|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
26-1622110
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
|
(I.R.S. Employer Identification No.)
|
|
|
|
|
|
480 Arsenal Way
|
|
|
|
Watertown, MA
|
|
02472
|
|
(Address Of Principal Executive Offices)
|
|
(Zip Code)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $0.0001 par value per share
|
NASDAQ Global Market
|
|
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
|
|
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Se-curities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
|
|
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any,every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
|
|
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter)is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
|
|
Large accelerated filer ☐
|
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒
|
|
Smaller reporting company ☐
|
|
TABLE OF CONTENTS
|
||
|
Item 1A.
|
Risk Factors
|
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
|
Item 2.
|
Properties
|
|
|
Item 3.
|
Legal Proceedings
|
|
|
Item 4.
|
Mine Safety Disclosures
|
|
|
Part II
|
||
|
Item 5.
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
Item 6.
|
Selected Consolidated Financial Data
|
|
|
Item 7.
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
|
|
Item 9A.
|
Controls and Procedures
|
|
|
Item 9B.
|
Other Information
|
|
|
Part III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
|
Item 11.
|
Executive Compensation
|
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
|
Item 14.
|
Principal Accounting Fees and Services
|
|
|
Part IV
|
||
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
|
|
Item 16.
|
Form 10-K Summary
|
|
|
SIGNATURES
|
||
|
EXHIBIT INDEX
|
||
|
-
|
our ability to enroll patients in clinical trials, timely and successfully complete those trials and receive necessary regulatory approvals;
|
|
-
|
our ability to establish our own manufacturing facilities and to receive or manufacture sufficient quantities of our product candidates;
|
|
•
|
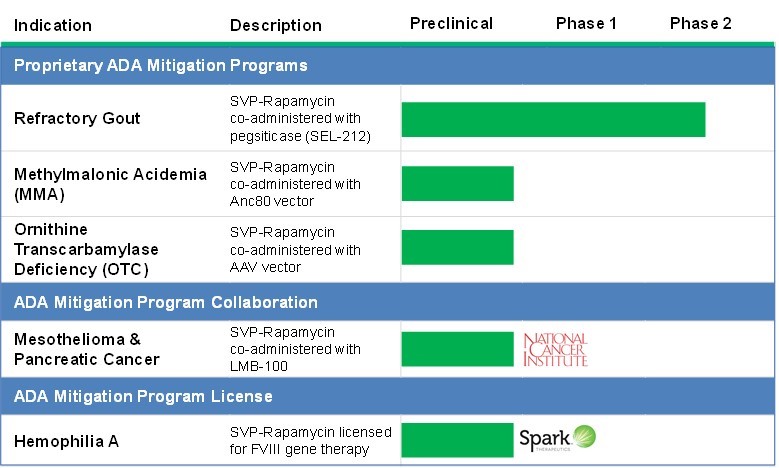
Rapidly advance the development of our lead product candidate, SEL-212, for the treatment of refractory and chronic tophaceous gout.
We believe SEL-212 has the potential to be the first biologic treatment that durably controls uric acid in refractory gout and dissolves and removes harmful deposits of uric acid crystals in chronic tophaceous gout in a majority of patients. We are currently conducting a comprehensive Phase 1/2 clinical program, comprised of two Phase 1 clinical studies, for which we received final data in December 2016, and an ongoing Phase 2 clinical trial, which commenced patient recruitment in October 2016 and for which we expect to announce initial data in the second quarter of 2017. We plan to advance this program through regulatory approval and commercialization, to the extent warranted by clinical trial results.
|
|
•
|
Leverage our SVP technology for immune tolerance to develop additional novel uses and classes of non-immunogenic biologics.
We intend to use our SVP technology to develop a range of proprietary, non-immunogenic biologic therapies. Our current pipeline includes SEL-212 as well as gene therapies designed to mitigate the formation of ADAs and therefore enable repeat administration. In addition, we intend to pursue opportunities to co-administer SVP-Rapamycin with other enzymes, gene therapies and immuno-oncology drugs. In each case SVP-Rapamycin would be intended to mitigate undesired immune response to the biologic therapy with the goal to improve efficacy and/or safety. Our strategy is to develop proprietary treatments for rare and serious diseases by combining SVP-Rapamycin with biologics that have been in licensed or provided through research collaborations.
|
|
•
|
Establish infrastructure and capabilities to commercialize our products in rare and orphan diseases.
While we believe our SVP technology may be broadly applicable across disease areas, we intend to focus our proprietary efforts on developing and commercializing proprietary SVP-enabled products for rare and serious diseases where there is high unmet medical need. Therapies for treating these diseases require focused commercial efforts and coordination with patient groups and investigators. As our product candidates advance towards commercialization, we intend to build a commercial infrastructure to capture the full value of our proprietary SVP products.
|
|
•
|
Selectively pursue collaborations and maximize the value of our SVP programs for immune tolerance.
In addition to our own proprietary product development efforts, we are in discussions with potential collaborators and licensees to pursue novel new therapies that would utilize our SVP technology. For example, in December 2016, we entered into the Spark License Agreement to develop gene therapies for certain targets utilizing our SVP technology. For more information, see “Business - Licenses and Collaborations.”
|
|
•
|
Opportunistically utilize our SVP technology to stimulate the immune system to fight disease.
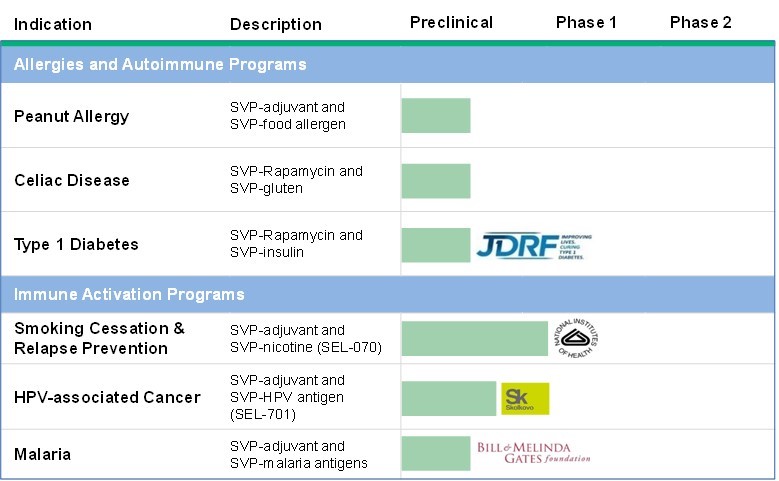
We are currently utilizing funding to develop prophylactic and therapeutic vaccines that activate the immune system to fight disease through our SVP immune stimulation programs. Our current pipeline includes a product candidate to treat HPV-associated cancers, a nicotine vaccine candidate for smoking cessation and relapse prevention and a product candidate for the prevention of malaria. We are developing our programs for HPV-associated cancers and smoking cessation and prevention on our own with grant funding from the Skolkovo Foundation for our HPV program and from the National Institute for Drug Abuse for our nicotine program. We are developing our malaria program under a sponsored research arrangement with The Bill and Melinda Gates Foundation.
|

|
•
|
Immunosuppressive therapies.
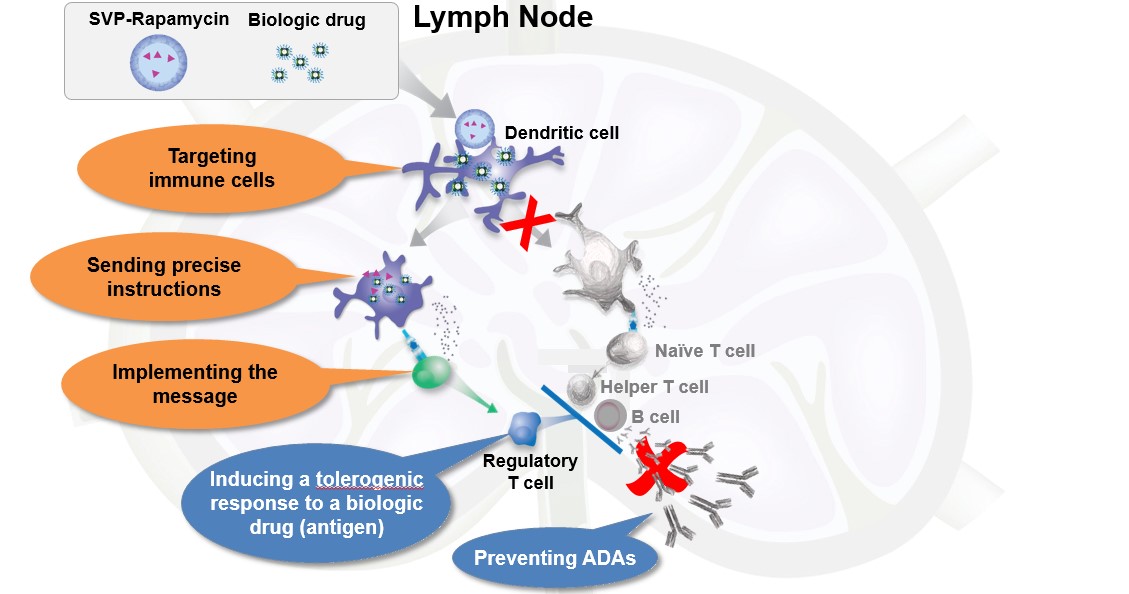
Immunosuppressive therapies are designed to suppress the immune system and inhibit an undesired immune response. However, many current therapies are not antigen-specific and, as a result, broadly suppress the immune system leading to undesired side effects that include opportunistic infections, skin cancer and lymphomas. We believe there is an opportunity to develop therapies that instruct the immune system to remain tolerant to a specific antigen and thereby avoid off-target effects of systemic immunosuppression.
|
|
•
|
Immunostimulatory therapies.
Immunostimulatory therapies are designed to stimulate the immune system to prevent or treat infections and cancers. The most common class of immunostimulatory therapies are vaccines, which are designed to simulate the body’s immune system to mount a defensive response to a specific antigen. While traditional vaccines have been successful for the prevention of infectious diseases, there has been limited success in developing therapeutic vaccines for the successful treatment of certain other diseases, including chronic infections and cancer. As a result, we believe there is a need for more effective vaccines to treat these diseases.
|
|
•
|
the treatment of chronic tophaceous and refractory gout;
|
|
•
|
antigen-specific immune tolerance for gene therapy involving gene augmentation, replacement or editing;
|
|
•
|
application with marketed products, product candidates in development and novel biologic drugs that would otherwise be too immunogenic to develop; and
|
|
•
|
immune stimulation programs to prevent and treat cancer, infectious diseases and other diseases.
|
|
•
|
tolerogenic response to mitigate the formation of ADAs against a biologic drug or treat allergies and autoimmune diseases; or
|
|
•
|
potent antigen-specific stimulatory response, such as an antibody response to a microbial antigen or a cytolytic T cell response to a tumor antigen.
|


|
•
|
for 43 currently marketed biologics, over 20% of the patients receiving the biologic are affected by immunogenicity, including Factor VIII products, such as Advate
®(antihemophilic factor)
, for the treatment of hemophilia A, antibody therapeutics with fully human sequences, such as Humira
®(adalimumab)
, and recently approved products such as the anti-IL-17 antibody Taltz
®(
ixekizumab
)
, and the enzyme replacement therapy Strensiq®(
asfotase alfa
);
|
|
•
|
improved versions of biologics that are in the same pharmaceutical class as marketed biologics, including pegsiticase, long-acting coagulation Factor VII and certain antibody drug conjugates, are affected by immunogenicity; and
|
|
•
|
novel platform technologies, such as gene therapy and gene editing, are fundamentally restricted by immunogenicity.
|

|
•
|
an empty nanoparticle, or Empty Nanoparticle, as indicated in blue;
|
|
•
|
a nanoparticle encapsulating rapamycin, or NP-Rapamycin, and administered without antigen, as indicated in red; or
|
|
•
|
SVP-Rapamycin encapsulating a peptide sequence from proteolipoprotein, or PLP, a myelin antigen associated with multiple sclerosis, or SVP-Rapamycin.PLP, as indicated in green.
|

|
•
|
the first group, referred to as the Delayed Immunization Group indicated in black, was not treated with anything during the treatment period;
|
|
•
|
the second group, referred to as the Daily Free Rapamycin Group indicated in red, was treated with doses of free unencapsulated rapamycin five days per week and weekly doses of the highly immunogenic antigen keyhole limpet hemocyanin, or KLH; and
|
|
•
|
the third group, referred to as the SVP-Rapamycin Group indicated in green, received three weekly doses of SVP-Rapamycin combined with KLH.
|

|
•
|
an empty nanoparticle, referred to as the Empty Nanoparticle Group and indicated in blue, or
|
|
•
|
SVP-Rapamycin, referred to as the SVP-Rapamycin Group and indicated in green.
|

|
•
|
Therapeutic enzymes
. Therapeutic enzymes are a frequently used class of biologic drugs to treat rare diseases. Through our analysis of biologic drugs, including our preclinical studies, we have observed that enzymes are especially prone to undesired immune responses. Our lead product candidate, SEL-212, includes pegsiticase, a pegylated uricase enzyme, which is an example of an immunogenic enzyme for which we are applying SVP-Rapamycin with the intention of improving the enzyme’s efficacy and safety. Other examples of immunogenic enzymes include acid alpha-glucosidase for the treatment of Pompe disease, alpha galactosidase A for the treatment of Fabry’s disease and microbial enzymes such as asparaginase for the treatment of cancers. We intend to seek opportunities to secure supply of and, if appropriate, licenses to these or other enzymes that we would pair with SVP-Rapamycin to enhance their efficacy, safety and use in their treatment of diseases.
|
|
•
|
Gene therapies
. We believe gene therapies have the potential to address key unmet medical needs for many rare genetic diseases, but that undesired immune responses to the viral vectors used for gene replacement, augmentation and editing may be restricting their broader use. Through our analysis of genetic diseases, we have identified applications and patient segments that we believe would benefit from our SVP technology. We intend to develop proprietary SVP-Rapamycin-enabled non-immunogenic gene therapies with viral vectors such as the Anc80 vector that we have licensed from MEE. We believe our product candidates have the potential to solve the problem of pre-existing immunogenicity to the gene therapy vector by using a novel engineered gene therapy vector, Anc80, and to prevent undesired immune responses to the vector and transgene that can occur with the first dose of gene therapy by using our SVP technology. Our initial areas of focus include lysosomal storage, genetic muscular and genetic metabolic diseases. Our proprietary gene therapy programs are focused on the use of vectors that have documented efficiency in delivery of the transgene in nonhuman primates such as Anc80. We believe we are the first company to systematically pursue the development of gene therapy products in combination with an immunotherapy with the goal of enabling repeat administration of the gene therapy. We have engaged third parties with experience in gene therapy and rare diseases to support the development of our proprietary products.
|
|
•
|
Oncology treatments
. While a variety of cancer treatments have demonstrated the ability potential to halt or reverse the course of tumor growth in patients, some of these therapies have proven to be highly immunogenic. One such example is LMB-100, an investigational immunotoxin being developed by the National Cancer Institute. The majority of mesothelioma patients treated with an earlier version of LMB-100, called SS1P, experienced dose-limiting immune responses despite the use of potent immunosuppressants. However, the few chemotherapy-refractory mesothelioma patients tolerating more than two treatment cycles in this Phase 1 clinical trial showed marked antitumor activity. We and NCI have generated preclinical data demonstrating that co-administration of SVP-Rapamycin with LMB-100 in mouse models prevented the formation of anti-LMB-100 antibodies and allowed for the administration of at least four
|
|
•
|
Other products and product candidates affected by undesired immune responses
. We have generated preclinical data demonstrating the broad potential benefit of SVP for immune tolerance. For many biologic drugs, undesired immune responses limit efficacy and cause safety concerns. This includes TNF-alpha-specific monoclonal antibodies for the treatment of rheumatoid arthritis and coagulation factor replacement therapies for the treatment of hemophilia. We intend to out-license SVP-Rapamycin technology for use with other products that are outside our focus to larger biopharmaceutical companies. We believe our SVP technology may also be of interest to biopharmaceutical companies with novel biologic development concepts or product candidates in clinical development that have demonstrated initial efficacy but are experiencing issues with safety or sustained efficacy due to inhibitory ADAs.
|





|
•
|
the first group, referred to as the Untreated Group and indicated in black, received no treatment;
|
|
•
|
the second group, referred to as the Pegsiticase Group and indicated in red, was treated with pegsiticase alone; and
|
|
•
|
the third group, referred to as the SVP-Rapamycin + Pegsiticase Group and indicated in green, was treated with SVP-Rapamycin co-administered with pegsiticase.
|

|
•
|
administered pegsiticase alone, referred to as the Empty Nanoparticle Group and indicated in blue, or
|
|
•
|
co-administered pegsiticase with one of two dose levels of SVP-Rapamycin, referred to as the SVP-Rapamycin 0.1X and SVP-Rapamycin 1X Groups and indicated in purple and green, respectively. The SVP-Rapamycin 0.1X Group received a dose level of SVP-Rapamycin of 0.3 mg/kg and the SVP-Rapamycin 1X Group received a dose level of SVP-Rapamycin of 3 mg/kg.
|






|
•
|
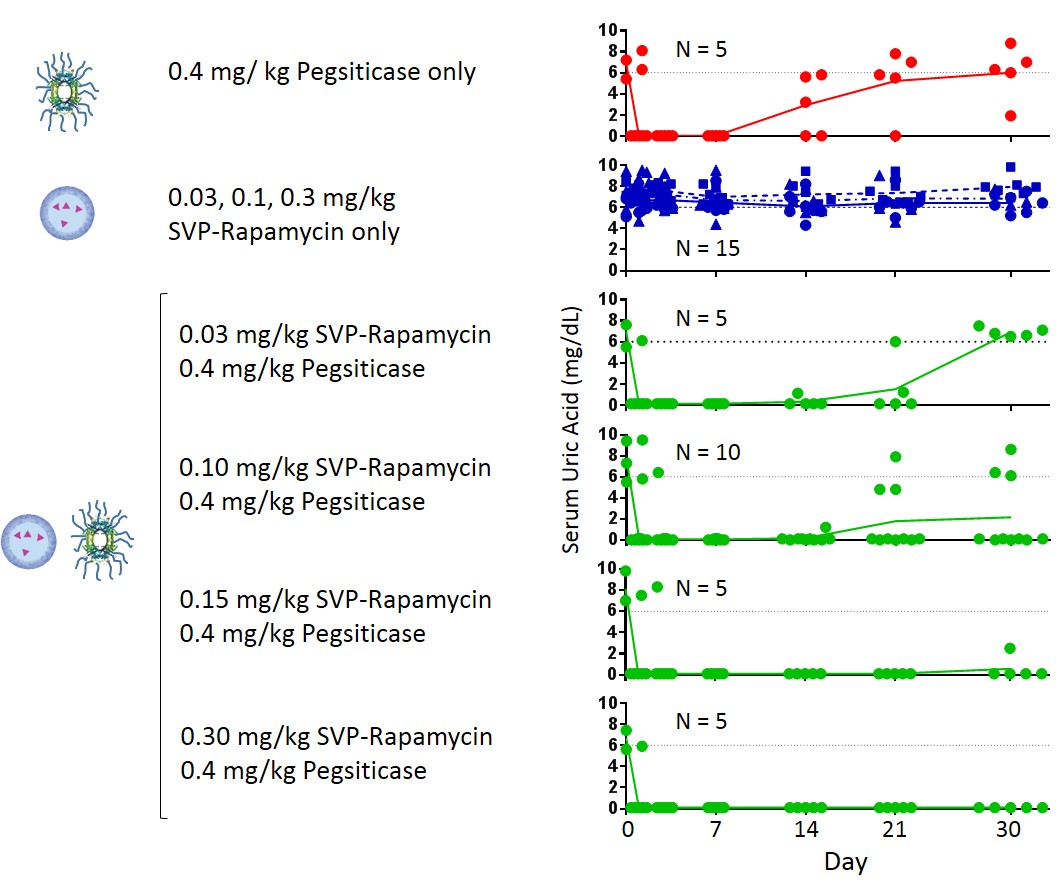
Mid-Dose Cohorts (three monthly doses of 0.08 mg/kg of SVP-Rapamycin + 0.2 mg/kg or 0.4 mg/kg of pegsiticase followed by two monthly doses of pegsiticase alone): Of the 13 patients in these cohorts, 11 patients continue to be dosed and all had maintained serum uric acid control through March 23. Nine of these patients had received three monthly doses and the other two patients had received two monthly doses. Of the two patients no longer being dosed, one dropped out of the trial and one reached the trial’s stopping rules.
|
|
•
|
Low-Dose Cohorts (three monthly doses of 0.05 mg/kg of SVP-Rapamycin + 0.2 mg/kg or 0.4 mg/kg of pegsiticase followed by two monthly doses of pegsiticase alone): Of the 19 patients in these cohorts, one patient had completed the full five-month regimen and maintained serum uric acid control for the duration of the trial, and three have received three monthly doses while maintaining serum uric acid and continue to be dosed. Of the 15 patients no longer being dosed, seven dropped out of the trial and eight reached the trial’s stopping rules.
|
|
•
|
Control Cohorts (five monthly doses of 0.2 mg/kg or 0.4 mg/kg of pegsiticase alone) - As expected, based upon the known immunogenicity of uricase enzymes, five of six patients who received pegsiticase alone were unable to maintain serum uric acid control. Consequently, enrollment in these control cohorts was stopped early.
|
|
•
|
empty nanoparticles, with such mice referred to as the Empty Nanoparticle Group and indicated in blue; or
|
|
•
|
SVP-Rapamycin, with such mice referred to as the SVP-Rapamycin Group and indicated in green.
|





|
•
|
one group of mice, referred to as the Untreated Group and indicated in black in Figure 26c below, were left untreated;
|
|
•
|
a second group of mice, referred to as the Humira Group and indicated in blue in Figures 26a through 24d below, were treated weekly with Humira alone from weeks 5 through 20; and
|
|
•
|
a third group of mice, referred to as the SVP-Rapamycin Group and indicated in green in Figures 26a through 26d below, were treated weekly with SVP-Rapamycin together with Humira from weeks five through 11 and then weekly with Humira alone from weeks 12 to 20.
|

|
•
|
the first group of mice, referred to as the Empty Nanoparticle Group and indicated in blue, received five weekly injections of empty nanoparticle together with Advate; and
|
|
•
|
the second group of mice, referred to as the SVP-Rapamycin Group and indicated in green, received five weekly doses of SVP-Rapamycin together with Advate.
|

|
•
|
Peanut Allergy.
In November 2012, we entered into an exclusive license agreement with Sanofi for the use of our SVP technology for peanut allergy, which will terminate no later than May 8, 2017. Our work with Sanofi focused on evaluating SVP encapsulating an adjuvant together with an allergen provided by Sanofi. In November 2016, we exercised our right to acquire this program under the Sanofi Agreement including certain intellectual rights and materials developed under the collaboration. After the termination date, we will be released from our exclusivity obligations under the Sanofi Agreement and may pursue development opportunities for this indication with third parties.
|
|
•
|
Celiac Disease.
In November 2014, Sanofi exercised its option to develop a SVP‑based therapy to treat celiac disease under similar financial and other terms as the peanut allergy program, which will likewise terminate on May 8, 2017. Our work with Sanofi focused on evaluating SVP encapsulating a rapamycin together with gluten antigens provided by Sanofi. In November 2016, we exercised our right to acquire this program under the Sanofi Agreement including certain intellectual rights and materials developed under the collaboration. After the termination date, we will be released from our exclusivity obligations under the Sanofi Agreement and may pursue development opportunities for this indication with third parties.
|
|
•
|
Type 1 Diabetes.
In September 2014, we received a grant from Sanofi, together with the JDRF for research on SVP formulations encapsulating Rapamycin and insulin or insulin peptides. The grant expired in September 2016. We continue to evaluate our strategic options to continue our research in type 1 diabetes.
|







|
•
|
the first group of mice, referred to as the Encapsulated TLR 7/8 Agonist Group and indicated in green, received a single dose of SVP encapsulating both an antigen and a TLR 7/8 agonist; and
|
|
•
|
the second group of mice, referred to as the Free TLR 7/8 Agonist Group and indicated in red, received a single dose of SVP encapsulating an antigen alone together with free TLR 7/8 agonist.
|

|
•
|
the first group of mice, referred to as the Empty Nanoparticle Group and indicated in blue in Figures 35a and 35c below, four doses of empty nanoparticles; and
|
|
•
|
the second group of mice, referred to as the SVP-HPV Group and indicated by in green in Figures 35b and 35c below, four doses of SVP-HPV.
|

|
•
|
first group of mice, referred to as the Empty Nanoparticle + Control Antibody Group and indicated in black, received empty nanoparticles with an isotype control antibody;
|
|
•
|
second group of mice, referred to as the Empty Nanoparticle + Anti-PD-L1 Antibody Group and indicated in red, received empty nanoparticles with a PD-L1-specific monoclonal antibody;
|
|
•
|
third group of mice, referred to as the SVP-TRP-2 + SVP-PO-CpG + Control Antibody Group and indicated in blue, received SVP encapsulating the TRP-2 peptide antigen and a TLR agonist with an isotype control antibody; and
|
|
•
|
fourth group of mice, referred to as the SVP-TRP-2 +SVP-PO-CpG + Anti-PD-L1 Antibody Group and indicated in green, received SVP encapsulating the TRP-2 peptide antigen and a TLR agonist with a PD-L1-specific monoclonal antibody.
|

|
•
|
first group of mice, referred to as the Empty Nanoparticle Group and indicated in black, received four injections of empty nanoparticles starting on day 14;
|
|
•
|
second group of mice, referred to as the Cisplatin Group and indicated in red, received cisplatin on days 5 and 12;
|
|
•
|
third group of mice, referred to as the SVP-E7*/E6* + SVP-CpG and indicated in blue, received four injections of SVP encapsulating the E7*/E6* fusion protein (comprising mutated E7 and E6 proteins of HPV) and a TLR agonist starting on day 14; and
|
|
•
|
fourth group of mice, referred to as the SVP-E7*/E6* + SVP-CpG + Cisplatin Antibody Group and indicated in green, received cisplatin on days 5 and 12 and four injections of SVP encapsulating the E7*/E6* fusion protein and a TLR agonist starting on day 14.
|

|
•
|
tolerance and cancer immunotherapy programs;
|
|
•
|
other immune stimulation programs;
|
|
•
|
methods and compositions incorporating our proprietary SVP nanoparticle in a variety of tolerance applications, including:
|
|
•
|
mitigating or treating anti-drug antibodies association with protein drugs (such as SEL-212), and
|
|
•
|
genetic therapies (such as viral delivery of genes);
|
|
•
|
development and commercialization of SEL-212, including both composition of matter and method of treatment claims (there are three patent families that cover the SEL-212 product, one of which is a licensed, issued U.S. patent that covers the SEL-212 product, which expires in 2021); and
|
|
•
|
methods and compositions incorporating our proprietary SVP nanoparticle in a variety of cancer immunotherapy applications, including:
|
|
•
|
creating various cancer vaccines, and
|
|
•
|
combination treatments, including co-treatment with PD-1/PDL-1 checkpoint inhibitors.
|
|
Program
|
Description
|
Patent
Family(1)
|
Expiration(2)
|
|||
|
Refractory and chronic tophaceous gout (SEL-212)
|
SVP-Rapamycin co-administered with pegsiticase
|
19
|
2032-2035
|
|||
|
Gene therapy
|
SVP-Rapamycin co-administered with AAV vector
|
19
|
2032-2035
|
|||
|
Peanut allergy
|
SVP-adjuvant and SVP-food allergen
|
13
|
2032-2035
|
|||
|
Celiac disease
|
SVP-Rapamycin and SVP-gluten
|
13
|
2032-2035
|
|||
|
Type 1 diabetes
|
SVP-Rapamycin and SVP-insulin
|
15
|
2032-2035
|
|||
|
Smoking cessation and relapse prevention (SEL-070)
|
SVP-adjuvant and SVP-nicotine
|
6
|
2030-2032
|
|||
|
HPV-associated cancer (SEL-701)
|
SVP-adjuvant and SVP-HPV antigen
|
6
|
2030-2032
|
|||
|
Malaria
|
SVP-adjuvant and SVP-malaria antigen
|
6
|
2030-2032
|
|||
|
(1)
|
Reflects number of relevant patent and patent application families.
|
|
(2)
|
Reflects expiration date and estimated expiration date ranges of issued patents and patent applications, respectively.
|
|
•
|
completion of extensive nonclinical testing, sometimes referred to as preclinical testing, including laboratory tests, animal trials and formulation studies in accordance with applicable regulations, including good laboratory practices, or GLPs, and applicable requirements for humane use of laboratory animals;
|
|
•
|
submission to the FDA of an investigational new drug, or IND, application, which must become effective before human clinical trials may begin;
|
|
•
|
performance of adequate and well-controlled human clinical trials according to the FDA’s regulations commonly referred to as good clinical practice, or GCP, regulations and any additional requirements for the protection of human research subjects and their health information, to establish the safety, purity and potency of the proposed biological product for its intended use;
|
|
•
|
submission to the FDA of a BLA for marketing approval that includes substantive evidence of safety, purity, and potency from results of nonclinical testing and clinical trials;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the biological product is produced to assess compliance with current good manufacturing practice, or cGMP, requirements to assure that the facilities, methods and controls are adequate to preserve the biological product’s identity, strength, quality and purity;
|
|
•
|
potential FDA audit of the nonclinical and clinical study sites that generated the data in support of the BLA; and
|
|
•
|
FDA review and approval, or licensure, of the BLA.
|
|
•
|
Phase I
. The biological product candidate is initially introduced into healthy human patients and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
|
|
•
|
Phase II
. The biological product candidate is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule.
|
|
•
|
Phase III
. Clinical trials are undertaken to further evaluate dosage, clinical efficacy, potency, and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labelling.
|
|
•
|
The Community MA, which is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use, or CHMP, of the European Medicines Agency, or EMA, and which is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, and medicinal products that contain a new active substance indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. Under the Centralized Procedure the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when the authorization of a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. Under the accelerated procedure the standard 210 days review period is reduced to 150 days.
|
|
•
|
National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member States through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure.
|
|
•
|
second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior;
|
|
•
|
applicant consents to a second orphan medicinal product application; or
|
|
•
|
applicant cannot supply enough orphan medicinal product.
|
|
-
|
continue the research and development of our other product candidates;
|
|
-
|
potentially establish a sales, marketing and distribution infrastructure and scale up external manufacturing capabilities to commercialize any products for which we may obtain regulatory approval;
|
|
-
|
scale up external manufacturing capabilities to commercialize any products for which we may obtain regulatory approval;
|
|
-
|
add clinical, scientific, operational, financial and management information systems and personnel, including personnel to support our product development and potential future commercialization efforts and to support our transition to a public company; and
|
|
-
|
experience any delays or encounter any issues with any of the above, including, but not limited to, failed studies, complex results, safety issues or other regulatory challenges.
|
|
-
|
our collaboration agreements remaining in effect, our entering into additional collaboration agreements and our ability to achieve milestones under these agreements;
|
|
-
|
the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other product candidates;
|
|
-
|
the costs and timing of future commercialization activities, including manufacturing, marketing, sales and distribution, for any of our product candidates for which we receive marketing approval;
|
|
-
|
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property‑related claims;
|
|
-
|
the extent to which we acquire or invest in businesses, products and technologies, including entering into licensing or collaboration arrangements for product candidates.
|
|
-
|
making arrangements with third party manufacturers for, or establishing, commercial manufacturing capabilities, or --establishing such capabilities ourselves;
|
|
-
|
our existing collaboration agreements remaining in effect and our entering into new collaborations throughout the development process as appropriate, from preclinical studies through to commercialization;
|
|
-
|
obtaining and maintaining coverage and adequate reimbursement by third‑party payors, including government payors, for our products, if approved;
|
|
-
|
maintaining and growing an organization of scientists and business people who can develop and commercialize our product candidates and technology.
|
|
-
|
due to the unproven nature of our SVP therapeutics, they may have different efficacy and safety rates in various indications;
|
|
-
|
the FDA or other regulatory agencies may lack experience in evaluating the efficacy and safety of products based on SVP or a biologic sourced from China or other jurisdictions, which could result in a longer‑than‑expected regulatory review process, increase our expected development costs or delay or prevent commercialization of our product candidates; and
|
|
-
|
in the event of a biologics license application for SEL‑212 or another product and a pre‑approval inspection by the FDA of the facilities of 3SBio or any other manufacturer of biologics we may use, the FDA may not approve the facility for production or may make observations that will take significant time for 3SBio or such other provider to address.
|
|
-
|
clinical trials of our product candidates may produce unfavorable, incomplete or inconclusive results;
|
|
-
|
regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
|
-
|
we may experience delays in reaching, or fail to reach, agreement on acceptable terms with contract research organizations, or CROs, or clinical trial sites;
|
|
-
|
we may be unable to recruit suitable patients to participate in a clinical trial, the number of patients required for clinical trials of our product candidates may be larger than we expect, enrollment in these clinical trials may be slower than we expect or participants may drop out of these clinical trials at a higher rate than we expect;
|
|
-
|
the number of clinical trial sites required for clinical trials of our product candidates may be larger than we expect;
|
|
-
|
our third‑party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
|
|
-
|
we may have to suspend or terminate clinical trials of our product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks;
|
|
-
|
investigators, regulators, data safety monitoring boards or institutional review boards may require that we or our investigators suspend or terminate clinical research, or we may decide to do so ourselves, for various reasons including noncompliance with regulatory requirements, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues such as a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions;
|
|
-
|
investigators may deviate from the trial protocol, fail to conduct the trial in accordance with regulatory requirements or misreport study data;
|
|
-
|
the cost of clinical trials of our product candidates may be greater than we expect;
|
|
-
|
the supply or quality of raw materials or manufactured product candidates (whether provided by us or third parties) or other materials necessary to conduct clinical trials of our product candidates may be insufficient, inadequate or not available at an acceptable cost, or we may experience interruptions in supply;
|
|
-
|
regulators may revise the requirements for approving our product candidates, or such requirements may not be as we expect;
|
|
-
|
the FDA or comparable foreign regulatory authorities may disagree with our clinical trial design or our interpretation of data from preclinical studies and clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design of our clinical trials; and
|
|
-
|
regarding trials managed by our existing or any future collaborators, our collaborators may face any of the above issues, and may conduct clinical trials in ways they view as advantageous to them but potentially suboptimal for us.
|
|
-
|
be delayed in obtaining marketing approval for our product candidates, if at all;
|
|
-
|
lose the support of collaborators, requiring us to bear more of the burden of research and development;
|
|
-
|
not obtain marketing approval at all;
|
|
-
|
obtain marketing approval in some countries and not in others;
|
|
-
|
obtain approval for indications or patient populations that are not as broad as intended or desired;
|
|
-
|
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings;
|
|
-
|
be subject to additional post‑marketing testing requirements; or
|
|
-
|
have a product removed from the market after obtaining marketing approval.
|
|
-
|
the severity of the disease under investigation;
|
|
-
|
the patient eligibility criteria for the study in question;
|
|
-
|
the perceived risks and benefits of the product candidate under study;
|
|
-
|
the availability of other treatments for the disease under investigation;
|
|
-
|
the existence of competing clinical trials;
|
|
-
|
our efforts to facilitate timely enrollment in clinical trials;
|
|
-
|
investigators engagement with, or enthusiasm about, the trial;
|
|
-
|
our payments for participating in clinical trials;
|
|
-
|
the patient referral practices of physicians;
|
|
-
|
the design of the trial;
|
|
-
|
the ability to monitor patients adequately during and after treatment; and
|
|
-
|
the proximity and availability of clinical trial sites for prospective patients.
|
|
-
|
regulatory authorities may withdraw approvals of such product;
|
|
-
|
regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication;
|
|
-
|
regulatory authorities may impose additional restrictions on the marketing of, or the manufacturing processes for, the particular product;
|
|
-
|
we may be required to create a medication guide outlining the risks of such side effects for distribution to patients;
|
|
-
|
we could be sued and held liable for harm caused to patients, or become subject to fines, injunctions or the imposition of civil or criminal penalties; and
|
|
-
|
our reputation may suffer.
|
|
-
|
inability, failure or unwillingness of third‑party manufacturers to comply with regulatory requirements, maintain quality assurance, meet our needs, specifications or schedules or continue to supply products to us;
|
|
-
|
reduced control we have over product development, including with respect to our lead product candidate, due to our reliance on such third‑party manufacturers,
|
|
-
|
breach of manufacturing agreements by the third‑party manufacturers;
|
|
-
|
misappropriation or disclosure of our proprietary information, including our trade secrets and know‑how;
|
|
-
|
relationships that the third party manufacturer may have with others, some of which may be our competitors, and, if it does not successfully carry out its contractual duties, does not meet expectations, experiences work stoppages, or needs to be replaced, we may need to enter into alternative arrangements, which may not be available, desirable or cost‑effective; and
|
|
-
|
termination or nonrenewal of agreements by third‑party manufacturers at times that are costly or inconvenient for us.
|
|
-
|
collaborators have significant discretion in determining the efforts and resources that they will apply to these -collaborations;
|
|
-
|
collaborators may not perform their obligations as expected;
|
|
-
|
collaborators may not pursue development and commercialization of any product candidates that achieve regulatory approval or may elect not to continue or renew development or commercialization programs based on preclinical or clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities;
|
|
-
|
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
|
|
-
|
collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours, which may cause collaborators to cease to devote resources to the commercialization of our product candidates;
|
|
-
|
a collaborator with marketing and distribution rights to one or more of our product candidates that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of such product or products;
|
|
-
|
disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time‑consuming and expensive;
|
|
-
|
collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
|
|
-
|
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
|
|
-
|
collaborations may be terminated for the convenience of the collaborator and, if terminated, we would potentially lose the right to pursue further development or commercialization of the applicable product candidates;
|
|
-
|
collaborators may learn about our technology and use this knowledge to compete with us in the future;
|
|
-
|
there may be conflicts between different collaborators that could negatively affect those collaborations and potentially others;
|
|
-
|
the number and type of our collaborations could adversely affect our attractiveness to future collaborators or acquirers; and
|
|
-
|
we currently have, and in the future may have, a limited number of collaborations and the loss of, or a disruption in our relationship with, any one or more of such collaborators may could harm our business.
|
|
-
|
their efficacy, safety and other potential advantages compared to alternative treatments;
|
|
-
|
the clinical indications for which our product candidates are approved;
|
|
-
|
our ability to offer them for sale at competitive prices;
|
|
-
|
their convenience and ease of administration compared to alternative treatments;
|
|
-
|
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
|
|
-
|
the strength of marketing and distribution support;
|
|
-
|
the availability of third‑party coverage and adequate reimbursement for our product candidates;
|
|
-
|
the prevalence and severity of their side effects and their overall safety profiles;
|
|
-
|
any restrictions on the use of our product candidates together with other medications;
|
|
-
|
interactions of our product candidates with other medicines patients are taking;
|
|
-
|
our ability to create awareness with patients and physicians about the harmful effects of uric acid deposits;
|
|
-
|
the timing of market introduction of any approved product candidates as well as competitive products and other therapies;
|
|
-
|
inability of certain types of patients to take our product candidates;
|
|
-
|
their ability to remain attractive in the event of changing treatment guidelines;
|
|
-
|
adverse publicity about the product or favorable publicity about competitive products; and
|
|
-
|
potential product liability claims.
|
|
-
|
our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
|
|
-
|
the inability of sales personnel to obtain access to or educate physicians on the benefits of our products;
|
|
-
|
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines;
|
|
-
|
unforeseen costs and expenses associated with creating an independent sales and marketing organization; and
|
|
-
|
inability to obtain sufficient coverage and reimbursement from third‑party payors and governmental agencies for our product candidates.
|
|
-
|
regulatory investigations, product recalls or withdrawals, or labeling, marketing or promotional restrictions;
|
|
-
|
decreased demand for any product candidates or products that we may develop;
|
|
-
|
injury to our reputation and significant negative media attention;
|
|
-
|
loss of clinical trial participants or increased difficulty in enrolling future participants;
|
|
-
|
significant costs to defend the related litigation or to reach a settlement;
|
|
-
|
substantial payments to trial participants or patients;
|
|
-
|
loss of revenue;
|
|
-
|
reduced resources of our management to pursue our business strategy; and
|
|
-
|
the inability to commercialize any products that we may develop.
|
|
-
|
the federal Anti‑Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti‑Kickback Statute or specific intent to violate it to have committed a violation; in addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti‑Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act);
|
|
-
|
the federal false claims and civil monetary penalties laws, including the civil False Claims Act, which impose criminal and civil penalties, through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
-
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti‑Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
|
|
-
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which also imposes obligations, including mandatory contractual terms, on certain types of people and entities with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
|
-
|
the federal Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the government information related to certain payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and requires applicable manufacturers to report annually to the government ownership and investment interests held by the physicians described above and their immediate family members and payments or other “transfers of value” to such physician owners; and
|
|
-
|
analogous state and foreign laws and regulations, such as state anti‑kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by third‑party payors, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and
|
|
-
|
an annual, nondeductible fee payable by any entity that manufactures or imports specified branded prescription drugs and biologic agents;
|
|
-
|
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
|
|
-
|
a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
|
|
-
|
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point‑of‑sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries under their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D;
|
|
-
|
extension of manufacturers’ Medicaid rebate liability to individuals enrolled in Medicaid managed care organizations;
|
|
-
|
expansion of eligibility criteria for Medicaid programs;
|
|
-
|
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
|
-
|
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and
|
|
-
|
a new Patient‑Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
|
|
-
|
litigation involving patients taking our products
|
|
-
|
restrictions on such products, manufacturers or manufacturing processes;
|
|
-
|
restrictions on the labeling or marketing of a product;
|
|
-
|
restrictions on product distribution or use;
|
|
-
|
requirements to conduct post‑marketing studies or clinical trials;
|
|
-
|
warning letters;
|
|
-
|
withdrawal of products from the market;
|
|
-
|
suspension or termination of ongoing clinical trials;
|
|
-
|
refusal to approve pending applications or supplements to approved applications that we submit;
|
|
-
|
recall of products;
|
|
-
|
fines, restitution or disgorgement of profits or revenues;
|
|
-
|
suspension or withdrawal of marketing approvals;
|
|
-
|
damage to relationships with existing and potential collaborators;
|
|
-
|
unfavorable press coverage and damage to our reputation;
|
|
-
|
refusal to permit the import or export of our products;
|
|
-
|
product seizure or detention;
|
|
-
|
injunctions; or
|
|
-
|
imposition of civil or criminal penalties.
|
|
-
|
others may be able to make compounds that are the same as or similar to our current or future product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed;
|
|
-
|
we or any of our licensors or collaborators might not have been the first to make the inventions covered by the patents or pending patent applications that we own or have exclusively licensed;
|
|
-
|
we or any of our licensors or collaborators might not have been the first to file patent applications covering certain of our inventions;
|
|
-
|
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
|
|
-
|
the prosecution of our pending patent applications may not result in granted patents;
|
|
-
|
granted patents that we own or have licensed may not cover our products or may be held not infringed, invalid or unenforceable, as a result of legal challenges by our competitors;
|
|
-
|
with respect to granted patents that we own or have licensed, especially patents that we either acquire or in‑license, if certain information was withheld from or misrepresented to the patent examiner, such patents might be held to be unenforceable;
|
|
-
|
patent protection on our product candidates may expire before we are able to develop and commercialize the product, or before we are able to recover our investment in the product candidates;
|
|
-
|
our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for such activities, as well as in countries in which we do not have patent rights, and may then use the information learned from such activities to develop competitive products for sale in markets where we intend to market our product candidates;
|
|
-
|
we may not develop additional proprietary technologies that are patentable;
|
|
-
|
the patents of others may have an adverse effect on our business; and
|
|
-
|
we may choose not to file a patent application for certain technologies, trade secrets or know‑how, and a third party may subsequently file a patent covering such intellectual property.
|
|
-
|
cease developing, selling or otherwise commercializing our product candidates;
|
|
-
|
pay substantial damages for past use of the asserted intellectual property;
|
|
-
|
obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all; and
|
|
-
|
in the case of trademark claims, redesign or rename some or all of our product candidates, or other brands to avoid infringing the intellectual property rights of third parties, which may not be possible and, even if possible, could be costly and time‑consuming.
|
|
-
|
multiple, conflicting and changing laws and regulations, such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
|
|
-
|
failure by us to obtain and maintain regulatory approvals for the use of our product candidates in various countries;
|
|
-
|
additional potentially relevant third‑party patent rights;
|
|
-
|
complexities and difficulties in obtaining protection of and enforcing our intellectual property rights;
|
|
-
|
difficulties in staffing and managing foreign operations;
|
|
-
|
complexities associated with managing multiple‑payor reimbursement regimes, government payors or patient self‑pay systems;
|
|
-
|
limits on our ability to penetrate international markets;
|
|
-
|
financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our product candidates and exposure to foreign currency exchange rate fluctuations, which could result in increased operating expenses and reduced revenues;
|
|
-
|
natural disasters, political and economic instability, including wars, events of terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions and economic weakness, including inflation;
|
|
-
|
changes in diplomatic and trade relationships;
|
|
-
|
challenges in enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the United States;
|
|
-
|
certain expenses including, among others, expenses for travel, translation and insurance;
|
|
-
|
legal risks, including use of the legal system by the government to benefit itself or affiliated entities at our expense, including expropriation of property; and
|
|
-
|
regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the FCPA its books and records provisions, or its anti‑bribery provisions.
|
|
-
|
disruption in our relationships with future customers or with current or future distributors or suppliers as a result of such a transaction;
|
|
-
|
unexpected liabilities related to acquired companies;
|
|
-
|
difficulties integrating acquired personnel, technologies and operations into our existing business;
|
|
-
|
diversion of management time and focus from operating our business to acquisition integration challenges;
|
|
-
|
increases in our expenses and reductions in our cash available for operations and other uses;
|
|
-
|
possible write‑offs or impairment charges relating to acquired businesses; and
|
|
-
|
inability to develop a sales force for any additional product candidates.
|
|
-
|
the success of competitive products or technologies;
|
|
-
|
results of clinical trials of our product candidates or those of our competitors;
|
|
-
|
failure or discontinuation of any of our development programs;
|
|
-
|
commencement of, termination of, or any development related to any collaboration or licensing arrangement;
|
|
-
|
regulatory or legal developments in the United States and other countries;
|
|
-
|
development of new product candidates that may address our markets and make our product candidates less attractive;
|
|
-
|
changes in physician, hospital or healthcare provider practices that may make our product candidates less useful;
|
|
-
|
announcements by us, our partners or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments;
|
|
-
|
announcement or market expectation of additional financing efforts;
|
|
-
|
developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
|
-
|
the recruitment or departure of key personnel;
|
|
-
|
the level of expenses related to any of our product candidates or clinical development programs;
|
|
-
|
failure to meet or exceed financial estimates, projections or development timelines of the investment community or that we provide to the public;
|
|
-
|
the results of our efforts to discover, develop, acquire or in‑license additional product candidates or products;
|
|
-
|
actual or expected changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
|
-
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
-
|
changes in the structure of healthcare payment systems;
|
|
-
|
sale of common stock by us or our stockholders in the future as well as the overall trading volume of our common stock;
|
|
-
|
market conditions in the pharmaceutical and biotechnology sectors;
|
|
-
|
general economic, industry and market conditions; and
|
|
-
|
the other factors described in this “Risk factors” section.
|
|
-
|
being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s discussion and analysis of financial condition and results of operations”;
|
|
-
|
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
|
|
-
|
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
|
-
|
reduced disclosure obligations regarding executive compensation; and
|
|
-
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
|
|
•
|
a classified board of directors with three‑year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors;
|
|
•
|
no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates;
|
|
•
|
the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from filling vacancies on our board of directors;
|
|
•
|
the ability of our board of directors to authorize the issuance of shares of preferred stock and to determine the terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquirer;
|
|
•
|
the ability of our board of directors to alter our bylaws without obtaining stockholder approval;
|
|
•
|
the required approval of the holders of at least two‑thirds of the shares entitled to vote at an election of directors to adopt, amend or repeal our bylaws or repeal the provisions of our restated certificate of incorporation regarding the election and removal of directors;
|
|
•
|
a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders;
|
|
•
|
the requirement that a special meeting of stockholders may be called only by the chairman of the board of directors, the chief executive officer, the president or the board of directors, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and
|
|
•
|
advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us.
|
|
2016:
|
High
|
Low
|
||||
|
Second Quarter (beginning June 21, 2016)
|
$
|
16.32
|
|
$
|
11.16
|
|
|
Third Quarter
|
$
|
18.88
|
|
$
|
10.26
|
|
|
Fourth Quarter
|
$
|
28.00
|
|
$
|
13.69
|
|

|
|
Year Ended December 31,
|
||||||||||
|
Consolidated Statement of Operations Data
|
2016
|
2015
|
2014
|
||||||||
|
(in thousands, except share and per share data)
|
|||||||||||
|
Grant and collaboration revenue
|
$
|
8,083
|
|
$
|
6,011
|
|
$
|
3,040
|
|
||
|
Operating expenses
|
42,753
|
|
31,315
|
|
18,439
|
|
|||||
|
Loss from operations
|
(34,670
|
)
|
(25,304
|
)
|
(15,399
|
)
|
|||||
|
Other income (expense)
|
(1,540
|
)
|
130
|
|
2,519
|
|
|||||
|
Net loss
|
$
|
(36,210
|
)
|
$
|
(25,174
|
)
|
$
|
(12,880
|
)
|
||
|
Net loss per share:
|
|||||||||||
|
Basic and diluted
|
$
|
(3.89
|
)
|
$
|
(15.13
|
)
|
$
|
(7.84
|
)
|
||
|
Weighted average common shares outstanding
|
|||||||||||
|
Basic and diluted
|
10,493,939
|
|
2,150,422
|
|
2,090,677
|
|
|||||
|
Balance Sheet Data
|
|||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
84,141
|
|
$
|
36,462
|
|
$
|
16,592
|
|
||
|
Total assets
|
$
|
89,301
|
|
$
|
42,824
|
|
$
|
22,228
|
|
||
|
Loans payable, net of current portion
|
$
|
7,977
|
|
$
|
11,855
|
|
$
|
4,824
|
|
||
|
Redeemable convertible preferred stock
|
$
|
—
|
|
$
|
137,482
|
|
$
|
94,033
|
|
||
|
Total stockholders' equity (deficit)
|
$
|
54,957
|
|
$
|
(116,493
|
)
|
$
|
(87,755
|
)
|
||
|
-
|
potentially establish a sales, marketing and distribution infrastructure and scale‑up external manufacturing capabilities to commercialize any products for which we may obtain regulatory approval;
|
|
-
|
hire additional staff, including clinical, scientific, operational and financial personnel, to execute our business plan; and
|
|
-
|
add personnel and clinical, scientific, operational, financial and management information systems to support our product development and potential future commercialization efforts, and to enable us to operate as a public company.
|
|
|
Year ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
External research and development expenses:
|
|||||||||||
|
SEL-212
|
$
|
7,442
|
|
$
|
9,335
|
|
$
|
474
|
|
||
|
Discovery and preclinical stage product programs, collectively
|
3,085
|
|
856
|
|
38
|
|
|||||
|
Internal research and development expenses
|
19,175
|
|
12,789
|
|
9,974
|
|
|||||
|
Total research and development expenses
|
$
|
29,702
|
|
|
$
|
22,980
|
|
|
$
|
10,486
|
|
|
•
|
fees payable to CROs and other third parties;
|
|
•
|
fees payable to vendors in connection with preclinical or clinical development activities; and
|
|
•
|
fees payable to vendors related to product manufacturing, development and distribution of clinical supplies.
|
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
Research and development
|
$
|
1,183
|
|
|
$
|
495
|
|
|
$
|
384
|
|
|
General and administrative
|
847
|
|
630
|
|
840
|
|
|||||
|
Total
|
$
|
2,030
|
|
$
|
1,125
|
|
$
|
1,224
|
|
||
|
|
Year Ended December 31,
|
Increase
|
||||||||||||
|
|
2016
|
2015
|
(decrease)
|
|||||||||||
|
Grant revenue
|
$
|
5,243
|
|
|
$
|
3,621
|
|
|
$
|
1,622
|
|
|
45
|
%
|
|
Collaboration revenue
|
2,840
|
|
2,390
|
|
450
|
|
|
19
|
%
|
|||||
|
Total revenue
|
$
|
8,083
|
|
$
|
6,011
|
|
$
|
2,072
|
|
|
34
|
%
|
||
|
|
Year Ended December 31,
|
Increase
|
||||||||||||
|
|
2016
|
2015
|
(decrease)
|
|||||||||||
|
Research and development
|
$
|
29,702
|
|
|
$
|
22,980
|
|
|
$
|
6,722
|
|
29
|
%
|
|
|
|
Year Ended December 31,
|
Increase
|
||||||||||||
|
|
2016
|
2015
|
(decrease)
|
|||||||||||
|
General and administrative
|
$
|
13,051
|
|
|
$
|
8,335
|
|
|
$
|
4,716
|
|
57
|
%
|
|
|
|
Year Ended December 31,
|
Increase
|
||||||||||||
|
|
2015
|
2014
|
(decrease)
|
|||||||||||
|
Grant revenue
|
$
|
3,620
|
|
|
$
|
948
|
|
|
$
|
2,672
|
|
282
|
%
|
|
|
Collaboration revenue
|
2,390
|
|
2,092
|
|
298
|
|
14
|
%
|
||||||
|
Total revenue
|
$
|
6,010
|
|
$
|
3,040
|
|
$
|
2,970
|
|
98
|
%
|
|||
|
|
Year Ended December 31,
|
Increase
|
||||||||||||
|
|
2015
|
2014
|
(decrease)
|
|||||||||||
|
Research and development
|
$
|
22,980
|
|
|
$
|
10,486
|
|
|
$
|
12,494
|
|
119
|
%
|
|
|
|
Year Ended December 31,
|
Increase
|
||||||||||||
|
|
2015
|
2014
|
(decrease)
|
|||||||||||
|
General and administrative
|
$
|
8,335
|
|
|
$
|
7,953
|
|
|
$
|
382
|
|
5
|
%
|
|
|
-
|
our collaboration agreements remaining in effect, our ability to enter into additional collaboration agreements and our ability to achieve milestones under these agreements;
|
|
-
|
the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other product candidates;
|
|
-
|
the costs and timing of future commercialization activities, including manufacturing, marketing, sales and distribution, for any of our product candidates for which we receive marketing approval;
|
|
-
|
the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval;
|
|
-
|
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and
|
|
|
Year Ended December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Beginning of the period
|
$
|
32,337
|
|
$
|
16,592
|
|
|
|
Net cash used in operating activities
|
(19,683
|
)
|
(22,463
|
)
|
|||
|
Net cash used in investing activities
|
(22,100
|
)
|
(4,679
|
)
|
|||
|
Net cash provided by financing activities
|
67,659
|
|
43,906
|
|
|||
|
Effect of exchange rate changes on cash
|
443
|
|
(1,019
|
)
|
|||
|
End of the period
|
$
|
58,656
|
|
$
|
32,337
|
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Beginning of the period
|
$
|
16,592
|
|
$
|
8,057
|
|
|
|
Net cash used in operating activities
|
(22,463
|
)
|
(12,686
|
)
|
|||
|
Net cash used in investing activities
|
(4,679
|
)
|
(227
|
)
|
|||
|
Net cash provided by financing activities
|
43,906
|
|
24,771
|
|
|||
|
Effect of exchange rate changes on cash
|
(1,019
|
)
|
(3,323
|
)
|
|||
|
End of the period
|
$
|
32,337
|
|
$
|
16,592
|
|
|
|
|
|
Less than
|
|
|
More than
|
||||||||||||||
|
Contractual Obligations
|
Total
|
1 year
|
1 to 3 years
|
3 to 5 years
|
5 years
|
||||||||||||||
|
Operating leases(1)
|
$
|
4,200
|
|
$
|
1,244
|
|
$
|
2,621
|
|
$
|
335
|
|
$
|
—
|
|
||||
|
Research and development contract obligations(2)
|
180
|
|
60
|
|
120
|
|
—
|
|
—
|
|
|||||||||
|
Long term debt(3)
|
14,016
|
|
5,319
|
|
8,697
|
|
—
|
|
—
|
|
|||||||||
|
Total obligations
|
$
|
18,396
|
|
$
|
6,623
|
|
$
|
11,438
|
|
$
|
335
|
|
$
|
—
|
|
||||
|
Name
|
Age
|
Position
|
|||
|
Directors
|
|||||
|
Werner Cautreels, Ph.D.
|
64
|
|
President and Chief Executive Officer and Director
|
||
|
Timothy C. Barabe (1)
|
63
|
|
Director
|
||
|
Omid Farokhzad, M.D.(3)
|
47
|
|
Director
|
||
|
Carl Gordon, Ph.D.(1)
|
52
|
|
Director
|
||
|
Peter Barton Hutt, LL.B., LL.M.(2)
|
82
|
|
Director
|
||
|
Edwin M. Kania(1)(2)
|
59
|
|
Director
|
||
|
Amir Nashat, Ph.D(1)(3)
|
43
|
|
Director
|
||
|
Aymeric Sallin, M.S.(2)
|
43
|
|
Director
|
||
|
Timothy A. Springer, Ph.D.(3)
|
68
|
|
Director
|
||
|
Executive Officers
|
|||||
|
Lloyd Johnston, Ph.D.
|
49
|
|
Chief Operating Officer and Senior Vice President, Research and Development
|
||
|
Takashi Kei Kishimoto, Ph.D.
|
56
|
|
Chief Scientific Officer
|
||
|
Peter Keller, M.Sci.
|
45
|
|
Chief Business Officer
|
||
|
David Abraham, J.D.
|
51
|
|
Chief Compliance Officer, General Counsel and Corporate Secretary
|
||
|
Earl Sands, M.D.
|
58
|
|
Chief Medical Officer
|
||
|
David Siewers, CPA
|
63
|
|
Chief Financial Officer
|
||
|
(1)
|
Member of the audit committee.
|
|
(2)
|
Member of the compensation committee.
|
|
(3)
|
Member of the nominating and corporate governance committee.
|
|
Name and principal position
|
Year
|
Salary ($)
|
Option awards ($)(1)
|
Non-equity incentive plan compensation ($)(2)
|
All other compensation ($)(3)
|
Total ($)
|
||||||||||
|
Werner Cautreels, Ph.D.
|
2016
|
425,000
|
|
1,286,295
|
|
258,000
|
|
—
|
|
1,969,295
|
|
|||||
|
President and Chief Executive Officer
|
2015
|
400,000
|
|
568,798
|
|
130,000
|
|
—
|
|
1,098,798
|
|
|||||
|
Earl Sands, M.D
|
2016
|
300,000
|
|
649,401
|
|
115,000
|
|
44,240
|
|
1,108,641
|
|
|||||
|
Chief Medical Officer
|
2015
|
143,231
|
|
388,147
|
|
40,000
|
|
22,847
|
|
594,225
|
|
|||||
|
Peter Keller, M.Sci.
|
2016
|
290,000
|
|
415,194
|
|
142,000
|
|
3,400
|
|
850,594
|
|
|||||
|
Chief Business Officer
|
2015
|
275,000
|
|
72,856
|
|
75,000
|
|
3,400
|
|
426,256
|
|
|||||
|
(1)
|
Represents the aggregate grant date fair value of stock options computed in accordance with ASC Topic 718, excluding the effect of estimated forfeitures. For a description of the assumptions used in valuing these awards, see Note 12 o our consolidated audited financial statements included elsewhere in this Annual Report.
|
|
(2)
|
Represents amounts earned under our annual performance based bonus program. For additional information, see “Performance Bonuses” below.
|
|
(3)
|
For Dr. Sands, the amount represents
$3,400
in our company’s matching contributions to his 401(k) plan account and
$40,840
in reimbursements for expenses incurred in 2016 for travel between his home in Georgia and our offices in Massachusetts. For Mr. Keller, the 2016 amount represents our company’s matching contributions to 401(k) plan accounts.
|
|
Named executive officer
|
Incentive Plan
|
Options Granted (#)
|
|||
|
Werner Cautreels, Ph.D.
|
2008 Plan
|
—
|
|
||
|
2016 Plan
|
115,384
|
|
|||
|
Earl Sands, M.D.
|
2008 Plan
|
10,256
|
|
||
|
2016 Plan
|
53,999
|
|
|||
|
Peter Keller, M.Sci.
|
2008 Plan
|
15,383
|
|
||
|
2016 Plan
|
30,841
|
|
|||
|
Option Awards
|
Stock awards
|
|||||||||||||||
|
Name
|
Number of
securities underlying unexercised options (#) exercisable(1) |
Number of
securities underlying unexercised options (#) unexercisable |
Option
exercise price ($) |
Option
expiration date |
Number of
securities that have not vested (#) |
Market
value of securities that have not vested ($) |
||||||||||
|
Werner Cautreels, Ph.D.
|
—
|
|
115,384
|
|
14.00
|
|
6/20/2026
|
—
|
|
—
|
|
|||||
|
123,076
|
|
(2)
|
—
|
|
6.40
|
|
|
12/4/2025
|
—
|
|
—
|
|
||||
|
57,692
|
|
(2)
|
—
|
|
8.97
|
|
|
4/7/2024
|
—
|
|
—
|
|
||||
|
16,613
|
|
(3)
|
—
|
|
2.77
|
|
|
6/13/2023
|
—
|
|
—
|
|
||||
|
41,025
|
|
(3)
|
—
|
|
3.44
|
|
|
3/29/2022
|
—
|
|
—
|
|
||||
|
Earl Sands, M.D.
|
—
|
|
53,999
|
|
14.00
|
|
6/20/2016
|
—
|
|
—
|
|
|||||
|
10,256
|
|
(2)
|
—
|
|
7.02
|
|
3/8/2026
|
—
|
|
—
|
|
|||||
|
102,563
|
|
(2)
|
—
|
|
9.36
|
|
|
9/7/2025
|
—
|
|
—
|
|
||||
|
Peter Keller, M.Sci.
|
—
|
|
30,841
|
|
14.00
|
|
|
6/20/2026
|
—
|
|
—
|
|
||||
|
15,383
|
|
(2)
|
—
|
|
7.02
|
|
3/8/2026
|
—
|
|
—
|
|
|||||
|
10,256
|
|
(2)
|
—
|
|
9.36
|
|
2/20/2025
|
—
|
|
—
|
|
|||||
|
8,461
|
|
(2)
|
—
|
|
8.97
|
|
4/7/2024
|
—
|
|
—
|
|
|||||
|
2,564
|
|
(2)
|
—
|
|
2.77
|
|
6/13/2023
|
—
|
|
—
|
|
|||||
|
94,183
|
|
(3)
|
—
|
|
0.63
|
|
|
2/16/2021
|
—
|
|
—
|
|
||||
|
(1)
|
All stock options issued under the 2008 Plan and held by our named executive officers, whether vested or unvested, are immediately exercisable on the date of grant. Shares purchased upon exercise of an unvested option become restricted stock and are subject to our right of repurchase in the event the option holder's service with us terminates prior to the date the shares vest for a purchase price equal to the exercise price paid for the shares.
|
|
(2)
|
The option vests as to 25% of the total shares underlying the option on the first anniversary of the vesting commencement date and in equal monthly installments over the ensuing 36 months, subject to the holder's continued employment with us through the applicable vesting date and potential accelerated vesting in the event of a termination without cause or resignation for good reason within 12 months following a change in control.
|
|
(3)
|
All shares underlying the option are fully vested.
|
|
•
|
annual director fee of $35,000,
|
|
•
|
chairman of the board or lead independent director, $15,000,
|
|
•
|
chairman of the audit committee, $15,000,
|
|
•
|
audit committee member other than the chairman, $7,500,
|
|
•
|
chairman of the compensation committee, $10,000,
|
|
•
|
compensation committee member other than the chairman, $5,000,
|
|
•
|
chairman of the nominating and corporate governance committee, $7,500, and
|
|
•
|
nominating and corporate governance committee member other than the chairman, $3,500.
|
|
Name
|
Fees earned or paid in cash ($)(1)
|
Option awards ($)(2)
|
All other compensation ($)(3)
|
Total ($)
|
|||||||
|
Omid Farokhzad, M.D.
|
20,319
|
|
|
—
|
|
|
110,000
|
|
|
130,319
|
|
|
Carl Gordon, Ph.D.
|
22,431
|
|
|
—
|
|
—
|
|
|
22,431
|
|
|
|
Peter Barton Hutt J.D., L.L.B., L.L.M
|
23,750
|
|
|
—
|
|
|
—
|
|
|
23,750
|
|
|
Edwin M. Kania
|
28,819
|
|
|
—
|
|
|
—
|
|
28,819
|
|
|
|
Timothy Barabe (4)
|
17,981
|
|
|
99,633
|
|
|
—
|
|
|
117,614
|
|
|
Amir Nashat, Ph.D
|
34,514
|
|
|
—
|
|
|
—
|
|
|
34,514
|
|
|
Aymeric Sallin, M.S.
|
21,111
|
|
|
—
|
|
|
—
|
|
|
21,111
|
|
|
Timothy Springer, Ph.D.
|
20,319
|
|
|
—
|
|
|
—
|
|
|
20,319
|
|
|
Robert Langer, Sc.D. (5)
|
—
|
|
—
|
|
33,750
|
|
33,750
|
|
|||
|
Leysan Shaydullina, M.D. (5)
|
—
|
|
—
|
|
—
|
|
—
|
|
|||
|
George Siber, M.D. (5)
|
—
|
|
—
|
|
20,100
|
|
20,100
|
|
|||
|
(1)
|
Represents cash retainers earned for services rendered as members of the Board of Directors and related Committees.
|
|
(2)
|
Represents the aggregate grant date fair value of stock options computed in accordance with ASC Topic 718, excluding the effect of estimated forfeitures. For a description of the assumptions used in valuing these awards, see Note 12 to our consolidated audited financial statements included elsewhere in this Annual Report.
|
|
(3)
|
Represents compensation earned in 2016 under consulting agreements with the company. For Robert Langer and George Siber, the amounts represent those fees earned only for the period for which they were directors. For additional information regarding this agreement, see "Certain relationships and related party transactions"
|
|
(4)
|
Timothy Barabe was elected to the Board of Directors in July 2016 and as such received an initial stock option award of
12,820
shares which will vest in equal monthly installments over a three year period following the date of grant.
|
|
(5)
|
Drs. Langer, Shaydullina and Siber resigned from our board of directors in June 2016.
|
|
Name
|
Options outstanding
at fiscal year end
|
||
|
Omid Farokhzad, M.D.
|
87,554
|
|
|
|
Carl Gordon, Ph.D.
|
—
|
|
|
|
Peter Barton Hutt J.D., L.L.B., L.L.M
|
44,870
|
|
|
|
Edwin M. Kania
|
—
|
|
|
|
Timothy Barabe (3)
|
12,820
|
|
|
|
Amir Nashat, Ph.D
|
—
|
|
|
|
Aymeric Sallin, M.S.
|
—
|
|
|
|
Timothy Springer
|
—
|
|
|
|
Name of Beneficial Owner
|
Number of Shares
|
Percent (%) of Total
|
||||
|
5% or Greater Stockholders
|
|
|
|
|||
|
Entities affiliated with Polaris Partners(1)
|
1,924,151
|
|
10.4
|
%
|
||
|
Entities affiliated with OrbiMed Advisors LLC(2)
|
1,849,371
|
|
10.0
|
%
|
||
|
TAS Partners LLC, and Leukon Partners, LP as affiliated entities (3)
|
1,692,234
|
|
9.2
|
%
|
||
|
RUSNANO(4)
|
1,489,280
|
|
8.1
|
%
|
||
|
Entities affiliated with Deerfield Partners, L.P. (5)
|
1,060,000
|
|
5.7
|
%
|
||
|
NanoDimension L.P.(6)
|
1,052,864
|
|
5.7
|
%
|
||
|
Named Executive Officers and Directors
|
||||||
|
Werner Cautreels, Ph.D. (8)
|
446,759
|
|
2.4
|
%
|
||
|
Lloyd Johnston, Ph.D.(9)
|
110,141
|
|
*
|
|
||
|
Earl Sands, M.D.(10)
|
47,867
|
|
*
|
|
||
|
Omid Farokhzad, M.D.(11)
|
449,704
|
|
2.4
|
%
|
||
|
Carl Gordon, Ph.D.(2)
|
1,849,371
|
|
10
|
%
|
||
|
Peter Barton Hutt (12)
|
40,173
|
|
*
|
|
||
|
Edwin M. Kania, Jr. (13)
|
847,124
|
|
4.6
|
%
|
||
|
Amir Nashat, Ph.D(1)
|
1,924,151
|
|
10.4
|
%
|
||
|
Aymeric Sallin, M.S.(6)
|
—
|
|
—
|
%
|
||
|
Timothy Barabe(7)
|
2,855
|
|
*
|
|
||
|
Timothy Springer(3)
|
1,692,234
|
|
9.2
|
%
|
||
|
All executive officers and directors as a group (14 persons)
|
7,828,752
|
|
42.4
|
%
|
||
|
(1)
|
Based on a Schedule 13D filed with the SEC on July 7, 2016, consists of (i) 1,836,703 shares of common stock held by Polaris Venture Partners V, L.P., or PVP V, (ii) 19,990 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by PVP V, (iii) 35,793 shares of common stock held by Polaris Venture Partners Entrepreneurs’ Fund V, L.P., or PVPE V, (iv) 389 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by PVPE V, (v) 12,577 shares of common stock held by Polaris Venture Partners Founders’ Fund V, L.P., or PVPFF V, (vi) 136 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by PVPFF V, (vii) 18,364 shares of common stock held by Polaris Venture Partners Special Founders’ Fund V, L.P., or PVPSFF V and, together with PVP V, PVPE V, PVPFF V and PVPSFF V, the Funds, and (viii) 199 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by PVPSFF V. The general partner of each of the Funds is Polaris Venture Management Co., V, L.L.C., or PVM V. PVM V may be deemed to have sole voting and investment power with respect to the shares held by the Funds. Jonathan A. Flint and Terrance G. McGuire are managing members of PVM V and Dr. Amir Nashat, our director, is the holder of an assignee interest in PMV V. As a result, each of Messrs. Flint and McGuire and Dr. Nashat may be
|
|
(2)
|
Based on a Schedule 13D filed with the SEC on July 7, 2016, consists of (i) 1,825,415 shares of common stock held by OrbiMed Private Investments III, LP, or OPI III, (ii) 17,382 shares of common stock held by OrbiMed Associates III, LP, or Associates III and, together with OPI III, the OrbiMed Funds, and (iii) 6,574 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017. OrbiMed Capital GP III LLC, or GP III, is the general partner of OPI III and OrbiMed Advisors LLC, or OrbiMed Advisors, is the managing member of GP III and the general partner of Associates III. Mr. Samuel D. Isaly is the managing member of and owner of a controlling interest in OrbiMed Advisors. By virtue of such relationships, GP III, OrbiMed Advisors and Mr. Isaly may be deemed to have shared voting and investment power over the shares held by OPI III and OrbiMed Advisors and Mr. Isaly may be deemed to have shared voting and investment power over the shares held by Associates III. The mailing address of the beneficial owners is 601 Lexington Avenue, 54th Floor, New York, NY 10022.
|
|
(3)
|
Consists of (i) 445,576 shares of common stock held by TAS Partners, LLC, or TAS, (ii) 4,304 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by TAS, (iii) 1,237,028 shares of common stock held by Leukon Investments LP, or Leukon, and (iv) 5,326 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017. LKST, Inc. is the general partner of Leukon. Timothy Springer, our director, is the president of LKST, Inc. and is also the manager of TAS. Mr. Springer disclaims beneficial ownership of the shares held by TAS and Leukon. Each of TAS and Leukon disclaim beneficial ownership of the shares held by the other. The address of TAS and Leukon is 36 Woodman Road, Chestnut Hill, MA 02467.
|
|
(4)
|
Based on a Schedule 13G filed with the SEC on July 5, 2016, consists of (i) 1,476,177 shares of common stock held by RUSNANO and (ii) 13,103 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by RUSNANO. RUSNANO is a joint stock company organized under the laws of the Russian Federation. The Russian Federation owns 100% of RUSNANO. RUSNANO is managed by RUSNANO Management Company LLC, the Executive Board of which has the power to vote and dispose of the securities held directly by RUSNANO below a certain amount, and is supervised by the Board of Directors of RUSNANO, which, along with the Executive Board of RUSNANO Management Company LLC, has the power to dispose of the securities held directly by RUSNANO above a certain amount. Anatoly Chubais, Vladimir Avetissian, German Pikhoya, Oleg Kiselev, Boris Podolsky and Yury Udaltsov, as the members of the Executive Board of RUSNANO Management Company LLC, and Arkadiy Dvorkovich, Anatoly Chubais, Igor Agamirzyan, Mikhail Alfimov, Oleg Fomichev, Andrey Ivanov, Denis Manturov, Vladislav Putilin, Pavel Teplukhin, Viktor Vekselberg and Ilya Yuzhanov, as the members of the Board of Directors of RUSNANO, may be deemed to have or share beneficial ownership of these securities. Each of them disclaims any such beneficial ownership. The address of each of RUSNANO and RUSNANO Management Company LLC is 10A prospect 60‑letiya Oktyabrya, Moscow, Russia 117036.
|
|
(6)
|
Based on a Schedule 13G filed with the SEC on February 24, 2017, consists of (i) 660,857 shares of common stock held by NanoDimension L.P., or ND LP, (ii) 350,000 shares of common stock held by NanoDimension L.P. II, or ND LP II, (iii) 35,000 shares of common stock held by NanoDimension Management Limited, or ND GP, and (iv) 7,007 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held by ND LP. ND GP serves as the general partner of ND LP and possesses power to direct the voting and disposition of the shares owned by ND LP and may be deemed to have indirect beneficial ownership of the shares held by ND LP. NanoDimension II Management Limited, or ND GP II, serves as the general partner of ND LP II and possesses the power to direct the voting and disposition of the shares owned by ND LP2 II and may be deemed to have indirect beneficial ownership of the shares held by ND LP II. Jonathan Nicholson and Richard Coles are the members of the board of directors of ND GP and ND GP II and share voting and dispositive power over the shares held by ND LP and ND LP II. Aymeric Sallin is a member of the investment advisory committee of ND GP that provides investment recommendation to ND GP. Each such person disclaims beneficial ownership of the shares reported herein. The address for the beneficial owners is Governor’s Square, Unit 3‑213‑6, 23 Lime Tree Bay Ave, Grand Cayman, Cayman Islands KY1‑1302.
|
|
(7)
|
Includes 2,855 shares of common stock underlying outstanding stock options exercisable within 60 days of February 15, 2017.
|
|
(8)
|
Includes 446,759 shares of common stock underlying outstanding stock options exercisable within 60 days of February 15, 2017.
|
|
(9)
|
Includes 110,141 shares of common stock underlying outstanding stock options exercisable within 60 days of February 15, 2017.
|
|
(10)
|
Includes 47,867 shares of common stock underlying stock options exercisable within 60 days of February 15, 2017.
|
|
(11)
|
Includes (i) 94,023 shares of common stock underlying outstanding stock options exercisable within 60 days of February 15, 2017; (ii) 202,051 shares of common stock held by a family trust for which Dr. Farokhzad’s wife serves as trustee; and (iii) 25,626 shares of common stock held by BioDynamics Core, L.P., which is managed by BioDynamics, LLC, of which Dr. Farokhzad is a member. Dr. Farokhzad disclaims beneficial ownership over the shares held by the family trust.
|
|
(12)
|
Includes 38,465 shares of common stock underlying outstanding stock options exercisable within 60 days of February 15, 2017.
|
|
(13)
|
Consists of (i) 826,862 shares of common stock held of record by Flagship Ventures Fund 2007, L.P., or Flagship Ventures 2007, and (ii) 20,262 shares of common stock underlying warrants exercisable within 60 days of February 15, 2017 held of record by Flagship Ventures 2007. Flagship Ventures 2007 General Partner, LLC, or Flagship 2007 LLC, is the general partner of Flagship Ventures 2007 and Noubar B. Afeyan Ph.D. and Edwin M. Kania, Jr. are the managers of Flagship 2007 LLC. Flagship 2007 LLC, Dr. Afeyan and Mr. Kania may be deemed to share voting and investment power with respect to all shares held by Flagship Ventures 2007. Flagship 2007 LLC, Dr. Afeyan and Mr. Kania expressly disclaim beneficial ownership of the securities listed above except to the extent of any pecuniary interest therein. The address for Flagship Ventures 2007 is One Memorial Drive, 7th Floor, Cambridge, MA 02142.
|
|
Number of securities
|
Number of securities
|
|||||||||
|
to be issued upon
|
Weighted-average
|
remaining available for
|
||||||||
|
exercise of outstanding
|
exercise price of
|
future issuance under
|
||||||||
|
stock options, warrants
|
outstanding options,
|
equity compensation
|
||||||||
|
Plan category
|
and rights
|
warrants and rights
|
plans (1)
|
|||||||
|
(a)
|
(b)
|
(c)
|
||||||||
|
Equity compensation plans approved by security holders
(2)
|
2,128,346
|
|
(3)
|
$
|
7.09
|
|
(4)
|
939,317
|
|
(5)
|
|
Equity compensation plans not approved by security holders
|
—
|
|
—
|
|
—
|
|
||||
|
Total
|
2,128,346
|
|
$
|
7.09
|
|
939,317
|
|
|||
|
(1)
|
Pursuant to the terms of the 2016 Plan, the number of shares of common stock available for issuance under the 2016 Plan automatically increases on each January 1, until and including January 1, 2026, by an amount equal to the lesser of: (a) 4% of the shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares as is determined by our board of directors. Pursuant to the terms of the 2016 Employee Stock Purchase Plan (the “2016 ESPP”), the number of shares of common stock available for issuance under the 2016 ESPP automatically increases on each January 1, until and including January 1, 2026, by an amount equal to the lesser of: (a) 1% of the shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares as is determined by our board of directors.
|
|
(2)
|
Includes the 2016 Plan, the 2008 Plan and the 2016 ESPP.
|
|
(3)
|
Includes
1,665,384
outstanding options to purchase stock under the 2008 Plan and
462,962
outstanding options to purchase stock under the 2016 Plan.
|
|
(4)
|
As of December 31, 2016, the weighted-average exercise price of outstanding options under the 2008 Plan was
$5.00
and the weighted-average exercise price of outstanding options under the 2016 Plan was
$14.62
.
|
|
(5)
|
Represents
18,947
shares available for issuance under the 2008 Plan,
747,294
shares available for issuance under the 2016 Plan and
173,076
shares available for issuance under the 2016 ESPP. To the extent outstanding stock options under the 2008 Plan are forfeited or lapse unexercised, the shares of common stock subject to such stock option awards will be available for issuance under the 2016 Plan.
No purchase rights were outstanding under the 2016 ESPP as of December 31, 2016.
|
|
Participants
|
Shares of Common Stock
|
||
|
5% or Greater Stockholders(1)
|
|||
|
Entities affiliated with Polaris Partners(2)
|
150,000
|
|
|
|
RUSNANO
|
107,143
|
|
|
|
Entities affiliated with OrbiMed Advisors LLC(3)
|
550,000
|
|
|
|
NanoDimension L.P.
|
385,000
|
|
|
|
TAS Partners LLC and Leukon Partners, LP, as affiliated entities(4)
|
710,000
|
|
|
|
(1)
|
Additional details regarding these stockholders and their equity holdings are provided in this Annual Report under the caption “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
|
|
(2)
|
Represents shares acquired by Polaris Venture Partners Entrepreneurs’ Fund V, L.P., Polaris Venture Partners Founders’ Fund V, L.P., Polaris Venture Partners Special Founders’ Fund V, L.P. and Polaris Venture Partners V, L.P.
|
|
(3)
|
Represents shares acquired by OrbiMed Associates III, LP and OrbiMed Private Investments III, LP.
|
|
(4)
|
Represents shares acquired by Leukon Investments LP.
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Balance Sheets at December 31, 2016 and 2015
|
F-2
|
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2016, 2015 and 2014
|
F-3
|
|
Consolidated Statements of Convertible Preferred Stock and Stockholders' Equity (Deficit) for the years ended December 31, 2016, 2015 and 2014
|
F-4
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2016, 2015 and 2014
|
F-5
|
|
Notes to Consolidated Financial Statements
|
F-6
|
|
|
December 31,
|
December 31,
|
|||||
|
|
2016
|
2015
|
|||||
|
|
|
||||||
|
Assets
|
|
|
|||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
58,656
|
|
$
|
32,337
|
|
|
|
Short-term deposits and investments
|
25,485
|
|
4,125
|
|
|||
|
Restricted cash
|
78
|
|
133
|
|
|||
|
Accounts receivable
|
215
|
|
824
|
|
|||
|
Prepaid expenses and other current assets
|
2,382
|
|
1,494
|
|
|||
|
Total current assets
|
86,816
|
|
|
38,913
|
|
||
|
Property and equipment, net
|
2,047
|
|
2,029
|
|
|||
|
Restricted cash and other deposits
|
316
|
|
316
|
|
|||
|
Other assets
|
122
|
|
1,566
|
|
|||
|
Total assets
|
$
|
89,301
|
|
|
$
|
42,824
|
|
|
Liabilities, redeemable convertible preferred stock, and stockholders’ equity (deficit)
|
|
|
|||||
|
Current liabilities:
|
|
|
|||||
|
Accounts payable
|
$
|
3,882
|
|
$
|
2,179
|
|
|
|
Accrued expenses
|
3,921
|
|
3,378
|
|
|||
|
Loans payable, current portion
|
4,067
|
|
—
|
|
|||
|
Deferred revenue, current portion
|
1,836
|
|
1,313
|
|
|||
|
Contingently repayable grant funding
|
—
|
|
420
|
|
|||
|
Total current liabilities
|
13,706
|
|
|
7,290
|
|
||
|
Non‑current liabilities:
|
|||||||
|
Deferred rent and lease incentive
|
222
|
|
105
|
|
|||
|
Loans payable, net of current portion
|
7,977
|
|
11,855
|
|
|||
|
Deferred revenue, net of current portion
|
12,439
|
|
2,295
|
|
|||
|
Other long‑term liabilities
|
—
|
|
290
|
|
|||
|
Total liabilities
|
34,344
|
|
|
21,835
|
|
||
|
Commitments and contingencies (Notes 8 and 13)
|
|
|
|||||
|
Redeemable Convertible Preferred Stock:
|
|
|
|||||
|
Series A redeemable convertible preferred stock, $0.0001 par value; 0 and 2,589,868 shares authorized; 0 and 2,589,868 shares issued and outstanding; as of December 31, 2016 and December 31, 2015 respectively.
|
—
|
|
3,644
|
|
|||
|
Series B redeemable convertible preferred stock, $0.0001 par value; 0 and 7,437,325 shares authorized; 0 and 7,437,325 shares issued and outstanding; as of December 31, 2016 and December 31, 2015 respectively.
|
—
|
|
21,448
|
|
|||
|
Series C redeemable convertible preferred stock, $0.0001 par value; 0 and 5,000,002 shares authorized; 0 and 5,000,002 shares issued and outstanding; as of December 31, 2016 and December 31, 2015 respectively.
|
—
|
|
20,178
|
|
|||
|
Series D redeemable convertible preferred stock, $0.0001 par value; 0 and 8,166,662 shares authorized; 0 and 8,099,994 shares issued and outstanding; as of December 31, 2016 and December 31, 2015 respectively.
|
—
|
|
42,902
|
|
|||
|
Series SRN redeemable convertible preferred stock, $0.0001 par value; 0 and 5,611,112 shares authorized; 0 and 2,111,109 shares issued and outstanding; as of December 31, 2016 and December 31, 2015 respectively.
|
—
|
|
12,082
|
|
|||
|
Series E redeemable convertible preferred stock, $0.0001 par value; 0 and 9,030,654 shares authorized; 0 and 8,888,888 shares issued and outstanding; as of December 31, 2016 and December 31, 2015 respectively.
|
—
|
|
37,228
|
|
|||
|
Total redeemable convertible preferred stock
|
—
|
|
|
137,482
|
|
||
|
Stockholders’ equity (deficit):
|
|
|
|||||
|
Preferred stock, $0.0001 par value; 10,000,000 and 0 shares authorized; 0 shares issued and outstanding at December 31, 2016 and December 31, 2015, respectively.
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value; 200,000,000 and 62,164,377 shares authorized at December 31, 2016 and December 31, 2015 respectively; 18,438,742 and 2,180,976 shares issued, 18,438,742 and 2,173,399 shares outstanding as of December 31, 2016 and December 31, 2015, respectively.
|
1
|
|
—
|
|
|||
|
Additional paid-in capital
|
211,125
|
|
1
|
|
|||
|
Receivable from stock option exercises
|
(75
|
)
|
—
|
|
|||
|
Accumulated deficit
|
(151,576
|
)
|
(111,508
|
)
|
|||
|
Accumulated other comprehensive loss
|
(4,518
|
)
|
(4,986
|
)
|
|||
|
Total stockholders’ equity (deficit)
|
54,957
|
|
(116,493
|
)
|
|||
|
Total liabilities, redeemable convertible preferred stock and stockholders’ equity
|
$
|
89,301
|
|
|
$
|
42,824
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Grant and collaboration revenue
|
$
|
8,083
|
|
$
|
6,011
|
|
$
|
3,040
|
|
||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
29,702
|
|
22,980
|
|
10,486
|
|
|||||
|
General and administrative
|
13,051
|
|
8,335
|
|
7,953
|
|
|||||
|
Total operating expenses
|
42,753
|
|
|
31,315
|
|
18,439
|
|
||||
|
Loss from operations
|
(34,670
|
)
|
(25,304
|
)
|
(15,399
|
)
|
|||||
|
Investment income
|
234
|
|
171
|
|
111
|
|
|||||
|
Foreign currency transaction gain (loss), net
|
(525
|
)
|
933
|
|
3,004
|
|
|||||
|
Interest expense
|
(1,253
|
)
|
(948
|
)
|
(552
|
)
|
|||||
|
Other expense, net
|
4
|
|
(26
|
)
|
(44
|
)
|
|||||
|
Net loss
|
(36,210
|
)
|
|
(25,174
|
)
|
(12,880
|
)
|
||||
|
Other comprehensive loss:
|
|||||||||||
|
Foreign currency translation adjustment
|
504
|
|
(1,110
|
)
|
(3,281
|
)
|
|||||
|
Unrealized gain (loss) on securities
|
(36
|
)
|
—
|
|
—
|
|
|||||
|
Comprehensive loss
|
$
|
(35,742
|
)
|
|
$
|
(26,284
|
)
|
$
|
(16,161
|
)
|
|
|
Net loss
|
(36,210
|
)
|
(25,174
|
)
|
(12,880
|
)
|
|||||
|
Accretion of redeemable convertible preferred stock
|
(4,566
|
)
|
(7,335
|
)
|
(4,951
|
)
|
|||||
|
Net effect of extinguishment of Series SRN redeemable convertible preferred stock
|
$
|
—
|
|
$
|
—
|
|
$
|
1,459
|
|
||
|
Net loss attributable to common stockholders
|
$
|
(40,776
|
)
|
|
$
|
(32,509
|
)
|
$
|
(16,372
|
)
|
|
|
Net loss per share attributable to common stockholders
|
|||||||||||
|
Basic and diluted
|
$
|
(3.89
|
)
|
$
|
(15.13
|
)
|
$
|
(7.84
|
)
|
||
|
Weighted average common shares outstanding
|
|||||||||||
|
Basic and diluted
|
10,493,939
|
|
2,150,422
|
|
2,090,677
|
|
|||||
|
|
Series A
|
Series B
|
Series C
|
Series D
|
Series SRN
|
Series E
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|
redeemable
|
redeemable
|
redeemable
|
redeemable
|
redeemable
|
redeemable
|
|
|
|
|
Accumulated
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|
convertible
|
convertible
|
convertible
|
convertible
|
convertible
|
convertible
|
|
|
Additional
|
Stock
|
|
other
|
Stockholders’
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
|
preferred stock
|
preferred stock
|
preferred stock
|
preferred stock
|
preferred stock
|
preferred stock
|
Common stock
|
paid‑In
|
option
|
Accumulated
|
comprehensive
|
Equity
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
receivable
|
deficit
|
loss
|
(Deficit)
|
||||||||||||||||||||||||||||||||||||||||||
|
Balance at December 31, 2013
|
2,589,868
|
|
$
|
3,350
|
|
7,437,325
|
|
$
|
19,662
|
|
5,000,002
|
|
$
|
18,381
|
|
4,888,889
|
|
$
|
24,366
|
|
777,777
|
|
$
|
4,643
|
|
—
|
|
$
|
—
|
|
2,027,254
|
|
$
|
—
|
|
$
|
1
|
|
$
|
—
|
|
$
|
(68,869
|
)
|
$
|
(595
|
)
|
$
|
(69,463
|
)
|
|||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
58,104
|
|
—
|
|
69
|
|
—
|
|
—
|
|
—
|
|
69
|
|
|||||||||||||||||||||||
|
Issuance of common stock upon exercise of options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
38,639
|
|
—
|
|
68
|
|
—
|
|
—
|
|
—
|
|
68
|
|
|||||||||||||||||||||||
|
Stock‑based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,224
|
|
—
|
|
—
|
|
—
|
|
1,224
|
|
|||||||||||||||||||||||
|
Accretion of preferred stock to redemption value
|
—
|
|
143
|
|
—
|
|
871
|
|
—
|
|
889
|
|
—
|
|
1,855
|
|
—
|
|
1,193
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,820
|
)
|
—
|
|
(2,131
|
)
|
—
|
|
(4,951
|
)
|
|||||||||||||||||||||||
|
Issuance of Series D redeemable convertible preferred stock, net of issuance costs of $100,734
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,211,105
|
|
14,349
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Net effect of extinguishment of Series SRN redeemable preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,459
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
1,459
|
|
—
|
|
—
|
|
—
|
|
1,459
|
|
|||||||||||||||||||||||
|
Issuance of Series SRN redeemable convertible preferred stock, net of issuance costs of $209,587
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,333,332
|
|
5,790
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(3,281
|
)
|
(3,281
|
)
|
|||||||||||||||||||||||
|
Unrealized gains (losses) on securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(12,880
|
)
|
—
|
|
(12,880
|
)
|
|||||||||||||||||||||||
|
Balance at December 31, 2014
|
2,589,868
|
|
$
|
3,493
|
|
7,437,325
|
|
$
|
20,533
|
|
5,000,002
|
|
$
|
19,270
|
|
8,099,994
|
|
$
|
40,570
|
|
2,111,109
|
|
$
|
10,167
|
|
—
|
|
$
|
—
|
|
2,123,997
|
|
$
|
—
|
|
$
|
1
|
|
$
|
—
|
|
$
|
(83,880
|
)
|
$
|
(3,876
|
)
|
$
|
(87,755
|
)
|
|||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7,688
|
|
—
|
|
23
|
|
—
|
|
—
|
|
—
|
|
23
|
|
|||||||||||||||||||||||
|
Issuance of common stock upon exercise of options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
41,714
|
|
—
|
|
86
|
|
—
|
|
—
|
|
—
|
|
86
|
|
|||||||||||||||||||||||
|
Stock‑based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,125
|
|
—
|
|
—
|
|
—
|
|
1,125
|
|
|||||||||||||||||||||||
|
Accretion of preferred stock to redemption value
|
—
|
|
151
|
|
—
|
|
915
|
|
—
|
|
908
|
|
—
|
|
2,332
|
|
—
|
|
1,915
|
|
—
|
|
1,114
|
|
—
|
|
—
|
|
(4,881
|
)
|
—
|
|
(2,454
|
)
|
—
|
|
(7,335
|
)
|
|||||||||||||||||||||||
|
Issuance of Series E redeemable convertible preferred stock, net of issuance costs of $213,469
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,888,888
|
|
36,114
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Issuance of common stock warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,647
|
|
—
|
|
—
|
|
—
|
|
3,647
|
|
|||||||||||||||||||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,110
|
)
|
(1,110
|
)
|
|||||||||||||||||||||||
|
Unrealized gains (losses) on securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(25,174
|
)
|
—
|
|
(25,174
|
)
|
|||||||||||||||||||||||
|
Balance at December 31, 2015
|
2,589,868
|
|
$
|
3,644
|
|
7,437,325
|
|
$
|
21,448
|
|
5,000,002
|
|
$
|
20,178
|
|
8,099,994
|
|
$
|
42,902
|
|
2,111,109
|
|
$
|
12,082
|
|
8,888,888
|
|
$
|
37,228
|
|
2,173,399
|
|
$
|
—
|
|
$
|
1
|
|
$
|
—
|
|
$
|
(111,508
|
)
|
$
|
(4,986
|
)
|
$
|
(116,493
|
)
|
|||||||||||
|
Vesting of restricted common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,535
|
|
—
|
|
21
|
|
—
|
|
—
|
|
—
|
|
21
|
|
|||||||||||||||||||||||
|
Issuance of common stock upon exercise of options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
71,816
|
|
—
|
|
224
|
|
(75
|
)
|
—
|
|
—
|
|
149
|
|
|||||||||||||||||||||||
|
Stock‑based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,030
|
|
—
|
|
—
|
|
—
|
|
2,030
|
|
|||||||||||||||||||||||
|
Accretion of preferred stock to redemption value
|
—
|
|
75
|
|
—
|
|
449
|
|
—
|
|
446
|
|
—
|
|
1,131
|
|
—
|
|
913
|
|
—
|
|
1,552
|
|
—
|
|
—
|
|
(708
|
)
|
—
|
|
(3,858
|
)
|
—
|
|
(4,566
|
)
|
|||||||||||||||||||||||
|
Exercise of common warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
572,003
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||
|
Conversion of convertible stock upon listing
|
(2,589,868
|
)
|
(3,719
|
)
|
(7,437,325
|
)
|
(21,897
|
)
|
(5,000,002
|
)
|
(20,624
|
)
|
(8,099,994
|
)
|
(44,033
|
)
|
(2,111,109
|
)
|
(12,995
|
)
|
(8,888,888
|
)
|
(38,780
|
)
|
10,126,118
|
|
1
|
|
142,047
|
|
—
|
|
—
|
|
—
|
|
142,048
|
|
|||||||||||||||||||||||
|
Issuance of common stock, Initial public offering net of issuance costs
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5,289,633
|
|
—
|
|
64,465
|
|
—
|
|
—
|
|
—
|
|
64,465
|
|
|||||||||||||||||||||||
|
Issuance of common stock, license agreement
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
197,238
|
|
—
|
|
2,743
|
|
—
|
|
—
|
|
—
|
|
2,743
|
|
|||||||||||||||||||||||
|
Conversion of series D preferred stock warrants into warrants for the purchase of common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
189
|
|
—
|
|
—
|
|
—
|
|
189
|
|
|||||||||||||||||||||||
|
Conversion of series E preferred stock warrants into warrants for the purchase of common stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
113
|
|
—
|
|
—
|
|
—
|
|
113
|
|
|||||||||||||||||||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
504
|
|
504
|
|
|||||||||||||||||||||||
|
Unrealized gains (losses) on securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(36
|
)
|
(36
|
)
|
|||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(36,210
|
)
|
—
|
|
(36,210
|
)
|
|||||||||||||||||||||||
|
Balance at December 31, 2016
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
18,438,742
|
|
$
|
1
|
|
$
|
211,125
|
|
$
|
(75
|
)
|
$
|
(151,576
|
)
|
$
|
(4,518
|
)
|
$
|
54,957
|
|
|||||||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Operating activities
|
|
|
|||||||||
|
Net loss
|
$
|
(36,210
|
)
|
$
|
(25,174
|
)
|
$
|
(12,880
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||||||
|
Depreciation
|
802
|
|
1,044
|
|
864
|
|
|||||
|
Amortization of premiums and accretion of discounts on investments
|
193
|
|
—
|
|
—
|
|
|||||
|
Loss on disposal of fixed assets
|
3
|
|
—
|
|
—
|
|
|||||
|
Stock‑based compensation expense
|
2,051
|
|
1,125
|
|
1,224
|
|
|||||
|
Non‑cash interest expense
|
263
|
|
198
|
|
155
|
|
|||||
|
Change in fair value of redeemable convertible preferred stock warrant
|
12
|
|
(83
|
)
|
38
|
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|||||||||
|
Accounts receivable
|
610
|
|
(153
|
)
|
(514
|
)
|
|||||
|
Prepaid expenses and other assets
|
(860
|
)
|
(1,011
|
)
|
(404
|
)
|
|||||
|
Restricted cash and other deposits
|
103
|
|
977
|
|
(1,779
|
)
|
|||||
|
Accounts payable
|
2,686
|
|
667
|
|
(90
|
)
|
|||||
|
Deferred revenue
|
10,409
|
|
(507
|
)
|
(20
|
)
|
|||||
|
Contingently repayable grant funding
|
(461
|
)
|
(805
|
)
|
305
|
|
|||||
|
Accrued expenses and other liabilities
|
716
|
|
1,259
|
|
415
|
|
|||||
|
Net cash used in operating activities
|
(19,683
|
)
|
(22,463
|
)
|
(12,686
|
)
|
|||||
|
Investing activities
|
|
|
|||||||||
|
Maturities of short term government obligations
|
6,900
|
|
—
|
|
—
|
|
|||||
|
Purchase of short term investments
|
(28,416
|
)
|
(3,516
|
)
|
—
|
|
|||||
|
Purchases of property and equipment
|
(586
|
)
|
(1,163
|
)
|
(227
|
)
|
|||||
|
Proceeds from the sale of property and equipment
|
2
|
|
—
|
|
—
|
|
|||||
|
Net cash used in investing activities
|
(22,100
|
)
|
(4,679
|
)
|
(227
|
)
|
|||||
|
Financing activities
|
|
|
|||||||||
|
Net proceeds from issuance of preferred stock and warrants
|
—
|
|
32,669
|
|
20,140
|
|
|||||
|
Principle payments on loan payable
|
—
|
|
(2,336
|
)
|
—
|
|
|||||
|
Deferred IPO costs paid
|
(4,103
|
)
|
(302
|
)
|
—
|
|
|||||
|
Issuance of convertible note
|
—
|
|
7,092
|
|
—
|
|
|||||
|
Proceeds from Initial Public Offering, net of underwriters' discounts and commissions
|
68,870
|
|
—
|
|
—
|
|
|||||
|
Issuance of common stock
|
2,743
|
|
—
|
|
—
|
|
|||||
|
Proceeds from loans payable, net of issuance costs
|
—
|
|
6,674
|
|
4,494
|
|
|||||
|
Exercise of stock options
|
149
|
|
109
|
|
137
|
|
|||||
|
Net cash provided by financing activities
|
67,659
|
|
43,906
|
|
24,771
|
|
|||||
|
Effect of exchange rate changes on cash
|
443
|
|
(1,019
|
)
|
(3,323
|
)
|
|||||
|
Net increase in cash and cash equivalents
|
26,319
|
|
15,745
|
|
8,535
|
|
|||||
|
Cash and cash equivalents at beginning of period
|
32,337
|
|
16,592
|
|
8,057
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
58,656
|
|
$
|
32,337
|
|
$
|
16,592
|
|
||
|
Cash paid during the year for:
|
|
|
|||||||||
|
Interest
|
$
|
972
|
|
$
|
531
|
|
$
|
366
|
|
||
|
Supplemental noncash investing and financing activities:
|
|
|
|||||||||
|
Purchase of property and equipment not yet paid
|
$
|
147
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Venture debt termination fee liability
|
—
|
|
270
|
|
270
|
|
|||||
|
Issuance of preferred warrants in connection with venture loans
|
—
|
|
137
|
|
121
|
|
|||||
|
Reclassification of deferred IPO costs from non-current assets to additional paid-in capital
|
4,404
|
|
—
|
|
—
|
|
|||||
|
Initial public offering costs in accounts payable and accrued liabilities
|
122
|
|
1,246
|
|
—
|
|
|||||
|
Accrued dividends and accretion of preferred stock to redemption value
|
4,566
|
|
7,335
|
|
4,951
|
|
|||||
|
Conversion of bridge loans into Series E preferred
|
—
|
|
7,288
|
|
—
|
|
|||||
|
Unrealized gain on marketable securities
|
$
|
(36
|
)
|
$
|
—
|
|
$
|
—
|
|
||
|
Foreign exchange translation adjustment
|
Unrealized gains (losses) on available-for-sale securities
|
Accumulated other comprehensive income (loss)
|
|||||||||
|
Balance as of December 31, 2015
|
$
|
(4,986
|
)
|
—
|
|
$
|
(4,986
|
)
|
|||
|
Other comprehensive gain (loss) during the period
|
504
|
|
(36
|
)
|
468
|
|
|||||
|
Balance as of December 31, 2016
|
$
|
(4,482
|
)
|
$
|
(36
|
)
|
$
|
(4,518
|
)
|
||
|
December 31, 2016
|
|||||||||||||||
|
Amortized Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Estimated Fair Value
|
||||||||||||
|
U.S. Treasury securities
|
$
|
—
|
|
|
$
|
—
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
Corporate bonds
|
24,821
|
|
|
17
|
|
|
(53
|
)
|
|
24,785
|
|
||||
|
Total available-for-sale marketable securities
|
$
|
24,821
|
|
$
|
17
|
|
$
|
(53
|
)
|
$
|
24,785
|
|
|||
|
December 31, 2015
|
|||||||||||||||
|
Amortized Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Estimated Fair Value
|
||||||||||||
|
U.S. Treasury securities
|
$
|
3,516
|
|
|
$
|
—
|
|
$
|
—
|
|
|
$
|
3,516
|
|
|
|
Corporate bonds
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
||||
|
Total available-for-sale marketable securities
|
$
|
3,516
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,516
|
|
|||
|
December 31, 2016
|
December 31, 2015
|
||||||||||||||
|
Fair Value
|
Amortized Cost
|
Fair Value
|
Amortized Cost
|
||||||||||||
|
Less than one year
|
$
|
24,785
|
|
$
|
24,821
|
|
$
|
3,516
|
|
$
|
3,516
|
|
|||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Numerator:
|
|
|
|
||||||||
|
Net (loss)
|
$
|
(36,210
|
)
|
$
|
(25,174
|
)
|
$
|
(12,880
|
)
|
||
|
Less: accretion on preferred stock
|
(4,566
|
)
|
(7,335
|
)
|
(4,951
|
)
|
|||||
|
Net effect of extinguishment of preferred stock
|
$
|
—
|
|
$
|
—
|
|
$
|
1,459
|
|
||
|
Net loss attributable to common stockholders
|
$
|
(40,776
|
)
|
$
|
(32,509
|
)
|
|
$
|
(16,372
|
)
|
|
|
Denominator:
|
|
|
|
||||||||
|
Weighted‑average common shares outstanding—basic and diluted
|
10,493,939
|
|
2,150,422
|
|
2,090,677
|
|
|||||
|
Net loss per share attributable to common stockholders—basic and diluted
|
$
|
(3.89
|
)
|
$
|
(15.13
|
)
|
$
|
(7.84
|
)
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Redeemable convertible preferred stock
|
—
|
|
9,890,580
|
|
6,471,358
|
|
||
|
Stock options to purchase common stock
|
2,128,346
|
|
1,569,379
|
|
1,143,237
|
|
||
|
Stock warrants to purchase common stock
|
97,302
|
|
650,618
|
|
—
|
|
||
|
Redeemable convertible preferred stock warrants
|
—
|
|
26,832
|
|
17,094
|
|
||
|
Total
|
2,225,648
|
|
|
12,137,409
|
|
7,631,689
|
|
|
|
|
December 31, 2016
|
||||||||||||||
|
|
(level 1)
|
(level 2)
|
(level 3)
|
Total
|
|||||||||||
|
Money market funds, included in cash equivalents
|
$
|
183
|
|
$
|
—
|
|
$
|
—
|
|
$
|
183
|
|
|||
|
Tri-party repurchase agreements, included in cash equivalents
|
$
|
—
|
|
$
|
27,000
|
|
$
|
—
|
|
$
|
27,000
|
|
|||
|
US Treasury obligations, included in investments
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Corporate bonds, included in investments
|
$
|
24,785
|
|
$
|
—
|
|
$
|
—
|
|
$
|
24,785
|
|
|||
|
Warrants to purchase redeemable convertible preferred stock, included in other long term liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
|
December 31, 2015
|
||||||||||||||
|
|
(level 1)
|
(level 2)
|
(level 3)
|
Total
|
|||||||||||
|
US Treasury obligations, included in cash equivalents
|
$
|
14,486
|
|
$
|
—
|
|
$
|
—
|
|
$
|
14,486
|
|
|||
|
US Treasury obligations, included in investments
|
$
|
3,516
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,516
|
|
|||
|
Corporate bonds, included in investments
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Warrants to purchase redeemable convertible preferred stock, included in other long term liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
290
|
|
$
|
290
|
|
|||
|
|
December 31, 2014
|
||||||||||
|
|
(level 1)
|
(level 2)
|
(level 3)
|
Total
|
|||||||
|
Warrants to purchase redeemable convertible preferred stock, included in other long term liabilities
|
—
|
|
—
|
|
236
|
|
236
|
|
|||
|
Warrant Liability
|
|||
|
Fair value as of December 31, 2014
|
$
|
236
|
|
|
Fair value of warrants issued
|
137
|
|
|
|
Change in fair value
|
(83
|
)
|
|
|
Fair value as of December 31, 2015
|
290
|
|
|
|
Fair value of warrants issued
|
—
|
|
|
|
Change in fair value
|
12
|
|
|
|
Reclassification to additional paid in capital at IPO
|
(302
|
)
|
|
|
Fair value as of December 31, 2016
|
$
|
—
|
|
|
|
December 31,
|
December 31,
|
|||||
|
|
2016
|
2015
|
|||||
|
Laboratory equipment
|
$
|
4,713
|
|
$
|
4,028
|
|
|
|
Computer equipment and software
|
562
|
|
409
|
|
|||
|
Leasehold improvements
|
222
|
|
91
|
|
|||
|
Furniture and fixtures
|
226
|
|
222
|
|
|||
|
Office equipment
|
64
|
|
62
|
|
|||
|
P,P&E—Construction in process
|
—
|
|
144
|
|
|||
|
Total property and equipment
|
5,787
|
|
|
4,956
|
|
||
|
Less accumulated depreciation
|
(3,740
|
)
|
(2,927
|
)
|
|||
|
Property and equipment, net
|
$
|
2,047
|
|
|
$
|
2,029
|
|
|
|
December 31,
|
December 31,
|
|||||
|
|
2016
|
2015
|
|||||
|
Payroll and employee related expenses
|
$
|
1,551
|
|
$
|
669
|
|
|
|
Legal
|
196
|
|
213
|
|
|||
|
Current portion of deferred rent and lease incentive
|
29
|
|
405
|
|
|||
|
Accrued patent fees
|
415
|
|
219
|
|
|||
|
Accrued external research and development costs
|
794
|
|
1,649
|
|
|||
|
Accrued audit fees
|
224
|
|
135
|
|
|||
|
Accrued grant refund
|
152
|
|
—
|
|
|||
|
Accrued interest
|
81
|
|
34
|
|
|||
|
Other
|
479
|
|
54
|
|
|||
|
Accrued expenses
|
$
|
3,921
|
|
|
$
|
3,378
|
|
|
Year ended December 31,
|
|||
|
2017
|
$
|
1,244
|
|
|
2018
|
1,291
|
|
|
|
2019
|
1,330
|
|
|
|
2020
|
335
|
|
|
|
Total minimum lease payments
|
$
|
4,200
|
|
|
Year ended December 31,
|
|
||
|
2017
|
$
|
5,319
|
|
|
2018
|
5,318
|
|
|
|
2019
|
3,379
|
|
|
|
Total minimum debt payments
|
14,016
|
|
|
|
Less: Amount representing interest
|
(1,296
|
)
|
|
|
Less: Debt discount and deferred charges
|
(621
|
)
|
|
|
Less: Current portion of loans payable
|
(4,122
|
)
|
|
|
Loans payable, net of current portion
|
$
|
7,977
|
|
|
|
Periods ending
|
||||
|
|
December 31, 2016
|
December 31, 2015
|
|||
|
Conversion of Series A Preferred
|
—
|
|
664,068
|
|
|
|
Conversion of Series B Preferred
|
—
|
|
1,907,006
|
|
|
|
Conversion of Series C Preferred
|
—
|
|
1,282,051
|
|
|
|
Conversion of Series D Preferred
|
—
|
|
2,191,412
|
|
|
|
Conversion of Series SRN Preferred
|
—
|
|
1,798,433
|
|
|
|
Conversion of Series E Preferred
|
—
|
|
2,662,885
|
|
|
|
Exercise of common warrants
|
97,302
|
|
651,618
|
|
|
|
Shares available for future stock incentive awards
|
939,317
|
|
100,034
|
|
|
|
Exercise of outstanding common stock options
|
2,128,346
|
|
1,569,379
|
|
|
|
Total
|
3,164,965
|
|
|
12,826,886
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Risk-free interest rate
|
1.42
|
%
|
1.79
|
%
|
1.93
|
%
|
|||||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
|||||
|
Expected life
|
6.04
|
|
6.02
|
|
5.94
|
|
|||||
|
Expected volatility
|
94.97
|
%
|
79.80
|
%
|
100.81
|
%
|
|||||
|
Weighted-average fair value of common stock
|
$
|
13.25
|
|
$
|
7.35
|
|
$
|
8.97
|
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Risk‑free interest rate
|
2.06
|
%
|
1.57
|
%
|
1.22
|
%
|
||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
||
|
Expected life (in years)
|
8.80
|
|
6.46
|
|
5.87
|
|
||
|
Expected volatility
|
87.33
|
%
|
98.18
|
%
|
98.25
|
%
|
||
|
|
|
|
Weighted‑average
|
|
||||||||
|
|
|
Weighted‑average
|
remaining
|
Aggregate
|
||||||||
|
|
Number of
|
exercise
|
contractual term
|
intrinsic value
|
||||||||
|
|
options
|
price
|
(in years)
|
(in thousands)
|
||||||||
|
Employee
|
|
|
|
|
||||||||
|
Outstanding at December 31, 2014
|
800,165
|
|
$
|
3.24
|
|
6.99
|
$
|
4,895
|
|
|||
|
Granted
|
485,833
|
|
$
|
7.91
|
|
|||||||
|
Exercised
|
(20,861
|
)
|
$
|
1.21
|
|
|||||||
|
Forfeited
|
(17,977
|
)
|
$
|
6.32
|
|
|||||||
|
Outstanding at December 31, 2015
|
1,247,160
|
|
$
|
5.05
|
|
7.41
|
$
|
3,130
|
|
|||
|
Vested at December 31, 2015
|
668,465
|
|
$
|
2.61
|
|
5.71
|
$
|
2,946
|
|
|||
|
Vested and expected to vest at December 31, 2015
|
1,171,380
|
|
$
|
4.84
|
|
7.26
|
$
|
3,138
|
|
|||
|
Outstanding at December 31, 2015
|
1,247,160
|
|
$
|
5.05
|
|
7.41
|
$
|
3,130
|
|
|||
|
Granted
|
543,595
|
|
$
|
13.25
|
|
|
|
|||||
|
Exercised
|
(57,850
|
)
|
$
|
2.00
|
|
|
|
|||||
|
Forfeited
|
(12,164
|
)
|
$
|
7.41
|
|
|
|
|||||
|
Outstanding at December 31, 2016
|
1,720,741
|
|
$
|
7.73
|
|
7.43
|
$
|
16,425
|
|
|||
|
Vested at December 31, 2016
|
830,833
|
|
$
|
4.05
|
|
5.65
|
$
|
10,885
|
|
|||
|
Vested and expected to vest at December 31, 2016
|
1,601,908
|
|
$
|
7.44
|
|
7.30
|
$
|
15,731
|
|
|||
|
Non‑Employee
|
|
|
|
|
||||||||
|
Outstanding at December 31, 2014
|
343,072
|
|
$
|
2.93
|
|
6.50
|
$
|
2,207
|
|
|||
|
Granted
|
—
|
|
$
|
—
|
|
|||||||
|
Exercised
|
(20,852
|
)
|
$
|
2.91
|
|
|||||||
|
Forfeited
|
—
|
|
$
|
—
|
|
|||||||
|
Outstanding at December 31, 2015
|
322,220
|
|
$
|
2.93
|
|
5.50
|
$
|
1,288
|
|
|||
|
Vested at December 31, 2015
|
310,680
|
|
$
|
2.70
|
|
5.40
|
$
|
1,288
|
|
|||
|
Vested and expected to vest at December 31, 2015
|
322,219
|
|
$
|
2.93
|
|
5.50
|
$
|
1,288
|
|
|||
|
Outstanding at December 31, 2015
|
322,220
|
|
$
|
2.93
|
|
5.50
|
$
|
1,288
|
|
|||
|
Granted
|
102,435
|
|
$
|
8.43
|
|
|
|
|||||
|
Exercised
|
(17,049
|
)
|
$
|
0.47
|
|
|
|
|||||
|
Forfeited
|
(1
|
)
|
$
|
3.44
|
|
|
|
|||||
|
Outstanding at December 31, 2016
|
407,605
|
|
$
|
4.42
|
|
5.79
|
$
|
5,224
|
|
|||
|
Vested at December 31, 2016
|
327,879
|
|
$
|
3.44
|
|
4.98
|
$
|
4,508
|
|
|||
|
Vested and expected to vest at December 31, 2016
|
406,200
|
|
$
|
4.40
|
|
5.77
|
$
|
5,215
|
|
|||
|
Year Ended December 31,
|
|||||||||||||||||
|
2016
|
2015
|
2014
|
|||||||||||||||
|
|
|
Weighted‑
|
|
Weighted‑
|
|
Weighted‑
|
|||||||||||
|
|
|
average
|
|
average
|
|
average
|
|||||||||||
|
|
|
exercise
|
|
exercise
|
|
exercise
|
|||||||||||
|
|
Shares
|
price
|
Shares
|
price
|
Shares
|
price
|
|||||||||||
|
Unvested at beginning of period
|
7,574
|
|
$
|
2.77
|
|
15,264
|
|
$
|
2.77
|
|
70,804
|
|
$
|
1.56
|
|
||
|
Issued
|
962
|
|
9.36
|
|
—
|
|
—
|
|
2,564
|
|
0.62
|
|
|||||
|
Vested
|
(8,536
|
)
|
3.51
|
|
(7,690
|
)
|
2.72
|
|
(58,104
|
)
|
1.44
|
|
|||||
|
Unvested at end of period
|
—
|
|
$
|
—
|
|
7,574
|
|
$
|
2.77
|
|
15,264
|
|
$
|
2.77
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
|
2015
|
|
2014
|
||||||
|
Research and development
|
$
|
1,183
|
|
$
|
495
|
|
$
|
384
|
|
||
|
General and administrative
|
847
|
|
630
|
|
840
|
|
|||||
|
Total
|
$
|
2,030
|
|
$
|
1,125
|
|
$
|
1,224
|
|
||
|
•
|
First Acquisition Right. During the period beginning on May 1, 2017 and ending on June 1, 2017, Spark will have the right (the “First Acquisition Right”) to purchase a number of shares of Common Stock equal to an aggregate price of
$5.0 million
.
|
|
•
|
Second Acquisition Right. During the period beginning on October 1, 2017 and ending on November 1, 2017, Spark will have the right (the “Second Acquisition Right”) to purchase a number of shares of Common Stock equal to an aggregate price of
$5.0 million
.
|
|
Year Ended December 31,
|
||||||||
|
2016
|
2015
|
2014
|
||||||
|
Statutory U.S. federal rate
|
34.0
|
%
|
34
|
%
|
34
|
%
|
||
|
State income taxes - net of federal benefit
|
5.8
|
%
|
5.7
|
%
|
5.9
|
%
|
||
|
Permanent items
|
(1.1
|
)%
|
(0.7
|
)%
|
(0.7
|
)%
|
||
|
Research tax credits/other
|
1.3
|
%
|
1.0
|
%
|
1.6
|
%
|
||
|
Valuation allowance, net
|
(39.8
|
)%
|
(40
|
)%
|
(33
|
)%
|
||
|
Other
|
(0.2
|
)%
|
—
|
%
|
(7.8
|
)%
|
||
|
Net deferred tax assets
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Year Ended December 31,
|
|||||||
|
2016
|
2015
|
||||||
|
Deferred Tax Assets
|
|||||||
|
Net operating loss carryforwards
|
$
|
39,420
|
|
$
|
31,958
|
|
|
|
Research and development credits
|
3,052
|
|
2,305
|
|
|||
|
Stock-based compensations expense
|
904
|
|
570
|
|
|||
|
Deferred rent and other expenses
|
713
|
|
493
|
|
|||
|
Deferred revenue
|
5,607
|
|
1,582
|
|
|||
|
Patent costs/amortization
|
4,934
|
|
3,500
|
|
|||
|
Tenant improvement allowance
|
—
|
|
—
|
|
|||
|
Warrant liability
|
—
|
|
114
|
|
|||
|
Gross deferred tax assets
|
54,630
|
|
40,522
|
|
|||
|
Deferred Tax Liabilities
|
|||||||
|
Depreciation
|
$
|
(131
|
)
|
$
|
(197
|
)
|
|
|
Debt discount
|
(69
|
)
|
(97
|
)
|
|||
|
Unrealized foreign exchange gain
|
(1,525
|
)
|
(1,728
|
)
|
|||
|
Gross deferred tax liabilities
|
(1,725
|
)
|
(2,022
|
)
|
|||
|
Net deferred tax assets
|
52,905
|
|
38,500
|
|
|||
|
Valuation allowance
|
(52,905
|
)
|
(38,500
|
)
|
|||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
|
|
Three Months Ended (unaudited)
|
|||||||||||||||
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
||||||||||||
|
2016
|
2016
|
2016
|
2016
|
||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Revenue
|
$
|
2,088
|
|
$
|
2,017
|
|
$
|
1,048
|
|
$
|
2,930
|
|
|||
|
Operating expenses
|
9,029
|
|
8,418
|
|
8,516
|
|
16,790
|
|
|||||||
|
Net (loss) income
|
$
|
(7,476
|
)
|
$
|
(6,923
|
)
|
$
|
(7,728
|
)
|
$
|
(14,083
|
)
|
|||
|
Accretion on preferred stock
|
(2,356
|
)
|
(2,210
|
)
|
—
|
|
—
|
|
|||||||
|
Net effect of extinguishment of preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Net (loss) income attributable to common stockholders - basic & diluted
|
$
|
(9,832
|
)
|
$
|
(9,133
|
)
|
$
|
(7,728
|
)
|
$
|
(14,083
|
)
|
|||
|
Net (loss) per share attributable to common stockholders:
|
|||||||||||||||
|
Basic & diluted
|
$
|
(4.52
|
)
|
$
|
(2.75
|
)
|
|
$
|
(0.43
|
)
|
$
|
(0.77
|
)
|
||
|
Weighted-average common shares outstanding used in net (loss) income per share attributable to common stockholders:
|
|||||||||||||||
|
Basic & diluted
|
2,175,037
|
|
3,322,546
|
|
|
18,108,014
|
|
18,265,771
|
|
||||||
|
Three Months Ended (unaudited)
|
|||||||||||||||
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
||||||||||||
|
2015
|
2015
|
2015
|
2015
|
||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Revenue
|
$
|
1,034
|
|
$
|
1,236
|
|
$
|
1,607
|
|
$
|
2,134
|
|
|||
|
Operating expenses
|
6,844
|
|
7,552
|
|
7,678
|
|
9,241
|
|
|||||||
|
Net (loss) income
|
$
|
(5,733
|
)
|
$
|
(6,867
|
)
|
$
|
(5,725
|
)
|
$
|
(6,849
|
)
|
|||
|
Accretion on preferred stock
|
(1,561
|
)
|
(1,562
|
)
|
(1,836
|
)
|
(2,376
|
)
|
|||||||
|
Net effect of extinguishment of preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Net (loss) income attributable to common stockholders - basic & diluted
|
$
|
(7,294
|
)
|
$
|
(8,429
|
)
|
$
|
(7,561
|
)
|
$
|
(9,225
|
)
|
|||
|
Net (loss) per share attributable to common stockholders:
|
|||||||||||||||
|
Basic & diluted
|
$
|
(3.43
|
)
|
$
|
(3.93
|
)
|
$
|
(3.50
|
)
|
$
|
(4.26
|
)
|
|||
|
Weighted-average common shares outstanding used in net (loss) income per share attributable to common stockholders:
|
|||||||||||||||
|
Basic & diluted
|
2,126,878
|
|
2,147,184
|
|
2,159,658
|
|
2,167,769
|
|
|||||||
|
|
SELECTA BIOSCIENCES, INC.
|
||
|
|
|
||
|
Date: March 27, 2017
|
By:
|
/s/ Werner Cautreels, Ph.D.
|
|
|
|
Werner Cautreels, Ph.D.
|
||
|
|
President and Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
|
/s/ Werner Cautreels, Ph.D.
|
President and Chief Executive Officer, and Director
|
March 27, 2017
|
|
Werner Cautreels, Ph.D.
|
(Principal Executive Officer)
|
|
|
/s/ David Siewers
|
Chief Financial Officer
|
March 27, 2017
|
|
David Siewers
|
(Principal Financial and Accounting Officer)
|
|
|
/s/ Timothy C. Barabe
|
Director
|
March 27, 2017
|
|
Timothy C. Barabe
|
||
|
/s/ Omid Farokhzad, M.D.
|
Director
|
March 27, 2017
|
|
Omid Farokhzad, M.D.
|
||
|
/s/ Carl Gordon, Ph.D.
|
Director
|
March 27, 2017
|
|
Carl Gordon, Ph.D.
|
||
|
/s/ Peter Barton Hutt
|
Director
|
March 27, 2017
|
|
Peter Barton Hutt
|
||
|
/s/ Edwin M. Kania, Jr.
|
Director
|
March 27, 2017
|
|
Edwin M. Kania, Jr.
|
||
|
/s/ Amir Nashat, Ph.D
|
Director
|
March 27, 2017
|
|
Amir Nashat, Ph.D
|
||
|
/s/ Aymeric Sallin
|
Director
|
March 27, 2017
|
|
Aymeric Sallin
|
||
|
/s/ Timothy Springer, Ph.D.
|
Director
|
March 27, 2017
|
|
Timothy Springer, Ph.D.
|
||
|
|
|
Incorporated by Reference
|
||||||||||
|
Exhibit
Number
|
Exhibit Description
|
Form
|
File No.
|
Exhibit
|
Filing
Date
|
Filed
Herewith
|
||||||
|
3.1
|
Restated Certificate of Incorporation of Selecta Biosciences, Inc.
|
8-K
|
001-37798
|
3.1
|
6/29/2016
|
|
||||||
|
3.2
|
Amended and Restated By-laws of Selecta Biosciences, Inc.
|
8-K
|
001-37798
|
3.2
|
6/29/2016
|
|
||||||
|
4.1
|
Fifth Amended and Restated Investors' Rights Agreement, dated as of August 26, 2015, by and between the Registrant and each of the stockholders party thereto, as amended by Amendment No. 1 to Fifth Amended and Restated Investors Rights Agreement, dated as of June 7, 2016
|
S-1/A
|
333-211555
|
4.1
|
6/8/2016
|
|||||||
|
4.2
|
Specimen Stock Certificate evidencing the shares of common stock
|
S-1
|
333-211555
|
4.2
|
5/24/2016
|
|||||||
|
4.3
|
Form of Warrant to Purchase Common Stock, dated July 24, 2015, issued by the Registrant to Investors in the Registrant's April 2015 Convertible Notes Financing, together with a schedule of warrant holders
|
S-1
|
333-211555
|
4.3
|
5/24/2016
|
|||||||
|
4.4
|
Form of Warrant to Purchase Shares of Series D Preferred Stock, dated August 9, 2013 or July 25, 2014, issued by the Registrant to Oxford Finance LLC and Square One Bank, together with a schedule of warrant holders
|
S-1
|
333-211555
|
4.5
|
5/24/2016
|
|||||||
|
4.5
|
Form of Warrant to Purchase Shares of Series E Preferred Stock, dated December 31, 2015, issued by the Registrant to Oxford Finance LLC and Square One Bank, together with a schedule of warrant holders
|
S-1
|
333-211555
|
4.6
|
5/24/2016
|
|||||||
|
10.1#
|
2016 Incentive Award Plan and form of award agreements thereunder
|
S-1/A
|
333-211555
|
10.2
|
6/8/2016
|
|||||||
|
10.2#
|
2016 Employee Stock Purchase Plan
|
S-1/A
|
333-211555
|
10.3
|
6/8/2016
|
|||||||
|
10.3#
|
Non-Employee Director Compensation Program
|
S-1/A
|
333-211555
|
10.4
|
6/8/2016
|
|||||||
|
10.4#
|
Form of Indemnification Agreement for Directors and Officers
|
S-1
|
333-211555
|
10.5
|
5/24/2016
|
|||||||
|
10.5
|
Amended and Restated Loan and Security Agreement, dated as of December 31, 2015, by and between the Registrant, Oxford Finance LLC and Pacific Western Bank
|
S-1
|
333-211555
|
10.6
|
5/24/2016
|
|||||||
|
10.6(a)†
|
Exclusive Patent License Agreement, dated as of November 25, 2008, by and between the Registrant and the Massachusetts Institute of Technology
|
S-1
|
333-211555
|
10.7(a)
|
5/24/2016
|
|||||||
|
10.6(b)†
|
First Amendment to Exclusive Patent License Agreement, dated as of January 12, 2010, by and between the Registrant and the Massachusetts Institute of Technology
|
S-1
|
333-211555
|
10.7(b)
|
5/24/2016
|
|||||||
|
10.6(c)†
|
Letter Agreement, dated as of November 27, 2012, by and among the Registrant, Massachusetts Institute of Technology and Sanofi
|
S-1
|
333-211555
|
10.7(c)
|
5/24/2016
|
|||||||
|
10.6(d)†
|
Letter Amendment, dated as of November 27, 2012, by and between the Registrant and the Massachusetts Institute of Technology
|
S-1
|
333-211555
|
10.7(d)
|
5/24/2016
|
|||||||
|
10.6(e)†
|
Second Amendment to Exclusive Patent License Agreement, dated as of August 29, 2013, by and between the Registrant and the Massachusetts Institute of Technology
|
S-1
|
333-211555
|
10.7(e)
|
5/24/2016
|
|||||||
|
10.7(a)†
|
License and Research Collaboration Agreement, dated as of November 27, 2012, by and between the Registrant and Sanofi
|
S-1
|
333-211555
|
10.8(a)
|
5/24/2016
|
|||||||
|
10.7(b)†
|
Supplemental Agreement No. 1 to License and Research Collaboration Agreement, dated as of May 7, 2015, by and between the Registrant and Sanofi
|
S-1
|
333-211555
|
10.8(b)
|
5/24/2016
|
|||||||
|
10.8†
|
License Agreement, dated as of May 12, 2014, by and between the Registrant and Shenyang Sunshine Pharmaceutical Co., Ltd.
|
S-1
|
333-211555
|
10.9
|
5/24/2016
|
|||||||
|
10.9†
|
Manufacturing Services Agreement, dated as of August 1, 2014, by and between the Registrant and Shenyang Sunshine Pharmaceutical Co., Ltd.
|
S-1
|
333-211555
|
10.10
|
5/24/2016
|
|||||||
|
10.10†
|
Patent Cross-License Agreement, dated as of December 18, 2008, by and between the Registrant and BIND Therapeutics, Inc. (formerly BIND Biosciences, Inc.)
|
S-1
|
333-211555
|
10.11
|
5/24/2016
|
|||||||
|
10.11†
|
Exclusive License Agreement, dated as of May 17, 2016, by and among the Registrant, the Massachusetts Eye and Ear Infirmary and The Schepens Eye Research Institute, Inc.
|
S-1
|
333-211555
|
10.12
|
5/24/2016
|
|||||||
|
10.12†
|
Lease, dated as of September 30, 2008, as amended by the First Amendment, dated as of July 12, 2011, the Second Amendment, dated as of October 11, 2011 and the Third Amendment, dated as of April 6, 2015, by and between the Registrant and ARE-480 Arsenal Street, LLC
|
S-1
|
333-211555
|
10.13
|
5/24/2016
|
|||||||
|
10.13
|
Fourth Amendment to Lease, dated August 21, 2016, by and between ARE-480 Arsenal Street LLC and Selecta Biosciences, Inc.
|
8-K
|
001-37798
|
10.1
|
9/14/2016
|
|||||||
|
10.14#
|
Consulting Agreement, dated as of March 10, 2008, as amended by the First Amendment to Consulting Agreement, dated as of January 1, 2012, by and between the Registrant and Omid Farokhzad
|
S-1
|
333-211555
|
10.15
|
5/24/2016
|
|||||||
|
10.15#
|
Summary of Oral Agreement, by and between the Registrant and Omid Farokhzad
|
S-1/A
|
333-211555
|
10.16
|
6/8/2016
|
|||||||
|
10.16#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and Werner Cautreels
|
S-1/A
|
333-211555
|
10.17
|
6/8/2016
|
|||||||
|
10.17#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and Takashi Kei Kishimoto
|
S-1/A
|
333-211555
|
10.18
|
6/8/2016
|
|||||||
|
10.18#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and Peter Keller
|
S-1/A
|
333-211555
|
10.19
|
6/8/2016
|
|||||||
|
10.19#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and Earl E. Sands
|
S-1/A
|
333-211555
|
10.20
|
6/8/2016
|
|||||||
|
10.20#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and Lloyd P. M. Johnston, Ph.D.
|
S-1/A
|
333-211555
|
10.21
|
6/8/2016
|
|||||||
|
10.21#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and David Abraham
|
S-1/A
|
333-211555
|
10.22
|
6/8/2016
|
|||||||
|
10.22#
|
Employment Agreement, dated as of June 6, 2016, by and between the Registrant and David Siewers
|
S-1/A
|
333-211555
|
10.23
|
6/8/2016
|
|||||||
|
10.23†
|
License and Option Agreement, dated as of December 2, 2016, by and between Spark Therapeutics, Inc. and the Registrant
|
8-K/A
|
001-37798
|
10.1
|
2/14/2017
|
|||||||
|
10.24†
|
Stock Purchase Agreement, dated as of December 2, 2016, by and between Spark Therapeutics, Inc. and the Registrant
|
8-K/A
|
001-37798
|
10.2
|
12/14/2016
|
|||||||
|
10.25(a)†
|
Third Amendment to Exclusive Patent License Agreement, entered into on November 21, 2016 and effective as of November 18, 2016, by and between the Massachusetts Institute of Technology and the Registrant
|
8-K/A
|
001-37798
|
10.3(a)
|
12/14/2016
|
|||||||
|
10.25(b)†
|
Letter Agreement, dated as of December 2, 2016, by and between the Massachusetts Institute of Technology and the Registrant
|
8-K/A
|
001-37798
|
10.3(b)
|
12/14/2016
|
|||||||
|
10.25(c)†
|
Letter Agreement, dated as of December 2, 2016, by and among Spark Therapeutics, Inc., the Massachusetts Institute of Technology and the Registrant
|
8-K/A
|
001-37798
|
10.3(c)
|
12/14/2016
|
|||||||
|
21.1
|
Subsidiaries of Selecta Biosciences, Inc.
|
S-1
|
333-211555
|
21.1
|
5/24/2016
|
|||||||
|
23.1
|
Consent of
Ernst & Young LLP, Independent Registered Public Accounting Firm
|
**
|
||||||||||
|
31.1
|
Rule 13a-14(a) / 15d-14(a) Certification of Chief Executive Officer
|
|
|
|
|
**
|
||||||
|
31.2
|
Rule 13a-14(a) / 15d-14(a) Certification of Chief Financial Officer
|
|
|
|
|
**
|
||||||
|
32.1
|
Section 1350 Certification of Chief Executive Officer
|
|
|
|
|
**
|
||||||
|
32.2
|
Section 1350 Certification of Chief Financial Officer
|
|
|
|
|
**
|
||||||
|
101.INS
|
XBRL Instance Document
|
|
|
|
|
*
|
||||||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
|
|
*
|
||||||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
|
|
*
|
||||||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
|
|
*
|
||||||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
|
|
*
|
||||||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
|
|
*
|
||||||