|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
56-2181648
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
3501 C Tricenter Boulevard
Durham, North Carolina
|
|
27713
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
Common Stock, par value $0.001 per share
|
|
The NASDAQ Stock Market LLC
|
|
Securities registered pursuant to section 12(g) of the Act: None
|
||
|
Large accelerated filer
|
|
¨
|
|
Accelerated filer
|
|
¨
|
|
Non-accelerated filer
|
¨
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
|
|
ý
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
ITEM 1.
|
BUSINESS
|
|
•
|
Drug resistant strains.
There are many invasive fungal strains resistant to azole drugs. High rates of morbidity and mortality, and extended hospital stays associated with infections from drug resistant strains, will make a strong argument for use of a branded-priced antifungal drug which is effective against these resistant strains.
|
|
•
|
Alternative to echinocandins.
Physicians are reluctant to prescribe azoles in hospitals where azole resistance is prevalent, as an ineffective course of therapy can compromise the patient’s survival. Thus, in these settings, physicians often prescribe echinocandins; but echinocandins are only available in IV formulation. Subsequent step down to an oral azole to allow release from the hospital risks relapse of an azole resistant infection if the original pathogen was not identified and susceptibility determined, leading some physicians to keep patients on IV echinocandins for the full course of therapy. If successfully developed, SCY-078 would provide an attractive alternative to echinocandin therapy by offering an IV-to-oral step-down within a single therapeutic class, thereby facilitating earlier discharge from the hospital and the resultant reduced exposure to the risk of hospital-acquired infections.
|
|
•
|
further develop SCY-078 to obtain regulatory approval in major commercial markets;
|
|
•
|
commercialize SCY-078 in the United States through a focused hospital-based sales force;
|
|
•
|
contract with commercial partners to develop and commercialize SCY-078 outside of the United States; and
|
|
•
|
leverage our strong scientific team and extensive in-house expertise in human and animal drug development to pursue the development of proprietary compounds.
|




|
Design/Objective
|
|
Clinical Endpoints
|
|
Subject
Population
|
|
Dosing Regimen
|
|
Results
|
|
Phase 1, randomized, double-blind, placebo-controlled, single ascending-dose, safety, tolerability, and PK study
|
|
Safety and tolerability by physical examination, vital signs, ECGs and laboratory safety evaluations (hematology, chemistry, urinalysis), gastrin levels;
PK data in fasted state and after high fat meal
|
|
16 healthy males (18–45 years)
|
|
Panel A: 8 subjects: single doses 10, 40, 150, 600, and 1600mg SCY-078
(6 active / 2 placebo for each dose)
Panel B: 8 subjects: single doses 20, 80, 300, and 800mg SCY-078
(6 active / 2 placebo for each dose)
|
|
Safety: SCY-078 up to 1600mg was generally safe and well tolerated; no serious adverse events (SAEs) reported.
Statistical analysis of PK parameters [AUC (“area under the curve”, a measure of cumulative drug exposure over a defined post-dose time interval), Tmax (time of maximum circulating drug concentration) and Cmax (maximum circulating drug exposure)] indicated that:
1) Dose proportionality was observed for doses up to 1600 mg
2) Dosing SCY-078 drug-filled capsules with a high fat meal increased drug exposure levels by ~20% compared to levels observed in fasted subjects, which was within intersubject variability
|
|
Phase 1, double-blind randomized, single dose study to evaluate the safety, tolerability, and PK in elderly subjects
|
|
Safety and tolerability by physical examination, vital signs, ECGs and laboratory safety evaluations (hematology, chemistry, urinalysis);
PK data
|
|
17 healthy males and females (65–85 years)
|
|
Panel A: 500 mg SCY-078/Placebo
Panel B: Placebo/500 mg SCY-078
(6 active / 2 placebo for each panel)
|
|
Safety: SCY-078 generally well tolerated. One non-drug -related SAE of metastatic carcinoid tumor was reported. The most common adverse events (AEs) were gastrointestinal disorders and nervous system disorders.
Statistical analysis of PK parameters (AUC, Tmax and Cmax) indicated that exposure levels were ~30% higher in elderly patients compared to young males.
|
|
Design/Objective
|
|
Clinical Endpoints
|
|
Subject
Population
|
|
Dosing Regimen
|
|
Results
|
|
Phase 1, Open label biocomparison study of two formulations of SCY-078 and a pantoprazole interaction study with SCY-078 in healthy subjects
|
|
Safety, tolerability and PK of fit-for-purpose (FFP) drug filled capsules compared to FFP compressed tablets; impact of multiple doses of a proton pump inhibitor on single doses of SCY-078; impact of high fat meal on FFP compressed tablets
|
|
16 healthy males (18–45 years)
|
|
Periods 1 and 2: Single doses of 500 mg SCY-078 (as five 100mg FFP dry filled capsules or two 250mg FFP compressed tablets)
Period 3: Pantoprazole 40mg X 5 days and 500 mg SCY-078 (two 250mg FFP compressed tablets)
Period 4: 500 mg SCY-078 (two 250mg FFP compressed tablets) administered after a high fat meal
|
|
Safety: SCY-078 generally well tolerated. One SAE of elevated liver enzymes that led to discontinuation was reported. The most common AEs were gastrointestinal disorders.
Statistical analysis of PK parameters (AUC, Tmax and Cmax) indicated that:
1) Exposure levels in patients who received compressed tablets were ~20% higher than in those who received drug filled capsules
2) Exposure levels of SCY-078 in patients were approximately 25% lower when administered with the proton pump inhibitor pantoprazole compared to SCY-078 administered alone
3) Dosing SCY-078 tablets with a high fat meal increased drug exposure levels by ~50%–60% compared to levels observed in fasted subjects
|
|
Phase 1, randomized, double-blind, placebo-controlled, multiple ascending-dose safety, tolerability and PK study
|
|
Safety and tolerability by physical examination, vital signs, ECGs and laboratory safety evaluations (hematology, chemistry, urinalysis), gastrin levels and gastric histology;
Plasma PK data and concentrations of intact drug in urine after multiple doses of SCY-078
|
|
32 healthy males (18–45 years)
|
|
300, 600, and 800 mg SCY-078 or matching placebo once daily for 10 days, or
800 mg SCY-078 or matching placebo once daily for 28 days.
(6 active /2 placebo in each panel)
|
|
Safety: SCY-078 was generally safe and well tolerated. Most common AEs were headache, lack of energy, dizziness, and gastrointestinal disorders.
Statistical analysis of PK parameters (AUC, Tmax and Cmax) indicated that:
1) The target drug exposure level (AUC of 17µM.hr) was approached after 10 days of dosing at 600mg per day
2) Two weeks were needed to reach steady state concentrations in many subjects
3) Exposure levels were ~2.3 fold (Cmax) to 3.3 fold (AUC) higher after 26 days of dosing compared to the first day
Insignificant concentrations of SCY-078 were found in urine.
|
|
Design/Objective
|
|
Clinical Endpoints
|
|
Subject
Population
|
|
Dosing Regimen
|
|
Results
|
|
Phase 1, randomized, partially-blind, placebo-controlled study of multiple doses of ketoconazole on single dose PK of SCY-078
|
|
Safety and tolerability of SCY-078
Single dose PK profile of SCY-078 after multiple doses of ketoconazole
|
|
12 healthy males (18–45 years)
|
|
Period 1: 100 mg SCY-078 or matching placebo
Period 2: Ketoconazole 400 mg once daily for 15 days starting on Day -1 with a single dose of 100 mg SCY-078 (or placebo) coadministered on Day 1.
12 Subjects (10 active / 2 placebo)
|
|
Safety: SCY-078 was generally well tolerated when dosed alone or with ketoconazole. The most common AEs were headache and increased ALT/AST.
Statistical analysis of PK parameters (AUC, Tmax and Cmax) indicated that in the presence of ketoconzaole
1) Drug exposure as measured by AUC was ~5.7 fold higher
2) Cmax increased 2.5 fold
|
|
Phase 1, randomized, double-blind, placebo controlled multiple dose study to assess the safety, tolerability, and PK of a loading dose of SCY-078
|
|
Safety and tolerability of SCY-078; PK profile of SCY-078 after a loading dose on day 1
|
|
8 healthy males (18–45 years)
|
|
1800 mg SCY-078 (or placebo) administered as 600 mg TID (three times a day) on Day 1, followed by 500 mg SCY-078 (or placebo) QD (once daily) on Days 2-7.
8 Subjects (6 active / 2 placebo)
|
|
Safety: SCY-078 was generally well tolerated. No SAEs or discontinuations. The most common AE was diarrhea; 1 subject had elevated bilirubin.
Statistical analysis of PK parameters (AUC, Tmax and Cmax) indicated that the loading dose on day 1 achieved a target drug exposure (AUC of ~20.8µM.hr). Drug exposures observed under the QD maintenance dosing regimen were ~20.8µM.hr on Day 3 and ~16µM.hr on Day 7.
|
|
Phase 1, open-label, fixed-sequence, multiple-dose study investigating the effect of diltiazem on the PK and safety of SCY-078 in healthy subjects
|
|
Safety and tolerability of SCY-078; PK profile of SCY-078 after multiple doses of diltiazem
|
|
16 males
(20-45 years)
|
|
Treatment A (Period 1), 200 mg SCY-078 q6h (total dose of 600 mg) on Day 1 and 100 mg SCY-078 QD Days 2 to 14.
Treatment B (Period 2), 240 mg of diltiazem QD on Days 1 to 14, 200 mg of SCY-078 q6h (total dose of 600 mg) on Day 1, and 100 mg SCY-078 QD Days 2 to 14.
|
|
Safety: SCY-078 generally well tolerated. The most common AE was headache. No SAEs; 1 discontinuation due to first degree heart block following administration of diltiazem only
Statistical analysis of PK parameters (AUC, Tmax and Cmax) indicates that in the presence of diltiazem:
1) Drug exposures as measured by AUC were ~2.5 fold higher
2) Cmax was increased 2 fold
|

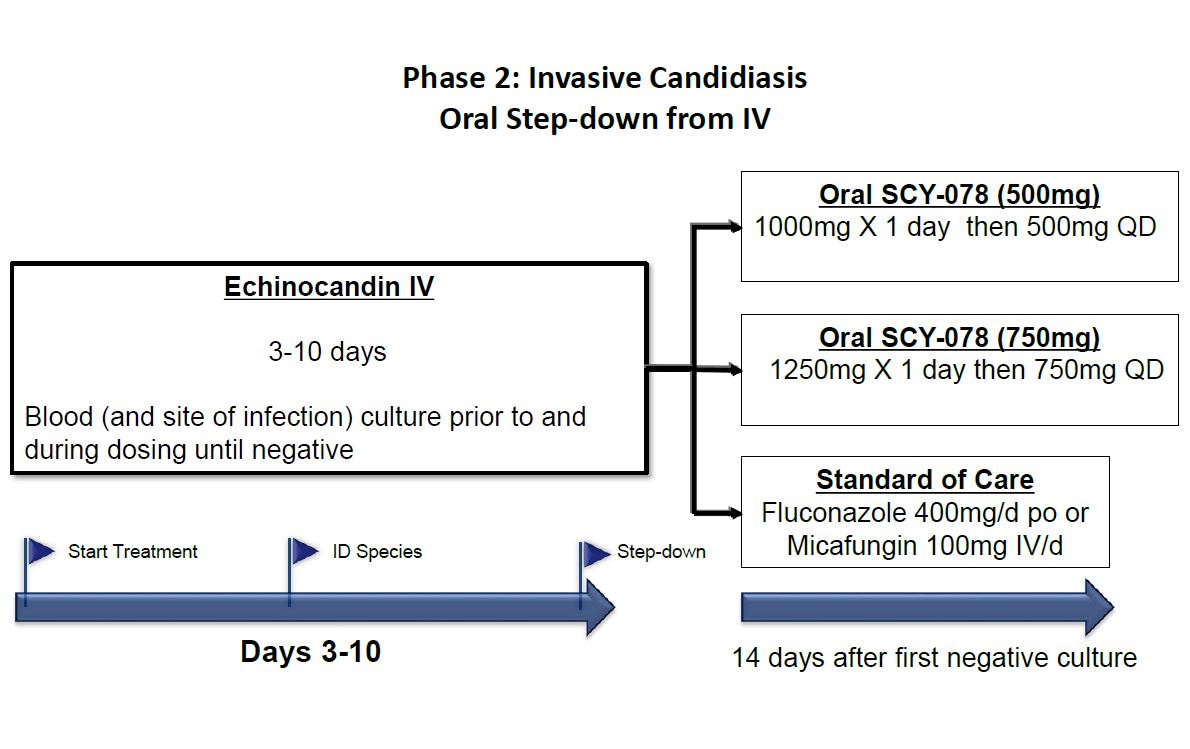
|
•
|
completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations;
|
|
•
|
submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin;
|
|
•
|
approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated;
|
|
•
|
performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug for each indication;
|
|
•
|
submission to the FDA of an NDA;
|
|
•
|
satisfactory completion of an FDA advisory committee review, if applicable;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and
|
|
•
|
FDA review and approval of the NDA.
|
|
(1)
|
caused by an antifungal resistant pathogen, including novel or emerging infectious pathogens; or
|
|
(2)
|
qualifying pathogens listed by the FDA in accordance with the GAIN Act.
|
|
•
|
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
|
|
•
|
warning letters or holds on post-approval clinical trials;
|
|
•
|
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of products; or
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
|
•
|
The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under federally funded healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the federal anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. The Affordable Care Act clarified that a person or entity need not have actual knowledge of the federal anti-kickback statute or specific intent to violate it. In addition, the Affordable Care Act amended the federal civil False Claims Act to provide that a claim that includes items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny.
|
|
•
|
The federal civil False Claims Act imposes civil penalties, and provides for whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent or knowingly making, or causing to be made, a false record or statement material to a false or fraudulent claim to avoid, decrease or conceal an obligation to pay money to the
|
|
•
|
The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
|
|
•
|
The federal criminal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact, making any materially false, fictitious, or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services.
|
|
•
|
The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires applicable pharmaceutical manufacturers of covered drugs to engage in extensive tracking of physician and teaching hospital payments, maintenance of a payments database, and public reporting of the payment data. Pharmaceutical manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program must perform such tracking and provide annual reports through the Open Payments Program. CMS posts such manufacturer disclosures on a searchable public website. Failure to comply with the reporting obligations may result in civil monetary penalties.
|
|
•
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by Medicaid or other state programs or, in several states, apply regardless of the payor. Several state laws require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to individual health care providers in those states. Some of these states also prohibit certain marketing related activities including the provision of gifts, meals or other items to certain health care providers. In addition, California, Connecticut, Nevada, and Massachusetts require pharmaceutical companies to implement compliance programs or marketing codes.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
•
|
continue the development of SCY-078;
|
|
•
|
conduct ongoing and initiate new clinical trials for SCY-078;
|
|
•
|
seek marketing approvals for SCY-078;
|
|
•
|
establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
|
|
•
|
maintain, expand and protect our intellectual property portfolio;
|
|
•
|
hire additional clinical, quality control and scientific personnel; and
|
|
•
|
maintain and create additional infrastructure to support our operations as a public company.
|
|
•
|
the costs associated with developing SCY-078, which are difficult for us to predict;
|
|
•
|
any delays in regulatory review and approval of SCY-078;
|
|
•
|
delays in the timing of submission of a new drug application, or NDA, as well as commencement, enrollment and the timing of clinical testing, of SCY-078 or any other product candidates we may seek to develop;
|
|
•
|
our ability to commercialize product candidates, both in the United States and overseas, if we are able to obtain regulatory approval to do so;
|
|
•
|
the costs associated with obtaining and maintaining regulatory approval and ongoing company compliance and product compliance for SCY-078;
|
|
•
|
the success of our providing contract research and development services;
|
|
•
|
market acceptance of SCY-078 and any future product candidates we may seek to develop;
|
|
•
|
changes in regulations and regulatory policies;
|
|
•
|
competition from existing products or new products that may emerge;
|
|
•
|
the ability of patients or healthcare providers to obtain coverage of, or sufficient reimbursement for, any products we are able to develop;
|
|
•
|
our ability to establish or maintain collaborations, licensing or other arrangements;
|
|
•
|
costs related to, and outcomes of, potential litigation;
|
|
•
|
potential product liability claims; and
|
|
•
|
potential liabilities associated with hazardous materials.
|
|
•
|
significantly delay, scale back or discontinue the development or commercialization of SCY-078 and any future product candidates we may seek to develop;
|
|
•
|
seek strategic alliances for research and development programs at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or
|
|
•
|
relinquish or license on unfavorable terms our rights to any product candidates that we otherwise would seek to develop or commercialize ourselves.
|
|
•
|
inability to reach agreements on acceptable terms with prospective clinical research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
difficulty identifying and engaging qualified clinical investigators;
|
|
•
|
regulatory objections to commencing a clinical trial or proceeding to the next phase of investigation, including inability to reach agreement with the FDA or non-U.S. regulators regarding the scope or design of our clinical trials or for other reasons such as safety concerns that might be identified during preclinical development or early stage clinical trials;
|
|
•
|
inability to identify and maintain a sufficient number of eligible trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication as our product candidates;
|
|
•
|
withdrawal of clinical trial sites from our clinical trials as a result of changing standards of care;
|
|
•
|
inability to obtain institutional review board (or ethics review committee) approval to conduct a clinical trial at prospective sites;
|
|
•
|
difficulty identifying, recruiting and enrolling eligible patients to participate in clinical trials for a variety of reasons, including meeting the enrollment criteria for our study and competition from other clinical trial programs for the same indication as product candidates we seek to commercialize;
|
|
•
|
inability to retain patients in clinical trials due to the treatment protocol, personal issues, side effects from the therapy or lack of efficacy; and
|
|
•
|
inability to obtain sufficient funding to commence a clinical trial.
|
|
•
|
failure by us, CROs or clinical investigators to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
|
|
•
|
failed inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities;
|
|
•
|
unforeseen safety or efficacy issues or any determination that a clinical trial presents unacceptable health risks; or
|
|
•
|
lack of adequate funding to continue the clinical trial due to unforeseen costs resulting from enrollment delays, requirements to conduct additional trials and studies, increased expenses associated with the services of our CROs and other third parties, or other reasons.
|
|
•
|
limitations or warnings contained in the FDA-approved labeling;
|
|
•
|
changes in the standard of care for the targeted indications;
|
|
•
|
limitations in the approved indications;
|
|
•
|
availability of alternative therapies with potentially advantageous results, or other products with similar results at similar or lower cost, including generics and over-the-counter products;
|
|
•
|
lower demonstrated clinical safety or efficacy compared to other products;
|
|
•
|
occurrence of significant adverse side effects;
|
|
•
|
ineffective sales, marketing and distribution support;
|
|
•
|
lack of availability of reimbursement from managed care plans and other third-party payors;
|
|
•
|
timing of market introduction and perceived effectiveness of competitive products;
|
|
•
|
lack of cost-effectiveness;
|
|
•
|
adverse publicity about our product candidates or favorable publicity about competitive products;
|
|
•
|
lack of convenience and ease of administration; and
|
|
•
|
potential product liability claims.
|
|
•
|
regulatory authorities may require the addition of labeling statements, specific warnings, precautions, contraindications or field alerts to physicians and pharmacies;
|
|
•
|
we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product;
|
|
•
|
we may have limitations on how we promote the product;
|
|
•
|
sales of the product may decrease significantly;
|
|
•
|
regulatory authorities may require us to take our approved product off the market;
|
|
•
|
we may be subject to litigation or product liability claims; and
|
|
•
|
our reputation may suffer.
|
|
•
|
resources, including capital, personnel and technology;
|
|
•
|
research and development capability;
|
|
•
|
clinical trial expertise;
|
|
•
|
regulatory expertise;
|
|
•
|
intellectual property portfolios;
|
|
•
|
expertise in prosecution of intellectual property rights;
|
|
•
|
manufacturing and distribution expertise; and
|
|
•
|
sales and marketing expertise.
|
|
•
|
SCY-078 and any future product candidates we may seek to develop may not generate preclinical or clinical data that are deemed sufficient by regulators in a given jurisdiction;
|
|
•
|
SCY-078 may not be approved for all indications requested, or any indications at all, in a given jurisdiction which could limit the uses of SCY-078 and any future product candidates we may seek to develop and have an adverse effect on product sales and potential royalties; and
|
|
•
|
such approval in a given jurisdiction may be subject to limitations on the indicated uses for which the product may be marketed or require costly post-marketing follow-up studies.
|
|
•
|
issue warning letters;
|
|
•
|
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners;
|
|
•
|
require us or our partners to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance;
|
|
•
|
impose other civil or criminal penalties;
|
|
•
|
suspend regulatory approval;
|
|
•
|
suspend any ongoing clinical trials;
|
|
•
|
refuse to approve pending applications or supplements to approved applications filed by us, our partners or our potential future partners;
|
|
•
|
impose restrictions on operations, including costly new manufacturing requirements; or
|
|
•
|
seize or detain products or require a product recall.
|
|
•
|
the possible breach of the manufacturing agreements or violation of regulatory standards by the third parties because of factors beyond our control; and
|
|
•
|
the possibility of termination or nonrenewal of the agreements by the third parties because of our breach of the manufacturing agreement or based on their own business priorities.
|
|
•
|
others may be able to make compounds that are similar to SCY-078 and any future product candidates we may seek to develop but that are not covered by the claims of our patents;
|
|
•
|
if we encounter delays in our clinical trials, the period of time during which we could market our drug candidates under patent protection would be reduced;
|
|
•
|
we might not have been the first to conceive, make or disclose the inventions covered by our patents or pending patent applications;
|
|
•
|
we might not have been the first to file patent applications for these inventions;
|
|
•
|
any patents that we obtain may be invalid or unenforceable or otherwise may not provide us with any competitive advantages; or
|
|
•
|
the patents of others may have a material adverse effect on our business.
|
|
•
|
successfully attract and recruit new employees with the expertise and experience we will require;
|
|
•
|
manage our clinical programs effectively, which we anticipate being conducted at numerous clinical sites;
|
|
•
|
develop a marketing and sales infrastructure; and
|
|
•
|
continue to develop our operational, financial and management controls, reporting systems and procedures.
|
|
•
|
withdrawal of clinical trial participants;
|
|
•
|
termination of clinical trial sites or entire trial programs;
|
|
•
|
costs of related litigation;
|
|
•
|
substantial monetary awards to patients or other claimants;
|
|
•
|
decreased demand for product candidates and loss of revenue;
|
|
•
|
impairment of our business reputation;
|
|
•
|
diversion of management and scientific resources from our business operations; and
|
|
•
|
the inability to commercialize product candidates.
|
|
•
|
the results of our preclinical testing or clinical trials;
|
|
•
|
the ability to obtain additional funding;
|
|
•
|
any delay in filing an NDA or similar foreign applications for SCY-078 and any future product candidate we may seek to develop or any adverse development or perceived adverse development with respect to the FDA’s review of that NDA or a foreign regulator’s review of a similar applications;
|
|
•
|
maintenance of our existing collaborations or ability to enter into new collaborations;
|
|
•
|
our collaboration partners’ election to develop or commercialize product candidates under our collaboration agreements or the termination of any programs under our collaboration agreements;
|
|
•
|
any intellectual property infringement actions in which we or our licensors and collaboration partners may become involved;
|
|
•
|
our ability to successfully develop and commercialize future product candidates;
|
|
•
|
changes in laws or regulations applicable to future products;
|
|
•
|
adverse regulatory decisions;
|
|
•
|
introduction of new products, services or technologies by our competitors;
|
|
•
|
achievement of financial projections we may provide to the public;
|
|
•
|
achievement of the estimates and projections of the investment community;
|
|
•
|
the perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community;
|
|
•
|
announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our collaboration partners or our competitors;
|
|
•
|
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
|
|
•
|
legislation or regulation that mandates or encourages the use of generic products;
|
|
•
|
additions or departures of key scientific or management personnel;
|
|
•
|
significant lawsuits, including patent or stockholder litigation;
|
|
•
|
changes in the market valuations of similar companies;
|
|
•
|
general economic and market conditions and overall fluctuations in the U.S. equity markets;
|
|
•
|
sales of our common stock by us, our executive officers and directors or our stockholders in the future; and
|
|
•
|
trading volume of our common stock.
|
|
•
|
the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting;
|
|
•
|
the obligation to provide three years of audited financial statements;
|
|
•
|
the “say on pay” provisions, requiring a non-binding stockholder vote to approve compensation of certain executive officers, and the “say on golden parachute” provisions, requiring a non-binding stockholder vote to
|
|
•
|
the requirement to provide detailed compensation discussion and analysis in proxy statements and reports filed under the Exchange Act, and instead provide a reduced level of disclosure concerning executive compensation; and
|
|
•
|
any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Year Ended December 31, 2014
|
High
|
Low
|
||||||
|
Second Quarter (beginning May 2, 2014)
|
$
|
9.89
|
|
$
|
7.78
|
|
||
|
Third Quarter
|
$
|
8.34
|
|
$
|
5.10
|
|
||
|
Fourth Quarter
|
$
|
11.98
|
|
$
|
5.70
|
|
||
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
ITEM 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
costs related to executing preclinical and clinical trials, including related drug formulation, manufacturing and other development;
|
|
•
|
salaries and personnel-related costs, including benefits and any stock-based compensation for personnel in research and development functions;
|
|
•
|
fees paid to CROs, vendors, consultants and other third parties who support our product candidate development and intellectual property protection;
|
|
•
|
other costs in seeking regulatory approval of our products; and
|
|
•
|
allocated overhead.
|
|
|
For the Year Ended December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
|
(dollars in thousands)
|
||||||
|
SCY-078
|
$
|
7,050
|
|
$
|
1,404
|
|
|
|
Cyclophilin Inhibitor Platform
|
1,237
|
|
2,953
|
|
|||
|
Other
|
—
|
|
6
|
|
|||
|
Total Research and Development
|
$
|
8,287
|
|
$
|
4,363
|
|
|
|
•
|
a related party guarantee of our outstanding credit facility;
|
|
•
|
interest on related party convertible debt;
|
|
•
|
interest expense related to a beneficial conversion feature associated with the conversion of related party convertible debt in December 2013;
|
|
•
|
fair value adjustments to our derivative liability for warrants issued in conjunction with the related party convertible debt; and
|
|
•
|
a loss on the extinguishment of debt.
|
|
|
Year Ended
|
|||||||||||||||||||
|
|
December 31, 2014
|
December 31, 2013
|
Period-to-Period
Change
|
|||||||||||||||||
|
|
Amount
|
Percentage of
Revenue
|
Amount
|
Percentage of
Revenue
|
Amount
|
Percentage
|
||||||||||||||
|
Total revenue
|
$
|
19,024
|
|
100.0
|
%
|
$
|
16,857
|
|
100.0
|
%
|
$
|
2,167
|
|
12.9
|
%
|
|||||
|
Cost of revenue
|
15,446
|
|
81.2
|
%
|
16,305
|
|
96.7
|
%
|
(859
|
)
|
(5.3
|
)%
|
||||||||
|
Gross profit
|
3,578
|
|
18.8
|
%
|
552
|
|
3.3
|
%
|
3,026
|
|
548.2
|
%
|
||||||||
|
Operating expenses:
|
|
|
||||||||||||||||||
|
Research and development
|
8,287
|
|
43.6
|
%
|
4,363
|
|
25.9
|
%
|
3,924
|
|
89.9
|
%
|
||||||||
|
Selling, general and administrative
|
7,568
|
|
39.8
|
%
|
4,381
|
|
26.0
|
%
|
3,187
|
|
72.7
|
%
|
||||||||
|
Gain on insurance recovery
|
(165
|
)
|
(0.9
|
)%
|
—
|
|
—
|
|
(165
|
)
|
*
|
|
||||||||
|
Gain on sale of asset
|
—
|
|
—
|
|
(988
|
)
|
(5.9
|
)%
|
988
|
|
(100.0
|
)%
|
||||||||
|
Total operating expenses
|
15,690
|
|
82.5
|
%
|
7,756
|
|
46.0
|
%
|
7,934
|
|
102.3
|
%
|
||||||||
|
Loss from operations
|
(12,112
|
)
|
(63.7
|
)%
|
(7,204
|
)
|
(42.7
|
)%
|
(4,908
|
)
|
68.1
|
%
|
||||||||
|
Other (income) expense:
|
|
|
|
|
||||||||||||||||
|
Amortization of deferred financing costs and debt discount
|
755
|
|
4.0
|
%
|
3,485
|
|
20.7
|
%
|
(2,730
|
)
|
(78.3
|
)%
|
||||||||
|
Loss on extinguishment of debt
|
1,389
|
|
7.3
|
%
|
—
|
|
—
|
|
1,389
|
|
*
|
|
||||||||
|
Interest expense on beneficial conversion feature
|
—
|
|
—
|
|
10,802
|
|
64.1
|
%
|
(10,802
|
)
|
(100.0
|
)%
|
||||||||
|
Interest expense — related party
|
—
|
|
—
|
|
892
|
|
5.3
|
%
|
(892
|
)
|
(100.0
|
)%
|
||||||||
|
Interest expense
|
48
|
|
—
|
|
192
|
|
1.1
|
%
|
(144
|
)
|
(75.0
|
)%
|
||||||||
|
Derivative fair value adjustment
|
(10,080
|
)
|
(53.0
|
)%
|
7,886
|
|
—
|
|
(17,966
|
)
|
(227.8
|
)%
|
||||||||
|
Other expense
|
10
|
|
0.1
|
%
|
—
|
|
—
|
|
10
|
|
*
|
|
||||||||
|
Total other (income) expense
|
(7,878
|
)
|
(41.4
|
)%
|
23,257
|
|
138.0
|
%
|
(31,135
|
)
|
(133.9
|
)%
|
||||||||
|
Net Loss
|
$
|
(4,234
|
)
|
(22.3
|
)%
|
$
|
(30,461
|
)
|
(180.7
|
)%
|
$
|
26,227
|
|
(86.1
|
)%
|
|||||
|
|
For the Year Ended December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
|
(unaudited; dollars in thousands)
|
||||||
|
Net cash used in operating activities
|
$
|
(9,472
|
)
|
$
|
(4,307
|
)
|
|
|
Net cash (used in) provided by investing activities
|
(488
|
)
|
557
|
|
|||
|
Net cash provided by financing activities
|
40,801
|
|
2,767
|
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
30,841
|
|
$
|
(983
|
)
|
|
|
•
|
the progress, costs, and the clinical development of SCY-078;
|
|
•
|
the outcome, costs and timing of seeking and obtaining FDA and any other regulatory approvals;
|
|
•
|
the ability of product candidates to progress through clinical development successfully;
|
|
•
|
our need to expand our research and development activities;
|
|
•
|
the costs associated with our continuing to support our ability to provide contract research and development services;
|
|
•
|
the costs associated with securing, establishing and maintaining commercialization and manufacturing capabilities;
|
|
•
|
our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights;
|
|
•
|
our need and ability to hire additional management and scientific and medical personnel;
|
|
•
|
our need to implement additional internal systems and infrastructure, including financial and reporting systems; and
|
|
•
|
the economic and other terms, timing and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future.
|
|
|
Years Ended
December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
|
(in thousands)
|
||||||
|
Cost of revenue
|
$
|
159
|
|
$
|
45
|
|
|
|
Research and development
|
394
|
|
28
|
|
|||
|
Selling, general and administrative
|
648
|
|
107
|
|
|||
|
Total
|
$
|
1,201
|
|
$
|
180
|
|
|
|
•
|
we do not have sufficient history to estimate the volatility of our common stock price. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies for which the historical information is available. For the purpose of identifying peer companies, we consider characteristics such as industry, length of trading history, similar vesting terms and in-the-money option status. We plan to continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants;
|
|
•
|
the assumed dividend yield is based on our expectation of not paying dividends for the foreseeable future;
|
|
•
|
we determine the average expected life of stock options based on the simplified method in accordance with SEC Staff Accounting Bulletin Nos. 107 and 110, as our common stock to date has not been publicly traded. We expect to use the simplified method until we have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term;
|
|
•
|
we determine the risk-free interest rate by reference to implied yields available from U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant; and
|
|
•
|
we estimate forfeitures based on our historical analysis of actual stock option forfeitures.
|
|
Employee Stock Options
|
Year Ended
December 31, |
||
|
|
2014
|
|
2013
|
|
Weighted average risk-free interest rate
|
2.05%
|
2.41%
|
|
|
Weighted average expected term (in years)
|
6.04
|
6.49
|
|
|
Weighted average expected volatility
|
68.57%
|
65.49%
|
|
|
Expected dividend yield
|
—%
|
—%
|
|
|
Forfeiture rate
|
5.00%
|
5.00%
|
|
|
Non-Employee Stock Options
|
Year Ended
December 31, |
||
|
|
2014
|
2013
|
|
|
Weighted average risk-free interest rate
|
1.75%
|
2.37%
|
|
|
Weighted average expected term (in years)
|
5.30
|
5.00
|
|
|
Weighted average expected volatility
|
64.10%
|
65.49%
|
|
|
Expected dividend yield
|
—%
|
—%
|
|
|
Forfeiture rate
|
5.00%
|
5.00%
|
|
|
•
|
our results of operations, financial position, status of our research and development efforts, stage of development and business strategy;
|
|
•
|
external market conditions affecting the life sciences and biotechnology industry sectors;
|
|
•
|
the prices at which we sold shares of convertible preferred stock to third-party investors;
|
|
•
|
the superior rights and preferences of the convertible preferred stock relative to our common stock at the time of each grant;
|
|
•
|
our stage of development and business strategy and the material risks related to our business and industry;
|
|
•
|
the valuation of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and acquisitions of peer companies;
|
|
•
|
the lack of an active public market for our common stock and convertible preferred stock;
|
|
•
|
the likelihood of achieving a liquidity event in light of prevailing market conditions, such as an initial public offering or sale of our company; and
|
|
•
|
any recent contemporaneous valuations prepared in accordance with methodologies outlined in the Practice Aid.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
December 31, 2014
|
December 31, 2013
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
32,243
|
|
$
|
1,402
|
|
|
|
Accounts receivable, net
|
1,118
|
|
719
|
|
|||
|
Unbilled services
|
383
|
|
343
|
|
|||
|
Prepaid expenses and other current assets
|
992
|
|
489
|
|
|||
|
Total current assets
|
34,736
|
|
2,953
|
|
|||
|
Property and equipment, net of accumulated depreciation
|
4,835
|
|
5,401
|
|
|||
|
Deferred financing costs
|
—
|
|
2,144
|
|
|||
|
Other assets
|
101
|
|
114
|
|
|||
|
Deferred offering costs
|
—
|
|
1,775
|
|
|||
|
Total assets
|
$
|
39,672
|
|
$
|
12,387
|
|
|
|
Liabilities, convertible preferred stock, and stockholders’ equity (deficit)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
855
|
|
$
|
1,932
|
|
|
|
Accrued expenses
|
2,497
|
|
1,058
|
|
|||
|
Deferred revenue, current portion
|
449
|
|
487
|
|
|||
|
Current portion of long-term debt
|
—
|
|
15,000
|
|
|||
|
Total current liabilities
|
3,801
|
|
18,477
|
|
|||
|
Deferred revenue, net of current portion
|
1,146
|
|
1,144
|
|
|||
|
Derivative liability
|
—
|
|
12,237
|
|
|||
|
Deferred rent
|
1,294
|
|
1,481
|
|
|||
|
Total liabilities
|
6,241
|
|
33,339
|
|
|||
|
Commitments and contingencies (Note 8)
|
|
|
|||||
|
Series A convertible preferred stock, $0.001 par value, authorized 0 and 31,410 shares as of December 31, 2014, and December 31, 2013; 0 and 31,407 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
—
|
|
250
|
|
|||
|
Series B convertible preferred stock, $0.001 par value, authorized 0 and 711,987 shares as of December 31, 2014, and December 31, 2013; 0 and 467,814 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
—
|
|
4,215
|
|
|||
|
Series C convertible preferred stock, $0.001 par value, authorized 0 and 2,967,678 shares as of December 31, 2014, and December 31, 2013; 0 and 2,770,633 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
—
|
|
28,121
|
|
|||
|
Series C-2 convertible preferred stock, $0.001 par value, authorized 0 and 2,347,826 shares as of December 31, 2014, and December 31, 2013; 0 and 2,347,826 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
—
|
|
13,500
|
|
|||
|
Series D-1 convertible preferred stock, $0.001 par value, authorized 0 and 10,000,000 shares as of December 31, 2014, and December 31, 2013; 0 and 6,054,255 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
—
|
|
16,952
|
|
|||
|
Series D-2 convertible preferred stock, $0.001 par value, authorized 0 and 10,000,000 shares as of December 31, 2014, and December 31, 2013; 0 and 5,742,697 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
—
|
|
24,119
|
|
|||
|
Stockholders’ equity (deficit):
|
|||||||
|
Common stock, $0.001 par value, authorized 125,000,000 and 70,000,000 shares as of December 31, 2014, and December 31, 2013; 8,512,103 and 334,068 shares issued and outstanding as of December 31, 2014, and December 31, 2013
|
8
|
|
—
|
|
|||
|
Additional paid-in capital
|
150,934
|
|
5,168
|
|
|||
|
Accumulated deficit
|
(117,511
|
)
|
(113,277
|
)
|
|||
|
Total stockholders’ equity (deficit)
|
33,431
|
|
(108,109
|
)
|
|||
|
Total liabilities, convertible preferred stock, and stockholders’ equity (deficit)
|
$
|
39,672
|
|
$
|
12,387
|
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Revenue — related party
|
$
|
7,288
|
|
$
|
7,288
|
|
|
|
Revenue
|
11,736
|
|
9,569
|
|
|||
|
Total revenue
|
19,024
|
|
16,857
|
|
|||
|
Cost of revenue
|
15,446
|
|
16,305
|
|
|||
|
Gross profit
|
3,578
|
|
552
|
|
|||
|
Operating expenses:
|
|||||||
|
Research and development
|
8,287
|
|
4,363
|
|
|||
|
Selling, general and administrative
|
7,568
|
|
4,381
|
|
|||
|
Gain on insurance recovery
|
(165
|
)
|
—
|
|
|||
|
Gain on sale of asset
|
—
|
|
(988
|
)
|
|||
|
Total operating expenses
|
15,690
|
|
7,756
|
|
|||
|
Loss from operations
|
(12,112
|
)
|
(7,204
|
)
|
|||
|
Other (income) expense:
|
|||||||
|
Amortization of deferred financing costs and debt discount
|
755
|
|
3,485
|
|
|||
|
Loss on extinguishment of debt
|
1,389
|
|
—
|
|
|||
|
Interest expense on beneficial conversion feature
|
—
|
|
10,802
|
|
|||
|
Interest expense — related party
|
—
|
|
892
|
|
|||
|
Interest expense
|
48
|
|
192
|
|
|||
|
Derivative fair value adjustment
|
(10,080
|
)
|
7,886
|
|
|||
|
Other expense
|
10
|
|
—
|
|
|||
|
Total other (income) expense:
|
(7,878
|
)
|
23,257
|
|
|||
|
Net loss
|
$
|
(4,234
|
)
|
$
|
(30,461
|
)
|
|
|
Deemed dividend for beneficial conversion feature on Series D-2 preferred stock
|
(909
|
)
|
(4,232
|
)
|
|||
|
Deemed dividend for antidilution adjustments to convertible preferred stock
|
(214
|
)
|
(6,402
|
)
|
|||
|
Accretion of convertible preferred stock
|
(510
|
)
|
(5,714
|
)
|
|||
|
Net loss attributable to common stockholders - basic
|
$
|
(5,867
|
)
|
$
|
(46,809
|
)
|
|
|
Derivative fair value adjustment
|
(10,080
|
)
|
—
|
|
|||
|
Net loss attributable to common stockholders - diluted
|
(15,947
|
)
|
(46,809
|
)
|
|||
|
Net loss per share attributable to common stockholders:
|
|||||||
|
Basic
|
$
|
(1.04
|
)
|
$
|
(139.47
|
)
|
|
|
Diluted
|
$
|
(2.69
|
)
|
$
|
(139.47
|
)
|
|
|
Weighted average common shares outstanding:
|
|||||||
|
Basic
|
5,663,311
|
|
335,612
|
|
|||
|
Diluted
|
5,937,087
|
|
335,612
|
|
|||
|
|
Series A
Convertible
Preferred
Stock
|
|
Series B
Convertible
Preferred
Stock
|
|
Series C
Convertible
Preferred
Stock
|
|
Series C-1
Convertible
Preferred
Stock
|
|
Series C-2
Convertible
Preferred
Stock
|
|
Series D-1
Convertible
Preferred
Stock
|
|
Series D-2
Convertible
Preferred
Stock
|
|
|
Common
Stock
|
|
Additional
Paid-in
Capital
|
|
Accumulated
Deficit
|
|
Total
Stockholders’
Equity (Deficit)
|
||||||||||||||||||||||
|
Balance as of December 31, 2012
|
$
|
250
|
|
$
|
4,215
|
|
$
|
28,121
|
|
$
|
—
|
|
$
|
13,500
|
|
—
|
|
—
|
|
$
|
—
|
|
$
|
17,401
|
|
$
|
(82,816
|
)
|
$
|
(65,415
|
)
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(30,461
|
)
|
(30,461
|
)
|
||||||||||||||||||||||
|
Exercise of stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5
|
|
—
|
|
5
|
|
||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
180
|
|
—
|
|
180
|
|
||||||||||||||||||||||
|
Deemed contribution on debt guarantee
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,930
|
|
—
|
|
3,930
|
|
||||||||||||||||||||||
|
Sale of preferred stock, net of issuance costs of $95 (Note 9)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,405
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||
|
Conversion of convertible notes into preferred stock (Note 7)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,476
|
|
5,540
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||
|
Reclassification of warrants issued with preferred stock to derivative liability (Note 9)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,500
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||
|
Beneficial conversion feature for antidilution adjustment (Note 9)
|
—
|
|
594
|
|
4,748
|
|
—
|
|
1,060
|
|
—
|
|
—
|
|
—
|
|
(6,402
|
)
|
—
|
|
(6,402
|
)
|
||||||||||||||||||||||
|
Beneficial conversion feature on sale of preferred stock (Note 9)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,232
|
|
—
|
|
(4,232
|
)
|
—
|
|
(4,232
|
)
|
||||||||||||||||||||||
|
Beneficial conversion feature on conversion of convertible notes (Note 7)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6,998
|
|
3,804
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||
|
Adjustment of preferred stock to liquidation value (Note 9)
|
—
|
|
(594
|
)
|
(4,748
|
)
|
—
|
|
(1,060
|
)
|
1,478
|
|
10,638
|
|
—
|
|
(5,714
|
)
|
—
|
|
(5,714
|
)
|
||||||||||||||||||||||
|
Balance as of December 31, 2013
|
$
|
250
|
|
$
|
4,215
|
|
$
|
28,121
|
|
$
|
—
|
|
$
|
13,500
|
|
$
|
16,952
|
|
$
|
24,119
|
|
$
|
—
|
|
$
|
5,168
|
|
$
|
(113,277
|
)
|
$
|
(108,109
|
)
|
|||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,234
|
)
|
(4,234
|
)
|
||||||||||||||||||||||
|
Exercise of stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
9
|
|
—
|
|
9
|
|
||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,201
|
|
—
|
|
1,201
|
|
||||||||||||||||||||||
|
Sale of preferred stock(Note 9)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
544
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||
|
Reclassification of warrants issued with preferred stock to derivative liability (Note 9)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(544
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||
|
Beneficial conversion feature for sale of preferred stock (Note 9)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
909
|
|
—
|
|
(909
|
)
|
—
|
|
(909
|
)
|
||||||||||||||||||||||
|
Beneficial conversion feature for antidilution adjustment (Note 9)
|
—
|
|
18
|
|
153
|
|
—
|
|
43
|
|
—
|
|
—
|
|
—
|
|
(214
|
)
|
—
|
|
(214
|
)
|
||||||||||||||||||||||
|
Adjustment of preferred stock to liquidation value
|
—
|
|
(18
|
)
|
(153
|
)
|
—
|
|
(43
|
)
|
—
|
|
724
|
|
—
|
|
(510
|
)
|
—
|
|
(510
|
)
|
||||||||||||||||||||||
|
Issuance of common stock from the IPO, net of underwriting discounts and commissions and offering expenses (Note 1)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6
|
|
54,577
|
|
—
|
|
54,583
|
|
||||||||||||||||||||||
|
Conversion of preferred stock into shares of common stock (Note 9)
|
(250
|
)
|
(4,215
|
)
|
(28,121
|
)
|
—
|
|
(13,500
|
)
|
(16,952
|
)
|
(25,752
|
)
|
2
|
|
88,788
|
|
—
|
|
88,790
|
|
||||||||||||||||||||||
|
Warrant derivative liability reclassified to additional paid-in capital (Note 10)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,701
|
|
—
|
|
2,701
|
|
||||||||||||||||||||||
|
Exercise of common stock warrants (Note 10)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
55
|
|
—
|
|
55
|
|
||||||||||||||||||||||
|
Issuance of common stock - ESPP
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
68
|
|
—
|
|
68
|
|
||||||||||||||||||||||
|
Balance as of December 31, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
8
|
|
$
|
150,934
|
|
$
|
(117,511
|
)
|
$
|
33,431
|
|
|||||||||||
|
|
Year Ended December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Cash flows from operating activities:
|
|||||||
|
Net loss
|
$
|
(4,234
|
)
|
$
|
(30,461
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||
|
Beneficial conversion feature on convertible notes — related party
|
—
|
|
10,802
|
|
|||
|
Gain on insurance recovery
|
(165
|
)
|
—
|
|
|||
|
Gain on sale of asset, net of transaction expenses
|
—
|
|
(988
|
)
|
|||
|
Loss on extinguishment of debt
|
1,389
|
|
—
|
|
|||
|
Allowance for bad debts
|
—
|
|
(10
|
)
|
|||
|
Depreciation
|
1,238
|
|
1,329
|
|
|||
|
Stock-based compensation expense
|
1,201
|
|
180
|
|
|||
|
Amortization of deferred financing costs and debt discount
|
755
|
|
3,485
|
|
|||
|
Change in fair value of derivative liability
|
(10,080
|
)
|
7,886
|
|
|||
|
Changes in deferred rent
|
(187
|
)
|
(53
|
)
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable and unbilled services
|
(439
|
)
|
1,366
|
|
|||
|
Prepaid expenses, other assets, and deferred costs
|
(490
|
)
|
(102
|
)
|
|||
|
Accounts payable and accrued expenses
|
1,575
|
|
(82
|
)
|
|||
|
Interest payable — related party
|
—
|
|
892
|
|
|||
|
Deferred revenue
|
(35
|
)
|
1,449
|
|
|||
|
Net cash used in operating activities
|
(9,472
|
)
|
(4,307
|
)
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Proceeds from insurance recovery
|
216
|
|
—
|
|
|||
|
Proceeds from sale of asset, net of transaction expenses
|
—
|
|
988
|
|
|||
|
Purchases of property and equipment
|
(704
|
)
|
(431
|
)
|
|||
|
Net cash (used in) provided by investing activities
|
(488
|
)
|
557
|
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Proceeds from initial public offering
|
62,000
|
|
|
|
|||
|
Proceeds from issuance of convertible notes
|
—
|
|
899
|
|
|||
|
Proceeds from sale of preferred stock
|
544
|
|
2,500
|
|
|||
|
Preferred stock issuance costs
|
—
|
|
(95
|
)
|
|||
|
Repayment of debt
|
(15,000
|
)
|
|
|
|||
|
Payments of deferred offering costs and underwriting discounts and commissions
|
(6,875
|
)
|
(542
|
)
|
|||
|
Proceeds from employee stock purchase plan issuance
|
68
|
|
—
|
|
|||
|
Proceeds from exercise of stock warrants
|
55
|
|
—
|
|
|||
|
Proceeds from exercise of stock options
|
9
|
|
5
|
|
|||
|
Net cash provided by financing activities
|
40,801
|
|
2,767
|
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
30,841
|
|
(983
|
)
|
|||
|
Cash and cash equivalents, beginning of period
|
1,402
|
|
2,385
|
|
|||
|
Cash and cash equivalents, end of period
|
$
|
32,243
|
|
$
|
1,402
|
|
|
|
Supplemental cash flow information:
|
|||||||
|
Cash paid for interest
|
$
|
49
|
|
$
|
197
|
|
|
|
Noncash financing and investing activities:
|
|||||||
|
Conversion of convertible notes into preferred stock
|
$
|
—
|
|
$
|
14,016
|
|
|
|
Beneficial conversion feature on sale of Series D-2 preferred stock
|
$
|
909
|
|
$
|
4,232
|
|
|
|
Beneficial conversion feature for antidilution adjustment
|
$
|
214
|
|
$
|
6,402
|
|
|
|
Adjustment of preferred stock to redemption value
|
$
|
510
|
|
$
|
5,714
|
|
|
|
Issuance of warrants allocated to debt discount
|
$
|
906
|
|
$
|
1,168
|
|
|
|
Deemed contribution of a loan guarantee
|
$
|
—
|
|
$
|
3,930
|
|
|
|
Issuance of warrants with preferred stock
|
$
|
—
|
|
$
|
2,500
|
|
|
|
Equipment purchase in accounts payable and accrued expenses
|
$
|
34
|
|
$
|
15
|
|
|
|
Impairment of fixed asset
|
$
|
51
|
|
$
|
—
|
|
|
|
Deferred offering costs included in accounts payable
|
$
|
—
|
|
$
|
1,233
|
|
|
|
Deferred offering costs reclassified to additional paid-in capital
|
$
|
4,126
|
|
$
|
—
|
|
|
|
Warrant derivative liability reclassified to additional paid-in capital
|
$
|
2,701
|
|
$
|
—
|
|
|
|
Conversion of convertible preferred stock to common stock
|
$
|
88,790
|
|
$
|
—
|
|
|
|
1.
|
Description of Business and Basis of Preparation
|
|
2.
|
Summary of Significant Accounting Policies
|
|
Year Ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Research and development expense, gross
|
$
|
8,513
|
|
$
|
4,363
|
|
||
|
Less: Reimbursement of research and development expense
|
226
|
|
—
|
|
||||
|
Research and development expense, net of reimbursements
|
$
|
8,287
|
|
$
|
4,363
|
|
||
|
•
|
Level 1 — Observable inputs that reflect quoted market prices (unadjusted) for identical assets or liabilities in active markets;
|
|
•
|
Level 2 — Observable inputs other than quoted prices in active markets that are observable either directly or indirectly in the marketplace for identical or similar assets and liabilities; and
|
|
•
|
Level 3 — Unobservable inputs that are supported by little or no market data, which require the Company to develop its own assumptions about the assumptions market participants would use in pricing the asset or liability based on the best information available in the circumstances.
|
|
|
Year Ended December 31,
|
||||||||||||
|
|
2014
|
2013
|
|||||||||||
|
United States
|
$
|
16,422
|
|
86
|
%
|
$
|
15,126
|
|
90
|
%
|
|||
|
Europe
|
1,235
|
|
7
|
%
|
1,626
|
|
10
|
%
|
|||||
|
Other non-US
|
1,367
|
|
7
|
%
|
105
|
|
—
|
|
|||||
|
Total
|
$
|
19,024
|
|
100
|
%
|
$
|
16,857
|
|
100
|
%
|
|||
|
Balance at
Beginning of
Period
|
Additions
Charged
to Expense
|
Deductions
|
Balance at
End of
Period
|
||||||||||||
|
Year ended December 31, 2013
|
$
|
251
|
|
$
|
—
|
|
$
|
(88
|
)
|
$
|
163
|
|
|||
|
Year ended December 31, 2014
|
$
|
163
|
|
$
|
—
|
|
$
|
(163
|
)
|
$
|
—
|
|
|||
|
|
December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Prepaid SCY-078 development services
|
$
|
149
|
|
$
|
—
|
|
|
|
Prepaid service contracts
|
67
|
|
86
|
|
|||
|
Prepaid insurance
|
295
|
|
97
|
|
|||
|
Other prepaid expenses
|
252
|
|
301
|
|
|||
|
Other receivable due from R-Pharm
|
226
|
|
—
|
|
|||
|
Other current assets
|
3
|
|
5
|
|
|||
|
$
|
992
|
|
$
|
489
|
|
||
|
5.
|
Property and Equipment
|
|
December 31,
2014 |
December 31,
2013 |
||||||
|
Equipment
|
$
|
8,552
|
|
$
|
9,577
|
|
|
|
Furniture and fixtures
|
375
|
|
378
|
|
|||
|
Leasehold improvements
|
13,193
|
|
13,115
|
|
|||
|
Total property and equipment
|
22,120
|
|
23,070
|
|
|||
|
Less accumulated depreciation
|
17,285
|
|
17,669
|
|
|||
|
Property and equipment, net
|
$
|
4,835
|
|
$
|
5,401
|
|
|
|
|
December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Accrued research and development expenses
|
$
|
293
|
|
$
|
102
|
|
|
|
Accrued employee bonus compensation
|
1,464
|
|
—
|
|
|||
|
Interest payable
|
—
|
|
23
|
|
|||
|
Employee withholdings
|
156
|
|
61
|
|
|||
|
Other accrued expenses
|
584
|
|
872
|
|
|||
|
$
|
2,497
|
|
$
|
1,058
|
|
||
|
7.
|
Debt Obligations
|
|
8.
|
Commitments and Contingencies
|
|
2015
|
$
|
1,075
|
|
|
2016
|
1,104
|
|
|
|
2017
|
1,123
|
|
|
|
2018
|
1,156
|
|
|
|
2019
|
291
|
|
|
|
Thereafter
|
—
|
|
|
|
Total
|
$
|
4,749
|
|
|
9.
|
Convertible Preferred Stock
|
|
Authorized
|
Issued and
Outstanding
|
||||
|
Series A Preferred
|
31,410
|
|
31,407
|
|
|
|
Series B Preferred
|
711,987
|
|
467,814
|
|
|
|
Series C Preferred
|
2,967,678
|
|
2,770,633
|
|
|
|
Series C-1 Preferred
|
3,076,923
|
|
—
|
|
|
|
Series C-2 Preferred
|
2,347,826
|
|
2,347,826
|
|
|
|
Series D-1 Preferred
|
10,000,000
|
|
6,054,255
|
|
|
|
Series D-2 Preferred
|
10,000,000
|
|
6,131,338
|
|
|
|
Total
|
29,135,824
|
|
17,803,273
|
|
|
|
|
Shares of
|
|||||||||||||||||||
|
|
Series A
Convertible
Preferred
Stock
|
Series B
Convertible
Preferred
Stock
|
Series C
Convertible
Preferred
Stock
|
Series C-1
Convertible
Preferred
Stock
|
Series C-2
Convertible
Preferred
Stock
|
Series D-1
Convertible
Preferred
Stock
|
Series D-2
Convertible
Preferred
Stock
|
|||||||||||||
|
Balance, December 31, 2012
|
31,407
|
|
467,814
|
|
2,770,633
|
|
—
|
|
2,347,826
|
|
—
|
|
—
|
|
||||||
|
Issuance of Series D-2 Preferred
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,785,712
|
|
||||||
|
Conversion of notes payable
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6,054,255
|
|
3,956,985
|
|
||||||
|
Balance, December 31, 2013
|
31,407
|
|
467,814
|
|
2,770,633
|
|
—
|
|
2,347,826
|
|
6,054,255
|
|
5,742,697
|
|
||||||
|
Issuance of Series D-2 Preferred
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
388,641
|
|
||||||
|
Automatic conversion to common stock
|
(31,407
|
)
|
(467,814
|
)
|
(2,770,633
|
)
|
—
|
|
(2,347,826
|
)
|
(6,054,255
|
)
|
(6,131,338
|
)
|
||||||
|
Balance, December 31, 2014
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||
|
10.
|
Common Stock
|
|
|
Shares of
Common Stock
|
|
|
Balance, December 31, 2012
|
335,782
|
|
|
Exercise of stock options
|
220
|
|
|
Repurchase of common stock
|
(1,934
|
)
|
|
Balance, December 31, 2013
|
334,068
|
|
|
Exercise of stock options
|
416
|
|
|
Conversion of preferred stock
|
1,691,884
|
|
|
Exercise of common stock warrants
|
275,687
|
|
|
Common stock issued through IPO
|
6,200,000
|
|
|
Common stock issued through employee stock purchase plan
|
10,048
|
|
|
Balance, December 31, 2014
|
8,512,103
|
|
|
As of
|
As of
|
||||
|
|
December 31,
2014 |
December 31,
2013 |
|||
|
For conversion of Series A Preferred, Series B Preferred, Series C Preferred, Series C-2 Preferred, Series D-1 Preferred, and Series D-2 Preferred and exercise of warrants to purchase Series C-1 Preferred and subsequent conversion of the shares purchased
|
—
|
|
1,675,812
|
|
|
|
Outstanding stock options
|
615,322
|
|
137,610
|
|
|
|
Outstanding common stock warrants
|
—
|
|
257,242
|
|
|
|
Outstanding Series C-1 convertible preferred stock warrants
|
14,033
|
|
13,879
|
|
|
|
For possible future issuance under stock option plan
|
180,610
|
|
49,734
|
|
|
|
For possible future issuance under employee stock purchase plan
|
37,746
|
|
—
|
|
|
|
Total common shares reserved for future issuance
|
847,711
|
|
2,134,277
|
|
|
|
11.
|
Stock-based Compensation
|
|
|
Employees
|
Nonemployees
|
|||||
|
|
Year Ended December 31,
|
Year Ended December 31,
|
|||||
|
|
2014
|
2013
|
2014
|
2013
|
|||
|
Expected dividend yield
|
—
|
—
|
—
|
—
|
|||
|
Weighted average expected volatility
|
68.57%
|
65.49%
|
64.10%
|
65.49%
|
|||
|
Weighted average risk-free interest rate
|
2.05%
|
2.41%
|
1.75%
|
2.37%
|
|||
|
Weighted average expected term (in years)
|
6.04
|
6.49
|
5.30
|
5.00
|
|||
|
Forfeiture rate
|
5.00%
|
5.00%
|
5.00%
|
5.00%
|
|||
|
Number
of
Shares
|
Weighted-
Average
Exercise
Price
|
Weighted-
Average
Remaining
Contractual
Life (in years)
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Outstanding — January 1, 2013
|
154,277
|
|
$
|
23.05
|
|
5.02
|
$
|
213
|
|
|||
|
Granted
|
11,525
|
|
50.39
|
|
||||||||
|
Exercised
|
(220
|
)
|
21.01
|
|
||||||||
|
Canceled
|
(27,972
|
)
|
20.81
|
|
||||||||
|
Outstanding — December 31, 2013
|
137,610
|
|
$
|
25.86
|
|
5.23
|
$
|
3,097
|
|
|||
|
Exercisable — December 31, 2013
|
115,475
|
|
$
|
23.26
|
|
4.50
|
$
|
2,898
|
|
|||
|
Vested or expected to vest — December 31, 2013
|
136,002
|
|
$
|
25.70
|
|
5.18
|
$
|
3,084
|
|
|||
|
Outstanding — January 1, 2014
|
137,610
|
|
$
|
25.86
|
|
5.23
|
$
|
3,097
|
|
|||
|
Granted
|
520,887
|
|
$
|
9.53
|
|
(1)
|
||||||
|
Exercised
|
(416
|
)
|
$
|
20.40
|
|
|||||||
|
Canceled
|
(42,759
|
)
|
$
|
9.57
|
|
|||||||
|
Outstanding — December 31, 2014
|
615,322
|
|
$
|
9.55
|
|
9.48
|
$
|
265
|
|
|||
|
Exercisable — December 31, 2014
|
192,916
|
|
$
|
9.44
|
|
9.48
|
$
|
88
|
|
|||
|
Vested or expected to vest —December 31, 2014
|
572,926
|
|
$
|
9.55
|
|
9.48
|
$
|
247
|
|
|||
|
(1)
|
The weighted average exercise price table takes into consideration the effect of the option award modifications approved by the Company's board of directors on June 18, 2014, and approved by the Company's shareholders on September 11, 2014. These option award modifications are described in further detail below.
|
|
|
Outstanding
|
Exercisable
|
||||||||
|
Exercise
Price
|
Number
of Shares
|
Weighted-
Average
Remaining
Contractual
Life (in years)
|
Number
of Shares
|
Weighted-
Average
Remaining
Contractual
Life (in years)
|
||||||
|
$6.77
|
8,000
|
|
9.47
|
—
|
|
0.00
|
||||
|
7.70
|
5,944
|
|
9.76
|
5,944
|
|
9.76
|
||||
|
8.03
|
5,700
|
|
9.50
|
5,700
|
|
9.50
|
||||
|
8.73
|
35,114
|
|
9.45
|
3,494
|
|
9.45
|
||||
|
9.64
|
543,374
|
|
9.47
|
176,248
|
|
9.47
|
||||
|
10.81
|
17,190
|
|
9.93
|
1,530
|
|
9.93
|
||||
|
615,322
|
|
|
192,916
|
|
|
|||||
|
As of December 31, 2014
|
As of December 31, 2013
|
|||||||
|
Exercise
Price
|
Number of
Unvested Shares
|
Exercise
Price
|
Number of
Unvested Shares
|
|||||
|
$6.77
|
8,000
|
|
$20.40
|
563
|
|
|||
|
7.70
|
—
|
|
24.48
|
7,318
|
|
|||
|
8.03
|
—
|
|
25.50
|
—
|
|
|||
|
8.73
|
31,620
|
|
25.91
|
—
|
|
|||
|
9.64
|
367,127
|
|
30.60
|
4,307
|
|
|||
|
10.81
|
15,660
|
|
55.08
|
9,947
|
|
|||
|
422,407
|
|
22,135
|
|
|||||
|
•
|
On April 29, 2014, the exercise price per share of certain options to purchase
53,404
shares of common stock under the 2009 Stock Option Plan was lowered to an amount equal to
$10.00
per share. The original exercise prices of such options ranged from
$20.40
to
$61.20
per share, with a weighted average exercise price of
$54.87
per share.
|
|
•
|
On June 18, 2014, the exercise price per share of all outstanding options to purchase shares of common stock under the 2009 Stock Option Plan was lowered to an amount equal to
$9.64
per share, the closing stock price on June 18, 2014. This modification lowered the exercise price of outstanding options to purchase
110,346
shares of common stock, including those options to purchase common stock that were previously modified on April 29, 2014. These outstanding stock options had exercise prices that ranged from
$20.40
to
$61.20
per share, with a weighted average exercise price of
$41.87
per share.
|
|
•
|
Also on June 18, 2014, the contractual term of all outstanding options to purchase shares of common stock under the 2009 Stock Option Plan was extended to June 17, 2024.
|
|
|
Year Ended December 31,
|
|||||||
|
|
2014
|
2013
|
||||||
|
Cost of revenue
|
$
|
159
|
|
$
|
45
|
|
||
|
Research and development
|
394
|
|
28
|
|
||||
|
Selling, general and administrative
|
648
|
|
107
|
|
||||
|
$
|
1,201
|
|
$
|
180
|
|
|||
|
12.
|
Income Taxes
|
|
|
Year Ended December 31,
|
||||||||||||
|
|
2014
|
2013
|
|||||||||||
|
|
Amount
|
Percent of
Pretax Income
|
Amount
|
Percent of
Pretax Income
|
|||||||||
|
Income taxes at statutory rate
|
$
|
(1,440
|
)
|
34.0
|
%
|
$
|
(10,356
|
)
|
34.0
|
%
|
|||
|
State income taxes
|
(467
|
)
|
11.0
|
%
|
(127
|
)
|
0.5
|
%
|
|||||
|
Beneficial conversion feature on convertible notes
|
—
|
|
—
|
%
|
3,673
|
|
(12.1
|
)%
|
|||||
|
Stock warrant derivative liability
|
(3,427
|
)
|
80.9
|
%
|
2,686
|
|
(8.8
|
)%
|
|||||
|
Debt discount amortization
|
—
|
|
—
|
%
|
392
|
|
(1.3
|
)%
|
|||||
|
Deemed contribution interest
|
—
|
|
—
|
%
|
1,516
|
|
(5.0
|
)%
|
|||||
|
Provision to return adjustments
|
—
|
|
—
|
%
|
(184
|
)
|
0.6
|
%
|
|||||
|
Stock compensation
|
322
|
|
(7.6
|
)%
|
57
|
|
(0.2
|
)%
|
|||||
|
Expiration of capital loss carryforward.
|
—
|
|
—
|
%
|
1,511
|
|
(5.0
|
)%
|
|||||
|
Change in statutory state income tax rate
|
—
|
|
—
|
%
|
1,000
|
|
(3.3
|
)%
|
|||||
|
Change in reserve for uncertain tax positions
|
—
|
|
—
|
%
|
623
|
|
(2.0
|
)%
|
|||||
|
R&D tax credits
|
(320
|
)
|
7.6
|
%
|
—
|
|
—
|
%
|
|||||
|
Other
|
57
|
|
(1.3
|
)%
|
3
|
|
—
|
%
|
|||||
|
Increase in valuation allowance
|
5,275
|
|
(124.6
|
)%
|
(794
|
)
|
2.6
|
%
|
|||||
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
||||
|
|
December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Current deferred tax assets (liabilities):
|
|||||||
|
Accrued expenses
|
$
|
1,701
|
|
$
|
1,286
|
|
|
|
Stock-based compensation
|
336
|
|
241
|
|
|||
|
Other
|
16
|
|
88
|
|
|||
|
2,053
|
|
1,615
|
|
||||
|
Noncurrent deferred tax assets (liabilities)
|
|||||||
|
Net operating loss carryforwards
|
29,981
|
|
26,286
|
|
|||
|
Research and development credits
|
2,684
|
|
2,373
|
|
|||
|
Depreciation
|
1,232
|
|
1,201
|
|
|||
|
Deferred financing costs
|
—
|
|
(800
|
)
|
|||
|
33,897
|
|
29,060
|
|
||||
|
Total deferred tax assets
|
35,950
|
|
30,675
|
|
|||
|
Valuation allowance
|
(35,950
|
)
|
(30,675
|
)
|
|||
|
Net deferred tax asset
|
$
|
—
|
|
$
|
—
|
|
|
|
|
December 31,
|
||||||
|
|
2014
|
|
2013
|
||||
|
Unrecognized tax benefit—January 1
|
$
|
623
|
|
$
|
—
|
|
|
|
Additions for tax positions of current period
|
—
|
|
333
|
|
|||
|
Additions for tax positions of prior periods
|
—
|
|
290
|
|
|||
|
Other
|
—
|
|
—
|
|
|||
|
Unrecognized tax benefit—December 31
|
$
|
623
|
|
$
|
623
|
|
|
|
13.
|
Net Loss Per Share
|
|
|
Year Ended
December 31, |
||||||
|
|
2014
|
2013
|
|||||
|
Net loss
|
$
|
(4,234
|
)
|
$
|
(30,461
|
)
|
|
|
Deemed dividend for beneficial conversion feature on Series D-2 Preferred
|
(909
|
)
|
(4,232
|
)
|
|||
|
Deemed dividend for antidilution adjustments to convertible preferred stock
|
(214
|
)
|
(6,402
|
)
|
|||
|
Accretion of convertible preferred stock
|
(510
|
)
|
(5,714
|
)
|
|||
|
Net loss attributable to common stock - basic
|
$
|
(5,867
|
)
|
$
|
(46,809
|
)
|
|
|
Derivative fair value adjustment
|
(10,080
|
)
|
—
|
|
|||
|
Net loss attributable to common stock - diluted
|
$
|
(15,947
|
)
|
$
|
(46,809
|
)
|
|
|
Weighted-average common shares outstanding - basic
|
5,663,311
|
|
335,612
|
|
|||
|
Incremental shares from assumed exercise of common stock warrants
|
273,776
|
|
—
|
|
|||
|
Weighted-average of outstanding common stock - diluted
|
5,937,087
|
|
335,612
|
|
|||
|
Net loss per share
|
|||||||
|
Basic
|
$
|
(1.04
|
)
|
$
|
(139.47
|
)
|
|
|
Diluted
|
$
|
(2.69
|
)
|
$
|
(139.47
|
)
|
|
|
|
Year Ended
December 31, |
||||
|
|
2014
|
2013
|
|||
|
Convertible preferred stock:
|
|||||
|
Series A Preferred
|
6,149
|
|
6,149
|
|
|
|
Series B Preferred
|
131,685
|
|
130,529
|
|
|
|
Series C Preferred
|
783,515
|
|
776,016
|
|
|
|
Series C-2 Preferred
|
173,213
|
|
170,967
|
|
|
|
Series D-1 Preferred
|
296,773
|
|
296,773
|
|
|
|
Series D-2 Preferred
|
300,549
|
|
281,500
|
|
|
|
Warrants to purchase Series C-1 Preferred
|
14,033
|
|
13,878
|
|
|
|
Warrants to purchase common stock
|
—
|
|
257,242
|
|
|
|
Stock options
|
615,322
|
|
137,610
|
|
|
|
ESPP
|
65,401
|
|
—
|
|
|
|
Convertible notes
|
—
|
|
—
|
|
|
|
14.
|
Related-Party Transactions
|
|
|
Year Ended December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Revenue
|
$
|
7,288
|
|
$
|
7,288
|
|
|
|
Selling, general and administrative expense
|
500
|
|
—
|
|
|||
|
17.
|
Fair Value Measurements
|
|
Balance as of
December 31, 2013
|
Quoted Prices in
Active Markets for
Identical Assets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
||||||||||||
|
Derivative liability — Series C-1 warrants
|
$
|
37
|
|
$
|
—
|
|
$
|
—
|
|
$
|
37
|
|
|||
|
Derivative liability — common stock warrants
|
12,200
|
|
—
|
|
—
|
|
12,200
|
|
|||||||
|
Total derivative liability
|
$
|
12,237
|
|
$
|
—
|
|
$
|
—
|
|
$
|
12,237
|
|
|||
|
Year Ended December 31,
|
|||||||
|
|
2014
|
2013
|
|||||
|
Balance at beginning of period
|
$
|
12,237
|
|
$
|
683
|
|
|
|
Issuance of warrants
|
544
|
|
5,382
|
|
|||
|
Excess of fair value of warrants over proceeds
|
362
|
|
(1,714
|
)
|
|||
|
Adjustment to fair value
|
(10,442
|
)
|
7,886
|
|
|||
|
Reclassification to additional paid-in capital upon exercise of warrants
|
(2,701
|
)
|
—
|
|
|||
|
Balance at end of period
|
$
|
—
|
|
$
|
12,237
|
|
|
|
18.
|
Significant Agreements
|
|
19.
|
Assessment of Strategic Alternatives
|
|
20.
|
Subsequent Events
|
|
•
|
Pursuant to the terms of the 2014 Plan (Note 11), on January 1, 2015, the Company automatically added
340,484
shares to the total number shares of common stock available for future issuance under the 2014 Plan.
|
|
•
|
In connection with the resignation of the Company's Chief Medical Officer, Dr. Carole Sable, effective as of February 20, 2015, the Company returned
57,452
shares to the total number shares of common stock available for future issuance under the 2014 Plan. The returned shares represent Dr. Sable's unvested shares as of the effective date of her resignation.
|
|
•
|
In February 2015, the board of directors appointed Dr. Marco Taglietti as Chief Executive Officer of the Company, effective as of April 1, 2015. In connection with Dr. Taglietti’s appointment as Chief Executive Officer, Dr. Taglietti will be granted an option to purchase
330,000
shares of the Company's common stock, subject to approval by the board of directors. The option shall be granted as of the effective date of Dr. Taglietti's employment, shall have a ten-year term and one-fourth of the shares subject to the option shall vest on the one-year anniversary of the date of grant with the remainder vesting in equal monthly installments for thirty-six
|
|
•
|
On February 25, 2015, the Company's board of directors approved an amendment of the 2014 Plan, subject to stockholder approval at the Company's 2015 annual meeting of stockholders, to increase the aggregate number of shares of common stock that may be issued pursuant to awards under the 2014 Plan by an additional
510,726
shares. All other material terms of the 2014 Plan otherwise remain unchanged.
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
Name
|
Age
|
Position
|
||
|
Yves J. Ribeill, Ph.D.*
|
55
|
President, Chief Executive Officer and Director (4)
|
||
|
Charles F. Osborne, Jr.*
|
49
|
Chief Financial Officer
|
||
|
Pamela J. Kirby, Ph.D.
|
61
|
Chairman of our Board of Directors (5)
|
||
|
Laurent Arthaud
|
52
|
Director (1)(2)(5)
|
||
|
Steven C. Gilman, Ph.D.
|
62
|
Director (1)(3)(6)
|
||
|
Ann F. Hanham, Ph.D.
|
62
|
Director (1)(3)
|
||
|
Patrick J. Langlois, Ph.D.
|
69
|
Director (1)(2)
|
||
|
Guy Macdonald
|
55
|
Director (2)
|
||
|
Jean-Yves Nothias, Ph.D.
|
53
|
Director (3)(5)
|
||
|
Edward E. Penhoet, Ph.D.
|
74
|
Director (2)(3)
|
||
|
Marco Taglietti, M.D.
|
55
|
Director (7)
|
||
|
(1)
|
Member of the audit committee
|
|
(2)
|
Member of the compensation committee
|
|
(3)
|
Member of the nominating and corporate governance committee
|
|
(4)
|
Dr. Ribeill is expected to resign as our Chief Executive Officer effective as of April 1, 2015 and continue employment with us as our President and a member of our board of directors.
|
|
(5)
|
In a meeting of our board of directors on March 26, 2015, the board determined to submit all current members as nominees, other than Pamela J. Kirby, Ph.D., Laurent Arthaud and Jean-Yves Nothias, Ph.D., to be elected by our stockholders at our 2015 annual meeting, to serve on our board until the 2016 annual meeting.
|
|
(6)
|
In February 2015, our board of directors appointed Dr. Gilman as a member of the board of directors, effective February 25, 2015.
|
|
(7)
|
In February 2015, our board of directors appointed Dr. Taglietti as our Chief Executive Officer, effective April 1, 2015.
|
|
•
|
reviewing and pre-approving the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services;
|
|
•
|
evaluating the performance of our independent registered public accounting firm and deciding whether to retain their services;
|
|
•
|
reviewing our annual and quarterly financial statements and reports and discussing the statements and reports with our independent registered public accounting firm and management, including a review of disclosures under the section of this annual report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations;”
|
|
•
|
considering and approving or disapproving of all related party transactions;
|
|
•
|
preparing the audit committee report required by the SEC to be included in our annual proxy statement;
|
|
•
|
reviewing, with our independent registered public accounting firm and management, significant issues that may arise regarding accounting principles and financial statement presentation, as well as matters concerning the scope, adequacy and effectiveness of our financial controls;
|
|
•
|
conducting an annual assessment of the performance of the audit committee and its members, and the adequacy of its charter; and
|
|
•
|
establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters.
|
|
•
|
determining the compensation and other terms of employment of our chief executive officer and our other executive officers and reviewing and approving corporate performance goals and objectives relevant to the compensation;
|
|
•
|
reviewing and recommending to the full board of directors the compensation of our non-employee directors;
|
|
•
|
evaluating, adopting and administering the equity incentive plans, compensation plans, and similar programs advisable for us, as well as modification or termination of existing plans and programs;
|
|
•
|
establishing policies with respect to equity compensation arrangements;
|
|
•
|
reviewing and discussing annually with management our “Compensation Discussion and Analysis” if required by SEC rules;
|
|
•
|
preparing the compensation committee report if required by the SEC to be included in our annual proxy statement; and
|
|
•
|
reviewing and evaluating, at least annually, the performance of the compensation committee and the adequacy of its charter.
|
|
•
|
reviewing periodically and evaluating director performance on our board of directors and its applicable committees, and recommending to our board of directors and management areas for improvement;
|
|
•
|
interviewing, evaluating, nominating and recommending individuals for membership on our board of directors;
|
|
•
|
reviewing and recommending to our board of directors any amendments to our corporate governance policies; and
|
|
•
|
reviewing and assessing, at least annually, the performance of the nominating and corporate governance committee and the adequacy of its charter.
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
Name and
Principal Position
|
Year
|
Salary
|
Bonus
|
Option
awards (1)
|
All other
compensation
|
Total
|
||||||||||||||||||||
|
Yves J. Ribeill, Ph.D.
|
2014
|
$
|
337,556
|
|
$
|
195,600
|
|
$
|
798,432
|
|
(2)
|
$
|
9,654
|
|
(5)
|
$
|
1,341,242
|
|
||||||||
|
President and Chief Executive Officer
|
2013
|
250,146
|
|
—
|
|
—
|
|
11,904
|
|
262,050
|
|
|||||||||||||||
|
Charles F. Osborne, Jr.
|
2014
|
272,127
|
|
59,999
|
|
237,771
|
|
(3)
|
9,172
|
|
579,069
|
|
||||||||||||||
|
Chief Financial Officer
|
2013
|
250,205
|
|
—
|
|
—
|
|
8,955
|
|
259,160
|
|
|||||||||||||||
|
Carole Sable
|
2014
|
336,762
|
|
86,520
|
|
1,579,657
|
|
(4)
|
4,483
|
|
2,007,422
|
|
||||||||||||||
|
Chief Medical Officer (6)
|
2013
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
(1)
|
The amounts in this column reflect the aggregate grant date fair value of each option award granted during the fiscal year, as computed in accordance with FASB ASC Topic 718. The amounts in this column also reflect the aggregate incremental fair value of all option awards modified during the fiscal year, as computed in accordance with FASB ASC Topic 718. The term modifications, which were approved by our board of directors on June 18, 2014 and by our shareholders on September 11, 2014, were made to all option awards granted pursuant to the 2009 Plan and 1999 Plan. The modified terms, the valuation methodologies, and assumptions used in determining grant date fair value or incremental fair value, are described in Note 11 to our financial statements included in this annual report.
|
|
(2)
|
This amount represents the sum of (i) $702,789, the aggregate grant date fair value of an option award granted during the fiscal year and (ii) $95,643, the aggregate incremental fair value of all option awards modified during the fiscal year. The grant date fair value per share of the option award granted during the fiscal year was $3.76 and the weighted average incremental fair value per share of option awards modified during the fiscal year was $3.10. Includes an option granted June 18, 2014, with an exercise price equal to $9.64 per share, of which 2.08% of the shares subject to the option vest monthly for 48 months as measured from the date of grant, provided executive continues to provide continuous services to us.
|
|
(3)
|
This amount represents the sum of (i) $215,738, the aggregate grant date fair value of an option award granted during the fiscal year and (ii) $22,033, the aggregate incremental fair value of all option awards modified during the fiscal year. The grant date fair value per share of the option award granted during the fiscal year was $3.76 and the weighted average incremental fair value per share of option awards modified during the fiscal year was $2.77. Includes an option granted June 18, 2014, with an exercise price equal to $9.64 per share, of which 2.08% of the shares subject to the option vest monthly for 48 months as measured from the date of grant, provided executive continues to provide continuous services to us.
|
|
(4)
|
This amount represents the sum of (i) $1,422,640, the aggregate grant date fair value of all option awards granted during the fiscal year and (ii) $157,017, the aggregate incremental fair value of an option award modified during the fiscal year. The weighted average grant date fair value of the option awards granted during the fiscal year was $19.33 and the incremental fair value per share of the option award modified during the fiscal year was $3.37. Includes an option granted January 16, 2014, and an option granted June 18, 2014. Both option grants have an exercise price equal to $9.64 per share, of which 2.08% of the shares subject to the option vest monthly for 48 months as measured from the date of grant, provided executive continues to provide continuous services to us.
|
|
(5)
|
Includes tax preparation payments in the amount of $600, short term/long term disability premiums in the amount of $1,130 and life insurance premiums in the amount of $420. Also includes $7,504 contributed to his 401(k) plan account.
|
|
(6)
|
Dr. Sable terminated her employment with us effective February 20, 2015.
|
|
|
Number of Securities Underlying
Unexercised options
|
|
Option
exercise
Price
|
Option
expiration
Date
|
||||||||
|
Name
|
Exercisable(1)
|
Unexercisable
|
|
|||||||||
|
Yves J. Ribeill, Ph.D.
|
7,352
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
||||
|
7,352
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
931
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
3,676
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
2,941
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
3,676
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
2,941
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
1,960
|
|
—
|
|
$
|
9.64
|
|
6/17/2024
|
|||||
|
27,278
|
|
159,774
|
|
(2)
|
$
|
9.64
|
|
6/17/2024
|
||||
|
Charles F. Osborne, Jr.
|
960
|
|
$
|
9.64
|
|
6/17/2024
|
||||||
|
934
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
490
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
1,225
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
808
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
1,225
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
1,470
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
833
|
|
$
|
9.64
|
|
6/17/2024
|
|||||||
|
8,373
|
|
49,047
|
|
(2)
|
$
|
9.64
|
|
6/17/2024
|
||||
|
Carole Sable, M.D.
|
—
|
|
46,636
|
|
(3)
|
$
|
9.64
|
|
6/17/2024
|
|||
|
3,933
|
|
23,037
|
|
(2)
|
$
|
9.64
|
|
6/17/2024
|
||||
|
(1)
|
The options listed are fully vested or are subject to an early exercise right and may be exercised in full prior to vesting of the shares underlying such options. Vesting of all options is subject to continued service on the applicable vesting date.
|
|
(2)
|
2.08% of the shares subject to the option vest monthly for 48 months as measured from the date of grant provided executive continues to provide continuous services to us.
|
|
(3)
|
25% of the shares subject to this option vested on January 16, 2015, and 6.25% of the shares subject to the option vest for each of the next 12 quarterly anniversaries thereafter, provided executive continues to provide continuous services to us. Dr. Sable terminated her employment with us effective February 20, 2015.
|
|
•
|
a cash amount equal to a portion (twelve months as was the case for Dr. Sable and is the case for Mr. Osborne, or 24 months in the case of Dr. Ribeill and Dr. Taglietti, upon his commencement of services as our CEO) of the executive officer’s then current base salary, which shall be paid over twelve months (as was the case for Dr. Sable and is the case for Mr. Osborne) or 24 months (in the case of Dr. Ribeill and Dr. Taglietti, upon his commencement of services as our CEO) commencing with the first payroll period following the termination date; and
|
|
•
|
payment of the same percentage of the COBRA premiums for continued medical, dental, and vision group health coverage as we paid prior to the executive officers termination, until the earlier of (a) twelve months (as was the case or Dr. Sable and is the case for Mr. Osborne) or 24 months (in the case of Dr. Ribeill and Dr. Taglietti, upon his commencement of services as our CEO) after termination of employment, (b) such time as the executive officer becomes enrolled in the group health insurance plan of another employer or (c) the executive officer becomes entitled to Medicare after the COBRA election.
|
|
•
|
a cash amount equal to a portion (six months as was the case for Dr. Sable and is the case for Mr. Osborne or twelve months in the case of Dr. Ribeill and Dr. Taglietti, upon his commencement of services as our CEO) of the executive officer’s then current base salary, which shall be paid over six months (as was the case or Dr. Sable and is the case for Mr. Osborne) or twelve months (in the case of Dr. Ribeill and Dr. Taglietti, upon his commencement of services as our CEO) commencing with the first payroll period following the termination date; and
|
|
•
|
payment of the same percentage of the COBRA premiums for continued medical, dental, and vision group health coverage as we paid prior to the executive officers termination, until the earlier of (a) six months (as was the case or Dr. Sable and is the case for Mr. Osborne) or twelve months (in the case of Dr. Ribeill and Dr. Taglietti, upon his commencement of services as our CEO) after termination of employment, (b) such time as the executive officer becomes enrolled in the group health insurance plan of another employer or (c) the executive officer becomes entitled to Medicare after the COBRA election.
|
|
Name
|
Fees Earned or Paid in Cash
|
Option Award(s) (1)
|
Total
|
|||||||||||
|
Pamela J. Kirby, Ph.D.
|
$
|
—
|
|
$
|
92,573
|
|
(4)
|
$
|
92,573
|
|
||||
|
Laurent Arthaud
|
27,666
|
|
27,544
|
|
(5)
|
55,210
|
|
|||||||
|
Mounia Chaoui, Ph.D. (2)
|
20,000
|
|
17,121
|
|
(6)
|
37,121
|
|
|||||||
|
Steven C. Gilman, Ph.D. (3)
|
—
|
|
—
|
|
—
|
|
||||||||
|
Ann F. Hanham, Ph.D.
|
—
|
|
39,921
|
|
(7)
|
39,921
|
|
|||||||
|
Patrick J. Langlois, Ph.D.
|
30,000
|
|
41,536
|
|
(8)
|
71,536
|
|
|||||||
|
Guy Macdonald
|
11,250
|
|
46,255
|
|
(9)
|
57,505
|
|
|||||||
|
Jean-Yves Nothias, Ph.D.
|
—
|
|
35,471
|
|
(10)
|
35,471
|
|
|||||||
|
Edward E. Penhoet, Ph.D.
|
27,000
|
|
17,121
|
|
(11)
|
44,121
|
|
|||||||
|
Marco Taglietti, Ph.D.
|
—
|
|
55,068
|
|
(12)
|
55,068
|
|
|||||||
|
(1)
|
The amounts in this column reflect the aggregate grant date fair value of each option award granted during the fiscal year, as computed in accordance with FASB ASC Topic 718. Additionally, for certain directors indicated in the following footnotes, the amounts in this column also reflect the aggregate incremental fair value of all option awards modified during the fiscal year, as computed in accordance with FASB ASC Topic 718. The valuation methodologies and assumptions used in determining such amounts are described in Note 11 to our financial statements included in this annual report. The table below lists the aggregate number of shares and additional information with respect to the outstanding option awards held by each of our non-employee directors as of December 31, 2014.
|
|
(2)
|
Dr. Chaoui resigned from the board of directors effective November 27, 2014.
|
|
(3)
|
Dr. Gilman was appointed to our board of directors effective February 25, 2015.
|
|
(4)
|
This amount represents the sum of (i) $50,701, the grant date fair value of all option awards granted during the fiscal year and (ii) $41,870, the aggregate incremental fair value of all option awards modified during the fiscal year. The
|
|
(5)
|
This amount represents the sum of (i) $17,121, the grant date fair value of an option award granted during the fiscal year and (ii) $10,423, the aggregate incremental fair value of all option awards modified during the fiscal year. The grant date fair value per share of the option award granted during the fiscal year was $4.92 and the weighted average incremental fair value per share of option awards modified during the fiscal year was $2.36.
|
|
(6)
|
This amount represents the grant date fair value of an option award granted during the fiscal year pursuant to our non-employee director compensation policy as more completely described below. The grant date fair value per share of the option award granted during the fiscal year was $4.92.
|
|
(7)
|
This amount represents the grant date fair value of all option awards granted during the fiscal year. The weighted average grant date fair value per share of option awards granted during the fiscal year was $4.59. Includes the aggregate grant date fair value of $22,800 for options, exercisable for an aggregate of 5,216 shares of our common stock issued in lieu of cash payments made on June 11, 2014, July 1, 2014, and October 1, 2014 pursuant to our non-employee director compensation policy as more completely described below.
|
|
(8)
|
This amount represents the sum of (i) $17,121, the grant date fair value of all option awards granted during the fiscal year pursuant to our non-employee director compensation policy as more completely described below and (ii) $24,415, the aggregate incremental fair value of all option awards modified during the fiscal year. The grant date fair value per share of the option award granted during the fiscal year was $4.92 and the weighted average incremental fair value per share of option awards modified during the fiscal year was $2.43.
|
|
(9)
|
This amount represents the grant date fair value of an option award granted during the fiscal year pursuant to our non-employee director compensation policy as more completely described below. The grant date fair value per share of the option award granted during the fiscal year was $5.91.
|
|
(10)
|
This amount represents the grant date fair value of all option awards granted during the fiscal year pursuant to our non-employee director compensation policy as more completely described below. The weighted average grant date fair value per share of option awards granted during the fiscal year was $4.62. Includes the aggregate grant date fair value of $18,350 for options, exercisable for an aggregate of 4,198 shares of our common stock issued in lieu of cash payments made on June 11, 2014, July 1, 2014, and October 1, 2014 pursuant to our non-employee director compensation policy as more completely described below.
|
|
(11)
|
This amount represents the grant date fair value of an option award granted during the fiscal year pursuant to our non-employee director compensation policy as more completely described below. The grant date fair value per share of the option award granted during the fiscal year was $4.92.
|
|
(12)
|
This amount represents the grant date fair value of all option awards granted during the fiscal year. The weighted average grant date fair value per share of option awards granted during the fiscal year was $5.88. Includes the aggregate grant date fair value of $8,812 for options, exercisable for an aggregate of 1,530 shares of our common stock issued in lieu of cash payments made on December 2, 2014 pursuant to our non-employee director compensation policy as more completely described below.
|
|
Name
|
Number of shares subject to outstanding options as of December 31, 2014
|
||
|
Pamela J. Kirby, Ph.D.
|
26,037
|
|
|
|
Laurent Arthaud
|
7,890
|
|
|
|
Mounia Chaoui, Ph.D. (1)
|
3,480
|
|
|
|
Steven C. Gilman, Ph.D. (2)
|
—
|
|
|
|
Ann F. Hanham, Ph.D.
|
8,696
|
|
|
|
Patrick J. Langlois, Ph.D.
|
13,525
|
|
|
|
Guy Macdonald
|
7,830
|
|
|
|
Jean-Yves Nothias, Ph.D.
|
7,678
|
|
|
|
Edward E. Penhoet, Ph.D.
|
3,480
|
|
|
|
Marco Taglietti, Ph.D.
|
9,360
|
|
|
|
(1)
|
Dr. Chaoui resigned from the board of directors effective November 27, 2014.
|
|
(2)
|
Dr. Gilman was appointed to our board of directors effective February 25, 2015.
|
|
a.
|
The chairperson of the audit committee receives an annual cash retainer of $15,000 for this service, paid quarterly, and each of the other members of the audit committee receives an annual cash retainer of $7,000, paid quarterly.
|
|
b.
|
The chairperson of the compensation committee receives an annual cash retainer of $10,000 for this service, paid quarterly, and each of the other members of the compensation committee receive an annual cash retainer of $5,000, paid quarterly.
|
|
c.
|
The chairperson of the nominating and corporate governance committee receive an annual cash retainer of $7,000 for this service, paid quarterly, and each of the other members of the nominating and corporate governance committee receive an annual cash retainer of $3,500, paid quarterly.
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
•
|
each of our directors and named executive officers;
|
|
•
|
all of our directors and executive officers as a group; and
|
|
•
|
each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock.
|
|
Name of Beneficial Owner
|
Number of Shares
Beneficially Owned
|
Percentage Total
|
|||
|
5% Stockholders:
|
|||||
|
Alta BioPharma Partners II, L.P. and
affiliate (1) |
1,286,311
|
|
15.11
|
%
|
|
|
Deerfield Management Company, L.P. (2)
|
500,000
|
|
5.87
|
%
|
|
|
Foresite Capital Fund II, L.P. (3)
|
662,945
|
|
7.79
|
%
|
|
|
GlaxoSmithKline plc (4)
|
545,009
|
|
6.40
|
%
|
|
|
RA Capital Management, LLC (5)
|
840,000
|
|
9.87
|
%
|
|
|
Sanofi (6)
|
1,677,057
|
|
19.70
|
%
|
|
|
Named Executive Officers and Directors:
|
|||||
|
Yves J. Ribeill, Ph.D. (7)
|
92,860
|
|
1.09
|
%
|
|
|
Charles F. Osborne, Jr.(8)
|
30,469
|
|
*
|
|
|
|
Carole Sable (9)
|
17,839
|
|
*
|
|
|
|
Pamela J. Kirby, Ph.D. (10)
|
48,045
|
|
*
|
|
|
|
Laurent Arthaud (11)
|
9,652
|
|
*
|
|
|
|
Steven C. Gilman, Ph.D. (12)
|
—
|
|
—
|
%
|
|
|
Ann F. Hanham, Ph.D. (13)
|
7,263
|
|
*
|
|
|
|
Patrick J. Langlois, Ph.D. (14)
|
21,417
|
|
*
|
|
|
|
Guy MacDonald (15)
|
1,738
|
|
*
|
|
|
|
Jean-Yves Nothias, Ph.D. (16)
|
300,549
|
|
3.53
|
%
|
|
|
Edward E. Penhoet, Ph.D.
|
—
|
|
—
|
%
|
|
|
Marco Taglietti (17)
|
3,190
|
|
*
|
|
|
|
All executive officers and directors as a group (12 persons)(18)
|
533,022
|
|
6.26
|
%
|
|
|
(1)
|
Based on a Schedule 13G filed with the SEC on May 14, 2014, reporting beneficial ownership as of May 7, 2014. Alta BioPharma Partners II, L.P. (“ABPII”) has sole voting and dispositive control over 1,240,575 shares, except that Alta BioPharma Management II, LLC (“ABMII”), the general partner of ABPII, and Farah Champsi (“Champsi”), the director of ABMII, may be deemed to share the right to direct the voting and dispositive control over such stock. Alta Embarcadero BioPharma Partners II, LLC (“AEBPII”) has sole voting and dispositive control over 45,736 shares of Common Stock, except that Champsi, the managing director of AEBPII, may be deemed to share the right to direct the voting and dispositive control over such stock. Champsi shares voting and dispositive control over the 1,240,575 shares of common stock beneficially owned by ABPII, and the 45,736 shares of Common Stock beneficially owned by AEBPII. The address for Alta Partners II, Inc. is One Embarcadero Center, 37th Floor, San Francisco, California 94111.
|
|
(2)
|
Based on a Schedule 13G filed with the SEC on February 17, 2015, reporting beneficial ownership as of December 31, 2014. Deerfield Mgmt, L.P. has shared voting investment power with respect to 500,000 shares, Deerfield Management Company, L.P. has shared voting investment power with respect to 500,000 shares, Deerfield Partners, L.P. has shared voting investment power with respect to 0 shares, Deerfield Special Situations Fund, L.P. has shared voting investment power with respect to 273,000 shares, Deerfield International Master Fund, L.P. has shared voting investment power with respect to 0 shares, Deerfield Special Situations International Master Fund, L.P. has shared voting investment power with respect to 227,000 shares, and James E. Flynn has shared voting and dispositive power with respect to 500,000 shares. The address for Deerfield Management Company is 780 Third Avenue, 37th Floor, New York, NY 10017.
|
|
(3)
|
Based on a Schedule 13G filed with the SEC on February 11, 2015, reporting beneficial ownership as of May 2, 2014. Foresite Capital Fund II, L.P. has sole voting investment power with respect to 662,945 shares, except that Foresite Capital Management II, LLC, the general partner of FCF II, may be deemed to have sole power to vote these shares, and James Tanabaum, the managing member of FCM II, may be deemed to have sole power to vote these shares. The address for Foresite Capital Management is 101 California Street, Suite 4100, San Francisco, 94111.
|
|
(4)
|
Based on a Schedule 13G filed with the SEC on May 15, 2014, reporting beneficial ownership as of May 7, 2014. Shares are held of record by S.R. One, Limited, an indirect, wholly-owned subsidiary. The address for GlaxoSmithKline plc is 980 Great West Road, Brentford, Middlesex, TW8 9GS England.
|
|
(5)
|
Based on a Schedule 13G filed with the SEC on February 17, 2015, reporting beneficial ownership as of December 31, 2014. RA Capital Management, LLC has shared voting investment power with respect to 840,000 shares, Peter Kolchinsky has shared voting investment power with respect to 840,000 shares, and RA Capital Healthcare Fund, L.P. has shared voting investment power with respect to 699,720 shares. Each of the Reporting Persons disclaims beneficial ownership of the shares reported herein except to the extent of its or his pecuniary interest therein. The address for RA Capital Management is 980 Great West Road, Brentford, Middlesex, TW8 9GS England.
|
|
(6)
|
Based on a Schedule 13G filed with the SEC on February 13, 2015, reporting beneficial ownership as of December 31, 2014. The address for Sanofi is 54 Rue La Boetie, 75008 Paris, France.
|
|
(7)
|
Includes 73,695 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015.
|
|
(8)
|
Includes 21,103 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015.
|
|
(9)
|
Includes 17,839 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015. Dr. Sable’s employment with us terminated on February 20, 2015.
|
|
(10)
|
Includes 22,555 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015.
|
|
(11)
|
Includes 4,410 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015, and 4,507 shares held by Mr. Arthaud’s spouse.
|
|
(12)
|
Dr. Gillman was appointed to our board of directors on February 25, 2015.
|
|
(13)
|
Includes 7,263 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015.
|
|
(14)
|
Consists of shares held by DFC Langlois and includes 10,914 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015 held by Dr. Langlois. Dr. Langlois is a general partner of DFC Langlois
|
|
(15)
|
Includes 1,738 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015.
|
|
(16)
|
Consists of shares held by FCPR Biotechnology and includes 5,472 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015 held by Dr. Nothias. Dr. Nothias disclaims beneficial ownership of the shares held by FCPR Biotechnology, except to the extent of his ability to direct the voting or disposition of such shares or his pecuniary interest therein.
|
|
(17)
|
Includes 3,190 shares of common stock issuable upon exercise of options exercisable within 60 days of January 31, 2015.
|
|
(18)
|
Consists of shares held by each executive officer and director, including the shares described in footnotes 7 through 17 above.
|
|
Plan Category
|
|
Number of
Securities to
be Issued upon Exercise of Outstanding Options |
|
Weighted Average
Exercise Price of Outstanding Options |
|
Number of Securities
Remaining Available for Future Issuances under Equity Compensation Plans (excluding securities reflected in column (a)) |
||||
|
|
|
(a)
|
|
(b)
|
|
(c)
|
||||
|
Equity Compensation Plans approved by security holders
|
|
615,322
|
|
9.55
|
|
218,356
|
|
(1)(2)
|
||
|
Equity Compensation Plans not approved by security holders
|
|
—
|
|
—
|
|
—
|
|
|||
|
Total
|
|
615,322
|
|
9.55
|
|
218,356
|
|
|||
|
(1)
|
Pursuant to terms of the SCYNEXIS, Inc. 2014 Equity Incentive Plan, the share reserve will automatically increase on January 1st of each year, for a period of not more than ten years, commencing on January 1, 2015, and ending on (and including) January 1, 2024, in an amount equal to 4.0% of the total number of shares of capital stock outstanding on December 31st of the preceding calendar year. Notwithstanding the foregoing, the Board may act prior to January 1st of a given year to provide that there will be no January 1st increase in the Share Reserve for such year or that the increase in the share reserve for such year will be a lesser number of shares of common stock than would otherwise occur pursuant to the preceding sentence. On January 1, 2015, pursuant to the previously described terms, the share reserve was increased by 340,484 shares.
|
|
(2)
|
Pursuant to terms of the SCYNEXIS, Inc. 2014 Employee Stock Purchase Plan, the maximum number of common stock shares available under the plan will automatically increase on January 1 of each year for a period of up to ten years, commencing on the first January 1 following the IPO Date and ending on (and including) January 1, 2024, in an amount equal to the lesser of (i) 0.8% of the total number of shares of Capital Stock outstanding on December 31 of the preceding fiscal year, and (ii) 29,411 shares of Common Stock. Notwithstanding the foregoing, the Board may act prior to the first day of any fiscal year to provide that there will be no January 1 increase in the share reserve for such fiscal year or that the increase in the share reserve for such fiscal year will be a lesser number of shares of common stock than would otherwise occur pursuant to the preceding sentence. On January 1, 2015, pursuant to the previously described terms, the maximum number of common stock shares available under the plan was increased by 29,411 shares.
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
•
|
the amounts involved exceeded or will exceed $120,000; and
|
|
•
|
any of our directors, executive officers, holders of more than 5% of our capital stock, or any affiliate of our directors, executive officers and holders of more than 5% of our capital stock, had or will have a direct or indirect material interest.
|
|
Purchasers (1)
|
Warrant Shares
|
Aggregate Purchase Price
|
|||||
|
Alta BioPharma Partners II, LP(2)
|
91,558
|
|
$
|
18,312
|
|
||
|
Alta Embarcadero BioPharma Partners II, LLC(2)
|
3,382
|
|
676
|
|
|||
|
F.C.P.R. Genavent
|
14,005
|
|
2,801
|
|
|||
|
FCPR Biotechnology Fund(3)
|
36,503
|
|
7,301
|
|
|||
|
Ventech Capital II(4)
|
71,715
|
|
14,343
|
|
|||
|
(1)
|
See “Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates.
|
|
(2)
|
Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABP II and manager of AEBP II.
|
|
(3)
|
FCPR Biotechnology Fund was a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund.
|
|
(4)
|
Ventech Capital II was a holder of more than 5% of our capital stock. Dr. Chaoui, was a member of our board of directors and was a venture partner of Ventech Capital II.
|
|
Purchasers(1)
|
Shares
Purchased
|
Warrant
Shares
|
Aggregate
Purchase Price
|
|||||||
|
Alta BioPharma Partners II, LP(2)
|
1,205,648
|
|
59,100
|
|
$
|
1,687,907
|
|
|||
|
Alta Embarcadero BioPharma Partners II, LLC(2)
|
44,352
|
|
2,174
|
|
62,093
|
|
||||
|
F.C.P.R. Genavent
|
71,428
|
|
3,501
|
|
99,999
|
|
||||
|
FCPR Biotechnology Fund(3)
|
107,142
|
|
5,251
|
|
149,999
|
|
||||
|
Ventech Capital II(4)
|
357,142
|
|
17,506
|
|
499,999
|
|
||||
|
(1)
|
See “Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates.
|
|
(2)
|
Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABP II and manager of AEBP II.
|
|
(3)
|
FCPR Biotechnology Fund was a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund.
|
|
(4)
|
Ventech Capital II was a holder of more than 5% of our capital stock. Dr. Chaoui, was a member of our board of directors and was a venture partner of Ventech Capital II.
|
|
Purchasers (1)
|
Shares
Purchased
|
Warrant
Shares
|
Aggregate
Purchase Price
|
|||||||
|
Pamela Kirby, Ph.D.
|
260,000
|
|
12,745
|
|
$
|
364,000
|
|
|||
|
DFC Langlois(2)
|
107,142
|
|
5,251
|
|
$
|
149,999
|
|
|||
|
Yves J. Ribeill, Ph.D.
|
12,142
|
|
595
|
|
$
|
16,999
|
|
|||
|
(1)
|
See “Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters” for more information about these directors and executive officers.
|
|
(2)
|
Patrick J. Langlois, Ph.D. is a member of our board of directors and is the general partner of DFC Langlois.
|
|
Purchasers(1)
|
Series D-1
Shares
|
Series D-2
Shares
|
Warrant
Shares
|
||||||
|
Alta BioPharma Partners II, LP(2)
|
1,024,876
|
|
211,667
|
|
32,456
|
|
|||
|
Alta Embarcadero BioPharma Partners II, LLC(2)
|
37,702
|
|
9,563
|
|
1,207
|
|
|||
|
Burrill Biotechnology Capital Fund(3)
|
885,481
|
|
94,712
|
|
8,402
|
|
|||
|
F.C.P.R. Genavent
|
955,215
|
|
270,028
|
|
10,502
|
|
|||
|
FCPR Biotechnology Fund(4)
|
863,672
|
|
516,738
|
|
31,249
|
|
|||
|
Ventech Capital II(5)
|
809,584
|
|
2,653,665
|
|
54,208
|
|
|||
|
S.R. One, Limited
|
762,944
|
|
185,570
|
|
24,609
|
|
|||
|
(1)
|
See “Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates.
|
|
(2)
|
Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABPII and manager of AEBP II.
|
|
(3)
|
Burrill Biotechnology Capital Fund, L.P. was a holder of more than 5% of our capital stock. Dr. Hanham, a member of our board of directors, is a former Managing Director and General Partner with Burrill & Company, an affiliate of Burrill Biotechnology Capital Fund, L.P.
|
|
(4)
|
FCPR Biotechnology Fund was a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund.
|
|
(5)
|
Ventech Capital II was a holder of more than 5% of our capital stock. Dr. Chaoui, was a member of our board of directors and was a venture partner of Ventech Capital II.
|
|
Purchasers(1)
|
Principal
Amount of
Notes
|
Initial
Warrant
Shares
|
Warrant Shares
After
Adjustment
|
||||||
|
Alta BioPharma Partners II, LP(2)
|
$
|
1,300,000
|
|
2,954
|
|
9,102
|
|
||
|
Alta Embarcadero BioPharma Partners II, LLC(2)
|
50,000
|
|
112
|
|
348
|
|
|||
|
Burrill Biotechnology Capital Fund, L.P.(3)
|
1,200,000
|
|
2,726
|
|
8,402
|
|
|||
|
F.C.P.R. Genavent
|
1,500,000
|
|
3,408
|
|
10,502
|
|
|||
|
FCPR Biotechnology Fund(4)
|
1,500,000
|
|
3,408
|
|
10,502
|
|
|||
|
Ventech Capital II(5)
|
4,000,000
|
|
9,092
|
|
28,010
|
|
|||
|
S.R. One, Limited
|
1,000,000
|
|
2,272
|
|
7,002
|
|
|||
|
(1)
|
See “Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates.
|
|
(2)
|
Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a managing director of Alta BioPharma Management II, LLC, the general partner of ABPII and manager of AEBP II.
|
|
(3)
|
Burrill Biotechnology Capital Fund, L.P. was a holder of more than 5% of our capital stock. Dr. Hanham, a member of our board of directors, is a former Managing Director and General Partner with Burrill & Company, an affiliate of Burrill Biotechnology Capital Fund, L.P.
|
|
(4)
|
FCPR Biotechnology Fund was a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund.
|
|
(5)
|
Ventech Capital II was a holder of more than 5% of our capital stock. Dr. Chaoui, was a member of our board of directors and was a venture partner of Ventech Capital II.
|
|
Purchaser(1)
|
Principal Amount
of Notes
|
Warrant
Shares
|
||||
|
Alta BioPharma Partners II, LP(2)
|
$
|
235,949
|
|
23,354
|
|
|
|
Alta Embarcadero BioPharma Partners II, LLC(2)
|
8,680
|
|
859
|
|
||
|
FCPR Biotechnology Fund(3)
|
209,609
|
|
20,747
|
|
||
|
Ventech Capital II(4)
|
264,676
|
|
26,198
|
|
||
|
S.R. One, Limited
|
177,889
|
|
17,607
|
|
||
|
(1)
|
See “Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters” for more information about these directors, executive officers, holders of more than 5% of our capital stock, and their affiliates.
|
|
(2)
|
Entities affiliated with Alta BioPharma Partners II, LP (“ABP II”) and Alta Embarcadero BioPharma Partners II, LLC (“AEBP II”) are holders of more than 5% of our capital stock. Dr. Penhoet, a member of our board of directors, is a director of Alta BioPharma Management II, LLC, the general partner of ABP II and manager of AEBP II.
|
|
(3)
|
FCPR Biotechnology Fund is a holder of more than 5% of our capital stock. Dr. Nothias, a member of our board of directors, is a member of the investment board of FCPR Biotechnology Fund.
|
|
(4)
|
Ventech Capital II was a holder of more than 5% of our capital stock. Dr. Chaoui, was a member of our board of directors and was a venture partner of Ventech Capital II.
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
1.
|
List of Financial Statements
|
|
2.
|
List of Financial Statement Schedules
|
|
3.
|
List of Exhibits
|
|
SCYNEXIS, INC.
|
||
|
By:
|
|
/s/ Yves J. Ribeill
|
|
|
Yves J. Ribeill
|
|
|
|
Chief Executive Officer
(Principal Executive Officer)
|
|
|
Date:
|
|
March 30, 2015
|
|
Signature
|
Title
|
Date
|
||
|
/s/ Yves J. Ribeill
|
Chief Executive Officer
|
March 30, 2015
|
||
|
Yves J. Ribeill
|
(Principal Executive Officer)
|
|||
|
/s/ Charles F. Osborne, Jr.
|
Chief Financial Officer
|
March 30, 2015
|
||
|
Charles F. Osborne, Jr.
|
(Principal Financial and Accounting Officer)
|
|||
|
/s/ Pamela J. Kirby, Ph.D.
|
Director
|
March 30, 2015
|
||
|
Pamela J. Kirby, Ph.D.
|
||||
|
/s/ Laurent Arthaud
|
Director
|
March 30, 2015
|
||
|
Laurent Arthaud
|
||||
|
/s/ Guy Macdonald
|
Director
|
March 30, 2015
|
||
|
Guy Macdonald
|
||||
|
/s/ Ann F. Hanham, Ph.D.
|
Director
|
March 30, 2015
|
||
|
Ann F. Hanham, Ph.D.
|
||||
|
/s/ Patrick J. Langlois, Ph.D.
|
Director
|
March 30, 2015
|
||
|
Patrick J. Langlois, Ph.D.
|
||||
|
/s/ Marco Taglietti
|
Director
|
March 30, 2015
|
||
|
Marco Taglietti
|
||||
|
/s/ Jean-Yves Nothias, Ph.D.
|
Director
|
March 30, 2015
|
||
|
Jean-Yves Nothias, Ph.D.
|
||||
|
/s/ Edward E. Penhoet, Ph.D.
|
Director
|
March 30, 2015
|
||
|
Edward E. Penhoet, Ph.D.
|
||||
|
/s/ Steven C. Gilman, Ph.D.
|
Director
|
March 30, 2015
|
||
|
Steven C. Gilman, Ph.D.
|
||||
|
Exhibit
Number
|
|
Description of Document
|
|
3.1
|
Amended and Restated Certificate of Incorporation. (Filed with the SEC as Exhibit 3.1 to our Current Report on Form 8-K, filed with the SEC on May 12, 2014, SEC File No. 001-36365).
|
|
|
3.3
|
Amended and Restated Bylaws, as amended and as currently in effect. (Filed with the SEC as Exhibit 3.4 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
4.1
|
Reference is made to Exhibits 3.1 and 3.2
|
|
|
4.2
|
Fifth Amended and Restated Investor Rights Agreement, dated December 11, 2013 (Filed with the SEC as Exhibit 10.21 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.1
|
Form of Indemnity Agreement between the Registrant and its directors and officers. (Filed with the SEC as Exhibit 10.1 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.2*
|
SCYNEXIS, Inc. Stock Option Plan, as amended, and Forms of Stock Option Grant Notice, Stock Option Agreement and Notice of Stock Option Exercise. (Filed with the SEC as Annex B to our Proxy Statement on Schedule 14A, filed with the SEC on August 1, 2014, SEC File No. 001-36365).
|
|
|
10.3*
|
SCYNEXIS, Inc. 2009 Stock Option Plan, as amended, and Forms of Stock Option Grant Notice, Stock Option Agreement and Notice of Stock Option Exercise. (Filed with the SEC as Exhibit 10.3 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.4*
|
SCYNEXIS, Inc. 2014 Equity Incentive Plan (Filed with the SEC as Annex A to our Proxy Statement on Schedule 14A, filed with the SEC on August 1, 2014, SEC File No. 001-36365).
|
|
|
10.5*
|
SCYNEXIS, Inc. 2014 Employee Stock Purchase Plan. (Filed with the SEC as Exhibit 99.4 to our Registration Statement on Form 8, filed with the SEC on May 16, 2014, SEC File No. 333-196007).
|
|
|
10.6*
|
Non-Employee Director Compensation Policy. (Filed with the SEC as Exhibit 10.1 to our Form 8-K, filed with the SEC on March 3, 3015, SEC File No. 001-36365).
|
|
|
10.7*
|
Amended and Restated Employment Agreement, dated December 7, 2012, between SCYNEXIS, Inc. and Charles F. Osborne, Jr. (Filed with the SEC as Exhibit 10.7 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.8*
|
Form of Stock Option Agreement and Form of Stock Option Grant Notice under the SCYNEXIS, Inc. 2014 Equity Incentive Plan (Filed with the SEC as Exhibit 99.3 to our Registration Statement on Form S-8, filed with the SEC on May 16, 2014, SEC File No. 333-196007).
|
|
|
10.9*
|
Amended and Restated Employment Agreement, dated December 7, 2012, between SCYNEXIS, Inc. and Yves J. Ribeill. (Filed with the SEC as Exhibit 10.9 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.10#
|
Development, License and Supply Agreement, dated August 1, 2013, between SCYNEXIS, Inc. and R-Pharm, CJSC. (Filed with the SEC as Exhibit 10.10 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.11#
|
License Agreement, dated August 7, 2012, as amended, between SCYNEXIS, Inc. and Dechra Ltd. (Filed with the SEC as Exhibit 10.11 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.12#
|
Termination and License Agreement, dated May 24, 2013, between SCYNEXIS. Inc. and Merck Sharp & Dohme Corp. (Filed with the SEC as Exhibit 10.12 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.13#
|
Agreement for the Assignment of Patents and Know How concerning Cyclosporin Derivatives, dated June 10, 2005, between SCYNEXIS, Inc. and C-CHEM AG. (Filed with the SEC as Exhibit 10.13 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.14#
|
Research Services Agreement, dated December 19, 2011 between SCYNEXIS, Inc. and Merial Limited. (Filed with the SEC as Exhibit 10.14 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.15#
|
Exclusive Worldwide License Agreement, dated May 10, 2005, between SCYNEXIS, Inc. and Aventis Pharma S.A. (Filed with the SEC as Exhibit 10.15 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.16
|
Amendment No. 1 to Exclusive Worldwide License Agreement, dated October 26, 2006, between SCYNEXIS, Inc. and Aventis Pharma S.A. (Filed with the SEC as Exhibit 10.16 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.17
|
Letter Agreement, dated April 9, 2010, as amended, between SCYNEXIS, Inc. and HSBC Bank USA, National Association. (Filed with the SEC as Exhibit 10.17 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.18
|
Stand Alone First Demand Guarantee, dated April 9, 2010, as amended, by the Guarantee Extension Agreement, dated March 5, 2013, by and between Sanofi-Aventis S.A. and HSBC Bank USA, National Association. (Filed with the SEC as Exhibit 10.18 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.19
|
Reimbursement & General Security Agreement, dated April 9, 2010, as amended, by the Guarantee Extension Agreement, dated March 5, 2013, between SCYNEXIS, Inc. and Sanofi-Aventis S.A. (Filed with the SEC as Exhibit 10.19 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.20
|
Guarantee Extension Agreement, dated March 5, 2013, between SCYNEXIS, Inc. and Sanofi-Aventis S.A. (Filed with the SEC as Exhibit 10.20 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.22
|
Industrial Building Lease, dated as of July 1, 2007, as amended, between SCYNEXIS, Inc. and Durham Research Tri-Center, LLC. (Filed with the SEC as Exhibit 10.22 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.23#
|
Amended and Restated License, Development and Commercialization Agreement, dated December 23, 2013, between SCYNEXIS, Inc. and Elanco Animal Health. (Filed with the SEC as Exhibit 10.23 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.24*
|
Employment Agreement, dated January 2014, between SCYNEXIS, Inc. and Carole A. Sable. (Filed with the SEC as Exhibit 10.24 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.25*
|
Offer Letter, dated September 29, 2013, from SCYNEXIS, Inc. to Vivian W. Doelling. (Filed with the SEC as Exhibit 10.25 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.26
|
Board Observation Rights Agreement, dated March 5, 2013, between SCYNEXIS, Inc. and Sanofi-Aventis S.A. (Filed with the SEC as Exhibit 10.26 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.27*
|
Employment Agreement, dated February 5, 2015, between SCYNEXIS, Inc. and Dr. Marco Taglietti.
|
|
|
10.28
|
Patent Assignment, dated January 28, 2014, between SCYNEXIS, Inc. and Merck Sharpe & Dohme Corp. (Filed with the SEC as Exhibit 3.4 to our Registration Statement on Form S-1, filed with the SEC on February 27, 2014, SEC File No. 333-194192).
|
|
|
10.29#
|
Research Services Agreement, dated December 24, 2014, between SCYNEXIS, Inc. and Merial Inc.
|
|
|
10.30
|
Series C-2 Preferred Stock Purchase Agreement, dated March 11, 2008 by and among SCYNEXIS, Inc., Merial Limited and S.R. One Limited. (Filed with the SEC as Exhibit 10.30 to our Amendment No. 1 to Registration Statement on Form S-1, filed with the SEC on March 19, 2014, SEC File No. 333-194192).
|
|
|
10.31
|
Addendums to Reimbursement Agreement, dated April 9, 2010, March 17, 2014 and April 29, 2014, between SCYNEXIS, Inc. and Sanofi. (Filed with the SEC as Exhibit 10.31 to our Amendment No. 3 to Registration Statement on Form S-1, filed with the SEC on April 30, 2014, SEC File No. 333-194192).
|
|
|
10.32#
|
Exclusive License Agreement, dated October 29, 2014, between SCYNEXIS, Inc. and Waterstone Pharmaceutical (HK Limited).
|
|
|
10.33#
|
Amendment to Termination and License Agreement, dated December 15, 2014, between SCYNEXIS, Inc. and Merck Sharp & Dohme Corp.
|
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
31.1
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a)/15d-14(a)
|
|
|
31.2
|
Certification of Chief Financial Officer pursuant to Rule 13(a)-14(a)/15d-14(a)
|
|
|
32.1
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350 as Adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS
|
|
XBRL Instance Document
|
|
101.SCH
|
|
XBRL Taxonomy Schema Linkbase Document
|
|
101.CAL
|
|
XBRL Taxonomy Calculation Linkbase Document
|
|
101.DEF
|
|
XBRL Taxonomy Definition Linkbase Document
|
|
101.LAB
|
|
XBRL Taxonomy Labels Linkbase Document
|
|
101.PRE
|
|
XBRL Taxonomy Presentation Linkbase Document
|
|
# Portions of this exhibit have been omitted pursuant to a request for confidential treatment, which portions were omitted and filed separately with the Securities and Exchange Commission.
|
||
|
*Designates management contract or compensatory plan or arrangement.
|
||