|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO
|
|
Delaware
|
26-2216351
|
|
(State or other jurisdiction
of incorporation or organization) |
(I.R.S. Employer
Identification No.) |
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, par value $0.0001 per share
|
SIBN
|
The Nasdaq Global Market
|
|
Large accelerated filer
o
|
Accelerated filer
x
|
Non-accelerated filer
o
|
|
Smaller reporting company
x
|
Emerging growth company
x
|
|
|
Page
|
|||
|
PART I
|
|
||
|
PART II
|
|||
|
PART III
|
|||
|
PART IV
|
|||
|
•
|
our expectation that a significant portion of our revenues will be derived from sales of the iFuse Implant System, or iFuse;
|
|
•
|
our ability to develop additional revenue opportunities, including new indications for use and new devices;
|
|
•
|
our ability to expand our sales team to increase demand for our products and expand geographically;
|
|
•
|
our ability to identify, train, and retain surgeons to perform procedures using our products;
|
|
•
|
our ability to obtain and maintain favorable coverage and reimbursement determinations from third-party payors;
|
|
•
|
our estimates of our market opportunity;
|
|
•
|
our expectations regarding the scope of protection from intellectual property rights covering our products;
|
|
•
|
developments or disputes concerning our intellectual property or other proprietary rights;
|
|
•
|
timing of and results from clinical and other trials;
|
|
•
|
marketing clearances and authorization from the U.S. Food and Drug Administration or FDA and regulators in other jurisdictions;
|
|
•
|
timing of regulatory filings and feedback;
|
|
•
|
competition in the markets we serve;
|
|
•
|
our expectations of the reliability and performance of our product;
|
|
•
|
our expectations of the benefits to patients, providers, and payors of our products;
|
|
•
|
our reliance on a limited number of suppliers, including sole source suppliers, which may impact the availability of instruments and materials;
|
|
•
|
our ability to sustain or increase demand for our products;
|
|
•
|
our estimates regarding our costs and risks associated with our international operations and expansion;
|
|
•
|
our expectations regarding our ability to retain and recruit key personnel;
|
|
•
|
our expectations regarding acquisitions and strategic operations;
|
|
•
|
our ability to fund our working capital requirements;
|
|
•
|
our compliance with, and the cost of, federal, state, and foreign regulatory requirements;
|
|
•
|
the factors that may impact our financial results; and
|
|
•
|
anticipated trends and challenges in our business and the markets in which we operate.
|
|
•
|
Medical therapy, including opiates and non-steroidal anti-inflammatory medications.
|
|
•
|
Physical therapy, which can involve exercises as well as massage.
|
|
•
|
Intra-articular injections of steroid medications, which are typically performed by physicians who specialize in pain treatment or anesthesia.
|
|
•
|
Radiofrequency ablation, or the cauterizing, of the lateral branches of the sacral nerve roots.
|
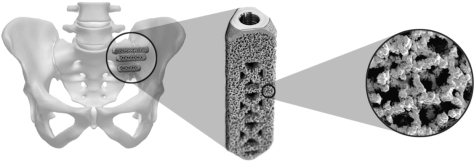
|
•
|
Pin
. The surgeon inserts a guide pin through the iliac bone, across the sacroiliac joint and into the sacrum.
|
|
•
|
Drill
. Surgeons drill over the guide pin, through the iliac bone, across the sacroiliac joint and just into the sacrum. This step is optional if using the sharp-tip broach.
|
|
•
|
Broach
. The surgeon impacts a triangular broach over the pin which prepares a triangular channel that is slightly smaller than the iFuse implant.
|
|
•
|
Implant
. The surgeon impacts the implant into the triangular channel thereby spanning the sacroiliac joint and docking the implant in the sacrum. The channel is slightly smaller than the implant, which produces an interference fit.
|
|
•
|
Reduction in Pain
. There was statistically significant and clinically important pain reduction in subjects treated with iFuse as compared to very small responses in the same measures in those treated with non-surgical management. Subjects surgically treated with iFuse had mean 52- 54- and 55-point reductions in sacroiliac joint pain at 6, 12 and 24 months, respectively, as measured by the Visual Analog Scale ("VAS"). By contrast, subjects in the non-surgical management group had only a mean 12-point reduction (p<0.0001) at six months. 12 points is below the commonly accepted 20-point threshold for clinically important improvement. In addition, the non-surgical management group subjects who elected after six months to cross over to have the iFuse procedure had pain reduction similar to that seen in subjects originally assigned to sacroiliac joint fusion with iFuse. At 24 months, the proportion of subjects with a reduction in VAS sacroiliac joint pain of 20 or more points due to the assigned treatment only was 83% in the iFuse group and 10% in the non-surgical management group.
|
|
•
|
Reduction in Disability
. There was a statistically significant and clinically important reduction in disability in subjects treated with iFuse as compared to very little response in those treated with non-surgical management. Subjects surgically treated with iFuse had a mean 27-point reduction in disability at six months, on the 0–100 Oswestry Disability Index ("ODI"), while subjects in the non-surgical management group had only a mean five-point reduction (p<0.0001). Five points is less than the commonly accepted 15-point threshold to denote a clinically important response. At 24 months, the iFuse group had a mean 28-point reduction in ODI. At six months, the proportion of subjects with ODI improvements of at least 15 points was 72.5% with iFuse treatment and only 13.0% in those undergoing non-surgical management (p<0.0001 for difference in response rate). In addition, the subjects who elected after six months to cross over to have the iFuse procedure had similar reduction in disability as the subjects originally assigned to sacroiliac joint fusion with iFuse. At 24 months, the proportion of subjects with an ODI improvement of at least 15 points with the assigned treatment only was 68.2% and 7.5% in the iFuse and non-surgical management groups, respectively (p<0.0001 for difference in response rate).
|
|
Covered Lives (in millions)
|
||
|
Commercial, exclusive
|
57.6
|
|
|
Commercial, non-exclusive
|
90.3
|
|
|
Medicaid
|
55.5
|
|
|
Medicare
|
64.5
|
|
|
Military/Federal
|
14.8
|
|
|
282.7
|
||
|
•
|
product and clinical procedure effectiveness;
|
|
•
|
ease of surgical technique and use of associated instruments;
|
|
•
|
safety;
|
|
•
|
published clinical outcomes and evidence;
|
|
•
|
sales force knowledge;
|
|
•
|
product support and service, and customer service;
|
|
•
|
comprehensive training, including disease, anatomy, diagnosis and treatment;
|
|
•
|
product innovation and the speed of innovation;
|
|
•
|
intellectual property;
|
|
•
|
accountability and responsiveness to customers’ demands;
|
|
•
|
pricing and reimbursement;
|
|
•
|
scientific (biomechanics) data; and
|
|
•
|
attracting and retaining key personnel.
|
|
•
|
product design, research, development, and manufacture;
|
|
•
|
product safety, testing, labeling, and storage;
|
|
•
|
record keeping procedures;
|
|
•
|
product marketing, promotion, advertising, sales, distribution, export, and import; and
|
|
•
|
post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions, and repair or recall of products.
|
|
•
|
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
|
|
•
|
investigational device exemptions to conduct premarket clinical trials, which include extensive monitoring, recordkeeping, and reporting requirements;
|
|
•
|
Quality System Regulation ("QSR"), which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;
|
|
•
|
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication;
|
|
•
|
clearance of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices;
|
|
•
|
approval of product modifications that affect the safety or effectiveness of one of our approved devices;
|
|
•
|
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
|
|
•
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
•
|
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
|
|
•
|
the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
|
|
•
|
regulations pertaining to voluntary recalls; and
|
|
•
|
notices of corrections or removals.
|
|
•
|
Product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
|
|
•
|
QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the manufacturing process;
|
|
•
|
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indications;
|
|
•
|
clearance of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices;
|
|
•
|
approval of product modifications that affect the safety or effectiveness of one of our approved devices;
|
|
•
|
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
|
|
•
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
•
|
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
|
|
•
|
the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
|
|
•
|
regulations pertaining to voluntary recalls; and
|
|
•
|
notices of corrections or removals.
|
|
•
|
untitled letters, warning letters, fines, injunctions, consent decrees, and civil penalties;
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
•
|
customer notifications for repair, replacement, refunds;
|
|
•
|
recall, detention or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying our requests for 510(k) clearance or PMA approval of new products or modified products;
|
|
•
|
operating restrictions;
|
|
•
|
withdrawing 510(k) clearances or PMA approvals that have already been granted;
|
|
•
|
refusal to grant export approval for our products; or
|
|
•
|
criminal prosecution.
|
|
•
|
registration of medical devices in individual EEA countries;
|
|
•
|
pricing and reimbursement of medical devices;
|
|
•
|
establishment of post-marketing surveillance and adverse event reporting procedures;
|
|
•
|
Field Safety Corrective Actions, including product recalls and withdrawals; and
|
|
•
|
interactions with physicians.
|
|
•
|
strengthen the rules on placing medical devices on the market and reinforce surveillance once they are available;
|
|
•
|
establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market;
|
|
•
|
improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number;
|
|
•
|
set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the European Union; and
|
|
•
|
strengthen the rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market.
|
|
•
|
the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order, arrangement for, or recommendation of, items or services for which payment may be made, in whole or in part, under federal healthcare programs, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value, and the government can establish a violation of the Anti-Kickback Statute without proving that a person or entity had actual knowledge of, or a specific intent to violate, the law;
|
|
•
|
the federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment of government funds; knowingly making, using, or causing to be made or used, a false record or statement to get a false claim paid or to avoid, decrease, or conceal an obligation to pay money to the federal government. A claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. Actions under the False Claims Act may be brought by the government or as a
qui tam
action by a private individual in the name of the government and to share in any monetary recovery. There are also criminal penalties for making or presenting a false or fictitious or fraudulent claim to the federal government;
|
|
•
|
the federal Health Insurance Portability and Accountability Act of 1996, which imposes criminal and civil liability for, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program including private third-party payors, or knowingly and willfully falsifying, concealing, or covering up a material fact or making a materially false, fictitious, or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious, or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items, or services;
|
|
•
|
the federal Physician Payment Sunshine Act, implemented by CMS as the Open Payments program, which requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to report annually to the CMS, information related to payments and other “transfers of value” made to physicians (currently defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and requires applicable manufacturers to report annually to CMS ownership and investment interests held by physicians and their immediate family members and payments or other “transfers of value” to such physician owners. Beginning in 2022, applicable manufacturers also will be required to report information regarding payments and transfers of value provided (beginning in 2021) to physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives; and
|
|
•
|
analogous state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers and patients; state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state beneficiary inducement laws, and state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
|
|
•
|
two years of income statements rather than three years as required by Rule 8-02 of Regulation S-X;
|
|
•
|
two-year management discussions and analysis comparison rather than three-year comparison as required by Item 303 of Regulation S-K;
|
|
•
|
no requirement to provide selected financial data table required by Item 301 of Regulation S-K;
|
|
•
|
no requirement to provide market risk disclosures required by Item 305 of Regulation S-K;
|
|
•
|
no requirement to provide supplemental financial information required by Item 302 of Regulation S-K; and
|
|
•
|
less extensive narrative disclosure than required of other reporting companies, particularly in the description of executive compensation as required by Item 402 of Regulation S-K.
|
|
•
|
lack of experience with minimally invasive procedures;
|
|
•
|
perceived liability risks generally associated with the use of new products and procedures;
|
|
•
|
costs associated with the purchase of new products; and
|
|
•
|
time commitment that may be required for training.
|
|
•
|
greater financial, human, and other resources for product research and development, sales and marketing, and legal matters;
|
|
•
|
significantly greater name recognition;
|
|
•
|
established relationships with surgeons, hospitals, and other healthcare providers;
|
|
•
|
large and established sales and marketing and distribution networks;
|
|
•
|
greater experience in obtaining and maintaining domestic and international regulatory clearances or approvals, or CE Certificates of Conformity for products and product enhancements;
|
|
•
|
more expansive portfolios of intellectual property rights; and
|
|
•
|
greater ability to cross-sell their products or to incentivize hospitals or surgeons to use their products.
|
|
•
|
sales of the product may decrease significantly, and we may not achieve the anticipated market share;
|
|
•
|
regulatory authorities or our Notified Body may require changes to the labeling of our product. This may include the addition of labeling statements, specific warnings, and contraindications and issuing field alerts to physicians and patients;
|
|
•
|
we may be required to change instructions regarding the way the product is implanted or conduct additional clinical trials;
|
|
•
|
we may be subject to limitations on how we may promote the product;
|
|
•
|
regulatory authorities may require us to take our approved product off the market (temporarily or permanently) or to conduct other field safety corrective actions;
|
|
•
|
we may be required to modify our product;
|
|
•
|
we may be subject to litigation fines or product liability claims; and
|
|
•
|
our reputation may suffer.
|
|
•
|
failure to complete sterilization on time or in compliance with the required regulatory standards;
|
|
•
|
transportation and import and export risk;
|
|
•
|
delays in analytical results or failure of analytical techniques that we will depend on for quality control and release of products;
|
|
•
|
natural disasters, labor disputes, financial distress, raw material availability, issues with facilities and equipment, or other forms of disruption to business operations affecting our manufacturers or suppliers; and
|
|
•
|
latent defects that may become apparent after products have been released and that may result in a recall of such products.
|
|
•
|
third-party contract manufacturers or suppliers may fail to comply with regulatory requirements or make errors in manufacturing that could negatively affect the safety or effectiveness of our products or cause delays in shipments of our products;
|
|
•
|
third-party contract manufacturers or suppliers may fail to maintain good manufacturing practices, leading to quality control problems or regulatory findings that could cause disruptions in their manufacturing processes and lead to delays in shipments of our products;
|
|
•
|
we or our third-party manufacturers and suppliers may not be able to respond to unanticipated changes in customer orders, and if orders do not match forecasts, we or our suppliers may have excess or inadequate inventory of materials and components;
|
|
•
|
we or our third-party manufacturers and suppliers may be subject to price fluctuations due to a lack of long-term supply arrangements for key components;
|
|
•
|
we or our third-party manufacturers and suppliers may lose access to critical services and components, resulting in an interruption in the manufacture, assembly and shipment of our systems;
|
|
•
|
we may experience delays in delivery by our third-party manufacturers and suppliers due to changes in demand from us or their other customers;
|
|
•
|
fluctuations in demand for products that our third-party manufacturers and suppliers manufacture for others may affect their ability or willingness to deliver components to us in a timely manner;
|
|
•
|
our third-party manufacturers and suppliers may wish to discontinue supplying components or services to us for risk management reasons;
|
|
•
|
we may not be able to find new or alternative components or reconfigure our system and manufacturing processes in a timely manner if the necessary components become unavailable; and
|
|
•
|
our third-party manufacturers and suppliers may encounter financial hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements.
|
|
•
|
managing production yields;
|
|
•
|
maintaining quality control and assurance;
|
|
•
|
providing component and service availability;
|
|
•
|
maintaining adequate control policies and procedures;
|
|
•
|
hiring and retaining qualified personnel; and
|
|
•
|
complying with state, federal, and foreign regulations.
|
|
•
|
properly identify and anticipate surgeon and patient needs;
|
|
•
|
develop and introduce new products or product enhancements in a timely manner;
|
|
•
|
adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third parties;
|
|
•
|
demonstrate the safety and effectiveness of new products; and
|
|
•
|
obtain the necessary domestic and international regulatory clearances or approvals and CE Certificates of Conformity for new products or product enhancements.
|
|
•
|
exposure to different legal and regulatory standards;
|
|
•
|
lack of stringent protection of intellectual property;
|
|
•
|
obstacles to obtaining domestic and foreign export, import, and other governmental approvals, permits, and licenses and compliance with foreign laws;
|
|
•
|
potentially adverse tax consequences and the complexities of foreign value-added tax systems;
|
|
•
|
adverse changes in tariffs and trade restrictions;
|
|
•
|
limitations on the repatriation of earnings;
|
|
•
|
difficulties in staffing and managing foreign operations;
|
|
•
|
transportation delays and difficulties of managing international distribution channels;
|
|
•
|
longer collection periods and difficulties in collecting receivables from foreign entities;
|
|
•
|
increased financing costs;
|
|
•
|
currency risks; and
|
|
•
|
political, social, and economic instability and increased security concerns.
|
|
•
|
sales and marketing, accounting, and financial functions;
|
|
•
|
inventory management;
|
|
•
|
engineering and product development tasks; and
|
|
•
|
our research and development data.
|
|
•
|
earthquakes, fires, floods, and other natural disasters;
|
|
•
|
terrorist attacks and attacks by computer viruses or hackers or breach of our cybersecurity;
|
|
•
|
power losses; and
|
|
•
|
computer systems, or Internet, telecommunications, or data network failures.
|
|
•
|
problems assimilating the purchased technologies, products, or business operations;
|
|
•
|
issues maintaining uniform standards, procedures, controls, and policies;
|
|
•
|
unanticipated costs and liabilities associated with acquisitions;
|
|
•
|
diversion of management’s attention from our core business;
|
|
•
|
adverse effects on existing business relationships with suppliers and customers;
|
|
•
|
risks associated with entering new markets in which we have limited or no experience;
|
|
•
|
potential loss of key employees of acquired businesses; and
|
|
•
|
increased legal and accounting compliance costs.
|
|
•
|
design, development, and manufacturing;
|
|
•
|
testing, labeling, content, and language of instructions for use and storage;
|
|
•
|
clinical trials;
|
|
•
|
product safety;
|
|
•
|
marketing, sales, and distribution;
|
|
•
|
premarket clearance and approval;
|
|
•
|
conformity assessment procedures;
|
|
•
|
record keeping procedures;
|
|
•
|
advertising and promotion;
|
|
•
|
compliance with good manufacturing practices requirements;
|
|
•
|
recalls and field safety corrective actions;
|
|
•
|
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury;
|
|
•
|
post-market approval studies; and
|
|
•
|
product import and export.
|
|
•
|
we may not be able to demonstrate to the FDA’s satisfaction that our products are safe and effective for their intended users;
|
|
•
|
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and
|
|
•
|
the manufacturing process or facilities we use may not meet applicable requirements.
|
|
•
|
warning letters;
|
|
•
|
fines;
|
|
•
|
injunctions;
|
|
•
|
civil penalties;
|
|
•
|
termination of distribution;
|
|
•
|
recalls or seizures of products;
|
|
•
|
delays in the introduction of products into the market;
|
|
•
|
total or partial suspension of production;
|
|
•
|
facility closures;
|
|
•
|
refusal of the FDA or our Notified Body or other regulator to grant future clearances or approvals or to issue CE Certificates of Conformity;
|
|
•
|
withdrawals, variation, or suspensions of current clearances or approvals and CE Certificates of Conformity, resulting in prohibitions on sales of our products; and
|
|
•
|
in the most serious cases, criminal penalties.
|
|
•
|
untitled letters, warning letters, fines, injunctions, consent, and civil penalties;
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
•
|
customer notifications for repair, replacement, refunds;
|
|
•
|
recall, detention, or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval and conformity assessments of new products or modified products;
|
|
•
|
limitations on the intended uses for which the product may be marketed;
|
|
•
|
operating restrictions;
|
|
•
|
withdrawing 510(k) clearances or PMA approvals that have already been granted;
|
|
•
|
suspension, variation or withdrawal of CE Certificates of Conformity;
|
|
•
|
refusal to grant export approval for our products; and
|
|
•
|
criminal prosecution.
|
|
•
|
additional scrutiny during the conformity assessment procedure for high risk medical devices;
|
|
•
|
strengthening of the clinical data requirements related to medical devices;
|
|
•
|
strengthening of the designation and monitoring processes governing notified bodies;
|
|
•
|
the obligation for manufacturers and authorized representative to have a person responsible for regulatory compliance continuously at their disposal;
|
|
•
|
authorized representatives would be held legally responsible and liable for defective products placed on the EU market;
|
|
•
|
increased traceability of medical devices following the introduction of a Unique Device Identification ("UDI"), system;
|
|
•
|
new rules governing the reprocessing of medical devices; and
|
|
•
|
increased transparency with the establishment of EUDAMED III as information from several databases concerning economic operators, CE Certificates of Conformity, conformity assessment, clinical investigations, the UDI system, adverse event reporting and market surveillance would be available to the public.
|
|
•
|
actual or anticipated changes or fluctuations in our results of operations;
|
|
•
|
results of our clinical trials and that of our competitors’ products;
|
|
•
|
regulatory actions with respect to our products or our competitor’s products;
|
|
•
|
announcements of new offerings, products, services or technologies, commercial relationships, acquisitions, or other events by us or our competitors;
|
|
•
|
price and volume fluctuations in the overall stock market from time to time;
|
|
•
|
significant volatility in the market price and trading volume of healthcare companies, in general, and of companies in the medical device industry in particular;
|
|
•
|
fluctuations in the trading volume of our shares or the size of our public float;
|
|
•
|
negative publicity;
|
|
•
|
whether our results of operations meet the expectations of securities analysts or investors or those expectations change;
|
|
•
|
litigation involving us, our industry, or both;
|
|
•
|
regulatory developments in the U.S., foreign countries, or both;
|
|
•
|
lock-up releases and sales of large blocks of our common stock;
|
|
•
|
additions or departures of key employees or scientific personnel; and
|
|
•
|
general economic conditions and trends.
|
|
•
|
payor coverage and reimbursement;
|
|
•
|
the number of products sold in the quarter and our ability to drive increased sales of our products;
|
|
•
|
our ability to establish and maintain an effective and dedicated sales force;
|
|
•
|
pricing pressure applicable to our products, including adverse third-party coverage and reimbursement outcomes;
|
|
•
|
results of clinical research and trials on our existing products and products in development;
|
|
•
|
the mix of our products sold because profit margins differ amongst our products;
|
|
•
|
timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors;
|
|
•
|
the ability of our suppliers to timely provide us with an adequate supply of materials and components;
|
|
•
|
the evolving product offerings of our competitors;
|
|
•
|
the demand for, and pricing of, our products and the products of our competitors;
|
|
•
|
factors that may affect the sale of our products, including seasonality and budgets of our customers;
|
|
•
|
domestic and international regulatory clearances or approvals, or CE Certificates of Conformity, and legislative changes affecting the products we may offer or those of our competitors;
|
|
•
|
interruption in the manufacturing or distribution of our products;
|
|
•
|
the effect of competing technological, industry and market developments;
|
|
•
|
our ability to expand the geographic reach of our sales and marketing efforts;
|
|
•
|
the costs of maintaining adequate insurance coverage, including product liability insurance;
|
|
•
|
the availability and cost of components and materials;
|
|
•
|
the number of selling days in the quarter;
|
|
•
|
fluctuation in foreign currency exchange rates; and
|
|
•
|
impairment and other special charges.
|
|
•
|
dispose of or sell assets;
|
|
•
|
make material changes in our business or management;
|
|
•
|
consolidate or merge with or acquire other entities;
|
|
•
|
incur additional indebtedness;
|
|
•
|
incur liens on our assets;
|
|
•
|
pay dividends or make distributions on our capital stock;
|
|
•
|
make certain investments;
|
|
•
|
enter into transactions with our affiliates;
|
|
•
|
make any payment in respect of any subordinated indebtedness; and
|
|
•
|
waive or amend any of our current intellectual property agreements or material contracts.
|
|
•
|
a classified board of directors with three-year staggered terms, which could delay the ability of stockholders to change the membership of a majority of our board of directors;
|
|
•
|
the ability of our board of directors to issue shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquiror;
|
|
•
|
the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of our board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors;
|
|
•
|
a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders;
|
|
•
|
the requirement that a special meeting of stockholders may be called only by a majority vote of our entire board of directors, the chairman of our board of directors, or our chief executive officer, which could delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors;
|
|
•
|
the requirement for the affirmative vote of holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of the voting stock, voting together as a single class, to amend the provisions of our amended and restated certificate of incorporation relating to the management of our business or our amended and restated bylaws, which may inhibit the ability of an acquiror to effect such amendments to facilitate an unsolicited takeover attempt; and
|
|
•
|
advance notice procedures with which stockholders must comply to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of us.
|
|
•
|
Covered lives
- As of
December 31, 2019
, of the U.S. payors covering
282.7
million lives that reimburse for iFuse,
147.9
million lives are covered by private payors, compared to 256.2 million covered lives, of which 121.7 million were covered by private payors as of
December 31, 2018
. We track the number of U.S. covered lives, or individuals whose healthcare is paid for by a private commercial or governmental payor that routinely reimburses for minimally invasive sacroiliac fusion, as a proxy for availability of the procedure within the U.S. healthcare payment system. As of
December 31, 2019
,
32
private payors have issued positive coverage policies exclusive to iFuse for sacroiliac joint fusion because of the clinical evidence, compared to 26 exclusive coverage policies as of
December 31, 2018
. These payors have based their exclusive coverage decisions on the quality of our data. Further, as of
December 31, 2019
and
2018
,
20
and 19, respectively, private payors are covering iFuse and other products for sacroiliac joint fusion. We believe that the full impact of each coverage decision grows over time as surgeons gain confidence that they will receive reimbursement for the majority of their diagnosed patients.
|
|
•
|
Surgeon payment
- The Center for Medicare & Medicaid Services ("CMS") announced in November 2019 that the U.S. national average physician fee reimbursement for minimally invasive sacroiliac joint fusion increased from $720, effective January 1, 2019 to $915, effective January 1, 2020. Many private payors set their payment amounts with reference to the Medicare payment, often approximately 10% to 33% higher than the Medicare payment for a procedure. We believe that expanded coverage for minimally invasive sacroiliac fusion and the increase in physician reimbursement for the procedure may enable surgeons to treat more patients diagnosed with sacroiliac joint dysfunction with iFuse.
|
|
Year ended December 31, 2019
|
Year ended December 31, 2018
|
||||||||||||
|
Amount
|
%
|
Amount
|
%
|
||||||||||
|
(in thousands, except for percentages)
|
|||||||||||||
|
Consolidated Statements of Operations Data:
|
|||||||||||||
|
Revenue
|
$
|
67,301
|
|
100
|
%
|
$
|
55,380
|
|
100
|
%
|
|||
|
Cost of goods sold
|
6,790
|
|
10
|
%
|
4,833
|
|
9
|
%
|
|||||
|
Gross profit
|
60,511
|
|
90
|
%
|
50,547
|
|
91
|
%
|
|||||
|
Operating expenses:
|
|||||||||||||
|
Sales and marketing
|
68,251
|
|
101
|
%
|
44,497
|
|
80
|
%
|
|||||
|
Research and development
|
7,279
|
|
11
|
%
|
5,376
|
|
10
|
%
|
|||||
|
General and administrative
|
20,984
|
|
31
|
%
|
12,639
|
|
23
|
%
|
|||||
|
Total operating expenses
|
96,514
|
|
143
|
%
|
62,512
|
|
113
|
%
|
|||||
|
Loss from operations
|
(36,003
|
)
|
(53
|
)%
|
(11,965
|
)
|
(22
|
)%
|
|||||
|
Interest and other income (expense), net:
|
|||||||||||||
|
Interest income
|
2,551
|
|
4
|
%
|
769
|
|
2
|
%
|
|||||
|
Interest expense
|
(4,949
|
)
|
(7
|
)%
|
(5,108
|
)
|
(9
|
)%
|
|||||
|
Other expense, net
|
(2
|
)
|
—
|
%
|
(1,149
|
)
|
(2
|
)%
|
|||||
|
Net loss
|
$
|
(38,403
|
)
|
(56
|
)%
|
$
|
(17,453
|
)
|
(31
|
)%
|
|||
|
Year ended December 31, 2019
|
Year ended December 31, 2018
|
||||||||||||
|
Amount
|
%
|
Amount
|
%
|
||||||||||
|
(in thousands except for percentages)
|
|||||||||||||
|
United States
|
$
|
61,843
|
|
92
|
%
|
$
|
50,137
|
|
91
|
%
|
|||
|
International
|
5,458
|
|
8
|
%
|
5,243
|
|
9
|
%
|
|||||
|
$
|
67,301
|
|
100
|
%
|
$
|
55,380
|
|
100
|
%
|
||||
|
Year ended December 31,
|
||||||||||||||
|
2019
|
2018
|
$ Change
|
% Change
|
|||||||||||
|
(in thousands except for percentages)
|
||||||||||||||
|
Revenue
|
$
|
67,301
|
|
$
|
55,380
|
|
$
|
11,921
|
|
22
|
%
|
|||
|
Cost of goods sold
|
6,790
|
|
4,833
|
|
1,957
|
|
40
|
%
|
||||||
|
Gross profit
|
$
|
60,511
|
|
$
|
50,547
|
|
$
|
9,964
|
|
20
|
%
|
|||
|
Gross margin
|
90
|
%
|
91
|
%
|
||||||||||
|
Year ended December 31,
|
||||||||||||||
|
2019
|
2018
|
$ Change
|
% Change
|
|||||||||||
|
(in thousands, except for percentages)
|
||||||||||||||
|
Sales and marketing
|
$
|
68,251
|
|
$
|
44,497
|
|
$
|
23,754
|
|
53
|
%
|
|||
|
Research and development
|
7,279
|
|
5,376
|
|
1,903
|
|
35
|
%
|
||||||
|
General and administrative
|
20,984
|
|
12,639
|
|
8,345
|
|
66
|
%
|
||||||
|
Total operating expenses
|
$
|
96,514
|
|
$
|
62,512
|
|
$
|
34,002
|
|
|||||
|
Year ended December 31,
|
$ Change
|
% Change
|
||||||||||||
|
2019
|
2018
|
|||||||||||||
|
(in thousands, except for percentages)
|
||||||||||||||
|
Interest income
|
$
|
2,551
|
|
$
|
769
|
|
$
|
1,782
|
|
232
|
%
|
|||
|
Interest expense
|
(4,949
|
)
|
(5,108
|
)
|
159
|
|
(3
|
)%
|
||||||
|
Other expense, net
|
(2
|
)
|
(1,149
|
)
|
1,147
|
|
(100
|
)%
|
||||||
|
Total interest and other expense, net
|
$
|
(2,400
|
)
|
$
|
(5,488
|
)
|
$
|
3,088
|
|
(56
|
)%
|
|||
|
Twelve Months Ending
|
Minimum Net Sales
|
Trailing 12-Month Consolidated EBITDA
|
|||||
|
(in thousands)
|
|||||||
|
March 31, 2020
|
$57,500
|
or
|
$1,000
|
||||
|
June 30, 2020
|
$58,500
|
or
|
$2,000
|
||||
|
thereafter, as applicable
|
$60,000
|
or
|
$3,000
|
||||
|
Payments Due By Period
|
|||||||||||||||||||
|
Total
|
Less than 1 year
|
1-3 years
|
4-5 years
|
More than 5 years
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Principal obligations on the debt arrangements
|
$
|
40,000
|
|
$
|
4,444
|
|
$
|
35,556
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Interest obligations on the debt arrangements
|
9,331
|
|
4,677
|
|
4,654
|
|
—
|
|
—
|
|
|||||||||
|
Operating leases
|
5,128
|
|
1,102
|
|
1,932
|
|
1,729
|
|
365
|
|
|||||||||
|
Purchase obligations
|
403
|
|
403
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total
|
$
|
54,862
|
|
$
|
10,626
|
|
$
|
42,142
|
|
$
|
1,729
|
|
$
|
365
|
|
||||
|
Year ended December 31,
|
||||||||||||||
|
2019
|
2018
|
$ Change
|
% Change
|
|||||||||||
|
(in thousands, except for percentages)
|
||||||||||||||
|
Net cash provided by (used in):
|
||||||||||||||
|
Operating activities
|
$
|
(31,627
|
)
|
$
|
(14,519
|
)
|
$
|
(17,108
|
)
|
118
|
%
|
|||
|
Investing activities
|
13,491
|
|
(97,825
|
)
|
111,316
|
|
(114
|
)%
|
||||||
|
Financing activities
|
3,488
|
|
115,150
|
|
(111,662
|
)
|
(97
|
)%
|
||||||
|
Effects of exchange rate changes on cash and cash equivalents
|
(37
|
)
|
(94
|
)
|
57
|
|
(61
|
)%
|
||||||
|
Net (decrease) increase in cash and cash equivalents
|
$
|
(14,685
|
)
|
$
|
2,712
|
|
$
|
(17,397
|
)
|
|||||
|
•
|
Expected Term -
The expected term represents the period that we expect the share-based awards to be outstanding. We use the simplified method to determine the expected term as permitted by the guidance since we have no sufficient historical exercise patterns to estimate the expected life. The simplified method is calculated as the average of the time to vesting and the contractual life of the options.
|
|
•
|
Expected Volatility -
Since we became public in October 2018 and have no sufficient trading history, we use stock price volatility using the average historical volatilities of publicly traded companies within our industry that we consider comparable to our business over a period approximately equal to the expected term of our stock options.
|
|
•
|
Risk-Free Interest Rate -
We base the risk-free interest rate on the U.S. Treasury zero coupon issued in effect at the time of grant for periods corresponding with the expected term of the option.
|
|
•
|
Dividend Yield -
We have not paid any dividends and we have no current plans to pay dividends on our common stock. As such, we use expected dividend yield of zero.
|
|
December 31, 2019
|
December 31, 2018
|
||||||
|
ASSETS
|
|||||||
|
CURRENT ASSETS
|
|||||||
|
Cash and cash equivalents
|
$
|
10,435
|
|
$
|
25,120
|
|
|
|
Short-term investments
|
81,345
|
|
97,103
|
|
|||
|
Accounts receivable, net of allowance for doubtful accounts of $238 and $263, respectively
|
11,720
|
|
8,486
|
|
|||
|
Inventory
|
5,452
|
|
3,343
|
|
|||
|
Prepaid expenses and other current assets
|
2,510
|
|
1,990
|
|
|||
|
Total current assets
|
111,462
|
|
136,042
|
|
|||
|
Long-term investments
|
1,278
|
|
—
|
|
|||
|
Property and equipment, net
|
3,954
|
|
2,154
|
|
|||
|
Other non-current assets
|
315
|
|
325
|
|
|||
|
TOTAL ASSETS
|
$
|
117,009
|
|
$
|
138,521
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
CURRENT LIABILITIES
|
|||||||
|
Accounts payable
|
$
|
2,811
|
|
$
|
2,146
|
|
|
|
Accrued liabilities and other
|
11,605
|
|
6,860
|
|
|||
|
Current portion of long-term borrowings
|
4,358
|
|
—
|
|
|||
|
Total current liabilities
|
18,774
|
|
9,006
|
|
|||
|
Long-term borrowings
|
34,865
|
|
38,963
|
|
|||
|
Other long-term liabilities
|
362
|
|
360
|
|
|||
|
TOTAL LIABILITIES
|
54,001
|
|
48,329
|
|
|||
|
Commitments and contingencies (Note 6)
|
|
|
|||||
|
STOCKHOLDERS’ EQUITY
|
|||||||
|
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value; 100,000,000 shares authorized; 25,163,803 and 24,450,757 shares issued and outstanding, respectively
|
3
|
|
3
|
|
|||
|
Additional paid-in capital
|
258,121
|
|
246,927
|
|
|||
|
Accumulated other comprehensive income
|
464
|
|
439
|
|
|||
|
Accumulated deficit
|
(195,580
|
)
|
(157,177
|
)
|
|||
|
TOTAL STOCKHOLDERS’ EQUITY
|
63,008
|
|
90,192
|
|
|||
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
117,009
|
|
$
|
138,521
|
|
|
|
Year ended December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Revenue
|
$
|
67,301
|
|
$
|
55,380
|
|
||
|
Cost of goods sold
|
6,790
|
|
4,833
|
|
||||
|
Gross profit
|
60,511
|
|
50,547
|
|
||||
|
Operating expenses:
|
||||||||
|
Sales and marketing
|
68,251
|
|
44,497
|
|
||||
|
Research and development
|
7,279
|
|
5,376
|
|
||||
|
General and administrative
|
20,984
|
|
12,639
|
|
||||
|
Total operating expenses
|
96,514
|
|
62,512
|
|
||||
|
Loss from operations
|
(36,003
|
)
|
(11,965
|
)
|
||||
|
Interest and other income (expense), net:
|
||||||||
|
Interest income
|
2,551
|
|
769
|
|
||||
|
Interest expense
|
(4,949
|
)
|
(5,108
|
)
|
||||
|
Other expense, net
|
(2
|
)
|
(1,149
|
)
|
||||
|
Net loss
|
(38,403
|
)
|
(17,453
|
)
|
||||
|
Other comprehensive income (loss):
|
||||||||
|
Unrealized gain of marketable securities
|
44
|
|
10
|
|
||||
|
Changes in foreign currency translation
|
(19
|
)
|
27
|
|
||||
|
Comprehensive loss
|
$
|
(38,378
|
)
|
$
|
(17,416
|
)
|
||
|
Net loss per share, basic and diluted
|
$
|
(1.55
|
)
|
$
|
(2.20
|
)
|
||
|
Weighted-average number of common shares used to compute basic and diluted net loss per share
|
24,705,980
|
|
7,950,284
|
|
||||
|
Redeemable
Convertible Preferred Stock |
Common Stock
|
Additional
Paid-in Capital |
Accumulated
Other Comprehensive Income (Loss) |
Accumulated
Deficit |
Total
Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||
|
Balances as of December 31, 2017
|
11,871,578
|
|
$
|
118,548
|
|
3,603,140
|
|
$
|
1
|
|
$
|
9,943
|
|
$
|
402
|
|
$
|
(139,724
|
)
|
$
|
(129,378
|
)
|
||||||||
|
Issuance of common stock upon exercise of stock options, net of shares withheld
|
—
|
|
—
|
|
289,077
|
|
—
|
|
1,136
|
|
—
|
|
—
|
|
1,136
|
|
||||||||||||||
|
Issuance of common stock upon exercise of unvested stock options
|
—
|
|
—
|
|
106,028
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Conversion from preferred stock to common stock
|
(11,871,578
|
)
|
(118,548
|
)
|
12,066,654
|
|
1
|
|
118,547
|
|
—
|
|
—
|
|
118,548
|
|
||||||||||||||
|
Conversion from preferred stock warrants to common stock warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
1,248
|
|
—
|
|
—
|
|
1,248
|
|
||||||||||||||
|
Issuance of common stock from warrants exercise
|
—
|
|
—
|
|
121,486
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Issuance of common stock from IPO proceeds, net
|
—
|
|
—
|
|
8,280,000
|
|
1
|
|
113,602
|
|
—
|
|
—
|
|
113,603
|
|
||||||||||||||
|
Repurchase of unvested early exercised stock options
|
—
|
|
—
|
|
(15,628
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
2,312
|
|
—
|
|
—
|
|
2,312
|
|
||||||||||||||
|
Vesting of early exercised stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
139
|
|
—
|
|
—
|
|
139
|
|
||||||||||||||
|
Foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
27
|
|
—
|
|
27
|
|
||||||||||||||
|
Unrealized gain of marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
10
|
|
—
|
|
10
|
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(17,453
|
)
|
(17,453
|
)
|
||||||||||||||
|
Balances as of December 31, 2018
|
—
|
|
—
|
|
24,450,757
|
|
3
|
|
246,927
|
|
439
|
|
(157,177
|
)
|
90,192
|
|
||||||||||||||
|
Issuance of common stock upon exercise of stock options, net of shares withheld
|
—
|
|
—
|
|
444,788
|
|
—
|
|
1,490
|
|
—
|
|
—
|
|
1,490
|
|
||||||||||||||
|
Issuance of common stock related to employee stock purchase plan
|
—
|
|
—
|
|
168,457
|
|
—
|
|
2,203
|
|
—
|
|
—
|
|
2,203
|
|
||||||||||||||
|
Issuance of common stock upon vesting of restricted stock units
|
—
|
|
—
|
|
108,631
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Repurchase of unvested early exercised stock options
|
—
|
|
—
|
|
(8,830
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
7,464
|
|
—
|
|
—
|
|
7,464
|
|
||||||||||||||
|
Vesting of early exercised stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
197
|
|
—
|
|
—
|
|
197
|
|
||||||||||||||
|
Additional accrual of IPO related costs
|
—
|
|
—
|
|
—
|
|
—
|
|
(160
|
)
|
—
|
|
—
|
|
(160
|
)
|
||||||||||||||
|
Foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(19
|
)
|
—
|
|
(19
|
)
|
||||||||||||||
|
Unrealized gain on marketable securities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
44
|
|
—
|
|
44
|
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(38,403
|
)
|
(38,403
|
)
|
||||||||||||||
|
Balances as of December 31, 2019
|
—
|
|
$
|
—
|
|
25,163,803
|
|
$
|
3
|
|
$
|
258,121
|
|
$
|
464
|
|
$
|
(195,580
|
)
|
$
|
63,008
|
|
||||||||
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Cash flows from operating activities
|
|||||||
|
Net loss
|
$
|
(38,403
|
)
|
$
|
(17,453
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities
|
|||||||
|
Stock-based compensation
|
7,464
|
|
2,312
|
|
|||
|
Depreciation and amortization
|
774
|
|
722
|
|
|||
|
Accretion on marketable securities
|
(1,413
|
)
|
(209
|
)
|
|||
|
Amortization of debt issuance costs
|
259
|
|
259
|
|
|||
|
Change in fair value of redeemable convertible preferred stock warrants
|
—
|
|
826
|
|
|||
|
Loss on sale and disposal of property and equipment
|
171
|
|
52
|
|
|||
|
Changes in operating assets and liabilities
|
|||||||
|
Accounts receivable
|
(3,236
|
)
|
(1,028
|
)
|
|||
|
Inventory
|
(2,105
|
)
|
(759
|
)
|
|||
|
Prepaid expenses and other assets
|
(515
|
)
|
(752
|
)
|
|||
|
Accounts payable
|
383
|
|
251
|
|
|||
|
Accrued liabilities and other
|
4,994
|
|
1,260
|
|
|||
|
Net cash used in operating activities
|
(31,627
|
)
|
(14,519
|
)
|
|||
|
Cash flows from investing activities
|
|||||||
|
Maturities of marketable securities
|
159,800
|
|
—
|
|
|||
|
Purchases of marketable securities
|
(143,864
|
)
|
(96,883
|
)
|
|||
|
Purchases of property and equipment
|
(2,445
|
)
|
(942
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
13,491
|
|
(97,825
|
)
|
|||
|
Cash flows from financing activities
|
|||||||
|
Proceeds from initial public offering, net of underwriting discounts and commissions
|
—
|
|
115,506
|
|
|||
|
Proceeds from the exercise of common stock options
|
1,490
|
|
1,614
|
|
|||
|
Proceeds from issuance of common stock under employee stock purchase plan
|
2,203
|
|
—
|
|
|||
|
Repurchase of unvested early exercised stock options
|
(38
|
)
|
(73
|
)
|
|||
|
Payments of public offering costs
|
(167
|
)
|
(1,897
|
)
|
|||
|
Net cash provided by financing activities
|
3,488
|
|
115,150
|
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
(37
|
)
|
(94
|
)
|
|||
|
Net (decrease) increase in cash and cash equivalents
|
(14,685
|
)
|
2,712
|
|
|||
|
Cash and cash equivalents at
|
|||||||
|
Beginning of year
|
25,120
|
|
22,408
|
|
|||
|
End of year
|
$
|
10,435
|
|
$
|
25,120
|
|
|
|
Supplemental disclosure of cash flow information
|
|||||||
|
Cash paid for interest
|
$
|
4,949
|
|
$
|
5,500
|
|
|
|
Supplemental disclosure of non-cash information
|
|||||||
|
Conversion of redeemable convertible preferred stock to common stock
|
—
|
|
118,547
|
|
|||
|
Conversion of preferred stock warrants to common stock warrants
|
—
|
|
1,248
|
|
|||
|
Vesting of early exercised stock options
|
197
|
|
139
|
|
|||
|
Purchases of property and equipment included in accounts payable and accrued liabilities
|
375
|
|
82
|
|
|||
|
Public offering costs included in accounts payable
|
—
|
|
7
|
|
|||
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
United States
|
$
|
61,843
|
|
$
|
50,137
|
|
|
|
International
|
5,458
|
|
5,243
|
|
|||
|
$
|
67,301
|
|
$
|
55,380
|
|
||
|
Computer and office equipment
|
3 – 5 years
|
|
Machinery and equipment
|
3 – 5 years
|
|
Furniture and fixtures
|
7 years
|
|
•
|
Expected Term -
The expected term represents the period that the share-based awards are expected to be outstanding. The Company uses the simplified method to determine the expected term as permitted by the guidance since the Company has no sufficient historical exercise patterns to estimate the expected life. The simplified method is calculated as the average of the time to vesting and the contractual life of the options.
|
|
•
|
Expected Volatility -
Since the Company became public in October 2018 and has no sufficient trading history, the Company uses stock price volatility using the average historical volatilities of publicly traded companies within its industry that the Company considers to be comparable to its business over a period approximately equal to the expected term for its stock options.
|
|
•
|
Risk-Free Interest Rate -
The risk-free interest rate is based on the U.S. Treasury zero coupon issued in effect at the time of grant for periods corresponding with the expected term of the option.
|
|
•
|
Dividend Yield -
The Company has not paid any dividends and has no current plans to pay dividends on its common stock. As such, the Company uses expected dividend yield of zero.
|
|
December 31, 2019
|
|||||||||||||||
|
Amortized Cost
|
Unrealized Gains
|
Unrealized Losses
|
Aggregate Fair Value
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Money market funds
|
$
|
3,068
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,068
|
|
|||
|
Commercial paper
|
2,495
|
|
—
|
|
—
|
|
2,495
|
|
|||||||
|
Cash equivalents
|
5,563
|
|
—
|
|
—
|
|
5,563
|
|
|||||||
|
U.S. treasury securities
|
67,051
|
|
34
|
|
(2
|
)
|
67,083
|
|
|||||||
|
Corporate bonds
|
9,075
|
|
24
|
|
(2
|
)
|
9,097
|
|
|||||||
|
Commercial paper
|
5,165
|
|
—
|
|
—
|
|
5,165
|
|
|||||||
|
Short-term investments
|
81,291
|
|
58
|
|
(4
|
)
|
81,345
|
|
|||||||
|
Corporate bonds
|
1,278
|
|
—
|
|
—
|
|
1,278
|
|
|||||||
|
Long-term investments
|
1,278
|
|
—
|
|
—
|
|
1,278
|
|
|||||||
|
Total marketable securities
|
$
|
88,132
|
|
$
|
58
|
|
$
|
(4
|
)
|
$
|
88,186
|
|
|||
|
December 31, 2018
|
|||||||||||||||
|
Amortized Cost
|
Unrealized Gains
|
Unrealized Losses
|
Aggregate Fair Value
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Money market funds
|
$
|
15,223
|
|
$
|
—
|
|
$
|
—
|
|
$
|
15,223
|
|
|||
|
U.S. treasury securities
|
1,000
|
|
—
|
|
—
|
|
1,000
|
|
|||||||
|
Commercial paper
|
6,635
|
|
—
|
|
—
|
|
6,635
|
|
|||||||
|
Cash equivalents
|
22,858
|
|
—
|
|
—
|
|
22,858
|
|
|||||||
|
U.S. treasury securities
|
65,491
|
|
2
|
|
(4
|
)
|
65,489
|
|
|||||||
|
Corporate bonds
|
19,708
|
|
15
|
|
(3
|
)
|
19,720
|
|
|||||||
|
Commercial paper
|
11,894
|
|
—
|
|
—
|
|
11,894
|
|
|||||||
|
Short-term investments
|
97,093
|
|
17
|
|
(7
|
)
|
97,103
|
|
|||||||
|
Total marketable securities
|
$
|
119,951
|
|
$
|
17
|
|
$
|
(7
|
)
|
$
|
119,961
|
|
|||
|
December 31, 2019
|
|||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Marketable securities
|
|||||||||||||||
|
Money market funds
|
$
|
3,068
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,068
|
|
|||
|
U.S. treasury securities
|
67,083
|
|
—
|
|
—
|
|
67,083
|
|
|||||||
|
Corporate bonds
|
—
|
|
10,375
|
|
—
|
|
10,375
|
|
|||||||
|
Commercial paper
|
—
|
|
7,660
|
|
—
|
|
7,660
|
|
|||||||
|
Total marketable securities
|
$
|
70,151
|
|
$
|
18,035
|
|
$
|
—
|
|
$
|
88,186
|
|
|||
|
December 31, 2018
|
|||||||||||||||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Marketable securities
|
|||||||||||||||
|
Money market funds
|
$
|
15,223
|
|
$
|
—
|
|
$
|
—
|
|
$
|
15,223
|
|
|||
|
U.S. treasury securities
|
66,489
|
|
—
|
|
—
|
|
66,489
|
|
|||||||
|
Corporate bonds
|
—
|
|
19,720
|
|
—
|
|
19,720
|
|
|||||||
|
Commercial paper
|
—
|
|
18,529
|
|
—
|
|
18,529
|
|
|||||||
|
Total marketable securities
|
$
|
81,712
|
|
$
|
38,249
|
|
$
|
—
|
|
$
|
119,961
|
|
|||
|
December 31, 2019
|
December 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Machinery and equipment
|
$
|
4,613
|
|
$
|
3,785
|
|
|
|
Construction in progress
|
1,854
|
|
730
|
|
|||
|
Computer and office equipment
|
598
|
|
407
|
|
|||
|
Leasehold improvements
|
497
|
|
448
|
|
|||
|
Furniture and fixtures
|
187
|
|
148
|
|
|||
|
7,749
|
|
5,518
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(3,795
|
)
|
(3,364
|
)
|
|||
|
$
|
3,954
|
|
$
|
2,154
|
|
||
|
December 31, 2019
|
December 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Accrued compensation and related expenses
|
$
|
7,274
|
|
$
|
5,425
|
|
|
|
Accrued litigation expense
|
3,200
|
|
—
|
|
|||
|
Accrued professional services
|
392
|
|
583
|
|
|||
|
Sales tax payable
|
370
|
|
388
|
|
|||
|
Liability for early exercise of unvested stock options
|
97
|
|
331
|
|
|||
|
Others
|
272
|
|
133
|
|
|||
|
$
|
11,605
|
|
$
|
6,860
|
|
||
|
Year Ending December 31,
|
(in thousands)
|
|
|
|
2020
|
$
|
1,102
|
|
|
2021
|
1,003
|
|
|
|
2022
|
929
|
|
|
|
2023
|
860
|
|
|
|
2024
|
869
|
|
|
|
Thereafter
|
365
|
|
|
|
$
|
5,128
|
|
|
|
December 31, 2019
|
December 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Principal outstanding
|
$
|
40,000
|
|
$
|
40,000
|
|
|
|
Less: unamortized debt issuance costs
|
(777
|
)
|
(1,037
|
)
|
|||
|
Outstanding debt, net of debt issuance costs
|
$
|
39,223
|
|
$
|
38,963
|
|
|
|
Classified as:
|
|||||||
|
Current portion of long-term borrowings
|
$
|
4,358
|
|
$
|
—
|
|
|
|
Long-term borrowings
|
$
|
34,865
|
|
$
|
38,963
|
|
|
|
Year ending December 31,
|
(in thousands)
|
||
|
2020
|
$
|
4,444
|
|
|
2021
|
17,778
|
|
|
|
2022
|
17,778
|
|
|
|
Total minimum principal payments
|
$
|
40,000
|
|
|
Twelve Months Ending
|
Minimum Net Sales
|
Trailing 12-Month Consolidated EBITDA
|
||
|
(in thousands)
|
||||
|
March 31, 2020
|
$57,500
|
or
|
$1,000
|
|
|
June 30, 2020
|
$58,500
|
or
|
$2,000
|
|
|
thereafter, as applicable
|
$60,000
|
or
|
$3,000
|
|
|
Date
|
Number of
Shares Underlying Warrants |
Price per
Share |
Fair Value
(in thousands)
|
||||||||
|
Issuance
|
Expiration
|
||||||||||
|
3/1/2017
|
3/1/2027
|
[a]
|
1,388
|
$5.94
|
$
|
5
|
|
[b]
|
|||
|
7/22/2013
|
7/22/2023
|
[a]
|
32,983
|
$9.10
|
122
|
|
[b]
|
||||
|
11/26/2014
|
11/26/2024
|
[a]
|
6,680
|
$16.47
|
49
|
|
[b]
|
||||
|
10/20/2015
|
10/20/2025
|
[a]
|
41,650
|
$16.47
|
396
|
|
[c]
|
||||
|
11/9/2015
|
11/9/2025
|
[a]
|
25,709
|
$16.47
|
244
|
|
[c]
|
||||
|
12/22/2016
|
12/22/2026
|
[a]
|
9,712
|
$10.03
|
45
|
|
[c]
|
||||
|
118,122
|
$
|
861
|
|
||||||||
|
[a]
|
Common stock warrants will remain outstanding until the earlier of the expiration date or the date exercised by the holder.
|
|
[b]
|
Fair value at the date of issuance.
|
|
Options Outstanding
|
||||||||||
|
Number of Shares
|
Weighted-Average Exercise Price
|
Weighted-Average Contractual Remaining Life (Years)
|
Aggregate Intrinsic Value (in thousands)
|
|||||||
|
Outstanding as of December 31, 2017
|
3,001,929
|
|
$4.15
|
|||||||
|
Granted
|
100,080
|
|
$8.88
|
|||||||
|
Exercised
|
(395,117
|
)
|
$4.08
|
|||||||
|
Canceled and forfeited
|
(65,694
|
)
|
$4.81
|
|||||||
|
Outstanding as of December 31, 2018
|
2,641,198
|
|
$4.27
|
|||||||
|
Granted
|
638,983
|
|
$20.89
|
|||||||
|
Exercised
|
(444,924
|
)
|
$3.36
|
|||||||
|
Canceled and forfeited
|
(116,286
|
)
|
$11.30
|
|||||||
|
Outstanding as of December 31, 2019
|
2,718,971
|
|
$8.02
|
6.83
|
$
|
36,872
|
|
|||
|
Options vested and exercisable as of December 31, 2019
|
2,126,781
|
|
$5.32
|
6.27
|
$
|
34,463
|
|
|||
|
Options vested and expected to vest as of December 31, 2019
|
2,652,883
|
|
$7.80
|
6.76
|
$
|
36,540
|
|
|||
|
Exercise Price
|
Options Outstanding
|
Options Vested and Exercisable
|
||||||||||||
|
Number of Shares
|
Average
Remaining Contractual Life (Years) |
Weighted-
Average Exercise Price |
Number of Shares
|
Weighted-
Average Exercise Price |
||||||||||
|
$0.84
|
-
|
$2.11
|
87,394
|
|
1.52
|
$1.56
|
87,394
|
|
$1.52
|
|||||
|
$3.24
|
-
|
$3.98
|
564,938
|
|
4.35
|
$3.47
|
564,938
|
|
$3.50
|
|||||
|
$4.32
|
-
|
$5.94
|
1,409,650
|
|
7.11
|
$4.63
|
1,306,033
|
|
$4.61
|
|||||
|
$6.84
|
-
|
$7.92
|
39,844
|
|
7.96
|
$7.32
|
20,298
|
|
$7.20
|
|||||
|
$17.72
|
-
|
$18.96
|
154,189
|
|
9.37
|
$17.85
|
51,110
|
|
$17.82
|
|||||
|
$19.02
|
-
|
$22.00
|
462,956
|
|
9.05
|
$21.91
|
97,008
|
|
$21.94
|
|||||
|
2,718,971
|
|
6.83
|
$8.02
|
2,126,781
|
|
$5.32
|
||||||||
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Expected term (years)
|
5.0
|
to
|
7.0
|
5.0
|
to
|
7.0
|
|
|
Expected volatility
|
41.7%
|
to
|
47.3%
|
42.0%
|
to
|
47.0%
|
|
|
Risk-free interest rate
|
1.3%
|
to
|
2.6%
|
2.4%
|
to
|
3.0%
|
|
|
Dividend yield
|
—%
|
—%
|
|||||
|
Number of
Shares |
Weighted-
Average Grant Date Fair Value |
||||
|
Outstanding as of December 31, 2017
|
—
|
|
$—
|
||
|
Granted
|
54,036
|
|
$11.79
|
||
|
Canceled and forfeited
|
(600
|
)
|
$20.60
|
||
|
Outstanding as of December 31, 2018
|
53,436
|
|
$11.69
|
||
|
Granted
|
639,726
|
|
$20.14
|
||
|
Vested
|
(108,631
|
)
|
$19.10
|
||
|
Canceled and forfeited
|
(41,490
|
)
|
$18.48
|
||
|
Outstanding as of December 31, 2019
|
543,041
|
|
$19.72
|
||
|
Year ended December 31,
|
|||||
|
2019
|
2018
|
||||
|
Expected term (years)
|
0.5
|
0.5
|
|||
|
Expected volatility
|
38.3%
|
to
|
58.4%
|
44.0%
|
|
|
Risk-free interest rate
|
1.6%
|
to
|
2.4%
|
2.5%
|
|
|
Dividend yield
|
—%
|
—%
|
|||
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Cost of goods sold
|
$
|
185
|
|
$
|
34
|
|
|
|
Sales and marketing
|
3,335
|
|
651
|
|
|||
|
Research and development
|
516
|
|
156
|
|
|||
|
General and administrative
|
3,428
|
|
1,471
|
|
|||
|
$
|
7,464
|
|
$
|
2,312
|
|
||
|
Year ended December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
(in thousands, except share and per share data)
|
||||||||
|
Net loss
|
$
|
(38,403
|
)
|
$
|
(17,453
|
)
|
||
|
Weighted-average shares used to compute basic and diluted net loss per share
|
24,705,980
|
|
7,950,284
|
|
*
|
|||
|
Net loss per share, basic and diluted
|
$
|
(1.55
|
)
|
$
|
(2.20
|
)
|
||
|
*
|
Calculated based on the 1-for-18 reverse stock split effected October 4, 2018.
|
|
Year ended December 31,
|
||||
|
2019
|
2018
|
|||
|
Stock options
|
2,718,971
|
2,641,198
|
||
|
Restricted stock units
|
543,041
|
53,436
|
||
|
Shares subject to repurchase
|
21,404
|
74,019
|
||
|
ESPP purchase rights
|
65,442
|
89,606
|
|
|
|
Common stock warrants
|
118,122
|
118,122
|
||
|
3,466,980
|
2,976,381
|
|||
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Domestic
|
$
|
(37,709
|
)
|
$
|
(16,835
|
)
|
|
|
Foreign
|
(694
|
)
|
(618
|
)
|
|||
|
Loss before income taxes
|
$
|
(38,403
|
)
|
$
|
(17,453
|
)
|
|
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Federal
|
$
|
8,523
|
|
$
|
3,555
|
|
|
|
State
|
1,569
|
|
822
|
|
|||
|
Foreign
|
(200
|
)
|
200
|
|
|||
|
Total deferred income taxes
|
9,892
|
|
4,577
|
|
|||
|
Change in deferred tax valuation allowance
|
(9,892
|
)
|
(4,577
|
)
|
|||
|
Net deferred income tax
|
$
|
—
|
|
$
|
—
|
|
|
|
Year ended December 31,
|
|||||
|
2019
|
2018
|
||||
|
Tax at statutory federal rate
|
(21.0
|
)%
|
(21.0
|
)%
|
|
|
State tax, net of federal benefit
|
(4.1
|
)%
|
(5.3
|
)%
|
|
|
Tax credits
|
(0.7
|
)%
|
(0.7
|
)%
|
|
|
Change in deferred tax valuation allowance
|
25.8
|
%
|
26.2
|
%
|
|
|
Other
|
—
|
%
|
0.8
|
%
|
|
|
Total income tax expense
|
—
|
%
|
—
|
%
|
|
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Net operating loss carryforwards
|
$
|
42,032
|
|
$
|
35,067
|
|
|
|
Research and development credits
|
2,428
|
|
2,255
|
|
|||
|
Accruals and reserves
|
4,222
|
|
2,059
|
|
|||
|
Stock compensation
|
1,512
|
|
899
|
|
|||
|
Depreciation and amortization
|
110
|
|
132
|
|
|||
|
Total deferred tax assets
|
50,304
|
|
40,412
|
|
|||
|
Less: Valuation allowance
|
(50,304
|
)
|
(40,412
|
)
|
|||
|
Total deferred tax asset, net of valuation allowance
|
$
|
—
|
|
$
|
—
|
|
|
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Beginning balance
|
$
|
40,412
|
|
$
|
35,835
|
|
|
|
Additions during the period
|
9,892
|
|
4,577
|
|
|||
|
Ending balance
|
$
|
50,304
|
|
$
|
40,412
|
|
|
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Balance at beginning of the year
|
$
|
1,084
|
|
$
|
993
|
|
|
|
Increases related to current year's tax positions
|
203
|
|
91
|
|
|||
|
Balance at end of the year
|
$
|
1,287
|
|
$
|
1,084
|
|
|
|
Incorporation By Reference
|
||||||||||
|
Exhibit
Number |
Description
|
Form
|
SEC File No.
|
Exhibit
|
Filing Date
|
|||||
|
3.1
|
8-K
|
001-38701
|
3.1
|
10/19/2018
|
||||||
|
3.2
|
S-1/A
|
333-227445
|
3.4
|
10/5/2018
|
||||||
|
4.1
|
S-1/A
|
333-227445
|
4.1
|
10/5/2018
|
||||||
|
4.2
|
||||||||||
|
4.3
|
||||||||||
|
10.1+
|
S-1
|
333-227445
|
10.1
|
9/20/2018
|
||||||
|
10.2+
|
S-1/A
|
333-227445
|
10.2
|
10/5/2018
|
||||||
|
10.3+
|
S-1/A
|
333-227445
|
10.3
|
10/5/2018
|
||||||
|
10.4+
|
S-1/A
|
333-227445
|
10.4
|
10/5/2018
|
||||||
|
10.5+
|
S-1/A
|
333-227445
|
10.5
|
10/5/2018
|
||||||
|
10.6+
|
S-1/A
|
333-227445
|
10.6
|
10/5/2018
|
||||||
|
10.7#
|
S-1
|
333-227445
|
10.6
|
9/20/2018
|
||||||
|
10.8+
|
S-1
|
333-227445
|
10.7
|
9/20/2018
|
||||||
|
10.9+
|
S-1
|
333-227445
|
10.8
|
9/20/2018
|
||||||
|
10.10+
|
S-1
|
333-227445
|
10.9
|
9/20/2018
|
||||||
|
10.11+
|
S-1
|
333-227445
|
10.10
|
9/20/2018
|
||||||
|
10.12+
|
S-1
|
333-227445
|
10.11
|
9/20/2018
|
||||||
|
10.13+
|
S-1
|
333-227445
|
10.12
|
9/20/2018
|
||||||
|
10.14+
|
S-1
|
333-227445
|
10.13
|
9/20/2018
|
||||||
|
10.15+
|
S-1
|
333-227445
|
10.14
|
9/20/2018
|
||||||
|
10.16+
|
S-1
|
333-227445
|
10.15
|
9/20/2018
|
||||||
|
10.17+
|
S-1
|
333-227445
|
10.16
|
9/20/2018
|
||||||
|
10.18+
|
S-1
|
333-227445
|
10.17
|
9/20/2018
|
||||||
|
10.19+
|
S-1
|
333-227445
|
10.18
|
9/20/2018
|
||||||
|
10.20
|
S-1/A
|
333-227445
|
10.21
|
10/5/2018
|
||||||
|
10.21
|
S-1
|
333-227445
|
10.20
|
9/20/2018
|
||||||
|
10.22
|
S-1
|
333-227445
|
10.21
|
9/20/2018
|
||||||
|
10.23
|
S-1
|
333-227445
|
10.22
|
9/20/2018
|
||||||
|
10.24
|
S-1
|
333-227445
|
10.23
|
9/20/2018
|
||||||
|
10.25
|
S-1
|
333-227445
|
10.24
|
9/20/2018
|
||||||
|
10.26
|
S-1
|
333-227445
|
10.25
|
9/20/2018
|
||||||
|
10.27
|
S-1
|
333-227445
|
10.26
|
9/20/2018
|
||||||
|
10.28
|
S-1
|
333-227445
|
10.27
|
9/20/2018
|
||||||
|
10.29
|
S-1
|
333-227445
|
10.28
|
9/20/2018
|
||||||
|
10.30
|
S-1
|
333-227445
|
10.29
|
9/20/2018
|
||||||
|
10.31+
|
S-1
|
333-227445
|
10.30
|
9/20/2018
|
||||||
|
10.32+
|
8-K
|
001-38701
|
Item 5.02
|
8/6/2019
|
||||||
|
10.33+
|
10-Q
|
001-38701
|
10.2
|
11/12/2019
|
||||||
|
10.34+
|
8-K
|
001-38701
|
Item 5.02
|
1/3/2020
|
||||||
|
10.35+
|
8-K
|
001-38701
|
Item 5.02
|
1/22/2019
|
||||||
|
10.36+*
|
||||||||||
|
21.1*
|
||||||||||
|
23.1*
|
||||||||||
|
24.1*
|
||||||||||
|
31.1*
|
||||||||||
|
31.2*
|
||||||||||
|
32.1**
|
||||||||||
|
101.INS
|
XBRL Instance Document
|
|||||||||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|||||||||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|||||||||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|||||||||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|||||||||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|||||||||
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith. Exhibit 32.1 is being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section, nor shall such exhibit be deemed to be incorporated by reference in any registration statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as otherwise specifically stated in such filing.
|
|
+
|
Indicates a management contract or compensatory plan.
|
|
#
|
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
|
|
SI-BONE, Inc.
|
||
|
By:
|
/s/ Jeffrey W. Dunn
|
|
|
Jeffrey W. Dunn
|
||
|
President and Chief Executive Officer
|
||
|
(Duly Authorized Officer and Principal Executive Officer
)
|
||
|
SI-BONE, Inc.
|
||
|
By:
|
/s/ Laura A. Francis
|
|
|
Laura A. Francis
|
||
|
Chief Operating Officer & Chief Financial Officer
|
||
|
(Principal Financial and Accounting Officer)
|
||
|
Signature
|
|
Title
|
|
Date
|
|
/s/ Jeffrey W. Dunn
|
President and Chief Executive Officer
|
March 11, 2020
|
||
|
Jeffrey W. Dunn
|
(Principal Executive Officer) and Director
|
|||
|
/s/ Laura A. Francis
|
Chief Operating Officer & Chief Financial Officer
|
March 11, 2020
|
||
|
Laura A. Francis
|
(Principal Financial and Accounting Officer)
|
|||
|
/s/ Timothy E. Davis, Jr.
|
Lead Independent Director, Director
|
March 11, 2020
|
||
|
Timothy E. Davis, Jr.
|
||||
|
/s/ Mark J. Foley
|
Director
|
March 11, 2020
|
||
|
Mark J. Foley
|
||||
|
/s/John G. Freund, M.D.
|
Director
|
March 11, 2020
|
||
|
John G. Freund, M.D.
|
||||
|
/s/ Jeryl L. Hilleman
|
Director
|
March 11, 2020
|
||
|
Jeryl L. Hilleman
|
||||
|
/s/ Gregory K. Hinckley
|
Director
|
March 11, 2020
|
||
|
Gregory K. Hinckley
|
||||
|
/s/ Karen A. Licitra
|
Director
|
March 11, 2020
|
||
|
Karen A. Licitra
|
||||
|
/s/ Keith C. Valentine
|
Director
|
March 11, 2020
|
||
|
Keith C. Valentine
|
||||