|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Colorado
|
|
20-5566275
|
|
(State
of other jurisdiction of incorporation)
|
|
(IRS
Employer ID No.)
|
|
|
Large
accelerated filer
☐
|
Accelerated
filer
☐
|
|
|
Non-accelerated
filer
☒
|
Smaller
reporting company
☒
|
|
|
|
Emerging
growth company
☒
|
|
|
TABLE OF
CONTENTS
|
|
|
|
PART I
FINANCIAL INFORMATION
|
Page No.
|
|
|
|
|
|
Item 1.
|
Financial Statements
|
3
|
|
|
Consolidated Balance Sheets as of March 31, 2021 and December 31,
2020 (Unaudited)
|
3
|
|
|
Consolidated Statements of Operations for the Three Month Periods
Ended March 31, 2021 and 2020 (Unaudited)
|
4
|
|
|
Consolidated Statements of Cash Flows for the Three Month Periods
Ended March 31, 2021 and 2020 (Unaudited)
|
5
|
|
|
Consolidated
Statement of
Shareholders' Equity (Unaudited)
|
6
|
|
|
Notes to Consolidated Financial Statements
|
7
|
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition
and Results of Operations
|
13
|
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market
Risk.
|
20
|
|
Item 4.
|
Controls and Procedures.
|
20
|
|
|
PART II
OTHER INFORMATION
|
|
|
|
|
|
|
Item 1.
|
Legal Proceedings
|
21
|
|
Item 1A.
|
Risk Factors
|
22
|
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of
Proceeds
|
22
|
|
Item 3.
|
Defaults Upon Senior Securities
|
22
|
|
Item 4.
|
Mine Safety Disclosures
|
22
|
|
Item 5.
|
Other Information
|
22
|
|
Item 6.
|
Exhibits
|
22
|
|
|
Signatures
|
23
|
|
|
March 31,
|
December 31,
|
|
|
2021
|
2020
|
|
|
|
|
|
ASSETS
|
|
|
|
Current
Assets:
|
|
|
|
Cash
and cash equivalents
|
$
1,796,596
|
$
989,888
|
|
Accounts
receivable
|
-
|
1,916
|
|
Inventory
|
26,660
|
23,771
|
|
Prepaid
expenses
|
10,993
|
2,778
|
|
Deposits
|
7,590
|
7,590
|
|
Total
Current Assets
|
1,841,839
|
1,025,943
|
|
|
|
|
|
Equipment
(net of $54,565 and $51,485 depreciation,
respectively)
|
16,629
|
19,531
|
|
Patents
(net of $58,918 amortization and $556,120 impairment)
|
-
|
-
|
|
|
|
|
|
TOTAL
ASSETS
|
$
1,858,468
|
$
1,045,474
|
|
|
|
|
|
LIABILITIES
|
|
|
|
Current
Liabilities:
|
|
|
|
Notes
payable
|
521,028
|
820,454
|
|
Notes
payable - related party
|
143,661
|
143,661
|
|
Accounts
payable & accrued expenses
|
148,564
|
62,870
|
|
Interest
payable
|
51,533
|
24,320
|
|
Total
Current Liabilities
|
864,786
|
1,051,305
|
|
|
|
|
|
Long-term
portion of notes payable
|
2,078,071
|
949,006
|
|
|
|
|
|
TOTAL
LIABILITIES
|
2,942,857
|
2,000,311
|
|
|
|
|
|
COMMITMENTS
AND CONTINGENCIES
|
|
|
|
|
|
|
|
SHAREHOLDERS'
EQUITY (DEFICIT)
|
|
|
|
Preferred Stock, Series B $0.10 par value per share; Authorized
1,000,000 shares;
|
|
|
|
Issued
and outstanding 1,000,000 shares
|
100,000
|
100,000
|
|
|
|
|
|
Common
Stock, $0.001 par value per share; Authorized 3,000,000,000
Shares;
|
|
|
|
Issued
and outstanding 465,005,925 and 346,419,296 March 31, 2021 and
December 31, 2020, respectively
|
465,005
|
346,418
|
|
|
|
|
|
Capital
paid in excess of par value
|
24,759,393
|
18,820,343
|
|
Accumulated
comprehensive income
|
(4,934
)
|
(2,871
)
|
|
Accumulated
(Deficit)
|
(26,403,853
)
|
(20,218,727
)
|
|
|
|
|
|
TOTAL
SHAREHOLDERS' EQUITY (DEFICIT)
|
(1,084,389
)
|
(954,837
)
|
|
|
|
|
|
TOTAL
LIABILITIES AND SHAREHOLDERS' EQUITY (DEFICIT)
|
$
1,858,468
|
$
1,045,474
|
|
|
3
Months
|
3
Months
|
|
|
Ended
|
Ended
|
|
|
March
31,
|
March
31,
|
|
|
2021
|
2020
|
|
|
|
|
|
Sales
|
$
40,058
|
$
11,102
|
|
Cost
of sales
|
18,520
|
3,883
|
|
Gross
profit
|
21,538
|
7,219
|
|
|
|
|
|
General
& Administrative Expenses:
|
|
|
|
Accounting
|
41,400
|
-
|
|
Consulting
|
10,893
|
1,724
|
|
Legal
|
7,117
|
19,347
|
|
Office
|
39,686
|
12,129
|
|
Officer
& director remuneration
|
1,021,927
|
3,830
|
|
Patenting
fees
|
6,193
|
4,377
|
|
R&D
|
166,786
|
-
|
|
Depreciation
|
3,182
|
3,511
|
|
Total
General & Administrative Expenses
|
1,297,184
|
44,918
|
|
|
|
|
|
Loss
from Operations
|
(1,275,646
)
|
(37,699
)
|
|
|
|
|
|
Other
Income (Expense):
|
|
|
|
Foreign
exchange
|
(14
)
|
10,896
|
|
Interest
expense
|
(49,711
)
|
(16,356
)
|
|
Debt
release
|
51,031
|
-
|
|
Loss
on debt conversions
|
(4,910,786
)
|
(51,100
)
|
|
Total
Other Income (Expense)
|
(4,909,480
)
|
(56,560
)
|
|
|
|
|
|
Net
Income (Loss) before income taxes
|
(6,185,126
)
|
(94,259
)
|
|
Provision
for income taxes
|
-
|
-
|
|
Net
Loss
|
(6,185,126
)
|
(94,259
)
|
|
|
|
|
|
Unrealized Loss from foreign exchange
translation
|
(2,063
)
|
(1,341
)
|
|
Comprehensive
Loss
|
(6,187,189
)
|
(95,600
)
|
|
|
|
|
|
|
|
|
|
Basic
Loss per Common Share
|
$
(0.01
)
|
$
(0.00
)
|
|
|
|
|
|
Weighted
Average Common Shares Outstanding
|
438,794,543
|
37,590,084
|
|
|
3 Months
Ended
March
31, 2021
|
3 Months
Ended
March
31, 2020
|
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
Net
Loss
|
$
(6,185,126
)
|
$
(94,259
)
|
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
|
Depreciation
and amortization
|
3,182
|
3,830
|
|
Foreign
exchange (gain) loss
|
14
|
(10,896
)
|
|
Stock
issued for services
|
918,000
|
-
|
|
Stock
issued for payment interest
|
6,851
|
4,486
|
|
Loss
on debt conversion
|
4,910,786
|
51,100
|
|
Debt
release
|
(51,031
)
|
-
|
|
(Increase)
decrease in accounts receivable
|
1,916
|
430
|
|
(Increase)
decrease in inventory
|
(2,889
)
|
2,198
|
|
(Increase)
in prepaid expenses
|
(8,215
)
|
(658
)
|
|
Increase
(decrease) in Accounts Payable & accrued expenses
|
81,568
|
1,158
|
|
Increase
(decrease) in interest payable
|
27,589
|
11,577
|
|
Net Cash Flows (Used) in Operations
|
(297,355
)
|
(31,034
)
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
|
-
|
-
|
|
Net Cash Flows (Used) in Investing Activities
|
-
|
-
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
Proceeds
from notes payable
|
1,150,000
|
-
|
|
Payments
of notes payable
|
(48,000
)
|
-
|
|
Net Cash Flows Provided by Financing Activities
|
1,102,000
|
-
|
|
|
|
|
|
Cash and Cash Equivalents at Beginning of Period
|
989,888
|
40,501
|
|
Net
increase (decrease) in cash and cash equivalents
|
804,645
|
(31,034
)
|
|
Foreign
currency translation adjustment
|
2,063
|
1,341
|
|
Cash and Cash Equivalents at End of Period
|
$
1,796,596
|
$
10,808
|
|
|
|
|
|
Supplementary Disclosure of Cash Flow Information:
|
-
|
|
|
Stock
issued for note conversions including interest
|
$
5,139,637
|
$
122,379
|
|
Cash
paid for interest
|
$
15,271
|
$
-
|
|
Cash
paid for income taxes
|
$
-
|
$
-
|
|
|
Number of Common Shares Issued
|
Common Stock
|
Capital Paid in Excess of Par Value
|
Number of Preferred Shares Issued
|
Preferred Stock
|
Comprehensive Income
|
Accumulated Deficit
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2019
|
35,319,990
|
35,320
|
16,616,426
|
500,000
|
50,000
|
(2,495
)
|
(17,434,636
)
|
(735,385
)
|
|
|
|
|
|
|
|
|
|
|
|
Common
Stock issued for the reduction of notes payable
|
|
|
|
|
|
|
|
|
|
and
payment of interest
|
24,355,427
|
24,355
|
98,024
|
|
|
|
|
122,379
|
|
|
|
|
|
|
|
|
|
|
|
Net
Loss
|
|
|
|
|
|
(1,341
)
|
(94,259
)
|
(95,600
)
|
|
|
|
|
|
|
|
|
|
|
|
Balance at March 31, 2020
|
59,675,417
|
$
59,675
|
$
16,714,450
|
500,000
|
$
50,000
|
$
(3,836
)
|
$
(17,528,895
)
|
(708,606
)
|
|
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2020
|
346,419,296
|
346,418
|
18,820,343
|
1,000,000
|
100,000
|
(2,871
)
|
(20,218,727
)
|
(954,837
)
|
|
|
|
|
|
|
|
|
|
|
|
Common
Stock issued for the reduction of notes payable
|
|
|
|
|
|
|
|
|
|
and
payment of interest
|
58,586,629
|
58,587
|
5,081,050
|
|
|
|
|
5,139,637
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued for services
|
60,000,000
|
60,000
|
858,000
|
|
|
|
|
918,000
|
|
|
|
|
|
|
|
|
|
|
|
Net
Loss
|
|
|
|
|
|
(2,063
)
|
(6,185,126
)
|
(6,187,189
)
|
|
|
|
|
|
|
|
|
|
|
|
Balance at March 31, 2021
|
465,005,925
|
$
465,005
|
$
24,759,393
|
1,000,000
|
$
100,000
|
$
(4,934
)
|
$
(26,403,853
)
|
(1,084,389
)
|
|
●
|
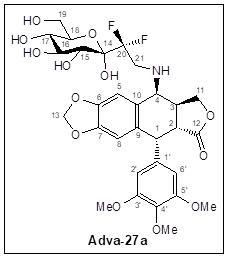
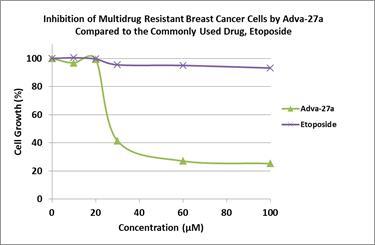
Adva-27a
clearance is independent of Cytochrome P450, a mechanism that is
less likely to produce toxic intermediates.
|
|
●
|
Adva-27a
is an excellent inhibitor of Topoisomerase II with an IC50 of only
13.7 micromolar (this number has recently been reduce to 1.44
micromolar as a result of resolving the two isomeric forms of
Adva-27a).
|
|
●
|
Adva-27a
has shown excellent pharmacokinetics profile as indicated by
studies done in rats.
|
|
●
|
Adva-27a
does not inhibit tubulin assembly.
|
|
●
|
GMP
Manufacturing of 2 kilogram for use in IND-Enabling Studies and
Phase I Clinical Trials
|
|
●
|
IND-Enabling
Studies
|
|
●
|
Regulatory
Filing (Fast-Track Status Anticipated)
|
|
●
|
Phase I
Clinical Trials (Pancreatic Cancer Indication)
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
|
Certification
of Chief Executive Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
Certification
of Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
Certification
of Chief Executive Officer and Chief Financial Officer Pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002
|
|
101.INS
|
|
XBRL
Instance Document*
|
|
101.SCH
|
|
XBRL
Schema Document*
|
|
101.CAL
|
|
XBRL
Calculation Linkbase Document*
|
|
101.DEF
|
|
XBRL
Definition Linkbase Document*
|
|
101.LAB
|
|
XBRL
Label Linkbase Document*
|
|
101.PRE
|
|
XBRL
Presentation Linkbase Document*
|
|
|
SUNSHINE BIOPHARMA, INC.
|
|
|
|
|
|
|
|
|
|
By:
|
s/ Dr.
Steve N. Slilaty
|
|
|
|
|
Dr.
Steve N. Slilaty,
|
|
|
|
|
Principal
Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
s/
Camille Sebaaly
|
|
|
|
|
Camille
Sebaaly,
Principal
Financial Officer and
|
|
|
|
|
Principal
Accounting Officer
|
|