|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
95-4078884
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
4B Cedar Brook Drive
Cranbury, New Jersey
|
08512
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, par value $.01 per share
|
NYSE MKT
|
|
Page
|
||
|
PART I
|
||
|
Item 1.
|
2
|
|
|
Item 1A.
|
12
|
|
|
Item 1B.
|
24
|
|
|
Item 2.
|
24
|
|
|
Item 3.
|
24
|
|
|
Item 4.
|
24
|
|
|
PART II
|
||
|
Item 5.
|
25
|
|
|
Item 6.
|
25
|
|
|
Item 7.
|
25
|
|
|
Item 7A.
|
30
|
|
|
Item 8.
|
31
|
|
|
Item 9.
|
50
|
|
|
Item 9A.
|
50
|
|
|
Item 9B.
|
50
|
|
|
PART III
|
||
|
Item 10.
|
51
|
|
|
Item 11.
|
55
|
|
|
Item 12.
|
60
|
|
|
Item 13.
|
65
|
|
|
Item 14.
|
66
|
|
|
PART IV
|
||
|
Item 15.
|
67
|
|
|
·
|
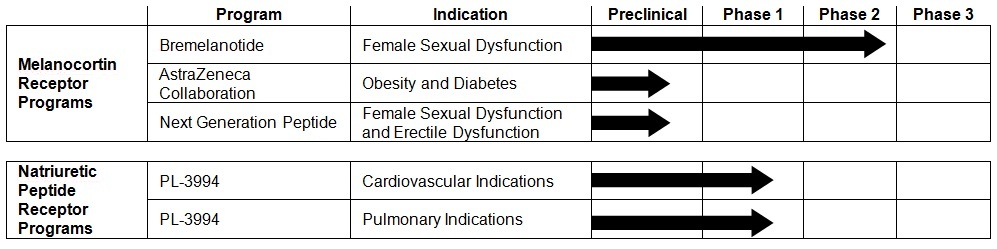
Bremelanotide, a peptide melanocortin receptor agonist, for treatment of FSD. This drug candidate is in Phase 2B clinical trials.
|
|
·
|
Melanocortin receptor-based compounds for treatment of obesity, under development by AstraZeneca AB (AstraZeneca) pursuant to our research collaboration and license agreement.
|
|
·
|
PL-3994, a peptide mimetic natriuretic peptide receptor A (NPR-A) agonist, for treatment of cardiovascular and pulmonary indications.
|
|
·
|
continuing to conduct preclinical development and clinical trials;
|
|
·
|
participating in regulatory approval processes;
|
|
·
|
formulating and manufacturing products, or having third parties formulate and manufacture products;
|
|
·
|
post-approval monitoring and surveillance of our products;
|
|
·
|
conducting sales and marketing activities, either alone or with a partner; and
|
|
·
|
obtaining additional capital.
|
|
·
|
the availability of sufficient capital to sustain operations and clinical trials;
|
|
·
|
timely completion of clinical site protocol approval and obtaining informed consent from subjects;
|
|
·
|
the rate of patient enrollment in clinical studies;
|
|
·
|
adverse medical events or side effects in treated patients; and
|
|
·
|
lack of effectiveness of the product being tested.
|
|
·
|
product approval or clearance;
|
|
·
|
regulatory compliance;
|
|
·
|
good manufacturing practices;
|
|
·
|
intellectual property rights;
|
|
·
|
product introduction; and
|
|
·
|
marketing and competition.
|
|
·
|
completion of non-clinical tests including preclinical laboratory and formulation studies and animal testing and toxicology;
|
|
·
|
submission to the FDA of an IND application, which must become effective before clinical trials may begin;
|
|
·
|
performance of adequate and well-controlled Phase 1, 2 and 3 human clinical trials to establish the safety and efficacy of the drug for each proposed indication;
|
|
·
|
submission to the FDA of an NDA;
|
|
·
|
FDA review and approval of the NDA before any commercial marketing or sale; and
|
|
·
|
Compliance with post-approval commitments and requirements.
|
|
·
|
perceptions by members of the healthcare community, including physicians, about its safety and effectiveness;
|
|
·
|
cost-effectiveness relative to competing products and technologies;
|
|
·
|
availability of reimbursement for our products from third party payors such as health insurers, health maintenance organizations and government programs such as Medicare and Medicaid; and
|
|
·
|
advantages over alternative treatment methods.
|
|
·
|
the degree and range of protection any patents will afford us against competitors, including whether third parties will find ways to invalidate or otherwise circumvent our patents;
|
|
·
|
if and when patents will be issued;
|
|
·
|
whether or not others will obtain patents claiming aspects similar to those covered by our patents and patent applications; and
|
|
·
|
whether we will need to initiate litigation or administrative proceedings, which may be costly whether we win or lose.
|
|
·
|
obtain licenses, which may not be available on commercially reasonable terms, if at all;
|
|
·
|
redesign our products or processes to avoid infringement;
|
|
·
|
stop using the subject matter claimed in the patents held by others;
|
|
·
|
pay damages; or
|
|
·
|
defend litigation or administrative proceedings, which may be costly whether we win or lose, and which could result in a substantial diversion of our management resources.
|
|
Redemption Price of All Series B 2012 Warrants (35,488,380 Shares)
|
|
|
Highest 10-Day Price of Common Stock
|
Redemption Price
|
|
$0.50
|
$17,744,190
|
|
$0.75
|
$26,616,285
|
|
$1.00
|
$35,488,380
|
|
$2.00
|
$70,976,760
|
|
July 3, 2012
|
June 30, 2013
|
|||||||
|
Aggregate fair value
|
$ | 17,034,422 | $ | 35,133,496 | ||||
|
Exercise price
|
$ | 0.01 | $ | 0.01 | ||||
|
Common stock price (per share)
|
$ | 0.49 | $ | 1.00 | ||||
|
·
|
52,834 shares issuable on the conversion of immediately convertible Series A Convertible preferred stock, subject to adjustment, for no further consideration;
|
|
·
|
2,758,633 shares issuable on the exercise of stock options, at exercise prices ranging from $0.65 to $42.10 per share;
|
|
·
|
472,500 shares issuable under restricted stock units which vest on dates between June 22, 2013 and July 17, 2014, subject to the fulfillment of service conditions; and
|
|
·
|
56,608,468 shares issuable on the exercise of warrants at exercise prices ranging from $0.01 to $4.12 per share.
|
|
·
|
publicity regarding actual or potential clinical results relating to products under development by our competitors or us;
|
|
·
|
delay or failure in initiating, completing or analyzing preclinical or clinical trials or unsatisfactory designs or results of these trials;
|
|
·
|
interim decisions by regulatory agencies, including the FDA, as to clinical trial designs, acceptable safety profiles and the benefit/risk ratio of products under development;
|
|
·
|
achievement or rejection of regulatory approvals by our competitors or by us;
|
|
·
|
announcements of technological innovations or new commercial products by our competitors or by us;
|
|
·
|
developments concerning proprietary rights, including patents;
|
|
·
|
developments concerning our collaborations;
|
|
·
|
regulatory developments in the United States and foreign countries;
|
|
·
|
whether our stockholders approve an increase in our authorized common stock, such that we avoid interest payments and certain other contractual obligations relating to our Series B 2012 warrants;
|
|
·
|
economic or other crises and other external factors;
|
|
·
|
period-to-period fluctuations in our revenue and other results of operations;
|
|
·
|
changes in financial estimates by securities analysts; and
|
|
·
|
sales of our common stock.
|
|
FISCAL YEAR ENDED JUNE 30, 2012
|
HIGH
|
LOW
|
||||||
|
Fourth Quarter
|
$ | 0.77 | $ | 0.40 | ||||
|
Third Quarter
|
0.75 | 0.39 | ||||||
|
Second Quarter
|
0.73 | 0.39 | ||||||
|
First Quarter
|
1.28 | 0.50 | ||||||
|
FISCAL YEAR ENDED JUNE 30, 2011
|
HIGH
|
LOW
|
||||||
|
Fourth Quarter
|
$ | 1.38 | $ | 0.79 | ||||
|
Third Quarter
|
1.45 | 0.78 | ||||||
|
Second Quarter
|
1.90 | 0.84 | ||||||
|
First Quarter
|
2.40 | 1.26 | ||||||
|
·
|
the development and testing of products in animals and humans;
|
|
·
|
product approval or clearance;
|
|
·
|
regulatory compliance;
|
|
·
|
good manufacturing practices;
|
|
·
|
intellectual property rights;
|
|
·
|
product introduction;
|
|
·
|
marketing, sales and competition; and
|
|
·
|
obtaining sufficient capital.
|
| Payments due by Period | ||||||||||||||||||||
|
Total
|
Less than
1 Year |
1 - 3 Years
|
3 - 5 Years
|
More than
5 Years |
||||||||||||||||
|
Facility operating leases
|
$ | 819,576 | $ | 346,906 | $ | 472,670 | $ | - | $ | - | ||||||||||
|
Capital lease obligations
|
45,353 | 24,738 | 20,615 | - | - | |||||||||||||||
|
Total contractual obligations
|
$ | 864,929 | $ | 371,644 | $ | 493,285 | $ | - | $ | - | ||||||||||
|
Page
|
||||
|
Report of Independent Registered Public Accounting Firm
|
32 | |||
|
Consolidated Balance Sheets
|
33 | |||
|
Consolidated Statements of Operations
|
34 | |||
|
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss
|
35 | |||
|
Consolidated Statements of Cash Flows
|
36 | |||
|
Notes to Consolidated Financial Statements
|
37 | |||
|
PALATIN TECHNOLOGIES, INC
.
|
|
and Subsidiary
|
|
|
|
June 30, 2012
|
June 30, 2011
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 3,827,198 | $ | 18,869,639 | ||||
|
Accounts receivable
|
27,631 | 131,149 | ||||||
|
Restricted cash
|
350,000 | - | ||||||
|
Prepaid expenses and other current assets
|
532,010 | 261,947 | ||||||
|
Total current assets
|
4,736,839 | 19,262,735 | ||||||
|
Property and equipment, net
|
318,653 | 1,305,331 | ||||||
|
Restricted cash
|
- | 350,000 | ||||||
|
Other assets
|
324,992 | 254,787 | ||||||
|
Total assets
|
$ | 5,380,484 | $ | 21,172,853 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Capital lease obligations
|
$ | 22,277 | $ | 34,923 | ||||
|
Accounts payable
|
294,894 | 496,908 | ||||||
|
Accrued expenses
|
2,706,496 | 1,854,007 | ||||||
|
Accrued compensation
|
433,333 | 374,094 | ||||||
|
Unearned revenue
|
- | 46,105 | ||||||
|
Total current liabilities
|
3,457,000 | 2,806,037 | ||||||
|
Capital lease obligations
|
19,909 | 42,186 | ||||||
|
Deferred rent
|
72,677 | 132,855 | ||||||
|
Total liabilities
|
3,549,586 | 2,981,078 | ||||||
|
Commitments and contingencies (Note 7)
|
||||||||
|
Stockholders’ equity:
|
||||||||
|
Preferred stock of $0.01 par value – authorized 10,000,000 shares;
|
||||||||
|
Series A Convertible; issued and outstanding 4,997 shares as of June 30, 2012 and 2011, respectively
|
50 | 50 | ||||||
|
Common stock of $0.01 par value – authorized 100,000,000 shares;
|
||||||||
|
issued and outstanding 34,900,591 shares as of June 30, 2012 and 2011, respectively
|
349,006 | 349,006 | ||||||
|
Additional paid-in capital
|
240,725,127 | 239,832,826 | ||||||
|
Accumulated deficit
|
(239,243,285 | ) | (221,990,107 | ) | ||||
|
Total stockholders’ equity
|
1,830,898 | 18,191,775 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 5,380,484 | $ | 21,172,853 | ||||
|
PALATIN TECHNOLOGIES, INC.
|
|
and Subsidiary
|
|
|
|
Year Ended June 30,
|
||||||||||||
|
2012
|
2011
|
2010
|
||||||||||
|
REVENUES:
|
||||||||||||
|
License and contract
|
$ | 73,736 | $ | 497,540 | $ | 14,180,727 | ||||||
|
Grant
|
- | 977,917 | - | |||||||||
|
Total revenues
|
73,736 | 1,475,457 | 14,180,727 | |||||||||
|
OPERATING EXPENSES:
|
||||||||||||
|
Research and development
|
13,813,376 | 10,377,019 | 12,293,910 | |||||||||
|
General and administrative
|
5,045,741 | 4,751,824 | 4,901,203 | |||||||||
|
Total operating expenses
|
18,859,117 | 15,128,843 | 17,195,113 | |||||||||
|
Loss from operations
|
(18,785,381 | ) | (13,653,386 | ) | (3,014,386 | ) | ||||||
|
OTHER INCOME (EXPENSE):
|
||||||||||||
|
Investment income
|
32,133 | 99,258 | 141,635 | |||||||||
|
Interest expense
|
(10,411 | ) | (10,606 | ) | (13,165 | ) | ||||||
|
Increase in fair value of warrants
|
- | (2,266 | ) | - | ||||||||
|
Gain on sale of securities
|
- | 119,346 | - | |||||||||
|
Gain (loss) on disposition of supplies and equipment
|
442,248 | (5,666 | ) | 95,000 | ||||||||
|
Total other income, net
|
463,970 | 200,066 | 223,470 | |||||||||
|
Loss before income taxes
|
(18,321,411 | ) | (13,453,320 | ) | (2,790,916 | ) | ||||||
|
Income tax benefit
|
1,068,233 | 637,391 | 998,408 | |||||||||
|
NET LOSS
|
$ | (17,253,178 | ) | $ | (12,815,929 | ) | $ | (1,792,508 | ) | |||
|
Basic and diluted net loss per common share
|
$ | (0.49 | ) | $ | (0.64 | ) | $ | (0.18 | ) | |||
|
Weighted average number of common shares outstanding used in computing basic and diluted net loss per common share
|
34,900,591 | 20,084,022 | 9,861,215 | |||||||||
|
PALATIN TECHNOLOGIES, INC.
|
|
and Subsidiary
|
|
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss
|
|
Accumulated
|
||||||||||||||||||||||||||||||||
|
Additional
|
Other
|
|||||||||||||||||||||||||||||||
|
Preferred Stock
|
Common Stock
|
Paid-in
|
Comprehensive
|
Accumulated
|
||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Income
|
Deficit
|
Total
|
|||||||||||||||||||||||||
|
Balance, June 30, 2009
|
4,997 | $ | 50 | 8,666,290 | $ | 86,663 | $ | 210,492,345 | $ | 116,111 | $ | (207,381,670 | ) | $ | 3,313,499 | |||||||||||||||||
|
Sale of common stock units, net of costs
|
- | - | 2,911,448 | 29,114 | 6,931,491 | - | - | 6,960,605 | ||||||||||||||||||||||||
|
Exercise of options
|
- | - | 6,725 | 67 | 11,371 | - | - | 11,438 | ||||||||||||||||||||||||
|
Stock-based compensation
|
- | - | 172,500 | 1,725 | 966,836 | - | - | 968,561 | ||||||||||||||||||||||||
|
Payment of withholding taxes related to
|
||||||||||||||||||||||||||||||||
|
restricted stock units
|
- | - | (54,145 | ) | (541 | ) | (165,320 | ) | (165,861 | ) | ||||||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||
|
Unrealized gain on investments
|
- | - | - | - | - | 22,539 | - | 22,539 | ||||||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | - | (1,792,508 | ) | (1,792,508 | ) | ||||||||||||||||||||||
|
Total comprehensive loss
|
(1,769,969 | ) | ||||||||||||||||||||||||||||||
|
Balance, June 30, 2010
|
4,997 | 50 | 11,702,818 | 117,028 | 218,236,723 | 138,650 | (209,174,178 | ) | 9,318,273 | |||||||||||||||||||||||
|
Stock split adjustment for fractional shares
|
- | - | (46 | ) | - | - | - | - | - | |||||||||||||||||||||||
|
Sale of common stock units, net of costs
|
- | - | 23,000,000 | 230,000 | 15,688,150 | - | - | 15,918,150 | ||||||||||||||||||||||||
|
Reclassification of warrants from liability
|
||||||||||||||||||||||||||||||||
|
to equity
|
- | - | - | - | 5,115,130 | - | - | 5,115,130 | ||||||||||||||||||||||||
|
Exercise of warrants
|
- | - | 32,200 | 322 | 64,078 | - | - | 64,400 | ||||||||||||||||||||||||
|
Stock-based compensation
|
- | - | 183,500 | 1,835 | 754,762 | - | - | 756,597 | ||||||||||||||||||||||||
|
Payment of withholding taxes related to
|
||||||||||||||||||||||||||||||||
|
restricted stock units
|
- | - | (17,881 | ) | (179 | ) | (26,017 | ) | - | - | (26,196 | ) | ||||||||||||||||||||
|
Realized gain on sale of securities
|
- | - | - | - | - | (119,346 | ) | - | (119,346 | ) | ||||||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||
|
Unrealized loss on investments
|
- | - | - | - | - | (19,304 | ) | - | (19,304 | ) | ||||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | - | (12,815,929 | ) | (12,815,929 | ) | ||||||||||||||||||||||
|
Total comprehensive loss
|
(12,835,233 | ) | ||||||||||||||||||||||||||||||
|
Balance, June 30, 2011
|
4,997 | 50 | 34,900,591 | 349,006 | 239,832,826 | - | (221,990,107 | ) | 18,191,775 | |||||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 892,301 | - | - | 892,301 | ||||||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | - | (17,253,178 | ) | (17,253,178 | ) | ||||||||||||||||||||||
|
Balance, June 30, 2012
|
4,997 | $ | 50 | 34,900,591 | $ | 349,006 | $ | 240,725,127 | $ | - | $ | (239,243,285 | ) | $ | 1,830,898 | |||||||||||||||||
|
PALATIN TECHNOLOGIES, INC.
|
|
and Subsidiary
|
|
|
|
Year Ended June 30,
|
||||||||||||
|
2012
|
2011
|
2010
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss
|
$ | (17,253,178 | ) | $ | (12,815,929 | ) | $ | (1,792,508 | ) | |||
|
Adjustments to reconcile net loss to net cash
|
||||||||||||
|
used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
949,542 | 1,138,183 | 1,269,413 | |||||||||
|
Loss (gain) on sale/disposition of supplies
|
||||||||||||
|
and equipment
|
(442,248 | ) | 5,666 | (95,000 | ) | |||||||
|
Gain on sale of available-for-sale investments
|
- | (119,346 | ) | - | ||||||||
|
Stock-based compensation
|
892,301 | 756,597 | 968,561 | |||||||||
|
Amortization of deferred revenue
|
- | - | (11,905,553 | ) | ||||||||
|
Increase in fair value of warrants
|
- | 2,266 | - | |||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
103,518 | (128,270 | ) | 505,649 | ||||||||
|
Prepaid expenses, restricted cash and other assets
|
(340,268 | ) | 263,280 | 92,174 | ||||||||
|
Accounts payable
|
(202,014 | ) | 341,113 | (50,568 | ) | |||||||
|
Accrued expenses, compensation and deferred rent
|
851,550 | (519,899 | ) | 278,088 | ||||||||
|
Deferred revenues
|
- | - | 5,000,000 | |||||||||
|
Unearned revenue
|
(46,105 | ) | 46,105 | - | ||||||||
|
Net cash used in operating activities
|
(15,486,902 | ) | (11,030,234 | ) | (5,729,744 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||
|
Proceed from sale of available-for-sale investments
|
- | 3,442,885 | - | |||||||||
|
Proceeds from sale of supplies and equipment
|
494,384 | 5,300 | 45,000 | |||||||||
|
Purchases of property and equipment
|
(15,000 | ) | - | (6,995 | ) | |||||||
|
Net cash provided by investing activities
|
479,384 | 3,448,185 | 38,005 | |||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||
|
Payments on capital lease obligations
|
(34,923 | ) | (22,960 | ) | (87,675 | ) | ||||||
|
Payment of withholding taxes related to restricted
|
||||||||||||
|
stock units
|
- | (26,196 | ) | (165,861 | ) | |||||||
|
Proceeds from sale of common stock units and exercise
|
||||||||||||
|
of common stock options and warrants
|
- | 21,095,414 | 6,972,043 | |||||||||
|
Net cash provided by (used in) financing activities
|
(34,923 | ) | 21,046,258 | 6,718,507 | ||||||||
|
NET INCREASE (DECREASE) IN CASH
|
||||||||||||
|
AND CASH EQUIVALENTS
|
(15,042,441 | ) | 13,464,209 | 1,026,768 | ||||||||
|
CASH AND CASH EQUIVALENTS, beginning of year
|
18,869,639 | 5,405,430 | 4,378,662 | |||||||||
|
CASH AND CASH EQUIVALENTS, end of year
|
$ | 3,827,198 | $ | 18,869,639 | $ | 5,405,430 | ||||||
|
SUPPLEMENTAL CASH FLOW INFORMATION:
|
||||||||||||
|
Cash paid for interest
|
$ | 9,984 | $ | 10,606 | $ | 13,165 | ||||||
|
Equipment acquired under financing arrangements
|
- | 66,115 | - | |||||||||
|
Unrealized gain (loss) on available-for-sale investments
|
- | (19,304 | ) | 22,539 | ||||||||
|
Fair Value
|
Quoted prices
in active markets (Level 1) |
Quoted prices
in active markets (Level 2) |
Quoted prices
in active markets (Level 3) |
|||||||||||||
|
June 30, 2012:
|
||||||||||||||||
|
Assets:
|
||||||||||||||||
|
Money Market Fund
|
$ | 3,344,146 | $ | 3,344,146 | $ | - | $ | - | ||||||||
|
June 30, 2011:
|
||||||||||||||||
|
Assets:
|
||||||||||||||||
|
Money Market Fund
|
$ | 18,383,284 | $ | 18,383,284 | $ | - | $ | - | ||||||||
|
June 30,
|
June 30,
|
|||||||
|
2012
|
2011
|
|||||||
|
Office equipment
|
$ | 1,157,553 | $ | 1,725,732 | ||||
|
Laboratory equipment
|
317,418 | 3,982,991 | ||||||
|
Leasehold improvements
|
7,088,462 | 7,088,462 | ||||||
| 8,563,433 | 12,797,185 | |||||||
|
Less: Accumulated depreciation and amortization
|
(8,244,780 | ) | (11,491,854 | ) | ||||
| $ | 318,653 | $ | 1,305,331 | |||||
|
June 30,
|
June 30,
|
|||||||
|
2012
|
2011
|
|||||||
|
Clinical study costs
|
$ | 1,752,392 | $ | 834,521 | ||||
|
Other research related expenses
|
253,968 | 124,819 | ||||||
|
Deferred rent, current portion
|
37,217 | 391,817 | ||||||
|
Professional services
|
444,601 | 175,500 | ||||||
|
Insurance premiums payable
|
130,973 | 131,631 | ||||||
|
Other
|
87,345 | 195,719 | ||||||
| $ | 2,706,496 | $ | 1,854,007 | |||||
|
Year Ending June 30,
|
||||
|
2013
|
$ | 346,906 | ||
|
2014
|
236,335 | |||
|
2015
|
236,335 | |||
| $ | 819,576 | |||
|
Year Ending June 30,
|
||||
|
2013
|
$ | 24,738 | ||
|
2014
|
20,615 | |||
| 45,353 | ||||
|
Amount representing interest
|
(3,167 | ) | ||
|
Net
|
$ | 42,186 | ||
|
March 1,
2011
|
May 11,
2011
|
|||||||
|
Aggregate fair value
|
$ | 5,112,864 | $ | 5,115,130 | ||||
|
Exercise price
|
$ | 1.00 | $ | 1.00 | ||||
|
Expected volatility
|
105 | % | 106 | % | ||||
|
Remaining contractual term (years)
|
6 | 5.83 | ||||||
|
Risk-free interest rate
|
2.47 | % | 2.22 | % | ||||
|
Expected dividend yield
|
0 | % | 0 | % | ||||
|
Common stock price (per share)
|
$ | 0.86 | $ | 0.88 | ||||
|
Shares of Common Stock
|
Exercise Price per Share
|
Latest Termination Date
|
|||||
| 100,000 | $ 0.50 |
July 24, 2012
|
|||||
| 50,000 | 2.50 |
November 26, 2012
|
|||||
| 48,148 | 3.38 |
November 26, 2012
|
|||||
| 47,424 | 4.13 |
November 26, 2012
|
|||||
| 317,776 | 3.00 |
August 30, 2013
|
|||||
| 50,000 | 0.75 |
January 24, 2014
|
|||||
| 331,969 | 3.30 |
August 12, 2014
|
|||||
| 50,000 | 0.60 |
November 9, 2014
|
|||||
| 50,000 | 1.00 |
November 9, 2014
|
|||||
| 100,000 | 1.50 |
November 9, 2014
|
|||||
| 575,000 | 1.00 |
February 23, 2016
|
|||||
| 2,000,000 | 1.00 |
March 1, 2016
|
|||||
| 21,000,000 | 1.00 |
March 2, 2017
|
|||||
| 24,720,317 | |||||||
|
2012
|
2011
|
2010
|
||||||||||||||||||||||
|
Number of
Shares |
Weighted
Average Exercise Price |
Number of
Shares |
Weighted
Average Exercise Price |
Number of
Shares |
Weighted
Average Exercise Price |
|||||||||||||||||||
|
Outstanding at beginning of year
|
2,231,898 | $ | 4.05 | 957,374 | $ | 13.20 | 882,862 | $ | 16.60 | |||||||||||||||
|
Granted
|
75,000 | 0.65 | 1,576,275 | 0.93 | 174,276 | 2.60 | ||||||||||||||||||
|
Forfeited
|
(90,870 | ) | 3.64 | (234,951 | ) | 10.02 | (34,303 | ) | 16.00 | |||||||||||||||
|
Exercised
|
- | - | - | - | (6,725 | ) | 1.70 | |||||||||||||||||
|
Expired
|
(34,175 | ) | 33.07 | (66,800 | ) | 41.14 | (58,736 | ) | 34.10 | |||||||||||||||
|
Outstanding at end of year
|
2,181,853 | 3.50 | 2,231,898 | 4.05 | 957,374 | 13.20 | ||||||||||||||||||
|
Exercisable at end of year
|
1,323,965 | 5.10 | 809,918 | 9.28 | 631,313 | 18.00 | ||||||||||||||||||
|
Weighted average grant-date fair value of options granted during the year
|
$ | 0.47 | $ | 0.77 | $ | 2.20 | ||||||||||||||||||
|
Number of
Shares |
Weighted
Average Exercise Price |
Weighted
Average Remaining Term in Years |
Aggregate
Intrinsic Value |
|||||||||||||
|
Options outstanding at end of year
|
2,181,853 | $ | 3.50 | 7.7 | $ | - | ||||||||||
|
Options vested and exercisable at end of year
|
1,323,965 | $ | 5.10 | 7.1 | $ | - | ||||||||||
|
Unvested options expected to vest
|
786,274 | $ | 1.04 | 8.8 | $ | - | ||||||||||
|
2012
|
2011
|
2010
|
||||||||||
|
Outstanding at beginning of year
|
500,000 | - | 172,500 | |||||||||
|
Granted
|
- | 705,000 | - | |||||||||
|
Forfeited
|
- | (21,500 | ) | - | ||||||||
|
Vested
|
(250,000 | ) | (183,500 | ) | (172,500 | ) | ||||||
|
Outstanding at end of year
|
250,000 | 500,000 | - | |||||||||
|
June 30,
|
June 30,
|
|||||||
|
2012
|
2011
|
|||||||
|
Net operating loss carryforwards
|
$ | 81,460,000 | $ | 76,813,000 | ||||
|
Research and development tax credits
|
6,364,000 | 5,853,000 | ||||||
|
Accrued expenses, deferred revenue and other
|
3,969,000 | 2,583,000 | ||||||
| 91,793,000 | 85,249,000 | |||||||
|
Valuation allowance
|
(91,793,000 | ) | (85,249,000 | ) | ||||
|
Net deferred tax assets
|
$ | - | $ | - | ||||
|
Three Months Ended
|
||||||||||||||||
|
June 30,
2012
|
March 31, 2012
|
December 31, 2011
|
September 30, 2011
|
|||||||||||||
|
(amounts in thousands, except per share data)
|
||||||||||||||||
|
Revenues
|
$ | 11 | $ | 24 | $ | 12 | $ | 27 | ||||||||
|
Operating expenses
|
5,702 | 6,050 | 3,713 | 3,394 | ||||||||||||
|
Other income/(expense), net
|
438 | 6 | 8 | 12 | ||||||||||||
|
Loss before income taxes
|
(5,253 | ) | (6,020 | ) | (3,693 | ) | (3,355 | ) | ||||||||
|
Income tax benefit
|
- | - | 1,068 | - | ||||||||||||
|
Net loss
|
$ | (5,253 | ) | $ | (6,020 | ) | $ | (2,625 | ) | $ | (3,355 | ) | ||||
|
Basic and diluted net loss per common share
|
$ | (0.14 | ) | $ | (0.17 | ) | $ | (0.08 | ) | $ | (0.10 | ) | ||||
|
Weighted average number of common shares outstanding used in computing basic and diluted net loss per common share
|
34,900,591 | 34,900,591 | 34,900,591 | 34,900,591 | ||||||||||||
|
Three Months Ended
|
||||||||||||||||
|
June 30,
2011
|
March 31, 2011
|
December 31, 2010
|
September 30, 2010
|
|||||||||||||
|
(amounts in thousands, except per share data)
|
||||||||||||||||
|
Revenues
|
$ | 156 | $ | 61 | $ | 1,042 | $ | 216 | ||||||||
|
Operating expenses
|
4,742 | 2,678 | 2,874 | 4,834 | ||||||||||||
|
Other income/(expense), net
|
1,277 | (1,189 | ) | 94 | 18 | |||||||||||
|
Loss before income taxes
|
(3,309 | ) | (3,806 | ) | (1,738 | ) | (4,600 | ) | ||||||||
|
Income tax benefit
|
- | - | 637 | - | ||||||||||||
|
Net loss
|
$ | (3,309 | ) | $ | (3,806 | ) | $ | (1,101 | ) | $ | (4,600 | ) | ||||
|
Basic and diluted net loss per common share
|
$ | (0.09 | ) | $ | (0.17 | ) | $ | (0.09 | ) | $ | (0.39 | ) | ||||
|
Weighted average number of common shares outstanding used in computing basic and diluted net loss per common share
|
34,900,591 | 22,832,109 | 11,839,309 | 11,730,308 | ||||||||||||
|
Name
|
Age
|
Position with Palatin
|
|||
|
Carl Spana, Ph.D.
|
50 |
Chief executive officer, president and a director
|
|||
|
John K.A. Prendergast, Ph.D.
|
58 |
Director, chairman of the board of directors
|
|||
|
Perry B. Molinoff, M.D. (1) (3)
|
72 |
Director
|
|||
|
Robert K. deVeer, Jr. (1) (2)
|
66 |
Director
|
|||
|
Zola P. Horovitz, Ph.D. (2) (3)
|
77 |
Director
|
|||
|
Robert I. Taber, Ph.D. (1) (2)
|
76 |
Director
|
|||
|
J. Stanley Hull (3)
|
60 |
Director
|
|||
|
Alan W. Dunton, M.D. (1) (2)
|
58 |
Director
|
|||
|
_________________________________
(1) Member of the Audit Committee.
(2) Member of the Compensation Committee.
(3) Member of the Nominating and Corporate Governance Committee.
|
|||||
|
Name
|
Age
|
Position with Palatin
|
|
Carl Spana, Ph.D.
|
50
|
Chief executive officer, president and director
|
|
Stephen T. Wills, MST, CPA
|
55
|
Chief financial officer, chief operating officer, executive vice president, secretary and treasurer
|
|
Name and Principal Position
|
Fiscal
Year
|
Salary
($)
|
Stock
awards (1)
($)
|
Option
awards (1)
($)
|
Nonequity incentive plan compensa-tion (2)
($)
|
All
other
compen-
sation (3)
($)
|
Total
($)
|
||||||||||||||||||
|
Carl Spana, Ph.D., chief executive officer and president
|
2012
|
420,000 | - | - | 112,500 | 12,750 | 545,250 | ||||||||||||||||||
|
2011
|
400,000 | 257,500 | 228,326 | 120,000 | 12,500 | 1,018,326 | |||||||||||||||||||
|
Stephen T. Wills, MST, CPA, chief financial officer, chief operating officer and executive vice president
|
2012
|
375,000 | - | - | 105,000 | 13,375 | 493,375 | ||||||||||||||||||
|
2011
|
330,000 | 227,500 | 190,271 | 100,000 | 12,475 | 860,246 | |||||||||||||||||||
|
Trevor Hallam, Ph.D., former executive vice president of research and development (4)
|
2012
|
- | - | - | - | - | - | ||||||||||||||||||
|
2011
|
165,000 | 34,000 | - | - | 169,225 | 368,225 | |||||||||||||||||||
|
(1)
|
Amounts in these columns represent the aggregate grant date fair value for stock awards and option awards computed using the Black-Scholes model. For a description of the assumptions we used to calculate these amounts, see Note 9 to the consolidated financial statements included in this Annual Report.
|
|
(2)
|
Bonus amounts earned in fiscal 2012 were paid in July 2012. Bonus amounts for fiscal 2011 were set by the board on June 22, 2011, but were not paid until July 15, 2011.
|
|
(3)
|
Consists of matching contributions to 401(k) plan accounts and, for fiscal 2011 for Dr. Hallam, includes severance payments of $165,000.
|
|
(4)
|
Dr. Hallam resigned effective December 31, 2010. All of his stock and option awards terminated prior to June 30, 2011.
|
|
·
|
annual discretionary bonus compensation, in an amount to be decided by the Compensation Committee and approved by the board, based on achievement of yearly objectives; and
|
|
·
|
participation in all benefit programs that we establish, to the extent the executive’s position, tenure, salary, age, health and other qualifications make him eligible to participate.
|
|
Option awards (1)
|
Stock awards (2)
|
|||||||
| Name (3) |
Option or
stock
award
grant
date
|
Number of
securities
underlying
unexercised
options
(#)
exercisable
|
Number of
securities
underlying
unexercised
options
(#)
unexercisable
|
Option
exercise
price
($)
|
Option
expiration
date
|
Number of shares or units of stock that have not vested
(#)
|
Market value of shares or units of stock that have not vested
($) (4)
|
|
| Carl Spana |
12/11/02
|
10,000
|
-
|
20.00
|
12/11/12
|
|||
|
07/16/03
|
10,000
|
-
|
32.40
|
07/16/13
|
||||
|
07/01/05
|
7,500
|
-
|
37.50
|
07/01/15
|
||||
|
07/01/05
|
8,300
|
-
|
17.50
|
07/01/15
|
||||
|
10/06/06
|
12,500
|
-
|
24.90
|
10/06/16
|
||||
|
03/26/08
|
28,125
|
-
|
2.80
|
03/26/18
|
||||
|
03/26/08
|
4,687
|
-
|
5.00
|
03/26/18
|
||||
|
03/26/08
|
4,688
|
-
|
6.60
|
03/26/18
|
||||
|
07/01/08
|
18,750
|
6,250
|
1.80
|
07/01/18
|
||||
|
07/01/09
|
12,500
|
12,500
|
2.80
|
07/01/19
|
||||
|
06/22/11
|
75,000
|
225,000
|
1.00
|
06/22/21
|
||||
|
06/22/11
|
125,000
|
62,500
|
||||||
| Stephen T. Wills |
12/11/02
|
8,000
|
-
|
20.00
|
12/11/12
|
|||
|
07/16/03
|
8,000
|
-
|
32.40
|
07/16/13
|
||||
|
07/01/05
|
5,000
|
-
|
37.50
|
07/01/15
|
||||
|
07/01/05
|
7,300
|
-
|
17.50
|
07/01/15
|
||||
|
10/06/06
|
10,000
|
-
|
24.90
|
10/06/16
|
||||
|
03/26/08
|
22,500
|
-
|
2.80
|
03/26/18
|
||||
|
03/26/08
|
3,750
|
-
|
5.00
|
03/26/18
|
||||
|
03/26/08
|
3,750
|
-
|
6.60
|
03/26/18
|
||||
|
07/01/08
|
15,000
|
5,000
|
1.80
|
07/01/18
|
||||
|
07/01/09
|
10,000
|
10,000
|
2.80
|
07/01/19
|
||||
|
06/22/11
|
62,500
|
187,500
|
1.00
|
06/22/21
|
||||
|
06/22/11
|
112,500
|
56,250
|
||||||
|
(1)
|
Stock option vesting schedules: all options granted on or before March 26, 2008 have fully vested. Options granted after March 26, 2008 vest over four years with 1/4 of the shares vesting per year starting on the first anniversary of the grant date.
|
|
(2)
|
Stock awards consist of restricted stock units granted on June 22, 2011, which vest as to 50% on June 22, 2012 and as to the remaining 50% on June 22, 2013, provided that the named executive officer remains an employee. The restricted stock units provide for accelerated vesting on a “change in control” or termination of employment other than for “cause” or at the election of the named executive officers (as these terms are defined in employment agreements with the named executive officers). If the named executive officer is terminated for cause or voluntarily terminates employment, all unvested restricted stock units are immediately forfeited.
|
|
(3)
|
Dr. Hallam, who resigned effective December 31, 2010, did not have any equity-based awards outstanding at fiscal year end.
|
|
(4)
|
Calculated by multiplying the number of restricted stock units by $0.50, the closing market price of our common stock on June 29, 2012, the last trading day of our most recently completed fiscal year.
|
|
|
(a)
|
some person or entity acquires more than 50% of the voting power of our outstanding securities;
|
|
|
(b)
|
the individuals who, during any twelve month period, constitute our board of directors cease to constitute at least a majority of the board of directors;
|
|
|
(c)
|
we enter into a merger or consolidation; or
|
|
|
The term “cause” means:
|
|
|
(a)
|
the occurrence of (i) the executive’s material breach of, or habitual neglect or failure to perform the material duties which he is required to perform under, the terms of his employment agreement; (ii) the executive’s material failure to follow the reasonable directives or policies established by or at the direction of our board of directors; or (iii) the executive’s engaging in conduct that is materially detrimental to our interests such that we sustain a material loss or injury as a result thereof, provided that the breach or failure of performance is not cured, to the extent cure is possible, within ten days of the delivery to the executive of written notice thereof;
|
|
|
(b)
|
the willful breach by the executive of his obligations to us with respect to confidentiality, invention and non-disclosure, non-competition or non-solicitation; or
|
|
|
(c)
|
the conviction of the executive of, or the entry of a pleading of guilty or nolo contendere by the executive to, any crime involving moral turpitude or any felony.
|
|
|
(a)
|
any material adverse change in the executive’s duties, authority or responsibilities, which causes the executive’s position with us to become of significantly less responsibility, or assignment of duties and responsibilities inconsistent with the executive’s position;
|
|
|
(b)
|
a material reduction in the executive’s salary;
|
|
|
(c)
|
our failure to continue in effect any material compensation or benefit plan in which the executive participates, unless an equitable arrangement has been made with respect to such plan, or our failure to continue the executive’s participation therein (or in a substitute or alternative plan) on a basis not materially less favorable, both in terms of the amount of benefits provided and the level of the executive’s participation relative to other participants;
|
|
|
(d)
|
our failure to continue to provide the executive with benefits substantially similar to those enjoyed by the executive under any of our health and welfare insurance, retirement and other fringe-benefit plans, the taking of any action by us which would directly or indirectly materially reduce any of such benefits, or our failure to provide the executive with the number of paid vacation days to which he is entitled; or
|
|
|
(e)
|
the relocation of the executive to a location which is a material distance from Cranbury, New Jersey.
|
|
Name (1)
|
Fees earned
or paid in cash ($) |
Total ($)
|
|
John K.A. Prendergast, Ph.D.
|
75,000
|
75,000
|
|
Perry B. Molinoff, M.D.
|
37,000
|
37,000
|
|
Robert K. deVeer, Jr.
|
43,500
|
43,500
|
|
Zola P. Horovitz, Ph.D.
|
37,500
|
37,500
|
|
Robert I. Taber, Ph.D.
|
42,000
|
42,000
|
|
J. Stanley Hull
|
32,000
|
32,000
|
|
Alan W. Dunton, M.D.
|
38,500
|
38,500
|
|
(1)
|
The aggregate number of shares underlying option awards outstanding at June 30, 2012 for each director was:
|
|
Dr. Prendergast
|
174,850
|
|
|
Dr. Molinoff
|
118,333
|
|
|
Mr. deVeer
|
114,500
|
|
|
Dr. Horovitz
|
111,000
|
|
|
Dr. Taber
|
111,000
|
|
|
Mr. Hull
|
107,166
|
|
|
Dr. Dunton
|
32,500
|
|
Equity Compensation Plan Information
as of June 30, 2012
|
||||||||||||
|
Plan category
|
Number of securities to be issued upon exercise of outstanding options,
warrants and rights
|
Weighted-average exercise price of outstanding options, warrants
and rights
|
Number of securities remaining available for future issuance
under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by security holders
|
2,431,853 | (1) | $ | 3.50 | (2) | 1,908,796 | ||||||
|
Equity compensation plans not approved by security holders
|
0 | 0 | 0 | |||||||||
|
Total
|
2,431,853 | $ | 3.50 | 1,908,796 | ||||||||
|
(1)
|
Consists of 1,541,750 options and 250,000 restricted stock units granted under our 2011 Stock Incentive Plan, 538,150 options granted under our 2005 Stock Plan and 101,953 options granted under our 1996 Stock Option Plan. Both our 2005 Stock Plan and 1996 Stock Option Plan have terminated, but termination does not affect awards that are currently outstanding under these plans. The shares subject to outstanding awards under the 2005 Stock Plan, if forfeited prior to exercise, will become available for issuance under the 2011 Stock Incentive Plan.
|
|
(2)
|
The amount in column (a) for equity compensation plans approved by security holders includes 250,000 shares reserved for issuance on vesting of outstanding restricted stock units, granted under our 2011 Stock Incentive Plan, which vest on June 22, 2013, subject to the fulfillment of service conditions. Because no exercise price is required for issuance of shares on vesting of the restricted stock units, the weighted-average exercise price in column (b) does not take the restricted stock units into account.
|
|
·
|
each director, each of the named executive officers, and all current directors and officers as a group; and
|
|
·
|
all persons who, to our knowledge, beneficially own more than five percent of the common stock or Series A preferred stock.
|
|
Class
|
Name of beneficial owner
|
Amount and nature of beneficial ownership
|
Percent
of class
|
Percent of total voting
power
|
|||||||||
|
Common
|
Carl Spana, Ph.D.
|
451,891 | (1) | 1.2 | % | * | |||||||
|
Common
|
Stephen T. Wills
|
399,742 | (2) | 1.0 | % | * | |||||||
|
Common
|
John K.A. Prendergast, Ph.D.
|
154,117 | (3) | * | * | ||||||||
|
Common
|
Perry B. Molinoff, M.D.
|
104,333 | (4) | * | * | ||||||||
|
Common
|
Robert K. deVeer, Jr.
|
98,725 | (5) | * | * | ||||||||
|
Common
|
Zola P. Horovitz, Ph.D.
|
96,500 | (6) | * | * | ||||||||
|
Common
|
Robert I. Taber, Ph.D.
|
96,500 | (7) | * | * | ||||||||
|
Common
|
J. Stanley Hull
|
92,166 | (8) | * | * | ||||||||
|
Common
|
Alan W. Dunton, M.D.
|
23,020 | (9) | * | * | ||||||||
|
All current directors and executive officers as a group (nine persons)
|
1,515,474 | (10) | 3.8 | % | 1.0 | % | |||||||
|
(1)
|
Includes 204,550 shares which Dr. Spana has the right to acquire under options, and 50,000 shares which he has the right to acquire under Series A and Series B 2011 warrants.
|
|
(2)
|
Includes 165,800 shares which Mr. Wills has the right to acquire under options, and 50,000 shares which he has the right to acquire under Series A and Series B 2011 warrants.
|
|
(3)
|
Includes 152,350 shares which Dr. Prendergast has the right to acquire under options.
|
|
(4)
|
Includes 103,333 shares which Dr. Molinoff has the right to acquire under options.
|
|
(5)
|
Includes 98,625 shares which Mr. deVeer has the right to acquire under options.
|
|
(6)
|
Includes 96,000 shares which Dr. Horovitz has the right to acquire under options.
|
|
(7)
|
Includes 96,000 shares which Dr. Taber has the right to acquire under options.
|
|
(8)
|
Shares which Mr. Hull has the right to acquire under options.
|
|
(9)
|
Includes 17,500 shares which Dr. Dunton has the right to acquire under options.
|
|
(10)
|
Includes 1,126,324 shares which directors and officers have the right to acquire under options and warrants.
|
|
Class
|
Name and address of beneficial owner
|
Amount and nature
of beneficial ownership (1) |
Percent
of class
|
Percent of
total voting
power
|
|||||||||
|
Common
|
Mark N. Lampert
BVF Inc.
BVF Partners L.P.
900 North Michigan Avenue
Suite 1100
Chicago, Illinois 60611
|
5,200,000 | (2) | 13.4 | % | 13.3 | % | ||||||
|
Common
|
QVT Financial LP
1177 Avenue of the Americas, 9th Floor
New York, New York 10036
|
3,892,882 | (3) | 9.9 | % | 9.9 | % | ||||||
|
Common
|
Great Point Partners LLC
Jeffrey R. Jay, M.D.
David Kroin
165 Mason Street, 3rd Floor
Greenwich, CT 06830
|
3,962,028 | (4) | 9.9 | % | 8.3 | % | ||||||
|
Common
|
Quogue Capital LLC
Wayne P. Rothbaum
50 West 57th Street 15th Floor
New York, NY 10019
|
4,000,000 | (5) | 9.8 | % | 5.1 | % | ||||||
|
Common
|
James E. Flynn
780 Third Avenue, 37th Floor
New York, NY 10017
|
3,987,321 | (6) | 9.9 | % | 7.7 | % | ||||||
|
Common
|
First Eagle Investment Management, LLC
1345 Avenue of the Americas
New York, NY 10105
|
3,187,563 | (7) | 7.9 | % | 4.3 | % | ||||||
|
Series A
Preferred
|
Tokenhouse PTE LTD
9 – 11 Reitergasse
Zurich 8027, Switzerland
|
667 | 14.2 | % | * | ||||||||
|
Series A
Preferred
|
Steven N. Ostrovsky
43 Nikki Ct.
Morganville, NJ 07751
|
500 | 10.6 | % | * | ||||||||
|
Series A
Preferred
|
Thomas L. Cassidy IRA Rollover
38 Canaan Close
New Canaan, CT 06840
|
500 | 10.6 | % | * | ||||||||
|
Series A
Preferred
|
Jonathan E. Rothschild
300 Mercer St., #28F
New York, NY 10003
|
500 | 10.6 | % | * | ||||||||
|
Series A
Preferred
|
Arthur J. Nagle
19 Garden Avenue
Bronxville, NY 10708
|
250 | 5.3 | % | * | ||||||||
|
Series A
Preferred
|
Thomas P. and Mary E. Heiser, JTWROS
10 Ridge Road
Hopkinton, MA 01748
|
250 | 5.3 | % | * | ||||||||
|
Series A
Preferred
|
Carl F. Schwartz
31 West 87th St.
New York, NY 10016
|
250 | 5.3 | % | * | ||||||||
|
Series A
Preferred
|
Michael J. Wrubel
3650 N. 36 Avenue, #39
Hollywood, FL 33021
|
250 | 5.3 | % | * |
|
Class
|
Name and address of beneficial owner
|
Amount and nature
of beneficial ownership (1) |
Percent
of class
|
Percent of
total voting
power
|
|||||||||
|
Series A
Preferred
|
Myron M. Teitelbaum, M.D.
175 Burton Lane
Lawrence, NY 11559
|
250 | 5.3 | % | * | ||||||||
|
Series A
Preferred
|
Laura Gold Galleries Ltd. Profit Sharing Trust Park South Gallery at Carnegie Hall
154 West 57th Street, Suite 114
New York, NY 10019-3321
|
250 | 5.3 | % | * | ||||||||
|
Series A
Preferred
|
Laura Gold
180 W. 58th Street
New York, NY 10019
|
250 | 5.3 | % | * |
|
No.
|
Description
|
|
3.01
|
Certificate of amendment of restated certificate of incorporation. Incorporated by reference to Exhibit 3.1 of our Current Report on Form 8-K, filed with the SEC on May 12, 2011.
|
|
3.02
|
Restated certificate of incorporation, as amended. Incorporated by reference to Exhibit 3.01 of our Annual Report on Form 10-K for the year ended June 30, 2010, filed with the SEC on September 27, 2010.
|
|
3.03
|
Bylaws. Incorporated by reference to Exhibit 3.1 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2007, filed with the SEC on February 8, 2008.
|
|
4.01
|
Form of warrant issued to purchasers in our August 2009 registered direct offering. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on August 13, 2009.
|
|
4.02
|
Form of Series A and Series B warrant issued to purchasers in our February 2010 registered direct offering. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on March 1, 2010.
|
|
4.03
|
Form of warrant issued to purchasers in our June 2010 registered direct offering. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on June 28, 2010.
|
|
4.04
|
Form of waiver agreement relating to our Series A and Series B warrants issued to purchasers in our February 2010 registered direct offering. Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K, filed with the SEC on June 28, 2010.
|
|
4.05
|
Warrant Agreement dated as of March 1, 2011, between Palatin and American Stock Transfer & Trust Company, a New York limited liability trust company. Incorporated by reference to Exhibit 4.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.06
|
Definitive form of Series A 2011 Warrant certificate pursuant to Palatin’s effective registration statement No. 333-170227 on Form S-1. Incorporated by reference to Exhibit 4.2 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.07
|
Definitive form of Series B 2011 Warrant certificate pursuant to Palatin’s effective registration statement No. 333-170227 on Form S-1. Incorporated by reference to Exhibit 4.3 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.08
|
Definitive form of underwriters’ warrant to purchase common stock pursuant to Palatin’s effective registration statement No. 333-170227 on Form S-1. Incorporated by reference to Exhibit 4.4 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.09
|
Warrant issued to Noble International Investments, Inc. at an exercise price of $0.60 per share in connection with entering into a contract for financial advisory services.
Incorporated by reference to Exhibit 4.1 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2011, filed with the SEC on February 14, 2012.
|
|
No.
|
Description
|
|
4.10
|
Form of warrant issued to Noble International Investments, Inc. at exercise prices of $1.00 and $1.50 per share in connection with entering into a contract for financial advisory services. Incorporated by reference to Exhibit 4.2 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2011, filed with the SEC on February 14, 2012.
|
|
4.11
|
Warrant issued to Chardan Capital Markets, LLC at an exercise price of $0.75 per share in connection with entering into a contract for financial advisory services. Incorporated by reference to Exhibit 4.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2012, filed with the SEC on May 14, 2012.
|
|
4.12
|
Form of Series A 2012 common stock purchase warrant. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
4.13
|
Form of Series B 2012 common stock purchase warrant. Incorporated by reference to Exhibit 4.2 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
10.01
|
1996 Stock Option Plan, as amended. Incorporated by reference to Exhibit 10.01 of our Annual Report on Form 10-K for the year ended June 30, 2009, filed with the SEC on September 28, 2009.†
|
|
10.02
|
Strategic Collaboration Agreement dated as of August 17, 1999, between Palatin and Mallinckrodt, Inc. Incorporated by reference to Exhibit 10.21 of our amended Annual Report on Form 10-KSB/A for the year ended June 30, 1999, filed with the SEC on December 28, 1999.
|
|
10.03
|
Amendment To Strategic Collaboration Agreement dated as of May 13, 2002 between Palatin and Mallinckrodt, Inc. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2002, filed with the SEC on May 15, 2002.
|
|
10.04
|
Amendment to Strategic Collaboration Agreement dated as of October 1, 2005, between Palatin and Mallinckrodt, Inc. Incorporated by reference to Exhibit 10.32 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2005, filed with the SEC on November 8, 2005. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.05
|
Form of Option Certificate (incentive option) under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.06
|
Form of Incentive Stock Option Agreement under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.07
|
Form of Option Certificate (non-qualified option) under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.3 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.08
|
Form of Non-Qualified Stock Option Agreement under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.4 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.09
|
Research Collaboration and License Agreement dated January 30, 2007, between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2006, filed with the SEC on February 8, 2007. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.10
|
Palatin Technologies, Inc. 2007 Change in Control Severance Plan. Incorporated by reference to Exhibit 10.4 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2007, filed with the SEC on February 8, 2008. †
|
|
10.11
|
2005 Stock Plan, as amended December 7, 2007, March 10, 2009 and May 13, 2009. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2009, filed with the SEC on May 15, 2009. †
|
|
10.12
|
Form of Executive Officer Option Certificate. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008, filed with the SEC on May 14, 2008. †
|
| No. |
Description
|
|
10.13
|
Form of Amended Restricted Stock Unit Agreement. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008, filed with the SEC on May 14, 2008. †
|
|
10.14
|
Form of Amended Option Certificate (incentive option) under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.3 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008, filed with the SEC on May 14, 2008. †
|
|
10.15
|
First Amendment dated June 27, 2008 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.28 of our Annual Report on Form 10-K for the year ended June 30, 2008, filed with the SEC on September 29, 2008. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.16
|
Second Amendment dated December 5, 2008 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2008, filed with the SEC on February 13, 2009. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.17
|
Clinical Trial Sponsored Research Agreement dated December 5, 2008 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.3 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2008, filed with the SEC on February 13, 2009. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.18
|
Form of securities purchase agreement for our August 2009 registered direct offering. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on August 13, 2009.
|
|
10.19
|
Form of securities purchase agreement for our February 2010 registered direct offering. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on March 1, 2010.
|
|
10.20
|
Form of securities purchase agreement for our June 2010 registered direct offering. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on June 28, 2010.
|
|
10.21
|
Employment Agreement, effective as of July 1, 2010, between Palatin and Carl Spana. Incorporated by reference to Exhibit 10.23 of our Annual Report on Form 10-K for year ended June 30, 2010, filed with the SEC on September 27, 2010. †
|
|
10.22
|
Employment Agreement, effective as of July 1, 2010, between Palatin and Stephen T. Wills. Incorporated by reference to Exhibit 10.24 of our Annual Report on Form 10-K for year ended June 30, 2010, filed with the SEC on September 27, 2010. †
|
|
10.23
|
Employment Agreement, effective as of July 1, 2010, between Palatin and Trevor Hallam. Incorporated by reference to Exhibit 10.25 of our Annual Report on Form 10-K for year ended June 30, 2010, filed with the SEC on September 27, 2010. †
|
|
10.24
|
Third Amendment dated September 24, 2009 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2009, filed with the SEC on November 13, 2009. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.25
|
Separation Agreement, Waiver and Release by and between Palatin and Trevor Hallam, dated November 14, 2010. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on November 19, 2010. †
|
|
10.26
|
Underwriting Agreement dated February 24, 2011 by and between Palatin and Roth Capital Partners, LLC. Incorporated by reference to Exhibit 1.1 of our Current Report on Form 8-K, filed with the SEC on February 24, 2011.
|
|
10.27
|
2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. † |
| No. |
Description
|
|
10.28
|
Form of Restricted Share Unit Agreement under the 2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. †
|
|
10.29
|
Form of Nonqualified Stock Option Agreement under the 2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.3 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. †
|
|
10.30
|
Form of Incentive Stock Option Agreement under the 2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.4 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. †
|
|
10.31
|
Letter agreement dated October 7, 2011 between Palatin and Biotechnology Value Fund, L.P. Incorporated by reference to Exhibit 10.01 of our Current Report on Form 8-K, filed with the SEC on October 7, 2011.
|
|
10.32
|
Purchase Agreement, dated July 2, 2012, by and between Palatin Technologies, Inc. and QVT Fund IV LP, QVT Fund V LP and Quintessence Fund L.P. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
10.33
|
Registration Rights Agreement, dated July 2, 2012, by and between Palatin Technologies, Inc. and QVT Fund IV LP, QVT Fund V LP and Quintessence Fund L.P. Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
21
|
Subsidiaries of the registrant. *
|
|
23
|
Consent of KPMG LLP. *
|
|
31.1
|
Certification of Chief Executive Officer. *
|
|
31.2
|
Certification of Chief Financial Officer. *
|
|
32.1
|
Certification of principal executive officer pursuant to U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
|
32.2
|
Certification of principal financial officer pursuant to U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
|
101.INS
|
XBRL Instance Document *
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document *
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document *
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document *
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document *
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document *
|
|
PALATIN TECHNOLOGIES, INC.
|
||
|
By:
|
/s/ Carl Spana
|
|
|
|
Carl Spana, Ph.D. | |
|
|
President and Chief Executive Officer
(principal executive officer)
|
|
|
Signature
|
Title
|
Date
|
|
|
/s/ Carl Spana
|
President, Chief Executive Officer and Director
|
September 10, 2012
|
|
|
Carl Spana
|
(principal executive officer)
|
||
|
/s/ Stephen T. Wills
|
Executive Vice President, Chief Financial Officer and
|
September 10, 2012
|
|
|
Stephen T. Wills
|
Chief Operating Officer
(principal financial and accounting officer)
|
||
|
/s/ John K.A. Prendergast
|
Chairman and Director
|
September 10, 2012
|
|
|
John K.A. Prendergast
|
|||
|
/s/ Perry B. Molinoff
|
Director
|
September 10, 2012
|
|
|
Perry B. Molinoff
|
|||
|
/s/ Robert K. deVeer, Jr.
|
Director
|
September 10, 2012
|
|
|
Robert K. deVeer, Jr.
|
|||
|
/s/ Zola P. Horovitz
|
Director
|
September 10, 2012
|
|
|
Zola P. Horovitz
|
|||
|
/s/ Robert I. Taber
|
Director
|
September 10, 2012
|
|
|
Robert I. Taber
|
|||
|
/s/ J. Stanley Hull
|
Director
|
September 10, 2012
|
|
|
J. Stanley Hull
|
|||
|
/s/ Alan W. Dunton
|
Director
|
September 10, 2012
|
|
|
Alan W. Dunton
|
|
No.
|
Description
|
|
3.01
|
Certificate of amendment of restated certificate of incorporation. Incorporated by reference to Exhibit 3.1 of our Current Report on Form 8-K, filed with the SEC on May 12, 2011.
|
|
3.02
|
Restated certificate of incorporation, as amended. Incorporated by reference to Exhibit 3.01 of our Annual Report on Form 10-K for the year ended June 30, 2010, filed with the SEC on September 27, 2010.
|
|
3.03
|
Bylaws. Incorporated by reference to Exhibit 3.1 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2007, filed with the SEC on February 8, 2008.
|
|
4.01
|
Form of warrant issued to purchasers in our August 2009 registered direct offering. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on August 13, 2009.
|
|
4.02
|
Form of Series A and Series B warrant issued to purchasers in our February 2010 registered direct offering. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on March 1, 2010.
|
|
4.03
|
Form of warrant issued to purchasers in our June 2010 registered direct offering. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on June 28, 2010.
|
|
4.04
|
Form of waiver agreement relating to our Series A and Series B warrants issued to purchasers in our February 2010 registered direct offering. Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K, filed with the SEC on June 28, 2010.
|
|
4.05
|
Warrant Agreement dated as of March 1, 2011, between Palatin and American Stock Transfer & Trust Company, a New York limited liability trust company. Incorporated by reference to Exhibit 4.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.06
|
Definitive form of Series A 2011 Warrant certificate pursuant to Palatin’s effective registration statement No. 333-170227 on Form S-1. Incorporated by reference to Exhibit 4.2 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.07
|
Definitive form of Series B 2011 Warrant certificate pursuant to Palatin’s effective registration statement No. 333-170227 on Form S-1. Incorporated by reference to Exhibit 4.3 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.08
|
Definitive form of underwriters’ warrant to purchase common stock pursuant to Palatin’s effective registration statement No. 333-170227 on Form S-1. Incorporated by reference to Exhibit 4.4 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011.
|
|
4.09
|
Warrant issued to Noble International Investments, Inc. at an exercise price of $0.60 per share in connection with entering into a contract for financial advisory services.
Incorporated by reference to Exhibit 4.1 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2011, filed with the SEC on February 14, 2012.
|
|
4.10
|
Form of warrant issued to Noble International Investments, Inc. at exercise prices of $1.00 and $1.50 per share in connection with entering into a contract for financial advisory services. Incorporated by reference to Exhibit 4.2 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2011, filed with the SEC on February 14, 2012.
|
|
4.11
|
Warrant issued to Chardan Capital Markets, LLC at an exercise price of $0.75 per share in connection with entering into a contract for financial advisory services. Incorporated by reference to Exhibit 4.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2012, filed with the SEC on May 14, 2012.
|
|
4.12
|
Form of Series A 2012 common stock purchase warrant. Incorporated by reference to Exhibit 4.1 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
4.13
|
Form of Series B 2012 common stock purchase warrant. Incorporated by reference to Exhibit 4.2 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
10.01
|
1996 Stock Option Plan, as amended. Incorporated by reference to Exhibit 10.01 of our Annual Report on Form 10-K for the year ended June 30, 2009, filed with the SEC on September 28, 2009.†
|
| No. |
Description
|
|
10.02
|
Strategic Collaboration Agreement dated as of August 17, 1999, between Palatin and Mallinckrodt, Inc. Incorporated by reference to Exhibit 10.21 of our amended Annual Report on Form 10-KSB/A for the year ended June 30, 1999, filed with the SEC on December 28, 1999.
|
|
10.03
|
Amendment To Strategic Collaboration Agreement dated as of May 13, 2002 between Palatin and Mallinckrodt, Inc. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2002, filed with the SEC on May 15, 2002.
|
|
10.04
|
Amendment to Strategic Collaboration Agreement dated as of October 1, 2005, between Palatin and Mallinckrodt, Inc. Incorporated by reference to Exhibit 10.32 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2005, filed with the SEC on November 8, 2005. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.05
|
Form of Option Certificate (incentive option) under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.06
|
Form of Incentive Stock Option Agreement under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.07
|
Form of Option Certificate (non-qualified option) under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.3 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.08
|
Form of Non-Qualified Stock Option Agreement under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.4 of our Current Report on Form 8-K, filed with the SEC on September 21, 2005. †
|
|
10.09
|
Research Collaboration and License Agreement dated January 30, 2007, between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2006, filed with the SEC on February 8, 2007. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.10
|
Palatin Technologies, Inc. 2007 Change in Control Severance Plan. Incorporated by reference to Exhibit 10.4 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2007, filed with the SEC on February 8, 2008. †
|
|
10.11
|
2005 Stock Plan, as amended December 7, 2007, March 10, 2009 and May 13, 2009. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2009, filed with the SEC on May 15, 2009. †
|
|
10.12
|
Form of Executive Officer Option Certificate. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008, filed with the SEC on May 14, 2008. †
|
|
10.13
|
Form of Amended Restricted Stock Unit Agreement. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008, filed with the SEC on May 14, 2008. †
|
|
10.14
|
Form of Amended Option Certificate (incentive option) under the 2005 Stock Plan. Incorporated by reference to Exhibit 10.3 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2008, filed with the SEC on May 14, 2008. †
|
|
10.15
|
First Amendment dated June 27, 2008 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.28 of our Annual Report on Form 10-K for the year
ended June 30, 2008, filed with the SEC on September 29, 2008. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.16
|
Second Amendment dated December 5, 2008 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2008, filed with the SEC on February 13, 2009. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
| No. |
Description
|
|
10.17
|
Clinical Trial Sponsored Research Agreement dated December 5, 2008 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.3 of our Quarterly Report on Form 10-Q for the quarter ended December 31, 2008, filed with the SEC on February 13, 2009. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.18
|
Form of securities purchase agreement for our August 2009 registered direct offering. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on August 13, 2009.
|
|
10.19
|
Form of securities purchase agreement for our February 2010 registered direct offering. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on March 1, 2010.
|
|
10.20
|
Form of securities purchase agreement for our June 2010 registered direct offering. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on June 28, 2010.
|
|
10.21
|
Employment Agreement, effective as of July 1, 2010, between Palatin and Carl Spana. Incorporated by reference to Exhibit 10.23 of our Annual Report on Form 10-K for year ended June 30, 2010, filed with the SEC on September 27, 2010. †
|
|
10.22
|
Employment Agreement, effective as of July 1, 2010, between Palatin and Stephen T. Wills. Incorporated by reference to Exhibit 10.24 of our Annual Report on Form 10-K for year ended June 30, 2010, filed with the SEC on September 27, 2010. †
|
|
10.23
|
Employment Agreement, effective as of July 1, 2010, between Palatin and Trevor Hallam. Incorporated by reference to Exhibit 10.25 of our Annual Report on Form 10-K for year ended June 30, 2010, filed with the SEC on September 27, 2010. †
|
|
10.24
|
Third Amendment dated September 24, 2009 to the Research Collaboration and License Agreement between Palatin and AstraZeneca AB. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2009, filed with the SEC on November 13, 2009. We have obtained confidential treatment of certain provisions contained in this exhibit. The copy filed as an exhibit omits the information subject to the confidentiality request.
|
|
10.25
|
Separation Agreement, Waiver and Release by and between Palatin and Trevor Hallam, dated November 14, 2010. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on November 19, 2010. †
|
|
10.26
|
Underwriting Agreement dated February 24, 2011 by and between Palatin and Roth Capital Partners, LLC. Incorporated by reference to Exhibit 1.1 of our Current Report on Form 8-K, filed with the SEC on February 24, 2011.
|
|
10.27
|
2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.1 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. †
|
|
10.28
|
Form of Restricted Share Unit Agreement under the 2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.2 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May
13, 2011. †
|
|
10.29
|
Form of Nonqualified Stock Option Agreement under the 2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.3 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. †
|
|
10.30
|
Form of Incentive Stock Option Agreement under the 2011 Stock Incentive Plan. Incorporated by reference to Exhibit 10.4 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed with the SEC on May 13, 2011. †
|
|
10.31
|
Letter agreement dated October 7, 2011 between Palatin and Biotechnology Value Fund, L.P. Incorporated by reference to Exhibit 10.01 of our Current Report on Form 8-K, filed with the SEC on October 7, 2011.
|
| No. |
Description
|
|
10.32
|
Purchase Agreement, dated July 2, 2012, by and between Palatin Technologies, Inc. and QVT Fund IV LP, QVT Fund V LP and Quintessence Fund L.P. Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
10.33
|
Registration Rights Agreement, dated July 2, 2012, by and between Palatin Technologies, Inc. and QVT Fund IV LP, QVT Fund V LP and Quintessence Fund L.P. Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K, filed with the SEC on July 6, 2012.
|
|
21
|
|
|
23
|
|
|
31.1
|
|
|
31.2
|
|
|
32.1
|
|
|
32.2
|
|
|
101.INS
|
XBRL Instance Document *
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document *
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document *
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document *
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document *
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document *
|