|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
DELAWARE
|
33-0827593
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
|
|
3020 CALLAN ROAD, SAN DIEGO, CALIFORNIA
|
92121
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Common stock, par value $0.001
|
NASDAQ Stock Market LLC
|
|
Large Accelerated Filer
☐
|
Accelerated Filer
☒
|
Non-Accelerated Filer
☐
|
Smaller reporting company
☐
|
|
(Do not check if a smaller reporting company)
|
|||
|
Page
|
||
|
PART I
|
||
|
Item 1.
|
3
|
|
|
Item 1A.
|
10
|
|
|
Item 1B.
|
24
|
|
|
Item 2.
|
24
|
|
|
Item 3.
|
24
|
|
|
Item 4.
|
24
|
|
|
PART II
|
||
|
Item 5.
|
25
|
|
|
Item 6.
|
27
|
|
|
Item 7.
|
29
|
|
|
Item 7A.
|
42
|
|
|
Item 8.
|
43
|
|
|
Item 9.
|
73
|
|
|
Item 9A.
|
73
|
|
|
Item 9B.
|
75
|
|
|
PART III
|
||
|
Item 10.
|
75
|
|
|
Item 11.
|
80
|
|
|
Item 12.
|
98
|
|
|
Item 13.
|
99
|
|
|
Item 14.
|
100
|
|
|
PART IV
|
||
|
Item 15.
|
101
|
| · | Supported enrollment in the ATHENA and ATHENA II trials; |
| · | Supported ongoing preclinical and other research activities towards BARDA contract milestones; |
| · | Continued patient follow-up and data analysis from the European ADVANCE trial assessing the effect of Cytori Cell Therapy compared to a placebo control in patients with chronic myocardial ischemia; |
| · | Supported FDA submission and approval of the STAR (scleroderma) and ACT-OA (osteoarthritis) trials; |
| · | Prepared and submitted multiple regulatory filings in the United States, Europe, Japan, and other regions related to various cell and tissue processing systems under development; |
| · | Developed new configurations and expanded functionality of our Celution ® platform to address the current Japan approval as a medical device (Japan Class I) and other markets; |
| · | Conducted adipose derived regenerative cells (ADRC) viability and transport studies in support of clinical trial requirements; |
| · | Conducted, presented, and published research efforts related to ADRC characterization and potency to further establish scientific leadership in the field; and |
| · | Continued to optimize and develop the Celution ® System family of products and next-generation devices, single-use consumables and related instrumentation. |
|
Region
|
Clinical Applications
|
Regulatory Status
|
|
Japan
|
Cell Banking
|
Approved
|
|
Celution® Centrifuge, Celbrush, Puregraft Bag and select components.
|
Class I Notification
|
|
|
Europe
|
Celution® 800 and Celution One: Cell Processing for re-implantation or re-infusion into same patient (General Processing)
|
CE Mark
|
|
Celution® 800 and Celution One: Breast reconstruction, healing of Crohn’s wounds and other cosmetic procedures
|
CE Mark
|
|
|
Celution® 800: Cryptoglandular fistula, tissue ischemia and other soft tissue procedures
|
CE Mark
|
|
|
Intravase® for use with Celution® 800
|
CE Mark
|
|
|
Cell Concentration
|
CE Mark
|
|
|
Celution® One cosmetic and reconstructive surgery claims
|
CE Mark
|
|
|
U.S.
|
Osteoarthritis
|
ACT-OA trial being initiated
|
|
U.S.
|
Scleroderma
|
STAR (full IDE approval granted in January 2015)
|
|
U.S.
|
Refractory Heart Failure
|
ATHENA and ATHENA II IDE trial underway
|
|
Australia
|
Celution 800 Cell Processing for re-implantation or re-infusion into same patient (general/plastic reconstruction), Puregraft, Instrument Sets
|
ARTG Certificate
|
|
Croatia
|
Celution 800 Cell Processing for re-implantation or re-infusion into same patient (general/plastic reconstruction), Puregraft
|
Approval Certificated from the Croatia Agency for Medicinal Products and Medical Devices
|
|
New Zealand
|
Celution 800, Puregraft, Instrument Sets
|
WAND Registered
|
|
Russia
|
Celution 800 Cell Processing for re-implantation or re-infusion into same patient (general/plastic reconstruction), Puregraft
|
Roszdravnadzor Certificate (Federal Service for Control of Healthcare and Social Development)
|
|
Serbia
|
Celution 800 Cell Processing for re-implantation or re-infusion into same patient (general/plastic reconstruction), Puregraft
|
ALIMS (Medicines and Medical Devices Agency of Serbia)
|
|
Singapore
|
Celution 800 Cell Processing for re-implantation or re-infusion into same patient (general/plastic reconstruction), Puregraft
|
HSA approved, SMDR Registered
|
| · | causing us to use a larger portion of our cash flow to fund interest and principal payments, reducing the availability of cash to fund working capital and capital expenditures and other business activities; |
| · | making it more difficult for us to take advantage of significant business opportunities, such as acquisition opportunities, and to react to changes in market or industry conditions; and |
| · | limiting our ability to borrow additional monies in the future to fund working capital, capital expenditures and other general corporate purposes. |
| • | restrictions on our products or manufacturing processes; |
| • | warning letters; |
| • | withdrawal of the products from the market; |
| • | voluntary or mandatory recall; |
| • | fines; |
| • | suspension or withdrawal of regulatory approvals; |
| • | suspension or termination of any of our ongoing clinical trials; |
| • | refusal to permit the import or export of our products; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | product seizure; |
| • | injunctions; or |
| • | imposition of civil or criminal penalties. |
| • | clinical results may not meet prescribed endpoints for the studies or otherwise provide sufficient data to support the efficacy of our products; |
| • | clinical and nonclinical test results may reveal side effects, adverse events or unexpected safety issues associated with the use of our products; |
| • | regulatory review may not find a product safe or effective enough to merit either continued testing or final approval; |
| • | regulatory review may not find that the data from preclinical testing and clinical trials justifies approval; |
| • | regulatory authorities may require that we change our studies or conduct additional studies which may significantly delay or make continued pursuit of approval commercially unattractive; |
| • | a regulatory agency may reject our trial data or disagree with our interpretations of either clinical trial data or applicable regulations; |
| • | the cost of clinical trials required for product approval may be greater than what we originally anticipate, and we may decide to not pursue regulatory approval for such a product; |
| • | a regulatory agency may identify problems or other deficiencies in our existing manufacturing processes or facilities, or the existing processes or facilities of our collaborators, our contract manufacturers or our raw material suppliers; |
| • | a regulatory agency may change its formal or informal approval requirements and policies, act contrary to previous guidance, adopt new regulations or raise new issues or concerns late in the approval process; |
| • | a product candidate may be approved only for indications that are narrow or under conditions that place the product at a competitive disadvantage, which may limit the sales and marketing activities for such products or otherwise adversely impact the commercial potential of a product; or |
| • | a regulatory agency may ask the company to put a clinical study on hold pending additional safety data; there is no guarantee that the company will be able to satisfy the regulator agencies requests in a timely manner, which can lead to significant uncertainty in the completion of a clinical study. |
| · | political unrest, terrorism and economic or financial instability; |
| · | unexpected changes and uncertainty in regulatory requirements; |
| · | nationalization programs that may be implemented by foreign governments; |
| · | import-export regulations; |
| · | difficulties in enforcing agreements and collecting receivables; |
| · | difficulties in ensuring compliance with the laws and regulations of multiple jurisdictions; |
| · | changes in labor practices, including wage inflation, labor unrest and unionization policies; |
| · | longer payment cycles by international customers; |
| · | currency exchange fluctuations; |
| · | disruptions of service from utilities or telecommunications providers, including electricity shortages; |
| · | difficulties in staffing foreign branches and subsidiaries and in managing an expatriate workforce, and differing employment practices and labor issues; and |
| · | potentially adverse tax consequences. |
| · | audit or object to our contract-related costs and fees, and require us to reimburse all such costs and fees; |
| · | suspend or prevent us for a set period of time from receiving new contracts or extending our existing contracts based on violations or suspected violations of laws or regulations; |
| · | cancel, terminate or suspend our contracts based on violations or suspected violations of laws or regulations; |
| · | terminate our contracts if in the Government’s best interest, including if funds become unavailable to the applicable governmental agency; |
| · | reduce the scope and value of our contracts; and |
| · | change certain terms and conditions in our contracts. |
| · | termination of contracts; |
| · | forfeiture of profits; |
| · | suspension of payments; |
| · | fines; and |
| · | suspension or prohibition from conducting business with the U.S. Government. |
| ● | fluctuations in our operating results or the operating results of our competitors; |
| ● | changes in estimates of our financial results or recommendations by securities analysts; |
| ● | variance in our financial performance from the expectations of securities analysts; |
| ● | changes in the estimates of the future size and growth rate of our markets; |
| ● | changes in accounting principles or changes in interpretations of existing principles, which could affect our financial results; |
| ● | conditions and trends in the markets we serve; |
| ● | changes in general economic, industry and market conditions; |
| ● | success of competitive products and services; |
| ● | changes in market valuations or earnings of our competitors; |
| ● | announcements of significant new products, contracts, acquisitions or strategic alliances by us or our competitors; |
| ● | the timing and outcome of regulatory reviews and approvals of our products; |
| ● | the commencement or outcome of litigation involving our company, our general industry or both; |
| ● | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| ● | actual or expected sales of our common stock by the holders of our common stock; and |
| ● | the trading volume of our common stock. |
| ● | authorize our Board of Directors to issue without stockholder approval up to 5,000,000 shares of preferred stock, the rights of which will be determined at the discretion of the Board of Directors; |
| ● | require that stockholder actions must be effected at a duly called stockholder meeting and cannot be taken by written consent; |
| ● | establish advance notice requirements for stockholder nominations to our Board of Directors or for stockholder proposals that can be acted on at stockholder meetings; and |
| ● | limit who may call stockholder meetings. |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
|
High
|
Low
|
|||||||
|
2013
|
||||||||
|
Quarter ended March 31, 2013
|
$
|
3.16
|
$
|
2.31
|
||||
|
Quarter ended June 30, 2013
|
$
|
2.89
|
$
|
2.20
|
||||
|
Quarter ended September 30, 2013
|
$
|
2.87
|
$
|
2.09
|
||||
|
Quarter ended December 31, 2013
|
$
|
3.93
|
$
|
2.00
|
||||
|
2014
|
||||||||
|
Quarter ended March 31, 2014
|
$
|
3.47
|
$
|
2.44
|
||||
|
Quarter ended June 30, 2014
|
$
|
2.88
|
$
|
2.14
|
||||
|
Quarter ended September 30, 2014
|
$
|
2.52
|
$
|
0.66
|
||||
|
Quarter ended December 31, 2014
|
$
|
0.70
|
$
|
0.36
|
||||
|
Plan Category
|
Number of securities to be issued
upon exercise of outstanding
options, warrants and rights
|
Weighted-average exercise price
of outstanding options, warrants
and rights
|
Number of securities remaining
available for future
issuance under equity compensation
plans (excluding securities reflected
in column(a))
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by security holders (1)
|
415,905
|
$
|
4.98
|
—
|
||||||||
|
Equity compensation plans not approved by security holders (2)
|
8,614,566
|
$
|
3.88
|
—
|
||||||||
|
Equity compensation plans not approved by security holders (3)
|
278,000
|
$
|
1.31
|
3,697,000
|
||||||||
|
Total
|
9,308,471
|
$
|
3.85
|
3,697,000
|
||||||||
| (1) | The 1997 Stock Option and Stock Purchase Plan expired in October 2007. |
| (2) | The 2004 Stock Option and Stock Purchase Plan expired in August 2014. |
| (3) | See Notes to the Consolidated Financial Statements included elsewhere herein for a description of our 2014 Equity Incentive Plan. |
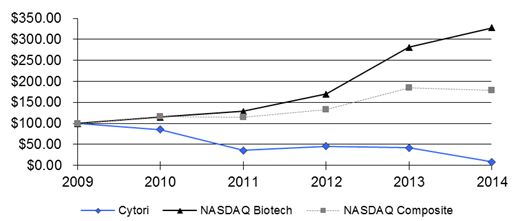
|
2014
|
2013
|
2012
|
2011
|
2010
|
||||||||||||||||
|
Statements of Operations Data:
|
||||||||||||||||||||
|
Product revenues:
|
||||||||||||||||||||
|
Sales to related party
|
$
|
—
|
$
|
1,845
|
$
|
—
|
$
|
—
|
$
|
590
|
||||||||||
|
Sales to third parties
|
4,953
|
5,277
|
8,709
|
7,983
|
7,664
|
|||||||||||||||
|
4,953
|
7,122
|
8,709
|
7,983
|
8,254
|
||||||||||||||||
|
Cost of product revenues
|
2,940
|
3,421
|
4,000
|
3,837
|
3,908
|
|||||||||||||||
|
Gross profit
|
2,013
|
3,701
|
4,709
|
4,146
|
4,346
|
|||||||||||||||
|
Development revenues:
|
||||||||||||||||||||
|
Development, related party
|
—
|
638
|
2,882
|
1,992
|
2,122
|
|||||||||||||||
|
Development
|
—
|
1,179
|
2,529
|
—
|
—
|
|||||||||||||||
|
Government contracts and other
|
2,645
|
3,257
|
381
|
21
|
251
|
|||||||||||||||
|
2,645
|
5,074
|
5,792
|
2,013
|
2,373
|
||||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
15,105
|
17,065
|
13,628
|
10,904
|
9,687
|
|||||||||||||||
|
Sales and marketing
|
6,406
|
9,026
|
9,488
|
13,560
|
11,040
|
|||||||||||||||
|
General and administrative
|
15,953
|
16,031
|
15,672
|
14,727
|
12,570
|
|||||||||||||||
|
Change in fair value of warrants
|
(369
|
)
|
(418
|
)
|
(209
|
)
|
(4,360
|
)
|
(1,285
|
)
|
||||||||||
|
Change in fair value of option liabilities
|
—
|
(2,250
|
)
|
340
|
740
|
30
|
||||||||||||||
|
Total operating expenses
|
37,095
|
39,454
|
38,919
|
35,571
|
32,042
|
|||||||||||||||
|
Total operating loss
|
(32,437
|
)
|
(30,679
|
)
|
(28,418
|
)
|
(29,412
|
)
|
(25,323
|
)
|
||||||||||
|
Other income (expense):
|
||||||||||||||||||||
|
Gain/loss on asset disposal
|
42
|
(257
|
)
|
—
|
—
|
—
|
||||||||||||||
|
Loss on debt extinguishment
|
—
|
(708
|
)
|
—
|
—
|
—
|
||||||||||||||
|
Interest income
|
6
|
4
|
4
|
9
|
9
|
|||||||||||||||
|
Interest expense
|
(4,371
|
)
|
(3,396
|
)
|
(3,386
|
)
|
(2,784
|
)
|
(2,052
|
)
|
||||||||||
|
Other income (expense), net
|
(608
|
)
|
(438
|
)
|
(314
|
)
|
(55
|
)
|
23
|
|||||||||||
|
Gain on Puregraft divestiture
|
—
|
4,453
|
—
|
—
|
—
|
|||||||||||||||
|
Gain on previously held equity interest in JV
|
—
|
4,892
|
—
|
—
|
—
|
|||||||||||||||
|
Equity loss in investments
|
—
|
(48
|
)
|
(165
|
)
|
(209
|
)
|
(151
|
)
|
|||||||||||
|
Net loss
|
$
|
(37,368
|
)
|
$
|
(26,177
|
)
|
$
|
(32,279
|
)
|
$
|
(32,451
|
)
|
$
|
(27,494
|
)
|
|||||
|
Beneficial conversion feature for convertible preferred stock
|
(1,169
|
)
|
—
|
—
|
—
|
—
|
||||||||||||||
|
Net loss allocable to common stockholders
|
$
|
(38,537
|
)
|
$
|
(26,177
|
)
|
$
|
(32,279
|
)
|
$
|
(32,451
|
)
|
$
|
(27,494
|
)
|
|||||
|
Basic and diluted net loss per share allocable to common stockholders
|
$
|
(0.48
|
)
|
$
|
(0.39
|
)
|
$
|
(0.55
|
)
|
$
|
(0.61
|
)
|
$
|
(0.60
|
)
|
|||||
|
Basic and diluted weighted average shares used in calculating net loss per share allocable to common stockholders
|
80,830,698
|
67,781,364
|
58,679,687
|
53,504,030
|
45,947,966
|
|||||||||||||||
|
Statements of Cash Flows Data:
|
||||||||||||||||||||
|
Net cash used in operating activities
|
$
|
(30,330
|
)
|
$
|
(34,563
|
)
|
$
|
(32,193
|
)
|
$
|
(35,323
|
)
|
$
|
(23,574
|
)
|
|||||
|
Net cash provided by(used in) investing activities
|
(1,343
|
)
|
3,686
|
(1,204
|
)
|
(560
|
)
|
(1,290
|
)
|
|||||||||||
|
Net cash provided by financing activities
|
30,874
|
20,772
|
22,192
|
20,137
|
64,678
|
|||||||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(85
|
)
|
(106
|
)
|
—
|
—
|
—
|
|||||||||||||
|
Net (decrease) increase in cash
|
(884
|
)
|
(10,211
|
)
|
(11,205
|
)
|
(15,746
|
)
|
39,814
|
|||||||||||
|
Cash and cash equivalents at beginning of year
|
15,506
|
25,717
|
36,922
|
52,668
|
12,854
|
|||||||||||||||
|
Cash and cash equivalents at end of year
|
$
|
14,622
|
$
|
15,506
|
$
|
25,717
|
$
|
36,922
|
$
|
52,668
|
||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
14,622
|
$
|
15,506
|
$
|
25,717
|
$
|
36,922
|
$
|
52,668
|
||||||||||
|
Working capital
|
5,769
|
9,671
|
16,366
|
35,516
|
45,730
|
|||||||||||||||
|
Total assets
|
38,719
|
42,060
|
43,250
|
51,534
|
66,347
|
|||||||||||||||
|
Deferred revenues, related party
|
—
|
—
|
638
|
3,520
|
5,512
|
|||||||||||||||
|
Deferred revenues
|
112
|
212
|
2,635
|
5,244
|
4,929
|
|||||||||||||||
|
Warrant liabilities, long-term
|
9,793
|
—
|
—
|
627
|
4,987
|
|||||||||||||||
|
Option liabilities
|
—
|
—
|
2,250
|
1,910
|
1,170
|
|||||||||||||||
|
Long-term deferred rent
|
558
|
710
|
756
|
504
|
398
|
|||||||||||||||
|
Long-term obligations, less current portion
|
18,041
|
23,100
|
12,903
|
21,962
|
13,255
|
|||||||||||||||
|
Total stockholders’ (deficit) equity
|
$
|
(5,702
|
)
|
$
|
3,132
|
$
|
6,455
|
$
|
9,946
|
$
|
22,873
|
|||||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Related party
|
$
|
—
|
$
|
1,845,000
|
$
|
—
|
||||||
|
Third party
|
4,953,000
|
5,277,000
|
8,709,000
|
|||||||||
|
Total product revenues
|
$
|
4,953,000
|
$
|
7,122,000
|
$
|
8,709,000
|
||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Cost of product revenues
|
$
|
2,856,000
|
$
|
3,338,000
|
$
|
3,923,000
|
||||||
|
Share-based compensation
|
84,000
|
83,000
|
77,000
|
|||||||||
|
Total cost of product revenues
|
$
|
2,940,000
|
$
|
3,421,000
|
$
|
4,000,000
|
||||||
|
Total cost of product revenues as % of product revenues
|
59
|
%
|
48
|
%
|
46
|
%
|
||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Government contract (BARDA) and other
|
2,645,000
|
$
|
3,257,000
|
381,000
|
||||||||
|
Development (Olympus)
|
$
|
—
|
638,000
|
$
|
2,882,000
|
|||||||
|
Development (Astellas)
|
—
|
—
|
2,529,000
|
|||||||||
|
Development (Senko)
|
—
|
1,179,000
|
—
|
|||||||||
|
Total development revenues
|
$
|
2,645,000
|
$
|
5,074,000
|
$
|
5,792,000
|
||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Research and development
|
$
|
14,527,000
|
$
|
16,444,000
|
$
|
12,784,000
|
||||||
|
Development milestone (Joint Venture)
|
—
|
16,000
|
219,000
|
|||||||||
|
Stock-based compensation
|
578,000
|
605,000
|
625,000
|
|||||||||
|
Total research and development expenses
|
$
|
15,105,000
|
$
|
17,065,000
|
$
|
13,628,000
|
||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Sales and marketing
|
$
|
5,946,000
|
$
|
8,329,000
|
$
|
8,764,000
|
||||||
|
Stock-based compensation
|
460,000
|
697,000
|
724,000
|
|||||||||
|
Total sales and marketing
|
$
|
6,406,000
|
$
|
9,026,000
|
$
|
9,488,000
|
||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
General and administrative
|
$
|
13,974,000
|
$
|
13,808,000
|
$
|
13,194,000
|
||||||
|
Stock-based compensation
|
1,979,000
|
2,223,000
|
2,478,000
|
|||||||||
|
Total general and administrative expenses
|
$
|
15,953,000
|
$
|
16,031,000
|
$
|
15,672,000
|
||||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Cost of product revenues
|
$
|
84,000
|
$
|
83,000
|
$
|
77,000
|
||||||
|
Research and development related
|
578,000
|
605,000
|
625,000
|
|||||||||
|
Sales and marketing related
|
460,000
|
697,000
|
724,000
|
|||||||||
|
General and administrative related
|
1,979,000
|
2,223,000
|
2,478,000
|
|||||||||
|
Total stock-based compensation
|
$
|
3,101,000
|
$
|
3,608,000
|
$
|
3,904,000
|
||||||
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Change in fair value of warrant liability
|
$
|
(369,000
|
)
|
$
|
(418,000
|
)
|
$
|
(209,000
|
)
|
|||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Change in fair value of option liability
|
$
|
—
|
$
|
(2,250,000
|
)
|
$
|
340,000
|
|||||
|
Years ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Loss on asset disposal
|
$
|
42,000
|
$
|
(257,000
|
)
|
$
|
—
|
|||||
|
Loss on debt extinguishment
|
—
|
(708,000
|
)
|
$
|
—
|
|||||||
|
Interest income
|
6,000
|
4,000
|
4,000
|
|||||||||
|
Interest expense
|
(4,371,000
|
)
|
(3,396,000
|
)
|
(3,386,000
|
)
|
||||||
|
Other income (expense), net
|
(608,000
|
)
|
(438,000
|
)
|
(314,000
|
)
|
||||||
|
Gain on Puregraft divestiture
|
—
|
4,453,000
|
—
|
|||||||||
|
Gain on previously held equity interest in joint venture
|
—
|
4,892,000
|
—
|
|||||||||
|
Total
|
$
|
(4,931,000
|
)
|
$
|
4,550,000
|
$
|
(3,696,000
|
)
|
||||
| · | Interest expense increased for the year ended December 31, 2014 as compared to 2013, due to cash interest and non-cash amortization of debt and warrant costs related to our $27.0 million Term Loan executed in June 2013, and increased accretion expense related to our Joint Venture liability. |
| · | We recorded a beneficial conversion feature of $1,169,000 in December of 2014, related to the issuance of our Series A 3.6% Convertible Preferred Stock. The fair value of the common stock into which the Series A 3.6% Preferred Stock was convertible on the respective dates of issuance of the preferred stock exceeded the proceeds allocated to the Series A 3.6% Convertible Preferred Stock, resulting in a beneficial conversion feature . |
| · | Interest expense increased for the year ended December 31, 2013 as compared to 2012 due to cash interest and non-cash amortization of debt issuance costs and debt discount for our $27.0 million term loan executed in June 2013 . |
| · | The changes in other income (expense) in 2014, 2013 and 2012 resulted primarily from changes in foreign currency exchange rates. |
| · | In connection with the June 2013 Loan Agreement, a loss on debt extinguishment was recorded that relates to the payoff of the prior loan obligation. See Note 11 to the Consolidated Financial Statements for further information. |
| · | Refer to Note 5 of the Notes to Consolidated Financial Statements for discussion of gain on Puregraft divestiture. |
| · | Refer to Note 4 of the Notes to Consolidated Financial Statements for discussion of gain on previously held equity interest in joint venture. |
|
As of December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Cash and cash equivalents
|
$
|
14,622,000
|
$
|
15,506,000
|
||||
|
Current assets
|
$
|
21,686,000
|
$
|
24,577,000
|
||||
|
Current liabilities
|
15,917,000
|
14,906,000
|
||||||
|
Working capital
|
$
|
5,769,000
|
$
|
9,671,000
|
||||
| · | In December 2012, we entered into an underwriting agreement with Lazard Capital Markets, LLC (underwriter), relating to the issuance and sale of 7,020,000 shares of our common stock. This price to the public in this offering was $2.85 per share and the underwriter purchased the shares from us at a price of $2.69 per share. The transaction was completed on December 19, 2012 raising approximately $20,007,000 in gross proceeds before deducting underwriting discounts and commissions and other offering expenses payable by us. |
| · | In January 2013, Lazard Capital Markets, LLC (underwriter) exercised its overallotment option and as a result we sold an additional 1,053,000 shares raising approximately $3,000,000 in gross proceeds before deducting underwriting discounts and commissions and other offering expenses payable by us. |
| · | On June 28, 2013 we entered into the Loan Agreement with Oxford Finance LLC and Silicon Valley Bank (together, the “Lenders”), pursuant to which the Lenders funded an aggregate principal amount of $27.0 million (Term Loan), subject to the terms and conditions set forth in the loan agreement. The Term Loan accrues interest at a fixed rate of 9.75% per annum. In connection with the Term Loan, on June 28, 2013, we issued to the Lenders warrants to purchase up to an aggregate of 596,553 shares of our common stock at an exercise price of $2.26 per share. These warrants are immediately exercisable and will expire on June 28, 2020. In connection with the Loan Agreement, we prepaid all outstanding amounts under the prior loan agreement, at which time our obligations under the prior loan agreement immediately terminated. The net proceeds of the Term Loans, after payment of lender fees and expenses and prepaying all the outstanding amounts relating to the prior loan agreement, were approximately $7.8 million. |
| · | On July 30, 2013, we entered into a Sale and Exclusive License/Supply Agreement with Bimini Technologies LLC (“Bimini”), pursuant to which we sold to Bimini substantially all of the assets (other than certain retained rights and licenses) of our Puregraft® product line, a series of standalone fat transplantation products that were developed to improve the predictability of outcomes for autologous fat grafting and aesthetic body contouring. The aggregate value of the consideration paid by Bimini at the execution of the agreement was $5.0 million. |
| · | On October 29, 2013, we entered into a partnership with Lorem Vascular, to commercialize Cytori Cell Therapy (OICH-D3) for the cardiovascular, renal and diabetes markets, in China, Hong Kong, Malaysia, Singapore and Australia (License/Supply Agreement), and a Common Stock Purchase Agreement. On January 30, 2014 we entered into the Amended and Restated License/Supply Agreement with Lorem Vascular (the “Restated Agreement”) expanding the licensed field to all uses excepting alopecia (hair loss). Under the Restated Agreement, Lorem Vascular committed to pay up to $500 million in license fees in the form of revenue milestones. In addition, Lorem is required to pay us 30% of their gross profits in China, Hong Kong and Malaysia for the term of the Restated Agreement. Cytori Cell Therapy is derived from our Celution® System, which enables access to a patient’s own adipose-derived regenerative cells (ADRCs) at the point-of-care. In addition, Lorem Vascular agreed to purchase our Celution® System and consumables under the Restated Agreement. Pursuant to the related Common Stock Purchase Agreement, we received $24.0 million in exchange for 8.0 million shares of our common stock issued to Lorem Vascular at $3.00 per share. The equity purchased was closed in two equal installments, in November 2013 and January 2014. |
| · | In May 2014, we and 47 holders of warrants to purchase a total of 3,156,238 shares of our common stock issued in a private offering in May 2009, agreed to extend the expiration date of the warrants from May 14, 2014 to May 14, 2015 and increase the exercise price of the warrants from $2.62 per share to $3.50 per share pursuant to an Amendment to Warrant to Purchase Common Stock. One holder of warrants did not agree to the Amendment, and their warrants, covering 38,500 shares of Common Stock, expired unexercised on May 14, 2014 in accordance with the original terms. |
| · | In May 2014, we entered into subscription agreements with certain institutional investors pursuant to which we sold a total of 4,048,584 units, with each unit consisting of one share of common stock and one warrant to purchase one share of common stock at a purchase price of $2.47 per unit, in a registered direct offering. Each warrant had an exercise price of $3.00 per share, was exercisable immediately after issuance and expires five years from the date of issuance. The transaction was completed on June 4, 2014 raising approximately $10,000,000 in gross proceeds before deducting any offering expenses or fees payable by us. Under the terms of our Placement Agent Agreement, we granted WBB Securities, LLC warrants to purchase 202,429 shares of common stock. The placement agent warrants have the same terms as the warrants issued to the purchasers in the offering, except that such warrants have an exercise price of $3.09. |
| · | In September 2014, we and 13 holders of warrants dated June 4, 2014 to purchase a total of 4,032,389 shares of our common stock agreed to amend the warrants in order to reduce the exercise price from $3.00 per share to $1.00 per share and change the expiration date from June 4, 2019 to September 10, 2014. We received proceeds of approximately $4,066,000 from the exercise of the warrants. In addition, pursuant to the terms of the amendment, upon each holder’s exercise of all warrants for cash prior to the amended expiration date, we issued additional warrants for the same number of common shares to the holders. The additional warrants have an exercise price of $2.00 per share, and are exercisable on the date that is six months and one day from the date of issuance and expire five years from the date of issuance. For those investors participating in the October 2014 issuance of Series A 3.6% Convertible Preferred Stock, we agreed to reduce the exercise price of 3,384,601 warrants from $2.00 per share to $0.5771 per share, conditioned upon shareholder approval which was obtained in January 2015. |
| · | In September 2014, we entered into a 2 nd Amendment to the Loan Agreement with the Lenders pursuant to the amended Loan Agreement, under which we were provided a conditional waiver of principal payments subject to meeting certain capital raise requirements, which we achieved in October. The waiver of principal payments continues from November 1, 2014 through April 1, 2015 and thereafter we are required to make payments of principal and accrued interest in equal monthly installments of $1.0 million, sufficient to amortize the Term Loans through the maturity date. |
| · | In October, 2014, we entered into a Securities Purchase Agreement with certain institutional investors pursuant to which we sold a total of 13,500 units for a purchase price of $1,000 per unit, with each unit consisting of one share of our Series A 3.6% Convertible Preferred Stock, which is convertible into shares of our common stock with a conversion price of $0.52, and warrants to purchase up to a number of shares of common stock equal to 100% of the conversion shares under the shares of preferred stock, in a registered direct offering. Each warrant has an exercise price of $0.5771 per share, is exercisable six months after the date of issuance and expires five years from the date on which it is initially exercisable. The preferred stock and the warrants were immediately separable and were issued separately. As of December 31, 2014, 8,189 units had been converted into 15,747,000 shares of common stock. |
|
Payments due by period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than 1
year
|
1 – 3 years
|
3 – 5 years
|
More than
5 years
|
|||||||||||||||
|
Long-term obligations
|
$
|
26,863,000
|
$
|
7,462,000
|
$
|
19,401,000
|
$
|
—
|
$
|
—
|
||||||||||
|
Interest commitment on long-term obligations
|
3,670,000
|
2,205,000
|
1,465,000
|
—
|
—
|
|||||||||||||||
|
Operating lease obligations
|
6,296,000
|
2,183,000
|
4,086,000
|
27,000
|
—
|
|||||||||||||||
|
Joint Venture purchase obligation*
|
3,297,000
|
3,297,000
|
—
|
—
|
—
|
|||||||||||||||
|
Clinical research study obligations
|
1,216,000
|
1,216,000
|
—
|
—
|
—
|
|||||||||||||||
|
Total
|
$
|
41,342,000
|
$
|
16,363,000
|
$
|
24,952,000
|
$
|
27,000
|
$
|
—
|
||||||||||
|
Years Ended
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Net cash used in operating activities
|
$
|
(30,330,000
|
)
|
$
|
(34,563,000
|
)
|
$
|
(32,193,000
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
(1,343,000
|
)
|
3,686,000
|
(1,204,000
|
)
|
|||||||
|
Net cash provided by financing activities
|
30,874,000
|
20,772,000
|
22,192,000
|
|||||||||
| · | initial consulting services; |
| · | license rights and standard operating procedures; |
| · | equipment and supplies; |
| · | installation services; |
| · | training services; |
| · | database hosting services; |
| · | technical support services; and |
| · | maintenance services. |
|
Page
|
|
|
Reports of KPMG LLP, Independent Registered Public Accounting Firm
|
44
|
|
Consolidated Balance Sheets as of December 31, 2014 and 2013
|
46
|
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2014, 2013 and 2012
|
47
|
|
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended December 31, 2014, 2013 and 2012
|
48
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2014, 2013 and 2012
|
49
|
|
Notes to Consolidated Financial Statements
|
51
|
| PART I. | FINANCIAL INFORMATION |
| Item 1. | Financial Statements |
|
|
/s/ KPMG LLP
|
|
|
|
|
San Diego, California
|
|
|
March 16, 2015
|
|
|
|
/s/ KPMG LLP
|
|
|
|
|
San Diego, California
|
|
|
March 16, 2015
|
|
|
|
|
|
As of December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
14,622,000
|
$
|
15,506,000
|
||||
|
Accounts receivable, net of reserves of $1,523,000 and of $1,445,000 in 2014 and 2013, respectively
|
1,243,000
|
4,152,000
|
||||||
|
Inventories, net
|
4,829,000
|
3,694,000
|
||||||
|
Other current assets
|
992,000
|
1,225,000
|
||||||
|
Total current assets
|
21,686,000
|
24,577,000
|
||||||
|
Property and equipment, net
|
1,583,000
|
1,054,000
|
||||||
|
Restricted cash and cash equivalents
|
350,000
|
350,000
|
||||||
|
Other assets
|
1,763,000
|
2,812,000
|
||||||
|
Intangibles, net
|
9,415,000
|
9,345,000
|
||||||
|
Goodwill
|
3,922,000
|
3,922,000
|
||||||
|
Total assets
|
$
|
38,719,000
|
$
|
42,060,000
|
||||
|
Liabilities and Stockholders’ (Deficit) Equity
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses
|
$
|
5,546,000
|
$
|
6,077,000
|
||||
|
Current portion of long-term obligations, net of discount
|
7,363,000
|
3,191,000
|
||||||
|
Termination fee obligation
|
—
|
400,000
|
||||||
|
Puregraft divestiture obligation
|
—
|
547,000
|
||||||
|
Joint Venture purchase obligation
|
3,008,000
|
4,691,000
|
||||||
|
Total current liabilities
|
15,917,000
|
14,906,000
|
||||||
|
Warrant liability
|
9,793,000
|
—
|
||||||
|
Deferred revenues
|
112,000
|
212,000
|
||||||
|
Long-term deferred rent
|
558,000
|
710,000
|
||||||
|
Long-term obligations, net of discount, less current portion
|
18,041,000
|
23,100,000
|
||||||
|
Total liabilities
|
44,421,000
|
38,928,000
|
||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders’ equity:
|
||||||||
|
Series A 3.6% convertible preferred stock, $0.001 par value; 5,000,000 shares authorized; 13,500 shares issued and 5,311 outstanding in 2014, and no shares issued and outstanding in 2013
|
—
|
—
|
||||||
|
Common stock, $0.001 par value; 145,000,000 shares authorized; 99,348,377 and 71,305,375 shares issued and outstanding in 2014 and 2013, respectively
|
99,000
|
71,000
|
||||||
|
Additional paid-in capital
|
331,772,000
|
303,710,000
|
||||||
|
Accumulated other comprehensive income
|
700,000
|
256,000
|
||||||
|
Accumulated deficit
|
(338,273,000
|
)
|
(300,905,000
|
)
|
||||
|
Total stockholders’ (deficit) equity
|
(5,702,000
|
)
|
3,132,000
|
|||||
|
Total liabilities and stockholders’ (deficit) equity
|
$
|
38,719,000
|
$
|
42,060,000
|
||||
|
For the Years Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Product revenues:
|
||||||||||||
|
Related party
|
$
|
—
|
$
|
1,845,000
|
$
|
—
|
||||||
|
Third party
|
4,953,000
|
5,277,000
|
8,709,000
|
|||||||||
|
4,953,000
|
7,122,000
|
8,709,000
|
||||||||||
|
Cost of product revenues
|
2,940,000
|
3,421,000
|
4,000,000
|
|||||||||
|
Gross profit
|
2,013,000
|
3,701,000
|
4,709,000
|
|||||||||
|
Development revenues:
|
||||||||||||
|
Development, related party
|
—
|
638,000
|
2,882,000
|
|||||||||
|
Development
|
—
|
1,179,000
|
2,529,000
|
|||||||||
|
Government contracts and other
|
2,645,000
|
3,257,000
|
381,000
|
|||||||||
|
2,645,000
|
5,074,000
|
5,792,000
|
||||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
15,105,000
|
17,065,000
|
13,628,000
|
|||||||||
|
Sales and marketing
|
6,406,000
|
9,026,000
|
9,488,000
|
|||||||||
|
General and administrative
|
15,953,000
|
16,031,000
|
15,672,000
|
|||||||||
|
Change in fair value of warrants
|
(369,000
|
)
|
(418,000
|
)
|
(209,000
|
)
|
||||||
|
Change in fair value of option liability
|
—
|
(2,250,000
|
)
|
340,000
|
||||||||
|
Total operating expenses
|
37,095,000
|
39,454,000
|
38,919,000
|
|||||||||
|
Operating loss
|
(32,437,000
|
)
|
(30,679,000
|
)
|
(28,418,000
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Gain (loss) on asset disposal
|
42,000
|
(257,000
|
)
|
—
|
||||||||
|
Loss on debt extinguishment
|
—
|
(708,000
|
)
|
—
|
||||||||
|
Interest income
|
6,000
|
4,000
|
4,000
|
|||||||||
|
Interest expense
|
(4,371,000
|
)
|
(3,396,000
|
)
|
(3,386,000
|
)
|
||||||
|
Other income (expense), net
|
(608,000
|
)
|
(438,000
|
)
|
(314,000
|
)
|
||||||
|
Gain on Puregraft divestiture
|
—
|
4,453,000
|
—
|
|||||||||
|
Gain on previously held equity interest in joint venture
|
—
|
4,892,000
|
—
|
|||||||||
|
Equity loss from investment in joint venture
|
—
|
(48,000
|
)
|
(165,000
|
)
|
|||||||
|
Total other income (expense)
|
(4,931,000
|
)
|
4,502,000
|
(3,861,000
|
)
|
|||||||
|
Net loss
|
(37,368,000
|
)
|
(26,177,000
|
)
|
(32,279,000
|
)
|
||||||
|
Beneficial conversion feature for
|
||||||||||||
|
convertible preferred stock
|
(1,169,000
|
)
|
—
|
—
|
||||||||
|
Net loss allocable to common stock holders
|
(38,537,000
|
)
|
(26,177,000
|
)
|
(32,279,000
|
)
|
||||||
|
Basic and diluted net loss per share allocable to common stockholders
|
$
|
(0.48
|
)
|
$
|
(0.39
|
)
|
$
|
(0.55
|
)
|
|||
|
Basic and diluted weighted average shares used in calculating net loss per share allocable to common stockholders
|
80,830,698
|
67,781,364
|
58,679,687
|
|||||||||
| Comprehensive loss: | ||||||||||||
| Net loss |
$
|
(37,368,000
|
)
|
$
|
(26,177,000
|
)
|
$
|
(32,279,000
|
)
|
|||
|
Other comprehensive income – foreign currency translation adjustments
|
444,000
|
256,000
|
—
|
|||||||||
|
Comprehensive loss
|
$
|
(36,924,000
|
)
|
$
|
(25,921,000
|
)
|
$
|
(32,279,000
|
)
|
|||
|
Convertible Preferred Stock
|
Common Stock
|
Additional
Paid-in
|
Accumulated
|
Accumulated Other Comprehensive Income
|
||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Deficit
|
(Loss)
|
Total
|
|||||||||||||||||||||||||
|
Balance at December 31, 2011
|
—
|
$
|
—
|
56,594,683
|
$
|
57,000
|
$
|
252,338,000
|
$
|
(242,449,000
|
)
|
$
|
—
|
$
|
9,946,000
|
|||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
3,904,000
|
—
|
—
|
3,904,000
|
||||||||||||||||||||||||
|
Issuance of common stock under stock option plan and employee stock purchase plan
|
—
|
—
|
450,512
|
—
|
1,157,000
|
—
|
—
|
1,157,000
|
||||||||||||||||||||||||
|
Issuance of common stock under stock warrant agreement
|
—
|
—
|
98,855
|
—
|
256,000
|
—
|
—
|
256,000
|
||||||||||||||||||||||||
|
Sale of common stock, net
|
—
|
—
|
8,770,000
|
9,000
|
23,462,000
|
—
|
—
|
23,471,000
|
||||||||||||||||||||||||
|
Net loss for the year ended December 31, 2012
|
—
|
—
|
—
|
—
|
—
|
(32,279,000
|
)
|
—
|
(32,279,000
|
)
|
||||||||||||||||||||||
|
Balance at December 31, 2012
|
—
|
—
|
65,914,050
|
$
|
66,000
|
$
|
281,117,000
|
$
|
(274,728,000
|
)
|
$
|
—
|
$
|
6,455,000
|
||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
3,608,000
|
—
|
—
|
3,608,000
|
||||||||||||||||||||||||
|
Issuance of common stock under stock option plan and employee stock purchase plan
|
—
|
—
|
338,325
|
—
|
225,000
|
—
|
—
|
225,000
|
||||||||||||||||||||||||
|
Sale of common stock, net
|
—
|
—
|
5,053,000
|
5,000
|
17,811,000
|
—
|
—
|
17,816,000
|
||||||||||||||||||||||||
|
Allocation of fair value for debt-related warrants
|
—
|
—
|
—
|
—
|
949,000
|
—
|
—
|
949,000
|
||||||||||||||||||||||||
|
Accumulated other comprehensive income (loss)
|
—
|
—
|
—
|
—
|
—
|
—
|
256,000
|
256,000
|
||||||||||||||||||||||||
|
Net loss for the year ended December 31, 2013
|
—
|
—
|
—
|
—
|
—
|
(26,177,000
|
)
|
—
|
(26,177,000
|
)
|
||||||||||||||||||||||
|
Balance at December 31, 2013
|
—
|
—
|
71,305,375
|
$
|
71,000
|
$
|
303,710,000
|
$
|
(300,905,000
|
)
|
$
|
256,000
|
$
|
3,132,000
|
||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
3,101,000
|
—
|
—
|
3,101,000
|
||||||||||||||||||||||||
|
Issuance of common stock under stock option plan and employee stock purchase plan
|
—
|
—
|
204,288
|
—
|
92,000
|
—
|
—
|
92,000
|
||||||||||||||||||||||||
|
Sale of common stock, net
|
—
|
—
|
8,048,584
|
8,000
|
18,582,000
|
—
|
—
|
18,590,000
|
||||||||||||||||||||||||
|
Issuance of Series A 3.6% Convertible Preferred Stock, net
|
13,500
|
—
|
—
|
—
|
2,235,000
|
—
|
—
|
2,235,000
|
||||||||||||||||||||||||
|
Conversion of Series A 3.6% Convertible Preferred Stock into common stock
|
(8,189
|
)
|
—
|
15,747,397
|
$
|
16,000
|
—
|
—
|
—
|
16,000
|
||||||||||||||||||||||
|
Issuance of common stock under stock warrant agreement
|
—
|
—
|
4,042,733
|
$
|
4,000
|
4,052,000
|
—
|
—
|
4,056,000
|
|||||||||||||||||||||||
|
Accumulated other comprehensive income (loss)
|
—
|
—
|
—
|
—
|
—
|
—
|
$
|
444,000
|
444,000
|
|||||||||||||||||||||||
|
Net loss for the year ended December 31, 2014
|
—
|
—
|
—
|
—
|
—
|
(37,368,000
|
)
|
—
|
(37,368,000
|
)
|
||||||||||||||||||||||
|
Balance at December 31, 2014
|
5,311
|
—
|
99,348,377
|
$
|
99,000
|
$
|
331,772,000
|
$
|
(338,273,000
|
)
|
$
|
700,000
|
$
|
(5,702,000
|
)
|
|||||||||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$
|
(37,368,000
|
)
|
$
|
(26,177,000
|
)
|
$
|
(32,279,000
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
779,000
|
1,630,000
|
933,000
|
|||||||||
|
Amortization of deferred financing costs and debt discount
|
1,220,000
|
893,000
|
930,000
|
|||||||||
|
Joint venture acquisition obligation accretion
|
579,000
|
204,000
|
—
|
|||||||||
|
Provision for doubtful accounts
|
1,084,000
|
1,141,000
|
144,000
|
|||||||||
|
Provision for expired enzymes
|
313,000
|
—
|
—
|
|||||||||
|
Change in fair value of warrants
|
(369,000
|
)
|
(418,000
|
)
|
(209,000
|
)
|
||||||
|
Change in fair value of option liability
|
—
|
(2,250,000
|
)
|
340,000
|
||||||||
|
Stock-based compensation
|
3,101,000
|
3,608,000
|
3,904,000
|
|||||||||
|
Equity loss from investment in joint venture
|
—
|
48,000
|
165,000
|
|||||||||
|
Loss on asset disposal
|
(33,000
|
)
|
257,000
|
—
|
||||||||
|
Gain on previously held equity interest in Joint Venture
|
—
|
(4,892,000
|
)
|
—
|
||||||||
|
Gain on sale of assets
|
—
|
(4,453,000
|
)
|
—
|
||||||||
|
Loss on debt extinguishment
|
—
|
708,000
|
—
|
|||||||||
|
Increases (decreases) in cash caused by changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
2,057,000
|
(1,209,000
|
)
|
(1,810,000
|
)
|
|||||||
|
Inventories
|
(815,000
|
)
|
(459,000
|
)
|
143,000
|
|||||||
|
Other current assets
|
510,000
|
(24,000
|
)
|
(324,000
|
)
|
|||||||
|
Other assets
|
11,000
|
(854,000
|
)
|
(74,000
|
)
|
|||||||
|
Accounts payable and accrued expenses
|
(1,147,000
|
)
|
(409,000
|
)
|
1,183,000
|
|||||||
|
Deferred revenues, related party
|
—
|
(638,000
|
)
|
(2,882,000
|
)
|
|||||||
|
Deferred revenues
|
(100,000
|
)
|
(1,223,000
|
)
|
(2,609,000
|
)
|
||||||
|
Long-term deferred rent
|
(152,000
|
)
|
(46,000
|
)
|
252,000
|
|||||||
|
Net cash used in operating activities
|
(30,330,000
|
)
|
(34,563,000
|
)
|
(32,193,000
|
)
|
||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchases of property and equipment
|
(764,000
|
)
|
(519,000
|
)
|
(1,204,000
|
)
|
||||||
|
Expenditures for intellectual property
|
(255,000
|
)
|
—
|
—
|
||||||||
|
Proceeds from sale of assets
|
76,000
|
5,000,000
|
—
|
|||||||||
|
License agreement termination fee
|
(400,000
|
)
|
(800,000
|
)
|
—
|
|||||||
|
Cash acquired in purchase of joint venture
|
—
|
5,000
|
—
|
|||||||||
|
Net cash (used in) provided by investing activities
|
(1,343,000
|
)
|
3,686,000
|
(1,204,000
|
)
|
|||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Principal payments on long-term debt obligations
|
(1,962,000
|
)
|
(22,304,000
|
)
|
(2,692,000
|
)
|
||||||
|
Proceeds from long-term obligations
|
—
|
27,000,000
|
—
|
|||||||||
|
Debt issuance costs and loan fees
|
—
|
(1,744,000
|
)
|
—
|
||||||||
|
Joint venture purchase payments
|
(2,262,000
|
)
|
(221,000
|
)
|
—
|
|||||||
|
Proceeds from exercise of employee stock options and warrants and stock purchase plan
|
4,151,000
|
225,000
|
1,413,000
|
|||||||||
|
Proceeds from issuance of common stock
|
19,001,000
|
18,000,000
|
24,953,000
|
|||||||||
|
Proceeds from issuance of preferred stock
|
13,500,000
|
—
|
—
|
|||||||||
|
Costs from sale of common stock
|
(425,000
|
)
|
(184,000
|
)
|
(1,482,000
|
)
|
||||||
|
Costs from sale of preferred stock
|
(1,129,000
|
)
|
—
|
—
|
||||||||
|
Net cash provided by financing activities
|
30,874,000
|
20,772,000
|
22,192,000
|
|||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(85,000
|
)
|
(106,000
|
)
|
—
|
|||||||
|
Net decrease in cash and cash equivalents
|
(884,000
|
)
|
(10,211,000
|
)
|
(11,205,000
|
)
|
||||||
|
Cash and cash equivalents at beginning of year
|
15,506,000
|
25,717,000
|
36,922,000
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
14,622,000
|
$
|
15,506,000
|
$
|
25,717,000
|
||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Supplemental disclosure of cash flows information:
|
||||||||||||
|
Cash paid during period for:
|
||||||||||||
|
Interest
|
$
|
2,588,000
|
$
|
2,252,000
|
$
|
2,497,000
|
||||||
|
Final payment fee on long-term debt
|
—
|
1,078,000
|
—
|
|||||||||
|
Supplemental schedule of non-cash investing and financing activities:
|
||||||||||||
|
Conversion of preferred stock into common stock
|
$
|
16,000
|
$
|
—
|
$
|
—
|
||||||
|
Declared dividend related to preferred stock
|
72,000
|
—
|
—
|
|||||||||
|
Fair value of warrants allocated to additional paid-in capital
|
—
|
949,000 |
—
|
|||||||||
|
Fair value of intangible assets acquired
|
—
|
9,394,000
|
—
|
|||||||||
|
Fair value of tangible assets acquired
|
—
|
260,000
|
—
|
|||||||||
|
Joint venture purchase obligation
|
—
|
4,709,000
|
—
|
|||||||||
|
Fair value of previously held equity interest at acquisition date
|
—
|
4,928,000
|
—
|
|||||||||
| 1. | Organization and Operations |
| 2. | Summary of Significant Accounting Policies |
|
December 31, 2014
|
||||
|
Other intangibles, net:
|
||||
|
Beginning balance
|
$
|
9,345,000
|
||
|
Increase
|
255,000
|
|||
|
Amortization
|
(185,000
|
)
|
||
|
Ending balance
|
9,415,000
|
|||
|
Goodwill, net:
|
||||
|
Beginning balance
|
3,922,000
|
|||
|
Increase (decrease)
|
—
|
|||
|
Ending balance
|
3,922,000
|
|||
|
Total goodwill and other intangibles, net
|
$
|
13,337,000
|
||
|
December 31, 2013
|
||||
|
Other intangibles, net:
|
||||
|
Beginning balance
|
$
|
—
|
||
|
Acquisition of JV Intangible
|
9,394,000
|
|||
|
Amortization
|
(49,000
|
)
|
||
|
Ending balance
|
9,345,000
|
|||
|
Goodwill, net:
|
||||
|
Beginning balance
|
3,922,000
|
|||
|
Increase (decrease)
|
—
|
|||
|
Ending balance
|
3,922,000
|
|||
|
Total goodwill and other intangibles, net
|
$
|
13,267,000
|
||
|
As of
December 31, 2014
|
||||
|
Expected term
|
5.3 years
|
|||
|
Common stock market price
|
$
|
0.49
|
||
|
Risk-free interest rate
|
1.65
|
%
|
||
|
Expected volatility
|
90.00
|
%
|
||
|
Resulting fair value (per warrant)
|
$
|
0.38
|
||
| · | initial consulting services; |
| · | license rights and standard operating procedures; |
| · | equipment and supplies; |
| · | installation services; |
| · | training services; |
| · | database hosting services; |
| · | technical support services; and |
| · | maintenance services. |
|
Years ended
|
||||||||||||||||||||||||
|
2014
|
2013
|
2012
|
||||||||||||||||||||||
|
Product Revenues
|
% of Total
|
Product Revenues
|
% of Total
|
Product Revenues
|
% of Total
|
|||||||||||||||||||
|
North America
|
$
|
894,000
|
18
|
%
|
$
|
1,079,000
|
15
|
%
|
$
|
1,143,000
|
13
|
%
|
||||||||||||
|
Japan
|
3,068,000
|
62
|
%
|
2,109,000
|
30
|
%
|
4,352,000
|
50
|
%
|
|||||||||||||||
|
Europe
|
506,000
|
10
|
%
|
1,240,000
|
17
|
%
|
2,004,000
|
23
|
%
|
|||||||||||||||
|
Other countries
|
485,000
|
10
|
%
|
2,694,000
|
38
|
%
|
1,210,000
|
14
|
%
|
|||||||||||||||
|
Total product revenues
|
$
|
4,953,000
|
100
|
%
|
$
|
7,122,000
|
100
|
%
|
$
|
8,709,000
|
100
|
%
|
||||||||||||
| 3. | Agreement with Lorem Vascular |
|
Useful Life
(in years)
|
Estimated
Fair Value
|
|||||||
|
Intangible assets:
|
||||||||
|
Developed technology
|
7
|
$
|
9,394,000
|
|||||
|
Estimated
Fair Value
|
||||
|
Current assets
|
$
|
236
|
||
|
Property and equipment
|
260
|
|||
|
Intangible assets
|
9,394
|
|||
|
Total assets acquired
|
9,890
|
|||
|
Accrued and other current liabilities
|
(33
|
)
|
||
|
Total fair value of the Joint Venture
|
$
|
9,857
|
||
|
5.
|
Sale and Exclusive License/Supply Agreement with Bimini Technologies LLC
|
|
6.
|
Fair Value Measurements
|
|
·
|
Level 1: Quoted prices in active markets for identical assets or liabilities.
|
|
·
|
Level 2: Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets.
|
|
·
|
Level 3: Valuations derived from valuation techniques in which one or more significant inputs are unobservable in active markets.
|
|
Balance as of
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
December 31, 2014
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash equivalents
|
$
|
8,144,000
|
$
|
8,144,000
|
$
|
—
|
$
|
—
|
||||||||
|
Liabilities:
|
||||||||||||||||
|
Warrant liability
|
$
|
9,793,000
|
$
|
—
|
$
|
—
|
$
|
9,793,000
|
||||||||
|
Balance as of
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
December 31, 2013
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash equivalents
|
$
|
4,644,000
|
$
|
4,644,000
|
$
|
—
|
$
|
—
|
||||||||
|
Warrant liability
|
December 31, 2014
|
December 31, 2013
|
||||||
|
Beginning balance
|
$
|
—
|
$
|
418,000
|
||||
|
Additions to warrant liability
|
10,162,000
|
—
|
||||||
|
Change in fair value
|
(369,000
|
)
|
(418,000
|
)
|
||||
|
Ending balance
|
$
|
9,793,000
|
$
|
—
|
||||
|
Put option liability
|
Year ended
December 31, 2013
|
|||
|
Beginning balance
|
$
|
(2,250,000
|
)
|
|
|
Decrease (increase) in fair value recognized in operating expenses
|
2,250,000
|
|||
|
Ending balance
|
$
|
—
|
||
|
7.
|
Fair Value
|
|
December 31, 2014
|
December 31, 2013
|
|||||||||||||||
|
Fair Value
|
Carrying Value
|
Fair Value
|
Carrying Value
|
|||||||||||||
|
Fixed rate long-term debt
|
$
|
25,206,000
|
$
|
25,373,000
|
$
|
26,207,000
|
$
|
26,241,000
|
||||||||
|
8.
|
Thin Film Japan Distribution Agreement
|
|
9
.
|
Composition of Certain Financial Statement Captions
|
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Raw materials
|
$
|
1,715,000
|
$
|
1,315,000
|
||||
|
Work in process
|
1,301,000
|
232,000
|
||||||
|
Finished goods
|
1,813,000
|
2,147,000
|
||||||
|
$
|
4,829,000
|
$
|
3,694,000
|
|||||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Prepaid insurance
|
$
|
200,000
|
$
|
264,000
|
||||
|
Prepaid other
|
675,000
|
850,000
|
||||||
|
Other receivables
|
117,000
|
111,000
|
||||||
|
$
|
992,000
|
$
|
1,225,000
|
|||||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Manufacturing and development equipment
|
$
|
5,674,000
|
$
|
5,059,000
|
||||
|
Office and computer equipment
|
2,006,000
|
2,274,000
|
||||||
|
Leasehold improvements
|
3,271,000
|
3,271,000
|
||||||
|
10,951,000
|
10,604,000
|
|||||||
|
Less accumulated depreciation and amortization
|
(9,368,000
|
)
|
(9,550,000
|
)
|
||||
|
$
|
1,583,000
|
$
|
1,054,000
|
|||||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Deposits
|
$
|
540,000
|
$
|
479,000
|
||||
|
Prepaid supplies, long-term
|
1,223,000
|
2,333,000
|
||||||
|
$
|
1,763,000
|
$
|
2,812,000
|
|||||
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Accrued legal fees
|
$
|
544,000
|
$
|
564,000
|
||||
|
Accrued R&D studies
|
273,000
|
376,000
|
||||||
|
Accounts payable
|
949,000
|
965,000
|
||||||
|
Accrued vacation
|
577,000
|
918,000
|
||||||
|
Accrued bonus
|
758,000
|
759,000
|
||||||
|
Accrued expenses
|
2,006,000
|
2,167,000
|
||||||
|
Deferred rent
|
191,000
|
138,000
|
||||||
|
Accrued accounting fees
|
130,000
|
140,000
|
||||||
|
Accrued payroll
|
118,000
|
50,000
|
||||||
|
$
|
5,546,000
|
$
|
6,077,000
|
|||||
|
10.
|
Commitments and Contingencies
|
|
Years Ending December 31,
|
Operating Leases
|
|||
|
2015
|
$
|
2,183,000
|
||
|
2016
|
2,241,000
|
|||
|
2017
|
1,790,000
|
|||
|
2018
|
55,000
|
|||
|
2019
|
27,000
|
|||
|
Total
|
$
|
6,296,000
|
||
| 11. | Long-term Obligations |
|
Origination Date
|
Original Loan
Amount
|
Interest
Rate
|
Current
Monthly
Payment*
|
Original Term
|
Remaining
Principal
(Face Value)
|
||||||||||||
|
June 2013
|
$
|
27,000,000
|
9.75
|
%
|
$
|
203,434
|
48 Months
|
$
|
25,038,125
|
||||||||
|
Years Ending December 31,
|
||||
|
2015
|
$
|
7,462,000
|
||
|
2016
|
10,805,000
|
|||
|
2017
|
8,596,000
|
|||
|
Total
|
$
|
26,863,000
|
||
|
Reconciliation of Face Value to Book Value as of December 31, 2014
|
||||
|
Total debt and lease obligations, including final payment fee (Face Value)
|
$
|
26,863,000
|
||
|
Less: Debt discount
|
(1,459,000
|
)
|
||
|
Total:
|
25,404,000
|
|||
|
Less: Current portion
|
(7,363,000
|
)
|
||
|
Long-term obligation
|
$
|
18,041,000
|
||
| 12. | Income Taxes |
|
2014
|
2013
|
2012
|
||||||||||
|
Income tax expense (benefit) at federal statutory rate
|
(34.00)
|
%
|
(34.00)
|
%
|
(34.00)
|
%
|
||||||
|
Income tax expense (benefit) at state statutory rate
|
(3.52)
|
%
|
(3.54)
|
%
|
(2.79)
|
%
|
||||||
|
Gain on previously held equity interest in joint venture
|
0.00
|
%
|
(7.02)
|
%
|
0.00
|
%
|
||||||
| Mark to market permanent adjustment |
(0.37)
|
%
|
(2.15)
|
%
|
(0.24)
|
%
|
||||||
|
Change in valuation allowance
|
27.12
|
%
|
80.13
|
%
|
35.86
|
%
|
||||||
|
Change in state rate
|
0.02
|
%
|
(1.01)
|
%
|
(8.36)
|
%
|
||||||
|
Permanent interest adjustments
|
4.17
|
%
|
0.00
|
%
|
0.00
|
%
|
||||||
|
Debt refinance permanent adjustment
|
3.92
|
%
|
0.00
|
%
|
0.00
|
%
|
||||||
|
Acquired NOL’s/Intangibles from joint venture
|
0.00
|
%
|
(33.40)
|
%
|
0.00
|
%
|
||||||
|
Foreign rate differential
|
0.00
|
%
|
2.48
|
%
|
(0.04)
|
%
|
||||||
|
Other, net
|
2.66
|
%
|
(1.49)
|
%
|
9.57
|
%
|
||||||
|
0.00
|
%
|
0.00
|
%
|
0.00
|
%
|
|||||||
|
2014
|
2013
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Allowances and reserves
|
$
|
825,000
|
$
|
639,000
|
||||
|
Accrued expenses
|
502,000
|
718,000
|
||||||
|
Deferred revenue and gain-on-sale
|
32,000
|
79,000
|
||||||
|
Stock based compensation
|
7,786,000
|
6,962,000
|
||||||
|
Net operating loss carryforwards
|
117,258,000
|
107,846,000
|
||||||
|
Income tax credit carryforwards
|
6,993,000
|
6,710,000
|
||||||
|
Property and equipment, principally due to differences in depreciation
|
926,000
|
804,000
|
||||||
|
Other,net
|
77,000
|
296,000
|
||||||
|
134,399,000
|
124,054,000
|
|||||||
|
Valuation allowance
|
(132,583,000
|
)
|
(122,450,000
|
)
|
||||
|
Total deferred tax assets, net of allowance
|
1,816,000
|
1,604,000
|
||||||
|
Deferred tax liabilities:
|
||||||||
|
Intangibles
|
(1,816,000
|
)
|
(1,604,000
|
)
|
||||
|
Total deferred tax liability
|
(1,816,000
|
)
|
(1,604,000
|
)
|
||||
|
Net deferred tax assets (liability)
|
$
|
—
|
$
|
—
|
||||
|
2014
|
2013
|
2012
|
||||||||||
|
Unrecognized Tax Benefits – Beginning
|
$
|
1,723,000
|
$
|
1,394,000
|
$
|
1,304,000
|
||||||
|
Gross increases – tax positions in prior period
|
—
|
69,000
|
—
|
|||||||||
|
Gross decreases – tax positions in prior period
|
—
|
—
|
—
|
|||||||||
|
Gross increase – current-period tax positions
|
129,000
|
260,000
|
90,000
|
|||||||||
|
Settlements
|
—
|
—
|
—
|
|||||||||
|
Lapse of statute of limitations
|
—
|
—
|
—
|
|||||||||
|
Unrecognized Tax Benefits – Ending
|
$
|
1,852,000
|
$
|
1,723,000
|
$
|
1,394,000
|
||||||
| 13. | Employee Benefit Plan |
| 14. | Stockholders’ Equity |
| 15. | Stockholders Rights Plan |
| 16. | Stock-based Compensation |
| · | 12/48 of a granted award will vest after one year of service, while an additional 1/48 of the award will vest at the end of each month thereafter for 36 months, or |
| · | 1/48 of the award will vest at the end of each month over a four-year period. |
|
Options
|
Weighted
Average
Exercise Price
|
|||||||
|
Balance as of January 1, 2014
|
8,322,289
|
$
|
4.55
|
|||||
|
Granted
|
3,058,190
|
$
|
1.99
|
|||||
|
Exercised
|
(2,667
|
)
|
$
|
2.62
|
||||
|
Expired
|
(396,328
|
)
|
$
|
4.14
|
||||
|
Cancelled/forfeited
|
(1,866,136
|
)
|
$
|
3.45
|
||||
|
Balance as of December 31, 2014
|
9,115,348
|
$
|
3.93
|
|||||
|
Options
|
Weighted
Average
Exercise Price
|
Weighted Average Remaining Contractual Term (years)
|
Aggregate
Intrinsic Value
|
|||||||||||||
|
Balance as of December 31, 2014
|
9,115,348
|
$
|
3.93
|
6.31
|
$
|
584.4
|
||||||||||
|
Vested and expected to vest at December 31, 2014
|
9,064,341
|
$
|
3.94
|
6.30
|
$
|
560.47
|
||||||||||
|
Exercisable at December 31, 2014
|
6,230,987
|
$
|
4.70
|
5.06
|
$
|
—
|
||||||||||
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Expected term
|
6.0 years
|
6.0 years
|
5.2 years
|
|||||||||
|
Risk-free interest rate
|
1.86
|
%
|
1.12
|
%
|
0.83
|
%
|
||||||
|
Volatility
|
77.52
|
%
|
75.27
|
%
|
75.63
|
%
|
||||||
|
Dividends
|
—
|
—
|
—
|
|||||||||
|
Resulting weighted average grant date fair value
|
$
|
1.35
|
$
|
1.72
|
$
|
1.96
|
||||||
|
Restricted
Stock Awards
|
Weighted
Average Grant
Date Fair Value
|
|||||||
|
Balance as of January 1, 2014
|
106,341
|
$
|
3.62
|
|||||
|
Granted
|
115,808
|
$
|
2.50
|
|||||
|
Exercised/Released
|
(12,200
|
)
|
$
|
2.80
|
||||
|
Cancelled/forfeited
|
(16,826
|
)
|
$
|
2.51
|
||||
|
Balance as of December 31, 2014
|
193,123
|
$
|
3.10
|
|||||
|
Restricted
Stock Awards
|
Weighted
Average Grant
Date Fair Value
|
Weighted
Average
Remaining
Contractual
Term (years)
|
||||||||||
|
Balance as of December 31, 2014
|
193,123
|
$
|
3.10
|
8.32
|
||||||||
|
Vested and expected to vest at December 31, 2014
|
193,123
|
$
|
3.10
|
7.21
|
||||||||
|
Exercisable at December 31, 2014
|
83,641
|
$
|
3.91
|
8.32
|
||||||||
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Total compensation cost for share-based payment arrangements recognized in the statement of operations (net of tax of $0)
|
$
|
3,101,000
|
$
|
3,608,000
|
$
|
3,904,000
|
||||||
|
17.
|
Related Party Transactions
|
|
18.
|
Quarterly Information (unaudited)
|
|
For the three months ended
|
||||||||||||||||
|
March 31,
2014
|
June 30,
2014
|
September 30,
2014
|
December 31,
2014
|
|||||||||||||
|
Product revenues
|
$
|
1,031,000
|
$
|
935,000
|
$
|
518,000
|
$
|
2,469,000
|
||||||||
|
Gross profit
|
610,000
|
169,000
|
181,000
|
1,053,000
|
||||||||||||
|
Development revenues
|
403,000
|
356,000
|
585,000
|
1,301,000
|
||||||||||||
|
Operating expenses
|
(10,560,000
|
)
|
(11,210,000
|
)
|
(8,656,000
|
)
|
(6,669,000
|
)
|
||||||||
|
Other income (expense)
|
(853,000
|
)
|
(1,143,000
|
)
|
(1,495,000
|
)
|
(1,440,000
|
)
|
||||||||
|
Net loss
|
$
|
(10,400,000
|
)
|
$
|
(11,828,000
|
)
|
$
|
(9,385,000
|
)
|
$
|
(5,755,000
|
)
|
||||
|
Beneficial conversion feature for convertible preferred stock
|
—
|
—
|
—
|
(1,169,000
|
)
|
|||||||||||
|
Net loss allocable to common stock holders…
|
(10,400,000
|
)
|
(11,828,000
|
)
|
(9,385,000
|
)
|
(6,924,000
|
)
|
||||||||
|
Basic and diluted net loss per share
|
$
|
(0.14
|
)
|
$
|
(0.15
|
)
|
$
|
(0.12
|
)
|
$
|
(0.08
|
)
|
||||
|
For the three months ended
|
||||||||||||||||
|
March 31,
2013
|
June 30,
2013
|
September 30,
2013
|
December 31,
2013
|
|||||||||||||
|
Product revenues
|
$
|
1,392,000
|
$
|
1,408,000
|
$
|
1,616,000
|
$
|
2,706,000
|
||||||||
|
Gross profit
|
636,000
|
800,000
|
685,000
|
1,580,000
|
||||||||||||
|
Development revenues
|
2,366,000
|
859,000
|
1,095,000
|
754,000
|
||||||||||||
|
Operating expenses
|
(9,739,000
|
)
|
(8,022,000
|
)
|
(10,241,000
|
)
|
(11,452,000
|
)
|
||||||||
|
Other income (expense)
|
(930,000
|
)
|
3,152,000
|
3,203,000
|
(923,000
|
)
|
||||||||||
|
Net loss
|
$
|
(7,667,000
|
)
|
$
|
(3,211,000
|
)
|
$
|
(5,258,000
|
)
|
$
|
(10,041,000
|
)
|
||||
|
Basic and diluted net loss per share
|
$
|
(0.11
|
)
|
$
|
(0.05
|
)
|
$
|
(0.08
|
)
|
$
|
(0.14
|
)
|
||||
| (a) | Evaluation of Disclosure Controls and Procedures |
| (b) | Management’s Report on Internal Control Over Financial Reporting |
| · | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| · | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and our Board of Directors; and |
| · | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
| (c) | Changes in Internal Control over Financial Reporting |
| (d) | Remediation of Material Weakness |
| 1. | reevaluation of our processes for the recognition of revenue, |
| 2. | hiring a qualified individual in Japan with appropriate experience to assist with our review of revenue arrangements and to help facilitate better communication with our Japan subsidiary, and |
| 3. | enhancing our assessment of collectability over our customers to ensure that adequate evidence of collectability is obtained prior to the recognition of revenue. For customers that have not developed an adequate payment history with us and a letter of credit is not in place at the time of the transaction, and assuming all other revenue recognition criteria have been met, we defer revenues until cash is received. Our remediation efforts, including design, implementation and testing continued throughout fiscal year 2014. |
|
Name
|
Age
|
Position
|
|
David M. Rickey
|
59
|
Chairman of the Board of Directors
|
|
Marc H. Hedrick, MD
|
52
|
President & CEO and Director
|
|
Richard J. Hawkins
|
66
|
Director
|
|
Paul W. Hawran
|
62
|
Director
|
|
Gary Lyons
|
63
|
Director
|
|
Tommy G. Thompson
|
73
|
Director
|
|
Gail K. Naughton, Ph.D.
|
59
|
Director
|
|
Name
|
Age
|
Position
|
|
Marc H. Hedrick, MD
|
52
|
President & CEO and Director
|
|
Tiago Girão
|
35
|
Vice President, Finance & Chief Financial Officer
|
|
Steven Kesten
|
56
|
Executive Vice President & Chief Medical Officer
|
|
Seijiro N. Shirahama
|
61
|
President—Asia Pacific
|
| · | Makes recommendations to the Compensation Committee regarding the base salary, bonus and stock option award levels for our other executive officers; and |
| · | Provides an annual recommendation to the Compensation Committee regarding overall Company performance objectives for the year and the individual performance objectives of each of our executive officers with respect to our Executive Management Incentive Compensation Plan, and reports to the Compensation Committee on the satisfaction of each such objective. |
| · | Base Salary; |
| · | Annual short-term performance–based cash incentives (The Executive Management Incentive Compensation Plan); |
| · | Long-term equity compensation in the form of Stock Options; |
| · | Short-term equity compensation in the potential forms as allowed by our respective 2004 Equity Incentive Plan and 2014 Equity Incentive Plan, including time, and performance vested restricted stock awards or units; |
| · | Personal benefits and perquisites; and |
| · | Change in control and severance agreements. |
|
Company
|
Market Capitalization as of October 14, 2013
|
|
Arena Pharmaceuticals
|
$953.8 Million
|
|
Athersys
|
$109.5 Million
|
|
AVEO Pharmaceuticals
|
$110.6 Million
|
|
BioCryst Pharmaceuticals
|
$386.6 Million
|
|
Cell Therapeutics
|
$218.9 Million
|
|
Cytokinetics
|
$206.6 Million
|
|
Dyax
|
$718.4 Million
|
|
Dynavax Technologies
|
$214.2 Million
|
|
Geron
|
$396.2 Million
|
|
Hansen Medical
|
$138.2 Million
|
|
Immunomedics
|
$448.7 Million
|
|
Ligand Pharmaceuticals
|
$971.3 Million
|
|
NeoStem
|
$188.1 Million
|
|
Neurocrine Biosciences
|
$688.7 Million
|
|
Novavax
|
$627.4 Million
|
|
Osiris Therapeutics
|
$594.2 Million
|
|
Pain Therapeutics
|
$126.3 Million
|
|
Peregrine Pharmaceuticals
|
$222.2 Million
|
|
Rigel Pharmaceutics
|
$296.4 Million
|
|
Sangamo BioSciences
|
$605.5 Million
|
|
Solta Medical
|
$170.7 Million
|
|
StemCells
|
$59.1 Million
|
|
Ziopharm Oncology
|
$375.9 Million
|
|
Company
|
Market Capitalization as of September 18, 2014
|
|
Aastrom Biosciences
|
$21.5 Million
|
|
Arqule
|
$72.8 Million
|
|
Athersys
|
$112.1 Million
|
|
AVEO Pharmaceuticals
|
$61.7 Million
|
|
CTI Biopharma
|
$400.3 Million
|
|
Cytokinetics
|
$141.7 Million
|
|
Dynavax Technologies
|
$357.5 Million
|
|
Fibrocell Science
|
$111.5 Million
|
|
Geron
|
$362.4 Million
|
|
Hansen Medical
|
$138 Million
|
|
Immunomedics
|
$302.6 Million
|
|
NeoStem
|
$183.3 Million
|
|
Neuralstem
|
$283.1 Million
|
|
Osiris Therapeutics
|
$451.2Million
|
|
Pain Therapeutics
|
$173.9 Million
|
|
Peregrine Pharmaceuticals
|
$251.3 Million
|
|
Rigel Pharmaceuticals
|
$196.7 Million
|
|
Senomyx
|
$378.2 Million
|
|
StemCells
|
$97.5 Million
|
|
Stereotaxis
|
$49.8 Million
|
|
Verastem
|
$248.6 Million
|
|
Vical
|
$102.8 Million
|
|
Ziopharm Oncology
|
$300.2 Million
|
|
Zogenix
|
$170.7 Million
|
|
2014 Base Salary
|
Target Bonus %
|
|||||||
|
Dr. Hedrick
|
$406,628 (increased to $450,000 upon appointment
to CEO in April 2014)
|
40% (Increased to 45%
upon appointment to CEO)
|
||||||
|
Mr. Girão
|
$
|
240,000
|
30
|
%
|
||||
|
Dr. Kesten
|
$
|
400,000
|
25
|
%
|
||||
|
Mr. Shirahama
(1)
|
$
|
366,978
|
25
|
%
|
||||
|
Mr. Shores
(2)
|
$
|
329,469
|
30
|
%
|
||||
|
Mr. Calhoun
(3)
|
$
|
467,900
|
50
|
%
|
||||
|
Mr. Saad
(4)
|
$
|
389,917
|
35
|
%
|
||||
|
(1)
|
Mr. Shirahama’s salary is in Japanese Yen. His base salary was calculated using the average exchange rate over the year.
|
| (2) | Mr. Shores resigned from the Company effective November 2014. |
| (3) | Mr. Calhoun retired as Chief Executive Officer effective April 2, 2014 and agreed to serve as Managing Director for a transition period beginning April 2, 2014 through July 1, 2014 to facilitate the orderly transfer of responsibilities |
|
(4)
|
Mr. Saad resigned from the Company effective August 2014.
|
| o | Financial Objectives |
| o | Manage operating loss to specified objectives |
| o | Achieve 2014 year end cash objectives |
| o | Clinical Development Objectives |
| o | Complete ATHENA I and ATHENA II trial and enrollment objectives |
| o | Identify new pipeline opportunities with development plans and budget for project initiation ready by year-end |
| o | Complete clinical partnership trial objectives |
| o | Government Contracting and Development Objectives |
| o | Transfer specified manufacturing to EU facility |
| o | Obtain BARDA funding to specified targets |
| o | Business Development Objectives |
| o | Bring licensing opportunity to term-sheet |
| o | Obtain certificate of foreign governments and CFDA class 1 approval |
| o | Research Sales and Marketing |
| o | Achieve specified product revenue targets |
| o | Develop operating plans for regional profitability in 2015. |
| · | Corporate cash burn reduction objectives |
| · | Capitalization objectives |
| · | Cash deal objectives |
| · | Investor relations objectives |
| · | Vendor cost reduction targets |
| · | Finance and accounting process improvement objectives |
| · | Compliance and reporting objectives |
| · | Bringing at least one substantive partnership to late stage discussion |
| · | Enrollment and/or Initiation goals for ATHENA I & ATHENA II, Development of new pipeline indications |
| · | Business development objectives |
| · | Revenue goal to specified targets |
| · | Achieve overall gross margin and consumable utilization objectives |
| • |
Development of new pipeline indications
|
| · | Achieve research sales and operational profitability objectives |
| · | Achieve business development deal objectives |
| · | Regulatory and clinical objectives |
|
Officer and Position
|
Target Bonus as a % of Salary
|
% of Target Bonus Awarded
|
Bonus Awarded as a % of Salary
|
Amount of 2014 Bonus Paid in 2015
(6)
|
||||||||||||
|
Marc H. Hedrick,
President & CEO
|
45
|
%
|
90
|
%
|
40.5
|
%
|
$
|
182,250
|
||||||||
|
Tiago M. Girão,
(1)
Chief Financial Officer
|
30
|
%
|
33
|
%
|
9.9
|
%
|
$
|
25,000
|
||||||||
|
Steven Kesten
Executive Vice President & Chief Medical Officer
|
25
|
%
|
63.8
|
%
|
15.9
|
%
|
$
|
63,750
|
||||||||
|
Seijiro Shirahama
(2)
President- Asia Pacific
|
25
|
%
|
55
|
%
|
13.8
|
%
|
$
|
50,460
|
||||||||
|
Clyde Shores,
(3)
Former Executive Vice President Marketing & Sales
|
N/
|
A
|
N/
|
A
|
N/
|
A
|
N/
|
A
|
||||||||
|
Christopher J. Calhoun,
(4)
Former Chief Executive Officer
|
N/
|
A
|
N/
|
A
|
N/
|
A
|
N/
|
A
|
||||||||
|
Mark Saad,
(5)
Former Chief Financial Officer
|
N/
|
A
|
N/
|
A
|
N/
|
A
|
N/
|
A
|
||||||||
| (1) | Mr. Girão did not formally participate in the 2014 EMIC program, but the Committee, in its discretion awarded Mr. Girão a $25,000 bonus. |
| (2) | Mr. Shirahama’s bonus was determined by the Committee as 13.8% of his base salary in US Dollars using the 2014 average annual foreign exchange rate. |
| (3) | Mr. Shores resigned from the Company effective November 2014, therefore his EMIC bonus determination is not applicable. |
| (4) | Mr. Calhoun retired as Chief Executive Officer effective April 2, 2014 , therefore his EMIC determination bonus is not applicable. |
| (5) | Mr. Saad resigned from the Company effective August 2014, therefore his EMIC bonus determination is not applicable. |
| (6) | The 2014 bonus has not been paid as of February 28, 2015. The bonus will be paid in quarterly installments starting April 1, 2015. |
|
Officer
|
Stock options at
fair market value
($
2.38)
|
|||
|
Dr. Hedrick
|
285,000
|
|||
|
Dr. Kesten
|
140,000
|
|||
|
Mr. Shirahama
|
100,000
|
|||
|
Mr. Shores
(1)
|
120,000
|
|||
|
Mr. Saad
(2)
|
170,000
|
|||
| (1) | Mr. Shores resigned from the Company effective November 2014, therefore his EMIC bonus determination is not applicable. |
| (2) | Mr. Saad resigned as CFO in August 2014. |
|
Officer
|
Stock options at
fair market value($
1.40)
|
|||
|
Dr. Hedrick
|
100,000
|
|||
|
Dr. Kesten
|
90,000
|
|||
|
Mr. Shirahama
|
50,000
|
|||
|
Mr. Shores
(1)
|
50,000
|
|||
| (1) | Mr. Shores resigned from the Company effective November 2014, therefore his EMIC bonus determination is not applicable. |
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
|||||||||||||||||||||
|
Name and Principal Position
|
Year
|
Salary
|
Stock Awards
(1)
|
Option Awards
(2)
|
Non-
Equity Incentive Plan
Comp.
(3)
|
All Other Comp-ensation
|
Total
|
|||||||||||||||||||||
| Marc H. Hedrick, President and Chief |
2014
|
$
|
437,350
|
—
|
$
|
554,307
|
$
|
182,250
|
—
|
(4) |
$
|
1,173,907
|
||||||||||||||||
| Executive Officer (PEO) |
2013
|
$
|
406,627
|
—
|
$
|
465,869
|
$
|
89,133
|
—
|
(4)
|
$
|
961,629
|
||||||||||||||||
|
2012
|
$
|
406,627
|
$
|
212,764
|
(12)
|
$
|
241,998
|
$
|
76,446
|
—
|
(4)
|
$
|
937,835
|
(8)
|
||||||||||||||
| Tiago M. Girão, |
2014
|
$
|
79,080
|
—
|
$
|
137,561
|
$
|
50,000
|
(5)
|
—
|
$
|
266,641
|
||||||||||||||||
| VP of Finance and Chief Financial |
2013
|
—
|
—
|
—
|
—
|
—
|
—
|
|||||||||||||||||||||
| Officer (PFO) (15) |
2012
|
—
|
—
|
—
|
—
|
—
|
—
|
|||||||||||||||||||||
| Steven Kesten, M.D., Executive Vice |
2014
|
$
|
400,000
|
—
|
$
|
310,888
|
$
|
63,750
|
—
|
(4)
|
$
|
774,638
|
||||||||||||||||
| President and Chief Medical Officer |
2013
|
$
|
333,333
|
—
|
$
|
271,174
|
$
|
113,880
|
$
|
68,401
|
(6)
|
$
|
786,788
|
|||||||||||||||
|
2012
|
—
|
—
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||
| Seijiro Shirahama, |
2014
|
$
|
366,978
|
(7)
|
—
|
$
|
208,574
|
$
|
50,460
|
—
|
(4)
|
$
|
626,012
|
(8)
|
||||||||||||||
| President- Asia Pacific |
2013
|
$
|
371,808
|
(7)
|
—
|
$
|
211,758
|
$
|
24,528
|
—
|
(4)
|
$
|
608,094
|
|||||||||||||||
|
2012
|
$
|
454,432
|
(7)
|
$
|
178,278
|
(12)
|
$
|
84,173
|
$
|
82,843
|
—
|
(4)
|
$
|
779,726
|
||||||||||||||
| Clyde W. Shores, |
2014
|
$
|
302,013
|
|
—
|
$
|
240,847
|
(11)
|
—
|
$
|
25,344
|
(10) |
$
|
568,204
|
||||||||||||||
| Former Executive Vice President |
2013
|
$
|
329,469
|
|
—
|
$
|
254,110
|
$
|
25,237
|
$
|
35,000
|
(10) |
$
|
643,816
|
||||||||||||||
| Marketing & Sales (9) |
2012
|
$
|
329,469
|
|
$
|
178,278
|
(12)
|
$
|
84,173
|
$
|
65,276
|
$
|
44,400
|
(10) |
$
|
701,596
|
||||||||||||
| Christopher J. Calhoun, Former |
2014
|
$
|
427,406
|
—
|
|
—
|
—
|
$
|
129,576
|
(14)
|
$
|
556,982
|
||||||||||||||||
| Chief Executive Officer (13) |
2013
|
$
|
467,900
|
—
|
$
|
635,276
|
$
|
82,467
|
—
|
(4)
|
$
|
1,185,643
|
||||||||||||||||
|
2012
|
$
|
467,900
|
$
|
293,260
|
(12)
|
$
|
483,996
|
$
|
109,956
|
—
|
(4)
|
$
|
1,355,112
|
(8)
|
||||||||||||||
| Mark E. Saad, |
2014
|
$
|
304,433
|
—
|
$
|
274,314
|
(16)
|
$
|
—
|
—
|
(4)
|
$
|
578,747
|
|||||||||||||||
| Former Chief Financial Officer (15) |
2013
|
$
|
389,917
|
—
|
$
|
381,165
|
$
|
35,660
|
—
|
(4)
|
$
|
806,742
|
||||||||||||||||
|
2012
|
$
|
389,917
|
$
|
184,040
|
(12)
|
$
|
84,173
|
$
|
64,141
|
—
|
(4)
|
$
|
722,271
|
(8)
|
||||||||||||||
| (1) | This column represents the dollar amount of the aggregate grant date fair value of stock awards, computed in accordance with FASB ASC Topic 718. For information relating to the assumptions made by us in valuing the stock awards made to our named executive officers in 2015, refer to Note 16 to our audited consolidated financial statements. |
| (2) | This column represents the dollar amount of the aggregate grant date fair value of awards, computed in accordance with FASB ASC Topic 718. For information relating to the assumptions made by us in valuing the option awards made to our named executive officers in 2015, refer to Note 16 to our audited consolidated financial statements. |
| (3) | The amounts in column (f) reflect the cash awards under our EMIC Plan, which is discussed in further detail in the CD&A under the heading “2015 NEO Compensation – Executive Management Incentive Compensation Plan .” |
| (4) | Dollar value of the Named Executive Officer’s perquisites and other personal benefits was less than $10,000 for the year reported. |
| (5) | Includes sign-on bonus ($25,000). |
| (6) | All Other Compensation for Dr. Kesten who was hired 2/26/2013 includes a relocation allowance ($68,401). |
| (7) | We pay Mr. Shirahama in Japanese Yen. During 2012, 2013, and 2014 his salary was recorded at the average exchange rate over the year. |
| (8) | Includes the value of RSA grants that did not vest in the timeframe required by the grants and therefore terminated in their entirety. |
| (9) | Mr. Shores resigned from the Company effective November 2014. |
| (10) | All Other Compensation for Mr. Shores include relocation allowance ($44,400) for 2012, additional relocation allowance ($35,000) for 2013 and severance ($24,344) for 2014. |
| (11) | Options awards were cancelled due to resignation. |
| (12) | On January 26, 2012, the Compensation Committee granted Restricted Stock Awards as well as Performance based RSAs with performance vesting requirement. In 2013, the Compensation Committee determined that one of the performance milestones was achieved and authorized to continue vesting the shares allocated to this milestone. The Compensation Committee used its discretion to continue vesting a portion of the awards allocated to the milestones that were not achieved by December 31, 2012. |
| (13) | Mr. Calhoun retired as Chief Executive Officer effective April 2, 2014 and agreed to serve as Managing Director for a transition period beginning April 2, 2014 through July 1, 2014 to facilitate the orderly transfer of responsibilities. From July 2, 2014 to September 29, 2014 Mr. Calhoun continued as a non-Board member Management Director with select Business Development responsibilities. |
| (14) | All Other Compensation for Mr. Calhoun severance of $129,576 for 2014. |
| (15) | Mr. Saad resigned as CFO in August 2014, and Mr. Girão joined the Company as Vice President Finance & Chief Financial Officer in September 2014. |
| (16) | Mr. Saad entered into a consulting services agreement on August 13, 2014. This agreement forfeited 170,000 vested options and 185,000 unvested options. The remaining unvested options continued to vest for the duration of the contract services; the remaining vested options continue to remain eligible for exercise. The fair value was decreased from $1.61 to $0.00 due to option modification revaluation at date of termination. |
|
(a)
|
(b)
|
(c-e) |
(f)
|
(g)
|
(h)
|
(i)
|
(j)
|
||||||||||||||||||||||||||
|
Estimated Future Payouts Under Non-Equity Incentive Plan Awards
(1)
|
All Other Stock Awards: Number of Shares of Stock or Units
|
All Other Option Awards: Number of Securities Underlying Options
|
Exercise or Base Price of Option Awards
|
Market Price on Date of Grant
|
Full Grant Date Fair Value of Stock and Option Awards
|
||||||||||||||||||||||||||||
|
Named Officers
|
Grant
|
Thre-shold
|
Target
|
Maxi-mum
|
|||||||||||||||||||||||||||||
| Date |
($)
|
($)
|
($)
|
(#)
|
(#)
|
($/Sh)
|
($/Sh)
|
($)
(2)
|
|||||||||||||||||||||||||
|
Marc H. Hedrick,
President and Chief Executive Officer
|
04/11/2014
08/21/2014
|
–
–
|
$
|
202,500
–
|
–
–
|
–
–
|
285,000
100,000
|
$
$
|
2.38
1.40
|
$
$
|
2.38
1.40
|
$
$
|
459,885
94,422
|
||||||||||||||||||||
|
Tiago M. Girão
,
VP of Finance Chief Financial Officer
|
09/02/2014
|
–
|
$
|
78,000
|
–
|
–
|
150,000
|
$
|
1.36
|
$
|
1.36
|
$
|
137,561
|
||||||||||||||||||||
|
Steven Kesten, M.D., Executive Vice President and Chief Medical Officer
|
04/11/2014
08/21/2014
|
–
–
|
$
|
100,000
–
|
–
–
|
–
–
|
140,000
90,000
|
$
$
|
2.38
1.40
|
$
$
|
2.38
1.40
|
$
$
|
225,908
84,979
|
||||||||||||||||||||
|
Seijiro Shirahama,
President- Asia Pacific
|
04/11/2014
08/21/2014
|
–
–
|
$
|
91,745
–
|
(3)
|
–
–
|
–
–
|
100,000
50,000
|
$
$
|
2.38
1.40
|
$
$
|
2.38
1.40
|
$
$
|
161,363
47,210
|
|||||||||||||||||||
|
Clyde W. Shores,
Former Executive Vice President Marketing & Sales
(4)
|
04/11/2014
08/21/2014
|
–
–
|
–
–
|
–
–
|
–
–
|
120,000
50,000
|
$
$
|
2.38
1.40
|
$
|
2.38
1.40
|
$
$
|
193,636
47,211
|
|||||||||||||||||||||
|
Christopher J. Calhoun, Former
Chief Executive Officer
(5)
|
–
|
–
|
–
|
–
|
–
|
–
|
–
|
–
|
–
|
||||||||||||||||||||||||
|
Mark E. Saad,
Former Chief Financial Officer
(6)
|
04/11/2014
|
–
|
–
|
–
|
–
|
170,000
|
$
|
2.38
|
$
|
2.38
|
$
|
274,317
|
|||||||||||||||||||||
| (1) | Amounts reported represent the target cash bonus amounts that could have been earned under the EMIC, as described under the heading Compensation Discussion & Analysis - Executive Compensation” above. |
| (2) | Computed in accordance with FASB ASC Topic 718. Refer to Note 16 to our audited consolidated financial statements regarding assumptions underlying valuation of equity awards. |
| (3) | Mr. Shirahama’s Estimated Future Payouts Under Non-Equity Incentive Plan Awards is based in US Dollars using the 2014 average annual foreign exchange rate. |
| (4) | Mr. Shores resigned from the Company effective November 2014. |
| (5) | Mr. Calhoun retired as Chief Executive Officer effective April 2, 2014 and agreed to serve as Managing Director for a transition period beginning April 2, 2014 through July 1, 2014 to facilitate the orderly transfer of responsibilities. |
| (6) | Mr. Saad resigned from the Company effective August 2014. |
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||
|
Name
|
Option Grant
Date
(1)
|
Number
of
Securities
Underlying Unexercised
Options
(#)
Exercisable
|
Number of
Securities
Underlying Unexercised
Options
(#) Un-
Exercisable
(2)
|
Option Exercise
Price
($)
|
Option Ex-
piration
Date
|
Number
of Shares
or Units
of Stock
That
Have
Not
Vested
(#)
|
Market
Value of
Shares or
Units of
Stock
That
Have Not
Vested
($)
|
|||||||||||||||
|
Marc H. Hedrick,
President and Chief Executive Officer
|
2/2/2005
1/24/2006
2/26/2007
1/31/2008
1/29/2009
2/5/2010
1/27/2011
1/26/2012
1/31/2013
1/31/2013
4/11/2014
8/21/2014
|
70,000
70,000
50,000
60,000
75,000
110,000
53,853
83,854
87,847
43,924
47,500
—
|
—
—
—
—
—
—
1,147
31,146
95,486
47,843
237,500
100,000
(6)
|
|
$3.12
$7.04
$5.44
$5.14
$4.80
$6.71
$5.57
$3.44
$2.74
$5.00
$2.38
$1.40
|
2/2/2015
1/24/2016
2/26/2017
1/31/2018
1/29/2019
2/5/2020
1/27/2021
1/26/2022
1/31/2023
1/31/2023
4/11/2024
8/21/2024
|
—
—
—
—
—
—
—
—
—
—
—
—
|
—
—
—
—
—
—
—
—
—
—
—
—
|
||||||||||||||
|
Tiago M. Girão
,
VP of Finance Chief Financial Officer
|
9/2/2014
|
—
|
150,000
|
|
$1.36
|
9/2/2024
|
—
|
—
|
||||||||||||||
|
Steven Kesten, M.D., Executive Vice President and Chief Medical Officer
|
1/31/2013
4/11/2014
8/21/2014
|
72,354
23,333
—
|
78,646
116,667
90,000
(6)
|
|
$2.74
$2.38
$1.40
|
1/31/2023
4/11/2024
8/21/2024
|
—
—
—
|
—
—
—
|
||||||||||||||
|
Seijiro Shirahama,
President- Asia Pacific
|
2/2/2005
12/08/2005
1/24/2006
2/26/2007
11/15/2007
1/31/2008
1/29/2009
2/5/2010
1/27/2011
1/26/2012
1/31/2013
1/31/2013
4/11/2014
8/21/2014
|
35,000
50,000
35,000
30,000
25,000
55,000
65,000
95,000
46,510
29,167
39,930
19,965
16,667
—
|
—
—
—
—
—
—
—
—
990
10,833
43,403
21,702
83,333
50,000
(6)
|
|
$3.12
$6.86
$7.04
$5.44
$5.35
$5.14
$4.80
$6.71
$5.57
$3.44
$2.74
$5.00
$2.38
$1.40
|
2/2/2015
12/08/2015
1/24/2016
2/26/2017
11/15/2017
1/31/2018
1/29/2019
2/5/2020
1/27/2021
1/26/2022
1/31/2023
1/31/2023
4/11/2024
8/21/2024
|
—
—
—
—
—
—
—
—
—
—
—
—
—
—
|
—
—
—
—
—
—
—
—
—
—
—
—
—
—
|
||||||||||||||
|
Clyde W. Shores,
Former Executive Vice President Marketing & Sales
(3)
|
5/19/2011
1/26/2012
1/31/2013
1/31/2013
4/11/2014
|
72,187
28,333
43,750
21,875
17,500
|
—
—
—
—
—
|
|
$5.37
$3.44
$2.74
$5.00
$2.38
|
5/19/2021
1/26/2022
1/31/2023
1/31/2023
4/11/2024
|
—
—
—
—
—
|
—
—
—
—
—
|
||||||||||||||
|
Christopher J. Calhoun,
Former Chief Executive Officer
(4)
|
2/2/2005
1/24/2006
2/26/2007
1/31/2008
1/29/2009
2/5/2010
1/27/2011
1/26/2012
1/31/2013
1/31/2013
|
100,000
100,000
70,000
85,000
100,000
150,000
67,186
148,542
250,000
49,479
|
—
—
—
—
—
—
—
—
—
—
|
|
$3.12
$7.04
$5.44
$5.14
$4.80
$6.71
$5.57
$3.44
$2.74
$5.00
|
2/2/2015
1/24/2016
2/26/2017
1/31/2018
1/29/2019
2/5/2020
1/27/2021
1/26/2022
1/31/2023
1/31/2023
|
—
—
—
—
—
—
—
—
—
—
|
—
—
—
—
—
—
—
—
—
—
|
||||||||||||||
|
Mark E. Saad,
Chief Former Financial Officer
(5)
|
1/24/2006
2/26/2007
1/31/2008
1/29/2009
1/27/2011
1/26/2012
1/31/2013
1/31/2013
4/11/2014
|
70,000
50,000
55,000
70,000
48,958
29,167
71,875
35,937
28,333
|
—
—
—
—
1,042
10,833
78,125
39,063
141,667
|
|
$7.04
$5.44
$5.14
$4.80
$5.57
$3.44
$2.74
$5.00
$2.38
|
1/24/2016
2/26/2017
1/31/2018
1/29/2019
1/27/2021
1/26/2022
1/31/2023
1/31/2023
4/11/2024
|
—
—
—
—
—
—
—
—
—
|
—
—
—
—
—
—
—
—
—
|
||||||||||||||
| (1) | For a better understanding of this table, we have included an additional column showing the grant date of the stock options. |
| (2) | Generally, awards issued under the 1997 or 2004 plans are subject to four-year vesting, and have a contractual term of 10 years. Awards presented in this table contain one of the following two vesting provisions: |
| · | 25% of a granted award vests after one year of service, while an additional 1/48 of the award vests at the end of each month thereafter for 36 months, or |
| · | 1/48 of the award vests at the end of each month over a four-year period. |
| (3) | Mr. Shores resigned from the Company effective November 2014. |
| (4) | Mr. Calhoun retired as Chief Executive Officer effective April 2, 2014 and agreed to serve as Managing Director for a transition period beginning April 2, 2014 through July 1, 2014 to facilitate the orderly transfer of responsibilities. |
| (5) | Mr. Saad resigned from the Company effective August 2014, per his separation agreement his options will continue to vest 12 months after resignation date. |
| (6) | The August 2014 grant schedule is, 50% of granted award vests after one year of service and the additional 50% on the second anniversary of the grant. |
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
||||||||||||
|
Option Awards
|
Stock Awards
|
|||||||||||||||
|
Name
|
Number of Shares Acquired on Exercise
(#)
|
Value Realized on Exercise
($)
|
Number of Shares Acquired on Vesting
(#)(1)
|
Value Realized on Vesting
($)
|
||||||||||||
|
Marc H. Hedrick,
President & Chief Executive Officer
|
—
|
—
|
21,373
|
$
|
62,623
|
|||||||||||
|
Tiago M. Girão
,
VP of Finance Chief Financial Officer
|
—
|
—
|
—
|
—
|
||||||||||||
|
Steven Kesten, M.D., Executive Vice President and Chief Medical Officer
|
—
|
—
|
—
|
—
|
||||||||||||
|
Seijiro Shirahama,
President- Asia Pacific
|
—
|
—
|
—
|
—
|
||||||||||||
|
Clyde W. Shores,
Former Executive Vice President Marketing & Sales
(3
)
|
—
|
—
|
18,458
|
$
|
54,082
|
|||||||||||
|
Christopher J. Calhoun,
Former Chief Executive Officer
(4
)
|
—
|
—
|
29,145
|
$
|
85,395
|
|||||||||||
|
Mark E. Saad,
Former Chief Financial Officer
(5
)
|
—
|
—
|
19,430
|
$
|
56,930
|
|||||||||||
| (1) | Represents time based restricted stock awards that vested on January 10, 2014. |
| (2) | The fair market value on January 10, 2014 was $2.93. Computed in accordance with FASB ASC Topic 718. Refer to Note 16 to our audited consolidated financial statements regarding assumptions underlying valuation of equity awards. |
| (3) | Mr. Shores resigned from the Company effective November 2014. |
| (4) | Mr. Calhoun retired as Chief Executive Officer effective April 2, 2014 and agreed to serve as Managing Director for a transition period beginning April 2, 2014 through July 1, 2014 to facilitate the orderly transfer of responsibilities |
| (5) | Mr. Saad resigned from the Company effective August 2014, per his separation agreement his options will continue to vest 12 months after resignation date. |
|
Change in
Control
(2)
|
Termination
(*)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
129,576
|
||||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
$
|
5,673
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
271,250
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
406,499
|
||||
|
Change in
Control
(2)
|
Termination
Following
Change in
Control
(3)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
675,000
|
||||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
$
|
33,701
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
—
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
708,701
|
||||
|
Change in Control
(2)
|
Termination
(*)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
50,000
|
||||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
$
|
—
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
—
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
50,000
|
||||
|
Change in
Control
(2)
|
Termination
Following
Change in
Control
(3)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
400,000
|
||||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
$
|
15,902
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
—
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
415,902
|
||||
|
Change in Control
(2)
|
Termination
(*)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
25,344
|
||||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
$
|
—
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
—
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
25,344
|
||||
|
Change in
Control
(2)
|
Termination
Following
Change in
Control
(3)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
366,978
|
(6)
|
|||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
N/
|
A
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
—
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
366,978
|
||||
|
Change in
Control
(2)
|
Termination
Following
Change in
Control
(3)
|
|||||||
|
PAYMENTS DUE UPON ACQUISITION / TERMINATION
(1)
:
|
||||||||
|
Cash Severance
|
||||||||
|
Base Salary
(4)
|
$
|
—
|
$
|
240,000
|
||||
|
Benefits
|
||||||||
|
COBRA Premiums
|
—
|
$
|
22,917
|
|||||
|
Long-Term Incentives
|
||||||||
|
Value of Accelerated Stock Options
(5)
|
$
|
—
|
$
|
—
|
||||
|
TOTAL VALUE
|
$
|
—
|
$
|
262,917
|
||||
| (1) | Assumes a triggering event occurred on December 31, 2014. |
| (2) | Based on the occurrence of a c hange in control of the Company, provided that the executive is at that time still in the service of the Company. |
| (3) | Based on the occurrence of either actual or constructive termination without good cause in the context of a change in control of the Company as described in detail in the section above titled, Company Acquisition/Post-Termination Compensation . |
| (4) | Based on the executive’s annual base salary on December 31, 2014, which was $450,000 for Dr. Hedrick; $400,000 for Dr. Kesten, and $366,978 for Mr. Shirahama. The base salary for Mr. Girão is based upon his salary as of joining the company in September of 2014. |
| (5) | Based on the difference between the aggregate exercise price of all accelerated in-the-money stock options and the aggregate market value of the underlying shares, calculated based on the per-share closing market price of our common stock on December 31, 2014, $0.49. |
| (6) | Mr. Shirahama’s salary is in Japanese Yen. His base salary was calculated using the average exchange rate over the current year. |
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
||||||||||||
|
Director Name
(1)
|
Fees Earned or Paid in Cash
(2)
($)
|
Stock Awards
($)
|
Option Awards
(11)
(12)
($)
|
Total
($)
|
||||||||||||
|
David M. Rickey,
Chairman
|
$
|
68,875
|
$
|
39,864
|
(3)
|
$
|
28,012
|
$
|
$136,751
|
|||||||
|
Lloyd H. Dean(13)
|
$
|
33,625
|
$
|
35,363
|
(4)
|
$
|
28,012
|
$
|
$97,000
|
|||||||
|
Richard J. Hawkins
|
$
|
50,976
|
$
|
39,676
|
(5)
|
$
|
28,012
|
$
|
$118,664
|
|||||||
|
Paul W. Hawran
|
$
|
63,875
|
$
|
41,300
|
(6)
|
$
|
28,012
|
$
|
$133,187
|
|||||||
|
E. Carmack Holmes, MD(13)
|
$
|
36,750
|
$
|
34,052
|
(7)
|
$
|
28,012
|
$
|
$98,814
|
|||||||
|
Gary A. Lyons
|
$
|
35,875
|
$
|
8,250
|
(8)
|
—
|
$
|
$44,125
|
||||||||
|
Gail K. Naughton, Ph.D.
|
$
|
25,375
|
$
|
50,400
|
(9)
|
$
|
34,039
|
(9)
|
$
|
$109,814
|
||||||
|
Tommy G. Thompson
|
$
|
51,438
|
$
|
40,426
|
(10)
|
$
|
28,012
|
$
|
$119,876
|
|||||||
|
Ruud JP Jona(15)
|
$
|
3,083
|
||||||||||||||
| (1) | Mr. Calhoun and Dr. Hedrick are not included in this table as they are employees of the Company and receive no extra compensation for their services as a Director. The compensation received by Mr. Calhoun and Dr. Hedrick as employees of the Company is shown in the 2014 Summary Compensation Table and the three equity-related tables above. |
| (2) | In fiscal year 2014, each non-employee director’s compensation included a $6,250 quarterly retainer, a fee of $2,000 per quarterly meeting attended, and a fee of $1,000 per special Board meeting attended in person. Attendance of telephonic meetings was compensated at $1,000 per meeting. Compensation Committee, Governance and Nominating Committee and Audit Committee members received $1,000 per meeting attended. Executive Committee members were exempt from receiving committee fees. The Chairman of the Board received an additional annual stipend of $25,000, the Chairman of the Audit Committee received an additional annual stipend of $15,000, and the Chairmen of the Compensation Committee and the Governance and Nominating Committee each received an additional annual stipend of $10,000 and $10,000, respectively. |
| (3) | David M. Rickey was granted 10,550 shares of restricted stock at a fair value of $2.57, effective on January 1, 2014 with shares cliff vesting on December 31, 2014. In addition he was granted 5,269 shares of restricted stock at a fair value of $2.42, effective on May 19, 2014 with shares cliff vesting on January 2, 2015 in exchange for forfeiting an amount estimated to be approximately 25% of his cash compensation for the second through fourth quarters of 2014 . |
| (4) | Lloyd H. Dean was granted 10,550 shares of restricted stock at a fair value of $2.57, effective on January 1, 2014 with shares cliff vesting on December 31, 2014. |
| (5) | Richard J. Hawkins was granted 10,550 shares of restricted stock at a fair value of $2.57, effective on January 1, 2014 with shares cliff vesting on December 31, 2014. In addition he was granted 5,862 shares of restricted stock at a fair value of $2.42, effective on May 19, 2014 with shares cliff vesting on January 2, 2015 in exchange for forfeiting an amount estimated to be approximately 25% of his cash compensation for the second through fourth quarters of 2014 .. |
| (6) | Paul W. Hawran was granted 10,550 shares of restricted stock at a fair value of $2.57, effective on January 1, 2014 with shares cliff vesting on December 31, 2014. In addition he was granted 5,191 shares of restricted stock at a fair value of $2.42, effective on May 19, 2014 with shares cliff vesting on January 2, 2015 in exchange for forfeiting an amount estimated to be approximately 25% of his cash compensation for the second through fourth quarters of 2014 .. |
| (7) | E. Carmack Holmes was granted 10,550 shares of restricted stock at a fair value of $2.57, effective on January 1, 2014 with shares cliff vesting on December 31, 2014. |
| (8) | Gary A. Lyons was granted 3,409 shares of restricted stock at a fair value of $2.42, effective on May 19, 2014 with shares cliff vesting on January 2, 2015 in exchange for forfeiting an amount estimated to be approximately 25% of his cash compensation for the second through fourth quarters of 2014 .. |
| (9) | Gail K. Naughton received an initial stock award and stock option grant upon joining the Board. She was granted 21,000 shares of restricted stock at a fair value of $2.42, effective on July 1, 2014 with 50% of the shares cliff vesting on July 1, 2015 and 50% of the shares cliff vesting on July 1, 2016. She was also awarded 21,000 options to purchase stock at a fair value of $1.62. |
| (10) | Tommy G. Thompson was granted 10,550 shares of restricted stock at a fair value of $2.57, effective on January 1, 2014 with shares cliff vesting on December 31, 2014. In addition he was granted 5,501 shares of restricted stock at a fair value of $2.42, effective on May 19, 2014 with shares cliff vesting on January 2, 2015 in exchange for forfeiting an amount estimated to be approximately 25% of his cash compensation for the second through fourth quarters of 2014 . |
| (11) | Column (d) represents the grant date fair value of the option awards, computed in accordance with FASB ASC Topic 718. For additional information on the valuation assumptions with respect to the 2014 grants, refer to Note 16 to our audited consolidated financial statements regarding assumptions underlying valuation of equity awards. |
| (12) | Directors were awarded 16,030 options to purchase stock at a fair value of $1.747 effective January 1, 2014. |
| (13) | Mr. Dean resigned from our Board of Directors effective November 1, 2014. |
| (14) | Dr. Holmes retired from the Company’s Board of Directors effective December 31, 2014. |
| (15) | Mr. Jona joined the Board of Directors on June 7, 2014 and resigned from the Board July 15, 2014. |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
Name and Address of Beneficial Owner
(1)
|
Number of Shares of Common Stock Owned
(2)
|
Number of Shares of Common Stock Subject to Options/Warrants Exercisable Within 60 Days
(3)
|
Total Number
of Shares of
Common Stock
Beneficially
Owned
(4)
|
Percent Ownership
|
||||||||||||
|
|
||||||||||||||||
|
Sabby Healthcare Master Fund, Ltd.
c/o Ogier Fiduciary Services (Cayman) Limited
89 Nexus Way, Camana Bay
Grand Cayman KY1-9007
Cayman Islands
|
9,235,675
|
-
|
9,235,675
|
8.5
|
%
|
|||||||||||
|
Kian Thiam Lim
(6)
|
8,000,000
|
-
|
8,000,000
|
7.4
|
%
|
|||||||||||
|
Level 12, 2 Queen Street
Melbourne, Victoria 3000,Australia
|
||||||||||||||||
|
BlackRock, Inc.
(5)
|
5,121,692
|
-
|
5,121,692
|
4.7
|
%
|
|||||||||||
|
55 East 52
nd
Street
New York, NY 10022
|
||||||||||||||||
|
Marc H. Hedrick, MD
|
515,711
|
778,646
|
1,294,357
|
1.2
|
%
|
|||||||||||
|
Christopher J. Calhoun
|
154,975
|
1,020,207
|
1,175,182
|
1.1
|
%
|
|||||||||||
|
Tiago M. Girão
|
60,000
|
5,000
|
65,000
|
*
|
||||||||||||
|
Steven Kesten, M.D.
|
-
|
123,459
|
123,459
|
*
|
||||||||||||
|
Seijiro Shirahama
|
48,659
|
531,042
|
579,701
|
*
|
||||||||||||
|
Clyde W. Shores
|
38,458
|
-
|
38,458
|
*
|
||||||||||||
|
Mark E. Saad
|
138,430
|
491,875
|
630,305
|
*
|
||||||||||||
|
David M. Rickey
|
592,444
|
153,411
|
745,855
|
*
|
||||||||||||
|
Paul W. Hawran
|
104,122
|
165,286
|
269,408
|
*
|
||||||||||||
|
Richard J. Hawkins
|
41,926
|
140,286
|
182,212
|
*
|
||||||||||||
|
Tommy Thompson
|
61,201
|
56,286
|
117,487
|
*
|
||||||||||||
|
Gary A. Lyons
|
24,409
|
19,917
|
44,326
|
*
|
||||||||||||
|
Gail Naughton
|
21,000
|
4,167
|
25,167
|
*
|
||||||||||||
|
All executive officers and directors as a group (13)
|
1,965,201
|
3,648,697
|
5,613,898
|
5.0
|
%
|
|||||||||||
|
*
|
Represents beneficial ownership of less than one percent (1%) of the outstanding shares as of February 28, 2015.
|
| (1) | Unless otherwise indicated, the address of each of the named individuals is c/o Cytori Therapeutics, Inc., 3020 Callan Road, San Diego, CA 92121. |
| (2) | Unless otherwise indicated, represents shares of outstanding common stock owned by the named parties as of February 28, 2015. |
| (3) | Shares of common stock subject to stock options or warrants currently exercisable or exercisable within 60 days of February 28, 2015 are deemed to be outstanding for computing the percentage ownership of the person holding such options and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person. |
| (4) | The amounts and percentages of common stock beneficially owned are reported on the basis of regulations of the Securities and Exchange Commission governing the determination of beneficial ownership of securities. Under the rules of the Commission, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or “investment power,” which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities for which that person has a right to acquire beneficial ownership within 60 days. |
| (5) | Information reported is based on a Schedule 13G/A as filed with the Securities and Exchange Commission on February 2, 2015. According to the Schedule 13G/A, BlackRock, Inc. has (i) sole power to vote or to direct the vote of 5,045,742 shares; and (ii) sole power to dispose or to direct the disposition of 5,121,692 shares. |
| (6) | Information reported is based on a Schedule 13D as filed with the Securities and Exchange Commission on February 28, 2014. According to the Schedule 13D, Kian Thiam Lim. has (i) sole power to vote or to direct the vote of 8,000,000 shares; and (ii) sole power to dispose or to direct the disposition of 8,000,000 shares. |
| (7) | Information reported is based on a Schedule 13G/A as filed with the Securities and Exchange Commission on January 16, 2015. According to the Schedule 13G/A, Sabby Heathcare Master Fund, Ltd. has (i) sole power to vote or to direct the vote of 9,235,675 shares; and (ii) sole power to dispose or to direct the disposition of 9,235,675 shares. |
|
2014
|
2013
|
|||||||
|
Audit fees
(1)
|
$
|
470,000
|
$
|
664,596
|
||||
|
Audit related fees
(2)
|
58,000
|
6,000
|
||||||
|
Tax Fees
(3)
|
56,000
|
87,640
|
||||||
|
All other fees
(4)
|
—
|
—
|
||||||
|
Total
|
$
|
584,000
|
$
|
758,236
|
||||
| (1) | Audit fees consist of fees for professional services performed by KPMG LLP for the integrated audit of our annual financial statements (and internal control over financial reporting) included in our Form 10-K filing and review of financial statements included in our quarterly Form 10-Q filings, reviews of registration statements and issuances of consents, and services that are normally provided in connection with statutory and regulatory filings or engagements. |
| (2) | Audit related fees consist of fees for assurance and related services, such as comfort letters, performed by KPMG LLP that are reasonably related to the performance of the audit or review of our financial statements. |
| (3) | Tax fees consist of fees for professional services performed by KPMG LLP with respect to tax compliance, tax advice, tax consulting and tax planning. |
| (4) | All other fees consist of fees for other permissible work performed by KPMG LLP that does not meet with the above category descriptions. No such fees were incurred in 2014 or 2013. |
|
(a) (1)
|
Financial Statements
|
Page
|
|
|
Reports of KPMG LLP, Independent Registered Public Accounting Firm
|
44
|
||
|
Consolidated Balance Sheets as of December 31, 2014 and 2013
|
46
|
||
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2014, 2013 and 2012
|
47
|
||
|
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended December 31, 2014, 2013 and 2012
|
48
|
||
|
Consolidated Statements of Cash Flows for the years ended December 31, 2014, 2013 and 2012
|
49
|
||
|
Notes to Consolidated Financial Statements
|
51
|
||
|
(a) (2)
|
Financial Statement Schedules
|
|
|
Balance at
beginning of
year
|
Additions (A)
|
Deductions (B)
|
Other (C)
|
Balance at
end of year
|
||||||||||||||||
|
Allowance for doubtful accounts
|
||||||||||||||||||||
|
Year ended December 31, 2014
|
$
|
1,445
|
$
|
1,084
|
$
|
(995
|
)
|
$
|
(11
|
)
|
$
|
1,523
|
||||||||
|
Year ended December 31, 2013
|
$
|
278
|
$
|
1,141
|
$
|
( 16
|
)
|
$
|
42
|
$
|
1,445
|
|||||||||
|
Year ended December 31, 2012
|
$
|
474
|
$
|
144
|
$
|
(313
|
)
|
$
|
(27
|
)
|
$
|
278
|
||||||||
| (A) | Includes charges to costs and expenses. |
| (B) | Includes deductions for uncollectible accounts receivable. |
| (C) | Miscellaneous activity. |
| (a)(3) | Exhibits |
| (b) | Exhibits |
|
CYTORI THERAPEUTICS, INC.
|
||
|
By:
|
/s/ Marc H. Hedrick, MD
|
|
|
Marc. H. Hedrick, MD
|
||
|
President & Chief Executive Officer
|
||
|
March 16, 2015
|
||
|
SIGNATURE
|
TITLE
|
DATE
|
||
|
/s/ David M. Rickey
|
Chairman of the Board of Directors
|
March 16, 2015
|
||
|
David M. Rickey
|
||||
|
/s/ Marc H. Hedrick, MD
|
President & Chief Executive Officer (Principal Executive Officer)
|
March 16, 2015
|
||
|
Marc H. Hedrick, MD
|
||||
|
/s/ Tiago M. Girão
|
VP of Finance and Chief Financial Officer (Principal Financial Officer)
|
March 16, 2015
|
||
|
Tiago M. Girão
|
||||
|
/s/ John W. Townsend
|
Chief Accounting Officer (Principal Accounting Officer)
|
March 16, 2015
|
||
|
John W. Townsend
|
||||
|
/s/ Paul W. Hawran
|
Director
|
March 16, 2015
|
||
|
Paul W. Hawran
|
||||
|
/s/ Gail K. Naughton, PhD
|
Director
|
March 16, 2015
|
||
|
Gail K. Naughton, PhD
|
||||
|
/s/ Richard J. Hawkins
|
Director
|
March 16, 2015
|
||
|
Richard J. Hawkins
|
||||
|
/s/ Tommy G. Thompson
|
Director
|
March 16, 2015
|
||
|
Tommy G. Thompson
|
|
/s/ Gary A. Lyons
|
Director
|
March 16, 2015
|
||
|
Gary A. Lyons
|
|
CYTORI THERAPEUTICS, INC.
|
|||||
|
EXHIBIT INDEX
|
|||||
|
Exhibit Number
|
Exhibit Title
|
Filed with this Form 10-K
|
Incorporated by Reference
|
||
|
Form
|
File No.
|
Date Filed
|
|||
|
Composite Certificate of Incorporation.
|
X | ||||
|
3.2
|
Amended and Restated Bylaws of Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 3.2
|
08/14/2003
|
|
|
4.2
|
Form of Warrant.
|
8-K
|
000-32501
Exhibit 4.2
|
03/10/2009
|
|
|
4.3
|
Form of Warrant to be dated February 28, 2007.
|
8-K
|
000-32501
Exhibit 10.4
|
02/26/2007
|
|
|
4.4
|
Form of Warrant to Purchase Common Stock issued on August 11, 2008 pursuant to the Securities Purchase Agreement, dated August 7, 2008, by and among the Company and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.34
|
08/08/2008
|
|
|
4.5
|
Registration Rights Agreement, dated August 7, 2008, by and among the Company and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.35
|
08/08/2008
|
|
|
4.6
|
Warrant to Purchase Common Stock issued by the Company on October 14, 2008 in favor of GE Capital Equity Investments, Inc., pursuant to the Loan and Security Agreement dated October 14, 2008.
|
10-K
|
000-32501
Exhibit 10.61
|
03/06/2009
|
|
|
4.7
|
Warrant to Purchase Common Stock issued by the Company on October 14, 2008 in favor of Silicon Valley Bank, pursuant to the Loan and Security Agreement dated October 14, 2008.
|
10-K
|
000-32501
Exhibit 10.62
|
03/06/2009
|
|
|
4.8
|
Form of Warrant to Purchase Common Stock to be issued on or about May 11, 2009.
|
8-K
|
000-32501
Exhibit 10.64
|
05/08/2009
|
|
|
4.9
|
Registration Rights Agreement, dated May 7, 2009, by and among Cytori Therapeutics, Inc. and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.65
|
05/08/2009
|
|
|
4.10
|
Warrant to Purchase Common Stock issued by the Company on June 11, 2010 in favor of GE Capital Equity Investments, Inc., pursuant to the Amended and Restated Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.73
|
06/17/2010
|
|
|
4.11
|
Warrant to Purchase Common Stock issued by the Company on June 11, 2010 in favor of Silicon Valley Bank, pursuant to the Amended and Restated Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.74
|
06/17/2010
|
|
|
4.12
|
Warrant to Purchase Common Stock issued by the Company on June 11, 2010 in favor of Oxford Financial Corporation, pursuant to the Amended and Restated Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.75
|
06/17/2010
|
|
|
4.13
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of GE Capital Equity Investments, Inc., pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.84
|
09/15/2011
|
|
|
4.14
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of Silicon Valley Bank, pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.85
|
09/15/2011
|
|
|
4.15
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of Oxford Financial Corporation, pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.86
|
09/15/2011
|
|
|
4.16
|
Warrant to Purchase Common Stock issued by the Company on September 9, 2011 in favor of Oxford Financial Corporation, pursuant to the Amended and Restated Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.87
|
09/15/2011
|
|
|
|
|||||
|
4.17
|
Warrant to Purchase Common Stock issued by the Company on June 28, 2013 in favor of Oxford Finance LLC pursuant to the Loan and Security Agreement dated June 28, 2013.
|
10-Q
|
001-34375
Exhibit 4.17
|
08/09/2013
|
|
|
4.18
|
Warrant to Purchase Common Stock issued by the Company on June 28, 2013 in favor of Oxford Finance LLC pursuant to the Loan and Security Agreement dated June 28, 2013.
|
10-Q
|
001-34375
Exhibit 4.18
|
08/09/2013
|
|
|
4.19
|
Warrant to Purchase Common Stock issued by the Company on June 28, 2013 in favor of Oxford Finance LLC pursuant to the Loan and Security Agreement dated June 28, 2013.
|
10-Q
|
001-34375
Exhibit 4.19
|
08/09/2013
|
|
|
4.20
|
Warrant to Purchase Common Stock issued by the Company on June 28, 2013 in favor of Oxford Finance LLC pursuant to the Loan and Security Agreement dated June 28, 2013.
|
10-Q
|
001-34375
Exhibit 4.20
|
08/09/2013
|
|
|
4.21
|
Warrant to Purchase Common Stock issued by the Company on June 28, 2013 in favor of Silicon Valley Bank pursuant to the Loan and Security Agreement dated June 28, 2013.
|
10-Q
|
001-34375
Exhibit 4.21
|
08/09/2013
|
|
|
4.22
|
Stock Purchase Agreement, effective October 29, 2013, by and between the Company and Lorem Vascular, Pty. Ltd.
|
S-3
|
333-192409
|
11-19-2013
|
|
|
10.1#
|
Amended and Restated 1997 Stock Option and Stock Purchase Plan.
|
10
|
000-32501
Exhibit 10.1
|
03/30/2001
|
|
|
10.1.1#
|
Board of Directors resolution adopted November 9, 2006 regarding determination of fair market value for stock option grant purposes (incorporated by reference to Exhibit 10.10.1 filed as Exhibit 10.10.1 to our Form 10-K Annual Report, as filed on March 30, 2007 and incorporated by reference herein)
|
10-K
|
000-32501
Exhibit 10.10.1
|
03/30/2007
|
|
|
10.10#
|
2004 Equity Incentive Plan of Cytori Therapeutics, Inc
|
8-K
|
000-32501
Exhibit 10.1
|
08/27/2004
|
|
|
10.10.1#
|
Board of Directors resolution adopted November 9, 2006 regarding determination of fair market value for stock option grant purposes.
|
10-K
|
000-32501
Exhibit 10.10.1
|
03/30/2007
|
|
|
10.12#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Nonstatutory).
|
10-Q
|
000-32501
Exhibit 10.19
|
11/15/2004
|
|
|
10.13#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Nonstatutory) with Cliff.
|
10-Q
|
000-32501
Exhibit 10.20
|
11/15/2004
|
|
|
10.14#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Incentive).
|
10-Q
|
000-32501
Exhibit 10.21
|
11/15/2004
|
|
|
10.15#
|
Notice and Agreement for Stock Options Grant Pursuant to Cytori Therapeutics, Inc. 1997 Stock Option and Stock Purchase Plan; (Incentive) with Cliff.
|
10-Q
|
000-32501
Exhibit 10.22
|
11/15/2004
|
|
|
10.16#
|
Form of Options Exercise and Stock Purchase Agreement Relating to the 2004 Equity Incentive Plan.
|
10-Q
|
000-32501
Exhibit 10.23
|
11/15/2004
|
|
|
10.17#
|
Form of Notice of Stock Options Grant Relating to the 2004 Equity Incentive Plan.
|
10-Q
|
000-32501
Exhibit 10.24
|
11/15/2004
|
|
|
10.22
|
Common Stock Purchase Agreement dated April 28, 2005, between Olympus Corporation and the Company.
|
10-Q
|
000-32501
Exhibit 10.21
|
08/15/2005
|
|
|
10.23
|
Sublease Agreement dated May 24, 2005, between Biogen Idec, Inc. and the Company.
|
10-Q
|
000-32501
Exhibit 10.21
|
08/15/2005
|
|
|
10.27+
|
Joint Venture Agreement dated November 4, 2005, between Olympus Corporation and the Company.
|
10-K
|
000-32501
Exhibit 10.27
|
03/30/2006
|
|
|
10.28+
|
License/ Commercial Agreement dated November 4, 2005, between Olympus-Cytori, Inc. and the Company
|
10-K
|
000-32501
Exhibit 10.28
|
03/30/2006
|
|
|
10.28.1
|
Amendment One to License/ Commercial Agreement dated November 14, 2007, between Olympus-Cytori, Inc. and the Company.
|
10-K
|
000-32501
Exhibit 10.28.1
|
03/14/2008
|
|
|
10.29+
|
License/ Joint Development Agreement dated November 4, 2005, between Olympus Corporation, Olympus-Cytori, Inc. and the Company.
|
10-K
|
000-32501
Exhibit 10.29
|
03/30/2006
|
|
10.29.1
|
Amendment No. 1 to License/ Joint Development Agreement dated May 20, 2008, between Olympus Corporation, Olympus-Cytori, Inc. and the Company.
|
10-Q
|
000-32501
Exhibit 10.29.1
|
08/11/2008
|
|
|
10.30+
|
Shareholders Agreement dated November 4, 2005, between Olympus Corporation and the Company.
|
10-K
|
000-32501
Exhibit 10.30
|
03/30/2006
|
|
|
10.32
|
Common Stock Purchase Agreement, dated August 9, 2006, by and between Cytori Therapeutics, Inc. and Olympus Corporation.
|
8-K
|
000-32501
Exhibit 10.32
|
08/15/2006
|
|
|
10.33
|
Form of Common Stock Subscription Agreement, dated August 9, 2006 (Agreements on this form were signed by Cytori and each of respective investors in the Institutional Offering).
|
8-K
|
000-32501
Exhibit 10.33
|
08/15/2006
|
|
|
10.43
|
Financial services advisory engagement letter agreement, dated February 16, 2007, between Cytori Therapeutics, Inc. and WBB Securities, LLC.
|
8-K
|
000-32501
Exhibit 10.2
|
02/26/2007
|
|
|
10.46
|
Common Stock Purchase Agreement, dated March 28, 2007, by and between Cytori Therapeutics, Inc. and Green Hospital Supply, Inc.
|
10-Q
|
000-32501
Exhibit 10.46
|
05/11/2007
|
|
|
10.47
|
Consulting Agreement, dated May 3, 2007, by and between Cytori Therapeutics, Inc. and Marshall G. Cox.
|
10-Q
|
000-32501
Exhibit 10.47
|
08/14/2007
|
|
|
10.48+
|
Master Cell Banking and Cryopreservation Agreement, effective August 13, 2007, by and between Green Hospital Supply, Inc.
and Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 10.48
|
11/13/2007
|
|
|
10.48.1
|
Amendment No. 1 to Master Cell Banking and Cryopreservation Agreement, effective June 4, 2008, by and between Green Hospital Supply, Inc. and the Company.
|
8-K
|
000-32501
Exhibit 10.48.1
|
06/10/2008
|
|
|
10.49+
|
License & Royalty Agreement, effective August 23, 2007, by and between Olympus-Cytori, Inc.
and Cytori Therapeutics, Inc.
|
10-Q
|
000-32501
Exhibit 10.49
|
11/13/2007
|
|
|
10.51
|
Common Stock Purchase Agreement, dated February 8, 2008, by and between Green Hospital Supply, Inc.
and Cytori Therapeutics, Inc.
|
8-K
|
000-32501
Exhibit 10.51
|
02/19/2008
|
|
|
10.51.1
|
Amendment No. 1 to Common Stock Purchase Agreement, dated February 29, 2008, by and between Green Hospital Supply, Inc. and Cytori Therapeutics, Inc.
|
8-K
|
000-32501
Exhibit 10.51.1
|
02/29/2008
|
|
|
10.52#
|
Agreement for Acceleration and/or Severance, dated January 31, 2008, by and between Christopher J. Calhoun
and Cytori Therapeutics, Inc.
|
10-K
|
000-32501
Exhibit 10.52
|
03/14/2008
|
|
|
10.53#
|
Agreement for Acceleration and/or Severance, dated January 31, 2008, by and between Marc H. Hedrick
and Cytori Therapeutics, Inc.
|
10-K
|
000-32501
Exhibit 10.53
|
03/14/2008
|
|
|
10.54#
|
Agreement for Acceleration and/or Severance, dated January 31, 2008, by and between Mark E. Saad
and Cytori Therapeutics, Inc.
|
10-K
|
000-32501
Exhibit 10.54
|
03/14/2008
|
|
|
10.55
|
Common Stock Purchase Agreement, dated August 7, 2008, by and between the Company and Olympus Corporation.
|
8-K
|
000-32501
Exhibit 10.32
|
08/08/2008
|
|
|
10.55.1
|
Amendment No. 1 to Common Stock Purchase Agreement, dated August 8, 2008, by and between the Company and Olympus Corporation.
|
8-K
|
000-32501
Exhibit 10.32.1
|
08/14/2008
|
|
|
10.56
|
Securities Purchase Agreement, dated August 7, 2008, by and among the Company and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.33
|
08/08/2008
|
|
|
10.59
|
Loan and Security Agreement, dated October 14, 2008, by and among the Company, General Electric Capital Corporation, and the other lenders signatory thereto.
|
10-K
|
000-32501
Exhibit 10.59
|
03/06/2009
|
|
|
10.60
|
Promissory Note issued by the Company in favor of General Electric Capital Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated October 14, 2008.
|
10-K
|
000-32501
Exhibit 10.60
|
03/06/2009
|
|
|
10.63
|
Form of Subscription Agreement by and between Cytori Therapeutics, Inc. and the Purchaser (as defined therein), dated as of March 9, 2009.
|
8-K
|
000-32501
Exhibit 10.63
|
03/10/2009
|
|
|
10.64
|
Placement Agency Agreement, dated March 9, 2009, between Cytori Therapeutics, Inc. and Piper Jaffray & Co.
|
8-K
|
000-32501
Exhibit 10.64
|
03/10/2009
|
|
10.65
|
Securities Purchase Agreement, dated May 7, 2009, by and among Cytori Therapeutics, Inc. and the Purchasers identified on the signature pages thereto.
|
8-K
|
000-32501
Exhibit 10.63
|
05/08/2009
|
|
|
10.68
|
Form of Common Stock Purchase Agreement by and between Cytori Therapeutics, Inc. and Seaside 88, LP, dated as of June 19, 2009.
|
8-K
|
001-34375
Exhibit 10.68
|
06/22/2009
|
|
|
10.69
|
Lease Agreement entered into on April 2, 2010, between HCP Callan Rd, LLC. and Cytori Therapeutics, Inc..
|
10-Q
|
001-34375
Exhibit 10.69
|
05/06/2010
|
|
|
10.70
|
Amended and Restated Loan and Security Agreement, dated June 11, 2010, by and among the Company, General Electric Capital Corporation, and the other lenders signatory thereto.
|
8-K
|
001-34375
Exhibit 10.70
|
06/17/2010
|
|
|
10.71
|
Promissory Note issued by the Company in favor of General Electric Capital Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.71
|
06/17/2010
|
|
|
10.72
|
Promissory Note issued by the Company in favor of Oxford Financial Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated June 11, 2010.
|
8-K
|
001-34375
Exhibit 10.72
|
06/17/2010
|
|
|
10.76
|
Common Stock Purchase Agreement, dated December 6, 2010, by and among Cytori Therapeutics, Inc. and Astellas Pharma Inc.
|
8-K
|
001-34375
Exhibit 10.76
|
12/09/2010
|
|
|
10.77
|
Form of Notice and Restricted Stock Award Agreement for grants of performance-based restricted stock awards under the 2004 Equity Incentive Plan.
|
8-K
|
001-34375
Exhibit 10.1
|
03/04/2011
|
|
|
10.78
|
Form of Common Stock Purchase Agreement by and between Cytori Therapeutics, Inc. and Seaside 88, LP, dated July 11, 2011
|
8-K
|
001-34375
Exhibit 10.78
|
07/12/2011
|
|
|
10.79
|
First Amendment to Amended and Restated Loan and Security Agreement, dated June 23, 2011, by and among the Company, Oxford Finance LLC, the other lenders party hereto and General Electric Capital Corporation.
|
10-Q
|
001-34375
Exhibit 10.79
|
08/09/2011
|
|
|
10.80
|
Second Amendment to the Amended and Restated Loan and Security Agreement, dated September 9, 2011, by and among the Company, General Electric Capital Corporation, and the other lenders signatory thereto.
|
8-K
|
001-34375
Exhibit 10.80
|
09/15/2011
|
|
|
10.81
|
Promissory Note issued by the Company in favor of General Electric Capital Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.81
|
09/15/2011
|
|
|
10.82
|
Promissory Note issued by the Company in favor of Silicon Valley Bank or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.82
|
09/15/2011
|
|
|
10.83
|
Promissory Note issued by the Company in favor of Oxford Financial Corporation or any subsequent holder thereof, pursuant to the Loan and Security Agreement dated September 9, 2011.
|
8-K
|
001-34375
Exhibit 10.83
|
09/15/2011
|
|
|
10.88
|
First Amendment to Lease Agreement entered into on November 4, 2011, between HCP Callan Rd, LLC. and the Company.
|
10-Q
|
001-34375
Exhibit 10.88
|
11/08/2011
|
|
|
10.89#
|
2011 Employee Stock Purchase Plan
|
DEF 14A
|
001-34375
Appendix A
|
05/02/2011
|
|
|
10.90+
|
Contract HHSO100201200008C dated September 27, 2012, by and between the Company and the U.S. Department of Health and Human Services Biomedical Advanced Research and Development Authority (portions of the exhibit have been omitted pursuant to a request for confidential treatment).
|
8-K
|
001-34375
Exhibit 10.90
|
10/03/2012
|
|
|
10.91
|
Joint Venture Termination Agreement dated May 8, 2013 by and between the Company and Olympus Corporation.
|
10-Q
|
001-34375
Exhibit 10.91
|
05/10/2013
|
|
|
10.92
|
Loan and Security Agreement, dated June 28, 2013, by and among the Company, Oxford Finance LLC and Silicon Valley Bank.
|
10-Q
|
001-34375
Exhibit 10.92
|
08/09/2013
|
|
|
10.93+
|
Puregraft Sale-License-Supply Agreement, dated July 30, 2013, by and among the Company and Bimini Technologies LLC.
|
10-Q/A
|
001-34375
Exhibit 10.93
|
11/12/2013
|
|
10.94+
|
Amended and Restated License and Supply Agreement dated January 30, 2014, by and between the Company and Lorem Vascular Pty. Ltd.
|
8-K
|
001-34375
|
02/04/2014
|
|
|
14.1
|
Code of Ethics.
|
10-K
|
000-32501
Exhibit 14.1
|
03/30/2004
|
|
|
Consent of KPMG LLP, Independent Registered Public Accounting Firm
|
X
|
||||
|
Certification of Principal Executive Officer Pursuant to Securities Exchange Act Rule 13a-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
Certification of Principal Financial Officer Pursuant to Securities Exchange Act Rule 13a-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
Certifications Pursuant to 18 U.S.C. Section 1350/ Securities Exchange Act Rule 13a-14(b), as adopted pursuant to Section 906 of the Sarbanes - Oxley Act of 2002
|
X
|
||||
|
101.INS
|
XBRL Instance Document
|
X
|
|||
|
101.SCH
|
XBRL Schema Document
|
X
|
|||
|
101.CAL
|
XBRL Calculation Linkbase Document
|
X
|
|||
|
101.DEF
|
XBRL Definition Linkbase Document
|
X
|
|||
|
101.LAB
|
XBRL Label Linkbase Document
|
X
|
|||
|
*
|
XBRL Presentation Linkbase Document
|
X
|