|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Colorado
|
|
20-5566275
|
|
(State
or other jurisdiction of Incorporation or
organization)
|
|
(I.R.S.
Employer Identification No.)
|
|
Title
of each class
|
|
Name of
each exchange on which registered
|
|
Common Stock, par value $0.001 per share
|
|
OTCQB
|
|
Large
accelerated filer
|
☐
|
Accelerated
filer
|
☐
|
|
Non-accelerated
filer
|
☐
|
Smaller
reporting company
|
☑
|
|
(Do not
check if a smaller reporting company)
|
|
|
|
|
Facing
Page
|
Page
No.
|
|
|
Index
|
|
|
|
|
|
|
|
PART
I
|
|
|
|
|
|
|
|
Item
1.
|
Business
|
3
|
|
|
|
|
|
Item
1A.
|
Risk
Factors
|
9
|
|
|
|
|
|
Item
1B.
|
Unresolved
Staff Comments
|
10
|
|
|
|
|
|
Item
2
|
Properties
|
10
|
|
|
|
|
|
Item
3.
|
Legal
Proceedings
|
10
|
|
|
|
|
|
Item
4.
|
Mine
Safety Disclosures
|
10
|
|
|
|
|
|
PART
II
|
|
|
|
|
|
|
|
Item
5.
|
Market
for the Registrant’s Common Equity and Related Stockholder
Matters and Issuer Purchases of Equity Securities
|
10
|
|
|
|
|
|
Item
6.
|
Selected
Financial Data
|
12
|
|
|
|
|
|
Item
7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
12
|
|
|
|
|
|
Item
7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
18
|
|
|
|
|
|
Item
8.
|
Financial
Statements and Supplementary Data
|
18
|
|
|
|
|
|
Item
9.
|
Changes
in and Disagreements on Accounting and Financial
Disclosure
|
21
|
|
|
|
|
|
Item
9A.
|
Controls
and Procedures
|
21
|
|
|
|
|
|
Item
9B.
|
Other
Information
|
22
|
|
|
|
|
|
PART
III
|
|
|
|
|
|
|
|
Item
10.
|
Directors,
Executive Officers and Corporate Governance
|
22
|
|
|
|
|
|
Item
11.
|
Executive
Compensation
|
24
|
|
|
|
|
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
25
|
|
|
|
|
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
26
|
|
|
|
|
|
Item
14.
|
Principal
Accounting Fees and Services
|
27
|
|
|
|
|
|
PART
IV
|
|
|
|
|
|
|
|
Item
15.
|
Exhibits,
Financial Statement Schedules
|
29
|
|
|
|
|
|
|
Signatures
|
30
|

|
Quarter
Ended
|
High
|
Low
|
|
|
|
|
|
March 31,
2015
|
$
0.0441
|
$
0.0121
|
|
June 30,
2015
|
$
0.0400
|
$
0.0120
|
|
September 31,
2015
|
$
0.0181
|
$
0.0061
|
|
December 31,
2015
|
$
0.0141
|
$
0.0023
|
|
March 31,
2016
|
$
0.0088
|
$
0.0052
|
|
June 30,
2016
|
$
0.0110
|
$
0.0061
|
|
September 30,
2016
|
$
0.0039
|
$
0.0030
|
|
December 31,
2016
|
$
0.0040
|
$
0.0032
|
|
|
●
|
contains
a description of the nature and level of risk in the market for
penny stocks in both public offerings and secondary
trading;
|
|
|
|
|
|
|
●
|
contains
a description of the broker's or dealer's duties to the customer
and of the rights and remedies available to the customer with
respect to a violation to such duties or other requirements of the
Securities Act of 1934, as amended;
|
|
|
|
|
|
|
●
|
contains
a brief, clear, narrative description of a dealer market, including
"bid" and "ask" prices for penny stocks and the significance of the
spread between the bid and ask price;
|
|
|
|
|
|
|
●
|
contains
a toll-free telephone number for inquiries on disciplinary
actions;
|
|
|
|
|
|
|
●
|
defines
significant terms in the disclosure document or in the conduct of
trading penny stocks; and
|
|
|
|
|
|
|
●
|
contains
such other information and is in such form (including language,
type, size and format) as the Securities and Exchange Commission
shall require by rule or regulation;
|
|
|
●
|
the bid
and offer quotations for the penny stock;
|
|
|
|
|
|
|
●
|
the
compensation of the broker-dealer and its salesperson in the
transaction;
|
|
|
|
|
|
|
●
|
the
number of shares to which such bid and ask prices apply, or other
comparable information relating to the depth and liquidity of the
market for such stock; and
|
|
|
|
|
|
|
●
|
monthly
account statements showing the market value of each penny stock
held in the customer's account.
|
|
|
Page
|
|
|
|
|
Independent
Accountant’s Audit Report
|
F-1
|
|
|
|
|
Consolidated
Balance Sheet
|
F-2
|
|
|
|
|
Consolidated
Statement of Operations
|
F-3
|
|
|
|
|
Consolidated
Statement of Cash Flows
|
F-4
|
|
|
|
|
Consolidated
Statement of Shareholders’ Equity
|
F-5
|
|
|
|
|
Notes
to Consolidated Financial Statements
|
F-6
|
|
Sunshine
Biopharma, Inc.
|
|
|
|
|
|
|
|
|
|
|
|
|
December
31,
|
December
31,
|
|
|
2016
|
2015
|
|
ASSETS
|
|
|
|
|
|
|
|
Current
Assets:
|
|
|
|
|
|
|
|
Cash and cash
equivalents
|
$
57,453
|
$
50,798
|
|
Refundable taxes
and Prepaid expenses
|
1,007
|
3,111
|
|
|
|
|
|
Total Current
Assets
|
58,460
|
53,909
|
|
|
|
|
|
Equipment (net of
$2,228 and $479 depreciation)
|
5,944
|
4,314
|
|
Patents (net of
$58,918 amortization and $556,120 impairment, and $3,772
amortization)
|
-
|
615,038
|
|
|
|
|
|
TOTAL
ASSETS
|
$
64,404
|
$
673,261
|
|
|
|
|
|
LIABILITIES AND
SHAREHOLDERS' EQUITY
|
|
|
|
|
|
|
|
Current
Liabilities:
|
|
|
|
|
|
|
|
Current portion of
notes payable
|
69,939
|
102,142
|
|
Current portion of
notes payable - related entity
|
167,032
|
1,038,430
|
|
Accounts
payable
|
28,122
|
46,591
|
|
Accounts payable -
related entity
|
-
|
80,487
|
|
Interest
payable
|
9,011
|
2,656
|
|
Total Current
Liabilities
|
274,104
|
1,270,306
|
|
Long-term
liabilities
|
-
|
-
|
|
|
|
|
|
TOTAL
LIABILITIES
|
274,104
|
1,270,306
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS'
EQUITY
|
|
|
|
|
|
|
|
Preferred Stock,
Series A, $0.10 par value per share; Authorized 850,000 Shares;
Issued and outstanding -0- shares at December 31, 2016 and
2015.
|
-
|
-
|
|
|
|
|
|
Preferred Stock,
Series B $0.10 par value per share; Authorized 500,000 Shares;
Issued and outstanding 500,000 and 500,000 shares at December 31,
2016 and 2015, respectively.
|
50,000
|
50,000
|
|
|
|
|
|
Common Stock,
$0.001 par value per share; Authorized 3,000,000,000 Shares; Issued
and outstanding 769,399,858 and 198,265,118 at December 31, 2016
and 2015, respectively
|
769,400
|
198,265
|
|
|
|
|
|
Capital paid in
excess of par value
|
11,548,460
|
8,235,217
|
|
|
|
|
|
Accumulated other
comprehensive (Loss)
|
394
|
740
|
|
|
|
|
|
Accumulated
(Deficit)
|
(12,577,954
)
|
(9,081,267
)
|
|
|
|
|
|
TOTAL SHAREHOLDERS'
EQUITY
|
(209,700
)
|
(597,045
)
|
|
|
|
|
|
TOTAL LIABILITIES
AND SHAREHOLDERS' EQUITY
|
$
64,404
|
$
673,261
|
|
|
December
31,
2016
|
December
31,
2015
|
|
|
|
|
|
Revenue:
|
$
-
|
1,708
|
|
|
|
|
|
General &
Administrative Expenses
|
|
|
|
|
|
|
|
Research and
Development
|
32,793
|
8,657
|
|
Accounting
|
70,413
|
70,972
|
|
Amortization
|
58,918
|
3,772
|
|
Consulting
|
207,401
|
87,290
|
|
Consulting –
officer
|
247,397
|
50,000
|
|
Depreciation
|
1,813
|
479
|
|
Director
fees
|
252,000
|
20,000
|
|
Legal
|
57,955
|
130,325
|
|
Licenses
|
19,203
|
384,581
|
|
Office
|
34,812
|
11,431
|
|
Stock Transfer
Fee
|
10,403
|
14,478
|
|
|
|
|
|
Total G &
A
|
993,108
|
781,985
|
|
|
|
|
|
(Loss) from
operations
|
(993,108
)
|
(780,277
)
|
|
|
|
|
|
Other
(expense):
|
|
|
|
Interest
expense
|
(34,732
)
|
(307,211
)
|
|
Loss on conversion
of notes payable
|
(1,945,898
)
|
(575,144
)
|
|
Loss on impairment
of patents
|
(556,120
)
|
-
|
|
Litigation
settlement proceeds
|
25,000
|
-
|
|
Gain from foreign
exchange transactions
|
-
|
204
|
|
Gain on interest
forgiveness
|
381
|
-
|
|
Debt
release
|
7,790
|
-
|
|
Gain on notes
payable interest write off
|
-
|
9,520
|
|
|
|
|
|
Total Other
(Expense)
|
(2,503,579
)
|
(872,631
)
|
|
|
|
|
|
Net
(loss)
|
$
(3,496,687
)
|
$
(1,652,908
)
|
|
|
|
|
|
Basic (Loss) per
common share
|
$
(0.01
)
|
$
(0.01
)
|
|
Weighted Average
Common Shares Outstanding
|
424,874,458
|
122,278,909
|
|
Net Income
(Loss)
|
$
(3,496,687
)
|
$
(1,652,908
)
|
|
Other comprehensive
income:
|
|
|
|
Unrealized foreign
currency gain (loss)
|
(346
)
|
740
|
|
Comprehensive
(Loss)
|
(3,497,033
)
|
(1,652,168
)
|
|
Basic (Loss) per
common share
|
(0.01
)
|
(0.01
)
|
|
Weighted Average
Common Shares Outstanding
|
424,874,458
|
122,278,909
|
|
Sunshine
Biopharma, Inc.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December
31,
|
December
31,
|
|
|
2016
|
2015
|
|
Cash Flows From
Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
Net
(Loss)
|
$
(3,496,687
)
|
$
(1,652,168
)
|
|
|
|
|
|
Adjustments to
reconcile net loss to net cash used in operating
activities:
|
|
|
|
Amortization &
Depreciation
|
60,731
|
4,251
|
|
Stock issued for
services
|
702,300
|
116,500
|
|
Loss on impairment
of patents
|
556,120
|
|
|
Loss on conversion
of notes payable
|
1,945,898
|
575.144
|
|
Stock issued for
payment of interest
|
9,270
|
12,886
|
|
Debt
forgiveness
|
(1,313
)
|
|
|
Increase (Decrease)
in prepaid expenses
|
2,104
|
(3,111
)
|
|
Increase (Decrease)
in Accounts Payable
|
(18,960
)
|
11,824
|
|
Increase (Decrease)
in Accounts Payable – related entity
|
(80,487
)
|
80,487
|
|
Increase (decrease)
in interest payable
|
6,355
|
(13,457
)
|
|
|
|
|
|
Net Cash Flows
(used) in operations
|
(314,182
)
|
(867,644
)
|
|
|
|
|
|
Cash Flows From
Investing Activities:
|
|
|
|
Purchase
equipment
|
(3,439
)
|
(4,793
)
|
|
Purchase of
patents
|
-
|
(618,810
)
|
|
|
|
|
|
Net Cash Flows
(used) in Investing activities
|
(3,439
)
|
(623,303
)
|
|
|
|
|
|
Cash Flows From
Financing Activities:
|
|
|
|
|
|
|
|
Proceed from note
payable
|
131,150
|
232,840
|
|
Note payable used
to pay expenses
|
-
|
12,160
|
|
Note payable
– Related entity
|
67,032
|
|
|
Note payable used
to pay origination fees & interest
|
22,312
|
81,678
|
|
Note payable
related entity for patent purchase
|
-
|
835,394
|
|
Sale of common
stock
|
104,128
|
236,550
|
|
|
|
|
|
Net Cash Flows
provided by financing activities
|
324,622
|
1,398,622
|
|
|
|
|
|
|
|
|
|
Net Increase
(Decrease) In Cash and cash equivalents
|
7,001
|
(92,625
)
|
|
Foreign currency
translation adjustment
|
(346
)
|
|
|
Cash and cash
equivalents at beginning of period
|
$
50,798
|
$
143,423
|
|
|
|
|
|
|
$
57,453
|
$
50,798
|
|
Supplementary
Disclosure Of Cash Flow Information:
|
|
|
|
Cash paid for
interest
|
$
5,264
|
$
-
|
|
Cash paid for
income taxes
|
$
-
|
$
-
|
|
|
|
|
|
Stock issued for
services
|
$
702,300
|
$
116,500
|
|
Stock issued for
note conversions
|
$
3,077,950
|
$
977,485
|
|
Stock issued for
interest
|
$
-
|
$
19,528
|
|
Stock issued for
payment of expenses
|
$
-
|
$
-
|
|
Loan proceeds used
to pay expenses
|
$
-
|
$
57,828
|
|
|
|
|
|
|
|
|
|
Deficit
accumulated
|
|
|
|
Number
Of
|
|
Capital
Paid
|
Number
Of
|
|
Stock
|
|
During
the
|
|
|
|
Common
|
Common
|
in
Excess
|
Preferred
|
Preferred
|
Subscription
|
Comprehensive
|
development
|
|
|
|
Shares Issued
|
Stock
|
of Par Value
|
Shares Issued
|
Stock
|
Receivable
|
Income
|
stage
|
Total
|
|
Balance at
December 31, 2014
|
73,551,041
|
$
73,551
|
$
6,967,228
|
-
|
$
-
|
$
-
|
$
-
|
$
(7,428,359
)
|
$
(387,580
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for cash
|
20,000,000
|
20,000
|
216,550
|
|
|
|
|
|
236,550
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for
services
|
1,800,000
|
1,800
|
64,700
|
|
|
|
|
|
66,500
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock series "B"
issued for services
|
|
|
|
500,000
|
50,000
|
|
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for the
reduction of note payable and payment of
interest
|
102,914,077
|
102,914
|
986,739
|
|
|
|
|
|
1,089,653
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income (Loss)
|
-
|
-
|
-
|
|
|
|
740
|
(1,652,908
)
|
(1,652,168
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31,
2015
|
198,265,118
|
$
198,265
|
$
8,235,217
|
500,000
|
$
50,000
|
$
-
|
$
740
|
$
(9,081,267
)
|
$
(597,045
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for
cash
|
12,555,556
|
12,556
|
91,572
|
|
|
|
|
|
104,128
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for
services
|
146,750,000
|
146,750
|
555,550
|
|
|
|
|
|
702,300
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for the
reduction of note payable and payment of
interest
|
411,829,184
|
411,829
|
2,666,121
|
|
|
|
|
|
3,077,950
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income
(Loss)
|
-
|
-
|
-
|
|
|
|
(346
)
|
(3,496,687
)
|
(3,497,033
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2016
|
769,399,858
|
$
769,400
|
$
11,548,460
|
500,000
|
$
50,000
|
$
-
|
$
394
|
$
(12,577,954
)
|
$
(209,700
)
|
|
|
Office
Equipment:
|
5-7
Years
|
|
|
Laboratory
Equipment
|
5
Years
|
|
|
Vehicles
|
5
Years
|
|
|
December
31,
|
December
31,
|
|
|
2016
|
2015
|
|
|
|
|
|
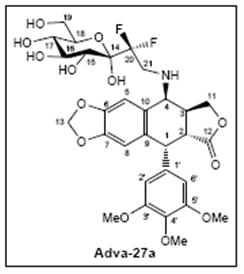
Adva-27a US
Patent*
|
$
155,940
|
$
155,940
|
|
Adva-27a Worldwide
Patents*
|
$
462,870
|
$
462,870
|
|
|
|
|
|
Total
|
$
618,810
|
$
618,810
|
|
|
|
|
|
Less accumulated
amortization (58,918 in 2016)
|
$
(62,690
)
|
$
(3,772
)
|
|
Net of
amortization
|
$
556,120
|
$
615,038
|
|
Less: impairment
loss
|
$
(556,120
)
|
$
-0-
|
|
|
|
|
|
Total at Year
End
|
$
-0-
|
$
615,038
|
|
|
2016
|
2015
|
|
|
|
|
|
Net (loss)
attributable to Common Stock
|
$
(3,496,687
)
|
$
(1,652,908
)
|
|
Basic weighted
average outstanding shares of Common Stock
|
424,874,458
|
122,278,909
|
|
Dilutive effects of
common share equivalents
|
-0-
|
-0-
|
|
Dilutive weighted
average outstanding shares of common stock
|
424,874,458
|
122,278,909
|
|
|
|
|
|
Net loss per share
of Common Stock
|
|
|
|
Basic and
Diluted
|
$
(0.01
)
|
$
(0.01
)
|
|
|
December
31,
|
December
31,
|
||
|
|
2016
|
2015
|
||
|
|
Temporary
|
Tax
|
Temporary
|
Tax
|
|
|
Difference
|
Effect
|
Difference
|
Effect
|
|
Deferred
tax assets:
|
|
|
|
|
|
Net operating
loss
|
$
10,182,607
|
$
3,773,674
|
$
9,081,267
|
$
6,172,980
|
|
Valuation
allowance
|
(10,182,607
)
|
(3,773,674
)
|
(9,081,267
)
|
(6,172,980
)
|
|
|
|
|
|
|
|
Total
deferred tax asset
|
-0-
|
-0-
|
-0-
|
-0-
|
|
|
|
|
|
|
|
Net
deferred tax asset
|
$
-0-
|
$
-0-
|
$
-
|
$
-
|
|
|
December 31,
|
|
|
|
2016
|
2015
|
|
U.S.
Federal statutory graduated rate …
|
34.00
%
|
34.00
%
|
|
State
income tax rate,
net
of federal benefit
|
3.06
%
|
4.63
%
|
|
Net
rate
|
37.06
%
|
38.63
%
|
|
Net
operating loss used
|
0.00
%
|
0.00
%
|
|
Net
operating loss for which no tax
benefit
is currently available
|
-37.06
%
|
-38.63
%
|
|
|
0.00
%
|
0.00
%
|
|
Notes payable
consist of the following:
|
2016
|
2015
|
|
|
|
|
|
Note Payable -
Original Face Value $12,500 with interest of 12% was renewed on
December 31, 2015 with the addition of accrued interest amounting
to $6,642 (“2015 Note”). The 2015 Note has a Face Value
of $19,142 and accrues interest at 12%. The Company renewed the
2015 Note when it became due on December 31, 2016 (“2016
Note”). The 2016 Note has a Face Value of $21,439 and accrues
interest at 12%. The Face Value amount includes $2,297 in accrued
interest. The 2016 Note is convertible anytime from the date of
issuance into $0.001 par value Common Stock at a 35% discount from
market price and is due December 31, 2017.
We estimate that the fair value of this
convertible debt approximates the face value, so no value has been
assigned to the beneficial conversion feature.
Any gain or
loss will be recognized at conversion.
|
$
21,439
|
$
19,142
|
|
|
|
|
|
In August 2015 the
Company received monies in exchange for a note having a Face Value
of $83,000 with interest at 8% is due May 7, 2016. The Note is
convertible after 180 days from issuance into $0.001 par value
Common Stock at
a price 35% below
market value. The Note, including
$83,000 of principal
and $3,120 in interest, was fully
converted into
$0.001 par value Common Stock during the
period ended December 31, 2016. In connection therewith, 9,906,049
shares of $0.001 par value Common Stock valued at $146,658 were
issued generating a loss of $60,538 on the conversion.
|
-0-
|
83,000
|
|
|
|
|
|
In February 2016,
the Company received monies in exchange for a note having a Face
Value of $85,000 with interest at 8% is due November 18, 2016. The
Note is convertible after 180 days from issuance into $0.001 par
value Common Stock at
a price 35%
below market value. The Note, including
$85,000 of principal
and $3,400 in interest, was fully
converted into
$0.001 par value Common Stock during the
period ended December 31, 2016. In connection therewith, 27,538,058
shares of $0.001 par value Common Stock valued at $172,433were
issued generating a loss of $84,033 on the conversion.
|
-0-
|
-0-
|
|
|
|
|
|
In June 2016 the
Company received monies in exchange for a note having a Face Value
of $55,000 with interest accruing at 10% is due April 1, 2017. The
Note is convertible after 180 days from issuance into $0.001 par
value Common Stock at
a price 40%
below market value.
In December 2016, $6,500 of the
principal was converted
into 5,000,000
shares of
$0.001 par value Common Stock valued at $20,000
and generating a loss of $13,500 on conversion. The Company
estimates that the fair value of the
convertible debt approximates the face value, so no value has been
assigned to the beneficial conversion feature
to be
recognized at conversion.
|
48,500
|
-0-
|
|
|
|
|
|
Total current
debt
|
$
69,939
|
$
102,142
|
|
Note Payable - Face
Value $128,000 with interest of 10% was due May 27, 2015. Issued on
November 27, 2014 at a premium and convertible from issuance into
$0.001 par value Common Stock at
a
price of $0.20 per share.
On June 30, 2015 the Company
renewed this note with the addition of accrued interest amounting
to $7,540 and an origination fee of $25,600. The new Note has a
Face Value of $161,140 and accrues interest at 12%. The new Note,
due December 31, 2015, is convertible any time from the date of
issuance into $0.001 par value Common Stock at a 35% discount from
market price. On December 31, 2015, the Company renewed this note
with the addition of accrued interest amounting to $9,668 and an
origination fee of $32,228. The new Note has a Face Value of
$203,036 and accrues interest at 12%. The new Note, due June 30,
2016, is convertible anytime from the date of issuance into $0.001
par value Common Stock at a 35% discount from market price. In
January 2016, $38,036 of the principal was converted, leaving a
principal balance of $165,000. In connection therewith, 7,705,186
shares of $0.001par value Common Stock valued $231,156 were issued
generating a loss on conversion of $193,120. The Company renewed
this note with the addition of accrued interest amounting to
$9,852. The renewed note has a Face Value of $174,852 and accrues
interest at 12%. It is due on March 31, 2017. In October 2016,
$74,852 of the principal amount was converted, leaving a principal
balance of $100,000, which was renewed with interest thereon for 90
days. In connection therewith, 40,374,475 shares of $0.001 par
value Common Stock valued $290,696 were issued generating a loss of
$215,844 on conversion.
The Company
estimates that the fair value of this convertible debt approximates
the face value, so no value has been assigned to the beneficial
conversion feature
to be recognized at
conversion.
|
$
100,000
|
$
203,036
|
|
|
|
|
|
On October 8, 2015
the Company acquired U.S. Patent Number 8,236,935 (the “US
Patent”) for the anticancer compound, Adva-27a, from
Advanomics Corporation (a related party), which includes all rights
to this intellectual property within the United States in exchange
for an interest-free note payable for $4,320,000 with annual
payments of $360,000 due and payable on or before December 31,
commencing in 2016 and continuing until paid in full. The note is
collateralized by the US Patent. Pursuant to an amended agreement
effective December 28, 2015, this note was cancelled and replaced
with a new note having a face value of $210,519, comprised of
$155,940 in principal amount which is Advanomics’ book value
of the US Patent plus $54,579 as an adjustment for the currency
exchange difference. This interest-free new note is automatically
convertible into 80,968,965 shares of the Company’s $0.001
par value Common Stock upon the Company completing an increase in
its authorized capital such that a sufficient number of Common
shares is available for issuance. In July 2016 the Company issued
the requisite 80,968,965 shares of $0.001 par value Common Stock
and this note was therefore automatically cancelled.
|
$
-0-
|
$
210,519
|
|
|
|
|
|
On December 28,
2015 the Company acquired the worldwide issued and pending patents
under PCT/FR2007/000697 and PCT/CA2014/000029 (the “Worldwide
Patents”) for the anticancer compound, Adva-27a, from
Advanomics Corporation (a related party), which include all
worldwide rights to this intellectual property in exchange for a
note payable for $12,822,499, with interest accruing at 2% per year
beginning January 1, 2016 and quarterly payments of $70,000 plus
interest commencing the end of March 2016 and continuing until
December 2020 when the entire principal balance and all accrued
interest will be due. The note is collateralized by the Worldwide
Patents. Pursuant to an amended agreement, effective December 28,
2015, this note was cancelled and replaced with a new convertible
note having a face value of $624,875, comprised of $462,870 in
principal amount which is Advanomics’ book value of the
Patents, plus a $162,005 amount as an adjustment for the currency
exchange difference. This interest-free new note is automatically
convertible into 240,336,451 shares of $0.001 par value Common
Stock upon the Company completing an increase in its authorized
capital such that a sufficient number of Common shares is available
for issuance. In July 2016 the Company issued the requisite
240,336,451 shares of $0.001 par value Common Stock and this note
was therefore automatically cancelled.
|
$
-0-
|
$
624,875
|
|
|
|
|
|
In December 2016,
the Company received monies from the Company’s CEO in
exchange for a note payable having a Face Value of $90,000 Canadian
($67,032 US) with interest at 12% due March 31, 2017. The Note is
convertible any time after the date of issuance into $0.001 par
value Common Stock at
a price 35%
below market value. The Company estimated that the fair value of
the convertible debt approximates the face value, so no value has
been assigned to the beneficial conversion feature.
Any gain
or loss will be recognized at conversion. This Note is
collateralized by the assets of the Company.
|
67,032
|
-0-
|
|
|
|
|
|
Total related
entity current debt
|
$
167,032
|
$
1,038,430
|
|
|
●
|
Pertain
to the maintenance of records that in reasonable detail accurately
and fairly reflect the transactions and dispositions of the assets
of the company;
|
|
|
●
|
Provide
reasonable assurance that transactions are recorded as necessary to
permit preparation of financial statements in accordance with
generally accepted accounting principles, and the receipts and
expenditures of the company are being made only in accordance with
authorizations of management and directors of the Company;
and
|
|
|
●
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisitions, use or disposition of the
company’s assets that could have a material effect on the
financial statements.
|
|
Name
|
|
Age
|
|
Position(s)
|
|
|
|
|
|
|
|
Dr.
Steve N. Slilaty
|
|
64
|
|
President,
Chief Executive Officer, and Chairman
|
|
|
|
|
|
|
|
Dr.
Abderrazzak Merzouki
|
|
53
|
|
Chief
Operating Officer and Director
|
|
|
|
|
|
|
|
Camille
Sebaaly
|
|
56
|
|
Chief
Financial Officer, Secretary and Director
|
|
Name and
Principal Position
|
|
Year
|
Salary
($)
|
Bonus
($)
|
Option
Awards
($)
|
All
Other
Compensation
($)
|
Total
($)
|
|
|
|
|
|
|
|
|
|
|
Dr. Steve N.
Slilaty, CEO
|
|
2016
|
1,000
|
-
|
-
|
164,600
(2)
|
165,600
|
|
|
|
2015
|
-
|
-
|
-
|
50,000
(1)
|
50,000
(1)
|
|
|
|
2014
|
-
|
-
|
-
|
$
-
|
$
-
|
|
|
|
|
|
|
|
|
|
|
Camille Sebaaly,
CFO
|
|
2016
|
4,597
|
|
|
164,600
(2)
|
$
169,187
(2)
|
|
|
|
2015
|
|
|
|
-
|
-
|
|
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abderrazzak
Merzouki, COO
|
|
2016
|
|
|
|
164,600
(2)
|
164,600
(2)
|
|
|
|
2015
|
|
|
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
Michele Di Turi,
COO(3)
|
|
|
|
|
|
|
|
|
|
|
2015
|
$
20,000
|
-
|
-
|
-
|
$
20,000
|
|
|
|
2014
|
$
15,000
|
-
|
-
|
|
$
15,000
|
|
Title of
Class
|
|
Name and Address
Of Beneficial Owner
|
Amount and
Nature Of Beneficial Ownership
|
Percent Of
Class
|
|
|
|
|
|
|
|
Common
|
|
Dr. Steve N.
Slilaty
(1)
579 rue
Lajeunesse
Laval,
Quebec
Canada H7X
3K4
|
253,398,597
(2)
|
29.8
%
|
|
|
|
|
|
|
|
Series B Preferred
(500,000 shares of 1,000 votes per share)
|
|
|
500,000,000
(3)
|
100
%
|
|
|
|
|
|
|
|
Common
|
|
Dr. Abderrazzak
Merzouki
(1)
731 Place de l'Eeau
Vive
Laval,
Quebec
Canada H7Y
2E1
|
39,467,000
|
4.6
%
|
|
|
|
|
|
|
|
Common
|
|
Camille
Sebaaly
())
14464 Gouin West,
#B
Montreal,
Quebec
Canada H9H
1B1
|
167,703,300
(4)
|
19.9
%
|
|
|
|
|
|
|
|
Common
|
|
Nabil
Dabar
150 Cote Vertu, Su,
200
Montreal, Quebec
H4N 1C6
Canada
|
49,219,656
|
5.8
%
|
|
|
|
|
|
|
|
Common
|
|
All Officers and
Directors
As a Group (3
persons)
|
960,568,897
|
71.1
%
|
|
|
|
|
|
|
|
Series B
Preferred
|
|
Dr, Steve N.
Silaty
|
500,000
(3)
|
100
%
|
|
(1)
|
Officer and Director of our
Company.
|
|
(2)
|
Includes 215,014,224 shares held in
the name of Advanomics Corporation. Dr. Slilaty is an
officer, director and principal shareholder of Advanomics and as a
result, controls the disposition of these
shares.
|
|
(3)
|
Comprised of 500,000 shares of
$0.10 par value Series “B” Preferred Stock having 1,000
votes per share. The Series “B” Preferred Stock is
non-convertible, non-redeemable, non-retractable and has a superior
liquidation value of $0.10 per share. See “Part
II, Item13 – Certain Relationships and Related Transactions
and Director Independence.”
|
| (4) | Includes 129,488,927 shares held in the name of 4019318 Canada, Inc. Mr. Sebaaly is the sole officer and director of this company and as a result, controls the disposition of these shares. |
|
|
December
31,
2015
|
December
31,
2016
|
|
Audit
Fees
|
$
13,000
|
$
21,600
|
|
|
-
|
|
|
Tax
Fees
|
-
|
|
|
All Other
Fees
|
-
|
|
|
Total
|
$
13,000
|
$
21,600
|
|
Exhibit
No.
|
|
Description
|
|
|
|
|
|
|
Certification
of Chief Executive Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
Certification
of Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
Certification
of Chief Executive Officer and Chief Financial Officer Pursuant to
18 U.S.C. Section 1350
|
|
101.INS
|
XBRL
Instances Document
|
|
|
|
|
101.SCH
|
XBRL
Taxonomy Extension Schema Document
|
|
|
|
|
101.CAL
|
XBRL
Taxonomy Extension Calculation Linkbase Document
|
|
|
|
|
101.DEF
|
XBRL
Taxonomy Extension Definition Linkbase Document
|
|
|
|
|
101.LAB
|
XBRL
Taxonomy Extension Label Linkbase Document
|
|
|
|
|
101.PRE
|
XBRL
Taxonomy Extension Presentation Linkbase Document
|
|
No.
|
|
Description
|
|
Filed With
|
|
Date
|
|
3.1
|
|
Articles
of Incorporation
|
|
Form
SB-2 Registration Statement
|
|
October
19, 2007
|
|
3.2
|
|
Bylaws
|
|
Form
SB-2 Registration Statement
|
|
October
19, 2007
|
|
3.3
|
|
Articles
of Amendment (Name Change)
|
|
Form
8-K Dated November 2, 2009
|
|
November
6, 2009
|
|
3.4
|
|
Statement
of Share and Equity Capital Exchange
|
|
Form
10-Q For Quarter Ended 06/30/10
|
|
August
4, 2010
|
|
3.5
|
|
Articles
of Amendment (Add Preferred and Series A Preferred to
Authorized)
|
|
Form
10-Q For Quarter Ended 06/30/10
|
|
August
4, 2010
|
|
10.1
|
|
Share
Exchange Agreement with Sunshine Biopharma, Inc.
|
|
Form
8-K Dated October 15, 2009
|
|
October
20, 2009
|
|
10.2
|
|
License
Agreement with Advanomics, Inc.
|
|
Form
8-K/A1 Dated October 15, 2009
|
|
January
19, 2010
|
|
10.3
|
|
Amendment
No. 1 to License Agreement with Advanomics, Inc.
|
|
Form
8-K/A1 Dated October 15, 2009
|
|
January
19, 2010
|
|
10.4
|
|
Research
Agreement with The Research Foundation of the State University of
New York
|
|
Form
8-K Dated January 17, 2011
|
|
January
19, 2011
|
|
10.5
|
|
Research
Agreement with Jewish General Hospital
|
|
Form
8-K Dated June 14, 2011
|
|
June
17, 2011
|
|
10.6
|
|
Amendment
No. 2 to License Agreement with Advanomics
|
|
Form
8-K Dated December 21, 2011
|
|
December
27, 2011
|
|
10.7
|
|
Investment
Agreement with Dutchess Investment Group II
|
|
Form
8-K dated April 23, 2015
|
|
April
28, 2015
|
|
10.8
|
|
Registration
Rights Agreement with Dutchess Investment Group II
|
|
“
|
|
“
|
|
10.9
|
|
Patent
Purchase Agreement with Advanomics Corporation
|
|
Form
8-K dated October 8, 2015
|
|
October
9, 2015
|
|
10.10
|
|
Second
Patent Purchase Agreement with Advanomics Corporation
|
|
Form
8-K dated December 28, 2015
|
|
December
28, 2015
|
|
10.11
|
|
Amendment
No. 1 to Patent Purchase Agreement with Advanomics Corporation
dated October 8, 2015, including Secured Convertible Promissory
Note.
|
|
Form
8-K dated March 14, 2016
|
|
March
14, 2016
|
|
10.12
|
|
Amendment
No. 1 to Patent Purchase Agreement with Advanomics Corporation
dated December 28, 2015, including Secured Convertible Promissory
Note
|
|
Form
8-K dated March 14, 2016
|
|
March
14, 2016
|
|
21.2
|
|
List of
Subsidiaries
|
|
Form
10-K For FYE December 31, 2010
|
|
March
30, 2011
|
|
|
SUNSHINE BIOPHARMA, INC.
|
|
|
|
|
|
|
|
|
Dated:
April 17, 2017
|
By:
|
/s/ Dr.
Steve N. Slilaty
|
|
|
|
|
Dr.
Steve N. Slilaty, Principal Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Camille Sebaaly
|
|
|
|
|
Camille
Sebaaly, Principal Financial and Accounting Officer
|
|
|
|
|
|
|