|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Mark One)
|
||
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the fiscal year ended: December 31, 2018
|
||
|
or
|
||
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
04-3416587
(I.R.S. Employer
Identification No.)
|
|
|
100 Corporate Court
South Plainfield, New Jersey
(Address of principal executive offices)
|
07080
(Zip Code)
|
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $0.001 par value
|
Nasdaq Global Select Market
|
|
|
Large accelerated filer
þ
|
|
Accelerated filer ☐
|
|
|
|
|
|
Non-accelerated filer ☐
|
|
Smaller reporting company ☐
|
|
Emerging growth company ☐
|
||
|
Page No.
|
|
|
•
|
our ability to realize the anticipated benefits of our acquisition of Agilis Biotherapeutics, Inc., or Agilis, including the possibility that the expected impact of benefits from the acquisition, including with respect to the business of Agilis and our expectations with respect to the potential achievement of development, regulatory and sales milestones and our contingent payments to the former Agilis equityholders with respect thereto, will not be realized or will not be realized within the expected time period, significant transaction costs, the integration of Agilis's operations and employees into our business, our ability to obtain marketing approval of our gene therapy for the treatment of Aromatic L-Amino Acid Decarboxylase, or AADC, deficiency, or PTC-AADC, and other product candidates we acquired from Agilis, unknown liabilities, the risk of litigation and/or regulatory actions related to the acquisition, and other business effects, including the effects of industry, market, economic, political or regulatory conditions;
|
|
•
|
our ability to negotiate, secure and maintain adequate pricing, coverage and reimbursement terms and processes on a timely basis, or at all, with third-party payors for Emflaza™ (deflazacort) for the treatment of Duchenne muscular dystrophy, or DMD, in the United States and for Translarna™ (ataluren) for the treatment of nonsense mutation DMD, or nmDMD, in the European Economic Area, or EEA, and other countries in which we have or may obtain regulatory approval, or in which there exist significant reimbursed early access programs, or EAP programs;
|
|
•
|
our ability to maintain our marketing authorization of Translarna for the treatment of nmDMD in the EEA (which is subject to the specific obligation to conduct and submit the results of Study 041 to the EMA and annual review and renewal by the European Commission following reassessment of the benefit-risk balance of the authorization by the European Medicines Agency, or EMA);
|
|
•
|
our ability to enroll, fund, and complete Study 041, a multicenter, randomized, double-blind, 18-month, placebo-controlled clinical trial of Translarna for the treatment of nmDMD followed by an 18-month open label extension, according to the protocol agreed with the EMA, and by the EMA’s deadline;
|
|
•
|
the anticipated period of market exclusivity for Emflaza for the treatment of DMD in the United States under the Orphan Drug Act of 1983, or Orphan Drug Act, the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act;
|
|
•
|
our ability to complete the United States Food and Drug Administration, or FDA, post-marketing requirements to the marketing authorization of Emflaza;
|
|
•
|
our ability to complete any dystrophin study necessary in order to resolve the matters set forth in the FDA's denial of our appeal to the Complete Response Letter we received from the FDA in connection with our New Drug Application, or NDA, for Translarna for the treatment of nmDMD, and our ability to perform additional clinical trials, non-clinical studies or CMC assessments or analyses at significant cost;
|
|
•
|
the timing and scope of our continued commercialization of Translarna as a treatment for nmDMD in the EEA or other territories outside of the United States;
|
|
•
|
our ability to obtain additional and maintain existing reimbursed named patient and cohort EAP programs for Translarna for the treatment of nmDMD on adequate terms, or at all;
|
|
•
|
our expectations and the potential financial impact and benefits related to our Collaboration and Licensing Agreement with Akcea Therapeutics, Inc., or Akcea, including with respect to the timing of regulatory approval of TegsediTM (inotersen) and WaylivraTM (volanesorsen) in countries in which we are licensed to commercialize them, the potential commercialization of Tegsedi and Waylivra, and our expectations with respect to contingent payments to Akcea based on the potential achievement of certain regulatory milestones and royalty payments by us to Akcea based on our potential achievement of certain net sales thresholds;
|
|
•
|
our estimates regarding the potential market opportunity for Translarna, Emflaza, PTC-AADC, Tegsedi, Waylivra, risdiplam or any other product candidate, including the size of eligible patient populations and our ability to identify such patients;
|
|
•
|
our estimates regarding expenses, future revenues, third-party discounts and rebates, capital requirements and needs for additional financing, including our ability to maintain the level of our expenses consistent with our internal budgets and forecasts and to secure additional funds on favorable terms or at all;
|
|
•
|
our expectations with respect to future revenue generation from Emflaza and contingent payments to Marathon Pharmaceuticals, LLC (now known as Complete Pharma Holdings, LLC), or Marathon, based on annual net sales;
|
|
•
|
the timing and conduct of our ongoing, planned and potential future clinical trials and studies of Translarna for the treatment of nmDMD, aniridia, and Dravet syndrome/CDKL5, each caused by nonsense mutations, and Emflaza for the treatment of limb-girdle 2I, as well as studies in our gene therapy, splicing and oncology programs, including the timing of initiation, enrollment and completion of the trials and the period during which the results of the trials will become available;
|
|
•
|
the rate and degree of market acceptance and clinical utility of Translarna, Emflaza, PTC-AADC, Tegsedi, Waylivra and risdiplam;
|
|
•
|
the ability and willingness of patients and healthcare professionals to access Translarna through alternative means if pricing and reimbursement negotiations in the applicable territory do not have a positive outcome;
|
|
•
|
the timing of, and our ability to obtain additional marketing authorizations for, Translarna, Tegsedi and our other product candidates;
|
|
•
|
the ability of Translarna, Emflaza, PTC-AADC, Tegsedi, Waylivra and risdiplam and our other product candidates to meet existing or future regulatory standards;
|
|
•
|
our ability to maintain the current labeling under the marketing authorization in the EEA or expand the approved product label of Translarna for the treatment of nmDMD in non-ambulatory patients or otherwise;
|
|
•
|
the potential receipt of revenues from future sales of Translarna, Emflaza and other product candidates, including our ability to earn a profit from sales or licenses of Translarna for the treatment of nmDMD in the countries in which we have or may obtain regulatory approval and of Emflaza for the treatment of DMD in the United States;
|
|
•
|
the potential impact that enrollment, funding and completion of Study 041 may have on our revenue growth;
|
|
•
|
our sales, marketing and distribution capabilities and strategy, including the ability of our third-party manufacturers to manufacture and deliver Translarna and Emflaza in clinically and commercially sufficient quantities and the ability of distributors to process orders in a timely manner and satisfy their other obligations to us;
|
|
•
|
our ability to establish and maintain arrangements for the manufacture of Translarna, Emflaza and our other product candidates that are sufficient to meet clinical trial and commercial launch requirements;
|
|
•
|
our ability to increase our manufacturing capabilities for our gene therapy platform;
|
|
•
|
our ability to satisfy our obligations under the terms of the credit and security agreement with MidCap Financial Trust, or MidCap Financial, as administrative agent and MidCap Financial and certain other financial institutions as lenders thereunder;
|
|
•
|
our other regulatory submissions, including with respect to timing and outcome of regulatory review;
|
|
•
|
our plans to pursue development of Translarna and Emflaza for additional indications;
|
|
•
|
our plans to advance our earlier stage programs and pursue research and development of other product candidates, including our splicing, gene therapy and oncology programs;
|
|
•
|
whether we may pursue business development opportunities, including potential collaborations, alliances, and acquisition or licensing of assets and our ability to successfully develop or commercialize any assets to which we may gain rights pursuant to such business development opportunities;
|
|
•
|
the potential advantages of Translarna, Emflaza, PTC-AADC, Tegsedi, Waylivra and risdiplam and any other product candidate;
|
|
•
|
our intellectual property position;
|
|
•
|
the impact of government laws and regulations;
|
|
•
|
the impact of litigation that has been or may be brought against us or of litigation that we are pursuing against others;
|
|
•
|
our competitive position; and
|
|
•
|
our expectations with respect to the development and regulatory status of our product candidates, including risdiplam, and program directed against spinal muscular atrophy in collaboration with F. Hoffmann La Roche Ltd and Hoffmann La Roche Inc., which we refer to collectively as Roche, and the Spinal Muscular Atrophy Foundation, or the SMA Foundation, and our estimates regarding future revenues from achievement of milestones in that program.
|
|
•
|
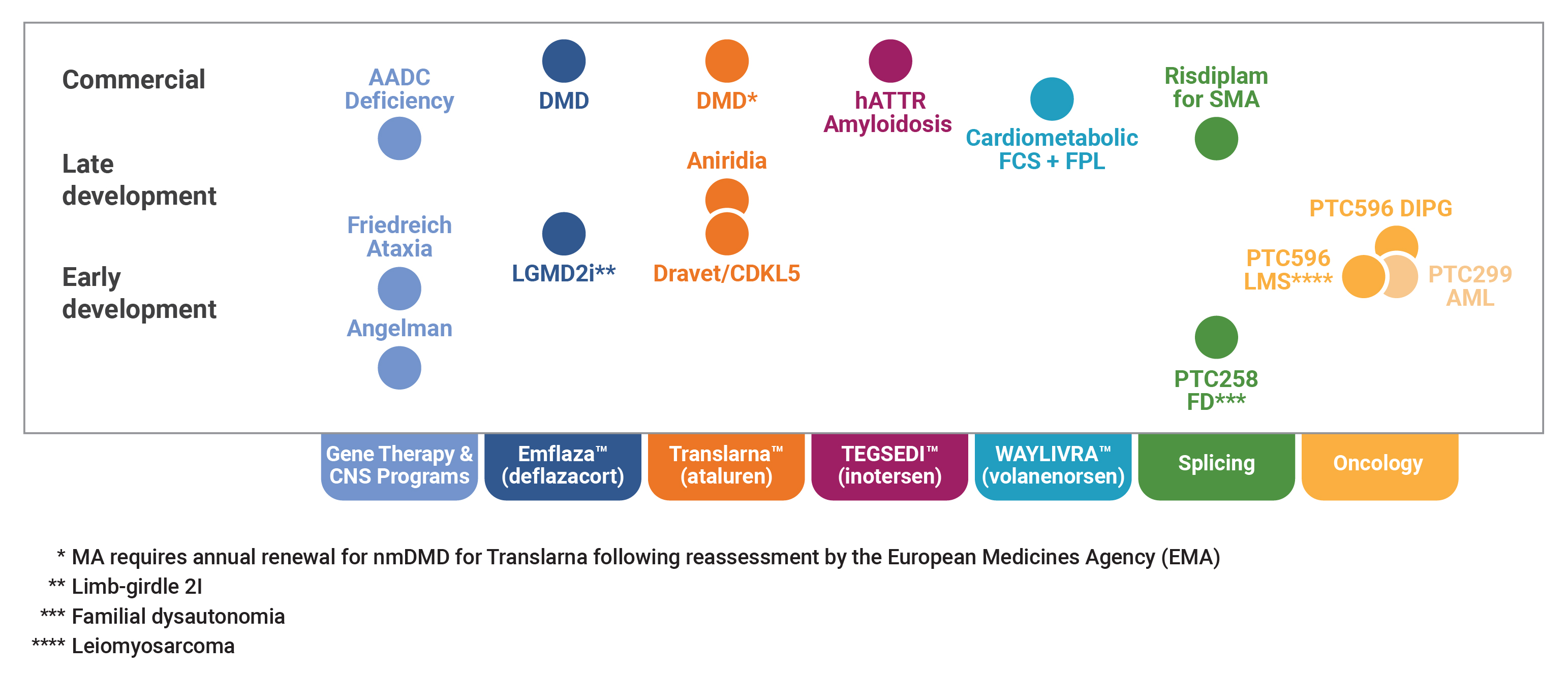
Global DMD Franchise
-
We have two products, Translarna™ (ataluren) and Emflaza™ (deflazacort), for the treatment of Duchenne muscular dystrophy, or DMD, a rare, life threatening disorder. Translarna received marketing authorization from the European Commission in August 2014 for the treatment of nonsense mutation Duchenne muscular dystrophy, or nmDMD, in ambulatory patients aged five years and older in the 31 member states of the European Economic Area, or EEA. In July 2018, the European Commission renewed our marketing authorization, which is subject to annual renewal and other conditions, and approved a label-extension to our marketing authorization to include patients from two to five years of age. During fiscal year 2018, Translarna achieved net sales of
$171.0 million
and is currently available for the treatment of nmDMD in over 40 countries. Emflaza is approved in the United States for the treatment of DMD in patients five years and older. During fiscal year 2018, Emflaza achieved net sales of
$92.0 million
.
|
|
•
|
Gene Therapy Platform -
We have a pipeline of gene therapy product candidates for rare monogenic diseases that affect the central nervous system, or CNS, including PTC-AADC for the treatment of Aromatic L-Amino Acid Decarboxylase, or AADC, deficiency, a rare CNS disorder arising from reductions in the enzyme AADC that result from mutations in the dopa decarboxylase gene. We are preparing a biologics license application, or BLA, for PTC-AADC for the treatment of AADC deficiency in the United States, which we anticipate submitting to the U.S. Food and Drug Administration, or FDA, in late 2019, with an anticipated commercial launch in the United States in 2020. We are also preparing a marketing authorization application, or MAA, for PTC-AADC for the treatment of AADC deficiency in the European Union, or EU, for submission to the European Medicines Agency, or EMA, which will follow our BLA submission to the FDA.
|
|
•
|
Akcea Partnership -
We hold the rights for the commercialization of Tegsedi™ (inotersen) and Waylivra™ (volanesorsen) for the treatment of rare diseases in countries in Latin America and the Caribbean pursuant to our Collaboration and License Agreement with Akcea Therapeutics, Inc., or Akcea. Tegsedi has received marketing authorization in the United States, EU and Canada for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin amyloidosis, or hATTR amyloidosis, and was recently granted priority review by Agência Nacional de Vigilância Sanitária, or ANVISA, the Brazilian health regulatory authority. Waylivra is currently under regulatory review in the EU for the treatment of familial chylomicronemia syndrome, or FCS.
|
|
•
|
Splicing Platform
-
We also have a spinal muscular atrophy, or SMA, collaboration with F. Hoffman-La Roche Ltd. and Hoffman-La Roche Inc., which we refer to collectively as Roche, and the Spinal Muscular Atrophy Foundation, or SMA Foundation. Currently, our collaboration has two pivotal clinical trials ongoing to evaluate the safety and effectiveness of risdiplam (RG7916, RO7034067), the lead compound in the SMA program. Roche is preparing an NDA and a MAA for risdiplam for the treatment of SMA in the United States and the EU, respectively, which Roche anticipates submitting to the FDA and the EMA in the second half of 2019.
|
|
•
|
Pursuing Value-Creation Opportunities -
In April 2017, we acquired all rights to Emflaza from Marathon Pharmaceuticals, LLC (now known as Complete Pharma Holdings, LLC), or Marathon. In August 2018, we acquired our gene therapy platform for rare monogenic diseases that affect the CNS through our acquisition of Agilis Biotherapeutics, Inc., or Agilis. Also in August 2018, we entered into a Collaboration and License Agreement with Akcea for the commercialization of Tegsedi
TM
(inotersen), Waylivra
TM
(volanesorsen) and products containing those compounds in countries in Latin America and the Caribbean. As part of our business strategy, we may engage in further strategic transactions to expand and diversify our product pipeline, including through the acquisition of assets, businesses, or rights to products, product candidates or technologies or through strategic alliances or collaborations.
|
|
Parameter
|
Placebo
N=115
|
Translarna
40 mg group
N=115
|
All
patients
N=230
|
||||||
|
Patients with ≥1 adverse event
|
101 (87.8)%
|
|
103 (89.6)%
|
|
204 (88.7)%
|
|
|||
|
Adverse events by severity
|
|||||||||
|
Grade 1 (mild)
|
54 (47.0)%
|
|
61 (53.0)%
|
|
115 (50.0)%
|
|
|||
|
Grade 2 (moderate)
|
37 (32.2)%
|
|
35 (30.4)%
|
|
72 (31.3)%
|
|
|||
|
Grade 3 (severe)
|
9 (7.8)%
|
|
7 (6.1)%
|
|
16 (7.0)%
|
|
|||
|
Grade 4 (life-threatening)
|
—
|
|
—
|
|
—
|
|
|||
|
Adverse events by relatedness
|
|||||||||
|
Unrelated
|
47 (40.9)%
|
|
44 (38.3)%
|
|
91 (39.6)%
|
|
|||
|
Unlikely
|
30 (26.1)%
|
|
20 (17.4)%
|
|
50 (21.7)%
|
|
|||
|
Possible
|
18 (15.7)%
|
|
27 (23.5)%
|
|
45 (19.6)%
|
|
|||
|
Probable
|
6 (5.2)%
|
|
12 (10.4)%
|
|
18 (7.8)%
|
|
|||
|
Discontinuations due to adverse events
|
1 (0.9)%
|
|
1 (0.9)%
|
|
2 (0.9)%
|
|
|||
|
Serious adverse events
|
4 (3.5)%
|
|
4 (3.5)%
|
|
8 (3.5)%
|
|
|||
|
Deaths
|
—
|
|
—
|
|
—
|
|
|||
|
•
|
Translarna for nmDMD.
There is currently no marketed therapy, other than Translarna in the EEA, which has received approval for the treatment of the underlying cause of nmDMD. Sarepta Therapeutics recently received approval in the United States for a treatment addressing the underlying cause of disease for different mutations in the DMD gene. Other biopharmaceutical companies are developing treatments addressing the underlying cause of disease for different mutations in the DMD gene (Sarepta, Daiichi Sankyo, Nippon Shinyaku, and Solid Biosciences).
|
|
•
|
Translarna for Other Indications.
Diacomit is marketed in the European Union by Laboratoires Biocodex for the treatment of Dravet syndrome. In the United States, both GW Pharmaceuticals and Zogenix have approved products for the treatment of Dravet syndrome. Aniridia therapeutic interventions, such as artificial iris implantation, are being developed by HumanOptics AG.
|
|
•
|
Emflaza for DMD.
The FDA has not approved a corticosteroid specifically for DMD in the United States other than Emflaza. However, prednisone/prednisolone, which is not approved for DMD in the United States, is generically available and has been prescribed off label for DMD patients.
|
|
•
|
Gene therapy product candidates.
Currently, no treatment options are available for the underlying cause of AADC deficiency, and care is limited to palliative options with significant burden on caregivers. Additionally, we are not aware of any late-stage development product candidates for AADC deficiency. While there is currently no treatment options
|
|
•
|
Risdiplam.
Risdiplam also faces competition. For example, in December 2016, the FDA approved nusinersen, a drug developed by Ionis Pharmaceuticals, Inc. and marketed by Biogen, to treat SMA. AveXis, Inc., (acquired by Novartis in 2018) is also evaluating a gene therapy product candidate for the treatment of SMA. Other companies are also pursuing product candidates for the treatment of SMA, including Trophos (also in collaboration with Roche), Kowa, Novartis Pharmaceuticals Corporation, and Cytokinetics.
|
|
•
|
Waylivra.
If approved, Waylivra could face competition from drugs like metreleptin. Metreleptin, produced by Novelion Therapeutics, Inc., is currently approved for use in generalized lipodystrophy patients.
|
|
•
|
Tegsedi.
Tegsedi could face competition from drugs like patisiran and ALN-TTRsc02 in development by Alnylam, tafamidis commercialized in some countries in LATAM by Pfizer and tolcapone in development by SOM Biotech.
|
|
•
|
completion of preclinical laboratory tests, animal studies and formulation studies under the FDA’s Good Laboratory Practice, or GLP, regulations and other applicable laws or regulations;
|
|
•
|
submission with the FDA of an investigational new drug application, or IND, for clinical testing, which must become effective before clinical trials may begin at United States clinical trial sites;
|
|
•
|
approval by an independent Institutional Review Board, or IRB, and in the case of gene therapy studies, IBC, prior to initiation and subject to continuing review;
|
|
•
|
completion of adequate and well-controlled clinical trials to establish safety and efficacy, in the case of a drug product candidate, or safety purity, and potency, in the case of a biologic product candidate for its intended use, performed in accordance with Good Clinical Practices, or GCP, and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, or ICH, E6 GCP guidelines. Gene therapy research must also be conducted in accordance with the NIH Guidelines;
|
|
•
|
development of manufacturing processes to ensure the product candidate’s identity, strength, quality, purity, and potency;
|
|
•
|
submission and FDA acceptance of an NDA, in the case of a drug product candidate, or BLA in the case of a biologic product candidate, and satisfactory completion of an FDA Advisory Committee meeting, if applicable;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current good manufacturing practices, or cGMPs, which require that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity, as well as satisfactory completion of an FDA inspection of selected clinical sites and selected clinical investigators to determine GCP compliance; and
|
|
•
|
FDA review and approval of the NDA or BLA to permit commercial marketing for particular indications for use.
|
|
•
|
Critical: Conditions, practices or processes that adversely affect the rights, safety or well-being of the subjects or the quality and integrity of data. Observations classified as critical may include a pattern of deviations classified as major.
|
|
•
|
Major: Conditions, practices or processes that might adversely affect the rights, safety or well-being of the subjects and/or the quality and integrity of data. Observations classified as major may include a pattern of deviations or numerous minor observations.
|
|
•
|
Minor: Conditions, practices or processes that would not be expected to adversely affect the rights, safety or wellbeing of the subjects or the quality and integrity of data. Minor observations indicate the need for improvement of conditions, practices and processes.
|
|
•
|
Comments: Suggestions on how to improve quality or reduce the potential for a deviation to occur in the future.
|
|
•
|
regulatory authorities may suspend or withdraw approvals of any product candidate that may receive regulatory approval, thereby preventing or delaying its commercialization;
|
|
•
|
regulatory authorities may require additional warnings or limitations of use in product labeling;
|
|
•
|
we may be required to change the way a product candidate is administered or conduct additional clinical trials;
|
|
•
|
we could be sued and held liable for harm caused by our products to patients; and
|
|
•
|
our reputation may suffer.
|
|
•
|
a covered benefit under its health plan;
|
|
•
|
safe, effective and medically necessary;
|
|
•
|
appropriate for the specific patient;
|
|
•
|
cost-effective; and
|
|
•
|
neither experimental nor investigational.
|
|
•
|
our ability to negotiate, secure and maintain adequate pricing, coverage and reimbursement terms on a timely basis, or at all;
|
|
•
|
the timing and scope of commercial launches;
|
|
•
|
the maintenance and expansion of a commercial infrastructure capable of supporting product sales, marketing and distribution;
|
|
•
|
the implementation and maintenance of marketing and distribution relationships with third parties in territories where we do not pursue direct commercialization;
|
|
•
|
our ability to establish and maintain commercial manufacturing arrangements with third-party manufacturers;
|
|
•
|
the ability of our third-party manufacturers to successfully produce commercial and clinical supply of drug on a timely basis sufficient to meet the needs of our commercial and clinical activities;
|
|
•
|
successful identification of eligible patients;
|
|
•
|
acceptance of the drug as a treatment for the approved indication by patients, the medical community and third-party payors;
|
|
•
|
effectively competing with other therapies;
|
|
•
|
a continued acceptable safety profile of the drug;
|
|
•
|
the costs, timing and outcome of post-marketing studies and trials for Emflaza and Translarna, including, with respect to Translarna, Study 041;
|
|
•
|
obtaining and maintaining patent and trade secret protection and regulatory exclusivity, including with respect to Emflaza, whether we are able to maintain market exclusivity periods under the Hatch-Waxman Act and Orphan Drug Act;
|
|
•
|
whether, with respect to Translarna, we are able to continue to satisfy our obligations under, and maintain, the marketing authorization in the EEA for Translarna for the treatment of nmDMD, including whether the EMA determines on an annual basis that the benefit-risk balance of Translarna supports renewal of our marketing authorization in the EEA, on the current approved label;
|
|
•
|
whether, and within what timeframe, we are able to advance Translarna for the treatment of nmDMD in the United States, including, whether we will be required to perform additional clinical trials, non-clinical studies or CMC assessments or analyses at significant cost which, if successful, may enable FDA review of an NDA submission by us and, ultimately, may support approval of Translarna for nmDMD in the United States;
|
|
•
|
the successful advancement of Translarna in additional indications, in particular, nonsense mutation aniridia and nonsense mutation Dravet syndrome/CDKL5;
|
|
•
|
the successful advancement of Emflaza in additional indications, in particular, limb-girdle 2I;
|
|
•
|
our ability to obtain additional and maintain existing reimbursed named patient and cohort EAP programs for Translarna for the treatment of nmDMD on adequate terms;
|
|
•
|
our ability to successfully prepare and advance regulatory submissions for marketing authorizations for Translarna in additional territories and for additional or expanded indications and whether and in what timeframe we may obtain such authorizations;
|
|
•
|
the ability and willingness of patients and healthcare professionals to access Translarna through alternative means if pricing and reimbursement negotiations in the applicable territory do not have a positive outcome; and
|
|
•
|
protecting our rights in our intellectual property portfolio.
|
|
•
|
be unable to successfully maintain our marketing authorization in the EEA for Translarna for the treatment of nmDMD, which is subject to annual review and renewal following reassessment of the benefit-risk balance of the authorization by the EMA;
|
|
•
|
be delayed in or unable to obtain marketing approval in the United States for Translarna or any other product candidates, including supplemental application approvals for any products that receive approval;
|
|
•
|
be delayed in obtaining additional marketing authorizations, or not obtain additional marketing authorizations at all, for Translarna for the treatment of nmDMD;
|
|
•
|
be delayed in obtaining marketing authorizations, or not obtain marketing authorizations at all, for Translarna for other indications, or for our other product candidates;
|
|
•
|
obtain approval for indications, uses or patient populations that are not as broad as intended or desired;
|
|
•
|
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, including boxed warnings;
|
|
•
|
obtain approval with labeling that does not include claims that are necessary or desirable for the successful commercialization of the product or product candidate;
|
|
•
|
be subject to additional post-marketing requirements or restrictions, such as post-approval studies or REMS;
|
|
•
|
have the product removed from markets after obtaining applicable marketing authorizations; or
|
|
•
|
not be permitted to sell Translarna under some or any reimbursed EAP programs.
|
|
•
|
clinical trials of our products or our product candidates may produce negative or inconclusive results for the necessary study endpoints, our studies may fail to reach the necessary level of statistical significance, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs;
|
|
•
|
there may be flaws in our clinical trials’ design that may not become apparent until the clinical trials are well advanced;
|
|
•
|
the number of patients required for clinical trials of our product and product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials or be lost to follow-up at a higher rate than we anticipate;
|
|
•
|
patients that enroll in our studies may misrepresent their eligibility or may otherwise not comply with the clinical trial protocol, resulting in the need to drop the patients from the study, increase the needed enrollment size for the study or extend the study’s duration;
|
|
•
|
we may be unable to enroll a sufficient number of patients in our clinical trials to ensure adequate statistical power to detect any statistically significant treatment effects;
|
|
•
|
we may enroll patients at clinical trial sites in countries that are inexperienced with clinical trials in general, or with the indication that is the subject of the trial;
|
|
•
|
we may enroll patients at clinical trial sites in countries that have a different standard of care for patients in general, or with respect to the indication that is the subject of the trial. Regulatory authorities, such as the FDA, may also not accept data generated at international clinical trial sites;
|
|
•
|
our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, or we may be required to engage in additional clinical trial site monitoring;
|
|
•
|
regulators, institutional review boards, institutional biosafety committees, or independent ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site or may require us to submit additional data, conduct additional studies or amend our investigational new drug application, or IND, or comparable application or protocols prior to commencing a clinical trial;
|
|
•
|
we may fail to reach an agreement with regulators, institutional review boards, institutional biosafety committees, or independent ethics committees regarding the scope, design, or implementation of our clinical trials. For instance, the FDA or comparable foreign regulatory authorities may require changes to our study design that make further study impractical or not financially prudent;
|
|
•
|
we may have delays in reaching or may fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites and contract research organizations;
|
|
•
|
we may have delays in adding new investigators or clinical trial sites, or we may experience a withdrawal of clinical trial sites;
|
|
•
|
we may have to suspend or terminate clinical trials of our products or our product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks;
|
|
•
|
regulators, institutional review boards, institutional biosafety committees, or independent ethics committees may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
|
|
•
|
the cost of clinical trials of our products or our product candidates may be greater than we anticipate or we may have insufficient funds for a clinical trial or to pay the substantial user fees required by the FDA upon the filing of a marketing application;
|
|
•
|
the supply or quality of our products or our product candidates or other materials necessary to conduct clinical trials of our products or our product candidates may be insufficient or inadequate;
|
|
•
|
our products or our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators, institutional review boards, institutional biosafety committees or independent ethics committees to suspend or terminate the trials;
|
|
•
|
regulators may require us to perform additional or unanticipated clinical or preclinical trials, develop additional manufacturing information, or make changes to our manufacturing process to obtain approval or we may be subject to additional post-marketing testing, surveillance, or REMS requirements to maintain regulatory approval;
|
|
•
|
there may be changes in the applicable regulatory authorities’ approval policies or review, statutes, or regulations, which may render our data insufficient to obtain marketing approval;
|
|
•
|
we may decide that it is no longer in our business interest to continue a development program;
|
|
•
|
there may be regulatory questions or disagreements regarding interpretations of data and results, or new information may emerge regarding our product candidates;
|
|
•
|
the FDA or comparable foreign regulatory authorities may disagree with our study design, including endpoints, or our interpretation of data from preclinical studies and clinical trials or find that a product candidate’s benefits do not outweigh its safety risks;
|
|
•
|
the FDA or comparable regulatory authorities may disagree with our intended indications;
|
|
•
|
the FDA or comparable foreign regulatory authorities may fail to approve or subsequently find fault with the manufacturing processes or our contract manufacturer’s manufacturing facility for clinical and future commercial supplies;
|
|
•
|
the data collected from clinical trials of our product candidates may not be sufficient to the satisfaction of the FDA or comparable foreign regulatory authorities to support the submission of a marketing application, or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere;
|
|
•
|
the FDA or comparable regulatory authorities may take longer than we anticipate to make a decision on our product candidates; or
|
|
•
|
we may not be able to demonstrate that a product candidate provides an advantage over current standards of care or current or future competitive therapies in development.
|
|
•
|
severity of the disease under investigation;
|
|
•
|
eligibility criteria for the study in question;
|
|
•
|
perceived benefits and risks of the product candidate under study;
|
|
•
|
efforts to facilitate timely enrollment in clinical trials;
|
|
•
|
patient referral practices of physicians;
|
|
•
|
the ability to monitor patients adequately during and after treatment; and
|
|
•
|
proximity and availability of clinical trial sites for prospective patients.
|
|
•
|
the efficacy and potential advantages compared to alternative treatments;
|
|
•
|
the prevalence and severity of any side effects;
|
|
•
|
the limitations or warnings contained in the product’s FDA-approved labeling
|
|
•
|
the claims we may make for a product, based on the approved label;
|
|
•
|
distribution and use restrictions imposed by the FDA with respect to such product candidates or to which we agree as part of a REMS or voluntary risk management plan
|
|
•
|
the ability to offer our products or product candidates for sale at competitive prices;
|
|
•
|
convenience and ease of administration compared to alternative treatments;
|
|
•
|
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
|
|
•
|
the strength of marketing and distribution support;
|
|
•
|
sufficient third-party coverage or reimbursement;
|
|
•
|
adverse publicity about our products or product candidates or favorable publicity about competitive products or product candidates; and
|
|
•
|
any restrictions on concomitant use of other medications.
|
|
•
|
our ability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
|
|
•
|
our ability to monitor the legal and regulatory compliance of sales and marketing personnel;
|
|
•
|
an inability to secure adequate coverage and reimbursement by government and private health plans;
|
|
•
|
reduced realization on government sales from mandatory discounts, rebates and fees, and from price concessions to private health plans and pharmacy benefit managers necessitated by competition for access to managed formularies;
|
|
•
|
the clinical indications for which the products are approved and the claims that we may make for the products;
|
|
•
|
limitations or warnings, including distribution or use restrictions, contained in the products’ approved labeling;
|
|
•
|
any distribution and use restrictions imposed by the FDA or to which we agree as part of a mandatory REMS or voluntary risk management plan;
|
|
•
|
liability for sales or marketing personnel who fail to comply with the applicable legal and regulatory requirements;
|
|
•
|
our ability to replace services being performed pursuant to a transition services agreement with Marathon before the termination of such agreement;
|
|
•
|
our ability to implement third-party marketing and distribution relationships on favorable terms, or at all, in territories where we do not pursue direct commercialization;
|
|
•
|
the ability of our commercial team to obtain access to or persuade adequate numbers of physicians to prescribe Translarna, Emflaza or any future products;
|
|
•
|
the lack of complementary products to be offered by our commercial team, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and
|
|
•
|
unforeseen costs and expenses associated with creating an independent commercial organization.
|
|
•
|
political, regulatory, compliance and economic developments that could restrict our ability to manufacture, market and sell our products;
|
|
•
|
financial risks such as longer payment cycles, difficulty collecting accounts receivable and exposure to fluctuations in foreign currency exchange rates;
|
|
•
|
difficulty in staffing and managing international operations;
|
|
•
|
potentially negative consequences from changes in or interpretations of tax laws;
|
|
•
|
changes in international medical reimbursement policies and programs;
|
|
•
|
unexpected changes in health care policies of foreign jurisdictions;
|
|
•
|
trade protection measures, including import or export licensing requirements and tariffs;
|
|
•
|
our ability to develop relationships with qualified local distributors and trading companies;
|
|
•
|
political and economic instability in particular foreign economies and markets, in particular in emerging markets, for example in Brazil;
|
|
•
|
diminished protection of intellectual property in some countries outside of the United States;
|
|
•
|
differing labor regulations and business practices; and
|
|
•
|
regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors’ and service providers’ activities that may fall within the purview of the Foreign Corrupt Practices Act, UK Bribery Act or similar local regulation.
|
|
•
|
seek to integrate Agilis's operations and employees into our business and seek to satisfy contractual and regulatory obligations we assumed in connection with the Agilis acquisition;
|
|
•
|
seek to satisfy contractual and regulatory obligations in conjunction with the Akcea Agreement, including the potential commercialization of Tegsedi and Waylivra in Latin America and the Caribbean, or the PTC Territory;
|
|
•
|
execute our strategy for Emflaza in the United States, including commercialization efforts;
|
|
•
|
satisfy contractual and regulatory obligations that we assumed through the Emflaza acquisition;
|
|
•
|
are required to complete any additional clinical trials, non-clinical studies or CMC assessments or analyses in order to advance Translarna for the treatment of nmDMD in the United States or elsewhere;
|
|
•
|
are required to take other steps, in addition to Study 041, to maintain our current marketing authorization in the EEA for Translarna for the treatment of nmDMD or to obtain further marketing authorizations for Translarna for the treatment of nmDMD or other indications;
|
|
•
|
initiate or continue the research and development of Translarna and Emflaza for additional indications and of our other product candidates, including for our gene therapy, splicing and oncology programs;
|
|
•
|
seek to discover and develop additional product candidates;
|
|
•
|
seek to expand and diversify our product pipeline through strategic transactions;
|
|
•
|
maintain, expand and protect our intellectual property portfolio; and
|
|
•
|
add operational, financial and management information systems and personnel, including personnel to support our product development and commercialization efforts.
|
|
•
|
commercializing and marketing Emflaza for the treatment of DMD in the United States;
|
|
•
|
negotiating, securing, and maintaining adequate pricing, coverage and reimbursement terms, on a timely basis, with third-party payors for Emflaza for the treatment of DMD in the United States and for Translarna for the treatment of nmDMD in the EEA and other territories outside of the United States;
|
|
•
|
maintaining orphan exclusivity for Emflaza and successfully completing all FDA post-marketing requirements with respect to Emflaza;
|
|
•
|
maintaining the marketing authorization of Translarna for the treatment of nmDMD in the EEA, including successfully obtaining annual renewals of the marketing authorization, fulfilling the specific obligation to conduct and report the results of Study 041 to the EMA, and meeting any ongoing requirements related to the marketing authorization;
|
|
•
|
advancing Translarna for the treatment of nmDMD in the United States, including, whether we will be required to perform additional clinical trials, non-clinical studies or CMC assessments or analyses at significant cost which, if successful, may enable FDA review of an NDA submission by us and, ultimately, may support approval of Translarna for nmDMD in the United States;
|
|
•
|
expanding the territories in which we are approved to market Translarna for the treatment of nmDMD;
|
|
•
|
minimizing the enrollment impact of Study 041 on commercialization efforts for Translarna for nmDMD;
|
|
•
|
commercializing and marketing Tegsedi and Waylivra in the PTC Territory;
|
|
•
|
developing Translarna and Emflaza for the treatment of additional indications, including nonsense mutation aniridia, nonsense mutation Dravet syndrome/CDKL5 and limb-girdle 2I and successfully advancing our other programs and collaborations, including our gene therapy, splicing and oncology programs;
|
|
•
|
establishing a global commercial infrastructure, including the sales, marketing and distribution capabilities to effectively market and sell Translarna for the treatment of nmDMD in the EEA and other parts of the world;
|
|
•
|
implementing marketing and distribution relationships with third parties in territories where we do not pursue direct commercialization;
|
|
•
|
launching commercial sales of Translarna for the treatment of nmDMD in accordance with our estimated timeline;
|
|
•
|
identifying patients eligible for treatment with our products and product candidates;
|
|
•
|
obtaining approval to market Translarna and Emflaza for the treatment of other indications;
|
|
•
|
expanding the approved product label of Translarna for the treatment of nmDMD;
|
|
•
|
successfully developing or commercializing any product candidate or product that we may in-license or acquire;
|
|
•
|
protecting our rights to our intellectual property portfolio related to Translarna; and
|
|
•
|
contracting for the manufacture and distribution of commercial quantities of Translarna, Emflaza and other product candidates.
|
|
•
|
our ability to commercialize and market Emflaza for the treatment of DMD in the United States;
|
|
•
|
our ability to negotiate, secure and maintain adequate pricing, coverage and reimbursement terms, on a timely basis, with third-party payors for Emflaza for the treatment of DMD in the United States and for Translarna for the treatment of nmDMD in the EEA and other territories outside of the United States;
|
|
•
|
our ability to maintain orphan exclusivity for, and successfully complete all FDA post-marketing requirements with respect to, Emflaza;
|
|
•
|
our ability to maintain the marketing authorization in the EEA for Translarna for the treatment of nmDMD, including whether the EMA determines on an annual basis that the benefit-risk balance of Translarna supports renewal of our marketing authorization in the EEA, on the current approved label;
|
|
•
|
the costs, timing and outcome of Study 041;
|
|
•
|
the costs, timing and outcome of our efforts to advance Translarna for the treatment of nmDMD in the United States, including, whether we will be required to perform additional clinical trials, non-clinical studies or CMC assessments or analyses at significant cost which, if successful, may enable FDA review of an NDA submission by us and, ultimately, may support approval of Translarna for nmDMD in the United States;
|
|
•
|
our ability to commercialize and market Tegsedi and Waylivra in the PTC Territory;
|
|
•
|
the progress and results of our open label extension clinical trials of Translarna for the treatment of nmDMD as well as our studies for nonsense mutation aniridia and nonsense mutation Dravet syndrome/CDKL5 and activities under our gene therapy and oncology programs;
|
|
•
|
the scope, costs and timing of our commercialization activities, including product sales, marketing, legal, regulatory, distribution and manufacturing, for both Emflaza for the treatment of DMD and Translarna for the treatment of nmDMD, for Tegsedi, for Waylivra and for any of our other product candidates that may receive marketing authorization or any additional indications or territories in which we receive authorization to market Translarna;
|
|
•
|
the costs, timing and outcome of regulatory review of our other product candidates, including those in our gene therapy and oncology programs, and Translarna in other territories or for indications other than nmDMD;
|
|
•
|
our ability to satisfy our obligations under the terms of the Credit Agreement with MidCap Financial;
|
|
•
|
the timing and scope of growth in our employee base;
|
|
•
|
the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for Translarna and Emflaza for additional indications and for our other product candidates, including those in our gene therapy and oncology programs;
|
|
•
|
revenue received from commercial sales of Translarna, Emflaza, Tegsedi, Waylivra, or any of our other product candidates;
|
|
•
|
our ability to obtain additional and maintain existing reimbursed named patient and cohort EAP programs for Translarna for the treatment of nmDMD on adequate terms, or at all;
|
|
•
|
the ability and willingness of patients and healthcare professionals to access Translarna through alternative means if pricing and reimbursement negotiations in the applicable territory do not have a positive outcome;
|
|
•
|
the costs of preparing, filing and prosecuting patent applications, maintaining, and protecting our intellectual property rights and defending against intellectual property-related claims;
|
|
•
|
the extent to which we acquire or invest in other businesses, products, product candidates, and technologies, including the success of any acquisition, in-licensing or other strategic transaction we may pursue, and the costs of subsequent development requirements and commercialization efforts, including with respect to our acquisition of Emflaza, our acquisition of Agilis, and our licensing of Tegsedi and Waylivra; and
|
|
•
|
our ability to establish and maintain collaborations, including our collaborations with Roche and the SMA Foundation, and our ability to obtain research funding and achieve milestones under these agreements.
|
|
•
|
requiring us to dedicate a substantial portion of cash and cash equivalents and marketable securities to the payment of interest on, and principal of, the term loans, which will reduce the amounts available to fund working capital, capital expenditures, product development efforts and other general corporate purposes;
|
|
•
|
obligating us to negative covenants restricting our activities, including limitations on dispositions, mergers or acquisitions, encumbering our intellectual property, incurring indebtedness or liens, paying dividends, making investments and engaging in certain other business transactions;
|
|
•
|
limiting our flexibility in planning for, or reacting to, changes in our business and our industry;
|
|
•
|
placing us at a competitive disadvantage compared to our competitors who have less debt or competitors with comparable debt at more favorable interest rates; and
|
|
•
|
limiting our ability to borrow additional amounts for working capital, capital expenditures, research and development efforts, acquisitions, debt service requirements, execution of our business strategy and other purposes.
|
|
•
|
incurrence of substantial debt, dilutive issuances of securities or depletion of cash to pay for acquisitions;
|
|
•
|
exposure to known and unknown liabilities, including possible intellectual property infringement claims, violations of laws, tax liabilities and commercial disputes;
|
|
•
|
higher than expected acquisition and integration costs;
|
|
•
|
difficulty in integrating operations and personnel of any acquired business;
|
|
•
|
increased amortization expenses or, in the event that we write-down the value of acquired assets, impairment losses;
|
|
•
|
impairment of relationships with key suppliers or customers of any acquired business due to changes in management and ownership;
|
|
•
|
inability to retain personnel, customers, distributors, vendors and other business partners integral to an in-licensed or acquired product, product candidate or technology;
|
|
•
|
potential failure of the due diligence processes to identify significant problems, liabilities or other shortcomings or challenges;
|
|
•
|
entry into indications or markets in which we have no or limited direct prior development or commercial experience and where competitors in such markets have stronger market positions; and
|
|
•
|
other challenges associated with managing an increasingly diversified business.
|
|
•
|
restrictions on such products, manufacturers or manufacturing processes;
|
|
•
|
changes to or restrictions on the labeling or marketing of a product;
|
|
•
|
modifications to promotional pieces;
|
|
•
|
issuance of corrective information;
|
|
•
|
clinical holds or termination of clinical trials;
|
|
•
|
changes in the way a product is administered;
|
|
•
|
liability for harm caused to patients or subjects;
|
|
•
|
adverse publicity, reputational harm, or the product becoming less competitive;
|
|
•
|
regulatory authority issuance of safety alerts, Dear Healthcare Provider letters, press releases, or other communications containing warnings or other safety information about the product;
|
|
•
|
restrictions on product distribution or use;
|
|
•
|
requirements to implement a REMS;
|
|
•
|
requirements to conduct post-marketing studies or clinical trials;
|
|
•
|
warning, cyber or untitled letters;
|
|
•
|
withdrawal of the products from the market or marketing suspensions;
|
|
•
|
refusal to approve pending applications or supplements to approved applications that we submit;
|
|
•
|
recall of products;
|
|
•
|
fines, restitution or disgorgement of profits or revenues;
|
|
•
|
suspension or withdrawal of marketing authorizations;
|
|
•
|
refusal to permit the import or export of our products;
|
|
•
|
product seizure or detention;
|
|
•
|
injunctions;
|
|
•
|
the imposition of civil or criminal penalties; or
|
|
•
|
FDA debarment, suspension and debarment from government contracts, and refusal of orders under existing government contracts, exclusion from federal healthcare programs, consent decrees, or corporate integrity agreements.
|
|
•
|
Anti-corruption and anti-bribery laws and regulations, such as the U.S. Foreign Corrupt Practices Act, or FCPA, the UK Bribery Act of 2010, or Bribery Act, and similar statutes which have been adopted, or may be adopted in the future, by other countries in which we operate and with which we are or may be required to comply.
|
|
•
|
Anti-kickback laws and regulations, including those applicable in the United States, the United Kingdom and other countries where we operate, which generally prohibit, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under government funded healthcare programs. The U.S. federal statute imposes criminal penalties and has been broadly interpreted to apply to manufacturer arrangements with prescribers, purchasers and formulary managers, among others and many states have enacted equivalent state laws that apply not only to government payors but to commercial payors as well.
|
|
•
|
False claim laws and regulations, including the U.S. False Claims Act and similar state laws, which may permit civil whistleblower or qui tam actions and may impose civil liability and criminal penalties on individuals and entities who submit, or cause to be submitted, false or fraudulent claims for payment to the government. Federal enforcement agencies have also showed increased interest in pharmaceutical companies' product and patient assistance programs, including reimbursement and co-pay support services, and a number of investigations into these programs have resulted in significant civil and criminal settlements.
|
|
•
|
Federal price reporting laws, including the Medicaid drug rebate statute, which requires manufacturers of covered outpatient drugs to calculate and submit complex pricing information that is used as the basis for reimbursement of certain drugs by, and payment of rebates to, the Medicaid program; the Medicare Modernization Act, which requires manufacturers to calculate and report a drug’s Average Sales Price used to reimburse providers for physician-administered drugs under Medicare Part B; and the Veterans Health Care Act of 1992, which requires, manufacturers of covered drugs (including all drugs approved under an NDA) to calculate and report a Federal Ceiling Price and offer their covered drugs for sale at no more than that price to the Department of Veterans Affairs, the Department of Defense, and other agencies. The Veterans Health Care Act also requires manufacturers to enter into pricing agreements with the Department of Health and Human Services to charge no more than a different ceiling price (derived from the Medicaid rebate percentage) to covered entities participating in the 340B drug discount program. Failure to accurately report drug pricing or provide the mandatory discounts may subject the manufacturer to specific civil monetary penalties. Failure to comply with the Veterans Health Care Act also jeopardizes payment by Medicaid for the manufacturer’s drugs. Certain states have also enacted drug price transparency laws that require reporting of pricing information.
|
|
•
|
Laws and regulations related to the privacy, security and transmission of individually identifiable health information, including the U.S. Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and similar state laws. For example, HIPAA, as amended by HITECH, along with its implementing regulations, which impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information, and may impose criminal and civil liability for violations of these obligations. In addition, international data protection laws including the European General Data Protection Regulation, and supplementary member state legislation may apply to some or all of the clinical or other protected data obtained, transmitted, or stored outside of the United States. Furthermore, certain privacy laws and genetic testing laws may apply directly to our operations and/or those of our collaborators and may impose restrictions on our use and dissemination of individuals’ health information.
|
|
•
|
HIPAA also imposes liability, including criminal liability, for, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, a healthcare benefit program, regardless of whether the payor is public or private, in connection with the delivery or payment for health care benefits, knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense and knowingly and willfully falsifying, concealing, or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items, or services relating to healthcare matters. Notably, the ACA amended the intent requirement of certain of these criminal statutes under HIPAA so that a person or entity no longer needs to have actual knowledge of the statute, or the specific intent to violate it, to have committed a violation.
|
|
•
|
Laws and regulations governing the advertising and promotion of medicinal products, interactions with physicians and patients, misleading and comparative advertising and unfair commercial practices. For example, legislation adopted by individual EU member states that may apply to the advertising and promotion of medicinal products require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities. The SmPC is the document that provides information to physicians concerning the safe and effective use of the medicinal product. Promotion of indications not covered by the SmPC is specifically prohibited.
|
|
•
|
Laws and regulations regulating off-label promotion of medicinal products, which is prohibited in the European Union. The applicable laws at European Union level and in the individual EU member states also prohibit the direct-to-consumer advertising of prescription-only medicinal products. Violations of the rules governing the promotion of medicinal products in the European Union could be penalized by administrative measures, fines and imprisonment. These laws may further limit or restrict the advertising and promotion of our products to the general public and may also impose limitations on our promotional activities with health care professionals.
|
|
•
|
Laws and regulations in the United States, including the Federal Food, Drug and Cosmetic Act and other laws and regulations, that prohibit us from promoting any of our FDA approved products for off-label uses and that require compliance with FDA’s advertising and promotional requirements. For example, the FDA requires that all product advertising and promotion be consistent with the FDA approved label, be truthful and non-misleading, be adequately substantiated, and have fair balance between product benefit claims and risks, among other requirements. This means, for example, that we cannot make claims about the use of our marketed products or their relative benefits compared to other treatments outside of their FDA approved indications and label and without adequate comparative studies, and we would not be able to discuss or provide information on off-label uses or safety benefits of such products in a
|
|
•
|
Laws and regulations requiring that we disclose publicly payments made to physicians, including in certain EU member states and the United States. For example, in the United States, under the federal Physician Payments Sunshine Act requirements, manufacturers of drugs, devices, biologics and medical supplies must report information related to payments and other transfers of value made to or at the request of covered recipients, such as physicians and teaching hospitals, as well as physician ownership and investment interests in such manufacturers. A number of U.S. states and other countries have enacted their own transparency requirements that obligate manufacturers to report different types of spending related to physicians, certain hospitals, and other covered recipients.
|
|
•
|
the diversion of management attention to integration matters;
|
|
•
|
difficulties in achieving anticipated business opportunities and growth prospects from our acquisition of Agilis;
|
|
•
|
challenges related to public and market perception of our acquisition of Agilis and gene therapy and increased regulatory scrutiny of gene therapy;
|
|
•
|
difficulties in managing the expanded operations of a larger and more complex company following our acquisition of Agilis;
|
|
•
|
difficulties in assimilating employees and in attracting and retaining key personnel; and
|
|
•
|
potential unknown liabilities, adverse consequences, unforeseen increased expenses or other unanticipated problems associated with the transaction.
|
|
•
|
reliance on the third party for regulatory compliance and quality assurance;
|
|
•
|
the possible breach of the manufacturing agreement by the third party;
|
|
•
|
the possible misappropriation of our proprietary information, including our trade secrets and know-how;
|
|
•
|
the possibility of commercial supplies of Translarna or Emflaza not being distributed to commercial vendors or end users in a timely manner, resulting in lost sales;
|
|
•
|
the possibility of clinical supplies not being delivered to clinical sites on time, leading to clinical trial interruptions;
|
|
•
|
the possibility of third-party resources not being devoted in the manner necessary to satisfy our requirements within the expected time frame;
|
|
•
|
the possibility of third parties not providing us with accurate or timely information regarding their inventories, the number of patients who are using Emflaza, or serious adverse events and/or product complaints regarding Emflaza;
|
|
•
|
the possibility of third parties being unable to satisfy their financial obligations to us or to others; and
|
|
•
|
the possible termination or nonrenewal of a critical agreement by the third party at a time that is costly or inconvenient to us.
|
|
•
|
collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations;
|
|
•
|
collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs, based on clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities;
|
|
•
|
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
|
|
•
|
collaborators could independently develop, or develop with third parties, products that replace or compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;
|
|
•
|
collaborators may fail to comply with the applicable regulatory requirements, subjecting them or us to potential regulatory enforcement action;
|
|
•
|
a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products;
|
|
•
|
collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
|
|
•
|
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
|
|
•
|
disputes may arise between the collaborator and us as to the ownership of intellectual property arising during the collaboration;
|
|
•
|
we may grant exclusive rights for our products or product candidates to our collaborators, which would prevent us from collaborating with others, or from using our products or product candidates ourselves;
|
|
•
|
disputes may arise between the collaborators and us that result in the delay or termination of the collaboration, which may include ending research, development or commercialization activities for our products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and
|
|
•
|
collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates.
|
|
•
|
reduced resources of our management to pursue our business strategy;
|
|
•
|
decreased demand for our products or any product candidates that we may develop;
|
|
•
|
injury to our reputation and significant negative media attention;
|
|
•
|
the inability to continue current clinical trials or begin planned clinical trials;
|
|
•
|
withdrawal or reduced enrollment of clinical trial participants;
|
|
•
|
significant costs to defend the related claims/litigation;
|
|
•
|
increased insurance costs, or an inability to maintain appropriate insurance coverage;
|
|
•
|
substantial monetary awards to trial participants, patients and/or their families;
|
|
•
|
loss of revenue;
|
|
•
|
the inability to commercialize or to continue commercializing any products or product candidates;
|
|
•
|
initiation of investigations and enforcement actions by regulators; and
|
|
•
|
the withdrawal of products from the market, product recalls, or the cessation of development or regulatory disapproval of product candidates or withdrawal of approvals, as well as labeling, marketing, or promotional restrictions.
|
|
•
|
provide for a classified board of directors such that not all members of the board are elected at one time;
|
|
•
|
allow the authorized number of our directors to be changed only by resolution of our board of directors;
|
|
•
|
limit the manner in which stockholders can remove directors from the board;
|
|
•
|
establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors;
|
|
•
|
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
|
|
•
|
limit who may call stockholder meetings;
|
|
•
|
authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and
|
|
•
|
require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws.
|
|
•
|
our ability to realize the anticipated benefits of our acquisition of Agilis, including our ability to obtain marketing approval for PTC-AADC;
|
|
•
|
any developments related to our ability or inability to execute our strategy for Emflaza for the treatment of DMD in the United States, in particular with respect to our commercialization efforts;
|
|
•
|
our ability to resolve the matters set forth in the FDA's denial of our appeal to the Complete Response Letter we received from the FDA in connection with our NDA for Translarna for the treatment of nmDMD, and our ability to perform additional clinical trials, non-clinical studies or CMC assessments or analyses at significant cost;
|
|
•
|
our ability to maintain our marketing authorization for Translarna for the treatment of nmDMD in the EEA, which is subject to the specific obligation to conduct Study 041 and is also subject to annual review and renewal by the European Commission following reassessment of the benefit-risk balance of the authorization by the EMA;
|
|
•
|
our ability to commercialize Tegsedi and Waylivra in the PTC Territory;
|
|
•
|
any developments related to Study 041, including with respect to design, timing, conduct, and enrollment, and developments with respect to any clinical or non-clinical trial required by other regulatory agencies, including the FDA for Translarna for the treatment of nmDMD;
|
|
•
|
results of clinical trials of Translarna and any other product candidate that we develop;
|
|
•
|
announcements by us or our competitors of significant acquisitions, licenses, strategic collaborations, joint ventures, collaborations or capital commitments;
|
|
•
|
negative publicity around our products or product candidates;
|
|
•
|
other developments concerning our regulatory submissions;
|
|
•
|
whether regulators in other territories agree with our interpretation of the results of ACT DMD;
|
|
•
|
our ability to advance the commercialization of Translarna for the treatment of nmDMD;
|
|
•
|
the success of competitive products or technologies;
|
|
•
|
the development and regulatory status of risdiplam and our SMA program with Roche and the SMA Foundation;
|
|
•
|
results of clinical trials of product candidates of our competitors;
|
|
•
|
regulatory or legal developments in the United States and other countries;
|
|
•
|
developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
|
•
|
the recruitment or departure of key personnel;
|
|
•
|
the level of expenses related to any of our products, product candidates or clinical development programs;
|
|
•
|
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
|
•
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
•
|
changes in the structure of healthcare payment systems;
|
|
•
|
market conditions in the pharmaceutical and biotechnology sectors;
|
|
•
|
general economic, industry and market conditions; and
|
|
•
|
the other factors described in this “Risk Factors” section.
|
|
Year ended December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
(In thousands, except per share data)
|
|||||||||||||||||||
|
Statement of Operations Data:
|
|||||||||||||||||||
|
Revenues:
|
|||||||||||||||||||
|
Net product revenue
|
$
|
263,005
|
|
$
|
174,066
|
|
$
|
81,447
|
|
$
|
33,696
|
|
$
|
717
|
|
||||
|
Collaboration and grant revenue
|
1,729
|
|
20,326
|
|
1,258
|
|
3,070
|
|
24,528
|
|
|||||||||
|
Total revenues
|
264,734
|
|
194,392
|
|
82,705
|
|
36,766
|
|
25,245
|
|
|||||||||
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Cost of product sales, excluding amortization of acquired intangible asset
|
12,670
|
|
4,577
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Amortization of acquired intangible asset
|
22,877
|
|
15,380
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Research and development
|
171,984
|
|
117,456
|
|
117,633
|
|
121,816
|
|
79,838
|
|
|||||||||
|
Selling, general and administrative
|
153,548
|
|
121,271
|
|
97,130
|
|
82,080
|
|
44,820
|
|
|||||||||
|
Change in the fair value of deferred and contingent consideration
|
19,340
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total operating expenses
|
380,419
|
|
258,684
|
|
214,763
|
|
203,896
|
|
124,658
|
|
|||||||||
|
Loss from operations
|
(115,685
|
)
|
(64,292
|
)
|
(132,058
|
)
|
(167,130
|
)
|
(99,413
|
)
|
|||||||||
|
Interest (expense) income, net
|
(12,554
|
)
|
(12,094
|
)
|
(8,276
|
)
|
(2,367
|
)
|
1,180
|
|
|||||||||
|
Other income (expense), net
|
129
|
|
(1,279
|
)
|
(1,207
|
)
|
(465
|
)
|
(213
|
)
|
|||||||||
|
Loss before income tax benefit (expense)
|
(128,110
|
)
|
(77,665
|
)
|
(141,541
|
)
|
(169,962
|
)
|
(98,446
|
)
|
|||||||||
|
Income tax benefit (expense)
|
29
|
|
(1,335
|
)
|
(569
|
)
|
(485
|
)
|
4,693
|
|
|||||||||
|
Net loss attributable to common stockholders
|
$
|
(128,081
|
)
|
$
|
(79,000
|
)
|
$
|
(142,110
|
)
|
$
|
(170,447
|
)
|
$
|
(93,753
|
)
|
||||
|
Net loss attributable to common stockholders per share:
|
|||||||||||||||||||
|
Basic
|
$
|
(2.75
|
)
|
$
|
(2.02
|
)
|
$
|
(4.17
|
)
|
$
|
(5.07
|
)
|
$
|
(2.97
|
)
|
||||
|
Diluted
|
$
|
(2.75
|
)
|
$
|
(2.02
|
)
|
$
|
(4.17
|
)
|
$
|
(5.07
|
)
|
$
|
(2.97
|
)
|
||||
|
Weighted-average shares outstanding:
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Basic
|
46,576,313
|
|
39,183,073
|
|
34,044,584
|
|
33,626,248
|
|
31,565,310
|
|
|||||||||
|
Diluted
|
46,576,313
|
|
39,183,073
|
|
34,044,584
|
|
33,626,248
|
|
31,565,310
|
|
|||||||||
|
As of December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
(In thousands)
|
|||||||||||||||||||
|
Balance Sheet Data:
|
|||||||||||||||||||
|
Cash, cash equivalents, and marketable securities
|
$
|
227,586
|
|
$
|
191,246
|
|
$
|
231,666
|
|
$
|
338,925
|
|
$
|
315,241
|
|
||||
|
Working Capital
|
154,061
|
|
167,015
|
|
211,662
|
|
310,563
|
|
291,096
|
|
|||||||||
|
Total assets*
|
1,119,222
|
|
391,653
|
|
269,345
|
|
365,281
|
|
333,219
|
|
|||||||||
|
Total debt*
|
153,014
|
|
144,971
|
|
98,216
|
|
91,848
|
|
—
|
|
|||||||||
|
Accumulated deficit
|
(938,923
|
)
|
(814,108
|
)
|
(735,108
|
)
|
(592,998
|
)
|
(422,551
|
)
|
|||||||||
|
Total stockholders’ equity
|
350,727
|
|
156,437
|
|
119,583
|
|
226,001
|
|
298,467
|
|
|||||||||
|
•
|
external research and development expenses incurred under agreements with third-party contract research organizations and investigative sites, third-party manufacturing organizations and consultants;
|
|
•
|
employee-related expenses, which include salaries and benefits, including share-based compensation, for the personnel involved in our drug discovery and development activities; and
|
|
•
|
facilities, depreciation and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, depreciation of leasehold improvements and equipment, and laboratory and other supplies.
|
|
Year ended
December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Translarna (nmDMD, nmCF, nmMPS I, aniridia and Dravet)
|
$
|
80,859
|
|
$
|
75,954
|
|
$
|
84,566
|
|
||
|
Gene therapy
|
6,534
|
|
—
|
|
—
|
|
|||||
|
Oncology
|
16,438
|
|
4,481
|
|
7,532
|
|
|||||
|
Next generation nonsense readthrough
|
6,735
|
|
5,609
|
|
6,428
|
|
|||||
|
Emflaza
|
16,461
|
|
7,053
|
|
—
|
|
|||||
|
Akcea
|
11,957
|
|
—
|
|
—
|
|
|||||
|
Other research and preclinical
|
33,000
|
|
24,359
|
|
19,107
|
|
|||||
|
Total research and development
|
$
|
171,984
|
|
$
|
117,456
|
|
$
|
117,633
|
|
||
|
•
|
the scope, rate of progress and expense of our clinical trials and other research and development activities;
|
|
•
|
the potential benefits of our product and product candidates over other therapies;
|
|
•
|
our ability to market, commercialize and achieve market acceptance for our product or any of our product candidates that we are developing or may develop in the future, including our ability to negotiate pricing and reimbursement terms acceptable to us;
|
|
•
|
clinical trial results;
|
|
•
|
the terms and timing of regulatory approvals; and
|
|
•
|
the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights.
|
|
•
|
Portfolio Approach - We applied the Portfolio Approach to contract reviews within identified revenue streams that have similar characteristics and we believe this approach would not differ materially than if applying ASC Topic 606 to each individual contract.
|
|
•
|
Significant Financing Component - We expect the period between when an we transfer a promised good or service to a customer and when the customer pays for the good or service to be one year or less.
|
|
•
|
Immaterial Performance Obligations - We disregard promises deemed to be immaterial in the context of the contract.
|
|
•
|
Shipping and Handling Activities - We consider any shipping and handling costs that are incurred after the customer has obtained control of the product as a cost to fulfill a promise.
|
|
•
|
fees paid to contract research organizations in connection with preclinical and toxicology studies and clinical trials;
|
|
•
|
fees paid to investigative sites in connection with clinical trials;
|
|
•
|
fees paid to contract manufacturers in connection with the production of clinical trial materials; and
|
|
•
|
professional service fees.
|
|
2018
|
2017
|
2016
|
|||
|
Risk-free interest rate
|
2.25% - 3.10%
|
1.84% - 2.45%
|
1.30% - 2.24%
|
||
|
Expected volatility
|
64% - 90%
|
76% - 81%
|
67% - 78%
|
||
|
Expected term
|
5.03 - 10.00 years
|
5.04 - 10.00 years
|
5.05 - 10.00 years
|
||
|
Restricted Stock Awards and Units
|
|||||||
|
Number of
Shares
|
Weighted
Average Grant
Date Fair Value
|
||||||
|
Unvested at December 31, 2017
|
393,011
|
|
$
|
15.64
|
|
||
|
Granted
|
354,691
|
|
$
|
19.09
|
|
||
|
Vested
|
(114,295
|
)
|
$
|
16.36
|
|
||
|
Forfeited
|
(61,928
|
)
|
$
|
17.24
|
|
||
|
Unvested at December 31, 2018
|
571,479
|
|
$
|
17.61
|
|
||
|
|
Year ended December 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
|||||||||
|
Research and development
|
$
|
16,096
|
|
$
|
15,456
|
|
$
|
16,812
|
|
|||
|
Selling, general and administrative
|
17,156
|
|
15,103
|
|
18,197
|
|
||||||
|
Total
|
$
|
33,252
|
|
$
|
30,559
|
|
$
|
35,009
|
|
|||
|
Year ended
December 31,
|
Change
2018 vs. 2017
|
||||||||||
|
(in thousands)
|
2018
|
2017
|
|||||||||
|
Net product revenue
|
$
|
263,005
|
|
$
|
174,066
|
|
$
|
88,939
|
|
||
|
Collaboration and grant revenue
|
1,729
|
|
20,326
|
|
$
|
(18,597
|
)
|
||||
|
Cost of product sales, excluding amortization of acquired intangible asset
|
12,670
|
|
4,577
|
|
$
|
8,093
|
|
||||
|
Amortization of acquired intangible asset
|
22,877
|
|
15,380
|
|
$
|
7,497
|
|
||||
|
Research and development expense
|
171,984
|
|
117,456
|
|
$
|
54,528
|
|
||||
|
Selling, general and administrative expense
|
153,548
|
|
121,271
|
|
$
|
32,277
|
|
||||
|
Change in the fair value of deferred and contingent consideration
|
19,340
|
|
—
|
|
$
|
19,340
|
|
||||
|
Interest expense, net
|
(12,554
|
)
|
(12,094
|
)
|
$
|
(460
|
)
|
||||
|
Other income (expense), net
|
129
|
|
(1,279
|
)
|
$
|
1,408
|
|
||||
|
Income tax benefit (expense)
|
29
|
|
(1,335
|
)
|
$
|
1,364
|
|
||||
|
Year ended
December 31,
|
Change
2017 vs. 2016
|
||||||||||
|
(in thousands)
|
2017
|
2016
|
|||||||||
|
Net product revenue
|
$
|
174,066
|
|
$
|
81,447
|
|
$
|
92,619
|
|
||
|
Collaboration and grant revenue
|
20,326
|
|
1,258
|
|
$
|
19,068
|
|
||||
|
Cost of product sales, excluding amortization of acquired intangible asset
|
4,577
|
|
—
|
|
$
|
4,577
|
|
||||
|
Amortization of acquired intangible asset
|
15,380
|
|
—
|
|
$
|
15,380
|
|
||||
|
Research and development expense
|
117,456
|
|
117,633
|
|
$
|
(177
|
)
|
||||
|
Selling, general and administrative expense
|
121,271
|
|
97,130
|
|
$
|
24,141
|
|
||||
|
Interest expense, net
|
(12,094
|
)
|
(8,276
|
)
|
$
|
(3,818
|
)
|
||||
|
Other expense, net
|
(1,279
|
)
|
(1,207
|
)
|
$
|
(72
|
)
|
||||
|
Income tax expense
|
(1,335
|
)
|
(569
|
)
|
$
|
(766
|
)
|
||||
|
Years ended
December 31,
|
||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
|||||
|
Cash (used in) provided by:
|
||||||||
|
Operating activities
|
(27,641
|
)
|
(10,063
|
)
|
(103,566
|
)
|
||
|
Investing activities
|
(42,613
|
)
|
13,117
|
|
104,481
|
|
||
|
Financing activities
|
131,571
|
|
44,218
|
|
968
|
|
||
|
•
|
seek to integrate Agilis's operations and employees into our business and seek to satisfy contractual and regulatory obligations we assumed in connection with the Agilis acquisition;
|
|
•
|
seek to satisfy contractual and regulatory obligations in conjunction with the Akcea Agreement, including the potential commercialization of Tegsedi and Waylivra in the PTC Territory;
|
|
•
|
execute our strategy for Emflaza in the United States, including commercialization and integration efforts;
|
|
•
|
satisfy contractual and regulatory obligations that we assumed through the Emflaza acquisition;
|
|
•
|
are required to complete any additional clinical trials, non-clinical studies or CMC assessments or analyses in order to advance Translarna for the treatment of nmDMD in the United States or elsewhere;
|
|
•
|
are required to take other steps, in addition to Study 041, to maintain our current marketing authorization in the EEA for Translarna for the treatment of nmDMD or to obtain further marketing authorizations for Translarna for the treatment of nmDMD or other indications;
|
|
•
|
initiate or continue the research and development of Translarna and Emflaza for additional indications and of our other product candidates;
|
|
•
|
seek to discover and develop additional product candidates;
|
|
•
|
seek to expand and diversify our product pipeline through strategic transactions;
|
|
•
|
maintain, expand and protect our intellectual property portfolio; and
|
|
•
|
add operational, financial and management information systems and personnel, including personnel to support our product development and commercialization efforts.
|
|
•
|
our ability to commercialize and market Emflaza for the treatment of DMD in the United States;
|
|
•
|
our ability to negotiate, secure and maintain adequate pricing, coverage and reimbursement terms, on a timely basis, with third-party payors for Emflaza for the treatment of DMD in the United States and for Translarna for the treatment of nmDMD in the EEA and other territories outside of the United States;
|
|
•
|
our ability to maintain orphan exclusivity for, and successfully complete all FDA post-marketing requirements with respect to, Emflaza;
|
|
•
|
our ability to maintain the marketing authorization in the EEA for Translarna for the treatment of nmDMD, including whether the EMA determines on an annual basis that the benefit-risk balance of Translarna supports renewal of our marketing authorization in the EEA, on the current approved label;
|
|
•
|
the costs, timing and outcome of Study 041;
|
|
•
|
the costs, timing and outcome of our efforts to advance Translarna for the treatment of nmDMD in the United States, including, whether we will be required to perform additional clinical trials, non-clinical studies or CMC assessments or analyses at significant cost which, if successful, may enable FDA review of an NDA submission by us and, ultimately, may support approval of Translarna for nmDMD in the United States;
|
|
•
|
our ability to commercialize and market Tegsedi and Waylivra in the PTC Territory;
|
|
•
|
the progress and results of our open label extension clinical trials of Translarna for the treatment of nmDMD as well as our studies for nonsense mutation aniridia and nonsense mutation Dravet syndrome/CDKL5 and activities under our gene therapy and oncology programs;
|
|
•
|
the scope, costs and timing of our commercialization activities, including product sales, marketing, legal, regulatory, distribution and manufacturing, for both Emflaza for the treatment of DMD and Translarna for the treatment of nmDMD, for Tegsedi, for Waylivra and for any of our other product candidates that may receive marketing authorization or any additional indications or territories in which we receive authorization to market Translarna;
|
|
•
|
the costs, timing and outcome of regulatory review of our other product candidates, including those in our gene therapy and oncology programs, and Translarna in other territories or for indications other than nmDMD;
|
|
•
|
our ability to satisfy our obligations under the terms of the Credit Agreement with MidCap Financial;
|
|
•
|
the timing and scope of growth in our employee base;
|
|
•
|
the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for Translarna and Emflaza for additional indications and for our other product candidates, including those in our gene therapy and oncology programs;
|
|
•
|
revenue received from commercial sales of Translarna, Emflaza, Tegsedi, Waylivra, or any of our other product candidates;
|
|
•
|
our ability to obtain additional and maintain existing reimbursed named patient and cohort EAP programs for Translarna for the treatment of nmDMD on adequate terms, or at all;
|
|
•
|
the ability and willingness of patients and healthcare professionals to access Translarna through alternative means if pricing and reimbursement negotiations in the applicable territory do not have a positive outcome;
|
|
•
|
the costs of preparing, filing and prosecuting patent applications, maintaining, and protecting our intellectual property rights and defending against intellectual property-related claims;
|
|
•
|
the extent to which we acquire or invest in other businesses, products, product candidates, and technologies, including the success of any acquisition, in-licensing or other strategic transaction we may pursue, and the costs of subsequent development requirements and commercialization efforts, including with respect to our acquisition of Emflaza, our acquisition of Agilis, and our licensing of Tegsedi and Waylivra; and
|
|
•
|
our ability to establish and maintain collaborations, including our collaborations with Roche and the SMA Foundation, and our ability to obtain research funding and achieve milestones under these agreements.
|
|
(in thousands)
|
Total
|
Less than
1 year
|
1 - 3 years
|
3 - 5 years
|
More than
5 years
|
||||||||||||||
|
Operating lease obligations (1)
|
$
|
13,928
|
|
3,216
|
|
5,309
|
|
3,965
|
|
1,438
|
|
||||||||
|
Long-term debt obligations, including interest (2)
|
$
|
168,000
|
|
4,500
|
|
9,000
|
|
154,500
|
|
—
|
|
||||||||
|
Minimum royalty (3)
|
$
|
8,588
|
|
1,718
|
|
3,435
|
|
3,435
|
|
—
|
|
||||||||
|
Credit agreement, including interest (4)
|
$
|
43,986
|
|
14,283
|
|
29,703
|
|
—
|
|
—
|
|
||||||||
|
Deferred Consideration payable (5)
|
$
|
40,000
|
|
20,000
|
|
20,000
|
|
—
|
|
—
|
|
||||||||
|
Total contractual obligations
|
$
|
274,502
|
|
$
|
43,717
|
|
$
|
67,447
|
|
$
|
161,900
|
|
$
|
1,438
|
|
||||
|
(1)
|
We lease office space for our principal office in South Plainfield, New Jersey under three non-cancelable operating leases with terms that extend through May 2022, August 2024 and October 2024. In addition, we lease office space in various countries for our international employees and operations.
|
|
(2)
|
Our long-term debt obligations reflect our obligations under the Convertible Notes to pay interest on the $150.0 million aggregate principal amount of the Convertible Notes and to make principal payments on the Convertible Notes at maturity or upon conversion.
|
|
(3)
|
Under an Exclusive License and Supply Agreement ("the Faes Agreement") with Faes Farma, S.A. ("Faes"), we are required to pay royalties as a percentage of or as a fixed payment with respect to net product sales by us allocable to the Emflaza oral suspension product. We are required to pay Faes an annual minimum royalty during the first seven calendar years with a fixed percentage royalty during the remainder of the Faes Agreement term. The amounts above reflect the minimum required payment based on the euro to U.S. dollar exchange rate as of
December 31, 2018
.
|
|
(4)
|
Under the terms of the Credit Agreement, we are required to make interest only payments through April 30, 2019. Commencing on May 1, 2019 and continuing for the remaining twenty-four months of the facility, we will be required to make monthly interest payments and monthly principal payments. The principal payments are to be made based on straight-line amortization of the principal over the twenty-four month period.
|
|
(5)
|
Pursuant to the Merger Agreement with Agilis, we are required to pay $40.0 million of development milestone payments, or deferred consideration payments, no later than the second anniversary of the closing of the Merger, regardless of whether the applicable milestones have been achieved. The payment schedule above reflects our expected timing of when the payments will be made as of
December 31, 2018
. The fair value of the deferred consideration payments at the
December 31, 2018
was estimated to be
$37.7 million
.
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
|
Consolidated Balance Sheets as of December 31, 2018 and 2017
|
|
|
|
Consolidated Statements of Operations for the years ended December 31, 2018, 2017 and 2016
|
|
|
|
Consolidated Statements of Comprehensive Loss for the years ended December 31, 2018, 2017 and 2016
|
|
|
|
Consolidated Statements Stockholders’ Equity for the years ended December 31, 2018, 2017 and 2016
|
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2018, 2017 and 2016
|
|
|
|
Notes to Consolidated Financial Statements
|
|
|
|
|
December 31,
|
||||||
|
|
2018
|
2017
|
|||||
|
Assets
|
|
|
|
|
|||
|
Current assets:
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
169,498
|
|
$
|
111,792
|
|
|
|
Marketable securities
|
58,088
|
|
79,454
|
|
|||
|
Trade receivables, net
|
67,907
|
|
40,394
|
|
|||
|
Inventory
|
16,117
|
|
10,754
|
|
|||
|
Prepaid expenses and other current assets
|
9,247
|
|
6,669
|
|
|||
|
Total current assets
|
320,857
|
|
249,063
|
|
|||
|
Fixed assets, net
|
12,694
|
|
8,376
|
|
|||
|
Intangible assets, net
|
701,031
|
|
132,993
|
|
|||
|
Goodwill
|
82,341
|
|
—
|
|
|||
|
Deposits and other assets
|
2,299
|
|
1,221
|
|
|||
|
Total assets
|
$
|
1,119,222
|
|
$
|
391,653
|
|
|
|
Liabilities and stockholders’ equity
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable and accrued expenses
|
128,199
|
|
76,446
|
|
|||
|
Current portion of long-term debt
|
11,667
|
|
—
|
|
|||
|
Deferred revenue
|
3,716
|
|
3,937
|
|
|||
|
Other current liabilities
|
3,814
|
|
1,665
|
|
|||
|
Deferred consideration payable- current
|
19,400
|
|
—
|
|
|||
|
Total current liabilities
|
166,796
|
|
82,048
|
|
|||
|
Deferred revenue - long-term
|
9,722
|
|
7,954
|
|
|||
|
Long-term debt
|
141,347
|
|
144,971
|
|
|||
|
Contingent consideration payable
|
310,240
|
|
—
|
|
|||
|
Deferred consideration payable- long-term
|
18,300
|
|
—
|
|
|||
|
Deferred tax liability
|
122,032
|
|
—
|
|
|||
|
Other long-term liabilities
|
58
|
|
243
|
|
|||
|
Total liabilities
|
768,495
|
|
235,216
|
|
|||
|
Stockholders’ equity:
|
|
|
|
|
|||
|
Common stock, $0.001 par value. Authorized 125,000,000 shares; issued and outstanding 50,606,147 shares at December 31, 2018. Authorized 125,000,000 shares; issued and outstanding 41,612,395 shares at December 31, 2017.
|
51
|
|
42
|
|
|||
|
Additional paid-in capital
|
1,288,137
|
|
966,534
|
|
|||
|
Accumulated other comprehensive income
|
1,462
|
|
3,969
|
|
|||
|
Accumulated deficit
|
(938,923
|
)
|
(814,108
|
)
|
|||
|
Total stockholders’ equity
|
350,727
|
|
156,437
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
1,119,222
|
|
$
|
391,653
|
|
|
|
|
Year ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Revenues:
|
|
|
|
|
|
|
|||||
|
Net product revenue
|
$
|
263,005
|
|
$
|
174,066
|
|
$
|
81,447
|
|
||
|
Collaboration and grant revenue
|
1,729
|
|
20,326
|
|
1,258
|
|
|||||
|
Total revenues
|
264,734
|
|
194,392
|
|
82,705
|
|
|||||
|
Operating expenses:
|
|
|
|
|
|
|
|||||
|
Cost of product sales, excluding amortization of acquired intangible asset
|
12,670
|
|
4,577
|
|
—
|
|
|||||
|
Amortization of acquired intangible asset
|
22,877
|
|
15,380
|
|
—
|
|
|||||
|
Research and development
|
171,984
|
|
117,456
|
|
117,633
|
|
|||||
|
Selling, general and administrative
|
153,548
|
|
121,271
|
|
97,130
|
|
|||||
|
Change in the fair value of deferred and contingent consideration
|
19,340
|
|
—
|
|
—
|
|
|||||
|
Total operating expenses
|
380,419
|
|
258,684
|
|
214,763
|
|
|||||
|
Loss from operations
|
(115,685
|
)
|
(64,292
|
)
|
(132,058
|
)
|
|||||
|
Interest expense, net
|
(12,554
|
)
|
(12,094
|
)
|
(8,276
|
)
|
|||||
|
Other income (expense), net
|
129
|
|
(1,279
|
)
|
(1,207
|
)
|
|||||
|
Loss before income tax expense
|
(128,110
|
)
|
(77,665
|
)
|
(141,541
|
)
|
|||||
|
Income tax benefit (expense)
|
29
|
|
(1,335
|
)
|
(569
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(128,081
|
)
|
$
|
(79,000
|
)
|
$
|
(142,110
|
)
|
||
|
Weighted-average shares outstanding:
|
|
|
|
|
|
|
|||||
|
Basic and diluted (in shares)
|
46,576,313
|
|
39,183,073
|
|
34,044,584
|
|
|||||
|
Net loss per share—basic and diluted (in dollars per share)
|
$
|
(2.75
|
)
|
$
|
(2.02
|
)
|
$
|
(4.17
|
)
|
||
|
|
Year ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Net loss
|
$
|
(128,081
|
)
|
$
|
(79,000
|
)
|
$
|
(142,110
|
)
|
||
|
Other comprehensive loss:
|
|
|
|
|
|
|
|||||
|
Unrealized gain on marketable securities, net of tax
|
9
|
|
225
|
|
386
|
|
|||||
|
Foreign currency translation (loss) gain
|
(2,516
|
)
|
5,229
|
|
(671
|
)
|
|||||
|
Comprehensive loss
|
$
|
(130,588
|
)
|
$
|
(73,546
|
)
|
$
|
(142,395
|
)
|
||
|
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
(loss) income
|
Accumulated
deficit
|
Total
stockholders’
equity
|
|||||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||||||
|
Balance, December 31, 2015
|
33,916,559
|
|
$
|
34
|
|
$
|
820,165
|
|
$
|
(1,200
|
)
|
$
|
(592,998
|
)
|
$
|
226,001
|
|
|||||
|
Exercise of options
|
89,216
|
|
—
|
|
968
|
|
—
|
|
—
|
|
968
|
|
||||||||||
|
Restricted stock vesting and issuance
|
163,635
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
35,009
|
|
—
|
|
—
|
|
35,009
|
|
||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(142,110
|
)
|
(142,110
|
)
|
||||||||||
|
Comprehensive loss
|
—
|
|
—
|
|
—
|
|
(285
|
)
|
—
|
|
(285
|
)
|
||||||||||
|
Balance, December 31, 2016
|
34,169,410
|
|
$
|
34
|
|
$
|
856,142
|
|
$
|
(1,485
|
)
|
$
|
(735,108
|
)
|
$
|
119,583
|
|
|||||
|
Issuance of common stock related to acquisition
|
6,683,598
|
|
7
|
|
75,184
|
|
—
|
|
—
|
|
75,191
|
|
||||||||||
|
Exercise of options
|
202,085
|
|
—
|
|
2,182
|
|
—
|
|
—
|
|
2,182
|
|
||||||||||
|
Restricted stock vesting and issuance
|
287,531
|
|
1
|
|
—
|
|
—
|
|
—
|
|
1
|
|
||||||||||
|
Issuance of common stock in connection with an employee stock purchase plan
|
269,771
|
|
—
|
|
2,467
|
|
—
|
|
—
|
|
2,467
|
|
||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
30,559
|
|
—
|
|
—
|
|
30,559
|
|
||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(79,000
|
)
|
(79,000
|
)
|
||||||||||
|
Comprehensive loss
|
—
|
|
—
|
|
—
|
|
5,454
|
|
—
|
|
5,454
|
|
||||||||||
|
Balance, December 31, 2017
|
41,612,395
|
|
$
|
42
|
|
$
|
966,534
|
|
$
|
3,969
|
|
$
|
(814,108
|
)
|
$
|
156,437
|
|
|||||
|
Adjustment to accumulated deficit
|
—
|
|
—
|
|
—
|
|
—
|
|
3,266
|
|
3,266
|
|
||||||||||
|
Issuance of common stock related to equity offering
|
4,600,000
|
|
5
|
|
117,911
|
|
—
|
|
—
|
|
117,916
|
|
||||||||||
|
Issuance of common stock related to acquisition
|
3,500,907
|
|
3
|
|
155,857
|
|
—
|
|
—
|
|
155,860
|
|
||||||||||
|
Exercise of options
|
633,973
|
|
1
|
|
10,867
|
|
—
|
|
—
|
|
10,868
|
|
||||||||||
|
Restricted stock vesting and issuance
|
119,691
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Issuance of common stock in connection with an employee stock purchase plan
|
139,181
|
|
—
|
|
2,787
|
|
—
|
|
—
|
|
2,787
|
|
||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
33,252
|
|
—
|
|
—
|
|
33,252
|
|
||||||||||
|
Receivable from investor
|
—
|
|
|
—
|
|
|
929
|
|
|
—
|
|
|
—
|
|
|
929
|
|
|||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(128,081
|
)
|
(128,081
|
)
|
||||||||||
|
Comprehensive loss
|
—
|
|
—
|
|
—
|
|
(2,507
|
)
|
—
|
|
(2,507
|
)
|
||||||||||
|
Balance, December 31, 2018
|
50,606,147
|
|
$
|
51
|
|
$
|
1,288,137
|
|
$
|
1,462
|
|
$
|
(938,923
|
)
|
$
|
350,727
|
|
|||||
|
|
Year ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Cash flows from operating activities
|
|
|
|
|
|
|
||||||
|
Net loss
|
$
|
(128,081
|
)
|
$
|
(79,000
|
)
|
$
|
(142,110
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
||||||
|
Depreciation and amortization
|
26,087
|
|
17,682
|
|
3,290
|
|
||||||
|
Change in valuation of warrant liability
|
—
|
|
—
|
|
(47
|
)
|
||||||
|
Change in valuation of deferred and contingent consideration
|
19,340
|
|
—
|
|
—
|
|
||||||
|
Amortization of (discounts) premiums on investments, net
|
(433
|
)
|
535
|
|
1,885
|
|
||||||
|
Amortization of debt issuance costs
|
524
|
|
433
|
|
302
|
|
||||||
|
Share-based compensation expense
|
33,252
|
|
30,559
|
|
35,009
|
|
||||||
|
Non-cash interest expense
|
7,518
|
|
6,755
|
|
6,065
|
|
||||||
|
Disposal of asset
|
2
|
|
5
|
|
—
|
|
||||||
|
Benefit for deferred income taxes
|
—
|
|
199
|
|
(199
|
)
|
||||||
|
Unrealized foreign currency transaction (gains) losses, net
|
(59
|
)
|
(459
|
)
|
1,202
|
|
||||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
||||||
|
Inventory, net
|
(5,823
|
)
|
(6,454
|
)
|
—
|
|
||||||
|
Prepaid expenses and other current assets
|
(1,609
|
)
|
(1,784
|
)
|
1,171
|
|
||||||
|
Trade receivables, net
|
(29,589
|
)
|
(12,203
|
)
|
(14,842
|
)
|
||||||
|
Deposits and other assets
|
(1,093
|
)
|
(544
|
)
|
(278
|
)
|
||||||
|
Accounts payable and accrued expenses
|
43,877
|
|
24,011
|
|
4,259
|
|
||||||
|
Other liabilities
|
1,932
|
|
733
|
|
(799
|
)
|
||||||
|
Deferred revenue
|
6,514
|
|
9,469
|
|
1,526
|
|
||||||
|
Net cash used in operating activities
|
(27,641
|
)
|
(10,063
|
)
|
(103,566
|
)
|
||||||
|
Cash flows from investing activities
|
|
|
|
|
|
|
||||||
|
Purchases of fixed assets
|
(7,097
|
)
|
(3,101
|
)
|
(1,776
|
)
|
||||||
|
Purchases of marketable securities
|
(68,614
|
)
|
(81,368
|
)
|
(85,377
|
)
|
||||||
|
Sale & redemption of marketable securities
|
90,423
|
|
174,749
|
|
191,634
|
|
||||||
|
Acquisition of product rights
|
(8,433
|
)
|
(77,163
|
)
|
—
|
|
||||||
|
Business acquisition, net of cash acquired
|
(48,892
|
)
|
—
|
|
—
|
|
||||||
|
Net cash (used in) provided by investing activities
|
(42,613
|
)
|
13,117
|
|
104,481
|
|
||||||
|
Cash flows from financing activities
|
|
|
|
|
|
|
||||||
|
Proceeds from exercise of options
|
10,868
|
|
2,182
|
|
968
|
|
||||||
|
Proceeds from shares issued under employee stock purchase plan
|
2,787
|
|
2,468
|
|
—
|
|
||||||
|
Debt issuance costs related to secured term loan
|
—
|
|
(432
|
)
|
—
|
|
||||||
|
Net proceeds from public offering
|
117,916
|
|
—
|
|
—
|
|
||||||
|
Proceeds from issuance of secured term loan
|
—
|
|
40,000
|
|
—
|
|
||||||
|
Net cash provided by financing activities
|
131,571
|
|
44,218
|
|
968
|
|
||||||
|
Effect of exchange rate changes on cash
|
(3,611
|
)
|
6,199
|
|
(1,584
|
)
|
||||||
|
Net increase in cash and cash equivalents
|
57,706
|
|
53,471
|
|
299
|
|
||||||
|
Cash and cash equivalents, beginning of period
|
111,792
|
|
58,321
|
|
58,022
|
|
||||||
|
Cash and cash equivalents, end of period
|
$
|
169,498
|
|
$
|
111,792
|
|
$
|
58,321
|
|
|||
|
Supplemental disclosure of cash information
|
|
|
|
|
|
|
||||||
|
Cash paid for interest
|
$
|
7,773
|
|
$
|
6,271
|
|
$
|
4,513
|
|
|||
|
Cash paid for income taxes
|
$
|
1,583
|
|
$
|
1,101
|
|
$
|
943
|
|
|||
|
Supplemental disclosures of non-cash information related to investing and financing activities
|
|
|
|
|
|
|
||||||
|
Change in unrealized gain on marketable securities, net of tax
|
$
|
9
|
|
$
|
225
|
|
$
|
386
|
|
|||
|
Acquisition of product rights and licenses
|
$
|
(5,981
|
)
|
$
|
—
|
|
$
|
—
|
|
|||
|
Leasehold improvements
|
Lesser of useful life or lease term
|
|
Computer equipment and software
|
3 years
|
|
Furniture, fixtures, and lab equipment
|
7 years
|
|
December 31, 2018
|
December 31, 2017
|
|||||||
|
Raw materials
|
$
|
1,431
|
|
$
|
452
|
|
||
|
Work in progress
|
9,324
|
|
3,912
|
|
||||
|
Finished goods
|
5,362
|
|
6,390
|
|
||||
|
Total inventory
|
$
|
16,117
|
|
$
|
10,754
|
|
||
|
•
|
Portfolio Approach - the Company applied the Portfolio Approach to contract reviews within its identified revenue streams that have similar characteristics and the Company believes this approach would not differ materially than if applying ASC Topic 606 to each individual contract.
|
|
•
|
Significant Financing Component - the Company expects the period between when it transfers a promised good to a customer and when the customer pays for the good or service to be one year or less.
|
|
•
|
Immaterial Performance Obligations - the Company disregards promises deemed to be immaterial in the context of the contract.
|
|
•
|
Shipping and Handling Activities - the Company considers any shipping and handling costs that are incurred after the customer has obtained control of the product as a cost to fulfill a promise.
|
|
•
|
Level 1—Unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the balance sheet date.
|
|
•
|
Level 2—Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets and liabilities in active markets, quoted prices
|
|
•
|
Level 3—Inputs are unobservable and reflect the Company’s assumptions as to what market participants would use in pricing the asset or liability. The Company develops these inputs based on the best information available.
|
|
Fair Value
|
|||
|
Cash consideration
|
$
|
49,221
|
|
|
Fair value of PTC common stock issued
|
155,860
|
|
|
|
Estimated fair value of deferred consideration payable
|
38,100
|
|
|
|
Estimated fair value of contingent consideration payable
|
290,500
|
|
|
|
Total consideration
|
$
|
533,681
|
|
|
|
Preliminary
Allocation as of the acquisition date of August 23, 2018 |
Measurement Period Adjustments
|
Final Allocation as of December 31, 2018
|
||||||||
|
Cash and cash equivalents
|
$
|
328
|
|
$
|
—
|
|
$
|
328
|
|
||
|
Prepaid expenses and other current assets
|
181
|
|
—
|
|
181
|
|
|||||
|
Fixed assets
|
153
|
|
—
|
|
153
|
|
|||||
|
Other assets
|
38
|
|
—
|
|
38
|
|
|||||
|
Intangible assets - IPR&D
|
480,000
|
|
96,500
|
|
576,500
|
|
|||||
|
Accounts payable and accrued expenses
|
(3,828
|
)
|
—
|
|
(3,828
|
)
|
|||||
|
Deferred tax liability
|
(115,200
|
)
|
(6,832
|
)
|
(122,032
|
)
|
|||||
|
Fair value of net assets acquired
|
$
|
361,672
|
|
$
|
89,668
|
|
$
|
451,340
|
|
||
|
Goodwill
|
100,309
|
|
(17,968
|
)
|
82,341
|
|
|||||
|
Total purchase price
|
$
|
461,981
|
|
$
|
71,700
|
|
$
|
533,681
|
|
||
|
Twelve Months Ended December 31,
|
||||||
|
2018
|
2017
|
|||||
|
Revenues
|
$
|
264,734
|
|
$
|
194,392
|
|
|
Net loss attributable to common stockholders
|
$
|
(138,083
|
)
|
$
|
(93,333
|
)
|
|
Cash consideration
|
$
|
75,000
|
|
|
|
Fair value of PTC common stock issued to Marathon (6,683,598 shares)
|
75,190
|
|
||
|
Acquisition costs
|
2,163
|
|
||
|
Total preliminary consideration transferred
|
$
|
152,353
|
|
|
|
Purchase price
|
$
|
152,353
|
|
|
|
Total fair value of tangible assets acquired and liabilities assumed:
|
||||
|
Inventory
|
3,980
|
|
||
|
Emflaza rights
|
$
|
148,373
|
|
|
|
|
December 31, 2018
|
|||||||||||||||
|
|
Total
|
Quoted prices
in active
markets for
identical assets
(level 1)
|
Significant
other
observable
inputs
(level 2)
|
Significant
unobservable
inputs
(level 3)
|
||||||||||||
|
Marketable securities
|
$
|
58,088
|
|
$
|
—
|
|
$
|
58,088
|
|
$
|
—
|
|
||||
|
Warrant liability
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Stock appreciation rights liability
|
$
|
3,814
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,814
|
|
||||
|
Deferred consideration payable
|
$
|
37,700
|
|
$
|
—
|
|
$
|
37,700
|
|
$
|
—
|
|
||||
|
Contingent consideration payable- development and regulatory milestones
|
$
|
257,040
|
|
$
|
—
|
|
$
|
—
|
|
$
|
257,040
|
|
||||
|
Contingent consideration payable- net sales milestones and royalties
|
$
|
53,200
|
|
$
|
—
|
|
$
|
—
|
|
$
|
53,200
|
|
||||
|
|
December 31, 2017
|
|||||||||||||||
|
|
Total
|
Quoted prices
in active
markets for
identical assets
(level 1)
|
Significant
other
observable
inputs
(level 2)
|
Significant
unobservable
inputs
(level 3)
|
||||||||||||
|
Marketable securities
|
$
|
79,454
|
|
$
|
—
|
|
$
|
79,454
|
|
$
|
—
|
|
||||
|
Warrant Liability
|
$
|
1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1
|
|
||||
|
Stock appreciation rights liability
|
$
|
1,665
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,665
|
|
||||
|
|
December 31, 2018
|
|||||||||||||||
|
|
Amortized
Cost
|
Gross
Unrealized
|
Fair
Value
|
|||||||||||||
|
|
||||||||||||||||
|
|
Gains
|
Losses
|
||||||||||||||
|
Commercial paper
|
$
|
31,657
|
|
$
|
43
|
|
$
|
(1
|
)
|
$
|
31,699
|
|
||||
|
Corporate debt securities
|
26,399
|
|
—
|
|
(10
|
)
|
26,389
|
|
||||||||
|
Total
|
$
|
58,056
|
|
$
|
43
|
|
$
|
(11
|
)
|
$
|
58,088
|
|
||||
|
|
December 31, 2017
|
|||||||||||||||
|
|
Amortized
Cost
|
Gross
Unrealized
|
Fair
Value
|
|||||||||||||
|
|
||||||||||||||||
|
|
Gains
|
Losses
|
||||||||||||||
|
Commercial paper
|
$
|
13,775
|
|
$
|
52
|
|
$
|
—
|
|
$
|
13,827
|
|
||||
|
Corporate debt securities
|
65,657
|
|
—
|
|
(30
|
)
|
65,627
|
|
||||||||
|
Total
|
$
|
79,432
|
|
$
|
52
|
|
$
|
(30
|
)
|
$
|
79,454
|
|
||||
|
December 31, 2018
|
||||||||||||||||||||||||
|
Securities in an unrealized loss position less than 12 months
|
Securities in an unrealized loss position greater than 12 months
|
Total
|
||||||||||||||||||||||
|
Unrealized losses
|
Fair Value
|
Unrealized losses
|
Fair Value
|
Unrealized losses
|
Fair Value
|
|||||||||||||||||||
|
Commercial Paper
|
$
|
(1
|
)
|
$
|
1,993
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(1
|
)
|
$
|
1,993
|
|
||||||
|
Corporate debt securities
|
(7
|
)
|
14,230
|
|
(3
|
)
|
10,087
|
|
$
|
(10
|
)
|
$
|
24,317
|
|
||||||||||
|
Total
|
$
|
(8
|
)
|
$
|
16,223
|
|
$
|
(3
|
)
|
$
|
10,087
|
|
$
|
(11
|
)
|
$
|
26,310
|
|
||||||
|
December 31, 2017
|
||||||||||||||||||||||||
|
Securities in an unrealized loss position less than 12 months
|
Securities in an unrealized loss position greater than 12 months
|
Total
|
||||||||||||||||||||||
|
Unrealized losses
|
Fair Value
|
Unrealized losses
|
Fair Value
|
Unrealized losses
|
Fair Value
|
|||||||||||||||||||
|
Corporate debt securities
|
$
|
(28
|
)
|
$
|
59,108
|
|
$
|
(2
|
)
|
$
|
6,519
|
|
$
|
(30
|
)
|
$
|
65,627
|
|
||||||
|
|
December 31, 2018
|
|||||||
|
|
Less Than
12 Months
|
More Than
12 Months
|
||||||
|
Commercial paper
|
$
|
31,699
|
|
$
|
—
|
|
||
|
Corporate debt securities
|
26,389
|
|
—
|
|
||||
|
Total Marketable securities
|
$
|
58,088
|
|
$
|
—
|
|
||
|
|
December 31, 2017
|
|||||||
|
|
Less Than
12 Months
|
More Than
12 Months
|
||||||
|
Commercial paper
|
$
|
13,827
|
|
$
|
—
|
|
||
|
Corporate debt securities
|
55,550
|
|
10,077
|
|
||||
|
Total Marketable securities
|
$
|
69,377
|
|
$
|
10,077
|
|
||
|
|
Level 3 liabilities
|
|||||||||||||||
|
Warrants
|
SARs
|
Contingent consideration payable- development and regulatory milestones
|
Contingent consideration payable- net sales milestones and royalties
|
|||||||||||||
|
Beginning balance as of December 31, 2016
|
$
|
1
|
|
$
|
865
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Change in fair value
|
—
|
|
1,864
|
|
—
|
|
—
|
|
||||||||
|
Payments
|
—
|
|
(1,064
|
)
|
—
|
|
—
|
|
||||||||
|
Ending balance as of December 31, 2017
|
$
|
1
|
|
$
|
1,665
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Additions
|
—
|
|
—
|
|
263,500
|
|
27,000
|
|
||||||||
|
Change in fair value
|
(1
|
)
|
4,140
|
|
(6,460
|
)
|
26,200
|
|
||||||||
|
Payments
|
—
|
|
(1,991
|
)
|
—
|
|
—
|
|
||||||||
|
Ending balance as of December 31, 2018
|
$
|
—
|
|
$
|
3,814
|
|
$
|
257,040
|
|
$
|
53,200
|
|
||||
|
December 31, 2018
|
||||||||
|
|
Fair Value
|
Valuation Technique
|
Unobservable Input
|
Range
|
||||
|
Warrants
|
—
|
Option-pricing model
|
Volatility
|
59.39% - 60.48%
|
||||
|
Risk free interest rate
|
2.6%
|
|||||||
|
Strike price
|
$128.00 - $2,520.00
|
|||||||
|
Fair value of common stock
|
$34.32
|
|||||||
|
Expected life
|
0.59 - 0.72 years
|
|||||||
|
SARs
|
$3,814
|
Option-pricing model
|
Volatility
|
46.53% - 59.59%
|
||||
|
Risk free interest rate
|
2.44% - 2.63%
|
|||||||
|
Strike price
|
$6.76 - $30.86
|
|||||||
|
Fair value of common stock
|
$34.32
|
|||||||
|
Expected life
|
0.01 - 1.01 years
|
|||||||
|
Contingent consideration payable- development and regulatory milestones
|
$257,040
|
Probability-adjusted discounted cash flow
|
Potential development and regulatory milestones
|
$0 - $555 million
|
||||
|
Probabilities of success
|
25% - 94%
|
|||||||
|
Discount rates
|
5.8% - 8.0%
|
|||||||
|
Projected years of payments
|
2020 - 2026
|
|||||||
|
Contingent considerable payable- net sales milestones and royalties
|
$53,200
|
Option-pricing model with Monte Carlo simulation
|
Potential net sales milestones
|
$0 - $150 million
|
||||
|
Probabilities of success
|
25% - 89%
|
|||||||
|
Potential percentage of net sales for royalties
|
2% - 6%
|
|||||||
|
Discount rate
|
14.0%
|
|||||||
|
Projected years of payments
|
2021 - 2038
|
|||||||
|
December 31, 2017
|
||||||||
|
Fair Value
|
Valuation Technique
|
Unobservable Input
|
Range
|
|||||
|
Warrants
|
$1
|
Option-pricing model
|
Volatility
|
69%
|
||||
|
Risk free interest rate
|
$1.89
|
|||||||
|
Strike price
|
$128.00 - $2,520.00
|
|||||||
|
Fair value of common stock
|
$16.68
|
|||||||
|
Expected life
|
1.60 - 1.70 years
|
|||||||
|
SARs
|
$1,665
|
Option-pricing model
|
Volatility
|
31% - 70%
|
||||
|
Risk free interest rate
|
1.28% - 1.89%
|
|||||||
|
Strike price
|
$6.76 - $30.86
|
|||||||
|
Fair value of common stock
|
$16.68
|
|||||||
|
Expected life
|
0.00 - 2.00 years
|
|||||||
|
|
December 31,
|
|||||||
|
|
2018
|
2017
|
||||||
|
Leasehold improvements
|
$
|
2,384
|
|
$
|
14,078
|
|
||
|
Computer equipment and software
|
4,609
|
|
5,471
|
|
||||
|
Furniture, fixtures, and lab equipment
|
9,965
|
|
20,776
|
|
||||
|
Assets in process
|
3,219
|
|
895
|
|
||||
|
20,177
|
|
41,220
|
|
|||||
|
Less accumulated depreciation and amortization
|
(7,483
|
)
|
(32,844
|
)
|
||||
|
Total
|
$
|
12,694
|
|
$
|
8,376
|
|
||
|
|
December 31,
|
|||||||
|
|
2018
|
2017
|
||||||
|
Employee compensation, benefits, and related accruals
|
$
|
27,629
|
|
$
|
17,711
|
|
||
|
Consulting and contracted research
|
11,267
|
|
5,137
|
|
||||
|
Professional fees
|
5,574
|
|
2,116
|
|
||||
|
Sales allowances and other related costs
|
29,417
|
|
22,257
|
|
||||
|
Royalties and rebates
|
31,874
|
|
11,657
|
|
||||
|
Accounts payable
|
6,001
|
|
15,282
|
|
||||
|
Other
|
16,437
|
|
2,286
|
|
||||
|
Total
|
$
|
128,199
|
|
$
|
76,446
|
|
||
|
•
|
during any calendar quarter commencing on or after September 30, 2015 (and only during such calendar quarter), if the last reported sale price of the Company’s common stock for at least
20
trading days (whether or not consecutive)
|
|
•
|
during the
five
business day period after any
five
consecutive trading day period (the “measurement period”) in which the trading price (as defined in the Convertible Notes Indenture) per
$1,000
principal amount of Convertible Notes for each trading day of the measurement period was less than
98%
of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such trading day;
|
|
•
|
during any period after the Company has issued notice of redemption until the close of business on the scheduled trading day immediately preceding the relevant redemption date; or
|
|
•
|
upon the occurrence of specified corporate events.
|
|
|
Year ended
December 31, |
|||||||
|
Liability component
|
2018
|
2017
|
||||||
|
Principal
|
$
|
150,000
|
|
$
|
150,000
|
|
||
|
Less: Debt issuance costs
|
(1,746
|
)
|
(2,121
|
)
|
||||
|
Less: Debt discount, net (1)
|
(35,054
|
)
|
(42,572
|
)
|
||||
|
Net carrying amount
|
$
|
113,200
|
|
$
|
105,307
|
|
||
|
(1)
|
Included in the consolidated balance sheets within convertible senior notes (due 2022) and amortized to interest expense over the remaining life of the Convertible Notes using the effective interest rate method.
|
|
|
Year ended
December 31, |
|||||||
|
|
2018
|
2017
|
||||||
|
Contractual interest expense
|
$
|
4,500
|
|
$
|
4,500
|
|
||
|
Amortization of debt issuance costs
|
375
|
|
337
|
|
||||
|
Amortization of debt discount
|
7,518
|
|
6,755
|
|
||||
|
Total
|
$
|
12,393
|
|
$
|
11,592
|
|
||
|
Effective interest rate of the liability component
|
11.0
|
%
|
11.0
|
%
|
||||
|
Warrant shares
|
Exercise price
|
Expiration
|
|||||||
|
Common stock
|
7,030
|
|
$
|
128.00
|
|
2019
|
|||
|
Common stock
|
130
|
|
$
|
2,520.00
|
|
2019
|
|||
|
Warrant shares
|
Exercise price
|
Expiration
|
|||||||
|
Common stock
|
7,030
|
|
$
|
128.00
|
|
2019
|
|||
|
Common stock
|
130
|
|
$
|
2,520.00
|
|
2019
|
|||
|
|
Year ended December 31,
|
||||||||||||
|
|
2018
|
2017
|
2016
|
||||||||||
|
Numerator
|
|
|
|
|
|
|
|||||||
|
Net loss
|
$
|
(128,081
|
)
|
$
|
(79,000
|
)
|
$
|
(142,110
|
)
|
||||
|
Denominator
|
|
|
|
|
|
|
|||||||
|
Denominator for basic and diluted net loss per share
|
46,576,313
|
|
39,183,073
|
|
34,044,584
|
|
|||||||
|
Net loss per share:
|
|
|
|
|
|
|
|||||||
|
Basic and diluted
|
$
|
(2.75
|
)
|
*
|
$
|
(2.02
|
)
|
*
|
$
|
(4.17
|
)
|
*
|
|
|
|
As of December 31,
|
||||||||
|
|
2018
|
2017
|
2016
|
||||||
|
Stock Options
|
8,534,358
|
|
6,448,642
|
|
5,854,316
|
|
|||
|
Unvested restricted stock
|
571,479
|
|
393,011
|
|
271,651
|
|
|||
|
Total
|
9,105,837
|
|
6,841,653
|
|
6,125,967
|
|
|||
|
|
Number of
options
|
Weighted-
average
exercise
price
|
Weighted-
average
remaining
contractual
term
|
Aggregate
intrinsic
value
|
|||||||||
|
|
(in thousands)
|
||||||||||||
|
Outstanding at December 31, 2015
|
4,826,477
|
|
$
|
37.20
|
|
|
|
|
|||||
|
Granted
|
1,500,645
|
|
$
|
27.90
|
|
|
|
|
|||||
|
Exercised
|
(89,216
|
)
|
$
|
10.85
|
|
|
|
|
|||||
|
Forfeited
|
(383,590
|
)
|
$
|
47.42
|
|
|
|
|
|||||
|
Outstanding at December 31, 2016
|
5,854,316
|
|
$
|
34.71
|
|
|
|
|
|||||
|
Granted
|
1,913,873
|
|
$
|
12.34
|
|
|
|
|
|||||
|
Exercised
|
(202,085
|
)
|
$
|
10.80
|
|
|
|
|
|||||
|
Forfeited
|
(1,117,462
|
)
|
$
|
33.65
|
|
|
|
|
|||||
|
Outstanding at December 31, 2017
|
6,448,642
|
|
$
|
29.00
|
|
|
|
|
|||||
|
Granted
|
3,181,623
|
|
$
|
26.64
|
|
|
|
|
|||||
|
Exercised
|
(633,973
|
)
|
$
|
18.61
|
|
|
|
|
|||||
|
Forfeited
|
(461,934
|
)
|
$
|
35.36
|
|
|
|
|
|||||
|
Outstanding at December 31, 2018
|
8,534,358
|
|
$
|
28.58
|
|
7.37
|
$
|
88,454
|
|
||||
|
Vested or expected to vest at December 31, 2018
|
3,881,360
|
|
$
|
24.81
|
|
8.79
|
$
|
44,893
|
|
||||
|
Exercisable at December 31, 2018
|
4,410,211
|
|
$
|
32.01
|
|
6.02
|
$
|
40,854
|
|
||||
|
2018
|
2017
|
2016
|
||||
|
Risk-free interest rate
|
2.25% - 3.10%
|
1.84% - 2.45%
|
1.30% - 2.24%
|
|||
|
Expected volatility
|
64% - 90%
|
76% - 81%
|
67% - 78%
|
|||
|
Expected term
|
5.03 - 10.00 years
|
5.04 - 10.00 years
|
5.05 - 10.00 years
|
|||
|
|
Restricted Stock Awards and Units
|
||||||
|
|
Number of
Shares
|
Weighted
Average
Grant
Date Fair
Value
|
|||||
|
Unvested at December 31, 2017
|
393,011
|
|
$
|
15.64
|
|
||
|
Granted
|
354,691
|
|
$
|
19.09
|
|
||
|
Vested
|
(114,295
|
)
|
$
|
16.36
|
|
||
|
Forfeited
|
(61,928
|
)
|
$
|
17.24
|
|
||
|
Unvested at December 31, 2018
|
571,479
|
|
$
|
17.61
|
|
||
|
|
Year ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Research and development
|
$
|
16,096
|
|
$
|
15,456
|
|
$
|
16,812
|
|
|||
|
Selling, general and administrative
|
17,156
|
|
15,103
|
|
18,197
|
|
||||||
|
Total
|
$
|
33,252
|
|
$
|
30,559
|
|
$
|
35,009
|
|
|||
|
|
Unrealized
(Losses)/Gains
On
Marketable
Securities, net of tax
|
Foreign
Currency
Translation
|
Total
Accumulated
Other
Comprehensive
Items
|
|||||||||
|
Balance at December 31, 2015
|
$
|
(589
|
)
|
$
|
(611
|
)
|
$
|
(1,200
|
)
|
|||
|
Other comprehensive income (loss) before reclassifications
|
386
|
|
(671
|
)
|
(285
|
)
|
||||||
|
Amounts reclassified from other comprehensive items
|
—
|
|
—
|
|
—
|
|
||||||
|
Other comprehensive income (loss)
|
386
|
|
(671
|
)
|
(285
|
)
|
||||||
|
Balance at December 31, 2016
|
$
|
(203
|
)
|
$
|
(1,282
|
)
|
$
|
(1,485
|
)
|
|||
|
Other comprehensive income before reclassifications
|
225
|
|
5,229
|
|
5,454
|
|
||||||
|
Amounts reclassified from other comprehensive items
|
—
|
|
—
|
|
—
|
|
||||||
|
Other comprehensive income
|
225
|
|
5,229
|
|
5,454
|
|
||||||
|
Balance at December 31, 2017
|
$
|
22
|
|
$
|
3,947
|
|
$
|
3,969
|
|
|||
|
Other comprehensive income (loss) before reclassifications
|
9
|
|
(2,516
|
)
|
(2,507
|
)
|
||||||
|
Amounts reclassified from other comprehensive items
|
—
|
|
—
|
|
—
|
|
||||||
|
Other comprehensive income (loss)
|
9
|
|
(2,516
|
)
|
(2,507
|
)
|
||||||
|
Balance at December 31, 2018
|
$
|
31
|
|
$
|
1,431
|
|
$
|
1,462
|
|
|||
|
Balance as of
December 31, 2017 |
Additions
|
Deductions
|
ASC 606 Adjustment
|
Balance as of
December 31, 2018 |
||||||||||||||||
|
Deferred Revenue
|
$
|
11,891
|
|
$
|
6,417
|
|
$
|
(1,433
|
)
|
$
|
(3,937
|
)
|
$
|
12,938
|
|
|||||
|
December 31,
2018 |
||||
|
Amounts included in contract liabilities at the beginning of the period
|
$
|
—
|
|
|
|
Performance obligations satisfied in previous period
|
—
|
|
||
|
Performance obligations satisfied in current period
|
263,005
|
|
||
|
Total product revenue
|
$
|
263,005
|
|
|
|
Impact of changes in accounting policies
|
||||||||||||
|
|
As reported December 31,
2018 |
Adjustments
|
As reported Balances without adoption of Topic 606
|
|||||||||
|
Assets
|
|
|
|
|||||||||
|
Current assets:
|
|
|
|
|||||||||
|
Cash and cash equivalents
|
$
|
169,498
|
|
$
|
—
|
|
$
|
169,498
|
|
|||
|
Marketable securities
|
58,088
|
|
—
|
|
58,088
|
|
||||||
|
Trade receivables, net
|
67,907
|
|
—
|
|
67,907
|
|
||||||
|
Inventory
|
16,117
|
|
(84
|
)
|
16,033
|
|
||||||
|
Prepaid expenses and other current assets
|
9,247
|
|
—
|
|
9,247
|
|
||||||
|
Total current assets
|
320,857
|
|
(84
|
)
|
320,773
|
|
||||||
|
Fixed assets, net
|
12,694
|
|
—
|
|
12,694
|
|
||||||
|
Intangible assets, net
|
701,031
|
|
—
|
|
701,031
|
|
||||||
|
Goodwill
|
82,341
|
|
—
|
|
82,341
|
|
||||||
|
Deposits and other assets
|
2,299
|
|
—
|
|
2,299
|
|
||||||
|
Total assets
|
$
|
1,119,222
|
|
$
|
(84
|
)
|
$
|
1,119,138
|
|
|||
|
Liabilities and stockholders’ equity
|
|
|
|
|||||||||
|
Current liabilities:
|
|
|
|
|||||||||
|
Accounts payable and accrued expenses
|
$
|
128,199
|
|
$
|
(2,120
|
)
|
$
|
126,079
|
|
|||
|
Current portion of long-term debt
|
11,667
|
|
—
|
|
11,667
|
|
||||||
|
Deferred revenue
|
3,716
|
|
10,563
|
|
14,279
|
|
||||||
|
Other current liabilities
|
3,814
|
|
—
|
|
3,814
|
|
||||||
|
Deferred consideration payable- current
|
19,400
|
|
—
|
|
19,400
|
|
||||||
|
Total current liabilities
|
166,796
|
|
8,443
|
|
175,239
|
|
||||||
|
Deferred revenue - long-term
|
9,722
|
|
—
|
|
9,722
|
|
||||||
|
Long-term debt
|
141,347
|
|
—
|
|
141,347
|
|
||||||
|
Contingent consideration payable
|
310,240
|
|
—
|
|
310,240
|
|
||||||
|
Deferred consideration payable - long-term
|
18,300
|
|
—
|
|
18,300
|
|
||||||
|
Deferred tax liability
|
122,032
|
|
—
|
|
122,032
|
|
||||||
|
Other long-term liabilities
|
58
|
|
—
|
|
58
|
|
||||||
|
Total liabilities
|
768,495
|
|
8,443
|
|
776,938
|
|
||||||
|
Stockholders’ equity:
|
|
|
|
|||||||||
|
Common stock
|
51
|
|
—
|
|
51
|
|
||||||
|
Additional paid-in capital
|
1,288,137
|
|
—
|
|
1,288,137
|
|
||||||
|
Accumulated other comprehensive income
|
1,462
|
|
—
|
|
1,462
|
|
||||||
|
Accumulated deficit
|
(938,923
|
)
|
(8,527
|
)
|
(947,450
|
)
|
||||||
|
Total stockholders’ equity
|
350,727
|
|
(8,527
|
)
|
342,200
|
|
||||||
|
Total liabilities and stockholders’ equity
|
$
|
1,119,222
|
|
$
|
(84
|
)
|
$
|
1,119,138
|
|
|||
|
|
Impact of changes in accounting policies
Twelve Months Ended
|
|||||||||||
|
|
As reported for the period ended December 31,
2018 |
Adjustments
|
As reported Balances without adoption of Topic 606
|
|||||||||
|
Revenues:
|
|
|
|
|
||||||||
|
Net product revenue
|
$
|
263,005
|
|
$
|
(5,177
|
)
|
$
|
257,828
|
|
|||
|
Collaboration and grant revenue
|
1,729
|
|
—
|
|
1,729
|
|
||||||
|
Total revenues
|
264,734
|
|
(5,177
|
)
|
259,557
|
|
||||||
|
Operating expenses:
|
|
|
|
|
||||||||
|
Cost of product sales, excluding amortization of acquired intangible asset
|
12,670
|
|
(84
|
)
|
12,586
|
|
||||||
|
Amortization of acquired intangible asset
|
22,877
|
|
—
|
|
22,877
|
|
||||||
|
Research and development
|
171,984
|
|
—
|
|
171,984
|
|
||||||
|
Selling, general and administrative
|
153,548
|
|
—
|
|
153,548
|
|
||||||
|
Change in the fair value of deferred and contingent consideration
|
19,340
|
|
—
|
|
19,340
|
|
||||||
|
Total operating expenses
|
380,419
|
|
(84
|
)
|
380,335
|
|
||||||
|
Loss from operations
|
(115,685
|
)
|
(5,093
|
)
|
(120,778
|
)
|
||||||
|
Interest expense, net
|
(12,554
|
)
|
—
|
|
(12,554
|
)
|
||||||
|
Other income, net
|
129
|
|
—
|
|
129
|
|
||||||
|
Loss before income tax expense
|
(128,110
|
)
|
(5,093
|
)
|
(133,203
|
)
|
||||||
|
Income tax benefit
|
29
|
|
—
|
|
29
|
|
||||||
|
Net loss attributable to common stockholders
|
$
|
(128,081
|
)
|
$
|
(5,093
|
)
|
$
|
(133,174
|
)
|
|||
|
|
Impact of changes in accounting policies
Twelve Months Ended
|
|||||||||||
|
|
As reported for the period ended December 31,
2018 |
Adjustments
|
As reported Balances without adoption of Topic 606
|
|||||||||
|
Net loss
|
$
|
(128,081
|
)
|
$
|
(5,093
|
)
|
$
|
(133,174
|
)
|
|||
|
Other comprehensive loss:
|
|
|
|
|
||||||||
|
Unrealized gain on marketable securities, net of tax
|
9
|
|
—
|
|
9
|
|
||||||
|
Foreign currency translation loss
|
(2,516
|
)
|
—
|
|
(2,516
|
)
|
||||||
|
Comprehensive loss
|
$
|
(130,588
|
)
|
$
|
(5,093
|
)
|
$
|
(135,681
|
)
|
|||
|
|
Impact of changes in accounting policies
|
|||||||||||
|
As reported for the period ended December 31,
2018 |
Adjustments
|
Balances without adoption of Topic 606
|
||||||||||
|
Cash flows from operating activities
|
|
|
|
|
||||||||
|
Net loss
|
$
|
(128,081
|
)
|
$
|
(5,093
|
)
|
$
|
(133,174
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
||||||||
|
Depreciation and amortization
|
26,087
|
|
—
|
|
26,087
|
|
||||||
|
Change in valuation of deferred and contingent consideration
|
19,340
|
|
—
|
|
19,340
|
|
||||||
|
Amortization of premiums on investments
|
(433
|
)
|
—
|
|
(433
|
)
|
||||||
|
Amortization of debt issuance costs
|
524
|
|
—
|
|
524
|
|
||||||
|
Share-based compensation expense
|
33,252
|
|
—
|
|
33,252
|
|
||||||
|
Non-cash interest expense
|
7,518
|
|
—
|
|
7,518
|
|
||||||
|
Disposal of asset
|
2
|
|
—
|
|
2
|
|
||||||
|
Unrealized foreign currency transaction (gains) losses, net
|
(59
|
)
|
—
|
|
(59
|
)
|
||||||
|
Changes in operating assets and liabilities:
|
0
|
|
||||||||||
|
Inventory, net
|
(5,823
|
)
|
(84
|
)
|
(5,907
|
)
|
||||||
|
Prepaid expenses and other current assets
|
(1,609
|
)
|
—
|
|
(1,609
|
)
|
||||||
|
Trade receivables, net
|
(29,589
|
)
|
—
|
|
(29,589
|
)
|
||||||
|
Deposits and other assets
|
(1,093
|
)
|
—
|
|
(1,093
|
)
|
||||||
|
Accounts payable and accrued expenses
|
43,877
|
|
(2,120
|
)
|
41,757
|
|
||||||
|
Other long-term liabilities
|
1,932
|
|
—
|
|
1,932
|
|
||||||
|
Deferred revenue
|
6,514
|
|
7,297
|
|
13,811
|
|
||||||
|
Net cash used in operating activities
|
(27,641
|
)
|
—
|
|
(27,641
|
)
|
||||||
|
Cash flows from investing activities
|
|
|
|
|
||||||||
|
Purchases of fixed assets
|
(7,097
|
)
|
—
|
|
(7,097
|
)
|
||||||
|
Purchases of marketable securities
|
(68,614
|
)
|
—
|
|
(68,614
|
)
|
||||||
|
Sale & redemption of marketable securities
|
90,423
|
|
—
|
|
90,423
|
|
||||||
|
Acquisition of product rights
|
(8,433
|
)
|
—
|
|
(8,433
|
)
|
||||||
|
Business acquisition, net of cash acquired
|
(48,892
|
)
|
—
|
|
(48,892
|
)
|
||||||
|
Net cash used in investing activities
|
(42,613
|
)
|
—
|
|
(42,613
|
)
|
||||||
|
Cash flows from financing activities
|
|
|
|
|
||||||||
|
Proceeds from exercise of options
|
10,868
|
|
—
|
|
10,868
|
|
||||||
|
Proceeds from shares issued under employee stock purchase plan
|
2,787
|
|
—
|
|
2,787
|
|
||||||
|
Net proceeds from public offerings
|
117,916
|
|
—
|
|
117,916
|
|
||||||
|
Net cash provided by financing activities
|
131,571
|
|
—
|
|
131,571
|
|
||||||
|
Effect of exchange rate changes on cash
|
(3,611
|
)
|
—
|
|
(3,611
|
)
|
||||||
|
Net increase in cash and cash equivalents
|
57,706
|
|
—
|
|
57,706
|
|
||||||
|
Cash and cash equivalents, beginning of period
|
111,792
|
|
—
|
|
111,792
|
|
||||||
|
Cash and cash equivalents, end of period
|
$
|
169,498
|
|
$
|
—
|
|
$
|
169,498
|
|
|||
|
2018
|
2017
|
2016
|
|||||||
|
Domestic
|
(68,461
|
)
|
(54,588
|
)
|
(61,446
|
)
|
|||
|
Foreign
|
(59,649
|
)
|
(23,077
|
)
|
(80,095
|
)
|
|||
|
Total
|
(128,110
|
)
|
(77,665
|
)
|
(141,541
|
)
|
|||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
|
|
|
|
||||||||
|
U.S. Federal
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S. State and Local
|
(38
|
)
|
(6
|
)
|
(2
|
)
|
||||||
|
Foreign
|
(669
|
)
|
(1,131
|
)
|
(766
|
)
|
||||||
|
Deferred:
|
||||||||||||
|
U.S. Federal
|
—
|
|
(198
|
)
|
199
|
|
||||||
|
U.S. State and Local
|
—
|
|
—
|
|
—
|
|
||||||
|
Foreign
|
736
|
|
—
|
|
—
|
|
||||||
|
Total tax benefit (expense)
|
$
|
29
|
|
$
|
(1,335
|
)
|
$
|
(569
|
)
|
|||
|
|
December 31,
|
||||||||
|
|
2018
|
2017
|
2016
|
||||||
|
Federal income tax provision at statutory rate
|
21.00
|
%
|
34.00
|
%
|
34.00
|
%
|
|||
|
State income tax provision, net of federal benefit
|
0.05
|
|
(1.01
|
)
|
3.05
|
|
|||
|
Permanent differences
|
(6.41
|
)
|
(8.48
|
)
|
(3.70
|
)
|
|||
|
Research and development
|
6.49
|
|
19.53
|
|
16.66
|
|
|||
|
Change in valuation allowances
|
2.20
|
|
29.10
|
|
(30.72
|
)
|
|||
|
Change in deferred tax assets
|
(14.22
|
)
|
(64.12
|
)
|
—
|
|
|||
|
Foreign tax rate differential
|
(9.10
|
)
|
(10.33
|
)
|
(19.84
|
)
|
|||
|
Benefit allocated from other comprehensive income
|
—
|
|
(0.26
|
)
|
0.14
|
|
|||
|
Other
|
0.01
|
|
(0.15
|
)
|
0.01
|
|
|||
|
Effective income tax rate
|
0.02
|
%
|
(1.72
|
)%
|
(0.40
|
)%
|
|||
|
2018
|
2017
|
|||||||
|
Assets:
|
||||||||
|
Noncurrent deferred income taxes
|
$
|
736
|
|
$
|
—
|
|
||
|
Liabilities:
|
||||||||
|
Noncurrent deferred income taxes
|
(122,032
|
)
|
—
|
|
||||
|
Deferred income taxes - net
|
$
|
(121,296
|
)
|
$
|
—
|
|
||
|
|
2018
|
2017
|
||||||
|
Deferred tax assets:
|
|
|
|
|
||||
|
Accrued expense
|
$
|
714
|
|
$
|
625
|
|
||
|
Amortization
|
5,148
|
|
2,116
|
|
||||
|
Depreciation
|
1,601
|
|
1,749
|
|
||||
|
Federal tax credits
|
89,070
|
|
80,961
|
|
||||
|
State tax credits
|
5,473
|
|
7,148
|
|
||||
|
Federal net operating losses
|
62,159
|
|
61,068
|
|
||||
|
State net operating losses
|
165
|
|
11,884
|
|
||||
|
Foreign net operating losses
|
736
|
|
—
|
|
||||
|
Capitalized research and development costs
|
2,093
|
|
3,332
|
|
||||
|
Share based compensation and other
|
21,411
|
|
18,815
|
|
||||
|
Total gross deferred tax assets
|
188,570
|
|
187,698
|
|
||||
|
Less valuation allowance
|
(180,481
|
)
|
(177,631
|
)
|
||||
|
Total deferred tax assets, net of valuation allowance
|
$
|
8,089
|
|
$
|
10,067
|
|
||
|
Deferred tax liabilities:
|
|
|
|
|
||||
|
Convertible debt
|
$
|
(7,353
|
)
|
$
|
(9,927
|
)
|
||
|
OCI unrealized (gains)/losses
|
—
|
|
(140
|
)
|
||||
|
Indefinite lived intangible
|
(122,032
|
)
|
—
|
|
||||
|
Total gross deferred tax liabilities
|
(129,385
|
)
|
(10,067
|
)
|
||||
|
Net deferred tax assets (liabilities)
|
$
|
(121,296
|
)
|
$
|
—
|
|
||
|
2019
|
$
|
3,216
|
|
|
|
2020
|
2,900
|
|
||
|
2021
|
2,409
|
|
||
|
2022
|
2,082
|
|
||
|
2023 and thereafter
|
3,321
|
|
||
|
Total
|
$
|
13,928
|
|
|
|
|
Year Ended December 31, 2018
|
|||||||||||
|
|
United States
|
Non-US
|
Total
|
|||||||||
|
Total assets
|
$
|
1,013,977
|
|
$
|
105,245
|
|
$
|
1,119,222
|
|
|||
|
Property and equipment, net
|
$
|
10,497
|
|
$
|
2,197
|
|
$
|
12,694
|
|
|||
|
Revenue
|
$
|
93,694
|
|
$
|
171,040
|
|
$
|
264,734
|
|
|||
|
|
Year Ended December 31, 2017
|
|||||||||||
|
|
United States
|
Non-US
|
Total
|
|||||||||
|
Total assets
|
$
|
278,108
|
|
$
|
113,545
|
|
$
|
391,653
|
|
|||
|
Property and equipment, net
|
$
|
6,272
|
|
$
|
2,104
|
|
$
|
8,376
|
|
|||
|
Revenue
|
$
|
49,155
|
|
$
|
145,237
|
|
$
|
194,392
|
|
|||
|
As of December 31, 2018
|
||||
|
2019
|
$
|
24,283
|
|
|
|
2020
|
24,276
|
|
||
|
2021
|
24,277
|
|
||
|
2022
|
24,277
|
|
||
|
2023 and thereafter
|
27,418
|
|
||
|
Total
|
$
|
124,531
|
|
|
|
|
For the quarters ending
|
|||||||||||||||
|
|
March 31
|
June 30
|
September 30
|
December 31
|
||||||||||||
|
2018:
|
|
|
|
|
|
|
|
|
||||||||
|
Net product revenue
|
$
|
55,981
|
|
$
|
68,170
|
|
$
|
53,021
|
|
$
|
85,833
|
|
||||
|
Collaboration and grant revenue
|
81
|
|
573
|
|
570
|
|
505
|
|
||||||||
|
Operating expenses
|
72,805
|
|
74,317
|
|
101,821
|
|
131,476
|
|
||||||||
|
Loss from operations
|
(16,743
|
)
|
(5,574
|
)
|
(48,230
|
)
|
(45,138
|
)
|
||||||||
|
Net loss
|
(19,263
|
)
|
(9,520
|
)
|
(50,969
|
)
|
(48,330
|
)
|
||||||||
|
Basic and diluted net loss per common share(1)
|
$
|
(0.46
|
)
|
$
|
(0.21
|
)
|
$
|
(1.06
|
)
|
$
|
(0.96
|
)
|
||||
|
2017:
|
|
|
|
|
|
|
|
|
||||||||
|
Net product revenue
|
$
|
26,442
|
|
$
|
47,891
|
|
$
|
41,780
|
|
$
|
57,953
|
|
||||
|
Collaboration and grant revenue
|
105
|
|
71
|
|
73
|
|
20,077
|
|
||||||||
|
Operating expenses
|
52,902
|
|
60,459
|
|
72,745
|
|
72,578
|
|
||||||||
|
(Loss) income from operations
|
(26,355
|
)
|
(12,497
|
)
|
(30,892
|
)
|
5,452
|
|
||||||||
|
Net (loss) income
|
(29,057
|
)
|
(17,475
|
)
|
(33,738
|
)
|
1,270
|
|
||||||||
|
Basic and diluted net (loss) income per common share(1)(2)
|
$
|
(0.85
|
)
|
$
|
(0.44
|
)
|
$
|
(0.82
|
)
|
$
|
0.03
|
|
||||
|
(1)
|
The amounts were computed independently for each quarter and the sum of the quarters may not total the annual amounts.
|
|
(2)
|
Diluted net income per common share for the quarter ending December 31, 2017 excludes the conversion of the Convertible Notes as the effect of their inclusion is anti-dilutive during the period.
|
|
•
|
Reports of independent registered public accounting firm
|
|
•
|
Consolidated Balance Sheets as of December 31, 2018 and 2017
|
|
•
|
Consolidated Statements of Operations for the years ended December 31, 2018, 2017 and 2016
|
|
•
|
Consolidated Statements of Comprehensive Loss for the years ended December 31, 2018, 2017 and 2016
|
|
•
|
Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2018, 2017 and 2016
|
|
•
|
Consolidated Statements of Cash Flows for the years ended December 31, 2018, 2017 and 2016
|
|
•
|
Notes to Consolidated Financial Statements
|
|
Exhibit
Number
|
|
Description of Exhibit
|
|
|
2.1†
|
|
||
|
2.2
|
|
||
|
2.3†
|
|
||
|
3.1
|
|
|
|
|
|
|
|
|
|
3.2
|
|
|
|
|
|
|
|
|
|
4.1
|
|
|
|
|
|
|
|
|
|
10.1+
|
|
||
|
|
|
|
|
|
10.2+
|
|
||
|
|
|
|
|
|
10.3+
|
|
||
|
|
|
|
|
|
10.4+
|
|
||
|
|
|
|
|
|
Exhibit
Number
|
|
Description of Exhibit
|
|
|
10.5+
|
|
||
|
|
|
|
|
|
10.6+
|
|
||
|
10.7+
|
|
||
|
|
|
|
|
|
10.8+
|
|
||
|
|
|||
|
10.9+
|
|
||
|
|
|
|
|
|
10.10+
|
|
||
|
|
|
|
|
|
10.11+
|
|
||
|
|
|
|
|
|
10.12+
|
|
||
|
|
|
|
|
|
10.13+
|
|
||
|
|
|
|
|
|
10.14+
|
|
||
|
|
|
|
|
|
10.15+
|
|
||
|
|
|
|
|
|
10.16+
|
|
||
|
|
|
|
|
|
10.17+
|
|
||
|
10.18+
|
|
||
|
10.19+
|
|
||
|
10.20+
|
|
||
|
10.21
|
|
|
|
|
|
|
|
|
|
10.22†
|
|
||
|
|
|
|
|
|
Exhibit
Number
|
|
Description of Exhibit
|
|
|
10.23†
|
|
||
|
|
|
|
|
|
10.24†
|
|
||
|
|
|
|
|
|
10.25†
|
|
||
|
|
|
|
|
|
10.26+
|
|
||
|
10.27+
|
|
||
|
|
|
|
|
|
10.28+
|
|
||
|
|
|
|
|
|
10.29+
|
|
||
|
|
|
|
|
|
10.30+
|
|
||
|
|
|
|
|
|
10.31†
|
|
||
|
10.32†
|
|
||
|
10.33
|
|
||
|
10.34
|
|
||
|
10.35
|
|
||
|
10.36
|
|
||
|
10.37+
|
|
||
|
10.38+
|
|
||
|
10.39†
|
|
||
|
Exhibit
Number
|
|
Description of Exhibit
|
|
|
10.40†
|
|
||
|
10.42†
|
|
||
|
10.43
|
|
||
|
10.44
|
|
||
|
21.1
|
|
|
|
|
|
|
|
|
|
23.1
|
|
|
|
|
|
|
|
|
|
24.1
|
|
|
|
|
|
|
|
|
|
31.1
|
|
|
|
|
|
|
|
|
|
31.2
|
|
|
|
|
|
|
|
|
|
32.1
|
|
|
|
|
|
|
|
|
|
32.2
|
|
|
|
|
|
|
||
|
101.INS
|
|
|
XBRL Instance Document
|
|
|
|
|
|
|
101.SCH
|
|
|
XBRL Taxonomy Extension Schema Document
|
|
101.CAL
|
|
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
|
|
|
|
101.LAB
|
|
|
XBRL Taxonomy Extension Label Linkbase Database
|
|
|
|
|
|
|
101.PRE
|
|
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
|
|
|
|
101.DEF
|
|
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
†
|
Confidential treatment has been granted for certain portions that are omitted from this exhibit. The omitted information has been filed separately with the U.S. Securities and Exchange Commission (the “SEC”) pursuant to the registrant’s application for confidential treatment. In addition, schedules have been omitted from this exhibit pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule will be furnished supplementally to the SEC upon request; provided, however, that the registrant may request confidential treatment for any document so furnished.
|
||
|
+
|
Management contract, compensatory plan or arrangement.
|
||
|
|
|
PTC THERAPEUTICS, INC.
|
|||
|
Date:
|
March 1, 2019
|
By:
|
/s/ STUART W. PELTZ
|
||
|
|
|
Stuart W. Peltz, Ph.D.
Chief Executive Officer
(Principal Executive Officer)
|
|||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ STUART W. PELTZ
|
|
Stuart W. Peltz
Chief Executive Officer and Director
|
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ CHRISTINE UTTER
|
|
Christine Utter
Principal Financial Officer
(Principal Financial and Accounting Officer)
|
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ MICHAEL SCHMERTZLER
|
|
Michael Schmertzler
Director
|
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ ALLAN JACOBSON
|
|
Allan Jacobson
Director |
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
/s/ STEPHANIE S. OKEY
|
|
|
Stephanie S. Okey
Director
|
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
/s/ EMMA REEVE
|
|
|
Emma Reeve
Director
|
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ DAVID P. SOUTHWELL
|
|
David P. Southwell
Director |
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ GLENN D. STEELE
|
|
Glenn D. Steele
Director |
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ DAWN SVORONOS
|
|
Dawn Svoronos
Director |
|||||
|
Dated:
|
March 1, 2019
|
|
By:
|
|
/s/ JEROME B. ZELDIS
|
|
Jerome B. Zeldis
Director |
|||||