|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¬
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
20-8099512
|
|
(State of incorporation)
|
|
(I.R.S. Employer Identification No.)
|
|
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
||
|
Title of Each Class
|
|
Name of Exchange on Which Registered
|
|
Common Stock, $0.0001 Par Value per Share
|
|
The NASDAQ Capital Market
|
|
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
||
|
None
|
||
|
Large accelerated filer
|
o
|
Accelerated filer
|
ý
|
|||
|
Non-accelerated filer
|
o
|
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
o
|
||
|
Part
No.
|
Item
No.
|
Description
|
Page
No.
|
||
|
I
|
1
|
Business
|
|||
|
1A
|
Risk Factors
|
||||
|
1B
|
Unresolved Staff Comments
|
||||
|
2
|
Properties
|
||||
|
3
|
Legal Proceedings
|
||||
|
4
|
Mine Safety Disclosures
|
||||
|
II
|
5
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|||
|
6
|
Selected Financial Data
|
||||
|
7
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
||||
|
7A
|
Quantitative and Qualitative Disclosures About Market Risk
|
||||
|
II
|
8
|
Financial Statements and Supplementary Data
|
|||
|
9
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures
|
||||
|
9A
|
Controls and Procedures
|
||||
|
9B
|
Other Information
|
||||
|
III
|
10
|
Directors, Executive Officers and Corporate Governance
|
|||
|
11
|
Executive Compensation
|
||||
|
12
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
||||
|
13
|
Certain Relationships and Related Transactions, and Director Independence
|
||||
|
14
|
Principal Accountant Fees and Services
|
||||
|
Index to Exhibits
|
|||||
|
EX-31.1
|
|||||
|
EX-31.2
|
|||||
|
EX-32.1
|
|||||
|
•
|
Develop novel cancer immunotherapies
to address unmet medical needs through the use of peptide based vaccines targeting well-established tumor antigens in the adjuvant, minimum residual disease setting, in high risk patients who are more likely to benefit from treatment via immunotherapy. Our immunotherapy programs currently seek to significantly decrease the risk of disease recurrence in breast cancer, gastric cancer, endometrial and ovarian cancers.
|
|
•
|
Expand our development pipeline
by enhancing the potential clinical and geographic footprint of our technologies. We can accomplish this through the initiation of additional clinical trials as well as through acquisition of additional development stage products in related oncology indications. We also seek to leverage valuable partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner.
|
|
•
|
Maintain commercial capabilities
to sell, market, and distribute oncology related pharmaceutical products in the U.S. through our established commercial infrastructure. This commercial strategy creates the opportunity to generate accretive cash flows to support our development programs, and also provides future leverage to support the potential commercialization of our clinical stage technologies in one of the world's largest economic markets.
|

|
•
|
Phase 3 Ongoing: Our Phase 3 PRESENT (
P
revention of
R
ecurrence in
E
arly-
S
tage, Node-Positive Breast Cancer with Low to Intermediate HER2
E
xpression with
NeuVax T
reatment) study is enrolling HER2 1+ and 2+ patients under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). The multinational, multicenter, randomized, double-blinded PRESENT trial is ongoing in North America, Western and Eastern Europe, and Israel. Additional information on the study can be found at
www.neuvax.com
.
|
|
•
|
Phase 2b Ongoing: A randomized, multicenter, investigator-sponsored, 300 patient Phase 2b clinical trial is enrolling HER2 1+/2+ node-positive and high-risk node-negative breast cancer patients to study NeuVax in combination with Herceptin
®
(trastuzumab; Genentech/Roche) in the adjuvant setting.
|
|
•
|
Phase 2 Ongoing: An investigator-sponsored trial is ongoing to study NeuVax in combination with Herceptin. The study will enroll 100 patients in neoadjuvant, node positive and negative HER2 IHC 3+ patients or HER2 gene-amplified breast cancer patients who are HLA A2+ or HLA A3+ and are determined to be at high-risk for recurrence. Partial funding for this trial comes from the Department of Defense (DoD) through the Congressionally Directed Medical Research Program (CDMRP) via legislation known as the Defense Appropriations Act. The grant was awarded under a Breast Cancer Research Program (BCRP) Breakthrough Award given to the lead investigator for the trial.
|
|
•
|
Phase 2 Planned: In January 2014, we partnered with Dr. Reddy’s Laboratories, Ltd. in India for the commercialization of NeuVax in that region. Dr. Reddy’s is responsible for running a Phase 2 gastric cancer trial of NeuVax in India that is expected to initiate in 2016.
|
|
Product
|
Indication
|
Scope
|
Strategic Partner
|
Estimated Exclusivity Period
|
||||
|
Abstral® (fentanyl) Sublingual Tablets
|
Breakthrough cancer pain
|
U.S.
|
Orexo AB
|
2019
|
||||
|
NeuVax™ (nelipepimut-S)
|
Breast cancer recurrence
|
Filed and pending or issued worldwide
|
University of Texas/MDACC/Henry M. Jackson Foundation
|
2028
|
||||
|
NeuVax™ in combination with Herceptin®
|
Breast cancer recurrence
|
Filed and pending or issued worldwide
|
Henry M. Jackson Foundation, Genentech/Roche
|
2026
|
||||
|
Folate Binding Protein (GALE-301)
|
Ovarian and endometrial cancer
|
Filed and pending or issued worldwide
|
Henry M. Jackson Foundation
|
2022
|
||||
|
Anagrelide Controlled Release (GALE-401)
|
Essential thrombocythemia
|
Filed and pending or issued worldwide
|
BioVascular, Inc.
|
2029
|
||||
|
•
|
our ability to communicate acceptable evidence of safety and efficacy;
|
|
•
|
acceptance by physicians and patients of the product as a safe and effective treatment;
|
|
•
|
the relative convenience and ease of administration;
|
|
•
|
the prevalence and severity of adverse side effects;
|
|
•
|
limitations or warnings contained in our products’ FDA-approved labeling;
|
|
•
|
the clinical indications for which our products are approved;
|
|
•
|
availability and perceived advantages of alternative treatments;
|
|
•
|
any negative publicity related to Abstral or Zuplenz or our competitors’ products;
|
|
•
|
the effectiveness of our or any current or future collaborators’ sales, marketing and distribution strategies;
|
|
•
|
pricing and cost effectiveness;
|
|
•
|
our ability to obtain sufficient third-party payor coverage and reimbursement;
|
|
•
|
the effectiveness of our patient assistance efforts; and
|
|
•
|
our ability to maintain compliance with regulatory requirements.
|
|
•
|
continue to improve our operational, financial, management and regulatory compliance controls and reporting systems and procedures;
|
|
•
|
attract and retain sufficient numbers of talented employees;
|
|
•
|
manage our commercialization activities in a cost-effective manner; and
|
|
•
|
carry out our contractual obligations to contractors and other third parties.
|
|
•
|
decreased demand for our products;
|
|
•
|
impairment of our business reputation;
|
|
•
|
product recall or withdrawal from the market;
|
|
•
|
costs of related litigation;
|
|
•
|
distraction of management’s attention from our primary business;
|
|
•
|
substantial monetary awards to patients or other claimants; or
|
|
•
|
loss of revenue.
|
|
•
|
impose restrictions on the marketing or manufacturing of our products, suspend or withdraw product approvals or revoke necessary licenses;
|
|
•
|
issue warning letters, show cause notices or untitled letters describing alleged violations, which may be publicly available;
|
|
•
|
commence criminal investigations and prosecutions;
|
|
•
|
impose injunctions, suspensions or revocations of necessary approvals or other licenses;
|
|
•
|
impose fines or other civil or criminal penalties;
|
|
•
|
deny or reduce quota allotments for the raw material for commercial production of our products;
|
|
•
|
suspend or impose restrictions on operations, including costly new manufacturing requirements; or
|
|
•
|
seize our products or require us to initiate a product recall.
|
|
•
|
regulatory authorities may withdraw its approval of our products;
|
|
•
|
regulatory authorities may require us to recall our products;
|
|
•
|
regulatory authorities may require the addition of warnings in the product label or narrowing of the indication in the product label;
|
|
•
|
we may be required to update our Medication Guide outlining the risks of such side effects for distribution to patients;
|
|
•
|
we may be required to change the way our products are administered or modify our products in other ways;
|
|
•
|
the FDA may require us to conduct additional clinical trials or costly post-marketing testing and surveillance to monitor the safety or efficacy of our products;
|
|
•
|
we could be sued and held liable for harm caused to patients; and
|
|
•
|
our business and results of operations and our reputation may suffer.
|
|
•
|
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to its market share in certain government healthcare programs;
|
|
•
|
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, retroactive to January 1, 2010, to 23% and 13% of the average manufacturer price for most branded and generic drugs, respectively;
|
|
•
|
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during its coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D;
|
|
•
|
extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations;
|
|
•
|
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing both the volume of sales and manufacturers’ Medicaid rebate liability;
|
|
•
|
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
|
•
|
new requirements to report certain financial arrangements with physicians and teaching hospitals, as defined in the PPACA and its implementing regulations, including reporting any “transfer of value” made or distributed to teaching hospitals, prescribers, and other healthcare providers and reporting any ownership and investment interests held by physicians and its immediate family members and applicable group purchasing organizations during the preceding calendar year, with data collection required and reporting to the Centers for Medicare & Medicaid Services (the “CMS”) required by the 90th day of each calendar year;
|
|
•
|
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians;
|
|
•
|
expansion of health care fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
|
|
•
|
a licensure framework for follow-on biologic products;
|
|
•
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
|
|
•
|
creation of the Independent Payment Advisory Board which, beginning in 2014, will have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription
|
|
•
|
establishment of a Center for Medicare Innovation at the CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
|
|
•
|
difficulties or delays in enrolling patients in our Phase 1/2 clinical trials of GALE-301 (folate binding protein (FBP) vaccine), our Phase 2 clinical trial of GALE-401 (anagrelide controlled release) or other clinical trials in conformity with required protocols or projected timeline or in our other NeuVax clinical trials;
|
|
•
|
conditions imposed on us by the FDA, including the possibility that the FDA would require an additional Phase 3 trial of NeuVax, or comparable foreign authorities regarding the scope or design of our clinical trials;
|
|
•
|
difficulties or delays in arranging for third parties to conduct clinical trials of our product candidates;
|
|
•
|
problems in engaging IRBs to oversee trials or problems in obtaining or maintaining IRB approval of studies;
|
|
•
|
third-party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner;
|
|
•
|
our drug candidates having very different chemical and pharmacological properties in humans than in laboratory testing and interacting with human biological systems in unforeseen, ineffective or harmful ways, and the possibility that our previous Phase 2 trials will not be indicative of our drug candidates’ performance in larger patient populations;
|
|
•
|
the need to suspend or terminate our clinical trials if the participants are being exposed to unacceptable health risks;
|
|
•
|
insufficient or inadequate supply or quality of our drug candidates or other necessary materials necessary to conduct our clinical trials;
|
|
•
|
disruption at our foreign clinical trial sites resulting from local social or political unrest or other geopolitical factors;
|
|
•
|
effects of our drug candidates not being the desired effects or including undesirable side effects or the drug candidates having other unexpected characteristics;
|
|
•
|
negative or inconclusive results from our clinical trials or the clinical trials of others for drug candidates similar to our own or inability to generate statistically significant data confirming the efficacy of the product being tested;
|
|
•
|
adverse results obtained by other companies developing similar drugs;
|
|
•
|
modification of the drug during testing;
|
|
•
|
changes in the FDA’s requirements for our testing during the course of that testing; and
|
|
•
|
reallocation of our financial and other resources to other clinical programs.
|
|
•
|
differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries;
|
|
•
|
unexpected changes in tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign taxes, including withholding of payroll taxes;
|
|
•
|
foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country;
|
|
•
|
workforce uncertainty in countries where labor unrest is more common than in the U.S.;
|
|
•
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
|
|
•
|
business interruptions resulting from geopolitical actions, including war and terrorism.
|
|
•
|
reports of the results of our clinical trials regarding the safety or efficacy of our product candidates and surrogate markers;
|
|
•
|
announcements of regulatory developments or technological innovations by us or our competitors;
|
|
•
|
announcements of business or strategic transactions;
|
|
•
|
announcements of legal or regulatory actions against us or any adverse outcome of any such actions;
|
|
•
|
changes in our relationship with our licensors, licensees and other strategic partners;
|
|
•
|
our quarterly operating results;
|
|
•
|
developments in patent or other technology ownership rights;
|
|
•
|
public concern regarding the safety of our Abstral product or our product candidates;
|
|
•
|
additional funds may not be available on terms that are favorable to us and, in the case of equity financings, may result in dilution to our stockholders;
|
|
•
|
government regulation of drug pricing; and
|
|
•
|
general changes in the economy, the financial markets or the pharmaceutical or biotechnology industries.
|
|
•
|
divide our board of directors into three classes, with members of each class to be elected for staggered three-year terms;
|
|
•
|
limit the right of security holders to remove directors;
|
|
•
|
prohibit stockholders from acting by written consent;
|
|
•
|
regulate how stockholders may present proposals or nominate directors for election at annual meetings of stockholders; and
|
|
•
|
authorize our board of directors to issue preferred stock in one or more series, without stockholder approval.
|
|
High
|
Low
|
||||||
|
2013
|
|||||||
|
First Quarter
|
$
|
2.18
|
|
$
|
1.55
|
|
|
|
Second Quarter
|
3.00
|
|
1.92
|
|
|||
|
Third Quarter
|
2.53
|
|
1.65
|
|
|||
|
Fourth Quarter
|
5.30
|
|
2.01
|
|
|||
|
2014
|
|||||||
|
First Quarter
|
$
|
7.77
|
|
$
|
2.15
|
|
|
|
Second Quarter
|
3.58
|
|
1.66
|
|
|||
|
Third Quarter
|
3.36
|
|
2.00
|
|
|||
|
Fourth Quarter
|
2.26
|
|
1.48
|
|
|||
|
(a)
|
(b)
|
Number of
Securities
Remaining
Available for
Issuance
Under Equity
Compensation
Plans
(Excluding
Securities
Reflected in
Column (a))
|
|||||||
|
Plan Category
|
Number of
Securities to be
Issued Upon
Exercise of
Outstanding
Options,
Warrants and
Rights
|
Weighted-
Average
Exercise Price
of Outstanding
Options,
Warrants and
Rights
|
|||||||
|
Equity compensation plans approved by our security holders:
|
|||||||||
|
Amended and Restated 2007 Incentive Plan
|
8,590,961
|
|
$
|
3.25
|
|
2,887,304
|
|
||
|
Equity compensation plans not approved by our security holders:
|
|||||||||
|
Employee Stock Purchase Plan
|
NA
|
|
NA
|
|
641,859
|
|
|||
|
Outstanding warrants
(1)
|
719,686
|
|
$
|
4.15
|
|
—
|
|
||
|
Total
|
9,310,647
|
|
$
|
3.32
|
|
3,529,163
|
|
||
|
(1)
|
The warrants shown were issued in discreet transactions from time to time as compensation for services rendered by consultants, advisers or other third parties, and do not include warrants sold in private placement or public offering transactions. The material terms of such warrants were determined based upon arm’s-length negotiations with the services providers. The warrant exercise prices approximated the market price of our common stock at or about the date of grant, and the warrant terms range from three to ten years from the grant date.
|
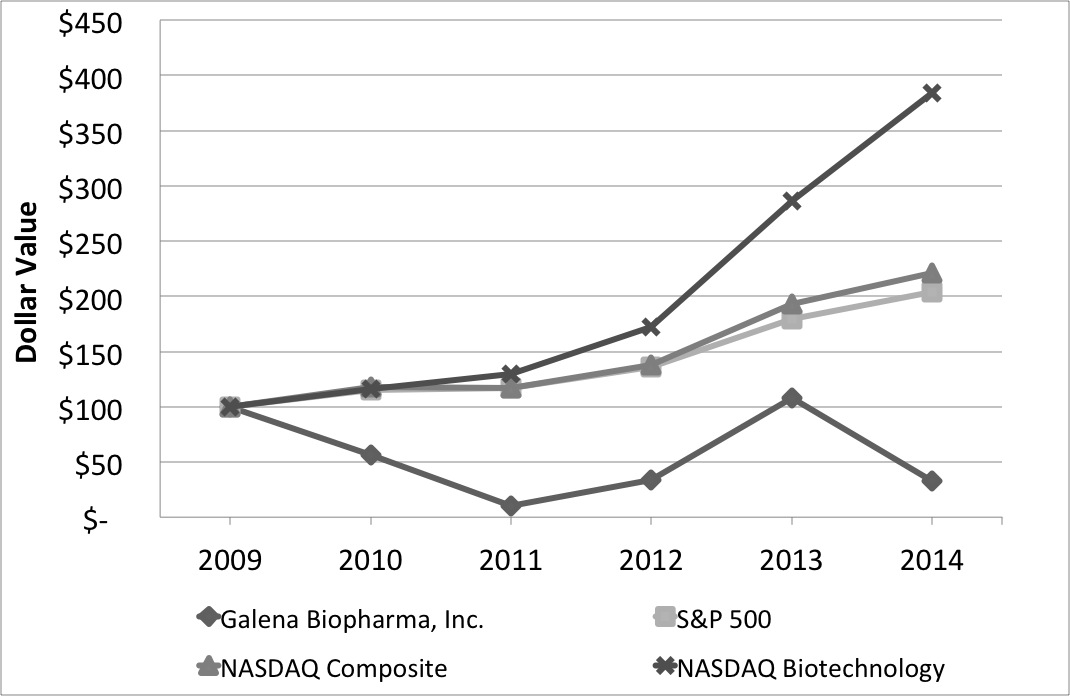
|
As of December 31,
|
|||||||||||||||||||||||
|
2009
|
2010
|
2011
|
2012
|
2013
|
2014
|
||||||||||||||||||
|
Galena Biopharma, Inc.
(1)
|
$
|
100.00
|
|
$
|
56.33
|
|
$
|
10.26
|
|
$
|
33.41
|
|
$
|
108.30
|
|
$
|
32.97
|
|
|||||
|
S&P 500
|
100.00
|
|
114.82
|
|
117.22
|
|
135.83
|
|
179.36
|
|
203.60
|
|
|||||||||||
|
NASDAQ Composite
|
100.00
|
|
117.99
|
|
117.08
|
|
137.81
|
|
192.78
|
|
221.15
|
|
|||||||||||
|
NASDAQ Biotechnology
|
100.00
|
|
115.95
|
|
129.93
|
|
172.44
|
|
286.14
|
|
384.45
|
|
|||||||||||
|
Years Ended December 31,
|
|||||||||||||||||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Net revenue
(1)
|
$
|
9,319
|
|
$
|
2,487
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Operating expenses:
|
|||||||||||||||||||
|
Research and development
(1)
|
28,354
|
|
21,076
|
|
14,614
|
|
3,851
|
|
7,873
|
|
|||||||||
|
Selling, general, and administrative
(1)
|
31,344
|
|
14,600
|
|
6,585
|
|
8,635
|
|
8,752
|
|
|||||||||
|
Non-operating income (loss)
(1)
|
15,616
|
|
(41,786
|
)
|
(13,178
|
)
|
9,079
|
|
4,632
|
|
|||||||||
|
Loss from continuing operations
(1)
|
(36,606
|
)
|
(76,678
|
)
|
(33,325
|
)
|
(3,407
|
)
|
(11,993
|
)
|
|||||||||
|
Loss from continuing operations per share
(1)
|
(0.31
|
)
|
(0.85
|
)
|
(0.53
|
)
|
(0.09
|
)
|
(0.67
|
)
|
|||||||||
|
As of December 31,
|
|||||||||||||||||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Total assets
(1)
|
$
|
80,488
|
|
$
|
87,976
|
|
$
|
54,986
|
|
$
|
30,968
|
|
$
|
7,476
|
|
||||
|
Total debt
(1)
|
8,402
|
|
9,892
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Other long-term obligations
(1)
|
11,704
|
|
11,874
|
|
11,311
|
|
9,654
|
|
20
|
|
|||||||||
|
Total stockholders' equity
(1)
|
37,059
|
|
5,886
|
|
27,756
|
|
10,112
|
|
2,430
|
|
|||||||||
|
•
|
Develop novel cancer immunotherapies
to address unmet medical needs through the use of peptide based vaccines targeting well-established tumor antigens in the adjuvant, minimum residual disease setting, in high risk patients who are more likely to benefit from treatment via immunotherapy. Our immunotherapy programs currently seek to significantly decrease the risk of disease recurrence in breast cancer, gastric cancer, endometrial and ovarian cancers.
|
|
•
|
Expand our development pipeline
by enhancing the potential clinical and geographic footprint of our technologies. We can accomplish this through the initiation of additional clinical trials as well as through acquisition of additional development stage products in related oncology indications. We also seek to leverage valuable partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner.
|
|
•
|
Maintain commercial capabilities
to sell, market, and distribute oncology related pharmaceutical products in the U.S. through our established commercial infrastructure. This commercial strategy creates the opportunity to generate accretive cash flows to support our development programs, and also provides future leverage to support the potential commercialization of our clinical stage technologies in one of the world's largest economic markets.
|
|
•
|
Phase 3 Ongoing: Our Phase 3 PRESENT (
P
revention of
R
ecurrence in
E
arly-
S
tage, Node-Positive Breast Cancer with Low to Intermediate HER2
E
xpression with
NeuVax T
reatment) study is enrolling HER2 1+ and 2+ patients under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). The multinational, multicenter, randomized, double-blinded PRESENT trial is ongoing in North America, Western and Eastern Europe, and Israel. Additional information on the study can be found at
www.neuvax.com
.
|
|
•
|
Phase 2b Ongoing: A randomized, multicenter, investigator-sponsored, 300 patient Phase 2b clinical trial is enrolling HER2 1+/2+ node-positive and high-risk node-negative breast cancer patients to study NeuVax in combination with Herceptin
®
(trastuzumab; Genentech/Roche) in the adjuvant setting.
|
|
•
|
Phase 2 Ongoing: An investigator-sponsored trial is ongoing to study NeuVax in combination with Herceptin. The study will enroll 100 patients in neoadjuvant, node positive and negative HER2 IHC 3+ patients or HER2 gene-amplified breast cancer patients who are HLA A2+ or HLA A3+ and are determined to be at high-risk for recurrence. Partial funding for this trial comes from the Department of Defense (DoD) through the Congressionally Directed Medical Research Program (CDMRP) via legislation known as the Defense Appropriations Act. The grant was awarded under a Breast Cancer Research Program (BCRP) Breakthrough Award given to the lead investigator for the trial.
|
|
•
|
Phase 2 Planned: In January 2014, we partnered with Dr. Reddy’s Laboratories, Ltd. in India for the commercialization of NeuVax in that region. Dr. Reddy’s is responsible for running a Phase 2 gastric cancer trial of NeuVax in India that is expected to initiate in 2016.
|
|
2014
|
2013
|
|||||
|
Risk free interest rate
|
2.01
|
%
|
1.57
|
%
|
||
|
Volatility
|
79.37
|
%
|
77.98
|
%
|
||
|
Expected lives (years)
|
6.16
|
|
6.25
|
|
||
|
Expected dividend yield
|
0.00
|
%
|
0.00
|
%
|
||
|
2014
|
2013
|
||||
|
Risk free interest rate
|
NA
|
0.11% – 1.61%
|
|
||
|
Volatility
|
NA
|
66.85% – 73.45%
|
|
||
|
Expected lives (years)
|
NA
|
0.59 – 4.72
|
|
||
|
Expected dividend yield
|
NA
|
0.00
|
%
|
||
|
•
|
significant changes in the manner of its use of acquired assets or the strategy for its overall business;
|
|
•
|
significant negative industry or economic trends;
|
|
•
|
significant decline in stock price for a sustained period; and
|
|
•
|
significant decline in market capitalization relative to net book value.
|
|
2014
|
2013
|
% Change
|
|||||||||
|
Net revenue
|
$
|
9,319
|
|
$
|
2,487
|
|
275
|
%
|
|||
|
Year Ended December 31,
|
||||||||||||||
|
2014
|
2013
|
|||||||||||||
|
$
|
% of Gross revenue
|
$
|
% of Gross revenue
|
|||||||||||
|
Gross revenue
|
$
|
16,224
|
|
100
|
%
|
$
|
7,569
|
|
100
|
%
|
||||
|
Gross to net deductions
|
6,905
|
|
43
|
%
|
5,082
|
|
67
|
%
|
||||||
|
Net revenue
|
$
|
9,319
|
|
57
|
%
|
$
|
2,487
|
|
33
|
%
|
||||
|
Year Ended December 31,
|
|||||||||||||
|
2014
|
% of net revenue
|
2013
|
% of net revenue
|
||||||||||
|
Cost of revenue (excluding amortization of certain acquired intangible assets:
|
|||||||||||||
|
Abstral royalties
|
$
|
1,143
|
|
12
|
%
|
$
|
298
|
|
12
|
%
|
|||
|
Direct product costs and related overhead
|
82
|
|
1
|
%
|
91
|
|
4
|
%
|
|||||
|
Other cost of revenue
|
178
|
|
2
|
%
|
131
|
|
5
|
%
|
|||||
|
Total cost of revenue (excluding amortization of certain acquired intangible assets
|
$
|
1,403
|
|
15
|
%
|
$
|
520
|
|
21
|
%
|
|||
|
Amortization of certain acquired intangible assets
|
$
|
440
|
|
5
|
%
|
$
|
131
|
|
5
|
%
|
|||
|
Year Ended December 31,
|
||||||||||
|
2014
|
2013
|
% Change
|
||||||||
|
Research and development expense
|
$
|
28,354
|
|
$
|
21,076
|
|
35
|
%
|
||
|
Year Ended December 31,
|
||||||||||
|
2013
|
2012
|
% Change
|
||||||||
|
Research and development expense
|
$
|
21,076
|
|
$
|
14,614
|
|
44
|
%
|
||
|
Year Ended December 31,
|
||||||||||
|
2014
|
2013
|
% Change
|
||||||||
|
Selling, general and administrative expense
|
$
|
31,344
|
|
$
|
14,600
|
|
115
|
%
|
||
|
Year Ended December 31,
|
||||||||||
|
2013
|
2012
|
% Change
|
||||||||
|
Selling, general and administrative expense
|
$
|
14,600
|
|
$
|
6,585
|
|
122
|
%
|
||
|
Year Ended December 31,
|
||||||||||
|
2014
|
2013
|
% Change
|
||||||||
|
Non-operating income (expense)
|
$
|
15,616
|
|
$
|
(41,786
|
)
|
(137
|
)%
|
||
|
Year Ended December 31,
|
||||||||||
|
2013
|
2012
|
% Change
|
||||||||
|
Non-operating income (expense)
|
$
|
(41,786
|
)
|
$
|
(13,178
|
)
|
217
|
%
|
||
|
Payment Due by Period
|
||||||||||||||||
|
Less than 1 Year
|
1 to 3 Years
|
3 to 5 Years
|
Total
|
|||||||||||||
|
Long-term debt
(1)
|
$
|
4,451
|
|
$
|
5,001
|
|
$
|
—
|
|
$
|
9,452
|
|
||||
|
Cancelable license agreements
(2)
|
325
|
|
700
|
|
7,515
|
|
8,540
|
|
||||||||
|
Non-cancelable employment agreements
(2)
|
300
|
|
928
|
|
—
|
|
1,228
|
|
||||||||
|
Non-cancelable operating leases
(2)
|
72
|
|
157
|
|
152
|
|
381
|
|
||||||||
|
Total
|
$
|
5,148
|
|
$
|
6,786
|
|
$
|
7,667
|
|
$
|
19,601
|
|
||||
|
|
Page No.
|
|
Index to Financial Statements
|
|
|
December 31, 2014
|
December 31, 2013
|
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
23,650
|
|
$
|
47,787
|
|
|
|
Restricted cash
|
200
|
|
200
|
|
|||
|
Accounts receivable
|
1,839
|
|
3,683
|
|
|||
|
Inventories
|
655
|
|
386
|
|
|||
|
Prepaid expenses
|
2,680
|
|
1,399
|
|
|||
|
Total current assets
|
29,024
|
|
53,455
|
|
|||
|
Equipment and furnishings, net
|
555
|
|
665
|
|
|||
|
Abstral rights, net
|
14,533
|
|
14,979
|
|
|||
|
Zuplenz rights
|
8,101
|
|
—
|
|
|||
|
GALE-401 rights
|
9,255
|
|
—
|
|
|||
|
In-process research and development
|
12,864
|
|
12,864
|
|
|||
|
Goodwill
|
6,069
|
|
5,898
|
|
|||
|
Deposits and other assets
|
87
|
|
115
|
|
|||
|
Total assets
|
$
|
80,488
|
|
$
|
87,976
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
2,271
|
|
$
|
2,660
|
|
|
|
Accrued expenses and other current liabilities
|
15,669
|
|
8,699
|
|
|||
|
Fair value of warrants potentially settleable in cash
|
5,383
|
|
48,965
|
|
|||
|
Current portion of long-term debt
|
3,910
|
|
2,149
|
|
|||
|
Total current liabilities
|
27,233
|
|
62,473
|
|
|||
|
Deferred tax liability
|
5,053
|
|
5,053
|
|
|||
|
Contingent purchase price consideration
|
6,651
|
|
6,821
|
|
|||
|
Long-term debt, net of current portion
|
4,492
|
|
7,743
|
|
|||
|
Total liabilities
|
43,429
|
|
82,090
|
|
|||
|
Commitments and contingencies
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value; 200,000,000 shares authorized, 130,146,341 shares issued and 129,471,341 shares outstanding at December 31, 2014; 125,000,000 shares authorized, 110,100,701 shares issued and 109,425,701 outstanding at December 31, 2013
|
12
|
|
10
|
|
|||
|
Additional paid-in capital
|
256,377
|
|
188,600
|
|
|||
|
Accumulated deficit
|
(215,481
|
)
|
(178,875
|
)
|
|||
|
Less treasury shares at cost, 675,000 shares
|
(3,849
|
)
|
(3,849
|
)
|
|||
|
Total stockholders’ equity
|
37,059
|
|
5,886
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
80,488
|
|
$
|
87,976
|
|
|
|
For the Year Ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Net revenue
|
$
|
9,319
|
|
$
|
2,487
|
|
$
|
—
|
|
||
|
Costs and expenses:
|
|||||||||||
|
Cost of revenue (excluding amortization of certain acquired intangible assets)
|
1,403
|
|
520
|
|
—
|
|
|||||
|
Research and development
|
28,354
|
|
21,076
|
|
14,614
|
|
|||||
|
Selling, general and administrative
|
31,344
|
|
14,600
|
|
6,585
|
|
|||||
|
Amortization of certain acquired intangible assets
|
440
|
|
131
|
|
—
|
|
|||||
|
Total costs and expenses
|
61,541
|
|
36,327
|
|
21,199
|
|
|||||
|
Operating loss
|
(52,222
|
)
|
(33,840
|
)
|
(21,199
|
)
|
|||||
|
Non-operating income (expense):
|
|||||||||||
|
Gain (loss) on warrant exchange
|
16,556
|
|
(44,001
|
)
|
(10,775
|
)
|
|||||
|
Interest income (expense), net
|
(1,110
|
)
|
(807
|
)
|
(33
|
)
|
|||||
|
Other income (expense)
|
170
|
|
3,022
|
|
(2,370
|
)
|
|||||
|
Total non-operating income (expense), net
|
15,616
|
|
(41,786
|
)
|
(13,178
|
)
|
|||||
|
Loss from continuing operations before income taxes
|
(36,606
|
)
|
(75,626
|
)
|
(34,377
|
)
|
|||||
|
Income tax expense (benefit)
|
—
|
|
1,052
|
|
(1,052
|
)
|
|||||
|
Loss from continuing operations
|
(36,606
|
)
|
(76,678
|
)
|
(33,325
|
)
|
|||||
|
Loss from discontinued operations
|
—
|
|
—
|
|
(1,644
|
)
|
|||||
|
Net loss
|
$
|
(36,606
|
)
|
$
|
(76,678
|
)
|
$
|
(34,969
|
)
|
||
|
Net loss per common share:
|
|||||||||||
|
Basic and diluted per share, continuing operations
|
$
|
(0.31
|
)
|
$
|
(0.85
|
)
|
$
|
(0.53
|
)
|
||
|
Basic and diluted loss per share, discontinued operations
|
$
|
—
|
|
$
|
—
|
|
$
|
(0.03
|
)
|
||
|
Basic and diluted net loss per share
|
$
|
(0.31
|
)
|
$
|
(0.85
|
)
|
$
|
(0.56
|
)
|
||
|
Weighted-average common shares outstanding: basic and diluted
|
119,388,366
|
|
90,181,501
|
|
62,480,666
|
|
|||||
|
Comprehensive loss
|
|||||||||||
|
Net loss
|
$
|
(36,606
|
)
|
$
|
(76,678
|
)
|
$
|
(34,969
|
)
|
||
|
Reclassification of unrealized gain upon sale of marketable securities
|
—
|
|
(2,678
|
)
|
—
|
|
|||||
|
Unrealized gain on marketable securities
|
—
|
|
—
|
|
2,678
|
|
|||||
|
Tax effect of reclassification of unrealized gain upon sale of marketable securities
|
—
|
|
1,052
|
|
—
|
|
|||||
|
Tax effect of unrealized gain on marketable securities
|
—
|
|
—
|
|
(1,052
|
)
|
|||||
|
Total comprehensive loss
|
$
|
(36,606
|
)
|
$
|
(78,304
|
)
|
$
|
(33,343
|
)
|
||
|
Common Stock
|
Additional Paid-In Capital
|
Accumulated Other Comprehensive Income (Loss)
|
Accumulated Deficit
|
Treasury Stock
|
Total
|
|||||||||||||||||||||
|
Shares Issued
|
Amount
|
|||||||||||||||||||||||||
|
Balance at December 31, 2011
|
47,811,453
|
|
$
|
5
|
|
$
|
81,184
|
|
$
|
—
|
|
$
|
(67,228
|
)
|
$
|
(3,849
|
)
|
$
|
10,112
|
|
||||||
|
Issuance of common stock
|
25,486,960
|
|
2
|
|
36,376
|
|
—
|
|
—
|
|
—
|
|
36,378
|
|
||||||||||||
|
Common stock warrants issued in connection with 2012 common stock offering
|
—
|
|
—
|
|
(7,286
|
)
|
—
|
|
—
|
|
—
|
|
(7,286
|
)
|
||||||||||||
|
Issuance of common stock in exchange for services
|
288,285
|
|
—
|
|
364
|
|
—
|
|
—
|
|
—
|
|
364
|
|
||||||||||||
|
Issuance of common stock upon the exchange and exercise of warrants including reclassification of $10,843 in warrant liability upon exercise
|
8,433,003
|
|
1
|
|
16,550
|
|
—
|
|
—
|
|
—
|
|
16,551
|
|
||||||||||||
|
Repurchase of common stock warrants
|
—
|
|
—
|
|
(266
|
)
|
—
|
|
—
|
|
—
|
|
(266
|
)
|
||||||||||||
|
Issuance of common stock in connection with employee stock purchase plan
|
234,350
|
|
—
|
|
93
|
|
—
|
|
—
|
|
—
|
|
93
|
|
||||||||||||
|
Stock based compensation for directors and employees
|
—
|
|
—
|
|
794
|
|
—
|
|
—
|
|
—
|
|
794
|
|
||||||||||||
|
Stock based compensation for services
|
—
|
|
—
|
|
600
|
|
—
|
|
—
|
|
—
|
|
600
|
|
||||||||||||
|
Exercise of stock options
|
25,937
|
|
—
|
|
21
|
|
—
|
|
—
|
|
—
|
|
21
|
|
||||||||||||
|
Issuance of common stock in settlement of contingent purchase price consideration
|
1,315,849
|
|
—
|
|
1,579
|
|
—
|
|
—
|
|
—
|
|
1,579
|
|
||||||||||||
|
Net liabilities distributed in connection with the RXi spin-off
|
—
|
|
—
|
|
2,159
|
|
—
|
|
—
|
|
—
|
|
2,159
|
|
||||||||||||
|
Unrealized gain on marketable securities, net of tax benefit of $1,052
|
—
|
|
—
|
|
—
|
|
1,626
|
|
—
|
|
—
|
|
1,626
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(34,969
|
)
|
—
|
|
(34,969
|
)
|
||||||||||||
|
Balance at December 31, 2012
|
83,595,837
|
|
$
|
8
|
|
$
|
132,168
|
|
$
|
1,626
|
|
$
|
(102,197
|
)
|
$
|
(3,849
|
)
|
$
|
27,756
|
|
||||||
|
Issuance of common stock
|
20,125,000
|
|
2
|
|
37,537
|
|
—
|
|
—
|
|
—
|
|
37,539
|
|
||||||||||||
|
Common stock warrants issued in connection with September 2013 common stock offering
|
—
|
|
—
|
|
(8,238
|
)
|
—
|
|
—
|
|
—
|
|
(8,238
|
)
|
||||||||||||
|
Issuance of common stock upon exercise of warrants
|
5,320,669
|
|
—
|
|
22,064
|
|
—
|
|
—
|
|
—
|
|
22,064
|
|
||||||||||||
|
Issuance of common stock in settlement of contingent purchase price consideration
|
492,988
|
|
—
|
|
1,247
|
|
—
|
|
—
|
|
—
|
|
1,247
|
|
||||||||||||
|
Issuance of common stock warrants with long-term debt financing
|
—
|
|
—
|
|
351
|
|
—
|
|
—
|
|
—
|
|
351
|
|
||||||||||||
|
Issuance of common stock in exchange for services
|
99,998
|
|
—
|
|
211
|
|
—
|
|
—
|
|
—
|
|
211
|
|
||||||||||||
|
Issuance of common stock in connection with employee stock purchase plan
|
52,532
|
|
—
|
|
163
|
|
—
|
|
—
|
|
—
|
|
163
|
|
||||||||||||
|
Stock based compensation for directors and employees
|
—
|
|
—
|
|
1,886
|
|
—
|
|
—
|
|
—
|
|
1,886
|
|
||||||||||||
|
Stock based compensation for services
|
—
|
|
—
|
|
644
|
|
—
|
|
—
|
|
—
|
|
644
|
|
||||||||||||
|
Reclassification of unrealized gain upon the sale of marketable securities, net of tax of $1,052
|
—
|
|
—
|
|
—
|
|
(1,626
|
)
|
—
|
|
—
|
|
(1,626
|
)
|
||||||||||||
|
Exercise of stock options
|
413,677
|
|
—
|
|
567
|
|
—
|
|
—
|
|
—
|
|
567
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(76,678
|
)
|
—
|
|
(76,678
|
)
|
||||||||||||
|
Balance at December 31, 2013
|
110,100,701
|
|
$
|
10
|
|
$
|
188,600
|
|
$
|
—
|
|
$
|
(178,875
|
)
|
$
|
(3,849
|
)
|
$
|
5,886
|
|
||||||
|
Common Stock
|
Additional Paid-In Capital
|
Accumulated Other Comprehensive Income (Loss)
|
Accumulated Deficit
|
Treasury Stock
|
Total
|
|||||||||||||||||||||
|
Shares Issued
|
Amount
|
|||||||||||||||||||||||||
|
Issuance of common stock
|
6,633,008
|
|
$
|
1
|
|
$
|
10,704
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
10,705
|
|
||||||
|
Issuance of common stock under milestone achievement
|
4,381,215
|
|
—
|
|
9,340
|
|
—
|
|
—
|
|
—
|
|
9,340
|
|
||||||||||||
|
Issuance of common stock upon exercise of warrants
|
5,467.027
|
|
1
|
|
37,741
|
|
—
|
|
—
|
|
—
|
|
37,742
|
|
||||||||||||
|
Issuance of common stock in connection with employee stock purchase plan
|
114,630
|
|
—
|
|
263
|
|
—
|
|
—
|
|
—
|
|
263
|
|
||||||||||||
|
Stock based compensation for directors and employees
|
—
|
|
—
|
|
5,253
|
|
—
|
|
—
|
|
—
|
|
5,253
|
|
||||||||||||
|
Stock based compensation for services
|
—
|
|
—
|
|
134
|
|
—
|
|
—
|
|
—
|
|
134
|
|
||||||||||||
|
Exercise of stock options
|
3,449,760
|
|
—
|
|
4,342
|
|
—
|
|
—
|
|
—
|
|
4,342
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(36,606
|
)
|
—
|
|
(36,606
|
)
|
||||||||||||
|
Balance at December 31, 2014
|
130,146,341
|
|
$
|
12
|
|
$
|
256,377
|
|
$
|
—
|
|
$
|
(215,481
|
)
|
$
|
(3,849
|
)
|
$
|
37,059
|
|
||||||
|
For the Year Ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net loss
|
$
|
(36,606
|
)
|
$
|
(76,678
|
)
|
$
|
(34,969
|
)
|
||
|
Adjustment to reconcile net loss to net cash used in operating activities:
|
|||||||||||
|
Depreciation and amortization expense
|
889
|
|
452
|
|
49
|
|
|||||
|
Gain on sale of marketable securities
|
—
|
|
(3,911
|
)
|
—
|
|
|||||
|
Allowance for doubtful accounts
|
200
|
|
—
|
|
—
|
|
|||||
|
Deferred taxes
|
—
|
|
1,052
|
|
(1,052
|
)
|
|||||
|
Non-cash stock-based compensation
|
5,387
|
|
2,530
|
|
1,394
|
|
|||||
|
Fair value of common stock issued in exchange for services
|
—
|
|
211
|
|
364
|
|
|||||
|
Change in fair value of common stock warrants
|
(16,556
|
)
|
44,001
|
|
10,775
|
|
|||||
|
Change in fair value of contingent consideration
|
(170
|
)
|
926
|
|
2,370
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
1,644
|
|
(3,683
|
)
|
—
|
|
|||||
|
Inventories
|
(269
|
)
|
(386
|
)
|
—
|
|
|||||
|
Prepaid expenses and other assets
|
(1,253
|
)
|
(832
|
)
|
(396
|
)
|
|||||
|
Accounts payable
|
(389
|
)
|
684
|
|
641
|
|
|||||
|
Accrued expenses and other current liabilities
|
4,254
|
|
6,701
|
|
(149
|
)
|
|||||
|
Net cash used in operating activities
|
(42,869
|
)
|
(28,933
|
)
|
(20,973
|
)
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Change in restricted cash
|
—
|
|
(99
|
)
|
—
|
|
|||||
|
Cash paid for acquisition of Abstral rights
|
—
|
|
(15,143
|
)
|
—
|
|
|||||
|
Cash paid for acquisition of Zuplenz rights
|
(3,056
|
)
|
—
|
|
—
|
|
|||||
|
Cash paid for acquisition of GALE-401
|
(2,415
|
)
|
—
|
|
—
|
|
|||||
|
Proceeds from sale of marketable securities
|
—
|
|
3,911
|
|
—
|
|
|||||
|
Cash paid for purchase of equipment and furnishings
|
(57
|
)
|
(705
|
)
|
—
|
|
|||||
|
Cash transferred with the RXi spin-off
|
—
|
|
—
|
|
(87
|
)
|
|||||
|
Net cash used in investing activities
|
(5,528
|
)
|
(12,036
|
)
|
(87
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Net proceeds from issuance of common stock
|
10,704
|
|
37,539
|
|
36,378
|
|
|||||
|
Cash paid for repurchase of warrants
|
—
|
|
—
|
|
(266
|
)
|
|||||
|
Net proceeds from exercise of stock options
|
4,342
|
|
567
|
|
21
|
|
|||||
|
Proceeds from exercise of warrants
|
10,717
|
|
7,815
|
|
5,708
|
|
|||||
|
Proceeds from common stock issued in connection with ESPP
|
263
|
|
163
|
|
93
|
|
|||||
|
Net proceeds from issuance of RXi convertible notes payable
|
—
|
|
—
|
|
500
|
|
|||||
|
Net proceeds from issuance of long-term debt
|
—
|
|
9,865
|
|
—
|
|
|||||
|
Principal payments on long-term debt
|
(1,766
|
)
|
—
|
|
—
|
|
|||||
|
Net cash provided by financing activities
|
24,260
|
|
55,949
|
|
42,434
|
|
|||||
|
Net increase in cash and cash equivalents
|
(24,137
|
)
|
14,980
|
|
21,374
|
|
|||||
|
Cash and cash equivalents at the beginning of period
|
47,787
|
|
32,807
|
|
11,433
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
23,650
|
|
$
|
47,787
|
|
$
|
32,807
|
|
||
|
For the Year Ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Supplemental disclosure of cash flow information:
|
|||||||||||
|
Cash received during the periods for interest
|
$
|
15
|
|
$
|
19
|
|
$
|
1
|
|
||
|
Cash paid during the periods for interest
|
$
|
800
|
|
$
|
547
|
|
$
|
1
|
|
||
|
Supplemental disclosure of non-cash investing and financing activities:
|
|||||||||||
|
Fair value of warrants issued in connection with common stock recorded as cost of equity
|
$
|
—
|
|
$
|
8,238
|
|
$
|
7,286
|
|
||
|
Net liabilities distributed to common stock holders in the RXi spin-off, net of cash transferred
|
$
|
—
|
|
$
|
—
|
|
$
|
2,246
|
|
||
|
Reclassification of warrant liabilities upon exercise
|
$
|
27,026
|
|
$
|
14,249
|
|
$
|
10,843
|
|
||
|
Common stock issued in settlement of contingent purchase price consideration
|
$
|
—
|
|
$
|
1,247
|
|
$
|
1,579
|
|
||
|
Change in fair value of marketable securities before settlement
|
$
|
—
|
|
$
|
(2,678
|
)
|
$
|
2,678
|
|
||
|
Issuance of common stock in settlement of GALE-401 milestone
|
$
|
6,840
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Fair value of shares issued to acquire Zuplenz rights
|
$
|
2,500
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Future obligations for Zuplenz rights included in accrued expenses
|
$
|
2,716
|
|
$
|
—
|
|
$
|
—
|
|
||
|
•
|
Phase 3 Ongoing: Our Phase 3 PRESENT (
P
revention of
R
ecurrence in
E
arly-
S
tage, Node-Positive Breast Cancer with Low to Intermediate HER2
E
xpression with
NeuVax T
reatment) study is enrolling HER2 1+ and 2+ patients under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). The multinational, multicenter, randomized, double-blinded PRESENT trial is ongoing in North America, Western and Eastern Europe, and Israel. Additional information on the study can be found at
www.neuvax.com
.
|
|
•
|
Phase 2b Ongoing: A randomized, multicenter, investigator-sponsored, 300 patient Phase 2b clinical trial is enrolling HER2 1+/2+ node-positive and high-risk node-negative breast cancer patients to study NeuVax in combination with Herceptin
®
(trastuzumab; Genentech/Roche) in the adjuvant setting.
|
|
•
|
Phase 2 Ongoing: An investigator-sponsored trial is ongoing to study NeuVax in combination with Herceptin. The study will enroll 100 patients in neoadjuvant, node positive and negative HER2 IHC 3+ patients or HER2 gene-amplified breast cancer patients who are HLA A2+ or HLA A3+ and are determined to be at high-risk for recurrence. Partial funding for this trial comes from the Department of Defense (DoD) through the Congressionally Directed Medical Research Program (CDMRP) via legislation known as the Defense Appropriations Act. The grant was awarded under a Breast Cancer Research Program (BCRP) Breakthrough Award given to the lead investigator for the trial.
|
|
•
|
Phase 2 Planned: In January 2014, we partnered with Dr. Reddy’s Laboratories, Ltd. in India for the commercialization of NeuVax in that region. Dr. Reddy’s is responsible for running a Phase 2 gastric cancer trial of NeuVax in India that is expected to initiate in 2016.
|
|
Total Acquisition Date Fair Value
|
||||
|
Purchase price consideration:
|
||||
|
Cash and cash equivalents
|
$
|
3,056
|
|
|
|
Common stock
|
2,482
|
|
||
|
Liabilities assumed:
|
||||
|
Future milestone payments
|
740
|
|
||
|
Credit memos for expiring channel inventory
|
1,995
|
|
||
|
Total consideration
|
$
|
8,273
|
|
|
|
Asset acquired:
|
||||
|
Zuplenz rights
|
$
|
8,101
|
|
|
|
Goodwill
|
172
|
|
||
|
Fair value of assets acquired
|
$
|
8,273
|
|
|
|
Description
|
December 31, 2014
|
Quoted Prices In
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
19,477
|
|
$
|
19,477
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total assets measured and recorded at fair value
|
$
|
19,477
|
|
$
|
19,477
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrants potentially settleable in cash
|
$
|
5,383
|
|
$
|
—
|
|
$
|
5,383
|
|
$
|
—
|
|
|||
|
Contingent purchase price consideration
|
6,651
|
|
—
|
|
—
|
|
6,651
|
|
|||||||
|
Total liabilities measured and recorded at fair value
|
$
|
12,034
|
|
$
|
—
|
|
$
|
5,383
|
|
$
|
6,651
|
|
|||
|
Description
|
December 31, 2013
|
Quoted Prices In
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
42,349
|
|
$
|
42,349
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total assets measured and recorded at fair value
|
$
|
42,349
|
|
$
|
42,349
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrants potentially settleable in cash
|
$
|
48,965
|
|
$
|
—
|
|
$
|
48,965
|
|
$
|
—
|
|
|||
|
Contingent purchase price consideration
|
6,821
|
|
—
|
|
—
|
|
6,821
|
|
|||||||
|
Total liabilities measured and recorded at fair value
|
$
|
55,786
|
|
$
|
—
|
|
$
|
48,965
|
|
$
|
6,821
|
|
|||
|
|
Fair Value
Measurements
Using Significant
Unobservable
Inputs
(Level 3)
|
||
|
Balance, January 1, 2013
|
$
|
7,142
|
|
|
Milestone payment
|
(1,247
|
)
|
|
|
Change in the estimated fair value of the contingent purchase price consideration
|
926
|
|
|
|
Balance, December 31, 2013
|
6,821
|
|
|
|
Change in the estimated fair value of the contingent purchase price consideration
|
(170
|
)
|
|
|
Balance at December 31, 2014
|
$
|
6,651
|
|
|
December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Clinical development expense
|
$
|
6,967
|
|
$
|
3,109
|
|
|
|
Patient assistance programs
|
2,444
|
|
2,618
|
|
|||
|
Compensation and related benefits
|
2,198
|
|
1,999
|
|
|||
|
Credit memos for expiring Zuplenz channel inventory
|
1,995
|
|
—
|
|
|||
|
Professional fees
|
860
|
|
745
|
|
|||
|
Zuplenz milestone payments
|
740
|
|
—
|
|
|||
|
Royalties
|
408
|
|
158
|
|
|||
|
Interest expense
|
57
|
|
70
|
|
|||
|
Accrued expenses and other current liabilities
|
$
|
15,669
|
|
$
|
8,699
|
|
|
|
For the year ending December 31, 2015
|
$
|
3,910
|
|
|
|
2016
|
4,254
|
|
||
|
Total future principal payments
|
8,164
|
|
||
|
Unamortized debt issuance costs (net of fair value of warrants issued)
|
238
|
|
||
|
Total debt
|
8,402
|
|
||
|
Less current portion
|
(3,910
|
)
|
||
|
Total long-term debt, net
|
$
|
4,492
|
|
|
|
Operating
Leases
(1)
|
Non-Cancelable
Employment
Agreements
(2)
|
Subtotal
|
Cancelable
License
Agreements
(3)
|
Total
|
|||||||||||||||
|
2015
|
$
|
74
|
|
$
|
828
|
|
$
|
902
|
|
$
|
350
|
|
$
|
1,252
|
|
||||
|
2016
|
83
|
|
100
|
|
183
|
|
350
|
|
533
|
|
|||||||||
|
2017
|
82
|
|
—
|
|
82
|
|
350
|
|
432
|
|
|||||||||
|
2018
|
70
|
|
—
|
|
70
|
|
350
|
|
420
|
|
|||||||||
|
2019 and thereafter
|
—
|
|
—
|
|
—
|
|
6,815
|
|
6,815
|
|
|||||||||
|
Total
|
$
|
309
|
|
$
|
928
|
|
$
|
1,237
|
|
$
|
8,215
|
|
$
|
9,452
|
|
||||
|
(1)
|
Operating leases are primarily facility and equipment related obligations with third party vendors. Operating lease expenses during the years ended December 31, 2014, 2013, and 2012 were approximately
$72,000
,
$77,000
and
$139,000
, respectively.
|
|
(2)
|
Employment agreement obligations include management contracts, as well as scientific advisory board member compensation agreements. Certain agreements, which have been revised from time to time, provide for minimum salary levels, adjusted annually at the discretion of the Compensation Committee, as well as for minimum bonuses that are payable.
|
|
(3)
|
License agreements generally relate to the company’s obligations with The Board of Regents, University of Texas and Henry M. Jackson Foundation for our oncology therapies. The company continually assesses the progress of its licensed technology and the progress of its research and development efforts as it relates to its licensed technology and may terminate with notice to the licensor at any time. In the event these licenses are terminated, no amounts will be due.
|
|
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
March
2011
Warrants
|
March
2010
Warrants
|
August
2009
Warrants
|
Consultant
and Oxford Warrants
|
Total
|
||||||||||||||||
|
Outstanding, January 1, 2013
|
—
|
|
7,578
|
|
2,846
|
|
361
|
|
360
|
|
978
|
|
1,093
|
|
13,216
|
|
|||||||
|
Granted
|
7,044
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
182
|
|
7,226
|
|
|||||||
|
Exercised
|
(602
|
)
|
(2,661
|
)
|
(1,688
|
)
|
(185
|
)
|
(70
|
)
|
—
|
|
(196
|
)
|
(5,402
|
)
|
|||||||
|
Expired
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(190
|
)
|
(190
|
)
|
|||||||
|
Outstanding, December 31, 2013
|
6,442
|
|
4,917
|
|
1,158
|
|
176
|
|
290
|
|
978
|
|
889
|
|
14,850
|
|
|||||||
|
Granted
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
300
|
|
300
|
|
|||||||
|
Exercised
|
(2,469
|
)
|
(1,886
|
)
|
(543
|
)
|
—
|
|
(265
|
)
|
(62
|
)
|
(469
|
)
|
(5,694
|
)
|
|||||||
|
Expired
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(916
|
)
|
—
|
|
(916
|
)
|
|||||||
|
Outstanding, December 31, 2014
|
3,973
|
|
3,031
|
|
615
|
|
176
|
|
25
|
|
—
|
|
720
|
|
8,540
|
|
|||||||
|
Expiration
|
September 2018
|
December 2017
|
April 2017
|
March 2016
|
March 2016
|
August 2014
|
Varies 2014-2020
|
||||||||||||||||
|
|
As of December 31, 2014
|
||||||||||||||||||||||
|
|
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
March
2011
Warrants
|
March
2010
Warrants
|
August
2009
Warrants
|
|||||||||||||||||
|
Strike price
|
$
|
2.50
|
|
$
|
1.90
|
|
$
|
0.65
|
|
$
|
0.65
|
|
$
|
2.15
|
|
$
|
4.50
|
|
|||||
|
Expected term (years)
|
3.72
|
|
2.98
|
|
2.31
|
|
1.18
|
|
1.24
|
|
0.00
|
|
|||||||||||
|
Volatility %
|
75.60
|
%
|
76.85
|
%
|
78.24
|
%
|
77.38
|
%
|
77.12
|
%
|
—
|
%
|
|||||||||||
|
Risk-free rate %
|
1.30
|
%
|
1.09
|
%
|
0.80
|
%
|
0.32
|
%
|
0.35
|
%
|
—
|
%
|
|||||||||||
|
|
As of December 31, 2013
|
||||||||||||||||||||||
|
|
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
March
2011
Warrants
|
March
2010
Warrants
|
August
2009
Warrants
|
|||||||||||||||||
|
Strike price
|
$
|
2.50
|
|
$
|
1.90
|
|
$
|
0.65
|
|
$
|
0.65
|
|
$
|
2.15
|
|
$
|
4.50
|
|
|||||
|
Expected term (years)
|
4.72
|
|
3.98
|
|
3.31
|
|
2.18
|
|
2.24
|
|
0.59
|
|
|||||||||||
|
Volatility %
|
71.97
|
%
|
71.38
|
%
|
71.71
|
%
|
73.45
|
%
|
73.36
|
%
|
66.85
|
%
|
|||||||||||
|
Risk-free rate %
|
1.61
|
%
|
1.25
|
%
|
0.93
|
%
|
0.45
|
%
|
0.47
|
%
|
0.11
|
%
|
|||||||||||
|
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
March
2011
Warrants
|
March
2010
Warrants
|
August
2009
Warrants
|
Total
|
|||||||||||||||||||||
|
Warrant liability, January 1, 2013
|
$
|
—
|
|
$
|
6,954
|
|
$
|
3,310
|
|
$
|
378
|
|
$
|
187
|
|
$
|
135
|
|
$
|
10,964
|
|
||||||
|
Fair value of warrants granted
|
8,238
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,238
|
|
|||||||||||||
|
Fair value of warrants exercised
|
(1,931
|
)
|
(8,482
|
)
|
(3,455
|
)
|
(260
|
)
|
(121
|
)
|
—
|
|
(14,249
|
)
|
|||||||||||||
|
Change in fair value of warrants
|
16,643
|
|
19,588
|
|
5,214
|
|
645
|
|
879
|
|
1,043
|
|
44,012
|
|
|||||||||||||
|
Warrant liability, December 31, 2013
|
22,950
|
|
18,060
|
|
5,069
|
|
763
|
|
945
|
|
1,178
|
|
48,965
|
|
|||||||||||||
|
Fair value of warrants granted
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Fair value of warrants exercised
|
(12,713
|
)
|
(10,086
|
)
|
(2,906
|
)
|
—
|
|
(1,159
|
)
|
(162
|
)
|
(27,026
|
)
|
|||||||||||||
|
Change in fair value of warrants
|
(7,677
|
)
|
(5,947
|
)
|
(1,538
|
)
|
(600
|
)
|
222
|
|
(1,016
|
)
|
(16,556
|
)
|
|||||||||||||
|
Warrant liability, December 31, 2014
|
$
|
2,560
|
|
$
|
2,027
|
|
$
|
625
|
|
$
|
163
|
|
$
|
8
|
|
$
|
—
|
|
$
|
5,383
|
|
||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Research and development
|
$
|
484
|
|
$
|
754
|
|
$
|
580
|
|
||
|
Selling, general, and administrative
|
4,903
|
|
2,150
|
|
1,179
|
|
|||||
|
Total stock-based compensation
|
$
|
5,387
|
|
$
|
2,904
|
|
$
|
1,759
|
|
||
|
2014
|
2013
|
2012
|
||||||
|
Risk free interest rate
|
2.01
|
%
|
1.57
|
%
|
1.05
|
%
|
||
|
Volatility
|
79.37
|
%
|
77.98
|
%
|
75.76
|
%
|
||
|
Expected lives (years)
|
6.16
|
|
6.25
|
|
6.13
|
|
||
|
Expected dividend yield
|
0.00
|
%
|
0.00
|
%
|
0.00
|
%
|
||
|
Total
Number of
Shares
(In Thousands)
|
Weighted
Average
Exercise
Price
|
|||||
|
Outstanding at December 31, 2013
|
13,159
|
|
$
|
2.73
|
|
|
|
Granted
|
1,375
|
|
2.50
|
|
||
|
Exercised
|
(3,608
|
)
|
1.31
|
|
||
|
Cancelled
|
(2,336
|
)
|
2.85
|
|
||
|
Outstanding at December 31, 2014
|
8,590
|
|
$
|
3.25
|
|
|
|
Options exercisable at December 31, 2014
|
5,544
|
|
$
|
3.56
|
|
|
|
Year Ended December 31,
|
||||||||||||
|
|
2014
|
2013
|
2012
|
|||||||||
|
Realized gain on sale of marketable securities
|
$
|
—
|
|
$
|
3,911
|
|
$
|
—
|
|
|||
|
Change in fair value of the contingent purchase price liability
|
170
|
|
(926
|
)
|
(2,370
|
)
|
||||||
|
Miscellaneous other income
|
—
|
|
37
|
|
—
|
|
||||||
|
Total other income (expense)
|
$
|
170
|
|
$
|
3,022
|
|
$
|
(2,370
|
)
|
|||
|
|
December 31,
|
||||
|
|
2014
|
2013
|
|||
|
Warrants to purchase common stock
|
8,540
|
|
14,850
|
|
|
|
Options to purchase common stock
|
8,590
|
|
13,159
|
|
|
|
Total
|
17,130
|
|
28,009
|
|
|
|
|
As of December 31,
|
|||||||||||
|
|
2014
|
2013
|
2012
|
|||||||||
|
Current
|
||||||||||||
|
Federal
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
State
|
—
|
|
—
|
|
—
|
|
||||||
|
Total current
|
—
|
|
—
|
|
—
|
|
||||||
|
Deferred expense (benefit)
|
||||||||||||
|
Federal
|
—
|
|
894
|
|
(894
|
)
|
||||||
|
State
|
—
|
|
158
|
|
(158
|
)
|
||||||
|
Total deferred
|
—
|
|
1,052
|
|
(1,052
|
)
|
||||||
|
Total income tax expense (benefit)
|
$
|
—
|
|
$
|
1,052
|
|
$
|
(1,052
|
)
|
|||
|
|
As of December 31,
|
|||||||
|
|
2014
|
2013
|
||||||
|
Net operating loss carryforwards
|
$
|
53,950
|
|
$
|
33,539
|
|
||
|
Tax credit carryforwards
|
3,590
|
|
3,549
|
|
||||
|
Stock based compensation
|
4,676
|
|
8,322
|
|
||||
|
Other
|
190
|
|
12
|
|
||||
|
Licensing deduction deferral
|
8,919
|
|
8,682
|
|
||||
|
Gross deferred tax assets
|
71,325
|
|
54,104
|
|
||||
|
Valuation allowance
|
(71,325
|
)
|
(54,104
|
)
|
||||
|
Net deferred tax asset
|
$
|
—
|
|
$
|
—
|
|
||
|
|
As of December 31,
|
|||||||
|
|
2014
|
2013
|
||||||
|
In-process research and development not subject to future amortization for tax purposes
|
$
|
5,053
|
|
$
|
5,053
|
|
||
|
Gross deferred tax liability
|
$
|
5,053
|
|
$
|
5,053
|
|
||
|
|
As of December 31,
|
|||||||||||
|
|
2014
|
2013
|
2012
|
|||||||||
|
Expected federal income tax benefit
|
$
|
(12,447
|
)
|
$
|
(25,713
|
)
|
$
|
(11,688
|
)
|
|||
|
State income taxes after credits
|
(1,283
|
)
|
(3,676
|
)
|
(1,067
|
)
|
||||||
|
Unrealized gain on marketable securities
|
—
|
|
1,052
|
|
(1,052
|
)
|
||||||
|
Changes in warrant value
|
(6,503
|
)
|
17,283
|
|
3,664
|
|
||||||
|
Stock compensation
|
3,996
|
|
813
|
|
152
|
|
||||||
|
Effect of change in valuation allowance
|
17,275
|
|
11,408
|
|
8,939
|
|
||||||
|
Income tax credits
|
(42
|
)
|
(240
|
)
|
—
|
|
||||||
|
Other
|
(996
|
)
|
125
|
|
—
|
|
||||||
|
$
|
—
|
|
$
|
1,052
|
|
$
|
(1,052
|
)
|
||||
|
Year ended December 31,
|
||||||
|
2014
|
2013
|
|||||
|
Customer A
|
43
|
%
|
25
|
%
|
||
|
Customer B
|
18
|
%
|
6
|
%
|
||
|
Customer C
|
14
|
%
|
26
|
%
|
||
|
Customer D
|
11
|
%
|
34
|
%
|
||
|
December 31,
|
||||||
|
2014
|
2013
|
|||||
|
Customer A
|
24
|
%
|
25
|
%
|
||
|
Customer B
|
31
|
%
|
1
|
%
|
||
|
Customer C
|
16
|
%
|
11
|
%
|
||
|
Customer D
|
21
|
%
|
54
|
%
|
||
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
|||||||||||||
|
2014
|
||||||||||||||||
|
Net revenue
|
$
|
2,173
|
|
$
|
2,331
|
|
$
|
1,620
|
|
$
|
3,195
|
|
||||
|
Gross profit on net revenue
(1)
|
$
|
1,751
|
|
$
|
1,886
|
|
$
|
1,303
|
|
$
|
2,536
|
|
||||
|
Net loss
|
$
|
(2,536
|
)
|
$
|
(19,941
|
)
|
$
|
(6,173
|
)
|
$
|
(7,506
|
)
|
||||
|
Net loss per share
|
$
|
(0.02
|
)
|
$
|
(0.17
|
)
|
$
|
(0.05
|
)
|
$
|
(0.06
|
)
|
||||
|
2013
|
||||||||||||||||
|
Net revenue
|
$
|
—
|
|
$
|
—
|
|
$
|
1,170
|
|
$
|
1,317
|
|
||||
|
Gross profit on net revenue
(1)
|
$
|
—
|
|
$
|
—
|
|
$
|
869
|
|
$
|
967
|
|
||||
|
Net loss
|
$
|
(9,293
|
)
|
$
|
(9,597
|
)
|
$
|
(9,287
|
)
|
$
|
(48,501
|
)
|
||||
|
Net loss per share
|
$
|
(0.11
|
)
|
$
|
(0.11
|
)
|
$
|
(0.11
|
)
|
$
|
(0.46
|
)
|
||||
|
(1)
|
Gross profit is calculated by taking net revenue less cost of revenue and amortization of certain acquired intangible assets.
|
|
Exhibit
Number
|
Description
|
||
|
1.1
|
At Market Issuance Sales Agreement dated May 24, 2013 between Registrant and Maxim Group LLC.(31)
|
||
|
1.2
|
At Market Issuance Sales agreements dated May 24, 2013 between Registrant and MLV & Co. LLC.(31)
|
||
|
1.3
|
Underwriting Agreement dated as of September 13, 2013 by and between Galena Biopharma, Inc. and Oppenheimer & Co. Inc. as representative of the several underwriters named in Schedule I thereto. (1)
|
||
|
1.4
|
Underwriting Agreement dated as of April 5, 2012 by and between Galena Biopharma, Inc. and Roth Capital Partners, LLC, as representative of the several underwriters named therein.(22)
|
||
|
1.5
|
Purchase Agreement dated as of December 18, 2012 by and between Galena Biopharma, Inc. and Piper Jaffray & Co.(20)
|
||
|
2.1
|
Unit Purchase Agreement, dated as of January 12, 2014, between Galena Biopharma, Inc. and Mills Pharmaceuticals, LLC.+(23)
|
||
|
3.1
|
Amended and Restated Certificate of Incorporation of Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), as amended as of June 28, 2013.(2)
|
||
|
3.2
|
Certificate of Ownership and Merger.(12)
|
||
|
3.3
|
Amended and Restated By-Laws of Galena Biopharma, Inc., as amended as of August 6, 2013. (2)
|
||
|
4.1
|
Form of Warrant Agreement by and Galena Biopharma, Inc., Computershare Inc. and Computershare Trust Company, N.A. (1)
|
||
|
4.2
|
Warrant No. A-1 in favor of J.P. Turner Partners, LP, dated August 7, 2008. (15)
|
||
|
4.3
|
Form of Common Stock Purchase Warrant issued in August 2009.(16)
|
||
|
4.4
|
Form of Common Stock Purchase Warrant issued in March 2010.(17)
|
||
|
4.5
|
Form of Five-Year Common Stock Purchase Warrant issued in March 2011.(18)
|
||
|
4.6
|
Form of Common Stock Purchase Warrant issued in April 2011.(19)
|
||
|
4.7
|
Warrant No. 2012-1 in favor of Legend Securities, Inc. issued in February 2012.(4)
|
||
|
4.8
|
Form of December 2012 Warrant.(20)
|
||
|
4.9
|
Registration Rights Agreement, dated January 12, 2014, between Galena Biopharma, Inc. and each former owner of membership units of Mills Pharmaceuticals, LLC. (23)
|
||
|
4.10
|
Form of warrants granted on May 8, 2013 under the Loan and Security Agreement set forth as Exhibit 10.25.(21)
|
||
|
10.1
|
Form of Contingent Value Rights Agreement among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Computershare Trust Company, N.A., Computershare Inc., and Robert E Kennedy, dated April 13, 2011.(3)
|
||
|
10.2
|
First Amendment to Contingent Value Rights Agreement among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Computershare Trust Company, N.A., Computershare Inc., and Robert E Kennedy, dated February 15, 2012.(4)
|
||
|
10.3
|
Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(8)
|
||
|
10.4
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(9)
|
||
|
10.5
|
Form of Incentive Stock Option.*(10)
|
||
|
10.6
|
Form of Non-qualified Stock Option.*(10)
|
||
|
10.7
|
Patent and Technology License Agreement, dated September 11, 2006, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).+(5))
|
||
|
10.8
|
Amendment No. 1 to Patent and Technology License Agreement, dated December 21, 2007, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).(5)
|
||
|
10.9
|
Amendment No. 2 to Patent and Technology License Agreement, dated September 3, 2008, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).(5)
|
||
|
10.10
|
Amendment No. 3 to Patent and Technology License Agreement, dated July 8, 2009, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).(5)
|
||
|
10.11
|
Amendment No. 4 to Patent and Technology License Agreement, dated February 11, 2010, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).+(5)
|
||
|
10.12
|
Amendment No. 5 to Patent and Technology License Agreement, dated January 10, 2011, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).+(5)
|
||
|
10.13
|
Scientific Advisory Agreement between Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) and George E. Peoples, Ph.D., dated May 1, 2011.(6)
|
||
|
10.14
|
Exclusive License Agreement, dated as of July 11, 2011, by and among The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) and its wholly-owned subsidiary, Apthera, Inc.+(5)
|
||
|
10.15
|
Agreement and Plan of Merger by and among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Diamondback Acquisition Corp., Apthera, Inc. and Robert E. Kennedy, in his capacity as the Stockholder Representative, dated March 31, 2011.(7)
|
||
|
10.16
|
Exclusive License Agreement, dated effective as of September 16, 2011, by and among The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), The Board of Regents of the University of Texas System and The University of Texas M.D. Anderson Cancer Center.+(11)
|
||
|
10.17
|
Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Employee Stock Purchase Plan.*(13)
|
||
|
10.18
|
License Agreement, effective as of April 30, 2009, between Kwangdong Pharmaceutical Co., Ltd. and Apthera, Inc.+(4)
|
||
|
10.19
|
Amendment No. 1 to License Agreement, dated as of January 13, 2012, by and among Apthera, Inc., Kwangdong Pharmaceutical Co., Ltd., and Galena Biopharma, Inc.(4)
|
||
|
10.20
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(27)
|
||
|
10.21
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(28)
|
||
|
10.22
|
License and Supply Agreement, effective December 3, 2012, between Galena Biopharma, Inc. and ABIC Marketing Limited, a subsidiary of Teva Pharmaceuticals.+(6)
|
||
|
10.23
|
Asset Purchase Agreement dated March 15, 2013 between Galena Biopharma, Inc. and Orexo AB.+(21)
|
||
|
10.24
|
License Agreement dated March 15, 2013 between Galena Biopharma, Inc. and Orexo AB.(21)
|
||
|
10.25
|
Loan and Security Agreement dated May 8, 2013 among Galena Biopharma, Inc., Apthera, Inc., Oxford Finance LLC and the Lenders listed on Schedule 1.1 thereto.(21)
|
||
|
10.26
|
Lease between Galena Biopharma, Inc. and Cameron Oregon properties LLC and Lucas Oregon Properties, LLC for Suite 270 in the Willamette Wharf Building at 4640 Macadam Avenue in Portland, Oregon dated April 25, 2013.(2)
|
||
|
10.27
|
License and Development Agreement, dated January 13, 2014, between Galena Biopharma, Inc. and Dr. Reddy’s Laboratories, Ltd.+(23)
|
||
|
10.28
|
Exclusive License Agreement, dated as of December 20, 2013, between Mills Pharmaceuticals, LLC and BioVascular, Inc.+(23)
|
||
|
10.29
|
Employment letter agreement, effective May 1, 2014, between Galena Biopharma, Inc. and Ryan M. Dunlap.*(26)
|
||
|
10.30
|
License and Supply Agreement dated as of July 17, 2014 between Galena Biopharma, Inc. and MonoSol Rx, LLC.+(25)
|
||
|
10.31
|
Employment Agreement, dated September 16, 2014, between Galena Biopharma, Inc. and Mark W. Schwartz, Ph.D.*(24)
|
||
|
10.32
|
Employment Agreement, dated July 28, 2014, between Galena Biopharma, Inc. and Margaret Kivinski.*(29)
|
||
|
10.33
|
Purchase Agreement, dated as of November 18, 2014, by and between Galena Biopharma, Inc. and Lincoln Park Capital Fund, LLC.(30)
|
||
|
14.1
|
Code of Ethics and Conduct.(14)
|
||
|
21.1
|
Subsidiaries of the Registrant.(23)
|
||
|
23.1
|
Consent of Moss Adams LLP, Independent Registered Public Accounting Firm.**
|
||
|
23.2
|
Consent of BDO USA LLP, Independent Registered Public Accounting Firm.**
|
||
|
31.1
|
Sarbanes-Oxley Act Section 302 Certification of Mark W. Schwartz, Ph.D.**
|
||
|
31.2
|
Sarbanes-Oxley Act Section 302 Certification of Ryan M. Dunlap.**
|
||
|
32.1
|
Sarbanes-Oxley Act Section 906 Certification of Mark W. Schwartz, Ph.D., and Ryan M. Dunlap.**
|
||
|
101.INS
|
XBRL Instance Document.
|
||
|
101.SCH
|
XBRL Taxonomy Extension Schema.
|
||
|
101.CAL
|
XBRL Taxonomy Extension Calculation.
|
||
|
101.DEF
|
XBRL Taxonomy Extension Definition.
|
||
|
101.LAB
|
XBRL Taxonomy Extension Label.
|
||
|
101.PRE
|
XBRL Taxonomy Extension Presentation.
|
||
|
101.PRE
|
XBRL Taxonomy Extension Presentation.
|
||
|
(1)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 13, 2013 (File No. 001-33958) and incorporated herein by reference.
|
|
(2)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 9, 2013 (File No. 001-33958) and incorporated herein by reference.
|
|
(3)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 14, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(4)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on March 28, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(5)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 15, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(6)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on March 12, 2013 (File No. 001-33958) and incorporated by reference herein.
|
|
(7)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 5, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(8)
|
Previously filed as Annex A to the Company’s Proxy Statement on Schedule 14A filed on April 23, 2010 (File No. 001-33958) and incorporated by reference herein.
|
|
(9)
|
Previously filed as Annex A to the Company’s Proxy Statement on Schedule 14A filed on May 31, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(10)
|
Previously filed as an Exhibit to the Company’s Registration Statement on Form S-1 filed on October 30, 2007 (File No. 333-147009) and incorporated by reference herein.
|
|
(11)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 21, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(12)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 26, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(13)
|
Previously filed as Annex B to the Company's Proxy Statement on Schedule 14A, filed on April 23, 2010 (File No. 001-33958) and incorporated by reference herein.
|
|
(14)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on April 15, 2008 (File No. 001-33958) and incorporated by reference herein.
|
|
(15)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on November 14, 2008 (File No. 001-33958) and incorporated by reference herein.
|
|
(16)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on July 31, 2009 (File No. 001-33958) and incorporated by reference herein.
|
|
(17)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on March 23, 2010 (File No. 001-33958) and incorporated by reference herein.
|
|
(18)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on March 1, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(19)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 15, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(20)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on December 19, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(21)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on May 9, 2013 (File No. 001-33958) and incorporated by reference herein.
|
|
(22)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 5, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(23)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on March 17, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(24)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 18, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(25)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 11, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(26)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on May 6, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(27)
|
Previously filed as Annex A to the Company’s Proxy Statement on Schedule 14A filed on April 30, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(28)
|
Previously filed as Annex B to the Company’s Proxy Statement on Schedule 14A filed on April 29, 2013 (File No. 001-33958) and incorporated by reference herein.
|
|
(29)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on November 5, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(30)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on November 20, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(31)
|
Previously filed as an Exhibit to the Company’s Registration Statement on Form S-3 filed on May 24, 2013 (File No. 333-188849) and incorporated by reference herein.
|
|
*
|
Indicates a management contract or compensatory plan or arrangement.
|
|
**
|
Filed herewith.
|
|
+
|
This exhibit was filed separately with the Commission pursuant to an application for confidential treatment. The confidential portions of the exhibit have been omitted and have been marked by an asterisk.
|
|
GALENA BIOPHARMA, INC.
|
|||
|
By:
|
/s/
Mark W. Schwartz
|
||
|
Mark W. Schwartz, Ph.D.
|
|||
|
President and Chief Executive Officer
|
|||
|
Date: March 5, 2015
|
|||
|
By:
|
/s/
Ryan M. Dunlap
|
||
|
Ryan M. Dunlap
|
|||
|
Vice President, Chief Financial Officer
|
|||
|
Date: March 5, 2015
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Mark W. Schwartz
|
President, Chief Executive Officer and Director (Principal Executive Officer)
|
March 5, 2015
|
||
|
Mark W. Schwartz, Ph. D.
|
||||
|
/s/ Ryan M. Dunlap
|
Vice President, Chief Financial Officer (Principal Financial and Accounting Officer)
|
March 5, 2015
|
||
|
Ryan M. Dunlap
|
||||
|
/s/ Sanford J. Hillsberg
|
Director, Chairman of the Board
|
March 5, 2015
|
||
|
Sanford J. Hillsberg
|
||||
|
/s/ William L. Ashton
|
Director
|
March 5, 2015
|
||
|
William L. Ashton
|
||||
|
/s/ Richard Chin
|
Director
|
March 5, 2015
|
||
|
Richard Chin, M.D.
|
||||
|
/s/ Irving M. Einhorn
|
Director
|
March 5, 2015
|
||
|
Irving M. Einhorn
|
||||
|
/s/ Stephen S. Galliker
|
Director
|
March 5, 2015
|
||
|
Stephen S. Galliker
|
||||
|
/s/ Steven A. Kriegsman
|
Director
|
March 5, 2015
|
||
|
Steven A. Kriegsman
|
||||
|
/s/ Rudolph Nisi
|
Director
|
March 5, 2015
|
||
|
Rudolph Nisi, M.D.
|
||||