|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
DELAWARE
|
33-0827593
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
|
3020 CALLAN ROAD, SAN DIEGO, CALIFORNIA
|
92121
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Large Accelerated Filer
o
|
Accelerated Filer
T
|
Non-Accelerated Filer
o
|
Smaller reporting company
o
|
|
(Do not check if a smaller reporting company)
|
|||
|
PART I
|
|||
|
Item 1.
|
4 | ||
|
Item 1A.
|
10 | ||
|
Item 1B.
|
18 | ||
|
Item 2.
|
18 | ||
|
Item 3.
|
18 | ||
|
Item 4.
|
19 | ||
|
PART II
|
|||
|
Item 5.
|
20 | ||
|
Item 6.
|
21 | ||
|
Item 7.
|
23 | ||
|
Item 7A.
|
38 | ||
|
Item 8.
|
40 | ||
|
Item 9.
|
74 | ||
|
Item 9A.
|
74 | ||
|
Item 9B.
|
75 | ||
|
PART III
|
|||
|
Item 10.
|
76 | ||
|
Item 11.
|
76 | ||
|
Item 12.
|
76 | ||
|
Item 13.
|
76 | ||
|
Item 14.
|
76 | ||
|
PART IV
|
|||
|
Item 15.
|
77 | ||
|
·
|
Made preparations for a prospective, multi-center safety and feasibility study (ATHENA Trial) in the US for the treatment of chronic myocardial ischemia. Prepared and filed Investigational Device Exemption (IDE) application with the FDA following a Pre-IDE meeting. In January 2012, Cytori received approval from the FDA to begin the ATHENA trial;
|
|
·
|
Initiated the ADVANCE multi-center clinical trial for acute heart attack patients in the EU; identified trial centers, sought hospital and country approvals, and began patient enrollment;
|
|
·
|
Prepared and submitted an application to our notified body in the EU to expand our CE Mark claims for the Celution® System to include no-option chronic myocardial ischemia patients and have maintained ongoing dialogue;
|
|
·
|
Continued patient follow-up from the APOLLO heart attack and PRECISE no-options chronic myocardial ischemia trials;
|
|
·
|
Reported final outcomes of RESTORE-2 lumpectomy defect reconstruction studies demonstrating high rate of patient and physician satisfaction with treatment results;
|
|
·
|
Obtained CE Mark claims for the next generation Celution® One System;
|
|
·
|
Prepared and submitted multiple regulatory filings in the United States, Europe, and Japan related to various cell and tissue processing systems under development;
|
|
·
|
Continued to optimize and develop the Celution
®
System family of products and next-generation devices, single-use consumables and related instrumentation;
|
|
Region
|
Clinical Applications
|
Regulatory Status
|
|
Japan
|
Cell Banking
|
Approved
|
|
Europe
|
Celution® 800 and Celution One: Cell Processing for re-implantation or re-infusion into same patient (General Processing)
|
CE Mark
|
|
Celution® 800 and Celution One: Breast reconstruction, healing of Crohn’s wounds and other cosmetic procedures
|
CE Mark
|
|
|
Celution® 800 Chronic myocardial ischemia
|
CE Mark submission for expanded claims under review
|
|
|
Acute Heart Attack
|
In clinical trial
|
|
|
Multiple specific surgical claims
|
Pre-clinical
|
|
|
Cell Concentration
|
CE Mark
|
|
|
Celution® One cosmetic and reconstructive surgery claims
|
CE Mark
|
|
|
U.S.
|
No option chronic myocardial ischemia
|
IDE/PMA safety and feasibility trial approved; to initiate in 2012
|
|
|
·
|
political unrest, terrorism and economic or financial instability;
|
|
|
·
|
unexpected changes and uncertainty in regulatory requirements and systems related
|
|
|
·
|
nationalization programs that may be implemented by foreign governments;
|
|
|
·
|
import-export regulations;
|
|
|
·
|
difficulties in enforcing agreements and collecting receivables;
|
|
|
·
|
difficulties in ensuring compliance with the laws and regulations of multiple jurisdictions;
|
|
|
·
|
changes in labor practices, including wage inflation, labor unrest and unionization policies;
|
|
|
·
|
longer payment cycles by international customers;
|
|
|
·
|
currency exchange fluctuations;
|
|
|
·
|
disruptions of service from utilities or telecommunications providers, including electricity shortages;
|
|
|
·
|
difficulties in staffing foreign branches and subsidiaries and in managing an expatriate workforce, and differing employment practices and labor issues;
|
|
|
·
|
potentially adverse tax consequences;
|
|
High
|
Low
|
|||||||
|
2010
|
||||||||
|
Quarter ended March 31, 2010
|
$ | 9.50 | $ | 4.40 | ||||
|
Quarter ended June 30, 2010
|
$ | 6.12 | $ | 3.42 | ||||
|
Quarter ended September 30, 2010
|
$ | 5.43 | $ | 3.15 | ||||
|
Quarter ended December 31, 2010
|
$ | 6.15 | $ | 4.07 | ||||
|
2011
|
||||||||
|
Quarter ended March 31, 2011
|
$ | 8.06 | $ | 5.18 | ||||
|
Quarter ended June 30, 2011
|
$ | 8.44 | $ | 4.50 | ||||
|
Quarter ended September 30, 2011
|
$ | 5.72 | $ | 2.32 | ||||
|
Quarter ended December 31, 2011
|
$ | 3.30 | $ | 1.90 | ||||
|
High
|
Low
|
|||||||
|
2010
|
||||||||
|
Quarter ended March 31, 2010
|
$ | 6.90 | $ | 2.62 | ||||
|
Quarter ended June 30, 2010
|
$ | 4.70 | $ | 1.94 | ||||
|
Quarter ended September 30, 2010
|
$ | 3.64 | $ | 2.05 | ||||
|
Quarter ended December 31, 2010
|
$ | 4.19 | $ | 2.99 | ||||
|
2011
|
||||||||
|
Quarter ended March 31, 2011
|
$ | 5.59 | $ | 3.39 | ||||
|
Quarter ended June 30, 2011
|
$ | 5.83 | $ | 2.68 | ||||
|
Quarter ended September 30, 2011
|
$ | 3.48 | $ | 1.49 | ||||
|
Quarter ended December 31, 2011
|
$ | 1.65 | $ | 0.78 | ||||
|
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
Weighted-average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column(a))
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by security holders (1)
|
2,226,053 | $ | 4.28 | — | ||||||||
|
Equity compensation plans not approved by security holders (2)
|
5,557,097 | $ | 5.18 | 1,050,036 | ||||||||
|
Total
|
7,783,150 | $ | 4.92 | 1,050,036 | ||||||||
|
|
(1)
|
The 1997 Stock Option and Stock Purchase Plan expired on October 22, 2007.
|
|
|
(2)
|
See Notes to our Consolidated Financial Statements included elsewhere herein for a description of our 2004 Equity Incentive Plan. The maximum number of shares shall be cumulatively increased on the first January 1 after the Effective Date, August 24, 2004, and each January 1 thereafter for 9 more years, by a number of shares equal to the lesser of (a) 2% of the number of shares issued and outstanding on the immediately preceding December 31, and (b) a number of shares set by the Board.
|
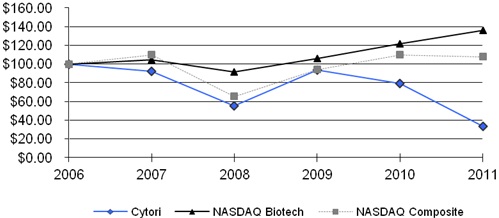
|
2011
|
2010
|
2009
|
2008
|
2007
|
||||||||||||||||
|
Statements of Operations Data:
|
||||||||||||||||||||
|
Product revenues:
|
||||||||||||||||||||
|
Sales to related party
|
$ | — | $ | 590 | $ | 591 | $ | 28 | $ | 792 | ||||||||||
|
Sales to third parties
|
7,983 | 7,664 | 5,246 | 4,500 | — | |||||||||||||||
| 7,983 | 8,254 | 5,837 | 4,528 | 792 | ||||||||||||||||
|
Cost of product revenues
|
3,837 | 3,908 | 3,394 | 1,854 | 422 | |||||||||||||||
|
Gross profit (loss)
|
4,146 | 4,346 | 2,443 | 2,674 | 370 | |||||||||||||||
|
Development revenues:
|
||||||||||||||||||||
|
Development, related party
|
1,992 | 2,122 | 8,840 | 774 | 5,158 | |||||||||||||||
|
Other, related party
|
— | — | — | 1,500 | — | |||||||||||||||
|
Research grants and other
|
21 | 251 | 53 | 51 | 99 | |||||||||||||||
| 2,013 | 2,373 | 8,893 | 2,325 | 5,257 | ||||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
10,904 | 9,687 | 12,231 | 17,371 | 20,020 | |||||||||||||||
|
Sales and marketing
|
13,560 | 11,040 | 6,583 | 4,602 | 2,673 | |||||||||||||||
|
General and administrative
|
14,727 | 12,570 | 10,415 | 11,727 | 14,184 | |||||||||||||||
|
Change in fair value of warrants
|
(4,360 | ) | (1,285 | ) | 4,574 | — | — | |||||||||||||
|
Change in fair value of option liabilities
|
740 | 30 | (920 | ) | 1,060 | 100 | ||||||||||||||
|
Total operating expenses
|
35,571 | 32,042 | 32,883 | 34,760 | 36,977 | |||||||||||||||
|
Total operating loss
|
(29,412 | ) | (25,323 | ) | (21,547 | ) | (29,761 | ) | (31,350 | ) | ||||||||||
|
Other income (expense):
|
||||||||||||||||||||
|
Gain on sale of assets
|
— | — | — | — | 1,858 | |||||||||||||||
|
Interest income
|
9 | 9 | 20 | 230 | 1,028 | |||||||||||||||
|
Interest expense
|
(2,784 | ) | (2,052 | ) | (1,427 | ) | (420 | ) | (155 | ) | ||||||||||
|
Other income (expense), net
|
(55 | ) | 23 | (218 | ) | (40 | ) | (46 | ) | |||||||||||
|
Equity loss in investments
|
(209 | ) | (151 | ) | (44 | ) | (45 | ) | (7 | ) | ||||||||||
|
Net loss
|
$ | (32,451 | ) | $ | (27,494 | ) | $ | (23,216 | ) | $ | (30,036 | ) | $ | (28,672 | ) | |||||
|
Basic and diluted net loss per share
|
$ | (0.61 | ) | $ | (0.60 | ) | $ | (0.65 | ) | $ | (1.12 | ) | $ | (1.25 | ) | |||||
|
Basic and diluted weighted average common shares
|
53,504,030 | 45,947,966 | 35,939,260 | 26,882,431 | 22,889,250 | |||||||||||||||
|
Statements of Cash Flows Data:
|
||||||||||||||||||||
|
Net cash used in operating activities
|
$ | (35,323 | ) | $ | (23,574 | ) | $ | (23,807 | ) | $ | (33,389 | ) | $ | (29,995 | ) | |||||
|
Net cash provided by (used in) investing activities
|
(560 | ) | (1,290 | ) | (221 | ) | (393 | ) | 5,982 | |||||||||||
|
Net cash provided by financing activities
|
20,137 | 64,678 | 24,271 | 34,928 | 26,576 | |||||||||||||||
|
Net increase (decrease) in cash
|
(15,746 | ) | 39,814 | 243 | 1,146 | 2,563 | ||||||||||||||
|
Cash and cash equivalents at beginning of year
|
52,668 | 12,854 | 12,611 | 11,465 | 8,902 | |||||||||||||||
|
Cash and cash equivalents at end of year
|
$ | 36,922 | $ | 52,668 | $ | 12,854 | $ | 12,611 | $ | 11,465 | ||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$ | 36,922 | $ | 52,668 | $ | 12,854 | $ | 12,611 | $ | 11,465 | ||||||||||
|
Working capital
|
35,516 | 45,730 | 9,915 | 10,090 | 4,168 | |||||||||||||||
|
Total assets
|
51,534 | 66,347 | 24,749 | 25,609 | 21,507 | |||||||||||||||
|
Deferred revenues, related party
|
3,520 | 5,512 | 7,634 | 16,474 | 18,748 | |||||||||||||||
|
Deferred revenues
|
5,244 | 4,929 | 2,388 | 2,445 | 2,379 | |||||||||||||||
|
Warrant liabilities
|
627 | 4,987 | 6,272 | — | — | |||||||||||||||
|
Option liabilities
|
1,910 | 1,170 | 1,140 | 2,060 | 1,000 | |||||||||||||||
|
Long-term deferred rent
|
504 | 398 | — | 168 | 473 | |||||||||||||||
|
Long-term obligations, less current portion
|
21,962 | 13,255 | 2,790 | 5,044 | 237 | |||||||||||||||
|
Total stockholders’ equity (deficit)
|
$ | 9,946 | $ | 22,873 | $ | (3,658 | ) | $ | (7,717 | ) | $ | (9,400 | ) | |||||||
|
|
·
|
Olympus paid $30,000,000 for its 50% interest in the Joint Venture. Moreover, Olympus simultaneously entered into a License/Joint Development Agreement with the Joint Venture and us to develop a second generation commercial system and manufacturing capabilities.
|
|
|
·
|
We licensed our device technology, including the Celution® System platform and certain related intellectual property, to the Joint Venture for use in future generation devices. These devices will process and purify adult stem and regenerative cells residing in adipose (fat) tissue for various therapeutic clinical applications. In exchange for this license, we received a 50% interest in the Joint Venture, as well as an initial $11,000,000 payment from the Joint Venture; the source of this payment was the $30,000,000 contributed to the Joint Venture by Olympus. Moreover, upon receipt of a CE mark for the first generation Celution® System platform in January 2006, we received an additional $11,000,000 development milestone payment from the Joint Venture.
|
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Related party
|
$ | — | $ | 590,000 | $ | 591,000 | ||||||
|
Third party
|
7,983,000 | 7,664,000 | 5,246,000 | |||||||||
|
Total product revenues
|
$ | 7,983,000 | $ | 8,254,000 | $ | 5,837,000 | ||||||
|
% attributable to Olympus
|
— | 0.1 | % | — | ||||||||
|
% attributable to Green Hospital Supply
|
— | 7.1 | % | 10.1 | % | |||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Cost of product revenues
|
$ | 3,772,000 | $ | 3,852,000 | $ | 3,340,000 | ||||||
|
Share-based compensation
|
65,000 | 56,000 | 54,000 | |||||||||
|
Total cost of product revenues
|
$ | 3,837,000 | $ | 3,908,000 | $ | 3,394,000 | ||||||
|
Total cost of product revenues as % of product revenues
|
48.1 | % | 47.3 | % | 58.1 | % | ||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Milestone revenue (Olympus)
|
$ | 1,992,000 | $ | 2,122,000 | $ | 8,840,000 | ||||||
|
Research grant (NIH)
|
— | — | 49,000 | |||||||||
|
Grant Revenue
|
— | 244,000 | — | |||||||||
|
Regenerative cell storage services
|
4,000 | 4,000 | 4,000 | |||||||||
|
Other
|
17,000 | 3,000 | — | |||||||||
|
Total development revenues
|
$ | 2,013,000 | $ | 2,373,000 | $ | 8,893,000 | ||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Research and development
|
$ | 10,021,000 | $ | 7,012,000 | $ | 9,007,000 | ||||||
|
Development milestone (Joint Venture)
|
396,000 | 2,221,000 | 2,713,000 | |||||||||
|
Research grants (NIH)
|
— | — | 49,000 | |||||||||
|
Stock-based compensation
|
487,000 | 454,000 | 462,000 | |||||||||
|
Total research and development expenses
|
$ | 10,904,000 | $ | 9,687,000 | $ | 12,231,000 | ||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Sales and marketing
|
$ | 12,674,000 | $ | 10,177,000 | $ | 6,076,000 | ||||||
|
Stock-based compensation
|
886,000 | 863,000 | 507,000 | |||||||||
|
Total sales and marketing
|
$ | 13,560,000 | $ | 11,040,000 | $ | 6,583,000 | ||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
General and administrative
|
$ | 12,849,000 | $ | 10,888,000 | $ | 8,789,000 | ||||||
|
Stock-based compensation
|
1,878,000 | 1,682,000 | 1,626,000 | |||||||||
|
Total general and administrative expenses
|
$ | 14,727,000 | $ | 12,570,000 | $ | 10,415,000 | ||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Cost of product revenues
|
$ | 65,000 | $ | 56,000 | $ | 54,000 | ||||||
|
Research and development related
|
487,000 | 454,000 | 462,000 | |||||||||
|
Sales and marketing related
|
886,000 | 863,000 | 507,000 | |||||||||
|
General and administrative related
|
1,878,000 | 1,682,000 | 1,626,000 | |||||||||
|
Total stock-based compensation
|
$ | 3,316,000 | $ | 3,055,000 | $ | 2,649,000 | ||||||
|
Options
|
Weighted Average Grant-Date Fair Value
|
|||||||
|
Outstanding at January 1, 2011
|
0 | |||||||
|
Granted
|
246,225 | $ | 5.82 | |||||
|
Vested
|
0 | |||||||
|
Cancelled/forfeited
|
0 | |||||||
|
Outstanding at December 31, 2011
|
246,225 | $ | 5.82 | |||||
|
Vested at December 31, 2011
|
0 | |||||||
|
Years ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Change in fair value of warrant liability
|
$ | (4,360,000 | ) | $ | (1,285,000 | ) | $ | 4,574,000 | ||||
|
|
As of
December 31, 2011
|
|
|
As of
December 31, 2010
|
|
|||
|
Expected term
|
|
1.61 years
|
|
|
2.61 years
|
|
||
|
Common stock market price
|
|
$
|
2.20
|
|
|
$
|
5.19
|
|
|
Risk-free interest rate
|
|
|
0.19
|
%
|
|
|
0.82
|
%
|
|
Expected volatility
|
|
|
69.98
|
%
|
|
|
86.03
|
%
|
|
Resulting fair value (per warrant)
|
|
$
|
0.32
|
|
|
$
|
2.50
|
|
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Change in fair value of option liability
|
$ | 740,000 | $ | 30,000 | $ | (920,000 | ) | |||||
|
December 31, 2011
|
December 31, 2010
|
December 31, 2009
|
||||||||||
|
Expected volatility of Cytori
|
76.07 | % | 73.00 | % | 72.00 | % | ||||||
|
Expected volatility of the Joint Venture
|
76.07 | % | 73.00 | % | 72.00 | % | ||||||
|
Bankruptcy recovery rate for Cytori
|
28.00 | % | 28.00 | % | 19.00 | % | ||||||
|
Bankruptcy threshold for Cytori
|
$ | 8,594,000 | $ | 5,842,000 | $ | 11,308,000 | ||||||
|
Probability of a change of control event for Cytori
|
3.33 | % | 3.43 | % | 2.95 | % | ||||||
|
Expected correlation between fair values of Cytori and the Joint Venture in the future
|
99.00 | % | 99.00 | % | 99.00 | % | ||||||
|
Risk free interest rate
|
1.89 | % | 3.30 | % | 3.85 | % | ||||||
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Interest income
|
$ | 9,000 | $ | 9,000 | $ | 20,000 | ||||||
|
Interest expense
|
(2,784,000 | ) | (2,052,000 | ) | (1,427,000 | ) | ||||||
|
Other income (expense), net
|
(55,000 | ) | 23,000 | (218,000 | ) | |||||||
|
Total
|
$ | (2,830,000 | ) | $ | (2,020,000 | ) | $ | (1,625,000 | ) | |||
|
|
·
|
Interest income remained comparable for the year ended December 31, 2011 as compared to the same period in 2010. Interest income decreased for the year December 31, 2010 as compared to the same period in 2009 and 2008 primarily due to a decrease in interest rates.
|
|
|
·
|
Interest expense increased for the year ended December 31, 2011 as compared to the same period in 2010 due to cash interest and non-cash amortization of debt issuance costs and debt discount for our $25.0 million term loan. In September 2011, we entered into a second amendment to the Amended and Restated Loan and Security Agreement, pursuant to which the lenders funded an additional principal increasing the total principal balance to $25.0 million. Interest expense increased in 2010 as compared to 2009 due to cash interest and non-cash amortization of debt issuance costs and debt discount for the $20.0 million term loan. During the second quarter of 2010, we entered into an Amended and Restated Loan and Security Agreement, pursuant to which the lenders funded a term loan in the amount of $20.0 million on June 14, 2010, and which refinanced the remaining balance of the term loan from 2008.
|
|
|
·
|
The changes in other income (expense) in 2011, 2010 and 2009 resulted primarily from changes in foreign currency exchange rates.
|
|
Years ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Equity loss from investment in joint venture
|
$ | (209,000 | ) | $ | (151,000 | ) | $ | (44,000 | ) | |||
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Cash and cash equivalents
|
$ | 36,922,000 | $ | 52,668,000 | ||||
|
Current assets
|
$ | 43,337,000 | $ | 58,953,000 | ||||
|
Current liabilities
|
7,821,000 | 13,223,000 | ||||||
|
Working capital
|
$ | 35,516,000 | $ | 45,730,000 | ||||
|
|
·
|
Receiving approximately $10,000,000 in gross proceeds from sale to institutional investors of a total of 4,771,174 shares of our common stock and warrants to purchase up to a total of 6,679,644 additional shares of our common stock with an exercise price of $2.59 per share in March 2009,
|
|
|
·
|
Receiving approximately $4,252,000 in gross proceeds from a private placement of 1,864,783 unregistered shares of common stock and 3,263,380 common stock warrants (with an exercise price of $2.62 per share) to a syndicate of investors in May 2009,
|
|
|
·
|
In June 2009, we entered into a common stock purchase agreement with Seaside 88, LP relating to the offering and sale of a total of up to 7,150,000 shares of our common stock.
The agreement required us to issue and Seaside to buy 275,000 shares of our common stock once every two weeks. Between June 2009 and June 2010, we raised an aggregate of approximately $30,172,000 in gross proceeds from the sale of 7,150,000 shares of our common stock,
|
|
|
·
|
In June 2010, we entered into an Amended and Restated Loan and Security Agreement with the GECC, SVB, and Oxford Finance Corporation (Lenders), pursuant to which the Lenders funded a term loan in the amount of $20,000,000 on June 14, 2010, which refinanced the remaining balance of the term loan entered into with GECC and SVB on October 14, 2008,
|
|
|
·
|
In October 2010, we entered into an underwriting agreement with Jefferies, relating to the issuance and sale of 4,600,000 shares of our common stock. This price to the public in this offering was $4.50 per share and Jefferies agreed to purchase the shares from us at a price of $4.23 per share. The transaction was completed on October 13, 2010 raising approximately $20,700,000 in gross proceeds before deducting underwriting discounts and commissions and other offering expenses payable by us, and
|
|
|
·
|
In December 2010, we raised $10,000,000 in gross proceeds from a sale of 1,428,571 shares of unregistered common stock to Astellas Pharma Inc. for $7.00 per share in a private stock placement.
|
|
|
·
|
In July 2011, we entered into a common stock purchase agreement with Seaside 88, LP relating to the offering and sale of a total of up to 6,326,262 shares of our common stock.
The agreement requires us to issue and Seaside to buy 1,326,262 shares of our common stock at an initial closing and 250,000 shares of our common stock once every two weeks, commencing 30 days after the initial closing, for up to an additional 20 closings, subject to the satisfaction of customary closing conditions. At the initial closing, the offering price was $4.52, which equaled to 88% of our common stock’s volume-weighted average trading prices, or VWAP, during the ten-day trading period immediately prior to the initial closing date, raising approximately $6,000,000 in gross proceeds. At subsequent closings, the offering price will equal 90.25% of our common stock’s volume-weighted average trading prices during the ten-day trading period immediately prior to each subsequent closing date. We raised an aggregate of approximately $13,286,000 in gross proceeds from the sale of 4,076,262 shares in our scheduled closings through December 31, 2011.
|
|
|
·
|
In September 2011, we entered into an Second Amendment to the Amended and Restated Loan and Security Agreement with the GECC, SVB, and Oxford Finance Corporation (Lenders), pursuant to which the Lenders increased the prior term loan made to the Company to a principal amount of $25.0 million.
|
|
Payments due by period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than 1
year
|
1 – 3 years
|
3 – 5 years
|
More than
5 years
|
|||||||||||||||
|
Long-term obligations
|
$ | 26,296,000 | $ | 2,693,000 | $ | 19,848,000 | $ | 3,755,000 | $ | — | ||||||||||
|
Interest commitment on long-term obligations
|
5,103,000 | 2,498,000 | 2,561,000 | 44,000 | — | |||||||||||||||
|
Operating lease obligations
|
10,836,000 | 1,879,000 | 3,679,000 | 3,688,000 | 1,590,000 | |||||||||||||||
|
Minimum purchase requirements
|
2,191,000 | 1,341,000 | 850,000 | — | — | |||||||||||||||
|
Pre-clinical research study obligations
|
60,000 | 60,000 | — | — | — | |||||||||||||||
|
Clinical research study obligations
|
13,800,000 | 3,250,000 | 8,650,000 | 1,900,000 | — | |||||||||||||||
|
Total
|
$ | 58,286,000 | $ | 11,721,000 | $ | 35,588,000 | $ | 9,387,000 | $ | 1,590,000 | ||||||||||
|
Years Ended
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Net cash used in operating activities
|
$ | (35,323,000 | ) | $ | (23,574,000 | ) | $ | (23,807,000 | ) | |||
|
Net cash used in investing activities
|
(560,000 | ) | (1,290,000 | ) | (221,000 | ) | ||||||
|
Net cash provided by financing activities
|
20,137,000 | 64,678,000 | 24,271,000 | |||||||||
|
Reports of Independent Registered Public Accounting Firm
|
41 |
|
Consolidated Balance Sheets as of December 31, 2011 and 2010
|
43 |
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2011, 2010 and 2009
|
44 |
|
Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2011, 2010 and 2009
|
45 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2011, 2010 and 2009
|
47 |
|
Notes to Consolidated Financial Statements
|
49 |
|
/s/ KPMG LLP
|
|
/s/ KPMG LLP
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 36,922,000 | $ | 52,668,000 | ||||
|
Accounts receivable, net of reserves of $474,000 and of $306,000 in 2011 and 2010, respectively
|
2,260,000 | 2,073,000 | ||||||
|
Inventories, net
|
3,318,000 | 3,378,000 | ||||||
|
Other current assets
|
837,000 | 834,000 | ||||||
|
Total current assets
|
43,337,000 | 58,953,000 | ||||||
|
Property and equipment, net
|
1,711,000 | 1,684,000 | ||||||
|
Restricted cash and cash equivalents
|
350,000 | 350,000 | ||||||
|
Investment in joint venture
|
250,000 | 459,000 | ||||||
|
Other assets
|
1,772,000 | 566,000 | ||||||
|
Intangibles, net
|
192,000 | 413,000 | ||||||
|
Goodwill
|
3,922,000 | 3,922,000 | ||||||
|
Total assets
|
$ | 51,534,000 | $ | 66,347,000 | ||||
|
Liabilities and Stockholders’ Equity (Deficit)
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses
|
$ | 5,334,000 | $ | 6,770,000 | ||||
|
Current portion of long-term obligations
|
2,487,000 | 6,453,000 | ||||||
|
Total current liabilities
|
7,821,000 | 13,223,000 | ||||||
|
Deferred revenues, related party
|
3,520,000 | 5,512,000 | ||||||
|
Deferred revenues
|
5,244,000 | 4,929,000 | ||||||
|
Warrant liability
|
627,000 | 4,987,000 | ||||||
|
Option liability
|
1,910,000 | 1,170,000 | ||||||
|
Long-term deferred rent
|
504,000 | 398,000 | ||||||
|
Long-term obligations, net of discount, less current portion
|
21,962,000 | 13,255,000 | ||||||
|
Total liabilities
|
41,588,000 | 43,474,000 | ||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders’ equity (deficit):
|
||||||||
|
Preferred stock, $0.001 par value; 5,000,000 shares authorized; -0- shares issued and outstanding in 2011 and 2010
|
— | — | ||||||
|
Common stock, $0.001 par value; 95,000,000 shares authorized; 56,594,683 and 51,955,265 shares issued and 56,594,683 and 51,955,265 shares outstanding in 2011 and 2010, respectively
|
57,000 | 52,000 | ||||||
|
Additional paid-in capital
|
252,338,000 | 232,819,000 | ||||||
|
Accumulated deficit
|
(242,449,000 | ) | (209,998,000 | ) | ||||
|
Total stockholders’ equity
|
9,946,000 | 22,873,000 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 51,534,000 | $ | 66,347,000 | ||||
|
For the Years Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Product revenues:
|
||||||||||||
|
Related party
|
$ | — | $ | 590,000 | $ | 591,000 | ||||||
|
Third party
|
7,983,000 | 7,664,000 | 5,246,000 | |||||||||
| 7,983,000 | 8,254,000 | 5,837,000 | ||||||||||
|
Cost of product revenues
|
3,837,000 | 3,908,000 | 3,394,000 | |||||||||
|
Gross profit
|
4,146,000 | 4,346,000 | 2,443,000 | |||||||||
|
Development revenues:
|
||||||||||||
|
Development, related party
|
1,992,000 | 2,122,000 | 8,840,000 | |||||||||
|
Research grants and other
|
21,000 | 251,000 | 53,000 | |||||||||
| 2,013,000 | 2,373,000 | 8,893,000 | ||||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
10,904,000 | 9,687,000 | 12,231,000 | |||||||||
|
Sales and marketing
|
13,560,000 | 11,040,000 | 6,583,000 | |||||||||
|
General and administrative
|
14,727,000 | 12,570,000 | 10,415,000 | |||||||||
|
Change in fair value of warrants
|
(4,360,000 | ) | (1,285,000 | ) | 4,574,000 | |||||||
|
Change in fair value of option liability
|
740,000 | 30,000 | (920,000 | ) | ||||||||
|
Total operating expenses
|
35,571,000 | 32,042,000 | 32,883,000 | |||||||||
|
Operating loss
|
(29,412,000 | ) | (25,323,000 | ) | (21,547,000 | ) | ||||||
|
Other income (expense):
|
||||||||||||
|
Interest income
|
9,000 | 9,000 | 20,000 | |||||||||
|
Interest expense
|
(2,784,000 | ) | (2,052,000 | ) | (1,427,000 | ) | ||||||
|
Other income (expense), net
|
(55,000 | ) | 23,000 | (218,000 | ) | |||||||
|
Equity loss from investment in joint venture
|
(209,000 | ) | (151,000 | ) | (44,000 | ) | ||||||
|
Total other income (expense)
|
(3,039,000 | ) | (2,171,000 | ) | (1,669,000 | ) | ||||||
|
Net loss
|
(32,451,000 | ) | (27,494,000 | ) | (23,216,000 | ) | ||||||
|
Basic and diluted net loss per common share
|
$ | (0.61 | ) | $ | (0.60 | ) | $ | (0.65 | ) | |||
|
Basic and diluted weighted average common shares
|
53,504,030 | 45,947,966 | 35,939,260 | |||||||||
|
Accumulated
|
Amount due
|
|||||||||||||||||||||||||||||||||||
|
Additional
|
Other
|
From
|
||||||||||||||||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Accumulated
|
Treasury Stock
|
Comprehensive
|
Exercises of
|
|||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Shares
|
Amount
|
Income (Loss)
|
Stock Options
|
Total
|
||||||||||||||||||||||||||||
|
Balance at December 31, 2008
|
31,176,275 | $ | 31,000 | $ | 161,214,000 | $ | (162,168,000 | ) | 1,872,834 | $ | (6,794,000 | ) | — | — | $ | (7,717,000 | ) | |||||||||||||||||||
|
Cumulative effect of change in accounting for certain warrants
|
— | — | (4,578,000 | ) | 2,880,000 | — | — | — | — | (1,698,000 | ) | |||||||||||||||||||||||||
|
Stock-based compensation expense
|
— | — | 2,649,000 | — | — | — | — | — | 2,649,000 | |||||||||||||||||||||||||||
|
Issuance of common stock under stock option plan
|
203,707 | — | 410,000 | — | — | — | — | — | 410,000 | |||||||||||||||||||||||||||
|
Issuance of common stock under stock warrant agreement
|
46,154 | 121,000 | — | — | — | — | — | 121,000 | ||||||||||||||||||||||||||||
|
Sale of common stock, net
|
8,613,123 | 9,000 | 21,851,000 | — | — | — | — | — | 21,860,000 | |||||||||||||||||||||||||||
|
Sale of treasury stock
|
— | — | (2,861,000 | ) | — | (1,872,834 | ) | 6,794,000 | — | — | 3,933,000 | |||||||||||||||||||||||||
|
Net loss for the year ended December 31, 2009
|
— | — | — | (23,216,000 | ) | — | — | — | — | (23,216,000 | ) | |||||||||||||||||||||||||
|
Balance at December 31, 2009
|
40,039,259 | 40,000 | 178,806,000 | (182,504,000 | ) | — | — | — | — | (3,658,000 | ) | |||||||||||||||||||||||||
|
Stock-based compensation expense
|
— | — | 3,055,000 | — | — | — | — | — | 3,055,000 | |||||||||||||||||||||||||||
|
Issuance of common stock under stock option plan
|
378,705 | — | 1,393,000 | — | — | — | — | — | 1,393,000 | |||||||||||||||||||||||||||
|
Issuance of common stock under stock warrant agreement
|
2,208,730 | 2,000 | 5,733,000 | — | — | — | — | — | 5,735,000 | |||||||||||||||||||||||||||
|
Accumulated
|
Amount due
|
|||||||||||||||||||||||||||||||||||
|
Additional
|
Other
|
From
|
||||||||||||||||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Accumulated
|
Treasury Stock
|
Comprehensive
|
Exercises of
|
|||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Shares
|
Amount
|
Income (Loss)
|
Stock Options
|
Total
|
||||||||||||||||||||||||||||
|
Sale of common stock, net
|
9,328,571 | 10,000 | 43,553,000 | — | — | — | — | — | 43,563,000 | |||||||||||||||||||||||||||
|
Allocation of fair value for debt-related warrants
|
— | — | 279,000 | — | — | — | — | — | 279,000 | |||||||||||||||||||||||||||
|
Net loss for the year ended December 31, 2010
|
— | — | — | (27,494,000 | ) | — | — | — | — | (27,494,000 | ) | |||||||||||||||||||||||||
|
Balance at December 31, 2010
|
51,955,265 | $ | 52,000 | $ | 232,819,000 | $ | (209,998,000 | ) | — | $ | — | $ | — | $ | — | $ | 22,873,000 | |||||||||||||||||||
|
Stock-based compensation expense
|
— | — | 3,316,000 | — | — | — | — | — | 3,316,000 | |||||||||||||||||||||||||||
|
Issuance of common stock under stock option plan
|
222,283 | — | 767,000 | — | — | — | — | — | 767,000 | |||||||||||||||||||||||||||
|
Issuance of common stock under stock warrant agreement
|
340,873 | 1,000 | 2,081,000 | — | — | — | — | — | 2,082,000 | |||||||||||||||||||||||||||
|
Sale of common stock, net
|
4,076,262 | 4,000 | 13,088,000 | — | — | — | — | — | 13,092,000 | |||||||||||||||||||||||||||
|
Allocation of fair value for debt-related warrants
|
— | — | 267,000 | — | — | — | — | — | 267,000 | |||||||||||||||||||||||||||
|
Net loss for the year ended December 31, 2011
|
— | — | — | (32,451,000 | ) | — | — | — | — | (32,451,000 | ) | |||||||||||||||||||||||||
|
Balance at December 31, 2011
|
56,594,683 | $ | 57,000 | $ | 252,338,000 | $ | (242,449,000 | ) | — | $ | — | $ | — | $ | — | $ | 9,946,000 | |||||||||||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$ | (32,451,000 | ) | $ | (27,494,000 | ) | $ | (23,216,000 | ) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
855,000 | 931,000 | 1,681,000 | |||||||||
|
Amortization of deferred financing costs and debt discount
|
711,000 | 703,000 | 709,000 | |||||||||
|
Warranty provision (reversal)
|
— | — | (23,000 | ) | ||||||||
|
Increase (reduction) in allowance for doubtful accounts
|
483,000 | 460,000 | 663,000 | |||||||||
|
Change in fair value of warrants
|
(4,360,000 | ) | (1,285,000 | ) | 4,574,000 | |||||||
|
Change in fair value of option liability
|
740,000 | 30,000 | (920,000 | ) | ||||||||
|
Stock-based compensation
|
3,316,000 | 3,055,000 | 2,649,000 | |||||||||
|
Equity loss from investment in joint venture
|
209,000 | 151,000 | 44,000 | |||||||||
|
Increases (decreases) in cash caused by changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
(670,000 | ) | (902,000 | ) | (986,000 | ) | ||||||
|
Inventories
|
60,000 | (777,000 | ) | (446,000 | ) | |||||||
|
Other current assets
|
(3,000 | ) | 36,000 | 41,000 | ||||||||
|
Other assets
|
(1,206,000 | ) | (110,000 | ) | 75,000 | |||||||
|
Accounts payable and accrued expenses
|
(1,436,000 | ) | 811,000 | 413,000 | ||||||||
|
Deferred revenues, related party
|
(1,992,000 | ) | (2,122,000 | ) | (8,840,000 | ) | ||||||
|
Deferred revenues
|
315,000 | 2,541,000 | (57,000 | ) | ||||||||
|
Long-term deferred rent
|
106,000 | 398,000 | (168,000 | ) | ||||||||
|
Net cash used in operating activities
|
(35,323,000 | ) | (23,574,000 | ) | (23,807,000 | ) | ||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchases of property and equipment
|
(560,000 | ) | (610,000 | ) | (221,000 | ) | ||||||
|
Cash invested in restricted cash
|
— | (350,000 | ) | — | ||||||||
|
Investment in joint venture
|
— | (330,000 | ) | — | ||||||||
|
Net cash used in investing activities
|
(560,000 | ) | (1,290,000 | ) | (221,000 | ) | ||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Principal payments on long-term obligations
|
(4,529,000 | ) | (5,454,000 | ) | (2,053,000 | ) | ||||||
|
Proceeds from long-term obligations
|
9,444,000 | 20,000,000 | — | |||||||||
|
Debt issuance costs and loan fees
|
(719,000 | ) | (559,000 | ) | — | |||||||
|
Proceeds from exercise of employee stock options and warrants
|
2,849,000 | 7,128,000 | 531,000 | |||||||||
|
Proceeds from sale of common stock
|
13,286,000 | 45,486,000 | 23,196,000 | |||||||||
|
Costs from sale of common stock
|
(194,000 | ) | (1,923,000 | ) | (1,336,000 | ) | ||||||
|
Proceeds from sale of treasury stock
|
— | — | 3,933,000 | |||||||||
|
Net cash provided by financing activities
|
20,137,000 | 64,678,000 | 24,271,000 | |||||||||
|
Net (decrease) increase in cash and cash equivalents
|
(15,746,000 | ) | 39,814,000 | 243,000 | ||||||||
|
Cash and cash equivalents at beginning of year
|
52,668,000 | 12,854,000 | 12,611,000 | |||||||||
|
Cash and cash equivalents at end of year
|
$ | 36,922,000 | $ | 52,668,000 | $ | 12,854,000 | ||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Supplemental disclosure of cash flows information:
|
||||||||||||
|
Cash paid during period for:
|
||||||||||||
|
Interest
|
$ | 2,031,000 | $ | 1,226,000 | $ | 739,000 | ||||||
|
Final payment fee on long-term debt
|
419,000 | 205,000 | — | |||||||||
|
Supplemental schedule of non-cash investing and financing activities:
|
||||||||||||
|
Fair value of warrants allocated to additional paid-in capital
|
$ | 267,000 | $ | 279,000 | $ | — | ||||||
|
Additions to fixed assets included in accounts payable and accrued expenses
|
— | 481,000 | — | |||||||||
|
Capital equipment lease
|
79,000 | — | — | |||||||||
|
1.
|
Organization and Operations
|
|
2.
|
Summary of Significant Accounting Policies
|
|
December 31, 2011
|
||||
|
Other intangibles, net:
|
||||
|
Beginning balance
|
$ | 413,000 | ||
|
Amortization
|
(221,000 | ) | ||
|
Ending balance
|
192,000 | |||
|
Goodwill, net:
|
||||
|
Beginning balance
|
3,922,000 | |||
|
Increase (decrease)
|
— | |||
|
Ending balance
|
3,922,000 | |||
|
Total goodwill and other intangibles, net
|
$ | 4,114,000 | ||
|
Cumulative amortization of other intangible assets
|
$ | 2,024,000 | ||
|
December 31, 2010
|
||||
|
Other intangibles, net:
|
||||
|
Beginning balance
|
$ | 635,000 | ||
|
Amortization
|
(222,000 | ) | ||
|
Ending balance
|
413,000 | |||
|
Goodwill, net:
|
||||
|
Beginning balance
|
3,922,000 | |||
|
Increase (decrease)
|
— | |||
|
Ending balance
|
3,922,000 | |||
|
Total goodwill and other intangibles, net
|
$ | 4,335,000 | ||
|
Cumulative amortization of other intangible assets
|
$ | 1,803,000 | ||
|
2012
|
$ |
192,000
|
|
|
|
$
|
192,000
|
|
|
As of
December 31, 2011
|
As of
December 31, 2010
|
|
|||||
|
Expected term
|
|
1.61 years
|
2.61 years
|
|
||||
|
Common stock market price
|
|
$
|
2.20
|
$
|
5.19
|
|
||
|
Risk-free interest rate
|
|
|
0.19
|
%
|
|
0.82
|
%
|
|
|
Expected volatility
|
|
|
69.98
|
%
|
|
86.03
|
%
|
|
|
Resulting fair value (per warrant)
|
|
$
|
0.32
|
$
|
2.50
|
|
||
|
3.
|
Transactions with Olympus Corporation
|
|
|
·
|
Olympus paid $30,000,000 for its 50% interest in the Joint Venture. Moreover, Olympus simultaneously entered into a License/Joint Development Agreement with the Joint Venture and us to develop a second generation commercial system and manufacturing capabilities.
|
|
|
·
|
We licensed our Celution® System device technology and certain related intellectual property, to the Joint Venture for use in future generation devices. These devices will process and purify regenerative cells residing in adipose tissue for various therapeutic clinical applications. In exchange for this license, we received a 50% interest in the Joint Venture, as well as an initial $11,000,000 payment from the Joint Venture; the source of this payment was the $30,000,000 contributed to the Joint Venture by Olympus. Moreover, upon receipt of a CE mark for the Celution® 600 in January 2006, we received an additional $11,000,000 development milestone payment from the Joint Venture.
|
|
December 31, 2011
|
December 31, 2010
|
November 4, 2005
|
||||||||||
|
Expected volatility of Cytori
|
76.07 | % | 73.00 | % | 63.20 | % | ||||||
|
Expected volatility of the Joint Venture
|
76.07 | % | 73.00 | % | 69.10 | % | ||||||
|
Bankruptcy recovery rate for Cytori
|
28.00 | % | 28.00 | % | 21.00 | % | ||||||
|
Bankruptcy threshold for Cytori
|
$ | 8,594,000 | $ | 5,842,000 | $ | 10,780,000 | ||||||
|
Probability of a change of control event for Cytori
|
3.33 | % | 3.43 | % | 3.04 | % | ||||||
|
Expected correlation between fair values of Cytori and the Joint Venture in the future
|
99.00 | % | 99.00 | % | 99.00 | % | ||||||
|
Risk free interest rate
|
1.89 | % | 3.30 | % | 4.66 | % | ||||||
|
December 31, 2011
|
December 31, 2010
|
|||||||
|
(Unaudited)
|
(Unaudited)
|
|||||||
|
Balance Sheets
|
||||||||
|
Assets:
|
||||||||
|
Cash
|
$ | 69,000 | $ | 183,000 | ||||
|
Amounts due from related party
|
104,000 | 632,000 | ||||||
|
Prepaid insurance
|
19,000 | 16,000 | ||||||
|
Computer equipment and software, net
|
797,000 | 995,000 | ||||||
|
Total assets
|
$ | 989,000 | $ | 1,826,000 | ||||
|
Liabilities and Stockholders’ Equity:
|
||||||||
|
Accrued expenses
|
$ | 48,000 | $ | 77,000 | ||||
|
Amounts due to related party
|
95,000 | 509,000 | ||||||
|
Stockholders’ equity
|
846,000 | 1,240,000 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 989,000 | $ | 1,826,000 | ||||
|
Years ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Statements of Operations
|
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
|||||||||
|
Product revenue
|
$ | 90,000 | $ | 458,000 | $ | — | ||||||
|
Cost of product revenue
|
87,000 | 458,000 | — | |||||||||
|
Gross profit
|
3,000 | — | — | |||||||||
|
Royalty revenue
|
166,000 | 253,000 | 242,000 | |||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
— | 14,000 | — | |||||||||
|
General and administrative:
|
||||||||||||
|
Accounting and other corporate services
|
164,000 | 88,000 | 75,000 | |||||||||
|
Quality system services
|
145,000 | 135,000 | 63,000 | |||||||||
|
Depreciation expense for tooling equipment
|
230,000 | 130,000 | — | |||||||||
|
Other
|
23,000 | 33,000 | 26,000 | |||||||||
|
Operating expenses
|
562,000 | 400,000 | 164,000 | |||||||||
|
Operating income (loss)
|
(393,000 | ) | (147,000 | ) | 78,000 | |||||||
|
Other income (expense):
|
||||||||||||
|
Interest income
|
— | 1,000 | 1,000 | |||||||||
|
Net income (loss)
|
$ | (393,000 | ) | $ | (146,000 | ) | $ | 79,000 | ||||
|
Reconciliation of net income (loss) to equity loss from investment in joint venture
|
||||||||||||
|
Net income (loss)
|
$ | (393,000 | ) | $ | (146,000 | ) | $ | 79,000 | ||||
|
Intercompany eliminations
|
25,000 | 156,000 | 167,000 | |||||||||
|
Net loss after intercompany eliminations
|
(418,000 | ) | (302,000 | ) | (88,000 | ) | ||||||
|
Cytori’s percentage of interest in joint venture
|
50 | % | 50 | % | 50 | % | ||||||
|
Cytori’s equity loss from investment in joint venture
|
$ | (209,000 | ) | $ | (151,000 | ) | $ | (44,000 | ) | |||
|
4.
|
Fair Value Measurements
|
|
Balance as of
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
December 31, 2011
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash equivalents
|
$ | 30,646,000 | $ | 30,646,000 | $ | — | $ | — | ||||||||
|
Liabilities:
|
||||||||||||||||
|
Put option liability
|
$ | (1,910,000 | ) | $ | — | $ | — | $ | (1,910,000 | ) | ||||||
|
Warrant liability
|
$ | (627,000 | ) | $ | — | $ | — | $ | (627,000 | ) | ||||||
|
Balance as of
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
December 31, 2010
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash equivalents
|
$ | 39,807,000 | $ | 39,807,000 | $ | — | $ | — | ||||||||
|
Liabilities:
|
||||||||||||||||
|
Put option liability
|
$ | (1,170,000 | ) | $ | — | $ | — | $ | (1,170,000 | ) | ||||||
|
Warrant liability
|
$ | (4,987,000 | ) | $ | — | $ | — | $ | (4,987,000 | ) | ||||||
|
Year ended
|
Year ended
|
|||||||
|
Put option liability
|
December 31, 2011
|
December 31, 2010
|
||||||
|
Beginning balance
|
$ | (1,170,000 | ) | $ | (1,140,000 | ) | ||
|
Decrease (increase) in fair value recognized in operating expenses
|
(740,000 | ) | (30,000 | ) | ||||
|
Ending balance
|
$ | (1,910,000 | ) | $ | (1,170,000 | ) | ||
|
Year ended
|
Year ended
|
|||||||
|
Warrant liability
|
December 31, 2011
|
December 31, 2010
|
||||||
|
Beginning balance
|
$ | (4,987,000 | ) | $ | (6,272,000 | ) | ||
|
Decrease (increase) in fair value recognized in operating expenses
|
4,360,000 | 1,285,000 | ||||||
|
Ending balance
|
$ | (627,000 | ) | $ | (4,987,000 | ) | ||
|
5.
|
Fair Value
|
|
December 31, 2011
|
December 31, 2010
|
|||||||||||||||
|
Fair Value
|
Carrying Value
|
Fair Value
|
Carrying Value
|
|||||||||||||
|
Fixed rate long-term debt
|
$ | 24,211,000 | $ | 24,341,000 | $ | 19,782,000 | $ | 19,679,000 | ||||||||
|
6.
|
Thin Film Japan Distribution Agreement
|
|
|
·
|
Anti-adhesion,
|
|
|
·
|
Soft tissue support, and
|
|
|
·
|
Minimization of the attachment of soft tissues throughout the body.
|
|
7.
|
Composition of Certain Financial Statement Captions
|
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Raw materials
|
$ | 1,503,000 | $ | 2,311,000 | ||||
|
Work in process
|
790,000 | 410,000 | ||||||
|
Finished goods
|
1,025,000 | 657,000 | ||||||
| $ | 3,318,000 | $ | 3,378,000 | |||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
|
|
|||||||
|
Prepaid insurance
|
$ | 234,000 | $ | 230,000 | ||||
|
Prepaid other
|
372,000 | 477,000 | ||||||
|
Other receivables
|
231,000 | 127,000 | ||||||
| $ | 837,000 | $ | 834,000 | |||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
|
|
|||||||
|
Manufacturing and development equipment
|
$ | 4,268,000 | $ | 4,035,000 | ||||
|
Office and computer equipment
|
2,177,000 | 2,137,000 | ||||||
|
Leasehold improvements
|
3,255,000 | 3,125,000 | ||||||
| 9,700,000 | 9,297,000 | |||||||
|
Less accumulated depreciation and amortization
|
(7,989,000 | ) | (7,613,000 | ) | ||||
| $ | 1,711,000 | $ | 1,684,000 | |||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
|
|
|||||||
|
Accrued legal fees
|
$ | 829,000 | $ | 646,000 | ||||
|
Accrued R&D studies
|
534,000 | 1,227,000 | ||||||
|
Accounts payable
|
272,000 | 601,000 | ||||||
|
Accrued vacation
|
908,000 | 760,000 | ||||||
|
Accrued bonus
|
866,000 | 1,045,000 | ||||||
|
Accrued expenses
|
1,572,000 | 2,077,000 | ||||||
|
Deferred rent
|
37,000 | 17,000 | ||||||
|
Accrued accounting fees
|
90,000 | 135,000 | ||||||
|
Accrued payroll
|
226,000 | 262,000 | ||||||
| $ | 5,334,000 | $ | 6,770,000 | |||||
|
8.
|
Commitments and Contingencies
|
|
Years Ending December 31,
|
Operating
Leases
|
|||
|
2012
|
$ | 1,879,000 | ||
|
2013
|
1,913,000 | |||
|
2014
|
1,766,000 | |||
|
2015
|
1,821,000 | |||
|
2016
|
1,867,000 | |||
|
2017
|
1,590,000 | |||
|
Total
|
$ | 10,836,000 | ||
|
9.
|
Long-term Obligations
|
|
Origination Date
|
Original Loan
Amount
|
Interest
Rate
|
Current
Monthly
Payment*
|
Term
|
Remaining
Principal
(Face Value)
|
||||||||||||
|
September 2011
|
$ | 25,000,000 | 9.87 | % | $ | 225,622 |
42 Months
|
$ | 24,938,000 | ||||||||
|
|
*
|
Current monthly payment is inclusive of interest and principal
|
|
Years Ending December 31,
|
||||
|
2012
|
$ | 2,693,000 | ||
|
2013
|
9,927,000 | |||
|
2014
|
9,921,000 | |||
|
2015
|
3,749,000 | |||
|
2016
|
6,000 | |||
|
Total
|
$ | 26,296,000 | ||
|
Reconciliation of Face Value to Book Value as of December 31, 2011
|
||||
|
Total debt and lease obligations, including final payment fee (Face Value)
|
$ | 26,296,000 | ||
|
Less: Debt discount
|
(1,847,000 | ) | ||
|
Total:
|
24,449,000 | |||
|
Less: Current portion
|
(2,487,000 | ) | ||
|
Long-term obligation
|
$ | 21,962,000 | ||
|
10.
|
Income Taxes
|
|
2011
|
2010
|
2009
|
||||||||||
|
Income tax expense (benefit) at federal statutory rate
|
(34.00 | ) % | (34.00 | ) % | (34.00 | ) % | ||||||
|
Income tax expense (benefit) at state statutory rate
|
(3.36 | ) % | (2.62 | ) % | (2.61 | ) % | ||||||
|
Mark to market permanent adjustment
|
(5.02 | ) % | (1.71 | ) % | 7.21 | % | ||||||
|
Change in federal valuation allowance
|
45.72 | % | 40.47 | % | 8.16 | % | ||||||
|
Change in State Rate
|
(3.29 | ) % | 0.00 | % | 24.55 | % | ||||||
|
Deferred revenue
|
(2.09 | ) % | (2.82 | ) % | (0.28 | ) % | ||||||
|
Other, net
|
2.04 | % | 0.68 | % | (3.03 | ) % | ||||||
| 0.00 | % | 0.00 | % | 0.00 | % | |||||||
|
2011
|
2010
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Allowances and reserves
|
$ | 292,000 | $ | 217,000 | ||||
|
Accrued expenses
|
587,000 | 540,000 | ||||||
|
Deferred revenue
|
3,276,000 | 3,164,000 | ||||||
|
Stock based compensation
|
4,886,000 | 3,860,000 | ||||||
|
Net operating loss carryforwards
|
73,774,000 | 61,398,000 | ||||||
|
Income tax credit carryforwards
|
5,569,000 | 5,242,000 | ||||||
|
Property and equipment, principally due to differences in depreciation
|
707,000 | 821,000 | ||||||
|
Other
|
181,000 | 0 | ||||||
| 89,272,000 | 75,242,000 | |||||||
|
Valuation allowance
|
(89,200,000 | ) | (74,994,000 | ) | ||||
|
Total deferred tax assets, net of allowance
|
72,000 | 248,000 | ||||||
|
Deferred tax liabilities:
|
||||||||
|
Intangibles
|
(72,000 | ) | (151,000 | ) | ||||
|
Capitalized Assets and other
|
0 | (97,000 | ) | |||||
|
Total deferred tax liability
|
(72,000 | ) | (248,000 | ) | ||||
|
Net deferred tax assets (liability)
|
$ | — | $ | — | ||||
|
2011
|
2010
|
2009
|
||||||||||
|
Unrecognized Tax Benefits – Beginning
|
$ | 1,166,000 | $ | 1,115,000 | $ | 952,000 | ||||||
|
Gross increases – tax positions in prior period
|
— | — | 4,000 | |||||||||
|
Gross decreases – tax positions in prior period
|
— | (49,000 | ) | — | ||||||||
|
Gross increase – current-period tax positions
|
138,000 | 100,000 | 159,000 | |||||||||
|
Settlements
|
— | — | — | |||||||||
|
Lapse of statute of limitations
|
— | — | — | |||||||||
|
Unrecognized Tax Benefits – Ending
|
$ | 1,304,000 | $ | 1,166,000 | $ | 1,115,000 | ||||||
|
11.
|
Employee Benefit Plan
|
|
12.
|
Stockholders’ Equity (Deficit)
|
|
13.
|
Stockholders Rights Plan
|
|
14.
|
Stock-based Compensation
|
|
|
·
|
12/48 of a granted award will vest after one year of service, while an additional 1/48 of the award will vest at the end of each month thereafter for 36 months, or
|
|
|
·
|
1/48 of the award will vest at the end of each month over a four-year period.
|
|
Options
|
Weighted
Average
Exercise Price
|
|||||||
|
Balance as of January 1, 2011
|
7,050,689 | $ | 5.19 | |||||
|
Granted
|
1,113,950 | $ | 5.20 | |||||
|
Exercised
|
(200,755 | ) | $ | 3. 82 | ||||
|
Expired
|
(397,496 | ) | $ | 7.12 | ||||
|
Cancelled/forfeited
|
(109,204 | ) | $ | 4.65 | ||||
|
Balance as of December 31, 2011
|
7,457,184 | $ | 5.13 | |||||
|
Options
|
Weighted
Average
Exercise Price
|
Weighted
Average
Remaining
Contractual
Term (years)
|
Aggregate
Intrinsic Value
|
|||||||||||||
|
Balance as of December 31, 2011
|
7,457,184 | $ | 5.13 | 5.53 | $ | 34,450 | ||||||||||
|
Vested and expected to vest at December 31, 2011
|
7,417,443 | $ | 5.13 | 5.52 | $ | 34,271 | ||||||||||
|
Exercisable at December 31, 2011
|
5,636,839 | $ | 5.02 | 4.57 | $ | 22,181 | ||||||||||
|
Years ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Expected term
|
5.5 years
|
5 years
|
5 years
|
|||||||||
|
Risk-free interest rate
|
1.95 | % | 2.22 | % | 1.94 | % | ||||||
|
Volatility
|
72.36 | % | 72.81 | % | 66.80 | % | ||||||
|
Dividends
|
— | — | — | |||||||||
|
Resulting weighted average grant date fair value
|
$ | 3.24 | $ | 4.02 | $ | 2.34 | ||||||
|
Restricted
Stock Awards
|
Weighted
Average Grant
Date Fair Value
|
|||||||
|
Balance as of January 1, 2011
|
40,269 | $ | 4.88 | |||||
|
Granted
|
61,000 | $ | 5.74 | |||||
|
Exercised/Released
|
(21,528 | ) | $ | 4.71 | ||||
|
Balance as of December 31, 2011
|
79,741 | $ | 5.59 | |||||
|
Options
|
Weighted
Average
Exercise Price
|
Weighted
Average
Remaining
Contractual
Term (years)
|
Aggregate
Intrinsic Value
|
|||||||||||||
|
Balance as of December 31, 2011
|
79,741 | $ | 5.59 | 9.0 | $ | 175,430 | ||||||||||
|
Vested and expected to vest at December 31, 2011
|
79,741 | $ | 5.59 | 9.0 | $ | 175,430 | ||||||||||
|
Exercisable at December 31, 2011
|
48,241 | $ | 4.94 | 8.9 | $ | 106,130 | ||||||||||
|
Restricted
Stock Awards
|
Weighted
Average Grant-
Date Fair Value
|
|||||||
|
Outstanding at January 1, 2011
|
0 | |||||||
|
Granted
|
246,225 | $ | 5.82 | |||||
|
Vested
|
0 | |||||||
|
Cancelled/forfeited
|
0 | |||||||
|
Outstanding at December 31, 2011
|
246,225 | $ | 5.82 | |||||
|
Vested at December 31, 2011
|
0 | |||||||
|
Years ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Total compensation cost for share-based payment arrangements recognized in the statement of operations (net of tax of $0)
|
$ | 3,316,000 | $ | 3,055,000 | $ | 2,649,000 | ||||||
|
15.
|
Related Party Transactions
|
|
16.
|
Subsequent Events
|
|
17.
|
Quarterly Information (unaudited)
|
|
For the three months ended
|
||||||||||||||||
|
March 31,
2011
|
June 30,
2011
|
September 30,
2011
|
December 31,
2011
|
|||||||||||||
|
Product revenues
|
$ | 1,362,000 | $ | 2,411,000 | $ | 2,134,000 | $ | 2,076,000 | ||||||||
|
Gross profit
|
520,000 | 1,302,000 | 1,192,000 | 1,132,000 | ||||||||||||
|
Development revenues
|
1,235,000 | 11,000 | 5,000 | 762,000 | ||||||||||||
|
Operating expenses
|
12,998,000 | 5,685,000 | 9,020,000 | 7,868,000 | ||||||||||||
|
Other income (expense)
|
(829,000 | ) | (766,000 | ) | (512,000 | ) | (932,000 | ) | ||||||||
|
Net loss
|
$ | (12,072,000 | ) | $ | (5,138,000 | ) | $ | (8,335,000 | ) | $ | (6,906,000 | ) | ||||
|
Basic and diluted net loss per share
|
$ | (0.23 | ) | $ | (0.10 | ) | $ | (0.15 | ) | $ | (0.13 | ) | ||||
|
For the three months ended
|
||||||||||||||||
|
March 31,
2010
|
June 30,
2010
|
September 30,
2010
|
December 31,
2010
|
|||||||||||||
|
Product revenues
|
$ | 2,266,000 | $ | 2,091,000 | $ | 1,519,000 | $ | 2,378,000 | ||||||||
|
Gross profit
|
1,336,000 | 1,208,000 | 599,000 | 1,203,000 | ||||||||||||
|
Development revenues
|
2,143,000 | 7,000 | 65,000 | 158,000 | ||||||||||||
|
Operating expenses
|
5,555,000 | 6,257,000 | 10,255,000 | 9,975,000 | ||||||||||||
|
Other income (expense)
|
(371,000 | ) | (335,000 | ) | (826,000 | ) | (639,000 | ) | ||||||||
|
Net loss
|
$ | (2,447,000 | ) | $ | (5,377,000 | ) | $ | (10,417,000 | ) | $ | (9,253,000 | ) | ||||
|
Basic and diluted net loss per share
|
$ | (0.06 | ) | $ | (0.12 | ) | $ | (0.23 | ) | $ | (0.19 | ) | ||||
|
|
·
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the Company’s assets;
|
|
|
·
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
|
|
|
·
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
|
|
(a) (1)
|
Financial Statements
|
|
Reports of KPMG LLP, Independent Registered Public Accounting Firm
|
41 |
|
Consolidated Balance Sheets as of December 31, 2011 and 2010
|
43 |
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2011, 2010 and 2009
|
44 |
|
Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2011, 2010 and 2009
|
45 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2011, 2010 and 2009
|
47 |
|
Notes to Consolidated Financial Statements
|
49 |
|
(a) (2)
|
Financial Statement Schedules
|
|
Balance at
beginning of
year
|
Additions (A)
|
Deductions (B)
|
Other (C)
|
Balance at
end of year
|
||||||||||||||||
|
Allowance for doubtful accounts
|
||||||||||||||||||||
|
Year ended December 31, 2011
|
$ | 306 | $ | 483 | $ | (256 | ) | $ | (59 | ) | $ | 474 | ||||||||
|
Year ended December 31, 2010
|
$ | 751 | $ | 460 | $ | ( 1,014 | ) | $ | 109 | $ | 306 | |||||||||
|
Year ended December 31, 2009
|
$ | 122 | $ | 663 | $ | (34 | ) | $ | — | $ | 751 | |||||||||
|
(A)
|
Includes increases to allowances for doubtful accounts, net of any equipment recovered
|
|
|
(B)
|
Includes write off of uncollectible accounts receivable, net of any equipment recovered
|
|
|
(C)
|
Includes collections fees incurred and product sales amounts deferred that do not meet revenue recognition criteria, net of cash received
|
|
(a)(3)
|
Exhibits
|
|
Filed
|
Incorporated by Reference
|
||||
|
with this
|
|||||
|
Exhibit
|
Form
|
||||
|
Number
|
Exhibit Title
|
10-K
|
Form
|
File No.
|
Date Filed
|
|
1.1
|
Underwriting Agreement, dated October 8, 2010, between Cytori Therapeutics, Inc. and Jefferies & Company.
|
8-K
|
001-34375
Exhibit 1.1
|
10/08/2010
|
|
|
2.5
|
Asset Purchase Agreement dated May 30, 2007, by and between Cytori Therapeutics, Inc. and MacroPore Acquisition Sub, Inc.
|
10-Q
|
000-32501
Exhibit 2.5
|
08/14/2007
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation.
|
10-Q
|
000-32501
Exhibit 3.1
|
08/13/2002
|
|
|
3.2
|
Amended and Restated Bylaws of Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 3.2
|
08/14/2003
|
|
|
3.3
|
Certificate of Ownership and Merger.
|
10-Q
|
000-32501
Exhibit 3.1.1
|
11/14/2005
|
|
|
4.1
|
Rights Agreement, dated as of May 19, 2003, between Cytori Therapeutics, Inc. and Computershare Trust Company, Inc. as Rights Agent, which includes: as Exhibit A thereto, the Form of Certificate of Designation, Preferences and Rights of Series RP Preferred Stock of Cytori Therapeutics, Inc.; as Exhibit B thereto, the Form of Right Certificate; and, as Exhibit C thereto, the Summary of Rights to Purchase Series RP Preferred Stock.
|
8-A
|
000-32501
Exhibit 4.1
|
05/30/2003
|
|
|
4.1.1
|
Amendment No. 1 to Rights Agreement dated as of May 12, 2005, between Cytori Therapeutics, Inc. and Computershare Trust Company, Inc. as Rights Agent.
|
8-K
|
000-32501
Exhibit 4.1.1
|
05/18/2005
|
|
|
4.1.2
|
Amendment No. 2 to Rights Agreement, dated as of August 28, 2007, between us and Computershare Trust Company, N.A. (as successor to Computershare Trust Company, Inc.), as Rights Agent.
|
8-K
|
000-32501
Exhibit 4.1.1
|
09/04/2007
|
|
|
4.2
|
Form of Warrant.
|
8-K
|
000-32501
Exhibit 4.2
|
03/10/2009
|
|
|
10.1#
|
Amended and Restated 1997 Stock Option and Stock Purchase Plan.
|
10
|
000-32501
Exhibit 10.1
|
03/30/2001
|
|
|
10.1.1#
|
Board of Directors resolution adopted November 9, 2006 regarding determination of fair market value for stock option grant purposes (incorporated by reference to Exhibit 10.10.1 filed as Exhibit 10.10.1 to our Form 10-K Annual Report, as filed on March 30, 2007 and incorporated by reference herein)
|
10-K
|
000-32501
Exhibit 10.10.1
|
03/30/2007
|
|
|
10.2+
|
Development and Supply Agreement, made and entered into as of January 5, 2000, by and between the Company and Medtronic.
|
10
|
000-32501
Exhibit 10.4
|
06/01/2001
|
|
|
10.3+
|
Amendment No. 1 to Development and Supply Agreement, effective as of December 22, 2000, by and between the Company and Medtronic, Inc..
|
10
|
000-32501
Exhibit 10.5
|
06/01/2001
|
|
|
10.5+
|
Amendment No. 2 to Development and Supply Agreement, effective as of September 30, 2002, by and between the Company and Medtronic, Inc.
|
8-K
|
000-32501
Exhibit 2.4
|
10/23/2002
|
|
|
10.7
|
Amended Master Security Agreement between the Company and General Electric Corporation, September, 2003.
|
10-Q
|
000-32501
Exhibit 10.1
|
11/12/2003
|
|
|
10.10#
|
2004 Equity Incentive Plan of Cytori Therapeutics, Inc
|
8-K
|
000-32501
Exhibit 10.1
|
08/27/2004
|
|
|
10.10.1#
|
Board of Directors resolution adopted November 9, 2006 regarding determination of fair market value for stock option grant purposes.
|
10-K
|
000-32501
Exhibit 10.10.1
|
03/30/2007
|
|
|
10.11
|
Exclusive Distribution Agreement, effective July 16, 2004 by and between the Company and Senko Medical Trading Co.
|
10-Q
|
000-32501
Exhibit 10.25
|
11/15/2004
|
|
|
10.12#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Nonstatutory).
|
10-Q
|
000-32501
Exhibit 10.19
|
11/15/2004
|
|
|
10.13#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Nonstatutory) with Cliff.
|
10-Q
|
000-32501
Exhibit 10.20
|
11/15/2004
|
|
|
10.14#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Incentive).
|
10-Q
|
000-32501
Exhibit 10.21
|
11/15/2004
|
|
|
10.15#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Incentive) with Cliff.
|
10-Q
|
000-32501
Exhibit 10.22
|
11/15/2004
|
|
|
10.16#
|
Form of Options Exercise and Stock Purchase Agreement Relating to the 2004 Equity Incentive Plan.
|
10-Q
|
000-32501
Exhibit 10.23
|
11/15/2004
|
|
|
10.17#
|
Form of Notice of Stock Options Grant Relating to the 2004 Equity Incentive Plan.
|
10-Q
|
000-32501
Exhibit 10.24
|
11/15/2004
|
|
|
10.22
|
Common Stock Purchase Agreement dated April 28, 2005, between Olympus Corporation and the Company.
|
10-Q
|
000-32501
Exhibit 10.21
|
08/15/2005
|
|
|
10.23
|
Sublease Agreement dated May 24, 2005, between Biogen Idec, Inc. and the Company.
|
10-Q
|
000-32501
Exhibit 10.21
|
08/15/2005
|
|
|
10.27+
|
Joint Venture Agreement dated November 4, 2005, between Olympus Corporation and the Company.
|
10-K
|
000-32501
Exhibit 10.27
|
03/30/2006
|
|
|
10.28+
|
License/ Commercial Agreement dated November 4, 2005, between Olympus-Cytori, Inc. and the Company
|
10-K
|
000-32501
Exhibit 10.28
|
03/30/2006
|
|
|
10.28.1
|
Amendment One to License/ Commercial Agreement dated November 14, 2007, between Olympus-Cytori, Inc. and the Company.
|
10-K
|
000-32501
Exhibit 10.28.1
|
03/14/2008
|
|
|
10.29+
|
License/ Joint Development Agreement dated November 4, 2005, between Olympus Corporation, Olympus-Cytori, Inc. and the Company.
|
10-K
|
000-32501
Exhibit 10.29
|
03/30/2006
|
|
|
10.29.1
|
Amendment No. 1 to License/ Joint Development Agreement dated May 20, 2008, between Olympus Corporation, Olympus-Cytori, Inc. and the Company.
|
10-Q
|
000-32501
Exhibit 10.29.1
|
08/11/2008
|
|
|
10.30+
|
Shareholders Agreement dated November 4, 2005, between Olympus Corporation and the Company.
|
10-K
|
000-32501
Exhibit 10.30
|
03/30/2006
|
|
|
10.32
|
Common Stock Purchase Agreement, dated August 9, 2006, by and between Cytori Therapeutics, Inc. and Olympus Corporation.
|
8-K
|
000-32501
Exhibit 10.32
|
08/15/2006
|
|
|
10.33
|
Form of Common Stock Subscription Agreement, dated August 9, 2006 (Agreements on this form were signed by Cytori and each of respective investors in the Institutional Offering).
|
8-K
|
000-32501
Exhibit 10.33
|
08/15/2006
|
|
|
10.34
|
Placement Agency Agreement, dated August 9, 2006, between Cytori Therapeutics, Inc. and Piper Jaffray & Co.
|
8-K
|
000-32501
Exhibit 10.34
|
08/15/2006
|
|
|
10.39+
|
Exclusive License Agreement between us and the Regents of the University of California dated October 16, 2001.
|
10-K
|
000-32501
Exhibit 10.10
|
03/31/2003
|
|
|
10.39.1 +
|
Amended and Restated Exclusive License Agreement, effective September 26, 2006, by and between The Regents of the University of California and Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 10.39
|
11/14/2006
|
|
|
10.42
|
Placement Agency Agreement, dated February 23, 2007, between Cytori Therapeutics, Inc. and Piper Jaffray & Co.
|
8-K
|
000-32501
Exhibit 10.1
|
02/26/2007
|
|
|
10.43
|
Financial services advisory engagement letter agreement, dated February 16, 2007, between Cytori Therapeutics, Inc. and WBB Securities, LLC.
|
8-K
|
000-32501
Exhibit 10.2
|
02/26/2007
|
|
|
10.44
|
Form of Subscription Agreement, dated February 23, 2007.
|
8-K
|
000-32501
Exhibit 10.3
|
02/26/2007
|
|
10.45
|
Form of Warrant to be dated February 28, 2007.
|
8-K
|
000-32501
Exhibit 10.4
|
02/26/2007
|
|
|
10.46
|
Common Stock Purchase Agreement, dated March 28, 2007, by and between Cytori Therapeutics, Inc. and Green Hospital Supply, Inc.
|
10-Q
|
000-32501
Exhibit 10.46
|
05/11/2007
|
|
|
10.47
|
Consulting Agreement, dated May 3, 2007, by and between Cytori Therapeutics, Inc. and Marshall G. Cox.
|
10-Q
|
000-32501
Exhibit 10.47
|
08/14/2007
|
|
|
10.48+
|
Master Cell Banking and Cryopreservation Agreement, effective August 13, 2007, by and between Green Hospital Supply, Inc.
and Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 10.48
|
11/13/2007
|
|
|
10.48.1
|
Amendment No. 1 to Master Cell Banking and Cryopreservation Agreement, effective June 4, 2008, by and between Green Hospital Supply, Inc. and the Company.
|
8-K
|
000-32501
Exhibit 10.48.1
|
06/10/2008
|
|
|
10.49+
|
License & Royalty Agreement, effective August 23, 2007, by and between Olympus-Cytori, Inc.
and Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 10.49
|
11/13/2007
|
|
|
10.50
|
General Release Agreement, dated August 13, 2007, between John Ransom and Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 10.49
|
11/13/2007
|
|
|
10.51
|
Common Stock Purchase Agreement, dated February 8, 2008, by and between Green Hospital Supply, Inc.
and Cytori Therapeutics, Inc.
|
8-K
|
000-32501
Exhibit 10.51
|
02/19/2008
|
|
|
10.51.1
|
Amendment No. 1 to Common Stock Purchase Agreement, dated February 29, 2008, by and between Green Hospital Supply, Inc. and Cytori Therapeutics, Inc.
|
8-K
|
000-32501
Exhibit 10.51.1
|
02/29/2008
|
|
|
10.52#
|
Agreement for Acceleration and/or Severance, dated January 31, 2008, by and between Christopher J. Calhoun
and Cytori Therapeutics, Inc.
|
10-K
|
000-32501
Exhibit 10.52
|
03/14/2008
|
|
|
10.53#
|
Agreement for Acceleration and/or Severance, dated January 31, 2008, by and between Marc H. Hedrick
and Cytori Therapeutics, Inc.
|
10-K
|
000-32501
Exhibit 10.53
|
03/14/2008
|
|
|
10.54#
|
Agreement for Acceleration and/or Severance, dated January 31, 2008, by and between Mark E. Saad
and Cytori Therapeutics, Inc.
|
10-K
|
000-32501
Exhibit 10.54
|
03/14/2008
|
|
|
10.55
|
Common Stock Purchase Agreement, dated August 7, 2008, by and between the Company and Olympus Corporation.
|
8-K
|
000-32501
Exhibit 10.32
|
08/08/2008
|
|
|
10.55.1
|
Amendment No. 1 to Common Stock Purchase Agreement, dated August 8, 2008, by and between the Company and Olympus Corporation.
|
8-K
|
000-32501
Exhibit 10.32.1
|
08/14/2008
|
|
|
10.56
|
Securities Purchase Agreement, dated August 7, 2008, by and among the Company and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.33
|
08/08/2008
|
|
|
10.57
|
Form of Warrant to Purchase Common Stock issued on August 11, 2008 pursuant to the Securities Purchase Agreement, dated August 7, 2008, by and among the Company and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.34
|
08/08/2008
|
|
|
10.58
|
Registration Rights Agreement, dated August 7, 2008, by and among the Company and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.35
|
08/08/2008
|
|
|
10.59
|
Loan and Security Agreement, dated October 14, 2008, by and among the Company, General Electric Capital Corporation, and the other lenders signatory thereto.
|
10-K
|
000-32501
Exhibit 10.59
|
03/06/2009
|
|
|
10.60
|
Promissory Note issued by the Company in favor of General Electric Capital Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated October 14, 2008.
|
10-K
|
000-32501
Exhibit 10.60
|
03/06/2009
|
|
|
10.61
|
Warrant to Purchase Common Stock issued by the Company on October 14, 2008 in favor of GE Capital Equity Investments, Inc., pursuant to the Loan and Security Agreement dated October 14, 2008.
|
10-K
|
000-32501
Exhibit 10.61
|
03/06/2009
|
|
|
10.62
|
Warrant to Purchase Common Stock issued by the Company on October 14, 2008 in favor of Silicon Valley Bank, pursuant to the Loan and Security Agreement dated October 14, 2008.
|
10-K
|
000-32501
Exhibit 10.62
|
03/06/2009
|
|
10.63
|
Form of Subscription Agreement by and between Cytori Therapeutics, Inc. and the Purchaser (as defined therein), dated as of March 9, 2009.
|
8-K
|
000-32501
Exhibit 10.63
|
03/10/2009
|
|
|
10.64
|
Placement Agency Agreement, dated March 9, 2009, between Cytori Therapeutics, Inc. and Piper Jaffray & Co.
|
8-K
|
000-32501
Exhibit 10.64
|
03/10/2009
|
|
|
10.65
|
Securities Purchase Agreement, dated May 7, 2009, by and among Cytori Therapeutics, Inc. and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.63
|
05/08/2009
|
|
|
10.66
|
Form of Warrant to Purchase Common Stock to be issued on or about May 11, 2009.
|
8-K
|
000-32501
Exhibit 10.64
|
05/08/2009
|
|
|
10.67
|
Registration Rights Agreement, dated May 7, 2009, by and among Cytori Therapeutics, Inc. and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.65
|
05/08/2009
|
|
|
10.68
|
Form of Common Stock Purchase Agreement by and between Cytori Therapeutics, Inc. and Seaside 88, LP, dated as of June 19, 2009.
|
8-K
|
001-34375
Exhibit 10.68
|
06/22/2009
|
|
|
10.69
|
Lease Agreement entered into on April 2, 2010, between HCP Callan Rd, LLC. and Cytori Therapeutics, Inc..
|
10-Q
|
001-34375
Exhibit 10.69
|
05/06/2010
|
|
|
10.70
|
Amended and Restated Loan and Security Agreement, dated June 11, 2010, by and among the Company, General Electric Capital Corporation, and the other lenders signatory thereto.
|
8-K
|
001-34375
Exhibit 10.70
|
06/17/2010
|
|
|
10.71
|
Promissory Note issued by the Company in favor of General Electric Capital Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.71
|
06/17/2010
|
|
|
10.72
|
Promissory Note issued by the Company in favor of Oxford Financial Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.72
|
06/17/2010
|
|
|
10.73
|
Warrant to Purchase Common Stock issued by the Company on June 11, 2010 in favor of GE Capital Equity Investments, Inc., pursuant to the Amended and Restated Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.73
|
06/17/2010
|
|
|
10.74
|
Warrant to Purchase Common Stock issued by the Company on June 11, 2010 in favor of Silicon Valley Bank, pursuant to the Amended and Restated Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.74
|
06/17/2010
|
|
|
10.75
|
Warrant to Purchase Common Stock issued by the Company on June 11, 2010 in favor of Oxford Financial Corporation, pursuant to the Amended and Restated Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.75
|
06/17/2010
|
|
|
10.76
|
Common Stock Purchase Agreement, dated December 6, 2010, by and among Cytori Therapeutics, Inc. and Astellas Pharma Inc.
|
8-K
|
001-34375
Exhibit 10.76
|
12/09/2010
|
|
|
10.77
|
Form of Notice and Restricted Stock Award Agreement for grants of performance-based restricted stock awards under the 2004 Equity Incentive Plan.
|
8-K
|
001-34375
Exhibit 10.1
|
03/04/2011
|
|
|
10.78
|
Form of Common Stock Purchase Agreement by and between Cytori Therapeutics, Inc. and Seaside 88, LP, dated July 11, 2011
|
8-K
|
001-34375
Exhibit 10.78
|
07/12/2011
|
|
|
10.79
|
First Amendment to Amended and Restated Loan and Security Agreement, dated June 23, 2011, by and among the Company, Oxford Finance LLC, the other lenders party hereto and General Electric Capital Corporation.
|
10-Q
|
001-34375
Exhibit 10.79
|
08/09/2011
|
|
|
10.80
|
Second Amendment to the Amended and Restated Loan and Security Agreement, dated September 9, 2011, by and among the Company, General Electric Capital Corporation, and the other lenders signatory thereto.
|
8-K
|
001-34375
Exhibit 10.80
|
09/15/2011
|
|
|
10.81
|
Promissory Note issued by the Company in favor of General Electric Capital Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.81
|
09/15/2011
|
|
|
10.82
|
Promissory Note issued by the Company in favor of Silicon Valley Bank or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.82
|
09/15/2011
|
|
10.83
|
Promissory Note issued by the Company in favor of Oxford Financial Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.83
|
09/15/2011
|
|
|
10.84
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of GE Capital Equity Investments, Inc., pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.84
|
09/15/2011
|
|
|
10.85
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of Silicon Valley Bank, pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.85
|
09/15/2011
|
|
|
10.86
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of Oxford Financial Corporation, pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.86
|
09/15/2011
|
|
|
10.87
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of Oxford Financial Corporation, pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.87
|
09/15/2011
|
|
|
10.88
|
First Amendment to Lease Agreement entered into on November 4, 2011, between HCP Callan Rd, LLC. and the Company.
|
10-Q
|
001-34375
Exhibit 10.88
|
11/08/2011
|
|
| 10.89# | 2011 Employee Stock Purchase Plan | DEF 14A |
001-34375
Appendix A |
05/02/2011 | |
|
14.1
|
Code of Ethics.
|
10-K
|
000-32501
Exhibit 14.1
|
03/30/2004
|
|
|
Consent of KPMG LLP, Independent Registered Public Accounting Firm (filed herewith).
|
X
|
||||
|
Certification of Principal Executive Officer Pursuant to Securities Exchange Act Rule 13a-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
X
|
||||
|
Certification of Principal Financial Officer Pursuant to Securities Exchange Act Rule 13a-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
X
|
||||
|
Certifications Pursuant to 18 U.S.C. Section 1350/ Securities Exchange Act Rule 13a-14(b), as adopted pursuant to Section 906 of the Sarbanes - Oxley Act of 2002 (filed herewith).
|
X
|
||||
| 101.INS |
XBRL Instance Document
|
X | |||
| 101.SCH |
XBRL Schema Document
|
X | |||
| 101.CAL |
XBRL Calculation Linkbase Document
|
X | |||
| 101.DEF |
XBRL Definition Linkbase Document
|
X | |||
| 101.LAB |
XBRL Label Linkbase Document
|
X | |||
| 101.PRE |
XBRL Presentation Linkbase Document
|
X |
|
|
+
|
Portions of these exhibits have been omitted pursuant to a request for confidential treatment.
|
|
|
#
|
Indicates management contract or compensatory plan or arrangement.
|
|
CYTORI THERAPEUTICS, INC.
|
||
|
By:
|
/s/
Christopher
J. Calhoun
|
|
|
Christopher J. Calhoun
|
||
|
Chief Executive Officer
|
||
|
March 13, 2012
|
||
|
SIGNATURE
|
TITLE
|
DATE
|
||
|
/s/ Lloyd H. Dean
|
Chairman of the Board of Directors
|
March 13, 2012
|
||
|
Lloyd H. Dean
|
||||
|
/s/ Christopher J. Calhoun
|
Chief Executive Officer, Vice-Chairman, Director (Principal Executive Officer)
|
March 13, 2012
|
||
|
Christopher J. Calhoun
|
||||
|
/s/ Marc H. Hedrick, MD
|
President, Director
|
March 13, 2012
|
||
|
Marc H. Hedrick, MD
|
||||
|
/s/ Mark E. Saad
|
Chief Financial Officer (Principal Financial Officer)
|
March 13, 2012
|
||
|
Mark E. Saad
|
||||
|
/s/ John W. Townsend
|
Chief Accounting Officer
|
March 13, 2012
|
||
|
John W. Townsend
|
||||
|
/s/ Richard J. Hawkins
|
Director
|
March 13, 2012
|
||
|
Richard J. Hawkins
|
||||
|
/s/ Paul W. Hawran
|
Director
|
March 13, 2012
|
||
|
Paul W. Hawran
|
||||
|
/s/ Ronald D. Henriksen
|
Director
|
March 13, 2012
|
||
|
Ronald D. Henriksen
|
||||
|
/s/ E. Carmack Holmes, MD
|
Director
|
March 13, 2012
|
||
|
E. Carmack Holmes, MD
|
||||
|
/s/ David M. Rickey
|
Director
|
March 13, 2012
|
||
|
David M. Rickey
|
||||
|
/s/ Tommy G. Thompson
|
Director
|
March 13, 2012
|
||
|
Tommy G. Thompson
|