|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
04-3475813
|
||
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
Accelerated filer ☒
|
|
|
Non-accelerated filer ☐
|
Smaller reporting company
☒
|
|
Emerging growth company ☒
|
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
|
Common Stock, $0.01 par value
|
RCKT
|
Nasdaq Global Market
|
|
Page
|
||
|
Item 1.
|
4 | |
|
4
|
||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 | ||
|
Item 2.
|
19 | |
|
Item 3.
|
28 | |
|
Item 4.
|
29 | |
|
PART II - OTHER INFORMATION
|
||
|
Item 1.
|
29 | |
|
Item 1A.
|
29 | |
|
Item 2.
|
55 | |
|
Item 3.
|
55 | |
|
Item 4.
|
55 | |
|
Item 5.
|
55 | |
|
Item 6.
|
56 | |
| 57 | ||
|
|
• |
federal, state, and non-U.S. regulatory requirements, including regulation of our current or any other future product candidates by the U.S. Food and Drug Administration (“FDA”);
|
|
|
• |
the safety, effectiveness and timing of product candidates that Rocket may develop, to treat Fanconi Anemia (FA), Leukocyte Adhesion Deficiency-I (LAD-I), Pyruvate Kinase Deficiency (PKD), Infantile Malignant Osteopetrosis (IMO) and Danon
Disease;
|
|
|
• |
the timing of and our ability to submit regulatory filings to the FDA and to obtain and maintain FDA or other regulatory authority approval, or other actions with respect to our product candidates;
|
|
|
• |
our competitors’ activities, including decisions as to the timing of competing product launches, generic entrants, pricing and discounting;
|
|
|
• |
whether safety and efficacy results of our clinical trials and other required tests for approval of our product candidates provide data to warrant progression of clinical trials, potential regulatory approval or further development of any
of our product candidates;
|
|
|
• |
our ability to develop, acquire and advance product candidates, enroll a sufficient number of patients into, and successfully complete, clinical studies, and our ability to apply for and obtain regulatory approval for such product
candidates, within currently anticipated timeframes, or at all;
|
|
|
• |
our ability to establish key collaborations and vendor relationships for our product candidates and any other future product candidates;
|
|
|
• |
our ability to successfully develop and commercialize any technology that we may in-license or products we may acquire;
|
|
|
• |
our ability to acquire additional businesses, form strategic alliances or create joint ventures and our ability to realize the benefit of such acquisitions, alliances or joint ventures;
|
|
|
• |
unanticipated delays due to manufacturing difficulties, including the development of our direct manufacturing capabilities for our AAV programs, and any supply constraints or changes in the regulatory environment;
|
|
|
• |
our ability to successfully operate in non-U.S. jurisdictions in which we currently or in the future may do business, including compliance with applicable regulatory requirements and laws;
|
|
|
• |
uncertainties associated with obtaining and enforcing patents to protect our product candidates, and our ability to successfully defend ourselves against unforeseen third-party infringement claims;
|
|
|
• |
anticipated trends and challenges in our business and the markets in which we operate;
|
|
|
• |
our estimates regarding our capital requirements;
|
|
|
• |
our ability to obtain additional financing and raise capital as necessary to fund operations or pursue business opportunities ; and
|
|
|
• |
the impact of the COVID-19 pandemic, including the scope thereof, on our business, operations, strategy, goals and anticipated milestones, including our ongoing and planned research and discovery activities, ability to conduct ongoing and
planned preclinical studies and clinical trials, clinical supply of current or future drug candidates.
|
|
March 31,
2020
|
December 31,
2019
|
|||||||
|
Assets
|
(unaudited)
|
|||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
190,520
|
$
|
185,383
|
||||
|
Investments
|
85,375
|
118,732
|
||||||
|
Prepaid expenses and other assets
|
3,910
|
3,639
|
||||||
|
Total current assets
|
279,805
|
307,754
|
||||||
|
Property and equipment, net
|
11,140
|
29,521
|
||||||
|
Goodwill
|
30,815
|
30,815
|
||||||
|
Restricted cash
|
1,525
|
1,525
|
||||||
|
Deposits
|
455
|
455
|
||||||
|
Operating lease right-of-use assets
|
1,470
|
2,051
|
||||||
|
Finance lease right-of-use asset
|
47,541
|
-
|
||||||
|
Total assets
|
$
|
372,751
|
$
|
372,121
|
||||
|
Liabilities and stockholders' equity
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses
|
$
|
18,819
|
$
|
17,352
|
||||
|
Operating lease liabilities, current
|
817
|
957
|
||||||
|
Finance lease liabilities, current
|
1,478
|
-
|
||||||
|
Total current liabilities
|
21,114
|
18,309
|
||||||
|
Convertible notes, net of unamortized discount
|
45,794
|
45,049
|
||||||
|
Operating lease liabilities, non-current
|
917
|
1,443
|
||||||
|
Finance lease liabilities, non-current
|
18,852
|
-
|
||||||
|
Other liabilities
|
23
|
23
|
||||||
|
Total liabilities
|
86,700
|
64,824
|
||||||
|
Commitments and contingencies (Note 11)
|
||||||||
|
Stockholders' equity:
|
||||||||
|
Preferred stock, $0.01 par value, authorized 5,000,000 shares:
|
||||||||
|
Series A convertible preferred stock; 300,000 shares designated as Series A; 0 shares issued and outstanding
|
-
|
-
|
||||||
|
Series B convertible preferred stock; 300,000 shares designated as Series B; 0 shares issued and outstanding
|
-
|
-
|
||||||
|
Common stock, $0.01 par value, 120,000,000 shares authorized; 55,157,035 and 54,773,061 shares issued and 55,126,474 and 54,769,030 shares outstanding at March 31, 2020 and December 31, 2019,
respectively
|
|
|
551
|
|
|
|
548
|
|
|
Treasury stock, at cost, 30,561 and 4,031 common shares at March 31, 2020 and December 31, 2019, respectively
|
(429
|
)
|
(53
|
)
|
||||
|
Additional paid-in capital
|
493,811
|
489,925
|
||||||
|
Accumulated other comprehensive loss (income)
|
(75
|
)
|
20
|
|||||
|
Accumulated deficit
|
(207,807
|
)
|
(183,143
|
)
|
||||
|
Total stockholders' equity
|
286,051
|
307,297
|
||||||
|
Total liabilities and stockholders' equity
|
$
|
372,751
|
$
|
372,121
|
||||
|
|
Three Months Ended March 31,
|
|||||||
|
|
2020
|
2019
|
||||||
|
Revenue
|
$
|
-
|
$
|
-
|
||||
|
Operating expenses:
|
||||||||
|
Research and development
|
16,957
|
15,137
|
||||||
|
General and administrative
|
7,163
|
3,808
|
||||||
|
Total operating expenses
|
24,120
|
18,945
|
||||||
|
Loss from operations
|
(24,120
|
)
|
(18,945
|
)
|
||||
|
Research and development incentives
|
-
|
250
|
||||||
|
Interest expense
|
(1,573
|
)
|
(1,604
|
)
|
||||
|
Interest and other income net
|
967
|
601
|
||||||
|
Accretion of discount on investments
|
62
|
247
|
||||||
|
Net loss
|
$
|
(24,664
|
)
|
$
|
(19,451
|
)
|
||
|
Net loss per share attributable to common stockholders - basic and diluted
|
$
|
(0.45
|
)
|
$
|
(0.43
|
)
|
||
|
Weighted-average common shares outstanding - basic and diluted
|
54,883,120
|
45,122,815
|
||||||
|
|
Three Months Ended March 31,
|
|||||||
|
|
2020
|
2019
|
||||||
|
Net loss
|
$
|
(24,664
|
)
|
$
|
(19,451
|
)
|
||
|
Other comprehensive loss
|
||||||||
|
Net unrealized gain (loss) on investments
|
(95
|
)
|
38
|
|||||
|
Total comprehensive loss
|
$
|
(24,759
|
)
|
$
|
(19,413
|
)
|
||
|
|
|
Treasury
Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
Income/(Loss)
|
Accumulated
Deficit
|
Total
Stockholders'
Equity
|
||||||||||||||||||||||
|
Common Stock
|
||||||||||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||||||||
|
Balance at December 31, 2019
|
54,773,061
|
$
|
548
|
$
|
(53
|
)
|
$
|
489,925
|
$
|
20
|
$
|
(183,143
|
)
|
$
|
307,297
|
|||||||||||||
|
Issuance of common stock pursuant to exercise of stock options
|
386,974
|
3
|
-
|
(3
|
)
|
-
|
-
|
-
|
||||||||||||||||||||
|
Common stock repurchase
|
(3,000
|
)
|
-
|
-
|
(72
|
)
|
-
|
-
|
(72
|
)
|
||||||||||||||||||
|
Sale of treasury stock
|
-
|
-
|
53
|
-
|
-
|
-
|
53
|
|||||||||||||||||||||
|
Issuance of treasury stock pursuant to exercise of stock options
|
-
|
-
|
(429
|
)
|
-
|
-
|
-
|
(429
|
)
|
|||||||||||||||||||
|
Unrealized comprehensive loss on marketable securities
|
-
|
-
|
-
|
-
|
(95
|
)
|
-
|
(95
|
)
|
|||||||||||||||||||
|
Stock-based compensation
|
-
|
-
|
-
|
3,961
|
-
|
-
|
3,961
|
|||||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
(24,664
|
)
|
(24,664
|
)
|
|||||||||||||||||||
|
Balance at March 31, 2020
|
55,157,035
|
$
|
551
|
(429
|
)
|
$
|
493,811
|
$
|
(75
|
)
|
$
|
(207,807
|
)
|
$
|
286,051
|
|||||||||||||
|
|
|
|
|
Treasury
Stock
|
|
|
Additional
Paid-In
Capital
|
|
|
Accumulated
Other
Comprehensive
Loss
|
|
|
Accumulated
Deficit
|
|
|
Total
Stockholders'
Equity
|
|
|||||||||||
|
Common Stock
|
||||||||||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||||||||
|
Balance at December 31, 2018
|
45,194,736
|
$
|
452
|
$
|
(668
|
)
|
$
|
300,253
|
$
|
(127
|
)
|
$
|
(105,873
|
)
|
$
|
194,037
|
||||||||||||
|
Issuance of common stock pursuant to exercise of stock options
|
19,701
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Stock repurchase
|
-
|
-
|
(725
|
)
|
(2
|
)
|
-
|
-
|
(727
|
)
|
||||||||||||||||||
|
Retirement of treasury stock
|
(100,000
|
)
|
(1
|
)
|
1,393
|
(1,392
|
)
|
-
|
-
|
-
|
||||||||||||||||||
|
Unrealized comprehensive gain on marketable securities
|
-
|
-
|
-
|
-
|
38
|
-
|
38
|
|||||||||||||||||||||
|
Stock-based compensation
|
-
|
-
|
-
|
3,180
|
-
|
-
|
3,180
|
|||||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
(19,451
|
)
|
(19,451
|
)
|
|||||||||||||||||||
|
Balance at March 31, 2019
|
45,114,437
|
$
|
451
|
-
|
$
|
302,039
|
$
|
(89
|
)
|
$
|
(125,324
|
)
|
$
|
177,077
|
||||||||||||||
|
|
Three Months Ended March 31,
|
|||||||
|
|
2020
|
2019
|
||||||
|
Operating Activities:
|
||||||||
|
Net loss
|
$
|
(24,664
|
)
|
$
|
(19,451
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||
|
Accretion of discount on convertible notes
|
844
|
850
|
||||||
|
Increase in lease liability
|
-
|
190
|
||||||
|
Depreciation expense
|
118
|
102
|
||||||
|
Write down of property and equipment
|
63
|
-
|
||||||
|
Stock-based compensation
|
3,961
|
3,180
|
||||||
|
Accretion of discount on investments
|
(63
|
)
|
(247
|
)
|
||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Prepaid expenses and other assets
|
(271
|
)
|
(307
|
)
|
||||
|
Accounts payable and accrued expenses
|
(1,932
|
)
|
(84
|
)
|
||||
|
Operating lease liabilities
|
(452
|
)
|
(210
|
)
|
||||
|
Finance lease liability
|
151
|
-
|
||||||
|
Net cash used in operating activities
|
(22,245
|
)
|
(15,977
|
)
|
||||
|
Investing activities:
|
||||||||
|
Purchases of investments
|
(23,481
|
)
|
-
|
|||||
|
Proceeds from maturities of investments
|
56,805
|
29,935
|
||||||
|
Payments made to acquire right of use asset
|
(530
|
)
|
-
|
|||||
|
Purchases of property and equipment
|
(5,207
|
)
|
(760
|
)
|
||||
|
Purchases of internal use software
|
(88
|
)
|
-
|
|||||
|
Net cash provided by investing activities
|
27,499
|
29,175
|
||||||
|
Financing activities:
|
||||||||
|
Proceeds from sale of treasury stock
|
53
|
-
|
||||||
|
Common stock repurchase
|
(72
|
)
|
-
|
|||||
|
Convertible notes refinancing costs to the lender
|
(98
|
)
|
-
|
|||||
|
Net cash used in financing activities
|
(117
|
)
|
-
|
|||||
|
Net change in cash, cash equivalents and restricted cash
|
5,137
|
13,198
|
||||||
|
Cash, cash equivalents and restricted cash at beginning of period
|
186,908
|
112,791
|
||||||
|
Cash, cash equivalents and restricted cash at end of period
|
$
|
192,045
|
$
|
125,989
|
||||
|
Supplemental disclosure of non-cash financing and investing activities:
|
||||||||
|
Accrued purchases of property and equipment
|
$
|
2,796
|
$
|
4,392
|
||||
|
Accrued purchases of internal use software
|
$
|
174
|
$
|
-
|
||||
|
Retirement of treasury stock
|
$
|
72
|
$
|
1,395
|
||||
|
Witholding tax payable on shares witheld in treasury stock
|
$
|
429
|
$
|
-
|
||||
|
Unrealized (loss) gain on investments
|
$
|
(95
|
)
|
$
|
38
|
|||
|
Finance lease right of use asset and lease liability
|
$
|
20,179
|
$
|
-
|
||||
|
Reclassification of construction in process of finance right of use asset
|
$
|
26,465
|
$
|
-
|
||||
|
Supplemental cash flow information:
|
||||||||
|
Cash paid for interest
|
$
|
1,495
|
$
|
1,495
|
||||
| 1. |
Nature of Business
|
| 2. |
Risks and Liquidity
|
| 3. |
Basis of Presentation, Principles of Consolidation and Summary of Significant Accounting Policies
|
|
|
March 31,
2020
|
December 31,
2019
|
||||||
|
|
||||||||
|
Cash and cash equivalents
|
$
|
190,520
|
$
|
185,383
|
||||
|
Restricted cash
|
1,525
|
1,525
|
||||||
|
|
$
|
192,045
|
$
|
186,908
|
||||
| 4. |
Fair Value of Financial Instruments
|
|
|
Fair Value Measurements as of
March 31, 2020 Using:
|
|||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market mutual funds (included in cash and cash equivalents)
|
$
|
106,221
|
$
|
-
|
$
|
-
|
$
|
106,221
|
||||||||
|
Cash
|
7,552
|
-
|
-
|
7,552
|
||||||||||||
|
113,773
|
-
|
-
|
113,773
|
|||||||||||||
|
United States Treasury securities
|
43,135
|
-
|
-
|
43,135
|
||||||||||||
|
Corporate Bonds
|
-
|
36,240
|
-
|
36,240
|
||||||||||||
|
Municipal Bonds
|
-
|
6,000
|
-
|
6,000
|
||||||||||||
|
Investments
|
43,135
|
42,240
|
-
|
85,375
|
||||||||||||
|
$
|
156,908
|
$
|
42,240
|
$
|
-
|
$
|
199,148
|
|||||||||
|
|
Fair Value Measurements as of
December 31, 2019 Using:
|
|||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market mutual funds (included in cash and cash equivalents)
|
$
|
72,114
|
$
|
-
|
$
|
-
|
$
|
72,114
|
||||||||
|
Cash
|
7,542
|
-
|
-
|
7,542
|
||||||||||||
|
79,656
|
-
|
-
|
79,656
|
|||||||||||||
|
United States Treasury securities
|
75,464
|
-
|
-
|
75,464
|
||||||||||||
|
Government Bonds
|
-
|
8,000
|
-
|
8,000
|
||||||||||||
|
Corporate Bonds
|
-
|
29,268
|
-
|
29,268
|
||||||||||||
|
Municipal Bonds
|
-
|
6,000
|
-
|
6,000
|
||||||||||||
|
Investments
|
75,464
|
43,268
|
-
|
118,732
|
||||||||||||
|
$
|
155,120
|
$
|
43,268
|
$
|
-
|
$
|
198,388
|
|||||||||
| 5. |
Property and Equipment
|
|
|
March 31,
2020
|
December 31,
2019
|
||||||
|
Laboratory equipment
|
$
|
9,724
|
$
|
1,910
|
||||
|
Computer equipment
|
179
|
179
|
||||||
|
Furniture and fixtures
|
1,743
|
273
|
||||||
|
Leasehold improvements
|
29
|
29
|
||||||
|
Internal use software
|
488
|
226
|
||||||
|
Construction costs in progress
|
-
|
27,809
|
||||||
|
|
12,163
|
30,426
|
||||||
|
Less: accumulated depreciation
|
(1,023
|
)
|
(905
|
)
|
||||
|
|
$
|
11,140
|
$
|
29,521
|
||||
| 6. |
Accounts Payable and Accrued Expenses
|
|
|
March 31,
2020
|
December 31,
2019
|
||||||
|
Research and development
|
$
|
12,142
|
$
|
7,418
|
||||
|
Property and equipment
|
2,796
|
4,424
|
||||||
|
Bonus
|
790
|
2,459
|
||||||
|
Accrued interest
|
500
|
1,241
|
||||||
|
Government grant payable
|
569
|
562
|
||||||
|
Professional fees
|
531
|
553
|
||||||
|
Accrued vacation
|
275
|
129
|
||||||
|
Internal use software
|
174
|
226
|
||||||
|
Debt exchange fees
|
283
|
-
|
||||||
|
Other
|
759
|
340
|
||||||
|
|
$
|
18,819
|
$
|
17,352
|
||||
| 7. |
Convertible Notes
|
|
Principal amount
|
$
|
12,650
|
||
|
Discount
|
(1,451
|
)
|
||
|
Carrying value as of March 31, 2020
|
$
|
11,199
|
|
Principal amount
|
$
|
39,350
|
||
|
Discount
|
(4,755
|
)
|
||
|
Carrying value as of March 31, 2020
|
$
|
34,595
|
| 8. |
Stock Based Compensation
|
|
|
Three Months Ended March 31,
|
|||||||
|
2020
|
2019
|
|||||||
|
Risk-free interest rate
|
1.48
|
%
|
2.61
|
%
|
||||
|
Expected term (in years)
|
5.87
|
5.77
|
||||||
|
Expected volatility
|
77.75
|
%
|
74.60
|
%
|
||||
|
Expected dividend yield
|
0.00
|
%
|
0.00
|
%
|
||||
|
Exercise price
|
$
|
22.47
|
$
|
14.61
|
||||
|
Fair value of common stock
|
$
|
22.47
|
$
|
14.61
|
||||
|
Number of
Shares
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Contractual
Term (Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding as of December 31, 2019
|
9,763,541
|
$
|
5.96
|
6.96
|
$
|
164,021
|
||||||||||
|
Granted
|
1,188,306
|
22.47
|
9.86
|
|||||||||||||
|
Exercised
|
(384,453
|
)
|
1.30
|
6,766
|
||||||||||||
|
Forfeited
|
(28,504
|
)
|
17.39
|
|||||||||||||
|
Outstanding as of March 31, 2020
|
10,538,890
|
$
|
7.96
|
7.09
|
$
|
80,643
|
||||||||||
|
Options vested and exercisable as of March 31, 2020
|
7,572,141
|
$
|
4.09
|
6.27
|
$
|
78,872
|
||||||||||
|
Options unvested as of March 31, 2020
|
2,966,749
|
$
|
17.85
|
9.18
|
||||||||||||
|
|
Three Months Ended March 31,
|
|||||||
|
2020
|
2019
|
|||||||
|
Research and development
|
$
|
1,735
|
$
|
1,565
|
||||
|
General and administrative
|
2,226
|
1,615
|
||||||
|
Total share based compensation expense
|
$
|
3,961
|
$
|
3,180
|
||||
| 9. |
Stockholders’ Equity
|
| 10. |
Net Loss Per Share
|
|
Three Months Ended March 31,
|
||||||||
|
2020
|
2019
|
|||||||
|
Numerator:
|
||||||||
|
Net loss attributable to common stockholders
|
$
|
(24,664
|
)
|
$
|
(19,451
|
)
|
||
|
Denominator:
|
||||||||
|
Weighted-average common shares outstanding - basic and diluted
|
54,883,120
|
45,122,815
|
||||||
|
Net loss per share attributable to common stockholders - basic and diluted
|
$
|
(0.45
|
)
|
$
|
(0.43
|
)
|
||
|
Three Months Ended March 31,
|
||||||||
|
2020
|
2019
|
|||||||
|
Shares issuable upon conversion of the 2021 Convertible Notes
|
394,327
|
1,620,848
|
||||||
|
Shares issuable upon conversion of the 2022 Convertible Notes
|
1,226,621
|
-
|
||||||
|
Warrants exercisable for common shares
|
14,102
|
14,102
|
||||||
|
Options to purchase common shares
|
10,538,890
|
9,392,821
|
||||||
|
12,173,940
|
11,027,771
|
|||||||
| 11. |
Commitments and Contingencies
|
|
Lease cost
|
March 31, 2020
|
|||
|
Operating lease cost
|
$
|
258
|
||
|
Finance lease cost
|
||||
|
Amortization of right of use assets
|
162
|
|||
|
Interest on lease liablities
|
151
|
|||
|
Total lease cost
|
$
|
571
|
||
|
Maturity of operating lease liabilities
|
March 31, 2020
|
|||
|
2020
|
$
|
690
|
||
|
2021
|
682
|
|||
|
2022
|
445
|
|||
|
2023
|
75
|
|||
|
Total lease payments
|
$
|
1,892
|
||
|
Less: interest
|
(158
|
)
|
||
|
Total operating lease liabilities
|
$
|
1,734
|
||
|
Maturity of finance lease liability
|
March 31, 2020
|
|||
|
2020
|
$
|
1,070
|
||
|
2021
|
1,644
|
|||
|
2022
|
1,689
|
|||
|
2023
|
1,736
|
|||
|
2024
|
1,791
|
|||
|
Thereafter
|
48,767
|
|||
|
Total lease payments
|
$
|
56,697
|
||
|
Less: interest
|
(36,367
|
)
|
||
|
Total finance lease liability
|
$
|
20,330
|
||
|
Leases
|
March 31, 2020
|
|||
|
Operating right-of-use assets
|
$
|
1,470
|
||
|
Operating current lease liabilities
|
817
|
|||
|
Operating noncurrent lease liabilities
|
917
|
|||
|
Total operating lease liabilities
|
$
|
1,734
|
||
|
Finance right-of-use assets
|
$
|
47,541
|
||
|
Finance current lease liability
|
1,478
|
|||
|
Finance noncurrent lease liability
|
18,852
|
|||
|
Total finance lease liability
|
$
|
20,330
|
||
|
Other information
|
||||
|
Cash paid for amounts included in the measurement of lease liabilities:
|
||||
|
Operating cash flows from operating leases
|
$
|
340
|
||
|
Cash flows from finance lease
|
-
|
|||
|
Weighted-average remaining lease term - operating leases
|
2.4 years
|
|||
|
Weighted-average remaining lease term - finance lease
|
24 years
|
|||
|
Weighted-average discount rate - operating leases
|
7.77
|
%
|
||
|
Weighted-average discount rate - finance lease
|
8.96
|
%
|
||
| 12. |
Agreements Related to Intellectual Property
|
| 13. |
Strategic Research Collaboration
|
| 14. |
CIRM Grant
|
| 15. |
Related Party Transactions
|
| 16. |
401(k) Savings Plan
|
| Item 2. |
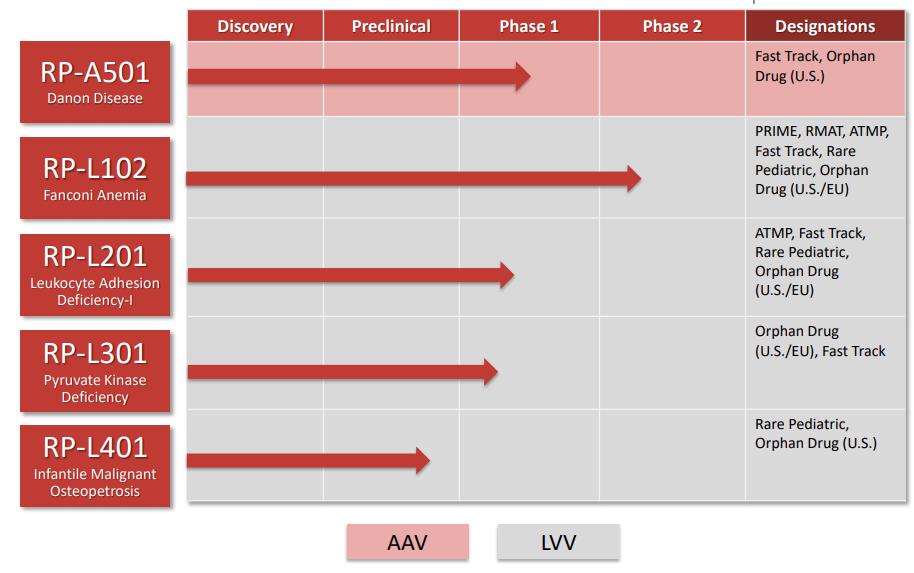
|
|
• |
expenses incurred under agreements with research institutions that conduct research and development activities including, process development, preclinical, and clinical activities on Rocket’s behalf;
|
|
|
• |
consultants supporting process development and regulatory activities; and
|
|
|
• |
costs related to in-licensing of rights to develop and commercialize our product candidate portfolio.
|
| • |
salaries and personnel-related costs, including benefits, travel and stock-based compensation, for our scientific personnel performing research and development activities;
|
| • |
facilities and other expenses, which include expenses for rent and maintenance of facilities, and depreciation expense and;
|
| • |
laboratory supplies and equipment used for internal research and development activities.
|
|
•
|
the scope, rate of progress, and expense of ongoing as well as any future clinical studies and other research and development activities that we undertake;
|
|
•
|
future clinical trial results;
|
| • |
uncertainties in clinical trial enrollment rates;
|
| • |
changing standards for regulatory approval; and
|
| • |
the timing and receipt of any regulatory approvals.
|
|
•
|
the scope, progress, outcome and costs of our clinical trials and other research and development activities;
|
|
•
|
the efficacy and potential advantages of our product candidates compared to alternative treatments, including any standard of care;
|
|
•
|
the market acceptance of our product candidates;
|
|
•
|
obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights;
|
|
•
|
significant and changing government regulation; and
|
|
•
|
the timing, receipt and terms of any marketing approvals.
|
|
|
Three Months Ended March 31,
|
|||||||||||
|
2020
|
2019
|
Change
|
||||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
$
|
16,957
|
$
|
15,137
|
$
|
1,820
|
||||||
|
General and administrative
|
7,163
|
3,808
|
3,355
|
|||||||||
|
Total operating expenses
|
24,120
|
18,945
|
5,175
|
|||||||||
|
Loss from operations
|
(24,120
|
)
|
(18,945
|
)
|
(5,175
|
)
|
||||||
|
Research and development incentives
|
-
|
250
|
(250
|
)
|
||||||||
|
Interest expense
|
(1,573
|
)
|
(1,604
|
)
|
31
|
|||||||
|
Interest and other income net
|
967
|
601
|
366
|
|||||||||
|
Accretion of discount on investments
|
62
|
247
|
(185
|
)
|
||||||||
|
Total other income (expense), net
|
(544
|
)
|
(506
|
)
|
(38
|
)
|
||||||
|
Net loss
|
$
|
(24,664
|
)
|
$
|
(19,451
|
)
|
$
|
(5,213
|
)
|
|||
|
|
Three Months Ended March 31,
|
|||||||
|
2020
|
2019
|
|||||||
|
Cash used in operating activities
|
$
|
(22,245
|
)
|
$
|
(15,977
|
)
|
||
|
Cash provided by investing activities
|
27,499
|
29,175
|
||||||
|
Cash used in financing activities
|
(117
|
)
|
-
|
|||||
|
Net change in cash, cash equivalents and restricted cash
|
$
|
5,137
|
$
|
13,198
|
||||
| • |
leverage our programs to advance other product candidates into preclinical and clinical development;
|
| • |
seek regulatory agreements to initiate clinical trials in the EU, US and ROW;
|
| • |
hire additional preclinical, clinical, regulatory, quality and scientific personnel;
|
| • |
maintain, expand and protect our intellectual property portfolio; and
|
| • |
acquire or in-license other product candidates and technologies.
|
| • |
the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials;
|
| • |
the costs, timing and outcome of regulatory review of our product candidates;
|
| • |
the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval;
|
| • |
the costs of manufacturing commercial-grade product to support commercial launch;
|
| • |
the ability to receive additional non-dilutive funding, including grants from organizations and foundations;
|
| • |
the revenue, if any, received from commercial sale of its products, should any of its product candidates receive marketing approval;
|
| • |
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims;
|
| • |
our ability to establish and maintain collaborations on favorable terms, if at all;
|
| • |
the extent to which we acquire or in-license other product candidates and technologies; and
|
| • |
the timing, receipt and amount of sales of, or milestone payments related to our royalties on, current or future product candidates, if any.
|
| Item 3. |
| Item 4. |
| Item 1. |
| Item 1A. |
|
|
• |
the timing of enrollment, commencement, completion and results of our clinical trials;
|
|
|
• |
the production of LVV and AAV gene therapy products to support preclinical and clinical needs;
|
|
|
• |
the results of our preclinical studies for our current product candidates and any subsequent clinical trials;
|
|
|
• |
the scope, progress, results and costs of drug discovery, laboratory testing, preclinical development and clinical trials, if any, for our internal product candidates; the costs associated with building out additional laboratory and
research capacity;
|
|
|
• |
the costs, timing and outcome of regulatory review of our product candidates;
|
|
|
• |
the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval;
|
|
|
• |
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims;
|
|
|
• |
our current licensing agreements or collaborations remaining in effect;
|
|
|
• |
our ability to establish and maintain additional licensing agreements or collaborations on favorable terms, if at all;
|
|
|
• |
the extent to which we acquire or in-license other product candidates and technologies;
|
|
|
• |
the costs associated with being a public company.
|
|
|
• |
completing research and preclinical and clinical development of our product candidates;
|
|
|
• |
seeking and obtaining regulatory and marketing approvals for product candidates for which we complete clinical studies;
|
|
|
• |
developing a sustainable, commercial-scale, reproducible, and transferable manufacturing process for our vectors and product candidates;
|
|
|
• |
establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products and services to support clinical development and the market demand for our product
candidates, if approved;
|
|
|
• |
launching and commercializing product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales force, marketing and distribution
infrastructure;
|
|
|
• |
obtaining sufficient pricing and reimbursement for our product candidates from private and governmental payors;
|
|
|
• |
obtaining market acceptance of our product candidates and gene therapy as a viable treatment option;
|
|
|
• |
addressing any competing technological and market developments;
|
|
|
• |
identifying and validating new gene therapy product candidates;
|
|
|
• |
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; and
|
|
|
• |
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how.
|
|
|
• |
failure of patients to enroll in the studies at the rate we expect;
|
|
|
• |
ineffectiveness of our product candidates;
|
|
|
• |
patients experiencing unexpected side effects or other safety concerns being raised during treatment;
|
|
|
• |
changes in governmental regulations or administrative actions;
|
|
|
• |
failure to conduct studies in accordance with required clinical practices;
|
|
|
• |
inspection of clinical study operations or study sites by the FDA, the EMA or other regulatory authorities, resulting in a clinical hold;
|
|
|
• |
insufficient financial resources;
|
|
|
• |
insufficient supplies of drug product to treat the patients in the studies;
|
|
|
• |
political unrest at foreign clinical sites;
|
|
|
• |
a shutdown of the U.S. government, including the FDA;
|
|
|
• |
public health crises such as pandemics and epidemics; or
|
|
|
• |
natural disasters at any of our clinical sites.
|
|
|
• |
difficulty in establishing or managing relationships with CROs, and physicians;
|
|
|
• |
different standards for the conduct of clinical trials;
|
|
|
• |
absence in some countries of established groups with sufficient regulatory expertise for review of LVV and AAV gene therapy protocols;
|
|
|
• |
our inability to locate qualified local partners or collaborators for such clinical trials; and
|
|
|
• |
the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatment.
|
|
|
• |
severity of the disease under investigation;
|
|
|
• |
design of the study protocol;
|
|
|
• |
size of the patient population;
|
|
|
• |
eligibility criteria for the study in question;
|
|
|
• |
perceived risks and benefits of the product candidate under study, including as a result of adverse effects observed in similar or competing therapies;
|
|
|
• |
proximity and availability of clinical study sites for prospective patients;
|
|
|
• |
availability of competing therapies and clinical studies;
|
|
|
• |
efforts to facilitate timely enrollment in clinical studies;
|
|
|
• |
patient referral practices of physicians; and
|
|
|
• |
ability to monitor patients adequately during and after treatment.
|
|
|
• |
issue a warning letter asserting that we are in violation of the law;
|
|
|
• |
seek an injunction or impose civil or criminal penalties or monetary fines;
|
|
|
• |
suspend any ongoing clinical studies;
|
|
|
• |
refuse to approve a pending marketing application, such as a BLA or supplements to a BLA submitted by us;
|
|
|
• |
seize products; or
|
|
|
• |
refuse to allow us to enter into supply contracts, including government contracts.
|
|
|
• |
regulatory authorities may suspend or withdraw approvals of such product candidate;
|
|
|
• |
regulatory authorities may require additional warnings on the label;
|
|
|
• |
we may be required to change the way a product candidate is administered or conduct additional clinical trials; and
|
|
|
• |
our reputation may suffer.
|
|
|
• |
some or all of our product candidates may be found to be unsafe or ineffective or otherwise fail to meet applicable regulatory standards or receive necessary regulatory clearances; our product candidates, if safe and effective, may
nonetheless not be able to be developed into commercially viable products;
|
|
|
• |
it may be difficult to manufacture or market our product candidates on a scale that is necessary to ultimately deliver our products to end-users;
|
|
|
• |
proprietary rights of third parties may preclude us from marketing our product candidates;
|
|
|
• |
the nature of our indications as rare diseases means that the potential market size may be limited; and
|
|
|
• |
third parties may market superior or equivalent drugs which could adversely affect the commercial viability and success of our product candidates.
|
|
|
• |
the inability to negotiate manufacturing agreements with third parties under commercially reasonable terms;
|
|
|
• |
reduced control as a result of using third-party manufacturers for certain aspects of manufacturing activities;
|
|
|
• |
the risk that these activities are not conducted in accordance with our study plans and protocols;
|
|
|
• |
termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; and
|
|
|
• |
disruptions to the operations of our third-party manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier.
|
|
|
• |
the efficacy and safety of such product candidates as demonstrated in preclinical studies and clinical trials;
|
|
|
• |
the potential and perceived advantages of product candidates over alternative treatments;
|
|
|
• |
the cost of our treatment relative to alternative treatments;
|
|
|
• |
the clinical indications for which the product candidate is approved by the FDA or the EMA;
|
|
|
• |
patient awareness of, and willingness to seek, gene therapy;
|
|
|
• |
the willingness of physicians to prescribe new therapies;
|
|
|
• |
the willingness of physicians to undergo specialized training with respect to administration of our product candidates;
|
|
|
• |
the willingness of the target patient population to try new therapies;
|
|
|
• |
the prevalence and severity of any side effects;
|
|
|
• |
product labeling or product insert requirements of the FDA, the EMA or other regulatory authorities, including any limitations or warnings contained in a product’s approved labeling;
|
|
|
• |
relative convenience and ease of administration;
|
|
|
• |
the strength of marketing and distribution support;
|
|
|
• |
the timing of market introduction of competitive products;
|
|
|
• |
publicity concerning our products or competing products and treatments; and
|
|
|
• |
sufficient third-party payor coverage and reimbursement.
|
|
|
• |
different regulatory requirements for approval of drugs and biologics in foreign countries;
|
|
|
• |
reduced protection for intellectual property rights;
|
|
|
• |
unexpected changes in tariffs, trade barriers and regulatory requirements;
|
|
|
• |
economic weakness, including inflation, or political instability in particular foreign economies and markets;
|
|
|
• |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
|
|
|
• |
foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
|
|
|
• |
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
|
|
• |
shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad;
|
|
|
• |
business interruptions resulting from geopolitical actions, including war and terrorism or natural disasters including earthquakes, typhoons, floods and fires, public health crises such as pandemics and epidemics, or from economic or
political instability;
|
|
|
• |
compliance with foreign laws, regulations, standards and regulatory guidance governing the collection, use, disclosure, retention, security and transfer of personal data, including the European Union General Data Privacy Regulation
(“GDPR”); and
|
|
|
• |
greater difficulty with enforcing our contracts in jurisdictions outside of the United States.
|
|
|
• |
diversion of management time and focus from operating our business to addressing acquisition integration challenges;
|
|
|
• |
coordination of R&D efforts;
|
|
|
• |
retention of key employees from any acquired company;
|
|
|
• |
changes in relationships with strategic partners as a result of any product acquisitions or strategic positioning resulting from the acquisition;
|
|
|
• |
cultural challenges associated with integrating employees from any acquired company into our organization or managing a strategic alliance or joint venture;
|
|
|
• |
the need to implement or improve controls, procedures, and policies at any acquired business that prior to the acquisition may have lacked sufficiently effective controls, procedures and policies;
|
|
|
• |
liability for activities of any acquired company before the acquisition, including intellectual property infringement claims, violation of laws, commercial disputes, tax liabilities, and other known liabilities;
|
|
|
• |
unanticipated write-offs or charges; and
|
|
|
• |
litigation or other claims in connection with any acquired company, including claims from terminated employees, customers, former stockholders or other third parties.
|
| • |
delays or difficulties in enrolling patients in our clinical trials;
|
| • |
delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff;
|
| • |
diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials;
|
| • |
interruption of key clinical trial activities, such as clinical trial site data monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others or interruption of clinical trial subject
visits and study procedures (particularly any procedures that may be deemed non-essential), which may impact the integrity of subject data and clinical study endpoints;
|
| • |
interruption or delays in the operations of the U.S. Food and Drug Administration and comparable foreign regulatory agencies, which may impact approval timelines;
|
|
|
• |
the scope of rights granted under the license agreement;
|
|
|
• |
whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
|
• |
our right to sublicense patent and other intellectual property rights to third parties under collaborative development relationships;
|
|
|
• |
our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of is product candidates, and what activities satisfy those diligence obligations;
|
|
|
• |
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
|
|
|
• |
whether and the extent to which inventors are able to contest the assignment of their rights to our licensors.
|
|
|
• |
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
|
• |
the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
|
• |
the sublicensing of patent and other rights under our collaborative development relationships;
|
|
|
• |
our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
|
|
• |
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
|
|
|
• |
the priority of invention of patented technology.
|
|
|
• |
results of clinical trials of our product candidates or those of our competitors;
|
|
|
• |
the success of competitive products or technologies;
|
|
|
• |
commencement or termination of collaborations;
|
|
|
• |
regulatory or legal developments in the United States and other countries;
|
|
|
• |
developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
|
|
• |
the recruitment or departure of key personnel;
|
|
|
• |
the level of expenses related to any of our product candidates or clinical development programs;
|
|
|
• |
the results of our efforts to discover, develop, acquire or in-license additional product candidates;
|
|
|
• |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
|
|
• |
negative publicity around gene therapy in general, or our product candidates;
|
|
|
• |
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
|
• |
changes in the structure of healthcare payment systems;
|
|
|
• |
market conditions in the pharmaceutical and biotechnology sectors; and
|
|
|
• |
general economic, industry and market conditions.
|
|
|
• |
permit only the board of directors to establish the number of directors;
|
|
|
• |
require super-majority voting to amend some provisions in our restated certificate of incorporation and restated bylaws;
|
|
|
• |
prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders; and
|
|
|
• |
establish advance notice requirements for nominations for election to our board or for proposing matters that can be acted upon by stockholders at annual stockholder meetings.
|
| Item 2. |
| Item 3. |
| Item 4. |
| Item 5. |
| Item 6. |
|
Number
|
Description of Exhibit
|
|
|
Agreement and Plan of Merger and Reorganization, dated as of September 12, 2017, by and among Inotek Pharmaceuticals Corporation, Rocket Pharmaceuticals, Ltd. and Rome Merger Sub (incorporated by reference to Exhibit 2.1 to the Company’s
Current Report on Form 8-K (001-36829), filed with the SEC on September 13, 2017)
|
||
|
Seventh Amended and Restated Certificate of Incorporation of Rocket Pharmaceuticals, Inc., effective as of February 23, 2015 (incorporated by reference to Exhibit 3.1 to the Company’s Annual Report on Form 10-K (001-36829), filed with the
SEC on March 31, 2015)
|
||
|
Certificate of Amendment (Reverse Stock Split) to the Seventh Amended and Restated Certificate of Incorporation of the Registrant, effective as of January 4, 2018 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on
Form 8-K (001-36829), filed with the SEC on January 5, 2018)
|
||
|
Certificate of Amendment (Name Change) to the Seventh Amended and Restated Certificate of Incorporation of the Registrant, effective January 4, 2018 (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K
(001-36829), filed with the SEC on January 5, 2018)
|
||
|
Certificate of Amendment to the Seventh Amended and Restated Certificate of Incorporation of the Registrant, effective as of June 25, 2018 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K (001-36829),
filed with the SEC on June 25, 2018)
|
||
|
Amended and Restated By-Laws of Rocket Pharmaceuticals, Inc., effective as of March 29, 2018 (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K (001-36829), filed with the SEC on April 4, 2018)
|
||
|
Amendment to First Supplemental Indenture, dated as of February 18, 2020, between Rocket Pharmaceuticals, Inc. and Wilmington Trust, National Association, as trustee (incorporated by reference to Exhibit 4.1 of the Company’s Current Report
on Form 8-K (001-36829), filed with the SEC on February 20, 2020)
|
||
|
Form of Exchange Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K (001-36829), filed with the SEC on February 11, 2020)
|
||
|
Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
||
|
Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
||
|
Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
||
|
101.INS
|
XBRL Instance Document.
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Document.
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document.
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Link Document.
|
| * |
Filed herewith.
|
|
ROCKET PHARMACEUTICALS, INC.
|
||
|
May 8, 2020
|
By:
|
/s/ Gaurav Shah, MD
|
|
Gaurav Shah, MD
|
||
|
President, Chief Executive Officer and Director
|
||
|
(Principal Executive Officer)
|
||
|
May 8, 2020
|
By:
|
/s/ Kamran Alam
|
|
Kamran Alam
|
||
|
SVP, Finance
|
||
|
(Principal Financial Officer)
|
||
57