
|
60
|
|
|||||
|
Accrued liabilities
|
332
|
|
92
|
|
(401
|
)
|
|||||
|
Other long term liabilities
|
401
|
|
(24
|
)
|
109
|
|
|||||
|
Long term deferred income taxes
|
(133
|
)
|
26
|
|
22
|
|
|||||
|
Net cash flows provided by (used in) operating activities
|
220
|
|
(1,718
|
)
|
1,267
|
|
|||||
|
CASH FLOWS USED IN INVESTING ACTIVITIES:
|
|||||||||||
|
Acquisitions
|
—
|
|
(6,000
|
)
|
—
|
|
|||||
|
Purchase of property and equipment
|
(231
|
)
|
(192
|
)
|
(351
|
)
|
|||||
|
Change in other assets
|
(277
|
)
|
(34
|
)
|
(26
|
)
|
|||||
|
Net cash flows used in investing activities
|
(508
|
)
|
(6,226
|
)
|
(377
|
)
|
|||||
|
CASH FLOWS PROVIDED BY (USED IN) FINANCING ACTIVITIES:
|
|||||||||||
|
Issuance of preferred stock and related warrants
|
—
|
|
6,000
|
|
—
|
|
|||||
|
Stock issuance costs
|
—
|
|
(209
|
)
|
—
|
|
|||||
|
Proceeds from note payable
|
3,000
|
|
—
|
|
—
|
|
|||||
|
Principal payments on capital lease obligations
|
(391
|
)
|
(72
|
)
|
—
|
|
|||||
|
Issuance of common stock
|
24
|
|
42
|
|
—
|
|
|||||
|
Principal payment on note payable
|
(907
|
)
|
—
|
|
—
|
|
|||||
|
Net cash flows provided by financing activities
|
1,726
|
|
5,761
|
|
—
|
|
|||||
|
EFFECT OF FOREIGN CURRENCY EXCHANGE RATE CHANGES ON CASH
|
54
|
|
(5
|
)
|
(19
|
)
|
|||||
|
NET CHANGE IN CASH AND CASH EQUIVALENTS
|
1,492
|
|
(2,188
|
)
|
871
|
|
|||||
|
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD
|
3,454
|
|
5,642
|
|
4,771
|
|
|||||
|
CASH AND CASH EQUIVALENTS AT END OF PERIOD
|
$
|
4,946
|
|
$
|
3,454
|
|
$
|
5,642
|
|
||
|
SUPPLEMENTAL CASH FLOW INFORMATION
|
|||||||||||
|
Cash paid during the period for:
|
|||||||||||
|
Interest
|
$
|
732
|
|
$
|
7
|
|
$
|
—
|
|
||
|
Income taxes, net
|
108
|
|
29
|
|
163
|
|
|||||
|
SUPPLEMENTAL DISCLOSURE OF NON-CASH INFORMATION
|
|||||||||||
|
Acquisition of equipment through capital leases
|
$
|
756
|
|
$
|
394
|
|
$
|
—
|
|
||
|
Dividends accrued on preferred stock
|
600
|
|
—
|
|
—
|
|
|||||
|
Common stock issued for elimination of derivatives on preferred stock
|
300
|
|
—
|
|
—
|
|
|||||
|
Goodwill purchase price adjustment
|
165
|
|
—
|
|
—
|
|
|||||
|
A.
|
BUSINESS DESCRIPTION
|
|
•
|
Clinical Laboratories. Our clinical laboratories specialize in genetic testing for cardiology, neurology, mitochondrial disorders, and oncology. Located in New Haven, Connecticut and Omaha, Nebraska the molecular clinical reference laboratories are certified under the Clinical Laboratory Improvement Amendment (CLIA) as high complexity labs and our Omaha facility is also accredited by the College of American Pathologists (CAP).
|
|
•
|
Pharmacogenomics Services. Our Contract Research Organization located in Omaha, Nebraska provides pharmacogenomics research services supporting Phase II and Phase III clinical trials conducted by our pharmaceutical customers. This lab specializes in pharmacogenomic, biomarker and mutation discovery research serving the pharmaceutical and biomedical industries world-wide for disease research, drug and diagnostic development and clinical trial support.
|
|
•
|
Diagnostic Tools. Our proprietary product is the WAVE
®
System which has broad applicability to genetic variation detection in both molecular genetic research and molecular diagnostics. There is a worldwide installed base of over 1,500 WAVE Systems as of
December 31, 2011
. We also distribute bioinstruments produced by other manufacturers (“OEM Equipment”) through our sales and distribution network. Service contracts to maintain installed systems are sold and supported by our technical support personnel. The installed WAVE base and some OEM Equipment platforms generate a demand for consumables that are required for the continued operation of the bioinstruments. We develop, manufacture and sell these consumable products. In addition, we manufacture and sell consumable products that can be used on multiple, independent platforms. These products include SURVEYOR
®
Nuclease and a range of chromatography columns.
|
|
B.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
|
|
Dollars in Thousands
|
||||||||||||||
|
|
Beginning
Balance
|
Provision
|
Write Offs
|
Ending
Balance
|
|||||||||||
|
Year Ended December 31, 2011
|
$
|
334
|
|
$
|
1,738
|
|
$
|
(984
|
)
|
$
|
1,088
|
|
|||
|
Year Ended December 31, 2010
|
$
|
310
|
|
$
|
28
|
|
$
|
(4
|
)
|
$
|
334
|
|
|||
|
Year Ended December 31, 2009
|
$
|
388
|
|
$
|
(8
|
)
|
$
|
(70
|
)
|
$
|
310
|
|
|||
|
|
Dollars in Thousands
|
||||||||||||||
|
|
Beginning
Balance
|
Provision
|
Write Offs
|
Ending
Balance
|
|||||||||||
|
Year Ended December 31, 2011
|
$
|
518
|
|
$
|
48
|
|
$
|
(55
|
)
|
$
|
511
|
|
|||
|
Year Ended December 31, 2010
|
$
|
507
|
|
$
|
100
|
|
$
|
(89
|
)
|
$
|
518
|
|
|||
|
Year Ended December 31, 2009
|
$
|
108
|
|
$
|
482
|
|
$
|
(83
|
)
|
$
|
507
|
|
|||
|
Leasehold improvements
|
1 to 10 years
|
|
Furniture and fixtures
|
3 to 7 years
|
|
Production equipment
|
3 to 7 years
|
|
Computer equipment
|
3 to 7 years
|
|
Research and development equipment
|
2 to 7 years
|
|
•
|
Persuasive evidence of an arrangement exists
|
|
•
|
Delivery has occurred or services have been rendered
|
|
•
|
The seller’s price to the buyer is fixed or determinable, and
|
|
•
|
Collectability is reasonably assured.
|
|
Consideration
|
Dollars in Thousands
|
||
|
Cash
|
$
|
6,000
|
|
|
Notes payable
|
9,628
|
|
|
|
Assumed liabilities
|
452
|
|
|
|
Contingent liabilities
|
2,736
|
|
|
|
|
|||
|
Fair value of consideration transferred
|
$
|
18,816
|
|
|
|
|||
|
Recognized Amounts of Identifiable Assets Acquired and Liabilities Assumed
|
Dollars in Thousands
|
||
|
Working capital, net
|
$
|
3,222
|
|
|
Property and Equipment
|
639
|
|
|
|
Identifiable intangible assets
|
8,680
|
|
|
|
|
|||
|
Total identifiable net assets
|
12,541
|
|
|
|
Goodwill
|
6,275
|
|
|
|
|
|||
|
Total purchase price
|
18,816
|
|
|
|
|
|||
|
Intangibles—acquired technology
|
Income Approach - Multi-period Excess Earnings Method
|
|
|
Intangibles—third party payor relationships
|
Cost Approach - Replacement Cost Method
|
|
|
Intangibles—assay royalties
|
Income Approach - Multi-period Excess Earnings Method
|
|
|
Intangibles—tradenames and trademarks
|
Income Approach - Relief from Royalty Method
|
|
|
|
Dollars in Thousands
|
|||||||
|
|
Year Ended December 31,
|
|||||||
|
|
2010
|
2009
|
||||||
|
Revenue—Supplemental pro-forma results
|
$
|
33,733
|
|
$
|
35,112
|
|
||
|
Net loss—Supplemental pro-forma results
|
(7,716
|
)
|
(13,071
|
)
|
||||
|
|
Dollars in Thousands
|
|||||||||||
|
Costs Incurred in the year ended December 31, 2011
|
Cumulative Costs
Incurred at
December 31, 2011
|
Total
Expected Costs
|
||||||||||
|
Severance and related costs
|
$
|
—
|
|
$
|
53
|
|
$
|
53
|
|
|||
|
Facility closure costs
|
28
|
|
74
|
|
74
|
|
||||||
|
Other
|
13
|
|
52
|
|
52
|
|
||||||
|
Restructuring charges
|
$
|
41
|
|
$
|
179
|
|
$
|
179
|
|
|||
|
|
Dollars in Thousands
|
||||||
|
|
December 31,
2011
|
|
December 31,
2010
|
|
|||
|
Finished goods
|
$
|
2,608
|
|
$
|
2,119
|
|
|
|
Raw materials and work in process
|
1,485
|
|
1,531
|
|
|||
|
Demonstration inventory
|
277
|
|
212
|
|
|||
|
$
|
4,370
|
|
$
|
3,862
|
|
||
|
Less allowance for obsolescence
|
(511
|
)
|
(518
|
)
|
|||
|
Total
|
$
|
3,859
|
|
$
|
3,344
|
|
|
|
|
Dollars in Thousands
|
||||||||||||||||||||||
|
|
December 31, 2011
|
December 31, 2010
|
|||||||||||||||||||||
|
|
Cost
|
Accumulated
Amortization
|
Net Book
Value
|
Cost
|
Accumulated
Amortization
|
Net Book
Value
|
|||||||||||||||||
|
Intangibles—acquired technology
|
$
|
6,535
|
|
$
|
911
|
|
$
|
5,624
|
|
$
|
6,535
|
|
$
|
—
|
|
$
|
6,535
|
|
|||||
|
Intangibles—assay royalties
|
1,434
|
|
205
|
|
1,229
|
|
1,434
|
|
—
|
|
1,434
|
|
|||||||||||
|
Intangibles—third party payor relationships
|
367
|
|
—
|
|
367
|
|
367
|
|
—
|
|
367
|
|
|||||||||||
|
Intangibles—tradenames and trademarks
|
344
|
|
49
|
|
295
|
|
344
|
|
—
|
|
344
|
|
|||||||||||
|
Patents
|
703
|
|
267
|
|
436
|
|
511
|
|
245
|
|
266
|
|
|||||||||||
|
Intellectual property
|
20
|
|
5
|
|
15
|
|
290
|
|
274
|
|
16
|
|
|||||||||||
|
$
|
9,403
|
|
$
|
1,437
|
|
$
|
7,966
|
|
$
|
9,481
|
|
$
|
519
|
|
$
|
8,962
|
|
||||||
|
|
Estimated Useful Life
|
|
Intellectual property
|
10 years
|
|
Patents
|
7 years
|
|
Intangibles—acquired technology
|
7 – 8 years
|
|
Intangibles—third party payor relationships
|
Indefinite
|
|
Intangibles—assay royalties
|
7 years
|
|
Intangibles—tradenames and trademarks
|
7 years
|
|
|
Dollars in Thousands
|
|||||||
|
|
Year Ended December 31,
|
|||||||
|
|
2011
|
2010
|
||||||
|
PGxHealth note payable (the "First Note")
(1)
|
$
|
8,640
|
|
$
|
8,640
|
|
||
|
PGxHealth note payable (the "Second Note")
(2)
|
82
|
|
989
|
|
||||
|
Third Security Convertible Promissory Notes
(3)
|
3,000
|
|
—
|
|
||||
|
Total debt, including short term debt
|
11,722
|
|
9,629
|
|
||||
|
Short term debt
|
(3,082
|
)
|
(989
|
)
|
||||
|
Current maturities of long term debt
|
(3,703
|
)
|
—
|
|
||||
|
Long-term debt, net of current maturities
|
$
|
4,937
|
|
$
|
8,640
|
|
||
|
(1)
|
The First Note is a three year senior secured promissory note to PGxHealth, LLC entered into on December 29, 2010 in conjunction with our acquisition of the FAMILION family of genetic tests from PGxHealth. Interest is payable at 10% per year with quarterly interest payments through March 29, 2012. Thereafter, quarterly installments will include both principal and interest through December 30, 2013.
|
|
(2)
|
The Second Note is a one year senior secured promissory note to PGxHealth, LLC entered into on December 31, 2010 for facility improvements made to the CLIA certified laboratory in New Haven, Connecticut. Interest is payable at 6.5% per year with the principal and interest payable in twelve monthly installments with the final payment made on January 3, 2012.
|
|
2012
|
$
|
3,703
|
|
|
2013
|
4,937
|
|
|
|
$
|
8,640
|
|
|
|
|
|||
|
|
Dollars in Thousands
|
||||||
|
|
Asset Balances at
|
||||||
|
Classes of Property
|
December 31,
2011
|
|
December 31,
2010
|
|
|||
|
Equipment
|
$
|
1,052
|
|
$
|
394
|
|
|
|
Less: Accumulated amortization
|
(164
|
)
|
(13
|
)
|
|||
|
Total
|
$
|
888
|
|
$
|
381
|
|
|
|
|
Dollars in Thousands
|
||
|
2012
|
$
|
378
|
|
|
2013
|
312
|
|
|
|
2014
|
97
|
|
|
|
2015
|
—
|
|
|
|
Total minimum lease payments
|
$
|
787
|
|
|
Less: Amount representing interest
|
(99
|
)
|
|
|
Present value of net minimum lease payments
|
$
|
688
|
|
|
|
Dollars in Thousands
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Benefit at federal rate
|
$
|
(3,311
|
)
|
$
|
(1,015
|
)
|
$
|
(639
|
)
|
|||
|
Increase (decrease) resulting from:
|
||||||||||||
|
State income taxes—net of federal benefit
|
2
|
|
20
|
|
(10
|
)
|
||||||
|
Foreign subsidiary tax rate difference
|
(94
|
)
|
(27
|
)
|
(50
|
)
|
||||||
|
Tax contingency
|
28
|
|
45
|
|
48
|
|
||||||
|
Net operating loss expiration
|
988
|
|
—
|
|
1,258
|
|
||||||
|
Earnings repatriation
|
—
|
|
1,479
|
|
—
|
|
||||||
|
Miscellaneous permanent differences
|
332
|
|
60
|
|
93
|
|
||||||
|
Other—net
|
(53
|
)
|
86
|
|
(33
|
)
|
||||||
|
Valuation allowance
|
2,153
|
|
(498
|
)
|
(625
|
)
|
||||||
|
Current income tax expense
|
$
|
45
|
|
$
|
150
|
|
$
|
42
|
|
|||
|
|
Dollars in Thousands
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Federal:
|
||||||||||||
|
Current
|
$
|
16
|
|
$
|
4
|
|
$
|
(58
|
)
|
|||
|
Deferred
|
—
|
|
—
|
|
—
|
|
||||||
|
Total Federal
|
$
|
16
|
|
$
|
3
|
|
$
|
(58
|
)
|
|||
|
State:
|
||||||||||||
|
Current
|
$
|
3
|
|
$
|
29
|
|
$
|
(16
|
)
|
|||
|
Deferred
|
—
|
|
—
|
|
—
|
|
||||||
|
Total State
|
$
|
3
|
|
$
|
29
|
|
$
|
(16
|
)
|
|||
|
Foreign:
|
||||||||||||
|
Current
|
$
|
159
|
|
$
|
111
|
|
$
|
(60
|
)
|
|||
|
Deferred
|
(133
|
)
|
6
|
|
176
|
|
||||||
|
Total Foreign
|
$
|
26
|
|
$
|
117
|
|
$
|
116
|
|
|||
|
Total Tax Provision
|
$
|
45
|
|
$
|
150
|
|
$
|
42
|
|
|||
|
|
Dollars in Thousands
|
|||||||
|
|
2011
|
2010
|
||||||
|
Deferred Tax Asset:
|
||||||||
|
Net operating loss carryforward
|
$
|
38,154
|
|
$
|
38,201
|
|
||
|
Unrealized gain
|
2,062
|
|
—
|
|
||||
|
Research and development credit carryforwards
|
1,232
|
|
1,232
|
|
||||
|
Deferred net sales
|
190
|
|
151
|
|
||||
|
Inventory
|
184
|
|
188
|
|
||||
|
Other
|
552
|
|
473
|
|
||||
|
42,374
|
|
40,245
|
|
|||||
|
Less valuation allowance
|
(42,294
|
)
|
(40,141
|
)
|
||||
|
Deferred Tax Asset
|
$
|
80
|
|
$
|
104
|
|
||
|
Deferred Tax Liability:
|
||||||||
|
Uninstalled instruments
|
$
|
2
|
|
$
|
159
|
|
||
|
Deferred Tax Liability
|
$
|
2
|
|
$
|
159
|
|
||
|
Net Deferred Asset (Liability)
|
$
|
78
|
|
$
|
(55
|
)
|
||
|
Warrant Holder
|
Issue Year
|
Expiration
|
Underlying
Shares
|
Exercise
Price
|
||||
|
Affiliates of Third Security, LLC (1)
|
2010
|
December 2015
|
5,172,408
|
$0.58
|
||||
|
(1)
|
Warrants issued in connection with the Financing. The number of shares shown reflects the post-conversion shares.
|
|
Number of
Options
|
Weighted Average
Exercise Price
|
||||||
|
Balance at January 1, 2011:
|
2,565,001
|
|
$
|
2.08
|
|
||
|
Granted
|
2,440,500
|
|
1.17
|
|
|||
|
Exercised
|
(30,000
|
)
|
(0.76
|
)
|
|||
|
Forfeited
|
(353,501
|
)
|
(1.64
|
)
|
|||
|
Expired
|
(450,000
|
)
|
(6.69
|
)
|
|||
|
Balance at December 31, 2011:
|
4,172,000
|
|
$
|
1.10
|
|
||
|
Exercisable at December 31, 2011
|
2,131,045
|
|
$
|
1.05
|
|
||
|
Number of
Options
|
Weighted Average
Exercise Price
|
||||||
|
Balance at January 1, 2010:
|
3,331,731
|
|
$
|
2.39
|
|
||
|
Granted
|
125,000
|
|
0.50
|
|
|||
|
Exercised
|
(100,000
|
)
|
(0.42
|
)
|
|||
|
Forfeited
|
(593,499
|
)
|
(0.73
|
)
|
|||
|
Expired
|
(198,231
|
)
|
(11.07
|
)
|
|||
|
Balance at December 31, 2010:
|
2,565,001
|
|
$
|
2.08
|
|
||
|
Exercisable at December 31, 2010
|
2,358,334
|
|
$
|
2.22
|
|
||
|
Number of
Options
|
Weighted Average
Exercise Price
|
||||||
|
Balance at January 1, 2009:
|
3,531,064
|
|
$
|
2.54
|
|
||
|
Granted
|
70,000
|
|
0.42
|
|
|||
|
Exercised
|
—
|
|
—
|
|
|||
|
Forfeited
|
(72,833
|
)
|
(1.39
|
)
|
|||
|
Expired
|
(196,500
|
)
|
(4.85
|
)
|
|||
|
Balance at December 31, 2009:
|
3,331,731
|
|
$
|
2.39
|
|
||
|
Exercisable at December 31, 2009
|
2,518,671
|
|
$
|
2.96
|
|
||
|
Number of
Options
|
Exercise Price
|
||||||
|
March 2, 2011
|
130,000
|
|
$
|
0.74
|
|
||
|
May 18, 2011
|
2,205,500
|
|
$
|
1.19
|
|
||
|
December 2, 2011
|
105,000
|
|
$
|
1.28
|
|
||
|
2,440,500
|
|
||||||
|
Exercise Price Range
|
Number of
Options
Outstanding
|
Remaining
Weighted-Average
Contractual Life
|
Weighted–Average
Exercise Price
|
Number of
Options
Exercisable
|
||||||
|
$ 0.00—$ 1.30
|
3,934,167
|
|
7.5 years
|
$1.03
|
1,893,212
|
|
||||
|
$ 1.31—$ 2.60
|
223,333
|
|
1.6 years
|
$1.89
|
223,333
|
|
||||
|
$ 5.21—$ 6.50
|
7,000
|
|
.3 years
|
$6.16
|
7,000
|
|
||||
|
$ 9.11—$10.00
|
7,500
|
|
.1 years
|
$9.63
|
7,500
|
|
||||
|
4,172,000
|
|
2,131,045
|
|
|||||||
|
Dollars in Thousands
|
|||||||||||
|
For the year ended
|
|||||||||||
|
December 31, 2011
|
|||||||||||
|
Preferred
Stock
Conversion
Feature
|
Preferred
Stock Warrant Liability |
Total
|
|||||||||
|
Beginning balance at January 1, 2011
|
$
|
1,983
|
|
$
|
2,351
|
|
$
|
4,334
|
|
||
|
Total gains or losses:
|
|||||||||||
|
Recognized in earnings
|
5,317
|
|
449
|
|
5,766
|
|
|||||
|
Balance as of November 8, 2011
|
7,300
|
|
2,800
|
|
10,100
|
|
|||||
|
Reclassification to shareholders' equity due to Amendment Agreement
|
$
|
(7,300
|
)
|
$
|
(2,800
|
)
|
$
|
(10,100
|
)
|
||
|
Balance as of December 31, 2011
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Dollars in Thousands
|
|||||||||||
|
For the year ended
|
|||||||||||
|
December 31, 2010
|
|||||||||||
|
Preferred
Stock
Conversion
Feature
|
Preferred
Stock Warrant Liability |
Total
|
|||||||||
|
Beginning balance at January 1, 2010
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Issuance
|
1,983
|
|
2,351
|
|
4,334
|
|
|||||
|
Balance at December 31, 2010
|
$
|
1,983
|
|
$
|
2,351
|
|
$
|
4,334
|
|
||
|
|
In thousands except per share data
|
||||||||||||||
|
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
2011
|
|||||||||||||||
|
Net Sales
|
$
|
7,480
|
|
|
$
|
7,667
|
|
|
$
|
8,253
|
|
|
$
|
8,571
|
|
|
Gross Profit
|
4,154
|
|
4,555
|
|
4,445
|
|
|
5,283
|
|
||||||
|
Net Income (Loss)
|
(2,778
|
)
|
(5,998
|
)
|
(1,270
|
)
|
264
|
|
|||||||
|
Basic and diluted loss per common share
|
$
|
(0.06
|
)
|
$
|
(0.13
|
)
|
$
|
(0.03
|
)
|
$
|
—
|
|
|||
|
|
|
||||||||||||||
|
2010
|
|||||||||||||||
|
Net Sales
|
$
|
5,442
|
|
$
|
5,095
|
|
$
|
4,419
|
|
$
|
5,092
|
|
|||
|
Gross Profit
|
2,884
|
|
2,487
|
|
2,017
|
|
2,376
|
|
|||||||
|
Net Loss
|
(324
|
)
|
(1,146
|
)
|
(898
|
)
|
(767
|
)
|
|||||||
|
Basic and diluted loss per common share
|
$
|
(0.01
|
)
|
$
|
(0.02
|
)
|
$
|
(0.02
|
)
|
$
|
(0.01
|
)
|
|||
|
|
Dollars in Thousands
|
||||||||||||||
|
|
2011
|
||||||||||||||
|
|
Clinical
Laboratories
|
Pharmacogenomic
Services
|
Diagnostic
Tools |
Total
|
|||||||||||
|
Net Sales
|
$
|
16,038
|
|
$
|
2,280
|
|
$
|
13,653
|
|
$
|
31,971
|
|
|||
|
Gross Profit
|
9,478
|
|
1,050
|
|
7,909
|
|
18,437
|
|
|||||||
|
Net Income (Loss) before Taxes
|
(11,016
|
)
|
(354
|
)
|
1,633
|
|
(9,737
|
)
|
|||||||
|
Income Tax Expense (Benefit)
|
—
|
|
—
|
|
45
|
|
45
|
|
|||||||
|
Net Income (Loss)
|
$
|
(11,016
|
)
|
$
|
(354
|
)
|
$
|
1,588
|
|
$
|
(9,782
|
)
|
|||
|
Depreciation/Amortization
|
1,568
|
|
242
|
|
235
|
|
2,045
|
|
|||||||
|
Restructure
|
29
|
|
—
|
|
12
|
|
41
|
|
|||||||
|
Interest Income (Expense)
|
(959
|
)
|
—
|
|
—
|
|
(959
|
)
|
|||||||
|
|
December 31, 2011
|
||||||||||||||
|
Total Assets
|
$
|
22,032
|
|
$
|
1,636
|
|
$
|
9,894
|
|
$
|
33,562
|
|
|||
|
Goodwill
|
6,440
|
|
$
|
—
|
|
$
|
—
|
|
$
|
6,440
|
|
||||
|
|
Dollars in Thousands
|
||||||||||||||
|
|
2010
|
||||||||||||||
|
|
Clinical
Laboratories |
Pharmacogenomic
Services |
Diagnostic
Tools |
Total
|
|||||||||||
|
Net Sales
|
$
|
3,606
|
|
$
|
1,373
|
|
$
|
15,069
|
|
$
|
20,048
|
|
|||
|
Gross Profit
|
1,481
|
|
(43
|
)
|
8,326
|
|
9,764
|
|
|||||||
|
Net Loss before Taxes
|
(1,829
|
)
|
(696
|
)
|
(459
|
)
|
(2,984
|
)
|
|||||||
|
Income Tax Expense (Benefit)
|
—
|
|
—
|
|
150
|
|
150
|
|
|||||||
|
Net Loss
|
$
|
(1,829
|
)
|
$
|
(696
|
)
|
$
|
(609
|
)
|
$
|
(3,134
|
)
|
|||
|
Depreciation/Amortization
|
119
|
|
186
|
|
190
|
|
495
|
|
|||||||
|
Restructure
|
65
|
|
—
|
|
73
|
|
138
|
|
|||||||
|
Interest Income (Expense)
|
(1
|
)
|
—
|
|
(3
|
)
|
(4
|
)
|
|||||||
|
|
December 31, 2010
|
||||||||||||||
|
Total Assets
|
$
|
22,945
|
|
$
|
1,686
|
|
$
|
7,396
|
|
$
|
32,027
|
|
|||
|
Goodwill
|
6,275
|
|
$
|
—
|
|
$
|
—
|
|
$
|
6,275
|
|
||||
|
|
Dollars in Thousands
|
||||||||||||||
|
|
2009
|
||||||||||||||
|
|
Clinical
Laboratories |
Pharmacogenomic
Services |
Diagnostic
Tools |
Total
|
|||||||||||
|
Net Sales
|
$
|
3,541
|
|
$
|
1,025
|
|
$
|
17,457
|
|
$
|
22,023
|
|
|||
|
Gross Profit
|
1,523
|
|
205
|
|
9,877
|
|
11,605
|
|
|||||||
|
Net Loss before Taxes
|
(1,763
|
)
|
(510
|
)
|
395
|
|
(1,878
|
)
|
|||||||
|
Income Tax Expense (Benefit)
|
—
|
|
—
|
|
42
|
|
42
|
|
|||||||
|
Net Loss
|
$
|
(1,763
|
)
|
$
|
(510
|
)
|
$
|
353
|
|
$
|
(1,920
|
)
|
|||
|
Depreciation/Amortization
|
146
|
|
151
|
|
450
|
|
747
|
|
|||||||
|
Restructure
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Interest Income (Expense)
|
4
|
|
—
|
|
11
|
|
15
|
|
|||||||
|
|
December 31, 2009
|
||||||||||||||
|
Total Assets
|
$
|
6,796
|
|
$
|
661
|
|
$
|
8,547
|
|
$
|
16,004
|
|
|||
|
|
Dollars in Thousands
|
||||||||||||
|
Years ended December 31,
|
|||||||||||||
|
2011
|
2010
|
2009
|
|||||||||||
|
United States
|
$
|
22,626
|
|
$
|
8,729
|
|
$
|
8,777
|
|
||||
|
Italy
|
3,152
|
|
3,294
|
|
3,683
|
|
|||||||
|
United Kingdom
|
778
|
|
1,412
|
|
842
|
|
|||||||
|
Germany
|
750
|
|
1,366
|
|
1,383
|
|
|||||||
|
France
|
758
|
|
1,160
|
|
1,545
|
|
|||||||
|
Netherlands
|
97
|
|
56
|
|
1,464
|
|
|||||||
|
All Other Countries
|
3,810
|
|
4,031
|
|
4,329
|
|
|||||||
|
Total
|
$
|
31,971
|
|
$
|
20,048
|
|
$
|
22,023
|
|
||||
|
Actual
|
Pro-Forma
|
|||||
|
Dollars in Thousands
|
||||||
|
December 31, 2011
|
December 31, 2011
|
|
||||
|
Total Assets
|
$33,562
|
$51,129
|
||||
|
Total Liabilities
|
22,514
|
|
19,514
|
|
||
|
Total Stockholders' Equity
|
11,048
|
|
31,615
|
|
||
|
$
|
33,562
|
|
51,129
|
|
||
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures.
|
|
Item 9A.
|
Controls and Procedures.
|
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets;
|
|
•
|
provide reasonable assurance that our transactions are recorded as necessary to permit preparation of our financial statements in accordance with accounting principles generally accepted in the United States of America, and that our receipts and expenditures are being made only in accordance with authorizations of our management and our directors; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
|
|
Item 9B.
|
Other Information.
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance.
|
|
Name
|
Age
|
Principal Occupation
|
Director Since
|
Term to Expire
|
||||
|
CLASS I DIRECTORS
|
||||||||
|
Robert M. Patzig, Preferred Stock Director
|
43
|
Senior Managing Director and Chief Investment Officer, Third Security, LLC
|
2010
|
2013
|
||||
|
Craig J. Tuttle, Common Stock Director
|
59
|
President and Chief Executive Officer of Transgenomic, Inc.
|
1997
|
2013
|
||||
|
CLASS II DIRECTORS
|
||||||||
|
Doit L. Koppler II, Preferred Stock Director
|
48
|
Managing Director and Treasurer, Third Security, LLC
|
2010
|
2014
|
||||
|
Antonius P Schuh, Ph.D, Common Stock Director
|
48
|
Chief Executive Officer of Sorrento Therapeutics, Inc.
|
2009
|
2014
|
||||
|
CLASS III DIRECTORS
|
||||||||
|
Rodney S. Markin, M.D., Ph.D, Common Stock Director
|
55
|
Chairman of the Board, Transgenomic, President of University of Nebraska Medical Center Physicians
|
2007
|
2015
|
||||
|
Item 11.
|
Executive Compensation.
|
|
•
|
align the interests of our executives and stockholders by motivating executives to increase stockholder value and rewarding executives when stockholder value increases;
|
|
•
|
motivate our executives to manage our business to meet our short and long-term objectives, and reward them for meeting these objectives.
|
|
•
|
Review and approve corporate goals and objectives relevant to CEO and other executive officers' compensation, evaluate performance in light of these goals and objectives, and determine and approve the compensation level for the CEO and other executive officers based on this evaluation.
|
|
•
|
Make recommendations to the Board of Directors with respect to incentive-compensation plans and equity-based plans.
|
|
•
|
Adoption of stock option and other long-term incentive plans and approval of individual grants and awards.
|
|
•
|
Adoption of executive annual incentive plans and approval of total incentive payments and individual awards to the President, CEO and other executive officers.
|
|
•
|
Adoption of benefit plans, including profit sharing and supplemental retirements plans.
|
|
•
|
Adoption of executive perquisite programs.
|
|
•
|
Annual evaluation and appraisal of President and CEO performance.
|
|
•
|
Approval of all employment agreements for President, CEO and other executives.
|
|
•
|
Annual review of non-employee Director compensation programs and recommendation of changes to the Board of Directors when appropriate.
|
|
|
•
|
the ability of the executive to drive results for the Company;
|
|
|
•
|
the executive's understanding of the Company's business and his/her organizational savvy;
|
|
|
•
|
the ability of the executive to make complex decisions and his/her strategic abilities;
|
|
|
•
|
the executive's ability to manage work process;
|
|
|
•
|
the communication skills of the executive; and
|
|
|
•
|
the executive's ability to manage diversity and ethics.
|
|
Element of Compensation
|
Purpose
|
Pay-for-Performance Considerations
|
|
Cash and Short-Term Variable Compensation:
|
||
|
Base Compensation
|
Provides competitive, fixed compensation to attract and retain exceptional executive talent
|
Adjustments to base salary consider the individual's overall performance, contribution to the business and internal and external comparisons
|
|
Cash Bonus
|
Encourages and rewards achievement of strong financial, operational and strategic performance by the Company
|
The amount of any discretionary bonus received by an executive officer, if any, depends on the degree we achieve strong annual financial, operational or strategic performance and the extent to which the executive officer contributes to the achievement
|
|
Long-Term Compensation:
|
||
|
Stock Options
|
Encourages executive officers to focus on the long-term performance of the Company, links an executive officer's incentives to our stockholders' interests in increasing our stockholder value, encourages significant ownership of our common stock and promotes long-term retention of our executives officers
|
The potential appreciation in our stock price above the exercise price for stock options motivates our executives to build stockholder value as the executive officer only realizes value from the stock option if the stock price appreciates
|
|
Other Elements:
|
||
|
Health, Retirement and Other Benefits
|
Provides broad-based market competitive employee benefits program such as participation in benefit plans generally available to our employees, including, employee stock purchase plan, 401(k) retirement plan, life, health and dental insurance and short-term and long-term disability plans
|
Not applicable
|
|
Target Attainment Percentage
|
Form of Payment
|
|
100%
|
Cash
|
|
Above 100%
|
50% Cash
50% Restricted Stock
|
|
MEMBERS OF THE COMPENSATION COMMITTEE:
|
|
Antonius P. Schuh, Ph.D.
|
|
Rodney .S. Markin, MD, Ph,D.
|
|
Robert M. Patzig
|
|
Name and Principal Position
|
Year
|
Salary ($)
|
Bonus ($)
|
Option Awards
(1)
$
|
All Other Compensation ($)
|
Total ($)
|
||||||||||||||||
|
Craig J. Tuttle
(2)
|
2011
|
$
|
325,000
|
|
$
|
10,000
|
|
$
|
457,950
|
|
$
|
12,102
|
|
$
|
805,052
|
|
||||||
|
President and
|
2010
|
325,000
|
|
—
|
|
—
|
|
18,377
|
|
(3)
|
343,377
|
|
||||||||||
|
Chief Executive Officer
|
2009
|
325,000
|
|
—
|
|
—
|
|
17,559
|
|
(3)
|
342,559
|
|
||||||||||
|
Brett L. Frevert
(4)
|
2011
|
—
|
|
5,000
|
|
228,975
|
|
242,250
|
|
(4)
|
476,225
|
|
||||||||||
|
Chief Financial Officer
|
2010
|
—
|
|
—
|
|
—
|
|
96,225
|
|
(4)
|
96,225
|
|
||||||||||
|
Chad M. Richards
(5)
|
2011
|
199,167
|
|
6,000
|
|
228,975
|
|
9,338
|
|
(6)
|
443,480
|
|
||||||||||
|
Chief Commercial Officer
|
2010
|
188,708
|
|
—
|
|
—
|
|
13,476
|
|
(6)
|
202,184
|
|
||||||||||
|
2009
|
182,250
|
|
—
|
|
—
|
|
11,340
|
|
(6)
|
193,590
|
|
|||||||||||
|
Name
|
Grant Date
|
All Other Option Awards: Number of Securities Underlying Options (#)
|
Exercise or Price of Option Awards ($/sh)
(1)
|
Grant Date Fair Value of Option Awards ($)
(2)
|
|||||||||
|
Craig J. Tuttle
|
5/18/2011
|
500,000
|
|
$
|
1.19
|
|
$
|
457,950
|
|
||||
|
Brett L. Frevert
|
5/18/2011
|
250,000
|
|
$
|
1.19
|
|
228,975
|
|
|||||
|
Chad M. Richards
|
5/18/2011
|
250,000
|
|
$
|
1.19
|
|
228,975
|
|
|||||
|
Option Awards
|
||||||||||||||
|
Name
|
Option Award / Grant Date
|
Number of Securities Underlying Unexercised Options (#) Exercisable
|
Number of Securities Underlying Unexercised Options (#) Unexercisable
|
Option Exercise Price ($)
|
Option Expiration Date
|
|||||||||
|
Craig J. Tuttle
|
9/1/2006
|
200,000
|
|
—
|
|
$
|
0.69
|
|
8/31/2016
|
|||||
|
Craig J. Tuttle
|
1/17/2007
|
200,000
|
|
—
|
|
0.75
|
|
1/16/2017
|
||||||
|
Craig J. Tuttle
|
7/12/2007
|
200,000
|
|
—
|
|
0.66
|
|
7/11/2017
|
||||||
|
Craig J. Tuttle
|
5/18/2011
|
—
|
|
500,000
|
|
1.19
|
|
5/17/2021
|
||||||
|
Brett L. Frevert
|
5/18/2011
|
—
|
|
250,000
|
|
1.19
|
|
5/17/2021
|
||||||
|
Chad M. Richards
|
10/8/2007
|
200,000
|
|
0.69
|
|
10/7/2017
|
||||||||
|
Chad M. Richards
|
5/18/2011
|
—
|
|
250,000
|
|
1.19
|
|
5/17/2021
|
||||||
|
Name
|
Benefit
|
Cause
|
Without Cause
(1)
|
Voluntary Termination
|
Change in Control
(2)
|
Death
(2)
|
Disability
(2)
|
Retirement
(2)
|
||||||||||||||||||||||
|
Craig. J. Tuttle
|
Cash
|
$
|
—
|
|
$
|
350,000
|
|
$
|
—
|
|
$
|
350,000
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||||||
|
Stock options
|
—
|
|
354,000
|
|
—
|
|
404,000
|
|
404,000
|
|
404,000
|
|
404,000
|
|
||||||||||||||||
|
Benefits
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Total
|
$
|
—
|
|
$
|
704,000
|
|
$
|
—
|
|
$
|
754,000
|
|
$
|
404,000
|
|
$
|
404,000
|
|
$
|
404,000
|
|
|||||||||
|
|
||||||||||||||||||||||||||||||
|
Brett L. Frevert
|
Cash
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||||||
|
Stock options
|
—
|
|
—
|
|
—
|
|
25,000
|
|
25,000
|
|
25,000
|
|
25,000
|
|
||||||||||||||||
|
Benefits
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Total
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
25,000
|
|
$
|
25,000
|
|
$
|
25,000
|
|
$
|
25,000
|
|
|||||||||
|
Chad M. Richards
|
Cash
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||||||
|
Stock options
|
—
|
|
120,000
|
|
—
|
|
145,000
|
|
145,000
|
|
145,000
|
|
145,000
|
|
||||||||||||||||
|
Benefits
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Total
|
$
|
—
|
|
$
|
120,000
|
|
$
|
—
|
|
$
|
145,000
|
|
$
|
145,000
|
|
$
|
145,000
|
|
$
|
145,000
|
|
|||||||||
|
Name
|
Fees Earned or Paid in Cash ($)
|
Option Awards $
(1)
|
Total ($)
|
||||
|
Doit Koppler
|
$21,000
|
$23,309
|
$44,309
|
||||
|
Robert Patzig
|
18,750
|
23,309
|
|
42,059
|
|||
|
Rodney Markin, M.D., Ph.D.
|
25,375
|
14,568
|
|
39,943
|
|||
|
Antonius Schuh, Ph.D
|
23,625
|
14,568
|
|
38,193
|
|||
|
Name
|
Vested Stock Option Awards
|
Unvested Stock Option Awards
|
Aggregate Stock Option Awards
|
||||||
|
Doit Koppler, II
|
—
|
|
40,000
|
|
40,000
|
|
|||
|
Robert Patzig
|
—
|
|
40,000
|
|
40,000
|
|
|||
|
Rodney Markin, M.D., Ph.D
|
20,000
|
|
35,000
|
|
55,000
|
|
|||
|
Antonius Schuh, Ph.D
|
10,000
|
|
35,000
|
|
45,000
|
|
|||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
|
(a)
|
(b)
|
(c)
|
|||||||||||||
|
PLAN CATEGORY
|
Number of
securities to be
issued upon
exercise of
outstanding options,
warrants and rights
|
Weighted-average
exercise price of
outstanding
options, warrants
and rights
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) )
|
||||||||||||
|
Equity compensation plans approved by security holders
(1)
|
4,172,000
|
|
$
|
1.10
|
|
4,944,231
|
|
||||||||
|
Equity compensation plans not approved by security holders
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total
|
4,172,000
|
|
$
|
1.10
|
|
4,944,231
|
|
||||||||
|
(1)
|
Consists of our 2006 Equity Compensation Plan
|
|
Name and Address of Beneficial Owner
(1)
|
Number of Shares Beneficially Owned
|
Percent of Class
|
||||
|
Directors and Executive Officers
|
||||||
|
Craig J. Tuttle, President and Chief Executive Officer, Director
|
600,000
|
|
(2)
|
*
|
||
|
Brett L. Frevert, Chief Financial Officer
|
—
|
|
(3)
|
*
|
||
|
Chad M. Richards, Chief Commercial Officer
|
243,800
|
|
(4)
|
*
|
||
|
Doit L. Koppler II, Director
|
107,500
|
|
(5)
|
*
|
||
|
Rodney S. Markin, M.D., Ph.D, Director
|
45,000
|
|
(6)
|
*
|
||
|
Robert M. Patzig, Director
|
92,500
|
|
(7)
|
*
|
||
|
Antonius P. Schuh, Ph.D, Director
|
35,000
|
|
(8)
|
*
|
||
|
All directors and executive officers as a group (7 persons)
|
1,123,800
|
|
(9)
|
1.5%
|
||
|
Other Stockholders
|
||||||
|
LeRoy C. Kopp
|
14,156,661
|
|
(10)
|
19.8%
|
||
|
AMH Equity, LLC and Leviticus Partners, L.P.
|
4,621,181
|
|
(11)
|
6.5%
|
||
|
Kevin Douglas
|
4,000,000
|
|
(12)
|
5.6%
|
||
|
Austin W. Marxe and David M. Greenhouse
|
5,250,000
|
|
(13)
|
7.2%
|
||
|
Randal J. Kirk
|
20,263,131
|
|
(14)
|
22.1%
|
||
|
Name and Address of Beneficial Owner
|
Number of Shares Beneficially Owned
|
Percent of Class
|
|||||
|
Randal J. Kirk
|
3,879,307
|
|
(1)
|
100
|
%
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
|
Item 14.
|
Principal Accountant Fees and Services.
|
|
2011
|
2010
|
|||||||
|
Audit fees
|
$
|
321,005
|
|
$
|
205,315
|
|
||
|
Audit-related fees
|
25,999
|
|
127,200
|
|
||||
|
Tax fees
|
30,190
|
|
34,055
|
|
||||
|
All other fees
|
—
|
|
—
|
|
||||
|
Total fees
|
$
|
377,194
|
|
$
|
366,572
|
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules.
|
|
(a)
|
The following documents are filed as part of this report:
|
|
1
|
Financial Statements. The following financial statements of the Registrant are included in response to Item 8 of this report:
|
|
2
|
Financial Statement Schedules.
|
|
3
|
Exhibits. The following exhibits were filed as required by Item 15(a)(3) of this report. Exhibit numbers refer to the paragraph numbers under Item 601 of Regulation S-K:
|
|
4.2
|
Certificate of Designation of Series A Convertible Preferred Stock dated as of December 28, 2010 (incorporated by reference to Exhibit 3.1 to the Registrant's Report on Form 8-K filed on January 4, 2011).
|
|
+10.25
|
Asset Purchase Agreement, dated November 29, 2010, by and among PGxHealth, LLC, Clinical Data, Inc. and Transgenomic, Inc. (incorporated by reference to Exhibit 2.1 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
+10.26
|
Amendment to Asset Purchase Agreement, dated December 29, 2010, by and among PGxHealth, LLC, Clinical Data, Inc. and Transgenomic, Inc. (incorporated by reference to Exhibit 2.2 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.28
|
Form of Series A Convertible Preferred Stock Warrant issued to Third Security Senior Staff 2008 LLC, Third Security Staff 2010 LLC, and Third Security Incentive 2010 LLC on December 29, 2010 (incorporated by reference to Exhibit 4.2 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.29
|
Registration Rights Agreement, dated December 29, 2010, by and among Transgenomic, Inc., Third Security Senior Staff 2008 LLC, Third Security Staff 2010 LLC, and Third Security Incentive 2010 LLC (incorporated by reference to Exhibit 4.3 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.30
|
Secured Promissory Note, issued December 29, 2010 by Transgenomic, Inc. in favor of PGxHealth, LLC (incorporated by reference to Exhibit 4.4 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.31
|
Secured Promissory Note, issued December 29, 2010 by Transgenomic, Inc. in favor of PGxHealth, LLC (incorporated by reference to Exhibit 4.5 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.32
|
Sublease Agreement, dated December 29, 2010, by and between Transgenomic, Inc. and Clinical Data, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.33
|
Noncompetition and Nonsolicitation Agreement, dated December 29, 2010, by and among PGxHealth, LLC, Clinical Data, Inc. and Transgenomic, Inc. (incorporated by reference to Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.34
|
Security Agreement, dated December 29, 2010, by and between PGxHealth, LLC and Transgenomic, Inc. (incorporated by reference to Exhibit 10.3 to the Registrant's Current Report on Form 8-K filed on January 4, 2011).
|
|
10.35
|
First Amendment to Registration Rights Agreement dated November 8, 2011 (incorporated by reference to Exhibit 4.2 to the Registrant's Current Report on Form 8-K filed on November 14, 2011).
|
|
10.36
|
Agreement Regarding Preferred Stock dated November 8, 2011 (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on November 14, 2011).
|
|
10.37
|
Convertible Promissory Note Purchase Agreement by and among the Company; Third Security Senior Staff 2008 LLC; Third Security Staff 2010 LLC; and Third Security Incentive 2010 LLC dated December 30, 2011 (incorporated by referenced to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on January 6, 2012).
|
|
10.38
|
Convertible Promissory Note by and between Transgenomic, Inc. and Third Security Senior Staff 2008
|
|
10.39
|
Convertible Promissory Note by and between Transgenomic, Inc. and Third Security Staff 2010 LLC dated December 30, 2011 (incorporated by referenced to Exhibit 10.3 to the Registrant's Current Report on Form 8-K filed on January 6, 2012).
|
|
10.40
|
Convertible Promissory Note by and between Transgenomic, Inc. and Third Security Incentive 2010 LLC dated December 30, 2011(incorporated by referenced to Exhibit 10.4 to the Registrant's Current Report on Form 8-K filed on January 6, 2012).
|
|
10.41
|
Securities Purchase Agreement entered into by and among the Company and the Investors dated February 2, 2012 (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on February 7, 2012).
|
|
10.42
|
Form of Warrant issued by the Company to the Third Securities Entities on February 7, 2012(incorporated by referenced to Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed on February 7, 2012).
|
|
10.43
|
Form of Warrant issued by the Company to the Investors on February 7, 2012 (incorporated by reference to Exhibit 10.3 to the Registrant's Current Report on Form 8-K filed on February 7, 2012).
|
|
10.44
|
Form of Registration Rights Agreement entered into by and among the Company, the Third Securities Entities and the Investors dated February 2, 2012 (incorporated by reference to Exhibit 10.4 to the Registrant's Current Report on Form 8-K filed on February 7, 2012).
|
|
101.INS
|
XBRL Instance Document ***
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document ***
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document ***
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document ***
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document ***
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document ***
|
|
|
*
|
Denotes exhibit that constitutes a management contract, or compensatory plan or arrangement.
|
|
**
|
This certification is not deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent that the Company specifically incorporates it by reference.
|
|
***
|
XBRL information is furnished and not filed for purposes of Sections 11 and 12 of the Securities Act of 1933 and Section 18 of the Securities Act of 1934, and is not subject to liability under those sections, is not part of any registration statement or prospectus to which it relates and is not incorporated or deemed to be incorporated by
|
|
+
|
Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
TRANSGENOMIC, INC.
|
||
|
By:
|
|
/s/ CRAIG J. TUTTLE
|
|
|
Craig J. Tuttle,
President and Chief Executive Officer
|
|
|
Signature
|
|
Title
|
|
/s/ CRAIG J. TUTTLE
Craig J. Tuttle
|
|
Director, President and Chief Executive Officer (Principal Executive Officer)
|
|
/s/ BRETT L. FREVERT
Brett L. Frevert
|
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer)
|
|
/s/ RODNEY S. MARKIN*
Rodney S. Markin
|
|
Director
|
|
/s/ ANTONIUS P. SCHUH*
Antonius P. Schuh
|
|
Director
|
|
/s/ ROBERT M. PATZIG*
Robert M. Patzig
|
|
Director
|
|
/s/ DOIT L. KOPPLER II*
Doit L. Koppler II
|
|
Director
|
|
*By Craig J. Tuttle, as attorney-in-fact
|
|
|
|
/s/ CRAIG J. TUTTLE
Craig J. Tuttle
Attorney-in-fact for the individuals as indicated.
|
|
|