|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Colorado
|
20-5566275
|
|
|
(State of other jurisdiction of incorporation)
|
(IRS Employer ID No.)
|
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
|
Non-accelerated filer
|
o
|
Smaller reporting company
|
þ
|
|
(Do not check if a smaller reporting company)
|
|
Page No.
|
||||
|
Item 1.
|
Financial Statements
|
4
|
||
|
Consolidated Balance Sheet as of September 30, 2014 (unaudited)
|
4
|
|||
|
Unaudited Statement of Operations for the Nine Month Period Ended September 30, 2014
|
5
|
|||
|
Unaudited Consolidated Statement of Cash Flows for the for the Nine Month Periods Ended September 30, 2014 and 2013
|
8
|
|||
|
Notes to Consolidated Financial Statements
|
9
|
|||
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations/Plan of Operation.
|
13
|
||
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
20
|
||
|
Item 4.
|
Controls and Procedures.
|
20
|
||
|
PART II
|
||||
|
OTHER INFORMATION
|
||||
|
Item
|
||||
|
Item 1.
|
Legal Proceedings
|
20
|
||
|
1A.
|
Risk Factors
|
20
|
||
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of Proceeds
|
20
|
||
|
Item 3.
|
Defaults Upon Senior Securities
|
21
|
||
|
Item 4.
|
Mine Safety Disclosures
|
21
|
||
|
Item 5.
|
Other Information
|
21
|
||
|
Item 6.
|
Exhibits
|
21
|
||
|
Signatures
|
22
|
|
Sunshine Biopharma, Inc.
|
||||||||
|
Balance Sheet
|
||||||||
|
Unaudited
|
Audited
|
|||||||
|
September 30,
|
December 31,
|
|||||||
|
2014
|
2013
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 173,857 | $ | 31,240 | ||||
|
Prepaid expenses
|
6,419 | - | ||||||
|
Total Current Assets
|
180,276 | 31,240 | ||||||
|
TOTAL ASSETS
|
$ | 180,276 | $ | 31,240 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Current portion of note payable
|
396,000 | 12,500 | ||||||
|
Accounts payable
|
51,320 | 23,809 | ||||||
|
Interest payable
|
9,320 | 2,641 | ||||||
|
TOTAL LIABILITIES
|
456,640 | 38,950 | ||||||
|
SHAREHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $0.10 par value per share;
|
||||||||
|
Authorized 5,000,000 Shares; Issued
|
||||||||
|
and outstanding -0- shares.
|
- | - | ||||||
|
Common Stock, $0.001 per share;
|
||||||||
|
Authorized 200,000,000 Shares; Issued
|
||||||||
|
and outstanding 68,911,041and 60,299,061 at
|
||||||||
|
September 30, 2014 and December 31, 2013 respectively
|
68,911 | 60,299 | ||||||
|
Capital paid in excess of par value
|
6,749,367 | 5,426,140 | ||||||
|
Accumulated other comprehesive (Loss)
|
- | - | ||||||
|
(Deficit) accumulated during the development stage
|
(7,094,642 | ) | (5,494,149 | ) | ||||
|
TOTAL SHAREHOLDERS' EQUITY
|
(276,364 | ) | (7,710 | ) | ||||
|
TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY
|
$ | 180,276 | $ | 31,240 | ||||
| See Accompanying Notes To These Financial Statements. | ||||||||
|
Sunshine Biopharma, Inc.
|
||||||||
|
Unaudited Statement Of Operations
|
||||||||
|
Unaudited
|
Unaudited
|
|||||||
|
3 Months
|
3 Months
|
|||||||
|
Ended
|
Ended
|
|||||||
|
September
|
September
|
|||||||
|
2014
|
20
13
|
|||||||
|
Revenue:
|
$ | - | $ | - | ||||
|
General & Administrative Expenses
|
||||||||
|
Accounting
|
1,620 | 1,620 | ||||||
|
Financial Consulting
|
215,500 | 95,000 | ||||||
|
Legal
|
20,152 | 27,873 | ||||||
|
Licenses & fees
|
30,000 | 50,000 | ||||||
|
Office
|
8,934 | 2,660 | ||||||
|
Public relations
|
- | - | ||||||
|
Research & development
|
- | - | ||||||
|
Stock Transfer Fee
|
1,221 | 530 | ||||||
|
Total G & A
|
277,427 | 177,683 | ||||||
|
(Loss) from operations
|
$ | (277,427 | ) | $ | (177,683 | ) | ||
|
Other (expense) interest
|
(3,422 | ) | (375 | ) | ||||
|
Net (loss)
|
$ | (280,849 | ) | $ | (178,058 | ) | ||
|
Basic (Loss) per common share
|
(0.00 | ) | (0.00 | ) | ||||
|
Weighted Average Common Shares Outstanding
|
67,820,603 | 56,914,063 | ||||||
| See Accompanying Notes To These Financial Statements. | ||||||||
|
Sunshine Biopharma, Inc.
|
||||||||
|
Unaudited Statement Of Operations
|
||||||||
|
Unaudited
|
Unaudited
|
|||||||
|
9 Months
|
9 Months
|
|||||||
|
Ended
|
Ended
|
|||||||
|
September 30,
|
September 30,
|
|||||||
|
2014
|
2013
|
|||||||
|
Revenue:
|
$ | - | $ | - | ||||
|
General & Administrative Expenses
|
||||||||
|
Research and Development
|
323,000 | 23,400 | ||||||
|
Accounting
|
38,820 | 7,020 | ||||||
|
Consulting
|
541,500 | 779,610 | ||||||
|
Legal
|
234,929 | 52,562 | ||||||
|
Licenses
|
183,333 | 375,000 | ||||||
|
Office
|
21,824 | 10,939 | ||||||
|
Merger Cost
|
- | - | ||||||
|
Public Relations
|
100,000 | - | ||||||
|
Stock Transfer Fee
|
5,408 | 2,136 | ||||||
|
Writedown of intangible assets
|
- | - | ||||||
|
Total G & A
|
1,448,814 | 1,250,667 | ||||||
|
(Loss) from operations
|
(1,448,814 | ) | (1,250,667 | ) | ||||
|
Other (expense):
|
||||||||
|
Interest expense
|
(151,679 | ) | (6,080 | ) | ||||
|
Beneficial conversion feature
|
- | (548,951 | ) | |||||
|
Total Other (Expense)
|
(151,679 | ) | (555,031 | ) | ||||
|
Net (loss)
|
$ | (1,600,493 | ) | $ | (1,805,698 | ) | ||
|
Basic (Loss) per common share
|
$ | (0.02 | ) | $ | (0.03 | ) | ||
|
Weighted Average Common Shares Outstanding
|
64,688,934 | 55,136,962 | ||||||
| See Accompanying Notes To These Financial Statements. | ||||||||
|
Sunshine Biopharma, Inc.
|
||||||||||||||||||||||||||||||
|
Statement of Shareholders' Equity
|
||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Number Of Common | Common | Capital Paid in Excess | Number Of Preferred | Preferred | Stock Subscription |
Comprehensive
|
Deficit accumulated During the development | |||||||||||||||||||||||
|
Shares Issued
|
Stock
|
of Par Value
|
Shares Issued
|
Stock
|
Receivable
|
Income
|
stage
|
Total
|
||||||||||||||||||||||
|
Balance at December 31, 2012
|
51,416,092 | $ | 51,416 | $ | 3,021,676 | - | $ | - | $ | - | $ | - | $ | 3,002,666 | $ | 70,426 | ||||||||||||||
|
January 11, 2013 issued 350,000 shares
|
|
|||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
|
|||||||||||||||||||||||||||||
|
valued at $ 136,500 or $0.39 per share
|
350,000 | 350 | 136,150 | 136,500 | ||||||||||||||||||||||||||
|
March 28, 2013 issued 918,500 shares
|
|
|||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
|
|||||||||||||||||||||||||||||
|
valued at $ 220,440 or $0.24 per share
|
918,500 | 919 | 219,522 | - | - | 220,440 | ||||||||||||||||||||||||
|
March 30, 2013 issued 259,043 shares
|
|
|||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
|
|||||||||||||||||||||||||||||
|
valued at $ 219,370 or $0.24 per share
|
914,043 | 914 | 218,456 | 219,370 | ||||||||||||||||||||||||||
|
March 30, 2013 issued 2,590,42
of par value $0.001 common stock for conversion
8 shares
of debt in the amount of $513,000 and interest
|
|
|||||||||||||||||||||||||||||
|
of $5,086 or $0.24 per share
|
2,590,426 | 2,590 | 515,496 | 518,086 | ||||||||||||||||||||||||||
|
Beneficial conversion feature
|
548,951 | 548,951 | ||||||||||||||||||||||||||||
|
May 14, 2013 issued 250,000 shares
of par value $0.001 common stock for services
|
250,000 | 250 | 59,750 | 60,000 | ||||||||||||||||||||||||||
|
August 1, 2013 issued 150,000 shares
of par value $0.001 common stock for services
valued at $ 30,000 or $0.20 per share
|
150,000 | 150 | 29,850 | 30,000 | ||||||||||||||||||||||||||
|
August 23, 2013 issued 250,000 shares
of par value $0.001 common stock for services
|
250,000 | 250 | 49,750 | 50,000 | ||||||||||||||||||||||||||
|
October 4, 2013 issued 60,0000 shares
of par value $0.001 common stock for services
|
60,000 | 60 | 14,940 | 15,000 | ||||||||||||||||||||||||||
|
November 4, 2013 issued 500,000 shares
of par value $0.001 common stock for cash
of $95,000 or $0.19 per share
|
500,000 | 500 | 94,500 | 95,000 | ||||||||||||||||||||||||||
|
November 20, 2013 issued 425,000 shares
of par value $0.001 common stock for cash
|
425,000 | 425 | 84,575 | 85,000 | ||||||||||||||||||||||||||
|
November 20, 2013 issued 600,000 shares
of par value $0.001 common stock for services
|
600,000 | 600 | 113,400 | 114,000 | ||||||||||||||||||||||||||
|
December 2, 2013 issued 75,000 shares
of par value $0.001 common stock for cash
|
75,000 | 75 | 14,925 | 15,000 | ||||||||||||||||||||||||||
|
December 27, 2013 issued 1,800,000 shares
of par value $0.001 common stock for services
|
1,800,000 | 1,800 | 304,200 | 306,000 | ||||||||||||||||||||||||||
|
Net (Loss)
|
- | - | - | (2,491,483 | ) | (2,491,483 | ) | |||||||||||||||||||||||
|
Balance at December 31, 2013
|
60,299,061 | $ | 60,299 | $ | 5,426,140 | - | $ | - | $ | - | $ | - | $ | 511,183 | $ | (7,710 | ) | |||||||||||||
|
January 6, 2014 issued 200,000 shares
of par value $0.001 common stock for cash
of $40,000 or $0.20 per share
|
200,000 | 200 | 39,800 | 40,000 | ||||||||||||||||||||||||||
|
January 30, 2014 issued 600,000 shares
of par value $0.001 common stock for services
valued at $ 96,000 or $0.16 per share
|
600,000 | 600 | 95,400 | 96,000 | ||||||||||||||||||||||||||
|
February 14, 2014 issued 66,667 shares
of par value $0.001 common stock for cash
of $13,333 or $0.20 per share
|
66,667 | 67 | 13,266 | 13,333 | ||||||||||||||||||||||||||
|
March 2, 2014 issued 10,000 shares
of par value $0.001 common stock for services
valued at $ 1,400 or $0.14 per share
|
10,000 | 10 | 1,390 | 1,400 | ||||||||||||||||||||||||||
|
March 14, 2014 issued 1,000,000 shares
of par value $0.001 common stock for services
valued at $ 170,000 or $0.17 per share
|
1,000,000 | 1,000 | 169,000 | 170,000 | ||||||||||||||||||||||||||
|
March 27, 2014 issued 500,000 shares
of par value $0.001 common stock for prepaid
interest valued at $ 75,000 or $0.15 per share
|
500,000 | 500 | 74,500 | 75,000 | ||||||||||||||||||||||||||
|
April 14, 2014 issued 500,000 shares
of par value $0.001 common stock for services
valued at $ 100,000 or $0.20 per share
|
500,000 | 500 | 99,500 | 100,000 | ||||||||||||||||||||||||||
|
April 17, 2014 issued 1,700 000 shares
of par value $0.001 common stock for services
valued at $323,000 or $0.19 per share
|
1,700,000 | 1,700 | 321,300 | 323,000 | ||||||||||||||||||||||||||
|
April 25, 2014 issued 400,000 shares
of par value $0.001 common stock for services
valued at $ 60,000 or $0.15 per share
|
400,000 | 400 | 59,600 | 60,000 | ||||||||||||||||||||||||||
|
May 15, 2014 issued 500,0000 shares
of par value $0.001 common stock for services
valued at $ 80,000 or $0.16 per share
|
500,000 | 500 | 79,500 | 80,000 | ||||||||||||||||||||||||||
|
June 12, 2014 issued 190,000 shares
of par value $0.001 common stock for services
valued at $ 30,400 or $0.16 per share
|
190,000 | 190 | 30,210 | 30,400 | ||||||||||||||||||||||||||
|
July 7, 2014 issued 975,000 shares
of par value $0.001 common stock for services
|
975,000 | 975 | 135,525 | 136,500 | ||||||||||||||||||||||||||
|
July 24, 2014 issued 400,000 shares
of par value $0.001 common stock for services
|
400,000 | 400 | 51,600 | 52,000 | ||||||||||||||||||||||||||
|
August 7, 2014 issued 930,233 shares
of par value $0.001 common stock for cash
|
930,233 | 930 | 99,070 | 100,000 | ||||||||||||||||||||||||||
|
September 8, 2014 issued 300,000 shares
of par value $0.001 common stock for services
|
300,000 | 300 | 26,700 | 27,000 | ||||||||||||||||||||||||||
|
September 19, 2014 issued 340,080 shares
of par value $0.001 common stock for services
|
340,080 | 340 | 26,866 | 27,206 | ||||||||||||||||||||||||||
|
Net (Loss)
|
- | - | - | (1,600,493 | ) | (1,600,493 | ) | |||||||||||||||||||||||
|
Balance at September 30, 2014 (Unaudited)
|
68,911,041 | $ | 68,911 | $ | 6,749,367 | - | $ | - | $ | - | $ | - | $ | (1,089,310 | ) | $ | (276,364 | ) | ||||||||||||
|
Sunshine Biopharma, Inc.
|
||||||||
|
Unaudited Statement Of Cash Flows
|
||||||||
|
Unaudited
|
Unaudited
|
|||||||
|
9 Months
|
9 Months
|
|||||||
|
Ended
|
Ended
|
|||||||
|
September 30,
|
September 30,
|
|||||||
|
2014
|
2013
|
|||||||
|
Cash Flows From Operating Activities:
|
||||||||
|
Net (Loss)
|
$ | (1,600,493 | ) | $ | (1,805,698 | ) | ||
|
Adjustments to reconcile net loss to net cash used in
|
||||||||
|
operating activities:
|
||||||||
|
Stock issued for licenses, services, and other assets
|
1,085,985 | 716,310 | ||||||
|
Stock issued for payment interest on notes payable
|
75,000 | 5,086 | ||||||
|
Stock issued for payment of expenses
|
60,854 | |||||||
|
Beneficial conversion feature on note conversion
|
- | 548,951 | ||||||
|
(Increase) Decrease in prepaid expenses
|
(6,419 | ) | 1,538 | |||||
|
Increase (Decrease) in Accounts Payable
|
27,511 | 8,168 | ||||||
|
Increase in interest payable
|
6,679 | 994 | ||||||
|
Net Cash Flows (used) in operations
|
(350,883 | ) | (524,651 | ) | ||||
|
Cash Flows From Investing Activities:
|
||||||||
|
Net Cash Flows (used) in Investing activities
|
- | - | ||||||
|
Cash Flows From Financing Activities:
|
||||||||
|
Proceed from note payable
|
273,500 | 463,000 | ||||||
|
Note payable used to pay expenses
|
60,000 | - | ||||||
|
Note payable used to pay origionation fees
|
50,000 | - | ||||||
|
Sale of common stock
|
110,000 | - | ||||||
|
Net Cash Flows provided by financing activities
|
493,500 | 463,000 | ||||||
|
|
||||||||
|
Net Increase (Decrease) In Cash and cash equivalents
|
142,617 | (61,651 | ) | |||||
|
Cash and cash equivalents at beginning of period
|
31,240 | 132,638 | ||||||
|
Cash and cash equivalents at end of period
|
$ | 173,857 | $ | 70,987 | ||||
|
Supplementary Disclosure Of Cash Flow Information:
|
$ | - | ||||||
|
Stock issued for services, licenses and other assets
|
$ | 818,585 | $ | 716,310 | ||||
|
Stock issued for note conversions
|
$ | - | $ | 513,000 | ||||
|
Stock issued for net deficit of MWBS
|
$ | - | $ | - | ||||
|
Stock issued for interest
|
$ | 170,000 | $ | - | ||||
|
Stock issued for payment of expenses
|
$ | 43,333 | $ | - | ||||
|
Loan proceeds used to pay expenses
|
$ | 40,000 | $ | - | ||||
|
Cash paid for interest
|
$ | - | $ | - | ||||
|
Cash paid for income taxes
|
||||||||
|
See Accompanying Notes To These Financial Statements.
|
||||||||
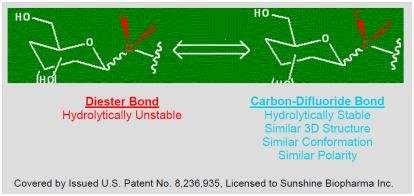
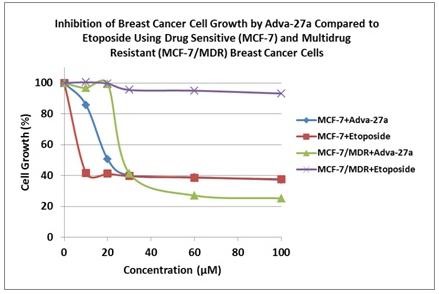
|
●
|
Adva-27a is effective at killing different types of multidrug resistant cancer cells, including:
|
|
|
-
|
Breast Cancer Cells (MCF-7/MDR)
|
|
|
-
|
Small Cell Lung Cancer Cells (H69AR)
|
|
-
|
Uterine Cancer (MES-SA/Dx5)
|
|
●
|
Adva-27a is unaffected by P-Glycoprotein, the enzyme responsible for making cancer cells resistant to anti-tumor drugs.
|
|
●
|
Adva-27a has excellent clearance time (half-life = 54 minutes) as indicated by human microsomes stability studies and pharmacokinetics data in rats.
|
|
●
|
Adva-27a clearance is independent of Cytochrome P450, a mechanism that is less likely to produce toxic intermediates.
|
|
●
|
Adva-27a is an excellent inhibitor of Topoisomerase II with an IC50 of only 13.7 micromolar (this number has recently been reduce to 1.44 micromolar as a result of resolving the two isomeric forms of Adva-27a).
|
|
●
|
Adva-27a has shown excellent pharmacokinetics profile as indicated by studies done in rats.
|
|
●
|
Adva-27a does not inhibit tubulin assembly.
|
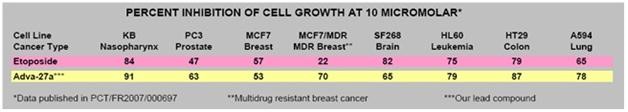
|
●
|
GMP Manufacturing of 1 kilogram for use in IND-Enabling Studies and Phase I Clinical Trials
|
|
●
|
IND-Enabling Studies
|
|
●
|
Regulatory Filing (Fast-Track Status Anticipated)
|
|
●
|
Phase I Clinical Trials (Multidrug Resistant Breast Cancer Indication)
|
Liquidity and Capital Resources
|
Exhibit No.
|
Description
|
|
|
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
||
|
Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
||
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
SUNSHINE BIOPHARMA, INC.
|
|||
|
By:
|
s/ Dr. Steve N. Slilaty
|
||
|
Dr. Steve N. Slilaty,
|
|||
|
Principal Executive Officer
|
|||
|
By:
|
s. Camille Sebaaly
|
||
|
Camille Sebaaly,
Principal Financial Officer and
|
|||
|
Principal Accounting Officer
|
|||